User login
Study: Pay one malpractice claim, expect second
If you pay a medical malpractice claim once, chances are you’ll pay a second claim in the future, according to a study published Jan. 28 in the New England Journal of Medicine (doi: 10.1056/NEJMsa1506137).
David M. Studdert Sc.D., of Stanford (Calif.) University and his colleagues analyzed 66,426 malpractice claims from the National Practitioner Data Bank that were paid against 54,099 physicians between 2005 through 2014. Investigators calculated the cumulative distribution of paid claims in two physician populations: U.S. doctors with one or more paid claims and all active U.S. physicians.
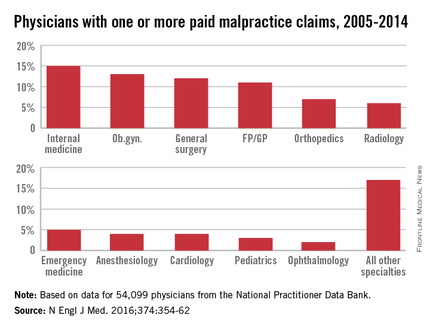
Of all paid claims, 82% involved male physicians. The specialists with the most paid claims were internists (15%), ob.gyns. (13%), general surgeons (12%), and family physicians (11%). Only 3% of the lawsuits were paid through trial verdicts. The remaining claims were paid by out-of-court settlements. The mean payment amount for claims was $371,054, while the median was $204,750.
When using all 915,564 active U.S. physicians as a denominator, only 6% of physicians had a paid claim against them and about 1% had at least two paid claims against them. When looking at physicians with at least one paid claim, 84% had only one paid claim over the study period, accounting for 68% of all paid claims. However, 16% (8,846 physicians) had at least two paid claims, accounting for 32% of all claims. A total of 4% of doctors (2,160 physicians) had at least three paid claims and accounted for 12% of all claims, and 1% (722 physicians) had at least four paid claims and accounted for 5% of all claims.
Specialties had a marked impact on the risk of future paid claims. Compared with internists, the risk of recurrence was double among neurosurgeons, orthopedic surgeons, general surgeons, plastic surgeons, and ob.gyns. Psychiatrists and pediatricians had the lowest risks of recurrence.
Mr. Studdert said that the investigators were surprised to learn the degree to which past paid claims predicted future claims.
“I think if you ask most people who work in the liability insurance industry, they would tell you that’s probably true,” he said in an interview. “But I don’ t think we’ve seen – at least not on this scale – research finding just how sharply claim risk rises with malpractice history. It does suggest for those multiclaim physicians, that there is something going on that is predisposing them to be the subject of malpractice litigation.”
He noted the analysis suggests that it is feasible to predict which physicians are at higher risk for future claims. The information could then be used to develop interventions to address malpractice claims.
“These multiclaim physicians do appear to have some distinctive characteristics, and that gives us some optimism that it might be possible to predict who is going to [become] a multiclaim physician,” Mr. Studdert said. “More work is needed to make sure that’s technically feasible, but if it is, that opens up a lot of possibilities for liability insurers, hospitals ,medical boards, and other regulators to use this information proactively rather than just reacting to events after they happen.”
On Twitter @legal_med
If you pay a medical malpractice claim once, chances are you’ll pay a second claim in the future, according to a study published Jan. 28 in the New England Journal of Medicine (doi: 10.1056/NEJMsa1506137).
David M. Studdert Sc.D., of Stanford (Calif.) University and his colleagues analyzed 66,426 malpractice claims from the National Practitioner Data Bank that were paid against 54,099 physicians between 2005 through 2014. Investigators calculated the cumulative distribution of paid claims in two physician populations: U.S. doctors with one or more paid claims and all active U.S. physicians.
Of all paid claims, 82% involved male physicians. The specialists with the most paid claims were internists (15%), ob.gyns. (13%), general surgeons (12%), and family physicians (11%). Only 3% of the lawsuits were paid through trial verdicts. The remaining claims were paid by out-of-court settlements. The mean payment amount for claims was $371,054, while the median was $204,750.
When using all 915,564 active U.S. physicians as a denominator, only 6% of physicians had a paid claim against them and about 1% had at least two paid claims against them. When looking at physicians with at least one paid claim, 84% had only one paid claim over the study period, accounting for 68% of all paid claims. However, 16% (8,846 physicians) had at least two paid claims, accounting for 32% of all claims. A total of 4% of doctors (2,160 physicians) had at least three paid claims and accounted for 12% of all claims, and 1% (722 physicians) had at least four paid claims and accounted for 5% of all claims.
Specialties had a marked impact on the risk of future paid claims. Compared with internists, the risk of recurrence was double among neurosurgeons, orthopedic surgeons, general surgeons, plastic surgeons, and ob.gyns. Psychiatrists and pediatricians had the lowest risks of recurrence.

Mr. Studdert said that the investigators were surprised to learn the degree to which past paid claims predicted future claims.
“I think if you ask most people who work in the liability insurance industry, they would tell you that’s probably true,” he said in an interview. “But I don’ t think we’ve seen – at least not on this scale – research finding just how sharply claim risk rises with malpractice history. It does suggest for those multiclaim physicians, that there is something going on that is predisposing them to be the subject of malpractice litigation.”
He noted the analysis suggests that it is feasible to predict which physicians are at higher risk for future claims. The information could then be used to develop interventions to address malpractice claims.
“These multiclaim physicians do appear to have some distinctive characteristics, and that gives us some optimism that it might be possible to predict who is going to [become] a multiclaim physician,” Mr. Studdert said. “More work is needed to make sure that’s technically feasible, but if it is, that opens up a lot of possibilities for liability insurers, hospitals ,medical boards, and other regulators to use this information proactively rather than just reacting to events after they happen.”
On Twitter @legal_med
If you pay a medical malpractice claim once, chances are you’ll pay a second claim in the future, according to a study published Jan. 28 in the New England Journal of Medicine (doi: 10.1056/NEJMsa1506137).
David M. Studdert Sc.D., of Stanford (Calif.) University and his colleagues analyzed 66,426 malpractice claims from the National Practitioner Data Bank that were paid against 54,099 physicians between 2005 through 2014. Investigators calculated the cumulative distribution of paid claims in two physician populations: U.S. doctors with one or more paid claims and all active U.S. physicians.
Of all paid claims, 82% involved male physicians. The specialists with the most paid claims were internists (15%), ob.gyns. (13%), general surgeons (12%), and family physicians (11%). Only 3% of the lawsuits were paid through trial verdicts. The remaining claims were paid by out-of-court settlements. The mean payment amount for claims was $371,054, while the median was $204,750.
When using all 915,564 active U.S. physicians as a denominator, only 6% of physicians had a paid claim against them and about 1% had at least two paid claims against them. When looking at physicians with at least one paid claim, 84% had only one paid claim over the study period, accounting for 68% of all paid claims. However, 16% (8,846 physicians) had at least two paid claims, accounting for 32% of all claims. A total of 4% of doctors (2,160 physicians) had at least three paid claims and accounted for 12% of all claims, and 1% (722 physicians) had at least four paid claims and accounted for 5% of all claims.
Specialties had a marked impact on the risk of future paid claims. Compared with internists, the risk of recurrence was double among neurosurgeons, orthopedic surgeons, general surgeons, plastic surgeons, and ob.gyns. Psychiatrists and pediatricians had the lowest risks of recurrence.

Mr. Studdert said that the investigators were surprised to learn the degree to which past paid claims predicted future claims.
“I think if you ask most people who work in the liability insurance industry, they would tell you that’s probably true,” he said in an interview. “But I don’ t think we’ve seen – at least not on this scale – research finding just how sharply claim risk rises with malpractice history. It does suggest for those multiclaim physicians, that there is something going on that is predisposing them to be the subject of malpractice litigation.”
He noted the analysis suggests that it is feasible to predict which physicians are at higher risk for future claims. The information could then be used to develop interventions to address malpractice claims.
“These multiclaim physicians do appear to have some distinctive characteristics, and that gives us some optimism that it might be possible to predict who is going to [become] a multiclaim physician,” Mr. Studdert said. “More work is needed to make sure that’s technically feasible, but if it is, that opens up a lot of possibilities for liability insurers, hospitals ,medical boards, and other regulators to use this information proactively rather than just reacting to events after they happen.”
On Twitter @legal_med
Key clinical point: Doctors with one paid medical malpractice claim have a greater chance of future paid claims.
Major finding: Of 54,099 physicians with at least one paid claim,16% had at least two paid claims. Physicians with two paid claims had almost twice the risk of having another paid claim compared with doctors who had only one paid claim against them.
Data source: Analysis of the National Practitioner Data Bank.
Disclosures: David Studdert, Sc.D., and Michelle Mello, Ph.D., reported grant support from the Risk Authority outside of this study.
Patient records requests: New regs clarify physician responsibilities
The age of the information-empowered patient is upon us. Not only do patients bring the results of their Internet research when they come to the office, they also want to take a record of the clinical encounter with them when they leave.
New HIPAA guidance issued in January by the Health & Human Services Department’s Office of Civil Rights (OCR) aims to help clinicians know how to respond and with what information; it also addresses when patients can be charged for the information.
In the past, physicians and other providers had to “wing it” when it came to unclear rules about patient’s data requests, said Dianne J. Bourque, a Boston health law and HIPAA compliance attorney. “Prior to this, there may not have been readily available guidance that would drill down” to address specific concerns.
When it comes to systems security, physicians and other health providers do not have to put their health IT systems at risk in an effort to meet a request for patient records. For example, Mrs. Smith requests that her protected health information (PHI) be copied onto a thumb drive that she has provided.
In most cases, a covered entity must provide data access in the manner requested by the patient. But the updated guidance states that health providers are not expected to tolerate “unacceptable levels of risk to the security of the PHI on its systems” in responding to requests.
Unlike system security, patient security does not trump patient access. If Mr. Black requests that his records by emailed to him, but a connection cannot be made secure, physicians are still required to send the data.
While OCR requires HIPAA-covered entities to implement reasonable safeguards to protect PHI while in transit, patients have a right to receive a copy of records by unencrypted email if they so wish.
To comply with the new rules, be sure to warn patients of the risks, and confirm that they still want their PHI by unencrypted email. If confirmed, you must comply with the request. This clarification relieves doctors of potential breach notification and liability if the data is intercepted in transit.
The guidance also clarifies how to deliver patients’ data. If PHI is maintained electronically, physicians and other HIPAA-covered entities must be able provide it to patients electronically.
“Because you hold it electronically, you can’t say, ‘Forget it, you have to have paper,’” Ms. Bourque said. “You lose that option when you keep [data] electronically. Maybe you have to go buy a scanner and scan [the document] and email it, but you can’t charge [patients] for the scanner.”
The new guidance also allows patients to get results directly from a clinical laboratory; however, labs are not required to interpret test results. Rather, patients are encouraged to reach out to their physician for such insights.
Overall, the access guidelines appear reasonable and hopefully will relieve hassles for patients in obtaining their health information, said Dr. Sam Slishman, an emergency physician for Sierra Vista Hospital in San Luis Obispo, Calif., and co-founder of Pre-R, a service that provides in-home visits. Dr. Slishman does not foresee the guidance having much impact on his practices.
“It’s crazy to me that patients have to struggle to retrieve their records at all,” he said in an interview. “I routinely send my patients home with at least their lab tests and copies of their radiology reports so they have something to bring to their [primary care physicians]. If they want more, I hand it to them.”
Dr. Rocky D. Bilhartz, an interventional cardiologist in private practice in College Station, Tex., said that he has concerns about the guidelines. Specifically, that doctors may charge a fee to cover the cost of copying records, but that they cannot charge for the cost of searching and retrieving data, said Dr. Bilhartz, who is founder of ECGsource, an online cardiovascular medical education resource.
“Record requests can take significant time for staff to filter through and gather,” he said in an interview. “That time should be reimbursable ... If updated provisions prohibit charging for time spent compiling records, it seems those provisions are a bit out of touch with understanding what those of us on the ground floor must do when a request is received.”
But Dr. Bilhartz acknowledged that he would be unlikely to charge patients for “reasonable” data requests.
“I’m in private practice ... and because of that, I have more market-driven accountability to all my patients,” he said. “Why would I nickel and dime people who I would want to be satisfied patients? For reasonable requests, I would just provide records for free.”
Ms. Bourque notes that while the clarifications are primarily positive for health providers, they present a double-edged sword.
“The good side is that, it has all this detail and it’s really helpful and makes things easier when you have a tricky access request and don’t know what to do,” she said. “The flip side is that once it’s out there, they expect you to read it and pay attention. You start running out of excuses for why you didn’t comply with the access right or why you got it wrong.”
On Twitter @legal_med
The age of the information-empowered patient is upon us. Not only do patients bring the results of their Internet research when they come to the office, they also want to take a record of the clinical encounter with them when they leave.
New HIPAA guidance issued in January by the Health & Human Services Department’s Office of Civil Rights (OCR) aims to help clinicians know how to respond and with what information; it also addresses when patients can be charged for the information.
In the past, physicians and other providers had to “wing it” when it came to unclear rules about patient’s data requests, said Dianne J. Bourque, a Boston health law and HIPAA compliance attorney. “Prior to this, there may not have been readily available guidance that would drill down” to address specific concerns.
When it comes to systems security, physicians and other health providers do not have to put their health IT systems at risk in an effort to meet a request for patient records. For example, Mrs. Smith requests that her protected health information (PHI) be copied onto a thumb drive that she has provided.
In most cases, a covered entity must provide data access in the manner requested by the patient. But the updated guidance states that health providers are not expected to tolerate “unacceptable levels of risk to the security of the PHI on its systems” in responding to requests.
Unlike system security, patient security does not trump patient access. If Mr. Black requests that his records by emailed to him, but a connection cannot be made secure, physicians are still required to send the data.
While OCR requires HIPAA-covered entities to implement reasonable safeguards to protect PHI while in transit, patients have a right to receive a copy of records by unencrypted email if they so wish.
To comply with the new rules, be sure to warn patients of the risks, and confirm that they still want their PHI by unencrypted email. If confirmed, you must comply with the request. This clarification relieves doctors of potential breach notification and liability if the data is intercepted in transit.
The guidance also clarifies how to deliver patients’ data. If PHI is maintained electronically, physicians and other HIPAA-covered entities must be able provide it to patients electronically.
“Because you hold it electronically, you can’t say, ‘Forget it, you have to have paper,’” Ms. Bourque said. “You lose that option when you keep [data] electronically. Maybe you have to go buy a scanner and scan [the document] and email it, but you can’t charge [patients] for the scanner.”
The new guidance also allows patients to get results directly from a clinical laboratory; however, labs are not required to interpret test results. Rather, patients are encouraged to reach out to their physician for such insights.
Overall, the access guidelines appear reasonable and hopefully will relieve hassles for patients in obtaining their health information, said Dr. Sam Slishman, an emergency physician for Sierra Vista Hospital in San Luis Obispo, Calif., and co-founder of Pre-R, a service that provides in-home visits. Dr. Slishman does not foresee the guidance having much impact on his practices.
“It’s crazy to me that patients have to struggle to retrieve their records at all,” he said in an interview. “I routinely send my patients home with at least their lab tests and copies of their radiology reports so they have something to bring to their [primary care physicians]. If they want more, I hand it to them.”
Dr. Rocky D. Bilhartz, an interventional cardiologist in private practice in College Station, Tex., said that he has concerns about the guidelines. Specifically, that doctors may charge a fee to cover the cost of copying records, but that they cannot charge for the cost of searching and retrieving data, said Dr. Bilhartz, who is founder of ECGsource, an online cardiovascular medical education resource.
“Record requests can take significant time for staff to filter through and gather,” he said in an interview. “That time should be reimbursable ... If updated provisions prohibit charging for time spent compiling records, it seems those provisions are a bit out of touch with understanding what those of us on the ground floor must do when a request is received.”
But Dr. Bilhartz acknowledged that he would be unlikely to charge patients for “reasonable” data requests.
“I’m in private practice ... and because of that, I have more market-driven accountability to all my patients,” he said. “Why would I nickel and dime people who I would want to be satisfied patients? For reasonable requests, I would just provide records for free.”
Ms. Bourque notes that while the clarifications are primarily positive for health providers, they present a double-edged sword.
“The good side is that, it has all this detail and it’s really helpful and makes things easier when you have a tricky access request and don’t know what to do,” she said. “The flip side is that once it’s out there, they expect you to read it and pay attention. You start running out of excuses for why you didn’t comply with the access right or why you got it wrong.”
On Twitter @legal_med
The age of the information-empowered patient is upon us. Not only do patients bring the results of their Internet research when they come to the office, they also want to take a record of the clinical encounter with them when they leave.
New HIPAA guidance issued in January by the Health & Human Services Department’s Office of Civil Rights (OCR) aims to help clinicians know how to respond and with what information; it also addresses when patients can be charged for the information.
In the past, physicians and other providers had to “wing it” when it came to unclear rules about patient’s data requests, said Dianne J. Bourque, a Boston health law and HIPAA compliance attorney. “Prior to this, there may not have been readily available guidance that would drill down” to address specific concerns.
When it comes to systems security, physicians and other health providers do not have to put their health IT systems at risk in an effort to meet a request for patient records. For example, Mrs. Smith requests that her protected health information (PHI) be copied onto a thumb drive that she has provided.
In most cases, a covered entity must provide data access in the manner requested by the patient. But the updated guidance states that health providers are not expected to tolerate “unacceptable levels of risk to the security of the PHI on its systems” in responding to requests.
Unlike system security, patient security does not trump patient access. If Mr. Black requests that his records by emailed to him, but a connection cannot be made secure, physicians are still required to send the data.
While OCR requires HIPAA-covered entities to implement reasonable safeguards to protect PHI while in transit, patients have a right to receive a copy of records by unencrypted email if they so wish.
To comply with the new rules, be sure to warn patients of the risks, and confirm that they still want their PHI by unencrypted email. If confirmed, you must comply with the request. This clarification relieves doctors of potential breach notification and liability if the data is intercepted in transit.
The guidance also clarifies how to deliver patients’ data. If PHI is maintained electronically, physicians and other HIPAA-covered entities must be able provide it to patients electronically.
“Because you hold it electronically, you can’t say, ‘Forget it, you have to have paper,’” Ms. Bourque said. “You lose that option when you keep [data] electronically. Maybe you have to go buy a scanner and scan [the document] and email it, but you can’t charge [patients] for the scanner.”
The new guidance also allows patients to get results directly from a clinical laboratory; however, labs are not required to interpret test results. Rather, patients are encouraged to reach out to their physician for such insights.
Overall, the access guidelines appear reasonable and hopefully will relieve hassles for patients in obtaining their health information, said Dr. Sam Slishman, an emergency physician for Sierra Vista Hospital in San Luis Obispo, Calif., and co-founder of Pre-R, a service that provides in-home visits. Dr. Slishman does not foresee the guidance having much impact on his practices.
“It’s crazy to me that patients have to struggle to retrieve their records at all,” he said in an interview. “I routinely send my patients home with at least their lab tests and copies of their radiology reports so they have something to bring to their [primary care physicians]. If they want more, I hand it to them.”
Dr. Rocky D. Bilhartz, an interventional cardiologist in private practice in College Station, Tex., said that he has concerns about the guidelines. Specifically, that doctors may charge a fee to cover the cost of copying records, but that they cannot charge for the cost of searching and retrieving data, said Dr. Bilhartz, who is founder of ECGsource, an online cardiovascular medical education resource.
“Record requests can take significant time for staff to filter through and gather,” he said in an interview. “That time should be reimbursable ... If updated provisions prohibit charging for time spent compiling records, it seems those provisions are a bit out of touch with understanding what those of us on the ground floor must do when a request is received.”
But Dr. Bilhartz acknowledged that he would be unlikely to charge patients for “reasonable” data requests.
“I’m in private practice ... and because of that, I have more market-driven accountability to all my patients,” he said. “Why would I nickel and dime people who I would want to be satisfied patients? For reasonable requests, I would just provide records for free.”
Ms. Bourque notes that while the clarifications are primarily positive for health providers, they present a double-edged sword.
“The good side is that, it has all this detail and it’s really helpful and makes things easier when you have a tricky access request and don’t know what to do,” she said. “The flip side is that once it’s out there, they expect you to read it and pay attention. You start running out of excuses for why you didn’t comply with the access right or why you got it wrong.”
On Twitter @legal_med
DME: New prior authorization requirements could up the ‘hassle factor’
Starting next month, Medicare won’t pay for certain durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) without prior authorization. The regulation could mean headaches for doctors in the form of extra paperwork and frustrated patients.
“Our initial reaction is one of concern,” said Dr. Wanda Filer, president of the American Academy of Family Physicians. “This new rule could prompt DMEPOS suppliers to request additional documentation from ordering/prescribing physicians to support the suppliers’ requests for prior authorization. Such requests could lead to administrative hassles for physicians.”
Under the new requirement – effective Feb. 29 – prior CMS authorization will be required for certain DMEPOS items that are frequently subject to “unnecessary utilization,” according to a Dec. 29 announcement. The prior authorization process requires the same information currently necessary for Medicare payment, but will happen earlier in the process.
The early evaluation will assure that all relevant coverage, coding, and clinical documentation is provided before the equipment is furnished to the patient and before the claim is submitted for payment. CMS hopes the prior review will reduce improper payments for DMEPOS.
Not every piece of durable medical equipment will be subject to prior authorization; instead, CMS will prescreen items from its master list of 135 costly and overprescribed items, especially those with an average purchase price of more than $1,000 or rental fee of $100. The complete master list was published within CMS’ final rule in the Federal Register. CMS will publish a “required prior authorization list” 60 days before implementation.
The final rule primarily impacts vendors paid by Medicare to supply durable medical equipment to patients, said Dr. Yul D. Ejnes, an internist in private practice and a past chair of the American College of Physicians Board of Regents. The prescriber is responsible for meeting all Medicare coverage, coding, and payment rules. However, doctors will likely be indirectly affected because of the clinical documentation required for CMS approval, Dr. Ejnes said.
“The documentation requirement could be burdensome depending on how DME vendors interpret the regulations, and then the whole issue of increasing the amount of chart documentation that’s going out to various places raises some concern,” Dr. Ejnes said in an interview. “Even though it may all be covered under HIPAA, [there’s] the issue of content in the notes that’s irrelevant to the DME request and how we handle that. Do we need to start redacting notes to meet the documentation requirements for prior authorization?”
The new requirements also may mean that patients wait longer for needed equipment, Dr. Ejnes added. “Oftentimes, there’s the finger-pointing exercise that occurs when things don’t happen quickly enough and patients are unhappy. “It just adds to the temperature of the environment, which is already pretty high because of patients unhappy about increasing copays and deductibles and everything else.”
To prepare for the rule, physicians should identify the DMEPOS items they order or prescribe most often and engage with suppliers early to ensure they understand what kind of documentation will be needed, Dr. Filer recommended.
“If the physician understands upfront what Medicare requires and is able to provide it to the DMEPOS supplier[s] at the time the DMEPOS items are ordered/prescribed, that may save time on the back-end preventing or otherwise dealing with additional documentation requests from the DMEPOS supplier[s] in support of prior authorization requests,” she said.
Be extremely thoughtful about prescribing durable medical equipment and make sure that equipment orders are placed that meet the patient’s needs rather than their desires, Dr. Ejnes recommended. In addition, it’s helpful for practices to consider work flow and how to efficiently respond to document requests. In some cases, office staff can locate records or precomplete basic information on forms, he said.
“Figure out a way to respond to these in terms of who in the office will take the first pass if there’s a form to fill out,” he said. “Be aware that there may be some delays in getting patients what they need. Some of these items are not emergency items. Educate patients to the fact that there’s a couple steps between writing the prescription and them picking up the item.”
On Twitter @legal_med
Starting next month, Medicare won’t pay for certain durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) without prior authorization. The regulation could mean headaches for doctors in the form of extra paperwork and frustrated patients.
“Our initial reaction is one of concern,” said Dr. Wanda Filer, president of the American Academy of Family Physicians. “This new rule could prompt DMEPOS suppliers to request additional documentation from ordering/prescribing physicians to support the suppliers’ requests for prior authorization. Such requests could lead to administrative hassles for physicians.”
Under the new requirement – effective Feb. 29 – prior CMS authorization will be required for certain DMEPOS items that are frequently subject to “unnecessary utilization,” according to a Dec. 29 announcement. The prior authorization process requires the same information currently necessary for Medicare payment, but will happen earlier in the process.
The early evaluation will assure that all relevant coverage, coding, and clinical documentation is provided before the equipment is furnished to the patient and before the claim is submitted for payment. CMS hopes the prior review will reduce improper payments for DMEPOS.
Not every piece of durable medical equipment will be subject to prior authorization; instead, CMS will prescreen items from its master list of 135 costly and overprescribed items, especially those with an average purchase price of more than $1,000 or rental fee of $100. The complete master list was published within CMS’ final rule in the Federal Register. CMS will publish a “required prior authorization list” 60 days before implementation.
The final rule primarily impacts vendors paid by Medicare to supply durable medical equipment to patients, said Dr. Yul D. Ejnes, an internist in private practice and a past chair of the American College of Physicians Board of Regents. The prescriber is responsible for meeting all Medicare coverage, coding, and payment rules. However, doctors will likely be indirectly affected because of the clinical documentation required for CMS approval, Dr. Ejnes said.
“The documentation requirement could be burdensome depending on how DME vendors interpret the regulations, and then the whole issue of increasing the amount of chart documentation that’s going out to various places raises some concern,” Dr. Ejnes said in an interview. “Even though it may all be covered under HIPAA, [there’s] the issue of content in the notes that’s irrelevant to the DME request and how we handle that. Do we need to start redacting notes to meet the documentation requirements for prior authorization?”
The new requirements also may mean that patients wait longer for needed equipment, Dr. Ejnes added. “Oftentimes, there’s the finger-pointing exercise that occurs when things don’t happen quickly enough and patients are unhappy. “It just adds to the temperature of the environment, which is already pretty high because of patients unhappy about increasing copays and deductibles and everything else.”
To prepare for the rule, physicians should identify the DMEPOS items they order or prescribe most often and engage with suppliers early to ensure they understand what kind of documentation will be needed, Dr. Filer recommended.
“If the physician understands upfront what Medicare requires and is able to provide it to the DMEPOS supplier[s] at the time the DMEPOS items are ordered/prescribed, that may save time on the back-end preventing or otherwise dealing with additional documentation requests from the DMEPOS supplier[s] in support of prior authorization requests,” she said.
Be extremely thoughtful about prescribing durable medical equipment and make sure that equipment orders are placed that meet the patient’s needs rather than their desires, Dr. Ejnes recommended. In addition, it’s helpful for practices to consider work flow and how to efficiently respond to document requests. In some cases, office staff can locate records or precomplete basic information on forms, he said.
“Figure out a way to respond to these in terms of who in the office will take the first pass if there’s a form to fill out,” he said. “Be aware that there may be some delays in getting patients what they need. Some of these items are not emergency items. Educate patients to the fact that there’s a couple steps between writing the prescription and them picking up the item.”
On Twitter @legal_med
Starting next month, Medicare won’t pay for certain durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) without prior authorization. The regulation could mean headaches for doctors in the form of extra paperwork and frustrated patients.
“Our initial reaction is one of concern,” said Dr. Wanda Filer, president of the American Academy of Family Physicians. “This new rule could prompt DMEPOS suppliers to request additional documentation from ordering/prescribing physicians to support the suppliers’ requests for prior authorization. Such requests could lead to administrative hassles for physicians.”
Under the new requirement – effective Feb. 29 – prior CMS authorization will be required for certain DMEPOS items that are frequently subject to “unnecessary utilization,” according to a Dec. 29 announcement. The prior authorization process requires the same information currently necessary for Medicare payment, but will happen earlier in the process.
The early evaluation will assure that all relevant coverage, coding, and clinical documentation is provided before the equipment is furnished to the patient and before the claim is submitted for payment. CMS hopes the prior review will reduce improper payments for DMEPOS.
Not every piece of durable medical equipment will be subject to prior authorization; instead, CMS will prescreen items from its master list of 135 costly and overprescribed items, especially those with an average purchase price of more than $1,000 or rental fee of $100. The complete master list was published within CMS’ final rule in the Federal Register. CMS will publish a “required prior authorization list” 60 days before implementation.
The final rule primarily impacts vendors paid by Medicare to supply durable medical equipment to patients, said Dr. Yul D. Ejnes, an internist in private practice and a past chair of the American College of Physicians Board of Regents. The prescriber is responsible for meeting all Medicare coverage, coding, and payment rules. However, doctors will likely be indirectly affected because of the clinical documentation required for CMS approval, Dr. Ejnes said.
“The documentation requirement could be burdensome depending on how DME vendors interpret the regulations, and then the whole issue of increasing the amount of chart documentation that’s going out to various places raises some concern,” Dr. Ejnes said in an interview. “Even though it may all be covered under HIPAA, [there’s] the issue of content in the notes that’s irrelevant to the DME request and how we handle that. Do we need to start redacting notes to meet the documentation requirements for prior authorization?”
The new requirements also may mean that patients wait longer for needed equipment, Dr. Ejnes added. “Oftentimes, there’s the finger-pointing exercise that occurs when things don’t happen quickly enough and patients are unhappy. “It just adds to the temperature of the environment, which is already pretty high because of patients unhappy about increasing copays and deductibles and everything else.”
To prepare for the rule, physicians should identify the DMEPOS items they order or prescribe most often and engage with suppliers early to ensure they understand what kind of documentation will be needed, Dr. Filer recommended.
“If the physician understands upfront what Medicare requires and is able to provide it to the DMEPOS supplier[s] at the time the DMEPOS items are ordered/prescribed, that may save time on the back-end preventing or otherwise dealing with additional documentation requests from the DMEPOS supplier[s] in support of prior authorization requests,” she said.
Be extremely thoughtful about prescribing durable medical equipment and make sure that equipment orders are placed that meet the patient’s needs rather than their desires, Dr. Ejnes recommended. In addition, it’s helpful for practices to consider work flow and how to efficiently respond to document requests. In some cases, office staff can locate records or precomplete basic information on forms, he said.
“Figure out a way to respond to these in terms of who in the office will take the first pass if there’s a form to fill out,” he said. “Be aware that there may be some delays in getting patients what they need. Some of these items are not emergency items. Educate patients to the fact that there’s a couple steps between writing the prescription and them picking up the item.”
On Twitter @legal_med
Noncompete clauses: Be wary, negotiate early
Noncompete clauses can severely limit a doctor’s business options and create serious financial challenges, so negotiate with employers early and watch out for tricky contract terms that could stifle future opportunities.
That is the advice from health law experts around the country. They point out that when it comes to noncompete clauses – employment contract language that limits where physicians can practice after employment ends or is terminated – doctors should pay close attention, especially to the following:
Geographical limitations
Distance requirements within noncompete provisions are a top issue that can trip up doctors, Bloomfield Hills, Mich., health law attorney Mark S. Kopson said. The clause typically specifies that a physician cannot practice within a certain radius of the former employer. However, if an employer has three offices for instance, that 10-mile radius can quickly become a 30-mile radius or more depending how the provision is worded. Mr. Kopson recalled a recent client who practiced for 5 years in one office and was transferred to an office in another town for 30 days. He was then terminated, and his employer attempted to enforce contract terms that would prevent him from practicing within a 10-mile radius of both offices. A court determined that the employer was acting in bad faith and sought an unfair competitive advantage.
“But No. 1, you don’t want to have to go to court,” Mr. Kopson said. “And No. 2, you can have the best lawyer, but once it’s in the hands of the judge or the jury, anything can happen. That’s why you really want to do the work on this up front.”
When negotiating noncompete clauses, be cognizant of where distances are being measured from and around, the legal experts stress. Also, be clear with employers about what defines a reasonable distance, based on the geographic spread of their patient base.
“What’s reasonable for a family practice physician is probably not going to be reasonable for a pediatric neurosurgeon, as they draw their patients from varying distances,” he said. “Also, negotiate to ensure that the length of time of the restriction is reasonable. Taking into account, both the distance and time period, the physician must still be able to earn a living.”
Time frame restrictions
Negotiate the shortest duration that you can, advises Greenbelt, Md., labor and employment attorney Jay P. Holland. Noncompete provisions typically limit a doctor from practicing around a certain radius for 1-5 years, but some employers may try to enforce longer time periods.
“Consider your career and lifestyle goals carefully prior to entering into a noncompete,” Mr. Holland said. “The first approach should always be an attempt to exclude the noncompete from your prospective agreement if you are joining a practice. If a noncompete is unavoidable, then strive to make it the least onerous possible. Ask yourself prior to signing an agreement, ‘If I were to leave this practice, what are the restrictions I could live with? Are the restrictions reasonable?’ ”
Knowing your state’s law is key. State regulation of noncompete provisions widely differ. States such as California broadly hold that noncompete contracts are per se invalid – even if narrowly tailored – unless necessary to protect trade secrets. States such as Maryland allow the provisions only if area and duration restrictions are reasonable and do not impose undue hardship on employees. Three states – Colorado, Delaware, and Massachusetts – have laws that strictly prohibit noncompete clauses in physician contracts.
“Most other states will generally enforce noncompete clauses so long as their terms are reasonable in light of the interests of the employer, the employee, and the general public,” Mr. Holland said. “Therefore, noncompete clauses should be no greater in scope than is necessary to protect the business or goodwill of the employer.”
Patient retention problems
Watch out for contract language referring to “trade secrets,” adds Los Angeles health law attorney Andrew H. Selesnick. Trade secret clauses are often lengthy and typically state that physicians cannot use or retain information from the employer that is considered confidential. Because patient lists are usually considered confidential, these terms could potentially prohibit patients from following their doctor.
“If you want to leave and take your patients with you, there may be some trade secret implications associated with that,” Mr. Selesnick said. “The ability to be able to move patients is significant and can have significant financial impacts. Know what you’re getting into.”
If bringing patients with them to a new practice, doctors should make sure the employment agreement excludes these patients from any nonsolicitation provision at the time the doctor leaves, notes Mr. Holland. Include language that states physicians can retain patients they originally brought to the practice when they depart without violating the agreement.
Make sure to review any proposed noncompete clauses in relation to proposed termination provisions, Mr. Kopson said. Doctors should negotiate language that ensures noncompete obligations will be null and void if physicians are terminated without cause (if such terminations are permitted by the contract), or if the employer breaches the contract.
Seeking the advice of an experienced contract attorney before signing a noncompete clause can save doctors significant time, money, and heartache in the long run, Mr. Kopson notes.
“The biggest risk is signing a contract that has such a clause with an expectation that it will not be enforced,” he said. “If [clauses are] properly drafted, they’re going to be binding. If you get the help up front, it’s going to be a lot less expensive than having your life turned upside down because you’re stuck with a noncompete that has bad terms in it.”
Unreasonable terms
Once signed, getting out of non-compete clauses can be tricky, Mr. Selesnick said. However, doctors can usually escape them if they can prove the terms are unreasonable.
“You can get out of them, especially if they’re very restrictive and say you can’t practice within an area that may prevent you from earning your livelihood,” Mr. Selesnick said. “Courts [generally] think that employees should be able to leave and be able to get a job elsewhere, even if it’s across the street.”
Courts are typically more favorable to physician-employees than independent contractors when it comes to noncompete clauses, Mr. Selesnick said. Independent contractors are generally viewed as having more power over their work than physician-employees. They may have a tougher time convincing a court that such provisions will harm their employment options.
When seeking to enforce a disputed noncompete agreement, employers frequently will request a court-ordered temporary restraining order or injunction to enforce the clause, Mr. Holland said. Judges consider general principles of fairness and equity, and balance the relative harm to the employer and the employee, when deciding whether to issue the injunction. The employee-physician can also try to beat the employer to the courthouse steps by filing a “declaratory judgment” lawsuit that seeks guidance from the court on the contract’s enforceability.
“Typically, employers attempt to do that which is in their best economic interest,” Mr. Holland said. “If a proposal can be negotiated where the employer’s economic well-being is not threatened, then the employer should have a strong interest in a compromise.”
On Twitter @legal_med
Noncompete clauses can severely limit a doctor’s business options and create serious financial challenges, so negotiate with employers early and watch out for tricky contract terms that could stifle future opportunities.
That is the advice from health law experts around the country. They point out that when it comes to noncompete clauses – employment contract language that limits where physicians can practice after employment ends or is terminated – doctors should pay close attention, especially to the following:
Geographical limitations
Distance requirements within noncompete provisions are a top issue that can trip up doctors, Bloomfield Hills, Mich., health law attorney Mark S. Kopson said. The clause typically specifies that a physician cannot practice within a certain radius of the former employer. However, if an employer has three offices for instance, that 10-mile radius can quickly become a 30-mile radius or more depending how the provision is worded. Mr. Kopson recalled a recent client who practiced for 5 years in one office and was transferred to an office in another town for 30 days. He was then terminated, and his employer attempted to enforce contract terms that would prevent him from practicing within a 10-mile radius of both offices. A court determined that the employer was acting in bad faith and sought an unfair competitive advantage.
“But No. 1, you don’t want to have to go to court,” Mr. Kopson said. “And No. 2, you can have the best lawyer, but once it’s in the hands of the judge or the jury, anything can happen. That’s why you really want to do the work on this up front.”
When negotiating noncompete clauses, be cognizant of where distances are being measured from and around, the legal experts stress. Also, be clear with employers about what defines a reasonable distance, based on the geographic spread of their patient base.
“What’s reasonable for a family practice physician is probably not going to be reasonable for a pediatric neurosurgeon, as they draw their patients from varying distances,” he said. “Also, negotiate to ensure that the length of time of the restriction is reasonable. Taking into account, both the distance and time period, the physician must still be able to earn a living.”
Time frame restrictions
Negotiate the shortest duration that you can, advises Greenbelt, Md., labor and employment attorney Jay P. Holland. Noncompete provisions typically limit a doctor from practicing around a certain radius for 1-5 years, but some employers may try to enforce longer time periods.
“Consider your career and lifestyle goals carefully prior to entering into a noncompete,” Mr. Holland said. “The first approach should always be an attempt to exclude the noncompete from your prospective agreement if you are joining a practice. If a noncompete is unavoidable, then strive to make it the least onerous possible. Ask yourself prior to signing an agreement, ‘If I were to leave this practice, what are the restrictions I could live with? Are the restrictions reasonable?’ ”
Knowing your state’s law is key. State regulation of noncompete provisions widely differ. States such as California broadly hold that noncompete contracts are per se invalid – even if narrowly tailored – unless necessary to protect trade secrets. States such as Maryland allow the provisions only if area and duration restrictions are reasonable and do not impose undue hardship on employees. Three states – Colorado, Delaware, and Massachusetts – have laws that strictly prohibit noncompete clauses in physician contracts.
“Most other states will generally enforce noncompete clauses so long as their terms are reasonable in light of the interests of the employer, the employee, and the general public,” Mr. Holland said. “Therefore, noncompete clauses should be no greater in scope than is necessary to protect the business or goodwill of the employer.”
Patient retention problems
Watch out for contract language referring to “trade secrets,” adds Los Angeles health law attorney Andrew H. Selesnick. Trade secret clauses are often lengthy and typically state that physicians cannot use or retain information from the employer that is considered confidential. Because patient lists are usually considered confidential, these terms could potentially prohibit patients from following their doctor.
“If you want to leave and take your patients with you, there may be some trade secret implications associated with that,” Mr. Selesnick said. “The ability to be able to move patients is significant and can have significant financial impacts. Know what you’re getting into.”
If bringing patients with them to a new practice, doctors should make sure the employment agreement excludes these patients from any nonsolicitation provision at the time the doctor leaves, notes Mr. Holland. Include language that states physicians can retain patients they originally brought to the practice when they depart without violating the agreement.
Make sure to review any proposed noncompete clauses in relation to proposed termination provisions, Mr. Kopson said. Doctors should negotiate language that ensures noncompete obligations will be null and void if physicians are terminated without cause (if such terminations are permitted by the contract), or if the employer breaches the contract.
Seeking the advice of an experienced contract attorney before signing a noncompete clause can save doctors significant time, money, and heartache in the long run, Mr. Kopson notes.
“The biggest risk is signing a contract that has such a clause with an expectation that it will not be enforced,” he said. “If [clauses are] properly drafted, they’re going to be binding. If you get the help up front, it’s going to be a lot less expensive than having your life turned upside down because you’re stuck with a noncompete that has bad terms in it.”
Unreasonable terms
Once signed, getting out of non-compete clauses can be tricky, Mr. Selesnick said. However, doctors can usually escape them if they can prove the terms are unreasonable.
“You can get out of them, especially if they’re very restrictive and say you can’t practice within an area that may prevent you from earning your livelihood,” Mr. Selesnick said. “Courts [generally] think that employees should be able to leave and be able to get a job elsewhere, even if it’s across the street.”
Courts are typically more favorable to physician-employees than independent contractors when it comes to noncompete clauses, Mr. Selesnick said. Independent contractors are generally viewed as having more power over their work than physician-employees. They may have a tougher time convincing a court that such provisions will harm their employment options.
When seeking to enforce a disputed noncompete agreement, employers frequently will request a court-ordered temporary restraining order or injunction to enforce the clause, Mr. Holland said. Judges consider general principles of fairness and equity, and balance the relative harm to the employer and the employee, when deciding whether to issue the injunction. The employee-physician can also try to beat the employer to the courthouse steps by filing a “declaratory judgment” lawsuit that seeks guidance from the court on the contract’s enforceability.
“Typically, employers attempt to do that which is in their best economic interest,” Mr. Holland said. “If a proposal can be negotiated where the employer’s economic well-being is not threatened, then the employer should have a strong interest in a compromise.”
On Twitter @legal_med
Noncompete clauses can severely limit a doctor’s business options and create serious financial challenges, so negotiate with employers early and watch out for tricky contract terms that could stifle future opportunities.
That is the advice from health law experts around the country. They point out that when it comes to noncompete clauses – employment contract language that limits where physicians can practice after employment ends or is terminated – doctors should pay close attention, especially to the following:
Geographical limitations
Distance requirements within noncompete provisions are a top issue that can trip up doctors, Bloomfield Hills, Mich., health law attorney Mark S. Kopson said. The clause typically specifies that a physician cannot practice within a certain radius of the former employer. However, if an employer has three offices for instance, that 10-mile radius can quickly become a 30-mile radius or more depending how the provision is worded. Mr. Kopson recalled a recent client who practiced for 5 years in one office and was transferred to an office in another town for 30 days. He was then terminated, and his employer attempted to enforce contract terms that would prevent him from practicing within a 10-mile radius of both offices. A court determined that the employer was acting in bad faith and sought an unfair competitive advantage.
“But No. 1, you don’t want to have to go to court,” Mr. Kopson said. “And No. 2, you can have the best lawyer, but once it’s in the hands of the judge or the jury, anything can happen. That’s why you really want to do the work on this up front.”
When negotiating noncompete clauses, be cognizant of where distances are being measured from and around, the legal experts stress. Also, be clear with employers about what defines a reasonable distance, based on the geographic spread of their patient base.
“What’s reasonable for a family practice physician is probably not going to be reasonable for a pediatric neurosurgeon, as they draw their patients from varying distances,” he said. “Also, negotiate to ensure that the length of time of the restriction is reasonable. Taking into account, both the distance and time period, the physician must still be able to earn a living.”
Time frame restrictions
Negotiate the shortest duration that you can, advises Greenbelt, Md., labor and employment attorney Jay P. Holland. Noncompete provisions typically limit a doctor from practicing around a certain radius for 1-5 years, but some employers may try to enforce longer time periods.
“Consider your career and lifestyle goals carefully prior to entering into a noncompete,” Mr. Holland said. “The first approach should always be an attempt to exclude the noncompete from your prospective agreement if you are joining a practice. If a noncompete is unavoidable, then strive to make it the least onerous possible. Ask yourself prior to signing an agreement, ‘If I were to leave this practice, what are the restrictions I could live with? Are the restrictions reasonable?’ ”
Knowing your state’s law is key. State regulation of noncompete provisions widely differ. States such as California broadly hold that noncompete contracts are per se invalid – even if narrowly tailored – unless necessary to protect trade secrets. States such as Maryland allow the provisions only if area and duration restrictions are reasonable and do not impose undue hardship on employees. Three states – Colorado, Delaware, and Massachusetts – have laws that strictly prohibit noncompete clauses in physician contracts.
“Most other states will generally enforce noncompete clauses so long as their terms are reasonable in light of the interests of the employer, the employee, and the general public,” Mr. Holland said. “Therefore, noncompete clauses should be no greater in scope than is necessary to protect the business or goodwill of the employer.”
Patient retention problems
Watch out for contract language referring to “trade secrets,” adds Los Angeles health law attorney Andrew H. Selesnick. Trade secret clauses are often lengthy and typically state that physicians cannot use or retain information from the employer that is considered confidential. Because patient lists are usually considered confidential, these terms could potentially prohibit patients from following their doctor.
“If you want to leave and take your patients with you, there may be some trade secret implications associated with that,” Mr. Selesnick said. “The ability to be able to move patients is significant and can have significant financial impacts. Know what you’re getting into.”
If bringing patients with them to a new practice, doctors should make sure the employment agreement excludes these patients from any nonsolicitation provision at the time the doctor leaves, notes Mr. Holland. Include language that states physicians can retain patients they originally brought to the practice when they depart without violating the agreement.
Make sure to review any proposed noncompete clauses in relation to proposed termination provisions, Mr. Kopson said. Doctors should negotiate language that ensures noncompete obligations will be null and void if physicians are terminated without cause (if such terminations are permitted by the contract), or if the employer breaches the contract.
Seeking the advice of an experienced contract attorney before signing a noncompete clause can save doctors significant time, money, and heartache in the long run, Mr. Kopson notes.
“The biggest risk is signing a contract that has such a clause with an expectation that it will not be enforced,” he said. “If [clauses are] properly drafted, they’re going to be binding. If you get the help up front, it’s going to be a lot less expensive than having your life turned upside down because you’re stuck with a noncompete that has bad terms in it.”
Unreasonable terms
Once signed, getting out of non-compete clauses can be tricky, Mr. Selesnick said. However, doctors can usually escape them if they can prove the terms are unreasonable.
“You can get out of them, especially if they’re very restrictive and say you can’t practice within an area that may prevent you from earning your livelihood,” Mr. Selesnick said. “Courts [generally] think that employees should be able to leave and be able to get a job elsewhere, even if it’s across the street.”
Courts are typically more favorable to physician-employees than independent contractors when it comes to noncompete clauses, Mr. Selesnick said. Independent contractors are generally viewed as having more power over their work than physician-employees. They may have a tougher time convincing a court that such provisions will harm their employment options.
When seeking to enforce a disputed noncompete agreement, employers frequently will request a court-ordered temporary restraining order or injunction to enforce the clause, Mr. Holland said. Judges consider general principles of fairness and equity, and balance the relative harm to the employer and the employee, when deciding whether to issue the injunction. The employee-physician can also try to beat the employer to the courthouse steps by filing a “declaratory judgment” lawsuit that seeks guidance from the court on the contract’s enforceability.
“Typically, employers attempt to do that which is in their best economic interest,” Mr. Holland said. “If a proposal can be negotiated where the employer’s economic well-being is not threatened, then the employer should have a strong interest in a compromise.”
On Twitter @legal_med
Physician groups to Supreme Court: Strike down Texas abortion law
The next Supreme Court showdown over abortion starts in March and some physician groups are already entering the fray in support of preserving abortion access.
In a joint brief sent to Supreme Court justices in early January, the American College of Obstetricians and Gynecologists, the American Medical Association, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American Osteopathic Association are all urging the Supreme Court to strike down a Texas law (HB2) that they say imposes unnecessary demands on abortion providers and creates restrictive requirements for abortion facilities.
“Restrictions such as those in HB2 that make it harder for women to access quality abortion care only make it less safe,” Dr. Hal C. Lawrence III, executive vice president and CEO of ACOG, said during a recent press conference. “How? By delaying women’s access until later in pregnancy, when the risk of complications associated with abortion – while still quite low – does increase. Worse, some women will simply not be able to access safe, legal abortion.”
The Supreme Court agreed to hear Whole Woman’s Health v. Cole in late November 2015. The case centers on two 2013 Texas regulations – both part of HB2 – mandating that abortion providers have admitting privileges at a hospital within 30 miles of an abortion clinic in order to provide the service, and that all abortion clinics meet the same requirements as ambulatory surgical centers (ASCs).
The plaintiffs, who are clinics and doctors, argue that both restrictions are unnecessary and limit access to abortion services. But defendant Kirk Cole, commissioner for the Texas Department of State Health Services, argues that the restrictions are reasonable and effective measures that raise the standard of care for abortion patients and ensure health and safety.
The case has ramifications for physicians and patients outside Texas. The plaintiffs are asking the Supreme Court to reaffirm prior rulings that outline when a new abortion law imposes an “undue burden” on a patient’s right to end a pregnancy. The standard results from a 1992 Supreme Court decision, Planned Parenthood of Southeastern Pennsylvania v. Casey, in which the justices affirmed abortion rights established in Roe v. Wade.
The plaintiffs are also asking the Supreme Court to instruct lower courts to weigh whether new state restrictions on abortions really serve to protect patient health. The 5th U.S. Circuit Court of Appeals refused to answer this question when it ruled in favor of Texas in 2015, stating that courts must accept that new laws brought before them would serve the public interest.
The 1992 Casey decision made it clear that the Constitution does not permit states to enact unnecessary health regulations that create undue burdens for women seeking abortion services, said Nancy Northup, president and CEO for the Center for Reproductive Rights, during the recent press conference.
“Texas has sought to sneak around the Casey decision by using the pretext of advancing women’s health as a cover for doing what the Constitution does not permit – blocking women’s access to safe and legal abortion,” Ms. Northup said. “So we’re back at the Supreme Court to ensure the rule of law prevails and the rights of women are respected.”
The Center for Reproductive Rights, as well as 45 other groups and organizations, issued friend-of-the-court briefs to the Supreme Court on Jan. 4 in support of the plaintiffs.
At press time, the state of Texas had not yet filed a brief following the Supreme Court’s acceptance of the case. In its initial brief requesting that the high court refuse the case, Texas officials argued that the state’s abortion regulations are rational and meet the state’s interest in patient health. The admitting-privileges requirement “ensures doctors are qualified, promotes continuity of care in the case of complications that require hospitalization, and reduces communication errors and time delays when a patient must be treated at a hospital,” the brief stated. The requirements placed on abortion clinics guarantee that “patients will not be relegated to substandard clinics, ensuring enhanced pain management options for patients and providing a sterile operating environment for surgical abortions,” according to the brief.
But ACOG and other physician groups counter that claim. In the Jan. 4 brief to the Supreme Court, the associations argue that the restrictions are inconsistent with accepted medical practice and provide no benefit to patient care.
“There is no medically sound reason to assume that abortions performed in a hospital or ASC setting are safer than those performed in a clinic or office, and requiring abortion clinics to meet the standards for ASCs has no medical purpose given the nature and simplicity of abortion procedures,” the groups wrote.
The Supreme Court justices are scheduled to hear arguments in Whole Woman’s Health v. Cole on March 2.
On Twitter @legal_med
The next Supreme Court showdown over abortion starts in March and some physician groups are already entering the fray in support of preserving abortion access.
In a joint brief sent to Supreme Court justices in early January, the American College of Obstetricians and Gynecologists, the American Medical Association, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American Osteopathic Association are all urging the Supreme Court to strike down a Texas law (HB2) that they say imposes unnecessary demands on abortion providers and creates restrictive requirements for abortion facilities.
“Restrictions such as those in HB2 that make it harder for women to access quality abortion care only make it less safe,” Dr. Hal C. Lawrence III, executive vice president and CEO of ACOG, said during a recent press conference. “How? By delaying women’s access until later in pregnancy, when the risk of complications associated with abortion – while still quite low – does increase. Worse, some women will simply not be able to access safe, legal abortion.”
The Supreme Court agreed to hear Whole Woman’s Health v. Cole in late November 2015. The case centers on two 2013 Texas regulations – both part of HB2 – mandating that abortion providers have admitting privileges at a hospital within 30 miles of an abortion clinic in order to provide the service, and that all abortion clinics meet the same requirements as ambulatory surgical centers (ASCs).
The plaintiffs, who are clinics and doctors, argue that both restrictions are unnecessary and limit access to abortion services. But defendant Kirk Cole, commissioner for the Texas Department of State Health Services, argues that the restrictions are reasonable and effective measures that raise the standard of care for abortion patients and ensure health and safety.
The case has ramifications for physicians and patients outside Texas. The plaintiffs are asking the Supreme Court to reaffirm prior rulings that outline when a new abortion law imposes an “undue burden” on a patient’s right to end a pregnancy. The standard results from a 1992 Supreme Court decision, Planned Parenthood of Southeastern Pennsylvania v. Casey, in which the justices affirmed abortion rights established in Roe v. Wade.
The plaintiffs are also asking the Supreme Court to instruct lower courts to weigh whether new state restrictions on abortions really serve to protect patient health. The 5th U.S. Circuit Court of Appeals refused to answer this question when it ruled in favor of Texas in 2015, stating that courts must accept that new laws brought before them would serve the public interest.
The 1992 Casey decision made it clear that the Constitution does not permit states to enact unnecessary health regulations that create undue burdens for women seeking abortion services, said Nancy Northup, president and CEO for the Center for Reproductive Rights, during the recent press conference.
“Texas has sought to sneak around the Casey decision by using the pretext of advancing women’s health as a cover for doing what the Constitution does not permit – blocking women’s access to safe and legal abortion,” Ms. Northup said. “So we’re back at the Supreme Court to ensure the rule of law prevails and the rights of women are respected.”
The Center for Reproductive Rights, as well as 45 other groups and organizations, issued friend-of-the-court briefs to the Supreme Court on Jan. 4 in support of the plaintiffs.
At press time, the state of Texas had not yet filed a brief following the Supreme Court’s acceptance of the case. In its initial brief requesting that the high court refuse the case, Texas officials argued that the state’s abortion regulations are rational and meet the state’s interest in patient health. The admitting-privileges requirement “ensures doctors are qualified, promotes continuity of care in the case of complications that require hospitalization, and reduces communication errors and time delays when a patient must be treated at a hospital,” the brief stated. The requirements placed on abortion clinics guarantee that “patients will not be relegated to substandard clinics, ensuring enhanced pain management options for patients and providing a sterile operating environment for surgical abortions,” according to the brief.
But ACOG and other physician groups counter that claim. In the Jan. 4 brief to the Supreme Court, the associations argue that the restrictions are inconsistent with accepted medical practice and provide no benefit to patient care.
“There is no medically sound reason to assume that abortions performed in a hospital or ASC setting are safer than those performed in a clinic or office, and requiring abortion clinics to meet the standards for ASCs has no medical purpose given the nature and simplicity of abortion procedures,” the groups wrote.
The Supreme Court justices are scheduled to hear arguments in Whole Woman’s Health v. Cole on March 2.
On Twitter @legal_med
The next Supreme Court showdown over abortion starts in March and some physician groups are already entering the fray in support of preserving abortion access.
In a joint brief sent to Supreme Court justices in early January, the American College of Obstetricians and Gynecologists, the American Medical Association, the American Academy of Family Physicians, the American Academy of Pediatrics, and the American Osteopathic Association are all urging the Supreme Court to strike down a Texas law (HB2) that they say imposes unnecessary demands on abortion providers and creates restrictive requirements for abortion facilities.
“Restrictions such as those in HB2 that make it harder for women to access quality abortion care only make it less safe,” Dr. Hal C. Lawrence III, executive vice president and CEO of ACOG, said during a recent press conference. “How? By delaying women’s access until later in pregnancy, when the risk of complications associated with abortion – while still quite low – does increase. Worse, some women will simply not be able to access safe, legal abortion.”
The Supreme Court agreed to hear Whole Woman’s Health v. Cole in late November 2015. The case centers on two 2013 Texas regulations – both part of HB2 – mandating that abortion providers have admitting privileges at a hospital within 30 miles of an abortion clinic in order to provide the service, and that all abortion clinics meet the same requirements as ambulatory surgical centers (ASCs).
The plaintiffs, who are clinics and doctors, argue that both restrictions are unnecessary and limit access to abortion services. But defendant Kirk Cole, commissioner for the Texas Department of State Health Services, argues that the restrictions are reasonable and effective measures that raise the standard of care for abortion patients and ensure health and safety.
The case has ramifications for physicians and patients outside Texas. The plaintiffs are asking the Supreme Court to reaffirm prior rulings that outline when a new abortion law imposes an “undue burden” on a patient’s right to end a pregnancy. The standard results from a 1992 Supreme Court decision, Planned Parenthood of Southeastern Pennsylvania v. Casey, in which the justices affirmed abortion rights established in Roe v. Wade.
The plaintiffs are also asking the Supreme Court to instruct lower courts to weigh whether new state restrictions on abortions really serve to protect patient health. The 5th U.S. Circuit Court of Appeals refused to answer this question when it ruled in favor of Texas in 2015, stating that courts must accept that new laws brought before them would serve the public interest.
The 1992 Casey decision made it clear that the Constitution does not permit states to enact unnecessary health regulations that create undue burdens for women seeking abortion services, said Nancy Northup, president and CEO for the Center for Reproductive Rights, during the recent press conference.
“Texas has sought to sneak around the Casey decision by using the pretext of advancing women’s health as a cover for doing what the Constitution does not permit – blocking women’s access to safe and legal abortion,” Ms. Northup said. “So we’re back at the Supreme Court to ensure the rule of law prevails and the rights of women are respected.”
The Center for Reproductive Rights, as well as 45 other groups and organizations, issued friend-of-the-court briefs to the Supreme Court on Jan. 4 in support of the plaintiffs.
At press time, the state of Texas had not yet filed a brief following the Supreme Court’s acceptance of the case. In its initial brief requesting that the high court refuse the case, Texas officials argued that the state’s abortion regulations are rational and meet the state’s interest in patient health. The admitting-privileges requirement “ensures doctors are qualified, promotes continuity of care in the case of complications that require hospitalization, and reduces communication errors and time delays when a patient must be treated at a hospital,” the brief stated. The requirements placed on abortion clinics guarantee that “patients will not be relegated to substandard clinics, ensuring enhanced pain management options for patients and providing a sterile operating environment for surgical abortions,” according to the brief.
But ACOG and other physician groups counter that claim. In the Jan. 4 brief to the Supreme Court, the associations argue that the restrictions are inconsistent with accepted medical practice and provide no benefit to patient care.
“There is no medically sound reason to assume that abortions performed in a hospital or ASC setting are safer than those performed in a clinic or office, and requiring abortion clinics to meet the standards for ASCs has no medical purpose given the nature and simplicity of abortion procedures,” the groups wrote.
The Supreme Court justices are scheduled to hear arguments in Whole Woman’s Health v. Cole on March 2.
On Twitter @legal_med
Candor laws growing, but are they effective?
Iowa is the latest state to launch a unique strategy that aims to reduce medical malpractice lawsuits and bolster doctor-patient communication after poor outcomes, while encouraging swift resolution. The Communication and Optimal Resolution (CANDOR) law, which took effect in July, permits privileged discussions between Iowa physicians and patients after medical errors and allows for compensation offers, when appropriate.
After years of failing to enact traditional tort reform, the alternative method is hoped to decrease litigation costs and keep doctors and patients out of a flawed court system, said Dr. Michael McCoy, chair of the Iowa Medical Society Ad Hoc Tort Reform Task Force and a West Burlington obstetrician-gynecologist.
“If there’s an untoward outcome, the current system creates isolation,” he said. “The patients get alienated, and the doctors get stressed. That’s the exact opposite of what should happen. You should be able to talk to patients and feel protection. That’s what this law does.”
About 36 states have so-called “apology laws” that prohibit certain statements, expressions, or other evidence from being admissible in a malpractice lawsuit, according to data from Sorry Works!, an advocacy group that tracks apology statutes. Most apology laws cover expressions of empathy or sympathy, while some statutes protect admissions of fault. At least three states – including Iowa – go a step further, laying the foundation for early disclosure of errors, communication between physicians and patients, and negotiation of potential resolutions. Oregon’s Early Discussion and Resolution (EDR) law, which passed in 2013, allows both providers and patients to initiate conversations after adverse events, discuss what happened, and resolve the issue outside of court. Massachusetts’ 2012 disclosure, apology, and offer legislation includes a 182-day waiting period before lawsuits can be filed to allow for the process. Other states, including Washington, Texas, California, and Utah have expressed interest in enacting similar laws.
Efforts in Iowa, Oregon, and Massachusetts were driven by the successes of similar programs at the University of Illinois Hospital and Health Sciences System, Chicago and at the University of Michigan Health System, Ann Arbor, among others. Since the University of Michigan began its CANDOR program in 2001, average legal expenses per case have been cut in half, according to the UM data. The health system’s presuit claims have gone from 260 annually to about 100 per year. Pilot efforts by the University of Illinois (Chicago) Hospital and Health Sciences System since 2006 have increased adverse event reports from 1,500 per year to 10,000 while decreasing malpractice premiums by $15 million dollars since 2010.
But questions remain as to whether such approaches are successful when implemented on a state level. So far, the majority of data on early discussion, apology, and offer programs have come from closed systems, such as academic medical centers.
“We’re starting to look at how this works in an open system when you try to disseminate it across a state,” said Dr. Alan C. Woodward, chair of the Massachusetts Medical Society Committee on Professional Liability and cofounder of the Massachusetts Alliance for Communication and Resolution following Medical Injury (MACRMI). “If we can show this is an effective model in a statewide distribution, the hope is that multiple other states will follow suit, because this is clearly a better alternative for patients and providers and for patient safety improvement efforts.”
Massachusetts program showing promise
In Massachusetts, the state’s CANDOR law enabled MACRMI to roll out its Communication, Apology, and Resolution (CARe) model at six hospital pilot sites. MACRMI was formed as part of a research initiative led by Beth Israel Deaconess Medical Center, Boston, and the Massachusetts Medical Society, Waltham, and funded by a 2010 grant from the Federal Agency for Health Care Research and Quality.
Under the CARe model, participating health providers communicate with patients and families after an unanticipated adverse outcome, investigate and explain what happened, and where appropriate, apologize and offer fair financial compensation. Data collection in the 3-year study trial closed in December 2015, and the alliance plans to publish its findings in spring 2016. The model has greatly expedited case resolutions, Dr. Woodward said. A case in the CARe program can be resolved in 6 months or less, compared with a lawsuit that can take up to 5 years to resolve. By April 2015, the alliance had screened 856 cases. In three-quarters of cases, the care provided was deemed appropriate, Dr. Woodward said. Of these cases, 615 were closed after full-disclosure meetings with patients. Of the total cases screened, 122 cases were referred to an insurer for resolution, and 93 cases were awaiting further evaluation.
“It’s doing extraordinarily well,” Dr. Woodward said. “We’re resolving cases very efficiently in a short period of time, rather than using litigation.”
Outside the pilot program, health providers in Massachusetts are benefiting from the state’s 182-day waiting period before malpractice lawsuits can be filed, said Elizabeth A. Cushing, vice president of claims for CRICO, a medical liability insurer for the Harvard University medical community. The law allows physicians and insurers the opportunity to review a case and decide whether the complaint requires compensation. In cases that are not considered malpractice, explaining the underlying reasoning to patients or plaintiffs’ attorneys prevents some lawsuits from being filed, she said.
However, Ms. Cushing notes that not every case fits smoothly into the alternative model. Cases in which a poor outcome is immediately known, such as a surgical mishap, lend themselves to quicker investigation, disclosure, and remedy, she said. Claims of misdiagnosed cancer for instance, where the alleged mistake occurred years before, are more challenging.
“A lot of what we are seeing are alleged failures to diagnose things sooner, so it’s 2 years later and someone says, ‘You should have picked up on the fact that I had lung cancer 2 years ago,’ Ms. Cushing said. “Those situations get tricky. What did you know, when? Those cases are not as easily amenable to this process.”
Oregon program gaining speed
Meanwhile, Oregon officials are hoping that more doctors will soon participate in the state’s Early Discussion and Resolution program. In 2014 – its first year in operation – no individual physicians initiated participation in the program. Because EDR is voluntary, both parties must agree to participate for EDR to begin and either party can choose to stop participating at any time.
In 2014, Oregon patients and health providers filed 29 EDR requests to participate in the program, according to data from the Oregon Patient Safety Commission. Patients filed 21 notices and health care professionals filed eight notices. A majority of the eight notices filed by health professionals were issued by hospital representatives. No requests were filed by individual health providers. In 9 of the 21 patient-filed notices, at least one involved health professional accepted the patient’s request to participate in EDR. In the remaining 12 patient-filed notices, the involved health provider(s) declined the request. Data is not yet available on resolution time or case outcomes.
EDR leaders are hopeful that more health professionals and patients will participate in the coming years, said Melissa Parkerton, director of the Early Discussion and Resolution program for the Oregon Patient Safety Commission.
“Widespread adoption of a new approach like EDR requires not just a new process, but a new mindset,” she said. “This kind of cultural shift takes time. In Oregon, our fervent hope is that EDR will be embraced by every health care organization and professional.”
Efforts to raise awareness about the program are ongoing, adds Dr. Robert Dannenhoffer, a Roseburg, Ore., pediatrician and past president of the Oregon Medical Association.
“It’s a little hard to get the word out,” he said in an interview. “Most [doctors] are not going to be dealing with this on a regular basis. It’s a slow uptake. We’re working on better educating physicians.”
A practical model for independent docs?
Questions remain about whether disclosure, apology, and offer models work for independent physicians in small practices.
Dr. Thad L. Anderson, an ob.gyn in private practice in Dubuque, Iowa, strongly supports his state’s law, but he does not foresee the process having much impact on his practice. Larger health systems are generally more situated to utilize the process, he said.
“A hospital system that employees physicians is probably better suited to take advantage of this law,” he said. Independent doctors “don’t have the infrastructure. You don’t have the in-house lawyer. It’s probably a little more problematic for an individual to take advantage of the process compared to a bigger system.”
With this issue in mind, MACRMI in Massachusetts recently added an 800-physician, multispecialty outpatient group to its program analysis. The goal is to evaluate how the process operates within this type of setting and identify challenges and impediments, Dr. Woodward said. In addition, the alliance is encouraging commercial insurers to assist independent physicians in participating in the CARe model.
“The independent or small practice all benefit from the statute we passed – they have the [182-day waiting period] – but if they don’t have an internal risk management structure, than we’ve been working with insurers to set up a support structure,” he said. That way, “when a patient is unhappy with their care, there is a process that can support even a small practice in going through this and working through it with a patient.”
*This story was updated 1/26/2016.
On Twitter @legal_med
Iowa is the latest state to launch a unique strategy that aims to reduce medical malpractice lawsuits and bolster doctor-patient communication after poor outcomes, while encouraging swift resolution. The Communication and Optimal Resolution (CANDOR) law, which took effect in July, permits privileged discussions between Iowa physicians and patients after medical errors and allows for compensation offers, when appropriate.
After years of failing to enact traditional tort reform, the alternative method is hoped to decrease litigation costs and keep doctors and patients out of a flawed court system, said Dr. Michael McCoy, chair of the Iowa Medical Society Ad Hoc Tort Reform Task Force and a West Burlington obstetrician-gynecologist.
“If there’s an untoward outcome, the current system creates isolation,” he said. “The patients get alienated, and the doctors get stressed. That’s the exact opposite of what should happen. You should be able to talk to patients and feel protection. That’s what this law does.”
About 36 states have so-called “apology laws” that prohibit certain statements, expressions, or other evidence from being admissible in a malpractice lawsuit, according to data from Sorry Works!, an advocacy group that tracks apology statutes. Most apology laws cover expressions of empathy or sympathy, while some statutes protect admissions of fault. At least three states – including Iowa – go a step further, laying the foundation for early disclosure of errors, communication between physicians and patients, and negotiation of potential resolutions. Oregon’s Early Discussion and Resolution (EDR) law, which passed in 2013, allows both providers and patients to initiate conversations after adverse events, discuss what happened, and resolve the issue outside of court. Massachusetts’ 2012 disclosure, apology, and offer legislation includes a 182-day waiting period before lawsuits can be filed to allow for the process. Other states, including Washington, Texas, California, and Utah have expressed interest in enacting similar laws.
Efforts in Iowa, Oregon, and Massachusetts were driven by the successes of similar programs at the University of Illinois Hospital and Health Sciences System, Chicago and at the University of Michigan Health System, Ann Arbor, among others. Since the University of Michigan began its CANDOR program in 2001, average legal expenses per case have been cut in half, according to the UM data. The health system’s presuit claims have gone from 260 annually to about 100 per year. Pilot efforts by the University of Illinois (Chicago) Hospital and Health Sciences System since 2006 have increased adverse event reports from 1,500 per year to 10,000 while decreasing malpractice premiums by $15 million dollars since 2010.
But questions remain as to whether such approaches are successful when implemented on a state level. So far, the majority of data on early discussion, apology, and offer programs have come from closed systems, such as academic medical centers.
“We’re starting to look at how this works in an open system when you try to disseminate it across a state,” said Dr. Alan C. Woodward, chair of the Massachusetts Medical Society Committee on Professional Liability and cofounder of the Massachusetts Alliance for Communication and Resolution following Medical Injury (MACRMI). “If we can show this is an effective model in a statewide distribution, the hope is that multiple other states will follow suit, because this is clearly a better alternative for patients and providers and for patient safety improvement efforts.”
Massachusetts program showing promise
In Massachusetts, the state’s CANDOR law enabled MACRMI to roll out its Communication, Apology, and Resolution (CARe) model at six hospital pilot sites. MACRMI was formed as part of a research initiative led by Beth Israel Deaconess Medical Center, Boston, and the Massachusetts Medical Society, Waltham, and funded by a 2010 grant from the Federal Agency for Health Care Research and Quality.
Under the CARe model, participating health providers communicate with patients and families after an unanticipated adverse outcome, investigate and explain what happened, and where appropriate, apologize and offer fair financial compensation. Data collection in the 3-year study trial closed in December 2015, and the alliance plans to publish its findings in spring 2016. The model has greatly expedited case resolutions, Dr. Woodward said. A case in the CARe program can be resolved in 6 months or less, compared with a lawsuit that can take up to 5 years to resolve. By April 2015, the alliance had screened 856 cases. In three-quarters of cases, the care provided was deemed appropriate, Dr. Woodward said. Of these cases, 615 were closed after full-disclosure meetings with patients. Of the total cases screened, 122 cases were referred to an insurer for resolution, and 93 cases were awaiting further evaluation.
“It’s doing extraordinarily well,” Dr. Woodward said. “We’re resolving cases very efficiently in a short period of time, rather than using litigation.”
Outside the pilot program, health providers in Massachusetts are benefiting from the state’s 182-day waiting period before malpractice lawsuits can be filed, said Elizabeth A. Cushing, vice president of claims for CRICO, a medical liability insurer for the Harvard University medical community. The law allows physicians and insurers the opportunity to review a case and decide whether the complaint requires compensation. In cases that are not considered malpractice, explaining the underlying reasoning to patients or plaintiffs’ attorneys prevents some lawsuits from being filed, she said.
However, Ms. Cushing notes that not every case fits smoothly into the alternative model. Cases in which a poor outcome is immediately known, such as a surgical mishap, lend themselves to quicker investigation, disclosure, and remedy, she said. Claims of misdiagnosed cancer for instance, where the alleged mistake occurred years before, are more challenging.
“A lot of what we are seeing are alleged failures to diagnose things sooner, so it’s 2 years later and someone says, ‘You should have picked up on the fact that I had lung cancer 2 years ago,’ Ms. Cushing said. “Those situations get tricky. What did you know, when? Those cases are not as easily amenable to this process.”
Oregon program gaining speed
Meanwhile, Oregon officials are hoping that more doctors will soon participate in the state’s Early Discussion and Resolution program. In 2014 – its first year in operation – no individual physicians initiated participation in the program. Because EDR is voluntary, both parties must agree to participate for EDR to begin and either party can choose to stop participating at any time.
In 2014, Oregon patients and health providers filed 29 EDR requests to participate in the program, according to data from the Oregon Patient Safety Commission. Patients filed 21 notices and health care professionals filed eight notices. A majority of the eight notices filed by health professionals were issued by hospital representatives. No requests were filed by individual health providers. In 9 of the 21 patient-filed notices, at least one involved health professional accepted the patient’s request to participate in EDR. In the remaining 12 patient-filed notices, the involved health provider(s) declined the request. Data is not yet available on resolution time or case outcomes.
EDR leaders are hopeful that more health professionals and patients will participate in the coming years, said Melissa Parkerton, director of the Early Discussion and Resolution program for the Oregon Patient Safety Commission.
“Widespread adoption of a new approach like EDR requires not just a new process, but a new mindset,” she said. “This kind of cultural shift takes time. In Oregon, our fervent hope is that EDR will be embraced by every health care organization and professional.”
Efforts to raise awareness about the program are ongoing, adds Dr. Robert Dannenhoffer, a Roseburg, Ore., pediatrician and past president of the Oregon Medical Association.
“It’s a little hard to get the word out,” he said in an interview. “Most [doctors] are not going to be dealing with this on a regular basis. It’s a slow uptake. We’re working on better educating physicians.”
A practical model for independent docs?
Questions remain about whether disclosure, apology, and offer models work for independent physicians in small practices.
Dr. Thad L. Anderson, an ob.gyn in private practice in Dubuque, Iowa, strongly supports his state’s law, but he does not foresee the process having much impact on his practice. Larger health systems are generally more situated to utilize the process, he said.
“A hospital system that employees physicians is probably better suited to take advantage of this law,” he said. Independent doctors “don’t have the infrastructure. You don’t have the in-house lawyer. It’s probably a little more problematic for an individual to take advantage of the process compared to a bigger system.”
With this issue in mind, MACRMI in Massachusetts recently added an 800-physician, multispecialty outpatient group to its program analysis. The goal is to evaluate how the process operates within this type of setting and identify challenges and impediments, Dr. Woodward said. In addition, the alliance is encouraging commercial insurers to assist independent physicians in participating in the CARe model.
“The independent or small practice all benefit from the statute we passed – they have the [182-day waiting period] – but if they don’t have an internal risk management structure, than we’ve been working with insurers to set up a support structure,” he said. That way, “when a patient is unhappy with their care, there is a process that can support even a small practice in going through this and working through it with a patient.”
*This story was updated 1/26/2016.
On Twitter @legal_med
Iowa is the latest state to launch a unique strategy that aims to reduce medical malpractice lawsuits and bolster doctor-patient communication after poor outcomes, while encouraging swift resolution. The Communication and Optimal Resolution (CANDOR) law, which took effect in July, permits privileged discussions between Iowa physicians and patients after medical errors and allows for compensation offers, when appropriate.
After years of failing to enact traditional tort reform, the alternative method is hoped to decrease litigation costs and keep doctors and patients out of a flawed court system, said Dr. Michael McCoy, chair of the Iowa Medical Society Ad Hoc Tort Reform Task Force and a West Burlington obstetrician-gynecologist.
“If there’s an untoward outcome, the current system creates isolation,” he said. “The patients get alienated, and the doctors get stressed. That’s the exact opposite of what should happen. You should be able to talk to patients and feel protection. That’s what this law does.”
About 36 states have so-called “apology laws” that prohibit certain statements, expressions, or other evidence from being admissible in a malpractice lawsuit, according to data from Sorry Works!, an advocacy group that tracks apology statutes. Most apology laws cover expressions of empathy or sympathy, while some statutes protect admissions of fault. At least three states – including Iowa – go a step further, laying the foundation for early disclosure of errors, communication between physicians and patients, and negotiation of potential resolutions. Oregon’s Early Discussion and Resolution (EDR) law, which passed in 2013, allows both providers and patients to initiate conversations after adverse events, discuss what happened, and resolve the issue outside of court. Massachusetts’ 2012 disclosure, apology, and offer legislation includes a 182-day waiting period before lawsuits can be filed to allow for the process. Other states, including Washington, Texas, California, and Utah have expressed interest in enacting similar laws.
Efforts in Iowa, Oregon, and Massachusetts were driven by the successes of similar programs at the University of Illinois Hospital and Health Sciences System, Chicago and at the University of Michigan Health System, Ann Arbor, among others. Since the University of Michigan began its CANDOR program in 2001, average legal expenses per case have been cut in half, according to the UM data. The health system’s presuit claims have gone from 260 annually to about 100 per year. Pilot efforts by the University of Illinois (Chicago) Hospital and Health Sciences System since 2006 have increased adverse event reports from 1,500 per year to 10,000 while decreasing malpractice premiums by $15 million dollars since 2010.
But questions remain as to whether such approaches are successful when implemented on a state level. So far, the majority of data on early discussion, apology, and offer programs have come from closed systems, such as academic medical centers.
“We’re starting to look at how this works in an open system when you try to disseminate it across a state,” said Dr. Alan C. Woodward, chair of the Massachusetts Medical Society Committee on Professional Liability and cofounder of the Massachusetts Alliance for Communication and Resolution following Medical Injury (MACRMI). “If we can show this is an effective model in a statewide distribution, the hope is that multiple other states will follow suit, because this is clearly a better alternative for patients and providers and for patient safety improvement efforts.”
Massachusetts program showing promise
In Massachusetts, the state’s CANDOR law enabled MACRMI to roll out its Communication, Apology, and Resolution (CARe) model at six hospital pilot sites. MACRMI was formed as part of a research initiative led by Beth Israel Deaconess Medical Center, Boston, and the Massachusetts Medical Society, Waltham, and funded by a 2010 grant from the Federal Agency for Health Care Research and Quality.
Under the CARe model, participating health providers communicate with patients and families after an unanticipated adverse outcome, investigate and explain what happened, and where appropriate, apologize and offer fair financial compensation. Data collection in the 3-year study trial closed in December 2015, and the alliance plans to publish its findings in spring 2016. The model has greatly expedited case resolutions, Dr. Woodward said. A case in the CARe program can be resolved in 6 months or less, compared with a lawsuit that can take up to 5 years to resolve. By April 2015, the alliance had screened 856 cases. In three-quarters of cases, the care provided was deemed appropriate, Dr. Woodward said. Of these cases, 615 were closed after full-disclosure meetings with patients. Of the total cases screened, 122 cases were referred to an insurer for resolution, and 93 cases were awaiting further evaluation.
“It’s doing extraordinarily well,” Dr. Woodward said. “We’re resolving cases very efficiently in a short period of time, rather than using litigation.”
Outside the pilot program, health providers in Massachusetts are benefiting from the state’s 182-day waiting period before malpractice lawsuits can be filed, said Elizabeth A. Cushing, vice president of claims for CRICO, a medical liability insurer for the Harvard University medical community. The law allows physicians and insurers the opportunity to review a case and decide whether the complaint requires compensation. In cases that are not considered malpractice, explaining the underlying reasoning to patients or plaintiffs’ attorneys prevents some lawsuits from being filed, she said.
However, Ms. Cushing notes that not every case fits smoothly into the alternative model. Cases in which a poor outcome is immediately known, such as a surgical mishap, lend themselves to quicker investigation, disclosure, and remedy, she said. Claims of misdiagnosed cancer for instance, where the alleged mistake occurred years before, are more challenging.
“A lot of what we are seeing are alleged failures to diagnose things sooner, so it’s 2 years later and someone says, ‘You should have picked up on the fact that I had lung cancer 2 years ago,’ Ms. Cushing said. “Those situations get tricky. What did you know, when? Those cases are not as easily amenable to this process.”
Oregon program gaining speed
Meanwhile, Oregon officials are hoping that more doctors will soon participate in the state’s Early Discussion and Resolution program. In 2014 – its first year in operation – no individual physicians initiated participation in the program. Because EDR is voluntary, both parties must agree to participate for EDR to begin and either party can choose to stop participating at any time.
In 2014, Oregon patients and health providers filed 29 EDR requests to participate in the program, according to data from the Oregon Patient Safety Commission. Patients filed 21 notices and health care professionals filed eight notices. A majority of the eight notices filed by health professionals were issued by hospital representatives. No requests were filed by individual health providers. In 9 of the 21 patient-filed notices, at least one involved health professional accepted the patient’s request to participate in EDR. In the remaining 12 patient-filed notices, the involved health provider(s) declined the request. Data is not yet available on resolution time or case outcomes.
EDR leaders are hopeful that more health professionals and patients will participate in the coming years, said Melissa Parkerton, director of the Early Discussion and Resolution program for the Oregon Patient Safety Commission.
“Widespread adoption of a new approach like EDR requires not just a new process, but a new mindset,” she said. “This kind of cultural shift takes time. In Oregon, our fervent hope is that EDR will be embraced by every health care organization and professional.”
Efforts to raise awareness about the program are ongoing, adds Dr. Robert Dannenhoffer, a Roseburg, Ore., pediatrician and past president of the Oregon Medical Association.
“It’s a little hard to get the word out,” he said in an interview. “Most [doctors] are not going to be dealing with this on a regular basis. It’s a slow uptake. We’re working on better educating physicians.”
A practical model for independent docs?
Questions remain about whether disclosure, apology, and offer models work for independent physicians in small practices.
Dr. Thad L. Anderson, an ob.gyn in private practice in Dubuque, Iowa, strongly supports his state’s law, but he does not foresee the process having much impact on his practice. Larger health systems are generally more situated to utilize the process, he said.
“A hospital system that employees physicians is probably better suited to take advantage of this law,” he said. Independent doctors “don’t have the infrastructure. You don’t have the in-house lawyer. It’s probably a little more problematic for an individual to take advantage of the process compared to a bigger system.”
With this issue in mind, MACRMI in Massachusetts recently added an 800-physician, multispecialty outpatient group to its program analysis. The goal is to evaluate how the process operates within this type of setting and identify challenges and impediments, Dr. Woodward said. In addition, the alliance is encouraging commercial insurers to assist independent physicians in participating in the CARe model.
“The independent or small practice all benefit from the statute we passed – they have the [182-day waiting period] – but if they don’t have an internal risk management structure, than we’ve been working with insurers to set up a support structure,” he said. That way, “when a patient is unhappy with their care, there is a process that can support even a small practice in going through this and working through it with a patient.”
*This story was updated 1/26/2016.
On Twitter @legal_med
Gun safety: HIPAA change allows providers to report on some mentally ill patients
As part of the Obama administration’s efforts to reduce gun violence, physicians and certain other health care providers will be permitted to disclose to the National Instant Criminal Background Check System (NICS) the identities of mentally ill patients who could be a danger to themselves or others.
NICS is the federal system by which firearms sellers determine whether a potential buyer has a criminal record or is otherwise ineligible to purchase firearms.
The change – a modification to the HIPAA Privacy Rule – was published in the Federal Register on Jan. 5, the same day President Obama announced new executive actions that aim to expand background checks for firearm purchases and increase federal enforcement of gun laws.
The HIPAA modification, which takes effect in February, makes clear that certain covered entities are permitted to disclose information to the NICS, including the minimum identifying information necessary about patients who have been involuntarily committed to a mental institution or who have otherwise been determined by a lawful authority to be a danger to themselves or others.
The modification is tailored to preserve the patient-provider relationship and ensure that patients are not discouraged from seeking voluntary treatment, according to officials at the U.S. Department of Health & Human Services.
The rule applies only to a small subset of HIPAA-covered entities that either make mental health determinations that disqualify patients from having firearms or are designated by states to report the information to NICS. The modification does not apply to most treating health providers and does not permit reporting of diagnostic, clinical, or other mental health treatment information.
The scope of disclosed information under the final rule is quite limited, according to Gerald “Jud” E. DeLoss, a Chicago health law attorney and cybersecurity expert. Disclosure is restricted to limited demographic data and other information. In addition, state statutes or regulations regarding mental health information disclosure that are more stringent than HIPAA would still apply regardless of the final rule, he added.
“Further, while the final rule would apply to mental health information, substance abuse treatment information held by a federally assisted program covered under the confidentiality regulations set forth under 42 CFR Part 2 would still be protected,” Mr. DeLoss said in an interview.
The HIPAA change finalizes a 2014 proposed rule that was recommended as part of President Obama’s plan to strengthen the national background check system and remove unnecessary legal barriers that prevent states from making data available to the background check system.
“Due to a history of underreporting, the NICS has lacked complete information about all individuals who are prohibited by federal law from possessing or receiving a firearm,” Jocelyn Samuels, director of the HHS Office for Civil Rights, wrote in a blog post. “The modification announced today better enables the reporting of the identities of prohibited individuals to the background check system and is an important step toward improving the public’s safety while continuing to strongly protect individuals’ privacy interests.”
Psychiatrist Dr. Daniel J. Carlat of Tufts University, Boston, said that he does not see the new rule posing a major threat to patient confidentiality.
“HIPAA already allows us to share clinical information with law enforcement under certain circumstances where a patient is thought to pose some type of danger,” Dr. Carlat said in an interview. “This new rule adds another limited instance to that list. This is certainly something that we would want to add to the HIPAA Privacy notices that we show our patients so that they are fully aware of this potential breach of confidentiality.”
Dr. Jane M. Orient, executive director of Association of American Physicians and Surgeons, said that she disagrees. She believes the modification will be harmful to both doctors and patients.
“My impression is that this supposedly narrow intrusion will get wider and wider, and will inevitably damage the patient-physician relationship,” she said in an interview. “If a person has been adjudicated by law to be ineligible to possess a firearm, then the legal authority should report. The NICS is not going to do much good anyway – if a person is an imminent danger, he needs to be restrained, not put into a database.”
In addition to the HIPAA rule change, President Obama unveiled several other initiatives to combat gun violence and asked Congress for $500 million to increase mental health care service capacity and the behavioral health workforce.
The President outlined stronger efforts by federal agencies to monitor and prevent illegal gun sales. This includes an overhaul of NICS and the hiring of 230 additional FBI examiners to process background checks. The President’s budget for fiscal 2017 will include funding for 200 new Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF) agents and investigators. The ATF plans to dedicate $4 million and additional personnel to enhance the National Integrated Ballistics Information Network (NIBIN), a database that links violent crimes across jurisdictions.
Physician associations praised President Obama’s efforts to reduce gun violence and improve public safety.
“Gun violence is a public health threat to children, and like any epidemic, it can be prevented with the right interventions,” Dr. Benard Dreyer, president of the American Academy of Pediatrics, said in a statement. “Preventing gun violence is complicated, but achievable, and President Obama’s new executive actions are a welcome and needed first step in an environment where we have yet to see the enactment of commonsense federal gun violence prevention legislation.”
Dr. Renée Binder, president of the American Psychiatric Association, said the announcement highlights needed investments in mental health and necessary efforts to curb the epidemic of gun violence.
“Gun violence is a public health problem and needs to be addressed as such,” Dr. Binder said in a statement. “We support the president’s efforts to expand background checks, propose policies that respect physician-patient confidentiality, and increase funding for mental health services by $500 million. We will work with Congress to make that funding proposal a reality, as we also work with allies in Congress who are championing comprehensive mental health reform.”
On Twitter @legal_med
As part of the Obama administration’s efforts to reduce gun violence, physicians and certain other health care providers will be permitted to disclose to the National Instant Criminal Background Check System (NICS) the identities of mentally ill patients who could be a danger to themselves or others.
NICS is the federal system by which firearms sellers determine whether a potential buyer has a criminal record or is otherwise ineligible to purchase firearms.
The change – a modification to the HIPAA Privacy Rule – was published in the Federal Register on Jan. 5, the same day President Obama announced new executive actions that aim to expand background checks for firearm purchases and increase federal enforcement of gun laws.
The HIPAA modification, which takes effect in February, makes clear that certain covered entities are permitted to disclose information to the NICS, including the minimum identifying information necessary about patients who have been involuntarily committed to a mental institution or who have otherwise been determined by a lawful authority to be a danger to themselves or others.
The modification is tailored to preserve the patient-provider relationship and ensure that patients are not discouraged from seeking voluntary treatment, according to officials at the U.S. Department of Health & Human Services.
The rule applies only to a small subset of HIPAA-covered entities that either make mental health determinations that disqualify patients from having firearms or are designated by states to report the information to NICS. The modification does not apply to most treating health providers and does not permit reporting of diagnostic, clinical, or other mental health treatment information.
The scope of disclosed information under the final rule is quite limited, according to Gerald “Jud” E. DeLoss, a Chicago health law attorney and cybersecurity expert. Disclosure is restricted to limited demographic data and other information. In addition, state statutes or regulations regarding mental health information disclosure that are more stringent than HIPAA would still apply regardless of the final rule, he added.
“Further, while the final rule would apply to mental health information, substance abuse treatment information held by a federally assisted program covered under the confidentiality regulations set forth under 42 CFR Part 2 would still be protected,” Mr. DeLoss said in an interview.
The HIPAA change finalizes a 2014 proposed rule that was recommended as part of President Obama’s plan to strengthen the national background check system and remove unnecessary legal barriers that prevent states from making data available to the background check system.
“Due to a history of underreporting, the NICS has lacked complete information about all individuals who are prohibited by federal law from possessing or receiving a firearm,” Jocelyn Samuels, director of the HHS Office for Civil Rights, wrote in a blog post. “The modification announced today better enables the reporting of the identities of prohibited individuals to the background check system and is an important step toward improving the public’s safety while continuing to strongly protect individuals’ privacy interests.”
Psychiatrist Dr. Daniel J. Carlat of Tufts University, Boston, said that he does not see the new rule posing a major threat to patient confidentiality.
“HIPAA already allows us to share clinical information with law enforcement under certain circumstances where a patient is thought to pose some type of danger,” Dr. Carlat said in an interview. “This new rule adds another limited instance to that list. This is certainly something that we would want to add to the HIPAA Privacy notices that we show our patients so that they are fully aware of this potential breach of confidentiality.”
Dr. Jane M. Orient, executive director of Association of American Physicians and Surgeons, said that she disagrees. She believes the modification will be harmful to both doctors and patients.
“My impression is that this supposedly narrow intrusion will get wider and wider, and will inevitably damage the patient-physician relationship,” she said in an interview. “If a person has been adjudicated by law to be ineligible to possess a firearm, then the legal authority should report. The NICS is not going to do much good anyway – if a person is an imminent danger, he needs to be restrained, not put into a database.”
In addition to the HIPAA rule change, President Obama unveiled several other initiatives to combat gun violence and asked Congress for $500 million to increase mental health care service capacity and the behavioral health workforce.
The President outlined stronger efforts by federal agencies to monitor and prevent illegal gun sales. This includes an overhaul of NICS and the hiring of 230 additional FBI examiners to process background checks. The President’s budget for fiscal 2017 will include funding for 200 new Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF) agents and investigators. The ATF plans to dedicate $4 million and additional personnel to enhance the National Integrated Ballistics Information Network (NIBIN), a database that links violent crimes across jurisdictions.
Physician associations praised President Obama’s efforts to reduce gun violence and improve public safety.
“Gun violence is a public health threat to children, and like any epidemic, it can be prevented with the right interventions,” Dr. Benard Dreyer, president of the American Academy of Pediatrics, said in a statement. “Preventing gun violence is complicated, but achievable, and President Obama’s new executive actions are a welcome and needed first step in an environment where we have yet to see the enactment of commonsense federal gun violence prevention legislation.”
Dr. Renée Binder, president of the American Psychiatric Association, said the announcement highlights needed investments in mental health and necessary efforts to curb the epidemic of gun violence.
“Gun violence is a public health problem and needs to be addressed as such,” Dr. Binder said in a statement. “We support the president’s efforts to expand background checks, propose policies that respect physician-patient confidentiality, and increase funding for mental health services by $500 million. We will work with Congress to make that funding proposal a reality, as we also work with allies in Congress who are championing comprehensive mental health reform.”
On Twitter @legal_med
As part of the Obama administration’s efforts to reduce gun violence, physicians and certain other health care providers will be permitted to disclose to the National Instant Criminal Background Check System (NICS) the identities of mentally ill patients who could be a danger to themselves or others.
NICS is the federal system by which firearms sellers determine whether a potential buyer has a criminal record or is otherwise ineligible to purchase firearms.
The change – a modification to the HIPAA Privacy Rule – was published in the Federal Register on Jan. 5, the same day President Obama announced new executive actions that aim to expand background checks for firearm purchases and increase federal enforcement of gun laws.
The HIPAA modification, which takes effect in February, makes clear that certain covered entities are permitted to disclose information to the NICS, including the minimum identifying information necessary about patients who have been involuntarily committed to a mental institution or who have otherwise been determined by a lawful authority to be a danger to themselves or others.
The modification is tailored to preserve the patient-provider relationship and ensure that patients are not discouraged from seeking voluntary treatment, according to officials at the U.S. Department of Health & Human Services.
The rule applies only to a small subset of HIPAA-covered entities that either make mental health determinations that disqualify patients from having firearms or are designated by states to report the information to NICS. The modification does not apply to most treating health providers and does not permit reporting of diagnostic, clinical, or other mental health treatment information.
The scope of disclosed information under the final rule is quite limited, according to Gerald “Jud” E. DeLoss, a Chicago health law attorney and cybersecurity expert. Disclosure is restricted to limited demographic data and other information. In addition, state statutes or regulations regarding mental health information disclosure that are more stringent than HIPAA would still apply regardless of the final rule, he added.
“Further, while the final rule would apply to mental health information, substance abuse treatment information held by a federally assisted program covered under the confidentiality regulations set forth under 42 CFR Part 2 would still be protected,” Mr. DeLoss said in an interview.
The HIPAA change finalizes a 2014 proposed rule that was recommended as part of President Obama’s plan to strengthen the national background check system and remove unnecessary legal barriers that prevent states from making data available to the background check system.
“Due to a history of underreporting, the NICS has lacked complete information about all individuals who are prohibited by federal law from possessing or receiving a firearm,” Jocelyn Samuels, director of the HHS Office for Civil Rights, wrote in a blog post. “The modification announced today better enables the reporting of the identities of prohibited individuals to the background check system and is an important step toward improving the public’s safety while continuing to strongly protect individuals’ privacy interests.”
Psychiatrist Dr. Daniel J. Carlat of Tufts University, Boston, said that he does not see the new rule posing a major threat to patient confidentiality.
“HIPAA already allows us to share clinical information with law enforcement under certain circumstances where a patient is thought to pose some type of danger,” Dr. Carlat said in an interview. “This new rule adds another limited instance to that list. This is certainly something that we would want to add to the HIPAA Privacy notices that we show our patients so that they are fully aware of this potential breach of confidentiality.”
Dr. Jane M. Orient, executive director of Association of American Physicians and Surgeons, said that she disagrees. She believes the modification will be harmful to both doctors and patients.
“My impression is that this supposedly narrow intrusion will get wider and wider, and will inevitably damage the patient-physician relationship,” she said in an interview. “If a person has been adjudicated by law to be ineligible to possess a firearm, then the legal authority should report. The NICS is not going to do much good anyway – if a person is an imminent danger, he needs to be restrained, not put into a database.”
In addition to the HIPAA rule change, President Obama unveiled several other initiatives to combat gun violence and asked Congress for $500 million to increase mental health care service capacity and the behavioral health workforce.
The President outlined stronger efforts by federal agencies to monitor and prevent illegal gun sales. This includes an overhaul of NICS and the hiring of 230 additional FBI examiners to process background checks. The President’s budget for fiscal 2017 will include funding for 200 new Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF) agents and investigators. The ATF plans to dedicate $4 million and additional personnel to enhance the National Integrated Ballistics Information Network (NIBIN), a database that links violent crimes across jurisdictions.
Physician associations praised President Obama’s efforts to reduce gun violence and improve public safety.
“Gun violence is a public health threat to children, and like any epidemic, it can be prevented with the right interventions,” Dr. Benard Dreyer, president of the American Academy of Pediatrics, said in a statement. “Preventing gun violence is complicated, but achievable, and President Obama’s new executive actions are a welcome and needed first step in an environment where we have yet to see the enactment of commonsense federal gun violence prevention legislation.”
Dr. Renée Binder, president of the American Psychiatric Association, said the announcement highlights needed investments in mental health and necessary efforts to curb the epidemic of gun violence.
“Gun violence is a public health problem and needs to be addressed as such,” Dr. Binder said in a statement. “We support the president’s efforts to expand background checks, propose policies that respect physician-patient confidentiality, and increase funding for mental health services by $500 million. We will work with Congress to make that funding proposal a reality, as we also work with allies in Congress who are championing comprehensive mental health reform.”
On Twitter @legal_med
Ask patients about military service, lawyers urge
Do you ask your patients, “Have you served in the military?”
That simple inquiry could have a major impact on whether military veterans get needed services and legal assistance, Patricia E. Roberts, director of the Lewis B. Puller Jr. Veterans Benefits Clinic at William & Mary Law School, Williamsburg, Va., said at a meeting sponsored by America Bar Association.
When encountering veterans in need of benefits or legal services, physicians also can help by contacting a local American Legion, VFW, or local U.S. Department of Veterans Affairs (VA) regional office, William A. Burke, a maritime lawyer based in Norfolk, Va., said at the meeting.
A growing number of law school clinics such as the Lewis B. Puller Jr. Benefits Clinic also address veterans’ legal needs, largely at no expense to the veteran. The newly created National Law School Veterans Clinic Coalition includes 30 such law school clinics that have veterans assistance programs.
Strong partnerships between physicians and lawyers are essential in linking injured veterans to needed medical treatment and financial assistance, Ms. Roberts and other legal experts said. The VA identifies legal needs as among the most significant unmet needs of homeless and poor veterans.
Physicians and lawyers need to work together or at least be in communication with one another, Kenneth J. Goldsmith, legislative counsel and director of state legislation for the ABA governmental affairs office, said at the meeting. Physicians are frequently the first stop, as many veterans have easier access to medical professionals or may have more trusting relationships with their doctors.
The lawyers acknowledged that physicians inexperienced with the process of helping patients apply for benefits through the VA can be hesitant to get involved. Common misconceptions include that the documentation process is lengthy, that the burden of evidence must be high, and that physicians may be called as witnesses.
Ms. Roberts stressed that the burden of evidence that a disability is linked to military service does not have to be absolute. In veterans’ law, a doctor has to conclude that there is a 50% likelihood that a disability is service connected or that it is “just as likely as not.”
“With that standard, which I think is lower than in other arenas ... medical professionals could have even more of an impact than they realize,” she said.
To receive disability compensation that a veteran earns through service, the veteran must prove: a current disability, that the incident that caused the injury occurred during active duty, and that there is a nexus between the current disability and the veteran’s active duty service.
In straightforward cases, a doctor needs to fill out the VA’s disability questionnaire. No further VA contact or participation is necessary, Ms. Roberts said. More time is required if a medical records review is needed or if a veteran is claiming multiple injuries linked to military service.
Not every case is simple, however. In some instances, it may be difficult for health providers to parse psychological injuries of conflict from a mental illness the veteran had before serving, Mr. Burke said. Making matters more complicated is the potential aggravation of a prior mental illness during service. “There’s really a latticework of comorbidities at play here – PTSD, depression, anxiety, sleep disorders, substance abuse problems – and ... substance abuse is seen as a symptom [by the VA] rather than a disorder in and of itself. It’s a real challenge.”
Veterans stuck in the backlog of claims often are halted because of delayed physician documentation, which can be significant in certain complex cases, according to Mr. Goldsmith.
“You’re talking about when a veteran comes in claiming 36 different kinds of injuries,” he said. “The doctor is not compensated to [fill out forms]. But doctors who are savvy, [who] do understand these issues, and what the VA’s interested in seeing, can go through it more expeditiously.”
*Correction, 1/5/2016: A previous version of this article misstated William A. Burke's title.
On Twitter @legal_med
Do you ask your patients, “Have you served in the military?”
That simple inquiry could have a major impact on whether military veterans get needed services and legal assistance, Patricia E. Roberts, director of the Lewis B. Puller Jr. Veterans Benefits Clinic at William & Mary Law School, Williamsburg, Va., said at a meeting sponsored by America Bar Association.
When encountering veterans in need of benefits or legal services, physicians also can help by contacting a local American Legion, VFW, or local U.S. Department of Veterans Affairs (VA) regional office, William A. Burke, a maritime lawyer based in Norfolk, Va., said at the meeting.
A growing number of law school clinics such as the Lewis B. Puller Jr. Benefits Clinic also address veterans’ legal needs, largely at no expense to the veteran. The newly created National Law School Veterans Clinic Coalition includes 30 such law school clinics that have veterans assistance programs.
Strong partnerships between physicians and lawyers are essential in linking injured veterans to needed medical treatment and financial assistance, Ms. Roberts and other legal experts said. The VA identifies legal needs as among the most significant unmet needs of homeless and poor veterans.
Physicians and lawyers need to work together or at least be in communication with one another, Kenneth J. Goldsmith, legislative counsel and director of state legislation for the ABA governmental affairs office, said at the meeting. Physicians are frequently the first stop, as many veterans have easier access to medical professionals or may have more trusting relationships with their doctors.
The lawyers acknowledged that physicians inexperienced with the process of helping patients apply for benefits through the VA can be hesitant to get involved. Common misconceptions include that the documentation process is lengthy, that the burden of evidence must be high, and that physicians may be called as witnesses.
Ms. Roberts stressed that the burden of evidence that a disability is linked to military service does not have to be absolute. In veterans’ law, a doctor has to conclude that there is a 50% likelihood that a disability is service connected or that it is “just as likely as not.”
“With that standard, which I think is lower than in other arenas ... medical professionals could have even more of an impact than they realize,” she said.
To receive disability compensation that a veteran earns through service, the veteran must prove: a current disability, that the incident that caused the injury occurred during active duty, and that there is a nexus between the current disability and the veteran’s active duty service.
In straightforward cases, a doctor needs to fill out the VA’s disability questionnaire. No further VA contact or participation is necessary, Ms. Roberts said. More time is required if a medical records review is needed or if a veteran is claiming multiple injuries linked to military service.
Not every case is simple, however. In some instances, it may be difficult for health providers to parse psychological injuries of conflict from a mental illness the veteran had before serving, Mr. Burke said. Making matters more complicated is the potential aggravation of a prior mental illness during service. “There’s really a latticework of comorbidities at play here – PTSD, depression, anxiety, sleep disorders, substance abuse problems – and ... substance abuse is seen as a symptom [by the VA] rather than a disorder in and of itself. It’s a real challenge.”
Veterans stuck in the backlog of claims often are halted because of delayed physician documentation, which can be significant in certain complex cases, according to Mr. Goldsmith.
“You’re talking about when a veteran comes in claiming 36 different kinds of injuries,” he said. “The doctor is not compensated to [fill out forms]. But doctors who are savvy, [who] do understand these issues, and what the VA’s interested in seeing, can go through it more expeditiously.”
*Correction, 1/5/2016: A previous version of this article misstated William A. Burke's title.
On Twitter @legal_med
Do you ask your patients, “Have you served in the military?”
That simple inquiry could have a major impact on whether military veterans get needed services and legal assistance, Patricia E. Roberts, director of the Lewis B. Puller Jr. Veterans Benefits Clinic at William & Mary Law School, Williamsburg, Va., said at a meeting sponsored by America Bar Association.
When encountering veterans in need of benefits or legal services, physicians also can help by contacting a local American Legion, VFW, or local U.S. Department of Veterans Affairs (VA) regional office, William A. Burke, a maritime lawyer based in Norfolk, Va., said at the meeting.
A growing number of law school clinics such as the Lewis B. Puller Jr. Benefits Clinic also address veterans’ legal needs, largely at no expense to the veteran. The newly created National Law School Veterans Clinic Coalition includes 30 such law school clinics that have veterans assistance programs.
Strong partnerships between physicians and lawyers are essential in linking injured veterans to needed medical treatment and financial assistance, Ms. Roberts and other legal experts said. The VA identifies legal needs as among the most significant unmet needs of homeless and poor veterans.
Physicians and lawyers need to work together or at least be in communication with one another, Kenneth J. Goldsmith, legislative counsel and director of state legislation for the ABA governmental affairs office, said at the meeting. Physicians are frequently the first stop, as many veterans have easier access to medical professionals or may have more trusting relationships with their doctors.
The lawyers acknowledged that physicians inexperienced with the process of helping patients apply for benefits through the VA can be hesitant to get involved. Common misconceptions include that the documentation process is lengthy, that the burden of evidence must be high, and that physicians may be called as witnesses.
Ms. Roberts stressed that the burden of evidence that a disability is linked to military service does not have to be absolute. In veterans’ law, a doctor has to conclude that there is a 50% likelihood that a disability is service connected or that it is “just as likely as not.”
“With that standard, which I think is lower than in other arenas ... medical professionals could have even more of an impact than they realize,” she said.
To receive disability compensation that a veteran earns through service, the veteran must prove: a current disability, that the incident that caused the injury occurred during active duty, and that there is a nexus between the current disability and the veteran’s active duty service.
In straightforward cases, a doctor needs to fill out the VA’s disability questionnaire. No further VA contact or participation is necessary, Ms. Roberts said. More time is required if a medical records review is needed or if a veteran is claiming multiple injuries linked to military service.
Not every case is simple, however. In some instances, it may be difficult for health providers to parse psychological injuries of conflict from a mental illness the veteran had before serving, Mr. Burke said. Making matters more complicated is the potential aggravation of a prior mental illness during service. “There’s really a latticework of comorbidities at play here – PTSD, depression, anxiety, sleep disorders, substance abuse problems – and ... substance abuse is seen as a symptom [by the VA] rather than a disorder in and of itself. It’s a real challenge.”
Veterans stuck in the backlog of claims often are halted because of delayed physician documentation, which can be significant in certain complex cases, according to Mr. Goldsmith.
“You’re talking about when a veteran comes in claiming 36 different kinds of injuries,” he said. “The doctor is not compensated to [fill out forms]. But doctors who are savvy, [who] do understand these issues, and what the VA’s interested in seeing, can go through it more expeditiously.”
*Correction, 1/5/2016: A previous version of this article misstated William A. Burke's title.
On Twitter @legal_med
EXPERT ANALYSIS FROM THE WASHINGTON HEALTH LAW SUMMIT
White House launches new plan to combat multidrug-resistant TB
The Obama administration has announced a new action plan to combat the rise of multidrug-resistant tuberculosis (MDR-TB).
The plan, published Dec. 22, outlines three primary goals to accomplish between 2016 and 2020: strengthening domestic capacity to treat the disease, improving international ability and collaboration, and accelerating research and treatment of MDR-TB.
To strengthen state and local capacity to prevent transmission of TB, the action plan proposes improving surge capacity for rapid response to individual patients, patient clusters, and larger outbreaks of drug-resistant TB, as well as advancing the treatment of latent TB infection and disease among vulnerable populations.
Disease surveillance will be upgraded to “gather, store, analyze, and report electronic data on drug-resistant TB.” As part of the effort, the Centers for Disease Control and Prevention will increase its capacity to use “whole-genome sequencing to elucidate paths of transmission, identify recent transmission, and identify emerging patterns of resistance,” according to the action plan.
Taken with other preventive activities, the measures are estimated to result in a 15% reduction in newly diagnosed MDR-TB cases by 2020, as specified in the National Action Plan for Combating Antibiotic-Resistant Bacteria.
Additionally, the U.S. government will work with partners including the World Health Organization and the Stop TB Partnership to support countries in developing new approaches to MDR-TB. The focus is expected to initiate treatment for an additional 200,000 people with MDR-TB during 2015-2019; an estimated 360,000 people are currently treated under the U.S. government’s Global TB Strategy. The action plan also aims to improve access to patient-centered diagnostic and treatment services in countries with limited health care resources.
Developing a TB vaccine is a key initiative. The plan details that the National Institutes of Health and the CDC will focus on expanding the dialogue among basic scientists, funders, and vaccine developers to identify strategies for vaccine development and preventive drugs.
The plan also urges focused research into the discovery of biological markers that indicate early response to therapy or protection against TB.
Pulmonologist Daniel R. Ouellette of Henry Ford Hospital in Detroit, said “the three goals are certainly worthy,” but the plan does not address the budgetary measures to be taken to increase domestic capacity to combat TB nor does it explain where resources will be found to encourage the development of new medications.
“How much money are we willing to spend in addition to what we spend now in treating general TB? he asked in an interview. “How are we going to incentivize industry to develop new drugs? It all sounds really good, but all of this is going to cost money. Where are the dollars that go with that?” said Dr. Ouellette, who is chair of the Guideline Oversight Committee for CHEST (the American College of Chest Physicians).
The national action plan states that all activities noted will be subject to budgetary constraints and other approvals, including the “weighing of priorities and available resources by the administration in formulating its annual budget and by Congress in legislating appropriations.”
TB causes the deaths of more than 1.5 million people worldwide annually, according to White House data. Nearly one-third of the world’s population is thought to be infected with Mycobacterium tuberculosis; each year, more than 9.5 million develop active TB and about 480,000 people develop MDR-TB. Fewer than 20% with MDR-TB receive appropriate therapy, and less than half are effectively treated.
On Twitter @legal_med
The Obama administration has announced a new action plan to combat the rise of multidrug-resistant tuberculosis (MDR-TB).
The plan, published Dec. 22, outlines three primary goals to accomplish between 2016 and 2020: strengthening domestic capacity to treat the disease, improving international ability and collaboration, and accelerating research and treatment of MDR-TB.
To strengthen state and local capacity to prevent transmission of TB, the action plan proposes improving surge capacity for rapid response to individual patients, patient clusters, and larger outbreaks of drug-resistant TB, as well as advancing the treatment of latent TB infection and disease among vulnerable populations.
Disease surveillance will be upgraded to “gather, store, analyze, and report electronic data on drug-resistant TB.” As part of the effort, the Centers for Disease Control and Prevention will increase its capacity to use “whole-genome sequencing to elucidate paths of transmission, identify recent transmission, and identify emerging patterns of resistance,” according to the action plan.
Taken with other preventive activities, the measures are estimated to result in a 15% reduction in newly diagnosed MDR-TB cases by 2020, as specified in the National Action Plan for Combating Antibiotic-Resistant Bacteria.
Additionally, the U.S. government will work with partners including the World Health Organization and the Stop TB Partnership to support countries in developing new approaches to MDR-TB. The focus is expected to initiate treatment for an additional 200,000 people with MDR-TB during 2015-2019; an estimated 360,000 people are currently treated under the U.S. government’s Global TB Strategy. The action plan also aims to improve access to patient-centered diagnostic and treatment services in countries with limited health care resources.
Developing a TB vaccine is a key initiative. The plan details that the National Institutes of Health and the CDC will focus on expanding the dialogue among basic scientists, funders, and vaccine developers to identify strategies for vaccine development and preventive drugs.
The plan also urges focused research into the discovery of biological markers that indicate early response to therapy or protection against TB.
Pulmonologist Daniel R. Ouellette of Henry Ford Hospital in Detroit, said “the three goals are certainly worthy,” but the plan does not address the budgetary measures to be taken to increase domestic capacity to combat TB nor does it explain where resources will be found to encourage the development of new medications.
“How much money are we willing to spend in addition to what we spend now in treating general TB? he asked in an interview. “How are we going to incentivize industry to develop new drugs? It all sounds really good, but all of this is going to cost money. Where are the dollars that go with that?” said Dr. Ouellette, who is chair of the Guideline Oversight Committee for CHEST (the American College of Chest Physicians).
The national action plan states that all activities noted will be subject to budgetary constraints and other approvals, including the “weighing of priorities and available resources by the administration in formulating its annual budget and by Congress in legislating appropriations.”
TB causes the deaths of more than 1.5 million people worldwide annually, according to White House data. Nearly one-third of the world’s population is thought to be infected with Mycobacterium tuberculosis; each year, more than 9.5 million develop active TB and about 480,000 people develop MDR-TB. Fewer than 20% with MDR-TB receive appropriate therapy, and less than half are effectively treated.
On Twitter @legal_med
The Obama administration has announced a new action plan to combat the rise of multidrug-resistant tuberculosis (MDR-TB).
The plan, published Dec. 22, outlines three primary goals to accomplish between 2016 and 2020: strengthening domestic capacity to treat the disease, improving international ability and collaboration, and accelerating research and treatment of MDR-TB.
To strengthen state and local capacity to prevent transmission of TB, the action plan proposes improving surge capacity for rapid response to individual patients, patient clusters, and larger outbreaks of drug-resistant TB, as well as advancing the treatment of latent TB infection and disease among vulnerable populations.
Disease surveillance will be upgraded to “gather, store, analyze, and report electronic data on drug-resistant TB.” As part of the effort, the Centers for Disease Control and Prevention will increase its capacity to use “whole-genome sequencing to elucidate paths of transmission, identify recent transmission, and identify emerging patterns of resistance,” according to the action plan.
Taken with other preventive activities, the measures are estimated to result in a 15% reduction in newly diagnosed MDR-TB cases by 2020, as specified in the National Action Plan for Combating Antibiotic-Resistant Bacteria.
Additionally, the U.S. government will work with partners including the World Health Organization and the Stop TB Partnership to support countries in developing new approaches to MDR-TB. The focus is expected to initiate treatment for an additional 200,000 people with MDR-TB during 2015-2019; an estimated 360,000 people are currently treated under the U.S. government’s Global TB Strategy. The action plan also aims to improve access to patient-centered diagnostic and treatment services in countries with limited health care resources.
Developing a TB vaccine is a key initiative. The plan details that the National Institutes of Health and the CDC will focus on expanding the dialogue among basic scientists, funders, and vaccine developers to identify strategies for vaccine development and preventive drugs.
The plan also urges focused research into the discovery of biological markers that indicate early response to therapy or protection against TB.
Pulmonologist Daniel R. Ouellette of Henry Ford Hospital in Detroit, said “the three goals are certainly worthy,” but the plan does not address the budgetary measures to be taken to increase domestic capacity to combat TB nor does it explain where resources will be found to encourage the development of new medications.
“How much money are we willing to spend in addition to what we spend now in treating general TB? he asked in an interview. “How are we going to incentivize industry to develop new drugs? It all sounds really good, but all of this is going to cost money. Where are the dollars that go with that?” said Dr. Ouellette, who is chair of the Guideline Oversight Committee for CHEST (the American College of Chest Physicians).
The national action plan states that all activities noted will be subject to budgetary constraints and other approvals, including the “weighing of priorities and available resources by the administration in formulating its annual budget and by Congress in legislating appropriations.”
TB causes the deaths of more than 1.5 million people worldwide annually, according to White House data. Nearly one-third of the world’s population is thought to be infected with Mycobacterium tuberculosis; each year, more than 9.5 million develop active TB and about 480,000 people develop MDR-TB. Fewer than 20% with MDR-TB receive appropriate therapy, and less than half are effectively treated.
On Twitter @legal_med
Key clinical point: A new action plan to combat the rise of multidrug-resistant tuberculosis focuses on strengthening the domestic capacity to treat the MDR-TB, improving international capacity and collaboration to combat the disease, and accelerating research and treatment developments.
Physician Compare: Expanded data cause concern
Potentially inaccurate data posted on the federal Physician Compare website could misinform patients and lead to incorrect assumptions about the quality of care individual doctors provide.
With the most recent update of Physician Compare, the Centers for Medicare & Medicaid Services for the first time has posted individual physician performance scores on the 40,000 individual health care professionals who are part of the Physician Quality Reporting System (PQRS).
“Given the widespread accuracy issues with the 2014 PQRS calculations, the newly released information is premature,” American Medical Association President Steven J. Stack said in a statement. “The data inaccuracies and difficulties with CMS’s processes grew over the last couple of months and, while CMS has acknowledged these problems, it has failed to address the underlying issues. Most importantly, consumers visiting the Physician Compare website are likely to get a false impression that it provides accurate quality information for all physicians, when in fact, due to significant data problems, the newly added information covers only about 40,000 physicians.”
Although the concept of Physician Compare makes sense, the CMS needs to resolve data inconsistencies and improve how the information is being presented before posting new information to the site, said Dr. Wanda Filer, president of the American Academy of Family Physicians (AAFP). Performance scores on each measure are displayed on Physician Compare as stars followed by a percent, with each star representing 20%.
“A star rating system is too simplistic to provide for informed decisions [and] doesn’t reflect the complexity or context of care that undergirds those measures,” Dr. Filer said in an interview. “Given that complexity, it is likely that inaccurate data will be attributed to a physician’s care.”
In an effort to reduce inaccuracies, the AAFP had called for an extended period – from the current 30 days to 90 – for physicians to review their data before the data are published, Dr. Filer said.
She added that the CMS takes too long to communicate with physicians about their performance scores. Doctors do not receive reports for 6-9 months, reducing the opportunity for them to improve their performance before the next reporting period, she said.
“CMS needs to provide feedback to physicians much sooner so improvement can take place before their data are posted on the next Physician Compare,” Dr. Filer said. “Moreover, CMS must do a better job educating physicians about this website and their opportunity to review and correct any accuracies in what is reported.”
In addition to posting new PQRS measures on Physician Compare, the CMS also has posted 2014 data from group practices that report patient experience measures through the Consumer Assessment of Healthcare Providers and Systems (CAHPS) for PQRS survey. The CAHPS survey measures Medicare patients’ feedback about their care experiences. Updated performance scores for accountable care organizations, including clinical quality of care and patient experience measures for Shared Savings Program ACOs and 20 Pioneer ACOs were also added.
The reporting of the new data may be welcome for some physicians or group practices that are achieving high scores under quality-reporting programs, noted Philadelphia health law attorney Karl A. Thallner Jr.
“However, a physician who does not participate or who does not have high scores may feel that the reported data does not provide an accurate picture of the quality of care that he/she provides to patients, and therefore may be misleading to consumers,” Mr. Thallner said in an interview. “Also, physicians may not report data consistently, making differences between physicians less relevant.”
On Twitter@legal_med
Potentially inaccurate data posted on the federal Physician Compare website could misinform patients and lead to incorrect assumptions about the quality of care individual doctors provide.
With the most recent update of Physician Compare, the Centers for Medicare & Medicaid Services for the first time has posted individual physician performance scores on the 40,000 individual health care professionals who are part of the Physician Quality Reporting System (PQRS).
“Given the widespread accuracy issues with the 2014 PQRS calculations, the newly released information is premature,” American Medical Association President Steven J. Stack said in a statement. “The data inaccuracies and difficulties with CMS’s processes grew over the last couple of months and, while CMS has acknowledged these problems, it has failed to address the underlying issues. Most importantly, consumers visiting the Physician Compare website are likely to get a false impression that it provides accurate quality information for all physicians, when in fact, due to significant data problems, the newly added information covers only about 40,000 physicians.”
Although the concept of Physician Compare makes sense, the CMS needs to resolve data inconsistencies and improve how the information is being presented before posting new information to the site, said Dr. Wanda Filer, president of the American Academy of Family Physicians (AAFP). Performance scores on each measure are displayed on Physician Compare as stars followed by a percent, with each star representing 20%.
“A star rating system is too simplistic to provide for informed decisions [and] doesn’t reflect the complexity or context of care that undergirds those measures,” Dr. Filer said in an interview. “Given that complexity, it is likely that inaccurate data will be attributed to a physician’s care.”
In an effort to reduce inaccuracies, the AAFP had called for an extended period – from the current 30 days to 90 – for physicians to review their data before the data are published, Dr. Filer said.
She added that the CMS takes too long to communicate with physicians about their performance scores. Doctors do not receive reports for 6-9 months, reducing the opportunity for them to improve their performance before the next reporting period, she said.
“CMS needs to provide feedback to physicians much sooner so improvement can take place before their data are posted on the next Physician Compare,” Dr. Filer said. “Moreover, CMS must do a better job educating physicians about this website and their opportunity to review and correct any accuracies in what is reported.”
In addition to posting new PQRS measures on Physician Compare, the CMS also has posted 2014 data from group practices that report patient experience measures through the Consumer Assessment of Healthcare Providers and Systems (CAHPS) for PQRS survey. The CAHPS survey measures Medicare patients’ feedback about their care experiences. Updated performance scores for accountable care organizations, including clinical quality of care and patient experience measures for Shared Savings Program ACOs and 20 Pioneer ACOs were also added.
The reporting of the new data may be welcome for some physicians or group practices that are achieving high scores under quality-reporting programs, noted Philadelphia health law attorney Karl A. Thallner Jr.
“However, a physician who does not participate or who does not have high scores may feel that the reported data does not provide an accurate picture of the quality of care that he/she provides to patients, and therefore may be misleading to consumers,” Mr. Thallner said in an interview. “Also, physicians may not report data consistently, making differences between physicians less relevant.”
On Twitter@legal_med
Potentially inaccurate data posted on the federal Physician Compare website could misinform patients and lead to incorrect assumptions about the quality of care individual doctors provide.
With the most recent update of Physician Compare, the Centers for Medicare & Medicaid Services for the first time has posted individual physician performance scores on the 40,000 individual health care professionals who are part of the Physician Quality Reporting System (PQRS).
“Given the widespread accuracy issues with the 2014 PQRS calculations, the newly released information is premature,” American Medical Association President Steven J. Stack said in a statement. “The data inaccuracies and difficulties with CMS’s processes grew over the last couple of months and, while CMS has acknowledged these problems, it has failed to address the underlying issues. Most importantly, consumers visiting the Physician Compare website are likely to get a false impression that it provides accurate quality information for all physicians, when in fact, due to significant data problems, the newly added information covers only about 40,000 physicians.”
Although the concept of Physician Compare makes sense, the CMS needs to resolve data inconsistencies and improve how the information is being presented before posting new information to the site, said Dr. Wanda Filer, president of the American Academy of Family Physicians (AAFP). Performance scores on each measure are displayed on Physician Compare as stars followed by a percent, with each star representing 20%.
“A star rating system is too simplistic to provide for informed decisions [and] doesn’t reflect the complexity or context of care that undergirds those measures,” Dr. Filer said in an interview. “Given that complexity, it is likely that inaccurate data will be attributed to a physician’s care.”
In an effort to reduce inaccuracies, the AAFP had called for an extended period – from the current 30 days to 90 – for physicians to review their data before the data are published, Dr. Filer said.
She added that the CMS takes too long to communicate with physicians about their performance scores. Doctors do not receive reports for 6-9 months, reducing the opportunity for them to improve their performance before the next reporting period, she said.
“CMS needs to provide feedback to physicians much sooner so improvement can take place before their data are posted on the next Physician Compare,” Dr. Filer said. “Moreover, CMS must do a better job educating physicians about this website and their opportunity to review and correct any accuracies in what is reported.”
In addition to posting new PQRS measures on Physician Compare, the CMS also has posted 2014 data from group practices that report patient experience measures through the Consumer Assessment of Healthcare Providers and Systems (CAHPS) for PQRS survey. The CAHPS survey measures Medicare patients’ feedback about their care experiences. Updated performance scores for accountable care organizations, including clinical quality of care and patient experience measures for Shared Savings Program ACOs and 20 Pioneer ACOs were also added.
The reporting of the new data may be welcome for some physicians or group practices that are achieving high scores under quality-reporting programs, noted Philadelphia health law attorney Karl A. Thallner Jr.
“However, a physician who does not participate or who does not have high scores may feel that the reported data does not provide an accurate picture of the quality of care that he/she provides to patients, and therefore may be misleading to consumers,” Mr. Thallner said in an interview. “Also, physicians may not report data consistently, making differences between physicians less relevant.”
On Twitter@legal_med