User login
Long-term antibiotic use may heighten stroke, CHD risk
, according to a study in the European Heart Journal.
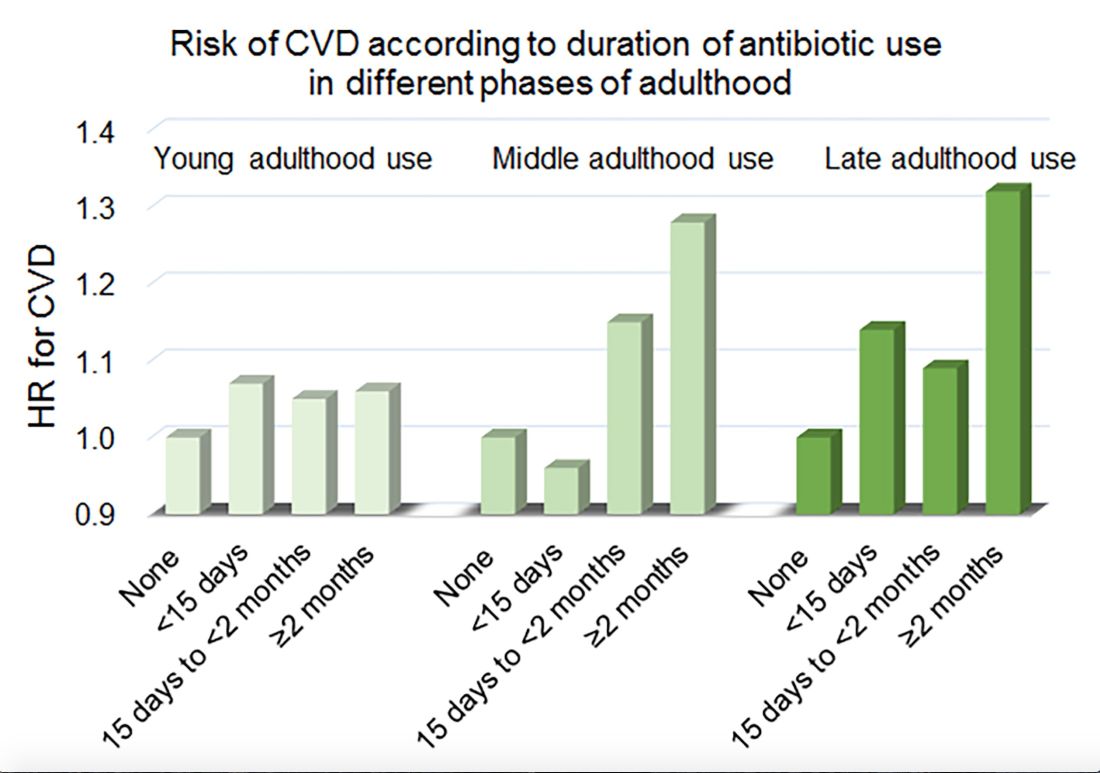
Women in the Nurses’ Health Study who used antibiotics for 2 or more months between ages 40 and 59 years or at age 60 years and older had a significantly increased risk of cardiovascular disease, compared with those who did not use antibiotics. Antibiotic use between 20 and 39 years old was not significantly related to cardiovascular disease.
Prior research has found that antibiotics may have long-lasting effects on gut microbiota and relate to cardiovascular disease risk.
“Antibiotic use is the most critical factor in altering the balance of microorganisms in the gut,” said lead investigator Lu Qi, MD, PhD, in a news release. “Previous studies have shown a link between alterations in the microbiotic environment of the gut and inflammation and narrowing of the blood vessels, stroke, and heart disease,” said Dr. Qi, who is the director of the Tulane University Obesity Research Center in New Orleans and an adjunct professor of nutrition at Harvard T.C. Chan School of Public Health in Boston.
To evaluate associations between life stage, antibiotic exposure, and subsequent cardiovascular disease, researchers analyzed data from 36,429 participants in the Nurses’ Health Study. The women were at least 60 years old and had no history of cardiovascular disease or cancer when they completed a 2004 questionnaire about antibiotic usage during young, middle, and late adulthood. The questionnaire asked participants to indicate the total time using antibiotics with eight categories ranging from none to 5 or more years.
The researchers defined incident cardiovascular disease as a composite endpoint of coronary heart disease (nonfatal myocardial infarction or fatal coronary heart disease) and stroke (nonfatal or fatal). They calculated person-years of follow-up from the questionnaire return date until date of cardiovascular disease diagnosis, death, or end of follow-up in 2012.
Women with longer duration of antibiotic use were more likely to use other medications and have unfavorable cardiovascular risk profiles, including family history of myocardial infarction and higher body mass index. Antibiotics most often were used to treat respiratory infections. During an average follow-up of 7.6 years, 1,056 participants developed cardiovascular disease.
In a multivariable model that adjusted for demographics, diet, lifestyle, reason for antibiotic use, medications, overweight status, and other factors, long-term antibiotic use – 2 months or more – in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Although antibiotic use was self-reported, which could lead to misclassification, the participants were health professionals, which may mitigate this limitation, the authors noted. Whether these findings apply to men and other populations requires further study, they said.
Because of the study’s observational design, the results “cannot show that antibiotics cause heart disease and stroke, only that there is a link between them,” Dr. Qi said. “It’s possible that women who reported more antibiotic use might be sicker in other ways that we were unable to measure, or there may be other factors that could affect the results that we have not been able take account of.”
“Our study suggests that antibiotics should be used only when they are absolutely needed,” he concluded. “Considering the potentially cumulative adverse effects, the shorter time of antibiotic use the better.”
The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
SOURCE: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
, according to a study in the European Heart Journal.

Women in the Nurses’ Health Study who used antibiotics for 2 or more months between ages 40 and 59 years or at age 60 years and older had a significantly increased risk of cardiovascular disease, compared with those who did not use antibiotics. Antibiotic use between 20 and 39 years old was not significantly related to cardiovascular disease.
Prior research has found that antibiotics may have long-lasting effects on gut microbiota and relate to cardiovascular disease risk.
“Antibiotic use is the most critical factor in altering the balance of microorganisms in the gut,” said lead investigator Lu Qi, MD, PhD, in a news release. “Previous studies have shown a link between alterations in the microbiotic environment of the gut and inflammation and narrowing of the blood vessels, stroke, and heart disease,” said Dr. Qi, who is the director of the Tulane University Obesity Research Center in New Orleans and an adjunct professor of nutrition at Harvard T.C. Chan School of Public Health in Boston.
To evaluate associations between life stage, antibiotic exposure, and subsequent cardiovascular disease, researchers analyzed data from 36,429 participants in the Nurses’ Health Study. The women were at least 60 years old and had no history of cardiovascular disease or cancer when they completed a 2004 questionnaire about antibiotic usage during young, middle, and late adulthood. The questionnaire asked participants to indicate the total time using antibiotics with eight categories ranging from none to 5 or more years.
The researchers defined incident cardiovascular disease as a composite endpoint of coronary heart disease (nonfatal myocardial infarction or fatal coronary heart disease) and stroke (nonfatal or fatal). They calculated person-years of follow-up from the questionnaire return date until date of cardiovascular disease diagnosis, death, or end of follow-up in 2012.
Women with longer duration of antibiotic use were more likely to use other medications and have unfavorable cardiovascular risk profiles, including family history of myocardial infarction and higher body mass index. Antibiotics most often were used to treat respiratory infections. During an average follow-up of 7.6 years, 1,056 participants developed cardiovascular disease.
In a multivariable model that adjusted for demographics, diet, lifestyle, reason for antibiotic use, medications, overweight status, and other factors, long-term antibiotic use – 2 months or more – in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Although antibiotic use was self-reported, which could lead to misclassification, the participants were health professionals, which may mitigate this limitation, the authors noted. Whether these findings apply to men and other populations requires further study, they said.
Because of the study’s observational design, the results “cannot show that antibiotics cause heart disease and stroke, only that there is a link between them,” Dr. Qi said. “It’s possible that women who reported more antibiotic use might be sicker in other ways that we were unable to measure, or there may be other factors that could affect the results that we have not been able take account of.”
“Our study suggests that antibiotics should be used only when they are absolutely needed,” he concluded. “Considering the potentially cumulative adverse effects, the shorter time of antibiotic use the better.”
The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
SOURCE: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
, according to a study in the European Heart Journal.

Women in the Nurses’ Health Study who used antibiotics for 2 or more months between ages 40 and 59 years or at age 60 years and older had a significantly increased risk of cardiovascular disease, compared with those who did not use antibiotics. Antibiotic use between 20 and 39 years old was not significantly related to cardiovascular disease.
Prior research has found that antibiotics may have long-lasting effects on gut microbiota and relate to cardiovascular disease risk.
“Antibiotic use is the most critical factor in altering the balance of microorganisms in the gut,” said lead investigator Lu Qi, MD, PhD, in a news release. “Previous studies have shown a link between alterations in the microbiotic environment of the gut and inflammation and narrowing of the blood vessels, stroke, and heart disease,” said Dr. Qi, who is the director of the Tulane University Obesity Research Center in New Orleans and an adjunct professor of nutrition at Harvard T.C. Chan School of Public Health in Boston.
To evaluate associations between life stage, antibiotic exposure, and subsequent cardiovascular disease, researchers analyzed data from 36,429 participants in the Nurses’ Health Study. The women were at least 60 years old and had no history of cardiovascular disease or cancer when they completed a 2004 questionnaire about antibiotic usage during young, middle, and late adulthood. The questionnaire asked participants to indicate the total time using antibiotics with eight categories ranging from none to 5 or more years.
The researchers defined incident cardiovascular disease as a composite endpoint of coronary heart disease (nonfatal myocardial infarction or fatal coronary heart disease) and stroke (nonfatal or fatal). They calculated person-years of follow-up from the questionnaire return date until date of cardiovascular disease diagnosis, death, or end of follow-up in 2012.
Women with longer duration of antibiotic use were more likely to use other medications and have unfavorable cardiovascular risk profiles, including family history of myocardial infarction and higher body mass index. Antibiotics most often were used to treat respiratory infections. During an average follow-up of 7.6 years, 1,056 participants developed cardiovascular disease.
In a multivariable model that adjusted for demographics, diet, lifestyle, reason for antibiotic use, medications, overweight status, and other factors, long-term antibiotic use – 2 months or more – in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Although antibiotic use was self-reported, which could lead to misclassification, the participants were health professionals, which may mitigate this limitation, the authors noted. Whether these findings apply to men and other populations requires further study, they said.
Because of the study’s observational design, the results “cannot show that antibiotics cause heart disease and stroke, only that there is a link between them,” Dr. Qi said. “It’s possible that women who reported more antibiotic use might be sicker in other ways that we were unable to measure, or there may be other factors that could affect the results that we have not been able take account of.”
“Our study suggests that antibiotics should be used only when they are absolutely needed,” he concluded. “Considering the potentially cumulative adverse effects, the shorter time of antibiotic use the better.”
The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
SOURCE: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
FROM THE EUROPEAN HEART JOURNAL
Key clinical point: Among middle-aged and older women, 2 or more months’ exposure to antibiotics is associated with an increased risk of coronary heart disease or stroke.
Major finding: Long-term antibiotic use in late adulthood was associated with significantly increased risk of cardiovascular disease (hazard ratio, 1.32), as was long-term antibiotic use in middle adulthood (HR, 1.28).
Study details: An analysis of data from nearly 36,500 women in the Nurses’ Health Study.
Disclosures: The study was supported by National Institutes of Health grants, the Boston Obesity Nutrition Research Center, and the United States–Israel Binational Science Foundation. One author received support from the Japan Society for the Promotion of Science. The authors had no conflicts of interest.
Source: Heianza Y et al. Eur Heart J. 2019 Apr 24. doi: 10.1093/eurheartj/ehz231.
Restricting opioids after knee surgery did not increase refills
according to a study in the Journal of Arthroplasty.
Contrary to concerns that restrictive opioid prescribing might increase the number of patient call-ins and refill requests, one academic institution had significantly fewer call-ins and refills after it implemented a strict postoperative opioid prescribing protocol on Jan. 1, 2018.
“Orthopedic surgeons might be reluctant to change practice without evidence that new, more-restrictive practice will not impede patient care,” the researchers wrote. “As the current study demonstrates, there is room to significantly decrease postoperative opioid prescriptions in total joint arthroplasty. This places patients at lower risk of opioid abuse and diversion without significantly altering the risk of postoperative complications or compromising postoperative pain control.”
Opioid overuse is a major public health concern, and orthopedic surgeons may overprescribe opioids after surgery. The University of Iowa Hospitals and Clinics in Iowa City implemented strict postoperative opioid prescription guidelines that are based on the American Academy of Orthopedic Surgeons Clinical Practice Guidelines. As part of the protocol, patients receive a preoperative education session that emphasizes risks associated with opioid use. Before initiating this protocol, postoperative drug choice and quantity had not been standardized.
To examine changes in opioid prescriptions and the number of call-ins, postoperative complications, and prescription refill requests after the implementation of the restrictive opioid prescribing protocol, investigators at the institution conducted a retrospective study.
Andrew J. Holte, a researcher in the department of orthopedics and rehabilitation, and his colleagues reviewed cases from June 2017 to February 2018. Their analysis included 399 patients who underwent total hip arthroplasty or total knee arthroplasty.
In all, 282 patients underwent surgery before the restrictive protocol (the historical cohort) and 117 after (the restrictive cohort). In the historical cohort, about 48% of the patients underwent total knee arthroplasty. In the restrictive cohort, about 44% underwent total knee arthroplasty. Patients had an average age of about 61 years, and approximately 52% were women.
According to comparisons of morphine mg equivalents (MME), the historical cohort received significantly larger mean initial opioid prescriptions (752 MME vs. 387 MME), significantly more refills per patient (0.5 vs. 0.3), and significantly more medication through refills (253 MME vs. 84 MME).
“For reference, 50 pills of 5 mg oxycodone is equivalent to 300 MMEs,” the authors noted.
A multivariable model found that younger age and total knee arthroplasty, compared with total hip arthroplasty, were associated with increased likelihood of requests for refills and patient call-ins.
“Surprisingly, there were significantly more patient call-ins and requests for refills of opioids in the historical cohort,” Mr. Holte and his colleagues said. “Although this study did not collect direct data on patient pain scores, we believe that call-ins and requests for refills are sufficient surrogate markers for inadequate pain control.”
The study does not account for prescriptions from other providers or whether patients took none, some, or all of their filled prescriptions. Future studies are needed to assess how reduced opioid prescriptions affect pain and functional outcomes in the long term, the researchers said.
One or more study authors disclosed potential conflicts of interest. The disclosures can be found in Appendix A, Supplementary Data, at the end of the journal article.
SOURCE: Holte AJ et al. J Arthroplasty. 2019 Feb 20. doi: 10.1016/j.arth.2019.02.022.
according to a study in the Journal of Arthroplasty.
Contrary to concerns that restrictive opioid prescribing might increase the number of patient call-ins and refill requests, one academic institution had significantly fewer call-ins and refills after it implemented a strict postoperative opioid prescribing protocol on Jan. 1, 2018.
“Orthopedic surgeons might be reluctant to change practice without evidence that new, more-restrictive practice will not impede patient care,” the researchers wrote. “As the current study demonstrates, there is room to significantly decrease postoperative opioid prescriptions in total joint arthroplasty. This places patients at lower risk of opioid abuse and diversion without significantly altering the risk of postoperative complications or compromising postoperative pain control.”
Opioid overuse is a major public health concern, and orthopedic surgeons may overprescribe opioids after surgery. The University of Iowa Hospitals and Clinics in Iowa City implemented strict postoperative opioid prescription guidelines that are based on the American Academy of Orthopedic Surgeons Clinical Practice Guidelines. As part of the protocol, patients receive a preoperative education session that emphasizes risks associated with opioid use. Before initiating this protocol, postoperative drug choice and quantity had not been standardized.
To examine changes in opioid prescriptions and the number of call-ins, postoperative complications, and prescription refill requests after the implementation of the restrictive opioid prescribing protocol, investigators at the institution conducted a retrospective study.
Andrew J. Holte, a researcher in the department of orthopedics and rehabilitation, and his colleagues reviewed cases from June 2017 to February 2018. Their analysis included 399 patients who underwent total hip arthroplasty or total knee arthroplasty.
In all, 282 patients underwent surgery before the restrictive protocol (the historical cohort) and 117 after (the restrictive cohort). In the historical cohort, about 48% of the patients underwent total knee arthroplasty. In the restrictive cohort, about 44% underwent total knee arthroplasty. Patients had an average age of about 61 years, and approximately 52% were women.
According to comparisons of morphine mg equivalents (MME), the historical cohort received significantly larger mean initial opioid prescriptions (752 MME vs. 387 MME), significantly more refills per patient (0.5 vs. 0.3), and significantly more medication through refills (253 MME vs. 84 MME).
“For reference, 50 pills of 5 mg oxycodone is equivalent to 300 MMEs,” the authors noted.
A multivariable model found that younger age and total knee arthroplasty, compared with total hip arthroplasty, were associated with increased likelihood of requests for refills and patient call-ins.
“Surprisingly, there were significantly more patient call-ins and requests for refills of opioids in the historical cohort,” Mr. Holte and his colleagues said. “Although this study did not collect direct data on patient pain scores, we believe that call-ins and requests for refills are sufficient surrogate markers for inadequate pain control.”
The study does not account for prescriptions from other providers or whether patients took none, some, or all of their filled prescriptions. Future studies are needed to assess how reduced opioid prescriptions affect pain and functional outcomes in the long term, the researchers said.
One or more study authors disclosed potential conflicts of interest. The disclosures can be found in Appendix A, Supplementary Data, at the end of the journal article.
SOURCE: Holte AJ et al. J Arthroplasty. 2019 Feb 20. doi: 10.1016/j.arth.2019.02.022.
according to a study in the Journal of Arthroplasty.
Contrary to concerns that restrictive opioid prescribing might increase the number of patient call-ins and refill requests, one academic institution had significantly fewer call-ins and refills after it implemented a strict postoperative opioid prescribing protocol on Jan. 1, 2018.
“Orthopedic surgeons might be reluctant to change practice without evidence that new, more-restrictive practice will not impede patient care,” the researchers wrote. “As the current study demonstrates, there is room to significantly decrease postoperative opioid prescriptions in total joint arthroplasty. This places patients at lower risk of opioid abuse and diversion without significantly altering the risk of postoperative complications or compromising postoperative pain control.”
Opioid overuse is a major public health concern, and orthopedic surgeons may overprescribe opioids after surgery. The University of Iowa Hospitals and Clinics in Iowa City implemented strict postoperative opioid prescription guidelines that are based on the American Academy of Orthopedic Surgeons Clinical Practice Guidelines. As part of the protocol, patients receive a preoperative education session that emphasizes risks associated with opioid use. Before initiating this protocol, postoperative drug choice and quantity had not been standardized.
To examine changes in opioid prescriptions and the number of call-ins, postoperative complications, and prescription refill requests after the implementation of the restrictive opioid prescribing protocol, investigators at the institution conducted a retrospective study.
Andrew J. Holte, a researcher in the department of orthopedics and rehabilitation, and his colleagues reviewed cases from June 2017 to February 2018. Their analysis included 399 patients who underwent total hip arthroplasty or total knee arthroplasty.
In all, 282 patients underwent surgery before the restrictive protocol (the historical cohort) and 117 after (the restrictive cohort). In the historical cohort, about 48% of the patients underwent total knee arthroplasty. In the restrictive cohort, about 44% underwent total knee arthroplasty. Patients had an average age of about 61 years, and approximately 52% were women.
According to comparisons of morphine mg equivalents (MME), the historical cohort received significantly larger mean initial opioid prescriptions (752 MME vs. 387 MME), significantly more refills per patient (0.5 vs. 0.3), and significantly more medication through refills (253 MME vs. 84 MME).
“For reference, 50 pills of 5 mg oxycodone is equivalent to 300 MMEs,” the authors noted.
A multivariable model found that younger age and total knee arthroplasty, compared with total hip arthroplasty, were associated with increased likelihood of requests for refills and patient call-ins.
“Surprisingly, there were significantly more patient call-ins and requests for refills of opioids in the historical cohort,” Mr. Holte and his colleagues said. “Although this study did not collect direct data on patient pain scores, we believe that call-ins and requests for refills are sufficient surrogate markers for inadequate pain control.”
The study does not account for prescriptions from other providers or whether patients took none, some, or all of their filled prescriptions. Future studies are needed to assess how reduced opioid prescriptions affect pain and functional outcomes in the long term, the researchers said.
One or more study authors disclosed potential conflicts of interest. The disclosures can be found in Appendix A, Supplementary Data, at the end of the journal article.
SOURCE: Holte AJ et al. J Arthroplasty. 2019 Feb 20. doi: 10.1016/j.arth.2019.02.022.
FROM THE JOURNAL OF ARTHROPLASTY
Can immune checkpoint inhibitors treat PML?
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
investigators reported in the New England Journal of Medicine.
Three research teams described 10 cases in which patients with PML received pembrolizumab or nivolumab.
In one study, researchers administered pembrolizumab to eight adults with PML. Five patients had clinical improvement or stabilization, whereas 3 patients did not. Among the patients with clinical improvement, treatment led to reduced JC viral load in cerebrospinal fluid (CSF) and increased CD4+ and CD8+ anti–JC virus activity in vitro. Among patients without clinical improvement, treatment did not meaningfully change viral load or antiviral cellular immune response.
In a separate letter, researchers in Germany described an additional patient with PML who had clinical stabilization and no disease progression on MRI after treatment with pembrolizumab.
In another letter, researchers in France described a patient with PML whose condition improved after treatment with nivolumab.
“Do pembrolizumab and nivolumab fit the bill for treatment of PML? The current reports are encouraging but suggest that the presence of JC virus–specific T cells in the blood is a prerequisite for their use,” said Igor J. Koralnik, MD, of the department of neurological sciences at Rush University Medical Center in Chicago, in an accompanying editorial. “A controlled trial may be needed to determine whether immune checkpoint inhibitors are indeed able to keep JC virus in check in patients with PML.”
Reinvigorating T cells
Both monoclonal antibodies target programmed cell death protein 1 (PD-1), which inhibits T-cell proliferation and cytokine production when it binds its associated ligand, Dr. Koralnik said. Pembrolizumab and nivolumab block this inhibition and have been used to spur T-cell activity against tumors in patients with cancer.
PML, an often fatal brain infection caused by the JC virus in patients with immunosuppression, has no specific treatment. Management hinges on “recovery of the immune system, either by treating the underlying cause of immunosuppression or by discontinuing the use of immunosuppressive medications,” said Dr. Koralnik.
Pembrolizumab
Prior studies have found that PD-1 expression is elevated on T lymphocytes of patients with PML. To determine whether PD-1 blockade with pembrolizumab reinvigorates anti–JC virus immune activity in patients with PML, Irene Cortese, MD, of the National Institutes of Health’s Neuroimmunology Clinic and her research colleagues administered pembrolizumab at a dose of 2 mg/kg of body weight every 4-6 weeks to eight adults with PML. The patients received 1-3 doses, and each patient had a different underlying condition.
In all patients, treatment induced down-regulation of PD-1 expression on lymphocytes in CSF and peripheral blood, and five of the eight patients had clinical stabilization or improvement. Of the other three patients who did not improve, one had stabilized prior to treatment and remained stable. The other two patients died from PML.
Additional reports
Separately, Sebastian Rauer, MD, of Albert Ludwigs University in Freiburg, Germany, and his colleagues reported that a patient with PML whose symptoms culminated in mutism in February 2018 began speaking again after receiving five infusions of pembrolizumab over 10 weeks. “In addition, the size and number of lesions on MRI decreased, and JCV was no longer detectable in CSF,” Dr. Rauer and his colleagues wrote. “The patient has remained stable as of the end of March 2019, with persistent but abating psychomotor slowing, aphasia, and disorientation.”
Finally, Ondine Walter, of Toulouse (France) University Hospital and colleagues described the case of a 60-year-old woman with PML who received nivolumab on a compassionate-use basis. Two weeks after treatment, JC viral load in CSF and blood had decreased. “Starting 8 weeks after the initiation of nivolumab therapy, the patient’s neurologic symptoms and signs stabilized, and subsequently she showed improved alertness, and the ptosis and hemiplegia abated.”
Reason for caution
Prior studies, however, give reasons for caution when considering the potential use of immune checkpoint inhibitors to treat PML, Dr. Koralnik noted. In one case, a patient developed an inflammatory form of PML known as immune reconstitution inflammatory syndrome after receiving nivolumab (J Neurovirol. 2019 March 12. doi: 10.1007/s13365-019-00738-x). In addition, researchers have reported a case of PML that occurred after 1 year of nivolumab treatment, and four cases of PML related to nivolumab have been reported in pharmacovigilance databases (Emerg Infect Dis. 2018;24:1594-6). The cost and safety profiles of the medications also may be considerations, Dr. Koralnik said.
The study by Dr. Cortese and colleagues was funded by the National Institutes of Health, and the authors had no relevant disclosures. Some of the research letter authors disclosed grants and personal fees from pharmaceutical companies.
SOURCES: Cortese I et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMoa1815039; Rauer S et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1817193; Walter O et al. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMc1816198; Koralnik IJ. N Engl J Med. 2019 Apr 10. doi: 10.1056/NEJMe1904140.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
A blood biomarker for MS: Coming to clinics soon?
DALLAS – Neurologists soon may use a blood biomarker of axonal damage to monitor patients with multiple sclerosis (MS) and guide treatment decisions, according to a lecture delivered at ACTRIMS Forum 2019.
said David Leppert, MD, senior research associate in the department of neurology at University Hopsital Basel (Switzerland).
Among patients with MS, blood NfL levels predict disability, brain volume loss, and spinal cord atrophy. In addition, studies have found that blood NfL decreases in response to disease-modifying therapy (DMT) and that second-line DMTs may decrease blood NfL more than first-line DMTs do.
The establishment of normative databases and reference biomarkers may allow neurologists to use NfL in their care of individual patients, Dr. Leppert predicted at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “I am very positive that we will make a breakthrough in the next 2 or 3 years for an individual use of neurofilaments,” he said.
Response to DMT
An analysis of blood NfL levels from patients with MS and from healthy controls in two phase 3 trials of fingolimod, FREEDOMS and TRANSFORMS, provides insights into NfL’s response to DMT (Neurology. 2019 Mar 5;92[10]:e1007-15). In FREEDOMS, which compared fingolimod with placebo, “fingolimod leads to a rapid decrease of neurofilament levels, close to normality, while placebo patients continue to have high levels,” said Dr. Leppert, a coauthor of the study.
TRANSFORMS compared interferon-beta and fingolimod. “The clinical experience that fingolimod is a more potent compound than interferon is actually reflected here by the NfL results,” Dr. Leppert said. “Both compounds lead to a decrease of neurofilaments – so, a decrease of neuronal damage – but one drug is more potent than the other one.”
Similarly, data from the observational EPIC study indicate that patients who do not receive DMT have a consistent increase in NfL levels, whereas those who receive platform therapies have a slight decrease in NfL and those who receive second-line therapies have a greater decrease, Dr. Leppert said.
Decades of research
For about 20 years, researchers have studied neurofilaments in cerebrospinal fluid (CSF) as a potential biomarker for MS and other diseases, including Alzheimer’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and head trauma.
“What prevented the emergence of NfL to clinical practice was the inability to measure it in blood because levels are 50-100 times lower [in blood] than in CSF,” Dr. Leppert said.
The development of single molecule array (SIMOA) technology enabled researchers to show a proper correlation between levels of NfL in CSF and those in blood, Dr. Leppert said. “That is now allowing repetitive testing in an accessible fluid compartment, meaning serum or plasma,” he said.
Compared with brain MRI, NfL may provide novel insights into MS disease activity. “MRI is measuring a structural deficit of the past,” Dr. Leppert said. “NfL is measuring online, real time what axonal damage is occurring.”
Correlation with outcomes
At the group level, patients with MS have higher levels of NfL, compared with controls, and levels are higher in patients with progressive forms of MS versus relapsing forms. “Levels increase dramatically in the wake of relapse,” he said.
Barro et al. found that patients with higher serum levels of NfL are more likely to experience Expanded Disability Status Scale (EDSS) worsening (Brain. 2018 Aug 1;141[8]:2382-91). Furthermore, MRI lesions are independently associated with serum NfL, and patients with higher levels of serum NfL have significantly greater average loss in brain volume and spinal cord volume over 5 years.
A treatment algorithm
NfL someday could be incorporated into MS treatment algorithms, Dr. Leppert suggested. For instance, if a patient has high levels of disease activity based on MRI or clinical grounds, then prescribe a high-efficacy therapy. If not, measure NfL. “If the levels are low, then you can be assured to use platform therapies or continue what the patient has. But if NfL levels are high, then you should choose high efficacious therapies or switch to high-efficacious therapies in the long run,” he said.
Limitations and next steps
Although NfL is a specific marker of neuronal damage, it is not specific for the cause of the damage. Further studies are needed to better understand NfL metabolism and confounding factors such as age. Reference biomarkers likely will be needed “to conceptualize whether the signal of NfL is due to acute disease or chronic disease,” Dr. Leppert said.
“We need to optimize the assay and come to a worldwide agreement on the platform. We need to have prospective studies, mainly to achieve regulatory acceptance. And we need to have a normative database” to determine which NfL values are pathologic at a particular age, he said.
Despite the biomarker’s potential, blood NfL levels will not replace clinical expertise. “Biomarkers cannot be of value without a clinical backing and a clinical evaluation,” Dr. Leppert said. “The idea that this will replace us or any other person who makes a clinical judgment is a big error. NfL will prevail as a biomarker. ... But interpretation of the clinical background is germane.”
Dr. Leppert has been an employee of pharmaceutical companies, most recently Novartis.
DALLAS – Neurologists soon may use a blood biomarker of axonal damage to monitor patients with multiple sclerosis (MS) and guide treatment decisions, according to a lecture delivered at ACTRIMS Forum 2019.
said David Leppert, MD, senior research associate in the department of neurology at University Hopsital Basel (Switzerland).
Among patients with MS, blood NfL levels predict disability, brain volume loss, and spinal cord atrophy. In addition, studies have found that blood NfL decreases in response to disease-modifying therapy (DMT) and that second-line DMTs may decrease blood NfL more than first-line DMTs do.
The establishment of normative databases and reference biomarkers may allow neurologists to use NfL in their care of individual patients, Dr. Leppert predicted at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “I am very positive that we will make a breakthrough in the next 2 or 3 years for an individual use of neurofilaments,” he said.
Response to DMT
An analysis of blood NfL levels from patients with MS and from healthy controls in two phase 3 trials of fingolimod, FREEDOMS and TRANSFORMS, provides insights into NfL’s response to DMT (Neurology. 2019 Mar 5;92[10]:e1007-15). In FREEDOMS, which compared fingolimod with placebo, “fingolimod leads to a rapid decrease of neurofilament levels, close to normality, while placebo patients continue to have high levels,” said Dr. Leppert, a coauthor of the study.
TRANSFORMS compared interferon-beta and fingolimod. “The clinical experience that fingolimod is a more potent compound than interferon is actually reflected here by the NfL results,” Dr. Leppert said. “Both compounds lead to a decrease of neurofilaments – so, a decrease of neuronal damage – but one drug is more potent than the other one.”
Similarly, data from the observational EPIC study indicate that patients who do not receive DMT have a consistent increase in NfL levels, whereas those who receive platform therapies have a slight decrease in NfL and those who receive second-line therapies have a greater decrease, Dr. Leppert said.
Decades of research
For about 20 years, researchers have studied neurofilaments in cerebrospinal fluid (CSF) as a potential biomarker for MS and other diseases, including Alzheimer’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and head trauma.
“What prevented the emergence of NfL to clinical practice was the inability to measure it in blood because levels are 50-100 times lower [in blood] than in CSF,” Dr. Leppert said.
The development of single molecule array (SIMOA) technology enabled researchers to show a proper correlation between levels of NfL in CSF and those in blood, Dr. Leppert said. “That is now allowing repetitive testing in an accessible fluid compartment, meaning serum or plasma,” he said.
Compared with brain MRI, NfL may provide novel insights into MS disease activity. “MRI is measuring a structural deficit of the past,” Dr. Leppert said. “NfL is measuring online, real time what axonal damage is occurring.”
Correlation with outcomes
At the group level, patients with MS have higher levels of NfL, compared with controls, and levels are higher in patients with progressive forms of MS versus relapsing forms. “Levels increase dramatically in the wake of relapse,” he said.
Barro et al. found that patients with higher serum levels of NfL are more likely to experience Expanded Disability Status Scale (EDSS) worsening (Brain. 2018 Aug 1;141[8]:2382-91). Furthermore, MRI lesions are independently associated with serum NfL, and patients with higher levels of serum NfL have significantly greater average loss in brain volume and spinal cord volume over 5 years.
A treatment algorithm
NfL someday could be incorporated into MS treatment algorithms, Dr. Leppert suggested. For instance, if a patient has high levels of disease activity based on MRI or clinical grounds, then prescribe a high-efficacy therapy. If not, measure NfL. “If the levels are low, then you can be assured to use platform therapies or continue what the patient has. But if NfL levels are high, then you should choose high efficacious therapies or switch to high-efficacious therapies in the long run,” he said.
Limitations and next steps
Although NfL is a specific marker of neuronal damage, it is not specific for the cause of the damage. Further studies are needed to better understand NfL metabolism and confounding factors such as age. Reference biomarkers likely will be needed “to conceptualize whether the signal of NfL is due to acute disease or chronic disease,” Dr. Leppert said.
“We need to optimize the assay and come to a worldwide agreement on the platform. We need to have prospective studies, mainly to achieve regulatory acceptance. And we need to have a normative database” to determine which NfL values are pathologic at a particular age, he said.
Despite the biomarker’s potential, blood NfL levels will not replace clinical expertise. “Biomarkers cannot be of value without a clinical backing and a clinical evaluation,” Dr. Leppert said. “The idea that this will replace us or any other person who makes a clinical judgment is a big error. NfL will prevail as a biomarker. ... But interpretation of the clinical background is germane.”
Dr. Leppert has been an employee of pharmaceutical companies, most recently Novartis.
DALLAS – Neurologists soon may use a blood biomarker of axonal damage to monitor patients with multiple sclerosis (MS) and guide treatment decisions, according to a lecture delivered at ACTRIMS Forum 2019.
said David Leppert, MD, senior research associate in the department of neurology at University Hopsital Basel (Switzerland).
Among patients with MS, blood NfL levels predict disability, brain volume loss, and spinal cord atrophy. In addition, studies have found that blood NfL decreases in response to disease-modifying therapy (DMT) and that second-line DMTs may decrease blood NfL more than first-line DMTs do.
The establishment of normative databases and reference biomarkers may allow neurologists to use NfL in their care of individual patients, Dr. Leppert predicted at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. “I am very positive that we will make a breakthrough in the next 2 or 3 years for an individual use of neurofilaments,” he said.
Response to DMT
An analysis of blood NfL levels from patients with MS and from healthy controls in two phase 3 trials of fingolimod, FREEDOMS and TRANSFORMS, provides insights into NfL’s response to DMT (Neurology. 2019 Mar 5;92[10]:e1007-15). In FREEDOMS, which compared fingolimod with placebo, “fingolimod leads to a rapid decrease of neurofilament levels, close to normality, while placebo patients continue to have high levels,” said Dr. Leppert, a coauthor of the study.
TRANSFORMS compared interferon-beta and fingolimod. “The clinical experience that fingolimod is a more potent compound than interferon is actually reflected here by the NfL results,” Dr. Leppert said. “Both compounds lead to a decrease of neurofilaments – so, a decrease of neuronal damage – but one drug is more potent than the other one.”
Similarly, data from the observational EPIC study indicate that patients who do not receive DMT have a consistent increase in NfL levels, whereas those who receive platform therapies have a slight decrease in NfL and those who receive second-line therapies have a greater decrease, Dr. Leppert said.
Decades of research
For about 20 years, researchers have studied neurofilaments in cerebrospinal fluid (CSF) as a potential biomarker for MS and other diseases, including Alzheimer’s disease, amyotrophic lateral sclerosis, Parkinson’s disease, and head trauma.
“What prevented the emergence of NfL to clinical practice was the inability to measure it in blood because levels are 50-100 times lower [in blood] than in CSF,” Dr. Leppert said.
The development of single molecule array (SIMOA) technology enabled researchers to show a proper correlation between levels of NfL in CSF and those in blood, Dr. Leppert said. “That is now allowing repetitive testing in an accessible fluid compartment, meaning serum or plasma,” he said.
Compared with brain MRI, NfL may provide novel insights into MS disease activity. “MRI is measuring a structural deficit of the past,” Dr. Leppert said. “NfL is measuring online, real time what axonal damage is occurring.”
Correlation with outcomes
At the group level, patients with MS have higher levels of NfL, compared with controls, and levels are higher in patients with progressive forms of MS versus relapsing forms. “Levels increase dramatically in the wake of relapse,” he said.
Barro et al. found that patients with higher serum levels of NfL are more likely to experience Expanded Disability Status Scale (EDSS) worsening (Brain. 2018 Aug 1;141[8]:2382-91). Furthermore, MRI lesions are independently associated with serum NfL, and patients with higher levels of serum NfL have significantly greater average loss in brain volume and spinal cord volume over 5 years.
A treatment algorithm
NfL someday could be incorporated into MS treatment algorithms, Dr. Leppert suggested. For instance, if a patient has high levels of disease activity based on MRI or clinical grounds, then prescribe a high-efficacy therapy. If not, measure NfL. “If the levels are low, then you can be assured to use platform therapies or continue what the patient has. But if NfL levels are high, then you should choose high efficacious therapies or switch to high-efficacious therapies in the long run,” he said.
Limitations and next steps
Although NfL is a specific marker of neuronal damage, it is not specific for the cause of the damage. Further studies are needed to better understand NfL metabolism and confounding factors such as age. Reference biomarkers likely will be needed “to conceptualize whether the signal of NfL is due to acute disease or chronic disease,” Dr. Leppert said.
“We need to optimize the assay and come to a worldwide agreement on the platform. We need to have prospective studies, mainly to achieve regulatory acceptance. And we need to have a normative database” to determine which NfL values are pathologic at a particular age, he said.
Despite the biomarker’s potential, blood NfL levels will not replace clinical expertise. “Biomarkers cannot be of value without a clinical backing and a clinical evaluation,” Dr. Leppert said. “The idea that this will replace us or any other person who makes a clinical judgment is a big error. NfL will prevail as a biomarker. ... But interpretation of the clinical background is germane.”
Dr. Leppert has been an employee of pharmaceutical companies, most recently Novartis.
EXPERT ANALYSIS FROM ACTRIMS FORUM 2019
Mindfulness yoga reduced stress and motor symptoms in patients with Parkinson’s disease
Among patients with mild or moderate Parkinson’s disease, mindfulness yoga was as effective as stretching and resistance training in improving motor function and mobility, a randomized trial found.
In addition, mindfulness yoga reduced anxiety and depressive symptoms and increased spiritual well-being and health-related quality of life more than stretching and resistance training, researchers reported in JAMA Neurology.
Although guidelines support exercise for patients with Parkinson’s disease, investigators had not examined whether yoga is superior to conventional exercise for stress and symptom management in this patient population. Jojo Y. Y. Kwok, PhD, a research assistant professor of nursing at the University of Hong Kong, and her colleagues conducted an assessor-masked, randomized trial that included 138 adults with idiopathic Parkinson’s disease who were able to stand on their own and walk with or without an assistive device. The trial was conducted at 4 community rehabilitation centers in Hong Kong between December 1, 2016, and May 31, 2017. Participants were randomized to 8 weeks of mindfulness yoga delivered weekly in 90-minute group sessions (71) or stretching and resistance training delivered in weekly 60-minute group sessions (67).
The primary outcomes was psychological distress in terms of anxiety and depressive symptoms assessed with the Hospital Anxiety and Depression Scale (HADS). Secondary outcomes included motor symptom severity, mobility, spiritual well-being in terms of perceived hardship and equanimity, and health-related quality of life. The researchers assessed patients at baseline, 8 weeks, and 20 weeks.
The average age of the participants was 63.7 years; 65 (47.1%) were men. Generalized estimating equation analyses found that patients in the yoga group had significantly better outcomes, including for anxiety (time-by-group interaction, beta, –1.79 at 8 weeks and –2.05 at 20 weeks), and depressive symptoms (beta, –2.75 at 8 weeks and –2.75 at 20 weeks). These improvements were considered “statistically and clinically significant, the authors wrote. There were no significant improvements in anxiety or depressive symptoms in the stretching and resistance training group at the different time points.
Outcomes in the yoga group were also better with regards to disease-specific health-related quality of life (beta, –7.77 at 8 weeks and –7.99 at 20 weeks). Those who were in the mindfulness yoga group also had greater improvements in measures of perceived hardship and equanimity, compared with the stretching and resistance training group.
Referring to the improved psychological outcomes in the yoga group, the authors wrote, “these benefits were remarkable because the participants who received the [mindfulness yoga] intervention attended a mean of only 6 sessions.”
There were significant reductions in motor symptoms in both groups, which were significantly higher among those undergoing stretching, but the differences in the mean scores between the two groups were “clinically insignificant,” they wrote.
Three participants in the yoga group and 2 in the control group reported temporary mild knee pain. No serious adverse events were reported.
Expectation bias, selection bias, and the dropout rates of 15.2% at 8 weeks and 18.8% at 20 weeks are limitations of the study, the authors noted.
“These findings suggest that ,” Dr. Kwok and her colleagues concluded. “Considering that PD is not only a physically limiting condition but also a psychologically distressing life event, health care professionals should adopt a holistic approach in PD rehabilitation. Future rehabilitation programs could consider integrating mindfulness skills into physical therapy to enhance the holistic well-being of people with neurodegenerative conditions.”
The trial was supported by the Professional Development Fund of the Association of Hong Kong Nursing Staff. The authors had no disclosures.
SOURCE: Kwok JYY et al. JAMA Neurol. 2019 Apr 8. doi: 10.1001/jamaneurol.2019.0534.
Among patients with mild or moderate Parkinson’s disease, mindfulness yoga was as effective as stretching and resistance training in improving motor function and mobility, a randomized trial found.
In addition, mindfulness yoga reduced anxiety and depressive symptoms and increased spiritual well-being and health-related quality of life more than stretching and resistance training, researchers reported in JAMA Neurology.
Although guidelines support exercise for patients with Parkinson’s disease, investigators had not examined whether yoga is superior to conventional exercise for stress and symptom management in this patient population. Jojo Y. Y. Kwok, PhD, a research assistant professor of nursing at the University of Hong Kong, and her colleagues conducted an assessor-masked, randomized trial that included 138 adults with idiopathic Parkinson’s disease who were able to stand on their own and walk with or without an assistive device. The trial was conducted at 4 community rehabilitation centers in Hong Kong between December 1, 2016, and May 31, 2017. Participants were randomized to 8 weeks of mindfulness yoga delivered weekly in 90-minute group sessions (71) or stretching and resistance training delivered in weekly 60-minute group sessions (67).
The primary outcomes was psychological distress in terms of anxiety and depressive symptoms assessed with the Hospital Anxiety and Depression Scale (HADS). Secondary outcomes included motor symptom severity, mobility, spiritual well-being in terms of perceived hardship and equanimity, and health-related quality of life. The researchers assessed patients at baseline, 8 weeks, and 20 weeks.
The average age of the participants was 63.7 years; 65 (47.1%) were men. Generalized estimating equation analyses found that patients in the yoga group had significantly better outcomes, including for anxiety (time-by-group interaction, beta, –1.79 at 8 weeks and –2.05 at 20 weeks), and depressive symptoms (beta, –2.75 at 8 weeks and –2.75 at 20 weeks). These improvements were considered “statistically and clinically significant, the authors wrote. There were no significant improvements in anxiety or depressive symptoms in the stretching and resistance training group at the different time points.
Outcomes in the yoga group were also better with regards to disease-specific health-related quality of life (beta, –7.77 at 8 weeks and –7.99 at 20 weeks). Those who were in the mindfulness yoga group also had greater improvements in measures of perceived hardship and equanimity, compared with the stretching and resistance training group.
Referring to the improved psychological outcomes in the yoga group, the authors wrote, “these benefits were remarkable because the participants who received the [mindfulness yoga] intervention attended a mean of only 6 sessions.”
There were significant reductions in motor symptoms in both groups, which were significantly higher among those undergoing stretching, but the differences in the mean scores between the two groups were “clinically insignificant,” they wrote.
Three participants in the yoga group and 2 in the control group reported temporary mild knee pain. No serious adverse events were reported.
Expectation bias, selection bias, and the dropout rates of 15.2% at 8 weeks and 18.8% at 20 weeks are limitations of the study, the authors noted.
“These findings suggest that ,” Dr. Kwok and her colleagues concluded. “Considering that PD is not only a physically limiting condition but also a psychologically distressing life event, health care professionals should adopt a holistic approach in PD rehabilitation. Future rehabilitation programs could consider integrating mindfulness skills into physical therapy to enhance the holistic well-being of people with neurodegenerative conditions.”
The trial was supported by the Professional Development Fund of the Association of Hong Kong Nursing Staff. The authors had no disclosures.
SOURCE: Kwok JYY et al. JAMA Neurol. 2019 Apr 8. doi: 10.1001/jamaneurol.2019.0534.
Among patients with mild or moderate Parkinson’s disease, mindfulness yoga was as effective as stretching and resistance training in improving motor function and mobility, a randomized trial found.
In addition, mindfulness yoga reduced anxiety and depressive symptoms and increased spiritual well-being and health-related quality of life more than stretching and resistance training, researchers reported in JAMA Neurology.
Although guidelines support exercise for patients with Parkinson’s disease, investigators had not examined whether yoga is superior to conventional exercise for stress and symptom management in this patient population. Jojo Y. Y. Kwok, PhD, a research assistant professor of nursing at the University of Hong Kong, and her colleagues conducted an assessor-masked, randomized trial that included 138 adults with idiopathic Parkinson’s disease who were able to stand on their own and walk with or without an assistive device. The trial was conducted at 4 community rehabilitation centers in Hong Kong between December 1, 2016, and May 31, 2017. Participants were randomized to 8 weeks of mindfulness yoga delivered weekly in 90-minute group sessions (71) or stretching and resistance training delivered in weekly 60-minute group sessions (67).
The primary outcomes was psychological distress in terms of anxiety and depressive symptoms assessed with the Hospital Anxiety and Depression Scale (HADS). Secondary outcomes included motor symptom severity, mobility, spiritual well-being in terms of perceived hardship and equanimity, and health-related quality of life. The researchers assessed patients at baseline, 8 weeks, and 20 weeks.
The average age of the participants was 63.7 years; 65 (47.1%) were men. Generalized estimating equation analyses found that patients in the yoga group had significantly better outcomes, including for anxiety (time-by-group interaction, beta, –1.79 at 8 weeks and –2.05 at 20 weeks), and depressive symptoms (beta, –2.75 at 8 weeks and –2.75 at 20 weeks). These improvements were considered “statistically and clinically significant, the authors wrote. There were no significant improvements in anxiety or depressive symptoms in the stretching and resistance training group at the different time points.
Outcomes in the yoga group were also better with regards to disease-specific health-related quality of life (beta, –7.77 at 8 weeks and –7.99 at 20 weeks). Those who were in the mindfulness yoga group also had greater improvements in measures of perceived hardship and equanimity, compared with the stretching and resistance training group.
Referring to the improved psychological outcomes in the yoga group, the authors wrote, “these benefits were remarkable because the participants who received the [mindfulness yoga] intervention attended a mean of only 6 sessions.”
There were significant reductions in motor symptoms in both groups, which were significantly higher among those undergoing stretching, but the differences in the mean scores between the two groups were “clinically insignificant,” they wrote.
Three participants in the yoga group and 2 in the control group reported temporary mild knee pain. No serious adverse events were reported.
Expectation bias, selection bias, and the dropout rates of 15.2% at 8 weeks and 18.8% at 20 weeks are limitations of the study, the authors noted.
“These findings suggest that ,” Dr. Kwok and her colleagues concluded. “Considering that PD is not only a physically limiting condition but also a psychologically distressing life event, health care professionals should adopt a holistic approach in PD rehabilitation. Future rehabilitation programs could consider integrating mindfulness skills into physical therapy to enhance the holistic well-being of people with neurodegenerative conditions.”
The trial was supported by the Professional Development Fund of the Association of Hong Kong Nursing Staff. The authors had no disclosures.
SOURCE: Kwok JYY et al. JAMA Neurol. 2019 Apr 8. doi: 10.1001/jamaneurol.2019.0534.
FROM JAMA NEUROLOGY
FDA concerned about e-cigs/seizures in youth
the agency announced April 3.
Between 2010 and early 2019, the FDA and poison control centers received 35 reports of seizures that mentioned the use of e-cigarettes. Most reports involved youth or young adults, and the reports have increased slightly since June 2018, the announcement says.
“We want to be clear that we don’t yet know if there’s a direct relationship between the use of e-cigarettes and a risk of seizure,” said FDA Commissioner Scott Gottlieb, MD, and Principal Deputy Commissioner Amy Abernethy, MD, PhD, in a statement. “We believe these 35 cases warrant scientific investigation into whether there is in fact a connection.”
In addition, the FDA is trying to determine whether any e-cigarette product-specific factors may be associated with the risk of seizures.
Seizures have been reported after a few puffs or up to 1 day after e-cigarette use and among first-time and experienced users. A few patients had a prior history of seizures or also used other substances, such as marijuana or amphetamines.
“While 35 cases may not seem like much compared to the total number of people using e-cigarettes, we are nonetheless concerned by these reported cases. We also recognized that not all of the cases may be reported,” Dr. Gottlieb and Dr. Abernethy said.
Although seizures are known side effects of nicotine toxicity and have been reported in the context of intentional or accidental swallowing of e-cigarette liquid, the voluntary reports of seizures occurring with vaping could represent a new safety issue, the FDA said.
The agency encouraged people to report cases via an online safety reporting portal. It also provided redacted case reports that involve vaping and seizures.
the agency announced April 3.
Between 2010 and early 2019, the FDA and poison control centers received 35 reports of seizures that mentioned the use of e-cigarettes. Most reports involved youth or young adults, and the reports have increased slightly since June 2018, the announcement says.
“We want to be clear that we don’t yet know if there’s a direct relationship between the use of e-cigarettes and a risk of seizure,” said FDA Commissioner Scott Gottlieb, MD, and Principal Deputy Commissioner Amy Abernethy, MD, PhD, in a statement. “We believe these 35 cases warrant scientific investigation into whether there is in fact a connection.”
In addition, the FDA is trying to determine whether any e-cigarette product-specific factors may be associated with the risk of seizures.
Seizures have been reported after a few puffs or up to 1 day after e-cigarette use and among first-time and experienced users. A few patients had a prior history of seizures or also used other substances, such as marijuana or amphetamines.
“While 35 cases may not seem like much compared to the total number of people using e-cigarettes, we are nonetheless concerned by these reported cases. We also recognized that not all of the cases may be reported,” Dr. Gottlieb and Dr. Abernethy said.
Although seizures are known side effects of nicotine toxicity and have been reported in the context of intentional or accidental swallowing of e-cigarette liquid, the voluntary reports of seizures occurring with vaping could represent a new safety issue, the FDA said.
The agency encouraged people to report cases via an online safety reporting portal. It also provided redacted case reports that involve vaping and seizures.
the agency announced April 3.
Between 2010 and early 2019, the FDA and poison control centers received 35 reports of seizures that mentioned the use of e-cigarettes. Most reports involved youth or young adults, and the reports have increased slightly since June 2018, the announcement says.
“We want to be clear that we don’t yet know if there’s a direct relationship between the use of e-cigarettes and a risk of seizure,” said FDA Commissioner Scott Gottlieb, MD, and Principal Deputy Commissioner Amy Abernethy, MD, PhD, in a statement. “We believe these 35 cases warrant scientific investigation into whether there is in fact a connection.”
In addition, the FDA is trying to determine whether any e-cigarette product-specific factors may be associated with the risk of seizures.
Seizures have been reported after a few puffs or up to 1 day after e-cigarette use and among first-time and experienced users. A few patients had a prior history of seizures or also used other substances, such as marijuana or amphetamines.
“While 35 cases may not seem like much compared to the total number of people using e-cigarettes, we are nonetheless concerned by these reported cases. We also recognized that not all of the cases may be reported,” Dr. Gottlieb and Dr. Abernethy said.
Although seizures are known side effects of nicotine toxicity and have been reported in the context of intentional or accidental swallowing of e-cigarette liquid, the voluntary reports of seizures occurring with vaping could represent a new safety issue, the FDA said.
The agency encouraged people to report cases via an online safety reporting portal. It also provided redacted case reports that involve vaping and seizures.
Cerebellar volume may predict disability in patients with relapsing-remitting MS
DALLAS – Among patients with relapsing-remitting multiple sclerosis (MS), cerebellar volume may independently predict clinical disability as measured by the 25-foot walk test, according to a retrospective analysis of data from a phase 3 trial. Baseline cerebellar gray matter volume was the only MRI metric that significantly predicted 25-foot walk test results at 36 months, researchers reported at ACTRIMS Forum 2019, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Investigators have found that demyelination in MS tends to occur in the cerebellum, and cerebellar atrophy contributes to clinical impairment in patients with the disease. In addition, cerebellar volume loss over one year may predict disease worsening in patients with progressive MS, said Maria Petracca, MD, PhD, a postdoctoral fellow at Icahn School of Medicine at Mount Sinai, New York, and her research colleagues. Prior studies, however, had not tested in a large group of patients whether baseline cerebellar volume predicts disability in relapsing-remitting MS.
To examine this question, Dr. Petracca and her colleagues analyzed MRI data from 838 of 1,008 patients in the phase 3 CombiRx trial. Patients in the multicenter, randomized trial had relapsing-remitting MS and received immunomodulatory treatment with glatiramer acetate, interferon beta-1a, or both. The researchers used an MRI analysis package to measure whole brain and cerebellar T2 and gadolinium-enhancing lesions, and they used statistical parametric mapping to measure gray matter fraction and cerebellar volume. Expanded Disability Status Scale (EDSS) scores and scores on the Multiple Sclerosis Functional Composite (MSFC) and its subcomponents were assessed at baseline and 36 months. The investigators assessed changes in clinical scores using repeated measure analysis of variance. They examined the relationship between MRI metrics and clinical disability at baseline and follow-up using ordinal and hierarchical multiple linear regression analysis.
At baseline, patients had a mean age of 37.7, and 72% were female. Median EDSS score was 2, and average cerebellar gray matter volume was 109.78 mL.
A regression model that included T2 and gadolinium-enhancing lesion volume, supratentorial gray matter volume, and cerebellar gray matter volume explained about 15% of the variance in EDSS and MSFC scores at baseline. Cerebellar volume was a significant predictor of MSFC (beta = 0.188).
The 25-foot walk test was the only clinical score that significantly worsened during follow-up – from an average of 4.94 seconds at baseline to 5.09 seconds at follow-up. “Baseline cerebellar gray matter volume was the only MRI metric to significantly predict 25-foot walk test scores at follow-up (beta = –0.172),” the researchers reported. “These results suggest that cerebellar volume is an independent predictor of clinical disability in MS patients as measured by 25-foot walk test.”
The researchers had no disclosures.
[email protected]
SOURCE: Petracca M et al. ACTRIMS Forum 2019, Abstract 160.
DALLAS – Among patients with relapsing-remitting multiple sclerosis (MS), cerebellar volume may independently predict clinical disability as measured by the 25-foot walk test, according to a retrospective analysis of data from a phase 3 trial. Baseline cerebellar gray matter volume was the only MRI metric that significantly predicted 25-foot walk test results at 36 months, researchers reported at ACTRIMS Forum 2019, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Investigators have found that demyelination in MS tends to occur in the cerebellum, and cerebellar atrophy contributes to clinical impairment in patients with the disease. In addition, cerebellar volume loss over one year may predict disease worsening in patients with progressive MS, said Maria Petracca, MD, PhD, a postdoctoral fellow at Icahn School of Medicine at Mount Sinai, New York, and her research colleagues. Prior studies, however, had not tested in a large group of patients whether baseline cerebellar volume predicts disability in relapsing-remitting MS.
To examine this question, Dr. Petracca and her colleagues analyzed MRI data from 838 of 1,008 patients in the phase 3 CombiRx trial. Patients in the multicenter, randomized trial had relapsing-remitting MS and received immunomodulatory treatment with glatiramer acetate, interferon beta-1a, or both. The researchers used an MRI analysis package to measure whole brain and cerebellar T2 and gadolinium-enhancing lesions, and they used statistical parametric mapping to measure gray matter fraction and cerebellar volume. Expanded Disability Status Scale (EDSS) scores and scores on the Multiple Sclerosis Functional Composite (MSFC) and its subcomponents were assessed at baseline and 36 months. The investigators assessed changes in clinical scores using repeated measure analysis of variance. They examined the relationship between MRI metrics and clinical disability at baseline and follow-up using ordinal and hierarchical multiple linear regression analysis.
At baseline, patients had a mean age of 37.7, and 72% were female. Median EDSS score was 2, and average cerebellar gray matter volume was 109.78 mL.
A regression model that included T2 and gadolinium-enhancing lesion volume, supratentorial gray matter volume, and cerebellar gray matter volume explained about 15% of the variance in EDSS and MSFC scores at baseline. Cerebellar volume was a significant predictor of MSFC (beta = 0.188).
The 25-foot walk test was the only clinical score that significantly worsened during follow-up – from an average of 4.94 seconds at baseline to 5.09 seconds at follow-up. “Baseline cerebellar gray matter volume was the only MRI metric to significantly predict 25-foot walk test scores at follow-up (beta = –0.172),” the researchers reported. “These results suggest that cerebellar volume is an independent predictor of clinical disability in MS patients as measured by 25-foot walk test.”
The researchers had no disclosures.
[email protected]
SOURCE: Petracca M et al. ACTRIMS Forum 2019, Abstract 160.
DALLAS – Among patients with relapsing-remitting multiple sclerosis (MS), cerebellar volume may independently predict clinical disability as measured by the 25-foot walk test, according to a retrospective analysis of data from a phase 3 trial. Baseline cerebellar gray matter volume was the only MRI metric that significantly predicted 25-foot walk test results at 36 months, researchers reported at ACTRIMS Forum 2019, the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Investigators have found that demyelination in MS tends to occur in the cerebellum, and cerebellar atrophy contributes to clinical impairment in patients with the disease. In addition, cerebellar volume loss over one year may predict disease worsening in patients with progressive MS, said Maria Petracca, MD, PhD, a postdoctoral fellow at Icahn School of Medicine at Mount Sinai, New York, and her research colleagues. Prior studies, however, had not tested in a large group of patients whether baseline cerebellar volume predicts disability in relapsing-remitting MS.
To examine this question, Dr. Petracca and her colleagues analyzed MRI data from 838 of 1,008 patients in the phase 3 CombiRx trial. Patients in the multicenter, randomized trial had relapsing-remitting MS and received immunomodulatory treatment with glatiramer acetate, interferon beta-1a, or both. The researchers used an MRI analysis package to measure whole brain and cerebellar T2 and gadolinium-enhancing lesions, and they used statistical parametric mapping to measure gray matter fraction and cerebellar volume. Expanded Disability Status Scale (EDSS) scores and scores on the Multiple Sclerosis Functional Composite (MSFC) and its subcomponents were assessed at baseline and 36 months. The investigators assessed changes in clinical scores using repeated measure analysis of variance. They examined the relationship between MRI metrics and clinical disability at baseline and follow-up using ordinal and hierarchical multiple linear regression analysis.
At baseline, patients had a mean age of 37.7, and 72% were female. Median EDSS score was 2, and average cerebellar gray matter volume was 109.78 mL.
A regression model that included T2 and gadolinium-enhancing lesion volume, supratentorial gray matter volume, and cerebellar gray matter volume explained about 15% of the variance in EDSS and MSFC scores at baseline. Cerebellar volume was a significant predictor of MSFC (beta = 0.188).
The 25-foot walk test was the only clinical score that significantly worsened during follow-up – from an average of 4.94 seconds at baseline to 5.09 seconds at follow-up. “Baseline cerebellar gray matter volume was the only MRI metric to significantly predict 25-foot walk test scores at follow-up (beta = –0.172),” the researchers reported. “These results suggest that cerebellar volume is an independent predictor of clinical disability in MS patients as measured by 25-foot walk test.”
The researchers had no disclosures.
[email protected]
SOURCE: Petracca M et al. ACTRIMS Forum 2019, Abstract 160.
REPORTING FROM ACTRIMS FORUM 2019
Key clinical point: In patients with relapsing-remitting MS, cerebellar volume may independently predict clinical disability as measured by the 25-foot walk test.
Major finding: Baseline cerebellar gray matter volume was the only MRI metric that significantly predicted 25-foot walk test results at 36 months (Beta = –0.172).
Study details: A retrospective analysis of MRI data from 838 patients in the phase 3 CombiRx trial.
Disclosures: The researchers had no disclosures.
Source: Petracca M et al. ACTRIMS Forum 2019, Abstract 160.
Many EMS protocols for status epilepticus do not follow evidence-based guidelines
“Many protocols did not follow evidence-based guidelines and did not accurately define generalized convulsive status epilepticus,” said John P. Betjemann, MD, associate professor of neurology at the University of California, San Francisco, and his colleagues. They reported their findings in the March 26 issue of JAMA.
Generalized convulsive status epilepticus is a neurologic emergency, and trials published in 2001 and 2012 found that benzodiazepines are effective prehospital treatments for patients with generalized convulsive status epilepticus. These trials informed a 2016 evidence-based guideline that cites level A evidence for intramuscular midazolam, IV lorazepam, and IV diazepam as initial treatment options for adults.
To determine whether EMS system protocols follow these recommendations, the investigators reviewed treatment protocols from 33 EMS systems that cover the 58 counties in California. The researchers reviewed EMS system protocols between May and June 2018 to determine when they were last updated and whether they defined generalized convulsive status epilepticus according to the guideline (namely, 5 or more minutes of continuous seizure or two or more discrete seizures between which a patient has incomplete recovery of consciousness). They also determined whether the protocols included any of the three benzodiazepines in the guideline and, if so, at what dose and using which route of administration.
Protocols’ most recent revision dates ranged between 2007 and 2018. Twenty-seven protocols (81.8%) were revised after the second clinical trial was published in 2012, and 17 (51.5%) were revised after the 2016 guideline. Seven EMS system protocols (21.2%) defined generalized convulsive status epilepticus according to the guideline. Thirty-two protocols (97.0%) included intramuscular midazolam, 2 (6.1%) included IV lorazepam, and 5 (15.2%) included IV diazepam.
Although the protocols “appropriately emphasized” intramuscular midazolam, the protocol doses often were lower than those used in the trials or recommended in the guideline. In addition, most protocols listed IV and intraosseous midazolam as options, although these treatments were not studied in the trials nor recommended in the guideline. In all, six of the protocols (18.2%) recommended at least one medication by the route and dose suggested in the trials or in the guideline.
“Why EMS system protocols deviate from the evidence and how this affects patient outcomes deserves further study,” the authors said.
The researchers noted that they examined EMS protocols in only one state and that “protocols may not necessarily reflect what emergency medical technicians actually do in practice.” In addition, the researchers accessed the most recent protocols by consulting EMS system websites rather than by contacting each EMS system for its most up-to-date protocol.
The authors reported personal compensation from JAMA Neurology and from Continuum Audio unrelated to the present study, as well as grants from the National Institutes of Health.
SOURCE: Betjemann JP et al. JAMA. 2019 Mar 26.
“Many protocols did not follow evidence-based guidelines and did not accurately define generalized convulsive status epilepticus,” said John P. Betjemann, MD, associate professor of neurology at the University of California, San Francisco, and his colleagues. They reported their findings in the March 26 issue of JAMA.
Generalized convulsive status epilepticus is a neurologic emergency, and trials published in 2001 and 2012 found that benzodiazepines are effective prehospital treatments for patients with generalized convulsive status epilepticus. These trials informed a 2016 evidence-based guideline that cites level A evidence for intramuscular midazolam, IV lorazepam, and IV diazepam as initial treatment options for adults.
To determine whether EMS system protocols follow these recommendations, the investigators reviewed treatment protocols from 33 EMS systems that cover the 58 counties in California. The researchers reviewed EMS system protocols between May and June 2018 to determine when they were last updated and whether they defined generalized convulsive status epilepticus according to the guideline (namely, 5 or more minutes of continuous seizure or two or more discrete seizures between which a patient has incomplete recovery of consciousness). They also determined whether the protocols included any of the three benzodiazepines in the guideline and, if so, at what dose and using which route of administration.
Protocols’ most recent revision dates ranged between 2007 and 2018. Twenty-seven protocols (81.8%) were revised after the second clinical trial was published in 2012, and 17 (51.5%) were revised after the 2016 guideline. Seven EMS system protocols (21.2%) defined generalized convulsive status epilepticus according to the guideline. Thirty-two protocols (97.0%) included intramuscular midazolam, 2 (6.1%) included IV lorazepam, and 5 (15.2%) included IV diazepam.
Although the protocols “appropriately emphasized” intramuscular midazolam, the protocol doses often were lower than those used in the trials or recommended in the guideline. In addition, most protocols listed IV and intraosseous midazolam as options, although these treatments were not studied in the trials nor recommended in the guideline. In all, six of the protocols (18.2%) recommended at least one medication by the route and dose suggested in the trials or in the guideline.
“Why EMS system protocols deviate from the evidence and how this affects patient outcomes deserves further study,” the authors said.
The researchers noted that they examined EMS protocols in only one state and that “protocols may not necessarily reflect what emergency medical technicians actually do in practice.” In addition, the researchers accessed the most recent protocols by consulting EMS system websites rather than by contacting each EMS system for its most up-to-date protocol.
The authors reported personal compensation from JAMA Neurology and from Continuum Audio unrelated to the present study, as well as grants from the National Institutes of Health.
SOURCE: Betjemann JP et al. JAMA. 2019 Mar 26.
“Many protocols did not follow evidence-based guidelines and did not accurately define generalized convulsive status epilepticus,” said John P. Betjemann, MD, associate professor of neurology at the University of California, San Francisco, and his colleagues. They reported their findings in the March 26 issue of JAMA.
Generalized convulsive status epilepticus is a neurologic emergency, and trials published in 2001 and 2012 found that benzodiazepines are effective prehospital treatments for patients with generalized convulsive status epilepticus. These trials informed a 2016 evidence-based guideline that cites level A evidence for intramuscular midazolam, IV lorazepam, and IV diazepam as initial treatment options for adults.
To determine whether EMS system protocols follow these recommendations, the investigators reviewed treatment protocols from 33 EMS systems that cover the 58 counties in California. The researchers reviewed EMS system protocols between May and June 2018 to determine when they were last updated and whether they defined generalized convulsive status epilepticus according to the guideline (namely, 5 or more minutes of continuous seizure or two or more discrete seizures between which a patient has incomplete recovery of consciousness). They also determined whether the protocols included any of the three benzodiazepines in the guideline and, if so, at what dose and using which route of administration.
Protocols’ most recent revision dates ranged between 2007 and 2018. Twenty-seven protocols (81.8%) were revised after the second clinical trial was published in 2012, and 17 (51.5%) were revised after the 2016 guideline. Seven EMS system protocols (21.2%) defined generalized convulsive status epilepticus according to the guideline. Thirty-two protocols (97.0%) included intramuscular midazolam, 2 (6.1%) included IV lorazepam, and 5 (15.2%) included IV diazepam.
Although the protocols “appropriately emphasized” intramuscular midazolam, the protocol doses often were lower than those used in the trials or recommended in the guideline. In addition, most protocols listed IV and intraosseous midazolam as options, although these treatments were not studied in the trials nor recommended in the guideline. In all, six of the protocols (18.2%) recommended at least one medication by the route and dose suggested in the trials or in the guideline.
“Why EMS system protocols deviate from the evidence and how this affects patient outcomes deserves further study,” the authors said.
The researchers noted that they examined EMS protocols in only one state and that “protocols may not necessarily reflect what emergency medical technicians actually do in practice.” In addition, the researchers accessed the most recent protocols by consulting EMS system websites rather than by contacting each EMS system for its most up-to-date protocol.
The authors reported personal compensation from JAMA Neurology and from Continuum Audio unrelated to the present study, as well as grants from the National Institutes of Health.
SOURCE: Betjemann JP et al. JAMA. 2019 Mar 26.
FROM JAMA
Key clinical point: Many emergency medical services (EMS) system protocols may not follow evidence-based guidelines or accurately define generalized convulsive status epilepticus.
Major finding: In all, 18.2% of the protocols recommended at least one medication by the route and at the dose suggested in clinical trials or in an evidence-based guideline.
Study details: A review of treatment protocols from 33 EMS systems that cover the 58 counties in California.
Disclosures: The authors reported personal compensation from JAMA Neurology and Continuum Audio unrelated to the present study and grants from the National Institutes of Health.
Source: Betjemann JP et al. JAMA. 2019 March 26.
Can intraputamenal infusions of GDNF treat Parkinson’s disease?
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”
Open-label extension
By week 80, when all participants had received GDNF, both groups showed moderate to large improvement in symptoms, compared with baseline. From baseline to week 80, percentage change in UPDRS motor score in the off state did not significantly differ between patients who received GDNF for 80 weeks and patients who received placebo followed by GDNF (26.7% vs. 27.6%). Secondary endpoints also did not differ between the groups. Treatment compliance was 97.8%; no patients discontinued the study.
The trials were funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. GDNF and additional resources and funding were provided by MedGenesis Therapeutix, which owns the license for GDNF and received funding from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw manufactured the convection-enhanced delivery device on behalf of North Bristol NHS Trust. The Gatsby Foundation provided a 3T MRI scanner. Some study authors are employed by and have shares or share options with MedGenesis Therapeutix. Other authors are employees of Renishaw. Dr. Gill is Renishaw’s medical director and may have a future royalty share from the drug delivery system that he invented.
SOURCES: Whone AL et al. Brain. 2019 Feb 26. doi: 10.1093/brain/awz023; Whone AL et al. J Parkinsons Dis. 2019 Feb 26. doi: 10.3233/JPD-191576.
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”
Open-label extension
By week 80, when all participants had received GDNF, both groups showed moderate to large improvement in symptoms, compared with baseline. From baseline to week 80, percentage change in UPDRS motor score in the off state did not significantly differ between patients who received GDNF for 80 weeks and patients who received placebo followed by GDNF (26.7% vs. 27.6%). Secondary endpoints also did not differ between the groups. Treatment compliance was 97.8%; no patients discontinued the study.
The trials were funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. GDNF and additional resources and funding were provided by MedGenesis Therapeutix, which owns the license for GDNF and received funding from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw manufactured the convection-enhanced delivery device on behalf of North Bristol NHS Trust. The Gatsby Foundation provided a 3T MRI scanner. Some study authors are employed by and have shares or share options with MedGenesis Therapeutix. Other authors are employees of Renishaw. Dr. Gill is Renishaw’s medical director and may have a future royalty share from the drug delivery system that he invented.
SOURCES: Whone AL et al. Brain. 2019 Feb 26. doi: 10.1093/brain/awz023; Whone AL et al. J Parkinsons Dis. 2019 Feb 26. doi: 10.3233/JPD-191576.
researchers reported. The investigational therapy, delivered through a skull-mounted port, was well tolerated in a 40-week, randomized, controlled trial and a 40-week, open-label extension.
Neither study met its primary endpoint, but post hoc analyses suggest possible clinical benefits. In addition, PET imaging after the 40-week, randomized trial found significantly increased 18F-DOPA uptake in patients who received GDNF. The randomized trial was published in the March 2019 issue of Brain; data from the open-label extension were published online ahead of print Feb. 26, 2019, in the Journal of Parkinson’s Disease.
“The spatial and relative magnitude of the improvement in the brain scans is beyond anything seen previously in trials of surgically delivered growth-factor treatments for Parkinson’s [disease],” said principal investigator Alan L. Whone, MBChB, PhD, of the University of Bristol (England) and North Bristol National Health Service Trust. “This represents some of the most compelling evidence yet that we may have a means to possibly reawaken and restore the dopamine brain cells that are gradually destroyed in Parkinson’s [disease].”
Nevertheless, the trial did not confirm clinical benefits. The hypothesis that growth factors can benefit patients with Parkinson’s disease may be incorrect, the researchers acknowledged. It also is possible that the hypothesis is valid and that a trial with a higher GDNF dose, longer treatment duration, patients with an earlier disease stage, or different outcome measures would yield positive results. GDNF warrants further study, they wrote.
The findings could have implications for other neurologic disorders as well.
“This trial has shown that we can safely and repeatedly infuse drugs directly into patients’ brains over months or years. This is a significant breakthrough in our ability to treat neurologic conditions ... because most drugs that might work cannot cross from the bloodstream into the brain,” said Steven Gill, MB, MS. Mr. Gill, of the North Bristol NHS Trust and the U.K.-based engineering firm Renishaw, designed the convection-enhanced delivery system used in the studies.
A neurotrophic protein
GDNF has neurorestorative and neuroprotective effects in animal models of Parkinson’s disease. In open-label studies, continuous, low-rate intraputamenal administration of GDNF has shown signs of potential efficacy, but a placebo-controlled trial did not replicate clinical benefits. In the present studies, the researchers assessed intermittent GDNF administration using convection-enhanced delivery, which can achieve wider and more even distribution of GDNF, compared with the previous approach.
The researchers conducted a single-center, randomized, double-blind, placebo-controlled trial to study this novel administration approach. Patients were aged 35-75 years, had motor symptoms for at least 5 years, and had moderate disease severity in the off state (that is, Hoehn and Yahr stage 2-3 and Unified Parkinson’s Disease Rating Scale motor score–part III [UPDRS-III] of 25-45).
In a pilot stage of the trial, six patients were randomized 2:1 to receive GDNF (120 mcg per putamen) or placebo. In the primary stage, another 35 patients were randomized 1:1 to GDNF or placebo. The primary outcome was the percentage change from baseline to week 40 in the off-state UPDRS-III among patients from the primary stage of the trial. Further analyses included all 41 patients from the pilot and primary stages.
Patients in the primary analysis had a mean age of 56.4 years and mean disease duration of 10.9 years. About half were female.
Results on primary and secondary clinical endpoints did not significantly differ between the groups. Average off state UPDRS motor score decreased by 17.3 in the active treatment group, compared with 11.8 in the placebo group.
A post hoc analysis, however, found that nine patients (43%) in the active-treatment group had a large, clinically important motor improvement of 10 or more points in the off state, whereas no placebo patients did. These “10-point responders in the GDNF group are a potential focus of interest; however, as this is a post hoc finding we would not wish to overinterpret its meaning,” Dr. Whone and his colleagues wrote. Among patients who received GDNF, PET imaging demonstrated significantly increased 18F-DOPA uptake throughout the putamen, ranging from a 25% increase in the left anterior putamen to a 100% increase in both posterior putamena, whereas patients who received placebo did not have significantly increased uptake.
No drug-related serious adverse events were reported. “The majority of device-related adverse events were port site associated, most commonly local hypertrophic scarring or infections, amenable to antibiotics,” the investigators wrote. “The frequency of these declined during the trial as surgical and device handling experience improved.”
Open-label extension
By week 80, when all participants had received GDNF, both groups showed moderate to large improvement in symptoms, compared with baseline. From baseline to week 80, percentage change in UPDRS motor score in the off state did not significantly differ between patients who received GDNF for 80 weeks and patients who received placebo followed by GDNF (26.7% vs. 27.6%). Secondary endpoints also did not differ between the groups. Treatment compliance was 97.8%; no patients discontinued the study.
The trials were funded by Parkinson’s UK with support from the Cure Parkinson’s Trust and in association with the North Bristol NHS Trust. GDNF and additional resources and funding were provided by MedGenesis Therapeutix, which owns the license for GDNF and received funding from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw manufactured the convection-enhanced delivery device on behalf of North Bristol NHS Trust. The Gatsby Foundation provided a 3T MRI scanner. Some study authors are employed by and have shares or share options with MedGenesis Therapeutix. Other authors are employees of Renishaw. Dr. Gill is Renishaw’s medical director and may have a future royalty share from the drug delivery system that he invented.
SOURCES: Whone AL et al. Brain. 2019 Feb 26. doi: 10.1093/brain/awz023; Whone AL et al. J Parkinsons Dis. 2019 Feb 26. doi: 10.3233/JPD-191576.
New data bolster latitude’s association with MS prevalence
DALLAS – Latitude continues to be associated with the prevalence of multiple sclerosis (MS), according to an updated meta-analysis presented at ACTRIMS Forum 2019, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
After integrating data from 94 new studies, “the latitudinal gradient in MS prevalence ... significantly enhanced in magnitude,” said presenting author Steve Simpson Jr., PhD, and his colleagues. Dr. Simpson is a researcher at the Melbourne School of Population & Global Health at the University of Melbourne and the Menzies Institute for Medical Research at the University of Tasmania.
The researchers’ original study, published in 2011, found a slope of 2.68.
Latitudinal variations in MS prevalence have given credence to the theory that sun exposure and vitamin D may play a role in MS onset. The researchers’ original meta-analysis – based on 650 prevalence estimates from 321 studies – “confirmed the existence of a robust latitudinal gradient,” they said. To examine whether the gradient has changed, the researchers identified relevant studies published between 2010 and 2018.
They included complete, peer-reviewed articles in their analysis and extracted data about the study area, prevalence year, MS diagnostic criteria used, sample size, source population, crude prevalence, and age-specific prevalence. They combined the new prevalence estimates with those from their original study. The estimates were log-transformed and weighted with the inverse of the variance. In addition, the researchers used random-effects meta-regression models that adjusted for prevalence year and method of case ascertainment.
Their literature review identified 126 new studies, 94 of which met inclusion criteria. The new studies yielded 230 additional prevalence points, predominantly in Scandinavia, France, and the Middle East.
“Latitude was consistently and significantly associated with MS prevalence in all analyses, increasing in magnitude on adjustment and persisting on age-standardization,” Dr. Simpson and his colleagues reported.
By region, strong and significant positive gradients continue to exist in Australasia, the United Kingdom/Ireland, and North America. Meanwhile, a significant inverse gradient continues to exist in the Italian region, which the researchers have said relates to the frequency of an MS-related HLA-DRB1 allele there. A negative gradient in the Scandinavian/North Atlantic region in the original meta-analysis, considered potentially related to dietary differences, was “markedly reduced” and no longer statistically significant in the updated meta-analysis.
“While there are potential intra-regional effects contributing to the latitudinal variation in MS prevalence, these results and the relative consistency across the whole of the globe continue to provide indirect evidence in support of the role of sun and vitamin D in MS etiology,” Dr. Simpson and his colleagues concluded.
The researchers had no disclosures.
SOURCE: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
DALLAS – Latitude continues to be associated with the prevalence of multiple sclerosis (MS), according to an updated meta-analysis presented at ACTRIMS Forum 2019, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
After integrating data from 94 new studies, “the latitudinal gradient in MS prevalence ... significantly enhanced in magnitude,” said presenting author Steve Simpson Jr., PhD, and his colleagues. Dr. Simpson is a researcher at the Melbourne School of Population & Global Health at the University of Melbourne and the Menzies Institute for Medical Research at the University of Tasmania.
The researchers’ original study, published in 2011, found a slope of 2.68.
Latitudinal variations in MS prevalence have given credence to the theory that sun exposure and vitamin D may play a role in MS onset. The researchers’ original meta-analysis – based on 650 prevalence estimates from 321 studies – “confirmed the existence of a robust latitudinal gradient,” they said. To examine whether the gradient has changed, the researchers identified relevant studies published between 2010 and 2018.
They included complete, peer-reviewed articles in their analysis and extracted data about the study area, prevalence year, MS diagnostic criteria used, sample size, source population, crude prevalence, and age-specific prevalence. They combined the new prevalence estimates with those from their original study. The estimates were log-transformed and weighted with the inverse of the variance. In addition, the researchers used random-effects meta-regression models that adjusted for prevalence year and method of case ascertainment.
Their literature review identified 126 new studies, 94 of which met inclusion criteria. The new studies yielded 230 additional prevalence points, predominantly in Scandinavia, France, and the Middle East.
“Latitude was consistently and significantly associated with MS prevalence in all analyses, increasing in magnitude on adjustment and persisting on age-standardization,” Dr. Simpson and his colleagues reported.
By region, strong and significant positive gradients continue to exist in Australasia, the United Kingdom/Ireland, and North America. Meanwhile, a significant inverse gradient continues to exist in the Italian region, which the researchers have said relates to the frequency of an MS-related HLA-DRB1 allele there. A negative gradient in the Scandinavian/North Atlantic region in the original meta-analysis, considered potentially related to dietary differences, was “markedly reduced” and no longer statistically significant in the updated meta-analysis.
“While there are potential intra-regional effects contributing to the latitudinal variation in MS prevalence, these results and the relative consistency across the whole of the globe continue to provide indirect evidence in support of the role of sun and vitamin D in MS etiology,” Dr. Simpson and his colleagues concluded.
The researchers had no disclosures.
SOURCE: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
DALLAS – Latitude continues to be associated with the prevalence of multiple sclerosis (MS), according to an updated meta-analysis presented at ACTRIMS Forum 2019, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
After integrating data from 94 new studies, “the latitudinal gradient in MS prevalence ... significantly enhanced in magnitude,” said presenting author Steve Simpson Jr., PhD, and his colleagues. Dr. Simpson is a researcher at the Melbourne School of Population & Global Health at the University of Melbourne and the Menzies Institute for Medical Research at the University of Tasmania.
The researchers’ original study, published in 2011, found a slope of 2.68.
Latitudinal variations in MS prevalence have given credence to the theory that sun exposure and vitamin D may play a role in MS onset. The researchers’ original meta-analysis – based on 650 prevalence estimates from 321 studies – “confirmed the existence of a robust latitudinal gradient,” they said. To examine whether the gradient has changed, the researchers identified relevant studies published between 2010 and 2018.
They included complete, peer-reviewed articles in their analysis and extracted data about the study area, prevalence year, MS diagnostic criteria used, sample size, source population, crude prevalence, and age-specific prevalence. They combined the new prevalence estimates with those from their original study. The estimates were log-transformed and weighted with the inverse of the variance. In addition, the researchers used random-effects meta-regression models that adjusted for prevalence year and method of case ascertainment.
Their literature review identified 126 new studies, 94 of which met inclusion criteria. The new studies yielded 230 additional prevalence points, predominantly in Scandinavia, France, and the Middle East.
“Latitude was consistently and significantly associated with MS prevalence in all analyses, increasing in magnitude on adjustment and persisting on age-standardization,” Dr. Simpson and his colleagues reported.
By region, strong and significant positive gradients continue to exist in Australasia, the United Kingdom/Ireland, and North America. Meanwhile, a significant inverse gradient continues to exist in the Italian region, which the researchers have said relates to the frequency of an MS-related HLA-DRB1 allele there. A negative gradient in the Scandinavian/North Atlantic region in the original meta-analysis, considered potentially related to dietary differences, was “markedly reduced” and no longer statistically significant in the updated meta-analysis.
“While there are potential intra-regional effects contributing to the latitudinal variation in MS prevalence, these results and the relative consistency across the whole of the globe continue to provide indirect evidence in support of the role of sun and vitamin D in MS etiology,” Dr. Simpson and his colleagues concluded.
The researchers had no disclosures.
SOURCE: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.
REPORTING FROM ACTRIMS FORUM 2019
Key clinical point: Latitude continues to be associated with the prevalence of multiple sclerosis (MS).
Major finding: For every degree of latitude increase, prevalence of MS per 100,000 people increased by 3.64 in a fully adjusted, age-standardized model.
Study details: A meta-analysis of data from more than 400 studies.
Disclosures: The investigators had no disclosures.
Source: Simpson S Jr et al. ACTRIMS Forum 2019, Abstract 126.