User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
How primary care physicians can hit the mark on contraceptive counseling
SAN FRANCISCO – When it comes to counseling women about contraceptive care and family planning, many primary care physicians fall short, according to Christine Dehlendorf, MD.
“Providing contraceptive care and family planning care is part of what we do as preventive care for women of reproductive age, but we don’t always do it as often as we should,” Dr. Dehlendorf said at the UCSF Annual Advances in Internal Medicine meeting. “We don’t often take the initiative of making sure that women’s contraceptive needs are being met at all visits when we engage with them.”
“Many of you might think that’s okay, because we’re only talking about it when women come in for family planning visits,” said Dr. Dehlendorf of the departments of family and community medicine and obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco. “In fact, this is something that is an ongoing need for women. We should be using every opportunity to make sure we’re helping them, even if it’s just by initiating the conversation and providing referrals as appropriate.”
Some might think that the best approach to contraceptive decision making involves recommending the most highly effective methods, such as long-acting reversible contraceptives, which have a risk of failure that’s 20 times lower than that of short-acting hormonal methods. Examples of counseling approaches used in that context include tiered effectiveness, in which the clinician presents methods in order of effectiveness, and motivational interviewing, a counseling approach developed for use in people with addictions.
However, Dr. Dehlendorf said she prefers to view contraceptive choice as a decision driven by women’s values and preferences.
“We know that effectiveness is very important to women, but we also know that things like side-effect profile and control over the [contraceptive] method are important as well,” she explained. “These strong features reflect women’s different assessments of the desirability of different outcomes associated with contraceptive use.
“For example, some women think that the possibility of amenorrhea with progestin IUDs or Depo-Provera is a great thing, and other people think it would be horrible,” Dr. Dehlendorf noted. “It has nothing to do with safety or effectiveness; this has to do with how women view that characteristic in the context of their own values and preferences.”
The differential value that women may place on contraceptive effectiveness also relates to different perceptions they have of the possibility of an unplanned pregnancy in their lives, and how important that is to avoid.
“In general, in the public health and clinical dialogue, the idea is that an unplanned pregnancy is an inherently bad pregnancy,” Dr. Dehlendorf said. “The conventional dialogue involves the notion of intentions and plans: Are they intending or planning to get pregnant? Intentions being timing-based ideas of when to get pregnant, and plans being concrete steps they take to act on those intentions.”
However, an emerging body of literature has shown that there are other dimensions of how women think about the possibility of pregnancy in their lives that are distinct from intentions and plans, she said, such as desire, which is how strongly they intend or don’t intend to have a pregnancy, and feelings, their emotional orientation around the potential for a pregnancy in their life.
“You could argue that this is overcomplicating things, but that is not true,” Dr. Dehlendorf said. “Plans, intentions, desires, and feelings are all different concepts, and some of them are more or less relevant to individual women, and they don’t always align with each other. That’s very much in conflict with how we conventionally talk about pregnancy in women’s lives.”
Examples from qualitative research have fleshed this out.
In one recently published study, researchers led by Jenny A. Higgins, PhD, of the department of gender and women’s studies at the University of Wisconsin–Madison, asked women about their views on IUDs. One woman said, “I guess one of the reasons that I haven’t gotten an IUD yet is like, I don’t know, having one kid already and being in a long-term committed relationship, it takes the element of surprise out of when we would have our next kid, which I kind of want. I’m in that weird position. I just don’t want to put too much thought and planning into when I have my next kid.”
Other women view an unplanned pregnancy as emotionally welcome.
In a longitudinal study that measured prospective pregnancy intentions and feelings among 403 women in Austin, Tex., one woman said, “Another pregnancy is definitely not the right path for me, and I’m being very careful with birth control. But if I somehow ended up pregnant, would I embrace it and think it’s for the best? Absolutely.”
Another study participant said, “I don’t want more kids and was hoping to get my tubes tied. We can’t afford another one. But if it happened, I’d still be happy. I’d be really excited. We’d rise to the occasion. Nothing would really change” (Soc Sci Med. 2015 May;132:149-155).
According to Dr. Dehlendorf, the lesson from such studies is that women are going to assess the importance of the efficacy of their contraceptive method differently, depending on how important it is for them to prevent an unintended pregnancy.
“They’re not going to make a decision about effectiveness the way we as clinicians might think that they should,” she said. “So, assuming that highly effective methods are the best methods for all women because of their effectiveness ignores the variability in preferences, and it also doesn’t take into account women’s strong feelings about other aspects of contraceptive use, such as bleeding profiles and control over their methods.”
Shared decision making may be the best way to help women make a choice based on their preferences. In a study that Dr. Dehlendorf and her associates conducted in 348 women who were seen for contraceptive care in the San Francisco Bay area, two habits were associated with contraceptive continuation: investing in the beginning, and eliciting the patient’s perspective (Am J Obstet Gynecol. 2016 Jul;215[1]:78.e1-9). “Investing in the beginning consists of greeting the patient warmly, making small talk, and treating the patient as a person,” she said. “That was the most highly influential aspect of the interaction. Building rapport and decision support helps women choose a method that’s a good fit for them.
“We also know that women like this method of counseling, but this approach might not be for everyone,” Dr. Dehlendorf cautioned. “Some women don’t want your suggestions, even if it’s grounded in their preferences. They just want to get the method that they came in for. The right thing is to acknowledge that, but you can also ask women if they want to hear about other methods, because some women might not know about all of their options.”
Dr. Dehlendorf reported having no relevant financial disclosures.
SAN FRANCISCO – When it comes to counseling women about contraceptive care and family planning, many primary care physicians fall short, according to Christine Dehlendorf, MD.
“Providing contraceptive care and family planning care is part of what we do as preventive care for women of reproductive age, but we don’t always do it as often as we should,” Dr. Dehlendorf said at the UCSF Annual Advances in Internal Medicine meeting. “We don’t often take the initiative of making sure that women’s contraceptive needs are being met at all visits when we engage with them.”
“Many of you might think that’s okay, because we’re only talking about it when women come in for family planning visits,” said Dr. Dehlendorf of the departments of family and community medicine and obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco. “In fact, this is something that is an ongoing need for women. We should be using every opportunity to make sure we’re helping them, even if it’s just by initiating the conversation and providing referrals as appropriate.”
Some might think that the best approach to contraceptive decision making involves recommending the most highly effective methods, such as long-acting reversible contraceptives, which have a risk of failure that’s 20 times lower than that of short-acting hormonal methods. Examples of counseling approaches used in that context include tiered effectiveness, in which the clinician presents methods in order of effectiveness, and motivational interviewing, a counseling approach developed for use in people with addictions.
However, Dr. Dehlendorf said she prefers to view contraceptive choice as a decision driven by women’s values and preferences.
“We know that effectiveness is very important to women, but we also know that things like side-effect profile and control over the [contraceptive] method are important as well,” she explained. “These strong features reflect women’s different assessments of the desirability of different outcomes associated with contraceptive use.
“For example, some women think that the possibility of amenorrhea with progestin IUDs or Depo-Provera is a great thing, and other people think it would be horrible,” Dr. Dehlendorf noted. “It has nothing to do with safety or effectiveness; this has to do with how women view that characteristic in the context of their own values and preferences.”
The differential value that women may place on contraceptive effectiveness also relates to different perceptions they have of the possibility of an unplanned pregnancy in their lives, and how important that is to avoid.
“In general, in the public health and clinical dialogue, the idea is that an unplanned pregnancy is an inherently bad pregnancy,” Dr. Dehlendorf said. “The conventional dialogue involves the notion of intentions and plans: Are they intending or planning to get pregnant? Intentions being timing-based ideas of when to get pregnant, and plans being concrete steps they take to act on those intentions.”
However, an emerging body of literature has shown that there are other dimensions of how women think about the possibility of pregnancy in their lives that are distinct from intentions and plans, she said, such as desire, which is how strongly they intend or don’t intend to have a pregnancy, and feelings, their emotional orientation around the potential for a pregnancy in their life.
“You could argue that this is overcomplicating things, but that is not true,” Dr. Dehlendorf said. “Plans, intentions, desires, and feelings are all different concepts, and some of them are more or less relevant to individual women, and they don’t always align with each other. That’s very much in conflict with how we conventionally talk about pregnancy in women’s lives.”
Examples from qualitative research have fleshed this out.
In one recently published study, researchers led by Jenny A. Higgins, PhD, of the department of gender and women’s studies at the University of Wisconsin–Madison, asked women about their views on IUDs. One woman said, “I guess one of the reasons that I haven’t gotten an IUD yet is like, I don’t know, having one kid already and being in a long-term committed relationship, it takes the element of surprise out of when we would have our next kid, which I kind of want. I’m in that weird position. I just don’t want to put too much thought and planning into when I have my next kid.”
Other women view an unplanned pregnancy as emotionally welcome.
In a longitudinal study that measured prospective pregnancy intentions and feelings among 403 women in Austin, Tex., one woman said, “Another pregnancy is definitely not the right path for me, and I’m being very careful with birth control. But if I somehow ended up pregnant, would I embrace it and think it’s for the best? Absolutely.”
Another study participant said, “I don’t want more kids and was hoping to get my tubes tied. We can’t afford another one. But if it happened, I’d still be happy. I’d be really excited. We’d rise to the occasion. Nothing would really change” (Soc Sci Med. 2015 May;132:149-155).
According to Dr. Dehlendorf, the lesson from such studies is that women are going to assess the importance of the efficacy of their contraceptive method differently, depending on how important it is for them to prevent an unintended pregnancy.
“They’re not going to make a decision about effectiveness the way we as clinicians might think that they should,” she said. “So, assuming that highly effective methods are the best methods for all women because of their effectiveness ignores the variability in preferences, and it also doesn’t take into account women’s strong feelings about other aspects of contraceptive use, such as bleeding profiles and control over their methods.”
Shared decision making may be the best way to help women make a choice based on their preferences. In a study that Dr. Dehlendorf and her associates conducted in 348 women who were seen for contraceptive care in the San Francisco Bay area, two habits were associated with contraceptive continuation: investing in the beginning, and eliciting the patient’s perspective (Am J Obstet Gynecol. 2016 Jul;215[1]:78.e1-9). “Investing in the beginning consists of greeting the patient warmly, making small talk, and treating the patient as a person,” she said. “That was the most highly influential aspect of the interaction. Building rapport and decision support helps women choose a method that’s a good fit for them.
“We also know that women like this method of counseling, but this approach might not be for everyone,” Dr. Dehlendorf cautioned. “Some women don’t want your suggestions, even if it’s grounded in their preferences. They just want to get the method that they came in for. The right thing is to acknowledge that, but you can also ask women if they want to hear about other methods, because some women might not know about all of their options.”
Dr. Dehlendorf reported having no relevant financial disclosures.
SAN FRANCISCO – When it comes to counseling women about contraceptive care and family planning, many primary care physicians fall short, according to Christine Dehlendorf, MD.
“Providing contraceptive care and family planning care is part of what we do as preventive care for women of reproductive age, but we don’t always do it as often as we should,” Dr. Dehlendorf said at the UCSF Annual Advances in Internal Medicine meeting. “We don’t often take the initiative of making sure that women’s contraceptive needs are being met at all visits when we engage with them.”
“Many of you might think that’s okay, because we’re only talking about it when women come in for family planning visits,” said Dr. Dehlendorf of the departments of family and community medicine and obstetrics, gynecology, and reproductive sciences at the University of California, San Francisco. “In fact, this is something that is an ongoing need for women. We should be using every opportunity to make sure we’re helping them, even if it’s just by initiating the conversation and providing referrals as appropriate.”
Some might think that the best approach to contraceptive decision making involves recommending the most highly effective methods, such as long-acting reversible contraceptives, which have a risk of failure that’s 20 times lower than that of short-acting hormonal methods. Examples of counseling approaches used in that context include tiered effectiveness, in which the clinician presents methods in order of effectiveness, and motivational interviewing, a counseling approach developed for use in people with addictions.
However, Dr. Dehlendorf said she prefers to view contraceptive choice as a decision driven by women’s values and preferences.
“We know that effectiveness is very important to women, but we also know that things like side-effect profile and control over the [contraceptive] method are important as well,” she explained. “These strong features reflect women’s different assessments of the desirability of different outcomes associated with contraceptive use.
“For example, some women think that the possibility of amenorrhea with progestin IUDs or Depo-Provera is a great thing, and other people think it would be horrible,” Dr. Dehlendorf noted. “It has nothing to do with safety or effectiveness; this has to do with how women view that characteristic in the context of their own values and preferences.”
The differential value that women may place on contraceptive effectiveness also relates to different perceptions they have of the possibility of an unplanned pregnancy in their lives, and how important that is to avoid.
“In general, in the public health and clinical dialogue, the idea is that an unplanned pregnancy is an inherently bad pregnancy,” Dr. Dehlendorf said. “The conventional dialogue involves the notion of intentions and plans: Are they intending or planning to get pregnant? Intentions being timing-based ideas of when to get pregnant, and plans being concrete steps they take to act on those intentions.”
However, an emerging body of literature has shown that there are other dimensions of how women think about the possibility of pregnancy in their lives that are distinct from intentions and plans, she said, such as desire, which is how strongly they intend or don’t intend to have a pregnancy, and feelings, their emotional orientation around the potential for a pregnancy in their life.
“You could argue that this is overcomplicating things, but that is not true,” Dr. Dehlendorf said. “Plans, intentions, desires, and feelings are all different concepts, and some of them are more or less relevant to individual women, and they don’t always align with each other. That’s very much in conflict with how we conventionally talk about pregnancy in women’s lives.”
Examples from qualitative research have fleshed this out.
In one recently published study, researchers led by Jenny A. Higgins, PhD, of the department of gender and women’s studies at the University of Wisconsin–Madison, asked women about their views on IUDs. One woman said, “I guess one of the reasons that I haven’t gotten an IUD yet is like, I don’t know, having one kid already and being in a long-term committed relationship, it takes the element of surprise out of when we would have our next kid, which I kind of want. I’m in that weird position. I just don’t want to put too much thought and planning into when I have my next kid.”
Other women view an unplanned pregnancy as emotionally welcome.
In a longitudinal study that measured prospective pregnancy intentions and feelings among 403 women in Austin, Tex., one woman said, “Another pregnancy is definitely not the right path for me, and I’m being very careful with birth control. But if I somehow ended up pregnant, would I embrace it and think it’s for the best? Absolutely.”
Another study participant said, “I don’t want more kids and was hoping to get my tubes tied. We can’t afford another one. But if it happened, I’d still be happy. I’d be really excited. We’d rise to the occasion. Nothing would really change” (Soc Sci Med. 2015 May;132:149-155).
According to Dr. Dehlendorf, the lesson from such studies is that women are going to assess the importance of the efficacy of their contraceptive method differently, depending on how important it is for them to prevent an unintended pregnancy.
“They’re not going to make a decision about effectiveness the way we as clinicians might think that they should,” she said. “So, assuming that highly effective methods are the best methods for all women because of their effectiveness ignores the variability in preferences, and it also doesn’t take into account women’s strong feelings about other aspects of contraceptive use, such as bleeding profiles and control over their methods.”
Shared decision making may be the best way to help women make a choice based on their preferences. In a study that Dr. Dehlendorf and her associates conducted in 348 women who were seen for contraceptive care in the San Francisco Bay area, two habits were associated with contraceptive continuation: investing in the beginning, and eliciting the patient’s perspective (Am J Obstet Gynecol. 2016 Jul;215[1]:78.e1-9). “Investing in the beginning consists of greeting the patient warmly, making small talk, and treating the patient as a person,” she said. “That was the most highly influential aspect of the interaction. Building rapport and decision support helps women choose a method that’s a good fit for them.
“We also know that women like this method of counseling, but this approach might not be for everyone,” Dr. Dehlendorf cautioned. “Some women don’t want your suggestions, even if it’s grounded in their preferences. They just want to get the method that they came in for. The right thing is to acknowledge that, but you can also ask women if they want to hear about other methods, because some women might not know about all of their options.”
Dr. Dehlendorf reported having no relevant financial disclosures.
AT THE ANNUAL ADVANCES IN INTERNAL MEDICINE
Optimal age for cardiovascular disease screening remains elusive
SAN FRANCISCO – In the United States, deaths from cardiovascular disease fell by about 50% in men and women between 1980 and 2000, and fell an additional 31% between 2000 and 2010, according to Robert Baron, MD.
That’s the good news, driven largely by the introduction of statin therapy and by risk-factor modifications. The not-so-great news? The optimal screening age for CVD risk remains elusive.
“We’ve been arguing about this for at least 30 years,” he said at the UCSF Annual Advances in Internal Medicine.
The 2013 American College of Cardiology/American Heart Association Guideline on the Assessment of Cardiovascular Risk recommends screening at age 21 years to identify those with an LDL cholesterol level greater than 190 mg/dL, while a more recent draft recommendation statement from the United States Preventive Services Task Force (USPSTF) recommends screening at age 35 in men and 45 in women for most cases, or at age 20 in people deemed to be at increased risk. Both sets of guidelines recommend the use of statins in patients with atherosclerotic cardiovascular disease, and LDL of 190 mg/dL or greater, and/or diabetes.
“In essence, the USPSTF guidelines are saying definitely use statins for primary prevention in high-risk people; they’re just creating a little more gray area as to what exactly constitutes high risk,” said Dr. Baron, an internist who is associate dean at UCSF Medical Center, San Francisco. “The question is, how do you draw the line? I would argue that depends on the patient preference.”
In a recently published analysis, researchers set out to determine how the USPSTF guidelines compare with the ACC/AHA guidelines in terms of the proportion of U.S. adults potentially treated (JAMA. 2017 Apr 18; 317[15]:1563-7). After using estimates based on data from 3,416 participants in the 2009-2014 National Health and Nutrition Examination Survey, the researchers found that USPSTF recommendations would be associated with statin initiation in 16% of U.S. adults aged 40-75 years without prior CVD, compared with 24% according to the ACC/AHA guidelines.
Among those adults for whom therapy would no longer be recommended under the USPSTF recommendations, 55% are aged 40-59 years with a mean 30-year cardiovascular risk exceeding 30%, and 28% have diabetes. According to Dr. Baron, the comparison “reinforces the primary prevention message. It gives the patient a bit more flexibility.”
After starting patients on a statin, the best available evidence recommends monitoring adherence but not treating to a specific LDL goal, he said. Statins should not be used in patients older than age 75 unless they have existing ASCVD. The addition of other lipid-modifying drugs is generally not recommended, but it may be needed in patients who demonstrate intolerance to statins. Patients should also avoid using NSAIDS, which raise CVD risk, and instead use medications that lower it, such as aspirin, he said.
Dr. Baron reported having no relevant financial disclosures.
SAN FRANCISCO – In the United States, deaths from cardiovascular disease fell by about 50% in men and women between 1980 and 2000, and fell an additional 31% between 2000 and 2010, according to Robert Baron, MD.
That’s the good news, driven largely by the introduction of statin therapy and by risk-factor modifications. The not-so-great news? The optimal screening age for CVD risk remains elusive.
“We’ve been arguing about this for at least 30 years,” he said at the UCSF Annual Advances in Internal Medicine.
The 2013 American College of Cardiology/American Heart Association Guideline on the Assessment of Cardiovascular Risk recommends screening at age 21 years to identify those with an LDL cholesterol level greater than 190 mg/dL, while a more recent draft recommendation statement from the United States Preventive Services Task Force (USPSTF) recommends screening at age 35 in men and 45 in women for most cases, or at age 20 in people deemed to be at increased risk. Both sets of guidelines recommend the use of statins in patients with atherosclerotic cardiovascular disease, and LDL of 190 mg/dL or greater, and/or diabetes.
“In essence, the USPSTF guidelines are saying definitely use statins for primary prevention in high-risk people; they’re just creating a little more gray area as to what exactly constitutes high risk,” said Dr. Baron, an internist who is associate dean at UCSF Medical Center, San Francisco. “The question is, how do you draw the line? I would argue that depends on the patient preference.”
In a recently published analysis, researchers set out to determine how the USPSTF guidelines compare with the ACC/AHA guidelines in terms of the proportion of U.S. adults potentially treated (JAMA. 2017 Apr 18; 317[15]:1563-7). After using estimates based on data from 3,416 participants in the 2009-2014 National Health and Nutrition Examination Survey, the researchers found that USPSTF recommendations would be associated with statin initiation in 16% of U.S. adults aged 40-75 years without prior CVD, compared with 24% according to the ACC/AHA guidelines.
Among those adults for whom therapy would no longer be recommended under the USPSTF recommendations, 55% are aged 40-59 years with a mean 30-year cardiovascular risk exceeding 30%, and 28% have diabetes. According to Dr. Baron, the comparison “reinforces the primary prevention message. It gives the patient a bit more flexibility.”
After starting patients on a statin, the best available evidence recommends monitoring adherence but not treating to a specific LDL goal, he said. Statins should not be used in patients older than age 75 unless they have existing ASCVD. The addition of other lipid-modifying drugs is generally not recommended, but it may be needed in patients who demonstrate intolerance to statins. Patients should also avoid using NSAIDS, which raise CVD risk, and instead use medications that lower it, such as aspirin, he said.
Dr. Baron reported having no relevant financial disclosures.
SAN FRANCISCO – In the United States, deaths from cardiovascular disease fell by about 50% in men and women between 1980 and 2000, and fell an additional 31% between 2000 and 2010, according to Robert Baron, MD.
That’s the good news, driven largely by the introduction of statin therapy and by risk-factor modifications. The not-so-great news? The optimal screening age for CVD risk remains elusive.
“We’ve been arguing about this for at least 30 years,” he said at the UCSF Annual Advances in Internal Medicine.
The 2013 American College of Cardiology/American Heart Association Guideline on the Assessment of Cardiovascular Risk recommends screening at age 21 years to identify those with an LDL cholesterol level greater than 190 mg/dL, while a more recent draft recommendation statement from the United States Preventive Services Task Force (USPSTF) recommends screening at age 35 in men and 45 in women for most cases, or at age 20 in people deemed to be at increased risk. Both sets of guidelines recommend the use of statins in patients with atherosclerotic cardiovascular disease, and LDL of 190 mg/dL or greater, and/or diabetes.
“In essence, the USPSTF guidelines are saying definitely use statins for primary prevention in high-risk people; they’re just creating a little more gray area as to what exactly constitutes high risk,” said Dr. Baron, an internist who is associate dean at UCSF Medical Center, San Francisco. “The question is, how do you draw the line? I would argue that depends on the patient preference.”
In a recently published analysis, researchers set out to determine how the USPSTF guidelines compare with the ACC/AHA guidelines in terms of the proportion of U.S. adults potentially treated (JAMA. 2017 Apr 18; 317[15]:1563-7). After using estimates based on data from 3,416 participants in the 2009-2014 National Health and Nutrition Examination Survey, the researchers found that USPSTF recommendations would be associated with statin initiation in 16% of U.S. adults aged 40-75 years without prior CVD, compared with 24% according to the ACC/AHA guidelines.
Among those adults for whom therapy would no longer be recommended under the USPSTF recommendations, 55% are aged 40-59 years with a mean 30-year cardiovascular risk exceeding 30%, and 28% have diabetes. According to Dr. Baron, the comparison “reinforces the primary prevention message. It gives the patient a bit more flexibility.”
After starting patients on a statin, the best available evidence recommends monitoring adherence but not treating to a specific LDL goal, he said. Statins should not be used in patients older than age 75 unless they have existing ASCVD. The addition of other lipid-modifying drugs is generally not recommended, but it may be needed in patients who demonstrate intolerance to statins. Patients should also avoid using NSAIDS, which raise CVD risk, and instead use medications that lower it, such as aspirin, he said.
Dr. Baron reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM THE ANNUAL ADVANCES IN INTERNAL MEDICINE
Study spotlights pediatric dermatology workplace trends
CHICAGO – Geographic maldistribution of trained pediatric dermatologists and long wait times for new-patient appointments are two key hurdles impacting patient access to pediatric dermatology care, according to a survey of workforce trends.
“There are large areas of the country that don’t have any pediatric dermatologists, which influences how we can train dermatologists, pediatricians, and medical students who want to do pediatric dermatology,” study author Brea Prindaville, MD, said in an interview at the World Congress for Pediatric Dermatology. “We’re trying to keep an eye on how that’s going, and how we can improve not only the geographic distribution, but also the numbers that we have in the workforce.”
In a project spearheaded by the Society for Pediatric Dermatology (SPD) workforce committee, Dr. Prindaville of Children’s Mercy-Kansas City (Mo.) and her associates emailed a nine-question survey to the 484 SPD members in the United States to determine practice location, number and types of patients seen, wait times, and association with advance practice providers between November and December of 2016. In all, 146 surveys were completed, for a response rate of 30%. Of these, 75% were from board-certified pediatric dermatologists. The majority of survey respondents were practicing in and around large cities, while 60% of all respondents and 68% of board-certified respondents were practicing full-time, seeing pediatric patients exclusively. An additional 30% were practicing part-time or also seeing adults. Board-certified pediatric dermatologists saw an average of 80 pediatric patients per week.
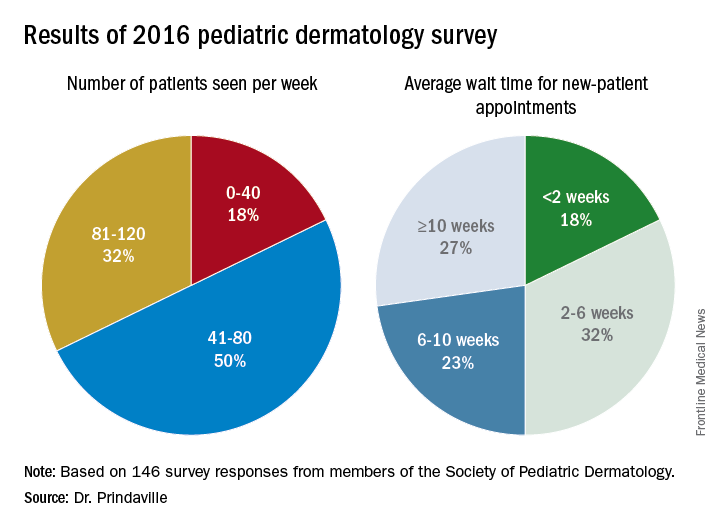
Only 10% of survey respondents did not accept Medicaid, and about 50% of patients seen by board-certified pediatric dermatologists were insured by Medicaid. In addition, about half of respondents worked with one to three advanced practice providers.
Dr. Prindaville, who helped conduct the study during her pediatric dermatology fellowship at the University of Massachusetts, Worcester, acknowledged certain limitations of the analysis, including its low response rate, the limited number of survey questions, and the question design. Going forward, she and her associates hope to keep a pulse on workplace trends by surveying SPD members annually, perhaps with membership renewal.
The survey was supported by the SPD. Dr. Prindaville reported having no relevant financial disclosures.
CHICAGO – Geographic maldistribution of trained pediatric dermatologists and long wait times for new-patient appointments are two key hurdles impacting patient access to pediatric dermatology care, according to a survey of workforce trends.
“There are large areas of the country that don’t have any pediatric dermatologists, which influences how we can train dermatologists, pediatricians, and medical students who want to do pediatric dermatology,” study author Brea Prindaville, MD, said in an interview at the World Congress for Pediatric Dermatology. “We’re trying to keep an eye on how that’s going, and how we can improve not only the geographic distribution, but also the numbers that we have in the workforce.”
In a project spearheaded by the Society for Pediatric Dermatology (SPD) workforce committee, Dr. Prindaville of Children’s Mercy-Kansas City (Mo.) and her associates emailed a nine-question survey to the 484 SPD members in the United States to determine practice location, number and types of patients seen, wait times, and association with advance practice providers between November and December of 2016. In all, 146 surveys were completed, for a response rate of 30%. Of these, 75% were from board-certified pediatric dermatologists. The majority of survey respondents were practicing in and around large cities, while 60% of all respondents and 68% of board-certified respondents were practicing full-time, seeing pediatric patients exclusively. An additional 30% were practicing part-time or also seeing adults. Board-certified pediatric dermatologists saw an average of 80 pediatric patients per week.

Only 10% of survey respondents did not accept Medicaid, and about 50% of patients seen by board-certified pediatric dermatologists were insured by Medicaid. In addition, about half of respondents worked with one to three advanced practice providers.
Dr. Prindaville, who helped conduct the study during her pediatric dermatology fellowship at the University of Massachusetts, Worcester, acknowledged certain limitations of the analysis, including its low response rate, the limited number of survey questions, and the question design. Going forward, she and her associates hope to keep a pulse on workplace trends by surveying SPD members annually, perhaps with membership renewal.
The survey was supported by the SPD. Dr. Prindaville reported having no relevant financial disclosures.
CHICAGO – Geographic maldistribution of trained pediatric dermatologists and long wait times for new-patient appointments are two key hurdles impacting patient access to pediatric dermatology care, according to a survey of workforce trends.
“There are large areas of the country that don’t have any pediatric dermatologists, which influences how we can train dermatologists, pediatricians, and medical students who want to do pediatric dermatology,” study author Brea Prindaville, MD, said in an interview at the World Congress for Pediatric Dermatology. “We’re trying to keep an eye on how that’s going, and how we can improve not only the geographic distribution, but also the numbers that we have in the workforce.”
In a project spearheaded by the Society for Pediatric Dermatology (SPD) workforce committee, Dr. Prindaville of Children’s Mercy-Kansas City (Mo.) and her associates emailed a nine-question survey to the 484 SPD members in the United States to determine practice location, number and types of patients seen, wait times, and association with advance practice providers between November and December of 2016. In all, 146 surveys were completed, for a response rate of 30%. Of these, 75% were from board-certified pediatric dermatologists. The majority of survey respondents were practicing in and around large cities, while 60% of all respondents and 68% of board-certified respondents were practicing full-time, seeing pediatric patients exclusively. An additional 30% were practicing part-time or also seeing adults. Board-certified pediatric dermatologists saw an average of 80 pediatric patients per week.

Only 10% of survey respondents did not accept Medicaid, and about 50% of patients seen by board-certified pediatric dermatologists were insured by Medicaid. In addition, about half of respondents worked with one to three advanced practice providers.
Dr. Prindaville, who helped conduct the study during her pediatric dermatology fellowship at the University of Massachusetts, Worcester, acknowledged certain limitations of the analysis, including its low response rate, the limited number of survey questions, and the question design. Going forward, she and her associates hope to keep a pulse on workplace trends by surveying SPD members annually, perhaps with membership renewal.
The survey was supported by the SPD. Dr. Prindaville reported having no relevant financial disclosures.
AT WCPD 2017
Key clinical point:
Major finding: The majority of survey respondents were practicing in and around large cities, and the average wait time for a new-patient appointment for all respondents was about 6 weeks.
Data source: Online survey completed by 146 Society for Pediatric Dermatology (SPD) members in the United States.
Disclosures: The survey was supported by the SPD. Dr. Prindaville reported having no relevant financial disclosures.
Standardization lacking in pediatric trials of atopic dermatitis
CHICAGO – There is considerable variability and poor documentation of severity assessments used for inclusion criteria and baseline severity evaluations in randomized, controlled pediatric atopic dermatitis (AD) trials, results from a systematic review showed.
“It is important for clinicians and investigators to recognize that these differences may limit our ability to reproduce trials, interpret individual studies, and compare results between studies of similar target populations for severity,” lead study author Rishi Chopra, MS, said in an interview in advance of the World Congress of Pediatric Dermatology. “Moreover, this heterogeneity should be considered when retroactively pooling results for meta-analyses of pediatric atopic dermatitis randomized, controlled trials.”
In an effort to evaluate the documentation and characterize the severity assessments used in inclusion criteria and baseline evaluations for randomized, controlled trials of pediatric AD internationally, the researchers performed a systematic review of relevant studies contained in the Cochrane Library, Embase, LILACS, GREAT, MEDLINE, and Scopus databases during 2007-2016. Inclusion criteria were RCT with a pharmacological intervention and any comparison with a control group, children, and males or females. In all, 89 studies met the inclusion/exclusion criteria. Most (70.8%) were studies of pediatric populations aged 0-17 years, and almost 17% were studies of infants aged 0-1 years. The most common target populations were mild-moderate AD (31.5%), moderate-severe AD (18.0%), or undefined (36.0%).
Mr. Chopra and his associates found that the most commonly used severity indices were Scoring AD (SCORAD) in 29.2%, Body Surface Area (BSA) in 16.9%, and global assessments in 13.4%, while the most common assessments of baseline severity were SCORAD in 43.8%, global assessments in 20.2%, Eczema Area and Severity Index in 17.9%, BSA in 14.6%, and visual itch in 13.5%. Only 85.4% of studies recorded the severity assessments used for recruiting the predefined target population and only 76.4% of studies documented baseline severity.
There was considerable heterogeneity across studies, as 16 unique assessments were used as inclusion criteria and 34 assessments were used to evaluate baseline severity. “In addition, even within an individual study, there was substantial discordance in their use as only 71.2% of studies used the same assessments for inclusion and documenting baseline disease severity,” Mr. Chopra said. “Altogether, this multidimensional lack of documentation and heterogeneity of inclusion criteria and baseline severity assessments limits our ability to assess whether the recruitment methods for patients were adequate and confirm whether the intended target population for severity was successfully enrolled.”
He acknowledged certain limitations of the study, including the fact that it may not be generalizable to nonpharmacological interventional trials or noninterventional studies. “In addition, we could only conduct an analysis for studies that provided adequate documentation of inclusion criteria and baseline severity,” Mr. Chopra said. “Thus, those studies that did not provide this information were left out. Nevertheless, across all studies, the uniformity and concordance between assessments likely are even more negatively impacted. It should also be noted that lack of documentation of assessments for inclusion criteria and baseline severity does not imply lack of their utilization. Finally, it is important to acknowledge that AD’s diverse phenotype and relapsing and remitting course may result in the unavoidable heterogeneity of severity assessment use. This may actually help to capture a broader range of disease and improve the external validity of results.”
He reported having no financial disclosures.
CHICAGO – There is considerable variability and poor documentation of severity assessments used for inclusion criteria and baseline severity evaluations in randomized, controlled pediatric atopic dermatitis (AD) trials, results from a systematic review showed.
“It is important for clinicians and investigators to recognize that these differences may limit our ability to reproduce trials, interpret individual studies, and compare results between studies of similar target populations for severity,” lead study author Rishi Chopra, MS, said in an interview in advance of the World Congress of Pediatric Dermatology. “Moreover, this heterogeneity should be considered when retroactively pooling results for meta-analyses of pediatric atopic dermatitis randomized, controlled trials.”
In an effort to evaluate the documentation and characterize the severity assessments used in inclusion criteria and baseline evaluations for randomized, controlled trials of pediatric AD internationally, the researchers performed a systematic review of relevant studies contained in the Cochrane Library, Embase, LILACS, GREAT, MEDLINE, and Scopus databases during 2007-2016. Inclusion criteria were RCT with a pharmacological intervention and any comparison with a control group, children, and males or females. In all, 89 studies met the inclusion/exclusion criteria. Most (70.8%) were studies of pediatric populations aged 0-17 years, and almost 17% were studies of infants aged 0-1 years. The most common target populations were mild-moderate AD (31.5%), moderate-severe AD (18.0%), or undefined (36.0%).
Mr. Chopra and his associates found that the most commonly used severity indices were Scoring AD (SCORAD) in 29.2%, Body Surface Area (BSA) in 16.9%, and global assessments in 13.4%, while the most common assessments of baseline severity were SCORAD in 43.8%, global assessments in 20.2%, Eczema Area and Severity Index in 17.9%, BSA in 14.6%, and visual itch in 13.5%. Only 85.4% of studies recorded the severity assessments used for recruiting the predefined target population and only 76.4% of studies documented baseline severity.
There was considerable heterogeneity across studies, as 16 unique assessments were used as inclusion criteria and 34 assessments were used to evaluate baseline severity. “In addition, even within an individual study, there was substantial discordance in their use as only 71.2% of studies used the same assessments for inclusion and documenting baseline disease severity,” Mr. Chopra said. “Altogether, this multidimensional lack of documentation and heterogeneity of inclusion criteria and baseline severity assessments limits our ability to assess whether the recruitment methods for patients were adequate and confirm whether the intended target population for severity was successfully enrolled.”
He acknowledged certain limitations of the study, including the fact that it may not be generalizable to nonpharmacological interventional trials or noninterventional studies. “In addition, we could only conduct an analysis for studies that provided adequate documentation of inclusion criteria and baseline severity,” Mr. Chopra said. “Thus, those studies that did not provide this information were left out. Nevertheless, across all studies, the uniformity and concordance between assessments likely are even more negatively impacted. It should also be noted that lack of documentation of assessments for inclusion criteria and baseline severity does not imply lack of their utilization. Finally, it is important to acknowledge that AD’s diverse phenotype and relapsing and remitting course may result in the unavoidable heterogeneity of severity assessment use. This may actually help to capture a broader range of disease and improve the external validity of results.”
He reported having no financial disclosures.
CHICAGO – There is considerable variability and poor documentation of severity assessments used for inclusion criteria and baseline severity evaluations in randomized, controlled pediatric atopic dermatitis (AD) trials, results from a systematic review showed.
“It is important for clinicians and investigators to recognize that these differences may limit our ability to reproduce trials, interpret individual studies, and compare results between studies of similar target populations for severity,” lead study author Rishi Chopra, MS, said in an interview in advance of the World Congress of Pediatric Dermatology. “Moreover, this heterogeneity should be considered when retroactively pooling results for meta-analyses of pediatric atopic dermatitis randomized, controlled trials.”
In an effort to evaluate the documentation and characterize the severity assessments used in inclusion criteria and baseline evaluations for randomized, controlled trials of pediatric AD internationally, the researchers performed a systematic review of relevant studies contained in the Cochrane Library, Embase, LILACS, GREAT, MEDLINE, and Scopus databases during 2007-2016. Inclusion criteria were RCT with a pharmacological intervention and any comparison with a control group, children, and males or females. In all, 89 studies met the inclusion/exclusion criteria. Most (70.8%) were studies of pediatric populations aged 0-17 years, and almost 17% were studies of infants aged 0-1 years. The most common target populations were mild-moderate AD (31.5%), moderate-severe AD (18.0%), or undefined (36.0%).
Mr. Chopra and his associates found that the most commonly used severity indices were Scoring AD (SCORAD) in 29.2%, Body Surface Area (BSA) in 16.9%, and global assessments in 13.4%, while the most common assessments of baseline severity were SCORAD in 43.8%, global assessments in 20.2%, Eczema Area and Severity Index in 17.9%, BSA in 14.6%, and visual itch in 13.5%. Only 85.4% of studies recorded the severity assessments used for recruiting the predefined target population and only 76.4% of studies documented baseline severity.
There was considerable heterogeneity across studies, as 16 unique assessments were used as inclusion criteria and 34 assessments were used to evaluate baseline severity. “In addition, even within an individual study, there was substantial discordance in their use as only 71.2% of studies used the same assessments for inclusion and documenting baseline disease severity,” Mr. Chopra said. “Altogether, this multidimensional lack of documentation and heterogeneity of inclusion criteria and baseline severity assessments limits our ability to assess whether the recruitment methods for patients were adequate and confirm whether the intended target population for severity was successfully enrolled.”
He acknowledged certain limitations of the study, including the fact that it may not be generalizable to nonpharmacological interventional trials or noninterventional studies. “In addition, we could only conduct an analysis for studies that provided adequate documentation of inclusion criteria and baseline severity,” Mr. Chopra said. “Thus, those studies that did not provide this information were left out. Nevertheless, across all studies, the uniformity and concordance between assessments likely are even more negatively impacted. It should also be noted that lack of documentation of assessments for inclusion criteria and baseline severity does not imply lack of their utilization. Finally, it is important to acknowledge that AD’s diverse phenotype and relapsing and remitting course may result in the unavoidable heterogeneity of severity assessment use. This may actually help to capture a broader range of disease and improve the external validity of results.”
He reported having no financial disclosures.
AT WCPD 2017
Key clinical point:
Major finding: Only 85.4% of studies recorded the severity assessments used for recruiting the predefined target population, and only 76.4% of studies documented baseline severity.
Data source: A systematic review of 89 pediatric atopic dermatitis randomized, controlled trials published during 2007-2016.
Disclosures: Mr. Chopra reported having no financial disclosures.
Look for comorbidities associated with hidradenitis suppurativa
CHICAGO – Hidradenitis suppurativa in children is often associated with comorbidities, especially obesity and endocrine abnormalities, a retrospective review of cases showed.
“When treating hidradenitis suppurativa, it is imperative to not only treat the skin but also to look for associated comorbidities,” Maria del Carmen Liy-Wong, MD, said in an interview in advance of the World Congress for Pediatric Dermatology.
Of the 41 patients, 78% were girls; the mean age of onset was 11 years, and the mean age at diagnosis was 14 years. A positive family history was found in 24% of cases. The most common cutaneous lesions were papules and pustules (51%), followed by scars (39%), and 88% of patients reported associated tenderness and pain.
After using the Hurley severity grade to classify disease severity, the researchers found that 56% of cases were mild, 32% were moderate, and 12% were severe. Comorbidities were identified in 92% of the cases; the most common was obesity (73%), followed by endocrine abnormalities (29%) and menstrual irregularities (20%). The researchers also found that 70% of patients were treated with a combination of topical and systemic antibiotics, and that early onset of disease correlated with more severe disease (P = .03).
Dr. Liy-Wong acknowledged that the study’s retrospective design is a limitation of the analysis, but she said that a prospective evaluation in planned for the near future.
The study was supported in part by a grant from AbbVie. Dr. Liy-Wong reported having no relevant financial disclosures.
[email protected]
CHICAGO – Hidradenitis suppurativa in children is often associated with comorbidities, especially obesity and endocrine abnormalities, a retrospective review of cases showed.
“When treating hidradenitis suppurativa, it is imperative to not only treat the skin but also to look for associated comorbidities,” Maria del Carmen Liy-Wong, MD, said in an interview in advance of the World Congress for Pediatric Dermatology.
Of the 41 patients, 78% were girls; the mean age of onset was 11 years, and the mean age at diagnosis was 14 years. A positive family history was found in 24% of cases. The most common cutaneous lesions were papules and pustules (51%), followed by scars (39%), and 88% of patients reported associated tenderness and pain.
After using the Hurley severity grade to classify disease severity, the researchers found that 56% of cases were mild, 32% were moderate, and 12% were severe. Comorbidities were identified in 92% of the cases; the most common was obesity (73%), followed by endocrine abnormalities (29%) and menstrual irregularities (20%). The researchers also found that 70% of patients were treated with a combination of topical and systemic antibiotics, and that early onset of disease correlated with more severe disease (P = .03).
Dr. Liy-Wong acknowledged that the study’s retrospective design is a limitation of the analysis, but she said that a prospective evaluation in planned for the near future.
The study was supported in part by a grant from AbbVie. Dr. Liy-Wong reported having no relevant financial disclosures.
[email protected]
CHICAGO – Hidradenitis suppurativa in children is often associated with comorbidities, especially obesity and endocrine abnormalities, a retrospective review of cases showed.
“When treating hidradenitis suppurativa, it is imperative to not only treat the skin but also to look for associated comorbidities,” Maria del Carmen Liy-Wong, MD, said in an interview in advance of the World Congress for Pediatric Dermatology.
Of the 41 patients, 78% were girls; the mean age of onset was 11 years, and the mean age at diagnosis was 14 years. A positive family history was found in 24% of cases. The most common cutaneous lesions were papules and pustules (51%), followed by scars (39%), and 88% of patients reported associated tenderness and pain.
After using the Hurley severity grade to classify disease severity, the researchers found that 56% of cases were mild, 32% were moderate, and 12% were severe. Comorbidities were identified in 92% of the cases; the most common was obesity (73%), followed by endocrine abnormalities (29%) and menstrual irregularities (20%). The researchers also found that 70% of patients were treated with a combination of topical and systemic antibiotics, and that early onset of disease correlated with more severe disease (P = .03).
Dr. Liy-Wong acknowledged that the study’s retrospective design is a limitation of the analysis, but she said that a prospective evaluation in planned for the near future.
The study was supported in part by a grant from AbbVie. Dr. Liy-Wong reported having no relevant financial disclosures.
[email protected]
AT WCPD 2017
Key clinical point:
Major finding: Comorbidities were identified in 92% of the cases, with obesity (73%) the most common.
Data source: A retrospective review of clinical characteristics, degree of severity, comorbidities, and management of hidradenitis suppurativa in 41 patients followed between January 1995 and January 2015.
Disclosures: Dr. Liy-Wong reported having no relevant financial disclosures.
Integrative approach to MS care underused, expert says
NEW ORLEANS – The integration of lifestyle, alternative, and conventional medicine into the care of patients with multiple sclerosis can be transformative for patients and clinicians alike, according to Allen C. Bowling, MD, PhD.
This approach not only emphasizes health and wellness of the whole person, it supports the clinician-patient relationship since neurologists serve as point persons for MS patients, Dr. Bowling said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “It’s critical to not just think about MS the disease, but to think about the patient’s overall health, maintaining health,” he said. “That’s a big mind shift. I think sometimes people hear the term ‘integrative medicine’ and they start walking out of the room or think it’s getting ‘woo-woo,’ but there is a very evidence-based approach to integrative medicine. The core of it still is not familiar to a lot of physicians.”
Harness ‘built-in’ resources
Dr. Bowling, author of “Optimal Health With Multiple Sclerosis: A Guide to Integrating Lifestyle, Alternative, and Conventional Medicine” (New York: Demos Medical Publishing, 2014) and developer of a website devoted to integrative care and MS (www.neurologycare.net), said that some of best treatment approaches may be those that use the “built-in” resources of the human body and do not require any medications, supplements, devices, or technology. “The most effective and long-lasting changes in lifestyle may be those that are small and consistent,” he said. For example, when he first started applying integrative care principles to his practice more than 16 years ago, some patients told him that MS was “one of the best things that happened to them, and that it was a gift in that it helped them clean up their lifestyle.” In the summer of 2001, one such patient came to see Dr. Bowling and said, “Since my last appointment I’ve lost 20 pounds, bought a bike. I exercise four times weekly. My wife and I have changed our diet. This is real; I’m on it.”
“I said. ‘Very impressive. How did that happen? What motivated you?’ ”
“He said, ‘You did.’ This was like a turning point in his life.”
As of May 2017, this patient has maintained his healthy lifestyle changes.
Dr. Bowling told meeting attendees that early in his neurology career he didn’t always consider other ways he could impact MS patients beyond helping them determine the best disease-modifying treatment and/or assisting them in managing symptoms. Thinking to counsel them in areas such as a balanced diet, exercise, and emotional wellness “was a mind shift for me, and made me realize how narrow my focus was,” he said. “It transformed me to go back to thinking more about general medicine. I’m a detail-oriented guy, but I don’t think that’s in the best interests of our patients. I think we need to combine very disease-specific advice with very general advice. We’re at a very important place with our patients where we can potentially have a very significant impact with our recommendations about MS as well as other medical conditions and health maintenance.”
Take baby steps
Dr. Bowling recommended that clinicians take baby steps to incorporate aspects of integrated care, including use of brief, strong supportive statements; focusing on only one issue per visit; referring patients to information resources; and sharing or transferring responsibility/accountability with other providers. Excessive focus on one therapy – including unusual and unproven CAM therapies – may detract from, or be used to avoid, other valuable approaches. For example, some of Dr. Bowling’s patients may come in very enchanted with a particular dietary supplement yet their own diet is unhealthy. “I don’t scold them, but I say, ‘I don’t think this supplement’s going to hurt you but there’s much more evidence that we should shift the focus of your motivation and drive to dietary approaches.’ ”
Use leverage to tackle emotional health
In the area of emotional health, Dr. Bowling said that an MS diagnosis often serves as a springboard to help young patients develop emotional literacy so that they don’t develop high levels of stress and anxiety. “What I find are people on the verge of entering into depression or anxiety and not really knowing how to address those emotional issues or even to do basic identification and processing of emotions, and getting attached to mind-body approaches, which I think have a clear benefit, but for many patients it does not lead to emotional literacy,” he said. “I emphasize ‘I can’t be with you 24/7. That’s your piece of the equation.’ You can also help patients identify things that give them joy, meaning, and fun in life. That’s a motivator for a lot of them – to keep doing what they find joyful, meaningful, and fun.” Dr. Bowling said that this kind of approach brings “plain old common sense” to the clinician-patient relationship. “I think we all have lots of leverage with our patients, but there’s no cookbook or algorithm here; you need to start where patients are,” he said. “Our younger patients in particular are not really listening to their primary care doctor too much. They’re paying very close attention to what we’re talking about: disease-modifying therapies and symptomatic management.”
In Dr. Bowling’s clinical experience, men often struggle with emotional health and emotional literacy issues. “I have seen amazing transformations of men in their early 20s with very low emotional literacy, and working with them, sometimes with psychodynamic psychotherapy and other methods, sometimes just talking with friends and family – really encouraging them before there’s serious anxiety or depression.” One patient told him that MS “forced me to do a year of psychotherapy and some intensive introspection for a few years. This helped me and also my family.” He recommended that clinicians tread carefully when advising men about tobacco and alcohol use, and when advising women about weight management. “Be prepared; it’s a little different every time you bring those potential issues up,” he said.
Dr. Bowling added that MS often affects how patients view the aging process itself. “It can be a crash course in how to understand the body and best use it before people technically get to the aging years,” he said. “There’s also more acceptance of disability in people who are older.” In the setting of patients who experience disease flare-ups, they may ask you if there’s anything they can do from a lifestyle standpoint to make them go away. “Be primed for these teaching moments and opportunities where there’s a seed of motivation,” he advised.
Approach treats, prevents clinician burnout
Dr. Bowling described the integrative approach to treating MS patients as “an ever-changing skill set. There’s all this talk about neurologist burnout, which is very real. If you practice this way, this is a great treatment and preventive approach for clinician burnout because your patients check you out to see if you ‘walk the walk’ on advice you give them. My success with my patients in some of these areas is less than 50%. But when there’s a real change, it’s for the life of that person. That’s the most rewarding part of my career at this point.”
Dr. Bowling disclosed that he has received research, consulting, advising, and speaking fees from Acorda, Biogen, EMD Serono, Genentech, Genzyme, Sanofi-Aventis, Teva, the American Academy of Neurology, the CMSC, the Mandell Center for Multiple Sclerosis, and the National MS Society.
NEW ORLEANS – The integration of lifestyle, alternative, and conventional medicine into the care of patients with multiple sclerosis can be transformative for patients and clinicians alike, according to Allen C. Bowling, MD, PhD.
This approach not only emphasizes health and wellness of the whole person, it supports the clinician-patient relationship since neurologists serve as point persons for MS patients, Dr. Bowling said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “It’s critical to not just think about MS the disease, but to think about the patient’s overall health, maintaining health,” he said. “That’s a big mind shift. I think sometimes people hear the term ‘integrative medicine’ and they start walking out of the room or think it’s getting ‘woo-woo,’ but there is a very evidence-based approach to integrative medicine. The core of it still is not familiar to a lot of physicians.”
Harness ‘built-in’ resources
Dr. Bowling, author of “Optimal Health With Multiple Sclerosis: A Guide to Integrating Lifestyle, Alternative, and Conventional Medicine” (New York: Demos Medical Publishing, 2014) and developer of a website devoted to integrative care and MS (www.neurologycare.net), said that some of best treatment approaches may be those that use the “built-in” resources of the human body and do not require any medications, supplements, devices, or technology. “The most effective and long-lasting changes in lifestyle may be those that are small and consistent,” he said. For example, when he first started applying integrative care principles to his practice more than 16 years ago, some patients told him that MS was “one of the best things that happened to them, and that it was a gift in that it helped them clean up their lifestyle.” In the summer of 2001, one such patient came to see Dr. Bowling and said, “Since my last appointment I’ve lost 20 pounds, bought a bike. I exercise four times weekly. My wife and I have changed our diet. This is real; I’m on it.”
“I said. ‘Very impressive. How did that happen? What motivated you?’ ”
“He said, ‘You did.’ This was like a turning point in his life.”
As of May 2017, this patient has maintained his healthy lifestyle changes.
Dr. Bowling told meeting attendees that early in his neurology career he didn’t always consider other ways he could impact MS patients beyond helping them determine the best disease-modifying treatment and/or assisting them in managing symptoms. Thinking to counsel them in areas such as a balanced diet, exercise, and emotional wellness “was a mind shift for me, and made me realize how narrow my focus was,” he said. “It transformed me to go back to thinking more about general medicine. I’m a detail-oriented guy, but I don’t think that’s in the best interests of our patients. I think we need to combine very disease-specific advice with very general advice. We’re at a very important place with our patients where we can potentially have a very significant impact with our recommendations about MS as well as other medical conditions and health maintenance.”
Take baby steps
Dr. Bowling recommended that clinicians take baby steps to incorporate aspects of integrated care, including use of brief, strong supportive statements; focusing on only one issue per visit; referring patients to information resources; and sharing or transferring responsibility/accountability with other providers. Excessive focus on one therapy – including unusual and unproven CAM therapies – may detract from, or be used to avoid, other valuable approaches. For example, some of Dr. Bowling’s patients may come in very enchanted with a particular dietary supplement yet their own diet is unhealthy. “I don’t scold them, but I say, ‘I don’t think this supplement’s going to hurt you but there’s much more evidence that we should shift the focus of your motivation and drive to dietary approaches.’ ”
Use leverage to tackle emotional health
In the area of emotional health, Dr. Bowling said that an MS diagnosis often serves as a springboard to help young patients develop emotional literacy so that they don’t develop high levels of stress and anxiety. “What I find are people on the verge of entering into depression or anxiety and not really knowing how to address those emotional issues or even to do basic identification and processing of emotions, and getting attached to mind-body approaches, which I think have a clear benefit, but for many patients it does not lead to emotional literacy,” he said. “I emphasize ‘I can’t be with you 24/7. That’s your piece of the equation.’ You can also help patients identify things that give them joy, meaning, and fun in life. That’s a motivator for a lot of them – to keep doing what they find joyful, meaningful, and fun.” Dr. Bowling said that this kind of approach brings “plain old common sense” to the clinician-patient relationship. “I think we all have lots of leverage with our patients, but there’s no cookbook or algorithm here; you need to start where patients are,” he said. “Our younger patients in particular are not really listening to their primary care doctor too much. They’re paying very close attention to what we’re talking about: disease-modifying therapies and symptomatic management.”
In Dr. Bowling’s clinical experience, men often struggle with emotional health and emotional literacy issues. “I have seen amazing transformations of men in their early 20s with very low emotional literacy, and working with them, sometimes with psychodynamic psychotherapy and other methods, sometimes just talking with friends and family – really encouraging them before there’s serious anxiety or depression.” One patient told him that MS “forced me to do a year of psychotherapy and some intensive introspection for a few years. This helped me and also my family.” He recommended that clinicians tread carefully when advising men about tobacco and alcohol use, and when advising women about weight management. “Be prepared; it’s a little different every time you bring those potential issues up,” he said.
Dr. Bowling added that MS often affects how patients view the aging process itself. “It can be a crash course in how to understand the body and best use it before people technically get to the aging years,” he said. “There’s also more acceptance of disability in people who are older.” In the setting of patients who experience disease flare-ups, they may ask you if there’s anything they can do from a lifestyle standpoint to make them go away. “Be primed for these teaching moments and opportunities where there’s a seed of motivation,” he advised.
Approach treats, prevents clinician burnout
Dr. Bowling described the integrative approach to treating MS patients as “an ever-changing skill set. There’s all this talk about neurologist burnout, which is very real. If you practice this way, this is a great treatment and preventive approach for clinician burnout because your patients check you out to see if you ‘walk the walk’ on advice you give them. My success with my patients in some of these areas is less than 50%. But when there’s a real change, it’s for the life of that person. That’s the most rewarding part of my career at this point.”
Dr. Bowling disclosed that he has received research, consulting, advising, and speaking fees from Acorda, Biogen, EMD Serono, Genentech, Genzyme, Sanofi-Aventis, Teva, the American Academy of Neurology, the CMSC, the Mandell Center for Multiple Sclerosis, and the National MS Society.
NEW ORLEANS – The integration of lifestyle, alternative, and conventional medicine into the care of patients with multiple sclerosis can be transformative for patients and clinicians alike, according to Allen C. Bowling, MD, PhD.
This approach not only emphasizes health and wellness of the whole person, it supports the clinician-patient relationship since neurologists serve as point persons for MS patients, Dr. Bowling said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “It’s critical to not just think about MS the disease, but to think about the patient’s overall health, maintaining health,” he said. “That’s a big mind shift. I think sometimes people hear the term ‘integrative medicine’ and they start walking out of the room or think it’s getting ‘woo-woo,’ but there is a very evidence-based approach to integrative medicine. The core of it still is not familiar to a lot of physicians.”
Harness ‘built-in’ resources
Dr. Bowling, author of “Optimal Health With Multiple Sclerosis: A Guide to Integrating Lifestyle, Alternative, and Conventional Medicine” (New York: Demos Medical Publishing, 2014) and developer of a website devoted to integrative care and MS (www.neurologycare.net), said that some of best treatment approaches may be those that use the “built-in” resources of the human body and do not require any medications, supplements, devices, or technology. “The most effective and long-lasting changes in lifestyle may be those that are small and consistent,” he said. For example, when he first started applying integrative care principles to his practice more than 16 years ago, some patients told him that MS was “one of the best things that happened to them, and that it was a gift in that it helped them clean up their lifestyle.” In the summer of 2001, one such patient came to see Dr. Bowling and said, “Since my last appointment I’ve lost 20 pounds, bought a bike. I exercise four times weekly. My wife and I have changed our diet. This is real; I’m on it.”
“I said. ‘Very impressive. How did that happen? What motivated you?’ ”
“He said, ‘You did.’ This was like a turning point in his life.”
As of May 2017, this patient has maintained his healthy lifestyle changes.
Dr. Bowling told meeting attendees that early in his neurology career he didn’t always consider other ways he could impact MS patients beyond helping them determine the best disease-modifying treatment and/or assisting them in managing symptoms. Thinking to counsel them in areas such as a balanced diet, exercise, and emotional wellness “was a mind shift for me, and made me realize how narrow my focus was,” he said. “It transformed me to go back to thinking more about general medicine. I’m a detail-oriented guy, but I don’t think that’s in the best interests of our patients. I think we need to combine very disease-specific advice with very general advice. We’re at a very important place with our patients where we can potentially have a very significant impact with our recommendations about MS as well as other medical conditions and health maintenance.”
Take baby steps
Dr. Bowling recommended that clinicians take baby steps to incorporate aspects of integrated care, including use of brief, strong supportive statements; focusing on only one issue per visit; referring patients to information resources; and sharing or transferring responsibility/accountability with other providers. Excessive focus on one therapy – including unusual and unproven CAM therapies – may detract from, or be used to avoid, other valuable approaches. For example, some of Dr. Bowling’s patients may come in very enchanted with a particular dietary supplement yet their own diet is unhealthy. “I don’t scold them, but I say, ‘I don’t think this supplement’s going to hurt you but there’s much more evidence that we should shift the focus of your motivation and drive to dietary approaches.’ ”
Use leverage to tackle emotional health
In the area of emotional health, Dr. Bowling said that an MS diagnosis often serves as a springboard to help young patients develop emotional literacy so that they don’t develop high levels of stress and anxiety. “What I find are people on the verge of entering into depression or anxiety and not really knowing how to address those emotional issues or even to do basic identification and processing of emotions, and getting attached to mind-body approaches, which I think have a clear benefit, but for many patients it does not lead to emotional literacy,” he said. “I emphasize ‘I can’t be with you 24/7. That’s your piece of the equation.’ You can also help patients identify things that give them joy, meaning, and fun in life. That’s a motivator for a lot of them – to keep doing what they find joyful, meaningful, and fun.” Dr. Bowling said that this kind of approach brings “plain old common sense” to the clinician-patient relationship. “I think we all have lots of leverage with our patients, but there’s no cookbook or algorithm here; you need to start where patients are,” he said. “Our younger patients in particular are not really listening to their primary care doctor too much. They’re paying very close attention to what we’re talking about: disease-modifying therapies and symptomatic management.”
In Dr. Bowling’s clinical experience, men often struggle with emotional health and emotional literacy issues. “I have seen amazing transformations of men in their early 20s with very low emotional literacy, and working with them, sometimes with psychodynamic psychotherapy and other methods, sometimes just talking with friends and family – really encouraging them before there’s serious anxiety or depression.” One patient told him that MS “forced me to do a year of psychotherapy and some intensive introspection for a few years. This helped me and also my family.” He recommended that clinicians tread carefully when advising men about tobacco and alcohol use, and when advising women about weight management. “Be prepared; it’s a little different every time you bring those potential issues up,” he said.
Dr. Bowling added that MS often affects how patients view the aging process itself. “It can be a crash course in how to understand the body and best use it before people technically get to the aging years,” he said. “There’s also more acceptance of disability in people who are older.” In the setting of patients who experience disease flare-ups, they may ask you if there’s anything they can do from a lifestyle standpoint to make them go away. “Be primed for these teaching moments and opportunities where there’s a seed of motivation,” he advised.
Approach treats, prevents clinician burnout
Dr. Bowling described the integrative approach to treating MS patients as “an ever-changing skill set. There’s all this talk about neurologist burnout, which is very real. If you practice this way, this is a great treatment and preventive approach for clinician burnout because your patients check you out to see if you ‘walk the walk’ on advice you give them. My success with my patients in some of these areas is less than 50%. But when there’s a real change, it’s for the life of that person. That’s the most rewarding part of my career at this point.”
Dr. Bowling disclosed that he has received research, consulting, advising, and speaking fees from Acorda, Biogen, EMD Serono, Genentech, Genzyme, Sanofi-Aventis, Teva, the American Academy of Neurology, the CMSC, the Mandell Center for Multiple Sclerosis, and the National MS Society.
EXPERT ANALYSIS FROM THE CMSC ANNUAL MEETING
Preliminary results promising from ublituximab phase II study for MS
NEW ORLEANS – The investigative agent ublituximab was well tolerated and demonstrated rapid B-cell depletion in patients with relapsing multiple sclerosis, results from an ongoing phase II trial showed.
“Ublituximab is a novel, chimeric monoclonal antibody targeting a unique epitope on the CD20 antigen, and glycoengineered to enhance affinity for all variants of FcyRIlla receptors, thereby demonstrating greater antibody-dependent cellular toxicity activity than rituximab and ofatumumab,” Amy Lovett-Racke, PhD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In a trial supported by TG Therapeutics, which is developing the agent, Dr. Lovett-Racke of the department of microbial infection and immunity at the Ohio State University Medical Center, Columbus, and her associates reported data from 24 patients who received ublituximab at doses markedly less than those used in ongoing phase III oncology studies, and at a range of infusion times, with a goal of rapid infusions.
The study ran for 48 weeks, and the primary endpoint was responders rate, defined as a percent of patients with at least a 95% reduction in peripheral CD19-positive B-cells within 2 weeks after the second infusion. All patients received the same total dose of 600 mg; only infusion times differed. (Ublituximab was administered on day 1, day 15, and week 24. Placebo IV infusion dose was administered on day 1 and day 15 only.)
The mean age of the study participants was 40 years, and the distribution of time from diagnosis was less than 5 years in 11 patients, 5-10 years in 7 patients, and greater than 10 years in 6 patients.
To date, ublituximab has been well tolerated, with only mild infusion reactions being observed, even with infusion times reduced to 1 hour. The researchers also found that ublituximab resulted in 99% B-cell depletion, meeting the study endpoint of greater than 95% depletion within 2 weeks of the second dose, which is comparable to ocrelizumab.
“Every patient has met the endpoint so far,” Dr. Lovett-Racke said. “Although there is a transient decrease in T cells after the initial dose of ublituximab, T-cell numbers are fairly stable over time. Memory B cells seem slightly more resistant to depletion, but are efficiently depleted in all patients. A comprehensive analysis of B- and T-cell profiles is being performed to understand how B-cell depletion influences T-cell profiles, and to characterize the B-cell repletion.”
TG Therapeutics funded the study. Dr. Lovett-Racke disclosed having received research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Strategic Pharmaceutical Academic Research Consortium.
NEW ORLEANS – The investigative agent ublituximab was well tolerated and demonstrated rapid B-cell depletion in patients with relapsing multiple sclerosis, results from an ongoing phase II trial showed.
“Ublituximab is a novel, chimeric monoclonal antibody targeting a unique epitope on the CD20 antigen, and glycoengineered to enhance affinity for all variants of FcyRIlla receptors, thereby demonstrating greater antibody-dependent cellular toxicity activity than rituximab and ofatumumab,” Amy Lovett-Racke, PhD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In a trial supported by TG Therapeutics, which is developing the agent, Dr. Lovett-Racke of the department of microbial infection and immunity at the Ohio State University Medical Center, Columbus, and her associates reported data from 24 patients who received ublituximab at doses markedly less than those used in ongoing phase III oncology studies, and at a range of infusion times, with a goal of rapid infusions.
The study ran for 48 weeks, and the primary endpoint was responders rate, defined as a percent of patients with at least a 95% reduction in peripheral CD19-positive B-cells within 2 weeks after the second infusion. All patients received the same total dose of 600 mg; only infusion times differed. (Ublituximab was administered on day 1, day 15, and week 24. Placebo IV infusion dose was administered on day 1 and day 15 only.)
The mean age of the study participants was 40 years, and the distribution of time from diagnosis was less than 5 years in 11 patients, 5-10 years in 7 patients, and greater than 10 years in 6 patients.
To date, ublituximab has been well tolerated, with only mild infusion reactions being observed, even with infusion times reduced to 1 hour. The researchers also found that ublituximab resulted in 99% B-cell depletion, meeting the study endpoint of greater than 95% depletion within 2 weeks of the second dose, which is comparable to ocrelizumab.
“Every patient has met the endpoint so far,” Dr. Lovett-Racke said. “Although there is a transient decrease in T cells after the initial dose of ublituximab, T-cell numbers are fairly stable over time. Memory B cells seem slightly more resistant to depletion, but are efficiently depleted in all patients. A comprehensive analysis of B- and T-cell profiles is being performed to understand how B-cell depletion influences T-cell profiles, and to characterize the B-cell repletion.”
TG Therapeutics funded the study. Dr. Lovett-Racke disclosed having received research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Strategic Pharmaceutical Academic Research Consortium.
NEW ORLEANS – The investigative agent ublituximab was well tolerated and demonstrated rapid B-cell depletion in patients with relapsing multiple sclerosis, results from an ongoing phase II trial showed.
“Ublituximab is a novel, chimeric monoclonal antibody targeting a unique epitope on the CD20 antigen, and glycoengineered to enhance affinity for all variants of FcyRIlla receptors, thereby demonstrating greater antibody-dependent cellular toxicity activity than rituximab and ofatumumab,” Amy Lovett-Racke, PhD, said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
In a trial supported by TG Therapeutics, which is developing the agent, Dr. Lovett-Racke of the department of microbial infection and immunity at the Ohio State University Medical Center, Columbus, and her associates reported data from 24 patients who received ublituximab at doses markedly less than those used in ongoing phase III oncology studies, and at a range of infusion times, with a goal of rapid infusions.
The study ran for 48 weeks, and the primary endpoint was responders rate, defined as a percent of patients with at least a 95% reduction in peripheral CD19-positive B-cells within 2 weeks after the second infusion. All patients received the same total dose of 600 mg; only infusion times differed. (Ublituximab was administered on day 1, day 15, and week 24. Placebo IV infusion dose was administered on day 1 and day 15 only.)
The mean age of the study participants was 40 years, and the distribution of time from diagnosis was less than 5 years in 11 patients, 5-10 years in 7 patients, and greater than 10 years in 6 patients.
To date, ublituximab has been well tolerated, with only mild infusion reactions being observed, even with infusion times reduced to 1 hour. The researchers also found that ublituximab resulted in 99% B-cell depletion, meeting the study endpoint of greater than 95% depletion within 2 weeks of the second dose, which is comparable to ocrelizumab.
“Every patient has met the endpoint so far,” Dr. Lovett-Racke said. “Although there is a transient decrease in T cells after the initial dose of ublituximab, T-cell numbers are fairly stable over time. Memory B cells seem slightly more resistant to depletion, but are efficiently depleted in all patients. A comprehensive analysis of B- and T-cell profiles is being performed to understand how B-cell depletion influences T-cell profiles, and to characterize the B-cell repletion.”
TG Therapeutics funded the study. Dr. Lovett-Racke disclosed having received research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Strategic Pharmaceutical Academic Research Consortium.
AT THE CMSC ANNUAL MEETING
Key clinical point:
Major finding: Use of ublituximab resulted in 99% B-cell depletion among patients with relapsing MS.
Data source: Preliminary results from a phase II placebo-controlled study of 24 MS patients who received ublituximab.
Disclosures: TG Therapeutics funded the study. Dr. Lovett-Racke disclosed having received research grants from the National Institutes of Health, the National Multiple Sclerosis Society, and the Strategic Pharmaceutical Academic Research Consortium.
New drug choices emerging to battle antibiotic resistance
SAN FRANCISCO – When the Infectious Diseases Society of America released the “Bad Bugs, No Drugs” report in 2004, its authors warned that effective antibiotics may not be available to treat seriously ill patients in the near future.
It also proposed legislative, regulatory, and funding solutions with a goal of developing and licensing 10 new antibiotics by the year 2020.
One such advancement was the Generating Antibiotics Incentives Now Act, which was signed into law in 2012 and created a designation for new antibiotics that are used to treat serious and/or life-threatening diseases due to certain pathogens. It also extends the patent life of these antibiotics and allows for fast-track Food and Drug Administration approval.
According to Dr. Erlich, chief of staff and medical director of infection control and antibiotic stewardship at Mills Peninsula Medical Center, Burlingame, Calif., increasingly common antibiotic-resistant pathogens besides MRSA and VRE include penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase–producing gram-negative rods, carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Mycobacterium tuberculosis, Salmonella enterica serotype Typhimurium DT 104, and drug-resistant Candida species.
Since 2010, several new antibiotics have been introduced to the market, including three second-generation lipoglycopeptide antibiotics with gram-positive coverage that are approved primarily for skin and soft tissue infections: dalbavancin (Dalvance), telavancin (Vibativ), and oritavancin (Orbactiv).
Compared with vancomycin, these new agents have more convenient dosing and a longer half life, “but they’re also more expensive,” said Dr. Erlich. Dalbavancin can be dosed once a week intravenously, telavancin can be dosed once daily intravenously, and oritavancin requires just one dose.
Another new agent is tedizolid phosphate (Sivextro), a second-generation oxazolidinone that is in the same drug class as linezolid (Zyvox). Tedizolid phosphate has gram-positive coverage including MRSA, but it is not approved for VRE. “It’s FDA approved for skin and soft-tissue infections (SSTI) but can be used for other locations as well,” Dr. Erlich said. “It features once-daily dosing IV or PO.”
Ceftaroline fosamil (Teflaro), ceftolozane/tazobactam (Zerbaxa), and ceftazidime/avibactam (Avycaz) are broad-spectrum cephalosporins with or without beta-lactamase inhibitors resulting in extended gram-negative coverage. FDA-approved indications include complicated urinary tract infections, complicated abdominal infections, SSTI, and pneumonia.
The primary advantage of these drugs, compared with other agents, is for multidrug-resistant gram-negative bacteria such as extended-spectrum beta-lactamase producers and CRE. “We’re not using a lot of these drugs in clinical practice, but they are available for patients with multidrug-resistant gram-negative rods who have no other options,” Dr. Erlich said.
Practical ways that clinicians can prevent antibiotic resistance include prescribing antibiotics only when necessary. “Be aware of local resistance patterns, avoid antibiotics for probable viral infections, use narrow-spectrum choices when possible, use shorter durations when appropriate, and consult published guidelines for optimal empiric antibiotic therapy,” Dr. Erlich advised.
In addition, “advocate infection control measures to keep patients from developing infections, including proper wound care, hand washing, respiratory etiquette, vaccinations, and social isolation for symptomatic individuals,” he noted.
Dr. Erlich reported having no relevant financial disclosures.
SAN FRANCISCO – When the Infectious Diseases Society of America released the “Bad Bugs, No Drugs” report in 2004, its authors warned that effective antibiotics may not be available to treat seriously ill patients in the near future.
It also proposed legislative, regulatory, and funding solutions with a goal of developing and licensing 10 new antibiotics by the year 2020.
One such advancement was the Generating Antibiotics Incentives Now Act, which was signed into law in 2012 and created a designation for new antibiotics that are used to treat serious and/or life-threatening diseases due to certain pathogens. It also extends the patent life of these antibiotics and allows for fast-track Food and Drug Administration approval.
According to Dr. Erlich, chief of staff and medical director of infection control and antibiotic stewardship at Mills Peninsula Medical Center, Burlingame, Calif., increasingly common antibiotic-resistant pathogens besides MRSA and VRE include penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase–producing gram-negative rods, carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Mycobacterium tuberculosis, Salmonella enterica serotype Typhimurium DT 104, and drug-resistant Candida species.
Since 2010, several new antibiotics have been introduced to the market, including three second-generation lipoglycopeptide antibiotics with gram-positive coverage that are approved primarily for skin and soft tissue infections: dalbavancin (Dalvance), telavancin (Vibativ), and oritavancin (Orbactiv).
Compared with vancomycin, these new agents have more convenient dosing and a longer half life, “but they’re also more expensive,” said Dr. Erlich. Dalbavancin can be dosed once a week intravenously, telavancin can be dosed once daily intravenously, and oritavancin requires just one dose.
Another new agent is tedizolid phosphate (Sivextro), a second-generation oxazolidinone that is in the same drug class as linezolid (Zyvox). Tedizolid phosphate has gram-positive coverage including MRSA, but it is not approved for VRE. “It’s FDA approved for skin and soft-tissue infections (SSTI) but can be used for other locations as well,” Dr. Erlich said. “It features once-daily dosing IV or PO.”
Ceftaroline fosamil (Teflaro), ceftolozane/tazobactam (Zerbaxa), and ceftazidime/avibactam (Avycaz) are broad-spectrum cephalosporins with or without beta-lactamase inhibitors resulting in extended gram-negative coverage. FDA-approved indications include complicated urinary tract infections, complicated abdominal infections, SSTI, and pneumonia.
The primary advantage of these drugs, compared with other agents, is for multidrug-resistant gram-negative bacteria such as extended-spectrum beta-lactamase producers and CRE. “We’re not using a lot of these drugs in clinical practice, but they are available for patients with multidrug-resistant gram-negative rods who have no other options,” Dr. Erlich said.
Practical ways that clinicians can prevent antibiotic resistance include prescribing antibiotics only when necessary. “Be aware of local resistance patterns, avoid antibiotics for probable viral infections, use narrow-spectrum choices when possible, use shorter durations when appropriate, and consult published guidelines for optimal empiric antibiotic therapy,” Dr. Erlich advised.
In addition, “advocate infection control measures to keep patients from developing infections, including proper wound care, hand washing, respiratory etiquette, vaccinations, and social isolation for symptomatic individuals,” he noted.
Dr. Erlich reported having no relevant financial disclosures.
SAN FRANCISCO – When the Infectious Diseases Society of America released the “Bad Bugs, No Drugs” report in 2004, its authors warned that effective antibiotics may not be available to treat seriously ill patients in the near future.
It also proposed legislative, regulatory, and funding solutions with a goal of developing and licensing 10 new antibiotics by the year 2020.
One such advancement was the Generating Antibiotics Incentives Now Act, which was signed into law in 2012 and created a designation for new antibiotics that are used to treat serious and/or life-threatening diseases due to certain pathogens. It also extends the patent life of these antibiotics and allows for fast-track Food and Drug Administration approval.
According to Dr. Erlich, chief of staff and medical director of infection control and antibiotic stewardship at Mills Peninsula Medical Center, Burlingame, Calif., increasingly common antibiotic-resistant pathogens besides MRSA and VRE include penicillin-resistant Streptococcus pneumoniae, extended-spectrum beta-lactamase–producing gram-negative rods, carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant Mycobacterium tuberculosis, Salmonella enterica serotype Typhimurium DT 104, and drug-resistant Candida species.
Since 2010, several new antibiotics have been introduced to the market, including three second-generation lipoglycopeptide antibiotics with gram-positive coverage that are approved primarily for skin and soft tissue infections: dalbavancin (Dalvance), telavancin (Vibativ), and oritavancin (Orbactiv).
Compared with vancomycin, these new agents have more convenient dosing and a longer half life, “but they’re also more expensive,” said Dr. Erlich. Dalbavancin can be dosed once a week intravenously, telavancin can be dosed once daily intravenously, and oritavancin requires just one dose.
Another new agent is tedizolid phosphate (Sivextro), a second-generation oxazolidinone that is in the same drug class as linezolid (Zyvox). Tedizolid phosphate has gram-positive coverage including MRSA, but it is not approved for VRE. “It’s FDA approved for skin and soft-tissue infections (SSTI) but can be used for other locations as well,” Dr. Erlich said. “It features once-daily dosing IV or PO.”
Ceftaroline fosamil (Teflaro), ceftolozane/tazobactam (Zerbaxa), and ceftazidime/avibactam (Avycaz) are broad-spectrum cephalosporins with or without beta-lactamase inhibitors resulting in extended gram-negative coverage. FDA-approved indications include complicated urinary tract infections, complicated abdominal infections, SSTI, and pneumonia.
The primary advantage of these drugs, compared with other agents, is for multidrug-resistant gram-negative bacteria such as extended-spectrum beta-lactamase producers and CRE. “We’re not using a lot of these drugs in clinical practice, but they are available for patients with multidrug-resistant gram-negative rods who have no other options,” Dr. Erlich said.
Practical ways that clinicians can prevent antibiotic resistance include prescribing antibiotics only when necessary. “Be aware of local resistance patterns, avoid antibiotics for probable viral infections, use narrow-spectrum choices when possible, use shorter durations when appropriate, and consult published guidelines for optimal empiric antibiotic therapy,” Dr. Erlich advised.
In addition, “advocate infection control measures to keep patients from developing infections, including proper wound care, hand washing, respiratory etiquette, vaccinations, and social isolation for symptomatic individuals,” he noted.
Dr. Erlich reported having no relevant financial disclosures.
AT THE ANNUAL ADVANCES IN INTERNAL MEDICINE
Bilateral cellulitis on legs? Think venous stasis dermatitis
SAN FRANCISCO – If a patient presents with bilateral cellulitis on both legs, think venous stasis dermatitis, which is the number one misdiagnosis of cellulitis and a frequent cause of unnecessary hospitalization for so-called “red leg,” according to Kanade Shinkai, MD, PhD.
“It’s easy to make that mistake, because you have a red, hot leg that’s painful, and the patient is having difficulty walking,” Dr. Shinkai said at the UCSF Annual Advances in Internal Medicine meeting. “Venous stasis dermatitis is one of the things you want to learn to recognize, as hospitalization is typically not needed.”
“That has to do with the venous return back to the heart,” said Dr. Shinkai, a dermatologist at UCSF Medical Center. “If it’s unilateral, it’s almost always on the left side.”
Patients often have features of venous insufficiency that cause stasis, including varicose veins and brawny hyperpigmentation on the medial aspects of the ankles. “They have almost no systemic features: no fever, no white count, no lymphadenopathy,” she said. “These patients need some kind of anti-inflammatory medication because the skin is very inflamed. If you happened to take a biopsy, you would see inflammation as well as lymphatic congestion.”
Dr. Shinkai recommends that patients apply a midpotency topical steroid such as triamcinolone to the affected area, followed by compression, ideally antiembolism stockings (TED hose) – but that can be a hard sell.
“When your legs are that swollen, they’re really painful to wear,” she said. “Patients will say, ‘Don’t you come near me with those TED hose.’ If you’re in that situation, tell them to use an Ace wrap with light compression and each day tighten the Ace wrap a little more until they are able to use TED hose with minimal discomfort.”
The differential diagnosis for venous stasis dermatitis includes cellulitis (which rarely presents bilaterally), deep vein thrombosis, asteatotic dermatitis, erysipelas (more superficial cellulitis that results in elevated, shiny plaques), pyomyositis, necrotizing fasciitis, leukocytoclastic vasculitis, and allergic contact dermatitis.
Dr. Shinkai reported having no relevant financial disclosures.
SAN FRANCISCO – If a patient presents with bilateral cellulitis on both legs, think venous stasis dermatitis, which is the number one misdiagnosis of cellulitis and a frequent cause of unnecessary hospitalization for so-called “red leg,” according to Kanade Shinkai, MD, PhD.
“It’s easy to make that mistake, because you have a red, hot leg that’s painful, and the patient is having difficulty walking,” Dr. Shinkai said at the UCSF Annual Advances in Internal Medicine meeting. “Venous stasis dermatitis is one of the things you want to learn to recognize, as hospitalization is typically not needed.”
“That has to do with the venous return back to the heart,” said Dr. Shinkai, a dermatologist at UCSF Medical Center. “If it’s unilateral, it’s almost always on the left side.”
Patients often have features of venous insufficiency that cause stasis, including varicose veins and brawny hyperpigmentation on the medial aspects of the ankles. “They have almost no systemic features: no fever, no white count, no lymphadenopathy,” she said. “These patients need some kind of anti-inflammatory medication because the skin is very inflamed. If you happened to take a biopsy, you would see inflammation as well as lymphatic congestion.”
Dr. Shinkai recommends that patients apply a midpotency topical steroid such as triamcinolone to the affected area, followed by compression, ideally antiembolism stockings (TED hose) – but that can be a hard sell.
“When your legs are that swollen, they’re really painful to wear,” she said. “Patients will say, ‘Don’t you come near me with those TED hose.’ If you’re in that situation, tell them to use an Ace wrap with light compression and each day tighten the Ace wrap a little more until they are able to use TED hose with minimal discomfort.”
The differential diagnosis for venous stasis dermatitis includes cellulitis (which rarely presents bilaterally), deep vein thrombosis, asteatotic dermatitis, erysipelas (more superficial cellulitis that results in elevated, shiny plaques), pyomyositis, necrotizing fasciitis, leukocytoclastic vasculitis, and allergic contact dermatitis.
Dr. Shinkai reported having no relevant financial disclosures.
SAN FRANCISCO – If a patient presents with bilateral cellulitis on both legs, think venous stasis dermatitis, which is the number one misdiagnosis of cellulitis and a frequent cause of unnecessary hospitalization for so-called “red leg,” according to Kanade Shinkai, MD, PhD.
“It’s easy to make that mistake, because you have a red, hot leg that’s painful, and the patient is having difficulty walking,” Dr. Shinkai said at the UCSF Annual Advances in Internal Medicine meeting. “Venous stasis dermatitis is one of the things you want to learn to recognize, as hospitalization is typically not needed.”
“That has to do with the venous return back to the heart,” said Dr. Shinkai, a dermatologist at UCSF Medical Center. “If it’s unilateral, it’s almost always on the left side.”
Patients often have features of venous insufficiency that cause stasis, including varicose veins and brawny hyperpigmentation on the medial aspects of the ankles. “They have almost no systemic features: no fever, no white count, no lymphadenopathy,” she said. “These patients need some kind of anti-inflammatory medication because the skin is very inflamed. If you happened to take a biopsy, you would see inflammation as well as lymphatic congestion.”
Dr. Shinkai recommends that patients apply a midpotency topical steroid such as triamcinolone to the affected area, followed by compression, ideally antiembolism stockings (TED hose) – but that can be a hard sell.
“When your legs are that swollen, they’re really painful to wear,” she said. “Patients will say, ‘Don’t you come near me with those TED hose.’ If you’re in that situation, tell them to use an Ace wrap with light compression and each day tighten the Ace wrap a little more until they are able to use TED hose with minimal discomfort.”
The differential diagnosis for venous stasis dermatitis includes cellulitis (which rarely presents bilaterally), deep vein thrombosis, asteatotic dermatitis, erysipelas (more superficial cellulitis that results in elevated, shiny plaques), pyomyositis, necrotizing fasciitis, leukocytoclastic vasculitis, and allergic contact dermatitis.
Dr. Shinkai reported having no relevant financial disclosures.
AT THE ANNUAL ADVANCES IN INTERNAL MEDICINE
Pregnancy and MS: How do they affect each other?
NEW ORLEANS – Multiple sclerosis has little to no impact on the ability to conceive, on pregnancy, or on fetal status, according to Patricia K. Coyle, MD.
“That’s very reassuring,” Dr. Coyle said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “We don’t see an increase in birth defects just because the mother has MS. There is no consistent increase in abortions, ectopic pregnancies, or assisted vaginal/cesarean deliveries.”
Dr. Coyle, director of the MS Comprehensive Care Center at Stony Brook (N.Y.) University Medical Center, said that the most dramatic changes for pregnant patients with MS occur in the final trimester and mainly involve rising levels of multiple hormones: estrogens, cortisol, progesterone, norepinephrine, and 1,25-dihydroxyvitamin D, which increase late in pregnancy, then rapidly drop off postpartum. This has led to the evaluation of sex hormone therapy for MS.
The impact of other pregnancy factors on MS disease activity remains unknown. One is microchimerism, a maternal-fetal exchange of cells and DNA. “These cells can last for a long time; you can find them in the blood, as well as in the [central nervous system],” Dr. Coyle said. “It’s been reported that fetal microchimerism may be increased in immune-mediated diseases like MS, but we really don’t have a lot of good data.”
Researchers also are studying the impact of changes in the gut microbiota that occur during pregnancy. “Could this be a potential target for MS therapy?” Dr. Coyle asked. “This is in its infancy.”
Counseling tips
She went on to share counseling tips for MS patients of childbearing age, including the fact that some studies report slightly smaller babies born to mothers with MS, while others have not found that association. “This is a question mark, but it doesn’t seem to be a major issue,” she said. One thing you can tell patients for certain is that MS is not inherited. “There are well over 230 genes linked to MS, so there’s a genetic enrichment that can make somebody vulnerable to MS, but there’s no gene that passes on MS,” Dr. Coyle said. “The risk is slightly higher for a first-degree relative, so when a parent has MS, the risk for the child is in the range of 2% to 2.5%, compared with the expected 0.13% in the general population. But there’s a slightly higher risk when you’re a sibling than when you’re a parent – 2.7% – which is speaking to environmental factors having an important impact on genes.”
Controversial data exist as to whether a maternal deficiency in vitamin D poses a risk of MS in the offspring. Dr. Coyle makes it a point to “normalize” vitamin D levels in pregnant MS patients, particularly in white patients. “You’d want to have them on prenatal vitamins and folic acid and tell them not to smoke, to limit their alcohol use, and advise them to have good sleep hygiene.”
Dr. Coyle, vice chair of clinical affairs at Stony Brook University Medical Center, said that up until the 1950s, physicians advised women with MS against having children. “They were told not to get pregnant or to have an abortion, because it was thought to make MS worse,” she said. “It turns out that was fiction. That was completely wrong. Pregnancy has no negative effect on long-term MS prognosis. It may have long-term benefits for relapsing MS, but there are not enough data to comment on its impact on progressive MS. Pregnancy makes it less likely that someone will develop a clinically isolated syndrome, but it may increase the radiologically isolated syndrome risk for clinical attack. That’s based on 7 pregnant patients out of a cohort of 60, so we need further data to explain that.”
Disease-modifying therapies
When it comes to washouts of disease-modifying therapies (DMTs), no one-size-fits-all approach exists. Interferon betas and glatiramer acetate have more than 1,000 pregnancy exposures that yield no evidence for teratogenicity or negative fetal impact. No washout is needed prior to pregnancy. “These agents can be used during pregnancy and breastfeeding,” she said.
The other DMTs paint a somewhat different picture. “There is insufficient pregnancy exposure to the three available oral DMTs to comment definitively on their safety, but there is no clear human teratogenicity to date,” Dr. Coyle said. The conventional washout for fingolimod is 8 weeks. In Dr. Coyle’s opinion, no washout is required with dimethyl fumarate. “The half-life is 40 minutes. There are no good signs of issues. For teriflunomide, it can hang around in individuals for 18-24 months. You should go through an accelerated elimination procedure with oral cholestyramine 8 mg three times a day for 11 days until blood level of the agent is less than 0.02 mcg/mL. Avoid all the orals with breastfeeding.”
Monoclonal antibodies – another form of DMTs – lack sufficient pregnancy exposures to merit comment on safety, but they should not be used during breastfeeding. Natalizumab is a humanized IgG4 antibody that crosses the placenta. “This has been used in several dozen pregnancies because the patients got so bad when they were taken off that it required reinstituting natalizumab even though they were pregnant,” Dr. Coyle said. “Human pregnancy exposures have been associated with transient hematologic issues in the newborn, including anemia, thrombocytopenia, and pancytopenia.” Data indicate that the rate of spontaneous abortion among pregnant women treated with natalizumab was 9%, the rate of major birth defects was 5.05%, and no malformation pattern was observed. The drug is detected in human breast milk and has a half-life of 11 days.
Alemtuzumab is a humanized IgG1 monoclonal antibody that crosses the placenta. The half-life elimination is about 14 days. In transgenic mice, giving alemtuzumab during organogenesis was found to be embryolethal. In human pregnancy, hypothyroidism is a concern. “The recommendation has been to wait 4 months after the last treatment before you try to become pregnant. Alemtuzumab is considered a two-cycle treatment. You don’t get the maximum benefit after the first cycle of 5 days. The complete treatment is the second cycle 3 days.”
Daclizumab, another humanized IgG1 monoclonal antibody, also crosses the placenta. Monkey exposure during gestation led to embryofetal death and decreased fetal growth, “but this was at greater than 30 times the human dose,” she said. “It was found to be excreted in monkey breast milk and the half-life is 21 days.” In humans, there have been 36 exposed women who had 38 pregnancies and 20 live births. The rate of spontaneous abortions/miscarriages was 11%, there were eight elective terminations, two ectopic pregnancies, and one congenital heart defect. “This is very limited data, but nothing that would raise the level of concern,” Dr. Coyle said.
Ocrelizumab, another humanized IgG1 monoclonal antibody, was approved by the Food and Drug Administration in March 2017. Prior studies of anti-CD20 antibodies in human pregnancy noted transient lymphocytopenia and peripheral B cell depletion in the newborns. In studies of pregnant monkeys that used 2 and 10 times human doses during organogenesis, it was associated with B cell depletion in spleen/lymph nodes, Dr. Coyle said. “During organogenesis and throughout the neonatal period, treatment could be associated with perinatal death, some associated with bacterial infection; glomerulonephropathy with inflammation; a decrease in circulating B cells, a decrease in testicular weight, and bone marrow lymphoid follicle formation.” Ocrelizumab is excreted in monkey breast milk and the prescription label suggests a 6-month delay in pregnancy. The drug’s half-life is 26 days.
Dr. Coyle reported that she has served as a consultant for Accordant, Acorda, Bayer, Biogen, Celgene, Genentech/Roche, Genzyme/Sanofi, Novartis, Serono, and Teva. She has also received research support from Actelion, Alkermes, Genentech/Roche, MedDay, the National Institute of Neurological Disorders and Stroke, and Novartis.
NEW ORLEANS – Multiple sclerosis has little to no impact on the ability to conceive, on pregnancy, or on fetal status, according to Patricia K. Coyle, MD.
“That’s very reassuring,” Dr. Coyle said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “We don’t see an increase in birth defects just because the mother has MS. There is no consistent increase in abortions, ectopic pregnancies, or assisted vaginal/cesarean deliveries.”
Dr. Coyle, director of the MS Comprehensive Care Center at Stony Brook (N.Y.) University Medical Center, said that the most dramatic changes for pregnant patients with MS occur in the final trimester and mainly involve rising levels of multiple hormones: estrogens, cortisol, progesterone, norepinephrine, and 1,25-dihydroxyvitamin D, which increase late in pregnancy, then rapidly drop off postpartum. This has led to the evaluation of sex hormone therapy for MS.
The impact of other pregnancy factors on MS disease activity remains unknown. One is microchimerism, a maternal-fetal exchange of cells and DNA. “These cells can last for a long time; you can find them in the blood, as well as in the [central nervous system],” Dr. Coyle said. “It’s been reported that fetal microchimerism may be increased in immune-mediated diseases like MS, but we really don’t have a lot of good data.”
Researchers also are studying the impact of changes in the gut microbiota that occur during pregnancy. “Could this be a potential target for MS therapy?” Dr. Coyle asked. “This is in its infancy.”
Counseling tips
She went on to share counseling tips for MS patients of childbearing age, including the fact that some studies report slightly smaller babies born to mothers with MS, while others have not found that association. “This is a question mark, but it doesn’t seem to be a major issue,” she said. One thing you can tell patients for certain is that MS is not inherited. “There are well over 230 genes linked to MS, so there’s a genetic enrichment that can make somebody vulnerable to MS, but there’s no gene that passes on MS,” Dr. Coyle said. “The risk is slightly higher for a first-degree relative, so when a parent has MS, the risk for the child is in the range of 2% to 2.5%, compared with the expected 0.13% in the general population. But there’s a slightly higher risk when you’re a sibling than when you’re a parent – 2.7% – which is speaking to environmental factors having an important impact on genes.”
Controversial data exist as to whether a maternal deficiency in vitamin D poses a risk of MS in the offspring. Dr. Coyle makes it a point to “normalize” vitamin D levels in pregnant MS patients, particularly in white patients. “You’d want to have them on prenatal vitamins and folic acid and tell them not to smoke, to limit their alcohol use, and advise them to have good sleep hygiene.”
Dr. Coyle, vice chair of clinical affairs at Stony Brook University Medical Center, said that up until the 1950s, physicians advised women with MS against having children. “They were told not to get pregnant or to have an abortion, because it was thought to make MS worse,” she said. “It turns out that was fiction. That was completely wrong. Pregnancy has no negative effect on long-term MS prognosis. It may have long-term benefits for relapsing MS, but there are not enough data to comment on its impact on progressive MS. Pregnancy makes it less likely that someone will develop a clinically isolated syndrome, but it may increase the radiologically isolated syndrome risk for clinical attack. That’s based on 7 pregnant patients out of a cohort of 60, so we need further data to explain that.”
Disease-modifying therapies
When it comes to washouts of disease-modifying therapies (DMTs), no one-size-fits-all approach exists. Interferon betas and glatiramer acetate have more than 1,000 pregnancy exposures that yield no evidence for teratogenicity or negative fetal impact. No washout is needed prior to pregnancy. “These agents can be used during pregnancy and breastfeeding,” she said.
The other DMTs paint a somewhat different picture. “There is insufficient pregnancy exposure to the three available oral DMTs to comment definitively on their safety, but there is no clear human teratogenicity to date,” Dr. Coyle said. The conventional washout for fingolimod is 8 weeks. In Dr. Coyle’s opinion, no washout is required with dimethyl fumarate. “The half-life is 40 minutes. There are no good signs of issues. For teriflunomide, it can hang around in individuals for 18-24 months. You should go through an accelerated elimination procedure with oral cholestyramine 8 mg three times a day for 11 days until blood level of the agent is less than 0.02 mcg/mL. Avoid all the orals with breastfeeding.”
Monoclonal antibodies – another form of DMTs – lack sufficient pregnancy exposures to merit comment on safety, but they should not be used during breastfeeding. Natalizumab is a humanized IgG4 antibody that crosses the placenta. “This has been used in several dozen pregnancies because the patients got so bad when they were taken off that it required reinstituting natalizumab even though they were pregnant,” Dr. Coyle said. “Human pregnancy exposures have been associated with transient hematologic issues in the newborn, including anemia, thrombocytopenia, and pancytopenia.” Data indicate that the rate of spontaneous abortion among pregnant women treated with natalizumab was 9%, the rate of major birth defects was 5.05%, and no malformation pattern was observed. The drug is detected in human breast milk and has a half-life of 11 days.
Alemtuzumab is a humanized IgG1 monoclonal antibody that crosses the placenta. The half-life elimination is about 14 days. In transgenic mice, giving alemtuzumab during organogenesis was found to be embryolethal. In human pregnancy, hypothyroidism is a concern. “The recommendation has been to wait 4 months after the last treatment before you try to become pregnant. Alemtuzumab is considered a two-cycle treatment. You don’t get the maximum benefit after the first cycle of 5 days. The complete treatment is the second cycle 3 days.”
Daclizumab, another humanized IgG1 monoclonal antibody, also crosses the placenta. Monkey exposure during gestation led to embryofetal death and decreased fetal growth, “but this was at greater than 30 times the human dose,” she said. “It was found to be excreted in monkey breast milk and the half-life is 21 days.” In humans, there have been 36 exposed women who had 38 pregnancies and 20 live births. The rate of spontaneous abortions/miscarriages was 11%, there were eight elective terminations, two ectopic pregnancies, and one congenital heart defect. “This is very limited data, but nothing that would raise the level of concern,” Dr. Coyle said.
Ocrelizumab, another humanized IgG1 monoclonal antibody, was approved by the Food and Drug Administration in March 2017. Prior studies of anti-CD20 antibodies in human pregnancy noted transient lymphocytopenia and peripheral B cell depletion in the newborns. In studies of pregnant monkeys that used 2 and 10 times human doses during organogenesis, it was associated with B cell depletion in spleen/lymph nodes, Dr. Coyle said. “During organogenesis and throughout the neonatal period, treatment could be associated with perinatal death, some associated with bacterial infection; glomerulonephropathy with inflammation; a decrease in circulating B cells, a decrease in testicular weight, and bone marrow lymphoid follicle formation.” Ocrelizumab is excreted in monkey breast milk and the prescription label suggests a 6-month delay in pregnancy. The drug’s half-life is 26 days.
Dr. Coyle reported that she has served as a consultant for Accordant, Acorda, Bayer, Biogen, Celgene, Genentech/Roche, Genzyme/Sanofi, Novartis, Serono, and Teva. She has also received research support from Actelion, Alkermes, Genentech/Roche, MedDay, the National Institute of Neurological Disorders and Stroke, and Novartis.
NEW ORLEANS – Multiple sclerosis has little to no impact on the ability to conceive, on pregnancy, or on fetal status, according to Patricia K. Coyle, MD.
“That’s very reassuring,” Dr. Coyle said at the annual meeting of the Consortium of Multiple Sclerosis Centers. “We don’t see an increase in birth defects just because the mother has MS. There is no consistent increase in abortions, ectopic pregnancies, or assisted vaginal/cesarean deliveries.”
Dr. Coyle, director of the MS Comprehensive Care Center at Stony Brook (N.Y.) University Medical Center, said that the most dramatic changes for pregnant patients with MS occur in the final trimester and mainly involve rising levels of multiple hormones: estrogens, cortisol, progesterone, norepinephrine, and 1,25-dihydroxyvitamin D, which increase late in pregnancy, then rapidly drop off postpartum. This has led to the evaluation of sex hormone therapy for MS.
The impact of other pregnancy factors on MS disease activity remains unknown. One is microchimerism, a maternal-fetal exchange of cells and DNA. “These cells can last for a long time; you can find them in the blood, as well as in the [central nervous system],” Dr. Coyle said. “It’s been reported that fetal microchimerism may be increased in immune-mediated diseases like MS, but we really don’t have a lot of good data.”
Researchers also are studying the impact of changes in the gut microbiota that occur during pregnancy. “Could this be a potential target for MS therapy?” Dr. Coyle asked. “This is in its infancy.”
Counseling tips
She went on to share counseling tips for MS patients of childbearing age, including the fact that some studies report slightly smaller babies born to mothers with MS, while others have not found that association. “This is a question mark, but it doesn’t seem to be a major issue,” she said. One thing you can tell patients for certain is that MS is not inherited. “There are well over 230 genes linked to MS, so there’s a genetic enrichment that can make somebody vulnerable to MS, but there’s no gene that passes on MS,” Dr. Coyle said. “The risk is slightly higher for a first-degree relative, so when a parent has MS, the risk for the child is in the range of 2% to 2.5%, compared with the expected 0.13% in the general population. But there’s a slightly higher risk when you’re a sibling than when you’re a parent – 2.7% – which is speaking to environmental factors having an important impact on genes.”
Controversial data exist as to whether a maternal deficiency in vitamin D poses a risk of MS in the offspring. Dr. Coyle makes it a point to “normalize” vitamin D levels in pregnant MS patients, particularly in white patients. “You’d want to have them on prenatal vitamins and folic acid and tell them not to smoke, to limit their alcohol use, and advise them to have good sleep hygiene.”
Dr. Coyle, vice chair of clinical affairs at Stony Brook University Medical Center, said that up until the 1950s, physicians advised women with MS against having children. “They were told not to get pregnant or to have an abortion, because it was thought to make MS worse,” she said. “It turns out that was fiction. That was completely wrong. Pregnancy has no negative effect on long-term MS prognosis. It may have long-term benefits for relapsing MS, but there are not enough data to comment on its impact on progressive MS. Pregnancy makes it less likely that someone will develop a clinically isolated syndrome, but it may increase the radiologically isolated syndrome risk for clinical attack. That’s based on 7 pregnant patients out of a cohort of 60, so we need further data to explain that.”
Disease-modifying therapies
When it comes to washouts of disease-modifying therapies (DMTs), no one-size-fits-all approach exists. Interferon betas and glatiramer acetate have more than 1,000 pregnancy exposures that yield no evidence for teratogenicity or negative fetal impact. No washout is needed prior to pregnancy. “These agents can be used during pregnancy and breastfeeding,” she said.
The other DMTs paint a somewhat different picture. “There is insufficient pregnancy exposure to the three available oral DMTs to comment definitively on their safety, but there is no clear human teratogenicity to date,” Dr. Coyle said. The conventional washout for fingolimod is 8 weeks. In Dr. Coyle’s opinion, no washout is required with dimethyl fumarate. “The half-life is 40 minutes. There are no good signs of issues. For teriflunomide, it can hang around in individuals for 18-24 months. You should go through an accelerated elimination procedure with oral cholestyramine 8 mg three times a day for 11 days until blood level of the agent is less than 0.02 mcg/mL. Avoid all the orals with breastfeeding.”
Monoclonal antibodies – another form of DMTs – lack sufficient pregnancy exposures to merit comment on safety, but they should not be used during breastfeeding. Natalizumab is a humanized IgG4 antibody that crosses the placenta. “This has been used in several dozen pregnancies because the patients got so bad when they were taken off that it required reinstituting natalizumab even though they were pregnant,” Dr. Coyle said. “Human pregnancy exposures have been associated with transient hematologic issues in the newborn, including anemia, thrombocytopenia, and pancytopenia.” Data indicate that the rate of spontaneous abortion among pregnant women treated with natalizumab was 9%, the rate of major birth defects was 5.05%, and no malformation pattern was observed. The drug is detected in human breast milk and has a half-life of 11 days.
Alemtuzumab is a humanized IgG1 monoclonal antibody that crosses the placenta. The half-life elimination is about 14 days. In transgenic mice, giving alemtuzumab during organogenesis was found to be embryolethal. In human pregnancy, hypothyroidism is a concern. “The recommendation has been to wait 4 months after the last treatment before you try to become pregnant. Alemtuzumab is considered a two-cycle treatment. You don’t get the maximum benefit after the first cycle of 5 days. The complete treatment is the second cycle 3 days.”
Daclizumab, another humanized IgG1 monoclonal antibody, also crosses the placenta. Monkey exposure during gestation led to embryofetal death and decreased fetal growth, “but this was at greater than 30 times the human dose,” she said. “It was found to be excreted in monkey breast milk and the half-life is 21 days.” In humans, there have been 36 exposed women who had 38 pregnancies and 20 live births. The rate of spontaneous abortions/miscarriages was 11%, there were eight elective terminations, two ectopic pregnancies, and one congenital heart defect. “This is very limited data, but nothing that would raise the level of concern,” Dr. Coyle said.
Ocrelizumab, another humanized IgG1 monoclonal antibody, was approved by the Food and Drug Administration in March 2017. Prior studies of anti-CD20 antibodies in human pregnancy noted transient lymphocytopenia and peripheral B cell depletion in the newborns. In studies of pregnant monkeys that used 2 and 10 times human doses during organogenesis, it was associated with B cell depletion in spleen/lymph nodes, Dr. Coyle said. “During organogenesis and throughout the neonatal period, treatment could be associated with perinatal death, some associated with bacterial infection; glomerulonephropathy with inflammation; a decrease in circulating B cells, a decrease in testicular weight, and bone marrow lymphoid follicle formation.” Ocrelizumab is excreted in monkey breast milk and the prescription label suggests a 6-month delay in pregnancy. The drug’s half-life is 26 days.
Dr. Coyle reported that she has served as a consultant for Accordant, Acorda, Bayer, Biogen, Celgene, Genentech/Roche, Genzyme/Sanofi, Novartis, Serono, and Teva. She has also received research support from Actelion, Alkermes, Genentech/Roche, MedDay, the National Institute of Neurological Disorders and Stroke, and Novartis.
EXPERT ANALYSIS AT THE CMSC ANNUAL MEETING