User login
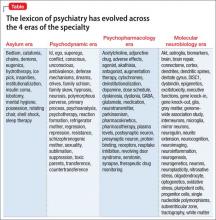
Psychoneurogastroenterology: The abdominal brain, the microbiome, and psychiatry
This nervous system is located inside the wall of the GI tract, extending from the esophagus to the rectum. Technically, it is known as the enteric nervous system, or ENS, but it has been given other labels, too: “second brain,”2 “abdominal brain,” “other brain,” and “back-up brain.” Its neurologic disorders include abdominal epilepsy, abdominal migraine, and autism with intestinal symptoms, such as chronic enterocolitis.3
Impressive brain-like features
The ENS includes 100 million neurons (same as the spinal cord) with glia-like support cells. It contains >30 neurotransmitters, including several closely linked to psychopathology (serotonin, dopamine, γ-aminobutyric acid, and acetylcholine). The ENS is not part of the autonomic nervous system. It communicates with the brain via the vagus nerve.
A vast system of gut bacteria
The ENS maintains close links with, and is influenced by, the microbiome, an extensive universe of commensal (that is, symbiotic) bacteria in the gut that play a vital role in immune health, brain function, and signaling systems within the CNS. The role of the microbiome in neuropsychiatric disorders has become a sizzling area of research.
The numbers of the microbiome are astonishing, including approximately 1,000 species of bacteria; 100 trillion total bacterial organisms (outnumbering cells of the body by 100-fold); 4 million bacterial genes (compared with 26,000 genes in the host human genome); and a density as high as 1 trillion bacteria in a cubic milliliter—higher than any known microbial system.4
Significant GI−brain connections
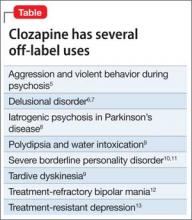
It is of great relevance to psychiatry that 90% of the body’s serotonin and 50% of dopamine are found in the GI brain. Selective serotonin reuptake inhibitors often are associated with GI symptoms, such as nausea and diarrhea; antipsychotics, which are dopamine antagonists, are known for antiemetic effects. Clozapine’s potent anticholinergic effects can cause serious ileus.
Things get more interesting when one considers the association of GI disorders and psychiatric symptoms:
Irritable bowel syndrome is associated with panic disorder, generalized anxiety disorder, social phobia, dysthymia, and major depression.
Inflammatory bowel disease (IBD)— such as Crohn’s disease and ulcerative colitis (prevalence ranging from 6% in Canada to 14% in the United States to 46% in Mexico5)—is commonly associated with mood and anxiety disorders and personality changes. The psychiatric manifestations of IBD are so common that the authors of a recent article in World Journal of Gastroenterology urged gastroenterologists to collaborate with psychiatrists when managing IBD.6
Celiac disease has been repeatedly associated with several neuropsychiatric disorders, including ataxia, epilepsy, peripheral neuropathy, headache, anxiety, attention-deficit/hyperactivity disorder, autism spectrum disorder, and schizophrenia.
New, exciting challenges for medical science
There potentially are important implications for possible exploitation of the ENS and the microbiome in the diagnosis and treatment of neuropsychiatric disorders. For example, consider these speculative challenges:
• Can intestinal biopsy reveal neurotransmitter pathology in schizophrenia?
• Can early dopamine deficiency predict Parkinson’s disease, enabling early intervention?
• Can β-amyloid deposits, the degenerative neurologic stigmata of Alzheimer’s disease, be detected in abdominal neurons years before onset of symptoms to allow early intervention?
• Can the ENS become a therapeutic pathway by targeting the various neurotransmitters found there or by engaging the enormous human microbiome to manipulate its beneficial properties?
• Can foods or probiotic supplements be prescribed as microbiomal adjuncts to improve the mood and anxiety spectrum?
One recommendation I came across is that ingesting 10 to 100 million beneficial bacteria, such as Lactobacillus plantarum and Bifidobacterium infantis, might be helpful. Such prescriptions obviously are speculative but also are reasonably testable hypotheses of ways to exploit the “other brain” and the microbiome.
We must summon the guts to seize this opportunity
An independent second brain and a remarkable microbiome appear to be significant evolutionary adaptations and advantages for humans. For too long, neuropsychiatric researchers have ignored the ENS and the microbiome; now, they must focus on how to exploit these entities to yield innovative diagnostic and therapeutic advances. Integrating the ENS and the microbiome and enmeshing them into neuropsychiatric research and clinical applications hold great promise.
The field of psychoneurogastroenterology is in its infancy, but its growth and relevance will be momentous for neuropsychiatry. A major intellectual peristalsis is underway.
1. Robinson B. The abdominal and pelvic brain. Hammond, IN: Frank S. Betz; 1907.
2. Gershon M. The second brain: a groundbreaking new understanding of nervous disorders of the stomach and intestine. New York, NY: HarperCollins Publishers; 1998.
3. McMillin DL, Richards DG, Mein EA, et al. The abdominal brain and enteric nervous system. J Altern Complement Med. 1999;5(6):575-586.
4. Hill JM, Bhattacharjee S, Pogue AI, et al. The gastrointestinal tract microbiome and potential link to Alzheimer’s disease. Front Neurol. 2014;5:43.
5. Olden KW, Lydiard RB. Gastrointestinal disorders. In: Rundell JR, Wise MG. Textbook of consultation-liaison psychiatry. Washington, DC: American Psychiatric Association; 1994.
6. Filipovic BR, Filipovic BF. World J Gastroenterol. 2014;20(13):3552-3563.
This nervous system is located inside the wall of the GI tract, extending from the esophagus to the rectum. Technically, it is known as the enteric nervous system, or ENS, but it has been given other labels, too: “second brain,”2 “abdominal brain,” “other brain,” and “back-up brain.” Its neurologic disorders include abdominal epilepsy, abdominal migraine, and autism with intestinal symptoms, such as chronic enterocolitis.3
Impressive brain-like features
The ENS includes 100 million neurons (same as the spinal cord) with glia-like support cells. It contains >30 neurotransmitters, including several closely linked to psychopathology (serotonin, dopamine, γ-aminobutyric acid, and acetylcholine). The ENS is not part of the autonomic nervous system. It communicates with the brain via the vagus nerve.
A vast system of gut bacteria
The ENS maintains close links with, and is influenced by, the microbiome, an extensive universe of commensal (that is, symbiotic) bacteria in the gut that play a vital role in immune health, brain function, and signaling systems within the CNS. The role of the microbiome in neuropsychiatric disorders has become a sizzling area of research.
The numbers of the microbiome are astonishing, including approximately 1,000 species of bacteria; 100 trillion total bacterial organisms (outnumbering cells of the body by 100-fold); 4 million bacterial genes (compared with 26,000 genes in the host human genome); and a density as high as 1 trillion bacteria in a cubic milliliter—higher than any known microbial system.4
Significant GI−brain connections
It is of great relevance to psychiatry that 90% of the body’s serotonin and 50% of dopamine are found in the GI brain. Selective serotonin reuptake inhibitors often are associated with GI symptoms, such as nausea and diarrhea; antipsychotics, which are dopamine antagonists, are known for antiemetic effects. Clozapine’s potent anticholinergic effects can cause serious ileus.
Things get more interesting when one considers the association of GI disorders and psychiatric symptoms:
Irritable bowel syndrome is associated with panic disorder, generalized anxiety disorder, social phobia, dysthymia, and major depression.
Inflammatory bowel disease (IBD)— such as Crohn’s disease and ulcerative colitis (prevalence ranging from 6% in Canada to 14% in the United States to 46% in Mexico5)—is commonly associated with mood and anxiety disorders and personality changes. The psychiatric manifestations of IBD are so common that the authors of a recent article in World Journal of Gastroenterology urged gastroenterologists to collaborate with psychiatrists when managing IBD.6
Celiac disease has been repeatedly associated with several neuropsychiatric disorders, including ataxia, epilepsy, peripheral neuropathy, headache, anxiety, attention-deficit/hyperactivity disorder, autism spectrum disorder, and schizophrenia.
New, exciting challenges for medical science
There potentially are important implications for possible exploitation of the ENS and the microbiome in the diagnosis and treatment of neuropsychiatric disorders. For example, consider these speculative challenges:
• Can intestinal biopsy reveal neurotransmitter pathology in schizophrenia?
• Can early dopamine deficiency predict Parkinson’s disease, enabling early intervention?
• Can β-amyloid deposits, the degenerative neurologic stigmata of Alzheimer’s disease, be detected in abdominal neurons years before onset of symptoms to allow early intervention?
• Can the ENS become a therapeutic pathway by targeting the various neurotransmitters found there or by engaging the enormous human microbiome to manipulate its beneficial properties?
• Can foods or probiotic supplements be prescribed as microbiomal adjuncts to improve the mood and anxiety spectrum?
One recommendation I came across is that ingesting 10 to 100 million beneficial bacteria, such as Lactobacillus plantarum and Bifidobacterium infantis, might be helpful. Such prescriptions obviously are speculative but also are reasonably testable hypotheses of ways to exploit the “other brain” and the microbiome.
We must summon the guts to seize this opportunity
An independent second brain and a remarkable microbiome appear to be significant evolutionary adaptations and advantages for humans. For too long, neuropsychiatric researchers have ignored the ENS and the microbiome; now, they must focus on how to exploit these entities to yield innovative diagnostic and therapeutic advances. Integrating the ENS and the microbiome and enmeshing them into neuropsychiatric research and clinical applications hold great promise.
The field of psychoneurogastroenterology is in its infancy, but its growth and relevance will be momentous for neuropsychiatry. A major intellectual peristalsis is underway.
This nervous system is located inside the wall of the GI tract, extending from the esophagus to the rectum. Technically, it is known as the enteric nervous system, or ENS, but it has been given other labels, too: “second brain,”2 “abdominal brain,” “other brain,” and “back-up brain.” Its neurologic disorders include abdominal epilepsy, abdominal migraine, and autism with intestinal symptoms, such as chronic enterocolitis.3
Impressive brain-like features
The ENS includes 100 million neurons (same as the spinal cord) with glia-like support cells. It contains >30 neurotransmitters, including several closely linked to psychopathology (serotonin, dopamine, γ-aminobutyric acid, and acetylcholine). The ENS is not part of the autonomic nervous system. It communicates with the brain via the vagus nerve.
A vast system of gut bacteria
The ENS maintains close links with, and is influenced by, the microbiome, an extensive universe of commensal (that is, symbiotic) bacteria in the gut that play a vital role in immune health, brain function, and signaling systems within the CNS. The role of the microbiome in neuropsychiatric disorders has become a sizzling area of research.
The numbers of the microbiome are astonishing, including approximately 1,000 species of bacteria; 100 trillion total bacterial organisms (outnumbering cells of the body by 100-fold); 4 million bacterial genes (compared with 26,000 genes in the host human genome); and a density as high as 1 trillion bacteria in a cubic milliliter—higher than any known microbial system.4
Significant GI−brain connections
It is of great relevance to psychiatry that 90% of the body’s serotonin and 50% of dopamine are found in the GI brain. Selective serotonin reuptake inhibitors often are associated with GI symptoms, such as nausea and diarrhea; antipsychotics, which are dopamine antagonists, are known for antiemetic effects. Clozapine’s potent anticholinergic effects can cause serious ileus.
Things get more interesting when one considers the association of GI disorders and psychiatric symptoms:
Irritable bowel syndrome is associated with panic disorder, generalized anxiety disorder, social phobia, dysthymia, and major depression.
Inflammatory bowel disease (IBD)— such as Crohn’s disease and ulcerative colitis (prevalence ranging from 6% in Canada to 14% in the United States to 46% in Mexico5)—is commonly associated with mood and anxiety disorders and personality changes. The psychiatric manifestations of IBD are so common that the authors of a recent article in World Journal of Gastroenterology urged gastroenterologists to collaborate with psychiatrists when managing IBD.6
Celiac disease has been repeatedly associated with several neuropsychiatric disorders, including ataxia, epilepsy, peripheral neuropathy, headache, anxiety, attention-deficit/hyperactivity disorder, autism spectrum disorder, and schizophrenia.
New, exciting challenges for medical science
There potentially are important implications for possible exploitation of the ENS and the microbiome in the diagnosis and treatment of neuropsychiatric disorders. For example, consider these speculative challenges:
• Can intestinal biopsy reveal neurotransmitter pathology in schizophrenia?
• Can early dopamine deficiency predict Parkinson’s disease, enabling early intervention?
• Can β-amyloid deposits, the degenerative neurologic stigmata of Alzheimer’s disease, be detected in abdominal neurons years before onset of symptoms to allow early intervention?
• Can the ENS become a therapeutic pathway by targeting the various neurotransmitters found there or by engaging the enormous human microbiome to manipulate its beneficial properties?
• Can foods or probiotic supplements be prescribed as microbiomal adjuncts to improve the mood and anxiety spectrum?
One recommendation I came across is that ingesting 10 to 100 million beneficial bacteria, such as Lactobacillus plantarum and Bifidobacterium infantis, might be helpful. Such prescriptions obviously are speculative but also are reasonably testable hypotheses of ways to exploit the “other brain” and the microbiome.
We must summon the guts to seize this opportunity
An independent second brain and a remarkable microbiome appear to be significant evolutionary adaptations and advantages for humans. For too long, neuropsychiatric researchers have ignored the ENS and the microbiome; now, they must focus on how to exploit these entities to yield innovative diagnostic and therapeutic advances. Integrating the ENS and the microbiome and enmeshing them into neuropsychiatric research and clinical applications hold great promise.
The field of psychoneurogastroenterology is in its infancy, but its growth and relevance will be momentous for neuropsychiatry. A major intellectual peristalsis is underway.
1. Robinson B. The abdominal and pelvic brain. Hammond, IN: Frank S. Betz; 1907.
2. Gershon M. The second brain: a groundbreaking new understanding of nervous disorders of the stomach and intestine. New York, NY: HarperCollins Publishers; 1998.
3. McMillin DL, Richards DG, Mein EA, et al. The abdominal brain and enteric nervous system. J Altern Complement Med. 1999;5(6):575-586.
4. Hill JM, Bhattacharjee S, Pogue AI, et al. The gastrointestinal tract microbiome and potential link to Alzheimer’s disease. Front Neurol. 2014;5:43.
5. Olden KW, Lydiard RB. Gastrointestinal disorders. In: Rundell JR, Wise MG. Textbook of consultation-liaison psychiatry. Washington, DC: American Psychiatric Association; 1994.
6. Filipovic BR, Filipovic BF. World J Gastroenterol. 2014;20(13):3552-3563.
1. Robinson B. The abdominal and pelvic brain. Hammond, IN: Frank S. Betz; 1907.
2. Gershon M. The second brain: a groundbreaking new understanding of nervous disorders of the stomach and intestine. New York, NY: HarperCollins Publishers; 1998.
3. McMillin DL, Richards DG, Mein EA, et al. The abdominal brain and enteric nervous system. J Altern Complement Med. 1999;5(6):575-586.
4. Hill JM, Bhattacharjee S, Pogue AI, et al. The gastrointestinal tract microbiome and potential link to Alzheimer’s disease. Front Neurol. 2014;5:43.
5. Olden KW, Lydiard RB. Gastrointestinal disorders. In: Rundell JR, Wise MG. Textbook of consultation-liaison psychiatry. Washington, DC: American Psychiatric Association; 1994.
6. Filipovic BR, Filipovic BF. World J Gastroenterol. 2014;20(13):3552-3563.
Unmet needs and hassles of psychiatric practice
Recently, my umbrage at these practices soared to a new height when a colleague told me that he had to fight for, and then wait to receive, permission from an insurance employee to increase, by a notch, the maintenance dosage of a long-acting antipsychotic for his patient.
Can anyone justify why an absentee person who has never met the patient should, sight-unseen, second-guess our clinical judgment that a patient’s symptoms are still not well-controlled and require upward adjustment of the dosage? Why are insurance companies allowed to micromanage clinical decision-making? Such outrageous intrusiveness is a signal that insurance companies have “jumped the shark” in their effort to push business interests ahead of the needs of their subscribers.
Whose interests are being put first?
I recall instances when I refused to buckle to pressure from a patient’s third-party payer to switch from 1 antidepressant to another, a move that would save the insurer money but put my patient at risk of relapse. I informed the insurance company representative that my attorney was going to file a lawsuit on behalf of my depressed patient if he were to relapse or attempt suicide because he had been switched from an antidepressant that was working to another that might not.
Fighting back paid off: In each case, I was told the payer would “make an exception” for that patient.
Frustrations of this kind have become commonplace in psychiatric practice. They tend to detract from the stimulating and gratifying aspects of the care we provide, and reinforce the perception that insurance companies’ primary goal is to fatten profits, not facilitate patients’ return to health.
Here are other reasons for chronic frustration in psychiatric practice. They reflect serious, unmet needs that we hope will be resolved soon.
Improved diagnostic schema. We need a valid—and more than simply reliable—evidence-based diagnostic system that is rooted in scientifically established pathophysiology. The basic clinical elements of DSM-5 should be gradually amalgamated with rapidly emerging genetics and biological endophenotypes. The continuum of and boundary between “normal” and “pathologic” human behavior should be further clarified.
Biomarkers. Our field eagerly awaits development of biomarkers (laboratory tests) to bolster psychiatric practice in several ways, including:
• confirming the clinical diagnosis
• identifying biological subtypes
• monitoring response
• guiding selection of drugs
• predicting side effects
• measuring severity of disease.
Elusive parity. We’ve been patient, but we’re tired and angry at empty promises of full parity for psychiatric care. We’ve also had it with the stigmatizing and discriminatory “carve-out” status that allows insurance companies to treat reimbursement for psychiatric services more restrictively than for other medical and surgical specialties. Policy makers must end the egregious discrimination that gives half a loaf to some brain disorders (psychiatric) and a full loaf to other brain disorders (neurologic).
Better medications. We need a stronger commitment from the pharmaceutical industry, on which we rely entirely for development of psychoactive drugs, to wage a relentless war on serious mental illness. The private sector should accelerate translation of groundbreaking neuroscientific discoveries—thanks to research funded by the public sector, such as the National Institutes of Health—into innovative new mechanisms of action. Patients who suffer from psychiatric brain disorders for which there are no approved treatments await that commitment and bold action.
Collaborative care. Psychiatry needs a more consistent, more productive bidirectional relationship with primary care. A stronger bond will improve the care of patients on both sides and would, I believe, increase the satisfaction of clinical practice for both specialists. Because structure can facilitate function, co-locating providers can help achieve this vision.
Legal entanglement. Psychiatry must be unshackled from an oppressive set of laws that tie our hands when we treat patients with brain pathology who are incapable of understanding their illness and their need to be treated. Those laws were imposed long before scientific advances showed that prolonged and untreated episodes of psychosis, mania, depression, and anxiety are associated with neurotoxic processes (neuroinflammation, oxidative and nitrosative stress). Medical urgency and patient protection must trump legalisms, just as unconscious stroke and myocardial infarction patients are treated immediately without filing multiple forms or waiting for a court order.
Another legal beef: We psychiatrists are exasperated with the expanding criminalization of our patients— hapless victims of brain diseases that impair their reality testing and behavior. Should a person who suffers a first epileptic seizure or a stroke while driving and kills the driver of an oncoming car be incarcerated with hardened murderers and rapists and treated for epilepsy in a prison instead of a neurology ward? Our patients belong in a secure hospital, a medical asylum, where they are given compassionate medical care, not the degrading treatment afforded to a felon.
More resources. There is a dire need for psychiatric hospital beds in many parts of the country, because many wards were closed and renovated into more profitable, procedure-oriented specialties. There also is a severe shortage of psychiatrists in our country, as I discussed in my editorial, “Signs, symptoms, and treatment of psychiatrynemia,” (December 2014). The 25% of the population who suffer a mental disorder are clearly underserved at this time.
Furthermore, because today’s research is tomorrow’s new treatment, funding for psychiatric research must increase substantially to find cures and to thus reduce huge direct and indirect costs of mental illness and addictions.
Public enlightenment. A well-informed populace would be a major boon to our sophisticated medical specialty, which remains shrouded by primitive beliefs and archaic attitudes. For many people who desperately need mental health care, negative perceptions of psychiatric disorders and their treatment are a major impediment to seeking help. Psychiatrists can catalyze the process of enlightenment by dedicating time to elevating public understanding of the biology and the medical basis of mental illness.
All this notwithstanding, our work is gratifying
Despite the hassles and unmet needs I’ve enumerated, psychiatry continues to be one of the most exciting fields in medicine. We provide more therapeutic face-time and verbal interactions with our patients than any other medical specialty. Imagine, then, how much more enjoyable psychiatric practice would be if these pesky obstacles were eliminated and the unmet needs of patients and practitioners were addressed.
Recently, my umbrage at these practices soared to a new height when a colleague told me that he had to fight for, and then wait to receive, permission from an insurance employee to increase, by a notch, the maintenance dosage of a long-acting antipsychotic for his patient.
Can anyone justify why an absentee person who has never met the patient should, sight-unseen, second-guess our clinical judgment that a patient’s symptoms are still not well-controlled and require upward adjustment of the dosage? Why are insurance companies allowed to micromanage clinical decision-making? Such outrageous intrusiveness is a signal that insurance companies have “jumped the shark” in their effort to push business interests ahead of the needs of their subscribers.
Whose interests are being put first?
I recall instances when I refused to buckle to pressure from a patient’s third-party payer to switch from 1 antidepressant to another, a move that would save the insurer money but put my patient at risk of relapse. I informed the insurance company representative that my attorney was going to file a lawsuit on behalf of my depressed patient if he were to relapse or attempt suicide because he had been switched from an antidepressant that was working to another that might not.
Fighting back paid off: In each case, I was told the payer would “make an exception” for that patient.
Frustrations of this kind have become commonplace in psychiatric practice. They tend to detract from the stimulating and gratifying aspects of the care we provide, and reinforce the perception that insurance companies’ primary goal is to fatten profits, not facilitate patients’ return to health.
Here are other reasons for chronic frustration in psychiatric practice. They reflect serious, unmet needs that we hope will be resolved soon.
Improved diagnostic schema. We need a valid—and more than simply reliable—evidence-based diagnostic system that is rooted in scientifically established pathophysiology. The basic clinical elements of DSM-5 should be gradually amalgamated with rapidly emerging genetics and biological endophenotypes. The continuum of and boundary between “normal” and “pathologic” human behavior should be further clarified.
Biomarkers. Our field eagerly awaits development of biomarkers (laboratory tests) to bolster psychiatric practice in several ways, including:
• confirming the clinical diagnosis
• identifying biological subtypes
• monitoring response
• guiding selection of drugs
• predicting side effects
• measuring severity of disease.
Elusive parity. We’ve been patient, but we’re tired and angry at empty promises of full parity for psychiatric care. We’ve also had it with the stigmatizing and discriminatory “carve-out” status that allows insurance companies to treat reimbursement for psychiatric services more restrictively than for other medical and surgical specialties. Policy makers must end the egregious discrimination that gives half a loaf to some brain disorders (psychiatric) and a full loaf to other brain disorders (neurologic).
Better medications. We need a stronger commitment from the pharmaceutical industry, on which we rely entirely for development of psychoactive drugs, to wage a relentless war on serious mental illness. The private sector should accelerate translation of groundbreaking neuroscientific discoveries—thanks to research funded by the public sector, such as the National Institutes of Health—into innovative new mechanisms of action. Patients who suffer from psychiatric brain disorders for which there are no approved treatments await that commitment and bold action.
Collaborative care. Psychiatry needs a more consistent, more productive bidirectional relationship with primary care. A stronger bond will improve the care of patients on both sides and would, I believe, increase the satisfaction of clinical practice for both specialists. Because structure can facilitate function, co-locating providers can help achieve this vision.
Legal entanglement. Psychiatry must be unshackled from an oppressive set of laws that tie our hands when we treat patients with brain pathology who are incapable of understanding their illness and their need to be treated. Those laws were imposed long before scientific advances showed that prolonged and untreated episodes of psychosis, mania, depression, and anxiety are associated with neurotoxic processes (neuroinflammation, oxidative and nitrosative stress). Medical urgency and patient protection must trump legalisms, just as unconscious stroke and myocardial infarction patients are treated immediately without filing multiple forms or waiting for a court order.
Another legal beef: We psychiatrists are exasperated with the expanding criminalization of our patients— hapless victims of brain diseases that impair their reality testing and behavior. Should a person who suffers a first epileptic seizure or a stroke while driving and kills the driver of an oncoming car be incarcerated with hardened murderers and rapists and treated for epilepsy in a prison instead of a neurology ward? Our patients belong in a secure hospital, a medical asylum, where they are given compassionate medical care, not the degrading treatment afforded to a felon.
More resources. There is a dire need for psychiatric hospital beds in many parts of the country, because many wards were closed and renovated into more profitable, procedure-oriented specialties. There also is a severe shortage of psychiatrists in our country, as I discussed in my editorial, “Signs, symptoms, and treatment of psychiatrynemia,” (December 2014). The 25% of the population who suffer a mental disorder are clearly underserved at this time.
Furthermore, because today’s research is tomorrow’s new treatment, funding for psychiatric research must increase substantially to find cures and to thus reduce huge direct and indirect costs of mental illness and addictions.
Public enlightenment. A well-informed populace would be a major boon to our sophisticated medical specialty, which remains shrouded by primitive beliefs and archaic attitudes. For many people who desperately need mental health care, negative perceptions of psychiatric disorders and their treatment are a major impediment to seeking help. Psychiatrists can catalyze the process of enlightenment by dedicating time to elevating public understanding of the biology and the medical basis of mental illness.
All this notwithstanding, our work is gratifying
Despite the hassles and unmet needs I’ve enumerated, psychiatry continues to be one of the most exciting fields in medicine. We provide more therapeutic face-time and verbal interactions with our patients than any other medical specialty. Imagine, then, how much more enjoyable psychiatric practice would be if these pesky obstacles were eliminated and the unmet needs of patients and practitioners were addressed.
Recently, my umbrage at these practices soared to a new height when a colleague told me that he had to fight for, and then wait to receive, permission from an insurance employee to increase, by a notch, the maintenance dosage of a long-acting antipsychotic for his patient.
Can anyone justify why an absentee person who has never met the patient should, sight-unseen, second-guess our clinical judgment that a patient’s symptoms are still not well-controlled and require upward adjustment of the dosage? Why are insurance companies allowed to micromanage clinical decision-making? Such outrageous intrusiveness is a signal that insurance companies have “jumped the shark” in their effort to push business interests ahead of the needs of their subscribers.
Whose interests are being put first?
I recall instances when I refused to buckle to pressure from a patient’s third-party payer to switch from 1 antidepressant to another, a move that would save the insurer money but put my patient at risk of relapse. I informed the insurance company representative that my attorney was going to file a lawsuit on behalf of my depressed patient if he were to relapse or attempt suicide because he had been switched from an antidepressant that was working to another that might not.
Fighting back paid off: In each case, I was told the payer would “make an exception” for that patient.
Frustrations of this kind have become commonplace in psychiatric practice. They tend to detract from the stimulating and gratifying aspects of the care we provide, and reinforce the perception that insurance companies’ primary goal is to fatten profits, not facilitate patients’ return to health.
Here are other reasons for chronic frustration in psychiatric practice. They reflect serious, unmet needs that we hope will be resolved soon.
Improved diagnostic schema. We need a valid—and more than simply reliable—evidence-based diagnostic system that is rooted in scientifically established pathophysiology. The basic clinical elements of DSM-5 should be gradually amalgamated with rapidly emerging genetics and biological endophenotypes. The continuum of and boundary between “normal” and “pathologic” human behavior should be further clarified.
Biomarkers. Our field eagerly awaits development of biomarkers (laboratory tests) to bolster psychiatric practice in several ways, including:
• confirming the clinical diagnosis
• identifying biological subtypes
• monitoring response
• guiding selection of drugs
• predicting side effects
• measuring severity of disease.
Elusive parity. We’ve been patient, but we’re tired and angry at empty promises of full parity for psychiatric care. We’ve also had it with the stigmatizing and discriminatory “carve-out” status that allows insurance companies to treat reimbursement for psychiatric services more restrictively than for other medical and surgical specialties. Policy makers must end the egregious discrimination that gives half a loaf to some brain disorders (psychiatric) and a full loaf to other brain disorders (neurologic).
Better medications. We need a stronger commitment from the pharmaceutical industry, on which we rely entirely for development of psychoactive drugs, to wage a relentless war on serious mental illness. The private sector should accelerate translation of groundbreaking neuroscientific discoveries—thanks to research funded by the public sector, such as the National Institutes of Health—into innovative new mechanisms of action. Patients who suffer from psychiatric brain disorders for which there are no approved treatments await that commitment and bold action.
Collaborative care. Psychiatry needs a more consistent, more productive bidirectional relationship with primary care. A stronger bond will improve the care of patients on both sides and would, I believe, increase the satisfaction of clinical practice for both specialists. Because structure can facilitate function, co-locating providers can help achieve this vision.
Legal entanglement. Psychiatry must be unshackled from an oppressive set of laws that tie our hands when we treat patients with brain pathology who are incapable of understanding their illness and their need to be treated. Those laws were imposed long before scientific advances showed that prolonged and untreated episodes of psychosis, mania, depression, and anxiety are associated with neurotoxic processes (neuroinflammation, oxidative and nitrosative stress). Medical urgency and patient protection must trump legalisms, just as unconscious stroke and myocardial infarction patients are treated immediately without filing multiple forms or waiting for a court order.
Another legal beef: We psychiatrists are exasperated with the expanding criminalization of our patients— hapless victims of brain diseases that impair their reality testing and behavior. Should a person who suffers a first epileptic seizure or a stroke while driving and kills the driver of an oncoming car be incarcerated with hardened murderers and rapists and treated for epilepsy in a prison instead of a neurology ward? Our patients belong in a secure hospital, a medical asylum, where they are given compassionate medical care, not the degrading treatment afforded to a felon.
More resources. There is a dire need for psychiatric hospital beds in many parts of the country, because many wards were closed and renovated into more profitable, procedure-oriented specialties. There also is a severe shortage of psychiatrists in our country, as I discussed in my editorial, “Signs, symptoms, and treatment of psychiatrynemia,” (December 2014). The 25% of the population who suffer a mental disorder are clearly underserved at this time.
Furthermore, because today’s research is tomorrow’s new treatment, funding for psychiatric research must increase substantially to find cures and to thus reduce huge direct and indirect costs of mental illness and addictions.
Public enlightenment. A well-informed populace would be a major boon to our sophisticated medical specialty, which remains shrouded by primitive beliefs and archaic attitudes. For many people who desperately need mental health care, negative perceptions of psychiatric disorders and their treatment are a major impediment to seeking help. Psychiatrists can catalyze the process of enlightenment by dedicating time to elevating public understanding of the biology and the medical basis of mental illness.
All this notwithstanding, our work is gratifying
Despite the hassles and unmet needs I’ve enumerated, psychiatry continues to be one of the most exciting fields in medicine. We provide more therapeutic face-time and verbal interactions with our patients than any other medical specialty. Imagine, then, how much more enjoyable psychiatric practice would be if these pesky obstacles were eliminated and the unmet needs of patients and practitioners were addressed.
10 Triggers of inflammation to be avoided, to reduce the risk of depression
Neuroinflammation is well-established as an underlying mechanism in depression, as well as in other neuropsychiatric disorders, including schizophrenia, multiple sclerosis, stroke, Parkinson’s disease, and sleep disorders.1 There is a dearth of prevention strategies for neuropsychiatric disorders but, given emerging scientific knowledge about immune dysregulation and the associated rise in inflammatory markers during the course of depression,2,3 it is logical to postulate that avoiding triggers of neuroinflammation might be a useful tactic to prevent depression or, perhaps, to minimize its severity.
Challenge your patients to avoid triggers of depression
What is known about what instigates the rise of inflammatory markers in the body and the brain? Actually, quite a substantial body of knowledge exists on the subject.4 Consider the 10 risk factors for depression that I enumerate here (Table), and advise patients to avoid them.
Sedentary lifestyle. Physical inactivity during childhood is associated with depression in adulthood. This is worrisome because video games seem ever more popular among children these days—more popular and prevalent than playing outdoors. Use this knowledge about the preventive benefit of exercise for long-range prevention in young patients.
Adults with a sedentary lifestyle usually have increased adiposity, which increases the risk of depression. Regular exercise has been shown to down-regulate systemic inflammation.
Smoking. Hundreds of toxic and inflammatory components in tobacco smoke (tars, metals, free radicals) can induce inflammation across the body and brain tissue, which explains not only depression but serious pulmonary and cerebrovascular diseases seen in smokers. People with depression are more likely to smoke than the general population, possibly because nicotine has a mild mood-elevating effect. Yet smoking might make depression worse by exacerbating inflammation, thus negating any mood-elevating effect of nicotine.
Poor diet. It is well known that the Western diet (processed meats, refined sugars, saturated fats) can increase the body’s level of inflammatory markers. The Mediterranean diet, on the other hand, which comprises fruits, vegetables, fish, legumes, and foods rich in omega-3 fatty acids (fish, nuts, leafy green vegetables), is anti-inflammatory. Furthermore, lycopene-containing foods (tomatoes, papaya, red cabbage, watermelon, carrots, asparagus) are rich in antioxidants and thus reduce inflammation.
The possible epigenetic effects of diet are an interesting phenomenon. Offspring of rats who were fed a diet rich in saturated fats have elevated levels of inflammatory markers, even when they had been fed a normal diet, suggesting a transgenerational effect. What parents eat before they conceive might doom their child’s health— regardless of what they feed them.
Tooth decay, gingivitis, periodontitis. Oral inflammation afflicts a large percentage of the population. These conditions can lead to systemic inflammation with elevated levels of C-reactive protein (CRP) and interleukins, which are conducive to depression.
Poor sleep hygiene. Sleep disorders, such as insomnia and insufficient sleep (which is epidemic in the United States), are risk factors for mood disorders. Sleep deprivation disrupts immune function and triggers the cascade of elevated cytokines, CRP, and tumor necrosis factor (TNF)-α. Just as depression is associated with impaired neurogenesis, so is chronic lack of sleep, suggesting a convergence of neurobiologic mechanisms.
Vitamin D deficiency. A link between vitamin D deficiency, now common in the United States, and depression and immune function has been recognized. Vitamin D has anti-inflammatory effects and can reduce oxidative stress, which culminates in inflammation. Vitamin D supplementation has been shown to alleviate neuro-immune disorders, such as multiple sclerosis.
Obesity. Obese people are >50% more likely to develop depression than non-obese people. Technically, obesity is a pro-inflammatory state, and inflammatory biomarkers, such as cytokines, are abundant in fat cells, especially abdominal (visceral or peri-omental) adiposity. When an obese person loses weight, levels of inflammatory markers (interleukin-6, TNF-α, leptin) decrease. We know that abdominal obesity is associated with neuroinflammation and early dementia.
Allergy involves inflammation triggered by the cascade of events consequent to the body’s fight against antigens, and the well-known hyper-sensitivity reaction, causing edema, coughing, sneezing, and itching. It is well-established that the incidence of atopy and allergy is high among people with depression.
Changes in gut permeability. Intestinal inflammatory diseases, such as ulcerative colitis, are recognized as pathways to depression. The mechanism is believed to be the immune response to lipopolysaccharides by commensal bacteria that live by the trillions in the gut. The result? Abnormal gut permeability, bacterial translocation, and depressed mood, possibly because serotonin is more abundant in the gut than in the CNS.
Stress. Arguably, the most common pathway to depression is stressful events of daily life. Stress-induced systemic inflammation hastens cardiovascular disease and leads to neuro-inflammation and neuropsychiatric disorders as well.
Especially malignant is the severe stress of childhood trauma (physical and sexual abuse, parental discord and death), which stimulates pro-inflammatory cytokines and detrimental neurobiological sensitization that lead to psychopathology, including depression and psychosis in adulthood. Childhood trauma has been reported to shorten life by 7 to 15 years.
Posttraumatic stress disorder is the best known clinical model of stress-induced depression and anxiety. The disorder is associated with a significant increase in pro-inflammatory cytokines and loss of brain tissue.
2-fold challenge: Reduce severity of disease, reduce risk before disease
We psychiatrists almost always see patients after they’ve developed depression and other psychiatric disorders in which neuroinflammation is already present. In addition to pharmacotherapy and psychotherapy (both reduce inflammation), educating patients about adopting a healthy lifestyle—not smoking, exercising, eating wisely, avoiding weight gain, getting enough sleep, maintaining good oral hygiene, and managing stress—might reduce psychiatric relapse and prolong their life.
We also should be challenged by the fact that the pathways to inflammation, including the 10 I’ve described here, are common among the population at large. Let’s increase our efforts to preemptively reduce the risk of brain disorders by encouraging parents and their children to adopt a healthy lifestyle and maintain wellness—and thus avoid falling victim to depression.
1. Baune BT. Inflammation and neurodegenerative disorders: is there still hope for therapeutic intervention? Curr Opin Psychiatry. 2015;28(2):148-154.
2. Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosc Biobehav Rev. 2012;36(2):764-785.
3. Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression [published online January 10, 2015]. Immunology. 2015. doi: 10.1111/imm.12443.
4. Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200.
Neuroinflammation is well-established as an underlying mechanism in depression, as well as in other neuropsychiatric disorders, including schizophrenia, multiple sclerosis, stroke, Parkinson’s disease, and sleep disorders.1 There is a dearth of prevention strategies for neuropsychiatric disorders but, given emerging scientific knowledge about immune dysregulation and the associated rise in inflammatory markers during the course of depression,2,3 it is logical to postulate that avoiding triggers of neuroinflammation might be a useful tactic to prevent depression or, perhaps, to minimize its severity.
Challenge your patients to avoid triggers of depression
What is known about what instigates the rise of inflammatory markers in the body and the brain? Actually, quite a substantial body of knowledge exists on the subject.4 Consider the 10 risk factors for depression that I enumerate here (Table), and advise patients to avoid them.
Sedentary lifestyle. Physical inactivity during childhood is associated with depression in adulthood. This is worrisome because video games seem ever more popular among children these days—more popular and prevalent than playing outdoors. Use this knowledge about the preventive benefit of exercise for long-range prevention in young patients.
Adults with a sedentary lifestyle usually have increased adiposity, which increases the risk of depression. Regular exercise has been shown to down-regulate systemic inflammation.
Smoking. Hundreds of toxic and inflammatory components in tobacco smoke (tars, metals, free radicals) can induce inflammation across the body and brain tissue, which explains not only depression but serious pulmonary and cerebrovascular diseases seen in smokers. People with depression are more likely to smoke than the general population, possibly because nicotine has a mild mood-elevating effect. Yet smoking might make depression worse by exacerbating inflammation, thus negating any mood-elevating effect of nicotine.
Poor diet. It is well known that the Western diet (processed meats, refined sugars, saturated fats) can increase the body’s level of inflammatory markers. The Mediterranean diet, on the other hand, which comprises fruits, vegetables, fish, legumes, and foods rich in omega-3 fatty acids (fish, nuts, leafy green vegetables), is anti-inflammatory. Furthermore, lycopene-containing foods (tomatoes, papaya, red cabbage, watermelon, carrots, asparagus) are rich in antioxidants and thus reduce inflammation.
The possible epigenetic effects of diet are an interesting phenomenon. Offspring of rats who were fed a diet rich in saturated fats have elevated levels of inflammatory markers, even when they had been fed a normal diet, suggesting a transgenerational effect. What parents eat before they conceive might doom their child’s health— regardless of what they feed them.
Tooth decay, gingivitis, periodontitis. Oral inflammation afflicts a large percentage of the population. These conditions can lead to systemic inflammation with elevated levels of C-reactive protein (CRP) and interleukins, which are conducive to depression.
Poor sleep hygiene. Sleep disorders, such as insomnia and insufficient sleep (which is epidemic in the United States), are risk factors for mood disorders. Sleep deprivation disrupts immune function and triggers the cascade of elevated cytokines, CRP, and tumor necrosis factor (TNF)-α. Just as depression is associated with impaired neurogenesis, so is chronic lack of sleep, suggesting a convergence of neurobiologic mechanisms.
Vitamin D deficiency. A link between vitamin D deficiency, now common in the United States, and depression and immune function has been recognized. Vitamin D has anti-inflammatory effects and can reduce oxidative stress, which culminates in inflammation. Vitamin D supplementation has been shown to alleviate neuro-immune disorders, such as multiple sclerosis.
Obesity. Obese people are >50% more likely to develop depression than non-obese people. Technically, obesity is a pro-inflammatory state, and inflammatory biomarkers, such as cytokines, are abundant in fat cells, especially abdominal (visceral or peri-omental) adiposity. When an obese person loses weight, levels of inflammatory markers (interleukin-6, TNF-α, leptin) decrease. We know that abdominal obesity is associated with neuroinflammation and early dementia.
Allergy involves inflammation triggered by the cascade of events consequent to the body’s fight against antigens, and the well-known hyper-sensitivity reaction, causing edema, coughing, sneezing, and itching. It is well-established that the incidence of atopy and allergy is high among people with depression.
Changes in gut permeability. Intestinal inflammatory diseases, such as ulcerative colitis, are recognized as pathways to depression. The mechanism is believed to be the immune response to lipopolysaccharides by commensal bacteria that live by the trillions in the gut. The result? Abnormal gut permeability, bacterial translocation, and depressed mood, possibly because serotonin is more abundant in the gut than in the CNS.
Stress. Arguably, the most common pathway to depression is stressful events of daily life. Stress-induced systemic inflammation hastens cardiovascular disease and leads to neuro-inflammation and neuropsychiatric disorders as well.
Especially malignant is the severe stress of childhood trauma (physical and sexual abuse, parental discord and death), which stimulates pro-inflammatory cytokines and detrimental neurobiological sensitization that lead to psychopathology, including depression and psychosis in adulthood. Childhood trauma has been reported to shorten life by 7 to 15 years.
Posttraumatic stress disorder is the best known clinical model of stress-induced depression and anxiety. The disorder is associated with a significant increase in pro-inflammatory cytokines and loss of brain tissue.
2-fold challenge: Reduce severity of disease, reduce risk before disease
We psychiatrists almost always see patients after they’ve developed depression and other psychiatric disorders in which neuroinflammation is already present. In addition to pharmacotherapy and psychotherapy (both reduce inflammation), educating patients about adopting a healthy lifestyle—not smoking, exercising, eating wisely, avoiding weight gain, getting enough sleep, maintaining good oral hygiene, and managing stress—might reduce psychiatric relapse and prolong their life.
We also should be challenged by the fact that the pathways to inflammation, including the 10 I’ve described here, are common among the population at large. Let’s increase our efforts to preemptively reduce the risk of brain disorders by encouraging parents and their children to adopt a healthy lifestyle and maintain wellness—and thus avoid falling victim to depression.
Neuroinflammation is well-established as an underlying mechanism in depression, as well as in other neuropsychiatric disorders, including schizophrenia, multiple sclerosis, stroke, Parkinson’s disease, and sleep disorders.1 There is a dearth of prevention strategies for neuropsychiatric disorders but, given emerging scientific knowledge about immune dysregulation and the associated rise in inflammatory markers during the course of depression,2,3 it is logical to postulate that avoiding triggers of neuroinflammation might be a useful tactic to prevent depression or, perhaps, to minimize its severity.
Challenge your patients to avoid triggers of depression
What is known about what instigates the rise of inflammatory markers in the body and the brain? Actually, quite a substantial body of knowledge exists on the subject.4 Consider the 10 risk factors for depression that I enumerate here (Table), and advise patients to avoid them.
Sedentary lifestyle. Physical inactivity during childhood is associated with depression in adulthood. This is worrisome because video games seem ever more popular among children these days—more popular and prevalent than playing outdoors. Use this knowledge about the preventive benefit of exercise for long-range prevention in young patients.
Adults with a sedentary lifestyle usually have increased adiposity, which increases the risk of depression. Regular exercise has been shown to down-regulate systemic inflammation.
Smoking. Hundreds of toxic and inflammatory components in tobacco smoke (tars, metals, free radicals) can induce inflammation across the body and brain tissue, which explains not only depression but serious pulmonary and cerebrovascular diseases seen in smokers. People with depression are more likely to smoke than the general population, possibly because nicotine has a mild mood-elevating effect. Yet smoking might make depression worse by exacerbating inflammation, thus negating any mood-elevating effect of nicotine.
Poor diet. It is well known that the Western diet (processed meats, refined sugars, saturated fats) can increase the body’s level of inflammatory markers. The Mediterranean diet, on the other hand, which comprises fruits, vegetables, fish, legumes, and foods rich in omega-3 fatty acids (fish, nuts, leafy green vegetables), is anti-inflammatory. Furthermore, lycopene-containing foods (tomatoes, papaya, red cabbage, watermelon, carrots, asparagus) are rich in antioxidants and thus reduce inflammation.
The possible epigenetic effects of diet are an interesting phenomenon. Offspring of rats who were fed a diet rich in saturated fats have elevated levels of inflammatory markers, even when they had been fed a normal diet, suggesting a transgenerational effect. What parents eat before they conceive might doom their child’s health— regardless of what they feed them.
Tooth decay, gingivitis, periodontitis. Oral inflammation afflicts a large percentage of the population. These conditions can lead to systemic inflammation with elevated levels of C-reactive protein (CRP) and interleukins, which are conducive to depression.
Poor sleep hygiene. Sleep disorders, such as insomnia and insufficient sleep (which is epidemic in the United States), are risk factors for mood disorders. Sleep deprivation disrupts immune function and triggers the cascade of elevated cytokines, CRP, and tumor necrosis factor (TNF)-α. Just as depression is associated with impaired neurogenesis, so is chronic lack of sleep, suggesting a convergence of neurobiologic mechanisms.
Vitamin D deficiency. A link between vitamin D deficiency, now common in the United States, and depression and immune function has been recognized. Vitamin D has anti-inflammatory effects and can reduce oxidative stress, which culminates in inflammation. Vitamin D supplementation has been shown to alleviate neuro-immune disorders, such as multiple sclerosis.
Obesity. Obese people are >50% more likely to develop depression than non-obese people. Technically, obesity is a pro-inflammatory state, and inflammatory biomarkers, such as cytokines, are abundant in fat cells, especially abdominal (visceral or peri-omental) adiposity. When an obese person loses weight, levels of inflammatory markers (interleukin-6, TNF-α, leptin) decrease. We know that abdominal obesity is associated with neuroinflammation and early dementia.
Allergy involves inflammation triggered by the cascade of events consequent to the body’s fight against antigens, and the well-known hyper-sensitivity reaction, causing edema, coughing, sneezing, and itching. It is well-established that the incidence of atopy and allergy is high among people with depression.
Changes in gut permeability. Intestinal inflammatory diseases, such as ulcerative colitis, are recognized as pathways to depression. The mechanism is believed to be the immune response to lipopolysaccharides by commensal bacteria that live by the trillions in the gut. The result? Abnormal gut permeability, bacterial translocation, and depressed mood, possibly because serotonin is more abundant in the gut than in the CNS.
Stress. Arguably, the most common pathway to depression is stressful events of daily life. Stress-induced systemic inflammation hastens cardiovascular disease and leads to neuro-inflammation and neuropsychiatric disorders as well.
Especially malignant is the severe stress of childhood trauma (physical and sexual abuse, parental discord and death), which stimulates pro-inflammatory cytokines and detrimental neurobiological sensitization that lead to psychopathology, including depression and psychosis in adulthood. Childhood trauma has been reported to shorten life by 7 to 15 years.
Posttraumatic stress disorder is the best known clinical model of stress-induced depression and anxiety. The disorder is associated with a significant increase in pro-inflammatory cytokines and loss of brain tissue.
2-fold challenge: Reduce severity of disease, reduce risk before disease
We psychiatrists almost always see patients after they’ve developed depression and other psychiatric disorders in which neuroinflammation is already present. In addition to pharmacotherapy and psychotherapy (both reduce inflammation), educating patients about adopting a healthy lifestyle—not smoking, exercising, eating wisely, avoiding weight gain, getting enough sleep, maintaining good oral hygiene, and managing stress—might reduce psychiatric relapse and prolong their life.
We also should be challenged by the fact that the pathways to inflammation, including the 10 I’ve described here, are common among the population at large. Let’s increase our efforts to preemptively reduce the risk of brain disorders by encouraging parents and their children to adopt a healthy lifestyle and maintain wellness—and thus avoid falling victim to depression.
1. Baune BT. Inflammation and neurodegenerative disorders: is there still hope for therapeutic intervention? Curr Opin Psychiatry. 2015;28(2):148-154.
2. Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosc Biobehav Rev. 2012;36(2):764-785.
3. Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression [published online January 10, 2015]. Immunology. 2015. doi: 10.1111/imm.12443.
4. Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200.
1. Baune BT. Inflammation and neurodegenerative disorders: is there still hope for therapeutic intervention? Curr Opin Psychiatry. 2015;28(2):148-154.
2. Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosc Biobehav Rev. 2012;36(2):764-785.
3. Bakunina N, Pariante CM, Zunszain PA. Immune mechanisms linked to depression via oxidative stress and neuroprogression [published online January 10, 2015]. Immunology. 2015. doi: 10.1111/imm.12443.
4. Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200.
10 Recent paradigm shifts in the neurobiology and treatment of depression
Nowhere is that change in landscape more apparent than in major depression, the No. 1 disabling condition in all of medicine, according to the World Health Organization. The past decade has generated at least 10 paradigm shifts in the neurobiology and pharmacotherapeutics of depression.
Clinging to simplistic tradition
Most contemporary clinicians continue to practice the traditional model of depression, which is based on the assumption that depression is caused by a deficiency of monoamines: serotonin (5-HT) and norepinephrine (NE). The entire antidepressant armamentarium approved for use by the FDA was designed according to the amine deficiency hypothesis. Depressed patients uniformly receive reuptake inhibitors of 5-HT and NE, but few achieve full remission, as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study showed.1
As scientific paradigm shifts infiltrate clinical practice, however, the tired notion of “chemical imbalance” will yield to more complex and evidence-based models.
Usually, it would be remarkable to witness a single paradigm shift in the understanding of a brain disorder. Imagine the disruptive impact of multiple scientific shifts within the past decade! Consider the following departures from the old dogma about the simplistic old explanation of depression.
1. From neurotransmitters to neuroplasticity
For half a century, our field tenaciously held to the monoamine theory, which posits that depression is caused by a deficiency of 5-HT or NE, or both. All antidepressants in use today were developed to increase brain monoamines by inhibiting their reuptake at the synaptic cleft. Now, research points to other causes:
• impaired neuroplasticity
• a decrement of neurogenesis
• synaptic deficits
• decreased neurotrophins (such as brain-derived neurotrophic factor)
• dendritic pathology.2,3
2. From ‘chemical imbalance’ to neuroinflammation
The simplistic notion that depression is a chemical imbalance, so to speak, in the brain is giving way to rapidly emerging evidence that depression is associated with neuroinflammation.4
Pro-inflammatory cytokines are elevated in the plasma of depressed patients, and subside when the acute episode is treated. Current antidepressants actually have anti-inflammatory effects that have gone unrecognized.5 A meta-analysis of the use of anti-inflammatory agents (such as nonsteroidal anti-inflammatory drugs and aspirin) in depression shows promising efficacy.6 Some inflammatory markers, such as C-reactive protein, already have been reported to predict response to some antidepressants, but not to others.7
3. From 5-HT and NE pathways to glutamate NMDA receptors
Recent landmark studies8 have, taken together, demonstrated that a single IV dose of the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine (a psychotogenic drug of abuse FDA-approved only as an anesthetic) can produce clinical improvement of severe depression and even full remission for several days. Such studies demonstrate that the old dogma of 5-HT and NE deficiency might not be a valid overarching hypothesis of the depression syndrome.
Long-term maintenance studies of ketamine to document its safety and continued efficacy need to be conducted. The mechanism of action of ketamine is believed to be a rapid trigger for enhancing neuroplasticity.
4. From oral to parenteral administration
Several studies have been published showing the efficacy of IV or intranasal administration of new agents for depression. Ketamine studies, for example, were conducted using an IV infusion of a 150-mg dose over 1 hour. Other IV studies used the anticholinergic scopolamine.9
Intranasal ketamine also has been shown to be clinically efficacious.10 Inhalable nitrous oxide (laughing gas, an NMDA antagonist) recently was reported to improve depression as well.11
It is possible that parenteral administration of antidepressant agents may exert a different neurobiological effect and provide a more rapid response than oral medication.
5. From delayed efficacy (weeks) to immediate onset (1 or 2 hours)
The widely entrenched notion that depression takes several weeks to improve with an antidepressant has collapsed with emerging evidence that symptoms of the disorder (even suicidal ideation) can be reversed within 1 or 2 hours.12 IV ketamine isn’t the only example; IV scopolamine,9 inhalable nitrous oxide,11 and overnight sleep deprivation13 also exert a rapid therapeutic effect. This is a major rethinking of how quickly the curtain of severe depression can be lifted, and is great news for patients and their family.
6. From psychological symptoms to cortical or subcortical changes
Depression traditionally has been recognized as a clinical syndrome of sadness, self-deprecation, cognitive dulling, and vegetative symptoms. In recent studies, however, researchers report that low hippocampus volume14 in healthy young girls predicts future depression. Patients with unremitting depression have been reported to have an abnormally shaped hippocampus.15
In addition, gray-matter volume in the subgenual anterior cingulate (Brodmann area 24) is hypoplastic in depressed persons,16 making that area a target for deep-brain stimulation (DBS). Brain morphological changes such as a hypoplastic hippocampus might become useful biomarkers for identifying persons at risk of severe depression, and might become a useful adjunctive biomarker for making a clinical diagnosis.
7. From healing the mind to repairing the brain
It is well-established that depression is associated with loss of dendritic spines and arborizations, loss of synapses, and diminishment of glial cells, especially in the hippocampus17 and anterior cingulate.18 Treating depression, whether pharmaceutical or somatic, involves reversing these changes by increasing neurotrophic factors, enhancing neurogenesis and gliogenesis, and restoring synaptic and dendritic health and cell survival in the hippocampus and frontal cortex.19,20 Treating depression involves brain repair, which is reflected, ultimately, in healing the mind.
8. From pharmacotherapy to neuromodulation
Although drugs remain the predominant treatment modality for depression, there is palpable escalation in the use of neuromodulation methods.
The oldest of these neuromodulatory techniques is electroconvulsive therapy, an excellent treatment for severe depression (and one that enhances hippocampal neurogenesis). In addition, several novel neuromodulation methods have been approved (transcranial magnetic stimulation and vagus nerve stimulation) or are in development (transcranial direct-current stimulation, cranial electrotherapy stimulation, and DBS).21 These somatic approaches to treating the brain directly to alleviate depression target regions involved in depression and reduce the needless risks associated with exposing other organ systems to a drug.
9. From monotherapy to combination therapy
The use of combination therapy for depression has escalated with FDA approval of adjunctive use of atypical antipsychotics in unipolar and bipolar depression. In addition, the landmark STAR*D study1 demonstrated the value of augmentation therapy with a second antidepressant when 1 agent fails. Other controlled studies have shown that combining 2 antidepressants is superior to administering 1.22
Just as other serious medical disorders—such as cancer and hypertension—are treated with 2 or 3 medications, severe depression might require a similar strategy. The field gradually is adopting that approach.
10. From cortical folds to wrinkles on the face
Last, a new (and unexpected) paradigm shift recently emerged, which is genuinely intriguing—even baffling. Using placebo-controlled designs, several researchers have reported significant, persistent improvement of depressive symptoms after injection of onabotulinumtoxinA in the corrugator muscles of the glabellar region of the face, where the omega sign often appears in a depressed person.23,24
The longest of the studies25 was 6 months; investigators reported that improvement continued even after the effect of the botulinum toxin on the omega sign wore off. The proposed mechanism of action is the facial feedback hypothesis, which suggests that, biologically, facial expression has an impact on one’s emotional state.
Big payoffs coming from research in neuroscience
These 10 paradigm shifts in a single psychiatric syndrome are emblematic of exciting clinical and research advances in our field. Like all syndromes, depression is associated with multiple genetic and environmental causes; it isn’t surprising that myriad treatment approaches are emerging.
The days of clinging to monolithic, serendipity-generated models surely are over. Evidence-based psychiatric brain research is shattering aging dogmas that have, for decades, stifled innovation in psychiatric therapeutics that is now moving in novel directions.
Take note, however, that the only paradigm shift that matters to depressed patients is the one that transcends mere control of their symptoms and restores their wellness, functional capacity, and quality of life. With the explosive momentum of neuroscience discovery, psychiatry is, at last, poised to deliver—in splendid, even seismic, fashion.
1. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12):1243-1252.
2. Serafini G, Hayley S, Pompili M, et al. Hippocampal neurogenesis, neurotrophic factors and depression: possible therapeutic targets [published online November 30, 2014]? CNS Neurol Disord Drug Targets. doi: 10.2174/1871527313666141130223723.
3. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68-72.
4. Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105-114.
5. Sacre S, Medghalichi M, Gregory B, et al. Fluoxetine and citalopram exhibit potent anti-inflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010;62(3):683-693.
6. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
7. Uher R, Tansey KE, Dew T, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortiptyline. Am J Psychiatry. 2014;171(14):1278-1286.
8. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics [published online October 17, 2014]. Annual Rev Med. doi: 10.1146/annurev-med-053013-062946.
9. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
10. Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014; 76(12):970-976.
11. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial [published December 14, 2014]. Biol Psychiatry. doi: http://dx.doi.org/10.1016/j.biopsych.2014.11.016.
12. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
13. Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73(12):1164-1171.
14. Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk for depression. Arch Gen Psychiatry. 2010;67(3):270-276.
15. Tae WS, Kim SS, Lee KU, et al. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am J Neuroradiol. 2011;32(4):671-676.
16. Hamani C, Mayberg H, Synder B, et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209-1215.
17. Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516-1518.
18. Redlich R, Almeoda JJ, Grotegerd D, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71(11):1222-1230.
19. Mendez-David I, Hen R, Gardier AM, et al. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Ann Pharm Fr. 2013;71(3):143-149.
20. Serafini G. Neuroplasticity and major depression, the role of modern antidepressant drugs. World J Psychiatry. 2012;2(3):49-57.
21. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
22. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
24. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
25. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
Nowhere is that change in landscape more apparent than in major depression, the No. 1 disabling condition in all of medicine, according to the World Health Organization. The past decade has generated at least 10 paradigm shifts in the neurobiology and pharmacotherapeutics of depression.
Clinging to simplistic tradition
Most contemporary clinicians continue to practice the traditional model of depression, which is based on the assumption that depression is caused by a deficiency of monoamines: serotonin (5-HT) and norepinephrine (NE). The entire antidepressant armamentarium approved for use by the FDA was designed according to the amine deficiency hypothesis. Depressed patients uniformly receive reuptake inhibitors of 5-HT and NE, but few achieve full remission, as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study showed.1
As scientific paradigm shifts infiltrate clinical practice, however, the tired notion of “chemical imbalance” will yield to more complex and evidence-based models.
Usually, it would be remarkable to witness a single paradigm shift in the understanding of a brain disorder. Imagine the disruptive impact of multiple scientific shifts within the past decade! Consider the following departures from the old dogma about the simplistic old explanation of depression.
1. From neurotransmitters to neuroplasticity
For half a century, our field tenaciously held to the monoamine theory, which posits that depression is caused by a deficiency of 5-HT or NE, or both. All antidepressants in use today were developed to increase brain monoamines by inhibiting their reuptake at the synaptic cleft. Now, research points to other causes:
• impaired neuroplasticity
• a decrement of neurogenesis
• synaptic deficits
• decreased neurotrophins (such as brain-derived neurotrophic factor)
• dendritic pathology.2,3
2. From ‘chemical imbalance’ to neuroinflammation
The simplistic notion that depression is a chemical imbalance, so to speak, in the brain is giving way to rapidly emerging evidence that depression is associated with neuroinflammation.4
Pro-inflammatory cytokines are elevated in the plasma of depressed patients, and subside when the acute episode is treated. Current antidepressants actually have anti-inflammatory effects that have gone unrecognized.5 A meta-analysis of the use of anti-inflammatory agents (such as nonsteroidal anti-inflammatory drugs and aspirin) in depression shows promising efficacy.6 Some inflammatory markers, such as C-reactive protein, already have been reported to predict response to some antidepressants, but not to others.7
3. From 5-HT and NE pathways to glutamate NMDA receptors
Recent landmark studies8 have, taken together, demonstrated that a single IV dose of the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine (a psychotogenic drug of abuse FDA-approved only as an anesthetic) can produce clinical improvement of severe depression and even full remission for several days. Such studies demonstrate that the old dogma of 5-HT and NE deficiency might not be a valid overarching hypothesis of the depression syndrome.
Long-term maintenance studies of ketamine to document its safety and continued efficacy need to be conducted. The mechanism of action of ketamine is believed to be a rapid trigger for enhancing neuroplasticity.
4. From oral to parenteral administration
Several studies have been published showing the efficacy of IV or intranasal administration of new agents for depression. Ketamine studies, for example, were conducted using an IV infusion of a 150-mg dose over 1 hour. Other IV studies used the anticholinergic scopolamine.9
Intranasal ketamine also has been shown to be clinically efficacious.10 Inhalable nitrous oxide (laughing gas, an NMDA antagonist) recently was reported to improve depression as well.11
It is possible that parenteral administration of antidepressant agents may exert a different neurobiological effect and provide a more rapid response than oral medication.
5. From delayed efficacy (weeks) to immediate onset (1 or 2 hours)
The widely entrenched notion that depression takes several weeks to improve with an antidepressant has collapsed with emerging evidence that symptoms of the disorder (even suicidal ideation) can be reversed within 1 or 2 hours.12 IV ketamine isn’t the only example; IV scopolamine,9 inhalable nitrous oxide,11 and overnight sleep deprivation13 also exert a rapid therapeutic effect. This is a major rethinking of how quickly the curtain of severe depression can be lifted, and is great news for patients and their family.
6. From psychological symptoms to cortical or subcortical changes
Depression traditionally has been recognized as a clinical syndrome of sadness, self-deprecation, cognitive dulling, and vegetative symptoms. In recent studies, however, researchers report that low hippocampus volume14 in healthy young girls predicts future depression. Patients with unremitting depression have been reported to have an abnormally shaped hippocampus.15
In addition, gray-matter volume in the subgenual anterior cingulate (Brodmann area 24) is hypoplastic in depressed persons,16 making that area a target for deep-brain stimulation (DBS). Brain morphological changes such as a hypoplastic hippocampus might become useful biomarkers for identifying persons at risk of severe depression, and might become a useful adjunctive biomarker for making a clinical diagnosis.
7. From healing the mind to repairing the brain
It is well-established that depression is associated with loss of dendritic spines and arborizations, loss of synapses, and diminishment of glial cells, especially in the hippocampus17 and anterior cingulate.18 Treating depression, whether pharmaceutical or somatic, involves reversing these changes by increasing neurotrophic factors, enhancing neurogenesis and gliogenesis, and restoring synaptic and dendritic health and cell survival in the hippocampus and frontal cortex.19,20 Treating depression involves brain repair, which is reflected, ultimately, in healing the mind.
8. From pharmacotherapy to neuromodulation
Although drugs remain the predominant treatment modality for depression, there is palpable escalation in the use of neuromodulation methods.
The oldest of these neuromodulatory techniques is electroconvulsive therapy, an excellent treatment for severe depression (and one that enhances hippocampal neurogenesis). In addition, several novel neuromodulation methods have been approved (transcranial magnetic stimulation and vagus nerve stimulation) or are in development (transcranial direct-current stimulation, cranial electrotherapy stimulation, and DBS).21 These somatic approaches to treating the brain directly to alleviate depression target regions involved in depression and reduce the needless risks associated with exposing other organ systems to a drug.
9. From monotherapy to combination therapy
The use of combination therapy for depression has escalated with FDA approval of adjunctive use of atypical antipsychotics in unipolar and bipolar depression. In addition, the landmark STAR*D study1 demonstrated the value of augmentation therapy with a second antidepressant when 1 agent fails. Other controlled studies have shown that combining 2 antidepressants is superior to administering 1.22
Just as other serious medical disorders—such as cancer and hypertension—are treated with 2 or 3 medications, severe depression might require a similar strategy. The field gradually is adopting that approach.
10. From cortical folds to wrinkles on the face
Last, a new (and unexpected) paradigm shift recently emerged, which is genuinely intriguing—even baffling. Using placebo-controlled designs, several researchers have reported significant, persistent improvement of depressive symptoms after injection of onabotulinumtoxinA in the corrugator muscles of the glabellar region of the face, where the omega sign often appears in a depressed person.23,24
The longest of the studies25 was 6 months; investigators reported that improvement continued even after the effect of the botulinum toxin on the omega sign wore off. The proposed mechanism of action is the facial feedback hypothesis, which suggests that, biologically, facial expression has an impact on one’s emotional state.
Big payoffs coming from research in neuroscience
These 10 paradigm shifts in a single psychiatric syndrome are emblematic of exciting clinical and research advances in our field. Like all syndromes, depression is associated with multiple genetic and environmental causes; it isn’t surprising that myriad treatment approaches are emerging.
The days of clinging to monolithic, serendipity-generated models surely are over. Evidence-based psychiatric brain research is shattering aging dogmas that have, for decades, stifled innovation in psychiatric therapeutics that is now moving in novel directions.
Take note, however, that the only paradigm shift that matters to depressed patients is the one that transcends mere control of their symptoms and restores their wellness, functional capacity, and quality of life. With the explosive momentum of neuroscience discovery, psychiatry is, at last, poised to deliver—in splendid, even seismic, fashion.
Nowhere is that change in landscape more apparent than in major depression, the No. 1 disabling condition in all of medicine, according to the World Health Organization. The past decade has generated at least 10 paradigm shifts in the neurobiology and pharmacotherapeutics of depression.
Clinging to simplistic tradition
Most contemporary clinicians continue to practice the traditional model of depression, which is based on the assumption that depression is caused by a deficiency of monoamines: serotonin (5-HT) and norepinephrine (NE). The entire antidepressant armamentarium approved for use by the FDA was designed according to the amine deficiency hypothesis. Depressed patients uniformly receive reuptake inhibitors of 5-HT and NE, but few achieve full remission, as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study showed.1
As scientific paradigm shifts infiltrate clinical practice, however, the tired notion of “chemical imbalance” will yield to more complex and evidence-based models.
Usually, it would be remarkable to witness a single paradigm shift in the understanding of a brain disorder. Imagine the disruptive impact of multiple scientific shifts within the past decade! Consider the following departures from the old dogma about the simplistic old explanation of depression.
1. From neurotransmitters to neuroplasticity
For half a century, our field tenaciously held to the monoamine theory, which posits that depression is caused by a deficiency of 5-HT or NE, or both. All antidepressants in use today were developed to increase brain monoamines by inhibiting their reuptake at the synaptic cleft. Now, research points to other causes:
• impaired neuroplasticity
• a decrement of neurogenesis
• synaptic deficits
• decreased neurotrophins (such as brain-derived neurotrophic factor)
• dendritic pathology.2,3
2. From ‘chemical imbalance’ to neuroinflammation
The simplistic notion that depression is a chemical imbalance, so to speak, in the brain is giving way to rapidly emerging evidence that depression is associated with neuroinflammation.4
Pro-inflammatory cytokines are elevated in the plasma of depressed patients, and subside when the acute episode is treated. Current antidepressants actually have anti-inflammatory effects that have gone unrecognized.5 A meta-analysis of the use of anti-inflammatory agents (such as nonsteroidal anti-inflammatory drugs and aspirin) in depression shows promising efficacy.6 Some inflammatory markers, such as C-reactive protein, already have been reported to predict response to some antidepressants, but not to others.7
3. From 5-HT and NE pathways to glutamate NMDA receptors
Recent landmark studies8 have, taken together, demonstrated that a single IV dose of the N-methyl-d-aspartate (NMDA) receptor antagonist ketamine (a psychotogenic drug of abuse FDA-approved only as an anesthetic) can produce clinical improvement of severe depression and even full remission for several days. Such studies demonstrate that the old dogma of 5-HT and NE deficiency might not be a valid overarching hypothesis of the depression syndrome.
Long-term maintenance studies of ketamine to document its safety and continued efficacy need to be conducted. The mechanism of action of ketamine is believed to be a rapid trigger for enhancing neuroplasticity.
4. From oral to parenteral administration
Several studies have been published showing the efficacy of IV or intranasal administration of new agents for depression. Ketamine studies, for example, were conducted using an IV infusion of a 150-mg dose over 1 hour. Other IV studies used the anticholinergic scopolamine.9
Intranasal ketamine also has been shown to be clinically efficacious.10 Inhalable nitrous oxide (laughing gas, an NMDA antagonist) recently was reported to improve depression as well.11
It is possible that parenteral administration of antidepressant agents may exert a different neurobiological effect and provide a more rapid response than oral medication.
5. From delayed efficacy (weeks) to immediate onset (1 or 2 hours)
The widely entrenched notion that depression takes several weeks to improve with an antidepressant has collapsed with emerging evidence that symptoms of the disorder (even suicidal ideation) can be reversed within 1 or 2 hours.12 IV ketamine isn’t the only example; IV scopolamine,9 inhalable nitrous oxide,11 and overnight sleep deprivation13 also exert a rapid therapeutic effect. This is a major rethinking of how quickly the curtain of severe depression can be lifted, and is great news for patients and their family.
6. From psychological symptoms to cortical or subcortical changes
Depression traditionally has been recognized as a clinical syndrome of sadness, self-deprecation, cognitive dulling, and vegetative symptoms. In recent studies, however, researchers report that low hippocampus volume14 in healthy young girls predicts future depression. Patients with unremitting depression have been reported to have an abnormally shaped hippocampus.15
In addition, gray-matter volume in the subgenual anterior cingulate (Brodmann area 24) is hypoplastic in depressed persons,16 making that area a target for deep-brain stimulation (DBS). Brain morphological changes such as a hypoplastic hippocampus might become useful biomarkers for identifying persons at risk of severe depression, and might become a useful adjunctive biomarker for making a clinical diagnosis.
7. From healing the mind to repairing the brain
It is well-established that depression is associated with loss of dendritic spines and arborizations, loss of synapses, and diminishment of glial cells, especially in the hippocampus17 and anterior cingulate.18 Treating depression, whether pharmaceutical or somatic, involves reversing these changes by increasing neurotrophic factors, enhancing neurogenesis and gliogenesis, and restoring synaptic and dendritic health and cell survival in the hippocampus and frontal cortex.19,20 Treating depression involves brain repair, which is reflected, ultimately, in healing the mind.
8. From pharmacotherapy to neuromodulation
Although drugs remain the predominant treatment modality for depression, there is palpable escalation in the use of neuromodulation methods.
The oldest of these neuromodulatory techniques is electroconvulsive therapy, an excellent treatment for severe depression (and one that enhances hippocampal neurogenesis). In addition, several novel neuromodulation methods have been approved (transcranial magnetic stimulation and vagus nerve stimulation) or are in development (transcranial direct-current stimulation, cranial electrotherapy stimulation, and DBS).21 These somatic approaches to treating the brain directly to alleviate depression target regions involved in depression and reduce the needless risks associated with exposing other organ systems to a drug.
9. From monotherapy to combination therapy
The use of combination therapy for depression has escalated with FDA approval of adjunctive use of atypical antipsychotics in unipolar and bipolar depression. In addition, the landmark STAR*D study1 demonstrated the value of augmentation therapy with a second antidepressant when 1 agent fails. Other controlled studies have shown that combining 2 antidepressants is superior to administering 1.22
Just as other serious medical disorders—such as cancer and hypertension—are treated with 2 or 3 medications, severe depression might require a similar strategy. The field gradually is adopting that approach.
10. From cortical folds to wrinkles on the face
Last, a new (and unexpected) paradigm shift recently emerged, which is genuinely intriguing—even baffling. Using placebo-controlled designs, several researchers have reported significant, persistent improvement of depressive symptoms after injection of onabotulinumtoxinA in the corrugator muscles of the glabellar region of the face, where the omega sign often appears in a depressed person.23,24
The longest of the studies25 was 6 months; investigators reported that improvement continued even after the effect of the botulinum toxin on the omega sign wore off. The proposed mechanism of action is the facial feedback hypothesis, which suggests that, biologically, facial expression has an impact on one’s emotional state.
Big payoffs coming from research in neuroscience
These 10 paradigm shifts in a single psychiatric syndrome are emblematic of exciting clinical and research advances in our field. Like all syndromes, depression is associated with multiple genetic and environmental causes; it isn’t surprising that myriad treatment approaches are emerging.
The days of clinging to monolithic, serendipity-generated models surely are over. Evidence-based psychiatric brain research is shattering aging dogmas that have, for decades, stifled innovation in psychiatric therapeutics that is now moving in novel directions.
Take note, however, that the only paradigm shift that matters to depressed patients is the one that transcends mere control of their symptoms and restores their wellness, functional capacity, and quality of life. With the explosive momentum of neuroscience discovery, psychiatry is, at last, poised to deliver—in splendid, even seismic, fashion.
1. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12):1243-1252.
2. Serafini G, Hayley S, Pompili M, et al. Hippocampal neurogenesis, neurotrophic factors and depression: possible therapeutic targets [published online November 30, 2014]? CNS Neurol Disord Drug Targets. doi: 10.2174/1871527313666141130223723.
3. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68-72.
4. Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105-114.
5. Sacre S, Medghalichi M, Gregory B, et al. Fluoxetine and citalopram exhibit potent anti-inflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010;62(3):683-693.
6. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
7. Uher R, Tansey KE, Dew T, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortiptyline. Am J Psychiatry. 2014;171(14):1278-1286.
8. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics [published online October 17, 2014]. Annual Rev Med. doi: 10.1146/annurev-med-053013-062946.
9. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
10. Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014; 76(12):970-976.
11. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial [published December 14, 2014]. Biol Psychiatry. doi: http://dx.doi.org/10.1016/j.biopsych.2014.11.016.
12. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
13. Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73(12):1164-1171.
14. Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk for depression. Arch Gen Psychiatry. 2010;67(3):270-276.
15. Tae WS, Kim SS, Lee KU, et al. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am J Neuroradiol. 2011;32(4):671-676.
16. Hamani C, Mayberg H, Synder B, et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209-1215.
17. Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516-1518.
18. Redlich R, Almeoda JJ, Grotegerd D, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71(11):1222-1230.
19. Mendez-David I, Hen R, Gardier AM, et al. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Ann Pharm Fr. 2013;71(3):143-149.
20. Serafini G. Neuroplasticity and major depression, the role of modern antidepressant drugs. World J Psychiatry. 2012;2(3):49-57.
21. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
22. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
24. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
25. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
1. Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Eng J Med. 2006;354(12):1243-1252.
2. Serafini G, Hayley S, Pompili M, et al. Hippocampal neurogenesis, neurotrophic factors and depression: possible therapeutic targets [published online November 30, 2014]? CNS Neurol Disord Drug Targets. doi: 10.2174/1871527313666141130223723.
3. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68-72.
4. Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105-114.
5. Sacre S, Medghalichi M, Gregory B, et al. Fluoxetine and citalopram exhibit potent anti-inflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis Rheum. 2010;62(3):683-693.
6. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
7. Uher R, Tansey KE, Dew T, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortiptyline. Am J Psychiatry. 2014;171(14):1278-1286.
8. Abdallah CG, Sanacora G, Duman RS, et al. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics [published online October 17, 2014]. Annual Rev Med. doi: 10.1146/annurev-med-053013-062946.
9. Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology. 2010;35(12):2479-2488.
10. Lapidus KA, Levitch CF, Perez AM, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014; 76(12):970-976.
11. Nagele P, Duma A, Kopec M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial [published December 14, 2014]. Biol Psychiatry. doi: http://dx.doi.org/10.1016/j.biopsych.2014.11.016.
12. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71(12): 1381-1391.
13. Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73(12):1164-1171.
14. Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk for depression. Arch Gen Psychiatry. 2010;67(3):270-276.
15. Tae WS, Kim SS, Lee KU, et al. Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am J Neuroradiol. 2011;32(4):671-676.
16. Hamani C, Mayberg H, Synder B, et al. Deep brain stimulation of the subcallosal cingulate gyrus for depression: anatomical location of active contacts in clinical responders and a suggested guideline for targeting. J Neurosurg. 2009;111(6):1209-1215.
17. Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516-1518.
18. Redlich R, Almeoda JJ, Grotegerd D, et al. Brain morphometric biomarkers distinguishing unipolar and bipolar depression. A voxel-based morphometry-pattern classification approach. JAMA Psychiatry. 2014;71(11):1222-1230.
19. Mendez-David I, Hen R, Gardier AM, et al. Adult hippocampal neurogenesis: an actor in the antidepressant-like action. Ann Pharm Fr. 2013;71(3):143-149.
20. Serafini G. Neuroplasticity and major depression, the role of modern antidepressant drugs. World J Psychiatry. 2012;2(3):49-57.
21. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102-116.
22. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281-288.
23. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
24. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
25. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
From bedlam to biomarkers: The transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes
Consider here my journey in psychiatry since my adolescence. Growing up in the 1960s and 1970s, I did not watch much television; my father was convinced TV would be “too distracting” for us children. At first, I was angry about his rule, and would occasionally watch programs such as Bonanza at sleep-overs.
The lure of psychoanalysis
Gradually, I became grateful to my father because—in contrast to my classmates, who sat passively for hours watching TV after school—I voraciously read the piles of fiction and nonfiction books that I checked out from the school library every week, expanding my general knowledge and perspectives. One of my favorite genres became psychology and psychiatry, including many of Sigmund Freud’s works.
I was enchanted by psychoanalysis and its explanation of mental illness because, growing up, I had been told that madness is caused by demonic spirits and bad behavior and it is completely untreatable. By the time I was in high school, I had decided to become a psychiatrist, and was practicing what I read by “counseling” my classmates about family conflicts, raging drives, and frustrating relationships with girlfriends.
Rising tide of psychopharmacology
My love for psychiatry never wavered during my undergraduate years. I focused not only on required pre-med courses but enthusiastically took many psychology, sociology, and anthropology electives to expand my understanding of human behavior. In medical school, I enjoyed all rotations, but psychiatry was simply sublime. Often, I offered (to my classmates’ delight) to take their weekend call at the psychiatric hospital so I could see more patients.
After my internship, I married my wife (a behavioral psychologist) and embarked on psychiatry residency training with gusto. I was far better prepared, I realized, than my fellow residents; my faculty supervisors noticed that I answered questions more often than many others during rounds and lectures. (Thanks, Dad, for banning television!) I relished every psychotherapy session and spent hours listening to audiotapes of my patients’ sessions to improve my skills and to discover the psychodynamic nuances of their psychopathology. Being supervised by expert psychoanalysts was the highlight of my week as I honed my psychodynamic psychotherapy skills.
But something interesting happened during my residency: Psychopharmacology and electroconvulsive therapy were helping my severely ill psychotic, manic, and depressed patients much faster than psychotherapy could. Length of stay in the wards typically was 30 days (there was no managed care back then to limit stay to an absurd 5 days), and I saw substantial improvement in many of my patients before discharge.
I was so enthralled by the rising tide of psychopharmacology that I decided in PGY-2 to conduct psychopharmacology research—which, I came to realize, was easier than research on psychotherapy. I secured a mentor from the department of pharmacology. In PGY-3, I presented my data at the Annual Meeting of the American Psychiatric Association; in PGY-4, the paper was published in the American Journal of Psychiatry.
By the end of residency, I had applied to the National Institute of Mental Health (NIMH) to pursue a research fellowship in the neuropharmacology of schizophrenia to prepare me for an academic career. I participated in numerous studies on the NIMH research ward, brimming with patients who had refractory schizophrenia (before the advent of clozapine in 1989), and I published many articles with mentors and fellow researchers.
Investigating brain biology
Then another funny thing happened: During my fellowship, one of my mentors shared with me some early studies about postmortem structural changes in the brain of schizophrenia patients. That prompted me to spend hours in the basement of the pathology department examining the brains of dozens of patients with schizophrenia, noting atrophic changes and performing measurements and histopathologic studies.
Consequently, I embarked on neuroimaging research to study the morphological abnormalities of cortical and subcortical regions in living patients. I found myself going beyond neuropsychopharmacology and diving into neuroanatomy books and neuroscience journals. I realized that I was continuously learning and using a new scientific language in my daily work.
After I left NIMH to begin a career of teaching, research, and patient care in a medical school setting, I was engulfed by meteoric advances in neuroscience producing unprecedented insights about the molecular biology of schizophrenia and other severe neuropsychiatric disorders, leading me to pursue new opportunities in neurobiology while continuing my psychopharmacology research.
The rate of transformation is mind-boggling
Looking back at the span of time from childhood through the exciting journey of my psychiatry career, I realize how massive a transformation I have witnessed and experienced. The specialty has shifted its clinical and scientific paradigms through several conceptual models—from demonic possession to psychoanalysis to psychopharmacology and, last, to molecular neurobiology. Four times in my life, the lexicon of psychiatry has undergone a complete makeover. This is a light-speed pace of scientific progress over a few decades—truly breathtaking! It’s like rewriting a dictionary over and over, with no 2 successive editions resembling each other whatsoever.
The Table shows 4 sets of examples of psychiatric terminology, each representing 1 of the 4 paradigmatic models that my generation of psychiatrists has had to adopt and use in clinical care and research. I cannot think of any other medical specialty that has come close to evolving and transforming its language and conceptual models of etiology and treatment at such a rapid pace.
When I embraced psychiatry in adolescence as my future career, I never imagined, in my wildest dreams, that I would experience such successive scientific earthquakes in my beloved medical specialty. Perhaps that’s what kept me stimulated and eager to come to work every day; I use all the models and treatment tools I have learned in understanding and helping my patients with evolving psychotherapeutic and biopharmaceutical tools; I also teach my students and residents about the multifaceted wonders of the human mind and the magnificent complexities of the brain in health and disease.
Psychiatry has been, and will continue to be, a Pandora’s box of medicine, full of stunning scientific twists and surprises and a transformative lexicon to match.
Consider here my journey in psychiatry since my adolescence. Growing up in the 1960s and 1970s, I did not watch much television; my father was convinced TV would be “too distracting” for us children. At first, I was angry about his rule, and would occasionally watch programs such as Bonanza at sleep-overs.
The lure of psychoanalysis
Gradually, I became grateful to my father because—in contrast to my classmates, who sat passively for hours watching TV after school—I voraciously read the piles of fiction and nonfiction books that I checked out from the school library every week, expanding my general knowledge and perspectives. One of my favorite genres became psychology and psychiatry, including many of Sigmund Freud’s works.
I was enchanted by psychoanalysis and its explanation of mental illness because, growing up, I had been told that madness is caused by demonic spirits and bad behavior and it is completely untreatable. By the time I was in high school, I had decided to become a psychiatrist, and was practicing what I read by “counseling” my classmates about family conflicts, raging drives, and frustrating relationships with girlfriends.
Rising tide of psychopharmacology
My love for psychiatry never wavered during my undergraduate years. I focused not only on required pre-med courses but enthusiastically took many psychology, sociology, and anthropology electives to expand my understanding of human behavior. In medical school, I enjoyed all rotations, but psychiatry was simply sublime. Often, I offered (to my classmates’ delight) to take their weekend call at the psychiatric hospital so I could see more patients.
After my internship, I married my wife (a behavioral psychologist) and embarked on psychiatry residency training with gusto. I was far better prepared, I realized, than my fellow residents; my faculty supervisors noticed that I answered questions more often than many others during rounds and lectures. (Thanks, Dad, for banning television!) I relished every psychotherapy session and spent hours listening to audiotapes of my patients’ sessions to improve my skills and to discover the psychodynamic nuances of their psychopathology. Being supervised by expert psychoanalysts was the highlight of my week as I honed my psychodynamic psychotherapy skills.
But something interesting happened during my residency: Psychopharmacology and electroconvulsive therapy were helping my severely ill psychotic, manic, and depressed patients much faster than psychotherapy could. Length of stay in the wards typically was 30 days (there was no managed care back then to limit stay to an absurd 5 days), and I saw substantial improvement in many of my patients before discharge.
I was so enthralled by the rising tide of psychopharmacology that I decided in PGY-2 to conduct psychopharmacology research—which, I came to realize, was easier than research on psychotherapy. I secured a mentor from the department of pharmacology. In PGY-3, I presented my data at the Annual Meeting of the American Psychiatric Association; in PGY-4, the paper was published in the American Journal of Psychiatry.
By the end of residency, I had applied to the National Institute of Mental Health (NIMH) to pursue a research fellowship in the neuropharmacology of schizophrenia to prepare me for an academic career. I participated in numerous studies on the NIMH research ward, brimming with patients who had refractory schizophrenia (before the advent of clozapine in 1989), and I published many articles with mentors and fellow researchers.
Investigating brain biology
Then another funny thing happened: During my fellowship, one of my mentors shared with me some early studies about postmortem structural changes in the brain of schizophrenia patients. That prompted me to spend hours in the basement of the pathology department examining the brains of dozens of patients with schizophrenia, noting atrophic changes and performing measurements and histopathologic studies.
Consequently, I embarked on neuroimaging research to study the morphological abnormalities of cortical and subcortical regions in living patients. I found myself going beyond neuropsychopharmacology and diving into neuroanatomy books and neuroscience journals. I realized that I was continuously learning and using a new scientific language in my daily work.
After I left NIMH to begin a career of teaching, research, and patient care in a medical school setting, I was engulfed by meteoric advances in neuroscience producing unprecedented insights about the molecular biology of schizophrenia and other severe neuropsychiatric disorders, leading me to pursue new opportunities in neurobiology while continuing my psychopharmacology research.
The rate of transformation is mind-boggling
Looking back at the span of time from childhood through the exciting journey of my psychiatry career, I realize how massive a transformation I have witnessed and experienced. The specialty has shifted its clinical and scientific paradigms through several conceptual models—from demonic possession to psychoanalysis to psychopharmacology and, last, to molecular neurobiology. Four times in my life, the lexicon of psychiatry has undergone a complete makeover. This is a light-speed pace of scientific progress over a few decades—truly breathtaking! It’s like rewriting a dictionary over and over, with no 2 successive editions resembling each other whatsoever.
The Table shows 4 sets of examples of psychiatric terminology, each representing 1 of the 4 paradigmatic models that my generation of psychiatrists has had to adopt and use in clinical care and research. I cannot think of any other medical specialty that has come close to evolving and transforming its language and conceptual models of etiology and treatment at such a rapid pace.
When I embraced psychiatry in adolescence as my future career, I never imagined, in my wildest dreams, that I would experience such successive scientific earthquakes in my beloved medical specialty. Perhaps that’s what kept me stimulated and eager to come to work every day; I use all the models and treatment tools I have learned in understanding and helping my patients with evolving psychotherapeutic and biopharmaceutical tools; I also teach my students and residents about the multifaceted wonders of the human mind and the magnificent complexities of the brain in health and disease.
Psychiatry has been, and will continue to be, a Pandora’s box of medicine, full of stunning scientific twists and surprises and a transformative lexicon to match.
Consider here my journey in psychiatry since my adolescence. Growing up in the 1960s and 1970s, I did not watch much television; my father was convinced TV would be “too distracting” for us children. At first, I was angry about his rule, and would occasionally watch programs such as Bonanza at sleep-overs.
The lure of psychoanalysis
Gradually, I became grateful to my father because—in contrast to my classmates, who sat passively for hours watching TV after school—I voraciously read the piles of fiction and nonfiction books that I checked out from the school library every week, expanding my general knowledge and perspectives. One of my favorite genres became psychology and psychiatry, including many of Sigmund Freud’s works.
I was enchanted by psychoanalysis and its explanation of mental illness because, growing up, I had been told that madness is caused by demonic spirits and bad behavior and it is completely untreatable. By the time I was in high school, I had decided to become a psychiatrist, and was practicing what I read by “counseling” my classmates about family conflicts, raging drives, and frustrating relationships with girlfriends.
Rising tide of psychopharmacology
My love for psychiatry never wavered during my undergraduate years. I focused not only on required pre-med courses but enthusiastically took many psychology, sociology, and anthropology electives to expand my understanding of human behavior. In medical school, I enjoyed all rotations, but psychiatry was simply sublime. Often, I offered (to my classmates’ delight) to take their weekend call at the psychiatric hospital so I could see more patients.
After my internship, I married my wife (a behavioral psychologist) and embarked on psychiatry residency training with gusto. I was far better prepared, I realized, than my fellow residents; my faculty supervisors noticed that I answered questions more often than many others during rounds and lectures. (Thanks, Dad, for banning television!) I relished every psychotherapy session and spent hours listening to audiotapes of my patients’ sessions to improve my skills and to discover the psychodynamic nuances of their psychopathology. Being supervised by expert psychoanalysts was the highlight of my week as I honed my psychodynamic psychotherapy skills.
But something interesting happened during my residency: Psychopharmacology and electroconvulsive therapy were helping my severely ill psychotic, manic, and depressed patients much faster than psychotherapy could. Length of stay in the wards typically was 30 days (there was no managed care back then to limit stay to an absurd 5 days), and I saw substantial improvement in many of my patients before discharge.
I was so enthralled by the rising tide of psychopharmacology that I decided in PGY-2 to conduct psychopharmacology research—which, I came to realize, was easier than research on psychotherapy. I secured a mentor from the department of pharmacology. In PGY-3, I presented my data at the Annual Meeting of the American Psychiatric Association; in PGY-4, the paper was published in the American Journal of Psychiatry.
By the end of residency, I had applied to the National Institute of Mental Health (NIMH) to pursue a research fellowship in the neuropharmacology of schizophrenia to prepare me for an academic career. I participated in numerous studies on the NIMH research ward, brimming with patients who had refractory schizophrenia (before the advent of clozapine in 1989), and I published many articles with mentors and fellow researchers.
Investigating brain biology
Then another funny thing happened: During my fellowship, one of my mentors shared with me some early studies about postmortem structural changes in the brain of schizophrenia patients. That prompted me to spend hours in the basement of the pathology department examining the brains of dozens of patients with schizophrenia, noting atrophic changes and performing measurements and histopathologic studies.
Consequently, I embarked on neuroimaging research to study the morphological abnormalities of cortical and subcortical regions in living patients. I found myself going beyond neuropsychopharmacology and diving into neuroanatomy books and neuroscience journals. I realized that I was continuously learning and using a new scientific language in my daily work.
After I left NIMH to begin a career of teaching, research, and patient care in a medical school setting, I was engulfed by meteoric advances in neuroscience producing unprecedented insights about the molecular biology of schizophrenia and other severe neuropsychiatric disorders, leading me to pursue new opportunities in neurobiology while continuing my psychopharmacology research.
The rate of transformation is mind-boggling
Looking back at the span of time from childhood through the exciting journey of my psychiatry career, I realize how massive a transformation I have witnessed and experienced. The specialty has shifted its clinical and scientific paradigms through several conceptual models—from demonic possession to psychoanalysis to psychopharmacology and, last, to molecular neurobiology. Four times in my life, the lexicon of psychiatry has undergone a complete makeover. This is a light-speed pace of scientific progress over a few decades—truly breathtaking! It’s like rewriting a dictionary over and over, with no 2 successive editions resembling each other whatsoever.
The Table shows 4 sets of examples of psychiatric terminology, each representing 1 of the 4 paradigmatic models that my generation of psychiatrists has had to adopt and use in clinical care and research. I cannot think of any other medical specialty that has come close to evolving and transforming its language and conceptual models of etiology and treatment at such a rapid pace.
When I embraced psychiatry in adolescence as my future career, I never imagined, in my wildest dreams, that I would experience such successive scientific earthquakes in my beloved medical specialty. Perhaps that’s what kept me stimulated and eager to come to work every day; I use all the models and treatment tools I have learned in understanding and helping my patients with evolving psychotherapeutic and biopharmaceutical tools; I also teach my students and residents about the multifaceted wonders of the human mind and the magnificent complexities of the brain in health and disease.
Psychiatry has been, and will continue to be, a Pandora’s box of medicine, full of stunning scientific twists and surprises and a transformative lexicon to match.
Treatment-resistant schizophrenia
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Signs, symptoms, and treatment of psychiatrynemia
Millions of people suffer because they lack access to psychiatric care that they desperately need. The shortage of psychiatrists grows worse every day.
As a result of this anemic supply of psychiatrists, the mental health of the country’s populace is in jeopardy.
The facts paint the picture
The landmark National Institutes of Health-funded Epidemiologic Catchment Area (ECA) study, published a quarter of a century ago, found that 25% of the population suffered from a psychiatric disorder at some point in their life.1 In 1990, that translated to 62 million people; today, the number would be 82 million.
Regrettably, the findings of the ECA were not followed by necessary action—simply, ensuring sufficient psychiatrists to meet the significant mental health needs of the nation.
Signs and symptoms
Manifestations of psychiatrynemia continue unabated:
• frustration by primary care providers when they try to refer a patient to a psychiatrist—with appointments often unavailable for 4 to 6 months
• lack of access to a psychiatrist within 100 miles in many rural areas
• a large number of unfilled positions for psychiatrists in many health care settings nationwide, which has created a thriving locum tenens industry
• emergency rooms packed with psychiatric patients
• a huge patient load in community mental health centers
• a large increase in the percentage of seriously mentally ill people in jails and prisons because of a lack of psychiatrists and psychiatric beds; according to Torrey et al,2 the percentage of mentally ill patients incarcerated in the United States today is the same as it was in 1840, the pre-asylum era—shameful for a civilized country
• an escalation of cash-only practices and concierge psychiatry
• a suicide rate that continues to rise (one wonders how many of the 40,600 deaths by suicide and 650,000 suicide attempts in 20123 could have been prevented by prompt access to psychiatric care)
• a severe shortage of psychiatric subspecialists (child, geriatric, addiction, psychosomatic); their numbers need to rise by 200% to 300% to meet the needs of those populations
• an alarming overreliance on absurdly brief 15-minute med-checks as a way to cope with a large patient load.
What can be done?
To boost the number of psychiatric clinicians and provide better access to care, I offer several prescriptions in the Box.4 (Yes, polytherapy is needed.)
Curing psychiatrynemia requires bold action on multiple fronts by different stakeholders. Considering the failure to act over the past 25 years, however, prospects for a quick remission are low.
We’re past needing an ounce of prevention; we need many pounds.
1. Robins LN, Regier DA. Psychiatric disorders in America: the Epidemiologic Catchment Area Study. New York, NY: The File Press; 1991.
2. Torrey EF, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than in hospitals: a survey of the states. http://www.treatmentadvocacycenter.org/ storage/documents/final_jails_v_hospitals_ study.pdf. Published May 2010. Accessed November 12, 2014.
3. Xu JQ, Kochanek KD, Murphy SL, et al. Mortality in the United States, 2012. NCHS data brief, no 168. Hyattsville, MD: National Center for Health Statistics; 2014.
4. Malowney M, Keltz S, Fischer D, et al. Availability of outpatient care from psychiatrists: a simulated-patient study in three U.S. cities [published online October 15, 2014]. Psychiatr Serv. doi: 10.1176/appi. ps.201400051.
Millions of people suffer because they lack access to psychiatric care that they desperately need. The shortage of psychiatrists grows worse every day.
As a result of this anemic supply of psychiatrists, the mental health of the country’s populace is in jeopardy.
The facts paint the picture
The landmark National Institutes of Health-funded Epidemiologic Catchment Area (ECA) study, published a quarter of a century ago, found that 25% of the population suffered from a psychiatric disorder at some point in their life.1 In 1990, that translated to 62 million people; today, the number would be 82 million.
Regrettably, the findings of the ECA were not followed by necessary action—simply, ensuring sufficient psychiatrists to meet the significant mental health needs of the nation.
Signs and symptoms
Manifestations of psychiatrynemia continue unabated:
• frustration by primary care providers when they try to refer a patient to a psychiatrist—with appointments often unavailable for 4 to 6 months
• lack of access to a psychiatrist within 100 miles in many rural areas
• a large number of unfilled positions for psychiatrists in many health care settings nationwide, which has created a thriving locum tenens industry
• emergency rooms packed with psychiatric patients
• a huge patient load in community mental health centers
• a large increase in the percentage of seriously mentally ill people in jails and prisons because of a lack of psychiatrists and psychiatric beds; according to Torrey et al,2 the percentage of mentally ill patients incarcerated in the United States today is the same as it was in 1840, the pre-asylum era—shameful for a civilized country
• an escalation of cash-only practices and concierge psychiatry
• a suicide rate that continues to rise (one wonders how many of the 40,600 deaths by suicide and 650,000 suicide attempts in 20123 could have been prevented by prompt access to psychiatric care)
• a severe shortage of psychiatric subspecialists (child, geriatric, addiction, psychosomatic); their numbers need to rise by 200% to 300% to meet the needs of those populations
• an alarming overreliance on absurdly brief 15-minute med-checks as a way to cope with a large patient load.
What can be done?
To boost the number of psychiatric clinicians and provide better access to care, I offer several prescriptions in the Box.4 (Yes, polytherapy is needed.)
Curing psychiatrynemia requires bold action on multiple fronts by different stakeholders. Considering the failure to act over the past 25 years, however, prospects for a quick remission are low.
We’re past needing an ounce of prevention; we need many pounds.
Millions of people suffer because they lack access to psychiatric care that they desperately need. The shortage of psychiatrists grows worse every day.
As a result of this anemic supply of psychiatrists, the mental health of the country’s populace is in jeopardy.
The facts paint the picture
The landmark National Institutes of Health-funded Epidemiologic Catchment Area (ECA) study, published a quarter of a century ago, found that 25% of the population suffered from a psychiatric disorder at some point in their life.1 In 1990, that translated to 62 million people; today, the number would be 82 million.
Regrettably, the findings of the ECA were not followed by necessary action—simply, ensuring sufficient psychiatrists to meet the significant mental health needs of the nation.
Signs and symptoms
Manifestations of psychiatrynemia continue unabated:
• frustration by primary care providers when they try to refer a patient to a psychiatrist—with appointments often unavailable for 4 to 6 months
• lack of access to a psychiatrist within 100 miles in many rural areas
• a large number of unfilled positions for psychiatrists in many health care settings nationwide, which has created a thriving locum tenens industry
• emergency rooms packed with psychiatric patients
• a huge patient load in community mental health centers
• a large increase in the percentage of seriously mentally ill people in jails and prisons because of a lack of psychiatrists and psychiatric beds; according to Torrey et al,2 the percentage of mentally ill patients incarcerated in the United States today is the same as it was in 1840, the pre-asylum era—shameful for a civilized country
• an escalation of cash-only practices and concierge psychiatry
• a suicide rate that continues to rise (one wonders how many of the 40,600 deaths by suicide and 650,000 suicide attempts in 20123 could have been prevented by prompt access to psychiatric care)
• a severe shortage of psychiatric subspecialists (child, geriatric, addiction, psychosomatic); their numbers need to rise by 200% to 300% to meet the needs of those populations
• an alarming overreliance on absurdly brief 15-minute med-checks as a way to cope with a large patient load.
What can be done?
To boost the number of psychiatric clinicians and provide better access to care, I offer several prescriptions in the Box.4 (Yes, polytherapy is needed.)
Curing psychiatrynemia requires bold action on multiple fronts by different stakeholders. Considering the failure to act over the past 25 years, however, prospects for a quick remission are low.
We’re past needing an ounce of prevention; we need many pounds.
1. Robins LN, Regier DA. Psychiatric disorders in America: the Epidemiologic Catchment Area Study. New York, NY: The File Press; 1991.
2. Torrey EF, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than in hospitals: a survey of the states. http://www.treatmentadvocacycenter.org/ storage/documents/final_jails_v_hospitals_ study.pdf. Published May 2010. Accessed November 12, 2014.
3. Xu JQ, Kochanek KD, Murphy SL, et al. Mortality in the United States, 2012. NCHS data brief, no 168. Hyattsville, MD: National Center for Health Statistics; 2014.
4. Malowney M, Keltz S, Fischer D, et al. Availability of outpatient care from psychiatrists: a simulated-patient study in three U.S. cities [published online October 15, 2014]. Psychiatr Serv. doi: 10.1176/appi. ps.201400051.
1. Robins LN, Regier DA. Psychiatric disorders in America: the Epidemiologic Catchment Area Study. New York, NY: The File Press; 1991.
2. Torrey EF, Kennard AD, Eslinger D, et al. More mentally ill persons are in jails and prisons than in hospitals: a survey of the states. http://www.treatmentadvocacycenter.org/ storage/documents/final_jails_v_hospitals_ study.pdf. Published May 2010. Accessed November 12, 2014.
3. Xu JQ, Kochanek KD, Murphy SL, et al. Mortality in the United States, 2012. NCHS data brief, no 168. Hyattsville, MD: National Center for Health Statistics; 2014.
4. Malowney M, Keltz S, Fischer D, et al. Availability of outpatient care from psychiatrists: a simulated-patient study in three U.S. cities [published online October 15, 2014]. Psychiatr Serv. doi: 10.1176/appi. ps.201400051.
The sins and peccadillos of psychiatric practice
But as professionals, and by virtue of our rigorous training, we continually reflect on our thoughts and feelings when we care for our patients, and we examine the effect of our behavior and communication patterns on them. Such self-reflection is especially important when it comes to countertransference while treating a person who has been made vulnerable by emotional turmoil and who develops strong transference feelings toward the treating psychiatrist.
Personal integrity is paramount in psychiatric practice; it’s an indispensable ingredient when dealing with the intimate thoughts, feelings, and impulses of people who are seeking psychiatric help. In addition, the wisdom to recognize one’s limitations as a provider of care is an important attributes of seasoned practitioners. Patients might perceive us as demigods, but we know better than to be carried away with hubris and pretend that we are.
Exercising sound judgment isn’t easy
This is especially true when dealing with the varying degrees of ambiguity that shroud complex psychiatric conditions. The overriding principle for good clinical judgment in any medical practice is the patient’s welfare, and that must dominate the moral, ethical, medical, and scientific decision-making of all psychiatrists. Those of us in charge of training medical students and residents emphasize this principle every day at the bedside and in the clinic. Upholding those principles, side by side with the knowledge and skills of psychiatric practice, are the hallmarks of good medical training.
But missteps occur. Peccadillos, infractions, and even transgressions happen—accidentally by competent, ethical psychiatrists; deliberately, sometimes, by a few unethical scoundrels. The sins of psychiatric practice come in a range of gravity and consequences. Here are some I’ve observed among colleagues over the years:
Becoming sexually intimate with one’s patient. Violating the sacred boundary of the doctor-patient relationship is unforgivable; it’s a sin that scars the patient and can destroy the psychiatrist’s reputation and career. We must respect and uphold that boundary—not only during active treatment but even after care is terminated.
Divulging clinical details to other without the patient’s consent. Breach of trust is not only an ethical misstep; it is a violation of the Health Insurance Portability and Accountability Act of 1996 (HIPAA), with substantial penalties attached.
Treating the mind while ignoring the body and brain. We are physicians first, psychiatrists second. We must fully assess patients who present with psychiatric signs and symptoms to rule out a general medical condition that could be generating behavioral symptoms. Such co-occurring medical conditions might involve various organ systems, such as endocrinopathies, or might emanate from brain lesions, whether traumatic, degenerative, demyelinating, infectious, or neoplastic. Without careful medical evaluation, the wrong diagnosis might be made and inappropriate, even harmful, treatment provided while necessary care is delayed.
Treatment plans can sometimes overlook potential harmful effects of some medications on physical health— whether metabolic, cardiovascular, neurologic, gastrointestinal, hormonal, hematologic, or dermatologic. An optimal treatment plan embarks on healing the mind without ignoring or harming the body.
Failing to obtain additional information from sources who know the patient or who can provide old records. Such information is vital in psychiatry, because the patient’s account often is incomplete, even distorted, because of cognitive deficits or psychopathologic factors. Additional information can be corroborative or contradictory, and is sometimes critical—significantly influencing the diagnosis or the treatment plan, or both.
Allowing personal beliefs to influence care. This transgression includes inserting one’s views about religion, politics, sexual orientation, ethnic origin, and socioeconomic class into medical care. The same unimpeachable, high caliber of care must be provided to every patient, and must not differ in any way from the care that we would recommend to our own family members.
Reducing psychiatry to prescribing a pill. However unacceptable and deficient this reductionist degradation of psychiatric management is, it is sometimes imposed by organizations in which the caseload is huge and the number of providers insufficient. We must resist the temptation to compromise, and must strive to address not only the biological aspects of illness but psychological and social dimensions as well. Patients will not have an optimal if we don’t.
Practicing with an outdated knowledge base—one acquired during residency years, often years or even decades, earlier. There is no medical or ethical justification for using 1985, or even 2005, standards of psychiatric care in 2015.
Psychiatry is rapidly evolving, with many ongoing changes and advances. Updating one’s practice pattern through lifelong learning is an absolute must for psychiatrists (and for all health care professionals). Utilizing the latest, evidence-based data to guide diagnosis and treatment is an indispensable component of good psychiatric care.
Neglecting to consider treatment options. Consider just a few scenarios: The recurrently relapsing patient with psychosis who is not switched to a long-acting injectable formulation; the persistently psychotic patient who does not receive a trial of clozapine; the treatment-resistant depression patient who is not referred for electroconvulsive therapy or transcranial magnetic stimulation; and the patient receiving an atypical antipsychotic who is monitored inconsistently for metabolic dysregulation.
Treating patients but not vigorously advocating for them—thus allowing a broken, convoluted mental health system to delay or prevent access to care; incarcerate relapsed patients instead of hospitalizing them; permit insurance companies to discriminate against coverage of mental illness; and tie the hands of psychiatrists who want to select medication they judge best for their patients.
None of us is 'without sin'
We all aspire to help our patients in the best way we can, and to avoid errors. However, even a seasoned psychiatrist can stumble unwittingly, and that is understandable and forgivable. It is willful, recurring neglect of the patient’s welfare that can be deleterious and that, in my opinion, qualifies as a cardinal sin. Fortunately, such neglect is a low-frequency event in psychiatric practice, but even a single occurrence is one too many.
But as professionals, and by virtue of our rigorous training, we continually reflect on our thoughts and feelings when we care for our patients, and we examine the effect of our behavior and communication patterns on them. Such self-reflection is especially important when it comes to countertransference while treating a person who has been made vulnerable by emotional turmoil and who develops strong transference feelings toward the treating psychiatrist.
Personal integrity is paramount in psychiatric practice; it’s an indispensable ingredient when dealing with the intimate thoughts, feelings, and impulses of people who are seeking psychiatric help. In addition, the wisdom to recognize one’s limitations as a provider of care is an important attributes of seasoned practitioners. Patients might perceive us as demigods, but we know better than to be carried away with hubris and pretend that we are.
Exercising sound judgment isn’t easy
This is especially true when dealing with the varying degrees of ambiguity that shroud complex psychiatric conditions. The overriding principle for good clinical judgment in any medical practice is the patient’s welfare, and that must dominate the moral, ethical, medical, and scientific decision-making of all psychiatrists. Those of us in charge of training medical students and residents emphasize this principle every day at the bedside and in the clinic. Upholding those principles, side by side with the knowledge and skills of psychiatric practice, are the hallmarks of good medical training.
But missteps occur. Peccadillos, infractions, and even transgressions happen—accidentally by competent, ethical psychiatrists; deliberately, sometimes, by a few unethical scoundrels. The sins of psychiatric practice come in a range of gravity and consequences. Here are some I’ve observed among colleagues over the years:
Becoming sexually intimate with one’s patient. Violating the sacred boundary of the doctor-patient relationship is unforgivable; it’s a sin that scars the patient and can destroy the psychiatrist’s reputation and career. We must respect and uphold that boundary—not only during active treatment but even after care is terminated.
Divulging clinical details to other without the patient’s consent. Breach of trust is not only an ethical misstep; it is a violation of the Health Insurance Portability and Accountability Act of 1996 (HIPAA), with substantial penalties attached.
Treating the mind while ignoring the body and brain. We are physicians first, psychiatrists second. We must fully assess patients who present with psychiatric signs and symptoms to rule out a general medical condition that could be generating behavioral symptoms. Such co-occurring medical conditions might involve various organ systems, such as endocrinopathies, or might emanate from brain lesions, whether traumatic, degenerative, demyelinating, infectious, or neoplastic. Without careful medical evaluation, the wrong diagnosis might be made and inappropriate, even harmful, treatment provided while necessary care is delayed.
Treatment plans can sometimes overlook potential harmful effects of some medications on physical health— whether metabolic, cardiovascular, neurologic, gastrointestinal, hormonal, hematologic, or dermatologic. An optimal treatment plan embarks on healing the mind without ignoring or harming the body.
Failing to obtain additional information from sources who know the patient or who can provide old records. Such information is vital in psychiatry, because the patient’s account often is incomplete, even distorted, because of cognitive deficits or psychopathologic factors. Additional information can be corroborative or contradictory, and is sometimes critical—significantly influencing the diagnosis or the treatment plan, or both.
Allowing personal beliefs to influence care. This transgression includes inserting one’s views about religion, politics, sexual orientation, ethnic origin, and socioeconomic class into medical care. The same unimpeachable, high caliber of care must be provided to every patient, and must not differ in any way from the care that we would recommend to our own family members.
Reducing psychiatry to prescribing a pill. However unacceptable and deficient this reductionist degradation of psychiatric management is, it is sometimes imposed by organizations in which the caseload is huge and the number of providers insufficient. We must resist the temptation to compromise, and must strive to address not only the biological aspects of illness but psychological and social dimensions as well. Patients will not have an optimal if we don’t.
Practicing with an outdated knowledge base—one acquired during residency years, often years or even decades, earlier. There is no medical or ethical justification for using 1985, or even 2005, standards of psychiatric care in 2015.
Psychiatry is rapidly evolving, with many ongoing changes and advances. Updating one’s practice pattern through lifelong learning is an absolute must for psychiatrists (and for all health care professionals). Utilizing the latest, evidence-based data to guide diagnosis and treatment is an indispensable component of good psychiatric care.
Neglecting to consider treatment options. Consider just a few scenarios: The recurrently relapsing patient with psychosis who is not switched to a long-acting injectable formulation; the persistently psychotic patient who does not receive a trial of clozapine; the treatment-resistant depression patient who is not referred for electroconvulsive therapy or transcranial magnetic stimulation; and the patient receiving an atypical antipsychotic who is monitored inconsistently for metabolic dysregulation.
Treating patients but not vigorously advocating for them—thus allowing a broken, convoluted mental health system to delay or prevent access to care; incarcerate relapsed patients instead of hospitalizing them; permit insurance companies to discriminate against coverage of mental illness; and tie the hands of psychiatrists who want to select medication they judge best for their patients.
None of us is 'without sin'
We all aspire to help our patients in the best way we can, and to avoid errors. However, even a seasoned psychiatrist can stumble unwittingly, and that is understandable and forgivable. It is willful, recurring neglect of the patient’s welfare that can be deleterious and that, in my opinion, qualifies as a cardinal sin. Fortunately, such neglect is a low-frequency event in psychiatric practice, but even a single occurrence is one too many.
But as professionals, and by virtue of our rigorous training, we continually reflect on our thoughts and feelings when we care for our patients, and we examine the effect of our behavior and communication patterns on them. Such self-reflection is especially important when it comes to countertransference while treating a person who has been made vulnerable by emotional turmoil and who develops strong transference feelings toward the treating psychiatrist.
Personal integrity is paramount in psychiatric practice; it’s an indispensable ingredient when dealing with the intimate thoughts, feelings, and impulses of people who are seeking psychiatric help. In addition, the wisdom to recognize one’s limitations as a provider of care is an important attributes of seasoned practitioners. Patients might perceive us as demigods, but we know better than to be carried away with hubris and pretend that we are.
Exercising sound judgment isn’t easy
This is especially true when dealing with the varying degrees of ambiguity that shroud complex psychiatric conditions. The overriding principle for good clinical judgment in any medical practice is the patient’s welfare, and that must dominate the moral, ethical, medical, and scientific decision-making of all psychiatrists. Those of us in charge of training medical students and residents emphasize this principle every day at the bedside and in the clinic. Upholding those principles, side by side with the knowledge and skills of psychiatric practice, are the hallmarks of good medical training.
But missteps occur. Peccadillos, infractions, and even transgressions happen—accidentally by competent, ethical psychiatrists; deliberately, sometimes, by a few unethical scoundrels. The sins of psychiatric practice come in a range of gravity and consequences. Here are some I’ve observed among colleagues over the years:
Becoming sexually intimate with one’s patient. Violating the sacred boundary of the doctor-patient relationship is unforgivable; it’s a sin that scars the patient and can destroy the psychiatrist’s reputation and career. We must respect and uphold that boundary—not only during active treatment but even after care is terminated.
Divulging clinical details to other without the patient’s consent. Breach of trust is not only an ethical misstep; it is a violation of the Health Insurance Portability and Accountability Act of 1996 (HIPAA), with substantial penalties attached.
Treating the mind while ignoring the body and brain. We are physicians first, psychiatrists second. We must fully assess patients who present with psychiatric signs and symptoms to rule out a general medical condition that could be generating behavioral symptoms. Such co-occurring medical conditions might involve various organ systems, such as endocrinopathies, or might emanate from brain lesions, whether traumatic, degenerative, demyelinating, infectious, or neoplastic. Without careful medical evaluation, the wrong diagnosis might be made and inappropriate, even harmful, treatment provided while necessary care is delayed.
Treatment plans can sometimes overlook potential harmful effects of some medications on physical health— whether metabolic, cardiovascular, neurologic, gastrointestinal, hormonal, hematologic, or dermatologic. An optimal treatment plan embarks on healing the mind without ignoring or harming the body.
Failing to obtain additional information from sources who know the patient or who can provide old records. Such information is vital in psychiatry, because the patient’s account often is incomplete, even distorted, because of cognitive deficits or psychopathologic factors. Additional information can be corroborative or contradictory, and is sometimes critical—significantly influencing the diagnosis or the treatment plan, or both.
Allowing personal beliefs to influence care. This transgression includes inserting one’s views about religion, politics, sexual orientation, ethnic origin, and socioeconomic class into medical care. The same unimpeachable, high caliber of care must be provided to every patient, and must not differ in any way from the care that we would recommend to our own family members.
Reducing psychiatry to prescribing a pill. However unacceptable and deficient this reductionist degradation of psychiatric management is, it is sometimes imposed by organizations in which the caseload is huge and the number of providers insufficient. We must resist the temptation to compromise, and must strive to address not only the biological aspects of illness but psychological and social dimensions as well. Patients will not have an optimal if we don’t.
Practicing with an outdated knowledge base—one acquired during residency years, often years or even decades, earlier. There is no medical or ethical justification for using 1985, or even 2005, standards of psychiatric care in 2015.
Psychiatry is rapidly evolving, with many ongoing changes and advances. Updating one’s practice pattern through lifelong learning is an absolute must for psychiatrists (and for all health care professionals). Utilizing the latest, evidence-based data to guide diagnosis and treatment is an indispensable component of good psychiatric care.
Neglecting to consider treatment options. Consider just a few scenarios: The recurrently relapsing patient with psychosis who is not switched to a long-acting injectable formulation; the persistently psychotic patient who does not receive a trial of clozapine; the treatment-resistant depression patient who is not referred for electroconvulsive therapy or transcranial magnetic stimulation; and the patient receiving an atypical antipsychotic who is monitored inconsistently for metabolic dysregulation.
Treating patients but not vigorously advocating for them—thus allowing a broken, convoluted mental health system to delay or prevent access to care; incarcerate relapsed patients instead of hospitalizing them; permit insurance companies to discriminate against coverage of mental illness; and tie the hands of psychiatrists who want to select medication they judge best for their patients.
None of us is 'without sin'
We all aspire to help our patients in the best way we can, and to avoid errors. However, even a seasoned psychiatrist can stumble unwittingly, and that is understandable and forgivable. It is willful, recurring neglect of the patient’s welfare that can be deleterious and that, in my opinion, qualifies as a cardinal sin. Fortunately, such neglect is a low-frequency event in psychiatric practice, but even a single occurrence is one too many.
Clozapine is a vastly underutilized, unique agent with multiple applications
Since clozapine was launched in 1989, miraculous improvements and “awakenings” have been reported in many patients afflicted with severe schizophrenia and considered hopelessly refractory to antipsychotic pharmacotherapy. Not only do severely disabled patients regain their sanity and return to normal functioning, but the joy that their family and treating psychiatrist experience is priceless.
That’s why I am perplexed by how infrequently clozapine is used in the United States (in about 5% of patients)—even though approximately 25% of patients who have schizophrenia are either treatment-resistant or have refractory hallucinations or delusions.
Consider Bethany’s case. She was one of my young patients, who, after taking clozapine, recovered fully and resumed a productive life, after years of homelessness during which she was controlled by auditory hallucinations.
Bethany’s story began well…
Bethany grew up in a loving home, smart and talented, an “A” student in high school and talented violinist. She received a scholarship to a prestigious private university at 16 and left her parent’s home in Ohio to major in molecular biology. Her goal was to attend medical school. She excelled during her first 3 years of college, and even published 2 papers in top-tier science journals.
In her senior year, after returning from a trip to Africa, Bethany began to change. She neglected her studies and focused on raising money for HIV clinics in Africa. She began getting F’s instead of A’s, lost her scholarship and her residence hall room, and had to drop out of college. Soon, she began hearing voices commanding her every action.
..but took a really bad turn
Bethany became homeless for the next 4.5 years. She ate discarded food from garbage cans, had no change of clothes, and slept on a concrete slab behind a downtown church in a major city in California. Her parents lost track of her, although her mother, a retired nurse, frantically and relentlessly tried to find out what happened to her only daughter during that time.
Eventually, Bethany was arrested when she was found screaming back at the voices, at midnight in a residential area of the city. She was hospitalized on a psychiatric ward and given antipsychotics, but with only modest improvement.
Her parents were contacted; immediately, they flew to California to see her. The treating psychiatrist told them that their daughter had schizophrenia, and that they should lower their expectations because she would be totally disabled for the rest of her life. They brought Bethany back to Ohio where, after a tumultuous year of failed trials of several antipsychotics to suppress the auditory hallucinations, we gave her clozapine.
Gradually, Bethany improved, but she still could not read a book or magazine (which I urged her to do) without the voices intensifying and preventing her from reading.
Bethany recovers
After 6 to 8 months on clozapine, however, Bethany’s auditory hallucinations faded away. With my encouragement, she enrolled at the University of Cincinnati and took 1 course at a time. She began to get A’s again—in advanced courses, such as genetics, physics, and molecular biology. She completed her degree requirements and graduated with honors, with a Bachelor of Science degree in molecular biology. She also served as a marshal in the commencement ceremony procession.
Over the next year, with strong encouragement, Bethany wrote a book about her remarkable recovery from refractory psychosis.1 In addition, her mother wrote a deeply emotional book that described the gut-wrenching ordeal that she and her husband went through during the years that Bethany disappeared.2 I urge you to read these inspiring books (Figure) about the remarkable recovery from refractory psychosis and the heavy family burden of schizophrenia.
Back to clozapine
Although the package insert for clozapine contains 5 black-box warnings (for agranulocytosis, seizures, myocarditis, respiratory effects, and increased mortality in geriatric patients with psychosis associated with dementia), the drug is a useful last-resort medication for several approved indications and off-label uses. In addition to the official, evidence-based indication for treatment-resistant and refractory schizophrenia,3 clozapine is FDA-approved for suicidality in schizophrenia.4 Clinically reported, but unapproved, uses are listed in the Table.5-13
A little-known advantage of clozapine is its salutary effect on mortality. In a Finnish study of 66,881 persons who had schizophrenia,14 those taking clozapine had, overall, lower mortality during the treatment period than those taking any of the 6 most commonly used antipsychotic drugs.
No doubt, clozapine is associated with serious side effects15—but so is chemotherapy for cancer, and oncologists do not hesitate to use it to save their patients from physical death. Severe schizophrenia is like a cancer of the mind, and clozapine is its chemotherapy.
Fortunately for Bethany, she had almost no physical adverse effects from clozapine except for intense sedation, which was mitigated with modafinil.
We should use clozapine more than we do
Clozapine has the potential to have a healing effect for many patients whose schizophrenia is resistant to treatment. Most such patients, however, never receive a trial of the drug. Furthermore, few practitioners use clozapine for schizophrenia patients with suicidal tendencies, despite the high rate of suicide completion in schizophrenia.16
Clozapine remains, regrettably, an underutilized agent in psychiatry. Until other breakthrough drugs are discovered, its use ought to be double or triple what it is now because there are many people like Bethany who are not being given a chance to recover from their illness.
1. Yeiser B. Mind estranged. My journey from schizophrenia and homelessness to recovery. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
2. Yeiser KS. Flight from reason: a mother’s story of schizophrenia, recovery and hope. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
3. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
4. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735.] Arch Gen Psychiatry. 2003;60(1):82-91.
5. Frogley C, Taylor D, Dickens G, et al. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15(9):1351-1371.
6. Margetié B, Aukst-Margetié B, Zarkovié-Palijan T. Successful treatment of polydipsia, water intoxication, and delusional jealousy in an alcohol dependent patient with clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1347-1349.
7. Cupina D, Boulton M. Secondary delusional parasitosis treated successfully with a combination of clozapine and citalopram. Psychosomatics. 2012;53(3):301-302.
8. Connolly BD, Lang AE. Pharmacolgoic treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670-1683.
9. Hazari N, Kate N, Grover S, et al. Clozapine and tardive movement disorders: a review. Asian J Psychiatry. 2013;6(6):439-451.
10. Zarzar T, McEvoy J. Clozapine for self-injurious behavior in individuals with borderline personality disorder. Ther Adv Psychopharmacol. 2013;3(5):272-274.
11. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
12. Ifteni P, Correll CU, Nielse J, et al. Rapid clozapine titration in treatment-refractory bipolar disorder. J Affect Disord. 2014;166:168-172.
13. Rogoz Z. Combined treatment with atypical antipsychotics and antidepressants in treatment-resistant depression: preclinical and clinical efficacy. Pharmacol Rep. 2013;65(6)1535-1544.
14. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
15. Raja M, Raja S. Clozapine safety, 40 years later [published online April 28, 2014]. Curr Drug Saf. doi: 10.2174/1574886309666140428115040.
16. Siris SG. Suicide and schizophrenia. J Psychopharmacol. 2001;15(2):127-135.
Since clozapine was launched in 1989, miraculous improvements and “awakenings” have been reported in many patients afflicted with severe schizophrenia and considered hopelessly refractory to antipsychotic pharmacotherapy. Not only do severely disabled patients regain their sanity and return to normal functioning, but the joy that their family and treating psychiatrist experience is priceless.
That’s why I am perplexed by how infrequently clozapine is used in the United States (in about 5% of patients)—even though approximately 25% of patients who have schizophrenia are either treatment-resistant or have refractory hallucinations or delusions.
Consider Bethany’s case. She was one of my young patients, who, after taking clozapine, recovered fully and resumed a productive life, after years of homelessness during which she was controlled by auditory hallucinations.
Bethany’s story began well…
Bethany grew up in a loving home, smart and talented, an “A” student in high school and talented violinist. She received a scholarship to a prestigious private university at 16 and left her parent’s home in Ohio to major in molecular biology. Her goal was to attend medical school. She excelled during her first 3 years of college, and even published 2 papers in top-tier science journals.
In her senior year, after returning from a trip to Africa, Bethany began to change. She neglected her studies and focused on raising money for HIV clinics in Africa. She began getting F’s instead of A’s, lost her scholarship and her residence hall room, and had to drop out of college. Soon, she began hearing voices commanding her every action.
..but took a really bad turn
Bethany became homeless for the next 4.5 years. She ate discarded food from garbage cans, had no change of clothes, and slept on a concrete slab behind a downtown church in a major city in California. Her parents lost track of her, although her mother, a retired nurse, frantically and relentlessly tried to find out what happened to her only daughter during that time.
Eventually, Bethany was arrested when she was found screaming back at the voices, at midnight in a residential area of the city. She was hospitalized on a psychiatric ward and given antipsychotics, but with only modest improvement.
Her parents were contacted; immediately, they flew to California to see her. The treating psychiatrist told them that their daughter had schizophrenia, and that they should lower their expectations because she would be totally disabled for the rest of her life. They brought Bethany back to Ohio where, after a tumultuous year of failed trials of several antipsychotics to suppress the auditory hallucinations, we gave her clozapine.
Gradually, Bethany improved, but she still could not read a book or magazine (which I urged her to do) without the voices intensifying and preventing her from reading.
Bethany recovers
After 6 to 8 months on clozapine, however, Bethany’s auditory hallucinations faded away. With my encouragement, she enrolled at the University of Cincinnati and took 1 course at a time. She began to get A’s again—in advanced courses, such as genetics, physics, and molecular biology. She completed her degree requirements and graduated with honors, with a Bachelor of Science degree in molecular biology. She also served as a marshal in the commencement ceremony procession.
Over the next year, with strong encouragement, Bethany wrote a book about her remarkable recovery from refractory psychosis.1 In addition, her mother wrote a deeply emotional book that described the gut-wrenching ordeal that she and her husband went through during the years that Bethany disappeared.2 I urge you to read these inspiring books (Figure) about the remarkable recovery from refractory psychosis and the heavy family burden of schizophrenia.
Back to clozapine
Although the package insert for clozapine contains 5 black-box warnings (for agranulocytosis, seizures, myocarditis, respiratory effects, and increased mortality in geriatric patients with psychosis associated with dementia), the drug is a useful last-resort medication for several approved indications and off-label uses. In addition to the official, evidence-based indication for treatment-resistant and refractory schizophrenia,3 clozapine is FDA-approved for suicidality in schizophrenia.4 Clinically reported, but unapproved, uses are listed in the Table.5-13
A little-known advantage of clozapine is its salutary effect on mortality. In a Finnish study of 66,881 persons who had schizophrenia,14 those taking clozapine had, overall, lower mortality during the treatment period than those taking any of the 6 most commonly used antipsychotic drugs.
No doubt, clozapine is associated with serious side effects15—but so is chemotherapy for cancer, and oncologists do not hesitate to use it to save their patients from physical death. Severe schizophrenia is like a cancer of the mind, and clozapine is its chemotherapy.
Fortunately for Bethany, she had almost no physical adverse effects from clozapine except for intense sedation, which was mitigated with modafinil.
We should use clozapine more than we do
Clozapine has the potential to have a healing effect for many patients whose schizophrenia is resistant to treatment. Most such patients, however, never receive a trial of the drug. Furthermore, few practitioners use clozapine for schizophrenia patients with suicidal tendencies, despite the high rate of suicide completion in schizophrenia.16
Clozapine remains, regrettably, an underutilized agent in psychiatry. Until other breakthrough drugs are discovered, its use ought to be double or triple what it is now because there are many people like Bethany who are not being given a chance to recover from their illness.
Since clozapine was launched in 1989, miraculous improvements and “awakenings” have been reported in many patients afflicted with severe schizophrenia and considered hopelessly refractory to antipsychotic pharmacotherapy. Not only do severely disabled patients regain their sanity and return to normal functioning, but the joy that their family and treating psychiatrist experience is priceless.
That’s why I am perplexed by how infrequently clozapine is used in the United States (in about 5% of patients)—even though approximately 25% of patients who have schizophrenia are either treatment-resistant or have refractory hallucinations or delusions.
Consider Bethany’s case. She was one of my young patients, who, after taking clozapine, recovered fully and resumed a productive life, after years of homelessness during which she was controlled by auditory hallucinations.
Bethany’s story began well…
Bethany grew up in a loving home, smart and talented, an “A” student in high school and talented violinist. She received a scholarship to a prestigious private university at 16 and left her parent’s home in Ohio to major in molecular biology. Her goal was to attend medical school. She excelled during her first 3 years of college, and even published 2 papers in top-tier science journals.
In her senior year, after returning from a trip to Africa, Bethany began to change. She neglected her studies and focused on raising money for HIV clinics in Africa. She began getting F’s instead of A’s, lost her scholarship and her residence hall room, and had to drop out of college. Soon, she began hearing voices commanding her every action.
..but took a really bad turn
Bethany became homeless for the next 4.5 years. She ate discarded food from garbage cans, had no change of clothes, and slept on a concrete slab behind a downtown church in a major city in California. Her parents lost track of her, although her mother, a retired nurse, frantically and relentlessly tried to find out what happened to her only daughter during that time.
Eventually, Bethany was arrested when she was found screaming back at the voices, at midnight in a residential area of the city. She was hospitalized on a psychiatric ward and given antipsychotics, but with only modest improvement.
Her parents were contacted; immediately, they flew to California to see her. The treating psychiatrist told them that their daughter had schizophrenia, and that they should lower their expectations because she would be totally disabled for the rest of her life. They brought Bethany back to Ohio where, after a tumultuous year of failed trials of several antipsychotics to suppress the auditory hallucinations, we gave her clozapine.
Gradually, Bethany improved, but she still could not read a book or magazine (which I urged her to do) without the voices intensifying and preventing her from reading.
Bethany recovers
After 6 to 8 months on clozapine, however, Bethany’s auditory hallucinations faded away. With my encouragement, she enrolled at the University of Cincinnati and took 1 course at a time. She began to get A’s again—in advanced courses, such as genetics, physics, and molecular biology. She completed her degree requirements and graduated with honors, with a Bachelor of Science degree in molecular biology. She also served as a marshal in the commencement ceremony procession.
Over the next year, with strong encouragement, Bethany wrote a book about her remarkable recovery from refractory psychosis.1 In addition, her mother wrote a deeply emotional book that described the gut-wrenching ordeal that she and her husband went through during the years that Bethany disappeared.2 I urge you to read these inspiring books (Figure) about the remarkable recovery from refractory psychosis and the heavy family burden of schizophrenia.
Back to clozapine
Although the package insert for clozapine contains 5 black-box warnings (for agranulocytosis, seizures, myocarditis, respiratory effects, and increased mortality in geriatric patients with psychosis associated with dementia), the drug is a useful last-resort medication for several approved indications and off-label uses. In addition to the official, evidence-based indication for treatment-resistant and refractory schizophrenia,3 clozapine is FDA-approved for suicidality in schizophrenia.4 Clinically reported, but unapproved, uses are listed in the Table.5-13
A little-known advantage of clozapine is its salutary effect on mortality. In a Finnish study of 66,881 persons who had schizophrenia,14 those taking clozapine had, overall, lower mortality during the treatment period than those taking any of the 6 most commonly used antipsychotic drugs.
No doubt, clozapine is associated with serious side effects15—but so is chemotherapy for cancer, and oncologists do not hesitate to use it to save their patients from physical death. Severe schizophrenia is like a cancer of the mind, and clozapine is its chemotherapy.
Fortunately for Bethany, she had almost no physical adverse effects from clozapine except for intense sedation, which was mitigated with modafinil.
We should use clozapine more than we do
Clozapine has the potential to have a healing effect for many patients whose schizophrenia is resistant to treatment. Most such patients, however, never receive a trial of the drug. Furthermore, few practitioners use clozapine for schizophrenia patients with suicidal tendencies, despite the high rate of suicide completion in schizophrenia.16
Clozapine remains, regrettably, an underutilized agent in psychiatry. Until other breakthrough drugs are discovered, its use ought to be double or triple what it is now because there are many people like Bethany who are not being given a chance to recover from their illness.
1. Yeiser B. Mind estranged. My journey from schizophrenia and homelessness to recovery. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
2. Yeiser KS. Flight from reason: a mother’s story of schizophrenia, recovery and hope. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
3. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
4. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735.] Arch Gen Psychiatry. 2003;60(1):82-91.
5. Frogley C, Taylor D, Dickens G, et al. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15(9):1351-1371.
6. Margetié B, Aukst-Margetié B, Zarkovié-Palijan T. Successful treatment of polydipsia, water intoxication, and delusional jealousy in an alcohol dependent patient with clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1347-1349.
7. Cupina D, Boulton M. Secondary delusional parasitosis treated successfully with a combination of clozapine and citalopram. Psychosomatics. 2012;53(3):301-302.
8. Connolly BD, Lang AE. Pharmacolgoic treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670-1683.
9. Hazari N, Kate N, Grover S, et al. Clozapine and tardive movement disorders: a review. Asian J Psychiatry. 2013;6(6):439-451.
10. Zarzar T, McEvoy J. Clozapine for self-injurious behavior in individuals with borderline personality disorder. Ther Adv Psychopharmacol. 2013;3(5):272-274.
11. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
12. Ifteni P, Correll CU, Nielse J, et al. Rapid clozapine titration in treatment-refractory bipolar disorder. J Affect Disord. 2014;166:168-172.
13. Rogoz Z. Combined treatment with atypical antipsychotics and antidepressants in treatment-resistant depression: preclinical and clinical efficacy. Pharmacol Rep. 2013;65(6)1535-1544.
14. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
15. Raja M, Raja S. Clozapine safety, 40 years later [published online April 28, 2014]. Curr Drug Saf. doi: 10.2174/1574886309666140428115040.
16. Siris SG. Suicide and schizophrenia. J Psychopharmacol. 2001;15(2):127-135.
1. Yeiser B. Mind estranged. My journey from schizophrenia and homelessness to recovery. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
2. Yeiser KS. Flight from reason: a mother’s story of schizophrenia, recovery and hope. North Charleston, SC: CreateSpace Independent Publishing Platform; 2014.
3. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
4. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) [Erratum in: Arch Gen Psychiatry. 2003;60(7):735.] Arch Gen Psychiatry. 2003;60(1):82-91.
5. Frogley C, Taylor D, Dickens G, et al. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15(9):1351-1371.
6. Margetié B, Aukst-Margetié B, Zarkovié-Palijan T. Successful treatment of polydipsia, water intoxication, and delusional jealousy in an alcohol dependent patient with clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1347-1349.
7. Cupina D, Boulton M. Secondary delusional parasitosis treated successfully with a combination of clozapine and citalopram. Psychosomatics. 2012;53(3):301-302.
8. Connolly BD, Lang AE. Pharmacolgoic treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670-1683.
9. Hazari N, Kate N, Grover S, et al. Clozapine and tardive movement disorders: a review. Asian J Psychiatry. 2013;6(6):439-451.
10. Zarzar T, McEvoy J. Clozapine for self-injurious behavior in individuals with borderline personality disorder. Ther Adv Psychopharmacol. 2013;3(5):272-274.
11. Vohra AK. Treatment of severe borderline personality disorder with clozapine. Indian J Psychiatry. 2010;52(3):267-269.
12. Ifteni P, Correll CU, Nielse J, et al. Rapid clozapine titration in treatment-refractory bipolar disorder. J Affect Disord. 2014;166:168-172.
13. Rogoz Z. Combined treatment with atypical antipsychotics and antidepressants in treatment-resistant depression: preclinical and clinical efficacy. Pharmacol Rep. 2013;65(6)1535-1544.
14. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
15. Raja M, Raja S. Clozapine safety, 40 years later [published online April 28, 2014]. Curr Drug Saf. doi: 10.2174/1574886309666140428115040.
16. Siris SG. Suicide and schizophrenia. J Psychopharmacol. 2001;15(2):127-135.
Can philanthropy fill the unmet needs of psychiatry?
Recent examples come quickly to mind:
• $100 million from the Lieber family to fund the Lieber Institute for Brain Development at Johns Hopkins University
• $300 million from Microsoft co-founder Paul G. Allen to create the Allen Institute for Brain Science
• $650 million from Ted Stanley for the Stanley Center at the Broad Institute.
Such generosity is cause for celebration by psychiatrists and their long-suffering patients who are disabled by a brain disorder. Private money supplements research funding by the National Institutes of Health and will bolster the war against mental illness,1 which costs >$300 billion annually (Box,2page 12).
Although philanthropy will help, many needs in psychiatry are unmet, and not all can be addressed with money. Consider a number of areas of need.
Unmet clinical needs
Models of disease. Psychiatry is in desperate need of an objective, valid diagnostic system that transcends the DSM model of symptom clusters. The Research Domain Criteria3 represents the effort to find an alternative. To achieve that goal, it’s necessary to identify biomarkers and establish their utility—a task that requires a huge amount of funding.
Therapeutics. Clinicians are hungry for innovative, safe pharmaceuticals and non-drug treatments that modify disease, not just alleviate symptoms. Obsessive-compulsive disorder always has lacked such therapies; so have dementia, schizophrenia, personality disorders, dissociative disorders, and sexual pathologies. In fact, >80% of DSM disorders do not have an FDA-approved, evidence-based treatment,4 and many available medications are only partially efficacious, poorly tolerated, or unsafe.
There are more unmet needs in therapeutics:
• Development of promising non-drug therapies, such as neuromodulation, proceeds slowly.
• Research into neurobiological mechanisms of psychotherapy is in its infancy.
• A foolproof method to monitor adherence does not exist, and countless patients relapse needlessly and deteriorate functionally.
• Effective, evidence-based rehabilitation for serious psychiatric disorders is used narrowly and vastly underfunded.
• Insurers continue to thumb their nose at laws that require parity for treating mental illness—thus impeding access to, delaying, or truncating psychiatric care.
Unmet scientific needs
Translational investigators. Despite increased funding for basic neuroscientific study and breathtaking discoveries in animal molecular neurobiology, a trickle of findings has been applied to clinical medicine. This translational gap has many causes, including a shortage of translational neuroscientists (MD-PhD psychiatrists and neurologists), insufficient long-term funding to develop such clinician-researchers, and complex regulatory oversight of human research.
Stalled progress in drug development. Development of novel-mechanism therapeutics for brain disorders is languishing. Some pharmaceutical manufacturers have abandoned the development of drugs that act on the CNS in favor of less complex, more lucrative areas such as oncology and cardiology; others have reduced their investment in CNS products. Developing treatments for knotty disorders of the most complex structure in the known universe requires mammoth investment. Why are stakeholders bailing out on the greatest challenge for science and medicine for easier endeavors?
Discovering new genes for every devastating neuropsychiatric syndrome, such as schizophrenia, is cause for celebration, but the champagne won’t flow until the coding of every gene is unscrambled so that specific biological interventions can be developed. The cost of the chase might be orders of magnitude greater than what is invested in research today. Conceptualizing new models of brain disorders is a critical part of scientific progress and an antidote to the inertia of perpetual group-think. Depression, for example, is being reconceptualized as a disorder of impaired neuroplasticity and neurotropic deficiency, rather than a shortage of serotonin and norepinephrine. Rapid reversal of severe depression to euthymia—in 1 or 2 hours—with IV ketamine shattered the dogma that depression takes weeks to lift, and is ushering in unprecedented new thinking and models likely to revolutionize treatment of severe depression. We need such breakthroughs for other psychiatric brain disorders.
Unmet professional and sociopolitical needs
Broadening of training. Psychiatrists have focused on the mind but insufficiently attended to the biology of the brain. For psychiatry to rise to the next level as a medical specialty and brain discipline, training must incorporate more neurology than it does now. The converse is true in neurology.
Hospitalization not incarceration. It is unconscionable that people suffering from a medical illness that impairs their judgment and behavior are locked up as criminals. Psychiatry must forcefully lobby so that the seriously mentally ill are treated in secure hospitals staffed by physicians, nurses, and mental health professionals.
That’s right: Bring back the asylum to address this unmet medical, political, and ethical need for psychiatric patients.a A serious mental disorder must be accepted as a fault-free illness.
aTo read more about this, I recommend my March 2008 editorial, “Bring back the asylums?,” at CurrentPsychiatry.com, and Dr. George Paulson’s excellent book, Closing the asylums: Causes and consequences of the deinstitutionalization movement (Jefferson, NC: McFarland & Co. Inc; 2012).
Full integration of psychiatry into the rest of medicine remains an unmet need, despite good progress. Because almost every medical illness can cause psychiatric symptoms, DSM-5 mandates that general medical conditions be ruled out before a primary psychiatric diagnosis is made.
Along the same lines, most severely mentally ill persons suffer from medical and neurologic ailments before their first episode,5 and many die prematurely from cardiovascular causes that often are the result of unhealthy lifestyle; iatrogenic complications; and lack of primary care interventions.6 Psychiatric patients must always receive standard general medical evaluation and management, side by side with their psychiatric care.
Philanthropy for psychiatry
Philanthropic support of psychiatry is a salutary trend. Some unmet needs in psychiatry, however, require not only money but a change in attitude (such as eliminating the absurd and discriminatory stigma of mental illness), better training, and forceful political activism by all of us.
1. Licinio J, Wong ML. Launching the ‘war on mental illness’. Mol Psychiatry. 2014;19(1):1-5.
2. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427.
3. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders. The majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
4. Cuthbert BN. The RDoC framework: facilitating transition from ICD/OSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28-35.
5. Sørensen HJ, Nielsen PR, Benros ME, et al. Somatic diseases and conditions before the first diagnosis of schizophrenia: a nationwide population-based cohort study in more than 900000 individuals [published online July 25, 2014]. Schizophr Bull. doi: 10.1093/schbul/sbu110.
6. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
Recent examples come quickly to mind:
• $100 million from the Lieber family to fund the Lieber Institute for Brain Development at Johns Hopkins University
• $300 million from Microsoft co-founder Paul G. Allen to create the Allen Institute for Brain Science
• $650 million from Ted Stanley for the Stanley Center at the Broad Institute.
Such generosity is cause for celebration by psychiatrists and their long-suffering patients who are disabled by a brain disorder. Private money supplements research funding by the National Institutes of Health and will bolster the war against mental illness,1 which costs >$300 billion annually (Box,2page 12).
Although philanthropy will help, many needs in psychiatry are unmet, and not all can be addressed with money. Consider a number of areas of need.
Unmet clinical needs
Models of disease. Psychiatry is in desperate need of an objective, valid diagnostic system that transcends the DSM model of symptom clusters. The Research Domain Criteria3 represents the effort to find an alternative. To achieve that goal, it’s necessary to identify biomarkers and establish their utility—a task that requires a huge amount of funding.
Therapeutics. Clinicians are hungry for innovative, safe pharmaceuticals and non-drug treatments that modify disease, not just alleviate symptoms. Obsessive-compulsive disorder always has lacked such therapies; so have dementia, schizophrenia, personality disorders, dissociative disorders, and sexual pathologies. In fact, >80% of DSM disorders do not have an FDA-approved, evidence-based treatment,4 and many available medications are only partially efficacious, poorly tolerated, or unsafe.
There are more unmet needs in therapeutics:
• Development of promising non-drug therapies, such as neuromodulation, proceeds slowly.
• Research into neurobiological mechanisms of psychotherapy is in its infancy.
• A foolproof method to monitor adherence does not exist, and countless patients relapse needlessly and deteriorate functionally.
• Effective, evidence-based rehabilitation for serious psychiatric disorders is used narrowly and vastly underfunded.
• Insurers continue to thumb their nose at laws that require parity for treating mental illness—thus impeding access to, delaying, or truncating psychiatric care.
Unmet scientific needs
Translational investigators. Despite increased funding for basic neuroscientific study and breathtaking discoveries in animal molecular neurobiology, a trickle of findings has been applied to clinical medicine. This translational gap has many causes, including a shortage of translational neuroscientists (MD-PhD psychiatrists and neurologists), insufficient long-term funding to develop such clinician-researchers, and complex regulatory oversight of human research.
Stalled progress in drug development. Development of novel-mechanism therapeutics for brain disorders is languishing. Some pharmaceutical manufacturers have abandoned the development of drugs that act on the CNS in favor of less complex, more lucrative areas such as oncology and cardiology; others have reduced their investment in CNS products. Developing treatments for knotty disorders of the most complex structure in the known universe requires mammoth investment. Why are stakeholders bailing out on the greatest challenge for science and medicine for easier endeavors?
Discovering new genes for every devastating neuropsychiatric syndrome, such as schizophrenia, is cause for celebration, but the champagne won’t flow until the coding of every gene is unscrambled so that specific biological interventions can be developed. The cost of the chase might be orders of magnitude greater than what is invested in research today. Conceptualizing new models of brain disorders is a critical part of scientific progress and an antidote to the inertia of perpetual group-think. Depression, for example, is being reconceptualized as a disorder of impaired neuroplasticity and neurotropic deficiency, rather than a shortage of serotonin and norepinephrine. Rapid reversal of severe depression to euthymia—in 1 or 2 hours—with IV ketamine shattered the dogma that depression takes weeks to lift, and is ushering in unprecedented new thinking and models likely to revolutionize treatment of severe depression. We need such breakthroughs for other psychiatric brain disorders.
Unmet professional and sociopolitical needs
Broadening of training. Psychiatrists have focused on the mind but insufficiently attended to the biology of the brain. For psychiatry to rise to the next level as a medical specialty and brain discipline, training must incorporate more neurology than it does now. The converse is true in neurology.
Hospitalization not incarceration. It is unconscionable that people suffering from a medical illness that impairs their judgment and behavior are locked up as criminals. Psychiatry must forcefully lobby so that the seriously mentally ill are treated in secure hospitals staffed by physicians, nurses, and mental health professionals.
That’s right: Bring back the asylum to address this unmet medical, political, and ethical need for psychiatric patients.a A serious mental disorder must be accepted as a fault-free illness.
aTo read more about this, I recommend my March 2008 editorial, “Bring back the asylums?,” at CurrentPsychiatry.com, and Dr. George Paulson’s excellent book, Closing the asylums: Causes and consequences of the deinstitutionalization movement (Jefferson, NC: McFarland & Co. Inc; 2012).
Full integration of psychiatry into the rest of medicine remains an unmet need, despite good progress. Because almost every medical illness can cause psychiatric symptoms, DSM-5 mandates that general medical conditions be ruled out before a primary psychiatric diagnosis is made.
Along the same lines, most severely mentally ill persons suffer from medical and neurologic ailments before their first episode,5 and many die prematurely from cardiovascular causes that often are the result of unhealthy lifestyle; iatrogenic complications; and lack of primary care interventions.6 Psychiatric patients must always receive standard general medical evaluation and management, side by side with their psychiatric care.
Philanthropy for psychiatry
Philanthropic support of psychiatry is a salutary trend. Some unmet needs in psychiatry, however, require not only money but a change in attitude (such as eliminating the absurd and discriminatory stigma of mental illness), better training, and forceful political activism by all of us.
Recent examples come quickly to mind:
• $100 million from the Lieber family to fund the Lieber Institute for Brain Development at Johns Hopkins University
• $300 million from Microsoft co-founder Paul G. Allen to create the Allen Institute for Brain Science
• $650 million from Ted Stanley for the Stanley Center at the Broad Institute.
Such generosity is cause for celebration by psychiatrists and their long-suffering patients who are disabled by a brain disorder. Private money supplements research funding by the National Institutes of Health and will bolster the war against mental illness,1 which costs >$300 billion annually (Box,2page 12).
Although philanthropy will help, many needs in psychiatry are unmet, and not all can be addressed with money. Consider a number of areas of need.
Unmet clinical needs
Models of disease. Psychiatry is in desperate need of an objective, valid diagnostic system that transcends the DSM model of symptom clusters. The Research Domain Criteria3 represents the effort to find an alternative. To achieve that goal, it’s necessary to identify biomarkers and establish their utility—a task that requires a huge amount of funding.
Therapeutics. Clinicians are hungry for innovative, safe pharmaceuticals and non-drug treatments that modify disease, not just alleviate symptoms. Obsessive-compulsive disorder always has lacked such therapies; so have dementia, schizophrenia, personality disorders, dissociative disorders, and sexual pathologies. In fact, >80% of DSM disorders do not have an FDA-approved, evidence-based treatment,4 and many available medications are only partially efficacious, poorly tolerated, or unsafe.
There are more unmet needs in therapeutics:
• Development of promising non-drug therapies, such as neuromodulation, proceeds slowly.
• Research into neurobiological mechanisms of psychotherapy is in its infancy.
• A foolproof method to monitor adherence does not exist, and countless patients relapse needlessly and deteriorate functionally.
• Effective, evidence-based rehabilitation for serious psychiatric disorders is used narrowly and vastly underfunded.
• Insurers continue to thumb their nose at laws that require parity for treating mental illness—thus impeding access to, delaying, or truncating psychiatric care.
Unmet scientific needs
Translational investigators. Despite increased funding for basic neuroscientific study and breathtaking discoveries in animal molecular neurobiology, a trickle of findings has been applied to clinical medicine. This translational gap has many causes, including a shortage of translational neuroscientists (MD-PhD psychiatrists and neurologists), insufficient long-term funding to develop such clinician-researchers, and complex regulatory oversight of human research.
Stalled progress in drug development. Development of novel-mechanism therapeutics for brain disorders is languishing. Some pharmaceutical manufacturers have abandoned the development of drugs that act on the CNS in favor of less complex, more lucrative areas such as oncology and cardiology; others have reduced their investment in CNS products. Developing treatments for knotty disorders of the most complex structure in the known universe requires mammoth investment. Why are stakeholders bailing out on the greatest challenge for science and medicine for easier endeavors?
Discovering new genes for every devastating neuropsychiatric syndrome, such as schizophrenia, is cause for celebration, but the champagne won’t flow until the coding of every gene is unscrambled so that specific biological interventions can be developed. The cost of the chase might be orders of magnitude greater than what is invested in research today. Conceptualizing new models of brain disorders is a critical part of scientific progress and an antidote to the inertia of perpetual group-think. Depression, for example, is being reconceptualized as a disorder of impaired neuroplasticity and neurotropic deficiency, rather than a shortage of serotonin and norepinephrine. Rapid reversal of severe depression to euthymia—in 1 or 2 hours—with IV ketamine shattered the dogma that depression takes weeks to lift, and is ushering in unprecedented new thinking and models likely to revolutionize treatment of severe depression. We need such breakthroughs for other psychiatric brain disorders.
Unmet professional and sociopolitical needs
Broadening of training. Psychiatrists have focused on the mind but insufficiently attended to the biology of the brain. For psychiatry to rise to the next level as a medical specialty and brain discipline, training must incorporate more neurology than it does now. The converse is true in neurology.
Hospitalization not incarceration. It is unconscionable that people suffering from a medical illness that impairs their judgment and behavior are locked up as criminals. Psychiatry must forcefully lobby so that the seriously mentally ill are treated in secure hospitals staffed by physicians, nurses, and mental health professionals.
That’s right: Bring back the asylum to address this unmet medical, political, and ethical need for psychiatric patients.a A serious mental disorder must be accepted as a fault-free illness.
aTo read more about this, I recommend my March 2008 editorial, “Bring back the asylums?,” at CurrentPsychiatry.com, and Dr. George Paulson’s excellent book, Closing the asylums: Causes and consequences of the deinstitutionalization movement (Jefferson, NC: McFarland & Co. Inc; 2012).
Full integration of psychiatry into the rest of medicine remains an unmet need, despite good progress. Because almost every medical illness can cause psychiatric symptoms, DSM-5 mandates that general medical conditions be ruled out before a primary psychiatric diagnosis is made.
Along the same lines, most severely mentally ill persons suffer from medical and neurologic ailments before their first episode,5 and many die prematurely from cardiovascular causes that often are the result of unhealthy lifestyle; iatrogenic complications; and lack of primary care interventions.6 Psychiatric patients must always receive standard general medical evaluation and management, side by side with their psychiatric care.
Philanthropy for psychiatry
Philanthropic support of psychiatry is a salutary trend. Some unmet needs in psychiatry, however, require not only money but a change in attitude (such as eliminating the absurd and discriminatory stigma of mental illness), better training, and forceful political activism by all of us.
1. Licinio J, Wong ML. Launching the ‘war on mental illness’. Mol Psychiatry. 2014;19(1):1-5.
2. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427.
3. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders. The majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
4. Cuthbert BN. The RDoC framework: facilitating transition from ICD/OSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28-35.
5. Sørensen HJ, Nielsen PR, Benros ME, et al. Somatic diseases and conditions before the first diagnosis of schizophrenia: a nationwide population-based cohort study in more than 900000 individuals [published online July 25, 2014]. Schizophr Bull. doi: 10.1093/schbul/sbu110.
6. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
1. Licinio J, Wong ML. Launching the ‘war on mental illness’. Mol Psychiatry. 2014;19(1):1-5.
2. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427.
3. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders. The majority of psychiatric diagnoses have no approved drug. Asian J Psychiatr. 2009;2(1):29-36.
4. Cuthbert BN. The RDoC framework: facilitating transition from ICD/OSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28-35.
5. Sørensen HJ, Nielsen PR, Benros ME, et al. Somatic diseases and conditions before the first diagnosis of schizophrenia: a nationwide population-based cohort study in more than 900000 individuals [published online July 25, 2014]. Schizophr Bull. doi: 10.1093/schbul/sbu110.
6. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.