User login
Damian McNamara is a journalist for Medscape Medical News and MDedge. He worked full-time for MDedge as the Miami Bureau covering a dozen medical specialties during 2001-2012, then as a freelancer for Medscape and MDedge, before being hired on staff by Medscape in 2018. Now the two companies are one. He uses what he learned in school – Damian has a BS in chemistry and an MS in science, health and environmental reporting/journalism. He works out of a home office in Miami, with a 100-pound chocolate lab known to snore under his desk during work hours.
Survey: More than half of gynecologic oncologists altered morcellation practices
ORLANDO – Following the Food and Drug Administration’s 2014 warning against use of laparoscopic uterine power morcellation, 13% of gynecologic oncologists decreased their use of the technique and another 39% discontinued it altogether, according to survey responses from 199 members of the Society of Gynecologic Oncology.
“This really gives a snapshot about how gynecologic oncologists feel about power morcellation in light of the FDA warning,” said Kerac N. Falk, MD, a resident at the Icahn School of Medicine at Mount Sinai, New York.
About 41% of gynecologic oncologists changed their surgical technique to minimally invasive without power morcellation. Another 20% of respondents who previously used power morcellation have switched to laparotomy.
A more rigorous informed consent process, better attention to patient selection, and enhanced protocols are positive effects emerging since the FDA Safety Communication was issued in April 2014, Dr. Falk said at the meeting sponsored by AAGL.
The 34-item survey included questions about demographics, institutional policies, and attitudes before and after the FDA warning. Among the respondents, 65% were men. Both “early” and “very seasoned” surgeons participated in the survey. The majority of the respondents were moderate- to high-volume surgeons.
Men were significantly more likely to decrease or discontinue use of power morcellation, compared with women (P = .0015). Region of practice, years in practice, or institution type did not significantly influence changes in practice. “Most said it was not a personal choice, but more about patient choice or an institutional policy change,” Dr. Falk said.
There is still a role for power morcellation in carefully selected patients, Dr. Falk added.
In July 2014, AAGL issued a statement in response to the FDA warning, stating that “we should improve but not abandon power morcellation, and that power morcellation with appropriate informed consent should remain available to appropriately screened, low-risk women.” In addition, the American College of Obstetricians and Gynecologists stated, “Although the worsening of an occult malignancy as a result of power morcellation is, of course, tragic, we believe that an approach that combines deliberate patient selection criteria with robust informed consent will help protect women from a negative outcome, while maintaining access to morcellation for women who would benefit from it.”
Dr. Falk is not a coauthor on the study. He presented the findings on behalf of a colleague unable to attend the meeting. Dr. Falk reported having no relevant financial disclosures.
ORLANDO – Following the Food and Drug Administration’s 2014 warning against use of laparoscopic uterine power morcellation, 13% of gynecologic oncologists decreased their use of the technique and another 39% discontinued it altogether, according to survey responses from 199 members of the Society of Gynecologic Oncology.
“This really gives a snapshot about how gynecologic oncologists feel about power morcellation in light of the FDA warning,” said Kerac N. Falk, MD, a resident at the Icahn School of Medicine at Mount Sinai, New York.
About 41% of gynecologic oncologists changed their surgical technique to minimally invasive without power morcellation. Another 20% of respondents who previously used power morcellation have switched to laparotomy.
A more rigorous informed consent process, better attention to patient selection, and enhanced protocols are positive effects emerging since the FDA Safety Communication was issued in April 2014, Dr. Falk said at the meeting sponsored by AAGL.
The 34-item survey included questions about demographics, institutional policies, and attitudes before and after the FDA warning. Among the respondents, 65% were men. Both “early” and “very seasoned” surgeons participated in the survey. The majority of the respondents were moderate- to high-volume surgeons.
Men were significantly more likely to decrease or discontinue use of power morcellation, compared with women (P = .0015). Region of practice, years in practice, or institution type did not significantly influence changes in practice. “Most said it was not a personal choice, but more about patient choice or an institutional policy change,” Dr. Falk said.
There is still a role for power morcellation in carefully selected patients, Dr. Falk added.
In July 2014, AAGL issued a statement in response to the FDA warning, stating that “we should improve but not abandon power morcellation, and that power morcellation with appropriate informed consent should remain available to appropriately screened, low-risk women.” In addition, the American College of Obstetricians and Gynecologists stated, “Although the worsening of an occult malignancy as a result of power morcellation is, of course, tragic, we believe that an approach that combines deliberate patient selection criteria with robust informed consent will help protect women from a negative outcome, while maintaining access to morcellation for women who would benefit from it.”
Dr. Falk is not a coauthor on the study. He presented the findings on behalf of a colleague unable to attend the meeting. Dr. Falk reported having no relevant financial disclosures.
ORLANDO – Following the Food and Drug Administration’s 2014 warning against use of laparoscopic uterine power morcellation, 13% of gynecologic oncologists decreased their use of the technique and another 39% discontinued it altogether, according to survey responses from 199 members of the Society of Gynecologic Oncology.
“This really gives a snapshot about how gynecologic oncologists feel about power morcellation in light of the FDA warning,” said Kerac N. Falk, MD, a resident at the Icahn School of Medicine at Mount Sinai, New York.
About 41% of gynecologic oncologists changed their surgical technique to minimally invasive without power morcellation. Another 20% of respondents who previously used power morcellation have switched to laparotomy.
A more rigorous informed consent process, better attention to patient selection, and enhanced protocols are positive effects emerging since the FDA Safety Communication was issued in April 2014, Dr. Falk said at the meeting sponsored by AAGL.
The 34-item survey included questions about demographics, institutional policies, and attitudes before and after the FDA warning. Among the respondents, 65% were men. Both “early” and “very seasoned” surgeons participated in the survey. The majority of the respondents were moderate- to high-volume surgeons.
Men were significantly more likely to decrease or discontinue use of power morcellation, compared with women (P = .0015). Region of practice, years in practice, or institution type did not significantly influence changes in practice. “Most said it was not a personal choice, but more about patient choice or an institutional policy change,” Dr. Falk said.
There is still a role for power morcellation in carefully selected patients, Dr. Falk added.
In July 2014, AAGL issued a statement in response to the FDA warning, stating that “we should improve but not abandon power morcellation, and that power morcellation with appropriate informed consent should remain available to appropriately screened, low-risk women.” In addition, the American College of Obstetricians and Gynecologists stated, “Although the worsening of an occult malignancy as a result of power morcellation is, of course, tragic, we believe that an approach that combines deliberate patient selection criteria with robust informed consent will help protect women from a negative outcome, while maintaining access to morcellation for women who would benefit from it.”
Dr. Falk is not a coauthor on the study. He presented the findings on behalf of a colleague unable to attend the meeting. Dr. Falk reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Key clinical point:
Major finding: Among 199 members of the Society of Gynecologic Oncology, 39% said they suspended use of power morcellation, and 13% decreased their use of the technique.
Data source: A survey sent to all members of the Society of Gynecologic Oncology with responses from 199 members.
Disclosures: Dr. Falk reported having no relevant financial disclosures.
Low rate of occult uterine malignancy with vaginal morcellation
ORLANDO – The Food and Drug Administration’s 2014 warning that laparoscopic power morcellation during hysterectomy or myomectomy could spread unsuspected cancerous tissue had a chilling effect across the specialty, but what about the risks associated with morcellation during vaginal hysterectomy?
“There is only one case of morcellation during vaginal hysterectomy with a leiomyosarcoma recorded in the literature,” Megan N. Wasson, DO, a fellow in minimally invasive gynecologic surgery at the Mayo Clinic in Phoenix said at the meeting sponsored by AAGL. “It is really unclear if vaginal and electromechanical morcellation carry the same inherent risk.”
To find out more, Dr. Wasson and her colleagues identified 2,296 patients who underwent total vaginal hysterectomy at one of three academic medical centers. A total of 611 of these women had uterine removal with uncontained morcellation via cold-knife wedge resection. The investigators assessed this group for incidence of occult malignancy, perioperative outcomes, and long-term survival in a retrospective cohort study.
Of the 611 women who underwent morcellation during the study, five patients had an occult malignancy, for a rate less than one percent, 0.82%. Three patients had a stage IA, grade I endometrial adenocarcinoma, and two patients had a low-grade stromal sarcoma. No patients had a leiomyosarcoma.
This group of five patients had a mean age of 49 years, a mean BMI of 32 kg/m2 and a median parity of two. Abnormal uterine bleeding was the indication for surgery for all five patients with a malignancy. The mean uterine weight was elevated at 231 g. One patient with endometrial adenocarcinoma later underwent pelvic lymphadenectomy and vaginal brachytherapy.
“So far, thankfully, all of these patients show no evidence of disease recurrence,” Dr. Wasson said. All five patients are alive, with a mean disease-free survival of 43 months among those with endometrial adenocarcinoma and 37 months for the low-grade stroma sarcoma patients.
“Overall, the incidence of occult uterine carcinoma at the time of vaginal hysterectomy is less than 1%,” Dr. Wasson said. “Thankfully, it does not appear to have a negative effect on patient outcomes when it occurs.”
More research is needed, however. “The risk is very limited in terms of what we know,” she said. “We investigated cancer in this study, but there is also a risk of dissemination of benign conditions.”
All patients underwent a preoperative evaluation that included sampling of the lining of the uterus and imaging. “Out of the five patients with carcinomas, two of the adenocarcinomas had completely benign preoperative sampling and one had hyperplasia, which unfortunately did develop into occult disease,” Dr. Wasson said. “We wouldn’t recommend morcellating any patient with hyperplasia. In the two patients with low-grade stromal sarcoma, neither had any hyperplasia on preoperative sampling.”
Following the 2014 FDA Safety Communication on power morcellation, the AAGL released its own guidance on morcellation during uterine tissue extraction. The AAGL recommended that clinicians avoid morcellation for any patient who had a premalignant or malignant condition or who was at risk for malignancy, and use caution when considering morcellation. “This was for all types of morcellation, including electromechanical and vaginal morcellation,” Dr. Wasson said.
“This was in response to studies and awareness of increased risk of disease with morcellation – specifically leiomyosarcomas – for dissemination of disease in the abdomen and pelvis, but also for an increased risk of recurrence,” she said. “This means, in turn, that patients can have decreased overall survival and disease-free survival, so this is very important when we are talking to our patients.”
Dr. Wasson reported having no relevant financial disclosures.
ORLANDO – The Food and Drug Administration’s 2014 warning that laparoscopic power morcellation during hysterectomy or myomectomy could spread unsuspected cancerous tissue had a chilling effect across the specialty, but what about the risks associated with morcellation during vaginal hysterectomy?
“There is only one case of morcellation during vaginal hysterectomy with a leiomyosarcoma recorded in the literature,” Megan N. Wasson, DO, a fellow in minimally invasive gynecologic surgery at the Mayo Clinic in Phoenix said at the meeting sponsored by AAGL. “It is really unclear if vaginal and electromechanical morcellation carry the same inherent risk.”
To find out more, Dr. Wasson and her colleagues identified 2,296 patients who underwent total vaginal hysterectomy at one of three academic medical centers. A total of 611 of these women had uterine removal with uncontained morcellation via cold-knife wedge resection. The investigators assessed this group for incidence of occult malignancy, perioperative outcomes, and long-term survival in a retrospective cohort study.
Of the 611 women who underwent morcellation during the study, five patients had an occult malignancy, for a rate less than one percent, 0.82%. Three patients had a stage IA, grade I endometrial adenocarcinoma, and two patients had a low-grade stromal sarcoma. No patients had a leiomyosarcoma.
This group of five patients had a mean age of 49 years, a mean BMI of 32 kg/m2 and a median parity of two. Abnormal uterine bleeding was the indication for surgery for all five patients with a malignancy. The mean uterine weight was elevated at 231 g. One patient with endometrial adenocarcinoma later underwent pelvic lymphadenectomy and vaginal brachytherapy.
“So far, thankfully, all of these patients show no evidence of disease recurrence,” Dr. Wasson said. All five patients are alive, with a mean disease-free survival of 43 months among those with endometrial adenocarcinoma and 37 months for the low-grade stroma sarcoma patients.
“Overall, the incidence of occult uterine carcinoma at the time of vaginal hysterectomy is less than 1%,” Dr. Wasson said. “Thankfully, it does not appear to have a negative effect on patient outcomes when it occurs.”
More research is needed, however. “The risk is very limited in terms of what we know,” she said. “We investigated cancer in this study, but there is also a risk of dissemination of benign conditions.”
All patients underwent a preoperative evaluation that included sampling of the lining of the uterus and imaging. “Out of the five patients with carcinomas, two of the adenocarcinomas had completely benign preoperative sampling and one had hyperplasia, which unfortunately did develop into occult disease,” Dr. Wasson said. “We wouldn’t recommend morcellating any patient with hyperplasia. In the two patients with low-grade stromal sarcoma, neither had any hyperplasia on preoperative sampling.”
Following the 2014 FDA Safety Communication on power morcellation, the AAGL released its own guidance on morcellation during uterine tissue extraction. The AAGL recommended that clinicians avoid morcellation for any patient who had a premalignant or malignant condition or who was at risk for malignancy, and use caution when considering morcellation. “This was for all types of morcellation, including electromechanical and vaginal morcellation,” Dr. Wasson said.
“This was in response to studies and awareness of increased risk of disease with morcellation – specifically leiomyosarcomas – for dissemination of disease in the abdomen and pelvis, but also for an increased risk of recurrence,” she said. “This means, in turn, that patients can have decreased overall survival and disease-free survival, so this is very important when we are talking to our patients.”
Dr. Wasson reported having no relevant financial disclosures.
ORLANDO – The Food and Drug Administration’s 2014 warning that laparoscopic power morcellation during hysterectomy or myomectomy could spread unsuspected cancerous tissue had a chilling effect across the specialty, but what about the risks associated with morcellation during vaginal hysterectomy?
“There is only one case of morcellation during vaginal hysterectomy with a leiomyosarcoma recorded in the literature,” Megan N. Wasson, DO, a fellow in minimally invasive gynecologic surgery at the Mayo Clinic in Phoenix said at the meeting sponsored by AAGL. “It is really unclear if vaginal and electromechanical morcellation carry the same inherent risk.”
To find out more, Dr. Wasson and her colleagues identified 2,296 patients who underwent total vaginal hysterectomy at one of three academic medical centers. A total of 611 of these women had uterine removal with uncontained morcellation via cold-knife wedge resection. The investigators assessed this group for incidence of occult malignancy, perioperative outcomes, and long-term survival in a retrospective cohort study.
Of the 611 women who underwent morcellation during the study, five patients had an occult malignancy, for a rate less than one percent, 0.82%. Three patients had a stage IA, grade I endometrial adenocarcinoma, and two patients had a low-grade stromal sarcoma. No patients had a leiomyosarcoma.
This group of five patients had a mean age of 49 years, a mean BMI of 32 kg/m2 and a median parity of two. Abnormal uterine bleeding was the indication for surgery for all five patients with a malignancy. The mean uterine weight was elevated at 231 g. One patient with endometrial adenocarcinoma later underwent pelvic lymphadenectomy and vaginal brachytherapy.
“So far, thankfully, all of these patients show no evidence of disease recurrence,” Dr. Wasson said. All five patients are alive, with a mean disease-free survival of 43 months among those with endometrial adenocarcinoma and 37 months for the low-grade stroma sarcoma patients.
“Overall, the incidence of occult uterine carcinoma at the time of vaginal hysterectomy is less than 1%,” Dr. Wasson said. “Thankfully, it does not appear to have a negative effect on patient outcomes when it occurs.”
More research is needed, however. “The risk is very limited in terms of what we know,” she said. “We investigated cancer in this study, but there is also a risk of dissemination of benign conditions.”
All patients underwent a preoperative evaluation that included sampling of the lining of the uterus and imaging. “Out of the five patients with carcinomas, two of the adenocarcinomas had completely benign preoperative sampling and one had hyperplasia, which unfortunately did develop into occult disease,” Dr. Wasson said. “We wouldn’t recommend morcellating any patient with hyperplasia. In the two patients with low-grade stromal sarcoma, neither had any hyperplasia on preoperative sampling.”
Following the 2014 FDA Safety Communication on power morcellation, the AAGL released its own guidance on morcellation during uterine tissue extraction. The AAGL recommended that clinicians avoid morcellation for any patient who had a premalignant or malignant condition or who was at risk for malignancy, and use caution when considering morcellation. “This was for all types of morcellation, including electromechanical and vaginal morcellation,” Dr. Wasson said.
“This was in response to studies and awareness of increased risk of disease with morcellation – specifically leiomyosarcomas – for dissemination of disease in the abdomen and pelvis, but also for an increased risk of recurrence,” she said. “This means, in turn, that patients can have decreased overall survival and disease-free survival, so this is very important when we are talking to our patients.”
Dr. Wasson reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Key clinical point:
Major finding: Of 611 patients who underwent morcellation during total vaginal hysterectomy, five patients (0.82%) had occult uterine carcinoma.
Data source: A retrospective cohort study of 611 women who had uterine removal with uncontained morcellation.
Disclosures: Dr. Wasson reported having no relevant financial disclosures.
Long-acting bupivacaine offers limited benefit in hysterectomy pain
ORLANDO – Port site infiltration during laparoscopic or robot-assisted hysterectomy with extended-release liposomal bupivacaine did not significantly improve most postoperative pain scores, compared with plain 0.25% bupivacaine.
In a randomized trial, the liposomal formulation was associated with 30% less pain on postoperative day 3, a significant difference not seen on postoperative day 1, 2, or 14.
“Liposomal bupivacaine is expected to last about 72 hours but it also comes at a cost,” Kenneth I. Barron, MD, a fellow in advanced minimally invasive gynecologic surgery at Florida Hospital Orlando, said at the meeting sponsored by AAGL. Extended-release bupivacaine costs $280, compared with $1.83 for plain bupivacaine, according to Dr. Barron.
“Based on this study, the routine use of liposomal bupivacaine as a port site local anesthetic in laparoscopic hysterectomy has limited usefulness and is not justified,” he said.
In the blinded study, surgeons at a tertiary-care community hospital performed pre-incision infiltration with undiluted liposomal extended-release bupivacaine for 32 surgery patients and with the short-acting formulation for another 32 surgery patients. All patients underwent either laparoscopic or robot-assisted total hysterectomy for benign indications. They were recruited for the study between July 2015 and January 2016 and there were no significant demographic differences between groups preoperatively.
For the primary outcome measure, investigators called each participant and asked them to rate their average overall pain on postoperative days 1, 2, 3, and 14. They used the Brief Pain Inventory 0-10 scale. There were no significant differences between groups on a composite score of their average and worst pain on days 1, 2, or 14. However, on day 3, the composite score was 3.26 in the extended-release group, compared with 4.83 for those receiving short-acting bupivacaine (P = .009).
“What this shows, if anything, is one method of local anesthetic is probably not enough to make a significant impact,” Dr. Barron said. What is needed instead is “probably more of a global approach to enhance recovery.”
There were no significant differences between groups in the secondary study outcomes: pain scores during the first 24 hours in the hospital, function based on pain interference scores, opioid use, or adverse events.
Dr. Barron reported having no relevant financial disclosures.
ORLANDO – Port site infiltration during laparoscopic or robot-assisted hysterectomy with extended-release liposomal bupivacaine did not significantly improve most postoperative pain scores, compared with plain 0.25% bupivacaine.
In a randomized trial, the liposomal formulation was associated with 30% less pain on postoperative day 3, a significant difference not seen on postoperative day 1, 2, or 14.
“Liposomal bupivacaine is expected to last about 72 hours but it also comes at a cost,” Kenneth I. Barron, MD, a fellow in advanced minimally invasive gynecologic surgery at Florida Hospital Orlando, said at the meeting sponsored by AAGL. Extended-release bupivacaine costs $280, compared with $1.83 for plain bupivacaine, according to Dr. Barron.
“Based on this study, the routine use of liposomal bupivacaine as a port site local anesthetic in laparoscopic hysterectomy has limited usefulness and is not justified,” he said.
In the blinded study, surgeons at a tertiary-care community hospital performed pre-incision infiltration with undiluted liposomal extended-release bupivacaine for 32 surgery patients and with the short-acting formulation for another 32 surgery patients. All patients underwent either laparoscopic or robot-assisted total hysterectomy for benign indications. They were recruited for the study between July 2015 and January 2016 and there were no significant demographic differences between groups preoperatively.
For the primary outcome measure, investigators called each participant and asked them to rate their average overall pain on postoperative days 1, 2, 3, and 14. They used the Brief Pain Inventory 0-10 scale. There were no significant differences between groups on a composite score of their average and worst pain on days 1, 2, or 14. However, on day 3, the composite score was 3.26 in the extended-release group, compared with 4.83 for those receiving short-acting bupivacaine (P = .009).
“What this shows, if anything, is one method of local anesthetic is probably not enough to make a significant impact,” Dr. Barron said. What is needed instead is “probably more of a global approach to enhance recovery.”
There were no significant differences between groups in the secondary study outcomes: pain scores during the first 24 hours in the hospital, function based on pain interference scores, opioid use, or adverse events.
Dr. Barron reported having no relevant financial disclosures.
ORLANDO – Port site infiltration during laparoscopic or robot-assisted hysterectomy with extended-release liposomal bupivacaine did not significantly improve most postoperative pain scores, compared with plain 0.25% bupivacaine.
In a randomized trial, the liposomal formulation was associated with 30% less pain on postoperative day 3, a significant difference not seen on postoperative day 1, 2, or 14.
“Liposomal bupivacaine is expected to last about 72 hours but it also comes at a cost,” Kenneth I. Barron, MD, a fellow in advanced minimally invasive gynecologic surgery at Florida Hospital Orlando, said at the meeting sponsored by AAGL. Extended-release bupivacaine costs $280, compared with $1.83 for plain bupivacaine, according to Dr. Barron.
“Based on this study, the routine use of liposomal bupivacaine as a port site local anesthetic in laparoscopic hysterectomy has limited usefulness and is not justified,” he said.
In the blinded study, surgeons at a tertiary-care community hospital performed pre-incision infiltration with undiluted liposomal extended-release bupivacaine for 32 surgery patients and with the short-acting formulation for another 32 surgery patients. All patients underwent either laparoscopic or robot-assisted total hysterectomy for benign indications. They were recruited for the study between July 2015 and January 2016 and there were no significant demographic differences between groups preoperatively.
For the primary outcome measure, investigators called each participant and asked them to rate their average overall pain on postoperative days 1, 2, 3, and 14. They used the Brief Pain Inventory 0-10 scale. There were no significant differences between groups on a composite score of their average and worst pain on days 1, 2, or 14. However, on day 3, the composite score was 3.26 in the extended-release group, compared with 4.83 for those receiving short-acting bupivacaine (P = .009).
“What this shows, if anything, is one method of local anesthetic is probably not enough to make a significant impact,” Dr. Barron said. What is needed instead is “probably more of a global approach to enhance recovery.”
There were no significant differences between groups in the secondary study outcomes: pain scores during the first 24 hours in the hospital, function based on pain interference scores, opioid use, or adverse events.
Dr. Barron reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Should surgeons change gloves during total laparoscopic hysterectomy?
ORLANDO – Many gynecologic surgeons change gloves, gowns, and even surgical drapes during total laparoscopic hysterectomy to prevent bacterial infections, but little data support the practice.
In a small study of women undergoing total laparoscopic hysterectomy, investigators found that the overall risk of infection from contaminated gowns, gloves, and instruments was very low, with bacterial growth below the infection threshold in 98.9% of samples and no surgical site infections reported during 6 weeks of follow-up after surgery.
“Tradition dictates that even after both fields have been prepped, we refer to the perineum and vagina as ‘dirty,’ and the abdomen as ‘clean,’ ” Dr. Shockley said, “And surgeons habitually change their gown and gloves when inadvertent contact with the perineum or vagina occurs.”
To elucidate the true pathogen picture, Dr. Shockley and her colleagues assessed 31 women undergoing total laparoscopic hysterectomy for a benign indication during 2016. They evaluated the type and quantity of bacteria found intraoperatively on the abdomen, vagina, surgical gloves, instrument tips, and uterus.
All patients received perioperative antibiotic prophylaxis and standard, separate perineovaginal and abdominal prep with chlorhexidine. Investigators swabbed the vaginal fornices and abdomen at six sites, as well as the surgeon’s gloves following placement of the uterine manipulator, tips of instruments used to close the vaginal cuff, uterine fundus after extraction, and surgeon’s gloves following removal of the uterus.
They detected no anaerobic bacterial growth from samples taken from the abdomen, in the vagina, or on the tips of instruments used for cuff closure. Similarly, there was no aerobic growth observed in the vagina of any patient. However, they did detect aerobic bacterial growth in the abdomen, which in all cases was consistent with skin flora.
Three patients demonstrated some growth with the surgeon’s gloves following manipulator placement. Nearly one-third – 32% – of surgeon’s gloves cultured bacteria after removal of the uterus. One sample yielded cumulative growth for a bacterial count considered high enough to potentially cause infection, defined as more than 5,000 colony-forming units (CFU) per milliliter. This was the highest growth sample out of the 180 samples collected.
Additionally, 39% of samples from the uterine fundus were positive, a higher percentage than at any other site, Dr. Shockley reported. “And the one sample with growth exceeding 5,000 CFU/mL – you guessed it – was from the same patient.”
Bacterial growth was scant on the instrument tips used to close the vaginal cuffs.
Overall, bacterial growth in 98.9% of samples was below the infection threshold. “We did not identify any post–surgical site infections during 6 weeks of follow-up,” Dr. Shockley said at the meeting sponsored by AAGL.
“This study does provide a good description and count of the bacteria encountered during total laparoscopic hysterectomy. They are unlikely to cause surgical site infections … but based on concentration and frequency of bacterial growth on the surgeon’s gloves after specimen extraction, we would recommend if you are going to change gloves, do it after this step, before turning your attention back to the abdomen for vaginal cuff closure,” she said.
But changing gloves after placing the Foley and uterine manipulator “seems to be a wasted exercise,” Dr. Shockley said. “There was no growth on the vaginal fornices of any patient.”
The bacteria on the gloves in those three cases developed very low colony counts. “Yes, there was growth after the removal of the specimen, but with the exception of one patient, the colony counts were all below 5,000,” she said. “I think we need more data to reassure ourselves [attire changes are] unnecessary at every step of the [total laparoscopic hysterectomy].”
The study was supported by an educational grant from the Foundation of the AAGL Jerome J. Hoffman Endowment. Dr. Shockley reported having no relevant financial disclosures.
ORLANDO – Many gynecologic surgeons change gloves, gowns, and even surgical drapes during total laparoscopic hysterectomy to prevent bacterial infections, but little data support the practice.
In a small study of women undergoing total laparoscopic hysterectomy, investigators found that the overall risk of infection from contaminated gowns, gloves, and instruments was very low, with bacterial growth below the infection threshold in 98.9% of samples and no surgical site infections reported during 6 weeks of follow-up after surgery.
“Tradition dictates that even after both fields have been prepped, we refer to the perineum and vagina as ‘dirty,’ and the abdomen as ‘clean,’ ” Dr. Shockley said, “And surgeons habitually change their gown and gloves when inadvertent contact with the perineum or vagina occurs.”
To elucidate the true pathogen picture, Dr. Shockley and her colleagues assessed 31 women undergoing total laparoscopic hysterectomy for a benign indication during 2016. They evaluated the type and quantity of bacteria found intraoperatively on the abdomen, vagina, surgical gloves, instrument tips, and uterus.
All patients received perioperative antibiotic prophylaxis and standard, separate perineovaginal and abdominal prep with chlorhexidine. Investigators swabbed the vaginal fornices and abdomen at six sites, as well as the surgeon’s gloves following placement of the uterine manipulator, tips of instruments used to close the vaginal cuff, uterine fundus after extraction, and surgeon’s gloves following removal of the uterus.
They detected no anaerobic bacterial growth from samples taken from the abdomen, in the vagina, or on the tips of instruments used for cuff closure. Similarly, there was no aerobic growth observed in the vagina of any patient. However, they did detect aerobic bacterial growth in the abdomen, which in all cases was consistent with skin flora.
Three patients demonstrated some growth with the surgeon’s gloves following manipulator placement. Nearly one-third – 32% – of surgeon’s gloves cultured bacteria after removal of the uterus. One sample yielded cumulative growth for a bacterial count considered high enough to potentially cause infection, defined as more than 5,000 colony-forming units (CFU) per milliliter. This was the highest growth sample out of the 180 samples collected.
Additionally, 39% of samples from the uterine fundus were positive, a higher percentage than at any other site, Dr. Shockley reported. “And the one sample with growth exceeding 5,000 CFU/mL – you guessed it – was from the same patient.”
Bacterial growth was scant on the instrument tips used to close the vaginal cuffs.
Overall, bacterial growth in 98.9% of samples was below the infection threshold. “We did not identify any post–surgical site infections during 6 weeks of follow-up,” Dr. Shockley said at the meeting sponsored by AAGL.
“This study does provide a good description and count of the bacteria encountered during total laparoscopic hysterectomy. They are unlikely to cause surgical site infections … but based on concentration and frequency of bacterial growth on the surgeon’s gloves after specimen extraction, we would recommend if you are going to change gloves, do it after this step, before turning your attention back to the abdomen for vaginal cuff closure,” she said.
But changing gloves after placing the Foley and uterine manipulator “seems to be a wasted exercise,” Dr. Shockley said. “There was no growth on the vaginal fornices of any patient.”
The bacteria on the gloves in those three cases developed very low colony counts. “Yes, there was growth after the removal of the specimen, but with the exception of one patient, the colony counts were all below 5,000,” she said. “I think we need more data to reassure ourselves [attire changes are] unnecessary at every step of the [total laparoscopic hysterectomy].”
The study was supported by an educational grant from the Foundation of the AAGL Jerome J. Hoffman Endowment. Dr. Shockley reported having no relevant financial disclosures.
ORLANDO – Many gynecologic surgeons change gloves, gowns, and even surgical drapes during total laparoscopic hysterectomy to prevent bacterial infections, but little data support the practice.
In a small study of women undergoing total laparoscopic hysterectomy, investigators found that the overall risk of infection from contaminated gowns, gloves, and instruments was very low, with bacterial growth below the infection threshold in 98.9% of samples and no surgical site infections reported during 6 weeks of follow-up after surgery.
“Tradition dictates that even after both fields have been prepped, we refer to the perineum and vagina as ‘dirty,’ and the abdomen as ‘clean,’ ” Dr. Shockley said, “And surgeons habitually change their gown and gloves when inadvertent contact with the perineum or vagina occurs.”
To elucidate the true pathogen picture, Dr. Shockley and her colleagues assessed 31 women undergoing total laparoscopic hysterectomy for a benign indication during 2016. They evaluated the type and quantity of bacteria found intraoperatively on the abdomen, vagina, surgical gloves, instrument tips, and uterus.
All patients received perioperative antibiotic prophylaxis and standard, separate perineovaginal and abdominal prep with chlorhexidine. Investigators swabbed the vaginal fornices and abdomen at six sites, as well as the surgeon’s gloves following placement of the uterine manipulator, tips of instruments used to close the vaginal cuff, uterine fundus after extraction, and surgeon’s gloves following removal of the uterus.
They detected no anaerobic bacterial growth from samples taken from the abdomen, in the vagina, or on the tips of instruments used for cuff closure. Similarly, there was no aerobic growth observed in the vagina of any patient. However, they did detect aerobic bacterial growth in the abdomen, which in all cases was consistent with skin flora.
Three patients demonstrated some growth with the surgeon’s gloves following manipulator placement. Nearly one-third – 32% – of surgeon’s gloves cultured bacteria after removal of the uterus. One sample yielded cumulative growth for a bacterial count considered high enough to potentially cause infection, defined as more than 5,000 colony-forming units (CFU) per milliliter. This was the highest growth sample out of the 180 samples collected.
Additionally, 39% of samples from the uterine fundus were positive, a higher percentage than at any other site, Dr. Shockley reported. “And the one sample with growth exceeding 5,000 CFU/mL – you guessed it – was from the same patient.”
Bacterial growth was scant on the instrument tips used to close the vaginal cuffs.
Overall, bacterial growth in 98.9% of samples was below the infection threshold. “We did not identify any post–surgical site infections during 6 weeks of follow-up,” Dr. Shockley said at the meeting sponsored by AAGL.
“This study does provide a good description and count of the bacteria encountered during total laparoscopic hysterectomy. They are unlikely to cause surgical site infections … but based on concentration and frequency of bacterial growth on the surgeon’s gloves after specimen extraction, we would recommend if you are going to change gloves, do it after this step, before turning your attention back to the abdomen for vaginal cuff closure,” she said.
But changing gloves after placing the Foley and uterine manipulator “seems to be a wasted exercise,” Dr. Shockley said. “There was no growth on the vaginal fornices of any patient.”
The bacteria on the gloves in those three cases developed very low colony counts. “Yes, there was growth after the removal of the specimen, but with the exception of one patient, the colony counts were all below 5,000,” she said. “I think we need more data to reassure ourselves [attire changes are] unnecessary at every step of the [total laparoscopic hysterectomy].”
The study was supported by an educational grant from the Foundation of the AAGL Jerome J. Hoffman Endowment. Dr. Shockley reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Key clinical point:
Major finding: Bacterial concentrations did not exceed thresholds required to trigger potential infection in almost 99% of cultures.
Data source: A study of 31 women undergoing total laparoscopic hysterectomy for benign indications in 2016.
Disclosures: The study was supported by an educational grant from the Foundation of the AAGL Jerome J. Hoffman Endowment. Dr. Shockley reported having no relevant financial disclosures.
‘Skip phenomenon’ could explain fluctuating positivity for S. aureus bacteremia
NEW ORLEANS – A proportion of patients treated appropriately with antibiotics for Staphylococcus aureus bacteremia can generate a negative blood culture followed by a positive one, a new clinical entity researchers are calling the “skip phenomenon.”
“This pattern is really in cases where people have known Staph. aureus bacteremia; it seems to clear; they’re on appropriate antibiotic therapy; and despite that, we see that the blood cultures come back positive again several days later,” explained Justin A. Fiala, MD, of Mayo Clinic in Rochester, Minn.
In a video interview, Dr. Fiala outlined the findings of the first study to identify and characterize this phenomenon.
Certain patients with S. aureus bacteremia could be at higher risk for skip phenomenon, for example. The nested case-control study identified these higher-risk patients, a population that might warrant more clinical testing.
Dr. Fiala also discussed associations with patient outcomes, as well as the overall prevalence of skip phenomenon in his research, which included more than 900 patients with S. aureus bacteremia treated at Mayo Clinic.
Dr. Fiala had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A proportion of patients treated appropriately with antibiotics for Staphylococcus aureus bacteremia can generate a negative blood culture followed by a positive one, a new clinical entity researchers are calling the “skip phenomenon.”
“This pattern is really in cases where people have known Staph. aureus bacteremia; it seems to clear; they’re on appropriate antibiotic therapy; and despite that, we see that the blood cultures come back positive again several days later,” explained Justin A. Fiala, MD, of Mayo Clinic in Rochester, Minn.
In a video interview, Dr. Fiala outlined the findings of the first study to identify and characterize this phenomenon.
Certain patients with S. aureus bacteremia could be at higher risk for skip phenomenon, for example. The nested case-control study identified these higher-risk patients, a population that might warrant more clinical testing.
Dr. Fiala also discussed associations with patient outcomes, as well as the overall prevalence of skip phenomenon in his research, which included more than 900 patients with S. aureus bacteremia treated at Mayo Clinic.
Dr. Fiala had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A proportion of patients treated appropriately with antibiotics for Staphylococcus aureus bacteremia can generate a negative blood culture followed by a positive one, a new clinical entity researchers are calling the “skip phenomenon.”
“This pattern is really in cases where people have known Staph. aureus bacteremia; it seems to clear; they’re on appropriate antibiotic therapy; and despite that, we see that the blood cultures come back positive again several days later,” explained Justin A. Fiala, MD, of Mayo Clinic in Rochester, Minn.
In a video interview, Dr. Fiala outlined the findings of the first study to identify and characterize this phenomenon.
Certain patients with S. aureus bacteremia could be at higher risk for skip phenomenon, for example. The nested case-control study identified these higher-risk patients, a population that might warrant more clinical testing.
Dr. Fiala also discussed associations with patient outcomes, as well as the overall prevalence of skip phenomenon in his research, which included more than 900 patients with S. aureus bacteremia treated at Mayo Clinic.
Dr. Fiala had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
In HIV, omega-3s significantly reduced triglycerides, CRP
NEW ORLEANS – Daily supplementation with high-dose omega-3 fatty acids significantly slashed levels of triglycerides and C-reactive protein (CRP) among people with HIV infection at 2 years, according to a randomized study.
“This is the longest randomized, controlled trial to date of high-dose omega-3 in the HIV infected to evaluate long-term effects on lipids, inflammation, and vascular function,” Gretchen Volpe, MD, said at an annual scientific meeting on infectious diseases. Prior studies were limited to 24 weeks or fewer of omega-3 supplementation, she added.
The trial is part of a bigger trend, one where investigators strive to improve duration and quality of life for people living with HIV/AIDS because of the field’s success in managing HIV infection itself.
“Cardiovascular disease is prevalent in persons with HIV,” said Dr. Volpe of Tufts Medical Center in Boston. “HIV increases cardiovascular disease through several pathways – including dyslipidemia, chronic inflammation, and vascular dysfunction.” Both HIV infection itself as well as antiretroviral therapy can have atherogenic effects on vasculature, she added.
Omega-3 fatty acids have been shown to reduce triglycerides in HIV-infected and non–HIV infected people, and they may reduce inflammation by decreasing the inflammatory mediator arachidonic acid, Dr. Volpe said.
She and her colleagues enrolled HIV-infected adults on stable antiretroviral therapy with fasting triglycerides between 150 mg/dL and 2,500 mg/dL at baseline. They randomized 43 people to 4 g omega-3 (Lovaza) daily, and another 40 people to placebo in an intent-to-treat analysis.
The mean age was 51 years, 21% were women, and there were no significant differences between groups at baseline on lipid parameters or statin use. Most patients (95%) were virologically suppressed, with a mean CD4+ count of 648 cells/mcL. The mean duration of HIV infection was 16 years.
The median decrease in triglycerides at 24 months was 68 mg/dL in the omega-3 group, compared with 22 mg/dL in the placebo group, a significant difference (P = .041). Another primary outcome, change in C-reactive protein as an indication of systemic inflammation, decreased 0.3 mg/L in treated participants, versus an increase of 0.6 mg/L in the placebo group. That difference also was statistically significant (P = .008).
In contrast, there were no significant differences in HDL-C changes, arterial stiffness, or brachial artery reactivity between groups at 2 years. Serious adverse event rates did not differ, either, Dr. Volpe reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society
“Adherence even over a 2-year trial is feasible, and efficacy may increase over time – we noted our 24-month data were more statistically significant than our 12-month data,” Dr. Volpe observed. Omega-3 fatty acid supplementation is well tolerated with limited side effects, she added. “Omega-3 fatty acid supplementation [also] may reduce inflammation as measured by CRP, even for those whose CRP was in the normal range at baseline.”
Total cholesterol did not significantly differ between groups at any time point, Dr. Volpe noted. “But we noticed a trend toward greater reduction in total cholesterol in the treatment group at 12 months and 24 months. There we no differences at any time point between groups in LDL cholesterol, which allays some concerns that fatty acids might increase LDL.”
The use of the prescription formulation of omega-3 fatty acids could be a limitation of the study, Dr. Volpe said, because not all people at risk may be able to access or afford it.
During a question session after the presentation, a meeting attendee asked about over-the-counter formulations that she could recommend to her patients. “We used the high dose, because some previous studies showed a dose-dependent response,” Dr. Volpe responded. “This was a purified formulation, so we could know what we were giving. ... I don’t know how to solve that problem in real life, but maybe use the best preparation that you are most familiar with.”
Another attendee asked about any differences between groups in statin use, alcohol consumption, or smoking. “We had about 30% statin users in either arm,” Dr. Volpe replied. “Some studies have shown that statins reduce or eliminate the effects of omega-3s, whereas other studies have shown that high-dose, high-efficacy statins improve the effects of omega-3s. Whatever the effect was, it was well distributed between groups.” Smoking and drinking rates did not significantly different between groups, she noted.
In a video interview at the meeting, Dr. Volpe discussed the study and its findings.
Dr. Volpe had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Daily supplementation with high-dose omega-3 fatty acids significantly slashed levels of triglycerides and C-reactive protein (CRP) among people with HIV infection at 2 years, according to a randomized study.
“This is the longest randomized, controlled trial to date of high-dose omega-3 in the HIV infected to evaluate long-term effects on lipids, inflammation, and vascular function,” Gretchen Volpe, MD, said at an annual scientific meeting on infectious diseases. Prior studies were limited to 24 weeks or fewer of omega-3 supplementation, she added.
The trial is part of a bigger trend, one where investigators strive to improve duration and quality of life for people living with HIV/AIDS because of the field’s success in managing HIV infection itself.
“Cardiovascular disease is prevalent in persons with HIV,” said Dr. Volpe of Tufts Medical Center in Boston. “HIV increases cardiovascular disease through several pathways – including dyslipidemia, chronic inflammation, and vascular dysfunction.” Both HIV infection itself as well as antiretroviral therapy can have atherogenic effects on vasculature, she added.
Omega-3 fatty acids have been shown to reduce triglycerides in HIV-infected and non–HIV infected people, and they may reduce inflammation by decreasing the inflammatory mediator arachidonic acid, Dr. Volpe said.
She and her colleagues enrolled HIV-infected adults on stable antiretroviral therapy with fasting triglycerides between 150 mg/dL and 2,500 mg/dL at baseline. They randomized 43 people to 4 g omega-3 (Lovaza) daily, and another 40 people to placebo in an intent-to-treat analysis.
The mean age was 51 years, 21% were women, and there were no significant differences between groups at baseline on lipid parameters or statin use. Most patients (95%) were virologically suppressed, with a mean CD4+ count of 648 cells/mcL. The mean duration of HIV infection was 16 years.
The median decrease in triglycerides at 24 months was 68 mg/dL in the omega-3 group, compared with 22 mg/dL in the placebo group, a significant difference (P = .041). Another primary outcome, change in C-reactive protein as an indication of systemic inflammation, decreased 0.3 mg/L in treated participants, versus an increase of 0.6 mg/L in the placebo group. That difference also was statistically significant (P = .008).
In contrast, there were no significant differences in HDL-C changes, arterial stiffness, or brachial artery reactivity between groups at 2 years. Serious adverse event rates did not differ, either, Dr. Volpe reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society
“Adherence even over a 2-year trial is feasible, and efficacy may increase over time – we noted our 24-month data were more statistically significant than our 12-month data,” Dr. Volpe observed. Omega-3 fatty acid supplementation is well tolerated with limited side effects, she added. “Omega-3 fatty acid supplementation [also] may reduce inflammation as measured by CRP, even for those whose CRP was in the normal range at baseline.”
Total cholesterol did not significantly differ between groups at any time point, Dr. Volpe noted. “But we noticed a trend toward greater reduction in total cholesterol in the treatment group at 12 months and 24 months. There we no differences at any time point between groups in LDL cholesterol, which allays some concerns that fatty acids might increase LDL.”
The use of the prescription formulation of omega-3 fatty acids could be a limitation of the study, Dr. Volpe said, because not all people at risk may be able to access or afford it.
During a question session after the presentation, a meeting attendee asked about over-the-counter formulations that she could recommend to her patients. “We used the high dose, because some previous studies showed a dose-dependent response,” Dr. Volpe responded. “This was a purified formulation, so we could know what we were giving. ... I don’t know how to solve that problem in real life, but maybe use the best preparation that you are most familiar with.”
Another attendee asked about any differences between groups in statin use, alcohol consumption, or smoking. “We had about 30% statin users in either arm,” Dr. Volpe replied. “Some studies have shown that statins reduce or eliminate the effects of omega-3s, whereas other studies have shown that high-dose, high-efficacy statins improve the effects of omega-3s. Whatever the effect was, it was well distributed between groups.” Smoking and drinking rates did not significantly different between groups, she noted.
In a video interview at the meeting, Dr. Volpe discussed the study and its findings.
Dr. Volpe had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Daily supplementation with high-dose omega-3 fatty acids significantly slashed levels of triglycerides and C-reactive protein (CRP) among people with HIV infection at 2 years, according to a randomized study.
“This is the longest randomized, controlled trial to date of high-dose omega-3 in the HIV infected to evaluate long-term effects on lipids, inflammation, and vascular function,” Gretchen Volpe, MD, said at an annual scientific meeting on infectious diseases. Prior studies were limited to 24 weeks or fewer of omega-3 supplementation, she added.
The trial is part of a bigger trend, one where investigators strive to improve duration and quality of life for people living with HIV/AIDS because of the field’s success in managing HIV infection itself.
“Cardiovascular disease is prevalent in persons with HIV,” said Dr. Volpe of Tufts Medical Center in Boston. “HIV increases cardiovascular disease through several pathways – including dyslipidemia, chronic inflammation, and vascular dysfunction.” Both HIV infection itself as well as antiretroviral therapy can have atherogenic effects on vasculature, she added.
Omega-3 fatty acids have been shown to reduce triglycerides in HIV-infected and non–HIV infected people, and they may reduce inflammation by decreasing the inflammatory mediator arachidonic acid, Dr. Volpe said.
She and her colleagues enrolled HIV-infected adults on stable antiretroviral therapy with fasting triglycerides between 150 mg/dL and 2,500 mg/dL at baseline. They randomized 43 people to 4 g omega-3 (Lovaza) daily, and another 40 people to placebo in an intent-to-treat analysis.
The mean age was 51 years, 21% were women, and there were no significant differences between groups at baseline on lipid parameters or statin use. Most patients (95%) were virologically suppressed, with a mean CD4+ count of 648 cells/mcL. The mean duration of HIV infection was 16 years.
The median decrease in triglycerides at 24 months was 68 mg/dL in the omega-3 group, compared with 22 mg/dL in the placebo group, a significant difference (P = .041). Another primary outcome, change in C-reactive protein as an indication of systemic inflammation, decreased 0.3 mg/L in treated participants, versus an increase of 0.6 mg/L in the placebo group. That difference also was statistically significant (P = .008).
In contrast, there were no significant differences in HDL-C changes, arterial stiffness, or brachial artery reactivity between groups at 2 years. Serious adverse event rates did not differ, either, Dr. Volpe reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society
“Adherence even over a 2-year trial is feasible, and efficacy may increase over time – we noted our 24-month data were more statistically significant than our 12-month data,” Dr. Volpe observed. Omega-3 fatty acid supplementation is well tolerated with limited side effects, she added. “Omega-3 fatty acid supplementation [also] may reduce inflammation as measured by CRP, even for those whose CRP was in the normal range at baseline.”
Total cholesterol did not significantly differ between groups at any time point, Dr. Volpe noted. “But we noticed a trend toward greater reduction in total cholesterol in the treatment group at 12 months and 24 months. There we no differences at any time point between groups in LDL cholesterol, which allays some concerns that fatty acids might increase LDL.”
The use of the prescription formulation of omega-3 fatty acids could be a limitation of the study, Dr. Volpe said, because not all people at risk may be able to access or afford it.
During a question session after the presentation, a meeting attendee asked about over-the-counter formulations that she could recommend to her patients. “We used the high dose, because some previous studies showed a dose-dependent response,” Dr. Volpe responded. “This was a purified formulation, so we could know what we were giving. ... I don’t know how to solve that problem in real life, but maybe use the best preparation that you are most familiar with.”
Another attendee asked about any differences between groups in statin use, alcohol consumption, or smoking. “We had about 30% statin users in either arm,” Dr. Volpe replied. “Some studies have shown that statins reduce or eliminate the effects of omega-3s, whereas other studies have shown that high-dose, high-efficacy statins improve the effects of omega-3s. Whatever the effect was, it was well distributed between groups.” Smoking and drinking rates did not significantly different between groups, she noted.
In a video interview at the meeting, Dr. Volpe discussed the study and its findings.
Dr. Volpe had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Key clinical point: Two years of high-dose omega-3 supplementation significantly reduced triglycerides and C-reactive protein levels in people with HIV infection.
Major finding: Triglyceride levels over 24 months dropped a median 68 mg/dL with omega-3 treatment, compared with 22 mg/dL with placebo. Similarly, C-reactive protein levels decreased 0.3 mg/L in treated patients, compared with an increase of 0.6 mg/mL in the placebo group.
Data source: A randomized, placebo-controlled trial of 83 patients.
Disclosures: Dr. Volpe had no relevant disclosures.
Novel antibiotic hits skin and soft tissue infections with one-two punch
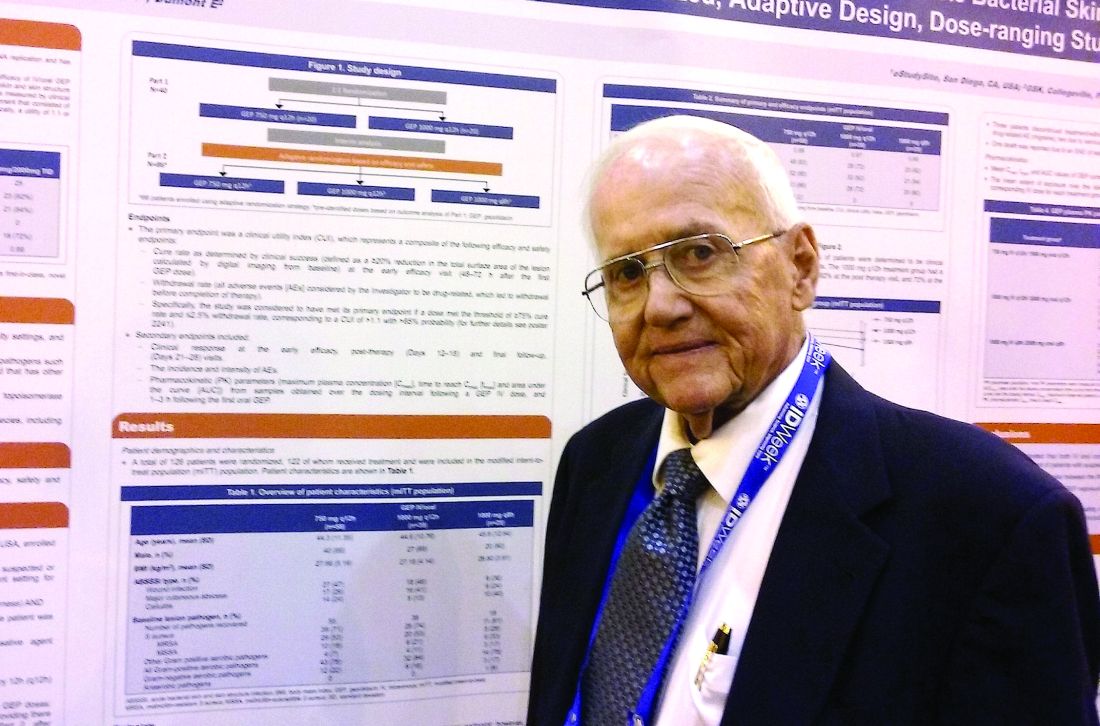
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
NEW ORLEANS – A novel antibiotic in development fared well in terms of efficacy and safety for patients hospitalized for suspected or confirmed Gram-positive acute skin and soft tissue infections, reveals the first reported findings of a phase II, randomized study.
Investigators randomized 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis to three different dosing intravenous/oral regimens of gepotidacin (GlaxoSmithKline). Patients in the 750-mg/1,500-mg q12h and 1,000-mg/2,000-mg q8h groups met the primary efficacy endpoint of an 80% or greater clinical success (83% and 92%, respectively) within 2-3 days. A third group, randomized to 1,000-mg/2,000-mg q12h, had a 72% early success rate.
All three groups of patients achieved the primary safety outcome, defined as less than a 2.5% withdrawal rate due to drug-related adverse events during gepotidacin treatment. One patient in the 750-mg q12h group withdrew because of a migraine related to the study drug.
Gepotidacin cleaves bacterial DNA in two places to block replication. “Because of its dual mechanism, there are a lot of potential applications,” Dr. O’Riordan said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Gepotidacin is also being assessed in ongoing gonorrhea, complicated intra-abdominal infections, and urinary tract infection studies.
The researchers in the current study also measured clinical success at post therapy days 12-18. They found 90% of the 750-mg/1,500-mg q12h group, 82% of the 1,000-mg/2,000-mg q8h, and 84% of the 1,000-mg/2,000-mg q12h group achieved the composite efficacy endpoint.
Overall, 84 or 69% of study participants experienced an adverse event. Nausea, diarrhea, and vomiting were the most common mild-to-moderate adverse events associated with the 10 days of gepotidacin treatment. Two serious adverse events not related to treatment also occurred during the study.
The “low adverse events and reproducible resolution of skin infections” in this phase II study support further development of gepotidacin, Dr. O’Riordan said.
Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
AT IDWEEK 2016
Key clinical point: A dual-mechanism-of-action antibiotic in development shows good efficacy and a low adverse event rate in a phase II study.
Major finding: A total 71 of 122 adult patients achieved clinical success within 48 to 72 hours with gepotidacin treatment.
Data source: 122 patients over 18 years of age with wound infections, major cutaneous abscesses, or cellulitis.
Disclosures: Dr. O’Riordan had no relevant disclosures. Some study coauthors are GlaxoSmithKline employees.
VIDEO: Biologic therapy for multidrug-resistant HIV offers new hope
NEW ORLEANS – Ibalizumab, the first monoclonal antibody to reach a phase III trial for multidrug resistant HIV therapy, has demonstrated efficacy, safety, and a novel mechanism of action, offering promise to many patients with few remaining options.
“The drug has now shown very significant antiretroviral activity, with 83% of patients demonstrating a half log decrease after 7 days, and a mean and median decrease of 1.1 log,” Jacob Lalezari, MD, lead author of the study and medical director of Quest Clinical Research in San Francisco, said at IDWeek 2016, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “The big message here is the novel mechanism of action provides new treatment to patients with limited options, really offering hope to those left behind by an otherwise amazing evolution of HIV treatment.”
Dr. Lalezari said there is currently no evidence of cross-resistance with existing antiretrovirals. “I think we got lucky with that,” he noted. “For the primary care HIV doc, who is increasingly overwhelmed by drug-drug interactions, the good news is ibalizumab does not have obvious drug-drug interactions with antiretrovirals or other [HIV] drugs in other classes.”
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, announced the findings with Dr. Lalezari at an IDWeek 2016 press conference. “This study is very important, because although we have terrific therapies for initial and second line treatment, we’ve really come to need treatment for the core group of patients who have developed resistance to all the drugs in the armamentarium,” he said.
Multidrug resistance emerges in less than 5% of people with HIV, affecting up to about 10,000 people in the United States. “It’s not a huge population, but it’s the most vulnerable and in need,” Dr. Lalezari said.
The 40 treatment-experienced patients in the study had their viral load and CD4+ counts measured at day 0. At day 7, they received a 2,000 mg IV loading dose of ibalizumab. At day 14, the response to ibalizumab monotherapy was measured and participants began an optimized background regimen with at least one other agent to which HIV showed sensitivity. Unfortunately, for about 50% of the patients, there was no such agent remaining, so researchers added BMS-663068, an investigational oral attachment inhibitor.
The cohort was 85% men, 45% nonwhite, and had a mean duration of HIV infection of 21 years. At study entry, patients’ mean viral load was approximately 100,000 copies/mL and mean CD4+ T-cell count was 160/mcL. However, half of the patients had T-cell counts below 100, and one third had counts below 10, “meaning they are at the very edge of sustainability,” Dr. Lalezari said.
Efficacy and safety
“The story is pretty simple – the drug worked,” Dr. Lalezari said. In addition to the 83% who met the primary endpoint of a half-life log decrease in HIV-1 RNA, 60% had a full log decrease or more at day 14. The mean and median HIV-1 RNA decrease for the entire cohort was 1.1 log10, a significant difference, compared with the day 0 to 7 control period (P less than .0001).
Putting the findings in perspective, Dr. Lalezari said, “So 1 log is not the most potent drug we see for HIV, but it’s pretty good. And in the setting of multidrug-resistant virus, it’s very good.” Although the agent is not potent enough for monotherapy, he said it is a strong candidate for combination therapy. The goal is to “give somebody a chance, potentially their last chance, to get control of the virus and prevent the progression of disease, and importantly, prevent them from spreading it to somebody else.”
“It’s a bit of a mystery” why 7 of the 40 patients did not meet the primary endpoint, Dr. Lalezari added. None of the factors the investigators compared between responders and nonresponders were significantly different.
Ibalizumab appears safe with no discontinuations and no treatment-related serious adverse events, Dr. Lalezari said. “We have not seen anything that strikes me as concerning at all in terms of safety, and it’s in a patient population that is quite ill.”
When asked during the press conference to address cost concerns, an issue in other specialties when biologics are introduced, Dr. Lalezari responded, “The one comment I will make is that whatever this drug is, it’s not a ‘me too drug.’ It’s unique and offers a unique mechanism of action. For those patients, the patients we saw whose T cells were under 10, whose health was failing and they were getting ready to die, their viral loads got suppressed and now they’re living, so it brings great value.”
Ibalizumab’s antiretroviral activity stems from blocking post-attachment conformational changes that are required to enable the HIV virus to bind with its co-receptors and ultimately gain entry into the target T cell. “Importantly the drug is away from the binding site for MHC class II molecules, and therefore not thought to cause T-cell depletion or be immunosuppressive,” Dr. Lalezari said.
In terms of the bigger picture, unlike most HIV drugs taken daily, ibalizumab is the first of the long-acting antiretrovirals, he noted. “I do think a paradigm shift is looming for at least some patients for whom long-acting therapy might be advantageous. There is also a movement toward IM and hopefully subcutaneous therapy as well, which would be very advantageous for patient self-administration.”
Although Dr. Lalezari and Dr. Kuritzkes presented the 14-day findings, the study is ongoing and participants continue to receive 800 mg ibalizumab intravenously every two weeks.
TaiMed Biologics, the sponsor of the study, has an expanded access program. Dr. Lalezari said, “So if you [have] a patient who is in trouble, in need of rescue therapy, there is an option for you now to consider.”
Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Ibalizumab, the first monoclonal antibody to reach a phase III trial for multidrug resistant HIV therapy, has demonstrated efficacy, safety, and a novel mechanism of action, offering promise to many patients with few remaining options.
“The drug has now shown very significant antiretroviral activity, with 83% of patients demonstrating a half log decrease after 7 days, and a mean and median decrease of 1.1 log,” Jacob Lalezari, MD, lead author of the study and medical director of Quest Clinical Research in San Francisco, said at IDWeek 2016, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “The big message here is the novel mechanism of action provides new treatment to patients with limited options, really offering hope to those left behind by an otherwise amazing evolution of HIV treatment.”
Dr. Lalezari said there is currently no evidence of cross-resistance with existing antiretrovirals. “I think we got lucky with that,” he noted. “For the primary care HIV doc, who is increasingly overwhelmed by drug-drug interactions, the good news is ibalizumab does not have obvious drug-drug interactions with antiretrovirals or other [HIV] drugs in other classes.”
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, announced the findings with Dr. Lalezari at an IDWeek 2016 press conference. “This study is very important, because although we have terrific therapies for initial and second line treatment, we’ve really come to need treatment for the core group of patients who have developed resistance to all the drugs in the armamentarium,” he said.
Multidrug resistance emerges in less than 5% of people with HIV, affecting up to about 10,000 people in the United States. “It’s not a huge population, but it’s the most vulnerable and in need,” Dr. Lalezari said.
The 40 treatment-experienced patients in the study had their viral load and CD4+ counts measured at day 0. At day 7, they received a 2,000 mg IV loading dose of ibalizumab. At day 14, the response to ibalizumab monotherapy was measured and participants began an optimized background regimen with at least one other agent to which HIV showed sensitivity. Unfortunately, for about 50% of the patients, there was no such agent remaining, so researchers added BMS-663068, an investigational oral attachment inhibitor.
The cohort was 85% men, 45% nonwhite, and had a mean duration of HIV infection of 21 years. At study entry, patients’ mean viral load was approximately 100,000 copies/mL and mean CD4+ T-cell count was 160/mcL. However, half of the patients had T-cell counts below 100, and one third had counts below 10, “meaning they are at the very edge of sustainability,” Dr. Lalezari said.
Efficacy and safety
“The story is pretty simple – the drug worked,” Dr. Lalezari said. In addition to the 83% who met the primary endpoint of a half-life log decrease in HIV-1 RNA, 60% had a full log decrease or more at day 14. The mean and median HIV-1 RNA decrease for the entire cohort was 1.1 log10, a significant difference, compared with the day 0 to 7 control period (P less than .0001).
Putting the findings in perspective, Dr. Lalezari said, “So 1 log is not the most potent drug we see for HIV, but it’s pretty good. And in the setting of multidrug-resistant virus, it’s very good.” Although the agent is not potent enough for monotherapy, he said it is a strong candidate for combination therapy. The goal is to “give somebody a chance, potentially their last chance, to get control of the virus and prevent the progression of disease, and importantly, prevent them from spreading it to somebody else.”
“It’s a bit of a mystery” why 7 of the 40 patients did not meet the primary endpoint, Dr. Lalezari added. None of the factors the investigators compared between responders and nonresponders were significantly different.
Ibalizumab appears safe with no discontinuations and no treatment-related serious adverse events, Dr. Lalezari said. “We have not seen anything that strikes me as concerning at all in terms of safety, and it’s in a patient population that is quite ill.”
When asked during the press conference to address cost concerns, an issue in other specialties when biologics are introduced, Dr. Lalezari responded, “The one comment I will make is that whatever this drug is, it’s not a ‘me too drug.’ It’s unique and offers a unique mechanism of action. For those patients, the patients we saw whose T cells were under 10, whose health was failing and they were getting ready to die, their viral loads got suppressed and now they’re living, so it brings great value.”
Ibalizumab’s antiretroviral activity stems from blocking post-attachment conformational changes that are required to enable the HIV virus to bind with its co-receptors and ultimately gain entry into the target T cell. “Importantly the drug is away from the binding site for MHC class II molecules, and therefore not thought to cause T-cell depletion or be immunosuppressive,” Dr. Lalezari said.
In terms of the bigger picture, unlike most HIV drugs taken daily, ibalizumab is the first of the long-acting antiretrovirals, he noted. “I do think a paradigm shift is looming for at least some patients for whom long-acting therapy might be advantageous. There is also a movement toward IM and hopefully subcutaneous therapy as well, which would be very advantageous for patient self-administration.”
Although Dr. Lalezari and Dr. Kuritzkes presented the 14-day findings, the study is ongoing and participants continue to receive 800 mg ibalizumab intravenously every two weeks.
TaiMed Biologics, the sponsor of the study, has an expanded access program. Dr. Lalezari said, “So if you [have] a patient who is in trouble, in need of rescue therapy, there is an option for you now to consider.”
Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Ibalizumab, the first monoclonal antibody to reach a phase III trial for multidrug resistant HIV therapy, has demonstrated efficacy, safety, and a novel mechanism of action, offering promise to many patients with few remaining options.
“The drug has now shown very significant antiretroviral activity, with 83% of patients demonstrating a half log decrease after 7 days, and a mean and median decrease of 1.1 log,” Jacob Lalezari, MD, lead author of the study and medical director of Quest Clinical Research in San Francisco, said at IDWeek 2016, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. “The big message here is the novel mechanism of action provides new treatment to patients with limited options, really offering hope to those left behind by an otherwise amazing evolution of HIV treatment.”
Dr. Lalezari said there is currently no evidence of cross-resistance with existing antiretrovirals. “I think we got lucky with that,” he noted. “For the primary care HIV doc, who is increasingly overwhelmed by drug-drug interactions, the good news is ibalizumab does not have obvious drug-drug interactions with antiretrovirals or other [HIV] drugs in other classes.”
Daniel Kuritzkes, MD, chief of infectious diseases at Brigham and Women’s Hospital and professor at Harvard Medical School, both in Boston, announced the findings with Dr. Lalezari at an IDWeek 2016 press conference. “This study is very important, because although we have terrific therapies for initial and second line treatment, we’ve really come to need treatment for the core group of patients who have developed resistance to all the drugs in the armamentarium,” he said.
Multidrug resistance emerges in less than 5% of people with HIV, affecting up to about 10,000 people in the United States. “It’s not a huge population, but it’s the most vulnerable and in need,” Dr. Lalezari said.
The 40 treatment-experienced patients in the study had their viral load and CD4+ counts measured at day 0. At day 7, they received a 2,000 mg IV loading dose of ibalizumab. At day 14, the response to ibalizumab monotherapy was measured and participants began an optimized background regimen with at least one other agent to which HIV showed sensitivity. Unfortunately, for about 50% of the patients, there was no such agent remaining, so researchers added BMS-663068, an investigational oral attachment inhibitor.
The cohort was 85% men, 45% nonwhite, and had a mean duration of HIV infection of 21 years. At study entry, patients’ mean viral load was approximately 100,000 copies/mL and mean CD4+ T-cell count was 160/mcL. However, half of the patients had T-cell counts below 100, and one third had counts below 10, “meaning they are at the very edge of sustainability,” Dr. Lalezari said.
Efficacy and safety
“The story is pretty simple – the drug worked,” Dr. Lalezari said. In addition to the 83% who met the primary endpoint of a half-life log decrease in HIV-1 RNA, 60% had a full log decrease or more at day 14. The mean and median HIV-1 RNA decrease for the entire cohort was 1.1 log10, a significant difference, compared with the day 0 to 7 control period (P less than .0001).
Putting the findings in perspective, Dr. Lalezari said, “So 1 log is not the most potent drug we see for HIV, but it’s pretty good. And in the setting of multidrug-resistant virus, it’s very good.” Although the agent is not potent enough for monotherapy, he said it is a strong candidate for combination therapy. The goal is to “give somebody a chance, potentially their last chance, to get control of the virus and prevent the progression of disease, and importantly, prevent them from spreading it to somebody else.”
“It’s a bit of a mystery” why 7 of the 40 patients did not meet the primary endpoint, Dr. Lalezari added. None of the factors the investigators compared between responders and nonresponders were significantly different.
Ibalizumab appears safe with no discontinuations and no treatment-related serious adverse events, Dr. Lalezari said. “We have not seen anything that strikes me as concerning at all in terms of safety, and it’s in a patient population that is quite ill.”
When asked during the press conference to address cost concerns, an issue in other specialties when biologics are introduced, Dr. Lalezari responded, “The one comment I will make is that whatever this drug is, it’s not a ‘me too drug.’ It’s unique and offers a unique mechanism of action. For those patients, the patients we saw whose T cells were under 10, whose health was failing and they were getting ready to die, their viral loads got suppressed and now they’re living, so it brings great value.”
Ibalizumab’s antiretroviral activity stems from blocking post-attachment conformational changes that are required to enable the HIV virus to bind with its co-receptors and ultimately gain entry into the target T cell. “Importantly the drug is away from the binding site for MHC class II molecules, and therefore not thought to cause T-cell depletion or be immunosuppressive,” Dr. Lalezari said.
In terms of the bigger picture, unlike most HIV drugs taken daily, ibalizumab is the first of the long-acting antiretrovirals, he noted. “I do think a paradigm shift is looming for at least some patients for whom long-acting therapy might be advantageous. There is also a movement toward IM and hopefully subcutaneous therapy as well, which would be very advantageous for patient self-administration.”
Although Dr. Lalezari and Dr. Kuritzkes presented the 14-day findings, the study is ongoing and participants continue to receive 800 mg ibalizumab intravenously every two weeks.
TaiMed Biologics, the sponsor of the study, has an expanded access program. Dr. Lalezari said, “So if you [have] a patient who is in trouble, in need of rescue therapy, there is an option for you now to consider.”
Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT IDWEEK 2016
Key clinical point: Ibalizumab, a new biologic in a phase III trial, significantly reduces viral loads in patients with multidrug-resistant HIV infection.
Major finding: At 14 days, 83% of 40 trial participants experienced at least a one-half log reduction in HIV viral load.
Data source: Single arm, 24-week study of ibalizumab plus optimized background regimen in treatment experienced patients with multidrug-resistant HIV-1.
Disclosures: TaiMed Biologics, the manufacturer of ibalizumab, sponsored the study. Dr. Lalezari receives research funding from TaiMed Biologics. Dr. Kuritzkes had no relevant financial disclosures.
Treated bacteremia that clears, then recurs, termed ‘skip phenomenon’
NEW ORLEANS – When Mayo Clinic physicians noticed some patients on appropriate antibiotic treatment for Staphylococcus aureus bacteremia cleared the infection, only to see it recur a few days later, Justin A. Fiala, MD and his colleagues grew curious.
Dr. Fiala, an infectious diseases internist at Mayo Clinic, Rochester, Minn., was intrigued by the possibility of fluctuating blood culture positivity in this subset of bacteremia patients.
“We wanted first to see whether or not this is a real entity and determine the prevalence of this ‘skip pattern,’” Dr. Fiala said at IDWeek 2016, the annual combined meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association and the Pediatric Infectious Diseases Society. He said identifying predictors and finding any differences in clinical outcomes compared to control S. aureus bacteremia (SAB) patients were additional aims.
Dr. Fiala and his colleagues assessed a hospitalized cohort of 726 adults with SAB at Mayo Clinic between July 2006 and June 2011. Patients with one or more negative blood cultures followed by a positive culture were identified within this group, and compared with 2 to 4 patients matched for age, sex and duration of bacteremia who served as controls.
The investigators found 29 patients – or 4% – of the 726 had this ‘skip pattern’ of infection, clearance, and reinfection. Those with the phenomenon were 90% male and tended to be older, with a mean age of 69 years, compared to the controls. They had index bacteremia about two days longer than controls. The study also revealed a significant difference in mean number of central venous catheters: 2.7 in the skip phenomenon group versus 1.7 in controls.
Given the predominance of the skip phenomenon in older, immunosuppressed males, “the takeaway … is that serial negative blood cultures may be warranted in these patient groups,” Dr. Fiala said.
The groups did not differ significantly by presence of implants or foreign bodies or by whether SAB was nosocomial or acquired in the community. “We thought it was interesting that 90% had immune suppression, although it was not statistically significant,” Dr. Fiala said.
With no prior reports in the medical literature, the researchers named this clinical entity “skip phenomenon.” Dr. Fiala noted that published studies have assessed recurrence of SAB after completion of antibiotics, but not specifically during treatment.
“We think this is a topic that is quite clinically prevalent and applicable,” Dr. Fiala said. He pointed out that SAB is common, accounting for about 20% of all nosocomial bacteremia cases. SAB also highly virulent with a mortality rate estimated between 20% and 35%. Although the study did not reveal significant mortality differences in the subgroup with skip phenomenon, “we can say there is increased morbidity.”
The most recent IDSA guidelines state that a single set of negative blood cultures is sufficient to demonstrate clearance of SAB, Dr. Fiala said. “Could this be falsely reassuring if Staphylococcus aureus does have a tendency to exhibit this fluctuating pattern?”
The retrospective design of the study and the relatively small number of patients with the skip phenomenon were limitations, the investigators acknowledged. Dr. Fiala had no relevant disclosures.
NEW ORLEANS – When Mayo Clinic physicians noticed some patients on appropriate antibiotic treatment for Staphylococcus aureus bacteremia cleared the infection, only to see it recur a few days later, Justin A. Fiala, MD and his colleagues grew curious.
Dr. Fiala, an infectious diseases internist at Mayo Clinic, Rochester, Minn., was intrigued by the possibility of fluctuating blood culture positivity in this subset of bacteremia patients.
“We wanted first to see whether or not this is a real entity and determine the prevalence of this ‘skip pattern,’” Dr. Fiala said at IDWeek 2016, the annual combined meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association and the Pediatric Infectious Diseases Society. He said identifying predictors and finding any differences in clinical outcomes compared to control S. aureus bacteremia (SAB) patients were additional aims.
Dr. Fiala and his colleagues assessed a hospitalized cohort of 726 adults with SAB at Mayo Clinic between July 2006 and June 2011. Patients with one or more negative blood cultures followed by a positive culture were identified within this group, and compared with 2 to 4 patients matched for age, sex and duration of bacteremia who served as controls.
The investigators found 29 patients – or 4% – of the 726 had this ‘skip pattern’ of infection, clearance, and reinfection. Those with the phenomenon were 90% male and tended to be older, with a mean age of 69 years, compared to the controls. They had index bacteremia about two days longer than controls. The study also revealed a significant difference in mean number of central venous catheters: 2.7 in the skip phenomenon group versus 1.7 in controls.
Given the predominance of the skip phenomenon in older, immunosuppressed males, “the takeaway … is that serial negative blood cultures may be warranted in these patient groups,” Dr. Fiala said.
The groups did not differ significantly by presence of implants or foreign bodies or by whether SAB was nosocomial or acquired in the community. “We thought it was interesting that 90% had immune suppression, although it was not statistically significant,” Dr. Fiala said.
With no prior reports in the medical literature, the researchers named this clinical entity “skip phenomenon.” Dr. Fiala noted that published studies have assessed recurrence of SAB after completion of antibiotics, but not specifically during treatment.
“We think this is a topic that is quite clinically prevalent and applicable,” Dr. Fiala said. He pointed out that SAB is common, accounting for about 20% of all nosocomial bacteremia cases. SAB also highly virulent with a mortality rate estimated between 20% and 35%. Although the study did not reveal significant mortality differences in the subgroup with skip phenomenon, “we can say there is increased morbidity.”
The most recent IDSA guidelines state that a single set of negative blood cultures is sufficient to demonstrate clearance of SAB, Dr. Fiala said. “Could this be falsely reassuring if Staphylococcus aureus does have a tendency to exhibit this fluctuating pattern?”
The retrospective design of the study and the relatively small number of patients with the skip phenomenon were limitations, the investigators acknowledged. Dr. Fiala had no relevant disclosures.
NEW ORLEANS – When Mayo Clinic physicians noticed some patients on appropriate antibiotic treatment for Staphylococcus aureus bacteremia cleared the infection, only to see it recur a few days later, Justin A. Fiala, MD and his colleagues grew curious.
Dr. Fiala, an infectious diseases internist at Mayo Clinic, Rochester, Minn., was intrigued by the possibility of fluctuating blood culture positivity in this subset of bacteremia patients.
“We wanted first to see whether or not this is a real entity and determine the prevalence of this ‘skip pattern,’” Dr. Fiala said at IDWeek 2016, the annual combined meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association and the Pediatric Infectious Diseases Society. He said identifying predictors and finding any differences in clinical outcomes compared to control S. aureus bacteremia (SAB) patients were additional aims.
Dr. Fiala and his colleagues assessed a hospitalized cohort of 726 adults with SAB at Mayo Clinic between July 2006 and June 2011. Patients with one or more negative blood cultures followed by a positive culture were identified within this group, and compared with 2 to 4 patients matched for age, sex and duration of bacteremia who served as controls.
The investigators found 29 patients – or 4% – of the 726 had this ‘skip pattern’ of infection, clearance, and reinfection. Those with the phenomenon were 90% male and tended to be older, with a mean age of 69 years, compared to the controls. They had index bacteremia about two days longer than controls. The study also revealed a significant difference in mean number of central venous catheters: 2.7 in the skip phenomenon group versus 1.7 in controls.
Given the predominance of the skip phenomenon in older, immunosuppressed males, “the takeaway … is that serial negative blood cultures may be warranted in these patient groups,” Dr. Fiala said.
The groups did not differ significantly by presence of implants or foreign bodies or by whether SAB was nosocomial or acquired in the community. “We thought it was interesting that 90% had immune suppression, although it was not statistically significant,” Dr. Fiala said.
With no prior reports in the medical literature, the researchers named this clinical entity “skip phenomenon.” Dr. Fiala noted that published studies have assessed recurrence of SAB after completion of antibiotics, but not specifically during treatment.
“We think this is a topic that is quite clinically prevalent and applicable,” Dr. Fiala said. He pointed out that SAB is common, accounting for about 20% of all nosocomial bacteremia cases. SAB also highly virulent with a mortality rate estimated between 20% and 35%. Although the study did not reveal significant mortality differences in the subgroup with skip phenomenon, “we can say there is increased morbidity.”
The most recent IDSA guidelines state that a single set of negative blood cultures is sufficient to demonstrate clearance of SAB, Dr. Fiala said. “Could this be falsely reassuring if Staphylococcus aureus does have a tendency to exhibit this fluctuating pattern?”
The retrospective design of the study and the relatively small number of patients with the skip phenomenon were limitations, the investigators acknowledged. Dr. Fiala had no relevant disclosures.
Key clinical point:
Major finding: About 4% of S. aureus bacteremia cases may not clear completely, as judged by one negative blood culture, contrary to recent IDSA guidelines.
Data source: Nested case-control study of 726 adult inpatients at Mayo Clinic between July 2006 and June 2011 with ≥3 days of S. aureus bacteremia.
Disclosures: Dr. Fiala had no relevant disclosures.
Surgical treatment tops medical management of prosthetic valve endocarditis
NEW ORLEANS – Over the years patients with prosthetic valve endocarditis treated at Cleveland Clinic tended to fare better with surgery compared to medical management, some clinicians noted. However, there was no data to confirm their observations.
“It was not recognized widely. A lot of our colleagues continued to believe it could be adequately treated with the right antibiotic,” Nabin K. Shrestha, MD, said at the IDWeek 2016 annual meeting on infectious diseases.
So Dr Shrestha and his colleagues conducted a retrospective cohort study to compare outcomes between 253 surgically treated adults and 77 others treated medically between April 2008 and December 2012. Survival from the time of treatment decision was the primary outcome.
The groups differed on some demographic and clinical factors. For example, the medically treated group was older, had fewer men, and more patients with mitral valves. “We might think the medical patients might be too sick for surgery, and that could certainly be true, but … they could have been too well for surgery too,” Dr. Shrestha said. To control for these differences between groups, the investigators performed a number of statistical analyses, including a propensity score adjusted model and reduced Cox proportion hazards model.
“Patients with PVE have a high hazard of death if treated medically,” Dr. Shrestha said, based on a 6.68 hazard ratio. The higher risk of death associated with medical treatment remained significant when adjusted for age, sex, and other factors. “Compared to surgical treatment, medical treatment was associated with a seven-fold higher hazard of death overall,” Dr. Shrestha said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The medical treatment group also fared worse on a number of secondary outcomes. For example, this group had a five-fold higher risk of death during hospitalization compared to the surgery group (odds ratio, 4.65); a 12-fold higher risk of death within one year (OR, 11.70); a seven-fold higher risk of subsequent surgery for infective endocarditis (OR, 6.57); and an eight-fold higher odds of surgery for the same episode of infective endocarditis at a subsequent hospitalization (OR, 8.02).
A large sample size and setting the date of management decision as time zero to avoid survival selection bias “give us confidence in our findings.” Limitations include an inability to look at some important variables because of the retrospective design.
A meeting attendee commented that surgeons often request a patient be optimized medically prior to surgery, and asked if investigators looked at time from hospitalization to the operation.
“The median date from admission to surgery was six days in our database,” said Dr. Shrestha, who is a staff physician at the Cleveland Clinic in Ohio.
“Medical treatment overall is associated with significantly poorer outcomes in patients with PVE compared with surgical treatment,” Dr. Shrestha said. “Although some patients are not candidates for surgery, a definite diagnosis of PVE should prompt a surgical evaluation in the majority of patients.”
Dr. Shrestha reported having no disclosures.
NEW ORLEANS – Over the years patients with prosthetic valve endocarditis treated at Cleveland Clinic tended to fare better with surgery compared to medical management, some clinicians noted. However, there was no data to confirm their observations.
“It was not recognized widely. A lot of our colleagues continued to believe it could be adequately treated with the right antibiotic,” Nabin K. Shrestha, MD, said at the IDWeek 2016 annual meeting on infectious diseases.
So Dr Shrestha and his colleagues conducted a retrospective cohort study to compare outcomes between 253 surgically treated adults and 77 others treated medically between April 2008 and December 2012. Survival from the time of treatment decision was the primary outcome.
The groups differed on some demographic and clinical factors. For example, the medically treated group was older, had fewer men, and more patients with mitral valves. “We might think the medical patients might be too sick for surgery, and that could certainly be true, but … they could have been too well for surgery too,” Dr. Shrestha said. To control for these differences between groups, the investigators performed a number of statistical analyses, including a propensity score adjusted model and reduced Cox proportion hazards model.
“Patients with PVE have a high hazard of death if treated medically,” Dr. Shrestha said, based on a 6.68 hazard ratio. The higher risk of death associated with medical treatment remained significant when adjusted for age, sex, and other factors. “Compared to surgical treatment, medical treatment was associated with a seven-fold higher hazard of death overall,” Dr. Shrestha said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The medical treatment group also fared worse on a number of secondary outcomes. For example, this group had a five-fold higher risk of death during hospitalization compared to the surgery group (odds ratio, 4.65); a 12-fold higher risk of death within one year (OR, 11.70); a seven-fold higher risk of subsequent surgery for infective endocarditis (OR, 6.57); and an eight-fold higher odds of surgery for the same episode of infective endocarditis at a subsequent hospitalization (OR, 8.02).
A large sample size and setting the date of management decision as time zero to avoid survival selection bias “give us confidence in our findings.” Limitations include an inability to look at some important variables because of the retrospective design.
A meeting attendee commented that surgeons often request a patient be optimized medically prior to surgery, and asked if investigators looked at time from hospitalization to the operation.
“The median date from admission to surgery was six days in our database,” said Dr. Shrestha, who is a staff physician at the Cleveland Clinic in Ohio.
“Medical treatment overall is associated with significantly poorer outcomes in patients with PVE compared with surgical treatment,” Dr. Shrestha said. “Although some patients are not candidates for surgery, a definite diagnosis of PVE should prompt a surgical evaluation in the majority of patients.”
Dr. Shrestha reported having no disclosures.
NEW ORLEANS – Over the years patients with prosthetic valve endocarditis treated at Cleveland Clinic tended to fare better with surgery compared to medical management, some clinicians noted. However, there was no data to confirm their observations.
“It was not recognized widely. A lot of our colleagues continued to believe it could be adequately treated with the right antibiotic,” Nabin K. Shrestha, MD, said at the IDWeek 2016 annual meeting on infectious diseases.
So Dr Shrestha and his colleagues conducted a retrospective cohort study to compare outcomes between 253 surgically treated adults and 77 others treated medically between April 2008 and December 2012. Survival from the time of treatment decision was the primary outcome.
The groups differed on some demographic and clinical factors. For example, the medically treated group was older, had fewer men, and more patients with mitral valves. “We might think the medical patients might be too sick for surgery, and that could certainly be true, but … they could have been too well for surgery too,” Dr. Shrestha said. To control for these differences between groups, the investigators performed a number of statistical analyses, including a propensity score adjusted model and reduced Cox proportion hazards model.
“Patients with PVE have a high hazard of death if treated medically,” Dr. Shrestha said, based on a 6.68 hazard ratio. The higher risk of death associated with medical treatment remained significant when adjusted for age, sex, and other factors. “Compared to surgical treatment, medical treatment was associated with a seven-fold higher hazard of death overall,” Dr. Shrestha said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The medical treatment group also fared worse on a number of secondary outcomes. For example, this group had a five-fold higher risk of death during hospitalization compared to the surgery group (odds ratio, 4.65); a 12-fold higher risk of death within one year (OR, 11.70); a seven-fold higher risk of subsequent surgery for infective endocarditis (OR, 6.57); and an eight-fold higher odds of surgery for the same episode of infective endocarditis at a subsequent hospitalization (OR, 8.02).
A large sample size and setting the date of management decision as time zero to avoid survival selection bias “give us confidence in our findings.” Limitations include an inability to look at some important variables because of the retrospective design.
A meeting attendee commented that surgeons often request a patient be optimized medically prior to surgery, and asked if investigators looked at time from hospitalization to the operation.
“The median date from admission to surgery was six days in our database,” said Dr. Shrestha, who is a staff physician at the Cleveland Clinic in Ohio.
“Medical treatment overall is associated with significantly poorer outcomes in patients with PVE compared with surgical treatment,” Dr. Shrestha said. “Although some patients are not candidates for surgery, a definite diagnosis of PVE should prompt a surgical evaluation in the majority of patients.”
Dr. Shrestha reported having no disclosures.
Key clinical point:
Major finding: Compared to surgery, odds of death within one year higher were almost 7 times greater with medical treatment (hazard ratio, 6.68).
Data source: Presentation at IDWeek 2016
Disclosures: Dr. Nabin K. Shrestha had no relevant disclosures.