User login
Colonoscopy lowers CRC risk and death, but not by much: NordICC
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM UEG 2022
Does COVID-19 cause type 1 diabetes in children? Time will tell
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – It remains inconclusive whether SARS-CoV-2 infection predisposes children and adolescents to a higher risk of type 1 diabetes. Data from two new studies and a recently published research letter add to the growing body of knowledge on the subject, but still can’t draw any definitive conclusions.
The latest results from a Norwegian and a Scottish study both examine incidence of type 1 diabetes in young people with a history of SARS-CoV-2 infection and were reported at the annual meeting of the European Association for the Study of Diabetes.
A 60% increased risk for type 1 diabetes at least 31 days after SARS-CoV-2 infection (adjusted hazard ratio, 1.63) was found in the Norwegian study, while in contrast, the Scottish study only found an increased risk in the first few months of the pandemic, in 2020, but importantly, no association over a much longer time period (March 2020–November 2021).
In a comment on Twitter on the two studies presented at EASD, session moderator Kamlesh Khunti, MD, professor of primary care diabetes and vascular medicine at the University of Leicester, (England), said: “In summary, two studies showing no or weak association of type 1 diabetes with COVID.”
But new data in the research letter published in JAMA Network Open, based on U.S. figures, also found an almost doubling of type 1 diabetes in children in the first few months after COVID-19 infection relative to infection with other respiratory viruses.
Lead author of the Scottish study, Helen Colhoun, PhD, honorary public health consultant at Public Health Scotland, commented: “Data in children are variable year on year, which emphasizes the need to be cautious over taking a tiny snapshot.”
Nevertheless, this is “a hugely important question and we must not drop the ball. [We must] keep looking at it and maintain scientific equipoise. ... [This] reinforces the need to carry on this analysis into the future to obtain an unequivocal picture,” she emphasized.
Norwegian study: If there is an association, the risk is small
German Tapia, PhD, from the Norwegian Institute of Public Health, Oslo, presented the results of a study of SARS-CoV-2 infection and subsequent risk of type 1 diabetes in 1.2 million children in Norway.
Of these, 424,354 children had been infected with SARS-CoV-2, and there were 990 incident cases of type 1 diabetes.
“What we do know about COVID-19 in children is that the symptoms are mild and only a small proportion are hospitalized with more serious symptoms. But we do not know the long-term effects of COVID-19 infection because this requires a longer follow-up period,” remarked Dr. Tapia, adding that other viral infections are thought to be linked to the development of type 1 diabetes, in particular, respiratory infections.
The data were sourced from the Norwegian Emergency Preparedness Register for COVID-19, which gathers daily data updates including infections (positive and negative results for free-of-charge testing), diagnoses (primary and secondary care), vaccinations (also free of charge), prescribed medications, and basic demographics.
“We link these data using the personal identification number that every Norwegian citizen has,” explained Dr. Tapia.
He presented results from two cohorts: firstly, results in children only, including those tested for SARS-CoV-2 infection, and secondly, a full national Norwegian population cohort.
Regarding the first cohort, those under 18 years who tested positive for SARS-CoV-2 infection, from March 2020 to March 2022, had a significantly increased risk of type 1 diabetes at least 31 days after infection, with an adjusted hazard ratio of 1.63 (95% confidence interval, 1.08-2.47; P = .02). Adjustments were made for age, sex, non-Nordic country of origin, geographic area, and socioeconomic factors.
For children who developed type 1 diabetes within 30 days of a SARS-CoV-2 infection, the HR was 1.26 (95% CI, 0.72-2.19; P = .42), which did not reach statistical significance.
“The fact that fewer people developed type 1 diabetes within 30 days is not surprising because we know that type 1 diabetes develops over a long period of time,” Dr. Tapia said.
“For this reason, we would not expect to find new cases of those people who develop type 1 diabetes within 30 days of COVID-19 infection,” he explained. In these cases, “it is most likely that they already had [type 1 diabetes], and the infection probably triggered clinical symptoms, so their type 1 diabetes was discovered.”
Turning to the full population cohort and diagnoses of type 1 diabetes over 30 days after SARS-CoV-2 infection, the Norwegian researchers found an association, with an HR of 1.57 (95% CI, 1.06-2.33; P = .03), while diagnosis of type 1 diabetes at 30 days or less generated a hazard ratio of 1.22 (95% CI, 0.72-2.19; P = .42).
“So very similar results were found, and after adjustment for confounders, results were still similar,” reported Dr. Tapia.
He also conducted a similar analysis with vaccination as an exposure but found no association between vaccination against SARS-CoV-2 and diagnosis of type 1 diabetes.
“From these results, we conclude that this suggests an increase in diagnosis of type 1 diabetes after SARS-CoV-2 infection, but it must be noted that the absolute risk of developing type 1 diabetes after infection in children is low, with most children not developing the disease,” he emphasized. “There are nearly half a million children who have been infected with SARS-CoV-2 in Norway, but only a very small proportion develop type 1 diabetes.”
Scottish study: No association found over longer term
Dr. Colhoun and colleagues looked at the relationship between incident type 1 diabetes and SARS-CoV-2 infection in children in Scotland using e-health record linkage.
The study involved 1.8 million people under 35 years of age and found very weak, if any, evidence of an association between incident type 1 diabetes and SARS-CoV-2.
Examining data between March 2020 and November 2021, Dr. Colhoun and colleagues identified 365,080 individuals up to age 35 with at least one detected SARS-CoV-2 infection during follow-up and 1,074 who developed type 1 diabetes.
“In children under 16 years, suspected cases of type 1 diabetes are admitted to hospital, and 97% of diagnosis dates are recorded in the Scottish Care Information – Diabetes Collaboration register [SCI-Diabetes] prior to or within 2 days of the first hospital admission for type 1 diabetes,” Dr. Colhoun said, stressing the timeliness of the data.
“We found the incidence of type 1 diabetes diagnosis increased 1.2-fold in those aged 0-14 years, but we did not find any association at an individual level of COVID-19 infection over 30 days prior to a type 1 diabetes diagnosis, in this particular dataset,” she reported. In young people aged 15-34, there was a linear increase in incident type 1 diabetes from 2015 to 2021 with no pandemic increase.
Referring to the 1.2-fold increase soon after the pandemic started, she explained that, in 0- to 14-year-olds, the increase followed a drop in the preceding months prepandemic in 2019. They also found that the seasonal pattern of type 1 diabetes diagnoses remained roughly the same across the pandemic months, with typical peaks in February and September.
In the cohort of under 35s, researchers also found a rate ratio of 2.62 (95% CI, 1.81-3.78) within a 30-day window of SARS-CoV-2 infection, but beyond 30 days, no evidence was seen of an association, with a RR of 0.86 (95% CI, 0.62-1.21; P = .40), she reported.
She explained her reasons for not considering diagnoses within 30 days of COVID-19 as causative. Echoing Dr. Tapia, Dr. Colhoun said the median time from symptom onset to diagnosis of type 1 diabetes is 25 days. “This suggests that 50% have had symptoms for over 25 days at diagnosis.”
She also stressed that when they compared the timing of SARS-CoV-2 testing with diagnosis, they found a much higher rate of COVID-19 testing around diagnosis. “This was not least because everyone admitted to hospital had to have a COVID-19 test.”
Latest U.S. data point to a link
Meanwhile, for the new data reported in JAMA Network Open, medical student Ellen K. Kendall of Case Western Reserve University, Cleveland, matched 571,256 pediatric patients: 285,628 with COVID-19 and 285,628 with non–COVID-19 respiratory infections.
By 6 months after COVID-19, 123 patients (0.043%) had received a new diagnosis of type 1 diabetes, but only 72 (0.025%) were diagnosed with type 1 diabetes within 6 months after non–COVID-19 respiratory infection.
At 1, 3, and 6 months after infection, risk of diagnosis of type 1 diabetes was greater among those infected with SARS-CoV-2, compared with those with non–COVID-19 respiratory infection (1 month: HR, 1.96; 3 months: HR, 2.10; and 6 months: HR, 1.83), and in subgroups of patients aged 0-9 years, a group unlikely to develop type 2 diabetes.
“In this study, new type 1 diabetes diagnoses were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections (or with other encounters with health systems),” noted Ms. Kendall and coauthors. “Respiratory infections have previously been associated with onset of type 1 diabetes, but this risk was even higher among those with COVID-19 in our study, raising concern for long-term, post–COVID-19 autoimmune complications among youths.”
“The increased risk of new-onset type 1 diabetes after COVID-19 adds an important consideration for risk–benefit discussions for prevention and treatment of SARS-CoV-2 infection in pediatric populations,” they concluded.
A study from the Centers for Disease Control and Prevention published in January 2022, also concluded there was a link between COVID-19 and diabetes in children, but not with other acute respiratory infections. Children were 2.5 times more likely to be diagnosed with diabetes following a SARS-CoV-2 infection, it found.
However, the study has been criticized because it pooled all types of diabetes together and did not account for other health conditions, medications that can increase blood glucose levels, race, obesity, and other social determinants of health that might influence a child’s risk of acquiring COVID-19 or diabetes.
“I’ve no doubt that the CDC data were incorrect because the incidence rate for ... diabetes, even in those never exposed to COVID-19 infection, was 10 times the rate ever reported in the U.S.,” Dr. Colhoun said. “There’s no way these data are correct. I believe there was a confusion between incidence and prevalence of diabetes.”
“This paper caused a great deal of panic, especially among those who have a child with type 1diabetes, so we need to be very careful not to cause undue alarm until we have more definitive evidence in this arena,” she stressed.
However, she also acknowledged that the new Norwegian study was well conducted, and she has no methodological concerns about it, so “I think we just have to wait and see.”
Given the inconclusiveness on the issue, there is an ongoing CoviDiab registry collecting data on this very subject.
Dr. Tapia presented on behalf of lead author Dr. Gulseth, who has reported no relevant financial relationships. Dr. Colhoun also reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Could cold exposure, especially shivering, combat type 2 diabetes?
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
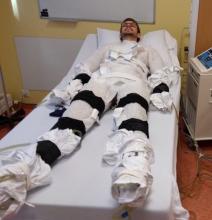
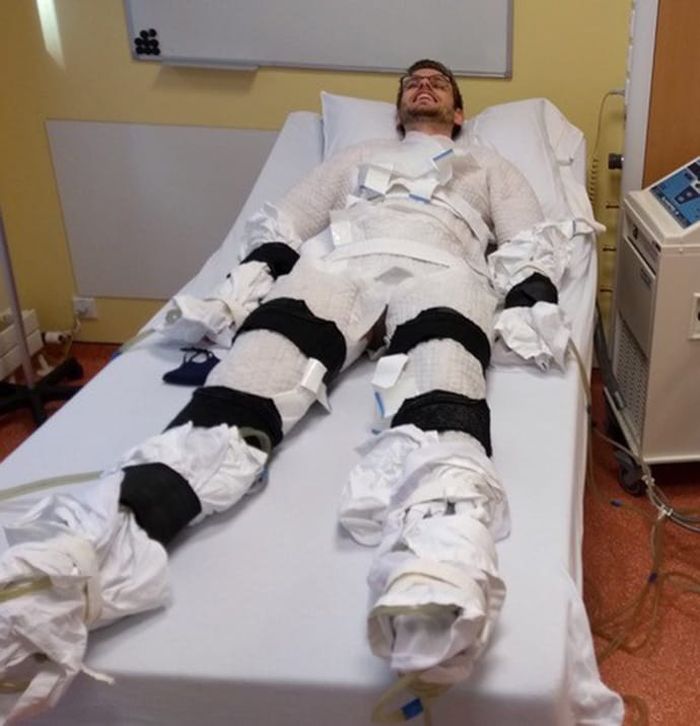
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Shivering upon repeated short exposures to cold improves glucose tolerance, decreases fasting blood glucose and lipid levels, and markedly reduces blood pressure, show new study results in adults with obesity and overweight.
Presenting the preliminary findings at the annual meeting of the European Association for the Study of Diabetes, Adam Sellers, a PhD student from Maastricht (the Netherlands) University, said: “The results are highly promising and may eventually suggest an alternative treatment or preventative measure for type 2 diabetes.”
Dr. Sellers found that 10 daily 1-hour sessions of shivering at 10° C led to 85% of participants showing a drop in fasting glucose, and a 32% drop in lipid levels, as well as a blood pressure drop of around 8% overall.
Although cold exposure is known to increase brown fat, Dr. Sellers doesn’t believe this explains his findings. “This research, in addition to two other prior studies, suggest that shivering and skeletal muscle may play a more important role than brown fat,” he said.
“Muscle can contract mechanically – [the concept of the] shivers – thereby generating heat, and there is considerably more muscle than brown fat in a human, so shivering can burn more calories and produce more heat,” he explained.
He added that, in the future, “in a similar way to saunas and steam rooms, there might be cold rooms where people go and sit in the cold room and shiver, or possibly patients attend hospital and shivering is induced.”
Audience member Anna Krook, PhD, professor of integrative physiology at the Karolinska Institute, Stockholm, commented on the work, saying the results are “potent” and demonstrate the metabolic effect of shivering. “One thing that struck me was, given the time the subject had to spend – 1 hour shivering over 10 days, I wonder if 1 hour of exercise would show similarly potent effects, and perhaps for those people who cannot perform exercise for whatever reason this might be a good alternative.”
She pointed out that, in terms of translation into practice, it “really depends on how tolerable this is. It also shows how important our muscle is in regulating metabolism. The study showed that you had to be shivering, and it wasn’t just enough to be cold, which has implications for the role of brown fat, especially when we consider the small amount of brown fat we have compared to muscle, which can be half of body weight.”
And Denis P. Blondin, PhD, said: “The reality is that we know it can be difficult and even painful for individuals with obesity to perform exercise, and therefore, cold exposure offers a passive way of improving our metabolic profile and cardiovascular health.”
“Some will argue that it is unrealistic to propose cold exposure as a therapy, but people overlook the fact that cold exposure [mostly through cold-water immersion] has increased in popularity over the past 5 years and has also been a cultural staple for many Nordic countries, albeit often performed with heat exposure as well [see the use of saunas and cold-water swimming in Finland and other Nordic countries],” added Dr. Blondin, of the faculty of medicine and health sciences, University of Sherbrooke (Que.)
“While it can certainly be uncomfortable at first (like starting an exercise program), we adapt very quickly,” he added.
1 hour in a cold-water suit to induce shivering
In the current study, Dr. Sellers exposed adults (aged 40-75 years; 11 men and 4 postmenopausal women) with overweight/obesity (body mass index, 27-35 kg/m2) to 10 consecutive cold exposures of at least 1 hour of shivering per cold exposure.
“The shivering in this new research was more intense [than in prior studies] and was induced with a different cold exposure method – a 10° C water-perfused suit [compared with a prior study of 14-15° C, 6 hours/day]. This facilitated a shorter cold exposure duration, deemed feasible for the participants,” explained Dr. Sellers.
“At baseline, participants had glucose and A1c levels at the upper end of the normal criteria [5.5 mmol/l and 5.4%, respectively],” he said, referring to measurements that were suggestive of possible progression to type 2 diabetes.
He explained how the cold exposure was applied. “We induced the cold with a water-perfused suit worn by the participant, through which water flows at 10° C, and this cools the participant. Eventually, the participant starts to shiver, and does so for at least 1 hour every morning for 10 days.”
Participants’ shivering-induced heat production was measured via surface electromyography and visual observation to confirm the presence of shivering. Both before and after the 10-day course of shivering, physiological measurements were taken in the morning while participants were at rest in an overnight fasted state, and under thermoneutral conditions. Blood pressure and fasting blood glucose were measured.
A 2-hour oral glucose tolerance test (OGTT) was conducted twice for each participant: on the morning before the 10-day course of shivering and again on the morning after the final 10th day of shivering.
The primary endpoint was change from before to after the 10-day shivering intervention, as represented by the total area under the curve of glucose levels over time during the OGTT.
“This provides a measure of the glucose concentrations in the blood before and after the 10 shivering sessions over the 10 days.”
Fasting glucose and blood lipids fall, glucose tolerance improves
After 10 shivering sessions, mean fasting plasma glucose decreased significantly in 13 out of the 15 participants, compared with before the first session (from 5.84 mmol/L to 5.67 mmol/L; P = .013).
Glucose tolerance during the OGTT improved by 6% (P = .041). “We can see that this was not due to a change in their insulin concentrations in the blood,” remarked Dr. Sellers, referring to the finding that plasma insulin concentrations at baseline and during the OGTT did not change.
Fasting plasma triglyceride and free-fatty acid concentrations also decreased significantly by 32% (P = .001) and 11% (P = .036), respectively.
“This is important because free-fatty acids are involved in the role of insulin resistance,” said Dr. Sellers. “In addition, the large reduction in serum triglycerides could have implications for atherosclerosis, which may also be beneficial.”
Dr. Sellers also found that systolic blood pressure decreased by 10 mm Hg or 7.4% (P < .001), while diastolic blood pressure decreased by 7 mm Hg or 8.1% (P < .001) on average. This lowering was seen in all participants.
“Again, quite strikingly, all participants showed” a reduction in blood pressure, said Dr. Sellers, which he noted relates to a decrease in resting heart rate (P = .062).
Brown fat or skeletal muscle contraction?
Dr. Sellers pointed out that, despite nonshivering thermogenesis being involved in mild cold acclimation, the data so far suggest that some level of mild muscle activity or shivering appears crucial in provoking the beneficial metabolic effects of cold acclimation.
“Brown fat is a metabolic heating system inside our bodies, burning calories”, explained Dr. Sellers. “This generates heat and prevents calories from being deposited as normal white fat. Brown fat is activated during cold and when we eat, but its activity is less in older adults and in individuals with obesity and diabetes.”
“Going forward, we might investigate the effects of shorter duration – so more intense shivering – to try and elucidate more precisely the optimum duration and intensity of shivering needed,” said Dr. Sellers.
“Our findings are promising and may have important health implications. In future studies, we plan to assess the effect of shivering in adults with type 2 diabetes,” he concluded.
Dr. Seller and Dr. Krook have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
‘Amazing’ data for cheap beta-blocker gel for diabetic foot ulcers
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Esmolol hydrochloride gel (Galnobax, NovoLead) appears to be a safe and effective novel topical treatment option for diabetic foot ulcers, according to results from a new trial of the drug, which is widely available as a generic and is inexpensive.
Of note, the proportion of participants achieving target ulcer closure at 12 weeks with esmolol (plus standard of care) was around 60% compared with just over 40% in patients who received standard of care alone.
Presenting the findings at this year’s annual meeting of the European Association for the Study of Diabetes was Ashu Rastogi, MD, a professor of endocrinology at the Postgraduate Institute of Medical Education and Research in Chandigarh, India.
“Esmolol can be given topically as a 14% gel and is a novel treatment option in diabetic foot ulcer,” said Dr. Rastogi.
Esmolol, a short-acting beta-adrenergic blocker, is currently approved by the U.S. Food and Drug Administration for cardiac indications only, such as short-term use for controlling supraventricular tachycardia. Beta-blockers are also used to treat hypertension.
However, esmolol has also been repurposed and formulated as a topical gel for the treatment of hard-to-heal diabetic foot ulcers (mainly neuropathic grade 1).
Audience member Ketan Dhatariya, MBBS, MD, PhD, a National Health Service consultant in diabetes, endocrinology, and general medicine and honorary senior lecturer at Norfolk and Norwich University Hospitals, England, enthused about the findings.
“This is an amazing study. I’m part of a working group looking at the updating of a guideline for the International Working Group of the Diabetic Foot, reviewing all the studies on wound healing, specifically pharmacological interventions. This is way beyond anything shown to date in terms of medical intervention. [The authors] should be congratulated; this is really astounding,” he told this news organization.
“Right now, there is very little out there in terms of pharmacological interventions that have shown benefit,” he added. “Once this study has been peer-reviewed and is published properly, it is potentially game-changing because it is a generic, worldwide, cheap, and freely available medication.”
Study across 27 sites in India
Prior phase 1/2 data have shown that 60% of ulcers completely closed with esmolol (14% gel) compared with 39% with standard of care. Encouraged by these findings, a phase 3 randomized, double-blind placebo-controlled study was conducted across 27 sites in India.
Patients were a mean age of 56 years, and had a body mass index (BMI) of 25-26 kg/m2 and mean hemoglobin A1c of 8.4%-8.7%. Around 70% of participants were men. Mean ulcer area was approximately 460-500 mm2, two-thirds of the ulcers were plantar, and mean ulcer duration was 40-50 weeks.
After screening and discontinuations (39 participants), a 12-week treatment phase began with patients randomized to one of three groups: esmolol (14% gel) along with standard of care administered twice daily (57 completers); standard of care only (63 completers); or vehicle gel (placebo) along with standard of care administered twice daily (17 completers).
Standard of care comprised wound cleaning, debridement, maintenance of moist wound environment, twice-daily fresh bandages, and off-loading footwear as needed, and was provided to all participants irrespective of study group.
The 12-week treatment period was followed by an observation period of 12 weeks up to the 24-week study endpoint.
The primary efficacy endpoint was the proportion of participants achieving target ulcer closure (100% re-epithelialization without drainage or dressing requirement) within the 12-week treatment phase.
Secondary endpoints included time to target ulcer closure during the 12-week treatment phase and proportion of participants achieving target ulcer closure by 24 weeks (end of study). Investigators were blinded throughout.
Subanalyses were conducted based on ulcer location, size, and age, as well as estimated glomerular filtration rate less than 90 mL/min and ankle-brachial index under 0.9 but greater than 0.7.
50% more patients on esmolol had complete ulcer closure
The proportion of participants with complete ulcer closure at 12 weeks was 60.3% in the esmolol plus standard of care group, compared with 41.7% with standard of care only, a difference of 18.6% (odds ratio, 2.13; P = .0276).
“The 24-week end-of-study data show what happened in the 12 weeks following end of treatment,” said Dr. Rastogi, turning to results showing that by 24 weeks the proportion of participants with complete ulcer closure was 77.2% versus 55.6%, respectively, with a difference of 21.6% (OR, 2.71; P = .013).
Time to ulcer closure (a secondary endpoint) was similar between the esmolol plus standard of care vs. standard of care groups (74.3 vs. 72.5 days).
The impact of ulcer location on complete ulcer closure, a subanalysis, showed a higher proportion of patients experienced complete ulcer closure with esmolol plus standard of care versus standard of care. For example, in plantar-based ulcers, esmolol led to complete closure in 58.7% vs. 43.1%, while for nonplantar ulcers, complete closure was found in 63.6% vs. 38.1%.
In wounds less than 5 cm2, the proportion of complete closures was 66.0% vs. 50.0% for esmolol compared with standard of care alone, while in wounds over 5 cm2, these proportions were 47.6% vs. 26.9%.
Subanalyses also showed that esmolol was substantially better in patients with BMI greater than 25, ulcer duration over 12 weeks, and A1c above 8%.
Also, a subanalysis stratified by “real-life” situations favored esmolol, showing a 50.9% difference in the proportion of patients with diabetic foot ulcer healing in those with a history of hypertension and a 31.8% difference favoring esmolol in those with an abnormal electrocardiogram.
Overall, the proportions of patients who had an adverse event were 13.2%, 18.4%, and 37.5% in the esmolol plus standard of care, standard of care alone, and vehicle plus standard of care groups, respectively, and the vast majority were unrelated to study drug. There were no serious adverse events in the esmolol plus standard of care group.
A class effect of beta blockers?
The proposed mechanism of action of esmolol relates to a sequence of reducing inflammation (via vasodilation, fibroblast migration, and cytokine reduction); proliferation by beta-blockade (improves keratinocyte migration and epithelialization); and remodeling (increases collagen turnover).
Asked by an audience member if the observations were a class effect and systemic effect of beta-blockers, Dr. Rastogi said he could not say for sure that it was a class effect, but they deliberately used a beta-1 adrenergic receptor antagonist.
“It may not be a systemic effect because we have some patients who use beta-blockers systemically and they still have diabetic foot ulcers,” he said.
Dr. Rastogi and Dr. Dhatariya have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EASD 2022
Whole grains may improve survival in people with type 2 diabetes
STOCKHOLM – Higher consumption of whole grains, fish, fiber, and omega-3 polyunsaturated fatty acids reduces deaths from all causes in people with type 2 diabetes, show new data.
Results from the systematic review and meta-analysis were presented at the annual meeting of the European Association for the Study of Diabetes by lead author Janett Barbaresko, PhD, a researcher from the German Diabetes Center in Düsseldorf.
Adding just one serving (around 20 g/day) of whole grains from foods such as brown bread, brown rice, or breakfast cereals was associated with about a 16% reduction in all-cause mortality, and each portion of fish consumed per week was associated with a 5% lower risk of all-cause mortality. In addition, eating 5 g/day of fiber was associated with a 14% reduction in all-cause mortality, and 0.1 g/day of omega-3 polyunsaturated fatty acids with a 13% reduction.
Diet also has role in improving survival in those with type 2 diabetes
Dr. Barbaresko explained that most dietary recommendations for people with type 2 diabetes are not evidence based or are derived from studies of the general population, and that the degree to which different components of diet are associated with all-cause mortality, or indeed the prevention of morbidity and mortality, remains unknown.
By way of example, she noted the American Diabetes Association 2022 guidelines for the prevention and management of diabetes complications advises limited intake of saturated and trans fatty acids, higher intake of polyunsaturated fatty acids, and following the Mediterranean or DASH (Dietary Approaches to Stop Hypertension) diets.
“Our findings show that dietary factors not only play a role in the prevention of type 2 diabetes, but also seem to be relevant for improving survival in people with diagnosed diabetes,” she said, adding that, “in particular, we found some key aspects of a healthy diet such as higher intakes of whole grains, fiber, fish, and omega-3 polyunsaturated fatty acids may improve survival of individuals with type 2 diabetes.”
She noted that individuals with type 2 diabetes are known to be more prone to circulatory diseases, dementia, cancer, and bone fractures, and that lifestyle modifications, including diet – with or without medications – underpin most management strategies.
“For the first time, we have provided a summary of all published studies on any dietary factor in association to all-cause mortality in individuals with type 2 diabetes,” said Dr. Barbaresko. “Moreover, the certainty of evidence has been evaluated for the first time.”
Matthias Schulze, MD, head of the German Institute of Human Nutrition, Berlin, moderated the session.
The new work “summarizes the available evidence, providing important dietary advice for patients with diabetes, for example, recommending whole grains,” he remarked. “However, the study also points to gaps in knowledge, so for many diet factors, we have either no or few studies, or study quality considered to be low, which calls for more research to fill the gap.”
High versus low intake of various dietary factors
The researchers performed meta-analyses based on published studies of all-cause mortality in individuals with type 2 diabetes aged 18 years and over, as associated with dietary patterns, macronutrients (carbohydrates, protein, fat), micronutrients (vitamins and minerals), secondary plant compounds (for example, polyphenols), and supplements.
Studies were conducted mainly in the United States and Europe with a mean follow-up of 10 years. Low and high intake were compared, and a dose-response relationship between different dietary factors and all-cause mortality was explored to generate summary risk ratios. The researchers also explored how the certainty of evidence was determined.
Decreased mortality from any cause was found for a higher intake of fish (SRR per serving/week, 0.95; over six studies); whole grain (SRR per 20 g/day, 0.84; two studies); fiber (SRR per 5 g/day, 0.86; three studies), and omega-3 polyunsaturated fatty acids (SRR per 0.1 g/day, 0.87; two studies).
A low certainty of evidence was found for an inverse association between all-cause mortality and vegetable consumption (SRR per 100 g/day, 0.88; two studies) and plant protein intake (SRR per 10 g/day, 0.91; three studies).
Eggs were associated with an increased risk of all-cause mortality (SRR per 10 g/day, 1.05; seven studies), as was dietary cholesterol (SRR per 300 mg/day, 1.19; two studies).
Regarding other dietary patterns, including the Mediterranean diet and low-carbohydrate diet, either no association was found and/or the evidence was very uncertain. Likewise, evidence was uncertain for foods including nuts, dairy, meat, sugar and sweets; macronutrients, including carbohydrates; and micronutrients, such as caffeine and vitamin D.
“With the Mediterranean diet, we saw an inverse association [with all-cause mortality] comparing high adherence with low adherence to the Mediterranean diet, but the certainty of evidence was very low, indicating a really uncertain meta-evidence,” remarked Dr. Barbaresko.
She concluded that a greater number of studies is needed to investigate the association of dietary factors with all-cause mortality in type 2 diabetes to strengthen the evidence for several other dietary factors. She also cautioned that meta-analyses are affected by unmeasured and residual confounding.
Dr. Barbaresko and Dr. Schulze reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Higher consumption of whole grains, fish, fiber, and omega-3 polyunsaturated fatty acids reduces deaths from all causes in people with type 2 diabetes, show new data.
Results from the systematic review and meta-analysis were presented at the annual meeting of the European Association for the Study of Diabetes by lead author Janett Barbaresko, PhD, a researcher from the German Diabetes Center in Düsseldorf.
Adding just one serving (around 20 g/day) of whole grains from foods such as brown bread, brown rice, or breakfast cereals was associated with about a 16% reduction in all-cause mortality, and each portion of fish consumed per week was associated with a 5% lower risk of all-cause mortality. In addition, eating 5 g/day of fiber was associated with a 14% reduction in all-cause mortality, and 0.1 g/day of omega-3 polyunsaturated fatty acids with a 13% reduction.
Diet also has role in improving survival in those with type 2 diabetes
Dr. Barbaresko explained that most dietary recommendations for people with type 2 diabetes are not evidence based or are derived from studies of the general population, and that the degree to which different components of diet are associated with all-cause mortality, or indeed the prevention of morbidity and mortality, remains unknown.
By way of example, she noted the American Diabetes Association 2022 guidelines for the prevention and management of diabetes complications advises limited intake of saturated and trans fatty acids, higher intake of polyunsaturated fatty acids, and following the Mediterranean or DASH (Dietary Approaches to Stop Hypertension) diets.
“Our findings show that dietary factors not only play a role in the prevention of type 2 diabetes, but also seem to be relevant for improving survival in people with diagnosed diabetes,” she said, adding that, “in particular, we found some key aspects of a healthy diet such as higher intakes of whole grains, fiber, fish, and omega-3 polyunsaturated fatty acids may improve survival of individuals with type 2 diabetes.”
She noted that individuals with type 2 diabetes are known to be more prone to circulatory diseases, dementia, cancer, and bone fractures, and that lifestyle modifications, including diet – with or without medications – underpin most management strategies.
“For the first time, we have provided a summary of all published studies on any dietary factor in association to all-cause mortality in individuals with type 2 diabetes,” said Dr. Barbaresko. “Moreover, the certainty of evidence has been evaluated for the first time.”
Matthias Schulze, MD, head of the German Institute of Human Nutrition, Berlin, moderated the session.
The new work “summarizes the available evidence, providing important dietary advice for patients with diabetes, for example, recommending whole grains,” he remarked. “However, the study also points to gaps in knowledge, so for many diet factors, we have either no or few studies, or study quality considered to be low, which calls for more research to fill the gap.”
High versus low intake of various dietary factors
The researchers performed meta-analyses based on published studies of all-cause mortality in individuals with type 2 diabetes aged 18 years and over, as associated with dietary patterns, macronutrients (carbohydrates, protein, fat), micronutrients (vitamins and minerals), secondary plant compounds (for example, polyphenols), and supplements.
Studies were conducted mainly in the United States and Europe with a mean follow-up of 10 years. Low and high intake were compared, and a dose-response relationship between different dietary factors and all-cause mortality was explored to generate summary risk ratios. The researchers also explored how the certainty of evidence was determined.
Decreased mortality from any cause was found for a higher intake of fish (SRR per serving/week, 0.95; over six studies); whole grain (SRR per 20 g/day, 0.84; two studies); fiber (SRR per 5 g/day, 0.86; three studies), and omega-3 polyunsaturated fatty acids (SRR per 0.1 g/day, 0.87; two studies).
A low certainty of evidence was found for an inverse association between all-cause mortality and vegetable consumption (SRR per 100 g/day, 0.88; two studies) and plant protein intake (SRR per 10 g/day, 0.91; three studies).
Eggs were associated with an increased risk of all-cause mortality (SRR per 10 g/day, 1.05; seven studies), as was dietary cholesterol (SRR per 300 mg/day, 1.19; two studies).
Regarding other dietary patterns, including the Mediterranean diet and low-carbohydrate diet, either no association was found and/or the evidence was very uncertain. Likewise, evidence was uncertain for foods including nuts, dairy, meat, sugar and sweets; macronutrients, including carbohydrates; and micronutrients, such as caffeine and vitamin D.
“With the Mediterranean diet, we saw an inverse association [with all-cause mortality] comparing high adherence with low adherence to the Mediterranean diet, but the certainty of evidence was very low, indicating a really uncertain meta-evidence,” remarked Dr. Barbaresko.
She concluded that a greater number of studies is needed to investigate the association of dietary factors with all-cause mortality in type 2 diabetes to strengthen the evidence for several other dietary factors. She also cautioned that meta-analyses are affected by unmeasured and residual confounding.
Dr. Barbaresko and Dr. Schulze reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
STOCKHOLM – Higher consumption of whole grains, fish, fiber, and omega-3 polyunsaturated fatty acids reduces deaths from all causes in people with type 2 diabetes, show new data.
Results from the systematic review and meta-analysis were presented at the annual meeting of the European Association for the Study of Diabetes by lead author Janett Barbaresko, PhD, a researcher from the German Diabetes Center in Düsseldorf.
Adding just one serving (around 20 g/day) of whole grains from foods such as brown bread, brown rice, or breakfast cereals was associated with about a 16% reduction in all-cause mortality, and each portion of fish consumed per week was associated with a 5% lower risk of all-cause mortality. In addition, eating 5 g/day of fiber was associated with a 14% reduction in all-cause mortality, and 0.1 g/day of omega-3 polyunsaturated fatty acids with a 13% reduction.
Diet also has role in improving survival in those with type 2 diabetes
Dr. Barbaresko explained that most dietary recommendations for people with type 2 diabetes are not evidence based or are derived from studies of the general population, and that the degree to which different components of diet are associated with all-cause mortality, or indeed the prevention of morbidity and mortality, remains unknown.
By way of example, she noted the American Diabetes Association 2022 guidelines for the prevention and management of diabetes complications advises limited intake of saturated and trans fatty acids, higher intake of polyunsaturated fatty acids, and following the Mediterranean or DASH (Dietary Approaches to Stop Hypertension) diets.
“Our findings show that dietary factors not only play a role in the prevention of type 2 diabetes, but also seem to be relevant for improving survival in people with diagnosed diabetes,” she said, adding that, “in particular, we found some key aspects of a healthy diet such as higher intakes of whole grains, fiber, fish, and omega-3 polyunsaturated fatty acids may improve survival of individuals with type 2 diabetes.”
She noted that individuals with type 2 diabetes are known to be more prone to circulatory diseases, dementia, cancer, and bone fractures, and that lifestyle modifications, including diet – with or without medications – underpin most management strategies.
“For the first time, we have provided a summary of all published studies on any dietary factor in association to all-cause mortality in individuals with type 2 diabetes,” said Dr. Barbaresko. “Moreover, the certainty of evidence has been evaluated for the first time.”
Matthias Schulze, MD, head of the German Institute of Human Nutrition, Berlin, moderated the session.
The new work “summarizes the available evidence, providing important dietary advice for patients with diabetes, for example, recommending whole grains,” he remarked. “However, the study also points to gaps in knowledge, so for many diet factors, we have either no or few studies, or study quality considered to be low, which calls for more research to fill the gap.”
High versus low intake of various dietary factors
The researchers performed meta-analyses based on published studies of all-cause mortality in individuals with type 2 diabetes aged 18 years and over, as associated with dietary patterns, macronutrients (carbohydrates, protein, fat), micronutrients (vitamins and minerals), secondary plant compounds (for example, polyphenols), and supplements.
Studies were conducted mainly in the United States and Europe with a mean follow-up of 10 years. Low and high intake were compared, and a dose-response relationship between different dietary factors and all-cause mortality was explored to generate summary risk ratios. The researchers also explored how the certainty of evidence was determined.
Decreased mortality from any cause was found for a higher intake of fish (SRR per serving/week, 0.95; over six studies); whole grain (SRR per 20 g/day, 0.84; two studies); fiber (SRR per 5 g/day, 0.86; three studies), and omega-3 polyunsaturated fatty acids (SRR per 0.1 g/day, 0.87; two studies).
A low certainty of evidence was found for an inverse association between all-cause mortality and vegetable consumption (SRR per 100 g/day, 0.88; two studies) and plant protein intake (SRR per 10 g/day, 0.91; three studies).
Eggs were associated with an increased risk of all-cause mortality (SRR per 10 g/day, 1.05; seven studies), as was dietary cholesterol (SRR per 300 mg/day, 1.19; two studies).
Regarding other dietary patterns, including the Mediterranean diet and low-carbohydrate diet, either no association was found and/or the evidence was very uncertain. Likewise, evidence was uncertain for foods including nuts, dairy, meat, sugar and sweets; macronutrients, including carbohydrates; and micronutrients, such as caffeine and vitamin D.
“With the Mediterranean diet, we saw an inverse association [with all-cause mortality] comparing high adherence with low adherence to the Mediterranean diet, but the certainty of evidence was very low, indicating a really uncertain meta-evidence,” remarked Dr. Barbaresko.
She concluded that a greater number of studies is needed to investigate the association of dietary factors with all-cause mortality in type 2 diabetes to strengthen the evidence for several other dietary factors. She also cautioned that meta-analyses are affected by unmeasured and residual confounding.
Dr. Barbaresko and Dr. Schulze reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT EASD 2022
Around 10% of back pain patients referred by chiropractors have undiagnosed SpA
GHENT, BELGIUM – Over 10% of patients referred by chiropractors to rheumatology had undiagnosed spondyloarthritis, with axial spondyloarthritis being the most common, according to new data. The U.S. study was aimed at understanding what proportion of back pain patients have undiagnosed spondyloarthritis.
The study also found that the most common cause for which patients see chiropractors is neck/cervical pain.
Atul Deodhar, MD, MRCP, rheumatologist and medical director of rheumatology clinics at Oregon Health & Science University, Portland, was senior author of the poster that was presented at the 13th International Congress of Spondyloarthritides.
“In the U.S., many people with back pain go to chiropractors, but many chiropractors are not aware of axial spondyloarthritis [axSpA] terminology, and very little – if anything – is published in chiropractic literature, “ he said in an interview.
He remarked that the study highlighted the need to develop a better strategy to identify undiagnosed patients, because the yield found in their study was poor (13%). “Patient-reported spondyloarthritis criteria are often poor, and do not match rheumatologist-inquired history,” he noted, adding that, “inflammatory back pain is in fact a poor ‘entry point.’ ”
Ulrich Weber, MD, rheumatologist from the Practice Buchsbaum in Schaffhausen, Switzerland, commented on the findings, saying he often receives delayed referrals from chiropractors, so
He added that he welcomed the study but noted, “the criteria used to identify patients in this study are broad and I’d worry that it would inundate our rheumatology practice. There remains a real need for a good method of identifying the patients.”
Referral to rheumatology
Back pain is highly prevalent in the general population, with a global mean lifetime prevalence of 38.9%. Chiropractors treat many patients with back pain of unknown cause.
“In this study, we wanted to see what percentage of patients in chiropractic practice have undiagnosed axial spondyloarthritis, and what are the common complaints. Our hypothesis was that chiropractors may be missing such patients,” Dr. Deodhar explained.
Dr. Deodhar and colleagues recruited chiropractors from four different parts of the city of Portland into the study. “We think Portland, Oregon, is a typical U.S. city, and our results could be generalized. However, this is our impression alone,” he remarked.
Adults, under the age of 45 years who attended a participating chiropractic clinics between November 2020 and November 2021 for chronic back pain and without a prior diagnosis of spondyloarthritis were eligible for inclusion.
If the patient reported at least one feature of spondyloarthritis in the screening questionnaire they were referred to a rheumatologist for a diagnostic assessment. This assessment involved taking history by telephone, both laboratory tests and imaging, and the patients were categorized as radiographic axSpA, nonradiographic axSpA, peripheral SpA, or no SpA.
The screening questionnaire included the following examples: If the patient was under 45 years and had chronic pain in back, hip or buttocks, then they were asked for more information including whether their pain was gradual (insidious) in onset; if the pain started before the age of 40; and if the pain improved with physical activities or movements. Use of drugs was investigated including whether the pain improved significantly with NSAIDs and whether the patient has current or past heel pains, particularly when waking up in the morning. They were also asked if they have experienced skin psoriasis. Other questions were asked about the presence of uveitis, iritis, family history of psoriasis, inflammatory bowel disease, or ankylosing spondylitis, and whether the patient had unexplained joint pains plus joint swelling.
Ten percent of patients referred to rheumatology
A total of 3,103 visits to chiropractor clinics were included, of which 115 patients were referred to a rheumatologist. Eventually, 63 patients were fully assessed by a rheumatologist.
Of those patients who were fully assessed, 12.7% has spondyloarthritis, with one having confirmed radiographic axSpA, five having nonradiographic SpA, and two having peripheral spondyloarthritis or psoriatic arthritis.
Based on the referral questionnaire, all patients reported at least four SpA criteria were met, said Dr. Deodhar.
Of those patients diagnosed with SpA, 14% (1) has elevated C-reactive protein (CRP) level, 14% (1) were HLA-B27 positive, and 14% (1) were identified as having both elevated CRP and HLA-B27 positivity. Sacroiliac joint inflammation was found in 14% (1) on MRI and one had sacroiliac joint inflammation according to modified New York criteria. One (14%) had both sacroiliac joint inflammation on MRI and elevated CRP, and 14% (1) had both sacroiliac joint inflammation and was HLA-B27 positive.
The top complaints reported by patients at chiropractor clinics were neck and cervical spine pain/spasm (16.8%); followed by acute low back pain (11.7%); acute upper back (7.1%); and chronic lower back pain (6.9%).
No patients with more than 10 SpA criteria
The performance of an initial diagnostic assessment based on patient reported SpA criteria, as compared with the outcome of the full diagnosis (by a rheumatologist) showed that patients with one to four SpA criteria had a sensitivity of 0.50 (95% confidence interval, 0.15-0.85), and specificity of 0.73 (95% CI, 0.61-0.84). This increased to sensitivity of 0.60 (95% CI, 0.17-1.03), and specificity of 0.61 (95% CI, 0.44-0.77) when six SpA criteria were present.
Dr. Deodhar said the results supported a need to further develop the chiropractor’s role in identifying the right patients for referral, and that the study showed that a referral strategy is required to find undiagnosed patients with spondyloarthritis from chiropractic offices. “Chiropractors need education for axSpA, when to suspect, and when to refer,” he asserted. “What referral strategy to use is for debate – the ASAS [Assessment in SpondyloArthritis international Society] strategy is too sensitive and not specific enough.”
Dr. Deodhar noted that SPARTAN (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Spondyloarthritis Research & Treatment Network) is working on a referral strategy that is likely to be more specific, and that more data would be forthcoming soon.
Dr. Deodhar declared affiliations with multiple companies involved in the field unrelated to the study. Dr. Weber declared no relevant disclosures.
GHENT, BELGIUM – Over 10% of patients referred by chiropractors to rheumatology had undiagnosed spondyloarthritis, with axial spondyloarthritis being the most common, according to new data. The U.S. study was aimed at understanding what proportion of back pain patients have undiagnosed spondyloarthritis.
The study also found that the most common cause for which patients see chiropractors is neck/cervical pain.
Atul Deodhar, MD, MRCP, rheumatologist and medical director of rheumatology clinics at Oregon Health & Science University, Portland, was senior author of the poster that was presented at the 13th International Congress of Spondyloarthritides.
“In the U.S., many people with back pain go to chiropractors, but many chiropractors are not aware of axial spondyloarthritis [axSpA] terminology, and very little – if anything – is published in chiropractic literature, “ he said in an interview.
He remarked that the study highlighted the need to develop a better strategy to identify undiagnosed patients, because the yield found in their study was poor (13%). “Patient-reported spondyloarthritis criteria are often poor, and do not match rheumatologist-inquired history,” he noted, adding that, “inflammatory back pain is in fact a poor ‘entry point.’ ”
Ulrich Weber, MD, rheumatologist from the Practice Buchsbaum in Schaffhausen, Switzerland, commented on the findings, saying he often receives delayed referrals from chiropractors, so
He added that he welcomed the study but noted, “the criteria used to identify patients in this study are broad and I’d worry that it would inundate our rheumatology practice. There remains a real need for a good method of identifying the patients.”
Referral to rheumatology
Back pain is highly prevalent in the general population, with a global mean lifetime prevalence of 38.9%. Chiropractors treat many patients with back pain of unknown cause.
“In this study, we wanted to see what percentage of patients in chiropractic practice have undiagnosed axial spondyloarthritis, and what are the common complaints. Our hypothesis was that chiropractors may be missing such patients,” Dr. Deodhar explained.
Dr. Deodhar and colleagues recruited chiropractors from four different parts of the city of Portland into the study. “We think Portland, Oregon, is a typical U.S. city, and our results could be generalized. However, this is our impression alone,” he remarked.
Adults, under the age of 45 years who attended a participating chiropractic clinics between November 2020 and November 2021 for chronic back pain and without a prior diagnosis of spondyloarthritis were eligible for inclusion.
If the patient reported at least one feature of spondyloarthritis in the screening questionnaire they were referred to a rheumatologist for a diagnostic assessment. This assessment involved taking history by telephone, both laboratory tests and imaging, and the patients were categorized as radiographic axSpA, nonradiographic axSpA, peripheral SpA, or no SpA.
The screening questionnaire included the following examples: If the patient was under 45 years and had chronic pain in back, hip or buttocks, then they were asked for more information including whether their pain was gradual (insidious) in onset; if the pain started before the age of 40; and if the pain improved with physical activities or movements. Use of drugs was investigated including whether the pain improved significantly with NSAIDs and whether the patient has current or past heel pains, particularly when waking up in the morning. They were also asked if they have experienced skin psoriasis. Other questions were asked about the presence of uveitis, iritis, family history of psoriasis, inflammatory bowel disease, or ankylosing spondylitis, and whether the patient had unexplained joint pains plus joint swelling.
Ten percent of patients referred to rheumatology
A total of 3,103 visits to chiropractor clinics were included, of which 115 patients were referred to a rheumatologist. Eventually, 63 patients were fully assessed by a rheumatologist.
Of those patients who were fully assessed, 12.7% has spondyloarthritis, with one having confirmed radiographic axSpA, five having nonradiographic SpA, and two having peripheral spondyloarthritis or psoriatic arthritis.
Based on the referral questionnaire, all patients reported at least four SpA criteria were met, said Dr. Deodhar.
Of those patients diagnosed with SpA, 14% (1) has elevated C-reactive protein (CRP) level, 14% (1) were HLA-B27 positive, and 14% (1) were identified as having both elevated CRP and HLA-B27 positivity. Sacroiliac joint inflammation was found in 14% (1) on MRI and one had sacroiliac joint inflammation according to modified New York criteria. One (14%) had both sacroiliac joint inflammation on MRI and elevated CRP, and 14% (1) had both sacroiliac joint inflammation and was HLA-B27 positive.
The top complaints reported by patients at chiropractor clinics were neck and cervical spine pain/spasm (16.8%); followed by acute low back pain (11.7%); acute upper back (7.1%); and chronic lower back pain (6.9%).
No patients with more than 10 SpA criteria
The performance of an initial diagnostic assessment based on patient reported SpA criteria, as compared with the outcome of the full diagnosis (by a rheumatologist) showed that patients with one to four SpA criteria had a sensitivity of 0.50 (95% confidence interval, 0.15-0.85), and specificity of 0.73 (95% CI, 0.61-0.84). This increased to sensitivity of 0.60 (95% CI, 0.17-1.03), and specificity of 0.61 (95% CI, 0.44-0.77) when six SpA criteria were present.
Dr. Deodhar said the results supported a need to further develop the chiropractor’s role in identifying the right patients for referral, and that the study showed that a referral strategy is required to find undiagnosed patients with spondyloarthritis from chiropractic offices. “Chiropractors need education for axSpA, when to suspect, and when to refer,” he asserted. “What referral strategy to use is for debate – the ASAS [Assessment in SpondyloArthritis international Society] strategy is too sensitive and not specific enough.”
Dr. Deodhar noted that SPARTAN (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Spondyloarthritis Research & Treatment Network) is working on a referral strategy that is likely to be more specific, and that more data would be forthcoming soon.
Dr. Deodhar declared affiliations with multiple companies involved in the field unrelated to the study. Dr. Weber declared no relevant disclosures.
GHENT, BELGIUM – Over 10% of patients referred by chiropractors to rheumatology had undiagnosed spondyloarthritis, with axial spondyloarthritis being the most common, according to new data. The U.S. study was aimed at understanding what proportion of back pain patients have undiagnosed spondyloarthritis.
The study also found that the most common cause for which patients see chiropractors is neck/cervical pain.
Atul Deodhar, MD, MRCP, rheumatologist and medical director of rheumatology clinics at Oregon Health & Science University, Portland, was senior author of the poster that was presented at the 13th International Congress of Spondyloarthritides.
“In the U.S., many people with back pain go to chiropractors, but many chiropractors are not aware of axial spondyloarthritis [axSpA] terminology, and very little – if anything – is published in chiropractic literature, “ he said in an interview.
He remarked that the study highlighted the need to develop a better strategy to identify undiagnosed patients, because the yield found in their study was poor (13%). “Patient-reported spondyloarthritis criteria are often poor, and do not match rheumatologist-inquired history,” he noted, adding that, “inflammatory back pain is in fact a poor ‘entry point.’ ”
Ulrich Weber, MD, rheumatologist from the Practice Buchsbaum in Schaffhausen, Switzerland, commented on the findings, saying he often receives delayed referrals from chiropractors, so
He added that he welcomed the study but noted, “the criteria used to identify patients in this study are broad and I’d worry that it would inundate our rheumatology practice. There remains a real need for a good method of identifying the patients.”
Referral to rheumatology
Back pain is highly prevalent in the general population, with a global mean lifetime prevalence of 38.9%. Chiropractors treat many patients with back pain of unknown cause.
“In this study, we wanted to see what percentage of patients in chiropractic practice have undiagnosed axial spondyloarthritis, and what are the common complaints. Our hypothesis was that chiropractors may be missing such patients,” Dr. Deodhar explained.
Dr. Deodhar and colleagues recruited chiropractors from four different parts of the city of Portland into the study. “We think Portland, Oregon, is a typical U.S. city, and our results could be generalized. However, this is our impression alone,” he remarked.
Adults, under the age of 45 years who attended a participating chiropractic clinics between November 2020 and November 2021 for chronic back pain and without a prior diagnosis of spondyloarthritis were eligible for inclusion.
If the patient reported at least one feature of spondyloarthritis in the screening questionnaire they were referred to a rheumatologist for a diagnostic assessment. This assessment involved taking history by telephone, both laboratory tests and imaging, and the patients were categorized as radiographic axSpA, nonradiographic axSpA, peripheral SpA, or no SpA.
The screening questionnaire included the following examples: If the patient was under 45 years and had chronic pain in back, hip or buttocks, then they were asked for more information including whether their pain was gradual (insidious) in onset; if the pain started before the age of 40; and if the pain improved with physical activities or movements. Use of drugs was investigated including whether the pain improved significantly with NSAIDs and whether the patient has current or past heel pains, particularly when waking up in the morning. They were also asked if they have experienced skin psoriasis. Other questions were asked about the presence of uveitis, iritis, family history of psoriasis, inflammatory bowel disease, or ankylosing spondylitis, and whether the patient had unexplained joint pains plus joint swelling.
Ten percent of patients referred to rheumatology
A total of 3,103 visits to chiropractor clinics were included, of which 115 patients were referred to a rheumatologist. Eventually, 63 patients were fully assessed by a rheumatologist.
Of those patients who were fully assessed, 12.7% has spondyloarthritis, with one having confirmed radiographic axSpA, five having nonradiographic SpA, and two having peripheral spondyloarthritis or psoriatic arthritis.
Based on the referral questionnaire, all patients reported at least four SpA criteria were met, said Dr. Deodhar.
Of those patients diagnosed with SpA, 14% (1) has elevated C-reactive protein (CRP) level, 14% (1) were HLA-B27 positive, and 14% (1) were identified as having both elevated CRP and HLA-B27 positivity. Sacroiliac joint inflammation was found in 14% (1) on MRI and one had sacroiliac joint inflammation according to modified New York criteria. One (14%) had both sacroiliac joint inflammation on MRI and elevated CRP, and 14% (1) had both sacroiliac joint inflammation and was HLA-B27 positive.
The top complaints reported by patients at chiropractor clinics were neck and cervical spine pain/spasm (16.8%); followed by acute low back pain (11.7%); acute upper back (7.1%); and chronic lower back pain (6.9%).
No patients with more than 10 SpA criteria
The performance of an initial diagnostic assessment based on patient reported SpA criteria, as compared with the outcome of the full diagnosis (by a rheumatologist) showed that patients with one to four SpA criteria had a sensitivity of 0.50 (95% confidence interval, 0.15-0.85), and specificity of 0.73 (95% CI, 0.61-0.84). This increased to sensitivity of 0.60 (95% CI, 0.17-1.03), and specificity of 0.61 (95% CI, 0.44-0.77) when six SpA criteria were present.
Dr. Deodhar said the results supported a need to further develop the chiropractor’s role in identifying the right patients for referral, and that the study showed that a referral strategy is required to find undiagnosed patients with spondyloarthritis from chiropractic offices. “Chiropractors need education for axSpA, when to suspect, and when to refer,” he asserted. “What referral strategy to use is for debate – the ASAS [Assessment in SpondyloArthritis international Society] strategy is too sensitive and not specific enough.”
Dr. Deodhar noted that SPARTAN (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Spondyloarthritis Research & Treatment Network) is working on a referral strategy that is likely to be more specific, and that more data would be forthcoming soon.
Dr. Deodhar declared affiliations with multiple companies involved in the field unrelated to the study. Dr. Weber declared no relevant disclosures.
AT THE 2022 SPA CONGRESS
Spondyloarthritis disease activity measurement with ASDAS not influenced by gender
GHENT, BELGIUM – The Ankylosing Spondylitis Disease Activity Score (ASDAS) should be the preferred tool for disease activity assessment in patients with axial spondyloarthritis (axSpA) because it is not influenced by gender, according to new data on gender and patient outcomes as assessed by commonly used scoring methods and indices across the spectrum of SpA disease subtypes.
In contrast, researchers led by Diego Benavent, MD, a rheumatologist at La Paz University Hospital, Madrid, found that gender influenced the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) in all three disease subtypes: axSpA, peripheral SpA (pSpA), and psoriatic arthritis (PsA).
In addition, data show that women with axSpA, pSpA, or PsA reported higher disease activity, functional limitation, and poorer overall health.
Dr. Benavent presented the results at the 13th International Congress on Spondyloarthritides. The study was also published online Sept. 12 in RMD Open.
“The ASDAS is more likely to be the activity score used because we are reassured that it performs well in both men and women. However, there is a need for more appropriate validated indices that are not affected by gender in peripheral spondyloarthropathies and psoriatic arthritis,” Dr. Benavent said.
So far, most data concerning gender differences have been described in patients with axSpA, and with various measurement instruments available to assess disease activity, function, and overall health. Dr. Benavent and his colleagues wanted to investigate the influence of gender on disease outcomes across not only axSpA but pSpA and PsA, too, to see if there were differences in the relationship between gender and these other disease subtypes.
In previous studies, ASDAS has shown better psychometric properties than the BASDAI for disease activity in axSpA. “But there is little validation in pSpA and PsA, and the influence of gender in the outcomes assessed by these instruments is unknown.
“Compared with men, women with an axSpA diagnosis tend to have more frequent peripheral and extramusculoskeletal manifestations, such as enthesitis and inflammatory bowel disease,” Dr. Benavent said in an interview. “However, males with axSpA present more radiographic damage and objective signs of inflammation.”
Martin Rudwaleit, MD, head of the department of internal medicine and rheumatology at Klinikum Bielefeld (Germany), who attended the talk, reflected on the findings.
“Decades ago, ankylosing spondylitis was largely considered a male disease as found in 80%-90% of cases. Later, with MRI, we started to diagnose patients earlier and learned that more females have the disease and that females have less structural damage in the spine than men. As such, male gender is a predictor for worse radiographic progression,” Dr. Rudwaleit said.
“The question is whether the female patients who are considered to have axSpA really have axSpA, or do they have other origins of their back pain?” he continued.
“Also, this study shows us that females report a wider spectrum of symptoms than males. For example, headache, general discomfort, and overall, a broader spectrum of symptoms than men. This might have contributed to the fact that, previously, diagnoses of axSpA might have been made later in females than males.”
Large study across SpA phenotypes and disease-scoring methods
A total of 4,185 patients from 24 countries participated, with 65% having axSpA, 10% pSpA, and 25% PsA. Females totaled 38.8% of patients across all three types of spondyloarthritis. The researchers drew the data from the Assessment of SpondyloArthritis International Society (ASAS)-perSpA study.
The researchers looked for associations between gender and disease activity as measured by ASDAS and BASDAI, C-reactive protein (CRP), physical function with the Bath Ankylosing Spondylitis Functional Index (BASFI), overall health with the ASAS Health Index (ASAS HI), and European Quality of Life Five Dimensions (EQ-5D) outcomes.
In axSpA, there was a split of 68% men vs. 32% women. The researchers observed certain factors that were more common among men: smoking (49% vs. 32%), HLA-B27 positivity (83% vs. 70%), and elevated CRP (75% vs. 66%). Women more often had enthesitis (45% vs. 39%) and fibromyalgia (17% vs. 3%).
In pSpA, the gender split was approximately equal at 47% men and 53% women. But compared with women, men had more inflammatory back pain (62% vs. 50%), HLA-B27 positivity (70% vs. 54%), and elevated CRP (75% vs. 66%). Women more frequently had inflammatory bowel disease (IBD, 8% vs. 3%) and fibromyalgia (18% vs. 3%).
An approximately equal gender split was also found with PsA (48.5% men vs. 51.5% women). Men more frequently reported ever drinking alcohol than did women (63% vs. 26%), whereas women had a greater family history of both spondyloarthritis (41% vs. 32%) and psoriasis (41% vs. 31%). Women also more often reported enthesitis (49% vs. 42%) and fibromyalgia (19% vs. 3%) than men.
“These data strongly suggest that female patients showed significantly more fibromyalgia across all disease subtypes, and the magnitude of the difference with men is notable,” Dr. Benavent said.”Fibromyalgia is associated with pain and worse patient-reported outcomes, which may bias outcomes with disease activity scores.”
When the researchers analyzed outcomes by the different scores and indices for each disease subtype, they found that females had worse scores for most indices (ASDAS, BASDAI, patient’s global assessment (PtGA), BASFI, ASAS HI, and EQ-5D). “However, for CRP, men presented worse scores across axSpA and pSpA, and no differences were found with women in PsA,” Dr. Benavent added.
Although there are differences between the genders according to the scores, these differences may be confounded and this will affect the score outcome: for example, confounding by fibromyalgia in women, he explained.
To avoid the confounding effect, multivariable regression models were used, including the dependent variable as the explored outcome: for example, with BASDAI or ASDAS serving as the dependent variable and gender as the main independent variable, along with adjustments for potential confounders. When the influence of gender on BASDAI was considered, Dr. Benavent and colleagues found that being female increased all scores across the spectrum: axSpA (0.39 units; 95% confidence interval, 0.2-0.58), pSpA (1.22 units; 95% CI, 0.77-1.69), and PsA (0.88 units; 95% CI, 0.59-1.19). When the influence of gender on ASDAS was considered, the researchers found that being female had no effect on axSpA (0.02 units; 95% CI, –0.07 to 0.11), but did for pSpA (0.36 units; 95% CI, 0.15-0.58) and PsA (0.25 units; 95% CI, 0.12-0.38).
“ASDAS is better than BASDAI because it is similar in males and females, but this only holds true in axSpA, not in pSpA or PsA,” Dr. Benavent concluded.
Dr. Benavent declared serving on speakers bureaus for Janssen, Galapagos, and AbbVie, and receiving grant or research support from Novartis outside the submitted work. Dr. Rudwaleit declared financial relationships with AbbVie, UCB, and Lilly.
GHENT, BELGIUM – The Ankylosing Spondylitis Disease Activity Score (ASDAS) should be the preferred tool for disease activity assessment in patients with axial spondyloarthritis (axSpA) because it is not influenced by gender, according to new data on gender and patient outcomes as assessed by commonly used scoring methods and indices across the spectrum of SpA disease subtypes.
In contrast, researchers led by Diego Benavent, MD, a rheumatologist at La Paz University Hospital, Madrid, found that gender influenced the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) in all three disease subtypes: axSpA, peripheral SpA (pSpA), and psoriatic arthritis (PsA).
In addition, data show that women with axSpA, pSpA, or PsA reported higher disease activity, functional limitation, and poorer overall health.
Dr. Benavent presented the results at the 13th International Congress on Spondyloarthritides. The study was also published online Sept. 12 in RMD Open.
“The ASDAS is more likely to be the activity score used because we are reassured that it performs well in both men and women. However, there is a need for more appropriate validated indices that are not affected by gender in peripheral spondyloarthropathies and psoriatic arthritis,” Dr. Benavent said.
So far, most data concerning gender differences have been described in patients with axSpA, and with various measurement instruments available to assess disease activity, function, and overall health. Dr. Benavent and his colleagues wanted to investigate the influence of gender on disease outcomes across not only axSpA but pSpA and PsA, too, to see if there were differences in the relationship between gender and these other disease subtypes.
In previous studies, ASDAS has shown better psychometric properties than the BASDAI for disease activity in axSpA. “But there is little validation in pSpA and PsA, and the influence of gender in the outcomes assessed by these instruments is unknown.
“Compared with men, women with an axSpA diagnosis tend to have more frequent peripheral and extramusculoskeletal manifestations, such as enthesitis and inflammatory bowel disease,” Dr. Benavent said in an interview. “However, males with axSpA present more radiographic damage and objective signs of inflammation.”
Martin Rudwaleit, MD, head of the department of internal medicine and rheumatology at Klinikum Bielefeld (Germany), who attended the talk, reflected on the findings.
“Decades ago, ankylosing spondylitis was largely considered a male disease as found in 80%-90% of cases. Later, with MRI, we started to diagnose patients earlier and learned that more females have the disease and that females have less structural damage in the spine than men. As such, male gender is a predictor for worse radiographic progression,” Dr. Rudwaleit said.
“The question is whether the female patients who are considered to have axSpA really have axSpA, or do they have other origins of their back pain?” he continued.
“Also, this study shows us that females report a wider spectrum of symptoms than males. For example, headache, general discomfort, and overall, a broader spectrum of symptoms than men. This might have contributed to the fact that, previously, diagnoses of axSpA might have been made later in females than males.”
Large study across SpA phenotypes and disease-scoring methods
A total of 4,185 patients from 24 countries participated, with 65% having axSpA, 10% pSpA, and 25% PsA. Females totaled 38.8% of patients across all three types of spondyloarthritis. The researchers drew the data from the Assessment of SpondyloArthritis International Society (ASAS)-perSpA study.
The researchers looked for associations between gender and disease activity as measured by ASDAS and BASDAI, C-reactive protein (CRP), physical function with the Bath Ankylosing Spondylitis Functional Index (BASFI), overall health with the ASAS Health Index (ASAS HI), and European Quality of Life Five Dimensions (EQ-5D) outcomes.
In axSpA, there was a split of 68% men vs. 32% women. The researchers observed certain factors that were more common among men: smoking (49% vs. 32%), HLA-B27 positivity (83% vs. 70%), and elevated CRP (75% vs. 66%). Women more often had enthesitis (45% vs. 39%) and fibromyalgia (17% vs. 3%).
In pSpA, the gender split was approximately equal at 47% men and 53% women. But compared with women, men had more inflammatory back pain (62% vs. 50%), HLA-B27 positivity (70% vs. 54%), and elevated CRP (75% vs. 66%). Women more frequently had inflammatory bowel disease (IBD, 8% vs. 3%) and fibromyalgia (18% vs. 3%).
An approximately equal gender split was also found with PsA (48.5% men vs. 51.5% women). Men more frequently reported ever drinking alcohol than did women (63% vs. 26%), whereas women had a greater family history of both spondyloarthritis (41% vs. 32%) and psoriasis (41% vs. 31%). Women also more often reported enthesitis (49% vs. 42%) and fibromyalgia (19% vs. 3%) than men.
“These data strongly suggest that female patients showed significantly more fibromyalgia across all disease subtypes, and the magnitude of the difference with men is notable,” Dr. Benavent said.”Fibromyalgia is associated with pain and worse patient-reported outcomes, which may bias outcomes with disease activity scores.”
When the researchers analyzed outcomes by the different scores and indices for each disease subtype, they found that females had worse scores for most indices (ASDAS, BASDAI, patient’s global assessment (PtGA), BASFI, ASAS HI, and EQ-5D). “However, for CRP, men presented worse scores across axSpA and pSpA, and no differences were found with women in PsA,” Dr. Benavent added.
Although there are differences between the genders according to the scores, these differences may be confounded and this will affect the score outcome: for example, confounding by fibromyalgia in women, he explained.
To avoid the confounding effect, multivariable regression models were used, including the dependent variable as the explored outcome: for example, with BASDAI or ASDAS serving as the dependent variable and gender as the main independent variable, along with adjustments for potential confounders. When the influence of gender on BASDAI was considered, Dr. Benavent and colleagues found that being female increased all scores across the spectrum: axSpA (0.39 units; 95% confidence interval, 0.2-0.58), pSpA (1.22 units; 95% CI, 0.77-1.69), and PsA (0.88 units; 95% CI, 0.59-1.19). When the influence of gender on ASDAS was considered, the researchers found that being female had no effect on axSpA (0.02 units; 95% CI, –0.07 to 0.11), but did for pSpA (0.36 units; 95% CI, 0.15-0.58) and PsA (0.25 units; 95% CI, 0.12-0.38).
“ASDAS is better than BASDAI because it is similar in males and females, but this only holds true in axSpA, not in pSpA or PsA,” Dr. Benavent concluded.
Dr. Benavent declared serving on speakers bureaus for Janssen, Galapagos, and AbbVie, and receiving grant or research support from Novartis outside the submitted work. Dr. Rudwaleit declared financial relationships with AbbVie, UCB, and Lilly.
GHENT, BELGIUM – The Ankylosing Spondylitis Disease Activity Score (ASDAS) should be the preferred tool for disease activity assessment in patients with axial spondyloarthritis (axSpA) because it is not influenced by gender, according to new data on gender and patient outcomes as assessed by commonly used scoring methods and indices across the spectrum of SpA disease subtypes.
In contrast, researchers led by Diego Benavent, MD, a rheumatologist at La Paz University Hospital, Madrid, found that gender influenced the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) in all three disease subtypes: axSpA, peripheral SpA (pSpA), and psoriatic arthritis (PsA).
In addition, data show that women with axSpA, pSpA, or PsA reported higher disease activity, functional limitation, and poorer overall health.
Dr. Benavent presented the results at the 13th International Congress on Spondyloarthritides. The study was also published online Sept. 12 in RMD Open.
“The ASDAS is more likely to be the activity score used because we are reassured that it performs well in both men and women. However, there is a need for more appropriate validated indices that are not affected by gender in peripheral spondyloarthropathies and psoriatic arthritis,” Dr. Benavent said.
So far, most data concerning gender differences have been described in patients with axSpA, and with various measurement instruments available to assess disease activity, function, and overall health. Dr. Benavent and his colleagues wanted to investigate the influence of gender on disease outcomes across not only axSpA but pSpA and PsA, too, to see if there were differences in the relationship between gender and these other disease subtypes.
In previous studies, ASDAS has shown better psychometric properties than the BASDAI for disease activity in axSpA. “But there is little validation in pSpA and PsA, and the influence of gender in the outcomes assessed by these instruments is unknown.
“Compared with men, women with an axSpA diagnosis tend to have more frequent peripheral and extramusculoskeletal manifestations, such as enthesitis and inflammatory bowel disease,” Dr. Benavent said in an interview. “However, males with axSpA present more radiographic damage and objective signs of inflammation.”
Martin Rudwaleit, MD, head of the department of internal medicine and rheumatology at Klinikum Bielefeld (Germany), who attended the talk, reflected on the findings.
“Decades ago, ankylosing spondylitis was largely considered a male disease as found in 80%-90% of cases. Later, with MRI, we started to diagnose patients earlier and learned that more females have the disease and that females have less structural damage in the spine than men. As such, male gender is a predictor for worse radiographic progression,” Dr. Rudwaleit said.
“The question is whether the female patients who are considered to have axSpA really have axSpA, or do they have other origins of their back pain?” he continued.
“Also, this study shows us that females report a wider spectrum of symptoms than males. For example, headache, general discomfort, and overall, a broader spectrum of symptoms than men. This might have contributed to the fact that, previously, diagnoses of axSpA might have been made later in females than males.”
Large study across SpA phenotypes and disease-scoring methods
A total of 4,185 patients from 24 countries participated, with 65% having axSpA, 10% pSpA, and 25% PsA. Females totaled 38.8% of patients across all three types of spondyloarthritis. The researchers drew the data from the Assessment of SpondyloArthritis International Society (ASAS)-perSpA study.
The researchers looked for associations between gender and disease activity as measured by ASDAS and BASDAI, C-reactive protein (CRP), physical function with the Bath Ankylosing Spondylitis Functional Index (BASFI), overall health with the ASAS Health Index (ASAS HI), and European Quality of Life Five Dimensions (EQ-5D) outcomes.
In axSpA, there was a split of 68% men vs. 32% women. The researchers observed certain factors that were more common among men: smoking (49% vs. 32%), HLA-B27 positivity (83% vs. 70%), and elevated CRP (75% vs. 66%). Women more often had enthesitis (45% vs. 39%) and fibromyalgia (17% vs. 3%).
In pSpA, the gender split was approximately equal at 47% men and 53% women. But compared with women, men had more inflammatory back pain (62% vs. 50%), HLA-B27 positivity (70% vs. 54%), and elevated CRP (75% vs. 66%). Women more frequently had inflammatory bowel disease (IBD, 8% vs. 3%) and fibromyalgia (18% vs. 3%).
An approximately equal gender split was also found with PsA (48.5% men vs. 51.5% women). Men more frequently reported ever drinking alcohol than did women (63% vs. 26%), whereas women had a greater family history of both spondyloarthritis (41% vs. 32%) and psoriasis (41% vs. 31%). Women also more often reported enthesitis (49% vs. 42%) and fibromyalgia (19% vs. 3%) than men.
“These data strongly suggest that female patients showed significantly more fibromyalgia across all disease subtypes, and the magnitude of the difference with men is notable,” Dr. Benavent said.”Fibromyalgia is associated with pain and worse patient-reported outcomes, which may bias outcomes with disease activity scores.”
When the researchers analyzed outcomes by the different scores and indices for each disease subtype, they found that females had worse scores for most indices (ASDAS, BASDAI, patient’s global assessment (PtGA), BASFI, ASAS HI, and EQ-5D). “However, for CRP, men presented worse scores across axSpA and pSpA, and no differences were found with women in PsA,” Dr. Benavent added.
Although there are differences between the genders according to the scores, these differences may be confounded and this will affect the score outcome: for example, confounding by fibromyalgia in women, he explained.
To avoid the confounding effect, multivariable regression models were used, including the dependent variable as the explored outcome: for example, with BASDAI or ASDAS serving as the dependent variable and gender as the main independent variable, along with adjustments for potential confounders. When the influence of gender on BASDAI was considered, Dr. Benavent and colleagues found that being female increased all scores across the spectrum: axSpA (0.39 units; 95% confidence interval, 0.2-0.58), pSpA (1.22 units; 95% CI, 0.77-1.69), and PsA (0.88 units; 95% CI, 0.59-1.19). When the influence of gender on ASDAS was considered, the researchers found that being female had no effect on axSpA (0.02 units; 95% CI, –0.07 to 0.11), but did for pSpA (0.36 units; 95% CI, 0.15-0.58) and PsA (0.25 units; 95% CI, 0.12-0.38).
“ASDAS is better than BASDAI because it is similar in males and females, but this only holds true in axSpA, not in pSpA or PsA,” Dr. Benavent concluded.
Dr. Benavent declared serving on speakers bureaus for Janssen, Galapagos, and AbbVie, and receiving grant or research support from Novartis outside the submitted work. Dr. Rudwaleit declared financial relationships with AbbVie, UCB, and Lilly.
AT THE 2022 SPA CONGRESS
Filgotinib reduces flare risk in uveitis in phase 2 study
GHENT, BELGIUM – Filgotinib (Jyseleca), a Janus kinase (JAK) inhibitor, reduced the risk of flare after withdrawal of glucocorticoids in patients with vision-threatening, noninfectious intermediate, posterior, or pan uveitis, data from a phase 2 study show.
Robin Besuyen, MD, clinical development leader in inflammatory diseases at Galapagos BV, Leiden, the Netherlands, presented the phase 2 results of the placebo-controlled HUMBOLDT trial at the 13th International Congress on Spondyloarthritides.
Treatment with filgotinib was well tolerated, with no new safety concerns for the immunosuppressed uveitis population.
Uveitis involves intraocular inflammation of the eye, accounts for 5%-20% of cases of blindness, and frequently requires long-term use of systemic therapy, mostly glucocorticoids or adalimumab (Humira).
Uveitis is documented to occur in 25%-40% of patients with spondyloarthritis, and its management is essential to prevent morbidity caused by vision loss and secondary complications. The majority of patients in HUMBOLDT had idiopathic uveitis (57%). One patient with spondyloarthritis was included.
Filgotinib is being investigated in the treatment of uveitis because Janus kinases have been found to play a role in the complex cytokine-signaling pathways implicated in immune-mediated diseases, including uveitis. “A preferential inhibitor of JAK 1 could play a role in managing this condition,” Dr. Besuyen said.
Session moderator Xenofon Baraliakos, MD, professor of internal medicine and rheumatology at Ruhr University Bochum (Germany), reflected on what he said was an “important study,” which, to his knowledge, was the first study of its kind in uveitis.
“The fact that they showed a significant decrease in uveitis in such a short period of time is very positive, especially for the uveitis we know of in spondyloarthritides,” he said in an interview. “The posterior uveitis was significantly impacted, and this is a very positive signal to move forward with further JAK studies in uveitis and apply them in patients with active uveitis and spondyloarthritis.”
With respect to patients with spondyloarthritis, he pointed out that, “if uveitis is fluctuating [in patients with spondyloarthritis] then patients can lose their vision. Uveitis is one of the most frequent extraskeletal manifestations of spondyloarthritis.”
However, he noted that the researchers did not show any correlation to HLA-B27 [human leukocyte antigen B27], which is “something we consider when we discuss uveitis in spondyloarthritides, but these data are convincing.”
Phase 2, randomized, double-blind trial – one of very few in uveitis
Participants in the randomized, double-blind trial were at least 18 years old and had intermediate, posterior, or pan uveitis that was active despite 2 weeks of treatment with glucocorticoids (oral prednisolone 10-60 mg/day). They were randomized 1:1 to filgotinib (200 mg once daily) or placebo and were assessed for evidence of treatment failure from week 6 onwards. Glucocorticoids in all participants were tapered off over 15 weeks.
The primary endpoint was the proportion of participants with treatment failure by week 24, defined as new, active, inflammatory chorioretinal and/or retinal vascular lesions at week 6 or later; worsening of best corrected visual acuity by 15 or more letters; or inability to achieve an anterior chamber cell or vitreous haze grade less than or equal to 0.5+ at week 6 or a 2-step grade increase after week 6. These effects had to be present in at least one eye.
Patients were stratified by the presence of sarcoidosis-related uveitis, baseline use of immunosuppressants, and prior use of anti–tumor necrosis factor (TNF) therapy. The mean patient age was 46 years, and around 60% were female, 57% had pan uveitis, and 22% had posterior uveitis. The mean number of uveitis flares in the previous 12 months was two.
A total of 37 patients received filgotinib and 35 received placebo, and together they composed the safety analysis set. Of these, 32 on filgotinib and 34 on placebo continued treatment to week 6, so 66 patients entered the efficacy analysis.
The study sponsor, Gilead, decided to stop the trial early for business reasons after the U.S. Food and Drug Administration rejected its application for filgotinib in the treatment of rheumatoid arthritis, and only 74 patients of the originally planned 248 participants were randomized. “Therefore, the conclusions that have been drawn from the study are limited, and results should be interpreted with caution,” Dr. Besuyen noted.
Primary endpoint of treatment failure favored filgotinib
The primary endpoint of treatment failure was met by 12 (38%) of 32 patients taking filgotinib and 23 (67%) of 34 patients taking placebo, generating an odds ratio of 0.23 favoring filgotinib, which was statistically significant (P = .008), Dr. Besuyen reported.
The median time to treatment failure on or after week 6, one of the trial’s secondary endpoints, was 22 weeks for placebo but could not be calculated for filgotinib because fewer than half of these patients failed treatment with filgotinib.
Filgotinib was safe and well tolerated, and the safety profile emerging from this study was similar to that seen in the indications for which it is marketed in the European Union, United Kingdom, and Japan (rheumatoid arthritis and ulcerative colitis). There were no deaths, no major adverse cardiovascular events, no malignancies, and no opportunistic infections. Treatment-emergent serious adverse events were seen in 13.5% with filgotinib and 5.7% with placebo.
Gilead Sciences and Galapagos NV sponsored and collaborated on the trial. Dr. Besuyen is an employee of Galapagos NV. Dr. Baraliakos has declared no relevant financial conflicts of interest.
GHENT, BELGIUM – Filgotinib (Jyseleca), a Janus kinase (JAK) inhibitor, reduced the risk of flare after withdrawal of glucocorticoids in patients with vision-threatening, noninfectious intermediate, posterior, or pan uveitis, data from a phase 2 study show.
Robin Besuyen, MD, clinical development leader in inflammatory diseases at Galapagos BV, Leiden, the Netherlands, presented the phase 2 results of the placebo-controlled HUMBOLDT trial at the 13th International Congress on Spondyloarthritides.
Treatment with filgotinib was well tolerated, with no new safety concerns for the immunosuppressed uveitis population.
Uveitis involves intraocular inflammation of the eye, accounts for 5%-20% of cases of blindness, and frequently requires long-term use of systemic therapy, mostly glucocorticoids or adalimumab (Humira).
Uveitis is documented to occur in 25%-40% of patients with spondyloarthritis, and its management is essential to prevent morbidity caused by vision loss and secondary complications. The majority of patients in HUMBOLDT had idiopathic uveitis (57%). One patient with spondyloarthritis was included.
Filgotinib is being investigated in the treatment of uveitis because Janus kinases have been found to play a role in the complex cytokine-signaling pathways implicated in immune-mediated diseases, including uveitis. “A preferential inhibitor of JAK 1 could play a role in managing this condition,” Dr. Besuyen said.
Session moderator Xenofon Baraliakos, MD, professor of internal medicine and rheumatology at Ruhr University Bochum (Germany), reflected on what he said was an “important study,” which, to his knowledge, was the first study of its kind in uveitis.
“The fact that they showed a significant decrease in uveitis in such a short period of time is very positive, especially for the uveitis we know of in spondyloarthritides,” he said in an interview. “The posterior uveitis was significantly impacted, and this is a very positive signal to move forward with further JAK studies in uveitis and apply them in patients with active uveitis and spondyloarthritis.”
With respect to patients with spondyloarthritis, he pointed out that, “if uveitis is fluctuating [in patients with spondyloarthritis] then patients can lose their vision. Uveitis is one of the most frequent extraskeletal manifestations of spondyloarthritis.”
However, he noted that the researchers did not show any correlation to HLA-B27 [human leukocyte antigen B27], which is “something we consider when we discuss uveitis in spondyloarthritides, but these data are convincing.”
Phase 2, randomized, double-blind trial – one of very few in uveitis
Participants in the randomized, double-blind trial were at least 18 years old and had intermediate, posterior, or pan uveitis that was active despite 2 weeks of treatment with glucocorticoids (oral prednisolone 10-60 mg/day). They were randomized 1:1 to filgotinib (200 mg once daily) or placebo and were assessed for evidence of treatment failure from week 6 onwards. Glucocorticoids in all participants were tapered off over 15 weeks.
The primary endpoint was the proportion of participants with treatment failure by week 24, defined as new, active, inflammatory chorioretinal and/or retinal vascular lesions at week 6 or later; worsening of best corrected visual acuity by 15 or more letters; or inability to achieve an anterior chamber cell or vitreous haze grade less than or equal to 0.5+ at week 6 or a 2-step grade increase after week 6. These effects had to be present in at least one eye.
Patients were stratified by the presence of sarcoidosis-related uveitis, baseline use of immunosuppressants, and prior use of anti–tumor necrosis factor (TNF) therapy. The mean patient age was 46 years, and around 60% were female, 57% had pan uveitis, and 22% had posterior uveitis. The mean number of uveitis flares in the previous 12 months was two.
A total of 37 patients received filgotinib and 35 received placebo, and together they composed the safety analysis set. Of these, 32 on filgotinib and 34 on placebo continued treatment to week 6, so 66 patients entered the efficacy analysis.
The study sponsor, Gilead, decided to stop the trial early for business reasons after the U.S. Food and Drug Administration rejected its application for filgotinib in the treatment of rheumatoid arthritis, and only 74 patients of the originally planned 248 participants were randomized. “Therefore, the conclusions that have been drawn from the study are limited, and results should be interpreted with caution,” Dr. Besuyen noted.
Primary endpoint of treatment failure favored filgotinib
The primary endpoint of treatment failure was met by 12 (38%) of 32 patients taking filgotinib and 23 (67%) of 34 patients taking placebo, generating an odds ratio of 0.23 favoring filgotinib, which was statistically significant (P = .008), Dr. Besuyen reported.
The median time to treatment failure on or after week 6, one of the trial’s secondary endpoints, was 22 weeks for placebo but could not be calculated for filgotinib because fewer than half of these patients failed treatment with filgotinib.
Filgotinib was safe and well tolerated, and the safety profile emerging from this study was similar to that seen in the indications for which it is marketed in the European Union, United Kingdom, and Japan (rheumatoid arthritis and ulcerative colitis). There were no deaths, no major adverse cardiovascular events, no malignancies, and no opportunistic infections. Treatment-emergent serious adverse events were seen in 13.5% with filgotinib and 5.7% with placebo.
Gilead Sciences and Galapagos NV sponsored and collaborated on the trial. Dr. Besuyen is an employee of Galapagos NV. Dr. Baraliakos has declared no relevant financial conflicts of interest.
GHENT, BELGIUM – Filgotinib (Jyseleca), a Janus kinase (JAK) inhibitor, reduced the risk of flare after withdrawal of glucocorticoids in patients with vision-threatening, noninfectious intermediate, posterior, or pan uveitis, data from a phase 2 study show.
Robin Besuyen, MD, clinical development leader in inflammatory diseases at Galapagos BV, Leiden, the Netherlands, presented the phase 2 results of the placebo-controlled HUMBOLDT trial at the 13th International Congress on Spondyloarthritides.
Treatment with filgotinib was well tolerated, with no new safety concerns for the immunosuppressed uveitis population.
Uveitis involves intraocular inflammation of the eye, accounts for 5%-20% of cases of blindness, and frequently requires long-term use of systemic therapy, mostly glucocorticoids or adalimumab (Humira).
Uveitis is documented to occur in 25%-40% of patients with spondyloarthritis, and its management is essential to prevent morbidity caused by vision loss and secondary complications. The majority of patients in HUMBOLDT had idiopathic uveitis (57%). One patient with spondyloarthritis was included.
Filgotinib is being investigated in the treatment of uveitis because Janus kinases have been found to play a role in the complex cytokine-signaling pathways implicated in immune-mediated diseases, including uveitis. “A preferential inhibitor of JAK 1 could play a role in managing this condition,” Dr. Besuyen said.
Session moderator Xenofon Baraliakos, MD, professor of internal medicine and rheumatology at Ruhr University Bochum (Germany), reflected on what he said was an “important study,” which, to his knowledge, was the first study of its kind in uveitis.
“The fact that they showed a significant decrease in uveitis in such a short period of time is very positive, especially for the uveitis we know of in spondyloarthritides,” he said in an interview. “The posterior uveitis was significantly impacted, and this is a very positive signal to move forward with further JAK studies in uveitis and apply them in patients with active uveitis and spondyloarthritis.”
With respect to patients with spondyloarthritis, he pointed out that, “if uveitis is fluctuating [in patients with spondyloarthritis] then patients can lose their vision. Uveitis is one of the most frequent extraskeletal manifestations of spondyloarthritis.”
However, he noted that the researchers did not show any correlation to HLA-B27 [human leukocyte antigen B27], which is “something we consider when we discuss uveitis in spondyloarthritides, but these data are convincing.”
Phase 2, randomized, double-blind trial – one of very few in uveitis
Participants in the randomized, double-blind trial were at least 18 years old and had intermediate, posterior, or pan uveitis that was active despite 2 weeks of treatment with glucocorticoids (oral prednisolone 10-60 mg/day). They were randomized 1:1 to filgotinib (200 mg once daily) or placebo and were assessed for evidence of treatment failure from week 6 onwards. Glucocorticoids in all participants were tapered off over 15 weeks.
The primary endpoint was the proportion of participants with treatment failure by week 24, defined as new, active, inflammatory chorioretinal and/or retinal vascular lesions at week 6 or later; worsening of best corrected visual acuity by 15 or more letters; or inability to achieve an anterior chamber cell or vitreous haze grade less than or equal to 0.5+ at week 6 or a 2-step grade increase after week 6. These effects had to be present in at least one eye.
Patients were stratified by the presence of sarcoidosis-related uveitis, baseline use of immunosuppressants, and prior use of anti–tumor necrosis factor (TNF) therapy. The mean patient age was 46 years, and around 60% were female, 57% had pan uveitis, and 22% had posterior uveitis. The mean number of uveitis flares in the previous 12 months was two.
A total of 37 patients received filgotinib and 35 received placebo, and together they composed the safety analysis set. Of these, 32 on filgotinib and 34 on placebo continued treatment to week 6, so 66 patients entered the efficacy analysis.
The study sponsor, Gilead, decided to stop the trial early for business reasons after the U.S. Food and Drug Administration rejected its application for filgotinib in the treatment of rheumatoid arthritis, and only 74 patients of the originally planned 248 participants were randomized. “Therefore, the conclusions that have been drawn from the study are limited, and results should be interpreted with caution,” Dr. Besuyen noted.
Primary endpoint of treatment failure favored filgotinib
The primary endpoint of treatment failure was met by 12 (38%) of 32 patients taking filgotinib and 23 (67%) of 34 patients taking placebo, generating an odds ratio of 0.23 favoring filgotinib, which was statistically significant (P = .008), Dr. Besuyen reported.
The median time to treatment failure on or after week 6, one of the trial’s secondary endpoints, was 22 weeks for placebo but could not be calculated for filgotinib because fewer than half of these patients failed treatment with filgotinib.
Filgotinib was safe and well tolerated, and the safety profile emerging from this study was similar to that seen in the indications for which it is marketed in the European Union, United Kingdom, and Japan (rheumatoid arthritis and ulcerative colitis). There were no deaths, no major adverse cardiovascular events, no malignancies, and no opportunistic infections. Treatment-emergent serious adverse events were seen in 13.5% with filgotinib and 5.7% with placebo.
Gilead Sciences and Galapagos NV sponsored and collaborated on the trial. Dr. Besuyen is an employee of Galapagos NV. Dr. Baraliakos has declared no relevant financial conflicts of interest.
AT THE 2022 SPA CONGRESS
COX-2, TNF inhibitor combo appear to have limited role in reducing axSpA spinal damage progression
GHENT, BELGIUM – A strong numerical signal suggests the addition of a selective cyclooxygenase-2 (COX-2) inhibitor to a tumor necrosis factor (TNF) inhibitor can reduce spinal radiographic progression in patients with active radiographic axial spondyloarthritis (axSpA) over 2 years, although results are not statistically significant.
Lead researcher and rheumatologist, Fabian Proft, MD, based at Charité University Medicine, Berlin, presented the findings of the study at the 13th International Congress on Spondyloarthritides.
Only 97 patients completed the study, and its follow-up period lasted 2 years, which is a relatively short period of time in which to determine the effects of an intervention that might affect structural progression of the spine, Dr. Proft said.
“Based on these data, I won’t treat all my patients with celecoxib,” he told this news organization. However, he added that, “If I have a patient with residual symptoms under biological DMARDs [disease-modifying antirheumatic drugs], and I feel they are at high risk of radiographic spinal progression and they still have symptoms, then I would add in an NSAID – and for that I’d choose a selective COX-2 inhibitor based on radiographic spinal progression data.”
Walter P. Maksymowych, MD, rheumatologist from the University of Alberta, Calgary, commented on the study findings in an interview. “This is an important clinical question because we want to know whether we should be adding an anti-inflammatory in patients who are on biologic therapies. There’s been a long debate and investigation into whether anti-inflammatories might prevent new bone formation and thereby prevent disease progression.”
He went on by acknowledging that there was no statistically significant difference in the primary endpoint (change in modified Stoke Ankylosing Spondylitis Spinal Score [mSASSS]) between the groups, but added that, “there was a sizable numerical difference, and I think this leaves the community somewhat hanging dry without a definitive answer. However, I do have concerns about whether there was an adequate sample size to address the study question.”
To add or not to add a selective COX-2 inhibitor to TNF inhibitor in axSpA treatment
The study aimed to investigate the effect of a selective COX-2 inhibitor when added to anti-TNF therapy with golimumab (Simponi), compared with golimumab therapy alone, on the progression of spinal structural damage over 2 years in patients with active radiographic axSpA.
“To date, we don’t have many treatments with evidence of reducing spinal radiographic progression in axSpA,” Dr. Proft said. “There was one study showing an effect of celecoxib, but another with diclofenac that failed to show any effect. As a result, there was a hypothesis that perhaps there was a selective COX-2 inhibitor effect.”
To investigate this further, Dr. Proft selected patients with high radiographic axSpA disease activity (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] ≥ 4) and with existing structural changes – both recognized risk factors for further progression. Participants had to have either an elevated C-reactive protein (CRP) > 5 mg/L and/or ≥ 1 syndesmophyte at screening, as well as a history of inadequate response to at least two DMARDs. Other patient risk factors for radiographic spinal progression included male gender and smoking. Duration of axSpA was unlimited.
Three radiographic readers were blinded for all clinical data and chronology. The primary endpoint was the change in mSASSS, while secondary endpoints were the presence of new syndesmophytes and clinical outcomes including activity, function, mobility, and health-related quality of life, as well as safety assessments.
Patients were treated with only golimumab (50 mg subcutaneous every 4 weeks) for the first 12 weeks and then only those patients with a good clinical response (n = 109) went into phase two of the study, at which point they were randomized 1:1 to golimumab monotherapy (control, 50 mg subcutaneous every 4 weeks), or golimumab (50 mg subcutaneous every 4 weeks) plus celecoxib (400 mg once daily) for 2 years. Radiographs were taken at baseline (week 0) and after 2 years. A total of 45 patients completed the combination therapy and 52 completed the monotherapy.
No statistical significance but a numerical difference found
“The primary outcome, which was change in mSASSS score, clearly shows a numerical difference between the combination arm at 1.1 and the monotherapy arm at 1.7 points, showing more structural progression in the monotherapy arm, compared to the combination arm,” Dr. Proft reported. However, he stressed that this difference did not reach statistical significance.
New syndesmophytes occurred in 25% with monotherapy and 11.1% with combination treatment. Again, this difference did not reach statistical significance.
“This might be due to sample size but also to the length of follow-up because a longer follow-up [given structural changes occur relatively slowly] might have shown a greater difference,” Dr. Proft pointed out.
Clinical data, according to Ankylosing Spondylitis Disease Activity Score with CRP and BASDAI, showed that both groups responded very well to therapy, and there were no differences seen between the two groups in terms of clinical parameters.
“It is important when we add a drug – and we know that NSAIDs can have safety concerns – that we do not see any statistically significant serious adverse events between patient groups,” Dr. Proft noted.
There were no significant differences in adverse events between monotherapy and combination therapy. There were 162 infections in the combination arm and 150 in the monotherapy arm. Combination therapy led to seven serious adverse events, and monotherapy occurred with five adverse events.
Dr. Proft added that four patients discontinued in the combination arm, compared with only one in the monotherapy arm, with a variety of different reasons for the discontinuations.
The study was supported by a grant from the German Ministry of Education and Research, and golimumab was provided free of charge by Merck Sharp & Dohme. Dr. Proft reported serving on speakers bureaus for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB; serving as a consultant to Novartis; and receiving grant or research support from Novartis, UCB, and Lilly. Dr. Maksymowych declared having no relevant conflicts of interest.
GHENT, BELGIUM – A strong numerical signal suggests the addition of a selective cyclooxygenase-2 (COX-2) inhibitor to a tumor necrosis factor (TNF) inhibitor can reduce spinal radiographic progression in patients with active radiographic axial spondyloarthritis (axSpA) over 2 years, although results are not statistically significant.
Lead researcher and rheumatologist, Fabian Proft, MD, based at Charité University Medicine, Berlin, presented the findings of the study at the 13th International Congress on Spondyloarthritides.
Only 97 patients completed the study, and its follow-up period lasted 2 years, which is a relatively short period of time in which to determine the effects of an intervention that might affect structural progression of the spine, Dr. Proft said.
“Based on these data, I won’t treat all my patients with celecoxib,” he told this news organization. However, he added that, “If I have a patient with residual symptoms under biological DMARDs [disease-modifying antirheumatic drugs], and I feel they are at high risk of radiographic spinal progression and they still have symptoms, then I would add in an NSAID – and for that I’d choose a selective COX-2 inhibitor based on radiographic spinal progression data.”
Walter P. Maksymowych, MD, rheumatologist from the University of Alberta, Calgary, commented on the study findings in an interview. “This is an important clinical question because we want to know whether we should be adding an anti-inflammatory in patients who are on biologic therapies. There’s been a long debate and investigation into whether anti-inflammatories might prevent new bone formation and thereby prevent disease progression.”
He went on by acknowledging that there was no statistically significant difference in the primary endpoint (change in modified Stoke Ankylosing Spondylitis Spinal Score [mSASSS]) between the groups, but added that, “there was a sizable numerical difference, and I think this leaves the community somewhat hanging dry without a definitive answer. However, I do have concerns about whether there was an adequate sample size to address the study question.”
To add or not to add a selective COX-2 inhibitor to TNF inhibitor in axSpA treatment
The study aimed to investigate the effect of a selective COX-2 inhibitor when added to anti-TNF therapy with golimumab (Simponi), compared with golimumab therapy alone, on the progression of spinal structural damage over 2 years in patients with active radiographic axSpA.
“To date, we don’t have many treatments with evidence of reducing spinal radiographic progression in axSpA,” Dr. Proft said. “There was one study showing an effect of celecoxib, but another with diclofenac that failed to show any effect. As a result, there was a hypothesis that perhaps there was a selective COX-2 inhibitor effect.”
To investigate this further, Dr. Proft selected patients with high radiographic axSpA disease activity (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] ≥ 4) and with existing structural changes – both recognized risk factors for further progression. Participants had to have either an elevated C-reactive protein (CRP) > 5 mg/L and/or ≥ 1 syndesmophyte at screening, as well as a history of inadequate response to at least two DMARDs. Other patient risk factors for radiographic spinal progression included male gender and smoking. Duration of axSpA was unlimited.
Three radiographic readers were blinded for all clinical data and chronology. The primary endpoint was the change in mSASSS, while secondary endpoints were the presence of new syndesmophytes and clinical outcomes including activity, function, mobility, and health-related quality of life, as well as safety assessments.
Patients were treated with only golimumab (50 mg subcutaneous every 4 weeks) for the first 12 weeks and then only those patients with a good clinical response (n = 109) went into phase two of the study, at which point they were randomized 1:1 to golimumab monotherapy (control, 50 mg subcutaneous every 4 weeks), or golimumab (50 mg subcutaneous every 4 weeks) plus celecoxib (400 mg once daily) for 2 years. Radiographs were taken at baseline (week 0) and after 2 years. A total of 45 patients completed the combination therapy and 52 completed the monotherapy.
No statistical significance but a numerical difference found
“The primary outcome, which was change in mSASSS score, clearly shows a numerical difference between the combination arm at 1.1 and the monotherapy arm at 1.7 points, showing more structural progression in the monotherapy arm, compared to the combination arm,” Dr. Proft reported. However, he stressed that this difference did not reach statistical significance.
New syndesmophytes occurred in 25% with monotherapy and 11.1% with combination treatment. Again, this difference did not reach statistical significance.
“This might be due to sample size but also to the length of follow-up because a longer follow-up [given structural changes occur relatively slowly] might have shown a greater difference,” Dr. Proft pointed out.
Clinical data, according to Ankylosing Spondylitis Disease Activity Score with CRP and BASDAI, showed that both groups responded very well to therapy, and there were no differences seen between the two groups in terms of clinical parameters.
“It is important when we add a drug – and we know that NSAIDs can have safety concerns – that we do not see any statistically significant serious adverse events between patient groups,” Dr. Proft noted.
There were no significant differences in adverse events between monotherapy and combination therapy. There were 162 infections in the combination arm and 150 in the monotherapy arm. Combination therapy led to seven serious adverse events, and monotherapy occurred with five adverse events.
Dr. Proft added that four patients discontinued in the combination arm, compared with only one in the monotherapy arm, with a variety of different reasons for the discontinuations.
The study was supported by a grant from the German Ministry of Education and Research, and golimumab was provided free of charge by Merck Sharp & Dohme. Dr. Proft reported serving on speakers bureaus for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB; serving as a consultant to Novartis; and receiving grant or research support from Novartis, UCB, and Lilly. Dr. Maksymowych declared having no relevant conflicts of interest.
GHENT, BELGIUM – A strong numerical signal suggests the addition of a selective cyclooxygenase-2 (COX-2) inhibitor to a tumor necrosis factor (TNF) inhibitor can reduce spinal radiographic progression in patients with active radiographic axial spondyloarthritis (axSpA) over 2 years, although results are not statistically significant.
Lead researcher and rheumatologist, Fabian Proft, MD, based at Charité University Medicine, Berlin, presented the findings of the study at the 13th International Congress on Spondyloarthritides.
Only 97 patients completed the study, and its follow-up period lasted 2 years, which is a relatively short period of time in which to determine the effects of an intervention that might affect structural progression of the spine, Dr. Proft said.
“Based on these data, I won’t treat all my patients with celecoxib,” he told this news organization. However, he added that, “If I have a patient with residual symptoms under biological DMARDs [disease-modifying antirheumatic drugs], and I feel they are at high risk of radiographic spinal progression and they still have symptoms, then I would add in an NSAID – and for that I’d choose a selective COX-2 inhibitor based on radiographic spinal progression data.”
Walter P. Maksymowych, MD, rheumatologist from the University of Alberta, Calgary, commented on the study findings in an interview. “This is an important clinical question because we want to know whether we should be adding an anti-inflammatory in patients who are on biologic therapies. There’s been a long debate and investigation into whether anti-inflammatories might prevent new bone formation and thereby prevent disease progression.”
He went on by acknowledging that there was no statistically significant difference in the primary endpoint (change in modified Stoke Ankylosing Spondylitis Spinal Score [mSASSS]) between the groups, but added that, “there was a sizable numerical difference, and I think this leaves the community somewhat hanging dry without a definitive answer. However, I do have concerns about whether there was an adequate sample size to address the study question.”
To add or not to add a selective COX-2 inhibitor to TNF inhibitor in axSpA treatment
The study aimed to investigate the effect of a selective COX-2 inhibitor when added to anti-TNF therapy with golimumab (Simponi), compared with golimumab therapy alone, on the progression of spinal structural damage over 2 years in patients with active radiographic axSpA.
“To date, we don’t have many treatments with evidence of reducing spinal radiographic progression in axSpA,” Dr. Proft said. “There was one study showing an effect of celecoxib, but another with diclofenac that failed to show any effect. As a result, there was a hypothesis that perhaps there was a selective COX-2 inhibitor effect.”
To investigate this further, Dr. Proft selected patients with high radiographic axSpA disease activity (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] ≥ 4) and with existing structural changes – both recognized risk factors for further progression. Participants had to have either an elevated C-reactive protein (CRP) > 5 mg/L and/or ≥ 1 syndesmophyte at screening, as well as a history of inadequate response to at least two DMARDs. Other patient risk factors for radiographic spinal progression included male gender and smoking. Duration of axSpA was unlimited.
Three radiographic readers were blinded for all clinical data and chronology. The primary endpoint was the change in mSASSS, while secondary endpoints were the presence of new syndesmophytes and clinical outcomes including activity, function, mobility, and health-related quality of life, as well as safety assessments.
Patients were treated with only golimumab (50 mg subcutaneous every 4 weeks) for the first 12 weeks and then only those patients with a good clinical response (n = 109) went into phase two of the study, at which point they were randomized 1:1 to golimumab monotherapy (control, 50 mg subcutaneous every 4 weeks), or golimumab (50 mg subcutaneous every 4 weeks) plus celecoxib (400 mg once daily) for 2 years. Radiographs were taken at baseline (week 0) and after 2 years. A total of 45 patients completed the combination therapy and 52 completed the monotherapy.
No statistical significance but a numerical difference found
“The primary outcome, which was change in mSASSS score, clearly shows a numerical difference between the combination arm at 1.1 and the monotherapy arm at 1.7 points, showing more structural progression in the monotherapy arm, compared to the combination arm,” Dr. Proft reported. However, he stressed that this difference did not reach statistical significance.
New syndesmophytes occurred in 25% with monotherapy and 11.1% with combination treatment. Again, this difference did not reach statistical significance.
“This might be due to sample size but also to the length of follow-up because a longer follow-up [given structural changes occur relatively slowly] might have shown a greater difference,” Dr. Proft pointed out.
Clinical data, according to Ankylosing Spondylitis Disease Activity Score with CRP and BASDAI, showed that both groups responded very well to therapy, and there were no differences seen between the two groups in terms of clinical parameters.
“It is important when we add a drug – and we know that NSAIDs can have safety concerns – that we do not see any statistically significant serious adverse events between patient groups,” Dr. Proft noted.
There were no significant differences in adverse events between monotherapy and combination therapy. There were 162 infections in the combination arm and 150 in the monotherapy arm. Combination therapy led to seven serious adverse events, and monotherapy occurred with five adverse events.
Dr. Proft added that four patients discontinued in the combination arm, compared with only one in the monotherapy arm, with a variety of different reasons for the discontinuations.
The study was supported by a grant from the German Ministry of Education and Research, and golimumab was provided free of charge by Merck Sharp & Dohme. Dr. Proft reported serving on speakers bureaus for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB; serving as a consultant to Novartis; and receiving grant or research support from Novartis, UCB, and Lilly. Dr. Maksymowych declared having no relevant conflicts of interest.
AT THE 2022 SPA CONGRESS
Bimekizumab effective for axSpA with or without prior TNFi treatment
GHENT, BELGIUM – Patients with nonradiographic or radiographic axial spondyloarthritis (axSpA) experienced clinically relevant treatment responses to bimekizumab (Bimzelx) at similar rates that significantly exceeded placebo, regardless of prior experience with a tumor necrosis factor (TNF) inhibitor, according to results from two phase 3 trials presented at the 13th International Congress on Spondyloarthritides.
In addition, around half of patients with either nonradiographic or radiographic disease achieved complete remission of enthesitis by week 16 of treatment with bimekizumab. The drug, a humanized, monoclonal antibody dually inhibiting interleukins (IL) 17A and 17F, is approved in the European Union for treating adults with moderate to severe plaque psoriasis.
“Bimekizumab blockade works independently of axial spondyloarthritis pretreatment, which means this drug specifically blocks something that other drugs do not reach,” said Xenofon Baraliakos, MD, professor of internal medicine and rheumatology at Ruhr University Bochum (Germany). He presented 24-week data on the use of bimekizumab.
The BE MOBILE 1 trial involved 256 patients with nonradiographic axSpA, whereas BE MOBILE 2 involved 232 patients with radiographic axSpA. In both trials, bimekizumab 160 mg was administered subcutaneously every 4 weeks, and at week 16, all patients, including those who had received placebo, received open-label bimekizumab for another 8 weeks. This news organization previously reported results from BE MOBILE 2 that were presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 annual meeting.
In Ghent, referring to the nonradiographic patients, Dr. Baraliakos said in an interview, “We saw a very clear response to the active drug even after 2 weeks. The curves separated out from placebo. The week 16 primary analysis showed patients on bimekizumab did significantly better, [and there was] a similar response in those who switched to [open-label] bimekizumab after placebo” at week 16.
In patients with nonradiographic disease at week 24, 52.3% on bimekizumab achieved the trial’s primary outcome of 40% improvement in Assessment in Spondyloarthritis International Society response criteria (ASAS 40), compared with 46.8% of patients who were receiving placebo and then switched to open-label bimekizumab at week 16, the latter rising from 21.4% at week 16. For comparison, 47.7% on bimekizumab achieved ASAS 40 at week 16.
At week 24 in BE MOBILE 2, 53.8% of patients with radiographic disease on continuous bimekizumab met ASAS 40 criteria, as did 56.8% of patients who switched from placebo to open-label bimekizumab, rising from 22.5% with placebo and 44.8% with bimekizumab at week 16.
Audience member Fabian Proft, MD, of Charité Medical University, Berlin, commented on the latest results as well as wider bimekizumab findings, including those relating to psoriasis. “When we compare this to drugs that are already approved and available, we can assume that bimekizumab is equally effective to existing ones,” he said, noting that “there is the additional option in patients with psoriasis, where it seems to be the most effective drug for this indication. If I had a patient with radiographic or nonradiographic axial SpA and who also had significant psoriasis, then bimekizumab would be my choice of treatment.”
Targeting IL-17A and IL-17F in one drug
In the BE MOBILE 1 study, Dr. Baraliakos and coinvestigators looked at whether inhibiting IL-17F as well as IL-17A “makes sense” in terms of clinical benefits in patients with axSpA.
“Previous experience with IL-17A inhibitors shows they work well but still miss some patients,” Dr. Baraliakos said, adding that, “the hope is that by blocking both IL-17A and IL-17F, the response will be a bit better in terms of both greater response and longevity of response than [with an] IL-17A [inhibitor] alone.”
Patients in BE MOBILE 1 were typical adult patients with nonradiographic axSpA who fulfilled ASAS classification criteria and had elevated C-reactive protein (CRP) and/or sacroiliitis on MRI. All patients were older than 18 years and had a mean age of 39 years. In each arm, 51%-57% were men. Overall, patients had a mean of 9 years of symptoms and a mean Ankylosing Spondylitis Disease Activity Score of 3.7 in both patient groups (placebo and bimekizumab).
All had active disease (Bath Ankylosing Spondylitis Disease Activity Index ≥ 4 and spinal pain ≥ 4) at baseline and demonstrated failure to respond to two different NSAIDs or had a history of intolerance to or contraindication to NSAIDs. Patients had previously received up to one TNF inhibitor (13.5% in the placebo group and 7.8% in the bimekizumab group).
The primary outcome compared rate of response to ASAS 40 criteria, which comprises patient global assessment of disease, spinal pain, function (as assessed by the Bath Ankylosing Spondylitis Functional Index [BASFI]), and inflammation (stiffness).
Early response seen regardless of previous TNF inhibitor experience
“We saw response to bimekizumab very early in our patients at 16 weeks. The amount of response was higher than that observed with IL-17A alone,” Dr. Baraliakos said in an interview. “It’s understood that IL-17A and IL-17F do not work together on the inflammatory cascade, but work separately, and this might explain the findings whereby this drug captures more inflammation.”
Dr. Baraliakos highlighted the unique response rates seen with bimekizumab regardless of past TNF inhibitor use. “The TNF inhibitor-experienced patients responded as well as the TNF inhibitor–naive ones. This is unusual because nonresponders to other drugs are usually more severely affected and have a lower chance of showing response to any drug. Also, we did not see this response in patients treated with IL-17A only.”
At 16 weeks, patients with nonradiographic disease without a past history of using a TNF inhibitor had ASAS 40 responses at rates of 46.6% with bimekizumab and 22.9% with placebo. These rates in patients with past TNF inhibitor use were 60% with bimekizumab and 11.8% with placebo.
Statistically significant differences between bimekizumab and placebo occurred for all primary and secondary outcomes. “This includes the MRI inflammation findings in bimekizumab-treated patients,” Dr. Baraliakos reported.
Complete resolution of enthesitis was also observed. By week 24, enthesitis completely resolved in 47.9% of patients with nonradiographic disease on continuous bimekizumab and 43.5% of those patients who switched from placebo to bimekizumab. In patients with radiographic disease, complete resolution occurred in 53% of those on continuous bimekizumab and 49.3% of patients who switched at week 16. “This was an excellent outcome,” Dr. Baraliakos said.
The safety profile at 24 weeks confirmed prior findings at 16 weeks in which the most common treatment-emergent adverse events with bimekizumab were nasopharyngitis (9.4%), upper respiratory tract infection (7%), and oral candidiasis (3.1%); fungal infections overall occurred in 7% taking bimekizumab.
“We saw slightly higher fungal infections, but this is because we block IL-17A and IL-17F, and [the risk for these infections] is linked to the mechanism of action. But we can deal with this,” Dr. Baraliakos said.
The trials were sponsored by UCB. Dr. Baraliakos disclosed serving on the speakers bureau and as a paid instructor and consultant for AbbVie, Bristol-Myers Squibb, Chugai, Eli Lilly, Galapagos, Gilead, Merck Sharp & Dohme, Novartis, Pfizer, and UCB. Dr. Proft disclosed serving on speakers bureaus for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB; being a consultant to Novartis; and receiving grant or research support from Novartis, UCB, and Lilly.
GHENT, BELGIUM – Patients with nonradiographic or radiographic axial spondyloarthritis (axSpA) experienced clinically relevant treatment responses to bimekizumab (Bimzelx) at similar rates that significantly exceeded placebo, regardless of prior experience with a tumor necrosis factor (TNF) inhibitor, according to results from two phase 3 trials presented at the 13th International Congress on Spondyloarthritides.
In addition, around half of patients with either nonradiographic or radiographic disease achieved complete remission of enthesitis by week 16 of treatment with bimekizumab. The drug, a humanized, monoclonal antibody dually inhibiting interleukins (IL) 17A and 17F, is approved in the European Union for treating adults with moderate to severe plaque psoriasis.
“Bimekizumab blockade works independently of axial spondyloarthritis pretreatment, which means this drug specifically blocks something that other drugs do not reach,” said Xenofon Baraliakos, MD, professor of internal medicine and rheumatology at Ruhr University Bochum (Germany). He presented 24-week data on the use of bimekizumab.
The BE MOBILE 1 trial involved 256 patients with nonradiographic axSpA, whereas BE MOBILE 2 involved 232 patients with radiographic axSpA. In both trials, bimekizumab 160 mg was administered subcutaneously every 4 weeks, and at week 16, all patients, including those who had received placebo, received open-label bimekizumab for another 8 weeks. This news organization previously reported results from BE MOBILE 2 that were presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 annual meeting.
In Ghent, referring to the nonradiographic patients, Dr. Baraliakos said in an interview, “We saw a very clear response to the active drug even after 2 weeks. The curves separated out from placebo. The week 16 primary analysis showed patients on bimekizumab did significantly better, [and there was] a similar response in those who switched to [open-label] bimekizumab after placebo” at week 16.
In patients with nonradiographic disease at week 24, 52.3% on bimekizumab achieved the trial’s primary outcome of 40% improvement in Assessment in Spondyloarthritis International Society response criteria (ASAS 40), compared with 46.8% of patients who were receiving placebo and then switched to open-label bimekizumab at week 16, the latter rising from 21.4% at week 16. For comparison, 47.7% on bimekizumab achieved ASAS 40 at week 16.
At week 24 in BE MOBILE 2, 53.8% of patients with radiographic disease on continuous bimekizumab met ASAS 40 criteria, as did 56.8% of patients who switched from placebo to open-label bimekizumab, rising from 22.5% with placebo and 44.8% with bimekizumab at week 16.
Audience member Fabian Proft, MD, of Charité Medical University, Berlin, commented on the latest results as well as wider bimekizumab findings, including those relating to psoriasis. “When we compare this to drugs that are already approved and available, we can assume that bimekizumab is equally effective to existing ones,” he said, noting that “there is the additional option in patients with psoriasis, where it seems to be the most effective drug for this indication. If I had a patient with radiographic or nonradiographic axial SpA and who also had significant psoriasis, then bimekizumab would be my choice of treatment.”
Targeting IL-17A and IL-17F in one drug
In the BE MOBILE 1 study, Dr. Baraliakos and coinvestigators looked at whether inhibiting IL-17F as well as IL-17A “makes sense” in terms of clinical benefits in patients with axSpA.
“Previous experience with IL-17A inhibitors shows they work well but still miss some patients,” Dr. Baraliakos said, adding that, “the hope is that by blocking both IL-17A and IL-17F, the response will be a bit better in terms of both greater response and longevity of response than [with an] IL-17A [inhibitor] alone.”
Patients in BE MOBILE 1 were typical adult patients with nonradiographic axSpA who fulfilled ASAS classification criteria and had elevated C-reactive protein (CRP) and/or sacroiliitis on MRI. All patients were older than 18 years and had a mean age of 39 years. In each arm, 51%-57% were men. Overall, patients had a mean of 9 years of symptoms and a mean Ankylosing Spondylitis Disease Activity Score of 3.7 in both patient groups (placebo and bimekizumab).
All had active disease (Bath Ankylosing Spondylitis Disease Activity Index ≥ 4 and spinal pain ≥ 4) at baseline and demonstrated failure to respond to two different NSAIDs or had a history of intolerance to or contraindication to NSAIDs. Patients had previously received up to one TNF inhibitor (13.5% in the placebo group and 7.8% in the bimekizumab group).
The primary outcome compared rate of response to ASAS 40 criteria, which comprises patient global assessment of disease, spinal pain, function (as assessed by the Bath Ankylosing Spondylitis Functional Index [BASFI]), and inflammation (stiffness).
Early response seen regardless of previous TNF inhibitor experience
“We saw response to bimekizumab very early in our patients at 16 weeks. The amount of response was higher than that observed with IL-17A alone,” Dr. Baraliakos said in an interview. “It’s understood that IL-17A and IL-17F do not work together on the inflammatory cascade, but work separately, and this might explain the findings whereby this drug captures more inflammation.”
Dr. Baraliakos highlighted the unique response rates seen with bimekizumab regardless of past TNF inhibitor use. “The TNF inhibitor-experienced patients responded as well as the TNF inhibitor–naive ones. This is unusual because nonresponders to other drugs are usually more severely affected and have a lower chance of showing response to any drug. Also, we did not see this response in patients treated with IL-17A only.”
At 16 weeks, patients with nonradiographic disease without a past history of using a TNF inhibitor had ASAS 40 responses at rates of 46.6% with bimekizumab and 22.9% with placebo. These rates in patients with past TNF inhibitor use were 60% with bimekizumab and 11.8% with placebo.
Statistically significant differences between bimekizumab and placebo occurred for all primary and secondary outcomes. “This includes the MRI inflammation findings in bimekizumab-treated patients,” Dr. Baraliakos reported.
Complete resolution of enthesitis was also observed. By week 24, enthesitis completely resolved in 47.9% of patients with nonradiographic disease on continuous bimekizumab and 43.5% of those patients who switched from placebo to bimekizumab. In patients with radiographic disease, complete resolution occurred in 53% of those on continuous bimekizumab and 49.3% of patients who switched at week 16. “This was an excellent outcome,” Dr. Baraliakos said.
The safety profile at 24 weeks confirmed prior findings at 16 weeks in which the most common treatment-emergent adverse events with bimekizumab were nasopharyngitis (9.4%), upper respiratory tract infection (7%), and oral candidiasis (3.1%); fungal infections overall occurred in 7% taking bimekizumab.
“We saw slightly higher fungal infections, but this is because we block IL-17A and IL-17F, and [the risk for these infections] is linked to the mechanism of action. But we can deal with this,” Dr. Baraliakos said.
The trials were sponsored by UCB. Dr. Baraliakos disclosed serving on the speakers bureau and as a paid instructor and consultant for AbbVie, Bristol-Myers Squibb, Chugai, Eli Lilly, Galapagos, Gilead, Merck Sharp & Dohme, Novartis, Pfizer, and UCB. Dr. Proft disclosed serving on speakers bureaus for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB; being a consultant to Novartis; and receiving grant or research support from Novartis, UCB, and Lilly.
GHENT, BELGIUM – Patients with nonradiographic or radiographic axial spondyloarthritis (axSpA) experienced clinically relevant treatment responses to bimekizumab (Bimzelx) at similar rates that significantly exceeded placebo, regardless of prior experience with a tumor necrosis factor (TNF) inhibitor, according to results from two phase 3 trials presented at the 13th International Congress on Spondyloarthritides.
In addition, around half of patients with either nonradiographic or radiographic disease achieved complete remission of enthesitis by week 16 of treatment with bimekizumab. The drug, a humanized, monoclonal antibody dually inhibiting interleukins (IL) 17A and 17F, is approved in the European Union for treating adults with moderate to severe plaque psoriasis.
“Bimekizumab blockade works independently of axial spondyloarthritis pretreatment, which means this drug specifically blocks something that other drugs do not reach,” said Xenofon Baraliakos, MD, professor of internal medicine and rheumatology at Ruhr University Bochum (Germany). He presented 24-week data on the use of bimekizumab.
The BE MOBILE 1 trial involved 256 patients with nonradiographic axSpA, whereas BE MOBILE 2 involved 232 patients with radiographic axSpA. In both trials, bimekizumab 160 mg was administered subcutaneously every 4 weeks, and at week 16, all patients, including those who had received placebo, received open-label bimekizumab for another 8 weeks. This news organization previously reported results from BE MOBILE 2 that were presented at the European Alliance of Associations for Rheumatology (EULAR) 2022 annual meeting.
In Ghent, referring to the nonradiographic patients, Dr. Baraliakos said in an interview, “We saw a very clear response to the active drug even after 2 weeks. The curves separated out from placebo. The week 16 primary analysis showed patients on bimekizumab did significantly better, [and there was] a similar response in those who switched to [open-label] bimekizumab after placebo” at week 16.
In patients with nonradiographic disease at week 24, 52.3% on bimekizumab achieved the trial’s primary outcome of 40% improvement in Assessment in Spondyloarthritis International Society response criteria (ASAS 40), compared with 46.8% of patients who were receiving placebo and then switched to open-label bimekizumab at week 16, the latter rising from 21.4% at week 16. For comparison, 47.7% on bimekizumab achieved ASAS 40 at week 16.
At week 24 in BE MOBILE 2, 53.8% of patients with radiographic disease on continuous bimekizumab met ASAS 40 criteria, as did 56.8% of patients who switched from placebo to open-label bimekizumab, rising from 22.5% with placebo and 44.8% with bimekizumab at week 16.
Audience member Fabian Proft, MD, of Charité Medical University, Berlin, commented on the latest results as well as wider bimekizumab findings, including those relating to psoriasis. “When we compare this to drugs that are already approved and available, we can assume that bimekizumab is equally effective to existing ones,” he said, noting that “there is the additional option in patients with psoriasis, where it seems to be the most effective drug for this indication. If I had a patient with radiographic or nonradiographic axial SpA and who also had significant psoriasis, then bimekizumab would be my choice of treatment.”
Targeting IL-17A and IL-17F in one drug
In the BE MOBILE 1 study, Dr. Baraliakos and coinvestigators looked at whether inhibiting IL-17F as well as IL-17A “makes sense” in terms of clinical benefits in patients with axSpA.
“Previous experience with IL-17A inhibitors shows they work well but still miss some patients,” Dr. Baraliakos said, adding that, “the hope is that by blocking both IL-17A and IL-17F, the response will be a bit better in terms of both greater response and longevity of response than [with an] IL-17A [inhibitor] alone.”
Patients in BE MOBILE 1 were typical adult patients with nonradiographic axSpA who fulfilled ASAS classification criteria and had elevated C-reactive protein (CRP) and/or sacroiliitis on MRI. All patients were older than 18 years and had a mean age of 39 years. In each arm, 51%-57% were men. Overall, patients had a mean of 9 years of symptoms and a mean Ankylosing Spondylitis Disease Activity Score of 3.7 in both patient groups (placebo and bimekizumab).
All had active disease (Bath Ankylosing Spondylitis Disease Activity Index ≥ 4 and spinal pain ≥ 4) at baseline and demonstrated failure to respond to two different NSAIDs or had a history of intolerance to or contraindication to NSAIDs. Patients had previously received up to one TNF inhibitor (13.5% in the placebo group and 7.8% in the bimekizumab group).
The primary outcome compared rate of response to ASAS 40 criteria, which comprises patient global assessment of disease, spinal pain, function (as assessed by the Bath Ankylosing Spondylitis Functional Index [BASFI]), and inflammation (stiffness).
Early response seen regardless of previous TNF inhibitor experience
“We saw response to bimekizumab very early in our patients at 16 weeks. The amount of response was higher than that observed with IL-17A alone,” Dr. Baraliakos said in an interview. “It’s understood that IL-17A and IL-17F do not work together on the inflammatory cascade, but work separately, and this might explain the findings whereby this drug captures more inflammation.”
Dr. Baraliakos highlighted the unique response rates seen with bimekizumab regardless of past TNF inhibitor use. “The TNF inhibitor-experienced patients responded as well as the TNF inhibitor–naive ones. This is unusual because nonresponders to other drugs are usually more severely affected and have a lower chance of showing response to any drug. Also, we did not see this response in patients treated with IL-17A only.”
At 16 weeks, patients with nonradiographic disease without a past history of using a TNF inhibitor had ASAS 40 responses at rates of 46.6% with bimekizumab and 22.9% with placebo. These rates in patients with past TNF inhibitor use were 60% with bimekizumab and 11.8% with placebo.
Statistically significant differences between bimekizumab and placebo occurred for all primary and secondary outcomes. “This includes the MRI inflammation findings in bimekizumab-treated patients,” Dr. Baraliakos reported.
Complete resolution of enthesitis was also observed. By week 24, enthesitis completely resolved in 47.9% of patients with nonradiographic disease on continuous bimekizumab and 43.5% of those patients who switched from placebo to bimekizumab. In patients with radiographic disease, complete resolution occurred in 53% of those on continuous bimekizumab and 49.3% of patients who switched at week 16. “This was an excellent outcome,” Dr. Baraliakos said.
The safety profile at 24 weeks confirmed prior findings at 16 weeks in which the most common treatment-emergent adverse events with bimekizumab were nasopharyngitis (9.4%), upper respiratory tract infection (7%), and oral candidiasis (3.1%); fungal infections overall occurred in 7% taking bimekizumab.
“We saw slightly higher fungal infections, but this is because we block IL-17A and IL-17F, and [the risk for these infections] is linked to the mechanism of action. But we can deal with this,” Dr. Baraliakos said.
The trials were sponsored by UCB. Dr. Baraliakos disclosed serving on the speakers bureau and as a paid instructor and consultant for AbbVie, Bristol-Myers Squibb, Chugai, Eli Lilly, Galapagos, Gilead, Merck Sharp & Dohme, Novartis, Pfizer, and UCB. Dr. Proft disclosed serving on speakers bureaus for Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and UCB; being a consultant to Novartis; and receiving grant or research support from Novartis, UCB, and Lilly.
AT THE 2022 SPA CONGRESS