User login
- Accurate differential diagnosis for women complaining of abnormal vaginal discharge requires in-office diagnostic testing at minimum, and laboratory testing in selected cases.
- Test for Chlamydia trachomatis and Neisseria gonorrhea when signs of purulent cervicitis are present (SOR: B).
- In suspected vulvovaginal candidiasis, culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR:B); rapid slide latex agglutination testing is not better than microscopy for diagnosing VVC (SOR: B).
In primary care practice, abnormal vaginal discharge is a common complaint. Signs and symptoms of vaginitis—the most common gynecologic diagnosis in primary care1 —are not specific for any single underlying cause.2 Officebased diagnostic testing, which is underused,3 must be employed to ensure accurate diagnosis and effective treatment. (An article on treatment by the same authors will appear in next month’s issue of The journal of family practice.)
In a primary-care study,4 vulvovaginal symptoms including vaginal discharge were due to vulvovaginal candidiasis (VVC) in 27% of patients, bacterial vaginosis (BV) in 21%, trichomoniasis in 8%, Chlamydia trachomatis in 2%, Neisseria gonorrhea (GC) in 1%, and no infection in 34%. Several pathogens may coexist.2 VVC, BV, and trichomoniasis account for at least 90% of infectious vaginitis.5 This review will therefore focus heavily on these causes of vaginal discharge among women of reproductive age, including pregnant women.
Cervicitis and physiologic cervical discharge
Some women may interpret a physiologic increase in cervical mucous production as abnormal. It occurs cyclically prior to ovulation, is typically transparent and colorless, and may be more pronounced in women with an everted cervix.
Chlamydial infection
In the clinical examination of the cervix, 3 characteristics have been associated with chlamydial infection: yellow endocervical discharge, easily induced cervical bleeding, and opaque cervical discharge.6 All 3 findings are statistically significant and independently associated with chlamydial infection (odds ratios 2.8, 2.3, and 2.9, respectively). In the primary care study cited above, purulent cervical discharge was found in 6% of women, most commonly testing positive for Chlamydia, less often for GC.4
Trichomonas vaginalis may cause cervicitis as well as vaginitis. Mycoplasma genitalium has been proposed as an additional possible pathogen. It was identified in 7% of more than 700 women with mucopurulent cervical discharge seen in a STD clinic with otherwise negative cultures.7 With cervical discharge that appears to be purulent, testing is warranted as a minimum for Chlamydia and GC (SOR: B). Screening of asymptomatic women less than 26 years of age for Chlamydia is recommended by the US Preventive Services Task Force (SOR: A).
Bacterial Vaginosis
Bacterial vaginosis (BV) is neither an inflammatory condition nor an STD, but is a shift in vaginal flora from the normal condition in which lactobacilli predominate, to a polymicrobial flora in which gram-positive anaerobes predominate. In addition to annoying vaginal symptoms, BV is associated with increased risks of more serious conditions such as pelvic inflammatory disease (PID), postoperative infections, and pregnancyrelated complications including prematurity. It also increases the likelihood of acquiring HIV in women exposed to the virus.8,9
Two principal factors put women at risk for acquiring BV: douching and exposure to a new sexual partner, both of which are thought to disrupt the vaginal ecosystem.10
Relative benefits of diagnostic tests
A gold standard test has not been established for BV. In about 50% of asymptomatic women, culture results are positive for flora such as Gardnerella vaginalis.5 While Amsel’s criteria are often used as a reference and generally suffice for the evaluation of symptomatic women, the best candidate for a gold standard test is probably Gram stain assessment using Nugent’s criteria (described in this section).11 Lack of leukocytes in the vaginal fluid supports a diagnosis of BV. A finding of white blood cells in excess of the number of vaginal epithelial cells suggests an inflammatory process (SOR: C).12
Amsel’s criteria with wet mount. The diagnostic approach most commonly used in the office is Amsel’s criteria—homogenous discharge, positive whiff-amine test, pH >4.5, and clue cells found on wet-mount microscopy (see How to perform a wet mount ).13 Three of 4 criteria deemed positive is considered diagnostic. If Gram stain is used as the reference standard, then Amsel’s criteria have 70% sensitivity and 94% specificity for diagnosing BV.14 An analysis of the individual criteria follows. The positive and negative predictive values of each compared with the whole group as reference standard is displayed in Table 1 .
Homogenous discharge. A thin, homogenous, grayish discharge is traditionally associated with BV. However, it is not specific to BV, being found commonly also in women with culture results positive for VVC or no diagnosis of vaginitis.2,15 It is the criterion least likely to be consistent with the whole group, seen in about half of women BVpositive and over one third of women BV-negative using Amsel’s criteria as the reference standard. 15
To perform a wet-mount preparation correctly, dilute the vaginal discharge with 1 or 2 drops of 0.9% saline and place it on a slide. Examine the slide under lowand high-powered fields for vaginal squamous cells, white blood cells (WBCs), lactobacilli, clue cells, and trichomonads. An increased number of WBCs can be defined as >5–10 WBC/HPF or WBCs exceeding the number vaginal epithelial cells.
To prepare the potassium hydroxide (KOH) slide, place a generous amount of vaginal discharge on a slide with 10% KOH solution. Air- or flame-drying before examination under low-power microscopy may improve sensitivity. A positive KOH preparation will have hyphae, mycelial tangles, or spores.
Whiff test. The whiff test is performed by adding drops of 10% potassium hydroxide solution to the vaginal fluid. A positive result is a “fishy” amine odor. In a study16 of 100 women complaining of malodorous discharge, a positive whiff test was predictive of positive culture results for anaerobic flora such as Bacteroides sp. with sensitivity 67%, specificity 94%, and a positive predictive value of 95%. The whiff test was not positive in any of the 5 cases with positive culture results for G vaginalis in the absence of anaerobes. There were also 12 cases positive for anaerobes without G vaginalis.
pH >4.5. Since the abnormal flora of BV is consistently associated with a vaginal pH >4.5, a normal pH excludes a diagnosis of BV.17,18 The determination of pH in the narrow range around 4.5 is not accurate using standard nitrazine paper. Narrower-range test paper is available and more accurate. Examples include pH paper for 4.5 to 5.5 (Micro Essential Laboratory), FemExam pH and Amines Test Card (Litmus Concepts), pHem-ALERT: pH paper on a stick (Imagyn Gynecology). Cervical mucous, semen, and blood are alkaline and can interfere with pH testing. Estrogen production is also necessary to maintain an acidic environment. A pH of 3.8 to 4.5 is consistent with normal vaginal flora in premenopausal women with normal estrogen production.17
Clue cells. Clue cells are vaginal epithelial cells coated with coccobacilli giving an appearance as if coated with ground black pepper. Clue cells on wet mount preparation is considered the most accurate of Amsel’s diagnostic criteria for BV.19 On the other hand, office evaluation of the wet mount is considered by some authors to be unreliable due to dependence on the clinician’s microscopy skills and lack of a durable record of the patient sample.
Gram stain a more objective test. A Gram stain evaluation using Nugent’s criteria has been adopted as the gold standard test for research purposes, including studies of prematurity. The Gram-stained vaginal specimen is scored from 0 to 10 based on semi-quantitative assessment of 3 classes of morphotypes ( Table 2 ): large gram-positive rods (Lactobacilli), small gram-negative rods (Gardnerella and Bacteroides spp.), and small curved gram-variable rods (Mobiluncus spp.).11
Diagnosis of BV is typically made when the Nugent score is 7 or more, which appears qualitatively as dominant morphotypes other than Lactobacilli. Gram staining is more objective and reproducible compared with wet-mount examination, with a sensitivity of 93% and specificity of 70% if Amsel’s criteria are used as the gold standard.14 It is useful for the evaluation of asymptomatic women. It also provides a durable record of the patient specimen. Compared with Gram stain, Amsel’s criteria tend to underdiagnose cases. We can expect that if screening for BV in pregnancy becomes a recommendation, Gram staining in a clinical laboratory will be the recommended method of diagnosis.
Other diagnostic tests for BV. DNA testing for Gardnerella is accurate for detection, but it is not synonymous with a diagnosis of BV, as described.20 DNA testing is further described under “Differential Diagnosis.” Gram staining is more reliable than gas-liquid chromatography21 and an assay for proline aminopeptidase (a metabolic product of some of the bacteria associated with BV).22 Latex agglutination testing for vaginal lactoferrin is a nonspecific marker for leukocytes, and thus inflammation. It is of little clinical utility in the diagnosis of vaginal discharge.23
TABLE 1
Predictive values of Amsel’s criteria (using 3 of 4 positive as diagnostic reference standard)
| Diagnostic criterion | Predictive value (%) | |
|---|---|---|
| Positive | Negative | |
| Homogeneous thin discharge seen at introitus | 42 | 89 |
| pH >4.5 | 53 | 94 |
| Odor on alkalinization | 94 | 93 |
| Clue cells on wet mount | 90 | 99 |
| Source: Thomason et al 1990.15 | ||
TABLE 2
How to use Nugent’s Gram stain criteria to diagnose bacterial vaginosis
| Lactobacillus morphophytes | Gardnerella and Bacteroides spp. morphophytes | Curved gram-variable rods | Points |
|---|---|---|---|
| 4+ | 0 | 0 | 0 |
| 3+ | 1+ | 1+ or 2+ | 1 |
| 2+ | 2+ | 3+ or 4+ | 2 |
| 1+ | 3+ | 3 | |
| 0 | 4+ | 4 | |
| Review each of the first 3 columns in turn, assigning points at far right according to your exam findings. | |||
| Add the points for all 3 columns for a final sum. A score of 7 or higher indicates bacterial vaginosis. Source: Nugent et al 1991.11 | |||
Vulvovaginal Candidiasis
Candidiasis is the second most commonly diagnosed vaginitis in the United States. Some experts estimate that 75% of women will have a yeast infection at some point in life and 5% will have recurrent infections.24 However, 10% to 30% of asymptomatic women with normal flora have positive culture results for Candida.25-29 The proportion of symptomatic women with positive culture results is 20% to 40%.4,30,31 Complications of VVC are rare,32 though vulvar vestibulitis33 and chorioamnionitis in pregnancy32 have been reported.
Risk factors. Symptomatic yeast vaginitis has been associated with condom and diaphragm use, recent antibiotic use, receptive oral sex, oral contraceptive use, spermicide use, diabetes, and immunosuppression including AIDS.31,34-37 The associations with antibiotic use and oral contraceptives are not consistent.30,38 Although pregnancy has been postulated as a risk factor for symptomatic VVC, prevalence of yeast on culture in pregnant women is similar to that of nonpregnant women.30
Suggestive symptoms. Among women with a culture result positive for Candida, the most common symptom is pruritus or burning.28 Abnormal discharge is a complaint for most symptomatic women with VVC confirmed by culture.2 In addition, women may complain of a thick, odorless, cottage cheese–like discharge.39 A thick, curdled-appearing discharge points to a diagnosis of Candida because it is rarely present with BV or trichomoniasis. In one study,28 a thick curdled discharge had a positive predictive value of 84% for diagnosis of VVC by culture (SOR: B). However, a thin discharge does not rule out VVC; in another study, clinicians described discharge as thin in about half of women ultimately diagnosed with VVC by culture in another study (SOR: B).2 On exam, vulvar and vaginal erythema are often present but are not specific findings. The accuracy of the clinical exam for VVC is poor compared with culture (SOR: A).2,30
Pathogens. Candida albicans is present in 80% to 90% of patients with VVC.5,40 remainder have non-albicans species, including C glabrata and others.28 An increase to almost 20% of non-Candida species in a vaginitis clinic by the mid-1990’s may be related to increased use of imidazoles available over-the-counter.40,41 Wet mount results are typically negative in the presence of non-Candida VVC.28
Diagnosis of VVC
The gold standard test for diagnosis of VVC is culture. The potassium hydroxide (KOH) wet mount is only 40% to 75% sensitive.28,29,42,43 False-positive results are also observed with variable frequency.44 The pH of the discharge is usually not more than 5.0 with Candida albicans, but may be higher with non-albicans species such as C glabrata.45 Culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR: B).5,28,46 Rapid slide latex agglutination testing is not better than microscopy (SOR: B).42
Trichomoniasis
Trichomonas, a motile protozoan with 4 flagella, causes the third most common form of vaginitis in the United States and is more common in some developing countries. Trichomoniasis accounts for no more than 10% of all cases of vaginitis, and it appears to be decreasing since the introduction of metronidazole.47,48 It is classified as an STD, although transmission is possible by other means if the organism is protected from desiccation—for example, in dirty washcloths or towels and contaminated water. Nonsexual transmission is thought to be uncommon.
Trichomoniasis is associated with GC and Chlamydia infections, and, like them, has been associated with seroconversion to HIV-positive status.49 Trichomonads are identified in 30% to 80% of male sexual partners of infected women. In men, trichomoniasis most often is an asymptomatic carrier state.50 However, it is the cause of about 10% of cases of nongonococcal urethritis in men.51
Our knowledge of the epidemiology of abnormal vaginal discharge is limited. Studies of vaginitis may exclude patients with vaginal discharge due to cervicitis; studies performed in sexually transmitted disease clinics are not representative of primary care practice; women who do not complain of abnormal vaginal discharge may have positive cultures for Gardnerella vaginalis and Candida albicans; and self-treatment of presumed yeast vaginitis with antifungals available over-the-counter further limits our knowledge of the prevalence and causes of vaginal discharge.
Clinical presentations. Women with trichomoniasis have variable presentations ranging from an asymptomatic carrier state to a malodorous, purulent discharge with vulvovaginal erythema. Punctate hemorrhagic cervical lesions are considered pathognomonic of trichomoniasis, but are seen in only about 2% of cases (SOR: B).52
Diagnosis. Culture for trichomoniasis is the gold standard. Several culture media have been used, most commonly the Diamond medium. Recently introduced is a transport and culture medium for detection of Trichomonas (InPouch TV), which performs as well as Diamond medium (SOR: A).53-55 A DNA probe is also available and accurate (SOR: A).
Motile trichomonads are seen on wet preparation in only 50% to 80% of culture-positive cases (SOR: B).50,54,56 Polymorphonuclear leukocytes can be dominant on wet mount, making visualization of trichomonads more difficult. The pH of the vaginal fluid is usually basic.
Trichomonas reported with cervical cytology
Trichomonas may also be reported on Pap smears. A meta-analysis57 comparing the pooled sensitivities and specificities of wet mounts and cytology demonstrated low sensitivities of 68% and 58%, respectively, and high specificities, 99.9% and 97%, respectively (SOR: A).
However, since cytology carries a 3% false-positive rate, its results are not diagnostic of trichomoniasis in low-risk, asymptomatic women.50,57 Treatment may be prescribed empirically based on positive cytology results. However, if an asymptomatic woman were concerned about whether she really has an STD, a positive wet prep would confirm the diagnosis. A negative wet prep should be followed up with culture to reliably rule out disease (SOR: B).
Trichomoniasis in pregnancy
Screening for asymptomatic trichomoniasis in pregnancy has not been recommended. In fact, some evidence suggests that treatment of trichomoniasis in pregnancy is associated with poorer pregnancy outcomes including lower birth weight and more prematurity (SOR: B).58,59
Aerobic vaginitis
Aerobic vaginitis is a term proposed to describe purulent vaginal discharge with predominance of abnormal aerobic flora.60 Aerobic vaginitis, which may be severe, has been reported as the cause of 5% of cases in a series from a specialty vaginitis clinic.61 The usual predominant microorganisms are group B streptococci, Escherichia coli, and Staphylococcus aureus. It is likely that less severe cases of aerobic vaginitis are not recognized in the primary care setting and are treated as BV or resolve spontaneously (SOR: C). The case series referred to above also reported good therapeutic response to 2% topical clindamycin (SOR: C).61
Noninfectious Vaginitis
Noninfectious causes of vaginal discharge include physiologic, irritant and allergic, cytolytic vaginitis, desquamative inflammatory vaginitis, collagen vascular disease, and idiopathic vaginitis.
Irritant and allergic vaginitis may result from sensitivities to topical medications, the active or base ingredients of spermicidal products, douching solutions, and the latex of condoms or diaphragms. If a woman with persistent symptoms has been using such intravaginal products, she should stop (SOR: C).
Cytolytic vaginitis is characterized by overgrowth of lactobacilli and cytolysis of squamous cells, including presence of cytoplasmic fragments and intact cells with naked nuclei.62 The cause is uncertain but may include a reaction to intravaginal medications or other products such as tampons. It can be found in up to 5% of women with symptoms and signs of vaginitis.62,63 Symptoms often mimic VVC and may include a white, cheesy discharge. Vaginal pH ranges from 3.5 to 5.5. Recurrences during luteal phase of the menstrual cycle have been described.64 Intravaginal antifungals should be discontinued. Baking soda sitz baths or douches are often used, but clinical trial data to support this practice are lacking (SOR: C).
Noninfectious desquamative inflammatory vaginitis (DIV) has also been described.65 DIV is an uncommon vaginitis characterized by profuse purulent discharge with epithelial cell exfoliation. It may occur at any time during the reproductive years or after menopause. There is probably a heterogeneous group of causes of DIV. Some cases may correspond to a disorder within the spectrum of lichen planus.66 Treatment is usually difficult, though there may be some response to local or systemic corticosteroid therapy (SOR: C).65
Differential diagnosis
A comparison of physical examination findings an diagnostic test results for various etiologies of vaginitis is summarized in Table 3 . An algorithmic approach to the differential diagnosis of abnormal vaginal discharge is presented in the Figure . Diagnosis is complicated in that signs and symptoms do little to help differentiate among BV, VVC, and trichomoniasis. A study2 of 22 genitourinary symptoms and signs showed that none differentiated among the 3 infections. This lack of clear-cut differences in symptoms also makes self-diagnosis and telephone triage inaccurate.67,68
A DNA probe testing system (Affirm VP III Microbial ID Test) for differential diagnosis is available but expensive. It identifies Gardnerella, Trichomonas, and Candida albicans with a sensitivity of 90% to 95%.54,66 The analyzer costs approximately $10,000 and would typically be purchased by a laboratory. Individual test kits cost about $27.
TABLE 3
Comparative findings among causes of vaginitis
| Cause | Physical exam findings* | Gold standard test | pH | Leukocytes | Wet mount | Alternative test |
|---|---|---|---|---|---|---|
| Bacterial vaginosis | Variable | Gram stain | >4.5 | No | Clue cells | Amsel’s criteria |
| Aerobic vaginitis | Abundant purulent discharge | Culture | >4.5 | Yes | Cocci or coarse rods | |
| Candida vaginitis | Adherent white disch. (thrush) | Culture | 3.8–4.5 | ± | Pseudohyphae or budding yeast | DNA testing |
| Non-Candida yeast vaginitis | Variable | Culture | Any | ± | Usually negative | |
| Trichomoniasis | Variable, occ. strawberry spots on cervix | Culture | >4.5 | ± | Motile trichomonads | DNA testing |
| Cytolytic vaginitis | Profuse discharge, often cheesy | Cytology and negative culture | 3.5–5.5 | ± | Overgrowth of lactobacilli and squamous cell fragments | |
| Desquamative inflammatory vaginitis | Abundant purulent discharge | Parabasal epithelial cells and negative culture | >4.5 | Yes | ||
| Irritant and allergic vaginitis | Variable, often erythema | None | Any | ± | ||
| * Helpful when present. | ||||||
FIGURE
Sequence of office tests to evaluate abnormal vaginal discharge
Corresponding author
Linda French, MD, Associate Professor, Department of Family Practice, College of Human Medicine, Michigan State University, B101 Clinical Center, East Lansing, MI 48824. E-mail: [email protected].
1. National Center for Health Statistics. National Ambulatory Medicine Care Survey. Available at: www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm.
2. Schaaf VM, Perez-Stable EJ, Borchardt K. The limited value of symptoms and signs in the diagnosis of vaginal infections. Arch Intern Med 1990;150:1929-1933.
3. Wiesenfeld HC, Macio I. The infrequent use of officebased diagnostic tests for vaginitis. Am J Obstet Gynecol 1999;181:39-41.
4. Berg AO, Heidrich FE, Fihn SD, et al. Establishing the cause of genitourinary symptoms in women in a family practice. Comparison of clinical examination and comprehensive microbiology. JAMA 1984;251:620-625.
5. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
6. Sellors JW, Walter SD, Howard M. A new visual indicator of chlamydial cervicitis? Sex Transm Infect 2000;76:46-48.
7. Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis 2003;187:650-657.
8. Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180:1863-1868.
9. Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses 1998;14Suppl 1:S17-21.
10. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogenperoxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. JClin Microbiol 1991;29:297-301.
12. Quan M. Vaginitis: meeting the clinical challenge. Clin Cornerstone 2000;3:36-47.
13. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14-22.
14. Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. ObstetGynecol 1996;88:573-576.
15. Thomason JL, Gelbart SM, Anderson RJ, Walt AK, Osypowski PJ, Broekhuizen FF. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am J Obstet Gynecol. 1990;162:155-160.
16. Erkkola R, Jarvinen H, Terho P, Meurman O. Microbial flora in women showing symptoms of nonspecific vaginosis: applicability of KOH test for diagnosis. Scand J Infect Dis Suppl. 1983;40:59-63.
17. Caillouette JC, Sharp CF, Jr, Zimmerman GJ, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. 1997;176:1270-1275discussion1275-1277.
18. Carr PL, Felsenstein D, Friedman RH. Evaluation and management of vaginitis. J Gen Intern Med 1998;13:335-346.
19. Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988;158:819-828.
20. Sheiness D, Dix K, Watanabe S, Hillier SL. Highlevels of Gardnerella vaginalis detected with an oligonucleotide probe combined with elevated pH as a diagnostic indicator of bacterial vaginosis. J Clin Microbiol 1992;30:642-648.
21. Thomason JL, Gelbart SM, James JA, Edwards JM, Hamilton PR. Is analysis of vaginal secretions for volatile organic acids to detect bacterial vaginosis of any diagnostic value? Am J Obstet Gynecol. 1988;159:1509-1511.
22. Thomason JL, Gelbart SM, Wilcoski LM, Peterson AK, Jilly BJ, Hamilton PR. Proline aminopeptidase activity as a rapid diagnostic test to confirm bacterial vaginosis. Obstet Gynecol 1988;71:607-611.
23. Rein MF, Shih LM, Miller JR, Guerrant RL. Use of a lactoferrin assay in the differential diagnosis of female genital tract infections and implications for the pathophysiology of bacterial vaginosis. Sex Transm Dis 1996;23:517-521.
24. Monif GR. Classification and pathogenesis of vulvovaginal candidiasis. Am J Obstet Gynecol 1985;152:935-939.
25. Giraldo P, von Nowaskonski A, Gomes FA, Linhares I, Neves NA, Witkin SS. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstet Gynecol 2000;95:413-416.
26. Bergman JJ, Berg AO. How useful are symptoms in the diagnosis of Candida vaginitis? J Fam Pract 1983;16:509-511.
27. Bro F. Patients with vaginal discharge in general practice. Acta Obstet Gynecol Scand 1989;68:41-43.
28. Eckert LO, Hawes SE, Stevens CE, Koutsky LA, Eschenbach DA, Holmes KK. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol 1998;92:757-765.
29. Bertholf ME, Stafford MJ. Colonization of Candida albicans in vagina, rectum, and mouth. J Fam Pract 1983;16:919-924.
30. Reed BD, Huck W, Zazove P. Differentiation of Gardnerella vaginalis, Candida albicans, and Trichomonas vaginalis infections of the vagina. J Fam Pract 1989;28:673-680.
31. Bro F. The diagnosis of candida vaginitis in general practice. Scand J Prim Health Care 1989;7:19-22.
32. Cotch MF, Hillier SL, Gibbs RS, Eschenbach DA. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 1998;178:374-380.
33. Pagano R. Vulvar vestibulitis syndrome: an often unrecognized cause of dyspareunia. Aust N Z J Obstet Gynaecol 1999;39:79-83.
34. Foxman B. The epidemiology of vulvovaginal candidiasis: risk factors. Am J Public Health 1990;80:329-331.
35. Geiger AM, Foxman B. Risk factors for vulvovaginal candidiasis: a case-control study among university students. Epidemiology 1996;7:182-187.
36. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
37. Spinillo A, Capuzzo E, Acciano S, De Santolo A, Zara F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am J Obstet Gynecol 1999;180:14-17.
38. Davidson F, Oates JK. The pill does not cause ‘thrush’. Br J Obstet Gynaecol Dec 1985;92:1265-1266.
39. Abbott J. Clinical and microscopic diagnosis of vaginal yeast infection: a prospective analysis. Ann Emerg Med 1995;25:587-591.
40. Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J Clin Pharmacol 1992;32:248-255.
41. Spinillo A, Capuzzo E, Gulminetti R, Marone P, Colonna L, Piazzi G. Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am J Obstet Gynecol 1997;176:138-141.
42. Reed BD, Pierson CL. Evaluation of a latex agglutination test for the identification of Candida species in vaginal discharge. J Am Board Fam Pract 1992;5:375-380.
43. Ferris DG, Hendrich J, Payne PM, et al. Office laboratoAE_French.1004.final 9/20/04 3:18 PM Page 813 ry diagnosis of vaginitis. Clinician-performed tests compared with a rapid nucleic acid hybridization test. J Fam Pract 1995;41:575-581.
44. Bergman JJ, Berg AO, Schneeweiss R, Heidrich FE. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J Fam Pract 1984;18:549-552.
45. Sobel JD. Vulvovaginitis due to Candida glabrata. An emerging problem. Mycoses. 1998;41:Suppl 2:18-22.
46. Zdolsek B, Hellberg D, Froman G, Nilsson S, Mardh PA. Culture and wet smear microscopy in the diagnosis of low-symptomatic vulvovaginal candidosis. Eur J Obstet Gynecol Reprod Biol 1995;58:47-51.
47. Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991;165:1217-1222.
48. Kent HL. Epidemiology of vaginitis. Am J Obstet Gynecol 1991;165:1168-1176.
49. Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 1993;7:95-102.
50. Krieger JN, Tam MR, Stevens CE, et al. Diagnosis of trichomoniasis. Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimens. JAMA 1988;259:1223-1227.
51. Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995;22:83-96.
52. Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis 1980;141:137-143.
53. Ohlemeyer CL, Hornberger LL, Lynch DA, Swierkosz EM. Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy. J Adolesc Health 1998;22:205-208.
54. Borchardt KA, Smith RF. An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Med 1991;67:149-152.
55. Levi MH, Torres J, Pina C, Klein RS. Comparison of the InPouch TV culture system and Diamond’s modified medium for detection of Trichomonas vaginalis. J Clin Microbiol 1997;35:3308-3310.
56. DeMeo LR, Draper DL, McGregor JA, et al. Evaluation of a deoxyribonucleic acid probe for the detection of Trichomonas vaginalis in vaginal secretions. Am J Obstet Gynecol 1996;174:1339-1342.
57. Wiese W, Patel SR, Patel SC, Ohl CA, Estrada CA. A meta-analysis of the Papanicolaou smear and wet mount for the diagnosis of vaginal trichomoniasis. Am J Med 2000;108:301-308.
58. Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001;345:487-493.
59. Kigozi GG, Brahmbhatt H, Wabwire-Mangen F, et al. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2003;189:1398-1400.
60. Donder GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Bjog 2002;109:34-43.
61. Sobel JD. Desquamative inflammatory vaginitis: a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol 1994;171:1215-1220.
62. Demirezen S. Cytolytic vaginosis: examination of 2947 vaginal smears. Cent Eur J Public Health 2003;11:23-24.
63. Wathne B, Holst E, Hovelius B, Mardh PA. Vaginal discharge—comparison of clinical, laboratory and microbiological findings. Acta Obstet Gynecol Scand 1994;73:802-808.
64. Secor RM. Cytolytic vaginosis: a common cause of cyclic vulvovaginitis. Nurse Pract Forum 1992;3:145-148.
65. Oates JK, Rowen D. Desquamative inflammatory vaginitis. A review. Genitourin Med 1990;66:275-279.
66. Pelisse M. The vulvo-vaginal-gingival syndrome. A new form of erosive lichen planus. Int J Dermatol 1989;28:381-384.
67. Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol 2002;99:419-425.
68. Allen-Davis JT, Beck A, Parker R, Ellis JL, Polley D. Assessment of vulvovaginal complaints: accuracy of telephone triage and in-office diagnosis. Obstet Gynecol 2002;99:18-22.
- Accurate differential diagnosis for women complaining of abnormal vaginal discharge requires in-office diagnostic testing at minimum, and laboratory testing in selected cases.
- Test for Chlamydia trachomatis and Neisseria gonorrhea when signs of purulent cervicitis are present (SOR: B).
- In suspected vulvovaginal candidiasis, culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR:B); rapid slide latex agglutination testing is not better than microscopy for diagnosing VVC (SOR: B).
In primary care practice, abnormal vaginal discharge is a common complaint. Signs and symptoms of vaginitis—the most common gynecologic diagnosis in primary care1 —are not specific for any single underlying cause.2 Officebased diagnostic testing, which is underused,3 must be employed to ensure accurate diagnosis and effective treatment. (An article on treatment by the same authors will appear in next month’s issue of The journal of family practice.)
In a primary-care study,4 vulvovaginal symptoms including vaginal discharge were due to vulvovaginal candidiasis (VVC) in 27% of patients, bacterial vaginosis (BV) in 21%, trichomoniasis in 8%, Chlamydia trachomatis in 2%, Neisseria gonorrhea (GC) in 1%, and no infection in 34%. Several pathogens may coexist.2 VVC, BV, and trichomoniasis account for at least 90% of infectious vaginitis.5 This review will therefore focus heavily on these causes of vaginal discharge among women of reproductive age, including pregnant women.
Cervicitis and physiologic cervical discharge
Some women may interpret a physiologic increase in cervical mucous production as abnormal. It occurs cyclically prior to ovulation, is typically transparent and colorless, and may be more pronounced in women with an everted cervix.
Chlamydial infection
In the clinical examination of the cervix, 3 characteristics have been associated with chlamydial infection: yellow endocervical discharge, easily induced cervical bleeding, and opaque cervical discharge.6 All 3 findings are statistically significant and independently associated with chlamydial infection (odds ratios 2.8, 2.3, and 2.9, respectively). In the primary care study cited above, purulent cervical discharge was found in 6% of women, most commonly testing positive for Chlamydia, less often for GC.4
Trichomonas vaginalis may cause cervicitis as well as vaginitis. Mycoplasma genitalium has been proposed as an additional possible pathogen. It was identified in 7% of more than 700 women with mucopurulent cervical discharge seen in a STD clinic with otherwise negative cultures.7 With cervical discharge that appears to be purulent, testing is warranted as a minimum for Chlamydia and GC (SOR: B). Screening of asymptomatic women less than 26 years of age for Chlamydia is recommended by the US Preventive Services Task Force (SOR: A).
Bacterial Vaginosis
Bacterial vaginosis (BV) is neither an inflammatory condition nor an STD, but is a shift in vaginal flora from the normal condition in which lactobacilli predominate, to a polymicrobial flora in which gram-positive anaerobes predominate. In addition to annoying vaginal symptoms, BV is associated with increased risks of more serious conditions such as pelvic inflammatory disease (PID), postoperative infections, and pregnancyrelated complications including prematurity. It also increases the likelihood of acquiring HIV in women exposed to the virus.8,9
Two principal factors put women at risk for acquiring BV: douching and exposure to a new sexual partner, both of which are thought to disrupt the vaginal ecosystem.10
Relative benefits of diagnostic tests
A gold standard test has not been established for BV. In about 50% of asymptomatic women, culture results are positive for flora such as Gardnerella vaginalis.5 While Amsel’s criteria are often used as a reference and generally suffice for the evaluation of symptomatic women, the best candidate for a gold standard test is probably Gram stain assessment using Nugent’s criteria (described in this section).11 Lack of leukocytes in the vaginal fluid supports a diagnosis of BV. A finding of white blood cells in excess of the number of vaginal epithelial cells suggests an inflammatory process (SOR: C).12
Amsel’s criteria with wet mount. The diagnostic approach most commonly used in the office is Amsel’s criteria—homogenous discharge, positive whiff-amine test, pH >4.5, and clue cells found on wet-mount microscopy (see How to perform a wet mount ).13 Three of 4 criteria deemed positive is considered diagnostic. If Gram stain is used as the reference standard, then Amsel’s criteria have 70% sensitivity and 94% specificity for diagnosing BV.14 An analysis of the individual criteria follows. The positive and negative predictive values of each compared with the whole group as reference standard is displayed in Table 1 .
Homogenous discharge. A thin, homogenous, grayish discharge is traditionally associated with BV. However, it is not specific to BV, being found commonly also in women with culture results positive for VVC or no diagnosis of vaginitis.2,15 It is the criterion least likely to be consistent with the whole group, seen in about half of women BVpositive and over one third of women BV-negative using Amsel’s criteria as the reference standard. 15
To perform a wet-mount preparation correctly, dilute the vaginal discharge with 1 or 2 drops of 0.9% saline and place it on a slide. Examine the slide under lowand high-powered fields for vaginal squamous cells, white blood cells (WBCs), lactobacilli, clue cells, and trichomonads. An increased number of WBCs can be defined as >5–10 WBC/HPF or WBCs exceeding the number vaginal epithelial cells.
To prepare the potassium hydroxide (KOH) slide, place a generous amount of vaginal discharge on a slide with 10% KOH solution. Air- or flame-drying before examination under low-power microscopy may improve sensitivity. A positive KOH preparation will have hyphae, mycelial tangles, or spores.
Whiff test. The whiff test is performed by adding drops of 10% potassium hydroxide solution to the vaginal fluid. A positive result is a “fishy” amine odor. In a study16 of 100 women complaining of malodorous discharge, a positive whiff test was predictive of positive culture results for anaerobic flora such as Bacteroides sp. with sensitivity 67%, specificity 94%, and a positive predictive value of 95%. The whiff test was not positive in any of the 5 cases with positive culture results for G vaginalis in the absence of anaerobes. There were also 12 cases positive for anaerobes without G vaginalis.
pH >4.5. Since the abnormal flora of BV is consistently associated with a vaginal pH >4.5, a normal pH excludes a diagnosis of BV.17,18 The determination of pH in the narrow range around 4.5 is not accurate using standard nitrazine paper. Narrower-range test paper is available and more accurate. Examples include pH paper for 4.5 to 5.5 (Micro Essential Laboratory), FemExam pH and Amines Test Card (Litmus Concepts), pHem-ALERT: pH paper on a stick (Imagyn Gynecology). Cervical mucous, semen, and blood are alkaline and can interfere with pH testing. Estrogen production is also necessary to maintain an acidic environment. A pH of 3.8 to 4.5 is consistent with normal vaginal flora in premenopausal women with normal estrogen production.17
Clue cells. Clue cells are vaginal epithelial cells coated with coccobacilli giving an appearance as if coated with ground black pepper. Clue cells on wet mount preparation is considered the most accurate of Amsel’s diagnostic criteria for BV.19 On the other hand, office evaluation of the wet mount is considered by some authors to be unreliable due to dependence on the clinician’s microscopy skills and lack of a durable record of the patient sample.
Gram stain a more objective test. A Gram stain evaluation using Nugent’s criteria has been adopted as the gold standard test for research purposes, including studies of prematurity. The Gram-stained vaginal specimen is scored from 0 to 10 based on semi-quantitative assessment of 3 classes of morphotypes ( Table 2 ): large gram-positive rods (Lactobacilli), small gram-negative rods (Gardnerella and Bacteroides spp.), and small curved gram-variable rods (Mobiluncus spp.).11
Diagnosis of BV is typically made when the Nugent score is 7 or more, which appears qualitatively as dominant morphotypes other than Lactobacilli. Gram staining is more objective and reproducible compared with wet-mount examination, with a sensitivity of 93% and specificity of 70% if Amsel’s criteria are used as the gold standard.14 It is useful for the evaluation of asymptomatic women. It also provides a durable record of the patient specimen. Compared with Gram stain, Amsel’s criteria tend to underdiagnose cases. We can expect that if screening for BV in pregnancy becomes a recommendation, Gram staining in a clinical laboratory will be the recommended method of diagnosis.
Other diagnostic tests for BV. DNA testing for Gardnerella is accurate for detection, but it is not synonymous with a diagnosis of BV, as described.20 DNA testing is further described under “Differential Diagnosis.” Gram staining is more reliable than gas-liquid chromatography21 and an assay for proline aminopeptidase (a metabolic product of some of the bacteria associated with BV).22 Latex agglutination testing for vaginal lactoferrin is a nonspecific marker for leukocytes, and thus inflammation. It is of little clinical utility in the diagnosis of vaginal discharge.23
TABLE 1
Predictive values of Amsel’s criteria (using 3 of 4 positive as diagnostic reference standard)
| Diagnostic criterion | Predictive value (%) | |
|---|---|---|
| Positive | Negative | |
| Homogeneous thin discharge seen at introitus | 42 | 89 |
| pH >4.5 | 53 | 94 |
| Odor on alkalinization | 94 | 93 |
| Clue cells on wet mount | 90 | 99 |
| Source: Thomason et al 1990.15 | ||
TABLE 2
How to use Nugent’s Gram stain criteria to diagnose bacterial vaginosis
| Lactobacillus morphophytes | Gardnerella and Bacteroides spp. morphophytes | Curved gram-variable rods | Points |
|---|---|---|---|
| 4+ | 0 | 0 | 0 |
| 3+ | 1+ | 1+ or 2+ | 1 |
| 2+ | 2+ | 3+ or 4+ | 2 |
| 1+ | 3+ | 3 | |
| 0 | 4+ | 4 | |
| Review each of the first 3 columns in turn, assigning points at far right according to your exam findings. | |||
| Add the points for all 3 columns for a final sum. A score of 7 or higher indicates bacterial vaginosis. Source: Nugent et al 1991.11 | |||
Vulvovaginal Candidiasis
Candidiasis is the second most commonly diagnosed vaginitis in the United States. Some experts estimate that 75% of women will have a yeast infection at some point in life and 5% will have recurrent infections.24 However, 10% to 30% of asymptomatic women with normal flora have positive culture results for Candida.25-29 The proportion of symptomatic women with positive culture results is 20% to 40%.4,30,31 Complications of VVC are rare,32 though vulvar vestibulitis33 and chorioamnionitis in pregnancy32 have been reported.
Risk factors. Symptomatic yeast vaginitis has been associated with condom and diaphragm use, recent antibiotic use, receptive oral sex, oral contraceptive use, spermicide use, diabetes, and immunosuppression including AIDS.31,34-37 The associations with antibiotic use and oral contraceptives are not consistent.30,38 Although pregnancy has been postulated as a risk factor for symptomatic VVC, prevalence of yeast on culture in pregnant women is similar to that of nonpregnant women.30
Suggestive symptoms. Among women with a culture result positive for Candida, the most common symptom is pruritus or burning.28 Abnormal discharge is a complaint for most symptomatic women with VVC confirmed by culture.2 In addition, women may complain of a thick, odorless, cottage cheese–like discharge.39 A thick, curdled-appearing discharge points to a diagnosis of Candida because it is rarely present with BV or trichomoniasis. In one study,28 a thick curdled discharge had a positive predictive value of 84% for diagnosis of VVC by culture (SOR: B). However, a thin discharge does not rule out VVC; in another study, clinicians described discharge as thin in about half of women ultimately diagnosed with VVC by culture in another study (SOR: B).2 On exam, vulvar and vaginal erythema are often present but are not specific findings. The accuracy of the clinical exam for VVC is poor compared with culture (SOR: A).2,30
Pathogens. Candida albicans is present in 80% to 90% of patients with VVC.5,40 remainder have non-albicans species, including C glabrata and others.28 An increase to almost 20% of non-Candida species in a vaginitis clinic by the mid-1990’s may be related to increased use of imidazoles available over-the-counter.40,41 Wet mount results are typically negative in the presence of non-Candida VVC.28
Diagnosis of VVC
The gold standard test for diagnosis of VVC is culture. The potassium hydroxide (KOH) wet mount is only 40% to 75% sensitive.28,29,42,43 False-positive results are also observed with variable frequency.44 The pH of the discharge is usually not more than 5.0 with Candida albicans, but may be higher with non-albicans species such as C glabrata.45 Culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR: B).5,28,46 Rapid slide latex agglutination testing is not better than microscopy (SOR: B).42
Trichomoniasis
Trichomonas, a motile protozoan with 4 flagella, causes the third most common form of vaginitis in the United States and is more common in some developing countries. Trichomoniasis accounts for no more than 10% of all cases of vaginitis, and it appears to be decreasing since the introduction of metronidazole.47,48 It is classified as an STD, although transmission is possible by other means if the organism is protected from desiccation—for example, in dirty washcloths or towels and contaminated water. Nonsexual transmission is thought to be uncommon.
Trichomoniasis is associated with GC and Chlamydia infections, and, like them, has been associated with seroconversion to HIV-positive status.49 Trichomonads are identified in 30% to 80% of male sexual partners of infected women. In men, trichomoniasis most often is an asymptomatic carrier state.50 However, it is the cause of about 10% of cases of nongonococcal urethritis in men.51
Our knowledge of the epidemiology of abnormal vaginal discharge is limited. Studies of vaginitis may exclude patients with vaginal discharge due to cervicitis; studies performed in sexually transmitted disease clinics are not representative of primary care practice; women who do not complain of abnormal vaginal discharge may have positive cultures for Gardnerella vaginalis and Candida albicans; and self-treatment of presumed yeast vaginitis with antifungals available over-the-counter further limits our knowledge of the prevalence and causes of vaginal discharge.
Clinical presentations. Women with trichomoniasis have variable presentations ranging from an asymptomatic carrier state to a malodorous, purulent discharge with vulvovaginal erythema. Punctate hemorrhagic cervical lesions are considered pathognomonic of trichomoniasis, but are seen in only about 2% of cases (SOR: B).52
Diagnosis. Culture for trichomoniasis is the gold standard. Several culture media have been used, most commonly the Diamond medium. Recently introduced is a transport and culture medium for detection of Trichomonas (InPouch TV), which performs as well as Diamond medium (SOR: A).53-55 A DNA probe is also available and accurate (SOR: A).
Motile trichomonads are seen on wet preparation in only 50% to 80% of culture-positive cases (SOR: B).50,54,56 Polymorphonuclear leukocytes can be dominant on wet mount, making visualization of trichomonads more difficult. The pH of the vaginal fluid is usually basic.
Trichomonas reported with cervical cytology
Trichomonas may also be reported on Pap smears. A meta-analysis57 comparing the pooled sensitivities and specificities of wet mounts and cytology demonstrated low sensitivities of 68% and 58%, respectively, and high specificities, 99.9% and 97%, respectively (SOR: A).
However, since cytology carries a 3% false-positive rate, its results are not diagnostic of trichomoniasis in low-risk, asymptomatic women.50,57 Treatment may be prescribed empirically based on positive cytology results. However, if an asymptomatic woman were concerned about whether she really has an STD, a positive wet prep would confirm the diagnosis. A negative wet prep should be followed up with culture to reliably rule out disease (SOR: B).
Trichomoniasis in pregnancy
Screening for asymptomatic trichomoniasis in pregnancy has not been recommended. In fact, some evidence suggests that treatment of trichomoniasis in pregnancy is associated with poorer pregnancy outcomes including lower birth weight and more prematurity (SOR: B).58,59
Aerobic vaginitis
Aerobic vaginitis is a term proposed to describe purulent vaginal discharge with predominance of abnormal aerobic flora.60 Aerobic vaginitis, which may be severe, has been reported as the cause of 5% of cases in a series from a specialty vaginitis clinic.61 The usual predominant microorganisms are group B streptococci, Escherichia coli, and Staphylococcus aureus. It is likely that less severe cases of aerobic vaginitis are not recognized in the primary care setting and are treated as BV or resolve spontaneously (SOR: C). The case series referred to above also reported good therapeutic response to 2% topical clindamycin (SOR: C).61
Noninfectious Vaginitis
Noninfectious causes of vaginal discharge include physiologic, irritant and allergic, cytolytic vaginitis, desquamative inflammatory vaginitis, collagen vascular disease, and idiopathic vaginitis.
Irritant and allergic vaginitis may result from sensitivities to topical medications, the active or base ingredients of spermicidal products, douching solutions, and the latex of condoms or diaphragms. If a woman with persistent symptoms has been using such intravaginal products, she should stop (SOR: C).
Cytolytic vaginitis is characterized by overgrowth of lactobacilli and cytolysis of squamous cells, including presence of cytoplasmic fragments and intact cells with naked nuclei.62 The cause is uncertain but may include a reaction to intravaginal medications or other products such as tampons. It can be found in up to 5% of women with symptoms and signs of vaginitis.62,63 Symptoms often mimic VVC and may include a white, cheesy discharge. Vaginal pH ranges from 3.5 to 5.5. Recurrences during luteal phase of the menstrual cycle have been described.64 Intravaginal antifungals should be discontinued. Baking soda sitz baths or douches are often used, but clinical trial data to support this practice are lacking (SOR: C).
Noninfectious desquamative inflammatory vaginitis (DIV) has also been described.65 DIV is an uncommon vaginitis characterized by profuse purulent discharge with epithelial cell exfoliation. It may occur at any time during the reproductive years or after menopause. There is probably a heterogeneous group of causes of DIV. Some cases may correspond to a disorder within the spectrum of lichen planus.66 Treatment is usually difficult, though there may be some response to local or systemic corticosteroid therapy (SOR: C).65
Differential diagnosis
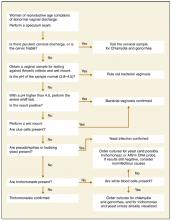
A comparison of physical examination findings an diagnostic test results for various etiologies of vaginitis is summarized in Table 3 . An algorithmic approach to the differential diagnosis of abnormal vaginal discharge is presented in the Figure . Diagnosis is complicated in that signs and symptoms do little to help differentiate among BV, VVC, and trichomoniasis. A study2 of 22 genitourinary symptoms and signs showed that none differentiated among the 3 infections. This lack of clear-cut differences in symptoms also makes self-diagnosis and telephone triage inaccurate.67,68
A DNA probe testing system (Affirm VP III Microbial ID Test) for differential diagnosis is available but expensive. It identifies Gardnerella, Trichomonas, and Candida albicans with a sensitivity of 90% to 95%.54,66 The analyzer costs approximately $10,000 and would typically be purchased by a laboratory. Individual test kits cost about $27.
TABLE 3
Comparative findings among causes of vaginitis
| Cause | Physical exam findings* | Gold standard test | pH | Leukocytes | Wet mount | Alternative test |
|---|---|---|---|---|---|---|
| Bacterial vaginosis | Variable | Gram stain | >4.5 | No | Clue cells | Amsel’s criteria |
| Aerobic vaginitis | Abundant purulent discharge | Culture | >4.5 | Yes | Cocci or coarse rods | |
| Candida vaginitis | Adherent white disch. (thrush) | Culture | 3.8–4.5 | ± | Pseudohyphae or budding yeast | DNA testing |
| Non-Candida yeast vaginitis | Variable | Culture | Any | ± | Usually negative | |
| Trichomoniasis | Variable, occ. strawberry spots on cervix | Culture | >4.5 | ± | Motile trichomonads | DNA testing |
| Cytolytic vaginitis | Profuse discharge, often cheesy | Cytology and negative culture | 3.5–5.5 | ± | Overgrowth of lactobacilli and squamous cell fragments | |
| Desquamative inflammatory vaginitis | Abundant purulent discharge | Parabasal epithelial cells and negative culture | >4.5 | Yes | ||
| Irritant and allergic vaginitis | Variable, often erythema | None | Any | ± | ||
| * Helpful when present. | ||||||
FIGURE
Sequence of office tests to evaluate abnormal vaginal discharge
Corresponding author
Linda French, MD, Associate Professor, Department of Family Practice, College of Human Medicine, Michigan State University, B101 Clinical Center, East Lansing, MI 48824. E-mail: [email protected].
- Accurate differential diagnosis for women complaining of abnormal vaginal discharge requires in-office diagnostic testing at minimum, and laboratory testing in selected cases.
- Test for Chlamydia trachomatis and Neisseria gonorrhea when signs of purulent cervicitis are present (SOR: B).
- In suspected vulvovaginal candidiasis, culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR:B); rapid slide latex agglutination testing is not better than microscopy for diagnosing VVC (SOR: B).
In primary care practice, abnormal vaginal discharge is a common complaint. Signs and symptoms of vaginitis—the most common gynecologic diagnosis in primary care1 —are not specific for any single underlying cause.2 Officebased diagnostic testing, which is underused,3 must be employed to ensure accurate diagnosis and effective treatment. (An article on treatment by the same authors will appear in next month’s issue of The journal of family practice.)
In a primary-care study,4 vulvovaginal symptoms including vaginal discharge were due to vulvovaginal candidiasis (VVC) in 27% of patients, bacterial vaginosis (BV) in 21%, trichomoniasis in 8%, Chlamydia trachomatis in 2%, Neisseria gonorrhea (GC) in 1%, and no infection in 34%. Several pathogens may coexist.2 VVC, BV, and trichomoniasis account for at least 90% of infectious vaginitis.5 This review will therefore focus heavily on these causes of vaginal discharge among women of reproductive age, including pregnant women.
Cervicitis and physiologic cervical discharge
Some women may interpret a physiologic increase in cervical mucous production as abnormal. It occurs cyclically prior to ovulation, is typically transparent and colorless, and may be more pronounced in women with an everted cervix.
Chlamydial infection
In the clinical examination of the cervix, 3 characteristics have been associated with chlamydial infection: yellow endocervical discharge, easily induced cervical bleeding, and opaque cervical discharge.6 All 3 findings are statistically significant and independently associated with chlamydial infection (odds ratios 2.8, 2.3, and 2.9, respectively). In the primary care study cited above, purulent cervical discharge was found in 6% of women, most commonly testing positive for Chlamydia, less often for GC.4
Trichomonas vaginalis may cause cervicitis as well as vaginitis. Mycoplasma genitalium has been proposed as an additional possible pathogen. It was identified in 7% of more than 700 women with mucopurulent cervical discharge seen in a STD clinic with otherwise negative cultures.7 With cervical discharge that appears to be purulent, testing is warranted as a minimum for Chlamydia and GC (SOR: B). Screening of asymptomatic women less than 26 years of age for Chlamydia is recommended by the US Preventive Services Task Force (SOR: A).
Bacterial Vaginosis
Bacterial vaginosis (BV) is neither an inflammatory condition nor an STD, but is a shift in vaginal flora from the normal condition in which lactobacilli predominate, to a polymicrobial flora in which gram-positive anaerobes predominate. In addition to annoying vaginal symptoms, BV is associated with increased risks of more serious conditions such as pelvic inflammatory disease (PID), postoperative infections, and pregnancyrelated complications including prematurity. It also increases the likelihood of acquiring HIV in women exposed to the virus.8,9
Two principal factors put women at risk for acquiring BV: douching and exposure to a new sexual partner, both of which are thought to disrupt the vaginal ecosystem.10
Relative benefits of diagnostic tests
A gold standard test has not been established for BV. In about 50% of asymptomatic women, culture results are positive for flora such as Gardnerella vaginalis.5 While Amsel’s criteria are often used as a reference and generally suffice for the evaluation of symptomatic women, the best candidate for a gold standard test is probably Gram stain assessment using Nugent’s criteria (described in this section).11 Lack of leukocytes in the vaginal fluid supports a diagnosis of BV. A finding of white blood cells in excess of the number of vaginal epithelial cells suggests an inflammatory process (SOR: C).12
Amsel’s criteria with wet mount. The diagnostic approach most commonly used in the office is Amsel’s criteria—homogenous discharge, positive whiff-amine test, pH >4.5, and clue cells found on wet-mount microscopy (see How to perform a wet mount ).13 Three of 4 criteria deemed positive is considered diagnostic. If Gram stain is used as the reference standard, then Amsel’s criteria have 70% sensitivity and 94% specificity for diagnosing BV.14 An analysis of the individual criteria follows. The positive and negative predictive values of each compared with the whole group as reference standard is displayed in Table 1 .
Homogenous discharge. A thin, homogenous, grayish discharge is traditionally associated with BV. However, it is not specific to BV, being found commonly also in women with culture results positive for VVC or no diagnosis of vaginitis.2,15 It is the criterion least likely to be consistent with the whole group, seen in about half of women BVpositive and over one third of women BV-negative using Amsel’s criteria as the reference standard. 15
To perform a wet-mount preparation correctly, dilute the vaginal discharge with 1 or 2 drops of 0.9% saline and place it on a slide. Examine the slide under lowand high-powered fields for vaginal squamous cells, white blood cells (WBCs), lactobacilli, clue cells, and trichomonads. An increased number of WBCs can be defined as >5–10 WBC/HPF or WBCs exceeding the number vaginal epithelial cells.
To prepare the potassium hydroxide (KOH) slide, place a generous amount of vaginal discharge on a slide with 10% KOH solution. Air- or flame-drying before examination under low-power microscopy may improve sensitivity. A positive KOH preparation will have hyphae, mycelial tangles, or spores.
Whiff test. The whiff test is performed by adding drops of 10% potassium hydroxide solution to the vaginal fluid. A positive result is a “fishy” amine odor. In a study16 of 100 women complaining of malodorous discharge, a positive whiff test was predictive of positive culture results for anaerobic flora such as Bacteroides sp. with sensitivity 67%, specificity 94%, and a positive predictive value of 95%. The whiff test was not positive in any of the 5 cases with positive culture results for G vaginalis in the absence of anaerobes. There were also 12 cases positive for anaerobes without G vaginalis.
pH >4.5. Since the abnormal flora of BV is consistently associated with a vaginal pH >4.5, a normal pH excludes a diagnosis of BV.17,18 The determination of pH in the narrow range around 4.5 is not accurate using standard nitrazine paper. Narrower-range test paper is available and more accurate. Examples include pH paper for 4.5 to 5.5 (Micro Essential Laboratory), FemExam pH and Amines Test Card (Litmus Concepts), pHem-ALERT: pH paper on a stick (Imagyn Gynecology). Cervical mucous, semen, and blood are alkaline and can interfere with pH testing. Estrogen production is also necessary to maintain an acidic environment. A pH of 3.8 to 4.5 is consistent with normal vaginal flora in premenopausal women with normal estrogen production.17
Clue cells. Clue cells are vaginal epithelial cells coated with coccobacilli giving an appearance as if coated with ground black pepper. Clue cells on wet mount preparation is considered the most accurate of Amsel’s diagnostic criteria for BV.19 On the other hand, office evaluation of the wet mount is considered by some authors to be unreliable due to dependence on the clinician’s microscopy skills and lack of a durable record of the patient sample.
Gram stain a more objective test. A Gram stain evaluation using Nugent’s criteria has been adopted as the gold standard test for research purposes, including studies of prematurity. The Gram-stained vaginal specimen is scored from 0 to 10 based on semi-quantitative assessment of 3 classes of morphotypes ( Table 2 ): large gram-positive rods (Lactobacilli), small gram-negative rods (Gardnerella and Bacteroides spp.), and small curved gram-variable rods (Mobiluncus spp.).11
Diagnosis of BV is typically made when the Nugent score is 7 or more, which appears qualitatively as dominant morphotypes other than Lactobacilli. Gram staining is more objective and reproducible compared with wet-mount examination, with a sensitivity of 93% and specificity of 70% if Amsel’s criteria are used as the gold standard.14 It is useful for the evaluation of asymptomatic women. It also provides a durable record of the patient specimen. Compared with Gram stain, Amsel’s criteria tend to underdiagnose cases. We can expect that if screening for BV in pregnancy becomes a recommendation, Gram staining in a clinical laboratory will be the recommended method of diagnosis.
Other diagnostic tests for BV. DNA testing for Gardnerella is accurate for detection, but it is not synonymous with a diagnosis of BV, as described.20 DNA testing is further described under “Differential Diagnosis.” Gram staining is more reliable than gas-liquid chromatography21 and an assay for proline aminopeptidase (a metabolic product of some of the bacteria associated with BV).22 Latex agglutination testing for vaginal lactoferrin is a nonspecific marker for leukocytes, and thus inflammation. It is of little clinical utility in the diagnosis of vaginal discharge.23
TABLE 1
Predictive values of Amsel’s criteria (using 3 of 4 positive as diagnostic reference standard)
| Diagnostic criterion | Predictive value (%) | |
|---|---|---|
| Positive | Negative | |
| Homogeneous thin discharge seen at introitus | 42 | 89 |
| pH >4.5 | 53 | 94 |
| Odor on alkalinization | 94 | 93 |
| Clue cells on wet mount | 90 | 99 |
| Source: Thomason et al 1990.15 | ||
TABLE 2
How to use Nugent’s Gram stain criteria to diagnose bacterial vaginosis
| Lactobacillus morphophytes | Gardnerella and Bacteroides spp. morphophytes | Curved gram-variable rods | Points |
|---|---|---|---|
| 4+ | 0 | 0 | 0 |
| 3+ | 1+ | 1+ or 2+ | 1 |
| 2+ | 2+ | 3+ or 4+ | 2 |
| 1+ | 3+ | 3 | |
| 0 | 4+ | 4 | |
| Review each of the first 3 columns in turn, assigning points at far right according to your exam findings. | |||
| Add the points for all 3 columns for a final sum. A score of 7 or higher indicates bacterial vaginosis. Source: Nugent et al 1991.11 | |||
Vulvovaginal Candidiasis
Candidiasis is the second most commonly diagnosed vaginitis in the United States. Some experts estimate that 75% of women will have a yeast infection at some point in life and 5% will have recurrent infections.24 However, 10% to 30% of asymptomatic women with normal flora have positive culture results for Candida.25-29 The proportion of symptomatic women with positive culture results is 20% to 40%.4,30,31 Complications of VVC are rare,32 though vulvar vestibulitis33 and chorioamnionitis in pregnancy32 have been reported.
Risk factors. Symptomatic yeast vaginitis has been associated with condom and diaphragm use, recent antibiotic use, receptive oral sex, oral contraceptive use, spermicide use, diabetes, and immunosuppression including AIDS.31,34-37 The associations with antibiotic use and oral contraceptives are not consistent.30,38 Although pregnancy has been postulated as a risk factor for symptomatic VVC, prevalence of yeast on culture in pregnant women is similar to that of nonpregnant women.30
Suggestive symptoms. Among women with a culture result positive for Candida, the most common symptom is pruritus or burning.28 Abnormal discharge is a complaint for most symptomatic women with VVC confirmed by culture.2 In addition, women may complain of a thick, odorless, cottage cheese–like discharge.39 A thick, curdled-appearing discharge points to a diagnosis of Candida because it is rarely present with BV or trichomoniasis. In one study,28 a thick curdled discharge had a positive predictive value of 84% for diagnosis of VVC by culture (SOR: B). However, a thin discharge does not rule out VVC; in another study, clinicians described discharge as thin in about half of women ultimately diagnosed with VVC by culture in another study (SOR: B).2 On exam, vulvar and vaginal erythema are often present but are not specific findings. The accuracy of the clinical exam for VVC is poor compared with culture (SOR: A).2,30
Pathogens. Candida albicans is present in 80% to 90% of patients with VVC.5,40 remainder have non-albicans species, including C glabrata and others.28 An increase to almost 20% of non-Candida species in a vaginitis clinic by the mid-1990’s may be related to increased use of imidazoles available over-the-counter.40,41 Wet mount results are typically negative in the presence of non-Candida VVC.28
Diagnosis of VVC
The gold standard test for diagnosis of VVC is culture. The potassium hydroxide (KOH) wet mount is only 40% to 75% sensitive.28,29,42,43 False-positive results are also observed with variable frequency.44 The pH of the discharge is usually not more than 5.0 with Candida albicans, but may be higher with non-albicans species such as C glabrata.45 Culture is recommended for patients with recurrent or persistent symptoms and a negative wet mount result (SOR: B).5,28,46 Rapid slide latex agglutination testing is not better than microscopy (SOR: B).42
Trichomoniasis
Trichomonas, a motile protozoan with 4 flagella, causes the third most common form of vaginitis in the United States and is more common in some developing countries. Trichomoniasis accounts for no more than 10% of all cases of vaginitis, and it appears to be decreasing since the introduction of metronidazole.47,48 It is classified as an STD, although transmission is possible by other means if the organism is protected from desiccation—for example, in dirty washcloths or towels and contaminated water. Nonsexual transmission is thought to be uncommon.
Trichomoniasis is associated with GC and Chlamydia infections, and, like them, has been associated with seroconversion to HIV-positive status.49 Trichomonads are identified in 30% to 80% of male sexual partners of infected women. In men, trichomoniasis most often is an asymptomatic carrier state.50 However, it is the cause of about 10% of cases of nongonococcal urethritis in men.51
Our knowledge of the epidemiology of abnormal vaginal discharge is limited. Studies of vaginitis may exclude patients with vaginal discharge due to cervicitis; studies performed in sexually transmitted disease clinics are not representative of primary care practice; women who do not complain of abnormal vaginal discharge may have positive cultures for Gardnerella vaginalis and Candida albicans; and self-treatment of presumed yeast vaginitis with antifungals available over-the-counter further limits our knowledge of the prevalence and causes of vaginal discharge.
Clinical presentations. Women with trichomoniasis have variable presentations ranging from an asymptomatic carrier state to a malodorous, purulent discharge with vulvovaginal erythema. Punctate hemorrhagic cervical lesions are considered pathognomonic of trichomoniasis, but are seen in only about 2% of cases (SOR: B).52
Diagnosis. Culture for trichomoniasis is the gold standard. Several culture media have been used, most commonly the Diamond medium. Recently introduced is a transport and culture medium for detection of Trichomonas (InPouch TV), which performs as well as Diamond medium (SOR: A).53-55 A DNA probe is also available and accurate (SOR: A).
Motile trichomonads are seen on wet preparation in only 50% to 80% of culture-positive cases (SOR: B).50,54,56 Polymorphonuclear leukocytes can be dominant on wet mount, making visualization of trichomonads more difficult. The pH of the vaginal fluid is usually basic.
Trichomonas reported with cervical cytology
Trichomonas may also be reported on Pap smears. A meta-analysis57 comparing the pooled sensitivities and specificities of wet mounts and cytology demonstrated low sensitivities of 68% and 58%, respectively, and high specificities, 99.9% and 97%, respectively (SOR: A).
However, since cytology carries a 3% false-positive rate, its results are not diagnostic of trichomoniasis in low-risk, asymptomatic women.50,57 Treatment may be prescribed empirically based on positive cytology results. However, if an asymptomatic woman were concerned about whether she really has an STD, a positive wet prep would confirm the diagnosis. A negative wet prep should be followed up with culture to reliably rule out disease (SOR: B).
Trichomoniasis in pregnancy
Screening for asymptomatic trichomoniasis in pregnancy has not been recommended. In fact, some evidence suggests that treatment of trichomoniasis in pregnancy is associated with poorer pregnancy outcomes including lower birth weight and more prematurity (SOR: B).58,59
Aerobic vaginitis
Aerobic vaginitis is a term proposed to describe purulent vaginal discharge with predominance of abnormal aerobic flora.60 Aerobic vaginitis, which may be severe, has been reported as the cause of 5% of cases in a series from a specialty vaginitis clinic.61 The usual predominant microorganisms are group B streptococci, Escherichia coli, and Staphylococcus aureus. It is likely that less severe cases of aerobic vaginitis are not recognized in the primary care setting and are treated as BV or resolve spontaneously (SOR: C). The case series referred to above also reported good therapeutic response to 2% topical clindamycin (SOR: C).61
Noninfectious Vaginitis
Noninfectious causes of vaginal discharge include physiologic, irritant and allergic, cytolytic vaginitis, desquamative inflammatory vaginitis, collagen vascular disease, and idiopathic vaginitis.
Irritant and allergic vaginitis may result from sensitivities to topical medications, the active or base ingredients of spermicidal products, douching solutions, and the latex of condoms or diaphragms. If a woman with persistent symptoms has been using such intravaginal products, she should stop (SOR: C).
Cytolytic vaginitis is characterized by overgrowth of lactobacilli and cytolysis of squamous cells, including presence of cytoplasmic fragments and intact cells with naked nuclei.62 The cause is uncertain but may include a reaction to intravaginal medications or other products such as tampons. It can be found in up to 5% of women with symptoms and signs of vaginitis.62,63 Symptoms often mimic VVC and may include a white, cheesy discharge. Vaginal pH ranges from 3.5 to 5.5. Recurrences during luteal phase of the menstrual cycle have been described.64 Intravaginal antifungals should be discontinued. Baking soda sitz baths or douches are often used, but clinical trial data to support this practice are lacking (SOR: C).
Noninfectious desquamative inflammatory vaginitis (DIV) has also been described.65 DIV is an uncommon vaginitis characterized by profuse purulent discharge with epithelial cell exfoliation. It may occur at any time during the reproductive years or after menopause. There is probably a heterogeneous group of causes of DIV. Some cases may correspond to a disorder within the spectrum of lichen planus.66 Treatment is usually difficult, though there may be some response to local or systemic corticosteroid therapy (SOR: C).65
Differential diagnosis
A comparison of physical examination findings an diagnostic test results for various etiologies of vaginitis is summarized in Table 3 . An algorithmic approach to the differential diagnosis of abnormal vaginal discharge is presented in the Figure . Diagnosis is complicated in that signs and symptoms do little to help differentiate among BV, VVC, and trichomoniasis. A study2 of 22 genitourinary symptoms and signs showed that none differentiated among the 3 infections. This lack of clear-cut differences in symptoms also makes self-diagnosis and telephone triage inaccurate.67,68
A DNA probe testing system (Affirm VP III Microbial ID Test) for differential diagnosis is available but expensive. It identifies Gardnerella, Trichomonas, and Candida albicans with a sensitivity of 90% to 95%.54,66 The analyzer costs approximately $10,000 and would typically be purchased by a laboratory. Individual test kits cost about $27.
TABLE 3
Comparative findings among causes of vaginitis
| Cause | Physical exam findings* | Gold standard test | pH | Leukocytes | Wet mount | Alternative test |
|---|---|---|---|---|---|---|
| Bacterial vaginosis | Variable | Gram stain | >4.5 | No | Clue cells | Amsel’s criteria |
| Aerobic vaginitis | Abundant purulent discharge | Culture | >4.5 | Yes | Cocci or coarse rods | |
| Candida vaginitis | Adherent white disch. (thrush) | Culture | 3.8–4.5 | ± | Pseudohyphae or budding yeast | DNA testing |
| Non-Candida yeast vaginitis | Variable | Culture | Any | ± | Usually negative | |
| Trichomoniasis | Variable, occ. strawberry spots on cervix | Culture | >4.5 | ± | Motile trichomonads | DNA testing |
| Cytolytic vaginitis | Profuse discharge, often cheesy | Cytology and negative culture | 3.5–5.5 | ± | Overgrowth of lactobacilli and squamous cell fragments | |
| Desquamative inflammatory vaginitis | Abundant purulent discharge | Parabasal epithelial cells and negative culture | >4.5 | Yes | ||
| Irritant and allergic vaginitis | Variable, often erythema | None | Any | ± | ||
| * Helpful when present. | ||||||
FIGURE
Sequence of office tests to evaluate abnormal vaginal discharge
Corresponding author
Linda French, MD, Associate Professor, Department of Family Practice, College of Human Medicine, Michigan State University, B101 Clinical Center, East Lansing, MI 48824. E-mail: [email protected].
1. National Center for Health Statistics. National Ambulatory Medicine Care Survey. Available at: www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm.
2. Schaaf VM, Perez-Stable EJ, Borchardt K. The limited value of symptoms and signs in the diagnosis of vaginal infections. Arch Intern Med 1990;150:1929-1933.
3. Wiesenfeld HC, Macio I. The infrequent use of officebased diagnostic tests for vaginitis. Am J Obstet Gynecol 1999;181:39-41.
4. Berg AO, Heidrich FE, Fihn SD, et al. Establishing the cause of genitourinary symptoms in women in a family practice. Comparison of clinical examination and comprehensive microbiology. JAMA 1984;251:620-625.
5. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
6. Sellors JW, Walter SD, Howard M. A new visual indicator of chlamydial cervicitis? Sex Transm Infect 2000;76:46-48.
7. Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis 2003;187:650-657.
8. Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180:1863-1868.
9. Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses 1998;14Suppl 1:S17-21.
10. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogenperoxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. JClin Microbiol 1991;29:297-301.
12. Quan M. Vaginitis: meeting the clinical challenge. Clin Cornerstone 2000;3:36-47.
13. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14-22.
14. Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. ObstetGynecol 1996;88:573-576.
15. Thomason JL, Gelbart SM, Anderson RJ, Walt AK, Osypowski PJ, Broekhuizen FF. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am J Obstet Gynecol. 1990;162:155-160.
16. Erkkola R, Jarvinen H, Terho P, Meurman O. Microbial flora in women showing symptoms of nonspecific vaginosis: applicability of KOH test for diagnosis. Scand J Infect Dis Suppl. 1983;40:59-63.
17. Caillouette JC, Sharp CF, Jr, Zimmerman GJ, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. 1997;176:1270-1275discussion1275-1277.
18. Carr PL, Felsenstein D, Friedman RH. Evaluation and management of vaginitis. J Gen Intern Med 1998;13:335-346.
19. Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988;158:819-828.
20. Sheiness D, Dix K, Watanabe S, Hillier SL. Highlevels of Gardnerella vaginalis detected with an oligonucleotide probe combined with elevated pH as a diagnostic indicator of bacterial vaginosis. J Clin Microbiol 1992;30:642-648.
21. Thomason JL, Gelbart SM, James JA, Edwards JM, Hamilton PR. Is analysis of vaginal secretions for volatile organic acids to detect bacterial vaginosis of any diagnostic value? Am J Obstet Gynecol. 1988;159:1509-1511.
22. Thomason JL, Gelbart SM, Wilcoski LM, Peterson AK, Jilly BJ, Hamilton PR. Proline aminopeptidase activity as a rapid diagnostic test to confirm bacterial vaginosis. Obstet Gynecol 1988;71:607-611.
23. Rein MF, Shih LM, Miller JR, Guerrant RL. Use of a lactoferrin assay in the differential diagnosis of female genital tract infections and implications for the pathophysiology of bacterial vaginosis. Sex Transm Dis 1996;23:517-521.
24. Monif GR. Classification and pathogenesis of vulvovaginal candidiasis. Am J Obstet Gynecol 1985;152:935-939.
25. Giraldo P, von Nowaskonski A, Gomes FA, Linhares I, Neves NA, Witkin SS. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstet Gynecol 2000;95:413-416.
26. Bergman JJ, Berg AO. How useful are symptoms in the diagnosis of Candida vaginitis? J Fam Pract 1983;16:509-511.
27. Bro F. Patients with vaginal discharge in general practice. Acta Obstet Gynecol Scand 1989;68:41-43.
28. Eckert LO, Hawes SE, Stevens CE, Koutsky LA, Eschenbach DA, Holmes KK. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol 1998;92:757-765.
29. Bertholf ME, Stafford MJ. Colonization of Candida albicans in vagina, rectum, and mouth. J Fam Pract 1983;16:919-924.
30. Reed BD, Huck W, Zazove P. Differentiation of Gardnerella vaginalis, Candida albicans, and Trichomonas vaginalis infections of the vagina. J Fam Pract 1989;28:673-680.
31. Bro F. The diagnosis of candida vaginitis in general practice. Scand J Prim Health Care 1989;7:19-22.
32. Cotch MF, Hillier SL, Gibbs RS, Eschenbach DA. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 1998;178:374-380.
33. Pagano R. Vulvar vestibulitis syndrome: an often unrecognized cause of dyspareunia. Aust N Z J Obstet Gynaecol 1999;39:79-83.
34. Foxman B. The epidemiology of vulvovaginal candidiasis: risk factors. Am J Public Health 1990;80:329-331.
35. Geiger AM, Foxman B. Risk factors for vulvovaginal candidiasis: a case-control study among university students. Epidemiology 1996;7:182-187.
36. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
37. Spinillo A, Capuzzo E, Acciano S, De Santolo A, Zara F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am J Obstet Gynecol 1999;180:14-17.
38. Davidson F, Oates JK. The pill does not cause ‘thrush’. Br J Obstet Gynaecol Dec 1985;92:1265-1266.
39. Abbott J. Clinical and microscopic diagnosis of vaginal yeast infection: a prospective analysis. Ann Emerg Med 1995;25:587-591.
40. Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J Clin Pharmacol 1992;32:248-255.
41. Spinillo A, Capuzzo E, Gulminetti R, Marone P, Colonna L, Piazzi G. Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am J Obstet Gynecol 1997;176:138-141.
42. Reed BD, Pierson CL. Evaluation of a latex agglutination test for the identification of Candida species in vaginal discharge. J Am Board Fam Pract 1992;5:375-380.
43. Ferris DG, Hendrich J, Payne PM, et al. Office laboratoAE_French.1004.final 9/20/04 3:18 PM Page 813 ry diagnosis of vaginitis. Clinician-performed tests compared with a rapid nucleic acid hybridization test. J Fam Pract 1995;41:575-581.
44. Bergman JJ, Berg AO, Schneeweiss R, Heidrich FE. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J Fam Pract 1984;18:549-552.
45. Sobel JD. Vulvovaginitis due to Candida glabrata. An emerging problem. Mycoses. 1998;41:Suppl 2:18-22.
46. Zdolsek B, Hellberg D, Froman G, Nilsson S, Mardh PA. Culture and wet smear microscopy in the diagnosis of low-symptomatic vulvovaginal candidosis. Eur J Obstet Gynecol Reprod Biol 1995;58:47-51.
47. Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991;165:1217-1222.
48. Kent HL. Epidemiology of vaginitis. Am J Obstet Gynecol 1991;165:1168-1176.
49. Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 1993;7:95-102.
50. Krieger JN, Tam MR, Stevens CE, et al. Diagnosis of trichomoniasis. Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimens. JAMA 1988;259:1223-1227.
51. Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995;22:83-96.
52. Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis 1980;141:137-143.
53. Ohlemeyer CL, Hornberger LL, Lynch DA, Swierkosz EM. Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy. J Adolesc Health 1998;22:205-208.
54. Borchardt KA, Smith RF. An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Med 1991;67:149-152.
55. Levi MH, Torres J, Pina C, Klein RS. Comparison of the InPouch TV culture system and Diamond’s modified medium for detection of Trichomonas vaginalis. J Clin Microbiol 1997;35:3308-3310.
56. DeMeo LR, Draper DL, McGregor JA, et al. Evaluation of a deoxyribonucleic acid probe for the detection of Trichomonas vaginalis in vaginal secretions. Am J Obstet Gynecol 1996;174:1339-1342.
57. Wiese W, Patel SR, Patel SC, Ohl CA, Estrada CA. A meta-analysis of the Papanicolaou smear and wet mount for the diagnosis of vaginal trichomoniasis. Am J Med 2000;108:301-308.
58. Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001;345:487-493.
59. Kigozi GG, Brahmbhatt H, Wabwire-Mangen F, et al. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2003;189:1398-1400.
60. Donder GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Bjog 2002;109:34-43.
61. Sobel JD. Desquamative inflammatory vaginitis: a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol 1994;171:1215-1220.
62. Demirezen S. Cytolytic vaginosis: examination of 2947 vaginal smears. Cent Eur J Public Health 2003;11:23-24.
63. Wathne B, Holst E, Hovelius B, Mardh PA. Vaginal discharge—comparison of clinical, laboratory and microbiological findings. Acta Obstet Gynecol Scand 1994;73:802-808.
64. Secor RM. Cytolytic vaginosis: a common cause of cyclic vulvovaginitis. Nurse Pract Forum 1992;3:145-148.
65. Oates JK, Rowen D. Desquamative inflammatory vaginitis. A review. Genitourin Med 1990;66:275-279.
66. Pelisse M. The vulvo-vaginal-gingival syndrome. A new form of erosive lichen planus. Int J Dermatol 1989;28:381-384.
67. Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol 2002;99:419-425.
68. Allen-Davis JT, Beck A, Parker R, Ellis JL, Polley D. Assessment of vulvovaginal complaints: accuracy of telephone triage and in-office diagnosis. Obstet Gynecol 2002;99:18-22.
1. National Center for Health Statistics. National Ambulatory Medicine Care Survey. Available at: www.cdc.gov/nchs/about/major/ahcd/ahcd1.htm.
2. Schaaf VM, Perez-Stable EJ, Borchardt K. The limited value of symptoms and signs in the diagnosis of vaginal infections. Arch Intern Med 1990;150:1929-1933.
3. Wiesenfeld HC, Macio I. The infrequent use of officebased diagnostic tests for vaginitis. Am J Obstet Gynecol 1999;181:39-41.
4. Berg AO, Heidrich FE, Fihn SD, et al. Establishing the cause of genitourinary symptoms in women in a family practice. Comparison of clinical examination and comprehensive microbiology. JAMA 1984;251:620-625.
5. Sobel JD. Vaginitis. N Engl J Med 1997;337:1896-1903.
6. Sellors JW, Walter SD, Howard M. A new visual indicator of chlamydial cervicitis? Sex Transm Infect 2000;76:46-48.
7. Manhart LE, Critchlow CW, Holmes KK, et al. Mucopurulent cervicitis and Mycoplasma genitalium. J Infect Dis 2003;187:650-657.
8. Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999;180:1863-1868.
9. Hillier SL. The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses 1998;14Suppl 1:S17-21.
10. Hawes SE, Hillier SL, Benedetti J, et al. Hydrogenperoxide-producing lactobacilli and acquisition of vaginal infections. J Infect Dis 1996;174:1058-1063.
11. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. JClin Microbiol 1991;29:297-301.
12. Quan M. Vaginitis: meeting the clinical challenge. Clin Cornerstone 2000;3:36-47.
13. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983;74:14-22.
14. Schwebke JR, Hillier SL, Sobel JD, McGregor JA, Sweet RL. Validity of the vaginal gram stain for the diagnosis of bacterial vaginosis. ObstetGynecol 1996;88:573-576.
15. Thomason JL, Gelbart SM, Anderson RJ, Walt AK, Osypowski PJ, Broekhuizen FF. Statistical evaluation of diagnostic criteria for bacterial vaginosis. Am J Obstet Gynecol. 1990;162:155-160.
16. Erkkola R, Jarvinen H, Terho P, Meurman O. Microbial flora in women showing symptoms of nonspecific vaginosis: applicability of KOH test for diagnosis. Scand J Infect Dis Suppl. 1983;40:59-63.
17. Caillouette JC, Sharp CF, Jr, Zimmerman GJ, Roy S. Vaginal pH as a marker for bacterial pathogens and menopausal status. Am J Obstet Gynecol. 1997;176:1270-1275discussion1275-1277.
18. Carr PL, Felsenstein D, Friedman RH. Evaluation and management of vaginitis. J Gen Intern Med 1998;13:335-346.
19. Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol 1988;158:819-828.
20. Sheiness D, Dix K, Watanabe S, Hillier SL. Highlevels of Gardnerella vaginalis detected with an oligonucleotide probe combined with elevated pH as a diagnostic indicator of bacterial vaginosis. J Clin Microbiol 1992;30:642-648.
21. Thomason JL, Gelbart SM, James JA, Edwards JM, Hamilton PR. Is analysis of vaginal secretions for volatile organic acids to detect bacterial vaginosis of any diagnostic value? Am J Obstet Gynecol. 1988;159:1509-1511.
22. Thomason JL, Gelbart SM, Wilcoski LM, Peterson AK, Jilly BJ, Hamilton PR. Proline aminopeptidase activity as a rapid diagnostic test to confirm bacterial vaginosis. Obstet Gynecol 1988;71:607-611.
23. Rein MF, Shih LM, Miller JR, Guerrant RL. Use of a lactoferrin assay in the differential diagnosis of female genital tract infections and implications for the pathophysiology of bacterial vaginosis. Sex Transm Dis 1996;23:517-521.
24. Monif GR. Classification and pathogenesis of vulvovaginal candidiasis. Am J Obstet Gynecol 1985;152:935-939.
25. Giraldo P, von Nowaskonski A, Gomes FA, Linhares I, Neves NA, Witkin SS. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstet Gynecol 2000;95:413-416.
26. Bergman JJ, Berg AO. How useful are symptoms in the diagnosis of Candida vaginitis? J Fam Pract 1983;16:509-511.
27. Bro F. Patients with vaginal discharge in general practice. Acta Obstet Gynecol Scand 1989;68:41-43.
28. Eckert LO, Hawes SE, Stevens CE, Koutsky LA, Eschenbach DA, Holmes KK. Vulvovaginal candidiasis: clinical manifestations, risk factors, management algorithm. Obstet Gynecol 1998;92:757-765.
29. Bertholf ME, Stafford MJ. Colonization of Candida albicans in vagina, rectum, and mouth. J Fam Pract 1983;16:919-924.
30. Reed BD, Huck W, Zazove P. Differentiation of Gardnerella vaginalis, Candida albicans, and Trichomonas vaginalis infections of the vagina. J Fam Pract 1989;28:673-680.
31. Bro F. The diagnosis of candida vaginitis in general practice. Scand J Prim Health Care 1989;7:19-22.
32. Cotch MF, Hillier SL, Gibbs RS, Eschenbach DA. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol 1998;178:374-380.
33. Pagano R. Vulvar vestibulitis syndrome: an often unrecognized cause of dyspareunia. Aust N Z J Obstet Gynaecol 1999;39:79-83.
34. Foxman B. The epidemiology of vulvovaginal candidiasis: risk factors. Am J Public Health 1990;80:329-331.
35. Geiger AM, Foxman B. Risk factors for vulvovaginal candidiasis: a case-control study among university students. Epidemiology 1996;7:182-187.
36. Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol 1998;178:203-211.
37. Spinillo A, Capuzzo E, Acciano S, De Santolo A, Zara F. Effect of antibiotic use on the prevalence of symptomatic vulvovaginal candidiasis. Am J Obstet Gynecol 1999;180:14-17.
38. Davidson F, Oates JK. The pill does not cause ‘thrush’. Br J Obstet Gynaecol Dec 1985;92:1265-1266.
39. Abbott J. Clinical and microscopic diagnosis of vaginal yeast infection: a prospective analysis. Ann Emerg Med 1995;25:587-591.
40. Horowitz BJ, Giaquinta D, Ito S. Evolving pathogens in vulvovaginal candidiasis: implications for patient care. J Clin Pharmacol 1992;32:248-255.
41. Spinillo A, Capuzzo E, Gulminetti R, Marone P, Colonna L, Piazzi G. Prevalence of and risk factors for fungal vaginitis caused by non-albicans species. Am J Obstet Gynecol 1997;176:138-141.
42. Reed BD, Pierson CL. Evaluation of a latex agglutination test for the identification of Candida species in vaginal discharge. J Am Board Fam Pract 1992;5:375-380.
43. Ferris DG, Hendrich J, Payne PM, et al. Office laboratoAE_French.1004.final 9/20/04 3:18 PM Page 813 ry diagnosis of vaginitis. Clinician-performed tests compared with a rapid nucleic acid hybridization test. J Fam Pract 1995;41:575-581.
44. Bergman JJ, Berg AO, Schneeweiss R, Heidrich FE. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J Fam Pract 1984;18:549-552.
45. Sobel JD. Vulvovaginitis due to Candida glabrata. An emerging problem. Mycoses. 1998;41:Suppl 2:18-22.
46. Zdolsek B, Hellberg D, Froman G, Nilsson S, Mardh PA. Culture and wet smear microscopy in the diagnosis of low-symptomatic vulvovaginal candidosis. Eur J Obstet Gynecol Reprod Biol 1995;58:47-51.
47. Lossick JG, Kent HL. Trichomoniasis: trends in diagnosis and management. Am J Obstet Gynecol 1991;165:1217-1222.
48. Kent HL. Epidemiology of vaginitis. Am J Obstet Gynecol 1991;165:1168-1176.
49. Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 1993;7:95-102.
50. Krieger JN, Tam MR, Stevens CE, et al. Diagnosis of trichomoniasis. Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimens. JAMA 1988;259:1223-1227.
51. Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995;22:83-96.
52. Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis 1980;141:137-143.
53. Ohlemeyer CL, Hornberger LL, Lynch DA, Swierkosz EM. Diagnosis of Trichomonas vaginalis in adolescent females: InPouch TV culture versus wet-mount microscopy. J Adolesc Health 1998;22:205-208.
54. Borchardt KA, Smith RF. An evaluation of an InPouch TV culture method for diagnosing Trichomonas vaginalis infection. Genitourin Med 1991;67:149-152.
55. Levi MH, Torres J, Pina C, Klein RS. Comparison of the InPouch TV culture system and Diamond’s modified medium for detection of Trichomonas vaginalis. J Clin Microbiol 1997;35:3308-3310.
56. DeMeo LR, Draper DL, McGregor JA, et al. Evaluation of a deoxyribonucleic acid probe for the detection of Trichomonas vaginalis in vaginal secretions. Am J Obstet Gynecol 1996;174:1339-1342.
57. Wiese W, Patel SR, Patel SC, Ohl CA, Estrada CA. A meta-analysis of the Papanicolaou smear and wet mount for the diagnosis of vaginal trichomoniasis. Am J Med 2000;108:301-308.
58. Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001;345:487-493.
59. Kigozi GG, Brahmbhatt H, Wabwire-Mangen F, et al. Treatment of Trichomonas in pregnancy and adverse outcomes of pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2003;189:1398-1400.
60. Donder GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Bjog 2002;109:34-43.
61. Sobel JD. Desquamative inflammatory vaginitis: a new subgroup of purulent vaginitis responsive to topical 2% clindamycin therapy. Am J Obstet Gynecol 1994;171:1215-1220.
62. Demirezen S. Cytolytic vaginosis: examination of 2947 vaginal smears. Cent Eur J Public Health 2003;11:23-24.
63. Wathne B, Holst E, Hovelius B, Mardh PA. Vaginal discharge—comparison of clinical, laboratory and microbiological findings. Acta Obstet Gynecol Scand 1994;73:802-808.
64. Secor RM. Cytolytic vaginosis: a common cause of cyclic vulvovaginitis. Nurse Pract Forum 1992;3:145-148.
65. Oates JK, Rowen D. Desquamative inflammatory vaginitis. A review. Genitourin Med 1990;66:275-279.
66. Pelisse M. The vulvo-vaginal-gingival syndrome. A new form of erosive lichen planus. Int J Dermatol 1989;28:381-384.
67. Ferris DG, Nyirjesy P, Sobel JD, Soper D, Pavletic A, Litaker MS. Over-the-counter antifungal drug misuse associated with patient-diagnosed vulvovaginal candidiasis. Obstet Gynecol 2002;99:419-425.
68. Allen-Davis JT, Beck A, Parker R, Ellis JL, Polley D. Assessment of vulvovaginal complaints: accuracy of telephone triage and in-office diagnosis. Obstet Gynecol 2002;99:18-22.