User login
For preventing AKs, 5-FU beats placebo for up to 3 years
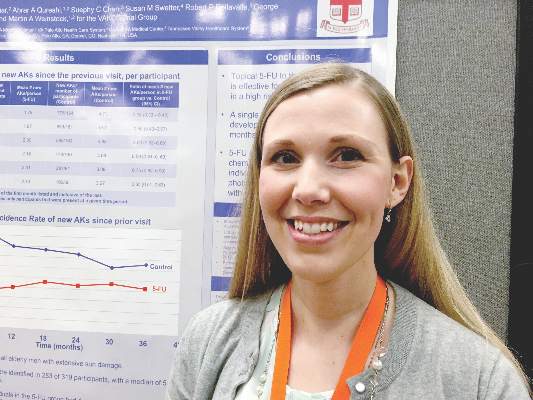
SCOTTSDALE – A single course of topical 5-fluorouracil (5-FU) prevented 62% more actinic keratoses than placebo, and this chemopreventive effect persisted for up to 3 years, according to an analysis of the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCCT) trial.
Other studies have shown that 5-FU effectively treats precancerous AKs, but have not examined whether 5-FU can prevent AKs, Dr. Joanna Walker said in an interview at the annual meeting of the Society for Investigative Dermatology.
Clinicians should consider preventive 5-FU in patients who are at high risk for basal cell and squamous cell carcinomas, especially if a skin check reveals multiple AKs, said Dr. Walker of the department of dermatology, Brown University, Providence, RI.
The VAKCCT was a randomized, double-blind, placebo-controlled study conducted at 12 Veterans Affairs dermatology clinics. The 319 patients in the analysis were nearly all elderly men with extensive sun damage, with a total of 2,386 AKs at baseline, for an average of five lesions per patient. Patients also had a history of at least two keratinocyte carcinomas in the past 5 years, including at least one lesion on the face or ears, and no recent history of 5-FU exposure.
The clinically and demographically similar study arms were randomized to either 5% topical 5-FU cream or a vehicle control cream, applied twice daily for 2-4 weeks. Both groups received cryotherapy for existing AKs, and were given free SPF 30 sunscreen. At each 6-month follow-up visit, the researchers counted existing AKs and new lesions.
At month 6, the treatment group had 62% fewer new AKs than the placebo group (average per patient, 1.78 and 4.73, respectively), a statistically significant difference. At months 12, 18, 24, 30, and 36, respectively, the treatment group had 50%, 40%, 41%, 25%, and 35% fewer new AKs than the placebo group, and these differences all were statistically significant. Furthermore, at month 6, only 56% of treated patients had at least one new AK, compared with 78% of the control group (incidence rate ratio, 0.72; 95% confidence interval, 0.54-0.95).
This chemopreventive effect remained significant for 24 months, the investigators reported. “Individuals with at least five AKs at the time of 5-FU treatment had an even more dramatic reduction in new AKs,” Dr. Walker noted. “There is now high-quality evidence supporting the use of topical 5-FU for AK chemoprevention. I think this is important information that we can take back to the clinic when we are trying to convince our patients to go through a course of 5-FU.”
The rate of new AKs in the placebo group fell during the first 2.5 years of the study and then stabilized. “For both groups, there was a dramatic increase in the use of sunscreen during the trial, and we hypothesized that the decrease in AKs in the control group was due to increased use of sun-protective measures,” Dr. Walker said.
The research was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
SCOTTSDALE – A single course of topical 5-fluorouracil (5-FU) prevented 62% more actinic keratoses than placebo, and this chemopreventive effect persisted for up to 3 years, according to an analysis of the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCCT) trial.
Other studies have shown that 5-FU effectively treats precancerous AKs, but have not examined whether 5-FU can prevent AKs, Dr. Joanna Walker said in an interview at the annual meeting of the Society for Investigative Dermatology.
Clinicians should consider preventive 5-FU in patients who are at high risk for basal cell and squamous cell carcinomas, especially if a skin check reveals multiple AKs, said Dr. Walker of the department of dermatology, Brown University, Providence, RI.
The VAKCCT was a randomized, double-blind, placebo-controlled study conducted at 12 Veterans Affairs dermatology clinics. The 319 patients in the analysis were nearly all elderly men with extensive sun damage, with a total of 2,386 AKs at baseline, for an average of five lesions per patient. Patients also had a history of at least two keratinocyte carcinomas in the past 5 years, including at least one lesion on the face or ears, and no recent history of 5-FU exposure.
The clinically and demographically similar study arms were randomized to either 5% topical 5-FU cream or a vehicle control cream, applied twice daily for 2-4 weeks. Both groups received cryotherapy for existing AKs, and were given free SPF 30 sunscreen. At each 6-month follow-up visit, the researchers counted existing AKs and new lesions.
At month 6, the treatment group had 62% fewer new AKs than the placebo group (average per patient, 1.78 and 4.73, respectively), a statistically significant difference. At months 12, 18, 24, 30, and 36, respectively, the treatment group had 50%, 40%, 41%, 25%, and 35% fewer new AKs than the placebo group, and these differences all were statistically significant. Furthermore, at month 6, only 56% of treated patients had at least one new AK, compared with 78% of the control group (incidence rate ratio, 0.72; 95% confidence interval, 0.54-0.95).
This chemopreventive effect remained significant for 24 months, the investigators reported. “Individuals with at least five AKs at the time of 5-FU treatment had an even more dramatic reduction in new AKs,” Dr. Walker noted. “There is now high-quality evidence supporting the use of topical 5-FU for AK chemoprevention. I think this is important information that we can take back to the clinic when we are trying to convince our patients to go through a course of 5-FU.”
The rate of new AKs in the placebo group fell during the first 2.5 years of the study and then stabilized. “For both groups, there was a dramatic increase in the use of sunscreen during the trial, and we hypothesized that the decrease in AKs in the control group was due to increased use of sun-protective measures,” Dr. Walker said.
The research was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
SCOTTSDALE – A single course of topical 5-fluorouracil (5-FU) prevented 62% more actinic keratoses than placebo, and this chemopreventive effect persisted for up to 3 years, according to an analysis of the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCCT) trial.
Other studies have shown that 5-FU effectively treats precancerous AKs, but have not examined whether 5-FU can prevent AKs, Dr. Joanna Walker said in an interview at the annual meeting of the Society for Investigative Dermatology.
Clinicians should consider preventive 5-FU in patients who are at high risk for basal cell and squamous cell carcinomas, especially if a skin check reveals multiple AKs, said Dr. Walker of the department of dermatology, Brown University, Providence, RI.
The VAKCCT was a randomized, double-blind, placebo-controlled study conducted at 12 Veterans Affairs dermatology clinics. The 319 patients in the analysis were nearly all elderly men with extensive sun damage, with a total of 2,386 AKs at baseline, for an average of five lesions per patient. Patients also had a history of at least two keratinocyte carcinomas in the past 5 years, including at least one lesion on the face or ears, and no recent history of 5-FU exposure.
The clinically and demographically similar study arms were randomized to either 5% topical 5-FU cream or a vehicle control cream, applied twice daily for 2-4 weeks. Both groups received cryotherapy for existing AKs, and were given free SPF 30 sunscreen. At each 6-month follow-up visit, the researchers counted existing AKs and new lesions.
At month 6, the treatment group had 62% fewer new AKs than the placebo group (average per patient, 1.78 and 4.73, respectively), a statistically significant difference. At months 12, 18, 24, 30, and 36, respectively, the treatment group had 50%, 40%, 41%, 25%, and 35% fewer new AKs than the placebo group, and these differences all were statistically significant. Furthermore, at month 6, only 56% of treated patients had at least one new AK, compared with 78% of the control group (incidence rate ratio, 0.72; 95% confidence interval, 0.54-0.95).
This chemopreventive effect remained significant for 24 months, the investigators reported. “Individuals with at least five AKs at the time of 5-FU treatment had an even more dramatic reduction in new AKs,” Dr. Walker noted. “There is now high-quality evidence supporting the use of topical 5-FU for AK chemoprevention. I think this is important information that we can take back to the clinic when we are trying to convince our patients to go through a course of 5-FU.”
The rate of new AKs in the placebo group fell during the first 2.5 years of the study and then stabilized. “For both groups, there was a dramatic increase in the use of sunscreen during the trial, and we hypothesized that the decrease in AKs in the control group was due to increased use of sun-protective measures,” Dr. Walker said.
The research was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: One course of topical 5-fluorouracil was effective and durable in preventing new actinic keratoses in high-risk patients.
Major finding: At month 6, the treatment group had 62% fewer new AKs than the placebo group, and the difference remained significant at month 36.
Data source: The double-blind controlled study evaluated 5-FU vs. a vehicle cream in 319 veterans, most of whom were elderly men.
Disclosures: The study was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
Serious Infections Are Increasing Among Psoriasis Inpatients
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Serious infections are increasing among psoriasis inpatients
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
SCOTTSDALE, ARIZ. – From enterocolitis to MRSA, serious infections are on the rise among inpatients with psoriasis, and psoriasis is an independent risk factor for serious infections, according to findings from large retrospective studies from the United States and the United Kingdom.
Inpatients with psoriasis in the United States also were at greater risk of serious infections, compared with nonpsoriatic inpatients at every time point studied, and serious infections were associated with increased hospital costs, length of stay, and risk of mortality, reported Derek Hsu, a medical student at Northwestern University, Chicago, and his associates. “Research is needed to determine how to reduce the risk of serious infections in patients with psoriasis,” the investigators emphasized.
Psoriasis affects some 7 million adults in the United States. Biologics, which are transforming the treatment landscape for moderate-to-severe psoriasis, “should reduce inherent infectious risk by controlling the inflammatory process and reducing disease severity, [but] these effects may be immunosuppressing and increase the risk of infection in other ways,” according to Mr. Hsu and his associates. For their study, they analyzed data for 2002-2012 from the Nationwide Inpatient Sample, which covers 20% of hospitalizations in the United States. They extracted validated ICD-9 codes for psoriasis and serious infections, and calculated costs of care after adjusting for 2014 inflation, based on the United States Consumer Price Index.
Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients with psoriasis between 2002 and 2012 (all P-values less than .05). Predictors of serious infections among inpatients with psoriasis included diabetes mellitus, obesity, and being of non-Caucasian race or ethnicity, female, older than 60 years, and on Medicare or Medicaid, the researchers reported at the annual meeting of the Society for Investigative Dermatology.
Furthermore, after controlling for age, sex, and race, psoriasis was a significant risk factor for many different types of serious infections. Among these were cellulitis, herpes simplex virus, infectious arthritis, osteomyelitis, meningitis, influenza, encephalitis, septicemia, enterocolitis, MRSA, methicillin-sensitive Staphylococcus aureus infections, and Clostridium difficile. Further, inpatients with psoriasis were more prone to urinary tract infection, peritonitis or intestinal abscess, appendicitis, tuberculosis, and viral and fungal infections (all P-values less than .05). The average cost of hospital stay for inpatients with psoriasis was more than $2,200 greater when they were diagnosed with one or more serious infections than otherwise, and their average length of hospital stay was 2 days longer.
The study in the United Kingdom included nearly 200,000 patients with psoriasis and almost 1 million patients without psoriasis from The Health Improvement Network electronic medical record database. Between 2002 and 2013, patients without psoriasis developed an estimated 78.5 serious infections per 100,000 person-years, compared with 88.9, 85.7, and 145.7 serious infections per 100,000 person-years, respectively, for all psoriasis patients, patients with mild disease, and patients with severe disease requiring systemic or phototherapy, said Dr. Junko Takeshita and her colleagues at the University of Pennsylvania in Philadelphia. After controlling for many potential demographic and clinical confounders, psoriasis increased the risk of serious infection by about 21% (hazard ratio, 1.21; 95% confidence interval, 1.18-1.23). Patients with severe psoriasis had a 63% greater risk of infection than patients without psoriasis, compared with an 18% increase for patients with mild psoriasis.
The findings show “serious infection, particularly respiratory and skin or soft tissue infections, to be an important and common cause of morbidity among patients with psoriasis, especially those with more severe disease,” Dr. Takeshita and her associates said. Notably, the link between psoriasis and risk of serious infection persisted after excluding patients on immunosuppressive therapies, suggesting “that the greater infection risk is at least partially attributable to more severe psoriasis, itself,” they added.
The analysis of Nationwide Inpatient Sample data was funded by the Agency for Healthcare Research and Quality and by the Dermatology Foundation. The analysis of Health Improvement Network data was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Psoriasis is an independent risk factor for serious infections, and serious infections are increasing among inpatients with psoriasis.
Major finding: Overall rates of serious infection and rates of pneumonia, MRSA, septicemia, diverticulitis, enterocolitis, encephalitis, and any viral or fungal infection rose significantly among inpatients in the United States with psoriasis between 2002 and 2012 (all P-values less than .05). In the United Kingdom during the same time period, patients with severe psoriasis had a 63% greater risk of serious infection than patients without psoriasis.
Data source: Analyses of data from the Nationwide Inpatient Sample for 2002 through 2012, and from The Health Improvement Network for 2003 through 2012.
Disclosures: The Nationwide Inpatient Sample analysis was funded by the Agency for Healthcare Research and Quality and the Dermatology Foundation. The analysis of The Health Improvement Network was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, which is part of the National Institutes of Health, and by the Dermatology Foundation. None of the investigators reported conflicts of interest.
Crisaborole’s safety holds up in long-term atopic dermatitis trial
SCOTTSDALE, ARIZ. – The phosphodiesterase-4 inhibitor crisaborole was well tolerated over 6 to 12 months, yielding no major safety signals during a multicenter, open-label extension study of patients with mild-to-moderate atopic dermatitis.
These safety results held up across age groups and over time, said Dr. Lawrence Eichenfield, a dermatologist at Children’s Hospital, San Diego, and at the University of San Diego School of Medicine. “The majority of treatment-emergent adverse events were considered mild to moderate and not related to treatment. There were no reports of long-term cutaneous reactions, such as atrophy or telangiectasia,” he and his associates added.
Atopic dermatitis has lacked widely accepted treatment options. Despite attempts to educate patients and parents about topical steroids, many are afraid to use them, and topical calcineurin inhibitors have a black box warning for cancer risk. Not surprisingly, therefore, a 2% ointment of crisaborole made headlines in 2015 after meeting its efficacy and safety endpoints in two pivotal phase III trials of patients with mild-to-moderate atopic dermatitis. Based on those results, Anacor Pharmaceuticals filed a new drug application for the novel boron-based small molecule in January 2016.
The pivotal trials lasted just 28 days, so to assess long-term safety, Dr. Eichenfield and his associates enrolled a subgroup of 517 patients aged 2 to 72 years into a single-arm, open-label, 48-week extension study of crisaborole. About 31% of participants had received the control vehicle during the pivotal trials, while the rest had received crisaborole and tolerated it well enough to continue using it. Patients applied crisaborole twice daily during treatment cycles of 28 days, and were evaluated on days 1, 8, and 29 for up to 12 treatment cycles. Patients whose skin became clear or almost clear went off treatment, but they were still assessed for adverse effects at the same frequency.
In all, 396 patients used crisaborole for at least 6 months, and 271 completed 12 months of treatment, the researchers reported at the annual meeting of the Society for Investigative Dermatology. Only nine (1.7%) patients stopped treatment during the extension study because of treatment-emergent adverse effects. A total of 65% of patients had at least one treatment-emergent adverse event during the initial phase III trials, the extension study, or both. These were usually mildly or moderately severe and included nasopharyngitis, upper respiratory infections, cough, and/or fever, all of which were considered unrelated to treatment.
Treatment-related adverse events included flares of atopic dermatitis, burning or stinging at the application site, and application site infection, which affected 3.1%, 2.3%, and 1.2%, respectively, of patients in the extension study. None of these events were considered serious. Notably, 11% of patients experienced atopic dermatitis flares in the original phase III trials, the researchers reported. Patients who could not tolerate crisaborole were excluded from the extension study, which might help explain the lower flare rate (3%) with long-term treatment.
“Crisaborole topical ointment, 2%, demonstrated a favorable long-term safety profile for the treatment of patients aged 2 years and older with mild-to-moderate atopic dermatitis,” the researchers concluded. The Food and Drug Administration accepted the new drug application in March.
Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
SCOTTSDALE, ARIZ. – The phosphodiesterase-4 inhibitor crisaborole was well tolerated over 6 to 12 months, yielding no major safety signals during a multicenter, open-label extension study of patients with mild-to-moderate atopic dermatitis.
These safety results held up across age groups and over time, said Dr. Lawrence Eichenfield, a dermatologist at Children’s Hospital, San Diego, and at the University of San Diego School of Medicine. “The majority of treatment-emergent adverse events were considered mild to moderate and not related to treatment. There were no reports of long-term cutaneous reactions, such as atrophy or telangiectasia,” he and his associates added.
Atopic dermatitis has lacked widely accepted treatment options. Despite attempts to educate patients and parents about topical steroids, many are afraid to use them, and topical calcineurin inhibitors have a black box warning for cancer risk. Not surprisingly, therefore, a 2% ointment of crisaborole made headlines in 2015 after meeting its efficacy and safety endpoints in two pivotal phase III trials of patients with mild-to-moderate atopic dermatitis. Based on those results, Anacor Pharmaceuticals filed a new drug application for the novel boron-based small molecule in January 2016.
The pivotal trials lasted just 28 days, so to assess long-term safety, Dr. Eichenfield and his associates enrolled a subgroup of 517 patients aged 2 to 72 years into a single-arm, open-label, 48-week extension study of crisaborole. About 31% of participants had received the control vehicle during the pivotal trials, while the rest had received crisaborole and tolerated it well enough to continue using it. Patients applied crisaborole twice daily during treatment cycles of 28 days, and were evaluated on days 1, 8, and 29 for up to 12 treatment cycles. Patients whose skin became clear or almost clear went off treatment, but they were still assessed for adverse effects at the same frequency.
In all, 396 patients used crisaborole for at least 6 months, and 271 completed 12 months of treatment, the researchers reported at the annual meeting of the Society for Investigative Dermatology. Only nine (1.7%) patients stopped treatment during the extension study because of treatment-emergent adverse effects. A total of 65% of patients had at least one treatment-emergent adverse event during the initial phase III trials, the extension study, or both. These were usually mildly or moderately severe and included nasopharyngitis, upper respiratory infections, cough, and/or fever, all of which were considered unrelated to treatment.
Treatment-related adverse events included flares of atopic dermatitis, burning or stinging at the application site, and application site infection, which affected 3.1%, 2.3%, and 1.2%, respectively, of patients in the extension study. None of these events were considered serious. Notably, 11% of patients experienced atopic dermatitis flares in the original phase III trials, the researchers reported. Patients who could not tolerate crisaborole were excluded from the extension study, which might help explain the lower flare rate (3%) with long-term treatment.
“Crisaborole topical ointment, 2%, demonstrated a favorable long-term safety profile for the treatment of patients aged 2 years and older with mild-to-moderate atopic dermatitis,” the researchers concluded. The Food and Drug Administration accepted the new drug application in March.
Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
SCOTTSDALE, ARIZ. – The phosphodiesterase-4 inhibitor crisaborole was well tolerated over 6 to 12 months, yielding no major safety signals during a multicenter, open-label extension study of patients with mild-to-moderate atopic dermatitis.
These safety results held up across age groups and over time, said Dr. Lawrence Eichenfield, a dermatologist at Children’s Hospital, San Diego, and at the University of San Diego School of Medicine. “The majority of treatment-emergent adverse events were considered mild to moderate and not related to treatment. There were no reports of long-term cutaneous reactions, such as atrophy or telangiectasia,” he and his associates added.
Atopic dermatitis has lacked widely accepted treatment options. Despite attempts to educate patients and parents about topical steroids, many are afraid to use them, and topical calcineurin inhibitors have a black box warning for cancer risk. Not surprisingly, therefore, a 2% ointment of crisaborole made headlines in 2015 after meeting its efficacy and safety endpoints in two pivotal phase III trials of patients with mild-to-moderate atopic dermatitis. Based on those results, Anacor Pharmaceuticals filed a new drug application for the novel boron-based small molecule in January 2016.
The pivotal trials lasted just 28 days, so to assess long-term safety, Dr. Eichenfield and his associates enrolled a subgroup of 517 patients aged 2 to 72 years into a single-arm, open-label, 48-week extension study of crisaborole. About 31% of participants had received the control vehicle during the pivotal trials, while the rest had received crisaborole and tolerated it well enough to continue using it. Patients applied crisaborole twice daily during treatment cycles of 28 days, and were evaluated on days 1, 8, and 29 for up to 12 treatment cycles. Patients whose skin became clear or almost clear went off treatment, but they were still assessed for adverse effects at the same frequency.
In all, 396 patients used crisaborole for at least 6 months, and 271 completed 12 months of treatment, the researchers reported at the annual meeting of the Society for Investigative Dermatology. Only nine (1.7%) patients stopped treatment during the extension study because of treatment-emergent adverse effects. A total of 65% of patients had at least one treatment-emergent adverse event during the initial phase III trials, the extension study, or both. These were usually mildly or moderately severe and included nasopharyngitis, upper respiratory infections, cough, and/or fever, all of which were considered unrelated to treatment.
Treatment-related adverse events included flares of atopic dermatitis, burning or stinging at the application site, and application site infection, which affected 3.1%, 2.3%, and 1.2%, respectively, of patients in the extension study. None of these events were considered serious. Notably, 11% of patients experienced atopic dermatitis flares in the original phase III trials, the researchers reported. Patients who could not tolerate crisaborole were excluded from the extension study, which might help explain the lower flare rate (3%) with long-term treatment.
“Crisaborole topical ointment, 2%, demonstrated a favorable long-term safety profile for the treatment of patients aged 2 years and older with mild-to-moderate atopic dermatitis,” the researchers concluded. The Food and Drug Administration accepted the new drug application in March.
Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: The topical phosphodiesterase-4 inhibitor crisaborole was safe and well tolerated for up to 48 weeks in patients with mild-to-moderate atopic dermatitis.
Major finding: The most common treatment–related adverse events were atopic dermatitis flare (3%), stinging and burning at the application site (2%), and application site infection (1%). None were serious.
Data source: A single-arm, multicenter, open-label, 48-week extension study of 517 patients with mild-to-moderate atopic dermatitis.
Disclosures: Anacor Pharmaceuticals makes crisaborole and funded the study. Dr. Eichenfield has served as an investigator and consultant to Anacor. Three coinvestigators also reported affiliations with Anacor.
Study Lays Groundwork for Refractory Cutaneous Lupus Treatment Algorithms
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Study lays groundwork for refractory cutaneous lupus treatment algorithms
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
SCOTTSDALE, ARIZ. – Thalidomide, methotrexate, dapsone, and belimumab may be the best treatment alternatives for cutaneous lupus erythematosus that is refractory to antimalarials, based on reviews of 15 years of medical records from three large tertiary care centers.
The study is the largest so far to take a comprehensive look at treatments and outcomes in hydroxychloroquine-refractory cutaneous lupus erythematosus (CLE), said Renee Fruchter, a medical student at New York University, who presented the findings at the annual meeting of the Society for Investigative Dermatology.
CLE has no approved treatments in the United States. For affected patients, quality of life is so poor that it resembles that reported by survivors of recent myocardial infarction, according to Ms. Fruchter. Patients with localized CLE can do reasonably well with sun protection and topical and intralesional treatments, but patients with more extensive disease typically need systemic treatment, most often with antimalarials, she noted. However, up to half of CLE patients are refractory to the first-line therapy, hydroxychloroquine (Plaquenil), and treatment options for this CLE subgroup are understudied.
Therefore, Ms. Fruchter and her associates reviewed medical records from patients with CLE treated between 2000 and 2015 at NYU Langone Medical Center, Brigham and Women’s Hospital, and Massachusetts General Hospital. Although the study was retrospective, they used clinical documents and medical photos, when available, to assess treatment response via the validated CLE Disease Area and Severity Index (CLASI).
Among 46 CLE patients who were refractory to hydroxychloroquine, 87% were female and 30% were African American, with an average age of 36 years, Ms. Fruchter said. Nearly three-quarters of patients (73%) had generalized CLE, while the rest had disease localized to the head and neck. As in prior studies, patients exhibited a wide range of CLE subtypes, but most commonly generalized discoid variant, Ms. Fruchter said. Nearly 30% of patients currently smoked, which also resembled prior studies of this risk factor.
Refractory patients received a wide range of systemic agents – most commonly chloroquine (Aralen) and mycophenolate mofetil (Cellcept), followed by quinacrine, dapsone, methotrexate, belimumab (Benlysta), azathioprine, thalidomide (Thalomid), lenalidomide (Revlimid), prednisone, and rituximab (Rituxan), Ms. Fruchter and her associates reported. Although treatment subgroups were small, thalidomide was most effective by far, improving disease by at least 50% in four of five treated patients. In contrast, 5 of 11 patients on methotrexate achieved at least a 50% improvement, as did 4 of 11 patients on dapsone and 3 of 9 patients on belimumab. “Other medications had lower rates of success,” Ms. Fruchter noted. Notably, CLE that failed to respond to antimalarials was also refractory to immunomodulatory therapies, she said. For example, azathioprine failed to induce a substantial clinical response in any of the patients, and only 20% of patients responded to mycophenolate mofetil.
The study also showed that switching to another antimalarial may be effective if patients do not respond to hydroxychloroquine. Fully 53% of hydroxychloroquine nonresponders had a substantial response to quinacrine, while 40% had a substantial response to chloroquine, Ms. Fruchter said.
She had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: Thalidomide, methotrexate, dapsone, and belimumab may be the best alternatives for patients whose cutaneous lupus erythematosus is refractory to antimalarials.
Major finding: Four of five patients treated with thalidomide achieved a response rate of at least 50%.
Data source: A retrospective study of 15 years of medical records from three tertiary care centers.
Disclosures: Ms. Fruchter had no disclosures.
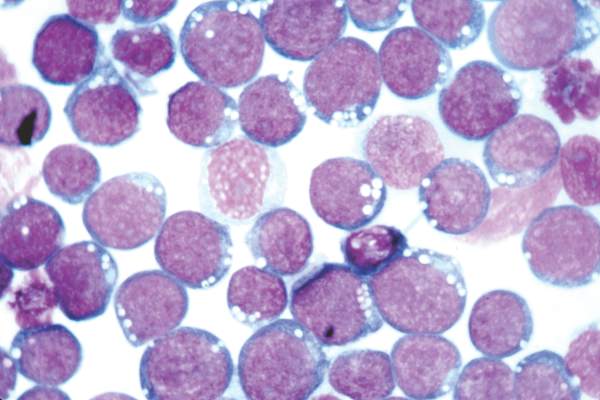
Epstein-Barr virus DNA in plasma reliably detects EBV-positive lymphoproliferative disorders
Detection of Epstein-Barr virus DNA in plasma reliably signaled “a broad range” of EBV+ diseases, according to investigators.
In contrast, the presence of EBV DNA in peripheral blood mononuclear cells did not reliably predict EBV diseases, said Dr. Jennifer A. Kanakry and her associates at Johns Hopkins University, Baltimore. Patients without EBV diseases can have EBV DNA in their PBMCs, particularly if they are immunocompromised, the researchers observed.
Latent EBV infection is associated with lymphomas, lymphoproliferative disorders, hemophagocytic lymphohistiocytosis, solid tumors, and other diseases. To characterize the relationship between these diseases and EBV DNA, the researchers studied viral quantitative real-time polymerase chain reaction assays of plasma and PBMCs from 2,146 patients tested at Johns Hopkins over 5 years. Patients were usually immunocompromised and hospitalized, the investigators noted (Blood 2016;127:2007-17).
A total of 535 patients (25%) had EBV detected in plasma or PBMCs. Notably, 69% of patients who did not have EBV diseases had EBV in PBMCs, but not in plasma. Among 105 patients with active systemic EBV+ diseases, 99% had EBV DNA in plasma, but only 54% had EBV in PBMCs. Furthermore, the number of copies of EBV DNA distinguished untreated EBV+ lymphoma, remitted EBV+ lymphoma, and EBV- lymphoma, and also distinguished untreated, EBV+ post-transplantation lymphoproliferative disorder (PTLD), EBV+ PTLD in remission, and EBV– PTLD.
“Cell-free (plasma) EBV DNA performs better than cellular EBV DNA as a marker of a broad range of EBV+ diseases,” the investigators concluded. “Within a largely immunocompromised and hospitalized cohort, detection of EBV DNA in plasma is uncommon in the absence of EBV+ disease.”
The National Cancer Institute, National Institutes of Health, and Center for AIDS Research funded the study. The researchers had no disclosures.
Detection of Epstein-Barr virus DNA in plasma reliably signaled “a broad range” of EBV+ diseases, according to investigators.
In contrast, the presence of EBV DNA in peripheral blood mononuclear cells did not reliably predict EBV diseases, said Dr. Jennifer A. Kanakry and her associates at Johns Hopkins University, Baltimore. Patients without EBV diseases can have EBV DNA in their PBMCs, particularly if they are immunocompromised, the researchers observed.
Latent EBV infection is associated with lymphomas, lymphoproliferative disorders, hemophagocytic lymphohistiocytosis, solid tumors, and other diseases. To characterize the relationship between these diseases and EBV DNA, the researchers studied viral quantitative real-time polymerase chain reaction assays of plasma and PBMCs from 2,146 patients tested at Johns Hopkins over 5 years. Patients were usually immunocompromised and hospitalized, the investigators noted (Blood 2016;127:2007-17).
A total of 535 patients (25%) had EBV detected in plasma or PBMCs. Notably, 69% of patients who did not have EBV diseases had EBV in PBMCs, but not in plasma. Among 105 patients with active systemic EBV+ diseases, 99% had EBV DNA in plasma, but only 54% had EBV in PBMCs. Furthermore, the number of copies of EBV DNA distinguished untreated EBV+ lymphoma, remitted EBV+ lymphoma, and EBV- lymphoma, and also distinguished untreated, EBV+ post-transplantation lymphoproliferative disorder (PTLD), EBV+ PTLD in remission, and EBV– PTLD.
“Cell-free (plasma) EBV DNA performs better than cellular EBV DNA as a marker of a broad range of EBV+ diseases,” the investigators concluded. “Within a largely immunocompromised and hospitalized cohort, detection of EBV DNA in plasma is uncommon in the absence of EBV+ disease.”
The National Cancer Institute, National Institutes of Health, and Center for AIDS Research funded the study. The researchers had no disclosures.
Detection of Epstein-Barr virus DNA in plasma reliably signaled “a broad range” of EBV+ diseases, according to investigators.
In contrast, the presence of EBV DNA in peripheral blood mononuclear cells did not reliably predict EBV diseases, said Dr. Jennifer A. Kanakry and her associates at Johns Hopkins University, Baltimore. Patients without EBV diseases can have EBV DNA in their PBMCs, particularly if they are immunocompromised, the researchers observed.
Latent EBV infection is associated with lymphomas, lymphoproliferative disorders, hemophagocytic lymphohistiocytosis, solid tumors, and other diseases. To characterize the relationship between these diseases and EBV DNA, the researchers studied viral quantitative real-time polymerase chain reaction assays of plasma and PBMCs from 2,146 patients tested at Johns Hopkins over 5 years. Patients were usually immunocompromised and hospitalized, the investigators noted (Blood 2016;127:2007-17).
A total of 535 patients (25%) had EBV detected in plasma or PBMCs. Notably, 69% of patients who did not have EBV diseases had EBV in PBMCs, but not in plasma. Among 105 patients with active systemic EBV+ diseases, 99% had EBV DNA in plasma, but only 54% had EBV in PBMCs. Furthermore, the number of copies of EBV DNA distinguished untreated EBV+ lymphoma, remitted EBV+ lymphoma, and EBV- lymphoma, and also distinguished untreated, EBV+ post-transplantation lymphoproliferative disorder (PTLD), EBV+ PTLD in remission, and EBV– PTLD.
“Cell-free (plasma) EBV DNA performs better than cellular EBV DNA as a marker of a broad range of EBV+ diseases,” the investigators concluded. “Within a largely immunocompromised and hospitalized cohort, detection of EBV DNA in plasma is uncommon in the absence of EBV+ disease.”
The National Cancer Institute, National Institutes of Health, and Center for AIDS Research funded the study. The researchers had no disclosures.
FROM BLOOD
Key clinical point: Epstein-Barr virus DNA was a more specific indicator of EBV+ diseases when detected in plasma, as opposed to peripheral blood mononuclear cells.
Major finding: Among 105 patients with active systemic EBV+ diseases, 99% had EBV DNA in plasma, but 54% had EBV in PBMCs.
Data source: Viral quantitative real-time polymerase chain reaction assays of plasma and PBMCs from 2,146 patients.
Disclosures: The National Cancer Institute, National Institutes of Health, and Center for AIDS Research funded the study. The researchers had no disclosures.
Calcineurin-targeted therapies eyed for multiple myeloma
Treating multiple myeloma cells with panobinostat and FK506 reduced their viability by inhibiting expression of the PPP3CA catalytic subunit of calcineurin, according to researchers.
“The development of new calcineurin-targeted therapies, which inhibit PPP3CA–NF-kappaB signaling by small molecules, is expected to profoundly improve the treatment of multiple myeloma. This will overcome drug resistance and improve osteolytic lesions in a wide range of patients, including those receiving reduced intensity–conditioned allogeneic stem cell transplantation, who may be treated with panobinostat and FK506,” wrote Dr. Yoichi Imai of Tokyo Women’s Medical University in Japan, and his associates (JCI Insight 2016 doi: 10.1172/jci.insight.85061). .
Adding panobinostat to bortezomib and dexamethasone has been shown to improve progression-free survival in relapsed and refractory multiple myeloma, the researchers noted. Other studies have linked calcineurin activation to the pathogenesis of T-cell cancers, and calcineurin inhibition seems to be involved in defective B-cell activation, they added.
Eight candidate oncogenes were identified by use of the Gene Expression Omnibus. PPP3CA was expressed at significantly higher levels in multiple myeloma cells from patients with stage III disease compared with those with stage I disease (P = .016). Furthermore, levels of serum lactate dehydrogenase correlated with PPP3CA expression and with poor overall and progression-free survival. Patients with high PPP3CA expression also had high levels of alpha4 integrins, which mediate resistance to bortezomib and conventional chemotherapy, the researchers noted.
When multiple myeloma cells were exposed to either panobinostat or a control agent, PPP3CA dropped in the panobinostat-treated cells. Adding the proteasome inhibitor lactacystin to the mixture counteracted this effect, “supporting the possibility that PPP3CA expression was reduced through protein degradation by panobinostat,” they said.
Levels of PPP3CA expression were lower in multiple myeloma cells that were cotreated with an HDAC inhibitor (panobinostat or ACY-1215) and the immunosuppressive agent FK506 than in multiple myeloma cells that were exposed only to an HDAC inhibitor. In addition, the combination regimen blocked the formation of osteoclasts, which are involved in osteolytic lesions, the researchers noted.
Also, significantly higher PPP3CA expression, which correlated with worse progression-free survival, was noted in bortezomib-resistant patients, compared with bortezomib-sensitive patients.
“The cytotoxic effect exerted by panobinostat on CD20+ cells was subtle, while the addition of FK506 did not increase their viability,” the researchers reported. “Moreover, development of T and B lineage cells was normal in PPP3CA-deficient mice, and panobinostat did not compromise donor lymphocyte reconstitution in a mouse BM transplantation model. These results suggest that calcineurin-targeting therapy exerts an antimyeloma effect without inducing significant side effects in normal lymphoid systems.”
The Japan Society for the Promotion of Science, the Takeda Science Foundation, the International Myeloma Foundation, and the Japan Leukemia Research Fund funded the study. The investigators had no disclosures.
Treating multiple myeloma cells with panobinostat and FK506 reduced their viability by inhibiting expression of the PPP3CA catalytic subunit of calcineurin, according to researchers.
“The development of new calcineurin-targeted therapies, which inhibit PPP3CA–NF-kappaB signaling by small molecules, is expected to profoundly improve the treatment of multiple myeloma. This will overcome drug resistance and improve osteolytic lesions in a wide range of patients, including those receiving reduced intensity–conditioned allogeneic stem cell transplantation, who may be treated with panobinostat and FK506,” wrote Dr. Yoichi Imai of Tokyo Women’s Medical University in Japan, and his associates (JCI Insight 2016 doi: 10.1172/jci.insight.85061). .
Adding panobinostat to bortezomib and dexamethasone has been shown to improve progression-free survival in relapsed and refractory multiple myeloma, the researchers noted. Other studies have linked calcineurin activation to the pathogenesis of T-cell cancers, and calcineurin inhibition seems to be involved in defective B-cell activation, they added.
Eight candidate oncogenes were identified by use of the Gene Expression Omnibus. PPP3CA was expressed at significantly higher levels in multiple myeloma cells from patients with stage III disease compared with those with stage I disease (P = .016). Furthermore, levels of serum lactate dehydrogenase correlated with PPP3CA expression and with poor overall and progression-free survival. Patients with high PPP3CA expression also had high levels of alpha4 integrins, which mediate resistance to bortezomib and conventional chemotherapy, the researchers noted.
When multiple myeloma cells were exposed to either panobinostat or a control agent, PPP3CA dropped in the panobinostat-treated cells. Adding the proteasome inhibitor lactacystin to the mixture counteracted this effect, “supporting the possibility that PPP3CA expression was reduced through protein degradation by panobinostat,” they said.
Levels of PPP3CA expression were lower in multiple myeloma cells that were cotreated with an HDAC inhibitor (panobinostat or ACY-1215) and the immunosuppressive agent FK506 than in multiple myeloma cells that were exposed only to an HDAC inhibitor. In addition, the combination regimen blocked the formation of osteoclasts, which are involved in osteolytic lesions, the researchers noted.
Also, significantly higher PPP3CA expression, which correlated with worse progression-free survival, was noted in bortezomib-resistant patients, compared with bortezomib-sensitive patients.
“The cytotoxic effect exerted by panobinostat on CD20+ cells was subtle, while the addition of FK506 did not increase their viability,” the researchers reported. “Moreover, development of T and B lineage cells was normal in PPP3CA-deficient mice, and panobinostat did not compromise donor lymphocyte reconstitution in a mouse BM transplantation model. These results suggest that calcineurin-targeting therapy exerts an antimyeloma effect without inducing significant side effects in normal lymphoid systems.”
The Japan Society for the Promotion of Science, the Takeda Science Foundation, the International Myeloma Foundation, and the Japan Leukemia Research Fund funded the study. The investigators had no disclosures.
Treating multiple myeloma cells with panobinostat and FK506 reduced their viability by inhibiting expression of the PPP3CA catalytic subunit of calcineurin, according to researchers.
“The development of new calcineurin-targeted therapies, which inhibit PPP3CA–NF-kappaB signaling by small molecules, is expected to profoundly improve the treatment of multiple myeloma. This will overcome drug resistance and improve osteolytic lesions in a wide range of patients, including those receiving reduced intensity–conditioned allogeneic stem cell transplantation, who may be treated with panobinostat and FK506,” wrote Dr. Yoichi Imai of Tokyo Women’s Medical University in Japan, and his associates (JCI Insight 2016 doi: 10.1172/jci.insight.85061). .
Adding panobinostat to bortezomib and dexamethasone has been shown to improve progression-free survival in relapsed and refractory multiple myeloma, the researchers noted. Other studies have linked calcineurin activation to the pathogenesis of T-cell cancers, and calcineurin inhibition seems to be involved in defective B-cell activation, they added.
Eight candidate oncogenes were identified by use of the Gene Expression Omnibus. PPP3CA was expressed at significantly higher levels in multiple myeloma cells from patients with stage III disease compared with those with stage I disease (P = .016). Furthermore, levels of serum lactate dehydrogenase correlated with PPP3CA expression and with poor overall and progression-free survival. Patients with high PPP3CA expression also had high levels of alpha4 integrins, which mediate resistance to bortezomib and conventional chemotherapy, the researchers noted.
When multiple myeloma cells were exposed to either panobinostat or a control agent, PPP3CA dropped in the panobinostat-treated cells. Adding the proteasome inhibitor lactacystin to the mixture counteracted this effect, “supporting the possibility that PPP3CA expression was reduced through protein degradation by panobinostat,” they said.
Levels of PPP3CA expression were lower in multiple myeloma cells that were cotreated with an HDAC inhibitor (panobinostat or ACY-1215) and the immunosuppressive agent FK506 than in multiple myeloma cells that were exposed only to an HDAC inhibitor. In addition, the combination regimen blocked the formation of osteoclasts, which are involved in osteolytic lesions, the researchers noted.
Also, significantly higher PPP3CA expression, which correlated with worse progression-free survival, was noted in bortezomib-resistant patients, compared with bortezomib-sensitive patients.
“The cytotoxic effect exerted by panobinostat on CD20+ cells was subtle, while the addition of FK506 did not increase their viability,” the researchers reported. “Moreover, development of T and B lineage cells was normal in PPP3CA-deficient mice, and panobinostat did not compromise donor lymphocyte reconstitution in a mouse BM transplantation model. These results suggest that calcineurin-targeting therapy exerts an antimyeloma effect without inducing significant side effects in normal lymphoid systems.”
The Japan Society for the Promotion of Science, the Takeda Science Foundation, the International Myeloma Foundation, and the Japan Leukemia Research Fund funded the study. The investigators had no disclosures.
FROM JCI INSIGHT
Key clinical point: Treating multiple myeloma cells with panobinostat and FK506 reduced their viability by inhibiting expression of the PPP3CA catalytic subunit of calcineurin.
Major finding: PPP3CA was associated with MM cell viability and osteoclast formation, and was degraded by panobinostat through HDAC inhibition.
Data source: An in vitro and in vivo laboratory study of MM in human and mouse models.
Disclosures: The Japan Society for the Promotion of Science, the Takeda Science Foundation, the International Myeloma Foundation, and the Japan Leukemia Research Fund funded the study. The investigators had no disclosures.
Low transformation rate in nodular lymphocyte–predominant Hodgkin lymphoma
Fewer than 8% of cases of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) transformed to diffuse large B-cell lymphoma (DLBCL), based on a large prospective single-center study with long-term follow-up.
This rate was lower than the risk of transformation reported for transformed follicular lymphoma or chronic lymphocytic leukemia, according to Dr. Saad Kenderian and his associates at the Mayo Clinic, Rochester, Minn. Transformation was significantly associated with splenic involvement at presentation and with prior chemotherapy exposure, but did not worsen overall survival, they added.
“To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL,” they said.
The study comprised 222 patients with newly diagnosed NLPHL who were treated at Mayo Clinic between 1970 and 2011. Median age at diagnosis was 40 years, and two-thirds of patients were men. The median follow-up period was 16 years (Blood 2016;12:1960-6. doi: 10.1182/blood-2015-08-665505).
During follow up, 17 cases (7.6%) transformed to DLBCL, for a transformation rate of 0.74 cases for every 100 patient-years, the investigators said. Median time to transformation was 35 months (range, 6-268 months). Predictors of transformation included any prior chemotherapy exposure (P = .04) and splenic involvement (P = .03). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement and 21% when the spleen was involved, and were 87% if radiation therapy was used as a single modality compared with 77% in patients treated with prior chemotherapy or chemoradiation.
Five-year overall survival was 76% in patients with transformed disease, which was similar to overall survival among patients whose disease did not transform to DLBCL, the researchers noted.
Other studies of NLPHL have reported anywhere from a 2% to a 17% transformation rate, but those studies had smaller sample sizes, shorter follow-up periods, and less rigorous enrollment criteria and methods to confirm transformation, the investigators noted. “The finding of splenic involvement as a risk factor for transformation was reported by previous investigators. Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies,” they added. “In contrast to follicular lymphoma, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation.”
The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
Kenderian et al. report a lower rate of transformation (7.6%) to diffuse large B-cell lymphoma for patients with nodular lymphocyte–predominant Hodgkin lymphoma compared with other series and found that transformation did not have a negative impact on overall survival. Reassuringly, even if transformation occurs, it is generally at a low rate. Also, these patients do well with additional treatment and do not have worse overall survival. At the MD Anderson Cancer Center, we have used a regimen based on R-CHOP and have not seen transformations. But only through large cooperative clinical trials can we determine whether R-CHOP or other more novel regimens are actually superior to ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or rituximab (R)-ABVD for patients at high risk of transformation.
Dr. Michelle Fanale is at the University of Texas MD Anderson Cancer Center, Houston. She had no disclosures. These comments are from her editorial (Blood 2016;1927:1946-7 doi: 10.1182/blood-2016-03-699108).
Kenderian et al. report a lower rate of transformation (7.6%) to diffuse large B-cell lymphoma for patients with nodular lymphocyte–predominant Hodgkin lymphoma compared with other series and found that transformation did not have a negative impact on overall survival. Reassuringly, even if transformation occurs, it is generally at a low rate. Also, these patients do well with additional treatment and do not have worse overall survival. At the MD Anderson Cancer Center, we have used a regimen based on R-CHOP and have not seen transformations. But only through large cooperative clinical trials can we determine whether R-CHOP or other more novel regimens are actually superior to ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or rituximab (R)-ABVD for patients at high risk of transformation.
Dr. Michelle Fanale is at the University of Texas MD Anderson Cancer Center, Houston. She had no disclosures. These comments are from her editorial (Blood 2016;1927:1946-7 doi: 10.1182/blood-2016-03-699108).
Kenderian et al. report a lower rate of transformation (7.6%) to diffuse large B-cell lymphoma for patients with nodular lymphocyte–predominant Hodgkin lymphoma compared with other series and found that transformation did not have a negative impact on overall survival. Reassuringly, even if transformation occurs, it is generally at a low rate. Also, these patients do well with additional treatment and do not have worse overall survival. At the MD Anderson Cancer Center, we have used a regimen based on R-CHOP and have not seen transformations. But only through large cooperative clinical trials can we determine whether R-CHOP or other more novel regimens are actually superior to ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or rituximab (R)-ABVD for patients at high risk of transformation.
Dr. Michelle Fanale is at the University of Texas MD Anderson Cancer Center, Houston. She had no disclosures. These comments are from her editorial (Blood 2016;1927:1946-7 doi: 10.1182/blood-2016-03-699108).
Fewer than 8% of cases of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) transformed to diffuse large B-cell lymphoma (DLBCL), based on a large prospective single-center study with long-term follow-up.
This rate was lower than the risk of transformation reported for transformed follicular lymphoma or chronic lymphocytic leukemia, according to Dr. Saad Kenderian and his associates at the Mayo Clinic, Rochester, Minn. Transformation was significantly associated with splenic involvement at presentation and with prior chemotherapy exposure, but did not worsen overall survival, they added.
“To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL,” they said.
The study comprised 222 patients with newly diagnosed NLPHL who were treated at Mayo Clinic between 1970 and 2011. Median age at diagnosis was 40 years, and two-thirds of patients were men. The median follow-up period was 16 years (Blood 2016;12:1960-6. doi: 10.1182/blood-2015-08-665505).
During follow up, 17 cases (7.6%) transformed to DLBCL, for a transformation rate of 0.74 cases for every 100 patient-years, the investigators said. Median time to transformation was 35 months (range, 6-268 months). Predictors of transformation included any prior chemotherapy exposure (P = .04) and splenic involvement (P = .03). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement and 21% when the spleen was involved, and were 87% if radiation therapy was used as a single modality compared with 77% in patients treated with prior chemotherapy or chemoradiation.
Five-year overall survival was 76% in patients with transformed disease, which was similar to overall survival among patients whose disease did not transform to DLBCL, the researchers noted.
Other studies of NLPHL have reported anywhere from a 2% to a 17% transformation rate, but those studies had smaller sample sizes, shorter follow-up periods, and less rigorous enrollment criteria and methods to confirm transformation, the investigators noted. “The finding of splenic involvement as a risk factor for transformation was reported by previous investigators. Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies,” they added. “In contrast to follicular lymphoma, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation.”
The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
Fewer than 8% of cases of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) transformed to diffuse large B-cell lymphoma (DLBCL), based on a large prospective single-center study with long-term follow-up.
This rate was lower than the risk of transformation reported for transformed follicular lymphoma or chronic lymphocytic leukemia, according to Dr. Saad Kenderian and his associates at the Mayo Clinic, Rochester, Minn. Transformation was significantly associated with splenic involvement at presentation and with prior chemotherapy exposure, but did not worsen overall survival, they added.
“To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL,” they said.
The study comprised 222 patients with newly diagnosed NLPHL who were treated at Mayo Clinic between 1970 and 2011. Median age at diagnosis was 40 years, and two-thirds of patients were men. The median follow-up period was 16 years (Blood 2016;12:1960-6. doi: 10.1182/blood-2015-08-665505).
During follow up, 17 cases (7.6%) transformed to DLBCL, for a transformation rate of 0.74 cases for every 100 patient-years, the investigators said. Median time to transformation was 35 months (range, 6-268 months). Predictors of transformation included any prior chemotherapy exposure (P = .04) and splenic involvement (P = .03). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement and 21% when the spleen was involved, and were 87% if radiation therapy was used as a single modality compared with 77% in patients treated with prior chemotherapy or chemoradiation.
Five-year overall survival was 76% in patients with transformed disease, which was similar to overall survival among patients whose disease did not transform to DLBCL, the researchers noted.
Other studies of NLPHL have reported anywhere from a 2% to a 17% transformation rate, but those studies had smaller sample sizes, shorter follow-up periods, and less rigorous enrollment criteria and methods to confirm transformation, the investigators noted. “The finding of splenic involvement as a risk factor for transformation was reported by previous investigators. Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies,” they added. “In contrast to follicular lymphoma, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation.”
The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
FROM BLOOD
Key clinical point: The risk of transformation to diffuse large B-cell lymphoma is low in patients with nodular lymphocyte–predominant Hodgkin lymphoma.
Major finding: Only 7.6% of cases transformed over a median of 16 years of follow-up, and transformation did not worsen overall survival.
Data source: A prospective single-center study of 222 consecutive adults with NLPHL.
Disclosures: The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
Session on failing organs encompasses strategy to regenerate liver function
BOSTON – More than half a century after the first successful kidney transplant, various new strategies are being pursued to intervene when major organs, such as the heart or kidney, are failing, according to experts at the 2016 AGA Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology.
Strategies include regenerating tissue and employing artificial devices to perform the function of the failing organ. For tissue regeneration, some of the most promising work is being done in the treatment of the decompensated liver. The unmet need is enormous.
Most patients are not candidates for liver transplant, and those with severe disease have a 30%-50% risk of dying within 90 days of diagnosis, explained Dr. Bill Frank, vice president of medical sciences at Vital Therapies in San Diego. Given the limitations of liver transplantation, he and fellow researchers at Vital Therapies designed an extracorporeal system that continuously delivers a proprietary line of human C3A liver cells. This immortal cell line expresses a number of proteins relevant to liver function, including cytochrome P450 isoenzymes, coagulation factors, growth factors, anti-inflammatory proteins, and anti-apoptotic factors.
The system, called ELAD®, has already been evaluated in a phase III trial conducted in patients with alcohol-induced liver decompensation (AILD). In the study, 203 patients were randomized to ELAD® or usual care. Although those randomized to ELAD® did not have a significantly improved survival at the end of 3 months compared with controls, which was the primary study endpoint, there was a trend toward a survival in benefit in the prespecified subgroups of younger patients (median age 46.9 years) and patients with lower MELD scores (less than 28). In these, the risk of death was reduced by more than 40% (P = .77) and more than 35% (P = .167), respectively.
The outcomes in these subgroups were sufficient for the Food and Drug Administration to permit further clinical trials, and Dr. Frank suggested that this approach remains promising.
“ELAD® treatment has the potential to modulate or reduce the inflammatory response and apoptosis and promote hepatic regeneration,” reported Dr. Frank, noting that it might be used for the treatment of a variety of types of liver decompensation, not just AILD. This includes small-for-size liver, liver damage due to hepatitis C virus (HCV) infection, and even septic liver disease.
A new phase III trial in patients with AILD is anticipated in the first half of 2016, and top-line data are expected by mid-2018, he added. Bringing those interested in GI technology up to speed on a key problem in solid organ transplantation, John J. Sninsky, Ph.D., chief scientific officer at CareDX, Inc. in Brisbane, Calif., reported progress in minimizing key factors involved in graft rejection. This continues to be one of the biggest hurdles in ensuring long-term survival.
“Suboptimal immunosuppressive dosing and the threat of allograft rejection must be balanced with excessive dosing and increased risks of infections and cancer,” Dr. Sninsky said. He described an array of approaches to reduce protein expression or other signals from donor grafts that prompt an immunologic response, but he focused on current initiatives to identify signals of rejection before they produce adverse clinical consequences so immunosuppressive therapy can be adjusted in time.
Of these strategies, cell-free DNA (cfDNA) is intriguing because it is released from dying cells in both the allograft and recipient, and next-generation sequencing can distinguish their respective genomes, Dr. Sninsky said. In a recent longitudinal study of heart and kidney transplant recipients, levels of donor cfDNA rose significantly before biopsy-confirmed transplant rejection. Moreover, donor cfDNA levels dropped after adjustment of immunosuppressive therapy.
“The cfDNA compartment also harbors information about specific infectious diseases and somatic mutations associated with cancer development,” Dr. Sninsky said. Such technology could one day help fulfill a “significant unmet need” for tools to help monitor how patients are responding to transplant, he added.
In the acutely failing kidney, transplantation may not be feasible, but an alternative strategy involving a bioartificial kidney is showing promise in clinical trials, according to Dr. H. David Humes, professor of nephrology, University of Michigan, Ann Arbor. He predicted that such a device might be commercially available sometime in 2017.
Describing the bioartificial kidney as a “renal tubule assist device,” Dr. Humes, who was instrumental in its development, said that living cells are employed to perform their normal filtration activities but also to regulate immune function. Dr. Humes said this extracorporeal device can “buy time” when patients are recovering from an acute insult. In particular, this device is promising for those with renal failure in the intensive care unit, where the mortality associated with this complication is approximately 50%.
Although the current work is focused on acute renal injury, Dr. Humes acknowledged that the ongoing work might eventually lead to an implantable device employing the same principles for patients in end-stage renal disease. This development, however, may be years away.
BOSTON – More than half a century after the first successful kidney transplant, various new strategies are being pursued to intervene when major organs, such as the heart or kidney, are failing, according to experts at the 2016 AGA Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology.
Strategies include regenerating tissue and employing artificial devices to perform the function of the failing organ. For tissue regeneration, some of the most promising work is being done in the treatment of the decompensated liver. The unmet need is enormous.
Most patients are not candidates for liver transplant, and those with severe disease have a 30%-50% risk of dying within 90 days of diagnosis, explained Dr. Bill Frank, vice president of medical sciences at Vital Therapies in San Diego. Given the limitations of liver transplantation, he and fellow researchers at Vital Therapies designed an extracorporeal system that continuously delivers a proprietary line of human C3A liver cells. This immortal cell line expresses a number of proteins relevant to liver function, including cytochrome P450 isoenzymes, coagulation factors, growth factors, anti-inflammatory proteins, and anti-apoptotic factors.
The system, called ELAD®, has already been evaluated in a phase III trial conducted in patients with alcohol-induced liver decompensation (AILD). In the study, 203 patients were randomized to ELAD® or usual care. Although those randomized to ELAD® did not have a significantly improved survival at the end of 3 months compared with controls, which was the primary study endpoint, there was a trend toward a survival in benefit in the prespecified subgroups of younger patients (median age 46.9 years) and patients with lower MELD scores (less than 28). In these, the risk of death was reduced by more than 40% (P = .77) and more than 35% (P = .167), respectively.
The outcomes in these subgroups were sufficient for the Food and Drug Administration to permit further clinical trials, and Dr. Frank suggested that this approach remains promising.
“ELAD® treatment has the potential to modulate or reduce the inflammatory response and apoptosis and promote hepatic regeneration,” reported Dr. Frank, noting that it might be used for the treatment of a variety of types of liver decompensation, not just AILD. This includes small-for-size liver, liver damage due to hepatitis C virus (HCV) infection, and even septic liver disease.
A new phase III trial in patients with AILD is anticipated in the first half of 2016, and top-line data are expected by mid-2018, he added. Bringing those interested in GI technology up to speed on a key problem in solid organ transplantation, John J. Sninsky, Ph.D., chief scientific officer at CareDX, Inc. in Brisbane, Calif., reported progress in minimizing key factors involved in graft rejection. This continues to be one of the biggest hurdles in ensuring long-term survival.
“Suboptimal immunosuppressive dosing and the threat of allograft rejection must be balanced with excessive dosing and increased risks of infections and cancer,” Dr. Sninsky said. He described an array of approaches to reduce protein expression or other signals from donor grafts that prompt an immunologic response, but he focused on current initiatives to identify signals of rejection before they produce adverse clinical consequences so immunosuppressive therapy can be adjusted in time.
Of these strategies, cell-free DNA (cfDNA) is intriguing because it is released from dying cells in both the allograft and recipient, and next-generation sequencing can distinguish their respective genomes, Dr. Sninsky said. In a recent longitudinal study of heart and kidney transplant recipients, levels of donor cfDNA rose significantly before biopsy-confirmed transplant rejection. Moreover, donor cfDNA levels dropped after adjustment of immunosuppressive therapy.
“The cfDNA compartment also harbors information about specific infectious diseases and somatic mutations associated with cancer development,” Dr. Sninsky said. Such technology could one day help fulfill a “significant unmet need” for tools to help monitor how patients are responding to transplant, he added.
In the acutely failing kidney, transplantation may not be feasible, but an alternative strategy involving a bioartificial kidney is showing promise in clinical trials, according to Dr. H. David Humes, professor of nephrology, University of Michigan, Ann Arbor. He predicted that such a device might be commercially available sometime in 2017.
Describing the bioartificial kidney as a “renal tubule assist device,” Dr. Humes, who was instrumental in its development, said that living cells are employed to perform their normal filtration activities but also to regulate immune function. Dr. Humes said this extracorporeal device can “buy time” when patients are recovering from an acute insult. In particular, this device is promising for those with renal failure in the intensive care unit, where the mortality associated with this complication is approximately 50%.
Although the current work is focused on acute renal injury, Dr. Humes acknowledged that the ongoing work might eventually lead to an implantable device employing the same principles for patients in end-stage renal disease. This development, however, may be years away.
BOSTON – More than half a century after the first successful kidney transplant, various new strategies are being pursued to intervene when major organs, such as the heart or kidney, are failing, according to experts at the 2016 AGA Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology.
Strategies include regenerating tissue and employing artificial devices to perform the function of the failing organ. For tissue regeneration, some of the most promising work is being done in the treatment of the decompensated liver. The unmet need is enormous.
Most patients are not candidates for liver transplant, and those with severe disease have a 30%-50% risk of dying within 90 days of diagnosis, explained Dr. Bill Frank, vice president of medical sciences at Vital Therapies in San Diego. Given the limitations of liver transplantation, he and fellow researchers at Vital Therapies designed an extracorporeal system that continuously delivers a proprietary line of human C3A liver cells. This immortal cell line expresses a number of proteins relevant to liver function, including cytochrome P450 isoenzymes, coagulation factors, growth factors, anti-inflammatory proteins, and anti-apoptotic factors.
The system, called ELAD®, has already been evaluated in a phase III trial conducted in patients with alcohol-induced liver decompensation (AILD). In the study, 203 patients were randomized to ELAD® or usual care. Although those randomized to ELAD® did not have a significantly improved survival at the end of 3 months compared with controls, which was the primary study endpoint, there was a trend toward a survival in benefit in the prespecified subgroups of younger patients (median age 46.9 years) and patients with lower MELD scores (less than 28). In these, the risk of death was reduced by more than 40% (P = .77) and more than 35% (P = .167), respectively.
The outcomes in these subgroups were sufficient for the Food and Drug Administration to permit further clinical trials, and Dr. Frank suggested that this approach remains promising.
“ELAD® treatment has the potential to modulate or reduce the inflammatory response and apoptosis and promote hepatic regeneration,” reported Dr. Frank, noting that it might be used for the treatment of a variety of types of liver decompensation, not just AILD. This includes small-for-size liver, liver damage due to hepatitis C virus (HCV) infection, and even septic liver disease.
A new phase III trial in patients with AILD is anticipated in the first half of 2016, and top-line data are expected by mid-2018, he added. Bringing those interested in GI technology up to speed on a key problem in solid organ transplantation, John J. Sninsky, Ph.D., chief scientific officer at CareDX, Inc. in Brisbane, Calif., reported progress in minimizing key factors involved in graft rejection. This continues to be one of the biggest hurdles in ensuring long-term survival.
“Suboptimal immunosuppressive dosing and the threat of allograft rejection must be balanced with excessive dosing and increased risks of infections and cancer,” Dr. Sninsky said. He described an array of approaches to reduce protein expression or other signals from donor grafts that prompt an immunologic response, but he focused on current initiatives to identify signals of rejection before they produce adverse clinical consequences so immunosuppressive therapy can be adjusted in time.
Of these strategies, cell-free DNA (cfDNA) is intriguing because it is released from dying cells in both the allograft and recipient, and next-generation sequencing can distinguish their respective genomes, Dr. Sninsky said. In a recent longitudinal study of heart and kidney transplant recipients, levels of donor cfDNA rose significantly before biopsy-confirmed transplant rejection. Moreover, donor cfDNA levels dropped after adjustment of immunosuppressive therapy.
“The cfDNA compartment also harbors information about specific infectious diseases and somatic mutations associated with cancer development,” Dr. Sninsky said. Such technology could one day help fulfill a “significant unmet need” for tools to help monitor how patients are responding to transplant, he added.
In the acutely failing kidney, transplantation may not be feasible, but an alternative strategy involving a bioartificial kidney is showing promise in clinical trials, according to Dr. H. David Humes, professor of nephrology, University of Michigan, Ann Arbor. He predicted that such a device might be commercially available sometime in 2017.
Describing the bioartificial kidney as a “renal tubule assist device,” Dr. Humes, who was instrumental in its development, said that living cells are employed to perform their normal filtration activities but also to regulate immune function. Dr. Humes said this extracorporeal device can “buy time” when patients are recovering from an acute insult. In particular, this device is promising for those with renal failure in the intensive care unit, where the mortality associated with this complication is approximately 50%.
Although the current work is focused on acute renal injury, Dr. Humes acknowledged that the ongoing work might eventually lead to an implantable device employing the same principles for patients in end-stage renal disease. This development, however, may be years away.
AT THE 2016 AGA TECH SUMMIT