User login
Lucas Franki is an associate editor for MDedge News, and has been with the company since 2014. He has a BA in English from Penn State University and is an Eagle Scout.
Werewolves of Vallejo and a haunted-house doctor’s note
A crappy excuse of a database
Have you ever been so impressed with your bowel movement that you’ve been compelled to record the incident for posterity? No? Just us? Well, you may want to reconsider, because a pair of AI tech companies are looking for a few good poop pictures.
It’s all part of the “Give a S--t” (you can probably guess what we’ve censored out) campaign, a joint venture from Auggi, a gut health start-up, and Seed Health. The companies hope to use photos sent in by regular people to build an app that would help people with chronic gut problems automatically track their own bowel movements. In addition, the photo library could also be used for research into gut-related diseases such as irritable bowl syndrome.
The two companies hope to collect 100,000 photos for their library, which is an absolutely prodigious amount of poop to sort through. But hey, that’s what the AI is for. They already know the AI works, as Auggi created a proof-of-concept library of 36,000 images of faux feces made from blue Play-Doh. The AI was able to recognize consistency according to the Bristol scale basically 100% of the time.
If you’ve been inspired, you can submit your lovely poop pictures here. Seed and Auggi expect contributers to send only one image each, but multiple submissions are welcome. They’ve already received a dozen from LOTME world headquarters. We love a good bowel movement here.
Criminal moon
“The Wolf Man.” “An American Werewolf in London.” “The Howling.” “Teen Wolf.” All terrifying Hollywood tales of bloodthirsty behavior and sanguinary slaughter. (Michael J. Fox as a hirsute homicidal lycan? Okay, maybe not “Teen Wolf.”)
And the propellant igniting all that criminal lycanthropy? The full moon.
Any teacher will swear a full moon portends the kind of student behavior that an entire pot of teachers’ lounge coffee can’t counter. And every cop knows it’s going to be a “Training Day” shift when the lunar light shines brightest.
But is the Thin Blue Line truly stretched to snapping during a full moon? New York University’s BetaGov research team looked at the purported “lunar effect” linking crime and the full moon. A lit review revealed mixed findings for and against a criminal lunar effect. The team then collaborated with the Vallejo, Calif., police department to match the moon’s phases with the city’s crime events. They did the same with departments in Canada and Mexico.
The results? A full moon had no effect on Vallejo’s crime rate, or anywhere else in North America.
While the finding eviscerates the moon-induced mayhem hypothesis, cops walking a full-moonlit beat can at least take comfort in this fact: Unlike London, Vallejo is clearly free of American werewolves.
A doctor’s note … of terror
With Halloween upon us, here’s a veddy scary riddle: When is a sports physical not a sports physical?
When it’s a haunted house physical.
Specifically, when the haunted house is McKamey Manor in Summertown, Tenn. … and in Huntsville, Ala. That’s right, it can be in two places at the same time. Terrifying.
McKamey Manor is considered by many to be the most terrifying haunted house in the United States, and by some to be a “torture chamber under disguise.”
The “Surivial [we think they misspelled it on purpose to make it even scarier] Horror Challenge” is so terrifying that management requires all participants to have a “completed ‘sports physical’ and doctor’s letter stating you are physically and mentally cleared,” as well as proof of medical insurance. Each paying customer also has to “pass a portable drug test on the day of the show,” according to the McKamey Manor website.
The manor also happens to be the subject of a petition, which currently has over 58,000 signatures, asking state officials in Alabama and Tennessee to shut it down because “some people have had to seek professional psychiatric help and medical care for extensive injuries.”
Ironically, we hear that some of the most traumatized customers have been actual physicians who succumbed to the horrors of Prior Approval Asylum, the EHR Torment Room, and the River of the Damned Maintenance of Certification.
A crappy excuse of a database
Have you ever been so impressed with your bowel movement that you’ve been compelled to record the incident for posterity? No? Just us? Well, you may want to reconsider, because a pair of AI tech companies are looking for a few good poop pictures.
It’s all part of the “Give a S--t” (you can probably guess what we’ve censored out) campaign, a joint venture from Auggi, a gut health start-up, and Seed Health. The companies hope to use photos sent in by regular people to build an app that would help people with chronic gut problems automatically track their own bowel movements. In addition, the photo library could also be used for research into gut-related diseases such as irritable bowl syndrome.
The two companies hope to collect 100,000 photos for their library, which is an absolutely prodigious amount of poop to sort through. But hey, that’s what the AI is for. They already know the AI works, as Auggi created a proof-of-concept library of 36,000 images of faux feces made from blue Play-Doh. The AI was able to recognize consistency according to the Bristol scale basically 100% of the time.
If you’ve been inspired, you can submit your lovely poop pictures here. Seed and Auggi expect contributers to send only one image each, but multiple submissions are welcome. They’ve already received a dozen from LOTME world headquarters. We love a good bowel movement here.
Criminal moon
“The Wolf Man.” “An American Werewolf in London.” “The Howling.” “Teen Wolf.” All terrifying Hollywood tales of bloodthirsty behavior and sanguinary slaughter. (Michael J. Fox as a hirsute homicidal lycan? Okay, maybe not “Teen Wolf.”)
And the propellant igniting all that criminal lycanthropy? The full moon.
Any teacher will swear a full moon portends the kind of student behavior that an entire pot of teachers’ lounge coffee can’t counter. And every cop knows it’s going to be a “Training Day” shift when the lunar light shines brightest.
But is the Thin Blue Line truly stretched to snapping during a full moon? New York University’s BetaGov research team looked at the purported “lunar effect” linking crime and the full moon. A lit review revealed mixed findings for and against a criminal lunar effect. The team then collaborated with the Vallejo, Calif., police department to match the moon’s phases with the city’s crime events. They did the same with departments in Canada and Mexico.
The results? A full moon had no effect on Vallejo’s crime rate, or anywhere else in North America.
While the finding eviscerates the moon-induced mayhem hypothesis, cops walking a full-moonlit beat can at least take comfort in this fact: Unlike London, Vallejo is clearly free of American werewolves.
A doctor’s note … of terror
With Halloween upon us, here’s a veddy scary riddle: When is a sports physical not a sports physical?
When it’s a haunted house physical.
Specifically, when the haunted house is McKamey Manor in Summertown, Tenn. … and in Huntsville, Ala. That’s right, it can be in two places at the same time. Terrifying.
McKamey Manor is considered by many to be the most terrifying haunted house in the United States, and by some to be a “torture chamber under disguise.”
The “Surivial [we think they misspelled it on purpose to make it even scarier] Horror Challenge” is so terrifying that management requires all participants to have a “completed ‘sports physical’ and doctor’s letter stating you are physically and mentally cleared,” as well as proof of medical insurance. Each paying customer also has to “pass a portable drug test on the day of the show,” according to the McKamey Manor website.
The manor also happens to be the subject of a petition, which currently has over 58,000 signatures, asking state officials in Alabama and Tennessee to shut it down because “some people have had to seek professional psychiatric help and medical care for extensive injuries.”
Ironically, we hear that some of the most traumatized customers have been actual physicians who succumbed to the horrors of Prior Approval Asylum, the EHR Torment Room, and the River of the Damned Maintenance of Certification.
A crappy excuse of a database
Have you ever been so impressed with your bowel movement that you’ve been compelled to record the incident for posterity? No? Just us? Well, you may want to reconsider, because a pair of AI tech companies are looking for a few good poop pictures.
It’s all part of the “Give a S--t” (you can probably guess what we’ve censored out) campaign, a joint venture from Auggi, a gut health start-up, and Seed Health. The companies hope to use photos sent in by regular people to build an app that would help people with chronic gut problems automatically track their own bowel movements. In addition, the photo library could also be used for research into gut-related diseases such as irritable bowl syndrome.
The two companies hope to collect 100,000 photos for their library, which is an absolutely prodigious amount of poop to sort through. But hey, that’s what the AI is for. They already know the AI works, as Auggi created a proof-of-concept library of 36,000 images of faux feces made from blue Play-Doh. The AI was able to recognize consistency according to the Bristol scale basically 100% of the time.
If you’ve been inspired, you can submit your lovely poop pictures here. Seed and Auggi expect contributers to send only one image each, but multiple submissions are welcome. They’ve already received a dozen from LOTME world headquarters. We love a good bowel movement here.
Criminal moon
“The Wolf Man.” “An American Werewolf in London.” “The Howling.” “Teen Wolf.” All terrifying Hollywood tales of bloodthirsty behavior and sanguinary slaughter. (Michael J. Fox as a hirsute homicidal lycan? Okay, maybe not “Teen Wolf.”)
And the propellant igniting all that criminal lycanthropy? The full moon.
Any teacher will swear a full moon portends the kind of student behavior that an entire pot of teachers’ lounge coffee can’t counter. And every cop knows it’s going to be a “Training Day” shift when the lunar light shines brightest.
But is the Thin Blue Line truly stretched to snapping during a full moon? New York University’s BetaGov research team looked at the purported “lunar effect” linking crime and the full moon. A lit review revealed mixed findings for and against a criminal lunar effect. The team then collaborated with the Vallejo, Calif., police department to match the moon’s phases with the city’s crime events. They did the same with departments in Canada and Mexico.
The results? A full moon had no effect on Vallejo’s crime rate, or anywhere else in North America.
While the finding eviscerates the moon-induced mayhem hypothesis, cops walking a full-moonlit beat can at least take comfort in this fact: Unlike London, Vallejo is clearly free of American werewolves.
A doctor’s note … of terror
With Halloween upon us, here’s a veddy scary riddle: When is a sports physical not a sports physical?
When it’s a haunted house physical.
Specifically, when the haunted house is McKamey Manor in Summertown, Tenn. … and in Huntsville, Ala. That’s right, it can be in two places at the same time. Terrifying.
McKamey Manor is considered by many to be the most terrifying haunted house in the United States, and by some to be a “torture chamber under disguise.”
The “Surivial [we think they misspelled it on purpose to make it even scarier] Horror Challenge” is so terrifying that management requires all participants to have a “completed ‘sports physical’ and doctor’s letter stating you are physically and mentally cleared,” as well as proof of medical insurance. Each paying customer also has to “pass a portable drug test on the day of the show,” according to the McKamey Manor website.
The manor also happens to be the subject of a petition, which currently has over 58,000 signatures, asking state officials in Alabama and Tennessee to shut it down because “some people have had to seek professional psychiatric help and medical care for extensive injuries.”
Ironically, we hear that some of the most traumatized customers have been actual physicians who succumbed to the horrors of Prior Approval Asylum, the EHR Torment Room, and the River of the Damned Maintenance of Certification.
Three companies issue recall for ranitidine because of NDMA impurities
The Food and Drug Administration has issued an alert to health care providers and patients about voluntary recalls of ranitidine (Zantac) from three separate companies because of the potential of N-nitrosodimethylamine (NDMA) in the medicine.
According to the FDA alert, Perrigo is recalling over-the-counter ranitidine tablets of all sizes, Novitium Pharma is recalling all unexpired quantities and lots of ranitidine hydrochloride capsules, and Lannett is recalling all unexpired lots of prescription ranitidine syrup (ranitidine oral solution (15 mg/mL).
Patients who are using over-the-counter ranitidine should consider switching to an alternative, such as famotidine, cimetidine, esomeprazole, lansoprazole, and omeprazole, the FDA noted. None of these medications have shown evidence of containing NDMA.
The alert is the fifth update on ranitidine since the initial FDA announcement that NDMA had been found in ranitidine on Sept. 13, 2019.
Lillian M. Beard, MD, who has a private pediatrics practice in Silver Spring, Md., commented, “We have been grappling with concerns about our patients currently taking ranitidine and how we might address the issue. In our practice, we have had very few questions so far. We anticipate that as their awareness is heightened, our parents will seek our advice. In querying our GI colleagues for recommendations on managing the conversations with parents about their concerns about possible carcinogen contamination in ranitidine samples, our practice group was advised that the FDA is not currently recommending that patients on the medication need to immediately stop, pending further investigation.”*
Dr. Beard, also an associate clinical professor of pediatrics at George Washington University in Washington, continued, “Our GI colleagues at Children’s National currently are not starting patients on ranitidine, but choosing a different H2 blocker, famotidine (Pepsid). They are not trying to reach out to patients on ranitidine to ‘switch,’ but when parents call in to refill prescriptions or for management advice, they are switching them to famotidine. Until further notification and or clarification, I will do the same.”
*Updated 11/5/2019
The Food and Drug Administration has issued an alert to health care providers and patients about voluntary recalls of ranitidine (Zantac) from three separate companies because of the potential of N-nitrosodimethylamine (NDMA) in the medicine.
According to the FDA alert, Perrigo is recalling over-the-counter ranitidine tablets of all sizes, Novitium Pharma is recalling all unexpired quantities and lots of ranitidine hydrochloride capsules, and Lannett is recalling all unexpired lots of prescription ranitidine syrup (ranitidine oral solution (15 mg/mL).
Patients who are using over-the-counter ranitidine should consider switching to an alternative, such as famotidine, cimetidine, esomeprazole, lansoprazole, and omeprazole, the FDA noted. None of these medications have shown evidence of containing NDMA.
The alert is the fifth update on ranitidine since the initial FDA announcement that NDMA had been found in ranitidine on Sept. 13, 2019.
Lillian M. Beard, MD, who has a private pediatrics practice in Silver Spring, Md., commented, “We have been grappling with concerns about our patients currently taking ranitidine and how we might address the issue. In our practice, we have had very few questions so far. We anticipate that as their awareness is heightened, our parents will seek our advice. In querying our GI colleagues for recommendations on managing the conversations with parents about their concerns about possible carcinogen contamination in ranitidine samples, our practice group was advised that the FDA is not currently recommending that patients on the medication need to immediately stop, pending further investigation.”*
Dr. Beard, also an associate clinical professor of pediatrics at George Washington University in Washington, continued, “Our GI colleagues at Children’s National currently are not starting patients on ranitidine, but choosing a different H2 blocker, famotidine (Pepsid). They are not trying to reach out to patients on ranitidine to ‘switch,’ but when parents call in to refill prescriptions or for management advice, they are switching them to famotidine. Until further notification and or clarification, I will do the same.”
*Updated 11/5/2019
The Food and Drug Administration has issued an alert to health care providers and patients about voluntary recalls of ranitidine (Zantac) from three separate companies because of the potential of N-nitrosodimethylamine (NDMA) in the medicine.
According to the FDA alert, Perrigo is recalling over-the-counter ranitidine tablets of all sizes, Novitium Pharma is recalling all unexpired quantities and lots of ranitidine hydrochloride capsules, and Lannett is recalling all unexpired lots of prescription ranitidine syrup (ranitidine oral solution (15 mg/mL).
Patients who are using over-the-counter ranitidine should consider switching to an alternative, such as famotidine, cimetidine, esomeprazole, lansoprazole, and omeprazole, the FDA noted. None of these medications have shown evidence of containing NDMA.
The alert is the fifth update on ranitidine since the initial FDA announcement that NDMA had been found in ranitidine on Sept. 13, 2019.
Lillian M. Beard, MD, who has a private pediatrics practice in Silver Spring, Md., commented, “We have been grappling with concerns about our patients currently taking ranitidine and how we might address the issue. In our practice, we have had very few questions so far. We anticipate that as their awareness is heightened, our parents will seek our advice. In querying our GI colleagues for recommendations on managing the conversations with parents about their concerns about possible carcinogen contamination in ranitidine samples, our practice group was advised that the FDA is not currently recommending that patients on the medication need to immediately stop, pending further investigation.”*
Dr. Beard, also an associate clinical professor of pediatrics at George Washington University in Washington, continued, “Our GI colleagues at Children’s National currently are not starting patients on ranitidine, but choosing a different H2 blocker, famotidine (Pepsid). They are not trying to reach out to patients on ranitidine to ‘switch,’ but when parents call in to refill prescriptions or for management advice, they are switching them to famotidine. Until further notification and or clarification, I will do the same.”
*Updated 11/5/2019
Three companies issue recall for ranitidine because of NDMA impurities
The Food and Drug Administration has issued an alert to health care providers and patients about voluntary recalls of ranitidine (Zantac) from three separate companies because of the potential of N-nitrosodimethylamine (NDMA) in the medicine.
According to the FDA alert, Perrigo is recalling over-the-counter ranitidine tablets of all sizes, Novitium Pharma is recalling all unexpired quantities and lots of ranitidine hydrochloride capsules, and Lannett is recalling all unexpired lots of prescription ranitidine syrup (ranitidine oral solution (15 mg/mL).
Patients who are using over-the-counter ranitidine should consider switching to an alternative, such as famotidine, cimetidine, esomeprazole, lansoprazole, and omeprazole, the FDA noted. None of these medications have shown evidence of containing NDMA.
The alert is the fifth update on ranitidine since the initial FDA announcement that NDMA had been found in ranitidine on Sept. 13, 2019.
The recent FDA safety alerts on ranitidine might be causing concern among your patients about their heartburn treatment. AGA offers key points that you can share with your patients at www.gastro.org/news/talking-to-your-patients-about-ranitidine.
The Food and Drug Administration has issued an alert to health care providers and patients about voluntary recalls of ranitidine (Zantac) from three separate companies because of the potential of N-nitrosodimethylamine (NDMA) in the medicine.
According to the FDA alert, Perrigo is recalling over-the-counter ranitidine tablets of all sizes, Novitium Pharma is recalling all unexpired quantities and lots of ranitidine hydrochloride capsules, and Lannett is recalling all unexpired lots of prescription ranitidine syrup (ranitidine oral solution (15 mg/mL).
Patients who are using over-the-counter ranitidine should consider switching to an alternative, such as famotidine, cimetidine, esomeprazole, lansoprazole, and omeprazole, the FDA noted. None of these medications have shown evidence of containing NDMA.
The alert is the fifth update on ranitidine since the initial FDA announcement that NDMA had been found in ranitidine on Sept. 13, 2019.
The recent FDA safety alerts on ranitidine might be causing concern among your patients about their heartburn treatment. AGA offers key points that you can share with your patients at www.gastro.org/news/talking-to-your-patients-about-ranitidine.
The Food and Drug Administration has issued an alert to health care providers and patients about voluntary recalls of ranitidine (Zantac) from three separate companies because of the potential of N-nitrosodimethylamine (NDMA) in the medicine.
According to the FDA alert, Perrigo is recalling over-the-counter ranitidine tablets of all sizes, Novitium Pharma is recalling all unexpired quantities and lots of ranitidine hydrochloride capsules, and Lannett is recalling all unexpired lots of prescription ranitidine syrup (ranitidine oral solution (15 mg/mL).
Patients who are using over-the-counter ranitidine should consider switching to an alternative, such as famotidine, cimetidine, esomeprazole, lansoprazole, and omeprazole, the FDA noted. None of these medications have shown evidence of containing NDMA.
The alert is the fifth update on ranitidine since the initial FDA announcement that NDMA had been found in ranitidine on Sept. 13, 2019.
The recent FDA safety alerts on ranitidine might be causing concern among your patients about their heartburn treatment. AGA offers key points that you can share with your patients at www.gastro.org/news/talking-to-your-patients-about-ranitidine.
NIH seeks gene-based cures for HIV, sickle cell disease
The National Institutes of Health and the Bill & Melinda Gates Foundation have announced that they plan to invest $100 million each over the next 4 years to develop affordable, gene-based cures for sickle cell disease (SCD) and HIV.
The initiative follows an announcement from President Trump that set a goal of ending the HIV epidemic in the United States in the next 10 years, seeking to reduce the number of diagnoses by 90% by 2030. The Trump administration has also identified SCD as an “intractable health challenge with the potential for dramatic advances in the coming years,” the NIH said in a statement.
Gene-based therapy has become a reality in recent years thanks to dramatic advances, but the cost is prohibitive in many parts of the world. “The collaboration between the NIH and the Gates Foundation sets out a bold goal of advancing safe, effective, and durable gene-based cures to clinical trials in the United States and relevant countries in sub-Saharan Africa within the next 7-10 years. The ultimate goal is to scale and implement these treatments globally in areas hardest hit by these diseases,” the NIH said.
Both diseases are a significant burden on low- and middle-income countries, as 95% of the 38 million people living with HIV globally are in the developing world, with 67% living in sub-Saharan Africa; about half of the HIV-infected population receives no treatment for the disease. An estimated 15 million children will be born with SCD over the next 30 years, with three-quarters of those births occurring in sub-Saharan Africa. About 50%-90% of children born with SCD will die before age 5 years.
The collaboration will focus on coordination in two areas: identifying potential candidate cures for SCD and HIV for preclinical and clinical evaluation, and defining long-term opportunities to work together and with African partners on advancing promising candidates to late-phase clinical trials, with funding to be determined as candidates progress.
“In recent years, gene-based treatments have been groundbreaking for rare genetic disorders and infectious diseases. While these treatments are exciting, people in low- and middle-income countries do not have access to these breakthroughs. By working with the NIH and scientists across Africa, we aim to ensure these approaches will improve the lives of those most in need and bring the incredible promise of gene-based treatments to the world of public health,” said Trevor Mundel, MD, PhD, president of the global health program at the Bill & Melinda Gates Foundation.
The National Institutes of Health and the Bill & Melinda Gates Foundation have announced that they plan to invest $100 million each over the next 4 years to develop affordable, gene-based cures for sickle cell disease (SCD) and HIV.
The initiative follows an announcement from President Trump that set a goal of ending the HIV epidemic in the United States in the next 10 years, seeking to reduce the number of diagnoses by 90% by 2030. The Trump administration has also identified SCD as an “intractable health challenge with the potential for dramatic advances in the coming years,” the NIH said in a statement.
Gene-based therapy has become a reality in recent years thanks to dramatic advances, but the cost is prohibitive in many parts of the world. “The collaboration between the NIH and the Gates Foundation sets out a bold goal of advancing safe, effective, and durable gene-based cures to clinical trials in the United States and relevant countries in sub-Saharan Africa within the next 7-10 years. The ultimate goal is to scale and implement these treatments globally in areas hardest hit by these diseases,” the NIH said.
Both diseases are a significant burden on low- and middle-income countries, as 95% of the 38 million people living with HIV globally are in the developing world, with 67% living in sub-Saharan Africa; about half of the HIV-infected population receives no treatment for the disease. An estimated 15 million children will be born with SCD over the next 30 years, with three-quarters of those births occurring in sub-Saharan Africa. About 50%-90% of children born with SCD will die before age 5 years.
The collaboration will focus on coordination in two areas: identifying potential candidate cures for SCD and HIV for preclinical and clinical evaluation, and defining long-term opportunities to work together and with African partners on advancing promising candidates to late-phase clinical trials, with funding to be determined as candidates progress.
“In recent years, gene-based treatments have been groundbreaking for rare genetic disorders and infectious diseases. While these treatments are exciting, people in low- and middle-income countries do not have access to these breakthroughs. By working with the NIH and scientists across Africa, we aim to ensure these approaches will improve the lives of those most in need and bring the incredible promise of gene-based treatments to the world of public health,” said Trevor Mundel, MD, PhD, president of the global health program at the Bill & Melinda Gates Foundation.
The National Institutes of Health and the Bill & Melinda Gates Foundation have announced that they plan to invest $100 million each over the next 4 years to develop affordable, gene-based cures for sickle cell disease (SCD) and HIV.
The initiative follows an announcement from President Trump that set a goal of ending the HIV epidemic in the United States in the next 10 years, seeking to reduce the number of diagnoses by 90% by 2030. The Trump administration has also identified SCD as an “intractable health challenge with the potential for dramatic advances in the coming years,” the NIH said in a statement.
Gene-based therapy has become a reality in recent years thanks to dramatic advances, but the cost is prohibitive in many parts of the world. “The collaboration between the NIH and the Gates Foundation sets out a bold goal of advancing safe, effective, and durable gene-based cures to clinical trials in the United States and relevant countries in sub-Saharan Africa within the next 7-10 years. The ultimate goal is to scale and implement these treatments globally in areas hardest hit by these diseases,” the NIH said.
Both diseases are a significant burden on low- and middle-income countries, as 95% of the 38 million people living with HIV globally are in the developing world, with 67% living in sub-Saharan Africa; about half of the HIV-infected population receives no treatment for the disease. An estimated 15 million children will be born with SCD over the next 30 years, with three-quarters of those births occurring in sub-Saharan Africa. About 50%-90% of children born with SCD will die before age 5 years.
The collaboration will focus on coordination in two areas: identifying potential candidate cures for SCD and HIV for preclinical and clinical evaluation, and defining long-term opportunities to work together and with African partners on advancing promising candidates to late-phase clinical trials, with funding to be determined as candidates progress.
“In recent years, gene-based treatments have been groundbreaking for rare genetic disorders and infectious diseases. While these treatments are exciting, people in low- and middle-income countries do not have access to these breakthroughs. By working with the NIH and scientists across Africa, we aim to ensure these approaches will improve the lives of those most in need and bring the incredible promise of gene-based treatments to the world of public health,” said Trevor Mundel, MD, PhD, president of the global health program at the Bill & Melinda Gates Foundation.
Liver abnormalities, disease common in patients with psoriatic arthritis
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
Liver abnormalities in patients with psoriatic arthritis are common and are associated with higher body mass index, more severe disease, and certain therapies, new research suggests.
Patients with psoriatic arthritis (PsA) often have comorbidities such as cardiovascular disease, metabolic syndrome, inflammatory bowel disease, osteoporosis, malignancy, and ophthalmic disease, and liver disease is no exception, wrote Rattapol Pakchotanon, MD, of the department of internal medicine at Phramongkutlao Hospital and College of Medicine, Bangkok, and associates. Their report is in the Journal of Rheumatology.
In psoriasis patients, the prevalence of liver abnormalities has been 24%-36% in previous research, but research regarding liver disease in PsA has been limited.
Of 1,061 patients from the University of Toronto Psoriatic Arthritis Clinic who were included in the study, 343 (32%) had liver abnormalities, including 256 who developed a liver abnormality or disease after their first evaluation at the clinic. Liver abnormality was defined as having aspartate transaminase, alanine transaminase, or alkaline phosphatase levels 1.5 times the upper limit of normal or greater, and liver diseases included drug-induced liver injury, fatty liver, viral hepatitis, autoimmune liver disease, alcoholic liver disease, liver fibrosis, and cirrhosis.
Among the patients with PsA who developed liver abnormalities or disease after their first visit, liver abnormalities occurred after an average of 8.3 years of follow-up and at a mean age of 50.5 years. The average BMI in this group was 29.7 kg/m2, and 11% of patients consumed alcohol daily. A total of 105 patients had recurrent liver abnormalities, and the rest had only one visit with an abnormality; those with transient abnormalities were significantly less likely to have evidence of liver disease (P less than .001).
The most common cause of liver disease was drug-induced hepatitis (14%) and fatty liver (13%). Alcohol-induced hepatitis occurred in 10 patients, and cirrhosis was reported in 2 patients.
In a multivariable analysis, factors found to be independently associated with liver abnormalities in PsA included BMI (odds ratio, 1.07; 95% confidence interval, 1.02-1.12; P = .007), daily alcohol intake (OR, 4.46; 95% CI, 1.30-15.28; P = .02), damaged joint count (OR, 1.04; 95% CI, 1.01-1.08; P = .01), elevated C-reactive protein (OR, 2.00; 95% CI, 1.04-3.85; P = .04), use of methotrexate or leflunomide (OR, 4.39; 95% CI, 1.67-11.54; P = .003), and use of tumor necrosis factor inhibitors (OR, 10.56; 95% CI, 3.63-30.69; P less than .0001).
“We recommend monitoring liver function tests in these high risk PsA patients,” the researchers concluded. “This is important in the management of patients with PsA as many of the therapeutic options may aggravate or even lead to liver abnormalities in this patient population.”
The study was funded in part by the Arthritis Society, the Canadian Institutes of Health Research, and the Krembil Foundation. The investigators reported that they had no conflicts of interest. Dr. Pakchotanon conducted the research while he was at the Centre for Prognosis Studies in the Rheumatic Diseases at Toronto Western Hospital.
SOURCE: Gladman DD et al. J Rheumatol. 2019 Oct 15. doi: 10.3899/jrheum.181312
FROM THE JOURNAL OF RHEUMATOLOGY
Electrified pathogens and urbanized mosquitoes
Microbes won’t believe this shocking truth!
Pathogens can be tough little critters. Always getting into places they don’t belong, and when they get there, they can be difficult to get rid of, what with their ability to quickly evolve resistance to our best medications. If only there was some shocking new way to tackle those nasty and annoying infections.
Thanks to some engineers and researchers from the University of Pittsburgh, if you’ve got an infection centered on a metal implant, that shocking new treatment won’t be just a figure of speech. They ran a weak electrical current through metal dental implants infected with recurring Candida albicans infection, which damaged the cell membranes of the offending fungal pathogens but left the healthy tissue around the infection alone. That damage increased the pathogen's permeability, making it more susceptible to antimicrobial treatment.
The treatment, also known as electrochemical therapy, is great if you’ve got a recurrent infection. The dormant pathogens responsible for the recurrence, not normally susceptible to treatment, are affected by antifungals or antibiotics after the shock wakes them up. Even bacteria that have evolved drug resistance become vulnerable again after a session with Dr. Electricity.
Unfortunately, while the therapy could certainly be expanded beyond dental implants, shocking yourself when you’ve got a regular old infection probably won’t work. We know – Watt a disappointment.
Blast from the brewing past
What separates a rich Belgian ale from its paler, mass-produced American competitors? Is it the sudsy je ne sais quoi produced by squabbling Walloons and Flemings debating the fine points of the brewing arts over open tanks? Perhaps it’s the signature warm-fermented ways of Trappist monks? Is it possible les Belges have hired the unemployed Artesians who once made Olympia Beer a household name across the American West?
Wrong, wrong, and faux. In fact, Belgium’s finest brews are driven by hybrids. Yeast hybrids. Specifically, rare and unusual forms of hybrid yeasts.
Or, as Belgian researcher and world beer hero Dr. Jan Steensels of VIB-KU Leuven Center for Microbiology explains, “Think of lions and tigers making a super-baby.”
Fearlessly, Dr. Steensels and his intrepid colleagues went hunting for these exotic creatures. They found that the yeasts behind many of Belgium’s finest brews combine the DNA of the traditional, domesticated ale yeast, Saccharomyces cerevisiae, with genetic material from wild yeasts such as Saccharomyces kudriavzevii.
The result? The mighty fermentation strengths of normal beer yeasts are paired with the stress resistance and alluring aromas of feral yeasts that survived mankind’s Medieval brewing endeavors and somehow stumbled into the modern brewery for a drink.
Dr. Steensels’ team is now using its knowledge to craft more yeasty lion-tiger super-babies. We at the Bureau of LOTME look forward to hoisting a pint of this “liger” elixir. We’re certain the brew will bear the name of ligers’ greatest cinematic fan, Napoleon Dynamite, and feature the subtle undertones of tater tots.
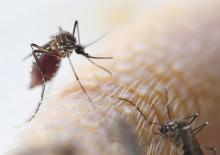
How can mosquitoes be even more fun?
If you’re anything like the gang at LOTME, you’ve spent quite a bit of time wondering which cliché is the best fit for a less-affluent Baltimore neighborhood.
The answer? When it rains, it pours.
We’ll explain. By definition, a less-affluent neighborhood is, well, less affluent, and that lack of affluence has many health consequences for the people who live in those neighborhoods. Today we’re focusing on everyone’s favorite winged disease vector, the mosquito.
It was already known that low-income urban neighborhoods have more mosquitoes than other neighborhoods, and now the Journal of Medical Entomology has published a survey of 13 residential blocks in Baltimore that shows low-income neighborhoods have larger mosquitoes as well.
Trapping took place in five socioeconomically diverse Baltimore neighborhoods during June and July of 2015-2017. (In case you were wondering, the researchers used BG-Sentinel traps baited with CO2 and a 2.0-mL Octenol Lure, which would have been our choice, too). It confirmed that lower affluence correlated with larger mosquito wing size. Wing size, the investigators said in a written statement, “is an accurate proxy for body size in mosquitoes, and body size influences traits that are important to disease transmission.”
So, it seems that larger mosquitoes are more efficient at transmitting diseases, which means more dengue fever, more Zika, more chikungunya, more eastern equine encephalitis, and more West Nile virus. To extend the original cliché a bit, when it rains in Baltimore, the poor neighborhoods get the wettest.
* Correction, 10/24/19: An earlier version of this story misstated the fungal target of the electrical therapy experiment.

Microbes won’t believe this shocking truth!
Pathogens can be tough little critters. Always getting into places they don’t belong, and when they get there, they can be difficult to get rid of, what with their ability to quickly evolve resistance to our best medications. If only there was some shocking new way to tackle those nasty and annoying infections.
Thanks to some engineers and researchers from the University of Pittsburgh, if you’ve got an infection centered on a metal implant, that shocking new treatment won’t be just a figure of speech. They ran a weak electrical current through metal dental implants infected with recurring Candida albicans infection, which damaged the cell membranes of the offending fungal pathogens but left the healthy tissue around the infection alone. That damage increased the pathogen's permeability, making it more susceptible to antimicrobial treatment.
The treatment, also known as electrochemical therapy, is great if you’ve got a recurrent infection. The dormant pathogens responsible for the recurrence, not normally susceptible to treatment, are affected by antifungals or antibiotics after the shock wakes them up. Even bacteria that have evolved drug resistance become vulnerable again after a session with Dr. Electricity.
Unfortunately, while the therapy could certainly be expanded beyond dental implants, shocking yourself when you’ve got a regular old infection probably won’t work. We know – Watt a disappointment.
Blast from the brewing past
What separates a rich Belgian ale from its paler, mass-produced American competitors? Is it the sudsy je ne sais quoi produced by squabbling Walloons and Flemings debating the fine points of the brewing arts over open tanks? Perhaps it’s the signature warm-fermented ways of Trappist monks? Is it possible les Belges have hired the unemployed Artesians who once made Olympia Beer a household name across the American West?
Wrong, wrong, and faux. In fact, Belgium’s finest brews are driven by hybrids. Yeast hybrids. Specifically, rare and unusual forms of hybrid yeasts.
Or, as Belgian researcher and world beer hero Dr. Jan Steensels of VIB-KU Leuven Center for Microbiology explains, “Think of lions and tigers making a super-baby.”
Fearlessly, Dr. Steensels and his intrepid colleagues went hunting for these exotic creatures. They found that the yeasts behind many of Belgium’s finest brews combine the DNA of the traditional, domesticated ale yeast, Saccharomyces cerevisiae, with genetic material from wild yeasts such as Saccharomyces kudriavzevii.
The result? The mighty fermentation strengths of normal beer yeasts are paired with the stress resistance and alluring aromas of feral yeasts that survived mankind’s Medieval brewing endeavors and somehow stumbled into the modern brewery for a drink.
Dr. Steensels’ team is now using its knowledge to craft more yeasty lion-tiger super-babies. We at the Bureau of LOTME look forward to hoisting a pint of this “liger” elixir. We’re certain the brew will bear the name of ligers’ greatest cinematic fan, Napoleon Dynamite, and feature the subtle undertones of tater tots.
How can mosquitoes be even more fun?
If you’re anything like the gang at LOTME, you’ve spent quite a bit of time wondering which cliché is the best fit for a less-affluent Baltimore neighborhood.
The answer? When it rains, it pours.
We’ll explain. By definition, a less-affluent neighborhood is, well, less affluent, and that lack of affluence has many health consequences for the people who live in those neighborhoods. Today we’re focusing on everyone’s favorite winged disease vector, the mosquito.
It was already known that low-income urban neighborhoods have more mosquitoes than other neighborhoods, and now the Journal of Medical Entomology has published a survey of 13 residential blocks in Baltimore that shows low-income neighborhoods have larger mosquitoes as well.
Trapping took place in five socioeconomically diverse Baltimore neighborhoods during June and July of 2015-2017. (In case you were wondering, the researchers used BG-Sentinel traps baited with CO2 and a 2.0-mL Octenol Lure, which would have been our choice, too). It confirmed that lower affluence correlated with larger mosquito wing size. Wing size, the investigators said in a written statement, “is an accurate proxy for body size in mosquitoes, and body size influences traits that are important to disease transmission.”
So, it seems that larger mosquitoes are more efficient at transmitting diseases, which means more dengue fever, more Zika, more chikungunya, more eastern equine encephalitis, and more West Nile virus. To extend the original cliché a bit, when it rains in Baltimore, the poor neighborhoods get the wettest.
* Correction, 10/24/19: An earlier version of this story misstated the fungal target of the electrical therapy experiment.

Microbes won’t believe this shocking truth!
Pathogens can be tough little critters. Always getting into places they don’t belong, and when they get there, they can be difficult to get rid of, what with their ability to quickly evolve resistance to our best medications. If only there was some shocking new way to tackle those nasty and annoying infections.
Thanks to some engineers and researchers from the University of Pittsburgh, if you’ve got an infection centered on a metal implant, that shocking new treatment won’t be just a figure of speech. They ran a weak electrical current through metal dental implants infected with recurring Candida albicans infection, which damaged the cell membranes of the offending fungal pathogens but left the healthy tissue around the infection alone. That damage increased the pathogen's permeability, making it more susceptible to antimicrobial treatment.
The treatment, also known as electrochemical therapy, is great if you’ve got a recurrent infection. The dormant pathogens responsible for the recurrence, not normally susceptible to treatment, are affected by antifungals or antibiotics after the shock wakes them up. Even bacteria that have evolved drug resistance become vulnerable again after a session with Dr. Electricity.
Unfortunately, while the therapy could certainly be expanded beyond dental implants, shocking yourself when you’ve got a regular old infection probably won’t work. We know – Watt a disappointment.
Blast from the brewing past
What separates a rich Belgian ale from its paler, mass-produced American competitors? Is it the sudsy je ne sais quoi produced by squabbling Walloons and Flemings debating the fine points of the brewing arts over open tanks? Perhaps it’s the signature warm-fermented ways of Trappist monks? Is it possible les Belges have hired the unemployed Artesians who once made Olympia Beer a household name across the American West?
Wrong, wrong, and faux. In fact, Belgium’s finest brews are driven by hybrids. Yeast hybrids. Specifically, rare and unusual forms of hybrid yeasts.
Or, as Belgian researcher and world beer hero Dr. Jan Steensels of VIB-KU Leuven Center for Microbiology explains, “Think of lions and tigers making a super-baby.”
Fearlessly, Dr. Steensels and his intrepid colleagues went hunting for these exotic creatures. They found that the yeasts behind many of Belgium’s finest brews combine the DNA of the traditional, domesticated ale yeast, Saccharomyces cerevisiae, with genetic material from wild yeasts such as Saccharomyces kudriavzevii.
The result? The mighty fermentation strengths of normal beer yeasts are paired with the stress resistance and alluring aromas of feral yeasts that survived mankind’s Medieval brewing endeavors and somehow stumbled into the modern brewery for a drink.
Dr. Steensels’ team is now using its knowledge to craft more yeasty lion-tiger super-babies. We at the Bureau of LOTME look forward to hoisting a pint of this “liger” elixir. We’re certain the brew will bear the name of ligers’ greatest cinematic fan, Napoleon Dynamite, and feature the subtle undertones of tater tots.
How can mosquitoes be even more fun?
If you’re anything like the gang at LOTME, you’ve spent quite a bit of time wondering which cliché is the best fit for a less-affluent Baltimore neighborhood.
The answer? When it rains, it pours.
We’ll explain. By definition, a less-affluent neighborhood is, well, less affluent, and that lack of affluence has many health consequences for the people who live in those neighborhoods. Today we’re focusing on everyone’s favorite winged disease vector, the mosquito.
It was already known that low-income urban neighborhoods have more mosquitoes than other neighborhoods, and now the Journal of Medical Entomology has published a survey of 13 residential blocks in Baltimore that shows low-income neighborhoods have larger mosquitoes as well.
Trapping took place in five socioeconomically diverse Baltimore neighborhoods during June and July of 2015-2017. (In case you were wondering, the researchers used BG-Sentinel traps baited with CO2 and a 2.0-mL Octenol Lure, which would have been our choice, too). It confirmed that lower affluence correlated with larger mosquito wing size. Wing size, the investigators said in a written statement, “is an accurate proxy for body size in mosquitoes, and body size influences traits that are important to disease transmission.”
So, it seems that larger mosquitoes are more efficient at transmitting diseases, which means more dengue fever, more Zika, more chikungunya, more eastern equine encephalitis, and more West Nile virus. To extend the original cliché a bit, when it rains in Baltimore, the poor neighborhoods get the wettest.
* Correction, 10/24/19: An earlier version of this story misstated the fungal target of the electrical therapy experiment.

FDA approves minocycline foam for moderate, severe acne
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
The Food and Drug Administration has vulgaris in adults and pediatric patients aged at least 9 years.
FDA approval was based on three phase 3, 12-week, multicenter, randomized, double-blind, vehicle-controlled studies of patients with moderate to severe acne vulgaris who were treated once daily with minocycline 4% plus vehicle or vehicle alone, according to a press release from the manufacturer, Foamix Pharmaceuticals. In all three studies, patients receiving minocycline had a reduction in the number of inflammatory lesions, compared with vehicle alone; in two studies, patients in the minocycline groups had significantly improved Investigator’s Global Assessment scores.
The most commonly reported adverse event reported during the trials was headache. No serious adverse events were reported. The company says it is expected to be available in January 2020.
Find the prescribing information on the FDA website.
FDA approves Trikafta for treatment of cystic fibrosis
in patients aged 12 years or older, the first triple-combination therapy approved for that indication.
Approval for Trikafta was based on results from two clinical trials in patients with cystic fibrosis with an F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In the first trial, a 24-week, randomized, double-blind, placebo-controlled study of 403 patients, the mean percent predicted forced expiratory volume in 1 second increased by 14% from baseline, compared with placebo. In the second trial, a 4-week, randomized, double-blind, active-controlled study of 107 patients, mean percent predicted forced expiratory volume in 1 second was increased 10% from baseline, compared with tezacaftor/ivacaftor, according to the FDA press release.
In the first trial, patients who received Trikafta also saw improvement in sweat chloride, reduction in the number of pulmonary exacerbations, and reduction of body mass index, compared with placebo.
The most common adverse events associated with Trikafta during the trials were headaches, upper respiratory tract infections, abdominal pains, diarrhea, rashes, and rhinorrhea, among others. The label includes a warning related to elevated liver function tests, use at the same time with products that induce or inhibit a liver enzyme called cytochrome P450 3A4, and cataract risk.
“At the FDA, we’re consistently looking for ways to help speed the development of new therapies for complex diseases, while maintaining our high standards of review. Today’s landmark approval is a testament to these efforts, making a novel treatment available to most cystic fibrosis patients, including adolescents, who previously had no options and giving others in the cystic fibrosis community access to an additional effective therapy,” said acting FDA Commissioner Ned Sharpless, MD.
Find the full press release on the FDA website.
in patients aged 12 years or older, the first triple-combination therapy approved for that indication.
Approval for Trikafta was based on results from two clinical trials in patients with cystic fibrosis with an F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In the first trial, a 24-week, randomized, double-blind, placebo-controlled study of 403 patients, the mean percent predicted forced expiratory volume in 1 second increased by 14% from baseline, compared with placebo. In the second trial, a 4-week, randomized, double-blind, active-controlled study of 107 patients, mean percent predicted forced expiratory volume in 1 second was increased 10% from baseline, compared with tezacaftor/ivacaftor, according to the FDA press release.
In the first trial, patients who received Trikafta also saw improvement in sweat chloride, reduction in the number of pulmonary exacerbations, and reduction of body mass index, compared with placebo.
The most common adverse events associated with Trikafta during the trials were headaches, upper respiratory tract infections, abdominal pains, diarrhea, rashes, and rhinorrhea, among others. The label includes a warning related to elevated liver function tests, use at the same time with products that induce or inhibit a liver enzyme called cytochrome P450 3A4, and cataract risk.
“At the FDA, we’re consistently looking for ways to help speed the development of new therapies for complex diseases, while maintaining our high standards of review. Today’s landmark approval is a testament to these efforts, making a novel treatment available to most cystic fibrosis patients, including adolescents, who previously had no options and giving others in the cystic fibrosis community access to an additional effective therapy,” said acting FDA Commissioner Ned Sharpless, MD.
Find the full press release on the FDA website.
in patients aged 12 years or older, the first triple-combination therapy approved for that indication.
Approval for Trikafta was based on results from two clinical trials in patients with cystic fibrosis with an F508del mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. In the first trial, a 24-week, randomized, double-blind, placebo-controlled study of 403 patients, the mean percent predicted forced expiratory volume in 1 second increased by 14% from baseline, compared with placebo. In the second trial, a 4-week, randomized, double-blind, active-controlled study of 107 patients, mean percent predicted forced expiratory volume in 1 second was increased 10% from baseline, compared with tezacaftor/ivacaftor, according to the FDA press release.
In the first trial, patients who received Trikafta also saw improvement in sweat chloride, reduction in the number of pulmonary exacerbations, and reduction of body mass index, compared with placebo.
The most common adverse events associated with Trikafta during the trials were headaches, upper respiratory tract infections, abdominal pains, diarrhea, rashes, and rhinorrhea, among others. The label includes a warning related to elevated liver function tests, use at the same time with products that induce or inhibit a liver enzyme called cytochrome P450 3A4, and cataract risk.
“At the FDA, we’re consistently looking for ways to help speed the development of new therapies for complex diseases, while maintaining our high standards of review. Today’s landmark approval is a testament to these efforts, making a novel treatment available to most cystic fibrosis patients, including adolescents, who previously had no options and giving others in the cystic fibrosis community access to an additional effective therapy,” said acting FDA Commissioner Ned Sharpless, MD.
Find the full press release on the FDA website.
Robot-assisted, gamelike tool effective for classifying ADHD
A novel robot-assisted, gamelike test accurately classified ADHD type in elementary school–aged children, according to Mun-Taek Choi, PhD, and associates.
A total of 326 children in the third and fourth grades were included in the study, 35 of whom had been diagnosed with ADHD and 26 of whom were at risk. For the 10- to 12-minute test, participants followed a robot on a path across a numbered mat while stimuli were shown on a TV with both images and sound, and completed a task at each numbered square, reported Dr. Choi, of Sungkyunkwan University, Suwan, South Korea, and associates. The study was published in the Journal of Intelligent & Robotic Systems.
Inattentive and hyperactive-impulsive behavior was measured by the number of omission and commission errors. Response time and task completion time contributed to the measure of inattentive and hyperactive-impulsive behavior. Working memory deficits were measured as deviations in the prescribed route.
This figure improved over the course of the study as the tool learned more, indicating that generalization errors were not a serious issue for the tool, the investigators noted.
“Unlike conventional questionnaire-based tests, the robot-assisted test increases the accuracy of ADHD diagnosis by directly reflecting the quality of children’s behavior during the activity game with the robot involved in the action. Since the test obtains behavioral patterns and levels using robotic sensing technologies, it can reliably determine the three key elements of ADHD diagnosis: hyperactivity, inattentive behavior, and working memory,” the investigators wrote. Ultimately, Dr. Choi and associates wrote, the tool could help clinicians diagnose childhood ADHD.
The study was funded by the South Korean Ministry of Trade, Industry, & Energy. No disclosures were reported.
SOURCE: Choi M-T et al. J Intell Robot Syst. 2018 Jun 19. doi: 10.1007/s10846-018-0890-9.
A novel robot-assisted, gamelike test accurately classified ADHD type in elementary school–aged children, according to Mun-Taek Choi, PhD, and associates.
A total of 326 children in the third and fourth grades were included in the study, 35 of whom had been diagnosed with ADHD and 26 of whom were at risk. For the 10- to 12-minute test, participants followed a robot on a path across a numbered mat while stimuli were shown on a TV with both images and sound, and completed a task at each numbered square, reported Dr. Choi, of Sungkyunkwan University, Suwan, South Korea, and associates. The study was published in the Journal of Intelligent & Robotic Systems.
Inattentive and hyperactive-impulsive behavior was measured by the number of omission and commission errors. Response time and task completion time contributed to the measure of inattentive and hyperactive-impulsive behavior. Working memory deficits were measured as deviations in the prescribed route.
This figure improved over the course of the study as the tool learned more, indicating that generalization errors were not a serious issue for the tool, the investigators noted.
“Unlike conventional questionnaire-based tests, the robot-assisted test increases the accuracy of ADHD diagnosis by directly reflecting the quality of children’s behavior during the activity game with the robot involved in the action. Since the test obtains behavioral patterns and levels using robotic sensing technologies, it can reliably determine the three key elements of ADHD diagnosis: hyperactivity, inattentive behavior, and working memory,” the investigators wrote. Ultimately, Dr. Choi and associates wrote, the tool could help clinicians diagnose childhood ADHD.
The study was funded by the South Korean Ministry of Trade, Industry, & Energy. No disclosures were reported.
SOURCE: Choi M-T et al. J Intell Robot Syst. 2018 Jun 19. doi: 10.1007/s10846-018-0890-9.
A novel robot-assisted, gamelike test accurately classified ADHD type in elementary school–aged children, according to Mun-Taek Choi, PhD, and associates.
A total of 326 children in the third and fourth grades were included in the study, 35 of whom had been diagnosed with ADHD and 26 of whom were at risk. For the 10- to 12-minute test, participants followed a robot on a path across a numbered mat while stimuli were shown on a TV with both images and sound, and completed a task at each numbered square, reported Dr. Choi, of Sungkyunkwan University, Suwan, South Korea, and associates. The study was published in the Journal of Intelligent & Robotic Systems.
Inattentive and hyperactive-impulsive behavior was measured by the number of omission and commission errors. Response time and task completion time contributed to the measure of inattentive and hyperactive-impulsive behavior. Working memory deficits were measured as deviations in the prescribed route.
This figure improved over the course of the study as the tool learned more, indicating that generalization errors were not a serious issue for the tool, the investigators noted.
“Unlike conventional questionnaire-based tests, the robot-assisted test increases the accuracy of ADHD diagnosis by directly reflecting the quality of children’s behavior during the activity game with the robot involved in the action. Since the test obtains behavioral patterns and levels using robotic sensing technologies, it can reliably determine the three key elements of ADHD diagnosis: hyperactivity, inattentive behavior, and working memory,” the investigators wrote. Ultimately, Dr. Choi and associates wrote, the tool could help clinicians diagnose childhood ADHD.
The study was funded by the South Korean Ministry of Trade, Industry, & Energy. No disclosures were reported.
SOURCE: Choi M-T et al. J Intell Robot Syst. 2018 Jun 19. doi: 10.1007/s10846-018-0890-9.
FROM THE JOURNAL OF INTELLIGENT & ROBOTIC SYSTEMS
Vaccination rates generally high in U.S. children in 2018
Vaccination rates among kindergartners during the 2018-2019 school year and children aged 24 months during 2016-2018 remained high, but several gaps in coverage remained, new research found.
The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.9% for DTaP, 94.7% for 2 doses of MMR, and 94.8% for state-required doses of varicella. The MMR vaccination rate fell just short of the recommended 95% vaccination rate threshold, according to Ranee Seither, MPH, of the immunization services division at the National Center for Immunization and Respiratory Diseases (NCIRD), and associates.
By state, Mississippi had the highest vaccination rate, achieving at least 99.2% coverage for DTaP, MMR, and varicella. Colorado had the lowest vaccination rate for MMR and varicella at 87.4% and 86.5%, respectively; Idaho had the lowest DTaP vaccination rate at 88.8%.
A total of 20 states had at least 95% MMR coverage while 2 had under 90%, 21 states had at least 95% DTaP coverage with only Idaho having below 90%, and 20 states had at least 95% varicella coverage with 4 states having below 90%.
The investigators noted that, if all nonexempt kindergartners were vaccinated in accordance with local and state vaccination policies, nearly all states could achieve the 95% MMR vaccination threshold.
“Recent measles outbreaks in states with high overall MMR coverage, such as New York, highlight the need for assessing vaccination coverage at the local level. [The Centers for Disease Control and Prevention] encourage programs to use their local-level school assessment data to identify populations of undervaccinated students and to partner with schools and providers to reduce barriers to vaccination and improve coverage,” Dr. Seither and associates wrote.
In a study published in the same issue of the Morbidity and Mortality Weekly Report, Holly A. Hill, MD, PhD, and associates from the immunization services division at NCIRD, found that, according to data collected from 25,059 participants in the National Immunization Survey–Child, national vaccination coverage in children aged 24 months was generally strong and stable.
The vaccines with coverage of at least 90% were poliovirus (92.7%), MMR (90.4%), hepatitis B (91%), and varicella (90%). Complete hepatitis A (74%), rotavirus (72.4%), influenza (53%), and combined seven-vaccine series (68.4%) rates were below 80%. Only 1.3% of children received no vaccinations.
In general, the highest rates of coverage were seen in children with private insurance, followed by those with other insurance, those with Medicaid, and finally those without insurance. Disparities also were seen depending on race/ethnicity, poverty level, and rural/urban location. Vaccination rates also varied by state; for example, 20 states had vaccination coverage for one dose of MMR below 90%, with 6 having coverage above 94% (Arkansas, Maine, Massachusetts, Mississippi, Rhode Island, Wisconsin).
“Improvements in childhood vaccination coverage will require that parents and other caregivers have access to vaccination providers and believe in the safety and effectiveness of vaccines. Increased opportunity for vaccination can be facilitated through expanded access to health insurance, greater promotion of available vaccines through the Vaccines for Children program, and solutions to logistical challenges such as transportation, child care, and time off from work. Providers can improve vaccination coverage overall and reduce disparities by administering all recommended vaccines during office visits,” Dr. Hill and associates wrote.
No conflicts of interest were reported by the investigators of either study.
SOURCES: Seither R et al. MMWR Morb Mortal Wkly Rep 2019;68:905-12; Hill HA et al. MMWR Morb Mortal Wkly Rep 2019;68:913-8.
Vaccination rates among kindergartners during the 2018-2019 school year and children aged 24 months during 2016-2018 remained high, but several gaps in coverage remained, new research found.
The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.9% for DTaP, 94.7% for 2 doses of MMR, and 94.8% for state-required doses of varicella. The MMR vaccination rate fell just short of the recommended 95% vaccination rate threshold, according to Ranee Seither, MPH, of the immunization services division at the National Center for Immunization and Respiratory Diseases (NCIRD), and associates.
By state, Mississippi had the highest vaccination rate, achieving at least 99.2% coverage for DTaP, MMR, and varicella. Colorado had the lowest vaccination rate for MMR and varicella at 87.4% and 86.5%, respectively; Idaho had the lowest DTaP vaccination rate at 88.8%.
A total of 20 states had at least 95% MMR coverage while 2 had under 90%, 21 states had at least 95% DTaP coverage with only Idaho having below 90%, and 20 states had at least 95% varicella coverage with 4 states having below 90%.
The investigators noted that, if all nonexempt kindergartners were vaccinated in accordance with local and state vaccination policies, nearly all states could achieve the 95% MMR vaccination threshold.
“Recent measles outbreaks in states with high overall MMR coverage, such as New York, highlight the need for assessing vaccination coverage at the local level. [The Centers for Disease Control and Prevention] encourage programs to use their local-level school assessment data to identify populations of undervaccinated students and to partner with schools and providers to reduce barriers to vaccination and improve coverage,” Dr. Seither and associates wrote.
In a study published in the same issue of the Morbidity and Mortality Weekly Report, Holly A. Hill, MD, PhD, and associates from the immunization services division at NCIRD, found that, according to data collected from 25,059 participants in the National Immunization Survey–Child, national vaccination coverage in children aged 24 months was generally strong and stable.
The vaccines with coverage of at least 90% were poliovirus (92.7%), MMR (90.4%), hepatitis B (91%), and varicella (90%). Complete hepatitis A (74%), rotavirus (72.4%), influenza (53%), and combined seven-vaccine series (68.4%) rates were below 80%. Only 1.3% of children received no vaccinations.
In general, the highest rates of coverage were seen in children with private insurance, followed by those with other insurance, those with Medicaid, and finally those without insurance. Disparities also were seen depending on race/ethnicity, poverty level, and rural/urban location. Vaccination rates also varied by state; for example, 20 states had vaccination coverage for one dose of MMR below 90%, with 6 having coverage above 94% (Arkansas, Maine, Massachusetts, Mississippi, Rhode Island, Wisconsin).
“Improvements in childhood vaccination coverage will require that parents and other caregivers have access to vaccination providers and believe in the safety and effectiveness of vaccines. Increased opportunity for vaccination can be facilitated through expanded access to health insurance, greater promotion of available vaccines through the Vaccines for Children program, and solutions to logistical challenges such as transportation, child care, and time off from work. Providers can improve vaccination coverage overall and reduce disparities by administering all recommended vaccines during office visits,” Dr. Hill and associates wrote.
No conflicts of interest were reported by the investigators of either study.
SOURCES: Seither R et al. MMWR Morb Mortal Wkly Rep 2019;68:905-12; Hill HA et al. MMWR Morb Mortal Wkly Rep 2019;68:913-8.
Vaccination rates among kindergartners during the 2018-2019 school year and children aged 24 months during 2016-2018 remained high, but several gaps in coverage remained, new research found.
The national vaccination rate for the almost 4 million kindergartners reported as enrolled in 2018-2019 was 94.9% for DTaP, 94.7% for 2 doses of MMR, and 94.8% for state-required doses of varicella. The MMR vaccination rate fell just short of the recommended 95% vaccination rate threshold, according to Ranee Seither, MPH, of the immunization services division at the National Center for Immunization and Respiratory Diseases (NCIRD), and associates.
By state, Mississippi had the highest vaccination rate, achieving at least 99.2% coverage for DTaP, MMR, and varicella. Colorado had the lowest vaccination rate for MMR and varicella at 87.4% and 86.5%, respectively; Idaho had the lowest DTaP vaccination rate at 88.8%.
A total of 20 states had at least 95% MMR coverage while 2 had under 90%, 21 states had at least 95% DTaP coverage with only Idaho having below 90%, and 20 states had at least 95% varicella coverage with 4 states having below 90%.
The investigators noted that, if all nonexempt kindergartners were vaccinated in accordance with local and state vaccination policies, nearly all states could achieve the 95% MMR vaccination threshold.
“Recent measles outbreaks in states with high overall MMR coverage, such as New York, highlight the need for assessing vaccination coverage at the local level. [The Centers for Disease Control and Prevention] encourage programs to use their local-level school assessment data to identify populations of undervaccinated students and to partner with schools and providers to reduce barriers to vaccination and improve coverage,” Dr. Seither and associates wrote.
In a study published in the same issue of the Morbidity and Mortality Weekly Report, Holly A. Hill, MD, PhD, and associates from the immunization services division at NCIRD, found that, according to data collected from 25,059 participants in the National Immunization Survey–Child, national vaccination coverage in children aged 24 months was generally strong and stable.
The vaccines with coverage of at least 90% were poliovirus (92.7%), MMR (90.4%), hepatitis B (91%), and varicella (90%). Complete hepatitis A (74%), rotavirus (72.4%), influenza (53%), and combined seven-vaccine series (68.4%) rates were below 80%. Only 1.3% of children received no vaccinations.
In general, the highest rates of coverage were seen in children with private insurance, followed by those with other insurance, those with Medicaid, and finally those without insurance. Disparities also were seen depending on race/ethnicity, poverty level, and rural/urban location. Vaccination rates also varied by state; for example, 20 states had vaccination coverage for one dose of MMR below 90%, with 6 having coverage above 94% (Arkansas, Maine, Massachusetts, Mississippi, Rhode Island, Wisconsin).
“Improvements in childhood vaccination coverage will require that parents and other caregivers have access to vaccination providers and believe in the safety and effectiveness of vaccines. Increased opportunity for vaccination can be facilitated through expanded access to health insurance, greater promotion of available vaccines through the Vaccines for Children program, and solutions to logistical challenges such as transportation, child care, and time off from work. Providers can improve vaccination coverage overall and reduce disparities by administering all recommended vaccines during office visits,” Dr. Hill and associates wrote.
No conflicts of interest were reported by the investigators of either study.
SOURCES: Seither R et al. MMWR Morb Mortal Wkly Rep 2019;68:905-12; Hill HA et al. MMWR Morb Mortal Wkly Rep 2019;68:913-8.
FROM THE MMWR