User login
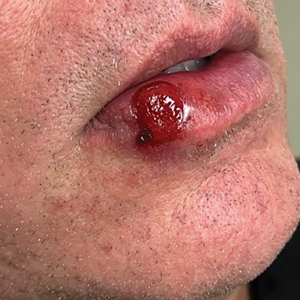
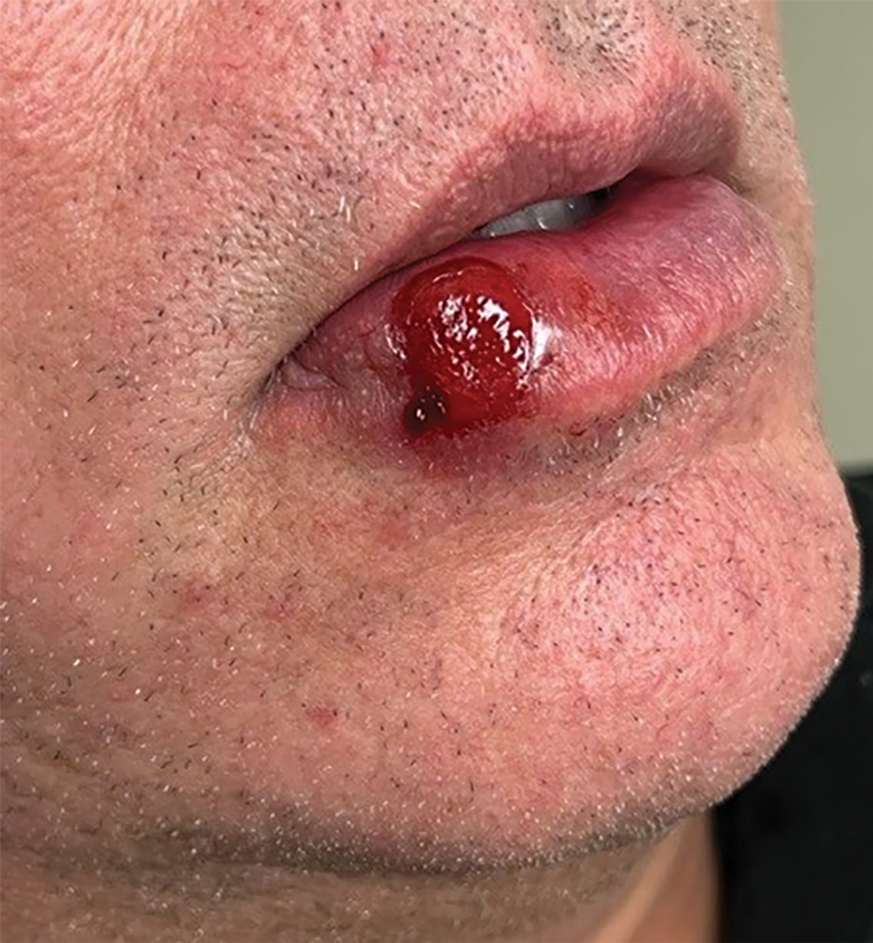
Nonhealing Ulcer on the Lower Lip
Nonhealing Ulcer on the Lower Lip
THE DIAGNOSIS: Syphilis
The differential diagnosis of oral lesions can be complex; in our patient, we considered conditions such as pyogenic granuloma, herpes simplex virus, and syphilis, despite the presence of pain. Immunohistochemical staining for spirochete antigens was positive, and serologic confirmation through a positive rapid plasma reagin (RPR) test confirmed the diagnosis of primary syphilis. The patient was promptly referred back to the primary care physician for treatment with intramuscular penicillin, leading to resolution of the lesion. At 3 months’ follow-up in our clinic, the lesion was fully resolved.
A primary syphilitic chancre is the initial lesion caused by Treponema pallidum, typically manifesting as a painless ulcer at the infection site, usually in the genital area; however, chancres also may manifest in other locations (eg, the anus or oral cavity) due to direct contact with infectious lesions on another individual. Our case represents an atypical presentation of an oral syphilitic chancre.
Syphilis is a sexually transmitted infection with various clinical manifestations. It is crucial to consider syphilis in the differential diagnosis of ulcerative lesions even when pain is present, especially in high-risk individuals such as those who engage in unprotected sex.1,2 Oral syphilitic chancres have been documented in the medical literature for more than a century, underscoring the importance of maintaining a high index of suspicion for diagnosis and a low threshold for obtaining an RPR test to facilitate early detection and treatment.2,3 Notably, the prevalence of syphilis is higher in men who have sex with men, particularly among those who engage in unprotected oral and anal sex. Increased screening and early treatment are essential to control the spread of disease within all populations. Doxycycline postexposure prophylaxis (doxyPEP) is used as a preventive measure for syphilis, chlamydia, and gonorrhea.4 This regimen consists of 200 mg of doxycycline taken within 24 hours but no later than 72 hours after unprotected anal, vaginal, or oral sex.
Our case highlights the importance of considering the differential diagnosis of oral ulcers, particularly in high-risk populations such as men who have sex with men. Prompt diagnosis, effective treatment, and preventive strategies such as doxyPEP are essential for controlling syphilis. Comprehensive patient education and regular follow-up appointments are critical components of successful management.
The United States has experienced a considerable rise in primary and congenital syphilis cases, with an 80% increase between 2018 and 2022.6 Serologic testing is the primary method for diagnosing, staging, and managing syphilis. Sexually active patients with suspected syphilis or unexplained symptoms should undergo testing. Prompt diagnosis and treatment can prevent systemic complications, including ocular involvement and permanent blindness.
Syphilis is transmitted through direct contact with a syphilitic ulcer or saliva or blood from an infected individual. Oral syphilitic ulcers can develop on the lips, tongue, oral mucosa, and tonsils. Chancres can range from a few millimeters to several centimeters, with an incubation period of 10 to 90 days (average, 21 days). The chancre lasts 3 to 6 weeks and heals spontaneously. Without treatment, primary syphilis can progress to secondary syphilis, characterized by a papulosquamous eruption and mucosal involvement, and potentially tertiary syphilis, which can affect the central nervous system, heart, bones, and skin.7
Immunocompromised patients, especially those diagnosed with HIV, face increased risks including altered clinical presentations (eg, multiple or deep chancres), delayed healing, overlapping stages of disease, and increased severity of organ involvement. All sexually active individuals should be screened for syphilis every 3 to 6 months, particularly those with unexplained oral ulcers.
Serologic testing is fundamental for syphilis diagnosis and management. Nontreponemal tests such as RPR and treponemal tests such as the fluorescent treponemal antibody absorption test provide comprehensive diagnostic information. Early diagnosis and empiric treatment are crucial in suspected cases. Ocular screening is recommended for suspected or confirmed syphilis cases.7
Management of syphilis includes treating all sexual partners and providing thorough patient education on the disease. Monitoring for the Jarisch-Herxheimer reaction—an acute febrile reaction following penicillin therapy—is important, especially in pregnant patients.5 Serologic evaluation at 6 and 12 months posttreatment is recommended, with more frequent evaluations if follow-up is uncertain, particularly for those with inconsistent access to health care or in whom reinfection is suspected. Guidelines from the Centers for Disease Control and Prevention advocate for intramuscular penicillin G benzathine as the preferred treatment, with specific dosing for adults and children.7 Due to the ongoing bicillin shortage, alternatives such as extencilline have temporarily been allowed for use in the United States.8
The rising incidence of syphilis in the United States underscores the critical need for enhanced public health initiatives focusing on education, screening, and early intervention. Comprehensive sexual education that includes information about syphilis and other sexually transmitted infections, proper use of prophylactic measures such as condoms, and the benefits of doxyPEP can considerably reduce transmission rates. Health care providers should routinely discuss these preventive measures with their patients, especially those in high-risk groups.
Our case highlights the importance of considering syphilis in the differential diagnosis of oral ulcers, particularly in high-risk populations. Timely diagnosis, effective treatment, and preventive measures such as doxyPEP are essential for managing and controlling syphilis. The rising incidence of syphilis in the United States warrants increased screening, patient education, and public health interventions to address this notable health challenge. The syphilis crisis calls for coordinated efforts from health care providers, public health officials, and community leaders to curb the spread of this infection and protect public health.
- Mayer KH, Traeger M, Marcus JL. Doxycycline postexposure prophylaxis and sexually transmitted infections. JAMA. 2023;330:1381-1382. doi:10.1001/jama.2023.16416
- Cossman JP, Fournier JB. Frequency of syphilis diagnoses by dermatologists. JAMA Dermatol. 2017;153:718-719. doi:10.1001 /jamadermatol.2017.0460
- Porterfield C, Brodell D, Dolohanty L, et al. Primary syphilis presenting as a chronic lip ulcer. Cureus. 2020;12:E7086. doi:10.7759 /cureus.7086
- Schamberg JF. An epidemic of chancres of the lip from kissing. JAMA. 1911;LVII:783-784. doi:10.1001/jama.1911.04260090005002
- Farmer TW. Jarisch-Herxheimer reaction in early syphilis. JAMA. 1948;138:480–485. doi:10.1001/jama.1948.02900070012003
- Winney A. Why is syphilis spiking in the U.S.? Johns Hopkins Bloomberg School of Public Health. Johns Hopkins Bloomberg School of Public Health. Published March 13, 2024. Accessed April 30, 2025. https://publichealth.jhu.edu/why-is-syphilis-spiking-in-the-us
- Koundanya VV, Tripathy K. Syphilis ocular manifestations. StatPearls Publishing; 2021. Updated August 25, 2023. Accessed May 6, 2025. https://www.ncbi.nlm.nih.gov/books/NBK558957/
- CDC. FDA announcement on availability of extencilline. National Center for HIV, Viral Hepatitis, STD, and Tuberculosis Prevention. Published July 19, 2024. Accessed April 30, 2025. https://www.cdc.gov/nchhstp/director-letters/extencilline-during-bicillin-l-a-shortage.html
THE DIAGNOSIS: Syphilis
The differential diagnosis of oral lesions can be complex; in our patient, we considered conditions such as pyogenic granuloma, herpes simplex virus, and syphilis, despite the presence of pain. Immunohistochemical staining for spirochete antigens was positive, and serologic confirmation through a positive rapid plasma reagin (RPR) test confirmed the diagnosis of primary syphilis. The patient was promptly referred back to the primary care physician for treatment with intramuscular penicillin, leading to resolution of the lesion. At 3 months’ follow-up in our clinic, the lesion was fully resolved.
A primary syphilitic chancre is the initial lesion caused by Treponema pallidum, typically manifesting as a painless ulcer at the infection site, usually in the genital area; however, chancres also may manifest in other locations (eg, the anus or oral cavity) due to direct contact with infectious lesions on another individual. Our case represents an atypical presentation of an oral syphilitic chancre.
Syphilis is a sexually transmitted infection with various clinical manifestations. It is crucial to consider syphilis in the differential diagnosis of ulcerative lesions even when pain is present, especially in high-risk individuals such as those who engage in unprotected sex.1,2 Oral syphilitic chancres have been documented in the medical literature for more than a century, underscoring the importance of maintaining a high index of suspicion for diagnosis and a low threshold for obtaining an RPR test to facilitate early detection and treatment.2,3 Notably, the prevalence of syphilis is higher in men who have sex with men, particularly among those who engage in unprotected oral and anal sex. Increased screening and early treatment are essential to control the spread of disease within all populations. Doxycycline postexposure prophylaxis (doxyPEP) is used as a preventive measure for syphilis, chlamydia, and gonorrhea.4 This regimen consists of 200 mg of doxycycline taken within 24 hours but no later than 72 hours after unprotected anal, vaginal, or oral sex.
Our case highlights the importance of considering the differential diagnosis of oral ulcers, particularly in high-risk populations such as men who have sex with men. Prompt diagnosis, effective treatment, and preventive strategies such as doxyPEP are essential for controlling syphilis. Comprehensive patient education and regular follow-up appointments are critical components of successful management.
The United States has experienced a considerable rise in primary and congenital syphilis cases, with an 80% increase between 2018 and 2022.6 Serologic testing is the primary method for diagnosing, staging, and managing syphilis. Sexually active patients with suspected syphilis or unexplained symptoms should undergo testing. Prompt diagnosis and treatment can prevent systemic complications, including ocular involvement and permanent blindness.
Syphilis is transmitted through direct contact with a syphilitic ulcer or saliva or blood from an infected individual. Oral syphilitic ulcers can develop on the lips, tongue, oral mucosa, and tonsils. Chancres can range from a few millimeters to several centimeters, with an incubation period of 10 to 90 days (average, 21 days). The chancre lasts 3 to 6 weeks and heals spontaneously. Without treatment, primary syphilis can progress to secondary syphilis, characterized by a papulosquamous eruption and mucosal involvement, and potentially tertiary syphilis, which can affect the central nervous system, heart, bones, and skin.7
Immunocompromised patients, especially those diagnosed with HIV, face increased risks including altered clinical presentations (eg, multiple or deep chancres), delayed healing, overlapping stages of disease, and increased severity of organ involvement. All sexually active individuals should be screened for syphilis every 3 to 6 months, particularly those with unexplained oral ulcers.
Serologic testing is fundamental for syphilis diagnosis and management. Nontreponemal tests such as RPR and treponemal tests such as the fluorescent treponemal antibody absorption test provide comprehensive diagnostic information. Early diagnosis and empiric treatment are crucial in suspected cases. Ocular screening is recommended for suspected or confirmed syphilis cases.7
Management of syphilis includes treating all sexual partners and providing thorough patient education on the disease. Monitoring for the Jarisch-Herxheimer reaction—an acute febrile reaction following penicillin therapy—is important, especially in pregnant patients.5 Serologic evaluation at 6 and 12 months posttreatment is recommended, with more frequent evaluations if follow-up is uncertain, particularly for those with inconsistent access to health care or in whom reinfection is suspected. Guidelines from the Centers for Disease Control and Prevention advocate for intramuscular penicillin G benzathine as the preferred treatment, with specific dosing for adults and children.7 Due to the ongoing bicillin shortage, alternatives such as extencilline have temporarily been allowed for use in the United States.8
The rising incidence of syphilis in the United States underscores the critical need for enhanced public health initiatives focusing on education, screening, and early intervention. Comprehensive sexual education that includes information about syphilis and other sexually transmitted infections, proper use of prophylactic measures such as condoms, and the benefits of doxyPEP can considerably reduce transmission rates. Health care providers should routinely discuss these preventive measures with their patients, especially those in high-risk groups.
Our case highlights the importance of considering syphilis in the differential diagnosis of oral ulcers, particularly in high-risk populations. Timely diagnosis, effective treatment, and preventive measures such as doxyPEP are essential for managing and controlling syphilis. The rising incidence of syphilis in the United States warrants increased screening, patient education, and public health interventions to address this notable health challenge. The syphilis crisis calls for coordinated efforts from health care providers, public health officials, and community leaders to curb the spread of this infection and protect public health.
THE DIAGNOSIS: Syphilis
The differential diagnosis of oral lesions can be complex; in our patient, we considered conditions such as pyogenic granuloma, herpes simplex virus, and syphilis, despite the presence of pain. Immunohistochemical staining for spirochete antigens was positive, and serologic confirmation through a positive rapid plasma reagin (RPR) test confirmed the diagnosis of primary syphilis. The patient was promptly referred back to the primary care physician for treatment with intramuscular penicillin, leading to resolution of the lesion. At 3 months’ follow-up in our clinic, the lesion was fully resolved.
A primary syphilitic chancre is the initial lesion caused by Treponema pallidum, typically manifesting as a painless ulcer at the infection site, usually in the genital area; however, chancres also may manifest in other locations (eg, the anus or oral cavity) due to direct contact with infectious lesions on another individual. Our case represents an atypical presentation of an oral syphilitic chancre.
Syphilis is a sexually transmitted infection with various clinical manifestations. It is crucial to consider syphilis in the differential diagnosis of ulcerative lesions even when pain is present, especially in high-risk individuals such as those who engage in unprotected sex.1,2 Oral syphilitic chancres have been documented in the medical literature for more than a century, underscoring the importance of maintaining a high index of suspicion for diagnosis and a low threshold for obtaining an RPR test to facilitate early detection and treatment.2,3 Notably, the prevalence of syphilis is higher in men who have sex with men, particularly among those who engage in unprotected oral and anal sex. Increased screening and early treatment are essential to control the spread of disease within all populations. Doxycycline postexposure prophylaxis (doxyPEP) is used as a preventive measure for syphilis, chlamydia, and gonorrhea.4 This regimen consists of 200 mg of doxycycline taken within 24 hours but no later than 72 hours after unprotected anal, vaginal, or oral sex.
Our case highlights the importance of considering the differential diagnosis of oral ulcers, particularly in high-risk populations such as men who have sex with men. Prompt diagnosis, effective treatment, and preventive strategies such as doxyPEP are essential for controlling syphilis. Comprehensive patient education and regular follow-up appointments are critical components of successful management.
The United States has experienced a considerable rise in primary and congenital syphilis cases, with an 80% increase between 2018 and 2022.6 Serologic testing is the primary method for diagnosing, staging, and managing syphilis. Sexually active patients with suspected syphilis or unexplained symptoms should undergo testing. Prompt diagnosis and treatment can prevent systemic complications, including ocular involvement and permanent blindness.
Syphilis is transmitted through direct contact with a syphilitic ulcer or saliva or blood from an infected individual. Oral syphilitic ulcers can develop on the lips, tongue, oral mucosa, and tonsils. Chancres can range from a few millimeters to several centimeters, with an incubation period of 10 to 90 days (average, 21 days). The chancre lasts 3 to 6 weeks and heals spontaneously. Without treatment, primary syphilis can progress to secondary syphilis, characterized by a papulosquamous eruption and mucosal involvement, and potentially tertiary syphilis, which can affect the central nervous system, heart, bones, and skin.7
Immunocompromised patients, especially those diagnosed with HIV, face increased risks including altered clinical presentations (eg, multiple or deep chancres), delayed healing, overlapping stages of disease, and increased severity of organ involvement. All sexually active individuals should be screened for syphilis every 3 to 6 months, particularly those with unexplained oral ulcers.
Serologic testing is fundamental for syphilis diagnosis and management. Nontreponemal tests such as RPR and treponemal tests such as the fluorescent treponemal antibody absorption test provide comprehensive diagnostic information. Early diagnosis and empiric treatment are crucial in suspected cases. Ocular screening is recommended for suspected or confirmed syphilis cases.7
Management of syphilis includes treating all sexual partners and providing thorough patient education on the disease. Monitoring for the Jarisch-Herxheimer reaction—an acute febrile reaction following penicillin therapy—is important, especially in pregnant patients.5 Serologic evaluation at 6 and 12 months posttreatment is recommended, with more frequent evaluations if follow-up is uncertain, particularly for those with inconsistent access to health care or in whom reinfection is suspected. Guidelines from the Centers for Disease Control and Prevention advocate for intramuscular penicillin G benzathine as the preferred treatment, with specific dosing for adults and children.7 Due to the ongoing bicillin shortage, alternatives such as extencilline have temporarily been allowed for use in the United States.8
The rising incidence of syphilis in the United States underscores the critical need for enhanced public health initiatives focusing on education, screening, and early intervention. Comprehensive sexual education that includes information about syphilis and other sexually transmitted infections, proper use of prophylactic measures such as condoms, and the benefits of doxyPEP can considerably reduce transmission rates. Health care providers should routinely discuss these preventive measures with their patients, especially those in high-risk groups.
Our case highlights the importance of considering syphilis in the differential diagnosis of oral ulcers, particularly in high-risk populations. Timely diagnosis, effective treatment, and preventive measures such as doxyPEP are essential for managing and controlling syphilis. The rising incidence of syphilis in the United States warrants increased screening, patient education, and public health interventions to address this notable health challenge. The syphilis crisis calls for coordinated efforts from health care providers, public health officials, and community leaders to curb the spread of this infection and protect public health.
- Mayer KH, Traeger M, Marcus JL. Doxycycline postexposure prophylaxis and sexually transmitted infections. JAMA. 2023;330:1381-1382. doi:10.1001/jama.2023.16416
- Cossman JP, Fournier JB. Frequency of syphilis diagnoses by dermatologists. JAMA Dermatol. 2017;153:718-719. doi:10.1001 /jamadermatol.2017.0460
- Porterfield C, Brodell D, Dolohanty L, et al. Primary syphilis presenting as a chronic lip ulcer. Cureus. 2020;12:E7086. doi:10.7759 /cureus.7086
- Schamberg JF. An epidemic of chancres of the lip from kissing. JAMA. 1911;LVII:783-784. doi:10.1001/jama.1911.04260090005002
- Farmer TW. Jarisch-Herxheimer reaction in early syphilis. JAMA. 1948;138:480–485. doi:10.1001/jama.1948.02900070012003
- Winney A. Why is syphilis spiking in the U.S.? Johns Hopkins Bloomberg School of Public Health. Johns Hopkins Bloomberg School of Public Health. Published March 13, 2024. Accessed April 30, 2025. https://publichealth.jhu.edu/why-is-syphilis-spiking-in-the-us
- Koundanya VV, Tripathy K. Syphilis ocular manifestations. StatPearls Publishing; 2021. Updated August 25, 2023. Accessed May 6, 2025. https://www.ncbi.nlm.nih.gov/books/NBK558957/
- CDC. FDA announcement on availability of extencilline. National Center for HIV, Viral Hepatitis, STD, and Tuberculosis Prevention. Published July 19, 2024. Accessed April 30, 2025. https://www.cdc.gov/nchhstp/director-letters/extencilline-during-bicillin-l-a-shortage.html
- Mayer KH, Traeger M, Marcus JL. Doxycycline postexposure prophylaxis and sexually transmitted infections. JAMA. 2023;330:1381-1382. doi:10.1001/jama.2023.16416
- Cossman JP, Fournier JB. Frequency of syphilis diagnoses by dermatologists. JAMA Dermatol. 2017;153:718-719. doi:10.1001 /jamadermatol.2017.0460
- Porterfield C, Brodell D, Dolohanty L, et al. Primary syphilis presenting as a chronic lip ulcer. Cureus. 2020;12:E7086. doi:10.7759 /cureus.7086
- Schamberg JF. An epidemic of chancres of the lip from kissing. JAMA. 1911;LVII:783-784. doi:10.1001/jama.1911.04260090005002
- Farmer TW. Jarisch-Herxheimer reaction in early syphilis. JAMA. 1948;138:480–485. doi:10.1001/jama.1948.02900070012003
- Winney A. Why is syphilis spiking in the U.S.? Johns Hopkins Bloomberg School of Public Health. Johns Hopkins Bloomberg School of Public Health. Published March 13, 2024. Accessed April 30, 2025. https://publichealth.jhu.edu/why-is-syphilis-spiking-in-the-us
- Koundanya VV, Tripathy K. Syphilis ocular manifestations. StatPearls Publishing; 2021. Updated August 25, 2023. Accessed May 6, 2025. https://www.ncbi.nlm.nih.gov/books/NBK558957/
- CDC. FDA announcement on availability of extencilline. National Center for HIV, Viral Hepatitis, STD, and Tuberculosis Prevention. Published July 19, 2024. Accessed April 30, 2025. https://www.cdc.gov/nchhstp/director-letters/extencilline-during-bicillin-l-a-shortage.html
Nonhealing Ulcer on the Lower Lip
Nonhealing Ulcer on the Lower Lip
A 54-year-old HIV-negative man with a history of having sex with men presented to his primary care physician with an ulcer on the lower lip of 3 weeks’ duration. The patient reported that the lesion had appeared as a typical cold sore with pain in the area. A 9-day course of oral valacyclovir prescribed by the primary care physician provided no relief or improvement. A 2-mm punch biopsy was performed.
Are You Up-to-date on Dermal Fillers?
The popularity of injectable fillers for aesthetic use continues to rise, and cosmetic injectors must select from an increasing range of options to achieve optimal outcomes. In addition to formulating a treatment plan and having an intimate knowledge of the facial anatomy, filler selection is critical along with an appreciation of both approved and off-label indications for these products. Appropriate patient selection and treatment technique can minimize adverse events (AEs) and allow for the best outcomes.
The US Food and Drug Administration (FDA) approved the first injectable hyaluronic acid (HA) filler in 2003, the first addition since the approval of bovine collagen in 1981. To date, there are now 4 groups of approved fillers: (1) HA (Belotero Balance [Merz North America, Inc], Juvèderm products [Allergan], Restylane products [Galderma Laboratories, LP], Resilient HA products [Revance Therapeutics Inc and Teoxane SA]), (2) calcium hydroxylapatite (Radiesse [Merz North America, Inc]), (3) poly-L-lactic acid (Sculptra Aesthetic [Galderma Laboratories, LP]), and (4) polymethylmethacrylate (Bellafill [Suneva Medical, Inc]).1-3 Given the versatility of this wide portfolio of products, which often are used in combination with one another, we have advanced from the early goals of filling isolated lines or wrinkles on the face to the 3-dimensional restructuring of an entire treatment area. The increasing diversity of products, particularly the range of rheologic properties of HA fillers, allows the injector to strategically select the type of filler and depth of injection to achieve the desired treatment outcome. The duration of the treatment effects also is related to the properties of the filler.4,5
Advancements in injectable fillers also have led to new applications both on and off the face. Many pivotal clinical trials of fillers were performed in isolated anatomic areas, most commonly the nasolabial folds, leading to FDA approval of this indication. Other FDA-approved indications for fillers include lip augmentation (Juvèderm Ultra, Juvèderm Volbella, Restylane, Restylane Silk, Restylane Kysse), human immunodeficiency virus–associated lipoatrophy (Sculptra Aesthetic, Radiesse), volumization of the dorsal hands (Radiesse, Restylane Lyft), acne scarring (Bellafill), and age-related volume loss of the midface (Juvèderm Voluma, Restylane Lyft). Although it is considered off label, treatment of the temples, brows, tear troughs, jawline, horizontal neck lines, and etched-in radial cheek lines has been reported.6-9 It is legal to use fillers to treat these areas, but data have not yet been evaluated by the FDA to officially grant their approval, which likely will change with the conclusion of many ongoing industry-sponsored trials.
Adverse events from filler injections range from the anticipated transient tenderness, swelling, and bruising, which are likely to resolve in a matter of days, to the most severe complications—intravascular occlusion with permanent sequelae, namely tissue necrosis, blindness or visual compromise, and stroke. It is critical to obtain written informed consent prior to proceeding with dermal filler injections. Masterful knowledge of the facial anatomy, in particular the location and depth of key vascular structures, is critical in minimizing these severe AEs. Injection technique, including use of a microcannula, can reduce the risk, in addition to administration of small volumes of filler at a time, aspiration prior to injection, and use of a retrograde injection technique. There also are variations in the predicted courses of vascular structures, as demonstrated in a cadaveric study showing 4 variants of the course of the angular artery.10
Hyaluronic acid fillers are the most commonly used of the available products, and hyaluronidase, which can dissolve the filler, can be utilized to manage emergent and nonemergent AEs.11 Physical examination findings related to impending necrosis include blanching of the skin in the distribution of a key vessel with a mottled or reticulated purple discoloration. Hyaluronidase, on the order of hundreds of units, may be injected into the area of vascular compromise until reperfusion is achieved, in addition to administering aspirin and applying warm compresses to the area.11,12 The most severe AEs are blindness and/or stroke, associated with findings such as immediate vision loss, pain, nausea, vomiting, and neurologic compromise. Although the glabella, nose, nasolabial folds, and forehead are the most common anatomic areas associated with these AEs (in order of frequency), injections in all areas of the face have been associated with blindness.13,14 Retrobulbar and/or peribulbar injection of hyaluronidase for management of vision changes has been reported, but in most cases vision loss associated with dermal filler injections is not reversible.14,15
Nonemergent uses of enzyme reversal of filler placement include correcting undesirable aesthetic outcomes, such as asymmetry, misplaced filler, or even delayed granulomatous reactions. Hyaluronidase dosage should be determined by the amount and type of filler that was delivered to the patient. All HA fillers are not created equally, and evidence from dosing studies indicates that higher cross-linked and more cohesive fillers require higher doses of hyaluronidase.11 For example, Juvèderm Voluma, created as a mixture of low- and high-molecular-weight HA, has a higher cross-linking ratio. Approximately 30 U of hyaluronidase are suggested to dissolve 0.1 cc of Juvèderm Voluma as compared to 10 U of hyaluronidase for 0.1 cc of Juvèderm Ultra and 5 U for 0.1 cc of Restylane.11
Treatment with dermal fillers generally is safe and effective, and as new fillers come to the market, we must identify how they will help further our goal of improving patient outcomes. The effects of coronavirus disease 19 on aesthetic medicine are still unclear, yet this uncertainty should not deflect treating clinicians from overlooking the fundamentals of dermal fillers. In addition to considering the appropriate use of each filler based on its unique characteristics and indications, we must be sure that we are prepared with the tools to manage any and all possible complications.
- Jiang B, Ramirez M, Ranjit-Reeves R, et al. Noncollagen dermal fillers: a summary of the clinical trials used for their FDA approval. Dermatol Surg. 2019;45:1585-1596.
- Monheit G, Kaufman-Janette J, Joseph J, et al. Efficacy and safety of two resilient hyaluronic acid fillers in the treatment of moderate-to-severe nasolabial folds [published online March 24, 2020]. Dermatol Surg. doi:10.1097/DSS0000000000002391.
- Kaufman-Janette J, Taylor SC, Cox SE, et al. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: a 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol. 2019;18:1244-1253.
- Jones D, Murphy D. Volumizing hyaluronic acid filler for midface volume deficit: 2 year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1611.
- Hausauer AK, Jones DH. Long-term correction of iatrogenic lipoatrophy with volumizing hyaluronic acid filler. Dermatol Surg. 2018;44(suppl 1):S60-S62.
- Black J, Jones D. Cohesive polydensified matrix hyaluronic acid for the treatment of etched-in fine facial lines: a 6-month, open-label clinical trial. Dermatol Surg. 2018;44:1002-1011.
- Breithaupt A, Jones D, Braz A, et al. Anatomic basis for safe and effective volumization of the temple. Dermatol Surg. 2015;41:S278-S283.
- Dallara JM, Baspeyras M, Bui P, et al. Calcium hydroxylapatite for jawline rejuvenation: consensus recommendations. J Cosmet Dermatol. 2014;13:3-14.
- Minokadeh A, Black J, Jones D. Effacement of transverse neck lines with VYC-15L and a cohesive polydensified matrix hyaluronic acid. Dermatol Surg. 2019;45:941-948.
- Kim YS, Choi DY, Gil YC, et al. The anatomical origin and course of the angular artery regarding its clinical implications. Dermatol Surg. 2014;40:1070-1076.
- Jones DH. Update on emergency and nonemergency use of hyaluronidase in aesthetic dermatology. JAMA Dermatol. 2018;154:763-764.
- Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthet Surg J. 2015;35:844-849.
- Beleznay K, Carruthers J, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Beleznay K, Carruthers J, Humphrey S, et al. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39:662-674.
- Chestnut C. Restoration of visual loss with retrobulblar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018;44:435-437.
The popularity of injectable fillers for aesthetic use continues to rise, and cosmetic injectors must select from an increasing range of options to achieve optimal outcomes. In addition to formulating a treatment plan and having an intimate knowledge of the facial anatomy, filler selection is critical along with an appreciation of both approved and off-label indications for these products. Appropriate patient selection and treatment technique can minimize adverse events (AEs) and allow for the best outcomes.
The US Food and Drug Administration (FDA) approved the first injectable hyaluronic acid (HA) filler in 2003, the first addition since the approval of bovine collagen in 1981. To date, there are now 4 groups of approved fillers: (1) HA (Belotero Balance [Merz North America, Inc], Juvèderm products [Allergan], Restylane products [Galderma Laboratories, LP], Resilient HA products [Revance Therapeutics Inc and Teoxane SA]), (2) calcium hydroxylapatite (Radiesse [Merz North America, Inc]), (3) poly-L-lactic acid (Sculptra Aesthetic [Galderma Laboratories, LP]), and (4) polymethylmethacrylate (Bellafill [Suneva Medical, Inc]).1-3 Given the versatility of this wide portfolio of products, which often are used in combination with one another, we have advanced from the early goals of filling isolated lines or wrinkles on the face to the 3-dimensional restructuring of an entire treatment area. The increasing diversity of products, particularly the range of rheologic properties of HA fillers, allows the injector to strategically select the type of filler and depth of injection to achieve the desired treatment outcome. The duration of the treatment effects also is related to the properties of the filler.4,5
Advancements in injectable fillers also have led to new applications both on and off the face. Many pivotal clinical trials of fillers were performed in isolated anatomic areas, most commonly the nasolabial folds, leading to FDA approval of this indication. Other FDA-approved indications for fillers include lip augmentation (Juvèderm Ultra, Juvèderm Volbella, Restylane, Restylane Silk, Restylane Kysse), human immunodeficiency virus–associated lipoatrophy (Sculptra Aesthetic, Radiesse), volumization of the dorsal hands (Radiesse, Restylane Lyft), acne scarring (Bellafill), and age-related volume loss of the midface (Juvèderm Voluma, Restylane Lyft). Although it is considered off label, treatment of the temples, brows, tear troughs, jawline, horizontal neck lines, and etched-in radial cheek lines has been reported.6-9 It is legal to use fillers to treat these areas, but data have not yet been evaluated by the FDA to officially grant their approval, which likely will change with the conclusion of many ongoing industry-sponsored trials.
Adverse events from filler injections range from the anticipated transient tenderness, swelling, and bruising, which are likely to resolve in a matter of days, to the most severe complications—intravascular occlusion with permanent sequelae, namely tissue necrosis, blindness or visual compromise, and stroke. It is critical to obtain written informed consent prior to proceeding with dermal filler injections. Masterful knowledge of the facial anatomy, in particular the location and depth of key vascular structures, is critical in minimizing these severe AEs. Injection technique, including use of a microcannula, can reduce the risk, in addition to administration of small volumes of filler at a time, aspiration prior to injection, and use of a retrograde injection technique. There also are variations in the predicted courses of vascular structures, as demonstrated in a cadaveric study showing 4 variants of the course of the angular artery.10
Hyaluronic acid fillers are the most commonly used of the available products, and hyaluronidase, which can dissolve the filler, can be utilized to manage emergent and nonemergent AEs.11 Physical examination findings related to impending necrosis include blanching of the skin in the distribution of a key vessel with a mottled or reticulated purple discoloration. Hyaluronidase, on the order of hundreds of units, may be injected into the area of vascular compromise until reperfusion is achieved, in addition to administering aspirin and applying warm compresses to the area.11,12 The most severe AEs are blindness and/or stroke, associated with findings such as immediate vision loss, pain, nausea, vomiting, and neurologic compromise. Although the glabella, nose, nasolabial folds, and forehead are the most common anatomic areas associated with these AEs (in order of frequency), injections in all areas of the face have been associated with blindness.13,14 Retrobulbar and/or peribulbar injection of hyaluronidase for management of vision changes has been reported, but in most cases vision loss associated with dermal filler injections is not reversible.14,15
Nonemergent uses of enzyme reversal of filler placement include correcting undesirable aesthetic outcomes, such as asymmetry, misplaced filler, or even delayed granulomatous reactions. Hyaluronidase dosage should be determined by the amount and type of filler that was delivered to the patient. All HA fillers are not created equally, and evidence from dosing studies indicates that higher cross-linked and more cohesive fillers require higher doses of hyaluronidase.11 For example, Juvèderm Voluma, created as a mixture of low- and high-molecular-weight HA, has a higher cross-linking ratio. Approximately 30 U of hyaluronidase are suggested to dissolve 0.1 cc of Juvèderm Voluma as compared to 10 U of hyaluronidase for 0.1 cc of Juvèderm Ultra and 5 U for 0.1 cc of Restylane.11
Treatment with dermal fillers generally is safe and effective, and as new fillers come to the market, we must identify how they will help further our goal of improving patient outcomes. The effects of coronavirus disease 19 on aesthetic medicine are still unclear, yet this uncertainty should not deflect treating clinicians from overlooking the fundamentals of dermal fillers. In addition to considering the appropriate use of each filler based on its unique characteristics and indications, we must be sure that we are prepared with the tools to manage any and all possible complications.
The popularity of injectable fillers for aesthetic use continues to rise, and cosmetic injectors must select from an increasing range of options to achieve optimal outcomes. In addition to formulating a treatment plan and having an intimate knowledge of the facial anatomy, filler selection is critical along with an appreciation of both approved and off-label indications for these products. Appropriate patient selection and treatment technique can minimize adverse events (AEs) and allow for the best outcomes.
The US Food and Drug Administration (FDA) approved the first injectable hyaluronic acid (HA) filler in 2003, the first addition since the approval of bovine collagen in 1981. To date, there are now 4 groups of approved fillers: (1) HA (Belotero Balance [Merz North America, Inc], Juvèderm products [Allergan], Restylane products [Galderma Laboratories, LP], Resilient HA products [Revance Therapeutics Inc and Teoxane SA]), (2) calcium hydroxylapatite (Radiesse [Merz North America, Inc]), (3) poly-L-lactic acid (Sculptra Aesthetic [Galderma Laboratories, LP]), and (4) polymethylmethacrylate (Bellafill [Suneva Medical, Inc]).1-3 Given the versatility of this wide portfolio of products, which often are used in combination with one another, we have advanced from the early goals of filling isolated lines or wrinkles on the face to the 3-dimensional restructuring of an entire treatment area. The increasing diversity of products, particularly the range of rheologic properties of HA fillers, allows the injector to strategically select the type of filler and depth of injection to achieve the desired treatment outcome. The duration of the treatment effects also is related to the properties of the filler.4,5
Advancements in injectable fillers also have led to new applications both on and off the face. Many pivotal clinical trials of fillers were performed in isolated anatomic areas, most commonly the nasolabial folds, leading to FDA approval of this indication. Other FDA-approved indications for fillers include lip augmentation (Juvèderm Ultra, Juvèderm Volbella, Restylane, Restylane Silk, Restylane Kysse), human immunodeficiency virus–associated lipoatrophy (Sculptra Aesthetic, Radiesse), volumization of the dorsal hands (Radiesse, Restylane Lyft), acne scarring (Bellafill), and age-related volume loss of the midface (Juvèderm Voluma, Restylane Lyft). Although it is considered off label, treatment of the temples, brows, tear troughs, jawline, horizontal neck lines, and etched-in radial cheek lines has been reported.6-9 It is legal to use fillers to treat these areas, but data have not yet been evaluated by the FDA to officially grant their approval, which likely will change with the conclusion of many ongoing industry-sponsored trials.
Adverse events from filler injections range from the anticipated transient tenderness, swelling, and bruising, which are likely to resolve in a matter of days, to the most severe complications—intravascular occlusion with permanent sequelae, namely tissue necrosis, blindness or visual compromise, and stroke. It is critical to obtain written informed consent prior to proceeding with dermal filler injections. Masterful knowledge of the facial anatomy, in particular the location and depth of key vascular structures, is critical in minimizing these severe AEs. Injection technique, including use of a microcannula, can reduce the risk, in addition to administration of small volumes of filler at a time, aspiration prior to injection, and use of a retrograde injection technique. There also are variations in the predicted courses of vascular structures, as demonstrated in a cadaveric study showing 4 variants of the course of the angular artery.10
Hyaluronic acid fillers are the most commonly used of the available products, and hyaluronidase, which can dissolve the filler, can be utilized to manage emergent and nonemergent AEs.11 Physical examination findings related to impending necrosis include blanching of the skin in the distribution of a key vessel with a mottled or reticulated purple discoloration. Hyaluronidase, on the order of hundreds of units, may be injected into the area of vascular compromise until reperfusion is achieved, in addition to administering aspirin and applying warm compresses to the area.11,12 The most severe AEs are blindness and/or stroke, associated with findings such as immediate vision loss, pain, nausea, vomiting, and neurologic compromise. Although the glabella, nose, nasolabial folds, and forehead are the most common anatomic areas associated with these AEs (in order of frequency), injections in all areas of the face have been associated with blindness.13,14 Retrobulbar and/or peribulbar injection of hyaluronidase for management of vision changes has been reported, but in most cases vision loss associated with dermal filler injections is not reversible.14,15
Nonemergent uses of enzyme reversal of filler placement include correcting undesirable aesthetic outcomes, such as asymmetry, misplaced filler, or even delayed granulomatous reactions. Hyaluronidase dosage should be determined by the amount and type of filler that was delivered to the patient. All HA fillers are not created equally, and evidence from dosing studies indicates that higher cross-linked and more cohesive fillers require higher doses of hyaluronidase.11 For example, Juvèderm Voluma, created as a mixture of low- and high-molecular-weight HA, has a higher cross-linking ratio. Approximately 30 U of hyaluronidase are suggested to dissolve 0.1 cc of Juvèderm Voluma as compared to 10 U of hyaluronidase for 0.1 cc of Juvèderm Ultra and 5 U for 0.1 cc of Restylane.11
Treatment with dermal fillers generally is safe and effective, and as new fillers come to the market, we must identify how they will help further our goal of improving patient outcomes. The effects of coronavirus disease 19 on aesthetic medicine are still unclear, yet this uncertainty should not deflect treating clinicians from overlooking the fundamentals of dermal fillers. In addition to considering the appropriate use of each filler based on its unique characteristics and indications, we must be sure that we are prepared with the tools to manage any and all possible complications.
- Jiang B, Ramirez M, Ranjit-Reeves R, et al. Noncollagen dermal fillers: a summary of the clinical trials used for their FDA approval. Dermatol Surg. 2019;45:1585-1596.
- Monheit G, Kaufman-Janette J, Joseph J, et al. Efficacy and safety of two resilient hyaluronic acid fillers in the treatment of moderate-to-severe nasolabial folds [published online March 24, 2020]. Dermatol Surg. doi:10.1097/DSS0000000000002391.
- Kaufman-Janette J, Taylor SC, Cox SE, et al. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: a 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol. 2019;18:1244-1253.
- Jones D, Murphy D. Volumizing hyaluronic acid filler for midface volume deficit: 2 year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1611.
- Hausauer AK, Jones DH. Long-term correction of iatrogenic lipoatrophy with volumizing hyaluronic acid filler. Dermatol Surg. 2018;44(suppl 1):S60-S62.
- Black J, Jones D. Cohesive polydensified matrix hyaluronic acid for the treatment of etched-in fine facial lines: a 6-month, open-label clinical trial. Dermatol Surg. 2018;44:1002-1011.
- Breithaupt A, Jones D, Braz A, et al. Anatomic basis for safe and effective volumization of the temple. Dermatol Surg. 2015;41:S278-S283.
- Dallara JM, Baspeyras M, Bui P, et al. Calcium hydroxylapatite for jawline rejuvenation: consensus recommendations. J Cosmet Dermatol. 2014;13:3-14.
- Minokadeh A, Black J, Jones D. Effacement of transverse neck lines with VYC-15L and a cohesive polydensified matrix hyaluronic acid. Dermatol Surg. 2019;45:941-948.
- Kim YS, Choi DY, Gil YC, et al. The anatomical origin and course of the angular artery regarding its clinical implications. Dermatol Surg. 2014;40:1070-1076.
- Jones DH. Update on emergency and nonemergency use of hyaluronidase in aesthetic dermatology. JAMA Dermatol. 2018;154:763-764.
- Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthet Surg J. 2015;35:844-849.
- Beleznay K, Carruthers J, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Beleznay K, Carruthers J, Humphrey S, et al. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39:662-674.
- Chestnut C. Restoration of visual loss with retrobulblar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018;44:435-437.
- Jiang B, Ramirez M, Ranjit-Reeves R, et al. Noncollagen dermal fillers: a summary of the clinical trials used for their FDA approval. Dermatol Surg. 2019;45:1585-1596.
- Monheit G, Kaufman-Janette J, Joseph J, et al. Efficacy and safety of two resilient hyaluronic acid fillers in the treatment of moderate-to-severe nasolabial folds [published online March 24, 2020]. Dermatol Surg. doi:10.1097/DSS0000000000002391.
- Kaufman-Janette J, Taylor SC, Cox SE, et al. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: a 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol. 2019;18:1244-1253.
- Jones D, Murphy D. Volumizing hyaluronic acid filler for midface volume deficit: 2 year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1611.
- Hausauer AK, Jones DH. Long-term correction of iatrogenic lipoatrophy with volumizing hyaluronic acid filler. Dermatol Surg. 2018;44(suppl 1):S60-S62.
- Black J, Jones D. Cohesive polydensified matrix hyaluronic acid for the treatment of etched-in fine facial lines: a 6-month, open-label clinical trial. Dermatol Surg. 2018;44:1002-1011.
- Breithaupt A, Jones D, Braz A, et al. Anatomic basis for safe and effective volumization of the temple. Dermatol Surg. 2015;41:S278-S283.
- Dallara JM, Baspeyras M, Bui P, et al. Calcium hydroxylapatite for jawline rejuvenation: consensus recommendations. J Cosmet Dermatol. 2014;13:3-14.
- Minokadeh A, Black J, Jones D. Effacement of transverse neck lines with VYC-15L and a cohesive polydensified matrix hyaluronic acid. Dermatol Surg. 2019;45:941-948.
- Kim YS, Choi DY, Gil YC, et al. The anatomical origin and course of the angular artery regarding its clinical implications. Dermatol Surg. 2014;40:1070-1076.
- Jones DH. Update on emergency and nonemergency use of hyaluronidase in aesthetic dermatology. JAMA Dermatol. 2018;154:763-764.
- Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthet Surg J. 2015;35:844-849.
- Beleznay K, Carruthers J, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Beleznay K, Carruthers J, Humphrey S, et al. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39:662-674.
- Chestnut C. Restoration of visual loss with retrobulblar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018;44:435-437.