User login
Patient and physician outreach boost CRC screening rates
Can outreach improve the globally low rates of adherence to colorectal cancer screening? Yes, according to two recent studies in JAMA; the studies found that both patient-focused and physician-focused outreach approaches can result in significantly better patient participation in colorectal cancer (CRC) screening.
The first study (JAMA. 2017;318[9]:806-15) compared a colonoscopy outreach program and a fecal immunochemical test (FIT) outreach program both with each other and with usual care. The results of the pragmatic, single-site, randomized, clinical trial showed that completed screenings were higher for both outreach groups, compared with the usual-care group.
The primary outcome measure of the study was completion of the screening process, wrote Amit Singal, MD, and his coauthors. This was defined as any adherence to colonoscopy completion, the completion of annual testing for patients who had a normal FIT test, or treatment evaluation if CRC was detected during the screening process. Screenings were considered complete even if, for example, a patient in the colonoscopy arm eventually went on to have three consecutive annual FIT tests rather than a colonoscopy.
A total of 5,999 patients eligible for screening were initially randomized to one of the three study arms. Across all study arms, approximately half were lost to follow-up. These patients were excluded from the primary analysis but were included in an additional intention-to-screen analysis. A total of 2,400 patients received a colonoscopy outreach mailing; 2,400 received FIT outreach, including a letter, the home FIT testing kit, and instructions; 1,199 received usual care. Patients in both intervention arms also received up to two phone calls if they didn’t respond to the initial mailing within 2 weeks. Mailings and phone calls were conducted in English or Spanish, according to the patients’ stated language preferences (those whose spoke neither language were excluded from the study).
Of the patients in the colonoscopy outreach group, 922 (38.4%) completed the screening process, compared with 671 (28.0%) in the FIT outreach group and 128 (10.7%) in the usual-care group.
Compared with the group receiving usual care, completion of the screening process was 27.7% higher in the colonoscopy outreach group and 17.3% higher in the FIT outreach group. Screening process completion was 10.4% higher for the colonoscopy outreach group, compared with the FIT outreach group (P less than .001 for all).
Dr. Singal, who is with the department of internal medicine at UT Southwestern Medical Center, Dallas, and his colleagues also performed several post-hoc secondary analyses. In one, they used a less-stringent definition of screening process completion in which biennial FIT testing was considered satisfactory. When this definition was applied, the colonoscopy outreach group had 0.5% lower screening process completion than the FIT outreach group. The chances of a patient receiving any screening during the study period was highest in the FIT group (65%), with 51.7% of those in the colonoscopy outreach group and 39% of those in the usual-care group receiving any screening.
“FIT has lower barriers to one-time participation but requires annual screening and diagnostic evaluation of abnormal results,” wrote Dr. Singal and his colleagues.
Strengths of the study, said Dr. Singal and his colleagues, included the fact that the study took place at a “safety net” institution with a racially and socioeconomically diverse population. Also, the study design avoided volunteer bias, and offered a pragmatic head-to-head comparison of colonoscopy and FIT.
The second study took place in western France, and targeted outreach to physicians rather than patients (JAMA. 2017;318[9];816-84). When physicians were given a list of their own patients who were not up to date on CRC screening, investigators saw a small, but significant, uptick in patient participation in FIT screening.
One year after the reminders went out, FIT screening had been initiated in 24.8% of patients whose physicians had received the list, compared with 21.7% of patients of physicians who had received a more generic notice and 20.6% of patients whose physicians received no notification, according to first author Cedric Rat, MD, and his colleagues.
The study examined which notification approach was most effective in increasing FIT screening among the physicians’ patient panels: sending general practitioners (GPs) letters that included a list of their own patients who had not undergone CRC screening, or sending them generic letters describing CRC screening adherence rates specific to their region. A usual-care group of practices received no notifications in this three-group randomized cluster design.
Patients in the patient-specific reminders group had an odds ratio of 1.27 for participation in FIT screening (P less than .001) compared to the usual-care group. The odds ratio for the generic-reminders group was 1.09, a nonsignificant difference.
Between-group comparison showed statistical significance for both the 3.1% difference between the patient-specific and generic-reminders groups, and for the 4.2% difference between the patient-specific and usual-care groups (P less than .001 for both). There was no significant difference between the generic- reminders group and the usual-care group.
Dr. Rat, professor of medicine at the Faculty of Medicine, Nantes, France, and his colleagues enrolled GPs in a total of 801 practices that included patients aged 50. Participating GPs cared for 33,044 patients who met study criteria.
Physician characteristics that were associated with higher FIT participation included younger age and an initially smaller number of unscreened patients. Patients with low socioeconomic status and those with a higher chronic disease burden were less likely to participate in FIT screening.
Dr. Rat and his colleagues noted that the busiest practices actually had higher CRC screening rates. The investigators hypothesized that a recent physician pay-for-performance grant for CRC completion might be more appealing for some busy physicians.
This was the largest study of CRC screening participation to date, according to Dr. Rat and his coauthors, and showed the small but detectable efficacy of an inexpensive intervention that, given complete patient records, is relatively easy to effect. Though the effect size was smaller than the 12% difference the investigators had anticipated seeing for the patient-specific reminders group, the study still showed that targeting physicians can be an effective public health intervention to increase CRC screening rates, said Dr. Rat and his colleagues.
None of the investigators in either study reported conflicts of interest.
The AGA Colorectal Cancer Clinical Service Line provides tools to help you become more efficient, understand quality standards and improve the process of care for patients. Learn more at www.gastro.org/crc.
Both studies, though they used different outreach interventions, highlight the same problem: the need to identify and execute effective colorectal cancer (CRC) screening programs. Effective screening has great lifesaving potential; if screening rates were elevated to greater than 80% in the United States, an estimated 200,000 deaths would be prevented within the next 2 decades.
The nature of CRC screening options means that a home fecal sample collection is inexpensive, and will result in an initial higher screening rate; however, complete screening via fecal occult blood testing requires annual repeats of negative tests, and patients with positive fecal occult blood tests still need colonoscopy.
Colonoscopy, although it’s burdensome for patients and perhaps cost prohibitive for those without health insurance, offers a one-time test that, if negative, provides patients with a 10-year window of screening coverage.
Any effective programs to increase CRC screening rates will need to use a systems change approach, with creative interventions that take patient education, and even delivery of preventive health services, out of the context of the already too-full office visit.
Staff supports, such as the follow-up telephone calls used in the patient-targeted intervention, are key to effective interventions, especially for vulnerable populations. Additionally, institutions must ensure that they have adequate physical and staff resources to support the increased screening they are seeking to achieve.
Michael Pignone, MD, MPH is a professor of medicine at the University of Texas at Austin. David Miller Jr., MD is a professor of internal medicine, Wake Forest University, Winston-Salem, N.C. Dr. Pignone is a medical director for Healthwise; Dr. Miller reported no relevant conflicts of interest. These remarks were drawn from an editorial accompanying the two clinical trials.
Both studies, though they used different outreach interventions, highlight the same problem: the need to identify and execute effective colorectal cancer (CRC) screening programs. Effective screening has great lifesaving potential; if screening rates were elevated to greater than 80% in the United States, an estimated 200,000 deaths would be prevented within the next 2 decades.
The nature of CRC screening options means that a home fecal sample collection is inexpensive, and will result in an initial higher screening rate; however, complete screening via fecal occult blood testing requires annual repeats of negative tests, and patients with positive fecal occult blood tests still need colonoscopy.
Colonoscopy, although it’s burdensome for patients and perhaps cost prohibitive for those without health insurance, offers a one-time test that, if negative, provides patients with a 10-year window of screening coverage.
Any effective programs to increase CRC screening rates will need to use a systems change approach, with creative interventions that take patient education, and even delivery of preventive health services, out of the context of the already too-full office visit.
Staff supports, such as the follow-up telephone calls used in the patient-targeted intervention, are key to effective interventions, especially for vulnerable populations. Additionally, institutions must ensure that they have adequate physical and staff resources to support the increased screening they are seeking to achieve.
Michael Pignone, MD, MPH is a professor of medicine at the University of Texas at Austin. David Miller Jr., MD is a professor of internal medicine, Wake Forest University, Winston-Salem, N.C. Dr. Pignone is a medical director for Healthwise; Dr. Miller reported no relevant conflicts of interest. These remarks were drawn from an editorial accompanying the two clinical trials.
Both studies, though they used different outreach interventions, highlight the same problem: the need to identify and execute effective colorectal cancer (CRC) screening programs. Effective screening has great lifesaving potential; if screening rates were elevated to greater than 80% in the United States, an estimated 200,000 deaths would be prevented within the next 2 decades.
The nature of CRC screening options means that a home fecal sample collection is inexpensive, and will result in an initial higher screening rate; however, complete screening via fecal occult blood testing requires annual repeats of negative tests, and patients with positive fecal occult blood tests still need colonoscopy.
Colonoscopy, although it’s burdensome for patients and perhaps cost prohibitive for those without health insurance, offers a one-time test that, if negative, provides patients with a 10-year window of screening coverage.
Any effective programs to increase CRC screening rates will need to use a systems change approach, with creative interventions that take patient education, and even delivery of preventive health services, out of the context of the already too-full office visit.
Staff supports, such as the follow-up telephone calls used in the patient-targeted intervention, are key to effective interventions, especially for vulnerable populations. Additionally, institutions must ensure that they have adequate physical and staff resources to support the increased screening they are seeking to achieve.
Michael Pignone, MD, MPH is a professor of medicine at the University of Texas at Austin. David Miller Jr., MD is a professor of internal medicine, Wake Forest University, Winston-Salem, N.C. Dr. Pignone is a medical director for Healthwise; Dr. Miller reported no relevant conflicts of interest. These remarks were drawn from an editorial accompanying the two clinical trials.
Can outreach improve the globally low rates of adherence to colorectal cancer screening? Yes, according to two recent studies in JAMA; the studies found that both patient-focused and physician-focused outreach approaches can result in significantly better patient participation in colorectal cancer (CRC) screening.
The first study (JAMA. 2017;318[9]:806-15) compared a colonoscopy outreach program and a fecal immunochemical test (FIT) outreach program both with each other and with usual care. The results of the pragmatic, single-site, randomized, clinical trial showed that completed screenings were higher for both outreach groups, compared with the usual-care group.
The primary outcome measure of the study was completion of the screening process, wrote Amit Singal, MD, and his coauthors. This was defined as any adherence to colonoscopy completion, the completion of annual testing for patients who had a normal FIT test, or treatment evaluation if CRC was detected during the screening process. Screenings were considered complete even if, for example, a patient in the colonoscopy arm eventually went on to have three consecutive annual FIT tests rather than a colonoscopy.
A total of 5,999 patients eligible for screening were initially randomized to one of the three study arms. Across all study arms, approximately half were lost to follow-up. These patients were excluded from the primary analysis but were included in an additional intention-to-screen analysis. A total of 2,400 patients received a colonoscopy outreach mailing; 2,400 received FIT outreach, including a letter, the home FIT testing kit, and instructions; 1,199 received usual care. Patients in both intervention arms also received up to two phone calls if they didn’t respond to the initial mailing within 2 weeks. Mailings and phone calls were conducted in English or Spanish, according to the patients’ stated language preferences (those whose spoke neither language were excluded from the study).
Of the patients in the colonoscopy outreach group, 922 (38.4%) completed the screening process, compared with 671 (28.0%) in the FIT outreach group and 128 (10.7%) in the usual-care group.
Compared with the group receiving usual care, completion of the screening process was 27.7% higher in the colonoscopy outreach group and 17.3% higher in the FIT outreach group. Screening process completion was 10.4% higher for the colonoscopy outreach group, compared with the FIT outreach group (P less than .001 for all).
Dr. Singal, who is with the department of internal medicine at UT Southwestern Medical Center, Dallas, and his colleagues also performed several post-hoc secondary analyses. In one, they used a less-stringent definition of screening process completion in which biennial FIT testing was considered satisfactory. When this definition was applied, the colonoscopy outreach group had 0.5% lower screening process completion than the FIT outreach group. The chances of a patient receiving any screening during the study period was highest in the FIT group (65%), with 51.7% of those in the colonoscopy outreach group and 39% of those in the usual-care group receiving any screening.
“FIT has lower barriers to one-time participation but requires annual screening and diagnostic evaluation of abnormal results,” wrote Dr. Singal and his colleagues.
Strengths of the study, said Dr. Singal and his colleagues, included the fact that the study took place at a “safety net” institution with a racially and socioeconomically diverse population. Also, the study design avoided volunteer bias, and offered a pragmatic head-to-head comparison of colonoscopy and FIT.
The second study took place in western France, and targeted outreach to physicians rather than patients (JAMA. 2017;318[9];816-84). When physicians were given a list of their own patients who were not up to date on CRC screening, investigators saw a small, but significant, uptick in patient participation in FIT screening.
One year after the reminders went out, FIT screening had been initiated in 24.8% of patients whose physicians had received the list, compared with 21.7% of patients of physicians who had received a more generic notice and 20.6% of patients whose physicians received no notification, according to first author Cedric Rat, MD, and his colleagues.
The study examined which notification approach was most effective in increasing FIT screening among the physicians’ patient panels: sending general practitioners (GPs) letters that included a list of their own patients who had not undergone CRC screening, or sending them generic letters describing CRC screening adherence rates specific to their region. A usual-care group of practices received no notifications in this three-group randomized cluster design.
Patients in the patient-specific reminders group had an odds ratio of 1.27 for participation in FIT screening (P less than .001) compared to the usual-care group. The odds ratio for the generic-reminders group was 1.09, a nonsignificant difference.
Between-group comparison showed statistical significance for both the 3.1% difference between the patient-specific and generic-reminders groups, and for the 4.2% difference between the patient-specific and usual-care groups (P less than .001 for both). There was no significant difference between the generic- reminders group and the usual-care group.
Dr. Rat, professor of medicine at the Faculty of Medicine, Nantes, France, and his colleagues enrolled GPs in a total of 801 practices that included patients aged 50. Participating GPs cared for 33,044 patients who met study criteria.
Physician characteristics that were associated with higher FIT participation included younger age and an initially smaller number of unscreened patients. Patients with low socioeconomic status and those with a higher chronic disease burden were less likely to participate in FIT screening.
Dr. Rat and his colleagues noted that the busiest practices actually had higher CRC screening rates. The investigators hypothesized that a recent physician pay-for-performance grant for CRC completion might be more appealing for some busy physicians.
This was the largest study of CRC screening participation to date, according to Dr. Rat and his coauthors, and showed the small but detectable efficacy of an inexpensive intervention that, given complete patient records, is relatively easy to effect. Though the effect size was smaller than the 12% difference the investigators had anticipated seeing for the patient-specific reminders group, the study still showed that targeting physicians can be an effective public health intervention to increase CRC screening rates, said Dr. Rat and his colleagues.
None of the investigators in either study reported conflicts of interest.
The AGA Colorectal Cancer Clinical Service Line provides tools to help you become more efficient, understand quality standards and improve the process of care for patients. Learn more at www.gastro.org/crc.
Can outreach improve the globally low rates of adherence to colorectal cancer screening? Yes, according to two recent studies in JAMA; the studies found that both patient-focused and physician-focused outreach approaches can result in significantly better patient participation in colorectal cancer (CRC) screening.
The first study (JAMA. 2017;318[9]:806-15) compared a colonoscopy outreach program and a fecal immunochemical test (FIT) outreach program both with each other and with usual care. The results of the pragmatic, single-site, randomized, clinical trial showed that completed screenings were higher for both outreach groups, compared with the usual-care group.
The primary outcome measure of the study was completion of the screening process, wrote Amit Singal, MD, and his coauthors. This was defined as any adherence to colonoscopy completion, the completion of annual testing for patients who had a normal FIT test, or treatment evaluation if CRC was detected during the screening process. Screenings were considered complete even if, for example, a patient in the colonoscopy arm eventually went on to have three consecutive annual FIT tests rather than a colonoscopy.
A total of 5,999 patients eligible for screening were initially randomized to one of the three study arms. Across all study arms, approximately half were lost to follow-up. These patients were excluded from the primary analysis but were included in an additional intention-to-screen analysis. A total of 2,400 patients received a colonoscopy outreach mailing; 2,400 received FIT outreach, including a letter, the home FIT testing kit, and instructions; 1,199 received usual care. Patients in both intervention arms also received up to two phone calls if they didn’t respond to the initial mailing within 2 weeks. Mailings and phone calls were conducted in English or Spanish, according to the patients’ stated language preferences (those whose spoke neither language were excluded from the study).
Of the patients in the colonoscopy outreach group, 922 (38.4%) completed the screening process, compared with 671 (28.0%) in the FIT outreach group and 128 (10.7%) in the usual-care group.
Compared with the group receiving usual care, completion of the screening process was 27.7% higher in the colonoscopy outreach group and 17.3% higher in the FIT outreach group. Screening process completion was 10.4% higher for the colonoscopy outreach group, compared with the FIT outreach group (P less than .001 for all).
Dr. Singal, who is with the department of internal medicine at UT Southwestern Medical Center, Dallas, and his colleagues also performed several post-hoc secondary analyses. In one, they used a less-stringent definition of screening process completion in which biennial FIT testing was considered satisfactory. When this definition was applied, the colonoscopy outreach group had 0.5% lower screening process completion than the FIT outreach group. The chances of a patient receiving any screening during the study period was highest in the FIT group (65%), with 51.7% of those in the colonoscopy outreach group and 39% of those in the usual-care group receiving any screening.
“FIT has lower barriers to one-time participation but requires annual screening and diagnostic evaluation of abnormal results,” wrote Dr. Singal and his colleagues.
Strengths of the study, said Dr. Singal and his colleagues, included the fact that the study took place at a “safety net” institution with a racially and socioeconomically diverse population. Also, the study design avoided volunteer bias, and offered a pragmatic head-to-head comparison of colonoscopy and FIT.
The second study took place in western France, and targeted outreach to physicians rather than patients (JAMA. 2017;318[9];816-84). When physicians were given a list of their own patients who were not up to date on CRC screening, investigators saw a small, but significant, uptick in patient participation in FIT screening.
One year after the reminders went out, FIT screening had been initiated in 24.8% of patients whose physicians had received the list, compared with 21.7% of patients of physicians who had received a more generic notice and 20.6% of patients whose physicians received no notification, according to first author Cedric Rat, MD, and his colleagues.
The study examined which notification approach was most effective in increasing FIT screening among the physicians’ patient panels: sending general practitioners (GPs) letters that included a list of their own patients who had not undergone CRC screening, or sending them generic letters describing CRC screening adherence rates specific to their region. A usual-care group of practices received no notifications in this three-group randomized cluster design.
Patients in the patient-specific reminders group had an odds ratio of 1.27 for participation in FIT screening (P less than .001) compared to the usual-care group. The odds ratio for the generic-reminders group was 1.09, a nonsignificant difference.
Between-group comparison showed statistical significance for both the 3.1% difference between the patient-specific and generic-reminders groups, and for the 4.2% difference between the patient-specific and usual-care groups (P less than .001 for both). There was no significant difference between the generic- reminders group and the usual-care group.
Dr. Rat, professor of medicine at the Faculty of Medicine, Nantes, France, and his colleagues enrolled GPs in a total of 801 practices that included patients aged 50. Participating GPs cared for 33,044 patients who met study criteria.
Physician characteristics that were associated with higher FIT participation included younger age and an initially smaller number of unscreened patients. Patients with low socioeconomic status and those with a higher chronic disease burden were less likely to participate in FIT screening.
Dr. Rat and his colleagues noted that the busiest practices actually had higher CRC screening rates. The investigators hypothesized that a recent physician pay-for-performance grant for CRC completion might be more appealing for some busy physicians.
This was the largest study of CRC screening participation to date, according to Dr. Rat and his coauthors, and showed the small but detectable efficacy of an inexpensive intervention that, given complete patient records, is relatively easy to effect. Though the effect size was smaller than the 12% difference the investigators had anticipated seeing for the patient-specific reminders group, the study still showed that targeting physicians can be an effective public health intervention to increase CRC screening rates, said Dr. Rat and his colleagues.
None of the investigators in either study reported conflicts of interest.
The AGA Colorectal Cancer Clinical Service Line provides tools to help you become more efficient, understand quality standards and improve the process of care for patients. Learn more at www.gastro.org/crc.
How to have a rational approach to the FUO work-up
CHICAGO – When a child’s worried parents bring him back to be rechecked on his 8th day of fever, what’s next? If the initial work-up is unrevealing, when is it time to consider hospitalization? And which children can safely be managed as outpatients?
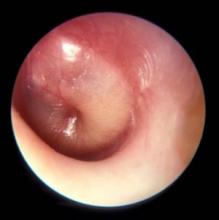
These tough scenarios are part of why “most pediatricians really don’t enjoy fever of unknown origin (FUO),” said Brian Williams, MD, speaking at a pediatric infectious disease update at the annual meeting of the American Academy of Pediatrics. “It can be really time consuming and frustrating to tease all of this out.”
Dr. Williams comes into the picture when, for the pediatrician, “something about that history, that physical exam, and that lab work has them concerned that the child needs to be hospitalized for closer monitoring and a more extensive work-up.”
“There’s lots of variability for inclusion criteria in the studies for pediatrics,” said Dr. Williams, but most characterize FUO as a fever of at least 100.4° F for 8 days or longer with no clear diagnosis.
he said. “I think it’s one of those diagnoses where a thorough history and exam can oftentimes give you some clues that can help lead to your diagnosis.”
“It’s a diagnosis that always gets my full attention because sometimes you can find some pretty significant infections – an osteomyelitis or a severe pelvic abscess,” he said. “And there’s always the concern of some of these more serious underlying diseases, like rheumatologic diseases; there’s plenty of case reports of [inflammatory bowel disease] presenting with FUO.” Of course, he said, even more dire diagnoses like Hodgkin’s lymphoma and leukemia have to remain in the differential as well.
Although the broad diagnostic differential includes noninfectious causes, they are rarer by far than infections. When the etiology of FUO has been studied in the United States, said Dr. Williams, “infections pretty consistently dominate as the most common cause of FUO … as general pediatricians, it’s our job to really do a good evaluation for infection before we start going after some of these less common diagnoses – the rheumatologic and cancer diagnoses.”
A systematic approach is important, he said. “A really good fever history can, a lot of times, provide important and valuable information.” Take the time to get granular detail: Find out how often the fever is being checked and by whom, what symptoms accompany the fever, and how the fever is being measured.
And don’t forget to ask if there are fever-free days, he said. In an otherwise well-appearing child, a few days’ respite from fever can increase the likelihood that you’re really seeing back-to-back viral illnesses rather than a protracted unexplained fever.
A thorough head-to-toe review of symptoms and history is critical, too. Dr. Williams related the story of a well-appearing 9-year-old boy who’d had many days of high fever with accompanying elevated inflammatory markers. His exam was unremarkable, and the only untoward symptom he could recall was a few days’ worth of left upper quadrant tenderness when running in gym class. The child, said Dr. Williams, turned out to have nephronia. “Sometimes, really subtle clues from the history can guide you.”
Ask about exposures, including travel, animals, foods, insects, and sick contacts. “Obviously, children can get into just about anything,” said Dr. Williams. A detailed family and social history also may turn up clues.
An infection-focused musculoskeletal exam, to include the spine, is a must, as is a top-to-bottom search for lymphadenopathy as part of a complete physical exam.
At this point in the pediatrics office, said Dr. Williams, you’ve come to a decision point: “Does this work-up need to be initiated in the inpatient setting, or is this something that can be started in the outpatient setting?”
“There’s a lot of data to support that, initially, a lot of these patients can be worked up in the outpatient setting with close follow-up,” he said. The outpatient FUO work-up begins with some basic screening labs. In addition to a complete blood count, chemistries, and a urinalysis, labs should include blood and urine cultures, erythrocyte sedimentation rate, and C-reactive protein levels.
“I’ll actually rely pretty heavily on my ESR and CRP,” said Dr. Williams. “If I have an otherwise well-appearing child with a normal CRP and an unremarkable exam, I think it’s a pretty tough argument to keep that child hospitalized and do a more invasive work-up.”
The advent of the viral polymerase chain reaction panel has helped streamline the FUO work-up as well. In the setting of a well-appearing child with an unremarkable initial work-up, “a positive adenovirus can provide a lot of reassurance to the families.”
Dr. Williams usually also gets a chest radiograph at this point, knowing that pneumonia is in the differential for FUO. He said he’s seen mediastinal masses, as well as picked up dense right upper lobe infiltrates that were missed on exam.
If the answer is still unclear at this point, exam and laboratory findings from the first-tier inquiry can help guide the next steps.
Some less common infectious etiologies can be considered now, said Dr. Williams. These can include Epstein-Barr virus, cytomegalovirus, and cat scratch fever; the latter, he’s found, is the third-most-common cause of FUO in some case series. For the real mystery cases, next-generation sequencing is an option: A blood sample is used to search for DNA fragments from a huge variety of microorganisms. “It’s a little overwhelming,” and very expensive, he said.
If an oncologic process is suspected, second-tier labs can include lactate dehydrogenase, uric acid, ferritin levels, and a peripheral smear. A rheumatologic work-up can be started, with antinuclear antibody and complement levels. At this point, though, a general pediatrician would be considering consults, he said.
Empiric antibiotics can be a tempting diagnostic strategy in some cases. “Is a trial of antibiotics warranted? Usually we advise against it,” but a case can be made for a time-limited trial in certain circumstances, said Dr. Williams.
Dr. Williams is a consultant for Zavante Therapeutics, which markets fosfomycin.
[email protected]
On Twitter @karioakes
CHICAGO – When a child’s worried parents bring him back to be rechecked on his 8th day of fever, what’s next? If the initial work-up is unrevealing, when is it time to consider hospitalization? And which children can safely be managed as outpatients?
These tough scenarios are part of why “most pediatricians really don’t enjoy fever of unknown origin (FUO),” said Brian Williams, MD, speaking at a pediatric infectious disease update at the annual meeting of the American Academy of Pediatrics. “It can be really time consuming and frustrating to tease all of this out.”
Dr. Williams comes into the picture when, for the pediatrician, “something about that history, that physical exam, and that lab work has them concerned that the child needs to be hospitalized for closer monitoring and a more extensive work-up.”
“There’s lots of variability for inclusion criteria in the studies for pediatrics,” said Dr. Williams, but most characterize FUO as a fever of at least 100.4° F for 8 days or longer with no clear diagnosis.
he said. “I think it’s one of those diagnoses where a thorough history and exam can oftentimes give you some clues that can help lead to your diagnosis.”
“It’s a diagnosis that always gets my full attention because sometimes you can find some pretty significant infections – an osteomyelitis or a severe pelvic abscess,” he said. “And there’s always the concern of some of these more serious underlying diseases, like rheumatologic diseases; there’s plenty of case reports of [inflammatory bowel disease] presenting with FUO.” Of course, he said, even more dire diagnoses like Hodgkin’s lymphoma and leukemia have to remain in the differential as well.
Although the broad diagnostic differential includes noninfectious causes, they are rarer by far than infections. When the etiology of FUO has been studied in the United States, said Dr. Williams, “infections pretty consistently dominate as the most common cause of FUO … as general pediatricians, it’s our job to really do a good evaluation for infection before we start going after some of these less common diagnoses – the rheumatologic and cancer diagnoses.”
A systematic approach is important, he said. “A really good fever history can, a lot of times, provide important and valuable information.” Take the time to get granular detail: Find out how often the fever is being checked and by whom, what symptoms accompany the fever, and how the fever is being measured.
And don’t forget to ask if there are fever-free days, he said. In an otherwise well-appearing child, a few days’ respite from fever can increase the likelihood that you’re really seeing back-to-back viral illnesses rather than a protracted unexplained fever.
A thorough head-to-toe review of symptoms and history is critical, too. Dr. Williams related the story of a well-appearing 9-year-old boy who’d had many days of high fever with accompanying elevated inflammatory markers. His exam was unremarkable, and the only untoward symptom he could recall was a few days’ worth of left upper quadrant tenderness when running in gym class. The child, said Dr. Williams, turned out to have nephronia. “Sometimes, really subtle clues from the history can guide you.”
Ask about exposures, including travel, animals, foods, insects, and sick contacts. “Obviously, children can get into just about anything,” said Dr. Williams. A detailed family and social history also may turn up clues.
An infection-focused musculoskeletal exam, to include the spine, is a must, as is a top-to-bottom search for lymphadenopathy as part of a complete physical exam.
At this point in the pediatrics office, said Dr. Williams, you’ve come to a decision point: “Does this work-up need to be initiated in the inpatient setting, or is this something that can be started in the outpatient setting?”
“There’s a lot of data to support that, initially, a lot of these patients can be worked up in the outpatient setting with close follow-up,” he said. The outpatient FUO work-up begins with some basic screening labs. In addition to a complete blood count, chemistries, and a urinalysis, labs should include blood and urine cultures, erythrocyte sedimentation rate, and C-reactive protein levels.
“I’ll actually rely pretty heavily on my ESR and CRP,” said Dr. Williams. “If I have an otherwise well-appearing child with a normal CRP and an unremarkable exam, I think it’s a pretty tough argument to keep that child hospitalized and do a more invasive work-up.”
The advent of the viral polymerase chain reaction panel has helped streamline the FUO work-up as well. In the setting of a well-appearing child with an unremarkable initial work-up, “a positive adenovirus can provide a lot of reassurance to the families.”
Dr. Williams usually also gets a chest radiograph at this point, knowing that pneumonia is in the differential for FUO. He said he’s seen mediastinal masses, as well as picked up dense right upper lobe infiltrates that were missed on exam.
If the answer is still unclear at this point, exam and laboratory findings from the first-tier inquiry can help guide the next steps.
Some less common infectious etiologies can be considered now, said Dr. Williams. These can include Epstein-Barr virus, cytomegalovirus, and cat scratch fever; the latter, he’s found, is the third-most-common cause of FUO in some case series. For the real mystery cases, next-generation sequencing is an option: A blood sample is used to search for DNA fragments from a huge variety of microorganisms. “It’s a little overwhelming,” and very expensive, he said.
If an oncologic process is suspected, second-tier labs can include lactate dehydrogenase, uric acid, ferritin levels, and a peripheral smear. A rheumatologic work-up can be started, with antinuclear antibody and complement levels. At this point, though, a general pediatrician would be considering consults, he said.
Empiric antibiotics can be a tempting diagnostic strategy in some cases. “Is a trial of antibiotics warranted? Usually we advise against it,” but a case can be made for a time-limited trial in certain circumstances, said Dr. Williams.
Dr. Williams is a consultant for Zavante Therapeutics, which markets fosfomycin.
[email protected]
On Twitter @karioakes
CHICAGO – When a child’s worried parents bring him back to be rechecked on his 8th day of fever, what’s next? If the initial work-up is unrevealing, when is it time to consider hospitalization? And which children can safely be managed as outpatients?
These tough scenarios are part of why “most pediatricians really don’t enjoy fever of unknown origin (FUO),” said Brian Williams, MD, speaking at a pediatric infectious disease update at the annual meeting of the American Academy of Pediatrics. “It can be really time consuming and frustrating to tease all of this out.”
Dr. Williams comes into the picture when, for the pediatrician, “something about that history, that physical exam, and that lab work has them concerned that the child needs to be hospitalized for closer monitoring and a more extensive work-up.”
“There’s lots of variability for inclusion criteria in the studies for pediatrics,” said Dr. Williams, but most characterize FUO as a fever of at least 100.4° F for 8 days or longer with no clear diagnosis.
he said. “I think it’s one of those diagnoses where a thorough history and exam can oftentimes give you some clues that can help lead to your diagnosis.”
“It’s a diagnosis that always gets my full attention because sometimes you can find some pretty significant infections – an osteomyelitis or a severe pelvic abscess,” he said. “And there’s always the concern of some of these more serious underlying diseases, like rheumatologic diseases; there’s plenty of case reports of [inflammatory bowel disease] presenting with FUO.” Of course, he said, even more dire diagnoses like Hodgkin’s lymphoma and leukemia have to remain in the differential as well.
Although the broad diagnostic differential includes noninfectious causes, they are rarer by far than infections. When the etiology of FUO has been studied in the United States, said Dr. Williams, “infections pretty consistently dominate as the most common cause of FUO … as general pediatricians, it’s our job to really do a good evaluation for infection before we start going after some of these less common diagnoses – the rheumatologic and cancer diagnoses.”
A systematic approach is important, he said. “A really good fever history can, a lot of times, provide important and valuable information.” Take the time to get granular detail: Find out how often the fever is being checked and by whom, what symptoms accompany the fever, and how the fever is being measured.
And don’t forget to ask if there are fever-free days, he said. In an otherwise well-appearing child, a few days’ respite from fever can increase the likelihood that you’re really seeing back-to-back viral illnesses rather than a protracted unexplained fever.
A thorough head-to-toe review of symptoms and history is critical, too. Dr. Williams related the story of a well-appearing 9-year-old boy who’d had many days of high fever with accompanying elevated inflammatory markers. His exam was unremarkable, and the only untoward symptom he could recall was a few days’ worth of left upper quadrant tenderness when running in gym class. The child, said Dr. Williams, turned out to have nephronia. “Sometimes, really subtle clues from the history can guide you.”
Ask about exposures, including travel, animals, foods, insects, and sick contacts. “Obviously, children can get into just about anything,” said Dr. Williams. A detailed family and social history also may turn up clues.
An infection-focused musculoskeletal exam, to include the spine, is a must, as is a top-to-bottom search for lymphadenopathy as part of a complete physical exam.
At this point in the pediatrics office, said Dr. Williams, you’ve come to a decision point: “Does this work-up need to be initiated in the inpatient setting, or is this something that can be started in the outpatient setting?”
“There’s a lot of data to support that, initially, a lot of these patients can be worked up in the outpatient setting with close follow-up,” he said. The outpatient FUO work-up begins with some basic screening labs. In addition to a complete blood count, chemistries, and a urinalysis, labs should include blood and urine cultures, erythrocyte sedimentation rate, and C-reactive protein levels.
“I’ll actually rely pretty heavily on my ESR and CRP,” said Dr. Williams. “If I have an otherwise well-appearing child with a normal CRP and an unremarkable exam, I think it’s a pretty tough argument to keep that child hospitalized and do a more invasive work-up.”
The advent of the viral polymerase chain reaction panel has helped streamline the FUO work-up as well. In the setting of a well-appearing child with an unremarkable initial work-up, “a positive adenovirus can provide a lot of reassurance to the families.”
Dr. Williams usually also gets a chest radiograph at this point, knowing that pneumonia is in the differential for FUO. He said he’s seen mediastinal masses, as well as picked up dense right upper lobe infiltrates that were missed on exam.
If the answer is still unclear at this point, exam and laboratory findings from the first-tier inquiry can help guide the next steps.
Some less common infectious etiologies can be considered now, said Dr. Williams. These can include Epstein-Barr virus, cytomegalovirus, and cat scratch fever; the latter, he’s found, is the third-most-common cause of FUO in some case series. For the real mystery cases, next-generation sequencing is an option: A blood sample is used to search for DNA fragments from a huge variety of microorganisms. “It’s a little overwhelming,” and very expensive, he said.
If an oncologic process is suspected, second-tier labs can include lactate dehydrogenase, uric acid, ferritin levels, and a peripheral smear. A rheumatologic work-up can be started, with antinuclear antibody and complement levels. At this point, though, a general pediatrician would be considering consults, he said.
Empiric antibiotics can be a tempting diagnostic strategy in some cases. “Is a trial of antibiotics warranted? Usually we advise against it,” but a case can be made for a time-limited trial in certain circumstances, said Dr. Williams.
Dr. Williams is a consultant for Zavante Therapeutics, which markets fosfomycin.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM AAP 2017
Thinking may be shifting about first-line AOM treatment
CHICAGO – Treating even uncomplicated acute otitis media (AOM) in 2017 may not be as simple as writing an amoxicillin prescription. Changes in pathogens may mean a shift in prescribing practices, Ellen Wald, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Wald, speaking to an audience that filled the lecture hall and an overflow room, said that there has long been good reason to turn to amoxicillin for AOM. “The reason that pediatricians and others like to reserve the use of amoxicillin as first-line therapy for children with AOM is that it is generally effective, it’s safe, it’s narrow in spectrum, and it’s relatively inexpensive. Those are all very desirable characteristics.”
However, the high-dose amoxicillin strategy is predicated on S. pneumoniae being the most likely cause of bacterial AOM. Since the seven-valent pneumococcal conjugate vaccine (PCV7), and then its successor PCV13, became part of the standard series of childhood immunizations, said Dr. Wald, the microbiology of AOM has shifted.
In 1990, S. pneumoniae was estimated to cause 35%-45% of AOM cases, with Haemophilus influenzae responsible for 25%-30% of cases. Moraxella catarrhalis was thought to cause 12%-15% of cases, with Streptococcus pyogenes–related AOM falling into the single digits.
In 2017, the balance has shifted, with S. pneumoniae only responsible for about a quarter of cases of AOM, and H. influenzae causing about half. The prevalence of M. catarrhalis and S. pyogenes cases hasn’t changed. This, said Dr. Wald, should prompt a shift in thinking about antibiotic strategy for AOM.
“The real problem with amoxicillin is that it’s not active against beta-lactamase–producing H. influenzae and M. catarrhalis. So my recommendation would be, rather than using amoxicillin, to use amoxicillin potassium clavulanate.”
Dr. Wald said she can’t currently recommend using azithromycin to treat AOM. “Azithromycin and the other macrolides have almost no activity against H. influenzae,” she said. “So given the current situation with the high prevalence of H. influenzae, azithromycin really should be avoided in the management of AOM.”
For children with non–type 1 penicillin hypersensitivity or mild type 1 hypersensitivity, a second- or third-generation cephalosporin, such as cefuroxime, cefpodoxime, or cefdinir, can be considered, she said.
“For life-threatening type 1 hypersensitivity reactions, we like to choose a drug of an entirely different class. For that reason, levofloxacin might be something you’d consider,” in those cases, said Dr. Wald, making clear that this is not a Food and Drug Administration–approved indication. Levofloxacin does have the antimicrobial spectrum to cover AOM pathogens, she said.
When parenteral therapy is indicated, as when a child isn’t tolerating oral medications or when nonadherence is likely, a single dose of ceftriaxone IM or IV, dosed at 50 mg/kg, remains a good option. “It’s a suitable agent because all middle ear pathogens are susceptible to ceftriaxone,” said Dr. Wald.
When oral antibiotics are used, how long should they be given? Some experts, she said, recommend a 5-day course for older children who have had infrequent previous episodes of AOM. In this age group, the shorter course can still yield an excellent response, she said.
However, a 2016 study that tried a shortened course of amoxicillin/clavulanate for children 6-23 months of age found that clinical failure occurred in 34% of the patients who received 5 days of antibiotics, compared with 16% of those who got the full 10-day course. “The recommendation is pretty clear that, for children under 2 years of age, a 10-day course of therapy is best,” said Dr. Wald.
Dr. Wald reported that she had no conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Treating even uncomplicated acute otitis media (AOM) in 2017 may not be as simple as writing an amoxicillin prescription. Changes in pathogens may mean a shift in prescribing practices, Ellen Wald, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Wald, speaking to an audience that filled the lecture hall and an overflow room, said that there has long been good reason to turn to amoxicillin for AOM. “The reason that pediatricians and others like to reserve the use of amoxicillin as first-line therapy for children with AOM is that it is generally effective, it’s safe, it’s narrow in spectrum, and it’s relatively inexpensive. Those are all very desirable characteristics.”
However, the high-dose amoxicillin strategy is predicated on S. pneumoniae being the most likely cause of bacterial AOM. Since the seven-valent pneumococcal conjugate vaccine (PCV7), and then its successor PCV13, became part of the standard series of childhood immunizations, said Dr. Wald, the microbiology of AOM has shifted.
In 1990, S. pneumoniae was estimated to cause 35%-45% of AOM cases, with Haemophilus influenzae responsible for 25%-30% of cases. Moraxella catarrhalis was thought to cause 12%-15% of cases, with Streptococcus pyogenes–related AOM falling into the single digits.
In 2017, the balance has shifted, with S. pneumoniae only responsible for about a quarter of cases of AOM, and H. influenzae causing about half. The prevalence of M. catarrhalis and S. pyogenes cases hasn’t changed. This, said Dr. Wald, should prompt a shift in thinking about antibiotic strategy for AOM.
“The real problem with amoxicillin is that it’s not active against beta-lactamase–producing H. influenzae and M. catarrhalis. So my recommendation would be, rather than using amoxicillin, to use amoxicillin potassium clavulanate.”
Dr. Wald said she can’t currently recommend using azithromycin to treat AOM. “Azithromycin and the other macrolides have almost no activity against H. influenzae,” she said. “So given the current situation with the high prevalence of H. influenzae, azithromycin really should be avoided in the management of AOM.”
For children with non–type 1 penicillin hypersensitivity or mild type 1 hypersensitivity, a second- or third-generation cephalosporin, such as cefuroxime, cefpodoxime, or cefdinir, can be considered, she said.
“For life-threatening type 1 hypersensitivity reactions, we like to choose a drug of an entirely different class. For that reason, levofloxacin might be something you’d consider,” in those cases, said Dr. Wald, making clear that this is not a Food and Drug Administration–approved indication. Levofloxacin does have the antimicrobial spectrum to cover AOM pathogens, she said.
When parenteral therapy is indicated, as when a child isn’t tolerating oral medications or when nonadherence is likely, a single dose of ceftriaxone IM or IV, dosed at 50 mg/kg, remains a good option. “It’s a suitable agent because all middle ear pathogens are susceptible to ceftriaxone,” said Dr. Wald.
When oral antibiotics are used, how long should they be given? Some experts, she said, recommend a 5-day course for older children who have had infrequent previous episodes of AOM. In this age group, the shorter course can still yield an excellent response, she said.
However, a 2016 study that tried a shortened course of amoxicillin/clavulanate for children 6-23 months of age found that clinical failure occurred in 34% of the patients who received 5 days of antibiotics, compared with 16% of those who got the full 10-day course. “The recommendation is pretty clear that, for children under 2 years of age, a 10-day course of therapy is best,” said Dr. Wald.
Dr. Wald reported that she had no conflicts of interest.
[email protected]
On Twitter @karioakes
CHICAGO – Treating even uncomplicated acute otitis media (AOM) in 2017 may not be as simple as writing an amoxicillin prescription. Changes in pathogens may mean a shift in prescribing practices, Ellen Wald, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Wald, speaking to an audience that filled the lecture hall and an overflow room, said that there has long been good reason to turn to amoxicillin for AOM. “The reason that pediatricians and others like to reserve the use of amoxicillin as first-line therapy for children with AOM is that it is generally effective, it’s safe, it’s narrow in spectrum, and it’s relatively inexpensive. Those are all very desirable characteristics.”
However, the high-dose amoxicillin strategy is predicated on S. pneumoniae being the most likely cause of bacterial AOM. Since the seven-valent pneumococcal conjugate vaccine (PCV7), and then its successor PCV13, became part of the standard series of childhood immunizations, said Dr. Wald, the microbiology of AOM has shifted.
In 1990, S. pneumoniae was estimated to cause 35%-45% of AOM cases, with Haemophilus influenzae responsible for 25%-30% of cases. Moraxella catarrhalis was thought to cause 12%-15% of cases, with Streptococcus pyogenes–related AOM falling into the single digits.
In 2017, the balance has shifted, with S. pneumoniae only responsible for about a quarter of cases of AOM, and H. influenzae causing about half. The prevalence of M. catarrhalis and S. pyogenes cases hasn’t changed. This, said Dr. Wald, should prompt a shift in thinking about antibiotic strategy for AOM.
“The real problem with amoxicillin is that it’s not active against beta-lactamase–producing H. influenzae and M. catarrhalis. So my recommendation would be, rather than using amoxicillin, to use amoxicillin potassium clavulanate.”
Dr. Wald said she can’t currently recommend using azithromycin to treat AOM. “Azithromycin and the other macrolides have almost no activity against H. influenzae,” she said. “So given the current situation with the high prevalence of H. influenzae, azithromycin really should be avoided in the management of AOM.”
For children with non–type 1 penicillin hypersensitivity or mild type 1 hypersensitivity, a second- or third-generation cephalosporin, such as cefuroxime, cefpodoxime, or cefdinir, can be considered, she said.
“For life-threatening type 1 hypersensitivity reactions, we like to choose a drug of an entirely different class. For that reason, levofloxacin might be something you’d consider,” in those cases, said Dr. Wald, making clear that this is not a Food and Drug Administration–approved indication. Levofloxacin does have the antimicrobial spectrum to cover AOM pathogens, she said.
When parenteral therapy is indicated, as when a child isn’t tolerating oral medications or when nonadherence is likely, a single dose of ceftriaxone IM or IV, dosed at 50 mg/kg, remains a good option. “It’s a suitable agent because all middle ear pathogens are susceptible to ceftriaxone,” said Dr. Wald.
When oral antibiotics are used, how long should they be given? Some experts, she said, recommend a 5-day course for older children who have had infrequent previous episodes of AOM. In this age group, the shorter course can still yield an excellent response, she said.
However, a 2016 study that tried a shortened course of amoxicillin/clavulanate for children 6-23 months of age found that clinical failure occurred in 34% of the patients who received 5 days of antibiotics, compared with 16% of those who got the full 10-day course. “The recommendation is pretty clear that, for children under 2 years of age, a 10-day course of therapy is best,” said Dr. Wald.
Dr. Wald reported that she had no conflicts of interest.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM AAP 2017
VA shares its best practices to achieve HCV ‘cascade of cure’
The U.S. Department of Veterans Affairs (VA) cares for more patients with hepatitis C virus than any other care provider in the country, and has achieved a rapid and sharp reduction in hepatitis C virus (HCV) infection among veterans over the past 3 years.
The VA has treated more than 92,000 HCV-infected individuals since the advent of oral direct-acting antiviral (DAA) therapy in 2014. The number of veterans with HCV who were eligible for treatment was 168,000 in 2014 and 51,000 in July 2017, according to an article in Annals of Internal Medicine (2017 Sep 26. doi: 10.7326/M17-1073).
Given that success in HCV treatment, with a cure rate that exceeds 90% for those prescribed DAAs, the VA is well positioned to share best practices with other organizations, according to Pamela S. Belperio, PharmD, and her coauthors. The best practices harmonize with the recently outlined national strategy to eliminate hepatitis B and C from the National Academies of Sciences, Engineering, and Medicine.
“The leveraging of health systems data transforms numbers into knowledge” that is used both by individual providers and the VA as a whole, wrote Dr. Belperio, national public health pharmacist in the VA’s public health division, and her coauthors.
Identifying veterans who were infected with HCV is simplified by means of electronic point-of-care reminders that prompt providers to conduct HCV risk assessment and testing. Veterans eligible for testing also receive automated letters that serve as a laboratory order form for HCV testing at a VA laboratory.
Adding HCV birth cohort screening as a performance measure and recommending reflex confirmatory HCV RNA testing for positive antibody tests were additional initiatives that contributed to the 3%-4% annual increase in veterans receiving HCV testing, “substantially higher than in other health care systems,” wrote Dr. Belperio and her colleagues.
To respect regional variations in practice within the VA system, multidisciplinary innovation teams meet in each region to identify opportunities for improving and streamlining HCV testing and treatment, using lean process improvement techniques. Regions also share innovations with each other.
The VA used a multipronged approach to raise awareness about HCV testing and treatment among VA health care providers and veterans. Provider reach has been expanded by extensive use of telemedicine, including real-time video calls between providers and patients or other providers at remote locations. Those telemedicine initiatives allow pharmacists and mental health providers to lend their expertise to physicians and other providers, who are themselves directly caring for HCV-infected individuals.
Patients at more than half of VA facilities receive care from physician assistants, nurse practitioners, and clinical pharmacy specialists, “who have been recognized as delivering the same quality of care and providing more timely access to HCV treatment,” wrote Dr. Belperio and her collaborators. Expanding the use of advanced practice providers allows more targeted use of specialists, and “is an important practice that can be adapted into other health care systems,” they noted.
Non–evidence-based criteria for HCV treatment eligibility remain a major barrier for HCV-infected individuals nationwide, despite VA studies showing “cure rates among veterans with alcohol, substance use, and mental health disorders that are similar to cure rates in those without these conditions,” said Dr. Belperio and her colleagues.
Within the VA, involving alcohol and substance use specialists, mental health providers, case managers, and social workers in the patient’s care team is an approach that can increase adherence and up the chance for a cure among the most vulnerable veterans.
The VA has been able to negotiate reduced pricing for the expensive DAAs, and has continued to receive additional appropriations to fund DAAs and other HCV-related resources. “Financing for HCV treatment and infrastructure resources, coupled with reduced drug prices, has been paramount to the VA’s success in curing HCV infection,” said Dr. Belperio and her colleagues.
However, they added, individual private insurers may not reap the benefits of achieving sweeping HCV cures within their particular patient panel, because patients frequently switch insurers. Still, “consistent leveraging of drug prices and removal of restrictions to HCV treatment among all insurers would help assuage this gap,” they wrote.
Dr. Belperio described the VA’s vision of a “hepatitis C cascade of cure” that occurs when individuals with chronic HCV are identified, linked to care, treated with DAAs, and achieve sustained viral response. The comprehensive strategies employed by the VA have meant that, from 2014 to 2016, the percentage of HCV-infected individuals who have been linked to care and treatment increased from 27% to 59%. Of those treated, SVR rates have risen from 51% to 84% during the same period, said Dr. Belperio and her coauthors.
It will not be easy to extend that success into the nongovernment sector, the article’s authors acknowledged. Still, “the VA recognizes the resources necessary to realize this goal and the innovations that could make it possible, and is poised to extend best practices to other health care organizations and providers delivering HCV care.”
None of the study authors reported conflicts of interest.
[email protected]
On Twitter @karioakes
The U.S. Department of Veterans Affairs (VA) cares for more patients with hepatitis C virus than any other care provider in the country, and has achieved a rapid and sharp reduction in hepatitis C virus (HCV) infection among veterans over the past 3 years.
The VA has treated more than 92,000 HCV-infected individuals since the advent of oral direct-acting antiviral (DAA) therapy in 2014. The number of veterans with HCV who were eligible for treatment was 168,000 in 2014 and 51,000 in July 2017, according to an article in Annals of Internal Medicine (2017 Sep 26. doi: 10.7326/M17-1073).
Given that success in HCV treatment, with a cure rate that exceeds 90% for those prescribed DAAs, the VA is well positioned to share best practices with other organizations, according to Pamela S. Belperio, PharmD, and her coauthors. The best practices harmonize with the recently outlined national strategy to eliminate hepatitis B and C from the National Academies of Sciences, Engineering, and Medicine.
“The leveraging of health systems data transforms numbers into knowledge” that is used both by individual providers and the VA as a whole, wrote Dr. Belperio, national public health pharmacist in the VA’s public health division, and her coauthors.
Identifying veterans who were infected with HCV is simplified by means of electronic point-of-care reminders that prompt providers to conduct HCV risk assessment and testing. Veterans eligible for testing also receive automated letters that serve as a laboratory order form for HCV testing at a VA laboratory.
Adding HCV birth cohort screening as a performance measure and recommending reflex confirmatory HCV RNA testing for positive antibody tests were additional initiatives that contributed to the 3%-4% annual increase in veterans receiving HCV testing, “substantially higher than in other health care systems,” wrote Dr. Belperio and her colleagues.
To respect regional variations in practice within the VA system, multidisciplinary innovation teams meet in each region to identify opportunities for improving and streamlining HCV testing and treatment, using lean process improvement techniques. Regions also share innovations with each other.
The VA used a multipronged approach to raise awareness about HCV testing and treatment among VA health care providers and veterans. Provider reach has been expanded by extensive use of telemedicine, including real-time video calls between providers and patients or other providers at remote locations. Those telemedicine initiatives allow pharmacists and mental health providers to lend their expertise to physicians and other providers, who are themselves directly caring for HCV-infected individuals.
Patients at more than half of VA facilities receive care from physician assistants, nurse practitioners, and clinical pharmacy specialists, “who have been recognized as delivering the same quality of care and providing more timely access to HCV treatment,” wrote Dr. Belperio and her collaborators. Expanding the use of advanced practice providers allows more targeted use of specialists, and “is an important practice that can be adapted into other health care systems,” they noted.
Non–evidence-based criteria for HCV treatment eligibility remain a major barrier for HCV-infected individuals nationwide, despite VA studies showing “cure rates among veterans with alcohol, substance use, and mental health disorders that are similar to cure rates in those without these conditions,” said Dr. Belperio and her colleagues.
Within the VA, involving alcohol and substance use specialists, mental health providers, case managers, and social workers in the patient’s care team is an approach that can increase adherence and up the chance for a cure among the most vulnerable veterans.
The VA has been able to negotiate reduced pricing for the expensive DAAs, and has continued to receive additional appropriations to fund DAAs and other HCV-related resources. “Financing for HCV treatment and infrastructure resources, coupled with reduced drug prices, has been paramount to the VA’s success in curing HCV infection,” said Dr. Belperio and her colleagues.
However, they added, individual private insurers may not reap the benefits of achieving sweeping HCV cures within their particular patient panel, because patients frequently switch insurers. Still, “consistent leveraging of drug prices and removal of restrictions to HCV treatment among all insurers would help assuage this gap,” they wrote.
Dr. Belperio described the VA’s vision of a “hepatitis C cascade of cure” that occurs when individuals with chronic HCV are identified, linked to care, treated with DAAs, and achieve sustained viral response. The comprehensive strategies employed by the VA have meant that, from 2014 to 2016, the percentage of HCV-infected individuals who have been linked to care and treatment increased from 27% to 59%. Of those treated, SVR rates have risen from 51% to 84% during the same period, said Dr. Belperio and her coauthors.
It will not be easy to extend that success into the nongovernment sector, the article’s authors acknowledged. Still, “the VA recognizes the resources necessary to realize this goal and the innovations that could make it possible, and is poised to extend best practices to other health care organizations and providers delivering HCV care.”
None of the study authors reported conflicts of interest.
[email protected]
On Twitter @karioakes
The U.S. Department of Veterans Affairs (VA) cares for more patients with hepatitis C virus than any other care provider in the country, and has achieved a rapid and sharp reduction in hepatitis C virus (HCV) infection among veterans over the past 3 years.
The VA has treated more than 92,000 HCV-infected individuals since the advent of oral direct-acting antiviral (DAA) therapy in 2014. The number of veterans with HCV who were eligible for treatment was 168,000 in 2014 and 51,000 in July 2017, according to an article in Annals of Internal Medicine (2017 Sep 26. doi: 10.7326/M17-1073).
Given that success in HCV treatment, with a cure rate that exceeds 90% for those prescribed DAAs, the VA is well positioned to share best practices with other organizations, according to Pamela S. Belperio, PharmD, and her coauthors. The best practices harmonize with the recently outlined national strategy to eliminate hepatitis B and C from the National Academies of Sciences, Engineering, and Medicine.
“The leveraging of health systems data transforms numbers into knowledge” that is used both by individual providers and the VA as a whole, wrote Dr. Belperio, national public health pharmacist in the VA’s public health division, and her coauthors.
Identifying veterans who were infected with HCV is simplified by means of electronic point-of-care reminders that prompt providers to conduct HCV risk assessment and testing. Veterans eligible for testing also receive automated letters that serve as a laboratory order form for HCV testing at a VA laboratory.
Adding HCV birth cohort screening as a performance measure and recommending reflex confirmatory HCV RNA testing for positive antibody tests were additional initiatives that contributed to the 3%-4% annual increase in veterans receiving HCV testing, “substantially higher than in other health care systems,” wrote Dr. Belperio and her colleagues.
To respect regional variations in practice within the VA system, multidisciplinary innovation teams meet in each region to identify opportunities for improving and streamlining HCV testing and treatment, using lean process improvement techniques. Regions also share innovations with each other.
The VA used a multipronged approach to raise awareness about HCV testing and treatment among VA health care providers and veterans. Provider reach has been expanded by extensive use of telemedicine, including real-time video calls between providers and patients or other providers at remote locations. Those telemedicine initiatives allow pharmacists and mental health providers to lend their expertise to physicians and other providers, who are themselves directly caring for HCV-infected individuals.
Patients at more than half of VA facilities receive care from physician assistants, nurse practitioners, and clinical pharmacy specialists, “who have been recognized as delivering the same quality of care and providing more timely access to HCV treatment,” wrote Dr. Belperio and her collaborators. Expanding the use of advanced practice providers allows more targeted use of specialists, and “is an important practice that can be adapted into other health care systems,” they noted.
Non–evidence-based criteria for HCV treatment eligibility remain a major barrier for HCV-infected individuals nationwide, despite VA studies showing “cure rates among veterans with alcohol, substance use, and mental health disorders that are similar to cure rates in those without these conditions,” said Dr. Belperio and her colleagues.
Within the VA, involving alcohol and substance use specialists, mental health providers, case managers, and social workers in the patient’s care team is an approach that can increase adherence and up the chance for a cure among the most vulnerable veterans.
The VA has been able to negotiate reduced pricing for the expensive DAAs, and has continued to receive additional appropriations to fund DAAs and other HCV-related resources. “Financing for HCV treatment and infrastructure resources, coupled with reduced drug prices, has been paramount to the VA’s success in curing HCV infection,” said Dr. Belperio and her colleagues.
However, they added, individual private insurers may not reap the benefits of achieving sweeping HCV cures within their particular patient panel, because patients frequently switch insurers. Still, “consistent leveraging of drug prices and removal of restrictions to HCV treatment among all insurers would help assuage this gap,” they wrote.
Dr. Belperio described the VA’s vision of a “hepatitis C cascade of cure” that occurs when individuals with chronic HCV are identified, linked to care, treated with DAAs, and achieve sustained viral response. The comprehensive strategies employed by the VA have meant that, from 2014 to 2016, the percentage of HCV-infected individuals who have been linked to care and treatment increased from 27% to 59%. Of those treated, SVR rates have risen from 51% to 84% during the same period, said Dr. Belperio and her coauthors.
It will not be easy to extend that success into the nongovernment sector, the article’s authors acknowledged. Still, “the VA recognizes the resources necessary to realize this goal and the innovations that could make it possible, and is poised to extend best practices to other health care organizations and providers delivering HCV care.”
None of the study authors reported conflicts of interest.
[email protected]
On Twitter @karioakes
FROM ANNALS OF INTERNAL MEDICINE
No benefit seen for routine low-dose oxygen after stroke
Routine use of low-dose oxygen supplementation in the first days after stroke doesn’t improve overall survival or reduce disability, according to a large new study.
The poststroke death and disability odds ratio was 0.97 for those receiving one of two continuous low-dose oxygen protocols, compared with the control group (95% confidence interval, 0.89-1.05; P = .47).
Participants, who were not hypoxic at enrollment, were randomized 1:1:1 to receive continuous oxygen supplementation for the first 72 hours after stroke, to receive supplementation only at night, or to receive oxygen when indicated by usual care protocols. The average participant age was 72 years and 55% were men in all study arms, and all stroke severity levels were included in the study.
Patients in the two intervention arms received 2 L of oxygen by nasal cannula when their baseline oxygen saturation was greater than 93%, and 3 L when oxygen saturation at baseline was 93% or less. Participation in the study did not preclude more intensive respiratory support when clinically indicated.
Nocturnal supplementation was included as a study arm for two reasons: Poststroke hypoxia is more common at night, and night-only supplementation would avoid any interference with early rehabilitation caused by cumbersome oxygen apparatus and tubing.
Not only was no benefit seen for patients in the pooled intervention arm cohorts, but no benefit was seen for night-time versus continuous oxygen as well. The odds ratio for a better outcome was 1.03 when comparing those receiving continuous oxygen to those who only received nocturnal supplementation (95% CI, 0.93-1.13; P = .61).
First author Christine Roffe, MD, and her collaborators in the Stroke Oxygen Study Collaborative Group also performed subgroup analyses and did not see benefit of oxygen supplementation for older or younger patients, or for patients with chronic obstructive pulmonary disease, heart failure, or more severe strokes.
“Supplemental oxygen could improve outcomes by preventing hypoxia and secondary brain damage but could also have adverse effects,” according to Dr. Roffe, consultant at Keele (England) University and her collaborators.
A much smaller SOS pilot study, they said, had shown improved early neurologic recovery for patients who received supplemental oxygen after stroke, but the pilot also “suggested that oxygen might adversely affect outcome in patients with mild strokes, possibly through formation of toxic free radicals,” wrote the investigators.
These were effects not seen in the larger SO2S study, which was designed to have statistical power to detect even small differences and to do detailed subgroup analysis. For patients like those included in the study, “These findings do not support low-dose oxygen in this setting,” wrote Dr. Roffe and her collaborators.
Dr. Roffe reported receiving compensation from Air Liquide. The study was funded by the United Kingdom’s National Institute for Health Research.
[email protected]
On Twitter @karioakes
Routine use of low-dose oxygen supplementation in the first days after stroke doesn’t improve overall survival or reduce disability, according to a large new study.
The poststroke death and disability odds ratio was 0.97 for those receiving one of two continuous low-dose oxygen protocols, compared with the control group (95% confidence interval, 0.89-1.05; P = .47).
Participants, who were not hypoxic at enrollment, were randomized 1:1:1 to receive continuous oxygen supplementation for the first 72 hours after stroke, to receive supplementation only at night, or to receive oxygen when indicated by usual care protocols. The average participant age was 72 years and 55% were men in all study arms, and all stroke severity levels were included in the study.
Patients in the two intervention arms received 2 L of oxygen by nasal cannula when their baseline oxygen saturation was greater than 93%, and 3 L when oxygen saturation at baseline was 93% or less. Participation in the study did not preclude more intensive respiratory support when clinically indicated.
Nocturnal supplementation was included as a study arm for two reasons: Poststroke hypoxia is more common at night, and night-only supplementation would avoid any interference with early rehabilitation caused by cumbersome oxygen apparatus and tubing.
Not only was no benefit seen for patients in the pooled intervention arm cohorts, but no benefit was seen for night-time versus continuous oxygen as well. The odds ratio for a better outcome was 1.03 when comparing those receiving continuous oxygen to those who only received nocturnal supplementation (95% CI, 0.93-1.13; P = .61).
First author Christine Roffe, MD, and her collaborators in the Stroke Oxygen Study Collaborative Group also performed subgroup analyses and did not see benefit of oxygen supplementation for older or younger patients, or for patients with chronic obstructive pulmonary disease, heart failure, or more severe strokes.
“Supplemental oxygen could improve outcomes by preventing hypoxia and secondary brain damage but could also have adverse effects,” according to Dr. Roffe, consultant at Keele (England) University and her collaborators.
A much smaller SOS pilot study, they said, had shown improved early neurologic recovery for patients who received supplemental oxygen after stroke, but the pilot also “suggested that oxygen might adversely affect outcome in patients with mild strokes, possibly through formation of toxic free radicals,” wrote the investigators.
These were effects not seen in the larger SO2S study, which was designed to have statistical power to detect even small differences and to do detailed subgroup analysis. For patients like those included in the study, “These findings do not support low-dose oxygen in this setting,” wrote Dr. Roffe and her collaborators.
Dr. Roffe reported receiving compensation from Air Liquide. The study was funded by the United Kingdom’s National Institute for Health Research.
[email protected]
On Twitter @karioakes
Routine use of low-dose oxygen supplementation in the first days after stroke doesn’t improve overall survival or reduce disability, according to a large new study.
The poststroke death and disability odds ratio was 0.97 for those receiving one of two continuous low-dose oxygen protocols, compared with the control group (95% confidence interval, 0.89-1.05; P = .47).
Participants, who were not hypoxic at enrollment, were randomized 1:1:1 to receive continuous oxygen supplementation for the first 72 hours after stroke, to receive supplementation only at night, or to receive oxygen when indicated by usual care protocols. The average participant age was 72 years and 55% were men in all study arms, and all stroke severity levels were included in the study.
Patients in the two intervention arms received 2 L of oxygen by nasal cannula when their baseline oxygen saturation was greater than 93%, and 3 L when oxygen saturation at baseline was 93% or less. Participation in the study did not preclude more intensive respiratory support when clinically indicated.
Nocturnal supplementation was included as a study arm for two reasons: Poststroke hypoxia is more common at night, and night-only supplementation would avoid any interference with early rehabilitation caused by cumbersome oxygen apparatus and tubing.
Not only was no benefit seen for patients in the pooled intervention arm cohorts, but no benefit was seen for night-time versus continuous oxygen as well. The odds ratio for a better outcome was 1.03 when comparing those receiving continuous oxygen to those who only received nocturnal supplementation (95% CI, 0.93-1.13; P = .61).
First author Christine Roffe, MD, and her collaborators in the Stroke Oxygen Study Collaborative Group also performed subgroup analyses and did not see benefit of oxygen supplementation for older or younger patients, or for patients with chronic obstructive pulmonary disease, heart failure, or more severe strokes.
“Supplemental oxygen could improve outcomes by preventing hypoxia and secondary brain damage but could also have adverse effects,” according to Dr. Roffe, consultant at Keele (England) University and her collaborators.
A much smaller SOS pilot study, they said, had shown improved early neurologic recovery for patients who received supplemental oxygen after stroke, but the pilot also “suggested that oxygen might adversely affect outcome in patients with mild strokes, possibly through formation of toxic free radicals,” wrote the investigators.
These were effects not seen in the larger SO2S study, which was designed to have statistical power to detect even small differences and to do detailed subgroup analysis. For patients like those included in the study, “These findings do not support low-dose oxygen in this setting,” wrote Dr. Roffe and her collaborators.
Dr. Roffe reported receiving compensation from Air Liquide. The study was funded by the United Kingdom’s National Institute for Health Research.
[email protected]
On Twitter @karioakes
FROM JAMA
Key clinical point:
Major finding: The poststroke death and disability odds ratio was 0.97 for those receiving continuous low-dose oxygen, compared with controls.
Data source: Single-blind, multisite, randomized, controlled trial of 8,003 patients admitted with acute stroke.
Disclosures: Dr. Roffe reported receiving compensation from Air Liquide. The study was funded by the United Kingdom’s National Institute for Health Research.
Few patients follow recommendation to use OTC benzoyl peroxide
Although benzoyl peroxide is a foundation of acne treatment, many patients are not following physician recommendations for its use, and its over-the-counter (OTC) availability may actually be a hindrance to adherence.
In a letter to the editor of the Journal of the American Academy of Dermatology, Andrea L. Zaenglein, MD, and Annie H. Huyler, of Penn State University, Hershey, reported the results of a telephone survey of 84 acne patients, aged 12-45 years. Fewer than a third (29%) recalled having received a recommendation for an OTC medication, and just 30% could recall that benzoyl peroxide (BP) was the recommended active ingredient (J Am Acad Dermatol. 2017 Oct;77[4]:763-4).
Curious about whether acne patients actually followed their treatment plans when it came to OTC BP, they arranged to have patients surveyed by telephone. New patients aged 12-45 years with an acne diagnosis who had been recommended BP were eligible.
The series of 10 survey questions began with more open-ended questions and moved to more close-ended questions. Of the 64% of patients who did buy an OTC product, further questioning revealed that half (32%) had actually purchased a BP-containing product. A total of 15% of patients had instead bought a face wash containing salicylic acid, and 17% of the products purchased had no active ingredient.
By contrast, the telephone survey revealed that all but one patient (93%) had filled the prescription for acne medication.
Benzoyl peroxide, which used to be available either by prescription or over the counter, has been available exclusively over the counter since 2011.
“The results from this study confirm that patient adherence to dermatologist-recommended BP is low,” they wrote. “Furthermore, of those who remembered BP by name, many were unable to find the correct product and instead had purchased an item with the wrong ingredient or no active ingredient,” they added. The findings are in line with other studies showing that patients are less likely to be adherent to recommendations to use OTC medications than they are to fill prescriptions for and take prescription medications, Ms. Huyler and Dr. Zaenglein wrote.
“Better education, in-office dispensing of BP, or fixed-dose combination prescription products are possible solutions,” they said.
The authors noted that their findings were limited by the exclusion of non-English speaking patients and by the fact that they used a nonvalidated telephone survey.
Ms. Huyler is a medical student and Dr. Zaenglein is a professor of dermatology at Penn State; they reported no conflicts of interest. The study had no external sources of funding.
Although benzoyl peroxide is a foundation of acne treatment, many patients are not following physician recommendations for its use, and its over-the-counter (OTC) availability may actually be a hindrance to adherence.
In a letter to the editor of the Journal of the American Academy of Dermatology, Andrea L. Zaenglein, MD, and Annie H. Huyler, of Penn State University, Hershey, reported the results of a telephone survey of 84 acne patients, aged 12-45 years. Fewer than a third (29%) recalled having received a recommendation for an OTC medication, and just 30% could recall that benzoyl peroxide (BP) was the recommended active ingredient (J Am Acad Dermatol. 2017 Oct;77[4]:763-4).
Curious about whether acne patients actually followed their treatment plans when it came to OTC BP, they arranged to have patients surveyed by telephone. New patients aged 12-45 years with an acne diagnosis who had been recommended BP were eligible.
The series of 10 survey questions began with more open-ended questions and moved to more close-ended questions. Of the 64% of patients who did buy an OTC product, further questioning revealed that half (32%) had actually purchased a BP-containing product. A total of 15% of patients had instead bought a face wash containing salicylic acid, and 17% of the products purchased had no active ingredient.
By contrast, the telephone survey revealed that all but one patient (93%) had filled the prescription for acne medication.
Benzoyl peroxide, which used to be available either by prescription or over the counter, has been available exclusively over the counter since 2011.
“The results from this study confirm that patient adherence to dermatologist-recommended BP is low,” they wrote. “Furthermore, of those who remembered BP by name, many were unable to find the correct product and instead had purchased an item with the wrong ingredient or no active ingredient,” they added. The findings are in line with other studies showing that patients are less likely to be adherent to recommendations to use OTC medications than they are to fill prescriptions for and take prescription medications, Ms. Huyler and Dr. Zaenglein wrote.
“Better education, in-office dispensing of BP, or fixed-dose combination prescription products are possible solutions,” they said.
The authors noted that their findings were limited by the exclusion of non-English speaking patients and by the fact that they used a nonvalidated telephone survey.
Ms. Huyler is a medical student and Dr. Zaenglein is a professor of dermatology at Penn State; they reported no conflicts of interest. The study had no external sources of funding.
Although benzoyl peroxide is a foundation of acne treatment, many patients are not following physician recommendations for its use, and its over-the-counter (OTC) availability may actually be a hindrance to adherence.
In a letter to the editor of the Journal of the American Academy of Dermatology, Andrea L. Zaenglein, MD, and Annie H. Huyler, of Penn State University, Hershey, reported the results of a telephone survey of 84 acne patients, aged 12-45 years. Fewer than a third (29%) recalled having received a recommendation for an OTC medication, and just 30% could recall that benzoyl peroxide (BP) was the recommended active ingredient (J Am Acad Dermatol. 2017 Oct;77[4]:763-4).
Curious about whether acne patients actually followed their treatment plans when it came to OTC BP, they arranged to have patients surveyed by telephone. New patients aged 12-45 years with an acne diagnosis who had been recommended BP were eligible.
The series of 10 survey questions began with more open-ended questions and moved to more close-ended questions. Of the 64% of patients who did buy an OTC product, further questioning revealed that half (32%) had actually purchased a BP-containing product. A total of 15% of patients had instead bought a face wash containing salicylic acid, and 17% of the products purchased had no active ingredient.
By contrast, the telephone survey revealed that all but one patient (93%) had filled the prescription for acne medication.
Benzoyl peroxide, which used to be available either by prescription or over the counter, has been available exclusively over the counter since 2011.
“The results from this study confirm that patient adherence to dermatologist-recommended BP is low,” they wrote. “Furthermore, of those who remembered BP by name, many were unable to find the correct product and instead had purchased an item with the wrong ingredient or no active ingredient,” they added. The findings are in line with other studies showing that patients are less likely to be adherent to recommendations to use OTC medications than they are to fill prescriptions for and take prescription medications, Ms. Huyler and Dr. Zaenglein wrote.
“Better education, in-office dispensing of BP, or fixed-dose combination prescription products are possible solutions,” they said.
The authors noted that their findings were limited by the exclusion of non-English speaking patients and by the fact that they used a nonvalidated telephone survey.
Ms. Huyler is a medical student and Dr. Zaenglein is a professor of dermatology at Penn State; they reported no conflicts of interest. The study had no external sources of funding.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: In a telephone interview, many acne patients said they had not obtained the recommended OTC benzoyl peroxide.
Major finding: Of the 64% of patients who had gotten an OTC acne medication, only 32% had purchased one containing benzoyl peroxide.
Study details: Single-center prospective study of 84 acne patients who received a physician recommendation for OTC benzoyl peroxide.
Disclosures: There was no funding source for the study, and the investigators had no conflicts of interest.
Full-spectrum family practice can still include obstetrics
For some patients in rural or otherwise underserved settings, the nearest obstetrician may be counties away. Other patients, though, seek the continuity of care that can come when one doctor cares for the whole family through all phases of life.
And physicians who choose to incorporate obstetrics into their range of practice do so to serve their communities but also because of the profound satisfaction they find in providing families care of this scope.

The American Academy of Family Practice makes it clear that full-spectrum family medicine still includes obstetrics. “We continue to support the full scope of practice for family physicians and training that supports that scope,” said Clif Knight, MD, senior vice president of education at the American Academy of Family Practice. “We believe that all family physicians should have a solid foundation of education in obstetrics, knowing that many will choose not to perform deliveries once they finish residency,” said Dr. Knight.
For those who really want to make obstetrics a focus or who are interested in teaching, a year-long postresidency obstetrics fellowship might make sense. “We absolutely are supportive of those family medicine grads who want to do additional training. That makes great sense to us,” said Dr. Knight.
For some family physicians, keeping an obstetrics practice, with its high level of engagement and procedural expertise, may actually ensure against burnout. Family physicians may have chosen the specialty because of the high priority they place on a wide scope of care that still puts human relationships first – and obstetrics certainly checks off those boxes, he said.
“We work hard to support our members who want to continue practicing obstetrics,” said Dr. Knight, noting that there’s an annual obstetrics-focused CME conference. When he attended the conference a few years ago, Dr. Knight “was struck by how mission-driven those family practice physicians are who continue to do obstetrics as part of their scope of practice,” he said. “They treasure that opportunity.”
Rural areas are the practice setting for many of the 17% of family physicians who report that they practice obstetrics. The family practice residency at the University of Wisconsin–Madison’s School of Medicine and Public Health includes an option for a rural training track; strong obstetrics training is woven through the residency curriculum.
The Baraboo approach
Since its inception in 1996, the Baraboo (Wisc.) rural residency has had 29 graduates, enrolling up to two residents per year. Two-thirds of the graduates now practice obstetrics, 69% are in rural areas, and about half have stayed in Wisconsin, said Sharilyn Munneke, MD, assistant program director for the residency. Dr. Munneke also heads up the obstetrics and women’s health curriculum for the residency.*
Residents who choose the Baraboo rural site for their training will spend their first postgraduate year in Madison, the state capital. The first year features a busy obstetrics rotation at a large community hospital, giving all residents a solid labor and delivery foundation. Rural track trainees also spend a day a week at a continuity clinic in Baraboo, a community of about 12,000 that’s an hour north of Madison.
Beginning in the second year, residents move to Baraboo; there, under supervision, “they essentially start functioning as a family doctor,” said Dr. Munneke in an interview, adding that the residents also have inpatient obstetrics and intensive care unit training at Baraboo’s 100-bed St. Clare Hospital.
The clinic setting gives residents an introduction to the multigenerational care that’s the hallmark of rural family medicine, as each succeeding class of residents inherits the graduating class’ panels. Patients come from the town, from surrounding agricultural and recreational areas, and from the Ho-Chunk Native American tribe, many of whose members live in the area. Residents begin to build their obstetrics practice from the clinic, she said, managing prenatal care, labor, and delivery under the supervision of family practice physicians who do obstetrics.
There is no obstetrician at St. Clare Hospital, but the rural-track residents still have the opportunity to assist at cesarean deliveries. “Our surgeons do our C-sections,” said Dr. Munneke, so residents will scrub in to assist the general surgeon on call for cesarean deliveries.
Dr. Munneke said that there are plenty of opportunities during training to learn other gynecologic procedures as well. “I teach colposcopies; I teach endometrial biopsies. I wrote a grant so we could get the equipment to do informal ultrasounds in the clinic, to assess for twins or for fetal viability,” she said.
Family practice physicians and residents in Baraboo have a good working relationship with Madison maternal-fetal medicine specialists and the referral hospital, she said, so that, even for high-risk pregnancies, as much care as possible can be delivered close to home. This is important for families whose farming obligations and family situations might make a woman’s prolonged absence incredibly difficult, said Dr. Munneke. Though women are referred to Madison for deliveries before 36 weeks, residents still receive neonatal resuscitation training, so they become comfortable stabilizing fragile neonates until transport is arranged.
For Rachel Hartline, MD, the Baraboo training experience was just what she’d been looking for. After completing medical school in her native Virginia, she realized that the family physicians she’d rotated with had been “excellent role models;” at the same time, she said, “I realized that their [practice] scope was not the scope I wanted to have.”
During her time in Baraboo, Dr. Hartline, who finished her residency in 2015, appreciated the opportunity for the “additional layer” that cesarean section training added for her. Whenever possible, she scrubbed in on scheduled cesareans. “There was also a C-section pager that was passed among those who were learning cesareans,” for additional opportunities when crash cesareans occurred, she said.
“My goal was 50 cesareans” during training, said Dr. Hartline. “I was a little shy of that,” she said, so her new partners in Dodgeville, Wisc. agreed to continue to mentor her through her first few cesarean deliveries.
Now, she is in a practice that includes obstetricians, with whom she splits obstetrics 1:4. Dodgeville’s Upland Hills Hospital is a critical-access hospital where approximately 300 babies are delivered yearly. Dr. Hartline said she’s also often called on to do deliveries for other physicians at one of the three groups who practice at Upland Hills.
Having a collaborative relationship with the community’s obstetricians is a real plus, said Dr. Hartline, who performs cesarean sections and is comfortable with vacuum deliveries, but doesn’t do forceps deliveries. “If I have a patient that seems too high, I might call one of my partners,” she said.
Upland Hills, like St. Clare, does not have a neonatal intensive care unit, so deliveries before 36 weeks are referred elsewhere whenever possible.
Dr. Hartline said that she also enjoys the full spectrum of family practice in her clinic. The agricultural area where she’s situated is home to many farm families, who she says can be reluctant to seek care, so chronic disease management can be a challenge. She also sees a growing number of undocumented immigrants as that population grows in rural Wisconsin. “I see all ages; I don’t say ‘no’ to much,” she said.
“But obstetrics is a big part of the reason why I’m a family doctor. It’s so cool to be a part of bringing someone’s child into the world and to be able to be there for them,” she said.
Cradle to grave
Dr. Hartline’s rural training classmate Rebecca Pfaff, MD, now lives and works in Vernon County, Wisc. The clinic and the small community hospital that are her practice home are in the small town of LaFarge. A native of Washington State, Dr. Pfaff thought she’d end up there after her undergraduate years at Wellesley College and her time at Meharry Medical College, a historically black medical school with a social justice focus in Nashville, Tenn.
She realized that in the right practice setting, she could find the scope she was seeking. “I became a family medicine doctor to do obstetrics, and to do family medicine,” she said.
Dr. Pfaff chose the Baraboo rural training track, still with the intention of eventually returning to practice in her home town of Port Angeles, Wash. There, the family practice group had already hired two Baraboo graduates, so they knew the strength of the program and recommended it to Dr. Pfaff.
Seeing patients living with the challenges of rural poverty, she said, helped her learn to care for women with substance use disorder and to gain experience caring for infants with neonatal abstinence syndrome. “That is the perfect setting for where family medicine thrives,” said Dr. Pfaff. Having established relationships with the mothers, she felt able to treat the mother-infant dyad as a unit, without judgment, and with natural opportunities for frequent follow-up.
Dr. Pfaff had a busy obstetrics practice in Baraboo and had the opportunity to perform cesarean sections. To feed her interest in low-intervention obstetrics, though, she sought and was able to secure a rotation in LaFarge, her eventual practice home.
Knowing she would return to LaFarge, Dr. Pfaff went on to complete a 1-year obstetrics fellowship at Swedish Hospital in Seattle. The obstetricians and family physicians there cared for high-risk patients from as far away as Alaska; her fellowship training, she said, was in many ways “the polar opposite” of the low-intervention, community-based work she’d done in Baraboo.
Still, she said, the fellowship gave her procedural expertise and boosted her confidence that she could handle many high-risk situations. She appreciated the perspective of some of her obstetrician instructors, who themselves had been solo practitioners in rural areas.
Her practice now, she says, is everything she’d hoped it would be. She sees patients in the clinic in a cradle-to-grave practice that includes many members of Vernon County, Wisconsin’s Amish community. Dr. Pfaff and her colleagues at the small clinic in LaFarge embrace the special challenges and rewards of obstetric care of a population that has traditionally had their babies at home.
The Amish community
Using low-intervention techniques is a priority, even when many Amish women have grand multiparity or present with other risk factors. “It’s not uncommon for us to see women on their twelfth or thirteenth pregnancy,” she said. Gestation dating is usually no more than a best guess, since women become pregnant while still amenorrheic from breastfeeding their last child.
Because the area’s Amish population has grown from a small group of founders who came to the hills of Vernon County in the late 1700s, there is an elevated rate of genetic disorders in the population, including metabolic disorders and congenital heart disease. Most Amish women return home within a few hours of delivery, and use no analgesia, even for breech and twin vaginal deliveries.
Though she’s comfortable with low-intervention care, Dr. Pfaff also performs cesarean sections at the community hospital when it’s indicated, sharing call duties with the community’s general surgeons. There’s no obstetrician in Vernon County.
The hospital is not equipped to care for preterm infants, so deliveries earlier than 36 weeks’ are referred, as are patients with abnormal placentation or fetal anomalies detected on ultrasound.
She loves the continuity that her practice now lets her have, said Dr. Pfaff. “When you’re seeing a woman in family medicine, instead of seeing her at 6 weeks, setting her up with contraception, and then seeing her again in a year, you’re seeing her every 2 weeks with that baby of hers,” she said. The care of mother and infant is so intertwined that sometimes it gives Dr. Pfaff pause, as when she tries to decide in whose chart she should be documenting. “They’re a unit at this time of their lives.”
When asked if she plans to stay put, Dr. Pfaff doesn’t hesitate. “Oh yes,” she said. “I’m so lucky.”
Dr. Hartline agreed. When asked what advice she’d give a trainee considering a rural, full-spectrum career path, she said, “This is a good life. Come and join us!”
Dr. Knight is an employee of AAFP. None of the physicians interviewed had financial conflicts of interest.
Correction, 9/22/17: An earlier version of this article misstated Dr. Munneke's title.
This article was updated 9/25/17.
[email protected]
On Twitter @karioakes
For some patients in rural or otherwise underserved settings, the nearest obstetrician may be counties away. Other patients, though, seek the continuity of care that can come when one doctor cares for the whole family through all phases of life.
And physicians who choose to incorporate obstetrics into their range of practice do so to serve their communities but also because of the profound satisfaction they find in providing families care of this scope.

The American Academy of Family Practice makes it clear that full-spectrum family medicine still includes obstetrics. “We continue to support the full scope of practice for family physicians and training that supports that scope,” said Clif Knight, MD, senior vice president of education at the American Academy of Family Practice. “We believe that all family physicians should have a solid foundation of education in obstetrics, knowing that many will choose not to perform deliveries once they finish residency,” said Dr. Knight.
For those who really want to make obstetrics a focus or who are interested in teaching, a year-long postresidency obstetrics fellowship might make sense. “We absolutely are supportive of those family medicine grads who want to do additional training. That makes great sense to us,” said Dr. Knight.
For some family physicians, keeping an obstetrics practice, with its high level of engagement and procedural expertise, may actually ensure against burnout. Family physicians may have chosen the specialty because of the high priority they place on a wide scope of care that still puts human relationships first – and obstetrics certainly checks off those boxes, he said.
“We work hard to support our members who want to continue practicing obstetrics,” said Dr. Knight, noting that there’s an annual obstetrics-focused CME conference. When he attended the conference a few years ago, Dr. Knight “was struck by how mission-driven those family practice physicians are who continue to do obstetrics as part of their scope of practice,” he said. “They treasure that opportunity.”
Rural areas are the practice setting for many of the 17% of family physicians who report that they practice obstetrics. The family practice residency at the University of Wisconsin–Madison’s School of Medicine and Public Health includes an option for a rural training track; strong obstetrics training is woven through the residency curriculum.
The Baraboo approach
Since its inception in 1996, the Baraboo (Wisc.) rural residency has had 29 graduates, enrolling up to two residents per year. Two-thirds of the graduates now practice obstetrics, 69% are in rural areas, and about half have stayed in Wisconsin, said Sharilyn Munneke, MD, assistant program director for the residency. Dr. Munneke also heads up the obstetrics and women’s health curriculum for the residency.*
Residents who choose the Baraboo rural site for their training will spend their first postgraduate year in Madison, the state capital. The first year features a busy obstetrics rotation at a large community hospital, giving all residents a solid labor and delivery foundation. Rural track trainees also spend a day a week at a continuity clinic in Baraboo, a community of about 12,000 that’s an hour north of Madison.
Beginning in the second year, residents move to Baraboo; there, under supervision, “they essentially start functioning as a family doctor,” said Dr. Munneke in an interview, adding that the residents also have inpatient obstetrics and intensive care unit training at Baraboo’s 100-bed St. Clare Hospital.
The clinic setting gives residents an introduction to the multigenerational care that’s the hallmark of rural family medicine, as each succeeding class of residents inherits the graduating class’ panels. Patients come from the town, from surrounding agricultural and recreational areas, and from the Ho-Chunk Native American tribe, many of whose members live in the area. Residents begin to build their obstetrics practice from the clinic, she said, managing prenatal care, labor, and delivery under the supervision of family practice physicians who do obstetrics.
There is no obstetrician at St. Clare Hospital, but the rural-track residents still have the opportunity to assist at cesarean deliveries. “Our surgeons do our C-sections,” said Dr. Munneke, so residents will scrub in to assist the general surgeon on call for cesarean deliveries.
Dr. Munneke said that there are plenty of opportunities during training to learn other gynecologic procedures as well. “I teach colposcopies; I teach endometrial biopsies. I wrote a grant so we could get the equipment to do informal ultrasounds in the clinic, to assess for twins or for fetal viability,” she said.
Family practice physicians and residents in Baraboo have a good working relationship with Madison maternal-fetal medicine specialists and the referral hospital, she said, so that, even for high-risk pregnancies, as much care as possible can be delivered close to home. This is important for families whose farming obligations and family situations might make a woman’s prolonged absence incredibly difficult, said Dr. Munneke. Though women are referred to Madison for deliveries before 36 weeks, residents still receive neonatal resuscitation training, so they become comfortable stabilizing fragile neonates until transport is arranged.
For Rachel Hartline, MD, the Baraboo training experience was just what she’d been looking for. After completing medical school in her native Virginia, she realized that the family physicians she’d rotated with had been “excellent role models;” at the same time, she said, “I realized that their [practice] scope was not the scope I wanted to have.”
During her time in Baraboo, Dr. Hartline, who finished her residency in 2015, appreciated the opportunity for the “additional layer” that cesarean section training added for her. Whenever possible, she scrubbed in on scheduled cesareans. “There was also a C-section pager that was passed among those who were learning cesareans,” for additional opportunities when crash cesareans occurred, she said.
“My goal was 50 cesareans” during training, said Dr. Hartline. “I was a little shy of that,” she said, so her new partners in Dodgeville, Wisc. agreed to continue to mentor her through her first few cesarean deliveries.
Now, she is in a practice that includes obstetricians, with whom she splits obstetrics 1:4. Dodgeville’s Upland Hills Hospital is a critical-access hospital where approximately 300 babies are delivered yearly. Dr. Hartline said she’s also often called on to do deliveries for other physicians at one of the three groups who practice at Upland Hills.
Having a collaborative relationship with the community’s obstetricians is a real plus, said Dr. Hartline, who performs cesarean sections and is comfortable with vacuum deliveries, but doesn’t do forceps deliveries. “If I have a patient that seems too high, I might call one of my partners,” she said.
Upland Hills, like St. Clare, does not have a neonatal intensive care unit, so deliveries before 36 weeks are referred elsewhere whenever possible.
Dr. Hartline said that she also enjoys the full spectrum of family practice in her clinic. The agricultural area where she’s situated is home to many farm families, who she says can be reluctant to seek care, so chronic disease management can be a challenge. She also sees a growing number of undocumented immigrants as that population grows in rural Wisconsin. “I see all ages; I don’t say ‘no’ to much,” she said.
“But obstetrics is a big part of the reason why I’m a family doctor. It’s so cool to be a part of bringing someone’s child into the world and to be able to be there for them,” she said.
Cradle to grave
Dr. Hartline’s rural training classmate Rebecca Pfaff, MD, now lives and works in Vernon County, Wisc. The clinic and the small community hospital that are her practice home are in the small town of LaFarge. A native of Washington State, Dr. Pfaff thought she’d end up there after her undergraduate years at Wellesley College and her time at Meharry Medical College, a historically black medical school with a social justice focus in Nashville, Tenn.
She realized that in the right practice setting, she could find the scope she was seeking. “I became a family medicine doctor to do obstetrics, and to do family medicine,” she said.
Dr. Pfaff chose the Baraboo rural training track, still with the intention of eventually returning to practice in her home town of Port Angeles, Wash. There, the family practice group had already hired two Baraboo graduates, so they knew the strength of the program and recommended it to Dr. Pfaff.
Seeing patients living with the challenges of rural poverty, she said, helped her learn to care for women with substance use disorder and to gain experience caring for infants with neonatal abstinence syndrome. “That is the perfect setting for where family medicine thrives,” said Dr. Pfaff. Having established relationships with the mothers, she felt able to treat the mother-infant dyad as a unit, without judgment, and with natural opportunities for frequent follow-up.
Dr. Pfaff had a busy obstetrics practice in Baraboo and had the opportunity to perform cesarean sections. To feed her interest in low-intervention obstetrics, though, she sought and was able to secure a rotation in LaFarge, her eventual practice home.
Knowing she would return to LaFarge, Dr. Pfaff went on to complete a 1-year obstetrics fellowship at Swedish Hospital in Seattle. The obstetricians and family physicians there cared for high-risk patients from as far away as Alaska; her fellowship training, she said, was in many ways “the polar opposite” of the low-intervention, community-based work she’d done in Baraboo.
Still, she said, the fellowship gave her procedural expertise and boosted her confidence that she could handle many high-risk situations. She appreciated the perspective of some of her obstetrician instructors, who themselves had been solo practitioners in rural areas.
Her practice now, she says, is everything she’d hoped it would be. She sees patients in the clinic in a cradle-to-grave practice that includes many members of Vernon County, Wisconsin’s Amish community. Dr. Pfaff and her colleagues at the small clinic in LaFarge embrace the special challenges and rewards of obstetric care of a population that has traditionally had their babies at home.
The Amish community
Using low-intervention techniques is a priority, even when many Amish women have grand multiparity or present with other risk factors. “It’s not uncommon for us to see women on their twelfth or thirteenth pregnancy,” she said. Gestation dating is usually no more than a best guess, since women become pregnant while still amenorrheic from breastfeeding their last child.
Because the area’s Amish population has grown from a small group of founders who came to the hills of Vernon County in the late 1700s, there is an elevated rate of genetic disorders in the population, including metabolic disorders and congenital heart disease. Most Amish women return home within a few hours of delivery, and use no analgesia, even for breech and twin vaginal deliveries.
Though she’s comfortable with low-intervention care, Dr. Pfaff also performs cesarean sections at the community hospital when it’s indicated, sharing call duties with the community’s general surgeons. There’s no obstetrician in Vernon County.
The hospital is not equipped to care for preterm infants, so deliveries earlier than 36 weeks’ are referred, as are patients with abnormal placentation or fetal anomalies detected on ultrasound.
She loves the continuity that her practice now lets her have, said Dr. Pfaff. “When you’re seeing a woman in family medicine, instead of seeing her at 6 weeks, setting her up with contraception, and then seeing her again in a year, you’re seeing her every 2 weeks with that baby of hers,” she said. The care of mother and infant is so intertwined that sometimes it gives Dr. Pfaff pause, as when she tries to decide in whose chart she should be documenting. “They’re a unit at this time of their lives.”
When asked if she plans to stay put, Dr. Pfaff doesn’t hesitate. “Oh yes,” she said. “I’m so lucky.”
Dr. Hartline agreed. When asked what advice she’d give a trainee considering a rural, full-spectrum career path, she said, “This is a good life. Come and join us!”
Dr. Knight is an employee of AAFP. None of the physicians interviewed had financial conflicts of interest.
Correction, 9/22/17: An earlier version of this article misstated Dr. Munneke's title.
This article was updated 9/25/17.
[email protected]
On Twitter @karioakes
For some patients in rural or otherwise underserved settings, the nearest obstetrician may be counties away. Other patients, though, seek the continuity of care that can come when one doctor cares for the whole family through all phases of life.
And physicians who choose to incorporate obstetrics into their range of practice do so to serve their communities but also because of the profound satisfaction they find in providing families care of this scope.

The American Academy of Family Practice makes it clear that full-spectrum family medicine still includes obstetrics. “We continue to support the full scope of practice for family physicians and training that supports that scope,” said Clif Knight, MD, senior vice president of education at the American Academy of Family Practice. “We believe that all family physicians should have a solid foundation of education in obstetrics, knowing that many will choose not to perform deliveries once they finish residency,” said Dr. Knight.
For those who really want to make obstetrics a focus or who are interested in teaching, a year-long postresidency obstetrics fellowship might make sense. “We absolutely are supportive of those family medicine grads who want to do additional training. That makes great sense to us,” said Dr. Knight.
For some family physicians, keeping an obstetrics practice, with its high level of engagement and procedural expertise, may actually ensure against burnout. Family physicians may have chosen the specialty because of the high priority they place on a wide scope of care that still puts human relationships first – and obstetrics certainly checks off those boxes, he said.
“We work hard to support our members who want to continue practicing obstetrics,” said Dr. Knight, noting that there’s an annual obstetrics-focused CME conference. When he attended the conference a few years ago, Dr. Knight “was struck by how mission-driven those family practice physicians are who continue to do obstetrics as part of their scope of practice,” he said. “They treasure that opportunity.”
Rural areas are the practice setting for many of the 17% of family physicians who report that they practice obstetrics. The family practice residency at the University of Wisconsin–Madison’s School of Medicine and Public Health includes an option for a rural training track; strong obstetrics training is woven through the residency curriculum.
The Baraboo approach
Since its inception in 1996, the Baraboo (Wisc.) rural residency has had 29 graduates, enrolling up to two residents per year. Two-thirds of the graduates now practice obstetrics, 69% are in rural areas, and about half have stayed in Wisconsin, said Sharilyn Munneke, MD, assistant program director for the residency. Dr. Munneke also heads up the obstetrics and women’s health curriculum for the residency.*
Residents who choose the Baraboo rural site for their training will spend their first postgraduate year in Madison, the state capital. The first year features a busy obstetrics rotation at a large community hospital, giving all residents a solid labor and delivery foundation. Rural track trainees also spend a day a week at a continuity clinic in Baraboo, a community of about 12,000 that’s an hour north of Madison.
Beginning in the second year, residents move to Baraboo; there, under supervision, “they essentially start functioning as a family doctor,” said Dr. Munneke in an interview, adding that the residents also have inpatient obstetrics and intensive care unit training at Baraboo’s 100-bed St. Clare Hospital.
The clinic setting gives residents an introduction to the multigenerational care that’s the hallmark of rural family medicine, as each succeeding class of residents inherits the graduating class’ panels. Patients come from the town, from surrounding agricultural and recreational areas, and from the Ho-Chunk Native American tribe, many of whose members live in the area. Residents begin to build their obstetrics practice from the clinic, she said, managing prenatal care, labor, and delivery under the supervision of family practice physicians who do obstetrics.
There is no obstetrician at St. Clare Hospital, but the rural-track residents still have the opportunity to assist at cesarean deliveries. “Our surgeons do our C-sections,” said Dr. Munneke, so residents will scrub in to assist the general surgeon on call for cesarean deliveries.
Dr. Munneke said that there are plenty of opportunities during training to learn other gynecologic procedures as well. “I teach colposcopies; I teach endometrial biopsies. I wrote a grant so we could get the equipment to do informal ultrasounds in the clinic, to assess for twins or for fetal viability,” she said.
Family practice physicians and residents in Baraboo have a good working relationship with Madison maternal-fetal medicine specialists and the referral hospital, she said, so that, even for high-risk pregnancies, as much care as possible can be delivered close to home. This is important for families whose farming obligations and family situations might make a woman’s prolonged absence incredibly difficult, said Dr. Munneke. Though women are referred to Madison for deliveries before 36 weeks, residents still receive neonatal resuscitation training, so they become comfortable stabilizing fragile neonates until transport is arranged.
For Rachel Hartline, MD, the Baraboo training experience was just what she’d been looking for. After completing medical school in her native Virginia, she realized that the family physicians she’d rotated with had been “excellent role models;” at the same time, she said, “I realized that their [practice] scope was not the scope I wanted to have.”
During her time in Baraboo, Dr. Hartline, who finished her residency in 2015, appreciated the opportunity for the “additional layer” that cesarean section training added for her. Whenever possible, she scrubbed in on scheduled cesareans. “There was also a C-section pager that was passed among those who were learning cesareans,” for additional opportunities when crash cesareans occurred, she said.
“My goal was 50 cesareans” during training, said Dr. Hartline. “I was a little shy of that,” she said, so her new partners in Dodgeville, Wisc. agreed to continue to mentor her through her first few cesarean deliveries.
Now, she is in a practice that includes obstetricians, with whom she splits obstetrics 1:4. Dodgeville’s Upland Hills Hospital is a critical-access hospital where approximately 300 babies are delivered yearly. Dr. Hartline said she’s also often called on to do deliveries for other physicians at one of the three groups who practice at Upland Hills.
Having a collaborative relationship with the community’s obstetricians is a real plus, said Dr. Hartline, who performs cesarean sections and is comfortable with vacuum deliveries, but doesn’t do forceps deliveries. “If I have a patient that seems too high, I might call one of my partners,” she said.
Upland Hills, like St. Clare, does not have a neonatal intensive care unit, so deliveries before 36 weeks are referred elsewhere whenever possible.
Dr. Hartline said that she also enjoys the full spectrum of family practice in her clinic. The agricultural area where she’s situated is home to many farm families, who she says can be reluctant to seek care, so chronic disease management can be a challenge. She also sees a growing number of undocumented immigrants as that population grows in rural Wisconsin. “I see all ages; I don’t say ‘no’ to much,” she said.
“But obstetrics is a big part of the reason why I’m a family doctor. It’s so cool to be a part of bringing someone’s child into the world and to be able to be there for them,” she said.
Cradle to grave
Dr. Hartline’s rural training classmate Rebecca Pfaff, MD, now lives and works in Vernon County, Wisc. The clinic and the small community hospital that are her practice home are in the small town of LaFarge. A native of Washington State, Dr. Pfaff thought she’d end up there after her undergraduate years at Wellesley College and her time at Meharry Medical College, a historically black medical school with a social justice focus in Nashville, Tenn.
She realized that in the right practice setting, she could find the scope she was seeking. “I became a family medicine doctor to do obstetrics, and to do family medicine,” she said.
Dr. Pfaff chose the Baraboo rural training track, still with the intention of eventually returning to practice in her home town of Port Angeles, Wash. There, the family practice group had already hired two Baraboo graduates, so they knew the strength of the program and recommended it to Dr. Pfaff.
Seeing patients living with the challenges of rural poverty, she said, helped her learn to care for women with substance use disorder and to gain experience caring for infants with neonatal abstinence syndrome. “That is the perfect setting for where family medicine thrives,” said Dr. Pfaff. Having established relationships with the mothers, she felt able to treat the mother-infant dyad as a unit, without judgment, and with natural opportunities for frequent follow-up.
Dr. Pfaff had a busy obstetrics practice in Baraboo and had the opportunity to perform cesarean sections. To feed her interest in low-intervention obstetrics, though, she sought and was able to secure a rotation in LaFarge, her eventual practice home.
Knowing she would return to LaFarge, Dr. Pfaff went on to complete a 1-year obstetrics fellowship at Swedish Hospital in Seattle. The obstetricians and family physicians there cared for high-risk patients from as far away as Alaska; her fellowship training, she said, was in many ways “the polar opposite” of the low-intervention, community-based work she’d done in Baraboo.
Still, she said, the fellowship gave her procedural expertise and boosted her confidence that she could handle many high-risk situations. She appreciated the perspective of some of her obstetrician instructors, who themselves had been solo practitioners in rural areas.
Her practice now, she says, is everything she’d hoped it would be. She sees patients in the clinic in a cradle-to-grave practice that includes many members of Vernon County, Wisconsin’s Amish community. Dr. Pfaff and her colleagues at the small clinic in LaFarge embrace the special challenges and rewards of obstetric care of a population that has traditionally had their babies at home.
The Amish community
Using low-intervention techniques is a priority, even when many Amish women have grand multiparity or present with other risk factors. “It’s not uncommon for us to see women on their twelfth or thirteenth pregnancy,” she said. Gestation dating is usually no more than a best guess, since women become pregnant while still amenorrheic from breastfeeding their last child.
Because the area’s Amish population has grown from a small group of founders who came to the hills of Vernon County in the late 1700s, there is an elevated rate of genetic disorders in the population, including metabolic disorders and congenital heart disease. Most Amish women return home within a few hours of delivery, and use no analgesia, even for breech and twin vaginal deliveries.
Though she’s comfortable with low-intervention care, Dr. Pfaff also performs cesarean sections at the community hospital when it’s indicated, sharing call duties with the community’s general surgeons. There’s no obstetrician in Vernon County.
The hospital is not equipped to care for preterm infants, so deliveries earlier than 36 weeks’ are referred, as are patients with abnormal placentation or fetal anomalies detected on ultrasound.
She loves the continuity that her practice now lets her have, said Dr. Pfaff. “When you’re seeing a woman in family medicine, instead of seeing her at 6 weeks, setting her up with contraception, and then seeing her again in a year, you’re seeing her every 2 weeks with that baby of hers,” she said. The care of mother and infant is so intertwined that sometimes it gives Dr. Pfaff pause, as when she tries to decide in whose chart she should be documenting. “They’re a unit at this time of their lives.”
When asked if she plans to stay put, Dr. Pfaff doesn’t hesitate. “Oh yes,” she said. “I’m so lucky.”
Dr. Hartline agreed. When asked what advice she’d give a trainee considering a rural, full-spectrum career path, she said, “This is a good life. Come and join us!”
Dr. Knight is an employee of AAFP. None of the physicians interviewed had financial conflicts of interest.
Correction, 9/22/17: An earlier version of this article misstated Dr. Munneke's title.
This article was updated 9/25/17.
[email protected]
On Twitter @karioakes
When is it really recurrent strep throat?
CHICAGO – When a child is sitting in your exam room with recurrent strep pharyngitis, the first question to ask yourself is “Is it real?”
According to pediatric infectious disease specialist John Bradley, MD, the answer to that question comes with careful attention to the history and clinical presentation, but titers and viral polymerase chain reaction tests can also help clarify the diagnosis.
Although that involves some detective work and perhaps some legwork by the provider or the office staff, it’s worth the effort, especially in an era of increased concerns about antimicrobial stewardship, said Dr. Bradley during an antimicrobial update session at the annual meeting of the American Academy of Pediatrics.
“Are the episodes really documented by you in your office?” asked Dr. Bradley. If so, the job is easier. If not, it’s important to differentiate whether documentation of the strep infection was done by culture, whether it was done by an extremely sensitive rapid test, or whether any testing has been done at all, said Dr. Bradley, chief of the division of infectious diseases at the University of California, San Diego.
Somehow, said Dr. Bradley, it’s still true that all group A streptococci are susceptible to penicillin, but penicillin does not always work. There’s about a 10% failure rate for reasons that are not completely understood. Perhaps some individuals have other oropharyngeal flora that produce beta-lactamases, thereby negating penicillin’s efficacy against the strep, he added.
One very good clue as to whether the child has recurrent strep is the appearance of the throat, said Dr. Bradley. A viral illness also can produce a very red posterior oropharynx, so – unless there’s frank pus – it’s unlikely to be strep pharyngitis.
Some patients will, in fact, have recurrent strep, but some patients who might even have positive rapid strep tests may actually be carriers.
So, “what the heck is the carrier state?” asked Dr. Bradley. Although a rapid strep test will occasionally be positive, he explained, the culture is only weakly positive, with growth that’s usually less than 1+. The child who’s a carrier is not symptomatic, will not have an elevated antistreptolysin O titer, and is not contagious. Also, the child will not respond to penicillin treatment.
How can clinicians differentiate recurrent strep from a child with frequent viral illnesses who’s a carrier?
“For the standard case of ‘recurrent strep,’ please get cultures and document the density of group A strep to rule out the carrier state,” said Dr. Bradley. Having parents text pictures of the throat during an episode – for which his facility has a secure portal – can save families an office visit. A negative antistreptolysin O titer can help rule out a recurrent infection, he added.
When a child is having recurrent bouts of pharyngitis, but the clinical picture isn’t clearly consistent with strep, physicians can consider submitting multiplex viral polymerase chain reaction tests. “This can give the family an alternative diagnosis” and reassure parents that it’s safe to hold off on antibiotics, noted Dr. Bradley.
Culturing between episodes of pharyngitis, when the patient is asymptomatic, can also help determine whether a child is a carrier. Sometimes, it makes sense to culture the whole family, and there have also been reports of family pets being Group A strep reservoirs, said Dr. Bradley.
For recurrent infection, choose a broad spectrum agent that will knock back both Group A strep and the oral flora that may be producing beta-lactamases or adhesion molecules that negate penicillin’s efficacy. One logical choice is clindamycin for 10 days, although some strains are resistant. Another good choice is amoxicillin/clavulanate for 10 days or 10 days of a cephalosporin. Penicillin can still be used if it’s augmented by oral rifampin during the last 4 days of the 10-day course.
Long-term prophylaxis can also be considered for stubborn recurrences, he noted.
Dr. Bradley reported no relevant conflicts of interest.
CHICAGO – When a child is sitting in your exam room with recurrent strep pharyngitis, the first question to ask yourself is “Is it real?”
According to pediatric infectious disease specialist John Bradley, MD, the answer to that question comes with careful attention to the history and clinical presentation, but titers and viral polymerase chain reaction tests can also help clarify the diagnosis.
Although that involves some detective work and perhaps some legwork by the provider or the office staff, it’s worth the effort, especially in an era of increased concerns about antimicrobial stewardship, said Dr. Bradley during an antimicrobial update session at the annual meeting of the American Academy of Pediatrics.
“Are the episodes really documented by you in your office?” asked Dr. Bradley. If so, the job is easier. If not, it’s important to differentiate whether documentation of the strep infection was done by culture, whether it was done by an extremely sensitive rapid test, or whether any testing has been done at all, said Dr. Bradley, chief of the division of infectious diseases at the University of California, San Diego.
Somehow, said Dr. Bradley, it’s still true that all group A streptococci are susceptible to penicillin, but penicillin does not always work. There’s about a 10% failure rate for reasons that are not completely understood. Perhaps some individuals have other oropharyngeal flora that produce beta-lactamases, thereby negating penicillin’s efficacy against the strep, he added.
One very good clue as to whether the child has recurrent strep is the appearance of the throat, said Dr. Bradley. A viral illness also can produce a very red posterior oropharynx, so – unless there’s frank pus – it’s unlikely to be strep pharyngitis.
Some patients will, in fact, have recurrent strep, but some patients who might even have positive rapid strep tests may actually be carriers.
So, “what the heck is the carrier state?” asked Dr. Bradley. Although a rapid strep test will occasionally be positive, he explained, the culture is only weakly positive, with growth that’s usually less than 1+. The child who’s a carrier is not symptomatic, will not have an elevated antistreptolysin O titer, and is not contagious. Also, the child will not respond to penicillin treatment.
How can clinicians differentiate recurrent strep from a child with frequent viral illnesses who’s a carrier?
“For the standard case of ‘recurrent strep,’ please get cultures and document the density of group A strep to rule out the carrier state,” said Dr. Bradley. Having parents text pictures of the throat during an episode – for which his facility has a secure portal – can save families an office visit. A negative antistreptolysin O titer can help rule out a recurrent infection, he added.
When a child is having recurrent bouts of pharyngitis, but the clinical picture isn’t clearly consistent with strep, physicians can consider submitting multiplex viral polymerase chain reaction tests. “This can give the family an alternative diagnosis” and reassure parents that it’s safe to hold off on antibiotics, noted Dr. Bradley.
Culturing between episodes of pharyngitis, when the patient is asymptomatic, can also help determine whether a child is a carrier. Sometimes, it makes sense to culture the whole family, and there have also been reports of family pets being Group A strep reservoirs, said Dr. Bradley.
For recurrent infection, choose a broad spectrum agent that will knock back both Group A strep and the oral flora that may be producing beta-lactamases or adhesion molecules that negate penicillin’s efficacy. One logical choice is clindamycin for 10 days, although some strains are resistant. Another good choice is amoxicillin/clavulanate for 10 days or 10 days of a cephalosporin. Penicillin can still be used if it’s augmented by oral rifampin during the last 4 days of the 10-day course.
Long-term prophylaxis can also be considered for stubborn recurrences, he noted.
Dr. Bradley reported no relevant conflicts of interest.
CHICAGO – When a child is sitting in your exam room with recurrent strep pharyngitis, the first question to ask yourself is “Is it real?”
According to pediatric infectious disease specialist John Bradley, MD, the answer to that question comes with careful attention to the history and clinical presentation, but titers and viral polymerase chain reaction tests can also help clarify the diagnosis.
Although that involves some detective work and perhaps some legwork by the provider or the office staff, it’s worth the effort, especially in an era of increased concerns about antimicrobial stewardship, said Dr. Bradley during an antimicrobial update session at the annual meeting of the American Academy of Pediatrics.
“Are the episodes really documented by you in your office?” asked Dr. Bradley. If so, the job is easier. If not, it’s important to differentiate whether documentation of the strep infection was done by culture, whether it was done by an extremely sensitive rapid test, or whether any testing has been done at all, said Dr. Bradley, chief of the division of infectious diseases at the University of California, San Diego.
Somehow, said Dr. Bradley, it’s still true that all group A streptococci are susceptible to penicillin, but penicillin does not always work. There’s about a 10% failure rate for reasons that are not completely understood. Perhaps some individuals have other oropharyngeal flora that produce beta-lactamases, thereby negating penicillin’s efficacy against the strep, he added.
One very good clue as to whether the child has recurrent strep is the appearance of the throat, said Dr. Bradley. A viral illness also can produce a very red posterior oropharynx, so – unless there’s frank pus – it’s unlikely to be strep pharyngitis.
Some patients will, in fact, have recurrent strep, but some patients who might even have positive rapid strep tests may actually be carriers.
So, “what the heck is the carrier state?” asked Dr. Bradley. Although a rapid strep test will occasionally be positive, he explained, the culture is only weakly positive, with growth that’s usually less than 1+. The child who’s a carrier is not symptomatic, will not have an elevated antistreptolysin O titer, and is not contagious. Also, the child will not respond to penicillin treatment.
How can clinicians differentiate recurrent strep from a child with frequent viral illnesses who’s a carrier?
“For the standard case of ‘recurrent strep,’ please get cultures and document the density of group A strep to rule out the carrier state,” said Dr. Bradley. Having parents text pictures of the throat during an episode – for which his facility has a secure portal – can save families an office visit. A negative antistreptolysin O titer can help rule out a recurrent infection, he added.
When a child is having recurrent bouts of pharyngitis, but the clinical picture isn’t clearly consistent with strep, physicians can consider submitting multiplex viral polymerase chain reaction tests. “This can give the family an alternative diagnosis” and reassure parents that it’s safe to hold off on antibiotics, noted Dr. Bradley.
Culturing between episodes of pharyngitis, when the patient is asymptomatic, can also help determine whether a child is a carrier. Sometimes, it makes sense to culture the whole family, and there have also been reports of family pets being Group A strep reservoirs, said Dr. Bradley.
For recurrent infection, choose a broad spectrum agent that will knock back both Group A strep and the oral flora that may be producing beta-lactamases or adhesion molecules that negate penicillin’s efficacy. One logical choice is clindamycin for 10 days, although some strains are resistant. Another good choice is amoxicillin/clavulanate for 10 days or 10 days of a cephalosporin. Penicillin can still be used if it’s augmented by oral rifampin during the last 4 days of the 10-day course.
Long-term prophylaxis can also be considered for stubborn recurrences, he noted.
Dr. Bradley reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAP 2017
How can pediatricians help prevent preschool expulsions?
CHICAGO –
Young African American boys are particularly at risk, said Claire Lerner, a senior parenting specialist with the nonprofit organization Zero to Three in Washington.
Speaking at the annual meeting of the American Academy of Pediatrics, Ms. Lerner cited data from 2005 showing that 10.4% of prekindergarten (preK) teachers had expelled at least one child for behavior problems during the previous year, yielding a preK expulsion rate of 6.7 per 1,000 students. This contrasts with a K-12 expulsion rate of 2.1 per 1,000 students.
“What I would say to you is that if a parent, or school, or caregiver comes to you and says, ‘The teacher’s concerned about X,’ don’t dismiss it. I cannot tell you how many times parents have come to me and said, ‘Well, the pediatrician says he’s totally fine. He’s just two.’”
This is not to blame pediatricians, said Ms. Lerner. Rather, “it’s to help you remember that teachers are smart, and they’re with these kids in a natural environment all day long. ...You are seeing such a random, finite, artificial experience in that room that you don’t see what’s going on in the classroom.” The child’s interactions with other children -- usually the impetus for disciplinary action -- will not necessarily be apparent in the pediatrician’s office.
Often, said Ms. Lerner, the child in question isn’t picking up on verbal and physical clues from other children, leading to behavior that’s seen as inappropriate or aggressive by other children -- and by staff. “A lot of these kids have motor planning problems, meaning they don’t know how to take their idea and execute it, so they’re the ones who are knocking down towers, and in kids’ faces. ...Those are the things that I’m talking about that are very unlikely to be picked up in a pediatric well-child visit,” she said.
“The evidence tells us that gender and race matter” in preschool suspensions and expulsions, said Ms. Lerner, noting that black preschool children are 3.6 times as likely as white children to be given an out-of-school suspension.
Black boys, she said, are 19% of male preschool attendees but make up 45% of the preschool children who receive out-of-school suspensions. Black girls make up 20% of female preschool attendees, but 54% of preschool girls who receive out-of-school suspensions are black.
In public preschools, 78% of those suspended are boys, though they make up just 54% of the attendees.
Aside from the home and work disruption caused by these suspensions, Ms. Lerner said that later outcomes are much worse for young students who are expelled or suspended, stacking the deck further against success. Rates of dropping out of high school, grade retention, and incarceration may be up to 10 times higher for these children.
Many school-related factors, including a higher child-teacher ratio and a longer school day, are associated with higher rates of preschool expulsion. Also, teachers who report more job stress are more likely to mete out suspensions or expulsions. By contrast, the availability of behavioral consultations reduces the risk of expulsion.
Within the realistic constraints of a busy pediatric practice, what can a physician do?
At the very least, said Ms. Lerner, pay attention when a parent comes to you and says, “My child’s getting in trouble in the classroom, and I’m afraid he’s going to get kicked out.” At that point, she said, the pediatrician should get in touch with the school. An approach that can be used in communicating with the school is to say, “We’re not seeing that, but we want to learn more,” said Ms. Lerner.
Just the fact that a pediatrician or his or her team members would reach out to the teacher for collaboration helps the teacher and school or daycare center realize that the family is taking the concerns seriously, said Ms. Lerner. “I will tell you that even just that phone call changes what happens for that kid,” she said.
Facilitating appropriate behavioral and mental health evaluations allows those professionals to become part of the problem-solving team for the child, school, and family.
Physicians can also help children and families by being familiar with the Americans with Disabilities Act safeguards for children with an Individualized Education Program (IEP) or a 504 Plan and making sure that families know about these protections.
Within the larger context of the school district and the community, a pediatrician’s voice can be a powerful advocacy tool to lobby against zero tolerance policies, and for stronger prevention and outreach strategies to help avoid out-of-school suspensions and expulsions for the very young. “There are things you can do -- starting tomorrow -- that will change the course for these children,” said Ms. Lerner.
Ms. Lerner is employed by Zero to Three. She reported no conflicts of interest.
CHICAGO –
Young African American boys are particularly at risk, said Claire Lerner, a senior parenting specialist with the nonprofit organization Zero to Three in Washington.
Speaking at the annual meeting of the American Academy of Pediatrics, Ms. Lerner cited data from 2005 showing that 10.4% of prekindergarten (preK) teachers had expelled at least one child for behavior problems during the previous year, yielding a preK expulsion rate of 6.7 per 1,000 students. This contrasts with a K-12 expulsion rate of 2.1 per 1,000 students.
“What I would say to you is that if a parent, or school, or caregiver comes to you and says, ‘The teacher’s concerned about X,’ don’t dismiss it. I cannot tell you how many times parents have come to me and said, ‘Well, the pediatrician says he’s totally fine. He’s just two.’”
This is not to blame pediatricians, said Ms. Lerner. Rather, “it’s to help you remember that teachers are smart, and they’re with these kids in a natural environment all day long. ...You are seeing such a random, finite, artificial experience in that room that you don’t see what’s going on in the classroom.” The child’s interactions with other children -- usually the impetus for disciplinary action -- will not necessarily be apparent in the pediatrician’s office.
Often, said Ms. Lerner, the child in question isn’t picking up on verbal and physical clues from other children, leading to behavior that’s seen as inappropriate or aggressive by other children -- and by staff. “A lot of these kids have motor planning problems, meaning they don’t know how to take their idea and execute it, so they’re the ones who are knocking down towers, and in kids’ faces. ...Those are the things that I’m talking about that are very unlikely to be picked up in a pediatric well-child visit,” she said.
“The evidence tells us that gender and race matter” in preschool suspensions and expulsions, said Ms. Lerner, noting that black preschool children are 3.6 times as likely as white children to be given an out-of-school suspension.
Black boys, she said, are 19% of male preschool attendees but make up 45% of the preschool children who receive out-of-school suspensions. Black girls make up 20% of female preschool attendees, but 54% of preschool girls who receive out-of-school suspensions are black.
In public preschools, 78% of those suspended are boys, though they make up just 54% of the attendees.
Aside from the home and work disruption caused by these suspensions, Ms. Lerner said that later outcomes are much worse for young students who are expelled or suspended, stacking the deck further against success. Rates of dropping out of high school, grade retention, and incarceration may be up to 10 times higher for these children.
Many school-related factors, including a higher child-teacher ratio and a longer school day, are associated with higher rates of preschool expulsion. Also, teachers who report more job stress are more likely to mete out suspensions or expulsions. By contrast, the availability of behavioral consultations reduces the risk of expulsion.
Within the realistic constraints of a busy pediatric practice, what can a physician do?
At the very least, said Ms. Lerner, pay attention when a parent comes to you and says, “My child’s getting in trouble in the classroom, and I’m afraid he’s going to get kicked out.” At that point, she said, the pediatrician should get in touch with the school. An approach that can be used in communicating with the school is to say, “We’re not seeing that, but we want to learn more,” said Ms. Lerner.
Just the fact that a pediatrician or his or her team members would reach out to the teacher for collaboration helps the teacher and school or daycare center realize that the family is taking the concerns seriously, said Ms. Lerner. “I will tell you that even just that phone call changes what happens for that kid,” she said.
Facilitating appropriate behavioral and mental health evaluations allows those professionals to become part of the problem-solving team for the child, school, and family.
Physicians can also help children and families by being familiar with the Americans with Disabilities Act safeguards for children with an Individualized Education Program (IEP) or a 504 Plan and making sure that families know about these protections.
Within the larger context of the school district and the community, a pediatrician’s voice can be a powerful advocacy tool to lobby against zero tolerance policies, and for stronger prevention and outreach strategies to help avoid out-of-school suspensions and expulsions for the very young. “There are things you can do -- starting tomorrow -- that will change the course for these children,” said Ms. Lerner.
Ms. Lerner is employed by Zero to Three. She reported no conflicts of interest.
CHICAGO –
Young African American boys are particularly at risk, said Claire Lerner, a senior parenting specialist with the nonprofit organization Zero to Three in Washington.
Speaking at the annual meeting of the American Academy of Pediatrics, Ms. Lerner cited data from 2005 showing that 10.4% of prekindergarten (preK) teachers had expelled at least one child for behavior problems during the previous year, yielding a preK expulsion rate of 6.7 per 1,000 students. This contrasts with a K-12 expulsion rate of 2.1 per 1,000 students.
“What I would say to you is that if a parent, or school, or caregiver comes to you and says, ‘The teacher’s concerned about X,’ don’t dismiss it. I cannot tell you how many times parents have come to me and said, ‘Well, the pediatrician says he’s totally fine. He’s just two.’”
This is not to blame pediatricians, said Ms. Lerner. Rather, “it’s to help you remember that teachers are smart, and they’re with these kids in a natural environment all day long. ...You are seeing such a random, finite, artificial experience in that room that you don’t see what’s going on in the classroom.” The child’s interactions with other children -- usually the impetus for disciplinary action -- will not necessarily be apparent in the pediatrician’s office.
Often, said Ms. Lerner, the child in question isn’t picking up on verbal and physical clues from other children, leading to behavior that’s seen as inappropriate or aggressive by other children -- and by staff. “A lot of these kids have motor planning problems, meaning they don’t know how to take their idea and execute it, so they’re the ones who are knocking down towers, and in kids’ faces. ...Those are the things that I’m talking about that are very unlikely to be picked up in a pediatric well-child visit,” she said.
“The evidence tells us that gender and race matter” in preschool suspensions and expulsions, said Ms. Lerner, noting that black preschool children are 3.6 times as likely as white children to be given an out-of-school suspension.
Black boys, she said, are 19% of male preschool attendees but make up 45% of the preschool children who receive out-of-school suspensions. Black girls make up 20% of female preschool attendees, but 54% of preschool girls who receive out-of-school suspensions are black.
In public preschools, 78% of those suspended are boys, though they make up just 54% of the attendees.
Aside from the home and work disruption caused by these suspensions, Ms. Lerner said that later outcomes are much worse for young students who are expelled or suspended, stacking the deck further against success. Rates of dropping out of high school, grade retention, and incarceration may be up to 10 times higher for these children.
Many school-related factors, including a higher child-teacher ratio and a longer school day, are associated with higher rates of preschool expulsion. Also, teachers who report more job stress are more likely to mete out suspensions or expulsions. By contrast, the availability of behavioral consultations reduces the risk of expulsion.
Within the realistic constraints of a busy pediatric practice, what can a physician do?
At the very least, said Ms. Lerner, pay attention when a parent comes to you and says, “My child’s getting in trouble in the classroom, and I’m afraid he’s going to get kicked out.” At that point, she said, the pediatrician should get in touch with the school. An approach that can be used in communicating with the school is to say, “We’re not seeing that, but we want to learn more,” said Ms. Lerner.
Just the fact that a pediatrician or his or her team members would reach out to the teacher for collaboration helps the teacher and school or daycare center realize that the family is taking the concerns seriously, said Ms. Lerner. “I will tell you that even just that phone call changes what happens for that kid,” she said.
Facilitating appropriate behavioral and mental health evaluations allows those professionals to become part of the problem-solving team for the child, school, and family.
Physicians can also help children and families by being familiar with the Americans with Disabilities Act safeguards for children with an Individualized Education Program (IEP) or a 504 Plan and making sure that families know about these protections.
Within the larger context of the school district and the community, a pediatrician’s voice can be a powerful advocacy tool to lobby against zero tolerance policies, and for stronger prevention and outreach strategies to help avoid out-of-school suspensions and expulsions for the very young. “There are things you can do -- starting tomorrow -- that will change the course for these children,” said Ms. Lerner.
Ms. Lerner is employed by Zero to Three. She reported no conflicts of interest.
EXPERT ANALYSIS FROM AAP 2017
Soccer-playing girls 5 times more likely to return to same-day play after concussion
CHICAGO – , according to a study presented at the annual meeting of the American Academy of Pediatrics.
Records from 87 soccer players aged 7-18 years (median, 14 years) were examined in a retrospective review of patients seen over a 2-year period by a single physician at a pediatric sports medicine center. Of these, two thirds (n = 58) were girls.
The soccer players included children participating in recreational, club, and school-sponsored soccer, said senior author Shane M. Miller, MD, in an interview. All patients were assessed according to a standardized concussion protocol that involved a neurologic exam and validated concussion evaluation testing, including the ImPACT and the Sports Concussion Assessment Tool (SCAT) tests.
As soccer has grown in popularity as a youth sport, so has the number of reported concussions. “The incidence of reported concussions has increased 1,600% from 1990 to 2014,” wrote Dr. Miller and his coauthors in the abstract accompanying the presentation. Dr. Miller said that girls are 1.5 times more likely than boys to sustain a concussion while playing soccer.
While seeing the patients who were the subject of the study, Dr. Miller realized that most of the soccer players had not come out of play for evaluation after the head impact. Rather, they had continued to play, only later reporting concussion symptoms to coaches, trainers, or parents.
“The athletes may have chosen not to say anything because they didn’t want to come out of the game,” said Dr. Miller, a sports medicine physician at Texas Scottish Rite Hospital for Children, Dallas.
“I was surprised by the significant degree of difference” between male and female soccer players, said Dr. Miller. The study was not designed to get at the reason for the discrepancy, so Dr. Miller could not say with certainty whether awareness of concussion symptoms is significantly lower for female athletes, or whether the athletic culture more strongly encourages minimization of symptoms for girls than boys. In any case, he said, there is room for education of players, coaches, and families to raise awareness of the importance to recognize and report concussion, and then remove the affected athlete from play,
Dr. Miller said that future research directions include collaboration with other facilities to conduct prospective research using a concussion registry. This will allow more robust statistical analysis, and help ascertain the degree of regional variation in pediatric sports concussion management.
“Current education efforts may not be enough to help athletes, parents, and coaches identify concussion symptoms, know the guidelines for immediate removal from play, and understand the risks of returning to play after an injury. More research is needed on how to better spread this message intended to protect the health of young athletes…” Aaron Zynda, the study’s first author and clinical research coordinator at Texas Scottish Rite, said in a press release accompanying the abstract. “Concussion recognition and identification is a team effort,” he said.
Neither Mr. Zynda nor Dr. Miller had any relevant conflicts of interest.
[email protected]
CHICAGO – , according to a study presented at the annual meeting of the American Academy of Pediatrics.
Records from 87 soccer players aged 7-18 years (median, 14 years) were examined in a retrospective review of patients seen over a 2-year period by a single physician at a pediatric sports medicine center. Of these, two thirds (n = 58) were girls.
The soccer players included children participating in recreational, club, and school-sponsored soccer, said senior author Shane M. Miller, MD, in an interview. All patients were assessed according to a standardized concussion protocol that involved a neurologic exam and validated concussion evaluation testing, including the ImPACT and the Sports Concussion Assessment Tool (SCAT) tests.
As soccer has grown in popularity as a youth sport, so has the number of reported concussions. “The incidence of reported concussions has increased 1,600% from 1990 to 2014,” wrote Dr. Miller and his coauthors in the abstract accompanying the presentation. Dr. Miller said that girls are 1.5 times more likely than boys to sustain a concussion while playing soccer.
While seeing the patients who were the subject of the study, Dr. Miller realized that most of the soccer players had not come out of play for evaluation after the head impact. Rather, they had continued to play, only later reporting concussion symptoms to coaches, trainers, or parents.
“The athletes may have chosen not to say anything because they didn’t want to come out of the game,” said Dr. Miller, a sports medicine physician at Texas Scottish Rite Hospital for Children, Dallas.
“I was surprised by the significant degree of difference” between male and female soccer players, said Dr. Miller. The study was not designed to get at the reason for the discrepancy, so Dr. Miller could not say with certainty whether awareness of concussion symptoms is significantly lower for female athletes, or whether the athletic culture more strongly encourages minimization of symptoms for girls than boys. In any case, he said, there is room for education of players, coaches, and families to raise awareness of the importance to recognize and report concussion, and then remove the affected athlete from play,
Dr. Miller said that future research directions include collaboration with other facilities to conduct prospective research using a concussion registry. This will allow more robust statistical analysis, and help ascertain the degree of regional variation in pediatric sports concussion management.
“Current education efforts may not be enough to help athletes, parents, and coaches identify concussion symptoms, know the guidelines for immediate removal from play, and understand the risks of returning to play after an injury. More research is needed on how to better spread this message intended to protect the health of young athletes…” Aaron Zynda, the study’s first author and clinical research coordinator at Texas Scottish Rite, said in a press release accompanying the abstract. “Concussion recognition and identification is a team effort,” he said.
Neither Mr. Zynda nor Dr. Miller had any relevant conflicts of interest.
[email protected]
CHICAGO – , according to a study presented at the annual meeting of the American Academy of Pediatrics.
Records from 87 soccer players aged 7-18 years (median, 14 years) were examined in a retrospective review of patients seen over a 2-year period by a single physician at a pediatric sports medicine center. Of these, two thirds (n = 58) were girls.
The soccer players included children participating in recreational, club, and school-sponsored soccer, said senior author Shane M. Miller, MD, in an interview. All patients were assessed according to a standardized concussion protocol that involved a neurologic exam and validated concussion evaluation testing, including the ImPACT and the Sports Concussion Assessment Tool (SCAT) tests.
As soccer has grown in popularity as a youth sport, so has the number of reported concussions. “The incidence of reported concussions has increased 1,600% from 1990 to 2014,” wrote Dr. Miller and his coauthors in the abstract accompanying the presentation. Dr. Miller said that girls are 1.5 times more likely than boys to sustain a concussion while playing soccer.
While seeing the patients who were the subject of the study, Dr. Miller realized that most of the soccer players had not come out of play for evaluation after the head impact. Rather, they had continued to play, only later reporting concussion symptoms to coaches, trainers, or parents.
“The athletes may have chosen not to say anything because they didn’t want to come out of the game,” said Dr. Miller, a sports medicine physician at Texas Scottish Rite Hospital for Children, Dallas.
“I was surprised by the significant degree of difference” between male and female soccer players, said Dr. Miller. The study was not designed to get at the reason for the discrepancy, so Dr. Miller could not say with certainty whether awareness of concussion symptoms is significantly lower for female athletes, or whether the athletic culture more strongly encourages minimization of symptoms for girls than boys. In any case, he said, there is room for education of players, coaches, and families to raise awareness of the importance to recognize and report concussion, and then remove the affected athlete from play,
Dr. Miller said that future research directions include collaboration with other facilities to conduct prospective research using a concussion registry. This will allow more robust statistical analysis, and help ascertain the degree of regional variation in pediatric sports concussion management.
“Current education efforts may not be enough to help athletes, parents, and coaches identify concussion symptoms, know the guidelines for immediate removal from play, and understand the risks of returning to play after an injury. More research is needed on how to better spread this message intended to protect the health of young athletes…” Aaron Zynda, the study’s first author and clinical research coordinator at Texas Scottish Rite, said in a press release accompanying the abstract. “Concussion recognition and identification is a team effort,” he said.
Neither Mr. Zynda nor Dr. Miller had any relevant conflicts of interest.
[email protected]
At AAP 2017
Key clinical point: Girls who played soccer were five times as likely as boys to return to play on the same day of concussion.
Major finding: Thirty of 58 girls with concussion returned to same-day play, compared with 5 of 29 boys.
Study details: Retrospective single-site study of 87 soccer players with concussion, median age 14 years.
Disclosures: None of the study authors had relevant conflicts of interest.