User login
Ileocecal resection possible first-line option in early Crohn’s disease
COPENHAGEN – Half of patients with ileocecal Crohn’s disease who undergo ileocecal resection within a year of diagnosis remain off drug treatment 5 years post procedure, challenging the current paradigm of reserving surgery for complicated Crohn’s disease, show real-world data comparing outcomes of surgery with anti–tumor necrosis factor inhibitor (TNFi) therapy.
“These data show that resection of inflamed bowel in early ileocecal Crohn’s disease effectively resets the clock,” said Manasi Agrawal, MD, of the department of gastroenterology at the Icahn School of Medicine at Mount Sinai, New York, and a research associate with the Center for Molecular Prediction of Inflammatory Bowel Disease, Aalborg University, Copenhagen, who presented the findings at the annual congress of the European Crohn’s and Colitis Organisation. “These data are in accordance with the LIR!C study data and suggest that ileocecal resection could be a first-line therapeutic option in early ileal or ileocecal Crohn’s disease that could be discussed with our patients.”
While ileocecal resection is accepted as a therapeutic option in early Crohn’s disease, most clinicians reserve it for complicated disease that is refractory or intolerant to medication.
A radical shift in approach might be justified, said Jean-Frederic Colombel, MD, a study coinvestigator and gastroenterologist at Mount Sinai Hospital.
“These data can be transforming. It confirms that there may be a subset of patients with limited ileal Crohn’s disease that is uncomplicated and in whom surgery may be almost ‘curative’ because after follow-up there was no need for drug therapy. We need to reposition surgery as one possible option at diagnosis in patients with limited Crohn’s disease,” he said.
The 2017 LIR!C trial demonstrated comparable 1-year outcomes with ileocecal resection and anti-TNFi therapy in limited, nonstricturing ileocecal Crohn’s disease. A retrospective analysis of 5-year data from this trial, further reinforced ileocecal resection as a first-line option for limited Crohn’s disease, such that of those patients who underwent resection, 26% continued on anti-TNFi therapy, compared with 38% in those patients who took anti-TNFi therapy only. In addition, no patient in the resection group underwent subsequent surgery, whereas 48% of patients on anti-TNF underwent surgery.
Dr. Agrawal and coinvestigators decided that more conclusive, real-world data were needed and would help to determine whether ileocecal resection offered better patient outcomes than staying on anti-TNFi therapy.
The new findings are based on an analysis of 16,443 adults living with ileal Crohn’s disease and were diagnosed between 2003 and 2018. The data is based on an analysis of nationwide Danish registries. It included individuals who underwent
Ileocecal resection or received anti-TNF drugs within 1 year of diagnosis. Of the 16,443 patients diagnosed with Crohn’s disease over the study period, 1,279 had ileal or ileocecal disease and were included with 581 (3.5%) undergoing resection and 698 (4.2%) anti-TNFi index therapy.
Outcomes were compared between the two groups, and the proportions of individuals initiated on immunomodulator, anti-TNFi therapy, or no therapy at 5 years after their ileocecal resection were determined.
The primary outcome was CD-related hospitalization, systemic corticosteroid exposure, CD-related surgery, and perianal CD diagnosis. Crohn’s disease–related hospitalization, systemic corticosteroid use, Crohn’s disease–related surgery or perianal Crohn’s disease occurred in 273 versus 318 people in the ileocecal resection and anti-TNFi therapy groups respectively, equating to a 33% lower risk in the resection group. Of those patients who underwent ileocecal resection, 50.3% were on no treatment 5 years later; 47.5% and 17.1% were on immunomodulators and on anti-TNFi therapy respectively.
Resection was found to be associated with a statistically significant reduced risk of systemic corticosteroid use with an adjusted hazard ratio of 0.71 (95% confidence interval, 0.54-0.92). No statistically significant reduced risk was found for Crohn’s disease-related hospitalization (aHR, 0.79; 95% CI, 0.61-1.01) or perianal Crohn’s disease diagnosis (aHR, 0.70; 95% CI, 0.38-1.30). Adjustments were made for demographic and clinical variables, for example, age, sex, year of treatment, number of hospital contacts for an indication all in the year prior to index treatment.
In comparison with the proportion of resection patients at 5 years who were on no treatment, immunomodulators or anti-TNFi therapy, there are no data on the 5-year outcomes of those patients who began on anti-TNFi therapy, but patients typically continue unless they become intolerant or response starts to fail, Dr. Agrawal said.
Willem Bemelmen, MD, a colorectal surgeon from the University of Amsterdam who served as a moderator after the presentation said: “These results could lead to a paradigm shift in the management of patients with Crohn’s disease. Prior studies gave us early signals that surgery in Crohn’s disease might benefit the patients, but now with these larger scale data, with many patients, we might finally convince gastroenterologists to send patients in for early surgery.”
Dr. Agrawal, Dr. Bemelmen, and Dr. Colombel declared no relevant conflicts of interest.
This article was updated 3/7/23.
COPENHAGEN – Half of patients with ileocecal Crohn’s disease who undergo ileocecal resection within a year of diagnosis remain off drug treatment 5 years post procedure, challenging the current paradigm of reserving surgery for complicated Crohn’s disease, show real-world data comparing outcomes of surgery with anti–tumor necrosis factor inhibitor (TNFi) therapy.
“These data show that resection of inflamed bowel in early ileocecal Crohn’s disease effectively resets the clock,” said Manasi Agrawal, MD, of the department of gastroenterology at the Icahn School of Medicine at Mount Sinai, New York, and a research associate with the Center for Molecular Prediction of Inflammatory Bowel Disease, Aalborg University, Copenhagen, who presented the findings at the annual congress of the European Crohn’s and Colitis Organisation. “These data are in accordance with the LIR!C study data and suggest that ileocecal resection could be a first-line therapeutic option in early ileal or ileocecal Crohn’s disease that could be discussed with our patients.”
While ileocecal resection is accepted as a therapeutic option in early Crohn’s disease, most clinicians reserve it for complicated disease that is refractory or intolerant to medication.
A radical shift in approach might be justified, said Jean-Frederic Colombel, MD, a study coinvestigator and gastroenterologist at Mount Sinai Hospital.
“These data can be transforming. It confirms that there may be a subset of patients with limited ileal Crohn’s disease that is uncomplicated and in whom surgery may be almost ‘curative’ because after follow-up there was no need for drug therapy. We need to reposition surgery as one possible option at diagnosis in patients with limited Crohn’s disease,” he said.
The 2017 LIR!C trial demonstrated comparable 1-year outcomes with ileocecal resection and anti-TNFi therapy in limited, nonstricturing ileocecal Crohn’s disease. A retrospective analysis of 5-year data from this trial, further reinforced ileocecal resection as a first-line option for limited Crohn’s disease, such that of those patients who underwent resection, 26% continued on anti-TNFi therapy, compared with 38% in those patients who took anti-TNFi therapy only. In addition, no patient in the resection group underwent subsequent surgery, whereas 48% of patients on anti-TNF underwent surgery.
Dr. Agrawal and coinvestigators decided that more conclusive, real-world data were needed and would help to determine whether ileocecal resection offered better patient outcomes than staying on anti-TNFi therapy.
The new findings are based on an analysis of 16,443 adults living with ileal Crohn’s disease and were diagnosed between 2003 and 2018. The data is based on an analysis of nationwide Danish registries. It included individuals who underwent
Ileocecal resection or received anti-TNF drugs within 1 year of diagnosis. Of the 16,443 patients diagnosed with Crohn’s disease over the study period, 1,279 had ileal or ileocecal disease and were included with 581 (3.5%) undergoing resection and 698 (4.2%) anti-TNFi index therapy.
Outcomes were compared between the two groups, and the proportions of individuals initiated on immunomodulator, anti-TNFi therapy, or no therapy at 5 years after their ileocecal resection were determined.
The primary outcome was CD-related hospitalization, systemic corticosteroid exposure, CD-related surgery, and perianal CD diagnosis. Crohn’s disease–related hospitalization, systemic corticosteroid use, Crohn’s disease–related surgery or perianal Crohn’s disease occurred in 273 versus 318 people in the ileocecal resection and anti-TNFi therapy groups respectively, equating to a 33% lower risk in the resection group. Of those patients who underwent ileocecal resection, 50.3% were on no treatment 5 years later; 47.5% and 17.1% were on immunomodulators and on anti-TNFi therapy respectively.
Resection was found to be associated with a statistically significant reduced risk of systemic corticosteroid use with an adjusted hazard ratio of 0.71 (95% confidence interval, 0.54-0.92). No statistically significant reduced risk was found for Crohn’s disease-related hospitalization (aHR, 0.79; 95% CI, 0.61-1.01) or perianal Crohn’s disease diagnosis (aHR, 0.70; 95% CI, 0.38-1.30). Adjustments were made for demographic and clinical variables, for example, age, sex, year of treatment, number of hospital contacts for an indication all in the year prior to index treatment.
In comparison with the proportion of resection patients at 5 years who were on no treatment, immunomodulators or anti-TNFi therapy, there are no data on the 5-year outcomes of those patients who began on anti-TNFi therapy, but patients typically continue unless they become intolerant or response starts to fail, Dr. Agrawal said.
Willem Bemelmen, MD, a colorectal surgeon from the University of Amsterdam who served as a moderator after the presentation said: “These results could lead to a paradigm shift in the management of patients with Crohn’s disease. Prior studies gave us early signals that surgery in Crohn’s disease might benefit the patients, but now with these larger scale data, with many patients, we might finally convince gastroenterologists to send patients in for early surgery.”
Dr. Agrawal, Dr. Bemelmen, and Dr. Colombel declared no relevant conflicts of interest.
This article was updated 3/7/23.
COPENHAGEN – Half of patients with ileocecal Crohn’s disease who undergo ileocecal resection within a year of diagnosis remain off drug treatment 5 years post procedure, challenging the current paradigm of reserving surgery for complicated Crohn’s disease, show real-world data comparing outcomes of surgery with anti–tumor necrosis factor inhibitor (TNFi) therapy.
“These data show that resection of inflamed bowel in early ileocecal Crohn’s disease effectively resets the clock,” said Manasi Agrawal, MD, of the department of gastroenterology at the Icahn School of Medicine at Mount Sinai, New York, and a research associate with the Center for Molecular Prediction of Inflammatory Bowel Disease, Aalborg University, Copenhagen, who presented the findings at the annual congress of the European Crohn’s and Colitis Organisation. “These data are in accordance with the LIR!C study data and suggest that ileocecal resection could be a first-line therapeutic option in early ileal or ileocecal Crohn’s disease that could be discussed with our patients.”
While ileocecal resection is accepted as a therapeutic option in early Crohn’s disease, most clinicians reserve it for complicated disease that is refractory or intolerant to medication.
A radical shift in approach might be justified, said Jean-Frederic Colombel, MD, a study coinvestigator and gastroenterologist at Mount Sinai Hospital.
“These data can be transforming. It confirms that there may be a subset of patients with limited ileal Crohn’s disease that is uncomplicated and in whom surgery may be almost ‘curative’ because after follow-up there was no need for drug therapy. We need to reposition surgery as one possible option at diagnosis in patients with limited Crohn’s disease,” he said.
The 2017 LIR!C trial demonstrated comparable 1-year outcomes with ileocecal resection and anti-TNFi therapy in limited, nonstricturing ileocecal Crohn’s disease. A retrospective analysis of 5-year data from this trial, further reinforced ileocecal resection as a first-line option for limited Crohn’s disease, such that of those patients who underwent resection, 26% continued on anti-TNFi therapy, compared with 38% in those patients who took anti-TNFi therapy only. In addition, no patient in the resection group underwent subsequent surgery, whereas 48% of patients on anti-TNF underwent surgery.
Dr. Agrawal and coinvestigators decided that more conclusive, real-world data were needed and would help to determine whether ileocecal resection offered better patient outcomes than staying on anti-TNFi therapy.
The new findings are based on an analysis of 16,443 adults living with ileal Crohn’s disease and were diagnosed between 2003 and 2018. The data is based on an analysis of nationwide Danish registries. It included individuals who underwent
Ileocecal resection or received anti-TNF drugs within 1 year of diagnosis. Of the 16,443 patients diagnosed with Crohn’s disease over the study period, 1,279 had ileal or ileocecal disease and were included with 581 (3.5%) undergoing resection and 698 (4.2%) anti-TNFi index therapy.
Outcomes were compared between the two groups, and the proportions of individuals initiated on immunomodulator, anti-TNFi therapy, or no therapy at 5 years after their ileocecal resection were determined.
The primary outcome was CD-related hospitalization, systemic corticosteroid exposure, CD-related surgery, and perianal CD diagnosis. Crohn’s disease–related hospitalization, systemic corticosteroid use, Crohn’s disease–related surgery or perianal Crohn’s disease occurred in 273 versus 318 people in the ileocecal resection and anti-TNFi therapy groups respectively, equating to a 33% lower risk in the resection group. Of those patients who underwent ileocecal resection, 50.3% were on no treatment 5 years later; 47.5% and 17.1% were on immunomodulators and on anti-TNFi therapy respectively.
Resection was found to be associated with a statistically significant reduced risk of systemic corticosteroid use with an adjusted hazard ratio of 0.71 (95% confidence interval, 0.54-0.92). No statistically significant reduced risk was found for Crohn’s disease-related hospitalization (aHR, 0.79; 95% CI, 0.61-1.01) or perianal Crohn’s disease diagnosis (aHR, 0.70; 95% CI, 0.38-1.30). Adjustments were made for demographic and clinical variables, for example, age, sex, year of treatment, number of hospital contacts for an indication all in the year prior to index treatment.
In comparison with the proportion of resection patients at 5 years who were on no treatment, immunomodulators or anti-TNFi therapy, there are no data on the 5-year outcomes of those patients who began on anti-TNFi therapy, but patients typically continue unless they become intolerant or response starts to fail, Dr. Agrawal said.
Willem Bemelmen, MD, a colorectal surgeon from the University of Amsterdam who served as a moderator after the presentation said: “These results could lead to a paradigm shift in the management of patients with Crohn’s disease. Prior studies gave us early signals that surgery in Crohn’s disease might benefit the patients, but now with these larger scale data, with many patients, we might finally convince gastroenterologists to send patients in for early surgery.”
Dr. Agrawal, Dr. Bemelmen, and Dr. Colombel declared no relevant conflicts of interest.
This article was updated 3/7/23.
AT ECCO 2023
Pediatric IBD patients wrestle with lingering gut pain
COPENHAGEN –
“A major finding of our small study was the impact of chronic pain on well-being and emotional health which was particularly significant in vulnerable children moving through adolescence towards adulthood,” said Dhamyanthi Thangarajah, MD, a consultant pediatric gastroenterologist at Chelsea and Westminster Hospital, London, in a presentation at the annual congress of the European Crohn’s and Colitis Organisation.
In the study of 41 children between 10 and 17 years old, chronic pain was found in 80% of participants who had established and extensive disease. Most participants had markers for fecal calprotectin, a sensitive marker for inflammation in the gastrointestinal tract, and others had Crohn’s disease and were prescribed biologics.
No relationship was found between chronic pain and IBD activity, but quality of life scores were negatively impacted in children with chronic pain.
“Moving forward, strategies should target screening for chronic pain in children with IBD and provide psychosocial interventions early on,” Dr. Thangarajah said. “We also need to understand more about internalizing pain and explore mood disorders.”
Many children with IBD also present with chronic abdominal pain, which was the impetus for conducting the study. “Essentially, we wondered whether this was a symptom of active disease, or were we missing something? In adult patients, chronic pain is prevalent, but in children we don’t necessarily screen for chronic pain, although it is part of active disease,” she said.
There is considerable patient and parental anxiety around the nature and origins of the chronic pain, Dr. Thangarajah said.
“We need to understand the prevalence and impact of chronic pain in children and adolescents, and as such we wanted to understand and characterize our cohort,” she said.
Dr. Thangarajah said clinicians tend to be very focused on disease activity and that screening for chronic pain is not usually carried out. “When we look at their clinical indices, the patients seem better, but the fact that it affects emotional health, and we don’t screen for it, means we need psychological help for these pediatric patients,” she said. “Patients need to be able to talk about their pain, and we need to understand if these children are having IBD-type symptoms, and this just isn’t asked about. It would be good to extend this study with a psychologist to understand more about this pain.”
How the study was conducted
The findings are based on the IMPACT III quality of life questionnaire for IBD. Chronic pain was defined as mild, moderate, or severe according to the van Korff scale.
“Patients had extensive and established disease, as expected in a pediatric cohort, the majority of whom were on immunosuppressant biologic drugs [64%-89%]. Among these patients, analgesic use was low, which is part of the education we give parents, and there was no opiate use in children, which differs from adults with IBD,” Dr. Thangarajah said.
A total of 33/41 (80%) of patients had chronic pain, and of these, abdominal chronic pain was most common in 30/33 (90%), joint pain was present in 2/33 (6%), and headache in 1/33, (3%). The majority 26/33 (79%) were on biologic agents, and analgesia use was low at 15/33 (45%). A total of 42% of children across the spectrum of chronic pain severity were on immunomodulators. Comorbidities were present in 42%-57% of patients with mild, and moderate-severe chronic pain respectively.
IBD disease activity in children with chronic pain was compared with those without chronic pain, as defined by Pediatric Crohn’s Disease Activity Index (PCDAI), Pediatric Ulcerative Colitis Activity Index (PUCAI), C-reactive protein (CRP), and faecal calprotectin. No difference was found.
Dr. Thangarajah highlighted the significantly lower quality of life score in children with chronic pain (69 and 51 in mild, and moderate-severe pain subgroups respectively, compared with 81 in those children without chronic pain, P < .05). Specifically, body image showed no difference between children with and without chronic pain (59-65 points across no pain, mild, moderate and severe chronic pain).
Chronic pain patients also commonly reported sleep disturbance with around 66% of patients with chronic pain, compared with around 11% in those without. Anemia was reported in 30% versus 21% respectively. However, nearly half of children with chronic pain had comorbidities 16/33 (48%), and 5/16 (31%) had diagnoses that may be associated with comorbid pain.
Psychosocial support within gastroenterology unavailable
Christine Norton, PhD, professor of nursing at Kings College London, also spoke at the conference on abdominal pain and the well-being of patients with IBD. She said that pain can still be a problem for some patients in remission from IBD.
“In adults we find pain is related to disease activity, however, 40%-50% of patients with IBD remission still report pain. Abdominal pain is dominant but it can be anywhere in the body. This is really poorly addressed in clinical consultations. It’s a ‘don’t ask, don’t tell’ situation where the nurse or doctor would do something if they could, but they just don’t ask the patients,” she said.
If patients volunteer the information that they still have pain during remission, it might get dismissed as irritable bowel syndrome (IBS), Dr. Norton said. “Some patients do fulfill these criteria for IBS, but it still needs to be managed. Here at ECCO, the focus is on getting patients into deep remission and inflammation under tight control, but what do we do with the jangling pain nerves although there’s nothing apparently triggering them, the gut-brain sensitivity – it’s so hard to live with it. They need support,” she said.
Dr. Norton said clinicians need a better way to validate chronic pain. “Sometimes people don’t feel believed, but even if the doctor believes them, they don’t know what to do anyway. There’s very few places with psychological support within the field of gastroenterology. Do we educate the gastroenterologist in this aspect? Do we develop the skills of IBD nurses?”
Dr. Thangarajah and Dr. Norton have no disclosures to declare.
COPENHAGEN –
“A major finding of our small study was the impact of chronic pain on well-being and emotional health which was particularly significant in vulnerable children moving through adolescence towards adulthood,” said Dhamyanthi Thangarajah, MD, a consultant pediatric gastroenterologist at Chelsea and Westminster Hospital, London, in a presentation at the annual congress of the European Crohn’s and Colitis Organisation.
In the study of 41 children between 10 and 17 years old, chronic pain was found in 80% of participants who had established and extensive disease. Most participants had markers for fecal calprotectin, a sensitive marker for inflammation in the gastrointestinal tract, and others had Crohn’s disease and were prescribed biologics.
No relationship was found between chronic pain and IBD activity, but quality of life scores were negatively impacted in children with chronic pain.
“Moving forward, strategies should target screening for chronic pain in children with IBD and provide psychosocial interventions early on,” Dr. Thangarajah said. “We also need to understand more about internalizing pain and explore mood disorders.”
Many children with IBD also present with chronic abdominal pain, which was the impetus for conducting the study. “Essentially, we wondered whether this was a symptom of active disease, or were we missing something? In adult patients, chronic pain is prevalent, but in children we don’t necessarily screen for chronic pain, although it is part of active disease,” she said.
There is considerable patient and parental anxiety around the nature and origins of the chronic pain, Dr. Thangarajah said.
“We need to understand the prevalence and impact of chronic pain in children and adolescents, and as such we wanted to understand and characterize our cohort,” she said.
Dr. Thangarajah said clinicians tend to be very focused on disease activity and that screening for chronic pain is not usually carried out. “When we look at their clinical indices, the patients seem better, but the fact that it affects emotional health, and we don’t screen for it, means we need psychological help for these pediatric patients,” she said. “Patients need to be able to talk about their pain, and we need to understand if these children are having IBD-type symptoms, and this just isn’t asked about. It would be good to extend this study with a psychologist to understand more about this pain.”
How the study was conducted
The findings are based on the IMPACT III quality of life questionnaire for IBD. Chronic pain was defined as mild, moderate, or severe according to the van Korff scale.
“Patients had extensive and established disease, as expected in a pediatric cohort, the majority of whom were on immunosuppressant biologic drugs [64%-89%]. Among these patients, analgesic use was low, which is part of the education we give parents, and there was no opiate use in children, which differs from adults with IBD,” Dr. Thangarajah said.
A total of 33/41 (80%) of patients had chronic pain, and of these, abdominal chronic pain was most common in 30/33 (90%), joint pain was present in 2/33 (6%), and headache in 1/33, (3%). The majority 26/33 (79%) were on biologic agents, and analgesia use was low at 15/33 (45%). A total of 42% of children across the spectrum of chronic pain severity were on immunomodulators. Comorbidities were present in 42%-57% of patients with mild, and moderate-severe chronic pain respectively.
IBD disease activity in children with chronic pain was compared with those without chronic pain, as defined by Pediatric Crohn’s Disease Activity Index (PCDAI), Pediatric Ulcerative Colitis Activity Index (PUCAI), C-reactive protein (CRP), and faecal calprotectin. No difference was found.
Dr. Thangarajah highlighted the significantly lower quality of life score in children with chronic pain (69 and 51 in mild, and moderate-severe pain subgroups respectively, compared with 81 in those children without chronic pain, P < .05). Specifically, body image showed no difference between children with and without chronic pain (59-65 points across no pain, mild, moderate and severe chronic pain).
Chronic pain patients also commonly reported sleep disturbance with around 66% of patients with chronic pain, compared with around 11% in those without. Anemia was reported in 30% versus 21% respectively. However, nearly half of children with chronic pain had comorbidities 16/33 (48%), and 5/16 (31%) had diagnoses that may be associated with comorbid pain.
Psychosocial support within gastroenterology unavailable
Christine Norton, PhD, professor of nursing at Kings College London, also spoke at the conference on abdominal pain and the well-being of patients with IBD. She said that pain can still be a problem for some patients in remission from IBD.
“In adults we find pain is related to disease activity, however, 40%-50% of patients with IBD remission still report pain. Abdominal pain is dominant but it can be anywhere in the body. This is really poorly addressed in clinical consultations. It’s a ‘don’t ask, don’t tell’ situation where the nurse or doctor would do something if they could, but they just don’t ask the patients,” she said.
If patients volunteer the information that they still have pain during remission, it might get dismissed as irritable bowel syndrome (IBS), Dr. Norton said. “Some patients do fulfill these criteria for IBS, but it still needs to be managed. Here at ECCO, the focus is on getting patients into deep remission and inflammation under tight control, but what do we do with the jangling pain nerves although there’s nothing apparently triggering them, the gut-brain sensitivity – it’s so hard to live with it. They need support,” she said.
Dr. Norton said clinicians need a better way to validate chronic pain. “Sometimes people don’t feel believed, but even if the doctor believes them, they don’t know what to do anyway. There’s very few places with psychological support within the field of gastroenterology. Do we educate the gastroenterologist in this aspect? Do we develop the skills of IBD nurses?”
Dr. Thangarajah and Dr. Norton have no disclosures to declare.
COPENHAGEN –
“A major finding of our small study was the impact of chronic pain on well-being and emotional health which was particularly significant in vulnerable children moving through adolescence towards adulthood,” said Dhamyanthi Thangarajah, MD, a consultant pediatric gastroenterologist at Chelsea and Westminster Hospital, London, in a presentation at the annual congress of the European Crohn’s and Colitis Organisation.
In the study of 41 children between 10 and 17 years old, chronic pain was found in 80% of participants who had established and extensive disease. Most participants had markers for fecal calprotectin, a sensitive marker for inflammation in the gastrointestinal tract, and others had Crohn’s disease and were prescribed biologics.
No relationship was found between chronic pain and IBD activity, but quality of life scores were negatively impacted in children with chronic pain.
“Moving forward, strategies should target screening for chronic pain in children with IBD and provide psychosocial interventions early on,” Dr. Thangarajah said. “We also need to understand more about internalizing pain and explore mood disorders.”
Many children with IBD also present with chronic abdominal pain, which was the impetus for conducting the study. “Essentially, we wondered whether this was a symptom of active disease, or were we missing something? In adult patients, chronic pain is prevalent, but in children we don’t necessarily screen for chronic pain, although it is part of active disease,” she said.
There is considerable patient and parental anxiety around the nature and origins of the chronic pain, Dr. Thangarajah said.
“We need to understand the prevalence and impact of chronic pain in children and adolescents, and as such we wanted to understand and characterize our cohort,” she said.
Dr. Thangarajah said clinicians tend to be very focused on disease activity and that screening for chronic pain is not usually carried out. “When we look at their clinical indices, the patients seem better, but the fact that it affects emotional health, and we don’t screen for it, means we need psychological help for these pediatric patients,” she said. “Patients need to be able to talk about their pain, and we need to understand if these children are having IBD-type symptoms, and this just isn’t asked about. It would be good to extend this study with a psychologist to understand more about this pain.”
How the study was conducted
The findings are based on the IMPACT III quality of life questionnaire for IBD. Chronic pain was defined as mild, moderate, or severe according to the van Korff scale.
“Patients had extensive and established disease, as expected in a pediatric cohort, the majority of whom were on immunosuppressant biologic drugs [64%-89%]. Among these patients, analgesic use was low, which is part of the education we give parents, and there was no opiate use in children, which differs from adults with IBD,” Dr. Thangarajah said.
A total of 33/41 (80%) of patients had chronic pain, and of these, abdominal chronic pain was most common in 30/33 (90%), joint pain was present in 2/33 (6%), and headache in 1/33, (3%). The majority 26/33 (79%) were on biologic agents, and analgesia use was low at 15/33 (45%). A total of 42% of children across the spectrum of chronic pain severity were on immunomodulators. Comorbidities were present in 42%-57% of patients with mild, and moderate-severe chronic pain respectively.
IBD disease activity in children with chronic pain was compared with those without chronic pain, as defined by Pediatric Crohn’s Disease Activity Index (PCDAI), Pediatric Ulcerative Colitis Activity Index (PUCAI), C-reactive protein (CRP), and faecal calprotectin. No difference was found.
Dr. Thangarajah highlighted the significantly lower quality of life score in children with chronic pain (69 and 51 in mild, and moderate-severe pain subgroups respectively, compared with 81 in those children without chronic pain, P < .05). Specifically, body image showed no difference between children with and without chronic pain (59-65 points across no pain, mild, moderate and severe chronic pain).
Chronic pain patients also commonly reported sleep disturbance with around 66% of patients with chronic pain, compared with around 11% in those without. Anemia was reported in 30% versus 21% respectively. However, nearly half of children with chronic pain had comorbidities 16/33 (48%), and 5/16 (31%) had diagnoses that may be associated with comorbid pain.
Psychosocial support within gastroenterology unavailable
Christine Norton, PhD, professor of nursing at Kings College London, also spoke at the conference on abdominal pain and the well-being of patients with IBD. She said that pain can still be a problem for some patients in remission from IBD.
“In adults we find pain is related to disease activity, however, 40%-50% of patients with IBD remission still report pain. Abdominal pain is dominant but it can be anywhere in the body. This is really poorly addressed in clinical consultations. It’s a ‘don’t ask, don’t tell’ situation where the nurse or doctor would do something if they could, but they just don’t ask the patients,” she said.
If patients volunteer the information that they still have pain during remission, it might get dismissed as irritable bowel syndrome (IBS), Dr. Norton said. “Some patients do fulfill these criteria for IBS, but it still needs to be managed. Here at ECCO, the focus is on getting patients into deep remission and inflammation under tight control, but what do we do with the jangling pain nerves although there’s nothing apparently triggering them, the gut-brain sensitivity – it’s so hard to live with it. They need support,” she said.
Dr. Norton said clinicians need a better way to validate chronic pain. “Sometimes people don’t feel believed, but even if the doctor believes them, they don’t know what to do anyway. There’s very few places with psychological support within the field of gastroenterology. Do we educate the gastroenterologist in this aspect? Do we develop the skills of IBD nurses?”
Dr. Thangarajah and Dr. Norton have no disclosures to declare.
AT ECCO 2023
IBD: More patients on vedolizumab vs. anti-TNFs at 2 years
COPENHAGEN – , according to the first meta-analysis of their real-world effectiveness.
The results mostly applied to bionaive subjects, and the benefit of vedolizumab over both TNFi’s – infliximab (Remicade) and adalimumab (Humira), was more evident in ulcerative colitis, compared with Crohn’s disease, noted the researchers, led by Tsz Hong Yiu, MD, a clinician and researcher at the University of Sydney.
“It appears that patients are more likely to stay on vedolizumab than either infliximab or adalimumab, especially in bionaive patients, which could suggest either a better tolerance to the treatment or a better response,” Dr. Yiu said in an interview at the annual Congress of the European Crohn’s and Colitis Organisation.
The 2-year follow up data were particularly encouraging, noted Dr. Yiu, with more patients persisting on vedolizumab than both anti-TNF alpha drugs overall with respect to both ulcerative colitis and Crohn’s disease.
In a head-to-head comparison, 15% more patients stayed on vedolizumab than anti-TNF alpha drugs overall, at 1-year follow-up for both ulcerative colitis and Crohn’s disease (risk ratio, 1.15). At 2 years of follow-up, 12% more patients remained on vedolizumab in comparison with anti-TNF alpha drugs overall (RR, 1.12), again for both forms of inflammatory bowel disease (IBD).
“This may provide early evidence that supports vedolizumab as a first-line biologic agent for inpatients with inflammatory bowel disease,” said Dr. Yiu, noting that further research was required to validate the correlation of persistence with clinical effectiveness.
Adding comment on the motivation for the study, senior author Rupert Leong, MD, a gastroenterologist at Concord RepatriaKon General Hospital, Sydney, said, “We wanted to identify the drug with the highest effectiveness, which is the real-world benefit of the drug to patients, rather than efficacy, which refers to clinical trial data.”
“Importantly, clinical trial data are usually only 1 year, whereas persistence collects data often for several years. This is relevant in chronic diseases that can affect patients over several decades, because the true benefit of a drug cannot be implied from a short-term clinical trial,” he explained.
Persistence was chosen as the primary end-point because it is a measure that incorporates a drug’s efficacy and side-effect profile but also the patient’s perspective, added Dr. Yiu. “So, a patient may value mild side effects over treatment effectiveness and decide to cease treatment.”
A prior meta-analysis looking at loss of response found that 33% of people taking infliximab and 41% of people taking adalimumab became resistant to the biologics after a median follow up of 1 year. “The most common cause of loss of response to anti-TNF inhibitors is due to immunogenicity,” remarked Dr. Yiu. “These findings suggested that alternative biologics with high effectiveness should be considered.”
Data from the 2019 VARSITY study also informed the researchers’ decision to conduct a real-world study. VARSITY investigators found vedolizumab had increased efficacy over adalimumab in ulcerative colitis, however, data on the real-world effectiveness of vedolizumab, compared with adalimumab and infliximab, in both ulcerative colitis and Crohn’s disease remained unknown.
Dr. Leong pointed out the difficulty in selecting the correct treatment given the increasing numbers of biological agents available. “The paucity of head-to-head studies meant use of cohort studies is considered both relevant and informative, not least because long-term follow-up data can reveal secondary loss of response of these monoclonal antibodies, while pooling data further increases the statistical power and determines consistency.”
As such, the researchers conducted a systematic review and meta-analysis of six observational studies evaluating persistence, as a surrogate marker for clinical response, of vedolizumab versus infliximab and adalimumab among participants aged over 18 years with a diagnosis of either ulcerative colitis or Crohn’s disease from 2017 to July 2022.
Overall, the study found that 1-year persistence of vedolizumab was 71.2% in ulcerative colitis and 76% in Crohn’s disease, which was significantly higher than with infliximab (56.4% in ulcerative colitis, 53.7% in Crohn’s disease), and likewise with adalimumab (53.7% in ulcerative colitis, 55.6% in Crohn’s disease).
Results of 2-year persistence were pooled from four studies and found that vedolizumab had a 2-year persistence of 66% in ulcerative colitis and 61% in Crohn’s disease. By comparison, infliximab had a persistence of 49.7% for ulcerative colitis and 59.1% for Crohn’s disease, and adalimumab had a persistence of 31.4% for ulcerative colitis and 56% for Crohn’s disease).
In ulcerative colitis specifically, vedolizumab performed better than both adalimumab and infliximab with an RR of 1.41 (95% confidence interval, 1.14-1.74) and 1.15 (95% CI, 1.06-1.25) respectively, and an RR of 1.23 (95% CI, 1.14-1.33) was generated when adalimumab and infliximab results were combined after 1 year of follow-up.
In Crohn’s disease specifically, vedolizumab had a slightly higher 1-year persistence over anti-TNF inhibitors combined (RR, 1.10; 95% CI, 1.02-1.19), but there were insufficient data to support individual analysis.
In a subgroup of bionaive patients, vedolizumab had a higher 1-year persistence (RR, 1.14; 95% CI, 1.07-1.22) but did not show a statistically significant advantage in bioexperienced patients (RR, 1.04; 95% CI, 0.80-1.35), compared with anti-TNF inhibitors.
Dr. Yiu remarked that they were unable to identify any randomized controlled trials (RCTs) directly comparing infliximab versus vedolizumab in IBD at the time of their systematic review. However, he drew attention to a recent research article that compared the effectiveness, persistence, and side-effect profile of vedolizumab and infliximab in a small cohort of ulcerative colitis patients. “ In this study, vedolizumab showed overall superiority over infliximab, which is in keeping with our study’s findings.”
Commenting on the study, Viraj Kariyawasam, MD, gastroenterologist and head of IBD at Blacktown and Mount Druitt hospital in Sydney, said the findings were “very important in defining the place of vedolizumab in the treatment of ulcerative colitis, and more so in Crohn’s disease.”
“Despite vedolizumab being considered a lower-efficacy drug, compared to infliximab, in Crohn’s disease by most practicing clinicians, and still favoring anti-TNF in the treatment of Crohn’s disease, the study highlights the superior persistence of vedolizumab,” he said in an interview.
“This is likely associated with efficacy over the two most used anti-TNF agents. With the knowledge we have about reduced efficacy of vedolizumab after the use of anti-TNF, or as a second- or third-line agent, and its superior persistence as a first-line biologic with already published safety data, vedolizumab should be considered and preferred as a first-line agent in the treatment of both ulcerative colitis and Crohn’s disease.”
Dr. Yiu has declared no conflicts of interest. Dr. Leong declares he is an advisory board member of AbbVie, Aspen, BMS, Celgene, Celltrion, Chiesi, Ferring, Glutagen, Hospira, Janssen, Lilly, MSD, Novartis, Pfizer, Prometheus Biosciences, Takeda; research grant recipient of Celltrion, Shire, Janssen, Takeda, Gastroenterological Society of Australia, NHMRC, Gutsy Group, Pfizer, Joanna Tiddy grant University of Sydney. One coauthor is an advisory board member of AbbVie and has received speaker fees from AbbVie and Takeda. Dr. Kariyawasam has educational grants and/or speaker fees from Janssen, AbbVie, and Takeda.
COPENHAGEN – , according to the first meta-analysis of their real-world effectiveness.
The results mostly applied to bionaive subjects, and the benefit of vedolizumab over both TNFi’s – infliximab (Remicade) and adalimumab (Humira), was more evident in ulcerative colitis, compared with Crohn’s disease, noted the researchers, led by Tsz Hong Yiu, MD, a clinician and researcher at the University of Sydney.
“It appears that patients are more likely to stay on vedolizumab than either infliximab or adalimumab, especially in bionaive patients, which could suggest either a better tolerance to the treatment or a better response,” Dr. Yiu said in an interview at the annual Congress of the European Crohn’s and Colitis Organisation.
The 2-year follow up data were particularly encouraging, noted Dr. Yiu, with more patients persisting on vedolizumab than both anti-TNF alpha drugs overall with respect to both ulcerative colitis and Crohn’s disease.
In a head-to-head comparison, 15% more patients stayed on vedolizumab than anti-TNF alpha drugs overall, at 1-year follow-up for both ulcerative colitis and Crohn’s disease (risk ratio, 1.15). At 2 years of follow-up, 12% more patients remained on vedolizumab in comparison with anti-TNF alpha drugs overall (RR, 1.12), again for both forms of inflammatory bowel disease (IBD).
“This may provide early evidence that supports vedolizumab as a first-line biologic agent for inpatients with inflammatory bowel disease,” said Dr. Yiu, noting that further research was required to validate the correlation of persistence with clinical effectiveness.
Adding comment on the motivation for the study, senior author Rupert Leong, MD, a gastroenterologist at Concord RepatriaKon General Hospital, Sydney, said, “We wanted to identify the drug with the highest effectiveness, which is the real-world benefit of the drug to patients, rather than efficacy, which refers to clinical trial data.”
“Importantly, clinical trial data are usually only 1 year, whereas persistence collects data often for several years. This is relevant in chronic diseases that can affect patients over several decades, because the true benefit of a drug cannot be implied from a short-term clinical trial,” he explained.
Persistence was chosen as the primary end-point because it is a measure that incorporates a drug’s efficacy and side-effect profile but also the patient’s perspective, added Dr. Yiu. “So, a patient may value mild side effects over treatment effectiveness and decide to cease treatment.”
A prior meta-analysis looking at loss of response found that 33% of people taking infliximab and 41% of people taking adalimumab became resistant to the biologics after a median follow up of 1 year. “The most common cause of loss of response to anti-TNF inhibitors is due to immunogenicity,” remarked Dr. Yiu. “These findings suggested that alternative biologics with high effectiveness should be considered.”
Data from the 2019 VARSITY study also informed the researchers’ decision to conduct a real-world study. VARSITY investigators found vedolizumab had increased efficacy over adalimumab in ulcerative colitis, however, data on the real-world effectiveness of vedolizumab, compared with adalimumab and infliximab, in both ulcerative colitis and Crohn’s disease remained unknown.
Dr. Leong pointed out the difficulty in selecting the correct treatment given the increasing numbers of biological agents available. “The paucity of head-to-head studies meant use of cohort studies is considered both relevant and informative, not least because long-term follow-up data can reveal secondary loss of response of these monoclonal antibodies, while pooling data further increases the statistical power and determines consistency.”
As such, the researchers conducted a systematic review and meta-analysis of six observational studies evaluating persistence, as a surrogate marker for clinical response, of vedolizumab versus infliximab and adalimumab among participants aged over 18 years with a diagnosis of either ulcerative colitis or Crohn’s disease from 2017 to July 2022.
Overall, the study found that 1-year persistence of vedolizumab was 71.2% in ulcerative colitis and 76% in Crohn’s disease, which was significantly higher than with infliximab (56.4% in ulcerative colitis, 53.7% in Crohn’s disease), and likewise with adalimumab (53.7% in ulcerative colitis, 55.6% in Crohn’s disease).
Results of 2-year persistence were pooled from four studies and found that vedolizumab had a 2-year persistence of 66% in ulcerative colitis and 61% in Crohn’s disease. By comparison, infliximab had a persistence of 49.7% for ulcerative colitis and 59.1% for Crohn’s disease, and adalimumab had a persistence of 31.4% for ulcerative colitis and 56% for Crohn’s disease).
In ulcerative colitis specifically, vedolizumab performed better than both adalimumab and infliximab with an RR of 1.41 (95% confidence interval, 1.14-1.74) and 1.15 (95% CI, 1.06-1.25) respectively, and an RR of 1.23 (95% CI, 1.14-1.33) was generated when adalimumab and infliximab results were combined after 1 year of follow-up.
In Crohn’s disease specifically, vedolizumab had a slightly higher 1-year persistence over anti-TNF inhibitors combined (RR, 1.10; 95% CI, 1.02-1.19), but there were insufficient data to support individual analysis.
In a subgroup of bionaive patients, vedolizumab had a higher 1-year persistence (RR, 1.14; 95% CI, 1.07-1.22) but did not show a statistically significant advantage in bioexperienced patients (RR, 1.04; 95% CI, 0.80-1.35), compared with anti-TNF inhibitors.
Dr. Yiu remarked that they were unable to identify any randomized controlled trials (RCTs) directly comparing infliximab versus vedolizumab in IBD at the time of their systematic review. However, he drew attention to a recent research article that compared the effectiveness, persistence, and side-effect profile of vedolizumab and infliximab in a small cohort of ulcerative colitis patients. “ In this study, vedolizumab showed overall superiority over infliximab, which is in keeping with our study’s findings.”
Commenting on the study, Viraj Kariyawasam, MD, gastroenterologist and head of IBD at Blacktown and Mount Druitt hospital in Sydney, said the findings were “very important in defining the place of vedolizumab in the treatment of ulcerative colitis, and more so in Crohn’s disease.”
“Despite vedolizumab being considered a lower-efficacy drug, compared to infliximab, in Crohn’s disease by most practicing clinicians, and still favoring anti-TNF in the treatment of Crohn’s disease, the study highlights the superior persistence of vedolizumab,” he said in an interview.
“This is likely associated with efficacy over the two most used anti-TNF agents. With the knowledge we have about reduced efficacy of vedolizumab after the use of anti-TNF, or as a second- or third-line agent, and its superior persistence as a first-line biologic with already published safety data, vedolizumab should be considered and preferred as a first-line agent in the treatment of both ulcerative colitis and Crohn’s disease.”
Dr. Yiu has declared no conflicts of interest. Dr. Leong declares he is an advisory board member of AbbVie, Aspen, BMS, Celgene, Celltrion, Chiesi, Ferring, Glutagen, Hospira, Janssen, Lilly, MSD, Novartis, Pfizer, Prometheus Biosciences, Takeda; research grant recipient of Celltrion, Shire, Janssen, Takeda, Gastroenterological Society of Australia, NHMRC, Gutsy Group, Pfizer, Joanna Tiddy grant University of Sydney. One coauthor is an advisory board member of AbbVie and has received speaker fees from AbbVie and Takeda. Dr. Kariyawasam has educational grants and/or speaker fees from Janssen, AbbVie, and Takeda.
COPENHAGEN – , according to the first meta-analysis of their real-world effectiveness.
The results mostly applied to bionaive subjects, and the benefit of vedolizumab over both TNFi’s – infliximab (Remicade) and adalimumab (Humira), was more evident in ulcerative colitis, compared with Crohn’s disease, noted the researchers, led by Tsz Hong Yiu, MD, a clinician and researcher at the University of Sydney.
“It appears that patients are more likely to stay on vedolizumab than either infliximab or adalimumab, especially in bionaive patients, which could suggest either a better tolerance to the treatment or a better response,” Dr. Yiu said in an interview at the annual Congress of the European Crohn’s and Colitis Organisation.
The 2-year follow up data were particularly encouraging, noted Dr. Yiu, with more patients persisting on vedolizumab than both anti-TNF alpha drugs overall with respect to both ulcerative colitis and Crohn’s disease.
In a head-to-head comparison, 15% more patients stayed on vedolizumab than anti-TNF alpha drugs overall, at 1-year follow-up for both ulcerative colitis and Crohn’s disease (risk ratio, 1.15). At 2 years of follow-up, 12% more patients remained on vedolizumab in comparison with anti-TNF alpha drugs overall (RR, 1.12), again for both forms of inflammatory bowel disease (IBD).
“This may provide early evidence that supports vedolizumab as a first-line biologic agent for inpatients with inflammatory bowel disease,” said Dr. Yiu, noting that further research was required to validate the correlation of persistence with clinical effectiveness.
Adding comment on the motivation for the study, senior author Rupert Leong, MD, a gastroenterologist at Concord RepatriaKon General Hospital, Sydney, said, “We wanted to identify the drug with the highest effectiveness, which is the real-world benefit of the drug to patients, rather than efficacy, which refers to clinical trial data.”
“Importantly, clinical trial data are usually only 1 year, whereas persistence collects data often for several years. This is relevant in chronic diseases that can affect patients over several decades, because the true benefit of a drug cannot be implied from a short-term clinical trial,” he explained.
Persistence was chosen as the primary end-point because it is a measure that incorporates a drug’s efficacy and side-effect profile but also the patient’s perspective, added Dr. Yiu. “So, a patient may value mild side effects over treatment effectiveness and decide to cease treatment.”
A prior meta-analysis looking at loss of response found that 33% of people taking infliximab and 41% of people taking adalimumab became resistant to the biologics after a median follow up of 1 year. “The most common cause of loss of response to anti-TNF inhibitors is due to immunogenicity,” remarked Dr. Yiu. “These findings suggested that alternative biologics with high effectiveness should be considered.”
Data from the 2019 VARSITY study also informed the researchers’ decision to conduct a real-world study. VARSITY investigators found vedolizumab had increased efficacy over adalimumab in ulcerative colitis, however, data on the real-world effectiveness of vedolizumab, compared with adalimumab and infliximab, in both ulcerative colitis and Crohn’s disease remained unknown.
Dr. Leong pointed out the difficulty in selecting the correct treatment given the increasing numbers of biological agents available. “The paucity of head-to-head studies meant use of cohort studies is considered both relevant and informative, not least because long-term follow-up data can reveal secondary loss of response of these monoclonal antibodies, while pooling data further increases the statistical power and determines consistency.”
As such, the researchers conducted a systematic review and meta-analysis of six observational studies evaluating persistence, as a surrogate marker for clinical response, of vedolizumab versus infliximab and adalimumab among participants aged over 18 years with a diagnosis of either ulcerative colitis or Crohn’s disease from 2017 to July 2022.
Overall, the study found that 1-year persistence of vedolizumab was 71.2% in ulcerative colitis and 76% in Crohn’s disease, which was significantly higher than with infliximab (56.4% in ulcerative colitis, 53.7% in Crohn’s disease), and likewise with adalimumab (53.7% in ulcerative colitis, 55.6% in Crohn’s disease).
Results of 2-year persistence were pooled from four studies and found that vedolizumab had a 2-year persistence of 66% in ulcerative colitis and 61% in Crohn’s disease. By comparison, infliximab had a persistence of 49.7% for ulcerative colitis and 59.1% for Crohn’s disease, and adalimumab had a persistence of 31.4% for ulcerative colitis and 56% for Crohn’s disease).
In ulcerative colitis specifically, vedolizumab performed better than both adalimumab and infliximab with an RR of 1.41 (95% confidence interval, 1.14-1.74) and 1.15 (95% CI, 1.06-1.25) respectively, and an RR of 1.23 (95% CI, 1.14-1.33) was generated when adalimumab and infliximab results were combined after 1 year of follow-up.
In Crohn’s disease specifically, vedolizumab had a slightly higher 1-year persistence over anti-TNF inhibitors combined (RR, 1.10; 95% CI, 1.02-1.19), but there were insufficient data to support individual analysis.
In a subgroup of bionaive patients, vedolizumab had a higher 1-year persistence (RR, 1.14; 95% CI, 1.07-1.22) but did not show a statistically significant advantage in bioexperienced patients (RR, 1.04; 95% CI, 0.80-1.35), compared with anti-TNF inhibitors.
Dr. Yiu remarked that they were unable to identify any randomized controlled trials (RCTs) directly comparing infliximab versus vedolizumab in IBD at the time of their systematic review. However, he drew attention to a recent research article that compared the effectiveness, persistence, and side-effect profile of vedolizumab and infliximab in a small cohort of ulcerative colitis patients. “ In this study, vedolizumab showed overall superiority over infliximab, which is in keeping with our study’s findings.”
Commenting on the study, Viraj Kariyawasam, MD, gastroenterologist and head of IBD at Blacktown and Mount Druitt hospital in Sydney, said the findings were “very important in defining the place of vedolizumab in the treatment of ulcerative colitis, and more so in Crohn’s disease.”
“Despite vedolizumab being considered a lower-efficacy drug, compared to infliximab, in Crohn’s disease by most practicing clinicians, and still favoring anti-TNF in the treatment of Crohn’s disease, the study highlights the superior persistence of vedolizumab,” he said in an interview.
“This is likely associated with efficacy over the two most used anti-TNF agents. With the knowledge we have about reduced efficacy of vedolizumab after the use of anti-TNF, or as a second- or third-line agent, and its superior persistence as a first-line biologic with already published safety data, vedolizumab should be considered and preferred as a first-line agent in the treatment of both ulcerative colitis and Crohn’s disease.”
Dr. Yiu has declared no conflicts of interest. Dr. Leong declares he is an advisory board member of AbbVie, Aspen, BMS, Celgene, Celltrion, Chiesi, Ferring, Glutagen, Hospira, Janssen, Lilly, MSD, Novartis, Pfizer, Prometheus Biosciences, Takeda; research grant recipient of Celltrion, Shire, Janssen, Takeda, Gastroenterological Society of Australia, NHMRC, Gutsy Group, Pfizer, Joanna Tiddy grant University of Sydney. One coauthor is an advisory board member of AbbVie and has received speaker fees from AbbVie and Takeda. Dr. Kariyawasam has educational grants and/or speaker fees from Janssen, AbbVie, and Takeda.
AT ECCO 2023
Cannabis tied to lower IBD mortality, hospital costs
COPENHAGEN – Mortality rate, length of hospital stay, and cost of hospitalization all drop significantly in patients with inflammatory bowel disease (IBD) concurrently using cannabis, shows a study that supports wider availability of the substance for specified medical use.
Inpatient mortality dropped by more than 70% in those patients concurrently using cannabis for another indication, compared with those not taking the drug, while total cost of hospitalization dropped by more than $11,000.
The findings were presented as a poster by Neethi Dasu, DO, PGY 6 Gastroenterology Fellow, at Jefferson Health Hospital, N.J., at the annual congress of the European Crohn’s and Colitis Organization. Dr. Dasu worked with coinvestigator Brian Blair, DO, FACOI, Gastroenterology Program Director, IBD specialist, at the same hospital.
Dr. Dasu said. “Not only do patients spend less time in hospital, but they also have a decrease in mortality and hospital cost, which can be significant for patients with IBD, a chronic condition, that often burdens them with high health care spend.”
The researcher also highlighted that with annual U.S. health care spending on IBD having increased significantly in recent years, getting patients well and out of the hospital in a timely manner is key and that “cannabis might help in this aim.”
Cannabis use is legalized in some U.S. states for medical treatment of several chronic, debilitating disorders, especially cancer. Currently, there is no direct Food and Drug Administration approval for use for IBD. “Utilizing it would be considered off-label and investigational,” Dr. Dasu pointed out.
Patients report cannabis, as a pain control treatment, is effective for acute flares and chronic IBD, said Dr. Dasu. “It is an excellent agent for pain control that is not a narcotic, as with opioids, which can cause dependence and addiction. These could ultimately harm patients in the long term,” she addedin an interview. “Opioids can also cause drowsiness and side effects, which harm a person’s quality of life.”
Patients with IBD using cannabis concurrently
Dr. Dasu and her coresearchers aimed to see if outcomes including mortality and pain could be modified with “a very accessible and cost-efficient agent that does not cause long term addiction or adverse events.”
She added that previous studies had evaluated the clinical response in patients with IBD and concomitant cannabis use, but that their study was novel because it looked at inpatient outcomes as well as overall hospital cost.
Dr. Dasu and colleagues analyzed data over the years 2015-2019, from the Nationwide Inpatient Sample (NIS), a large publicly available all-payer inpatient care database, which encompasses approximately 7 million inpatient hospitalizations annually in the United States.
All included patients had IBD, either ulcerative colitis or Crohn’s disease, were aged 18 years and over, and used cannabis for a concurrent indication.
Odds ratios were calculated for in-hospital mortality, average length of hospital stay, and hospital charges, after adjusting for age, gender, race, primary insurance payer status, hospital type and size (number of beds), hospital region, hospital teaching status, and other demographic characteristics.
Of the 1,198,839 patients with IBD, 29,445 used cannabis for a different indication. Participants had an average age of 38.7 years.
Highly significant drop in mortality and hospital costs
Inpatient mortality shows a significant decrease of 72% (odds ratio, 0.28; confidence interval, 0.19-0.41, P < .0001) in those who concurrently used cannabis, compared with those who did not. Hospital length of stay also dropped by –0.17 days (95% CI, –0.35 to –0.01; P < .041), and this translated into a significant drop in the total cost of hospitalization from $39,309.00 (IBD without cannabis use) to $28,254.30 (IBD with cannabis use), resulting in an $11,054.70 savings (95% CI, –$13,681.15 to –$8,427.24; P < .0001).
As a chronic inflammatory disease, IBD involves immune dysregulation leading to symptoms of nausea, vomiting, bleeding, and abdominal pain; however, the pathophysiologic mechanism is not fully understood. She added that studies in mice had shown that cannabis acts via cannabinoid 1 and 2 receptors, located in the nervous system, to decrease pain, nausea, and vomiting. “Mechanisms of cannabis’s analgesic effect also involves inhibition of the release of neurotransmitters involved in pain and inflammation.”
Asked how she felt about the future for cannabis treatment in IBD, Dr. Dasu remarked that it would most likely require decriminalizing marijuana use on a federal level, although individual states currently offer exemptions.
“Further research should be done to evaluate the medical benefits of cannabis use in patients with IBD, with studies warranted to investigate the factors that may be driving these differences, as well warranted to investigations into the effect of cannabis on remission rates, rates of hospitalization, potential complications, and quality of life,” concluded Dr. Dasu.
Commenting on the study, Mary-Jane Williams, MD, a gastroenterology fellow at East Carolina University Health Medical Center, Greenville, N.C., told this news organization that the study was “a pleasant breath of information on the topic of cannabis use in IBD,” adding that providers often face questions about cannabis use from patients.
“Modulation of the endocannabinoid system ... plays a key role in the pathogenesis of IBD including pain control, limiting intestinal inflammation, and decreasing intestinal motility,” Dr. Williams said, adding that, “Its use in IBD has promising improvement in the therapeutic effect and overall quality of life.”
“This study highlights and supports substantial therapeutic effects of cannabis in the management of IBD patients, be it their pain control, improving nausea, appetite and sleep, remission rates, earlier time to recovery, shortened hospitalization and faster endoscopic improvement,” she pointed out, noting the need for further studies, but also that most organizations, including the Crohn’s and Colitis Foundation, support policies that facilitate the conduct of clinical research using objective parameters and the potential development of cannabinoid-based medications in the management of our patients with IBD.
Dr. Dasu, Dr. Blair, and Dr. Williams have declared no financial disclosures.
COPENHAGEN – Mortality rate, length of hospital stay, and cost of hospitalization all drop significantly in patients with inflammatory bowel disease (IBD) concurrently using cannabis, shows a study that supports wider availability of the substance for specified medical use.
Inpatient mortality dropped by more than 70% in those patients concurrently using cannabis for another indication, compared with those not taking the drug, while total cost of hospitalization dropped by more than $11,000.
The findings were presented as a poster by Neethi Dasu, DO, PGY 6 Gastroenterology Fellow, at Jefferson Health Hospital, N.J., at the annual congress of the European Crohn’s and Colitis Organization. Dr. Dasu worked with coinvestigator Brian Blair, DO, FACOI, Gastroenterology Program Director, IBD specialist, at the same hospital.
Dr. Dasu said. “Not only do patients spend less time in hospital, but they also have a decrease in mortality and hospital cost, which can be significant for patients with IBD, a chronic condition, that often burdens them with high health care spend.”
The researcher also highlighted that with annual U.S. health care spending on IBD having increased significantly in recent years, getting patients well and out of the hospital in a timely manner is key and that “cannabis might help in this aim.”
Cannabis use is legalized in some U.S. states for medical treatment of several chronic, debilitating disorders, especially cancer. Currently, there is no direct Food and Drug Administration approval for use for IBD. “Utilizing it would be considered off-label and investigational,” Dr. Dasu pointed out.
Patients report cannabis, as a pain control treatment, is effective for acute flares and chronic IBD, said Dr. Dasu. “It is an excellent agent for pain control that is not a narcotic, as with opioids, which can cause dependence and addiction. These could ultimately harm patients in the long term,” she addedin an interview. “Opioids can also cause drowsiness and side effects, which harm a person’s quality of life.”
Patients with IBD using cannabis concurrently
Dr. Dasu and her coresearchers aimed to see if outcomes including mortality and pain could be modified with “a very accessible and cost-efficient agent that does not cause long term addiction or adverse events.”
She added that previous studies had evaluated the clinical response in patients with IBD and concomitant cannabis use, but that their study was novel because it looked at inpatient outcomes as well as overall hospital cost.
Dr. Dasu and colleagues analyzed data over the years 2015-2019, from the Nationwide Inpatient Sample (NIS), a large publicly available all-payer inpatient care database, which encompasses approximately 7 million inpatient hospitalizations annually in the United States.
All included patients had IBD, either ulcerative colitis or Crohn’s disease, were aged 18 years and over, and used cannabis for a concurrent indication.
Odds ratios were calculated for in-hospital mortality, average length of hospital stay, and hospital charges, after adjusting for age, gender, race, primary insurance payer status, hospital type and size (number of beds), hospital region, hospital teaching status, and other demographic characteristics.
Of the 1,198,839 patients with IBD, 29,445 used cannabis for a different indication. Participants had an average age of 38.7 years.
Highly significant drop in mortality and hospital costs
Inpatient mortality shows a significant decrease of 72% (odds ratio, 0.28; confidence interval, 0.19-0.41, P < .0001) in those who concurrently used cannabis, compared with those who did not. Hospital length of stay also dropped by –0.17 days (95% CI, –0.35 to –0.01; P < .041), and this translated into a significant drop in the total cost of hospitalization from $39,309.00 (IBD without cannabis use) to $28,254.30 (IBD with cannabis use), resulting in an $11,054.70 savings (95% CI, –$13,681.15 to –$8,427.24; P < .0001).
As a chronic inflammatory disease, IBD involves immune dysregulation leading to symptoms of nausea, vomiting, bleeding, and abdominal pain; however, the pathophysiologic mechanism is not fully understood. She added that studies in mice had shown that cannabis acts via cannabinoid 1 and 2 receptors, located in the nervous system, to decrease pain, nausea, and vomiting. “Mechanisms of cannabis’s analgesic effect also involves inhibition of the release of neurotransmitters involved in pain and inflammation.”
Asked how she felt about the future for cannabis treatment in IBD, Dr. Dasu remarked that it would most likely require decriminalizing marijuana use on a federal level, although individual states currently offer exemptions.
“Further research should be done to evaluate the medical benefits of cannabis use in patients with IBD, with studies warranted to investigate the factors that may be driving these differences, as well warranted to investigations into the effect of cannabis on remission rates, rates of hospitalization, potential complications, and quality of life,” concluded Dr. Dasu.
Commenting on the study, Mary-Jane Williams, MD, a gastroenterology fellow at East Carolina University Health Medical Center, Greenville, N.C., told this news organization that the study was “a pleasant breath of information on the topic of cannabis use in IBD,” adding that providers often face questions about cannabis use from patients.
“Modulation of the endocannabinoid system ... plays a key role in the pathogenesis of IBD including pain control, limiting intestinal inflammation, and decreasing intestinal motility,” Dr. Williams said, adding that, “Its use in IBD has promising improvement in the therapeutic effect and overall quality of life.”
“This study highlights and supports substantial therapeutic effects of cannabis in the management of IBD patients, be it their pain control, improving nausea, appetite and sleep, remission rates, earlier time to recovery, shortened hospitalization and faster endoscopic improvement,” she pointed out, noting the need for further studies, but also that most organizations, including the Crohn’s and Colitis Foundation, support policies that facilitate the conduct of clinical research using objective parameters and the potential development of cannabinoid-based medications in the management of our patients with IBD.
Dr. Dasu, Dr. Blair, and Dr. Williams have declared no financial disclosures.
COPENHAGEN – Mortality rate, length of hospital stay, and cost of hospitalization all drop significantly in patients with inflammatory bowel disease (IBD) concurrently using cannabis, shows a study that supports wider availability of the substance for specified medical use.
Inpatient mortality dropped by more than 70% in those patients concurrently using cannabis for another indication, compared with those not taking the drug, while total cost of hospitalization dropped by more than $11,000.
The findings were presented as a poster by Neethi Dasu, DO, PGY 6 Gastroenterology Fellow, at Jefferson Health Hospital, N.J., at the annual congress of the European Crohn’s and Colitis Organization. Dr. Dasu worked with coinvestigator Brian Blair, DO, FACOI, Gastroenterology Program Director, IBD specialist, at the same hospital.
Dr. Dasu said. “Not only do patients spend less time in hospital, but they also have a decrease in mortality and hospital cost, which can be significant for patients with IBD, a chronic condition, that often burdens them with high health care spend.”
The researcher also highlighted that with annual U.S. health care spending on IBD having increased significantly in recent years, getting patients well and out of the hospital in a timely manner is key and that “cannabis might help in this aim.”
Cannabis use is legalized in some U.S. states for medical treatment of several chronic, debilitating disorders, especially cancer. Currently, there is no direct Food and Drug Administration approval for use for IBD. “Utilizing it would be considered off-label and investigational,” Dr. Dasu pointed out.
Patients report cannabis, as a pain control treatment, is effective for acute flares and chronic IBD, said Dr. Dasu. “It is an excellent agent for pain control that is not a narcotic, as with opioids, which can cause dependence and addiction. These could ultimately harm patients in the long term,” she addedin an interview. “Opioids can also cause drowsiness and side effects, which harm a person’s quality of life.”
Patients with IBD using cannabis concurrently
Dr. Dasu and her coresearchers aimed to see if outcomes including mortality and pain could be modified with “a very accessible and cost-efficient agent that does not cause long term addiction or adverse events.”
She added that previous studies had evaluated the clinical response in patients with IBD and concomitant cannabis use, but that their study was novel because it looked at inpatient outcomes as well as overall hospital cost.
Dr. Dasu and colleagues analyzed data over the years 2015-2019, from the Nationwide Inpatient Sample (NIS), a large publicly available all-payer inpatient care database, which encompasses approximately 7 million inpatient hospitalizations annually in the United States.
All included patients had IBD, either ulcerative colitis or Crohn’s disease, were aged 18 years and over, and used cannabis for a concurrent indication.
Odds ratios were calculated for in-hospital mortality, average length of hospital stay, and hospital charges, after adjusting for age, gender, race, primary insurance payer status, hospital type and size (number of beds), hospital region, hospital teaching status, and other demographic characteristics.
Of the 1,198,839 patients with IBD, 29,445 used cannabis for a different indication. Participants had an average age of 38.7 years.
Highly significant drop in mortality and hospital costs
Inpatient mortality shows a significant decrease of 72% (odds ratio, 0.28; confidence interval, 0.19-0.41, P < .0001) in those who concurrently used cannabis, compared with those who did not. Hospital length of stay also dropped by –0.17 days (95% CI, –0.35 to –0.01; P < .041), and this translated into a significant drop in the total cost of hospitalization from $39,309.00 (IBD without cannabis use) to $28,254.30 (IBD with cannabis use), resulting in an $11,054.70 savings (95% CI, –$13,681.15 to –$8,427.24; P < .0001).
As a chronic inflammatory disease, IBD involves immune dysregulation leading to symptoms of nausea, vomiting, bleeding, and abdominal pain; however, the pathophysiologic mechanism is not fully understood. She added that studies in mice had shown that cannabis acts via cannabinoid 1 and 2 receptors, located in the nervous system, to decrease pain, nausea, and vomiting. “Mechanisms of cannabis’s analgesic effect also involves inhibition of the release of neurotransmitters involved in pain and inflammation.”
Asked how she felt about the future for cannabis treatment in IBD, Dr. Dasu remarked that it would most likely require decriminalizing marijuana use on a federal level, although individual states currently offer exemptions.
“Further research should be done to evaluate the medical benefits of cannabis use in patients with IBD, with studies warranted to investigate the factors that may be driving these differences, as well warranted to investigations into the effect of cannabis on remission rates, rates of hospitalization, potential complications, and quality of life,” concluded Dr. Dasu.
Commenting on the study, Mary-Jane Williams, MD, a gastroenterology fellow at East Carolina University Health Medical Center, Greenville, N.C., told this news organization that the study was “a pleasant breath of information on the topic of cannabis use in IBD,” adding that providers often face questions about cannabis use from patients.
“Modulation of the endocannabinoid system ... plays a key role in the pathogenesis of IBD including pain control, limiting intestinal inflammation, and decreasing intestinal motility,” Dr. Williams said, adding that, “Its use in IBD has promising improvement in the therapeutic effect and overall quality of life.”
“This study highlights and supports substantial therapeutic effects of cannabis in the management of IBD patients, be it their pain control, improving nausea, appetite and sleep, remission rates, earlier time to recovery, shortened hospitalization and faster endoscopic improvement,” she pointed out, noting the need for further studies, but also that most organizations, including the Crohn’s and Colitis Foundation, support policies that facilitate the conduct of clinical research using objective parameters and the potential development of cannabinoid-based medications in the management of our patients with IBD.
Dr. Dasu, Dr. Blair, and Dr. Williams have declared no financial disclosures.
AT ECCO 2023
In families with gout, obesity and alcohol add to personal risk
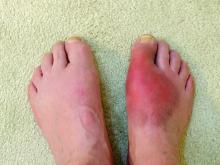
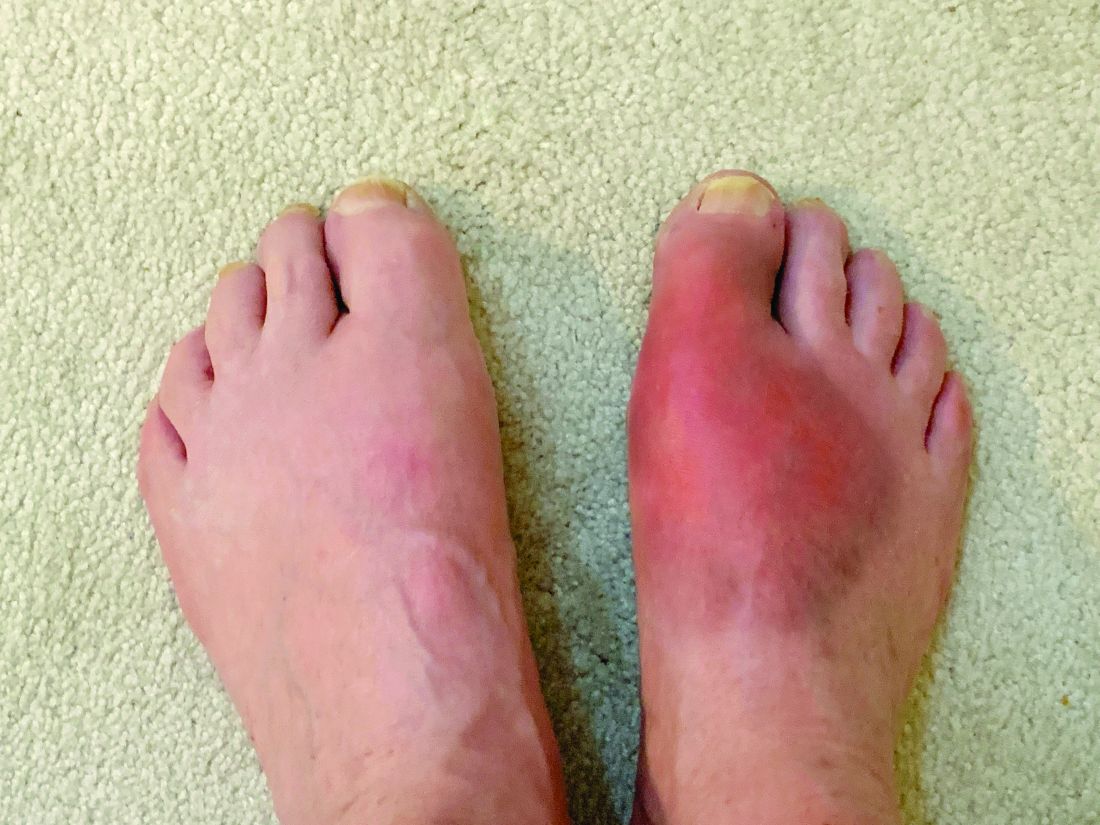
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE AND RESEARCH
Highly processed foods ‘as addictive’ as tobacco
according to a new U.S. study that proposes a set of criteria to assess the addictive potential of some foods.
The research suggests that health care professionals are taking steps toward framing food addiction as a clinical entity in its own right; it currently lacks validated treatment protocols and recognition as a clinical diagnosis.
Meanwhile, other data, reported by researchers at the 2022 Diabetes Professional Care conference in London also add support to the clinical recognition of food addiction.
Clinical psychologist Jen Unwin, PhD, from Southport, England, showed that a 3-month online program of low-carbohydrate diet together with psychoeducational support significantly reduced food addiction symptoms among a varied group of individuals, not all of whom were overweight or had obesity.
Dr. Unwin said her new data represent the first wide-scale clinical audit of its kind, other than a prior report of three patients with food addiction who were successfully treated with a ketogenic diet.
“Food addiction explains so much of what we see in clinical practice, where intelligent people understand what we tell them about the physiology associated with a low-carb diet, and they follow it for a while, but then they relapse,” said Dr. Unwin, explaining the difficulties faced by around 20% of her patients who are considered to have food addiction.
Meanwhile, the authors of the U.S. study, led by Ashley N. Gearhardt, PhD, a psychologist from the University of Michigan, Ann Arbor, wrote that the ability of highly processed foods (HPFs) “to rapidly deliver high doses of refined carbohydrates and/or fat appear key to their addictive potential. Thus, we conclude that HPFs can be considered addictive substances based on scientifically established criteria.”
They asserted that the contribution to preventable deaths by a diet dominated by highly processed foods is comparable with that of tobacco products, and as such, like Dr. Unwin, the authors sought clinical recognition and a more formalized protocol to manage food addiction.
“Understanding whether addiction contributes to HPF intake may lead to new treatments, as preliminary research finds that behavioral and pharmacological interventions that target addictive mechanisms may reduce compulsive HPF intake,” they stated.
The study led by Dr. Gearhardt was published in the journal Addiction, and the study led by Unwin was also recently published in Frontiers in Psychiatry.
Addiction criteria similar to tobacco
HPFs can be associated with an eating phenotype “that reflects the hallmarks of addiction,” said Dr. Gearhardt and coauthors; typically, loss of control over intake, intense cravings, inability to cut down, and continued use despite negative consequences.
Acknowledging the lack of a single addictive agent, they explain that food addiction reflects mechanisms implicated in other addictive disorders such as smoking.
As such, in their study, Dr. Gearhardt and colleagues proposed a set of scientifically based criteria for the evaluation of whether certain foods are addictive. “Specifically, we propose the primary criteria used to resolve one of the last major controversies over whether a substance, tobacco products, was addictive.”
They consider certain foods according to the primary criteria that have stood the test of time after being proposed in 1988 by the U.S. Surgeon General to establish the addictive potential of tobacco: they trigger compulsive use, they have psychoactive effects, and they are reinforcing.
They have updated these criteria to include the ability to trigger urges and cravings, and added that “both these products [tobacco and HPFs] are legal, easily accessible, inexpensive, lack an intoxication syndrome, and are major causes of preventable death.”
For example, with compulsive use, tobacco meets this criterion because evidence suggests that most smokers would like to quit but are unable to do so.
Likewise, wrote Dr. Gearhardt and colleagues, even “in the face of significant diet-related health consequences (e.g., diabetes and cardiovascular disease), the majority of patients are unable to adhere to medically recommended dietary plans that require a reduction in HPF intake.”
Reinforcement, through tobacco use, is demonstrated by its ‘being sufficiently rewarding to maintain self-administration” because of its ability to deliver nicotine, they said, quoting the Surgeon General’s report, and likewise, with food addiction, “both adults and children will self-administer HPFs (e.g., potato chips, candy, and cookies) even when satiated.”
Online group food addiction intervention study
Dr. Unwin and coauthors want people with food addiction to be able to access a validated treatment protocol. Their study aimed to evaluate an online group intervention across multiple sites in the United States, Canada, and the United Kingdom, involving an abstinent, low-carbohydrate diet and biopsychosocial education focused on addiction and recovery in people self-identifying as having food addiction.
“Lots of people with food addiction go to GPs who don’t clinically recognize this, or if they attend addiction services and psychiatry, then they tend to only specialize in drugs, alcohol, and gambling. Eating disorder services are linked but their programs mostly don’t work for a food addict,” Dr. Unwin remarked in an interview.
“We feel running groups, as well as training professionals to run groups, is the best way to manage food addiction,” she said, reflecting on the scale of the problem, with around 10% of adults in the U.K. general population considered to have food addiction. In Dr. Unwin’s study, some people had type 2 diabetes and some overweight/obesity, but she added that some participants were underweight or of normal weight.
Initially, the 103 participants received weekly group (8-24 people) sessions for 10-14 weeks, and then monthly maintenance comprising follow-up that involved coaching participants on how to cope with relapse and get back on track.
Food addiction symptoms were assessed pre- and post program using the modified Yale Food Addiction Scale (mYFAS) 2.0; ICD-10 symptoms of food-related substance use disorder (CRAVED); and mental health well-being measured using the short version of the Warwick Edinburgh Mental Wellbeing scale and body weight.
“The program eliminates processed foods with a personalized, abstinence food plan that involves education around mechanisms involved,” said Dr. Unwin, who explained that processed foods deliver a dopamine high, and in response to this, the brain lowers the number of dopamine receptors to effectively counteract the increase in dopamine. This drop in dopamine receptors explains the depression often associated with food addiction.
Dr. Unwin reported that food addiction symptoms were significantly reduced, with the mYFAS dropping by 1.52, the CRAVED score by 1.53, and body weight by 2.34 kg (5.2 lb). Mental health, as measured by the Warwick Edinburgh Mental Wellbeing scale, improved by 2.37 points.
“We were very interested in mental health and well-being because it impacts so much across our lives, and we saw significant improvements here, but we were less interested in weight because food addicts come in all shapes and sizes with some people underweight,” said Dr. Unwin. “Food addiction symptoms were significantly improved in the group, but we now need to look at the longer-term outcomes.”
Dr. Unwin runs a low-carbohydrate program for type 2 diabetes with her husband David Unwin, MD, who is a GP in Southport, England. She said that they ask patients if they think they have food addiction, and most say they do.
“I always try to explain to patients about the dopamine high, and how this starts the craving which makes people wonder when and where they can find the next sugar hit. Just thinking about the next chocolate bar gets the dopamine running for many people, and the more they tread this path then the worse it gets because the dopamine receptors keep reducing.”
Lorraine Avery, RN, a diabetes nurse specialist for Solent NHS Trust, who attended the DPC conference, welcomed Dr. Unwin’s presentation.
“My concern as a diabetes nurse specialist is that I’m unsure all our patients recognize their food addiction, and there are often more drivers to eating than just the food in front of them,” she said in an interview. “I think there’s an emotional element, too. These people are often ‘yo-yo’ dieters, and they join lots of expert companies to help them lose weight, but these companies want them to regain and re-join their programs,” she said.
“I think there is something about helping patients recognize they have a food addiction and they need to consider that other approaches might be helpful.”
Dr. Unwin reported no relevant financial relationships; some other authors have fee-paying clients with food addiction. Dr. Gearhardt and Ms. Avery reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new U.S. study that proposes a set of criteria to assess the addictive potential of some foods.
The research suggests that health care professionals are taking steps toward framing food addiction as a clinical entity in its own right; it currently lacks validated treatment protocols and recognition as a clinical diagnosis.
Meanwhile, other data, reported by researchers at the 2022 Diabetes Professional Care conference in London also add support to the clinical recognition of food addiction.
Clinical psychologist Jen Unwin, PhD, from Southport, England, showed that a 3-month online program of low-carbohydrate diet together with psychoeducational support significantly reduced food addiction symptoms among a varied group of individuals, not all of whom were overweight or had obesity.
Dr. Unwin said her new data represent the first wide-scale clinical audit of its kind, other than a prior report of three patients with food addiction who were successfully treated with a ketogenic diet.
“Food addiction explains so much of what we see in clinical practice, where intelligent people understand what we tell them about the physiology associated with a low-carb diet, and they follow it for a while, but then they relapse,” said Dr. Unwin, explaining the difficulties faced by around 20% of her patients who are considered to have food addiction.
Meanwhile, the authors of the U.S. study, led by Ashley N. Gearhardt, PhD, a psychologist from the University of Michigan, Ann Arbor, wrote that the ability of highly processed foods (HPFs) “to rapidly deliver high doses of refined carbohydrates and/or fat appear key to their addictive potential. Thus, we conclude that HPFs can be considered addictive substances based on scientifically established criteria.”
They asserted that the contribution to preventable deaths by a diet dominated by highly processed foods is comparable with that of tobacco products, and as such, like Dr. Unwin, the authors sought clinical recognition and a more formalized protocol to manage food addiction.
“Understanding whether addiction contributes to HPF intake may lead to new treatments, as preliminary research finds that behavioral and pharmacological interventions that target addictive mechanisms may reduce compulsive HPF intake,” they stated.
The study led by Dr. Gearhardt was published in the journal Addiction, and the study led by Unwin was also recently published in Frontiers in Psychiatry.
Addiction criteria similar to tobacco
HPFs can be associated with an eating phenotype “that reflects the hallmarks of addiction,” said Dr. Gearhardt and coauthors; typically, loss of control over intake, intense cravings, inability to cut down, and continued use despite negative consequences.
Acknowledging the lack of a single addictive agent, they explain that food addiction reflects mechanisms implicated in other addictive disorders such as smoking.
As such, in their study, Dr. Gearhardt and colleagues proposed a set of scientifically based criteria for the evaluation of whether certain foods are addictive. “Specifically, we propose the primary criteria used to resolve one of the last major controversies over whether a substance, tobacco products, was addictive.”
They consider certain foods according to the primary criteria that have stood the test of time after being proposed in 1988 by the U.S. Surgeon General to establish the addictive potential of tobacco: they trigger compulsive use, they have psychoactive effects, and they are reinforcing.
They have updated these criteria to include the ability to trigger urges and cravings, and added that “both these products [tobacco and HPFs] are legal, easily accessible, inexpensive, lack an intoxication syndrome, and are major causes of preventable death.”
For example, with compulsive use, tobacco meets this criterion because evidence suggests that most smokers would like to quit but are unable to do so.
Likewise, wrote Dr. Gearhardt and colleagues, even “in the face of significant diet-related health consequences (e.g., diabetes and cardiovascular disease), the majority of patients are unable to adhere to medically recommended dietary plans that require a reduction in HPF intake.”
Reinforcement, through tobacco use, is demonstrated by its ‘being sufficiently rewarding to maintain self-administration” because of its ability to deliver nicotine, they said, quoting the Surgeon General’s report, and likewise, with food addiction, “both adults and children will self-administer HPFs (e.g., potato chips, candy, and cookies) even when satiated.”
Online group food addiction intervention study
Dr. Unwin and coauthors want people with food addiction to be able to access a validated treatment protocol. Their study aimed to evaluate an online group intervention across multiple sites in the United States, Canada, and the United Kingdom, involving an abstinent, low-carbohydrate diet and biopsychosocial education focused on addiction and recovery in people self-identifying as having food addiction.
“Lots of people with food addiction go to GPs who don’t clinically recognize this, or if they attend addiction services and psychiatry, then they tend to only specialize in drugs, alcohol, and gambling. Eating disorder services are linked but their programs mostly don’t work for a food addict,” Dr. Unwin remarked in an interview.
“We feel running groups, as well as training professionals to run groups, is the best way to manage food addiction,” she said, reflecting on the scale of the problem, with around 10% of adults in the U.K. general population considered to have food addiction. In Dr. Unwin’s study, some people had type 2 diabetes and some overweight/obesity, but she added that some participants were underweight or of normal weight.
Initially, the 103 participants received weekly group (8-24 people) sessions for 10-14 weeks, and then monthly maintenance comprising follow-up that involved coaching participants on how to cope with relapse and get back on track.
Food addiction symptoms were assessed pre- and post program using the modified Yale Food Addiction Scale (mYFAS) 2.0; ICD-10 symptoms of food-related substance use disorder (CRAVED); and mental health well-being measured using the short version of the Warwick Edinburgh Mental Wellbeing scale and body weight.
“The program eliminates processed foods with a personalized, abstinence food plan that involves education around mechanisms involved,” said Dr. Unwin, who explained that processed foods deliver a dopamine high, and in response to this, the brain lowers the number of dopamine receptors to effectively counteract the increase in dopamine. This drop in dopamine receptors explains the depression often associated with food addiction.
Dr. Unwin reported that food addiction symptoms were significantly reduced, with the mYFAS dropping by 1.52, the CRAVED score by 1.53, and body weight by 2.34 kg (5.2 lb). Mental health, as measured by the Warwick Edinburgh Mental Wellbeing scale, improved by 2.37 points.
“We were very interested in mental health and well-being because it impacts so much across our lives, and we saw significant improvements here, but we were less interested in weight because food addicts come in all shapes and sizes with some people underweight,” said Dr. Unwin. “Food addiction symptoms were significantly improved in the group, but we now need to look at the longer-term outcomes.”
Dr. Unwin runs a low-carbohydrate program for type 2 diabetes with her husband David Unwin, MD, who is a GP in Southport, England. She said that they ask patients if they think they have food addiction, and most say they do.
“I always try to explain to patients about the dopamine high, and how this starts the craving which makes people wonder when and where they can find the next sugar hit. Just thinking about the next chocolate bar gets the dopamine running for many people, and the more they tread this path then the worse it gets because the dopamine receptors keep reducing.”
Lorraine Avery, RN, a diabetes nurse specialist for Solent NHS Trust, who attended the DPC conference, welcomed Dr. Unwin’s presentation.
“My concern as a diabetes nurse specialist is that I’m unsure all our patients recognize their food addiction, and there are often more drivers to eating than just the food in front of them,” she said in an interview. “I think there’s an emotional element, too. These people are often ‘yo-yo’ dieters, and they join lots of expert companies to help them lose weight, but these companies want them to regain and re-join their programs,” she said.
“I think there is something about helping patients recognize they have a food addiction and they need to consider that other approaches might be helpful.”
Dr. Unwin reported no relevant financial relationships; some other authors have fee-paying clients with food addiction. Dr. Gearhardt and Ms. Avery reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new U.S. study that proposes a set of criteria to assess the addictive potential of some foods.
The research suggests that health care professionals are taking steps toward framing food addiction as a clinical entity in its own right; it currently lacks validated treatment protocols and recognition as a clinical diagnosis.
Meanwhile, other data, reported by researchers at the 2022 Diabetes Professional Care conference in London also add support to the clinical recognition of food addiction.
Clinical psychologist Jen Unwin, PhD, from Southport, England, showed that a 3-month online program of low-carbohydrate diet together with psychoeducational support significantly reduced food addiction symptoms among a varied group of individuals, not all of whom were overweight or had obesity.
Dr. Unwin said her new data represent the first wide-scale clinical audit of its kind, other than a prior report of three patients with food addiction who were successfully treated with a ketogenic diet.
“Food addiction explains so much of what we see in clinical practice, where intelligent people understand what we tell them about the physiology associated with a low-carb diet, and they follow it for a while, but then they relapse,” said Dr. Unwin, explaining the difficulties faced by around 20% of her patients who are considered to have food addiction.
Meanwhile, the authors of the U.S. study, led by Ashley N. Gearhardt, PhD, a psychologist from the University of Michigan, Ann Arbor, wrote that the ability of highly processed foods (HPFs) “to rapidly deliver high doses of refined carbohydrates and/or fat appear key to their addictive potential. Thus, we conclude that HPFs can be considered addictive substances based on scientifically established criteria.”
They asserted that the contribution to preventable deaths by a diet dominated by highly processed foods is comparable with that of tobacco products, and as such, like Dr. Unwin, the authors sought clinical recognition and a more formalized protocol to manage food addiction.
“Understanding whether addiction contributes to HPF intake may lead to new treatments, as preliminary research finds that behavioral and pharmacological interventions that target addictive mechanisms may reduce compulsive HPF intake,” they stated.
The study led by Dr. Gearhardt was published in the journal Addiction, and the study led by Unwin was also recently published in Frontiers in Psychiatry.
Addiction criteria similar to tobacco
HPFs can be associated with an eating phenotype “that reflects the hallmarks of addiction,” said Dr. Gearhardt and coauthors; typically, loss of control over intake, intense cravings, inability to cut down, and continued use despite negative consequences.
Acknowledging the lack of a single addictive agent, they explain that food addiction reflects mechanisms implicated in other addictive disorders such as smoking.
As such, in their study, Dr. Gearhardt and colleagues proposed a set of scientifically based criteria for the evaluation of whether certain foods are addictive. “Specifically, we propose the primary criteria used to resolve one of the last major controversies over whether a substance, tobacco products, was addictive.”
They consider certain foods according to the primary criteria that have stood the test of time after being proposed in 1988 by the U.S. Surgeon General to establish the addictive potential of tobacco: they trigger compulsive use, they have psychoactive effects, and they are reinforcing.
They have updated these criteria to include the ability to trigger urges and cravings, and added that “both these products [tobacco and HPFs] are legal, easily accessible, inexpensive, lack an intoxication syndrome, and are major causes of preventable death.”
For example, with compulsive use, tobacco meets this criterion because evidence suggests that most smokers would like to quit but are unable to do so.
Likewise, wrote Dr. Gearhardt and colleagues, even “in the face of significant diet-related health consequences (e.g., diabetes and cardiovascular disease), the majority of patients are unable to adhere to medically recommended dietary plans that require a reduction in HPF intake.”
Reinforcement, through tobacco use, is demonstrated by its ‘being sufficiently rewarding to maintain self-administration” because of its ability to deliver nicotine, they said, quoting the Surgeon General’s report, and likewise, with food addiction, “both adults and children will self-administer HPFs (e.g., potato chips, candy, and cookies) even when satiated.”
Online group food addiction intervention study
Dr. Unwin and coauthors want people with food addiction to be able to access a validated treatment protocol. Their study aimed to evaluate an online group intervention across multiple sites in the United States, Canada, and the United Kingdom, involving an abstinent, low-carbohydrate diet and biopsychosocial education focused on addiction and recovery in people self-identifying as having food addiction.
“Lots of people with food addiction go to GPs who don’t clinically recognize this, or if they attend addiction services and psychiatry, then they tend to only specialize in drugs, alcohol, and gambling. Eating disorder services are linked but their programs mostly don’t work for a food addict,” Dr. Unwin remarked in an interview.
“We feel running groups, as well as training professionals to run groups, is the best way to manage food addiction,” she said, reflecting on the scale of the problem, with around 10% of adults in the U.K. general population considered to have food addiction. In Dr. Unwin’s study, some people had type 2 diabetes and some overweight/obesity, but she added that some participants were underweight or of normal weight.
Initially, the 103 participants received weekly group (8-24 people) sessions for 10-14 weeks, and then monthly maintenance comprising follow-up that involved coaching participants on how to cope with relapse and get back on track.
Food addiction symptoms were assessed pre- and post program using the modified Yale Food Addiction Scale (mYFAS) 2.0; ICD-10 symptoms of food-related substance use disorder (CRAVED); and mental health well-being measured using the short version of the Warwick Edinburgh Mental Wellbeing scale and body weight.
“The program eliminates processed foods with a personalized, abstinence food plan that involves education around mechanisms involved,” said Dr. Unwin, who explained that processed foods deliver a dopamine high, and in response to this, the brain lowers the number of dopamine receptors to effectively counteract the increase in dopamine. This drop in dopamine receptors explains the depression often associated with food addiction.
Dr. Unwin reported that food addiction symptoms were significantly reduced, with the mYFAS dropping by 1.52, the CRAVED score by 1.53, and body weight by 2.34 kg (5.2 lb). Mental health, as measured by the Warwick Edinburgh Mental Wellbeing scale, improved by 2.37 points.
“We were very interested in mental health and well-being because it impacts so much across our lives, and we saw significant improvements here, but we were less interested in weight because food addicts come in all shapes and sizes with some people underweight,” said Dr. Unwin. “Food addiction symptoms were significantly improved in the group, but we now need to look at the longer-term outcomes.”
Dr. Unwin runs a low-carbohydrate program for type 2 diabetes with her husband David Unwin, MD, who is a GP in Southport, England. She said that they ask patients if they think they have food addiction, and most say they do.
“I always try to explain to patients about the dopamine high, and how this starts the craving which makes people wonder when and where they can find the next sugar hit. Just thinking about the next chocolate bar gets the dopamine running for many people, and the more they tread this path then the worse it gets because the dopamine receptors keep reducing.”
Lorraine Avery, RN, a diabetes nurse specialist for Solent NHS Trust, who attended the DPC conference, welcomed Dr. Unwin’s presentation.
“My concern as a diabetes nurse specialist is that I’m unsure all our patients recognize their food addiction, and there are often more drivers to eating than just the food in front of them,” she said in an interview. “I think there’s an emotional element, too. These people are often ‘yo-yo’ dieters, and they join lots of expert companies to help them lose weight, but these companies want them to regain and re-join their programs,” she said.
“I think there is something about helping patients recognize they have a food addiction and they need to consider that other approaches might be helpful.”
Dr. Unwin reported no relevant financial relationships; some other authors have fee-paying clients with food addiction. Dr. Gearhardt and Ms. Avery reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sexual issues common for GI patients, but docs often avoid topic
VIENNA – Sexual dysfunction in patients with gastrointestinal disorders is undermanaged, with a lack of clinician education, time constraints, and embarrassment preventing constructive discussions to improve patient care and quality of life, according to a new survey.
Overall, 71% of gastroenterologists do not ask their patients about sexual dysfunction, the survey finds.
“While patients with gastrointestinal disorders often experience sexual dysfunction, discussions around the matter are not routine in gastroenterological care,” said Marco Romano, MD, from the University of Campania “Luigi Vanvitelli,” Naples, Italy.
Romano presented the survey findings at this year’s United European Gastroenterology Week meeting.
The research shows not only a clear need for better awareness but also a need to build gastroenterologists’ confidence in addressing sexual dysfunction with their patients, Dr. Romano added.
“Most felt that sexual medicine education and improvement of communication skills within the context of their residency training might be important in order to increase the awareness of sexual dysfunction, to overcome barriers, and to improve care and quality of life for their patients,” reported Dr. Romano. “This will lead to prompt diagnosis and treatment of any sexual problems.”
Respectfully asking the patients if their gastrointestinal disorders interfere with their intimate relationships “is often considered a relief to patients who find that the gastrointestinal problem and the sexual dysfunction are interlinked,” he added.
The findings
The survey was needed because the question of whether gastroenterologists inquire about their patients’ sexual issues had never been assessed, Dr. Romano said.
The researchers sent a cross-sectional, anonymous online survey to members of the Italian Society of Gastroenterology and Digestive Endoscopy. The questionnaire, designed and informed by a literature review, consisted of 29 single multiple-choice and open-ended questions.
A total of 426 surveys were returned: 335 from experienced gastroenterologists and 91 from residents (less experienced). Of all respondents, 54.7% were men and 45.3% were women.
Even though most gastroenterologists do not ask their patients about sexual dysfunction, the majority want to learn how to manage the issue, the survey found. Of the survey respondents, 80% agreed that it would be useful for gastroenterologists to attend courses dedicated to the problem of sexual dysfunction.
Only 4% of patients report (initiate a dialogue about) the problem, the survey found. Among women aged 40-50 years, the most common complaint reported was dyspareunia (pain on intercourse). In men, the most frequent complaints reported were in the over-40s age group, with 75% citing erectile dysfunction and 45% reporting loss of libido.
The most common gastrointestinal disorders associated with sexual dysfunction are inflammatory bowel diseases (37% of cases), chronic liver diseases (28%), and irritable bowel syndrome (26%), according to the survey.
On the question of whether medications played a role in patients’ sexual dysfunction, nearly 15% of respondents said that prokinetic agents were involved, and 18% thought proton pump inhibitors affect sexual function. Both drug classes are considered responsible for sexual disturbances.
Few gastroenterologists prescribe phosphodiesterase type 5 inhibitors (PDE5i), e.g., Viagra, to treat sexual dysfunction, the survey found. Approximately 90% of respondents said that they never prescribed this class of drugs, preferring to refer patients to an andrologist. Of those who did prescribe PDE5i, significantly fewer residents did compared with experienced gastroenterologists (1.1% vs. 8.8%, respectively; P = .01).
Finally, the biggest reasons why gastroenterologists do not discuss sexual dysfunction are lack of knowledge (80%), insufficient experience (58%), time (44%), and embarrassment (30%).
Practice experience matters
There were some differences among respondents in the experienced group vs. the residents. More men were in the experienced group compared with residents (57.6% vs. 44%, respectively); mean age was 47 years vs. 29 years, respectively; and 71% had 5 or more years of experience in the experienced gastroenterologist group, whereas 78% had 1-5 years of experience among residents.
The survey found that more residents than experienced gastroenterologists “never discussed sexual dysfunction” (38.5% vs. 21.3%, respectively; P = .001) and that more residents than experienced gastroenterologists reported that “patients did not relate their sexual dysfunction to the prescribed therapy” (47.8% vs. 32.5%, respectively; P = .007).
The two groups varied regarding prescription drugs’ role in sexual dysfunction. More experienced gastroenterologists than residents felt that proton pump inhibitors (5.8% vs. 0%, respectively; P = .018) or prokinetics (19.8% vs. 9.5%, respectively; P = .028) might be responsible for some degree of sexual dysfunction.
More residents than experienced doctors felt that other (nongastroenterologic) drugs might contribute to sexual dysfunction in their patients (57.1% vs. 44.7%, respectively; P = .043).
Dr. Romano reported that fewer residents than experienced gastroenterologists referred male patients with sexual dysfunction to an andrologist (frequently/always: 28.1% vs. 44.4%, respectively; P = .004). However, more residents than experienced gastroenterologists disagreed that discussing sexual dysfunction with patients pertains only to specialists (andrologists and gynecologists; 83.5% vs. 71.2%, respectively; P = .018).
Time to step up
Asma Fikree, BMBCh, PhD, of Royal London Hospital, Barts Health NHS Trust, London, moderated the session. The survey highlights that asking patients about sexual dysfunction is an area for improvement for gastroenterologists, she said.
“We might do it in men and ask about erectile dysfunction, but we are very poor about asking in women,” Dr. Fikree noted.
The pros and cons of different medications should be discussed with patients, she said.
Gastroenterologists need to do a better job of considering how medications can lead to sexual dysfunction and interfere with quality of life, and training would help, she added.
“Some patients might not be very bothered by sexual dysfunction, but others might consider it very important,” Dr. Fikree said. “We should be considering this as part of their treatment and care.”
Dr. Romano and Dr. Fikree report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – Sexual dysfunction in patients with gastrointestinal disorders is undermanaged, with a lack of clinician education, time constraints, and embarrassment preventing constructive discussions to improve patient care and quality of life, according to a new survey.
Overall, 71% of gastroenterologists do not ask their patients about sexual dysfunction, the survey finds.
“While patients with gastrointestinal disorders often experience sexual dysfunction, discussions around the matter are not routine in gastroenterological care,” said Marco Romano, MD, from the University of Campania “Luigi Vanvitelli,” Naples, Italy.
Romano presented the survey findings at this year’s United European Gastroenterology Week meeting.
The research shows not only a clear need for better awareness but also a need to build gastroenterologists’ confidence in addressing sexual dysfunction with their patients, Dr. Romano added.
“Most felt that sexual medicine education and improvement of communication skills within the context of their residency training might be important in order to increase the awareness of sexual dysfunction, to overcome barriers, and to improve care and quality of life for their patients,” reported Dr. Romano. “This will lead to prompt diagnosis and treatment of any sexual problems.”
Respectfully asking the patients if their gastrointestinal disorders interfere with their intimate relationships “is often considered a relief to patients who find that the gastrointestinal problem and the sexual dysfunction are interlinked,” he added.
The findings
The survey was needed because the question of whether gastroenterologists inquire about their patients’ sexual issues had never been assessed, Dr. Romano said.
The researchers sent a cross-sectional, anonymous online survey to members of the Italian Society of Gastroenterology and Digestive Endoscopy. The questionnaire, designed and informed by a literature review, consisted of 29 single multiple-choice and open-ended questions.
A total of 426 surveys were returned: 335 from experienced gastroenterologists and 91 from residents (less experienced). Of all respondents, 54.7% were men and 45.3% were women.
Even though most gastroenterologists do not ask their patients about sexual dysfunction, the majority want to learn how to manage the issue, the survey found. Of the survey respondents, 80% agreed that it would be useful for gastroenterologists to attend courses dedicated to the problem of sexual dysfunction.
Only 4% of patients report (initiate a dialogue about) the problem, the survey found. Among women aged 40-50 years, the most common complaint reported was dyspareunia (pain on intercourse). In men, the most frequent complaints reported were in the over-40s age group, with 75% citing erectile dysfunction and 45% reporting loss of libido.
The most common gastrointestinal disorders associated with sexual dysfunction are inflammatory bowel diseases (37% of cases), chronic liver diseases (28%), and irritable bowel syndrome (26%), according to the survey.
On the question of whether medications played a role in patients’ sexual dysfunction, nearly 15% of respondents said that prokinetic agents were involved, and 18% thought proton pump inhibitors affect sexual function. Both drug classes are considered responsible for sexual disturbances.
Few gastroenterologists prescribe phosphodiesterase type 5 inhibitors (PDE5i), e.g., Viagra, to treat sexual dysfunction, the survey found. Approximately 90% of respondents said that they never prescribed this class of drugs, preferring to refer patients to an andrologist. Of those who did prescribe PDE5i, significantly fewer residents did compared with experienced gastroenterologists (1.1% vs. 8.8%, respectively; P = .01).
Finally, the biggest reasons why gastroenterologists do not discuss sexual dysfunction are lack of knowledge (80%), insufficient experience (58%), time (44%), and embarrassment (30%).
Practice experience matters
There were some differences among respondents in the experienced group vs. the residents. More men were in the experienced group compared with residents (57.6% vs. 44%, respectively); mean age was 47 years vs. 29 years, respectively; and 71% had 5 or more years of experience in the experienced gastroenterologist group, whereas 78% had 1-5 years of experience among residents.
The survey found that more residents than experienced gastroenterologists “never discussed sexual dysfunction” (38.5% vs. 21.3%, respectively; P = .001) and that more residents than experienced gastroenterologists reported that “patients did not relate their sexual dysfunction to the prescribed therapy” (47.8% vs. 32.5%, respectively; P = .007).
The two groups varied regarding prescription drugs’ role in sexual dysfunction. More experienced gastroenterologists than residents felt that proton pump inhibitors (5.8% vs. 0%, respectively; P = .018) or prokinetics (19.8% vs. 9.5%, respectively; P = .028) might be responsible for some degree of sexual dysfunction.
More residents than experienced doctors felt that other (nongastroenterologic) drugs might contribute to sexual dysfunction in their patients (57.1% vs. 44.7%, respectively; P = .043).
Dr. Romano reported that fewer residents than experienced gastroenterologists referred male patients with sexual dysfunction to an andrologist (frequently/always: 28.1% vs. 44.4%, respectively; P = .004). However, more residents than experienced gastroenterologists disagreed that discussing sexual dysfunction with patients pertains only to specialists (andrologists and gynecologists; 83.5% vs. 71.2%, respectively; P = .018).
Time to step up
Asma Fikree, BMBCh, PhD, of Royal London Hospital, Barts Health NHS Trust, London, moderated the session. The survey highlights that asking patients about sexual dysfunction is an area for improvement for gastroenterologists, she said.
“We might do it in men and ask about erectile dysfunction, but we are very poor about asking in women,” Dr. Fikree noted.
The pros and cons of different medications should be discussed with patients, she said.
Gastroenterologists need to do a better job of considering how medications can lead to sexual dysfunction and interfere with quality of life, and training would help, she added.
“Some patients might not be very bothered by sexual dysfunction, but others might consider it very important,” Dr. Fikree said. “We should be considering this as part of their treatment and care.”
Dr. Romano and Dr. Fikree report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – Sexual dysfunction in patients with gastrointestinal disorders is undermanaged, with a lack of clinician education, time constraints, and embarrassment preventing constructive discussions to improve patient care and quality of life, according to a new survey.
Overall, 71% of gastroenterologists do not ask their patients about sexual dysfunction, the survey finds.
“While patients with gastrointestinal disorders often experience sexual dysfunction, discussions around the matter are not routine in gastroenterological care,” said Marco Romano, MD, from the University of Campania “Luigi Vanvitelli,” Naples, Italy.
Romano presented the survey findings at this year’s United European Gastroenterology Week meeting.
The research shows not only a clear need for better awareness but also a need to build gastroenterologists’ confidence in addressing sexual dysfunction with their patients, Dr. Romano added.
“Most felt that sexual medicine education and improvement of communication skills within the context of their residency training might be important in order to increase the awareness of sexual dysfunction, to overcome barriers, and to improve care and quality of life for their patients,” reported Dr. Romano. “This will lead to prompt diagnosis and treatment of any sexual problems.”
Respectfully asking the patients if their gastrointestinal disorders interfere with their intimate relationships “is often considered a relief to patients who find that the gastrointestinal problem and the sexual dysfunction are interlinked,” he added.
The findings
The survey was needed because the question of whether gastroenterologists inquire about their patients’ sexual issues had never been assessed, Dr. Romano said.
The researchers sent a cross-sectional, anonymous online survey to members of the Italian Society of Gastroenterology and Digestive Endoscopy. The questionnaire, designed and informed by a literature review, consisted of 29 single multiple-choice and open-ended questions.
A total of 426 surveys were returned: 335 from experienced gastroenterologists and 91 from residents (less experienced). Of all respondents, 54.7% were men and 45.3% were women.
Even though most gastroenterologists do not ask their patients about sexual dysfunction, the majority want to learn how to manage the issue, the survey found. Of the survey respondents, 80% agreed that it would be useful for gastroenterologists to attend courses dedicated to the problem of sexual dysfunction.
Only 4% of patients report (initiate a dialogue about) the problem, the survey found. Among women aged 40-50 years, the most common complaint reported was dyspareunia (pain on intercourse). In men, the most frequent complaints reported were in the over-40s age group, with 75% citing erectile dysfunction and 45% reporting loss of libido.
The most common gastrointestinal disorders associated with sexual dysfunction are inflammatory bowel diseases (37% of cases), chronic liver diseases (28%), and irritable bowel syndrome (26%), according to the survey.
On the question of whether medications played a role in patients’ sexual dysfunction, nearly 15% of respondents said that prokinetic agents were involved, and 18% thought proton pump inhibitors affect sexual function. Both drug classes are considered responsible for sexual disturbances.
Few gastroenterologists prescribe phosphodiesterase type 5 inhibitors (PDE5i), e.g., Viagra, to treat sexual dysfunction, the survey found. Approximately 90% of respondents said that they never prescribed this class of drugs, preferring to refer patients to an andrologist. Of those who did prescribe PDE5i, significantly fewer residents did compared with experienced gastroenterologists (1.1% vs. 8.8%, respectively; P = .01).
Finally, the biggest reasons why gastroenterologists do not discuss sexual dysfunction are lack of knowledge (80%), insufficient experience (58%), time (44%), and embarrassment (30%).
Practice experience matters
There were some differences among respondents in the experienced group vs. the residents. More men were in the experienced group compared with residents (57.6% vs. 44%, respectively); mean age was 47 years vs. 29 years, respectively; and 71% had 5 or more years of experience in the experienced gastroenterologist group, whereas 78% had 1-5 years of experience among residents.
The survey found that more residents than experienced gastroenterologists “never discussed sexual dysfunction” (38.5% vs. 21.3%, respectively; P = .001) and that more residents than experienced gastroenterologists reported that “patients did not relate their sexual dysfunction to the prescribed therapy” (47.8% vs. 32.5%, respectively; P = .007).
The two groups varied regarding prescription drugs’ role in sexual dysfunction. More experienced gastroenterologists than residents felt that proton pump inhibitors (5.8% vs. 0%, respectively; P = .018) or prokinetics (19.8% vs. 9.5%, respectively; P = .028) might be responsible for some degree of sexual dysfunction.
More residents than experienced doctors felt that other (nongastroenterologic) drugs might contribute to sexual dysfunction in their patients (57.1% vs. 44.7%, respectively; P = .043).
Dr. Romano reported that fewer residents than experienced gastroenterologists referred male patients with sexual dysfunction to an andrologist (frequently/always: 28.1% vs. 44.4%, respectively; P = .004). However, more residents than experienced gastroenterologists disagreed that discussing sexual dysfunction with patients pertains only to specialists (andrologists and gynecologists; 83.5% vs. 71.2%, respectively; P = .018).
Time to step up
Asma Fikree, BMBCh, PhD, of Royal London Hospital, Barts Health NHS Trust, London, moderated the session. The survey highlights that asking patients about sexual dysfunction is an area for improvement for gastroenterologists, she said.
“We might do it in men and ask about erectile dysfunction, but we are very poor about asking in women,” Dr. Fikree noted.
The pros and cons of different medications should be discussed with patients, she said.
Gastroenterologists need to do a better job of considering how medications can lead to sexual dysfunction and interfere with quality of life, and training would help, she added.
“Some patients might not be very bothered by sexual dysfunction, but others might consider it very important,” Dr. Fikree said. “We should be considering this as part of their treatment and care.”
Dr. Romano and Dr. Fikree report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT UEG WEEK 2022
FMT in IBS: ‘We’ve been targeting the wrong part of the intestine’
VIENNA – , vs. it being administered into the large intestine, according to a new study.
Patients also reported an improvement in symptoms and quality of life with repeated doses of FMT (two doses, given 1 week apart), compared with a single dose in the small intestine, although statistical significance was not met.
“Administering a fecal transplant to the small intestine leads to long-term – up to 1 year in this analysis – colonization of beneficial bacteria, whereas administrating the fecal transplant to the large intestine results in the effect only lasting for the first 3 months,” said Magdy El-Salhy, MD, from the University of Bergen, Norway.
Dr. El-Salhy presented the results at the annual United European Gastroenterology Week meeting.
“It seems that bacteria in the small intestine play a more central role in IBS, as well as its associated fatigue, than bacteria in the large intestine,” Dr. El-Salhy said in an interview.
“Until now, we’ve been targeting the wrong part of the intestine,” he said.
The findings are the first to show that the small intestine is a more effective location for administering FMT than the large intestine for IBS. “It would be worthwhile doing similar [studies] in other diseases, especially in inflammatory bowel diseases,” said Dr. El-Salhy.
Researchers also didn’t expect the repeated dose to improve symptoms for a longer duration. “It really was revolutionary to see,” he added.
Some of Dr. El-Salhy’s patients have had up to 5 years of follow-up, although these results were not presented at this year’s UEG, he said.
“Around 75% of my patients have shown duration of response up to 3 years, and a few up to 5 years, on a 60-g dose from an earlier study group,” he said. “It’s an incredible result after a 10-minute treatment.”
In Dr. El-Salhy’s previous work, he found that increasing the dose from 30 g to 60 g increased the response from about 75% to about 90%. However, in this study presented, he found that increasing the dose to 90 g did not further increase the response. He also noted that while repeating the FMT dose improved symptoms and quality of life more than a single transplantation, it did not increase the response.
Targeting the small intestine
FMT has been widely investigated for the treatment of such conditions as psoriatic arthritis, Clostridioides difficile infection, and ulcerative colitis.
In this study, Dr. El-Salhy built on prior work (seven randomized controlled studies with varied outcomes) by asking whether the transplant dose increases FMT efficacy, which route of administration is more effective, and whether repeating FMT increases efficacy in patients with IBS.
A total of 186 patients were randomized to one of three groups: 90 g of frozen transplant into the large intestine (n = 62), 90 g of frozen transplant into the small intestine (n = 62), or 90 g of frozen transplant into the small intestine twice (with a 1-week interval; n = 62). FMT was administered via nasoduodenal tube and colonoscopy into the small and large intestines, respectively.
Outcomes were measured at 3, 6, and 12 months. The 12-month analysis of outcomes via patient questionnaire included 60, 61, and 60 patients, respectively.
The patient questionnaires included in the study were the IBS-SSS (a composite score of abdominal pain, duration of abdominal pain, bloating/distention, satisfaction with bowel habits, and IBS-related quality of life), the Birmingham IBS Symptom questionnaire, the Fatigue Assessment Scale questionnaire, the IBS-Quality of Life assessment, and the Short-Form Nepean Dyspepsia Index.
Fecal samples were taken and tested for bacterial loads. The bacterial profile and dysbiosis index were determined using the 16S rRNA gene.
At 3 months, patients had similar response rates, around 80%, across single dose in large intestine, single dose in small intestine, and repeat doses in small intestine.
At 6 months, the differences in response rates started to become noticeable, with 67.9% for single dose in large intestine, 71.4% for single dose in small intestine, and 86% for repeat doses in small intestine.
By 12 months, the difference in response rate between the single dose in the large and small intestines was statistically significant at 51.9% and 75.5%, respectively. The response rate to the repeat doses in the small intestine at 12 months (80.9%) was similar to that at 3 months (80.8%).
Side effects, including mild abdominal pain, diarrhea, and constipation, after FMT were seen for the first 5 days after treatment. “People who generally suffer from constipation get diarrhea after FMT and vice versa,” Dr. El-Salhy reported.
“Long-term side effects, as monitored up to 3 years, were not observed,” he added.
Treatment reduced IBS symptoms in all patient groups as measured by IBS-SSS scores. By 12 months, the score fell from around 350 to around 220 in patients who received a single dose in the large intestine, from around 300 to around 200 in patients who received a single dose in the small intestine, and from around 350 to around 170 in patients who received repeat doses in the small intestine.
Quality of life showed a statistically significant difference at 3 months between single and repeated doses in the small intestine and similarly at 6 and 12 months.
Chronic fatigue, experienced by many patients with IBS, was substantially reduced after FMT, Dr. El-Salhy noted. “This surge in energy is often more important to them than the gastrointestinal symptoms.”
Location affects bacterial success
Certain beneficial bacteria were found to thrive more when the donor transplant was administered to the small intestine than to the large intestine.
Of note, Lactobacillus species and Holdemanella biformis grew and then dropped off sharply after 3 months in patients who received a single-dose fecal transplant in the large intestine, while they grew after 3 months and continued to grow after 6 and 12 months in the groups who received a fecal transplant in the small intestine.
“We think bacteria in the small intestine have different characteristics to those in the large intestine,” Dr. El-Salhy said. “This is relatively new, because many years ago it was thought that bile acids prevented bacterial survival. Now we know lots can thrive in the small intestine.”
“It might be viral or some other component that is most effective here. We don’t know yet, but so far we have identified 11 bacteria of interest,” he added.
Broader questions
“Rather than focusing on a specific, single strain microbe as a predictor of success in a disease, the global equilibrium of microbiota is more important, and microbial ecology parameters would be interesting to assess,” remarked Gianluca Ianiro, MD, from the Università Cattolica del Sacro Cuore, Rome, who comoderated the session. “Selected survival of some bacteria through the gut may be the response.”
FMT emerged in response to the challenges posed by recurrent C. difficile infections, noted Alexander Khoruts, MD, a professor of medicine in the division of gastroenterology, hepatology, and nutrition at the University of Minnesota, Minneapolis, who was not involved in the research.
“It is much harder to achieve remodeling of the gut microbiome in non–C. difficile conditions where there is an intact and resilient indigenous microbiota,” he said in an interview. “Therefore, regimens using antibiotic preconditioning and repeated administrations of microbiota are generally more efficacious in achieving this objective.”
The specificity of the bacteria according to disease type targeted was important, said Dr. Khoruts, who has a special interest in gut microbiota.
“The big question in non–C. difficile indications is the composition of donor microbiota. It is critical that we understand the mechanisms involved in each target disease to design appropriate microbiota-based therapeutics,” he said.
Dr. Khoruts sounded a note of caution with respect to establishing the pharmacokinetic and dynamic data related to FMT, which is classified as a drug in the United States.
“It’s imperative that we develop the pharmacology discipline appropriate for this class of therapeutics, including their pharmacokinetics and pharmacodynamics, and an understanding of their potential toxicity and drug-drug interactions,” he said.
Drug distribution data are needed to determine host-microbiota interactions.
“This includes the small bowel microbiome, which continues to be woefully understudied,” Dr. Khoruts said.
Dr. El-Salhy reports no relevant financial relationships. Dr. Ianiro reports receiving personal fees for acting as speaker for Biocodex, Sofar, Malesci, and Tillotts Pharma, and for acting as consultant/advisor for Ferring Therapeutics, Biocodex, Tillotts Pharma, and Zambon. Dr. Khoruts reports he has patents pertaining to fecal microbiota separation from stool and their cryopreservation and lyopreservation.
Through the AGA Center for Gut Microbiome Research and Education, AGA is committed to keeping you up-to-speed on the latest news, research and policy updates related to the gut microbiome: www.gastro.org/microbiome.
A version of this article first appeared on Medscape.com.
VIENNA – , vs. it being administered into the large intestine, according to a new study.
Patients also reported an improvement in symptoms and quality of life with repeated doses of FMT (two doses, given 1 week apart), compared with a single dose in the small intestine, although statistical significance was not met.
“Administering a fecal transplant to the small intestine leads to long-term – up to 1 year in this analysis – colonization of beneficial bacteria, whereas administrating the fecal transplant to the large intestine results in the effect only lasting for the first 3 months,” said Magdy El-Salhy, MD, from the University of Bergen, Norway.
Dr. El-Salhy presented the results at the annual United European Gastroenterology Week meeting.
“It seems that bacteria in the small intestine play a more central role in IBS, as well as its associated fatigue, than bacteria in the large intestine,” Dr. El-Salhy said in an interview.
“Until now, we’ve been targeting the wrong part of the intestine,” he said.
The findings are the first to show that the small intestine is a more effective location for administering FMT than the large intestine for IBS. “It would be worthwhile doing similar [studies] in other diseases, especially in inflammatory bowel diseases,” said Dr. El-Salhy.
Researchers also didn’t expect the repeated dose to improve symptoms for a longer duration. “It really was revolutionary to see,” he added.
Some of Dr. El-Salhy’s patients have had up to 5 years of follow-up, although these results were not presented at this year’s UEG, he said.
“Around 75% of my patients have shown duration of response up to 3 years, and a few up to 5 years, on a 60-g dose from an earlier study group,” he said. “It’s an incredible result after a 10-minute treatment.”
In Dr. El-Salhy’s previous work, he found that increasing the dose from 30 g to 60 g increased the response from about 75% to about 90%. However, in this study presented, he found that increasing the dose to 90 g did not further increase the response. He also noted that while repeating the FMT dose improved symptoms and quality of life more than a single transplantation, it did not increase the response.
Targeting the small intestine
FMT has been widely investigated for the treatment of such conditions as psoriatic arthritis, Clostridioides difficile infection, and ulcerative colitis.
In this study, Dr. El-Salhy built on prior work (seven randomized controlled studies with varied outcomes) by asking whether the transplant dose increases FMT efficacy, which route of administration is more effective, and whether repeating FMT increases efficacy in patients with IBS.
A total of 186 patients were randomized to one of three groups: 90 g of frozen transplant into the large intestine (n = 62), 90 g of frozen transplant into the small intestine (n = 62), or 90 g of frozen transplant into the small intestine twice (with a 1-week interval; n = 62). FMT was administered via nasoduodenal tube and colonoscopy into the small and large intestines, respectively.
Outcomes were measured at 3, 6, and 12 months. The 12-month analysis of outcomes via patient questionnaire included 60, 61, and 60 patients, respectively.
The patient questionnaires included in the study were the IBS-SSS (a composite score of abdominal pain, duration of abdominal pain, bloating/distention, satisfaction with bowel habits, and IBS-related quality of life), the Birmingham IBS Symptom questionnaire, the Fatigue Assessment Scale questionnaire, the IBS-Quality of Life assessment, and the Short-Form Nepean Dyspepsia Index.
Fecal samples were taken and tested for bacterial loads. The bacterial profile and dysbiosis index were determined using the 16S rRNA gene.
At 3 months, patients had similar response rates, around 80%, across single dose in large intestine, single dose in small intestine, and repeat doses in small intestine.
At 6 months, the differences in response rates started to become noticeable, with 67.9% for single dose in large intestine, 71.4% for single dose in small intestine, and 86% for repeat doses in small intestine.
By 12 months, the difference in response rate between the single dose in the large and small intestines was statistically significant at 51.9% and 75.5%, respectively. The response rate to the repeat doses in the small intestine at 12 months (80.9%) was similar to that at 3 months (80.8%).
Side effects, including mild abdominal pain, diarrhea, and constipation, after FMT were seen for the first 5 days after treatment. “People who generally suffer from constipation get diarrhea after FMT and vice versa,” Dr. El-Salhy reported.
“Long-term side effects, as monitored up to 3 years, were not observed,” he added.
Treatment reduced IBS symptoms in all patient groups as measured by IBS-SSS scores. By 12 months, the score fell from around 350 to around 220 in patients who received a single dose in the large intestine, from around 300 to around 200 in patients who received a single dose in the small intestine, and from around 350 to around 170 in patients who received repeat doses in the small intestine.
Quality of life showed a statistically significant difference at 3 months between single and repeated doses in the small intestine and similarly at 6 and 12 months.
Chronic fatigue, experienced by many patients with IBS, was substantially reduced after FMT, Dr. El-Salhy noted. “This surge in energy is often more important to them than the gastrointestinal symptoms.”
Location affects bacterial success
Certain beneficial bacteria were found to thrive more when the donor transplant was administered to the small intestine than to the large intestine.
Of note, Lactobacillus species and Holdemanella biformis grew and then dropped off sharply after 3 months in patients who received a single-dose fecal transplant in the large intestine, while they grew after 3 months and continued to grow after 6 and 12 months in the groups who received a fecal transplant in the small intestine.
“We think bacteria in the small intestine have different characteristics to those in the large intestine,” Dr. El-Salhy said. “This is relatively new, because many years ago it was thought that bile acids prevented bacterial survival. Now we know lots can thrive in the small intestine.”
“It might be viral or some other component that is most effective here. We don’t know yet, but so far we have identified 11 bacteria of interest,” he added.
Broader questions
“Rather than focusing on a specific, single strain microbe as a predictor of success in a disease, the global equilibrium of microbiota is more important, and microbial ecology parameters would be interesting to assess,” remarked Gianluca Ianiro, MD, from the Università Cattolica del Sacro Cuore, Rome, who comoderated the session. “Selected survival of some bacteria through the gut may be the response.”
FMT emerged in response to the challenges posed by recurrent C. difficile infections, noted Alexander Khoruts, MD, a professor of medicine in the division of gastroenterology, hepatology, and nutrition at the University of Minnesota, Minneapolis, who was not involved in the research.
“It is much harder to achieve remodeling of the gut microbiome in non–C. difficile conditions where there is an intact and resilient indigenous microbiota,” he said in an interview. “Therefore, regimens using antibiotic preconditioning and repeated administrations of microbiota are generally more efficacious in achieving this objective.”
The specificity of the bacteria according to disease type targeted was important, said Dr. Khoruts, who has a special interest in gut microbiota.
“The big question in non–C. difficile indications is the composition of donor microbiota. It is critical that we understand the mechanisms involved in each target disease to design appropriate microbiota-based therapeutics,” he said.
Dr. Khoruts sounded a note of caution with respect to establishing the pharmacokinetic and dynamic data related to FMT, which is classified as a drug in the United States.
“It’s imperative that we develop the pharmacology discipline appropriate for this class of therapeutics, including their pharmacokinetics and pharmacodynamics, and an understanding of their potential toxicity and drug-drug interactions,” he said.
Drug distribution data are needed to determine host-microbiota interactions.
“This includes the small bowel microbiome, which continues to be woefully understudied,” Dr. Khoruts said.
Dr. El-Salhy reports no relevant financial relationships. Dr. Ianiro reports receiving personal fees for acting as speaker for Biocodex, Sofar, Malesci, and Tillotts Pharma, and for acting as consultant/advisor for Ferring Therapeutics, Biocodex, Tillotts Pharma, and Zambon. Dr. Khoruts reports he has patents pertaining to fecal microbiota separation from stool and their cryopreservation and lyopreservation.
Through the AGA Center for Gut Microbiome Research and Education, AGA is committed to keeping you up-to-speed on the latest news, research and policy updates related to the gut microbiome: www.gastro.org/microbiome.
A version of this article first appeared on Medscape.com.
VIENNA – , vs. it being administered into the large intestine, according to a new study.
Patients also reported an improvement in symptoms and quality of life with repeated doses of FMT (two doses, given 1 week apart), compared with a single dose in the small intestine, although statistical significance was not met.
“Administering a fecal transplant to the small intestine leads to long-term – up to 1 year in this analysis – colonization of beneficial bacteria, whereas administrating the fecal transplant to the large intestine results in the effect only lasting for the first 3 months,” said Magdy El-Salhy, MD, from the University of Bergen, Norway.
Dr. El-Salhy presented the results at the annual United European Gastroenterology Week meeting.
“It seems that bacteria in the small intestine play a more central role in IBS, as well as its associated fatigue, than bacteria in the large intestine,” Dr. El-Salhy said in an interview.
“Until now, we’ve been targeting the wrong part of the intestine,” he said.
The findings are the first to show that the small intestine is a more effective location for administering FMT than the large intestine for IBS. “It would be worthwhile doing similar [studies] in other diseases, especially in inflammatory bowel diseases,” said Dr. El-Salhy.
Researchers also didn’t expect the repeated dose to improve symptoms for a longer duration. “It really was revolutionary to see,” he added.
Some of Dr. El-Salhy’s patients have had up to 5 years of follow-up, although these results were not presented at this year’s UEG, he said.
“Around 75% of my patients have shown duration of response up to 3 years, and a few up to 5 years, on a 60-g dose from an earlier study group,” he said. “It’s an incredible result after a 10-minute treatment.”
In Dr. El-Salhy’s previous work, he found that increasing the dose from 30 g to 60 g increased the response from about 75% to about 90%. However, in this study presented, he found that increasing the dose to 90 g did not further increase the response. He also noted that while repeating the FMT dose improved symptoms and quality of life more than a single transplantation, it did not increase the response.
Targeting the small intestine
FMT has been widely investigated for the treatment of such conditions as psoriatic arthritis, Clostridioides difficile infection, and ulcerative colitis.
In this study, Dr. El-Salhy built on prior work (seven randomized controlled studies with varied outcomes) by asking whether the transplant dose increases FMT efficacy, which route of administration is more effective, and whether repeating FMT increases efficacy in patients with IBS.
A total of 186 patients were randomized to one of three groups: 90 g of frozen transplant into the large intestine (n = 62), 90 g of frozen transplant into the small intestine (n = 62), or 90 g of frozen transplant into the small intestine twice (with a 1-week interval; n = 62). FMT was administered via nasoduodenal tube and colonoscopy into the small and large intestines, respectively.
Outcomes were measured at 3, 6, and 12 months. The 12-month analysis of outcomes via patient questionnaire included 60, 61, and 60 patients, respectively.
The patient questionnaires included in the study were the IBS-SSS (a composite score of abdominal pain, duration of abdominal pain, bloating/distention, satisfaction with bowel habits, and IBS-related quality of life), the Birmingham IBS Symptom questionnaire, the Fatigue Assessment Scale questionnaire, the IBS-Quality of Life assessment, and the Short-Form Nepean Dyspepsia Index.
Fecal samples were taken and tested for bacterial loads. The bacterial profile and dysbiosis index were determined using the 16S rRNA gene.
At 3 months, patients had similar response rates, around 80%, across single dose in large intestine, single dose in small intestine, and repeat doses in small intestine.
At 6 months, the differences in response rates started to become noticeable, with 67.9% for single dose in large intestine, 71.4% for single dose in small intestine, and 86% for repeat doses in small intestine.
By 12 months, the difference in response rate between the single dose in the large and small intestines was statistically significant at 51.9% and 75.5%, respectively. The response rate to the repeat doses in the small intestine at 12 months (80.9%) was similar to that at 3 months (80.8%).
Side effects, including mild abdominal pain, diarrhea, and constipation, after FMT were seen for the first 5 days after treatment. “People who generally suffer from constipation get diarrhea after FMT and vice versa,” Dr. El-Salhy reported.
“Long-term side effects, as monitored up to 3 years, were not observed,” he added.
Treatment reduced IBS symptoms in all patient groups as measured by IBS-SSS scores. By 12 months, the score fell from around 350 to around 220 in patients who received a single dose in the large intestine, from around 300 to around 200 in patients who received a single dose in the small intestine, and from around 350 to around 170 in patients who received repeat doses in the small intestine.
Quality of life showed a statistically significant difference at 3 months between single and repeated doses in the small intestine and similarly at 6 and 12 months.
Chronic fatigue, experienced by many patients with IBS, was substantially reduced after FMT, Dr. El-Salhy noted. “This surge in energy is often more important to them than the gastrointestinal symptoms.”
Location affects bacterial success
Certain beneficial bacteria were found to thrive more when the donor transplant was administered to the small intestine than to the large intestine.
Of note, Lactobacillus species and Holdemanella biformis grew and then dropped off sharply after 3 months in patients who received a single-dose fecal transplant in the large intestine, while they grew after 3 months and continued to grow after 6 and 12 months in the groups who received a fecal transplant in the small intestine.
“We think bacteria in the small intestine have different characteristics to those in the large intestine,” Dr. El-Salhy said. “This is relatively new, because many years ago it was thought that bile acids prevented bacterial survival. Now we know lots can thrive in the small intestine.”
“It might be viral or some other component that is most effective here. We don’t know yet, but so far we have identified 11 bacteria of interest,” he added.
Broader questions
“Rather than focusing on a specific, single strain microbe as a predictor of success in a disease, the global equilibrium of microbiota is more important, and microbial ecology parameters would be interesting to assess,” remarked Gianluca Ianiro, MD, from the Università Cattolica del Sacro Cuore, Rome, who comoderated the session. “Selected survival of some bacteria through the gut may be the response.”
FMT emerged in response to the challenges posed by recurrent C. difficile infections, noted Alexander Khoruts, MD, a professor of medicine in the division of gastroenterology, hepatology, and nutrition at the University of Minnesota, Minneapolis, who was not involved in the research.
“It is much harder to achieve remodeling of the gut microbiome in non–C. difficile conditions where there is an intact and resilient indigenous microbiota,” he said in an interview. “Therefore, regimens using antibiotic preconditioning and repeated administrations of microbiota are generally more efficacious in achieving this objective.”
The specificity of the bacteria according to disease type targeted was important, said Dr. Khoruts, who has a special interest in gut microbiota.
“The big question in non–C. difficile indications is the composition of donor microbiota. It is critical that we understand the mechanisms involved in each target disease to design appropriate microbiota-based therapeutics,” he said.
Dr. Khoruts sounded a note of caution with respect to establishing the pharmacokinetic and dynamic data related to FMT, which is classified as a drug in the United States.
“It’s imperative that we develop the pharmacology discipline appropriate for this class of therapeutics, including their pharmacokinetics and pharmacodynamics, and an understanding of their potential toxicity and drug-drug interactions,” he said.
Drug distribution data are needed to determine host-microbiota interactions.
“This includes the small bowel microbiome, which continues to be woefully understudied,” Dr. Khoruts said.
Dr. El-Salhy reports no relevant financial relationships. Dr. Ianiro reports receiving personal fees for acting as speaker for Biocodex, Sofar, Malesci, and Tillotts Pharma, and for acting as consultant/advisor for Ferring Therapeutics, Biocodex, Tillotts Pharma, and Zambon. Dr. Khoruts reports he has patents pertaining to fecal microbiota separation from stool and their cryopreservation and lyopreservation.
Through the AGA Center for Gut Microbiome Research and Education, AGA is committed to keeping you up-to-speed on the latest news, research and policy updates related to the gut microbiome: www.gastro.org/microbiome.
A version of this article first appeared on Medscape.com.
First RCT evaluating benefits of colonoscopy screening rocks GI: NordICC
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This study’s data show that colonoscopy is effective – if it is completed. Only 42% of patients randomized to colonoscopy completed the test; among patients who actually got a colonoscopy, results are much more impressive in colorectal cancer (CRC) prevention (31% decrease) and mortality (50% decrease). In this study, many endoscopists had ADRs below the 25% benchmark, and low ADRs are associated with a higher risk of postcolonoscopy CRC. Differences between the two groups may increase with longer follow-up, which is planned, because detection and removal of polyps via colonoscopy prevents future cancers.
Remind your patients that they shouldn’t let media headlines guide your health care decisions. You should also explain how colonoscopy can detect and remove polyps, which prevents those polyps from developing into cancer. Most of the patients in the Norway study skipped their colonoscopy, but the test can’t prevent cancers if it isn’t performed! Lastly, colonoscopy is effective in a U.S. population and can cut their risk of dying from CRC.
David Lieberman, MD, is a professor of medicine and chief of the division of gastroenterology and hepatology at Oregon Health and Science University, Portland. He disclosed being a consultant for Freenome and Check-Cap.
This study’s data show that colonoscopy is effective – if it is completed. Only 42% of patients randomized to colonoscopy completed the test; among patients who actually got a colonoscopy, results are much more impressive in colorectal cancer (CRC) prevention (31% decrease) and mortality (50% decrease). In this study, many endoscopists had ADRs below the 25% benchmark, and low ADRs are associated with a higher risk of postcolonoscopy CRC. Differences between the two groups may increase with longer follow-up, which is planned, because detection and removal of polyps via colonoscopy prevents future cancers.
Remind your patients that they shouldn’t let media headlines guide your health care decisions. You should also explain how colonoscopy can detect and remove polyps, which prevents those polyps from developing into cancer. Most of the patients in the Norway study skipped their colonoscopy, but the test can’t prevent cancers if it isn’t performed! Lastly, colonoscopy is effective in a U.S. population and can cut their risk of dying from CRC.
David Lieberman, MD, is a professor of medicine and chief of the division of gastroenterology and hepatology at Oregon Health and Science University, Portland. He disclosed being a consultant for Freenome and Check-Cap.
This study’s data show that colonoscopy is effective – if it is completed. Only 42% of patients randomized to colonoscopy completed the test; among patients who actually got a colonoscopy, results are much more impressive in colorectal cancer (CRC) prevention (31% decrease) and mortality (50% decrease). In this study, many endoscopists had ADRs below the 25% benchmark, and low ADRs are associated with a higher risk of postcolonoscopy CRC. Differences between the two groups may increase with longer follow-up, which is planned, because detection and removal of polyps via colonoscopy prevents future cancers.
Remind your patients that they shouldn’t let media headlines guide your health care decisions. You should also explain how colonoscopy can detect and remove polyps, which prevents those polyps from developing into cancer. Most of the patients in the Norway study skipped their colonoscopy, but the test can’t prevent cancers if it isn’t performed! Lastly, colonoscopy is effective in a U.S. population and can cut their risk of dying from CRC.
David Lieberman, MD, is a professor of medicine and chief of the division of gastroenterology and hepatology at Oregon Health and Science University, Portland. He disclosed being a consultant for Freenome and Check-Cap.
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
VIENNA – the 10-year follow-up of the large, multicenter, randomized Northern-European Initiative on Colorectal Cancer (NordICC) trial shows.
In effect, this means the number needed to invite to undergo screening to prevent one case of colorectal cancer is 455 (95% confidence interval, 270-1,429), the researchers determined.
The results were presented at the United European Gastroenterology Week 2022 meeting and were published simultaneously in The New England Journal of Medicine.
The results of the study, which was designed to be truly population based and to mimic national colorectal cancer screening programs, provide an estimate of the effect of screening colonoscopy in the general population.
The primary outcome was determined on an intention-to-screen basis. All persons who were invited to undergo colonoscopy screening were compared with people who received usual care (that is, received no invitation or screening). At UEG 2022, the researchers presented the interim 10-year colorectal cancer risk, which was found to be 0.98%, compared to 1.20%. This represents a risk reduction of 18% among colonoscopy invitees (risk ratio, 0.82; 95% CI, 0.70-0.93). During the study period, 259 cases of colorectal cancer were diagnosed in the invited group versus622 in the usual-care group.
The risk of death from colorectal cancer was 0.28% in the invited group and 0.31% in the usual-care group (RR, 0.90; 95% CI, 0.64-1.16). The risk of death from any cause was similar in both the invited group and the usual-care group, at 11.03% and 11.04%, respectively (RR, 0.99; 95% CI, 0.96-1.04).
The authors noted that the benefit would have been greater had more people undergone screening; only 42% of those who were invited actually underwent colonoscopy. In an adjusted analysis, had all those who had been invited to undergo screening undergone colonoscopy, the 10-year risk of colorectal cancer would have decreased from 1.22% to 0.84%, and the risk of colorectal cancer–related death would have fallen from 0.30% to 0.15%.
The researchers, led by gastroenterologist Michael Bretthauer, MD, from the department of medicine, gastrointestinal endoscopy, University of Oslo, who presented the data at UEG 2022 on behalf of the NordICC study group, acknowledged that, despite the “observed appreciable reductions in relative risks, the absolute risks of the risk of colorectal cancer and even more so of colorectal cancer–related death were lower than those in previous screening trials and lower than what we anticipated when the trial was planned.”
However, they add that “optimism related to the effects of screening on colorectal cancer–related death may be warranted in light of the 50% decrease observed in adjusted per-protocol analyses.”
With his coauthors, Dr. Bretthauer wrote that even their adjusted findings “probably underestimated the benefit because, as in most other large-scale trials of colorectal cancer screening, we could not adjust for all important confounders in all countries.”
Dr. Bretthauer also noted that results were similar to those achieved through sigmoidoscopy screening. By close comparison, sigmoidoscopy studies show the risk of colorectal cancer is reduced between 33% and 40%, according to per protocol analyses. “These results suggest that colonoscopy screening might not be substantially better in reducing the risk of colorectal cancer than sigmoidoscopy.”
Real-world, population-based study
NordICC is an ongoing, pragmatic study and is the first randomized trial to quantify the possible benefit of colonoscopy screening on risk of colorectal cancer and related death.
Researchers recruited healthy men and women from registries in Poland, Norway, Sweden, and the Netherlands between 2009 and 2014. Most participants came from Poland (54,258), followed by Norway (26,411) and Sweden (3,646). Data from the Netherlands could not be included owing to data protection law.
At baseline, 84,585 participants aged 55-64 years were randomly assigned in a 1:2 ratio either to receive an invitation to undergo a single screening colonoscopy (28,220; invited) or to undergo usual care in each participant country (56,365; no invitation or screening).
Any colorectal cancer lesions detected were removed, whenever possible. The primary endpoints were the risks of colorectal cancer and colorectal cancer–related death. The secondary endpoint was death from any cause.
‘Modest effectiveness,’ but longer follow-up to give fuller picture
In an editorial that accompanied publication of the study, Jason A. Dominitz, MD, from the division of gastroenterology, University of Washington, Seattle, and Douglas J. Robertson, MD, from White River Junction (Vt.) Veterans Affairs Medical Center, commented on the possible reasons for the low reduction in incident cancer and deaths seen in NordICC.
They pointed out that cohort studies suggest a 40%-69% decrease in the incidence of colorectal cancer and a 29%-88% decrease in the risk of death with colonoscopy. However, they noted that “cohort studies probably overestimate the real-world effectiveness of colonoscopy because of the inability to adjust for important factors such as incomplete adherence to testing and the tendency of healthier persons to seek preventive care.”
Referring to Dr. Bretthauer’s point about attendance to screening, Dr. Dominitz and Dr. Robertson added that, in the United States, colonoscopy is the predominant form of screening for colorectal cancer and that in countries where colonoscopy is less established, participation may be very different.
“The actual effectiveness of colonoscopy in populations that are more accepting of colonoscopy could more closely resemble the effectiveness shown in the per-protocol analysis in this trial,” they wrote.
The editorialists also pointed out that the benefits of screening colonoscopy take time to be realized “because the incidence of colorectal cancer is initially increased when presymptomatic cancers are identified.” A repeat and final analysis of the NordICC data is due at 15 years’ follow-up.
In addition, they noted that “colonoscopy is highly operator dependent” and that the adenoma detection rate is variable and affects cancer risk and related mortality.
Given the “modest effectiveness” of screening colonoscopy in the trial, they asserted that, “if the trial truly represents the real-world performance of population-based screening colonoscopy, it might be hard to justify the risk and expense of this form of screening when simpler, less-invasive strategies (e.g., sigmoidoscopy and FIT [fecal immunochemical test]) are available.”
However, they also noted that “additional analyses, including longer follow up and results from other ongoing comparative effectiveness trials, will help us to fully understand the benefits of this test.”
Also commenting on the study was Michiel Maas, MD, from the department of gastroenterology and hepatology, Radboud UMC, Nijmegen, the Netherlands, told this news organization that he agreed that the absolute effect on colorectal cancer risk or colorectal cancer–related death was not as high as expected and may be disappointing.
But Dr. Maas said that “around half of the patients in the study did not undergo colonoscopy, which may have negatively impacted the results.
“An additional factor, which can be influential in colonoscopy studies, is the potential variability in detection rates between operators/endoscopists,” he said.
Looking to the future, Dr. Maas noted that “AI [artificial intelligence] or computer-aided detection can level this playing field in detection rates.
“Nevertheless, this is a very interesting study, which sheds a new light on the efficacy on screening colonoscopies,” he said.
Dr. Bretthauer has relationships with Paion, Cybernet, and the Norwegian Council of Research. Dr. Dominitz is cochair of VA Cooperative Studies Program #577: “Colonoscopy vs. Fecal Immunochemical Test (FIT) in Reducing Mortality from Colorectal Cancer” (the CONFIRM Study), which is funded by the Department of Veterans Affairs. Dr. Robertson is national cochair (with Dr. Dominitz) of the CONFIRM trial and has received personal fees from Freenome outside of the submitted work. Dr. Maas reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM UEG 2022
FMT in IBS: ‘We’ve been targeting the wrong part of the intestine’
VIENNA – , vs. it being administered into the large intestine, according to a new study.
Patients also reported an improvement in symptoms and quality of life with repeated doses of FMT (two doses, given 1 week apart), compared with a single dose in the small intestine, although statistical significance was not met.
“Administering a fecal transplant to the small intestine leads to long-term – up to 1 year in this analysis – colonization of beneficial bacteria, whereas administrating the fecal transplant to the large intestine results in the effect only lasting for the first 3 months,” said Magdy El-Salhy, MD, from the University of Bergen, Norway.
Dr. El-Salhy presented the results at the annual United European Gastroenterology Week meeting.
“It seems that bacteria in the small intestine play a more central role in IBS, as well as its associated fatigue, than bacteria in the large intestine,” Dr. El-Salhy said in an interview.
“Until now, we’ve been targeting the wrong part of the intestine,” he said.
The findings are the first to show that the small intestine is a more effective location for administering FMT than the large intestine for IBS. “It would be worthwhile doing similar [studies] in other diseases, especially in inflammatory bowel diseases,” said Dr. El-Salhy.
Researchers also didn’t expect the repeated dose to improve symptoms for a longer duration. “It really was revolutionary to see,” he added.
Some of Dr. El-Salhy’s patients have had up to 5 years of follow-up, although these results were not presented at this year’s UEG, he said.
“Around 75% of my patients have shown duration of response up to 3 years, and a few up to 5 years, on a 60-g dose from an earlier study group,” he said. “It’s an incredible result after a 10-minute treatment.”
In Dr. El-Salhy’s previous work, he found that increasing the dose from 30 g to 60 g increased the response from about 75% to about 90%. However, in this study presented, he found that increasing the dose to 90 g did not further increase the response. He also noted that while repeating the FMT dose improved symptoms and quality of life more than a single transplantation, it did not increase the response.
Targeting the small intestine
FMT has been widely investigated for the treatment of such conditions as psoriatic arthritis, Clostridioides difficile infection, and ulcerative colitis.
In this study, Dr. El-Salhy built on prior work (seven randomized controlled studies with varied outcomes) by asking whether the transplant dose increases FMT efficacy, which route of administration is more effective, and whether repeating FMT increases efficacy in patients with IBS.
A total of 186 patients were randomized to one of three groups: 90 g of frozen transplant into the large intestine (n = 62), 90 g of frozen transplant into the small intestine (n = 62), or 90 g of frozen transplant into the small intestine twice (with a 1-week interval; n = 62). FMT was administered via nasoduodenal tube and colonoscopy into the small and large intestines, respectively.
Outcomes were measured at 3, 6, and 12 months. The 12-month analysis of outcomes via patient questionnaire included 60, 61, and 60 patients, respectively.
The patient questionnaires included in the study were the IBS-SSS (a composite score of abdominal pain, duration of abdominal pain, bloating/distention, satisfaction with bowel habits, and IBS-related quality of life), the Birmingham IBS Symptom questionnaire, the Fatigue Assessment Scale questionnaire, the IBS-Quality of Life assessment, and the Short-Form Nepean Dyspepsia Index.
Fecal samples were taken and tested for bacterial loads. The bacterial profile and dysbiosis index were determined using the 16S rRNA gene.
At 3 months, patients had similar response rates, around 80%, across single dose in large intestine, single dose in small intestine, and repeat doses in small intestine.
At 6 months, the differences in response rates started to become noticeable, with 67.9% for single dose in large intestine, 71.4% for single dose in small intestine, and 86% for repeat doses in small intestine.
By 12 months, the difference in response rate between the single dose in the large and small intestines was statistically significant at 51.9% and 75.5%, respectively. The response rate to the repeat doses in the small intestine at 12 months (80.9%) was similar to that at 3 months (80.8%).
Side effects, including mild abdominal pain, diarrhea, and constipation, after FMT were seen for the first 5 days after treatment. “People who generally suffer from constipation get diarrhea after FMT and vice versa,” Dr. El-Salhy reported.
“Long-term side effects, as monitored up to 3 years, were not observed,” he added.
Treatment reduced IBS symptoms in all patient groups as measured by IBS-SSS scores. By 12 months, the score fell from around 350 to around 220 in patients who received a single dose in the large intestine, from around 300 to around 200 in patients who received a single dose in the small intestine, and from around 350 to around 170 in patients who received repeat doses in the small intestine.
Quality of life showed a statistically significant difference at 3 months between single and repeated doses in the small intestine and similarly at 6 and 12 months.
Chronic fatigue, experienced by many patients with IBS, was substantially reduced after FMT, Dr. El-Salhy noted. “This surge in energy is often more important to them than the gastrointestinal symptoms.”
Location affects bacterial success
Certain beneficial bacteria were found to thrive more when the donor transplant was administered to the small intestine than to the large intestine.
Of note, Lactobacillus species and Holdemanella biformis grew and then dropped off sharply after 3 months in patients who received a single-dose fecal transplant in the large intestine, while they grew after 3 months and continued to grow after 6 and 12 months in the groups who received a fecal transplant in the small intestine.
“We think bacteria in the small intestine have different characteristics to those in the large intestine,” Dr. El-Salhy said. “This is relatively new, because many years ago it was thought that bile acids prevented bacterial survival. Now we know lots can thrive in the small intestine.”
“It might be viral or some other component that is most effective here. We don’t know yet, but so far we have identified 11 bacteria of interest,” he added.
Broader questions
“Rather than focusing on a specific, single strain microbe as a predictor of success in a disease, the global equilibrium of microbiota is more important, and microbial ecology parameters would be interesting to assess,” remarked Gianluca Ianiro, MD, from the Università Cattolica del Sacro Cuore, Rome, who comoderated the session. “Selected survival of some bacteria through the gut may be the response.”
FMT emerged in response to the challenges posed by recurrent C. difficile infections, noted Alexander Khoruts, MD, a professor of medicine in the division of gastroenterology, hepatology, and nutrition at the University of Minnesota, Minneapolis, who was not involved in the research.
“It is much harder to achieve remodeling of the gut microbiome in non–C. difficile conditions where there is an intact and resilient indigenous microbiota,” he said in an interview. “Therefore, regimens using antibiotic preconditioning and repeated administrations of microbiota are generally more efficacious in achieving this objective.”
The specificity of the bacteria according to disease type targeted was important, said Dr. Khoruts, who has a special interest in gut microbiota.
“The big question in non–C. difficile indications is the composition of donor microbiota. It is critical that we understand the mechanisms involved in each target disease to design appropriate microbiota-based therapeutics,” he said.
Dr. Khoruts sounded a note of caution with respect to establishing the pharmacokinetic and dynamic data related to FMT, which is classified as a drug in the United States.
“It’s imperative that we develop the pharmacology discipline appropriate for this class of therapeutics, including their pharmacokinetics and pharmacodynamics, and an understanding of their potential toxicity and drug-drug interactions,” he said.
Drug distribution data are needed to determine host-microbiota interactions.
“This includes the small bowel microbiome, which continues to be woefully understudied,” Dr. Khoruts said.
Dr. El-Salhy reports no relevant financial relationships. Dr. Ianiro reports receiving personal fees for acting as speaker for Biocodex, Sofar, Malesci, and Tillotts Pharma, and for acting as consultant/advisor for Ferring Therapeutics, Biocodex, Tillotts Pharma, and Zambon. Dr. Khoruts reports he has patents pertaining to fecal microbiota separation from stool and their cryopreservation and lyopreservation.
A version of this article first appeared on Medscape.com.
VIENNA – , vs. it being administered into the large intestine, according to a new study.
Patients also reported an improvement in symptoms and quality of life with repeated doses of FMT (two doses, given 1 week apart), compared with a single dose in the small intestine, although statistical significance was not met.
“Administering a fecal transplant to the small intestine leads to long-term – up to 1 year in this analysis – colonization of beneficial bacteria, whereas administrating the fecal transplant to the large intestine results in the effect only lasting for the first 3 months,” said Magdy El-Salhy, MD, from the University of Bergen, Norway.
Dr. El-Salhy presented the results at the annual United European Gastroenterology Week meeting.
“It seems that bacteria in the small intestine play a more central role in IBS, as well as its associated fatigue, than bacteria in the large intestine,” Dr. El-Salhy said in an interview.
“Until now, we’ve been targeting the wrong part of the intestine,” he said.
The findings are the first to show that the small intestine is a more effective location for administering FMT than the large intestine for IBS. “It would be worthwhile doing similar [studies] in other diseases, especially in inflammatory bowel diseases,” said Dr. El-Salhy.
Researchers also didn’t expect the repeated dose to improve symptoms for a longer duration. “It really was revolutionary to see,” he added.
Some of Dr. El-Salhy’s patients have had up to 5 years of follow-up, although these results were not presented at this year’s UEG, he said.
“Around 75% of my patients have shown duration of response up to 3 years, and a few up to 5 years, on a 60-g dose from an earlier study group,” he said. “It’s an incredible result after a 10-minute treatment.”
In Dr. El-Salhy’s previous work, he found that increasing the dose from 30 g to 60 g increased the response from about 75% to about 90%. However, in this study presented, he found that increasing the dose to 90 g did not further increase the response. He also noted that while repeating the FMT dose improved symptoms and quality of life more than a single transplantation, it did not increase the response.
Targeting the small intestine
FMT has been widely investigated for the treatment of such conditions as psoriatic arthritis, Clostridioides difficile infection, and ulcerative colitis.
In this study, Dr. El-Salhy built on prior work (seven randomized controlled studies with varied outcomes) by asking whether the transplant dose increases FMT efficacy, which route of administration is more effective, and whether repeating FMT increases efficacy in patients with IBS.
A total of 186 patients were randomized to one of three groups: 90 g of frozen transplant into the large intestine (n = 62), 90 g of frozen transplant into the small intestine (n = 62), or 90 g of frozen transplant into the small intestine twice (with a 1-week interval; n = 62). FMT was administered via nasoduodenal tube and colonoscopy into the small and large intestines, respectively.
Outcomes were measured at 3, 6, and 12 months. The 12-month analysis of outcomes via patient questionnaire included 60, 61, and 60 patients, respectively.
The patient questionnaires included in the study were the IBS-SSS (a composite score of abdominal pain, duration of abdominal pain, bloating/distention, satisfaction with bowel habits, and IBS-related quality of life), the Birmingham IBS Symptom questionnaire, the Fatigue Assessment Scale questionnaire, the IBS-Quality of Life assessment, and the Short-Form Nepean Dyspepsia Index.
Fecal samples were taken and tested for bacterial loads. The bacterial profile and dysbiosis index were determined using the 16S rRNA gene.
At 3 months, patients had similar response rates, around 80%, across single dose in large intestine, single dose in small intestine, and repeat doses in small intestine.
At 6 months, the differences in response rates started to become noticeable, with 67.9% for single dose in large intestine, 71.4% for single dose in small intestine, and 86% for repeat doses in small intestine.
By 12 months, the difference in response rate between the single dose in the large and small intestines was statistically significant at 51.9% and 75.5%, respectively. The response rate to the repeat doses in the small intestine at 12 months (80.9%) was similar to that at 3 months (80.8%).
Side effects, including mild abdominal pain, diarrhea, and constipation, after FMT were seen for the first 5 days after treatment. “People who generally suffer from constipation get diarrhea after FMT and vice versa,” Dr. El-Salhy reported.
“Long-term side effects, as monitored up to 3 years, were not observed,” he added.
Treatment reduced IBS symptoms in all patient groups as measured by IBS-SSS scores. By 12 months, the score fell from around 350 to around 220 in patients who received a single dose in the large intestine, from around 300 to around 200 in patients who received a single dose in the small intestine, and from around 350 to around 170 in patients who received repeat doses in the small intestine.
Quality of life showed a statistically significant difference at 3 months between single and repeated doses in the small intestine and similarly at 6 and 12 months.
Chronic fatigue, experienced by many patients with IBS, was substantially reduced after FMT, Dr. El-Salhy noted. “This surge in energy is often more important to them than the gastrointestinal symptoms.”
Location affects bacterial success
Certain beneficial bacteria were found to thrive more when the donor transplant was administered to the small intestine than to the large intestine.
Of note, Lactobacillus species and Holdemanella biformis grew and then dropped off sharply after 3 months in patients who received a single-dose fecal transplant in the large intestine, while they grew after 3 months and continued to grow after 6 and 12 months in the groups who received a fecal transplant in the small intestine.
“We think bacteria in the small intestine have different characteristics to those in the large intestine,” Dr. El-Salhy said. “This is relatively new, because many years ago it was thought that bile acids prevented bacterial survival. Now we know lots can thrive in the small intestine.”
“It might be viral or some other component that is most effective here. We don’t know yet, but so far we have identified 11 bacteria of interest,” he added.
Broader questions
“Rather than focusing on a specific, single strain microbe as a predictor of success in a disease, the global equilibrium of microbiota is more important, and microbial ecology parameters would be interesting to assess,” remarked Gianluca Ianiro, MD, from the Università Cattolica del Sacro Cuore, Rome, who comoderated the session. “Selected survival of some bacteria through the gut may be the response.”
FMT emerged in response to the challenges posed by recurrent C. difficile infections, noted Alexander Khoruts, MD, a professor of medicine in the division of gastroenterology, hepatology, and nutrition at the University of Minnesota, Minneapolis, who was not involved in the research.
“It is much harder to achieve remodeling of the gut microbiome in non–C. difficile conditions where there is an intact and resilient indigenous microbiota,” he said in an interview. “Therefore, regimens using antibiotic preconditioning and repeated administrations of microbiota are generally more efficacious in achieving this objective.”
The specificity of the bacteria according to disease type targeted was important, said Dr. Khoruts, who has a special interest in gut microbiota.
“The big question in non–C. difficile indications is the composition of donor microbiota. It is critical that we understand the mechanisms involved in each target disease to design appropriate microbiota-based therapeutics,” he said.
Dr. Khoruts sounded a note of caution with respect to establishing the pharmacokinetic and dynamic data related to FMT, which is classified as a drug in the United States.
“It’s imperative that we develop the pharmacology discipline appropriate for this class of therapeutics, including their pharmacokinetics and pharmacodynamics, and an understanding of their potential toxicity and drug-drug interactions,” he said.
Drug distribution data are needed to determine host-microbiota interactions.
“This includes the small bowel microbiome, which continues to be woefully understudied,” Dr. Khoruts said.
Dr. El-Salhy reports no relevant financial relationships. Dr. Ianiro reports receiving personal fees for acting as speaker for Biocodex, Sofar, Malesci, and Tillotts Pharma, and for acting as consultant/advisor for Ferring Therapeutics, Biocodex, Tillotts Pharma, and Zambon. Dr. Khoruts reports he has patents pertaining to fecal microbiota separation from stool and their cryopreservation and lyopreservation.
A version of this article first appeared on Medscape.com.
VIENNA – , vs. it being administered into the large intestine, according to a new study.
Patients also reported an improvement in symptoms and quality of life with repeated doses of FMT (two doses, given 1 week apart), compared with a single dose in the small intestine, although statistical significance was not met.
“Administering a fecal transplant to the small intestine leads to long-term – up to 1 year in this analysis – colonization of beneficial bacteria, whereas administrating the fecal transplant to the large intestine results in the effect only lasting for the first 3 months,” said Magdy El-Salhy, MD, from the University of Bergen, Norway.
Dr. El-Salhy presented the results at the annual United European Gastroenterology Week meeting.
“It seems that bacteria in the small intestine play a more central role in IBS, as well as its associated fatigue, than bacteria in the large intestine,” Dr. El-Salhy said in an interview.
“Until now, we’ve been targeting the wrong part of the intestine,” he said.
The findings are the first to show that the small intestine is a more effective location for administering FMT than the large intestine for IBS. “It would be worthwhile doing similar [studies] in other diseases, especially in inflammatory bowel diseases,” said Dr. El-Salhy.
Researchers also didn’t expect the repeated dose to improve symptoms for a longer duration. “It really was revolutionary to see,” he added.
Some of Dr. El-Salhy’s patients have had up to 5 years of follow-up, although these results were not presented at this year’s UEG, he said.
“Around 75% of my patients have shown duration of response up to 3 years, and a few up to 5 years, on a 60-g dose from an earlier study group,” he said. “It’s an incredible result after a 10-minute treatment.”
In Dr. El-Salhy’s previous work, he found that increasing the dose from 30 g to 60 g increased the response from about 75% to about 90%. However, in this study presented, he found that increasing the dose to 90 g did not further increase the response. He also noted that while repeating the FMT dose improved symptoms and quality of life more than a single transplantation, it did not increase the response.
Targeting the small intestine
FMT has been widely investigated for the treatment of such conditions as psoriatic arthritis, Clostridioides difficile infection, and ulcerative colitis.
In this study, Dr. El-Salhy built on prior work (seven randomized controlled studies with varied outcomes) by asking whether the transplant dose increases FMT efficacy, which route of administration is more effective, and whether repeating FMT increases efficacy in patients with IBS.
A total of 186 patients were randomized to one of three groups: 90 g of frozen transplant into the large intestine (n = 62), 90 g of frozen transplant into the small intestine (n = 62), or 90 g of frozen transplant into the small intestine twice (with a 1-week interval; n = 62). FMT was administered via nasoduodenal tube and colonoscopy into the small and large intestines, respectively.
Outcomes were measured at 3, 6, and 12 months. The 12-month analysis of outcomes via patient questionnaire included 60, 61, and 60 patients, respectively.
The patient questionnaires included in the study were the IBS-SSS (a composite score of abdominal pain, duration of abdominal pain, bloating/distention, satisfaction with bowel habits, and IBS-related quality of life), the Birmingham IBS Symptom questionnaire, the Fatigue Assessment Scale questionnaire, the IBS-Quality of Life assessment, and the Short-Form Nepean Dyspepsia Index.
Fecal samples were taken and tested for bacterial loads. The bacterial profile and dysbiosis index were determined using the 16S rRNA gene.
At 3 months, patients had similar response rates, around 80%, across single dose in large intestine, single dose in small intestine, and repeat doses in small intestine.
At 6 months, the differences in response rates started to become noticeable, with 67.9% for single dose in large intestine, 71.4% for single dose in small intestine, and 86% for repeat doses in small intestine.
By 12 months, the difference in response rate between the single dose in the large and small intestines was statistically significant at 51.9% and 75.5%, respectively. The response rate to the repeat doses in the small intestine at 12 months (80.9%) was similar to that at 3 months (80.8%).
Side effects, including mild abdominal pain, diarrhea, and constipation, after FMT were seen for the first 5 days after treatment. “People who generally suffer from constipation get diarrhea after FMT and vice versa,” Dr. El-Salhy reported.
“Long-term side effects, as monitored up to 3 years, were not observed,” he added.
Treatment reduced IBS symptoms in all patient groups as measured by IBS-SSS scores. By 12 months, the score fell from around 350 to around 220 in patients who received a single dose in the large intestine, from around 300 to around 200 in patients who received a single dose in the small intestine, and from around 350 to around 170 in patients who received repeat doses in the small intestine.
Quality of life showed a statistically significant difference at 3 months between single and repeated doses in the small intestine and similarly at 6 and 12 months.
Chronic fatigue, experienced by many patients with IBS, was substantially reduced after FMT, Dr. El-Salhy noted. “This surge in energy is often more important to them than the gastrointestinal symptoms.”
Location affects bacterial success
Certain beneficial bacteria were found to thrive more when the donor transplant was administered to the small intestine than to the large intestine.
Of note, Lactobacillus species and Holdemanella biformis grew and then dropped off sharply after 3 months in patients who received a single-dose fecal transplant in the large intestine, while they grew after 3 months and continued to grow after 6 and 12 months in the groups who received a fecal transplant in the small intestine.
“We think bacteria in the small intestine have different characteristics to those in the large intestine,” Dr. El-Salhy said. “This is relatively new, because many years ago it was thought that bile acids prevented bacterial survival. Now we know lots can thrive in the small intestine.”
“It might be viral or some other component that is most effective here. We don’t know yet, but so far we have identified 11 bacteria of interest,” he added.
Broader questions
“Rather than focusing on a specific, single strain microbe as a predictor of success in a disease, the global equilibrium of microbiota is more important, and microbial ecology parameters would be interesting to assess,” remarked Gianluca Ianiro, MD, from the Università Cattolica del Sacro Cuore, Rome, who comoderated the session. “Selected survival of some bacteria through the gut may be the response.”
FMT emerged in response to the challenges posed by recurrent C. difficile infections, noted Alexander Khoruts, MD, a professor of medicine in the division of gastroenterology, hepatology, and nutrition at the University of Minnesota, Minneapolis, who was not involved in the research.
“It is much harder to achieve remodeling of the gut microbiome in non–C. difficile conditions where there is an intact and resilient indigenous microbiota,” he said in an interview. “Therefore, regimens using antibiotic preconditioning and repeated administrations of microbiota are generally more efficacious in achieving this objective.”
The specificity of the bacteria according to disease type targeted was important, said Dr. Khoruts, who has a special interest in gut microbiota.
“The big question in non–C. difficile indications is the composition of donor microbiota. It is critical that we understand the mechanisms involved in each target disease to design appropriate microbiota-based therapeutics,” he said.
Dr. Khoruts sounded a note of caution with respect to establishing the pharmacokinetic and dynamic data related to FMT, which is classified as a drug in the United States.
“It’s imperative that we develop the pharmacology discipline appropriate for this class of therapeutics, including their pharmacokinetics and pharmacodynamics, and an understanding of their potential toxicity and drug-drug interactions,” he said.
Drug distribution data are needed to determine host-microbiota interactions.
“This includes the small bowel microbiome, which continues to be woefully understudied,” Dr. Khoruts said.
Dr. El-Salhy reports no relevant financial relationships. Dr. Ianiro reports receiving personal fees for acting as speaker for Biocodex, Sofar, Malesci, and Tillotts Pharma, and for acting as consultant/advisor for Ferring Therapeutics, Biocodex, Tillotts Pharma, and Zambon. Dr. Khoruts reports he has patents pertaining to fecal microbiota separation from stool and their cryopreservation and lyopreservation.
A version of this article first appeared on Medscape.com.
AT UEG WEEK 2022