User login
Dermatofibrosarcoma Protuberans
To the Editor:
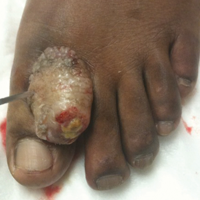
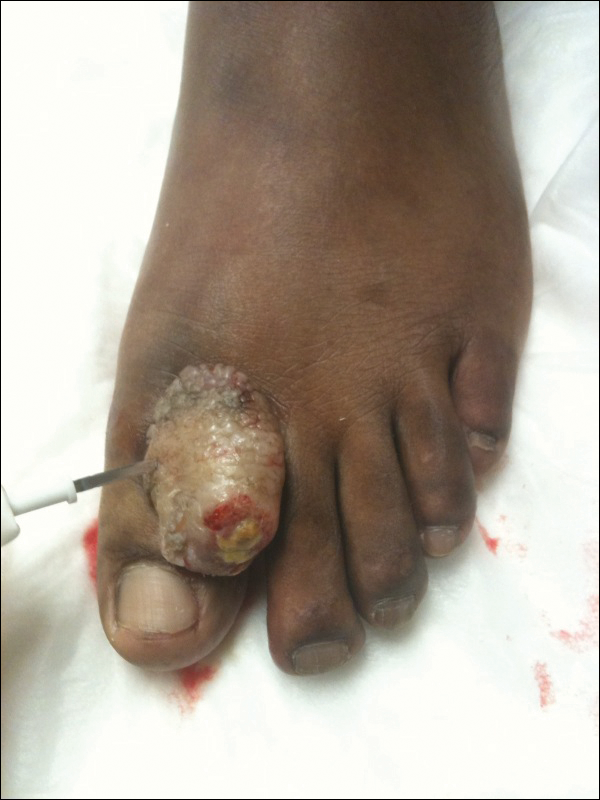
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.
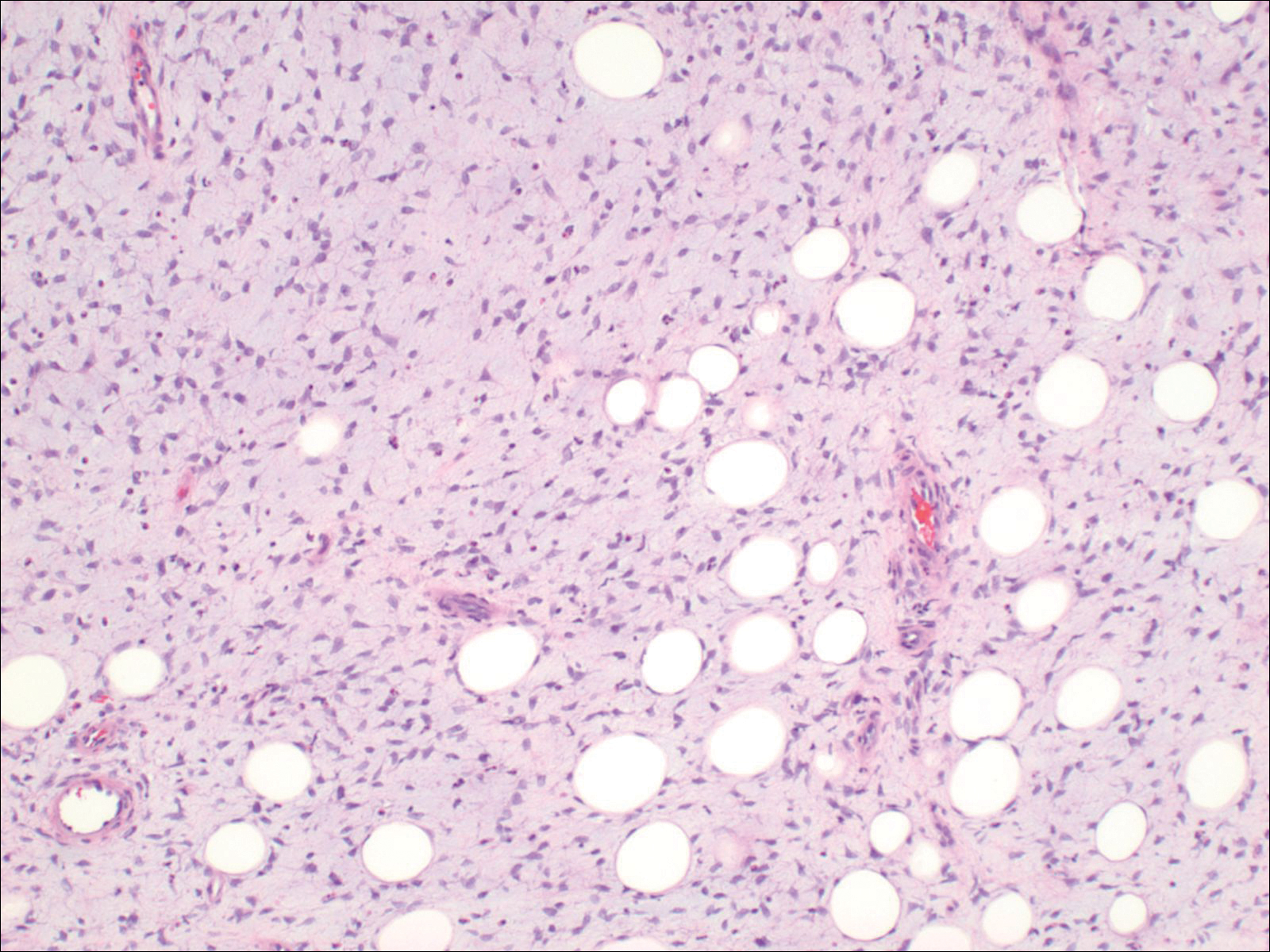
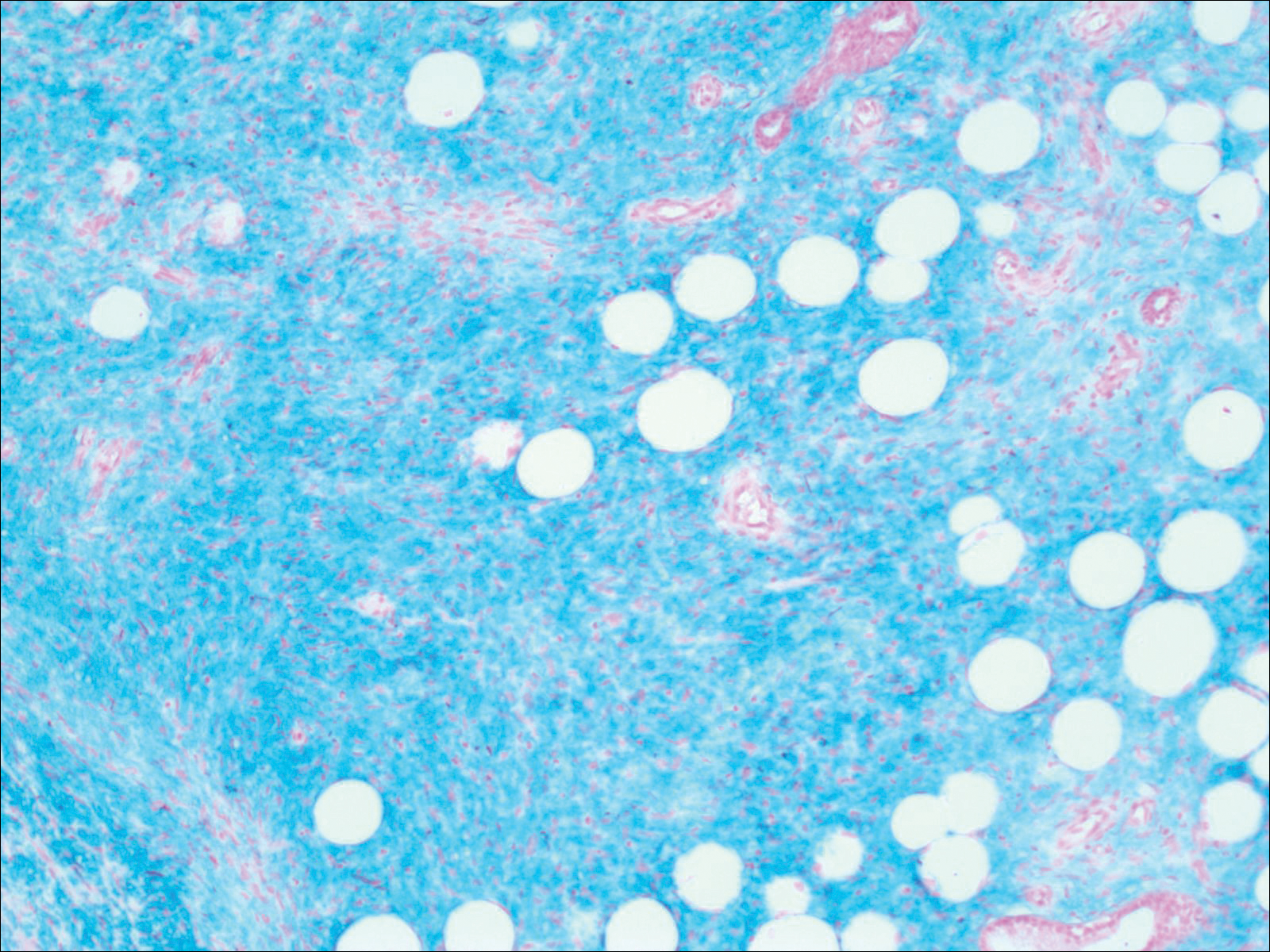
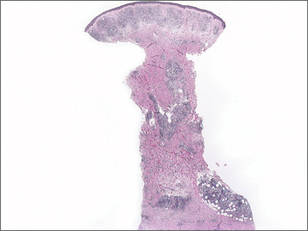
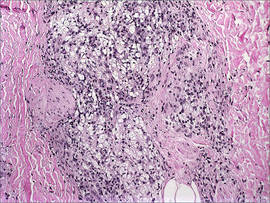
Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.
Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
To the Editor:
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.

Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.


Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
To the Editor:
A 41-year-old man presented with a slowly enlarging, tender, firm lesion on the left hallux of approximately 5 months' duration that initially appeared to be a blister. He reported no history of keloids or trauma to the left foot. On examination, a 3.5-cm, flesh-colored, pedunculated, firm nodule was present on the lateral aspect of the left great hallux (Figure 1). No lymphadenopathy was found. The lesion was diagnosed at that time as a keloid and treated with intralesional steroids without response. The patient was lost to follow-up, and after 5 months he presented again with pain and drainage from the lesion. Acute drainage resolved after antibiotic therapy. A shave biopsy was performed, which revealed findings consistent with a dermatofibrosarcoma protuberans (DFSP). A chest radiograph was unremarkable. Re-excision was performed with negative margins on frozen section but with positive peripheral and deep margins on permanent sections. The patient subsequently underwent amputation of the left great toe and was lost to follow-up after the initial postoperative period.

Histopathologic examination demonstrated a polypoid spindle cell tumor that filled the dermis and invaded into the subcutaneous adipose tissue (Figure 2). The spindle cells had tapered nuclei in a honeycomb arrangement with only mild nuclear pleomorphism arranged in fascicles with a herringbone formation. Areas showed a myxoid stroma with abundant mucin (Figure 3). Immunostaining demonstrated cells strongly positive for CD34 and negative for MART (melanoma-associated antigen recognized by T cells), S-100, and smooth muscle actin immunostains.


Dermatofibrosarcoma protuberans is a sarcoma that is locally aggressive and tends to recur after surgical excision, though rare cases of metastasis involving the lungs have been reported.12 Dermatofibrosarcoma protuberans usually affects young to middle-aged adults. Acral DFSP is rare in adults, with tumors most commonly occurring on the trunk (50%-60%), proximal extremities (20%-30%), or the head and neck (10%-15%).1,2 A higher rate of acral DFSP has been found in children, which may be due to the increased rate of extremity trauma. Dermatofibrosarcoma protuberans commonly presents as an asymptomatic, slowly growing, indurated plaque that may be flesh colored or hyperpigmented, followed by development of erythematous firm nodules of up to several centimeters.1,3 Dermatofibrosarcoma protuberans may be associated with a purulent exudate or ulceration, and pain may develop as the lesion grows.
Histopathologic evaluation shows an early plaque stage characterized by low cellularity, minimal nuclear atypia, and rare mitotic figures.4 In the nodular stage, the spindle cells are arranged as short fascicles in a storiform arrangement and infiltrate the subcutaneous tissue in a honeycomb pattern with hyperchromatic nuclei and mitotic figures. The nodules may develop myxomatous areas as well as less-differentiated foci with intersecting fascicles in a herringbone pattern. Anti-CD34 antibody immunostaining demonstrates strongly positive spindle cells, while DFSP is negative for stromelysin 3, factor XIIIa, and D2-40, which can help to differentiate DFSP from dermatofibroma.5 The myxoid subtype of DFSP does not differ clinically or prognostically from conventional DFSP, though its recognition can be of use in differentiating other myxoid tumors. Myxoid DFSP is nearly always positive for CD34 and negative for the neural marker S-100 protein.6
Some reports have demonstrated that Mohs micrographic surgery is superior to wide local excision in treatment of DFSP, as it results in fewer local recurrences and metastases.7,8 Because of cytogenic abnormalities such as a reciprocal chromosomal (17;22) translocation or supernumerary ring chromosome derived from t(17;22) that place the PDGFB gene under the control of COL1A1 promoter, imatinib mesylate has been tested in DFSP and resulted in dramatic responses in both adults and children.9,10 Suggested uses of imatinib include metastatic disease and locally invasive disease not suitable for surgical excision as well as a method to debulk tumors prior to resection.11
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
- Gloster HM Jr. Dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(3, pt 1):355-374; quiz 375-376.
- Do AN, Goleno K, Geisse JK. Mohs micrographic surgery and partial amputation preserving function and aesthetics in digits: case reports of invasive melanoma and digital dermatofibrosarcoma protuberans. Dermatol Surg. 2006;32:1516-1521.
- Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans: a study of 115 cases. Cancer. 1962;15:717-725.
- Kamino H, Reddy VB, Pui J. Dermatofibrosarcoma protuberans. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 3rd ed. London, England: Elsevier; 2012:1961-1977.
- Cohen PR, Rapini RP, Farhood AI. Dermatofibroma and dermatofibrosarcoma protuberans: differential expression of CD34 and factor XIIIa. Am J Dermatopathol. 1994;16:573-574.
- Llombart B, Serra-Guillén C, Monteagudo C, et al. Dermatofibrosarcoma protuberans: a comprehensive review and update of diagnosis and management. Semin Diagn Pathol. 2013;30:13-28.
- Paradisi A, Abeni D, Rusciani A, et al. Dermatofibrosarcoma protuberans: wide local excision vs. Mohs micrographic surgery. Cancer Treat Rev. 2008;34:728-736.
- Foroozan M, Sei JF, Amini M, et al. Efficacy of Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans: systematic review. Arch Dermatol. 2012;148:1055-1063.
- Patel KU, Szaebo SS, Hernandez VS, et al. Dermatofibrosarcoma protuberans COL1A1-PDGFB fusion is identified in virtually all dermatofibrosarcoma protuberans cases when investigated by newly developed multiplex reverse transcription polymerase chain reaction and fluorescence in situ hybridization assays. Hum Pathol. 2008;39:184-193.
- McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866-873.
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al; European Organisation for Research and Treatment of Cancer Soft Tissue/Bone Sarcoma Group, Southwest Oncology Group. Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials [published online March 1, 2010]. J Clin Oncol. 2010;28:1772-1779.
- Mentzel T, Beham A, Katenkamp D, et al. Fibrosarcomatous ("high-grade") dermatofibrosarcoma protuberans: clinicopathologic and immunohistochemical study of a series of 41 cases with emphasis on prognostic significance. Am J Surg Pathol. 1998;22:576-587.
Practice Points
- Consider dermatofibrosarcoma protuberans for a keloidlike enlarging lesion when there is no history of trauma or prior keloid formation.
- Treatments such as Mohs micrographic surgery or oral imatinib mesylate can provide lower recurrence rates in appropriate patients as stand-alone or adjuvant therapy.
Lepromatous Leprosy Associated With Erythema Nodosum Leprosum
To the Editor:
A 41-year-old man presented to our clinic with concerns of ulceration, skin thickening, and loss of eyebrows. The ulceration developed on the knees approximately 1 year prior to presentation; however, he reported loss of sensation in the knees after an accident involving his back 7 years prior. Loss of the eyebrows and skin thickening on the abdomen occurred within the same time frame of the knee ulceration. He also experienced generalized loss of skin sensation. The patient had a history of hunting and fishing but denied any contact with an armadillo.
On clinical examination thin yellow plaques were present on both cheeks, as well as typical leonine facies, a saddle nose deformity, loss of bilateral eyebrows, slate gray color on the face, and infiltrated plaques with telangiectases on the nose. Subcutaneous nodules developed on the arms, chest, and abdomen. He also had angulated ulcers with granulation tissue on the knees.
Laboratory data collected included a complete metabolic panel and complete blood cell count with differential. The complete metabolic panel was within reference range excluding a moderately high glucose level of 114 mg/dL (reference range, 70–100 mg/dL) and a low creatinine level of 0.7 mg/dL (reference range, 0.5–1.2 mg/dL). The complete blood cell count showed mild anemia with a hemoglobin to hematocrit ratio of 11.0 g/dL (reference range, 12.0–16.0 g/dL) to 32.6% (reference range, 37.0%–48.5%) and a nonspecific elevated mean corpuscular volume of 94.4 fL (reference range, 82–95 fL). The differential revealed a low percentage of lymphocytes (11.4% [reference range, 21%–44%]) and a high percentage of granulocytes (85.6% [reference range, 38%–73%]).
A total of 3 biopsies were taken from the abdomen, upper chest, and right wrist. Histology showed a diffuse infiltrate of epithelioid histiocytes forming ill-defined nodules in the dermis, extending into the represented subcutis (Figure 1). Many of the histiocytes had prominent foamy cytoplasms (Figure 2). Scattered lymphocytes and plasma cells also were present. Fite-modified acid-fast staining was performed on all of the biopsies and yielded large numbers of acid-fast bacilli dispersed throughout the specimens (Figure 3), which confirmed the diagnosis of lepromatous leprosy. The patient was referred to the National Hansen’s Disease (Leprosy) Program in Baton Rouge, Louisiana. The physician team started him on high-dose clofazimine, dapsone, and rifampin. He also was diagnosed with erythema nodosum leprosum for which he was given thalidomide.
Hansen disease, also known as leprosy, is a chronic inflammatory disease caused by Mycobacterium leprae and Mycobacterium lepromatosis. The mode of transmission is postulated to be through respiratory droplets and nasal secretions. Armadillo exposure, poor sanitary conditions, endemic location, and infected family members are considered risk factors for the development of leprosy. The incubation period is approximately 5 years, with symptoms arising up to 20 years after acquisition of the bacillus. Replication tends to begin in cooler regions of the body. The disease spectrum ranges from lepromatous to tuberculoid.1-3 Lepromatous leprosy consists of an uncontrolled high-titer replication of the mycobacteria, resulting mostly in cutaneous changes and late nerve damage. Histologically, foamy or undifferentiated macrophages predominate the field; they often include many bacilli that can be demonstrated by the modified Fite-Faraco method, a clinically relevant carbol-fuchsin stain.3-5 Acid-fast organisms stain red. A Ziehl-Neelsen stain and Harada modified Allochrome method also may be used.
The damage to peripheral nerves and the cutis can cause substantial loss of function and lead to painless ulceration of the skin. Changes in the skin may include nodules, hypopigmented macules, sores, and skin thickening. Leonine facies and disfigurement also may be observed. A type III hypersensitivity reaction may occur, resulting in erythema nodosum leprosum, a type II lepra reaction characterized by elevated tumor necrosis factor α.6
Treatment requires multidrug therapy involving dapsone, rifampin, and clofazimine.4 The World Health Organization is targeted at eliminating the disease and providing free multidrug therapy to patients.7

- Leprosy (Hansen’s disease): Technical Information. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/hansens_disease/technical.html/. Updated May 17, 2010. Accessed July 2, 2014.
- Esfandbod M. Images in clinical medicine. tuberculoid leprosy. N Engl J Med. 2011;364:1657.
- Robati RM, Rahimi H, Asadi-Kani Z, et al. Photoclinic. lepromatous leprosy. Arch Iran Med. 2010;13:443-444.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
- Wolff K, Johnson RA, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Walker SL, Lockwood DN. Leprosy. Clin Dermatol. 2007;25:165-172.
- World Health Organization Committee on Leprosy. WHO Expert Committee on Leprosy: Seventh Report. Geneva, Switzerland: World Health Organization Committee on Leprosy; 1997.
To the Editor:
A 41-year-old man presented to our clinic with concerns of ulceration, skin thickening, and loss of eyebrows. The ulceration developed on the knees approximately 1 year prior to presentation; however, he reported loss of sensation in the knees after an accident involving his back 7 years prior. Loss of the eyebrows and skin thickening on the abdomen occurred within the same time frame of the knee ulceration. He also experienced generalized loss of skin sensation. The patient had a history of hunting and fishing but denied any contact with an armadillo.
On clinical examination thin yellow plaques were present on both cheeks, as well as typical leonine facies, a saddle nose deformity, loss of bilateral eyebrows, slate gray color on the face, and infiltrated plaques with telangiectases on the nose. Subcutaneous nodules developed on the arms, chest, and abdomen. He also had angulated ulcers with granulation tissue on the knees.
Laboratory data collected included a complete metabolic panel and complete blood cell count with differential. The complete metabolic panel was within reference range excluding a moderately high glucose level of 114 mg/dL (reference range, 70–100 mg/dL) and a low creatinine level of 0.7 mg/dL (reference range, 0.5–1.2 mg/dL). The complete blood cell count showed mild anemia with a hemoglobin to hematocrit ratio of 11.0 g/dL (reference range, 12.0–16.0 g/dL) to 32.6% (reference range, 37.0%–48.5%) and a nonspecific elevated mean corpuscular volume of 94.4 fL (reference range, 82–95 fL). The differential revealed a low percentage of lymphocytes (11.4% [reference range, 21%–44%]) and a high percentage of granulocytes (85.6% [reference range, 38%–73%]).
A total of 3 biopsies were taken from the abdomen, upper chest, and right wrist. Histology showed a diffuse infiltrate of epithelioid histiocytes forming ill-defined nodules in the dermis, extending into the represented subcutis (Figure 1). Many of the histiocytes had prominent foamy cytoplasms (Figure 2). Scattered lymphocytes and plasma cells also were present. Fite-modified acid-fast staining was performed on all of the biopsies and yielded large numbers of acid-fast bacilli dispersed throughout the specimens (Figure 3), which confirmed the diagnosis of lepromatous leprosy. The patient was referred to the National Hansen’s Disease (Leprosy) Program in Baton Rouge, Louisiana. The physician team started him on high-dose clofazimine, dapsone, and rifampin. He also was diagnosed with erythema nodosum leprosum for which he was given thalidomide.

Hansen disease, also known as leprosy, is a chronic inflammatory disease caused by Mycobacterium leprae and Mycobacterium lepromatosis. The mode of transmission is postulated to be through respiratory droplets and nasal secretions. Armadillo exposure, poor sanitary conditions, endemic location, and infected family members are considered risk factors for the development of leprosy. The incubation period is approximately 5 years, with symptoms arising up to 20 years after acquisition of the bacillus. Replication tends to begin in cooler regions of the body. The disease spectrum ranges from lepromatous to tuberculoid.1-3 Lepromatous leprosy consists of an uncontrolled high-titer replication of the mycobacteria, resulting mostly in cutaneous changes and late nerve damage. Histologically, foamy or undifferentiated macrophages predominate the field; they often include many bacilli that can be demonstrated by the modified Fite-Faraco method, a clinically relevant carbol-fuchsin stain.3-5 Acid-fast organisms stain red. A Ziehl-Neelsen stain and Harada modified Allochrome method also may be used.

The damage to peripheral nerves and the cutis can cause substantial loss of function and lead to painless ulceration of the skin. Changes in the skin may include nodules, hypopigmented macules, sores, and skin thickening. Leonine facies and disfigurement also may be observed. A type III hypersensitivity reaction may occur, resulting in erythema nodosum leprosum, a type II lepra reaction characterized by elevated tumor necrosis factor α.6
Treatment requires multidrug therapy involving dapsone, rifampin, and clofazimine.4 The World Health Organization is targeted at eliminating the disease and providing free multidrug therapy to patients.7

To the Editor:
A 41-year-old man presented to our clinic with concerns of ulceration, skin thickening, and loss of eyebrows. The ulceration developed on the knees approximately 1 year prior to presentation; however, he reported loss of sensation in the knees after an accident involving his back 7 years prior. Loss of the eyebrows and skin thickening on the abdomen occurred within the same time frame of the knee ulceration. He also experienced generalized loss of skin sensation. The patient had a history of hunting and fishing but denied any contact with an armadillo.
On clinical examination thin yellow plaques were present on both cheeks, as well as typical leonine facies, a saddle nose deformity, loss of bilateral eyebrows, slate gray color on the face, and infiltrated plaques with telangiectases on the nose. Subcutaneous nodules developed on the arms, chest, and abdomen. He also had angulated ulcers with granulation tissue on the knees.
Laboratory data collected included a complete metabolic panel and complete blood cell count with differential. The complete metabolic panel was within reference range excluding a moderately high glucose level of 114 mg/dL (reference range, 70–100 mg/dL) and a low creatinine level of 0.7 mg/dL (reference range, 0.5–1.2 mg/dL). The complete blood cell count showed mild anemia with a hemoglobin to hematocrit ratio of 11.0 g/dL (reference range, 12.0–16.0 g/dL) to 32.6% (reference range, 37.0%–48.5%) and a nonspecific elevated mean corpuscular volume of 94.4 fL (reference range, 82–95 fL). The differential revealed a low percentage of lymphocytes (11.4% [reference range, 21%–44%]) and a high percentage of granulocytes (85.6% [reference range, 38%–73%]).
A total of 3 biopsies were taken from the abdomen, upper chest, and right wrist. Histology showed a diffuse infiltrate of epithelioid histiocytes forming ill-defined nodules in the dermis, extending into the represented subcutis (Figure 1). Many of the histiocytes had prominent foamy cytoplasms (Figure 2). Scattered lymphocytes and plasma cells also were present. Fite-modified acid-fast staining was performed on all of the biopsies and yielded large numbers of acid-fast bacilli dispersed throughout the specimens (Figure 3), which confirmed the diagnosis of lepromatous leprosy. The patient was referred to the National Hansen’s Disease (Leprosy) Program in Baton Rouge, Louisiana. The physician team started him on high-dose clofazimine, dapsone, and rifampin. He also was diagnosed with erythema nodosum leprosum for which he was given thalidomide.

Hansen disease, also known as leprosy, is a chronic inflammatory disease caused by Mycobacterium leprae and Mycobacterium lepromatosis. The mode of transmission is postulated to be through respiratory droplets and nasal secretions. Armadillo exposure, poor sanitary conditions, endemic location, and infected family members are considered risk factors for the development of leprosy. The incubation period is approximately 5 years, with symptoms arising up to 20 years after acquisition of the bacillus. Replication tends to begin in cooler regions of the body. The disease spectrum ranges from lepromatous to tuberculoid.1-3 Lepromatous leprosy consists of an uncontrolled high-titer replication of the mycobacteria, resulting mostly in cutaneous changes and late nerve damage. Histologically, foamy or undifferentiated macrophages predominate the field; they often include many bacilli that can be demonstrated by the modified Fite-Faraco method, a clinically relevant carbol-fuchsin stain.3-5 Acid-fast organisms stain red. A Ziehl-Neelsen stain and Harada modified Allochrome method also may be used.

The damage to peripheral nerves and the cutis can cause substantial loss of function and lead to painless ulceration of the skin. Changes in the skin may include nodules, hypopigmented macules, sores, and skin thickening. Leonine facies and disfigurement also may be observed. A type III hypersensitivity reaction may occur, resulting in erythema nodosum leprosum, a type II lepra reaction characterized by elevated tumor necrosis factor α.6
Treatment requires multidrug therapy involving dapsone, rifampin, and clofazimine.4 The World Health Organization is targeted at eliminating the disease and providing free multidrug therapy to patients.7

- Leprosy (Hansen’s disease): Technical Information. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/hansens_disease/technical.html/. Updated May 17, 2010. Accessed July 2, 2014.
- Esfandbod M. Images in clinical medicine. tuberculoid leprosy. N Engl J Med. 2011;364:1657.
- Robati RM, Rahimi H, Asadi-Kani Z, et al. Photoclinic. lepromatous leprosy. Arch Iran Med. 2010;13:443-444.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
- Wolff K, Johnson RA, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Walker SL, Lockwood DN. Leprosy. Clin Dermatol. 2007;25:165-172.
- World Health Organization Committee on Leprosy. WHO Expert Committee on Leprosy: Seventh Report. Geneva, Switzerland: World Health Organization Committee on Leprosy; 1997.
- Leprosy (Hansen’s disease): Technical Information. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/hansens_disease/technical.html/. Updated May 17, 2010. Accessed July 2, 2014.
- Esfandbod M. Images in clinical medicine. tuberculoid leprosy. N Engl J Med. 2011;364:1657.
- Robati RM, Rahimi H, Asadi-Kani Z, et al. Photoclinic. lepromatous leprosy. Arch Iran Med. 2010;13:443-444.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
- Wolff K, Johnson RA, Saavedra AP, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013.
- Walker SL, Lockwood DN. Leprosy. Clin Dermatol. 2007;25:165-172.
- World Health Organization Committee on Leprosy. WHO Expert Committee on Leprosy: Seventh Report. Geneva, Switzerland: World Health Organization Committee on Leprosy; 1997.