User login
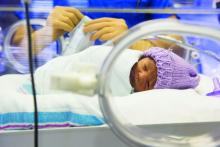
No reduction in preterm bronchopulmonary dysplasia with inhaled NO
Inhaled nitric oxide (NO) therapy does not appear to achieve reduction in the incidence of bronchopulmonary dysplasia in preterm infants, according to data published online Sept. 25 in JAMA Pediatrics.
Shabih U. Hasan, MD, from the Cumming School of Medicine at the University of Calgary, and his coauthors wrote that inhaled nitric oxide is currently approved for the treatment of hypoxic respiratory failure in infants with pulmonary hypertension. Animal studies have prompted interest in its potential to prevent bronchopulmonary dysplasia in preterm infants, but randomized trials so far have shown mixed results (JAMA Pediatr. 2017 Sep 25. doi: 10.1001/jamapediatrics.2017.2618).
The dosage selected was higher, and the treatment was given for a longer period and initiated later than in some previous studies, which the authors hypothesized might improve outcomes.
However, there was no significant difference between the placebo and inhaled NO groups in the primary outcome of survival to 36 weeks postmenstrual age without bronchopulmonary dysplasia (31.5% vs. 34.9%).
Similarly, the rate of severe bronchopulmonary dysplasia was similar for placebo and inhaled nitric oxide (26.6% vs. 20.5%), as was the rate of postnatal corticosteroid use (41.0% vs. 41.5%), mean days of positive pressure respiratory support (55 vs. 54), mean days of oxygen therapy (88 vs. 91) and mean days of hospitalization (105 vs. 108).
The subgroup analysis revealed that characteristics such as birth weight, gestational age, sex, postnatal age at study entry, maternal race or mode of respiratory support also did not influence the outcomes.
While the rates of severe bronchopulmonary dysplasia were similar between the placebo and inhaled nitric oxide groups, the inhaled NO group had a larger number of infants whose mothers were white and a higher rate of rupture of membranes for more than 7 days, compared with the placebo group.
The two groups had similar incidence of prematurity complications, such as sepsis, patent ductus arteriosus, necrotizing enterocolitis, retinopathy, intraventricular hemorrhage, and pulmonary air leak.
There were also no significant differences in neurodevelopmental or respiratory outcomes at 18-24 months postmenstrual age.
The authors commented that they had hoped their results would be similar to the earlier NO CLD trial, which hinted at a substantial increase in survival without bronchopulmonary dysplasia, compared with placebo in infants aged 7-14 days at the start of treatment.
“The NO CLD trial was not powered to assess the primary outcome in the subgroup enrolled between ages 7 and 14 days, whereas our study was powered specifically for that purpose and included twice as many infants in each treatment arm,” the authors wrote.
Despite this, and a lack of any obvious differences between the study populations, the authors could not identify a reason for the lack of efficacy seen in their own study, compared with this earlier study.
The authors noted that their findings of a lack of benefit from prophylactic but delayed NO on bronchopulmonary dysplasia were consistent with previous meta-analyses, and with a consensus statement from the National Institutes of Health.
The study was sponsored by Mallinckrodt Pharmaceuticals. Four authors declared honorarium, speaking engagements, advisory positions or consultancies with Mallinckrodt Pharmaceuticals. No other conflicts of interest were declared.
Inhaled nitric oxide (NO) therapy does not appear to achieve reduction in the incidence of bronchopulmonary dysplasia in preterm infants, according to data published online Sept. 25 in JAMA Pediatrics.
Shabih U. Hasan, MD, from the Cumming School of Medicine at the University of Calgary, and his coauthors wrote that inhaled nitric oxide is currently approved for the treatment of hypoxic respiratory failure in infants with pulmonary hypertension. Animal studies have prompted interest in its potential to prevent bronchopulmonary dysplasia in preterm infants, but randomized trials so far have shown mixed results (JAMA Pediatr. 2017 Sep 25. doi: 10.1001/jamapediatrics.2017.2618).
The dosage selected was higher, and the treatment was given for a longer period and initiated later than in some previous studies, which the authors hypothesized might improve outcomes.
However, there was no significant difference between the placebo and inhaled NO groups in the primary outcome of survival to 36 weeks postmenstrual age without bronchopulmonary dysplasia (31.5% vs. 34.9%).
Similarly, the rate of severe bronchopulmonary dysplasia was similar for placebo and inhaled nitric oxide (26.6% vs. 20.5%), as was the rate of postnatal corticosteroid use (41.0% vs. 41.5%), mean days of positive pressure respiratory support (55 vs. 54), mean days of oxygen therapy (88 vs. 91) and mean days of hospitalization (105 vs. 108).
The subgroup analysis revealed that characteristics such as birth weight, gestational age, sex, postnatal age at study entry, maternal race or mode of respiratory support also did not influence the outcomes.
While the rates of severe bronchopulmonary dysplasia were similar between the placebo and inhaled nitric oxide groups, the inhaled NO group had a larger number of infants whose mothers were white and a higher rate of rupture of membranes for more than 7 days, compared with the placebo group.
The two groups had similar incidence of prematurity complications, such as sepsis, patent ductus arteriosus, necrotizing enterocolitis, retinopathy, intraventricular hemorrhage, and pulmonary air leak.
There were also no significant differences in neurodevelopmental or respiratory outcomes at 18-24 months postmenstrual age.
The authors commented that they had hoped their results would be similar to the earlier NO CLD trial, which hinted at a substantial increase in survival without bronchopulmonary dysplasia, compared with placebo in infants aged 7-14 days at the start of treatment.
“The NO CLD trial was not powered to assess the primary outcome in the subgroup enrolled between ages 7 and 14 days, whereas our study was powered specifically for that purpose and included twice as many infants in each treatment arm,” the authors wrote.
Despite this, and a lack of any obvious differences between the study populations, the authors could not identify a reason for the lack of efficacy seen in their own study, compared with this earlier study.
The authors noted that their findings of a lack of benefit from prophylactic but delayed NO on bronchopulmonary dysplasia were consistent with previous meta-analyses, and with a consensus statement from the National Institutes of Health.
The study was sponsored by Mallinckrodt Pharmaceuticals. Four authors declared honorarium, speaking engagements, advisory positions or consultancies with Mallinckrodt Pharmaceuticals. No other conflicts of interest were declared.
Inhaled nitric oxide (NO) therapy does not appear to achieve reduction in the incidence of bronchopulmonary dysplasia in preterm infants, according to data published online Sept. 25 in JAMA Pediatrics.
Shabih U. Hasan, MD, from the Cumming School of Medicine at the University of Calgary, and his coauthors wrote that inhaled nitric oxide is currently approved for the treatment of hypoxic respiratory failure in infants with pulmonary hypertension. Animal studies have prompted interest in its potential to prevent bronchopulmonary dysplasia in preterm infants, but randomized trials so far have shown mixed results (JAMA Pediatr. 2017 Sep 25. doi: 10.1001/jamapediatrics.2017.2618).
The dosage selected was higher, and the treatment was given for a longer period and initiated later than in some previous studies, which the authors hypothesized might improve outcomes.
However, there was no significant difference between the placebo and inhaled NO groups in the primary outcome of survival to 36 weeks postmenstrual age without bronchopulmonary dysplasia (31.5% vs. 34.9%).
Similarly, the rate of severe bronchopulmonary dysplasia was similar for placebo and inhaled nitric oxide (26.6% vs. 20.5%), as was the rate of postnatal corticosteroid use (41.0% vs. 41.5%), mean days of positive pressure respiratory support (55 vs. 54), mean days of oxygen therapy (88 vs. 91) and mean days of hospitalization (105 vs. 108).
The subgroup analysis revealed that characteristics such as birth weight, gestational age, sex, postnatal age at study entry, maternal race or mode of respiratory support also did not influence the outcomes.
While the rates of severe bronchopulmonary dysplasia were similar between the placebo and inhaled nitric oxide groups, the inhaled NO group had a larger number of infants whose mothers were white and a higher rate of rupture of membranes for more than 7 days, compared with the placebo group.
The two groups had similar incidence of prematurity complications, such as sepsis, patent ductus arteriosus, necrotizing enterocolitis, retinopathy, intraventricular hemorrhage, and pulmonary air leak.
There were also no significant differences in neurodevelopmental or respiratory outcomes at 18-24 months postmenstrual age.
The authors commented that they had hoped their results would be similar to the earlier NO CLD trial, which hinted at a substantial increase in survival without bronchopulmonary dysplasia, compared with placebo in infants aged 7-14 days at the start of treatment.
“The NO CLD trial was not powered to assess the primary outcome in the subgroup enrolled between ages 7 and 14 days, whereas our study was powered specifically for that purpose and included twice as many infants in each treatment arm,” the authors wrote.
Despite this, and a lack of any obvious differences between the study populations, the authors could not identify a reason for the lack of efficacy seen in their own study, compared with this earlier study.
The authors noted that their findings of a lack of benefit from prophylactic but delayed NO on bronchopulmonary dysplasia were consistent with previous meta-analyses, and with a consensus statement from the National Institutes of Health.
The study was sponsored by Mallinckrodt Pharmaceuticals. Four authors declared honorarium, speaking engagements, advisory positions or consultancies with Mallinckrodt Pharmaceuticals. No other conflicts of interest were declared.
FROM JAMA PEDIATRICS
Key clinical point: Treatment with inhaled nitric oxide does not reduce the incidence of bronchopulmonary dysplasia in preterm infants.
Major finding: The incidence of bronchopulmonary dysplasia in preterm infants was not reduced with inhaled nitric oxide therapy.
Data source: Prospective randomized placebo-controlled trial in 451 preterm infants of less than 30 weeks gestation.
Disclosures: The study was sponsored by Mallinckrodt Pharmaceuticals. Four authors declared honorarium, speakers fees, advisory positions, or consultancies with Mallinckrodt Pharmaceuticals. No other conflicts of interest were declared.
Rheumatoid arthritis characteristics make large contribution to cardiovascular risk
Nearly one-third of cardiovascular events in patients with rheumatoid arthritis can be attributed to their rheumatoid arthritis characteristics, such as Disease Activity Score and rheumatoid factor or anticitrullinated protein antibody positivity, research suggests.
A prospective, international cohort study published in Annals of the Rheumatic Diseases followed 5,638 patients with rheumatoid arthritis (RA) and no history of cardiovascular disease for a mean of 5.8 years to look at their risk of myocardial infarction, angina, revascularization, stroke, peripheral vascular disease, and death from cardiovascular disease.
Overall, the 10-year cumulative incidence of cardiovascular events was 20.9% in men and 11.1% in women.
Smoking and hypertension were the strongest predictors of cardiovascular disease in both men and women and had the highest population attributable risk (PAR), even after adjustment for other cardiovascular risk factors.
The PAR for triglycerides was 11.5% overall, but it was 12.6% for Disease Activity Score in 28 joints (DAS28) and 12.2% for rheumatoid factor (RF)/anticitrullinated protein antibody (ACPA) positivity. Other RA-related factors, such as erythrocyte sedimentation rate (ESR) and C-reactive protein, did not have a significant effect on cardiovascular event risk.
When combined, cardiovascular risk factors such as blood pressure, cholesterol levels, smoking, body mass index, diabetes, and family history accounted for 49% of the PAR of cardiovascular events in people with RA, and the RA characteristics explained 30.3% of the risk.
Together, the cardiovascular and RA risk factors accounted for 69.6% of the risk of cardiovascular events, and the remaining 30.4% could not be explained.
While the PAR associated with the combined cardiovascular risk factors was higher in men than in women, the contribution of all the RA characteristics combined proved to be greater in women than in men. However, neither sex difference was statistically significant.
“While the prevalence of RF/ACPA positivity and DAS28 levels was similar between the sexes, the effect sizes of RA characteristics appeared to be larger among women than men, despite lack of statistical significance,” the authors wrote.
“Moreover, higher levels of ESR in women than men may partially explain this apparent difference in PAR [and] RA disease duration was longer among women, and more women than men were receiving biological [disease-modifying antirheumatic drugs] at baseline.”
Eli Lilly, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Norwegian South East Health Authority supported the study. Two authors declared honoraria, fees, and grants from the pharmaceutical industry, including Eli Lilly. No other conflicts of interest were declared.
Nearly one-third of cardiovascular events in patients with rheumatoid arthritis can be attributed to their rheumatoid arthritis characteristics, such as Disease Activity Score and rheumatoid factor or anticitrullinated protein antibody positivity, research suggests.
A prospective, international cohort study published in Annals of the Rheumatic Diseases followed 5,638 patients with rheumatoid arthritis (RA) and no history of cardiovascular disease for a mean of 5.8 years to look at their risk of myocardial infarction, angina, revascularization, stroke, peripheral vascular disease, and death from cardiovascular disease.
Overall, the 10-year cumulative incidence of cardiovascular events was 20.9% in men and 11.1% in women.
Smoking and hypertension were the strongest predictors of cardiovascular disease in both men and women and had the highest population attributable risk (PAR), even after adjustment for other cardiovascular risk factors.
The PAR for triglycerides was 11.5% overall, but it was 12.6% for Disease Activity Score in 28 joints (DAS28) and 12.2% for rheumatoid factor (RF)/anticitrullinated protein antibody (ACPA) positivity. Other RA-related factors, such as erythrocyte sedimentation rate (ESR) and C-reactive protein, did not have a significant effect on cardiovascular event risk.
When combined, cardiovascular risk factors such as blood pressure, cholesterol levels, smoking, body mass index, diabetes, and family history accounted for 49% of the PAR of cardiovascular events in people with RA, and the RA characteristics explained 30.3% of the risk.
Together, the cardiovascular and RA risk factors accounted for 69.6% of the risk of cardiovascular events, and the remaining 30.4% could not be explained.
While the PAR associated with the combined cardiovascular risk factors was higher in men than in women, the contribution of all the RA characteristics combined proved to be greater in women than in men. However, neither sex difference was statistically significant.
“While the prevalence of RF/ACPA positivity and DAS28 levels was similar between the sexes, the effect sizes of RA characteristics appeared to be larger among women than men, despite lack of statistical significance,” the authors wrote.
“Moreover, higher levels of ESR in women than men may partially explain this apparent difference in PAR [and] RA disease duration was longer among women, and more women than men were receiving biological [disease-modifying antirheumatic drugs] at baseline.”
Eli Lilly, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Norwegian South East Health Authority supported the study. Two authors declared honoraria, fees, and grants from the pharmaceutical industry, including Eli Lilly. No other conflicts of interest were declared.
Nearly one-third of cardiovascular events in patients with rheumatoid arthritis can be attributed to their rheumatoid arthritis characteristics, such as Disease Activity Score and rheumatoid factor or anticitrullinated protein antibody positivity, research suggests.
A prospective, international cohort study published in Annals of the Rheumatic Diseases followed 5,638 patients with rheumatoid arthritis (RA) and no history of cardiovascular disease for a mean of 5.8 years to look at their risk of myocardial infarction, angina, revascularization, stroke, peripheral vascular disease, and death from cardiovascular disease.
Overall, the 10-year cumulative incidence of cardiovascular events was 20.9% in men and 11.1% in women.
Smoking and hypertension were the strongest predictors of cardiovascular disease in both men and women and had the highest population attributable risk (PAR), even after adjustment for other cardiovascular risk factors.
The PAR for triglycerides was 11.5% overall, but it was 12.6% for Disease Activity Score in 28 joints (DAS28) and 12.2% for rheumatoid factor (RF)/anticitrullinated protein antibody (ACPA) positivity. Other RA-related factors, such as erythrocyte sedimentation rate (ESR) and C-reactive protein, did not have a significant effect on cardiovascular event risk.
When combined, cardiovascular risk factors such as blood pressure, cholesterol levels, smoking, body mass index, diabetes, and family history accounted for 49% of the PAR of cardiovascular events in people with RA, and the RA characteristics explained 30.3% of the risk.
Together, the cardiovascular and RA risk factors accounted for 69.6% of the risk of cardiovascular events, and the remaining 30.4% could not be explained.
While the PAR associated with the combined cardiovascular risk factors was higher in men than in women, the contribution of all the RA characteristics combined proved to be greater in women than in men. However, neither sex difference was statistically significant.
“While the prevalence of RF/ACPA positivity and DAS28 levels was similar between the sexes, the effect sizes of RA characteristics appeared to be larger among women than men, despite lack of statistical significance,” the authors wrote.
“Moreover, higher levels of ESR in women than men may partially explain this apparent difference in PAR [and] RA disease duration was longer among women, and more women than men were receiving biological [disease-modifying antirheumatic drugs] at baseline.”
Eli Lilly, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Norwegian South East Health Authority supported the study. Two authors declared honoraria, fees, and grants from the pharmaceutical industry, including Eli Lilly. No other conflicts of interest were declared.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Rheumatoid arthritis characteristics explained 30.3% of the risk of cardiovascular events in individuals with RA.
Data source: A prospective, international cohort study of 5,638 patients with RA.
Disclosures: Eli Lilly, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Norwegian South East Health Authority supported the study. Two authors declared honoraria, fees, and grants from the pharmaceutical industry, including Eli Lilly. No other conflicts of interest were declared.
Hospital-led interventions cut pediatric asthma hospitalizations
Hospital-driven interventions designed to improve management of asthma in children achieved significant reductions in monthly asthma-related hospitalizations and emergency department visits, according to a paper published online Sept. 18 in JAMA Pediatrics.
Long-term management of pediatric asthma is challenging, and around 40% of children and adolescents hospitalized with the disease tend to be rehospitalized or revisit the emergency department (ED) within 12 months, according to Carolyn M. Kercsmar, MD, of Children’s Hospital Medical Center in Cincinnati, and her coauthors.
“Traditional care models do not adequately address underlying risk factors, propagating disparities and costly health care use,” they wrote (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2600).
This study, initiated by Cincinnati Children’s Hospital Medical Center, involved a range of interventions implemented with inpatients and outpatients and through the community setting, targeting the region’s more than 36,000 children and adolescents with asthma, approximately 13,000 of whom were Medicaid insured.
Over the 5-year study, researchers saw a 41.8% relative reduction in asthma-related hospitalizations – from 8.1 to 4.7 per 10,000 Medicaid patients per month. Asthma-related visits to the ED decreased by 42.4%, from 21.5 to 12.4 per 10,000 Medicaid patients per month, and the percentage of patients rehospitalized or who returned to the ED for asthma within 30 days declined from 12% to 7%, “within 3 years of implementation of the inpatient care interventions,” the researchers noted.
There was also a significant increase in the percentage of patients discharged with a 30-day supply of inhaled controller medications, from 50% in May 2008 to 90% in May 2010, and the percentage of patients discharged with a short course of oral corticosteroids increased from 0% to 70% by March 2011.
Outpatient processes ensured that Asthma Control Test scores were collected and that patients were provided with asthma action plans. This was associated with an increase in the percentage of patients with well-controlled asthma from 48% to 54%.
“Implementation of an integrated, multilevel approach focused on enhancing availability and accessibility of treatments, removing barriers to adherence, mitigating risks related to adverse exposures, and augmenting self-management and collaborative relationships between the family and the health care system was associated with improved asthma outcomes,” the authors wrote.
Noting that previous research has found 38%-70% of patients do not get their prescribed medications at hospital discharge, the authors said they believed giving a 30-day supply of all daily asthma medications at discharge was a key part of their success.
The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
Of importance, any future efforts to replicate this work in a patient-centered way should include consideration of how information on asthma management is communicated to and understood by patients. Standard tools such as asthma action plans often contain language and other information that is inaccessible to populations with low health literacy levels.
After years of elevated morbidity, the work of Kercsmar et al. is a demonstration of how interdisciplinary care focused within a biopsychosocial model can improve outcomes for vulnerable children. Future efforts to replicate these results in other communities should continue to emphasize this patient-centered, biopsychosocial philosophy, with heightened attention to the challenges that remain for children and families.
Dr. Sean M. Frey and Dr. Jill S. Halterman are in the department of pediatrics at the University of Rochester (N.Y.) School of Medicine and Dentistry. These comments are taken from an accompanying editorial (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2609). No conflicts of interest were declared.
Of importance, any future efforts to replicate this work in a patient-centered way should include consideration of how information on asthma management is communicated to and understood by patients. Standard tools such as asthma action plans often contain language and other information that is inaccessible to populations with low health literacy levels.
After years of elevated morbidity, the work of Kercsmar et al. is a demonstration of how interdisciplinary care focused within a biopsychosocial model can improve outcomes for vulnerable children. Future efforts to replicate these results in other communities should continue to emphasize this patient-centered, biopsychosocial philosophy, with heightened attention to the challenges that remain for children and families.
Dr. Sean M. Frey and Dr. Jill S. Halterman are in the department of pediatrics at the University of Rochester (N.Y.) School of Medicine and Dentistry. These comments are taken from an accompanying editorial (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2609). No conflicts of interest were declared.
Of importance, any future efforts to replicate this work in a patient-centered way should include consideration of how information on asthma management is communicated to and understood by patients. Standard tools such as asthma action plans often contain language and other information that is inaccessible to populations with low health literacy levels.
After years of elevated morbidity, the work of Kercsmar et al. is a demonstration of how interdisciplinary care focused within a biopsychosocial model can improve outcomes for vulnerable children. Future efforts to replicate these results in other communities should continue to emphasize this patient-centered, biopsychosocial philosophy, with heightened attention to the challenges that remain for children and families.
Dr. Sean M. Frey and Dr. Jill S. Halterman are in the department of pediatrics at the University of Rochester (N.Y.) School of Medicine and Dentistry. These comments are taken from an accompanying editorial (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2609). No conflicts of interest were declared.
Hospital-driven interventions designed to improve management of asthma in children achieved significant reductions in monthly asthma-related hospitalizations and emergency department visits, according to a paper published online Sept. 18 in JAMA Pediatrics.
Long-term management of pediatric asthma is challenging, and around 40% of children and adolescents hospitalized with the disease tend to be rehospitalized or revisit the emergency department (ED) within 12 months, according to Carolyn M. Kercsmar, MD, of Children’s Hospital Medical Center in Cincinnati, and her coauthors.
“Traditional care models do not adequately address underlying risk factors, propagating disparities and costly health care use,” they wrote (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2600).
This study, initiated by Cincinnati Children’s Hospital Medical Center, involved a range of interventions implemented with inpatients and outpatients and through the community setting, targeting the region’s more than 36,000 children and adolescents with asthma, approximately 13,000 of whom were Medicaid insured.
Over the 5-year study, researchers saw a 41.8% relative reduction in asthma-related hospitalizations – from 8.1 to 4.7 per 10,000 Medicaid patients per month. Asthma-related visits to the ED decreased by 42.4%, from 21.5 to 12.4 per 10,000 Medicaid patients per month, and the percentage of patients rehospitalized or who returned to the ED for asthma within 30 days declined from 12% to 7%, “within 3 years of implementation of the inpatient care interventions,” the researchers noted.
There was also a significant increase in the percentage of patients discharged with a 30-day supply of inhaled controller medications, from 50% in May 2008 to 90% in May 2010, and the percentage of patients discharged with a short course of oral corticosteroids increased from 0% to 70% by March 2011.
Outpatient processes ensured that Asthma Control Test scores were collected and that patients were provided with asthma action plans. This was associated with an increase in the percentage of patients with well-controlled asthma from 48% to 54%.
“Implementation of an integrated, multilevel approach focused on enhancing availability and accessibility of treatments, removing barriers to adherence, mitigating risks related to adverse exposures, and augmenting self-management and collaborative relationships between the family and the health care system was associated with improved asthma outcomes,” the authors wrote.
Noting that previous research has found 38%-70% of patients do not get their prescribed medications at hospital discharge, the authors said they believed giving a 30-day supply of all daily asthma medications at discharge was a key part of their success.
The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
Hospital-driven interventions designed to improve management of asthma in children achieved significant reductions in monthly asthma-related hospitalizations and emergency department visits, according to a paper published online Sept. 18 in JAMA Pediatrics.
Long-term management of pediatric asthma is challenging, and around 40% of children and adolescents hospitalized with the disease tend to be rehospitalized or revisit the emergency department (ED) within 12 months, according to Carolyn M. Kercsmar, MD, of Children’s Hospital Medical Center in Cincinnati, and her coauthors.
“Traditional care models do not adequately address underlying risk factors, propagating disparities and costly health care use,” they wrote (JAMA Pediatrics 2017, Sep 18. doi: 10.1001/jamapediatrics.2017.2600).
This study, initiated by Cincinnati Children’s Hospital Medical Center, involved a range of interventions implemented with inpatients and outpatients and through the community setting, targeting the region’s more than 36,000 children and adolescents with asthma, approximately 13,000 of whom were Medicaid insured.
Over the 5-year study, researchers saw a 41.8% relative reduction in asthma-related hospitalizations – from 8.1 to 4.7 per 10,000 Medicaid patients per month. Asthma-related visits to the ED decreased by 42.4%, from 21.5 to 12.4 per 10,000 Medicaid patients per month, and the percentage of patients rehospitalized or who returned to the ED for asthma within 30 days declined from 12% to 7%, “within 3 years of implementation of the inpatient care interventions,” the researchers noted.
There was also a significant increase in the percentage of patients discharged with a 30-day supply of inhaled controller medications, from 50% in May 2008 to 90% in May 2010, and the percentage of patients discharged with a short course of oral corticosteroids increased from 0% to 70% by March 2011.
Outpatient processes ensured that Asthma Control Test scores were collected and that patients were provided with asthma action plans. This was associated with an increase in the percentage of patients with well-controlled asthma from 48% to 54%.
“Implementation of an integrated, multilevel approach focused on enhancing availability and accessibility of treatments, removing barriers to adherence, mitigating risks related to adverse exposures, and augmenting self-management and collaborative relationships between the family and the health care system was associated with improved asthma outcomes,” the authors wrote.
Noting that previous research has found 38%-70% of patients do not get their prescribed medications at hospital discharge, the authors said they believed giving a 30-day supply of all daily asthma medications at discharge was a key part of their success.
The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
FROM JAMA PEDIATRICS
Key clinical point: A hospital-driven intervention to improve management of asthma in children has achieved significant reductions in asthma-related hospitalizations and emergency department visits and increased medication uptake.
Major finding: A multifactorial intervention to improve asthma management in children was associated with a 41.8% relative reduction in asthma-related hospitalizations and a 42.4% reduction in emergency department visits.
Data source: A hospital-based intervention.
Disclosures: The study was supported by the Cincinnati Children’s Hospital Medical Center and one author received a grant from the National Institutes of Health. One author declared compensation for a committee role on a study of asthma treatments in children. No other conflicts of interest were declared.
Patent foramen ovale closure reduces risk of stroke in three trials
Three separate trials examining the impact of closing a patent foramen ovale on the risk of stroke all point to endovascular closure offering a greater reduction in risk than with anticoagulant or antiplatelet therapy alone.
However, this benefit may be evident only in patients at higher risk of stroke associated with patent foramen ovale (PFO), and comes at the cost of an increased risk of atrial fibrillation and procedure-related adverse events, according to papers published in the Sept. 14 issue of the New England Journal of Medicine.
Closure plus anticoagulation vs. anticoagulation or antiplatelets alone
In the CLOSE trial, 663 patients aged 16-60 years who had experienced a recent stroke attributed to PFO and who had an associated atrial septal aneurysm or large interatrial shunt were randomized to transcatheter closure plus long-term antiplatelet therapy, antiplatelets alone, or oral anticoagulation alone.
After a mean follow-up of 5.3 years, Jean‑Louis Mas, MD, of Sainte-Anne Hospital, Paris, and his colleagues reported that there were no recurrent strokes in the closure group, but 14 of the 235 patients in the antiplatelets-only group experienced a stroke, representing a 97% reduction in the risk of stroke with endovascular closure (P less than .001). Patients in the antiplatelets-only group had a 4.9% overall probability of stroke, and no explanation other than PFO could be found for their recurrent stroke (N Engl J Med. 2017;377:1011-21), reported .
The study was not adequately powered to compare the outcomes of anticoagulant therapy with antiplatelet therapy alone.
The closure group also had a 61% lower risk of the secondary composite outcome of stroke, transient ischemic attack, or systemic embolism, compared with the antiplatelet-only group (P = .01).
However, closure of the PFO was associated with a higher rate of new-onset atrial fibrillation or flutter than antiplatelet therapy alone (4.6% vs. 0.9%, P = .02), and major procedural complications occurred in 5.9% of patients.
Closure plus antiplatelets vs. antiplatelets alone
In the second study – REDUCE – 664 patients with PFO who had experienced an ischemic stroke with no other obvious cause were randomized either to closure plus antiplatelet therapy or antiplatelet therapy alone (N Engl J Med. 2017;377:1033-42).
Over the median follow-up of 3.2 years, there were ischemic strokes in 1.4% of patients in the closure group, compared with 5.4% in the antiplatelet-only group; this was a 77% reduction in risk (P = .002), Lars Søndergaard, MD, of the University of Copenhagen, and his coauthors reported.
The closure group also had a 49% lower incidence of new brain infarctions, compared with the antiplatelet-only group, although the incidence of silent brain infarction was similar between the two groups.
While the risks of major bleeding, deep-vein thrombosis, and pulmonary embolism and serious adverse events were similar between the two groups, the closure group had a 2.5% rate of procedure-related serious adverse events and 1.4% rate of device-related serious adverse events. The closure group also had a significantly higher incidence of atrial fibrillation when compared with the control group (6.6% vs. 0.4%; P less than .001).
Closure vs. medical therapy alone
Finally, in the third paper, Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates reported the long-term outcomes of the RESPECT trial of PFO closure versus medical therapy alone in 980 patients with PFO who had experienced a cryptogenic ischemic stroke (N Engl J Med. 2017; 377:1022-32).
After a median follow-up of 5.9 years, there was a 45% lower risk of recurrent ischemic stroke in the closure group, compared with the medical therapy alone group. The overall incidence of recurrent ischemic stroke was 0.58 events per 100 patient-years after closure, compared against 1.07 events with medical therapy, which included aspirin, warfarin, clopidogrel, or aspirin combined with extended-release dipyridamole.
However, the rate of pulmonary embolism was more than threefold higher, and the rate of deep vein thrombosis was more than fourfold higher in the closure group, compared with the medical therapy group, although the latter was not statistically significant.
FDA perspective
In an accompanying perspective on the three studies, Andrew Farb, MD, and his colleagues from the Center for Devices and Radiological Health at the Food and Drug Administration noted that the clinical benefit of closing a PFO has been an ongoing question for several decades, but the data on the Amplatzer PFO Occluder – a device for PFO closure – had met the agency’s approval threshold.
“The FDA concluded that although there were few recurrent strokes in both groups and some uncertainty regarding the reduction in stroke risk attributable to the device, preventing recurrent stroke is of high value,” the authors wrote (N Engl J Med. 2017;377:1006-9).
However, they stressed that because of the high prevalence of PFO, patients being considered for surgical closure should undergo comprehensive clinical assessment by a neurologist and cardiologist to ensure the ischemic stroke did not have any other possible cause.
The CLOSE trial was supported by the French Ministry of Health. Fourteen authors reported funding, grants, consultancies, and other support from the pharmaceutical industry. The REDUCE trial was supported by W.L. Gore and Associates, and 11 authors declared grants or fees from W.L. Gore and Associates. The RESPECT trial was supported by St. Jude Medical. Eight authors declared grants, fees, or nonfinancial support from St. Jude Medical. One also declared grants and fees from the pharmaceutical industry. The authors of the accompanying perspective had no conflicts of interest to declare.
The evidence for causation of embolic stroke in any given person is, of course, circumstantial (for example, atrial fibrillation or carotid stenosis), and it seems reasonable that the presence of a PFO and a sizable interatrial shunt should similarly no longer result in the categorization of a stroke as cryptogenic.
One conclusion from previous trials of closure of patent foramen ovale is that the potential benefit from closure is determined on the basis of the positive characteristics of the PFO rather than on the basis of exclusionary factors that make a stroke cryptogenic. Restricting PFO closure entirely to patients with high-risk characteristics of the PFO may perhaps be too conservative, but the boundaries of the features that support the procedure are becoming clearer.
Dr. Allan H. Ropper is deputy editor of the New England Journal of Medicine. These comments are taken from an accompanying editorial (N Engl J Med. 2017; 377:1093-5). He had no conflicts of interest to declare.
The evidence for causation of embolic stroke in any given person is, of course, circumstantial (for example, atrial fibrillation or carotid stenosis), and it seems reasonable that the presence of a PFO and a sizable interatrial shunt should similarly no longer result in the categorization of a stroke as cryptogenic.
One conclusion from previous trials of closure of patent foramen ovale is that the potential benefit from closure is determined on the basis of the positive characteristics of the PFO rather than on the basis of exclusionary factors that make a stroke cryptogenic. Restricting PFO closure entirely to patients with high-risk characteristics of the PFO may perhaps be too conservative, but the boundaries of the features that support the procedure are becoming clearer.
Dr. Allan H. Ropper is deputy editor of the New England Journal of Medicine. These comments are taken from an accompanying editorial (N Engl J Med. 2017; 377:1093-5). He had no conflicts of interest to declare.
The evidence for causation of embolic stroke in any given person is, of course, circumstantial (for example, atrial fibrillation or carotid stenosis), and it seems reasonable that the presence of a PFO and a sizable interatrial shunt should similarly no longer result in the categorization of a stroke as cryptogenic.
One conclusion from previous trials of closure of patent foramen ovale is that the potential benefit from closure is determined on the basis of the positive characteristics of the PFO rather than on the basis of exclusionary factors that make a stroke cryptogenic. Restricting PFO closure entirely to patients with high-risk characteristics of the PFO may perhaps be too conservative, but the boundaries of the features that support the procedure are becoming clearer.
Dr. Allan H. Ropper is deputy editor of the New England Journal of Medicine. These comments are taken from an accompanying editorial (N Engl J Med. 2017; 377:1093-5). He had no conflicts of interest to declare.
Three separate trials examining the impact of closing a patent foramen ovale on the risk of stroke all point to endovascular closure offering a greater reduction in risk than with anticoagulant or antiplatelet therapy alone.
However, this benefit may be evident only in patients at higher risk of stroke associated with patent foramen ovale (PFO), and comes at the cost of an increased risk of atrial fibrillation and procedure-related adverse events, according to papers published in the Sept. 14 issue of the New England Journal of Medicine.
Closure plus anticoagulation vs. anticoagulation or antiplatelets alone
In the CLOSE trial, 663 patients aged 16-60 years who had experienced a recent stroke attributed to PFO and who had an associated atrial septal aneurysm or large interatrial shunt were randomized to transcatheter closure plus long-term antiplatelet therapy, antiplatelets alone, or oral anticoagulation alone.
After a mean follow-up of 5.3 years, Jean‑Louis Mas, MD, of Sainte-Anne Hospital, Paris, and his colleagues reported that there were no recurrent strokes in the closure group, but 14 of the 235 patients in the antiplatelets-only group experienced a stroke, representing a 97% reduction in the risk of stroke with endovascular closure (P less than .001). Patients in the antiplatelets-only group had a 4.9% overall probability of stroke, and no explanation other than PFO could be found for their recurrent stroke (N Engl J Med. 2017;377:1011-21), reported .
The study was not adequately powered to compare the outcomes of anticoagulant therapy with antiplatelet therapy alone.
The closure group also had a 61% lower risk of the secondary composite outcome of stroke, transient ischemic attack, or systemic embolism, compared with the antiplatelet-only group (P = .01).
However, closure of the PFO was associated with a higher rate of new-onset atrial fibrillation or flutter than antiplatelet therapy alone (4.6% vs. 0.9%, P = .02), and major procedural complications occurred in 5.9% of patients.
Closure plus antiplatelets vs. antiplatelets alone
In the second study – REDUCE – 664 patients with PFO who had experienced an ischemic stroke with no other obvious cause were randomized either to closure plus antiplatelet therapy or antiplatelet therapy alone (N Engl J Med. 2017;377:1033-42).
Over the median follow-up of 3.2 years, there were ischemic strokes in 1.4% of patients in the closure group, compared with 5.4% in the antiplatelet-only group; this was a 77% reduction in risk (P = .002), Lars Søndergaard, MD, of the University of Copenhagen, and his coauthors reported.
The closure group also had a 49% lower incidence of new brain infarctions, compared with the antiplatelet-only group, although the incidence of silent brain infarction was similar between the two groups.
While the risks of major bleeding, deep-vein thrombosis, and pulmonary embolism and serious adverse events were similar between the two groups, the closure group had a 2.5% rate of procedure-related serious adverse events and 1.4% rate of device-related serious adverse events. The closure group also had a significantly higher incidence of atrial fibrillation when compared with the control group (6.6% vs. 0.4%; P less than .001).
Closure vs. medical therapy alone
Finally, in the third paper, Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates reported the long-term outcomes of the RESPECT trial of PFO closure versus medical therapy alone in 980 patients with PFO who had experienced a cryptogenic ischemic stroke (N Engl J Med. 2017; 377:1022-32).
After a median follow-up of 5.9 years, there was a 45% lower risk of recurrent ischemic stroke in the closure group, compared with the medical therapy alone group. The overall incidence of recurrent ischemic stroke was 0.58 events per 100 patient-years after closure, compared against 1.07 events with medical therapy, which included aspirin, warfarin, clopidogrel, or aspirin combined with extended-release dipyridamole.
However, the rate of pulmonary embolism was more than threefold higher, and the rate of deep vein thrombosis was more than fourfold higher in the closure group, compared with the medical therapy group, although the latter was not statistically significant.
FDA perspective
In an accompanying perspective on the three studies, Andrew Farb, MD, and his colleagues from the Center for Devices and Radiological Health at the Food and Drug Administration noted that the clinical benefit of closing a PFO has been an ongoing question for several decades, but the data on the Amplatzer PFO Occluder – a device for PFO closure – had met the agency’s approval threshold.
“The FDA concluded that although there were few recurrent strokes in both groups and some uncertainty regarding the reduction in stroke risk attributable to the device, preventing recurrent stroke is of high value,” the authors wrote (N Engl J Med. 2017;377:1006-9).
However, they stressed that because of the high prevalence of PFO, patients being considered for surgical closure should undergo comprehensive clinical assessment by a neurologist and cardiologist to ensure the ischemic stroke did not have any other possible cause.
The CLOSE trial was supported by the French Ministry of Health. Fourteen authors reported funding, grants, consultancies, and other support from the pharmaceutical industry. The REDUCE trial was supported by W.L. Gore and Associates, and 11 authors declared grants or fees from W.L. Gore and Associates. The RESPECT trial was supported by St. Jude Medical. Eight authors declared grants, fees, or nonfinancial support from St. Jude Medical. One also declared grants and fees from the pharmaceutical industry. The authors of the accompanying perspective had no conflicts of interest to declare.
Three separate trials examining the impact of closing a patent foramen ovale on the risk of stroke all point to endovascular closure offering a greater reduction in risk than with anticoagulant or antiplatelet therapy alone.
However, this benefit may be evident only in patients at higher risk of stroke associated with patent foramen ovale (PFO), and comes at the cost of an increased risk of atrial fibrillation and procedure-related adverse events, according to papers published in the Sept. 14 issue of the New England Journal of Medicine.
Closure plus anticoagulation vs. anticoagulation or antiplatelets alone
In the CLOSE trial, 663 patients aged 16-60 years who had experienced a recent stroke attributed to PFO and who had an associated atrial septal aneurysm or large interatrial shunt were randomized to transcatheter closure plus long-term antiplatelet therapy, antiplatelets alone, or oral anticoagulation alone.
After a mean follow-up of 5.3 years, Jean‑Louis Mas, MD, of Sainte-Anne Hospital, Paris, and his colleagues reported that there were no recurrent strokes in the closure group, but 14 of the 235 patients in the antiplatelets-only group experienced a stroke, representing a 97% reduction in the risk of stroke with endovascular closure (P less than .001). Patients in the antiplatelets-only group had a 4.9% overall probability of stroke, and no explanation other than PFO could be found for their recurrent stroke (N Engl J Med. 2017;377:1011-21), reported .
The study was not adequately powered to compare the outcomes of anticoagulant therapy with antiplatelet therapy alone.
The closure group also had a 61% lower risk of the secondary composite outcome of stroke, transient ischemic attack, or systemic embolism, compared with the antiplatelet-only group (P = .01).
However, closure of the PFO was associated with a higher rate of new-onset atrial fibrillation or flutter than antiplatelet therapy alone (4.6% vs. 0.9%, P = .02), and major procedural complications occurred in 5.9% of patients.
Closure plus antiplatelets vs. antiplatelets alone
In the second study – REDUCE – 664 patients with PFO who had experienced an ischemic stroke with no other obvious cause were randomized either to closure plus antiplatelet therapy or antiplatelet therapy alone (N Engl J Med. 2017;377:1033-42).
Over the median follow-up of 3.2 years, there were ischemic strokes in 1.4% of patients in the closure group, compared with 5.4% in the antiplatelet-only group; this was a 77% reduction in risk (P = .002), Lars Søndergaard, MD, of the University of Copenhagen, and his coauthors reported.
The closure group also had a 49% lower incidence of new brain infarctions, compared with the antiplatelet-only group, although the incidence of silent brain infarction was similar between the two groups.
While the risks of major bleeding, deep-vein thrombosis, and pulmonary embolism and serious adverse events were similar between the two groups, the closure group had a 2.5% rate of procedure-related serious adverse events and 1.4% rate of device-related serious adverse events. The closure group also had a significantly higher incidence of atrial fibrillation when compared with the control group (6.6% vs. 0.4%; P less than .001).
Closure vs. medical therapy alone
Finally, in the third paper, Jeffrey L. Saver, MD, of the University of California, Los Angeles, and his associates reported the long-term outcomes of the RESPECT trial of PFO closure versus medical therapy alone in 980 patients with PFO who had experienced a cryptogenic ischemic stroke (N Engl J Med. 2017; 377:1022-32).
After a median follow-up of 5.9 years, there was a 45% lower risk of recurrent ischemic stroke in the closure group, compared with the medical therapy alone group. The overall incidence of recurrent ischemic stroke was 0.58 events per 100 patient-years after closure, compared against 1.07 events with medical therapy, which included aspirin, warfarin, clopidogrel, or aspirin combined with extended-release dipyridamole.
However, the rate of pulmonary embolism was more than threefold higher, and the rate of deep vein thrombosis was more than fourfold higher in the closure group, compared with the medical therapy group, although the latter was not statistically significant.
FDA perspective
In an accompanying perspective on the three studies, Andrew Farb, MD, and his colleagues from the Center for Devices and Radiological Health at the Food and Drug Administration noted that the clinical benefit of closing a PFO has been an ongoing question for several decades, but the data on the Amplatzer PFO Occluder – a device for PFO closure – had met the agency’s approval threshold.
“The FDA concluded that although there were few recurrent strokes in both groups and some uncertainty regarding the reduction in stroke risk attributable to the device, preventing recurrent stroke is of high value,” the authors wrote (N Engl J Med. 2017;377:1006-9).
However, they stressed that because of the high prevalence of PFO, patients being considered for surgical closure should undergo comprehensive clinical assessment by a neurologist and cardiologist to ensure the ischemic stroke did not have any other possible cause.
The CLOSE trial was supported by the French Ministry of Health. Fourteen authors reported funding, grants, consultancies, and other support from the pharmaceutical industry. The REDUCE trial was supported by W.L. Gore and Associates, and 11 authors declared grants or fees from W.L. Gore and Associates. The RESPECT trial was supported by St. Jude Medical. Eight authors declared grants, fees, or nonfinancial support from St. Jude Medical. One also declared grants and fees from the pharmaceutical industry. The authors of the accompanying perspective had no conflicts of interest to declare.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Patent foramen ovale closure was associated with a 45%-97% reduction in the incidence of recurrent ischemic stroke, compared with treatment with antiplatelets or anticoagulants alone.
Data source: The CLOSE, REDUCE, and RESPECT randomized, controlled trials in patients with patent foramen ovale who had experienced a cryptogenic ischemic stroke.
Disclosures: The CLOSE trial was supported by the French Ministry of Health. Fourteen authors reported funding, grants, consultancies and other support from the pharmaceutical industry. The REDUCE trial was supported by W.L. Gore and Associates, and 11 authors declared grants or fees from W.L. Gore and Associates. The RESPECT trial was supported by St. Jude Medical. Eight authors declared grants, fees, or nonfinancial support from St. Jude Medical. One also declared grants and fees from the pharmaceutical industry.
Tezepelumab reduces exacerbations in persistent, treatment-resistant asthma
Patients whose asthma remains uncontrolled despite treatment may benefit from a new monoclonal antibody that targets an inflammatory cytokine known to be promoted in asthmatic airways, according to data presented at the annual congress of the European Respiratory Society.
Writing in the Sept. 7 issue of the New England Journal of Medicine, researchers reported on a phase 2, randomized placebo-controlled trial of three dosing regimens of subcutaneous tezepelumab, which targets the epithelial cell–derived cytokine thymic stromal lymphopoietin (TSLP). The trial involved 584 patients with uncontrolled asthma, despite treatment with long-acting beta-agonists and medium to high doses of inhaled glucocorticoids.
At 70 mg every 4 weeks, exacerbation rates were 61% lower than in the placebo group; at 210 mg every 4 weeks, they were 71% lower; and at 280 mg every 2 weeks, they were 66% lower (P was less than 001 in comparisons between each group and the placebo).
The overall annualized exacerbation rates by week 52 were 0.26 for the 70-mg group, 0.19 for the 210-mg group, and 0.22 for the 280-mg group, compared with 0.67 in the placebo group, regardless of a patient’s baseline eosinophil count. Patients treated with tezepelumab had a longer time to first asthma exacerbation. They also experienced a significantly higher change from baseline in their prebronchodilator forced expiratory volume in 1 second at week 52, when compared with patients on the placebo.
“The observed improvements in disease control in patients who received tezepelumab highlight the potential pathogenic role of TSLP across different asthma phenotypes,” reported Jonathan Corren, MD, of the University of California, Los Angeles, and his coauthors. “... Although TSLP is central to the regulation of type 2 immunity, many cell types that are activated by or respond to TSLP, such as mast cells, basophils, natural killer T cells, innate lymphoid cells, and neutrophils, may play a role in inflammation in asthma beyond type 2 inflammation.”
The incidences of adverse events and serious adverse events were similar across all groups in the study. Three serious adverse events – pneumonia and stroke in the same patient and one case of Guillain-Barré syndrome – in patients taking tezepelumab, were deemed to be related to the treatment.
The study was supported by tezepelumab manufacturers MedImmune (a member of the AstraZeneca group) and Amgen. Six of the seven authors are employees of MedImmune or Amgen. One author declared support and honoraria from several pharmaceutical companies, one declared a related patent, and five also had stock options in either MedImmune or Amgen.
Tezepelumab is the first biologic that has a substantial positive effect on two important markers of the inflammation of asthma – namely, blood eosinophil counts and the fraction of exhaled nitric oxide. It appears to be the broadest and most promising biologic for the treatment of persistent uncontrolled asthma to date.
The observation that tezepelumab reduces the level of both inflammatory markers shows that it hits a more upstream target and that it blocks at least two relevant inflammatory pathways in asthma. This is likely to be clinically relevant, since simultaneously increased exhaled nitric oxide levels and blood eosinophil counts are related to increased morbidity due to asthma.
Elisabeth H. Bel, MD, PhD, is with the department of respiratory medicine, Academic Medical Center, the University of Amsterdam. These comments were taken from an accompanying editorial (N Engl J Med. 2017;377:989-91). Dr. Bel declared consultancies and grants from pharmaceutical companies including AstraZeneca.
Tezepelumab is the first biologic that has a substantial positive effect on two important markers of the inflammation of asthma – namely, blood eosinophil counts and the fraction of exhaled nitric oxide. It appears to be the broadest and most promising biologic for the treatment of persistent uncontrolled asthma to date.
The observation that tezepelumab reduces the level of both inflammatory markers shows that it hits a more upstream target and that it blocks at least two relevant inflammatory pathways in asthma. This is likely to be clinically relevant, since simultaneously increased exhaled nitric oxide levels and blood eosinophil counts are related to increased morbidity due to asthma.
Elisabeth H. Bel, MD, PhD, is with the department of respiratory medicine, Academic Medical Center, the University of Amsterdam. These comments were taken from an accompanying editorial (N Engl J Med. 2017;377:989-91). Dr. Bel declared consultancies and grants from pharmaceutical companies including AstraZeneca.
Tezepelumab is the first biologic that has a substantial positive effect on two important markers of the inflammation of asthma – namely, blood eosinophil counts and the fraction of exhaled nitric oxide. It appears to be the broadest and most promising biologic for the treatment of persistent uncontrolled asthma to date.
The observation that tezepelumab reduces the level of both inflammatory markers shows that it hits a more upstream target and that it blocks at least two relevant inflammatory pathways in asthma. This is likely to be clinically relevant, since simultaneously increased exhaled nitric oxide levels and blood eosinophil counts are related to increased morbidity due to asthma.
Elisabeth H. Bel, MD, PhD, is with the department of respiratory medicine, Academic Medical Center, the University of Amsterdam. These comments were taken from an accompanying editorial (N Engl J Med. 2017;377:989-91). Dr. Bel declared consultancies and grants from pharmaceutical companies including AstraZeneca.
Patients whose asthma remains uncontrolled despite treatment may benefit from a new monoclonal antibody that targets an inflammatory cytokine known to be promoted in asthmatic airways, according to data presented at the annual congress of the European Respiratory Society.
Writing in the Sept. 7 issue of the New England Journal of Medicine, researchers reported on a phase 2, randomized placebo-controlled trial of three dosing regimens of subcutaneous tezepelumab, which targets the epithelial cell–derived cytokine thymic stromal lymphopoietin (TSLP). The trial involved 584 patients with uncontrolled asthma, despite treatment with long-acting beta-agonists and medium to high doses of inhaled glucocorticoids.
At 70 mg every 4 weeks, exacerbation rates were 61% lower than in the placebo group; at 210 mg every 4 weeks, they were 71% lower; and at 280 mg every 2 weeks, they were 66% lower (P was less than 001 in comparisons between each group and the placebo).
The overall annualized exacerbation rates by week 52 were 0.26 for the 70-mg group, 0.19 for the 210-mg group, and 0.22 for the 280-mg group, compared with 0.67 in the placebo group, regardless of a patient’s baseline eosinophil count. Patients treated with tezepelumab had a longer time to first asthma exacerbation. They also experienced a significantly higher change from baseline in their prebronchodilator forced expiratory volume in 1 second at week 52, when compared with patients on the placebo.
“The observed improvements in disease control in patients who received tezepelumab highlight the potential pathogenic role of TSLP across different asthma phenotypes,” reported Jonathan Corren, MD, of the University of California, Los Angeles, and his coauthors. “... Although TSLP is central to the regulation of type 2 immunity, many cell types that are activated by or respond to TSLP, such as mast cells, basophils, natural killer T cells, innate lymphoid cells, and neutrophils, may play a role in inflammation in asthma beyond type 2 inflammation.”
The incidences of adverse events and serious adverse events were similar across all groups in the study. Three serious adverse events – pneumonia and stroke in the same patient and one case of Guillain-Barré syndrome – in patients taking tezepelumab, were deemed to be related to the treatment.
The study was supported by tezepelumab manufacturers MedImmune (a member of the AstraZeneca group) and Amgen. Six of the seven authors are employees of MedImmune or Amgen. One author declared support and honoraria from several pharmaceutical companies, one declared a related patent, and five also had stock options in either MedImmune or Amgen.
Patients whose asthma remains uncontrolled despite treatment may benefit from a new monoclonal antibody that targets an inflammatory cytokine known to be promoted in asthmatic airways, according to data presented at the annual congress of the European Respiratory Society.
Writing in the Sept. 7 issue of the New England Journal of Medicine, researchers reported on a phase 2, randomized placebo-controlled trial of three dosing regimens of subcutaneous tezepelumab, which targets the epithelial cell–derived cytokine thymic stromal lymphopoietin (TSLP). The trial involved 584 patients with uncontrolled asthma, despite treatment with long-acting beta-agonists and medium to high doses of inhaled glucocorticoids.
At 70 mg every 4 weeks, exacerbation rates were 61% lower than in the placebo group; at 210 mg every 4 weeks, they were 71% lower; and at 280 mg every 2 weeks, they were 66% lower (P was less than 001 in comparisons between each group and the placebo).
The overall annualized exacerbation rates by week 52 were 0.26 for the 70-mg group, 0.19 for the 210-mg group, and 0.22 for the 280-mg group, compared with 0.67 in the placebo group, regardless of a patient’s baseline eosinophil count. Patients treated with tezepelumab had a longer time to first asthma exacerbation. They also experienced a significantly higher change from baseline in their prebronchodilator forced expiratory volume in 1 second at week 52, when compared with patients on the placebo.
“The observed improvements in disease control in patients who received tezepelumab highlight the potential pathogenic role of TSLP across different asthma phenotypes,” reported Jonathan Corren, MD, of the University of California, Los Angeles, and his coauthors. “... Although TSLP is central to the regulation of type 2 immunity, many cell types that are activated by or respond to TSLP, such as mast cells, basophils, natural killer T cells, innate lymphoid cells, and neutrophils, may play a role in inflammation in asthma beyond type 2 inflammation.”
The incidences of adverse events and serious adverse events were similar across all groups in the study. Three serious adverse events – pneumonia and stroke in the same patient and one case of Guillain-Barré syndrome – in patients taking tezepelumab, were deemed to be related to the treatment.
The study was supported by tezepelumab manufacturers MedImmune (a member of the AstraZeneca group) and Amgen. Six of the seven authors are employees of MedImmune or Amgen. One author declared support and honoraria from several pharmaceutical companies, one declared a related patent, and five also had stock options in either MedImmune or Amgen.
FROM THE ERS CONGRESS 2017
Key clinical point: The monoclonal antibody tezepelumab is associated with a significant reduction in asthma exacerbations in patients with treatment-resistant and persistent disease.
Major finding: Patients treated with tezepelumab had a 34% reduction in the risk of asthma exacerbations, compared with those on placebo.
Data source: A phase 2, randomized placebo-controlled trial in 584 patients with persistent asthma.
Disclosures: The study was supported by tezepelumab manufacturers MedImmune (a member of the AstraZeneca group) and Amgen. Six of the seven authors are employees of MedImmune or Amgen. One author declared support and honoraria from several pharmaceutical companies, one declared a related patent, and five also had stock options in either MedImmune or Amgen.
Most type 2 diabetes patients skipping metformin as first-line therapy
Few patients who are initiated on second-line treatment for type 2 diabetes mellitus show evidence of recommended use of first-line treatment with metformin, according to research published online Sept. 13 in Diabetes Care.
A retrospective cross-sectional study examined Aetna member claims data from 52,544 individuals with type 2 diabetes. It showed that of the 22,956 individuals given second-line treatment, only 8.2% had claims evidence of recommended use of metformin in the previous 60 days.
Furthermore, 28% had no claims evidence at all of having taken metformin, and only a small number of these patients had evidence of contraindications to metformin, such as heart failure (2.9%), chronic obstructive pulmonary disease (3.1%), liver diseases (4.3%), or renal diseases (4.1%).
“Although gastrointestinal adverse effects related to metformin therapy might lead to guideline nonadherence and early second-line medication initiation, we did not find evidence for gastrointestinal upset in the claims data,” wrote Yi-Ju Tseng, PhD, of the Computational Health Informatics Program at Boston Children’s Hospital, and her coauthors.
Even at the top range of sensitivity, researchers argued that less than half of the patients on second-line treatment could have had prior recommended use of metformin as the first-line treatment, while at the lower end of sensitivity, that figure was less than 10% (Diabetes Care. 2017 Sep 13. doi: 10.2337/dc17-0213).
Around one-third of patients received some metformin before beginning a second-line treatment, but the duration of metformin treatment was less than the 2 months recommended by current guidelines. Of these patients, just over half were prescribed both metformin and the second-line medication on the same day.
“What may be taken as evidence of treatment failure by clinicians may instead represent failure of adherence to established treatment guidelines, which in turn may lead to the use of insulin or additional second-line medications,” the authors wrote. “Point-of-care decision support and population health–level approaches should focus on improving adherence to first-line therapy.”
The study also found that patients who were given a second-line treatment without evidence of recommended first-line use of metformin were significantly more likely to be given insulin or an additional second-line antihyperglycemic medication. They were also more likely to be male.
However, the authors acknowledged that retrospective claims-based analyses were limited by the exclusion of uninsured patients, and a lack of detailed clinical or behavioral information, or information on out-of-pocket medications.
The study was supported by the National Institute of General Medical Sciences, and one author was supported by grants from the Ministry of Science and Technology, Taiwan and Chang Gung Memorial Hospital, Linkou, Taiwan. No conflicts of interest were declared.
Few patients who are initiated on second-line treatment for type 2 diabetes mellitus show evidence of recommended use of first-line treatment with metformin, according to research published online Sept. 13 in Diabetes Care.
A retrospective cross-sectional study examined Aetna member claims data from 52,544 individuals with type 2 diabetes. It showed that of the 22,956 individuals given second-line treatment, only 8.2% had claims evidence of recommended use of metformin in the previous 60 days.
Furthermore, 28% had no claims evidence at all of having taken metformin, and only a small number of these patients had evidence of contraindications to metformin, such as heart failure (2.9%), chronic obstructive pulmonary disease (3.1%), liver diseases (4.3%), or renal diseases (4.1%).
“Although gastrointestinal adverse effects related to metformin therapy might lead to guideline nonadherence and early second-line medication initiation, we did not find evidence for gastrointestinal upset in the claims data,” wrote Yi-Ju Tseng, PhD, of the Computational Health Informatics Program at Boston Children’s Hospital, and her coauthors.
Even at the top range of sensitivity, researchers argued that less than half of the patients on second-line treatment could have had prior recommended use of metformin as the first-line treatment, while at the lower end of sensitivity, that figure was less than 10% (Diabetes Care. 2017 Sep 13. doi: 10.2337/dc17-0213).
Around one-third of patients received some metformin before beginning a second-line treatment, but the duration of metformin treatment was less than the 2 months recommended by current guidelines. Of these patients, just over half were prescribed both metformin and the second-line medication on the same day.
“What may be taken as evidence of treatment failure by clinicians may instead represent failure of adherence to established treatment guidelines, which in turn may lead to the use of insulin or additional second-line medications,” the authors wrote. “Point-of-care decision support and population health–level approaches should focus on improving adherence to first-line therapy.”
The study also found that patients who were given a second-line treatment without evidence of recommended first-line use of metformin were significantly more likely to be given insulin or an additional second-line antihyperglycemic medication. They were also more likely to be male.
However, the authors acknowledged that retrospective claims-based analyses were limited by the exclusion of uninsured patients, and a lack of detailed clinical or behavioral information, or information on out-of-pocket medications.
The study was supported by the National Institute of General Medical Sciences, and one author was supported by grants from the Ministry of Science and Technology, Taiwan and Chang Gung Memorial Hospital, Linkou, Taiwan. No conflicts of interest were declared.
Few patients who are initiated on second-line treatment for type 2 diabetes mellitus show evidence of recommended use of first-line treatment with metformin, according to research published online Sept. 13 in Diabetes Care.
A retrospective cross-sectional study examined Aetna member claims data from 52,544 individuals with type 2 diabetes. It showed that of the 22,956 individuals given second-line treatment, only 8.2% had claims evidence of recommended use of metformin in the previous 60 days.
Furthermore, 28% had no claims evidence at all of having taken metformin, and only a small number of these patients had evidence of contraindications to metformin, such as heart failure (2.9%), chronic obstructive pulmonary disease (3.1%), liver diseases (4.3%), or renal diseases (4.1%).
“Although gastrointestinal adverse effects related to metformin therapy might lead to guideline nonadherence and early second-line medication initiation, we did not find evidence for gastrointestinal upset in the claims data,” wrote Yi-Ju Tseng, PhD, of the Computational Health Informatics Program at Boston Children’s Hospital, and her coauthors.
Even at the top range of sensitivity, researchers argued that less than half of the patients on second-line treatment could have had prior recommended use of metformin as the first-line treatment, while at the lower end of sensitivity, that figure was less than 10% (Diabetes Care. 2017 Sep 13. doi: 10.2337/dc17-0213).
Around one-third of patients received some metformin before beginning a second-line treatment, but the duration of metformin treatment was less than the 2 months recommended by current guidelines. Of these patients, just over half were prescribed both metformin and the second-line medication on the same day.
“What may be taken as evidence of treatment failure by clinicians may instead represent failure of adherence to established treatment guidelines, which in turn may lead to the use of insulin or additional second-line medications,” the authors wrote. “Point-of-care decision support and population health–level approaches should focus on improving adherence to first-line therapy.”
The study also found that patients who were given a second-line treatment without evidence of recommended first-line use of metformin were significantly more likely to be given insulin or an additional second-line antihyperglycemic medication. They were also more likely to be male.
However, the authors acknowledged that retrospective claims-based analyses were limited by the exclusion of uninsured patients, and a lack of detailed clinical or behavioral information, or information on out-of-pocket medications.
The study was supported by the National Institute of General Medical Sciences, and one author was supported by grants from the Ministry of Science and Technology, Taiwan and Chang Gung Memorial Hospital, Linkou, Taiwan. No conflicts of interest were declared.
FROM DIABETES CARE
Key clinical point: Most patients with type 2 diabetes are not taking the recommended first-line metformin treatment or are skipping straight to second-line therapies altogether.
Major finding: Fewer than 1 in 10 patients with type 2 diabetes have evidence of recommended use of metformin as a first-line treatment for type 2 diabetes.
Data source: A retrospective cross-sectional study of member claims data from 52,544 individuals with type 2 diabetes.
Disclosures: The study was supported by the National Institute of General Medical Sciences, and one author was supported by grants from the Ministry of Science and Technology, Taiwan and Chang Gung Memorial Hospital. No conflicts of interest were declared.
Lack of lupus ‘gold standard’ definition hampers estimates of its incidence, prevalence
Two new studies representing the latest efforts to determine the incidence and prevalence of systemic lupus erythematosus in the United States have revealed the difficulty of ascertaining cases when definitions of the disease vary.
The two population-based registries on which the studies were based – the California Lupus Surveillance Project and the Manhattan Lupus Surveillance Program (MLSP) – confirmed that black women represent the highest risk group for systemic lupus erythematosus (SLE) and also reaffirmed the elevated risk observed in Hispanic and Asian women, compared with white women.
The MLSP researchers applied the updated 1997 American College of Rheumatology (ACR) classification criteria, the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria, or a treating rheumatologist’s diagnosis to records from hospitals, rheumatologists, and administrative databases, and looked at both the prevalence of the disease in 2007 and the incidence from the period of 2007-2009. Using the ACR’s definition of SLE, they found an age-standardized prevalence of 62.2 and an incidence rate of 4.6 per 100,000 person-years (Arthritis Rheumatol. 2017 Sep 11. doi: 10.1002/art.40192).
The prevalence was significantly higher in women than in men (107.4 vs. 12.5 per 100,000 persons), and was highest overall in non-Hispanic black women (210.9), followed by Hispanic women (138.3), non-Hispanic Asian women (91.2), and non-Hispanic white women (64.3).
However, use of the SLICC classification criteria increased the age-standardized prevalence by 17%-19%, to a prevalence of 73.8 per 100,000 person-years and resulted in a 35% increase in incidence, to 6.2 per 100,000 person-years, compared with the ACR rates.
“The small number of cases that met the ACR but not the SLICC case definition is reassuring as it suggests that few cases met ACR criteria for SLE without the presence of autoantibodies,” wrote Peter M. Izmirly, MD, of New York (N.Y.) University, and his coauthors.
“However, given the descriptive nature of the MLSP and the absence of a gold standard test that would unambiguously identify SLE, this project cannot assess which set of classification criteria is more sensitive or specific.”
Meanwhile, the California Lupus Surveillance Project, a registry of people with lupus living in San Francisco County between 2007 and 2009, used the ACR definition to record an age-standardized annual incidence rate of 4.6 per 100,000 person-years, and an average annual prevalence of 84.8 per 100,000 persons (Arthritis Rheumatol. 2017 Sep 11. doi: 10.1002/art.40191).
As with the MLSP, the prevalence was highest in black women (458.1), followed by Hispanic women (177.9), Asian women (149.7) and white women (109.8). The incidence was 30.5 per 100,000 person-years in black women, 8.9 in Hispanic women, 7.2 in Asian women, and 5.3 in white women.
Looking at the clinical manifestations of disease, researchers found hematologic was the most common, affecting 84% of patients, while neurologic disorder affected only 8% of the population. Immunologic manifestations were present in 80%, arthritis in 57%, renal disorders in 45%, pleuritis or pericarditis in 41%, and malar rash in 33%.
There were racial variations in manifestations, with renal manifestations being more common in black, Asian/Pacific Islander, and Hispanic patients, compared with whites. Discoid rash was most common among black patients, while it was not evident at all in Hispanic patients.
Maria Dall’Era, MD, of the Russell/Engleman Research Center at the University of California, San Francisco, and her coauthors said that given the changing demographic of the diverse San Francisco County, a reliable estimate of the burden of lupus in different racial and ethnic groups was essential for health care planning.
“Up until the recent completion of the Georgia and Michigan surveillance projects, most previous epidemiologic studies were limited by small geographic areas, homogeneous populations, varying case definitions, and incomplete case ascertainment that relied on administrative codes or patient self-reported diagnosis,” they wrote.
To overcome the previous lack of data in Asian and Hispanic populations, the authors worked with physicians serving these populations and performed extensive case-finding in hospitals and health care clinics.
“Our approach of partnering with the community and engaging culturally and linguistically concordant community members led to successful case ascertainment of these traditionally understudied populations,” they wrote. “Had we not taken these extra steps, we would have missed SLE cases in the Asian and Hispanic populations.”
Both studies noted that racial and ethnic data were determined from medical records, and may not have accurately reflected the patient’s race or ethnicity.
The Manhattan study was supported by the Centers for Disease Control and Prevention and the New York City Department of Health and Mental Hygiene. The San Francisco study was supported by the Centers for Disease Control and Prevention and the Russell/Engleman Rheumatology Research Center at UCSF. No conflict of interest disclosures were available.
Together, the five national lupus registries funded by the Centers for Disease Control and Prevention provide important information about lupus in populations totaling over 6.5 million from four states and three selected Indian Health Service regions, including mixed urban and rural areas. Studies of this magnitude hold great value because case definitions and case ascertainment methodologies have been standardized, and they incorporate capture-recapture analyses to estimate the completeness of case finding.
Susan Manzi, MD, is with the department of medicine at the Allegheny Health Network, Pittsburgh, and Joan Merrill, MD, is with the department of arthritis and clinical immunology at the Oklahoma Medical Research Foundation, Oklahoma City. These comments are taken from an accompanying editorial (Arthritis Rheumatol. 2017 Sep 11. doi: 10.1002/art.40190). No conflicts of interest were available.
Together, the five national lupus registries funded by the Centers for Disease Control and Prevention provide important information about lupus in populations totaling over 6.5 million from four states and three selected Indian Health Service regions, including mixed urban and rural areas. Studies of this magnitude hold great value because case definitions and case ascertainment methodologies have been standardized, and they incorporate capture-recapture analyses to estimate the completeness of case finding.
Susan Manzi, MD, is with the department of medicine at the Allegheny Health Network, Pittsburgh, and Joan Merrill, MD, is with the department of arthritis and clinical immunology at the Oklahoma Medical Research Foundation, Oklahoma City. These comments are taken from an accompanying editorial (Arthritis Rheumatol. 2017 Sep 11. doi: 10.1002/art.40190). No conflicts of interest were available.
Together, the five national lupus registries funded by the Centers for Disease Control and Prevention provide important information about lupus in populations totaling over 6.5 million from four states and three selected Indian Health Service regions, including mixed urban and rural areas. Studies of this magnitude hold great value because case definitions and case ascertainment methodologies have been standardized, and they incorporate capture-recapture analyses to estimate the completeness of case finding.
Susan Manzi, MD, is with the department of medicine at the Allegheny Health Network, Pittsburgh, and Joan Merrill, MD, is with the department of arthritis and clinical immunology at the Oklahoma Medical Research Foundation, Oklahoma City. These comments are taken from an accompanying editorial (Arthritis Rheumatol. 2017 Sep 11. doi: 10.1002/art.40190). No conflicts of interest were available.
Two new studies representing the latest efforts to determine the incidence and prevalence of systemic lupus erythematosus in the United States have revealed the difficulty of ascertaining cases when definitions of the disease vary.
The two population-based registries on which the studies were based – the California Lupus Surveillance Project and the Manhattan Lupus Surveillance Program (MLSP) – confirmed that black women represent the highest risk group for systemic lupus erythematosus (SLE) and also reaffirmed the elevated risk observed in Hispanic and Asian women, compared with white women.
The MLSP researchers applied the updated 1997 American College of Rheumatology (ACR) classification criteria, the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria, or a treating rheumatologist’s diagnosis to records from hospitals, rheumatologists, and administrative databases, and looked at both the prevalence of the disease in 2007 and the incidence from the period of 2007-2009. Using the ACR’s definition of SLE, they found an age-standardized prevalence of 62.2 and an incidence rate of 4.6 per 100,000 person-years (Arthritis Rheumatol. 2017 Sep 11. doi: 10.1002/art.40192).
The prevalence was significantly higher in women than in men (107.4 vs. 12.5 per 100,000 persons), and was highest overall in non-Hispanic black women (210.9), followed by Hispanic women (138.3), non-Hispanic Asian women (91.2), and non-Hispanic white women (64.3).
However, use of the SLICC classification criteria increased the age-standardized prevalence by 17%-19%, to a prevalence of 73.8 per 100,000 person-years and resulted in a 35% increase in incidence, to 6.2 per 100,000 person-years, compared with the ACR rates.
“The small number of cases that met the ACR but not the SLICC case definition is reassuring as it suggests that few cases met ACR criteria for SLE without the presence of autoantibodies,” wrote Peter M. Izmirly, MD, of New York (N.Y.) University, and his coauthors.
“However, given the descriptive nature of the MLSP and the absence of a gold standard test that would unambiguously identify SLE, this project cannot assess which set of classification criteria is more sensitive or specific.”
Meanwhile, the California Lupus Surveillance Project, a registry of people with lupus living in San Francisco County between 2007 and 2009, used the ACR definition to record an age-standardized annual incidence rate of 4.6 per 100,000 person-years, and an average annual prevalence of 84.8 per 100,000 persons (Arthritis Rheumatol. 2017 Sep 11. doi: 10.1002/art.40191).
As with the MLSP, the prevalence was highest in black women (458.1), followed by Hispanic women (177.9), Asian women (149.7) and white women (109.8). The incidence was 30.5 per 100,000 person-years in black women, 8.9 in Hispanic women, 7.2 in Asian women, and 5.3 in white women.
Looking at the clinical manifestations of disease, researchers found hematologic was the most common, affecting 84% of patients, while neurologic disorder affected only 8% of the population. Immunologic manifestations were present in 80%, arthritis in 57%, renal disorders in 45%, pleuritis or pericarditis in 41%, and malar rash in 33%.
There were racial variations in manifestations, with renal manifestations being more common in black, Asian/Pacific Islander, and Hispanic patients, compared with whites. Discoid rash was most common among black patients, while it was not evident at all in Hispanic patients.
Maria Dall’Era, MD, of the Russell/Engleman Research Center at the University of California, San Francisco, and her coauthors said that given the changing demographic of the diverse San Francisco County, a reliable estimate of the burden of lupus in different racial and ethnic groups was essential for health care planning.
“Up until the recent completion of the Georgia and Michigan surveillance projects, most previous epidemiologic studies were limited by small geographic areas, homogeneous populations, varying case definitions, and incomplete case ascertainment that relied on administrative codes or patient self-reported diagnosis,” they wrote.
To overcome the previous lack of data in Asian and Hispanic populations, the authors worked with physicians serving these populations and performed extensive case-finding in hospitals and health care clinics.
“Our approach of partnering with the community and engaging culturally and linguistically concordant community members led to successful case ascertainment of these traditionally understudied populations,” they wrote. “Had we not taken these extra steps, we would have missed SLE cases in the Asian and Hispanic populations.”
Both studies noted that racial and ethnic data were determined from medical records, and may not have accurately reflected the patient’s race or ethnicity.
The Manhattan study was supported by the Centers for Disease Control and Prevention and the New York City Department of Health and Mental Hygiene. The San Francisco study was supported by the Centers for Disease Control and Prevention and the Russell/Engleman Rheumatology Research Center at UCSF. No conflict of interest disclosures were available.
Two new studies representing the latest efforts to determine the incidence and prevalence of systemic lupus erythematosus in the United States have revealed the difficulty of ascertaining cases when definitions of the disease vary.
The two population-based registries on which the studies were based – the California Lupus Surveillance Project and the Manhattan Lupus Surveillance Program (MLSP) – confirmed that black women represent the highest risk group for systemic lupus erythematosus (SLE) and also reaffirmed the elevated risk observed in Hispanic and Asian women, compared with white women.
The MLSP researchers applied the updated 1997 American College of Rheumatology (ACR) classification criteria, the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria, or a treating rheumatologist’s diagnosis to records from hospitals, rheumatologists, and administrative databases, and looked at both the prevalence of the disease in 2007 and the incidence from the period of 2007-2009. Using the ACR’s definition of SLE, they found an age-standardized prevalence of 62.2 and an incidence rate of 4.6 per 100,000 person-years (Arthritis Rheumatol. 2017 Sep 11. doi: 10.1002/art.40192).
The prevalence was significantly higher in women than in men (107.4 vs. 12.5 per 100,000 persons), and was highest overall in non-Hispanic black women (210.9), followed by Hispanic women (138.3), non-Hispanic Asian women (91.2), and non-Hispanic white women (64.3).
However, use of the SLICC classification criteria increased the age-standardized prevalence by 17%-19%, to a prevalence of 73.8 per 100,000 person-years and resulted in a 35% increase in incidence, to 6.2 per 100,000 person-years, compared with the ACR rates.
“The small number of cases that met the ACR but not the SLICC case definition is reassuring as it suggests that few cases met ACR criteria for SLE without the presence of autoantibodies,” wrote Peter M. Izmirly, MD, of New York (N.Y.) University, and his coauthors.
“However, given the descriptive nature of the MLSP and the absence of a gold standard test that would unambiguously identify SLE, this project cannot assess which set of classification criteria is more sensitive or specific.”
Meanwhile, the California Lupus Surveillance Project, a registry of people with lupus living in San Francisco County between 2007 and 2009, used the ACR definition to record an age-standardized annual incidence rate of 4.6 per 100,000 person-years, and an average annual prevalence of 84.8 per 100,000 persons (Arthritis Rheumatol. 2017 Sep 11. doi: 10.1002/art.40191).
As with the MLSP, the prevalence was highest in black women (458.1), followed by Hispanic women (177.9), Asian women (149.7) and white women (109.8). The incidence was 30.5 per 100,000 person-years in black women, 8.9 in Hispanic women, 7.2 in Asian women, and 5.3 in white women.
Looking at the clinical manifestations of disease, researchers found hematologic was the most common, affecting 84% of patients, while neurologic disorder affected only 8% of the population. Immunologic manifestations were present in 80%, arthritis in 57%, renal disorders in 45%, pleuritis or pericarditis in 41%, and malar rash in 33%.
There were racial variations in manifestations, with renal manifestations being more common in black, Asian/Pacific Islander, and Hispanic patients, compared with whites. Discoid rash was most common among black patients, while it was not evident at all in Hispanic patients.
Maria Dall’Era, MD, of the Russell/Engleman Research Center at the University of California, San Francisco, and her coauthors said that given the changing demographic of the diverse San Francisco County, a reliable estimate of the burden of lupus in different racial and ethnic groups was essential for health care planning.
“Up until the recent completion of the Georgia and Michigan surveillance projects, most previous epidemiologic studies were limited by small geographic areas, homogeneous populations, varying case definitions, and incomplete case ascertainment that relied on administrative codes or patient self-reported diagnosis,” they wrote.
To overcome the previous lack of data in Asian and Hispanic populations, the authors worked with physicians serving these populations and performed extensive case-finding in hospitals and health care clinics.
“Our approach of partnering with the community and engaging culturally and linguistically concordant community members led to successful case ascertainment of these traditionally understudied populations,” they wrote. “Had we not taken these extra steps, we would have missed SLE cases in the Asian and Hispanic populations.”
Both studies noted that racial and ethnic data were determined from medical records, and may not have accurately reflected the patient’s race or ethnicity.
The Manhattan study was supported by the Centers for Disease Control and Prevention and the New York City Department of Health and Mental Hygiene. The San Francisco study was supported by the Centers for Disease Control and Prevention and the Russell/Engleman Rheumatology Research Center at UCSF. No conflict of interest disclosures were available.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Black women have the highest prevalence and incidence of systemic lupus erythematosus.
Major finding: Use of the SLICC classification criteria increased the age-standardized prevalence by 17%-19% and resulted in a 35% increase in incidence, compared with the ACR criteria rates.
Data source: The Manhattan and California Lupus Surveillance Projects population-based registries.
Disclosures: The Manhattan study was supported by the Centers for Disease Control and Prevention and the New York City Department of Health and Mental Hygiene. The San Francisco study was supported by the Centers for Disease Control and Prevention and the Russell/Engleman Rheumatology Research Center at UCSF. No conflict of interest disclosures were available.
Metabolically healthy obese still at elevated cardiovascular risk
Obese individuals with no metabolic abnormalities, such as dyslipidemia, high blood pressure, or high blood sugar levels, still have a higher risk of cardiovascular disease than do metabolically healthy people of normal weight, new data suggests.
“Our study robustly challenges the assertion that MHO [metabolically healthy obese] is a benign condition and adds to the evidence base that MHO is a high-risk state for future CVD events,” wrote Rishi Caleyachetty, MD, of the University of Birmingham, England, and his coauthors online (J Am Coll Cardiol. 2017, Sep 11. doi. 10.1016/j.jacc.2017.07.763).
Dr. Caleyachetty and his associates reported findings from a population-based study using the electronic health records of nearly 3.5 million individuals aged 18 years or older who were free of cardiovascular disease at baseline.
Overall, 15% of the population were classified as being metabolically healthy obese, meaning that they had a body mass index (BMI) of at least 30 kg/m2 with no sign of diabetes, hypertension, or hyperlipidemia, and 26% were overweight with no metabolic abnormalities. Despite their lack of metabolic disease, these obese individuals still had a significant 49% higher risk of coronary heart disease, 7% higher risk of cerebrovascular disease, and 96% higher risk of heart failure, compared with normal-weight individuals with no metabolic disease, after researchers adjusted for age, sex, smoking status, and social deprivation.
Individuals who were overweight but metabolically healthy had a 30% increased risk of ischemic heart disease, 11% increased risk of heart failure, and the same risk of cerebrovascular disease as normal-weight, healthy individuals.
They also saw an increasing risk of ischemic heart disease, cerebrovascular disease, heart failure, and peripheral vascular disease with each additional metabolic abnormality, even among underweight and normal-weight individuals, and suggested that a focus on screening overweight and obese individuals only could miss metabolic abnormalities in many patients.
Overweight and obese individuals without metabolic disease had a significantly lower risk of peripheral vascular disease, compared with healthy normal-weight individuals. The authors said this was a surprising finding but suggested cigarette smoking could be a confounding factor, as this is associated with both peripheral vascular disease and lower BMI.
“In sensitivity analyses restricted to individuals who were obese with no metabolic abnormalities and reported never smoking cigarettes, risk for PVD [peripheral vascular disease] was increased, compared [with] normal-weight individuals with no metabolic abnormalities,” Dr. Caleyachetty and his coinvestigators wrote.
Over the mean follow-up of 5.4 years, 5.6% of initially metabolically healthy obese individuals developed diabetes, 11.5% developed hyperlipidemia and 10.5% developed hypertension. In contrast, among the metabolically healthy overweight individuals at baseline, 1.9% developed diabetes, 9.4% developed hyperlipidemia, and 7.2% developed hypertension.
While the analysis adjusted for sex, the authors did note that women who were overweight or obese but metabolically healthy had stronger positive associations than did males with cerebrovascular disease and heart failure.
“Clinicians need to be aware that individuals who would otherwise be considered nonobese, based on a normal BMI, can have metabolic abnormalities, and therefore also be at high risk for CVD events,” the investigators concluded.
No conflicts of interest were declared.
Recently, studies have consistently placed metabolically healthy obese individuals between metabolically healthy lean and metabolically unhealthy obese individuals in terms of cardiovascular disease risk, occult cardiac dysfunction, and type 2 diabetes. Thus, either metabolic dysfunction or elevated body mass index appears to increases CVD risk factors.
Often, one or two metabolic risk factors in normal weight individuals are dismissed as unimportant because they are of healthy weight; however, these data suggest that the normal-weight group is at similar risk, compared with overweight, and at times, obese individuals, when metabolic abnormalities are present. The study not only definitively counters the concept of metabolically benign obesity but also demonstrates great risk to normal weight individuals if metabolic dysfunction is present. Thus, we would suggest an increased need for screening in the normal-weight population.
Jennifer W. Bea, PhD, is from the Collaboratory for Metabolic Disease Prevention and Treatment in Tucson, Ariz., and Nancy K. Sweitzer, MD, is chief of the division of cardiology at the Sarver Heart Center. These comments are taken from an accompanying editorial (J Am Coll Cardiol. 2017 Sep 19;70:1438-40. doi. org/10.1016/j.jacc.2017.07.742). No conflicts of interest were declared.
Recently, studies have consistently placed metabolically healthy obese individuals between metabolically healthy lean and metabolically unhealthy obese individuals in terms of cardiovascular disease risk, occult cardiac dysfunction, and type 2 diabetes. Thus, either metabolic dysfunction or elevated body mass index appears to increases CVD risk factors.
Often, one or two metabolic risk factors in normal weight individuals are dismissed as unimportant because they are of healthy weight; however, these data suggest that the normal-weight group is at similar risk, compared with overweight, and at times, obese individuals, when metabolic abnormalities are present. The study not only definitively counters the concept of metabolically benign obesity but also demonstrates great risk to normal weight individuals if metabolic dysfunction is present. Thus, we would suggest an increased need for screening in the normal-weight population.
Jennifer W. Bea, PhD, is from the Collaboratory for Metabolic Disease Prevention and Treatment in Tucson, Ariz., and Nancy K. Sweitzer, MD, is chief of the division of cardiology at the Sarver Heart Center. These comments are taken from an accompanying editorial (J Am Coll Cardiol. 2017 Sep 19;70:1438-40. doi. org/10.1016/j.jacc.2017.07.742). No conflicts of interest were declared.
Recently, studies have consistently placed metabolically healthy obese individuals between metabolically healthy lean and metabolically unhealthy obese individuals in terms of cardiovascular disease risk, occult cardiac dysfunction, and type 2 diabetes. Thus, either metabolic dysfunction or elevated body mass index appears to increases CVD risk factors.
Often, one or two metabolic risk factors in normal weight individuals are dismissed as unimportant because they are of healthy weight; however, these data suggest that the normal-weight group is at similar risk, compared with overweight, and at times, obese individuals, when metabolic abnormalities are present. The study not only definitively counters the concept of metabolically benign obesity but also demonstrates great risk to normal weight individuals if metabolic dysfunction is present. Thus, we would suggest an increased need for screening in the normal-weight population.
Jennifer W. Bea, PhD, is from the Collaboratory for Metabolic Disease Prevention and Treatment in Tucson, Ariz., and Nancy K. Sweitzer, MD, is chief of the division of cardiology at the Sarver Heart Center. These comments are taken from an accompanying editorial (J Am Coll Cardiol. 2017 Sep 19;70:1438-40. doi. org/10.1016/j.jacc.2017.07.742). No conflicts of interest were declared.
Obese individuals with no metabolic abnormalities, such as dyslipidemia, high blood pressure, or high blood sugar levels, still have a higher risk of cardiovascular disease than do metabolically healthy people of normal weight, new data suggests.
“Our study robustly challenges the assertion that MHO [metabolically healthy obese] is a benign condition and adds to the evidence base that MHO is a high-risk state for future CVD events,” wrote Rishi Caleyachetty, MD, of the University of Birmingham, England, and his coauthors online (J Am Coll Cardiol. 2017, Sep 11. doi. 10.1016/j.jacc.2017.07.763).
Dr. Caleyachetty and his associates reported findings from a population-based study using the electronic health records of nearly 3.5 million individuals aged 18 years or older who were free of cardiovascular disease at baseline.
Overall, 15% of the population were classified as being metabolically healthy obese, meaning that they had a body mass index (BMI) of at least 30 kg/m2 with no sign of diabetes, hypertension, or hyperlipidemia, and 26% were overweight with no metabolic abnormalities. Despite their lack of metabolic disease, these obese individuals still had a significant 49% higher risk of coronary heart disease, 7% higher risk of cerebrovascular disease, and 96% higher risk of heart failure, compared with normal-weight individuals with no metabolic disease, after researchers adjusted for age, sex, smoking status, and social deprivation.
Individuals who were overweight but metabolically healthy had a 30% increased risk of ischemic heart disease, 11% increased risk of heart failure, and the same risk of cerebrovascular disease as normal-weight, healthy individuals.
They also saw an increasing risk of ischemic heart disease, cerebrovascular disease, heart failure, and peripheral vascular disease with each additional metabolic abnormality, even among underweight and normal-weight individuals, and suggested that a focus on screening overweight and obese individuals only could miss metabolic abnormalities in many patients.
Overweight and obese individuals without metabolic disease had a significantly lower risk of peripheral vascular disease, compared with healthy normal-weight individuals. The authors said this was a surprising finding but suggested cigarette smoking could be a confounding factor, as this is associated with both peripheral vascular disease and lower BMI.
“In sensitivity analyses restricted to individuals who were obese with no metabolic abnormalities and reported never smoking cigarettes, risk for PVD [peripheral vascular disease] was increased, compared [with] normal-weight individuals with no metabolic abnormalities,” Dr. Caleyachetty and his coinvestigators wrote.
Over the mean follow-up of 5.4 years, 5.6% of initially metabolically healthy obese individuals developed diabetes, 11.5% developed hyperlipidemia and 10.5% developed hypertension. In contrast, among the metabolically healthy overweight individuals at baseline, 1.9% developed diabetes, 9.4% developed hyperlipidemia, and 7.2% developed hypertension.
While the analysis adjusted for sex, the authors did note that women who were overweight or obese but metabolically healthy had stronger positive associations than did males with cerebrovascular disease and heart failure.
“Clinicians need to be aware that individuals who would otherwise be considered nonobese, based on a normal BMI, can have metabolic abnormalities, and therefore also be at high risk for CVD events,” the investigators concluded.
No conflicts of interest were declared.
Obese individuals with no metabolic abnormalities, such as dyslipidemia, high blood pressure, or high blood sugar levels, still have a higher risk of cardiovascular disease than do metabolically healthy people of normal weight, new data suggests.
“Our study robustly challenges the assertion that MHO [metabolically healthy obese] is a benign condition and adds to the evidence base that MHO is a high-risk state for future CVD events,” wrote Rishi Caleyachetty, MD, of the University of Birmingham, England, and his coauthors online (J Am Coll Cardiol. 2017, Sep 11. doi. 10.1016/j.jacc.2017.07.763).
Dr. Caleyachetty and his associates reported findings from a population-based study using the electronic health records of nearly 3.5 million individuals aged 18 years or older who were free of cardiovascular disease at baseline.
Overall, 15% of the population were classified as being metabolically healthy obese, meaning that they had a body mass index (BMI) of at least 30 kg/m2 with no sign of diabetes, hypertension, or hyperlipidemia, and 26% were overweight with no metabolic abnormalities. Despite their lack of metabolic disease, these obese individuals still had a significant 49% higher risk of coronary heart disease, 7% higher risk of cerebrovascular disease, and 96% higher risk of heart failure, compared with normal-weight individuals with no metabolic disease, after researchers adjusted for age, sex, smoking status, and social deprivation.
Individuals who were overweight but metabolically healthy had a 30% increased risk of ischemic heart disease, 11% increased risk of heart failure, and the same risk of cerebrovascular disease as normal-weight, healthy individuals.
They also saw an increasing risk of ischemic heart disease, cerebrovascular disease, heart failure, and peripheral vascular disease with each additional metabolic abnormality, even among underweight and normal-weight individuals, and suggested that a focus on screening overweight and obese individuals only could miss metabolic abnormalities in many patients.
Overweight and obese individuals without metabolic disease had a significantly lower risk of peripheral vascular disease, compared with healthy normal-weight individuals. The authors said this was a surprising finding but suggested cigarette smoking could be a confounding factor, as this is associated with both peripheral vascular disease and lower BMI.
“In sensitivity analyses restricted to individuals who were obese with no metabolic abnormalities and reported never smoking cigarettes, risk for PVD [peripheral vascular disease] was increased, compared [with] normal-weight individuals with no metabolic abnormalities,” Dr. Caleyachetty and his coinvestigators wrote.
Over the mean follow-up of 5.4 years, 5.6% of initially metabolically healthy obese individuals developed diabetes, 11.5% developed hyperlipidemia and 10.5% developed hypertension. In contrast, among the metabolically healthy overweight individuals at baseline, 1.9% developed diabetes, 9.4% developed hyperlipidemia, and 7.2% developed hypertension.
While the analysis adjusted for sex, the authors did note that women who were overweight or obese but metabolically healthy had stronger positive associations than did males with cerebrovascular disease and heart failure.
“Clinicians need to be aware that individuals who would otherwise be considered nonobese, based on a normal BMI, can have metabolic abnormalities, and therefore also be at high risk for CVD events,” the investigators concluded.
No conflicts of interest were declared.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Obese individuals without any sign of metabolic disease, such as hypertension or dyslipidemia, still have a greater risk of cardiovascular disease than do normal weight individuals.
Major finding: Metabolically healthy obese individuals have a 49% higher risk of coronary heart disease and 96% higher risk of heart failure than metabolically healthy normal weight individuals.
Data source: Population-based electronic health record study of 3.5 million people.
Disclosures: No conflicts of interest were declared.
Prenatal antidepressant use linked to psychiatric illness in offspring
Antidepressant use before and during pregnancy may be associated with an increased risk of psychiatric disorders in offspring, according to the results of a population-based cohort study published Sept. 7 in the BMJ.
There have been contradictory findings in the literature about whether in utero exposure to SSRIs is associated with autism spectrum disorder and ADHD. “However, these studies did not investigate the overall risk of psychiatric disorders, which is important because differentiating between overlapping symptoms and diagnosing specific disorders are challenging in children and adolescents,” Xiaoqin Liu, MD, PhD, of Aarhus University in Denmark, and her coauthors wrote (BMJ 2017;358:j3668. doi: 10.1136/bmj.j3668).
Participants were categorized into four groups based on maternal use of antidepressants; unexposed, antidepressant discontinuation (if the mother had used in the 2 years before but not during pregnancy), antidepressant continuation (if the use happened in the 2 years before pregnancy and during it), and new users (if antidepressant use happened only during pregnancy).
The study found that children whose mothers used antidepressants both in the 2 years before pregnancy and during pregnancy had a 27% higher incidence of any psychiatric disorder, compared with children whose mothers had used antidepressants but discontinued them before becoming pregnant (95% confidence interval, 1.17-1.38).
This figure was adjusted for factors such as maternal age and psychiatric history at delivery, psychiatric treatment in the 2 years before pregnancy, other psychotropic medications used during pregnancy, and paternal psychiatric history at the time of delivery.
Any maternal antidepressant use was associated with an increased risk of psychiatric disorders in the offspring, compared with the unexposed group. The 15-year cumulative incidence of psychiatric disorders in offspring was 8% in the unexposed group, 11.5% in the discontinuation group, 13.6% in the continuation group, and 14.5% in the new user group.
There were no differences in risk between children exposed to SSRI monotherapy and those exposed to non-SSRI monotherapy, although the statistical precision for the latter was low, the researchers noted. However, they did see a lower risk of psychiatric disorder in children who were exposed only during the first trimester, compared with those exposed in the second or third trimesters.
The researchers suggested that the association between in utero exposure and the risk of psychiatric disorders in offspring may be the result of a combination of underlying maternal disorders and in utero antidepressant exposure. “We speculated that this increased risk could be due to the severity of underlying maternal psychiatric disorders because mothers with severe symptoms are more likely to continue treatment during pregnancy,” they wrote.
The researchers cautioned that discontinuation of treatment could lead to psychiatric episodes that could have long-lasting effects on both mother and child.
The investigators reported support from several research foundations, as well as institutional grants from Sage Therapeutics and Janssen. No conflicts of interest were declared.
Only the most severely sick women have drugs prescribed during pregnancy. Consequently, confounding by indication is a major challenge in pharmacoepidemiological studies. Including a disease comparison group with women discontinuing antidepressants before pregnancy, as in Liu and her colleagues’ study, offers an important advantage over studies that use only healthy comparison groups because it allows researchers to disentangle the effect of antidepressants from the underlying maternal psychiatric disease.
It is important that researchers report absolute risks to facilitate communication between clinicians and pregnant women. For example, if prenatal exposure to antidepressants is associated with a 23% increased risk of autism in children, and if we assume a baseline prevalence of autism of 1%, then for every 10,000 women who continue treatment during pregnancy, 23 additional cases of autism would occur. This number may be alarming to some patients and reassuring to others.
Hedvig Nordeng, PhD, Angela Lupattelli, PhD, and Mollie Wood, PhD, are from the University of Oslo. These comments are adapted from an accompanying editorial (BMJ 2017;358:j3950 doi: 10.1136/bmj.j3950). No conflicts of interest were declared.
Only the most severely sick women have drugs prescribed during pregnancy. Consequently, confounding by indication is a major challenge in pharmacoepidemiological studies. Including a disease comparison group with women discontinuing antidepressants before pregnancy, as in Liu and her colleagues’ study, offers an important advantage over studies that use only healthy comparison groups because it allows researchers to disentangle the effect of antidepressants from the underlying maternal psychiatric disease.
It is important that researchers report absolute risks to facilitate communication between clinicians and pregnant women. For example, if prenatal exposure to antidepressants is associated with a 23% increased risk of autism in children, and if we assume a baseline prevalence of autism of 1%, then for every 10,000 women who continue treatment during pregnancy, 23 additional cases of autism would occur. This number may be alarming to some patients and reassuring to others.
Hedvig Nordeng, PhD, Angela Lupattelli, PhD, and Mollie Wood, PhD, are from the University of Oslo. These comments are adapted from an accompanying editorial (BMJ 2017;358:j3950 doi: 10.1136/bmj.j3950). No conflicts of interest were declared.
Only the most severely sick women have drugs prescribed during pregnancy. Consequently, confounding by indication is a major challenge in pharmacoepidemiological studies. Including a disease comparison group with women discontinuing antidepressants before pregnancy, as in Liu and her colleagues’ study, offers an important advantage over studies that use only healthy comparison groups because it allows researchers to disentangle the effect of antidepressants from the underlying maternal psychiatric disease.
It is important that researchers report absolute risks to facilitate communication between clinicians and pregnant women. For example, if prenatal exposure to antidepressants is associated with a 23% increased risk of autism in children, and if we assume a baseline prevalence of autism of 1%, then for every 10,000 women who continue treatment during pregnancy, 23 additional cases of autism would occur. This number may be alarming to some patients and reassuring to others.
Hedvig Nordeng, PhD, Angela Lupattelli, PhD, and Mollie Wood, PhD, are from the University of Oslo. These comments are adapted from an accompanying editorial (BMJ 2017;358:j3950 doi: 10.1136/bmj.j3950). No conflicts of interest were declared.
Antidepressant use before and during pregnancy may be associated with an increased risk of psychiatric disorders in offspring, according to the results of a population-based cohort study published Sept. 7 in the BMJ.
There have been contradictory findings in the literature about whether in utero exposure to SSRIs is associated with autism spectrum disorder and ADHD. “However, these studies did not investigate the overall risk of psychiatric disorders, which is important because differentiating between overlapping symptoms and diagnosing specific disorders are challenging in children and adolescents,” Xiaoqin Liu, MD, PhD, of Aarhus University in Denmark, and her coauthors wrote (BMJ 2017;358:j3668. doi: 10.1136/bmj.j3668).
Participants were categorized into four groups based on maternal use of antidepressants; unexposed, antidepressant discontinuation (if the mother had used in the 2 years before but not during pregnancy), antidepressant continuation (if the use happened in the 2 years before pregnancy and during it), and new users (if antidepressant use happened only during pregnancy).
The study found that children whose mothers used antidepressants both in the 2 years before pregnancy and during pregnancy had a 27% higher incidence of any psychiatric disorder, compared with children whose mothers had used antidepressants but discontinued them before becoming pregnant (95% confidence interval, 1.17-1.38).
This figure was adjusted for factors such as maternal age and psychiatric history at delivery, psychiatric treatment in the 2 years before pregnancy, other psychotropic medications used during pregnancy, and paternal psychiatric history at the time of delivery.
Any maternal antidepressant use was associated with an increased risk of psychiatric disorders in the offspring, compared with the unexposed group. The 15-year cumulative incidence of psychiatric disorders in offspring was 8% in the unexposed group, 11.5% in the discontinuation group, 13.6% in the continuation group, and 14.5% in the new user group.
There were no differences in risk between children exposed to SSRI monotherapy and those exposed to non-SSRI monotherapy, although the statistical precision for the latter was low, the researchers noted. However, they did see a lower risk of psychiatric disorder in children who were exposed only during the first trimester, compared with those exposed in the second or third trimesters.
The researchers suggested that the association between in utero exposure and the risk of psychiatric disorders in offspring may be the result of a combination of underlying maternal disorders and in utero antidepressant exposure. “We speculated that this increased risk could be due to the severity of underlying maternal psychiatric disorders because mothers with severe symptoms are more likely to continue treatment during pregnancy,” they wrote.
The researchers cautioned that discontinuation of treatment could lead to psychiatric episodes that could have long-lasting effects on both mother and child.
The investigators reported support from several research foundations, as well as institutional grants from Sage Therapeutics and Janssen. No conflicts of interest were declared.
Antidepressant use before and during pregnancy may be associated with an increased risk of psychiatric disorders in offspring, according to the results of a population-based cohort study published Sept. 7 in the BMJ.
There have been contradictory findings in the literature about whether in utero exposure to SSRIs is associated with autism spectrum disorder and ADHD. “However, these studies did not investigate the overall risk of psychiatric disorders, which is important because differentiating between overlapping symptoms and diagnosing specific disorders are challenging in children and adolescents,” Xiaoqin Liu, MD, PhD, of Aarhus University in Denmark, and her coauthors wrote (BMJ 2017;358:j3668. doi: 10.1136/bmj.j3668).
Participants were categorized into four groups based on maternal use of antidepressants; unexposed, antidepressant discontinuation (if the mother had used in the 2 years before but not during pregnancy), antidepressant continuation (if the use happened in the 2 years before pregnancy and during it), and new users (if antidepressant use happened only during pregnancy).
The study found that children whose mothers used antidepressants both in the 2 years before pregnancy and during pregnancy had a 27% higher incidence of any psychiatric disorder, compared with children whose mothers had used antidepressants but discontinued them before becoming pregnant (95% confidence interval, 1.17-1.38).
This figure was adjusted for factors such as maternal age and psychiatric history at delivery, psychiatric treatment in the 2 years before pregnancy, other psychotropic medications used during pregnancy, and paternal psychiatric history at the time of delivery.
Any maternal antidepressant use was associated with an increased risk of psychiatric disorders in the offspring, compared with the unexposed group. The 15-year cumulative incidence of psychiatric disorders in offspring was 8% in the unexposed group, 11.5% in the discontinuation group, 13.6% in the continuation group, and 14.5% in the new user group.
There were no differences in risk between children exposed to SSRI monotherapy and those exposed to non-SSRI monotherapy, although the statistical precision for the latter was low, the researchers noted. However, they did see a lower risk of psychiatric disorder in children who were exposed only during the first trimester, compared with those exposed in the second or third trimesters.
The researchers suggested that the association between in utero exposure and the risk of psychiatric disorders in offspring may be the result of a combination of underlying maternal disorders and in utero antidepressant exposure. “We speculated that this increased risk could be due to the severity of underlying maternal psychiatric disorders because mothers with severe symptoms are more likely to continue treatment during pregnancy,” they wrote.
The researchers cautioned that discontinuation of treatment could lead to psychiatric episodes that could have long-lasting effects on both mother and child.
The investigators reported support from several research foundations, as well as institutional grants from Sage Therapeutics and Janssen. No conflicts of interest were declared.
FROM THE BMJ
Key clinical point:
Major finding: Children whose mothers took antidepressants both before and during pregnancy are 27% more likely to develop psychiatric illness than are those whose mothers stopped taking antidepressants before pregnancy.
Data source: A population-based cohort study in 905,383 liveborn singletons.
Disclosures: The investigators reported support from several research foundations, as well as institutional grants from Sage Therapeutics and Janssen. No conflicts of interest were declared.
Annual mammograms from age 40 linked with greatest reductions in mortality
Annual mammograms starting at age 40 and continuing until age 84 will achieve the greatest reductions in breast cancer deaths, according to a modeling study published in Cancer.
Researchers used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models to compare three current mammography-based screening approaches for women at average risk of breast cancer. The first approach is for annual screening from ages 40-84 years, the second is a hybrid approach with annual screening from ages 45 to 54 then biennial screening from ages 55 to 79, and the third approach involves biennial mammograms from ages 50 to 74.
The modeling suggested that the largest mean reduction in mortality – 36.9% – was associated with annual screening starting at age 40. The second largest reduction of 30.8% occurred with the hybrid screening approach starting at age 45, while the smallest reduction in mortality – 23.2% – occurred with biennial screening starting at age 50 (Cancer 2017 Aug 21. doi: 10.1002/cncr.30842).
Annual screening also averted the most deaths from breast cancer – 11.9 per 1,000 women screened – and gained the most life-years (189 per 1,000 women screened, compared with 149 for the hybrid approach and 110 for biennial screening), reported Elizabeth K. Arleo, MD, of Weill Cornell Imaging at New York–Presbyterian, New York, and her coauthors.
However, the annual screening approach was also associated with the largest number of benign recalls and benign biopsies. The model suggested that a woman starting annual screening from age 40 could expect on average to be recalled for a benign diagnostic work-up every 13 years and undergo a benign biopsy every 187 years.
Overall, using a single-year cohort of women born in 1960 who were 100% compliant with screening, the model predicted that 44,671 would still die of breast cancer with annual screening, 51,211 would die under the hybrid screening model, and 56,887 would die under the biennial screening model.
“If the goal is to avert the most breast cancer deaths and gain the most life-years, CISNET modeling shows that the optimal age of initiation for screening mammography is 40 years, the optimal screening frequency is annual, and the optimal stopping age is when a woman’s life expectancy is less than 5-7 years,” the researchers wrote. “Individual women should continue to have the choice to reduce their risk of dying from breast cancer as much as possible, and as CISNET models show, annual mammography starting at age 40 years is the best way to do so.”
Two of the researchers reported consulting or institutional support from GE Healthcare. No other conflicts of interest were reported.
Annual mammograms starting at age 40 and continuing until age 84 will achieve the greatest reductions in breast cancer deaths, according to a modeling study published in Cancer.
Researchers used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models to compare three current mammography-based screening approaches for women at average risk of breast cancer. The first approach is for annual screening from ages 40-84 years, the second is a hybrid approach with annual screening from ages 45 to 54 then biennial screening from ages 55 to 79, and the third approach involves biennial mammograms from ages 50 to 74.
The modeling suggested that the largest mean reduction in mortality – 36.9% – was associated with annual screening starting at age 40. The second largest reduction of 30.8% occurred with the hybrid screening approach starting at age 45, while the smallest reduction in mortality – 23.2% – occurred with biennial screening starting at age 50 (Cancer 2017 Aug 21. doi: 10.1002/cncr.30842).
Annual screening also averted the most deaths from breast cancer – 11.9 per 1,000 women screened – and gained the most life-years (189 per 1,000 women screened, compared with 149 for the hybrid approach and 110 for biennial screening), reported Elizabeth K. Arleo, MD, of Weill Cornell Imaging at New York–Presbyterian, New York, and her coauthors.
However, the annual screening approach was also associated with the largest number of benign recalls and benign biopsies. The model suggested that a woman starting annual screening from age 40 could expect on average to be recalled for a benign diagnostic work-up every 13 years and undergo a benign biopsy every 187 years.
Overall, using a single-year cohort of women born in 1960 who were 100% compliant with screening, the model predicted that 44,671 would still die of breast cancer with annual screening, 51,211 would die under the hybrid screening model, and 56,887 would die under the biennial screening model.
“If the goal is to avert the most breast cancer deaths and gain the most life-years, CISNET modeling shows that the optimal age of initiation for screening mammography is 40 years, the optimal screening frequency is annual, and the optimal stopping age is when a woman’s life expectancy is less than 5-7 years,” the researchers wrote. “Individual women should continue to have the choice to reduce their risk of dying from breast cancer as much as possible, and as CISNET models show, annual mammography starting at age 40 years is the best way to do so.”
Two of the researchers reported consulting or institutional support from GE Healthcare. No other conflicts of interest were reported.
Annual mammograms starting at age 40 and continuing until age 84 will achieve the greatest reductions in breast cancer deaths, according to a modeling study published in Cancer.
Researchers used Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer models to compare three current mammography-based screening approaches for women at average risk of breast cancer. The first approach is for annual screening from ages 40-84 years, the second is a hybrid approach with annual screening from ages 45 to 54 then biennial screening from ages 55 to 79, and the third approach involves biennial mammograms from ages 50 to 74.
The modeling suggested that the largest mean reduction in mortality – 36.9% – was associated with annual screening starting at age 40. The second largest reduction of 30.8% occurred with the hybrid screening approach starting at age 45, while the smallest reduction in mortality – 23.2% – occurred with biennial screening starting at age 50 (Cancer 2017 Aug 21. doi: 10.1002/cncr.30842).
Annual screening also averted the most deaths from breast cancer – 11.9 per 1,000 women screened – and gained the most life-years (189 per 1,000 women screened, compared with 149 for the hybrid approach and 110 for biennial screening), reported Elizabeth K. Arleo, MD, of Weill Cornell Imaging at New York–Presbyterian, New York, and her coauthors.
However, the annual screening approach was also associated with the largest number of benign recalls and benign biopsies. The model suggested that a woman starting annual screening from age 40 could expect on average to be recalled for a benign diagnostic work-up every 13 years and undergo a benign biopsy every 187 years.
Overall, using a single-year cohort of women born in 1960 who were 100% compliant with screening, the model predicted that 44,671 would still die of breast cancer with annual screening, 51,211 would die under the hybrid screening model, and 56,887 would die under the biennial screening model.
“If the goal is to avert the most breast cancer deaths and gain the most life-years, CISNET modeling shows that the optimal age of initiation for screening mammography is 40 years, the optimal screening frequency is annual, and the optimal stopping age is when a woman’s life expectancy is less than 5-7 years,” the researchers wrote. “Individual women should continue to have the choice to reduce their risk of dying from breast cancer as much as possible, and as CISNET models show, annual mammography starting at age 40 years is the best way to do so.”
Two of the researchers reported consulting or institutional support from GE Healthcare. No other conflicts of interest were reported.
FROM CANCER
Key clinical point:
Major finding: Annual mammograms from age 40 are associated with a 36.9% mean reduction in mortality, compared with a 23.2% reduction with biennial screening from age 50.
Data source: A study using Cancer Intervention and Surveillance Modeling Network models.
Disclosures: Two of the researchers reported consulting or institutional support from GE Healthcare. No other conflicts of interest were reported.