User login
Lucas Franki is an associate editor for MDedge News, and has been with the company since 2014. He has a BA in English from Penn State University and is an Eagle Scout.
Seeing is bleeding, and smelling is perceiving
True Blood casting call!
If you’ve seen the show True Blood on HBO, you’re probably familiar with blood coming out instead of tears when any of the vampires start crying. Apparently, this interesting phenomenon isn’t unique to vampires on TV.
If you know about female anatomy, you know that the eyes aren’t usually involved in the menstrual cycle. However, a 25-year-old woman went to the ED when she experienced haemolacria, the term for blood tears, for the second time in 2 months during her cycle. She did not appear to have any injuries or illnesses that caused the eye bleeding, but physicians noted that both times she had eye bleeding, she also had her period.
Menstrual bleeding outside of the uterus, called vicarious menstruation, can occur, and it seems that the patient may have had that condition.
Since there are rumors of a True Blood remake circling, perhaps the show’s writers could blend in a little medical fact with vampire fiction.
What does skinny smell like? Lemons apparently
When you smell a lemon, what comes to your mind? How does it make you feel? Now think of the scent of vanilla. How does that one make you feel? Current research suggests certain smells may have an effect on how you perceive your body image.
Researchers from the University of Sussex (England) have found that certain olfactory stimuli (such as lemons and vanilla) and audio stimuli (light steps vs. heavy steps), have a moderate effect on self-image.
During their study, participants were put through a series of auditory and olfactory tests, from listening to stilettos and boots walking across the floor, to being exposed to certain essential oils with different sound pitches.
Exposure to lemon and higher-pitched sounds (like stilettos) made participants feel lighter and was associated with thin, spiky shapes. Exposure to vanilla and lower-pitched sounds was more associated with thicker, rounded shapes. This made researchers believe that multisensory stimuli, such as scents and sounds, can have a bigger role in treating eating disorders.
Our brain functions with multiple “mental models” of ourselves. Based on sensory stimuli from our day-to-day lives, those images and perceptions of ourselves change. Someone complimenting your snazzy new sweater provokes one self-perception, while someone letting you know that your fly is down provokes another.
Well, the researchers believe that, through a sense of smell, we can alter that perception of ourselves when paired with positive influence. Doing this through wearable “interactive clothes” could help boost the confidence and self-esteem of patients struggling with body image. Light smells equals light feelings. Of course, this won’t help the nearly 5% of the world who have some kind of smell disorder.
The researchers said that more research needs to be done, but you can do your own little experiment at home. Think about yourself and how you react to certain smells. How do they make you feel? If it makes you feel good, stop and smell more often.
Pregnancy with a side of pregnancy
It was a great day when Rebecca Roberts and her partner went to the obstetrician to confirm their positive pregnancy test. They’d been trying for more than a year without success, and now they would be having a baby. Note the usage of the singular there. That will become important in a moment.
When Ms. Roberts went back for her 12-week ultrasound appointment, there was an unexpected complication: Baby had become babies. The original fetus was there and doing fine, but there was now a second, less-developed fetus who’d invited herself in unannounced. While they were technically twins, the second fetus did not form at the same time, like normal fraternal twins, instead forming from an egg that was released weeks after the first egg was fertilized.
The phenomenon, called superfetation, is incredibly rare. Prior to 2008, there were fewer than 10 reported cases in the world, according to the European Journal of Obstetrics & Gynecology and Reproductive Biology. The odds of an egg being released during pregnancy, something that’s not supposed to happen, and then having that egg also become fertilized and successfully implanted in the uterus, is astronomically small.
It was not an easy pregnancy for Ms. Roberts, and at 33 weeks into the first pregnancy, the younger fetus’s umbilical cord began to malfunction, so delivery for both was induced in September 2020. Both infants spent time in the neonatal ICU, with the younger baby being in for 3 months, but after 6 months both are doing well and developing quickly. It’s always nice to have a happy ending to one of these weird medical phenomena, especially one with such an unpleasant-sounding name. If we didn’t know better, we’d think superfetation was something really, really smelly.
What’s a little misinformation among neighbors?
Vaccination will, hopefully, get the COVID-19 pandemic under control at some point, but the related misinformation floating around the Internet is another story. Already rampant in the United States, it’s now spreading … to Canada.
Investigators from that northern land took a look at the Twitter accounts of the platform’s 187,000 most active Canadian users and eventually ended up with a database of 147 million tweets, of which 154,000 contained terms associated with misinformation.
The Canadian social media users had more exposure to information from the United States than from Canada, and the exposure to U.S. outlets was more likely to involve misperceptions about COVID-19. “Most of the misinformation circulating on Twitter shared by Canadians was retweeted from U.S. sources,” the researchers said, and “Canadians who followed more American users were more likely to post misinformation.”
The study’s lead investigator, Aengus Bridgman of McGill University in Montreal, put it this way: “It’s hard for Canadian journalists, scientists, and public health experts to be heard by the average Canadian, given all the noise generated by American sources.”
People generally don’t take the time to read the fine print on contracts, and it looks like the Canadians have fallen into that trap. Not entirely their fault, of course, because most people coming from Canada to America don’t pass the Statue of Liberty, but she’s got some fine print of her own.
That poem written on the pedestal, the one that says, “Give me your tired, your poor, your huddled masses yearning to breathe free”? It’s actually a contract, and at the bottom, in very small print, it says, “In return for acceptance of the aforementioned ‘huddled masses,’ countries of origin agree to accept all of the social media noise generated by American sources.”
Sorry, Canada, but we gotcha.
True Blood casting call!
If you’ve seen the show True Blood on HBO, you’re probably familiar with blood coming out instead of tears when any of the vampires start crying. Apparently, this interesting phenomenon isn’t unique to vampires on TV.
If you know about female anatomy, you know that the eyes aren’t usually involved in the menstrual cycle. However, a 25-year-old woman went to the ED when she experienced haemolacria, the term for blood tears, for the second time in 2 months during her cycle. She did not appear to have any injuries or illnesses that caused the eye bleeding, but physicians noted that both times she had eye bleeding, she also had her period.
Menstrual bleeding outside of the uterus, called vicarious menstruation, can occur, and it seems that the patient may have had that condition.
Since there are rumors of a True Blood remake circling, perhaps the show’s writers could blend in a little medical fact with vampire fiction.
What does skinny smell like? Lemons apparently
When you smell a lemon, what comes to your mind? How does it make you feel? Now think of the scent of vanilla. How does that one make you feel? Current research suggests certain smells may have an effect on how you perceive your body image.
Researchers from the University of Sussex (England) have found that certain olfactory stimuli (such as lemons and vanilla) and audio stimuli (light steps vs. heavy steps), have a moderate effect on self-image.
During their study, participants were put through a series of auditory and olfactory tests, from listening to stilettos and boots walking across the floor, to being exposed to certain essential oils with different sound pitches.
Exposure to lemon and higher-pitched sounds (like stilettos) made participants feel lighter and was associated with thin, spiky shapes. Exposure to vanilla and lower-pitched sounds was more associated with thicker, rounded shapes. This made researchers believe that multisensory stimuli, such as scents and sounds, can have a bigger role in treating eating disorders.
Our brain functions with multiple “mental models” of ourselves. Based on sensory stimuli from our day-to-day lives, those images and perceptions of ourselves change. Someone complimenting your snazzy new sweater provokes one self-perception, while someone letting you know that your fly is down provokes another.
Well, the researchers believe that, through a sense of smell, we can alter that perception of ourselves when paired with positive influence. Doing this through wearable “interactive clothes” could help boost the confidence and self-esteem of patients struggling with body image. Light smells equals light feelings. Of course, this won’t help the nearly 5% of the world who have some kind of smell disorder.
The researchers said that more research needs to be done, but you can do your own little experiment at home. Think about yourself and how you react to certain smells. How do they make you feel? If it makes you feel good, stop and smell more often.
Pregnancy with a side of pregnancy
It was a great day when Rebecca Roberts and her partner went to the obstetrician to confirm their positive pregnancy test. They’d been trying for more than a year without success, and now they would be having a baby. Note the usage of the singular there. That will become important in a moment.
When Ms. Roberts went back for her 12-week ultrasound appointment, there was an unexpected complication: Baby had become babies. The original fetus was there and doing fine, but there was now a second, less-developed fetus who’d invited herself in unannounced. While they were technically twins, the second fetus did not form at the same time, like normal fraternal twins, instead forming from an egg that was released weeks after the first egg was fertilized.
The phenomenon, called superfetation, is incredibly rare. Prior to 2008, there were fewer than 10 reported cases in the world, according to the European Journal of Obstetrics & Gynecology and Reproductive Biology. The odds of an egg being released during pregnancy, something that’s not supposed to happen, and then having that egg also become fertilized and successfully implanted in the uterus, is astronomically small.
It was not an easy pregnancy for Ms. Roberts, and at 33 weeks into the first pregnancy, the younger fetus’s umbilical cord began to malfunction, so delivery for both was induced in September 2020. Both infants spent time in the neonatal ICU, with the younger baby being in for 3 months, but after 6 months both are doing well and developing quickly. It’s always nice to have a happy ending to one of these weird medical phenomena, especially one with such an unpleasant-sounding name. If we didn’t know better, we’d think superfetation was something really, really smelly.
What’s a little misinformation among neighbors?
Vaccination will, hopefully, get the COVID-19 pandemic under control at some point, but the related misinformation floating around the Internet is another story. Already rampant in the United States, it’s now spreading … to Canada.
Investigators from that northern land took a look at the Twitter accounts of the platform’s 187,000 most active Canadian users and eventually ended up with a database of 147 million tweets, of which 154,000 contained terms associated with misinformation.
The Canadian social media users had more exposure to information from the United States than from Canada, and the exposure to U.S. outlets was more likely to involve misperceptions about COVID-19. “Most of the misinformation circulating on Twitter shared by Canadians was retweeted from U.S. sources,” the researchers said, and “Canadians who followed more American users were more likely to post misinformation.”
The study’s lead investigator, Aengus Bridgman of McGill University in Montreal, put it this way: “It’s hard for Canadian journalists, scientists, and public health experts to be heard by the average Canadian, given all the noise generated by American sources.”
People generally don’t take the time to read the fine print on contracts, and it looks like the Canadians have fallen into that trap. Not entirely their fault, of course, because most people coming from Canada to America don’t pass the Statue of Liberty, but she’s got some fine print of her own.
That poem written on the pedestal, the one that says, “Give me your tired, your poor, your huddled masses yearning to breathe free”? It’s actually a contract, and at the bottom, in very small print, it says, “In return for acceptance of the aforementioned ‘huddled masses,’ countries of origin agree to accept all of the social media noise generated by American sources.”
Sorry, Canada, but we gotcha.
True Blood casting call!
If you’ve seen the show True Blood on HBO, you’re probably familiar with blood coming out instead of tears when any of the vampires start crying. Apparently, this interesting phenomenon isn’t unique to vampires on TV.
If you know about female anatomy, you know that the eyes aren’t usually involved in the menstrual cycle. However, a 25-year-old woman went to the ED when she experienced haemolacria, the term for blood tears, for the second time in 2 months during her cycle. She did not appear to have any injuries or illnesses that caused the eye bleeding, but physicians noted that both times she had eye bleeding, she also had her period.
Menstrual bleeding outside of the uterus, called vicarious menstruation, can occur, and it seems that the patient may have had that condition.
Since there are rumors of a True Blood remake circling, perhaps the show’s writers could blend in a little medical fact with vampire fiction.
What does skinny smell like? Lemons apparently
When you smell a lemon, what comes to your mind? How does it make you feel? Now think of the scent of vanilla. How does that one make you feel? Current research suggests certain smells may have an effect on how you perceive your body image.
Researchers from the University of Sussex (England) have found that certain olfactory stimuli (such as lemons and vanilla) and audio stimuli (light steps vs. heavy steps), have a moderate effect on self-image.
During their study, participants were put through a series of auditory and olfactory tests, from listening to stilettos and boots walking across the floor, to being exposed to certain essential oils with different sound pitches.
Exposure to lemon and higher-pitched sounds (like stilettos) made participants feel lighter and was associated with thin, spiky shapes. Exposure to vanilla and lower-pitched sounds was more associated with thicker, rounded shapes. This made researchers believe that multisensory stimuli, such as scents and sounds, can have a bigger role in treating eating disorders.
Our brain functions with multiple “mental models” of ourselves. Based on sensory stimuli from our day-to-day lives, those images and perceptions of ourselves change. Someone complimenting your snazzy new sweater provokes one self-perception, while someone letting you know that your fly is down provokes another.
Well, the researchers believe that, through a sense of smell, we can alter that perception of ourselves when paired with positive influence. Doing this through wearable “interactive clothes” could help boost the confidence and self-esteem of patients struggling with body image. Light smells equals light feelings. Of course, this won’t help the nearly 5% of the world who have some kind of smell disorder.
The researchers said that more research needs to be done, but you can do your own little experiment at home. Think about yourself and how you react to certain smells. How do they make you feel? If it makes you feel good, stop and smell more often.
Pregnancy with a side of pregnancy
It was a great day when Rebecca Roberts and her partner went to the obstetrician to confirm their positive pregnancy test. They’d been trying for more than a year without success, and now they would be having a baby. Note the usage of the singular there. That will become important in a moment.
When Ms. Roberts went back for her 12-week ultrasound appointment, there was an unexpected complication: Baby had become babies. The original fetus was there and doing fine, but there was now a second, less-developed fetus who’d invited herself in unannounced. While they were technically twins, the second fetus did not form at the same time, like normal fraternal twins, instead forming from an egg that was released weeks after the first egg was fertilized.
The phenomenon, called superfetation, is incredibly rare. Prior to 2008, there were fewer than 10 reported cases in the world, according to the European Journal of Obstetrics & Gynecology and Reproductive Biology. The odds of an egg being released during pregnancy, something that’s not supposed to happen, and then having that egg also become fertilized and successfully implanted in the uterus, is astronomically small.
It was not an easy pregnancy for Ms. Roberts, and at 33 weeks into the first pregnancy, the younger fetus’s umbilical cord began to malfunction, so delivery for both was induced in September 2020. Both infants spent time in the neonatal ICU, with the younger baby being in for 3 months, but after 6 months both are doing well and developing quickly. It’s always nice to have a happy ending to one of these weird medical phenomena, especially one with such an unpleasant-sounding name. If we didn’t know better, we’d think superfetation was something really, really smelly.
What’s a little misinformation among neighbors?
Vaccination will, hopefully, get the COVID-19 pandemic under control at some point, but the related misinformation floating around the Internet is another story. Already rampant in the United States, it’s now spreading … to Canada.
Investigators from that northern land took a look at the Twitter accounts of the platform’s 187,000 most active Canadian users and eventually ended up with a database of 147 million tweets, of which 154,000 contained terms associated with misinformation.
The Canadian social media users had more exposure to information from the United States than from Canada, and the exposure to U.S. outlets was more likely to involve misperceptions about COVID-19. “Most of the misinformation circulating on Twitter shared by Canadians was retweeted from U.S. sources,” the researchers said, and “Canadians who followed more American users were more likely to post misinformation.”
The study’s lead investigator, Aengus Bridgman of McGill University in Montreal, put it this way: “It’s hard for Canadian journalists, scientists, and public health experts to be heard by the average Canadian, given all the noise generated by American sources.”
People generally don’t take the time to read the fine print on contracts, and it looks like the Canadians have fallen into that trap. Not entirely their fault, of course, because most people coming from Canada to America don’t pass the Statue of Liberty, but she’s got some fine print of her own.
That poem written on the pedestal, the one that says, “Give me your tired, your poor, your huddled masses yearning to breathe free”? It’s actually a contract, and at the bottom, in very small print, it says, “In return for acceptance of the aforementioned ‘huddled masses,’ countries of origin agree to accept all of the social media noise generated by American sources.”
Sorry, Canada, but we gotcha.
Life after death, and the case of the disappearing digit
It’s alive!!!
Calling all “The Walking Dead” fans! Did you know that, after death, certain cells in the brain can stay active and even become colossal?
Researchers evaluated brain tissue to feign the gene expression during autopsy and death. By doing this, they found that these inflammatory cells, called glial cells, can increase gene expression and “grow and sprout long arm-like appendages for many hours after death.”
According to Dr. Jeffrey Loeb, the study’s senior author, the continued growth after death doesn’t come as a shock since these are the cells that do damage control after certain brain injuries, such as stroke.
Maybe those mindless zombies aren’t so mindless after all. We’re not sure if we should be more scared of a zombie that can think, or a zombie that can’t. We’re sensing a spin-off!
Beam me up, Doc!
In the realm of Star Trek, Dr. Leonard “Bones” McCoy isn’t the only physician who seems to find merit in the adventures of the starship Enterprise.
Pediatric cardiologist Victor Grech, it was reported, has been so influenced by the generational hit that the show made special guest appearances in his medical writing.
The alarm was sounded by a student at Oxford University who had suspicions about more than 100 articles published in Early Human Development. Of the articles eventually withdrawn by the journal’s publisher, Elsevier, 26 were on COVID-19 alone.
Just like a Romulan cloaking device, where the stories once stood Elsevier has left a “withdrawn” statement, making the articles vanish out of thin air.
Along with articles on COVID-19, Dr. Grech’s 48-article series with coauthors on how to write a scientific paper rightfully came into question. Elsevier’s statement on the incident says that the journal’s editorial work flow has been redesigned “to ensure that this will not happen again in the future.”
The number of retracted articles boldly puts Dr. Grech in a lane where few men have gone before.
Something’s wrong, but I can’t put my finger on it
Mixed martial arts is not a sport for the faint of heart. However, we doubt fans who were watching the Khetag Pliev/Devin Goodale fight on April 1 were prepared for the announcement that a search was commencing for a missing finger. Not broken, in case you think that was a misprint. Completely 100% removed from the rest of the hand.
One would think that pinpointing the exact moment when the finger, belonging to Mr. Pliev, was severed would be easy, but the video evidence is unclear, with the best guess being that a kick in the first round broke the finger and a grapple in the second severed it completely. Mr. Pliev was not helpful in clearing up the matter; not only did he fail to immediately notice the fact that his finger had broken or severed, he tried to keep the fight going after the second round when the referee noticed some blood where his left ring finger should have been. He thought he was winning. Unfortunately, the doctor on hand, who was clearly a complete drag, felt differently, ending the fight and awarding it to Mr. Goodale in a technical knockout.
Rest assured, there is a happy ending to this gruesome story. After a frantic search, the missing finger was found deep within Mr. Pliev’s glove and was successfully reattached in a Philadelphia emergency room.
The LOTME team commends Mr. Pliev’s commitment to his craft by wanting to continue the fight, but we respectfully disagree with his assertion that he was winning. We’re fairly confident that body part removal is an automatic loss (pun intended), unless you’re the Black Knight from “Monty Python and the Holy Grail.” Then it’s a draw.
Take two cookies and call me in the morning
The placebo effect is a well-known phenomenon. A pharmacologically inactive treatment can help people if they don’t know it’s pharmacologically inactive. But what if they did know? Would it still work?
That’s what researchers at Beth Israel Deaconess Medical Center in Boston wanted to find out. They divided a cohort of patients with irritable bowel syndrome into three groups. One group got pill bottles containing “open-label placebo,” so the subjects knew they were getting a placebo. The second received bottles labeled “double-blind placebo or peppermint oil.” The third got no pills but followed the rest of the study protocol.
Can you see where this is going? Two-thirds of the open-label placebo group had meaningful improvement of their symptoms, there was no difference in improvement between the two placebo groups, and both did significantly better than the no-pill group.
“If the presumption that deception is necessary for placebos to be effective is false, then many theories about the mechanisms that drive placebo effects may need modification,” investigator Ted J. Kaptchuk said in a written statement.
In other words, this changes everything. Who needs real drugs when anything that a doctor gives to a patient will help? Someone who has trouble swallowing pills can get a milkshake instead. Kid doesn’t like the taste of amoxicillin? Prescribe a slice of therapeutic pizza. Vaccine deniers can get a shot of vitamin C … or bourbon. And just imagine all the good that can be done in this crazy, mixed up world with a batch of chocolate chip cookies.
It’s alive!!!
Calling all “The Walking Dead” fans! Did you know that, after death, certain cells in the brain can stay active and even become colossal?
Researchers evaluated brain tissue to feign the gene expression during autopsy and death. By doing this, they found that these inflammatory cells, called glial cells, can increase gene expression and “grow and sprout long arm-like appendages for many hours after death.”
According to Dr. Jeffrey Loeb, the study’s senior author, the continued growth after death doesn’t come as a shock since these are the cells that do damage control after certain brain injuries, such as stroke.
Maybe those mindless zombies aren’t so mindless after all. We’re not sure if we should be more scared of a zombie that can think, or a zombie that can’t. We’re sensing a spin-off!
Beam me up, Doc!
In the realm of Star Trek, Dr. Leonard “Bones” McCoy isn’t the only physician who seems to find merit in the adventures of the starship Enterprise.
Pediatric cardiologist Victor Grech, it was reported, has been so influenced by the generational hit that the show made special guest appearances in his medical writing.
The alarm was sounded by a student at Oxford University who had suspicions about more than 100 articles published in Early Human Development. Of the articles eventually withdrawn by the journal’s publisher, Elsevier, 26 were on COVID-19 alone.
Just like a Romulan cloaking device, where the stories once stood Elsevier has left a “withdrawn” statement, making the articles vanish out of thin air.
Along with articles on COVID-19, Dr. Grech’s 48-article series with coauthors on how to write a scientific paper rightfully came into question. Elsevier’s statement on the incident says that the journal’s editorial work flow has been redesigned “to ensure that this will not happen again in the future.”
The number of retracted articles boldly puts Dr. Grech in a lane where few men have gone before.
Something’s wrong, but I can’t put my finger on it
Mixed martial arts is not a sport for the faint of heart. However, we doubt fans who were watching the Khetag Pliev/Devin Goodale fight on April 1 were prepared for the announcement that a search was commencing for a missing finger. Not broken, in case you think that was a misprint. Completely 100% removed from the rest of the hand.
One would think that pinpointing the exact moment when the finger, belonging to Mr. Pliev, was severed would be easy, but the video evidence is unclear, with the best guess being that a kick in the first round broke the finger and a grapple in the second severed it completely. Mr. Pliev was not helpful in clearing up the matter; not only did he fail to immediately notice the fact that his finger had broken or severed, he tried to keep the fight going after the second round when the referee noticed some blood where his left ring finger should have been. He thought he was winning. Unfortunately, the doctor on hand, who was clearly a complete drag, felt differently, ending the fight and awarding it to Mr. Goodale in a technical knockout.
Rest assured, there is a happy ending to this gruesome story. After a frantic search, the missing finger was found deep within Mr. Pliev’s glove and was successfully reattached in a Philadelphia emergency room.
The LOTME team commends Mr. Pliev’s commitment to his craft by wanting to continue the fight, but we respectfully disagree with his assertion that he was winning. We’re fairly confident that body part removal is an automatic loss (pun intended), unless you’re the Black Knight from “Monty Python and the Holy Grail.” Then it’s a draw.
Take two cookies and call me in the morning
The placebo effect is a well-known phenomenon. A pharmacologically inactive treatment can help people if they don’t know it’s pharmacologically inactive. But what if they did know? Would it still work?
That’s what researchers at Beth Israel Deaconess Medical Center in Boston wanted to find out. They divided a cohort of patients with irritable bowel syndrome into three groups. One group got pill bottles containing “open-label placebo,” so the subjects knew they were getting a placebo. The second received bottles labeled “double-blind placebo or peppermint oil.” The third got no pills but followed the rest of the study protocol.
Can you see where this is going? Two-thirds of the open-label placebo group had meaningful improvement of their symptoms, there was no difference in improvement between the two placebo groups, and both did significantly better than the no-pill group.
“If the presumption that deception is necessary for placebos to be effective is false, then many theories about the mechanisms that drive placebo effects may need modification,” investigator Ted J. Kaptchuk said in a written statement.
In other words, this changes everything. Who needs real drugs when anything that a doctor gives to a patient will help? Someone who has trouble swallowing pills can get a milkshake instead. Kid doesn’t like the taste of amoxicillin? Prescribe a slice of therapeutic pizza. Vaccine deniers can get a shot of vitamin C … or bourbon. And just imagine all the good that can be done in this crazy, mixed up world with a batch of chocolate chip cookies.
It’s alive!!!
Calling all “The Walking Dead” fans! Did you know that, after death, certain cells in the brain can stay active and even become colossal?
Researchers evaluated brain tissue to feign the gene expression during autopsy and death. By doing this, they found that these inflammatory cells, called glial cells, can increase gene expression and “grow and sprout long arm-like appendages for many hours after death.”
According to Dr. Jeffrey Loeb, the study’s senior author, the continued growth after death doesn’t come as a shock since these are the cells that do damage control after certain brain injuries, such as stroke.
Maybe those mindless zombies aren’t so mindless after all. We’re not sure if we should be more scared of a zombie that can think, or a zombie that can’t. We’re sensing a spin-off!
Beam me up, Doc!
In the realm of Star Trek, Dr. Leonard “Bones” McCoy isn’t the only physician who seems to find merit in the adventures of the starship Enterprise.
Pediatric cardiologist Victor Grech, it was reported, has been so influenced by the generational hit that the show made special guest appearances in his medical writing.
The alarm was sounded by a student at Oxford University who had suspicions about more than 100 articles published in Early Human Development. Of the articles eventually withdrawn by the journal’s publisher, Elsevier, 26 were on COVID-19 alone.
Just like a Romulan cloaking device, where the stories once stood Elsevier has left a “withdrawn” statement, making the articles vanish out of thin air.
Along with articles on COVID-19, Dr. Grech’s 48-article series with coauthors on how to write a scientific paper rightfully came into question. Elsevier’s statement on the incident says that the journal’s editorial work flow has been redesigned “to ensure that this will not happen again in the future.”
The number of retracted articles boldly puts Dr. Grech in a lane where few men have gone before.
Something’s wrong, but I can’t put my finger on it
Mixed martial arts is not a sport for the faint of heart. However, we doubt fans who were watching the Khetag Pliev/Devin Goodale fight on April 1 were prepared for the announcement that a search was commencing for a missing finger. Not broken, in case you think that was a misprint. Completely 100% removed from the rest of the hand.
One would think that pinpointing the exact moment when the finger, belonging to Mr. Pliev, was severed would be easy, but the video evidence is unclear, with the best guess being that a kick in the first round broke the finger and a grapple in the second severed it completely. Mr. Pliev was not helpful in clearing up the matter; not only did he fail to immediately notice the fact that his finger had broken or severed, he tried to keep the fight going after the second round when the referee noticed some blood where his left ring finger should have been. He thought he was winning. Unfortunately, the doctor on hand, who was clearly a complete drag, felt differently, ending the fight and awarding it to Mr. Goodale in a technical knockout.
Rest assured, there is a happy ending to this gruesome story. After a frantic search, the missing finger was found deep within Mr. Pliev’s glove and was successfully reattached in a Philadelphia emergency room.
The LOTME team commends Mr. Pliev’s commitment to his craft by wanting to continue the fight, but we respectfully disagree with his assertion that he was winning. We’re fairly confident that body part removal is an automatic loss (pun intended), unless you’re the Black Knight from “Monty Python and the Holy Grail.” Then it’s a draw.
Take two cookies and call me in the morning
The placebo effect is a well-known phenomenon. A pharmacologically inactive treatment can help people if they don’t know it’s pharmacologically inactive. But what if they did know? Would it still work?
That’s what researchers at Beth Israel Deaconess Medical Center in Boston wanted to find out. They divided a cohort of patients with irritable bowel syndrome into three groups. One group got pill bottles containing “open-label placebo,” so the subjects knew they were getting a placebo. The second received bottles labeled “double-blind placebo or peppermint oil.” The third got no pills but followed the rest of the study protocol.
Can you see where this is going? Two-thirds of the open-label placebo group had meaningful improvement of their symptoms, there was no difference in improvement between the two placebo groups, and both did significantly better than the no-pill group.
“If the presumption that deception is necessary for placebos to be effective is false, then many theories about the mechanisms that drive placebo effects may need modification,” investigator Ted J. Kaptchuk said in a written statement.
In other words, this changes everything. Who needs real drugs when anything that a doctor gives to a patient will help? Someone who has trouble swallowing pills can get a milkshake instead. Kid doesn’t like the taste of amoxicillin? Prescribe a slice of therapeutic pizza. Vaccine deniers can get a shot of vitamin C … or bourbon. And just imagine all the good that can be done in this crazy, mixed up world with a batch of chocolate chip cookies.
Match Day 2021: Pediatrics experiences slow, steady growth
Match Day 2021 was another record breaker, despite the pandemic, and pediatrics played its part, adding nearly 40 more slots than 2020 and filling nearly 50 more, according to the National Resident Matching Program (NRMP).
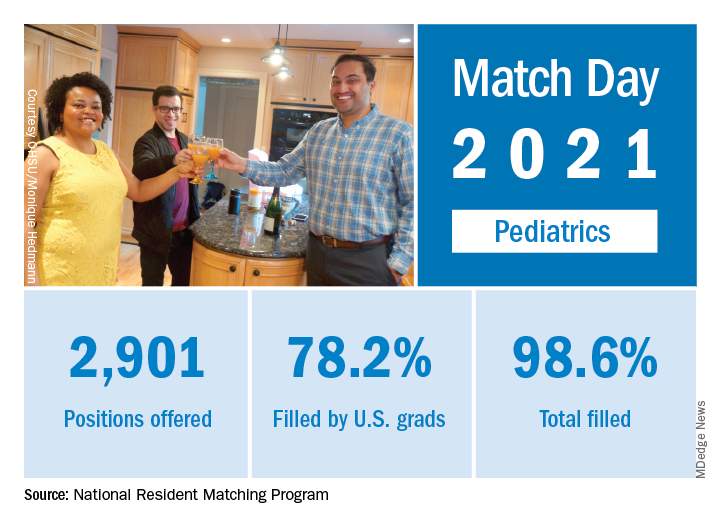
“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a press release. Overall, 35,194 first-year (PGY-1) slots were offered and 33,353 were filled, both more than ever before, for a fill rate of 94.8%, a slight increase from the 94.6% fill rate last year.
Pediatrics offered 2,901 slots in 2021, up from 2,864 in 2020, though the proportion of pediatrics slots in the overall total fell slightly to 8.2% from 8.4% in 2020. Of those 2,901 slots, 2,860 were filled, for a fill rate of 98.6%, up from 98.2% last year. Of those filled positions, 60.3% were filled by MD seniors, and 78.2% were filled by U.S. graduates.
Since 2017, pediatrics has offered more slots every year, rising from 2,738 in 2017 up to the 2,901 in 2021, an overall growth rate of just under 6%.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized, [as] growth in registration was seen in every applicant group,” the NRMP noted. Rank-order lists submissions in 2021 were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen international medical graduates, and 15.0% for non–U.S.-citizen IMGs, compared with 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. ... The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in the press release.
Match Day 2021 was another record breaker, despite the pandemic, and pediatrics played its part, adding nearly 40 more slots than 2020 and filling nearly 50 more, according to the National Resident Matching Program (NRMP).

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a press release. Overall, 35,194 first-year (PGY-1) slots were offered and 33,353 were filled, both more than ever before, for a fill rate of 94.8%, a slight increase from the 94.6% fill rate last year.
Pediatrics offered 2,901 slots in 2021, up from 2,864 in 2020, though the proportion of pediatrics slots in the overall total fell slightly to 8.2% from 8.4% in 2020. Of those 2,901 slots, 2,860 were filled, for a fill rate of 98.6%, up from 98.2% last year. Of those filled positions, 60.3% were filled by MD seniors, and 78.2% were filled by U.S. graduates.
Since 2017, pediatrics has offered more slots every year, rising from 2,738 in 2017 up to the 2,901 in 2021, an overall growth rate of just under 6%.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized, [as] growth in registration was seen in every applicant group,” the NRMP noted. Rank-order lists submissions in 2021 were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen international medical graduates, and 15.0% for non–U.S.-citizen IMGs, compared with 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. ... The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in the press release.
Match Day 2021 was another record breaker, despite the pandemic, and pediatrics played its part, adding nearly 40 more slots than 2020 and filling nearly 50 more, according to the National Resident Matching Program (NRMP).

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a press release. Overall, 35,194 first-year (PGY-1) slots were offered and 33,353 were filled, both more than ever before, for a fill rate of 94.8%, a slight increase from the 94.6% fill rate last year.
Pediatrics offered 2,901 slots in 2021, up from 2,864 in 2020, though the proportion of pediatrics slots in the overall total fell slightly to 8.2% from 8.4% in 2020. Of those 2,901 slots, 2,860 were filled, for a fill rate of 98.6%, up from 98.2% last year. Of those filled positions, 60.3% were filled by MD seniors, and 78.2% were filled by U.S. graduates.
Since 2017, pediatrics has offered more slots every year, rising from 2,738 in 2017 up to the 2,901 in 2021, an overall growth rate of just under 6%.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized, [as] growth in registration was seen in every applicant group,” the NRMP noted. Rank-order lists submissions in 2021 were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen international medical graduates, and 15.0% for non–U.S.-citizen IMGs, compared with 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. ... The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in the press release.
Match Day 2021: Dermatology holds steady
Despite the pandemic, , according to the National Resident Matching Program (NRMP).
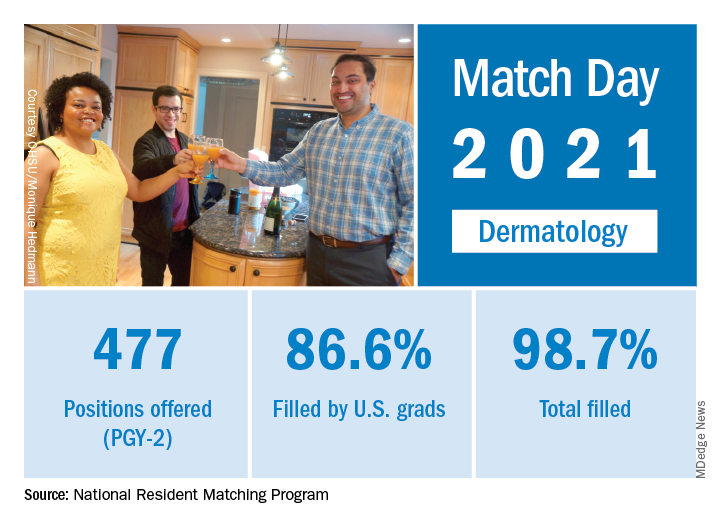
Available dermatology PGY-2 slots fell by 0.2% from 478 in 2020 to 477 in 2021, but 471 slots were filled in 2021, 2 more than last year, for an increase of 0.4%. The overall fill rate was 98.7% in 2021, with 86.6% being filled by U.S. graduates. Just under 88% of filled positions went to MD and DO seniors.
While Match Day 2021 set a record for positions offered and filled at 38,106 slots and 36,179, respectively, an overall total of 2,699 PGY-2 slots were offered in 2021, which is a decrease of 1.6% from last year’s total of 2,742 slots. Of the available PGY-2 slots, 97.6% were filled in 2021, compared with 96.6% in 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. We admire all the Match participants for their hard work and their commitment to train and serve alongside their peers. The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in a press release.
Despite the pandemic, , according to the National Resident Matching Program (NRMP).

Available dermatology PGY-2 slots fell by 0.2% from 478 in 2020 to 477 in 2021, but 471 slots were filled in 2021, 2 more than last year, for an increase of 0.4%. The overall fill rate was 98.7% in 2021, with 86.6% being filled by U.S. graduates. Just under 88% of filled positions went to MD and DO seniors.
While Match Day 2021 set a record for positions offered and filled at 38,106 slots and 36,179, respectively, an overall total of 2,699 PGY-2 slots were offered in 2021, which is a decrease of 1.6% from last year’s total of 2,742 slots. Of the available PGY-2 slots, 97.6% were filled in 2021, compared with 96.6% in 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. We admire all the Match participants for their hard work and their commitment to train and serve alongside their peers. The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in a press release.
Despite the pandemic, , according to the National Resident Matching Program (NRMP).

Available dermatology PGY-2 slots fell by 0.2% from 478 in 2020 to 477 in 2021, but 471 slots were filled in 2021, 2 more than last year, for an increase of 0.4%. The overall fill rate was 98.7% in 2021, with 86.6% being filled by U.S. graduates. Just under 88% of filled positions went to MD and DO seniors.
While Match Day 2021 set a record for positions offered and filled at 38,106 slots and 36,179, respectively, an overall total of 2,699 PGY-2 slots were offered in 2021, which is a decrease of 1.6% from last year’s total of 2,742 slots. Of the available PGY-2 slots, 97.6% were filled in 2021, compared with 96.6% in 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. We admire all the Match participants for their hard work and their commitment to train and serve alongside their peers. The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in a press release.
FDA approves Qwo for treatment of cellulite
. The drug is the first injectable treatment for cellulite to receive regulatory approval.
In cellulite, fibrous septae are a primary contributing factor. The septae make up the fibrous connective tissue that connects the skin perpendicularly to the fascia below and tether the skin, drawing it downward and leading to a mattress-like appearance, commonly referred to as “dimpling.” When injected into the treatment area, Qwo is thought to release the fibrous septae enzymatically by specifically targeting types 1 and 3 collagen, which may result in smoothing of the skin and an improved appearance of cellulite.
The most common side effects of Qwo include injection site bruising, pain, areas of hardness, itching, redness, discoloration, swelling, and warmth in the treatment area.
Qwo is expected to be available throughout the United States at aesthetic health care practitioner’s offices starting in Spring 2021.
“Qwo could be a game-changer for many women with cellulite,” Anne Chapas, MD, a board-certified dermatologist at Union Square Laser Dermatology in New York, said in a press release. “I am thrilled there will now be an FDA-approved injectable treatment option proven to address a root cause of cellulite. What is exciting about Qwo is that it is a cutting-edge cellulite treatment, without the cutting,” Dr. Chapas, said.
. The drug is the first injectable treatment for cellulite to receive regulatory approval.
In cellulite, fibrous septae are a primary contributing factor. The septae make up the fibrous connective tissue that connects the skin perpendicularly to the fascia below and tether the skin, drawing it downward and leading to a mattress-like appearance, commonly referred to as “dimpling.” When injected into the treatment area, Qwo is thought to release the fibrous septae enzymatically by specifically targeting types 1 and 3 collagen, which may result in smoothing of the skin and an improved appearance of cellulite.
The most common side effects of Qwo include injection site bruising, pain, areas of hardness, itching, redness, discoloration, swelling, and warmth in the treatment area.
Qwo is expected to be available throughout the United States at aesthetic health care practitioner’s offices starting in Spring 2021.
“Qwo could be a game-changer for many women with cellulite,” Anne Chapas, MD, a board-certified dermatologist at Union Square Laser Dermatology in New York, said in a press release. “I am thrilled there will now be an FDA-approved injectable treatment option proven to address a root cause of cellulite. What is exciting about Qwo is that it is a cutting-edge cellulite treatment, without the cutting,” Dr. Chapas, said.
. The drug is the first injectable treatment for cellulite to receive regulatory approval.
In cellulite, fibrous septae are a primary contributing factor. The septae make up the fibrous connective tissue that connects the skin perpendicularly to the fascia below and tether the skin, drawing it downward and leading to a mattress-like appearance, commonly referred to as “dimpling.” When injected into the treatment area, Qwo is thought to release the fibrous septae enzymatically by specifically targeting types 1 and 3 collagen, which may result in smoothing of the skin and an improved appearance of cellulite.
The most common side effects of Qwo include injection site bruising, pain, areas of hardness, itching, redness, discoloration, swelling, and warmth in the treatment area.
Qwo is expected to be available throughout the United States at aesthetic health care practitioner’s offices starting in Spring 2021.
“Qwo could be a game-changer for many women with cellulite,” Anne Chapas, MD, a board-certified dermatologist at Union Square Laser Dermatology in New York, said in a press release. “I am thrilled there will now be an FDA-approved injectable treatment option proven to address a root cause of cellulite. What is exciting about Qwo is that it is a cutting-edge cellulite treatment, without the cutting,” Dr. Chapas, said.
FDA approves Cosentyx for treatment of active nr-axSpA
The Food and Drug Administration has approved secukinumab (Cosentyx) for the treatment of active nonradiographic axial spondyloarthritis (nr-axSpA), according to an announcement from the drug’s manufacturer, Novartis.
FDA approval was based on results of the 2-year PREVENT trial, a randomized, double-blind, placebo-controlled, phase 3 study in 555 adults with active nr-axSpA who received a loading dose of 150 mg secukinumab subcutaneously weekly for 4 weeks, then maintenance dosing with 150 mg secukinumab monthly; 150 mg secukinumab monthly with no loading dose; or placebo. Patients were included if they were aged at least 18 years with 6 months or more of inflammatory back pain, had objective signs of inflammation (sacroiliitis on MRI and/or C-reactive protein at 5.0 mg/dL or higher), had active disease and spinal pain according to the Bath Ankylosing Spondylitis Disease Activity Index, had total back pain with a visual analog scale of 40 mm or greater, and had not received a tumor necrosis factor (TNF) inhibitor or had an inadequate response to no more than one TNF inhibitor. A total of 501 patients had not previously taken a biologic medication.
A significantly greater proportion of biologic-naive patients taking secukinumab in both active treatment arm met the trial’s primary endpoint of at least a 40% improvement in the Assessment of Spondyloarthritis International Society response criteria versus placebo after 52 weeks. Both loading and nonloading arms saw significant improvements in Ankylosing Spondylitis Quality of Life scores, compared with those in the placebo group.
The safety profile of secukinumab in PREVENT was shown to be consistent with previous clinical trials, with no new safety signals detected.
Secukinumab, a fully human monoclonal antibody that directly inhibits interleukin-17A, also received European Medicines Agency approval for the treatment of nr-axSpA in April 2020. It is already approved by the FDA for the treatment of moderate to severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis.
The Food and Drug Administration has approved secukinumab (Cosentyx) for the treatment of active nonradiographic axial spondyloarthritis (nr-axSpA), according to an announcement from the drug’s manufacturer, Novartis.
FDA approval was based on results of the 2-year PREVENT trial, a randomized, double-blind, placebo-controlled, phase 3 study in 555 adults with active nr-axSpA who received a loading dose of 150 mg secukinumab subcutaneously weekly for 4 weeks, then maintenance dosing with 150 mg secukinumab monthly; 150 mg secukinumab monthly with no loading dose; or placebo. Patients were included if they were aged at least 18 years with 6 months or more of inflammatory back pain, had objective signs of inflammation (sacroiliitis on MRI and/or C-reactive protein at 5.0 mg/dL or higher), had active disease and spinal pain according to the Bath Ankylosing Spondylitis Disease Activity Index, had total back pain with a visual analog scale of 40 mm or greater, and had not received a tumor necrosis factor (TNF) inhibitor or had an inadequate response to no more than one TNF inhibitor. A total of 501 patients had not previously taken a biologic medication.
A significantly greater proportion of biologic-naive patients taking secukinumab in both active treatment arm met the trial’s primary endpoint of at least a 40% improvement in the Assessment of Spondyloarthritis International Society response criteria versus placebo after 52 weeks. Both loading and nonloading arms saw significant improvements in Ankylosing Spondylitis Quality of Life scores, compared with those in the placebo group.
The safety profile of secukinumab in PREVENT was shown to be consistent with previous clinical trials, with no new safety signals detected.
Secukinumab, a fully human monoclonal antibody that directly inhibits interleukin-17A, also received European Medicines Agency approval for the treatment of nr-axSpA in April 2020. It is already approved by the FDA for the treatment of moderate to severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis.
The Food and Drug Administration has approved secukinumab (Cosentyx) for the treatment of active nonradiographic axial spondyloarthritis (nr-axSpA), according to an announcement from the drug’s manufacturer, Novartis.
FDA approval was based on results of the 2-year PREVENT trial, a randomized, double-blind, placebo-controlled, phase 3 study in 555 adults with active nr-axSpA who received a loading dose of 150 mg secukinumab subcutaneously weekly for 4 weeks, then maintenance dosing with 150 mg secukinumab monthly; 150 mg secukinumab monthly with no loading dose; or placebo. Patients were included if they were aged at least 18 years with 6 months or more of inflammatory back pain, had objective signs of inflammation (sacroiliitis on MRI and/or C-reactive protein at 5.0 mg/dL or higher), had active disease and spinal pain according to the Bath Ankylosing Spondylitis Disease Activity Index, had total back pain with a visual analog scale of 40 mm or greater, and had not received a tumor necrosis factor (TNF) inhibitor or had an inadequate response to no more than one TNF inhibitor. A total of 501 patients had not previously taken a biologic medication.
A significantly greater proportion of biologic-naive patients taking secukinumab in both active treatment arm met the trial’s primary endpoint of at least a 40% improvement in the Assessment of Spondyloarthritis International Society response criteria versus placebo after 52 weeks. Both loading and nonloading arms saw significant improvements in Ankylosing Spondylitis Quality of Life scores, compared with those in the placebo group.
The safety profile of secukinumab in PREVENT was shown to be consistent with previous clinical trials, with no new safety signals detected.
Secukinumab, a fully human monoclonal antibody that directly inhibits interleukin-17A, also received European Medicines Agency approval for the treatment of nr-axSpA in April 2020. It is already approved by the FDA for the treatment of moderate to severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis.
FDA approves Uplizna for treatment of anti-AQP4 antibody–positive NMOSD
The Food and Drug Administration has approved Uplizna (inebilizumab-cdon) for the treatment of adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are anti-AQP4 antibody positive. Uplizna is the second approved treatment for the disorder.
Approval was based on results from the global, placebo-controlled N-MOmentum trial, which included 213 anti-AQP4 antibody–positive patients and 17 anti-AQP4 antibody–negative patients who received inebilizumab-cdon or placebo. Just under 90% of patients in the positive group remained relapse free 6 months after the initial dosing, compared with 58% of patients taking placebo. People who took inebilizumab also saw a reduction in NMOSD-related hospitalizations. There was no evidence of a benefit in patients who were anti-AQP4 antibody negative.
Inebilizumab-cdon was safe and well tolerated during the trial, with the most common adverse events being urinary tract infection (20%), nasopharyngitis (13%), infusion reaction (12%), arthralgia (11%), and headache (10%). The drug is approved as twice-yearly maintenance after initial dosing. The prescribing information for Uplizna includes a warning for infusion reactions, potential depletion of certain proteins (hypogammaglobulinemia), and potential increased risk of infection—including progressive multifocal leukoencephalopathy—and potential reactivation of hepatitis B and tuberculosis.
“NMOSD is an extremely challenging disease to treat. Patients experience unpredictable attacks that can lead to permanent disability from blindness and paralysis. In addition, each subsequent attack may result in a cumulative worsening of disability,” Bruce Cree, MD, PhD, lead investigator for the N-MOmentum trial and professor of clinical neurology at the University of California, San Francisco, said in a press release. “Uplizna is an important new treatment option that provides prescribing physicians and patients living with NMOSD a therapy with proven efficacy, a favorable safety profile and a twice-a-year maintenance dosing schedule.”
The Food and Drug Administration has approved Uplizna (inebilizumab-cdon) for the treatment of adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are anti-AQP4 antibody positive. Uplizna is the second approved treatment for the disorder.
Approval was based on results from the global, placebo-controlled N-MOmentum trial, which included 213 anti-AQP4 antibody–positive patients and 17 anti-AQP4 antibody–negative patients who received inebilizumab-cdon or placebo. Just under 90% of patients in the positive group remained relapse free 6 months after the initial dosing, compared with 58% of patients taking placebo. People who took inebilizumab also saw a reduction in NMOSD-related hospitalizations. There was no evidence of a benefit in patients who were anti-AQP4 antibody negative.
Inebilizumab-cdon was safe and well tolerated during the trial, with the most common adverse events being urinary tract infection (20%), nasopharyngitis (13%), infusion reaction (12%), arthralgia (11%), and headache (10%). The drug is approved as twice-yearly maintenance after initial dosing. The prescribing information for Uplizna includes a warning for infusion reactions, potential depletion of certain proteins (hypogammaglobulinemia), and potential increased risk of infection—including progressive multifocal leukoencephalopathy—and potential reactivation of hepatitis B and tuberculosis.
“NMOSD is an extremely challenging disease to treat. Patients experience unpredictable attacks that can lead to permanent disability from blindness and paralysis. In addition, each subsequent attack may result in a cumulative worsening of disability,” Bruce Cree, MD, PhD, lead investigator for the N-MOmentum trial and professor of clinical neurology at the University of California, San Francisco, said in a press release. “Uplizna is an important new treatment option that provides prescribing physicians and patients living with NMOSD a therapy with proven efficacy, a favorable safety profile and a twice-a-year maintenance dosing schedule.”
The Food and Drug Administration has approved Uplizna (inebilizumab-cdon) for the treatment of adult patients with neuromyelitis optica spectrum disorder (NMOSD) who are anti-AQP4 antibody positive. Uplizna is the second approved treatment for the disorder.
Approval was based on results from the global, placebo-controlled N-MOmentum trial, which included 213 anti-AQP4 antibody–positive patients and 17 anti-AQP4 antibody–negative patients who received inebilizumab-cdon or placebo. Just under 90% of patients in the positive group remained relapse free 6 months after the initial dosing, compared with 58% of patients taking placebo. People who took inebilizumab also saw a reduction in NMOSD-related hospitalizations. There was no evidence of a benefit in patients who were anti-AQP4 antibody negative.
Inebilizumab-cdon was safe and well tolerated during the trial, with the most common adverse events being urinary tract infection (20%), nasopharyngitis (13%), infusion reaction (12%), arthralgia (11%), and headache (10%). The drug is approved as twice-yearly maintenance after initial dosing. The prescribing information for Uplizna includes a warning for infusion reactions, potential depletion of certain proteins (hypogammaglobulinemia), and potential increased risk of infection—including progressive multifocal leukoencephalopathy—and potential reactivation of hepatitis B and tuberculosis.
“NMOSD is an extremely challenging disease to treat. Patients experience unpredictable attacks that can lead to permanent disability from blindness and paralysis. In addition, each subsequent attack may result in a cumulative worsening of disability,” Bruce Cree, MD, PhD, lead investigator for the N-MOmentum trial and professor of clinical neurology at the University of California, San Francisco, said in a press release. “Uplizna is an important new treatment option that provides prescribing physicians and patients living with NMOSD a therapy with proven efficacy, a favorable safety profile and a twice-a-year maintenance dosing schedule.”
Baloxavir effective, well tolerated for influenza treatment in children
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
according to Jeffrey Baker, MD, of Clinical Research Prime, Idaho Falls, and associates.
In the double-blind, randomized, controlled MiniSTONE-2 phase 3 trial, the investigators randomized 112 children aged 1-12 years to baloxavir and 57 to oseltamivir. The predominant influenza A subtype was H3N2 for both groups, followed by H1N1pdm09. Demographics and baseline characteristics were similar between treatment groups, the investigators wrote in the Pediatric Infectious Disease Journal.
The time to alleviation of signs and symptoms was a median 138 hours for those receiving baloxavir and 150 hours for those receiving oseltamivir, a nonsignificant difference. Duration of fever and of all symptoms also were similar between groups, as was the time to return to normal health and activity.
A total of 122 adverse events were reported in 84 children, with 95% of adverse events being resolved or resolving by the end of the study. The incidence of adverse events was 46% in those receiving baloxavir and 53% in those receiving oseltamivir, a nonsignificant difference, with the most common adverse event in both groups being gastrointestinal disorders. No deaths, serious adverse events, or hospitalizations were reported, but two patients receiving oseltamivir discontinued because of adverse events.
The study was funded by F. Hoffmann-La Roche. Dr. Baker and a coauthor received funding through their institutions for the conduct of the study; several coauthors reported being employed by and owning stocks in F. Hoffmann–La Roche. One coauthor reported receiving consultancy fees from F. Hoffmann–La Roche and grants from Shionogi.
SOURCE: Baker J et al. Pediatr Infect Dis J. 2020 Jun 5. doi: 10.1097/INF.0000000000002747.
FROM THE PEDIATRIC INFECTIOUS DISEASE JOURNAL
FDA approves new antibiotic for HABP/VABP treatment
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
in people aged 18 years and older.
Approval for Recarbrio was based on results of a randomized, controlled clinical trial of 535 hospitalized adults with hospital-acquired and ventilator-associated bacterial pneumonia who received either Recarbrio or piperacillin-tazobactam. After 28 days, 16% of patients who received Recarbrio and 21% of patients who received piperacillin-tazobactam had died.
The most common adverse events associated with Recarbrio are increased alanine aminotransferase/ aspartate aminotransferase, anemia, diarrhea, hypokalemia, and hyponatremia. Recarbrio was previously approved by the FDA to treat patients with complicated urinary tract infections and complicated intra-abdominal infections who have limited or no alternative treatment options, according to an FDA press release.
“As a public health agency, the FDA addresses the threat of antimicrobial-resistant infections by facilitating the development of safe and effective new treatments. These efforts provide more options to fight serious bacterial infections and get new, safe and effective therapies to patients as soon as possible,” said Sumathi Nambiar, MD, MPH, director of the division of anti-infectives within the office of infectious disease at the Center for Drug Evaluation and Research.
FDA approves ramucirumab-erlotinib combo for metastatic NSCLC
The approval was supported by results from the phase 3 RELAY trial (Lancet Oncol. 2019 Dec;20[12]:1655-69). The trial enrolled 449 patients with previously untreated, EGFR-mutated, metastatic NSCLC.
Patients received either ramucirumab at 10 mg/kg or placebo every 2 weeks as an intravenous infusion in combination with erlotinib at 150 mg orally once daily. Patients continued treatment until they progressed or developed unacceptable toxicity. The median progression-free survival was 19.4 months in the ramucirumab-erlotinib arm, compared with 12.4 months in the placebo-erlotinib arm (hazard ratio, 0.59; 95% confidence interval, 0.46-0.76; P < .0001). The overall response rate was 76% in the ramucirumab arm and 75% in the placebo arm. The median duration of response was 18.0 months and 11.1 months, respectively. Overall survival data were not mature at the final analysis.
Adverse events that were more common in the ramucirumab arm were infections, hypertension, stomatitis, proteinuria, alopecia, epistaxis, and peripheral edema. Full prescribing information is available on the FDA website.
The approval was supported by results from the phase 3 RELAY trial (Lancet Oncol. 2019 Dec;20[12]:1655-69). The trial enrolled 449 patients with previously untreated, EGFR-mutated, metastatic NSCLC.
Patients received either ramucirumab at 10 mg/kg or placebo every 2 weeks as an intravenous infusion in combination with erlotinib at 150 mg orally once daily. Patients continued treatment until they progressed or developed unacceptable toxicity. The median progression-free survival was 19.4 months in the ramucirumab-erlotinib arm, compared with 12.4 months in the placebo-erlotinib arm (hazard ratio, 0.59; 95% confidence interval, 0.46-0.76; P < .0001). The overall response rate was 76% in the ramucirumab arm and 75% in the placebo arm. The median duration of response was 18.0 months and 11.1 months, respectively. Overall survival data were not mature at the final analysis.
Adverse events that were more common in the ramucirumab arm were infections, hypertension, stomatitis, proteinuria, alopecia, epistaxis, and peripheral edema. Full prescribing information is available on the FDA website.
The approval was supported by results from the phase 3 RELAY trial (Lancet Oncol. 2019 Dec;20[12]:1655-69). The trial enrolled 449 patients with previously untreated, EGFR-mutated, metastatic NSCLC.
Patients received either ramucirumab at 10 mg/kg or placebo every 2 weeks as an intravenous infusion in combination with erlotinib at 150 mg orally once daily. Patients continued treatment until they progressed or developed unacceptable toxicity. The median progression-free survival was 19.4 months in the ramucirumab-erlotinib arm, compared with 12.4 months in the placebo-erlotinib arm (hazard ratio, 0.59; 95% confidence interval, 0.46-0.76; P < .0001). The overall response rate was 76% in the ramucirumab arm and 75% in the placebo arm. The median duration of response was 18.0 months and 11.1 months, respectively. Overall survival data were not mature at the final analysis.
Adverse events that were more common in the ramucirumab arm were infections, hypertension, stomatitis, proteinuria, alopecia, epistaxis, and peripheral edema. Full prescribing information is available on the FDA website.