User login
Damian McNamara is a journalist for Medscape Medical News and MDedge. He worked full-time for MDedge as the Miami Bureau covering a dozen medical specialties during 2001-2012, then as a freelancer for Medscape and MDedge, before being hired on staff by Medscape in 2018. Now the two companies are one. He uses what he learned in school – Damian has a BS in chemistry and an MS in science, health and environmental reporting/journalism. He works out of a home office in Miami, with a 100-pound chocolate lab known to snore under his desk during work hours.
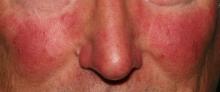
Rosacea research reveals advances, promising therapies
MIAMI – Management of rosacea continues to challenge dermatologists and patients alike, although new advances and recent studies shine a light on promising new therapies to target this inflammatory skin condition.
Linda Stein Gold, MD, who directs dermatology clinical trials at the Henry Ford Hospital in Detroit, shared new information about the pathophysiology of rosacea and the controversial associations with cardiovascular disease and addressed the rosacea “genes versus environment” etiology question at the Orlando Dermatology Aesthetic and Clinical Conference.
The topical vasoconstrictor of cutaneous vasculature, oxymetazoline hydrochloride cream 1%, showed a statistically significant improvement in erythema, compared with vehicle only in people with rosacea in a phase III study, Dr. Stein Gold said. The outcome was strict, requiring both physician and patient assessment of at least a two-point improvement on the Erythema Assessment Scale. Investigators observed responses over 12 hours on the same day. “It’s actually kind of fun to do these studies,” she added. “You get to see what happens with patients across a whole day.”
A long-term analysis showed the efficacy of oxymetazoline “actually increased over the course of 52 weeks,” Dr. Stein Gold said. A total of 43% of participants experienced a two-grade improvement in erythema during this time. The agent was generally well tolerated, with dermatitis, pruritus, and headaches the most common treatment-related adverse events reported. (In January, the Food and Drug Administration approved oxymetazoline cream for the treatment of “persistent facial erythema associated with rosacea in adults.”)
Sometimes, a new formulation can make a difference in terms of treatment tolerability, a major consideration for patients with rosacea, Dr. Stein Gold said. Recent evidence suggests azelaic acid foam, 15% (Finacea), approved by the FDA in 2015, provides a well-tolerated option with only 6.2% of patients experiencing any application site pain, compared with 1.5% on vehicle alone, she added.
Cardiovascular comorbidities
“We’ve heard a lot about psoriasis and cardiovascular comorbidities, and we worry that other skin diseases may have similar associations,” Dr. Stein Gold said. New revelations in the pathogenesis of rosacea suggest a comparable association, she added, including findings related to matrix metalloproteinases (MMPs). MMPs have a key role in rosacea, for example, and are also important in the pathogenesis of cardiovascular disease, she noted. Several studies have confirmed this association as well as other links, including to Parkinson’s disease.
Although these studies support associations, more evidence is needed to prove any causal relationship between rosacea and other conditions where inflammation plays a prominent role, she added.
Translating findings into action
Given this emerging evidence, “what are we going to do about it?” Dr. Stein Gold asked attendees at the meeting. Research suggests tetracycline might be protective, she said, because this antibiotic can inhibit MMP activity. In a retrospective cohort study, investigators discovered rosacea patients on tetracycline therapy were at lower risk for developing vascular disease (J Invest Dermatol. 2014 Aug;134[8]:2267-9).
Nature or nurture?
Researchers and clinicians frequently debate the precise etiology of rosacea and whether the underlying causes are primarily genetic versus environmental. Investigators conducted a twin cohort study to find a more concrete answer, specifically looking at identical and fraternal twin pairs to determine how much genetics or environment likely contributes to factors on the National Rosacea Society grading system (JAMA Dermatol. 2015 Nov;151[11]:1213-9).
“The bottom line is it’s really about half and half – about half were associated with genetics, the other half with environment,” Dr. Stein Gold said.
No matter what the etiology, it’s important to diagnose and treat rosacea, Dr. Stein Gold said. Although patients tend to be middle-aged white women, the condition is not limited to this patient population, and “you have to think about it to diagnose it in skin of color,” she added.
Rosacea, which has a high emotional impact, presents an opportunity for dermatologists to improve quality of life, Dr. Stein Gold said. “When people walk around with papules and pustules, [other] people think there is something wrong with them.”
Dr. Stein Gold disclosed that she is a consultant, member of the advisory boards and speaker’s bureaus for, and receives research grants from Galderma, Leo, Novan, Valeant, Novartis, Celgene and Allergan. She is also a consultant, advisory board member, and receives research grants from Dermira and Foamix. She is a consultant to Sol-Gel, Promis, Anacor, and Medimetriks. She is on the advisory board for Promis.
MIAMI – Management of rosacea continues to challenge dermatologists and patients alike, although new advances and recent studies shine a light on promising new therapies to target this inflammatory skin condition.
Linda Stein Gold, MD, who directs dermatology clinical trials at the Henry Ford Hospital in Detroit, shared new information about the pathophysiology of rosacea and the controversial associations with cardiovascular disease and addressed the rosacea “genes versus environment” etiology question at the Orlando Dermatology Aesthetic and Clinical Conference.
The topical vasoconstrictor of cutaneous vasculature, oxymetazoline hydrochloride cream 1%, showed a statistically significant improvement in erythema, compared with vehicle only in people with rosacea in a phase III study, Dr. Stein Gold said. The outcome was strict, requiring both physician and patient assessment of at least a two-point improvement on the Erythema Assessment Scale. Investigators observed responses over 12 hours on the same day. “It’s actually kind of fun to do these studies,” she added. “You get to see what happens with patients across a whole day.”
A long-term analysis showed the efficacy of oxymetazoline “actually increased over the course of 52 weeks,” Dr. Stein Gold said. A total of 43% of participants experienced a two-grade improvement in erythema during this time. The agent was generally well tolerated, with dermatitis, pruritus, and headaches the most common treatment-related adverse events reported. (In January, the Food and Drug Administration approved oxymetazoline cream for the treatment of “persistent facial erythema associated with rosacea in adults.”)
Sometimes, a new formulation can make a difference in terms of treatment tolerability, a major consideration for patients with rosacea, Dr. Stein Gold said. Recent evidence suggests azelaic acid foam, 15% (Finacea), approved by the FDA in 2015, provides a well-tolerated option with only 6.2% of patients experiencing any application site pain, compared with 1.5% on vehicle alone, she added.
Cardiovascular comorbidities
“We’ve heard a lot about psoriasis and cardiovascular comorbidities, and we worry that other skin diseases may have similar associations,” Dr. Stein Gold said. New revelations in the pathogenesis of rosacea suggest a comparable association, she added, including findings related to matrix metalloproteinases (MMPs). MMPs have a key role in rosacea, for example, and are also important in the pathogenesis of cardiovascular disease, she noted. Several studies have confirmed this association as well as other links, including to Parkinson’s disease.
Although these studies support associations, more evidence is needed to prove any causal relationship between rosacea and other conditions where inflammation plays a prominent role, she added.
Translating findings into action
Given this emerging evidence, “what are we going to do about it?” Dr. Stein Gold asked attendees at the meeting. Research suggests tetracycline might be protective, she said, because this antibiotic can inhibit MMP activity. In a retrospective cohort study, investigators discovered rosacea patients on tetracycline therapy were at lower risk for developing vascular disease (J Invest Dermatol. 2014 Aug;134[8]:2267-9).
Nature or nurture?
Researchers and clinicians frequently debate the precise etiology of rosacea and whether the underlying causes are primarily genetic versus environmental. Investigators conducted a twin cohort study to find a more concrete answer, specifically looking at identical and fraternal twin pairs to determine how much genetics or environment likely contributes to factors on the National Rosacea Society grading system (JAMA Dermatol. 2015 Nov;151[11]:1213-9).
“The bottom line is it’s really about half and half – about half were associated with genetics, the other half with environment,” Dr. Stein Gold said.
No matter what the etiology, it’s important to diagnose and treat rosacea, Dr. Stein Gold said. Although patients tend to be middle-aged white women, the condition is not limited to this patient population, and “you have to think about it to diagnose it in skin of color,” she added.
Rosacea, which has a high emotional impact, presents an opportunity for dermatologists to improve quality of life, Dr. Stein Gold said. “When people walk around with papules and pustules, [other] people think there is something wrong with them.”
Dr. Stein Gold disclosed that she is a consultant, member of the advisory boards and speaker’s bureaus for, and receives research grants from Galderma, Leo, Novan, Valeant, Novartis, Celgene and Allergan. She is also a consultant, advisory board member, and receives research grants from Dermira and Foamix. She is a consultant to Sol-Gel, Promis, Anacor, and Medimetriks. She is on the advisory board for Promis.
MIAMI – Management of rosacea continues to challenge dermatologists and patients alike, although new advances and recent studies shine a light on promising new therapies to target this inflammatory skin condition.
Linda Stein Gold, MD, who directs dermatology clinical trials at the Henry Ford Hospital in Detroit, shared new information about the pathophysiology of rosacea and the controversial associations with cardiovascular disease and addressed the rosacea “genes versus environment” etiology question at the Orlando Dermatology Aesthetic and Clinical Conference.
The topical vasoconstrictor of cutaneous vasculature, oxymetazoline hydrochloride cream 1%, showed a statistically significant improvement in erythema, compared with vehicle only in people with rosacea in a phase III study, Dr. Stein Gold said. The outcome was strict, requiring both physician and patient assessment of at least a two-point improvement on the Erythema Assessment Scale. Investigators observed responses over 12 hours on the same day. “It’s actually kind of fun to do these studies,” she added. “You get to see what happens with patients across a whole day.”
A long-term analysis showed the efficacy of oxymetazoline “actually increased over the course of 52 weeks,” Dr. Stein Gold said. A total of 43% of participants experienced a two-grade improvement in erythema during this time. The agent was generally well tolerated, with dermatitis, pruritus, and headaches the most common treatment-related adverse events reported. (In January, the Food and Drug Administration approved oxymetazoline cream for the treatment of “persistent facial erythema associated with rosacea in adults.”)
Sometimes, a new formulation can make a difference in terms of treatment tolerability, a major consideration for patients with rosacea, Dr. Stein Gold said. Recent evidence suggests azelaic acid foam, 15% (Finacea), approved by the FDA in 2015, provides a well-tolerated option with only 6.2% of patients experiencing any application site pain, compared with 1.5% on vehicle alone, she added.
Cardiovascular comorbidities
“We’ve heard a lot about psoriasis and cardiovascular comorbidities, and we worry that other skin diseases may have similar associations,” Dr. Stein Gold said. New revelations in the pathogenesis of rosacea suggest a comparable association, she added, including findings related to matrix metalloproteinases (MMPs). MMPs have a key role in rosacea, for example, and are also important in the pathogenesis of cardiovascular disease, she noted. Several studies have confirmed this association as well as other links, including to Parkinson’s disease.
Although these studies support associations, more evidence is needed to prove any causal relationship between rosacea and other conditions where inflammation plays a prominent role, she added.
Translating findings into action
Given this emerging evidence, “what are we going to do about it?” Dr. Stein Gold asked attendees at the meeting. Research suggests tetracycline might be protective, she said, because this antibiotic can inhibit MMP activity. In a retrospective cohort study, investigators discovered rosacea patients on tetracycline therapy were at lower risk for developing vascular disease (J Invest Dermatol. 2014 Aug;134[8]:2267-9).
Nature or nurture?
Researchers and clinicians frequently debate the precise etiology of rosacea and whether the underlying causes are primarily genetic versus environmental. Investigators conducted a twin cohort study to find a more concrete answer, specifically looking at identical and fraternal twin pairs to determine how much genetics or environment likely contributes to factors on the National Rosacea Society grading system (JAMA Dermatol. 2015 Nov;151[11]:1213-9).
“The bottom line is it’s really about half and half – about half were associated with genetics, the other half with environment,” Dr. Stein Gold said.
No matter what the etiology, it’s important to diagnose and treat rosacea, Dr. Stein Gold said. Although patients tend to be middle-aged white women, the condition is not limited to this patient population, and “you have to think about it to diagnose it in skin of color,” she added.
Rosacea, which has a high emotional impact, presents an opportunity for dermatologists to improve quality of life, Dr. Stein Gold said. “When people walk around with papules and pustules, [other] people think there is something wrong with them.”
Dr. Stein Gold disclosed that she is a consultant, member of the advisory boards and speaker’s bureaus for, and receives research grants from Galderma, Leo, Novan, Valeant, Novartis, Celgene and Allergan. She is also a consultant, advisory board member, and receives research grants from Dermira and Foamix. She is a consultant to Sol-Gel, Promis, Anacor, and Medimetriks. She is on the advisory board for Promis.
EXPERT ANALYSIS FROM ODAC 2017
Tips to maximize minimally invasive lower facial lift procedure
MIAMI – Lifting the lower face using minimally invasive barbed sutures can yield rejuvenation results that last 12-18 months, providing an option for patients who do not wish to undergo full facelift surgery, according to a presentation at ODAC 2017.
“We have something new in our toolbox. The Silhouette InstaLift is a “good new thing to consider adding to your practice. It works really nice for me in combination with other facial rejuvenation strategies,” said Susan Weinkle, MD, a private practice Mohs surgeon and aesthetic dermatologist in Bradenton, Fla.
“Now we can reposition the skin. It works quite nicely in skin of color also,” Dr. Weinkle said during a lecture and live demonstration at the Orlando Dermatology Aesthetic and Clinical Conference.
Procedure tips
Begin by marking where on the patient’s face you intend to place the sutures. The proper vector is a straight lift back toward the upper ear in many cases. Also, take a photo before you start, so you know the location of the sutures in case the patient comes back for more, Dr. Weinkle recommended.
“What I like to do next is to sit the patient up and look at them head-on,” Dr. Weinkle said. “It helps to tweak the placement of the suture markings.” If a patient’s face appears asymmetrical, the dermatologist can place more sutures on one side than the other. Also, when there are neck issues, you can put an exit point for the sutures 1.5 cm below the mandible, she added.
Be patient after you anesthetize the entry and exit points because the epinephrine does not work immediately, Dr. Weinkle advised.
She does not anesthetize along the entire suture tract because the aim is to thread the sutures through the subcutaneous tissue. “You do not want to anesthetize the tract area between entry and exit points. If the patient feels it, you’re probably too superficial and you’re in the dermis.”
The next step is using an 18G needle to form an entry point for the 23G needle. “Pinch the skin, insert and stretch the skin just a bit. If you don’t pinch at the entry point, the cones can be difficult to pop in.” Dr. Weinkle said. Also, if you establish all entry points before inserting the sutures, it can save time.
Dr. Weinkle generally uses a number 8 InstaLift suture, but it also comes in sizes 12 and 16. After you open the package, gently pull the suture to tighten the knots between the cones. “Don’t pull hard from both ends – you can break the suture.”
When inserting the long needle to place the suture, it’s “almost like playing the violin with a bow.” As the clinician advances along tract line, he or she should run the needle between thumb and forefinger to check the positioning. Once the suture is placed and the needle removed, pull medially on the suture until the cones audibly “pop” – they are self anchoring. “I’ve seen some people break the sutures. You can pull them out and start again,” she noted.
“InstaLift is a bit of a misnomer,” Dr. Weinkle said. “Fibroblast stimulation and collagen improves final result over the next 2-3 months.” Improvements can last 12-18 months – it’s not permanent. “I think I would make a great candidate,” she joked. “Unfortunately, this is one thing I cannot do myself.”
There is minimal bruising after the procedure in Dr. Weinkle’s experience. Potential complications include swelling, dimpling at entry points, misplacement, and ecchymosis.
Dr. Weinkle is a consultant and principal investigator for Sinclair Pharma, manufacturer of InstaLift.
MIAMI – Lifting the lower face using minimally invasive barbed sutures can yield rejuvenation results that last 12-18 months, providing an option for patients who do not wish to undergo full facelift surgery, according to a presentation at ODAC 2017.
“We have something new in our toolbox. The Silhouette InstaLift is a “good new thing to consider adding to your practice. It works really nice for me in combination with other facial rejuvenation strategies,” said Susan Weinkle, MD, a private practice Mohs surgeon and aesthetic dermatologist in Bradenton, Fla.
“Now we can reposition the skin. It works quite nicely in skin of color also,” Dr. Weinkle said during a lecture and live demonstration at the Orlando Dermatology Aesthetic and Clinical Conference.
Procedure tips
Begin by marking where on the patient’s face you intend to place the sutures. The proper vector is a straight lift back toward the upper ear in many cases. Also, take a photo before you start, so you know the location of the sutures in case the patient comes back for more, Dr. Weinkle recommended.
“What I like to do next is to sit the patient up and look at them head-on,” Dr. Weinkle said. “It helps to tweak the placement of the suture markings.” If a patient’s face appears asymmetrical, the dermatologist can place more sutures on one side than the other. Also, when there are neck issues, you can put an exit point for the sutures 1.5 cm below the mandible, she added.
Be patient after you anesthetize the entry and exit points because the epinephrine does not work immediately, Dr. Weinkle advised.
She does not anesthetize along the entire suture tract because the aim is to thread the sutures through the subcutaneous tissue. “You do not want to anesthetize the tract area between entry and exit points. If the patient feels it, you’re probably too superficial and you’re in the dermis.”
The next step is using an 18G needle to form an entry point for the 23G needle. “Pinch the skin, insert and stretch the skin just a bit. If you don’t pinch at the entry point, the cones can be difficult to pop in.” Dr. Weinkle said. Also, if you establish all entry points before inserting the sutures, it can save time.
Dr. Weinkle generally uses a number 8 InstaLift suture, but it also comes in sizes 12 and 16. After you open the package, gently pull the suture to tighten the knots between the cones. “Don’t pull hard from both ends – you can break the suture.”
When inserting the long needle to place the suture, it’s “almost like playing the violin with a bow.” As the clinician advances along tract line, he or she should run the needle between thumb and forefinger to check the positioning. Once the suture is placed and the needle removed, pull medially on the suture until the cones audibly “pop” – they are self anchoring. “I’ve seen some people break the sutures. You can pull them out and start again,” she noted.
“InstaLift is a bit of a misnomer,” Dr. Weinkle said. “Fibroblast stimulation and collagen improves final result over the next 2-3 months.” Improvements can last 12-18 months – it’s not permanent. “I think I would make a great candidate,” she joked. “Unfortunately, this is one thing I cannot do myself.”
There is minimal bruising after the procedure in Dr. Weinkle’s experience. Potential complications include swelling, dimpling at entry points, misplacement, and ecchymosis.
Dr. Weinkle is a consultant and principal investigator for Sinclair Pharma, manufacturer of InstaLift.
MIAMI – Lifting the lower face using minimally invasive barbed sutures can yield rejuvenation results that last 12-18 months, providing an option for patients who do not wish to undergo full facelift surgery, according to a presentation at ODAC 2017.
“We have something new in our toolbox. The Silhouette InstaLift is a “good new thing to consider adding to your practice. It works really nice for me in combination with other facial rejuvenation strategies,” said Susan Weinkle, MD, a private practice Mohs surgeon and aesthetic dermatologist in Bradenton, Fla.
“Now we can reposition the skin. It works quite nicely in skin of color also,” Dr. Weinkle said during a lecture and live demonstration at the Orlando Dermatology Aesthetic and Clinical Conference.
Procedure tips
Begin by marking where on the patient’s face you intend to place the sutures. The proper vector is a straight lift back toward the upper ear in many cases. Also, take a photo before you start, so you know the location of the sutures in case the patient comes back for more, Dr. Weinkle recommended.
“What I like to do next is to sit the patient up and look at them head-on,” Dr. Weinkle said. “It helps to tweak the placement of the suture markings.” If a patient’s face appears asymmetrical, the dermatologist can place more sutures on one side than the other. Also, when there are neck issues, you can put an exit point for the sutures 1.5 cm below the mandible, she added.
Be patient after you anesthetize the entry and exit points because the epinephrine does not work immediately, Dr. Weinkle advised.
She does not anesthetize along the entire suture tract because the aim is to thread the sutures through the subcutaneous tissue. “You do not want to anesthetize the tract area between entry and exit points. If the patient feels it, you’re probably too superficial and you’re in the dermis.”
The next step is using an 18G needle to form an entry point for the 23G needle. “Pinch the skin, insert and stretch the skin just a bit. If you don’t pinch at the entry point, the cones can be difficult to pop in.” Dr. Weinkle said. Also, if you establish all entry points before inserting the sutures, it can save time.
Dr. Weinkle generally uses a number 8 InstaLift suture, but it also comes in sizes 12 and 16. After you open the package, gently pull the suture to tighten the knots between the cones. “Don’t pull hard from both ends – you can break the suture.”
When inserting the long needle to place the suture, it’s “almost like playing the violin with a bow.” As the clinician advances along tract line, he or she should run the needle between thumb and forefinger to check the positioning. Once the suture is placed and the needle removed, pull medially on the suture until the cones audibly “pop” – they are self anchoring. “I’ve seen some people break the sutures. You can pull them out and start again,” she noted.
“InstaLift is a bit of a misnomer,” Dr. Weinkle said. “Fibroblast stimulation and collagen improves final result over the next 2-3 months.” Improvements can last 12-18 months – it’s not permanent. “I think I would make a great candidate,” she joked. “Unfortunately, this is one thing I cannot do myself.”
There is minimal bruising after the procedure in Dr. Weinkle’s experience. Potential complications include swelling, dimpling at entry points, misplacement, and ecchymosis.
Dr. Weinkle is a consultant and principal investigator for Sinclair Pharma, manufacturer of InstaLift.
EXPERT ANALYSIS AT ODAC 2017
New auto-grafting techniques could advance wound healing
MIAMI – Pinch grafting can accelerate the healing of chronic, treatment-resistant wounds such as leg ulcers, while at the same time reducing morbidity to the donor skin site. A new epidermal harvesting device also is showing promise, as is a new tool that minces autologous skin grafts prior to application to promote wound healing.
These and other advances in wound healing were presented at the Orlando Dermatology Aesthetic and Clinical Conference. The pinch grafts and minced grafts each rely on the newly added skin to stimulate cytokines. Interestingly, there is evidence that grafts taken from hair-bearing donor sites could be superior for stimulating cytokines and accelerating wound healing, said Robert Kirsner, MD, PhD, of the University of Miami Health System.
Islands of regrowth
Physicians perform pinch grafting by taking small punches of skin from a donor site on the thigh, abdomen, or elsewhere, and then transferring the grafts to serve as islands of regrowth in a wound. Pinch grafting can be faster and less expensive than techniques typically performed in an operating room, such as meshed auto-grafting. In contrast, pinch grafting can be accomplished in an office setting “and patients can do quite well.” Dr. Kirsner said. In terms of outcomes, “our data is typical,” he added. “About 50% of refractory ulcers heal, 25% improve, and a percentage recur.”
Spreadable skin grafts
Another autologous grafting technique that can be performed at the bedside uses the Xpansion Micro-Autografting Kit, which minces autologous, split thickness skin grafts. “Then you apply them like peanut butter to bread,” Dr. Kirsner said.
The micro-autografts can help heal both acute and chronic wounds, including full thickness wounds from trauma, some burn wounds, diabetic foot ulcers, and venous ulcers, according to the manufacturer’s website.
Epidermal harvesting (without anesthesia)
Epidermal grafting can make sense because the epidermis regenerates. “You can lift off just the epidermis with heat or suction, “ Dr. Kirsner said. For the first time, he added, a new tool allows epidermal grafting without the need for anesthesia (Cellutome Epidermal Harvesting System). The device raises little microdomes of epidermis down to the basal layer, including basal keratinocytes and melanocytes, and a dermatologist can use a sterile dressing to transfer them to the wound. Confocal microscopy shows the dermoepidermal junction healing as early as within 2 days.
The epidermal harvesting was initially developed for pigment problems, such as piebaldism. (Dermatol Surg. 2017 Jan;43[1]:159-60). “We quickly realized it might have applicability for nonhealing wounds,” Dr. Kirsner said.
Deeper wound healing
A novel strategy for triggering deeper wound healing evolved from fractional laser technology, which remove columns of skin to generate healing. Instead, Rox Anderson, MD, of Massachusetts General Hospital, Boston, “envisioned pulling up microcolumns of full thickness epidermis, all the way to the fat, placing them into a wound, and the wound would heal with very little donor site morbidity,” Dr. Kirsner said.
This tool is coming out in spring of this year, he noted. It will resemble a fractional laser, “but now you have the skin available to place in another wound.” Prior animal studies revealed a healing benefit with very little scarring, he added.
Is hairier better?
Does the donor site matter? Dr. Kirsner asked. Although dermatologists typically graft skin from an abdomen or thigh, a hair-bearing site may be a better option because of the presence of pluripotent stem cells, according to a case report (Wounds. 2016 Apr;28[4]:109-11). J.D. Fox of the University of Miami, Dr. Kirsner, and their colleagues treated a large, chronic venous leg ulcer, almost 60 cm2, with punch grafts from a variety of donor sites.
“The side that got scalp punch grafts healed better, suggesting with skin taken from richly hairy area, you’ll get better results,” Dr. Kirsner said.
Another study supports this strategy (J Am Acad Dermatol. 2016 Nov;75[5]:1007-14). These researchers reported greater wound size reduction using grafts containing hair follicles versus nonhairy areas, again suggesting follicular stem cells play a role in better wound healing, Dr. Kirsner said. “This may be a better source of donor skin in the future.”
Dr. Kirsner is a consultant for Cardinal Health, Mölnlycke, Amniox, Organogenesis, Kerecis, Keretec, and KCI, an Acelity company.
MIAMI – Pinch grafting can accelerate the healing of chronic, treatment-resistant wounds such as leg ulcers, while at the same time reducing morbidity to the donor skin site. A new epidermal harvesting device also is showing promise, as is a new tool that minces autologous skin grafts prior to application to promote wound healing.
These and other advances in wound healing were presented at the Orlando Dermatology Aesthetic and Clinical Conference. The pinch grafts and minced grafts each rely on the newly added skin to stimulate cytokines. Interestingly, there is evidence that grafts taken from hair-bearing donor sites could be superior for stimulating cytokines and accelerating wound healing, said Robert Kirsner, MD, PhD, of the University of Miami Health System.
Islands of regrowth
Physicians perform pinch grafting by taking small punches of skin from a donor site on the thigh, abdomen, or elsewhere, and then transferring the grafts to serve as islands of regrowth in a wound. Pinch grafting can be faster and less expensive than techniques typically performed in an operating room, such as meshed auto-grafting. In contrast, pinch grafting can be accomplished in an office setting “and patients can do quite well.” Dr. Kirsner said. In terms of outcomes, “our data is typical,” he added. “About 50% of refractory ulcers heal, 25% improve, and a percentage recur.”
Spreadable skin grafts
Another autologous grafting technique that can be performed at the bedside uses the Xpansion Micro-Autografting Kit, which minces autologous, split thickness skin grafts. “Then you apply them like peanut butter to bread,” Dr. Kirsner said.
The micro-autografts can help heal both acute and chronic wounds, including full thickness wounds from trauma, some burn wounds, diabetic foot ulcers, and venous ulcers, according to the manufacturer’s website.
Epidermal harvesting (without anesthesia)
Epidermal grafting can make sense because the epidermis regenerates. “You can lift off just the epidermis with heat or suction, “ Dr. Kirsner said. For the first time, he added, a new tool allows epidermal grafting without the need for anesthesia (Cellutome Epidermal Harvesting System). The device raises little microdomes of epidermis down to the basal layer, including basal keratinocytes and melanocytes, and a dermatologist can use a sterile dressing to transfer them to the wound. Confocal microscopy shows the dermoepidermal junction healing as early as within 2 days.
The epidermal harvesting was initially developed for pigment problems, such as piebaldism. (Dermatol Surg. 2017 Jan;43[1]:159-60). “We quickly realized it might have applicability for nonhealing wounds,” Dr. Kirsner said.
Deeper wound healing
A novel strategy for triggering deeper wound healing evolved from fractional laser technology, which remove columns of skin to generate healing. Instead, Rox Anderson, MD, of Massachusetts General Hospital, Boston, “envisioned pulling up microcolumns of full thickness epidermis, all the way to the fat, placing them into a wound, and the wound would heal with very little donor site morbidity,” Dr. Kirsner said.
This tool is coming out in spring of this year, he noted. It will resemble a fractional laser, “but now you have the skin available to place in another wound.” Prior animal studies revealed a healing benefit with very little scarring, he added.
Is hairier better?
Does the donor site matter? Dr. Kirsner asked. Although dermatologists typically graft skin from an abdomen or thigh, a hair-bearing site may be a better option because of the presence of pluripotent stem cells, according to a case report (Wounds. 2016 Apr;28[4]:109-11). J.D. Fox of the University of Miami, Dr. Kirsner, and their colleagues treated a large, chronic venous leg ulcer, almost 60 cm2, with punch grafts from a variety of donor sites.
“The side that got scalp punch grafts healed better, suggesting with skin taken from richly hairy area, you’ll get better results,” Dr. Kirsner said.
Another study supports this strategy (J Am Acad Dermatol. 2016 Nov;75[5]:1007-14). These researchers reported greater wound size reduction using grafts containing hair follicles versus nonhairy areas, again suggesting follicular stem cells play a role in better wound healing, Dr. Kirsner said. “This may be a better source of donor skin in the future.”
Dr. Kirsner is a consultant for Cardinal Health, Mölnlycke, Amniox, Organogenesis, Kerecis, Keretec, and KCI, an Acelity company.
MIAMI – Pinch grafting can accelerate the healing of chronic, treatment-resistant wounds such as leg ulcers, while at the same time reducing morbidity to the donor skin site. A new epidermal harvesting device also is showing promise, as is a new tool that minces autologous skin grafts prior to application to promote wound healing.
These and other advances in wound healing were presented at the Orlando Dermatology Aesthetic and Clinical Conference. The pinch grafts and minced grafts each rely on the newly added skin to stimulate cytokines. Interestingly, there is evidence that grafts taken from hair-bearing donor sites could be superior for stimulating cytokines and accelerating wound healing, said Robert Kirsner, MD, PhD, of the University of Miami Health System.
Islands of regrowth
Physicians perform pinch grafting by taking small punches of skin from a donor site on the thigh, abdomen, or elsewhere, and then transferring the grafts to serve as islands of regrowth in a wound. Pinch grafting can be faster and less expensive than techniques typically performed in an operating room, such as meshed auto-grafting. In contrast, pinch grafting can be accomplished in an office setting “and patients can do quite well.” Dr. Kirsner said. In terms of outcomes, “our data is typical,” he added. “About 50% of refractory ulcers heal, 25% improve, and a percentage recur.”
Spreadable skin grafts
Another autologous grafting technique that can be performed at the bedside uses the Xpansion Micro-Autografting Kit, which minces autologous, split thickness skin grafts. “Then you apply them like peanut butter to bread,” Dr. Kirsner said.
The micro-autografts can help heal both acute and chronic wounds, including full thickness wounds from trauma, some burn wounds, diabetic foot ulcers, and venous ulcers, according to the manufacturer’s website.
Epidermal harvesting (without anesthesia)
Epidermal grafting can make sense because the epidermis regenerates. “You can lift off just the epidermis with heat or suction, “ Dr. Kirsner said. For the first time, he added, a new tool allows epidermal grafting without the need for anesthesia (Cellutome Epidermal Harvesting System). The device raises little microdomes of epidermis down to the basal layer, including basal keratinocytes and melanocytes, and a dermatologist can use a sterile dressing to transfer them to the wound. Confocal microscopy shows the dermoepidermal junction healing as early as within 2 days.
The epidermal harvesting was initially developed for pigment problems, such as piebaldism. (Dermatol Surg. 2017 Jan;43[1]:159-60). “We quickly realized it might have applicability for nonhealing wounds,” Dr. Kirsner said.
Deeper wound healing
A novel strategy for triggering deeper wound healing evolved from fractional laser technology, which remove columns of skin to generate healing. Instead, Rox Anderson, MD, of Massachusetts General Hospital, Boston, “envisioned pulling up microcolumns of full thickness epidermis, all the way to the fat, placing them into a wound, and the wound would heal with very little donor site morbidity,” Dr. Kirsner said.
This tool is coming out in spring of this year, he noted. It will resemble a fractional laser, “but now you have the skin available to place in another wound.” Prior animal studies revealed a healing benefit with very little scarring, he added.
Is hairier better?
Does the donor site matter? Dr. Kirsner asked. Although dermatologists typically graft skin from an abdomen or thigh, a hair-bearing site may be a better option because of the presence of pluripotent stem cells, according to a case report (Wounds. 2016 Apr;28[4]:109-11). J.D. Fox of the University of Miami, Dr. Kirsner, and their colleagues treated a large, chronic venous leg ulcer, almost 60 cm2, with punch grafts from a variety of donor sites.
“The side that got scalp punch grafts healed better, suggesting with skin taken from richly hairy area, you’ll get better results,” Dr. Kirsner said.
Another study supports this strategy (J Am Acad Dermatol. 2016 Nov;75[5]:1007-14). These researchers reported greater wound size reduction using grafts containing hair follicles versus nonhairy areas, again suggesting follicular stem cells play a role in better wound healing, Dr. Kirsner said. “This may be a better source of donor skin in the future.”
Dr. Kirsner is a consultant for Cardinal Health, Mölnlycke, Amniox, Organogenesis, Kerecis, Keretec, and KCI, an Acelity company.
Evidence supports efficacy of topical skin products for photoaging reversal
MIAMI – Topical skin care products are evolving along with evidence in the literature supporting their efficacy; these products include serums, lotions, and cleansers with DNA repair enzymes or epidermal growth factor as active ingredients, according to Ron Moy, MD.
Importantly, some of these formulations not only show efficacy to reverse the signs of photodamage to skin and to promote rejuvenation, but may have a role in skin cancer prevention as well, said Dr. Moy, a dermatologist and facial plastic surgeon in private practice in Beverly Hills, Calif.
More investigators are assessing the mechanisms and potential advantages of DNA repair, Dr. Moy said at the Orlando Dermatology Aesthetic and Clinical Conference. In 2015, the Nobel Prize in Chemistry was awarded to scientists who discovered key mechanisms underlying DNA repair. “Since then, DNA repair has gotten a lot more attention,” he said.
Evidence suggests a person’s DNA repair capability can modulate colon cancer risk. Also, smokers with a low level of DNA repair enzymes carry a higher risk for lung cancer, and DNA repair genes can predict ovarian cancer and lung cancer survival, Dr. Moy said.
“It is still not entirely understood how everything ties together,” he said. “But the work on DNA repair is convincing, more convincing than work on antioxidants or retinoids.” Although antioxidants look favorable in experimental and animal studies, for example, “generally the published work on antioxidants does not give you good clinical results,” he said.
DNA repair enzymes have a beneficial effect on the proto-oncogene hyperexpression in human skin and ultraviolet light telomere shortening, according to an experimental pilot study in the Journal of Drugs in Dermatology (2013 Sep;12[9]:1017-21).
Also, a DNA repair enzyme derived from bacteria, T4 endonuclease V, showed promise in an older study of 30 patients with xeroderma pigmentosum (Lancet. 2001 Mar 24;357[9260]:926-9). Those affected carry a higher risk overall for any skin cancer, compared with the general population. The DNA repair enzyme group had fewer new actinic keratoses and fewer new basal cell carcinoma, squamous cell carcinomas, and melanoma lesions at 1 year, compared with a vehicle-only group at 1 year.
Another class of DNA repair molecules, photolyases, is newer than T4 endonuclease V and “might work even better,” Dr. Moy said.
“There is evidence that DNA enzymes are very effective and helpful in preventing skin cancer,” he pointed out. One mechanism is the ability of exogenous DNA repair enzymes to bolster intrinsic DNA in the fight against carcinogenesis, according to a review article Dr. Moy and his colleagues published in the Journal of Drugs in Dermatology (2015 Mar;14[3]:297-303).
The role of human epidermal growth factor for improving skin appearance and tightening is another area of active research and promise.
“It basically thickens and tightens skin,” Dr. Moy explained. “It works better on thinner skin, including the eyelids and neck.” Ultrasound objectively demonstrates gains in skin thickness. Studies also show improvement in acne scars following application of epidermal growth factor.
In addition, epidermal growth factor can enhance the appearance of solar purpura. In fact, this is the dermatologic condition with the most convincing evidence supporting its use so far, Dr. Moy said. Clinical studies have shown that epidermal growth factor along with other active ingredients also can improve acne scars and eye bags.
During the question and answer session at the ODAC conference, moderator Susan Weinkle, MD, a dermatologist and Mohs surgeon in private practice in Bradenton, Fla., asked Dr. Moy which epidermal growth factor product he recommends.
“There are a lot of different growth factors. Epidermal growth factors are not all the same,” said Dr. Moy, who is the founder of DNA EGF Renewal, a company that manufactures skin care products containing epidermal growth factor and DNA repair enzymes.
When pressed for the name of his growth factor product by Dr. Weinkle and an uproar from the audience, Dr. Moy chose to stay noncommercial, pointing attendees to his website instead: www.dnaegfrenewal.com.
Dr. Moy is founder of DNA EGF Renewal in Beverly Hills, Calif.
MIAMI – Topical skin care products are evolving along with evidence in the literature supporting their efficacy; these products include serums, lotions, and cleansers with DNA repair enzymes or epidermal growth factor as active ingredients, according to Ron Moy, MD.
Importantly, some of these formulations not only show efficacy to reverse the signs of photodamage to skin and to promote rejuvenation, but may have a role in skin cancer prevention as well, said Dr. Moy, a dermatologist and facial plastic surgeon in private practice in Beverly Hills, Calif.
More investigators are assessing the mechanisms and potential advantages of DNA repair, Dr. Moy said at the Orlando Dermatology Aesthetic and Clinical Conference. In 2015, the Nobel Prize in Chemistry was awarded to scientists who discovered key mechanisms underlying DNA repair. “Since then, DNA repair has gotten a lot more attention,” he said.
Evidence suggests a person’s DNA repair capability can modulate colon cancer risk. Also, smokers with a low level of DNA repair enzymes carry a higher risk for lung cancer, and DNA repair genes can predict ovarian cancer and lung cancer survival, Dr. Moy said.
“It is still not entirely understood how everything ties together,” he said. “But the work on DNA repair is convincing, more convincing than work on antioxidants or retinoids.” Although antioxidants look favorable in experimental and animal studies, for example, “generally the published work on antioxidants does not give you good clinical results,” he said.
DNA repair enzymes have a beneficial effect on the proto-oncogene hyperexpression in human skin and ultraviolet light telomere shortening, according to an experimental pilot study in the Journal of Drugs in Dermatology (2013 Sep;12[9]:1017-21).
Also, a DNA repair enzyme derived from bacteria, T4 endonuclease V, showed promise in an older study of 30 patients with xeroderma pigmentosum (Lancet. 2001 Mar 24;357[9260]:926-9). Those affected carry a higher risk overall for any skin cancer, compared with the general population. The DNA repair enzyme group had fewer new actinic keratoses and fewer new basal cell carcinoma, squamous cell carcinomas, and melanoma lesions at 1 year, compared with a vehicle-only group at 1 year.
Another class of DNA repair molecules, photolyases, is newer than T4 endonuclease V and “might work even better,” Dr. Moy said.
“There is evidence that DNA enzymes are very effective and helpful in preventing skin cancer,” he pointed out. One mechanism is the ability of exogenous DNA repair enzymes to bolster intrinsic DNA in the fight against carcinogenesis, according to a review article Dr. Moy and his colleagues published in the Journal of Drugs in Dermatology (2015 Mar;14[3]:297-303).
The role of human epidermal growth factor for improving skin appearance and tightening is another area of active research and promise.
“It basically thickens and tightens skin,” Dr. Moy explained. “It works better on thinner skin, including the eyelids and neck.” Ultrasound objectively demonstrates gains in skin thickness. Studies also show improvement in acne scars following application of epidermal growth factor.
In addition, epidermal growth factor can enhance the appearance of solar purpura. In fact, this is the dermatologic condition with the most convincing evidence supporting its use so far, Dr. Moy said. Clinical studies have shown that epidermal growth factor along with other active ingredients also can improve acne scars and eye bags.
During the question and answer session at the ODAC conference, moderator Susan Weinkle, MD, a dermatologist and Mohs surgeon in private practice in Bradenton, Fla., asked Dr. Moy which epidermal growth factor product he recommends.
“There are a lot of different growth factors. Epidermal growth factors are not all the same,” said Dr. Moy, who is the founder of DNA EGF Renewal, a company that manufactures skin care products containing epidermal growth factor and DNA repair enzymes.
When pressed for the name of his growth factor product by Dr. Weinkle and an uproar from the audience, Dr. Moy chose to stay noncommercial, pointing attendees to his website instead: www.dnaegfrenewal.com.
Dr. Moy is founder of DNA EGF Renewal in Beverly Hills, Calif.
MIAMI – Topical skin care products are evolving along with evidence in the literature supporting their efficacy; these products include serums, lotions, and cleansers with DNA repair enzymes or epidermal growth factor as active ingredients, according to Ron Moy, MD.
Importantly, some of these formulations not only show efficacy to reverse the signs of photodamage to skin and to promote rejuvenation, but may have a role in skin cancer prevention as well, said Dr. Moy, a dermatologist and facial plastic surgeon in private practice in Beverly Hills, Calif.
More investigators are assessing the mechanisms and potential advantages of DNA repair, Dr. Moy said at the Orlando Dermatology Aesthetic and Clinical Conference. In 2015, the Nobel Prize in Chemistry was awarded to scientists who discovered key mechanisms underlying DNA repair. “Since then, DNA repair has gotten a lot more attention,” he said.
Evidence suggests a person’s DNA repair capability can modulate colon cancer risk. Also, smokers with a low level of DNA repair enzymes carry a higher risk for lung cancer, and DNA repair genes can predict ovarian cancer and lung cancer survival, Dr. Moy said.
“It is still not entirely understood how everything ties together,” he said. “But the work on DNA repair is convincing, more convincing than work on antioxidants or retinoids.” Although antioxidants look favorable in experimental and animal studies, for example, “generally the published work on antioxidants does not give you good clinical results,” he said.
DNA repair enzymes have a beneficial effect on the proto-oncogene hyperexpression in human skin and ultraviolet light telomere shortening, according to an experimental pilot study in the Journal of Drugs in Dermatology (2013 Sep;12[9]:1017-21).
Also, a DNA repair enzyme derived from bacteria, T4 endonuclease V, showed promise in an older study of 30 patients with xeroderma pigmentosum (Lancet. 2001 Mar 24;357[9260]:926-9). Those affected carry a higher risk overall for any skin cancer, compared with the general population. The DNA repair enzyme group had fewer new actinic keratoses and fewer new basal cell carcinoma, squamous cell carcinomas, and melanoma lesions at 1 year, compared with a vehicle-only group at 1 year.
Another class of DNA repair molecules, photolyases, is newer than T4 endonuclease V and “might work even better,” Dr. Moy said.
“There is evidence that DNA enzymes are very effective and helpful in preventing skin cancer,” he pointed out. One mechanism is the ability of exogenous DNA repair enzymes to bolster intrinsic DNA in the fight against carcinogenesis, according to a review article Dr. Moy and his colleagues published in the Journal of Drugs in Dermatology (2015 Mar;14[3]:297-303).
The role of human epidermal growth factor for improving skin appearance and tightening is another area of active research and promise.
“It basically thickens and tightens skin,” Dr. Moy explained. “It works better on thinner skin, including the eyelids and neck.” Ultrasound objectively demonstrates gains in skin thickness. Studies also show improvement in acne scars following application of epidermal growth factor.
In addition, epidermal growth factor can enhance the appearance of solar purpura. In fact, this is the dermatologic condition with the most convincing evidence supporting its use so far, Dr. Moy said. Clinical studies have shown that epidermal growth factor along with other active ingredients also can improve acne scars and eye bags.
During the question and answer session at the ODAC conference, moderator Susan Weinkle, MD, a dermatologist and Mohs surgeon in private practice in Bradenton, Fla., asked Dr. Moy which epidermal growth factor product he recommends.
“There are a lot of different growth factors. Epidermal growth factors are not all the same,” said Dr. Moy, who is the founder of DNA EGF Renewal, a company that manufactures skin care products containing epidermal growth factor and DNA repair enzymes.
When pressed for the name of his growth factor product by Dr. Weinkle and an uproar from the audience, Dr. Moy chose to stay noncommercial, pointing attendees to his website instead: www.dnaegfrenewal.com.
Dr. Moy is founder of DNA EGF Renewal in Beverly Hills, Calif.
EXPERT ANALYSIS FROM ODAC 2017
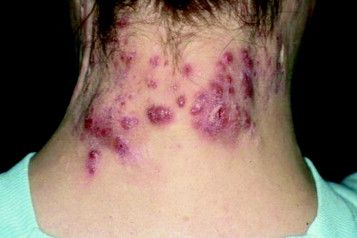
Recognize, treat, and teach others to spot hidradenitis suppurativa
MIAMI – Clinicians have many options to treat and help people manage hidradenitis suppurativa, but for most patients, an early and accurate diagnosis remains elusive.
“The problem here is because it has so many mimickers, the diagnosis is often delayed, patients can be [treated for an incorrect diagnosis] and in many ways that treatment can be harmful,” said Adam Friedman, MD, of the George Washington University in Washington. Missed or ignored diagnoses can lead to more pain, impaired function, and wasted time and money.
“There are – no question – gaps in clinical care. Patients seek care outside of dermatology and go to urgent care centers, emergency rooms, [and other settings]. That’s why it’s not only important for us to recognize this, but to teach everyone else as well,” Dr. Friedman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Tips for early detection
Look for chronicity in the disease’s presentation, Dr. Friedman said. “Chronicity is key, but the morphology will change, and lesions will look different over time.” Therefore, “the clinical presentation can be challenging, depending on when you catch the patient.”
Hidradenitis suppurativa is characterized by very purulent, indurated, abscesslike structures often on the underarms and groin. Ask patients where and how often they see lesions. Lesions in certain locations can be very disabling for patients not only because of pain, but also from a psychosocial impact. The groin and chest are prime examples. Also, there is a genetic predisposition so it is important to ask patients about family history as well.
Use combination therapy to hit disease ‘from all angles’
It is imperative to treat patients even when they present at a time of mild disease, Dr. Friedman said. “This is a chronic, snowballing disease that will get worse over time, because inflammation begets inflammation. Even if it’s mild disease, you still want to treat. Combinations are king, and we dermatologists are the synergy masters.”
Effective treatment strategies include medications that curtail inflammation and block hormonal influences; dietary changes (a minimal-dairy, low-carbohydrate diet helps some patients, for example); environmental changes and/or eliminating the invasive proliferative gelatinous mass (IPGM). “This is not step therapy,” Dr. Friedman emphasized. “You want to hit all these angles at once.”
In terms of nutritional support, “I usually put my patients on a combination of zinc and vitamin C, both anti-inflammatories, but also good for wound healing,” Dr. Friedman said. “I also get them on board with V-8, which can be a tough sell sometimes.” He recommends patients drink three small cans per week, adding that he has no financial disclosure related to the vegetable juice.
Patient education, smoking cessation, and keeping affected areas dry and cool are other important management strategies. Instruct patients that stress, friction, and obesity can each worsen the condition “I think obesity is an independent risk factor here, like it is in psoriasis, where obesity alone has been shown to increase the risk of psoriasis later on,” he said.
Ease the inflammation
Hidradenitis suppurativa is a disease of “inappropriate inflammation,” Dr. Friedman said, which explains why anti-inflammatory agents remain the mainstay of treatment. These include antibiotics, classic corticosteroids, and biologics, which all can have a role in therapy. He highlighted the potential role of the cutaneous microbiota and possible dysbiosis associated with disease activity. “I also use a lot chlorhexidine washes to wipe the microbial slate clean in high-risk areas; just be careful to avoid the face.”
Intralesional Kenalog (triamcinolone acetonide), in particular, is useful for its rapid results. “This is such a great and easy trick, and it really works quickly,” Dr. Friedman said. He added that a recent case series provides evidence for its efficacy as well (J Amer Acad Dermatol. 2016 Dec;75:1151-5).
Three take-home treatment pointers
Dr. Friedman shared these three take-aways for treatment of hidradenitis suppurativa with antibiotics:
- The combination of oral clindamycin 300 mg twice daily and oral rifampin 300 mg twice daily carries the most evidence for efficacy and safety, with no evidence of resistance.
- Rifampin also acts against Clostridium difficile infections, which decreases the risk of associated colitis.
- Do not give a tetracycline antibiotic as monotherapy.
In terms of retinoids, “I’ve been pretty disappointed. I find them effective [in other conditions], but for hidradenitis suppurativa, I’m just not impressed, unfortunately,” Dr. Friedman said. “Antihormonal therapies such as oral contraceptives and spironolactone for women and finasteride for men have been a useful adjuncts in my practice, with evidence supporting their use in the literature, he added. Biologics are among the new treatment options, but there are cost and insurance coverage issues, Dr. Friedman said. There are small case series evaluating biologics such as infliximab, which are very supportive – and he himself has had good responses – although the Food and Drug Administration indication has been the hurdle.
One exception is the recent FDA approval of adalimumab (Humira) for hidradenitis suppurativa. “Make sure you realize that the dosing is different, more like a ‘whopping’ Crohn’s disease dose. This is a very important medication in our armamentarium – the issue is when you start it,” Dr. Friedman said. He also cautioned, “if a patient has sinus tracts and scarring, it’s not going to be enough. You still need to address that. Adalimumab is only going to get rid of the inflammation. This is why early diagnosis is so important!”
When it comes to surgery, “my philosophy is it’s all or none. If you’re going to do surgery, do surgery. You better cut these things out. Do a wide global excision,” Dr. Friedman emphasized.
Dr. Friedman is a member of the Dermatology News editorial advisory board.
Dr. Friedman had no relevant financial disclosures.
MIAMI – Clinicians have many options to treat and help people manage hidradenitis suppurativa, but for most patients, an early and accurate diagnosis remains elusive.
“The problem here is because it has so many mimickers, the diagnosis is often delayed, patients can be [treated for an incorrect diagnosis] and in many ways that treatment can be harmful,” said Adam Friedman, MD, of the George Washington University in Washington. Missed or ignored diagnoses can lead to more pain, impaired function, and wasted time and money.
“There are – no question – gaps in clinical care. Patients seek care outside of dermatology and go to urgent care centers, emergency rooms, [and other settings]. That’s why it’s not only important for us to recognize this, but to teach everyone else as well,” Dr. Friedman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Tips for early detection
Look for chronicity in the disease’s presentation, Dr. Friedman said. “Chronicity is key, but the morphology will change, and lesions will look different over time.” Therefore, “the clinical presentation can be challenging, depending on when you catch the patient.”
Hidradenitis suppurativa is characterized by very purulent, indurated, abscesslike structures often on the underarms and groin. Ask patients where and how often they see lesions. Lesions in certain locations can be very disabling for patients not only because of pain, but also from a psychosocial impact. The groin and chest are prime examples. Also, there is a genetic predisposition so it is important to ask patients about family history as well.
Use combination therapy to hit disease ‘from all angles’
It is imperative to treat patients even when they present at a time of mild disease, Dr. Friedman said. “This is a chronic, snowballing disease that will get worse over time, because inflammation begets inflammation. Even if it’s mild disease, you still want to treat. Combinations are king, and we dermatologists are the synergy masters.”
Effective treatment strategies include medications that curtail inflammation and block hormonal influences; dietary changes (a minimal-dairy, low-carbohydrate diet helps some patients, for example); environmental changes and/or eliminating the invasive proliferative gelatinous mass (IPGM). “This is not step therapy,” Dr. Friedman emphasized. “You want to hit all these angles at once.”
In terms of nutritional support, “I usually put my patients on a combination of zinc and vitamin C, both anti-inflammatories, but also good for wound healing,” Dr. Friedman said. “I also get them on board with V-8, which can be a tough sell sometimes.” He recommends patients drink three small cans per week, adding that he has no financial disclosure related to the vegetable juice.
Patient education, smoking cessation, and keeping affected areas dry and cool are other important management strategies. Instruct patients that stress, friction, and obesity can each worsen the condition “I think obesity is an independent risk factor here, like it is in psoriasis, where obesity alone has been shown to increase the risk of psoriasis later on,” he said.
Ease the inflammation
Hidradenitis suppurativa is a disease of “inappropriate inflammation,” Dr. Friedman said, which explains why anti-inflammatory agents remain the mainstay of treatment. These include antibiotics, classic corticosteroids, and biologics, which all can have a role in therapy. He highlighted the potential role of the cutaneous microbiota and possible dysbiosis associated with disease activity. “I also use a lot chlorhexidine washes to wipe the microbial slate clean in high-risk areas; just be careful to avoid the face.”
Intralesional Kenalog (triamcinolone acetonide), in particular, is useful for its rapid results. “This is such a great and easy trick, and it really works quickly,” Dr. Friedman said. He added that a recent case series provides evidence for its efficacy as well (J Amer Acad Dermatol. 2016 Dec;75:1151-5).
Three take-home treatment pointers
Dr. Friedman shared these three take-aways for treatment of hidradenitis suppurativa with antibiotics:
- The combination of oral clindamycin 300 mg twice daily and oral rifampin 300 mg twice daily carries the most evidence for efficacy and safety, with no evidence of resistance.
- Rifampin also acts against Clostridium difficile infections, which decreases the risk of associated colitis.
- Do not give a tetracycline antibiotic as monotherapy.
In terms of retinoids, “I’ve been pretty disappointed. I find them effective [in other conditions], but for hidradenitis suppurativa, I’m just not impressed, unfortunately,” Dr. Friedman said. “Antihormonal therapies such as oral contraceptives and spironolactone for women and finasteride for men have been a useful adjuncts in my practice, with evidence supporting their use in the literature, he added. Biologics are among the new treatment options, but there are cost and insurance coverage issues, Dr. Friedman said. There are small case series evaluating biologics such as infliximab, which are very supportive – and he himself has had good responses – although the Food and Drug Administration indication has been the hurdle.
One exception is the recent FDA approval of adalimumab (Humira) for hidradenitis suppurativa. “Make sure you realize that the dosing is different, more like a ‘whopping’ Crohn’s disease dose. This is a very important medication in our armamentarium – the issue is when you start it,” Dr. Friedman said. He also cautioned, “if a patient has sinus tracts and scarring, it’s not going to be enough. You still need to address that. Adalimumab is only going to get rid of the inflammation. This is why early diagnosis is so important!”
When it comes to surgery, “my philosophy is it’s all or none. If you’re going to do surgery, do surgery. You better cut these things out. Do a wide global excision,” Dr. Friedman emphasized.
Dr. Friedman is a member of the Dermatology News editorial advisory board.
Dr. Friedman had no relevant financial disclosures.
MIAMI – Clinicians have many options to treat and help people manage hidradenitis suppurativa, but for most patients, an early and accurate diagnosis remains elusive.
“The problem here is because it has so many mimickers, the diagnosis is often delayed, patients can be [treated for an incorrect diagnosis] and in many ways that treatment can be harmful,” said Adam Friedman, MD, of the George Washington University in Washington. Missed or ignored diagnoses can lead to more pain, impaired function, and wasted time and money.
“There are – no question – gaps in clinical care. Patients seek care outside of dermatology and go to urgent care centers, emergency rooms, [and other settings]. That’s why it’s not only important for us to recognize this, but to teach everyone else as well,” Dr. Friedman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Tips for early detection
Look for chronicity in the disease’s presentation, Dr. Friedman said. “Chronicity is key, but the morphology will change, and lesions will look different over time.” Therefore, “the clinical presentation can be challenging, depending on when you catch the patient.”
Hidradenitis suppurativa is characterized by very purulent, indurated, abscesslike structures often on the underarms and groin. Ask patients where and how often they see lesions. Lesions in certain locations can be very disabling for patients not only because of pain, but also from a psychosocial impact. The groin and chest are prime examples. Also, there is a genetic predisposition so it is important to ask patients about family history as well.
Use combination therapy to hit disease ‘from all angles’
It is imperative to treat patients even when they present at a time of mild disease, Dr. Friedman said. “This is a chronic, snowballing disease that will get worse over time, because inflammation begets inflammation. Even if it’s mild disease, you still want to treat. Combinations are king, and we dermatologists are the synergy masters.”
Effective treatment strategies include medications that curtail inflammation and block hormonal influences; dietary changes (a minimal-dairy, low-carbohydrate diet helps some patients, for example); environmental changes and/or eliminating the invasive proliferative gelatinous mass (IPGM). “This is not step therapy,” Dr. Friedman emphasized. “You want to hit all these angles at once.”
In terms of nutritional support, “I usually put my patients on a combination of zinc and vitamin C, both anti-inflammatories, but also good for wound healing,” Dr. Friedman said. “I also get them on board with V-8, which can be a tough sell sometimes.” He recommends patients drink three small cans per week, adding that he has no financial disclosure related to the vegetable juice.
Patient education, smoking cessation, and keeping affected areas dry and cool are other important management strategies. Instruct patients that stress, friction, and obesity can each worsen the condition “I think obesity is an independent risk factor here, like it is in psoriasis, where obesity alone has been shown to increase the risk of psoriasis later on,” he said.
Ease the inflammation
Hidradenitis suppurativa is a disease of “inappropriate inflammation,” Dr. Friedman said, which explains why anti-inflammatory agents remain the mainstay of treatment. These include antibiotics, classic corticosteroids, and biologics, which all can have a role in therapy. He highlighted the potential role of the cutaneous microbiota and possible dysbiosis associated with disease activity. “I also use a lot chlorhexidine washes to wipe the microbial slate clean in high-risk areas; just be careful to avoid the face.”
Intralesional Kenalog (triamcinolone acetonide), in particular, is useful for its rapid results. “This is such a great and easy trick, and it really works quickly,” Dr. Friedman said. He added that a recent case series provides evidence for its efficacy as well (J Amer Acad Dermatol. 2016 Dec;75:1151-5).
Three take-home treatment pointers
Dr. Friedman shared these three take-aways for treatment of hidradenitis suppurativa with antibiotics:
- The combination of oral clindamycin 300 mg twice daily and oral rifampin 300 mg twice daily carries the most evidence for efficacy and safety, with no evidence of resistance.
- Rifampin also acts against Clostridium difficile infections, which decreases the risk of associated colitis.
- Do not give a tetracycline antibiotic as monotherapy.
In terms of retinoids, “I’ve been pretty disappointed. I find them effective [in other conditions], but for hidradenitis suppurativa, I’m just not impressed, unfortunately,” Dr. Friedman said. “Antihormonal therapies such as oral contraceptives and spironolactone for women and finasteride for men have been a useful adjuncts in my practice, with evidence supporting their use in the literature, he added. Biologics are among the new treatment options, but there are cost and insurance coverage issues, Dr. Friedman said. There are small case series evaluating biologics such as infliximab, which are very supportive – and he himself has had good responses – although the Food and Drug Administration indication has been the hurdle.
One exception is the recent FDA approval of adalimumab (Humira) for hidradenitis suppurativa. “Make sure you realize that the dosing is different, more like a ‘whopping’ Crohn’s disease dose. This is a very important medication in our armamentarium – the issue is when you start it,” Dr. Friedman said. He also cautioned, “if a patient has sinus tracts and scarring, it’s not going to be enough. You still need to address that. Adalimumab is only going to get rid of the inflammation. This is why early diagnosis is so important!”
When it comes to surgery, “my philosophy is it’s all or none. If you’re going to do surgery, do surgery. You better cut these things out. Do a wide global excision,” Dr. Friedman emphasized.
Dr. Friedman is a member of the Dermatology News editorial advisory board.
Dr. Friedman had no relevant financial disclosures.
EXPERT ANALYSIS FROM ODAC 2017
Clinicians underusing statins, aspirin in HIV patients
NEW ORLEANS – Clinicians may not be prescribing enough statins and/or aspirin therapy for the HIV-infected population at highest risk for atherosclerotic cardiovascular disease, according to the results of a large retrospective study from an HIV clinic in New York.
“We need to shift our paradigm for care – from ‘take antiretrovirals and that’s it’ – to a focus on the whole patient and chronic conditions,” Emma Kaplan-Lewis, MD, said during a poster presentation at an annual scientific meeting on infectious diseases. Risk of cardiovascular events increases 1.5-fold among people with HIV treated with antiretroviral therapy, compared with the uninfected population, she noted.
The investigators examined prescriptions for a statin or aspirin therapy in three high-risk groups, defined by 2013 American College of Cardiology/American Heart Association guidelines and 2011 AHA/American College of Cardiology Foundation guidelines. They classified patients with an ICD-9 code indicating a history of atherosclerotic cardiovascular disease, 40- to 75-year-olds with diabetes and LDL cholesterol greater than 70 mg/dL, and those with an LDL cholesterol level greater than 190 mg/dL.
Almost two-thirds of the higher-risk patients, 141 (61%), had a history of atherosclerotic cardiovascular disease. In this cohort, 56% were prescribed statin therapy, and 100 (71%) were prescribed aspirin.
Of the 85 high-risk patients with diabetes and an LDL greater than 70 mg/dL, 48 (57%) were on statin therapy (aspirin not indicated), and of the 5 high-risk patients with an LDL cholesterol level greater than 190 mg/dL, 3 (60%) were on statin therapy, and 1 (20%) was on aspirin.
The investigators also found 37% of the higher-risk patients were active cigarette smokers. There was a trend toward lower statin use among smokers, 33% versus 44% for nonsmokers. “Smoking was not significantly associated with statin prescription, but this is a modifiable risk factor – after which they may not need a statin,” Dr. Kaplan-Lewis said.
The findings support risk-reduction interventions for people with HIV infection who are at higher risk for cardiovascular disease, Dr. Kaplan-Lewis said. The results support previous reports in the literature, including a study that found 51% of 13,579 veterans infected with HIV had an indication for statin use, but 22% of this group was not prescribed the therapy (Clin Infect Dis. 2016;63:407-13. doi: 10.1093/cid/ciw289).
In 2017, the investigators plan to share the study data with providers. “Each provider will get a list of their patients who should be on a statin. This is about awareness and making it more of a priority,” Dr. Kaplan-Lewis said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The findings of additional research assessing lower thresholds for statin use among people with HIV infection are still pending, Dr. Kaplan-Lewis added.
The study was supported in part by the New York State Department of Health Empire Clinical Research Investigator Program. Dr. Kaplan-Lewis had no relevant disclosures.
NEW ORLEANS – Clinicians may not be prescribing enough statins and/or aspirin therapy for the HIV-infected population at highest risk for atherosclerotic cardiovascular disease, according to the results of a large retrospective study from an HIV clinic in New York.
“We need to shift our paradigm for care – from ‘take antiretrovirals and that’s it’ – to a focus on the whole patient and chronic conditions,” Emma Kaplan-Lewis, MD, said during a poster presentation at an annual scientific meeting on infectious diseases. Risk of cardiovascular events increases 1.5-fold among people with HIV treated with antiretroviral therapy, compared with the uninfected population, she noted.
The investigators examined prescriptions for a statin or aspirin therapy in three high-risk groups, defined by 2013 American College of Cardiology/American Heart Association guidelines and 2011 AHA/American College of Cardiology Foundation guidelines. They classified patients with an ICD-9 code indicating a history of atherosclerotic cardiovascular disease, 40- to 75-year-olds with diabetes and LDL cholesterol greater than 70 mg/dL, and those with an LDL cholesterol level greater than 190 mg/dL.
Almost two-thirds of the higher-risk patients, 141 (61%), had a history of atherosclerotic cardiovascular disease. In this cohort, 56% were prescribed statin therapy, and 100 (71%) were prescribed aspirin.
Of the 85 high-risk patients with diabetes and an LDL greater than 70 mg/dL, 48 (57%) were on statin therapy (aspirin not indicated), and of the 5 high-risk patients with an LDL cholesterol level greater than 190 mg/dL, 3 (60%) were on statin therapy, and 1 (20%) was on aspirin.
The investigators also found 37% of the higher-risk patients were active cigarette smokers. There was a trend toward lower statin use among smokers, 33% versus 44% for nonsmokers. “Smoking was not significantly associated with statin prescription, but this is a modifiable risk factor – after which they may not need a statin,” Dr. Kaplan-Lewis said.
The findings support risk-reduction interventions for people with HIV infection who are at higher risk for cardiovascular disease, Dr. Kaplan-Lewis said. The results support previous reports in the literature, including a study that found 51% of 13,579 veterans infected with HIV had an indication for statin use, but 22% of this group was not prescribed the therapy (Clin Infect Dis. 2016;63:407-13. doi: 10.1093/cid/ciw289).
In 2017, the investigators plan to share the study data with providers. “Each provider will get a list of their patients who should be on a statin. This is about awareness and making it more of a priority,” Dr. Kaplan-Lewis said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The findings of additional research assessing lower thresholds for statin use among people with HIV infection are still pending, Dr. Kaplan-Lewis added.
The study was supported in part by the New York State Department of Health Empire Clinical Research Investigator Program. Dr. Kaplan-Lewis had no relevant disclosures.
NEW ORLEANS – Clinicians may not be prescribing enough statins and/or aspirin therapy for the HIV-infected population at highest risk for atherosclerotic cardiovascular disease, according to the results of a large retrospective study from an HIV clinic in New York.
“We need to shift our paradigm for care – from ‘take antiretrovirals and that’s it’ – to a focus on the whole patient and chronic conditions,” Emma Kaplan-Lewis, MD, said during a poster presentation at an annual scientific meeting on infectious diseases. Risk of cardiovascular events increases 1.5-fold among people with HIV treated with antiretroviral therapy, compared with the uninfected population, she noted.
The investigators examined prescriptions for a statin or aspirin therapy in three high-risk groups, defined by 2013 American College of Cardiology/American Heart Association guidelines and 2011 AHA/American College of Cardiology Foundation guidelines. They classified patients with an ICD-9 code indicating a history of atherosclerotic cardiovascular disease, 40- to 75-year-olds with diabetes and LDL cholesterol greater than 70 mg/dL, and those with an LDL cholesterol level greater than 190 mg/dL.
Almost two-thirds of the higher-risk patients, 141 (61%), had a history of atherosclerotic cardiovascular disease. In this cohort, 56% were prescribed statin therapy, and 100 (71%) were prescribed aspirin.
Of the 85 high-risk patients with diabetes and an LDL greater than 70 mg/dL, 48 (57%) were on statin therapy (aspirin not indicated), and of the 5 high-risk patients with an LDL cholesterol level greater than 190 mg/dL, 3 (60%) were on statin therapy, and 1 (20%) was on aspirin.
The investigators also found 37% of the higher-risk patients were active cigarette smokers. There was a trend toward lower statin use among smokers, 33% versus 44% for nonsmokers. “Smoking was not significantly associated with statin prescription, but this is a modifiable risk factor – after which they may not need a statin,” Dr. Kaplan-Lewis said.
The findings support risk-reduction interventions for people with HIV infection who are at higher risk for cardiovascular disease, Dr. Kaplan-Lewis said. The results support previous reports in the literature, including a study that found 51% of 13,579 veterans infected with HIV had an indication for statin use, but 22% of this group was not prescribed the therapy (Clin Infect Dis. 2016;63:407-13. doi: 10.1093/cid/ciw289).
In 2017, the investigators plan to share the study data with providers. “Each provider will get a list of their patients who should be on a statin. This is about awareness and making it more of a priority,” Dr. Kaplan-Lewis said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The findings of additional research assessing lower thresholds for statin use among people with HIV infection are still pending, Dr. Kaplan-Lewis added.
The study was supported in part by the New York State Department of Health Empire Clinical Research Investigator Program. Dr. Kaplan-Lewis had no relevant disclosures.
AT IDWEEK 2016
Key clinical point: Statins and aspirin therapy are underutilized in a proportion of the HIV positive population at higher risk for cardiovascular disease.
Major finding: Of the 141 high-risk people with HIV infection and a history of atherosclerotic cardiovascular disease, only 56% were prescribed a statin and 71% were prescribed aspirin.
Data source: Poster presentation at IDWeek 2016.
Disclosures: The study was supported in part by the New York State Department of Health Empire Clinical Research Investigator Program. Dr. Kaplan-Lewis had no relevant disclosures.
Fixed combination topical shows promise for psoriasis
MIAMI – In an ongoing drive to identify an alternative to the mainstay treatment of psoriasis with topical corticosteroids, researchers evaluated a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion compared to its monads and vehicle in 212 people with moderate to severe psoriasis.
After 8 weeks of once-daily applications, combination therapy yielded significantly greater reductions in erythema, plaque elevation, and scaling at the target lesion, compared with the other groups in the phase II trial. The investigators also reported at safety profile consistent with halobetasol propionate or tazarotene monotherapy, meaning no new safety concerns emerged with the combination.
Participants were randomized to the combination, halobetasol propionate alone, tazarotene monotherapy, or vehicle in the multicenter, double blind study.
Efficacy evaluation
At 8 weeks, 53% of the combination group achieved treatment success, defined as at least a 2-point gain in Investigator’s Global Assessment of Disease Severity and a score of “clear” or “almost clear.” In contrast, 33% of the halobetasol propionate group, 19% in the tazarotene, and 10% of the vehicle only group achieved those endpoints in the intent-to-treat-analysis. The difference in efficacy between the combination group and vehicle was statistically significant (P less than .001).
On individual clinical efficacy measures, more than half of the patients in the combination treatment group achieved at least a 2-point improvement from baseline: 54% for erythema, 68% for plaque elevation, 65% for scaling. Each of these outcomes was significantly superior compared to the tazarotene or vehicle group (P less than or equal to .001).
“I was not surprised by the findings as the drugs work by complimentary mechanisms of action – so combination therapy should be effective,” Dr. Stein Gold said.
At baseline, IGA severity score of 3 was most common in each group and for each psoriasis sign at the target lesions. In each group, the mean age of participants ranged from 48 to 56 years; the proportion of men ranged from 59% to 68%; and 87% to 95% were white. The median target lesion sizes ranged from 25 to 32 cm2.
Safety outcomes
One patient in the vehicle-only group died. Three other participants experienced serious adverse events – one in each of the noncombination groups. None of these events were related to the study medications, the investigators noted. Application site reactions were the most common treatment-associated adverse events. Some skin atrophy was reported.
“These results show that the fixed combination of halobetasol propionate and tazarotene was effective and well tolerated,” said Dr. Stein Gold, who serves as division head of dermatology at Henry Ford Health System, West Bloomfield, Mich. “This would be a nice addition to our treatment armamentarium for patients with plaque psoriasis.”
Dr. Stein Gold is a speaker, consultant, and study investigator for Valeant Pharmaceuticals, which supported the study.
MIAMI – In an ongoing drive to identify an alternative to the mainstay treatment of psoriasis with topical corticosteroids, researchers evaluated a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion compared to its monads and vehicle in 212 people with moderate to severe psoriasis.
After 8 weeks of once-daily applications, combination therapy yielded significantly greater reductions in erythema, plaque elevation, and scaling at the target lesion, compared with the other groups in the phase II trial. The investigators also reported at safety profile consistent with halobetasol propionate or tazarotene monotherapy, meaning no new safety concerns emerged with the combination.
Participants were randomized to the combination, halobetasol propionate alone, tazarotene monotherapy, or vehicle in the multicenter, double blind study.
Efficacy evaluation
At 8 weeks, 53% of the combination group achieved treatment success, defined as at least a 2-point gain in Investigator’s Global Assessment of Disease Severity and a score of “clear” or “almost clear.” In contrast, 33% of the halobetasol propionate group, 19% in the tazarotene, and 10% of the vehicle only group achieved those endpoints in the intent-to-treat-analysis. The difference in efficacy between the combination group and vehicle was statistically significant (P less than .001).
On individual clinical efficacy measures, more than half of the patients in the combination treatment group achieved at least a 2-point improvement from baseline: 54% for erythema, 68% for plaque elevation, 65% for scaling. Each of these outcomes was significantly superior compared to the tazarotene or vehicle group (P less than or equal to .001).
“I was not surprised by the findings as the drugs work by complimentary mechanisms of action – so combination therapy should be effective,” Dr. Stein Gold said.
At baseline, IGA severity score of 3 was most common in each group and for each psoriasis sign at the target lesions. In each group, the mean age of participants ranged from 48 to 56 years; the proportion of men ranged from 59% to 68%; and 87% to 95% were white. The median target lesion sizes ranged from 25 to 32 cm2.
Safety outcomes
One patient in the vehicle-only group died. Three other participants experienced serious adverse events – one in each of the noncombination groups. None of these events were related to the study medications, the investigators noted. Application site reactions were the most common treatment-associated adverse events. Some skin atrophy was reported.
“These results show that the fixed combination of halobetasol propionate and tazarotene was effective and well tolerated,” said Dr. Stein Gold, who serves as division head of dermatology at Henry Ford Health System, West Bloomfield, Mich. “This would be a nice addition to our treatment armamentarium for patients with plaque psoriasis.”
Dr. Stein Gold is a speaker, consultant, and study investigator for Valeant Pharmaceuticals, which supported the study.
MIAMI – In an ongoing drive to identify an alternative to the mainstay treatment of psoriasis with topical corticosteroids, researchers evaluated a fixed-dose combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion compared to its monads and vehicle in 212 people with moderate to severe psoriasis.
After 8 weeks of once-daily applications, combination therapy yielded significantly greater reductions in erythema, plaque elevation, and scaling at the target lesion, compared with the other groups in the phase II trial. The investigators also reported at safety profile consistent with halobetasol propionate or tazarotene monotherapy, meaning no new safety concerns emerged with the combination.
Participants were randomized to the combination, halobetasol propionate alone, tazarotene monotherapy, or vehicle in the multicenter, double blind study.
Efficacy evaluation
At 8 weeks, 53% of the combination group achieved treatment success, defined as at least a 2-point gain in Investigator’s Global Assessment of Disease Severity and a score of “clear” or “almost clear.” In contrast, 33% of the halobetasol propionate group, 19% in the tazarotene, and 10% of the vehicle only group achieved those endpoints in the intent-to-treat-analysis. The difference in efficacy between the combination group and vehicle was statistically significant (P less than .001).
On individual clinical efficacy measures, more than half of the patients in the combination treatment group achieved at least a 2-point improvement from baseline: 54% for erythema, 68% for plaque elevation, 65% for scaling. Each of these outcomes was significantly superior compared to the tazarotene or vehicle group (P less than or equal to .001).
“I was not surprised by the findings as the drugs work by complimentary mechanisms of action – so combination therapy should be effective,” Dr. Stein Gold said.
At baseline, IGA severity score of 3 was most common in each group and for each psoriasis sign at the target lesions. In each group, the mean age of participants ranged from 48 to 56 years; the proportion of men ranged from 59% to 68%; and 87% to 95% were white. The median target lesion sizes ranged from 25 to 32 cm2.
Safety outcomes
One patient in the vehicle-only group died. Three other participants experienced serious adverse events – one in each of the noncombination groups. None of these events were related to the study medications, the investigators noted. Application site reactions were the most common treatment-associated adverse events. Some skin atrophy was reported.
“These results show that the fixed combination of halobetasol propionate and tazarotene was effective and well tolerated,” said Dr. Stein Gold, who serves as division head of dermatology at Henry Ford Health System, West Bloomfield, Mich. “This would be a nice addition to our treatment armamentarium for patients with plaque psoriasis.”
Dr. Stein Gold is a speaker, consultant, and study investigator for Valeant Pharmaceuticals, which supported the study.
AT ODAC 2017
Key clinical point: A fixed dose combination of topical halobetasol propionate 0.01% and tazarotene 0.045% proved superior to either monotherapy or vehicle.
Major finding: Treatment success was achieved by 53% of a combination group compared with 33% of a halobetasol propionate group, 19% of a tazarotene group, and 10% of the vehicle-only group in an intent-to-treat-analysis.
Data source: A multicenter, randomized, double blind phase II study of 212 people with moderate to severe psoriasis.
Disclosures: Dr. Stein Gold is a speaker, consultant, and study investigator for Valeant Pharmaceuticals, which supported the study.
VIDEO: HIV PrEP effective, but adoption lags among high-risk patients
NEW ORLEANS – The adoption and use of HIV preexposure prophylaxis (PrEP) continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men, says a clinician who works with these patients.
John Krotchko, MD, a family practitioner at Denver Health and Hospital Authority, has studied the impact of a community outreach program – targeting HIV-negative men who have sex with men – that educates about HIV PrEP and offers HIV testing. He and his colleagues have examined the effectiveness of this approach, how comfortable participants are discussing their behaviors, and have tried to determine whether availability of HIV PrEP increases high-risk behaviors.
Dr. Krotchko and his colleagues also studied overall trends in awareness about PrEP over time, as well as the acceptability of participants to take a once-daily emtricitabine/tenofovir pill. He explained in a video interview why he believes it’s important to manage these patients in a primary care setting. The interview took place at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Krotchko had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – The adoption and use of HIV preexposure prophylaxis (PrEP) continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men, says a clinician who works with these patients.
John Krotchko, MD, a family practitioner at Denver Health and Hospital Authority, has studied the impact of a community outreach program – targeting HIV-negative men who have sex with men – that educates about HIV PrEP and offers HIV testing. He and his colleagues have examined the effectiveness of this approach, how comfortable participants are discussing their behaviors, and have tried to determine whether availability of HIV PrEP increases high-risk behaviors.
Dr. Krotchko and his colleagues also studied overall trends in awareness about PrEP over time, as well as the acceptability of participants to take a once-daily emtricitabine/tenofovir pill. He explained in a video interview why he believes it’s important to manage these patients in a primary care setting. The interview took place at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Krotchko had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – The adoption and use of HIV preexposure prophylaxis (PrEP) continues to lag behind studies showing its effectiveness in the high-risk population of men who have sex with men, says a clinician who works with these patients.
John Krotchko, MD, a family practitioner at Denver Health and Hospital Authority, has studied the impact of a community outreach program – targeting HIV-negative men who have sex with men – that educates about HIV PrEP and offers HIV testing. He and his colleagues have examined the effectiveness of this approach, how comfortable participants are discussing their behaviors, and have tried to determine whether availability of HIV PrEP increases high-risk behaviors.
Dr. Krotchko and his colleagues also studied overall trends in awareness about PrEP over time, as well as the acceptability of participants to take a once-daily emtricitabine/tenofovir pill. He explained in a video interview why he believes it’s important to manage these patients in a primary care setting. The interview took place at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Krotchko had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT IDWEEK 2016
Why ustekinumab dosing differs in Crohn’s disease
ORLANDO – Preclinical studies and years of clinical experience using the monoclonal antibody ustekinumab (Stelara, Janssen Biotech) in psoriasis and psoriatic arthritis offer important clues to any gastroenterologist perplexed by the official Food and Drug Administration indication, dosing frequency, and intensity for Crohn’s disease. Phase II and phase III findings also reveal where the monoclonal antibody may offer particular advantages, compared with other agents.
“Ustekinumab landed in your lap in September. You’re probably all trying to figure out how to get the ID formulation paid for with insurance,” William J. Sanborn, MD, professor and chief of the division of gastroenterology at the University of California, San Diego, said at the Advances in Inflammatory Bowel Diseases meeting. “But this is now the reality that you have this in your Crohn’s practice.”
The FDA approved ustekinumab to treat adults with moderately to severely active Crohn’s disease who 1) failed or were intolerant to immune modulators or corticosteroids but did not fail tumor necrosis factor (TNF) blockers or 2) failed or were intolerant to one or more TNF blockers. Dr. Sanborn and colleagues observed a significant induction of clinical response in a subgroup of patients who previously failed a TNF blocker in an early efficacy study (Gastroenterology. 2008;135:1130-41). “This is where the idea of initially focusing on TNF failures came from,” he added at the meeting sponsored by the Crohn’s & Colitis Foundation of America.
Induction dosing in Crohn’s disease is intravenous versus subcutaneous in psoriasis and psoriatic arthritis, in part because of the same study. “It looked like relatively better bioavailability and relatively better effect with intravenous dosing,” Dr. Sanborn said. “In Crohn’s disease, it’s a completely different animal.”
Official induction dosing is approximately 6 mg/kg in three fixed doses according to patient weight in Crohn’s disease. The 6-mg/kg dose yielded the most consistent response, compared with 1-mg/kg or 3-mg/kg doses in a subsequent phase IIb study (N Engl J Med. 2012;367:1519-28).
The most consistent induction results at weeks 6 and 8 were observed with 6 mg/kg ustekinumab versus 1 mg/kg or 3 mg/kg.
Dr. Sanborn and coinvestigators also saw “numeric differences in drug versus placebo for remission at 6 and 8 weeks “but it was not that clear from the phase II trial what the remission efficacy was, so that needed more exploration to really understand.”
Another distinction for ustekinumab in Crohn’s disease is the approved maintenance dosing of 90 mg subcutaneously every 8 weeks versus a 12-week interval recommended for psoriasis. “Why so much more in Crohn’s disease, and is that necessary?” Dr. Sanborn asked.
Based on changes in C-reactive protein levels and a “rapid drop” in Crohn’s Disease Activity Index scores by 4 weeks, “clearly efficacy was there for induction,” he said. Ustekinumab has a “quick onset – analogous to the TNF blockers.”
“These were quite encouraging data, and paved the way to move on to phase III [studies],” Dr. Sanborn said. The preclinical studies up to this point focused on patients with Crohn’s disease who previously failed TNF blockers. However, “in clinical practice, we would be interested to know if it would work in anti-TNF naive or nonfailures as well.”
So two subsequent studies assessed safety and efficacy in a TNF blocker–failure population (UNITI-1 trial. Inflamm. Bowel Dis. 2016 Mar;22 Suppl 1:S1) and a non-TNF failure population of patients who did fail previous conventional therapy such as steroids or immunomodulators (UNITI-2 trial).
Clinical response and remission steadily rose following induction up to a significant difference versus placebo at 8 weeks in the non–TNF failure population. “Remember, in the phase IIa study, the remission rates were not as clear-cut, so this really nails down this as a good drug in both patient populations,” Dr. Sanborn said.
To evaluate long-term maintenance, investigators rerandomized all participants in the UNITI-1 and UNITI-2 studies. They saw a 15% gain in clinical remission out to week 44, compared with placebo. Dr. Sanborn noted that ustekinumab has a relatively long half-life, so the difference in patients switched to placebo may not have been as striking. “In practice it’s important to know the on-time and off-time of this agent, and I think the clinical trials make that clear.”
The trials also show that 12-week dosing works, Dr. Sanborn said. “You see about 20% gain for every 8-week dosing. You get extra 5% or 10% extra on all outcome measures at 8 weeks, compared to 12 weeks dosing, with no difference in safety signals.” He added, “So more intensive dosing of 90 mg every 8 weeks is what ended up getting approved in the United States.”
Safety profile
So what does all the preclinical evidence suggest about safety of ustekinumab? The UNITI trials combined included more than 1,000 patients, and there were no deaths, Dr. Sanborn said. “Usually with TNF blockers in 1,000 patients you would see a few deaths.”
Patient withdrawals from the preclinical studies were also relatively low, Dr. Sanborn reported. “With ustekinumab monotherapy, drug withdrawal is only 3% or 4%, so it seems to be different from TNF blockers in that sense [too].”
In addition, the rates of adverse events were similar between placebo (83.5%) and ustekinumab’s combined every 8 week and every 12 week dosing groups through 44 weeks (81.0%), Dr. Sanborn said. The rates of serious adverse events were likewise similar, 15.0% and 11.0%, respectively. Reported malignancy included two cases of basal cell skin cancers, one in the placebo group and one in the every-8-week dosing group, he added.
“So all those black box warnings you’re used to worrying about with TNF blockers – serious infections, about opportunistic infections, malignancy – there is no black box warning with this agent around that.”
Dr. Sanborn noted that the FDA labeling reports infections. “We know Crohn’s disease patients are [also] getting azathioprine, steroids, methotrexate, so you will see some infections, but there wasn’t a consistent opportunistic infection signal.”
One case of reversible posterior leukoencephalopathy syndrome is included on the labeling. Dr. Sanborn also put this in perspective: “With all the experience in psoriasis and psoriatic arthritis, and the clinical trials [in IBD], there is just one case. So the relationship is not very clear.”
“The safety signals with ustekinumab are really very good. It seems to be an extremely safe agent – we really don’t see much in terms of infections,” Brian Feagan, MD, an internist and gastroenterologist at the University of Western Ontario in London, said in a separate presentation at the conference. “We don’t have a lot of long-term experience with ustekinumab in Crohn’s disease, but we have a lot of experience in psoriasis, and it’s a safe drug.”
“Ustekinumab may be our first really valid monotherapy, with less immunogenicity,” Dr. Feagan said.
AGA Resource
AGA offers an IBD Clinical Service Line that provides tools to help you become more efficient, understand quality standards, and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/ibd
ORLANDO – Preclinical studies and years of clinical experience using the monoclonal antibody ustekinumab (Stelara, Janssen Biotech) in psoriasis and psoriatic arthritis offer important clues to any gastroenterologist perplexed by the official Food and Drug Administration indication, dosing frequency, and intensity for Crohn’s disease. Phase II and phase III findings also reveal where the monoclonal antibody may offer particular advantages, compared with other agents.
“Ustekinumab landed in your lap in September. You’re probably all trying to figure out how to get the ID formulation paid for with insurance,” William J. Sanborn, MD, professor and chief of the division of gastroenterology at the University of California, San Diego, said at the Advances in Inflammatory Bowel Diseases meeting. “But this is now the reality that you have this in your Crohn’s practice.”
The FDA approved ustekinumab to treat adults with moderately to severely active Crohn’s disease who 1) failed or were intolerant to immune modulators or corticosteroids but did not fail tumor necrosis factor (TNF) blockers or 2) failed or were intolerant to one or more TNF blockers. Dr. Sanborn and colleagues observed a significant induction of clinical response in a subgroup of patients who previously failed a TNF blocker in an early efficacy study (Gastroenterology. 2008;135:1130-41). “This is where the idea of initially focusing on TNF failures came from,” he added at the meeting sponsored by the Crohn’s & Colitis Foundation of America.
Induction dosing in Crohn’s disease is intravenous versus subcutaneous in psoriasis and psoriatic arthritis, in part because of the same study. “It looked like relatively better bioavailability and relatively better effect with intravenous dosing,” Dr. Sanborn said. “In Crohn’s disease, it’s a completely different animal.”
Official induction dosing is approximately 6 mg/kg in three fixed doses according to patient weight in Crohn’s disease. The 6-mg/kg dose yielded the most consistent response, compared with 1-mg/kg or 3-mg/kg doses in a subsequent phase IIb study (N Engl J Med. 2012;367:1519-28).
The most consistent induction results at weeks 6 and 8 were observed with 6 mg/kg ustekinumab versus 1 mg/kg or 3 mg/kg.
Dr. Sanborn and coinvestigators also saw “numeric differences in drug versus placebo for remission at 6 and 8 weeks “but it was not that clear from the phase II trial what the remission efficacy was, so that needed more exploration to really understand.”
Another distinction for ustekinumab in Crohn’s disease is the approved maintenance dosing of 90 mg subcutaneously every 8 weeks versus a 12-week interval recommended for psoriasis. “Why so much more in Crohn’s disease, and is that necessary?” Dr. Sanborn asked.
Based on changes in C-reactive protein levels and a “rapid drop” in Crohn’s Disease Activity Index scores by 4 weeks, “clearly efficacy was there for induction,” he said. Ustekinumab has a “quick onset – analogous to the TNF blockers.”
“These were quite encouraging data, and paved the way to move on to phase III [studies],” Dr. Sanborn said. The preclinical studies up to this point focused on patients with Crohn’s disease who previously failed TNF blockers. However, “in clinical practice, we would be interested to know if it would work in anti-TNF naive or nonfailures as well.”
So two subsequent studies assessed safety and efficacy in a TNF blocker–failure population (UNITI-1 trial. Inflamm. Bowel Dis. 2016 Mar;22 Suppl 1:S1) and a non-TNF failure population of patients who did fail previous conventional therapy such as steroids or immunomodulators (UNITI-2 trial).
Clinical response and remission steadily rose following induction up to a significant difference versus placebo at 8 weeks in the non–TNF failure population. “Remember, in the phase IIa study, the remission rates were not as clear-cut, so this really nails down this as a good drug in both patient populations,” Dr. Sanborn said.
To evaluate long-term maintenance, investigators rerandomized all participants in the UNITI-1 and UNITI-2 studies. They saw a 15% gain in clinical remission out to week 44, compared with placebo. Dr. Sanborn noted that ustekinumab has a relatively long half-life, so the difference in patients switched to placebo may not have been as striking. “In practice it’s important to know the on-time and off-time of this agent, and I think the clinical trials make that clear.”
The trials also show that 12-week dosing works, Dr. Sanborn said. “You see about 20% gain for every 8-week dosing. You get extra 5% or 10% extra on all outcome measures at 8 weeks, compared to 12 weeks dosing, with no difference in safety signals.” He added, “So more intensive dosing of 90 mg every 8 weeks is what ended up getting approved in the United States.”
Safety profile
So what does all the preclinical evidence suggest about safety of ustekinumab? The UNITI trials combined included more than 1,000 patients, and there were no deaths, Dr. Sanborn said. “Usually with TNF blockers in 1,000 patients you would see a few deaths.”
Patient withdrawals from the preclinical studies were also relatively low, Dr. Sanborn reported. “With ustekinumab monotherapy, drug withdrawal is only 3% or 4%, so it seems to be different from TNF blockers in that sense [too].”
In addition, the rates of adverse events were similar between placebo (83.5%) and ustekinumab’s combined every 8 week and every 12 week dosing groups through 44 weeks (81.0%), Dr. Sanborn said. The rates of serious adverse events were likewise similar, 15.0% and 11.0%, respectively. Reported malignancy included two cases of basal cell skin cancers, one in the placebo group and one in the every-8-week dosing group, he added.
“So all those black box warnings you’re used to worrying about with TNF blockers – serious infections, about opportunistic infections, malignancy – there is no black box warning with this agent around that.”
Dr. Sanborn noted that the FDA labeling reports infections. “We know Crohn’s disease patients are [also] getting azathioprine, steroids, methotrexate, so you will see some infections, but there wasn’t a consistent opportunistic infection signal.”
One case of reversible posterior leukoencephalopathy syndrome is included on the labeling. Dr. Sanborn also put this in perspective: “With all the experience in psoriasis and psoriatic arthritis, and the clinical trials [in IBD], there is just one case. So the relationship is not very clear.”
“The safety signals with ustekinumab are really very good. It seems to be an extremely safe agent – we really don’t see much in terms of infections,” Brian Feagan, MD, an internist and gastroenterologist at the University of Western Ontario in London, said in a separate presentation at the conference. “We don’t have a lot of long-term experience with ustekinumab in Crohn’s disease, but we have a lot of experience in psoriasis, and it’s a safe drug.”
“Ustekinumab may be our first really valid monotherapy, with less immunogenicity,” Dr. Feagan said.
AGA Resource
AGA offers an IBD Clinical Service Line that provides tools to help you become more efficient, understand quality standards, and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/ibd
ORLANDO – Preclinical studies and years of clinical experience using the monoclonal antibody ustekinumab (Stelara, Janssen Biotech) in psoriasis and psoriatic arthritis offer important clues to any gastroenterologist perplexed by the official Food and Drug Administration indication, dosing frequency, and intensity for Crohn’s disease. Phase II and phase III findings also reveal where the monoclonal antibody may offer particular advantages, compared with other agents.
“Ustekinumab landed in your lap in September. You’re probably all trying to figure out how to get the ID formulation paid for with insurance,” William J. Sanborn, MD, professor and chief of the division of gastroenterology at the University of California, San Diego, said at the Advances in Inflammatory Bowel Diseases meeting. “But this is now the reality that you have this in your Crohn’s practice.”
The FDA approved ustekinumab to treat adults with moderately to severely active Crohn’s disease who 1) failed or were intolerant to immune modulators or corticosteroids but did not fail tumor necrosis factor (TNF) blockers or 2) failed or were intolerant to one or more TNF blockers. Dr. Sanborn and colleagues observed a significant induction of clinical response in a subgroup of patients who previously failed a TNF blocker in an early efficacy study (Gastroenterology. 2008;135:1130-41). “This is where the idea of initially focusing on TNF failures came from,” he added at the meeting sponsored by the Crohn’s & Colitis Foundation of America.
Induction dosing in Crohn’s disease is intravenous versus subcutaneous in psoriasis and psoriatic arthritis, in part because of the same study. “It looked like relatively better bioavailability and relatively better effect with intravenous dosing,” Dr. Sanborn said. “In Crohn’s disease, it’s a completely different animal.”
Official induction dosing is approximately 6 mg/kg in three fixed doses according to patient weight in Crohn’s disease. The 6-mg/kg dose yielded the most consistent response, compared with 1-mg/kg or 3-mg/kg doses in a subsequent phase IIb study (N Engl J Med. 2012;367:1519-28).
The most consistent induction results at weeks 6 and 8 were observed with 6 mg/kg ustekinumab versus 1 mg/kg or 3 mg/kg.
Dr. Sanborn and coinvestigators also saw “numeric differences in drug versus placebo for remission at 6 and 8 weeks “but it was not that clear from the phase II trial what the remission efficacy was, so that needed more exploration to really understand.”
Another distinction for ustekinumab in Crohn’s disease is the approved maintenance dosing of 90 mg subcutaneously every 8 weeks versus a 12-week interval recommended for psoriasis. “Why so much more in Crohn’s disease, and is that necessary?” Dr. Sanborn asked.
Based on changes in C-reactive protein levels and a “rapid drop” in Crohn’s Disease Activity Index scores by 4 weeks, “clearly efficacy was there for induction,” he said. Ustekinumab has a “quick onset – analogous to the TNF blockers.”
“These were quite encouraging data, and paved the way to move on to phase III [studies],” Dr. Sanborn said. The preclinical studies up to this point focused on patients with Crohn’s disease who previously failed TNF blockers. However, “in clinical practice, we would be interested to know if it would work in anti-TNF naive or nonfailures as well.”
So two subsequent studies assessed safety and efficacy in a TNF blocker–failure population (UNITI-1 trial. Inflamm. Bowel Dis. 2016 Mar;22 Suppl 1:S1) and a non-TNF failure population of patients who did fail previous conventional therapy such as steroids or immunomodulators (UNITI-2 trial).
Clinical response and remission steadily rose following induction up to a significant difference versus placebo at 8 weeks in the non–TNF failure population. “Remember, in the phase IIa study, the remission rates were not as clear-cut, so this really nails down this as a good drug in both patient populations,” Dr. Sanborn said.
To evaluate long-term maintenance, investigators rerandomized all participants in the UNITI-1 and UNITI-2 studies. They saw a 15% gain in clinical remission out to week 44, compared with placebo. Dr. Sanborn noted that ustekinumab has a relatively long half-life, so the difference in patients switched to placebo may not have been as striking. “In practice it’s important to know the on-time and off-time of this agent, and I think the clinical trials make that clear.”
The trials also show that 12-week dosing works, Dr. Sanborn said. “You see about 20% gain for every 8-week dosing. You get extra 5% or 10% extra on all outcome measures at 8 weeks, compared to 12 weeks dosing, with no difference in safety signals.” He added, “So more intensive dosing of 90 mg every 8 weeks is what ended up getting approved in the United States.”
Safety profile
So what does all the preclinical evidence suggest about safety of ustekinumab? The UNITI trials combined included more than 1,000 patients, and there were no deaths, Dr. Sanborn said. “Usually with TNF blockers in 1,000 patients you would see a few deaths.”
Patient withdrawals from the preclinical studies were also relatively low, Dr. Sanborn reported. “With ustekinumab monotherapy, drug withdrawal is only 3% or 4%, so it seems to be different from TNF blockers in that sense [too].”
In addition, the rates of adverse events were similar between placebo (83.5%) and ustekinumab’s combined every 8 week and every 12 week dosing groups through 44 weeks (81.0%), Dr. Sanborn said. The rates of serious adverse events were likewise similar, 15.0% and 11.0%, respectively. Reported malignancy included two cases of basal cell skin cancers, one in the placebo group and one in the every-8-week dosing group, he added.
“So all those black box warnings you’re used to worrying about with TNF blockers – serious infections, about opportunistic infections, malignancy – there is no black box warning with this agent around that.”
Dr. Sanborn noted that the FDA labeling reports infections. “We know Crohn’s disease patients are [also] getting azathioprine, steroids, methotrexate, so you will see some infections, but there wasn’t a consistent opportunistic infection signal.”
One case of reversible posterior leukoencephalopathy syndrome is included on the labeling. Dr. Sanborn also put this in perspective: “With all the experience in psoriasis and psoriatic arthritis, and the clinical trials [in IBD], there is just one case. So the relationship is not very clear.”
“The safety signals with ustekinumab are really very good. It seems to be an extremely safe agent – we really don’t see much in terms of infections,” Brian Feagan, MD, an internist and gastroenterologist at the University of Western Ontario in London, said in a separate presentation at the conference. “We don’t have a lot of long-term experience with ustekinumab in Crohn’s disease, but we have a lot of experience in psoriasis, and it’s a safe drug.”
“Ustekinumab may be our first really valid monotherapy, with less immunogenicity,” Dr. Feagan said.
AGA Resource
AGA offers an IBD Clinical Service Line that provides tools to help you become more efficient, understand quality standards, and improve the process of care for patients. Learn more at http://www.gastro.org/patient-care/conditions-diseases/ibd
Meta-analysis compares infliximab with tacrolimus in ulcerative colitis
ORLANDO – Infliximab and tacrolimus each demonstrate efficacy for treatment of moderate to severe active ulcerative colitis in published studies. However, it remains unknown if one agent offers greater efficacy than the other in this patient population, so researchers in Japan conducted a meta-analysis to find out more.
Lead investigator Shinichi Kawano, MD, at Kyushu University, Fukuoka, Japan, and his colleagues searched PubMed for relevant studies up until August 2016. They conducted a systematic review and identified 79 potential studies of tumor necrosis factor blocker infliximab (Remicade, Janssen) and the calcineurin inhibitor tacrolimus (various brands) in this patient population. They ruled out the vast majority, 75 studies, for not directly comparing therapeutic efficacy. They also excluded one additional study for insufficient data on their five outcomes of interest: rates of clinical remission, clinical response, freedom from colectomy, adverse events, and serious adverse events. They focused on three 2016 retrospective studies with a total of 244 patients, and added 13 of their own patients, 5 taking infliximab and 8 taking tacrolimus, to their dataset.
Similarly, the short-term clinical response rate favored infliximab (overall RR, 1.55), but again the difference was not statistically significant.
The investigators found the rate of colectomy was comparable between patients taking infliximab and tacrolimus (overall RR, 1.01). They reported their findings in a poster presentation at the Advances in Inflammatory Bowel Diseases meeting, sponsored by the Crohn’s & Colitis Foundation of America.
The adverse event rate favored tacrolimus over infliximab, but it was not significant (overall RR, 0.23). The serious adverse event rate slightly favored tacrolimus as well (overall RR, 0.88). The incidences of adverse events and serious adverse events were comparable between the two groups, the study authors wrote.
The trials included in the meta-analysis looked at either adults only or adults and pediatric patients with active ulcerative colitis. In one study, there were 40 patients taking infliximab and 50 taking tacrolimus (Aliment Pharmacol Ther. 2016;43:705-16); in another 48 patients took infliximab and 47 took tacrolimus (Gastroenterol Res Pract. 2016;2016:3162595); and in the third study 30 were treated with infliximab and 29 with tacrolimus (Scand J Gastroenterol. 2016;51:700-5).
“This meta-analysis demonstrates equivalent therapeutic efficacy and safety between infliximab and tacrolimus,” the authors continued. Dr. Kawano said he was not surprised by the findings. “Because there are only three retrospective studies, it is reasonable that there is no significant difference in efficacy between infliximab and tacrolimus.”
However, he added, “We think that further prospective, comparative trials are needed.”
Dr. Kawano had no relevant financial disclosures.
ORLANDO – Infliximab and tacrolimus each demonstrate efficacy for treatment of moderate to severe active ulcerative colitis in published studies. However, it remains unknown if one agent offers greater efficacy than the other in this patient population, so researchers in Japan conducted a meta-analysis to find out more.
Lead investigator Shinichi Kawano, MD, at Kyushu University, Fukuoka, Japan, and his colleagues searched PubMed for relevant studies up until August 2016. They conducted a systematic review and identified 79 potential studies of tumor necrosis factor blocker infliximab (Remicade, Janssen) and the calcineurin inhibitor tacrolimus (various brands) in this patient population. They ruled out the vast majority, 75 studies, for not directly comparing therapeutic efficacy. They also excluded one additional study for insufficient data on their five outcomes of interest: rates of clinical remission, clinical response, freedom from colectomy, adverse events, and serious adverse events. They focused on three 2016 retrospective studies with a total of 244 patients, and added 13 of their own patients, 5 taking infliximab and 8 taking tacrolimus, to their dataset.
Similarly, the short-term clinical response rate favored infliximab (overall RR, 1.55), but again the difference was not statistically significant.
The investigators found the rate of colectomy was comparable between patients taking infliximab and tacrolimus (overall RR, 1.01). They reported their findings in a poster presentation at the Advances in Inflammatory Bowel Diseases meeting, sponsored by the Crohn’s & Colitis Foundation of America.
The adverse event rate favored tacrolimus over infliximab, but it was not significant (overall RR, 0.23). The serious adverse event rate slightly favored tacrolimus as well (overall RR, 0.88). The incidences of adverse events and serious adverse events were comparable between the two groups, the study authors wrote.
The trials included in the meta-analysis looked at either adults only or adults and pediatric patients with active ulcerative colitis. In one study, there were 40 patients taking infliximab and 50 taking tacrolimus (Aliment Pharmacol Ther. 2016;43:705-16); in another 48 patients took infliximab and 47 took tacrolimus (Gastroenterol Res Pract. 2016;2016:3162595); and in the third study 30 were treated with infliximab and 29 with tacrolimus (Scand J Gastroenterol. 2016;51:700-5).
“This meta-analysis demonstrates equivalent therapeutic efficacy and safety between infliximab and tacrolimus,” the authors continued. Dr. Kawano said he was not surprised by the findings. “Because there are only three retrospective studies, it is reasonable that there is no significant difference in efficacy between infliximab and tacrolimus.”
However, he added, “We think that further prospective, comparative trials are needed.”
Dr. Kawano had no relevant financial disclosures.
ORLANDO – Infliximab and tacrolimus each demonstrate efficacy for treatment of moderate to severe active ulcerative colitis in published studies. However, it remains unknown if one agent offers greater efficacy than the other in this patient population, so researchers in Japan conducted a meta-analysis to find out more.
Lead investigator Shinichi Kawano, MD, at Kyushu University, Fukuoka, Japan, and his colleagues searched PubMed for relevant studies up until August 2016. They conducted a systematic review and identified 79 potential studies of tumor necrosis factor blocker infliximab (Remicade, Janssen) and the calcineurin inhibitor tacrolimus (various brands) in this patient population. They ruled out the vast majority, 75 studies, for not directly comparing therapeutic efficacy. They also excluded one additional study for insufficient data on their five outcomes of interest: rates of clinical remission, clinical response, freedom from colectomy, adverse events, and serious adverse events. They focused on three 2016 retrospective studies with a total of 244 patients, and added 13 of their own patients, 5 taking infliximab and 8 taking tacrolimus, to their dataset.
Similarly, the short-term clinical response rate favored infliximab (overall RR, 1.55), but again the difference was not statistically significant.
The investigators found the rate of colectomy was comparable between patients taking infliximab and tacrolimus (overall RR, 1.01). They reported their findings in a poster presentation at the Advances in Inflammatory Bowel Diseases meeting, sponsored by the Crohn’s & Colitis Foundation of America.
The adverse event rate favored tacrolimus over infliximab, but it was not significant (overall RR, 0.23). The serious adverse event rate slightly favored tacrolimus as well (overall RR, 0.88). The incidences of adverse events and serious adverse events were comparable between the two groups, the study authors wrote.
The trials included in the meta-analysis looked at either adults only or adults and pediatric patients with active ulcerative colitis. In one study, there were 40 patients taking infliximab and 50 taking tacrolimus (Aliment Pharmacol Ther. 2016;43:705-16); in another 48 patients took infliximab and 47 took tacrolimus (Gastroenterol Res Pract. 2016;2016:3162595); and in the third study 30 were treated with infliximab and 29 with tacrolimus (Scand J Gastroenterol. 2016;51:700-5).
“This meta-analysis demonstrates equivalent therapeutic efficacy and safety between infliximab and tacrolimus,” the authors continued. Dr. Kawano said he was not surprised by the findings. “Because there are only three retrospective studies, it is reasonable that there is no significant difference in efficacy between infliximab and tacrolimus.”
However, he added, “We think that further prospective, comparative trials are needed.”
Dr. Kawano had no relevant financial disclosures.
AT AIBD 2016
Key clinical point: Infliximab and tacrolimus both demonstrate efficacy for active ulcerative colitis in published studies, but few direct comparisons exist.
Major finding: The reported rate of clinical remission with infliximab was higher than with tacrolimus (risk ratio, 1.17), but the difference was not statistically significant.
Data source: Meta-analysis of relevant articles identified in a PubMed search through August 2016.
Disclosures: Dr. Kawano had no relevant financial disclosures.