User login
Abnormal vaginal discharge: What does and does not work in treating underlying causes
- Treat bacterial vaginosis with oral or intravaginal metronidazole or with clindamycin (SOR: A);recurrences are common (SOR:C).
- Oral and intravaginal imidazoles are equally effective in the treatment of candidiasis (SOR:A); alternate therapies for resistant cases have been little studied. Oral metronidazole is the standard therapy for trichomoniasis (SOR: A).
- Oral tinidazole, newly available in the US in 2004, should be used in resistant cases (SOR: B).
Antifungal medications for intravaginal use have been available in the United States for more than a decade. Women may be inclined to self-diagnose yeast infections with any vaginal discharge or other vulvovaginal symptoms that they deem abnormal. As we saw in the first part of this article, “Abnormal vaginal discharge: Using office diagnostic testing more effectively” (JFP2004; 53[10]:805–814), abnormal discharge is more likely to be bacterial vaginosis or no pathogen at all. Potential delay in diagnosis and treatment of a sexually transmitted disease is also a concern. Increasing resistance of Candida sp. to imidazoles is associated with indiscriminate use of over-the-counter products.
Bacterial vaginosis
The standard treatment for bacterial vaginosis (BV) has been oral metronidazole (Flagyl) 500 mg twice daily for 5 to 7 days. Intravaginal 0.75% metronidazole gel (MetroGel) has been shown to be as effective as oral metronidazole (SOR: A).1,2
Oral metronidazole can cause nausea and abdominal pain in some patients; vaginal treatment may be preferable for them. A meta-analysis of 52 studies of regimens of oral metronidazole at a dose of 2 g daily of varying duration showed similar initial cure rates of 85%, 87%, 86%, and 87% for 1, 2, 5, and 7 days, respectively (strength of recommendation [SOR]: A).3 Single-dose therapy may improve adherence (SOR: C).
Clindamycin (Cleocin), orally or in vaginal cream, for 5 days is also effective for BV (SOR: A).4-8 Clindamycin cream is used at a dose of 5 g daily and a concentration of 2%. Lower concentration (1%) has been less effective.6 Oral regimens range from 300 mg twice daily to 450 mg 3 times daily. Oral and vaginal preparations have shown equal efficacy in direct comparisons (SOR: A).8 A 3-day course of vaginal clindamycin is as effective as a 5-day course (SOR: B).9
Several studies have compared clindamycin and metronidazole head to head. They have shown similar cure rates that were not statistically different in the 75% to 90% range (SOR: A).4,5,10,11 Other antibiotics that have shown in vitro efficacy for treating the spectrum of microbes associated with BV are amoxicillin-clavulanate (Augmentin), imipenem (Primaxin), and cefmetazole (Zefazone) (SOR: C).8,12 Some Mobiluncus strains show resistance to metronidazole (SOR: C).12
Recurrences of BV are common. The initial regimen or an alternative regimen may be used. A longer, 10- to 14-day, course of antibiotic therapy has been recommended by one expert for treating relapses (SOR: C).13 Recolonizing the vagina with lactobacilli by eating yogurt or using bacteria-containing suppositories is an approach that deserves further study (SOR: C).14 Suppressive therapy such as intravaginal metronidazole twice weekly may also be considered as maintenance therapy to prevent recurrences (SOR: C).
BV and pregnancy
A number of studies have been published on screening for BV in pregnancy using Gram stain and on treating positive cases with antibiotics. While studies that used metronidazole for treatment have not shown consistently good results, more recent studies using clindamycin orally or intravaginally have been promising (SOR: B).7,15 Oral dosing at 300 mg twice daily, at 12 to 22 weeks gestation, has reduced preterm delivery for pregnant women with BV diagnosed by Nugent’s criteria (number needed to treat [NNT]=10).7 Likewise, for women with BV treated at 13 to 20 weeks gestation, intravaginal clindamycin therapy for 3 days has reduced the incidence of preterm births (NNT=17).15
Clindamycin appears to be the treatment of choice for BV in pregnancy (SOR: C) since it is considered safe (category B) throughout pregnancy, and because use of metronidazole in the first trimester is controversial.
Candidiasis
Treating vulvovaginal candidiasis (VVC) with intravaginal imidazoles reduces symptoms with NNT=3 after 1 month (SOR: A) ( Table ).16 No difference has been seen in outcomes with the various imidazoles or with treatment durations of 1 to 14 days. Intravaginal nystatin also decreases symptoms of VVC, with a NNT of 3 after 1 week compared with placebo (SOR: B).
Data showing that imidazoles are more effective than nystatin are not strong (SOR: B). A Clinical Evidencereview16 identified 1 trial comparing intravaginal miconazole, clotrimazole, econazole, and nystatin; symptomatic relapse was lower with intravaginal imidazoles than with nystatin. Another trial comparing clotrimazole and nystatin showed no difference in the proportion of women with persistent symptoms after 4 weeks. An open label study17 comparing econazole, miconazole, and nystatin showed that the imidazoles had more antifungal activity, but there was no difference in clinical outcome assessment.
Oral treatments are popular, most commonly a single dose of fluconazole. Oral itraconazole and ketoconazole have also been used successfully (SOR: A).18-21 A systematic review of oral vs vaginal azoles showed similar efficacy, but more side effects occurred with oral therapy (SOR: A).22 Gastrointestinal side effects occur in up to 15% of women.23
TABLE
Antifungal medications used to treat vulvovaginal candidiasis
| Generic name | Trade name | Dose | Duration | Cost per course of treatment* |
|---|---|---|---|---|
| Over the counter | ||||
| Butoconazole 2% cream | Femstat-3 Mycelex-3 generic | 5 g every night | 3 days | $5–$34 |
| Clotrimazole 1% cream | Mycelex-7 generic | 5 g every night | 7–10 days | $2–$7 |
| Clotrimazole 200 mg suppository | Gyne-Lotrimin generic | 1 every night | 3 days | $2–$9 |
| Miconazole 100 mg suppository | Monistat generic | 1 every night | 7 days | $2–$14 |
| Miconazole 2% cream | Monistat generic | 5 g every night | 7–10 days | $2–$11 |
| Miconazole 200 mg suppository | Monistat-3 | 1 every night | 3 days | $3–$22 |
| Miconazole 100 mg suppository plus 2% external cream | Gyne-Lotrimin generic | 1 every night | 5 days | $5–$12 |
| Tioconazole 6.5% ointment | Vagistat-1 generic | 4.6 g | 1 day | $2–$19 |
| Prescription | ||||
| Econazole 1% cream | Spectazole | 5 g | 3–6 days | $18 for 15 g $31 for 30 g |
| Terconazole 0.4% cream | Terazol-7 | 5 g | 7 days | $41 |
| Terconazole 0.8% cream | Terazol-3 | 5 g | 3 days | $41 |
| Terconazole 80 mg suppository | Terazol-3 | 1 every night | 3 days | $41 |
| Nystatin 100,000 U vaginal tablets | Generic | 1 every night | 7–14 days | $14–$35 |
| Fluconazole 150 mg tablet | Diflucan | 1 orally daily | 1 day | $14 |
| Itraconazole 100 mg tablet | Sporonox | 2 orally daily 4 orally | 3 days 1 day | $56 $37 ($281 for 30 tablets) |
| Ketoconazole 200 mg tablet | Nizoral generic | 2 orally daily | 5 days | $32–$43 ($95 for 30 generic tablets) |
| *Average wholesale price for entire regimen in US dollars. | ||||
Treating complicated VVC
About 5% of women diagnosed with VVC will have frequent recurrences, 4 or more per year.24 Current therapies are fungistatic rather than fungicidal, so the yeast are reduced but not eradicated. Hypersensitivity and allergic reactions to topical preparations may be confused with recurrences. Experts recommend that, if wet mount or culture results confirm recurrent vaginitis, topical therapy should be increased from 5 to 7 days up to 10 to 14 days, or that a second oral fluconazole tablet be given 3 days after the first (SOR: C).24 Women with severe cases of VVC also benefit from 2 sequential doses of fluconazole given 3 days apart (SOR: B).25
Suppressive therapy may be used after initial treatment for 6 months or more (SOR: B). Suppressive therapy options include oral fluconazole 150 mg or vaginal clotrimazole 500 mg once a week, oral or intravaginal nystatin twice weekly, and oral itraconazole 200 mg monthly.24,26
Non-albicans species tend to be more resistant to oral and topical azoles (SOR: B).27-29 If this species is detected on culture, a long course of suppressive therapy should be attempted (SOR: C).24 If imidazole therapy fails to control symptoms, suspect resistance.
Resistance to azoles may be demonstrated by in vitro susceptibility testing. Cross-resistance to topical and oral (fluconazole) azoles has been documented.30 There have been few studies of alternatives to azoles for treatment of resistant yeast vaginitis. One little-studied alternative is intravaginal boric acid, which may be used as a 14-day course of 600 mg daily in gelatin capsules (SOR: C).31 Nystatin and flucytosine (Ancoban) for 7 to 14 days are other alternatives (SOR: C).24
Candida in pregnancy
Commonly used topical imidazoles are classified as category C in pregnancy and have not been associated with increased risk of birth defects.
Trichomoniasis
Current treatment for trichomoniasis is oral metronidazole, given as a 2-g single dose, 250 mg 3 times daily for 7 days or 500 mg twice a day for 7 days. Treatment should also be given to the woman’s partner (SOR: A).32 Intravaginal therapy is not effective, probably due to the parasite’s presence in inaccessible areas such as the vaginal glands and urethra.33,34 Short-term treatment is comparable with long-term treatment, with similar rates of nausea and vomiting (SOR: A).32 A 1.5-g single-dose treatment has been shown to be equivalent to 2 g (SOR: B).35
The incidence metronidazole-resistant trichomoniasis has been estimated at 5%.36 In such cases, higher-dose therapy may be still be effective. For low to moderate resistance, 2 too 2.5 g daily for 3 to 10 days has been recommended (SOR: B).37 Intravenous high-dose metronidazole, 2 g every 8 hours for 3 days, has been reported to successfully treat highly resistant trichomonas (SOR: C).38 Another case report of 2 women with presumed allergy to metronidazole were successfully treated with incremental dosing of IV metronidazole (SOR: C).39 A small case series of women with metronidazole allergy or resistance treated with paromomycin cream intravaginally showed cure in 6 of 9 cases (SOR: C).40 Oral tinidazole has been approved in 2004 for use in the treatment of metronidazole-resistant trichomoniasis (SOR: B).41
Corresponding author
Linda French, MD, Associate Professor, Department of Family Practice, College of Human Medicine, Michigan State University, B101 Clinical Center, East Lansing, MI 48824. E-mail: [email protected].
1. Hillier SL, Lipinski C, Briselden AM, Eschenbach DA. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol 1993;81:963-967.
2. Hanson JM, McGregor JA, Hillier SL, et al. Metronidazole for bacterial vaginosis. A comparison of vaginal gel vs. oral therapy. J Reprod Med 2000;45:889-896.
3. Lugo-Miro VI, Green M, Mazur L. Comparison of different metronidazole therapeutic regimens for bacterial vaginosis. A meta-analysis. JAMA 1992;268:92-95.
4. Ferris DG, Litaker MS, Woodward L, Mathis D, Hendrich J. Treatment of bacterial vaginosis: a comparison of oral metronidazole, metronidazole vaginal gel, and clindamycin vaginal cream. J Fam Pract 1995;41:443-449.
5. Fischbach F, Petersen EE, Weissenbacher ER, Martius J, Hosmann J, Mayer H. Efficacy of clindamycin vaginal cream versus oral metronidazole in the treatment of bacterial vaginosis. Obstet Gynecol 1993;82:405-410.
6. Hillier S, Krohn MA, Watts DH, Wolner-Hanssen P, Eschenbach D. Microbiologic efficacy of intravaginal clindamycin cream for the treatment of bacterial vaginosis. Obstet Gynecol 1990;76:407-413.
7. Ugwumadu A, Manyonda I, Reid F, Hay P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet 2003;361:983-988.
8. Mikamo H, Kawazoe K, Izumi K, Watanabe K, Ueno K, Tamaya T. Comparative study on vaginal or oral treatment of bacterial vaginosis. Chemotherapy 1997;43:60-68.
9. Ahmed-Jushuf IH, Shahmanesh M, Arya OP. The treatment of bacterial vaginosis with a 3 day course of 2% clindamycin cream: results of a multicentre, double blind, placebo controlled trial. B V Investigators Group. Genitourin Med 1995;71:254-256.
10. Schmitt C, Sobel JD, Meriwether C. Bacterial vaginosis: treatment with clindamycin cream versus oral metronidazole. Obstet Gynecol 1992;79:1020-1023.
11. Paavonen J, Mangioni C, Martin MA, Wajszczuk CP. Vaginal clindamycin and oral metronidazole for bacterial vaginosis: a randomized trial. Obstet Gynecol 2000;96:256-260.
12. Puapermpoonsiri S, Watanabe K, Kato N, Ueno K. In vitro activities of 10 antimicrobial agents against bacterial vaginosis-associated anaerobic isolates from pregnant Japanese and Thai women. Antimicrob Agents Chemother 1997;41:2297-2299.
13. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
14. Shalev E, Battino S, Weiner E, Colodner R, Keness Y. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med 1996;5:593-596.
15. Lamont RF, Jones BM, Mandal D, Hay PE, Sheehan M. The efficacy of vaginal clindamycin for the treatment of abnormal genital tract flora in pregnancy. Infect Dis Obstet Gynecol 2003;11:181-189.
16. Marrazzo J. Vulvovaginal candidiasis. BMJ 2002;325:586.
17. Emele FE, Fadahunsi AA, Anyiwo CE, Ogunleye O. A comparative clinical evaluation of econazole nitrate, miconazole, and nystatin in the treatment of vaginal candidiasis. West Afr J Med 2000;19:12-15.
18. Stein GE, Mummaw N. Placebo controlled trial of itraconazole for treatment of acute vaginal candidiasis. Antimicrob Agents Chemother 1993;37:89-92.
19. Van der Pas H, Peeters F, Janssens D, Snauwaert E, Van Cutsem J. Treatment of vaginal candidosis with oral ketoconazole. Eur J Obstet Gynecol Reprod Biol 1983;14:399-404.
20. Sanz Sanz F, del Palacio Hernanz A. Randomized comparative trial of three regimens of itraconazole for treatment of vaginal mycoses. Rev Infect Dis 1987;9 Suppl 1:S139-S142.
21. Silva-Cruz A, Andrade L, Sobral L, Francisca A. Itraconazole versus placebo in the management of vaginal candidiasis. Int J Gynaecol Obstet 1991;36:229-232.
22. Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intravaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): a systematic review. BJOG 2002;109:85-95.
23. van Heusden AM, Merkus HM, Corbeij RS, et al. Single-dose oral fluconazole versus single-dose topical miconazole for the treatment of acute vulvovaginal candidosis. Acta Obstet Gynecol Scand 1990;69:417-422.
24. Sobel JD. Vulvovaginitis. When Candida becomes a problem. Dermatol Clin 1998;16:763-768,xii.-
25. Sobel JD, Kapernick PS, Zervos M, et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001;185:363-369.
26. Creatsas GC, Charalambidis VM, Zagotzidou EH, Anthopoulou HN, Michailidis DC, Aravantinos DI. Chronic or recurrent vaginal candidosis: short-term treatment and prophylaxis with itraconazole. Clin Ther 1993;15:662-671.
27. Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J Clin Pharmacol 1992;32:248-255.
28. del Palacio A, Sanz F, Sanchez-Alor G, et al. Double-blind randomized dose-finding study in acute vulvovaginal can-didosis. Comparison of flutrimazole site-release cream (1, 2 and 4%) with placebo site-release vaginal cream. Mycoses 2000;43:355-365.
29. Singh S, Sobel JD, Bhargava P, Boikov D, Vazquez JA. Vaginitis due to Candida krusei: epidemiology, clinical aspects, and therapy. Clin Infect Dis 2002;35:1066-1070.
30. Cross EW, Park S, Perlin DS. Cross-Resistance of clinical isolates of Candida albicans and Candida glabrata to over-the-counter azoles used in the treatment of vaginitis. Microb Drug Resist 2000;6:155-161.
31. Sobel JD, Chaim W. Treatment of Torulopsis glabrata vaginitis: retrospective review of boric acid therapy. Clin Infect Dis 1997;24:649-652.
32. Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003(2);CD000218.
33. duBouchet L, McGregor JA, Ismail M, McCormack WM. A pilot study of metronidazole vaginal gel versus oral metronidazole for the treatment of Trichomonas vaginalis vaginitis. Sex Transm Dis 1998;25:176-179.
34. Tidwell BH, Lushbaugh WB, Laughlin MD, Cleary JD, Finley RW. A double-blind placebo-controlled trial of single-dose intravaginal versus single-dose oral metronidazole in the treatment of trichomonal vaginitis. J Infect Dis 1994;170:242-246.
35. Spence MR, Harwell TS, Davies MC, Smith JL. The minimum single oral metronidazole dose for treating trichomoniasis: a randomized, blinded study. Obstet Gynecol 1997;89:699-703.
36. Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev 1998;11:300-317.
37. Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991;165:1217-1222.
38. Dombrowski MP, Sokol RJ, Brown WJ, Bronsteen RA. Intravenous therapy of metronidazole-resistant Trichomonas vaginalis. Obstet Gynecol 1987;69:524-525.
39. Pearlman MD, Yashar C, Ernst S, Solomon W. An incremental dosing protocol for women with severe vaginal trichomoniasis and adverse reaction to metronidazole. Am J Obstet Gynecol 1996;174:934-936.
40. Nyirjesy P, Sobel JD, Weitz MV, Leaman DJ, Gelone SP. Difficult-to-treat trichomoniasis: results with paromomycin cream. Clin Infect Dis. 1998;26:986-988.
41. Sobel JD, Nyirjesy P, Brown W. Tinidazole therapy for metronidazole resistant vaginal trichomoniasis. Clin Infect Dis 2001;33:1341-1346.
- Treat bacterial vaginosis with oral or intravaginal metronidazole or with clindamycin (SOR: A);recurrences are common (SOR:C).
- Oral and intravaginal imidazoles are equally effective in the treatment of candidiasis (SOR:A); alternate therapies for resistant cases have been little studied. Oral metronidazole is the standard therapy for trichomoniasis (SOR: A).
- Oral tinidazole, newly available in the US in 2004, should be used in resistant cases (SOR: B).
Antifungal medications for intravaginal use have been available in the United States for more than a decade. Women may be inclined to self-diagnose yeast infections with any vaginal discharge or other vulvovaginal symptoms that they deem abnormal. As we saw in the first part of this article, “Abnormal vaginal discharge: Using office diagnostic testing more effectively” (JFP2004; 53[10]:805–814), abnormal discharge is more likely to be bacterial vaginosis or no pathogen at all. Potential delay in diagnosis and treatment of a sexually transmitted disease is also a concern. Increasing resistance of Candida sp. to imidazoles is associated with indiscriminate use of over-the-counter products.
Bacterial vaginosis
The standard treatment for bacterial vaginosis (BV) has been oral metronidazole (Flagyl) 500 mg twice daily for 5 to 7 days. Intravaginal 0.75% metronidazole gel (MetroGel) has been shown to be as effective as oral metronidazole (SOR: A).1,2
Oral metronidazole can cause nausea and abdominal pain in some patients; vaginal treatment may be preferable for them. A meta-analysis of 52 studies of regimens of oral metronidazole at a dose of 2 g daily of varying duration showed similar initial cure rates of 85%, 87%, 86%, and 87% for 1, 2, 5, and 7 days, respectively (strength of recommendation [SOR]: A).3 Single-dose therapy may improve adherence (SOR: C).
Clindamycin (Cleocin), orally or in vaginal cream, for 5 days is also effective for BV (SOR: A).4-8 Clindamycin cream is used at a dose of 5 g daily and a concentration of 2%. Lower concentration (1%) has been less effective.6 Oral regimens range from 300 mg twice daily to 450 mg 3 times daily. Oral and vaginal preparations have shown equal efficacy in direct comparisons (SOR: A).8 A 3-day course of vaginal clindamycin is as effective as a 5-day course (SOR: B).9
Several studies have compared clindamycin and metronidazole head to head. They have shown similar cure rates that were not statistically different in the 75% to 90% range (SOR: A).4,5,10,11 Other antibiotics that have shown in vitro efficacy for treating the spectrum of microbes associated with BV are amoxicillin-clavulanate (Augmentin), imipenem (Primaxin), and cefmetazole (Zefazone) (SOR: C).8,12 Some Mobiluncus strains show resistance to metronidazole (SOR: C).12
Recurrences of BV are common. The initial regimen or an alternative regimen may be used. A longer, 10- to 14-day, course of antibiotic therapy has been recommended by one expert for treating relapses (SOR: C).13 Recolonizing the vagina with lactobacilli by eating yogurt or using bacteria-containing suppositories is an approach that deserves further study (SOR: C).14 Suppressive therapy such as intravaginal metronidazole twice weekly may also be considered as maintenance therapy to prevent recurrences (SOR: C).
BV and pregnancy
A number of studies have been published on screening for BV in pregnancy using Gram stain and on treating positive cases with antibiotics. While studies that used metronidazole for treatment have not shown consistently good results, more recent studies using clindamycin orally or intravaginally have been promising (SOR: B).7,15 Oral dosing at 300 mg twice daily, at 12 to 22 weeks gestation, has reduced preterm delivery for pregnant women with BV diagnosed by Nugent’s criteria (number needed to treat [NNT]=10).7 Likewise, for women with BV treated at 13 to 20 weeks gestation, intravaginal clindamycin therapy for 3 days has reduced the incidence of preterm births (NNT=17).15
Clindamycin appears to be the treatment of choice for BV in pregnancy (SOR: C) since it is considered safe (category B) throughout pregnancy, and because use of metronidazole in the first trimester is controversial.
Candidiasis
Treating vulvovaginal candidiasis (VVC) with intravaginal imidazoles reduces symptoms with NNT=3 after 1 month (SOR: A) ( Table ).16 No difference has been seen in outcomes with the various imidazoles or with treatment durations of 1 to 14 days. Intravaginal nystatin also decreases symptoms of VVC, with a NNT of 3 after 1 week compared with placebo (SOR: B).
Data showing that imidazoles are more effective than nystatin are not strong (SOR: B). A Clinical Evidencereview16 identified 1 trial comparing intravaginal miconazole, clotrimazole, econazole, and nystatin; symptomatic relapse was lower with intravaginal imidazoles than with nystatin. Another trial comparing clotrimazole and nystatin showed no difference in the proportion of women with persistent symptoms after 4 weeks. An open label study17 comparing econazole, miconazole, and nystatin showed that the imidazoles had more antifungal activity, but there was no difference in clinical outcome assessment.
Oral treatments are popular, most commonly a single dose of fluconazole. Oral itraconazole and ketoconazole have also been used successfully (SOR: A).18-21 A systematic review of oral vs vaginal azoles showed similar efficacy, but more side effects occurred with oral therapy (SOR: A).22 Gastrointestinal side effects occur in up to 15% of women.23
TABLE
Antifungal medications used to treat vulvovaginal candidiasis
| Generic name | Trade name | Dose | Duration | Cost per course of treatment* |
|---|---|---|---|---|
| Over the counter | ||||
| Butoconazole 2% cream | Femstat-3 Mycelex-3 generic | 5 g every night | 3 days | $5–$34 |
| Clotrimazole 1% cream | Mycelex-7 generic | 5 g every night | 7–10 days | $2–$7 |
| Clotrimazole 200 mg suppository | Gyne-Lotrimin generic | 1 every night | 3 days | $2–$9 |
| Miconazole 100 mg suppository | Monistat generic | 1 every night | 7 days | $2–$14 |
| Miconazole 2% cream | Monistat generic | 5 g every night | 7–10 days | $2–$11 |
| Miconazole 200 mg suppository | Monistat-3 | 1 every night | 3 days | $3–$22 |
| Miconazole 100 mg suppository plus 2% external cream | Gyne-Lotrimin generic | 1 every night | 5 days | $5–$12 |
| Tioconazole 6.5% ointment | Vagistat-1 generic | 4.6 g | 1 day | $2–$19 |
| Prescription | ||||
| Econazole 1% cream | Spectazole | 5 g | 3–6 days | $18 for 15 g $31 for 30 g |
| Terconazole 0.4% cream | Terazol-7 | 5 g | 7 days | $41 |
| Terconazole 0.8% cream | Terazol-3 | 5 g | 3 days | $41 |
| Terconazole 80 mg suppository | Terazol-3 | 1 every night | 3 days | $41 |
| Nystatin 100,000 U vaginal tablets | Generic | 1 every night | 7–14 days | $14–$35 |
| Fluconazole 150 mg tablet | Diflucan | 1 orally daily | 1 day | $14 |
| Itraconazole 100 mg tablet | Sporonox | 2 orally daily 4 orally | 3 days 1 day | $56 $37 ($281 for 30 tablets) |
| Ketoconazole 200 mg tablet | Nizoral generic | 2 orally daily | 5 days | $32–$43 ($95 for 30 generic tablets) |
| *Average wholesale price for entire regimen in US dollars. | ||||
Treating complicated VVC
About 5% of women diagnosed with VVC will have frequent recurrences, 4 or more per year.24 Current therapies are fungistatic rather than fungicidal, so the yeast are reduced but not eradicated. Hypersensitivity and allergic reactions to topical preparations may be confused with recurrences. Experts recommend that, if wet mount or culture results confirm recurrent vaginitis, topical therapy should be increased from 5 to 7 days up to 10 to 14 days, or that a second oral fluconazole tablet be given 3 days after the first (SOR: C).24 Women with severe cases of VVC also benefit from 2 sequential doses of fluconazole given 3 days apart (SOR: B).25
Suppressive therapy may be used after initial treatment for 6 months or more (SOR: B). Suppressive therapy options include oral fluconazole 150 mg or vaginal clotrimazole 500 mg once a week, oral or intravaginal nystatin twice weekly, and oral itraconazole 200 mg monthly.24,26
Non-albicans species tend to be more resistant to oral and topical azoles (SOR: B).27-29 If this species is detected on culture, a long course of suppressive therapy should be attempted (SOR: C).24 If imidazole therapy fails to control symptoms, suspect resistance.
Resistance to azoles may be demonstrated by in vitro susceptibility testing. Cross-resistance to topical and oral (fluconazole) azoles has been documented.30 There have been few studies of alternatives to azoles for treatment of resistant yeast vaginitis. One little-studied alternative is intravaginal boric acid, which may be used as a 14-day course of 600 mg daily in gelatin capsules (SOR: C).31 Nystatin and flucytosine (Ancoban) for 7 to 14 days are other alternatives (SOR: C).24
Candida in pregnancy
Commonly used topical imidazoles are classified as category C in pregnancy and have not been associated with increased risk of birth defects.
Trichomoniasis
Current treatment for trichomoniasis is oral metronidazole, given as a 2-g single dose, 250 mg 3 times daily for 7 days or 500 mg twice a day for 7 days. Treatment should also be given to the woman’s partner (SOR: A).32 Intravaginal therapy is not effective, probably due to the parasite’s presence in inaccessible areas such as the vaginal glands and urethra.33,34 Short-term treatment is comparable with long-term treatment, with similar rates of nausea and vomiting (SOR: A).32 A 1.5-g single-dose treatment has been shown to be equivalent to 2 g (SOR: B).35
The incidence metronidazole-resistant trichomoniasis has been estimated at 5%.36 In such cases, higher-dose therapy may be still be effective. For low to moderate resistance, 2 too 2.5 g daily for 3 to 10 days has been recommended (SOR: B).37 Intravenous high-dose metronidazole, 2 g every 8 hours for 3 days, has been reported to successfully treat highly resistant trichomonas (SOR: C).38 Another case report of 2 women with presumed allergy to metronidazole were successfully treated with incremental dosing of IV metronidazole (SOR: C).39 A small case series of women with metronidazole allergy or resistance treated with paromomycin cream intravaginally showed cure in 6 of 9 cases (SOR: C).40 Oral tinidazole has been approved in 2004 for use in the treatment of metronidazole-resistant trichomoniasis (SOR: B).41
Corresponding author
Linda French, MD, Associate Professor, Department of Family Practice, College of Human Medicine, Michigan State University, B101 Clinical Center, East Lansing, MI 48824. E-mail: [email protected].
- Treat bacterial vaginosis with oral or intravaginal metronidazole or with clindamycin (SOR: A);recurrences are common (SOR:C).
- Oral and intravaginal imidazoles are equally effective in the treatment of candidiasis (SOR:A); alternate therapies for resistant cases have been little studied. Oral metronidazole is the standard therapy for trichomoniasis (SOR: A).
- Oral tinidazole, newly available in the US in 2004, should be used in resistant cases (SOR: B).
Antifungal medications for intravaginal use have been available in the United States for more than a decade. Women may be inclined to self-diagnose yeast infections with any vaginal discharge or other vulvovaginal symptoms that they deem abnormal. As we saw in the first part of this article, “Abnormal vaginal discharge: Using office diagnostic testing more effectively” (JFP2004; 53[10]:805–814), abnormal discharge is more likely to be bacterial vaginosis or no pathogen at all. Potential delay in diagnosis and treatment of a sexually transmitted disease is also a concern. Increasing resistance of Candida sp. to imidazoles is associated with indiscriminate use of over-the-counter products.
Bacterial vaginosis
The standard treatment for bacterial vaginosis (BV) has been oral metronidazole (Flagyl) 500 mg twice daily for 5 to 7 days. Intravaginal 0.75% metronidazole gel (MetroGel) has been shown to be as effective as oral metronidazole (SOR: A).1,2
Oral metronidazole can cause nausea and abdominal pain in some patients; vaginal treatment may be preferable for them. A meta-analysis of 52 studies of regimens of oral metronidazole at a dose of 2 g daily of varying duration showed similar initial cure rates of 85%, 87%, 86%, and 87% for 1, 2, 5, and 7 days, respectively (strength of recommendation [SOR]: A).3 Single-dose therapy may improve adherence (SOR: C).
Clindamycin (Cleocin), orally or in vaginal cream, for 5 days is also effective for BV (SOR: A).4-8 Clindamycin cream is used at a dose of 5 g daily and a concentration of 2%. Lower concentration (1%) has been less effective.6 Oral regimens range from 300 mg twice daily to 450 mg 3 times daily. Oral and vaginal preparations have shown equal efficacy in direct comparisons (SOR: A).8 A 3-day course of vaginal clindamycin is as effective as a 5-day course (SOR: B).9
Several studies have compared clindamycin and metronidazole head to head. They have shown similar cure rates that were not statistically different in the 75% to 90% range (SOR: A).4,5,10,11 Other antibiotics that have shown in vitro efficacy for treating the spectrum of microbes associated with BV are amoxicillin-clavulanate (Augmentin), imipenem (Primaxin), and cefmetazole (Zefazone) (SOR: C).8,12 Some Mobiluncus strains show resistance to metronidazole (SOR: C).12
Recurrences of BV are common. The initial regimen or an alternative regimen may be used. A longer, 10- to 14-day, course of antibiotic therapy has been recommended by one expert for treating relapses (SOR: C).13 Recolonizing the vagina with lactobacilli by eating yogurt or using bacteria-containing suppositories is an approach that deserves further study (SOR: C).14 Suppressive therapy such as intravaginal metronidazole twice weekly may also be considered as maintenance therapy to prevent recurrences (SOR: C).
BV and pregnancy
A number of studies have been published on screening for BV in pregnancy using Gram stain and on treating positive cases with antibiotics. While studies that used metronidazole for treatment have not shown consistently good results, more recent studies using clindamycin orally or intravaginally have been promising (SOR: B).7,15 Oral dosing at 300 mg twice daily, at 12 to 22 weeks gestation, has reduced preterm delivery for pregnant women with BV diagnosed by Nugent’s criteria (number needed to treat [NNT]=10).7 Likewise, for women with BV treated at 13 to 20 weeks gestation, intravaginal clindamycin therapy for 3 days has reduced the incidence of preterm births (NNT=17).15
Clindamycin appears to be the treatment of choice for BV in pregnancy (SOR: C) since it is considered safe (category B) throughout pregnancy, and because use of metronidazole in the first trimester is controversial.
Candidiasis
Treating vulvovaginal candidiasis (VVC) with intravaginal imidazoles reduces symptoms with NNT=3 after 1 month (SOR: A) ( Table ).16 No difference has been seen in outcomes with the various imidazoles or with treatment durations of 1 to 14 days. Intravaginal nystatin also decreases symptoms of VVC, with a NNT of 3 after 1 week compared with placebo (SOR: B).
Data showing that imidazoles are more effective than nystatin are not strong (SOR: B). A Clinical Evidencereview16 identified 1 trial comparing intravaginal miconazole, clotrimazole, econazole, and nystatin; symptomatic relapse was lower with intravaginal imidazoles than with nystatin. Another trial comparing clotrimazole and nystatin showed no difference in the proportion of women with persistent symptoms after 4 weeks. An open label study17 comparing econazole, miconazole, and nystatin showed that the imidazoles had more antifungal activity, but there was no difference in clinical outcome assessment.
Oral treatments are popular, most commonly a single dose of fluconazole. Oral itraconazole and ketoconazole have also been used successfully (SOR: A).18-21 A systematic review of oral vs vaginal azoles showed similar efficacy, but more side effects occurred with oral therapy (SOR: A).22 Gastrointestinal side effects occur in up to 15% of women.23
TABLE
Antifungal medications used to treat vulvovaginal candidiasis
| Generic name | Trade name | Dose | Duration | Cost per course of treatment* |
|---|---|---|---|---|
| Over the counter | ||||
| Butoconazole 2% cream | Femstat-3 Mycelex-3 generic | 5 g every night | 3 days | $5–$34 |
| Clotrimazole 1% cream | Mycelex-7 generic | 5 g every night | 7–10 days | $2–$7 |
| Clotrimazole 200 mg suppository | Gyne-Lotrimin generic | 1 every night | 3 days | $2–$9 |
| Miconazole 100 mg suppository | Monistat generic | 1 every night | 7 days | $2–$14 |
| Miconazole 2% cream | Monistat generic | 5 g every night | 7–10 days | $2–$11 |
| Miconazole 200 mg suppository | Monistat-3 | 1 every night | 3 days | $3–$22 |
| Miconazole 100 mg suppository plus 2% external cream | Gyne-Lotrimin generic | 1 every night | 5 days | $5–$12 |
| Tioconazole 6.5% ointment | Vagistat-1 generic | 4.6 g | 1 day | $2–$19 |
| Prescription | ||||
| Econazole 1% cream | Spectazole | 5 g | 3–6 days | $18 for 15 g $31 for 30 g |
| Terconazole 0.4% cream | Terazol-7 | 5 g | 7 days | $41 |
| Terconazole 0.8% cream | Terazol-3 | 5 g | 3 days | $41 |
| Terconazole 80 mg suppository | Terazol-3 | 1 every night | 3 days | $41 |
| Nystatin 100,000 U vaginal tablets | Generic | 1 every night | 7–14 days | $14–$35 |
| Fluconazole 150 mg tablet | Diflucan | 1 orally daily | 1 day | $14 |
| Itraconazole 100 mg tablet | Sporonox | 2 orally daily 4 orally | 3 days 1 day | $56 $37 ($281 for 30 tablets) |
| Ketoconazole 200 mg tablet | Nizoral generic | 2 orally daily | 5 days | $32–$43 ($95 for 30 generic tablets) |
| *Average wholesale price for entire regimen in US dollars. | ||||
Treating complicated VVC
About 5% of women diagnosed with VVC will have frequent recurrences, 4 or more per year.24 Current therapies are fungistatic rather than fungicidal, so the yeast are reduced but not eradicated. Hypersensitivity and allergic reactions to topical preparations may be confused with recurrences. Experts recommend that, if wet mount or culture results confirm recurrent vaginitis, topical therapy should be increased from 5 to 7 days up to 10 to 14 days, or that a second oral fluconazole tablet be given 3 days after the first (SOR: C).24 Women with severe cases of VVC also benefit from 2 sequential doses of fluconazole given 3 days apart (SOR: B).25
Suppressive therapy may be used after initial treatment for 6 months or more (SOR: B). Suppressive therapy options include oral fluconazole 150 mg or vaginal clotrimazole 500 mg once a week, oral or intravaginal nystatin twice weekly, and oral itraconazole 200 mg monthly.24,26
Non-albicans species tend to be more resistant to oral and topical azoles (SOR: B).27-29 If this species is detected on culture, a long course of suppressive therapy should be attempted (SOR: C).24 If imidazole therapy fails to control symptoms, suspect resistance.
Resistance to azoles may be demonstrated by in vitro susceptibility testing. Cross-resistance to topical and oral (fluconazole) azoles has been documented.30 There have been few studies of alternatives to azoles for treatment of resistant yeast vaginitis. One little-studied alternative is intravaginal boric acid, which may be used as a 14-day course of 600 mg daily in gelatin capsules (SOR: C).31 Nystatin and flucytosine (Ancoban) for 7 to 14 days are other alternatives (SOR: C).24
Candida in pregnancy
Commonly used topical imidazoles are classified as category C in pregnancy and have not been associated with increased risk of birth defects.
Trichomoniasis
Current treatment for trichomoniasis is oral metronidazole, given as a 2-g single dose, 250 mg 3 times daily for 7 days or 500 mg twice a day for 7 days. Treatment should also be given to the woman’s partner (SOR: A).32 Intravaginal therapy is not effective, probably due to the parasite’s presence in inaccessible areas such as the vaginal glands and urethra.33,34 Short-term treatment is comparable with long-term treatment, with similar rates of nausea and vomiting (SOR: A).32 A 1.5-g single-dose treatment has been shown to be equivalent to 2 g (SOR: B).35
The incidence metronidazole-resistant trichomoniasis has been estimated at 5%.36 In such cases, higher-dose therapy may be still be effective. For low to moderate resistance, 2 too 2.5 g daily for 3 to 10 days has been recommended (SOR: B).37 Intravenous high-dose metronidazole, 2 g every 8 hours for 3 days, has been reported to successfully treat highly resistant trichomonas (SOR: C).38 Another case report of 2 women with presumed allergy to metronidazole were successfully treated with incremental dosing of IV metronidazole (SOR: C).39 A small case series of women with metronidazole allergy or resistance treated with paromomycin cream intravaginally showed cure in 6 of 9 cases (SOR: C).40 Oral tinidazole has been approved in 2004 for use in the treatment of metronidazole-resistant trichomoniasis (SOR: B).41
Corresponding author
Linda French, MD, Associate Professor, Department of Family Practice, College of Human Medicine, Michigan State University, B101 Clinical Center, East Lansing, MI 48824. E-mail: [email protected].
1. Hillier SL, Lipinski C, Briselden AM, Eschenbach DA. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol 1993;81:963-967.
2. Hanson JM, McGregor JA, Hillier SL, et al. Metronidazole for bacterial vaginosis. A comparison of vaginal gel vs. oral therapy. J Reprod Med 2000;45:889-896.
3. Lugo-Miro VI, Green M, Mazur L. Comparison of different metronidazole therapeutic regimens for bacterial vaginosis. A meta-analysis. JAMA 1992;268:92-95.
4. Ferris DG, Litaker MS, Woodward L, Mathis D, Hendrich J. Treatment of bacterial vaginosis: a comparison of oral metronidazole, metronidazole vaginal gel, and clindamycin vaginal cream. J Fam Pract 1995;41:443-449.
5. Fischbach F, Petersen EE, Weissenbacher ER, Martius J, Hosmann J, Mayer H. Efficacy of clindamycin vaginal cream versus oral metronidazole in the treatment of bacterial vaginosis. Obstet Gynecol 1993;82:405-410.
6. Hillier S, Krohn MA, Watts DH, Wolner-Hanssen P, Eschenbach D. Microbiologic efficacy of intravaginal clindamycin cream for the treatment of bacterial vaginosis. Obstet Gynecol 1990;76:407-413.
7. Ugwumadu A, Manyonda I, Reid F, Hay P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet 2003;361:983-988.
8. Mikamo H, Kawazoe K, Izumi K, Watanabe K, Ueno K, Tamaya T. Comparative study on vaginal or oral treatment of bacterial vaginosis. Chemotherapy 1997;43:60-68.
9. Ahmed-Jushuf IH, Shahmanesh M, Arya OP. The treatment of bacterial vaginosis with a 3 day course of 2% clindamycin cream: results of a multicentre, double blind, placebo controlled trial. B V Investigators Group. Genitourin Med 1995;71:254-256.
10. Schmitt C, Sobel JD, Meriwether C. Bacterial vaginosis: treatment with clindamycin cream versus oral metronidazole. Obstet Gynecol 1992;79:1020-1023.
11. Paavonen J, Mangioni C, Martin MA, Wajszczuk CP. Vaginal clindamycin and oral metronidazole for bacterial vaginosis: a randomized trial. Obstet Gynecol 2000;96:256-260.
12. Puapermpoonsiri S, Watanabe K, Kato N, Ueno K. In vitro activities of 10 antimicrobial agents against bacterial vaginosis-associated anaerobic isolates from pregnant Japanese and Thai women. Antimicrob Agents Chemother 1997;41:2297-2299.
13. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
14. Shalev E, Battino S, Weiner E, Colodner R, Keness Y. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med 1996;5:593-596.
15. Lamont RF, Jones BM, Mandal D, Hay PE, Sheehan M. The efficacy of vaginal clindamycin for the treatment of abnormal genital tract flora in pregnancy. Infect Dis Obstet Gynecol 2003;11:181-189.
16. Marrazzo J. Vulvovaginal candidiasis. BMJ 2002;325:586.
17. Emele FE, Fadahunsi AA, Anyiwo CE, Ogunleye O. A comparative clinical evaluation of econazole nitrate, miconazole, and nystatin in the treatment of vaginal candidiasis. West Afr J Med 2000;19:12-15.
18. Stein GE, Mummaw N. Placebo controlled trial of itraconazole for treatment of acute vaginal candidiasis. Antimicrob Agents Chemother 1993;37:89-92.
19. Van der Pas H, Peeters F, Janssens D, Snauwaert E, Van Cutsem J. Treatment of vaginal candidosis with oral ketoconazole. Eur J Obstet Gynecol Reprod Biol 1983;14:399-404.
20. Sanz Sanz F, del Palacio Hernanz A. Randomized comparative trial of three regimens of itraconazole for treatment of vaginal mycoses. Rev Infect Dis 1987;9 Suppl 1:S139-S142.
21. Silva-Cruz A, Andrade L, Sobral L, Francisca A. Itraconazole versus placebo in the management of vaginal candidiasis. Int J Gynaecol Obstet 1991;36:229-232.
22. Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intravaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): a systematic review. BJOG 2002;109:85-95.
23. van Heusden AM, Merkus HM, Corbeij RS, et al. Single-dose oral fluconazole versus single-dose topical miconazole for the treatment of acute vulvovaginal candidosis. Acta Obstet Gynecol Scand 1990;69:417-422.
24. Sobel JD. Vulvovaginitis. When Candida becomes a problem. Dermatol Clin 1998;16:763-768,xii.-
25. Sobel JD, Kapernick PS, Zervos M, et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001;185:363-369.
26. Creatsas GC, Charalambidis VM, Zagotzidou EH, Anthopoulou HN, Michailidis DC, Aravantinos DI. Chronic or recurrent vaginal candidosis: short-term treatment and prophylaxis with itraconazole. Clin Ther 1993;15:662-671.
27. Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J Clin Pharmacol 1992;32:248-255.
28. del Palacio A, Sanz F, Sanchez-Alor G, et al. Double-blind randomized dose-finding study in acute vulvovaginal can-didosis. Comparison of flutrimazole site-release cream (1, 2 and 4%) with placebo site-release vaginal cream. Mycoses 2000;43:355-365.
29. Singh S, Sobel JD, Bhargava P, Boikov D, Vazquez JA. Vaginitis due to Candida krusei: epidemiology, clinical aspects, and therapy. Clin Infect Dis 2002;35:1066-1070.
30. Cross EW, Park S, Perlin DS. Cross-Resistance of clinical isolates of Candida albicans and Candida glabrata to over-the-counter azoles used in the treatment of vaginitis. Microb Drug Resist 2000;6:155-161.
31. Sobel JD, Chaim W. Treatment of Torulopsis glabrata vaginitis: retrospective review of boric acid therapy. Clin Infect Dis 1997;24:649-652.
32. Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003(2);CD000218.
33. duBouchet L, McGregor JA, Ismail M, McCormack WM. A pilot study of metronidazole vaginal gel versus oral metronidazole for the treatment of Trichomonas vaginalis vaginitis. Sex Transm Dis 1998;25:176-179.
34. Tidwell BH, Lushbaugh WB, Laughlin MD, Cleary JD, Finley RW. A double-blind placebo-controlled trial of single-dose intravaginal versus single-dose oral metronidazole in the treatment of trichomonal vaginitis. J Infect Dis 1994;170:242-246.
35. Spence MR, Harwell TS, Davies MC, Smith JL. The minimum single oral metronidazole dose for treating trichomoniasis: a randomized, blinded study. Obstet Gynecol 1997;89:699-703.
36. Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev 1998;11:300-317.
37. Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991;165:1217-1222.
38. Dombrowski MP, Sokol RJ, Brown WJ, Bronsteen RA. Intravenous therapy of metronidazole-resistant Trichomonas vaginalis. Obstet Gynecol 1987;69:524-525.
39. Pearlman MD, Yashar C, Ernst S, Solomon W. An incremental dosing protocol for women with severe vaginal trichomoniasis and adverse reaction to metronidazole. Am J Obstet Gynecol 1996;174:934-936.
40. Nyirjesy P, Sobel JD, Weitz MV, Leaman DJ, Gelone SP. Difficult-to-treat trichomoniasis: results with paromomycin cream. Clin Infect Dis. 1998;26:986-988.
41. Sobel JD, Nyirjesy P, Brown W. Tinidazole therapy for metronidazole resistant vaginal trichomoniasis. Clin Infect Dis 2001;33:1341-1346.
1. Hillier SL, Lipinski C, Briselden AM, Eschenbach DA. Efficacy of intravaginal 0.75% metronidazole gel for the treatment of bacterial vaginosis. Obstet Gynecol 1993;81:963-967.
2. Hanson JM, McGregor JA, Hillier SL, et al. Metronidazole for bacterial vaginosis. A comparison of vaginal gel vs. oral therapy. J Reprod Med 2000;45:889-896.
3. Lugo-Miro VI, Green M, Mazur L. Comparison of different metronidazole therapeutic regimens for bacterial vaginosis. A meta-analysis. JAMA 1992;268:92-95.
4. Ferris DG, Litaker MS, Woodward L, Mathis D, Hendrich J. Treatment of bacterial vaginosis: a comparison of oral metronidazole, metronidazole vaginal gel, and clindamycin vaginal cream. J Fam Pract 1995;41:443-449.
5. Fischbach F, Petersen EE, Weissenbacher ER, Martius J, Hosmann J, Mayer H. Efficacy of clindamycin vaginal cream versus oral metronidazole in the treatment of bacterial vaginosis. Obstet Gynecol 1993;82:405-410.
6. Hillier S, Krohn MA, Watts DH, Wolner-Hanssen P, Eschenbach D. Microbiologic efficacy of intravaginal clindamycin cream for the treatment of bacterial vaginosis. Obstet Gynecol 1990;76:407-413.
7. Ugwumadu A, Manyonda I, Reid F, Hay P. Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet 2003;361:983-988.
8. Mikamo H, Kawazoe K, Izumi K, Watanabe K, Ueno K, Tamaya T. Comparative study on vaginal or oral treatment of bacterial vaginosis. Chemotherapy 1997;43:60-68.
9. Ahmed-Jushuf IH, Shahmanesh M, Arya OP. The treatment of bacterial vaginosis with a 3 day course of 2% clindamycin cream: results of a multicentre, double blind, placebo controlled trial. B V Investigators Group. Genitourin Med 1995;71:254-256.
10. Schmitt C, Sobel JD, Meriwether C. Bacterial vaginosis: treatment with clindamycin cream versus oral metronidazole. Obstet Gynecol 1992;79:1020-1023.
11. Paavonen J, Mangioni C, Martin MA, Wajszczuk CP. Vaginal clindamycin and oral metronidazole for bacterial vaginosis: a randomized trial. Obstet Gynecol 2000;96:256-260.
12. Puapermpoonsiri S, Watanabe K, Kato N, Ueno K. In vitro activities of 10 antimicrobial agents against bacterial vaginosis-associated anaerobic isolates from pregnant Japanese and Thai women. Antimicrob Agents Chemother 1997;41:2297-2299.
13. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
14. Shalev E, Battino S, Weiner E, Colodner R, Keness Y. Ingestion of yogurt containing Lactobacillus acidophilus compared with pasteurized yogurt as prophylaxis for recurrent candidal vaginitis and bacterial vaginosis. Arch Fam Med 1996;5:593-596.
15. Lamont RF, Jones BM, Mandal D, Hay PE, Sheehan M. The efficacy of vaginal clindamycin for the treatment of abnormal genital tract flora in pregnancy. Infect Dis Obstet Gynecol 2003;11:181-189.
16. Marrazzo J. Vulvovaginal candidiasis. BMJ 2002;325:586.
17. Emele FE, Fadahunsi AA, Anyiwo CE, Ogunleye O. A comparative clinical evaluation of econazole nitrate, miconazole, and nystatin in the treatment of vaginal candidiasis. West Afr J Med 2000;19:12-15.
18. Stein GE, Mummaw N. Placebo controlled trial of itraconazole for treatment of acute vaginal candidiasis. Antimicrob Agents Chemother 1993;37:89-92.
19. Van der Pas H, Peeters F, Janssens D, Snauwaert E, Van Cutsem J. Treatment of vaginal candidosis with oral ketoconazole. Eur J Obstet Gynecol Reprod Biol 1983;14:399-404.
20. Sanz Sanz F, del Palacio Hernanz A. Randomized comparative trial of three regimens of itraconazole for treatment of vaginal mycoses. Rev Infect Dis 1987;9 Suppl 1:S139-S142.
21. Silva-Cruz A, Andrade L, Sobral L, Francisca A. Itraconazole versus placebo in the management of vaginal candidiasis. Int J Gynaecol Obstet 1991;36:229-232.
22. Watson MC, Grimshaw JM, Bond CM, Mollison J, Ludbrook A. Oral versus intravaginal imidazole and triazole anti-fungal agents for the treatment of uncomplicated vulvovaginal candidiasis (thrush): a systematic review. BJOG 2002;109:85-95.
23. van Heusden AM, Merkus HM, Corbeij RS, et al. Single-dose oral fluconazole versus single-dose topical miconazole for the treatment of acute vulvovaginal candidosis. Acta Obstet Gynecol Scand 1990;69:417-422.
24. Sobel JD. Vulvovaginitis. When Candida becomes a problem. Dermatol Clin 1998;16:763-768,xii.-
25. Sobel JD, Kapernick PS, Zervos M, et al. Treatment of complicated Candida vaginitis: comparison of single and sequential doses of fluconazole. Am J Obstet Gynecol 2001;185:363-369.
26. Creatsas GC, Charalambidis VM, Zagotzidou EH, Anthopoulou HN, Michailidis DC, Aravantinos DI. Chronic or recurrent vaginal candidosis: short-term treatment and prophylaxis with itraconazole. Clin Ther 1993;15:662-671.
27. Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J Clin Pharmacol 1992;32:248-255.
28. del Palacio A, Sanz F, Sanchez-Alor G, et al. Double-blind randomized dose-finding study in acute vulvovaginal can-didosis. Comparison of flutrimazole site-release cream (1, 2 and 4%) with placebo site-release vaginal cream. Mycoses 2000;43:355-365.
29. Singh S, Sobel JD, Bhargava P, Boikov D, Vazquez JA. Vaginitis due to Candida krusei: epidemiology, clinical aspects, and therapy. Clin Infect Dis 2002;35:1066-1070.
30. Cross EW, Park S, Perlin DS. Cross-Resistance of clinical isolates of Candida albicans and Candida glabrata to over-the-counter azoles used in the treatment of vaginitis. Microb Drug Resist 2000;6:155-161.
31. Sobel JD, Chaim W. Treatment of Torulopsis glabrata vaginitis: retrospective review of boric acid therapy. Clin Infect Dis 1997;24:649-652.
32. Forna F, Gulmezoglu AM. Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev 2003(2);CD000218.
33. duBouchet L, McGregor JA, Ismail M, McCormack WM. A pilot study of metronidazole vaginal gel versus oral metronidazole for the treatment of Trichomonas vaginalis vaginitis. Sex Transm Dis 1998;25:176-179.
34. Tidwell BH, Lushbaugh WB, Laughlin MD, Cleary JD, Finley RW. A double-blind placebo-controlled trial of single-dose intravaginal versus single-dose oral metronidazole in the treatment of trichomonal vaginitis. J Infect Dis 1994;170:242-246.
35. Spence MR, Harwell TS, Davies MC, Smith JL. The minimum single oral metronidazole dose for treating trichomoniasis: a randomized, blinded study. Obstet Gynecol 1997;89:699-703.
36. Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev 1998;11:300-317.
37. Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991;165:1217-1222.
38. Dombrowski MP, Sokol RJ, Brown WJ, Bronsteen RA. Intravenous therapy of metronidazole-resistant Trichomonas vaginalis. Obstet Gynecol 1987;69:524-525.
39. Pearlman MD, Yashar C, Ernst S, Solomon W. An incremental dosing protocol for women with severe vaginal trichomoniasis and adverse reaction to metronidazole. Am J Obstet Gynecol 1996;174:934-936.
40. Nyirjesy P, Sobel JD, Weitz MV, Leaman DJ, Gelone SP. Difficult-to-treat trichomoniasis: results with paromomycin cream. Clin Infect Dis. 1998;26:986-988.
41. Sobel JD, Nyirjesy P, Brown W. Tinidazole therapy for metronidazole resistant vaginal trichomoniasis. Clin Infect Dis 2001;33:1341-1346.
Abnormal vaginal discharge: Using office diagnostic testing more effectively
- Accurate differential diagnosis for women complaining of abnormal vaginal discharge requires in-office diagnostic testing at minimum, and laboratory testing in selected cases.
- Test for Chlamydia trachomatis and Neisseria gonorrhea when signs of purulent cervicitis are present (SOR: B).
- In suspected vulvovaginal candidiasis, culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR:B); rapid slide latex agglutination testing is not better than microscopy for diagnosing VVC (SOR: B).
In primary care practice, abnormal vaginal discharge is a common complaint. Signs and symptoms of vaginitis—the most common gynecologic diagnosis in primary care1 —are not specific for any single underlying cause.2 Officebased diagnostic testing, which is underused,3 must be employed to ensure accurate diagnosis and effective treatment. (An article on treatment by the same authors will appear in next month’s issue of The journal of family practice.)
In a primary-care study,4 vulvovaginal symptoms including vaginal discharge were due to vulvovaginal candidiasis (VVC) in 27% of patients, bacterial vaginosis (BV) in 21%, trichomoniasis in 8%, Chlamydia trachomatis in 2%, Neisseria gonorrhea (GC) in 1%, and no infection in 34%. Several pathogens may coexist.2 VVC, BV, and trichomoniasis account for at least 90% of infectious vaginitis.5 This review will therefore focus heavily on these causes of vaginal discharge among women of reproductive age, including pregnant women.
Cervicitis and physiologic cervical discharge
Some women may interpret a physiologic increase in cervical mucous production as abnormal. It occurs cyclically prior to ovulation, is typically transparent and colorless, and may be more pronounced in women with an everted cervix.
Chlamydial infection
In the clinical examination of the cervix, 3 characteristics have been associated with chlamydial infection: yellow endocervical discharge, easily induced cervical bleeding, and opaque cervical discharge.6 All 3 findings are statistically significant and independently associated with chlamydial infection (odds ratios 2.8, 2.3, and 2.9, respectively). In the primary care study cited above, purulent cervical discharge was found in 6% of women, most commonly testing positive for Chlamydia, less often for GC.4
Trichomonas vaginalis may cause cervicitis as well as vaginitis. Mycoplasma genitalium has been proposed as an additional possible pathogen. It was identified in 7% of more than 700 women with mucopurulent cervical discharge seen in a STD clinic with otherwise negative cultures.7 With cervical discharge that appears to be purulent, testing is warranted as a minimum for Chlamydia and GC (SOR: B). Screening of asymptomatic women less than 26 years of age for Chlamydia is recommended by the US Preventive Services Task Force (SOR: A).
Bacterial Vaginosis
Bacterial vaginosis (BV) is neither an inflammatory condition nor an STD, but is a shift in vaginal flora from the normal condition in which lactobacilli predominate, to a polymicrobial flora in which gram-positive anaerobes predominate. In addition to annoying vaginal symptoms, BV is associated with increased risks of more serious conditions such as pelvic inflammatory disease (PID), postoperative infections, and pregnancyrelated complications including prematurity. It also increases the likelihood of acquiring HIV in women exposed to the virus.8,9
Two principal factors put women at risk for acquiring BV: douching and exposure to a new sexual partner, both of which are thought to disrupt the vaginal ecosystem.10
Relative benefits of diagnostic tests
A gold standard test has not been established for BV. In about 50% of asymptomatic women, culture results are positive for flora such as Gardnerella vaginalis.5 While Amsel’s criteria are often used as a reference and generally suffice for the evaluation of symptomatic women, the best candidate for a gold standard test is probably Gram stain assessment using Nugent’s criteria (described in this section).11 Lack of leukocytes in the vaginal fluid supports a diagnosis of BV. A finding of white blood cells in excess of the number of vaginal epithelial cells suggests an inflammatory process (SOR: C).12
Amsel’s criteria with wet mount. The diagnostic approach most commonly used in the office is Amsel’s criteria—homogenous discharge, positive whiff-amine test, pH >4.5, and clue cells found on wet-mount microscopy (see How to perform a wet mount ).13 Three of 4 criteria deemed positive is considered diagnostic. If Gram stain is used as the reference standard, then Amsel’s criteria have 70% sensitivity and 94% specificity for diagnosing BV.14 An analysis of the individual criteria follows. The positive and negative predictive values of each compared with the whole group as reference standard is displayed in Table 1 .
Homogenous discharge. A thin, homogenous, grayish discharge is traditionally associated with BV. However, it is not specific to BV, being found commonly also in women with culture results positive for VVC or no diagnosis of vaginitis.2,15 It is the criterion least likely to be consistent with the whole group, seen in about half of women BVpositive and over one third of women BV-negative using Amsel’s criteria as the reference standard. 15
To perform a wet-mount preparation correctly, dilute the vaginal discharge with 1 or 2 drops of 0.9% saline and place it on a slide. Examine the slide under lowand high-powered fields for vaginal squamous cells, white blood cells (WBCs), lactobacilli, clue cells, and trichomonads. An increased number of WBCs can be defined as >5–10 WBC/HPF or WBCs exceeding the number vaginal epithelial cells.
To prepare the potassium hydroxide (KOH) slide, place a generous amount of vaginal discharge on a slide with 10% KOH solution. Air- or flame-drying before examination under low-power microscopy may improve sensitivity. A positive KOH preparation will have hyphae, mycelial tangles, or spores.
Whiff test. The whiff test is performed by adding drops of 10% potassium hydroxide solution to the vaginal fluid. A positive result is a “fishy” amine odor. In a study16 of 100 women complaining of malodorous discharge, a positive whiff test was predictive of positive culture results for anaerobic flora such as Bacteroides sp. with sensitivity 67%, specificity 94%, and a positive predictive value of 95%. The whiff test was not positive in any of the 5 cases with positive culture results for G vaginalis in the absence of anaerobes. There were also 12 cases positive for anaerobes without G vaginalis.
pH >4.5. Since the abnormal flora of BV is consistently associated with a vaginal pH >4.5, a normal pH excludes a diagnosis of BV.17,18 The determination of pH in the narrow range around 4.5 is not accurate using standard nitrazine paper. Narrower-range test paper is available and more accurate. Examples include pH paper for 4.5 to 5.5 (Micro Essential Laboratory), FemExam pH and Amines Test Card (Litmus Concepts), pHem-ALERT: pH paper on a stick (Imagyn Gynecology). Cervical mucous, semen, and blood are alkaline and can interfere with pH testing. Estrogen production is also necessary to maintain an acidic environment. A pH of 3.8 to 4.5 is consistent with normal vaginal flora in premenopausal women with normal estrogen production.17
Clue cells. Clue cells are vaginal epithelial cells coated with coccobacilli giving an appearance as if coated with ground black pepper. Clue cells on wet mount preparation is considered the most accurate of Amsel’s diagnostic criteria for BV.19 On the other hand, office evaluation of the wet mount is considered by some authors to be unreliable due to dependence on the clinician’s microscopy skills and lack of a durable record of the patient sample.
Gram stain a more objective test. A Gram stain evaluation using Nugent’s criteria has been adopted as the gold standard test for research purposes, including studies of prematurity. The Gram-stained vaginal specimen is scored from 0 to 10 based on semi-quantitative assessment of 3 classes of morphotypes ( Table 2 ): large gram-positive rods (Lactobacilli), small gram-negative rods (Gardnerella and Bacteroides spp.), and small curved gram-variable rods (Mobiluncus spp.).11
Diagnosis of BV is typically made when the Nugent score is 7 or more, which appears qualitatively as dominant morphotypes other than Lactobacilli. Gram staining is more objective and reproducible compared with wet-mount examination, with a sensitivity of 93% and specificity of 70% if Amsel’s criteria are used as the gold standard.14 It is useful for the evaluation of asymptomatic women. It also provides a durable record of the patient specimen. Compared with Gram stain, Amsel’s criteria tend to underdiagnose cases. We can expect that if screening for BV in pregnancy becomes a recommendation, Gram staining in a clinical laboratory will be the recommended method of diagnosis.
Other diagnostic tests for BV. DNA testing for Gardnerella is accurate for detection, but it is not synonymous with a diagnosis of BV, as described.20 DNA testing is further described under “Differential Diagnosis.” Gram staining is more reliable than gas-liquid chromatography21 and an assay for proline aminopeptidase (a metabolic product of some of the bacteria associated with BV).22 Latex agglutination testing for vaginal lactoferrin is a nonspecific marker for leukocytes, and thus inflammation. It is of little clinical utility in the diagnosis of vaginal discharge.23
TABLE 1
Predictive values of Amsel’s criteria (using 3 of 4 positive as diagnostic reference standard)
| Diagnostic criterion | Predictive value (%) | |
|---|---|---|
| Positive | Negative | |
| Homogeneous thin discharge seen at introitus | 42 | 89 |
| pH >4.5 | 53 | 94 |
| Odor on alkalinization | 94 | 93 |
| Clue cells on wet mount | 90 | 99 |
| Source: Thomason et al 1990.15 | ||
TABLE 2
How to use Nugent’s Gram stain criteria to diagnose bacterial vaginosis
| Lactobacillus morphophytes | Gardnerella and Bacteroides spp. morphophytes | Curved gram-variable rods | Points |
|---|---|---|---|
| 4+ | 0 | 0 | 0 |
| 3+ | 1+ | 1+ or 2+ | 1 |
| 2+ | 2+ | 3+ or 4+ | 2 |
| 1+ | 3+ | 3 | |
| 0 | 4+ | 4 | |
| Review each of the first 3 columns in turn, assigning points at far right according to your exam findings. | |||
| Add the points for all 3 columns for a final sum. A score of 7 or higher indicates bacterial vaginosis. Source: Nugent et al 1991.11 | |||
Vulvovaginal Candidiasis
Candidiasis is the second most commonly diagnosed vaginitis in the United States. Some experts estimate that 75% of women will have a yeast infection at some point in life and 5% will have recurrent infections.24 However, 10% to 30% of asymptomatic women with normal flora have positive culture results for Candida.25-29 The proportion of symptomatic women with positive culture results is 20% to 40%.4,30,31 Complications of VVC are rare,32 though vulvar vestibulitis33 and chorioamnionitis in pregnancy32 have been reported.
Risk factors. Symptomatic yeast vaginitis has been associated with condom and diaphragm use, recent antibiotic use, receptive oral sex, oral contraceptive use, spermicide use, diabetes, and immunosuppression including AIDS.31,34-37 The associations with antibiotic use and oral contraceptives are not consistent.30,38 Although pregnancy has been postulated as a risk factor for symptomatic VVC, prevalence of yeast on culture in pregnant women is similar to that of nonpregnant women.30
Suggestive symptoms. Among women with a culture result positive for Candida, the most common symptom is pruritus or burning.28 Abnormal discharge is a complaint for most symptomatic women with VVC confirmed by culture.2 In addition, women may complain of a thick, odorless, cottage cheese–like discharge.39 A thick, curdled-appearing discharge points to a diagnosis of Candida because it is rarely present with BV or trichomoniasis. In one study,28 a thick curdled discharge had a positive predictive value of 84% for diagnosis of VVC by culture (SOR: B). However, a thin discharge does not rule out VVC; in another study, clinicians described discharge as thin in about half of women ultimately diagnosed with VVC by culture in another study (SOR: B).2 On exam, vulvar and vaginal erythema are often present but are not specific findings. The accuracy of the clinical exam for VVC is poor compared with culture (SOR: A).2,30
Pathogens. Candida albicans is present in 80% to 90% of patients with VVC.5,40 remainder have non-albicans species, including C glabrata and others.28 An increase to almost 20% of non-Candida species in a vaginitis clinic by the mid-1990’s may be related to increased use of imidazoles available over-the-counter.40,41 Wet mount results are typically negative in the presence of non-Candida VVC.28
Diagnosis of VVC
The gold standard test for diagnosis of VVC is culture. The potassium hydroxide (KOH) wet mount is only 40% to 75% sensitive.28,29,42,43 False-positive results are also observed with variable frequency.44 The pH of the discharge is usually not more than 5.0 with Candida albicans, but may be higher with non-albicans species such as C glabrata.45 Culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR: B).5,28,46 Rapid slide latex agglutination testing is not better than microscopy (SOR: B).42
Trichomoniasis
Trichomonas, a motile protozoan with 4 flagella, causes the third most common form of vaginitis in the United States and is more common in some developing countries. Trichomoniasis accounts for no more than 10% of all cases of vaginitis, and it appears to be decreasing since the introduction of metronidazole.47,48 It is classified as an STD, although transmission is possible by other means if the organism is protected from desiccation—for example, in dirty washcloths or towels and contaminated water. Nonsexual transmission is thought to be uncommon.
Trichomoniasis is associated with GC and Chlamydia infections, and, like them, has been associated with seroconversion to HIV-positive status.49 Trichomonads are identified in 30% to 80% of male sexual partners of infected women. In men, trichomoniasis most often is an asymptomatic carrier state.50 However, it is the cause of about 10% of cases of nongonococcal urethritis in men.51
Our knowledge of the epidemiology of abnormal vaginal discharge is limited. Studies of vaginitis may exclude patients with vaginal discharge due to cervicitis; studies performed in sexually transmitted disease clinics are not representative of primary care practice; women who do not complain of abnormal vaginal discharge may have positive cultures for Gardnerella vaginalis and Candida albicans; and self-treatment of presumed yeast vaginitis with antifungals available over-the-counter further limits our knowledge of the prevalence and causes of vaginal discharge.
Clinical presentations. Women with trichomoniasis have variable presentations ranging from an asymptomatic carrier state to a malodorous, purulent discharge with vulvovaginal erythema. Punctate hemorrhagic cervical lesions are considered pathognomonic of trichomoniasis, but are seen in only about 2% of cases (SOR: B).52
Diagnosis. Culture for trichomoniasis is the gold standard. Several culture media have been used, most commonly the Diamond medium. Recently introduced is a transport and culture medium for detection of Trichomonas (InPouch TV), which performs as well as Diamond medium (SOR: A).53-55 A DNA probe is also available and accurate (SOR: A).
Motile trichomonads are seen on wet preparation in only 50% to 80% of culture-positive cases (SOR: B).50,54,56 Polymorphonuclear leukocytes can be dominant on wet mount, making visualization of trichomonads more difficult. The pH of the vaginal fluid is usually basic.
Trichomonas reported with cervical cytology
Trichomonas may also be reported on Pap smears. A meta-analysis57 comparing the pooled sensitivities and specificities of wet mounts and cytology demonstrated low sensitivities of 68% and 58%, respectively, and high specificities, 99.9% and 97%, respectively (SOR: A).
However, since cytology carries a 3% false-positive rate, its results are not diagnostic of trichomoniasis in low-risk, asymptomatic women.50,57 Treatment may be prescribed empirically based on positive cytology results. However, if an asymptomatic woman were concerned about whether she really has an STD, a positive wet prep would confirm the diagnosis. A negative wet prep should be followed up with culture to reliably rule out disease (SOR: B).
Trichomoniasis in pregnancy
Screening for asymptomatic trichomoniasis in pregnancy has not been recommended. In fact, some evidence suggests that treatment of trichomoniasis in pregnancy is associated with poorer pregnancy outcomes including lower birth weight and more prematurity (SOR: B).58,59
Aerobic vaginitis
Aerobic vaginitis is a term proposed to describe purulent vaginal discharge with predominance of abnormal aerobic flora.60 Aerobic vaginitis, which may be severe, has been reported as the cause of 5% of cases in a series from a specialty vaginitis clinic.61 The usual predominant microorganisms are group B streptococci, Escherichia coli, and Staphylococcus aureus. It is likely that less severe cases of aerobic vaginitis are not recognized in the primary care setting and are treated as BV or resolve spontaneously (SOR: C). The case series referred to above also reported good therapeutic response to 2% topical clindamycin (SOR: C).61
Noninfectious Vaginitis
Noninfectious causes of vaginal discharge include physiologic, irritant and allergic, cytolytic vaginitis, desquamative inflammatory vaginitis, collagen vascular disease, and idiopathic vaginitis.
Irritant and allergic vaginitis may result from sensitivities to topical medications, the active or base ingredients of spermicidal products, douching solutions, and the latex of condoms or diaphragms. If a woman with persistent symptoms has been using such intravaginal products, she should stop (SOR: C).
Cytolytic vaginitis is characterized by overgrowth of lactobacilli and cytolysis of squamous cells, including presence of cytoplasmic fragments and intact cells with naked nuclei.62 The cause is uncertain but may include a reaction to intravaginal medications or other products such as tampons. It can be found in up to 5% of women with symptoms and signs of vaginitis.62,63 Symptoms often mimic VVC and may include a white, cheesy discharge. Vaginal pH ranges from 3.5 to 5.5. Recurrences during luteal phase of the menstrual cycle have been described.64 Intravaginal antifungals should be discontinued. Baking soda sitz baths or douches are often used, but clinical trial data to support this practice are lacking (SOR: C).
Noninfectious desquamative inflammatory vaginitis (DIV) has also been described.65 DIV is an uncommon vaginitis characterized by profuse purulent discharge with epithelial cell exfoliation. It may occur at any time during the reproductive years or after menopause. There is probably a heterogeneous group of causes of DIV. Some cases may correspond to a disorder within the spectrum of lichen planus.66 Treatment is usually difficult, though there may be some response to local or systemic corticosteroid therapy (SOR: C).65
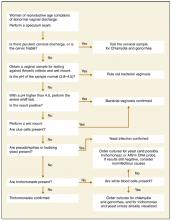
Differential diagnosis
A comparison of physical examination findings an diagnostic test results for various etiologies of vaginitis is summarized in Table 3 . An algorithmic approach to the differential diagnosis of abnormal vaginal discharge is presented in the Figure . Diagnosis is complicated in that signs and symptoms do little to help differentiate among BV, VVC, and trichomoniasis. A study2 of 22 genitourinary symptoms and signs showed that none differentiated among the 3 infections. This lack of clear-cut differences in symptoms also makes self-diagnosis and telephone triage inaccurate.67,68
A DNA probe testing system (Affirm VP III Microbial ID Test) for differential diagnosis is available but expensive. It identifies Gardnerella, Trichomonas, and Candida albicans with a sensitivity of 90% to 95%.54,66 The analyzer costs approximately $10,000 and would typically be purchased by a laboratory. Individual test kits cost about $27.
TABLE 3
Comparative findings among causes of vaginitis
| Cause | Physical exam findings* | Gold standard test | pH | Leukocytes | Wet mount | Alternative test |
|---|---|---|---|---|---|---|
| Bacterial vaginosis | Variable | Gram stain | >4.5 | No | Clue cells | Amsel’s criteria |
| Aerobic vaginitis | Abundant purulent discharge | Culture | >4.5 | Yes | Cocci or coarse rods | |
| Candida vaginitis | Adherent white disch. (thrush) | Culture | 3.8–4.5 | ± | Pseudohyphae or budding yeast | DNA testing |
| Non-Candida yeast vaginitis | Variable | Culture | Any | ± | Usually negative | |
| Trichomoniasis | Variable, occ. strawberry spots on cervix | Culture | >4.5 | ± | Motile trichomonads | DNA testing |
| Cytolytic vaginitis | Profuse discharge, often cheesy | Cytology and negative culture | 3.5–5.5 | ± | Overgrowth of lactobacilli and squamous cell fragments | |
| Desquamative inflammatory vaginitis | Abundant purulent discharge | Parabasal epithelial cells and negative culture | >4.5 | Yes | ||
| Irritant and allergic vaginitis | Variable, often erythema | None | Any | ± | ||
| * Helpful when present. | ||||||
FIGURE
Sequence of office tests to evaluate abnormal vaginal discharge
Corresponding author
Linda French, MD, Associate Professor, Department of Family Practice, College of Human Medicine, Michigan State University, B101 Clinical Center, East Lansing, MI 48824. E-mail: [email protected].
1. National Center for Health Statistics. National Ambulatory Medicine Care Survey. Available at: www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm.
2. Schaaf VM, Perez-Stable EJ, Borchardt K. The limited value of symptoms and signs in the diagnosis of vaginal infections. Arch Intern Med 1990;150:1929-1933.
3. Wiesenfeld HC, Macio I. The infrequent use of officebased diagnostic tests for vaginitis. Am J Obstet Gynecol 1999;181:39-41.
4. Berg AO, Heidrich FE, Fihn SD, et al. Establishing the cause of genitourinary symptoms in women in a family practice. Comparison of clinical examination and comprehensive microbiology. JAMA 1984;251:620-625.
5. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
6. Sellors JW, Walter SD, Howard M. A new visual indicator of chlamydial cervicitis? Sex Transm Infect 2000;76:46-48.
7. Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis 2003;187:650-657.
8. Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180:1863-1868.
9. Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses 1998;14Suppl 1:S17-21.
10. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogenperoxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. JClin Microbiol 1991;29:297-301.
12. Quan M. Vaginitis: meeting the clinical challenge. Clin Cornerstone 2000;3:36-47.
13. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14-22.
14. Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. ObstetGynecol 1996;88:573-576.
15. Thomason JL, Gelbart SM, Anderson RJ, Walt AK, Osypowski PJ, Broekhuizen FF. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am J Obstet Gynecol. 1990;162:155-160.
16. Erkkola R, Jarvinen H, Terho P, Meurman O. Microbial flora in women showing symptoms of nonspecific vaginosis: applicability of KOH test for diagnosis. Scand J Infect Dis Suppl. 1983;40:59-63.
17. Caillouette JC, Sharp CF, Jr, Zimmerman GJ, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. 1997;176:1270-1275discussion1275-1277.
18. Carr PL, Felsenstein D, Friedman RH. Evaluation and management of vaginitis. J Gen Intern Med 1998;13:335-346.
19. Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988;158:819-828.
20. Sheiness D, Dix K, Watanabe S, Hillier SL. Highlevels of Gardnerella vaginalis detected with an oligonucleotide probe combined with elevated pH as a diagnostic indicator of bacterial vaginosis. J Clin Microbiol 1992;30:642-648.
21. Thomason JL, Gelbart SM, James JA, Edwards JM, Hamilton PR. Is analysis of vaginal secretions for volatile organic acids to detect bacterial vaginosis of any diagnostic value? Am J Obstet Gynecol. 1988;159:1509-1511.
22. Thomason JL, Gelbart SM, Wilcoski LM, Peterson AK, Jilly BJ, Hamilton PR. Proline aminopeptidase activity as a rapid diagnostic test to confirm bacterial vaginosis. Obstet Gynecol 1988;71:607-611.
23. Rein MF, Shih LM, Miller JR, Guerrant RL. Use of a lactoferrin assay in the differential diagnosis of female genital tract infections and implications for the pathophysiology of bacterial vaginosis. Sex Transm Dis 1996;23:517-521.
24. Monif GR. Classification and pathogenesis of vulvovaginal candidiasis. Am J Obstet Gynecol 1985;152:935-939.
25. Giraldo P, von Nowaskonski A, Gomes FA, Linhares I, Neves NA, Witkin SS. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstet Gynecol 2000;95:413-416.
26. Bergman JJ, Berg AO. How useful are symptoms in the diagnosis of Candida vaginitis? J Fam Pract 1983;16:509-511.
27. Bro F. Patients with vaginal discharge in general practice. Acta Obstet Gynecol Scand 1989;68:41-43.
28. Eckert LO, Hawes SE, Stevens CE, Koutsky LA, Eschenbach DA, Holmes KK. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol 1998;92:757-765.
29. Bertholf ME, Stafford MJ. Colonization of Candida albicans in vagina, rectum, and mouth. J Fam Pract 1983;16:919-924.
30. Reed BD, Huck W, Zazove P. Differentiation of Gardnerella vaginalis, Candida albicans, and Trichomonas vaginalis infections of the vagina. J Fam Pract 1989;28:673-680.
31. Bro F. The diagnosis of candida vaginitis in general practice. Scand J Prim Health Care 1989;7:19-22.
32. Cotch MF, Hillier SL, Gibbs RS, Eschenbach DA. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 1998;178:374-380.
33. Pagano R. Vulvar vestibulitis syndrome: an often unrecognized cause of dyspareunia. Aust N Z J Obstet Gynaecol 1999;39:79-83.
34. Foxman B. The epidemiology of vulvovaginal candidiasis: risk factors. Am J Public Health 1990;80:329-331.
35. Geiger AM, Foxman B. Risk factors for vulvovaginal candidiasis: a case-control study among university students. Epidemiology 1996;7:182-187.
36. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
37. Spinillo A, Capuzzo E, Acciano S, De Santolo A, Zara F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am J Obstet Gynecol 1999;180:14-17.
38. Davidson F, Oates JK. The pill does not cause ‘thrush’. Br J Obstet Gynaecol Dec 1985;92:1265-1266.
39. Abbott J. Clinical and microscopic diagnosis of vaginal yeast infection: a prospective analysis. Ann Emerg Med 1995;25:587-591.
40. Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J Clin Pharmacol 1992;32:248-255.
41. Spinillo A, Capuzzo E, Gulminetti R, Marone P, Colonna L, Piazzi G. Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am J Obstet Gynecol 1997;176:138-141.
42. Reed BD, Pierson CL. Evaluation of a latex agglutination test for the identification of Candida species in vaginal discharge. J Am Board Fam Pract 1992;5:375-380.
43. Ferris DG, Hendrich J, Payne PM, et al. Office laboratoAE_French.1004.final 9/20/04 3:18 PM Page 813 ry diagnosis of vaginitis. Clinician-performed tests compared with a rapid nucleic acid hybridization test. J Fam Pract 1995;41:575-581.
44. Bergman JJ, Berg AO, Schneeweiss R, Heidrich FE. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J Fam Pract 1984;18:549-552.
45. Sobel JD. Vulvovaginitis due to Candida glabrata. An emerging problem. Mycoses. 1998;41:Suppl 2:18-22.
46. Zdolsek B, Hellberg D, Froman G, Nilsson S, Mardh PA. Culture and wet smear microscopy in the diagnosis of low-symptomatic vulvovaginal candidosis. Eur J Obstet Gynecol Reprod Biol 1995;58:47-51.
47. Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991;165:1217-1222.
48. Kent HL. Epidemiology of vaginitis. Am J Obstet Gynecol 1991;165:1168-1176.
49. Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 1993;7:95-102.
50. Krieger JN, Tam MR, Stevens CE, et al. Diagnosis of trichomoniasis. Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimens. JAMA 1988;259:1223-1227.
51. Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995;22:83-96.
52. Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis 1980;141:137-143.
53. Ohlemeyer CL, Hornberger LL, Lynch DA, Swierkosz EM. Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy. J Adolesc Health 1998;22:205-208.
54. Borchardt KA, Smith RF. An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Med 1991;67:149-152.
55. Levi MH, Torres J, Pina C, Klein RS. Comparison of the InPouch TV culture system and Diamond’s modified medium for detection of Trichomonas vaginalis. J Clin Microbiol 1997;35:3308-3310.
56. DeMeo LR, Draper DL, McGregor JA, et al. Evaluation of a deoxyribonucleic acid probe for the detection of Trichomonas vaginalis in vaginal secretions. Am J Obstet Gynecol 1996;174:1339-1342.
57. Wiese W, Patel SR, Patel SC, Ohl CA, Estrada CA. A meta-analysis of the Papanicolaou smear and wet mount for the diagnosis of vaginal trichomoniasis. Am J Med 2000;108:301-308.
58. Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001;345:487-493.
59. Kigozi GG, Brahmbhatt H, Wabwire-Mangen F, et al. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2003;189:1398-1400.
60. Donder GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Bjog 2002;109:34-43.
61. Sobel JD. Desquamative inflammatory vaginitis: a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol 1994;171:1215-1220.
62. Demirezen S. Cytolytic vaginosis: examination of 2947 vaginal smears. Cent Eur J Public Health 2003;11:23-24.
63. Wathne B, Holst E, Hovelius B, Mardh PA. Vaginal discharge—comparison of clinical, laboratory and microbiological findings. Acta Obstet Gynecol Scand 1994;73:802-808.
64. Secor RM. Cytolytic vaginosis: a common cause of cyclic vulvovaginitis. Nurse Pract Forum 1992;3:145-148.
65. Oates JK, Rowen D. Desquamative inflammatory vaginitis. A review. Genitourin Med 1990;66:275-279.
66. Pelisse M. The vulvo-vaginal-gingival syndrome. A new form of erosive lichen planus. Int J Dermatol 1989;28:381-384.
67. Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol 2002;99:419-425.
68. Allen-Davis JT, Beck A, Parker R, Ellis JL, Polley D. Assessment of vulvovaginal complaints: accuracy of telephone triage and in-office diagnosis. Obstet Gynecol 2002;99:18-22.
- Accurate differential diagnosis for women complaining of abnormal vaginal discharge requires in-office diagnostic testing at minimum, and laboratory testing in selected cases.
- Test for Chlamydia trachomatis and Neisseria gonorrhea when signs of purulent cervicitis are present (SOR: B).
- In suspected vulvovaginal candidiasis, culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR:B); rapid slide latex agglutination testing is not better than microscopy for diagnosing VVC (SOR: B).
In primary care practice, abnormal vaginal discharge is a common complaint. Signs and symptoms of vaginitis—the most common gynecologic diagnosis in primary care1 —are not specific for any single underlying cause.2 Officebased diagnostic testing, which is underused,3 must be employed to ensure accurate diagnosis and effective treatment. (An article on treatment by the same authors will appear in next month’s issue of The journal of family practice.)
In a primary-care study,4 vulvovaginal symptoms including vaginal discharge were due to vulvovaginal candidiasis (VVC) in 27% of patients, bacterial vaginosis (BV) in 21%, trichomoniasis in 8%, Chlamydia trachomatis in 2%, Neisseria gonorrhea (GC) in 1%, and no infection in 34%. Several pathogens may coexist.2 VVC, BV, and trichomoniasis account for at least 90% of infectious vaginitis.5 This review will therefore focus heavily on these causes of vaginal discharge among women of reproductive age, including pregnant women.
Cervicitis and physiologic cervical discharge
Some women may interpret a physiologic increase in cervical mucous production as abnormal. It occurs cyclically prior to ovulation, is typically transparent and colorless, and may be more pronounced in women with an everted cervix.
Chlamydial infection
In the clinical examination of the cervix, 3 characteristics have been associated with chlamydial infection: yellow endocervical discharge, easily induced cervical bleeding, and opaque cervical discharge.6 All 3 findings are statistically significant and independently associated with chlamydial infection (odds ratios 2.8, 2.3, and 2.9, respectively). In the primary care study cited above, purulent cervical discharge was found in 6% of women, most commonly testing positive for Chlamydia, less often for GC.4
Trichomonas vaginalis may cause cervicitis as well as vaginitis. Mycoplasma genitalium has been proposed as an additional possible pathogen. It was identified in 7% of more than 700 women with mucopurulent cervical discharge seen in a STD clinic with otherwise negative cultures.7 With cervical discharge that appears to be purulent, testing is warranted as a minimum for Chlamydia and GC (SOR: B). Screening of asymptomatic women less than 26 years of age for Chlamydia is recommended by the US Preventive Services Task Force (SOR: A).
Bacterial Vaginosis
Bacterial vaginosis (BV) is neither an inflammatory condition nor an STD, but is a shift in vaginal flora from the normal condition in which lactobacilli predominate, to a polymicrobial flora in which gram-positive anaerobes predominate. In addition to annoying vaginal symptoms, BV is associated with increased risks of more serious conditions such as pelvic inflammatory disease (PID), postoperative infections, and pregnancyrelated complications including prematurity. It also increases the likelihood of acquiring HIV in women exposed to the virus.8,9
Two principal factors put women at risk for acquiring BV: douching and exposure to a new sexual partner, both of which are thought to disrupt the vaginal ecosystem.10
Relative benefits of diagnostic tests
A gold standard test has not been established for BV. In about 50% of asymptomatic women, culture results are positive for flora such as Gardnerella vaginalis.5 While Amsel’s criteria are often used as a reference and generally suffice for the evaluation of symptomatic women, the best candidate for a gold standard test is probably Gram stain assessment using Nugent’s criteria (described in this section).11 Lack of leukocytes in the vaginal fluid supports a diagnosis of BV. A finding of white blood cells in excess of the number of vaginal epithelial cells suggests an inflammatory process (SOR: C).12
Amsel’s criteria with wet mount. The diagnostic approach most commonly used in the office is Amsel’s criteria—homogenous discharge, positive whiff-amine test, pH >4.5, and clue cells found on wet-mount microscopy (see How to perform a wet mount ).13 Three of 4 criteria deemed positive is considered diagnostic. If Gram stain is used as the reference standard, then Amsel’s criteria have 70% sensitivity and 94% specificity for diagnosing BV.14 An analysis of the individual criteria follows. The positive and negative predictive values of each compared with the whole group as reference standard is displayed in Table 1 .
Homogenous discharge. A thin, homogenous, grayish discharge is traditionally associated with BV. However, it is not specific to BV, being found commonly also in women with culture results positive for VVC or no diagnosis of vaginitis.2,15 It is the criterion least likely to be consistent with the whole group, seen in about half of women BVpositive and over one third of women BV-negative using Amsel’s criteria as the reference standard. 15
To perform a wet-mount preparation correctly, dilute the vaginal discharge with 1 or 2 drops of 0.9% saline and place it on a slide. Examine the slide under lowand high-powered fields for vaginal squamous cells, white blood cells (WBCs), lactobacilli, clue cells, and trichomonads. An increased number of WBCs can be defined as >5–10 WBC/HPF or WBCs exceeding the number vaginal epithelial cells.
To prepare the potassium hydroxide (KOH) slide, place a generous amount of vaginal discharge on a slide with 10% KOH solution. Air- or flame-drying before examination under low-power microscopy may improve sensitivity. A positive KOH preparation will have hyphae, mycelial tangles, or spores.
Whiff test. The whiff test is performed by adding drops of 10% potassium hydroxide solution to the vaginal fluid. A positive result is a “fishy” amine odor. In a study16 of 100 women complaining of malodorous discharge, a positive whiff test was predictive of positive culture results for anaerobic flora such as Bacteroides sp. with sensitivity 67%, specificity 94%, and a positive predictive value of 95%. The whiff test was not positive in any of the 5 cases with positive culture results for G vaginalis in the absence of anaerobes. There were also 12 cases positive for anaerobes without G vaginalis.
pH >4.5. Since the abnormal flora of BV is consistently associated with a vaginal pH >4.5, a normal pH excludes a diagnosis of BV.17,18 The determination of pH in the narrow range around 4.5 is not accurate using standard nitrazine paper. Narrower-range test paper is available and more accurate. Examples include pH paper for 4.5 to 5.5 (Micro Essential Laboratory), FemExam pH and Amines Test Card (Litmus Concepts), pHem-ALERT: pH paper on a stick (Imagyn Gynecology). Cervical mucous, semen, and blood are alkaline and can interfere with pH testing. Estrogen production is also necessary to maintain an acidic environment. A pH of 3.8 to 4.5 is consistent with normal vaginal flora in premenopausal women with normal estrogen production.17
Clue cells. Clue cells are vaginal epithelial cells coated with coccobacilli giving an appearance as if coated with ground black pepper. Clue cells on wet mount preparation is considered the most accurate of Amsel’s diagnostic criteria for BV.19 On the other hand, office evaluation of the wet mount is considered by some authors to be unreliable due to dependence on the clinician’s microscopy skills and lack of a durable record of the patient sample.
Gram stain a more objective test. A Gram stain evaluation using Nugent’s criteria has been adopted as the gold standard test for research purposes, including studies of prematurity. The Gram-stained vaginal specimen is scored from 0 to 10 based on semi-quantitative assessment of 3 classes of morphotypes ( Table 2 ): large gram-positive rods (Lactobacilli), small gram-negative rods (Gardnerella and Bacteroides spp.), and small curved gram-variable rods (Mobiluncus spp.).11
Diagnosis of BV is typically made when the Nugent score is 7 or more, which appears qualitatively as dominant morphotypes other than Lactobacilli. Gram staining is more objective and reproducible compared with wet-mount examination, with a sensitivity of 93% and specificity of 70% if Amsel’s criteria are used as the gold standard.14 It is useful for the evaluation of asymptomatic women. It also provides a durable record of the patient specimen. Compared with Gram stain, Amsel’s criteria tend to underdiagnose cases. We can expect that if screening for BV in pregnancy becomes a recommendation, Gram staining in a clinical laboratory will be the recommended method of diagnosis.
Other diagnostic tests for BV. DNA testing for Gardnerella is accurate for detection, but it is not synonymous with a diagnosis of BV, as described.20 DNA testing is further described under “Differential Diagnosis.” Gram staining is more reliable than gas-liquid chromatography21 and an assay for proline aminopeptidase (a metabolic product of some of the bacteria associated with BV).22 Latex agglutination testing for vaginal lactoferrin is a nonspecific marker for leukocytes, and thus inflammation. It is of little clinical utility in the diagnosis of vaginal discharge.23
TABLE 1
Predictive values of Amsel’s criteria (using 3 of 4 positive as diagnostic reference standard)
| Diagnostic criterion | Predictive value (%) | |
|---|---|---|
| Positive | Negative | |
| Homogeneous thin discharge seen at introitus | 42 | 89 |
| pH >4.5 | 53 | 94 |
| Odor on alkalinization | 94 | 93 |
| Clue cells on wet mount | 90 | 99 |
| Source: Thomason et al 1990.15 | ||
TABLE 2
How to use Nugent’s Gram stain criteria to diagnose bacterial vaginosis
| Lactobacillus morphophytes | Gardnerella and Bacteroides spp. morphophytes | Curved gram-variable rods | Points |
|---|---|---|---|
| 4+ | 0 | 0 | 0 |
| 3+ | 1+ | 1+ or 2+ | 1 |
| 2+ | 2+ | 3+ or 4+ | 2 |
| 1+ | 3+ | 3 | |
| 0 | 4+ | 4 | |
| Review each of the first 3 columns in turn, assigning points at far right according to your exam findings. | |||
| Add the points for all 3 columns for a final sum. A score of 7 or higher indicates bacterial vaginosis. Source: Nugent et al 1991.11 | |||
Vulvovaginal Candidiasis
Candidiasis is the second most commonly diagnosed vaginitis in the United States. Some experts estimate that 75% of women will have a yeast infection at some point in life and 5% will have recurrent infections.24 However, 10% to 30% of asymptomatic women with normal flora have positive culture results for Candida.25-29 The proportion of symptomatic women with positive culture results is 20% to 40%.4,30,31 Complications of VVC are rare,32 though vulvar vestibulitis33 and chorioamnionitis in pregnancy32 have been reported.
Risk factors. Symptomatic yeast vaginitis has been associated with condom and diaphragm use, recent antibiotic use, receptive oral sex, oral contraceptive use, spermicide use, diabetes, and immunosuppression including AIDS.31,34-37 The associations with antibiotic use and oral contraceptives are not consistent.30,38 Although pregnancy has been postulated as a risk factor for symptomatic VVC, prevalence of yeast on culture in pregnant women is similar to that of nonpregnant women.30
Suggestive symptoms. Among women with a culture result positive for Candida, the most common symptom is pruritus or burning.28 Abnormal discharge is a complaint for most symptomatic women with VVC confirmed by culture.2 In addition, women may complain of a thick, odorless, cottage cheese–like discharge.39 A thick, curdled-appearing discharge points to a diagnosis of Candida because it is rarely present with BV or trichomoniasis. In one study,28 a thick curdled discharge had a positive predictive value of 84% for diagnosis of VVC by culture (SOR: B). However, a thin discharge does not rule out VVC; in another study, clinicians described discharge as thin in about half of women ultimately diagnosed with VVC by culture in another study (SOR: B).2 On exam, vulvar and vaginal erythema are often present but are not specific findings. The accuracy of the clinical exam for VVC is poor compared with culture (SOR: A).2,30
Pathogens. Candida albicans is present in 80% to 90% of patients with VVC.5,40 remainder have non-albicans species, including C glabrata and others.28 An increase to almost 20% of non-Candida species in a vaginitis clinic by the mid-1990’s may be related to increased use of imidazoles available over-the-counter.40,41 Wet mount results are typically negative in the presence of non-Candida VVC.28
Diagnosis of VVC
The gold standard test for diagnosis of VVC is culture. The potassium hydroxide (KOH) wet mount is only 40% to 75% sensitive.28,29,42,43 False-positive results are also observed with variable frequency.44 The pH of the discharge is usually not more than 5.0 with Candida albicans, but may be higher with non-albicans species such as C glabrata.45 Culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR: B).5,28,46 Rapid slide latex agglutination testing is not better than microscopy (SOR: B).42
Trichomoniasis
Trichomonas, a motile protozoan with 4 flagella, causes the third most common form of vaginitis in the United States and is more common in some developing countries. Trichomoniasis accounts for no more than 10% of all cases of vaginitis, and it appears to be decreasing since the introduction of metronidazole.47,48 It is classified as an STD, although transmission is possible by other means if the organism is protected from desiccation—for example, in dirty washcloths or towels and contaminated water. Nonsexual transmission is thought to be uncommon.
Trichomoniasis is associated with GC and Chlamydia infections, and, like them, has been associated with seroconversion to HIV-positive status.49 Trichomonads are identified in 30% to 80% of male sexual partners of infected women. In men, trichomoniasis most often is an asymptomatic carrier state.50 However, it is the cause of about 10% of cases of nongonococcal urethritis in men.51
Our knowledge of the epidemiology of abnormal vaginal discharge is limited. Studies of vaginitis may exclude patients with vaginal discharge due to cervicitis; studies performed in sexually transmitted disease clinics are not representative of primary care practice; women who do not complain of abnormal vaginal discharge may have positive cultures for Gardnerella vaginalis and Candida albicans; and self-treatment of presumed yeast vaginitis with antifungals available over-the-counter further limits our knowledge of the prevalence and causes of vaginal discharge.
Clinical presentations. Women with trichomoniasis have variable presentations ranging from an asymptomatic carrier state to a malodorous, purulent discharge with vulvovaginal erythema. Punctate hemorrhagic cervical lesions are considered pathognomonic of trichomoniasis, but are seen in only about 2% of cases (SOR: B).52
Diagnosis. Culture for trichomoniasis is the gold standard. Several culture media have been used, most commonly the Diamond medium. Recently introduced is a transport and culture medium for detection of Trichomonas (InPouch TV), which performs as well as Diamond medium (SOR: A).53-55 A DNA probe is also available and accurate (SOR: A).
Motile trichomonads are seen on wet preparation in only 50% to 80% of culture-positive cases (SOR: B).50,54,56 Polymorphonuclear leukocytes can be dominant on wet mount, making visualization of trichomonads more difficult. The pH of the vaginal fluid is usually basic.
Trichomonas reported with cervical cytology
Trichomonas may also be reported on Pap smears. A meta-analysis57 comparing the pooled sensitivities and specificities of wet mounts and cytology demonstrated low sensitivities of 68% and 58%, respectively, and high specificities, 99.9% and 97%, respectively (SOR: A).
However, since cytology carries a 3% false-positive rate, its results are not diagnostic of trichomoniasis in low-risk, asymptomatic women.50,57 Treatment may be prescribed empirically based on positive cytology results. However, if an asymptomatic woman were concerned about whether she really has an STD, a positive wet prep would confirm the diagnosis. A negative wet prep should be followed up with culture to reliably rule out disease (SOR: B).
Trichomoniasis in pregnancy
Screening for asymptomatic trichomoniasis in pregnancy has not been recommended. In fact, some evidence suggests that treatment of trichomoniasis in pregnancy is associated with poorer pregnancy outcomes including lower birth weight and more prematurity (SOR: B).58,59
Aerobic vaginitis
Aerobic vaginitis is a term proposed to describe purulent vaginal discharge with predominance of abnormal aerobic flora.60 Aerobic vaginitis, which may be severe, has been reported as the cause of 5% of cases in a series from a specialty vaginitis clinic.61 The usual predominant microorganisms are group B streptococci, Escherichia coli, and Staphylococcus aureus. It is likely that less severe cases of aerobic vaginitis are not recognized in the primary care setting and are treated as BV or resolve spontaneously (SOR: C). The case series referred to above also reported good therapeutic response to 2% topical clindamycin (SOR: C).61
Noninfectious Vaginitis
Noninfectious causes of vaginal discharge include physiologic, irritant and allergic, cytolytic vaginitis, desquamative inflammatory vaginitis, collagen vascular disease, and idiopathic vaginitis.
Irritant and allergic vaginitis may result from sensitivities to topical medications, the active or base ingredients of spermicidal products, douching solutions, and the latex of condoms or diaphragms. If a woman with persistent symptoms has been using such intravaginal products, she should stop (SOR: C).
Cytolytic vaginitis is characterized by overgrowth of lactobacilli and cytolysis of squamous cells, including presence of cytoplasmic fragments and intact cells with naked nuclei.62 The cause is uncertain but may include a reaction to intravaginal medications or other products such as tampons. It can be found in up to 5% of women with symptoms and signs of vaginitis.62,63 Symptoms often mimic VVC and may include a white, cheesy discharge. Vaginal pH ranges from 3.5 to 5.5. Recurrences during luteal phase of the menstrual cycle have been described.64 Intravaginal antifungals should be discontinued. Baking soda sitz baths or douches are often used, but clinical trial data to support this practice are lacking (SOR: C).
Noninfectious desquamative inflammatory vaginitis (DIV) has also been described.65 DIV is an uncommon vaginitis characterized by profuse purulent discharge with epithelial cell exfoliation. It may occur at any time during the reproductive years or after menopause. There is probably a heterogeneous group of causes of DIV. Some cases may correspond to a disorder within the spectrum of lichen planus.66 Treatment is usually difficult, though there may be some response to local or systemic corticosteroid therapy (SOR: C).65
Differential diagnosis
A comparison of physical examination findings an diagnostic test results for various etiologies of vaginitis is summarized in Table 3 . An algorithmic approach to the differential diagnosis of abnormal vaginal discharge is presented in the Figure . Diagnosis is complicated in that signs and symptoms do little to help differentiate among BV, VVC, and trichomoniasis. A study2 of 22 genitourinary symptoms and signs showed that none differentiated among the 3 infections. This lack of clear-cut differences in symptoms also makes self-diagnosis and telephone triage inaccurate.67,68
A DNA probe testing system (Affirm VP III Microbial ID Test) for differential diagnosis is available but expensive. It identifies Gardnerella, Trichomonas, and Candida albicans with a sensitivity of 90% to 95%.54,66 The analyzer costs approximately $10,000 and would typically be purchased by a laboratory. Individual test kits cost about $27.
TABLE 3
Comparative findings among causes of vaginitis
| Cause | Physical exam findings* | Gold standard test | pH | Leukocytes | Wet mount | Alternative test |
|---|---|---|---|---|---|---|
| Bacterial vaginosis | Variable | Gram stain | >4.5 | No | Clue cells | Amsel’s criteria |
| Aerobic vaginitis | Abundant purulent discharge | Culture | >4.5 | Yes | Cocci or coarse rods | |
| Candida vaginitis | Adherent white disch. (thrush) | Culture | 3.8–4.5 | ± | Pseudohyphae or budding yeast | DNA testing |
| Non-Candida yeast vaginitis | Variable | Culture | Any | ± | Usually negative | |
| Trichomoniasis | Variable, occ. strawberry spots on cervix | Culture | >4.5 | ± | Motile trichomonads | DNA testing |
| Cytolytic vaginitis | Profuse discharge, often cheesy | Cytology and negative culture | 3.5–5.5 | ± | Overgrowth of lactobacilli and squamous cell fragments | |
| Desquamative inflammatory vaginitis | Abundant purulent discharge | Parabasal epithelial cells and negative culture | >4.5 | Yes | ||
| Irritant and allergic vaginitis | Variable, often erythema | None | Any | ± | ||
| * Helpful when present. | ||||||
FIGURE
Sequence of office tests to evaluate abnormal vaginal discharge
Corresponding author
Linda French, MD, Associate Professor, Department of Family Practice, College of Human Medicine, Michigan State University, B101 Clinical Center, East Lansing, MI 48824. E-mail: [email protected].
- Accurate differential diagnosis for women complaining of abnormal vaginal discharge requires in-office diagnostic testing at minimum, and laboratory testing in selected cases.
- Test for Chlamydia trachomatis and Neisseria gonorrhea when signs of purulent cervicitis are present (SOR: B).
- In suspected vulvovaginal candidiasis, culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR:B); rapid slide latex agglutination testing is not better than microscopy for diagnosing VVC (SOR: B).
In primary care practice, abnormal vaginal discharge is a common complaint. Signs and symptoms of vaginitis—the most common gynecologic diagnosis in primary care1 —are not specific for any single underlying cause.2 Officebased diagnostic testing, which is underused,3 must be employed to ensure accurate diagnosis and effective treatment. (An article on treatment by the same authors will appear in next month’s issue of The journal of family practice.)
In a primary-care study,4 vulvovaginal symptoms including vaginal discharge were due to vulvovaginal candidiasis (VVC) in 27% of patients, bacterial vaginosis (BV) in 21%, trichomoniasis in 8%, Chlamydia trachomatis in 2%, Neisseria gonorrhea (GC) in 1%, and no infection in 34%. Several pathogens may coexist.2 VVC, BV, and trichomoniasis account for at least 90% of infectious vaginitis.5 This review will therefore focus heavily on these causes of vaginal discharge among women of reproductive age, including pregnant women.
Cervicitis and physiologic cervical discharge
Some women may interpret a physiologic increase in cervical mucous production as abnormal. It occurs cyclically prior to ovulation, is typically transparent and colorless, and may be more pronounced in women with an everted cervix.
Chlamydial infection
In the clinical examination of the cervix, 3 characteristics have been associated with chlamydial infection: yellow endocervical discharge, easily induced cervical bleeding, and opaque cervical discharge.6 All 3 findings are statistically significant and independently associated with chlamydial infection (odds ratios 2.8, 2.3, and 2.9, respectively). In the primary care study cited above, purulent cervical discharge was found in 6% of women, most commonly testing positive for Chlamydia, less often for GC.4
Trichomonas vaginalis may cause cervicitis as well as vaginitis. Mycoplasma genitalium has been proposed as an additional possible pathogen. It was identified in 7% of more than 700 women with mucopurulent cervical discharge seen in a STD clinic with otherwise negative cultures.7 With cervical discharge that appears to be purulent, testing is warranted as a minimum for Chlamydia and GC (SOR: B). Screening of asymptomatic women less than 26 years of age for Chlamydia is recommended by the US Preventive Services Task Force (SOR: A).
Bacterial Vaginosis
Bacterial vaginosis (BV) is neither an inflammatory condition nor an STD, but is a shift in vaginal flora from the normal condition in which lactobacilli predominate, to a polymicrobial flora in which gram-positive anaerobes predominate. In addition to annoying vaginal symptoms, BV is associated with increased risks of more serious conditions such as pelvic inflammatory disease (PID), postoperative infections, and pregnancyrelated complications including prematurity. It also increases the likelihood of acquiring HIV in women exposed to the virus.8,9
Two principal factors put women at risk for acquiring BV: douching and exposure to a new sexual partner, both of which are thought to disrupt the vaginal ecosystem.10
Relative benefits of diagnostic tests
A gold standard test has not been established for BV. In about 50% of asymptomatic women, culture results are positive for flora such as Gardnerella vaginalis.5 While Amsel’s criteria are often used as a reference and generally suffice for the evaluation of symptomatic women, the best candidate for a gold standard test is probably Gram stain assessment using Nugent’s criteria (described in this section).11 Lack of leukocytes in the vaginal fluid supports a diagnosis of BV. A finding of white blood cells in excess of the number of vaginal epithelial cells suggests an inflammatory process (SOR: C).12
Amsel’s criteria with wet mount. The diagnostic approach most commonly used in the office is Amsel’s criteria—homogenous discharge, positive whiff-amine test, pH >4.5, and clue cells found on wet-mount microscopy (see How to perform a wet mount ).13 Three of 4 criteria deemed positive is considered diagnostic. If Gram stain is used as the reference standard, then Amsel’s criteria have 70% sensitivity and 94% specificity for diagnosing BV.14 An analysis of the individual criteria follows. The positive and negative predictive values of each compared with the whole group as reference standard is displayed in Table 1 .
Homogenous discharge. A thin, homogenous, grayish discharge is traditionally associated with BV. However, it is not specific to BV, being found commonly also in women with culture results positive for VVC or no diagnosis of vaginitis.2,15 It is the criterion least likely to be consistent with the whole group, seen in about half of women BVpositive and over one third of women BV-negative using Amsel’s criteria as the reference standard. 15
To perform a wet-mount preparation correctly, dilute the vaginal discharge with 1 or 2 drops of 0.9% saline and place it on a slide. Examine the slide under lowand high-powered fields for vaginal squamous cells, white blood cells (WBCs), lactobacilli, clue cells, and trichomonads. An increased number of WBCs can be defined as >5–10 WBC/HPF or WBCs exceeding the number vaginal epithelial cells.
To prepare the potassium hydroxide (KOH) slide, place a generous amount of vaginal discharge on a slide with 10% KOH solution. Air- or flame-drying before examination under low-power microscopy may improve sensitivity. A positive KOH preparation will have hyphae, mycelial tangles, or spores.
Whiff test. The whiff test is performed by adding drops of 10% potassium hydroxide solution to the vaginal fluid. A positive result is a “fishy” amine odor. In a study16 of 100 women complaining of malodorous discharge, a positive whiff test was predictive of positive culture results for anaerobic flora such as Bacteroides sp. with sensitivity 67%, specificity 94%, and a positive predictive value of 95%. The whiff test was not positive in any of the 5 cases with positive culture results for G vaginalis in the absence of anaerobes. There were also 12 cases positive for anaerobes without G vaginalis.
pH >4.5. Since the abnormal flora of BV is consistently associated with a vaginal pH >4.5, a normal pH excludes a diagnosis of BV.17,18 The determination of pH in the narrow range around 4.5 is not accurate using standard nitrazine paper. Narrower-range test paper is available and more accurate. Examples include pH paper for 4.5 to 5.5 (Micro Essential Laboratory), FemExam pH and Amines Test Card (Litmus Concepts), pHem-ALERT: pH paper on a stick (Imagyn Gynecology). Cervical mucous, semen, and blood are alkaline and can interfere with pH testing. Estrogen production is also necessary to maintain an acidic environment. A pH of 3.8 to 4.5 is consistent with normal vaginal flora in premenopausal women with normal estrogen production.17
Clue cells. Clue cells are vaginal epithelial cells coated with coccobacilli giving an appearance as if coated with ground black pepper. Clue cells on wet mount preparation is considered the most accurate of Amsel’s diagnostic criteria for BV.19 On the other hand, office evaluation of the wet mount is considered by some authors to be unreliable due to dependence on the clinician’s microscopy skills and lack of a durable record of the patient sample.
Gram stain a more objective test. A Gram stain evaluation using Nugent’s criteria has been adopted as the gold standard test for research purposes, including studies of prematurity. The Gram-stained vaginal specimen is scored from 0 to 10 based on semi-quantitative assessment of 3 classes of morphotypes ( Table 2 ): large gram-positive rods (Lactobacilli), small gram-negative rods (Gardnerella and Bacteroides spp.), and small curved gram-variable rods (Mobiluncus spp.).11
Diagnosis of BV is typically made when the Nugent score is 7 or more, which appears qualitatively as dominant morphotypes other than Lactobacilli. Gram staining is more objective and reproducible compared with wet-mount examination, with a sensitivity of 93% and specificity of 70% if Amsel’s criteria are used as the gold standard.14 It is useful for the evaluation of asymptomatic women. It also provides a durable record of the patient specimen. Compared with Gram stain, Amsel’s criteria tend to underdiagnose cases. We can expect that if screening for BV in pregnancy becomes a recommendation, Gram staining in a clinical laboratory will be the recommended method of diagnosis.
Other diagnostic tests for BV. DNA testing for Gardnerella is accurate for detection, but it is not synonymous with a diagnosis of BV, as described.20 DNA testing is further described under “Differential Diagnosis.” Gram staining is more reliable than gas-liquid chromatography21 and an assay for proline aminopeptidase (a metabolic product of some of the bacteria associated with BV).22 Latex agglutination testing for vaginal lactoferrin is a nonspecific marker for leukocytes, and thus inflammation. It is of little clinical utility in the diagnosis of vaginal discharge.23
TABLE 1
Predictive values of Amsel’s criteria (using 3 of 4 positive as diagnostic reference standard)
| Diagnostic criterion | Predictive value (%) | |
|---|---|---|
| Positive | Negative | |
| Homogeneous thin discharge seen at introitus | 42 | 89 |
| pH >4.5 | 53 | 94 |
| Odor on alkalinization | 94 | 93 |
| Clue cells on wet mount | 90 | 99 |
| Source: Thomason et al 1990.15 | ||
TABLE 2
How to use Nugent’s Gram stain criteria to diagnose bacterial vaginosis
| Lactobacillus morphophytes | Gardnerella and Bacteroides spp. morphophytes | Curved gram-variable rods | Points |
|---|---|---|---|
| 4+ | 0 | 0 | 0 |
| 3+ | 1+ | 1+ or 2+ | 1 |
| 2+ | 2+ | 3+ or 4+ | 2 |
| 1+ | 3+ | 3 | |
| 0 | 4+ | 4 | |
| Review each of the first 3 columns in turn, assigning points at far right according to your exam findings. | |||
| Add the points for all 3 columns for a final sum. A score of 7 or higher indicates bacterial vaginosis. Source: Nugent et al 1991.11 | |||
Vulvovaginal Candidiasis
Candidiasis is the second most commonly diagnosed vaginitis in the United States. Some experts estimate that 75% of women will have a yeast infection at some point in life and 5% will have recurrent infections.24 However, 10% to 30% of asymptomatic women with normal flora have positive culture results for Candida.25-29 The proportion of symptomatic women with positive culture results is 20% to 40%.4,30,31 Complications of VVC are rare,32 though vulvar vestibulitis33 and chorioamnionitis in pregnancy32 have been reported.
Risk factors. Symptomatic yeast vaginitis has been associated with condom and diaphragm use, recent antibiotic use, receptive oral sex, oral contraceptive use, spermicide use, diabetes, and immunosuppression including AIDS.31,34-37 The associations with antibiotic use and oral contraceptives are not consistent.30,38 Although pregnancy has been postulated as a risk factor for symptomatic VVC, prevalence of yeast on culture in pregnant women is similar to that of nonpregnant women.30
Suggestive symptoms. Among women with a culture result positive for Candida, the most common symptom is pruritus or burning.28 Abnormal discharge is a complaint for most symptomatic women with VVC confirmed by culture.2 In addition, women may complain of a thick, odorless, cottage cheese–like discharge.39 A thick, curdled-appearing discharge points to a diagnosis of Candida because it is rarely present with BV or trichomoniasis. In one study,28 a thick curdled discharge had a positive predictive value of 84% for diagnosis of VVC by culture (SOR: B). However, a thin discharge does not rule out VVC; in another study, clinicians described discharge as thin in about half of women ultimately diagnosed with VVC by culture in another study (SOR: B).2 On exam, vulvar and vaginal erythema are often present but are not specific findings. The accuracy of the clinical exam for VVC is poor compared with culture (SOR: A).2,30
Pathogens. Candida albicans is present in 80% to 90% of patients with VVC.5,40 remainder have non-albicans species, including C glabrata and others.28 An increase to almost 20% of non-Candida species in a vaginitis clinic by the mid-1990’s may be related to increased use of imidazoles available over-the-counter.40,41 Wet mount results are typically negative in the presence of non-Candida VVC.28
Diagnosis of VVC
The gold standard test for diagnosis of VVC is culture. The potassium hydroxide (KOH) wet mount is only 40% to 75% sensitive.28,29,42,43 False-positive results are also observed with variable frequency.44 The pH of the discharge is usually not more than 5.0 with Candida albicans, but may be higher with non-albicans species such as C glabrata.45 Culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR: B).5,28,46 Rapid slide latex agglutination testing is not better than microscopy (SOR: B).42
Trichomoniasis
Trichomonas, a motile protozoan with 4 flagella, causes the third most common form of vaginitis in the United States and is more common in some developing countries. Trichomoniasis accounts for no more than 10% of all cases of vaginitis, and it appears to be decreasing since the introduction of metronidazole.47,48 It is classified as an STD, although transmission is possible by other means if the organism is protected from desiccation—for example, in dirty washcloths or towels and contaminated water. Nonsexual transmission is thought to be uncommon.
Trichomoniasis is associated with GC and Chlamydia infections, and, like them, has been associated with seroconversion to HIV-positive status.49 Trichomonads are identified in 30% to 80% of male sexual partners of infected women. In men, trichomoniasis most often is an asymptomatic carrier state.50 However, it is the cause of about 10% of cases of nongonococcal urethritis in men.51
Our knowledge of the epidemiology of abnormal vaginal discharge is limited. Studies of vaginitis may exclude patients with vaginal discharge due to cervicitis; studies performed in sexually transmitted disease clinics are not representative of primary care practice; women who do not complain of abnormal vaginal discharge may have positive cultures for Gardnerella vaginalis and Candida albicans; and self-treatment of presumed yeast vaginitis with antifungals available over-the-counter further limits our knowledge of the prevalence and causes of vaginal discharge.
Clinical presentations. Women with trichomoniasis have variable presentations ranging from an asymptomatic carrier state to a malodorous, purulent discharge with vulvovaginal erythema. Punctate hemorrhagic cervical lesions are considered pathognomonic of trichomoniasis, but are seen in only about 2% of cases (SOR: B).52
Diagnosis. Culture for trichomoniasis is the gold standard. Several culture media have been used, most commonly the Diamond medium. Recently introduced is a transport and culture medium for detection of Trichomonas (InPouch TV), which performs as well as Diamond medium (SOR: A).53-55 A DNA probe is also available and accurate (SOR: A).
Motile trichomonads are seen on wet preparation in only 50% to 80% of culture-positive cases (SOR: B).50,54,56 Polymorphonuclear leukocytes can be dominant on wet mount, making visualization of trichomonads more difficult. The pH of the vaginal fluid is usually basic.
Trichomonas reported with cervical cytology
Trichomonas may also be reported on Pap smears. A meta-analysis57 comparing the pooled sensitivities and specificities of wet mounts and cytology demonstrated low sensitivities of 68% and 58%, respectively, and high specificities, 99.9% and 97%, respectively (SOR: A).
However, since cytology carries a 3% false-positive rate, its results are not diagnostic of trichomoniasis in low-risk, asymptomatic women.50,57 Treatment may be prescribed empirically based on positive cytology results. However, if an asymptomatic woman were concerned about whether she really has an STD, a positive wet prep would confirm the diagnosis. A negative wet prep should be followed up with culture to reliably rule out disease (SOR: B).
Trichomoniasis in pregnancy
Screening for asymptomatic trichomoniasis in pregnancy has not been recommended. In fact, some evidence suggests that treatment of trichomoniasis in pregnancy is associated with poorer pregnancy outcomes including lower birth weight and more prematurity (SOR: B).58,59
Aerobic vaginitis
Aerobic vaginitis is a term proposed to describe purulent vaginal discharge with predominance of abnormal aerobic flora.60 Aerobic vaginitis, which may be severe, has been reported as the cause of 5% of cases in a series from a specialty vaginitis clinic.61 The usual predominant microorganisms are group B streptococci, Escherichia coli, and Staphylococcus aureus. It is likely that less severe cases of aerobic vaginitis are not recognized in the primary care setting and are treated as BV or resolve spontaneously (SOR: C). The case series referred to above also reported good therapeutic response to 2% topical clindamycin (SOR: C).61
Noninfectious Vaginitis
Noninfectious causes of vaginal discharge include physiologic, irritant and allergic, cytolytic vaginitis, desquamative inflammatory vaginitis, collagen vascular disease, and idiopathic vaginitis.
Irritant and allergic vaginitis may result from sensitivities to topical medications, the active or base ingredients of spermicidal products, douching solutions, and the latex of condoms or diaphragms. If a woman with persistent symptoms has been using such intravaginal products, she should stop (SOR: C).
Cytolytic vaginitis is characterized by overgrowth of lactobacilli and cytolysis of squamous cells, including presence of cytoplasmic fragments and intact cells with naked nuclei.62 The cause is uncertain but may include a reaction to intravaginal medications or other products such as tampons. It can be found in up to 5% of women with symptoms and signs of vaginitis.62,63 Symptoms often mimic VVC and may include a white, cheesy discharge. Vaginal pH ranges from 3.5 to 5.5. Recurrences during luteal phase of the menstrual cycle have been described.64 Intravaginal antifungals should be discontinued. Baking soda sitz baths or douches are often used, but clinical trial data to support this practice are lacking (SOR: C).
Noninfectious desquamative inflammatory vaginitis (DIV) has also been described.65 DIV is an uncommon vaginitis characterized by profuse purulent discharge with epithelial cell exfoliation. It may occur at any time during the reproductive years or after menopause. There is probably a heterogeneous group of causes of DIV. Some cases may correspond to a disorder within the spectrum of lichen planus.66 Treatment is usually difficult, though there may be some response to local or systemic corticosteroid therapy (SOR: C).65
Differential diagnosis
A comparison of physical examination findings an diagnostic test results for various etiologies of vaginitis is summarized in Table 3 . An algorithmic approach to the differential diagnosis of abnormal vaginal discharge is presented in the Figure . Diagnosis is complicated in that signs and symptoms do little to help differentiate among BV, VVC, and trichomoniasis. A study2 of 22 genitourinary symptoms and signs showed that none differentiated among the 3 infections. This lack of clear-cut differences in symptoms also makes self-diagnosis and telephone triage inaccurate.67,68
A DNA probe testing system (Affirm VP III Microbial ID Test) for differential diagnosis is available but expensive. It identifies Gardnerella, Trichomonas, and Candida albicans with a sensitivity of 90% to 95%.54,66 The analyzer costs approximately $10,000 and would typically be purchased by a laboratory. Individual test kits cost about $27.
TABLE 3
Comparative findings among causes of vaginitis
| Cause | Physical exam findings* | Gold standard test | pH | Leukocytes | Wet mount | Alternative test |
|---|---|---|---|---|---|---|
| Bacterial vaginosis | Variable | Gram stain | >4.5 | No | Clue cells | Amsel’s criteria |
| Aerobic vaginitis | Abundant purulent discharge | Culture | >4.5 | Yes | Cocci or coarse rods | |
| Candida vaginitis | Adherent white disch. (thrush) | Culture | 3.8–4.5 | ± | Pseudohyphae or budding yeast | DNA testing |
| Non-Candida yeast vaginitis | Variable | Culture | Any | ± | Usually negative | |
| Trichomoniasis | Variable, occ. strawberry spots on cervix | Culture | >4.5 | ± | Motile trichomonads | DNA testing |
| Cytolytic vaginitis | Profuse discharge, often cheesy | Cytology and negative culture | 3.5–5.5 | ± | Overgrowth of lactobacilli and squamous cell fragments | |
| Desquamative inflammatory vaginitis | Abundant purulent discharge | Parabasal epithelial cells and negative culture | >4.5 | Yes | ||
| Irritant and allergic vaginitis | Variable, often erythema | None | Any | ± | ||
| * Helpful when present. | ||||||
FIGURE
Sequence of office tests to evaluate abnormal vaginal discharge
Corresponding author
Linda French, MD, Associate Professor, Department of Family Practice, College of Human Medicine, Michigan State University, B101 Clinical Center, East Lansing, MI 48824. E-mail: [email protected].
1. National Center for Health Statistics. National Ambulatory Medicine Care Survey. Available at: www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm.
2. Schaaf VM, Perez-Stable EJ, Borchardt K. The limited value of symptoms and signs in the diagnosis of vaginal infections. Arch Intern Med 1990;150:1929-1933.
3. Wiesenfeld HC, Macio I. The infrequent use of officebased diagnostic tests for vaginitis. Am J Obstet Gynecol 1999;181:39-41.
4. Berg AO, Heidrich FE, Fihn SD, et al. Establishing the cause of genitourinary symptoms in women in a family practice. Comparison of clinical examination and comprehensive microbiology. JAMA 1984;251:620-625.
5. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
6. Sellors JW, Walter SD, Howard M. A new visual indicator of chlamydial cervicitis? Sex Transm Infect 2000;76:46-48.
7. Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis 2003;187:650-657.
8. Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180:1863-1868.
9. Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses 1998;14Suppl 1:S17-21.
10. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogenperoxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. JClin Microbiol 1991;29:297-301.
12. Quan M. Vaginitis: meeting the clinical challenge. Clin Cornerstone 2000;3:36-47.
13. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14-22.
14. Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. ObstetGynecol 1996;88:573-576.
15. Thomason JL, Gelbart SM, Anderson RJ, Walt AK, Osypowski PJ, Broekhuizen FF. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am J Obstet Gynecol. 1990;162:155-160.
16. Erkkola R, Jarvinen H, Terho P, Meurman O. Microbial flora in women showing symptoms of nonspecific vaginosis: applicability of KOH test for diagnosis. Scand J Infect Dis Suppl. 1983;40:59-63.
17. Caillouette JC, Sharp CF, Jr, Zimmerman GJ, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. 1997;176:1270-1275discussion1275-1277.
18. Carr PL, Felsenstein D, Friedman RH. Evaluation and management of vaginitis. J Gen Intern Med 1998;13:335-346.
19. Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988;158:819-828.
20. Sheiness D, Dix K, Watanabe S, Hillier SL. Highlevels of Gardnerella vaginalis detected with an oligonucleotide probe combined with elevated pH as a diagnostic indicator of bacterial vaginosis. J Clin Microbiol 1992;30:642-648.
21. Thomason JL, Gelbart SM, James JA, Edwards JM, Hamilton PR. Is analysis of vaginal secretions for volatile organic acids to detect bacterial vaginosis of any diagnostic value? Am J Obstet Gynecol. 1988;159:1509-1511.
22. Thomason JL, Gelbart SM, Wilcoski LM, Peterson AK, Jilly BJ, Hamilton PR. Proline aminopeptidase activity as a rapid diagnostic test to confirm bacterial vaginosis. Obstet Gynecol 1988;71:607-611.
23. Rein MF, Shih LM, Miller JR, Guerrant RL. Use of a lactoferrin assay in the differential diagnosis of female genital tract infections and implications for the pathophysiology of bacterial vaginosis. Sex Transm Dis 1996;23:517-521.
24. Monif GR. Classification and pathogenesis of vulvovaginal candidiasis. Am J Obstet Gynecol 1985;152:935-939.
25. Giraldo P, von Nowaskonski A, Gomes FA, Linhares I, Neves NA, Witkin SS. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstet Gynecol 2000;95:413-416.
26. Bergman JJ, Berg AO. How useful are symptoms in the diagnosis of Candida vaginitis? J Fam Pract 1983;16:509-511.
27. Bro F. Patients with vaginal discharge in general practice. Acta Obstet Gynecol Scand 1989;68:41-43.
28. Eckert LO, Hawes SE, Stevens CE, Koutsky LA, Eschenbach DA, Holmes KK. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol 1998;92:757-765.
29. Bertholf ME, Stafford MJ. Colonization of Candida albicans in vagina, rectum, and mouth. J Fam Pract 1983;16:919-924.
30. Reed BD, Huck W, Zazove P. Differentiation of Gardnerella vaginalis, Candida albicans, and Trichomonas vaginalis infections of the vagina. J Fam Pract 1989;28:673-680.
31. Bro F. The diagnosis of candida vaginitis in general practice. Scand J Prim Health Care 1989;7:19-22.
32. Cotch MF, Hillier SL, Gibbs RS, Eschenbach DA. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 1998;178:374-380.
33. Pagano R. Vulvar vestibulitis syndrome: an often unrecognized cause of dyspareunia. Aust N Z J Obstet Gynaecol 1999;39:79-83.
34. Foxman B. The epidemiology of vulvovaginal candidiasis: risk factors. Am J Public Health 1990;80:329-331.
35. Geiger AM, Foxman B. Risk factors for vulvovaginal candidiasis: a case-control study among university students. Epidemiology 1996;7:182-187.
36. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
37. Spinillo A, Capuzzo E, Acciano S, De Santolo A, Zara F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am J Obstet Gynecol 1999;180:14-17.
38. Davidson F, Oates JK. The pill does not cause ‘thrush’. Br J Obstet Gynaecol Dec 1985;92:1265-1266.
39. Abbott J. Clinical and microscopic diagnosis of vaginal yeast infection: a prospective analysis. Ann Emerg Med 1995;25:587-591.
40. Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J Clin Pharmacol 1992;32:248-255.
41. Spinillo A, Capuzzo E, Gulminetti R, Marone P, Colonna L, Piazzi G. Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am J Obstet Gynecol 1997;176:138-141.
42. Reed BD, Pierson CL. Evaluation of a latex agglutination test for the identification of Candida species in vaginal discharge. J Am Board Fam Pract 1992;5:375-380.
43. Ferris DG, Hendrich J, Payne PM, et al. Office laboratoAE_French.1004.final 9/20/04 3:18 PM Page 813 ry diagnosis of vaginitis. Clinician-performed tests compared with a rapid nucleic acid hybridization test. J Fam Pract 1995;41:575-581.
44. Bergman JJ, Berg AO, Schneeweiss R, Heidrich FE. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J Fam Pract 1984;18:549-552.
45. Sobel JD. Vulvovaginitis due to Candida glabrata. An emerging problem. Mycoses. 1998;41:Suppl 2:18-22.
46. Zdolsek B, Hellberg D, Froman G, Nilsson S, Mardh PA. Culture and wet smear microscopy in the diagnosis of low-symptomatic vulvovaginal candidosis. Eur J Obstet Gynecol Reprod Biol 1995;58:47-51.
47. Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991;165:1217-1222.
48. Kent HL. Epidemiology of vaginitis. Am J Obstet Gynecol 1991;165:1168-1176.
49. Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 1993;7:95-102.
50. Krieger JN, Tam MR, Stevens CE, et al. Diagnosis of trichomoniasis. Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimens. JAMA 1988;259:1223-1227.
51. Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995;22:83-96.
52. Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis 1980;141:137-143.
53. Ohlemeyer CL, Hornberger LL, Lynch DA, Swierkosz EM. Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy. J Adolesc Health 1998;22:205-208.
54. Borchardt KA, Smith RF. An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Med 1991;67:149-152.
55. Levi MH, Torres J, Pina C, Klein RS. Comparison of the InPouch TV culture system and Diamond’s modified medium for detection of Trichomonas vaginalis. J Clin Microbiol 1997;35:3308-3310.
56. DeMeo LR, Draper DL, McGregor JA, et al. Evaluation of a deoxyribonucleic acid probe for the detection of Trichomonas vaginalis in vaginal secretions. Am J Obstet Gynecol 1996;174:1339-1342.
57. Wiese W, Patel SR, Patel SC, Ohl CA, Estrada CA. A meta-analysis of the Papanicolaou smear and wet mount for the diagnosis of vaginal trichomoniasis. Am J Med 2000;108:301-308.
58. Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001;345:487-493.
59. Kigozi GG, Brahmbhatt H, Wabwire-Mangen F, et al. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2003;189:1398-1400.
60. Donder GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Bjog 2002;109:34-43.
61. Sobel JD. Desquamative inflammatory vaginitis: a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol 1994;171:1215-1220.
62. Demirezen S. Cytolytic vaginosis: examination of 2947 vaginal smears. Cent Eur J Public Health 2003;11:23-24.
63. Wathne B, Holst E, Hovelius B, Mardh PA. Vaginal discharge—comparison of clinical, laboratory and microbiological findings. Acta Obstet Gynecol Scand 1994;73:802-808.
64. Secor RM. Cytolytic vaginosis: a common cause of cyclic vulvovaginitis. Nurse Pract Forum 1992;3:145-148.
65. Oates JK, Rowen D. Desquamative inflammatory vaginitis. A review. Genitourin Med 1990;66:275-279.
66. Pelisse M. The vulvo-vaginal-gingival syndrome. A new form of erosive lichen planus. Int J Dermatol 1989;28:381-384.
67. Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol 2002;99:419-425.
68. Allen-Davis JT, Beck A, Parker R, Ellis JL, Polley D. Assessment of vulvovaginal complaints: accuracy of telephone triage and in-office diagnosis. Obstet Gynecol 2002;99:18-22.
1. National Center for Health Statistics. National Ambulatory Medicine Care Survey. Available at: www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm.
2. Schaaf VM, Perez-Stable EJ, Borchardt K. The limited value of symptoms and signs in the diagnosis of vaginal infections. Arch Intern Med 1990;150:1929-1933.
3. Wiesenfeld HC, Macio I. The infrequent use of officebased diagnostic tests for vaginitis. Am J Obstet Gynecol 1999;181:39-41.
4. Berg AO, Heidrich FE, Fihn SD, et al. Establishing the cause of genitourinary symptoms in women in a family practice. Comparison of clinical examination and comprehensive microbiology. JAMA 1984;251:620-625.
5. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
6. Sellors JW, Walter SD, Howard M. A new visual indicator of chlamydial cervicitis? Sex Transm Infect 2000;76:46-48.
7. Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis 2003;187:650-657.
8. Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180:1863-1868.
9. Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses 1998;14Suppl 1:S17-21.
10. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogenperoxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. JClin Microbiol 1991;29:297-301.
12. Quan M. Vaginitis: meeting the clinical challenge. Clin Cornerstone 2000;3:36-47.
13. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14-22.
14. Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. ObstetGynecol 1996;88:573-576.
15. Thomason JL, Gelbart SM, Anderson RJ, Walt AK, Osypowski PJ, Broekhuizen FF. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am J Obstet Gynecol. 1990;162:155-160.
16. Erkkola R, Jarvinen H, Terho P, Meurman O. Microbial flora in women showing symptoms of nonspecific vaginosis: applicability of KOH test for diagnosis. Scand J Infect Dis Suppl. 1983;40:59-63.
17. Caillouette JC, Sharp CF, Jr, Zimmerman GJ, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. 1997;176:1270-1275discussion1275-1277.
18. Carr PL, Felsenstein D, Friedman RH. Evaluation and management of vaginitis. J Gen Intern Med 1998;13:335-346.
19. Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988;158:819-828.
20. Sheiness D, Dix K, Watanabe S, Hillier SL. Highlevels of Gardnerella vaginalis detected with an oligonucleotide probe combined with elevated pH as a diagnostic indicator of bacterial vaginosis. J Clin Microbiol 1992;30:642-648.
21. Thomason JL, Gelbart SM, James JA, Edwards JM, Hamilton PR. Is analysis of vaginal secretions for volatile organic acids to detect bacterial vaginosis of any diagnostic value? Am J Obstet Gynecol. 1988;159:1509-1511.
22. Thomason JL, Gelbart SM, Wilcoski LM, Peterson AK, Jilly BJ, Hamilton PR. Proline aminopeptidase activity as a rapid diagnostic test to confirm bacterial vaginosis. Obstet Gynecol 1988;71:607-611.
23. Rein MF, Shih LM, Miller JR, Guerrant RL. Use of a lactoferrin assay in the differential diagnosis of female genital tract infections and implications for the pathophysiology of bacterial vaginosis. Sex Transm Dis 1996;23:517-521.
24. Monif GR. Classification and pathogenesis of vulvovaginal candidiasis. Am J Obstet Gynecol 1985;152:935-939.
25. Giraldo P, von Nowaskonski A, Gomes FA, Linhares I, Neves NA, Witkin SS. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstet Gynecol 2000;95:413-416.
26. Bergman JJ, Berg AO. How useful are symptoms in the diagnosis of Candida vaginitis? J Fam Pract 1983;16:509-511.
27. Bro F. Patients with vaginal discharge in general practice. Acta Obstet Gynecol Scand 1989;68:41-43.
28. Eckert LO, Hawes SE, Stevens CE, Koutsky LA, Eschenbach DA, Holmes KK. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol 1998;92:757-765.
29. Bertholf ME, Stafford MJ. Colonization of Candida albicans in vagina, rectum, and mouth. J Fam Pract 1983;16:919-924.
30. Reed BD, Huck W, Zazove P. Differentiation of Gardnerella vaginalis, Candida albicans, and Trichomonas vaginalis infections of the vagina. J Fam Pract 1989;28:673-680.
31. Bro F. The diagnosis of candida vaginitis in general practice. Scand J Prim Health Care 1989;7:19-22.
32. Cotch MF, Hillier SL, Gibbs RS, Eschenbach DA. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 1998;178:374-380.
33. Pagano R. Vulvar vestibulitis syndrome: an often unrecognized cause of dyspareunia. Aust N Z J Obstet Gynaecol 1999;39:79-83.
34. Foxman B. The epidemiology of vulvovaginal candidiasis: risk factors. Am J Public Health 1990;80:329-331.
35. Geiger AM, Foxman B. Risk factors for vulvovaginal candidiasis: a case-control study among university students. Epidemiology 1996;7:182-187.
36. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
37. Spinillo A, Capuzzo E, Acciano S, De Santolo A, Zara F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am J Obstet Gynecol 1999;180:14-17.
38. Davidson F, Oates JK. The pill does not cause ‘thrush’. Br J Obstet Gynaecol Dec 1985;92:1265-1266.
39. Abbott J. Clinical and microscopic diagnosis of vaginal yeast infection: a prospective analysis. Ann Emerg Med 1995;25:587-591.
40. Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J Clin Pharmacol 1992;32:248-255.
41. Spinillo A, Capuzzo E, Gulminetti R, Marone P, Colonna L, Piazzi G. Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am J Obstet Gynecol 1997;176:138-141.
42. Reed BD, Pierson CL. Evaluation of a latex agglutination test for the identification of Candida species in vaginal discharge. J Am Board Fam Pract 1992;5:375-380.
43. Ferris DG, Hendrich J, Payne PM, et al. Office laboratoAE_French.1004.final 9/20/04 3:18 PM Page 813 ry diagnosis of vaginitis. Clinician-performed tests compared with a rapid nucleic acid hybridization test. J Fam Pract 1995;41:575-581.
44. Bergman JJ, Berg AO, Schneeweiss R, Heidrich FE. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J Fam Pract 1984;18:549-552.
45. Sobel JD. Vulvovaginitis due to Candida glabrata. An emerging problem. Mycoses. 1998;41:Suppl 2:18-22.
46. Zdolsek B, Hellberg D, Froman G, Nilsson S, Mardh PA. Culture and wet smear microscopy in the diagnosis of low-symptomatic vulvovaginal candidosis. Eur J Obstet Gynecol Reprod Biol 1995;58:47-51.
47. Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991;165:1217-1222.
48. Kent HL. Epidemiology of vaginitis. Am J Obstet Gynecol 1991;165:1168-1176.
49. Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 1993;7:95-102.
50. Krieger JN, Tam MR, Stevens CE, et al. Diagnosis of trichomoniasis. Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimens. JAMA 1988;259:1223-1227.
51. Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995;22:83-96.
52. Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis 1980;141:137-143.
53. Ohlemeyer CL, Hornberger LL, Lynch DA, Swierkosz EM. Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy. J Adolesc Health 1998;22:205-208.
54. Borchardt KA, Smith RF. An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Med 1991;67:149-152.
55. Levi MH, Torres J, Pina C, Klein RS. Comparison of the InPouch TV culture system and Diamond’s modified medium for detection of Trichomonas vaginalis. J Clin Microbiol 1997;35:3308-3310.
56. DeMeo LR, Draper DL, McGregor JA, et al. Evaluation of a deoxyribonucleic acid probe for the detection of Trichomonas vaginalis in vaginal secretions. Am J Obstet Gynecol 1996;174:1339-1342.
57. Wiese W, Patel SR, Patel SC, Ohl CA, Estrada CA. A meta-analysis of the Papanicolaou smear and wet mount for the diagnosis of vaginal trichomoniasis. Am J Med 2000;108:301-308.
58. Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001;345:487-493.
59. Kigozi GG, Brahmbhatt H, Wabwire-Mangen F, et al. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2003;189:1398-1400.
60. Donder GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Bjog 2002;109:34-43.
61. Sobel JD. Desquamative inflammatory vaginitis: a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol 1994;171:1215-1220.
62. Demirezen S. Cytolytic vaginosis: examination of 2947 vaginal smears. Cent Eur J Public Health 2003;11:23-24.
63. Wathne B, Holst E, Hovelius B, Mardh PA. Vaginal discharge—comparison of clinical, laboratory and microbiological findings. Acta Obstet Gynecol Scand 1994;73:802-808.
64. Secor RM. Cytolytic vaginosis: a common cause of cyclic vulvovaginitis. Nurse Pract Forum 1992;3:145-148.
65. Oates JK, Rowen D. Desquamative inflammatory vaginitis. A review. Genitourin Med 1990;66:275-279.
66. Pelisse M. The vulvo-vaginal-gingival syndrome. A new form of erosive lichen planus. Int J Dermatol 1989;28:381-384.
67. Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol 2002;99:419-425.
68. Allen-Davis JT, Beck A, Parker R, Ellis JL, Polley D. Assessment of vulvovaginal complaints: accuracy of telephone triage and in-office diagnosis. Obstet Gynecol 2002;99:18-22.
Which cytology results predict cervical intraepithelial neoplasia?
For women with cervical cytology results showing atypical squamous cell of undetermined significance (ASCUS) and positive results on DNA testing of a cervical sample for human papillomavirus (HPV) high-risk types, about 1 out of 8 progress to cervical intraepithelial neoplasia (CIN) grades 2 or 3 within 2 years of initial colposcopy results showing not more than CIN 1. Since women with cytology results of lowgrade squamous intraepithelial lesion (LSIL) progress to CIN 2 or 3 in the same proportion, management protocols for HPV-positive ASCUS and LSIL should be identical.
For women with cervical cytology results showing atypical squamous cell of undetermined significance (ASCUS) and positive results on DNA testing of a cervical sample for human papillomavirus (HPV) high-risk types, about 1 out of 8 progress to cervical intraepithelial neoplasia (CIN) grades 2 or 3 within 2 years of initial colposcopy results showing not more than CIN 1. Since women with cytology results of lowgrade squamous intraepithelial lesion (LSIL) progress to CIN 2 or 3 in the same proportion, management protocols for HPV-positive ASCUS and LSIL should be identical.
For women with cervical cytology results showing atypical squamous cell of undetermined significance (ASCUS) and positive results on DNA testing of a cervical sample for human papillomavirus (HPV) high-risk types, about 1 out of 8 progress to cervical intraepithelial neoplasia (CIN) grades 2 or 3 within 2 years of initial colposcopy results showing not more than CIN 1. Since women with cytology results of lowgrade squamous intraepithelial lesion (LSIL) progress to CIN 2 or 3 in the same proportion, management protocols for HPV-positive ASCUS and LSIL should be identical.
Does a high-fiber diet prevent colon cancer in at-risk patients?
There is no direct evidence of an effect of dietary fiber on colon cancer incidence. A diet high in fiber has not been shown to be effective in the short-term (2- to 4-year) prevention of recurrent colon polyps (strength of recommendation [SOR]=A, based on consistent randomized clinical trials). Furthermore, epidemiological evidence is inconsistent in demonstrating an association between dietary fiber consumption and the occurrence of colon cancer (SOR=C).
Evidence summary
The term “dietary fiber” refers to a heterogeneous group of substances that may vary in their biologic effects. Fiber is thought to reduce the risk of colon cancer through the following proposed mechanisms—decreased gastrointestinal transit time, increased stool bulk, and fermentation of volatile fatty acids. Other aspects of diet such as fat content, red meat, and micronutrients may also play a role in the development of colon cancer.
Additional proposed risk factors include sedentary lifestyle, obesity, tobacco use, and alcohol consumption1 ; while the commonly accepted high-risk groups for colon cancer are those aged >60 years, those with a positive family history of colorectal cancer, and those with familial polyposis syndrome. In summary, it appears that the cause of colon cancer is complex and multifactorial.
No randomized controlled trials of interventions test whether increase dietary fiber affects the development of colon cancer. Recent randomized controlled trials of interventions have used colon polyps as a surrogate endpoint, since it is believed that polyps are precursors to cancer. A Cochrane meta-analysis2 of 5 trials (including 4349 subjects) of increased dietary fiber to prevent recurrence of colon adenomas found no difference between intervention and control groups for development of at least 1 adenoma (relative risk [RR]=1.04; 95% confidence interval [CI], 0.95–1.13). In a trial3 of ispaghula husk fiber, the intervention group actually had significantly more recurrent adenomas after 3 years (29.3% vs 20.2%; RR=1.67; 95% CI, 1.01–2.76; P=.04).
Other evidence comes from epidemiological studies, which have limited ability to demonstrate causation. Immigrants to Westernized countries from ethnic groups with lower risk of colon cancer develop colon cancer rates similar to the host country over time. Such data support environmental factors in the risk for colon cancer.
Dietary fiber is 1 of several possible factors, yet epidemiological evidence has not been consistent. A systematic review4 of dietary fiber and colorectal neoplasia (which included case-control and cohort studies as well as randomized controlled trials) showed that 13 of 24 case-control studies found an association with high dietary fiber as a possible protective factor, while only 3 of 13 longitudinal studies found such an association.
Recommendations from others
The American Gastroenterological Association states that “currently available evidence from epidemiological, animal, and intervention studies does not unequivocally support the protective role of fiber against development of colorectal cancer.”5 They recommend dietary fiber consumption of at least 30–35 g/d from a variety of sources. The intake level of most studies that demonstrate protective effects are in that range, and it is not certain what the best source(s) may be. They state that a high-fiber diet should begin before age 30, because the impact of dietary change may require decades; they also note that a high-fiber diet has other established health benefits.
The American Dietetic Association recommends a diet rich in dietary fiber through consumption of a variety of fruits, vegetables, whole and high-fiber grain products, and legumes for a daily intake of 20–35 g/d for healthy adults and, for children, a daily intake of 5 plus the child’s age in grams.6 They cite the epidemiological association of a high-fiber diet and lower colorectal cancer risk as well as many other health benefits.
Dietary fiber has benefits, but is no panacea
Mark B. Stephens, MD, MS
Uniformed Services University of the Health Sciences, Bethesda, MD
Given colorectal cancer’s multifactorial nature, it comes as no surprise that dietary fiber is not the panacea for primary or secondary prevention in high-risk patients. These data are specific only to high-risk patients, however, and should not be misinterpreted as reason to abandon recommendations for patients to consume an adequate bulk of fiber on a daily basis. Routine preventive counseling for reducing rates of colorectal cancer should also emphasize the benefits of adequate physical activity and a low-fat diet.
1. Le Marchand L, Wilkens LR, Kolonel LN, Hankin JH, Lyu LC. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res 1997;57:4787-4794.
2. Asano T, McLeod RS. Dietary fiber for the prevention of colorectal adenomas and carcinomas. Cochrane Database Syst Rev 2002;CD003430. Updated quarterly.-
3. Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet 2000;356:1300-1306.
4. Sengupta S, Tjandra JJ, Gibson PR. Dietary fiber and colorectal neoplasia. Dis Colon Rectum 2001;44:1016-1033.
5. American Gastroenterological Association medical position statement: Impact of dietary fiber on colon cancer occurrence. American College of Gastroenterology. Gastroenterology 2000;118:1233-1234.
6. Marlett JA, McBurney MI, Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc 2002;102:993-1000.
There is no direct evidence of an effect of dietary fiber on colon cancer incidence. A diet high in fiber has not been shown to be effective in the short-term (2- to 4-year) prevention of recurrent colon polyps (strength of recommendation [SOR]=A, based on consistent randomized clinical trials). Furthermore, epidemiological evidence is inconsistent in demonstrating an association between dietary fiber consumption and the occurrence of colon cancer (SOR=C).
Evidence summary
The term “dietary fiber” refers to a heterogeneous group of substances that may vary in their biologic effects. Fiber is thought to reduce the risk of colon cancer through the following proposed mechanisms—decreased gastrointestinal transit time, increased stool bulk, and fermentation of volatile fatty acids. Other aspects of diet such as fat content, red meat, and micronutrients may also play a role in the development of colon cancer.
Additional proposed risk factors include sedentary lifestyle, obesity, tobacco use, and alcohol consumption1 ; while the commonly accepted high-risk groups for colon cancer are those aged >60 years, those with a positive family history of colorectal cancer, and those with familial polyposis syndrome. In summary, it appears that the cause of colon cancer is complex and multifactorial.
No randomized controlled trials of interventions test whether increase dietary fiber affects the development of colon cancer. Recent randomized controlled trials of interventions have used colon polyps as a surrogate endpoint, since it is believed that polyps are precursors to cancer. A Cochrane meta-analysis2 of 5 trials (including 4349 subjects) of increased dietary fiber to prevent recurrence of colon adenomas found no difference between intervention and control groups for development of at least 1 adenoma (relative risk [RR]=1.04; 95% confidence interval [CI], 0.95–1.13). In a trial3 of ispaghula husk fiber, the intervention group actually had significantly more recurrent adenomas after 3 years (29.3% vs 20.2%; RR=1.67; 95% CI, 1.01–2.76; P=.04).
Other evidence comes from epidemiological studies, which have limited ability to demonstrate causation. Immigrants to Westernized countries from ethnic groups with lower risk of colon cancer develop colon cancer rates similar to the host country over time. Such data support environmental factors in the risk for colon cancer.
Dietary fiber is 1 of several possible factors, yet epidemiological evidence has not been consistent. A systematic review4 of dietary fiber and colorectal neoplasia (which included case-control and cohort studies as well as randomized controlled trials) showed that 13 of 24 case-control studies found an association with high dietary fiber as a possible protective factor, while only 3 of 13 longitudinal studies found such an association.
Recommendations from others
The American Gastroenterological Association states that “currently available evidence from epidemiological, animal, and intervention studies does not unequivocally support the protective role of fiber against development of colorectal cancer.”5 They recommend dietary fiber consumption of at least 30–35 g/d from a variety of sources. The intake level of most studies that demonstrate protective effects are in that range, and it is not certain what the best source(s) may be. They state that a high-fiber diet should begin before age 30, because the impact of dietary change may require decades; they also note that a high-fiber diet has other established health benefits.
The American Dietetic Association recommends a diet rich in dietary fiber through consumption of a variety of fruits, vegetables, whole and high-fiber grain products, and legumes for a daily intake of 20–35 g/d for healthy adults and, for children, a daily intake of 5 plus the child’s age in grams.6 They cite the epidemiological association of a high-fiber diet and lower colorectal cancer risk as well as many other health benefits.
Dietary fiber has benefits, but is no panacea
Mark B. Stephens, MD, MS
Uniformed Services University of the Health Sciences, Bethesda, MD
Given colorectal cancer’s multifactorial nature, it comes as no surprise that dietary fiber is not the panacea for primary or secondary prevention in high-risk patients. These data are specific only to high-risk patients, however, and should not be misinterpreted as reason to abandon recommendations for patients to consume an adequate bulk of fiber on a daily basis. Routine preventive counseling for reducing rates of colorectal cancer should also emphasize the benefits of adequate physical activity and a low-fat diet.
There is no direct evidence of an effect of dietary fiber on colon cancer incidence. A diet high in fiber has not been shown to be effective in the short-term (2- to 4-year) prevention of recurrent colon polyps (strength of recommendation [SOR]=A, based on consistent randomized clinical trials). Furthermore, epidemiological evidence is inconsistent in demonstrating an association between dietary fiber consumption and the occurrence of colon cancer (SOR=C).
Evidence summary
The term “dietary fiber” refers to a heterogeneous group of substances that may vary in their biologic effects. Fiber is thought to reduce the risk of colon cancer through the following proposed mechanisms—decreased gastrointestinal transit time, increased stool bulk, and fermentation of volatile fatty acids. Other aspects of diet such as fat content, red meat, and micronutrients may also play a role in the development of colon cancer.
Additional proposed risk factors include sedentary lifestyle, obesity, tobacco use, and alcohol consumption1 ; while the commonly accepted high-risk groups for colon cancer are those aged >60 years, those with a positive family history of colorectal cancer, and those with familial polyposis syndrome. In summary, it appears that the cause of colon cancer is complex and multifactorial.
No randomized controlled trials of interventions test whether increase dietary fiber affects the development of colon cancer. Recent randomized controlled trials of interventions have used colon polyps as a surrogate endpoint, since it is believed that polyps are precursors to cancer. A Cochrane meta-analysis2 of 5 trials (including 4349 subjects) of increased dietary fiber to prevent recurrence of colon adenomas found no difference between intervention and control groups for development of at least 1 adenoma (relative risk [RR]=1.04; 95% confidence interval [CI], 0.95–1.13). In a trial3 of ispaghula husk fiber, the intervention group actually had significantly more recurrent adenomas after 3 years (29.3% vs 20.2%; RR=1.67; 95% CI, 1.01–2.76; P=.04).
Other evidence comes from epidemiological studies, which have limited ability to demonstrate causation. Immigrants to Westernized countries from ethnic groups with lower risk of colon cancer develop colon cancer rates similar to the host country over time. Such data support environmental factors in the risk for colon cancer.
Dietary fiber is 1 of several possible factors, yet epidemiological evidence has not been consistent. A systematic review4 of dietary fiber and colorectal neoplasia (which included case-control and cohort studies as well as randomized controlled trials) showed that 13 of 24 case-control studies found an association with high dietary fiber as a possible protective factor, while only 3 of 13 longitudinal studies found such an association.
Recommendations from others
The American Gastroenterological Association states that “currently available evidence from epidemiological, animal, and intervention studies does not unequivocally support the protective role of fiber against development of colorectal cancer.”5 They recommend dietary fiber consumption of at least 30–35 g/d from a variety of sources. The intake level of most studies that demonstrate protective effects are in that range, and it is not certain what the best source(s) may be. They state that a high-fiber diet should begin before age 30, because the impact of dietary change may require decades; they also note that a high-fiber diet has other established health benefits.
The American Dietetic Association recommends a diet rich in dietary fiber through consumption of a variety of fruits, vegetables, whole and high-fiber grain products, and legumes for a daily intake of 20–35 g/d for healthy adults and, for children, a daily intake of 5 plus the child’s age in grams.6 They cite the epidemiological association of a high-fiber diet and lower colorectal cancer risk as well as many other health benefits.
Dietary fiber has benefits, but is no panacea
Mark B. Stephens, MD, MS
Uniformed Services University of the Health Sciences, Bethesda, MD
Given colorectal cancer’s multifactorial nature, it comes as no surprise that dietary fiber is not the panacea for primary or secondary prevention in high-risk patients. These data are specific only to high-risk patients, however, and should not be misinterpreted as reason to abandon recommendations for patients to consume an adequate bulk of fiber on a daily basis. Routine preventive counseling for reducing rates of colorectal cancer should also emphasize the benefits of adequate physical activity and a low-fat diet.
1. Le Marchand L, Wilkens LR, Kolonel LN, Hankin JH, Lyu LC. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res 1997;57:4787-4794.
2. Asano T, McLeod RS. Dietary fiber for the prevention of colorectal adenomas and carcinomas. Cochrane Database Syst Rev 2002;CD003430. Updated quarterly.-
3. Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet 2000;356:1300-1306.
4. Sengupta S, Tjandra JJ, Gibson PR. Dietary fiber and colorectal neoplasia. Dis Colon Rectum 2001;44:1016-1033.
5. American Gastroenterological Association medical position statement: Impact of dietary fiber on colon cancer occurrence. American College of Gastroenterology. Gastroenterology 2000;118:1233-1234.
6. Marlett JA, McBurney MI, Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc 2002;102:993-1000.
1. Le Marchand L, Wilkens LR, Kolonel LN, Hankin JH, Lyu LC. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res 1997;57:4787-4794.
2. Asano T, McLeod RS. Dietary fiber for the prevention of colorectal adenomas and carcinomas. Cochrane Database Syst Rev 2002;CD003430. Updated quarterly.-
3. Bonithon-Kopp C, Kronborg O, Giacosa A, Rath U, Faivre J. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet 2000;356:1300-1306.
4. Sengupta S, Tjandra JJ, Gibson PR. Dietary fiber and colorectal neoplasia. Dis Colon Rectum 2001;44:1016-1033.
5. American Gastroenterological Association medical position statement: Impact of dietary fiber on colon cancer occurrence. American College of Gastroenterology. Gastroenterology 2000;118:1233-1234.
6. Marlett JA, McBurney MI, Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc 2002;102:993-1000.
Evidence-based answers from the Family Physicians Inquiries Network
Prevention and Treatment of Osteoporosis in Postmenopausal Women
The last decade has witnessed important technological advances in the diagnosis of osteoporosis and an increase in therapeutic options. However, there is still considerable uncertainty about optimal strategies for screening and primary preventive treatment.
In 1994, a World Health Organization working group proposed that the diagnosis of osteoporosis be made when BMD, assessed by a dual-energy x-ray absorptiometry (DXA), is at least 2.5 standard deviations below the mean for young adult women (T-score) at the spine, hip, or wrist, or when a history of a traumatic fracture is present.2 A T-score between −1 and −2.5 is designated as osteopenia.
Osteoporosis is defined as “a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture.”1 While no accurate overall measurement of bone strength exists, bone mineral density (BMD) is frequently used as a proxy.
These facts underscore the importance of osteoporotic fractures:
- Only one third of patients regain their prior level of functioning after hip fracture, and one third are discharged to nursing homes.3
- About 1 in 5 patients dies within a year after a hip fracture.
- Vertebral fracture may result in chronic back pain and disability.4
- Existence of fracture greatly increases risk of subsequent fracture.5
- Direct medical costs for osteoporotic fractures are estimated at $13.8 billion in 1995 dollars.6
Prevalence of osteoporosis and fractures
Of American women over age 50 of all races, an estimated 15%, or 5 million, have osteoporosis (based on DXA T-score at the femoral neck) and an additional 40%, or 14 million, have osteopenia.7 In African Americans, the prevalence is about half that of whites.8 The prevalence of osteoporosis assessed by BMD testing increases with age—from 4% of white women aged 50 to 59 to 48% of women aged 80 to 89.9
At least 1 vertebral fracture, as indicated by radiographic criteria, has occurred in 5% of white women aged 50 to 59, and in 25% at age 80.3 The lifetime risk of hip fracture for 50-year-old white women and men is 14% and 5%, respectively; for African American women and men, 6% and 3%, respectively.3 Hip and symptomatic vertebral fractures occur mainly in women over 75,3,10 and the risk for wrist fractures increases starting in the late 50s.11
Age is a particularly important risk factor for hip fractures, reflecting deterioration in bone strength beyond that detectable with BMD testing. The National Osteoporosis Foundation12 observed that the 5-year risk of hip fracture for women with the same T-score (−3) increases dramatically with advancing age (Figure): from 2.4% at age 50 to 9.7% at age 90, with the steepest increase occurring during the 10 years between ages 70 (5.5%) and 80 (9%).
FIGURE
Five-year risk for hip fracture for women with T-score of −3 by age12
Bone mineral density testing
Screening recommendations
The clinical value of different screening strategies is not established, although recommendations have been made within guidelines and consensus statements that discuss prevention and treatment of osteoporosis. Guidelines are consistent in recommending that BMD screening be done only if results will influence treatment decisions. The US Preventive Services Task Force,13 The National Osteoporosis Foundation,14 and American Association of Clinical Endocrinologists15 recommend screening all women over 65, as well as younger women with risk factors for osteoporosis. The National Institutes of Health3 and the North American Menopause Society16 recommend an individualized decision-making approach to screening. The National Osteoporosis Foundation developed nomograms that integrate risk factors into decision-making for testing and treatment,12 which seem promising and merit testing in prospective studies.
Diagnostic testing
DXA. Although several technologies are available, DXA of the hip is considered the best predictor of hip fracture and an equivalent predictor of other fractures.10 The likelihood of making a diagnosis of osteoporosis based on BMD, however, varies and is related to type of test, equipment, anatomic site tested, number of sites tested, technique, and relevance of the reference range to the local population. For example, when the same group of people is tested with DXA equipment from different manufacturers, the proportion diagnosed with osteoporosis varies by as much as 15%.11
Quantitative ultrasound (QUS) and radiographic absorptiometry (RA). Testing by QUS of the heel and RA of the hand are less expensive than DXA and have become popular. While QUS of the heel has been shown to predict hip fracture and all nonvertebral fractures nearly as well as DXA,3,10 it does not highly correlate with DXA and appears to reflect other aspects of bone quality.10 Since QUS and DXA results frequently disagree and can cause confusion, DXA is the most appropriate test of BMD at present. If QUS and RA are used for screening, confirmation with DXA is recommended before therapy is initiated.
Calculations based on risk factors. In a comparison of strategies using risk factors to predict low BMD in postmenopausal women, 2 decision rules performed well: the Osteoporosis Risk Assessment Instrument, which is based on age and weight (Table 1),17 and the Simple Calculated Osteoporosis Risk Estimation (SCORE).17 Research to test these instruments with fracture rather than BMD as outcome is needed.18
Biochemical markers. Levels of markers in serum and/or urine reflect bone turnover and have potential use in diagnosing and monitoring therapy of osteoporosis. They are not yet widely available and have not been consistently associated with identifying patients at risk for fracture.10 They are not recommended at this time.
TABLE 1
Osteoporosis risk assessment instrument17
| Patient characteristic | Points |
|---|---|
| Age (years) | |
| 75 or older | 15 |
| 65 to 74 | 9 |
| 55 to 64 | 5 |
| 54 or younger | 0 |
| Weight | |
| <132 lb (60 kg) | 9 |
| 132 to 153.9 lb (60 to 70 kg) | 3 |
| >154 lb (>70 kg) | 0 |
| No current estrogen use | 2 |
| Total: | |
| Patients with a score of 9 or higher are at risk for diagnosis of osteoporosis by bone mineral density measurement. Sensitivity 97.5%, specificity 28%, positive predictive value 28%, negative predictive value 99.6%, given a 10% baseline risk of a bone mineral density 2.5 SD less than the mean. | |
Importance of primary prevention
At least half of bone strength is attributable to genetic factors12; modifiable factors may contribute almost equally as a group, and therefore warrant attention. Genetic risk factors include age, family history, female sex, low weight, small frame, and white or Asian race. Primary prevention efforts should begin in childhood and continue throughout the life span to maximize bone mass.3
Prevention efforts that target the modifiable factors described below should be a routine part of the health-maintenance visit.
Fall reduction
Falls are the direct cause of more than 90% of osteoporotic hip fractures,19 and the tendency to fall increases with age. Some studies have shown that, for women over age 70, the most important predictors of hip fractures are fall-related factors20,21 such as poor cognitive function, slow gait and otherwise impaired mobility, poor vision, drugs that impair alertness or balance, and history of falls. In women over 75, age and slow gait are equal to low BMD of the femoral neck as predictors of hip fracture.22 Unfortunately, labeling women as osteopenic or osteoporotic can cause fear of falling and lack of activity, leading to further acceleration of bone loss.10
Medications that interfere with balance or alertness should be avoided if possible. Environmental hazards such as loose rugs and uneven or slippery surfaces are also well-recognized modifiable risks for falls23,24 that should be eliminated. Hip protectors effectively reduce fractures in the frail elderly25 and can boost confidence for beneficial increases in physical activity levels,26 but they are often poorly accepted by patients.25,27 Other options include referral for gait training, home visits by a physician or nurse to identify problems in the home that increase the risk of falls, or providing information on home modification (such as installing bathtub rails, removing throw rugs, etc.).
Improvement of nutritional intake
Adequate consumption of calcium is essential for bone health. Calcium balance also can be adversely affected by dietary habits, including high intake of protein, phosphorus, and sodium, although these effects appear to be less important when dietary calcium is sufficient.3 The recommended calcium intake for postmenopausal women (1200–1500 mg/day)28 can be met with food sources, but supplements should be added if needed. Most postmenopausal women in the United States consume only about 600 mg/day.28 High-calcium foods include milk (290–300 mg/cup), sardines in oil, with bones (370 mg/3 oz), yogurt (300–500 mg depending on container size), cheese (165–270 mg/slice), canned salmon, with bones (170–210 mg/3 oz), broccoli (160–180 mg/cup), and tofu (144–155 mg/4 oz).15
Vitamin D is essential for intestinal absorption of calcium. The recommended intake for women is 400 IU/day for ages 51 to 70, 600 IU/day over age 70, and 800 IU/day for all high-risk women, including those who are homebound, institutionalized, on chronic glucocorticoids, or who live in northern latitudes and therefore have limited exposure to sunlight.29 Sources of vitamin D include sunlight, vitamin D–fortified foods, fish oils, and supplements. Multivitamins typically contain 400 IU of vitamin D.
Phytoestrogens, particularly in the form of soy products, have received attention for bone health. Overall, studies do not support the use of soy foods to prevent osteoporosis.3 A well-designed trial in postmenopausal women found that ipriflavone, a synthetic phytoestrogen, did not decrease bone loss.30 Furthermore, use was associated with subclinical lymphocytopenia.
Regular exercise
Weight-bearing physical activity such as walking or running in early life contributes to higher peak bone mass. Limited data suggest weight-bearing exercise in postmenopausal women produces small increases in bone density at the hip31 and improvement in balance and strength.32 For women with established osteoporosis, activities that place an anterior load on the vertebral bodies, such as forward flexion exercises, are associated with an increased incidence of new vertebral deformities, and patients should be advised to avoid them.33
Avoidance of adverse health habits
Current smoking, compared with never smoking, doubles the risk of hip fracture.34 Consumption of more than 1 alcoholic drink/day or more than 7/week is associated with osteoporosis and fracture, while moderate consumption of 1 drink/day or less is associated with decreased risk.35 Excessive caffeine intake is also associated with increased osteoporosis risk and should be avoided. This effect appears to result from substitution of calcium-containing beverages such as milk or fortified orange juice with caffeinated, non–calcium-containing beverages such as colas.
Treatment for fracture prevention and pain relief
The goals of therapy for osteoporosis are fracture prevention and pain relief to maximize physical function.15 Prior fracture is associated with a fivefold risk of future fractures.5 About 20% of women who experience a vertebral fracture have another fracture within 1 year.36 Currently available therapies (Table 2) are antiresorptive: they slow bone turnover and allow bone formation to exceed resorption. Trials of antiresorptive agents in elderly women with osteoporosis and baseline vertebral fracture demonstrate that 1 new vertebral fracture is prevented for each 12 to 35 women treated for 2 to 3 years.14,37Table 3 summarizes the results of key treatment studies and provides information on the number of women that need to be treated (NNT) for the study period to prevent 1 fracture.
TABLE 2
Drug therapy for prevention and treatment of postmenopausal osteoporosis
| Drug (trade name) | Indication and dosage | Possible side effects (% of patients) | Cost per month* |
|---|---|---|---|
| Calcium and vitamin D (generic, Tums, Citracal, and others) | Prevention and treatment: 1200–1500 mg/day calcium and 800 IU/day vitamin D | Nausea, dyspepsia (uncommon), constipation (10%) | $5 (both) |
| Estrogen† (Premarin, Ogen, Estrace, Estraderm, and others) | Prevention: 0.625 mg/day conjugated equine estrogen or the equivalent; 0.3 mg/day may be effective | Nausea, breast tenderness, vaginal bleeding, mood alterations, headache, bloating | $14–$28 |
| Alendronate (Fosamax) | Prevention and treatment: 5 mg/day or 35 mg/week | Nausea, dyspepsia esophageal irritation | $67 |
| Risedronate (Actonel) | Prevention and treatment: 5 mg/day or 35 mg/week | Abdominal pain, nausea, diarrhea | $67 |
| Raloxifene (Evista) | Treatment: 60 mg/day | Hot flashes (6%), leg cramps (3%) | $70 |
| Calcitonin nasal spray (Miacalcin) | Treatment: 200 IU/day (1 spray in 1 nostril per day) | Rhinitis (5%), epistaxis, sinusitis | $66 |
| *Average wholesale cost to the pharmacy for 30 days of therapy; (Drug Topics Red Book. Montvale, NJ; Medical Economics Co., Inc, 2002.) | |||
| †Women with a uterus need to take a progestin such as medroxyprogesterone acetate (Provera $30/month, generic $9/month) or a combination estrogen/progestin product (Prempro $33/month, FemHRT $26/month). | |||
TABLE 3
Clinical trials of drug therapy for the prevention of fracture in postmenopausal women with osteoporosis
| Trial, year | Therapy | Outcome prevented | Number needed to treat for n years |
|---|---|---|---|
| Elderly, postmenopausal women | |||
| Chapuy, 199267 | Calcium/vitamin D | Hip fracture | 48 women for 1.5 years |
| Postmenopausal women | |||
| WHI, 200242 | Hormone replacement therapy | Hip fracture | 2000 women for 5 years |
| Postmenopausal women with osteoporosis | |||
| Ettinger, 199956 | Raloxifene | Vertebral fracture | 29 women for 3 years |
| Liberman, 199554 | Alendronate | Vertebral fracture | 34 women for 3 years |
| Heaney, 200253 | Risendronate | Vertebral fracture | 15 women for 3 years |
| McClung, 200170 | Risedronate | Hip fracture | 91 women for 3 years |
| Postmenopausal women with osteoporosis and previous vertebral racture | |||
| Harris, 199951 | Risedronate | Vertebral fracture | 20 women for 3 years |
| Black, 199652 | Alendronate | Vertebral fracture | 35 women for 3 years |
| Black, 199652 | Alendronate | Hip fracture | 86 women for 3 years |
Calcium and vitamin D
Calcium with or without vitamin D has been reported to positively affect fracture incidence.14 Vitamin D alone does not decrease the incidence of hip fractures.38 Calcium, 1200 to 1500 mg/day, and vitamin D, 800 IU/day, should be used concurrently with other forms of pharmacologic treatment. Calcium supplements are best absorbed with meals; for maximum absorption, calcium should be taken in doses of 500 mg or less.29,39
Minor gastrointestinal adverse effects may occur (most often constipation, 10%),14 which is often resolved by switching to a different preparation.40 Calcium in doses up to 1500 mg/day does not increase the risk for renal calculi and may, in fact, decrease risk.40,41 Calcium interferes with the absorption of certain medications, including tetracycline and quinolone antibiotics, which should be taken several hours apart from calcium. Calcium carbonate requires an acidic environment to dissolve. Patients taking stomach acid–suppressant therapy should use calcium citrate because it does not require an acidic environment for dissolution or should take their calcium supplement with meals. Traces of lead may be present in natural sources of calcium (bone meal, oyster shell, limestone, and dolomite), but can be avoided by use of over-the-counter calcium carbonate tablets (Tums).
Estrogen
Data from the Women’s Health Initiative (WHI) demonstrated that hormone replacement therapy (HRT) combining an estrogen and a progestin reduced hip and vertebral fractures by 1 in 2000 women per year and reduced all fractures by 1 in 333 women per year.42 Estrogen has a positive effect on BMD whether given in early or late postmenopause.43 Rapid bone loss as assessed by BMD does not occur after stopping HRT.44 However, in elderly women who have never used HRT, BMD is similar to those who have used it for 10 years and then stopped for at least 10 years.43 The effect of short-term (< 5 years) HRT during perimenopause on lifetime risk of osteoporotic fracture is unknown.
Common side effects (Table 2) can often be addressed by altering dosages, specific products, or regimens. The risk for breast cancer increases with duration of treatment and with combination HRT, compared with estrogen-only preparations.42,45,46 Combination HRT products are, however, essential for endometrial protection in women who have a uterus. The WHI reported an increase in breast cancer cases of 1 in 1250 women per year during an average 5-year follow-up.42 An increased risk for venous thromboembolism of 1 in 555 women per year was also observed with HRT use. Both the Heart and Estrogen/Progestin Study47 and the WHI studies found that venous thromboembolism occurred more frequently in the first 2 years of HRT use. An increased risk of myocardial infarction in the first 2 years of use was also noted among women with coronary heart disease47 and those without heart disease (1 in 1429 women per year).42 A small increase in stroke risk has also been documented.42,48-50 Contraindications to estrogen use include active thromboembolism, estrogen-related cancers, and liver disease.
Bisphosphonates
Two bisphosphonates, alendronate and risedronate, are approved in the United States for both prevention and treatment of postmenopausal osteoporosis. Clinical trials have demonstrated that both rapidly reduce the risk for symptomatic fractures in women with previous fracture and osteoporosis.37,51,52 The extent of fracture reduction is significant: Recent studies have shown that, over 3 years, the number of patients who would need to be treated with risedronate to prevent a vertebral fracture is 15, with alendronate, 34.53,54 Prevention of fractures among women without a prior vertebral fracture is less well established. No published data demonstrate that one bisphosphonate is more effective than another at preventing clinical fractures. A bisphosphonate is, therefore, the drug of choice for severe osteoporosis.
Bisphosphonates are generally well tolerated.55,56 However, postmarketing surveillance has demonstrated esophagitis and esophageal ulcer associated with alendronate.55 A pooled analysis of trials of risedronate found no increase in upper gastrointestinal (GI) adverse events even in patients with history of peptic ulcers, heartburn, and esophagitis, or among those taking nonsteroidal anti-inflammatory drugs, including aspirin.57
Oral bisphosphonates are not well absorbed (less than 1% of each dose).55,56 Therefore, to maximize absorption and to decrease the likelihood of adverse GI effects, the manufacturers of both bisphosphonates recommend that patients take the medication with a full glass of water, remain upright (sitting or standing) for at least 30 minutes following the dose, and not recline until food is consumed. Both bisphosphonates should be used with caution in patients with active GI disorders. Bisphosphonates are eliminated via the kidney and are not recommended for patients with a creatinine clearance below 30 mL/min.
Selective estrogen receptor modulators (SERMs)
Raloxifene is currently the only selective estrogen receptor modulator approved in the United States for prevention and treatment of osteoporosis. It has been shown to significantly decrease new vertebral fractures in women with a previous history of fracture and osteoporosis.58 The magnitude of fracture reduction is similar to that of bisphosphonates, although improvement in BMD is less marked.59 Raloxifene may confer other benefits. It has been shown to reduce the risk of breast cancer60,61 except in women with low estradiol levels,60 and may reduce the risk of myocardial infarction in women at high risk.60 Raloxifene does not increase the risk for endometrial cancer.60
Hot flashes and leg cramps are relatively common side effects of raloxifene.60 The observed risk of venous thromboembolism, 1 in 465 women per year during 3 years of treatment, is similar to that observed with HRT.60 Raloxifene is teratogenic and should not be used in premenopausal women.
Salmon calcitonin
Salmon calcitonin has demonstrated an analgesic effect for osteoporotic fracture.63,64 A large trial of salmon calcitonin at dosages of 100, 200, and 400 IU/day versus placebo found that salmon calcitonin at 200 IU/day decreased new vertebral fractures among women with a previous osteoporotic vertebral fracture based on radiographic assessment.65 No benefit was observed at the 100 and 400 IU/day dosages. The effect of calcitonin on clinical (symptomatic) fractures has not been reported. Calcitonin is approved for use in treatment, but not prevention, of osteoporosis.
Nasal calcitonin can cause minor rhinitis symptoms.30 Saline nasal solution may be useful to prevent or resolve irritation and dryness. Administration using alternate nostrils helps minimize local side effects. Unopened bottles (14 doses) must be stored in the refrigerator. Open bottles are stable at room temperature for up to 30 days.
Monitoring therapy
The value of serial densitometry to monitor the therapy of individual patients has not been established by randomized trials comparing different monitoring intervals or monitoring versus no monitoring.10,66 One important limitation is the relative imprecision of BMD testing: it takes almost a year to detect a 3% change in BMD.10 Disconcerting decreases in BMD scores are seen in yearly testing and may be offset by larger increases later, without a change in therapy. In studies of alendronate and raloxifene, disproportionately large fracture reductions cannot be explained by improvement in BMD alone.66,68 Bone densitometry is therefore not recommended until the patient has been treated for 2 years, and is of uncertain value beyond that point.
1. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement Online 2000 March 27-29; [cited 9/2/2]; 17(1):1-36.
2. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843. Geneva: World Health Organization; 1994.
3. NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
4. Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(suppl 3):185S-189S.
5. Black DM, Arden NK, Palermo I, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821-828.
6. Ray NF, Chan JK, Thamer M, Melton LJ, 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24-35.
7. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468-489.
8. Aloia JF, Vaswani A, Yeh JK, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int. 1996;59:415-423.
9. Melton LJ. How many women have osteoporosis now? J Bone Miner Res. 1995;10:175-177.
10. Nelson HD, Morris CD, Mahon S, Carney N, Nygren PM, Helfand M. Osteoporosis in postmenopausal women: diagnosis and monitoring. Evidence Report/Technology Assessment Number 28. Rockville, MD: Agency for Healthcare Research and Quality; November 2001. 01-E032.
11. Melton LJ, 3rd, Amadio PC, Crowson CS, O’Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8:341-348.
12. National Osteoporosis Foundation. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Osteoporos Int. 1998;8(suppl):S7-S80.
13. U.S. Preventive Services Task Force. Screening for Osteoporosis in Postmenopausal Women. September 2002. Originally in Annals of Internal Medicine 2002;137:526-8.Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/3rduspstf/osteoporosis/osteorr.htm.
14. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 1998.
15. American Association of Clinical Endocrinologists 2001 medical guidelines for clinical practice for the prevention and management of osteoporosis. Endocr Pract. 2001;7:293-312.
16. North American Menopause Society. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
17. Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual energy x-ray absorptiometry. JAMA. 2001;286:57-63.
18. Wasnich RD. Consensus and the T-score fallacy. Clin Rheumatol. 1997;16:337-339.
19. Grisso JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of hip fracture in women.The Northeast Hip Fracture Study Group. N Engl J Med. 1991;324:1326-1331.
20. Cooper C, Barker DJ, Morris J, Briggs RS. Osteoporosis, falls, and age in fracture of the proximal femur. Br Med J (Clin Res Ed). 1987;295:13-15.
21. Gardsell P, Johnell O, Nilsson BE, Nilsson JA. The predictive value of fracture, disease, and falling tendency for fragility fractures in women. Calcif Tissue Int. 1989;45:327-330.
22. Dargent-Molina P, Schott AM, Hans D, et al. Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Epidemiologie de l’Osteoporose. Osteoporos Int. 1999;9:188-192.
23. Clemson L, Cumming RG, Roland M. Case-control study of hazards in the home and risk of falls and hip fractures. Age Ageing. 1996;25:97-101.
24. Norton R, Campbell AJ, Lee-Joe T, Robinson E, Butler M. Circumstances of falls resulting in hip fractures among older people. J Am Geriatr Soc 1997;45:1108-1112.
25. Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000;343:1506-1513.
26. Cameron ID, Stafford B, Cumming RG, et al. Hip protectors improve falls self-efficacy. Age Ageing. 2000;29:57-62.
27. Hubacher M, Wettstein A. Acceptance of hip protectors for hip fracture prevention in nursing homes. Osteoporos Int. 2001;12:794-799.
28. NIH Consensus Development Panel on Optimal Calcium Intake. Optimal Calcium Intake. JAMA. 1994;272:1942-1948.
29. Morgan SL. Calcium and vitamin D in osteoporosis. Rheum Dis Clin North Am. 2001;27:101-130.
30. Tsourounis C. Clinical effects of phytoestrogens. Clin Obstet Gynecol. 2001;44:836-842.
31. Kelley GA. Aerobic exercise and bone density at the hip in postmenopausal women: a meta-analysis. Prev Med. 1998;27:798-807.
32. Marcus R. Role of exercise in preventing and treating osteoporosis. Rheum Dis Clin North Am. 2001;27:131-141,vi.-
33. Sinaki M, Mikkelsen BA. Postmenopausal spinal osteoporosis: flexion versus extension exercises. Arch Phys Med Rehabil. 1984;65:593-596.
34. Law M, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of major effect. BMJ. 1997;315:841-846.
35. Felson DT, Zhang Y, Hannan MT, Kannel WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142:485-492.
36. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
37. Marcus R, Wong M, Heath H, 3rd, Stock JL. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocr Rev. 2002;23:16-37.
38. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124:400-406.
39. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77-84.
40. North American Menopause Society. The role of calcium in peri- and postmenopausal women; consensus opinion of The North American Menopause Society. Menopause. 2001;8:84-95.
41. Williams CP, Child DF, Hudson PR, et al. Why oral calcium supplements may reduce renal stone disease: report of a clinical pilot study. J Clin Pathol. 2001;54:54-62.
42. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results for the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-333.
43. Barrett-Connor E, Hendrix S, Ettinger B. International position paper on women’s health and menopause: a comprehensive approach. National Heart, Lung, and Blood Institute; 2002.
44. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
45. Schairer C, Lubin J, Triosi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485-491.
46. Ross R, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328-332.
47. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
48. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Annals d’Endocrinologie. 1999;60:232-241.
49. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen/Progestin Replacement Study (HERS). Circulation. 2001;103:638-642.
50. Viscoli C, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243-1249.
51. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352.
52. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
53. Heaney RP, Zizic TM, Fogelman I, et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int. 2002;13:501-505.
54. Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437-1443.
55. Sharpe M, Noble S, Spencer CM. Alendronate: an update of its use in osteoporosis. Drugs. 2001;61:999-1039.
56. Dunn CJ, Goa KL. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 2001;61:685-712.
57. Taggart H, Bolognese MA, Lindsay R, et al. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc. 2002;77:262-270.
58. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-645.
59. Sarkar S, Mitlak B, Wong M, Stock J, Black D, Harper K. Raloxifene-induced fracture reductions not directly associated with BMD changes. J Bone Miner Res. 2002;1:1-10.
60. Barrett-Connor E. Raloxifene: risks and benefits. Ann N Y Acad Sci. 2001;949:295-303.
61. Cummings S, Duong T, Kenyon E, Cauley J, Whitehead M, Krueger K. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
62. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287:847-857.
63. Lyritis GP, Paspati I, Karachalios T, Ioakimidis D, Skarantavos G, Lyritis PG. Pain relief from nasal salmon calcitonin in osteoporotic vertebral crush fractures. A double blind, placebo-controlled clinical study. Acta Orthop Scand Suppl. 1997;275:112-114.
64. Pun KK, Chan LW. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures. Clin Ther. 1989;11:205-209.
65. Chesnut CH, 3rd. Calcitonin in the prevention and treatment of osteoporosis. Osteoporos Int. 1993;3(suppl 1):206-207.
66. Crandall C. The role of serial bone mineral density testing for osteoporosis. J Womens Health Gend Based Med. 2001;10:887-895.
67. Cummings SR. The paradox of small changes in bone density and reductions in risk of fracture with raloxifene. Ann N Y Acad Sci. 2001;949:198-201.
68. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281-289.
69. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637-1642.
70. McClung M, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333-340.
The last decade has witnessed important technological advances in the diagnosis of osteoporosis and an increase in therapeutic options. However, there is still considerable uncertainty about optimal strategies for screening and primary preventive treatment.
In 1994, a World Health Organization working group proposed that the diagnosis of osteoporosis be made when BMD, assessed by a dual-energy x-ray absorptiometry (DXA), is at least 2.5 standard deviations below the mean for young adult women (T-score) at the spine, hip, or wrist, or when a history of a traumatic fracture is present.2 A T-score between −1 and −2.5 is designated as osteopenia.
Osteoporosis is defined as “a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture.”1 While no accurate overall measurement of bone strength exists, bone mineral density (BMD) is frequently used as a proxy.
These facts underscore the importance of osteoporotic fractures:
- Only one third of patients regain their prior level of functioning after hip fracture, and one third are discharged to nursing homes.3
- About 1 in 5 patients dies within a year after a hip fracture.
- Vertebral fracture may result in chronic back pain and disability.4
- Existence of fracture greatly increases risk of subsequent fracture.5
- Direct medical costs for osteoporotic fractures are estimated at $13.8 billion in 1995 dollars.6
Prevalence of osteoporosis and fractures
Of American women over age 50 of all races, an estimated 15%, or 5 million, have osteoporosis (based on DXA T-score at the femoral neck) and an additional 40%, or 14 million, have osteopenia.7 In African Americans, the prevalence is about half that of whites.8 The prevalence of osteoporosis assessed by BMD testing increases with age—from 4% of white women aged 50 to 59 to 48% of women aged 80 to 89.9
At least 1 vertebral fracture, as indicated by radiographic criteria, has occurred in 5% of white women aged 50 to 59, and in 25% at age 80.3 The lifetime risk of hip fracture for 50-year-old white women and men is 14% and 5%, respectively; for African American women and men, 6% and 3%, respectively.3 Hip and symptomatic vertebral fractures occur mainly in women over 75,3,10 and the risk for wrist fractures increases starting in the late 50s.11
Age is a particularly important risk factor for hip fractures, reflecting deterioration in bone strength beyond that detectable with BMD testing. The National Osteoporosis Foundation12 observed that the 5-year risk of hip fracture for women with the same T-score (−3) increases dramatically with advancing age (Figure): from 2.4% at age 50 to 9.7% at age 90, with the steepest increase occurring during the 10 years between ages 70 (5.5%) and 80 (9%).
FIGURE
Five-year risk for hip fracture for women with T-score of −3 by age12
Bone mineral density testing
Screening recommendations
The clinical value of different screening strategies is not established, although recommendations have been made within guidelines and consensus statements that discuss prevention and treatment of osteoporosis. Guidelines are consistent in recommending that BMD screening be done only if results will influence treatment decisions. The US Preventive Services Task Force,13 The National Osteoporosis Foundation,14 and American Association of Clinical Endocrinologists15 recommend screening all women over 65, as well as younger women with risk factors for osteoporosis. The National Institutes of Health3 and the North American Menopause Society16 recommend an individualized decision-making approach to screening. The National Osteoporosis Foundation developed nomograms that integrate risk factors into decision-making for testing and treatment,12 which seem promising and merit testing in prospective studies.
Diagnostic testing
DXA. Although several technologies are available, DXA of the hip is considered the best predictor of hip fracture and an equivalent predictor of other fractures.10 The likelihood of making a diagnosis of osteoporosis based on BMD, however, varies and is related to type of test, equipment, anatomic site tested, number of sites tested, technique, and relevance of the reference range to the local population. For example, when the same group of people is tested with DXA equipment from different manufacturers, the proportion diagnosed with osteoporosis varies by as much as 15%.11
Quantitative ultrasound (QUS) and radiographic absorptiometry (RA). Testing by QUS of the heel and RA of the hand are less expensive than DXA and have become popular. While QUS of the heel has been shown to predict hip fracture and all nonvertebral fractures nearly as well as DXA,3,10 it does not highly correlate with DXA and appears to reflect other aspects of bone quality.10 Since QUS and DXA results frequently disagree and can cause confusion, DXA is the most appropriate test of BMD at present. If QUS and RA are used for screening, confirmation with DXA is recommended before therapy is initiated.
Calculations based on risk factors. In a comparison of strategies using risk factors to predict low BMD in postmenopausal women, 2 decision rules performed well: the Osteoporosis Risk Assessment Instrument, which is based on age and weight (Table 1),17 and the Simple Calculated Osteoporosis Risk Estimation (SCORE).17 Research to test these instruments with fracture rather than BMD as outcome is needed.18
Biochemical markers. Levels of markers in serum and/or urine reflect bone turnover and have potential use in diagnosing and monitoring therapy of osteoporosis. They are not yet widely available and have not been consistently associated with identifying patients at risk for fracture.10 They are not recommended at this time.
TABLE 1
Osteoporosis risk assessment instrument17
| Patient characteristic | Points |
|---|---|
| Age (years) | |
| 75 or older | 15 |
| 65 to 74 | 9 |
| 55 to 64 | 5 |
| 54 or younger | 0 |
| Weight | |
| <132 lb (60 kg) | 9 |
| 132 to 153.9 lb (60 to 70 kg) | 3 |
| >154 lb (>70 kg) | 0 |
| No current estrogen use | 2 |
| Total: | |
| Patients with a score of 9 or higher are at risk for diagnosis of osteoporosis by bone mineral density measurement. Sensitivity 97.5%, specificity 28%, positive predictive value 28%, negative predictive value 99.6%, given a 10% baseline risk of a bone mineral density 2.5 SD less than the mean. | |
Importance of primary prevention
At least half of bone strength is attributable to genetic factors12; modifiable factors may contribute almost equally as a group, and therefore warrant attention. Genetic risk factors include age, family history, female sex, low weight, small frame, and white or Asian race. Primary prevention efforts should begin in childhood and continue throughout the life span to maximize bone mass.3
Prevention efforts that target the modifiable factors described below should be a routine part of the health-maintenance visit.
Fall reduction
Falls are the direct cause of more than 90% of osteoporotic hip fractures,19 and the tendency to fall increases with age. Some studies have shown that, for women over age 70, the most important predictors of hip fractures are fall-related factors20,21 such as poor cognitive function, slow gait and otherwise impaired mobility, poor vision, drugs that impair alertness or balance, and history of falls. In women over 75, age and slow gait are equal to low BMD of the femoral neck as predictors of hip fracture.22 Unfortunately, labeling women as osteopenic or osteoporotic can cause fear of falling and lack of activity, leading to further acceleration of bone loss.10
Medications that interfere with balance or alertness should be avoided if possible. Environmental hazards such as loose rugs and uneven or slippery surfaces are also well-recognized modifiable risks for falls23,24 that should be eliminated. Hip protectors effectively reduce fractures in the frail elderly25 and can boost confidence for beneficial increases in physical activity levels,26 but they are often poorly accepted by patients.25,27 Other options include referral for gait training, home visits by a physician or nurse to identify problems in the home that increase the risk of falls, or providing information on home modification (such as installing bathtub rails, removing throw rugs, etc.).
Improvement of nutritional intake
Adequate consumption of calcium is essential for bone health. Calcium balance also can be adversely affected by dietary habits, including high intake of protein, phosphorus, and sodium, although these effects appear to be less important when dietary calcium is sufficient.3 The recommended calcium intake for postmenopausal women (1200–1500 mg/day)28 can be met with food sources, but supplements should be added if needed. Most postmenopausal women in the United States consume only about 600 mg/day.28 High-calcium foods include milk (290–300 mg/cup), sardines in oil, with bones (370 mg/3 oz), yogurt (300–500 mg depending on container size), cheese (165–270 mg/slice), canned salmon, with bones (170–210 mg/3 oz), broccoli (160–180 mg/cup), and tofu (144–155 mg/4 oz).15
Vitamin D is essential for intestinal absorption of calcium. The recommended intake for women is 400 IU/day for ages 51 to 70, 600 IU/day over age 70, and 800 IU/day for all high-risk women, including those who are homebound, institutionalized, on chronic glucocorticoids, or who live in northern latitudes and therefore have limited exposure to sunlight.29 Sources of vitamin D include sunlight, vitamin D–fortified foods, fish oils, and supplements. Multivitamins typically contain 400 IU of vitamin D.
Phytoestrogens, particularly in the form of soy products, have received attention for bone health. Overall, studies do not support the use of soy foods to prevent osteoporosis.3 A well-designed trial in postmenopausal women found that ipriflavone, a synthetic phytoestrogen, did not decrease bone loss.30 Furthermore, use was associated with subclinical lymphocytopenia.
Regular exercise
Weight-bearing physical activity such as walking or running in early life contributes to higher peak bone mass. Limited data suggest weight-bearing exercise in postmenopausal women produces small increases in bone density at the hip31 and improvement in balance and strength.32 For women with established osteoporosis, activities that place an anterior load on the vertebral bodies, such as forward flexion exercises, are associated with an increased incidence of new vertebral deformities, and patients should be advised to avoid them.33
Avoidance of adverse health habits
Current smoking, compared with never smoking, doubles the risk of hip fracture.34 Consumption of more than 1 alcoholic drink/day or more than 7/week is associated with osteoporosis and fracture, while moderate consumption of 1 drink/day or less is associated with decreased risk.35 Excessive caffeine intake is also associated with increased osteoporosis risk and should be avoided. This effect appears to result from substitution of calcium-containing beverages such as milk or fortified orange juice with caffeinated, non–calcium-containing beverages such as colas.
Treatment for fracture prevention and pain relief
The goals of therapy for osteoporosis are fracture prevention and pain relief to maximize physical function.15 Prior fracture is associated with a fivefold risk of future fractures.5 About 20% of women who experience a vertebral fracture have another fracture within 1 year.36 Currently available therapies (Table 2) are antiresorptive: they slow bone turnover and allow bone formation to exceed resorption. Trials of antiresorptive agents in elderly women with osteoporosis and baseline vertebral fracture demonstrate that 1 new vertebral fracture is prevented for each 12 to 35 women treated for 2 to 3 years.14,37Table 3 summarizes the results of key treatment studies and provides information on the number of women that need to be treated (NNT) for the study period to prevent 1 fracture.
TABLE 2
Drug therapy for prevention and treatment of postmenopausal osteoporosis
| Drug (trade name) | Indication and dosage | Possible side effects (% of patients) | Cost per month* |
|---|---|---|---|
| Calcium and vitamin D (generic, Tums, Citracal, and others) | Prevention and treatment: 1200–1500 mg/day calcium and 800 IU/day vitamin D | Nausea, dyspepsia (uncommon), constipation (10%) | $5 (both) |
| Estrogen† (Premarin, Ogen, Estrace, Estraderm, and others) | Prevention: 0.625 mg/day conjugated equine estrogen or the equivalent; 0.3 mg/day may be effective | Nausea, breast tenderness, vaginal bleeding, mood alterations, headache, bloating | $14–$28 |
| Alendronate (Fosamax) | Prevention and treatment: 5 mg/day or 35 mg/week | Nausea, dyspepsia esophageal irritation | $67 |
| Risedronate (Actonel) | Prevention and treatment: 5 mg/day or 35 mg/week | Abdominal pain, nausea, diarrhea | $67 |
| Raloxifene (Evista) | Treatment: 60 mg/day | Hot flashes (6%), leg cramps (3%) | $70 |
| Calcitonin nasal spray (Miacalcin) | Treatment: 200 IU/day (1 spray in 1 nostril per day) | Rhinitis (5%), epistaxis, sinusitis | $66 |
| *Average wholesale cost to the pharmacy for 30 days of therapy; (Drug Topics Red Book. Montvale, NJ; Medical Economics Co., Inc, 2002.) | |||
| †Women with a uterus need to take a progestin such as medroxyprogesterone acetate (Provera $30/month, generic $9/month) or a combination estrogen/progestin product (Prempro $33/month, FemHRT $26/month). | |||
TABLE 3
Clinical trials of drug therapy for the prevention of fracture in postmenopausal women with osteoporosis
| Trial, year | Therapy | Outcome prevented | Number needed to treat for n years |
|---|---|---|---|
| Elderly, postmenopausal women | |||
| Chapuy, 199267 | Calcium/vitamin D | Hip fracture | 48 women for 1.5 years |
| Postmenopausal women | |||
| WHI, 200242 | Hormone replacement therapy | Hip fracture | 2000 women for 5 years |
| Postmenopausal women with osteoporosis | |||
| Ettinger, 199956 | Raloxifene | Vertebral fracture | 29 women for 3 years |
| Liberman, 199554 | Alendronate | Vertebral fracture | 34 women for 3 years |
| Heaney, 200253 | Risendronate | Vertebral fracture | 15 women for 3 years |
| McClung, 200170 | Risedronate | Hip fracture | 91 women for 3 years |
| Postmenopausal women with osteoporosis and previous vertebral racture | |||
| Harris, 199951 | Risedronate | Vertebral fracture | 20 women for 3 years |
| Black, 199652 | Alendronate | Vertebral fracture | 35 women for 3 years |
| Black, 199652 | Alendronate | Hip fracture | 86 women for 3 years |
Calcium and vitamin D
Calcium with or without vitamin D has been reported to positively affect fracture incidence.14 Vitamin D alone does not decrease the incidence of hip fractures.38 Calcium, 1200 to 1500 mg/day, and vitamin D, 800 IU/day, should be used concurrently with other forms of pharmacologic treatment. Calcium supplements are best absorbed with meals; for maximum absorption, calcium should be taken in doses of 500 mg or less.29,39
Minor gastrointestinal adverse effects may occur (most often constipation, 10%),14 which is often resolved by switching to a different preparation.40 Calcium in doses up to 1500 mg/day does not increase the risk for renal calculi and may, in fact, decrease risk.40,41 Calcium interferes with the absorption of certain medications, including tetracycline and quinolone antibiotics, which should be taken several hours apart from calcium. Calcium carbonate requires an acidic environment to dissolve. Patients taking stomach acid–suppressant therapy should use calcium citrate because it does not require an acidic environment for dissolution or should take their calcium supplement with meals. Traces of lead may be present in natural sources of calcium (bone meal, oyster shell, limestone, and dolomite), but can be avoided by use of over-the-counter calcium carbonate tablets (Tums).
Estrogen
Data from the Women’s Health Initiative (WHI) demonstrated that hormone replacement therapy (HRT) combining an estrogen and a progestin reduced hip and vertebral fractures by 1 in 2000 women per year and reduced all fractures by 1 in 333 women per year.42 Estrogen has a positive effect on BMD whether given in early or late postmenopause.43 Rapid bone loss as assessed by BMD does not occur after stopping HRT.44 However, in elderly women who have never used HRT, BMD is similar to those who have used it for 10 years and then stopped for at least 10 years.43 The effect of short-term (< 5 years) HRT during perimenopause on lifetime risk of osteoporotic fracture is unknown.
Common side effects (Table 2) can often be addressed by altering dosages, specific products, or regimens. The risk for breast cancer increases with duration of treatment and with combination HRT, compared with estrogen-only preparations.42,45,46 Combination HRT products are, however, essential for endometrial protection in women who have a uterus. The WHI reported an increase in breast cancer cases of 1 in 1250 women per year during an average 5-year follow-up.42 An increased risk for venous thromboembolism of 1 in 555 women per year was also observed with HRT use. Both the Heart and Estrogen/Progestin Study47 and the WHI studies found that venous thromboembolism occurred more frequently in the first 2 years of HRT use. An increased risk of myocardial infarction in the first 2 years of use was also noted among women with coronary heart disease47 and those without heart disease (1 in 1429 women per year).42 A small increase in stroke risk has also been documented.42,48-50 Contraindications to estrogen use include active thromboembolism, estrogen-related cancers, and liver disease.
Bisphosphonates
Two bisphosphonates, alendronate and risedronate, are approved in the United States for both prevention and treatment of postmenopausal osteoporosis. Clinical trials have demonstrated that both rapidly reduce the risk for symptomatic fractures in women with previous fracture and osteoporosis.37,51,52 The extent of fracture reduction is significant: Recent studies have shown that, over 3 years, the number of patients who would need to be treated with risedronate to prevent a vertebral fracture is 15, with alendronate, 34.53,54 Prevention of fractures among women without a prior vertebral fracture is less well established. No published data demonstrate that one bisphosphonate is more effective than another at preventing clinical fractures. A bisphosphonate is, therefore, the drug of choice for severe osteoporosis.
Bisphosphonates are generally well tolerated.55,56 However, postmarketing surveillance has demonstrated esophagitis and esophageal ulcer associated with alendronate.55 A pooled analysis of trials of risedronate found no increase in upper gastrointestinal (GI) adverse events even in patients with history of peptic ulcers, heartburn, and esophagitis, or among those taking nonsteroidal anti-inflammatory drugs, including aspirin.57
Oral bisphosphonates are not well absorbed (less than 1% of each dose).55,56 Therefore, to maximize absorption and to decrease the likelihood of adverse GI effects, the manufacturers of both bisphosphonates recommend that patients take the medication with a full glass of water, remain upright (sitting or standing) for at least 30 minutes following the dose, and not recline until food is consumed. Both bisphosphonates should be used with caution in patients with active GI disorders. Bisphosphonates are eliminated via the kidney and are not recommended for patients with a creatinine clearance below 30 mL/min.
Selective estrogen receptor modulators (SERMs)
Raloxifene is currently the only selective estrogen receptor modulator approved in the United States for prevention and treatment of osteoporosis. It has been shown to significantly decrease new vertebral fractures in women with a previous history of fracture and osteoporosis.58 The magnitude of fracture reduction is similar to that of bisphosphonates, although improvement in BMD is less marked.59 Raloxifene may confer other benefits. It has been shown to reduce the risk of breast cancer60,61 except in women with low estradiol levels,60 and may reduce the risk of myocardial infarction in women at high risk.60 Raloxifene does not increase the risk for endometrial cancer.60
Hot flashes and leg cramps are relatively common side effects of raloxifene.60 The observed risk of venous thromboembolism, 1 in 465 women per year during 3 years of treatment, is similar to that observed with HRT.60 Raloxifene is teratogenic and should not be used in premenopausal women.
Salmon calcitonin
Salmon calcitonin has demonstrated an analgesic effect for osteoporotic fracture.63,64 A large trial of salmon calcitonin at dosages of 100, 200, and 400 IU/day versus placebo found that salmon calcitonin at 200 IU/day decreased new vertebral fractures among women with a previous osteoporotic vertebral fracture based on radiographic assessment.65 No benefit was observed at the 100 and 400 IU/day dosages. The effect of calcitonin on clinical (symptomatic) fractures has not been reported. Calcitonin is approved for use in treatment, but not prevention, of osteoporosis.
Nasal calcitonin can cause minor rhinitis symptoms.30 Saline nasal solution may be useful to prevent or resolve irritation and dryness. Administration using alternate nostrils helps minimize local side effects. Unopened bottles (14 doses) must be stored in the refrigerator. Open bottles are stable at room temperature for up to 30 days.
Monitoring therapy
The value of serial densitometry to monitor the therapy of individual patients has not been established by randomized trials comparing different monitoring intervals or monitoring versus no monitoring.10,66 One important limitation is the relative imprecision of BMD testing: it takes almost a year to detect a 3% change in BMD.10 Disconcerting decreases in BMD scores are seen in yearly testing and may be offset by larger increases later, without a change in therapy. In studies of alendronate and raloxifene, disproportionately large fracture reductions cannot be explained by improvement in BMD alone.66,68 Bone densitometry is therefore not recommended until the patient has been treated for 2 years, and is of uncertain value beyond that point.
The last decade has witnessed important technological advances in the diagnosis of osteoporosis and an increase in therapeutic options. However, there is still considerable uncertainty about optimal strategies for screening and primary preventive treatment.
In 1994, a World Health Organization working group proposed that the diagnosis of osteoporosis be made when BMD, assessed by a dual-energy x-ray absorptiometry (DXA), is at least 2.5 standard deviations below the mean for young adult women (T-score) at the spine, hip, or wrist, or when a history of a traumatic fracture is present.2 A T-score between −1 and −2.5 is designated as osteopenia.
Osteoporosis is defined as “a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture.”1 While no accurate overall measurement of bone strength exists, bone mineral density (BMD) is frequently used as a proxy.
These facts underscore the importance of osteoporotic fractures:
- Only one third of patients regain their prior level of functioning after hip fracture, and one third are discharged to nursing homes.3
- About 1 in 5 patients dies within a year after a hip fracture.
- Vertebral fracture may result in chronic back pain and disability.4
- Existence of fracture greatly increases risk of subsequent fracture.5
- Direct medical costs for osteoporotic fractures are estimated at $13.8 billion in 1995 dollars.6
Prevalence of osteoporosis and fractures
Of American women over age 50 of all races, an estimated 15%, or 5 million, have osteoporosis (based on DXA T-score at the femoral neck) and an additional 40%, or 14 million, have osteopenia.7 In African Americans, the prevalence is about half that of whites.8 The prevalence of osteoporosis assessed by BMD testing increases with age—from 4% of white women aged 50 to 59 to 48% of women aged 80 to 89.9
At least 1 vertebral fracture, as indicated by radiographic criteria, has occurred in 5% of white women aged 50 to 59, and in 25% at age 80.3 The lifetime risk of hip fracture for 50-year-old white women and men is 14% and 5%, respectively; for African American women and men, 6% and 3%, respectively.3 Hip and symptomatic vertebral fractures occur mainly in women over 75,3,10 and the risk for wrist fractures increases starting in the late 50s.11
Age is a particularly important risk factor for hip fractures, reflecting deterioration in bone strength beyond that detectable with BMD testing. The National Osteoporosis Foundation12 observed that the 5-year risk of hip fracture for women with the same T-score (−3) increases dramatically with advancing age (Figure): from 2.4% at age 50 to 9.7% at age 90, with the steepest increase occurring during the 10 years between ages 70 (5.5%) and 80 (9%).
FIGURE
Five-year risk for hip fracture for women with T-score of −3 by age12
Bone mineral density testing
Screening recommendations
The clinical value of different screening strategies is not established, although recommendations have been made within guidelines and consensus statements that discuss prevention and treatment of osteoporosis. Guidelines are consistent in recommending that BMD screening be done only if results will influence treatment decisions. The US Preventive Services Task Force,13 The National Osteoporosis Foundation,14 and American Association of Clinical Endocrinologists15 recommend screening all women over 65, as well as younger women with risk factors for osteoporosis. The National Institutes of Health3 and the North American Menopause Society16 recommend an individualized decision-making approach to screening. The National Osteoporosis Foundation developed nomograms that integrate risk factors into decision-making for testing and treatment,12 which seem promising and merit testing in prospective studies.
Diagnostic testing
DXA. Although several technologies are available, DXA of the hip is considered the best predictor of hip fracture and an equivalent predictor of other fractures.10 The likelihood of making a diagnosis of osteoporosis based on BMD, however, varies and is related to type of test, equipment, anatomic site tested, number of sites tested, technique, and relevance of the reference range to the local population. For example, when the same group of people is tested with DXA equipment from different manufacturers, the proportion diagnosed with osteoporosis varies by as much as 15%.11
Quantitative ultrasound (QUS) and radiographic absorptiometry (RA). Testing by QUS of the heel and RA of the hand are less expensive than DXA and have become popular. While QUS of the heel has been shown to predict hip fracture and all nonvertebral fractures nearly as well as DXA,3,10 it does not highly correlate with DXA and appears to reflect other aspects of bone quality.10 Since QUS and DXA results frequently disagree and can cause confusion, DXA is the most appropriate test of BMD at present. If QUS and RA are used for screening, confirmation with DXA is recommended before therapy is initiated.
Calculations based on risk factors. In a comparison of strategies using risk factors to predict low BMD in postmenopausal women, 2 decision rules performed well: the Osteoporosis Risk Assessment Instrument, which is based on age and weight (Table 1),17 and the Simple Calculated Osteoporosis Risk Estimation (SCORE).17 Research to test these instruments with fracture rather than BMD as outcome is needed.18
Biochemical markers. Levels of markers in serum and/or urine reflect bone turnover and have potential use in diagnosing and monitoring therapy of osteoporosis. They are not yet widely available and have not been consistently associated with identifying patients at risk for fracture.10 They are not recommended at this time.
TABLE 1
Osteoporosis risk assessment instrument17
| Patient characteristic | Points |
|---|---|
| Age (years) | |
| 75 or older | 15 |
| 65 to 74 | 9 |
| 55 to 64 | 5 |
| 54 or younger | 0 |
| Weight | |
| <132 lb (60 kg) | 9 |
| 132 to 153.9 lb (60 to 70 kg) | 3 |
| >154 lb (>70 kg) | 0 |
| No current estrogen use | 2 |
| Total: | |
| Patients with a score of 9 or higher are at risk for diagnosis of osteoporosis by bone mineral density measurement. Sensitivity 97.5%, specificity 28%, positive predictive value 28%, negative predictive value 99.6%, given a 10% baseline risk of a bone mineral density 2.5 SD less than the mean. | |
Importance of primary prevention
At least half of bone strength is attributable to genetic factors12; modifiable factors may contribute almost equally as a group, and therefore warrant attention. Genetic risk factors include age, family history, female sex, low weight, small frame, and white or Asian race. Primary prevention efforts should begin in childhood and continue throughout the life span to maximize bone mass.3
Prevention efforts that target the modifiable factors described below should be a routine part of the health-maintenance visit.
Fall reduction
Falls are the direct cause of more than 90% of osteoporotic hip fractures,19 and the tendency to fall increases with age. Some studies have shown that, for women over age 70, the most important predictors of hip fractures are fall-related factors20,21 such as poor cognitive function, slow gait and otherwise impaired mobility, poor vision, drugs that impair alertness or balance, and history of falls. In women over 75, age and slow gait are equal to low BMD of the femoral neck as predictors of hip fracture.22 Unfortunately, labeling women as osteopenic or osteoporotic can cause fear of falling and lack of activity, leading to further acceleration of bone loss.10
Medications that interfere with balance or alertness should be avoided if possible. Environmental hazards such as loose rugs and uneven or slippery surfaces are also well-recognized modifiable risks for falls23,24 that should be eliminated. Hip protectors effectively reduce fractures in the frail elderly25 and can boost confidence for beneficial increases in physical activity levels,26 but they are often poorly accepted by patients.25,27 Other options include referral for gait training, home visits by a physician or nurse to identify problems in the home that increase the risk of falls, or providing information on home modification (such as installing bathtub rails, removing throw rugs, etc.).
Improvement of nutritional intake
Adequate consumption of calcium is essential for bone health. Calcium balance also can be adversely affected by dietary habits, including high intake of protein, phosphorus, and sodium, although these effects appear to be less important when dietary calcium is sufficient.3 The recommended calcium intake for postmenopausal women (1200–1500 mg/day)28 can be met with food sources, but supplements should be added if needed. Most postmenopausal women in the United States consume only about 600 mg/day.28 High-calcium foods include milk (290–300 mg/cup), sardines in oil, with bones (370 mg/3 oz), yogurt (300–500 mg depending on container size), cheese (165–270 mg/slice), canned salmon, with bones (170–210 mg/3 oz), broccoli (160–180 mg/cup), and tofu (144–155 mg/4 oz).15
Vitamin D is essential for intestinal absorption of calcium. The recommended intake for women is 400 IU/day for ages 51 to 70, 600 IU/day over age 70, and 800 IU/day for all high-risk women, including those who are homebound, institutionalized, on chronic glucocorticoids, or who live in northern latitudes and therefore have limited exposure to sunlight.29 Sources of vitamin D include sunlight, vitamin D–fortified foods, fish oils, and supplements. Multivitamins typically contain 400 IU of vitamin D.
Phytoestrogens, particularly in the form of soy products, have received attention for bone health. Overall, studies do not support the use of soy foods to prevent osteoporosis.3 A well-designed trial in postmenopausal women found that ipriflavone, a synthetic phytoestrogen, did not decrease bone loss.30 Furthermore, use was associated with subclinical lymphocytopenia.
Regular exercise
Weight-bearing physical activity such as walking or running in early life contributes to higher peak bone mass. Limited data suggest weight-bearing exercise in postmenopausal women produces small increases in bone density at the hip31 and improvement in balance and strength.32 For women with established osteoporosis, activities that place an anterior load on the vertebral bodies, such as forward flexion exercises, are associated with an increased incidence of new vertebral deformities, and patients should be advised to avoid them.33
Avoidance of adverse health habits
Current smoking, compared with never smoking, doubles the risk of hip fracture.34 Consumption of more than 1 alcoholic drink/day or more than 7/week is associated with osteoporosis and fracture, while moderate consumption of 1 drink/day or less is associated with decreased risk.35 Excessive caffeine intake is also associated with increased osteoporosis risk and should be avoided. This effect appears to result from substitution of calcium-containing beverages such as milk or fortified orange juice with caffeinated, non–calcium-containing beverages such as colas.
Treatment for fracture prevention and pain relief
The goals of therapy for osteoporosis are fracture prevention and pain relief to maximize physical function.15 Prior fracture is associated with a fivefold risk of future fractures.5 About 20% of women who experience a vertebral fracture have another fracture within 1 year.36 Currently available therapies (Table 2) are antiresorptive: they slow bone turnover and allow bone formation to exceed resorption. Trials of antiresorptive agents in elderly women with osteoporosis and baseline vertebral fracture demonstrate that 1 new vertebral fracture is prevented for each 12 to 35 women treated for 2 to 3 years.14,37Table 3 summarizes the results of key treatment studies and provides information on the number of women that need to be treated (NNT) for the study period to prevent 1 fracture.
TABLE 2
Drug therapy for prevention and treatment of postmenopausal osteoporosis
| Drug (trade name) | Indication and dosage | Possible side effects (% of patients) | Cost per month* |
|---|---|---|---|
| Calcium and vitamin D (generic, Tums, Citracal, and others) | Prevention and treatment: 1200–1500 mg/day calcium and 800 IU/day vitamin D | Nausea, dyspepsia (uncommon), constipation (10%) | $5 (both) |
| Estrogen† (Premarin, Ogen, Estrace, Estraderm, and others) | Prevention: 0.625 mg/day conjugated equine estrogen or the equivalent; 0.3 mg/day may be effective | Nausea, breast tenderness, vaginal bleeding, mood alterations, headache, bloating | $14–$28 |
| Alendronate (Fosamax) | Prevention and treatment: 5 mg/day or 35 mg/week | Nausea, dyspepsia esophageal irritation | $67 |
| Risedronate (Actonel) | Prevention and treatment: 5 mg/day or 35 mg/week | Abdominal pain, nausea, diarrhea | $67 |
| Raloxifene (Evista) | Treatment: 60 mg/day | Hot flashes (6%), leg cramps (3%) | $70 |
| Calcitonin nasal spray (Miacalcin) | Treatment: 200 IU/day (1 spray in 1 nostril per day) | Rhinitis (5%), epistaxis, sinusitis | $66 |
| *Average wholesale cost to the pharmacy for 30 days of therapy; (Drug Topics Red Book. Montvale, NJ; Medical Economics Co., Inc, 2002.) | |||
| †Women with a uterus need to take a progestin such as medroxyprogesterone acetate (Provera $30/month, generic $9/month) or a combination estrogen/progestin product (Prempro $33/month, FemHRT $26/month). | |||
TABLE 3
Clinical trials of drug therapy for the prevention of fracture in postmenopausal women with osteoporosis
| Trial, year | Therapy | Outcome prevented | Number needed to treat for n years |
|---|---|---|---|
| Elderly, postmenopausal women | |||
| Chapuy, 199267 | Calcium/vitamin D | Hip fracture | 48 women for 1.5 years |
| Postmenopausal women | |||
| WHI, 200242 | Hormone replacement therapy | Hip fracture | 2000 women for 5 years |
| Postmenopausal women with osteoporosis | |||
| Ettinger, 199956 | Raloxifene | Vertebral fracture | 29 women for 3 years |
| Liberman, 199554 | Alendronate | Vertebral fracture | 34 women for 3 years |
| Heaney, 200253 | Risendronate | Vertebral fracture | 15 women for 3 years |
| McClung, 200170 | Risedronate | Hip fracture | 91 women for 3 years |
| Postmenopausal women with osteoporosis and previous vertebral racture | |||
| Harris, 199951 | Risedronate | Vertebral fracture | 20 women for 3 years |
| Black, 199652 | Alendronate | Vertebral fracture | 35 women for 3 years |
| Black, 199652 | Alendronate | Hip fracture | 86 women for 3 years |
Calcium and vitamin D
Calcium with or without vitamin D has been reported to positively affect fracture incidence.14 Vitamin D alone does not decrease the incidence of hip fractures.38 Calcium, 1200 to 1500 mg/day, and vitamin D, 800 IU/day, should be used concurrently with other forms of pharmacologic treatment. Calcium supplements are best absorbed with meals; for maximum absorption, calcium should be taken in doses of 500 mg or less.29,39
Minor gastrointestinal adverse effects may occur (most often constipation, 10%),14 which is often resolved by switching to a different preparation.40 Calcium in doses up to 1500 mg/day does not increase the risk for renal calculi and may, in fact, decrease risk.40,41 Calcium interferes with the absorption of certain medications, including tetracycline and quinolone antibiotics, which should be taken several hours apart from calcium. Calcium carbonate requires an acidic environment to dissolve. Patients taking stomach acid–suppressant therapy should use calcium citrate because it does not require an acidic environment for dissolution or should take their calcium supplement with meals. Traces of lead may be present in natural sources of calcium (bone meal, oyster shell, limestone, and dolomite), but can be avoided by use of over-the-counter calcium carbonate tablets (Tums).
Estrogen
Data from the Women’s Health Initiative (WHI) demonstrated that hormone replacement therapy (HRT) combining an estrogen and a progestin reduced hip and vertebral fractures by 1 in 2000 women per year and reduced all fractures by 1 in 333 women per year.42 Estrogen has a positive effect on BMD whether given in early or late postmenopause.43 Rapid bone loss as assessed by BMD does not occur after stopping HRT.44 However, in elderly women who have never used HRT, BMD is similar to those who have used it for 10 years and then stopped for at least 10 years.43 The effect of short-term (< 5 years) HRT during perimenopause on lifetime risk of osteoporotic fracture is unknown.
Common side effects (Table 2) can often be addressed by altering dosages, specific products, or regimens. The risk for breast cancer increases with duration of treatment and with combination HRT, compared with estrogen-only preparations.42,45,46 Combination HRT products are, however, essential for endometrial protection in women who have a uterus. The WHI reported an increase in breast cancer cases of 1 in 1250 women per year during an average 5-year follow-up.42 An increased risk for venous thromboembolism of 1 in 555 women per year was also observed with HRT use. Both the Heart and Estrogen/Progestin Study47 and the WHI studies found that venous thromboembolism occurred more frequently in the first 2 years of HRT use. An increased risk of myocardial infarction in the first 2 years of use was also noted among women with coronary heart disease47 and those without heart disease (1 in 1429 women per year).42 A small increase in stroke risk has also been documented.42,48-50 Contraindications to estrogen use include active thromboembolism, estrogen-related cancers, and liver disease.
Bisphosphonates
Two bisphosphonates, alendronate and risedronate, are approved in the United States for both prevention and treatment of postmenopausal osteoporosis. Clinical trials have demonstrated that both rapidly reduce the risk for symptomatic fractures in women with previous fracture and osteoporosis.37,51,52 The extent of fracture reduction is significant: Recent studies have shown that, over 3 years, the number of patients who would need to be treated with risedronate to prevent a vertebral fracture is 15, with alendronate, 34.53,54 Prevention of fractures among women without a prior vertebral fracture is less well established. No published data demonstrate that one bisphosphonate is more effective than another at preventing clinical fractures. A bisphosphonate is, therefore, the drug of choice for severe osteoporosis.
Bisphosphonates are generally well tolerated.55,56 However, postmarketing surveillance has demonstrated esophagitis and esophageal ulcer associated with alendronate.55 A pooled analysis of trials of risedronate found no increase in upper gastrointestinal (GI) adverse events even in patients with history of peptic ulcers, heartburn, and esophagitis, or among those taking nonsteroidal anti-inflammatory drugs, including aspirin.57
Oral bisphosphonates are not well absorbed (less than 1% of each dose).55,56 Therefore, to maximize absorption and to decrease the likelihood of adverse GI effects, the manufacturers of both bisphosphonates recommend that patients take the medication with a full glass of water, remain upright (sitting or standing) for at least 30 minutes following the dose, and not recline until food is consumed. Both bisphosphonates should be used with caution in patients with active GI disorders. Bisphosphonates are eliminated via the kidney and are not recommended for patients with a creatinine clearance below 30 mL/min.
Selective estrogen receptor modulators (SERMs)
Raloxifene is currently the only selective estrogen receptor modulator approved in the United States for prevention and treatment of osteoporosis. It has been shown to significantly decrease new vertebral fractures in women with a previous history of fracture and osteoporosis.58 The magnitude of fracture reduction is similar to that of bisphosphonates, although improvement in BMD is less marked.59 Raloxifene may confer other benefits. It has been shown to reduce the risk of breast cancer60,61 except in women with low estradiol levels,60 and may reduce the risk of myocardial infarction in women at high risk.60 Raloxifene does not increase the risk for endometrial cancer.60
Hot flashes and leg cramps are relatively common side effects of raloxifene.60 The observed risk of venous thromboembolism, 1 in 465 women per year during 3 years of treatment, is similar to that observed with HRT.60 Raloxifene is teratogenic and should not be used in premenopausal women.
Salmon calcitonin
Salmon calcitonin has demonstrated an analgesic effect for osteoporotic fracture.63,64 A large trial of salmon calcitonin at dosages of 100, 200, and 400 IU/day versus placebo found that salmon calcitonin at 200 IU/day decreased new vertebral fractures among women with a previous osteoporotic vertebral fracture based on radiographic assessment.65 No benefit was observed at the 100 and 400 IU/day dosages. The effect of calcitonin on clinical (symptomatic) fractures has not been reported. Calcitonin is approved for use in treatment, but not prevention, of osteoporosis.
Nasal calcitonin can cause minor rhinitis symptoms.30 Saline nasal solution may be useful to prevent or resolve irritation and dryness. Administration using alternate nostrils helps minimize local side effects. Unopened bottles (14 doses) must be stored in the refrigerator. Open bottles are stable at room temperature for up to 30 days.
Monitoring therapy
The value of serial densitometry to monitor the therapy of individual patients has not been established by randomized trials comparing different monitoring intervals or monitoring versus no monitoring.10,66 One important limitation is the relative imprecision of BMD testing: it takes almost a year to detect a 3% change in BMD.10 Disconcerting decreases in BMD scores are seen in yearly testing and may be offset by larger increases later, without a change in therapy. In studies of alendronate and raloxifene, disproportionately large fracture reductions cannot be explained by improvement in BMD alone.66,68 Bone densitometry is therefore not recommended until the patient has been treated for 2 years, and is of uncertain value beyond that point.
1. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement Online 2000 March 27-29; [cited 9/2/2]; 17(1):1-36.
2. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843. Geneva: World Health Organization; 1994.
3. NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
4. Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(suppl 3):185S-189S.
5. Black DM, Arden NK, Palermo I, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821-828.
6. Ray NF, Chan JK, Thamer M, Melton LJ, 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24-35.
7. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468-489.
8. Aloia JF, Vaswani A, Yeh JK, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int. 1996;59:415-423.
9. Melton LJ. How many women have osteoporosis now? J Bone Miner Res. 1995;10:175-177.
10. Nelson HD, Morris CD, Mahon S, Carney N, Nygren PM, Helfand M. Osteoporosis in postmenopausal women: diagnosis and monitoring. Evidence Report/Technology Assessment Number 28. Rockville, MD: Agency for Healthcare Research and Quality; November 2001. 01-E032.
11. Melton LJ, 3rd, Amadio PC, Crowson CS, O’Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8:341-348.
12. National Osteoporosis Foundation. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Osteoporos Int. 1998;8(suppl):S7-S80.
13. U.S. Preventive Services Task Force. Screening for Osteoporosis in Postmenopausal Women. September 2002. Originally in Annals of Internal Medicine 2002;137:526-8.Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/3rduspstf/osteoporosis/osteorr.htm.
14. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 1998.
15. American Association of Clinical Endocrinologists 2001 medical guidelines for clinical practice for the prevention and management of osteoporosis. Endocr Pract. 2001;7:293-312.
16. North American Menopause Society. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
17. Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual energy x-ray absorptiometry. JAMA. 2001;286:57-63.
18. Wasnich RD. Consensus and the T-score fallacy. Clin Rheumatol. 1997;16:337-339.
19. Grisso JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of hip fracture in women.The Northeast Hip Fracture Study Group. N Engl J Med. 1991;324:1326-1331.
20. Cooper C, Barker DJ, Morris J, Briggs RS. Osteoporosis, falls, and age in fracture of the proximal femur. Br Med J (Clin Res Ed). 1987;295:13-15.
21. Gardsell P, Johnell O, Nilsson BE, Nilsson JA. The predictive value of fracture, disease, and falling tendency for fragility fractures in women. Calcif Tissue Int. 1989;45:327-330.
22. Dargent-Molina P, Schott AM, Hans D, et al. Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Epidemiologie de l’Osteoporose. Osteoporos Int. 1999;9:188-192.
23. Clemson L, Cumming RG, Roland M. Case-control study of hazards in the home and risk of falls and hip fractures. Age Ageing. 1996;25:97-101.
24. Norton R, Campbell AJ, Lee-Joe T, Robinson E, Butler M. Circumstances of falls resulting in hip fractures among older people. J Am Geriatr Soc 1997;45:1108-1112.
25. Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000;343:1506-1513.
26. Cameron ID, Stafford B, Cumming RG, et al. Hip protectors improve falls self-efficacy. Age Ageing. 2000;29:57-62.
27. Hubacher M, Wettstein A. Acceptance of hip protectors for hip fracture prevention in nursing homes. Osteoporos Int. 2001;12:794-799.
28. NIH Consensus Development Panel on Optimal Calcium Intake. Optimal Calcium Intake. JAMA. 1994;272:1942-1948.
29. Morgan SL. Calcium and vitamin D in osteoporosis. Rheum Dis Clin North Am. 2001;27:101-130.
30. Tsourounis C. Clinical effects of phytoestrogens. Clin Obstet Gynecol. 2001;44:836-842.
31. Kelley GA. Aerobic exercise and bone density at the hip in postmenopausal women: a meta-analysis. Prev Med. 1998;27:798-807.
32. Marcus R. Role of exercise in preventing and treating osteoporosis. Rheum Dis Clin North Am. 2001;27:131-141,vi.-
33. Sinaki M, Mikkelsen BA. Postmenopausal spinal osteoporosis: flexion versus extension exercises. Arch Phys Med Rehabil. 1984;65:593-596.
34. Law M, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of major effect. BMJ. 1997;315:841-846.
35. Felson DT, Zhang Y, Hannan MT, Kannel WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142:485-492.
36. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
37. Marcus R, Wong M, Heath H, 3rd, Stock JL. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocr Rev. 2002;23:16-37.
38. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124:400-406.
39. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77-84.
40. North American Menopause Society. The role of calcium in peri- and postmenopausal women; consensus opinion of The North American Menopause Society. Menopause. 2001;8:84-95.
41. Williams CP, Child DF, Hudson PR, et al. Why oral calcium supplements may reduce renal stone disease: report of a clinical pilot study. J Clin Pathol. 2001;54:54-62.
42. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results for the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-333.
43. Barrett-Connor E, Hendrix S, Ettinger B. International position paper on women’s health and menopause: a comprehensive approach. National Heart, Lung, and Blood Institute; 2002.
44. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
45. Schairer C, Lubin J, Triosi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485-491.
46. Ross R, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328-332.
47. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
48. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Annals d’Endocrinologie. 1999;60:232-241.
49. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen/Progestin Replacement Study (HERS). Circulation. 2001;103:638-642.
50. Viscoli C, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243-1249.
51. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352.
52. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
53. Heaney RP, Zizic TM, Fogelman I, et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int. 2002;13:501-505.
54. Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437-1443.
55. Sharpe M, Noble S, Spencer CM. Alendronate: an update of its use in osteoporosis. Drugs. 2001;61:999-1039.
56. Dunn CJ, Goa KL. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 2001;61:685-712.
57. Taggart H, Bolognese MA, Lindsay R, et al. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc. 2002;77:262-270.
58. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-645.
59. Sarkar S, Mitlak B, Wong M, Stock J, Black D, Harper K. Raloxifene-induced fracture reductions not directly associated with BMD changes. J Bone Miner Res. 2002;1:1-10.
60. Barrett-Connor E. Raloxifene: risks and benefits. Ann N Y Acad Sci. 2001;949:295-303.
61. Cummings S, Duong T, Kenyon E, Cauley J, Whitehead M, Krueger K. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
62. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287:847-857.
63. Lyritis GP, Paspati I, Karachalios T, Ioakimidis D, Skarantavos G, Lyritis PG. Pain relief from nasal salmon calcitonin in osteoporotic vertebral crush fractures. A double blind, placebo-controlled clinical study. Acta Orthop Scand Suppl. 1997;275:112-114.
64. Pun KK, Chan LW. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures. Clin Ther. 1989;11:205-209.
65. Chesnut CH, 3rd. Calcitonin in the prevention and treatment of osteoporosis. Osteoporos Int. 1993;3(suppl 1):206-207.
66. Crandall C. The role of serial bone mineral density testing for osteoporosis. J Womens Health Gend Based Med. 2001;10:887-895.
67. Cummings SR. The paradox of small changes in bone density and reductions in risk of fracture with raloxifene. Ann N Y Acad Sci. 2001;949:198-201.
68. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281-289.
69. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637-1642.
70. McClung M, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333-340.
1. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consensus Statement Online 2000 March 27-29; [cited 9/2/2]; 17(1):1-36.
2. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report Series 843. Geneva: World Health Organization; 1994.
3. NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785-795.
4. Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone. 1996;18(suppl 3):185S-189S.
5. Black DM, Arden NK, Palermo I, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821-828.
6. Ray NF, Chan JK, Thamer M, Melton LJ, 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12:24-35.
7. Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468-489.
8. Aloia JF, Vaswani A, Yeh JK, Flaster E. Risk for osteoporosis in black women. Calcif Tissue Int. 1996;59:415-423.
9. Melton LJ. How many women have osteoporosis now? J Bone Miner Res. 1995;10:175-177.
10. Nelson HD, Morris CD, Mahon S, Carney N, Nygren PM, Helfand M. Osteoporosis in postmenopausal women: diagnosis and monitoring. Evidence Report/Technology Assessment Number 28. Rockville, MD: Agency for Healthcare Research and Quality; November 2001. 01-E032.
11. Melton LJ, 3rd, Amadio PC, Crowson CS, O’Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8:341-348.
12. National Osteoporosis Foundation. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Osteoporos Int. 1998;8(suppl):S7-S80.
13. U.S. Preventive Services Task Force. Screening for Osteoporosis in Postmenopausal Women. September 2002. Originally in Annals of Internal Medicine 2002;137:526-8.Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/3rduspstf/osteoporosis/osteorr.htm.
14. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 1998.
15. American Association of Clinical Endocrinologists 2001 medical guidelines for clinical practice for the prevention and management of osteoporosis. Endocr Pract. 2001;7:293-312.
16. North American Menopause Society. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
17. Cadarette SM, Jaglal SB, Murray TM, McIsaac WJ, Joseph L, Brown JP. Evaluation of decision rules for referring women for bone densitometry by dual energy x-ray absorptiometry. JAMA. 2001;286:57-63.
18. Wasnich RD. Consensus and the T-score fallacy. Clin Rheumatol. 1997;16:337-339.
19. Grisso JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of hip fracture in women.The Northeast Hip Fracture Study Group. N Engl J Med. 1991;324:1326-1331.
20. Cooper C, Barker DJ, Morris J, Briggs RS. Osteoporosis, falls, and age in fracture of the proximal femur. Br Med J (Clin Res Ed). 1987;295:13-15.
21. Gardsell P, Johnell O, Nilsson BE, Nilsson JA. The predictive value of fracture, disease, and falling tendency for fragility fractures in women. Calcif Tissue Int. 1989;45:327-330.
22. Dargent-Molina P, Schott AM, Hans D, et al. Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Epidemiologie de l’Osteoporose. Osteoporos Int. 1999;9:188-192.
23. Clemson L, Cumming RG, Roland M. Case-control study of hazards in the home and risk of falls and hip fractures. Age Ageing. 1996;25:97-101.
24. Norton R, Campbell AJ, Lee-Joe T, Robinson E, Butler M. Circumstances of falls resulting in hip fractures among older people. J Am Geriatr Soc 1997;45:1108-1112.
25. Kannus P, Parkkari J, Niemi S, et al. Prevention of hip fracture in elderly people with use of a hip protector. N Engl J Med. 2000;343:1506-1513.
26. Cameron ID, Stafford B, Cumming RG, et al. Hip protectors improve falls self-efficacy. Age Ageing. 2000;29:57-62.
27. Hubacher M, Wettstein A. Acceptance of hip protectors for hip fracture prevention in nursing homes. Osteoporos Int. 2001;12:794-799.
28. NIH Consensus Development Panel on Optimal Calcium Intake. Optimal Calcium Intake. JAMA. 1994;272:1942-1948.
29. Morgan SL. Calcium and vitamin D in osteoporosis. Rheum Dis Clin North Am. 2001;27:101-130.
30. Tsourounis C. Clinical effects of phytoestrogens. Clin Obstet Gynecol. 2001;44:836-842.
31. Kelley GA. Aerobic exercise and bone density at the hip in postmenopausal women: a meta-analysis. Prev Med. 1998;27:798-807.
32. Marcus R. Role of exercise in preventing and treating osteoporosis. Rheum Dis Clin North Am. 2001;27:131-141,vi.-
33. Sinaki M, Mikkelsen BA. Postmenopausal spinal osteoporosis: flexion versus extension exercises. Arch Phys Med Rehabil. 1984;65:593-596.
34. Law M, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of major effect. BMJ. 1997;315:841-846.
35. Felson DT, Zhang Y, Hannan MT, Kannel WB, Kiel DP. Alcohol intake and bone mineral density in elderly men and women. The Framingham Study. Am J Epidemiol. 1995;142:485-492.
36. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320-323.
37. Marcus R, Wong M, Heath H, 3rd, Stock JL. Antiresorptive treatment of postmenopausal osteoporosis: comparison of study designs and outcomes in large clinical trials with fracture as an endpoint. Endocr Rev. 2002;23:16-37.
38. Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124:400-406.
39. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med. 2002;346:77-84.
40. North American Menopause Society. The role of calcium in peri- and postmenopausal women; consensus opinion of The North American Menopause Society. Menopause. 2001;8:84-95.
41. Williams CP, Child DF, Hudson PR, et al. Why oral calcium supplements may reduce renal stone disease: report of a clinical pilot study. J Clin Pathol. 2001;54:54-62.
42. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results for the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321-333.
43. Barrett-Connor E, Hendrix S, Ettinger B. International position paper on women’s health and menopause: a comprehensive approach. National Heart, Lung, and Blood Institute; 2002.
44. Greendale GA, Espeland M, Slone S, Marcus R, Barrett-Connor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
45. Schairer C, Lubin J, Triosi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485-491.
46. Ross R, Paganini-Hill A, Wan PC, Pike MC. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst. 2000;92:328-332.
47. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605-613.
48. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Annals d’Endocrinologie. 1999;60:232-241.
49. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen/Progestin Replacement Study (HERS). Circulation. 2001;103:638-642.
50. Viscoli C, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243-1249.
51. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344-1352.
52. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535-1541.
53. Heaney RP, Zizic TM, Fogelman I, et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int. 2002;13:501-505.
54. Liberman UA, Weiss SR, Broll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995;333:1437-1443.
55. Sharpe M, Noble S, Spencer CM. Alendronate: an update of its use in osteoporosis. Drugs. 2001;61:999-1039.
56. Dunn CJ, Goa KL. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 2001;61:685-712.
57. Taggart H, Bolognese MA, Lindsay R, et al. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc. 2002;77:262-270.
58. Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637-645.
59. Sarkar S, Mitlak B, Wong M, Stock J, Black D, Harper K. Raloxifene-induced fracture reductions not directly associated with BMD changes. J Bone Miner Res. 2002;1:1-10.
60. Barrett-Connor E. Raloxifene: risks and benefits. Ann N Y Acad Sci. 2001;949:295-303.
61. Cummings S, Duong T, Kenyon E, Cauley J, Whitehead M, Krueger K. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216-220.
62. Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002;287:847-857.
63. Lyritis GP, Paspati I, Karachalios T, Ioakimidis D, Skarantavos G, Lyritis PG. Pain relief from nasal salmon calcitonin in osteoporotic vertebral crush fractures. A double blind, placebo-controlled clinical study. Acta Orthop Scand Suppl. 1997;275:112-114.
64. Pun KK, Chan LW. Analgesic effect of intranasal salmon calcitonin in the treatment of osteoporotic vertebral fractures. Clin Ther. 1989;11:205-209.
65. Chesnut CH, 3rd. Calcitonin in the prevention and treatment of osteoporosis. Osteoporos Int. 1993;3(suppl 1):206-207.
66. Crandall C. The role of serial bone mineral density testing for osteoporosis. J Womens Health Gend Based Med. 2001;10:887-895.
67. Cummings SR. The paradox of small changes in bone density and reductions in risk of fracture with raloxifene. Ann N Y Acad Sci. 2001;949:198-201.
68. Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med. 2002;112:281-289.
69. Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327:1637-1642.
70. McClung M, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333-340.
What is the most effective treatment for external genital warts?
Podofilox (Condylox), imiquimod (Aldara), cryotherapy, and surgical options all seem reasonable alternatives that are superior to podophyllin. (Grade of recommendation: B, based on systematic review.) No studies of surgical options versus home use preparations have been reported. Trichloroacetic acid and 5-fluorouracil (5-FU) have not been sufficiently studied.
Evidence summary
Nonsurgical treatments that are beneficial in eradicating genital warts are podofilox (Condylox) (8 randomized controlled trials [RCTs] with 1035 participants), imiquimod (Aldara) (2 RCTs with 968 participants), and intralesional interferon (8 RCTs). Cryotherapy is equivalent to trichloroacetic acid1,2 and electrosurgery.3 Although surgical treatments have not been compared with placebo or no treatment, both electrosurgery and surgical excision are superior to podophyllin in clinical trials.4,5 Laser surgery is as effective as surgical excision.6 Studies of topical interferon show conflicting results.7 Systemic interferon is not beneficial.7 Topical 5-FU has not been studied with RCTs. Wart clearance rates are summarized in the Table. Treatment duration for nonsurgical options is 4 to 8 weeks. Treatment of genital warts has not been shown to reduce transmission to sex partners.7
Two RCTs4,5 showed more frequent recurrence with podophyllin (60% to 65%) than with surgical excision (19% to 20%). Another trial1 showed recurrence in 22% of participants receiving electrosurgery, in 21% of those receiving cryotherapy, and in 44% of those receiving podophyllin treatment. Data are lacking on recurrence rates with imiquimod, podofilox, and intralesional interferon.
Pain occurs in less than 20% of people with imiquimod, cryotherapy, podophyllin, and electrosurgery; 39% with topical interferon; 44% with electrosurgery; 75% with podofilox; and 100% with surgical excision or laser surgery.7 However, pain has been measured using methods that are unlikely to be comparable across studies. Flulike symptoms, leukopenia, thrombocytopenia, and elevated aspartate transaminase levels are associated with intralesional interferon.7 Topical medications have not been studied in pregnant patients. Cryotherapy is safe in pregnancy based on case series, if only 3 or 4 treatments are given.7
Direct comparisons between home therapies (imiquimod, podofilox) and other treatments are needed. Products for home use are relatively expensive: a 1-month supply of imiquimod costs approximately $150; a 1-month supply of podofilox, $110 to $130. These are average wholesale prices, rounded to the nearest $10, as of Feb. 15, 2002.
TABLE
CLEARANCE RATES REPORTED IN CLINICAL TRIALS
| Therapy | Clearance Rate (%) |
|---|---|
| Cryotherapy | 63–88 |
| Electrosurgery | 61–94 |
| Imiquimod | 37–56 |
| Interferon (topical) | 6–90 |
| Interferon (intralesional) | 17–63 |
| Laser surgery | 23–52 |
| Podofilox | 45–77 |
| Podophyllin | 32–79 |
| Surgical excision | 35–72 |
| Trichloroacetic acid | 50–81 |
| Placebo or no treatment | 0–56 |
Recommendations from others
The CDC endorses podophyllin, bi- and trichloroacetic acid, podofilox, imiquimod, cryotherapy, intralesional interferon, electrosurgery, laser surgery, and surgical excision.8 A United Kingdom guideline on anogenital warts recommends physical ablative methods such as cryotherapy and surgical options for keratinized lesions and topical medications for soft lesions. The guideline also recommends ablative therapy for persons with a small number of warts regardless of type. Interferon and 5-FU are not recommended.9
Clinical Commentary by David White, MD, at http://www.fpin.org.
1. Abdullah AN, Walzman M, Wade A. Sex Transm Dis 1993;20:344-5.
2. Godley MJ, Bradbeer CS, Gellan M, Thin RN. Genitourin Med 1987;63:390-2.
3. Stone KM, Becker TM, Hadgu A, Kraus SJ. Genitourin Med 1990;66:16-9.
4. Khawaja HT. J Reprod Med 1990;35:1019-22.
5. Jensen SL. Lancet 1985;2:1146-8.
6. Duus BR, Philipsen T, Christensen JD, et al. Genitourin Med 1985;61:59-61.
7. Wiley DJ. Genital warts. Clin Evidence Issue 4, December 2000;910-8.
8. Centers for Disease Control and Prevention. Morbid Mortal Weekly Rep MMWR 1998;47(RR-1):91-4.
9. Clinical Effectiveness Group (Association of Genitourinary Medicine and the Medical Society for the Study of Venereal Diseases). Sex Transm Infect 1999;75(suppl 1):71-5S.
Podofilox (Condylox), imiquimod (Aldara), cryotherapy, and surgical options all seem reasonable alternatives that are superior to podophyllin. (Grade of recommendation: B, based on systematic review.) No studies of surgical options versus home use preparations have been reported. Trichloroacetic acid and 5-fluorouracil (5-FU) have not been sufficiently studied.
Evidence summary
Nonsurgical treatments that are beneficial in eradicating genital warts are podofilox (Condylox) (8 randomized controlled trials [RCTs] with 1035 participants), imiquimod (Aldara) (2 RCTs with 968 participants), and intralesional interferon (8 RCTs). Cryotherapy is equivalent to trichloroacetic acid1,2 and electrosurgery.3 Although surgical treatments have not been compared with placebo or no treatment, both electrosurgery and surgical excision are superior to podophyllin in clinical trials.4,5 Laser surgery is as effective as surgical excision.6 Studies of topical interferon show conflicting results.7 Systemic interferon is not beneficial.7 Topical 5-FU has not been studied with RCTs. Wart clearance rates are summarized in the Table. Treatment duration for nonsurgical options is 4 to 8 weeks. Treatment of genital warts has not been shown to reduce transmission to sex partners.7
Two RCTs4,5 showed more frequent recurrence with podophyllin (60% to 65%) than with surgical excision (19% to 20%). Another trial1 showed recurrence in 22% of participants receiving electrosurgery, in 21% of those receiving cryotherapy, and in 44% of those receiving podophyllin treatment. Data are lacking on recurrence rates with imiquimod, podofilox, and intralesional interferon.
Pain occurs in less than 20% of people with imiquimod, cryotherapy, podophyllin, and electrosurgery; 39% with topical interferon; 44% with electrosurgery; 75% with podofilox; and 100% with surgical excision or laser surgery.7 However, pain has been measured using methods that are unlikely to be comparable across studies. Flulike symptoms, leukopenia, thrombocytopenia, and elevated aspartate transaminase levels are associated with intralesional interferon.7 Topical medications have not been studied in pregnant patients. Cryotherapy is safe in pregnancy based on case series, if only 3 or 4 treatments are given.7
Direct comparisons between home therapies (imiquimod, podofilox) and other treatments are needed. Products for home use are relatively expensive: a 1-month supply of imiquimod costs approximately $150; a 1-month supply of podofilox, $110 to $130. These are average wholesale prices, rounded to the nearest $10, as of Feb. 15, 2002.
TABLE
CLEARANCE RATES REPORTED IN CLINICAL TRIALS
| Therapy | Clearance Rate (%) |
|---|---|
| Cryotherapy | 63–88 |
| Electrosurgery | 61–94 |
| Imiquimod | 37–56 |
| Interferon (topical) | 6–90 |
| Interferon (intralesional) | 17–63 |
| Laser surgery | 23–52 |
| Podofilox | 45–77 |
| Podophyllin | 32–79 |
| Surgical excision | 35–72 |
| Trichloroacetic acid | 50–81 |
| Placebo or no treatment | 0–56 |
Recommendations from others
The CDC endorses podophyllin, bi- and trichloroacetic acid, podofilox, imiquimod, cryotherapy, intralesional interferon, electrosurgery, laser surgery, and surgical excision.8 A United Kingdom guideline on anogenital warts recommends physical ablative methods such as cryotherapy and surgical options for keratinized lesions and topical medications for soft lesions. The guideline also recommends ablative therapy for persons with a small number of warts regardless of type. Interferon and 5-FU are not recommended.9
Clinical Commentary by David White, MD, at http://www.fpin.org.
Podofilox (Condylox), imiquimod (Aldara), cryotherapy, and surgical options all seem reasonable alternatives that are superior to podophyllin. (Grade of recommendation: B, based on systematic review.) No studies of surgical options versus home use preparations have been reported. Trichloroacetic acid and 5-fluorouracil (5-FU) have not been sufficiently studied.
Evidence summary
Nonsurgical treatments that are beneficial in eradicating genital warts are podofilox (Condylox) (8 randomized controlled trials [RCTs] with 1035 participants), imiquimod (Aldara) (2 RCTs with 968 participants), and intralesional interferon (8 RCTs). Cryotherapy is equivalent to trichloroacetic acid1,2 and electrosurgery.3 Although surgical treatments have not been compared with placebo or no treatment, both electrosurgery and surgical excision are superior to podophyllin in clinical trials.4,5 Laser surgery is as effective as surgical excision.6 Studies of topical interferon show conflicting results.7 Systemic interferon is not beneficial.7 Topical 5-FU has not been studied with RCTs. Wart clearance rates are summarized in the Table. Treatment duration for nonsurgical options is 4 to 8 weeks. Treatment of genital warts has not been shown to reduce transmission to sex partners.7
Two RCTs4,5 showed more frequent recurrence with podophyllin (60% to 65%) than with surgical excision (19% to 20%). Another trial1 showed recurrence in 22% of participants receiving electrosurgery, in 21% of those receiving cryotherapy, and in 44% of those receiving podophyllin treatment. Data are lacking on recurrence rates with imiquimod, podofilox, and intralesional interferon.
Pain occurs in less than 20% of people with imiquimod, cryotherapy, podophyllin, and electrosurgery; 39% with topical interferon; 44% with electrosurgery; 75% with podofilox; and 100% with surgical excision or laser surgery.7 However, pain has been measured using methods that are unlikely to be comparable across studies. Flulike symptoms, leukopenia, thrombocytopenia, and elevated aspartate transaminase levels are associated with intralesional interferon.7 Topical medications have not been studied in pregnant patients. Cryotherapy is safe in pregnancy based on case series, if only 3 or 4 treatments are given.7
Direct comparisons between home therapies (imiquimod, podofilox) and other treatments are needed. Products for home use are relatively expensive: a 1-month supply of imiquimod costs approximately $150; a 1-month supply of podofilox, $110 to $130. These are average wholesale prices, rounded to the nearest $10, as of Feb. 15, 2002.
TABLE
CLEARANCE RATES REPORTED IN CLINICAL TRIALS
| Therapy | Clearance Rate (%) |
|---|---|
| Cryotherapy | 63–88 |
| Electrosurgery | 61–94 |
| Imiquimod | 37–56 |
| Interferon (topical) | 6–90 |
| Interferon (intralesional) | 17–63 |
| Laser surgery | 23–52 |
| Podofilox | 45–77 |
| Podophyllin | 32–79 |
| Surgical excision | 35–72 |
| Trichloroacetic acid | 50–81 |
| Placebo or no treatment | 0–56 |
Recommendations from others
The CDC endorses podophyllin, bi- and trichloroacetic acid, podofilox, imiquimod, cryotherapy, intralesional interferon, electrosurgery, laser surgery, and surgical excision.8 A United Kingdom guideline on anogenital warts recommends physical ablative methods such as cryotherapy and surgical options for keratinized lesions and topical medications for soft lesions. The guideline also recommends ablative therapy for persons with a small number of warts regardless of type. Interferon and 5-FU are not recommended.9
Clinical Commentary by David White, MD, at http://www.fpin.org.
1. Abdullah AN, Walzman M, Wade A. Sex Transm Dis 1993;20:344-5.
2. Godley MJ, Bradbeer CS, Gellan M, Thin RN. Genitourin Med 1987;63:390-2.
3. Stone KM, Becker TM, Hadgu A, Kraus SJ. Genitourin Med 1990;66:16-9.
4. Khawaja HT. J Reprod Med 1990;35:1019-22.
5. Jensen SL. Lancet 1985;2:1146-8.
6. Duus BR, Philipsen T, Christensen JD, et al. Genitourin Med 1985;61:59-61.
7. Wiley DJ. Genital warts. Clin Evidence Issue 4, December 2000;910-8.
8. Centers for Disease Control and Prevention. Morbid Mortal Weekly Rep MMWR 1998;47(RR-1):91-4.
9. Clinical Effectiveness Group (Association of Genitourinary Medicine and the Medical Society for the Study of Venereal Diseases). Sex Transm Infect 1999;75(suppl 1):71-5S.
1. Abdullah AN, Walzman M, Wade A. Sex Transm Dis 1993;20:344-5.
2. Godley MJ, Bradbeer CS, Gellan M, Thin RN. Genitourin Med 1987;63:390-2.
3. Stone KM, Becker TM, Hadgu A, Kraus SJ. Genitourin Med 1990;66:16-9.
4. Khawaja HT. J Reprod Med 1990;35:1019-22.
5. Jensen SL. Lancet 1985;2:1146-8.
6. Duus BR, Philipsen T, Christensen JD, et al. Genitourin Med 1985;61:59-61.
7. Wiley DJ. Genital warts. Clin Evidence Issue 4, December 2000;910-8.
8. Centers for Disease Control and Prevention. Morbid Mortal Weekly Rep MMWR 1998;47(RR-1):91-4.
9. Clinical Effectiveness Group (Association of Genitourinary Medicine and the Medical Society for the Study of Venereal Diseases). Sex Transm Infect 1999;75(suppl 1):71-5S.
Evidence-based answers from the Family Physicians Inquiries Network
Approach to the Perimenopausal Patient
- Laboratory testing is not indicated to initiate treatment of perimenopausal symptoms.
- While estrogens are the best established of the options to treat vasomotor symptoms at perimenopause, they are not a proven treatment for major depression or poor libido.
- Little evidence exists regarding the benefits and risks of androgens for perimenopausal women, suggesting a cautious approach to their use.
- Routine use of hormone replacement therapy, especially beyond 5 years’ duration, is not recommended because of uncertainties regarding risks and benefits.
Menopause has been successfully promoted as an estrogen-deficient state. Prescriptions in the United States for noncontraceptive estrogen formulations increased from 16 million to 39 million between 1982 and 1992; progestin sales reached 4.7 million by 1992 after their introduction in 1986.1 A condition for which half of the population becomes eligible for pharmacologic treatment for 30 years or more of their life spans is worthy of family physicians’ attention. Counseling of women regarding menopause has also been incorporated into the Health Employer Data Information Set (HEDIS) for measuring the quality of care provided by health care plans.
The women of the generation born from 1946 to 1965 are now 36 to 55 years old. About half will at some time seek medical attention for relief of symptoms believed to be related to the menopausal transition.2 The clinical picture, however, can be confusing: women at midlife are susceptible to diseases that may affect or be affected by the menopausal transition. Life cycle changes can also provoke dysphoric symptoms similar to those of menopause or aggravate symptoms that already exist.
Natural history
A woman’s hormonal rhythm changes gradually, usually in the early to middle forties. Ovarian mass decreases progressively; production of ovarian hormones decreases as well. The menstrual cycles tend to be somewhat shorter. Follicle-stimulating hormone (FSH) and estrogen levels fluctuate. Estrogen levels may be transiently higher than in former years in response to higher FSH levels, recruiting more ovarian follicles. Anovulatory cycles are more frequent. Perimenopausal menstrual irregularity typically lasts for approximately 4 years; the large majority of women experience such irregularity for 1 to 7 years.2 For 10% of women, menses simply cease without prior menstrual irregularity.
The best estimate of mean age at menopause in the United States, based on a cohort of primarily Caucasian women, is 51.3 years.2 Smokers experience menopause 1.8 years earlier than nonsmokers (50.2 versus 52.0 years). Less than 10% of women reach menopause before age 46, while approximately 30% do so before age 50.2 A recent review3 concluded that the lifetime number of ovulatory cycles is predictive of age at menopause: earlier for women with shorter cycles and nulliparous women, later for multigravid women and those with a history of oral contraceptive use. A familial tendency toward similarity in age at menopause has been noted.
Premature menopause or premature ovarian failure is defined as cessation of menstrual periods before 40 years of age. The prevalence of premature ovarian failure is approximately 1% by age 40 and 0.1% by 30 years of age.4 Premature ovarian failure is frequently an autoimmune disorder.5
Diagnosis of menopause
The gold standard for diagnosing menopause is to do so retrospectively, 1 year after the last menstrual period. In general, a diagnosis of menopause based on menstrual history or hormone levels is not considered necessary to begin treatment for perimenopausal symptoms, which often begin several years before the onset of menopause.
Laboratory diagnosis
The extent to which FSH or other serologic markers can be used to diagnose menopause is controversial. The most important clinical reason to do so is to discontinue contraceptive methods safely. Some consider an FSH level greater than 40 mIU/mL to be diagnostic. This value was chosen because it is about 2 standard deviations above the periovulatory peak in FSH levels in regularly cycling women. However, longitudinal studies6,7 during the perimenopausal years have demonstrated that hormonal patterns that include FSH values greater than 40 mIU/mL often abruptly revert to premenopausal patterns and are accompanied by ovulatory cycles. For the individual patient, hormone levels do not appear to rule out fertility reliably.8 Studies defining test characteristics (sensitivity, specificity, likelihood ratios) of hormone assays for the diagnosis of menopause are needed.
History and physical examination
A large population-based survey of Swedish women9 found that the most common climacteric symptoms are, in order of frequency, vasomotor symptoms (hot flashes), mood disturbances, sleep disturbances, decreased libido, and vaginal dryness. Several observational studies10-13 have shown that vasomotor symptoms have the clearest temporal association with the menstrual cycle changes of the climacteric. These symptoms result from a sudden change in the hypothalamic control of temperature regulation,14 although the precise triggers have not been elucidated. Hot flashes occur commonly among women in their late thirties and forties who have regular menstrual cycles.15 Several studies2,10,13,16 have shown that the prevalence of hot flashes peaks in the year immediately following the final menstrual period. A typical pattern prevalence of hot flashes is 25% in premenopausal women, 69% in perimenopausal women, and 39% in late-postmenopausal women (more than 4.5 years).17 Fifteen years after menopause, 10% of women may continue to have moderate to severe hot flashes,18 which can be lifelong.
Irritability and mood swings are common climacteric complaints. Women often compare them with their earlier premenstrual symptoms. Studies of depressive symptoms in menopausal women indicate that menopause is not associated with increased rates of major depression.19 Stressful life context and poor health status appear to be more important risk factors for depression than symptoms of menopause in climacteric women.20
Many perimenopausal women complain of poor sleep, often attributed to nocturnal hot flashes. Subjective impairment of sleep quality that is associated with climacteric vasomotor symptoms does not manifest as abnormalities in polysomnographic sleep recordings.21 It does not appear to be related to sleep apnea.
Sexual dysfunction is common in women at midlife and beyond. Dyspareunia, associated with vaginal dryness, increases in frequency with increasing time after menopause.9 The other complaint is decreased libido. Multiple factors may contribute to lack of sexual interest. Both aging and the menopause are independently associated with decreases in sexual responsiveness.22 The roles of declining endogenous sex steroid hormones in this process have not been elucidated.
Treatment
Vasomotor symptoms
Table 1 summarizes treatment options for vasomotor symptoms. Numerous well-designed clinical trials have demonstrated the effectiveness of oral or transdermal estrogen replacement therapy (ERT) for hot flashes.18,23-25 Low-dose oral contraceptive formulations are approved until 50 years of age for nonsmoking women.26 In a well-designed randomized controlled trial (RCT) of 93 women, low-dose estrogen (0.625 mg conjugated equine estrogens daily) plus 1.25 mg methyltestosterone daily was shown to be more effective than low-dose estrogen only and as effective as high-dose estrogen (1.25 mg conjugated estrogens daily).27
Phytoestrogens may be helpful, but have not yet been studied extensively. One RCT28 of 104 postmenopausal women comparing ingestion of 60 g soy protein daily with that of 60 g casein (placebo) daily showed a 45% relative reduction of hot flashes at 12 weeks in the group taking soy versus the control group. A second RCT29 of 51 women comparing soy protein with carbohydrate placebo showed a decrease in severity, but not frequency, of hot flashes. Another well-designed RCT30 including 69 women treated with 40 g soy daily versus whey protein for 24 weeks showed no difference between treatment groups and improvement in symptom scores over time in both groups. It is difficult to include a 40-g to 60-g protein supplement in the daily diet because of the accompanying caloric intake required. Recent reports of randomized placebo-controlled trials of black cohosh31 and dong quai32 and a systematic review33 of controlled trials of red clover have found no benefit.
Alternatives to estrogen for treatment of hot flashes include methyldopa, clonidine, transdermal progesterone, and megestrol acetate. Megestrol, which reduces symptoms by 70%, appears to be the most effective of these.34 Although long-term use of megestrol acetate by cancer survivors for the treatment of hot flashes has been demonstrated to be effective and well tolerated,35 it is not customarily used at menopause. A 20% reduction in hot flashes can be expected with clonidine at a dose of 0.1 to 0.2 mg daily,34,36 although this regimen may cause an increase in difficulty sleeping37 as well as dry mouth, constipation, and low blood pressure. Transdermal progesterone cream alone has been shown to improve vasomotor symptoms, although without protective effect regarding bone loss.38 One small study39 of behavioral approaches showed symptom reduction with deep-breathing relaxation techniques. Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 show promise in the treatment of hot flashes.
The remainder of this article focuses on hormonal treatment effects and risks for menopausal women. A summary appears in Table 2.
TABLE 1
TREATMENT OF VASOMOTOR SYMPTOMS
| Strength of Recommendation | Treatment | Comment |
|---|---|---|
| A | Estrogens | Many preparations with both oral and transdermal delivery have been studied |
| A | Estrogen + MPA | Other progestins not well studied |
| B | Transdermal progesterone | One RCT38 |
| B | Estrogen + testosterone | One RCT27; long-term safety is a theoretical concern |
| B | Megasterol | Cohort35; long experience with cancer patients gives some assurance of safety |
| C | Behavioral approaches | One small RCT39; deep breathing was beneficial |
| C | Clonidine | Small RCTs36,37 with important loss of subjects because of side effects |
| D | Antidepressants | Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 |
| D | Phytoestrogens | Conflicting RCT results |
| D | Exercise | Weak observational studies suggest benefit74,76 |
| No benefit seen | ||
| B | Black cohosh | No benefit seen in one RCT31 |
| B | Dong quai | No benefit seen in one RCT32 |
| B | Red clover | No benefit seen in systematic review33 |
| Grades of recommendation are based on Oxford Centre for Evidence-Based Medicine guidelines. | ||
| MPA denotes medroxyprogesterone; RCT, randomized clinical trial. | ||
TABLE 2
SUMMARY OF RISKS AND BENEFITS OF TREATMENTS FOR PERIMENOPAUSAL SYMPTOMS
| Treatment | VMS | Mood | Libido | Bone | CAD | Breast CA |
|---|---|---|---|---|---|---|
| Estrogens | Benefit | Benefit | No benefit | Benefit | Uncertain | Risk |
| Estrogen + MPA | Benefit | ? benefit | No benefit | Benefit | Uncertain* | Risk† |
| Progesterone | Benefit | Risk | No benefit | NS | Uncertain | NS |
| Testosterone | Benefit | NS | ? benefit | Benefit | NS | NS |
| Phytoestrogens | ? benefit | NS | NS | No benefit | NS | NS |
| DHEA | NS | ? benefit | ? benefit | NS | NS | NS |
| * Not beneficial for secondary prevention. † Increased risk over estrogen alone. | ||||||
| CA denotes cancer; CAD, coronary artery disease; DHEA, dihydroepiandrosterone; MPA, medroxyprogesterone acetate; NS, not studied; VMS, vasomotor symptoms. | ||||||
Mood disorders
In a meta-analysis43 including 26 RCTs of the effects of hormone replacement therapy (HRT) on depressed mood, estrogen showed limited effectiveness in improving mood. The addition of synthetic progestins reduced the estrogen effect. More recent short trials of unopposed transdermal estrogen showed benefit.44,45 Other reviews46,47 have concluded that ERT or HRT has little effect in the treatment of psychological symptoms, including anxiety, cognitive, and affective symptoms. As an adjuvant to psychotropic therapy, it may have limited effect. There is insufficient evidence to support prophylactic ERT or HRT to prevent depression in women whose medical history includes prior postpartum depression.47 Estrogens do not affect the ability of a woman with moderate to severe vasomotor symptoms to cope with stress.48 Clinical trials reporting the effects of testosterone treatment on mood in women were not identified.
Women with mild psychological and predominantly vasomotor symptoms may benefit from a trial of HRT before psychotropic medication. For women who meet criteria for a diagnosis of major depression, initial treatment with an antidepressant alone or concurrent with HRT is advisable.
Sleep disturbance
In a survey of more than 6000 women aged 40 to 64 years, 30% of HRT users reported sleep improvement that they attributed to therapy.49 Other standard approaches to insomnia, such as sleep hygiene measures and progressive relaxation techniques, can also be used. If sleep apnea is suspected, a sleep study may be indicated.
Sexual dysfunction
In a systematic review50 of HRT for climacteric sexual dysfunction, vaginal dryness improved with ERT in 7 of 8 studies. Dyspareunia improved in only 1 of 6 studies using transdermal 17-beta-estradiol. Orgasm increased in only 1 of 5 trials using ethinyl estradiol. Sexual interest increased in none of 7 studies that used conjugated estrogens. However, taking testosterone appeared to increase sexual interest. The evidence regarding the safety and efficacy of androgens (testosterone and dehydroepiandrosterone [DHEA]) for the treatment of sexual dysfunction in perimenopause is incomplete; therefore, these drugs should not routinely be prescribed.51
Bone
Although HRT prevents the rapid bone loss observed in the early menopausal period, this effect is lost when treatment is stopped. The positive effect of estrogen alone on bone mineral density was not diminished by medroxyprogesterone acetate (MPA) or micronized progesterone over a 3-year follow-up period.52 The long-term effects of MPA on fracture risk in postmenopausal women have not been reported. Use of transdermal progesterone alone does not prevent bone loss.38
Cancer
Estrogen alone for women with an intact uterus is currently considered unacceptable because adding the hormone poses endometrial cancer risk. An exception is low-dose estrogen administered intravaginally; this method does not alter the endometrium.53
Estrogen alone or in combination with progestins has been associated with an increased risk of breast cancer in many observational studies and meta-analyses. A comprehensive reanalysis54 of 51 mostly observational studies, including 52,705 cases of breast cancer and more than 100,000 controls, examined the association of breast cancer with HRT, predominantly unopposed estrogen. These authors concluded that there is an increase in incidence of breast cancer of 0.2%, 0.6%. and 1.2% with 5, 10, and 15 years of use, respectively. Thus, 1 additional case of breast cancer occurs for every 167 women treated for 10 years (number needed to harm [NNH] = 167). Two recent observational studies have documented up to a fourfold increase in breast cancer with estrogen plus progesterone over estrogen alone.55,56
Cardiovascular disease
HRT has been widely advocated for prevention of coronary artery disease (CAD), based on many observational studies. A meta-analysis57 of 25 studies published through 1997 gave a relative risk (RR) of 0.7 (CI 0.65-0.75) for coronary events in women using HRT. However, a consistent bias in these studies of selecting healthy, compliant women for inclusion may explain the observed benefit.
A meta-analysis58 of 22 trials of 4124 women comparing HRT with placebo, no therapy, or vitamins, in which cardiovascular events were secondary endpoints, revealed that there was no benefit regarding cardiac events and there were small increases in absolute risk of stroke and venous thromboembolism (VET). In the Heart and Estrogen/Progestin Replacement (HERS) study,59 conjugated equine estrogens (CEE) plus MPA, administered to women with established CAD for a mean of 4.1 years, did not reduce risk of cardiovascular events. An increase in events, particularly VET and stroke,60 occurred in the first year of use. Small increases in the absolute risks of stroke61,62 and VTE63,64 have also been described in observational studies.
Randomized trial evidence is currently lacking for a role of HRT in the primary prevention of cardiovascular disease. A large study of low-risk postmenopausal women, the Women’s Health Initiative,65 is currently under way. Its objective is to investigate strategies for the prevention and control of some of the most common causes of morbidity and mortality in postmenopausal women. The study includes 27,000 women randomized to CEE plus MPA or placebo. Results are expected in 2007. The American Heart Association now recommends against estrogen therapy with or without progestin solely for the prevention of heart disease.66 Long-term effects of androgens on cardiovascular risk have not been studied; concerns exist about their use.51
Other effects
A meta-analysis67 of trials of HRT for urinary incontinence showed no benefit. The HERS study68 showed an increase in urinary incontinence episodes with combined HRT for women with incontinence at baseline (NNH = 8). HRT also increases the risk of gallbladder disease69 and may worsen cognitive function for women with mild to moderate dementia.70
Prognosis
The symptoms of perimenopause are not life threatening and are usually limited in time. Climacteric symptoms are generally more severe and difficult to treat in women who have undergone bilateral oophorectomy before experiencing natural menopause.71 Women with multiple chronic medical conditions,13,72,73 psychiatric illnesses,15,74 or a history of premenstrual syndrome11,12,75 are also likely to experience more difficulty with symptoms attributed to the menopausal transition. Table 3 provides a list of resources for patient education regarding menopause.
TABLE 3
RESOURCES FOR PATIENT EDUCATION ABOUT MENOPAUSE
| Organization | Contact Information | Description |
|---|---|---|
| Ottawa Health Decision Centre | [email protected] 613-798-5555 | Making Choices: Hormones After Menopause (audiotape and workbook) |
| American Academy of Family Physicians | www.aafp.org 1-800-274-2237 | Brochures: “Menopause: What to Expect When Your Body Is Changing”; “Osteoporosis: Keeping Your Bones Healthy and Strong” |
| American College of Obstetricians and Gynecologists | www.acog.org 1-800-410-ACOG | Brochures: “Midlife Transitions: A Guide to the Menopause Years”; “Hormone Replacement Therapy” |
1. Wysowski D, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982-1992. Obstet Gynecol 1995;85:6-10.
2. McKinlay S, Brambilla PJ, Posnere JG. The normal menopause transition. Maturitas 1992;14:13-5.
3. Harlow B, Signorello LB. Factors associated with early menopause. Maturitas 2000;35:3-9.
4. Coulam C, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol 1986;67:604-6.
5. Kalantaridou S, Nelson LM. Premature ovarian failure is not premature menopause. Ann NY Acad Sci 2000;900:393-402.
6. Hee J, MacNaughton J, Bangah M, Burger HG. Perimenopausal patterns of gonadotropins, immunoreactive inhibin, oestradiol and progesterone. Maturitas 1993;18:9-20.
7. Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone moneral density. Maturitas 1995;21:103-13.
8. Burger H. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition—an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol 1994;130:38-42.
9. Stadberg E, Mattson LA, Milsom I. The prevalence and severity of climacteric symptoms and the use of different treatment regimens in a Swedish population. Acta Obstet Gynecol Scand 1997;76:442-8.
10. Holte A, Mikkelsen A. The menopausal syndrome: a factor analytic replication. Maturitas 1991;13:193-203.
11. Hunter M. The South-East England Longitudinal Study of the climacteric and postmenopause. Maturitas 1992;14:217-28.
12. Collins A, Landgren BM. Reproductive health, use of estrogen and experience of symptoms in perimenopausal women: a population-based study. Maturitas 1995;20:101-11.
13. Dennerstein L, Smith AMA, Morse C, Burger H, Green A, Hopper J, et al. Menopausal symptoms in Australian women. Med J Aust 1993;159:232-6.
14. Mashchak C, Kletsky QA, Artel R, et al. The relation of physiological changes to subjective symptoms in postmenopausal women with and without hot flushes. Maturitas 1985;6:301-8.
15. Grisso J, Freeman EW, Maurin E, Garcia-Espans B, Berlin JA. Racial differences in menopause information and the experience of hot flashes. J Gen Intern Med 1999;14:98-103.
16. Oldenhave A, Jaszmann LJB, Haspels AA, et al. Impact of climacteric on well-being. Am J Obstet Gynecol 1993;168:772-80.
17. Barentsen R, Groeneveld FP, Bareman FP, et al. Women’s opinion on withdrawal bleeding with hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol 1993;51:203-7.
18. Bachman G. Vasomotor flushes in menopausal women. Am J Obstet Gynecol 1999;180:S312-6.
19. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocr Metab Clin North Am 1997;26:279-94.
20. Woods N, Mitchell ES. Pathways to depressed mood for midlife women: observations from the Seattle midlife women’s health study. Res Nurs Health 1997;20:119-29.
21. Polo-Kantola P, Erkkola R. Climacteric symptoms and sleep quality. Obstet Gynecol 1999;94:219-24.
22. Dennerstein L, Dudley E, Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril 2001;76:456-60.
23. Notelovitz M, Cassel D, Hille D, et al. Efficacy of continuous sequential transdermal estradiol and norethindrone acetate in relieving vasomotor symptoms associated with menopause. Am J Obstet Gynecol 2000;182:7-12.
24. Utian W, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. Am J Obstet Gynecol 1999;181:71-9.
25. Greendale G, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the postmenopausal estrogen/progestin interventions trial. Obstet Gynecol 1998;92:982-8.
26. World Health Organization. Improving access to quality care in family planning:medical eligibility criteria for contraceptive use. Geneva, Switzerland; 1996.
27. Simon J, Kaiber E, Wiita B, Bowen YHA. Differential effects of estrogen-androgen and estrogen-only therapy on vasomotor symptoms, gonadotropin secretion, and endogenous androgen bioavailability in postmenopausal women. Menopause 1999;6:138-46.
28. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, de Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998;91:6-11.
29. Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause 1999;6:7-13.
30. St Germaine A, Peterson CT, Robinson JG. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause 2001;8:17-26.
31. Jacobson J, Troxel AB, Evans J. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739-45.
32. Hirata J, Swiersz LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril 1997;68:981-6.
33. Fugh-Berman A, Kronenberg F. Red clover (trifolium pratense) for menopausal women: current state of knowledge. Menopause 2001;8:333-7.
34. Greendale G, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571-80.
35. Quella S, Loprinzi CL, Sloan JA, et al. Long term use of megestrol acetate by cancer survivors for the treatment of hot flashes. Cancer 1998;82:1784-8.
36. Laufer L, Erlik Y, Meldrum DR, Judd HL. Effect of clonidine on hot flashes in postmenopausal women. Obstet Gynecol 1982;60:583-6.
37. Pandya K, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Int Med 2000;132:788-93.
38. Leonetti H, Longo S, Anasti JN. Transdermal progresterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol 1999;94:225-8.
39. Freedman R, Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am J Obstet Gynecol 1992;167436-9.
40. Roth A, Sacher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology 1998;7:129-32.
41. Loprinzi C, Pisansky TM, Fonseca R, et al. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol 1998;16:2377-81.
42. Stearns V, Isaacs C, Rowland J, et al. A pilot trial of paroxetine hydrochloride in controlling hot flashes in breast cancer survivors. Ann Oncol 2000;11:17-22.
43. Zweifel J, O’Brien WH. Meta-analysis of the effect of hormone replacement therapy on depressed mood. Psychoneuroendocrinology 1997;22:189-212.
44. Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58:529-34.
45. Schmidt P, Neiman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000;183:414-20.
46. Bech P, Munk-Jensen N, Obel EB, et al. Combined versus sequential hormonal replacement therapy: a double-blind, placebo-controlled study on quality of life-relationed outcome measures. Psychother Psychosom 1998;7:259-65.
47. Joffe H, Cohen LS. Estrogen, serotonin and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44:798-811.
48. Nedstrand E, Wijma K, Lindgren M, Hammar M. The relationship between stress-coping and vasomotor symptoms in postmenopausal women. Maturitas 1998;31:29-34.
49. Asplund R, Aberg HE. Body mass index and sleep in women aged 40-64 years. Maturitas 1995;22:1-8.
50. McCoy N. Sexual issues for postmenopausal women. Top Geriatr Rehab 1997;12:28-39.
51. American College of Obstetricians and Gynecologists Committee on Gyecologic Practice. Androgen treatment of decreased libido. Obstet Gynecol 2000;96:244-5.
52. Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density. JAMA 1996;276:1389.-
53. Botsis D, Kassanos D, Kalogiro D, et al. Vaginal ultrasound of the endometrium in postmenopausal women with symptoms of uro genital atrophy on low-dose estrogen or tibolone treatment: a comparison. Maturitas 1997;26:57-62.
54. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997;350:1047-59.
55. Ross R, Paganini-Hill A, Wan PC, et al. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst 2000;92:328-32.
56. Schairer C, Lubin J, Triosi R, et al. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 2000;283:485-91.
57. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other conditions. Ann Rev Public Health 1998;19:55-72.
58. Hemminki E, McPherson K. Impact of postmenopausal therapy on cardiovascular events and cancer: pooled data from clinical trials. BMJ 1997;315:149-53.
59. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
60. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen/Progestin Replacement Study (HERS). Circulation 2001;103:638-42.
61. Wilson P, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. N Engl J Med 1985;313:1038-45.
62. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Ann d’Endocrinol 1999;60:232-41.
63. Gutthann S, Rodriguez LA, Castellsague J, Oliart AD. Hormone replacement therapy and risk of thromboembolism: population based case-control study. BMJ 1997;314:796-800.
64. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996;348:977-80.
65. Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61-109.
66. Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S. AHA/ACC scientific statement: consensus panel statement. Guide to preventive cardiology for women. J Am Coll Cardiol 1999;33:1751-5.
67. Fantl J, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. Obstet Gynecol 1994;83:12-8.
68. Grady D, Brown JS, Vittinghoff E, Applegate W, Warner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol 2001;97:116-20.
69. Uhler M, Marks JW, Judd HL. Estrogen replacement therapy and gallbladder disease in postmenopausal women. Menopause 2000;7:162-7.
70. Mulnard R, Cotman CW, Kawas C, et al. Estrogen replacement therapy for mild to moderate Alzheimer’s disease: a randomized controlled trial. JAMA 2000;283:1007-15.
71. Langenberg P, Kjerulff KH, Stolley PD. Hormone replacement and menopausal symptoms following hysterectomy. Am J Epidemiol 1997;146:870-80.
72. Kuh D, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol 1997;104:923-33.
73. Kirchengast S. Relations between anthropometric characteristics and degree of severity of the climacteric syndrome in Austrian women. Maturitas 1993;17:167-80.
74. Stadberg E, Mattson LA, Milsom I. Factors associated with climacteric symptoms and the use of hormone replacement therapy. Acta Obstet Gynecol Scand 2000;79:286-92.
75. Guthrie J, Dennerstein L, Hopper JL, Burger HG. Hot flushes, menstrual status, and hormone levels in a population-based sample of midlife women. Obstet Gynecol 1996;88:437-42.
76. Hammar M, Berg G, Lindgren R. Does physical exercise influence the frequency of postmenopausal hot flushes? Acta Obstet Gynecol Scand 1990;69:409-12.
- Laboratory testing is not indicated to initiate treatment of perimenopausal symptoms.
- While estrogens are the best established of the options to treat vasomotor symptoms at perimenopause, they are not a proven treatment for major depression or poor libido.
- Little evidence exists regarding the benefits and risks of androgens for perimenopausal women, suggesting a cautious approach to their use.
- Routine use of hormone replacement therapy, especially beyond 5 years’ duration, is not recommended because of uncertainties regarding risks and benefits.
Menopause has been successfully promoted as an estrogen-deficient state. Prescriptions in the United States for noncontraceptive estrogen formulations increased from 16 million to 39 million between 1982 and 1992; progestin sales reached 4.7 million by 1992 after their introduction in 1986.1 A condition for which half of the population becomes eligible for pharmacologic treatment for 30 years or more of their life spans is worthy of family physicians’ attention. Counseling of women regarding menopause has also been incorporated into the Health Employer Data Information Set (HEDIS) for measuring the quality of care provided by health care plans.
The women of the generation born from 1946 to 1965 are now 36 to 55 years old. About half will at some time seek medical attention for relief of symptoms believed to be related to the menopausal transition.2 The clinical picture, however, can be confusing: women at midlife are susceptible to diseases that may affect or be affected by the menopausal transition. Life cycle changes can also provoke dysphoric symptoms similar to those of menopause or aggravate symptoms that already exist.
Natural history
A woman’s hormonal rhythm changes gradually, usually in the early to middle forties. Ovarian mass decreases progressively; production of ovarian hormones decreases as well. The menstrual cycles tend to be somewhat shorter. Follicle-stimulating hormone (FSH) and estrogen levels fluctuate. Estrogen levels may be transiently higher than in former years in response to higher FSH levels, recruiting more ovarian follicles. Anovulatory cycles are more frequent. Perimenopausal menstrual irregularity typically lasts for approximately 4 years; the large majority of women experience such irregularity for 1 to 7 years.2 For 10% of women, menses simply cease without prior menstrual irregularity.
The best estimate of mean age at menopause in the United States, based on a cohort of primarily Caucasian women, is 51.3 years.2 Smokers experience menopause 1.8 years earlier than nonsmokers (50.2 versus 52.0 years). Less than 10% of women reach menopause before age 46, while approximately 30% do so before age 50.2 A recent review3 concluded that the lifetime number of ovulatory cycles is predictive of age at menopause: earlier for women with shorter cycles and nulliparous women, later for multigravid women and those with a history of oral contraceptive use. A familial tendency toward similarity in age at menopause has been noted.
Premature menopause or premature ovarian failure is defined as cessation of menstrual periods before 40 years of age. The prevalence of premature ovarian failure is approximately 1% by age 40 and 0.1% by 30 years of age.4 Premature ovarian failure is frequently an autoimmune disorder.5
Diagnosis of menopause
The gold standard for diagnosing menopause is to do so retrospectively, 1 year after the last menstrual period. In general, a diagnosis of menopause based on menstrual history or hormone levels is not considered necessary to begin treatment for perimenopausal symptoms, which often begin several years before the onset of menopause.
Laboratory diagnosis
The extent to which FSH or other serologic markers can be used to diagnose menopause is controversial. The most important clinical reason to do so is to discontinue contraceptive methods safely. Some consider an FSH level greater than 40 mIU/mL to be diagnostic. This value was chosen because it is about 2 standard deviations above the periovulatory peak in FSH levels in regularly cycling women. However, longitudinal studies6,7 during the perimenopausal years have demonstrated that hormonal patterns that include FSH values greater than 40 mIU/mL often abruptly revert to premenopausal patterns and are accompanied by ovulatory cycles. For the individual patient, hormone levels do not appear to rule out fertility reliably.8 Studies defining test characteristics (sensitivity, specificity, likelihood ratios) of hormone assays for the diagnosis of menopause are needed.
History and physical examination
A large population-based survey of Swedish women9 found that the most common climacteric symptoms are, in order of frequency, vasomotor symptoms (hot flashes), mood disturbances, sleep disturbances, decreased libido, and vaginal dryness. Several observational studies10-13 have shown that vasomotor symptoms have the clearest temporal association with the menstrual cycle changes of the climacteric. These symptoms result from a sudden change in the hypothalamic control of temperature regulation,14 although the precise triggers have not been elucidated. Hot flashes occur commonly among women in their late thirties and forties who have regular menstrual cycles.15 Several studies2,10,13,16 have shown that the prevalence of hot flashes peaks in the year immediately following the final menstrual period. A typical pattern prevalence of hot flashes is 25% in premenopausal women, 69% in perimenopausal women, and 39% in late-postmenopausal women (more than 4.5 years).17 Fifteen years after menopause, 10% of women may continue to have moderate to severe hot flashes,18 which can be lifelong.
Irritability and mood swings are common climacteric complaints. Women often compare them with their earlier premenstrual symptoms. Studies of depressive symptoms in menopausal women indicate that menopause is not associated with increased rates of major depression.19 Stressful life context and poor health status appear to be more important risk factors for depression than symptoms of menopause in climacteric women.20
Many perimenopausal women complain of poor sleep, often attributed to nocturnal hot flashes. Subjective impairment of sleep quality that is associated with climacteric vasomotor symptoms does not manifest as abnormalities in polysomnographic sleep recordings.21 It does not appear to be related to sleep apnea.
Sexual dysfunction is common in women at midlife and beyond. Dyspareunia, associated with vaginal dryness, increases in frequency with increasing time after menopause.9 The other complaint is decreased libido. Multiple factors may contribute to lack of sexual interest. Both aging and the menopause are independently associated with decreases in sexual responsiveness.22 The roles of declining endogenous sex steroid hormones in this process have not been elucidated.
Treatment
Vasomotor symptoms
Table 1 summarizes treatment options for vasomotor symptoms. Numerous well-designed clinical trials have demonstrated the effectiveness of oral or transdermal estrogen replacement therapy (ERT) for hot flashes.18,23-25 Low-dose oral contraceptive formulations are approved until 50 years of age for nonsmoking women.26 In a well-designed randomized controlled trial (RCT) of 93 women, low-dose estrogen (0.625 mg conjugated equine estrogens daily) plus 1.25 mg methyltestosterone daily was shown to be more effective than low-dose estrogen only and as effective as high-dose estrogen (1.25 mg conjugated estrogens daily).27
Phytoestrogens may be helpful, but have not yet been studied extensively. One RCT28 of 104 postmenopausal women comparing ingestion of 60 g soy protein daily with that of 60 g casein (placebo) daily showed a 45% relative reduction of hot flashes at 12 weeks in the group taking soy versus the control group. A second RCT29 of 51 women comparing soy protein with carbohydrate placebo showed a decrease in severity, but not frequency, of hot flashes. Another well-designed RCT30 including 69 women treated with 40 g soy daily versus whey protein for 24 weeks showed no difference between treatment groups and improvement in symptom scores over time in both groups. It is difficult to include a 40-g to 60-g protein supplement in the daily diet because of the accompanying caloric intake required. Recent reports of randomized placebo-controlled trials of black cohosh31 and dong quai32 and a systematic review33 of controlled trials of red clover have found no benefit.
Alternatives to estrogen for treatment of hot flashes include methyldopa, clonidine, transdermal progesterone, and megestrol acetate. Megestrol, which reduces symptoms by 70%, appears to be the most effective of these.34 Although long-term use of megestrol acetate by cancer survivors for the treatment of hot flashes has been demonstrated to be effective and well tolerated,35 it is not customarily used at menopause. A 20% reduction in hot flashes can be expected with clonidine at a dose of 0.1 to 0.2 mg daily,34,36 although this regimen may cause an increase in difficulty sleeping37 as well as dry mouth, constipation, and low blood pressure. Transdermal progesterone cream alone has been shown to improve vasomotor symptoms, although without protective effect regarding bone loss.38 One small study39 of behavioral approaches showed symptom reduction with deep-breathing relaxation techniques. Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 show promise in the treatment of hot flashes.
The remainder of this article focuses on hormonal treatment effects and risks for menopausal women. A summary appears in Table 2.
TABLE 1
TREATMENT OF VASOMOTOR SYMPTOMS
| Strength of Recommendation | Treatment | Comment |
|---|---|---|
| A | Estrogens | Many preparations with both oral and transdermal delivery have been studied |
| A | Estrogen + MPA | Other progestins not well studied |
| B | Transdermal progesterone | One RCT38 |
| B | Estrogen + testosterone | One RCT27; long-term safety is a theoretical concern |
| B | Megasterol | Cohort35; long experience with cancer patients gives some assurance of safety |
| C | Behavioral approaches | One small RCT39; deep breathing was beneficial |
| C | Clonidine | Small RCTs36,37 with important loss of subjects because of side effects |
| D | Antidepressants | Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 |
| D | Phytoestrogens | Conflicting RCT results |
| D | Exercise | Weak observational studies suggest benefit74,76 |
| No benefit seen | ||
| B | Black cohosh | No benefit seen in one RCT31 |
| B | Dong quai | No benefit seen in one RCT32 |
| B | Red clover | No benefit seen in systematic review33 |
| Grades of recommendation are based on Oxford Centre for Evidence-Based Medicine guidelines. | ||
| MPA denotes medroxyprogesterone; RCT, randomized clinical trial. | ||
TABLE 2
SUMMARY OF RISKS AND BENEFITS OF TREATMENTS FOR PERIMENOPAUSAL SYMPTOMS
| Treatment | VMS | Mood | Libido | Bone | CAD | Breast CA |
|---|---|---|---|---|---|---|
| Estrogens | Benefit | Benefit | No benefit | Benefit | Uncertain | Risk |
| Estrogen + MPA | Benefit | ? benefit | No benefit | Benefit | Uncertain* | Risk† |
| Progesterone | Benefit | Risk | No benefit | NS | Uncertain | NS |
| Testosterone | Benefit | NS | ? benefit | Benefit | NS | NS |
| Phytoestrogens | ? benefit | NS | NS | No benefit | NS | NS |
| DHEA | NS | ? benefit | ? benefit | NS | NS | NS |
| * Not beneficial for secondary prevention. † Increased risk over estrogen alone. | ||||||
| CA denotes cancer; CAD, coronary artery disease; DHEA, dihydroepiandrosterone; MPA, medroxyprogesterone acetate; NS, not studied; VMS, vasomotor symptoms. | ||||||
Mood disorders
In a meta-analysis43 including 26 RCTs of the effects of hormone replacement therapy (HRT) on depressed mood, estrogen showed limited effectiveness in improving mood. The addition of synthetic progestins reduced the estrogen effect. More recent short trials of unopposed transdermal estrogen showed benefit.44,45 Other reviews46,47 have concluded that ERT or HRT has little effect in the treatment of psychological symptoms, including anxiety, cognitive, and affective symptoms. As an adjuvant to psychotropic therapy, it may have limited effect. There is insufficient evidence to support prophylactic ERT or HRT to prevent depression in women whose medical history includes prior postpartum depression.47 Estrogens do not affect the ability of a woman with moderate to severe vasomotor symptoms to cope with stress.48 Clinical trials reporting the effects of testosterone treatment on mood in women were not identified.
Women with mild psychological and predominantly vasomotor symptoms may benefit from a trial of HRT before psychotropic medication. For women who meet criteria for a diagnosis of major depression, initial treatment with an antidepressant alone or concurrent with HRT is advisable.
Sleep disturbance
In a survey of more than 6000 women aged 40 to 64 years, 30% of HRT users reported sleep improvement that they attributed to therapy.49 Other standard approaches to insomnia, such as sleep hygiene measures and progressive relaxation techniques, can also be used. If sleep apnea is suspected, a sleep study may be indicated.
Sexual dysfunction
In a systematic review50 of HRT for climacteric sexual dysfunction, vaginal dryness improved with ERT in 7 of 8 studies. Dyspareunia improved in only 1 of 6 studies using transdermal 17-beta-estradiol. Orgasm increased in only 1 of 5 trials using ethinyl estradiol. Sexual interest increased in none of 7 studies that used conjugated estrogens. However, taking testosterone appeared to increase sexual interest. The evidence regarding the safety and efficacy of androgens (testosterone and dehydroepiandrosterone [DHEA]) for the treatment of sexual dysfunction in perimenopause is incomplete; therefore, these drugs should not routinely be prescribed.51
Bone
Although HRT prevents the rapid bone loss observed in the early menopausal period, this effect is lost when treatment is stopped. The positive effect of estrogen alone on bone mineral density was not diminished by medroxyprogesterone acetate (MPA) or micronized progesterone over a 3-year follow-up period.52 The long-term effects of MPA on fracture risk in postmenopausal women have not been reported. Use of transdermal progesterone alone does not prevent bone loss.38
Cancer
Estrogen alone for women with an intact uterus is currently considered unacceptable because adding the hormone poses endometrial cancer risk. An exception is low-dose estrogen administered intravaginally; this method does not alter the endometrium.53
Estrogen alone or in combination with progestins has been associated with an increased risk of breast cancer in many observational studies and meta-analyses. A comprehensive reanalysis54 of 51 mostly observational studies, including 52,705 cases of breast cancer and more than 100,000 controls, examined the association of breast cancer with HRT, predominantly unopposed estrogen. These authors concluded that there is an increase in incidence of breast cancer of 0.2%, 0.6%. and 1.2% with 5, 10, and 15 years of use, respectively. Thus, 1 additional case of breast cancer occurs for every 167 women treated for 10 years (number needed to harm [NNH] = 167). Two recent observational studies have documented up to a fourfold increase in breast cancer with estrogen plus progesterone over estrogen alone.55,56
Cardiovascular disease
HRT has been widely advocated for prevention of coronary artery disease (CAD), based on many observational studies. A meta-analysis57 of 25 studies published through 1997 gave a relative risk (RR) of 0.7 (CI 0.65-0.75) for coronary events in women using HRT. However, a consistent bias in these studies of selecting healthy, compliant women for inclusion may explain the observed benefit.
A meta-analysis58 of 22 trials of 4124 women comparing HRT with placebo, no therapy, or vitamins, in which cardiovascular events were secondary endpoints, revealed that there was no benefit regarding cardiac events and there were small increases in absolute risk of stroke and venous thromboembolism (VET). In the Heart and Estrogen/Progestin Replacement (HERS) study,59 conjugated equine estrogens (CEE) plus MPA, administered to women with established CAD for a mean of 4.1 years, did not reduce risk of cardiovascular events. An increase in events, particularly VET and stroke,60 occurred in the first year of use. Small increases in the absolute risks of stroke61,62 and VTE63,64 have also been described in observational studies.
Randomized trial evidence is currently lacking for a role of HRT in the primary prevention of cardiovascular disease. A large study of low-risk postmenopausal women, the Women’s Health Initiative,65 is currently under way. Its objective is to investigate strategies for the prevention and control of some of the most common causes of morbidity and mortality in postmenopausal women. The study includes 27,000 women randomized to CEE plus MPA or placebo. Results are expected in 2007. The American Heart Association now recommends against estrogen therapy with or without progestin solely for the prevention of heart disease.66 Long-term effects of androgens on cardiovascular risk have not been studied; concerns exist about their use.51
Other effects
A meta-analysis67 of trials of HRT for urinary incontinence showed no benefit. The HERS study68 showed an increase in urinary incontinence episodes with combined HRT for women with incontinence at baseline (NNH = 8). HRT also increases the risk of gallbladder disease69 and may worsen cognitive function for women with mild to moderate dementia.70
Prognosis
The symptoms of perimenopause are not life threatening and are usually limited in time. Climacteric symptoms are generally more severe and difficult to treat in women who have undergone bilateral oophorectomy before experiencing natural menopause.71 Women with multiple chronic medical conditions,13,72,73 psychiatric illnesses,15,74 or a history of premenstrual syndrome11,12,75 are also likely to experience more difficulty with symptoms attributed to the menopausal transition. Table 3 provides a list of resources for patient education regarding menopause.
TABLE 3
RESOURCES FOR PATIENT EDUCATION ABOUT MENOPAUSE
| Organization | Contact Information | Description |
|---|---|---|
| Ottawa Health Decision Centre | [email protected] 613-798-5555 | Making Choices: Hormones After Menopause (audiotape and workbook) |
| American Academy of Family Physicians | www.aafp.org 1-800-274-2237 | Brochures: “Menopause: What to Expect When Your Body Is Changing”; “Osteoporosis: Keeping Your Bones Healthy and Strong” |
| American College of Obstetricians and Gynecologists | www.acog.org 1-800-410-ACOG | Brochures: “Midlife Transitions: A Guide to the Menopause Years”; “Hormone Replacement Therapy” |
- Laboratory testing is not indicated to initiate treatment of perimenopausal symptoms.
- While estrogens are the best established of the options to treat vasomotor symptoms at perimenopause, they are not a proven treatment for major depression or poor libido.
- Little evidence exists regarding the benefits and risks of androgens for perimenopausal women, suggesting a cautious approach to their use.
- Routine use of hormone replacement therapy, especially beyond 5 years’ duration, is not recommended because of uncertainties regarding risks and benefits.
Menopause has been successfully promoted as an estrogen-deficient state. Prescriptions in the United States for noncontraceptive estrogen formulations increased from 16 million to 39 million between 1982 and 1992; progestin sales reached 4.7 million by 1992 after their introduction in 1986.1 A condition for which half of the population becomes eligible for pharmacologic treatment for 30 years or more of their life spans is worthy of family physicians’ attention. Counseling of women regarding menopause has also been incorporated into the Health Employer Data Information Set (HEDIS) for measuring the quality of care provided by health care plans.
The women of the generation born from 1946 to 1965 are now 36 to 55 years old. About half will at some time seek medical attention for relief of symptoms believed to be related to the menopausal transition.2 The clinical picture, however, can be confusing: women at midlife are susceptible to diseases that may affect or be affected by the menopausal transition. Life cycle changes can also provoke dysphoric symptoms similar to those of menopause or aggravate symptoms that already exist.
Natural history
A woman’s hormonal rhythm changes gradually, usually in the early to middle forties. Ovarian mass decreases progressively; production of ovarian hormones decreases as well. The menstrual cycles tend to be somewhat shorter. Follicle-stimulating hormone (FSH) and estrogen levels fluctuate. Estrogen levels may be transiently higher than in former years in response to higher FSH levels, recruiting more ovarian follicles. Anovulatory cycles are more frequent. Perimenopausal menstrual irregularity typically lasts for approximately 4 years; the large majority of women experience such irregularity for 1 to 7 years.2 For 10% of women, menses simply cease without prior menstrual irregularity.
The best estimate of mean age at menopause in the United States, based on a cohort of primarily Caucasian women, is 51.3 years.2 Smokers experience menopause 1.8 years earlier than nonsmokers (50.2 versus 52.0 years). Less than 10% of women reach menopause before age 46, while approximately 30% do so before age 50.2 A recent review3 concluded that the lifetime number of ovulatory cycles is predictive of age at menopause: earlier for women with shorter cycles and nulliparous women, later for multigravid women and those with a history of oral contraceptive use. A familial tendency toward similarity in age at menopause has been noted.
Premature menopause or premature ovarian failure is defined as cessation of menstrual periods before 40 years of age. The prevalence of premature ovarian failure is approximately 1% by age 40 and 0.1% by 30 years of age.4 Premature ovarian failure is frequently an autoimmune disorder.5
Diagnosis of menopause
The gold standard for diagnosing menopause is to do so retrospectively, 1 year after the last menstrual period. In general, a diagnosis of menopause based on menstrual history or hormone levels is not considered necessary to begin treatment for perimenopausal symptoms, which often begin several years before the onset of menopause.
Laboratory diagnosis
The extent to which FSH or other serologic markers can be used to diagnose menopause is controversial. The most important clinical reason to do so is to discontinue contraceptive methods safely. Some consider an FSH level greater than 40 mIU/mL to be diagnostic. This value was chosen because it is about 2 standard deviations above the periovulatory peak in FSH levels in regularly cycling women. However, longitudinal studies6,7 during the perimenopausal years have demonstrated that hormonal patterns that include FSH values greater than 40 mIU/mL often abruptly revert to premenopausal patterns and are accompanied by ovulatory cycles. For the individual patient, hormone levels do not appear to rule out fertility reliably.8 Studies defining test characteristics (sensitivity, specificity, likelihood ratios) of hormone assays for the diagnosis of menopause are needed.
History and physical examination
A large population-based survey of Swedish women9 found that the most common climacteric symptoms are, in order of frequency, vasomotor symptoms (hot flashes), mood disturbances, sleep disturbances, decreased libido, and vaginal dryness. Several observational studies10-13 have shown that vasomotor symptoms have the clearest temporal association with the menstrual cycle changes of the climacteric. These symptoms result from a sudden change in the hypothalamic control of temperature regulation,14 although the precise triggers have not been elucidated. Hot flashes occur commonly among women in their late thirties and forties who have regular menstrual cycles.15 Several studies2,10,13,16 have shown that the prevalence of hot flashes peaks in the year immediately following the final menstrual period. A typical pattern prevalence of hot flashes is 25% in premenopausal women, 69% in perimenopausal women, and 39% in late-postmenopausal women (more than 4.5 years).17 Fifteen years after menopause, 10% of women may continue to have moderate to severe hot flashes,18 which can be lifelong.
Irritability and mood swings are common climacteric complaints. Women often compare them with their earlier premenstrual symptoms. Studies of depressive symptoms in menopausal women indicate that menopause is not associated with increased rates of major depression.19 Stressful life context and poor health status appear to be more important risk factors for depression than symptoms of menopause in climacteric women.20
Many perimenopausal women complain of poor sleep, often attributed to nocturnal hot flashes. Subjective impairment of sleep quality that is associated with climacteric vasomotor symptoms does not manifest as abnormalities in polysomnographic sleep recordings.21 It does not appear to be related to sleep apnea.
Sexual dysfunction is common in women at midlife and beyond. Dyspareunia, associated with vaginal dryness, increases in frequency with increasing time after menopause.9 The other complaint is decreased libido. Multiple factors may contribute to lack of sexual interest. Both aging and the menopause are independently associated with decreases in sexual responsiveness.22 The roles of declining endogenous sex steroid hormones in this process have not been elucidated.
Treatment
Vasomotor symptoms
Table 1 summarizes treatment options for vasomotor symptoms. Numerous well-designed clinical trials have demonstrated the effectiveness of oral or transdermal estrogen replacement therapy (ERT) for hot flashes.18,23-25 Low-dose oral contraceptive formulations are approved until 50 years of age for nonsmoking women.26 In a well-designed randomized controlled trial (RCT) of 93 women, low-dose estrogen (0.625 mg conjugated equine estrogens daily) plus 1.25 mg methyltestosterone daily was shown to be more effective than low-dose estrogen only and as effective as high-dose estrogen (1.25 mg conjugated estrogens daily).27
Phytoestrogens may be helpful, but have not yet been studied extensively. One RCT28 of 104 postmenopausal women comparing ingestion of 60 g soy protein daily with that of 60 g casein (placebo) daily showed a 45% relative reduction of hot flashes at 12 weeks in the group taking soy versus the control group. A second RCT29 of 51 women comparing soy protein with carbohydrate placebo showed a decrease in severity, but not frequency, of hot flashes. Another well-designed RCT30 including 69 women treated with 40 g soy daily versus whey protein for 24 weeks showed no difference between treatment groups and improvement in symptom scores over time in both groups. It is difficult to include a 40-g to 60-g protein supplement in the daily diet because of the accompanying caloric intake required. Recent reports of randomized placebo-controlled trials of black cohosh31 and dong quai32 and a systematic review33 of controlled trials of red clover have found no benefit.
Alternatives to estrogen for treatment of hot flashes include methyldopa, clonidine, transdermal progesterone, and megestrol acetate. Megestrol, which reduces symptoms by 70%, appears to be the most effective of these.34 Although long-term use of megestrol acetate by cancer survivors for the treatment of hot flashes has been demonstrated to be effective and well tolerated,35 it is not customarily used at menopause. A 20% reduction in hot flashes can be expected with clonidine at a dose of 0.1 to 0.2 mg daily,34,36 although this regimen may cause an increase in difficulty sleeping37 as well as dry mouth, constipation, and low blood pressure. Transdermal progesterone cream alone has been shown to improve vasomotor symptoms, although without protective effect regarding bone loss.38 One small study39 of behavioral approaches showed symptom reduction with deep-breathing relaxation techniques. Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 show promise in the treatment of hot flashes.
The remainder of this article focuses on hormonal treatment effects and risks for menopausal women. A summary appears in Table 2.
TABLE 1
TREATMENT OF VASOMOTOR SYMPTOMS
| Strength of Recommendation | Treatment | Comment |
|---|---|---|
| A | Estrogens | Many preparations with both oral and transdermal delivery have been studied |
| A | Estrogen + MPA | Other progestins not well studied |
| B | Transdermal progesterone | One RCT38 |
| B | Estrogen + testosterone | One RCT27; long-term safety is a theoretical concern |
| B | Megasterol | Cohort35; long experience with cancer patients gives some assurance of safety |
| C | Behavioral approaches | One small RCT39; deep breathing was beneficial |
| C | Clonidine | Small RCTs36,37 with important loss of subjects because of side effects |
| D | Antidepressants | Pilot studies of sertraline,40 venlafaxine,41 and paroxetine42 |
| D | Phytoestrogens | Conflicting RCT results |
| D | Exercise | Weak observational studies suggest benefit74,76 |
| No benefit seen | ||
| B | Black cohosh | No benefit seen in one RCT31 |
| B | Dong quai | No benefit seen in one RCT32 |
| B | Red clover | No benefit seen in systematic review33 |
| Grades of recommendation are based on Oxford Centre for Evidence-Based Medicine guidelines. | ||
| MPA denotes medroxyprogesterone; RCT, randomized clinical trial. | ||
TABLE 2
SUMMARY OF RISKS AND BENEFITS OF TREATMENTS FOR PERIMENOPAUSAL SYMPTOMS
| Treatment | VMS | Mood | Libido | Bone | CAD | Breast CA |
|---|---|---|---|---|---|---|
| Estrogens | Benefit | Benefit | No benefit | Benefit | Uncertain | Risk |
| Estrogen + MPA | Benefit | ? benefit | No benefit | Benefit | Uncertain* | Risk† |
| Progesterone | Benefit | Risk | No benefit | NS | Uncertain | NS |
| Testosterone | Benefit | NS | ? benefit | Benefit | NS | NS |
| Phytoestrogens | ? benefit | NS | NS | No benefit | NS | NS |
| DHEA | NS | ? benefit | ? benefit | NS | NS | NS |
| * Not beneficial for secondary prevention. † Increased risk over estrogen alone. | ||||||
| CA denotes cancer; CAD, coronary artery disease; DHEA, dihydroepiandrosterone; MPA, medroxyprogesterone acetate; NS, not studied; VMS, vasomotor symptoms. | ||||||
Mood disorders
In a meta-analysis43 including 26 RCTs of the effects of hormone replacement therapy (HRT) on depressed mood, estrogen showed limited effectiveness in improving mood. The addition of synthetic progestins reduced the estrogen effect. More recent short trials of unopposed transdermal estrogen showed benefit.44,45 Other reviews46,47 have concluded that ERT or HRT has little effect in the treatment of psychological symptoms, including anxiety, cognitive, and affective symptoms. As an adjuvant to psychotropic therapy, it may have limited effect. There is insufficient evidence to support prophylactic ERT or HRT to prevent depression in women whose medical history includes prior postpartum depression.47 Estrogens do not affect the ability of a woman with moderate to severe vasomotor symptoms to cope with stress.48 Clinical trials reporting the effects of testosterone treatment on mood in women were not identified.
Women with mild psychological and predominantly vasomotor symptoms may benefit from a trial of HRT before psychotropic medication. For women who meet criteria for a diagnosis of major depression, initial treatment with an antidepressant alone or concurrent with HRT is advisable.
Sleep disturbance
In a survey of more than 6000 women aged 40 to 64 years, 30% of HRT users reported sleep improvement that they attributed to therapy.49 Other standard approaches to insomnia, such as sleep hygiene measures and progressive relaxation techniques, can also be used. If sleep apnea is suspected, a sleep study may be indicated.
Sexual dysfunction
In a systematic review50 of HRT for climacteric sexual dysfunction, vaginal dryness improved with ERT in 7 of 8 studies. Dyspareunia improved in only 1 of 6 studies using transdermal 17-beta-estradiol. Orgasm increased in only 1 of 5 trials using ethinyl estradiol. Sexual interest increased in none of 7 studies that used conjugated estrogens. However, taking testosterone appeared to increase sexual interest. The evidence regarding the safety and efficacy of androgens (testosterone and dehydroepiandrosterone [DHEA]) for the treatment of sexual dysfunction in perimenopause is incomplete; therefore, these drugs should not routinely be prescribed.51
Bone
Although HRT prevents the rapid bone loss observed in the early menopausal period, this effect is lost when treatment is stopped. The positive effect of estrogen alone on bone mineral density was not diminished by medroxyprogesterone acetate (MPA) or micronized progesterone over a 3-year follow-up period.52 The long-term effects of MPA on fracture risk in postmenopausal women have not been reported. Use of transdermal progesterone alone does not prevent bone loss.38
Cancer
Estrogen alone for women with an intact uterus is currently considered unacceptable because adding the hormone poses endometrial cancer risk. An exception is low-dose estrogen administered intravaginally; this method does not alter the endometrium.53
Estrogen alone or in combination with progestins has been associated with an increased risk of breast cancer in many observational studies and meta-analyses. A comprehensive reanalysis54 of 51 mostly observational studies, including 52,705 cases of breast cancer and more than 100,000 controls, examined the association of breast cancer with HRT, predominantly unopposed estrogen. These authors concluded that there is an increase in incidence of breast cancer of 0.2%, 0.6%. and 1.2% with 5, 10, and 15 years of use, respectively. Thus, 1 additional case of breast cancer occurs for every 167 women treated for 10 years (number needed to harm [NNH] = 167). Two recent observational studies have documented up to a fourfold increase in breast cancer with estrogen plus progesterone over estrogen alone.55,56
Cardiovascular disease
HRT has been widely advocated for prevention of coronary artery disease (CAD), based on many observational studies. A meta-analysis57 of 25 studies published through 1997 gave a relative risk (RR) of 0.7 (CI 0.65-0.75) for coronary events in women using HRT. However, a consistent bias in these studies of selecting healthy, compliant women for inclusion may explain the observed benefit.
A meta-analysis58 of 22 trials of 4124 women comparing HRT with placebo, no therapy, or vitamins, in which cardiovascular events were secondary endpoints, revealed that there was no benefit regarding cardiac events and there were small increases in absolute risk of stroke and venous thromboembolism (VET). In the Heart and Estrogen/Progestin Replacement (HERS) study,59 conjugated equine estrogens (CEE) plus MPA, administered to women with established CAD for a mean of 4.1 years, did not reduce risk of cardiovascular events. An increase in events, particularly VET and stroke,60 occurred in the first year of use. Small increases in the absolute risks of stroke61,62 and VTE63,64 have also been described in observational studies.
Randomized trial evidence is currently lacking for a role of HRT in the primary prevention of cardiovascular disease. A large study of low-risk postmenopausal women, the Women’s Health Initiative,65 is currently under way. Its objective is to investigate strategies for the prevention and control of some of the most common causes of morbidity and mortality in postmenopausal women. The study includes 27,000 women randomized to CEE plus MPA or placebo. Results are expected in 2007. The American Heart Association now recommends against estrogen therapy with or without progestin solely for the prevention of heart disease.66 Long-term effects of androgens on cardiovascular risk have not been studied; concerns exist about their use.51
Other effects
A meta-analysis67 of trials of HRT for urinary incontinence showed no benefit. The HERS study68 showed an increase in urinary incontinence episodes with combined HRT for women with incontinence at baseline (NNH = 8). HRT also increases the risk of gallbladder disease69 and may worsen cognitive function for women with mild to moderate dementia.70
Prognosis
The symptoms of perimenopause are not life threatening and are usually limited in time. Climacteric symptoms are generally more severe and difficult to treat in women who have undergone bilateral oophorectomy before experiencing natural menopause.71 Women with multiple chronic medical conditions,13,72,73 psychiatric illnesses,15,74 or a history of premenstrual syndrome11,12,75 are also likely to experience more difficulty with symptoms attributed to the menopausal transition. Table 3 provides a list of resources for patient education regarding menopause.
TABLE 3
RESOURCES FOR PATIENT EDUCATION ABOUT MENOPAUSE
| Organization | Contact Information | Description |
|---|---|---|
| Ottawa Health Decision Centre | [email protected] 613-798-5555 | Making Choices: Hormones After Menopause (audiotape and workbook) |
| American Academy of Family Physicians | www.aafp.org 1-800-274-2237 | Brochures: “Menopause: What to Expect When Your Body Is Changing”; “Osteoporosis: Keeping Your Bones Healthy and Strong” |
| American College of Obstetricians and Gynecologists | www.acog.org 1-800-410-ACOG | Brochures: “Midlife Transitions: A Guide to the Menopause Years”; “Hormone Replacement Therapy” |
1. Wysowski D, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982-1992. Obstet Gynecol 1995;85:6-10.
2. McKinlay S, Brambilla PJ, Posnere JG. The normal menopause transition. Maturitas 1992;14:13-5.
3. Harlow B, Signorello LB. Factors associated with early menopause. Maturitas 2000;35:3-9.
4. Coulam C, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol 1986;67:604-6.
5. Kalantaridou S, Nelson LM. Premature ovarian failure is not premature menopause. Ann NY Acad Sci 2000;900:393-402.
6. Hee J, MacNaughton J, Bangah M, Burger HG. Perimenopausal patterns of gonadotropins, immunoreactive inhibin, oestradiol and progesterone. Maturitas 1993;18:9-20.
7. Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone moneral density. Maturitas 1995;21:103-13.
8. Burger H. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition—an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol 1994;130:38-42.
9. Stadberg E, Mattson LA, Milsom I. The prevalence and severity of climacteric symptoms and the use of different treatment regimens in a Swedish population. Acta Obstet Gynecol Scand 1997;76:442-8.
10. Holte A, Mikkelsen A. The menopausal syndrome: a factor analytic replication. Maturitas 1991;13:193-203.
11. Hunter M. The South-East England Longitudinal Study of the climacteric and postmenopause. Maturitas 1992;14:217-28.
12. Collins A, Landgren BM. Reproductive health, use of estrogen and experience of symptoms in perimenopausal women: a population-based study. Maturitas 1995;20:101-11.
13. Dennerstein L, Smith AMA, Morse C, Burger H, Green A, Hopper J, et al. Menopausal symptoms in Australian women. Med J Aust 1993;159:232-6.
14. Mashchak C, Kletsky QA, Artel R, et al. The relation of physiological changes to subjective symptoms in postmenopausal women with and without hot flushes. Maturitas 1985;6:301-8.
15. Grisso J, Freeman EW, Maurin E, Garcia-Espans B, Berlin JA. Racial differences in menopause information and the experience of hot flashes. J Gen Intern Med 1999;14:98-103.
16. Oldenhave A, Jaszmann LJB, Haspels AA, et al. Impact of climacteric on well-being. Am J Obstet Gynecol 1993;168:772-80.
17. Barentsen R, Groeneveld FP, Bareman FP, et al. Women’s opinion on withdrawal bleeding with hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol 1993;51:203-7.
18. Bachman G. Vasomotor flushes in menopausal women. Am J Obstet Gynecol 1999;180:S312-6.
19. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocr Metab Clin North Am 1997;26:279-94.
20. Woods N, Mitchell ES. Pathways to depressed mood for midlife women: observations from the Seattle midlife women’s health study. Res Nurs Health 1997;20:119-29.
21. Polo-Kantola P, Erkkola R. Climacteric symptoms and sleep quality. Obstet Gynecol 1999;94:219-24.
22. Dennerstein L, Dudley E, Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril 2001;76:456-60.
23. Notelovitz M, Cassel D, Hille D, et al. Efficacy of continuous sequential transdermal estradiol and norethindrone acetate in relieving vasomotor symptoms associated with menopause. Am J Obstet Gynecol 2000;182:7-12.
24. Utian W, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. Am J Obstet Gynecol 1999;181:71-9.
25. Greendale G, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the postmenopausal estrogen/progestin interventions trial. Obstet Gynecol 1998;92:982-8.
26. World Health Organization. Improving access to quality care in family planning:medical eligibility criteria for contraceptive use. Geneva, Switzerland; 1996.
27. Simon J, Kaiber E, Wiita B, Bowen YHA. Differential effects of estrogen-androgen and estrogen-only therapy on vasomotor symptoms, gonadotropin secretion, and endogenous androgen bioavailability in postmenopausal women. Menopause 1999;6:138-46.
28. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, de Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998;91:6-11.
29. Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause 1999;6:7-13.
30. St Germaine A, Peterson CT, Robinson JG. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause 2001;8:17-26.
31. Jacobson J, Troxel AB, Evans J. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739-45.
32. Hirata J, Swiersz LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril 1997;68:981-6.
33. Fugh-Berman A, Kronenberg F. Red clover (trifolium pratense) for menopausal women: current state of knowledge. Menopause 2001;8:333-7.
34. Greendale G, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571-80.
35. Quella S, Loprinzi CL, Sloan JA, et al. Long term use of megestrol acetate by cancer survivors for the treatment of hot flashes. Cancer 1998;82:1784-8.
36. Laufer L, Erlik Y, Meldrum DR, Judd HL. Effect of clonidine on hot flashes in postmenopausal women. Obstet Gynecol 1982;60:583-6.
37. Pandya K, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Int Med 2000;132:788-93.
38. Leonetti H, Longo S, Anasti JN. Transdermal progresterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol 1999;94:225-8.
39. Freedman R, Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am J Obstet Gynecol 1992;167436-9.
40. Roth A, Sacher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology 1998;7:129-32.
41. Loprinzi C, Pisansky TM, Fonseca R, et al. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol 1998;16:2377-81.
42. Stearns V, Isaacs C, Rowland J, et al. A pilot trial of paroxetine hydrochloride in controlling hot flashes in breast cancer survivors. Ann Oncol 2000;11:17-22.
43. Zweifel J, O’Brien WH. Meta-analysis of the effect of hormone replacement therapy on depressed mood. Psychoneuroendocrinology 1997;22:189-212.
44. Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58:529-34.
45. Schmidt P, Neiman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000;183:414-20.
46. Bech P, Munk-Jensen N, Obel EB, et al. Combined versus sequential hormonal replacement therapy: a double-blind, placebo-controlled study on quality of life-relationed outcome measures. Psychother Psychosom 1998;7:259-65.
47. Joffe H, Cohen LS. Estrogen, serotonin and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44:798-811.
48. Nedstrand E, Wijma K, Lindgren M, Hammar M. The relationship between stress-coping and vasomotor symptoms in postmenopausal women. Maturitas 1998;31:29-34.
49. Asplund R, Aberg HE. Body mass index and sleep in women aged 40-64 years. Maturitas 1995;22:1-8.
50. McCoy N. Sexual issues for postmenopausal women. Top Geriatr Rehab 1997;12:28-39.
51. American College of Obstetricians and Gynecologists Committee on Gyecologic Practice. Androgen treatment of decreased libido. Obstet Gynecol 2000;96:244-5.
52. Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density. JAMA 1996;276:1389.-
53. Botsis D, Kassanos D, Kalogiro D, et al. Vaginal ultrasound of the endometrium in postmenopausal women with symptoms of uro genital atrophy on low-dose estrogen or tibolone treatment: a comparison. Maturitas 1997;26:57-62.
54. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997;350:1047-59.
55. Ross R, Paganini-Hill A, Wan PC, et al. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst 2000;92:328-32.
56. Schairer C, Lubin J, Triosi R, et al. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 2000;283:485-91.
57. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other conditions. Ann Rev Public Health 1998;19:55-72.
58. Hemminki E, McPherson K. Impact of postmenopausal therapy on cardiovascular events and cancer: pooled data from clinical trials. BMJ 1997;315:149-53.
59. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
60. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen/Progestin Replacement Study (HERS). Circulation 2001;103:638-42.
61. Wilson P, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. N Engl J Med 1985;313:1038-45.
62. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Ann d’Endocrinol 1999;60:232-41.
63. Gutthann S, Rodriguez LA, Castellsague J, Oliart AD. Hormone replacement therapy and risk of thromboembolism: population based case-control study. BMJ 1997;314:796-800.
64. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996;348:977-80.
65. Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61-109.
66. Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S. AHA/ACC scientific statement: consensus panel statement. Guide to preventive cardiology for women. J Am Coll Cardiol 1999;33:1751-5.
67. Fantl J, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. Obstet Gynecol 1994;83:12-8.
68. Grady D, Brown JS, Vittinghoff E, Applegate W, Warner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol 2001;97:116-20.
69. Uhler M, Marks JW, Judd HL. Estrogen replacement therapy and gallbladder disease in postmenopausal women. Menopause 2000;7:162-7.
70. Mulnard R, Cotman CW, Kawas C, et al. Estrogen replacement therapy for mild to moderate Alzheimer’s disease: a randomized controlled trial. JAMA 2000;283:1007-15.
71. Langenberg P, Kjerulff KH, Stolley PD. Hormone replacement and menopausal symptoms following hysterectomy. Am J Epidemiol 1997;146:870-80.
72. Kuh D, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol 1997;104:923-33.
73. Kirchengast S. Relations between anthropometric characteristics and degree of severity of the climacteric syndrome in Austrian women. Maturitas 1993;17:167-80.
74. Stadberg E, Mattson LA, Milsom I. Factors associated with climacteric symptoms and the use of hormone replacement therapy. Acta Obstet Gynecol Scand 2000;79:286-92.
75. Guthrie J, Dennerstein L, Hopper JL, Burger HG. Hot flushes, menstrual status, and hormone levels in a population-based sample of midlife women. Obstet Gynecol 1996;88:437-42.
76. Hammar M, Berg G, Lindgren R. Does physical exercise influence the frequency of postmenopausal hot flushes? Acta Obstet Gynecol Scand 1990;69:409-12.
1. Wysowski D, Golden L, Burke L. Use of menopausal estrogens and medroxyprogesterone in the United States, 1982-1992. Obstet Gynecol 1995;85:6-10.
2. McKinlay S, Brambilla PJ, Posnere JG. The normal menopause transition. Maturitas 1992;14:13-5.
3. Harlow B, Signorello LB. Factors associated with early menopause. Maturitas 2000;35:3-9.
4. Coulam C, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol 1986;67:604-6.
5. Kalantaridou S, Nelson LM. Premature ovarian failure is not premature menopause. Ann NY Acad Sci 2000;900:393-402.
6. Hee J, MacNaughton J, Bangah M, Burger HG. Perimenopausal patterns of gonadotropins, immunoreactive inhibin, oestradiol and progesterone. Maturitas 1993;18:9-20.
7. Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone moneral density. Maturitas 1995;21:103-13.
8. Burger H. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition—an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol 1994;130:38-42.
9. Stadberg E, Mattson LA, Milsom I. The prevalence and severity of climacteric symptoms and the use of different treatment regimens in a Swedish population. Acta Obstet Gynecol Scand 1997;76:442-8.
10. Holte A, Mikkelsen A. The menopausal syndrome: a factor analytic replication. Maturitas 1991;13:193-203.
11. Hunter M. The South-East England Longitudinal Study of the climacteric and postmenopause. Maturitas 1992;14:217-28.
12. Collins A, Landgren BM. Reproductive health, use of estrogen and experience of symptoms in perimenopausal women: a population-based study. Maturitas 1995;20:101-11.
13. Dennerstein L, Smith AMA, Morse C, Burger H, Green A, Hopper J, et al. Menopausal symptoms in Australian women. Med J Aust 1993;159:232-6.
14. Mashchak C, Kletsky QA, Artel R, et al. The relation of physiological changes to subjective symptoms in postmenopausal women with and without hot flushes. Maturitas 1985;6:301-8.
15. Grisso J, Freeman EW, Maurin E, Garcia-Espans B, Berlin JA. Racial differences in menopause information and the experience of hot flashes. J Gen Intern Med 1999;14:98-103.
16. Oldenhave A, Jaszmann LJB, Haspels AA, et al. Impact of climacteric on well-being. Am J Obstet Gynecol 1993;168:772-80.
17. Barentsen R, Groeneveld FP, Bareman FP, et al. Women’s opinion on withdrawal bleeding with hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol 1993;51:203-7.
18. Bachman G. Vasomotor flushes in menopausal women. Am J Obstet Gynecol 1999;180:S312-6.
19. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocr Metab Clin North Am 1997;26:279-94.
20. Woods N, Mitchell ES. Pathways to depressed mood for midlife women: observations from the Seattle midlife women’s health study. Res Nurs Health 1997;20:119-29.
21. Polo-Kantola P, Erkkola R. Climacteric symptoms and sleep quality. Obstet Gynecol 1999;94:219-24.
22. Dennerstein L, Dudley E, Burger H. Are changes in sexual functioning during midlife due to aging or menopause? Fertil Steril 2001;76:456-60.
23. Notelovitz M, Cassel D, Hille D, et al. Efficacy of continuous sequential transdermal estradiol and norethindrone acetate in relieving vasomotor symptoms associated with menopause. Am J Obstet Gynecol 2000;182:7-12.
24. Utian W, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high dosages of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. Am J Obstet Gynecol 1999;181:71-9.
25. Greendale G, Reboussin BA, Hogan P, et al. Symptom relief and side effects of postmenopausal hormones: results from the postmenopausal estrogen/progestin interventions trial. Obstet Gynecol 1998;92:982-8.
26. World Health Organization. Improving access to quality care in family planning:medical eligibility criteria for contraceptive use. Geneva, Switzerland; 1996.
27. Simon J, Kaiber E, Wiita B, Bowen YHA. Differential effects of estrogen-androgen and estrogen-only therapy on vasomotor symptoms, gonadotropin secretion, and endogenous androgen bioavailability in postmenopausal women. Menopause 1999;6:138-46.
28. Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, de Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol 1998;91:6-11.
29. Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause 1999;6:7-13.
30. St Germaine A, Peterson CT, Robinson JG. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause 2001;8:17-26.
31. Jacobson J, Troxel AB, Evans J. Randomized trial of black cohosh for the treatment of hot flashes among women with a history of breast cancer. J Clin Oncol 2001;19:2739-45.
32. Hirata J, Swiersz LM, Zell B, Small R, Ettinger B. Does dong quai have estrogenic effects in postmenopausal women? A double-blind, placebo-controlled trial. Fertil Steril 1997;68:981-6.
33. Fugh-Berman A, Kronenberg F. Red clover (trifolium pratense) for menopausal women: current state of knowledge. Menopause 2001;8:333-7.
34. Greendale G, Lee NP, Arriola ER. The menopause. Lancet 1999;353:571-80.
35. Quella S, Loprinzi CL, Sloan JA, et al. Long term use of megestrol acetate by cancer survivors for the treatment of hot flashes. Cancer 1998;82:1784-8.
36. Laufer L, Erlik Y, Meldrum DR, Judd HL. Effect of clonidine on hot flashes in postmenopausal women. Obstet Gynecol 1982;60:583-6.
37. Pandya K, Raubertas RF, Flynn PJ, et al. Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Int Med 2000;132:788-93.
38. Leonetti H, Longo S, Anasti JN. Transdermal progresterone cream for vasomotor symptoms and postmenopausal bone loss. Obstet Gynecol 1999;94:225-8.
39. Freedman R, Woodward S. Behavioral treatment of menopausal hot flushes: evaluation by ambulatory monitoring. Am J Obstet Gynecol 1992;167436-9.
40. Roth A, Sacher HI. Sertraline relieves hot flashes secondary to medical castration as treatment of advanced prostate cancer. Psychooncology 1998;7:129-32.
41. Loprinzi C, Pisansky TM, Fonseca R, et al. Pilot evaluation of venlafaxine hydrochloride for the therapy of hot flashes in cancer survivors. J Clin Oncol 1998;16:2377-81.
42. Stearns V, Isaacs C, Rowland J, et al. A pilot trial of paroxetine hydrochloride in controlling hot flashes in breast cancer survivors. Ann Oncol 2000;11:17-22.
43. Zweifel J, O’Brien WH. Meta-analysis of the effect of hormone replacement therapy on depressed mood. Psychoneuroendocrinology 1997;22:189-212.
44. Soares C, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58:529-34.
45. Schmidt P, Neiman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000;183:414-20.
46. Bech P, Munk-Jensen N, Obel EB, et al. Combined versus sequential hormonal replacement therapy: a double-blind, placebo-controlled study on quality of life-relationed outcome measures. Psychother Psychosom 1998;7:259-65.
47. Joffe H, Cohen LS. Estrogen, serotonin and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44:798-811.
48. Nedstrand E, Wijma K, Lindgren M, Hammar M. The relationship between stress-coping and vasomotor symptoms in postmenopausal women. Maturitas 1998;31:29-34.
49. Asplund R, Aberg HE. Body mass index and sleep in women aged 40-64 years. Maturitas 1995;22:1-8.
50. McCoy N. Sexual issues for postmenopausal women. Top Geriatr Rehab 1997;12:28-39.
51. American College of Obstetricians and Gynecologists Committee on Gyecologic Practice. Androgen treatment of decreased libido. Obstet Gynecol 2000;96:244-5.
52. Writing Group for the PEPI Trial. Effects of hormone therapy on bone mineral density. JAMA 1996;276:1389.-
53. Botsis D, Kassanos D, Kalogiro D, et al. Vaginal ultrasound of the endometrium in postmenopausal women with symptoms of uro genital atrophy on low-dose estrogen or tibolone treatment: a comparison. Maturitas 1997;26:57-62.
54. Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997;350:1047-59.
55. Ross R, Paganini-Hill A, Wan PC, et al. Effect of hormone replacement therapy on breast cancer risk: estrogen versus estrogen plus progestin. J Natl Cancer Inst 2000;92:328-32.
56. Schairer C, Lubin J, Triosi R, et al. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA 2000;283:485-91.
57. Barrett-Connor E, Grady D. Hormone replacement therapy, heart disease, and other conditions. Ann Rev Public Health 1998;19:55-72.
58. Hemminki E, McPherson K. Impact of postmenopausal therapy on cardiovascular events and cancer: pooled data from clinical trials. BMJ 1997;315:149-53.
59. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progestin Replacement Study (HERS) Research Group. JAMA 1998;280:605-13.
60. Simon J, Hsia J, Cauley JA, et al. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen/Progestin Replacement Study (HERS). Circulation 2001;103:638-42.
61. Wilson P, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. N Engl J Med 1985;313:1038-45.
62. Oger E, Scarabin PY. Risk of stroke among users of hormone replacement therapy. Ann d’Endocrinol 1999;60:232-41.
63. Gutthann S, Rodriguez LA, Castellsague J, Oliart AD. Hormone replacement therapy and risk of thromboembolism: population based case-control study. BMJ 1997;314:796-800.
64. Daly E, Vessey MP, Hawkins MM, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet 1996;348:977-80.
65. Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61-109.
66. Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S. AHA/ACC scientific statement: consensus panel statement. Guide to preventive cardiology for women. J Am Coll Cardiol 1999;33:1751-5.
67. Fantl J, Cardozo L, McClish DK. Estrogen therapy in the management of urinary incontinence in postmenopausal women: a meta-analysis. Obstet Gynecol 1994;83:12-8.
68. Grady D, Brown JS, Vittinghoff E, Applegate W, Warner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol 2001;97:116-20.
69. Uhler M, Marks JW, Judd HL. Estrogen replacement therapy and gallbladder disease in postmenopausal women. Menopause 2000;7:162-7.
70. Mulnard R, Cotman CW, Kawas C, et al. Estrogen replacement therapy for mild to moderate Alzheimer’s disease: a randomized controlled trial. JAMA 2000;283:1007-15.
71. Langenberg P, Kjerulff KH, Stolley PD. Hormone replacement and menopausal symptoms following hysterectomy. Am J Epidemiol 1997;146:870-80.
72. Kuh D, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol 1997;104:923-33.
73. Kirchengast S. Relations between anthropometric characteristics and degree of severity of the climacteric syndrome in Austrian women. Maturitas 1993;17:167-80.
74. Stadberg E, Mattson LA, Milsom I. Factors associated with climacteric symptoms and the use of hormone replacement therapy. Acta Obstet Gynecol Scand 2000;79:286-92.
75. Guthrie J, Dennerstein L, Hopper JL, Burger HG. Hot flushes, menstrual status, and hormone levels in a population-based sample of midlife women. Obstet Gynecol 1996;88:437-42.
76. Hammar M, Berg G, Lindgren R. Does physical exercise influence the frequency of postmenopausal hot flushes? Acta Obstet Gynecol Scand 1990;69:409-12.
Bed Rest Ineffective as Therapy
CLINICAL QUESTION: Should bed rest be prescribed for any condition?
BACKGROUND: Bed rest is a traditional and frequently prescribed treatment for various medical conditions. Its therapeutic value should be critically assessed just as any other treatment modality.
POPULATION STUDIED: The authors identified a total of 39 randomized controlled trials of bed rest as a therapeutic intervention published between January 1966 and June 1998. The studies represented 17 conditions and a total of 5777 patients. Therapeutic uses of bed rest were used as prophylactic treatment after medical procedures and as primary treatment. Procedures included lumbar puncture (2 studies), spinal anesthesia (4), radiculography (4), cardiac catheterization (9), skin graft of burn (1), liver biopsy (1), fixation of femoral fracture (1), pressure sore surgery (1), and gastric surgery (1). The conditions for primary treatment included acute low back pain (5), spontaneous labor (1), proteinuric hypertension during pregnancy (2), early threatened abortion (1), uncomplicated myocardial infarction (4), pulmonary tuberculosis (1), rheumatoid arthritis (1), and acute infectious hepatitis (1). Control groups had to receive the same treatment other than bed rest and in the same environment (eg, hospital, home).
STUDY DESIGN AND VALIDITY: This was a systematic review of the literature with well-described methodology regarding search strategy and selection criteria. The authors did not present an assessment of the methodologic quality of the included studies. They determined that no pooled analyses were possible and presented their results appropriately in tables.
OUTCOMES MEASURED: The measure of interest for this review was presence or absence of statistically significant differences between treatment groups in the studies identified.
RESULTS: There were a total of 64 outcomes reported in the included studies. These were classified as better or worse with bed rest. In the 24 trials of bed rest as prophylaxis after procedures, there were 7 outcomes that were better with bed rest, none significantly. There were 26 outcomes worse with bed rest, 9 significantly. The significantly worse outcomes included nausea after lumbar puncture, headache after spinal anesthesia, dizziness after radiculography, hematoma, pain, back pain after cardiac catheterization, and time to normal bowel function after gastric surgery. In the 15 trials of bed rest as primary treatment, 6 outcomes were better with treatment (none significantly) and 25 outcomes were worse with treatment (8 significantly). The significantly worse outcomes included disability index at day 1 for acute low back pain, length of first stage of labor, contraction strength, assisted delivery, analgesia required during labor, 5-minute Apgar score, venous thrombosis after myocardial infarction, and time for recovery from acute infectious hepatitis.
Bed rest has not been proven beneficial as a therapeutic intervention for any condition. It should not be prescribed after lumbar puncture or spinal anesthesia or for treatment of acute low back pain, myocardial infarction, pulmonary tuberculosis, acute infectious hepatitis, or management of the first stage of labor. For other conditions, we should not assume that bed rest, beyond that imposed by symptoms, is beneficial treatment without evidence from clinical trials. Appropriate indications for bed rest as primary therapy are yet to be defined.
CLINICAL QUESTION: Should bed rest be prescribed for any condition?
BACKGROUND: Bed rest is a traditional and frequently prescribed treatment for various medical conditions. Its therapeutic value should be critically assessed just as any other treatment modality.
POPULATION STUDIED: The authors identified a total of 39 randomized controlled trials of bed rest as a therapeutic intervention published between January 1966 and June 1998. The studies represented 17 conditions and a total of 5777 patients. Therapeutic uses of bed rest were used as prophylactic treatment after medical procedures and as primary treatment. Procedures included lumbar puncture (2 studies), spinal anesthesia (4), radiculography (4), cardiac catheterization (9), skin graft of burn (1), liver biopsy (1), fixation of femoral fracture (1), pressure sore surgery (1), and gastric surgery (1). The conditions for primary treatment included acute low back pain (5), spontaneous labor (1), proteinuric hypertension during pregnancy (2), early threatened abortion (1), uncomplicated myocardial infarction (4), pulmonary tuberculosis (1), rheumatoid arthritis (1), and acute infectious hepatitis (1). Control groups had to receive the same treatment other than bed rest and in the same environment (eg, hospital, home).
STUDY DESIGN AND VALIDITY: This was a systematic review of the literature with well-described methodology regarding search strategy and selection criteria. The authors did not present an assessment of the methodologic quality of the included studies. They determined that no pooled analyses were possible and presented their results appropriately in tables.
OUTCOMES MEASURED: The measure of interest for this review was presence or absence of statistically significant differences between treatment groups in the studies identified.
RESULTS: There were a total of 64 outcomes reported in the included studies. These were classified as better or worse with bed rest. In the 24 trials of bed rest as prophylaxis after procedures, there were 7 outcomes that were better with bed rest, none significantly. There were 26 outcomes worse with bed rest, 9 significantly. The significantly worse outcomes included nausea after lumbar puncture, headache after spinal anesthesia, dizziness after radiculography, hematoma, pain, back pain after cardiac catheterization, and time to normal bowel function after gastric surgery. In the 15 trials of bed rest as primary treatment, 6 outcomes were better with treatment (none significantly) and 25 outcomes were worse with treatment (8 significantly). The significantly worse outcomes included disability index at day 1 for acute low back pain, length of first stage of labor, contraction strength, assisted delivery, analgesia required during labor, 5-minute Apgar score, venous thrombosis after myocardial infarction, and time for recovery from acute infectious hepatitis.
Bed rest has not been proven beneficial as a therapeutic intervention for any condition. It should not be prescribed after lumbar puncture or spinal anesthesia or for treatment of acute low back pain, myocardial infarction, pulmonary tuberculosis, acute infectious hepatitis, or management of the first stage of labor. For other conditions, we should not assume that bed rest, beyond that imposed by symptoms, is beneficial treatment without evidence from clinical trials. Appropriate indications for bed rest as primary therapy are yet to be defined.
CLINICAL QUESTION: Should bed rest be prescribed for any condition?
BACKGROUND: Bed rest is a traditional and frequently prescribed treatment for various medical conditions. Its therapeutic value should be critically assessed just as any other treatment modality.
POPULATION STUDIED: The authors identified a total of 39 randomized controlled trials of bed rest as a therapeutic intervention published between January 1966 and June 1998. The studies represented 17 conditions and a total of 5777 patients. Therapeutic uses of bed rest were used as prophylactic treatment after medical procedures and as primary treatment. Procedures included lumbar puncture (2 studies), spinal anesthesia (4), radiculography (4), cardiac catheterization (9), skin graft of burn (1), liver biopsy (1), fixation of femoral fracture (1), pressure sore surgery (1), and gastric surgery (1). The conditions for primary treatment included acute low back pain (5), spontaneous labor (1), proteinuric hypertension during pregnancy (2), early threatened abortion (1), uncomplicated myocardial infarction (4), pulmonary tuberculosis (1), rheumatoid arthritis (1), and acute infectious hepatitis (1). Control groups had to receive the same treatment other than bed rest and in the same environment (eg, hospital, home).
STUDY DESIGN AND VALIDITY: This was a systematic review of the literature with well-described methodology regarding search strategy and selection criteria. The authors did not present an assessment of the methodologic quality of the included studies. They determined that no pooled analyses were possible and presented their results appropriately in tables.
OUTCOMES MEASURED: The measure of interest for this review was presence or absence of statistically significant differences between treatment groups in the studies identified.
RESULTS: There were a total of 64 outcomes reported in the included studies. These were classified as better or worse with bed rest. In the 24 trials of bed rest as prophylaxis after procedures, there were 7 outcomes that were better with bed rest, none significantly. There were 26 outcomes worse with bed rest, 9 significantly. The significantly worse outcomes included nausea after lumbar puncture, headache after spinal anesthesia, dizziness after radiculography, hematoma, pain, back pain after cardiac catheterization, and time to normal bowel function after gastric surgery. In the 15 trials of bed rest as primary treatment, 6 outcomes were better with treatment (none significantly) and 25 outcomes were worse with treatment (8 significantly). The significantly worse outcomes included disability index at day 1 for acute low back pain, length of first stage of labor, contraction strength, assisted delivery, analgesia required during labor, 5-minute Apgar score, venous thrombosis after myocardial infarction, and time for recovery from acute infectious hepatitis.
Bed rest has not been proven beneficial as a therapeutic intervention for any condition. It should not be prescribed after lumbar puncture or spinal anesthesia or for treatment of acute low back pain, myocardial infarction, pulmonary tuberculosis, acute infectious hepatitis, or management of the first stage of labor. For other conditions, we should not assume that bed rest, beyond that imposed by symptoms, is beneficial treatment without evidence from clinical trials. Appropriate indications for bed rest as primary therapy are yet to be defined.
Estrogen Replacement After Breast Cancer May Be Helpful
CLINICAL QUESTION: Is estrogen replacement therapy (ERT) beneficial for women after treatment for breast cancer?
BACKGROUND: A history of breast cancer is considered a contraindication for ERT, on the assumption that this treatment promotes carcinoma of the breast and may therefore hasten recurrences and metastases. However, evidence of this is lacking. The authors of this study challenged the assumptions.
POPULATION STUDIED: All women (N = 76) with a history of breast cancer seen in an academic practice from 1978 through 1998 were included. Age ranged from 34 to 83 years with a mean of 61.8 ± 2.6 years. Information regarding ethnicity was not provided.
STUDY DESIGN AND VALIDITY: This is a retrospective case series (chart review). The methods section of the paper consists entirely of a description of the Population studied and the fact that most of the information was obtained from charts and supplemented by the hospital tumor registry. Additionally, telephone calls were made to determine the current status of those women whose records had not been updated in the past year.
OUTCOMES MEASURED: The number and percentage of each group that died during the study period and the cause of death were the main results reported. The length of time each woman was receiving ERT was a secondary measure.
RESULTS: Of the women receiving ERT, 3 (6%) died: 2 of breast cancer and 1 of myocardial infarction. This group included the one patient with advanced disease at baseline. Of the women receiving nonestrogen hormone replacement therapy, one (12.5%) died of breast cancer. Of the group receiving no hormones, 6 (33.3%) died: 5 of breast cancer and 1 of a stroke. Mean duration of ERT was 5.5 ± 2.5 years, with a range of 6 months to 32 years.
It cannot be assumed that ERT is contraindicated for women who have been treated for stage I breast cancer. At this point, it is not known whether ERT has any influence on the prognosis of breast cancer. This study and several other observational studies cited by the authors suggest that it may even improve the prognosis. A well-designed randomized controlled trial of ERT after treatment for breast cancer is warranted. Definitive results will take many years to obtain. In the meantime, individual patients previously treated for breast cancer should be counseled regarding what is known about the risks and benefits of ERT and should be given the option of using it.
CLINICAL QUESTION: Is estrogen replacement therapy (ERT) beneficial for women after treatment for breast cancer?
BACKGROUND: A history of breast cancer is considered a contraindication for ERT, on the assumption that this treatment promotes carcinoma of the breast and may therefore hasten recurrences and metastases. However, evidence of this is lacking. The authors of this study challenged the assumptions.
POPULATION STUDIED: All women (N = 76) with a history of breast cancer seen in an academic practice from 1978 through 1998 were included. Age ranged from 34 to 83 years with a mean of 61.8 ± 2.6 years. Information regarding ethnicity was not provided.
STUDY DESIGN AND VALIDITY: This is a retrospective case series (chart review). The methods section of the paper consists entirely of a description of the Population studied and the fact that most of the information was obtained from charts and supplemented by the hospital tumor registry. Additionally, telephone calls were made to determine the current status of those women whose records had not been updated in the past year.
OUTCOMES MEASURED: The number and percentage of each group that died during the study period and the cause of death were the main results reported. The length of time each woman was receiving ERT was a secondary measure.
RESULTS: Of the women receiving ERT, 3 (6%) died: 2 of breast cancer and 1 of myocardial infarction. This group included the one patient with advanced disease at baseline. Of the women receiving nonestrogen hormone replacement therapy, one (12.5%) died of breast cancer. Of the group receiving no hormones, 6 (33.3%) died: 5 of breast cancer and 1 of a stroke. Mean duration of ERT was 5.5 ± 2.5 years, with a range of 6 months to 32 years.
It cannot be assumed that ERT is contraindicated for women who have been treated for stage I breast cancer. At this point, it is not known whether ERT has any influence on the prognosis of breast cancer. This study and several other observational studies cited by the authors suggest that it may even improve the prognosis. A well-designed randomized controlled trial of ERT after treatment for breast cancer is warranted. Definitive results will take many years to obtain. In the meantime, individual patients previously treated for breast cancer should be counseled regarding what is known about the risks and benefits of ERT and should be given the option of using it.
CLINICAL QUESTION: Is estrogen replacement therapy (ERT) beneficial for women after treatment for breast cancer?
BACKGROUND: A history of breast cancer is considered a contraindication for ERT, on the assumption that this treatment promotes carcinoma of the breast and may therefore hasten recurrences and metastases. However, evidence of this is lacking. The authors of this study challenged the assumptions.
POPULATION STUDIED: All women (N = 76) with a history of breast cancer seen in an academic practice from 1978 through 1998 were included. Age ranged from 34 to 83 years with a mean of 61.8 ± 2.6 years. Information regarding ethnicity was not provided.
STUDY DESIGN AND VALIDITY: This is a retrospective case series (chart review). The methods section of the paper consists entirely of a description of the Population studied and the fact that most of the information was obtained from charts and supplemented by the hospital tumor registry. Additionally, telephone calls were made to determine the current status of those women whose records had not been updated in the past year.
OUTCOMES MEASURED: The number and percentage of each group that died during the study period and the cause of death were the main results reported. The length of time each woman was receiving ERT was a secondary measure.
RESULTS: Of the women receiving ERT, 3 (6%) died: 2 of breast cancer and 1 of myocardial infarction. This group included the one patient with advanced disease at baseline. Of the women receiving nonestrogen hormone replacement therapy, one (12.5%) died of breast cancer. Of the group receiving no hormones, 6 (33.3%) died: 5 of breast cancer and 1 of a stroke. Mean duration of ERT was 5.5 ± 2.5 years, with a range of 6 months to 32 years.
It cannot be assumed that ERT is contraindicated for women who have been treated for stage I breast cancer. At this point, it is not known whether ERT has any influence on the prognosis of breast cancer. This study and several other observational studies cited by the authors suggest that it may even improve the prognosis. A well-designed randomized controlled trial of ERT after treatment for breast cancer is warranted. Definitive results will take many years to obtain. In the meantime, individual patients previously treated for breast cancer should be counseled regarding what is known about the risks and benefits of ERT and should be given the option of using it.