User login
Palivizumab cut odds of RSV hospitalization, belying AAP recommendations
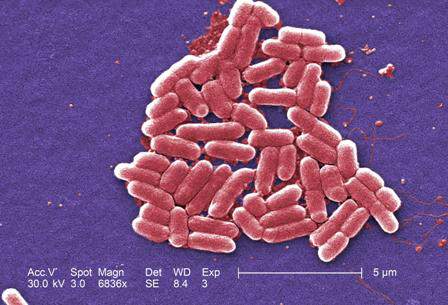
SAN DIEGO – Prophylactic palivizumab cut the odds of hospitalization for severe respiratory syncytial virus (RSV) infection by about 75% among preterm infants born at more than 29 weeks’ gestational age – even those without congenital heart disease or chronic lung disease, investigators reported.
The finding belies the American Academy of Pediatrics’ recommendation to limit use of the humanized monoclonal antibody to infants born before 29 weeks’ gestation and to children who have other risk factors for severe RSV infection, Dr. Ram Yogev of Ann and Robert H. Lurie Children’s Hospital, Chicago, said in an interview.
“Our results validate the older studies, except this was done in real life,” added lead investigator Dr. Eric Simões of Children’s Hospital Colorado, Aurora, who presented the findings at an annual scientific meeting on infectious diseases.
RSV usually causes mild upper respiratory tract infections, but premature infants and children who have comorbid cardiac or pulmonary disease can develop severe infections of the lower respiratory tract. Weekly palivizumab dosing was 45%-80% effective in preventing RSV-related hospitalizations in clinical trials of these high-risk patients, noted Dr. Simões and his associates.
But in 2014, the American Academy of Pediatrics reviewed the literature and revised its guidance to limit palivizumab to preterm infants born before 29 weeks’ gestation and to infants with comorbid risk conditions. The biologic “has been shown to have a limited effect on reducing RSV hospitalization,” the academy concluded. The update drew criticism from some pediatric infectious disease experts, who contended that AAP cited observational studies that actually contradicted its conclusions.
To further examine the issue, Dr. Simões and associates analyzed data from a multicenter study of high-risk infants and children under the age of 2 years who had been hospitalized with lower respiratory tract infections. Between 2002 and 2006, 849 of these patients had a nasopharyngeal wash or endotracheal aspirate tested for RSV, and 403 were positive. The investigators determined that the odds of a positive RSV test were 58% lower for patients who had received prophylactic palivizumab, compared with patients who had not (95% confidence interval for efficacy, 43%-69%; P less than .0001).
Furthermore, palivizumab was 75% effective against severe RSV disease in preterm patients born at 29-35 weeks’ gestation who were chronologically younger than 6 months and had no congenital heart disease or chronic lung disease, said the investigators. Based on that finding, AAP should reconsider its recommendations on palivizumab, said Dr. Yogev.
“This study should answer some of the issues raised in the AAP recommendations,” added Dr. Simões.
Palivizumab did not prevent hospitalizations for human metapneumovirus (hMPV) infection, which further validated the results on RSV, said Dr. Simões. The test-negative case-control design of the study “cuts out the whole issue of [bias due to] care-seeking behavior,” he added. “But children on palivizumab would be more seriously ill, so how do you correct for that? Traditional methods are based on multivariate analysis, but we used propensity scores.” A goodness-of-fit test showed that this approach controlled for all variables except palivizumab exposure and age greater than 6 months, meaning that only these two factors were significantly protective against RSV infection, he said.
IDWeek marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
MedImmune sponsored the original study of hMPV tract infections, from which these data were obtained. Dr. Simões reported having received funding support and consulting fees from MedImmune.
SAN DIEGO – Prophylactic palivizumab cut the odds of hospitalization for severe respiratory syncytial virus (RSV) infection by about 75% among preterm infants born at more than 29 weeks’ gestational age – even those without congenital heart disease or chronic lung disease, investigators reported.
The finding belies the American Academy of Pediatrics’ recommendation to limit use of the humanized monoclonal antibody to infants born before 29 weeks’ gestation and to children who have other risk factors for severe RSV infection, Dr. Ram Yogev of Ann and Robert H. Lurie Children’s Hospital, Chicago, said in an interview.
“Our results validate the older studies, except this was done in real life,” added lead investigator Dr. Eric Simões of Children’s Hospital Colorado, Aurora, who presented the findings at an annual scientific meeting on infectious diseases.
RSV usually causes mild upper respiratory tract infections, but premature infants and children who have comorbid cardiac or pulmonary disease can develop severe infections of the lower respiratory tract. Weekly palivizumab dosing was 45%-80% effective in preventing RSV-related hospitalizations in clinical trials of these high-risk patients, noted Dr. Simões and his associates.
But in 2014, the American Academy of Pediatrics reviewed the literature and revised its guidance to limit palivizumab to preterm infants born before 29 weeks’ gestation and to infants with comorbid risk conditions. The biologic “has been shown to have a limited effect on reducing RSV hospitalization,” the academy concluded. The update drew criticism from some pediatric infectious disease experts, who contended that AAP cited observational studies that actually contradicted its conclusions.
To further examine the issue, Dr. Simões and associates analyzed data from a multicenter study of high-risk infants and children under the age of 2 years who had been hospitalized with lower respiratory tract infections. Between 2002 and 2006, 849 of these patients had a nasopharyngeal wash or endotracheal aspirate tested for RSV, and 403 were positive. The investigators determined that the odds of a positive RSV test were 58% lower for patients who had received prophylactic palivizumab, compared with patients who had not (95% confidence interval for efficacy, 43%-69%; P less than .0001).
Furthermore, palivizumab was 75% effective against severe RSV disease in preterm patients born at 29-35 weeks’ gestation who were chronologically younger than 6 months and had no congenital heart disease or chronic lung disease, said the investigators. Based on that finding, AAP should reconsider its recommendations on palivizumab, said Dr. Yogev.
“This study should answer some of the issues raised in the AAP recommendations,” added Dr. Simões.
Palivizumab did not prevent hospitalizations for human metapneumovirus (hMPV) infection, which further validated the results on RSV, said Dr. Simões. The test-negative case-control design of the study “cuts out the whole issue of [bias due to] care-seeking behavior,” he added. “But children on palivizumab would be more seriously ill, so how do you correct for that? Traditional methods are based on multivariate analysis, but we used propensity scores.” A goodness-of-fit test showed that this approach controlled for all variables except palivizumab exposure and age greater than 6 months, meaning that only these two factors were significantly protective against RSV infection, he said.
IDWeek marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
MedImmune sponsored the original study of hMPV tract infections, from which these data were obtained. Dr. Simões reported having received funding support and consulting fees from MedImmune.
SAN DIEGO – Prophylactic palivizumab cut the odds of hospitalization for severe respiratory syncytial virus (RSV) infection by about 75% among preterm infants born at more than 29 weeks’ gestational age – even those without congenital heart disease or chronic lung disease, investigators reported.
The finding belies the American Academy of Pediatrics’ recommendation to limit use of the humanized monoclonal antibody to infants born before 29 weeks’ gestation and to children who have other risk factors for severe RSV infection, Dr. Ram Yogev of Ann and Robert H. Lurie Children’s Hospital, Chicago, said in an interview.
“Our results validate the older studies, except this was done in real life,” added lead investigator Dr. Eric Simões of Children’s Hospital Colorado, Aurora, who presented the findings at an annual scientific meeting on infectious diseases.
RSV usually causes mild upper respiratory tract infections, but premature infants and children who have comorbid cardiac or pulmonary disease can develop severe infections of the lower respiratory tract. Weekly palivizumab dosing was 45%-80% effective in preventing RSV-related hospitalizations in clinical trials of these high-risk patients, noted Dr. Simões and his associates.
But in 2014, the American Academy of Pediatrics reviewed the literature and revised its guidance to limit palivizumab to preterm infants born before 29 weeks’ gestation and to infants with comorbid risk conditions. The biologic “has been shown to have a limited effect on reducing RSV hospitalization,” the academy concluded. The update drew criticism from some pediatric infectious disease experts, who contended that AAP cited observational studies that actually contradicted its conclusions.
To further examine the issue, Dr. Simões and associates analyzed data from a multicenter study of high-risk infants and children under the age of 2 years who had been hospitalized with lower respiratory tract infections. Between 2002 and 2006, 849 of these patients had a nasopharyngeal wash or endotracheal aspirate tested for RSV, and 403 were positive. The investigators determined that the odds of a positive RSV test were 58% lower for patients who had received prophylactic palivizumab, compared with patients who had not (95% confidence interval for efficacy, 43%-69%; P less than .0001).
Furthermore, palivizumab was 75% effective against severe RSV disease in preterm patients born at 29-35 weeks’ gestation who were chronologically younger than 6 months and had no congenital heart disease or chronic lung disease, said the investigators. Based on that finding, AAP should reconsider its recommendations on palivizumab, said Dr. Yogev.
“This study should answer some of the issues raised in the AAP recommendations,” added Dr. Simões.
Palivizumab did not prevent hospitalizations for human metapneumovirus (hMPV) infection, which further validated the results on RSV, said Dr. Simões. The test-negative case-control design of the study “cuts out the whole issue of [bias due to] care-seeking behavior,” he added. “But children on palivizumab would be more seriously ill, so how do you correct for that? Traditional methods are based on multivariate analysis, but we used propensity scores.” A goodness-of-fit test showed that this approach controlled for all variables except palivizumab exposure and age greater than 6 months, meaning that only these two factors were significantly protective against RSV infection, he said.
IDWeek marked the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
MedImmune sponsored the original study of hMPV tract infections, from which these data were obtained. Dr. Simões reported having received funding support and consulting fees from MedImmune.
AT IDWEEK 2015
Key clinical point: Palivizumab cut the odds of severe RSV infection by nearly 75% among patients who AAP has recommended not routinely receive the prophylactic biologic.
Major finding: The odds of confirmed RSV infection were 74% lower among treated patients, compared with untreated patients (P less than .0001).
Data source: Multicenter case-control study of 849 infants and children who had been hospitalized with severe lower respiratory tract infections.
Disclosures: MedImmune sponsored the original study of human metapneumovirus respiratory tract infections, from which these data were obtained. Dr. Simões reported funding support and consulting fees from MedImmune.
IDWeek: VA found no evidence of CRE transmission through duodenoscopes
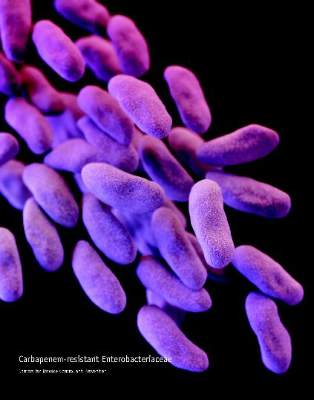
SAN DIEGO – Researchers found “no compelling evidence” that carbapenem-resistant enterobacteriaceae were transmitted to Veterans Affairs patients during more than 55,000 endoscopic retrograde cholangiopancreatography (ERCP) and ultrasonography (EUS) procedures, Dr. Russell Ryono said at an annual meeting on infectious diseases.
The few clusters of cases uncovered by the 5-year retrospective analysis were separated not only in time, but by culture-negative patients treated with the same duodenoscopes at the same facilities, said Dr. Ryono of the public health surveillance and research office at the Department of Veterans Affairs in Palo Alto, Calif. “Our findings do not provide evidence of CRE transmission related to ERCP or EUS in Veterans Affairs,” Dr. Ryono and his associates concluded.
Contaminated duodenoscopes made headlines across the country this year after they caused outbreaks of fatal CRE infections in Los Angeles County. The Food and Drug Administration later acknowledged that the “complex design of the devices makes it difficult to remove contaminants compared to other types of endoscopes,” and both the Centers for Disease Control and Prevention and FDA recommended surveillance and reprocessing practices to help keep the scopes from transmitting serious infections.
In response to these concerns, the VA mined its data warehouses for ERCP and EUS procedures and CRE isolates recovered between January 2010 and the end of February 2015. Investigators looked for patients who were culture positive for the same type of CRE, underwent ERCP/EUS at the same facility within 6 months of each other, and were treated with the same scope or with an unidentified scope. Within this group, researchers honed in on “pairs” – or clusters – of patients in which the second patient exposed to the scope was CRE negative before the procedure and CRE positive afterward.
The initial query yielded more than 55,600 procedures among 40,329 veterans at 38 VA centers, as well as nearly 5,000 CRE isolates, more than half of which were cultured from urine samples, said Dr. Ryono. However, only 81 ERCP/EUS patients had CRE cultured from any anatomical site at any time. Among them, 57 patients were CRE negative before their procedure but CRE positive afterward, Dr. Ryono said.
There were 10 pairs of culture-positive patients treated at four facilities, one of which did not track serial numbers of duodenoscopes, according to Dr. Ryono. At that facility, procedure dates for pairs were 68-143 days apart, he said. Procedure dates for pairs at the other three facilities were spaced at least 82 days apart. Based on such extensive temporal spacing and the fact that the same scopes were used on culture-negative patients during intervening times, the investigators concluded that duodenoscopes were unlikely to have caused CRE infection or colonization.
Dr. Ryono noted several study limitations, however. Not only were CRE isolates unavailable for epidemiologic testing, but researchers also could not determine the extent to which facilities had implemented the 2010 guidelines for CLSI breakpoints. “Certainly, those facilities that have implemented those guidelines are more likely to catch CRE,” he said. Also, VA does not routinely screen patients for CRE before they undergo ERCP or EUS, “so it is very possible that some of our patients might have been colonized with CRE before the procedure, and we would not have been able to capture that,” he added. “Given the limitations of this review, the possibility that there was some transmission certainly cannot be completely excluded.”
Dr. Ryono reported these findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The researchers declared no funding sources or conflicts of interest.
SAN DIEGO – Researchers found “no compelling evidence” that carbapenem-resistant enterobacteriaceae were transmitted to Veterans Affairs patients during more than 55,000 endoscopic retrograde cholangiopancreatography (ERCP) and ultrasonography (EUS) procedures, Dr. Russell Ryono said at an annual meeting on infectious diseases.
The few clusters of cases uncovered by the 5-year retrospective analysis were separated not only in time, but by culture-negative patients treated with the same duodenoscopes at the same facilities, said Dr. Ryono of the public health surveillance and research office at the Department of Veterans Affairs in Palo Alto, Calif. “Our findings do not provide evidence of CRE transmission related to ERCP or EUS in Veterans Affairs,” Dr. Ryono and his associates concluded.
Contaminated duodenoscopes made headlines across the country this year after they caused outbreaks of fatal CRE infections in Los Angeles County. The Food and Drug Administration later acknowledged that the “complex design of the devices makes it difficult to remove contaminants compared to other types of endoscopes,” and both the Centers for Disease Control and Prevention and FDA recommended surveillance and reprocessing practices to help keep the scopes from transmitting serious infections.
In response to these concerns, the VA mined its data warehouses for ERCP and EUS procedures and CRE isolates recovered between January 2010 and the end of February 2015. Investigators looked for patients who were culture positive for the same type of CRE, underwent ERCP/EUS at the same facility within 6 months of each other, and were treated with the same scope or with an unidentified scope. Within this group, researchers honed in on “pairs” – or clusters – of patients in which the second patient exposed to the scope was CRE negative before the procedure and CRE positive afterward.
The initial query yielded more than 55,600 procedures among 40,329 veterans at 38 VA centers, as well as nearly 5,000 CRE isolates, more than half of which were cultured from urine samples, said Dr. Ryono. However, only 81 ERCP/EUS patients had CRE cultured from any anatomical site at any time. Among them, 57 patients were CRE negative before their procedure but CRE positive afterward, Dr. Ryono said.
There were 10 pairs of culture-positive patients treated at four facilities, one of which did not track serial numbers of duodenoscopes, according to Dr. Ryono. At that facility, procedure dates for pairs were 68-143 days apart, he said. Procedure dates for pairs at the other three facilities were spaced at least 82 days apart. Based on such extensive temporal spacing and the fact that the same scopes were used on culture-negative patients during intervening times, the investigators concluded that duodenoscopes were unlikely to have caused CRE infection or colonization.
Dr. Ryono noted several study limitations, however. Not only were CRE isolates unavailable for epidemiologic testing, but researchers also could not determine the extent to which facilities had implemented the 2010 guidelines for CLSI breakpoints. “Certainly, those facilities that have implemented those guidelines are more likely to catch CRE,” he said. Also, VA does not routinely screen patients for CRE before they undergo ERCP or EUS, “so it is very possible that some of our patients might have been colonized with CRE before the procedure, and we would not have been able to capture that,” he added. “Given the limitations of this review, the possibility that there was some transmission certainly cannot be completely excluded.”
Dr. Ryono reported these findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The researchers declared no funding sources or conflicts of interest.
SAN DIEGO – Researchers found “no compelling evidence” that carbapenem-resistant enterobacteriaceae were transmitted to Veterans Affairs patients during more than 55,000 endoscopic retrograde cholangiopancreatography (ERCP) and ultrasonography (EUS) procedures, Dr. Russell Ryono said at an annual meeting on infectious diseases.
The few clusters of cases uncovered by the 5-year retrospective analysis were separated not only in time, but by culture-negative patients treated with the same duodenoscopes at the same facilities, said Dr. Ryono of the public health surveillance and research office at the Department of Veterans Affairs in Palo Alto, Calif. “Our findings do not provide evidence of CRE transmission related to ERCP or EUS in Veterans Affairs,” Dr. Ryono and his associates concluded.
Contaminated duodenoscopes made headlines across the country this year after they caused outbreaks of fatal CRE infections in Los Angeles County. The Food and Drug Administration later acknowledged that the “complex design of the devices makes it difficult to remove contaminants compared to other types of endoscopes,” and both the Centers for Disease Control and Prevention and FDA recommended surveillance and reprocessing practices to help keep the scopes from transmitting serious infections.
In response to these concerns, the VA mined its data warehouses for ERCP and EUS procedures and CRE isolates recovered between January 2010 and the end of February 2015. Investigators looked for patients who were culture positive for the same type of CRE, underwent ERCP/EUS at the same facility within 6 months of each other, and were treated with the same scope or with an unidentified scope. Within this group, researchers honed in on “pairs” – or clusters – of patients in which the second patient exposed to the scope was CRE negative before the procedure and CRE positive afterward.
The initial query yielded more than 55,600 procedures among 40,329 veterans at 38 VA centers, as well as nearly 5,000 CRE isolates, more than half of which were cultured from urine samples, said Dr. Ryono. However, only 81 ERCP/EUS patients had CRE cultured from any anatomical site at any time. Among them, 57 patients were CRE negative before their procedure but CRE positive afterward, Dr. Ryono said.
There were 10 pairs of culture-positive patients treated at four facilities, one of which did not track serial numbers of duodenoscopes, according to Dr. Ryono. At that facility, procedure dates for pairs were 68-143 days apart, he said. Procedure dates for pairs at the other three facilities were spaced at least 82 days apart. Based on such extensive temporal spacing and the fact that the same scopes were used on culture-negative patients during intervening times, the investigators concluded that duodenoscopes were unlikely to have caused CRE infection or colonization.
Dr. Ryono noted several study limitations, however. Not only were CRE isolates unavailable for epidemiologic testing, but researchers also could not determine the extent to which facilities had implemented the 2010 guidelines for CLSI breakpoints. “Certainly, those facilities that have implemented those guidelines are more likely to catch CRE,” he said. Also, VA does not routinely screen patients for CRE before they undergo ERCP or EUS, “so it is very possible that some of our patients might have been colonized with CRE before the procedure, and we would not have been able to capture that,” he added. “Given the limitations of this review, the possibility that there was some transmission certainly cannot be completely excluded.”
Dr. Ryono reported these findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The researchers declared no funding sources or conflicts of interest.
AT IDWEEK 2015
Key clinical point: In a large retrospective review, the Department of Veterans Affairs found no evidence of transmission of carbapenem-resistant enterobacteriaceae through ERCP or EUS.
Major finding: The review found no compelling evidence of CRE transmission.
Data source: Retrospective study of 55,676 ERCP/EUS procedures in 40,329 veterans and 4,914 CRE isolates from 2,383 patients.
Disclosures:. The researchers reported no funding sources or conflicts of interest.
IDWeek: Despite better drugs, HCV deaths keep rising
SAN DIEGO – Despite better therapies, deaths from hepatitis C virus (HCV) infection continue to rise, indicating poor penetrance of medications and care to patients who need them, Dr. Scott Holmberg said at an annual scientific meeting on infectious diseases.
“Deaths in chronic HCV–infected persons, even when grossly under-enumerated on death certificates, far outstrip deaths from 60 other infectious conditions reportable to CDC,” said Dr. Holmberg of the Centers for Disease Control and Prevention Division of Viral Hepatitis in Atlanta.
Drugs for chronic HCV infection have vastly improved in the past several years, yielding far better rates of sustained viral response (SVR) and high chances of cure after 8-24 weeks of treatment. To see if better antiviral therapies have affected HCV mortality rates, Dr. Holmberg and his associates studied ICD-9 data from Multiple Cause of Death records for all U.S. death certificates between 2003 and 2013. They divided deaths that were linked to HCV or 60 other nationally notifiable infectious diseases by U.S. Census numbers for the same year. They also examined data from the Chronic Hepatitis Cohort Study (Clin Infect Dis. 2014;58:1055-61), which includes patients presumed to have adequate access to HCV treatment.
Chronic HCV-related deaths climbed from about 12,000 annually in 2003 to more than 19,000 in 2013, said Dr. Holmberg. In contrast, deaths from the 60 other reportable infectious diseases dropped from about 25,000 annually to below 20,000 per year. Annual deaths tied to HIV infection ranked second behind HCV at about 8,800, followed by Staphylococcus aureus (including MRSA), hepatitis B virus, tuberculosis, and pneumococcal disease. “This does not include 4,444 adult influenza deaths, but does include 165 childhood influenza deaths in 2013,” Dr. Holmberg noted.
The analysis of the Chronic Hepatitis Cohort Study revealed a doubling in mortality from chronic HCV infection among patients who should have had adequate access to treatment, according to Dr. Holmberg. For every 100 person-years of observation, about 2.5 people died from consequences of chronic HCV infection in 2007, compared with about 5.5 in 2013, he said. “Hidden mortality from HCV is considerable,” he added. “Only 19% of HCV patients who died had their infection noted anywhere on their death certificates, despite the fact that more than 75% had premortem evidence of liver disease.”
Uptake of sofosbuvir-based regimens more than quintupled in the second quarter of 2015, compared with a year earlier, according to data from Gilead Sciences presented by Dr. Holmberg. But high drug costs have spurred state Medicaid programs and private payers to stipulate many preapproval requirements, he noted. Patients must be drug and alcohol free for at least 6 months, and in many states, must provide evidence of liver scarring from a recent biopsy or FibroScan, which is not always easy to access. “This is often a barrier,” Dr. Holmberg said. “For those in more rural areas, finding a specialist, as required by many state Medicaid offices, can be very difficult.”
And there are even more obstacles. Many clinicians still see HCV as a “benign condition,” and patients often have other urgent health, social, or financial problems, Dr. Holmberg said. The public, for its part, may not prioritize infectious diseases. “These patients lack a strong advocacy group,” he added. “Most are former injection drug users, and the public is often reluctant to help them.”
So what are the measurable results of these barriers? Among about 3.2 million individuals in the United States with chronic HCV infection, only half were ever tested for HCV, 38% received some sort of care related to their infection, 11% were treated, and 6% achieved SVR, Dr. Holmberg and his associates noted in a perspective piece (N Engl J Med. 2013;368:1859-861).
At the same time, the United States faces an emerging epidemic of new HCV infections in nonurban areas among young persons who inject drugs (MMWR. 64;453-8). “This is really a tale of two epidemics,” he added. “Control of the chronic and the acute outbreaks will require a multipronged approach, with interventions along a testing to cure continuum of care.”
Dr. Holmberg and his associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported no funding sources and had no financial disclosures.
SAN DIEGO – Despite better therapies, deaths from hepatitis C virus (HCV) infection continue to rise, indicating poor penetrance of medications and care to patients who need them, Dr. Scott Holmberg said at an annual scientific meeting on infectious diseases.
“Deaths in chronic HCV–infected persons, even when grossly under-enumerated on death certificates, far outstrip deaths from 60 other infectious conditions reportable to CDC,” said Dr. Holmberg of the Centers for Disease Control and Prevention Division of Viral Hepatitis in Atlanta.
Drugs for chronic HCV infection have vastly improved in the past several years, yielding far better rates of sustained viral response (SVR) and high chances of cure after 8-24 weeks of treatment. To see if better antiviral therapies have affected HCV mortality rates, Dr. Holmberg and his associates studied ICD-9 data from Multiple Cause of Death records for all U.S. death certificates between 2003 and 2013. They divided deaths that were linked to HCV or 60 other nationally notifiable infectious diseases by U.S. Census numbers for the same year. They also examined data from the Chronic Hepatitis Cohort Study (Clin Infect Dis. 2014;58:1055-61), which includes patients presumed to have adequate access to HCV treatment.
Chronic HCV-related deaths climbed from about 12,000 annually in 2003 to more than 19,000 in 2013, said Dr. Holmberg. In contrast, deaths from the 60 other reportable infectious diseases dropped from about 25,000 annually to below 20,000 per year. Annual deaths tied to HIV infection ranked second behind HCV at about 8,800, followed by Staphylococcus aureus (including MRSA), hepatitis B virus, tuberculosis, and pneumococcal disease. “This does not include 4,444 adult influenza deaths, but does include 165 childhood influenza deaths in 2013,” Dr. Holmberg noted.
The analysis of the Chronic Hepatitis Cohort Study revealed a doubling in mortality from chronic HCV infection among patients who should have had adequate access to treatment, according to Dr. Holmberg. For every 100 person-years of observation, about 2.5 people died from consequences of chronic HCV infection in 2007, compared with about 5.5 in 2013, he said. “Hidden mortality from HCV is considerable,” he added. “Only 19% of HCV patients who died had their infection noted anywhere on their death certificates, despite the fact that more than 75% had premortem evidence of liver disease.”
Uptake of sofosbuvir-based regimens more than quintupled in the second quarter of 2015, compared with a year earlier, according to data from Gilead Sciences presented by Dr. Holmberg. But high drug costs have spurred state Medicaid programs and private payers to stipulate many preapproval requirements, he noted. Patients must be drug and alcohol free for at least 6 months, and in many states, must provide evidence of liver scarring from a recent biopsy or FibroScan, which is not always easy to access. “This is often a barrier,” Dr. Holmberg said. “For those in more rural areas, finding a specialist, as required by many state Medicaid offices, can be very difficult.”
And there are even more obstacles. Many clinicians still see HCV as a “benign condition,” and patients often have other urgent health, social, or financial problems, Dr. Holmberg said. The public, for its part, may not prioritize infectious diseases. “These patients lack a strong advocacy group,” he added. “Most are former injection drug users, and the public is often reluctant to help them.”
So what are the measurable results of these barriers? Among about 3.2 million individuals in the United States with chronic HCV infection, only half were ever tested for HCV, 38% received some sort of care related to their infection, 11% were treated, and 6% achieved SVR, Dr. Holmberg and his associates noted in a perspective piece (N Engl J Med. 2013;368:1859-861).
At the same time, the United States faces an emerging epidemic of new HCV infections in nonurban areas among young persons who inject drugs (MMWR. 64;453-8). “This is really a tale of two epidemics,” he added. “Control of the chronic and the acute outbreaks will require a multipronged approach, with interventions along a testing to cure continuum of care.”
Dr. Holmberg and his associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported no funding sources and had no financial disclosures.
SAN DIEGO – Despite better therapies, deaths from hepatitis C virus (HCV) infection continue to rise, indicating poor penetrance of medications and care to patients who need them, Dr. Scott Holmberg said at an annual scientific meeting on infectious diseases.
“Deaths in chronic HCV–infected persons, even when grossly under-enumerated on death certificates, far outstrip deaths from 60 other infectious conditions reportable to CDC,” said Dr. Holmberg of the Centers for Disease Control and Prevention Division of Viral Hepatitis in Atlanta.
Drugs for chronic HCV infection have vastly improved in the past several years, yielding far better rates of sustained viral response (SVR) and high chances of cure after 8-24 weeks of treatment. To see if better antiviral therapies have affected HCV mortality rates, Dr. Holmberg and his associates studied ICD-9 data from Multiple Cause of Death records for all U.S. death certificates between 2003 and 2013. They divided deaths that were linked to HCV or 60 other nationally notifiable infectious diseases by U.S. Census numbers for the same year. They also examined data from the Chronic Hepatitis Cohort Study (Clin Infect Dis. 2014;58:1055-61), which includes patients presumed to have adequate access to HCV treatment.
Chronic HCV-related deaths climbed from about 12,000 annually in 2003 to more than 19,000 in 2013, said Dr. Holmberg. In contrast, deaths from the 60 other reportable infectious diseases dropped from about 25,000 annually to below 20,000 per year. Annual deaths tied to HIV infection ranked second behind HCV at about 8,800, followed by Staphylococcus aureus (including MRSA), hepatitis B virus, tuberculosis, and pneumococcal disease. “This does not include 4,444 adult influenza deaths, but does include 165 childhood influenza deaths in 2013,” Dr. Holmberg noted.
The analysis of the Chronic Hepatitis Cohort Study revealed a doubling in mortality from chronic HCV infection among patients who should have had adequate access to treatment, according to Dr. Holmberg. For every 100 person-years of observation, about 2.5 people died from consequences of chronic HCV infection in 2007, compared with about 5.5 in 2013, he said. “Hidden mortality from HCV is considerable,” he added. “Only 19% of HCV patients who died had their infection noted anywhere on their death certificates, despite the fact that more than 75% had premortem evidence of liver disease.”
Uptake of sofosbuvir-based regimens more than quintupled in the second quarter of 2015, compared with a year earlier, according to data from Gilead Sciences presented by Dr. Holmberg. But high drug costs have spurred state Medicaid programs and private payers to stipulate many preapproval requirements, he noted. Patients must be drug and alcohol free for at least 6 months, and in many states, must provide evidence of liver scarring from a recent biopsy or FibroScan, which is not always easy to access. “This is often a barrier,” Dr. Holmberg said. “For those in more rural areas, finding a specialist, as required by many state Medicaid offices, can be very difficult.”
And there are even more obstacles. Many clinicians still see HCV as a “benign condition,” and patients often have other urgent health, social, or financial problems, Dr. Holmberg said. The public, for its part, may not prioritize infectious diseases. “These patients lack a strong advocacy group,” he added. “Most are former injection drug users, and the public is often reluctant to help them.”
So what are the measurable results of these barriers? Among about 3.2 million individuals in the United States with chronic HCV infection, only half were ever tested for HCV, 38% received some sort of care related to their infection, 11% were treated, and 6% achieved SVR, Dr. Holmberg and his associates noted in a perspective piece (N Engl J Med. 2013;368:1859-861).
At the same time, the United States faces an emerging epidemic of new HCV infections in nonurban areas among young persons who inject drugs (MMWR. 64;453-8). “This is really a tale of two epidemics,” he added. “Control of the chronic and the acute outbreaks will require a multipronged approach, with interventions along a testing to cure continuum of care.”
Dr. Holmberg and his associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. The researchers reported no funding sources and had no financial disclosures.
AT IDWEEK 2015
Key clinical point: Mortality from chronic hepatitis C virus infection continues to rise, despite significant improvements in antiviral therapies.
Major finding: Even with substantial underreporting, in 2013, deaths tied to chronic HCV infection exceeded mortality from 60 other reportable infectious diseases.
Data source: Analysis of 10 years of national death certificate data and 7 years of data from the Chronic Hepatitis Cohort Study.
Disclosures: The researchers reported no funding sources and made no financial disclosures.
IDWeek: Testing delays linked to misclassification of hospital-onset C. difficile
SAN DIEGO – Delays in laboratory testing led a hospital to misclassify the origin of nearly a quarter of Clostridium difficile infections, Dr. Christopher Polage said at an annual scientific meeting on infectious diseases.
“Many patients with symptoms and risk factors are not being tested within 3 days of admission, leading to overreporting of hospital-onset [Clostridium difficile infections] and underreporting of community-onset CDI,” said Dr. Polage of the UC Davis (Calif.) Health System. By testing patients who have diarrhea and risk factors for CDI sooner after admission, hospitals can prevent outbreaks and improve their standardized infection ratio for CDI, he added.
Clostridium difficile is implicated in about 29,000 deaths every year in the United States. The vast majority of such cases are classified as hospital onset, based on the “3-day rule,” meaning that patients were tested more than 3 days after admission. “This is the preventable hospital outcome that we’re all trying to bring down,” Dr. Polage said.
As part of that effort, he and his colleagues studied 11 hospital units that were considered high risk for CDI. To identify toxigenic C. difficile, they performed culture and polymerase chain reaction testing on perianal swabs collected from all adult patients admitted to these units. They also analyzed toxin immunoassays of stool samples when physicians requested them for patients with diarrhea.
“The question was, how often was misclassification happening?” said Dr. Polage. Among 48 cases that the laboratory reported as hospital onset, based on the “3-day rule,” close to half (44%) actually had CD-positive perianal swabs when admitted, he said. And half of these swab-positive patients waited more than 3 days for a CD stool test even though they had current or recent diarrhea, he added. In fact, swab-positive patients with diarrhea went a median of 6 days without a CD stool test, and some went untested for 10 days, Dr. Polage said.
“Anyone who has tried to determine if a hospitalized patient is having diarrhea knows that this can be very hard to pin down,” Dr. Polage noted. But some patients with positive swabs on admission met three different definitions for diarrhea, “making it pretty clear that they had community-onset CDI,” he said. This most conservative approach found that 23% of “hospital-onset” cases were actually community onset, he said.
Thus far, UC Davis Health System seems not to have had an increase in antibiotic prescriptions in response to greater detection of community-onset CDI, Dr. Polage said. “This is not something we take lightly,” he added. “We put together a lot of educational materials for patients, physicians, and providers, and work with our antibiotic stewardship team. We found that we might be able to focus on patients who had CDI at time of admission, and intervene with them individually to more carefully monitor what antibiotic they were using.”
Dr. Polage reported these findings at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The Gordon and Betty Moore Foundation helped fund the study. Dr. Polage reported having received research materials or honoraria from Cepheid, TechLab, Alere, Meridian, and ACEA Biosciences.
SAN DIEGO – Delays in laboratory testing led a hospital to misclassify the origin of nearly a quarter of Clostridium difficile infections, Dr. Christopher Polage said at an annual scientific meeting on infectious diseases.
“Many patients with symptoms and risk factors are not being tested within 3 days of admission, leading to overreporting of hospital-onset [Clostridium difficile infections] and underreporting of community-onset CDI,” said Dr. Polage of the UC Davis (Calif.) Health System. By testing patients who have diarrhea and risk factors for CDI sooner after admission, hospitals can prevent outbreaks and improve their standardized infection ratio for CDI, he added.
Clostridium difficile is implicated in about 29,000 deaths every year in the United States. The vast majority of such cases are classified as hospital onset, based on the “3-day rule,” meaning that patients were tested more than 3 days after admission. “This is the preventable hospital outcome that we’re all trying to bring down,” Dr. Polage said.
As part of that effort, he and his colleagues studied 11 hospital units that were considered high risk for CDI. To identify toxigenic C. difficile, they performed culture and polymerase chain reaction testing on perianal swabs collected from all adult patients admitted to these units. They also analyzed toxin immunoassays of stool samples when physicians requested them for patients with diarrhea.
“The question was, how often was misclassification happening?” said Dr. Polage. Among 48 cases that the laboratory reported as hospital onset, based on the “3-day rule,” close to half (44%) actually had CD-positive perianal swabs when admitted, he said. And half of these swab-positive patients waited more than 3 days for a CD stool test even though they had current or recent diarrhea, he added. In fact, swab-positive patients with diarrhea went a median of 6 days without a CD stool test, and some went untested for 10 days, Dr. Polage said.
“Anyone who has tried to determine if a hospitalized patient is having diarrhea knows that this can be very hard to pin down,” Dr. Polage noted. But some patients with positive swabs on admission met three different definitions for diarrhea, “making it pretty clear that they had community-onset CDI,” he said. This most conservative approach found that 23% of “hospital-onset” cases were actually community onset, he said.
Thus far, UC Davis Health System seems not to have had an increase in antibiotic prescriptions in response to greater detection of community-onset CDI, Dr. Polage said. “This is not something we take lightly,” he added. “We put together a lot of educational materials for patients, physicians, and providers, and work with our antibiotic stewardship team. We found that we might be able to focus on patients who had CDI at time of admission, and intervene with them individually to more carefully monitor what antibiotic they were using.”
Dr. Polage reported these findings at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The Gordon and Betty Moore Foundation helped fund the study. Dr. Polage reported having received research materials or honoraria from Cepheid, TechLab, Alere, Meridian, and ACEA Biosciences.
SAN DIEGO – Delays in laboratory testing led a hospital to misclassify the origin of nearly a quarter of Clostridium difficile infections, Dr. Christopher Polage said at an annual scientific meeting on infectious diseases.
“Many patients with symptoms and risk factors are not being tested within 3 days of admission, leading to overreporting of hospital-onset [Clostridium difficile infections] and underreporting of community-onset CDI,” said Dr. Polage of the UC Davis (Calif.) Health System. By testing patients who have diarrhea and risk factors for CDI sooner after admission, hospitals can prevent outbreaks and improve their standardized infection ratio for CDI, he added.
Clostridium difficile is implicated in about 29,000 deaths every year in the United States. The vast majority of such cases are classified as hospital onset, based on the “3-day rule,” meaning that patients were tested more than 3 days after admission. “This is the preventable hospital outcome that we’re all trying to bring down,” Dr. Polage said.
As part of that effort, he and his colleagues studied 11 hospital units that were considered high risk for CDI. To identify toxigenic C. difficile, they performed culture and polymerase chain reaction testing on perianal swabs collected from all adult patients admitted to these units. They also analyzed toxin immunoassays of stool samples when physicians requested them for patients with diarrhea.
“The question was, how often was misclassification happening?” said Dr. Polage. Among 48 cases that the laboratory reported as hospital onset, based on the “3-day rule,” close to half (44%) actually had CD-positive perianal swabs when admitted, he said. And half of these swab-positive patients waited more than 3 days for a CD stool test even though they had current or recent diarrhea, he added. In fact, swab-positive patients with diarrhea went a median of 6 days without a CD stool test, and some went untested for 10 days, Dr. Polage said.
“Anyone who has tried to determine if a hospitalized patient is having diarrhea knows that this can be very hard to pin down,” Dr. Polage noted. But some patients with positive swabs on admission met three different definitions for diarrhea, “making it pretty clear that they had community-onset CDI,” he said. This most conservative approach found that 23% of “hospital-onset” cases were actually community onset, he said.
Thus far, UC Davis Health System seems not to have had an increase in antibiotic prescriptions in response to greater detection of community-onset CDI, Dr. Polage said. “This is not something we take lightly,” he added. “We put together a lot of educational materials for patients, physicians, and providers, and work with our antibiotic stewardship team. We found that we might be able to focus on patients who had CDI at time of admission, and intervene with them individually to more carefully monitor what antibiotic they were using.”
Dr. Polage reported these findings at IDWeek, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The Gordon and Betty Moore Foundation helped fund the study. Dr. Polage reported having received research materials or honoraria from Cepheid, TechLab, Alere, Meridian, and ACEA Biosciences.
AT IDWEEK 2015
Key clinical point: Delays in laboratory testing led to misclassification of Clostridium difficile infections.
Major finding: At least 23% of cases that were reported as hospital onset were actually community onset.
Data source: An analysis of 12 months of C. difficile infection surveillance data from one academic medical center.
Disclosures: The Gordon and Betty Moore Foundation helped fund the study. Dr. Polage reported having received research materials or honoraria from Cepheid, TechLab, Alere, Meridian, and ACEA Biosciences.
IDWEEK: Antibiotic ‘time-out’ cut vancomycin use
SAN DIEGO – A “time-out” to review antibiotic therapy 72-96 hours into treatment increased appropriate discontinuations of vancomycin by 31%, researchers said at an annual scientific meeting on infectious diseases.
The practice fit into hospital work flow, streamlined other antibiotic stewardship practices, and respected provider autonomy, said Dr. Christopher Graber of VA Greater Los Angeles Healthcare System and the University of California, Los Angeles. It also was durable, persisting into the second 6 months of the program even though active research support had ended, he and his associates said.
The Centers for Disease Control and Prevention endorses the antibiotic time-out as a way to encourage providers to switch from empirical treatment with broad-spectrum antibiotics to a more tailored plan after culture and other laboratory results become available. The Veterans Affairs teaching hospital in greater Los Angeles created a self-directed time-out program to encourage reconsideration of broad-spectrum therapy with vancomycin and piperacillin-tazobactam after the first 3 days of empirical treatment. The program included an antimicrobial “dashboard” report, an electronic template to document time-outs, and a marketing program consisting of educational documents, lectures, “clinical champions,” and reminder notes and fliers posted near computers.
To evaluate early and late responses to the program, Dr. Graber and his associates tracked discontinuations of vancomycin and piperacillin-tazobactam, as well as decisions to continue these antibiotics through day 5 when doing so contradicted guidelines. Among 276 vancomycin episodes during the program, clinicians discontinued 175 (63%) – significantly more than before the program started (96 of 199; 48%; P = .001). They documented vancomycin time-outs 46% of the time, continued patients on vancomycin through day 5 without a time-out in 7.6% of cases, and allowed vancomycin to expire without a time-out 46% of the time.
The rate of inappropriate discontinuations of vancomycin during the program exceeded baseline (4.7% vs. none; P = .001), but this trend was balanced out by the overall rise in vancomycin discontinuations, the researchers said. Furthermore, providers documented vancomycin time-outs more often during the second 6 months, compared with the first (54% vs. 37%; P = .005). The increase in vancomycin discontinuations also was durable (about 63% throughout the two 6-month periods), and inappropriate continuations remained stable at about 4.5%.
“Piperacillin-tazobactam did not see any change in discontinuations between the two study periods,” said the researchers. The discontinuation rate was about 62% before and during the program. Clinicians performed time-outs about half the time, and inappropriately continued the antibiotics beyond day 5 in 10% of cases, compared with 2% before the program started (P = .02). Clinicians continued performing piperacillin-tazobactam time-outs at the same rate in the second 6 months of the program as during the beginning, and rates of discontinuation and inappropriate continuations also remained similar.
The investigators reported these results at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
CDC funded the research with a grant to the University of Utah. The researchers reported no conflicts of interest.
SAN DIEGO – A “time-out” to review antibiotic therapy 72-96 hours into treatment increased appropriate discontinuations of vancomycin by 31%, researchers said at an annual scientific meeting on infectious diseases.
The practice fit into hospital work flow, streamlined other antibiotic stewardship practices, and respected provider autonomy, said Dr. Christopher Graber of VA Greater Los Angeles Healthcare System and the University of California, Los Angeles. It also was durable, persisting into the second 6 months of the program even though active research support had ended, he and his associates said.
The Centers for Disease Control and Prevention endorses the antibiotic time-out as a way to encourage providers to switch from empirical treatment with broad-spectrum antibiotics to a more tailored plan after culture and other laboratory results become available. The Veterans Affairs teaching hospital in greater Los Angeles created a self-directed time-out program to encourage reconsideration of broad-spectrum therapy with vancomycin and piperacillin-tazobactam after the first 3 days of empirical treatment. The program included an antimicrobial “dashboard” report, an electronic template to document time-outs, and a marketing program consisting of educational documents, lectures, “clinical champions,” and reminder notes and fliers posted near computers.
To evaluate early and late responses to the program, Dr. Graber and his associates tracked discontinuations of vancomycin and piperacillin-tazobactam, as well as decisions to continue these antibiotics through day 5 when doing so contradicted guidelines. Among 276 vancomycin episodes during the program, clinicians discontinued 175 (63%) – significantly more than before the program started (96 of 199; 48%; P = .001). They documented vancomycin time-outs 46% of the time, continued patients on vancomycin through day 5 without a time-out in 7.6% of cases, and allowed vancomycin to expire without a time-out 46% of the time.
The rate of inappropriate discontinuations of vancomycin during the program exceeded baseline (4.7% vs. none; P = .001), but this trend was balanced out by the overall rise in vancomycin discontinuations, the researchers said. Furthermore, providers documented vancomycin time-outs more often during the second 6 months, compared with the first (54% vs. 37%; P = .005). The increase in vancomycin discontinuations also was durable (about 63% throughout the two 6-month periods), and inappropriate continuations remained stable at about 4.5%.
“Piperacillin-tazobactam did not see any change in discontinuations between the two study periods,” said the researchers. The discontinuation rate was about 62% before and during the program. Clinicians performed time-outs about half the time, and inappropriately continued the antibiotics beyond day 5 in 10% of cases, compared with 2% before the program started (P = .02). Clinicians continued performing piperacillin-tazobactam time-outs at the same rate in the second 6 months of the program as during the beginning, and rates of discontinuation and inappropriate continuations also remained similar.
The investigators reported these results at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
CDC funded the research with a grant to the University of Utah. The researchers reported no conflicts of interest.
SAN DIEGO – A “time-out” to review antibiotic therapy 72-96 hours into treatment increased appropriate discontinuations of vancomycin by 31%, researchers said at an annual scientific meeting on infectious diseases.
The practice fit into hospital work flow, streamlined other antibiotic stewardship practices, and respected provider autonomy, said Dr. Christopher Graber of VA Greater Los Angeles Healthcare System and the University of California, Los Angeles. It also was durable, persisting into the second 6 months of the program even though active research support had ended, he and his associates said.
The Centers for Disease Control and Prevention endorses the antibiotic time-out as a way to encourage providers to switch from empirical treatment with broad-spectrum antibiotics to a more tailored plan after culture and other laboratory results become available. The Veterans Affairs teaching hospital in greater Los Angeles created a self-directed time-out program to encourage reconsideration of broad-spectrum therapy with vancomycin and piperacillin-tazobactam after the first 3 days of empirical treatment. The program included an antimicrobial “dashboard” report, an electronic template to document time-outs, and a marketing program consisting of educational documents, lectures, “clinical champions,” and reminder notes and fliers posted near computers.
To evaluate early and late responses to the program, Dr. Graber and his associates tracked discontinuations of vancomycin and piperacillin-tazobactam, as well as decisions to continue these antibiotics through day 5 when doing so contradicted guidelines. Among 276 vancomycin episodes during the program, clinicians discontinued 175 (63%) – significantly more than before the program started (96 of 199; 48%; P = .001). They documented vancomycin time-outs 46% of the time, continued patients on vancomycin through day 5 without a time-out in 7.6% of cases, and allowed vancomycin to expire without a time-out 46% of the time.
The rate of inappropriate discontinuations of vancomycin during the program exceeded baseline (4.7% vs. none; P = .001), but this trend was balanced out by the overall rise in vancomycin discontinuations, the researchers said. Furthermore, providers documented vancomycin time-outs more often during the second 6 months, compared with the first (54% vs. 37%; P = .005). The increase in vancomycin discontinuations also was durable (about 63% throughout the two 6-month periods), and inappropriate continuations remained stable at about 4.5%.
“Piperacillin-tazobactam did not see any change in discontinuations between the two study periods,” said the researchers. The discontinuation rate was about 62% before and during the program. Clinicians performed time-outs about half the time, and inappropriately continued the antibiotics beyond day 5 in 10% of cases, compared with 2% before the program started (P = .02). Clinicians continued performing piperacillin-tazobactam time-outs at the same rate in the second 6 months of the program as during the beginning, and rates of discontinuation and inappropriate continuations also remained similar.
The investigators reported these results at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
CDC funded the research with a grant to the University of Utah. The researchers reported no conflicts of interest.
AT IDWEEK 2015
Key clinical point: A self-directed antibiotic time-out performed 72-96 hours into treatment was tied to increases in appropriate discontinuations of vancomycin.
Major finding: Discontinuation of vancomycin was higher than before the intervention (63% vs. 48%; P = .001).
Data source: Prospective single-center study.
Disclosures: CDC helped fund the study with a grant to the University of Utah. The researchers reported no conflicts of interest.
Study linked three genetic risk variants to alcohol-related cirrhosis
A genome-wide association study uncovered two new genetic risk variants for alcohol-related liver cirrhosis and confirmed a third previously identified susceptibility locus, researchers reported online Oct. 16 in Nature Genetics.
The new variants affect the MBOAT7 and TM6SF2 genes, while the third is rs738409, a single-nucleotide polymorphism of the PNPLA3 gene that was previously implicated in nonalcoholic fatty liver disease (NAFLD). “These three loci have a role in lipid processing, suggesting that lipid turnover is important in the pathogenesis of alcohol-related cirrhosis,” said Dr. Stephan Buch of the Dresden (Germany) University of Technology and his associates.
Most heavy drinkers develop hepatic steatosis (fatty liver), but a minority ultimately progress to cirrhosis. To assess genetic risk factors for alcohol-related cirrhosis, the researchers compared genome-wide association data for 712 cirrhotic alcoholics in Belgium, the United Kingdom, and Germany with 1,426 alcohol misusers from these countries who did not have cirrhosis. They validated the results in two separate cohorts of 1,148 cirrhotic alcoholics (cases) and 2,315 noncirrhotic alcoholic controls (Nat. Genet. 2015 Oct. 16. doi: 10.1038/ng.3417).
Variants in MBOAT7 and TM6SF2 significantly increased the risk of alcohol-related cirrhosis (P = 1.03 × 10-9 and 7.89 × 10-10, respectively), the investigators found. The rs738409 SNP in the PNPLA3 gene also was an important risk factor for alcohol-related cirrhosis (P = 1.54 × 10-48), as it is in NAFLD, added the researchers. Variants in both PNPLA3 and TM6SF2 have been linked to increased intrahepatic fat and potential loss of liver function. “Thus, we hypothesize that the genetic variants in these loci confer risk via dysfunctional lipid turnover,” they wrote.
Overlapping results from this study of alcohol-related cirrhosis and the prior genome-wide association study of NAFLD indicate that the two conditions might have similar pathogeneses, and that therapies targeting these genes might improve outcomes in both disorders. “The variants at the three identified loci may also help to define high-risk populations for targeted abstinence intervention and hepatic surveillance programs,” they concluded.
The investigators reported many funding sources, including the Germany Ministry of Education and Research, PopGen 2.0 network biobank, TU Dresden, Christian Albrechts University Kiel, Swiss National Funds, Ministry of Cultural Affairs, Social Ministry of the Federal State of Mecklenburg–West Pomerania, European Union, EFRE–State Ministry of Economics, Deutsche Krebshilfe, DFG Excellence Cluster 306, Foundation for Experimental Medicine (Switzerland), University College London, the Belgian Federal Science Policy Office, and the Fund for Scientific Research. The researchers declared no competing financial interests.
A genome-wide association study uncovered two new genetic risk variants for alcohol-related liver cirrhosis and confirmed a third previously identified susceptibility locus, researchers reported online Oct. 16 in Nature Genetics.
The new variants affect the MBOAT7 and TM6SF2 genes, while the third is rs738409, a single-nucleotide polymorphism of the PNPLA3 gene that was previously implicated in nonalcoholic fatty liver disease (NAFLD). “These three loci have a role in lipid processing, suggesting that lipid turnover is important in the pathogenesis of alcohol-related cirrhosis,” said Dr. Stephan Buch of the Dresden (Germany) University of Technology and his associates.
Most heavy drinkers develop hepatic steatosis (fatty liver), but a minority ultimately progress to cirrhosis. To assess genetic risk factors for alcohol-related cirrhosis, the researchers compared genome-wide association data for 712 cirrhotic alcoholics in Belgium, the United Kingdom, and Germany with 1,426 alcohol misusers from these countries who did not have cirrhosis. They validated the results in two separate cohorts of 1,148 cirrhotic alcoholics (cases) and 2,315 noncirrhotic alcoholic controls (Nat. Genet. 2015 Oct. 16. doi: 10.1038/ng.3417).
Variants in MBOAT7 and TM6SF2 significantly increased the risk of alcohol-related cirrhosis (P = 1.03 × 10-9 and 7.89 × 10-10, respectively), the investigators found. The rs738409 SNP in the PNPLA3 gene also was an important risk factor for alcohol-related cirrhosis (P = 1.54 × 10-48), as it is in NAFLD, added the researchers. Variants in both PNPLA3 and TM6SF2 have been linked to increased intrahepatic fat and potential loss of liver function. “Thus, we hypothesize that the genetic variants in these loci confer risk via dysfunctional lipid turnover,” they wrote.
Overlapping results from this study of alcohol-related cirrhosis and the prior genome-wide association study of NAFLD indicate that the two conditions might have similar pathogeneses, and that therapies targeting these genes might improve outcomes in both disorders. “The variants at the three identified loci may also help to define high-risk populations for targeted abstinence intervention and hepatic surveillance programs,” they concluded.
The investigators reported many funding sources, including the Germany Ministry of Education and Research, PopGen 2.0 network biobank, TU Dresden, Christian Albrechts University Kiel, Swiss National Funds, Ministry of Cultural Affairs, Social Ministry of the Federal State of Mecklenburg–West Pomerania, European Union, EFRE–State Ministry of Economics, Deutsche Krebshilfe, DFG Excellence Cluster 306, Foundation for Experimental Medicine (Switzerland), University College London, the Belgian Federal Science Policy Office, and the Fund for Scientific Research. The researchers declared no competing financial interests.
A genome-wide association study uncovered two new genetic risk variants for alcohol-related liver cirrhosis and confirmed a third previously identified susceptibility locus, researchers reported online Oct. 16 in Nature Genetics.
The new variants affect the MBOAT7 and TM6SF2 genes, while the third is rs738409, a single-nucleotide polymorphism of the PNPLA3 gene that was previously implicated in nonalcoholic fatty liver disease (NAFLD). “These three loci have a role in lipid processing, suggesting that lipid turnover is important in the pathogenesis of alcohol-related cirrhosis,” said Dr. Stephan Buch of the Dresden (Germany) University of Technology and his associates.
Most heavy drinkers develop hepatic steatosis (fatty liver), but a minority ultimately progress to cirrhosis. To assess genetic risk factors for alcohol-related cirrhosis, the researchers compared genome-wide association data for 712 cirrhotic alcoholics in Belgium, the United Kingdom, and Germany with 1,426 alcohol misusers from these countries who did not have cirrhosis. They validated the results in two separate cohorts of 1,148 cirrhotic alcoholics (cases) and 2,315 noncirrhotic alcoholic controls (Nat. Genet. 2015 Oct. 16. doi: 10.1038/ng.3417).
Variants in MBOAT7 and TM6SF2 significantly increased the risk of alcohol-related cirrhosis (P = 1.03 × 10-9 and 7.89 × 10-10, respectively), the investigators found. The rs738409 SNP in the PNPLA3 gene also was an important risk factor for alcohol-related cirrhosis (P = 1.54 × 10-48), as it is in NAFLD, added the researchers. Variants in both PNPLA3 and TM6SF2 have been linked to increased intrahepatic fat and potential loss of liver function. “Thus, we hypothesize that the genetic variants in these loci confer risk via dysfunctional lipid turnover,” they wrote.
Overlapping results from this study of alcohol-related cirrhosis and the prior genome-wide association study of NAFLD indicate that the two conditions might have similar pathogeneses, and that therapies targeting these genes might improve outcomes in both disorders. “The variants at the three identified loci may also help to define high-risk populations for targeted abstinence intervention and hepatic surveillance programs,” they concluded.
The investigators reported many funding sources, including the Germany Ministry of Education and Research, PopGen 2.0 network biobank, TU Dresden, Christian Albrechts University Kiel, Swiss National Funds, Ministry of Cultural Affairs, Social Ministry of the Federal State of Mecklenburg–West Pomerania, European Union, EFRE–State Ministry of Economics, Deutsche Krebshilfe, DFG Excellence Cluster 306, Foundation for Experimental Medicine (Switzerland), University College London, the Belgian Federal Science Policy Office, and the Fund for Scientific Research. The researchers declared no competing financial interests.
FROM NATURE GENETICS
Key clinical point: A genome-wide association study identified two new gene variants and confirmed a third as risk factors for alcohol-related cirrhosis.
Major finding: Variants in MBOAT7 and TM6SF2 significantly increased the risk of alcohol-related cirrhosis (P = 1.03 × 10-9 and 7.89 × 10-10, respectively). A single-nucleotide polymorphism (rs738409) in the PNPLA3 gene also was implicated (P = 1.54 × 10-48), as it has been in non–alcohol-related fatty liver disease.
Data source: Genome-wide association case-control study of 2,138 individuals of European descent.
Disclosures: The investigators reported many funding sources, including the Germany Ministry of Education and Research, PopGen 2.0 network biobank, TU Dresden, Christian Albrechts University Kiel, Swiss National Funds, Ministry of Cultural Affairs, Social Ministry of the Federal State of Mecklenburg–West Pomerania, European Union, EFRE–State Ministry of Economics, Deutsche Krebshilfe, DFG Excellence Cluster 306, Foundation for Ex-perimental Medicine (Switzerland), University College London, the Belgian Federal Science Policy Office, and the Fund for Scientific Research. The researchers declared no competing financial interests.
IDWeek: Rifapentine had best completion rates for health care workers with latent TB
SAN DIEGO – Health care workers with latent tuberculosis infection (LTBI) were significantly more likely to continue a shorter course of weekly rifapentine plus isoniazid (INH) than daily INH monotherapy, researchers reported at an annual scientific meeting on infectious diseases.
“Consideration should be given to no longer routinely recommending INH for the treatment of LTBI among health care workers,” said Dr. Esther Arguello Perez of Memorial Sloan Kettering Cancer Center, New York.
Health care workers face a greater risk of TB infection than the general population, regardless of the income level in the country where they live; patients with undiagnosed laryngeal or pulmonary TB usually pose the greatest risk, especially during procedures that cause coughing, such as sputum induction and bronchoscopy (Int J Tuberc Lung Dis. 2007;11[6]:593-605).
Although occupational TB testing is routine in U.S. health care organizations, more than half of health care workers who start treatment for LTBI historically have failed to finish (Chest. 2010;137[2]:401-9. doi: 10.1378/chest.09-0394). The standard LTBI regimen – 300 mg INH daily for 9 months – has been linked to potentially intolerable adverse effects such as hepatotoxicity, persistent gastrointestinal symptoms, rash, and neuropsychiatric problems (Drug Healthc Patient Saf. 2014;6:145-9. doi: 10.2147/DHPS.S68837).
In a 2011 multicenter trial, investigators reported a significantly higher completion rate for weekly rifapentine plus INH (900 mg each; 82% vs. 69% for daily INH; P < .001). Rates of adverse effects were significantly lower with weekly rifapentine plus INH, although grade 3-4 events and risk of death did not differ between the groups (N Engl J Med. 2011;365:2155-66. doi: 10.1056/NEJMoa1104875). The results of that trial quickly transformed recommendations for LTBI treatment (MMWR. 2011:60(48);1650-53).
Memorial Sloan Kettering implemented weekly rifapentine plus INH for its LTBI personnel in 2011. By 2014, about three-quarters of personnel with LTBI received rifapentine plus INH, while the rest were evenly split between rifampin and INH monotherapy, Dr. Arguello Perez reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
To understand how health care workers’ attitudes and treatment acceptance shifted along with practice, the investigators reviewed records from all health care workers at Memorial Sloan Kettering who were diagnosed with LTBI for 2005-2014. Among 930 patients, only 357 (38%) accepted treatment, although 76% of these individuals finished the regimen they started, she noted. Rifapentine plus INH had the highest completion rate (88%), significantly exceeding rates for a 4-month course of daily rifampin (84%) and for 9 months of INH monotherapy (70%; P < .01 for both differences). In contrast, completion rates for rifampin and INH did not significantly differ, Dr. Arguello Perez said.
Notably, LTBI treatment completion rates among health care workers rose by 26% between 2013, when most prescriptions were for rifampin or INH monotherapy, and 2014, when most were for rifapentine plus INH. “Health care workers might be more likely to accept treatment for LTBI if they know about alternatives to INH,” she concluded.
Dr. Arguello Perez and her associates reported no funding sources and had no financial disclosures.
SAN DIEGO – Health care workers with latent tuberculosis infection (LTBI) were significantly more likely to continue a shorter course of weekly rifapentine plus isoniazid (INH) than daily INH monotherapy, researchers reported at an annual scientific meeting on infectious diseases.
“Consideration should be given to no longer routinely recommending INH for the treatment of LTBI among health care workers,” said Dr. Esther Arguello Perez of Memorial Sloan Kettering Cancer Center, New York.
Health care workers face a greater risk of TB infection than the general population, regardless of the income level in the country where they live; patients with undiagnosed laryngeal or pulmonary TB usually pose the greatest risk, especially during procedures that cause coughing, such as sputum induction and bronchoscopy (Int J Tuberc Lung Dis. 2007;11[6]:593-605).
Although occupational TB testing is routine in U.S. health care organizations, more than half of health care workers who start treatment for LTBI historically have failed to finish (Chest. 2010;137[2]:401-9. doi: 10.1378/chest.09-0394). The standard LTBI regimen – 300 mg INH daily for 9 months – has been linked to potentially intolerable adverse effects such as hepatotoxicity, persistent gastrointestinal symptoms, rash, and neuropsychiatric problems (Drug Healthc Patient Saf. 2014;6:145-9. doi: 10.2147/DHPS.S68837).
In a 2011 multicenter trial, investigators reported a significantly higher completion rate for weekly rifapentine plus INH (900 mg each; 82% vs. 69% for daily INH; P < .001). Rates of adverse effects were significantly lower with weekly rifapentine plus INH, although grade 3-4 events and risk of death did not differ between the groups (N Engl J Med. 2011;365:2155-66. doi: 10.1056/NEJMoa1104875). The results of that trial quickly transformed recommendations for LTBI treatment (MMWR. 2011:60(48);1650-53).
Memorial Sloan Kettering implemented weekly rifapentine plus INH for its LTBI personnel in 2011. By 2014, about three-quarters of personnel with LTBI received rifapentine plus INH, while the rest were evenly split between rifampin and INH monotherapy, Dr. Arguello Perez reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
To understand how health care workers’ attitudes and treatment acceptance shifted along with practice, the investigators reviewed records from all health care workers at Memorial Sloan Kettering who were diagnosed with LTBI for 2005-2014. Among 930 patients, only 357 (38%) accepted treatment, although 76% of these individuals finished the regimen they started, she noted. Rifapentine plus INH had the highest completion rate (88%), significantly exceeding rates for a 4-month course of daily rifampin (84%) and for 9 months of INH monotherapy (70%; P < .01 for both differences). In contrast, completion rates for rifampin and INH did not significantly differ, Dr. Arguello Perez said.
Notably, LTBI treatment completion rates among health care workers rose by 26% between 2013, when most prescriptions were for rifampin or INH monotherapy, and 2014, when most were for rifapentine plus INH. “Health care workers might be more likely to accept treatment for LTBI if they know about alternatives to INH,” she concluded.
Dr. Arguello Perez and her associates reported no funding sources and had no financial disclosures.
SAN DIEGO – Health care workers with latent tuberculosis infection (LTBI) were significantly more likely to continue a shorter course of weekly rifapentine plus isoniazid (INH) than daily INH monotherapy, researchers reported at an annual scientific meeting on infectious diseases.
“Consideration should be given to no longer routinely recommending INH for the treatment of LTBI among health care workers,” said Dr. Esther Arguello Perez of Memorial Sloan Kettering Cancer Center, New York.
Health care workers face a greater risk of TB infection than the general population, regardless of the income level in the country where they live; patients with undiagnosed laryngeal or pulmonary TB usually pose the greatest risk, especially during procedures that cause coughing, such as sputum induction and bronchoscopy (Int J Tuberc Lung Dis. 2007;11[6]:593-605).
Although occupational TB testing is routine in U.S. health care organizations, more than half of health care workers who start treatment for LTBI historically have failed to finish (Chest. 2010;137[2]:401-9. doi: 10.1378/chest.09-0394). The standard LTBI regimen – 300 mg INH daily for 9 months – has been linked to potentially intolerable adverse effects such as hepatotoxicity, persistent gastrointestinal symptoms, rash, and neuropsychiatric problems (Drug Healthc Patient Saf. 2014;6:145-9. doi: 10.2147/DHPS.S68837).
In a 2011 multicenter trial, investigators reported a significantly higher completion rate for weekly rifapentine plus INH (900 mg each; 82% vs. 69% for daily INH; P < .001). Rates of adverse effects were significantly lower with weekly rifapentine plus INH, although grade 3-4 events and risk of death did not differ between the groups (N Engl J Med. 2011;365:2155-66. doi: 10.1056/NEJMoa1104875). The results of that trial quickly transformed recommendations for LTBI treatment (MMWR. 2011:60(48);1650-53).
Memorial Sloan Kettering implemented weekly rifapentine plus INH for its LTBI personnel in 2011. By 2014, about three-quarters of personnel with LTBI received rifapentine plus INH, while the rest were evenly split between rifampin and INH monotherapy, Dr. Arguello Perez reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
To understand how health care workers’ attitudes and treatment acceptance shifted along with practice, the investigators reviewed records from all health care workers at Memorial Sloan Kettering who were diagnosed with LTBI for 2005-2014. Among 930 patients, only 357 (38%) accepted treatment, although 76% of these individuals finished the regimen they started, she noted. Rifapentine plus INH had the highest completion rate (88%), significantly exceeding rates for a 4-month course of daily rifampin (84%) and for 9 months of INH monotherapy (70%; P < .01 for both differences). In contrast, completion rates for rifampin and INH did not significantly differ, Dr. Arguello Perez said.
Notably, LTBI treatment completion rates among health care workers rose by 26% between 2013, when most prescriptions were for rifampin or INH monotherapy, and 2014, when most were for rifapentine plus INH. “Health care workers might be more likely to accept treatment for LTBI if they know about alternatives to INH,” she concluded.
Dr. Arguello Perez and her associates reported no funding sources and had no financial disclosures.
AT IDweek 2015
Key clinical point: Isoniazid monotherapy should not be recommended routinely for health care workers with latent tuberculosis infections.
Major finding: Rates of treatment completion were significantly higher for weekly rifapentine plus isoniazid than for daily isoniazid monotherapy (P < .01).
Data source: Retrospective analysis of all health care workers with LTBI at Memorial Sloan Kettering Cancer Center during 2005-2014.
Disclosures: The researchers reported no funding sources and made no financial disclosures.
Software picked up HAI clusters faster than hospitals
SAN DIEGO – An automated outbreak detection system was popular among infection preventionists and detected pathogenic clusters about 5 days sooner than did hospitals themselves, researchers said at an annual scientific meeting on infectious diseases.
“The vast majority of hospitals were very pleased to expand surveillance beyond multidrug-resistant organisms, and most thought this system would improve their ability to detect outbreaks and streamline their work,” said Dr. Meghan Baker of Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
Patients in acute-care hospitals acquired almost 782,000 healthcare-associated infections (HAIs) in 2011, according to the Centers for Disease Control and Prevention.
Traditionally, infection preventionists at these hospitals look for HAIs by identifying temporal or spatial clusters of “a limited number of prespecified pathogens,” Dr. Baker said. But some real clusters do not meet these empirical rules, leading to more cases and potentially severe consequences for patients. Automated HAI outbreak detection systems can help, but not if they constantly trigger false alarms or, conversely, are so specific that they miss outbreaks.
Using the Premier SafetySurveillor infection control tool and free statistical software, Dr. Baker and her associates analyzed 83 years of historical microbiology data from 44 hospitals. The system signaled for any cluster involving at least three cases, even if cases occurred by chance less than once a year, she said. The researchers compared the results with outbreak data submitted by a convenience sample of hospitals that used their usual surveillance methods.
The automated approach identified 230 clusters. Most were detected based on antimicrobial resistance, but others shared the same ward or specialty service and some produced more than one signal. Clusters most often involved Staphylococcus aureus, Enterococcus, Pseudomonas, Escherichia coli, and Klebsiella, but most organisms were not under routine surveillance. As a result, 89% of clusters detected by the automated tool were not detected by hospitals using their usual methods, she emphasized. “Some were not real breaks, as determined later by genetic typing,” she added. “Most real clusters were [methicillin-resistant S. aureus], Clostridium difficile, and some were resistant gram-negative rods.”
When surveyed, 76% of infection preventionists said the automated tool moderately or greatly improved their ability to detect outbreaks, Dr. Baker reported. They would have wanted notification about 81% of the clusters, and considered 47% to be moderately or highly concerning. Notably, 51% of clusters expanded after detection.
“If these had been detected in real time, it would have been possible to intervene and possibly curtail the outbreak,” and 237 (42%) of 559 infections might have been avoided if these interventions were successful, she said.
Dr. Baker and her associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Baker reported no competing interests. Two of her associates reported financial and relationships with Premier, Sage, Molnycke, and 3M.
SAN DIEGO – An automated outbreak detection system was popular among infection preventionists and detected pathogenic clusters about 5 days sooner than did hospitals themselves, researchers said at an annual scientific meeting on infectious diseases.
“The vast majority of hospitals were very pleased to expand surveillance beyond multidrug-resistant organisms, and most thought this system would improve their ability to detect outbreaks and streamline their work,” said Dr. Meghan Baker of Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
Patients in acute-care hospitals acquired almost 782,000 healthcare-associated infections (HAIs) in 2011, according to the Centers for Disease Control and Prevention.
Traditionally, infection preventionists at these hospitals look for HAIs by identifying temporal or spatial clusters of “a limited number of prespecified pathogens,” Dr. Baker said. But some real clusters do not meet these empirical rules, leading to more cases and potentially severe consequences for patients. Automated HAI outbreak detection systems can help, but not if they constantly trigger false alarms or, conversely, are so specific that they miss outbreaks.
Using the Premier SafetySurveillor infection control tool and free statistical software, Dr. Baker and her associates analyzed 83 years of historical microbiology data from 44 hospitals. The system signaled for any cluster involving at least three cases, even if cases occurred by chance less than once a year, she said. The researchers compared the results with outbreak data submitted by a convenience sample of hospitals that used their usual surveillance methods.
The automated approach identified 230 clusters. Most were detected based on antimicrobial resistance, but others shared the same ward or specialty service and some produced more than one signal. Clusters most often involved Staphylococcus aureus, Enterococcus, Pseudomonas, Escherichia coli, and Klebsiella, but most organisms were not under routine surveillance. As a result, 89% of clusters detected by the automated tool were not detected by hospitals using their usual methods, she emphasized. “Some were not real breaks, as determined later by genetic typing,” she added. “Most real clusters were [methicillin-resistant S. aureus], Clostridium difficile, and some were resistant gram-negative rods.”
When surveyed, 76% of infection preventionists said the automated tool moderately or greatly improved their ability to detect outbreaks, Dr. Baker reported. They would have wanted notification about 81% of the clusters, and considered 47% to be moderately or highly concerning. Notably, 51% of clusters expanded after detection.
“If these had been detected in real time, it would have been possible to intervene and possibly curtail the outbreak,” and 237 (42%) of 559 infections might have been avoided if these interventions were successful, she said.
Dr. Baker and her associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Baker reported no competing interests. Two of her associates reported financial and relationships with Premier, Sage, Molnycke, and 3M.
SAN DIEGO – An automated outbreak detection system was popular among infection preventionists and detected pathogenic clusters about 5 days sooner than did hospitals themselves, researchers said at an annual scientific meeting on infectious diseases.
“The vast majority of hospitals were very pleased to expand surveillance beyond multidrug-resistant organisms, and most thought this system would improve their ability to detect outbreaks and streamline their work,” said Dr. Meghan Baker of Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
Patients in acute-care hospitals acquired almost 782,000 healthcare-associated infections (HAIs) in 2011, according to the Centers for Disease Control and Prevention.
Traditionally, infection preventionists at these hospitals look for HAIs by identifying temporal or spatial clusters of “a limited number of prespecified pathogens,” Dr. Baker said. But some real clusters do not meet these empirical rules, leading to more cases and potentially severe consequences for patients. Automated HAI outbreak detection systems can help, but not if they constantly trigger false alarms or, conversely, are so specific that they miss outbreaks.
Using the Premier SafetySurveillor infection control tool and free statistical software, Dr. Baker and her associates analyzed 83 years of historical microbiology data from 44 hospitals. The system signaled for any cluster involving at least three cases, even if cases occurred by chance less than once a year, she said. The researchers compared the results with outbreak data submitted by a convenience sample of hospitals that used their usual surveillance methods.
The automated approach identified 230 clusters. Most were detected based on antimicrobial resistance, but others shared the same ward or specialty service and some produced more than one signal. Clusters most often involved Staphylococcus aureus, Enterococcus, Pseudomonas, Escherichia coli, and Klebsiella, but most organisms were not under routine surveillance. As a result, 89% of clusters detected by the automated tool were not detected by hospitals using their usual methods, she emphasized. “Some were not real breaks, as determined later by genetic typing,” she added. “Most real clusters were [methicillin-resistant S. aureus], Clostridium difficile, and some were resistant gram-negative rods.”
When surveyed, 76% of infection preventionists said the automated tool moderately or greatly improved their ability to detect outbreaks, Dr. Baker reported. They would have wanted notification about 81% of the clusters, and considered 47% to be moderately or highly concerning. Notably, 51% of clusters expanded after detection.
“If these had been detected in real time, it would have been possible to intervene and possibly curtail the outbreak,” and 237 (42%) of 559 infections might have been avoided if these interventions were successful, she said.
Dr. Baker and her associates reported their findings at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Dr. Baker reported no competing interests. Two of her associates reported financial and relationships with Premier, Sage, Molnycke, and 3M.
AT IDWEEK 2015
Key clinical point: An automated outbreak detection system can augment traditional methods for detecting pathogenic clusters.
Major finding: The software tool identified clusters about 5 days sooner than did hospitals themselves.
Data source: Comparison of an automated outbreak detection tool with usual hospital surveillance methods, and surveys of infection preventionists.
Disclosures: Dr. Baker reported no competing interests. Two of her associates reported financial and relationships with Premier, Sage, Molnycke, and 3M.
Self-assessed mental health scores tied to radical cystectomy complications
Patients with bladder cancer and low scores on self-assessed mental health testing had more high-grade complications after radical cystectomy than their peers with better scores, even though their physical health was comparable, according to research published in the Journal of Urology.
The retrospective study mirrors past work linking preoperative mental disorders to adverse outcomes after major abdominal surgery, bariatric surgery, cardiac surgery, and kidney transplantation, said Dr. Pranav Sharma and his associates at Moffitt Cancer Center in Tampa. This study is the first to examine the association for bladder cancer patients who undergo radical cystectomy, according to the researchers.
They examined 30-day rates of Clavien grade IIIa or greater complications among 274 patients who filled out the Short Form 12 self-assessment of health status less than 6 months before surgery as part of a “new patient” questionnaire (J Urol. 2015. doi:10.1016/j.juro.2015.07.095). This included only 58% of radical cystectomy patients treated at Moffitt, and these patients had higher rates of positive soft-tissue margins and more aggressive pathology than did radical cystectomy patients who did not fill out the SF-12, the researchers said.
In all, 17% of patients experienced at least one high-grade complication within 30 days of their surgery. The median mental health composite score among patients with high-grade complications was 44.8 on a 100-point scale – significantly lower than the 49.8 score for patients who did not experience 30-day high-grade complications (P = .004). Physical composite scores did not significantly differ among patients who did and did not experience 30-day high-grade complications (median, 39.2 for patients with complications vs. 43.8 for those without; P = .06), the investigators said.
A model that incorporated known risk factors for postoperative complications – including age, body mass index, age-adjusted Charlson Comorbidity Index, American Society of Anesthesiologists score, preoperative albumin, and pathologic tumor stage – was not significantly linked with 30-day high-grade complications, even after including the SF-12 physical composite score (P = .14), Dr. Sharma and his associates reported. The association, however, became significant after they added the mental health composite score to the model (odds ratio, 0.96; 95% confidence interval, 0.93-0.99; P = .01).
“Although there is responder subjectivity associated with survey-based health-related [quality of life] measures, this finding suggests that measuring baseline mental health before surgery may provide additional information that can improve the risk stratification of patients undergoing radical cystectomy,” they wrote.
The study might have lacked statistical power to detect some associations, and the small sample size precluded the researchers from testing associations between self-reported health and specific types of complications, they noted. Also, “since we evaluated patients retrospectively, only a non-causal association between preoperative SF-12 mental composite score and high-grade 30-day complications after radical cystectomy can be suggested,” they cautioned.
The research was partly supported by the Collaborative Data Services Core at the H. Lee Moffitt Cancer Center & Research Institute. The investigators declared no conflicts of interest.
Patients with bladder cancer and low scores on self-assessed mental health testing had more high-grade complications after radical cystectomy than their peers with better scores, even though their physical health was comparable, according to research published in the Journal of Urology.
The retrospective study mirrors past work linking preoperative mental disorders to adverse outcomes after major abdominal surgery, bariatric surgery, cardiac surgery, and kidney transplantation, said Dr. Pranav Sharma and his associates at Moffitt Cancer Center in Tampa. This study is the first to examine the association for bladder cancer patients who undergo radical cystectomy, according to the researchers.
They examined 30-day rates of Clavien grade IIIa or greater complications among 274 patients who filled out the Short Form 12 self-assessment of health status less than 6 months before surgery as part of a “new patient” questionnaire (J Urol. 2015. doi:10.1016/j.juro.2015.07.095). This included only 58% of radical cystectomy patients treated at Moffitt, and these patients had higher rates of positive soft-tissue margins and more aggressive pathology than did radical cystectomy patients who did not fill out the SF-12, the researchers said.
In all, 17% of patients experienced at least one high-grade complication within 30 days of their surgery. The median mental health composite score among patients with high-grade complications was 44.8 on a 100-point scale – significantly lower than the 49.8 score for patients who did not experience 30-day high-grade complications (P = .004). Physical composite scores did not significantly differ among patients who did and did not experience 30-day high-grade complications (median, 39.2 for patients with complications vs. 43.8 for those without; P = .06), the investigators said.
A model that incorporated known risk factors for postoperative complications – including age, body mass index, age-adjusted Charlson Comorbidity Index, American Society of Anesthesiologists score, preoperative albumin, and pathologic tumor stage – was not significantly linked with 30-day high-grade complications, even after including the SF-12 physical composite score (P = .14), Dr. Sharma and his associates reported. The association, however, became significant after they added the mental health composite score to the model (odds ratio, 0.96; 95% confidence interval, 0.93-0.99; P = .01).
“Although there is responder subjectivity associated with survey-based health-related [quality of life] measures, this finding suggests that measuring baseline mental health before surgery may provide additional information that can improve the risk stratification of patients undergoing radical cystectomy,” they wrote.
The study might have lacked statistical power to detect some associations, and the small sample size precluded the researchers from testing associations between self-reported health and specific types of complications, they noted. Also, “since we evaluated patients retrospectively, only a non-causal association between preoperative SF-12 mental composite score and high-grade 30-day complications after radical cystectomy can be suggested,” they cautioned.
The research was partly supported by the Collaborative Data Services Core at the H. Lee Moffitt Cancer Center & Research Institute. The investigators declared no conflicts of interest.
Patients with bladder cancer and low scores on self-assessed mental health testing had more high-grade complications after radical cystectomy than their peers with better scores, even though their physical health was comparable, according to research published in the Journal of Urology.
The retrospective study mirrors past work linking preoperative mental disorders to adverse outcomes after major abdominal surgery, bariatric surgery, cardiac surgery, and kidney transplantation, said Dr. Pranav Sharma and his associates at Moffitt Cancer Center in Tampa. This study is the first to examine the association for bladder cancer patients who undergo radical cystectomy, according to the researchers.
They examined 30-day rates of Clavien grade IIIa or greater complications among 274 patients who filled out the Short Form 12 self-assessment of health status less than 6 months before surgery as part of a “new patient” questionnaire (J Urol. 2015. doi:10.1016/j.juro.2015.07.095). This included only 58% of radical cystectomy patients treated at Moffitt, and these patients had higher rates of positive soft-tissue margins and more aggressive pathology than did radical cystectomy patients who did not fill out the SF-12, the researchers said.
In all, 17% of patients experienced at least one high-grade complication within 30 days of their surgery. The median mental health composite score among patients with high-grade complications was 44.8 on a 100-point scale – significantly lower than the 49.8 score for patients who did not experience 30-day high-grade complications (P = .004). Physical composite scores did not significantly differ among patients who did and did not experience 30-day high-grade complications (median, 39.2 for patients with complications vs. 43.8 for those without; P = .06), the investigators said.
A model that incorporated known risk factors for postoperative complications – including age, body mass index, age-adjusted Charlson Comorbidity Index, American Society of Anesthesiologists score, preoperative albumin, and pathologic tumor stage – was not significantly linked with 30-day high-grade complications, even after including the SF-12 physical composite score (P = .14), Dr. Sharma and his associates reported. The association, however, became significant after they added the mental health composite score to the model (odds ratio, 0.96; 95% confidence interval, 0.93-0.99; P = .01).
“Although there is responder subjectivity associated with survey-based health-related [quality of life] measures, this finding suggests that measuring baseline mental health before surgery may provide additional information that can improve the risk stratification of patients undergoing radical cystectomy,” they wrote.
The study might have lacked statistical power to detect some associations, and the small sample size precluded the researchers from testing associations between self-reported health and specific types of complications, they noted. Also, “since we evaluated patients retrospectively, only a non-causal association between preoperative SF-12 mental composite score and high-grade 30-day complications after radical cystectomy can be suggested,” they cautioned.
The research was partly supported by the Collaborative Data Services Core at the H. Lee Moffitt Cancer Center & Research Institute. The investigators declared no conflicts of interest.
FROM THE JOURNAL OF UROLOGY
Key clinical point: Measuring baseline mental health before surgery may provide additional information that can improve the risk stratification of patients undergoing radical cystectomy.
Major finding: The median mental health composite score among patients with high-grade complications was 44.8 on a 100-point scale – significantly lower than the 49.8 median score for patients with no 30-day high-grade complications (P = .004).
Data source: Single-center retrospective study of patients who completed the Short Form 12 less than 6 months before undergoing surgery.
Disclosures: The research was partly supported by the Collaborative Data Services Core at the H. Lee Moffitt Cancer Center and Research Institute. The investigators declared no conflicts of interest.
Federally funded prevention programs tied to drop in suicide attempts
Federally funded suicide prevention programs might have helped avert more than 79,000 suicide attempts by young people over a 3-year period, said authors of a national observational study.
Counties that implemented the comprehensive, community-based programs reported about 5 fewer suicide attempts for every 1,000 young people aged 16-23 years, compared with counties that were otherwise similar but lacked the programs, said Lucas Godoy Garraza of ICF International, New York, and his associates.
Since 2004, the Garrett Lee Smith (GLS) Memorial Act has channeled competitive federal grants to states, colleges, and tribes for youth suicide prevention programs. These initiatives include activities aimed at increasing suicide awareness; screening at-risk youth; training gatekeepers; and connecting young people and families with mental health care, survivor support programs, and crisis hotlines, the investigators said. A prior study tied the programs to short-term drops in rates of completed suicides, they noted (JAMA Psychiatry. 2015 Oct 14. doi: 10.1001/jamapsychiatry.2015.1933). To explore the effects of the GLS program on youth suicide attempts, the investigators compared National Survey on Drug Use and Health data for 466 counties that had implemented the programs and 1,161 counties that had not but were otherwise similar. The final dataset included 46 states and 12 tribal communities, the researchers said.
A year after implementing GLS-funded programs, counties averaged 4.9 fewer suicide attempts per 1,000 youths than did control counties (95% confidence interval, 1.8-8.0; P = .003). “Although causality cannot be definitively inferred from our study owing to a lack of random assignment, these results suggest that more than 79,000 attempts were avoided between 2008 and 2011 following implementation of the GLS program,” the investigators added.
The results, however, were not durable. Two years after program implementation, rates of suicide attempts in GLS counties again resembled those of non-GLS counties, mirroring findings from the study of suicide mortality, the investigators said. “Many factors not directly addressed by the present study likely contribute to these results,” they added. “Assessments of comprehensive suicide prevention programs in other contexts have suggested that adherence and focus on comprehensive suicide prevention activities may fade over time. The present results do not suggest that the GLS program activities were effective only once but, rather, that effectively preventing suicides requires continued implementation of program activities.”
The Substance Abuse and Mental Health Services Administration funded the study. Richard McKeon, Ph.D., one of the authors, is employed by SAMHSA. The other investigators declared no conflicts of interest.
Federally funded suicide prevention programs might have helped avert more than 79,000 suicide attempts by young people over a 3-year period, said authors of a national observational study.
Counties that implemented the comprehensive, community-based programs reported about 5 fewer suicide attempts for every 1,000 young people aged 16-23 years, compared with counties that were otherwise similar but lacked the programs, said Lucas Godoy Garraza of ICF International, New York, and his associates.
Since 2004, the Garrett Lee Smith (GLS) Memorial Act has channeled competitive federal grants to states, colleges, and tribes for youth suicide prevention programs. These initiatives include activities aimed at increasing suicide awareness; screening at-risk youth; training gatekeepers; and connecting young people and families with mental health care, survivor support programs, and crisis hotlines, the investigators said. A prior study tied the programs to short-term drops in rates of completed suicides, they noted (JAMA Psychiatry. 2015 Oct 14. doi: 10.1001/jamapsychiatry.2015.1933). To explore the effects of the GLS program on youth suicide attempts, the investigators compared National Survey on Drug Use and Health data for 466 counties that had implemented the programs and 1,161 counties that had not but were otherwise similar. The final dataset included 46 states and 12 tribal communities, the researchers said.
A year after implementing GLS-funded programs, counties averaged 4.9 fewer suicide attempts per 1,000 youths than did control counties (95% confidence interval, 1.8-8.0; P = .003). “Although causality cannot be definitively inferred from our study owing to a lack of random assignment, these results suggest that more than 79,000 attempts were avoided between 2008 and 2011 following implementation of the GLS program,” the investigators added.
The results, however, were not durable. Two years after program implementation, rates of suicide attempts in GLS counties again resembled those of non-GLS counties, mirroring findings from the study of suicide mortality, the investigators said. “Many factors not directly addressed by the present study likely contribute to these results,” they added. “Assessments of comprehensive suicide prevention programs in other contexts have suggested that adherence and focus on comprehensive suicide prevention activities may fade over time. The present results do not suggest that the GLS program activities were effective only once but, rather, that effectively preventing suicides requires continued implementation of program activities.”
The Substance Abuse and Mental Health Services Administration funded the study. Richard McKeon, Ph.D., one of the authors, is employed by SAMHSA. The other investigators declared no conflicts of interest.
Federally funded suicide prevention programs might have helped avert more than 79,000 suicide attempts by young people over a 3-year period, said authors of a national observational study.
Counties that implemented the comprehensive, community-based programs reported about 5 fewer suicide attempts for every 1,000 young people aged 16-23 years, compared with counties that were otherwise similar but lacked the programs, said Lucas Godoy Garraza of ICF International, New York, and his associates.
Since 2004, the Garrett Lee Smith (GLS) Memorial Act has channeled competitive federal grants to states, colleges, and tribes for youth suicide prevention programs. These initiatives include activities aimed at increasing suicide awareness; screening at-risk youth; training gatekeepers; and connecting young people and families with mental health care, survivor support programs, and crisis hotlines, the investigators said. A prior study tied the programs to short-term drops in rates of completed suicides, they noted (JAMA Psychiatry. 2015 Oct 14. doi: 10.1001/jamapsychiatry.2015.1933). To explore the effects of the GLS program on youth suicide attempts, the investigators compared National Survey on Drug Use and Health data for 466 counties that had implemented the programs and 1,161 counties that had not but were otherwise similar. The final dataset included 46 states and 12 tribal communities, the researchers said.
A year after implementing GLS-funded programs, counties averaged 4.9 fewer suicide attempts per 1,000 youths than did control counties (95% confidence interval, 1.8-8.0; P = .003). “Although causality cannot be definitively inferred from our study owing to a lack of random assignment, these results suggest that more than 79,000 attempts were avoided between 2008 and 2011 following implementation of the GLS program,” the investigators added.
The results, however, were not durable. Two years after program implementation, rates of suicide attempts in GLS counties again resembled those of non-GLS counties, mirroring findings from the study of suicide mortality, the investigators said. “Many factors not directly addressed by the present study likely contribute to these results,” they added. “Assessments of comprehensive suicide prevention programs in other contexts have suggested that adherence and focus on comprehensive suicide prevention activities may fade over time. The present results do not suggest that the GLS program activities were effective only once but, rather, that effectively preventing suicides requires continued implementation of program activities.”
The Substance Abuse and Mental Health Services Administration funded the study. Richard McKeon, Ph.D., one of the authors, is employed by SAMHSA. The other investigators declared no conflicts of interest.
FROM JAMA PSYCHIATRY
Key clinical point: Federally funded suicide prevention programs may have helped avert more than 79,000 suicide attempts by young people over 3 years.
Major finding: At 1 year, program counties averaged 4.9 fewer suicide attempts per 1,000 youths than control counties (P = .003).
Data source: National observational study comparing 466 counties that had implemented the programs with 1,161 counties that had not but were otherwise similar.
Disclosures: The Substance Abuse and Mental Health Services Administration funded the study. Richard McKeon, Ph.D., one of the authors, is employed by SAMHSA. The other investigators declared no conflicts of interest.