User login
Endoscopic ultrasound survives the sharks at AGA Tech Summit
After a 3-year, pandemic-induced hiatus, the American Gastroenterological Association’s Tech Summit returned to a live meeting in San Francisco. As usual, the highlight of the 2-day event, which is sponsored by the AGA Center for GI Innovation and Technology, was the Shark Tank, where selected companies presented lightning-round overviews of their technology and business plans. A panel of sharks and the audience voted for their favorite.
The contestants presented technologies such as a cell phone app to improve gut health (Agora Health), a polypectomy suite (IzoMed), an implantable weight-loss device (Lean Medical), a device to alleviate gastric obstruction in pancreatic cancer (Myka Labs), a pill designed to map out the gastrointestinal system to aid in diagnosis (Rock West Medical Devices), and an endoscopic ultrasound device (EndoSound).
Six finalists were selected from 20 submissions, and EndoSound was the winner. According to Raman Muthusamy, MD, medical director of endoscopy at UCLA Health and professor of clinical medicine at the University of California, Los Angeles, and past chair of the AGA Center for GI Innovation and Technology, the quality of presentations and the sophistication of the companies have increased year after year. “This was really the very best,” said Dr. Muthusamy.
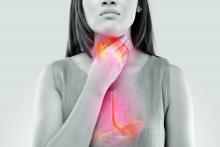
Both the judges and the audience chose EndoSound. Endoscopic ultrasound (EUS) focuses on diagnosis and treatment of chest and abdomen disorders, particularly the pancreas. The EndoSound device attaches to an upper endoscope and converts it to a fully therapeutic endoscope that can perform all standard EUS procedures. Moreover, it does not use an elevator, which has been linked to infection risk.
Most clinical facilities lack EUS capability: 97% of ambulatory surgical centers and 80% of hospitals. EUS systems have hardly changed since the late 20th century, and they cost about $450,000. The projected cost of the EndoSound device is closer to $50,000.
“Just like colonoscopies and upper endoscopies, most endoscopic ultrasounds ought to be done in surgical centers. The idea that they can do them efficiently, and at lower cost and greater convenience to their patients and themselves, seems to me the way everything is going, and the way this procedure ought to go as well. The only obstacle to that has been the cost of the equipment. If we can take away that obstacle, then people who are already doing procedures in hospitals where it’s not convenient and not efficient, will be able to do the procedures in surgical centers,” said Stephen Steinberg, MD, founder and President of EndoSound.
“It’s a radical redesign. You’ve cut cost and you’ve cut space. And it’s something that could be put on at a moment’s notice. Rather than referring the patient for [ultrasound], it could allow you to do it on the spot, and perhaps save a second trip for a patient. It allows flexibility in terms of site of service,” said Dr. Muthusamy.
Dr. Muthusamy called it a “godsend” for low-resource institutions in the United States or abroad who have the expertise, but not the equipment, to perform EUS. “There’s no question that more EUS procedures could be done than are currently being done because of issues of availability, and this device takes a significant step to alleviate that.”
The Food and Drug Administration has granted a breakthrough device designation to EndoSound, which allows the company to forgo human clinical trials to support the application. “We’re hoping and expecting to have our application in the beginning of the fourth quarter, and with a little bit of luck to be approved by the end of the year. That’s our goal,” said Dr. Steinberg.
The technology started out as a challenge that Dr. Steinberg set for himself. His career overlapped with some of the earliest innovators of therapeutic endoscopy. “They were the stars. I wasn’t, but I was there,” said Dr. Steinberg. In his practice, Dr. Steinberg was doing procedures that included endoscopic ultrasound.
By the new millennium, EUS had gained a lot of interest, but there was a problem. “It was expensive, and it could only be done in hospitals. I started wondering if we couldn’t get it into a different environment by having a simpler solution,” said Dr. Steinberg.
But success didn’t come quickly. “I started drawing on the back of napkins to see if there wasn’t some solution,” said Dr. Steinberg. It wasn’t until a serendipitous meeting occurred that the concept took shape. Dr. Steinberg’s wife was the CEO and provost of Oregon Health Sciences University, Portland, as well as head of the technology transfer program. Dr. Steinberg’s practice, however, was in Florida so he commuted to Oregon every weekend.
One day, she told him about a presentation by Scott Corbett, MD. “My wife said: ‘Hey, they’re doing ultrasound. Why don’t you come and sit in [on the meeting] because I don’t know anything about it.’ [Dr.] Corbett was working with Sonivate, a point-of-care ultrasound company that was developing an ultrasound that could be placed over the end of the finger, to be used in battlefield triage. I thought, well, if you could put it on a finger, why couldn’t you put it on a scope? So, Scott and I got to talking, and went through a couple of iterations that didn’t work, and then finally came up with one that seemed like it was suitable.”
The device has been tested in five animal models with 20 EUS physicians who concluded that the images were equivalent to legacy devices and that they could be adopted quickly. The company also presented results from a human study that demonstrated noninferiority to the latest EUS system from Pentax.
Dr. Steinberg is an employee and stockholder of Sonivate. Dr. Muthusamy has no relevant financial disclosures. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
*Correction, 5/17/22: An earlier version of this article stated that Geneoscopy was a finalist in the competition. It was not. Also, EndoSound should have been listed as a finalist in this paragraph.
After a 3-year, pandemic-induced hiatus, the American Gastroenterological Association’s Tech Summit returned to a live meeting in San Francisco. As usual, the highlight of the 2-day event, which is sponsored by the AGA Center for GI Innovation and Technology, was the Shark Tank, where selected companies presented lightning-round overviews of their technology and business plans. A panel of sharks and the audience voted for their favorite.
The contestants presented technologies such as a cell phone app to improve gut health (Agora Health), a polypectomy suite (IzoMed), an implantable weight-loss device (Lean Medical), a device to alleviate gastric obstruction in pancreatic cancer (Myka Labs), a pill designed to map out the gastrointestinal system to aid in diagnosis (Rock West Medical Devices), and an endoscopic ultrasound device (EndoSound).
Six finalists were selected from 20 submissions, and EndoSound was the winner. According to Raman Muthusamy, MD, medical director of endoscopy at UCLA Health and professor of clinical medicine at the University of California, Los Angeles, and past chair of the AGA Center for GI Innovation and Technology, the quality of presentations and the sophistication of the companies have increased year after year. “This was really the very best,” said Dr. Muthusamy.
Both the judges and the audience chose EndoSound. Endoscopic ultrasound (EUS) focuses on diagnosis and treatment of chest and abdomen disorders, particularly the pancreas. The EndoSound device attaches to an upper endoscope and converts it to a fully therapeutic endoscope that can perform all standard EUS procedures. Moreover, it does not use an elevator, which has been linked to infection risk.
Most clinical facilities lack EUS capability: 97% of ambulatory surgical centers and 80% of hospitals. EUS systems have hardly changed since the late 20th century, and they cost about $450,000. The projected cost of the EndoSound device is closer to $50,000.
“Just like colonoscopies and upper endoscopies, most endoscopic ultrasounds ought to be done in surgical centers. The idea that they can do them efficiently, and at lower cost and greater convenience to their patients and themselves, seems to me the way everything is going, and the way this procedure ought to go as well. The only obstacle to that has been the cost of the equipment. If we can take away that obstacle, then people who are already doing procedures in hospitals where it’s not convenient and not efficient, will be able to do the procedures in surgical centers,” said Stephen Steinberg, MD, founder and President of EndoSound.
“It’s a radical redesign. You’ve cut cost and you’ve cut space. And it’s something that could be put on at a moment’s notice. Rather than referring the patient for [ultrasound], it could allow you to do it on the spot, and perhaps save a second trip for a patient. It allows flexibility in terms of site of service,” said Dr. Muthusamy.
Dr. Muthusamy called it a “godsend” for low-resource institutions in the United States or abroad who have the expertise, but not the equipment, to perform EUS. “There’s no question that more EUS procedures could be done than are currently being done because of issues of availability, and this device takes a significant step to alleviate that.”
The Food and Drug Administration has granted a breakthrough device designation to EndoSound, which allows the company to forgo human clinical trials to support the application. “We’re hoping and expecting to have our application in the beginning of the fourth quarter, and with a little bit of luck to be approved by the end of the year. That’s our goal,” said Dr. Steinberg.
The technology started out as a challenge that Dr. Steinberg set for himself. His career overlapped with some of the earliest innovators of therapeutic endoscopy. “They were the stars. I wasn’t, but I was there,” said Dr. Steinberg. In his practice, Dr. Steinberg was doing procedures that included endoscopic ultrasound.
By the new millennium, EUS had gained a lot of interest, but there was a problem. “It was expensive, and it could only be done in hospitals. I started wondering if we couldn’t get it into a different environment by having a simpler solution,” said Dr. Steinberg.
But success didn’t come quickly. “I started drawing on the back of napkins to see if there wasn’t some solution,” said Dr. Steinberg. It wasn’t until a serendipitous meeting occurred that the concept took shape. Dr. Steinberg’s wife was the CEO and provost of Oregon Health Sciences University, Portland, as well as head of the technology transfer program. Dr. Steinberg’s practice, however, was in Florida so he commuted to Oregon every weekend.
One day, she told him about a presentation by Scott Corbett, MD. “My wife said: ‘Hey, they’re doing ultrasound. Why don’t you come and sit in [on the meeting] because I don’t know anything about it.’ [Dr.] Corbett was working with Sonivate, a point-of-care ultrasound company that was developing an ultrasound that could be placed over the end of the finger, to be used in battlefield triage. I thought, well, if you could put it on a finger, why couldn’t you put it on a scope? So, Scott and I got to talking, and went through a couple of iterations that didn’t work, and then finally came up with one that seemed like it was suitable.”
The device has been tested in five animal models with 20 EUS physicians who concluded that the images were equivalent to legacy devices and that they could be adopted quickly. The company also presented results from a human study that demonstrated noninferiority to the latest EUS system from Pentax.
Dr. Steinberg is an employee and stockholder of Sonivate. Dr. Muthusamy has no relevant financial disclosures. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
*Correction, 5/17/22: An earlier version of this article stated that Geneoscopy was a finalist in the competition. It was not. Also, EndoSound should have been listed as a finalist in this paragraph.
After a 3-year, pandemic-induced hiatus, the American Gastroenterological Association’s Tech Summit returned to a live meeting in San Francisco. As usual, the highlight of the 2-day event, which is sponsored by the AGA Center for GI Innovation and Technology, was the Shark Tank, where selected companies presented lightning-round overviews of their technology and business plans. A panel of sharks and the audience voted for their favorite.
The contestants presented technologies such as a cell phone app to improve gut health (Agora Health), a polypectomy suite (IzoMed), an implantable weight-loss device (Lean Medical), a device to alleviate gastric obstruction in pancreatic cancer (Myka Labs), a pill designed to map out the gastrointestinal system to aid in diagnosis (Rock West Medical Devices), and an endoscopic ultrasound device (EndoSound).
Six finalists were selected from 20 submissions, and EndoSound was the winner. According to Raman Muthusamy, MD, medical director of endoscopy at UCLA Health and professor of clinical medicine at the University of California, Los Angeles, and past chair of the AGA Center for GI Innovation and Technology, the quality of presentations and the sophistication of the companies have increased year after year. “This was really the very best,” said Dr. Muthusamy.
Both the judges and the audience chose EndoSound. Endoscopic ultrasound (EUS) focuses on diagnosis and treatment of chest and abdomen disorders, particularly the pancreas. The EndoSound device attaches to an upper endoscope and converts it to a fully therapeutic endoscope that can perform all standard EUS procedures. Moreover, it does not use an elevator, which has been linked to infection risk.
Most clinical facilities lack EUS capability: 97% of ambulatory surgical centers and 80% of hospitals. EUS systems have hardly changed since the late 20th century, and they cost about $450,000. The projected cost of the EndoSound device is closer to $50,000.
“Just like colonoscopies and upper endoscopies, most endoscopic ultrasounds ought to be done in surgical centers. The idea that they can do them efficiently, and at lower cost and greater convenience to their patients and themselves, seems to me the way everything is going, and the way this procedure ought to go as well. The only obstacle to that has been the cost of the equipment. If we can take away that obstacle, then people who are already doing procedures in hospitals where it’s not convenient and not efficient, will be able to do the procedures in surgical centers,” said Stephen Steinberg, MD, founder and President of EndoSound.
“It’s a radical redesign. You’ve cut cost and you’ve cut space. And it’s something that could be put on at a moment’s notice. Rather than referring the patient for [ultrasound], it could allow you to do it on the spot, and perhaps save a second trip for a patient. It allows flexibility in terms of site of service,” said Dr. Muthusamy.
Dr. Muthusamy called it a “godsend” for low-resource institutions in the United States or abroad who have the expertise, but not the equipment, to perform EUS. “There’s no question that more EUS procedures could be done than are currently being done because of issues of availability, and this device takes a significant step to alleviate that.”
The Food and Drug Administration has granted a breakthrough device designation to EndoSound, which allows the company to forgo human clinical trials to support the application. “We’re hoping and expecting to have our application in the beginning of the fourth quarter, and with a little bit of luck to be approved by the end of the year. That’s our goal,” said Dr. Steinberg.
The technology started out as a challenge that Dr. Steinberg set for himself. His career overlapped with some of the earliest innovators of therapeutic endoscopy. “They were the stars. I wasn’t, but I was there,” said Dr. Steinberg. In his practice, Dr. Steinberg was doing procedures that included endoscopic ultrasound.
By the new millennium, EUS had gained a lot of interest, but there was a problem. “It was expensive, and it could only be done in hospitals. I started wondering if we couldn’t get it into a different environment by having a simpler solution,” said Dr. Steinberg.
But success didn’t come quickly. “I started drawing on the back of napkins to see if there wasn’t some solution,” said Dr. Steinberg. It wasn’t until a serendipitous meeting occurred that the concept took shape. Dr. Steinberg’s wife was the CEO and provost of Oregon Health Sciences University, Portland, as well as head of the technology transfer program. Dr. Steinberg’s practice, however, was in Florida so he commuted to Oregon every weekend.
One day, she told him about a presentation by Scott Corbett, MD. “My wife said: ‘Hey, they’re doing ultrasound. Why don’t you come and sit in [on the meeting] because I don’t know anything about it.’ [Dr.] Corbett was working with Sonivate, a point-of-care ultrasound company that was developing an ultrasound that could be placed over the end of the finger, to be used in battlefield triage. I thought, well, if you could put it on a finger, why couldn’t you put it on a scope? So, Scott and I got to talking, and went through a couple of iterations that didn’t work, and then finally came up with one that seemed like it was suitable.”
The device has been tested in five animal models with 20 EUS physicians who concluded that the images were equivalent to legacy devices and that they could be adopted quickly. The company also presented results from a human study that demonstrated noninferiority to the latest EUS system from Pentax.
Dr. Steinberg is an employee and stockholder of Sonivate. Dr. Muthusamy has no relevant financial disclosures. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
*Correction, 5/17/22: An earlier version of this article stated that Geneoscopy was a finalist in the competition. It was not. Also, EndoSound should have been listed as a finalist in this paragraph.
FROM 2022 AGA TECH SUMMIT
Endoscopic obesity treatments offer alternatives to surgery
SAN FRANCISCO – Endoscopic treatments for obesity are under-utilized but represent an opportunity for gastroenterologists to help address the metabolic epidemic that affects up to 40% of people in the United States, according to a presentation reviewing these techniques.
Lifestyle modification is the first intervention, but results in just a 5% average weight loss, according to Allison Schulman, MD, MPH, who discussed these options at the 2022 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. Although surgical interventions induce more weight loss and greater improvement of metabolic outcomes, they come with significant risks and many patients are reluctant to pursue them, she added. In fact, fewer than 1% of obese individuals who qualify for bariatric surgery ultimately undergo it.
Dr. Schulman emphasized another option: Endoscopic bariatric therapies fill this void in between those two extremes, as they are clearly less invasive” said Dr. Schulman, who is an assistant professor of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “They may appeal to those who do not qualify or do not want bariatric surgery. They also could bridge a critical gap in the treatment of obesity, as they reach patients earlier, at BMIs [body mass indexes] where they may not be surgical candidates. Furthermore, these therapies are oftentimes repeatable and commonly can be used in combination [with other weight loss approaches].”
Endoscopic therapies for obesity include devices that occupy space in the stomach, such as intragastric balloons, gastric remodeling procedures like endoscopic sleeve gastroplasty (ESG), and aspiration therapy.
Potential candidates for noninvasive approaches include patients with a BMI over 30 kg/m2 who have not lost sufficient weight through nonsurgical methods or those who do not want to undergo surgery or require a bridge therapy to surgery.
Fluid-filled balloons can be placed and filled to an appropriate volume. One network meta-analysis found that fluid-filled balloons were more likely to lead to weight loss, but also more likely to be removed due to intolerance. She also noted that the Elipse balloon (Allurion Technologies) is designed to be swallowed and thus avoid procedures entirely; it is currently under review by Food and Drug Administration.
Although balloons are linked to 7%-10% weight loss in some studies and reviews, Dr. Schulman said, “we know … that the majority of these lead to much more weight loss in clinical practice, oftentimes closer to 13%-%15.”
One review found that balloons also lead to improvement in obesity-related comorbidities, compared with conventional nonsurgical approaches, and this benefit extends past 1 year. A study of 21 patients with nonalcoholic steatohepatitis (NASH) treated with intragastric balloons found that 90% had an improvement in nonalcoholic fatty liver disease activity score, with a median drop of 3 points, and 80% had a drop of at least 2 points. Of these patients, 50% also had an improvement in fibrosis determined by magnetic resonance elastography.
Balloon therapy should be highly individualized, according to Dr. Schulman.
Dr. Schulman also described ESG, which uses sutures to remodel the stomach and reduce volume by up to 70%. She outlined studies and reviews, such as those from Sharaiha and colleagues and Hedjoudje and colleagues, showing that ESG leads to significant and sustained weight loss. The procedure was also quite safe, with one large, single-center study showing that both fever and significant blood loss each occurred in less than 1% of patients (Gastrointest Endosc. 2019 Jun;89[6]:1132-8), while the systematic review and meta-analysis from Hedjoudje and colleagues found an adverse event frequency of 2.2%.
In a matched control study, laparoscopic sleeve gastrectomy led to more weight loss, but ESG had fewer adverse events (5.2% versus 16.9%; P < .01) and had a greater effect on gastroesophageal reflux disease.
ESG can be effective when repeated, while surgical revisions are associated with much higher morbidity, according to Dr. Schulman.
During her presentation, Dr. Schulman mentioned the AspireAssist device developed by Aspire Bariatrics, which is similar to a percutaneous endoscopic gastrostomy (PEG) tube. It leads to the removal of about 30% of calories consumed during a meal, with patients instructed to aspirate 20-30 minutes after a meal, two to three times a day. It gained Food and Drug Administration approval on the strength of the PATHWAY study, which showed significant weight loss.
“But perhaps more impressive is the overall patient satisfaction and willingness to recommend this device to others,” said Dr. Schulman.
Another approach she described is the transpyloric shuttle (TPS), which leads to faster filling times and delayed gastric emptying, though it must be removed endoscopically at 12 months.
Dr. Schulman also discussed endoscopic bariatric and metabolic therapy. This approach is currently a primary therapy for obesity, and is in development for the treatment of diabetes and non-alcoholic fatty liver disease. The approach is predicated on the idea that obesity is a disorder of energy homeostasis, and that enteric neurons in the small bowel are key players, possibly through reduced production of as yet unknown signaling molecules, leading to insulin resistance. It’s also known that diets high in fat and sugar alter the duodenum, which causes changes in nutrient signaling to the brain.
“It’s thought that this leads to duodenal endocrine hyperactivity and ultimately metabolic disease,” said Dr. Schulman.
Finally, she described small-bowel therapies like endobarrier sleeves, duodenal mucosal resurfacing, and an incisionless anastomosis system designed to improve glycemic control by altering the gut through noninvasive means.
Dr. Schulman has consulted for Apollo Endosurgery, Boston Scientific, Olympus, and MicroTech, and has received research support from GI Dynamics.
SAN FRANCISCO – Endoscopic treatments for obesity are under-utilized but represent an opportunity for gastroenterologists to help address the metabolic epidemic that affects up to 40% of people in the United States, according to a presentation reviewing these techniques.
Lifestyle modification is the first intervention, but results in just a 5% average weight loss, according to Allison Schulman, MD, MPH, who discussed these options at the 2022 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. Although surgical interventions induce more weight loss and greater improvement of metabolic outcomes, they come with significant risks and many patients are reluctant to pursue them, she added. In fact, fewer than 1% of obese individuals who qualify for bariatric surgery ultimately undergo it.
Dr. Schulman emphasized another option: Endoscopic bariatric therapies fill this void in between those two extremes, as they are clearly less invasive” said Dr. Schulman, who is an assistant professor of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “They may appeal to those who do not qualify or do not want bariatric surgery. They also could bridge a critical gap in the treatment of obesity, as they reach patients earlier, at BMIs [body mass indexes] where they may not be surgical candidates. Furthermore, these therapies are oftentimes repeatable and commonly can be used in combination [with other weight loss approaches].”
Endoscopic therapies for obesity include devices that occupy space in the stomach, such as intragastric balloons, gastric remodeling procedures like endoscopic sleeve gastroplasty (ESG), and aspiration therapy.
Potential candidates for noninvasive approaches include patients with a BMI over 30 kg/m2 who have not lost sufficient weight through nonsurgical methods or those who do not want to undergo surgery or require a bridge therapy to surgery.
Fluid-filled balloons can be placed and filled to an appropriate volume. One network meta-analysis found that fluid-filled balloons were more likely to lead to weight loss, but also more likely to be removed due to intolerance. She also noted that the Elipse balloon (Allurion Technologies) is designed to be swallowed and thus avoid procedures entirely; it is currently under review by Food and Drug Administration.
Although balloons are linked to 7%-10% weight loss in some studies and reviews, Dr. Schulman said, “we know … that the majority of these lead to much more weight loss in clinical practice, oftentimes closer to 13%-%15.”
One review found that balloons also lead to improvement in obesity-related comorbidities, compared with conventional nonsurgical approaches, and this benefit extends past 1 year. A study of 21 patients with nonalcoholic steatohepatitis (NASH) treated with intragastric balloons found that 90% had an improvement in nonalcoholic fatty liver disease activity score, with a median drop of 3 points, and 80% had a drop of at least 2 points. Of these patients, 50% also had an improvement in fibrosis determined by magnetic resonance elastography.
Balloon therapy should be highly individualized, according to Dr. Schulman.
Dr. Schulman also described ESG, which uses sutures to remodel the stomach and reduce volume by up to 70%. She outlined studies and reviews, such as those from Sharaiha and colleagues and Hedjoudje and colleagues, showing that ESG leads to significant and sustained weight loss. The procedure was also quite safe, with one large, single-center study showing that both fever and significant blood loss each occurred in less than 1% of patients (Gastrointest Endosc. 2019 Jun;89[6]:1132-8), while the systematic review and meta-analysis from Hedjoudje and colleagues found an adverse event frequency of 2.2%.
In a matched control study, laparoscopic sleeve gastrectomy led to more weight loss, but ESG had fewer adverse events (5.2% versus 16.9%; P < .01) and had a greater effect on gastroesophageal reflux disease.
ESG can be effective when repeated, while surgical revisions are associated with much higher morbidity, according to Dr. Schulman.
During her presentation, Dr. Schulman mentioned the AspireAssist device developed by Aspire Bariatrics, which is similar to a percutaneous endoscopic gastrostomy (PEG) tube. It leads to the removal of about 30% of calories consumed during a meal, with patients instructed to aspirate 20-30 minutes after a meal, two to three times a day. It gained Food and Drug Administration approval on the strength of the PATHWAY study, which showed significant weight loss.
“But perhaps more impressive is the overall patient satisfaction and willingness to recommend this device to others,” said Dr. Schulman.
Another approach she described is the transpyloric shuttle (TPS), which leads to faster filling times and delayed gastric emptying, though it must be removed endoscopically at 12 months.
Dr. Schulman also discussed endoscopic bariatric and metabolic therapy. This approach is currently a primary therapy for obesity, and is in development for the treatment of diabetes and non-alcoholic fatty liver disease. The approach is predicated on the idea that obesity is a disorder of energy homeostasis, and that enteric neurons in the small bowel are key players, possibly through reduced production of as yet unknown signaling molecules, leading to insulin resistance. It’s also known that diets high in fat and sugar alter the duodenum, which causes changes in nutrient signaling to the brain.
“It’s thought that this leads to duodenal endocrine hyperactivity and ultimately metabolic disease,” said Dr. Schulman.
Finally, she described small-bowel therapies like endobarrier sleeves, duodenal mucosal resurfacing, and an incisionless anastomosis system designed to improve glycemic control by altering the gut through noninvasive means.
Dr. Schulman has consulted for Apollo Endosurgery, Boston Scientific, Olympus, and MicroTech, and has received research support from GI Dynamics.
SAN FRANCISCO – Endoscopic treatments for obesity are under-utilized but represent an opportunity for gastroenterologists to help address the metabolic epidemic that affects up to 40% of people in the United States, according to a presentation reviewing these techniques.
Lifestyle modification is the first intervention, but results in just a 5% average weight loss, according to Allison Schulman, MD, MPH, who discussed these options at the 2022 AGA Tech Summit sponsored by the AGA Center for GI Innovation and Technology. Although surgical interventions induce more weight loss and greater improvement of metabolic outcomes, they come with significant risks and many patients are reluctant to pursue them, she added. In fact, fewer than 1% of obese individuals who qualify for bariatric surgery ultimately undergo it.
Dr. Schulman emphasized another option: Endoscopic bariatric therapies fill this void in between those two extremes, as they are clearly less invasive” said Dr. Schulman, who is an assistant professor of gastroenterology and hepatology at the University of Michigan, Ann Arbor. “They may appeal to those who do not qualify or do not want bariatric surgery. They also could bridge a critical gap in the treatment of obesity, as they reach patients earlier, at BMIs [body mass indexes] where they may not be surgical candidates. Furthermore, these therapies are oftentimes repeatable and commonly can be used in combination [with other weight loss approaches].”
Endoscopic therapies for obesity include devices that occupy space in the stomach, such as intragastric balloons, gastric remodeling procedures like endoscopic sleeve gastroplasty (ESG), and aspiration therapy.
Potential candidates for noninvasive approaches include patients with a BMI over 30 kg/m2 who have not lost sufficient weight through nonsurgical methods or those who do not want to undergo surgery or require a bridge therapy to surgery.
Fluid-filled balloons can be placed and filled to an appropriate volume. One network meta-analysis found that fluid-filled balloons were more likely to lead to weight loss, but also more likely to be removed due to intolerance. She also noted that the Elipse balloon (Allurion Technologies) is designed to be swallowed and thus avoid procedures entirely; it is currently under review by Food and Drug Administration.
Although balloons are linked to 7%-10% weight loss in some studies and reviews, Dr. Schulman said, “we know … that the majority of these lead to much more weight loss in clinical practice, oftentimes closer to 13%-%15.”
One review found that balloons also lead to improvement in obesity-related comorbidities, compared with conventional nonsurgical approaches, and this benefit extends past 1 year. A study of 21 patients with nonalcoholic steatohepatitis (NASH) treated with intragastric balloons found that 90% had an improvement in nonalcoholic fatty liver disease activity score, with a median drop of 3 points, and 80% had a drop of at least 2 points. Of these patients, 50% also had an improvement in fibrosis determined by magnetic resonance elastography.
Balloon therapy should be highly individualized, according to Dr. Schulman.
Dr. Schulman also described ESG, which uses sutures to remodel the stomach and reduce volume by up to 70%. She outlined studies and reviews, such as those from Sharaiha and colleagues and Hedjoudje and colleagues, showing that ESG leads to significant and sustained weight loss. The procedure was also quite safe, with one large, single-center study showing that both fever and significant blood loss each occurred in less than 1% of patients (Gastrointest Endosc. 2019 Jun;89[6]:1132-8), while the systematic review and meta-analysis from Hedjoudje and colleagues found an adverse event frequency of 2.2%.
In a matched control study, laparoscopic sleeve gastrectomy led to more weight loss, but ESG had fewer adverse events (5.2% versus 16.9%; P < .01) and had a greater effect on gastroesophageal reflux disease.
ESG can be effective when repeated, while surgical revisions are associated with much higher morbidity, according to Dr. Schulman.
During her presentation, Dr. Schulman mentioned the AspireAssist device developed by Aspire Bariatrics, which is similar to a percutaneous endoscopic gastrostomy (PEG) tube. It leads to the removal of about 30% of calories consumed during a meal, with patients instructed to aspirate 20-30 minutes after a meal, two to three times a day. It gained Food and Drug Administration approval on the strength of the PATHWAY study, which showed significant weight loss.
“But perhaps more impressive is the overall patient satisfaction and willingness to recommend this device to others,” said Dr. Schulman.
Another approach she described is the transpyloric shuttle (TPS), which leads to faster filling times and delayed gastric emptying, though it must be removed endoscopically at 12 months.
Dr. Schulman also discussed endoscopic bariatric and metabolic therapy. This approach is currently a primary therapy for obesity, and is in development for the treatment of diabetes and non-alcoholic fatty liver disease. The approach is predicated on the idea that obesity is a disorder of energy homeostasis, and that enteric neurons in the small bowel are key players, possibly through reduced production of as yet unknown signaling molecules, leading to insulin resistance. It’s also known that diets high in fat and sugar alter the duodenum, which causes changes in nutrient signaling to the brain.
“It’s thought that this leads to duodenal endocrine hyperactivity and ultimately metabolic disease,” said Dr. Schulman.
Finally, she described small-bowel therapies like endobarrier sleeves, duodenal mucosal resurfacing, and an incisionless anastomosis system designed to improve glycemic control by altering the gut through noninvasive means.
Dr. Schulman has consulted for Apollo Endosurgery, Boston Scientific, Olympus, and MicroTech, and has received research support from GI Dynamics.
AT 2022 AGA TECH SUMMIT
Two rounds of FIT vs. single colonoscopy as a one-time CRC screening
A fecal immunochemical test (FIT) conducted 2 years apart could form the basis for population-based colorectal cancer screening if this approach goes on to show a mortality benefit, according to a preliminary analysis.
Researchers are investigating the effect of this time frame of FIT screening as well as a single colonoscopy on colorectal cancer incidence and mortality as the primary endpoints of a randomized controlled trial called SCREESCO. Both were compared to a control of no intervention.
“The rationale is to have the FIT test for a 2-year interval and then no more [colorectal cancer screening] after that,” Anna Forsberg, MD, PhD, lead author and a researcher at the Karolinska Institute in Stockholm, told this news organization.
In addition to the spacing of the FIT screening, the cutoff value in the stool sample of 10 micrograms of hemoglobin per gram is also unique to SCREESCO.
“In most other studies, the cutoff is either 20 micrograms or 40 micrograms,” Dr. Forsberg said. “With this very low cutoff, we hope that we find cancer, including advanced adenomas that are precancerous and can be removed.”
The preliminary analysis reporting the trial’s baseline findings was published online in The Lancet Gastroenterology & Hepatology.
Comparing two interventions
Between 2014 and 2020, the study included 278,280 individuals, with 31,140 assigned to the colonoscopy group, 60,300 to the FIT group, and 186,840 to the control group. Both colonoscopy and FIT screening occurred at age 60 years.
Of the individuals in the colonoscopy group, 35.1% had a colonoscopy, compared with 55.5% in the FIT group who participated in at least one round of testing; 41.4% completed both rounds of FIT screening.
In the FIT group, 6.3% of the test results were positive, and 90.8% of individuals with a positive FIT test underwent a follow-up colonoscopy.
Polyps were found in 45.2% of colonoscopies in the colonoscopy group and in 58.2% in the FIT group. The median adenoma detection rate was 20% in the colonoscopy group and 34% in the FIT group.
The intention-to-treat analysis found a colorectal cancer detection rate of 0.16% in the colonoscopy group and 0.20% in the FIT group, but it was not statistically significant. Advanced adenomas were detected more often in the colonoscopy group than in the FIT group (2.05% vs. 1.61%; relative risk, 1.27; 95% confidence interval, 1.15-1.41).
The number of colonoscopies needed to detect one cancer was 218 in the colonoscopy group and 49 in the FIT group; to detect a single advanced adenoma, 17 and 6 colonoscopies had to be done, respectively.
Too early to change screening strategies
When asked to comment on the study, David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, said that the results should be understood as preliminary.
“This is only a description of what they have to date and not of what they proposed for their endpoints,” said Dr. Johnson, who wasn’t associated with the research. “A recommendation for changing screening strategies based on these findings would be very inappropriate at this point.”
Dr. Johnson raised concerns about the quality of the colonoscopies, noting that the procedures were carried out by a mix of gastroenterologists and surgeons. He said that several measures, including the surprisingly low yields for the colonoscopy group and even more so for the FIT-positive group, were concerning.
“They are much lower than even the minimum standard that we would use as benchmark quality indicators in the United States,” he said. “That raises a considerable flag to me that the quality of the colonoscopy confirmation [after a positive FIT test] and screening were both low.”
Dr. Johnson said the ultimate goal of colorectal cancer screening is to detect and remove polyps, a strategy that has been shown to reduce mortality. But FIT tests are not well suited to detecting adenomatous polyps and are of little value in detecting sessile serrated lesions.
“FIT is a detection for cancer, not a detection for polyps. The true value of screening is not just detection but prevention by identification and removal of precancerous polyps,” he said.
Still, Dr. Forsberg emphasized the importance of maximizing uptake of colorectal cancer screening.
“When we pooled the results from the two interventions, the participation in the FIT arm was 56%, which is good. If you look at international figures, that is also high for participation in colonoscopy screening,” Dr. Forsberg said.
“Participation is the main thing. You can have the most fantastic test, but if people don’t participate, then it’s not worth much.”
SCREESCO is one of several ongoing studies comparing screening strategies that employ colonoscopy or FIT. Other studies of colon cancer surveillance methods include the COLONPREV and CONFIRM trials comparing colonoscopy to FIT, and the NordICC trial comparing colonoscopy to no intervention. None of these trials have yet published their final results on colorectal cancer mortality.
Dr. Forsberg and Dr. Johnson have disclosed no relevant financial disclosures. The study was funded by Swedish regions, County Council, Regional Cancer Center Mellansverige, Swedish Cancer Society, Aleris Research and Development Fund, and Eiken Chemical.
A version of this article first appeared on Medscape.com.
A fecal immunochemical test (FIT) conducted 2 years apart could form the basis for population-based colorectal cancer screening if this approach goes on to show a mortality benefit, according to a preliminary analysis.
Researchers are investigating the effect of this time frame of FIT screening as well as a single colonoscopy on colorectal cancer incidence and mortality as the primary endpoints of a randomized controlled trial called SCREESCO. Both were compared to a control of no intervention.
“The rationale is to have the FIT test for a 2-year interval and then no more [colorectal cancer screening] after that,” Anna Forsberg, MD, PhD, lead author and a researcher at the Karolinska Institute in Stockholm, told this news organization.
In addition to the spacing of the FIT screening, the cutoff value in the stool sample of 10 micrograms of hemoglobin per gram is also unique to SCREESCO.
“In most other studies, the cutoff is either 20 micrograms or 40 micrograms,” Dr. Forsberg said. “With this very low cutoff, we hope that we find cancer, including advanced adenomas that are precancerous and can be removed.”
The preliminary analysis reporting the trial’s baseline findings was published online in The Lancet Gastroenterology & Hepatology.
Comparing two interventions
Between 2014 and 2020, the study included 278,280 individuals, with 31,140 assigned to the colonoscopy group, 60,300 to the FIT group, and 186,840 to the control group. Both colonoscopy and FIT screening occurred at age 60 years.
Of the individuals in the colonoscopy group, 35.1% had a colonoscopy, compared with 55.5% in the FIT group who participated in at least one round of testing; 41.4% completed both rounds of FIT screening.
In the FIT group, 6.3% of the test results were positive, and 90.8% of individuals with a positive FIT test underwent a follow-up colonoscopy.
Polyps were found in 45.2% of colonoscopies in the colonoscopy group and in 58.2% in the FIT group. The median adenoma detection rate was 20% in the colonoscopy group and 34% in the FIT group.
The intention-to-treat analysis found a colorectal cancer detection rate of 0.16% in the colonoscopy group and 0.20% in the FIT group, but it was not statistically significant. Advanced adenomas were detected more often in the colonoscopy group than in the FIT group (2.05% vs. 1.61%; relative risk, 1.27; 95% confidence interval, 1.15-1.41).
The number of colonoscopies needed to detect one cancer was 218 in the colonoscopy group and 49 in the FIT group; to detect a single advanced adenoma, 17 and 6 colonoscopies had to be done, respectively.
Too early to change screening strategies
When asked to comment on the study, David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, said that the results should be understood as preliminary.
“This is only a description of what they have to date and not of what they proposed for their endpoints,” said Dr. Johnson, who wasn’t associated with the research. “A recommendation for changing screening strategies based on these findings would be very inappropriate at this point.”
Dr. Johnson raised concerns about the quality of the colonoscopies, noting that the procedures were carried out by a mix of gastroenterologists and surgeons. He said that several measures, including the surprisingly low yields for the colonoscopy group and even more so for the FIT-positive group, were concerning.
“They are much lower than even the minimum standard that we would use as benchmark quality indicators in the United States,” he said. “That raises a considerable flag to me that the quality of the colonoscopy confirmation [after a positive FIT test] and screening were both low.”
Dr. Johnson said the ultimate goal of colorectal cancer screening is to detect and remove polyps, a strategy that has been shown to reduce mortality. But FIT tests are not well suited to detecting adenomatous polyps and are of little value in detecting sessile serrated lesions.
“FIT is a detection for cancer, not a detection for polyps. The true value of screening is not just detection but prevention by identification and removal of precancerous polyps,” he said.
Still, Dr. Forsberg emphasized the importance of maximizing uptake of colorectal cancer screening.
“When we pooled the results from the two interventions, the participation in the FIT arm was 56%, which is good. If you look at international figures, that is also high for participation in colonoscopy screening,” Dr. Forsberg said.
“Participation is the main thing. You can have the most fantastic test, but if people don’t participate, then it’s not worth much.”
SCREESCO is one of several ongoing studies comparing screening strategies that employ colonoscopy or FIT. Other studies of colon cancer surveillance methods include the COLONPREV and CONFIRM trials comparing colonoscopy to FIT, and the NordICC trial comparing colonoscopy to no intervention. None of these trials have yet published their final results on colorectal cancer mortality.
Dr. Forsberg and Dr. Johnson have disclosed no relevant financial disclosures. The study was funded by Swedish regions, County Council, Regional Cancer Center Mellansverige, Swedish Cancer Society, Aleris Research and Development Fund, and Eiken Chemical.
A version of this article first appeared on Medscape.com.
A fecal immunochemical test (FIT) conducted 2 years apart could form the basis for population-based colorectal cancer screening if this approach goes on to show a mortality benefit, according to a preliminary analysis.
Researchers are investigating the effect of this time frame of FIT screening as well as a single colonoscopy on colorectal cancer incidence and mortality as the primary endpoints of a randomized controlled trial called SCREESCO. Both were compared to a control of no intervention.
“The rationale is to have the FIT test for a 2-year interval and then no more [colorectal cancer screening] after that,” Anna Forsberg, MD, PhD, lead author and a researcher at the Karolinska Institute in Stockholm, told this news organization.
In addition to the spacing of the FIT screening, the cutoff value in the stool sample of 10 micrograms of hemoglobin per gram is also unique to SCREESCO.
“In most other studies, the cutoff is either 20 micrograms or 40 micrograms,” Dr. Forsberg said. “With this very low cutoff, we hope that we find cancer, including advanced adenomas that are precancerous and can be removed.”
The preliminary analysis reporting the trial’s baseline findings was published online in The Lancet Gastroenterology & Hepatology.
Comparing two interventions
Between 2014 and 2020, the study included 278,280 individuals, with 31,140 assigned to the colonoscopy group, 60,300 to the FIT group, and 186,840 to the control group. Both colonoscopy and FIT screening occurred at age 60 years.
Of the individuals in the colonoscopy group, 35.1% had a colonoscopy, compared with 55.5% in the FIT group who participated in at least one round of testing; 41.4% completed both rounds of FIT screening.
In the FIT group, 6.3% of the test results were positive, and 90.8% of individuals with a positive FIT test underwent a follow-up colonoscopy.
Polyps were found in 45.2% of colonoscopies in the colonoscopy group and in 58.2% in the FIT group. The median adenoma detection rate was 20% in the colonoscopy group and 34% in the FIT group.
The intention-to-treat analysis found a colorectal cancer detection rate of 0.16% in the colonoscopy group and 0.20% in the FIT group, but it was not statistically significant. Advanced adenomas were detected more often in the colonoscopy group than in the FIT group (2.05% vs. 1.61%; relative risk, 1.27; 95% confidence interval, 1.15-1.41).
The number of colonoscopies needed to detect one cancer was 218 in the colonoscopy group and 49 in the FIT group; to detect a single advanced adenoma, 17 and 6 colonoscopies had to be done, respectively.
Too early to change screening strategies
When asked to comment on the study, David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, said that the results should be understood as preliminary.
“This is only a description of what they have to date and not of what they proposed for their endpoints,” said Dr. Johnson, who wasn’t associated with the research. “A recommendation for changing screening strategies based on these findings would be very inappropriate at this point.”
Dr. Johnson raised concerns about the quality of the colonoscopies, noting that the procedures were carried out by a mix of gastroenterologists and surgeons. He said that several measures, including the surprisingly low yields for the colonoscopy group and even more so for the FIT-positive group, were concerning.
“They are much lower than even the minimum standard that we would use as benchmark quality indicators in the United States,” he said. “That raises a considerable flag to me that the quality of the colonoscopy confirmation [after a positive FIT test] and screening were both low.”
Dr. Johnson said the ultimate goal of colorectal cancer screening is to detect and remove polyps, a strategy that has been shown to reduce mortality. But FIT tests are not well suited to detecting adenomatous polyps and are of little value in detecting sessile serrated lesions.
“FIT is a detection for cancer, not a detection for polyps. The true value of screening is not just detection but prevention by identification and removal of precancerous polyps,” he said.
Still, Dr. Forsberg emphasized the importance of maximizing uptake of colorectal cancer screening.
“When we pooled the results from the two interventions, the participation in the FIT arm was 56%, which is good. If you look at international figures, that is also high for participation in colonoscopy screening,” Dr. Forsberg said.
“Participation is the main thing. You can have the most fantastic test, but if people don’t participate, then it’s not worth much.”
SCREESCO is one of several ongoing studies comparing screening strategies that employ colonoscopy or FIT. Other studies of colon cancer surveillance methods include the COLONPREV and CONFIRM trials comparing colonoscopy to FIT, and the NordICC trial comparing colonoscopy to no intervention. None of these trials have yet published their final results on colorectal cancer mortality.
Dr. Forsberg and Dr. Johnson have disclosed no relevant financial disclosures. The study was funded by Swedish regions, County Council, Regional Cancer Center Mellansverige, Swedish Cancer Society, Aleris Research and Development Fund, and Eiken Chemical.
A version of this article first appeared on Medscape.com.
Antibody reduces amyloid, induces symptom decline
SEATTLE – The researchers identified two plasma biomarkers that correlate well with established amyloid PET standard uptake value ratio (SUVr) changes, potentially paving the way for monitoring lecanemab treatment effects. The researchers also found evidence that the plasma biomarker could be used to allow dose frequency reduction after initial reduction in amyloid plaques.
Lecanemab preferably targets aggregated species of amyloid called protofibrils, which is unique among anti-amyloid antibodies, and these are also among the most toxic manifestations of amyloid, according to Chad Swanson, PhD, who presented the study at the 2022 annual meeting of the American Academy of Neurology.
Are amyloid plaques a key driver of Alzheimer’s disease?
The study could help answer the question of whether amyloid plaques drive the cognitive decline seen in Alzheimer’s disease, in part because the antibody is so effective at what it was designed to do, according to Fernando Testai, MD, PhD, who comoderated the session where the study was presented. “The effect on amyloid content that they measured was persistent over a number of months. Cognition may follow along, so more studies have to be done, but the medication seems to be quite effective doing what it’s supposed to do. If it has something to do with disease, these treatments actually should give us the answer, because the effect on amyloid is pretty significant. After so many years of thinking amyloid probably has nothing to do (with Alzheimer’s symptoms, these results suggest) it may have something to do with the disease,” Dr. Testai said in an interview. He is a professor of neurology at the University of Illinois at Chicago.
Dr. Swanson is confident that amyloid plaques are a key driver of disease. “I’d say a number of companies now with anti-amyloid agents have shown that targeting amyloid can produce some slowing of clinical decline, as well as a robust reduction in amyloid, supporting this idea that that amyloid is meaningful. And it’s very clear that amyloid [deposition] tends to trigger tau pathology,” said Dr. Swanson in an interview. He is executive director of the neurology business group at Eisai Pharmaceuticals, which is developing the anti-amyloid antibody, called lecanemab.
Searching for the best dose and dose frequency
Dr. Swanson noted that Eisai has already conducted a large phase 2b study which informed the current phase 3 ClarityAD study design. The phase 2b study utilized a Bayesian adaptive design, which allocated more patients in a fully blinded way to doses that had the most potential for slowing clinical decline. “The intent was to maximize the efficiency of the design so that more subjects would go to a dose that looks like it could be the best dose according to the Bayesian algorithm,” said Dr. Swanson.
The study also included a gap period that followed the randomized phase of the trial, where subjects were not being dosed with the antibody from 9 to 59 months (mean, 2 years), before reinitiation at the start of the open-label extension phase. “[That] allowed us to answer some really important questions about what happens when you remove lecanemab after reducing amyloid, and then what happens when you reintroduce lecanemab in the open-label extension,” said Dr. Swanson.
The researchers found that amyloid reaccumulated during the treatment gap, and soluble biomarkers were potentially the most sensitive to the change. “Taking all of this information together with clinical data that suggest potential disease-modifying effects, it suggests that we need to continue to treat these individuals, but we may be able to treat with a less-frequent dosing interval once amyloid is removed from the brain. It’s a biweekly infusion. Following 18 months of treatment, we may be able to go in once every month or once every 3 months to maintain low levels of amyloid. We’re going to be testing that soon in the current phase 2 open-label extension,” said Dr. Swanson.
The study included 856 patients who were randomized to biweekly placebo or lecanemab 2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, or 10 mg/kg biweekly. The primary endpoint was the Alzheimer’s disease composite score at 12 months; secondary endpoints included ADCOMS and various biomarker levels at 18 months.
At 18 months, the 10-mg/kg biweekly group had an adjusted mean change from placebo in brain amyloid of –0.31 SUVr units, with more than 80% of the subjects converting from amyloid positive to amyloid negative by visual read. Most subjects remained amyloid negative at open-label extension baseline following the gap period despite a slow reaccumulation of amyloid plaque in the treated group. Out to 18 months in the core study, the same group had a 30% reduction in cognitive decline, compared with placebo, as measured by ADCOMS (P < .05).
There was a correlation between PET SUVr, clinical outcomes, and the Abeta42/40 ratio and plasma p-tau181. During the gap period, treatment discontinuation was associated with changes in the plasma biomarkers that echoed amyloid re-accumulation and clinical decline. Change from baseline in both plasma biomarkers were associated with a change from baseline in PET SUVr at 18 months.
Amyloid-related imaging abnormalities related to brain edema or sulcal effusion (ARIA-E) occurred in 9.9% of patients during the randomized phase of the trial, and 7.8% during the open-label extension phase. About 2% were symptomatic in both the randomization and open-label extension phases. The majority of ARIA-E cases appeared within 3 months of treatment initiation, and generally resolved in 4-16 weeks. 80% were mild to moderate by radiography.
Dr. Swanson is an employee of Eisai Pharmaceuticals, which sponsored the study. Dr. Testai has no relevant financial disclosures.
SEATTLE – The researchers identified two plasma biomarkers that correlate well with established amyloid PET standard uptake value ratio (SUVr) changes, potentially paving the way for monitoring lecanemab treatment effects. The researchers also found evidence that the plasma biomarker could be used to allow dose frequency reduction after initial reduction in amyloid plaques.
Lecanemab preferably targets aggregated species of amyloid called protofibrils, which is unique among anti-amyloid antibodies, and these are also among the most toxic manifestations of amyloid, according to Chad Swanson, PhD, who presented the study at the 2022 annual meeting of the American Academy of Neurology.
Are amyloid plaques a key driver of Alzheimer’s disease?
The study could help answer the question of whether amyloid plaques drive the cognitive decline seen in Alzheimer’s disease, in part because the antibody is so effective at what it was designed to do, according to Fernando Testai, MD, PhD, who comoderated the session where the study was presented. “The effect on amyloid content that they measured was persistent over a number of months. Cognition may follow along, so more studies have to be done, but the medication seems to be quite effective doing what it’s supposed to do. If it has something to do with disease, these treatments actually should give us the answer, because the effect on amyloid is pretty significant. After so many years of thinking amyloid probably has nothing to do (with Alzheimer’s symptoms, these results suggest) it may have something to do with the disease,” Dr. Testai said in an interview. He is a professor of neurology at the University of Illinois at Chicago.
Dr. Swanson is confident that amyloid plaques are a key driver of disease. “I’d say a number of companies now with anti-amyloid agents have shown that targeting amyloid can produce some slowing of clinical decline, as well as a robust reduction in amyloid, supporting this idea that that amyloid is meaningful. And it’s very clear that amyloid [deposition] tends to trigger tau pathology,” said Dr. Swanson in an interview. He is executive director of the neurology business group at Eisai Pharmaceuticals, which is developing the anti-amyloid antibody, called lecanemab.
Searching for the best dose and dose frequency
Dr. Swanson noted that Eisai has already conducted a large phase 2b study which informed the current phase 3 ClarityAD study design. The phase 2b study utilized a Bayesian adaptive design, which allocated more patients in a fully blinded way to doses that had the most potential for slowing clinical decline. “The intent was to maximize the efficiency of the design so that more subjects would go to a dose that looks like it could be the best dose according to the Bayesian algorithm,” said Dr. Swanson.
The study also included a gap period that followed the randomized phase of the trial, where subjects were not being dosed with the antibody from 9 to 59 months (mean, 2 years), before reinitiation at the start of the open-label extension phase. “[That] allowed us to answer some really important questions about what happens when you remove lecanemab after reducing amyloid, and then what happens when you reintroduce lecanemab in the open-label extension,” said Dr. Swanson.
The researchers found that amyloid reaccumulated during the treatment gap, and soluble biomarkers were potentially the most sensitive to the change. “Taking all of this information together with clinical data that suggest potential disease-modifying effects, it suggests that we need to continue to treat these individuals, but we may be able to treat with a less-frequent dosing interval once amyloid is removed from the brain. It’s a biweekly infusion. Following 18 months of treatment, we may be able to go in once every month or once every 3 months to maintain low levels of amyloid. We’re going to be testing that soon in the current phase 2 open-label extension,” said Dr. Swanson.
The study included 856 patients who were randomized to biweekly placebo or lecanemab 2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, or 10 mg/kg biweekly. The primary endpoint was the Alzheimer’s disease composite score at 12 months; secondary endpoints included ADCOMS and various biomarker levels at 18 months.
At 18 months, the 10-mg/kg biweekly group had an adjusted mean change from placebo in brain amyloid of –0.31 SUVr units, with more than 80% of the subjects converting from amyloid positive to amyloid negative by visual read. Most subjects remained amyloid negative at open-label extension baseline following the gap period despite a slow reaccumulation of amyloid plaque in the treated group. Out to 18 months in the core study, the same group had a 30% reduction in cognitive decline, compared with placebo, as measured by ADCOMS (P < .05).
There was a correlation between PET SUVr, clinical outcomes, and the Abeta42/40 ratio and plasma p-tau181. During the gap period, treatment discontinuation was associated with changes in the plasma biomarkers that echoed amyloid re-accumulation and clinical decline. Change from baseline in both plasma biomarkers were associated with a change from baseline in PET SUVr at 18 months.
Amyloid-related imaging abnormalities related to brain edema or sulcal effusion (ARIA-E) occurred in 9.9% of patients during the randomized phase of the trial, and 7.8% during the open-label extension phase. About 2% were symptomatic in both the randomization and open-label extension phases. The majority of ARIA-E cases appeared within 3 months of treatment initiation, and generally resolved in 4-16 weeks. 80% were mild to moderate by radiography.
Dr. Swanson is an employee of Eisai Pharmaceuticals, which sponsored the study. Dr. Testai has no relevant financial disclosures.
SEATTLE – The researchers identified two plasma biomarkers that correlate well with established amyloid PET standard uptake value ratio (SUVr) changes, potentially paving the way for monitoring lecanemab treatment effects. The researchers also found evidence that the plasma biomarker could be used to allow dose frequency reduction after initial reduction in amyloid plaques.
Lecanemab preferably targets aggregated species of amyloid called protofibrils, which is unique among anti-amyloid antibodies, and these are also among the most toxic manifestations of amyloid, according to Chad Swanson, PhD, who presented the study at the 2022 annual meeting of the American Academy of Neurology.
Are amyloid plaques a key driver of Alzheimer’s disease?
The study could help answer the question of whether amyloid plaques drive the cognitive decline seen in Alzheimer’s disease, in part because the antibody is so effective at what it was designed to do, according to Fernando Testai, MD, PhD, who comoderated the session where the study was presented. “The effect on amyloid content that they measured was persistent over a number of months. Cognition may follow along, so more studies have to be done, but the medication seems to be quite effective doing what it’s supposed to do. If it has something to do with disease, these treatments actually should give us the answer, because the effect on amyloid is pretty significant. After so many years of thinking amyloid probably has nothing to do (with Alzheimer’s symptoms, these results suggest) it may have something to do with the disease,” Dr. Testai said in an interview. He is a professor of neurology at the University of Illinois at Chicago.
Dr. Swanson is confident that amyloid plaques are a key driver of disease. “I’d say a number of companies now with anti-amyloid agents have shown that targeting amyloid can produce some slowing of clinical decline, as well as a robust reduction in amyloid, supporting this idea that that amyloid is meaningful. And it’s very clear that amyloid [deposition] tends to trigger tau pathology,” said Dr. Swanson in an interview. He is executive director of the neurology business group at Eisai Pharmaceuticals, which is developing the anti-amyloid antibody, called lecanemab.
Searching for the best dose and dose frequency
Dr. Swanson noted that Eisai has already conducted a large phase 2b study which informed the current phase 3 ClarityAD study design. The phase 2b study utilized a Bayesian adaptive design, which allocated more patients in a fully blinded way to doses that had the most potential for slowing clinical decline. “The intent was to maximize the efficiency of the design so that more subjects would go to a dose that looks like it could be the best dose according to the Bayesian algorithm,” said Dr. Swanson.
The study also included a gap period that followed the randomized phase of the trial, where subjects were not being dosed with the antibody from 9 to 59 months (mean, 2 years), before reinitiation at the start of the open-label extension phase. “[That] allowed us to answer some really important questions about what happens when you remove lecanemab after reducing amyloid, and then what happens when you reintroduce lecanemab in the open-label extension,” said Dr. Swanson.
The researchers found that amyloid reaccumulated during the treatment gap, and soluble biomarkers were potentially the most sensitive to the change. “Taking all of this information together with clinical data that suggest potential disease-modifying effects, it suggests that we need to continue to treat these individuals, but we may be able to treat with a less-frequent dosing interval once amyloid is removed from the brain. It’s a biweekly infusion. Following 18 months of treatment, we may be able to go in once every month or once every 3 months to maintain low levels of amyloid. We’re going to be testing that soon in the current phase 2 open-label extension,” said Dr. Swanson.
The study included 856 patients who were randomized to biweekly placebo or lecanemab 2.5 mg/kg biweekly, 5 mg/kg monthly, 5 mg/kg biweekly, 10 mg/kg monthly, or 10 mg/kg biweekly. The primary endpoint was the Alzheimer’s disease composite score at 12 months; secondary endpoints included ADCOMS and various biomarker levels at 18 months.
At 18 months, the 10-mg/kg biweekly group had an adjusted mean change from placebo in brain amyloid of –0.31 SUVr units, with more than 80% of the subjects converting from amyloid positive to amyloid negative by visual read. Most subjects remained amyloid negative at open-label extension baseline following the gap period despite a slow reaccumulation of amyloid plaque in the treated group. Out to 18 months in the core study, the same group had a 30% reduction in cognitive decline, compared with placebo, as measured by ADCOMS (P < .05).
There was a correlation between PET SUVr, clinical outcomes, and the Abeta42/40 ratio and plasma p-tau181. During the gap period, treatment discontinuation was associated with changes in the plasma biomarkers that echoed amyloid re-accumulation and clinical decline. Change from baseline in both plasma biomarkers were associated with a change from baseline in PET SUVr at 18 months.
Amyloid-related imaging abnormalities related to brain edema or sulcal effusion (ARIA-E) occurred in 9.9% of patients during the randomized phase of the trial, and 7.8% during the open-label extension phase. About 2% were symptomatic in both the randomization and open-label extension phases. The majority of ARIA-E cases appeared within 3 months of treatment initiation, and generally resolved in 4-16 weeks. 80% were mild to moderate by radiography.
Dr. Swanson is an employee of Eisai Pharmaceuticals, which sponsored the study. Dr. Testai has no relevant financial disclosures.
AT AAN 2022
Neighborhood-level data sheds new light on racial and ethnic diversity in MS
SEATTLE – These populations often have more severe disease, likely driven by socioeconomic factors and health care access, according to a new study that examined neighborhood-level data and disease severity in the United States.
“It has previously been thought that MS is less common among non-European Caucasian White populations, driven partly by the well-known association of incidence with latitude. It is abundantly clear at this point that this idea is not true,” said Christopher Orlando, MD, during a presentation at the 2022 annual meeting of the American Academy of Neurology.
He noted that several U.S. studies with large sample sizes have shown greater disease severity and a higher disability burden among Hispanic and Black patients. “Black patients in particular appear to have a higher incidence of disease and a greater proportion of progressive disease phenotypes,” said Dr. Orlando.
Race and ethnicity are unlikely explanations for this disparity, according to Dr. Orlando. “While much remains to be discovered of the genetic underpinnings of MS, what we do know does not support the idea that minorities would have a predilection to more severe disease. For example, the well-known high-risk allele HLA DRB1*1501 appears to have a lower frequency in African populations, compared with European [populations].”
Instead, evidence suggests that interrelated social causes include access to resources, environmental exposures, and psychosocial stress. “These affect health via a number of pathways including direct physical injury, allostatic load, and access to health care,” said Dr. Orlando.
Probing racial and ethnic disparities
Previous studies that corrected for social determinants of health such as socioeconomic and insurance status reduce the association between MS disability and race, but they do not completely explain it.
To get a better understanding of the impacts of these factors, researchers have used neighborhood-level data combined with information on socioeconomic status and social deprivation to identify associations with MS severity.
At the conference, Dr. Orlando presented a new study that is the first to use this methodology in the United States, and it is the first to apply it to the study of racial and ethnic disparities in MS.
The study confirmed more severe disability in Hispanic and Black patients than in White patients. Clinical factors associated with more severe disease were similar across the three groups, with some small differences among individual traits. “More stark differences appeared when we compared social determinants of health. Hispanic patients were less likely to speak English as a primary language or to complete 12 years of education. Black patients were less likely to live in a rural county and more likely to be unemployed. One particularly stark difference was in the number of unemployed specifically due to their MS, with only 1 White patient [1.1%], 7 Hispanic patients [7.8%] and 27 Black patients [31.0%],” said Dr. Orlando.
The researchers found that Black and Hispanic patients tend to live in more vulnerable neighborhoods than White patients. The researchers found no significant association between social vulnerability index (SVI) values and MS severity, though there was an association in a separate analysis that only included White patients. The SVI uses 15 measures taken from the U.S. Census to identify communities that might require additional support during natural disasters.
“It would appear that the sheer complexity both in variety and magnitude of the social determinants of health are such that by far the stronger association is with race and ethnicity, which are surrogates for any number of social determinants and societal inequities,” said Dr. Orlando.
What drives the inequity?
Dr. Orlando acknowledged that some might wonder if these results indicate a true biologically intrinsic factor such as genetic predisposition. “I want to warn against that kind of thinking in the strongest possible terms. It is implausible on several levels. It’s not biologically plausible based on our understanding that race and ethnicity are not genetic constructs. And it’s also not numerically plausible based on these data,” said Dr. Orlando.
While some of the drivers of this inequity have been partially examined, many have not been studied. “As long as this is the case, our ability to fulfill our roles as physicians will be limited in several important ways. Our ability to assess our patients’ individual risk will be missing key information, which will limit the efficacy of shared decision-making, which of course is the cornerstone of MS treatment. In addition, we will continue to struggle to include minority patients in our research studies, and the very design and results of those studies may be misguided, as we will either fail to include these populations, or we will fail to adjust for important confounders,” he said.
New answers, new questions
The neighborhood-level data examined by Dr. Orlando’s group “brings extra information in terms of the negative impact of social determinants of health. The disparity seen in neighborhood living is quite striking,” said Lilyana Amezcua, MD, who served as a discussant for Dr. Orlando’s presentation. The study reinforces findings of her own group in Hispanic and Latinx individuals with MS. Some comorbidities are more common among these groups, which is exacerbated by poor health access.
“We have noted that almost 30% of them also have this comorbidity of hypertension, but what is also observed is that only 7% of them are aware [that they have hypertension],” said Dr. Amezcua, who is an associate professor of neurology at the University of Southern California, Los Angeles.
The findings should prompt further research to understand the impact of systemic racism and neighborhood factors, such as disinvestment in the public and private sectors, underresourced hospitals and clinics, and negative infrastructure. “We need to start discussing the (patient’s) environment so we can better understand the community resources they may have available, as well as create innovative transitional care services. We need to also recognize and accept that structural racism and imbalanced distribution of resources and neighborhoods does restrict educational and economic opportunities, as well as health care access and the safety of these marginalized communities,” said Dr. Amezcua.
Dr. Amezcua has consulted for, received speaking fees from, or served on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono. She has received research support from the Bristol-Myers Squibb Foundation and Biogen Idec. Dr. Orlando has no relevant financial disclosures.
SEATTLE – These populations often have more severe disease, likely driven by socioeconomic factors and health care access, according to a new study that examined neighborhood-level data and disease severity in the United States.
“It has previously been thought that MS is less common among non-European Caucasian White populations, driven partly by the well-known association of incidence with latitude. It is abundantly clear at this point that this idea is not true,” said Christopher Orlando, MD, during a presentation at the 2022 annual meeting of the American Academy of Neurology.
He noted that several U.S. studies with large sample sizes have shown greater disease severity and a higher disability burden among Hispanic and Black patients. “Black patients in particular appear to have a higher incidence of disease and a greater proportion of progressive disease phenotypes,” said Dr. Orlando.
Race and ethnicity are unlikely explanations for this disparity, according to Dr. Orlando. “While much remains to be discovered of the genetic underpinnings of MS, what we do know does not support the idea that minorities would have a predilection to more severe disease. For example, the well-known high-risk allele HLA DRB1*1501 appears to have a lower frequency in African populations, compared with European [populations].”
Instead, evidence suggests that interrelated social causes include access to resources, environmental exposures, and psychosocial stress. “These affect health via a number of pathways including direct physical injury, allostatic load, and access to health care,” said Dr. Orlando.
Probing racial and ethnic disparities
Previous studies that corrected for social determinants of health such as socioeconomic and insurance status reduce the association between MS disability and race, but they do not completely explain it.
To get a better understanding of the impacts of these factors, researchers have used neighborhood-level data combined with information on socioeconomic status and social deprivation to identify associations with MS severity.
At the conference, Dr. Orlando presented a new study that is the first to use this methodology in the United States, and it is the first to apply it to the study of racial and ethnic disparities in MS.
The study confirmed more severe disability in Hispanic and Black patients than in White patients. Clinical factors associated with more severe disease were similar across the three groups, with some small differences among individual traits. “More stark differences appeared when we compared social determinants of health. Hispanic patients were less likely to speak English as a primary language or to complete 12 years of education. Black patients were less likely to live in a rural county and more likely to be unemployed. One particularly stark difference was in the number of unemployed specifically due to their MS, with only 1 White patient [1.1%], 7 Hispanic patients [7.8%] and 27 Black patients [31.0%],” said Dr. Orlando.
The researchers found that Black and Hispanic patients tend to live in more vulnerable neighborhoods than White patients. The researchers found no significant association between social vulnerability index (SVI) values and MS severity, though there was an association in a separate analysis that only included White patients. The SVI uses 15 measures taken from the U.S. Census to identify communities that might require additional support during natural disasters.
“It would appear that the sheer complexity both in variety and magnitude of the social determinants of health are such that by far the stronger association is with race and ethnicity, which are surrogates for any number of social determinants and societal inequities,” said Dr. Orlando.
What drives the inequity?
Dr. Orlando acknowledged that some might wonder if these results indicate a true biologically intrinsic factor such as genetic predisposition. “I want to warn against that kind of thinking in the strongest possible terms. It is implausible on several levels. It’s not biologically plausible based on our understanding that race and ethnicity are not genetic constructs. And it’s also not numerically plausible based on these data,” said Dr. Orlando.
While some of the drivers of this inequity have been partially examined, many have not been studied. “As long as this is the case, our ability to fulfill our roles as physicians will be limited in several important ways. Our ability to assess our patients’ individual risk will be missing key information, which will limit the efficacy of shared decision-making, which of course is the cornerstone of MS treatment. In addition, we will continue to struggle to include minority patients in our research studies, and the very design and results of those studies may be misguided, as we will either fail to include these populations, or we will fail to adjust for important confounders,” he said.
New answers, new questions
The neighborhood-level data examined by Dr. Orlando’s group “brings extra information in terms of the negative impact of social determinants of health. The disparity seen in neighborhood living is quite striking,” said Lilyana Amezcua, MD, who served as a discussant for Dr. Orlando’s presentation. The study reinforces findings of her own group in Hispanic and Latinx individuals with MS. Some comorbidities are more common among these groups, which is exacerbated by poor health access.
“We have noted that almost 30% of them also have this comorbidity of hypertension, but what is also observed is that only 7% of them are aware [that they have hypertension],” said Dr. Amezcua, who is an associate professor of neurology at the University of Southern California, Los Angeles.
The findings should prompt further research to understand the impact of systemic racism and neighborhood factors, such as disinvestment in the public and private sectors, underresourced hospitals and clinics, and negative infrastructure. “We need to start discussing the (patient’s) environment so we can better understand the community resources they may have available, as well as create innovative transitional care services. We need to also recognize and accept that structural racism and imbalanced distribution of resources and neighborhoods does restrict educational and economic opportunities, as well as health care access and the safety of these marginalized communities,” said Dr. Amezcua.
Dr. Amezcua has consulted for, received speaking fees from, or served on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono. She has received research support from the Bristol-Myers Squibb Foundation and Biogen Idec. Dr. Orlando has no relevant financial disclosures.
SEATTLE – These populations often have more severe disease, likely driven by socioeconomic factors and health care access, according to a new study that examined neighborhood-level data and disease severity in the United States.
“It has previously been thought that MS is less common among non-European Caucasian White populations, driven partly by the well-known association of incidence with latitude. It is abundantly clear at this point that this idea is not true,” said Christopher Orlando, MD, during a presentation at the 2022 annual meeting of the American Academy of Neurology.
He noted that several U.S. studies with large sample sizes have shown greater disease severity and a higher disability burden among Hispanic and Black patients. “Black patients in particular appear to have a higher incidence of disease and a greater proportion of progressive disease phenotypes,” said Dr. Orlando.
Race and ethnicity are unlikely explanations for this disparity, according to Dr. Orlando. “While much remains to be discovered of the genetic underpinnings of MS, what we do know does not support the idea that minorities would have a predilection to more severe disease. For example, the well-known high-risk allele HLA DRB1*1501 appears to have a lower frequency in African populations, compared with European [populations].”
Instead, evidence suggests that interrelated social causes include access to resources, environmental exposures, and psychosocial stress. “These affect health via a number of pathways including direct physical injury, allostatic load, and access to health care,” said Dr. Orlando.
Probing racial and ethnic disparities
Previous studies that corrected for social determinants of health such as socioeconomic and insurance status reduce the association between MS disability and race, but they do not completely explain it.
To get a better understanding of the impacts of these factors, researchers have used neighborhood-level data combined with information on socioeconomic status and social deprivation to identify associations with MS severity.
At the conference, Dr. Orlando presented a new study that is the first to use this methodology in the United States, and it is the first to apply it to the study of racial and ethnic disparities in MS.
The study confirmed more severe disability in Hispanic and Black patients than in White patients. Clinical factors associated with more severe disease were similar across the three groups, with some small differences among individual traits. “More stark differences appeared when we compared social determinants of health. Hispanic patients were less likely to speak English as a primary language or to complete 12 years of education. Black patients were less likely to live in a rural county and more likely to be unemployed. One particularly stark difference was in the number of unemployed specifically due to their MS, with only 1 White patient [1.1%], 7 Hispanic patients [7.8%] and 27 Black patients [31.0%],” said Dr. Orlando.
The researchers found that Black and Hispanic patients tend to live in more vulnerable neighborhoods than White patients. The researchers found no significant association between social vulnerability index (SVI) values and MS severity, though there was an association in a separate analysis that only included White patients. The SVI uses 15 measures taken from the U.S. Census to identify communities that might require additional support during natural disasters.
“It would appear that the sheer complexity both in variety and magnitude of the social determinants of health are such that by far the stronger association is with race and ethnicity, which are surrogates for any number of social determinants and societal inequities,” said Dr. Orlando.
What drives the inequity?
Dr. Orlando acknowledged that some might wonder if these results indicate a true biologically intrinsic factor such as genetic predisposition. “I want to warn against that kind of thinking in the strongest possible terms. It is implausible on several levels. It’s not biologically plausible based on our understanding that race and ethnicity are not genetic constructs. And it’s also not numerically plausible based on these data,” said Dr. Orlando.
While some of the drivers of this inequity have been partially examined, many have not been studied. “As long as this is the case, our ability to fulfill our roles as physicians will be limited in several important ways. Our ability to assess our patients’ individual risk will be missing key information, which will limit the efficacy of shared decision-making, which of course is the cornerstone of MS treatment. In addition, we will continue to struggle to include minority patients in our research studies, and the very design and results of those studies may be misguided, as we will either fail to include these populations, or we will fail to adjust for important confounders,” he said.
New answers, new questions
The neighborhood-level data examined by Dr. Orlando’s group “brings extra information in terms of the negative impact of social determinants of health. The disparity seen in neighborhood living is quite striking,” said Lilyana Amezcua, MD, who served as a discussant for Dr. Orlando’s presentation. The study reinforces findings of her own group in Hispanic and Latinx individuals with MS. Some comorbidities are more common among these groups, which is exacerbated by poor health access.
“We have noted that almost 30% of them also have this comorbidity of hypertension, but what is also observed is that only 7% of them are aware [that they have hypertension],” said Dr. Amezcua, who is an associate professor of neurology at the University of Southern California, Los Angeles.
The findings should prompt further research to understand the impact of systemic racism and neighborhood factors, such as disinvestment in the public and private sectors, underresourced hospitals and clinics, and negative infrastructure. “We need to start discussing the (patient’s) environment so we can better understand the community resources they may have available, as well as create innovative transitional care services. We need to also recognize and accept that structural racism and imbalanced distribution of resources and neighborhoods does restrict educational and economic opportunities, as well as health care access and the safety of these marginalized communities,” said Dr. Amezcua.
Dr. Amezcua has consulted for, received speaking fees from, or served on steering committees or advisory boards for Biogen Idec, Novartis, Genentech, and EMD Serono. She has received research support from the Bristol-Myers Squibb Foundation and Biogen Idec. Dr. Orlando has no relevant financial disclosures.
AT AAN 2022
Mutation testing recommended for advanced and refractory thyroid cancer
A focuses on a definition of advanced thyroid cancer and outlines strategies for mutation testing and targeted treatment.
Mutation testing should not be pursued if cancer burden and disease threat is low, since most thyroid cancers have a very good prognosis and are highly treatable. But 15% of differentiated thyroid cancer cases are locally advanced, and radioiodine refractory differentiated thyroid cancer has a 10-year survival below 50%.
More generally, advanced thyroid cancer has not been well defined clinically. Physicians with experience diagnosing advanced disease may recognize it, but there is no widely accepted definition. “This may be the first time that an expert group of physicians has attempted to define what advanced thyroid cancer is,” said David Shonka, MD, who is a coauthor of the consensus statement, which was published online in Head & Neck. He is an associate professor of otolaryngology/head and neck surgery at the University of Virginia, Charlottesville.
“All patients with advanced thyroid disease and most patients with incurable radioiodine refractory differentiated thyroid cancer should undergo somatic mutational testing,” the authors wrote. “Next-generation sequencing can reveal targetable mutations and potentially give patients affected by advanced thyroid carcinoma systemic treatment options that can prolong survival. These new innovative approaches are changing the landscape of clinical care for patients with advanced thyroid cancer.”
For differentiated thyroid cancer and medullary thyroid carcinoma, the authors created a definition that combines structural factors on imaging, along with surgical findings, and biochemical, histologic, and molecular factors. Anaplastic thyroid cancer should always be considered advanced, even after a complete resection and incidental pathological identification.
The statement also summarizes recent advances in thyroid cancer that have revealed molecular markers which contribute to oncogenesis. Initially, those approaches were applied to indeterminate fine needle biopsies to improve diagnosis. More recent studies used them to match patients to targeted therapies. There are Food and Drug Administration–approved therapies targeting the BRAF and RET mutations, but advanced thyroid cancer is also included in some “basket” trials that test targeted agents against driver mutations across multiple tumor types.
Radioiodine refractory differentiated thyroid cancer had few treatments as recently as 10 years ago. But recent research has shown that multikinase inhibitors improve outcomes, and a range of mutations have been found in this type of thyroid cancer, including BRAF V600E, RET, PIK3CA, and PTEN, and fusions involving RET, NTRK, and ALK. Other mutations have been linked to more aggressive disease. Efforts to personalize treatment also include microsatellite stability status, tumor mutational burden, and programmed death–ligand 1 status as indicators for immunotherapy. “With discovery of many other molecular targets, and emerging literature showcasing promise of matched targeted therapies, we recommend that all patients with advanced thyroid cancer have comprehensive genomic profiling on tumor tissue through (next generation sequencing),” the authors wrote.
These newer and novel therapies have presented physicians with options outside of surgery, chemotherapy, or radiotherapy, which have low efficacy against advanced thyroid cancer. “It is an area in which there has been substantial change. Even 5-7 years ago, patients with advanced thyroid cancer that was not responsive to radioactive iodine or surgery really didn’t have a lot of options. This is a really an exciting and growing field,” Dr. Shonka said.
He specifically cited anaplastic thyroid cancer, which like radioiodine refractory differentiated thyroid cancer has had few treatment options until recently. “Now, if you see a patient with anaplastic thyroid cancer, your knee-jerk reaction should be ‘let’s do molecular testing on this, this is definitely advanced disease.’ If they have a BRAF mutation, that’s targetable, and we can treat this patient with combination therapy that actually improves their survival. So, there’s some exciting stuff happening and probably more coming down the road as we develop new drugs that can target these mutations that we’re identifying.”
Dr. Shonka has no relevant financial disclosures.
A focuses on a definition of advanced thyroid cancer and outlines strategies for mutation testing and targeted treatment.
Mutation testing should not be pursued if cancer burden and disease threat is low, since most thyroid cancers have a very good prognosis and are highly treatable. But 15% of differentiated thyroid cancer cases are locally advanced, and radioiodine refractory differentiated thyroid cancer has a 10-year survival below 50%.
More generally, advanced thyroid cancer has not been well defined clinically. Physicians with experience diagnosing advanced disease may recognize it, but there is no widely accepted definition. “This may be the first time that an expert group of physicians has attempted to define what advanced thyroid cancer is,” said David Shonka, MD, who is a coauthor of the consensus statement, which was published online in Head & Neck. He is an associate professor of otolaryngology/head and neck surgery at the University of Virginia, Charlottesville.
“All patients with advanced thyroid disease and most patients with incurable radioiodine refractory differentiated thyroid cancer should undergo somatic mutational testing,” the authors wrote. “Next-generation sequencing can reveal targetable mutations and potentially give patients affected by advanced thyroid carcinoma systemic treatment options that can prolong survival. These new innovative approaches are changing the landscape of clinical care for patients with advanced thyroid cancer.”
For differentiated thyroid cancer and medullary thyroid carcinoma, the authors created a definition that combines structural factors on imaging, along with surgical findings, and biochemical, histologic, and molecular factors. Anaplastic thyroid cancer should always be considered advanced, even after a complete resection and incidental pathological identification.
The statement also summarizes recent advances in thyroid cancer that have revealed molecular markers which contribute to oncogenesis. Initially, those approaches were applied to indeterminate fine needle biopsies to improve diagnosis. More recent studies used them to match patients to targeted therapies. There are Food and Drug Administration–approved therapies targeting the BRAF and RET mutations, but advanced thyroid cancer is also included in some “basket” trials that test targeted agents against driver mutations across multiple tumor types.
Radioiodine refractory differentiated thyroid cancer had few treatments as recently as 10 years ago. But recent research has shown that multikinase inhibitors improve outcomes, and a range of mutations have been found in this type of thyroid cancer, including BRAF V600E, RET, PIK3CA, and PTEN, and fusions involving RET, NTRK, and ALK. Other mutations have been linked to more aggressive disease. Efforts to personalize treatment also include microsatellite stability status, tumor mutational burden, and programmed death–ligand 1 status as indicators for immunotherapy. “With discovery of many other molecular targets, and emerging literature showcasing promise of matched targeted therapies, we recommend that all patients with advanced thyroid cancer have comprehensive genomic profiling on tumor tissue through (next generation sequencing),” the authors wrote.
These newer and novel therapies have presented physicians with options outside of surgery, chemotherapy, or radiotherapy, which have low efficacy against advanced thyroid cancer. “It is an area in which there has been substantial change. Even 5-7 years ago, patients with advanced thyroid cancer that was not responsive to radioactive iodine or surgery really didn’t have a lot of options. This is a really an exciting and growing field,” Dr. Shonka said.
He specifically cited anaplastic thyroid cancer, which like radioiodine refractory differentiated thyroid cancer has had few treatment options until recently. “Now, if you see a patient with anaplastic thyroid cancer, your knee-jerk reaction should be ‘let’s do molecular testing on this, this is definitely advanced disease.’ If they have a BRAF mutation, that’s targetable, and we can treat this patient with combination therapy that actually improves their survival. So, there’s some exciting stuff happening and probably more coming down the road as we develop new drugs that can target these mutations that we’re identifying.”
Dr. Shonka has no relevant financial disclosures.
A focuses on a definition of advanced thyroid cancer and outlines strategies for mutation testing and targeted treatment.
Mutation testing should not be pursued if cancer burden and disease threat is low, since most thyroid cancers have a very good prognosis and are highly treatable. But 15% of differentiated thyroid cancer cases are locally advanced, and radioiodine refractory differentiated thyroid cancer has a 10-year survival below 50%.
More generally, advanced thyroid cancer has not been well defined clinically. Physicians with experience diagnosing advanced disease may recognize it, but there is no widely accepted definition. “This may be the first time that an expert group of physicians has attempted to define what advanced thyroid cancer is,” said David Shonka, MD, who is a coauthor of the consensus statement, which was published online in Head & Neck. He is an associate professor of otolaryngology/head and neck surgery at the University of Virginia, Charlottesville.
“All patients with advanced thyroid disease and most patients with incurable radioiodine refractory differentiated thyroid cancer should undergo somatic mutational testing,” the authors wrote. “Next-generation sequencing can reveal targetable mutations and potentially give patients affected by advanced thyroid carcinoma systemic treatment options that can prolong survival. These new innovative approaches are changing the landscape of clinical care for patients with advanced thyroid cancer.”
For differentiated thyroid cancer and medullary thyroid carcinoma, the authors created a definition that combines structural factors on imaging, along with surgical findings, and biochemical, histologic, and molecular factors. Anaplastic thyroid cancer should always be considered advanced, even after a complete resection and incidental pathological identification.
The statement also summarizes recent advances in thyroid cancer that have revealed molecular markers which contribute to oncogenesis. Initially, those approaches were applied to indeterminate fine needle biopsies to improve diagnosis. More recent studies used them to match patients to targeted therapies. There are Food and Drug Administration–approved therapies targeting the BRAF and RET mutations, but advanced thyroid cancer is also included in some “basket” trials that test targeted agents against driver mutations across multiple tumor types.
Radioiodine refractory differentiated thyroid cancer had few treatments as recently as 10 years ago. But recent research has shown that multikinase inhibitors improve outcomes, and a range of mutations have been found in this type of thyroid cancer, including BRAF V600E, RET, PIK3CA, and PTEN, and fusions involving RET, NTRK, and ALK. Other mutations have been linked to more aggressive disease. Efforts to personalize treatment also include microsatellite stability status, tumor mutational burden, and programmed death–ligand 1 status as indicators for immunotherapy. “With discovery of many other molecular targets, and emerging literature showcasing promise of matched targeted therapies, we recommend that all patients with advanced thyroid cancer have comprehensive genomic profiling on tumor tissue through (next generation sequencing),” the authors wrote.
These newer and novel therapies have presented physicians with options outside of surgery, chemotherapy, or radiotherapy, which have low efficacy against advanced thyroid cancer. “It is an area in which there has been substantial change. Even 5-7 years ago, patients with advanced thyroid cancer that was not responsive to radioactive iodine or surgery really didn’t have a lot of options. This is a really an exciting and growing field,” Dr. Shonka said.
He specifically cited anaplastic thyroid cancer, which like radioiodine refractory differentiated thyroid cancer has had few treatment options until recently. “Now, if you see a patient with anaplastic thyroid cancer, your knee-jerk reaction should be ‘let’s do molecular testing on this, this is definitely advanced disease.’ If they have a BRAF mutation, that’s targetable, and we can treat this patient with combination therapy that actually improves their survival. So, there’s some exciting stuff happening and probably more coming down the road as we develop new drugs that can target these mutations that we’re identifying.”
Dr. Shonka has no relevant financial disclosures.
FROM HEAD & NECK
Poverty-related stress linked to aggressive head and neck cancer
A humanized mouse model suggests that head and neck cancer growth may stem from chronic stress. The study found that animals had immunophenotypic changes and a greater propensity towards tumor growth and metastasis.
Other studies have shown this may be caused by the lack of access to health care services or poor quality care. but the difference remains even after adjusting for these factors, according to researchers writing in Head and Neck.
Led by Heather A. Himburg, PhD, associate professor of radiation oncology with the Medical College of Wisconsin, Milwaukee, researchers conducted a study of head and neck cancer models in which tumor cells were implanted into a mouse with a humanized immune system.
Their theory was that psychosocial stress may contribute to the growth of head and neck tumors. The stress of poverty, social deprivation and social isolation can lead to the up-regulation of proinflammatory markers in circulating blood leukocytes, and this has been tied to worse outcomes in hematologic malignancies and breast cancer. Many such studies examined social adversity and found an association with greater tumor growth rates and treatment resistance.
Other researchers have used mouse models to study the phenomenon, but the results have been inconclusive. For example, some research linked the beta-adrenergic pathway to head and neck cancer, but clinical trials of beta-blockers showed no benefit, and even potential harm, for patients with head and neck cancers. Those results imply that this pathway does not drive tumor growth and metastasis in the presence of chronic stress.
Previous research used immunocompromised or nonhumanized mice. However, neither type of model reproduces the human tumor microenvironment, which may contribute to ensuing clinical failures. In the new study, researchers describe results from a preclinical model created using a human head and neck cancer xenograft in a mouse with a humanized immune system.
How the study was conducted
The animals were randomly assigned to normal housing of two or three animals from the same litter to a cage, or social isolation from littermates. There were five male and five female animals in each arm, and the animals were housed in their separate conditions for 4 weeks before tumor implantation.
The isolated animals experienced increased growth and metastasis of the xenografts, compared with controls. The results are consistent with findings in immunodeficient or syngeneic mice, but the humanized nature of the new model could lead to better translation of findings into clinical studies. “The humanized model system in this study demonstrated the presence of both human myeloid and lymphoid lineages as well as expression of at least 40 human cytokines. These data indicate that our model is likely to well-represent the human condition and better predict human clinical responses as compared to both immunodeficient and syngeneic models,” the authors wrote.
The researchers also found that chronic stress may act through an immunoregulatory effect, since there was greater human immune infiltrate into the tumors of stressed animals. Increased presence of regulatory components like myeloid-derived suppressor cells or regulatory T cells, or eroded function of tumor-infiltrating lymphocytes, might explain this finding. The researchers also identified a proinflammatory change in peripheral blood monocular cells in the stressed group. When they analyzed samples from patients who were low income earners of less than $45,000 in annual household income, they found a similar pattern. “This suggests that chronic socioeconomic stress may induce a similar proinflammatory immune state as our chronic stress model system,” the authors wrote.
Tumors were also different between the two groups of mice. Tumors in stressed animals had a higher percentage of cancer stem cells, which is associated with more aggressive tumors and worse disease-free survival. The researchers suggested that up-regulated levels of the chemokine SDF-1 seen in the stressed animals may be driving the higher proportion of stem cells through its effects on the CXCR4 receptor, which is expressed by stem cells in various organs and may cause migration, proliferation, and cell survival.
The study was funded by an endowment from Advancing a Healthier Wisconsin and a grant from the National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
A humanized mouse model suggests that head and neck cancer growth may stem from chronic stress. The study found that animals had immunophenotypic changes and a greater propensity towards tumor growth and metastasis.
Other studies have shown this may be caused by the lack of access to health care services or poor quality care. but the difference remains even after adjusting for these factors, according to researchers writing in Head and Neck.
Led by Heather A. Himburg, PhD, associate professor of radiation oncology with the Medical College of Wisconsin, Milwaukee, researchers conducted a study of head and neck cancer models in which tumor cells were implanted into a mouse with a humanized immune system.
Their theory was that psychosocial stress may contribute to the growth of head and neck tumors. The stress of poverty, social deprivation and social isolation can lead to the up-regulation of proinflammatory markers in circulating blood leukocytes, and this has been tied to worse outcomes in hematologic malignancies and breast cancer. Many such studies examined social adversity and found an association with greater tumor growth rates and treatment resistance.
Other researchers have used mouse models to study the phenomenon, but the results have been inconclusive. For example, some research linked the beta-adrenergic pathway to head and neck cancer, but clinical trials of beta-blockers showed no benefit, and even potential harm, for patients with head and neck cancers. Those results imply that this pathway does not drive tumor growth and metastasis in the presence of chronic stress.
Previous research used immunocompromised or nonhumanized mice. However, neither type of model reproduces the human tumor microenvironment, which may contribute to ensuing clinical failures. In the new study, researchers describe results from a preclinical model created using a human head and neck cancer xenograft in a mouse with a humanized immune system.
How the study was conducted
The animals were randomly assigned to normal housing of two or three animals from the same litter to a cage, or social isolation from littermates. There were five male and five female animals in each arm, and the animals were housed in their separate conditions for 4 weeks before tumor implantation.
The isolated animals experienced increased growth and metastasis of the xenografts, compared with controls. The results are consistent with findings in immunodeficient or syngeneic mice, but the humanized nature of the new model could lead to better translation of findings into clinical studies. “The humanized model system in this study demonstrated the presence of both human myeloid and lymphoid lineages as well as expression of at least 40 human cytokines. These data indicate that our model is likely to well-represent the human condition and better predict human clinical responses as compared to both immunodeficient and syngeneic models,” the authors wrote.
The researchers also found that chronic stress may act through an immunoregulatory effect, since there was greater human immune infiltrate into the tumors of stressed animals. Increased presence of regulatory components like myeloid-derived suppressor cells or regulatory T cells, or eroded function of tumor-infiltrating lymphocytes, might explain this finding. The researchers also identified a proinflammatory change in peripheral blood monocular cells in the stressed group. When they analyzed samples from patients who were low income earners of less than $45,000 in annual household income, they found a similar pattern. “This suggests that chronic socioeconomic stress may induce a similar proinflammatory immune state as our chronic stress model system,” the authors wrote.
Tumors were also different between the two groups of mice. Tumors in stressed animals had a higher percentage of cancer stem cells, which is associated with more aggressive tumors and worse disease-free survival. The researchers suggested that up-regulated levels of the chemokine SDF-1 seen in the stressed animals may be driving the higher proportion of stem cells through its effects on the CXCR4 receptor, which is expressed by stem cells in various organs and may cause migration, proliferation, and cell survival.
The study was funded by an endowment from Advancing a Healthier Wisconsin and a grant from the National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
A humanized mouse model suggests that head and neck cancer growth may stem from chronic stress. The study found that animals had immunophenotypic changes and a greater propensity towards tumor growth and metastasis.
Other studies have shown this may be caused by the lack of access to health care services or poor quality care. but the difference remains even after adjusting for these factors, according to researchers writing in Head and Neck.
Led by Heather A. Himburg, PhD, associate professor of radiation oncology with the Medical College of Wisconsin, Milwaukee, researchers conducted a study of head and neck cancer models in which tumor cells were implanted into a mouse with a humanized immune system.
Their theory was that psychosocial stress may contribute to the growth of head and neck tumors. The stress of poverty, social deprivation and social isolation can lead to the up-regulation of proinflammatory markers in circulating blood leukocytes, and this has been tied to worse outcomes in hematologic malignancies and breast cancer. Many such studies examined social adversity and found an association with greater tumor growth rates and treatment resistance.
Other researchers have used mouse models to study the phenomenon, but the results have been inconclusive. For example, some research linked the beta-adrenergic pathway to head and neck cancer, but clinical trials of beta-blockers showed no benefit, and even potential harm, for patients with head and neck cancers. Those results imply that this pathway does not drive tumor growth and metastasis in the presence of chronic stress.
Previous research used immunocompromised or nonhumanized mice. However, neither type of model reproduces the human tumor microenvironment, which may contribute to ensuing clinical failures. In the new study, researchers describe results from a preclinical model created using a human head and neck cancer xenograft in a mouse with a humanized immune system.
How the study was conducted
The animals were randomly assigned to normal housing of two or three animals from the same litter to a cage, or social isolation from littermates. There were five male and five female animals in each arm, and the animals were housed in their separate conditions for 4 weeks before tumor implantation.
The isolated animals experienced increased growth and metastasis of the xenografts, compared with controls. The results are consistent with findings in immunodeficient or syngeneic mice, but the humanized nature of the new model could lead to better translation of findings into clinical studies. “The humanized model system in this study demonstrated the presence of both human myeloid and lymphoid lineages as well as expression of at least 40 human cytokines. These data indicate that our model is likely to well-represent the human condition and better predict human clinical responses as compared to both immunodeficient and syngeneic models,” the authors wrote.
The researchers also found that chronic stress may act through an immunoregulatory effect, since there was greater human immune infiltrate into the tumors of stressed animals. Increased presence of regulatory components like myeloid-derived suppressor cells or regulatory T cells, or eroded function of tumor-infiltrating lymphocytes, might explain this finding. The researchers also identified a proinflammatory change in peripheral blood monocular cells in the stressed group. When they analyzed samples from patients who were low income earners of less than $45,000 in annual household income, they found a similar pattern. “This suggests that chronic socioeconomic stress may induce a similar proinflammatory immune state as our chronic stress model system,” the authors wrote.
Tumors were also different between the two groups of mice. Tumors in stressed animals had a higher percentage of cancer stem cells, which is associated with more aggressive tumors and worse disease-free survival. The researchers suggested that up-regulated levels of the chemokine SDF-1 seen in the stressed animals may be driving the higher proportion of stem cells through its effects on the CXCR4 receptor, which is expressed by stem cells in various organs and may cause migration, proliferation, and cell survival.
The study was funded by an endowment from Advancing a Healthier Wisconsin and a grant from the National Center for Advancing Translational Sciences. The authors reported no conflicts of interest.
FROM HEAD & NECK
Steroids counter ataxia telangiectasia
SEATTLE –
The disease is an autosomal recessive disorder caused by mutations in the ATM gene, which is critical to the response to cellular insults such as DNA breaks, oxidative damage, and other forms of stress. The result is clinical manifestations that range from a suppressed immune system to organ damage and neurological symptoms that typically lead patients to be wheelchair bound by their teenage years.
“It’s really multisystem and a very, very difficult disease for people to live with,” Howard M. Lederman, MD, PhD, said in an interview. Dr. Lederman is a coauthor of the study, which was presented by Stefan Zielen, PhD, professor at the University of Goethe, at the 2022 annual meeting of the American Academy of Neurology.
Various therapies have been developed to improve immunodeficiency, lung disease, and some of the other clinical aspects of the condition, but there is no treatment for its neurological effects. “There’s not really been a good animal model, which has been a big problem in trying to test drugs and design treatment trials,” said Dr. Lederman, professor of pediatrics and medicine at Johns Hopkins University, Baltimore.
The new results may change that. “In the children under the age of 9, there was really a very clear slowdown in the neurodegeneration, and specifically the time that it took for them to lose the ability to ambulate. It’s very exciting, because it’s the first time that anybody has really shown in a double-blind, placebo-controlled, large phase 3 study that any drug has been able to do this. And there were really no steroid side effects, which is the other really remarkable thing about this study,” said Dr. Lederman.
The therapy grew out of a study by researchers in Italy who treated pediatric ataxia telangiectasia patients with corticosteroids and found some transitory improvements in gross motor function, but concerns about long-term exposure to steroids limited its application. EryDel, which specializes in encapsulating therapeutics in red blood cells, became interested and developed a formulation using the patient’s own red blood cells infused with DSP. Reinfused to the patients, the red blood cells slowly release the steroid.
It isn’t clear how dexamethasone works. There are data suggesting that it might lead to transcription of small pieces of the ATM protein, “but that has really not been nailed down in any way at this point. Corticosteroids act on all kinds of cells in all kinds of ways, and so there might be a little bit of this so-called mini-ATM that’s produced, but that may or may not be related to the way in which corticosteroids have a beneficial effect on the rate of neurodegeneration,” said Dr. Lederman.
The treatment process is not easy. Children must have 50-60 cc of blood removed. Red blood cells treated to become porous are exposed to DSP, and then resealed. Then the cells are reinfused. “The whole process takes from beginning to end probably about 3 hours, with a really experienced team of people doing it. And it’s limiting because it’s not easy to put in an IV and take 50 or 60 cc of blood out of children much younger than 5 or 6. The process is now being modified to see whether we could do it with 20 to 30 cc instead,” said Dr. Lederman.
A ‘promising and impressive’ study
The study is promising, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “They were able to show a slower rate of neurological degeneration or duration on both the lower and higher dose compared with the placebo. This is promising and impressive, in the sense that it’s a really large (trial) for a rare condition,” Dr. Johnson, vice chair of research at Virginia Commonwealth University, Richmond, said in an interview.
The study included 164 patients Europe, Australia, Israel, Tunisia, India, and the United States, who received 5-10 mg dexamethasone, 14-22 mg DSP, or placebo. Mean ages in each group ranged from 9.6 to 10.4 years.
In an intention-to-treat analysis, modified International Cooperative Ataxia Rating Scale (mICARS) scores trended toward improvement in the low-dose (–1.37; P = .0847) and high-dose groups (–1.40; P = .0765) when determined by central raters during the COVID-19 pandemic. There was also a trend toward improvement when determined by local raters in the low dose group (–1.73; P = .0720) and a statistically significant change in the high dose group (–2.11; P = .0277). The researchers noted some inconsistency between local and central raters, due to inconsistency of videography and language challenges for central raters.
An intention-to-treat analysis of a subgroup of 89 patients age 6-9, who were compared with natural history data from 245 patients, found a deterioration of mICARS of 3.7 per year, compared with 0.92 in the high-dose group, for a reduction of 75% (P = .020). In the high-dose group, 51.7% had a minimal or significant improvement compared with baseline according to the Clinical Global Impression of Change, as did 29.0% on low dose, and 27.6% in the placebo group.
SEATTLE –
The disease is an autosomal recessive disorder caused by mutations in the ATM gene, which is critical to the response to cellular insults such as DNA breaks, oxidative damage, and other forms of stress. The result is clinical manifestations that range from a suppressed immune system to organ damage and neurological symptoms that typically lead patients to be wheelchair bound by their teenage years.
“It’s really multisystem and a very, very difficult disease for people to live with,” Howard M. Lederman, MD, PhD, said in an interview. Dr. Lederman is a coauthor of the study, which was presented by Stefan Zielen, PhD, professor at the University of Goethe, at the 2022 annual meeting of the American Academy of Neurology.
Various therapies have been developed to improve immunodeficiency, lung disease, and some of the other clinical aspects of the condition, but there is no treatment for its neurological effects. “There’s not really been a good animal model, which has been a big problem in trying to test drugs and design treatment trials,” said Dr. Lederman, professor of pediatrics and medicine at Johns Hopkins University, Baltimore.
The new results may change that. “In the children under the age of 9, there was really a very clear slowdown in the neurodegeneration, and specifically the time that it took for them to lose the ability to ambulate. It’s very exciting, because it’s the first time that anybody has really shown in a double-blind, placebo-controlled, large phase 3 study that any drug has been able to do this. And there were really no steroid side effects, which is the other really remarkable thing about this study,” said Dr. Lederman.
The therapy grew out of a study by researchers in Italy who treated pediatric ataxia telangiectasia patients with corticosteroids and found some transitory improvements in gross motor function, but concerns about long-term exposure to steroids limited its application. EryDel, which specializes in encapsulating therapeutics in red blood cells, became interested and developed a formulation using the patient’s own red blood cells infused with DSP. Reinfused to the patients, the red blood cells slowly release the steroid.
It isn’t clear how dexamethasone works. There are data suggesting that it might lead to transcription of small pieces of the ATM protein, “but that has really not been nailed down in any way at this point. Corticosteroids act on all kinds of cells in all kinds of ways, and so there might be a little bit of this so-called mini-ATM that’s produced, but that may or may not be related to the way in which corticosteroids have a beneficial effect on the rate of neurodegeneration,” said Dr. Lederman.
The treatment process is not easy. Children must have 50-60 cc of blood removed. Red blood cells treated to become porous are exposed to DSP, and then resealed. Then the cells are reinfused. “The whole process takes from beginning to end probably about 3 hours, with a really experienced team of people doing it. And it’s limiting because it’s not easy to put in an IV and take 50 or 60 cc of blood out of children much younger than 5 or 6. The process is now being modified to see whether we could do it with 20 to 30 cc instead,” said Dr. Lederman.
A ‘promising and impressive’ study
The study is promising, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “They were able to show a slower rate of neurological degeneration or duration on both the lower and higher dose compared with the placebo. This is promising and impressive, in the sense that it’s a really large (trial) for a rare condition,” Dr. Johnson, vice chair of research at Virginia Commonwealth University, Richmond, said in an interview.
The study included 164 patients Europe, Australia, Israel, Tunisia, India, and the United States, who received 5-10 mg dexamethasone, 14-22 mg DSP, or placebo. Mean ages in each group ranged from 9.6 to 10.4 years.
In an intention-to-treat analysis, modified International Cooperative Ataxia Rating Scale (mICARS) scores trended toward improvement in the low-dose (–1.37; P = .0847) and high-dose groups (–1.40; P = .0765) when determined by central raters during the COVID-19 pandemic. There was also a trend toward improvement when determined by local raters in the low dose group (–1.73; P = .0720) and a statistically significant change in the high dose group (–2.11; P = .0277). The researchers noted some inconsistency between local and central raters, due to inconsistency of videography and language challenges for central raters.
An intention-to-treat analysis of a subgroup of 89 patients age 6-9, who were compared with natural history data from 245 patients, found a deterioration of mICARS of 3.7 per year, compared with 0.92 in the high-dose group, for a reduction of 75% (P = .020). In the high-dose group, 51.7% had a minimal or significant improvement compared with baseline according to the Clinical Global Impression of Change, as did 29.0% on low dose, and 27.6% in the placebo group.
SEATTLE –
The disease is an autosomal recessive disorder caused by mutations in the ATM gene, which is critical to the response to cellular insults such as DNA breaks, oxidative damage, and other forms of stress. The result is clinical manifestations that range from a suppressed immune system to organ damage and neurological symptoms that typically lead patients to be wheelchair bound by their teenage years.
“It’s really multisystem and a very, very difficult disease for people to live with,” Howard M. Lederman, MD, PhD, said in an interview. Dr. Lederman is a coauthor of the study, which was presented by Stefan Zielen, PhD, professor at the University of Goethe, at the 2022 annual meeting of the American Academy of Neurology.
Various therapies have been developed to improve immunodeficiency, lung disease, and some of the other clinical aspects of the condition, but there is no treatment for its neurological effects. “There’s not really been a good animal model, which has been a big problem in trying to test drugs and design treatment trials,” said Dr. Lederman, professor of pediatrics and medicine at Johns Hopkins University, Baltimore.
The new results may change that. “In the children under the age of 9, there was really a very clear slowdown in the neurodegeneration, and specifically the time that it took for them to lose the ability to ambulate. It’s very exciting, because it’s the first time that anybody has really shown in a double-blind, placebo-controlled, large phase 3 study that any drug has been able to do this. And there were really no steroid side effects, which is the other really remarkable thing about this study,” said Dr. Lederman.
The therapy grew out of a study by researchers in Italy who treated pediatric ataxia telangiectasia patients with corticosteroids and found some transitory improvements in gross motor function, but concerns about long-term exposure to steroids limited its application. EryDel, which specializes in encapsulating therapeutics in red blood cells, became interested and developed a formulation using the patient’s own red blood cells infused with DSP. Reinfused to the patients, the red blood cells slowly release the steroid.
It isn’t clear how dexamethasone works. There are data suggesting that it might lead to transcription of small pieces of the ATM protein, “but that has really not been nailed down in any way at this point. Corticosteroids act on all kinds of cells in all kinds of ways, and so there might be a little bit of this so-called mini-ATM that’s produced, but that may or may not be related to the way in which corticosteroids have a beneficial effect on the rate of neurodegeneration,” said Dr. Lederman.
The treatment process is not easy. Children must have 50-60 cc of blood removed. Red blood cells treated to become porous are exposed to DSP, and then resealed. Then the cells are reinfused. “The whole process takes from beginning to end probably about 3 hours, with a really experienced team of people doing it. And it’s limiting because it’s not easy to put in an IV and take 50 or 60 cc of blood out of children much younger than 5 or 6. The process is now being modified to see whether we could do it with 20 to 30 cc instead,” said Dr. Lederman.
A ‘promising and impressive’ study
The study is promising, according to Nicholas Johnson, MD, who comoderated the session where the study was presented. “They were able to show a slower rate of neurological degeneration or duration on both the lower and higher dose compared with the placebo. This is promising and impressive, in the sense that it’s a really large (trial) for a rare condition,” Dr. Johnson, vice chair of research at Virginia Commonwealth University, Richmond, said in an interview.
The study included 164 patients Europe, Australia, Israel, Tunisia, India, and the United States, who received 5-10 mg dexamethasone, 14-22 mg DSP, or placebo. Mean ages in each group ranged from 9.6 to 10.4 years.
In an intention-to-treat analysis, modified International Cooperative Ataxia Rating Scale (mICARS) scores trended toward improvement in the low-dose (–1.37; P = .0847) and high-dose groups (–1.40; P = .0765) when determined by central raters during the COVID-19 pandemic. There was also a trend toward improvement when determined by local raters in the low dose group (–1.73; P = .0720) and a statistically significant change in the high dose group (–2.11; P = .0277). The researchers noted some inconsistency between local and central raters, due to inconsistency of videography and language challenges for central raters.
An intention-to-treat analysis of a subgroup of 89 patients age 6-9, who were compared with natural history data from 245 patients, found a deterioration of mICARS of 3.7 per year, compared with 0.92 in the high-dose group, for a reduction of 75% (P = .020). In the high-dose group, 51.7% had a minimal or significant improvement compared with baseline according to the Clinical Global Impression of Change, as did 29.0% on low dose, and 27.6% in the placebo group.
AT AAN 2022
AGA Clinical Practice Update: Expert review on personalizing GERD management
A recent American Gastroenterological Association Clinical Practice Update for evaluation and management of gastroesophageal reflux disease (GERD) focuses on delivering personalized diagnostic and therapeutic strategies.
The document includes new advice on use of upfront objective testing for isolated extraesophageal symptoms, confirmation of GERD diagnosis prior to long-term GERD therapy even in PPI responders, as well as important elements focused on personalization of therapy.
Although GERD is common, with an estimated 30% of people in the United States experiencing symptoms, up to half of all individuals on proton pump inhibitor (PPI) therapy report incomplete symptom improvement. That could be due to the heterogeneous nature of symptoms, which may include heartburn and regurgitation, chest pain, and cough or sore throat, among others. Other conditions may produce similar symptoms or could be exacerbated by the presence of GERD.
The authors of the expert review, published in Clinical Gastroenterology and Hepatology, note that these considerations have driven increased interest in personalized approaches to the management of GERD. The practice update includes sections on how to approach GERD symptoms in the clinic, personalized diagnosis related to GERD symptoms, and precision management.
In the initial management, the authors offer advice on involving the patient in creating a care plan, patient education, and conducting a 4- to 8-week PPI trial in patients with heartburn, regurgitation, or noncardiac chest pains without accompanying alarm signals. If symptoms don’t improve to the patient’s satisfaction, dosing can be boosted to twice per day, or a more effective acid suppressor can be substituted and continued at a once-daily dose. When the response to PPIs is adequate, the dose should be reduced until the lowest effective dose is reached, or the patient could potentially be moved to H2 receptor antagonists or other antacids. However, patients with erosive esophagitis, biopsy-confirmed Barrett’s esophagus, or peptic stricture must stay on long-term PPI therapy.
The authors also gave advice on when to conduct objective testing. When a PPI trial doesn’t adequately address troublesome heartburn, regurgitation, and/or noncardiac chest pain, or if alarm systems are present, endoscopy should be employed to look for erosive reflux disease or long-segment Barrett’s esophagus as conclusive evidence for GERD. If these are absent, prolonged wireless pH monitoring while a patient is off medication is suggested. In addition, patients with extraesophageal symptoms suspected to be caused by reflux should undergo upfront objective reflux testing while off PPI therapy rather than doing an empiric PPI trial.
The authors advise that, if patients don’t have proven GERD and are continued on PPI therapy, they should be evaluated within 12 months to ensure that the therapy and dose are appropriate. Physicians should offer endoscopy with prolonged wireless reflux monitoring in the absence of PPI therapy (ideally after 2-4 weeks of withdrawal) to confirm that long-term PPI therapy is needed.
In the section on personalization of disease management, the authors note that ambulatory reflux monitoring and upper gastrointestinal endoscopy can be used to guide management of GERD. When upper GI endoscopy reveals no erosive findings and esophageal acid exposure time (AET) is less than 4% throughout all days of prolonged wireless pH monitoring, the physician can conclude that the patient has no pathologic gastroesophageal reflux and is likely to have a functional esophageal disorder. In contrast, erosive findings during upper GI endoscopy and/or AET more than 4% across at least 1 day of wireless pH monitoring suggests a GERD diagnosis.
Optimization of PPI is important among patients with GERD, and the authors stress that patients should be educated about the safety of PPI use.
Adjunctive pharmacotherapy is useful and can include alginate antacids for breakthrough symptoms, H2RAs for nocturnal symptoms, baclofen to counter regurgitation or belching, and prokinetics for accompanying gastroparesis. The choice of medications depends on the phenotype, and they should not be used empirically.
For patients with functional heartburn or reflux disease linked to esophageal hypervigilance, reflux sensitivity, or behavioral disorders, options include pharmacologic neuromodulation, hypnotherapy provided by a behavioral therapist, cognitive behavioral therapy, and diaphragmatic breathing and relaxation.
If symptoms persist despite efforts at optimization of treatments and lifestyle factors, ambulatory 24-hour pH-impedance monitoring on PPI can be used to investigate mechanistic causes, especially when there is no known antireflux barrier abnormality, but the technique requires expertise to correctly interpret. This can ensure that the symptoms are not due to reflux hypersensitivity, rumination syndrome, or a belching disorder. When symptoms are confirmed to be treatment resistant, therapy should be escalated, using a strategy that incorporates a pattern of reflux, integrity of the antireflux barrier, obesity if present, and psychological factors.
Surgical options for confirmed GERD include laparoscopic fundoplication and magnetic sphincter augmentation. Transoral incisionless fundoplication can be performed endoscopically in selected patients. For obese patients with confirmed GERD, Roux-en-Y gastric bypass is effective at reducing reflux and can be used as a salvage treatment for nonobese patients. Sleeve gastrectomy may exacerbate GERD.
The authors reported relationships with Medtronic, Diversatek, Ironwood, and Takeda. The authors also reported funding from National Institutes of Health grants.
A recent American Gastroenterological Association Clinical Practice Update for evaluation and management of gastroesophageal reflux disease (GERD) focuses on delivering personalized diagnostic and therapeutic strategies.
The document includes new advice on use of upfront objective testing for isolated extraesophageal symptoms, confirmation of GERD diagnosis prior to long-term GERD therapy even in PPI responders, as well as important elements focused on personalization of therapy.
Although GERD is common, with an estimated 30% of people in the United States experiencing symptoms, up to half of all individuals on proton pump inhibitor (PPI) therapy report incomplete symptom improvement. That could be due to the heterogeneous nature of symptoms, which may include heartburn and regurgitation, chest pain, and cough or sore throat, among others. Other conditions may produce similar symptoms or could be exacerbated by the presence of GERD.
The authors of the expert review, published in Clinical Gastroenterology and Hepatology, note that these considerations have driven increased interest in personalized approaches to the management of GERD. The practice update includes sections on how to approach GERD symptoms in the clinic, personalized diagnosis related to GERD symptoms, and precision management.
In the initial management, the authors offer advice on involving the patient in creating a care plan, patient education, and conducting a 4- to 8-week PPI trial in patients with heartburn, regurgitation, or noncardiac chest pains without accompanying alarm signals. If symptoms don’t improve to the patient’s satisfaction, dosing can be boosted to twice per day, or a more effective acid suppressor can be substituted and continued at a once-daily dose. When the response to PPIs is adequate, the dose should be reduced until the lowest effective dose is reached, or the patient could potentially be moved to H2 receptor antagonists or other antacids. However, patients with erosive esophagitis, biopsy-confirmed Barrett’s esophagus, or peptic stricture must stay on long-term PPI therapy.
The authors also gave advice on when to conduct objective testing. When a PPI trial doesn’t adequately address troublesome heartburn, regurgitation, and/or noncardiac chest pain, or if alarm systems are present, endoscopy should be employed to look for erosive reflux disease or long-segment Barrett’s esophagus as conclusive evidence for GERD. If these are absent, prolonged wireless pH monitoring while a patient is off medication is suggested. In addition, patients with extraesophageal symptoms suspected to be caused by reflux should undergo upfront objective reflux testing while off PPI therapy rather than doing an empiric PPI trial.
The authors advise that, if patients don’t have proven GERD and are continued on PPI therapy, they should be evaluated within 12 months to ensure that the therapy and dose are appropriate. Physicians should offer endoscopy with prolonged wireless reflux monitoring in the absence of PPI therapy (ideally after 2-4 weeks of withdrawal) to confirm that long-term PPI therapy is needed.
In the section on personalization of disease management, the authors note that ambulatory reflux monitoring and upper gastrointestinal endoscopy can be used to guide management of GERD. When upper GI endoscopy reveals no erosive findings and esophageal acid exposure time (AET) is less than 4% throughout all days of prolonged wireless pH monitoring, the physician can conclude that the patient has no pathologic gastroesophageal reflux and is likely to have a functional esophageal disorder. In contrast, erosive findings during upper GI endoscopy and/or AET more than 4% across at least 1 day of wireless pH monitoring suggests a GERD diagnosis.
Optimization of PPI is important among patients with GERD, and the authors stress that patients should be educated about the safety of PPI use.
Adjunctive pharmacotherapy is useful and can include alginate antacids for breakthrough symptoms, H2RAs for nocturnal symptoms, baclofen to counter regurgitation or belching, and prokinetics for accompanying gastroparesis. The choice of medications depends on the phenotype, and they should not be used empirically.
For patients with functional heartburn or reflux disease linked to esophageal hypervigilance, reflux sensitivity, or behavioral disorders, options include pharmacologic neuromodulation, hypnotherapy provided by a behavioral therapist, cognitive behavioral therapy, and diaphragmatic breathing and relaxation.
If symptoms persist despite efforts at optimization of treatments and lifestyle factors, ambulatory 24-hour pH-impedance monitoring on PPI can be used to investigate mechanistic causes, especially when there is no known antireflux barrier abnormality, but the technique requires expertise to correctly interpret. This can ensure that the symptoms are not due to reflux hypersensitivity, rumination syndrome, or a belching disorder. When symptoms are confirmed to be treatment resistant, therapy should be escalated, using a strategy that incorporates a pattern of reflux, integrity of the antireflux barrier, obesity if present, and psychological factors.
Surgical options for confirmed GERD include laparoscopic fundoplication and magnetic sphincter augmentation. Transoral incisionless fundoplication can be performed endoscopically in selected patients. For obese patients with confirmed GERD, Roux-en-Y gastric bypass is effective at reducing reflux and can be used as a salvage treatment for nonobese patients. Sleeve gastrectomy may exacerbate GERD.
The authors reported relationships with Medtronic, Diversatek, Ironwood, and Takeda. The authors also reported funding from National Institutes of Health grants.
A recent American Gastroenterological Association Clinical Practice Update for evaluation and management of gastroesophageal reflux disease (GERD) focuses on delivering personalized diagnostic and therapeutic strategies.
The document includes new advice on use of upfront objective testing for isolated extraesophageal symptoms, confirmation of GERD diagnosis prior to long-term GERD therapy even in PPI responders, as well as important elements focused on personalization of therapy.
Although GERD is common, with an estimated 30% of people in the United States experiencing symptoms, up to half of all individuals on proton pump inhibitor (PPI) therapy report incomplete symptom improvement. That could be due to the heterogeneous nature of symptoms, which may include heartburn and regurgitation, chest pain, and cough or sore throat, among others. Other conditions may produce similar symptoms or could be exacerbated by the presence of GERD.
The authors of the expert review, published in Clinical Gastroenterology and Hepatology, note that these considerations have driven increased interest in personalized approaches to the management of GERD. The practice update includes sections on how to approach GERD symptoms in the clinic, personalized diagnosis related to GERD symptoms, and precision management.
In the initial management, the authors offer advice on involving the patient in creating a care plan, patient education, and conducting a 4- to 8-week PPI trial in patients with heartburn, regurgitation, or noncardiac chest pains without accompanying alarm signals. If symptoms don’t improve to the patient’s satisfaction, dosing can be boosted to twice per day, or a more effective acid suppressor can be substituted and continued at a once-daily dose. When the response to PPIs is adequate, the dose should be reduced until the lowest effective dose is reached, or the patient could potentially be moved to H2 receptor antagonists or other antacids. However, patients with erosive esophagitis, biopsy-confirmed Barrett’s esophagus, or peptic stricture must stay on long-term PPI therapy.
The authors also gave advice on when to conduct objective testing. When a PPI trial doesn’t adequately address troublesome heartburn, regurgitation, and/or noncardiac chest pain, or if alarm systems are present, endoscopy should be employed to look for erosive reflux disease or long-segment Barrett’s esophagus as conclusive evidence for GERD. If these are absent, prolonged wireless pH monitoring while a patient is off medication is suggested. In addition, patients with extraesophageal symptoms suspected to be caused by reflux should undergo upfront objective reflux testing while off PPI therapy rather than doing an empiric PPI trial.
The authors advise that, if patients don’t have proven GERD and are continued on PPI therapy, they should be evaluated within 12 months to ensure that the therapy and dose are appropriate. Physicians should offer endoscopy with prolonged wireless reflux monitoring in the absence of PPI therapy (ideally after 2-4 weeks of withdrawal) to confirm that long-term PPI therapy is needed.
In the section on personalization of disease management, the authors note that ambulatory reflux monitoring and upper gastrointestinal endoscopy can be used to guide management of GERD. When upper GI endoscopy reveals no erosive findings and esophageal acid exposure time (AET) is less than 4% throughout all days of prolonged wireless pH monitoring, the physician can conclude that the patient has no pathologic gastroesophageal reflux and is likely to have a functional esophageal disorder. In contrast, erosive findings during upper GI endoscopy and/or AET more than 4% across at least 1 day of wireless pH monitoring suggests a GERD diagnosis.
Optimization of PPI is important among patients with GERD, and the authors stress that patients should be educated about the safety of PPI use.
Adjunctive pharmacotherapy is useful and can include alginate antacids for breakthrough symptoms, H2RAs for nocturnal symptoms, baclofen to counter regurgitation or belching, and prokinetics for accompanying gastroparesis. The choice of medications depends on the phenotype, and they should not be used empirically.
For patients with functional heartburn or reflux disease linked to esophageal hypervigilance, reflux sensitivity, or behavioral disorders, options include pharmacologic neuromodulation, hypnotherapy provided by a behavioral therapist, cognitive behavioral therapy, and diaphragmatic breathing and relaxation.
If symptoms persist despite efforts at optimization of treatments and lifestyle factors, ambulatory 24-hour pH-impedance monitoring on PPI can be used to investigate mechanistic causes, especially when there is no known antireflux barrier abnormality, but the technique requires expertise to correctly interpret. This can ensure that the symptoms are not due to reflux hypersensitivity, rumination syndrome, or a belching disorder. When symptoms are confirmed to be treatment resistant, therapy should be escalated, using a strategy that incorporates a pattern of reflux, integrity of the antireflux barrier, obesity if present, and psychological factors.
Surgical options for confirmed GERD include laparoscopic fundoplication and magnetic sphincter augmentation. Transoral incisionless fundoplication can be performed endoscopically in selected patients. For obese patients with confirmed GERD, Roux-en-Y gastric bypass is effective at reducing reflux and can be used as a salvage treatment for nonobese patients. Sleeve gastrectomy may exacerbate GERD.
The authors reported relationships with Medtronic, Diversatek, Ironwood, and Takeda. The authors also reported funding from National Institutes of Health grants.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
AGA Clinical Practice Update: Expert review on deprescribing PPIs
An American Gastroenterological Association practice update on deprescribing proton-pump inhibitors (PPIs) delineates conditions under which drug withdrawal should be considered, and acknowledges that conversations between physicians and patients can be complicated. An inappropriate decision to discontinue PPI therapy can have significant consequences for the patient, while continued inappropriate use raises health care costs and may rarely lead to adverse effects.
One purpose of the update is to provide guidance when patients and providers don’t have the resources to systematically examine the issue, especially when other medical concerns may be in play. The authors also suggested that physicians include pharmacists in the employment of the best practices advice.
“None of these statements represents a radical departure from previously published guidance on PPI appropriateness and deprescribing: Our [recommendations] simply seek to summarize the evidence and to provide the clinician with a single document which distills the evidence down into clinically applicable guidance statements,” Laura Targownik, MD, associate professor of medicine at the University of Toronto and corresponding author of the practice update published in Gastroenterology said in an interview.
“PPIs are highly effective medications for specific gastrointestinal conditions, and are largely safe. However, PPIs are often used in situations where they have minimal and no proven benefit, leading to unnecessary health care spending and unnecessary exposure to drugs. Our paper helps clinicians identify which patients require long-term PPI use as well as those who may be using them unnecessarily, and provides actionable advice on how to deprescribe PPIs from those deemed to be using them without clear benefit,” said Dr. Targownik.
An estimated 7%-15% of health care patients in general and 40% of those over 70 use PPIs at any given time, making them among the most commonly used drugs. About one in four patients who start PPIs will use them for a year or more. Aside from their use for acid-mediated upper gastrointestinal conditions, PPIs often find use for less well-defined complaints. Since PPIs are available over the counter, physicians may not even be involved in a patient’s decision to use them.
Although PPI use has been associated with adverse events, including chronic kidney disease, fractures, dementia, and greater risk of COVID-19 infection, there is not high-quality evidence to suggest that PPIs are directly responsible for any of these adverse events.
The authors suggested the primary care provider should periodically review and document the complaints or indications that prompt PPI use. When a patient is found to have no chronic condition that PPIs could reasonably address, the physician should consider a trial withdrawal. Patients who take PPIs twice daily for a known chronic condition should be considered for a reduction to a once-daily dose.
In general, PPI discontinuation is not a good option for most patients with complicated gastroesophageal reflux disease, such as those with a history of severe erosive esophagitis, esophageal ulcer, or peptic stricture. The same is true for patients with Barrett’s esophagus, eosinophilic esophagitis, or idiopathic pulmonary fibrosis.
Before any deprescribing is considered, the patient should be evaluated for risk of upper gastrointestinal bleeding, and those at high risk are not candidates for PPI deprescribing.
When the decision is made to withdraw PPIs, the patient should be advised of an increased risk of transient upper gastrointestinal symptoms caused by rebound acid hypersecretion.
The withdrawal of PPIs can be done abruptly, or the dose can be tapered gradually.
PPI-associated adverse events should not be a consideration when discussing the option of withdrawing from PPIs. Instead, the decision should be based on the absence of a specific reason for their use. A history of such adverse events, or a current adverse event, should not be a sole reason for discontinuation, nor should risk factors associated with risk of adverse events. Concerns about adverse events have driven recent interest in reducing use of PPIs, but those adverse events were identified through retrospective studies and may be only associated with PPI use rather than caused by it. In many cases there is no plausible mechanistic cause, and no clinical trials have demonstrated increased adverse events in PPI users.
Three-quarters of physicians say they have altered treatment plans for patients because of concerns about PPI adverse events, and 80% say they would advise patients to withdraw PPIs if they learned the patient was at increased risk of upper gastrointestinal bleeding. Unnecessary withdrawal can lead to recurrent symptoms and complications when PPIs are effective treatments. “Therefore, physicians should not use concern about unproven complications of PPI use as a justification for PPI deprescribing if there remain ongoing valid indications for PPI use,” the authors wrote.
Dr. Targownik has received investigator-initiated funding from Janssen Canada and served on advisory boards for AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Janssen Canada, Roche Canada, and Sandoz Canada. She is the lead on an IBD registry supported by AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Amgen Canada, Roche Canada, and Sandoz Canada. None of the companies with whom Dr. Targownik has a relation are involved in the manufacturing, distribution, or sales of PPIs or any other agents mentioned in the manuscript.
An American Gastroenterological Association practice update on deprescribing proton-pump inhibitors (PPIs) delineates conditions under which drug withdrawal should be considered, and acknowledges that conversations between physicians and patients can be complicated. An inappropriate decision to discontinue PPI therapy can have significant consequences for the patient, while continued inappropriate use raises health care costs and may rarely lead to adverse effects.
One purpose of the update is to provide guidance when patients and providers don’t have the resources to systematically examine the issue, especially when other medical concerns may be in play. The authors also suggested that physicians include pharmacists in the employment of the best practices advice.
“None of these statements represents a radical departure from previously published guidance on PPI appropriateness and deprescribing: Our [recommendations] simply seek to summarize the evidence and to provide the clinician with a single document which distills the evidence down into clinically applicable guidance statements,” Laura Targownik, MD, associate professor of medicine at the University of Toronto and corresponding author of the practice update published in Gastroenterology said in an interview.
“PPIs are highly effective medications for specific gastrointestinal conditions, and are largely safe. However, PPIs are often used in situations where they have minimal and no proven benefit, leading to unnecessary health care spending and unnecessary exposure to drugs. Our paper helps clinicians identify which patients require long-term PPI use as well as those who may be using them unnecessarily, and provides actionable advice on how to deprescribe PPIs from those deemed to be using them without clear benefit,” said Dr. Targownik.
An estimated 7%-15% of health care patients in general and 40% of those over 70 use PPIs at any given time, making them among the most commonly used drugs. About one in four patients who start PPIs will use them for a year or more. Aside from their use for acid-mediated upper gastrointestinal conditions, PPIs often find use for less well-defined complaints. Since PPIs are available over the counter, physicians may not even be involved in a patient’s decision to use them.
Although PPI use has been associated with adverse events, including chronic kidney disease, fractures, dementia, and greater risk of COVID-19 infection, there is not high-quality evidence to suggest that PPIs are directly responsible for any of these adverse events.
The authors suggested the primary care provider should periodically review and document the complaints or indications that prompt PPI use. When a patient is found to have no chronic condition that PPIs could reasonably address, the physician should consider a trial withdrawal. Patients who take PPIs twice daily for a known chronic condition should be considered for a reduction to a once-daily dose.
In general, PPI discontinuation is not a good option for most patients with complicated gastroesophageal reflux disease, such as those with a history of severe erosive esophagitis, esophageal ulcer, or peptic stricture. The same is true for patients with Barrett’s esophagus, eosinophilic esophagitis, or idiopathic pulmonary fibrosis.
Before any deprescribing is considered, the patient should be evaluated for risk of upper gastrointestinal bleeding, and those at high risk are not candidates for PPI deprescribing.
When the decision is made to withdraw PPIs, the patient should be advised of an increased risk of transient upper gastrointestinal symptoms caused by rebound acid hypersecretion.
The withdrawal of PPIs can be done abruptly, or the dose can be tapered gradually.
PPI-associated adverse events should not be a consideration when discussing the option of withdrawing from PPIs. Instead, the decision should be based on the absence of a specific reason for their use. A history of such adverse events, or a current adverse event, should not be a sole reason for discontinuation, nor should risk factors associated with risk of adverse events. Concerns about adverse events have driven recent interest in reducing use of PPIs, but those adverse events were identified through retrospective studies and may be only associated with PPI use rather than caused by it. In many cases there is no plausible mechanistic cause, and no clinical trials have demonstrated increased adverse events in PPI users.
Three-quarters of physicians say they have altered treatment plans for patients because of concerns about PPI adverse events, and 80% say they would advise patients to withdraw PPIs if they learned the patient was at increased risk of upper gastrointestinal bleeding. Unnecessary withdrawal can lead to recurrent symptoms and complications when PPIs are effective treatments. “Therefore, physicians should not use concern about unproven complications of PPI use as a justification for PPI deprescribing if there remain ongoing valid indications for PPI use,” the authors wrote.
Dr. Targownik has received investigator-initiated funding from Janssen Canada and served on advisory boards for AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Janssen Canada, Roche Canada, and Sandoz Canada. She is the lead on an IBD registry supported by AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Amgen Canada, Roche Canada, and Sandoz Canada. None of the companies with whom Dr. Targownik has a relation are involved in the manufacturing, distribution, or sales of PPIs or any other agents mentioned in the manuscript.
An American Gastroenterological Association practice update on deprescribing proton-pump inhibitors (PPIs) delineates conditions under which drug withdrawal should be considered, and acknowledges that conversations between physicians and patients can be complicated. An inappropriate decision to discontinue PPI therapy can have significant consequences for the patient, while continued inappropriate use raises health care costs and may rarely lead to adverse effects.
One purpose of the update is to provide guidance when patients and providers don’t have the resources to systematically examine the issue, especially when other medical concerns may be in play. The authors also suggested that physicians include pharmacists in the employment of the best practices advice.
“None of these statements represents a radical departure from previously published guidance on PPI appropriateness and deprescribing: Our [recommendations] simply seek to summarize the evidence and to provide the clinician with a single document which distills the evidence down into clinically applicable guidance statements,” Laura Targownik, MD, associate professor of medicine at the University of Toronto and corresponding author of the practice update published in Gastroenterology said in an interview.
“PPIs are highly effective medications for specific gastrointestinal conditions, and are largely safe. However, PPIs are often used in situations where they have minimal and no proven benefit, leading to unnecessary health care spending and unnecessary exposure to drugs. Our paper helps clinicians identify which patients require long-term PPI use as well as those who may be using them unnecessarily, and provides actionable advice on how to deprescribe PPIs from those deemed to be using them without clear benefit,” said Dr. Targownik.
An estimated 7%-15% of health care patients in general and 40% of those over 70 use PPIs at any given time, making them among the most commonly used drugs. About one in four patients who start PPIs will use them for a year or more. Aside from their use for acid-mediated upper gastrointestinal conditions, PPIs often find use for less well-defined complaints. Since PPIs are available over the counter, physicians may not even be involved in a patient’s decision to use them.
Although PPI use has been associated with adverse events, including chronic kidney disease, fractures, dementia, and greater risk of COVID-19 infection, there is not high-quality evidence to suggest that PPIs are directly responsible for any of these adverse events.
The authors suggested the primary care provider should periodically review and document the complaints or indications that prompt PPI use. When a patient is found to have no chronic condition that PPIs could reasonably address, the physician should consider a trial withdrawal. Patients who take PPIs twice daily for a known chronic condition should be considered for a reduction to a once-daily dose.
In general, PPI discontinuation is not a good option for most patients with complicated gastroesophageal reflux disease, such as those with a history of severe erosive esophagitis, esophageal ulcer, or peptic stricture. The same is true for patients with Barrett’s esophagus, eosinophilic esophagitis, or idiopathic pulmonary fibrosis.
Before any deprescribing is considered, the patient should be evaluated for risk of upper gastrointestinal bleeding, and those at high risk are not candidates for PPI deprescribing.
When the decision is made to withdraw PPIs, the patient should be advised of an increased risk of transient upper gastrointestinal symptoms caused by rebound acid hypersecretion.
The withdrawal of PPIs can be done abruptly, or the dose can be tapered gradually.
PPI-associated adverse events should not be a consideration when discussing the option of withdrawing from PPIs. Instead, the decision should be based on the absence of a specific reason for their use. A history of such adverse events, or a current adverse event, should not be a sole reason for discontinuation, nor should risk factors associated with risk of adverse events. Concerns about adverse events have driven recent interest in reducing use of PPIs, but those adverse events were identified through retrospective studies and may be only associated with PPI use rather than caused by it. In many cases there is no plausible mechanistic cause, and no clinical trials have demonstrated increased adverse events in PPI users.
Three-quarters of physicians say they have altered treatment plans for patients because of concerns about PPI adverse events, and 80% say they would advise patients to withdraw PPIs if they learned the patient was at increased risk of upper gastrointestinal bleeding. Unnecessary withdrawal can lead to recurrent symptoms and complications when PPIs are effective treatments. “Therefore, physicians should not use concern about unproven complications of PPI use as a justification for PPI deprescribing if there remain ongoing valid indications for PPI use,” the authors wrote.
Dr. Targownik has received investigator-initiated funding from Janssen Canada and served on advisory boards for AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Janssen Canada, Roche Canada, and Sandoz Canada. She is the lead on an IBD registry supported by AbbVie Canada, Takeda Canada, Merck Canada, Pfizer Canada, Amgen Canada, Roche Canada, and Sandoz Canada. None of the companies with whom Dr. Targownik has a relation are involved in the manufacturing, distribution, or sales of PPIs or any other agents mentioned in the manuscript.
FROM GASTROENTEROLOGY