User login
Heavy menstrual bleeding in teens often linked to bleeding disorders
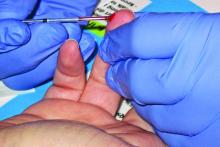
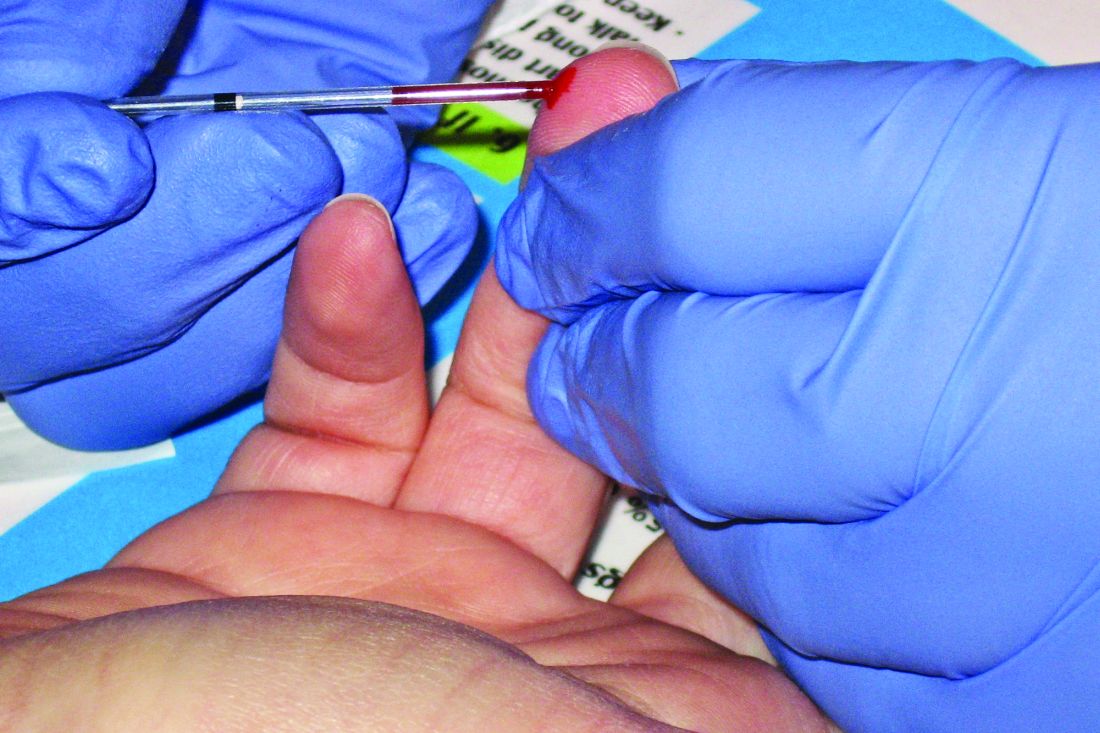
Over one-third of adolescents presenting with heavy menstrual bleeding were diagnosed with a bleeding disorder after screening, according to results of a retrospective study.
The high incidence of bleeding disorders detected argues for routine screening of adolescents with heavy menstrual bleeding (HMB), Brooke O’Brien, MD, of the University of Queensland, Brisbane, Australia, and her colleagues wrote in the Journal of Pediatric & Adolescent Gynecology.
“These findings support comprehensive and systematic hemostatic evaluation in adolescents with HMB,” Dr. O’Brien and her colleagues wrote. “A higher level of awareness of bleeding disorders as a cause for HMB in adolescence, especially [von Willebrand disease] and platelet function disorders, is needed and close multidisciplinary collaboration between the pediatric and adolescent gynecologist and hematologist in a specialized tertiary center should be established in the management of these patients.”
In their study, Dr. O’Brien and her colleagues retrospectively evaluated 124 adolescents with HMB at a pediatric and adolescent gynecology tertiary care center between July 2007 and July 2017. Of these, 77 patients (62.1%) underwent screening for blood disorders.
The researchers found 27 adolescents overall were diagnosed with a blood disorder, which consisted of 35.0% of patients screened and 21.7% of all patients studied. Specifically, 14 of 27 patients (51.6%) screened were diagnosed with von Willebrand disease, 9 of 27 patients (33.3%) screened were found to have inherited platelet function disorders, 3 of 27 patients (11.1%) had inherited or acquired thrombocytopenia, and 1 of 27 patients (3.7%) had factor IX deficiency. The researchers also screened for iron deficiency and/or anemia and found 53 of 107 patients (49.5%) who were screened received a diagnosis, and 19 of 27 patients (70.3%) who were diagnosed with a bleeding disorder also had iron deficiency and/or anemia.
“In adolescents who are already known to have a bleeding disorder, consultation with a pediatric gynecologist and/or hematologist prior to menarche may be helpful to outline abnormal patterns of menstrual bleeding and to discuss options of treatment in the event of heavy menstrual bleeding,” Dr. O’Brien and her colleagues wrote.
Potential limitations in the study include the refractory nature of referrals at a tertiary care center potentially overestimating the prevalence of HMB in this population as well as the study’s retrospective design when investigating and measuring heavy menstrual bleeding, but researchers noted patients were reviewed and classified by a specialist pediatric hematologist.
The authors reported no relevant conflicts of interest.
SOURCE: O’Brien B et al. J Pediatr Adolesc Gynecol. 2018 Nov 22. doi: 10.1016/j.jpag.2018.11.005.
Over one-third of adolescents presenting with heavy menstrual bleeding were diagnosed with a bleeding disorder after screening, according to results of a retrospective study.
The high incidence of bleeding disorders detected argues for routine screening of adolescents with heavy menstrual bleeding (HMB), Brooke O’Brien, MD, of the University of Queensland, Brisbane, Australia, and her colleagues wrote in the Journal of Pediatric & Adolescent Gynecology.
“These findings support comprehensive and systematic hemostatic evaluation in adolescents with HMB,” Dr. O’Brien and her colleagues wrote. “A higher level of awareness of bleeding disorders as a cause for HMB in adolescence, especially [von Willebrand disease] and platelet function disorders, is needed and close multidisciplinary collaboration between the pediatric and adolescent gynecologist and hematologist in a specialized tertiary center should be established in the management of these patients.”
In their study, Dr. O’Brien and her colleagues retrospectively evaluated 124 adolescents with HMB at a pediatric and adolescent gynecology tertiary care center between July 2007 and July 2017. Of these, 77 patients (62.1%) underwent screening for blood disorders.
The researchers found 27 adolescents overall were diagnosed with a blood disorder, which consisted of 35.0% of patients screened and 21.7% of all patients studied. Specifically, 14 of 27 patients (51.6%) screened were diagnosed with von Willebrand disease, 9 of 27 patients (33.3%) screened were found to have inherited platelet function disorders, 3 of 27 patients (11.1%) had inherited or acquired thrombocytopenia, and 1 of 27 patients (3.7%) had factor IX deficiency. The researchers also screened for iron deficiency and/or anemia and found 53 of 107 patients (49.5%) who were screened received a diagnosis, and 19 of 27 patients (70.3%) who were diagnosed with a bleeding disorder also had iron deficiency and/or anemia.
“In adolescents who are already known to have a bleeding disorder, consultation with a pediatric gynecologist and/or hematologist prior to menarche may be helpful to outline abnormal patterns of menstrual bleeding and to discuss options of treatment in the event of heavy menstrual bleeding,” Dr. O’Brien and her colleagues wrote.
Potential limitations in the study include the refractory nature of referrals at a tertiary care center potentially overestimating the prevalence of HMB in this population as well as the study’s retrospective design when investigating and measuring heavy menstrual bleeding, but researchers noted patients were reviewed and classified by a specialist pediatric hematologist.
The authors reported no relevant conflicts of interest.
SOURCE: O’Brien B et al. J Pediatr Adolesc Gynecol. 2018 Nov 22. doi: 10.1016/j.jpag.2018.11.005.
Over one-third of adolescents presenting with heavy menstrual bleeding were diagnosed with a bleeding disorder after screening, according to results of a retrospective study.
The high incidence of bleeding disorders detected argues for routine screening of adolescents with heavy menstrual bleeding (HMB), Brooke O’Brien, MD, of the University of Queensland, Brisbane, Australia, and her colleagues wrote in the Journal of Pediatric & Adolescent Gynecology.
“These findings support comprehensive and systematic hemostatic evaluation in adolescents with HMB,” Dr. O’Brien and her colleagues wrote. “A higher level of awareness of bleeding disorders as a cause for HMB in adolescence, especially [von Willebrand disease] and platelet function disorders, is needed and close multidisciplinary collaboration between the pediatric and adolescent gynecologist and hematologist in a specialized tertiary center should be established in the management of these patients.”
In their study, Dr. O’Brien and her colleagues retrospectively evaluated 124 adolescents with HMB at a pediatric and adolescent gynecology tertiary care center between July 2007 and July 2017. Of these, 77 patients (62.1%) underwent screening for blood disorders.
The researchers found 27 adolescents overall were diagnosed with a blood disorder, which consisted of 35.0% of patients screened and 21.7% of all patients studied. Specifically, 14 of 27 patients (51.6%) screened were diagnosed with von Willebrand disease, 9 of 27 patients (33.3%) screened were found to have inherited platelet function disorders, 3 of 27 patients (11.1%) had inherited or acquired thrombocytopenia, and 1 of 27 patients (3.7%) had factor IX deficiency. The researchers also screened for iron deficiency and/or anemia and found 53 of 107 patients (49.5%) who were screened received a diagnosis, and 19 of 27 patients (70.3%) who were diagnosed with a bleeding disorder also had iron deficiency and/or anemia.
“In adolescents who are already known to have a bleeding disorder, consultation with a pediatric gynecologist and/or hematologist prior to menarche may be helpful to outline abnormal patterns of menstrual bleeding and to discuss options of treatment in the event of heavy menstrual bleeding,” Dr. O’Brien and her colleagues wrote.
Potential limitations in the study include the refractory nature of referrals at a tertiary care center potentially overestimating the prevalence of HMB in this population as well as the study’s retrospective design when investigating and measuring heavy menstrual bleeding, but researchers noted patients were reviewed and classified by a specialist pediatric hematologist.
The authors reported no relevant conflicts of interest.
SOURCE: O’Brien B et al. J Pediatr Adolesc Gynecol. 2018 Nov 22. doi: 10.1016/j.jpag.2018.11.005.
FROM THE JOURNAL OF PEDIATRIC & ADOLESCENT GYNECOLOGY
Key clinical point: More than one-third of adolescents with heavy menstrual bleeding were diagnosed with a bleeding disorder.
Major finding: After screening, 35% of women with heavy menstrual bleeding had a bleeding disorder; over half of those screened had von Willebrand disease.
Study details: A retrospective study of 124 adolescents at the Queensland Paediatric and Adolescent Gynaecology Service between July 2007 and July 2017.
Disclosures: The authors reported no relevant conflicts of interest.
Source: O’Brien B et al. J Pediatr Adolesc Gynecol. 2018 Nov 22 . doi: 10.1016/j.jpag.2018.11.005.
Omega-3 fatty acid supplementation reduces risk of preterm birth
Taking omega-3 long-chain polyunsaturated fatty acids during pregnancy was associated with reduced risk of preterm birth, and also may reduce the risk of babies born at a low birth weight and risk of requiring neonatal intensive care, according to a Cochrane review of 70 randomized controlled trials.
“There are not many options for preventing premature birth, so these new findings are very important for pregnant women, babies, and the health professionals who care for them,” Philippa Middleton, MPH, PhD, of Cochrane Pregnancy and Childbirth Group and the South Australian Health and Medical Research Institute, in Adelaide, stated in a press release. “We don’t yet fully understand the causes of premature labor, so predicting and preventing early birth has always been a challenge. This is one of the reasons omega-3 supplementation in pregnancy is of such great interest to researchers around the world.”
Dr. Middleton and her colleagues performed a search of the Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform and identified 70 randomized controlled trials (RCTs) where 19,927 women at varying levels of risk for preterm birth received omega-3 long-chain polyunsaturated fatty acids (LCPUFA), placebo, or no omega-3.
“Many pregnant women in the UK are already taking omega-3 supplements by personal choice rather than as a result of advice from health professionals,” Dr. Middleton said in the release. “It’s worth noting though that many supplements currently on the market don’t contain the optimal dose or type of omega-3 for preventing premature birth. Our review found the optimum dose was a daily supplement containing between 500 and 1,000 milligrams of long-chain omega-3 fats (containing at least 500 mg of DHA [docosahexaenoic acid]) starting at 12 weeks of pregnancy.”
In 26 RCTs (10,304 women), the risk of preterm birth under 37 weeks was 11% lower for women who took omega-3 LCPUFA compared with women who did not take omega-3 (relative risk, 0.89; 95% confidence interval, 0.81-0.97), while the risk for preterm birth under 34 weeks in 9 RCTs (5,204 women) was 42% lower for women compared with women who did not take omega-3 (RR, 0.58; 95% CI, 0.44-0.77).
With regard to infant health, use of omega-3 LCPUFA during pregnancy was associated in 10 RCTs (7,416 women) with a potential reduced risk of perinatal mortality (RR, 0.75; 95% CI, 0.54-1.03) and, in 9 RCTs (6,920 women), a reduced risk of neonatal intensive care admission (RR, 0.92; 95% CI, 0.83-1.03). The researchers noted that omega-3 use in 15 trials (8,449 women) was potentially associated with a reduced number of babies with low birth weight (RR, 0.90; 95% CI, 0.82-0.99), but an increase in babies who were large for their gestational age in 3,722 women from 6 RCTs (RR, 1.15; 95% CI, 0.97-1.36). There was no significant difference among groups with regard to babies who were born small for their gestational age or in uterine growth restriction, they said.
While maternal outcomes were examined, Dr. Middleton and her colleagues found no significant differences between groups in factors such as postterm induction, serious adverse events, admission to intensive care, and postnatal depression.
“Ultimately, we hope this review will make a real contribution to the evidence base we need to reduce premature births, which continue to be one of the most pressing and intractable maternal and child health problems in every country around the world,” Dr. Middleton said.
The National Institutes of Health funded the review. The authors reported no conflicts of interest.
SOURCE: Middleton P et al. Cochrane Database Syst Rev. 2018; doi: 10.1002/14651858.CD003402.pub3.
Taking omega-3 long-chain polyunsaturated fatty acids during pregnancy was associated with reduced risk of preterm birth, and also may reduce the risk of babies born at a low birth weight and risk of requiring neonatal intensive care, according to a Cochrane review of 70 randomized controlled trials.
“There are not many options for preventing premature birth, so these new findings are very important for pregnant women, babies, and the health professionals who care for them,” Philippa Middleton, MPH, PhD, of Cochrane Pregnancy and Childbirth Group and the South Australian Health and Medical Research Institute, in Adelaide, stated in a press release. “We don’t yet fully understand the causes of premature labor, so predicting and preventing early birth has always been a challenge. This is one of the reasons omega-3 supplementation in pregnancy is of such great interest to researchers around the world.”
Dr. Middleton and her colleagues performed a search of the Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform and identified 70 randomized controlled trials (RCTs) where 19,927 women at varying levels of risk for preterm birth received omega-3 long-chain polyunsaturated fatty acids (LCPUFA), placebo, or no omega-3.
“Many pregnant women in the UK are already taking omega-3 supplements by personal choice rather than as a result of advice from health professionals,” Dr. Middleton said in the release. “It’s worth noting though that many supplements currently on the market don’t contain the optimal dose or type of omega-3 for preventing premature birth. Our review found the optimum dose was a daily supplement containing between 500 and 1,000 milligrams of long-chain omega-3 fats (containing at least 500 mg of DHA [docosahexaenoic acid]) starting at 12 weeks of pregnancy.”
In 26 RCTs (10,304 women), the risk of preterm birth under 37 weeks was 11% lower for women who took omega-3 LCPUFA compared with women who did not take omega-3 (relative risk, 0.89; 95% confidence interval, 0.81-0.97), while the risk for preterm birth under 34 weeks in 9 RCTs (5,204 women) was 42% lower for women compared with women who did not take omega-3 (RR, 0.58; 95% CI, 0.44-0.77).
With regard to infant health, use of omega-3 LCPUFA during pregnancy was associated in 10 RCTs (7,416 women) with a potential reduced risk of perinatal mortality (RR, 0.75; 95% CI, 0.54-1.03) and, in 9 RCTs (6,920 women), a reduced risk of neonatal intensive care admission (RR, 0.92; 95% CI, 0.83-1.03). The researchers noted that omega-3 use in 15 trials (8,449 women) was potentially associated with a reduced number of babies with low birth weight (RR, 0.90; 95% CI, 0.82-0.99), but an increase in babies who were large for their gestational age in 3,722 women from 6 RCTs (RR, 1.15; 95% CI, 0.97-1.36). There was no significant difference among groups with regard to babies who were born small for their gestational age or in uterine growth restriction, they said.
While maternal outcomes were examined, Dr. Middleton and her colleagues found no significant differences between groups in factors such as postterm induction, serious adverse events, admission to intensive care, and postnatal depression.
“Ultimately, we hope this review will make a real contribution to the evidence base we need to reduce premature births, which continue to be one of the most pressing and intractable maternal and child health problems in every country around the world,” Dr. Middleton said.
The National Institutes of Health funded the review. The authors reported no conflicts of interest.
SOURCE: Middleton P et al. Cochrane Database Syst Rev. 2018; doi: 10.1002/14651858.CD003402.pub3.
Taking omega-3 long-chain polyunsaturated fatty acids during pregnancy was associated with reduced risk of preterm birth, and also may reduce the risk of babies born at a low birth weight and risk of requiring neonatal intensive care, according to a Cochrane review of 70 randomized controlled trials.
“There are not many options for preventing premature birth, so these new findings are very important for pregnant women, babies, and the health professionals who care for them,” Philippa Middleton, MPH, PhD, of Cochrane Pregnancy and Childbirth Group and the South Australian Health and Medical Research Institute, in Adelaide, stated in a press release. “We don’t yet fully understand the causes of premature labor, so predicting and preventing early birth has always been a challenge. This is one of the reasons omega-3 supplementation in pregnancy is of such great interest to researchers around the world.”
Dr. Middleton and her colleagues performed a search of the Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform and identified 70 randomized controlled trials (RCTs) where 19,927 women at varying levels of risk for preterm birth received omega-3 long-chain polyunsaturated fatty acids (LCPUFA), placebo, or no omega-3.
“Many pregnant women in the UK are already taking omega-3 supplements by personal choice rather than as a result of advice from health professionals,” Dr. Middleton said in the release. “It’s worth noting though that many supplements currently on the market don’t contain the optimal dose or type of omega-3 for preventing premature birth. Our review found the optimum dose was a daily supplement containing between 500 and 1,000 milligrams of long-chain omega-3 fats (containing at least 500 mg of DHA [docosahexaenoic acid]) starting at 12 weeks of pregnancy.”
In 26 RCTs (10,304 women), the risk of preterm birth under 37 weeks was 11% lower for women who took omega-3 LCPUFA compared with women who did not take omega-3 (relative risk, 0.89; 95% confidence interval, 0.81-0.97), while the risk for preterm birth under 34 weeks in 9 RCTs (5,204 women) was 42% lower for women compared with women who did not take omega-3 (RR, 0.58; 95% CI, 0.44-0.77).
With regard to infant health, use of omega-3 LCPUFA during pregnancy was associated in 10 RCTs (7,416 women) with a potential reduced risk of perinatal mortality (RR, 0.75; 95% CI, 0.54-1.03) and, in 9 RCTs (6,920 women), a reduced risk of neonatal intensive care admission (RR, 0.92; 95% CI, 0.83-1.03). The researchers noted that omega-3 use in 15 trials (8,449 women) was potentially associated with a reduced number of babies with low birth weight (RR, 0.90; 95% CI, 0.82-0.99), but an increase in babies who were large for their gestational age in 3,722 women from 6 RCTs (RR, 1.15; 95% CI, 0.97-1.36). There was no significant difference among groups with regard to babies who were born small for their gestational age or in uterine growth restriction, they said.
While maternal outcomes were examined, Dr. Middleton and her colleagues found no significant differences between groups in factors such as postterm induction, serious adverse events, admission to intensive care, and postnatal depression.
“Ultimately, we hope this review will make a real contribution to the evidence base we need to reduce premature births, which continue to be one of the most pressing and intractable maternal and child health problems in every country around the world,” Dr. Middleton said.
The National Institutes of Health funded the review. The authors reported no conflicts of interest.
SOURCE: Middleton P et al. Cochrane Database Syst Rev. 2018; doi: 10.1002/14651858.CD003402.pub3.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point:
Major finding: In 26 randomized controlled trials, the risk of preterm birth at 37 weeks (10,304 women) was 11% lower and the risk of preterm birth at 34 weeks (5,204 women) in 9 RCTs was 42% lower for women taking omega-3, compared with women not taking omega-3.
Study details: A Cochrane review of 70 RCTs with a total of 19,927 women at varying levels of risk for preterm birth who received omega-3 long-chain polyunsaturated fatty acids, placebo, or no omega-3.
Disclosures: The National Institutes of Health funded the review. The authors reported no conflicts of interest.
Source: Middleton P et al. Cochrane Database Syst Rev. 2018. doi: 10.1002/14651858.CD003402.pub3.
AAP speaker emphasizes importance of understanding patients’ ‘lived experience’
ORLANDO – Pediatricians who learn about their patients’ lived experience have the potential to encourage patients and help them overcome biases, assumptions, and barriers of opioid use disorder, Tamela Milan said at the American Academy of Pediatrics annual meeting.
After her five children were taken into state welfare custody and she began a sixth pregnancy while struggling with opioid use disorder and as a survivor of domestic violence, Ms. Milan’s pediatrician was the one to encourage her to take steps to improve her life. She went on to regain custody of her children and complete college, and has given back by working in community health programs for over 20 years.
In a video interview, Ms. Milan said she would not have been able to overcome these barriers had it not been for the support of her pediatrician, who saw her as a person instead of a mother with opioid use disorder.
“I’ve been on both sides of the fence,” Ms. Milan said. “As someone who’s had to receive treatment and to provide it, it’s really important that we start looking at people for who they are and where they are.”
Tamela Milan has no relevant conflicts of interest.
ORLANDO – Pediatricians who learn about their patients’ lived experience have the potential to encourage patients and help them overcome biases, assumptions, and barriers of opioid use disorder, Tamela Milan said at the American Academy of Pediatrics annual meeting.
After her five children were taken into state welfare custody and she began a sixth pregnancy while struggling with opioid use disorder and as a survivor of domestic violence, Ms. Milan’s pediatrician was the one to encourage her to take steps to improve her life. She went on to regain custody of her children and complete college, and has given back by working in community health programs for over 20 years.
In a video interview, Ms. Milan said she would not have been able to overcome these barriers had it not been for the support of her pediatrician, who saw her as a person instead of a mother with opioid use disorder.
“I’ve been on both sides of the fence,” Ms. Milan said. “As someone who’s had to receive treatment and to provide it, it’s really important that we start looking at people for who they are and where they are.”
Tamela Milan has no relevant conflicts of interest.
ORLANDO – Pediatricians who learn about their patients’ lived experience have the potential to encourage patients and help them overcome biases, assumptions, and barriers of opioid use disorder, Tamela Milan said at the American Academy of Pediatrics annual meeting.
After her five children were taken into state welfare custody and she began a sixth pregnancy while struggling with opioid use disorder and as a survivor of domestic violence, Ms. Milan’s pediatrician was the one to encourage her to take steps to improve her life. She went on to regain custody of her children and complete college, and has given back by working in community health programs for over 20 years.
In a video interview, Ms. Milan said she would not have been able to overcome these barriers had it not been for the support of her pediatrician, who saw her as a person instead of a mother with opioid use disorder.
“I’ve been on both sides of the fence,” Ms. Milan said. “As someone who’s had to receive treatment and to provide it, it’s really important that we start looking at people for who they are and where they are.”
Tamela Milan has no relevant conflicts of interest.
REPORTING FROM AAP 2018
Rheumatologists underscreened RA patients for hyperlipidemia
Patients with rheumatoid arthritis had low rates of screening for primary hyperlipidemia, but patients who visited both a rheumatologist and a nonrheumatology clinician for follow-up were more likely to be screened, according to research published in Arthritis Care & Research.
Iris Navarro-Millán, MD, MSPH, from Cornell University, New York, and her colleagues performed a retrospective study of roughly 244,000 participants with private insurance, Medicare, or Medicaid who had rheumatoid arthritis (13,000 patients), diabetes (63,000), both rheumatoid arthritis (RA) and diabetes (1,000), and neither RA nor diabetes (168,000) during 2006-2010. Patients were between the ages of 41 years and 85 years. There were similar rates of hypertension among the RA patients (40%) and patients with neither RA nor diabetes (39%), as well as among the diabetes patients (79%) and the both patients with both RA and diabetes (70%).
The researchers found 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2 years’ follow-up. For RA-only patients, 22% saw a rheumatologist only, while 56% saw both a rheumatologist and then a nonrheumatology clinician at 2-years’ follow-up. Patients who visited a rheumatologist and a nonrheumatology clinician for follow-up were 55% more likely to receive primary lipid screening, and RA patients who visited a nonrheumatology clinician only were 22% more likely to receive screening than RA patients who saw only a rheumatologist.
Dr. Navarro-Millán and her colleagues said European RA patients may be more likely to receive screening for hyperlipidemia because their health care systems follow European League Against Rheumatism (EULAR) recommendations for management of cardiovascular disease (CVD) risk, while many rheumatologists based in the United States are “still reluctant to take responsibility to assess and mitigate (if needed) CVD risk for patients with RA.”
“Measures to achieve this goal must be implemented and may include defining specific roles for rheumatologists, nonrheumatology practitioners, and patients to determine who should be responsible for hyperlipidemia screening and treatment for patients with RA,” Dr. Navarro-Millán and her colleagues wrote.
Limitations to the study include lack of information on uninsured patients, lack of some clinical and demographic data, and potential misclassification of some clinicians who may have provided rheumatology-specific care but may have been in primary care settings.
This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
SOURCE: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
Patients with rheumatoid arthritis had low rates of screening for primary hyperlipidemia, but patients who visited both a rheumatologist and a nonrheumatology clinician for follow-up were more likely to be screened, according to research published in Arthritis Care & Research.
Iris Navarro-Millán, MD, MSPH, from Cornell University, New York, and her colleagues performed a retrospective study of roughly 244,000 participants with private insurance, Medicare, or Medicaid who had rheumatoid arthritis (13,000 patients), diabetes (63,000), both rheumatoid arthritis (RA) and diabetes (1,000), and neither RA nor diabetes (168,000) during 2006-2010. Patients were between the ages of 41 years and 85 years. There were similar rates of hypertension among the RA patients (40%) and patients with neither RA nor diabetes (39%), as well as among the diabetes patients (79%) and the both patients with both RA and diabetes (70%).
The researchers found 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2 years’ follow-up. For RA-only patients, 22% saw a rheumatologist only, while 56% saw both a rheumatologist and then a nonrheumatology clinician at 2-years’ follow-up. Patients who visited a rheumatologist and a nonrheumatology clinician for follow-up were 55% more likely to receive primary lipid screening, and RA patients who visited a nonrheumatology clinician only were 22% more likely to receive screening than RA patients who saw only a rheumatologist.
Dr. Navarro-Millán and her colleagues said European RA patients may be more likely to receive screening for hyperlipidemia because their health care systems follow European League Against Rheumatism (EULAR) recommendations for management of cardiovascular disease (CVD) risk, while many rheumatologists based in the United States are “still reluctant to take responsibility to assess and mitigate (if needed) CVD risk for patients with RA.”
“Measures to achieve this goal must be implemented and may include defining specific roles for rheumatologists, nonrheumatology practitioners, and patients to determine who should be responsible for hyperlipidemia screening and treatment for patients with RA,” Dr. Navarro-Millán and her colleagues wrote.
Limitations to the study include lack of information on uninsured patients, lack of some clinical and demographic data, and potential misclassification of some clinicians who may have provided rheumatology-specific care but may have been in primary care settings.
This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
SOURCE: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
Patients with rheumatoid arthritis had low rates of screening for primary hyperlipidemia, but patients who visited both a rheumatologist and a nonrheumatology clinician for follow-up were more likely to be screened, according to research published in Arthritis Care & Research.
Iris Navarro-Millán, MD, MSPH, from Cornell University, New York, and her colleagues performed a retrospective study of roughly 244,000 participants with private insurance, Medicare, or Medicaid who had rheumatoid arthritis (13,000 patients), diabetes (63,000), both rheumatoid arthritis (RA) and diabetes (1,000), and neither RA nor diabetes (168,000) during 2006-2010. Patients were between the ages of 41 years and 85 years. There were similar rates of hypertension among the RA patients (40%) and patients with neither RA nor diabetes (39%), as well as among the diabetes patients (79%) and the both patients with both RA and diabetes (70%).
The researchers found 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2 years’ follow-up. For RA-only patients, 22% saw a rheumatologist only, while 56% saw both a rheumatologist and then a nonrheumatology clinician at 2-years’ follow-up. Patients who visited a rheumatologist and a nonrheumatology clinician for follow-up were 55% more likely to receive primary lipid screening, and RA patients who visited a nonrheumatology clinician only were 22% more likely to receive screening than RA patients who saw only a rheumatologist.
Dr. Navarro-Millán and her colleagues said European RA patients may be more likely to receive screening for hyperlipidemia because their health care systems follow European League Against Rheumatism (EULAR) recommendations for management of cardiovascular disease (CVD) risk, while many rheumatologists based in the United States are “still reluctant to take responsibility to assess and mitigate (if needed) CVD risk for patients with RA.”
“Measures to achieve this goal must be implemented and may include defining specific roles for rheumatologists, nonrheumatology practitioners, and patients to determine who should be responsible for hyperlipidemia screening and treatment for patients with RA,” Dr. Navarro-Millán and her colleagues wrote.
Limitations to the study include lack of information on uninsured patients, lack of some clinical and demographic data, and potential misclassification of some clinicians who may have provided rheumatology-specific care but may have been in primary care settings.
This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
SOURCE: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Patients with rheumatoid arthritis who saw only a rheumatologist were less likely to receive primary lipid screening than those who saw both a rheumatologist and then a clinician at 2-year follow-up.
Major finding: 37% of RA patients, 60% of diabetes patients, 55% of patients with both RA and diabetes, and 41% of patients with neither RA nor diabetes received primary lipid screening over 2-year follow-up.
Study details: A retrospective study of claims data from 243,909 participants aged 41-85 years in private and public health plans during 2006-2010.
Disclosures: This study is funded by grants and contracts from the National Institutes of Health and the Department of Health and Human Services. The authors report no relevant financial disclosures.
Source: Navarro-Millán I et al. Arthritis Care Res. 2018. doi: 10.1002/acr.23810.
ADA releases guidelines for type 2 diabetes in children, youth
The American Diabetes Association’s guidelines for the evaluation and management of pediatric patients with type 2 diabetes differ from those for adults.
“Puberty-related physiologic insulin resistance, particularly in obese youth, may play a role” in the fact that youth are more insulin resistant than adults. Also, type 2 diabetes apparently is “more aggressive in youth than adults, with a faster rate of deterioration of beta-cell function and poorer response to glucose-lowering medications,” wrote Silva Arslanian, MD, from the division of pediatric endocrinology, metabolism, and diabetes mellitus at the University of Pittsburgh, and her colleagues. “Even though our knowledge of youth-onset type 2 diabetes has increased tremendously over the last 2 decades, robust and evidence-based data are still limited regarding diagnostic and therapeutic approaches and prevention of complications.”
The ADA position statement by Dr. Arslanian and her colleagues outlines management of type 2 diabetes in children and youth.
Diagnosis
and repeat testing should occur at least every 3 years for these patients. Pancreatic autoantibody tests should also be considered in this patient population to rule out autoimmune type 1 diabetes, and genetic evaluation should be performed to test for monogenic diabetes, “based on clinical characteristics and presentation,” they wrote.
Use fasting plasma glucose, 2-hour fasting plasma glucose after a 75-g oral glucose tolerance test, or glycosylated hemoglobin (HbA1C) to test for diabetes or prediabetes. Also consider factors like medication adherence and treatment effects when prescribing glucose-lowering or other medications for overweight or obese children and adolescents with type 2 diabetes.
Lifestyle management
With regard to lifestyle management programs, the intervention should be introduced as a part of diabetes care – aimed at reducing between 7% and 10% of body weight – and be based on a chronic care model. The intervention should include 30-60 minutes of moderate to intense physical activity for 5 days each week, strength training 3 days per week, and incorporate healthy eating plans. Dr. Arslanian and her associates noted there was limited evidence for pharmacotherapy for weight reduction in children and adolescents with type 2 diabetes.
Pharmacologic therapy
Pharmacologic therapy should be started together with lifestyle therapy once a diagnosis is made, according to the recommendations.
Metformin is the preferred initial pharmacologic treatment for patients with normal renal function who are asymptomatic and with HbA1C levels of less than 8.5%.
Patients with blood glucose greater than or equal to 250 mg/dL and HbA1C greater than or equal to 8.5% with symptoms such as weight loss, polydipsia, polyuria, or nocturia should receive basal insulin during initiation and titration of metformin.
Patients with ketosis or ketoacidosis should receive intravenous insulin to address hyperglycemia. Once the acidosis is corrected, initiate metformin with subcutaneous insulin therapy. For patients who are reaching home-based glucose monitoring targets, consider tapering the dose over 2-6 weeks with a 10%-30% reduction in insulin every few days.
In patients where metformin alone is not meeting the glycemic target, consider basal insulin therapy and, if that fails to help achieve glycemic targets, more intensive approaches should be considered, such as metabolic surgery.
Jay Cohen, MD, FACE, medical director at the Endocrine Clinic in Memphis, said in an interview that he agreed with the ADA position statement except for the pharmacologic therapy recommendations.
“The pharmacology therapy is 4 years outdated,” he said. “We routinely use all of the medications that are not Food and Drug Administration [approved] for kids, but are FDA approved for adults.”
He also questioned the ADA’s recommendation to give basal insulin to patients who are insulin resistant.
“Why give insulin if these people are insulin resistant?” said Dr. Cohen, who also is a Clinical Endocrinology News editorial board member. “The oral and injectable noninsulins work fabulously with less weight gain – already a problem for these patients – and less hypoglycemia, less side effects, and better compliance.”
Treatment goals
The HbA1C goal for children and adolescents with type 2 diabetes is less than 7% when treated with oral agents alone. HbA1C should be tested every 3 months and should be individualized, according to the ADA recommendations. In some patients, such as those with a shorter diabetes duration, lesser degrees of beta-cell dysfunction, and those who achieve significant weight improvement through lifestyle changes or taking metformin, consider lowering the HbA1C goal to less than 6.5%.
Give individualized care with regard to home self-monitoring of blood glucose. Also provide patients and their families with “culturally competent” diabetes self-management tools and lifestyle programs. Consider social factors such as housing stability, food insecurity, and financial barriers when making treatment decisions.
Screening for complications
To screen for nephropathy, take BP measurements at every visit and promote lifestyle management to reduce risk of diabetic kidney disease and improve weight loss. After 6 months, if a patient’s BP remains greater than the 95th percentile for their age, gender, and height, ACE inhibitors, or angiotensin receptor blockers are initial therapeutic options, according to the position statement. Other BP-lowering treatments may be added as necessary.
Also monitor protein intake (0.8 g/kg per day) as well as urine albumin/creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) annually. Patients with diabetes and hypertension who are not pregnant should receive an ACE inhibitor or angiotensin receptor blocker if their UACR is modestly elevated (30-299 mg/g creatinine). Such a regimen is strongly recommended if their UACR is above 300 mg/g creatinine and/or if their eGFR is less than 60 mL/min per 1.73 m2.
Screening issues
When considering diabetes distress and mental or behavioral health in children and adolescents with type 2 diabetes, use standardized and validated tools to assess symptoms such as depression and disordered eating behaviors. Regularly screen for smoking and alcohol use and provide preconception counseling for female patients of child-bearing age.
Screen for neuropathy, retinopathy, and nonalcoholic fatty liver disease annually and for obstructive sleep apnea at each visit. Lipid testing should be performed annually once patients have achieved glycemic control. Polycystic ovary syndrome should be considered in female patients with type 2 diabetes and treated with metformin together with lifestyle changes to address menstrual cyclicity and hyperandrogenism, the authors recommended.
Dr. Arslanian is on a data monitoring committee for AstraZeneca; data safety monitoring board for Boehringer Ingelheim; and advisory boards for Eli Lilly, Novo Nordisk, and Sanofi-Aventis; and has received research grants from Eli Lilly and Novo Nordisk. Other authors reported various relationships with a number of pharmaceutical companies.
SOURCE: Arslanian S et al. Diabetes Care. 2018 Nov 13. doi: 10.2337/dci18-0052.
The American Diabetes Association’s guidelines for the evaluation and management of pediatric patients with type 2 diabetes differ from those for adults.
“Puberty-related physiologic insulin resistance, particularly in obese youth, may play a role” in the fact that youth are more insulin resistant than adults. Also, type 2 diabetes apparently is “more aggressive in youth than adults, with a faster rate of deterioration of beta-cell function and poorer response to glucose-lowering medications,” wrote Silva Arslanian, MD, from the division of pediatric endocrinology, metabolism, and diabetes mellitus at the University of Pittsburgh, and her colleagues. “Even though our knowledge of youth-onset type 2 diabetes has increased tremendously over the last 2 decades, robust and evidence-based data are still limited regarding diagnostic and therapeutic approaches and prevention of complications.”
The ADA position statement by Dr. Arslanian and her colleagues outlines management of type 2 diabetes in children and youth.
Diagnosis
and repeat testing should occur at least every 3 years for these patients. Pancreatic autoantibody tests should also be considered in this patient population to rule out autoimmune type 1 diabetes, and genetic evaluation should be performed to test for monogenic diabetes, “based on clinical characteristics and presentation,” they wrote.
Use fasting plasma glucose, 2-hour fasting plasma glucose after a 75-g oral glucose tolerance test, or glycosylated hemoglobin (HbA1C) to test for diabetes or prediabetes. Also consider factors like medication adherence and treatment effects when prescribing glucose-lowering or other medications for overweight or obese children and adolescents with type 2 diabetes.
Lifestyle management
With regard to lifestyle management programs, the intervention should be introduced as a part of diabetes care – aimed at reducing between 7% and 10% of body weight – and be based on a chronic care model. The intervention should include 30-60 minutes of moderate to intense physical activity for 5 days each week, strength training 3 days per week, and incorporate healthy eating plans. Dr. Arslanian and her associates noted there was limited evidence for pharmacotherapy for weight reduction in children and adolescents with type 2 diabetes.
Pharmacologic therapy
Pharmacologic therapy should be started together with lifestyle therapy once a diagnosis is made, according to the recommendations.
Metformin is the preferred initial pharmacologic treatment for patients with normal renal function who are asymptomatic and with HbA1C levels of less than 8.5%.
Patients with blood glucose greater than or equal to 250 mg/dL and HbA1C greater than or equal to 8.5% with symptoms such as weight loss, polydipsia, polyuria, or nocturia should receive basal insulin during initiation and titration of metformin.
Patients with ketosis or ketoacidosis should receive intravenous insulin to address hyperglycemia. Once the acidosis is corrected, initiate metformin with subcutaneous insulin therapy. For patients who are reaching home-based glucose monitoring targets, consider tapering the dose over 2-6 weeks with a 10%-30% reduction in insulin every few days.
In patients where metformin alone is not meeting the glycemic target, consider basal insulin therapy and, if that fails to help achieve glycemic targets, more intensive approaches should be considered, such as metabolic surgery.
Jay Cohen, MD, FACE, medical director at the Endocrine Clinic in Memphis, said in an interview that he agreed with the ADA position statement except for the pharmacologic therapy recommendations.
“The pharmacology therapy is 4 years outdated,” he said. “We routinely use all of the medications that are not Food and Drug Administration [approved] for kids, but are FDA approved for adults.”
He also questioned the ADA’s recommendation to give basal insulin to patients who are insulin resistant.
“Why give insulin if these people are insulin resistant?” said Dr. Cohen, who also is a Clinical Endocrinology News editorial board member. “The oral and injectable noninsulins work fabulously with less weight gain – already a problem for these patients – and less hypoglycemia, less side effects, and better compliance.”
Treatment goals
The HbA1C goal for children and adolescents with type 2 diabetes is less than 7% when treated with oral agents alone. HbA1C should be tested every 3 months and should be individualized, according to the ADA recommendations. In some patients, such as those with a shorter diabetes duration, lesser degrees of beta-cell dysfunction, and those who achieve significant weight improvement through lifestyle changes or taking metformin, consider lowering the HbA1C goal to less than 6.5%.
Give individualized care with regard to home self-monitoring of blood glucose. Also provide patients and their families with “culturally competent” diabetes self-management tools and lifestyle programs. Consider social factors such as housing stability, food insecurity, and financial barriers when making treatment decisions.
Screening for complications
To screen for nephropathy, take BP measurements at every visit and promote lifestyle management to reduce risk of diabetic kidney disease and improve weight loss. After 6 months, if a patient’s BP remains greater than the 95th percentile for their age, gender, and height, ACE inhibitors, or angiotensin receptor blockers are initial therapeutic options, according to the position statement. Other BP-lowering treatments may be added as necessary.
Also monitor protein intake (0.8 g/kg per day) as well as urine albumin/creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) annually. Patients with diabetes and hypertension who are not pregnant should receive an ACE inhibitor or angiotensin receptor blocker if their UACR is modestly elevated (30-299 mg/g creatinine). Such a regimen is strongly recommended if their UACR is above 300 mg/g creatinine and/or if their eGFR is less than 60 mL/min per 1.73 m2.
Screening issues
When considering diabetes distress and mental or behavioral health in children and adolescents with type 2 diabetes, use standardized and validated tools to assess symptoms such as depression and disordered eating behaviors. Regularly screen for smoking and alcohol use and provide preconception counseling for female patients of child-bearing age.
Screen for neuropathy, retinopathy, and nonalcoholic fatty liver disease annually and for obstructive sleep apnea at each visit. Lipid testing should be performed annually once patients have achieved glycemic control. Polycystic ovary syndrome should be considered in female patients with type 2 diabetes and treated with metformin together with lifestyle changes to address menstrual cyclicity and hyperandrogenism, the authors recommended.
Dr. Arslanian is on a data monitoring committee for AstraZeneca; data safety monitoring board for Boehringer Ingelheim; and advisory boards for Eli Lilly, Novo Nordisk, and Sanofi-Aventis; and has received research grants from Eli Lilly and Novo Nordisk. Other authors reported various relationships with a number of pharmaceutical companies.
SOURCE: Arslanian S et al. Diabetes Care. 2018 Nov 13. doi: 10.2337/dci18-0052.
The American Diabetes Association’s guidelines for the evaluation and management of pediatric patients with type 2 diabetes differ from those for adults.
“Puberty-related physiologic insulin resistance, particularly in obese youth, may play a role” in the fact that youth are more insulin resistant than adults. Also, type 2 diabetes apparently is “more aggressive in youth than adults, with a faster rate of deterioration of beta-cell function and poorer response to glucose-lowering medications,” wrote Silva Arslanian, MD, from the division of pediatric endocrinology, metabolism, and diabetes mellitus at the University of Pittsburgh, and her colleagues. “Even though our knowledge of youth-onset type 2 diabetes has increased tremendously over the last 2 decades, robust and evidence-based data are still limited regarding diagnostic and therapeutic approaches and prevention of complications.”
The ADA position statement by Dr. Arslanian and her colleagues outlines management of type 2 diabetes in children and youth.
Diagnosis
and repeat testing should occur at least every 3 years for these patients. Pancreatic autoantibody tests should also be considered in this patient population to rule out autoimmune type 1 diabetes, and genetic evaluation should be performed to test for monogenic diabetes, “based on clinical characteristics and presentation,” they wrote.
Use fasting plasma glucose, 2-hour fasting plasma glucose after a 75-g oral glucose tolerance test, or glycosylated hemoglobin (HbA1C) to test for diabetes or prediabetes. Also consider factors like medication adherence and treatment effects when prescribing glucose-lowering or other medications for overweight or obese children and adolescents with type 2 diabetes.
Lifestyle management
With regard to lifestyle management programs, the intervention should be introduced as a part of diabetes care – aimed at reducing between 7% and 10% of body weight – and be based on a chronic care model. The intervention should include 30-60 minutes of moderate to intense physical activity for 5 days each week, strength training 3 days per week, and incorporate healthy eating plans. Dr. Arslanian and her associates noted there was limited evidence for pharmacotherapy for weight reduction in children and adolescents with type 2 diabetes.
Pharmacologic therapy
Pharmacologic therapy should be started together with lifestyle therapy once a diagnosis is made, according to the recommendations.
Metformin is the preferred initial pharmacologic treatment for patients with normal renal function who are asymptomatic and with HbA1C levels of less than 8.5%.
Patients with blood glucose greater than or equal to 250 mg/dL and HbA1C greater than or equal to 8.5% with symptoms such as weight loss, polydipsia, polyuria, or nocturia should receive basal insulin during initiation and titration of metformin.
Patients with ketosis or ketoacidosis should receive intravenous insulin to address hyperglycemia. Once the acidosis is corrected, initiate metformin with subcutaneous insulin therapy. For patients who are reaching home-based glucose monitoring targets, consider tapering the dose over 2-6 weeks with a 10%-30% reduction in insulin every few days.
In patients where metformin alone is not meeting the glycemic target, consider basal insulin therapy and, if that fails to help achieve glycemic targets, more intensive approaches should be considered, such as metabolic surgery.
Jay Cohen, MD, FACE, medical director at the Endocrine Clinic in Memphis, said in an interview that he agreed with the ADA position statement except for the pharmacologic therapy recommendations.
“The pharmacology therapy is 4 years outdated,” he said. “We routinely use all of the medications that are not Food and Drug Administration [approved] for kids, but are FDA approved for adults.”
He also questioned the ADA’s recommendation to give basal insulin to patients who are insulin resistant.
“Why give insulin if these people are insulin resistant?” said Dr. Cohen, who also is a Clinical Endocrinology News editorial board member. “The oral and injectable noninsulins work fabulously with less weight gain – already a problem for these patients – and less hypoglycemia, less side effects, and better compliance.”
Treatment goals
The HbA1C goal for children and adolescents with type 2 diabetes is less than 7% when treated with oral agents alone. HbA1C should be tested every 3 months and should be individualized, according to the ADA recommendations. In some patients, such as those with a shorter diabetes duration, lesser degrees of beta-cell dysfunction, and those who achieve significant weight improvement through lifestyle changes or taking metformin, consider lowering the HbA1C goal to less than 6.5%.
Give individualized care with regard to home self-monitoring of blood glucose. Also provide patients and their families with “culturally competent” diabetes self-management tools and lifestyle programs. Consider social factors such as housing stability, food insecurity, and financial barriers when making treatment decisions.
Screening for complications
To screen for nephropathy, take BP measurements at every visit and promote lifestyle management to reduce risk of diabetic kidney disease and improve weight loss. After 6 months, if a patient’s BP remains greater than the 95th percentile for their age, gender, and height, ACE inhibitors, or angiotensin receptor blockers are initial therapeutic options, according to the position statement. Other BP-lowering treatments may be added as necessary.
Also monitor protein intake (0.8 g/kg per day) as well as urine albumin/creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) annually. Patients with diabetes and hypertension who are not pregnant should receive an ACE inhibitor or angiotensin receptor blocker if their UACR is modestly elevated (30-299 mg/g creatinine). Such a regimen is strongly recommended if their UACR is above 300 mg/g creatinine and/or if their eGFR is less than 60 mL/min per 1.73 m2.
Screening issues
When considering diabetes distress and mental or behavioral health in children and adolescents with type 2 diabetes, use standardized and validated tools to assess symptoms such as depression and disordered eating behaviors. Regularly screen for smoking and alcohol use and provide preconception counseling for female patients of child-bearing age.
Screen for neuropathy, retinopathy, and nonalcoholic fatty liver disease annually and for obstructive sleep apnea at each visit. Lipid testing should be performed annually once patients have achieved glycemic control. Polycystic ovary syndrome should be considered in female patients with type 2 diabetes and treated with metformin together with lifestyle changes to address menstrual cyclicity and hyperandrogenism, the authors recommended.
Dr. Arslanian is on a data monitoring committee for AstraZeneca; data safety monitoring board for Boehringer Ingelheim; and advisory boards for Eli Lilly, Novo Nordisk, and Sanofi-Aventis; and has received research grants from Eli Lilly and Novo Nordisk. Other authors reported various relationships with a number of pharmaceutical companies.
SOURCE: Arslanian S et al. Diabetes Care. 2018 Nov 13. doi: 10.2337/dci18-0052.
FROM DIABETES CARE
Levonorgestrel implant right after delivery does not affect breastfeeding, infant growth
Researchers found no significant differences in infant growth, changes in breastfeeding initiation or breastfeeding continuation at 3-month and 6-month follow-up among women who received a levonorgestrel contraception implant very soon after delivery, compared with women who waited to receive the implant.
“These findings are consistent with the preponderance of literature supporting the hypothesis that progestin-containing contraceptives do not compromise a woman’s ability to initiate or sustain breastfeeding and do not adversely affect infant growth,” Sarah Averbach, MD, of the University of California, San Francisco, and her colleagues wrote in their study published in Contraception.
Dr. Averbach and her colleagues randomized 96 women to receive a two-rod levonorgestrel (LNG)–releasing subdermal contraceptive implant within 5 days of delivery (mean time, 36 hours post delivery) and 87 women to delay the implant to between 6 and 8 weeks at a postpartum follow-up visit (mean time, 68 days). The women were a minimum of 18 years old with a recent vaginal or cesarean section delivery at a Ugandan hospital; 55% of the women had at least three children, and 73% said they had prior experience breastfeeding. The researchers then examined infant weight change and infant head circumference change at 6 months from birth, time to lactogenesis, and whether mothers continued to breastfeed at 3 months and 6 months after birth.
Infant weight was similar in the immediate-implant group (4,632 g), compared with the delayed-implant group (4,407 g; P = .26); infant head circumference was similar between both groups (9.3 cm vs. 9.5 cm; P = .70) at 6 months as well. The time to lactogenesis was not significantly different in the immediate-implant (65 hours) and delayed-implant (63 hours; P = .84) groups. At 3 months, 74% of immediate-implant participants and 71% of delayed-implant participants said they were breastfeeding exclusively (P = .74); at 6 months, 48% of immediate implant participants and 52% of delayed implant participants reported exclusive breastfeeding (P equals .58).
Limitations of the study included follow-up to only 6 months and selection of participants with previous breastfeeding experience. Researchers also noted better measurements of infant and maternal breast milk intake also could be used and limit generalization of the results.
This study was funded by the Society of Family Planning Research Fund. Dr. Averbach is supported by an award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The other authors had no relevant financial disclosures.
SOURCE: Averbach S et al. Contraception. 2018. doi: 10.1016/j.contraception.2018.10.008.
Researchers found no significant differences in infant growth, changes in breastfeeding initiation or breastfeeding continuation at 3-month and 6-month follow-up among women who received a levonorgestrel contraception implant very soon after delivery, compared with women who waited to receive the implant.
“These findings are consistent with the preponderance of literature supporting the hypothesis that progestin-containing contraceptives do not compromise a woman’s ability to initiate or sustain breastfeeding and do not adversely affect infant growth,” Sarah Averbach, MD, of the University of California, San Francisco, and her colleagues wrote in their study published in Contraception.
Dr. Averbach and her colleagues randomized 96 women to receive a two-rod levonorgestrel (LNG)–releasing subdermal contraceptive implant within 5 days of delivery (mean time, 36 hours post delivery) and 87 women to delay the implant to between 6 and 8 weeks at a postpartum follow-up visit (mean time, 68 days). The women were a minimum of 18 years old with a recent vaginal or cesarean section delivery at a Ugandan hospital; 55% of the women had at least three children, and 73% said they had prior experience breastfeeding. The researchers then examined infant weight change and infant head circumference change at 6 months from birth, time to lactogenesis, and whether mothers continued to breastfeed at 3 months and 6 months after birth.
Infant weight was similar in the immediate-implant group (4,632 g), compared with the delayed-implant group (4,407 g; P = .26); infant head circumference was similar between both groups (9.3 cm vs. 9.5 cm; P = .70) at 6 months as well. The time to lactogenesis was not significantly different in the immediate-implant (65 hours) and delayed-implant (63 hours; P = .84) groups. At 3 months, 74% of immediate-implant participants and 71% of delayed-implant participants said they were breastfeeding exclusively (P = .74); at 6 months, 48% of immediate implant participants and 52% of delayed implant participants reported exclusive breastfeeding (P equals .58).
Limitations of the study included follow-up to only 6 months and selection of participants with previous breastfeeding experience. Researchers also noted better measurements of infant and maternal breast milk intake also could be used and limit generalization of the results.
This study was funded by the Society of Family Planning Research Fund. Dr. Averbach is supported by an award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The other authors had no relevant financial disclosures.
SOURCE: Averbach S et al. Contraception. 2018. doi: 10.1016/j.contraception.2018.10.008.
Researchers found no significant differences in infant growth, changes in breastfeeding initiation or breastfeeding continuation at 3-month and 6-month follow-up among women who received a levonorgestrel contraception implant very soon after delivery, compared with women who waited to receive the implant.
“These findings are consistent with the preponderance of literature supporting the hypothesis that progestin-containing contraceptives do not compromise a woman’s ability to initiate or sustain breastfeeding and do not adversely affect infant growth,” Sarah Averbach, MD, of the University of California, San Francisco, and her colleagues wrote in their study published in Contraception.
Dr. Averbach and her colleagues randomized 96 women to receive a two-rod levonorgestrel (LNG)–releasing subdermal contraceptive implant within 5 days of delivery (mean time, 36 hours post delivery) and 87 women to delay the implant to between 6 and 8 weeks at a postpartum follow-up visit (mean time, 68 days). The women were a minimum of 18 years old with a recent vaginal or cesarean section delivery at a Ugandan hospital; 55% of the women had at least three children, and 73% said they had prior experience breastfeeding. The researchers then examined infant weight change and infant head circumference change at 6 months from birth, time to lactogenesis, and whether mothers continued to breastfeed at 3 months and 6 months after birth.
Infant weight was similar in the immediate-implant group (4,632 g), compared with the delayed-implant group (4,407 g; P = .26); infant head circumference was similar between both groups (9.3 cm vs. 9.5 cm; P = .70) at 6 months as well. The time to lactogenesis was not significantly different in the immediate-implant (65 hours) and delayed-implant (63 hours; P = .84) groups. At 3 months, 74% of immediate-implant participants and 71% of delayed-implant participants said they were breastfeeding exclusively (P = .74); at 6 months, 48% of immediate implant participants and 52% of delayed implant participants reported exclusive breastfeeding (P equals .58).
Limitations of the study included follow-up to only 6 months and selection of participants with previous breastfeeding experience. Researchers also noted better measurements of infant and maternal breast milk intake also could be used and limit generalization of the results.
This study was funded by the Society of Family Planning Research Fund. Dr. Averbach is supported by an award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The other authors had no relevant financial disclosures.
SOURCE: Averbach S et al. Contraception. 2018. doi: 10.1016/j.contraception.2018.10.008.
FROM CONTRACEPTION
Key clinical point:
Major finding: Infant weight was similar in the immediate-implant group (4,632 g), compared with the delayed-implant group (4,407 g; P = .26); infant head circumference was similar between both groups (9.3 cm vs. 9.5 cm; P = .70) at 6 months as well.
Study details: A randomized trial of 96 women in Uganda who received a contraceptive implant less than 5 days after delivery and 86 women who received the implant between 6 and 8 weeks post partum.
Disclosures: This study was funded by the Society of Family Planning Research Fund. Dr. Averbach is supported by an award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The other authors had no relevant financial disclosures.
Source: Averbach S et al. Contraception. 2018. doi: 10.1016/j.contraception.2018.10.008.
Assess ADHD at developmental age, rather than chronological
ORLANDO – said David O. Childers, MD, chief of the division of developmental pediatrics at the University of Florida in Jacksonville.
“That is a huge consideration, and the vast majority of pediatricians don’t understand that,” Dr. Childers said at the annual meeting of the American Academy of Pediatrics.
A diagnosis of ADHD in children should be reserved for situations in which the symptoms clearly interfere with how they function in social, academic, and occupational settings. ADHD should not be diagnosed in children who exhibit hostility, defiance, a failure to understand, or who have oppositional defiant disorder (ODD). In addition, ADHD in children often overlaps with other conditions, such as learning disabilities, ODD, anxiety, depression, poor self-esteem, conduct disorder, motor coordination problems, and encopresis and enuresis.
One mistake you can make is starting the medication too young in patients who exhibit symptoms of ADHD. Receptive language relative to developmental age should be taken into consideration when deciding whether to treat very young patients. “If the voice in your head is at a 3-year-old level, that’s going to be your behavior picture regardless of your chronological age,” Dr. Childers said.
You also can misdiagnose ADHD in young patients because you don’t recognize behavioral phenotypes and diagnoses that are similar to ADHD. Extreme prematurity, global developmental delay, fetal alcohol syndrome, spina bifida, genetic syndrome, intellectual and learning disabilities, closed head injuries, and depression all can mimic ADHD symptoms. “Everything that wheezes is not asthma, and everything that is ‘hyper’ or ‘drifty’ is not ADHD,” he noted.
Another mistake is considering mixed amphetamine salts (MAS) the same as methylphenidate (MPH), Dr. Childers said. MAS has 5 mg of active isomer versus 2.5 mg in MPH doses, and patients could see side effects from the increased potency in MAS in the form of appetite suppression and tics. You should start with a low dose of the medication and titrate up until patients get the most out of the medication to avoid situations in which patients report a change in personality or that the treatment is not effective.
“The medicine should never change a kid’s personality,” Dr. Childers said. “We are not here to treat the kid to the point of hyperactivity management; we’re here to treat for attention.”
In cases where patients ask for the short-acting medication rather than long-acting stimulant, remember that short-acting medication for ADHD has a higher potential for abuse. “Medication diversion is real. It does occur,” he said.
Establishing a basal sleep history and whether the child has insomnia is important before prescribing ADHD medication. Dr. Childers noted he uses a short-acting stimulant as an off-label treatment to treat some of his patients with insomnia, but he said to be aware of side effects like headache, abdominal pain, anorexia, and tics, as well as cardiac issues.
You also should rethink your diagnosis if the medication is not having an effect after two trials of a stimulant, Dr. Childers noted, and when a child has conditions like anxiety and depression that can mimic ADHD symptoms, a stimulant will increase irritability and have a paradoxical response to the medication. Inattentive ADHD can be missed in adolescent patients who struggle academically until they fall behind their peers, which can result in developing depression that complicates treatment, he said.
A learning disorder also can be mistaken for ADHD in children and presents in different ways depending on what grade the child is in. As a child ages and learns how to read, he or she will begin to fall behind as learning through reading becomes more complex. Learning disorders should be ruled out before treating for ADHD because, if the child improves after taking a stimulant, it could be more difficult to find a diagnosis of learning disorder later, Dr. Childers noted.
Bipolar disorder can be overdiagnosed in children; in these patients, the most likely diagnoses are ADHD and ODD, he said. According to a 2004 National Alliance of Mental Illness fact sheet, approximately 7% of patients in caseloads for federally funded studies in psychiatric facilities met the criteria for bipolar disorder, but the lifelong prevalence of the disease is between 1% and 1.6%.
“Bipolar disorder is frequently oppositional defiant disorder, and the word ‘no’ can be a trigger,” Dr. Childers said.
Dr. Childers reported no relevant conflicts of interest.
ORLANDO – said David O. Childers, MD, chief of the division of developmental pediatrics at the University of Florida in Jacksonville.
“That is a huge consideration, and the vast majority of pediatricians don’t understand that,” Dr. Childers said at the annual meeting of the American Academy of Pediatrics.
A diagnosis of ADHD in children should be reserved for situations in which the symptoms clearly interfere with how they function in social, academic, and occupational settings. ADHD should not be diagnosed in children who exhibit hostility, defiance, a failure to understand, or who have oppositional defiant disorder (ODD). In addition, ADHD in children often overlaps with other conditions, such as learning disabilities, ODD, anxiety, depression, poor self-esteem, conduct disorder, motor coordination problems, and encopresis and enuresis.
One mistake you can make is starting the medication too young in patients who exhibit symptoms of ADHD. Receptive language relative to developmental age should be taken into consideration when deciding whether to treat very young patients. “If the voice in your head is at a 3-year-old level, that’s going to be your behavior picture regardless of your chronological age,” Dr. Childers said.
You also can misdiagnose ADHD in young patients because you don’t recognize behavioral phenotypes and diagnoses that are similar to ADHD. Extreme prematurity, global developmental delay, fetal alcohol syndrome, spina bifida, genetic syndrome, intellectual and learning disabilities, closed head injuries, and depression all can mimic ADHD symptoms. “Everything that wheezes is not asthma, and everything that is ‘hyper’ or ‘drifty’ is not ADHD,” he noted.
Another mistake is considering mixed amphetamine salts (MAS) the same as methylphenidate (MPH), Dr. Childers said. MAS has 5 mg of active isomer versus 2.5 mg in MPH doses, and patients could see side effects from the increased potency in MAS in the form of appetite suppression and tics. You should start with a low dose of the medication and titrate up until patients get the most out of the medication to avoid situations in which patients report a change in personality or that the treatment is not effective.
“The medicine should never change a kid’s personality,” Dr. Childers said. “We are not here to treat the kid to the point of hyperactivity management; we’re here to treat for attention.”
In cases where patients ask for the short-acting medication rather than long-acting stimulant, remember that short-acting medication for ADHD has a higher potential for abuse. “Medication diversion is real. It does occur,” he said.
Establishing a basal sleep history and whether the child has insomnia is important before prescribing ADHD medication. Dr. Childers noted he uses a short-acting stimulant as an off-label treatment to treat some of his patients with insomnia, but he said to be aware of side effects like headache, abdominal pain, anorexia, and tics, as well as cardiac issues.
You also should rethink your diagnosis if the medication is not having an effect after two trials of a stimulant, Dr. Childers noted, and when a child has conditions like anxiety and depression that can mimic ADHD symptoms, a stimulant will increase irritability and have a paradoxical response to the medication. Inattentive ADHD can be missed in adolescent patients who struggle academically until they fall behind their peers, which can result in developing depression that complicates treatment, he said.
A learning disorder also can be mistaken for ADHD in children and presents in different ways depending on what grade the child is in. As a child ages and learns how to read, he or she will begin to fall behind as learning through reading becomes more complex. Learning disorders should be ruled out before treating for ADHD because, if the child improves after taking a stimulant, it could be more difficult to find a diagnosis of learning disorder later, Dr. Childers noted.
Bipolar disorder can be overdiagnosed in children; in these patients, the most likely diagnoses are ADHD and ODD, he said. According to a 2004 National Alliance of Mental Illness fact sheet, approximately 7% of patients in caseloads for federally funded studies in psychiatric facilities met the criteria for bipolar disorder, but the lifelong prevalence of the disease is between 1% and 1.6%.
“Bipolar disorder is frequently oppositional defiant disorder, and the word ‘no’ can be a trigger,” Dr. Childers said.
Dr. Childers reported no relevant conflicts of interest.
ORLANDO – said David O. Childers, MD, chief of the division of developmental pediatrics at the University of Florida in Jacksonville.
“That is a huge consideration, and the vast majority of pediatricians don’t understand that,” Dr. Childers said at the annual meeting of the American Academy of Pediatrics.
A diagnosis of ADHD in children should be reserved for situations in which the symptoms clearly interfere with how they function in social, academic, and occupational settings. ADHD should not be diagnosed in children who exhibit hostility, defiance, a failure to understand, or who have oppositional defiant disorder (ODD). In addition, ADHD in children often overlaps with other conditions, such as learning disabilities, ODD, anxiety, depression, poor self-esteem, conduct disorder, motor coordination problems, and encopresis and enuresis.
One mistake you can make is starting the medication too young in patients who exhibit symptoms of ADHD. Receptive language relative to developmental age should be taken into consideration when deciding whether to treat very young patients. “If the voice in your head is at a 3-year-old level, that’s going to be your behavior picture regardless of your chronological age,” Dr. Childers said.
You also can misdiagnose ADHD in young patients because you don’t recognize behavioral phenotypes and diagnoses that are similar to ADHD. Extreme prematurity, global developmental delay, fetal alcohol syndrome, spina bifida, genetic syndrome, intellectual and learning disabilities, closed head injuries, and depression all can mimic ADHD symptoms. “Everything that wheezes is not asthma, and everything that is ‘hyper’ or ‘drifty’ is not ADHD,” he noted.
Another mistake is considering mixed amphetamine salts (MAS) the same as methylphenidate (MPH), Dr. Childers said. MAS has 5 mg of active isomer versus 2.5 mg in MPH doses, and patients could see side effects from the increased potency in MAS in the form of appetite suppression and tics. You should start with a low dose of the medication and titrate up until patients get the most out of the medication to avoid situations in which patients report a change in personality or that the treatment is not effective.
“The medicine should never change a kid’s personality,” Dr. Childers said. “We are not here to treat the kid to the point of hyperactivity management; we’re here to treat for attention.”
In cases where patients ask for the short-acting medication rather than long-acting stimulant, remember that short-acting medication for ADHD has a higher potential for abuse. “Medication diversion is real. It does occur,” he said.
Establishing a basal sleep history and whether the child has insomnia is important before prescribing ADHD medication. Dr. Childers noted he uses a short-acting stimulant as an off-label treatment to treat some of his patients with insomnia, but he said to be aware of side effects like headache, abdominal pain, anorexia, and tics, as well as cardiac issues.
You also should rethink your diagnosis if the medication is not having an effect after two trials of a stimulant, Dr. Childers noted, and when a child has conditions like anxiety and depression that can mimic ADHD symptoms, a stimulant will increase irritability and have a paradoxical response to the medication. Inattentive ADHD can be missed in adolescent patients who struggle academically until they fall behind their peers, which can result in developing depression that complicates treatment, he said.
A learning disorder also can be mistaken for ADHD in children and presents in different ways depending on what grade the child is in. As a child ages and learns how to read, he or she will begin to fall behind as learning through reading becomes more complex. Learning disorders should be ruled out before treating for ADHD because, if the child improves after taking a stimulant, it could be more difficult to find a diagnosis of learning disorder later, Dr. Childers noted.
Bipolar disorder can be overdiagnosed in children; in these patients, the most likely diagnoses are ADHD and ODD, he said. According to a 2004 National Alliance of Mental Illness fact sheet, approximately 7% of patients in caseloads for federally funded studies in psychiatric facilities met the criteria for bipolar disorder, but the lifelong prevalence of the disease is between 1% and 1.6%.
“Bipolar disorder is frequently oppositional defiant disorder, and the word ‘no’ can be a trigger,” Dr. Childers said.
Dr. Childers reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAP 18
New pediatric therapies show promise for influenza, multidrug-resistant pathogens
ORLANDO – John S. Bradley, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Bradley, director of the division of infectious diseases at Rady Children’s Hospital–San Diego, discussed a therapy for influenza, baloxavir, which was recently approved as a fast-acting single-dose medication and currently is under study in children. Also, a recent double-blind, phase 3 trial in the New England Journal of Medicine recruited patients as young as 12 years old. In the study, patients in the intervention group resolved their fever in median 25 hours, compared with 42 hours in the placebo group. Baloxavir better reduced viral load at day 2, compared with oseltamivir and placebo, but there was a similar alleviation of symptoms between both groups. There was a greater incidence of nausea and vomiting among the oseltamivir group, while the baloxavir group had a higher rate of diarrhea (N Engl J Med 2018;379:913-23).
However, Dr. Bradley noted baloxavir is much more expensive than oseltamivir, which may not justify the better tolerance of the drug for influenza treatment.
You don’t get better with it faster, so I’m not going to be recommending you all run to baloxavir this flu season for kids 12 years of age and older,” Dr. Bradley said. “I think oseltamivir is still fine, unless we end up with oseltamivir resistance.”
Solithromycin, an intravenous and oral fluoroketolide, has shown promising results against gram-positive and gram-negative pathogens for community-acquired pneumonia and other infections. During the drug’s study period, Cempra sold solithromycin to Melinta. However, one trial showed elevated liver functions in a higher number of patients than expected, and the Food and Drug Administration asked Melinta to conduct additional studies. Investigations on solithromycin have currently stopped until Melinta secures funding. “Until they get better resources, this particular drug is on hold, but you’ll see it again, I’m sure,” said Dr. Bradley, who also is professor and chief of the division of infectious diseases at the University of California, San Diego.
Dr. Bradley also discussed the efficacy of tedizolid, a protein synthesis inhibitor similar to linezolid approved in adults for the treatment of skin infections. He noted tedizolid is more active than linezolid, but the treatment course is a shorter dose for a shorter amount of time. Compared with linezolid, which can cause thrombocytopenia or neutropenia if taken for more than 10 days to 14 days, there also are fewer side effects.
“The tedizolid is much, much safer,” Dr. Bradley said, who added that trials for efficacy of tedizolid are currently underway in pediatric patients. “We’re hoping that will end up being the pediatric oxazolidinone.”
Other investigative therapies approved for adults and under study for use in children include ceftazidime/avibactam for treatment of urinary tract and complicated intra-abdominal infections, which is effective against meropenem-resistant Enterobacteriaceae and resistant Escherichia coli with extended-spectrum beta-lactamases (ESBL); ceftolozane/tazobactam has also been approved for adults, is pending approval in pediatric patients, and is active against ESBLs such as Pseudomonas; and meropenem/vaborbactam, which is active against Klebsiella pneumoniae carbapenemase (KPC)–producing isolates. Plazomicin, an aminoglycoside similar to gentamicin used to treat KPC-producing isolates, is stable against enzymes that degrade gentamicin and tobramycin.
Therapies currently under study for adults and being considered for children include imipenem/relebactam for treatment against E. coli, Enterobacter species, and KPC-producing isolates, and cefiderocol, a siderophore cephalosporin antibiotic – commonly described as a “Trojan horse” antibiotic because it binds to iron and is actively transported into the organism – is effective against Pseudomonas and has finished phase 2 trials in adults, with researchers looking to do single-dose trials in children, Dr. Bradley noted.
More experimentally, phage therapy for multidrug-resistant Acinetobacter baumannii proved effective in a 68-year-old patient with necrotizing pancreatitis who continued to deteriorate over a 4-month period despite multiple courses of antibiotics and attempted drainage of a pancreatic pseudocyst. Researchers selected a phage-specific bacterium with specificity for A. baumannii and cured him. “This is like science fiction,” Dr. Bradley said.
Dr. Bradley reported no relevant conflicts of interest.
ORLANDO – John S. Bradley, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Bradley, director of the division of infectious diseases at Rady Children’s Hospital–San Diego, discussed a therapy for influenza, baloxavir, which was recently approved as a fast-acting single-dose medication and currently is under study in children. Also, a recent double-blind, phase 3 trial in the New England Journal of Medicine recruited patients as young as 12 years old. In the study, patients in the intervention group resolved their fever in median 25 hours, compared with 42 hours in the placebo group. Baloxavir better reduced viral load at day 2, compared with oseltamivir and placebo, but there was a similar alleviation of symptoms between both groups. There was a greater incidence of nausea and vomiting among the oseltamivir group, while the baloxavir group had a higher rate of diarrhea (N Engl J Med 2018;379:913-23).
However, Dr. Bradley noted baloxavir is much more expensive than oseltamivir, which may not justify the better tolerance of the drug for influenza treatment.
You don’t get better with it faster, so I’m not going to be recommending you all run to baloxavir this flu season for kids 12 years of age and older,” Dr. Bradley said. “I think oseltamivir is still fine, unless we end up with oseltamivir resistance.”
Solithromycin, an intravenous and oral fluoroketolide, has shown promising results against gram-positive and gram-negative pathogens for community-acquired pneumonia and other infections. During the drug’s study period, Cempra sold solithromycin to Melinta. However, one trial showed elevated liver functions in a higher number of patients than expected, and the Food and Drug Administration asked Melinta to conduct additional studies. Investigations on solithromycin have currently stopped until Melinta secures funding. “Until they get better resources, this particular drug is on hold, but you’ll see it again, I’m sure,” said Dr. Bradley, who also is professor and chief of the division of infectious diseases at the University of California, San Diego.
Dr. Bradley also discussed the efficacy of tedizolid, a protein synthesis inhibitor similar to linezolid approved in adults for the treatment of skin infections. He noted tedizolid is more active than linezolid, but the treatment course is a shorter dose for a shorter amount of time. Compared with linezolid, which can cause thrombocytopenia or neutropenia if taken for more than 10 days to 14 days, there also are fewer side effects.
“The tedizolid is much, much safer,” Dr. Bradley said, who added that trials for efficacy of tedizolid are currently underway in pediatric patients. “We’re hoping that will end up being the pediatric oxazolidinone.”
Other investigative therapies approved for adults and under study for use in children include ceftazidime/avibactam for treatment of urinary tract and complicated intra-abdominal infections, which is effective against meropenem-resistant Enterobacteriaceae and resistant Escherichia coli with extended-spectrum beta-lactamases (ESBL); ceftolozane/tazobactam has also been approved for adults, is pending approval in pediatric patients, and is active against ESBLs such as Pseudomonas; and meropenem/vaborbactam, which is active against Klebsiella pneumoniae carbapenemase (KPC)–producing isolates. Plazomicin, an aminoglycoside similar to gentamicin used to treat KPC-producing isolates, is stable against enzymes that degrade gentamicin and tobramycin.
Therapies currently under study for adults and being considered for children include imipenem/relebactam for treatment against E. coli, Enterobacter species, and KPC-producing isolates, and cefiderocol, a siderophore cephalosporin antibiotic – commonly described as a “Trojan horse” antibiotic because it binds to iron and is actively transported into the organism – is effective against Pseudomonas and has finished phase 2 trials in adults, with researchers looking to do single-dose trials in children, Dr. Bradley noted.
More experimentally, phage therapy for multidrug-resistant Acinetobacter baumannii proved effective in a 68-year-old patient with necrotizing pancreatitis who continued to deteriorate over a 4-month period despite multiple courses of antibiotics and attempted drainage of a pancreatic pseudocyst. Researchers selected a phage-specific bacterium with specificity for A. baumannii and cured him. “This is like science fiction,” Dr. Bradley said.
Dr. Bradley reported no relevant conflicts of interest.
ORLANDO – John S. Bradley, MD, said at the annual meeting of the American Academy of Pediatrics.
Dr. Bradley, director of the division of infectious diseases at Rady Children’s Hospital–San Diego, discussed a therapy for influenza, baloxavir, which was recently approved as a fast-acting single-dose medication and currently is under study in children. Also, a recent double-blind, phase 3 trial in the New England Journal of Medicine recruited patients as young as 12 years old. In the study, patients in the intervention group resolved their fever in median 25 hours, compared with 42 hours in the placebo group. Baloxavir better reduced viral load at day 2, compared with oseltamivir and placebo, but there was a similar alleviation of symptoms between both groups. There was a greater incidence of nausea and vomiting among the oseltamivir group, while the baloxavir group had a higher rate of diarrhea (N Engl J Med 2018;379:913-23).
However, Dr. Bradley noted baloxavir is much more expensive than oseltamivir, which may not justify the better tolerance of the drug for influenza treatment.
You don’t get better with it faster, so I’m not going to be recommending you all run to baloxavir this flu season for kids 12 years of age and older,” Dr. Bradley said. “I think oseltamivir is still fine, unless we end up with oseltamivir resistance.”
Solithromycin, an intravenous and oral fluoroketolide, has shown promising results against gram-positive and gram-negative pathogens for community-acquired pneumonia and other infections. During the drug’s study period, Cempra sold solithromycin to Melinta. However, one trial showed elevated liver functions in a higher number of patients than expected, and the Food and Drug Administration asked Melinta to conduct additional studies. Investigations on solithromycin have currently stopped until Melinta secures funding. “Until they get better resources, this particular drug is on hold, but you’ll see it again, I’m sure,” said Dr. Bradley, who also is professor and chief of the division of infectious diseases at the University of California, San Diego.
Dr. Bradley also discussed the efficacy of tedizolid, a protein synthesis inhibitor similar to linezolid approved in adults for the treatment of skin infections. He noted tedizolid is more active than linezolid, but the treatment course is a shorter dose for a shorter amount of time. Compared with linezolid, which can cause thrombocytopenia or neutropenia if taken for more than 10 days to 14 days, there also are fewer side effects.
“The tedizolid is much, much safer,” Dr. Bradley said, who added that trials for efficacy of tedizolid are currently underway in pediatric patients. “We’re hoping that will end up being the pediatric oxazolidinone.”
Other investigative therapies approved for adults and under study for use in children include ceftazidime/avibactam for treatment of urinary tract and complicated intra-abdominal infections, which is effective against meropenem-resistant Enterobacteriaceae and resistant Escherichia coli with extended-spectrum beta-lactamases (ESBL); ceftolozane/tazobactam has also been approved for adults, is pending approval in pediatric patients, and is active against ESBLs such as Pseudomonas; and meropenem/vaborbactam, which is active against Klebsiella pneumoniae carbapenemase (KPC)–producing isolates. Plazomicin, an aminoglycoside similar to gentamicin used to treat KPC-producing isolates, is stable against enzymes that degrade gentamicin and tobramycin.
Therapies currently under study for adults and being considered for children include imipenem/relebactam for treatment against E. coli, Enterobacter species, and KPC-producing isolates, and cefiderocol, a siderophore cephalosporin antibiotic – commonly described as a “Trojan horse” antibiotic because it binds to iron and is actively transported into the organism – is effective against Pseudomonas and has finished phase 2 trials in adults, with researchers looking to do single-dose trials in children, Dr. Bradley noted.
More experimentally, phage therapy for multidrug-resistant Acinetobacter baumannii proved effective in a 68-year-old patient with necrotizing pancreatitis who continued to deteriorate over a 4-month period despite multiple courses of antibiotics and attempted drainage of a pancreatic pseudocyst. Researchers selected a phage-specific bacterium with specificity for A. baumannii and cured him. “This is like science fiction,” Dr. Bradley said.
Dr. Bradley reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAP 18
Team approach needed to tackle complex care for fostered, adopted children
ORLANDO – Children in foster care or who are adopted are at a higher risk of developing medical conditions, and a team approach is needed when caring for these children with a focus on medical, developmental, and mental health, Judith K. Eckerle, MD, said at the annual meeting of the American Academy of Pediatrics.
“One of the things that I’m advocating more these days is [that] we don’t operate as physicians or medical people in a vacuum. We certainly have to operate within a team approach, and that really is the best approach for these kids,” said Dr. Eckerle, a pediatrician who specializes in neurobehavioral development and is director of the Adoption Medicine Clinic at the University of Minnesota, Minneapolis.
Recognizing how early adversity is associated with conditions such as growth, brain development, motor skills, cognition, mental health, attachment, stress sensitivity, sensory processing, language, and chromosome structure can help in the medical assessment of these patients. Dr. Eckerle noted that these issues can even present in children with no early adversity who are adopted or placed into healthy foster environments.
“What we’ve learned is, we’ve just seen dozens if not hundreds of kids now in the exact same scenario who are having exactly the same types of bonding, or attachment or stress issues, as kids who did spend those few years in a really neglectful or orphanage scenarios,” Dr. Eckerle said.
“The majority of adopted and foster care kids do great in the end,” she emphasized. “As a group, they can do beautifully, but we just have to be cognizant of the things that we should be looking for and not just gloss over them so we’re not missing things along the way.”
At her institution, Dr. Eckerle said a medical assessment for new foster care or adoption patients begins with explaining the exam process with the parent and child before the child is screened by an occupational therapist. During the screening, the occupational therapist will screen the child for signs of developmental delay such as delay in gross or fine motor skills, speech, cognition, musculoskeletal, psychosocial, and sensory processing, as well as in school-based, self-care, and functional skills.
While the child is out of the room, Dr. Eckerle will ask whether the parent has any concerns about the child. After that, she will check the child’s eyes, heart, and ears to make sure they are healthy; during the end of the exam, she may order lab tests based on any concerns about prenatal or foster care trauma. Adolescents and children in whom sexual abuse is suspected should be screened for hepatitis B, hepatitis C, syphilis, and HIV.
International adoptees should undergo ova and parasite screening and the Giardia antigen test. International adoptees as well as those who had contact with the homeless or prison systems should undergo QuantiFERON-TB Gold testing for tuberculosis, and children aged under 5 years should receive targeted tuberculin skin testing. She said her center also screens children for intellectual disability, fetal alcohol spectrum disorder, short stature, and precocious puberty.
Lastly, the child is seen by a pediatric psychologist for additional screening. Dr. Eckerle noted the most common diagnoses seen at her center are reactive attachment disorder, oppositional defiant disorder, conduct disorder, pervasive development delay, autism, learning disabilities, emotional or behavioral problems, and ADHD. With regard to mental health, doctors should help a caregiver understand a child with a mental health conditions and consider pairing with a pediatric psychologist to offer evidence-based interventions for children aged under 5 years, such as child-parent psychotherapy, circle of security, and attachment biobehavioral catch-up, and offer trauma-focused cognitive behavioral therapy in children aged between 4 and 5 years.
Bad behavior often is misinterpreted as dishonesty and willful misconduct on the part of the child, but instead could be a neurodevelopmental or mental health problem, Dr. Eckerle noted. For example, a parent might interpret a child who takes a sparkling watch off of a teacher’s desk as stealing, but the child may not yet understand the concept of ownership. “Reframing these things will lead to the goal of helping that parent understand the child.”
Above all, Dr. Eckerle said she emphasizes what will happen after the exam with children she sees to establish a routine, as many children she sees have gone through multiple foster environments. It also is important to let parents know the exam is just a first step and they likely will not receive a diagnosis that day, particularly with out-of-state or international families. “I want them to have appropriate expectations that we’re not going to cure or solve or fix everything the second that they leave that room, but that we are starting the process and we are on the right track.”
Dr. Eckerle reported no conflicts of interest.
ORLANDO – Children in foster care or who are adopted are at a higher risk of developing medical conditions, and a team approach is needed when caring for these children with a focus on medical, developmental, and mental health, Judith K. Eckerle, MD, said at the annual meeting of the American Academy of Pediatrics.
“One of the things that I’m advocating more these days is [that] we don’t operate as physicians or medical people in a vacuum. We certainly have to operate within a team approach, and that really is the best approach for these kids,” said Dr. Eckerle, a pediatrician who specializes in neurobehavioral development and is director of the Adoption Medicine Clinic at the University of Minnesota, Minneapolis.
Recognizing how early adversity is associated with conditions such as growth, brain development, motor skills, cognition, mental health, attachment, stress sensitivity, sensory processing, language, and chromosome structure can help in the medical assessment of these patients. Dr. Eckerle noted that these issues can even present in children with no early adversity who are adopted or placed into healthy foster environments.
“What we’ve learned is, we’ve just seen dozens if not hundreds of kids now in the exact same scenario who are having exactly the same types of bonding, or attachment or stress issues, as kids who did spend those few years in a really neglectful or orphanage scenarios,” Dr. Eckerle said.
“The majority of adopted and foster care kids do great in the end,” she emphasized. “As a group, they can do beautifully, but we just have to be cognizant of the things that we should be looking for and not just gloss over them so we’re not missing things along the way.”
At her institution, Dr. Eckerle said a medical assessment for new foster care or adoption patients begins with explaining the exam process with the parent and child before the child is screened by an occupational therapist. During the screening, the occupational therapist will screen the child for signs of developmental delay such as delay in gross or fine motor skills, speech, cognition, musculoskeletal, psychosocial, and sensory processing, as well as in school-based, self-care, and functional skills.
While the child is out of the room, Dr. Eckerle will ask whether the parent has any concerns about the child. After that, she will check the child’s eyes, heart, and ears to make sure they are healthy; during the end of the exam, she may order lab tests based on any concerns about prenatal or foster care trauma. Adolescents and children in whom sexual abuse is suspected should be screened for hepatitis B, hepatitis C, syphilis, and HIV.
International adoptees should undergo ova and parasite screening and the Giardia antigen test. International adoptees as well as those who had contact with the homeless or prison systems should undergo QuantiFERON-TB Gold testing for tuberculosis, and children aged under 5 years should receive targeted tuberculin skin testing. She said her center also screens children for intellectual disability, fetal alcohol spectrum disorder, short stature, and precocious puberty.
Lastly, the child is seen by a pediatric psychologist for additional screening. Dr. Eckerle noted the most common diagnoses seen at her center are reactive attachment disorder, oppositional defiant disorder, conduct disorder, pervasive development delay, autism, learning disabilities, emotional or behavioral problems, and ADHD. With regard to mental health, doctors should help a caregiver understand a child with a mental health conditions and consider pairing with a pediatric psychologist to offer evidence-based interventions for children aged under 5 years, such as child-parent psychotherapy, circle of security, and attachment biobehavioral catch-up, and offer trauma-focused cognitive behavioral therapy in children aged between 4 and 5 years.
Bad behavior often is misinterpreted as dishonesty and willful misconduct on the part of the child, but instead could be a neurodevelopmental or mental health problem, Dr. Eckerle noted. For example, a parent might interpret a child who takes a sparkling watch off of a teacher’s desk as stealing, but the child may not yet understand the concept of ownership. “Reframing these things will lead to the goal of helping that parent understand the child.”
Above all, Dr. Eckerle said she emphasizes what will happen after the exam with children she sees to establish a routine, as many children she sees have gone through multiple foster environments. It also is important to let parents know the exam is just a first step and they likely will not receive a diagnosis that day, particularly with out-of-state or international families. “I want them to have appropriate expectations that we’re not going to cure or solve or fix everything the second that they leave that room, but that we are starting the process and we are on the right track.”
Dr. Eckerle reported no conflicts of interest.
ORLANDO – Children in foster care or who are adopted are at a higher risk of developing medical conditions, and a team approach is needed when caring for these children with a focus on medical, developmental, and mental health, Judith K. Eckerle, MD, said at the annual meeting of the American Academy of Pediatrics.
“One of the things that I’m advocating more these days is [that] we don’t operate as physicians or medical people in a vacuum. We certainly have to operate within a team approach, and that really is the best approach for these kids,” said Dr. Eckerle, a pediatrician who specializes in neurobehavioral development and is director of the Adoption Medicine Clinic at the University of Minnesota, Minneapolis.
Recognizing how early adversity is associated with conditions such as growth, brain development, motor skills, cognition, mental health, attachment, stress sensitivity, sensory processing, language, and chromosome structure can help in the medical assessment of these patients. Dr. Eckerle noted that these issues can even present in children with no early adversity who are adopted or placed into healthy foster environments.
“What we’ve learned is, we’ve just seen dozens if not hundreds of kids now in the exact same scenario who are having exactly the same types of bonding, or attachment or stress issues, as kids who did spend those few years in a really neglectful or orphanage scenarios,” Dr. Eckerle said.
“The majority of adopted and foster care kids do great in the end,” she emphasized. “As a group, they can do beautifully, but we just have to be cognizant of the things that we should be looking for and not just gloss over them so we’re not missing things along the way.”
At her institution, Dr. Eckerle said a medical assessment for new foster care or adoption patients begins with explaining the exam process with the parent and child before the child is screened by an occupational therapist. During the screening, the occupational therapist will screen the child for signs of developmental delay such as delay in gross or fine motor skills, speech, cognition, musculoskeletal, psychosocial, and sensory processing, as well as in school-based, self-care, and functional skills.
While the child is out of the room, Dr. Eckerle will ask whether the parent has any concerns about the child. After that, she will check the child’s eyes, heart, and ears to make sure they are healthy; during the end of the exam, she may order lab tests based on any concerns about prenatal or foster care trauma. Adolescents and children in whom sexual abuse is suspected should be screened for hepatitis B, hepatitis C, syphilis, and HIV.
International adoptees should undergo ova and parasite screening and the Giardia antigen test. International adoptees as well as those who had contact with the homeless or prison systems should undergo QuantiFERON-TB Gold testing for tuberculosis, and children aged under 5 years should receive targeted tuberculin skin testing. She said her center also screens children for intellectual disability, fetal alcohol spectrum disorder, short stature, and precocious puberty.
Lastly, the child is seen by a pediatric psychologist for additional screening. Dr. Eckerle noted the most common diagnoses seen at her center are reactive attachment disorder, oppositional defiant disorder, conduct disorder, pervasive development delay, autism, learning disabilities, emotional or behavioral problems, and ADHD. With regard to mental health, doctors should help a caregiver understand a child with a mental health conditions and consider pairing with a pediatric psychologist to offer evidence-based interventions for children aged under 5 years, such as child-parent psychotherapy, circle of security, and attachment biobehavioral catch-up, and offer trauma-focused cognitive behavioral therapy in children aged between 4 and 5 years.
Bad behavior often is misinterpreted as dishonesty and willful misconduct on the part of the child, but instead could be a neurodevelopmental or mental health problem, Dr. Eckerle noted. For example, a parent might interpret a child who takes a sparkling watch off of a teacher’s desk as stealing, but the child may not yet understand the concept of ownership. “Reframing these things will lead to the goal of helping that parent understand the child.”
Above all, Dr. Eckerle said she emphasizes what will happen after the exam with children she sees to establish a routine, as many children she sees have gone through multiple foster environments. It also is important to let parents know the exam is just a first step and they likely will not receive a diagnosis that day, particularly with out-of-state or international families. “I want them to have appropriate expectations that we’re not going to cure or solve or fix everything the second that they leave that room, but that we are starting the process and we are on the right track.”
Dr. Eckerle reported no conflicts of interest.
EXPERT ANALYSIS FROM AAP 18
Children with headache disorders may benefit from anti-CGRP mAb treatment
Use of anti–calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) may benefit children with more than 8 headache days each month, a high Pediatric Migraine Disability Assessment (PedMIDAS) score, and failure of other treatments; however, researchers cautioned that long-term safety outcomes for the treatment are not yet known, according to a recent set of recommendations published in the journal Headache.
Christina L. Szperka, MD, MSCE, of the division of neurology at the Children’s Hospital of Philadelphia and members of the Pediatric and Adolescent Headache special interest group of the American Headache Society discussed the topic of anti-CGRP mAbs at the 2018 Annual Scientific Meeting of the American Headache Society. They noted clinical outcomes for anti-CGRP mAbs in pediatric patients will likely not be available for several years and created a set of recommendations based on expert opinion of anti-CGRP mAb use in children and adolescents.
Their recommendations support using anti-CGRP mAbs for children with migraine if patients meet the following criteria: headache frequency exceeding 8 headache days per month; a PedMIDAS score of 30 or greater; failure of two or more therapies, such as pharmacologic, nonpharmacologic, or nutraceutical ones; and in patients who are past puberty.
The special interest group recommended against use of anti-CGRP mAbs in children and adolescents with recent meningitis, recent peripheral nerve injury, neurosurgery, or a central nervous system injury caused by a potentially compromised blood-brain barrier. Children and adolescents with immunodeficiency, receiving immunosuppressive medications, with structural heart defects, with pulmonary hypertension, with coronary artery disease, with cardiomyopathy, or at risk for stroke should also avoid use of anti-CGRP mAbs. Anti-CGRP mAbs are also potentially teratogenic and should not be used by adolescents or women who are pregnant, breastfeeding, or have a pregnancy wish.
Pediatric patients with significant osteoporosis or bone disease should be monitored when prescribed anti-CGRP mAbs, and the recommendations specified monitoring height and linear growth or waiting until after puberty to prescribe anti-CGRP mAbs. Although there is currently no evidence that use of anti-CGRP mAb requires pituitary hormone monitoring, the recommendations noted that weight and body mass index should also be observed.
“Pediatric and adolescent trials of anti-CGRP mAbs should be designed to maximize the chances of determining efficacy in these age groups and should focus on those who have not been successful with current multidisciplinary care,” Dr. Szperka and her colleagues wrote in the recommendations. “In the interim, the use of anti-CGRP mAbs for the treatment of headache disorders in children and adolescents may be considered in appropriate cases but should be done with close follow-up and attention to patient characteristics such as age, pubertal state, and medical comorbidities.”
Dr. Szperka receives grant support from Pfizer and Amgen and research funding from NIH. Other authors have reported grants, consulting fees, speaking fees, royalties, advisory board memberships, speaker’s bureau memberships and travel funds from Alder, Allergan, American Academy of Neurology, Amgen, Aralez, Avanir, Autonomic Technologies Inc., Biohaven, Cambridge University Press, Curelator, Depomed, Dr. Reddy’s Laboratories, Electrocore, eNeura, Genentech, Healint, Impax, JAMA Neurology, Journal Watch, Lilly, Massachusetts Medical Society, MedDay, MedicoLegal, Merck, NIH, Novartis, Oxford University Press, Quest Diagnostics, Scion, Supernus, Teva, Trigemina Inc., Upsher-Smith, UpToDate, Wolters Kluwer and Zosano.
SOURCE: Szperka CL et al. Headache. 2018. doi: 10.1111/head.13414.
Use of anti–calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) may benefit children with more than 8 headache days each month, a high Pediatric Migraine Disability Assessment (PedMIDAS) score, and failure of other treatments; however, researchers cautioned that long-term safety outcomes for the treatment are not yet known, according to a recent set of recommendations published in the journal Headache.
Christina L. Szperka, MD, MSCE, of the division of neurology at the Children’s Hospital of Philadelphia and members of the Pediatric and Adolescent Headache special interest group of the American Headache Society discussed the topic of anti-CGRP mAbs at the 2018 Annual Scientific Meeting of the American Headache Society. They noted clinical outcomes for anti-CGRP mAbs in pediatric patients will likely not be available for several years and created a set of recommendations based on expert opinion of anti-CGRP mAb use in children and adolescents.
Their recommendations support using anti-CGRP mAbs for children with migraine if patients meet the following criteria: headache frequency exceeding 8 headache days per month; a PedMIDAS score of 30 or greater; failure of two or more therapies, such as pharmacologic, nonpharmacologic, or nutraceutical ones; and in patients who are past puberty.
The special interest group recommended against use of anti-CGRP mAbs in children and adolescents with recent meningitis, recent peripheral nerve injury, neurosurgery, or a central nervous system injury caused by a potentially compromised blood-brain barrier. Children and adolescents with immunodeficiency, receiving immunosuppressive medications, with structural heart defects, with pulmonary hypertension, with coronary artery disease, with cardiomyopathy, or at risk for stroke should also avoid use of anti-CGRP mAbs. Anti-CGRP mAbs are also potentially teratogenic and should not be used by adolescents or women who are pregnant, breastfeeding, or have a pregnancy wish.
Pediatric patients with significant osteoporosis or bone disease should be monitored when prescribed anti-CGRP mAbs, and the recommendations specified monitoring height and linear growth or waiting until after puberty to prescribe anti-CGRP mAbs. Although there is currently no evidence that use of anti-CGRP mAb requires pituitary hormone monitoring, the recommendations noted that weight and body mass index should also be observed.
“Pediatric and adolescent trials of anti-CGRP mAbs should be designed to maximize the chances of determining efficacy in these age groups and should focus on those who have not been successful with current multidisciplinary care,” Dr. Szperka and her colleagues wrote in the recommendations. “In the interim, the use of anti-CGRP mAbs for the treatment of headache disorders in children and adolescents may be considered in appropriate cases but should be done with close follow-up and attention to patient characteristics such as age, pubertal state, and medical comorbidities.”
Dr. Szperka receives grant support from Pfizer and Amgen and research funding from NIH. Other authors have reported grants, consulting fees, speaking fees, royalties, advisory board memberships, speaker’s bureau memberships and travel funds from Alder, Allergan, American Academy of Neurology, Amgen, Aralez, Avanir, Autonomic Technologies Inc., Biohaven, Cambridge University Press, Curelator, Depomed, Dr. Reddy’s Laboratories, Electrocore, eNeura, Genentech, Healint, Impax, JAMA Neurology, Journal Watch, Lilly, Massachusetts Medical Society, MedDay, MedicoLegal, Merck, NIH, Novartis, Oxford University Press, Quest Diagnostics, Scion, Supernus, Teva, Trigemina Inc., Upsher-Smith, UpToDate, Wolters Kluwer and Zosano.
SOURCE: Szperka CL et al. Headache. 2018. doi: 10.1111/head.13414.
Use of anti–calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) may benefit children with more than 8 headache days each month, a high Pediatric Migraine Disability Assessment (PedMIDAS) score, and failure of other treatments; however, researchers cautioned that long-term safety outcomes for the treatment are not yet known, according to a recent set of recommendations published in the journal Headache.
Christina L. Szperka, MD, MSCE, of the division of neurology at the Children’s Hospital of Philadelphia and members of the Pediatric and Adolescent Headache special interest group of the American Headache Society discussed the topic of anti-CGRP mAbs at the 2018 Annual Scientific Meeting of the American Headache Society. They noted clinical outcomes for anti-CGRP mAbs in pediatric patients will likely not be available for several years and created a set of recommendations based on expert opinion of anti-CGRP mAb use in children and adolescents.
Their recommendations support using anti-CGRP mAbs for children with migraine if patients meet the following criteria: headache frequency exceeding 8 headache days per month; a PedMIDAS score of 30 or greater; failure of two or more therapies, such as pharmacologic, nonpharmacologic, or nutraceutical ones; and in patients who are past puberty.
The special interest group recommended against use of anti-CGRP mAbs in children and adolescents with recent meningitis, recent peripheral nerve injury, neurosurgery, or a central nervous system injury caused by a potentially compromised blood-brain barrier. Children and adolescents with immunodeficiency, receiving immunosuppressive medications, with structural heart defects, with pulmonary hypertension, with coronary artery disease, with cardiomyopathy, or at risk for stroke should also avoid use of anti-CGRP mAbs. Anti-CGRP mAbs are also potentially teratogenic and should not be used by adolescents or women who are pregnant, breastfeeding, or have a pregnancy wish.
Pediatric patients with significant osteoporosis or bone disease should be monitored when prescribed anti-CGRP mAbs, and the recommendations specified monitoring height and linear growth or waiting until after puberty to prescribe anti-CGRP mAbs. Although there is currently no evidence that use of anti-CGRP mAb requires pituitary hormone monitoring, the recommendations noted that weight and body mass index should also be observed.
“Pediatric and adolescent trials of anti-CGRP mAbs should be designed to maximize the chances of determining efficacy in these age groups and should focus on those who have not been successful with current multidisciplinary care,” Dr. Szperka and her colleagues wrote in the recommendations. “In the interim, the use of anti-CGRP mAbs for the treatment of headache disorders in children and adolescents may be considered in appropriate cases but should be done with close follow-up and attention to patient characteristics such as age, pubertal state, and medical comorbidities.”
Dr. Szperka receives grant support from Pfizer and Amgen and research funding from NIH. Other authors have reported grants, consulting fees, speaking fees, royalties, advisory board memberships, speaker’s bureau memberships and travel funds from Alder, Allergan, American Academy of Neurology, Amgen, Aralez, Avanir, Autonomic Technologies Inc., Biohaven, Cambridge University Press, Curelator, Depomed, Dr. Reddy’s Laboratories, Electrocore, eNeura, Genentech, Healint, Impax, JAMA Neurology, Journal Watch, Lilly, Massachusetts Medical Society, MedDay, MedicoLegal, Merck, NIH, Novartis, Oxford University Press, Quest Diagnostics, Scion, Supernus, Teva, Trigemina Inc., Upsher-Smith, UpToDate, Wolters Kluwer and Zosano.
SOURCE: Szperka CL et al. Headache. 2018. doi: 10.1111/head.13414.
FROM HEADACHE
Key clinical point: Treatment of headache disorders with anti–calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAbs) in children and adolescents may be indicated in some cases.
Major finding: Pediatric patients with more than eight headache days per month, PedMIDAS score of 30 or higher, and failure of other pharmacological, nonpharmacological, and nutraceutical treatments may benefit from anti-CGRP mAb treatment.
Study details: Expert opinion from the members of the Pediatric and Adolescent Headache special interest group based on recommendations made at the 2018 Annual Scientific Meeting of the American Headache Society.
Disclosures: Dr. Szperka receives grant support from Pfizer and Amgen and research funding from NIH. Other authors have reported grants, consulting fees, speaking fees, royalties, advisory board memberships, speaker’s bureau memberships, and travel funds from Alder, Allergan, American Academy of Neurology, Amgen, Aralez, Avanir, Autonomic Technologies, Biohaven, Cambridge University Press, Curelator, Depomed, Dr. Reddy’s Laboratories, Electrocore, eNeura, Genentech, Healint, Impax, JAMA Neurology, Journal Watch, Lilly, Massachusetts Medical Society, MedDay, MedicoLegal, Merck, NIH, Novartis, Oxford University Press, Quest Diagnostics, Scion, Supernus, Teva, Trigemina, Upsher-Smith, UpToDate, Wolters Kluwer, and Zosano.
Source: Szperka CL et al. Headache. 2018. doi: 10.1111/head.13414.