User login
Rituximab for Acquired Hemophilia A in the Setting of Bullous Pemphigoid
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by the formation of antihemidesmosomal antibodies, resulting in tense bullae concentrated on the extremities and trunk that often are preceded by a pruritic urticarial phase.1 A rare complication of BP is the subsequent development of acquired hemophilia A. We report a case of BP with associated factor VIII–neutralizing antibodies in a patient who improved with prednisone and rituximab therapy.
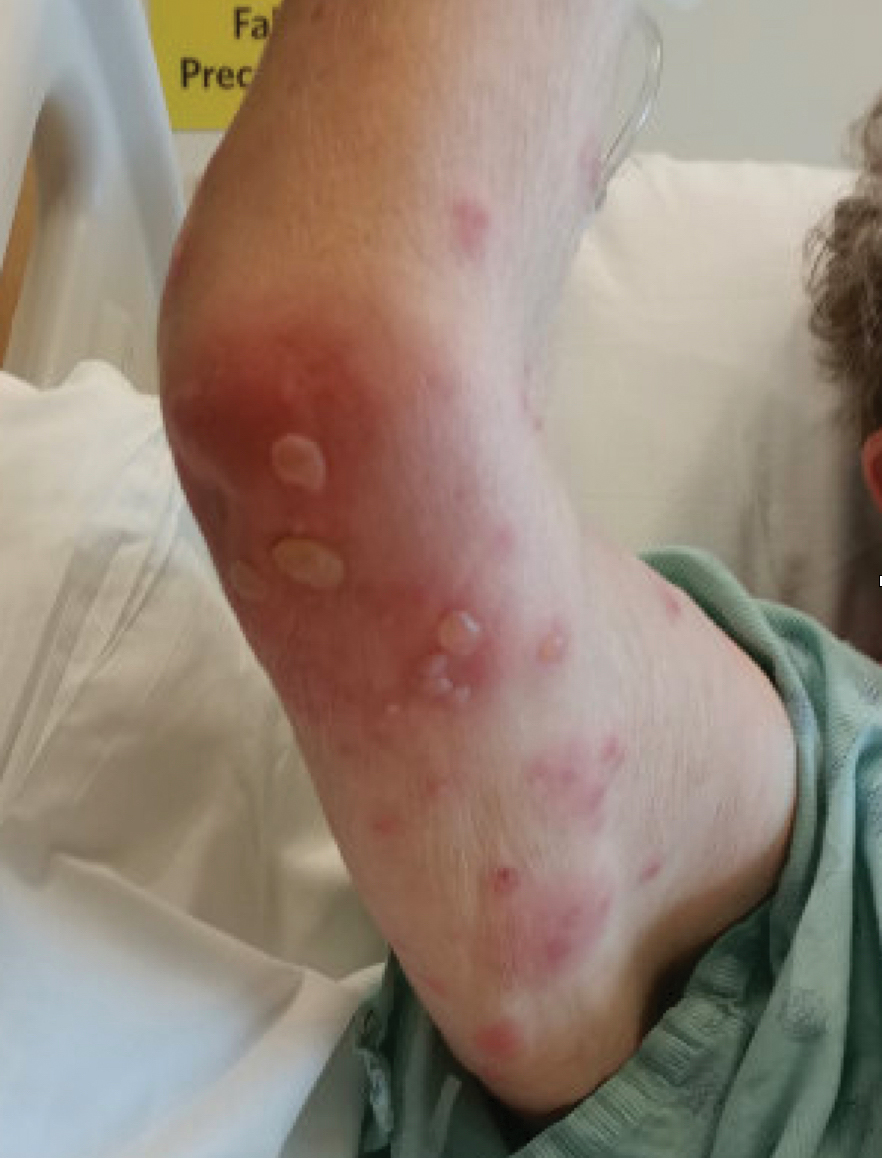
A 78-year-old woman presented with red-orange pruritic plaques on the right heel that spread to involve the arms and legs, abdomen, and trunk with new-onset bullae over the course of 2 weeks (Figure 1). Dermatology was consulted, and a diagnosis of BP was confirmed via biopsy and direct immunofluorescence.
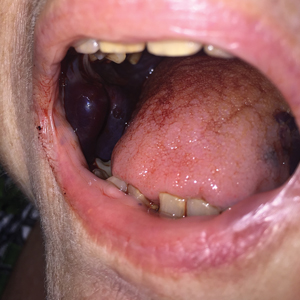
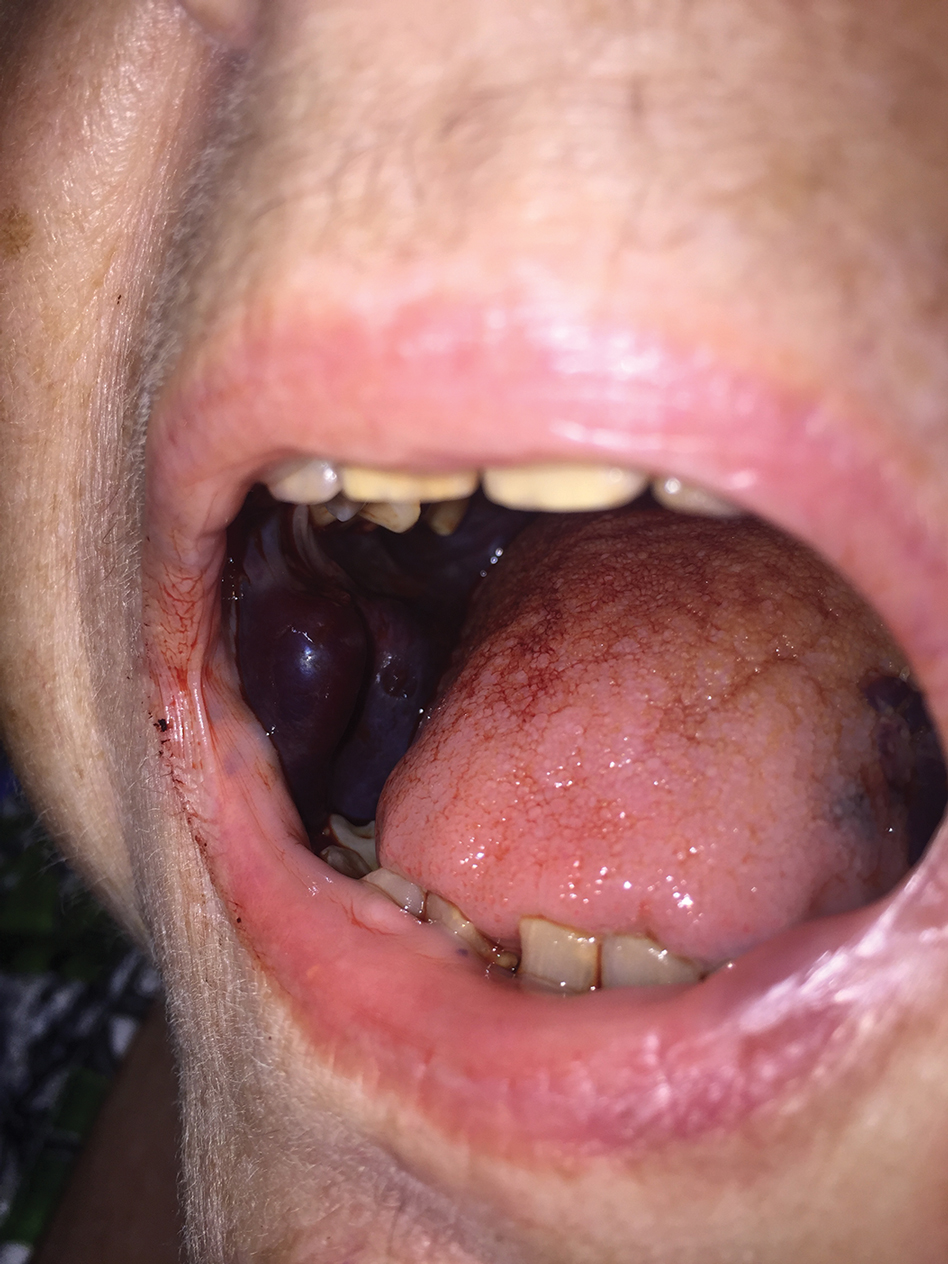
Despite treatment with prednisone 40 mg/d and clobetasol ointment 0.05%, she continued to develop extensive cutaneous bullae and new hemorrhagic bullae on the buccal mucosae (Figure 2), necessitating hospital admission. She clinically improved after prednisone was increased to 60 mg/d and mycophenolate mofetil 500 mg twice daily was added; however, she returned 8 days after discharge from the hospital with altered mental status, new-onset hematomas of the abdomen and right leg, and a hemoglobin level of 5.8 g/dL (reference range, 14.0–17.5 g/dL). Activated prothrombin time was prolonged without correction on mixing studies, raising concern for coagulation factor inhibition. Factor VIII activity was diminished to 9% and then 1% three days later. Mycophenolate mofetil was discontinued, and the patient was acutely stabilized with blood transfusions, intravenous immunoglobulin, tranexamic acid, and aminocaproic acid. Rituximab was initiated at 1000 mg and then administered again 2 weeks later. At 7-week follow-up, coagulation studies normalized, and there was no evidence of blistering dermatosis on examination.
Bullous pemphigoid generally is seen in patients older than 60 years, and the incidence increases with age. The disease course follows formation of IgG antibodies against BP180 or BP230, leading to localized activation of the complement cascade at the basement membrane zone.1 Medications, vaccinations, UV radiation, and burns have been implicated in disease induction.2
Identification of antihemidesmosomal antibodies on lesional biopsy via direct immunofluorescence is the gold standard for diagnosis, though indirect antibodies measured via enzyme-linked immunosorbent assay may provide information regarding disease severity.1 Patients with milder disease may be treated with topical corticosteroids, doxycycline, and nicotinamide; however, severe disease requires treatment with systemic glucocorticoids and steroid-sparing agents.3 Rituximab initially was approved by the US Food and Drug Administration for the treatment of pemphigus vulgaris, and mounting evidence for the use of rituximab in BP is promising. Although data are limited to retrospective studies, rituximab has shown notable remission rates and steroid-sparing effects in those with moderate to severe BP.4
Acquired hemophilia A (AHA) is caused by the production of IgG autoantibodies, which block physiologic interactions between factor VIII and factor IX, phospholipids, and von Willebrand factor.5 Acquired hemophilia A often is diagnosed by prolonged activated prothrombin time and decreased factor VIII activity after a previously unaffected patient develops severe bleeding. Treatment involves re-establishing hemostasis and the use of corticosteroids and immunosuppressive agents to diminish autoantibody production.4
Bullous pemphigoid–associated AHA likely is due to antigenic similarity between BP180 and factor VIII, leading to concomitant neutralization of factor VIII with the production of BP-associated autoantibodies.5 Bullous pemphigoid–associated AHA has been reported with manifestations of bleeding concurrent with or after the development of dermatologic disease. Rituximab use has been reported with clinical efficacy in several cases, including our patient.6 Continued hematologic monitoring is recommended, as recurrences are common within the first 2 years.5
- Bağcı IS, Horváth ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Schiavo AL, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013;31:391-399.
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320-332.
- Cho Y, Chu C, Wang L. First-line combination therapy with rituximab and corticosteroids provides a high complete remission rate in moderate-to-severe bullous pemphigoid. Br J Dermatol. 2015;173:302-304.
- Zdziarska J, Musial J. Acquired hemophilia A: an underdiagnosed severe bleeding disorder. Pol Arch Med Wewn. 2014;124:200-206.
- Binet Q, Lambert C, Sacré L, et al. Successful management of acquired hemophilia associated with bullous pemphigoid: a case report and review of the literature [published online March 28, 2017]. Case Rep Hematol. 2017;2017:2057019.
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by the formation of antihemidesmosomal antibodies, resulting in tense bullae concentrated on the extremities and trunk that often are preceded by a pruritic urticarial phase.1 A rare complication of BP is the subsequent development of acquired hemophilia A. We report a case of BP with associated factor VIII–neutralizing antibodies in a patient who improved with prednisone and rituximab therapy.
A 78-year-old woman presented with red-orange pruritic plaques on the right heel that spread to involve the arms and legs, abdomen, and trunk with new-onset bullae over the course of 2 weeks (Figure 1). Dermatology was consulted, and a diagnosis of BP was confirmed via biopsy and direct immunofluorescence.

Despite treatment with prednisone 40 mg/d and clobetasol ointment 0.05%, she continued to develop extensive cutaneous bullae and new hemorrhagic bullae on the buccal mucosae (Figure 2), necessitating hospital admission. She clinically improved after prednisone was increased to 60 mg/d and mycophenolate mofetil 500 mg twice daily was added; however, she returned 8 days after discharge from the hospital with altered mental status, new-onset hematomas of the abdomen and right leg, and a hemoglobin level of 5.8 g/dL (reference range, 14.0–17.5 g/dL). Activated prothrombin time was prolonged without correction on mixing studies, raising concern for coagulation factor inhibition. Factor VIII activity was diminished to 9% and then 1% three days later. Mycophenolate mofetil was discontinued, and the patient was acutely stabilized with blood transfusions, intravenous immunoglobulin, tranexamic acid, and aminocaproic acid. Rituximab was initiated at 1000 mg and then administered again 2 weeks later. At 7-week follow-up, coagulation studies normalized, and there was no evidence of blistering dermatosis on examination.

Bullous pemphigoid generally is seen in patients older than 60 years, and the incidence increases with age. The disease course follows formation of IgG antibodies against BP180 or BP230, leading to localized activation of the complement cascade at the basement membrane zone.1 Medications, vaccinations, UV radiation, and burns have been implicated in disease induction.2
Identification of antihemidesmosomal antibodies on lesional biopsy via direct immunofluorescence is the gold standard for diagnosis, though indirect antibodies measured via enzyme-linked immunosorbent assay may provide information regarding disease severity.1 Patients with milder disease may be treated with topical corticosteroids, doxycycline, and nicotinamide; however, severe disease requires treatment with systemic glucocorticoids and steroid-sparing agents.3 Rituximab initially was approved by the US Food and Drug Administration for the treatment of pemphigus vulgaris, and mounting evidence for the use of rituximab in BP is promising. Although data are limited to retrospective studies, rituximab has shown notable remission rates and steroid-sparing effects in those with moderate to severe BP.4
Acquired hemophilia A (AHA) is caused by the production of IgG autoantibodies, which block physiologic interactions between factor VIII and factor IX, phospholipids, and von Willebrand factor.5 Acquired hemophilia A often is diagnosed by prolonged activated prothrombin time and decreased factor VIII activity after a previously unaffected patient develops severe bleeding. Treatment involves re-establishing hemostasis and the use of corticosteroids and immunosuppressive agents to diminish autoantibody production.4
Bullous pemphigoid–associated AHA likely is due to antigenic similarity between BP180 and factor VIII, leading to concomitant neutralization of factor VIII with the production of BP-associated autoantibodies.5 Bullous pemphigoid–associated AHA has been reported with manifestations of bleeding concurrent with or after the development of dermatologic disease. Rituximab use has been reported with clinical efficacy in several cases, including our patient.6 Continued hematologic monitoring is recommended, as recurrences are common within the first 2 years.5
To the Editor:
Bullous pemphigoid (BP) is an autoimmune blistering disease characterized by the formation of antihemidesmosomal antibodies, resulting in tense bullae concentrated on the extremities and trunk that often are preceded by a pruritic urticarial phase.1 A rare complication of BP is the subsequent development of acquired hemophilia A. We report a case of BP with associated factor VIII–neutralizing antibodies in a patient who improved with prednisone and rituximab therapy.
A 78-year-old woman presented with red-orange pruritic plaques on the right heel that spread to involve the arms and legs, abdomen, and trunk with new-onset bullae over the course of 2 weeks (Figure 1). Dermatology was consulted, and a diagnosis of BP was confirmed via biopsy and direct immunofluorescence.

Despite treatment with prednisone 40 mg/d and clobetasol ointment 0.05%, she continued to develop extensive cutaneous bullae and new hemorrhagic bullae on the buccal mucosae (Figure 2), necessitating hospital admission. She clinically improved after prednisone was increased to 60 mg/d and mycophenolate mofetil 500 mg twice daily was added; however, she returned 8 days after discharge from the hospital with altered mental status, new-onset hematomas of the abdomen and right leg, and a hemoglobin level of 5.8 g/dL (reference range, 14.0–17.5 g/dL). Activated prothrombin time was prolonged without correction on mixing studies, raising concern for coagulation factor inhibition. Factor VIII activity was diminished to 9% and then 1% three days later. Mycophenolate mofetil was discontinued, and the patient was acutely stabilized with blood transfusions, intravenous immunoglobulin, tranexamic acid, and aminocaproic acid. Rituximab was initiated at 1000 mg and then administered again 2 weeks later. At 7-week follow-up, coagulation studies normalized, and there was no evidence of blistering dermatosis on examination.

Bullous pemphigoid generally is seen in patients older than 60 years, and the incidence increases with age. The disease course follows formation of IgG antibodies against BP180 or BP230, leading to localized activation of the complement cascade at the basement membrane zone.1 Medications, vaccinations, UV radiation, and burns have been implicated in disease induction.2
Identification of antihemidesmosomal antibodies on lesional biopsy via direct immunofluorescence is the gold standard for diagnosis, though indirect antibodies measured via enzyme-linked immunosorbent assay may provide information regarding disease severity.1 Patients with milder disease may be treated with topical corticosteroids, doxycycline, and nicotinamide; however, severe disease requires treatment with systemic glucocorticoids and steroid-sparing agents.3 Rituximab initially was approved by the US Food and Drug Administration for the treatment of pemphigus vulgaris, and mounting evidence for the use of rituximab in BP is promising. Although data are limited to retrospective studies, rituximab has shown notable remission rates and steroid-sparing effects in those with moderate to severe BP.4
Acquired hemophilia A (AHA) is caused by the production of IgG autoantibodies, which block physiologic interactions between factor VIII and factor IX, phospholipids, and von Willebrand factor.5 Acquired hemophilia A often is diagnosed by prolonged activated prothrombin time and decreased factor VIII activity after a previously unaffected patient develops severe bleeding. Treatment involves re-establishing hemostasis and the use of corticosteroids and immunosuppressive agents to diminish autoantibody production.4
Bullous pemphigoid–associated AHA likely is due to antigenic similarity between BP180 and factor VIII, leading to concomitant neutralization of factor VIII with the production of BP-associated autoantibodies.5 Bullous pemphigoid–associated AHA has been reported with manifestations of bleeding concurrent with or after the development of dermatologic disease. Rituximab use has been reported with clinical efficacy in several cases, including our patient.6 Continued hematologic monitoring is recommended, as recurrences are common within the first 2 years.5
- Bağcı IS, Horváth ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Schiavo AL, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013;31:391-399.
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320-332.
- Cho Y, Chu C, Wang L. First-line combination therapy with rituximab and corticosteroids provides a high complete remission rate in moderate-to-severe bullous pemphigoid. Br J Dermatol. 2015;173:302-304.
- Zdziarska J, Musial J. Acquired hemophilia A: an underdiagnosed severe bleeding disorder. Pol Arch Med Wewn. 2014;124:200-206.
- Binet Q, Lambert C, Sacré L, et al. Successful management of acquired hemophilia associated with bullous pemphigoid: a case report and review of the literature [published online March 28, 2017]. Case Rep Hematol. 2017;2017:2057019.
- Bağcı IS, Horváth ON, Ruzicka T, et al. Bullous pemphigoid. Autoimmun Rev. 2017;16:445-455.
- Schiavo AL, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013;31:391-399.
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320-332.
- Cho Y, Chu C, Wang L. First-line combination therapy with rituximab and corticosteroids provides a high complete remission rate in moderate-to-severe bullous pemphigoid. Br J Dermatol. 2015;173:302-304.
- Zdziarska J, Musial J. Acquired hemophilia A: an underdiagnosed severe bleeding disorder. Pol Arch Med Wewn. 2014;124:200-206.
- Binet Q, Lambert C, Sacré L, et al. Successful management of acquired hemophilia associated with bullous pemphigoid: a case report and review of the literature [published online March 28, 2017]. Case Rep Hematol. 2017;2017:2057019.
Practice Points
- Physicians must be aware of the potential for acquired hemophilia A in patients with bullous pemphigoid (BP).
- Rituximab is an effective therapy for BP and should be considered for patients in this cohort.
Seborrhea Herpeticum: Cutaneous Herpes Simplex Virus Infection Within Infantile Seborrheic Dermatitis
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
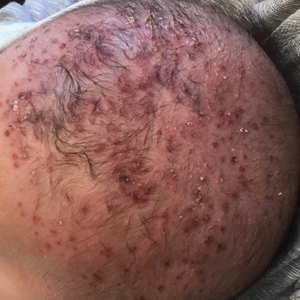
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.
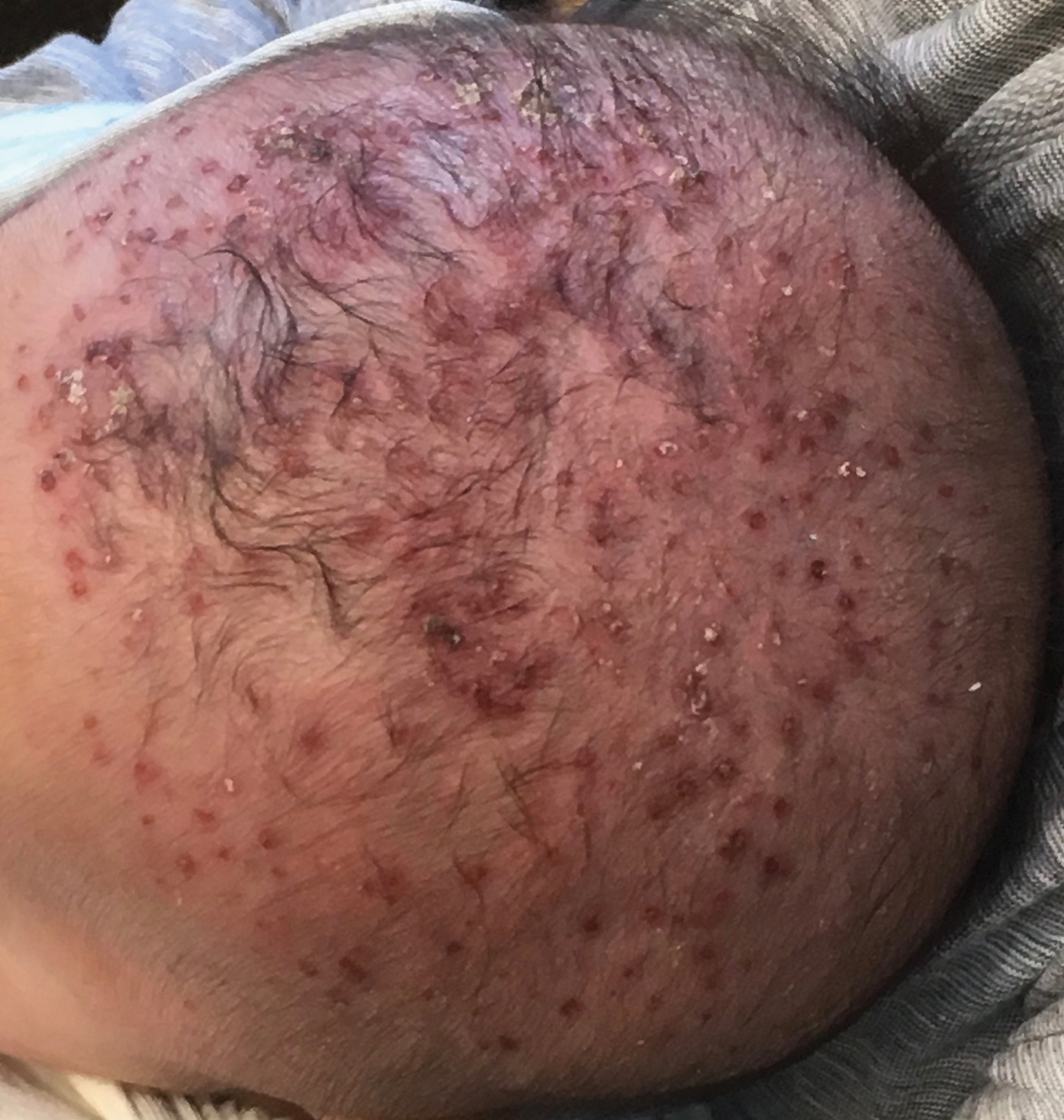
Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.

Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
Classically, eczema herpeticum is associated with atopic dermatitis (AD), but it also has been previously reported in the setting of pemphigus vulgaris, Darier disease, ichthyosis vulgaris, burns, psoriasis, and irritant contact dermatitis.1,2 Descriptions of cutaneous herpes simplex virus (HSV) in the setting of seborrheic dermatitis are lacking.
Case Report
A 2-month-old infant boy who was otherwise healthy presented to the emergency department with a new rash on the scalp. Initially there were a few clusters of small fluid-filled lesions that evolved over several days into diffuse clusters covering the scalp and extending onto the forehead and upper chest (Figure). The patient’s medical history was notable for infantile seborrheic dermatitis and a family history of AD. His grandmother, who was his primary caretaker, had a recent history of herpes labialis.

Physical examination revealed numerous discrete, erythematous, and punched-out erosions diffusely on the scalp. There were fewer similar erosions on the forehead and upper chest. There were no oral or periocular lesions. There were no areas of lichenification or eczematous plaques on the remainder of the trunk or extremities. Laboratory testing was positive for HSV type 1 polymerase chain reaction and positive for HSV type 1 viral culture. Liver enzymes were elevated with alanine aminotransferase at 107 U/L (reference range, 7–52 U/L) and aspartate aminotransferase at 94 U/L (reference range, 13–39 U/L).
The patient was admitted to the hospital and was treated by the dermatology and infectious disease services. Intravenous acyclovir 60 mg/kg daily was administered for 3 days until all lesions had crusted over. On the day of discharge, the patient was transitioned to oral valacyclovir 20 mg/kg daily for 7 days with resolution. One month later he developed a recurrence that was within his existing seborrheic dermatitis. After a repeat 7-day course of oral valacyclovir 20 mg/kg daily, he was placed on prophylaxis therapy of oral acyclovir 10 mg/kg daily. Gentle skin care precautions also were recommended.
Comment
Eczema herpeticum refers to disseminated cutaneous infection with HSV types 1 or 2 in the setting of underlying dermatosis.2 Although it is classically associated with AD, it has been reported in a number of other chronic skin disorders and can lead to serious complications, including hepatitis, keratoconjunctivitis, and meningitis. In those with AD who develop HSV, presentation may occur in active dermatitis locations because of skin barrier disruption, which may lead to increased susceptibility to viral infection.3
Herpes simplex virus in a background of seborrheic dermatitis has not been well described. Although the pathogenesis of seborrheic dermatitis has not been fully reported, several gene mutations and protein deficiencies have been identified in patients and animal models that are associated with immune response or epidermal differentiation.4 Therefore, it is possible that, as with AD, a disruption in the skin barrier increases susceptibility to viral infection.
It also has been suggested that infantile seborrheic dermatitis and AD represent the same spectrum of disease.5 Given our patient’s family history of AD, it is possible his presentation represents early underlying AD. Providers should be aware that cutaneous HSV can be confined to a seborrheic distribution and may represent underlying epidermal dysfunction secondary to seborrheic dermatitis.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
- Wheeler CE, Abele DC. Eczema herpeticum, primary and recurrent. Arch Dermatol. 1966;93:162-173.
- Santmyire-Rosenberger BR, Nigra TP. Psoriasis herpeticum: three cases of Kaposi’s varicelliform eruption in psoriasis. J Am Acad Dermatol. 2005;53:52-56.
- Wollenberg A, Wetzel S, Burgdorf WH, et al. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667-674.
- Karakadze M, Hirt P, Wikramanayake T. The genetic basis of seborrhoeic dermatitis: a review. J Eur Acad Dermatol Venereol. 2017;32:529-536.
- Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis. Pediatr Dermatol. 2013;31:125-130.
Practice Points
- Cutaneous herpes simplex virus may present in a seborrheic distribution within infantile seborrheic dermatitis, suggesting underlying dysfunction secondary to seborrheic dermatitis.
- Treatment of seborrhea herpeticum involves antiviral therapy to treat the secondary viral infection and gentle skin care precautions for the primary condition.