User login
‘Staggering’ weight loss and benefits in body composition with tirzepatide
DUBLIN – , according to the latest results of the SURMOUNT-1 study.
The new analysis showed that up to 63% of participants achieved a reduction in body weight of at least 20%, and all three tirzepatide doses (5 mg, 10 mg, and 15 mg) led to substantial, clinically meaningful, and sustained body-weight reduction, compared with placebo at 72 weeks of follow-up.
Mean weight loss was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for placebo (all P < .001 vs. placebo). And among participants taking the highest 15-mg dose of tirzepatide, 96%, 90%, and 78% of patients achieved weight reductions of at least 5%, 10%, and 15%.
Tirzepatide is approved in the United States and the European Union for the treatment of type 2 diabetes but is not yet approved for obesity in any country. The manufacturer of tirzepatide, Eli Lilly, intends to seek approval for the drug as an obesity treatment from the U.S. Food and Drug Administration, European Medicines Agency, and in other territories beginning in 2023.
Regardless of baseline BMI category, 9 out of 10 people achieved the greater than or equal to 5% body weight reduction threshold across all doses of tirzepatide, and at the higher doses, over one-third achieved weight loss of 25% or more.
“Similar to lifestyle and surgical treatments, participants on tirzepatide had around a threefold greater percent reduction in fat mass, compared with lean mass, resulting in an overall improvement in body composition,” reported SURMOUNT-1 co-investigator Louis Aronne, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, New York.
“This is staggering weight loss,” remarked Dr. Aronne. “To put it in perspective, mean weight loss in people having Lap-Band surgery is 17%, mean weight loss for sleeve gastrectomy is 25%, and gastric bypass is 33%, which puts the effects of tirzepatide squarely in the realm of bariatric surgery.”
“Something we have sought for decades, we have finally been able to achieve,” he asserted. “I still remember exactly where I was when I saw these results for the first time last April. I knew something big was happening,” declared Dr. Aronne when presenting the latest analyses at the 2023 European Congress on Obesity. Full study results were published in the New England Journal of Medicine.
Moderator Gabriella Lieberman, MD, endocrinologist and head of the Israeli Center for Weight Management, Sheba Medical Center, Ramat-Gan, Israel, welcomed the study but also expressed caution. “It’s very potent, but as we see generally with potent therapies, I think it will change how we look at nutritional advice and the role of the dietician will change. I’m a bit worried the drug is running fast and the support, which is crucial with these treatments, is not keeping up, and we’ll have to deal with some effects later, such as sarcopenia,” she pointed out in an interview.
“We have to treat these drugs as if they are bariatric surgery. I see patients on these types of drugs in clinic and their appetite is so suppressed that they think they can afford to eat things that are unhealthy because they lose weight, and that’s what they want. There has to be a responsible adult looking at what they’re eating, and not just clapping their hands for the weight loss, but ensuring they are not deprived of anything,” she said.
Weight loss and body composition explored
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that works to activate the GIP and GLP-1 receptors, respectively, found in areas of the brain important for appetite regulation, decreasing food intake, and modulating fat utilization.
The phase 3, double-blind, randomized, controlled trial included data from 2,539 adults with a BMI greater than or equal to 30 kg/m2 (class I, II, III obesity) or greater than or equal to 27 kg/m2 (overweight) with one or more weight-related complications, excluding diabetes. At baseline, mean body weight was 104.8 kg, mean BMI was 38.0 kg/m2, and 94.5% of participants had BMI greater than or equal to 30 kg/m2.
Patients were randomized to once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. The primary objective was to show that tirzepatide was superior to placebo in terms of percentage change in body weight and proportion of participants with body-weight reduction of greater than or equal to 5%. The percentage change from baseline body weight and proportion of participants with body weight reduction greater than or equal to 5% were also assessed across BMI categories of greater than or equal to 27 to less than 30 kg/m2, greater than or equal to 30 to less than 35 kg/m2 (class 1 obesity), greater than or equal to 35 to less than 40 kg/m2 (class 2 obesity), and greater than or equal to 40 kg/m2 (class 3 obesity).
In addition, in a retrospective subanalysis, body composition was evaluated in a subpopulation that underwent dual-energy x-ray absorptiometry, assessing change from baseline body composition within age subgroups less than 50 years (n = 99), 50-64.9 years (n = 41), and greater than or equal to 65 years (n = 20).
The average weight reduction over the 72 weeks of follow-up was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for participants taking placebo (all P < .001 vs. placebo).
The percentages of participants reaching target weight reductions of greater than or equal to 5%, greater than or equal to 10%, greater than or equal to 15%, greater than or equal to 20%, and greater than or equal to 25% were recorded. Over 90% achieved greater than or equal to 5% weight loss, irrespective of BMI and tirzepatide dose, while 55.5% and 62.9% in the 10-mg and 15-mg groups achieved greater than or equal to 20% weight loss, and 35.0% and 39.7% in the 10-mg and 15-mg groups achieved greater than or equal to 25% weight loss, respectively.
By increasing BMI category, in the 10-mg group, weight loss was –18.2 kg, –21.9 kg, –22.0, and –20.7 kg; and in the 15-mg group, weight loss was –18.1kg, –21.2 kg, –24.5 kg, and –22.8 kg. Weight loss in the 5-mg group ranged from –16.6 kg to –15.9 kg from lowest to highest BMI category.
“In the lower-weight categories, there is less weight to lose, so we see a flattening of the curve [with a] maximum of around 18%, so it may be that as we learn more about a drug that is so potent, we recognize that we don’t need to use such a high dose in people with BMI 27-30 kg/m2,” he explained. “It’s the higher BMI categories where we need the higher dose.”
As with lifestyle and surgical treatments, participants taking tirzepatide had around a three times greater percentage reduction in fat mass than lean mass, resulting in an overall improvement in body composition, reported Dr. Aronne.
“We want loss of fat, not lean mass, and we know that we lose around one part lean to three parts fat mass when on a diet and exercise regimen,” he went on to explain. “We see exactly this [balance of lean-to-fat-mass loss] here with 33.9% total fat mass reduction in the treatment group, compared with 8.2% in the placebo group.”
Visceral fat mass reduction was 40% in the treatment group, compared with 7.3% with placebo. “It’s good to see there’s more loss of visceral fat,” said Dr. Aronne. Lean mass loss was 10.9%. “So around three times greater reduction in fat over lean mass loss, resulting in overall improvement of body composition,” he reported.
Also, in older people (≥ 65 years) there was approximately no difference in fat versus lean mass loss, compared with younger people, despite older people being more likely to lose more lean mass.
With respect to patient-reported outcomes based on the 36-item Short-Form Health Survey (SF-36), Dr. Aronne said that physical functioning scores significantly improved at 72 weeks, compared with placebo, particularly in participants with physical function limitations at baseline.
“In an interesting subanalysis, those with physical limitations at baseline showed a significant improvement versus placebo of over 5% difference [considered significant],” he added.
Safety and tolerability were previously reported in the NEJM article. The most common adverse events with tirzepatide were gastrointestinal, and adverse events causing treatment discontinuation occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg doses or placebo, respectively.
“A revolution is coming in the treatment of obesity and cardiometabolic disease, and most physicians cannot grasp this. We’re finally getting the efficacy we’ve been looking for that will produce benefits in every realm,” concluded Dr. Aronne. “These data show that we are now hitting all the secondary endpoints and making our patients better.”
“I think this bodes well. I always envisioned a time when the treatment of obesity would come first before the treatment of cardiometabolic complications of obesity, and I think we’re on the verge of that era with semaglutide, tirzepatide, and the very exciting treatments to come.”
The SURMOUNT-1 trial was sponsored by Lilly. Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly.
A version of this article first appeared on Medscape.com.
DUBLIN – , according to the latest results of the SURMOUNT-1 study.
The new analysis showed that up to 63% of participants achieved a reduction in body weight of at least 20%, and all three tirzepatide doses (5 mg, 10 mg, and 15 mg) led to substantial, clinically meaningful, and sustained body-weight reduction, compared with placebo at 72 weeks of follow-up.
Mean weight loss was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for placebo (all P < .001 vs. placebo). And among participants taking the highest 15-mg dose of tirzepatide, 96%, 90%, and 78% of patients achieved weight reductions of at least 5%, 10%, and 15%.
Tirzepatide is approved in the United States and the European Union for the treatment of type 2 diabetes but is not yet approved for obesity in any country. The manufacturer of tirzepatide, Eli Lilly, intends to seek approval for the drug as an obesity treatment from the U.S. Food and Drug Administration, European Medicines Agency, and in other territories beginning in 2023.
Regardless of baseline BMI category, 9 out of 10 people achieved the greater than or equal to 5% body weight reduction threshold across all doses of tirzepatide, and at the higher doses, over one-third achieved weight loss of 25% or more.
“Similar to lifestyle and surgical treatments, participants on tirzepatide had around a threefold greater percent reduction in fat mass, compared with lean mass, resulting in an overall improvement in body composition,” reported SURMOUNT-1 co-investigator Louis Aronne, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, New York.
“This is staggering weight loss,” remarked Dr. Aronne. “To put it in perspective, mean weight loss in people having Lap-Band surgery is 17%, mean weight loss for sleeve gastrectomy is 25%, and gastric bypass is 33%, which puts the effects of tirzepatide squarely in the realm of bariatric surgery.”
“Something we have sought for decades, we have finally been able to achieve,” he asserted. “I still remember exactly where I was when I saw these results for the first time last April. I knew something big was happening,” declared Dr. Aronne when presenting the latest analyses at the 2023 European Congress on Obesity. Full study results were published in the New England Journal of Medicine.
Moderator Gabriella Lieberman, MD, endocrinologist and head of the Israeli Center for Weight Management, Sheba Medical Center, Ramat-Gan, Israel, welcomed the study but also expressed caution. “It’s very potent, but as we see generally with potent therapies, I think it will change how we look at nutritional advice and the role of the dietician will change. I’m a bit worried the drug is running fast and the support, which is crucial with these treatments, is not keeping up, and we’ll have to deal with some effects later, such as sarcopenia,” she pointed out in an interview.
“We have to treat these drugs as if they are bariatric surgery. I see patients on these types of drugs in clinic and their appetite is so suppressed that they think they can afford to eat things that are unhealthy because they lose weight, and that’s what they want. There has to be a responsible adult looking at what they’re eating, and not just clapping their hands for the weight loss, but ensuring they are not deprived of anything,” she said.
Weight loss and body composition explored
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that works to activate the GIP and GLP-1 receptors, respectively, found in areas of the brain important for appetite regulation, decreasing food intake, and modulating fat utilization.
The phase 3, double-blind, randomized, controlled trial included data from 2,539 adults with a BMI greater than or equal to 30 kg/m2 (class I, II, III obesity) or greater than or equal to 27 kg/m2 (overweight) with one or more weight-related complications, excluding diabetes. At baseline, mean body weight was 104.8 kg, mean BMI was 38.0 kg/m2, and 94.5% of participants had BMI greater than or equal to 30 kg/m2.
Patients were randomized to once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. The primary objective was to show that tirzepatide was superior to placebo in terms of percentage change in body weight and proportion of participants with body-weight reduction of greater than or equal to 5%. The percentage change from baseline body weight and proportion of participants with body weight reduction greater than or equal to 5% were also assessed across BMI categories of greater than or equal to 27 to less than 30 kg/m2, greater than or equal to 30 to less than 35 kg/m2 (class 1 obesity), greater than or equal to 35 to less than 40 kg/m2 (class 2 obesity), and greater than or equal to 40 kg/m2 (class 3 obesity).
In addition, in a retrospective subanalysis, body composition was evaluated in a subpopulation that underwent dual-energy x-ray absorptiometry, assessing change from baseline body composition within age subgroups less than 50 years (n = 99), 50-64.9 years (n = 41), and greater than or equal to 65 years (n = 20).
The average weight reduction over the 72 weeks of follow-up was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for participants taking placebo (all P < .001 vs. placebo).
The percentages of participants reaching target weight reductions of greater than or equal to 5%, greater than or equal to 10%, greater than or equal to 15%, greater than or equal to 20%, and greater than or equal to 25% were recorded. Over 90% achieved greater than or equal to 5% weight loss, irrespective of BMI and tirzepatide dose, while 55.5% and 62.9% in the 10-mg and 15-mg groups achieved greater than or equal to 20% weight loss, and 35.0% and 39.7% in the 10-mg and 15-mg groups achieved greater than or equal to 25% weight loss, respectively.
By increasing BMI category, in the 10-mg group, weight loss was –18.2 kg, –21.9 kg, –22.0, and –20.7 kg; and in the 15-mg group, weight loss was –18.1kg, –21.2 kg, –24.5 kg, and –22.8 kg. Weight loss in the 5-mg group ranged from –16.6 kg to –15.9 kg from lowest to highest BMI category.
“In the lower-weight categories, there is less weight to lose, so we see a flattening of the curve [with a] maximum of around 18%, so it may be that as we learn more about a drug that is so potent, we recognize that we don’t need to use such a high dose in people with BMI 27-30 kg/m2,” he explained. “It’s the higher BMI categories where we need the higher dose.”
As with lifestyle and surgical treatments, participants taking tirzepatide had around a three times greater percentage reduction in fat mass than lean mass, resulting in an overall improvement in body composition, reported Dr. Aronne.
“We want loss of fat, not lean mass, and we know that we lose around one part lean to three parts fat mass when on a diet and exercise regimen,” he went on to explain. “We see exactly this [balance of lean-to-fat-mass loss] here with 33.9% total fat mass reduction in the treatment group, compared with 8.2% in the placebo group.”
Visceral fat mass reduction was 40% in the treatment group, compared with 7.3% with placebo. “It’s good to see there’s more loss of visceral fat,” said Dr. Aronne. Lean mass loss was 10.9%. “So around three times greater reduction in fat over lean mass loss, resulting in overall improvement of body composition,” he reported.
Also, in older people (≥ 65 years) there was approximately no difference in fat versus lean mass loss, compared with younger people, despite older people being more likely to lose more lean mass.
With respect to patient-reported outcomes based on the 36-item Short-Form Health Survey (SF-36), Dr. Aronne said that physical functioning scores significantly improved at 72 weeks, compared with placebo, particularly in participants with physical function limitations at baseline.
“In an interesting subanalysis, those with physical limitations at baseline showed a significant improvement versus placebo of over 5% difference [considered significant],” he added.
Safety and tolerability were previously reported in the NEJM article. The most common adverse events with tirzepatide were gastrointestinal, and adverse events causing treatment discontinuation occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg doses or placebo, respectively.
“A revolution is coming in the treatment of obesity and cardiometabolic disease, and most physicians cannot grasp this. We’re finally getting the efficacy we’ve been looking for that will produce benefits in every realm,” concluded Dr. Aronne. “These data show that we are now hitting all the secondary endpoints and making our patients better.”
“I think this bodes well. I always envisioned a time when the treatment of obesity would come first before the treatment of cardiometabolic complications of obesity, and I think we’re on the verge of that era with semaglutide, tirzepatide, and the very exciting treatments to come.”
The SURMOUNT-1 trial was sponsored by Lilly. Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly.
A version of this article first appeared on Medscape.com.
DUBLIN – , according to the latest results of the SURMOUNT-1 study.
The new analysis showed that up to 63% of participants achieved a reduction in body weight of at least 20%, and all three tirzepatide doses (5 mg, 10 mg, and 15 mg) led to substantial, clinically meaningful, and sustained body-weight reduction, compared with placebo at 72 weeks of follow-up.
Mean weight loss was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for placebo (all P < .001 vs. placebo). And among participants taking the highest 15-mg dose of tirzepatide, 96%, 90%, and 78% of patients achieved weight reductions of at least 5%, 10%, and 15%.
Tirzepatide is approved in the United States and the European Union for the treatment of type 2 diabetes but is not yet approved for obesity in any country. The manufacturer of tirzepatide, Eli Lilly, intends to seek approval for the drug as an obesity treatment from the U.S. Food and Drug Administration, European Medicines Agency, and in other territories beginning in 2023.
Regardless of baseline BMI category, 9 out of 10 people achieved the greater than or equal to 5% body weight reduction threshold across all doses of tirzepatide, and at the higher doses, over one-third achieved weight loss of 25% or more.
“Similar to lifestyle and surgical treatments, participants on tirzepatide had around a threefold greater percent reduction in fat mass, compared with lean mass, resulting in an overall improvement in body composition,” reported SURMOUNT-1 co-investigator Louis Aronne, MD, Comprehensive Weight Control Center, Weill Cornell Medicine, New York.
“This is staggering weight loss,” remarked Dr. Aronne. “To put it in perspective, mean weight loss in people having Lap-Band surgery is 17%, mean weight loss for sleeve gastrectomy is 25%, and gastric bypass is 33%, which puts the effects of tirzepatide squarely in the realm of bariatric surgery.”
“Something we have sought for decades, we have finally been able to achieve,” he asserted. “I still remember exactly where I was when I saw these results for the first time last April. I knew something big was happening,” declared Dr. Aronne when presenting the latest analyses at the 2023 European Congress on Obesity. Full study results were published in the New England Journal of Medicine.
Moderator Gabriella Lieberman, MD, endocrinologist and head of the Israeli Center for Weight Management, Sheba Medical Center, Ramat-Gan, Israel, welcomed the study but also expressed caution. “It’s very potent, but as we see generally with potent therapies, I think it will change how we look at nutritional advice and the role of the dietician will change. I’m a bit worried the drug is running fast and the support, which is crucial with these treatments, is not keeping up, and we’ll have to deal with some effects later, such as sarcopenia,” she pointed out in an interview.
“We have to treat these drugs as if they are bariatric surgery. I see patients on these types of drugs in clinic and their appetite is so suppressed that they think they can afford to eat things that are unhealthy because they lose weight, and that’s what they want. There has to be a responsible adult looking at what they’re eating, and not just clapping their hands for the weight loss, but ensuring they are not deprived of anything,” she said.
Weight loss and body composition explored
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that works to activate the GIP and GLP-1 receptors, respectively, found in areas of the brain important for appetite regulation, decreasing food intake, and modulating fat utilization.
The phase 3, double-blind, randomized, controlled trial included data from 2,539 adults with a BMI greater than or equal to 30 kg/m2 (class I, II, III obesity) or greater than or equal to 27 kg/m2 (overweight) with one or more weight-related complications, excluding diabetes. At baseline, mean body weight was 104.8 kg, mean BMI was 38.0 kg/m2, and 94.5% of participants had BMI greater than or equal to 30 kg/m2.
Patients were randomized to once-weekly subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks. The primary objective was to show that tirzepatide was superior to placebo in terms of percentage change in body weight and proportion of participants with body-weight reduction of greater than or equal to 5%. The percentage change from baseline body weight and proportion of participants with body weight reduction greater than or equal to 5% were also assessed across BMI categories of greater than or equal to 27 to less than 30 kg/m2, greater than or equal to 30 to less than 35 kg/m2 (class 1 obesity), greater than or equal to 35 to less than 40 kg/m2 (class 2 obesity), and greater than or equal to 40 kg/m2 (class 3 obesity).
In addition, in a retrospective subanalysis, body composition was evaluated in a subpopulation that underwent dual-energy x-ray absorptiometry, assessing change from baseline body composition within age subgroups less than 50 years (n = 99), 50-64.9 years (n = 41), and greater than or equal to 65 years (n = 20).
The average weight reduction over the 72 weeks of follow-up was –16.0%, –21.4%, and –22.5% with tirzepatide 5 mg, 10 mg, and 15 mg, compared with –2.4% for participants taking placebo (all P < .001 vs. placebo).
The percentages of participants reaching target weight reductions of greater than or equal to 5%, greater than or equal to 10%, greater than or equal to 15%, greater than or equal to 20%, and greater than or equal to 25% were recorded. Over 90% achieved greater than or equal to 5% weight loss, irrespective of BMI and tirzepatide dose, while 55.5% and 62.9% in the 10-mg and 15-mg groups achieved greater than or equal to 20% weight loss, and 35.0% and 39.7% in the 10-mg and 15-mg groups achieved greater than or equal to 25% weight loss, respectively.
By increasing BMI category, in the 10-mg group, weight loss was –18.2 kg, –21.9 kg, –22.0, and –20.7 kg; and in the 15-mg group, weight loss was –18.1kg, –21.2 kg, –24.5 kg, and –22.8 kg. Weight loss in the 5-mg group ranged from –16.6 kg to –15.9 kg from lowest to highest BMI category.
“In the lower-weight categories, there is less weight to lose, so we see a flattening of the curve [with a] maximum of around 18%, so it may be that as we learn more about a drug that is so potent, we recognize that we don’t need to use such a high dose in people with BMI 27-30 kg/m2,” he explained. “It’s the higher BMI categories where we need the higher dose.”
As with lifestyle and surgical treatments, participants taking tirzepatide had around a three times greater percentage reduction in fat mass than lean mass, resulting in an overall improvement in body composition, reported Dr. Aronne.
“We want loss of fat, not lean mass, and we know that we lose around one part lean to three parts fat mass when on a diet and exercise regimen,” he went on to explain. “We see exactly this [balance of lean-to-fat-mass loss] here with 33.9% total fat mass reduction in the treatment group, compared with 8.2% in the placebo group.”
Visceral fat mass reduction was 40% in the treatment group, compared with 7.3% with placebo. “It’s good to see there’s more loss of visceral fat,” said Dr. Aronne. Lean mass loss was 10.9%. “So around three times greater reduction in fat over lean mass loss, resulting in overall improvement of body composition,” he reported.
Also, in older people (≥ 65 years) there was approximately no difference in fat versus lean mass loss, compared with younger people, despite older people being more likely to lose more lean mass.
With respect to patient-reported outcomes based on the 36-item Short-Form Health Survey (SF-36), Dr. Aronne said that physical functioning scores significantly improved at 72 weeks, compared with placebo, particularly in participants with physical function limitations at baseline.
“In an interesting subanalysis, those with physical limitations at baseline showed a significant improvement versus placebo of over 5% difference [considered significant],” he added.
Safety and tolerability were previously reported in the NEJM article. The most common adverse events with tirzepatide were gastrointestinal, and adverse events causing treatment discontinuation occurred in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg doses or placebo, respectively.
“A revolution is coming in the treatment of obesity and cardiometabolic disease, and most physicians cannot grasp this. We’re finally getting the efficacy we’ve been looking for that will produce benefits in every realm,” concluded Dr. Aronne. “These data show that we are now hitting all the secondary endpoints and making our patients better.”
“I think this bodes well. I always envisioned a time when the treatment of obesity would come first before the treatment of cardiometabolic complications of obesity, and I think we’re on the verge of that era with semaglutide, tirzepatide, and the very exciting treatments to come.”
The SURMOUNT-1 trial was sponsored by Lilly. Dr. Aronne is cofounder, chief scientific advisor, and a member of the board of directors for Intellihealth. He is also a paid scientific advisory board member for Eli Lilly.
A version of this article first appeared on Medscape.com.
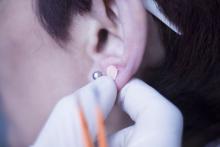
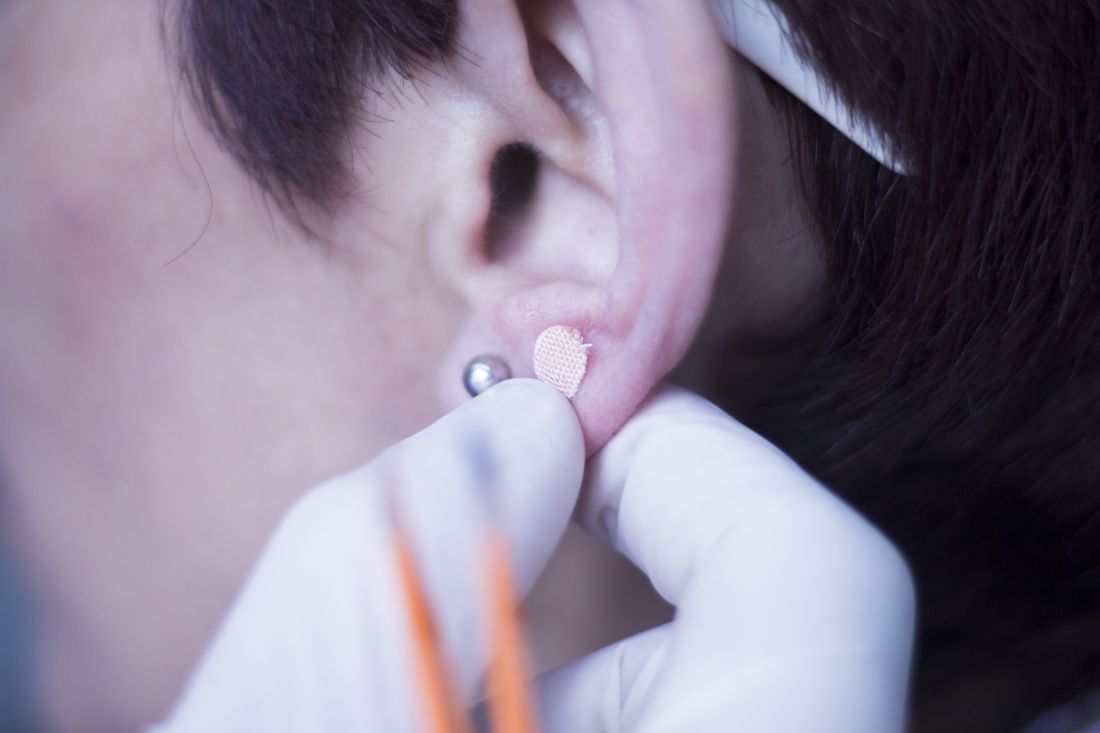
Ear acupuncture with diet aids weight loss
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
DUBLIN – with high levels of visceral fat and overweight/obesity.
Three months of auricular acupuncture stimulation and dietary restriction led to a mean weight loss of nearly 9 kg plus a drop in waist circumference of more than 10 cm.
According to the researchers, acupuncture beads, used in Japan to augment weight loss for more than 30 years, are thought to stimulate nerves and organs that regulate appetite, satiety, hunger, and food cravings.
Findings of the observational study were presented by Takahiro Fujimoto, MD, PhD, Clinic F, Tokyo, at this year’s European Congress on Obesity.
Together with a prior study using the same intervention in women, Dr. Fujimoto and colleagues have now gathered data in more than 1,000 individuals, he said. “We wanted a method that was simple and noninvasive that would serve as a support to exercise and dietary therapy,” Dr. Fujimoto said in an interview.
“We believe there is an effect,” he asserted. “Acupuncture’s effect lies in stimulating the satiety center with benefits in helping individuals to control their food cravings and intake when reducing meals,” he said, pointing out that similar techniques have been used in patients undergoing withdrawal from drug addiction and in smoking cessation. He explained that acupuncture beads are believed to help individuals change their lifestyle habits, and added that “the relapse rate after 6 months is addressed in another paper, and it is very low.”
Professor Jason C.G. Halford, PhD, head of school at the University of Leeds, England, and president of the European Association for the Study of Obesity, commented on the findings. “There is no control group here receiving everything but the acupuncture,” he noted. “As such, it could be other elements of the intervention driving this [effect] including the act of keeping a food diary increasing awareness of one’s diet. A randomized controlled trial would be the next step.”
In women, the technique led to significantly more weight loss than in those who were untreated, and weight loss was maintained for 6 months after the end of treatment.
The researchers added that acupuncture stimulation with beads was a simpler method than traditional use of intradermal needles requiring expert acupuncturists. The stimulation is applied with 1.5-mm metal ear beads on 6 points of the outer ear (shen men, food pipe, upper stomach opening, stomach, lungs, and endocrine system) that correspond to meridian lines, and as such, restores the flow of qi by resolving any blockages or disruption. This may help with a variety of health conditions, according to the researchers. Placed on both ears, surgical tape was used to keep the beads in place to ensure participants continuously received uniform pressure on each of the six acupuncture points.
Dietary guidance was provided to participants to help reduce food intake by half, and nutritional supplements were given to compensate for any deficiencies. Participants attended twice-weekly clinic visits for bead sticking and diet progress monitoring. Body weight, body fat percentage, fat mass, lean mass, muscle mass, body mass index (BMI), and abdominal fat were assessed at the start and end of the study period.
“Since these tiny metal beads are attached to six points on the outer ear that stimulate nerves and organs which regulate appetite, satiety, and hunger, this type of acupuncture does not require complex knowledge or skill,” explained Dr. Fujimoto.
The results of the latest study, in men only, build on a prior study of more than 1,300 women who also received auricular acupuncture stimulation with beads as well as a halving of their food intake. In women, the weight loss program led to total body weight loss of 11.2% over 3 months.
At baseline, the 81 male participants, ages 21-78 years, had a mean BMI of 28.4 kg/m2 and mean waist circumference of 98.4 cm. Body fat percentage was 28.2%.
After 3 months, participants lost a mean of 8.6 kg (P < .001), decreased waist circumference by a mean of 10.4 cm (P < .001), and lost a mean of 4.0% of total body fat (P < .001). Visceral fat levels also fell by 2.2 points (P < .001), from 15.2 points at baseline to 13.0 points after 3 months. (A healthy visceral fat rating is between 1 and 12 points.) BMI decreased by almost 3 kg/m2 (from 28.4 at baseline to 25.5 at 3 months; P < .001).
Improvement in muscle-to-fat ratio was greater in men than women, whereas women had a greater decrease in percentage body fat than men.
“Whilst receiving ear acupuncture, the investigators asked participants to cut their food intake by half. It’s not unreasonable to expect that this major dietary change was the main reason participants lost weight,” remarked Graham Wheeler, PhD, statistical ambassador at the Royal Statistical Society, United Kingdom.
He also commented on the lack of a control group: “This study does not show us the impact of ear acupuncture on weight loss.”
Dr. Fujimoto and Dr. Halford have reported no relevant financial relationships. Dr. Wheeler is a statistical ambassador for the Royal Statistical Society, is employed by GSK, and holds an honorary senior lecturer post at Imperial College London.
A version of this article first appeared on Medscape.com.
AT ECO 2023
Half of teens drop below obesity cutoff with semaglutide
DUBLIN – according to a secondary analysis of the STEP TEENS (Semaglutide Treatment Effect in People With Obesity) trial.
By comparison, only 12.1% of adolescents with obesity taking placebo in the trial dropped below the obesity threshold.
The study also found that 74% of participants shifted down by at least one body mass index (BMI) category after receiving the GLP-1 agonist, compared with 19% of those taking placebo.
“In a practical sense, we see that semaglutide reduced weight to a level below what is defined as clinical obesity in nearly 50% of the teens in our trial, which is historically unprecedented with treatments other than bariatric surgery,” remarked Aaron S. Kelly, MD, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota, Minneapolis, who presented the latest data at this year’s European Congress on Obesity.
“There was a 22.7-higher odds of dropping below the obesity threshold if assigned to semaglutide versus odds on placebo (P < .0001), and a 23.5-fold higher odds of dropping BMI by one category if on semaglutide (P < .0001),” he reported.
This analysis follows the 2022 publication of the main results of STEP TEENS published in the New England Journal of Medicine, which showed semaglutide helped adolescents lose weight. The drug was subsequently approved by the U.S. Food and Drug Administration for the treatment of obesity in those aged 12 and over in January of this year.
The new analysis was presented at ECO and simultaneously published in Obesity.
Grace O’Malley, PhD, Child & Adolescent Obesity Service, Children’s Health Ireland, Dublin, commented on the findings, noting that adolescents’ access to comprehensive health care is essential for the proper treatment of obesity.
“Treatment requires a long-term, multidisciplinary chronic-care approach, and usually, when treatment stops, the biological mechanisms driving the obesity begin again to drive the build-up of adipose tissue,” she said. This means that “long-term treatment including nutrition therapy, exercise ... behavioral support, and sleep therapy needs to be available to families in combination with pharmacotherapy and surgical intervention where required.”
“The results of the STEP TEENS study represent a promising development for the treatment of adolescent obesity and for associated complications related to liver function,” she added. “The observed improvements in obesity category and [liver enzyme] alanine transaminase will help clinicians plan more tailored care for adolescents with obesity,” she noted.
Semaglutide shifts BMI category
In this new secondary analysis of STEP TEENS, the authors examined the effect of subcutaneous semaglutide 2.4 mg on moving adolescents from one BMI category to another, including dropping below the obesity threshold into the overweight or normal weight categories.
The study also looked at the effect of semaglutide on glucose metabolism and cardiovascular risk factors, as well as safety and tolerability. However, this particular analysis only examined adolescents with obesity (only one person had overweight, and so they were excluded), who were divided into three further subclasses: obesity class I (BMI ≥ 95th to < 20% above the 95th percentile); obesity class II (BMI ≥ 20% to < 40% above 95th percentile); and obesity class III (BMI ≥ 40% above the 95th percentile).
After a 12-week run-in period of lifestyle intervention only, a total of 200 adolescents (12-18 years) with obesity (in the top 5% of BMI) were randomized (2:1) to once-weekly subcutaneous semaglutide 2.4 mg or placebo for 68 weeks, after a 16-week titration period. All participants continued to receive counseling about healthy nutrition and were set a goal of 60 minutes per day of moderate- to high-intensity physical activity.
Dr. Kelly and colleagues determined levels of improvement in BMI category and attainment of normal weight, or overweight, BMI category by week 68.
At baseline, the percentage of participants in obesity class I, II, or III, in those taking placebo was 39.7%, 41.4%, and 19.0%, or taking semaglutide was 31.4%, 31.4%, and 37.3%, respectively.
“After 68 weeks, not a lot happened [in placebo participants]; however, 12.1% of placebo participants did drop below the obesity threshold into overweight or normal-weight categories,” reported Dr. Kelly.
Referring to participants taking semaglutide, he added that “a total of 45% of patients on semaglutide dropped below the clinical BMI cut point for obesity, such that 19.5% dropped into the overweight category and 25.4% reduced their BMI into the normal-weight category.”
Turning to obesity class, Dr. Kelly reported that of those initially with obesity class III taking placebo, 91% remained in that class and 9.1% dropped to obesity class II at week 68. For those adolescents with obesity class III taking semaglutide, 36.4% dropped to obesity class II, 18.2% dropped to obesity class I, 11% dropped below the obesity threshold, and 34.1% remained in obesity class III, he added.
For obesity class II specifically, 71% of placebo participants stayed in that category, while 12% moved up a category. “On semaglutide, over 50% (51.2%) reduced their BMI below the obesity cut point,” noted Dr. Kelly.
In obesity class I, 26% of patients taking placebo reduced their BMI below the obesity cut point. “On semaglutide, nearly 80% reduced their BMI below the obesity threshold, with 57% dropping their BMI into the normal category,” he said.
“When we looked at baseline factors that might predict the response to semaglutide or placebo, we did not find any factors that were ... significant due to small sample sizes,” he said. However, he pointed out that “females tended to respond better to semaglutide, likewise younger adolescents, and middle body weights tended to respond better to the drug, and there was a similar pattern with obesity classes.”
Commenting on the study, Jesse Bittman, MD, University of British Columbia, Vancouver, said: “Good to see more data on different populations that some semaglutide is used in and the variability in response to it. The focus on BMI was interesting because in obesity medicine we spend a lot of time telling our patients not to focus on BMIs and ‘normals’ because there are more important tools, and we see that when these become the focus of research outcomes they can become problematic.”
Asked whether rapid weight loss in adolescents might be problematic in some respects, Dr. Bittman pointed out that “one concern with these medications is whether people are going to have loss of muscle mass or malnutrition, or whether they develop eating disorders and other disturbed eating behaviors.”
Dr. Kelly has reported engaging in unpaid consulting and educational activities for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus, and receiving donated drug/placebo from Novo Nordisk and Vivus for National Institutes of Health–funded clinical trials. Dr. O’Malley has declared having received grants in the past 3 years from the Health Research Board, Department of Health, Ireland, European Association for the Study of Obesity (via a Novo Nordisk educational grant), Healthy Ireland fund, and the Royal College of Surgeons in Ireland Strategic Academic Recruitment (StAR) Programme. Dr. Bittman has reported receiving funding from Novo Nordisk, Bayer, and Bausch Health.
A version of this article first appeared on Medscape.com.
DUBLIN – according to a secondary analysis of the STEP TEENS (Semaglutide Treatment Effect in People With Obesity) trial.
By comparison, only 12.1% of adolescents with obesity taking placebo in the trial dropped below the obesity threshold.
The study also found that 74% of participants shifted down by at least one body mass index (BMI) category after receiving the GLP-1 agonist, compared with 19% of those taking placebo.
“In a practical sense, we see that semaglutide reduced weight to a level below what is defined as clinical obesity in nearly 50% of the teens in our trial, which is historically unprecedented with treatments other than bariatric surgery,” remarked Aaron S. Kelly, MD, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota, Minneapolis, who presented the latest data at this year’s European Congress on Obesity.
“There was a 22.7-higher odds of dropping below the obesity threshold if assigned to semaglutide versus odds on placebo (P < .0001), and a 23.5-fold higher odds of dropping BMI by one category if on semaglutide (P < .0001),” he reported.
This analysis follows the 2022 publication of the main results of STEP TEENS published in the New England Journal of Medicine, which showed semaglutide helped adolescents lose weight. The drug was subsequently approved by the U.S. Food and Drug Administration for the treatment of obesity in those aged 12 and over in January of this year.
The new analysis was presented at ECO and simultaneously published in Obesity.
Grace O’Malley, PhD, Child & Adolescent Obesity Service, Children’s Health Ireland, Dublin, commented on the findings, noting that adolescents’ access to comprehensive health care is essential for the proper treatment of obesity.
“Treatment requires a long-term, multidisciplinary chronic-care approach, and usually, when treatment stops, the biological mechanisms driving the obesity begin again to drive the build-up of adipose tissue,” she said. This means that “long-term treatment including nutrition therapy, exercise ... behavioral support, and sleep therapy needs to be available to families in combination with pharmacotherapy and surgical intervention where required.”
“The results of the STEP TEENS study represent a promising development for the treatment of adolescent obesity and for associated complications related to liver function,” she added. “The observed improvements in obesity category and [liver enzyme] alanine transaminase will help clinicians plan more tailored care for adolescents with obesity,” she noted.
Semaglutide shifts BMI category
In this new secondary analysis of STEP TEENS, the authors examined the effect of subcutaneous semaglutide 2.4 mg on moving adolescents from one BMI category to another, including dropping below the obesity threshold into the overweight or normal weight categories.
The study also looked at the effect of semaglutide on glucose metabolism and cardiovascular risk factors, as well as safety and tolerability. However, this particular analysis only examined adolescents with obesity (only one person had overweight, and so they were excluded), who were divided into three further subclasses: obesity class I (BMI ≥ 95th to < 20% above the 95th percentile); obesity class II (BMI ≥ 20% to < 40% above 95th percentile); and obesity class III (BMI ≥ 40% above the 95th percentile).
After a 12-week run-in period of lifestyle intervention only, a total of 200 adolescents (12-18 years) with obesity (in the top 5% of BMI) were randomized (2:1) to once-weekly subcutaneous semaglutide 2.4 mg or placebo for 68 weeks, after a 16-week titration period. All participants continued to receive counseling about healthy nutrition and were set a goal of 60 minutes per day of moderate- to high-intensity physical activity.
Dr. Kelly and colleagues determined levels of improvement in BMI category and attainment of normal weight, or overweight, BMI category by week 68.
At baseline, the percentage of participants in obesity class I, II, or III, in those taking placebo was 39.7%, 41.4%, and 19.0%, or taking semaglutide was 31.4%, 31.4%, and 37.3%, respectively.
“After 68 weeks, not a lot happened [in placebo participants]; however, 12.1% of placebo participants did drop below the obesity threshold into overweight or normal-weight categories,” reported Dr. Kelly.
Referring to participants taking semaglutide, he added that “a total of 45% of patients on semaglutide dropped below the clinical BMI cut point for obesity, such that 19.5% dropped into the overweight category and 25.4% reduced their BMI into the normal-weight category.”
Turning to obesity class, Dr. Kelly reported that of those initially with obesity class III taking placebo, 91% remained in that class and 9.1% dropped to obesity class II at week 68. For those adolescents with obesity class III taking semaglutide, 36.4% dropped to obesity class II, 18.2% dropped to obesity class I, 11% dropped below the obesity threshold, and 34.1% remained in obesity class III, he added.
For obesity class II specifically, 71% of placebo participants stayed in that category, while 12% moved up a category. “On semaglutide, over 50% (51.2%) reduced their BMI below the obesity cut point,” noted Dr. Kelly.
In obesity class I, 26% of patients taking placebo reduced their BMI below the obesity cut point. “On semaglutide, nearly 80% reduced their BMI below the obesity threshold, with 57% dropping their BMI into the normal category,” he said.
“When we looked at baseline factors that might predict the response to semaglutide or placebo, we did not find any factors that were ... significant due to small sample sizes,” he said. However, he pointed out that “females tended to respond better to semaglutide, likewise younger adolescents, and middle body weights tended to respond better to the drug, and there was a similar pattern with obesity classes.”
Commenting on the study, Jesse Bittman, MD, University of British Columbia, Vancouver, said: “Good to see more data on different populations that some semaglutide is used in and the variability in response to it. The focus on BMI was interesting because in obesity medicine we spend a lot of time telling our patients not to focus on BMIs and ‘normals’ because there are more important tools, and we see that when these become the focus of research outcomes they can become problematic.”
Asked whether rapid weight loss in adolescents might be problematic in some respects, Dr. Bittman pointed out that “one concern with these medications is whether people are going to have loss of muscle mass or malnutrition, or whether they develop eating disorders and other disturbed eating behaviors.”
Dr. Kelly has reported engaging in unpaid consulting and educational activities for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus, and receiving donated drug/placebo from Novo Nordisk and Vivus for National Institutes of Health–funded clinical trials. Dr. O’Malley has declared having received grants in the past 3 years from the Health Research Board, Department of Health, Ireland, European Association for the Study of Obesity (via a Novo Nordisk educational grant), Healthy Ireland fund, and the Royal College of Surgeons in Ireland Strategic Academic Recruitment (StAR) Programme. Dr. Bittman has reported receiving funding from Novo Nordisk, Bayer, and Bausch Health.
A version of this article first appeared on Medscape.com.
DUBLIN – according to a secondary analysis of the STEP TEENS (Semaglutide Treatment Effect in People With Obesity) trial.
By comparison, only 12.1% of adolescents with obesity taking placebo in the trial dropped below the obesity threshold.
The study also found that 74% of participants shifted down by at least one body mass index (BMI) category after receiving the GLP-1 agonist, compared with 19% of those taking placebo.
“In a practical sense, we see that semaglutide reduced weight to a level below what is defined as clinical obesity in nearly 50% of the teens in our trial, which is historically unprecedented with treatments other than bariatric surgery,” remarked Aaron S. Kelly, MD, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota, Minneapolis, who presented the latest data at this year’s European Congress on Obesity.
“There was a 22.7-higher odds of dropping below the obesity threshold if assigned to semaglutide versus odds on placebo (P < .0001), and a 23.5-fold higher odds of dropping BMI by one category if on semaglutide (P < .0001),” he reported.
This analysis follows the 2022 publication of the main results of STEP TEENS published in the New England Journal of Medicine, which showed semaglutide helped adolescents lose weight. The drug was subsequently approved by the U.S. Food and Drug Administration for the treatment of obesity in those aged 12 and over in January of this year.
The new analysis was presented at ECO and simultaneously published in Obesity.
Grace O’Malley, PhD, Child & Adolescent Obesity Service, Children’s Health Ireland, Dublin, commented on the findings, noting that adolescents’ access to comprehensive health care is essential for the proper treatment of obesity.
“Treatment requires a long-term, multidisciplinary chronic-care approach, and usually, when treatment stops, the biological mechanisms driving the obesity begin again to drive the build-up of adipose tissue,” she said. This means that “long-term treatment including nutrition therapy, exercise ... behavioral support, and sleep therapy needs to be available to families in combination with pharmacotherapy and surgical intervention where required.”
“The results of the STEP TEENS study represent a promising development for the treatment of adolescent obesity and for associated complications related to liver function,” she added. “The observed improvements in obesity category and [liver enzyme] alanine transaminase will help clinicians plan more tailored care for adolescents with obesity,” she noted.
Semaglutide shifts BMI category
In this new secondary analysis of STEP TEENS, the authors examined the effect of subcutaneous semaglutide 2.4 mg on moving adolescents from one BMI category to another, including dropping below the obesity threshold into the overweight or normal weight categories.
The study also looked at the effect of semaglutide on glucose metabolism and cardiovascular risk factors, as well as safety and tolerability. However, this particular analysis only examined adolescents with obesity (only one person had overweight, and so they were excluded), who were divided into three further subclasses: obesity class I (BMI ≥ 95th to < 20% above the 95th percentile); obesity class II (BMI ≥ 20% to < 40% above 95th percentile); and obesity class III (BMI ≥ 40% above the 95th percentile).
After a 12-week run-in period of lifestyle intervention only, a total of 200 adolescents (12-18 years) with obesity (in the top 5% of BMI) were randomized (2:1) to once-weekly subcutaneous semaglutide 2.4 mg or placebo for 68 weeks, after a 16-week titration period. All participants continued to receive counseling about healthy nutrition and were set a goal of 60 minutes per day of moderate- to high-intensity physical activity.
Dr. Kelly and colleagues determined levels of improvement in BMI category and attainment of normal weight, or overweight, BMI category by week 68.
At baseline, the percentage of participants in obesity class I, II, or III, in those taking placebo was 39.7%, 41.4%, and 19.0%, or taking semaglutide was 31.4%, 31.4%, and 37.3%, respectively.
“After 68 weeks, not a lot happened [in placebo participants]; however, 12.1% of placebo participants did drop below the obesity threshold into overweight or normal-weight categories,” reported Dr. Kelly.
Referring to participants taking semaglutide, he added that “a total of 45% of patients on semaglutide dropped below the clinical BMI cut point for obesity, such that 19.5% dropped into the overweight category and 25.4% reduced their BMI into the normal-weight category.”
Turning to obesity class, Dr. Kelly reported that of those initially with obesity class III taking placebo, 91% remained in that class and 9.1% dropped to obesity class II at week 68. For those adolescents with obesity class III taking semaglutide, 36.4% dropped to obesity class II, 18.2% dropped to obesity class I, 11% dropped below the obesity threshold, and 34.1% remained in obesity class III, he added.
For obesity class II specifically, 71% of placebo participants stayed in that category, while 12% moved up a category. “On semaglutide, over 50% (51.2%) reduced their BMI below the obesity cut point,” noted Dr. Kelly.
In obesity class I, 26% of patients taking placebo reduced their BMI below the obesity cut point. “On semaglutide, nearly 80% reduced their BMI below the obesity threshold, with 57% dropping their BMI into the normal category,” he said.
“When we looked at baseline factors that might predict the response to semaglutide or placebo, we did not find any factors that were ... significant due to small sample sizes,” he said. However, he pointed out that “females tended to respond better to semaglutide, likewise younger adolescents, and middle body weights tended to respond better to the drug, and there was a similar pattern with obesity classes.”
Commenting on the study, Jesse Bittman, MD, University of British Columbia, Vancouver, said: “Good to see more data on different populations that some semaglutide is used in and the variability in response to it. The focus on BMI was interesting because in obesity medicine we spend a lot of time telling our patients not to focus on BMIs and ‘normals’ because there are more important tools, and we see that when these become the focus of research outcomes they can become problematic.”
Asked whether rapid weight loss in adolescents might be problematic in some respects, Dr. Bittman pointed out that “one concern with these medications is whether people are going to have loss of muscle mass or malnutrition, or whether they develop eating disorders and other disturbed eating behaviors.”
Dr. Kelly has reported engaging in unpaid consulting and educational activities for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Vivus, and receiving donated drug/placebo from Novo Nordisk and Vivus for National Institutes of Health–funded clinical trials. Dr. O’Malley has declared having received grants in the past 3 years from the Health Research Board, Department of Health, Ireland, European Association for the Study of Obesity (via a Novo Nordisk educational grant), Healthy Ireland fund, and the Royal College of Surgeons in Ireland Strategic Academic Recruitment (StAR) Programme. Dr. Bittman has reported receiving funding from Novo Nordisk, Bayer, and Bausch Health.
A version of this article first appeared on Medscape.com.
FROM ECO 2023
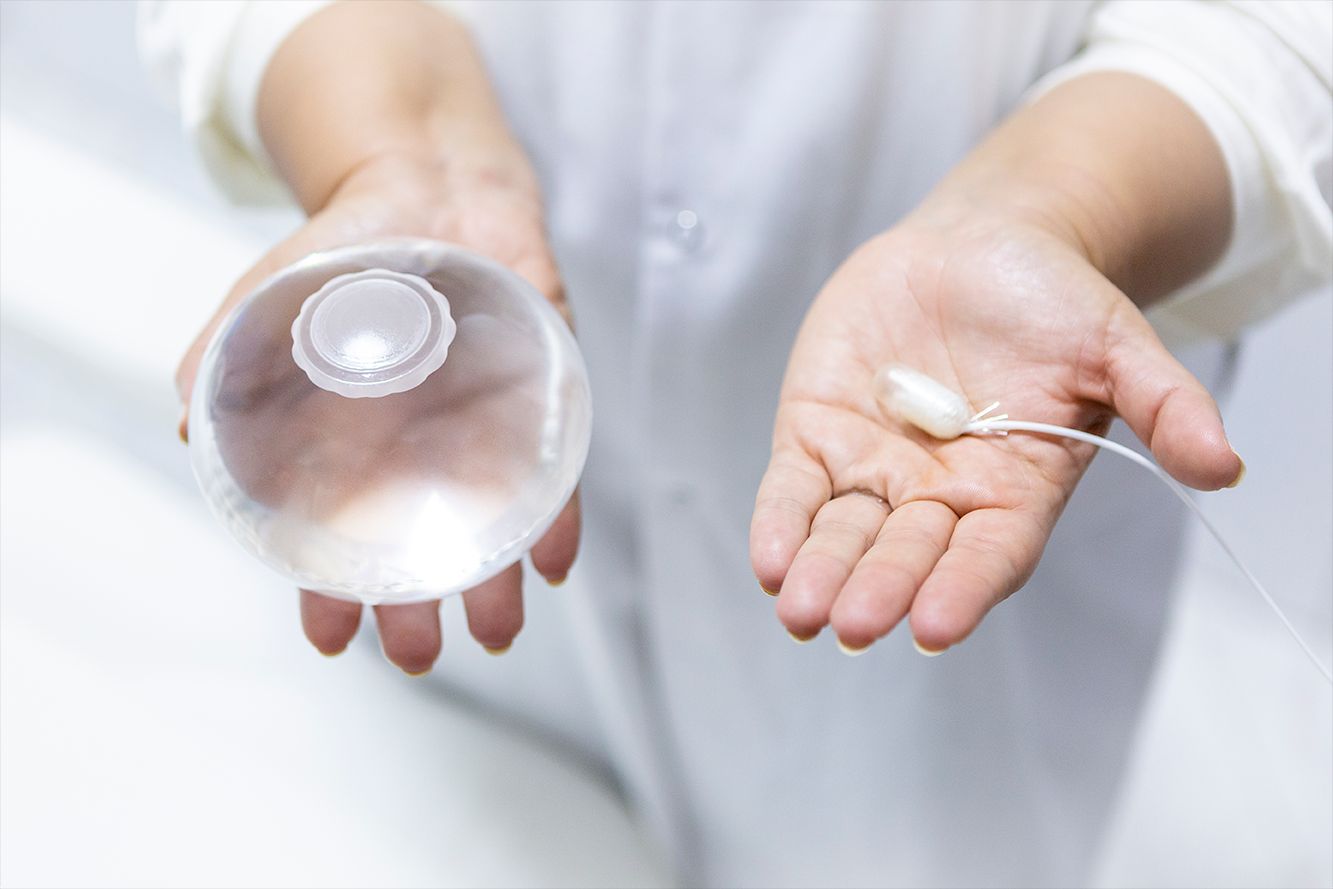
Obesity drug with swallowable balloon boosts weight loss
DUBLIN – A swallowable gastric balloon (Allurion Balloon, formerly known as Elipse) combined with daily subcutaneous injections of the glucagonlike peptide 1 (GLP-1) agonist liraglutide (Saxenda, Novo Nordisk), leads to a significant average total body weight loss of 19% (18 kg or 40 lb) after around 4 months in people with obesity.
said Roberta Ienca, MD, from the Clinica Nuova Villa Claudia, Rome, who presented the findings at this year’s European Congress on Obesity.
“Despite both the balloon and liraglutide working on the early satiety feeling, the introduction of liraglutide around 1 month after [swallowing the balloon] or more frequently after 3-4 months, could sustain these feelings for a longer period of time,” she said in an interview.
“The addition of the GLP-1 agonist therapy (liraglutide) to patients treated with the Allurion program [gastric balloon] is feasible, safe, and effective in those who need additional weight loss,” she emphasized.
The balloon stayed inside participants’ stomachs for an average of 16 weeks and liraglutide was continued for an average of 4 months, resulting in a mean reduction in body mass index (BMI) of 6.4 kg/m2.
The Allurion is the world’s first and only swallowable gastric balloon placed without surgery, endoscopy, or anesthesia, and is excreted naturally after around 16 weeks.
The Allurion program delivered “excellent weight loss in individuals with overweight and obesity without going under the knife, and liraglutide has the potential to further safely enhance weight loss in cases of suboptimal adherence with the program,” Dr. Ienca said. “These two treatment approaches appear to have complementary mechanisms of action in a geographically and demographically diverse population.”
Adelardo Caballero, MD, director of the Institute of Obesity, Madrid, said that he had over 6 years of experience with the Allurion balloon in around 2,500 cases. “Over the last 3 years, we have been using Allurion balloons in combination with GLP-1 agonists. In Europe, use of the swallowable gastric balloon is common, the results are good, and it is a safe tool.”
“Using liraglutide daily in subcutaneous form is authorized in Europe and is useful in overweight and mild obesity, while use in the combination [with the balloon] is also very popular,” he explained. “In the future, the combined use of semaglutide once-weekly GLP-1 agonist or the use of dual GLP-1/gastric inhibitory polypeptide agonists [such as tirzepatide] with the swallowable intragastric balloon Allurion program or endoscopic sleeve gastroplasty will improve results,” he added.
Average 40-lb weight loss with balloon and liraglutide
For the current study, data from three international multidisciplinary obesity centers (in Italy, Spain, and Egypt) were retrospectively analyzed. All 181 patients received the combination of the Allurion balloon and liraglutide, with the latter added 4-16 weeks after swallowing the balloon.
During a 20-minute outpatient visit, participants swallowed the balloon, which was filled with liquid after reaching the stomach, and placement was confirmed by x-ray. The balloon remained inserted for around 15-17 weeks (mean 16 weeks) before natural excretion. All patients received liraglutide once daily for 1-6 months (mean 4 months). After excreting the balloon, patients started the Mediterranean diet for weight maintenance and were followed for at least 6 months.
Patients were monitored for weight loss, percentage total body weight loss, percentage excess weight loss, and BMI reduction. The timing of combining drug therapy with the Allurion program, metabolic results, and adverse event data were collected. However, Dr. Ienca explained that “the study was preliminary and aimed to evaluate feasibility and results of a combined treatment, so we didn’t collect long-term data.”
Liraglutide was mostly added in cases of unsatisfactory weight loss to boost weight reduction in patients with high BMIs, to sustain weight maintenance, and to aid diabetes control in patients with satisfactory weight loss. There were no criteria for time of onset of drug therapy in terms of a time point or percentage weight loss.
Before treatment, mean weight was 94.8 ± 21 kg and mean BMI was 33.7 ± 6.2 kg/m2. After 4 months of balloon treatment, weight loss, percentage total body weight loss, percentage excess weight loss, and decrease in BMI were 13.1 ± 7 kg, 13.9% ± 7.7%, 74.3% ± 57.1%, and 4.5 ±1.4 kg/m2 respectively.
After a mean duration of 4 months of liraglutide treatment (in addition to the gastric balloon), participants lost on average 18.1 ± 12.1 kg overall and 18.7% ± 12% of their initial total body weight. They shed 99.4% ± 84.9% of excess weight and reduced BMI by 6.4 ± 5.9 kg/m2.
Dr. Ienca explained that the study did not explore the separate contributions of the balloon or drug therapy to weight loss. “However, existing literature shows that the Allurion program leads to a weight loss of approximately 14% of total body weight after 4 months, while liraglutide studies report 12% of total body weight loss at 1 year,” he noted.
When describing the mechanism of action, Dr. Ienca said the Allurion balloon induces satiety and delays gastric emptying but the feeling of satiety starts to decrease after the first month. “For a few patients, this feeling of satiety decreases more rapidly or they have more difficulty putting in place new alimentary habits. In these patients, the addition of liraglutide gives an additional boost to support this behavioral change.”
Liraglutide-related adverse events included nausea (16.5%), diarrhea (3.3%), constipation (2.2%), and headache (1.7%), as well as drug discontinuation due to tachycardia/chest pain (1.1%) and gastrointestinal symptoms (1.1%).
Balloon removal because of intolerance occurred in 1.1% of patients, gastric dilation in 0.5%, and early balloon deflation in 0.5%. Other expected balloon-related adverse events included nausea, vomiting, and abdominal cramps.
The researchers note that the Allurion program offers a more acceptable option to balloon placement by endoscopy.
“The ease of use, low rate of adverse events, and potentially lower cost of the Allurion Program could enable much wider application of this critical intervention, and ultimately, help the millions who struggle with obesity and its associated health complications.”
A version of this article originally appeared on Medscape.com.
DUBLIN – A swallowable gastric balloon (Allurion Balloon, formerly known as Elipse) combined with daily subcutaneous injections of the glucagonlike peptide 1 (GLP-1) agonist liraglutide (Saxenda, Novo Nordisk), leads to a significant average total body weight loss of 19% (18 kg or 40 lb) after around 4 months in people with obesity.
said Roberta Ienca, MD, from the Clinica Nuova Villa Claudia, Rome, who presented the findings at this year’s European Congress on Obesity.
“Despite both the balloon and liraglutide working on the early satiety feeling, the introduction of liraglutide around 1 month after [swallowing the balloon] or more frequently after 3-4 months, could sustain these feelings for a longer period of time,” she said in an interview.
“The addition of the GLP-1 agonist therapy (liraglutide) to patients treated with the Allurion program [gastric balloon] is feasible, safe, and effective in those who need additional weight loss,” she emphasized.
The balloon stayed inside participants’ stomachs for an average of 16 weeks and liraglutide was continued for an average of 4 months, resulting in a mean reduction in body mass index (BMI) of 6.4 kg/m2.
The Allurion is the world’s first and only swallowable gastric balloon placed without surgery, endoscopy, or anesthesia, and is excreted naturally after around 16 weeks.
The Allurion program delivered “excellent weight loss in individuals with overweight and obesity without going under the knife, and liraglutide has the potential to further safely enhance weight loss in cases of suboptimal adherence with the program,” Dr. Ienca said. “These two treatment approaches appear to have complementary mechanisms of action in a geographically and demographically diverse population.”
Adelardo Caballero, MD, director of the Institute of Obesity, Madrid, said that he had over 6 years of experience with the Allurion balloon in around 2,500 cases. “Over the last 3 years, we have been using Allurion balloons in combination with GLP-1 agonists. In Europe, use of the swallowable gastric balloon is common, the results are good, and it is a safe tool.”
“Using liraglutide daily in subcutaneous form is authorized in Europe and is useful in overweight and mild obesity, while use in the combination [with the balloon] is also very popular,” he explained. “In the future, the combined use of semaglutide once-weekly GLP-1 agonist or the use of dual GLP-1/gastric inhibitory polypeptide agonists [such as tirzepatide] with the swallowable intragastric balloon Allurion program or endoscopic sleeve gastroplasty will improve results,” he added.
Average 40-lb weight loss with balloon and liraglutide
For the current study, data from three international multidisciplinary obesity centers (in Italy, Spain, and Egypt) were retrospectively analyzed. All 181 patients received the combination of the Allurion balloon and liraglutide, with the latter added 4-16 weeks after swallowing the balloon.
During a 20-minute outpatient visit, participants swallowed the balloon, which was filled with liquid after reaching the stomach, and placement was confirmed by x-ray. The balloon remained inserted for around 15-17 weeks (mean 16 weeks) before natural excretion. All patients received liraglutide once daily for 1-6 months (mean 4 months). After excreting the balloon, patients started the Mediterranean diet for weight maintenance and were followed for at least 6 months.
Patients were monitored for weight loss, percentage total body weight loss, percentage excess weight loss, and BMI reduction. The timing of combining drug therapy with the Allurion program, metabolic results, and adverse event data were collected. However, Dr. Ienca explained that “the study was preliminary and aimed to evaluate feasibility and results of a combined treatment, so we didn’t collect long-term data.”
Liraglutide was mostly added in cases of unsatisfactory weight loss to boost weight reduction in patients with high BMIs, to sustain weight maintenance, and to aid diabetes control in patients with satisfactory weight loss. There were no criteria for time of onset of drug therapy in terms of a time point or percentage weight loss.
Before treatment, mean weight was 94.8 ± 21 kg and mean BMI was 33.7 ± 6.2 kg/m2. After 4 months of balloon treatment, weight loss, percentage total body weight loss, percentage excess weight loss, and decrease in BMI were 13.1 ± 7 kg, 13.9% ± 7.7%, 74.3% ± 57.1%, and 4.5 ±1.4 kg/m2 respectively.
After a mean duration of 4 months of liraglutide treatment (in addition to the gastric balloon), participants lost on average 18.1 ± 12.1 kg overall and 18.7% ± 12% of their initial total body weight. They shed 99.4% ± 84.9% of excess weight and reduced BMI by 6.4 ± 5.9 kg/m2.
Dr. Ienca explained that the study did not explore the separate contributions of the balloon or drug therapy to weight loss. “However, existing literature shows that the Allurion program leads to a weight loss of approximately 14% of total body weight after 4 months, while liraglutide studies report 12% of total body weight loss at 1 year,” he noted.
When describing the mechanism of action, Dr. Ienca said the Allurion balloon induces satiety and delays gastric emptying but the feeling of satiety starts to decrease after the first month. “For a few patients, this feeling of satiety decreases more rapidly or they have more difficulty putting in place new alimentary habits. In these patients, the addition of liraglutide gives an additional boost to support this behavioral change.”
Liraglutide-related adverse events included nausea (16.5%), diarrhea (3.3%), constipation (2.2%), and headache (1.7%), as well as drug discontinuation due to tachycardia/chest pain (1.1%) and gastrointestinal symptoms (1.1%).
Balloon removal because of intolerance occurred in 1.1% of patients, gastric dilation in 0.5%, and early balloon deflation in 0.5%. Other expected balloon-related adverse events included nausea, vomiting, and abdominal cramps.
The researchers note that the Allurion program offers a more acceptable option to balloon placement by endoscopy.
“The ease of use, low rate of adverse events, and potentially lower cost of the Allurion Program could enable much wider application of this critical intervention, and ultimately, help the millions who struggle with obesity and its associated health complications.”
A version of this article originally appeared on Medscape.com.
DUBLIN – A swallowable gastric balloon (Allurion Balloon, formerly known as Elipse) combined with daily subcutaneous injections of the glucagonlike peptide 1 (GLP-1) agonist liraglutide (Saxenda, Novo Nordisk), leads to a significant average total body weight loss of 19% (18 kg or 40 lb) after around 4 months in people with obesity.
said Roberta Ienca, MD, from the Clinica Nuova Villa Claudia, Rome, who presented the findings at this year’s European Congress on Obesity.
“Despite both the balloon and liraglutide working on the early satiety feeling, the introduction of liraglutide around 1 month after [swallowing the balloon] or more frequently after 3-4 months, could sustain these feelings for a longer period of time,” she said in an interview.
“The addition of the GLP-1 agonist therapy (liraglutide) to patients treated with the Allurion program [gastric balloon] is feasible, safe, and effective in those who need additional weight loss,” she emphasized.
The balloon stayed inside participants’ stomachs for an average of 16 weeks and liraglutide was continued for an average of 4 months, resulting in a mean reduction in body mass index (BMI) of 6.4 kg/m2.
The Allurion is the world’s first and only swallowable gastric balloon placed without surgery, endoscopy, or anesthesia, and is excreted naturally after around 16 weeks.
The Allurion program delivered “excellent weight loss in individuals with overweight and obesity without going under the knife, and liraglutide has the potential to further safely enhance weight loss in cases of suboptimal adherence with the program,” Dr. Ienca said. “These two treatment approaches appear to have complementary mechanisms of action in a geographically and demographically diverse population.”
Adelardo Caballero, MD, director of the Institute of Obesity, Madrid, said that he had over 6 years of experience with the Allurion balloon in around 2,500 cases. “Over the last 3 years, we have been using Allurion balloons in combination with GLP-1 agonists. In Europe, use of the swallowable gastric balloon is common, the results are good, and it is a safe tool.”
“Using liraglutide daily in subcutaneous form is authorized in Europe and is useful in overweight and mild obesity, while use in the combination [with the balloon] is also very popular,” he explained. “In the future, the combined use of semaglutide once-weekly GLP-1 agonist or the use of dual GLP-1/gastric inhibitory polypeptide agonists [such as tirzepatide] with the swallowable intragastric balloon Allurion program or endoscopic sleeve gastroplasty will improve results,” he added.
Average 40-lb weight loss with balloon and liraglutide
For the current study, data from three international multidisciplinary obesity centers (in Italy, Spain, and Egypt) were retrospectively analyzed. All 181 patients received the combination of the Allurion balloon and liraglutide, with the latter added 4-16 weeks after swallowing the balloon.
During a 20-minute outpatient visit, participants swallowed the balloon, which was filled with liquid after reaching the stomach, and placement was confirmed by x-ray. The balloon remained inserted for around 15-17 weeks (mean 16 weeks) before natural excretion. All patients received liraglutide once daily for 1-6 months (mean 4 months). After excreting the balloon, patients started the Mediterranean diet for weight maintenance and were followed for at least 6 months.
Patients were monitored for weight loss, percentage total body weight loss, percentage excess weight loss, and BMI reduction. The timing of combining drug therapy with the Allurion program, metabolic results, and adverse event data were collected. However, Dr. Ienca explained that “the study was preliminary and aimed to evaluate feasibility and results of a combined treatment, so we didn’t collect long-term data.”
Liraglutide was mostly added in cases of unsatisfactory weight loss to boost weight reduction in patients with high BMIs, to sustain weight maintenance, and to aid diabetes control in patients with satisfactory weight loss. There were no criteria for time of onset of drug therapy in terms of a time point or percentage weight loss.
Before treatment, mean weight was 94.8 ± 21 kg and mean BMI was 33.7 ± 6.2 kg/m2. After 4 months of balloon treatment, weight loss, percentage total body weight loss, percentage excess weight loss, and decrease in BMI were 13.1 ± 7 kg, 13.9% ± 7.7%, 74.3% ± 57.1%, and 4.5 ±1.4 kg/m2 respectively.
After a mean duration of 4 months of liraglutide treatment (in addition to the gastric balloon), participants lost on average 18.1 ± 12.1 kg overall and 18.7% ± 12% of their initial total body weight. They shed 99.4% ± 84.9% of excess weight and reduced BMI by 6.4 ± 5.9 kg/m2.
Dr. Ienca explained that the study did not explore the separate contributions of the balloon or drug therapy to weight loss. “However, existing literature shows that the Allurion program leads to a weight loss of approximately 14% of total body weight after 4 months, while liraglutide studies report 12% of total body weight loss at 1 year,” he noted.
When describing the mechanism of action, Dr. Ienca said the Allurion balloon induces satiety and delays gastric emptying but the feeling of satiety starts to decrease after the first month. “For a few patients, this feeling of satiety decreases more rapidly or they have more difficulty putting in place new alimentary habits. In these patients, the addition of liraglutide gives an additional boost to support this behavioral change.”
Liraglutide-related adverse events included nausea (16.5%), diarrhea (3.3%), constipation (2.2%), and headache (1.7%), as well as drug discontinuation due to tachycardia/chest pain (1.1%) and gastrointestinal symptoms (1.1%).
Balloon removal because of intolerance occurred in 1.1% of patients, gastric dilation in 0.5%, and early balloon deflation in 0.5%. Other expected balloon-related adverse events included nausea, vomiting, and abdominal cramps.
The researchers note that the Allurion program offers a more acceptable option to balloon placement by endoscopy.
“The ease of use, low rate of adverse events, and potentially lower cost of the Allurion Program could enable much wider application of this critical intervention, and ultimately, help the millions who struggle with obesity and its associated health complications.”
A version of this article originally appeared on Medscape.com.
AT ECO 2023
Metabolic abnormalities boost obesity-related cancer risk
, and an even higher risk, two- to threefold higher, for specific cancers, such as endometrial, liver, and renal cell cancers, compared with metabolically healthy normal weight.
Even in people with so-called “metabolically healthy” obesity, the risk for overall obesity-related cancer is increased, compared with normal-weight, metabolically healthy individuals; however, the associations here are weaker than in people with metabolically unhealthy obesity.
“The type of metabolic obesity phenotype is important when assessing obesity-related cancer risk,” lead researcher Ming Sun, PhD, from Lund University, Malmö, Sweden, said in an interview. “In general, metabolic aberrations further increased the obesity-induced cancer risk, suggesting that obesity and metabolic aberrations are useful targets for prevention.”
“This synergy means that when obesity and metabolic unhealth occur together, that’s particularly bad,” added Tanja Stocks, PhD, senior author, also of Lund University.
“But the data also highlight that even obesity and overweight alone comprise an increased risk of cancer,” Dr. Stocks noted.
Dr. Sun said the findings have important public health implications, suggesting that “a significant number of cancer cases could potentially be prevented by targeting the coexistence of metabolic problems and obesity, in particular for obesity-related cancers among men.”
The results will be presented as a poster by Dr. Sun at the European Congress on Obesity 2023, being held in Dublin, and have been published in the Journal of the National Cancer Institute.
Metabolically unhealthy obesity worst for cancer risks
Andrew G. Renehan, PhD, FRCS, professor of cancer studies and surgery, University of Manchester, England, welcomed the new work, saying it addresses the issue with very large study numbers. “[It] nicely demonstrates that there are clear examples where metabolically unhealthy overweight and obese phenotypes have increased cancer risk relative to [metabolically] healthy overweight and obese phenotypes,” he said.
“There is a clear need for clinically based research addressing these hypotheses ... but these studies will additionally need to factor in other dimensions such as the selection of treatment for metabolic aberrations, both medical and surgical, and the consequent metabolic control resulting from these interventions,” Dr. Renehan observed.
Vibhu Chittajallu, MD, a gastroenterologist based at University Hospitals Cleveland Medical Center, said it was beneficial to see another study further validating the association of obesity with the development of obesity-associated cancers.
“This is an interesting study [because it focuses] on the role of metabolic syndrome in obesity and how it affects the risk of development of obesity-associated cancers,” he said in an interview.
“I believe that the results of this study further strengthen the need for improved management of obesity and metabolic syndrome to reduce the risk of obesity-associated cancer formation that plays a role in preventable and premature deaths in adult patients with obesity.”
Synergy between metabolic aberrations and obesity, and cancer risk
Dr. Sun and colleagues note that obesity is an established risk factor for several cancers. It is often accompanied by metabolic aberrations, which have been a commonly proposed mechanism to link obesity with cancer. During the last decade, obesity with or without metabolic aberrations – commonly termed “metabolically unhealthy” or “healthy obesity” – has been extensively investigated in the cardiovascular field; however, studies regarding cancer are limited.
According to Dr. Sun, this new study is the first to look at the synergistic effect of unhealthy metabolism and body mass index – the latter was further categorized as normal weight (BMI < 25 kg/m2), overweight (BMI < 30) and obesity (BMI ≤ 30) – and the association with cancer risk, both overall and in relation to site-specific cancers.
Data were drawn from 797,193 European individuals (in Norway, Sweden, and Austria), of whom 23,630 developed an obesity-related cancer during the follow-up period. A metabolic score comprising mid-blood pressure, plasma glucose, and triglycerides was used to provide a measure of healthy or unhealthy metabolic status. Relative risks (hazard ratios) for overall and site-specific cancers were determined. Comparisons were made with metabolically healthy people of normal weight (effectively controls).
When different metabolic scores and BMIs were combined, participants fell into six categories: metabolically unhealthy obesity (6.8% of participants); metabolically healthy obesity (3.4%), metabolically unhealthy overweight (15.4%), metabolically healthy overweight (19.8%), metabolically unhealthy normal weight (12.5%), and metabolically healthy normal weight (42.0%).
Metabolically unhealthy women with obesity had a hazard ratio of 1.43 for overall obesity-related cancers, compared with metabolically healthy women of normal weight. Of particular note were risks of two cancer types in women with metabolically unhealthy obesity: renal cancer, with an HR of 2.43, and endometrial cancer, with an HR of 3.0, compared with controls.
Even in metabolically healthy women with obesity, compared with metabolically healthy women of normal weight, there was an increased risk of endometrial cancer, with an HR of 2.36.
“If you look at individual cancers, in particular endometrial cancer, this seems to be very much driven by obesity and not so much by the metabolic factor,” remarked Dr. Stocks.
In males, compared with metabolically healthy men of normal weight, metabolically unhealthy men with obesity had an overall obesity-related cancer risk HR of 1.91. Specifically, the risk of renal cell cancer was more than doubled, with an HR of 2.59. The HR for colon cancer was 1.85, and that for rectal cancer and pancreatic cancer was similar, both having HRs of 1.32.
Again, risk was lower in metabolically healthy men with obesity, although still higher than for metabolically healthy normal-weight men.
A version of this article first appeared on Medscape.com.
, and an even higher risk, two- to threefold higher, for specific cancers, such as endometrial, liver, and renal cell cancers, compared with metabolically healthy normal weight.
Even in people with so-called “metabolically healthy” obesity, the risk for overall obesity-related cancer is increased, compared with normal-weight, metabolically healthy individuals; however, the associations here are weaker than in people with metabolically unhealthy obesity.
“The type of metabolic obesity phenotype is important when assessing obesity-related cancer risk,” lead researcher Ming Sun, PhD, from Lund University, Malmö, Sweden, said in an interview. “In general, metabolic aberrations further increased the obesity-induced cancer risk, suggesting that obesity and metabolic aberrations are useful targets for prevention.”
“This synergy means that when obesity and metabolic unhealth occur together, that’s particularly bad,” added Tanja Stocks, PhD, senior author, also of Lund University.
“But the data also highlight that even obesity and overweight alone comprise an increased risk of cancer,” Dr. Stocks noted.
Dr. Sun said the findings have important public health implications, suggesting that “a significant number of cancer cases could potentially be prevented by targeting the coexistence of metabolic problems and obesity, in particular for obesity-related cancers among men.”
The results will be presented as a poster by Dr. Sun at the European Congress on Obesity 2023, being held in Dublin, and have been published in the Journal of the National Cancer Institute.
Metabolically unhealthy obesity worst for cancer risks
Andrew G. Renehan, PhD, FRCS, professor of cancer studies and surgery, University of Manchester, England, welcomed the new work, saying it addresses the issue with very large study numbers. “[It] nicely demonstrates that there are clear examples where metabolically unhealthy overweight and obese phenotypes have increased cancer risk relative to [metabolically] healthy overweight and obese phenotypes,” he said.
“There is a clear need for clinically based research addressing these hypotheses ... but these studies will additionally need to factor in other dimensions such as the selection of treatment for metabolic aberrations, both medical and surgical, and the consequent metabolic control resulting from these interventions,” Dr. Renehan observed.
Vibhu Chittajallu, MD, a gastroenterologist based at University Hospitals Cleveland Medical Center, said it was beneficial to see another study further validating the association of obesity with the development of obesity-associated cancers.
“This is an interesting study [because it focuses] on the role of metabolic syndrome in obesity and how it affects the risk of development of obesity-associated cancers,” he said in an interview.
“I believe that the results of this study further strengthen the need for improved management of obesity and metabolic syndrome to reduce the risk of obesity-associated cancer formation that plays a role in preventable and premature deaths in adult patients with obesity.”
Synergy between metabolic aberrations and obesity, and cancer risk
Dr. Sun and colleagues note that obesity is an established risk factor for several cancers. It is often accompanied by metabolic aberrations, which have been a commonly proposed mechanism to link obesity with cancer. During the last decade, obesity with or without metabolic aberrations – commonly termed “metabolically unhealthy” or “healthy obesity” – has been extensively investigated in the cardiovascular field; however, studies regarding cancer are limited.
According to Dr. Sun, this new study is the first to look at the synergistic effect of unhealthy metabolism and body mass index – the latter was further categorized as normal weight (BMI < 25 kg/m2), overweight (BMI < 30) and obesity (BMI ≤ 30) – and the association with cancer risk, both overall and in relation to site-specific cancers.
Data were drawn from 797,193 European individuals (in Norway, Sweden, and Austria), of whom 23,630 developed an obesity-related cancer during the follow-up period. A metabolic score comprising mid-blood pressure, plasma glucose, and triglycerides was used to provide a measure of healthy or unhealthy metabolic status. Relative risks (hazard ratios) for overall and site-specific cancers were determined. Comparisons were made with metabolically healthy people of normal weight (effectively controls).
When different metabolic scores and BMIs were combined, participants fell into six categories: metabolically unhealthy obesity (6.8% of participants); metabolically healthy obesity (3.4%), metabolically unhealthy overweight (15.4%), metabolically healthy overweight (19.8%), metabolically unhealthy normal weight (12.5%), and metabolically healthy normal weight (42.0%).
Metabolically unhealthy women with obesity had a hazard ratio of 1.43 for overall obesity-related cancers, compared with metabolically healthy women of normal weight. Of particular note were risks of two cancer types in women with metabolically unhealthy obesity: renal cancer, with an HR of 2.43, and endometrial cancer, with an HR of 3.0, compared with controls.
Even in metabolically healthy women with obesity, compared with metabolically healthy women of normal weight, there was an increased risk of endometrial cancer, with an HR of 2.36.
“If you look at individual cancers, in particular endometrial cancer, this seems to be very much driven by obesity and not so much by the metabolic factor,” remarked Dr. Stocks.
In males, compared with metabolically healthy men of normal weight, metabolically unhealthy men with obesity had an overall obesity-related cancer risk HR of 1.91. Specifically, the risk of renal cell cancer was more than doubled, with an HR of 2.59. The HR for colon cancer was 1.85, and that for rectal cancer and pancreatic cancer was similar, both having HRs of 1.32.
Again, risk was lower in metabolically healthy men with obesity, although still higher than for metabolically healthy normal-weight men.
A version of this article first appeared on Medscape.com.
, and an even higher risk, two- to threefold higher, for specific cancers, such as endometrial, liver, and renal cell cancers, compared with metabolically healthy normal weight.
Even in people with so-called “metabolically healthy” obesity, the risk for overall obesity-related cancer is increased, compared with normal-weight, metabolically healthy individuals; however, the associations here are weaker than in people with metabolically unhealthy obesity.
“The type of metabolic obesity phenotype is important when assessing obesity-related cancer risk,” lead researcher Ming Sun, PhD, from Lund University, Malmö, Sweden, said in an interview. “In general, metabolic aberrations further increased the obesity-induced cancer risk, suggesting that obesity and metabolic aberrations are useful targets for prevention.”
“This synergy means that when obesity and metabolic unhealth occur together, that’s particularly bad,” added Tanja Stocks, PhD, senior author, also of Lund University.
“But the data also highlight that even obesity and overweight alone comprise an increased risk of cancer,” Dr. Stocks noted.
Dr. Sun said the findings have important public health implications, suggesting that “a significant number of cancer cases could potentially be prevented by targeting the coexistence of metabolic problems and obesity, in particular for obesity-related cancers among men.”
The results will be presented as a poster by Dr. Sun at the European Congress on Obesity 2023, being held in Dublin, and have been published in the Journal of the National Cancer Institute.
Metabolically unhealthy obesity worst for cancer risks
Andrew G. Renehan, PhD, FRCS, professor of cancer studies and surgery, University of Manchester, England, welcomed the new work, saying it addresses the issue with very large study numbers. “[It] nicely demonstrates that there are clear examples where metabolically unhealthy overweight and obese phenotypes have increased cancer risk relative to [metabolically] healthy overweight and obese phenotypes,” he said.
“There is a clear need for clinically based research addressing these hypotheses ... but these studies will additionally need to factor in other dimensions such as the selection of treatment for metabolic aberrations, both medical and surgical, and the consequent metabolic control resulting from these interventions,” Dr. Renehan observed.
Vibhu Chittajallu, MD, a gastroenterologist based at University Hospitals Cleveland Medical Center, said it was beneficial to see another study further validating the association of obesity with the development of obesity-associated cancers.
“This is an interesting study [because it focuses] on the role of metabolic syndrome in obesity and how it affects the risk of development of obesity-associated cancers,” he said in an interview.
“I believe that the results of this study further strengthen the need for improved management of obesity and metabolic syndrome to reduce the risk of obesity-associated cancer formation that plays a role in preventable and premature deaths in adult patients with obesity.”
Synergy between metabolic aberrations and obesity, and cancer risk
Dr. Sun and colleagues note that obesity is an established risk factor for several cancers. It is often accompanied by metabolic aberrations, which have been a commonly proposed mechanism to link obesity with cancer. During the last decade, obesity with or without metabolic aberrations – commonly termed “metabolically unhealthy” or “healthy obesity” – has been extensively investigated in the cardiovascular field; however, studies regarding cancer are limited.
According to Dr. Sun, this new study is the first to look at the synergistic effect of unhealthy metabolism and body mass index – the latter was further categorized as normal weight (BMI < 25 kg/m2), overweight (BMI < 30) and obesity (BMI ≤ 30) – and the association with cancer risk, both overall and in relation to site-specific cancers.
Data were drawn from 797,193 European individuals (in Norway, Sweden, and Austria), of whom 23,630 developed an obesity-related cancer during the follow-up period. A metabolic score comprising mid-blood pressure, plasma glucose, and triglycerides was used to provide a measure of healthy or unhealthy metabolic status. Relative risks (hazard ratios) for overall and site-specific cancers were determined. Comparisons were made with metabolically healthy people of normal weight (effectively controls).
When different metabolic scores and BMIs were combined, participants fell into six categories: metabolically unhealthy obesity (6.8% of participants); metabolically healthy obesity (3.4%), metabolically unhealthy overweight (15.4%), metabolically healthy overweight (19.8%), metabolically unhealthy normal weight (12.5%), and metabolically healthy normal weight (42.0%).
Metabolically unhealthy women with obesity had a hazard ratio of 1.43 for overall obesity-related cancers, compared with metabolically healthy women of normal weight. Of particular note were risks of two cancer types in women with metabolically unhealthy obesity: renal cancer, with an HR of 2.43, and endometrial cancer, with an HR of 3.0, compared with controls.
Even in metabolically healthy women with obesity, compared with metabolically healthy women of normal weight, there was an increased risk of endometrial cancer, with an HR of 2.36.
“If you look at individual cancers, in particular endometrial cancer, this seems to be very much driven by obesity and not so much by the metabolic factor,” remarked Dr. Stocks.
In males, compared with metabolically healthy men of normal weight, metabolically unhealthy men with obesity had an overall obesity-related cancer risk HR of 1.91. Specifically, the risk of renal cell cancer was more than doubled, with an HR of 2.59. The HR for colon cancer was 1.85, and that for rectal cancer and pancreatic cancer was similar, both having HRs of 1.32.
Again, risk was lower in metabolically healthy men with obesity, although still higher than for metabolically healthy normal-weight men.
A version of this article first appeared on Medscape.com.
FROM ECO 2023
Dried blood spot test validated for HIV, hep B, and hep C
A test that uses a single drop of dried blood to detect HIV, hepatitis B virus, and HCV has been validated and is now in use in some high-risk settings in Denmark, according to research presented at the annual European Congress of Clinical Microbiology & Infectious Diseases.
Molecular biologist Stephen Nilsson-Møller, MSc, and colleagues at the department of clinical microbiology, Copenhagen University Hospital, developed and validated the test, known as the Dried Blood Spot (DBS), for HIV, HBV, and HCV.
The “test that can detect low viral loads for all three viruses from a single drop of blood, and can be done using existing hospital equipment,” Mr. Nilsson-Møller said in an interview. “Importantly, it does not require venipuncture, but can be done from a drop of dried blood from the finger.”
He highlighted the utility of the new test in more challenging settings. “This method is particularly useful in high-risk settings such as homeless shelters, drug rehabilitation centers, and prisons, where needles might be misused, and it can be difficult to convince people to have the more invasive test.”
“Also, in some places – such as in low- and middle-income settings – there is a distinct risk of ruining blood samples before analysis due to limited refrigeration for transit and storage,” he added. “[Standard] blood samples need to be analyzed within 6 hours when kept at room temperature, while dried blood spots can last for 9 months at room temperature and can be mailed to a laboratory with the right equipment to analyze it.”
Tiny amounts of virus detected
Mr. Nilsson-Møller was tasked with developing a test for use by the university’s department of infectious diseases to screen people in high-risk settings in the capital region of Copenhagen. The work forms part of a PhD project by Jonas Demant at the University of Copenhagen, for which he is screening for HIV, HBV, and HCV in drug rehabilitation centers, prisons, and homeless shelters.
The study is the first to use the Hologic Panther system (a nucleic acid amplification test) combining all three viruses, Mr. Nilsson-Møller pointed out. “A tiny amount of virus can be detected because it is a very sensitive platform using transcription-mediated amplification.”
“If it detects low amounts of virus, it will create many copies very quickly, creating a signal that tells us that the sample is positive,” he explained.
The researchers collected whole blood from a finger prick, dried it out on a protein saver card (filter paper), and cut out a 1.2-cm diameter dry blood spot which was then prepared for analysis.
Twenty blood samples with known amounts of HIV, HBV, and HCV were analyzed via the DBS method (60 in total) and the viruses were detected in all of the samples.
To validate the method, the researchers used plasma with a known viral load, and a series of dilutions were performed to determine the lower limit for positive detection of all three viruses.
“Untreated patients typically have above 1 million IU/mL of viral loads in their plasma, and we found that we can detect much lower levels,” said Mr. Nilsson-Møller. “Ideally, 40 mcL of blood is good, but less should be sufficient if the test is on untreated patients.”
Early testing and treatment reduces morbidity and mortality
Elimination of HBV, HCV, and HIV by 2030 is a global health strategy set by the World Health Organization, but to meet this goal, new approaches for diagnostic testing are required. The DBS test for HIV, HBV, and HCV promises to make a significant contribution toward this goal.
“One in two people currently living with HIV is diagnosed late in the course of their infection, and an even larger proportion of the estimated 6 million Europeans living with chronic hepatitis B or C are not aware that they are infected,” said Anastasia Pharris, PhD, from the European Center for Disease Prevention and Control Principal Expert Infectious Diseases.
“Increasing testing coverage and uptake, especially for those most at risk, is an essential element of any strategy to eliminate HBV, HCV, and HIV in the European Union and European Economic Area,” she pointed out.
Dr. Pharris also highlighted that, while HIV, and often HBV infection, require lifelong treatment, HCV infection is now curable within a few weeks. “To maximize the benefits of individual treatment for all three infections, it is critical to test and diagnose people as soon as possible – in itself a challenge given that these infections can typically be asymptomatic for years.
“Early diagnosis of HBV, HCV, or HIV is vital as it allows people to access treatment, which significantly reduces associated long-term morbidity and mortality.
“In many cases, those most at risk of one of these infections are also more vulnerable to infection with one or both of the other viruses, making the argument for integrated testing even stronger,” she said in an interview.
Mr. Nilsson-Møller and Dr. Pharris reported no relevant financial relationships. Aptima kits for validation were provided by Hologic.
A version of this article first appeared on Medscape.com.
A test that uses a single drop of dried blood to detect HIV, hepatitis B virus, and HCV has been validated and is now in use in some high-risk settings in Denmark, according to research presented at the annual European Congress of Clinical Microbiology & Infectious Diseases.
Molecular biologist Stephen Nilsson-Møller, MSc, and colleagues at the department of clinical microbiology, Copenhagen University Hospital, developed and validated the test, known as the Dried Blood Spot (DBS), for HIV, HBV, and HCV.
The “test that can detect low viral loads for all three viruses from a single drop of blood, and can be done using existing hospital equipment,” Mr. Nilsson-Møller said in an interview. “Importantly, it does not require venipuncture, but can be done from a drop of dried blood from the finger.”
He highlighted the utility of the new test in more challenging settings. “This method is particularly useful in high-risk settings such as homeless shelters, drug rehabilitation centers, and prisons, where needles might be misused, and it can be difficult to convince people to have the more invasive test.”
“Also, in some places – such as in low- and middle-income settings – there is a distinct risk of ruining blood samples before analysis due to limited refrigeration for transit and storage,” he added. “[Standard] blood samples need to be analyzed within 6 hours when kept at room temperature, while dried blood spots can last for 9 months at room temperature and can be mailed to a laboratory with the right equipment to analyze it.”
Tiny amounts of virus detected
Mr. Nilsson-Møller was tasked with developing a test for use by the university’s department of infectious diseases to screen people in high-risk settings in the capital region of Copenhagen. The work forms part of a PhD project by Jonas Demant at the University of Copenhagen, for which he is screening for HIV, HBV, and HCV in drug rehabilitation centers, prisons, and homeless shelters.
The study is the first to use the Hologic Panther system (a nucleic acid amplification test) combining all three viruses, Mr. Nilsson-Møller pointed out. “A tiny amount of virus can be detected because it is a very sensitive platform using transcription-mediated amplification.”
“If it detects low amounts of virus, it will create many copies very quickly, creating a signal that tells us that the sample is positive,” he explained.
The researchers collected whole blood from a finger prick, dried it out on a protein saver card (filter paper), and cut out a 1.2-cm diameter dry blood spot which was then prepared for analysis.
Twenty blood samples with known amounts of HIV, HBV, and HCV were analyzed via the DBS method (60 in total) and the viruses were detected in all of the samples.
To validate the method, the researchers used plasma with a known viral load, and a series of dilutions were performed to determine the lower limit for positive detection of all three viruses.
“Untreated patients typically have above 1 million IU/mL of viral loads in their plasma, and we found that we can detect much lower levels,” said Mr. Nilsson-Møller. “Ideally, 40 mcL of blood is good, but less should be sufficient if the test is on untreated patients.”
Early testing and treatment reduces morbidity and mortality
Elimination of HBV, HCV, and HIV by 2030 is a global health strategy set by the World Health Organization, but to meet this goal, new approaches for diagnostic testing are required. The DBS test for HIV, HBV, and HCV promises to make a significant contribution toward this goal.
“One in two people currently living with HIV is diagnosed late in the course of their infection, and an even larger proportion of the estimated 6 million Europeans living with chronic hepatitis B or C are not aware that they are infected,” said Anastasia Pharris, PhD, from the European Center for Disease Prevention and Control Principal Expert Infectious Diseases.
“Increasing testing coverage and uptake, especially for those most at risk, is an essential element of any strategy to eliminate HBV, HCV, and HIV in the European Union and European Economic Area,” she pointed out.
Dr. Pharris also highlighted that, while HIV, and often HBV infection, require lifelong treatment, HCV infection is now curable within a few weeks. “To maximize the benefits of individual treatment for all three infections, it is critical to test and diagnose people as soon as possible – in itself a challenge given that these infections can typically be asymptomatic for years.
“Early diagnosis of HBV, HCV, or HIV is vital as it allows people to access treatment, which significantly reduces associated long-term morbidity and mortality.
“In many cases, those most at risk of one of these infections are also more vulnerable to infection with one or both of the other viruses, making the argument for integrated testing even stronger,” she said in an interview.
Mr. Nilsson-Møller and Dr. Pharris reported no relevant financial relationships. Aptima kits for validation were provided by Hologic.
A version of this article first appeared on Medscape.com.
A test that uses a single drop of dried blood to detect HIV, hepatitis B virus, and HCV has been validated and is now in use in some high-risk settings in Denmark, according to research presented at the annual European Congress of Clinical Microbiology & Infectious Diseases.
Molecular biologist Stephen Nilsson-Møller, MSc, and colleagues at the department of clinical microbiology, Copenhagen University Hospital, developed and validated the test, known as the Dried Blood Spot (DBS), for HIV, HBV, and HCV.
The “test that can detect low viral loads for all three viruses from a single drop of blood, and can be done using existing hospital equipment,” Mr. Nilsson-Møller said in an interview. “Importantly, it does not require venipuncture, but can be done from a drop of dried blood from the finger.”
He highlighted the utility of the new test in more challenging settings. “This method is particularly useful in high-risk settings such as homeless shelters, drug rehabilitation centers, and prisons, where needles might be misused, and it can be difficult to convince people to have the more invasive test.”
“Also, in some places – such as in low- and middle-income settings – there is a distinct risk of ruining blood samples before analysis due to limited refrigeration for transit and storage,” he added. “[Standard] blood samples need to be analyzed within 6 hours when kept at room temperature, while dried blood spots can last for 9 months at room temperature and can be mailed to a laboratory with the right equipment to analyze it.”
Tiny amounts of virus detected
Mr. Nilsson-Møller was tasked with developing a test for use by the university’s department of infectious diseases to screen people in high-risk settings in the capital region of Copenhagen. The work forms part of a PhD project by Jonas Demant at the University of Copenhagen, for which he is screening for HIV, HBV, and HCV in drug rehabilitation centers, prisons, and homeless shelters.
The study is the first to use the Hologic Panther system (a nucleic acid amplification test) combining all three viruses, Mr. Nilsson-Møller pointed out. “A tiny amount of virus can be detected because it is a very sensitive platform using transcription-mediated amplification.”
“If it detects low amounts of virus, it will create many copies very quickly, creating a signal that tells us that the sample is positive,” he explained.
The researchers collected whole blood from a finger prick, dried it out on a protein saver card (filter paper), and cut out a 1.2-cm diameter dry blood spot which was then prepared for analysis.
Twenty blood samples with known amounts of HIV, HBV, and HCV were analyzed via the DBS method (60 in total) and the viruses were detected in all of the samples.
To validate the method, the researchers used plasma with a known viral load, and a series of dilutions were performed to determine the lower limit for positive detection of all three viruses.
“Untreated patients typically have above 1 million IU/mL of viral loads in their plasma, and we found that we can detect much lower levels,” said Mr. Nilsson-Møller. “Ideally, 40 mcL of blood is good, but less should be sufficient if the test is on untreated patients.”
Early testing and treatment reduces morbidity and mortality
Elimination of HBV, HCV, and HIV by 2030 is a global health strategy set by the World Health Organization, but to meet this goal, new approaches for diagnostic testing are required. The DBS test for HIV, HBV, and HCV promises to make a significant contribution toward this goal.
“One in two people currently living with HIV is diagnosed late in the course of their infection, and an even larger proportion of the estimated 6 million Europeans living with chronic hepatitis B or C are not aware that they are infected,” said Anastasia Pharris, PhD, from the European Center for Disease Prevention and Control Principal Expert Infectious Diseases.
“Increasing testing coverage and uptake, especially for those most at risk, is an essential element of any strategy to eliminate HBV, HCV, and HIV in the European Union and European Economic Area,” she pointed out.
Dr. Pharris also highlighted that, while HIV, and often HBV infection, require lifelong treatment, HCV infection is now curable within a few weeks. “To maximize the benefits of individual treatment for all three infections, it is critical to test and diagnose people as soon as possible – in itself a challenge given that these infections can typically be asymptomatic for years.
“Early diagnosis of HBV, HCV, or HIV is vital as it allows people to access treatment, which significantly reduces associated long-term morbidity and mortality.
“In many cases, those most at risk of one of these infections are also more vulnerable to infection with one or both of the other viruses, making the argument for integrated testing even stronger,” she said in an interview.
Mr. Nilsson-Møller and Dr. Pharris reported no relevant financial relationships. Aptima kits for validation were provided by Hologic.
A version of this article first appeared on Medscape.com.
FROM ECCMID 2023
Sleep disturbances linked to post-COVID dyspnea
according to data from the U.K.’s CircCOVID study.
The researchers, led by John Blaikley, MRCP, PhD, respiratory physician and clinical scientist from the University of Manchester (England), found that sleep disturbance is a common problem after hospital admission for COVID-19 and may last for at least 1 year.
The study also showed that sleep disturbance after COVID hospitalization was associated with dyspnea and lower lung function. Further in-depth analysis revealed that the effects of sleep disturbance on dyspnea were partially mediated through both anxiety and muscle weakness; however, “this does not fully explain the association, suggesting other pathways are involved,” said Dr. Blaikley.
The study was jointly conducted by researchers from the University of Leicester (England), as well as 20 other U.K. institutes and the University of Helsinki. It was presented at the European Congress of Clinical Microbiology & Infectious Diseases and was simultaneously published in The Lancet Respiratory Medicine.
“Sleep disturbance is a common problem after hospitalization for COVID-19 and is associated with several symptoms in the post-COVID syndrome,” said Dr. Blaikley. “Clinicians should be aware of this association in their post-COVID syndrome clinics.”
He added that further work needs to be done to define the mechanism and to see whether the links are causal. “However, if they are, then treating sleep disturbance could have beneficial effects beyond improving sleep quality,” he said in an interview.
A large study recently showed that 4 in 10 people with post-COVID syndrome had moderate to severe sleep problems. Black people were at least three times more likely than White people to experience sleep problems. A total of 59% of all participants with long COVID reported having normal sleep or mild sleep disturbances, and 41% reported having moderate to severe sleep disturbances.
Unlike prior studies that evaluated sleep quality after COVID-19, which used either objective or subjective measures of sleep disturbance, the current study used both. “Using both measures revealed previously poorly described associations between sleep disturbance, breathlessness, reduced lung function, anxiety, and muscle weakness,” Dr. Blaikley pointed out.
Subjective and objective measures of sleep
The multicenter CircCOVID cohort study aimed to shed light on the prevalence and nature of sleep disturbance after patients are discharged from hospital for COVID-19 and to assess whether this was associated with dyspnea.
The study recruited a total of 2,320 participants who were part of a larger parent PHOSP-COVID study. After attending an early follow-up visit (at a median of 5 months after discharge from 83 U.K. hospitals for COVID-19), 638 participants provided data for analysis as measured by the Pittsburgh Sleep Quality Index (a subjective measure of sleep quality); 729 participants provided data for analysis as measured by actigraphy (an objective, wrist-worn, device-based measure of sleep quality) at a median of 7 months.
Breathlessness, the primary outcome, was assessed using the Dyspnea-12 validated questionnaire.
Actigraphy measurements were compared with an age-matched, sex-matched, body mass index (BMI)–matched, and time from discharge–matched cohort from the UK Biobank (a prepandemic comparator longitudinal cohort of 502,540 individuals, one-fifth of whom wore actigraphy devices). Sleep regularity was found to be 19% less in previously hospitalized patients with post-COVID syndrome, compared with matched controls who had been hospitalized for other reasons.
This “revealed that the actigraphy changes may be, in part, due to COVID-19 rather than hospitalization alone,” said Dr. Blaikley.
Data were collected at two time points after hospital discharge: 2-7 months (early), and 10-14 months (late). At the early time point, participants were clinically assessed with respect to anxiety, muscle function, and dyspnea, and lung function.
After discharge from hospital, the majority (62%) of post–COVID-19 participants reported poor sleep quality on the Pittsburgh Sleep Quality Index questionnaire. A “comparable” proportion (53%) felt that their quality of sleep had deteriorated following hospital discharge according to the numerical rating scale (subjective measure).
Also, sleep disturbance was found likely to persist for at least 12 months, since subjective sleep quality hardly changed between the early and late time points after hospital discharge.
Both subjective metrics (sleep quality and sleep quality deterioration after hospital discharge) and objective, device-based metrics (sleep regularity) were found to be associated with dyspnea and reduced lung function in patients with post-COVID syndrome.
“One of the striking findings in our study is the consistency with breathlessness and reduced lung function across different methods used to evaluate sleep,” highlighted Dr. Blaikley.
“The other striking finding was that participants following COVID-19 hospitalization actually slept longer [65 min; 95% confidence interval, 59-71 min] than participants hospitalized for non-COVID; however, their bedtimes were irregular, and it was this irregularity that was associated with breathlessness,” he added.
In comparison with nonhospitalized controls, also from the UK Biobank, study participants with lower sleep regularity had higher Dyspnea-12 scores (unadjusted effect estimate, 4.38; 95%: CI, 2.10-6.65). Those with poor sleep quality overall also had higher Dyspnea-12 scores (unadjusted effect estimate, 3.94; 95% CI, 2.78-5.10), and those who reported sleep quality deterioration had higher Dyspnea-12 scores (unadjusted effect estimate, 3,00; 95% CI, 1.82-4.28).
In comparison with hospitalized controls, CircCOVID participants had lower sleep regularity index (–19%; 95% CI, –20 to –16) and lower sleep efficiency (3.83 percentage points; 95% CI, 3.40-4.26).
Sleep disturbance after COVID hospitalization was also associated with lower lung function, from a 7% to a 14% reduction in predicted forced vital capacity, depending on which sleep measure used.
In an analysis of mediating factors active in the relationship between sleep disturbance and dyspnea/decreased lung function, the researchers found that reduced muscle function and anxiety, which are both recognized causes of dyspnea, could partially contribute to the association.
Regarding anxiety, and depending on the sleep metric, anxiety mediated 18%-39% of the effect of sleep disturbance on dyspnea, while muscle weakness mediated 27%-41% of this effect, reported Dr. Blaikley. Those with poor sleep quality were more likely to have mild, moderate, or severe anxiety, compared with participants who reported good-quality sleep.
A similar association was observed between anxiety and sleep quality deterioration.
“Two key questions are raised by our study: Do sleep interventions have a beneficial effect in post–COVID-19 syndrome, and are the associations causal?” asked Dr. Blaikley. “We hope to do a sleep intervention trial to answer these questions to explore if this is an effective treatment for post–COVID-19 syndrome.”
‘Underlying mechanisms remain unclear’
Amitava Banerjee, MD, professor of clinical data science and honorary consultant cardiologist, Institute of Health Informatics, UCL, London, welcomed the study but noted that it did not include nonhospitalized post-COVID patients.
“The majority of people with long COVID were not hospitalized for COVID, so the results may not be generalizable to this larger group,” she said in an interview. “Good-quality sleep is important for health and reduces risk of chronic diseases; quality of sleep is therefore likely to be important for those with long COVID in reducing their risk of chronic disease, but the role of sleep in the mechanism of long COVID needs further research.”
In a commentary also published in The Lancet Respiratory Medicine, W. Cameron McGuire, MD, pulmonary and critical care specialist from San Diego, California, and colleagues wrote: “These findings suggest that sleep disturbance, dyspnea, and anxiety are common after COVID-19 and are associated with one another, although the underlying mechanisms remain unclear.”
The commentators “applauded” the work overall but noted that the findings represent correlation rather than causation. “It is unclear whether sleep disturbance is causing anxiety or whether anxiety is contributing to poor sleep. ... For the sleep disturbances, increased BMI in the cohort reporting poor sleep, compared with those reporting good sleep might suggest underlying obstructive sleep apnea,” they wrote.
Dr. McGuire and colleagues added that many questions remain for researchers and clinicians, including “whether anxiety and dyspnoea are contributing to a low arousal threshold [disrupting sleep] ... whether the observed abnormalities (e.g., in dyspnea score) are clinically significant,” and “whether therapies such as glucocorticoids, anticoagulants, or previous vaccinations mitigate the observed abnormalities during COVID-19 recovery.”
Dr. Blaikley has received support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; grants to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; and support from TEVA and Therakos for attending meetings. He is a committee member of the Royal Society of Medicine. A coauthor received funding from the National Institutes of Health and income for medical education from Zoll, Livanova, Jazz, and Eli Lilly. Dr. Banerjee is the chief investigator of STIMULATE-ICP (an NIHR-funded study) and has received research funding from AstraZeneca.
A version of this article first appeared on Medscape.com.
according to data from the U.K.’s CircCOVID study.
The researchers, led by John Blaikley, MRCP, PhD, respiratory physician and clinical scientist from the University of Manchester (England), found that sleep disturbance is a common problem after hospital admission for COVID-19 and may last for at least 1 year.
The study also showed that sleep disturbance after COVID hospitalization was associated with dyspnea and lower lung function. Further in-depth analysis revealed that the effects of sleep disturbance on dyspnea were partially mediated through both anxiety and muscle weakness; however, “this does not fully explain the association, suggesting other pathways are involved,” said Dr. Blaikley.
The study was jointly conducted by researchers from the University of Leicester (England), as well as 20 other U.K. institutes and the University of Helsinki. It was presented at the European Congress of Clinical Microbiology & Infectious Diseases and was simultaneously published in The Lancet Respiratory Medicine.
“Sleep disturbance is a common problem after hospitalization for COVID-19 and is associated with several symptoms in the post-COVID syndrome,” said Dr. Blaikley. “Clinicians should be aware of this association in their post-COVID syndrome clinics.”
He added that further work needs to be done to define the mechanism and to see whether the links are causal. “However, if they are, then treating sleep disturbance could have beneficial effects beyond improving sleep quality,” he said in an interview.
A large study recently showed that 4 in 10 people with post-COVID syndrome had moderate to severe sleep problems. Black people were at least three times more likely than White people to experience sleep problems. A total of 59% of all participants with long COVID reported having normal sleep or mild sleep disturbances, and 41% reported having moderate to severe sleep disturbances.
Unlike prior studies that evaluated sleep quality after COVID-19, which used either objective or subjective measures of sleep disturbance, the current study used both. “Using both measures revealed previously poorly described associations between sleep disturbance, breathlessness, reduced lung function, anxiety, and muscle weakness,” Dr. Blaikley pointed out.
Subjective and objective measures of sleep
The multicenter CircCOVID cohort study aimed to shed light on the prevalence and nature of sleep disturbance after patients are discharged from hospital for COVID-19 and to assess whether this was associated with dyspnea.
The study recruited a total of 2,320 participants who were part of a larger parent PHOSP-COVID study. After attending an early follow-up visit (at a median of 5 months after discharge from 83 U.K. hospitals for COVID-19), 638 participants provided data for analysis as measured by the Pittsburgh Sleep Quality Index (a subjective measure of sleep quality); 729 participants provided data for analysis as measured by actigraphy (an objective, wrist-worn, device-based measure of sleep quality) at a median of 7 months.
Breathlessness, the primary outcome, was assessed using the Dyspnea-12 validated questionnaire.
Actigraphy measurements were compared with an age-matched, sex-matched, body mass index (BMI)–matched, and time from discharge–matched cohort from the UK Biobank (a prepandemic comparator longitudinal cohort of 502,540 individuals, one-fifth of whom wore actigraphy devices). Sleep regularity was found to be 19% less in previously hospitalized patients with post-COVID syndrome, compared with matched controls who had been hospitalized for other reasons.
This “revealed that the actigraphy changes may be, in part, due to COVID-19 rather than hospitalization alone,” said Dr. Blaikley.
Data were collected at two time points after hospital discharge: 2-7 months (early), and 10-14 months (late). At the early time point, participants were clinically assessed with respect to anxiety, muscle function, and dyspnea, and lung function.
After discharge from hospital, the majority (62%) of post–COVID-19 participants reported poor sleep quality on the Pittsburgh Sleep Quality Index questionnaire. A “comparable” proportion (53%) felt that their quality of sleep had deteriorated following hospital discharge according to the numerical rating scale (subjective measure).
Also, sleep disturbance was found likely to persist for at least 12 months, since subjective sleep quality hardly changed between the early and late time points after hospital discharge.
Both subjective metrics (sleep quality and sleep quality deterioration after hospital discharge) and objective, device-based metrics (sleep regularity) were found to be associated with dyspnea and reduced lung function in patients with post-COVID syndrome.
“One of the striking findings in our study is the consistency with breathlessness and reduced lung function across different methods used to evaluate sleep,” highlighted Dr. Blaikley.
“The other striking finding was that participants following COVID-19 hospitalization actually slept longer [65 min; 95% confidence interval, 59-71 min] than participants hospitalized for non-COVID; however, their bedtimes were irregular, and it was this irregularity that was associated with breathlessness,” he added.
In comparison with nonhospitalized controls, also from the UK Biobank, study participants with lower sleep regularity had higher Dyspnea-12 scores (unadjusted effect estimate, 4.38; 95%: CI, 2.10-6.65). Those with poor sleep quality overall also had higher Dyspnea-12 scores (unadjusted effect estimate, 3.94; 95% CI, 2.78-5.10), and those who reported sleep quality deterioration had higher Dyspnea-12 scores (unadjusted effect estimate, 3,00; 95% CI, 1.82-4.28).
In comparison with hospitalized controls, CircCOVID participants had lower sleep regularity index (–19%; 95% CI, –20 to –16) and lower sleep efficiency (3.83 percentage points; 95% CI, 3.40-4.26).
Sleep disturbance after COVID hospitalization was also associated with lower lung function, from a 7% to a 14% reduction in predicted forced vital capacity, depending on which sleep measure used.
In an analysis of mediating factors active in the relationship between sleep disturbance and dyspnea/decreased lung function, the researchers found that reduced muscle function and anxiety, which are both recognized causes of dyspnea, could partially contribute to the association.
Regarding anxiety, and depending on the sleep metric, anxiety mediated 18%-39% of the effect of sleep disturbance on dyspnea, while muscle weakness mediated 27%-41% of this effect, reported Dr. Blaikley. Those with poor sleep quality were more likely to have mild, moderate, or severe anxiety, compared with participants who reported good-quality sleep.
A similar association was observed between anxiety and sleep quality deterioration.
“Two key questions are raised by our study: Do sleep interventions have a beneficial effect in post–COVID-19 syndrome, and are the associations causal?” asked Dr. Blaikley. “We hope to do a sleep intervention trial to answer these questions to explore if this is an effective treatment for post–COVID-19 syndrome.”
‘Underlying mechanisms remain unclear’
Amitava Banerjee, MD, professor of clinical data science and honorary consultant cardiologist, Institute of Health Informatics, UCL, London, welcomed the study but noted that it did not include nonhospitalized post-COVID patients.
“The majority of people with long COVID were not hospitalized for COVID, so the results may not be generalizable to this larger group,” she said in an interview. “Good-quality sleep is important for health and reduces risk of chronic diseases; quality of sleep is therefore likely to be important for those with long COVID in reducing their risk of chronic disease, but the role of sleep in the mechanism of long COVID needs further research.”
In a commentary also published in The Lancet Respiratory Medicine, W. Cameron McGuire, MD, pulmonary and critical care specialist from San Diego, California, and colleagues wrote: “These findings suggest that sleep disturbance, dyspnea, and anxiety are common after COVID-19 and are associated with one another, although the underlying mechanisms remain unclear.”
The commentators “applauded” the work overall but noted that the findings represent correlation rather than causation. “It is unclear whether sleep disturbance is causing anxiety or whether anxiety is contributing to poor sleep. ... For the sleep disturbances, increased BMI in the cohort reporting poor sleep, compared with those reporting good sleep might suggest underlying obstructive sleep apnea,” they wrote.
Dr. McGuire and colleagues added that many questions remain for researchers and clinicians, including “whether anxiety and dyspnoea are contributing to a low arousal threshold [disrupting sleep] ... whether the observed abnormalities (e.g., in dyspnea score) are clinically significant,” and “whether therapies such as glucocorticoids, anticoagulants, or previous vaccinations mitigate the observed abnormalities during COVID-19 recovery.”
Dr. Blaikley has received support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; grants to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; and support from TEVA and Therakos for attending meetings. He is a committee member of the Royal Society of Medicine. A coauthor received funding from the National Institutes of Health and income for medical education from Zoll, Livanova, Jazz, and Eli Lilly. Dr. Banerjee is the chief investigator of STIMULATE-ICP (an NIHR-funded study) and has received research funding from AstraZeneca.
A version of this article first appeared on Medscape.com.
according to data from the U.K.’s CircCOVID study.
The researchers, led by John Blaikley, MRCP, PhD, respiratory physician and clinical scientist from the University of Manchester (England), found that sleep disturbance is a common problem after hospital admission for COVID-19 and may last for at least 1 year.
The study also showed that sleep disturbance after COVID hospitalization was associated with dyspnea and lower lung function. Further in-depth analysis revealed that the effects of sleep disturbance on dyspnea were partially mediated through both anxiety and muscle weakness; however, “this does not fully explain the association, suggesting other pathways are involved,” said Dr. Blaikley.
The study was jointly conducted by researchers from the University of Leicester (England), as well as 20 other U.K. institutes and the University of Helsinki. It was presented at the European Congress of Clinical Microbiology & Infectious Diseases and was simultaneously published in The Lancet Respiratory Medicine.
“Sleep disturbance is a common problem after hospitalization for COVID-19 and is associated with several symptoms in the post-COVID syndrome,” said Dr. Blaikley. “Clinicians should be aware of this association in their post-COVID syndrome clinics.”
He added that further work needs to be done to define the mechanism and to see whether the links are causal. “However, if they are, then treating sleep disturbance could have beneficial effects beyond improving sleep quality,” he said in an interview.
A large study recently showed that 4 in 10 people with post-COVID syndrome had moderate to severe sleep problems. Black people were at least three times more likely than White people to experience sleep problems. A total of 59% of all participants with long COVID reported having normal sleep or mild sleep disturbances, and 41% reported having moderate to severe sleep disturbances.
Unlike prior studies that evaluated sleep quality after COVID-19, which used either objective or subjective measures of sleep disturbance, the current study used both. “Using both measures revealed previously poorly described associations between sleep disturbance, breathlessness, reduced lung function, anxiety, and muscle weakness,” Dr. Blaikley pointed out.
Subjective and objective measures of sleep
The multicenter CircCOVID cohort study aimed to shed light on the prevalence and nature of sleep disturbance after patients are discharged from hospital for COVID-19 and to assess whether this was associated with dyspnea.
The study recruited a total of 2,320 participants who were part of a larger parent PHOSP-COVID study. After attending an early follow-up visit (at a median of 5 months after discharge from 83 U.K. hospitals for COVID-19), 638 participants provided data for analysis as measured by the Pittsburgh Sleep Quality Index (a subjective measure of sleep quality); 729 participants provided data for analysis as measured by actigraphy (an objective, wrist-worn, device-based measure of sleep quality) at a median of 7 months.
Breathlessness, the primary outcome, was assessed using the Dyspnea-12 validated questionnaire.
Actigraphy measurements were compared with an age-matched, sex-matched, body mass index (BMI)–matched, and time from discharge–matched cohort from the UK Biobank (a prepandemic comparator longitudinal cohort of 502,540 individuals, one-fifth of whom wore actigraphy devices). Sleep regularity was found to be 19% less in previously hospitalized patients with post-COVID syndrome, compared with matched controls who had been hospitalized for other reasons.
This “revealed that the actigraphy changes may be, in part, due to COVID-19 rather than hospitalization alone,” said Dr. Blaikley.
Data were collected at two time points after hospital discharge: 2-7 months (early), and 10-14 months (late). At the early time point, participants were clinically assessed with respect to anxiety, muscle function, and dyspnea, and lung function.
After discharge from hospital, the majority (62%) of post–COVID-19 participants reported poor sleep quality on the Pittsburgh Sleep Quality Index questionnaire. A “comparable” proportion (53%) felt that their quality of sleep had deteriorated following hospital discharge according to the numerical rating scale (subjective measure).
Also, sleep disturbance was found likely to persist for at least 12 months, since subjective sleep quality hardly changed between the early and late time points after hospital discharge.
Both subjective metrics (sleep quality and sleep quality deterioration after hospital discharge) and objective, device-based metrics (sleep regularity) were found to be associated with dyspnea and reduced lung function in patients with post-COVID syndrome.
“One of the striking findings in our study is the consistency with breathlessness and reduced lung function across different methods used to evaluate sleep,” highlighted Dr. Blaikley.
“The other striking finding was that participants following COVID-19 hospitalization actually slept longer [65 min; 95% confidence interval, 59-71 min] than participants hospitalized for non-COVID; however, their bedtimes were irregular, and it was this irregularity that was associated with breathlessness,” he added.
In comparison with nonhospitalized controls, also from the UK Biobank, study participants with lower sleep regularity had higher Dyspnea-12 scores (unadjusted effect estimate, 4.38; 95%: CI, 2.10-6.65). Those with poor sleep quality overall also had higher Dyspnea-12 scores (unadjusted effect estimate, 3.94; 95% CI, 2.78-5.10), and those who reported sleep quality deterioration had higher Dyspnea-12 scores (unadjusted effect estimate, 3,00; 95% CI, 1.82-4.28).
In comparison with hospitalized controls, CircCOVID participants had lower sleep regularity index (–19%; 95% CI, –20 to –16) and lower sleep efficiency (3.83 percentage points; 95% CI, 3.40-4.26).
Sleep disturbance after COVID hospitalization was also associated with lower lung function, from a 7% to a 14% reduction in predicted forced vital capacity, depending on which sleep measure used.
In an analysis of mediating factors active in the relationship between sleep disturbance and dyspnea/decreased lung function, the researchers found that reduced muscle function and anxiety, which are both recognized causes of dyspnea, could partially contribute to the association.
Regarding anxiety, and depending on the sleep metric, anxiety mediated 18%-39% of the effect of sleep disturbance on dyspnea, while muscle weakness mediated 27%-41% of this effect, reported Dr. Blaikley. Those with poor sleep quality were more likely to have mild, moderate, or severe anxiety, compared with participants who reported good-quality sleep.
A similar association was observed between anxiety and sleep quality deterioration.
“Two key questions are raised by our study: Do sleep interventions have a beneficial effect in post–COVID-19 syndrome, and are the associations causal?” asked Dr. Blaikley. “We hope to do a sleep intervention trial to answer these questions to explore if this is an effective treatment for post–COVID-19 syndrome.”
‘Underlying mechanisms remain unclear’
Amitava Banerjee, MD, professor of clinical data science and honorary consultant cardiologist, Institute of Health Informatics, UCL, London, welcomed the study but noted that it did not include nonhospitalized post-COVID patients.
“The majority of people with long COVID were not hospitalized for COVID, so the results may not be generalizable to this larger group,” she said in an interview. “Good-quality sleep is important for health and reduces risk of chronic diseases; quality of sleep is therefore likely to be important for those with long COVID in reducing their risk of chronic disease, but the role of sleep in the mechanism of long COVID needs further research.”
In a commentary also published in The Lancet Respiratory Medicine, W. Cameron McGuire, MD, pulmonary and critical care specialist from San Diego, California, and colleagues wrote: “These findings suggest that sleep disturbance, dyspnea, and anxiety are common after COVID-19 and are associated with one another, although the underlying mechanisms remain unclear.”
The commentators “applauded” the work overall but noted that the findings represent correlation rather than causation. “It is unclear whether sleep disturbance is causing anxiety or whether anxiety is contributing to poor sleep. ... For the sleep disturbances, increased BMI in the cohort reporting poor sleep, compared with those reporting good sleep might suggest underlying obstructive sleep apnea,” they wrote.
Dr. McGuire and colleagues added that many questions remain for researchers and clinicians, including “whether anxiety and dyspnoea are contributing to a low arousal threshold [disrupting sleep] ... whether the observed abnormalities (e.g., in dyspnea score) are clinically significant,” and “whether therapies such as glucocorticoids, anticoagulants, or previous vaccinations mitigate the observed abnormalities during COVID-19 recovery.”
Dr. Blaikley has received support to his institute from an MRC Transition Fellowship, Asthma + Lung UK, NIHR Manchester BRC, and UKRI; grants to his institution from the Small Business Research Initiative Home Spirometer and the National Institute of Academic Anaesthesia; and support from TEVA and Therakos for attending meetings. He is a committee member of the Royal Society of Medicine. A coauthor received funding from the National Institutes of Health and income for medical education from Zoll, Livanova, Jazz, and Eli Lilly. Dr. Banerjee is the chief investigator of STIMULATE-ICP (an NIHR-funded study) and has received research funding from AstraZeneca.
A version of this article first appeared on Medscape.com.
FROM ECCMID 2023
Over-the-scope clips in routine nonvariceal bleed still uncertain
when used as primary treatment in patients with high-risk nonvariceal upper gastrointestinal lesions, shows a randomized controlled trial (RCT).
However, noted the investigators, writing in Annals of Internal Medicine, and physicians who wrote an accompanying editorial, reservations remain about first-line use of OTSCs, but mostly relate to method, technique, and cost.
“The absolute difference in the rate of further bleeding was 11.4 percentage points. We should however be cautious in our recommendation of using OTSC as first-line treatment,” wrote researchers who were led by James Y.W. Lau, MD, from Prince of Wales Hospital, Chinese University of Hong Kong.
“The primary use of OTSCs may find a role in the treatment of ulcers predicted to fail standard endoscopic treatment,” the authors wrote. However, they emphasized that, “We are not advocating routine primary use of OTSCs. These clips are costly, and a formal cost analysis is not available in the literature. The use of OTSCs involves scope withdrawal, mounting of the OTSCs, and scope reinsertion, which increase the procedure time. Endoscopists also require training before using OTSCs.”
Alan N. Barkun, MD, gastroenterologist and professor of medicine with McGill University, Montreal, who cowrote the editorial accompanying the research paper, said the study investigators were highly experienced surgeon-scientists, pointing out that, overall, first-line use of OTSC in this patient group improved patient outcomes.
“The main message here is that if you can position the clip properly, then it is likely to stay in place, better than standard approaches,” he said, adding that, “I support it fully for second-line use but there currently still exists uncertainty for routine first-line adoption in nonvariceal bleeding. Clinicians fail to position the clip properly in around 5% of patients which is higher than standard endoscopic approaches, and nobody has yet clearly defined the lesions that are difficult to clip with the OTSC.
“If you’re going to tell people to use it, then you need to tell them with which particular lesions OTSC works best as first-line approach,” he added.
Lesions of concern include upon leaving the stomach and entering the duodenum, and in passing from the first to the second stage of the duodenum. “These are tight areas, and these larger full-thickness bite OTSC may create pseudo-polyps, even possibly causing obstruction. Perforation is also a risk.” One of each of these complications were noted in this study.
The study included 190 adult patients with active bleeding or a nonbleeding visible vessel from a nonvariceal cause on upper gastrointestinal endoscopy. Of these, 97 patients received standard hemostatic treatment and 93 received OTSC. The primary endpoint of a 30-day probability of further bleeding was 14.6% in the standard treatment and 3.2% in the OTSC group (risk difference, 11.4 percentage points [95% confidence interval (CI), 3.3-20.0 percentage points]; P = .006). Failure to control bleeding after assigned endoscopic treatment in the standard treatment and OTSC groups was 6 versus 1 in the standard treatment and OTSC groups, respectively. Thirty-day recurrent bleeding was 8 versus 2 in the standard treatment and OTSC groups, respectively. Eight patients in the standard treatment group needed further intervention compared with two in the OTSC group. Thirty-day mortality was four versus two, respectively.
“First-line OTSC has a role to play but whether it is the best approach is hard to say due to methodological limitations that were seen in this and earlier studies, however if you can position the clip properly it likely does well,” Dr. Barkun said.
Dr. Lau declares that he received honorarium for a lecture from OVESCO. Dr. Li has no disclosures. Dr. Barkun has no relevant disclosures.
when used as primary treatment in patients with high-risk nonvariceal upper gastrointestinal lesions, shows a randomized controlled trial (RCT).
However, noted the investigators, writing in Annals of Internal Medicine, and physicians who wrote an accompanying editorial, reservations remain about first-line use of OTSCs, but mostly relate to method, technique, and cost.
“The absolute difference in the rate of further bleeding was 11.4 percentage points. We should however be cautious in our recommendation of using OTSC as first-line treatment,” wrote researchers who were led by James Y.W. Lau, MD, from Prince of Wales Hospital, Chinese University of Hong Kong.
“The primary use of OTSCs may find a role in the treatment of ulcers predicted to fail standard endoscopic treatment,” the authors wrote. However, they emphasized that, “We are not advocating routine primary use of OTSCs. These clips are costly, and a formal cost analysis is not available in the literature. The use of OTSCs involves scope withdrawal, mounting of the OTSCs, and scope reinsertion, which increase the procedure time. Endoscopists also require training before using OTSCs.”
Alan N. Barkun, MD, gastroenterologist and professor of medicine with McGill University, Montreal, who cowrote the editorial accompanying the research paper, said the study investigators were highly experienced surgeon-scientists, pointing out that, overall, first-line use of OTSC in this patient group improved patient outcomes.
“The main message here is that if you can position the clip properly, then it is likely to stay in place, better than standard approaches,” he said, adding that, “I support it fully for second-line use but there currently still exists uncertainty for routine first-line adoption in nonvariceal bleeding. Clinicians fail to position the clip properly in around 5% of patients which is higher than standard endoscopic approaches, and nobody has yet clearly defined the lesions that are difficult to clip with the OTSC.
“If you’re going to tell people to use it, then you need to tell them with which particular lesions OTSC works best as first-line approach,” he added.
Lesions of concern include upon leaving the stomach and entering the duodenum, and in passing from the first to the second stage of the duodenum. “These are tight areas, and these larger full-thickness bite OTSC may create pseudo-polyps, even possibly causing obstruction. Perforation is also a risk.” One of each of these complications were noted in this study.
The study included 190 adult patients with active bleeding or a nonbleeding visible vessel from a nonvariceal cause on upper gastrointestinal endoscopy. Of these, 97 patients received standard hemostatic treatment and 93 received OTSC. The primary endpoint of a 30-day probability of further bleeding was 14.6% in the standard treatment and 3.2% in the OTSC group (risk difference, 11.4 percentage points [95% confidence interval (CI), 3.3-20.0 percentage points]; P = .006). Failure to control bleeding after assigned endoscopic treatment in the standard treatment and OTSC groups was 6 versus 1 in the standard treatment and OTSC groups, respectively. Thirty-day recurrent bleeding was 8 versus 2 in the standard treatment and OTSC groups, respectively. Eight patients in the standard treatment group needed further intervention compared with two in the OTSC group. Thirty-day mortality was four versus two, respectively.
“First-line OTSC has a role to play but whether it is the best approach is hard to say due to methodological limitations that were seen in this and earlier studies, however if you can position the clip properly it likely does well,” Dr. Barkun said.
Dr. Lau declares that he received honorarium for a lecture from OVESCO. Dr. Li has no disclosures. Dr. Barkun has no relevant disclosures.
when used as primary treatment in patients with high-risk nonvariceal upper gastrointestinal lesions, shows a randomized controlled trial (RCT).
However, noted the investigators, writing in Annals of Internal Medicine, and physicians who wrote an accompanying editorial, reservations remain about first-line use of OTSCs, but mostly relate to method, technique, and cost.
“The absolute difference in the rate of further bleeding was 11.4 percentage points. We should however be cautious in our recommendation of using OTSC as first-line treatment,” wrote researchers who were led by James Y.W. Lau, MD, from Prince of Wales Hospital, Chinese University of Hong Kong.
“The primary use of OTSCs may find a role in the treatment of ulcers predicted to fail standard endoscopic treatment,” the authors wrote. However, they emphasized that, “We are not advocating routine primary use of OTSCs. These clips are costly, and a formal cost analysis is not available in the literature. The use of OTSCs involves scope withdrawal, mounting of the OTSCs, and scope reinsertion, which increase the procedure time. Endoscopists also require training before using OTSCs.”
Alan N. Barkun, MD, gastroenterologist and professor of medicine with McGill University, Montreal, who cowrote the editorial accompanying the research paper, said the study investigators were highly experienced surgeon-scientists, pointing out that, overall, first-line use of OTSC in this patient group improved patient outcomes.
“The main message here is that if you can position the clip properly, then it is likely to stay in place, better than standard approaches,” he said, adding that, “I support it fully for second-line use but there currently still exists uncertainty for routine first-line adoption in nonvariceal bleeding. Clinicians fail to position the clip properly in around 5% of patients which is higher than standard endoscopic approaches, and nobody has yet clearly defined the lesions that are difficult to clip with the OTSC.
“If you’re going to tell people to use it, then you need to tell them with which particular lesions OTSC works best as first-line approach,” he added.
Lesions of concern include upon leaving the stomach and entering the duodenum, and in passing from the first to the second stage of the duodenum. “These are tight areas, and these larger full-thickness bite OTSC may create pseudo-polyps, even possibly causing obstruction. Perforation is also a risk.” One of each of these complications were noted in this study.
The study included 190 adult patients with active bleeding or a nonbleeding visible vessel from a nonvariceal cause on upper gastrointestinal endoscopy. Of these, 97 patients received standard hemostatic treatment and 93 received OTSC. The primary endpoint of a 30-day probability of further bleeding was 14.6% in the standard treatment and 3.2% in the OTSC group (risk difference, 11.4 percentage points [95% confidence interval (CI), 3.3-20.0 percentage points]; P = .006). Failure to control bleeding after assigned endoscopic treatment in the standard treatment and OTSC groups was 6 versus 1 in the standard treatment and OTSC groups, respectively. Thirty-day recurrent bleeding was 8 versus 2 in the standard treatment and OTSC groups, respectively. Eight patients in the standard treatment group needed further intervention compared with two in the OTSC group. Thirty-day mortality was four versus two, respectively.
“First-line OTSC has a role to play but whether it is the best approach is hard to say due to methodological limitations that were seen in this and earlier studies, however if you can position the clip properly it likely does well,” Dr. Barkun said.
Dr. Lau declares that he received honorarium for a lecture from OVESCO. Dr. Li has no disclosures. Dr. Barkun has no relevant disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Could a baby’s gut health be an early predictor of future type 1 diabetes?
Microbial biomarkers for type 1 diabetes may be present in infants as young as 12 months old, suggesting the potential to mitigate disease onset by nurturing a healthy gut microbiome early, show data from the Swedish general population.
“Our findings indicate that the gut of infants who go on to develop type 1 diabetes is notably different from healthy babies,” said Malin Bélteky, MD, from the Crown Princess Victoria’s Children’s Hospital, Linköping, Sweden, who jointly led the work, which was recently published in Diabetologia, alongside Patricia L. Milletich, PhD candidate, from the University of Florida, Gainesville.
“This discovery could be used to help identity infants at [the] highest risk of developing type 1 diabetes before or during the first stage of disease and could offer the opportunity to bolster a healthy gut microbiome to prevent the disease from becoming established,” added Dr. Bélteky.
Currently, beta-cell autoantibodies are used to predict disease, which are usually only identifiable between 9 and 36 months of age.
Marian Rewers, MD, PhD, professor of pediatrics & medicine, University of Colorado, Denver, and principal investigator of The Environmental Determinants of Diabetes in the Young (TEDDY) study, welcomed the findings, saying it is a well-designed study from a strong group of investigators.
“While the effective number of cases was very small [n = 16], the results were apparently adjusted for multiple comparisons, and significant differences were noted in the microbiome of cases versus controls at 1 year of age. This was 12 years prior to the average age of type 1 diabetes diagnosis in the cases,” he said.
“The differences in diversity and abundances of specific bacteria need to be interpreted with caution; however, the study results are consistent with several previous reports,” he noted.
Differences in microbial diversity and function
Data were drawn from children participating in the longitudinal, general population All Babies In Southeast Sweden (ABIS) study. Microbiota from stool samples, taken at age 1 year, were sequenced and analyzed to establish diversity, abundance, and functional status of the component bacteria. Questionnaires were completed at birth and at 1 year of age, allowing for the study of environmental factors that might influence the microbiota or type 1 diabetes risk independently. Parent diaries provided information on pregnancy, nutrition, and lifestyle factors.
Of the cohort of 167 children who developed type 1 diabetes by 2020, stool samples were available for 16 of these participants, which were compared with 268 healthy controls. The microbiomes of the 16 infants who later developed type 1 diabetes were compared with 100 iterations of 32 matched control infants (matched by geographical region, siblings at birth, residence type, duration of breastfeeding, and month of stool collection) who didn’t develop type 1 diabetes by the age of 20.
Specific bacteria found in greater abundance in children who later developed type 1 diabetes, compared with those who didn’t, included Firmicutes (Enterococcus, Gemella, and Hungatella), as well as Bacteroides (Bacteroides and Porphyromonas), known to promote inflammation and be involved in the immune response.
Bacteria with greater abundance in children who didn’t develop type 1 diabetes, compared with those who did, were Firmicutes (Anaerostipes, Flavonifractor, and Ruminococcaceae UBA1819, and Eubacterium). These species help maintain metabolic and immune health and produce butyrate, an important short-chain fatty acid that helps prevent inflammation and fuels the cells of the gut lining.
Alistipes were more abundant in infants who didn’t develop type 1 diabetes, and various abundances of Fusicatenibacter were the strongest factors for differentiating future type 1 diabetes, reported the researchers.
“Gut microbial biomarkers at 12 months would benefit the prediction opportunity well before the onset of multiple autoantibodies,” write the authors.
The youngest age at type 1 diabetes diagnosis was aged 1 year, 4 months, and the oldest was aged 21 years, 4 months. The mean age at diagnosis was 13.3 years.
The microbial differences found between infants who go on to develop type 1 diabetes and those who don’t also shed light on interactions between the developing immune system and short-chain fatty acid production and metabolism in childhood autoimmunity, write the authors.
Prior studies have found fewer short-chain fatty acid–producing microbiota in the gut of children with early-onset autoantibody development. This study confirmed these data, finding a decrease in butyrate-producing bacteria (Anaerostipes, Flavonifractor, Ruminococcaceae UBA1819, and Eubacterium) in infants who went on to develop type 1 diabetes. Likewise, a reduction in pyruvate fermentation was found in those infants with future disease.
According to coauthor Eric Triplett, PhD, from the University of Florida, Gainesville: “The autoimmune processes usually begin long before any clinical signs of disease appear, highlighting how differences in the makeup of the infant gut microbiome could shed important light on the complex interaction between the developing immune system, environmental exposures in childhood, and autoimmunity. Studies with much larger cohorts of prospectively traced individuals will be required to establish which are the strongest biomarkers and how effectively they can predict disease.”
The authors and Dr. Rewers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Microbial biomarkers for type 1 diabetes may be present in infants as young as 12 months old, suggesting the potential to mitigate disease onset by nurturing a healthy gut microbiome early, show data from the Swedish general population.
“Our findings indicate that the gut of infants who go on to develop type 1 diabetes is notably different from healthy babies,” said Malin Bélteky, MD, from the Crown Princess Victoria’s Children’s Hospital, Linköping, Sweden, who jointly led the work, which was recently published in Diabetologia, alongside Patricia L. Milletich, PhD candidate, from the University of Florida, Gainesville.
“This discovery could be used to help identity infants at [the] highest risk of developing type 1 diabetes before or during the first stage of disease and could offer the opportunity to bolster a healthy gut microbiome to prevent the disease from becoming established,” added Dr. Bélteky.
Currently, beta-cell autoantibodies are used to predict disease, which are usually only identifiable between 9 and 36 months of age.
Marian Rewers, MD, PhD, professor of pediatrics & medicine, University of Colorado, Denver, and principal investigator of The Environmental Determinants of Diabetes in the Young (TEDDY) study, welcomed the findings, saying it is a well-designed study from a strong group of investigators.
“While the effective number of cases was very small [n = 16], the results were apparently adjusted for multiple comparisons, and significant differences were noted in the microbiome of cases versus controls at 1 year of age. This was 12 years prior to the average age of type 1 diabetes diagnosis in the cases,” he said.
“The differences in diversity and abundances of specific bacteria need to be interpreted with caution; however, the study results are consistent with several previous reports,” he noted.
Differences in microbial diversity and function
Data were drawn from children participating in the longitudinal, general population All Babies In Southeast Sweden (ABIS) study. Microbiota from stool samples, taken at age 1 year, were sequenced and analyzed to establish diversity, abundance, and functional status of the component bacteria. Questionnaires were completed at birth and at 1 year of age, allowing for the study of environmental factors that might influence the microbiota or type 1 diabetes risk independently. Parent diaries provided information on pregnancy, nutrition, and lifestyle factors.
Of the cohort of 167 children who developed type 1 diabetes by 2020, stool samples were available for 16 of these participants, which were compared with 268 healthy controls. The microbiomes of the 16 infants who later developed type 1 diabetes were compared with 100 iterations of 32 matched control infants (matched by geographical region, siblings at birth, residence type, duration of breastfeeding, and month of stool collection) who didn’t develop type 1 diabetes by the age of 20.
Specific bacteria found in greater abundance in children who later developed type 1 diabetes, compared with those who didn’t, included Firmicutes (Enterococcus, Gemella, and Hungatella), as well as Bacteroides (Bacteroides and Porphyromonas), known to promote inflammation and be involved in the immune response.
Bacteria with greater abundance in children who didn’t develop type 1 diabetes, compared with those who did, were Firmicutes (Anaerostipes, Flavonifractor, and Ruminococcaceae UBA1819, and Eubacterium). These species help maintain metabolic and immune health and produce butyrate, an important short-chain fatty acid that helps prevent inflammation and fuels the cells of the gut lining.
Alistipes were more abundant in infants who didn’t develop type 1 diabetes, and various abundances of Fusicatenibacter were the strongest factors for differentiating future type 1 diabetes, reported the researchers.
“Gut microbial biomarkers at 12 months would benefit the prediction opportunity well before the onset of multiple autoantibodies,” write the authors.
The youngest age at type 1 diabetes diagnosis was aged 1 year, 4 months, and the oldest was aged 21 years, 4 months. The mean age at diagnosis was 13.3 years.
The microbial differences found between infants who go on to develop type 1 diabetes and those who don’t also shed light on interactions between the developing immune system and short-chain fatty acid production and metabolism in childhood autoimmunity, write the authors.
Prior studies have found fewer short-chain fatty acid–producing microbiota in the gut of children with early-onset autoantibody development. This study confirmed these data, finding a decrease in butyrate-producing bacteria (Anaerostipes, Flavonifractor, Ruminococcaceae UBA1819, and Eubacterium) in infants who went on to develop type 1 diabetes. Likewise, a reduction in pyruvate fermentation was found in those infants with future disease.
According to coauthor Eric Triplett, PhD, from the University of Florida, Gainesville: “The autoimmune processes usually begin long before any clinical signs of disease appear, highlighting how differences in the makeup of the infant gut microbiome could shed important light on the complex interaction between the developing immune system, environmental exposures in childhood, and autoimmunity. Studies with much larger cohorts of prospectively traced individuals will be required to establish which are the strongest biomarkers and how effectively they can predict disease.”
The authors and Dr. Rewers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Microbial biomarkers for type 1 diabetes may be present in infants as young as 12 months old, suggesting the potential to mitigate disease onset by nurturing a healthy gut microbiome early, show data from the Swedish general population.
“Our findings indicate that the gut of infants who go on to develop type 1 diabetes is notably different from healthy babies,” said Malin Bélteky, MD, from the Crown Princess Victoria’s Children’s Hospital, Linköping, Sweden, who jointly led the work, which was recently published in Diabetologia, alongside Patricia L. Milletich, PhD candidate, from the University of Florida, Gainesville.
“This discovery could be used to help identity infants at [the] highest risk of developing type 1 diabetes before or during the first stage of disease and could offer the opportunity to bolster a healthy gut microbiome to prevent the disease from becoming established,” added Dr. Bélteky.
Currently, beta-cell autoantibodies are used to predict disease, which are usually only identifiable between 9 and 36 months of age.
Marian Rewers, MD, PhD, professor of pediatrics & medicine, University of Colorado, Denver, and principal investigator of The Environmental Determinants of Diabetes in the Young (TEDDY) study, welcomed the findings, saying it is a well-designed study from a strong group of investigators.
“While the effective number of cases was very small [n = 16], the results were apparently adjusted for multiple comparisons, and significant differences were noted in the microbiome of cases versus controls at 1 year of age. This was 12 years prior to the average age of type 1 diabetes diagnosis in the cases,” he said.
“The differences in diversity and abundances of specific bacteria need to be interpreted with caution; however, the study results are consistent with several previous reports,” he noted.
Differences in microbial diversity and function
Data were drawn from children participating in the longitudinal, general population All Babies In Southeast Sweden (ABIS) study. Microbiota from stool samples, taken at age 1 year, were sequenced and analyzed to establish diversity, abundance, and functional status of the component bacteria. Questionnaires were completed at birth and at 1 year of age, allowing for the study of environmental factors that might influence the microbiota or type 1 diabetes risk independently. Parent diaries provided information on pregnancy, nutrition, and lifestyle factors.
Of the cohort of 167 children who developed type 1 diabetes by 2020, stool samples were available for 16 of these participants, which were compared with 268 healthy controls. The microbiomes of the 16 infants who later developed type 1 diabetes were compared with 100 iterations of 32 matched control infants (matched by geographical region, siblings at birth, residence type, duration of breastfeeding, and month of stool collection) who didn’t develop type 1 diabetes by the age of 20.
Specific bacteria found in greater abundance in children who later developed type 1 diabetes, compared with those who didn’t, included Firmicutes (Enterococcus, Gemella, and Hungatella), as well as Bacteroides (Bacteroides and Porphyromonas), known to promote inflammation and be involved in the immune response.
Bacteria with greater abundance in children who didn’t develop type 1 diabetes, compared with those who did, were Firmicutes (Anaerostipes, Flavonifractor, and Ruminococcaceae UBA1819, and Eubacterium). These species help maintain metabolic and immune health and produce butyrate, an important short-chain fatty acid that helps prevent inflammation and fuels the cells of the gut lining.
Alistipes were more abundant in infants who didn’t develop type 1 diabetes, and various abundances of Fusicatenibacter were the strongest factors for differentiating future type 1 diabetes, reported the researchers.
“Gut microbial biomarkers at 12 months would benefit the prediction opportunity well before the onset of multiple autoantibodies,” write the authors.
The youngest age at type 1 diabetes diagnosis was aged 1 year, 4 months, and the oldest was aged 21 years, 4 months. The mean age at diagnosis was 13.3 years.
The microbial differences found between infants who go on to develop type 1 diabetes and those who don’t also shed light on interactions between the developing immune system and short-chain fatty acid production and metabolism in childhood autoimmunity, write the authors.
Prior studies have found fewer short-chain fatty acid–producing microbiota in the gut of children with early-onset autoantibody development. This study confirmed these data, finding a decrease in butyrate-producing bacteria (Anaerostipes, Flavonifractor, Ruminococcaceae UBA1819, and Eubacterium) in infants who went on to develop type 1 diabetes. Likewise, a reduction in pyruvate fermentation was found in those infants with future disease.
According to coauthor Eric Triplett, PhD, from the University of Florida, Gainesville: “The autoimmune processes usually begin long before any clinical signs of disease appear, highlighting how differences in the makeup of the infant gut microbiome could shed important light on the complex interaction between the developing immune system, environmental exposures in childhood, and autoimmunity. Studies with much larger cohorts of prospectively traced individuals will be required to establish which are the strongest biomarkers and how effectively they can predict disease.”
The authors and Dr. Rewers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Crohn’s disease remission rates ‘remarkably higher’ with vedolizumab
COPENHAGEN –
The study was presented at the annual congress of the European Crohn’s and Colitis Organization by Wolfgang Mohl, MD, of the Center for Gastroenterology in Saarbrucken, Germany, who suggested this biologic, which is a monoclonal antibody, could possibly be used as a first-line treatment instead of as a second or third choice. Currently, TNF inhibitors are generally prescribed first.
“Compared to previous research clinical trials, this prospective 2-year real-world study comparing vedolizumab with anti-TNF showed that, in biologic-naive Crohn’s disease patients, remission rates at 2 years with vedolizumab were remarkably higher than with anti-TNF [therapy],” Dr. Mohl and colleagues wrote in the study abstract.
“Now we know vedolizumab is a good first-line drug and that patients can stay on it for a long time,” he said in an interview. “These data also suggest that we are wrong in thinking TNF inhibitors should be standard. I don’t think this belief holds true anymore.”
The study included 63 biologic-naive patients who were treated with vedolizumab and 197 patients who were treated with anti-TNF agents adalimumab (58.4%) and infliximab (41.6%).
After 2 years, approximately 83% of patients who were treated with vedolizumab were still receiving treatment, but only 56% of patients who received anti-TNF therapy were still undergoing therapy with either adalimumab or infliximab. After 2 years of treatment, 64.2% of patients who were treated with vedolizumab were in clinical remission, compared with 44.7% of patients who were treated with anti-TNF therapy. And, 62.5% of patients who were treated with vedolizumab were not receiving steroid treatment, compared with 41.6% of patients in the anti-TNF therapy group. This, Dr. Mohl said, was a statistically significant difference (P < .05).
“It is clinically relevant to achieve remission without steroids because this is hard to obtain,” he said. “Patients really don’t want to have to take steroids because they can experience lots of side effects including osteoporosis. It’s good to be in remission, but to be in steroid-free remission is so much better.”
Vedolizumab is a relatively new drug, compared with infliximab and adalimumab, which were approved by the Food and Drug Administration in 1998 and 2008, respectively. “We wanted real-world data to help us understand the pattern of outcomes outside of the clinical trial environment,” Dr. Mohl said.
From 45 treatment centers across Germany, researchers prospectively enrolled 1,200 biologic-naive and biologic-experienced patients with either Crohn’s disease or ulcerative colitis between 2017 and 2020 into the VEDOIBD study. This analysis was limited to 260 patients with Crohn’s disease.
In addition to a higher proportion of patients on vedolizumab continuing on treatment, compared with patients on anti–TNF inhibitor therapy, there was a significantly higher clinical remission rate with vedolizumab (64.2%), compared with anti-TNFi therapy (44.7%) after 2 years (P < .05). Researchers used a statistical method to determine the effect of 2-year maintenance in only those patients who responded to a 3-month induction, and they found a significantly better response in terms of clinical remission in patients on vedolizumab (88.6%), compared with anti-TNF inhibitors (45.8%) (P = .0001), and likewise in steroid-free remission with 86.8% for vedolizumab, compared with 44.1% for anti-TNF inhibitors (P < .001).
Dr. Mohl described his experience with vedolizumab in clinical practice. “Vedolizumab may take a little longer to work but then we don’t lose patients due to side effects, which we see more often with anti-TNF therapy,” he said, adding that around 60% of patients experience side effects but around 10% actually stop anti-TNF because of side effects.
“We often lose patients because they develop antidrug antibodies, but also due to escape mechanisms, as well as dermatological side effects including psoriasis which is really annoying for patients. We also find that anti-TNF drugs just stop working after 12-18 months, and then we need to use steroids which patients dislike,” he said.
Andreas Stallmach, MD, director of gastroenterology, Friedrich Schiller University Jena (Germany), described the findings as important.
“I see this as a really important real-world data study and to summarize, vedolizumab in Crohn’s disease is better than expected. The main explanation for the difference is due to loss of response in the anti-TNF group and this could be explained by the development of autoantibodies against anti-TNF drugs. Now, vedolizumab could be a first-line treatment in patients with Crohn’s disease, especially patients with risk factors for, or history of infections, of comorbidities,” he said.
As a modern monoclonal antibody, vedolizumab uses fewer autoantibodies, compared with infliximab, which is much older, Dr. Stallmach said. “If we combine infliximab with an immunosuppressant agent, such as azathioprine, then we can prevent autoantibody development and increase the efficacy and adherence rate, but with this comes the increased risk of infections and malignancies.”
Dr. Mohl receives research support from companies involved in making biologics for inflammatory bowel disease. Dr. Stallmach is on the advisory boards of most companies that make biologics, including Takeda, which sponsored this study.
* This article was updated March 10, 2023.
COPENHAGEN –
The study was presented at the annual congress of the European Crohn’s and Colitis Organization by Wolfgang Mohl, MD, of the Center for Gastroenterology in Saarbrucken, Germany, who suggested this biologic, which is a monoclonal antibody, could possibly be used as a first-line treatment instead of as a second or third choice. Currently, TNF inhibitors are generally prescribed first.
“Compared to previous research clinical trials, this prospective 2-year real-world study comparing vedolizumab with anti-TNF showed that, in biologic-naive Crohn’s disease patients, remission rates at 2 years with vedolizumab were remarkably higher than with anti-TNF [therapy],” Dr. Mohl and colleagues wrote in the study abstract.
“Now we know vedolizumab is a good first-line drug and that patients can stay on it for a long time,” he said in an interview. “These data also suggest that we are wrong in thinking TNF inhibitors should be standard. I don’t think this belief holds true anymore.”
The study included 63 biologic-naive patients who were treated with vedolizumab and 197 patients who were treated with anti-TNF agents adalimumab (58.4%) and infliximab (41.6%).
After 2 years, approximately 83% of patients who were treated with vedolizumab were still receiving treatment, but only 56% of patients who received anti-TNF therapy were still undergoing therapy with either adalimumab or infliximab. After 2 years of treatment, 64.2% of patients who were treated with vedolizumab were in clinical remission, compared with 44.7% of patients who were treated with anti-TNF therapy. And, 62.5% of patients who were treated with vedolizumab were not receiving steroid treatment, compared with 41.6% of patients in the anti-TNF therapy group. This, Dr. Mohl said, was a statistically significant difference (P < .05).
“It is clinically relevant to achieve remission without steroids because this is hard to obtain,” he said. “Patients really don’t want to have to take steroids because they can experience lots of side effects including osteoporosis. It’s good to be in remission, but to be in steroid-free remission is so much better.”
Vedolizumab is a relatively new drug, compared with infliximab and adalimumab, which were approved by the Food and Drug Administration in 1998 and 2008, respectively. “We wanted real-world data to help us understand the pattern of outcomes outside of the clinical trial environment,” Dr. Mohl said.
From 45 treatment centers across Germany, researchers prospectively enrolled 1,200 biologic-naive and biologic-experienced patients with either Crohn’s disease or ulcerative colitis between 2017 and 2020 into the VEDOIBD study. This analysis was limited to 260 patients with Crohn’s disease.
In addition to a higher proportion of patients on vedolizumab continuing on treatment, compared with patients on anti–TNF inhibitor therapy, there was a significantly higher clinical remission rate with vedolizumab (64.2%), compared with anti-TNFi therapy (44.7%) after 2 years (P < .05). Researchers used a statistical method to determine the effect of 2-year maintenance in only those patients who responded to a 3-month induction, and they found a significantly better response in terms of clinical remission in patients on vedolizumab (88.6%), compared with anti-TNF inhibitors (45.8%) (P = .0001), and likewise in steroid-free remission with 86.8% for vedolizumab, compared with 44.1% for anti-TNF inhibitors (P < .001).
Dr. Mohl described his experience with vedolizumab in clinical practice. “Vedolizumab may take a little longer to work but then we don’t lose patients due to side effects, which we see more often with anti-TNF therapy,” he said, adding that around 60% of patients experience side effects but around 10% actually stop anti-TNF because of side effects.
“We often lose patients because they develop antidrug antibodies, but also due to escape mechanisms, as well as dermatological side effects including psoriasis which is really annoying for patients. We also find that anti-TNF drugs just stop working after 12-18 months, and then we need to use steroids which patients dislike,” he said.
Andreas Stallmach, MD, director of gastroenterology, Friedrich Schiller University Jena (Germany), described the findings as important.
“I see this as a really important real-world data study and to summarize, vedolizumab in Crohn’s disease is better than expected. The main explanation for the difference is due to loss of response in the anti-TNF group and this could be explained by the development of autoantibodies against anti-TNF drugs. Now, vedolizumab could be a first-line treatment in patients with Crohn’s disease, especially patients with risk factors for, or history of infections, of comorbidities,” he said.
As a modern monoclonal antibody, vedolizumab uses fewer autoantibodies, compared with infliximab, which is much older, Dr. Stallmach said. “If we combine infliximab with an immunosuppressant agent, such as azathioprine, then we can prevent autoantibody development and increase the efficacy and adherence rate, but with this comes the increased risk of infections and malignancies.”
Dr. Mohl receives research support from companies involved in making biologics for inflammatory bowel disease. Dr. Stallmach is on the advisory boards of most companies that make biologics, including Takeda, which sponsored this study.
* This article was updated March 10, 2023.
COPENHAGEN –
The study was presented at the annual congress of the European Crohn’s and Colitis Organization by Wolfgang Mohl, MD, of the Center for Gastroenterology in Saarbrucken, Germany, who suggested this biologic, which is a monoclonal antibody, could possibly be used as a first-line treatment instead of as a second or third choice. Currently, TNF inhibitors are generally prescribed first.
“Compared to previous research clinical trials, this prospective 2-year real-world study comparing vedolizumab with anti-TNF showed that, in biologic-naive Crohn’s disease patients, remission rates at 2 years with vedolizumab were remarkably higher than with anti-TNF [therapy],” Dr. Mohl and colleagues wrote in the study abstract.
“Now we know vedolizumab is a good first-line drug and that patients can stay on it for a long time,” he said in an interview. “These data also suggest that we are wrong in thinking TNF inhibitors should be standard. I don’t think this belief holds true anymore.”
The study included 63 biologic-naive patients who were treated with vedolizumab and 197 patients who were treated with anti-TNF agents adalimumab (58.4%) and infliximab (41.6%).
After 2 years, approximately 83% of patients who were treated with vedolizumab were still receiving treatment, but only 56% of patients who received anti-TNF therapy were still undergoing therapy with either adalimumab or infliximab. After 2 years of treatment, 64.2% of patients who were treated with vedolizumab were in clinical remission, compared with 44.7% of patients who were treated with anti-TNF therapy. And, 62.5% of patients who were treated with vedolizumab were not receiving steroid treatment, compared with 41.6% of patients in the anti-TNF therapy group. This, Dr. Mohl said, was a statistically significant difference (P < .05).
“It is clinically relevant to achieve remission without steroids because this is hard to obtain,” he said. “Patients really don’t want to have to take steroids because they can experience lots of side effects including osteoporosis. It’s good to be in remission, but to be in steroid-free remission is so much better.”
Vedolizumab is a relatively new drug, compared with infliximab and adalimumab, which were approved by the Food and Drug Administration in 1998 and 2008, respectively. “We wanted real-world data to help us understand the pattern of outcomes outside of the clinical trial environment,” Dr. Mohl said.
From 45 treatment centers across Germany, researchers prospectively enrolled 1,200 biologic-naive and biologic-experienced patients with either Crohn’s disease or ulcerative colitis between 2017 and 2020 into the VEDOIBD study. This analysis was limited to 260 patients with Crohn’s disease.
In addition to a higher proportion of patients on vedolizumab continuing on treatment, compared with patients on anti–TNF inhibitor therapy, there was a significantly higher clinical remission rate with vedolizumab (64.2%), compared with anti-TNFi therapy (44.7%) after 2 years (P < .05). Researchers used a statistical method to determine the effect of 2-year maintenance in only those patients who responded to a 3-month induction, and they found a significantly better response in terms of clinical remission in patients on vedolizumab (88.6%), compared with anti-TNF inhibitors (45.8%) (P = .0001), and likewise in steroid-free remission with 86.8% for vedolizumab, compared with 44.1% for anti-TNF inhibitors (P < .001).
Dr. Mohl described his experience with vedolizumab in clinical practice. “Vedolizumab may take a little longer to work but then we don’t lose patients due to side effects, which we see more often with anti-TNF therapy,” he said, adding that around 60% of patients experience side effects but around 10% actually stop anti-TNF because of side effects.
“We often lose patients because they develop antidrug antibodies, but also due to escape mechanisms, as well as dermatological side effects including psoriasis which is really annoying for patients. We also find that anti-TNF drugs just stop working after 12-18 months, and then we need to use steroids which patients dislike,” he said.
Andreas Stallmach, MD, director of gastroenterology, Friedrich Schiller University Jena (Germany), described the findings as important.
“I see this as a really important real-world data study and to summarize, vedolizumab in Crohn’s disease is better than expected. The main explanation for the difference is due to loss of response in the anti-TNF group and this could be explained by the development of autoantibodies against anti-TNF drugs. Now, vedolizumab could be a first-line treatment in patients with Crohn’s disease, especially patients with risk factors for, or history of infections, of comorbidities,” he said.
As a modern monoclonal antibody, vedolizumab uses fewer autoantibodies, compared with infliximab, which is much older, Dr. Stallmach said. “If we combine infliximab with an immunosuppressant agent, such as azathioprine, then we can prevent autoantibody development and increase the efficacy and adherence rate, but with this comes the increased risk of infections and malignancies.”
Dr. Mohl receives research support from companies involved in making biologics for inflammatory bowel disease. Dr. Stallmach is on the advisory boards of most companies that make biologics, including Takeda, which sponsored this study.
* This article was updated March 10, 2023.
AT ECCO 2023