User login
Bortezomib-based regimen + transplant increased progression-free survival in primary plasma cell leukemia
In a prospective study of 40 patients with primary plasma cell leukemia, upfront autotransplantation followed by allotransplant for younger patients and by consolidation/maintenance for older patients was associated with a median overall survival of 36.3 months and a median progression-free survival of 15.1 months.
Patients with this aggressive form of multiple myeloma received a regimen that combined standard chemotherapy, a proteasome inhibitor, high-dose melphalan followed by autologous stem cell transplantation, and allogeneic transplantation or immunomodulatory drugs, reported Dr. Bruno Royer of University Hospital in Amiens, France, and his associates.
Induction therapy consisted of four 21-day cycles: Cycles 1 and 3 included subcutaneous bortezomib, intravenous pegylated doxorubicin, and oral dexamethasone; cycles 2 and 4 included subcutaneous bortezomib, oral cyclophosphamide, and oral dexamethasone. Of 39 patients – one patient died 24 hours after study inclusion – 35 completed the four cycles. The overall response rate to induction was 69%: 10% of patients had a complete response and 26% had a very good partial response. Of 27 responding patients, 25 underwent high-dose melphalan followed by autologous stem cell transplantation.
The high response rates allowed 16 patients who were younger than 66 years and had an HLA-matched donor to then receive high-dose melphalan followed by autologous stem cell transplantation followed by consolidation with either an reduced-intensity conditioning allograft or a second high-dose melphalan followed by autologous stem cell transplantation and subsequent maintenance with lenalidomide, bortezomib, and dexamethasone for 1 year, the researchers said (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.63.1929).
A total of 20% of patients had a complete response to the entire treatment protocol, 13% had a stringent complete response, 26% had a very good partial response, 5% had stable disease, and 5% had progressive disease. Thirteen patients died of progressive disease and four died of infections, including three that occurred during induction or after allograft.
This is only the second prospective trial in patients with primary plasma cell leukemia, an aggressive form of multiple myeloma that accounts for 2%-4% of cases, the researchers said. Future prospective trials should seek to optimize induction with newer combinations, such as carfilzomib, lenalidomide, dexamethasone, or monoclonal anti-CD38 antibodies. Also, optimizing the stem cell conditioning procedure and the postallograft immunomodulation may further benefit younger patients.
Dr. Royer reported receiving honoraria from Amgen and having served as a consultant or advisor for Octapharma Plasma. Fifteen coinvestigators also reported financial relationships with a number of pharmaceutical companies.
The study by Dr. Royer and associates is the first prospective trial to confirm that bortezomib-based regimens combined with a transplantation program may be effective and feasible in a significant proportion of patients with primary plasma cell leukemia. Response to induction therapy, however, was not remarkable; thus, although both cyclophosphamide and doxorubicin have demonstrated efficacy in primary plasma cell leukemia, the introduction of lenalidomide and/or incorporation of newer agents such as pomalidomide, carfilzomib, or daratumumab could hopefully optimize the induction phase and increase the rate and quality of response in future studies.
Hopefully, sequential phases of induction therapy, multiple transplantations (if applicable), further consolidation, and maintenance should ensure rapid disease control and reduction of early deaths from initial complications, a contrasting of clonal evolution that may induce drug resistance, and activity on residual disease by decreasing the risk of relapse. Feasibility of these approaches, however, may be limited, especially for older and frail patients who are unable to tolerate intensive induction or prolonged treatments. Personalized therapies with acceptable toxicities should be considered for these patients.
Dr. Pellegrino Musto is at Referral Cancer Center of Basilicata, Rionero in Vulture, Italy. He reported receiving honoraria from Celgene, Janssen-Cilag, Novartis, Sanofi, and Bristol-Myers Squibb. These comments are from an editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2016.66.6115) that accompanied the published study.
The study by Dr. Royer and associates is the first prospective trial to confirm that bortezomib-based regimens combined with a transplantation program may be effective and feasible in a significant proportion of patients with primary plasma cell leukemia. Response to induction therapy, however, was not remarkable; thus, although both cyclophosphamide and doxorubicin have demonstrated efficacy in primary plasma cell leukemia, the introduction of lenalidomide and/or incorporation of newer agents such as pomalidomide, carfilzomib, or daratumumab could hopefully optimize the induction phase and increase the rate and quality of response in future studies.
Hopefully, sequential phases of induction therapy, multiple transplantations (if applicable), further consolidation, and maintenance should ensure rapid disease control and reduction of early deaths from initial complications, a contrasting of clonal evolution that may induce drug resistance, and activity on residual disease by decreasing the risk of relapse. Feasibility of these approaches, however, may be limited, especially for older and frail patients who are unable to tolerate intensive induction or prolonged treatments. Personalized therapies with acceptable toxicities should be considered for these patients.
Dr. Pellegrino Musto is at Referral Cancer Center of Basilicata, Rionero in Vulture, Italy. He reported receiving honoraria from Celgene, Janssen-Cilag, Novartis, Sanofi, and Bristol-Myers Squibb. These comments are from an editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2016.66.6115) that accompanied the published study.
The study by Dr. Royer and associates is the first prospective trial to confirm that bortezomib-based regimens combined with a transplantation program may be effective and feasible in a significant proportion of patients with primary plasma cell leukemia. Response to induction therapy, however, was not remarkable; thus, although both cyclophosphamide and doxorubicin have demonstrated efficacy in primary plasma cell leukemia, the introduction of lenalidomide and/or incorporation of newer agents such as pomalidomide, carfilzomib, or daratumumab could hopefully optimize the induction phase and increase the rate and quality of response in future studies.
Hopefully, sequential phases of induction therapy, multiple transplantations (if applicable), further consolidation, and maintenance should ensure rapid disease control and reduction of early deaths from initial complications, a contrasting of clonal evolution that may induce drug resistance, and activity on residual disease by decreasing the risk of relapse. Feasibility of these approaches, however, may be limited, especially for older and frail patients who are unable to tolerate intensive induction or prolonged treatments. Personalized therapies with acceptable toxicities should be considered for these patients.
Dr. Pellegrino Musto is at Referral Cancer Center of Basilicata, Rionero in Vulture, Italy. He reported receiving honoraria from Celgene, Janssen-Cilag, Novartis, Sanofi, and Bristol-Myers Squibb. These comments are from an editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2016.66.6115) that accompanied the published study.
In a prospective study of 40 patients with primary plasma cell leukemia, upfront autotransplantation followed by allotransplant for younger patients and by consolidation/maintenance for older patients was associated with a median overall survival of 36.3 months and a median progression-free survival of 15.1 months.
Patients with this aggressive form of multiple myeloma received a regimen that combined standard chemotherapy, a proteasome inhibitor, high-dose melphalan followed by autologous stem cell transplantation, and allogeneic transplantation or immunomodulatory drugs, reported Dr. Bruno Royer of University Hospital in Amiens, France, and his associates.
Induction therapy consisted of four 21-day cycles: Cycles 1 and 3 included subcutaneous bortezomib, intravenous pegylated doxorubicin, and oral dexamethasone; cycles 2 and 4 included subcutaneous bortezomib, oral cyclophosphamide, and oral dexamethasone. Of 39 patients – one patient died 24 hours after study inclusion – 35 completed the four cycles. The overall response rate to induction was 69%: 10% of patients had a complete response and 26% had a very good partial response. Of 27 responding patients, 25 underwent high-dose melphalan followed by autologous stem cell transplantation.
The high response rates allowed 16 patients who were younger than 66 years and had an HLA-matched donor to then receive high-dose melphalan followed by autologous stem cell transplantation followed by consolidation with either an reduced-intensity conditioning allograft or a second high-dose melphalan followed by autologous stem cell transplantation and subsequent maintenance with lenalidomide, bortezomib, and dexamethasone for 1 year, the researchers said (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.63.1929).
A total of 20% of patients had a complete response to the entire treatment protocol, 13% had a stringent complete response, 26% had a very good partial response, 5% had stable disease, and 5% had progressive disease. Thirteen patients died of progressive disease and four died of infections, including three that occurred during induction or after allograft.
This is only the second prospective trial in patients with primary plasma cell leukemia, an aggressive form of multiple myeloma that accounts for 2%-4% of cases, the researchers said. Future prospective trials should seek to optimize induction with newer combinations, such as carfilzomib, lenalidomide, dexamethasone, or monoclonal anti-CD38 antibodies. Also, optimizing the stem cell conditioning procedure and the postallograft immunomodulation may further benefit younger patients.
Dr. Royer reported receiving honoraria from Amgen and having served as a consultant or advisor for Octapharma Plasma. Fifteen coinvestigators also reported financial relationships with a number of pharmaceutical companies.
In a prospective study of 40 patients with primary plasma cell leukemia, upfront autotransplantation followed by allotransplant for younger patients and by consolidation/maintenance for older patients was associated with a median overall survival of 36.3 months and a median progression-free survival of 15.1 months.
Patients with this aggressive form of multiple myeloma received a regimen that combined standard chemotherapy, a proteasome inhibitor, high-dose melphalan followed by autologous stem cell transplantation, and allogeneic transplantation or immunomodulatory drugs, reported Dr. Bruno Royer of University Hospital in Amiens, France, and his associates.
Induction therapy consisted of four 21-day cycles: Cycles 1 and 3 included subcutaneous bortezomib, intravenous pegylated doxorubicin, and oral dexamethasone; cycles 2 and 4 included subcutaneous bortezomib, oral cyclophosphamide, and oral dexamethasone. Of 39 patients – one patient died 24 hours after study inclusion – 35 completed the four cycles. The overall response rate to induction was 69%: 10% of patients had a complete response and 26% had a very good partial response. Of 27 responding patients, 25 underwent high-dose melphalan followed by autologous stem cell transplantation.
The high response rates allowed 16 patients who were younger than 66 years and had an HLA-matched donor to then receive high-dose melphalan followed by autologous stem cell transplantation followed by consolidation with either an reduced-intensity conditioning allograft or a second high-dose melphalan followed by autologous stem cell transplantation and subsequent maintenance with lenalidomide, bortezomib, and dexamethasone for 1 year, the researchers said (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.63.1929).
A total of 20% of patients had a complete response to the entire treatment protocol, 13% had a stringent complete response, 26% had a very good partial response, 5% had stable disease, and 5% had progressive disease. Thirteen patients died of progressive disease and four died of infections, including three that occurred during induction or after allograft.
This is only the second prospective trial in patients with primary plasma cell leukemia, an aggressive form of multiple myeloma that accounts for 2%-4% of cases, the researchers said. Future prospective trials should seek to optimize induction with newer combinations, such as carfilzomib, lenalidomide, dexamethasone, or monoclonal anti-CD38 antibodies. Also, optimizing the stem cell conditioning procedure and the postallograft immunomodulation may further benefit younger patients.
Dr. Royer reported receiving honoraria from Amgen and having served as a consultant or advisor for Octapharma Plasma. Fifteen coinvestigators also reported financial relationships with a number of pharmaceutical companies.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Progression-free survival was improved in patients who had primary plasma cell leukemia and underwent four cycles of induction; high-dose melphalan followed by autologous stem cell transplantation; consolidation with either a reduced-intensity conditioning allograft or a second high-dose melphalan followed by autologous stem cell transplantation; and subsequent maintenance with lenalidomide, bortezomib, and dexamethasone for 1 year.
Major finding: Median overall survival was 36.3 months and median progression-free survival was 15.1 months.
Data source: A prospective phase II study of 40 adults with newly diagnosed primary plasma cell leukemia.
Disclosures: Dr. Royer reported receiving honoraria from Amgen and having served as a consultant or advisor for Octapharma Plasma. Fifteen coinvestigators also reported financial relationships with various drug companies.
Psoriasis Tied to Abdominal Aortic Aneurysm in Nationwide Study
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Psoriasis tied to abdominal aortic aneurysm in nationwide study
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Key clinical point: Psoriasis predicted abdominal aortic aneurysm in a large, population-based study.
Major finding: The adjusted risk of abdominal aortic aneurysm was 1.67 times greater among patients with severe psoriasis than in the reference population.
Data source: A retrospective cohort study of 5.5 million Danish adults, including 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis.
Disclosures: The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
HIV-1C predicts virologic failure, especially with ritonavir-boosted PIs
Patients infected with HIV-1 subtype C failed antiretroviral therapy significantly earlier than did HIV-1B–infected patients, especially when they received ritonavir-boosted protease inhibitors instead of nonnucleoside reverse transcriptase inhibitors, according to a Swedish national cohort study.
“As low- and middle-income countries are poised to scale up second-line antiretroviral therapy containing ritonavir-boosted PIs [protease inhibitors], a concern is that PIs will be less efficient in patients with HIV-1C,” said Dr. Ujjwal Neogi of the Karolinska Institute in Stockholm and his associates. “Studies of new drugs such as integrase inhibitors should be done in patients with HIV-1C and other non-B subtypes, which are responsible for the greatest global HIV burden,” they wrote online in the Lancet HIV.
HIV-infected patients in low-income and middle-income countries often receive second-line boosted PI-based regimens, but few studies have rigorously looked at treatment response for the HIV-1C subtype, which causes most infections in countries such as Ethiopia, India, and South Africa, the investigators noted. They studied more than 99% of Sweden’s HIV-positive population to identify predictors of virologic failure. The dataset included 1,077 HIV-1B–infected patients and 596 HIV-1C–infected patients; 90% of the latter were immigrants (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00023-0).
Predictors of primary virologic failure included higher baseline viral load (odds ratio, 1.8; 95% confidence interval, 1.5-2.2); subtype C infection (OR, 1.75; 1.1-2.9), and boosted PI-based regimens (OR, 1.55; 95% CI, 1.5-2.1). After adjustment for transmission route and duration of antiretroviral therapy, time to secondary virologic failure on boosted PI-based regimens was significantly earlier for HIV-1C–infected patients, compared with HIV-1B patients (hazard ratio, 1.8; 95% CI, 1.3-2.9). However, HIV-1 subtype did not significantly predict time to virologic failure for nonnucleoside reverse transcriptase inhibitor–based regimens.
“The poorer treatment outcome occurred in the patients with HIV-1C despite developed clinical care, modern laboratory monitoring, and focused adherence support at highly HIV-specialized infectious disease clinics in a high-income country,” the researchers wrote. Self-reported adherence to therapy did not differ by treatment regimen or HIV-1 subtype, although adherence data were available for only about a third of HIV-1C–infected patients, they added.
Homology-based molecular modeling also seemed to show a lower affinity between the PIs darunavir and lopinavir and the HIV-1C protease, compared with the HIV-1B protease, said the researchers. “Naturally occurring polymorphisms in HIV-1C protease might affect the binding of at least some protease inhibitors, potentially contributing to the differences we observed,” they concluded.
The study was funded by the Karolinska Institutet Research Foundation, Swedish Research Council, Stockholm County Council, Swedish Physicians against AIDS, National Institutes of Health, and University of Missouri. The investigators had no relevant financial disclosures.
HIV-1 is diverse worldwide, but antiretroviral drug development has typically used subtype B as a standard. This study highlights the importance of doing studies in different populations representative of the global diversity of subtypes.
We acknowledge that failure rates might be relatively high in real-life settings, [in contrast to] a very low incidence of failure and protease-inhibitor–associated drug resistance in randomized controlled trials. However, a clinically important decreased enzyme affinity for protease inhibitors in HIV-1C will result in decreased susceptibility and will contribute to an increased risk of drug resistance mutations and failure, which currently is not observed in clinical practice.
In our opinion, substantiating impaired enzyme to protease inhibitor affinity as a cause of failure needs evidence of a significant reduction in phenotypic susceptibility and should be investigated further. Adherence is likely a more important predictor of virologic failure than are viral factors, but investigating this conclusively is challenging, given the difficulty of objective assessment of adherence. Other patient-related factors, in addition to adherence, might affect drug concentrations: Data are increasing for interindividual pharmacokinetic variability, which includes pharmacogenetics, concomitant drug interactions, and health and nutritional status of patients. Nevertheless, a better understanding of ritonavir-boosted lopinavir regimen failure remains a priority because these regimens are a last line for many patients in low-resource settings.
Dr. Gert U van Zyl and Dr. Eric H. Decloedt are in the division of medical virology at Stellenbosch University in Cape Town, South Africa. Dr. van Zyl reported receiving an honorarium from Abbvie; Dr. Decloedt reported no conflicts of interest. These comments are from their editorial accompanying the study (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00040-0).
HIV-1 is diverse worldwide, but antiretroviral drug development has typically used subtype B as a standard. This study highlights the importance of doing studies in different populations representative of the global diversity of subtypes.
We acknowledge that failure rates might be relatively high in real-life settings, [in contrast to] a very low incidence of failure and protease-inhibitor–associated drug resistance in randomized controlled trials. However, a clinically important decreased enzyme affinity for protease inhibitors in HIV-1C will result in decreased susceptibility and will contribute to an increased risk of drug resistance mutations and failure, which currently is not observed in clinical practice.
In our opinion, substantiating impaired enzyme to protease inhibitor affinity as a cause of failure needs evidence of a significant reduction in phenotypic susceptibility and should be investigated further. Adherence is likely a more important predictor of virologic failure than are viral factors, but investigating this conclusively is challenging, given the difficulty of objective assessment of adherence. Other patient-related factors, in addition to adherence, might affect drug concentrations: Data are increasing for interindividual pharmacokinetic variability, which includes pharmacogenetics, concomitant drug interactions, and health and nutritional status of patients. Nevertheless, a better understanding of ritonavir-boosted lopinavir regimen failure remains a priority because these regimens are a last line for many patients in low-resource settings.
Dr. Gert U van Zyl and Dr. Eric H. Decloedt are in the division of medical virology at Stellenbosch University in Cape Town, South Africa. Dr. van Zyl reported receiving an honorarium from Abbvie; Dr. Decloedt reported no conflicts of interest. These comments are from their editorial accompanying the study (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00040-0).
HIV-1 is diverse worldwide, but antiretroviral drug development has typically used subtype B as a standard. This study highlights the importance of doing studies in different populations representative of the global diversity of subtypes.
We acknowledge that failure rates might be relatively high in real-life settings, [in contrast to] a very low incidence of failure and protease-inhibitor–associated drug resistance in randomized controlled trials. However, a clinically important decreased enzyme affinity for protease inhibitors in HIV-1C will result in decreased susceptibility and will contribute to an increased risk of drug resistance mutations and failure, which currently is not observed in clinical practice.
In our opinion, substantiating impaired enzyme to protease inhibitor affinity as a cause of failure needs evidence of a significant reduction in phenotypic susceptibility and should be investigated further. Adherence is likely a more important predictor of virologic failure than are viral factors, but investigating this conclusively is challenging, given the difficulty of objective assessment of adherence. Other patient-related factors, in addition to adherence, might affect drug concentrations: Data are increasing for interindividual pharmacokinetic variability, which includes pharmacogenetics, concomitant drug interactions, and health and nutritional status of patients. Nevertheless, a better understanding of ritonavir-boosted lopinavir regimen failure remains a priority because these regimens are a last line for many patients in low-resource settings.
Dr. Gert U van Zyl and Dr. Eric H. Decloedt are in the division of medical virology at Stellenbosch University in Cape Town, South Africa. Dr. van Zyl reported receiving an honorarium from Abbvie; Dr. Decloedt reported no conflicts of interest. These comments are from their editorial accompanying the study (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00040-0).
Patients infected with HIV-1 subtype C failed antiretroviral therapy significantly earlier than did HIV-1B–infected patients, especially when they received ritonavir-boosted protease inhibitors instead of nonnucleoside reverse transcriptase inhibitors, according to a Swedish national cohort study.
“As low- and middle-income countries are poised to scale up second-line antiretroviral therapy containing ritonavir-boosted PIs [protease inhibitors], a concern is that PIs will be less efficient in patients with HIV-1C,” said Dr. Ujjwal Neogi of the Karolinska Institute in Stockholm and his associates. “Studies of new drugs such as integrase inhibitors should be done in patients with HIV-1C and other non-B subtypes, which are responsible for the greatest global HIV burden,” they wrote online in the Lancet HIV.
HIV-infected patients in low-income and middle-income countries often receive second-line boosted PI-based regimens, but few studies have rigorously looked at treatment response for the HIV-1C subtype, which causes most infections in countries such as Ethiopia, India, and South Africa, the investigators noted. They studied more than 99% of Sweden’s HIV-positive population to identify predictors of virologic failure. The dataset included 1,077 HIV-1B–infected patients and 596 HIV-1C–infected patients; 90% of the latter were immigrants (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00023-0).
Predictors of primary virologic failure included higher baseline viral load (odds ratio, 1.8; 95% confidence interval, 1.5-2.2); subtype C infection (OR, 1.75; 1.1-2.9), and boosted PI-based regimens (OR, 1.55; 95% CI, 1.5-2.1). After adjustment for transmission route and duration of antiretroviral therapy, time to secondary virologic failure on boosted PI-based regimens was significantly earlier for HIV-1C–infected patients, compared with HIV-1B patients (hazard ratio, 1.8; 95% CI, 1.3-2.9). However, HIV-1 subtype did not significantly predict time to virologic failure for nonnucleoside reverse transcriptase inhibitor–based regimens.
“The poorer treatment outcome occurred in the patients with HIV-1C despite developed clinical care, modern laboratory monitoring, and focused adherence support at highly HIV-specialized infectious disease clinics in a high-income country,” the researchers wrote. Self-reported adherence to therapy did not differ by treatment regimen or HIV-1 subtype, although adherence data were available for only about a third of HIV-1C–infected patients, they added.
Homology-based molecular modeling also seemed to show a lower affinity between the PIs darunavir and lopinavir and the HIV-1C protease, compared with the HIV-1B protease, said the researchers. “Naturally occurring polymorphisms in HIV-1C protease might affect the binding of at least some protease inhibitors, potentially contributing to the differences we observed,” they concluded.
The study was funded by the Karolinska Institutet Research Foundation, Swedish Research Council, Stockholm County Council, Swedish Physicians against AIDS, National Institutes of Health, and University of Missouri. The investigators had no relevant financial disclosures.
Patients infected with HIV-1 subtype C failed antiretroviral therapy significantly earlier than did HIV-1B–infected patients, especially when they received ritonavir-boosted protease inhibitors instead of nonnucleoside reverse transcriptase inhibitors, according to a Swedish national cohort study.
“As low- and middle-income countries are poised to scale up second-line antiretroviral therapy containing ritonavir-boosted PIs [protease inhibitors], a concern is that PIs will be less efficient in patients with HIV-1C,” said Dr. Ujjwal Neogi of the Karolinska Institute in Stockholm and his associates. “Studies of new drugs such as integrase inhibitors should be done in patients with HIV-1C and other non-B subtypes, which are responsible for the greatest global HIV burden,” they wrote online in the Lancet HIV.
HIV-infected patients in low-income and middle-income countries often receive second-line boosted PI-based regimens, but few studies have rigorously looked at treatment response for the HIV-1C subtype, which causes most infections in countries such as Ethiopia, India, and South Africa, the investigators noted. They studied more than 99% of Sweden’s HIV-positive population to identify predictors of virologic failure. The dataset included 1,077 HIV-1B–infected patients and 596 HIV-1C–infected patients; 90% of the latter were immigrants (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00023-0).
Predictors of primary virologic failure included higher baseline viral load (odds ratio, 1.8; 95% confidence interval, 1.5-2.2); subtype C infection (OR, 1.75; 1.1-2.9), and boosted PI-based regimens (OR, 1.55; 95% CI, 1.5-2.1). After adjustment for transmission route and duration of antiretroviral therapy, time to secondary virologic failure on boosted PI-based regimens was significantly earlier for HIV-1C–infected patients, compared with HIV-1B patients (hazard ratio, 1.8; 95% CI, 1.3-2.9). However, HIV-1 subtype did not significantly predict time to virologic failure for nonnucleoside reverse transcriptase inhibitor–based regimens.
“The poorer treatment outcome occurred in the patients with HIV-1C despite developed clinical care, modern laboratory monitoring, and focused adherence support at highly HIV-specialized infectious disease clinics in a high-income country,” the researchers wrote. Self-reported adherence to therapy did not differ by treatment regimen or HIV-1 subtype, although adherence data were available for only about a third of HIV-1C–infected patients, they added.
Homology-based molecular modeling also seemed to show a lower affinity between the PIs darunavir and lopinavir and the HIV-1C protease, compared with the HIV-1B protease, said the researchers. “Naturally occurring polymorphisms in HIV-1C protease might affect the binding of at least some protease inhibitors, potentially contributing to the differences we observed,” they concluded.
The study was funded by the Karolinska Institutet Research Foundation, Swedish Research Council, Stockholm County Council, Swedish Physicians against AIDS, National Institutes of Health, and University of Missouri. The investigators had no relevant financial disclosures.
FROM THE LANCET HIV
Key clinical point: HIV-1 subtype C infection predicted primary and secondary virologic failure, compared with HIV-1B infection, especially with ritonavir-boosted protease inhibitor–based regimens.
Major finding: Time to secondary virologic failure was significantly earlier among HIV-1C patients, compared with HIV-1B patients, on boosted PI-based regimens (hazard ratio, 1.8).
Data source: A cohort study of 1,077 HIV-1B–infected patients and 596 HIV-1C–infected patients.
Disclosures: The study was funded by the Karolinska Institutet Research Foundation, Swedish Research Council, Stockholm County Council, Swedish Physicians Against AIDS, National Institutes of Health, and University of Missouri. The investigators had no relevant financial disclosures.
HIV-1C predicts virologic failure, especially with ritonavir-boosted PIs
Patients infected with HIV-1 subtype C failed antiretroviral therapy significantly earlier than did HIV-1B–infected patients, especially when they received ritonavir-boosted protease inhibitors instead of nonnucleoside reverse transcriptase inhibitors, according to a Swedish national cohort study.
“As low- and middle-income countries are poised to scale up second-line antiretroviral therapy containing ritonavir-boosted PIs [protease inhibitors], a concern is that PIs will be less efficient in patients with HIV-1C,” said Dr. Ujjwal Neogi of the Karolinska Institute in Stockholm and his associates. “Studies of new drugs such as integrase inhibitors should be done in patients with HIV-1C and other non-B subtypes, which are responsible for the greatest global HIV burden,” they wrote online in the Lancet HIV.
HIV-infected patients in low-income and middle-income countries often receive second-line boosted PI-based regimens, but few studies have rigorously looked at treatment response for the HIV-1C subtype, which causes most infections in countries such as Ethiopia, India, and South Africa, the investigators noted. They studied more than 99% of Sweden’s HIV-positive population to identify predictors of virologic failure. The dataset included 1,077 HIV-1B–infected patients and 596 HIV-1C–infected patients; 90% of the latter were immigrants (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00023-0).
Predictors of primary virologic failure included higher baseline viral load (odds ratio, 1.8; 95% confidence interval, 1.5-2.2); subtype C infection (OR, 1.75; 1.1-2.9), and boosted PI-based regimens (OR, 1.55; 95% CI, 1.5-2.1). After adjustment for transmission route and duration of antiretroviral therapy, time to secondary virologic failure on boosted PI-based regimens was significantly earlier for HIV-1C–infected patients, compared with HIV-1B patients (hazard ratio, 1.8; 95% CI, 1.3-2.9). However, HIV-1 subtype did not significantly predict time to virologic failure for nonnucleoside reverse transcriptase inhibitor–based regimens.
“The poorer treatment outcome occurred in the patients with HIV-1C despite developed clinical care, modern laboratory monitoring, and focused adherence support at highly HIV-specialized infectious disease clinics in a high-income country,” the researchers wrote. Self-reported adherence to therapy did not differ by treatment regimen or HIV-1 subtype, although adherence data were available for only about a third of HIV-1C–infected patients, they added.
Homology-based molecular modeling also seemed to show a lower affinity between the PIs darunavir and lopinavir and the HIV-1C protease, compared with the HIV-1B protease, said the researchers. “Naturally occurring polymorphisms in HIV-1C protease might affect the binding of at least some protease inhibitors, potentially contributing to the differences we observed,” they concluded.
The study was funded by the Karolinska Institutet Research Foundation, Swedish Research Council, Stockholm County Council, Swedish Physicians against AIDS, National Institutes of Health, and University of Missouri. The investigators had no relevant financial disclosures.
HIV-1 is diverse worldwide, but antiretroviral drug development has typically used subtype B as a standard. This study highlights the importance of doing studies in different populations representative of the global diversity of subtypes.
We acknowledge that failure rates might be relatively high in real-life settings, [in contrast to] a very low incidence of failure and protease-inhibitor–associated drug resistance in randomized controlled trials. However, a clinically important decreased enzyme affinity for protease inhibitors in HIV-1C will result in decreased susceptibility and will contribute to an increased risk of drug resistance mutations and failure, which currently is not observed in clinical practice.
In our opinion, substantiating impaired enzyme to protease inhibitor affinity as a cause of failure needs evidence of a significant reduction in phenotypic susceptibility and should be investigated further. Adherence is likely a more important predictor of virologic failure than are viral factors, but investigating this conclusively is challenging, given the difficulty of objective assessment of adherence. Other patient-related factors, in addition to adherence, might affect drug concentrations: Data are increasing for interindividual pharmacokinetic variability, which includes pharmacogenetics, concomitant drug interactions, and health and nutritional status of patients. Nevertheless, a better understanding of ritonavir-boosted lopinavir regimen failure remains a priority because these regimens are a last line for many patients in low-resource settings.
Dr. Gert U van Zyl and Dr. Eric H. Decloedt are in the division of medical virology at Stellenbosch University in Cape Town, South Africa. Dr. van Zyl reported receiving an honorarium from Abbvie; Dr. Decloedt reported no conflicts of interest. These comments are from their editorial accompanying the study (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00040-0).
HIV-1 is diverse worldwide, but antiretroviral drug development has typically used subtype B as a standard. This study highlights the importance of doing studies in different populations representative of the global diversity of subtypes.
We acknowledge that failure rates might be relatively high in real-life settings, [in contrast to] a very low incidence of failure and protease-inhibitor–associated drug resistance in randomized controlled trials. However, a clinically important decreased enzyme affinity for protease inhibitors in HIV-1C will result in decreased susceptibility and will contribute to an increased risk of drug resistance mutations and failure, which currently is not observed in clinical practice.
In our opinion, substantiating impaired enzyme to protease inhibitor affinity as a cause of failure needs evidence of a significant reduction in phenotypic susceptibility and should be investigated further. Adherence is likely a more important predictor of virologic failure than are viral factors, but investigating this conclusively is challenging, given the difficulty of objective assessment of adherence. Other patient-related factors, in addition to adherence, might affect drug concentrations: Data are increasing for interindividual pharmacokinetic variability, which includes pharmacogenetics, concomitant drug interactions, and health and nutritional status of patients. Nevertheless, a better understanding of ritonavir-boosted lopinavir regimen failure remains a priority because these regimens are a last line for many patients in low-resource settings.
Dr. Gert U van Zyl and Dr. Eric H. Decloedt are in the division of medical virology at Stellenbosch University in Cape Town, South Africa. Dr. van Zyl reported receiving an honorarium from Abbvie; Dr. Decloedt reported no conflicts of interest. These comments are from their editorial accompanying the study (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00040-0).
HIV-1 is diverse worldwide, but antiretroviral drug development has typically used subtype B as a standard. This study highlights the importance of doing studies in different populations representative of the global diversity of subtypes.
We acknowledge that failure rates might be relatively high in real-life settings, [in contrast to] a very low incidence of failure and protease-inhibitor–associated drug resistance in randomized controlled trials. However, a clinically important decreased enzyme affinity for protease inhibitors in HIV-1C will result in decreased susceptibility and will contribute to an increased risk of drug resistance mutations and failure, which currently is not observed in clinical practice.
In our opinion, substantiating impaired enzyme to protease inhibitor affinity as a cause of failure needs evidence of a significant reduction in phenotypic susceptibility and should be investigated further. Adherence is likely a more important predictor of virologic failure than are viral factors, but investigating this conclusively is challenging, given the difficulty of objective assessment of adherence. Other patient-related factors, in addition to adherence, might affect drug concentrations: Data are increasing for interindividual pharmacokinetic variability, which includes pharmacogenetics, concomitant drug interactions, and health and nutritional status of patients. Nevertheless, a better understanding of ritonavir-boosted lopinavir regimen failure remains a priority because these regimens are a last line for many patients in low-resource settings.
Dr. Gert U van Zyl and Dr. Eric H. Decloedt are in the division of medical virology at Stellenbosch University in Cape Town, South Africa. Dr. van Zyl reported receiving an honorarium from Abbvie; Dr. Decloedt reported no conflicts of interest. These comments are from their editorial accompanying the study (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00040-0).
Patients infected with HIV-1 subtype C failed antiretroviral therapy significantly earlier than did HIV-1B–infected patients, especially when they received ritonavir-boosted protease inhibitors instead of nonnucleoside reverse transcriptase inhibitors, according to a Swedish national cohort study.
“As low- and middle-income countries are poised to scale up second-line antiretroviral therapy containing ritonavir-boosted PIs [protease inhibitors], a concern is that PIs will be less efficient in patients with HIV-1C,” said Dr. Ujjwal Neogi of the Karolinska Institute in Stockholm and his associates. “Studies of new drugs such as integrase inhibitors should be done in patients with HIV-1C and other non-B subtypes, which are responsible for the greatest global HIV burden,” they wrote online in the Lancet HIV.
HIV-infected patients in low-income and middle-income countries often receive second-line boosted PI-based regimens, but few studies have rigorously looked at treatment response for the HIV-1C subtype, which causes most infections in countries such as Ethiopia, India, and South Africa, the investigators noted. They studied more than 99% of Sweden’s HIV-positive population to identify predictors of virologic failure. The dataset included 1,077 HIV-1B–infected patients and 596 HIV-1C–infected patients; 90% of the latter were immigrants (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00023-0).
Predictors of primary virologic failure included higher baseline viral load (odds ratio, 1.8; 95% confidence interval, 1.5-2.2); subtype C infection (OR, 1.75; 1.1-2.9), and boosted PI-based regimens (OR, 1.55; 95% CI, 1.5-2.1). After adjustment for transmission route and duration of antiretroviral therapy, time to secondary virologic failure on boosted PI-based regimens was significantly earlier for HIV-1C–infected patients, compared with HIV-1B patients (hazard ratio, 1.8; 95% CI, 1.3-2.9). However, HIV-1 subtype did not significantly predict time to virologic failure for nonnucleoside reverse transcriptase inhibitor–based regimens.
“The poorer treatment outcome occurred in the patients with HIV-1C despite developed clinical care, modern laboratory monitoring, and focused adherence support at highly HIV-specialized infectious disease clinics in a high-income country,” the researchers wrote. Self-reported adherence to therapy did not differ by treatment regimen or HIV-1 subtype, although adherence data were available for only about a third of HIV-1C–infected patients, they added.
Homology-based molecular modeling also seemed to show a lower affinity between the PIs darunavir and lopinavir and the HIV-1C protease, compared with the HIV-1B protease, said the researchers. “Naturally occurring polymorphisms in HIV-1C protease might affect the binding of at least some protease inhibitors, potentially contributing to the differences we observed,” they concluded.
The study was funded by the Karolinska Institutet Research Foundation, Swedish Research Council, Stockholm County Council, Swedish Physicians against AIDS, National Institutes of Health, and University of Missouri. The investigators had no relevant financial disclosures.
Patients infected with HIV-1 subtype C failed antiretroviral therapy significantly earlier than did HIV-1B–infected patients, especially when they received ritonavir-boosted protease inhibitors instead of nonnucleoside reverse transcriptase inhibitors, according to a Swedish national cohort study.
“As low- and middle-income countries are poised to scale up second-line antiretroviral therapy containing ritonavir-boosted PIs [protease inhibitors], a concern is that PIs will be less efficient in patients with HIV-1C,” said Dr. Ujjwal Neogi of the Karolinska Institute in Stockholm and his associates. “Studies of new drugs such as integrase inhibitors should be done in patients with HIV-1C and other non-B subtypes, which are responsible for the greatest global HIV burden,” they wrote online in the Lancet HIV.
HIV-infected patients in low-income and middle-income countries often receive second-line boosted PI-based regimens, but few studies have rigorously looked at treatment response for the HIV-1C subtype, which causes most infections in countries such as Ethiopia, India, and South Africa, the investigators noted. They studied more than 99% of Sweden’s HIV-positive population to identify predictors of virologic failure. The dataset included 1,077 HIV-1B–infected patients and 596 HIV-1C–infected patients; 90% of the latter were immigrants (Lancet HIV. 2016 Mar 14. doi: 10.1016/S2352-3018[16]00023-0).
Predictors of primary virologic failure included higher baseline viral load (odds ratio, 1.8; 95% confidence interval, 1.5-2.2); subtype C infection (OR, 1.75; 1.1-2.9), and boosted PI-based regimens (OR, 1.55; 95% CI, 1.5-2.1). After adjustment for transmission route and duration of antiretroviral therapy, time to secondary virologic failure on boosted PI-based regimens was significantly earlier for HIV-1C–infected patients, compared with HIV-1B patients (hazard ratio, 1.8; 95% CI, 1.3-2.9). However, HIV-1 subtype did not significantly predict time to virologic failure for nonnucleoside reverse transcriptase inhibitor–based regimens.
“The poorer treatment outcome occurred in the patients with HIV-1C despite developed clinical care, modern laboratory monitoring, and focused adherence support at highly HIV-specialized infectious disease clinics in a high-income country,” the researchers wrote. Self-reported adherence to therapy did not differ by treatment regimen or HIV-1 subtype, although adherence data were available for only about a third of HIV-1C–infected patients, they added.
Homology-based molecular modeling also seemed to show a lower affinity between the PIs darunavir and lopinavir and the HIV-1C protease, compared with the HIV-1B protease, said the researchers. “Naturally occurring polymorphisms in HIV-1C protease might affect the binding of at least some protease inhibitors, potentially contributing to the differences we observed,” they concluded.
The study was funded by the Karolinska Institutet Research Foundation, Swedish Research Council, Stockholm County Council, Swedish Physicians against AIDS, National Institutes of Health, and University of Missouri. The investigators had no relevant financial disclosures.
FROM THE LANCET HIV
Key clinical point: HIV-1 subtype C infection predicted primary and secondary virologic failure, compared with HIV-1B infection, especially with ritonavir-boosted protease inhibitor–based regimens.
Major finding: Time to secondary virologic failure was significantly earlier among HIV-1C patients, compared with HIV-1B patients, on boosted PI-based regimens (hazard ratio, 1.8).
Data source: A cohort study of 1,077 HIV-1B–infected patients and 596 HIV-1C–infected patients.
Disclosures: The study was funded by the Karolinska Institutet Research Foundation, Swedish Research Council, Stockholm County Council, Swedish Physicians Against AIDS, National Institutes of Health, and University of Missouri. The investigators had no relevant financial disclosures.
Zika caused rash, pruritus more than high fever
Zika virus infection in adults often causes pruritic maculopapular rash, but rarely leads to clinically significant fever, according to two cohort studies in Rio de Janeiro.
The findings raise questions about case definitions of Zika virus disease (ZVD).
“In our opinion, pruritus, the second most common clinical sign presented by the confirmed cases, should be added to the Pan American Health Organization case definition [for Zika virus disease], while fever could be given less emphasis,” Dr. Patricia Brasil of the Oswaldo Cruz Foundation in Rio de Janeiro and her associates wrote online April 12 in PLoS Neglected Tropical Diseases.
Watching for a combination of itching and rash also could help distinguish Zika virus disease from infections of Chikungunya and Dengue, co-circulating arboviruses in Brazil that tend to cause nonpruritic rash, the investigators noted. Distinguishing these infections is crucial because Dengue, in particular, can be devastating without appropriate treatment (doi: 10.1371/journal.pntd.0004636).
Brazil confirmed Zika virus disease in the northeastern state of Bahia in May 2015. Cases in Rio de Janeiro soon followed, triggering worries because of its dense population and status as host of the 2016 Olympic and Paralympic Games. To better characterize Zika virus disease in Rio de Janeiro, Dr. Brasil and her associates studied 364 cases of acute rash, with or without fever, among adults with clinical onset during the first half of 2015. Quantitative reverse transcription–polymerase chain reaction detected Zika viral RNA in blood samples from 119 (45%) of 262 patients tested.
The first 4 days of confirmed ZVD were marked by rash (97%), itching (79%), prostration (73%), headache (66%), arthralgias (63%), and myalgia (61%). Just 36% of patients were febrile, and fevers usually were short-lived and low-grade, in contrast to other arboviral infections. Partial sequencing of the Zika virus gene from 10 randomly selected positive samples showed that it resembled Asian strains of Zika virus, supporting the hypothesis that Pacific Islanders brought Zika to Rio de Janeiro during a canoe championship in 2014, the researchers added.
The researchers also determined that Zika virus was circulating in Rio de Janeiro as early as January 2015 – “at least five months before its detection was announced by the health authorities, which must be taken into consideration for future design and implementation of effective syndromic surveillance systems,” they wrote. Surprisingly, an assay for Dengue was negative in all 250 patients tested, which might indicate “explosive transmission dynamics of Zika virus disease,” the investigators added.
A retrospective cohort study of confirmed Zika virus cases in Rio de Janeiro also reported a much higher prevalence of rash compared with fever (Emerg Infect Dis. 2016 Jul. doi: 10.3201/eid2207.160375).
This was a retrospective convenience sample of 57 patients, including 98% with rash, 56% with pruritus, and 67% with measured or self-reported fever. Most fevers were low-grade, and the median recorded temperature was 30.0 degrees Celsius (within normal limits). Other common presentations included headache (67%), arthralgias (58%), myalgias (49%), and joint swelling (23%), according to Dr. Jose Cerbino-Neto of the Oswaldo Cruz Foundation in Rio de Janeiro and his associates. Their findings support emphasizing rash, fever, or both as “primary characteristics” of ZVD, they added. Currently, the interim case definition from the World Health Organization defines a suspected Zika virus disease case as rash, fever, or both, plus at least one of three other symptoms – arthralgia, arthritis, or conjunctivitis.
The study by Dr. Brasil and her associates was funded by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, the Fundacao de Amparo a Pesquisa no Estado do Rio de Janeiro, and the European Commission. Dr. Brasil and her coinvestigators had no disclosures. Dr. Cerbino-Neto reported no funding sources or conflicts of interest.
Zika virus infection in adults often causes pruritic maculopapular rash, but rarely leads to clinically significant fever, according to two cohort studies in Rio de Janeiro.
The findings raise questions about case definitions of Zika virus disease (ZVD).
“In our opinion, pruritus, the second most common clinical sign presented by the confirmed cases, should be added to the Pan American Health Organization case definition [for Zika virus disease], while fever could be given less emphasis,” Dr. Patricia Brasil of the Oswaldo Cruz Foundation in Rio de Janeiro and her associates wrote online April 12 in PLoS Neglected Tropical Diseases.
Watching for a combination of itching and rash also could help distinguish Zika virus disease from infections of Chikungunya and Dengue, co-circulating arboviruses in Brazil that tend to cause nonpruritic rash, the investigators noted. Distinguishing these infections is crucial because Dengue, in particular, can be devastating without appropriate treatment (doi: 10.1371/journal.pntd.0004636).
Brazil confirmed Zika virus disease in the northeastern state of Bahia in May 2015. Cases in Rio de Janeiro soon followed, triggering worries because of its dense population and status as host of the 2016 Olympic and Paralympic Games. To better characterize Zika virus disease in Rio de Janeiro, Dr. Brasil and her associates studied 364 cases of acute rash, with or without fever, among adults with clinical onset during the first half of 2015. Quantitative reverse transcription–polymerase chain reaction detected Zika viral RNA in blood samples from 119 (45%) of 262 patients tested.
The first 4 days of confirmed ZVD were marked by rash (97%), itching (79%), prostration (73%), headache (66%), arthralgias (63%), and myalgia (61%). Just 36% of patients were febrile, and fevers usually were short-lived and low-grade, in contrast to other arboviral infections. Partial sequencing of the Zika virus gene from 10 randomly selected positive samples showed that it resembled Asian strains of Zika virus, supporting the hypothesis that Pacific Islanders brought Zika to Rio de Janeiro during a canoe championship in 2014, the researchers added.
The researchers also determined that Zika virus was circulating in Rio de Janeiro as early as January 2015 – “at least five months before its detection was announced by the health authorities, which must be taken into consideration for future design and implementation of effective syndromic surveillance systems,” they wrote. Surprisingly, an assay for Dengue was negative in all 250 patients tested, which might indicate “explosive transmission dynamics of Zika virus disease,” the investigators added.
A retrospective cohort study of confirmed Zika virus cases in Rio de Janeiro also reported a much higher prevalence of rash compared with fever (Emerg Infect Dis. 2016 Jul. doi: 10.3201/eid2207.160375).
This was a retrospective convenience sample of 57 patients, including 98% with rash, 56% with pruritus, and 67% with measured or self-reported fever. Most fevers were low-grade, and the median recorded temperature was 30.0 degrees Celsius (within normal limits). Other common presentations included headache (67%), arthralgias (58%), myalgias (49%), and joint swelling (23%), according to Dr. Jose Cerbino-Neto of the Oswaldo Cruz Foundation in Rio de Janeiro and his associates. Their findings support emphasizing rash, fever, or both as “primary characteristics” of ZVD, they added. Currently, the interim case definition from the World Health Organization defines a suspected Zika virus disease case as rash, fever, or both, plus at least one of three other symptoms – arthralgia, arthritis, or conjunctivitis.
The study by Dr. Brasil and her associates was funded by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, the Fundacao de Amparo a Pesquisa no Estado do Rio de Janeiro, and the European Commission. Dr. Brasil and her coinvestigators had no disclosures. Dr. Cerbino-Neto reported no funding sources or conflicts of interest.
Zika virus infection in adults often causes pruritic maculopapular rash, but rarely leads to clinically significant fever, according to two cohort studies in Rio de Janeiro.
The findings raise questions about case definitions of Zika virus disease (ZVD).
“In our opinion, pruritus, the second most common clinical sign presented by the confirmed cases, should be added to the Pan American Health Organization case definition [for Zika virus disease], while fever could be given less emphasis,” Dr. Patricia Brasil of the Oswaldo Cruz Foundation in Rio de Janeiro and her associates wrote online April 12 in PLoS Neglected Tropical Diseases.
Watching for a combination of itching and rash also could help distinguish Zika virus disease from infections of Chikungunya and Dengue, co-circulating arboviruses in Brazil that tend to cause nonpruritic rash, the investigators noted. Distinguishing these infections is crucial because Dengue, in particular, can be devastating without appropriate treatment (doi: 10.1371/journal.pntd.0004636).
Brazil confirmed Zika virus disease in the northeastern state of Bahia in May 2015. Cases in Rio de Janeiro soon followed, triggering worries because of its dense population and status as host of the 2016 Olympic and Paralympic Games. To better characterize Zika virus disease in Rio de Janeiro, Dr. Brasil and her associates studied 364 cases of acute rash, with or without fever, among adults with clinical onset during the first half of 2015. Quantitative reverse transcription–polymerase chain reaction detected Zika viral RNA in blood samples from 119 (45%) of 262 patients tested.
The first 4 days of confirmed ZVD were marked by rash (97%), itching (79%), prostration (73%), headache (66%), arthralgias (63%), and myalgia (61%). Just 36% of patients were febrile, and fevers usually were short-lived and low-grade, in contrast to other arboviral infections. Partial sequencing of the Zika virus gene from 10 randomly selected positive samples showed that it resembled Asian strains of Zika virus, supporting the hypothesis that Pacific Islanders brought Zika to Rio de Janeiro during a canoe championship in 2014, the researchers added.
The researchers also determined that Zika virus was circulating in Rio de Janeiro as early as January 2015 – “at least five months before its detection was announced by the health authorities, which must be taken into consideration for future design and implementation of effective syndromic surveillance systems,” they wrote. Surprisingly, an assay for Dengue was negative in all 250 patients tested, which might indicate “explosive transmission dynamics of Zika virus disease,” the investigators added.
A retrospective cohort study of confirmed Zika virus cases in Rio de Janeiro also reported a much higher prevalence of rash compared with fever (Emerg Infect Dis. 2016 Jul. doi: 10.3201/eid2207.160375).
This was a retrospective convenience sample of 57 patients, including 98% with rash, 56% with pruritus, and 67% with measured or self-reported fever. Most fevers were low-grade, and the median recorded temperature was 30.0 degrees Celsius (within normal limits). Other common presentations included headache (67%), arthralgias (58%), myalgias (49%), and joint swelling (23%), according to Dr. Jose Cerbino-Neto of the Oswaldo Cruz Foundation in Rio de Janeiro and his associates. Their findings support emphasizing rash, fever, or both as “primary characteristics” of ZVD, they added. Currently, the interim case definition from the World Health Organization defines a suspected Zika virus disease case as rash, fever, or both, plus at least one of three other symptoms – arthralgia, arthritis, or conjunctivitis.
The study by Dr. Brasil and her associates was funded by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, the Fundacao de Amparo a Pesquisa no Estado do Rio de Janeiro, and the European Commission. Dr. Brasil and her coinvestigators had no disclosures. Dr. Cerbino-Neto reported no funding sources or conflicts of interest.
FROM PLOS NEGLECTED TROPICAL DISEASES AND EMERGING INFECTIOUS DISEASES
Key clinical point: Rash was much more common than fever in two separate studies of Zika virus cases among adults in Rio de Janeiro.
Major finding: In the first study, 97% of laboratory-confirmed cases had rash, while only 36% had fever. In the second study, 98% of patients had rash and 67% had fever. In contrast to other arboviral infections, patients often described the rashes as pruritic.
Data source: A prospective study of 119 PCR-confirmed cases and a retrospective study of 57 confirmed cases, all in Rio de Janeiro.
Disclosures: The study by Dr. Brasil and her associates was funded by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, the Fundacao de Amparo a Pesquisa no Estado do Rio de Janeiro, and the European Commission. Dr. Brasil and her coinvestigators had no disclosures. Dr. Cerbino-Neto reported no funding sources or conflicts of interest.
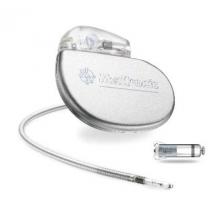
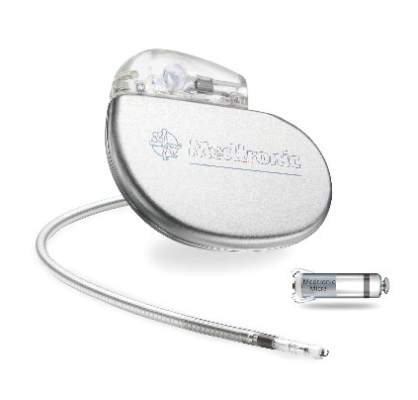
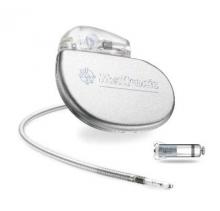
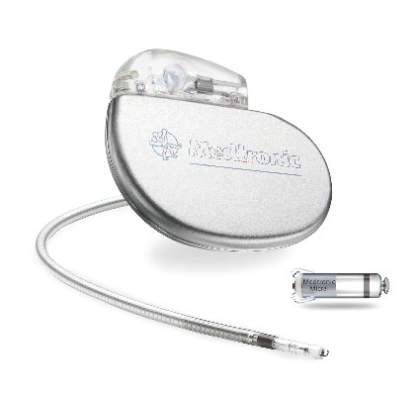
FDA Approves First Leadless Pacemaker
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
FDA approves first leadless pacemaker
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
Report laid groundwork for ending the public health problem of viral hepatitis
The United States can end hepatitis B and C transmission and prevent related morbidity and mortality given enough time, resources, and will to surmount a variety of barriers, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
A more feasible short-term goal is to control viral hepatitis by reducing the incidence and prevalence of hepatitis B (HBV) and C virus (HCV) infections, states the report, which is the first of a two-phase study. The second part, to be published in early 2017, will outline a strategy to achieve the goals.
“Disease elimination” once meant stopping all new infections in a population, but eliminating a public health problem “can be a less absolute goal,” Dr. Brian Strom, chancellor of Rutgers Biomedical and Health Sciences, wrote along with his associates. “The WHO has accepted a non-zero target in its work against viral hepatitis,” they added. “The organization’s provisional target is a 90% reduction in incidence, and a 65% reduction in mortality by 2030.”
Furthermore, those are global targets – countries should base their own strategies on their specific viral hepatitis disease burdens and epidemiologic features, Dr. Strom and his associates emphasized. For example, universal vaccination could eradicate HBV in the United States in two generations, but there is no cure for the 700,000-1.4 million Americans who are already infected. Mother-to-child HBV transmission is rare here, but better screening and surveillance could help prevent the 800-1,000 cases of vertical transmission that still occur annually.
More than a third of newborns in the United States do not receive the HBV vaccination on the day they are born, and 28% remain unvaccinated when they are 3 days old, “but childhood catch-up is possible,” the experts also wrote. Vaccinating adults is more complicated, but targeting high-risk groups such as prison inmates or patients at sexually transmitted disease clinics might work, they added. Vaccination is especially important, because chronically infected HBV patients need lifelong medical follow-up to prevent progression and death from cirrhosis and liver cancer, they emphasized.
The hepatitis C virus presents a different picture – there is no HCV vaccine, while the novel direct-acting antivirals can theoretically cure nearly all chronic infections. But in reality, high drug costs and the massive burden of undiagnosed HCV mean that only a small fraction of patients can be cured, the authors acknowledged. On an individual basis, the benefits of direct-acting antivirals outweigh the costs of treating genotype 1 HCV and associated morbidity, they add. But on a population level, “eliminating HCV infection would require near universal access to treatment, something that appears infeasible given the current pricing and policy environment.”
Several other barriers also impede progress against HBV and HCV. For example, only five states and two large cities receive enough funding for comprehensive viral hepatitis surveillance, Dr. Strom and his associates noted. If local and state public health departments cannot track infections, they cannot characterize their epidemics. In addition, viral hepatitis remains a source of stigma, adding to fear and avoidance of testing and undermining elimination efforts. Stigma, avoidance, and the fact that HBV and HCV are asymptomatic in the early stages all contribute to a large population of chronically infected patients who do not know their status.
Moreover, many state Medicaid programs will not cover foreigners until they have lived in the United States for 5 years, and the Affordable Care Act does not cover care for short-term residents and undocumented immigrants, the report stated. Newly infected HCV patients tend to have fewer resources, lower educational levels, and more often use injection drugs, making them harder to reach and screen through the health system. “Prisons are a promising venue in which to treat HCV, but treating HCV is expensive for the prison system. The cost of the direct-acting antivirals is high, and the staff time required to manage an inmate in treatment often far exceed the available resources,” the report stated.
The study was sponsored by the Centers for Disease Control and Prevention Office of Viral Hepatitis and the U.S. Department of Health and Human Services Office of Minority Health. The authors disclosed no conflicts of interest.

|
Dr. Sean Koppe |
The report offers a comprehensive view of the challenges and opportunities in regard to eliminating hepatitis B and C. As is well articulated in the report, we currently have excellent treatment for both hepatitis B and hepatitis C and a highly effective vaccine for eliminating transmission of hepatitis B. It is disappointing that we essentially already have the tools necessary to eliminate viral hepatitis as a top 10 cause of mortality worldwide, but currently lack the will and resources to accomplish the task.
I hope this report will serve to stimulate public health efforts to enhance screening and vaccination programs and also prod insurers and drug makers to make medications more affordable and accessible.
Dr. Sean Koppe is director of hepatology and medical director of transplantation, University of Illinois Hospital & Health Sciences System, Chicago. He has no conflicts of interest.

|
Dr. Sean Koppe |
The report offers a comprehensive view of the challenges and opportunities in regard to eliminating hepatitis B and C. As is well articulated in the report, we currently have excellent treatment for both hepatitis B and hepatitis C and a highly effective vaccine for eliminating transmission of hepatitis B. It is disappointing that we essentially already have the tools necessary to eliminate viral hepatitis as a top 10 cause of mortality worldwide, but currently lack the will and resources to accomplish the task.
I hope this report will serve to stimulate public health efforts to enhance screening and vaccination programs and also prod insurers and drug makers to make medications more affordable and accessible.
Dr. Sean Koppe is director of hepatology and medical director of transplantation, University of Illinois Hospital & Health Sciences System, Chicago. He has no conflicts of interest.

|
Dr. Sean Koppe |
The report offers a comprehensive view of the challenges and opportunities in regard to eliminating hepatitis B and C. As is well articulated in the report, we currently have excellent treatment for both hepatitis B and hepatitis C and a highly effective vaccine for eliminating transmission of hepatitis B. It is disappointing that we essentially already have the tools necessary to eliminate viral hepatitis as a top 10 cause of mortality worldwide, but currently lack the will and resources to accomplish the task.
I hope this report will serve to stimulate public health efforts to enhance screening and vaccination programs and also prod insurers and drug makers to make medications more affordable and accessible.
Dr. Sean Koppe is director of hepatology and medical director of transplantation, University of Illinois Hospital & Health Sciences System, Chicago. He has no conflicts of interest.
The United States can end hepatitis B and C transmission and prevent related morbidity and mortality given enough time, resources, and will to surmount a variety of barriers, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
A more feasible short-term goal is to control viral hepatitis by reducing the incidence and prevalence of hepatitis B (HBV) and C virus (HCV) infections, states the report, which is the first of a two-phase study. The second part, to be published in early 2017, will outline a strategy to achieve the goals.
“Disease elimination” once meant stopping all new infections in a population, but eliminating a public health problem “can be a less absolute goal,” Dr. Brian Strom, chancellor of Rutgers Biomedical and Health Sciences, wrote along with his associates. “The WHO has accepted a non-zero target in its work against viral hepatitis,” they added. “The organization’s provisional target is a 90% reduction in incidence, and a 65% reduction in mortality by 2030.”
Furthermore, those are global targets – countries should base their own strategies on their specific viral hepatitis disease burdens and epidemiologic features, Dr. Strom and his associates emphasized. For example, universal vaccination could eradicate HBV in the United States in two generations, but there is no cure for the 700,000-1.4 million Americans who are already infected. Mother-to-child HBV transmission is rare here, but better screening and surveillance could help prevent the 800-1,000 cases of vertical transmission that still occur annually.
More than a third of newborns in the United States do not receive the HBV vaccination on the day they are born, and 28% remain unvaccinated when they are 3 days old, “but childhood catch-up is possible,” the experts also wrote. Vaccinating adults is more complicated, but targeting high-risk groups such as prison inmates or patients at sexually transmitted disease clinics might work, they added. Vaccination is especially important, because chronically infected HBV patients need lifelong medical follow-up to prevent progression and death from cirrhosis and liver cancer, they emphasized.
The hepatitis C virus presents a different picture – there is no HCV vaccine, while the novel direct-acting antivirals can theoretically cure nearly all chronic infections. But in reality, high drug costs and the massive burden of undiagnosed HCV mean that only a small fraction of patients can be cured, the authors acknowledged. On an individual basis, the benefits of direct-acting antivirals outweigh the costs of treating genotype 1 HCV and associated morbidity, they add. But on a population level, “eliminating HCV infection would require near universal access to treatment, something that appears infeasible given the current pricing and policy environment.”
Several other barriers also impede progress against HBV and HCV. For example, only five states and two large cities receive enough funding for comprehensive viral hepatitis surveillance, Dr. Strom and his associates noted. If local and state public health departments cannot track infections, they cannot characterize their epidemics. In addition, viral hepatitis remains a source of stigma, adding to fear and avoidance of testing and undermining elimination efforts. Stigma, avoidance, and the fact that HBV and HCV are asymptomatic in the early stages all contribute to a large population of chronically infected patients who do not know their status.
Moreover, many state Medicaid programs will not cover foreigners until they have lived in the United States for 5 years, and the Affordable Care Act does not cover care for short-term residents and undocumented immigrants, the report stated. Newly infected HCV patients tend to have fewer resources, lower educational levels, and more often use injection drugs, making them harder to reach and screen through the health system. “Prisons are a promising venue in which to treat HCV, but treating HCV is expensive for the prison system. The cost of the direct-acting antivirals is high, and the staff time required to manage an inmate in treatment often far exceed the available resources,” the report stated.
The study was sponsored by the Centers for Disease Control and Prevention Office of Viral Hepatitis and the U.S. Department of Health and Human Services Office of Minority Health. The authors disclosed no conflicts of interest.
The United States can end hepatitis B and C transmission and prevent related morbidity and mortality given enough time, resources, and will to surmount a variety of barriers, according to a new report from the National Academies of Sciences, Engineering, and Medicine.
A more feasible short-term goal is to control viral hepatitis by reducing the incidence and prevalence of hepatitis B (HBV) and C virus (HCV) infections, states the report, which is the first of a two-phase study. The second part, to be published in early 2017, will outline a strategy to achieve the goals.
“Disease elimination” once meant stopping all new infections in a population, but eliminating a public health problem “can be a less absolute goal,” Dr. Brian Strom, chancellor of Rutgers Biomedical and Health Sciences, wrote along with his associates. “The WHO has accepted a non-zero target in its work against viral hepatitis,” they added. “The organization’s provisional target is a 90% reduction in incidence, and a 65% reduction in mortality by 2030.”
Furthermore, those are global targets – countries should base their own strategies on their specific viral hepatitis disease burdens and epidemiologic features, Dr. Strom and his associates emphasized. For example, universal vaccination could eradicate HBV in the United States in two generations, but there is no cure for the 700,000-1.4 million Americans who are already infected. Mother-to-child HBV transmission is rare here, but better screening and surveillance could help prevent the 800-1,000 cases of vertical transmission that still occur annually.
More than a third of newborns in the United States do not receive the HBV vaccination on the day they are born, and 28% remain unvaccinated when they are 3 days old, “but childhood catch-up is possible,” the experts also wrote. Vaccinating adults is more complicated, but targeting high-risk groups such as prison inmates or patients at sexually transmitted disease clinics might work, they added. Vaccination is especially important, because chronically infected HBV patients need lifelong medical follow-up to prevent progression and death from cirrhosis and liver cancer, they emphasized.
The hepatitis C virus presents a different picture – there is no HCV vaccine, while the novel direct-acting antivirals can theoretically cure nearly all chronic infections. But in reality, high drug costs and the massive burden of undiagnosed HCV mean that only a small fraction of patients can be cured, the authors acknowledged. On an individual basis, the benefits of direct-acting antivirals outweigh the costs of treating genotype 1 HCV and associated morbidity, they add. But on a population level, “eliminating HCV infection would require near universal access to treatment, something that appears infeasible given the current pricing and policy environment.”
Several other barriers also impede progress against HBV and HCV. For example, only five states and two large cities receive enough funding for comprehensive viral hepatitis surveillance, Dr. Strom and his associates noted. If local and state public health departments cannot track infections, they cannot characterize their epidemics. In addition, viral hepatitis remains a source of stigma, adding to fear and avoidance of testing and undermining elimination efforts. Stigma, avoidance, and the fact that HBV and HCV are asymptomatic in the early stages all contribute to a large population of chronically infected patients who do not know their status.
Moreover, many state Medicaid programs will not cover foreigners until they have lived in the United States for 5 years, and the Affordable Care Act does not cover care for short-term residents and undocumented immigrants, the report stated. Newly infected HCV patients tend to have fewer resources, lower educational levels, and more often use injection drugs, making them harder to reach and screen through the health system. “Prisons are a promising venue in which to treat HCV, but treating HCV is expensive for the prison system. The cost of the direct-acting antivirals is high, and the staff time required to manage an inmate in treatment often far exceed the available resources,” the report stated.
The study was sponsored by the Centers for Disease Control and Prevention Office of Viral Hepatitis and the U.S. Department of Health and Human Services Office of Minority Health. The authors disclosed no conflicts of interest.
FROM THE NATIONAL ACADEMIES OF SCIENCES, ENGINEERING, AND MEDICINE
Hepatitis C virus transmission peaked in 1950
Iatrogenic factors, not high-risk behaviors, were the main cause of the exponential spread of hepatitis C virus infections in North America, according to a phylogenetic study published online in the Lancet Infectious Diseases.
The study shifted the epicenter of spread back by more than 15 years, to about 1950, when medical procedures were expanding after World War II at the same time that clinicians continued to reuse metal and glass syringes, said Dr. Jeffrey Joy of the British Columbia Centre for Excellence in HIV/AIDS, Vancouver, and his associates.
Exponential HCV transmission had mostly ended by 1965, negating “the idea that the epidemic among baby boomers and other demographic groups in North America is primarily due to injection drug use, unsafe tattooing, high-risk sex, and travel to high endemic areas during youth,” the researchers added.
The hepatitis C virus evolves rapidly, enabling researchers to characterize its spread based on the genetic divergence of infections. Dr. Joy and his associates studied 45,316 HCV genotype 1a sequences from the National Center for Biotechnology Information GenBank nucleotide database to carry out phylogenetic analyses of five HCV genes – E1, E2, NS2, NS4B, and NS5B (Lancet Infect Dis. 2016 Mar 30. doi: 10.1016/S1473-3099[16]00124-9).
The combined results for all gene regions suggested that the North American HCV epidemic expanded the most between 1940 and 1965, and that transmission rates peaked in 1950, when the oldest baby boomers were only 5 years old. Thus, nosocomial transmission rather than risky behaviors was the likely driver behind most infections, the researchers said.
“Our results could help to reduce stigma related to screening and diagnosis of hepatitis C virus, potentially increasing the numbers of providers offering screening, patients accepting testing, and if positive, presenting for care and treatment,” the authors noted.
The researchers also found that HCV transmission rates plateaued between 1965 and 1989, and dropped in the early 1990s, coinciding with increases in blood product testing and needle exchange programs aimed at preventing HIV infections. Furthermore, HCV infections rose slightly after 2000, mirroring epidemiologic reports of outbreaks among young injection drug users in nonurban areas and among men who have sex with men, the researchers said.
The Canadian Institutes of Health Research, Michael Smith Foundation for Health Research, and BC Centre for Excellence in HIV/AIDS funded the study. The investigators disclosed no competing interests.
Dr. Jeffrey Joy and colleagues offer evidence that the era of high rates of transmission and rapid expansion of hepatitis C virus infections was from 1940 to 1960, when reuse of glass and metal syringes was common.
The authors conclude that attributing the spread of hepatitis C virus to the medical establishment, rather than youthful behavior long outgrown by most baby boomers, could justify changing the message around screening of baby boomers. Baby boomers came of age when nosocomial transmission could have been rampant. For this additional reason, rather than just individual behavior, all Americans born between 1945 and 1965 should be tested, say the authors.
This change of message, while effective, could backfire. By accepting responsibility for the pre-1960 spread, the medical establishment might increase stigma for patients born after the uptake of disposable syringes and safer clinical practices. Also, screening by birth cohort might already be outlasting its usefulness in some populations. Lowering the cost of hepatitis C virus testing could lead to routine, universal testing becoming the norm, making birth cohort testing obsolete.
Last, the novel method used in this study could be replaced by an even newer, more precise technology; would our message need to change once more?
Here are the facts: Hepatitis C virus is common among Americans born between 1945 and 1965. A current Centers for Disease Control and Prevention fact sheet asserts that most people infected with hepatitis C virus are unaware of their diagnosis, despite aggressive campaigns since 2012 to find cases. Let’s just go ahead and test our baby boomers.
Dr. Anne C. Spaulding and Dr. Lesley S. Miller are at the Rollins School of Public Health, Emory University, Atlanta. They reported receiving grants and other funding from Gilead Sciences and Bristol-Myers Squibb. These comments are from their editorial (Lancet Infect Dis. 2016 Mar 30. doi: 10.1016/S1473-3099[16]30002-0).
Dr. Jeffrey Joy and colleagues offer evidence that the era of high rates of transmission and rapid expansion of hepatitis C virus infections was from 1940 to 1960, when reuse of glass and metal syringes was common.
The authors conclude that attributing the spread of hepatitis C virus to the medical establishment, rather than youthful behavior long outgrown by most baby boomers, could justify changing the message around screening of baby boomers. Baby boomers came of age when nosocomial transmission could have been rampant. For this additional reason, rather than just individual behavior, all Americans born between 1945 and 1965 should be tested, say the authors.
This change of message, while effective, could backfire. By accepting responsibility for the pre-1960 spread, the medical establishment might increase stigma for patients born after the uptake of disposable syringes and safer clinical practices. Also, screening by birth cohort might already be outlasting its usefulness in some populations. Lowering the cost of hepatitis C virus testing could lead to routine, universal testing becoming the norm, making birth cohort testing obsolete.
Last, the novel method used in this study could be replaced by an even newer, more precise technology; would our message need to change once more?
Here are the facts: Hepatitis C virus is common among Americans born between 1945 and 1965. A current Centers for Disease Control and Prevention fact sheet asserts that most people infected with hepatitis C virus are unaware of their diagnosis, despite aggressive campaigns since 2012 to find cases. Let’s just go ahead and test our baby boomers.
Dr. Anne C. Spaulding and Dr. Lesley S. Miller are at the Rollins School of Public Health, Emory University, Atlanta. They reported receiving grants and other funding from Gilead Sciences and Bristol-Myers Squibb. These comments are from their editorial (Lancet Infect Dis. 2016 Mar 30. doi: 10.1016/S1473-3099[16]30002-0).
Dr. Jeffrey Joy and colleagues offer evidence that the era of high rates of transmission and rapid expansion of hepatitis C virus infections was from 1940 to 1960, when reuse of glass and metal syringes was common.
The authors conclude that attributing the spread of hepatitis C virus to the medical establishment, rather than youthful behavior long outgrown by most baby boomers, could justify changing the message around screening of baby boomers. Baby boomers came of age when nosocomial transmission could have been rampant. For this additional reason, rather than just individual behavior, all Americans born between 1945 and 1965 should be tested, say the authors.
This change of message, while effective, could backfire. By accepting responsibility for the pre-1960 spread, the medical establishment might increase stigma for patients born after the uptake of disposable syringes and safer clinical practices. Also, screening by birth cohort might already be outlasting its usefulness in some populations. Lowering the cost of hepatitis C virus testing could lead to routine, universal testing becoming the norm, making birth cohort testing obsolete.
Last, the novel method used in this study could be replaced by an even newer, more precise technology; would our message need to change once more?
Here are the facts: Hepatitis C virus is common among Americans born between 1945 and 1965. A current Centers for Disease Control and Prevention fact sheet asserts that most people infected with hepatitis C virus are unaware of their diagnosis, despite aggressive campaigns since 2012 to find cases. Let’s just go ahead and test our baby boomers.
Dr. Anne C. Spaulding and Dr. Lesley S. Miller are at the Rollins School of Public Health, Emory University, Atlanta. They reported receiving grants and other funding from Gilead Sciences and Bristol-Myers Squibb. These comments are from their editorial (Lancet Infect Dis. 2016 Mar 30. doi: 10.1016/S1473-3099[16]30002-0).
Iatrogenic factors, not high-risk behaviors, were the main cause of the exponential spread of hepatitis C virus infections in North America, according to a phylogenetic study published online in the Lancet Infectious Diseases.
The study shifted the epicenter of spread back by more than 15 years, to about 1950, when medical procedures were expanding after World War II at the same time that clinicians continued to reuse metal and glass syringes, said Dr. Jeffrey Joy of the British Columbia Centre for Excellence in HIV/AIDS, Vancouver, and his associates.
Exponential HCV transmission had mostly ended by 1965, negating “the idea that the epidemic among baby boomers and other demographic groups in North America is primarily due to injection drug use, unsafe tattooing, high-risk sex, and travel to high endemic areas during youth,” the researchers added.
The hepatitis C virus evolves rapidly, enabling researchers to characterize its spread based on the genetic divergence of infections. Dr. Joy and his associates studied 45,316 HCV genotype 1a sequences from the National Center for Biotechnology Information GenBank nucleotide database to carry out phylogenetic analyses of five HCV genes – E1, E2, NS2, NS4B, and NS5B (Lancet Infect Dis. 2016 Mar 30. doi: 10.1016/S1473-3099[16]00124-9).
The combined results for all gene regions suggested that the North American HCV epidemic expanded the most between 1940 and 1965, and that transmission rates peaked in 1950, when the oldest baby boomers were only 5 years old. Thus, nosocomial transmission rather than risky behaviors was the likely driver behind most infections, the researchers said.
“Our results could help to reduce stigma related to screening and diagnosis of hepatitis C virus, potentially increasing the numbers of providers offering screening, patients accepting testing, and if positive, presenting for care and treatment,” the authors noted.
The researchers also found that HCV transmission rates plateaued between 1965 and 1989, and dropped in the early 1990s, coinciding with increases in blood product testing and needle exchange programs aimed at preventing HIV infections. Furthermore, HCV infections rose slightly after 2000, mirroring epidemiologic reports of outbreaks among young injection drug users in nonurban areas and among men who have sex with men, the researchers said.
The Canadian Institutes of Health Research, Michael Smith Foundation for Health Research, and BC Centre for Excellence in HIV/AIDS funded the study. The investigators disclosed no competing interests.
Iatrogenic factors, not high-risk behaviors, were the main cause of the exponential spread of hepatitis C virus infections in North America, according to a phylogenetic study published online in the Lancet Infectious Diseases.
The study shifted the epicenter of spread back by more than 15 years, to about 1950, when medical procedures were expanding after World War II at the same time that clinicians continued to reuse metal and glass syringes, said Dr. Jeffrey Joy of the British Columbia Centre for Excellence in HIV/AIDS, Vancouver, and his associates.
Exponential HCV transmission had mostly ended by 1965, negating “the idea that the epidemic among baby boomers and other demographic groups in North America is primarily due to injection drug use, unsafe tattooing, high-risk sex, and travel to high endemic areas during youth,” the researchers added.
The hepatitis C virus evolves rapidly, enabling researchers to characterize its spread based on the genetic divergence of infections. Dr. Joy and his associates studied 45,316 HCV genotype 1a sequences from the National Center for Biotechnology Information GenBank nucleotide database to carry out phylogenetic analyses of five HCV genes – E1, E2, NS2, NS4B, and NS5B (Lancet Infect Dis. 2016 Mar 30. doi: 10.1016/S1473-3099[16]00124-9).
The combined results for all gene regions suggested that the North American HCV epidemic expanded the most between 1940 and 1965, and that transmission rates peaked in 1950, when the oldest baby boomers were only 5 years old. Thus, nosocomial transmission rather than risky behaviors was the likely driver behind most infections, the researchers said.
“Our results could help to reduce stigma related to screening and diagnosis of hepatitis C virus, potentially increasing the numbers of providers offering screening, patients accepting testing, and if positive, presenting for care and treatment,” the authors noted.
The researchers also found that HCV transmission rates plateaued between 1965 and 1989, and dropped in the early 1990s, coinciding with increases in blood product testing and needle exchange programs aimed at preventing HIV infections. Furthermore, HCV infections rose slightly after 2000, mirroring epidemiologic reports of outbreaks among young injection drug users in nonurban areas and among men who have sex with men, the researchers said.
The Canadian Institutes of Health Research, Michael Smith Foundation for Health Research, and BC Centre for Excellence in HIV/AIDS funded the study. The investigators disclosed no competing interests.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point: Iatrogenic factors, rather than high-risk behaviors, were the most likely cause of the exponential spread of hepatitis C virus infections in North America.
Major finding: Transmission of HCV genotype 1a peaked in North America in 1950, when the oldest baby boomers were 5 years old.
Data source: A retrospective phylogenetic study of five HCV genotype 1a genes from 45,316 publicly available sequences.
Disclosures: The Canadian Institutes of Health Research, Michael Smith Foundation for Health Research, and BC Centre for Excellence in HIV/AIDS funded the study. The investigators disclosed no competing interests.