User login
AI algorithm detects erosions, ankylosis with high accuracy in patients with sacroiliitis
Erosions and ankylosis in patients with sacroiliitis are detectable to a high degree of accuracy on CT images using an artificial intelligence (AI)–based algorithm, according to research presented at the 13th International Congress on Spondyloarthritides.
Lennart Jans, MD, head of clinics in musculoskeletal imaging in the department of radiology at Ghent (Belgium) University Hospital, shared data on the development and validation of the algorithm for automatic detection of erosion and ankylosis on CT images of the sacroiliac (SI) joints.
“Essentially, in terms of statistics, this AI algorithm has 95% sensitivity for picking up erosions in patients with clinical symptoms of sacroiliitis, and if this is further developed as a tool, it could aid detection in people with erosions that would otherwise go undetected and undiagnosed,” Dr. Jans said in an interview, stressing that the results were still preliminary.
“We want to move from reporting one patient at a time to a system that detects and helps to diagnose larger numbers of patients and makes a larger impact on patient outcomes.”
He stressed that, with thousands of images per patient, it is an impossible workload for any radiology department to read every image necessary to inform diagnoses, and this is only exacerbated by the shortage of rheumatologists, especially in the United States.
Denis Poddubnyy, MD, head of rheumatology at Charité University Hospital, Berlin, acknowledged that AI has potential to improve the recognition of changes indicative of spondyloarthritis (SpA) on imaging. “A standardized, valid, and reliable detection of those changes is relevant for both diagnosis, including differential diagnosis, and classification of SpA.”
Dr. Poddubnyy added that the AI-based algorithm developed by Dr. Jans and associates is designed to detect very specific SpA structural changes in the SI joints on CT. “CT is usually applied in the clinical practice after MRI ... normally in cases where MRI does not provide conclusive results,” he said. Since MRI scans have also been recently used to develop an AI-based algorithm for the detection of active inflammation – not captured by CT – and structural changes in SI joints, he noted that the “generated data on CT should be, therefore, seen in a broader context toward standardization of imaging findings detection.”
Proof-of-concept findings are due for scale-up
Dr. Jans acknowledged that the current data only establish proof of concept. Among the study’s 145 patients, 60% were used for training the AI algorithm and 40% for testing it. All patients who had clinical symptoms of sacroiliitis and had undergone a SI joint CT scan were included from two hospitals: Ghent University Hospital and the University of Alberta Hospital, Edmonton. The majority of patients were female (81 of 145). They had a mean age of 40 years, 84 had diagnosed axial SpA, 15 had mechanical back pain, and 46 did not have a final diagnosis.
CT images were examined by three independent and blinded radiologists who annotated erosions more than 1 mm and ankylosis more than 2 mm, while a type of AI algorithm known as a neural network pipeline was developed to segment the SI joints and detect structural lesions.
In the first instance, Dr. Jans explained, examination of CT images using the AI algorithm from patients who enter the hospital for other reasons, such as trauma, rheumatic diseases, kidney stones, or appendicitis, might lead to the detection of otherwise unknown erosions. “Often patients have complained of backache for years, seeing various physiotherapists and similar, but had no idea what might be causing it,” he said. “We just don’t have the time for examining all the thousands of images separately. We need some kind of aid here. We need an extra pair of eyes. This is what AI software does.”
Dr. Jans said rheumatologists who ultimately want to detect and diagnose patients with SI erosions want to reduce the false-negative findings. “They want the system to pick up all the patients who have erosions. Here, the most important parameter is sensitivity, and we find that our algorithm shows a very high sensitivity. Optimization of the AI algorithm to reduce false negatives resulted in a sensitivity of 95% for detection of erosions on CT of the sacroiliac joints on a patient level.”
While overall accuracy was over 90%, Dr. Jans acknowledged that the algorithm was run in a relatively select population of dedicated CT scans of the joints. He is also aware that a good AI algorithm needs to work well across locations and populations. “If you make something within your institution alone, it will not work in a hospital on the other side of the street.”
However, he added, the researchers used images from four different CT scanners and images from two different institutions – one in Canada and their own in Belgium, providing a case mix that makes their algorithm more refined.
Next step: Test in an unselected population
When asked to comment on the study, Mikael Boesen, MD, PhD, of Bispebjerg and Frederiksberg Hospital, Copenhagen, congratulated Dr. Jans on the work and remarked that he found the research potentially clinically useful.
“The next steps would be to test the performance of the model in an unselected population of patients who have CT scans of the abdomen for other reasons to test the model’s ability to flag potential SI joint disease to the reader, which is often overlooked, as well as [to see] how the model performs in larger datasets from other hospitals, vendors, and CT-reconstruction algorithms.”
Finally, Dr. Boesen pointed out that it would be interesting to see if the AI algorithm can detect different reasons for erosions. “Especially [for] separation between mechanical and inflammatory courses. This could potentially be done by automatically mapping the location of the erosions in the SI joints.”
Dr. Jans has now opened up the project to other radiologists to collaborate and provide images to train and test the algorithm further. “We now have 2.4 million images that have been enriched, and we will use these in the near future as we move beyond the proof-of-concept stage.
He is looking for as for as many partners as possible to help collect enriched images and develop this into a real tool for use in hospitals worldwide on clinical patients. “We have joined forces with several hospitals but continue looking for further collaborations.
“We need, just like self-driving cars, not just thousands, but tens of thousands or millions of images to develop this.”
Dr. Jans declared receiving speaker fees from UCB, AbbVie, Lilly, and Novartis, and that he is cofounder of a future spin-off of Ghent University RheumaFinder. Dr. Poddubnyy and Dr. Boesen declared no relevant disclosures.
Erosions and ankylosis in patients with sacroiliitis are detectable to a high degree of accuracy on CT images using an artificial intelligence (AI)–based algorithm, according to research presented at the 13th International Congress on Spondyloarthritides.
Lennart Jans, MD, head of clinics in musculoskeletal imaging in the department of radiology at Ghent (Belgium) University Hospital, shared data on the development and validation of the algorithm for automatic detection of erosion and ankylosis on CT images of the sacroiliac (SI) joints.
“Essentially, in terms of statistics, this AI algorithm has 95% sensitivity for picking up erosions in patients with clinical symptoms of sacroiliitis, and if this is further developed as a tool, it could aid detection in people with erosions that would otherwise go undetected and undiagnosed,” Dr. Jans said in an interview, stressing that the results were still preliminary.
“We want to move from reporting one patient at a time to a system that detects and helps to diagnose larger numbers of patients and makes a larger impact on patient outcomes.”
He stressed that, with thousands of images per patient, it is an impossible workload for any radiology department to read every image necessary to inform diagnoses, and this is only exacerbated by the shortage of rheumatologists, especially in the United States.
Denis Poddubnyy, MD, head of rheumatology at Charité University Hospital, Berlin, acknowledged that AI has potential to improve the recognition of changes indicative of spondyloarthritis (SpA) on imaging. “A standardized, valid, and reliable detection of those changes is relevant for both diagnosis, including differential diagnosis, and classification of SpA.”
Dr. Poddubnyy added that the AI-based algorithm developed by Dr. Jans and associates is designed to detect very specific SpA structural changes in the SI joints on CT. “CT is usually applied in the clinical practice after MRI ... normally in cases where MRI does not provide conclusive results,” he said. Since MRI scans have also been recently used to develop an AI-based algorithm for the detection of active inflammation – not captured by CT – and structural changes in SI joints, he noted that the “generated data on CT should be, therefore, seen in a broader context toward standardization of imaging findings detection.”
Proof-of-concept findings are due for scale-up
Dr. Jans acknowledged that the current data only establish proof of concept. Among the study’s 145 patients, 60% were used for training the AI algorithm and 40% for testing it. All patients who had clinical symptoms of sacroiliitis and had undergone a SI joint CT scan were included from two hospitals: Ghent University Hospital and the University of Alberta Hospital, Edmonton. The majority of patients were female (81 of 145). They had a mean age of 40 years, 84 had diagnosed axial SpA, 15 had mechanical back pain, and 46 did not have a final diagnosis.
CT images were examined by three independent and blinded radiologists who annotated erosions more than 1 mm and ankylosis more than 2 mm, while a type of AI algorithm known as a neural network pipeline was developed to segment the SI joints and detect structural lesions.
In the first instance, Dr. Jans explained, examination of CT images using the AI algorithm from patients who enter the hospital for other reasons, such as trauma, rheumatic diseases, kidney stones, or appendicitis, might lead to the detection of otherwise unknown erosions. “Often patients have complained of backache for years, seeing various physiotherapists and similar, but had no idea what might be causing it,” he said. “We just don’t have the time for examining all the thousands of images separately. We need some kind of aid here. We need an extra pair of eyes. This is what AI software does.”
Dr. Jans said rheumatologists who ultimately want to detect and diagnose patients with SI erosions want to reduce the false-negative findings. “They want the system to pick up all the patients who have erosions. Here, the most important parameter is sensitivity, and we find that our algorithm shows a very high sensitivity. Optimization of the AI algorithm to reduce false negatives resulted in a sensitivity of 95% for detection of erosions on CT of the sacroiliac joints on a patient level.”
While overall accuracy was over 90%, Dr. Jans acknowledged that the algorithm was run in a relatively select population of dedicated CT scans of the joints. He is also aware that a good AI algorithm needs to work well across locations and populations. “If you make something within your institution alone, it will not work in a hospital on the other side of the street.”
However, he added, the researchers used images from four different CT scanners and images from two different institutions – one in Canada and their own in Belgium, providing a case mix that makes their algorithm more refined.
Next step: Test in an unselected population
When asked to comment on the study, Mikael Boesen, MD, PhD, of Bispebjerg and Frederiksberg Hospital, Copenhagen, congratulated Dr. Jans on the work and remarked that he found the research potentially clinically useful.
“The next steps would be to test the performance of the model in an unselected population of patients who have CT scans of the abdomen for other reasons to test the model’s ability to flag potential SI joint disease to the reader, which is often overlooked, as well as [to see] how the model performs in larger datasets from other hospitals, vendors, and CT-reconstruction algorithms.”
Finally, Dr. Boesen pointed out that it would be interesting to see if the AI algorithm can detect different reasons for erosions. “Especially [for] separation between mechanical and inflammatory courses. This could potentially be done by automatically mapping the location of the erosions in the SI joints.”
Dr. Jans has now opened up the project to other radiologists to collaborate and provide images to train and test the algorithm further. “We now have 2.4 million images that have been enriched, and we will use these in the near future as we move beyond the proof-of-concept stage.
He is looking for as for as many partners as possible to help collect enriched images and develop this into a real tool for use in hospitals worldwide on clinical patients. “We have joined forces with several hospitals but continue looking for further collaborations.
“We need, just like self-driving cars, not just thousands, but tens of thousands or millions of images to develop this.”
Dr. Jans declared receiving speaker fees from UCB, AbbVie, Lilly, and Novartis, and that he is cofounder of a future spin-off of Ghent University RheumaFinder. Dr. Poddubnyy and Dr. Boesen declared no relevant disclosures.
Erosions and ankylosis in patients with sacroiliitis are detectable to a high degree of accuracy on CT images using an artificial intelligence (AI)–based algorithm, according to research presented at the 13th International Congress on Spondyloarthritides.
Lennart Jans, MD, head of clinics in musculoskeletal imaging in the department of radiology at Ghent (Belgium) University Hospital, shared data on the development and validation of the algorithm for automatic detection of erosion and ankylosis on CT images of the sacroiliac (SI) joints.
“Essentially, in terms of statistics, this AI algorithm has 95% sensitivity for picking up erosions in patients with clinical symptoms of sacroiliitis, and if this is further developed as a tool, it could aid detection in people with erosions that would otherwise go undetected and undiagnosed,” Dr. Jans said in an interview, stressing that the results were still preliminary.
“We want to move from reporting one patient at a time to a system that detects and helps to diagnose larger numbers of patients and makes a larger impact on patient outcomes.”
He stressed that, with thousands of images per patient, it is an impossible workload for any radiology department to read every image necessary to inform diagnoses, and this is only exacerbated by the shortage of rheumatologists, especially in the United States.
Denis Poddubnyy, MD, head of rheumatology at Charité University Hospital, Berlin, acknowledged that AI has potential to improve the recognition of changes indicative of spondyloarthritis (SpA) on imaging. “A standardized, valid, and reliable detection of those changes is relevant for both diagnosis, including differential diagnosis, and classification of SpA.”
Dr. Poddubnyy added that the AI-based algorithm developed by Dr. Jans and associates is designed to detect very specific SpA structural changes in the SI joints on CT. “CT is usually applied in the clinical practice after MRI ... normally in cases where MRI does not provide conclusive results,” he said. Since MRI scans have also been recently used to develop an AI-based algorithm for the detection of active inflammation – not captured by CT – and structural changes in SI joints, he noted that the “generated data on CT should be, therefore, seen in a broader context toward standardization of imaging findings detection.”
Proof-of-concept findings are due for scale-up
Dr. Jans acknowledged that the current data only establish proof of concept. Among the study’s 145 patients, 60% were used for training the AI algorithm and 40% for testing it. All patients who had clinical symptoms of sacroiliitis and had undergone a SI joint CT scan were included from two hospitals: Ghent University Hospital and the University of Alberta Hospital, Edmonton. The majority of patients were female (81 of 145). They had a mean age of 40 years, 84 had diagnosed axial SpA, 15 had mechanical back pain, and 46 did not have a final diagnosis.
CT images were examined by three independent and blinded radiologists who annotated erosions more than 1 mm and ankylosis more than 2 mm, while a type of AI algorithm known as a neural network pipeline was developed to segment the SI joints and detect structural lesions.
In the first instance, Dr. Jans explained, examination of CT images using the AI algorithm from patients who enter the hospital for other reasons, such as trauma, rheumatic diseases, kidney stones, or appendicitis, might lead to the detection of otherwise unknown erosions. “Often patients have complained of backache for years, seeing various physiotherapists and similar, but had no idea what might be causing it,” he said. “We just don’t have the time for examining all the thousands of images separately. We need some kind of aid here. We need an extra pair of eyes. This is what AI software does.”
Dr. Jans said rheumatologists who ultimately want to detect and diagnose patients with SI erosions want to reduce the false-negative findings. “They want the system to pick up all the patients who have erosions. Here, the most important parameter is sensitivity, and we find that our algorithm shows a very high sensitivity. Optimization of the AI algorithm to reduce false negatives resulted in a sensitivity of 95% for detection of erosions on CT of the sacroiliac joints on a patient level.”
While overall accuracy was over 90%, Dr. Jans acknowledged that the algorithm was run in a relatively select population of dedicated CT scans of the joints. He is also aware that a good AI algorithm needs to work well across locations and populations. “If you make something within your institution alone, it will not work in a hospital on the other side of the street.”
However, he added, the researchers used images from four different CT scanners and images from two different institutions – one in Canada and their own in Belgium, providing a case mix that makes their algorithm more refined.
Next step: Test in an unselected population
When asked to comment on the study, Mikael Boesen, MD, PhD, of Bispebjerg and Frederiksberg Hospital, Copenhagen, congratulated Dr. Jans on the work and remarked that he found the research potentially clinically useful.
“The next steps would be to test the performance of the model in an unselected population of patients who have CT scans of the abdomen for other reasons to test the model’s ability to flag potential SI joint disease to the reader, which is often overlooked, as well as [to see] how the model performs in larger datasets from other hospitals, vendors, and CT-reconstruction algorithms.”
Finally, Dr. Boesen pointed out that it would be interesting to see if the AI algorithm can detect different reasons for erosions. “Especially [for] separation between mechanical and inflammatory courses. This could potentially be done by automatically mapping the location of the erosions in the SI joints.”
Dr. Jans has now opened up the project to other radiologists to collaborate and provide images to train and test the algorithm further. “We now have 2.4 million images that have been enriched, and we will use these in the near future as we move beyond the proof-of-concept stage.
He is looking for as for as many partners as possible to help collect enriched images and develop this into a real tool for use in hospitals worldwide on clinical patients. “We have joined forces with several hospitals but continue looking for further collaborations.
“We need, just like self-driving cars, not just thousands, but tens of thousands or millions of images to develop this.”
Dr. Jans declared receiving speaker fees from UCB, AbbVie, Lilly, and Novartis, and that he is cofounder of a future spin-off of Ghent University RheumaFinder. Dr. Poddubnyy and Dr. Boesen declared no relevant disclosures.
FROM THE 2022 SPA CONGRESS
Comments open for U.K.’s transgender care guideline
Gynecologic and obstetric health care needs of transgender and gender-diverse adults, including fertility preservation, ending masculinizing hormones in pregnancy, and support for “chest-feeding” are proposed in a novel draft guideline issued by the U.K.’s Royal College of Obstetricians and Gynaecologists.
The draft Green-top Guideline on Care of Trans and Gender Diverse Adults in Obstetrics and Gynaecology is open for consultation and comment until Sept. 6. It aims to address the specific needs of transgender and gender-diverse individuals that, according to the guideline, are currently not consistently included in specialist training programs or in continuing professional development.
With a rise in the number of people seeking to transition, obstetricians and gynecologists are seeing more transgender and gender-diverse patients. Phil Rolland, MD, consultant gynecological oncologist from Gloucestershire Hospitals NHS Foundation Trust, Cheltenham, and member of the guideline committee, said that, “It is highly likely that if an obstetrician or gynaecologist hasn’t already consulted or treated a trans or gender-diverse patient then it is only a matter of time before they do.”
He stressed the importance of ensuring inclusivity in obstetric and gynecologic care. “We know that trans people are more likely to have poor experiences when accessing health care, and we can do better.”
The U.K.-based guideline follows a similar document from the American College of Obstetricians and Gynecologists, put in place in March 2021, as reported by this news organization. It called for greater “awareness, knowledge, and sensitivity” in caring for these patients and noted that “bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients.”
Guideline addresses fertility preservation, obstetric care, and more
Regarding fertility preservation, discussions around protecting future options should be held before endocrine interventions and/or gender-affirming genital or pelvic surgery procedures, says the guideline. In addition, gynecologic problems that can be experienced need to be explained.
The guideline also addresses obstetric care, advising that trans men on long-acting masculinizing hormone therapy should stop therapy 3 months prior to conception. People who conceive while taking masculinizing hormone therapy should discontinue the therapy as soon as possible.
Birth mode should be discussed with all trans men who plan to conceive, ideally at a prepregnancy counseling appointment, but at minimum, before the third trimester. Choice of feeding manner should also be addressed in the antenatal period, with trans men who wish to chest feed offered chest-feeding support, similar to that given to cis women.
The RCOG guideline comes in the wake of the U.K. government’s new Women’s Health Strategy for England, released in July, which notes that trans men (with female reproductive organs) should be able to access screening services for cervical and breast cancer, a position upheld by the RCOG guideline.
Other key recommendations include that obstetricians and gynecologists, when approached by transgender and gender-diverse people to help with identity-related issues, should liaise with gender-identity specialist services to provide appropriate care.
Removing bias, providing affirming care
Asha Kasliwal, MD, consultant in Community Gynaecology and Reproductive Health Care, Manchester, England, and president of the Faculty of Sexual and Reproductive Healthcare, also reflected on how transgender and gender-diverse people often feel uncomfortable accessing care, which could lead to, “many people failing to seek or continue health care because of concerns over how they will be treated,” adding that there were associated reports of poor clinical outcomes.
She highlighted that the draft guideline pointed out the importance of language during consultation with transgender and gender-diverse people, noting that “misuse of language, and particularly deliberate misuse of language associated with the sex assigned at birth (misgendering), may cause profound offence.”
Dr. Kasliwal cited the example of “using the correct pronouns when addressing someone and receiving any information about a person’s gender diversity neutrally and nonjudgementally.”
Edward Morris, MD, president of the Royal College of Obstetricians and Gynaecologists, acknowledged that trans and gender-diverse individuals say they often feel judged and misunderstood by the health service. “This can act as a barrier for them when it comes to accessing vital care, and we as health care professionals have a role to play in making them feel listened to and recognized.”
“This draft guideline is our first attempt to ensure we are providing personalised care for all our patients,” said Dr. Morris. “We welcome feedback on this draft to ensure the guideline is the best as it can be for clinicians and the trans and gender-diverse individuals who use our services.”
The draft guideline as peer-review draft, Care of Trans and Gender Diverse Adults in Obstetrics and Gynaecology is available on the RCOG website. Consultation is open until Sept. 6, 2022.
A version of this article first appeared on Medscape.com.
Gynecologic and obstetric health care needs of transgender and gender-diverse adults, including fertility preservation, ending masculinizing hormones in pregnancy, and support for “chest-feeding” are proposed in a novel draft guideline issued by the U.K.’s Royal College of Obstetricians and Gynaecologists.
The draft Green-top Guideline on Care of Trans and Gender Diverse Adults in Obstetrics and Gynaecology is open for consultation and comment until Sept. 6. It aims to address the specific needs of transgender and gender-diverse individuals that, according to the guideline, are currently not consistently included in specialist training programs or in continuing professional development.
With a rise in the number of people seeking to transition, obstetricians and gynecologists are seeing more transgender and gender-diverse patients. Phil Rolland, MD, consultant gynecological oncologist from Gloucestershire Hospitals NHS Foundation Trust, Cheltenham, and member of the guideline committee, said that, “It is highly likely that if an obstetrician or gynaecologist hasn’t already consulted or treated a trans or gender-diverse patient then it is only a matter of time before they do.”
He stressed the importance of ensuring inclusivity in obstetric and gynecologic care. “We know that trans people are more likely to have poor experiences when accessing health care, and we can do better.”
The U.K.-based guideline follows a similar document from the American College of Obstetricians and Gynecologists, put in place in March 2021, as reported by this news organization. It called for greater “awareness, knowledge, and sensitivity” in caring for these patients and noted that “bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients.”
Guideline addresses fertility preservation, obstetric care, and more
Regarding fertility preservation, discussions around protecting future options should be held before endocrine interventions and/or gender-affirming genital or pelvic surgery procedures, says the guideline. In addition, gynecologic problems that can be experienced need to be explained.
The guideline also addresses obstetric care, advising that trans men on long-acting masculinizing hormone therapy should stop therapy 3 months prior to conception. People who conceive while taking masculinizing hormone therapy should discontinue the therapy as soon as possible.
Birth mode should be discussed with all trans men who plan to conceive, ideally at a prepregnancy counseling appointment, but at minimum, before the third trimester. Choice of feeding manner should also be addressed in the antenatal period, with trans men who wish to chest feed offered chest-feeding support, similar to that given to cis women.
The RCOG guideline comes in the wake of the U.K. government’s new Women’s Health Strategy for England, released in July, which notes that trans men (with female reproductive organs) should be able to access screening services for cervical and breast cancer, a position upheld by the RCOG guideline.
Other key recommendations include that obstetricians and gynecologists, when approached by transgender and gender-diverse people to help with identity-related issues, should liaise with gender-identity specialist services to provide appropriate care.
Removing bias, providing affirming care
Asha Kasliwal, MD, consultant in Community Gynaecology and Reproductive Health Care, Manchester, England, and president of the Faculty of Sexual and Reproductive Healthcare, also reflected on how transgender and gender-diverse people often feel uncomfortable accessing care, which could lead to, “many people failing to seek or continue health care because of concerns over how they will be treated,” adding that there were associated reports of poor clinical outcomes.
She highlighted that the draft guideline pointed out the importance of language during consultation with transgender and gender-diverse people, noting that “misuse of language, and particularly deliberate misuse of language associated with the sex assigned at birth (misgendering), may cause profound offence.”
Dr. Kasliwal cited the example of “using the correct pronouns when addressing someone and receiving any information about a person’s gender diversity neutrally and nonjudgementally.”
Edward Morris, MD, president of the Royal College of Obstetricians and Gynaecologists, acknowledged that trans and gender-diverse individuals say they often feel judged and misunderstood by the health service. “This can act as a barrier for them when it comes to accessing vital care, and we as health care professionals have a role to play in making them feel listened to and recognized.”
“This draft guideline is our first attempt to ensure we are providing personalised care for all our patients,” said Dr. Morris. “We welcome feedback on this draft to ensure the guideline is the best as it can be for clinicians and the trans and gender-diverse individuals who use our services.”
The draft guideline as peer-review draft, Care of Trans and Gender Diverse Adults in Obstetrics and Gynaecology is available on the RCOG website. Consultation is open until Sept. 6, 2022.
A version of this article first appeared on Medscape.com.
Gynecologic and obstetric health care needs of transgender and gender-diverse adults, including fertility preservation, ending masculinizing hormones in pregnancy, and support for “chest-feeding” are proposed in a novel draft guideline issued by the U.K.’s Royal College of Obstetricians and Gynaecologists.
The draft Green-top Guideline on Care of Trans and Gender Diverse Adults in Obstetrics and Gynaecology is open for consultation and comment until Sept. 6. It aims to address the specific needs of transgender and gender-diverse individuals that, according to the guideline, are currently not consistently included in specialist training programs or in continuing professional development.
With a rise in the number of people seeking to transition, obstetricians and gynecologists are seeing more transgender and gender-diverse patients. Phil Rolland, MD, consultant gynecological oncologist from Gloucestershire Hospitals NHS Foundation Trust, Cheltenham, and member of the guideline committee, said that, “It is highly likely that if an obstetrician or gynaecologist hasn’t already consulted or treated a trans or gender-diverse patient then it is only a matter of time before they do.”
He stressed the importance of ensuring inclusivity in obstetric and gynecologic care. “We know that trans people are more likely to have poor experiences when accessing health care, and we can do better.”
The U.K.-based guideline follows a similar document from the American College of Obstetricians and Gynecologists, put in place in March 2021, as reported by this news organization. It called for greater “awareness, knowledge, and sensitivity” in caring for these patients and noted that “bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients.”
Guideline addresses fertility preservation, obstetric care, and more
Regarding fertility preservation, discussions around protecting future options should be held before endocrine interventions and/or gender-affirming genital or pelvic surgery procedures, says the guideline. In addition, gynecologic problems that can be experienced need to be explained.
The guideline also addresses obstetric care, advising that trans men on long-acting masculinizing hormone therapy should stop therapy 3 months prior to conception. People who conceive while taking masculinizing hormone therapy should discontinue the therapy as soon as possible.
Birth mode should be discussed with all trans men who plan to conceive, ideally at a prepregnancy counseling appointment, but at minimum, before the third trimester. Choice of feeding manner should also be addressed in the antenatal period, with trans men who wish to chest feed offered chest-feeding support, similar to that given to cis women.
The RCOG guideline comes in the wake of the U.K. government’s new Women’s Health Strategy for England, released in July, which notes that trans men (with female reproductive organs) should be able to access screening services for cervical and breast cancer, a position upheld by the RCOG guideline.
Other key recommendations include that obstetricians and gynecologists, when approached by transgender and gender-diverse people to help with identity-related issues, should liaise with gender-identity specialist services to provide appropriate care.
Removing bias, providing affirming care
Asha Kasliwal, MD, consultant in Community Gynaecology and Reproductive Health Care, Manchester, England, and president of the Faculty of Sexual and Reproductive Healthcare, also reflected on how transgender and gender-diverse people often feel uncomfortable accessing care, which could lead to, “many people failing to seek or continue health care because of concerns over how they will be treated,” adding that there were associated reports of poor clinical outcomes.
She highlighted that the draft guideline pointed out the importance of language during consultation with transgender and gender-diverse people, noting that “misuse of language, and particularly deliberate misuse of language associated with the sex assigned at birth (misgendering), may cause profound offence.”
Dr. Kasliwal cited the example of “using the correct pronouns when addressing someone and receiving any information about a person’s gender diversity neutrally and nonjudgementally.”
Edward Morris, MD, president of the Royal College of Obstetricians and Gynaecologists, acknowledged that trans and gender-diverse individuals say they often feel judged and misunderstood by the health service. “This can act as a barrier for them when it comes to accessing vital care, and we as health care professionals have a role to play in making them feel listened to and recognized.”
“This draft guideline is our first attempt to ensure we are providing personalised care for all our patients,” said Dr. Morris. “We welcome feedback on this draft to ensure the guideline is the best as it can be for clinicians and the trans and gender-diverse individuals who use our services.”
The draft guideline as peer-review draft, Care of Trans and Gender Diverse Adults in Obstetrics and Gynaecology is available on the RCOG website. Consultation is open until Sept. 6, 2022.
A version of this article first appeared on Medscape.com.
Bulevirtide reduces hepatitis D viral load in difficult-to-treat patients
Bulevirtide (Hepcludex) monotherapy significantly reduces the load of hepatitis delta virus (HDV) and is safe in difficult-to-treat patients with compensated cirrhosis and clinically significant portal hypertension, according to the results of an ongoing 1-year study.
In presenting a poster with these findings at the annual International Liver Congress, sponsored by the European Association for the Study of the Liver, lead author Elisabetta Degasperi, MD, from the Grand Hospital Maggiore Policlinico in Milan, said that they were important “because they confirm the safety of this drug in real life.”
Dr. Degasperi and colleagues showed that bulevirtide leads to a significant viral response in 78% of patients by week 48, which was measured using the outcome of greater than 2 log decline in HDV RNA from baseline.
Dr. Degasperi added that the research still needed to assess the longer-term benefits, but
Addressing an immense, unmet therapeutic need
HDV requires the presence of hepatitis B virus to replicate. Bulevirtide blocks the entry of HDV and hepatitis B virus into hepatocytes.
In July 2020, it was conditionally approved in the European Economic Area for use to treat chronic HDV infection in adults with compensated liver disease upon confirmation of HDV RNA in the blood. It currently remains an investigational agent in the United States, as well as outside of the EEA.
The ongoing trial led by Dr. Degasperi is specifically conducted in patients with compensated cirrhosis who also have clinically significant portal hypertension, where safety and efficacy are unknown.
Dr. Degasperi said in an interview that, although HDV was rare, there is nonetheless an “immense” need for effective therapies against it, especially in young patients with advanced liver disease.
“We have a lot of patients with hepatitis D who have not responded to other antiviral treatment. Right now, the only other available treatment is pegylated interferon,” she said. “Unfortunately, rates of sustained viral response to pegylated interferon are extremely low at around 30% of patients.”
Chronic HDV is the most severe form of viral hepatitis and can have mortality rates as high as 50% within 5 years in patients with cirrhosis.
The management of hepatitis D is also complicated by the fact that patients with advanced cirrhosis and clinically significant portal hypertension cannot be treated with pegylated interferon owing to lack of efficacy and safety reasons, including a high risk for decompensation and liver-related complications. Pegylated interferon is contraindicated in these patients.
Bulevirtide at 48 weeks: A closer look at the findings
Eighteen patients with HDV, compensated cirrhosis, and clinically significant portal hypertension were consecutively enrolled in this single-center, longitudinal study.
All received bulevirtide monotherapy at 2 mg/day and underwent monitoring every 2 months. They were also treated with nucleotide analogs for their hepatitis B virus, which was suppressed when they began bulevirtide.
Clinical and virologic characteristics were collected at baseline, at weeks 4 and 8, and then every 8 weeks thereafter.
Bulevirtide led to a significant viral response such that by week 48, HDV RNA declined by 3.1 log IU/mL (range, 0.2-4.6 log IU/mL), was undetectable in six patients (33%), and was less than 100 IU/L in 50% of patients. Two patients were nonresponders. In addition, 78% of patients achieved at least an HDV RNA 2 log decline from baseline.
There was also a normalization of biochemical response in the majority of patients.
Alanine aminotransferase normalization was seen in 89% of patients and declined by a median of 34 U/L (range, 15-76 U/L) over 48 weeks. Aspartate aminotransferase declined to 39 U/L (range, 21-92 U/L). A combined response was seen in 72% of patients, reported Dr. Degasperi.
“Previously, we only had results from a phase 2 study, so we had no idea of the results over such a long treatment period,” said Dr. Degasperi. “It is also the first time we have been able to treat these patients with such advanced disease that is so difficult to manage.”
“Real-world results are typically inferior to those from clinical trials, but the viral decline is comparable to phase 2 trials, and the first report of the phase 3 trial,” said Dr. Degasperi.
Gamma-glutamyltransferase, alpha-fetoprotein, immunoglobulin G, and gamma-globulin levels also improved, whereas hepatitis B surface antigen, hepatitis B virus RNA, hepatitis B core-related antigen, platelet, and bilirubin values did not significantly change.
“All patients were Child-Pugh score A, so well-compensated [disease]. However, they increased a little bit in liver function by week 48,” Dr. Degasperi said. “This was important for this very advanced disease population.”
She added that the safety profile was very favorable, with no adverse events, including no injection-site reactions.
There was an asymptomatic increase in serum bile acids. “No patients complained about itching or pruritus,” Dr. Degasperi said.
What’s ahead for bulevirtide?
In a comment, Marc Bourlière, MD, from Saint Joseph Hospital in Marseilles, France, welcomed the decrease in viral load.
“This is known to be beneficial in terms of reducing morbidity and mortality in hepatitis D,” he said. “Remember that this disease is very difficult to treat, and until now, we have had no drug available. Pegylated interferon achieves cure in only 30% of patients, and half of these relapse, so actually only 15% have a meaningful response from pegylated interferon.”
“The main issue is its use as a daily subcutaneous injection. In clinical practice, it is a little bit complicated to set up, but once done, it is quite well accepted,” he said.
“I’m impressed with these results to date because there are no other compounds that have, as yet, achieved such results. This is impressive,” he added. “But whether it translates into a long-term response we don’t yet know.”
Dr. Bourlière also noted the meaningful 2-point log decline, noting that “HDV RNA negativity where treatment can be stopped would be really meaningful, but this endpoint is hard to obtain.”
Dr. Bourlière is awaiting results of the current ongoing phase 2/3 study, which would help determine a possible final treatment duration. He is also curious to settle the ongoing debate about whether bulevirtide should be used alone or in combination.
“We need to combine bulevirtide with pegylated interferon in less-advanced patients, because we know it is more potent and active against the HDV RNA,” he said.
Dr. Degasperi has previously declared she was on the advisory board for AbbVie and has spoken and taught for Gilead, MSD, and AbbVie. Dr. Bourlière declared interests with all companies involved in the R&D of liver therapies.
A version of this article first appeared on Medscape.com.
Bulevirtide (Hepcludex) monotherapy significantly reduces the load of hepatitis delta virus (HDV) and is safe in difficult-to-treat patients with compensated cirrhosis and clinically significant portal hypertension, according to the results of an ongoing 1-year study.
In presenting a poster with these findings at the annual International Liver Congress, sponsored by the European Association for the Study of the Liver, lead author Elisabetta Degasperi, MD, from the Grand Hospital Maggiore Policlinico in Milan, said that they were important “because they confirm the safety of this drug in real life.”
Dr. Degasperi and colleagues showed that bulevirtide leads to a significant viral response in 78% of patients by week 48, which was measured using the outcome of greater than 2 log decline in HDV RNA from baseline.
Dr. Degasperi added that the research still needed to assess the longer-term benefits, but
Addressing an immense, unmet therapeutic need
HDV requires the presence of hepatitis B virus to replicate. Bulevirtide blocks the entry of HDV and hepatitis B virus into hepatocytes.
In July 2020, it was conditionally approved in the European Economic Area for use to treat chronic HDV infection in adults with compensated liver disease upon confirmation of HDV RNA in the blood. It currently remains an investigational agent in the United States, as well as outside of the EEA.
The ongoing trial led by Dr. Degasperi is specifically conducted in patients with compensated cirrhosis who also have clinically significant portal hypertension, where safety and efficacy are unknown.
Dr. Degasperi said in an interview that, although HDV was rare, there is nonetheless an “immense” need for effective therapies against it, especially in young patients with advanced liver disease.
“We have a lot of patients with hepatitis D who have not responded to other antiviral treatment. Right now, the only other available treatment is pegylated interferon,” she said. “Unfortunately, rates of sustained viral response to pegylated interferon are extremely low at around 30% of patients.”
Chronic HDV is the most severe form of viral hepatitis and can have mortality rates as high as 50% within 5 years in patients with cirrhosis.
The management of hepatitis D is also complicated by the fact that patients with advanced cirrhosis and clinically significant portal hypertension cannot be treated with pegylated interferon owing to lack of efficacy and safety reasons, including a high risk for decompensation and liver-related complications. Pegylated interferon is contraindicated in these patients.
Bulevirtide at 48 weeks: A closer look at the findings
Eighteen patients with HDV, compensated cirrhosis, and clinically significant portal hypertension were consecutively enrolled in this single-center, longitudinal study.
All received bulevirtide monotherapy at 2 mg/day and underwent monitoring every 2 months. They were also treated with nucleotide analogs for their hepatitis B virus, which was suppressed when they began bulevirtide.
Clinical and virologic characteristics were collected at baseline, at weeks 4 and 8, and then every 8 weeks thereafter.
Bulevirtide led to a significant viral response such that by week 48, HDV RNA declined by 3.1 log IU/mL (range, 0.2-4.6 log IU/mL), was undetectable in six patients (33%), and was less than 100 IU/L in 50% of patients. Two patients were nonresponders. In addition, 78% of patients achieved at least an HDV RNA 2 log decline from baseline.
There was also a normalization of biochemical response in the majority of patients.
Alanine aminotransferase normalization was seen in 89% of patients and declined by a median of 34 U/L (range, 15-76 U/L) over 48 weeks. Aspartate aminotransferase declined to 39 U/L (range, 21-92 U/L). A combined response was seen in 72% of patients, reported Dr. Degasperi.
“Previously, we only had results from a phase 2 study, so we had no idea of the results over such a long treatment period,” said Dr. Degasperi. “It is also the first time we have been able to treat these patients with such advanced disease that is so difficult to manage.”
“Real-world results are typically inferior to those from clinical trials, but the viral decline is comparable to phase 2 trials, and the first report of the phase 3 trial,” said Dr. Degasperi.
Gamma-glutamyltransferase, alpha-fetoprotein, immunoglobulin G, and gamma-globulin levels also improved, whereas hepatitis B surface antigen, hepatitis B virus RNA, hepatitis B core-related antigen, platelet, and bilirubin values did not significantly change.
“All patients were Child-Pugh score A, so well-compensated [disease]. However, they increased a little bit in liver function by week 48,” Dr. Degasperi said. “This was important for this very advanced disease population.”
She added that the safety profile was very favorable, with no adverse events, including no injection-site reactions.
There was an asymptomatic increase in serum bile acids. “No patients complained about itching or pruritus,” Dr. Degasperi said.
What’s ahead for bulevirtide?
In a comment, Marc Bourlière, MD, from Saint Joseph Hospital in Marseilles, France, welcomed the decrease in viral load.
“This is known to be beneficial in terms of reducing morbidity and mortality in hepatitis D,” he said. “Remember that this disease is very difficult to treat, and until now, we have had no drug available. Pegylated interferon achieves cure in only 30% of patients, and half of these relapse, so actually only 15% have a meaningful response from pegylated interferon.”
“The main issue is its use as a daily subcutaneous injection. In clinical practice, it is a little bit complicated to set up, but once done, it is quite well accepted,” he said.
“I’m impressed with these results to date because there are no other compounds that have, as yet, achieved such results. This is impressive,” he added. “But whether it translates into a long-term response we don’t yet know.”
Dr. Bourlière also noted the meaningful 2-point log decline, noting that “HDV RNA negativity where treatment can be stopped would be really meaningful, but this endpoint is hard to obtain.”
Dr. Bourlière is awaiting results of the current ongoing phase 2/3 study, which would help determine a possible final treatment duration. He is also curious to settle the ongoing debate about whether bulevirtide should be used alone or in combination.
“We need to combine bulevirtide with pegylated interferon in less-advanced patients, because we know it is more potent and active against the HDV RNA,” he said.
Dr. Degasperi has previously declared she was on the advisory board for AbbVie and has spoken and taught for Gilead, MSD, and AbbVie. Dr. Bourlière declared interests with all companies involved in the R&D of liver therapies.
A version of this article first appeared on Medscape.com.
Bulevirtide (Hepcludex) monotherapy significantly reduces the load of hepatitis delta virus (HDV) and is safe in difficult-to-treat patients with compensated cirrhosis and clinically significant portal hypertension, according to the results of an ongoing 1-year study.
In presenting a poster with these findings at the annual International Liver Congress, sponsored by the European Association for the Study of the Liver, lead author Elisabetta Degasperi, MD, from the Grand Hospital Maggiore Policlinico in Milan, said that they were important “because they confirm the safety of this drug in real life.”
Dr. Degasperi and colleagues showed that bulevirtide leads to a significant viral response in 78% of patients by week 48, which was measured using the outcome of greater than 2 log decline in HDV RNA from baseline.
Dr. Degasperi added that the research still needed to assess the longer-term benefits, but
Addressing an immense, unmet therapeutic need
HDV requires the presence of hepatitis B virus to replicate. Bulevirtide blocks the entry of HDV and hepatitis B virus into hepatocytes.
In July 2020, it was conditionally approved in the European Economic Area for use to treat chronic HDV infection in adults with compensated liver disease upon confirmation of HDV RNA in the blood. It currently remains an investigational agent in the United States, as well as outside of the EEA.
The ongoing trial led by Dr. Degasperi is specifically conducted in patients with compensated cirrhosis who also have clinically significant portal hypertension, where safety and efficacy are unknown.
Dr. Degasperi said in an interview that, although HDV was rare, there is nonetheless an “immense” need for effective therapies against it, especially in young patients with advanced liver disease.
“We have a lot of patients with hepatitis D who have not responded to other antiviral treatment. Right now, the only other available treatment is pegylated interferon,” she said. “Unfortunately, rates of sustained viral response to pegylated interferon are extremely low at around 30% of patients.”
Chronic HDV is the most severe form of viral hepatitis and can have mortality rates as high as 50% within 5 years in patients with cirrhosis.
The management of hepatitis D is also complicated by the fact that patients with advanced cirrhosis and clinically significant portal hypertension cannot be treated with pegylated interferon owing to lack of efficacy and safety reasons, including a high risk for decompensation and liver-related complications. Pegylated interferon is contraindicated in these patients.
Bulevirtide at 48 weeks: A closer look at the findings
Eighteen patients with HDV, compensated cirrhosis, and clinically significant portal hypertension were consecutively enrolled in this single-center, longitudinal study.
All received bulevirtide monotherapy at 2 mg/day and underwent monitoring every 2 months. They were also treated with nucleotide analogs for their hepatitis B virus, which was suppressed when they began bulevirtide.
Clinical and virologic characteristics were collected at baseline, at weeks 4 and 8, and then every 8 weeks thereafter.
Bulevirtide led to a significant viral response such that by week 48, HDV RNA declined by 3.1 log IU/mL (range, 0.2-4.6 log IU/mL), was undetectable in six patients (33%), and was less than 100 IU/L in 50% of patients. Two patients were nonresponders. In addition, 78% of patients achieved at least an HDV RNA 2 log decline from baseline.
There was also a normalization of biochemical response in the majority of patients.
Alanine aminotransferase normalization was seen in 89% of patients and declined by a median of 34 U/L (range, 15-76 U/L) over 48 weeks. Aspartate aminotransferase declined to 39 U/L (range, 21-92 U/L). A combined response was seen in 72% of patients, reported Dr. Degasperi.
“Previously, we only had results from a phase 2 study, so we had no idea of the results over such a long treatment period,” said Dr. Degasperi. “It is also the first time we have been able to treat these patients with such advanced disease that is so difficult to manage.”
“Real-world results are typically inferior to those from clinical trials, but the viral decline is comparable to phase 2 trials, and the first report of the phase 3 trial,” said Dr. Degasperi.
Gamma-glutamyltransferase, alpha-fetoprotein, immunoglobulin G, and gamma-globulin levels also improved, whereas hepatitis B surface antigen, hepatitis B virus RNA, hepatitis B core-related antigen, platelet, and bilirubin values did not significantly change.
“All patients were Child-Pugh score A, so well-compensated [disease]. However, they increased a little bit in liver function by week 48,” Dr. Degasperi said. “This was important for this very advanced disease population.”
She added that the safety profile was very favorable, with no adverse events, including no injection-site reactions.
There was an asymptomatic increase in serum bile acids. “No patients complained about itching or pruritus,” Dr. Degasperi said.
What’s ahead for bulevirtide?
In a comment, Marc Bourlière, MD, from Saint Joseph Hospital in Marseilles, France, welcomed the decrease in viral load.
“This is known to be beneficial in terms of reducing morbidity and mortality in hepatitis D,” he said. “Remember that this disease is very difficult to treat, and until now, we have had no drug available. Pegylated interferon achieves cure in only 30% of patients, and half of these relapse, so actually only 15% have a meaningful response from pegylated interferon.”
“The main issue is its use as a daily subcutaneous injection. In clinical practice, it is a little bit complicated to set up, but once done, it is quite well accepted,” he said.
“I’m impressed with these results to date because there are no other compounds that have, as yet, achieved such results. This is impressive,” he added. “But whether it translates into a long-term response we don’t yet know.”
Dr. Bourlière also noted the meaningful 2-point log decline, noting that “HDV RNA negativity where treatment can be stopped would be really meaningful, but this endpoint is hard to obtain.”
Dr. Bourlière is awaiting results of the current ongoing phase 2/3 study, which would help determine a possible final treatment duration. He is also curious to settle the ongoing debate about whether bulevirtide should be used alone or in combination.
“We need to combine bulevirtide with pegylated interferon in less-advanced patients, because we know it is more potent and active against the HDV RNA,” he said.
Dr. Degasperi has previously declared she was on the advisory board for AbbVie and has spoken and taught for Gilead, MSD, and AbbVie. Dr. Bourlière declared interests with all companies involved in the R&D of liver therapies.
A version of this article first appeared on Medscape.com.
FROM ILC 2022
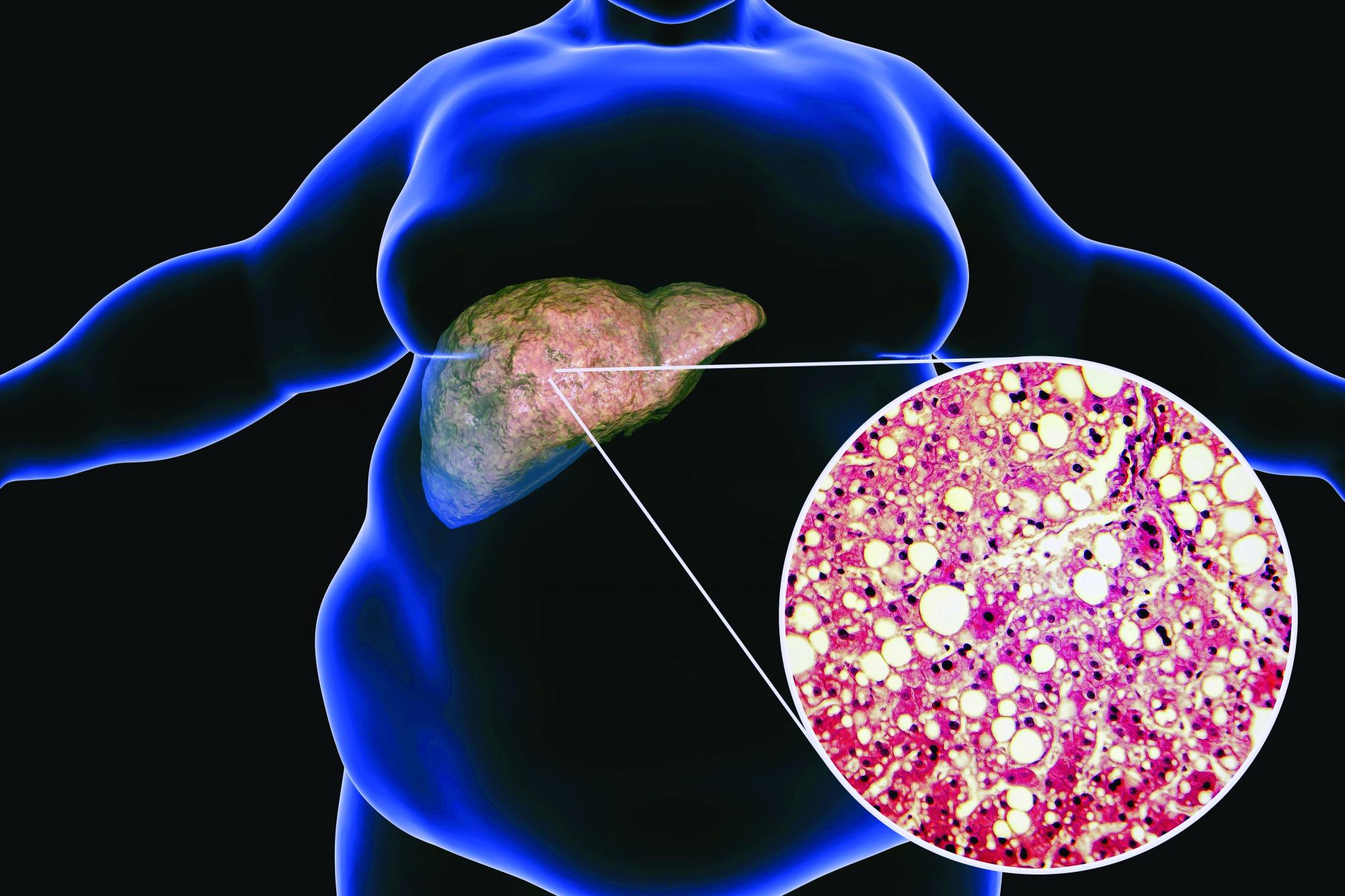
Low-carb, high-fat diet improves A1c, reduces liver fat
LONDON – A low-carbohydrate, high-fat (LCHF) diet reduced the progression of nonalcoholic fatty liver disease (NAFLD), and despite no calorie restriction, participants with both NAFLD and type 2 diabetes lost 5.8% of their body weight, according to a randomized controlled study.
“Based on these results, the LCHF diet may be recommended to people with NAFLD and type 2 diabetes,” said Camilla Dalby Hansen, MD, department of gastroenterology and hepatology, Odense University Hospital, Denmark, who presented the data at the International Liver Congress (ILC) 2022.
“Basically, if you have fat in your liver, you will benefit from eating fat,” she said.
The LCHF diet was compared with a low-fat, high-carbohydrate diet more typically followed for these conditions. The low-fat diet was also found to reduce the progression of NAFLD, but to a lesser extent than the LCHF diet.
Dr. Dalby Hansen called their study one of the most extensive investigations of the LCHF diet in patients with type 2 diabetes and fatty liver disease.
“Combining this [reduction in NAFLD score] with the huge weight loss, the lower HbA1c [blood sugar], the lowering of blood pressure in women, the rise in HDL levels, and reduction in triglycerides – all in all, this diet is very promising,” she said.
Stephen Harrison, MD, visiting professor, University of Oxford, United Kingdom, medical director of Pinnacle Clinical Research and president of Summit Clinical Research, San Antonio, commended Dr. Dalby Hansen on her methodology, which included before-and-after liver biopsies. “It’s a heinous effort to do paired liver biopsies in a lifestyle modification trial. That’s huge.”
“This study tells me that the way we manage patients doesn’t change – it is still lifestyle modification,” said Dr. Harrison, who was not involved with the study. “It’s eat less [rather] than more. It’s exercise and try to lose weight. In the long term, we give patients benefit, and we show that the disease has improved, and we offer something that means they can maintain a healthy life.”
He added that the relatively small and short trial was informative.
“They improved the NAFLD activity score [NAS],” he said. “I don’t know by how much. There was no change in fibrosis, but we wouldn’t expect this at 6 months.”
“It’s provocative work, and it gives us healthy information about how we can help manage our patients from a lifestyle perspective,” he concluded.
‘Do not lose weight. Eat until you are full’
In the study, 110 participants with type 2 diabetes and NAFLD, aged 18-78 years, were allocated to the LCHF diet, and 55 were allocated to the low-fat diet for 6 months.
The researchers performed liver biopsies at baseline and 6 months, which were blinded for scoring.
Participants had ongoing dietitian consultations, with follow-up visits at 3 and 6 months. Compliance was reported continuously through an online food diary platform.
The primary endpoint was change in glycemic control as measured by A1c level over 6 months. The secondary endpoints comprised the proportion of participants with changes in the NAS of at least 2 points over 6 months. Both these measures were compared between the two dietary groups.
The two groups were matched at baseline, with a mean age of 55-57 years, 58% were women, 89% with metabolic syndrome, and a mean BMI 34 kg/m2.
In baseline liver disease, F1 level fibrosis was the most common (58%), followed by hepatic steatosis (S1, 47%; S2, 32%), with a median NAS of 3, and 19% had nonalcoholic steatohepatitis.
The special thing about these diets was that participants were told to “not lose weight, but eat until you are full,” remarked Dr. Dalby Hansen.
Those on the LCHF diet consumed an average of 61% energy from fat, 13% from carbohydrates, and 23% from protein, compared with the low-fat diet, which comprised an average of 29% energy from fat, 46% from carbohydrates, and 21% from protein.
“It’s a lot of fat and corresponds to a quarter of a liter of olive oil per day,” said Dr. Dalby Hansen. “They really had to change their mindset a lot, because it was difficult for them to start eating all these fats, especially since we’ve all been told for decades that it isn’t good. But we supported them, and they got into it.”
The LCHF diet was primarily comprised of unsaturated fats – for example, avocado, oil, nuts, and seeds – but also included saturated fats, such as cheese, cream, and high-fat dairy products. Participants were free to eat unsaturated and saturated fats, but Dr. Dalby Hansen and her team advised participants that “good” unsaturated fats were preferable.
“Also, this diet contained vegetables but no bread, no potatoes, no rice, and no pasta. It was low in carbohydrates, below 20%,” she added.
Improved glycemic control, reduced liver fat
“We found that the LCHF diet improved diabetes control, it reduced the fat in the liver, and, even though they’re eating as many calories as they were used to until they were full, they lost 5.8% of body weight,” said Dr. Dalby Hansen in reporting the results. Participants in the low-fat group lost only 1.8% of body weight.
However, mean calorie intake dropped in both groups, by –2.2% in the LCHF group and –8.7% in the low-fat group.
“The LCHF diet improved the primary outcome of A1c by 9.5 mmol/mol, which is similar to some anti-diabetic medications, such as DPP-4 inhibitors and SGLT2 inhibitors,” she said.
The low-fat group reduced A1c by 3.4 mmol/mol, resulting in a between-group difference of 6.1 mmol/mol.
“Upon follow-up of 3 months, after stopping the diets, on average the participants in both groups returned their HbA1c levels to nearly baseline values,” she said. Results were adjusted for weight loss and baseline values.
Both diets also improved the NAS. The proportion of participants who improved their NAS score by 2 or more points was 22% in the LCHF group versus 17% in the low-fat group (P = 0.58). Additionally, in the LCHF group, 70% of participants improved their score by 1 or more points, compared with 49% in the low-fat group and fewer in the LCHF group experienced a worsening of their score (1% vs. 23%, respectively).
One participant on LCHF had high triglycerides of 12 mmol/L after 3 months. Overall, the low-density lipoprotein increased marginally by 0.2 mmol per liter in the high-fat group, said Dr. Dalby Hansen.
Dr. Dalby Hansen noted some limitations. The findings might not be applicable in more severe NAFLD, dietary assessment relied on self-reporting, no food was provided, and participants had to cook themselves. It was also an open-label study because of the nature of the intervention.
Some hope for more sustainable dieting
Many diets are difficult to adhere to, remarked Dr. Dalby Hansen. “We thought this [diet] might be easier to comply with in the longer term, and we hope that these results might provide patients with more options.”
She added that most people who started the diet adapted and complied with it. “However, it might not be for everyone, but I think we can say that if people try, and it fits into their lives, then they go for it.”
However, “it is not about going out and eating whatever fat and how much of it you want. It’s important that you cut the carbohydrates too,” she said. “With this approach, we really saw amazing results.”
Dr. Dalby Hansen added that having various diets available, including the LCHF one, meant that as clinicians they could empower patients to take control of their metabolic health.
“We can ask them directly, ‘What would fit into their life?’” she said. “We know that one size does not fit at all, and I believe that if we could engage patients more, then they can take control of their own situation.”
Asked whether these findings were enough to change guidelines, Zobair Younossi, MD, professor and chairman, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., remarked that it was the sugar at work here.
“Dietary fat – it’s not the same as fat in the liver, and this diet has more to do with the sugar levels,” he said.
“I’m always reluctant to take results from a short-term study without long-term follow-up,” Dr. Younossi said. “I want to know will patients live longer, and long-term data are needed for this. Until I have that strong evidence that outcomes are going to change, or at least some sign that the outcome is going to change, it is too early to change any guidelines.”
Dr. Dalby Hansen reports no relevant financial relationships. Dr. Harrison reported financial relationships with numerous pharmaceutical companies. Dr. Younossi reports the following financial relationships: research funds and/or consultant to Abbott, Allergan, Bristol Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
LONDON – A low-carbohydrate, high-fat (LCHF) diet reduced the progression of nonalcoholic fatty liver disease (NAFLD), and despite no calorie restriction, participants with both NAFLD and type 2 diabetes lost 5.8% of their body weight, according to a randomized controlled study.
“Based on these results, the LCHF diet may be recommended to people with NAFLD and type 2 diabetes,” said Camilla Dalby Hansen, MD, department of gastroenterology and hepatology, Odense University Hospital, Denmark, who presented the data at the International Liver Congress (ILC) 2022.
“Basically, if you have fat in your liver, you will benefit from eating fat,” she said.
The LCHF diet was compared with a low-fat, high-carbohydrate diet more typically followed for these conditions. The low-fat diet was also found to reduce the progression of NAFLD, but to a lesser extent than the LCHF diet.
Dr. Dalby Hansen called their study one of the most extensive investigations of the LCHF diet in patients with type 2 diabetes and fatty liver disease.
“Combining this [reduction in NAFLD score] with the huge weight loss, the lower HbA1c [blood sugar], the lowering of blood pressure in women, the rise in HDL levels, and reduction in triglycerides – all in all, this diet is very promising,” she said.
Stephen Harrison, MD, visiting professor, University of Oxford, United Kingdom, medical director of Pinnacle Clinical Research and president of Summit Clinical Research, San Antonio, commended Dr. Dalby Hansen on her methodology, which included before-and-after liver biopsies. “It’s a heinous effort to do paired liver biopsies in a lifestyle modification trial. That’s huge.”
“This study tells me that the way we manage patients doesn’t change – it is still lifestyle modification,” said Dr. Harrison, who was not involved with the study. “It’s eat less [rather] than more. It’s exercise and try to lose weight. In the long term, we give patients benefit, and we show that the disease has improved, and we offer something that means they can maintain a healthy life.”
He added that the relatively small and short trial was informative.
“They improved the NAFLD activity score [NAS],” he said. “I don’t know by how much. There was no change in fibrosis, but we wouldn’t expect this at 6 months.”
“It’s provocative work, and it gives us healthy information about how we can help manage our patients from a lifestyle perspective,” he concluded.
‘Do not lose weight. Eat until you are full’
In the study, 110 participants with type 2 diabetes and NAFLD, aged 18-78 years, were allocated to the LCHF diet, and 55 were allocated to the low-fat diet for 6 months.
The researchers performed liver biopsies at baseline and 6 months, which were blinded for scoring.
Participants had ongoing dietitian consultations, with follow-up visits at 3 and 6 months. Compliance was reported continuously through an online food diary platform.
The primary endpoint was change in glycemic control as measured by A1c level over 6 months. The secondary endpoints comprised the proportion of participants with changes in the NAS of at least 2 points over 6 months. Both these measures were compared between the two dietary groups.
The two groups were matched at baseline, with a mean age of 55-57 years, 58% were women, 89% with metabolic syndrome, and a mean BMI 34 kg/m2.
In baseline liver disease, F1 level fibrosis was the most common (58%), followed by hepatic steatosis (S1, 47%; S2, 32%), with a median NAS of 3, and 19% had nonalcoholic steatohepatitis.
The special thing about these diets was that participants were told to “not lose weight, but eat until you are full,” remarked Dr. Dalby Hansen.
Those on the LCHF diet consumed an average of 61% energy from fat, 13% from carbohydrates, and 23% from protein, compared with the low-fat diet, which comprised an average of 29% energy from fat, 46% from carbohydrates, and 21% from protein.
“It’s a lot of fat and corresponds to a quarter of a liter of olive oil per day,” said Dr. Dalby Hansen. “They really had to change their mindset a lot, because it was difficult for them to start eating all these fats, especially since we’ve all been told for decades that it isn’t good. But we supported them, and they got into it.”
The LCHF diet was primarily comprised of unsaturated fats – for example, avocado, oil, nuts, and seeds – but also included saturated fats, such as cheese, cream, and high-fat dairy products. Participants were free to eat unsaturated and saturated fats, but Dr. Dalby Hansen and her team advised participants that “good” unsaturated fats were preferable.
“Also, this diet contained vegetables but no bread, no potatoes, no rice, and no pasta. It was low in carbohydrates, below 20%,” she added.
Improved glycemic control, reduced liver fat
“We found that the LCHF diet improved diabetes control, it reduced the fat in the liver, and, even though they’re eating as many calories as they were used to until they were full, they lost 5.8% of body weight,” said Dr. Dalby Hansen in reporting the results. Participants in the low-fat group lost only 1.8% of body weight.
However, mean calorie intake dropped in both groups, by –2.2% in the LCHF group and –8.7% in the low-fat group.
“The LCHF diet improved the primary outcome of A1c by 9.5 mmol/mol, which is similar to some anti-diabetic medications, such as DPP-4 inhibitors and SGLT2 inhibitors,” she said.
The low-fat group reduced A1c by 3.4 mmol/mol, resulting in a between-group difference of 6.1 mmol/mol.
“Upon follow-up of 3 months, after stopping the diets, on average the participants in both groups returned their HbA1c levels to nearly baseline values,” she said. Results were adjusted for weight loss and baseline values.
Both diets also improved the NAS. The proportion of participants who improved their NAS score by 2 or more points was 22% in the LCHF group versus 17% in the low-fat group (P = 0.58). Additionally, in the LCHF group, 70% of participants improved their score by 1 or more points, compared with 49% in the low-fat group and fewer in the LCHF group experienced a worsening of their score (1% vs. 23%, respectively).
One participant on LCHF had high triglycerides of 12 mmol/L after 3 months. Overall, the low-density lipoprotein increased marginally by 0.2 mmol per liter in the high-fat group, said Dr. Dalby Hansen.
Dr. Dalby Hansen noted some limitations. The findings might not be applicable in more severe NAFLD, dietary assessment relied on self-reporting, no food was provided, and participants had to cook themselves. It was also an open-label study because of the nature of the intervention.
Some hope for more sustainable dieting
Many diets are difficult to adhere to, remarked Dr. Dalby Hansen. “We thought this [diet] might be easier to comply with in the longer term, and we hope that these results might provide patients with more options.”
She added that most people who started the diet adapted and complied with it. “However, it might not be for everyone, but I think we can say that if people try, and it fits into their lives, then they go for it.”
However, “it is not about going out and eating whatever fat and how much of it you want. It’s important that you cut the carbohydrates too,” she said. “With this approach, we really saw amazing results.”
Dr. Dalby Hansen added that having various diets available, including the LCHF one, meant that as clinicians they could empower patients to take control of their metabolic health.
“We can ask them directly, ‘What would fit into their life?’” she said. “We know that one size does not fit at all, and I believe that if we could engage patients more, then they can take control of their own situation.”
Asked whether these findings were enough to change guidelines, Zobair Younossi, MD, professor and chairman, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., remarked that it was the sugar at work here.
“Dietary fat – it’s not the same as fat in the liver, and this diet has more to do with the sugar levels,” he said.
“I’m always reluctant to take results from a short-term study without long-term follow-up,” Dr. Younossi said. “I want to know will patients live longer, and long-term data are needed for this. Until I have that strong evidence that outcomes are going to change, or at least some sign that the outcome is going to change, it is too early to change any guidelines.”
Dr. Dalby Hansen reports no relevant financial relationships. Dr. Harrison reported financial relationships with numerous pharmaceutical companies. Dr. Younossi reports the following financial relationships: research funds and/or consultant to Abbott, Allergan, Bristol Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
LONDON – A low-carbohydrate, high-fat (LCHF) diet reduced the progression of nonalcoholic fatty liver disease (NAFLD), and despite no calorie restriction, participants with both NAFLD and type 2 diabetes lost 5.8% of their body weight, according to a randomized controlled study.
“Based on these results, the LCHF diet may be recommended to people with NAFLD and type 2 diabetes,” said Camilla Dalby Hansen, MD, department of gastroenterology and hepatology, Odense University Hospital, Denmark, who presented the data at the International Liver Congress (ILC) 2022.
“Basically, if you have fat in your liver, you will benefit from eating fat,” she said.
The LCHF diet was compared with a low-fat, high-carbohydrate diet more typically followed for these conditions. The low-fat diet was also found to reduce the progression of NAFLD, but to a lesser extent than the LCHF diet.
Dr. Dalby Hansen called their study one of the most extensive investigations of the LCHF diet in patients with type 2 diabetes and fatty liver disease.
“Combining this [reduction in NAFLD score] with the huge weight loss, the lower HbA1c [blood sugar], the lowering of blood pressure in women, the rise in HDL levels, and reduction in triglycerides – all in all, this diet is very promising,” she said.
Stephen Harrison, MD, visiting professor, University of Oxford, United Kingdom, medical director of Pinnacle Clinical Research and president of Summit Clinical Research, San Antonio, commended Dr. Dalby Hansen on her methodology, which included before-and-after liver biopsies. “It’s a heinous effort to do paired liver biopsies in a lifestyle modification trial. That’s huge.”
“This study tells me that the way we manage patients doesn’t change – it is still lifestyle modification,” said Dr. Harrison, who was not involved with the study. “It’s eat less [rather] than more. It’s exercise and try to lose weight. In the long term, we give patients benefit, and we show that the disease has improved, and we offer something that means they can maintain a healthy life.”
He added that the relatively small and short trial was informative.
“They improved the NAFLD activity score [NAS],” he said. “I don’t know by how much. There was no change in fibrosis, but we wouldn’t expect this at 6 months.”
“It’s provocative work, and it gives us healthy information about how we can help manage our patients from a lifestyle perspective,” he concluded.
‘Do not lose weight. Eat until you are full’
In the study, 110 participants with type 2 diabetes and NAFLD, aged 18-78 years, were allocated to the LCHF diet, and 55 were allocated to the low-fat diet for 6 months.
The researchers performed liver biopsies at baseline and 6 months, which were blinded for scoring.
Participants had ongoing dietitian consultations, with follow-up visits at 3 and 6 months. Compliance was reported continuously through an online food diary platform.
The primary endpoint was change in glycemic control as measured by A1c level over 6 months. The secondary endpoints comprised the proportion of participants with changes in the NAS of at least 2 points over 6 months. Both these measures were compared between the two dietary groups.
The two groups were matched at baseline, with a mean age of 55-57 years, 58% were women, 89% with metabolic syndrome, and a mean BMI 34 kg/m2.
In baseline liver disease, F1 level fibrosis was the most common (58%), followed by hepatic steatosis (S1, 47%; S2, 32%), with a median NAS of 3, and 19% had nonalcoholic steatohepatitis.
The special thing about these diets was that participants were told to “not lose weight, but eat until you are full,” remarked Dr. Dalby Hansen.
Those on the LCHF diet consumed an average of 61% energy from fat, 13% from carbohydrates, and 23% from protein, compared with the low-fat diet, which comprised an average of 29% energy from fat, 46% from carbohydrates, and 21% from protein.
“It’s a lot of fat and corresponds to a quarter of a liter of olive oil per day,” said Dr. Dalby Hansen. “They really had to change their mindset a lot, because it was difficult for them to start eating all these fats, especially since we’ve all been told for decades that it isn’t good. But we supported them, and they got into it.”
The LCHF diet was primarily comprised of unsaturated fats – for example, avocado, oil, nuts, and seeds – but also included saturated fats, such as cheese, cream, and high-fat dairy products. Participants were free to eat unsaturated and saturated fats, but Dr. Dalby Hansen and her team advised participants that “good” unsaturated fats were preferable.
“Also, this diet contained vegetables but no bread, no potatoes, no rice, and no pasta. It was low in carbohydrates, below 20%,” she added.
Improved glycemic control, reduced liver fat
“We found that the LCHF diet improved diabetes control, it reduced the fat in the liver, and, even though they’re eating as many calories as they were used to until they were full, they lost 5.8% of body weight,” said Dr. Dalby Hansen in reporting the results. Participants in the low-fat group lost only 1.8% of body weight.
However, mean calorie intake dropped in both groups, by –2.2% in the LCHF group and –8.7% in the low-fat group.
“The LCHF diet improved the primary outcome of A1c by 9.5 mmol/mol, which is similar to some anti-diabetic medications, such as DPP-4 inhibitors and SGLT2 inhibitors,” she said.
The low-fat group reduced A1c by 3.4 mmol/mol, resulting in a between-group difference of 6.1 mmol/mol.
“Upon follow-up of 3 months, after stopping the diets, on average the participants in both groups returned their HbA1c levels to nearly baseline values,” she said. Results were adjusted for weight loss and baseline values.
Both diets also improved the NAS. The proportion of participants who improved their NAS score by 2 or more points was 22% in the LCHF group versus 17% in the low-fat group (P = 0.58). Additionally, in the LCHF group, 70% of participants improved their score by 1 or more points, compared with 49% in the low-fat group and fewer in the LCHF group experienced a worsening of their score (1% vs. 23%, respectively).
One participant on LCHF had high triglycerides of 12 mmol/L after 3 months. Overall, the low-density lipoprotein increased marginally by 0.2 mmol per liter in the high-fat group, said Dr. Dalby Hansen.
Dr. Dalby Hansen noted some limitations. The findings might not be applicable in more severe NAFLD, dietary assessment relied on self-reporting, no food was provided, and participants had to cook themselves. It was also an open-label study because of the nature of the intervention.
Some hope for more sustainable dieting
Many diets are difficult to adhere to, remarked Dr. Dalby Hansen. “We thought this [diet] might be easier to comply with in the longer term, and we hope that these results might provide patients with more options.”
She added that most people who started the diet adapted and complied with it. “However, it might not be for everyone, but I think we can say that if people try, and it fits into their lives, then they go for it.”
However, “it is not about going out and eating whatever fat and how much of it you want. It’s important that you cut the carbohydrates too,” she said. “With this approach, we really saw amazing results.”
Dr. Dalby Hansen added that having various diets available, including the LCHF one, meant that as clinicians they could empower patients to take control of their metabolic health.
“We can ask them directly, ‘What would fit into their life?’” she said. “We know that one size does not fit at all, and I believe that if we could engage patients more, then they can take control of their own situation.”
Asked whether these findings were enough to change guidelines, Zobair Younossi, MD, professor and chairman, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., remarked that it was the sugar at work here.
“Dietary fat – it’s not the same as fat in the liver, and this diet has more to do with the sugar levels,” he said.
“I’m always reluctant to take results from a short-term study without long-term follow-up,” Dr. Younossi said. “I want to know will patients live longer, and long-term data are needed for this. Until I have that strong evidence that outcomes are going to change, or at least some sign that the outcome is going to change, it is too early to change any guidelines.”
Dr. Dalby Hansen reports no relevant financial relationships. Dr. Harrison reported financial relationships with numerous pharmaceutical companies. Dr. Younossi reports the following financial relationships: research funds and/or consultant to Abbott, Allergan, Bristol Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk.
A version of this article first appeared on Medscape.com.
AT ILC 2022
Acute hepatitis cases in children show declining trend; adenovirus, COVID-19 remain key leads
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Case numbers of acute hepatitis in children show “a declining trajectory,” and COVID-19 and adenovirus remain the most likely, but as yet unproven, causative agents, said experts in an update at the annual International Liver Congress sponsored by the European Association for the Study of the Liver.
Philippa Easterbrook, MD, medical expert at the World Health Organization Global HIV, Hepatitis, and STI Programme, shared the latest case numbers and working hypotheses of possible causative agents in the outbreak of acute hepatitis among children in Europe and beyond.
Global data across the five WHO regions show there were 244 cases in the past month, bringing the total to 894 probable cases reported since October 2021 from 33 countries.
“It’s important to remember that this includes new cases, as well as retrospectively identified cases,” Dr.Easterbrook said. “Over half (52%) are from the European region, while 262 cases (30% of the global total) are from the United Kingdom.”
Data from Europe and the United States show a declining trajectory of reports of new cases. “This is a positive development,” she said.
The second highest reporting region is the Americas, she said, with 368 cases total, 290 cases of which come from the United States, accounting for 35% of the global total.
“Together the United Kingdom and the United States make up 65% of the global total,” she said.
Dr. Easterbrook added that 17 of the 33 reporting countries had more than five cases. Most cases (75%) are in young children under 5 years of age.
Serious cases are relatively few, but 44 (5%) children have required liver transplantation. Data from the European region show that 30% have required intensive care at some point during their hospitalization. There have been 18 (2%) reported deaths.
Possible post-COVID phenomenon, adenovirus most commonly reported
Dr. Easterbrook acknowledged the emerging hypothesis of a post-COVID phenomenon.
“Is this a variant of the rare but recognized multisystem inflammatory syndrome condition in children that’s been reported, often 1-2 months after COVID, causing widespread organ damage?” But she pointed out that the reported COVID cases with hepatitis “don’t seem to fit these features.”
Adenovirus remains the most commonly detected virus in acute hepatitis in children, found in 53% of cases overall, she said. The adenovirus detection rate is higher in the United Kingdom, at 68%.
“There are quite high rates of detection, but they’re not in all cases. There does seem to be a high rate of detection in the younger age groups and in those who are developing severe disease, so perhaps there is some link to severity,” Dr. Easterbrook said.
The working hypotheses continue to favor adenovirus together with past or current SARS-CoV-2 infection, as proposed early in the outbreak, she said. “These either work independently or work together as cofactors in some way to result in hepatitis. And there has been some clear progress on this. WHO is bringing together the data from different countries on some of these working hypotheses.”
Dr. Easterbrook highlighted the importance of procuring global data, especially given that two countries are reporting the majority of cases and in high numbers. “It’s a mixed picture with different rates of adenovirus detection and of COVID,” she said. “We need good-quality data collected in a standardized way.” WHO is requesting that countries provide these data.
She also highlighted the need for good in-depth studies, citing the UK Health Security Agency as an example of this. “There’s only a few countries that have the capacity or the patient numbers to look at this in detail, for example, the U.K. and the UKHSA.”
She noted that the UKHSA had laid out a comprehensive, systematic set of further investigations. For example, a case-control study is trying to establish whether there is a difference in the rate of adenovirus detection in children with hepatitis compared with other hospitalized children at the same time. “This aims to really tease out whether adenovirus is a cause or just a bystander,” she said.
She added that there were also genetic studies investigating whether genes were predisposing some children to develop a more severe form of disease. Other studies are evaluating the immune response of the patients.
Dr. Easterbrook added that the WHO will soon launch a global survey asking whether the reports of acute hepatitis are greater than the expected background rate for cases of hepatitis of unknown etiology.
Acute hepatitis is not new, but high caseload is
Also speaking at the ILC special briefing was Maria Buti, MD, PhD, policy and public health chair for the European Association for the Study of the Liver, and chief of the internal medicine and hepatology department at Hospital General Universitari Valle Hebron in Barcelona.
Dr. Buti drew attention to the fact that severe acute hepatitis of unknown etiology in children is not new.
“We have cases of acute hepatitis that even needed liver transplantation some years ago, and every year in our clinics we see these type of patients,” Dr. Buti remarked. What is really new, she added, is the amount of cases, particularly in the United Kingdom.
Dr. Easterbrook and Dr. Buti have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
Fatty liver disease drives rise in liver cancer deaths
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the International Liver Congress (ILC) 2022.
“NAFLD and NASH [nonalcoholic steatohepatitis] are rapidly becoming the main causes of cirrhosis and liver cancer in the world,” Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009-2019, the annual percent change (APC) of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense Liver Research Centre at SDU and Odense University Hospital, Denmark, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around two billion cases of chronic liver disease globally, he said. He noted that over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that globally, the overall prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policymakers is very low. “From my perspective, raising awareness of NAFLD is the number one priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The WHO [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Krag has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the International Liver Congress (ILC) 2022.
“NAFLD and NASH [nonalcoholic steatohepatitis] are rapidly becoming the main causes of cirrhosis and liver cancer in the world,” Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009-2019, the annual percent change (APC) of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense Liver Research Centre at SDU and Odense University Hospital, Denmark, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around two billion cases of chronic liver disease globally, he said. He noted that over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that globally, the overall prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policymakers is very low. “From my perspective, raising awareness of NAFLD is the number one priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The WHO [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Krag has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the International Liver Congress (ILC) 2022.
“NAFLD and NASH [nonalcoholic steatohepatitis] are rapidly becoming the main causes of cirrhosis and liver cancer in the world,” Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009-2019, the annual percent change (APC) of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense Liver Research Centre at SDU and Odense University Hospital, Denmark, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around two billion cases of chronic liver disease globally, he said. He noted that over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that globally, the overall prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policymakers is very low. “From my perspective, raising awareness of NAFLD is the number one priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The WHO [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Krag has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
Fatty liver disease drives rise in liver cancer deaths
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the meeting sponsored by the European Association for the Study of the Liver.
Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009–2019, the annual percent change of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense (Denmark) Liver Research Centre at SDU and Odense University Hospital, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around 2 billion cases of chronic liver disease globally, he said. He noted that, over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that the overall global prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policy makers is very low. “From my perspective, raising awareness of NAFLD is the No. 1 priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Karg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the meeting sponsored by the European Association for the Study of the Liver.
Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009–2019, the annual percent change of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense (Denmark) Liver Research Centre at SDU and Odense University Hospital, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around 2 billion cases of chronic liver disease globally, he said. He noted that, over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that the overall global prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policy makers is very low. “From my perspective, raising awareness of NAFLD is the No. 1 priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Karg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
LONDON – Around the world, nonalcoholic fatty liver disease (NAFLD) has driven an increase in deaths from liver cancer over the past decade, overtaking alcoholic liver disease, hepatitis B, and hepatitis C, according to an analysis of the Global Burden of Disease Study 2019.
A global rise in liver cancer deaths and chronic liver disease reflects changes in underlying health patterns, said Zobair Younossi, MD, MPH, professor and chair, department of medicine, Inova Fairfax Medical Campus, Falls Church, Va., who presented the analysis at the meeting sponsored by the European Association for the Study of the Liver.
Dr. Younossi told this news organization. “We have known about the increasing prevalence for some time, but now the outcomes in terms of mortality are catching up,” he said.
“The bottom line of this study is that the burden of this disease [NAFLD] is going up, and it will be the most important disease of the next decade or so,” he said, adding that “the largest annual percentage increase in rates of mortality from liver cancer or chronic liver disease cirrhosis is related to NAFLD.”
Specifically, during the decade of 2009–2019, the annual percent change of +1.33% in the global liver cancer death rate was driven by the fact that the APC for NAFLD was +2.47%. By comparison, the APC for alcoholic liver disease was +1.91%; for hepatitis B, the APC was +0.21%; and for hepatitis C, the APC was +1.12%.
Aleksander Krag, MD, PhD, professor and senior consultant of hepatology and director of Odense (Denmark) Liver Research Centre at SDU and Odense University Hospital, who chaired the session in which this presentation was a part, acknowledged the importance of recognizing the contribution of NAFLD to liver cancer mortality.
“Liver diseases are on the rise. They are the fastest rising cause of death in the United Kingdom, faster than heart disease and other cancers. NAFLD in particular is the fastest growing cause of liver cancer, and the leading cause in France and the United States,” he remarked.
Dr. Krag also highlighted the costs of disease management.
“Managing fatty liver disease in Europe is estimated at €35 billion in direct health care, so we need to do something now,” he stressed.
“The global burden of NAFLD is so high that we need both prevention and treatment tools,” Dr. Krag said. “Change to lifestyle is a ‘no-brainer’ and costs governments very little. For the sake of our young people, we need to take this very seriously. At a political level, we can easily implement this, for example, by banning junk food advertisements, but also educating young people and their families. Good drugs will also help.”
NAFLD: The liver manifestation of type 2 diabetes
About 25%-30% of the global population have NAFLD, and 3%-5% have NASH. Dr. Younossi highlighted that the U.S. transplant database shows that NAFLD was the second indication for all liver transplants in the country. NAFLD also was a leading cause of liver transplants for patients with hepatocellular carcinoma.
There are around 2 billion cases of chronic liver disease globally, he said. He noted that, over time, there has been an increase in all kinds of liver diseases, as reflected in the annual percent change.
“The global epidemic of obesity and type 2 diabetes is driving the rise in NAFLD, but even among lean people, the prevalence of NAFLD is around 9%,” Dr. Younossi said. “Alongside the eye and kidney complications of diabetes, this is the liver manifestation of type 2 diabetes.”
To assess global liver disease and death, Dr. Younossi and his colleagues turned to the Global Burden of Disease Study, which gathered data from around 7,000 investigators located across 22 different regions of the world, comprising 156 countries.
They calculated the incidence, prevalence, mortality, and disability-adjusted life-years (DALYs) in relation to liver cancer and chronic liver disease, including the APC. They linked the data to changes in four liver diseases: NAFLD, alcoholic liver disease, hepatitis B infection, and hepatitis C infection.
The cases of NAFLD reported in the study had been diagnosed by ultrasound or other imaging. Importantly, the prevalence of NAFLD was adjusted for alcohol use in the various national populations, explained Dr. Younossi.
In 2019, they reported that the overall global prevalence of liver disease reached 1.69 billion (liver cancer, 0.04%; chronic liver disease, 99.96%), with an incidence of 2.59 million (liver cancer, 20.7%; chronic liver disease, 79.3%), mortality of 1.95 million (liver cancer, 24.8%; chronic liver disease, 75.3%), and DALYs of 58.7 million (liver cancer, 21.3%; chronic liver disease, 78.7%).
Between 2009 and 2019, deaths from liver cancer rose by 27.2%, and deaths from chronic liver disease rose by 10.6%. DALYs from liver cancer rose by 21.9%, and DALYs from chronic liver disease were up by 5.1%.
In contrast to the increase in liver cancer deaths, deaths from chronic liver disease decreased (APC, –0.18%). The decrease was driven by a decrease in hepatitis B (APC, –1.83%). APCs for hepatitis C (+0.37%), alcoholic liver disease (+0.45%), and NAFLD (+1.33%) increased.
“The burden of hepatitis B–related mortality has decreased because we have been so good at vaccinating people,” Dr. Younossi remarked.
NAFLD ‘exploding’ in Middle East, North Africa, and East Asia
The increase in NAFLD has been seen in all regions of the world, but a breakdown by region shows that NAFLD is primarily “exploding” with highest prevalence and mortality in the Middle East (mostly Egypt, Iran, and Turkey), North Africa, and East Asia, said Dr. Younossi. In addition, there are large increases in the West and South America.
“We knew that the prevalence was high in the Middle East, but we now know that mortality is also high, so we are connecting these data,” said Dr. Younossi.
Awareness lacking
Dr. Younossi pressed the fact that awareness among the general population, primary care providers, and policy makers is very low. “From my perspective, raising awareness of NAFLD is the No. 1 priority, and that is the value of this study.”
He added that more people will become aware as testing becomes more manageable.
“There are some noninvasive tests being developed, so in the future, we won’t have to do liver biopsies to diagnose these patients,” he said. “Currently, there are some excellent treatments being developed.”
“The [World Health Organization] does not mention NAFLD as an important noncommunicable disease, and this too has to change,” Dr. Younossi added.
Dr. Younossi has received research funds and/or has consulted for Abbott, Allergan, Bristol-Myers Squibb, Echosens, Genfit, Gilead Sciences, Intercept, Madrigal, Merck, and Novo Nordisk. Dr. Karg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ILC 2022
Stopping immunosuppressives in lupus nephritis isn’t noninferior to continuing
COPENHAGEN – Discontinuing maintenance immunosuppressive therapy (IST) in patients with proliferative lupus nephritis in remission proved less effective than continuing it in terms of the rates of renal relapse and severe systemic lupus erythematosus (SLE) flare, results from the WIN-Lupus trial show.
Lead researcher Noemie Jourde-Chiche, MD, assistant professor at Aix-Marseille (France) University, presented the results at the annual European Congress of Rheumatology.
The randomized, controlled trial aimed to determine the optimal duration of maintenance IST for proliferative lupus nephritis, by asking whether discontinuation of such therapy after 2-3 years was noninferior to IST continuation for 2 more years.
“This is the first randomized IST trial in proliferative lupus nephritis,” Dr. Jourde-Chiche reported. “We found that noninferiority of IST discontinuation was not demonstrated, and those who discontinued had a higher risk of SLE flares, but the majority of patients who discontinued did not experience flare.”
Regarding the incidence of renal relapse, no statistically significant difference was found between patients who continued versus those who discontinued IST.
Rheumatologist Christophe Richez, MD, of the Groupe Hospitalier Pellegrin-CHU de Bordeaux (France) welcomed the trial. “The work does not find a significant difference but suggests that with more power, the difference would have been significant,” he said in an interview.
He added that a significant number of patients refused to enter the study for several reasons, including scheduling a pregnancy and fear of relapse after stopping the treatment. “This in itself shows that we need this type of study to know if we can stop the treatment to plan a pregnancy or if a relay treatment is necessary. These data also mean we can now better inform our patients about the benefit-risk [profile] of continuing treatment or not.”
Dr. Richez, who was not involved in the trial, noted that the data provide information that strongly suggest more research is needed to better determine which patients are at risk of relapse, and consequently, for which patients is discontinuation possible. “There’s also a need for further analysis of tolerance to the immunosuppressive drugs according to the strategy.”
Finally, he referred to the issue around therapeutic adherence that is often faced with management of lupus. “The data from this study will allow us to better explain to patients the need to continue or discontinue their treatments, including hydroxychloroquine, and also the possibility of decreasing the immunosuppressive drugs to half-dose.”
Maintenance therapy in lupus nephritis: To continue or not?
Conducted in 28 French centers, participants had class III or IV lupus nephritis with active lesions and had previously received induction IST of cyclophosphamide or mycophenolate mofetil with hydroxychloroquine and glucocorticoids. Maintenance IST was azathioprine or mycophenolate mofetil, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day).
A total of 96 patients were randomized into two groups: continuation of maintenance therapy (azathioprine or mycophenolate mofetil for 2-3 more years, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day), or discontinuation of maintenance therapy (azathioprine or mycophenolate mofetil) over 3 months.
Both study arms were similar with a mean age of 36-37 years, and 82%-86% of patients were female, 59%-67% were White. They had a mean disease duration of 7-9 years, and 72%-80% had experienced their first flare of proliferative nephritis. A total of 54%-65% had received cyclophosphamide induction therapy, and 75%-81% were on mycophenolate mofetil maintenance therapy that had been ongoing for a mean of 2.8 years. Tests of patients’ kidney function revealed a mean serum creatinine of 67-72 micromol/L and estimated glomerular filtration rate (eGFR) of 94-101 mL/min per 1.73 m2. The mean SLE Disease Activity Index score was around 2.
Follow-up visits were conducted every 3 months for 2 years, and the trial had a primary end point of renal relapse rate at 24 months (confirmed by kidney biopsy), while secondary endpoints included rate of severe SLE flares, survival without renal relapse, or severe flare and adverse events, among others.
Patients were excluded if they were not taking hydroxychloroquine, had extrarenal SLE, an eGFR less than 30 mL/min per 1.73 m2 or stage VI disease. After some participants did not finish the trial because of pregnancy, wish for pregnancy, or adverse events, data from a total of 40 in the IST continuation group and 44 in the discontinuation group were analyzed.
Noninferiority of discontinuation versus continuation not shown
A total of 12.5% in the continuation arm experienced renal relapse over 24 months, compared with 27.3% in the discontinuation arm (P = .079).
“The endpoint of noninferiority of discontinuation of immunosuppressive therapy was not shown, but no statistically significant difference was found between groups for renal relapse,” Dr. Jourde-Chiche said.
Severe SLE flares (renal or extrarenal) occurred significantly less often over 24 months in the continuation arm at 12.5% versus 31.8% in the discontinuation arm (P = .034).
However, Dr. Jourde-Chiche pointed out that “the majority of patients who discontinued treatment did not experience a flare.”
No differences were seen between groups with respect to any secondary endpoints. “Fortunately, no patients died or reached end-stage renal disease, and overall, the adverse events did not differ between groups,” she reported.
The study identified several risk factors for renal relapse, among which were low complement component 3 and higher baseline urinary protein to creatinine ratio.
Longer immunosuppressive therapy prediscontinuation leads to better results
Gabriella Moroni, MD, of the nephrology unit at Ospedale Maggiore Policlinico, Milan, authored a review of studies that attempted to interrupt glucocorticoids and other immunosuppressive agents in lupus nephritis and in SLE. Her review concluded that “the available data suggest that therapy withdrawal is feasible at least in patients enjoying a complete clinical remission after a prolonged therapy.”
Asked to comment on the French study, Dr. Moroni said the trial was very welcome. “In our long-term experience, we have stopped IST in 73 patients with lupus nephritis, followed for a mean of 23 years. Of these, 32 never reassumed therapy and 20 had at least one flare.”
Dr. Moroni noted that those participants who did not experience flares had significantly longer IST and longer remission before discontinuation and took hydroxychloroquine more frequently.
“We feel that, to prevent severe flares, firstly, lupus nephritis should be in complete remission for at least 3 years, and secondly, patients should have received IST for at least 5 years before discontinuation; immunosuppressive drugs should be tapered off very slowly and after strict clinical surveillance, and finally, hydroxychloroquine can prevent extrarenal flares.
Dr. Jourde-Chiche reported serving on a speakers bureau for Vifor Pharma and receiving grant/research support from Fresenius Medical Care. Some coauthors reported financial ties to many pharmaceutical companies. Dr. Richez said he has received fees for lectures or boards from GlaxoSmithKline, AstraZeneca, and Novartis. Dr. Moroni had no relevant disclosures.
COPENHAGEN – Discontinuing maintenance immunosuppressive therapy (IST) in patients with proliferative lupus nephritis in remission proved less effective than continuing it in terms of the rates of renal relapse and severe systemic lupus erythematosus (SLE) flare, results from the WIN-Lupus trial show.
Lead researcher Noemie Jourde-Chiche, MD, assistant professor at Aix-Marseille (France) University, presented the results at the annual European Congress of Rheumatology.
The randomized, controlled trial aimed to determine the optimal duration of maintenance IST for proliferative lupus nephritis, by asking whether discontinuation of such therapy after 2-3 years was noninferior to IST continuation for 2 more years.
“This is the first randomized IST trial in proliferative lupus nephritis,” Dr. Jourde-Chiche reported. “We found that noninferiority of IST discontinuation was not demonstrated, and those who discontinued had a higher risk of SLE flares, but the majority of patients who discontinued did not experience flare.”
Regarding the incidence of renal relapse, no statistically significant difference was found between patients who continued versus those who discontinued IST.
Rheumatologist Christophe Richez, MD, of the Groupe Hospitalier Pellegrin-CHU de Bordeaux (France) welcomed the trial. “The work does not find a significant difference but suggests that with more power, the difference would have been significant,” he said in an interview.
He added that a significant number of patients refused to enter the study for several reasons, including scheduling a pregnancy and fear of relapse after stopping the treatment. “This in itself shows that we need this type of study to know if we can stop the treatment to plan a pregnancy or if a relay treatment is necessary. These data also mean we can now better inform our patients about the benefit-risk [profile] of continuing treatment or not.”
Dr. Richez, who was not involved in the trial, noted that the data provide information that strongly suggest more research is needed to better determine which patients are at risk of relapse, and consequently, for which patients is discontinuation possible. “There’s also a need for further analysis of tolerance to the immunosuppressive drugs according to the strategy.”
Finally, he referred to the issue around therapeutic adherence that is often faced with management of lupus. “The data from this study will allow us to better explain to patients the need to continue or discontinue their treatments, including hydroxychloroquine, and also the possibility of decreasing the immunosuppressive drugs to half-dose.”
Maintenance therapy in lupus nephritis: To continue or not?
Conducted in 28 French centers, participants had class III or IV lupus nephritis with active lesions and had previously received induction IST of cyclophosphamide or mycophenolate mofetil with hydroxychloroquine and glucocorticoids. Maintenance IST was azathioprine or mycophenolate mofetil, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day).
A total of 96 patients were randomized into two groups: continuation of maintenance therapy (azathioprine or mycophenolate mofetil for 2-3 more years, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day), or discontinuation of maintenance therapy (azathioprine or mycophenolate mofetil) over 3 months.
Both study arms were similar with a mean age of 36-37 years, and 82%-86% of patients were female, 59%-67% were White. They had a mean disease duration of 7-9 years, and 72%-80% had experienced their first flare of proliferative nephritis. A total of 54%-65% had received cyclophosphamide induction therapy, and 75%-81% were on mycophenolate mofetil maintenance therapy that had been ongoing for a mean of 2.8 years. Tests of patients’ kidney function revealed a mean serum creatinine of 67-72 micromol/L and estimated glomerular filtration rate (eGFR) of 94-101 mL/min per 1.73 m2. The mean SLE Disease Activity Index score was around 2.
Follow-up visits were conducted every 3 months for 2 years, and the trial had a primary end point of renal relapse rate at 24 months (confirmed by kidney biopsy), while secondary endpoints included rate of severe SLE flares, survival without renal relapse, or severe flare and adverse events, among others.
Patients were excluded if they were not taking hydroxychloroquine, had extrarenal SLE, an eGFR less than 30 mL/min per 1.73 m2 or stage VI disease. After some participants did not finish the trial because of pregnancy, wish for pregnancy, or adverse events, data from a total of 40 in the IST continuation group and 44 in the discontinuation group were analyzed.
Noninferiority of discontinuation versus continuation not shown
A total of 12.5% in the continuation arm experienced renal relapse over 24 months, compared with 27.3% in the discontinuation arm (P = .079).
“The endpoint of noninferiority of discontinuation of immunosuppressive therapy was not shown, but no statistically significant difference was found between groups for renal relapse,” Dr. Jourde-Chiche said.
Severe SLE flares (renal or extrarenal) occurred significantly less often over 24 months in the continuation arm at 12.5% versus 31.8% in the discontinuation arm (P = .034).
However, Dr. Jourde-Chiche pointed out that “the majority of patients who discontinued treatment did not experience a flare.”
No differences were seen between groups with respect to any secondary endpoints. “Fortunately, no patients died or reached end-stage renal disease, and overall, the adverse events did not differ between groups,” she reported.
The study identified several risk factors for renal relapse, among which were low complement component 3 and higher baseline urinary protein to creatinine ratio.
Longer immunosuppressive therapy prediscontinuation leads to better results
Gabriella Moroni, MD, of the nephrology unit at Ospedale Maggiore Policlinico, Milan, authored a review of studies that attempted to interrupt glucocorticoids and other immunosuppressive agents in lupus nephritis and in SLE. Her review concluded that “the available data suggest that therapy withdrawal is feasible at least in patients enjoying a complete clinical remission after a prolonged therapy.”
Asked to comment on the French study, Dr. Moroni said the trial was very welcome. “In our long-term experience, we have stopped IST in 73 patients with lupus nephritis, followed for a mean of 23 years. Of these, 32 never reassumed therapy and 20 had at least one flare.”
Dr. Moroni noted that those participants who did not experience flares had significantly longer IST and longer remission before discontinuation and took hydroxychloroquine more frequently.
“We feel that, to prevent severe flares, firstly, lupus nephritis should be in complete remission for at least 3 years, and secondly, patients should have received IST for at least 5 years before discontinuation; immunosuppressive drugs should be tapered off very slowly and after strict clinical surveillance, and finally, hydroxychloroquine can prevent extrarenal flares.
Dr. Jourde-Chiche reported serving on a speakers bureau for Vifor Pharma and receiving grant/research support from Fresenius Medical Care. Some coauthors reported financial ties to many pharmaceutical companies. Dr. Richez said he has received fees for lectures or boards from GlaxoSmithKline, AstraZeneca, and Novartis. Dr. Moroni had no relevant disclosures.
COPENHAGEN – Discontinuing maintenance immunosuppressive therapy (IST) in patients with proliferative lupus nephritis in remission proved less effective than continuing it in terms of the rates of renal relapse and severe systemic lupus erythematosus (SLE) flare, results from the WIN-Lupus trial show.
Lead researcher Noemie Jourde-Chiche, MD, assistant professor at Aix-Marseille (France) University, presented the results at the annual European Congress of Rheumatology.
The randomized, controlled trial aimed to determine the optimal duration of maintenance IST for proliferative lupus nephritis, by asking whether discontinuation of such therapy after 2-3 years was noninferior to IST continuation for 2 more years.
“This is the first randomized IST trial in proliferative lupus nephritis,” Dr. Jourde-Chiche reported. “We found that noninferiority of IST discontinuation was not demonstrated, and those who discontinued had a higher risk of SLE flares, but the majority of patients who discontinued did not experience flare.”
Regarding the incidence of renal relapse, no statistically significant difference was found between patients who continued versus those who discontinued IST.
Rheumatologist Christophe Richez, MD, of the Groupe Hospitalier Pellegrin-CHU de Bordeaux (France) welcomed the trial. “The work does not find a significant difference but suggests that with more power, the difference would have been significant,” he said in an interview.
He added that a significant number of patients refused to enter the study for several reasons, including scheduling a pregnancy and fear of relapse after stopping the treatment. “This in itself shows that we need this type of study to know if we can stop the treatment to plan a pregnancy or if a relay treatment is necessary. These data also mean we can now better inform our patients about the benefit-risk [profile] of continuing treatment or not.”
Dr. Richez, who was not involved in the trial, noted that the data provide information that strongly suggest more research is needed to better determine which patients are at risk of relapse, and consequently, for which patients is discontinuation possible. “There’s also a need for further analysis of tolerance to the immunosuppressive drugs according to the strategy.”
Finally, he referred to the issue around therapeutic adherence that is often faced with management of lupus. “The data from this study will allow us to better explain to patients the need to continue or discontinue their treatments, including hydroxychloroquine, and also the possibility of decreasing the immunosuppressive drugs to half-dose.”
Maintenance therapy in lupus nephritis: To continue or not?
Conducted in 28 French centers, participants had class III or IV lupus nephritis with active lesions and had previously received induction IST of cyclophosphamide or mycophenolate mofetil with hydroxychloroquine and glucocorticoids. Maintenance IST was azathioprine or mycophenolate mofetil, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day).
A total of 96 patients were randomized into two groups: continuation of maintenance therapy (azathioprine or mycophenolate mofetil for 2-3 more years, hydroxychloroquine, and possibly low-dose glucocorticoids (below 10 mg/day), or discontinuation of maintenance therapy (azathioprine or mycophenolate mofetil) over 3 months.
Both study arms were similar with a mean age of 36-37 years, and 82%-86% of patients were female, 59%-67% were White. They had a mean disease duration of 7-9 years, and 72%-80% had experienced their first flare of proliferative nephritis. A total of 54%-65% had received cyclophosphamide induction therapy, and 75%-81% were on mycophenolate mofetil maintenance therapy that had been ongoing for a mean of 2.8 years. Tests of patients’ kidney function revealed a mean serum creatinine of 67-72 micromol/L and estimated glomerular filtration rate (eGFR) of 94-101 mL/min per 1.73 m2. The mean SLE Disease Activity Index score was around 2.
Follow-up visits were conducted every 3 months for 2 years, and the trial had a primary end point of renal relapse rate at 24 months (confirmed by kidney biopsy), while secondary endpoints included rate of severe SLE flares, survival without renal relapse, or severe flare and adverse events, among others.
Patients were excluded if they were not taking hydroxychloroquine, had extrarenal SLE, an eGFR less than 30 mL/min per 1.73 m2 or stage VI disease. After some participants did not finish the trial because of pregnancy, wish for pregnancy, or adverse events, data from a total of 40 in the IST continuation group and 44 in the discontinuation group were analyzed.
Noninferiority of discontinuation versus continuation not shown
A total of 12.5% in the continuation arm experienced renal relapse over 24 months, compared with 27.3% in the discontinuation arm (P = .079).
“The endpoint of noninferiority of discontinuation of immunosuppressive therapy was not shown, but no statistically significant difference was found between groups for renal relapse,” Dr. Jourde-Chiche said.
Severe SLE flares (renal or extrarenal) occurred significantly less often over 24 months in the continuation arm at 12.5% versus 31.8% in the discontinuation arm (P = .034).
However, Dr. Jourde-Chiche pointed out that “the majority of patients who discontinued treatment did not experience a flare.”
No differences were seen between groups with respect to any secondary endpoints. “Fortunately, no patients died or reached end-stage renal disease, and overall, the adverse events did not differ between groups,” she reported.
The study identified several risk factors for renal relapse, among which were low complement component 3 and higher baseline urinary protein to creatinine ratio.
Longer immunosuppressive therapy prediscontinuation leads to better results
Gabriella Moroni, MD, of the nephrology unit at Ospedale Maggiore Policlinico, Milan, authored a review of studies that attempted to interrupt glucocorticoids and other immunosuppressive agents in lupus nephritis and in SLE. Her review concluded that “the available data suggest that therapy withdrawal is feasible at least in patients enjoying a complete clinical remission after a prolonged therapy.”
Asked to comment on the French study, Dr. Moroni said the trial was very welcome. “In our long-term experience, we have stopped IST in 73 patients with lupus nephritis, followed for a mean of 23 years. Of these, 32 never reassumed therapy and 20 had at least one flare.”
Dr. Moroni noted that those participants who did not experience flares had significantly longer IST and longer remission before discontinuation and took hydroxychloroquine more frequently.
“We feel that, to prevent severe flares, firstly, lupus nephritis should be in complete remission for at least 3 years, and secondly, patients should have received IST for at least 5 years before discontinuation; immunosuppressive drugs should be tapered off very slowly and after strict clinical surveillance, and finally, hydroxychloroquine can prevent extrarenal flares.
Dr. Jourde-Chiche reported serving on a speakers bureau for Vifor Pharma and receiving grant/research support from Fresenius Medical Care. Some coauthors reported financial ties to many pharmaceutical companies. Dr. Richez said he has received fees for lectures or boards from GlaxoSmithKline, AstraZeneca, and Novartis. Dr. Moroni had no relevant disclosures.
AT THE EULAR 2022 CONGRESS
Immunosuppressed rheumatic patients not at high risk of breakthrough COVID-19
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
AT THE EULAR 2022 CONGRESS
‘Encouraging’ results of baricitinib in juvenile idiopathic arthritis
COPENHAGEN – Baricitinib (Olumiant), a Janus kinase (JAK) inhibitor, significantly increases time to disease flare and decreases frequency of flares in patients with juvenile idiopathic arthritis (JIA), according to the results of a phase 3, placebo-controlled study.
The results support use of baricitinib when biologic or conventional synthetic disease-modifying antirheumatic drugs (DMARDs) fail.
The difference in the proportion of patients who flared between baricitinib and placebo was seen as soon as 4 weeks after half of the patients switched from active drug to placebo, at 3.7% versus 23.5% respectively, reported Athimalaipet Ramanan, MD, from the University of Bristol (England) who presented the findings of the withdrawal, efficacy, and safety study at the annual European Congress of Rheumatology.
“Our patients and parents have been waiting for alternative drugs for JIA, so JAK inhibitors have come at the right time,” he said. “These are really very encouraging findings for families, caregivers, and patients with JIA, to have an effective oral JAK inhibitor for managing these children.”
In reporting the key findings, Dr. Ramanan added that the majority of patients (76%) achieved a JIA-ACR (American College of Rheumatology) 30 score during the 12-week open-label phase and went on to enter the double-blind withdrawal phase of the trial.
Baricitinib 2-mg tablets are already Food and Drug Administration approved for the treatment of adults with moderately to severely active rheumatoid arthritis. This study, sponsored by the drug manufacturer Eli Lilly, aimed to investigate the efficacy and safety in pediatric patients with JIA who have shown an inadequate response to conventional synthetic or biologic DMARDs.
“For juvenile patients we need to make a dose adjustment [from the adult dosing], especially because we don’t have long-term safety data from JAK inhibitors in general,” said Osama Elfayad, MD, rheumatologist from Mouwasat Hospital, Dammam, Saudi Arabia who attended the presentation and commented on the findings.
He emphasized that safety was of primary concern in the pediatric population who have a long life expectancy. “For me it is essential to have good long-term safety data in juvenile patients. If we start with 4 mg and if the patient is controlled, we should shift to 2 mg which will be much better. I understand some clinicians are asking for 1 mg.”
Study details
The study population included patients aged from 2 to 17 years old with extended oligo- or polyarticular JIA, enthesitis-related juvenile idiopathic arthritis (ERA) and juvenile psoriatic arthritis.
The trial was divided into three periods: a 2-week safety assessment, a 12-week open-label lead-in phase, and an up-to 32-week double-blind withdrawal phase. After confirmation of dose and safety, children were enrolled in the open-label phase receiving age-based, oral, once daily doses of baricitinib.
“The primary endpoint is really concerned with the next phase of the study [double-blind withdrawal phase] looking at the proportion of patients who have shown a response at week 12 [achieved JIA-ACR30] but when switched from active drug to placebo have a flare,” explained Dr. Ramanan.
Patients were randomized 1:1 to continuing baricitinib or newly starting placebo until disease flare or up to week 32. The time to flare during the double-blind phase was the primary endpoint, while secondary endpoints included JIA-ACR30/50/70/90 response rates at week 12, and the proportion of patients with a flare during the double-blind phase.
“These secondary endpoints are more relevant to the clinic,” noted Dr. Ramanan.
A total of 219 patients entered the open-label phase, and of these, 163 achieved a JIA-ACR 30. These 163 children entered the double-blind stage and were randomized to baricitinib four times a day (56 completed), or placebo (32 completed).
Two-thirds of patients were female, which is typical of the disease, explained Dr. Ramanan, and over two-thirds were White. “Most patients had had disease for around 4 years, and about half had had prior biologic therapy. About half were on baseline methotrexate and almost one-third had used corticosteroids although at doses of under 0.2mg/kg.
“It’s gratifying to see that over 75% achieved a JIA-ACR 30 [76.3%]. More importantly, two-thirds of the patients have a JIA-ACR 50 [63.5%], and almost half of the patients have a JIA-ACR 70 [46.1%]. This is pretty significant at 12 weeks only,” he remarked.
The key finding, however, was in the withdrawal phase, said Dr. Ramanan. “We see that those patients who had a response at week 12 and were then switched to placebo, about half [50.6%] flared on placebo, compared to only 17% of those who continued with baricitinib. So not only do those who switch to placebo have a higher frequency of flares but they are more likely to flare quickly, as early as 4 weeks.”
With respect to safety, he said: “This shows short-term safety, but what we really need is medium and long-term safety data. It is no surprise that most of the events seen were as expected in children including nasopharyngitis, upper respiratory tract infections, and nausea.”
In the baricitinib versus placebo phase, 4.9% had serious adverse events in the baricitinib group compared to 3.7% in the placebo group. “There was nothing we didn’t expect to see which was mainly infection,” said Dr. Ramanan.
Dr. Elfayad has no disclosures. Professor Ramanan is a consultant for Eli Lilly, Abbvie, Roche, UCB, Novartis, Pfizer, and Sobi. He has received grant/research support from Eli Lilly.
COPENHAGEN – Baricitinib (Olumiant), a Janus kinase (JAK) inhibitor, significantly increases time to disease flare and decreases frequency of flares in patients with juvenile idiopathic arthritis (JIA), according to the results of a phase 3, placebo-controlled study.
The results support use of baricitinib when biologic or conventional synthetic disease-modifying antirheumatic drugs (DMARDs) fail.
The difference in the proportion of patients who flared between baricitinib and placebo was seen as soon as 4 weeks after half of the patients switched from active drug to placebo, at 3.7% versus 23.5% respectively, reported Athimalaipet Ramanan, MD, from the University of Bristol (England) who presented the findings of the withdrawal, efficacy, and safety study at the annual European Congress of Rheumatology.
“Our patients and parents have been waiting for alternative drugs for JIA, so JAK inhibitors have come at the right time,” he said. “These are really very encouraging findings for families, caregivers, and patients with JIA, to have an effective oral JAK inhibitor for managing these children.”
In reporting the key findings, Dr. Ramanan added that the majority of patients (76%) achieved a JIA-ACR (American College of Rheumatology) 30 score during the 12-week open-label phase and went on to enter the double-blind withdrawal phase of the trial.
Baricitinib 2-mg tablets are already Food and Drug Administration approved for the treatment of adults with moderately to severely active rheumatoid arthritis. This study, sponsored by the drug manufacturer Eli Lilly, aimed to investigate the efficacy and safety in pediatric patients with JIA who have shown an inadequate response to conventional synthetic or biologic DMARDs.
“For juvenile patients we need to make a dose adjustment [from the adult dosing], especially because we don’t have long-term safety data from JAK inhibitors in general,” said Osama Elfayad, MD, rheumatologist from Mouwasat Hospital, Dammam, Saudi Arabia who attended the presentation and commented on the findings.
He emphasized that safety was of primary concern in the pediatric population who have a long life expectancy. “For me it is essential to have good long-term safety data in juvenile patients. If we start with 4 mg and if the patient is controlled, we should shift to 2 mg which will be much better. I understand some clinicians are asking for 1 mg.”
Study details
The study population included patients aged from 2 to 17 years old with extended oligo- or polyarticular JIA, enthesitis-related juvenile idiopathic arthritis (ERA) and juvenile psoriatic arthritis.
The trial was divided into three periods: a 2-week safety assessment, a 12-week open-label lead-in phase, and an up-to 32-week double-blind withdrawal phase. After confirmation of dose and safety, children were enrolled in the open-label phase receiving age-based, oral, once daily doses of baricitinib.
“The primary endpoint is really concerned with the next phase of the study [double-blind withdrawal phase] looking at the proportion of patients who have shown a response at week 12 [achieved JIA-ACR30] but when switched from active drug to placebo have a flare,” explained Dr. Ramanan.
Patients were randomized 1:1 to continuing baricitinib or newly starting placebo until disease flare or up to week 32. The time to flare during the double-blind phase was the primary endpoint, while secondary endpoints included JIA-ACR30/50/70/90 response rates at week 12, and the proportion of patients with a flare during the double-blind phase.
“These secondary endpoints are more relevant to the clinic,” noted Dr. Ramanan.
A total of 219 patients entered the open-label phase, and of these, 163 achieved a JIA-ACR 30. These 163 children entered the double-blind stage and were randomized to baricitinib four times a day (56 completed), or placebo (32 completed).
Two-thirds of patients were female, which is typical of the disease, explained Dr. Ramanan, and over two-thirds were White. “Most patients had had disease for around 4 years, and about half had had prior biologic therapy. About half were on baseline methotrexate and almost one-third had used corticosteroids although at doses of under 0.2mg/kg.
“It’s gratifying to see that over 75% achieved a JIA-ACR 30 [76.3%]. More importantly, two-thirds of the patients have a JIA-ACR 50 [63.5%], and almost half of the patients have a JIA-ACR 70 [46.1%]. This is pretty significant at 12 weeks only,” he remarked.
The key finding, however, was in the withdrawal phase, said Dr. Ramanan. “We see that those patients who had a response at week 12 and were then switched to placebo, about half [50.6%] flared on placebo, compared to only 17% of those who continued with baricitinib. So not only do those who switch to placebo have a higher frequency of flares but they are more likely to flare quickly, as early as 4 weeks.”
With respect to safety, he said: “This shows short-term safety, but what we really need is medium and long-term safety data. It is no surprise that most of the events seen were as expected in children including nasopharyngitis, upper respiratory tract infections, and nausea.”
In the baricitinib versus placebo phase, 4.9% had serious adverse events in the baricitinib group compared to 3.7% in the placebo group. “There was nothing we didn’t expect to see which was mainly infection,” said Dr. Ramanan.
Dr. Elfayad has no disclosures. Professor Ramanan is a consultant for Eli Lilly, Abbvie, Roche, UCB, Novartis, Pfizer, and Sobi. He has received grant/research support from Eli Lilly.
COPENHAGEN – Baricitinib (Olumiant), a Janus kinase (JAK) inhibitor, significantly increases time to disease flare and decreases frequency of flares in patients with juvenile idiopathic arthritis (JIA), according to the results of a phase 3, placebo-controlled study.
The results support use of baricitinib when biologic or conventional synthetic disease-modifying antirheumatic drugs (DMARDs) fail.
The difference in the proportion of patients who flared between baricitinib and placebo was seen as soon as 4 weeks after half of the patients switched from active drug to placebo, at 3.7% versus 23.5% respectively, reported Athimalaipet Ramanan, MD, from the University of Bristol (England) who presented the findings of the withdrawal, efficacy, and safety study at the annual European Congress of Rheumatology.
“Our patients and parents have been waiting for alternative drugs for JIA, so JAK inhibitors have come at the right time,” he said. “These are really very encouraging findings for families, caregivers, and patients with JIA, to have an effective oral JAK inhibitor for managing these children.”
In reporting the key findings, Dr. Ramanan added that the majority of patients (76%) achieved a JIA-ACR (American College of Rheumatology) 30 score during the 12-week open-label phase and went on to enter the double-blind withdrawal phase of the trial.
Baricitinib 2-mg tablets are already Food and Drug Administration approved for the treatment of adults with moderately to severely active rheumatoid arthritis. This study, sponsored by the drug manufacturer Eli Lilly, aimed to investigate the efficacy and safety in pediatric patients with JIA who have shown an inadequate response to conventional synthetic or biologic DMARDs.
“For juvenile patients we need to make a dose adjustment [from the adult dosing], especially because we don’t have long-term safety data from JAK inhibitors in general,” said Osama Elfayad, MD, rheumatologist from Mouwasat Hospital, Dammam, Saudi Arabia who attended the presentation and commented on the findings.
He emphasized that safety was of primary concern in the pediatric population who have a long life expectancy. “For me it is essential to have good long-term safety data in juvenile patients. If we start with 4 mg and if the patient is controlled, we should shift to 2 mg which will be much better. I understand some clinicians are asking for 1 mg.”
Study details
The study population included patients aged from 2 to 17 years old with extended oligo- or polyarticular JIA, enthesitis-related juvenile idiopathic arthritis (ERA) and juvenile psoriatic arthritis.
The trial was divided into three periods: a 2-week safety assessment, a 12-week open-label lead-in phase, and an up-to 32-week double-blind withdrawal phase. After confirmation of dose and safety, children were enrolled in the open-label phase receiving age-based, oral, once daily doses of baricitinib.
“The primary endpoint is really concerned with the next phase of the study [double-blind withdrawal phase] looking at the proportion of patients who have shown a response at week 12 [achieved JIA-ACR30] but when switched from active drug to placebo have a flare,” explained Dr. Ramanan.
Patients were randomized 1:1 to continuing baricitinib or newly starting placebo until disease flare or up to week 32. The time to flare during the double-blind phase was the primary endpoint, while secondary endpoints included JIA-ACR30/50/70/90 response rates at week 12, and the proportion of patients with a flare during the double-blind phase.
“These secondary endpoints are more relevant to the clinic,” noted Dr. Ramanan.
A total of 219 patients entered the open-label phase, and of these, 163 achieved a JIA-ACR 30. These 163 children entered the double-blind stage and were randomized to baricitinib four times a day (56 completed), or placebo (32 completed).
Two-thirds of patients were female, which is typical of the disease, explained Dr. Ramanan, and over two-thirds were White. “Most patients had had disease for around 4 years, and about half had had prior biologic therapy. About half were on baseline methotrexate and almost one-third had used corticosteroids although at doses of under 0.2mg/kg.
“It’s gratifying to see that over 75% achieved a JIA-ACR 30 [76.3%]. More importantly, two-thirds of the patients have a JIA-ACR 50 [63.5%], and almost half of the patients have a JIA-ACR 70 [46.1%]. This is pretty significant at 12 weeks only,” he remarked.
The key finding, however, was in the withdrawal phase, said Dr. Ramanan. “We see that those patients who had a response at week 12 and were then switched to placebo, about half [50.6%] flared on placebo, compared to only 17% of those who continued with baricitinib. So not only do those who switch to placebo have a higher frequency of flares but they are more likely to flare quickly, as early as 4 weeks.”
With respect to safety, he said: “This shows short-term safety, but what we really need is medium and long-term safety data. It is no surprise that most of the events seen were as expected in children including nasopharyngitis, upper respiratory tract infections, and nausea.”
In the baricitinib versus placebo phase, 4.9% had serious adverse events in the baricitinib group compared to 3.7% in the placebo group. “There was nothing we didn’t expect to see which was mainly infection,” said Dr. Ramanan.
Dr. Elfayad has no disclosures. Professor Ramanan is a consultant for Eli Lilly, Abbvie, Roche, UCB, Novartis, Pfizer, and Sobi. He has received grant/research support from Eli Lilly.
AT THE EULAR 2022 CONGRESS