User login
House budget includes SGR patch; permanent fix sails through committees
WASHINGTON - Congress has moved the ball forward on permanently replacing the Medicare Sustainable Growth Rate formula, but with time short for a fix by year's end the House has voted to approve a temporary 3-month reprieve from the 20% cut due to take effect Jan. 1.
In a 332-94 vote, with eight abstentions, the House on Dec. 12 approved the Bipartisan Budget Act of 2013, a wide-ranging budget agreement that includes the 3-month patch. The bill also would increase physician pay by 0.5% through March.
The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. The fix would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
It also would extend the 2% sequestration cuts for Medicare providers by 2 years, from 2021 to 2023.
The Senate has yet to consider the budget package including the SGR patch; it is expected to do so before its holiday recess. President Obama has said that he supports the deal.
The agreement, brokered by House Budget Committee Chairman Paul Ryan (R-Wisc.) and Senate Budget Committee Chairman Patty Murray (D-Wash.), adds about $63 billion in discretionary federal spending over 2 years and makes targeted cuts and fee hikes to bring about overall deficit reduction of about $23 billion.
Although physician groups aren't thrilled about the continuation of the Medicare cuts under sequestration, most favor the temporary SGR reprieve and the restoration of some funding to federal health programs.
Dr. Ardis Dee Hoven, president of the American Medical Association, said that the continuation of the sequester cuts in Medicare is "frustrating" and poses the risk of destabilizing physician practices. "The concept of the sequester is probably not the best way to rein in spending," she said.
Dr. Clifford A. Hudis, president of the American Society of Clinical Oncology, praised the agreement for providing funds that could restore cuts in medical research and cancer care at the National Institutes of Health. He expressed disappointment, however, that the bill does not reverse cuts to Medicare, including reductions in the payments for physician-administered drugs under Medicare Part B.
"Oncologists are doing everything possible to continue providing care for Medicare patients, but this reduction has forced many in private practice to send patients to hospitals for chemotherapy because they cannot afford to administer these drugs in their office," Dr. Hudis said in a statement.
Physician organizations viewed the patch as necessary while Congress continues to work on a permanent SGR fix. Both the House and Senate took steps toward that goal on Dec. 12.
The House Ways and Means Committee voted 39-0 to approve its replacement proposal, which essentially adds on to the bill approved by the House Energy and Commerce Committee in July.
"This may not be the final step, it's a very important step forward," said Rep. Kevin Brady (R-Tex.), chairman of the Ways and Means Health Subcommittee.
The House did not address how to pay for the permanent replacement. House Ways and Means Chairman Dave Camp (R-Mich.) noted that the Congressional Budget Office has estimated that it will cost $116 billion over 10 years to repeal the SGR, which is "more than half the cost 2 years ago." Even though that is the lowest estimate ever, "I am of no illusion that finding pay-fors will be an easy task," he said.
The Senate Finance Committee also did not include a way to pay for repeal in its proposal.
The bill had widespread bipartisan support in the committee, but some Senators raised concerns about the lack of a funding mechanism. Sen. Pat Roberts (R-Kan.) said that he wouldn’t support the bill until he could see how it would be funded.
Sen. Orrin Hatch (R- Utah), the committee's top-ranking Republican, said that the offsets would be worked out once the bill had cleared the initial policymaking phase.
"This bill will be offset, period, or it's not going to go through both houses," he said. "This bill will be paid for."
The panel agreed to add a provision aimed at expanding access to community mental health services. The amendment, offered by Sen. Debbie Stabenow (D-Mich.) and Sen. Roy Blunt (R-Mo.), would create pilot programs in 10 states to ensure that community behavioral health clinics offer a full range of mental health services, including 24-hour crisis care, substance abuse treatment, and expanded support for families.
Physician groups praised the continued congressional action.
The AMA "strongly commends members of the House Ways and Means Committee and the Senate Finance Committee for the tremendous progress they have made toward repealing Medicare's failed Sustainable Growth Rate (SGR) formula and creating a stronger Medicare program," Dr. Hoven said in a statement. "The AMA will continue to work collaboratively with Congress so that a bipartisan agreement can be signed into law early next year to repeal the failed SGR payment formula."
The American College of Physicians said that it, too, would work to ensure that a bill moves through Congress and gets to the White House for approval soon.
"The bills reported today ... will help ensure that Medicare patients continue to have access to their physicians," said Dr. Charles Cutler, chairman of the ACP Board of Regents. "Their efforts will work to stabilize payments, provide multiple pathways for physicians to qualify for positive updates and to participate in alternative payment models, create positive incentives for patient-centered medical homes, provide assistance to small practices, and needed funding for development of quality measures."
The American College of Cardiology said in a statement that the proposals accomplished two of its highest priorities: eliminating the SGR and including provisions that will emphasize quality of care, including "provisions that emphasize the importance of clinical data registries, quality measure development, and appropriate use criteria to promote evidence-based care."
"We caution that our final support rests upon the caveat that paying for this legislation must not cause harm to patients and the physicians who care for them," Dr. John Gordon Harold, ACC president, said in the statement.
Legislators from the Finance Committee and the Ways and Means Committee celebrated their votes in a joint statement. In the statement, Sen. Hatch also issued a word of caution.
"Now that this legislation moves out of Committee and onto the floor, we need to continue to work together to ensure that this smart policy becomes law and ensure that it doesn't add one dime to our nation's debt."
WASHINGTON - Congress has moved the ball forward on permanently replacing the Medicare Sustainable Growth Rate formula, but with time short for a fix by year's end the House has voted to approve a temporary 3-month reprieve from the 20% cut due to take effect Jan. 1.
In a 332-94 vote, with eight abstentions, the House on Dec. 12 approved the Bipartisan Budget Act of 2013, a wide-ranging budget agreement that includes the 3-month patch. The bill also would increase physician pay by 0.5% through March.
The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. The fix would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
It also would extend the 2% sequestration cuts for Medicare providers by 2 years, from 2021 to 2023.
The Senate has yet to consider the budget package including the SGR patch; it is expected to do so before its holiday recess. President Obama has said that he supports the deal.
The agreement, brokered by House Budget Committee Chairman Paul Ryan (R-Wisc.) and Senate Budget Committee Chairman Patty Murray (D-Wash.), adds about $63 billion in discretionary federal spending over 2 years and makes targeted cuts and fee hikes to bring about overall deficit reduction of about $23 billion.
Although physician groups aren't thrilled about the continuation of the Medicare cuts under sequestration, most favor the temporary SGR reprieve and the restoration of some funding to federal health programs.
Dr. Ardis Dee Hoven, president of the American Medical Association, said that the continuation of the sequester cuts in Medicare is "frustrating" and poses the risk of destabilizing physician practices. "The concept of the sequester is probably not the best way to rein in spending," she said.
Dr. Clifford A. Hudis, president of the American Society of Clinical Oncology, praised the agreement for providing funds that could restore cuts in medical research and cancer care at the National Institutes of Health. He expressed disappointment, however, that the bill does not reverse cuts to Medicare, including reductions in the payments for physician-administered drugs under Medicare Part B.
"Oncologists are doing everything possible to continue providing care for Medicare patients, but this reduction has forced many in private practice to send patients to hospitals for chemotherapy because they cannot afford to administer these drugs in their office," Dr. Hudis said in a statement.
Physician organizations viewed the patch as necessary while Congress continues to work on a permanent SGR fix. Both the House and Senate took steps toward that goal on Dec. 12.
The House Ways and Means Committee voted 39-0 to approve its replacement proposal, which essentially adds on to the bill approved by the House Energy and Commerce Committee in July.
"This may not be the final step, it's a very important step forward," said Rep. Kevin Brady (R-Tex.), chairman of the Ways and Means Health Subcommittee.
The House did not address how to pay for the permanent replacement. House Ways and Means Chairman Dave Camp (R-Mich.) noted that the Congressional Budget Office has estimated that it will cost $116 billion over 10 years to repeal the SGR, which is "more than half the cost 2 years ago." Even though that is the lowest estimate ever, "I am of no illusion that finding pay-fors will be an easy task," he said.
The Senate Finance Committee also did not include a way to pay for repeal in its proposal.
The bill had widespread bipartisan support in the committee, but some Senators raised concerns about the lack of a funding mechanism. Sen. Pat Roberts (R-Kan.) said that he wouldn’t support the bill until he could see how it would be funded.
Sen. Orrin Hatch (R- Utah), the committee's top-ranking Republican, said that the offsets would be worked out once the bill had cleared the initial policymaking phase.
"This bill will be offset, period, or it's not going to go through both houses," he said. "This bill will be paid for."
The panel agreed to add a provision aimed at expanding access to community mental health services. The amendment, offered by Sen. Debbie Stabenow (D-Mich.) and Sen. Roy Blunt (R-Mo.), would create pilot programs in 10 states to ensure that community behavioral health clinics offer a full range of mental health services, including 24-hour crisis care, substance abuse treatment, and expanded support for families.
Physician groups praised the continued congressional action.
The AMA "strongly commends members of the House Ways and Means Committee and the Senate Finance Committee for the tremendous progress they have made toward repealing Medicare's failed Sustainable Growth Rate (SGR) formula and creating a stronger Medicare program," Dr. Hoven said in a statement. "The AMA will continue to work collaboratively with Congress so that a bipartisan agreement can be signed into law early next year to repeal the failed SGR payment formula."
The American College of Physicians said that it, too, would work to ensure that a bill moves through Congress and gets to the White House for approval soon.
"The bills reported today ... will help ensure that Medicare patients continue to have access to their physicians," said Dr. Charles Cutler, chairman of the ACP Board of Regents. "Their efforts will work to stabilize payments, provide multiple pathways for physicians to qualify for positive updates and to participate in alternative payment models, create positive incentives for patient-centered medical homes, provide assistance to small practices, and needed funding for development of quality measures."
The American College of Cardiology said in a statement that the proposals accomplished two of its highest priorities: eliminating the SGR and including provisions that will emphasize quality of care, including "provisions that emphasize the importance of clinical data registries, quality measure development, and appropriate use criteria to promote evidence-based care."
"We caution that our final support rests upon the caveat that paying for this legislation must not cause harm to patients and the physicians who care for them," Dr. John Gordon Harold, ACC president, said in the statement.
Legislators from the Finance Committee and the Ways and Means Committee celebrated their votes in a joint statement. In the statement, Sen. Hatch also issued a word of caution.
"Now that this legislation moves out of Committee and onto the floor, we need to continue to work together to ensure that this smart policy becomes law and ensure that it doesn't add one dime to our nation's debt."
WASHINGTON - Congress has moved the ball forward on permanently replacing the Medicare Sustainable Growth Rate formula, but with time short for a fix by year's end the House has voted to approve a temporary 3-month reprieve from the 20% cut due to take effect Jan. 1.
In a 332-94 vote, with eight abstentions, the House on Dec. 12 approved the Bipartisan Budget Act of 2013, a wide-ranging budget agreement that includes the 3-month patch. The bill also would increase physician pay by 0.5% through March.
The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. The fix would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
It also would extend the 2% sequestration cuts for Medicare providers by 2 years, from 2021 to 2023.
The Senate has yet to consider the budget package including the SGR patch; it is expected to do so before its holiday recess. President Obama has said that he supports the deal.
The agreement, brokered by House Budget Committee Chairman Paul Ryan (R-Wisc.) and Senate Budget Committee Chairman Patty Murray (D-Wash.), adds about $63 billion in discretionary federal spending over 2 years and makes targeted cuts and fee hikes to bring about overall deficit reduction of about $23 billion.
Although physician groups aren't thrilled about the continuation of the Medicare cuts under sequestration, most favor the temporary SGR reprieve and the restoration of some funding to federal health programs.
Dr. Ardis Dee Hoven, president of the American Medical Association, said that the continuation of the sequester cuts in Medicare is "frustrating" and poses the risk of destabilizing physician practices. "The concept of the sequester is probably not the best way to rein in spending," she said.
Dr. Clifford A. Hudis, president of the American Society of Clinical Oncology, praised the agreement for providing funds that could restore cuts in medical research and cancer care at the National Institutes of Health. He expressed disappointment, however, that the bill does not reverse cuts to Medicare, including reductions in the payments for physician-administered drugs under Medicare Part B.
"Oncologists are doing everything possible to continue providing care for Medicare patients, but this reduction has forced many in private practice to send patients to hospitals for chemotherapy because they cannot afford to administer these drugs in their office," Dr. Hudis said in a statement.
Physician organizations viewed the patch as necessary while Congress continues to work on a permanent SGR fix. Both the House and Senate took steps toward that goal on Dec. 12.
The House Ways and Means Committee voted 39-0 to approve its replacement proposal, which essentially adds on to the bill approved by the House Energy and Commerce Committee in July.
"This may not be the final step, it's a very important step forward," said Rep. Kevin Brady (R-Tex.), chairman of the Ways and Means Health Subcommittee.
The House did not address how to pay for the permanent replacement. House Ways and Means Chairman Dave Camp (R-Mich.) noted that the Congressional Budget Office has estimated that it will cost $116 billion over 10 years to repeal the SGR, which is "more than half the cost 2 years ago." Even though that is the lowest estimate ever, "I am of no illusion that finding pay-fors will be an easy task," he said.
The Senate Finance Committee also did not include a way to pay for repeal in its proposal.
The bill had widespread bipartisan support in the committee, but some Senators raised concerns about the lack of a funding mechanism. Sen. Pat Roberts (R-Kan.) said that he wouldn’t support the bill until he could see how it would be funded.
Sen. Orrin Hatch (R- Utah), the committee's top-ranking Republican, said that the offsets would be worked out once the bill had cleared the initial policymaking phase.
"This bill will be offset, period, or it's not going to go through both houses," he said. "This bill will be paid for."
The panel agreed to add a provision aimed at expanding access to community mental health services. The amendment, offered by Sen. Debbie Stabenow (D-Mich.) and Sen. Roy Blunt (R-Mo.), would create pilot programs in 10 states to ensure that community behavioral health clinics offer a full range of mental health services, including 24-hour crisis care, substance abuse treatment, and expanded support for families.
Physician groups praised the continued congressional action.
The AMA "strongly commends members of the House Ways and Means Committee and the Senate Finance Committee for the tremendous progress they have made toward repealing Medicare's failed Sustainable Growth Rate (SGR) formula and creating a stronger Medicare program," Dr. Hoven said in a statement. "The AMA will continue to work collaboratively with Congress so that a bipartisan agreement can be signed into law early next year to repeal the failed SGR payment formula."
The American College of Physicians said that it, too, would work to ensure that a bill moves through Congress and gets to the White House for approval soon.
"The bills reported today ... will help ensure that Medicare patients continue to have access to their physicians," said Dr. Charles Cutler, chairman of the ACP Board of Regents. "Their efforts will work to stabilize payments, provide multiple pathways for physicians to qualify for positive updates and to participate in alternative payment models, create positive incentives for patient-centered medical homes, provide assistance to small practices, and needed funding for development of quality measures."
The American College of Cardiology said in a statement that the proposals accomplished two of its highest priorities: eliminating the SGR and including provisions that will emphasize quality of care, including "provisions that emphasize the importance of clinical data registries, quality measure development, and appropriate use criteria to promote evidence-based care."
"We caution that our final support rests upon the caveat that paying for this legislation must not cause harm to patients and the physicians who care for them," Dr. John Gordon Harold, ACC president, said in the statement.
Legislators from the Finance Committee and the Ways and Means Committee celebrated their votes in a joint statement. In the statement, Sen. Hatch also issued a word of caution.
"Now that this legislation moves out of Committee and onto the floor, we need to continue to work together to ensure that this smart policy becomes law and ensure that it doesn't add one dime to our nation's debt."
FDA Wants Proof Antibacterial Soaps are Safe, Effective
The Food and Drug Administration is proposing that manufacturers of antibacterial soaps prove by 2016 that the products are safe and effective, or they must be reformulated or relabeled.
The agency said it currently is looking only at consumer products. It will address antibacterial soaps used in the health care and food preparation settings separately. The proposal also does not affect alcohol-based hand sanitizers, which the agency has already recognized as generally safe and effective.
The Dec. 16 proposed rule is the result of a settlement with the Natural Resources Defense Council (NRDC). That nonprofit environmental group sued the agency in 2010 to force it to issue a final rule on a 1978 proposal to remove the antibacterial agent triclosan from certain consumer products.
The FDA now says that manufacturers will have to prove that antibacterial soaps and body washes – primarily made with triclosan and triclocarban – are safer and more effective than soap and water in preventing infection and the spread of germs. The agency estimates that at least 2,200 antibacterial soaps are on the market. Manufacturers have provided no evidence supporting their use, Dr. Sandra Kweder, deputy director of the Office of New Drugs at the FDA’s Center for Drug Evaluation and Research, said in a call with reporters. Those manufacturers will now be required to conduct studies to prove safety and effectiveness in humans.
There has been evidence in animal and in vitro studies that triclosan and triclocarban are endocrine disruptors, Dr. Kweder said.
With the antibacterial soaps, "there are unknowns and even a slight potential for a risk," she said. "We think consumers ought to know, and we ought to know, what the benefits of these products are."
The rule is open for comment until June 2014. The antibacterial soaps are allowed to remain on the market until the agency finalizes its proposal – not likely until September 2016, Dr. Kweder said. If manufacturers cannot demonstrate safety and effectiveness, the soaps will have to be reformulated without triclosan or relabeled to remove any antibacterial claims, Dr. Kweder said.
Manufacturers, however, said that they already have complied with agency requests for more data.
"We are perplexed that the Agency would suggest there is no evidence that antibacterial soaps are beneficial, as industry has long provided data and information about the safety and efficacy of these products," said the American Cleaning Institute and the Personal Care Products Council in a joint statement. "Our industry’s Topical Antimicrobial Coalition has submitted to FDA in-depth data showing that antibacterial soaps are more effective in killing germs when compared with non-antibacterial soaps," the statement said.
The groups said that they "will continue to operate in good faith to submit any new data that is available," but that manufacturers will submit comments to FDA "reaffirming that the use of antibacterial wash products in the home environment does not contribute to antibiotic or antibacterial resistance."
Consumer groups such as the NRDC, the Environmental Working Group, and others that have pressured the agency to more strictly regulate triclosan or remove it from the market applauded the FDA proposal, but noted that the examination of the ingredient has already been 30 years in the making.
Since the FDA first proposed regulating triclosan in 1978, it has revisited the topic multiple times, including at a 2005 advisory committee meeting. The panel said that the antibacterial soaps were ineffective and raised numerous safety concerns.
Mae Wu, an attorney with the NRDC’s health program, said in a statement that the agency’s proposal was "a good first step toward getting unsafe triclosan off the market."
Sen. Ed Markey (D-Mass.), who has urged the FDA to finalize its triclosan ruling and also has encouraged manufacturers to stop using it, said in a statement that "chemicals like triclosan existed in a regulatory black hole" for decades. "I applaud the FDA for taking a step to restrict the use of this harmful and ineffective chemical that continues to pollute our bodies," he said.
The FDA and the Environmental Protection Agency, among other agencies, have acknowledged triclosan’s potential to interfere with the normal functioning of estrogen, testosterone, and thyroid hormone. The National Toxicology Program continues to study the chemical, but the FDA wants manufacturers to come forward with information, too, Dr. Kweder said.
The FDA did approve the use of triclosan in Colgate Total toothpaste in 1997. The NRDC recently sued the FDA for access to its full records on that approval, saying that the ingredient should not be in toothpaste, either.
The FDA is not as concerned about the use in toothpaste, because it received data from the manufacturer showing that triclosan was safe and effective in that setting, Dr. Kweder said.
On Twitter @aliciaault
The Food and Drug Administration is proposing that manufacturers of antibacterial soaps prove by 2016 that the products are safe and effective, or they must be reformulated or relabeled.
The agency said it currently is looking only at consumer products. It will address antibacterial soaps used in the health care and food preparation settings separately. The proposal also does not affect alcohol-based hand sanitizers, which the agency has already recognized as generally safe and effective.
The Dec. 16 proposed rule is the result of a settlement with the Natural Resources Defense Council (NRDC). That nonprofit environmental group sued the agency in 2010 to force it to issue a final rule on a 1978 proposal to remove the antibacterial agent triclosan from certain consumer products.
The FDA now says that manufacturers will have to prove that antibacterial soaps and body washes – primarily made with triclosan and triclocarban – are safer and more effective than soap and water in preventing infection and the spread of germs. The agency estimates that at least 2,200 antibacterial soaps are on the market. Manufacturers have provided no evidence supporting their use, Dr. Sandra Kweder, deputy director of the Office of New Drugs at the FDA’s Center for Drug Evaluation and Research, said in a call with reporters. Those manufacturers will now be required to conduct studies to prove safety and effectiveness in humans.
There has been evidence in animal and in vitro studies that triclosan and triclocarban are endocrine disruptors, Dr. Kweder said.
With the antibacterial soaps, "there are unknowns and even a slight potential for a risk," she said. "We think consumers ought to know, and we ought to know, what the benefits of these products are."
The rule is open for comment until June 2014. The antibacterial soaps are allowed to remain on the market until the agency finalizes its proposal – not likely until September 2016, Dr. Kweder said. If manufacturers cannot demonstrate safety and effectiveness, the soaps will have to be reformulated without triclosan or relabeled to remove any antibacterial claims, Dr. Kweder said.
Manufacturers, however, said that they already have complied with agency requests for more data.
"We are perplexed that the Agency would suggest there is no evidence that antibacterial soaps are beneficial, as industry has long provided data and information about the safety and efficacy of these products," said the American Cleaning Institute and the Personal Care Products Council in a joint statement. "Our industry’s Topical Antimicrobial Coalition has submitted to FDA in-depth data showing that antibacterial soaps are more effective in killing germs when compared with non-antibacterial soaps," the statement said.
The groups said that they "will continue to operate in good faith to submit any new data that is available," but that manufacturers will submit comments to FDA "reaffirming that the use of antibacterial wash products in the home environment does not contribute to antibiotic or antibacterial resistance."
Consumer groups such as the NRDC, the Environmental Working Group, and others that have pressured the agency to more strictly regulate triclosan or remove it from the market applauded the FDA proposal, but noted that the examination of the ingredient has already been 30 years in the making.
Since the FDA first proposed regulating triclosan in 1978, it has revisited the topic multiple times, including at a 2005 advisory committee meeting. The panel said that the antibacterial soaps were ineffective and raised numerous safety concerns.
Mae Wu, an attorney with the NRDC’s health program, said in a statement that the agency’s proposal was "a good first step toward getting unsafe triclosan off the market."
Sen. Ed Markey (D-Mass.), who has urged the FDA to finalize its triclosan ruling and also has encouraged manufacturers to stop using it, said in a statement that "chemicals like triclosan existed in a regulatory black hole" for decades. "I applaud the FDA for taking a step to restrict the use of this harmful and ineffective chemical that continues to pollute our bodies," he said.
The FDA and the Environmental Protection Agency, among other agencies, have acknowledged triclosan’s potential to interfere with the normal functioning of estrogen, testosterone, and thyroid hormone. The National Toxicology Program continues to study the chemical, but the FDA wants manufacturers to come forward with information, too, Dr. Kweder said.
The FDA did approve the use of triclosan in Colgate Total toothpaste in 1997. The NRDC recently sued the FDA for access to its full records on that approval, saying that the ingredient should not be in toothpaste, either.
The FDA is not as concerned about the use in toothpaste, because it received data from the manufacturer showing that triclosan was safe and effective in that setting, Dr. Kweder said.
On Twitter @aliciaault
The Food and Drug Administration is proposing that manufacturers of antibacterial soaps prove by 2016 that the products are safe and effective, or they must be reformulated or relabeled.
The agency said it currently is looking only at consumer products. It will address antibacterial soaps used in the health care and food preparation settings separately. The proposal also does not affect alcohol-based hand sanitizers, which the agency has already recognized as generally safe and effective.
The Dec. 16 proposed rule is the result of a settlement with the Natural Resources Defense Council (NRDC). That nonprofit environmental group sued the agency in 2010 to force it to issue a final rule on a 1978 proposal to remove the antibacterial agent triclosan from certain consumer products.
The FDA now says that manufacturers will have to prove that antibacterial soaps and body washes – primarily made with triclosan and triclocarban – are safer and more effective than soap and water in preventing infection and the spread of germs. The agency estimates that at least 2,200 antibacterial soaps are on the market. Manufacturers have provided no evidence supporting their use, Dr. Sandra Kweder, deputy director of the Office of New Drugs at the FDA’s Center for Drug Evaluation and Research, said in a call with reporters. Those manufacturers will now be required to conduct studies to prove safety and effectiveness in humans.
There has been evidence in animal and in vitro studies that triclosan and triclocarban are endocrine disruptors, Dr. Kweder said.
With the antibacterial soaps, "there are unknowns and even a slight potential for a risk," she said. "We think consumers ought to know, and we ought to know, what the benefits of these products are."
The rule is open for comment until June 2014. The antibacterial soaps are allowed to remain on the market until the agency finalizes its proposal – not likely until September 2016, Dr. Kweder said. If manufacturers cannot demonstrate safety and effectiveness, the soaps will have to be reformulated without triclosan or relabeled to remove any antibacterial claims, Dr. Kweder said.
Manufacturers, however, said that they already have complied with agency requests for more data.
"We are perplexed that the Agency would suggest there is no evidence that antibacterial soaps are beneficial, as industry has long provided data and information about the safety and efficacy of these products," said the American Cleaning Institute and the Personal Care Products Council in a joint statement. "Our industry’s Topical Antimicrobial Coalition has submitted to FDA in-depth data showing that antibacterial soaps are more effective in killing germs when compared with non-antibacterial soaps," the statement said.
The groups said that they "will continue to operate in good faith to submit any new data that is available," but that manufacturers will submit comments to FDA "reaffirming that the use of antibacterial wash products in the home environment does not contribute to antibiotic or antibacterial resistance."
Consumer groups such as the NRDC, the Environmental Working Group, and others that have pressured the agency to more strictly regulate triclosan or remove it from the market applauded the FDA proposal, but noted that the examination of the ingredient has already been 30 years in the making.
Since the FDA first proposed regulating triclosan in 1978, it has revisited the topic multiple times, including at a 2005 advisory committee meeting. The panel said that the antibacterial soaps were ineffective and raised numerous safety concerns.
Mae Wu, an attorney with the NRDC’s health program, said in a statement that the agency’s proposal was "a good first step toward getting unsafe triclosan off the market."
Sen. Ed Markey (D-Mass.), who has urged the FDA to finalize its triclosan ruling and also has encouraged manufacturers to stop using it, said in a statement that "chemicals like triclosan existed in a regulatory black hole" for decades. "I applaud the FDA for taking a step to restrict the use of this harmful and ineffective chemical that continues to pollute our bodies," he said.
The FDA and the Environmental Protection Agency, among other agencies, have acknowledged triclosan’s potential to interfere with the normal functioning of estrogen, testosterone, and thyroid hormone. The National Toxicology Program continues to study the chemical, but the FDA wants manufacturers to come forward with information, too, Dr. Kweder said.
The FDA did approve the use of triclosan in Colgate Total toothpaste in 1997. The NRDC recently sued the FDA for access to its full records on that approval, saying that the ingredient should not be in toothpaste, either.
The FDA is not as concerned about the use in toothpaste, because it received data from the manufacturer showing that triclosan was safe and effective in that setting, Dr. Kweder said.
On Twitter @aliciaault
FDA wants manufacturers to show antibacterial soaps are safe, effective
The Food and Drug Administration is proposing that manufacturers of antibacterial soaps prove by 2016 that the products are safe and effective, or they must be reformulated or relabeled.
The agency said it currently is looking only at consumer products. It will address antibacterial soaps used in the health care and food preparation settings separately. The proposal also does not affect alcohol-based hand sanitizers, which the agency has already recognized as generally safe and effective.
The Dec. 16 proposed rule is the result of a settlement with the Natural Resources Defense Council (NRDC). That nonprofit environmental group sued the agency in 2010 to force it to issue a final rule on a 1978 proposal to remove the antibacterial agent triclosan from certain consumer products.
The FDA now says that manufacturers will have to prove that antibacterial soaps and body washes – primarily made with triclosan and triclocarban – are safer and more effective than soap and water in preventing infection and the spread of germs. The agency estimates that at least 2,200 antibacterial soaps are on the market. Manufacturers have provided no evidence supporting their use, Dr. Sandra Kweder, deputy director of the Office of New Drugs at the FDA’s Center for Drug Evaluation and Research, said in a call with reporters. Those manufacturers will now be required to conduct studies to prove safety and effectiveness in humans.
There has been evidence in animal and in vitro studies that triclosan and triclocarban are endocrine disruptors, Dr. Kweder said.
With the antibacterial soaps, "there are unknowns and even a slight potential for a risk," she said. "We think consumers ought to know, and we ought to know, what the benefits of these products are."
The rule is open for comment until June 2014. The antibacterial soaps are allowed to remain on the market until the agency finalizes its proposal – not likely until September 2016, Dr. Kweder said. If manufacturers cannot demonstrate safety and effectiveness, the soaps will have to be reformulated without triclosan or relabeled to remove any antibacterial claims, Dr. Kweder said.
Manufacturers, however, said that they already have complied with agency requests for more data.
"We are perplexed that the Agency would suggest there is no evidence that antibacterial soaps are beneficial, as industry has long provided data and information about the safety and efficacy of these products," said the American Cleaning Institute and the Personal Care Products Council in a joint statement. "Our industry’s Topical Antimicrobial Coalition has submitted to FDA in-depth data showing that antibacterial soaps are more effective in killing germs when compared with non-antibacterial soaps," the statement said.
The groups said that they "will continue to operate in good faith to submit any new data that is available," but that manufacturers will submit comments to FDA "reaffirming that the use of antibacterial wash products in the home environment does not contribute to antibiotic or antibacterial resistance."
Consumer groups such as the NRDC, the Environmental Working Group, and others that have pressured the agency to more strictly regulate triclosan or remove it from the market applauded the FDA proposal, but noted that the examination of the ingredient has already been 30 years in the making.
Since the FDA first proposed regulating triclosan in 1978, it has revisited the topic multiple times, including at a 2005 advisory committee meeting. The panel said that the antibacterial soaps were ineffective and raised numerous safety concerns.
Mae Wu, an attorney with the NRDC’s health program, said in a statement that the agency’s proposal was "a good first step toward getting unsafe triclosan off the market."
Sen. Ed Markey (D-Mass.), who has urged the FDA to finalize its triclosan ruling and also has encouraged manufacturers to stop using it, said in a statement that "chemicals like triclosan existed in a regulatory black hole" for decades. "I applaud the FDA for taking a step to restrict the use of this harmful and ineffective chemical that continues to pollute our bodies," he said.
The FDA and the Environmental Protection Agency, among other agencies, have acknowledged triclosan’s potential to interfere with the normal functioning of estrogen, testosterone, and thyroid hormone. The National Toxicology Program continues to study the chemical, but the FDA wants manufacturers to come forward with information, too, Dr. Kweder said.
The FDA did approve the use of triclosan in Colgate Total toothpaste in 1997. The NRDC recently sued the FDA for access to its full records on that approval, saying that the ingredient should not be in toothpaste, either.
The FDA is not as concerned about the use in toothpaste, because it received data from the manufacturer showing that triclosan was safe and effective in that setting, Dr. Kweder said.
On Twitter @aliciaault
The Food and Drug Administration is proposing that manufacturers of antibacterial soaps prove by 2016 that the products are safe and effective, or they must be reformulated or relabeled.
The agency said it currently is looking only at consumer products. It will address antibacterial soaps used in the health care and food preparation settings separately. The proposal also does not affect alcohol-based hand sanitizers, which the agency has already recognized as generally safe and effective.
The Dec. 16 proposed rule is the result of a settlement with the Natural Resources Defense Council (NRDC). That nonprofit environmental group sued the agency in 2010 to force it to issue a final rule on a 1978 proposal to remove the antibacterial agent triclosan from certain consumer products.
The FDA now says that manufacturers will have to prove that antibacterial soaps and body washes – primarily made with triclosan and triclocarban – are safer and more effective than soap and water in preventing infection and the spread of germs. The agency estimates that at least 2,200 antibacterial soaps are on the market. Manufacturers have provided no evidence supporting their use, Dr. Sandra Kweder, deputy director of the Office of New Drugs at the FDA’s Center for Drug Evaluation and Research, said in a call with reporters. Those manufacturers will now be required to conduct studies to prove safety and effectiveness in humans.
There has been evidence in animal and in vitro studies that triclosan and triclocarban are endocrine disruptors, Dr. Kweder said.
With the antibacterial soaps, "there are unknowns and even a slight potential for a risk," she said. "We think consumers ought to know, and we ought to know, what the benefits of these products are."
The rule is open for comment until June 2014. The antibacterial soaps are allowed to remain on the market until the agency finalizes its proposal – not likely until September 2016, Dr. Kweder said. If manufacturers cannot demonstrate safety and effectiveness, the soaps will have to be reformulated without triclosan or relabeled to remove any antibacterial claims, Dr. Kweder said.
Manufacturers, however, said that they already have complied with agency requests for more data.
"We are perplexed that the Agency would suggest there is no evidence that antibacterial soaps are beneficial, as industry has long provided data and information about the safety and efficacy of these products," said the American Cleaning Institute and the Personal Care Products Council in a joint statement. "Our industry’s Topical Antimicrobial Coalition has submitted to FDA in-depth data showing that antibacterial soaps are more effective in killing germs when compared with non-antibacterial soaps," the statement said.
The groups said that they "will continue to operate in good faith to submit any new data that is available," but that manufacturers will submit comments to FDA "reaffirming that the use of antibacterial wash products in the home environment does not contribute to antibiotic or antibacterial resistance."
Consumer groups such as the NRDC, the Environmental Working Group, and others that have pressured the agency to more strictly regulate triclosan or remove it from the market applauded the FDA proposal, but noted that the examination of the ingredient has already been 30 years in the making.
Since the FDA first proposed regulating triclosan in 1978, it has revisited the topic multiple times, including at a 2005 advisory committee meeting. The panel said that the antibacterial soaps were ineffective and raised numerous safety concerns.
Mae Wu, an attorney with the NRDC’s health program, said in a statement that the agency’s proposal was "a good first step toward getting unsafe triclosan off the market."
Sen. Ed Markey (D-Mass.), who has urged the FDA to finalize its triclosan ruling and also has encouraged manufacturers to stop using it, said in a statement that "chemicals like triclosan existed in a regulatory black hole" for decades. "I applaud the FDA for taking a step to restrict the use of this harmful and ineffective chemical that continues to pollute our bodies," he said.
The FDA and the Environmental Protection Agency, among other agencies, have acknowledged triclosan’s potential to interfere with the normal functioning of estrogen, testosterone, and thyroid hormone. The National Toxicology Program continues to study the chemical, but the FDA wants manufacturers to come forward with information, too, Dr. Kweder said.
The FDA did approve the use of triclosan in Colgate Total toothpaste in 1997. The NRDC recently sued the FDA for access to its full records on that approval, saying that the ingredient should not be in toothpaste, either.
The FDA is not as concerned about the use in toothpaste, because it received data from the manufacturer showing that triclosan was safe and effective in that setting, Dr. Kweder said.
On Twitter @aliciaault
The Food and Drug Administration is proposing that manufacturers of antibacterial soaps prove by 2016 that the products are safe and effective, or they must be reformulated or relabeled.
The agency said it currently is looking only at consumer products. It will address antibacterial soaps used in the health care and food preparation settings separately. The proposal also does not affect alcohol-based hand sanitizers, which the agency has already recognized as generally safe and effective.
The Dec. 16 proposed rule is the result of a settlement with the Natural Resources Defense Council (NRDC). That nonprofit environmental group sued the agency in 2010 to force it to issue a final rule on a 1978 proposal to remove the antibacterial agent triclosan from certain consumer products.
The FDA now says that manufacturers will have to prove that antibacterial soaps and body washes – primarily made with triclosan and triclocarban – are safer and more effective than soap and water in preventing infection and the spread of germs. The agency estimates that at least 2,200 antibacterial soaps are on the market. Manufacturers have provided no evidence supporting their use, Dr. Sandra Kweder, deputy director of the Office of New Drugs at the FDA’s Center for Drug Evaluation and Research, said in a call with reporters. Those manufacturers will now be required to conduct studies to prove safety and effectiveness in humans.
There has been evidence in animal and in vitro studies that triclosan and triclocarban are endocrine disruptors, Dr. Kweder said.
With the antibacterial soaps, "there are unknowns and even a slight potential for a risk," she said. "We think consumers ought to know, and we ought to know, what the benefits of these products are."
The rule is open for comment until June 2014. The antibacterial soaps are allowed to remain on the market until the agency finalizes its proposal – not likely until September 2016, Dr. Kweder said. If manufacturers cannot demonstrate safety and effectiveness, the soaps will have to be reformulated without triclosan or relabeled to remove any antibacterial claims, Dr. Kweder said.
Manufacturers, however, said that they already have complied with agency requests for more data.
"We are perplexed that the Agency would suggest there is no evidence that antibacterial soaps are beneficial, as industry has long provided data and information about the safety and efficacy of these products," said the American Cleaning Institute and the Personal Care Products Council in a joint statement. "Our industry’s Topical Antimicrobial Coalition has submitted to FDA in-depth data showing that antibacterial soaps are more effective in killing germs when compared with non-antibacterial soaps," the statement said.
The groups said that they "will continue to operate in good faith to submit any new data that is available," but that manufacturers will submit comments to FDA "reaffirming that the use of antibacterial wash products in the home environment does not contribute to antibiotic or antibacterial resistance."
Consumer groups such as the NRDC, the Environmental Working Group, and others that have pressured the agency to more strictly regulate triclosan or remove it from the market applauded the FDA proposal, but noted that the examination of the ingredient has already been 30 years in the making.
Since the FDA first proposed regulating triclosan in 1978, it has revisited the topic multiple times, including at a 2005 advisory committee meeting. The panel said that the antibacterial soaps were ineffective and raised numerous safety concerns.
Mae Wu, an attorney with the NRDC’s health program, said in a statement that the agency’s proposal was "a good first step toward getting unsafe triclosan off the market."
Sen. Ed Markey (D-Mass.), who has urged the FDA to finalize its triclosan ruling and also has encouraged manufacturers to stop using it, said in a statement that "chemicals like triclosan existed in a regulatory black hole" for decades. "I applaud the FDA for taking a step to restrict the use of this harmful and ineffective chemical that continues to pollute our bodies," he said.
The FDA and the Environmental Protection Agency, among other agencies, have acknowledged triclosan’s potential to interfere with the normal functioning of estrogen, testosterone, and thyroid hormone. The National Toxicology Program continues to study the chemical, but the FDA wants manufacturers to come forward with information, too, Dr. Kweder said.
The FDA did approve the use of triclosan in Colgate Total toothpaste in 1997. The NRDC recently sued the FDA for access to its full records on that approval, saying that the ingredient should not be in toothpaste, either.
The FDA is not as concerned about the use in toothpaste, because it received data from the manufacturer showing that triclosan was safe and effective in that setting, Dr. Kweder said.
On Twitter @aliciaault
HHS call for insurer flexibility meets some doctors’ concerns
Changes designed to make it easier for consumers to buy insurance through the health insurance exchanges address some concerns raised by physicians.
In a rule issued Dec. 12, the Health and Human Services department formalized a requirement that insurers give consumers until Dec. 23 to choose a health insurance plan and until midnight Dec. 31 to pay for it, in order to be considered covered on Jan. 1. State exchanges can set their own rules about Jan. 1 coverage dates, and in some cases, already have, Chiquita Brooks-Lasure, policy director for the HHS center for consumer information and insurance oversight, said during a press briefing.
Some states may elect to allow payment later than Dec. 31, she said. The HHS is asking insurers to consider accepting payment after Jan. 1 and retroactively cover services. Ms. Brooks-Lasure said that Aetna has already said it will accept payment until Jan. 8.
Insurers are also being asked to accept partial payment for the first month’s premium. Payment flexibility "will reduce the risk that patients will start the year without coverage," said Bob Doherty, senior vice president for governmental affairs and public policy at the American College of Physicians.
The agency is also "strongly encouraging" insurers to consider out-of-network providers as in network and to pay for prescription refills even if the drug is not covered under its plan – at least for the month of January.
The request has precedents, Michael Hash, director of the HHS office of health reform, said during the briefing. "This transition opportunity is quite common in the insurance world."
Mr. Doherty said the ACP hopes that "the insurance industry does its part to help ease the transition, especially by allowing patients who are in a course of treatment to keep seeing their doctors even when they are not in the plan’s network and to continue to get the drugs prescribed for them if not on the plan’s formulary."
Physician groups have asked that such a transition period be considered. At a White House meeting on Nov. 26, they expressed concern that patients in the midst of expensive or specialized treatment might experience a major disruption in care if forced to give up a particular physician or therapy when switching to a new plan.
Mr. Hash said insurers were open to accommodating new enrollees. He said the HHS and insurance companies are working together to "make sure that everyone seeking coverage effective Jan. 1 gets coverage."
The HHS also announced on Dec. 12 that the almost 86,000 individuals in the federal high-risk pool, known as the Pre-Existing Condition Insurance Plan (PCIP), have had their coverage extended to Jan. 31. Those policies had been set to expire Dec. 31.
The program has had its share of financial troubles, and in February, the HHS temporarily stopped enrolling new patients. House Republicans charged that the PCIP had been mismanaged and sought to give it more money. That never happened. On the call with reporters, Ms. Brooks Lasure said the program had spent only $4.74 billion of the $5 billion allotted to it under the Affordable Care Act (ACA), and thus, could afford to pay for the additional month.
The American Cancer Society Cancer Action Network (ACSCAN) applauded the PCIP extension. "Extending coverage under PCIP gives patients valuable additional time to select the marketplace plan that best meets their unique needs," ACSCAN president Chris Hansen said in a statement.
House Republicans, however, portrayed the PCIP policy change as "another delay" in ACA implementation. "The administration has known for many months that this law was not ready for prime time, and Americans who depend on high-risk pools would have been better served by the administration admitting their failures sooner and working with the Congress to protect these and other Americans being harmed by the health care law," House Energy and Commerce Committee Chairman Fred Upton (R-Mich.) said in a statement.
On Twitter @aliciaault
Changes designed to make it easier for consumers to buy insurance through the health insurance exchanges address some concerns raised by physicians.
In a rule issued Dec. 12, the Health and Human Services department formalized a requirement that insurers give consumers until Dec. 23 to choose a health insurance plan and until midnight Dec. 31 to pay for it, in order to be considered covered on Jan. 1. State exchanges can set their own rules about Jan. 1 coverage dates, and in some cases, already have, Chiquita Brooks-Lasure, policy director for the HHS center for consumer information and insurance oversight, said during a press briefing.
Some states may elect to allow payment later than Dec. 31, she said. The HHS is asking insurers to consider accepting payment after Jan. 1 and retroactively cover services. Ms. Brooks-Lasure said that Aetna has already said it will accept payment until Jan. 8.
Insurers are also being asked to accept partial payment for the first month’s premium. Payment flexibility "will reduce the risk that patients will start the year without coverage," said Bob Doherty, senior vice president for governmental affairs and public policy at the American College of Physicians.
The agency is also "strongly encouraging" insurers to consider out-of-network providers as in network and to pay for prescription refills even if the drug is not covered under its plan – at least for the month of January.
The request has precedents, Michael Hash, director of the HHS office of health reform, said during the briefing. "This transition opportunity is quite common in the insurance world."
Mr. Doherty said the ACP hopes that "the insurance industry does its part to help ease the transition, especially by allowing patients who are in a course of treatment to keep seeing their doctors even when they are not in the plan’s network and to continue to get the drugs prescribed for them if not on the plan’s formulary."
Physician groups have asked that such a transition period be considered. At a White House meeting on Nov. 26, they expressed concern that patients in the midst of expensive or specialized treatment might experience a major disruption in care if forced to give up a particular physician or therapy when switching to a new plan.
Mr. Hash said insurers were open to accommodating new enrollees. He said the HHS and insurance companies are working together to "make sure that everyone seeking coverage effective Jan. 1 gets coverage."
The HHS also announced on Dec. 12 that the almost 86,000 individuals in the federal high-risk pool, known as the Pre-Existing Condition Insurance Plan (PCIP), have had their coverage extended to Jan. 31. Those policies had been set to expire Dec. 31.
The program has had its share of financial troubles, and in February, the HHS temporarily stopped enrolling new patients. House Republicans charged that the PCIP had been mismanaged and sought to give it more money. That never happened. On the call with reporters, Ms. Brooks Lasure said the program had spent only $4.74 billion of the $5 billion allotted to it under the Affordable Care Act (ACA), and thus, could afford to pay for the additional month.
The American Cancer Society Cancer Action Network (ACSCAN) applauded the PCIP extension. "Extending coverage under PCIP gives patients valuable additional time to select the marketplace plan that best meets their unique needs," ACSCAN president Chris Hansen said in a statement.
House Republicans, however, portrayed the PCIP policy change as "another delay" in ACA implementation. "The administration has known for many months that this law was not ready for prime time, and Americans who depend on high-risk pools would have been better served by the administration admitting their failures sooner and working with the Congress to protect these and other Americans being harmed by the health care law," House Energy and Commerce Committee Chairman Fred Upton (R-Mich.) said in a statement.
On Twitter @aliciaault
Changes designed to make it easier for consumers to buy insurance through the health insurance exchanges address some concerns raised by physicians.
In a rule issued Dec. 12, the Health and Human Services department formalized a requirement that insurers give consumers until Dec. 23 to choose a health insurance plan and until midnight Dec. 31 to pay for it, in order to be considered covered on Jan. 1. State exchanges can set their own rules about Jan. 1 coverage dates, and in some cases, already have, Chiquita Brooks-Lasure, policy director for the HHS center for consumer information and insurance oversight, said during a press briefing.
Some states may elect to allow payment later than Dec. 31, she said. The HHS is asking insurers to consider accepting payment after Jan. 1 and retroactively cover services. Ms. Brooks-Lasure said that Aetna has already said it will accept payment until Jan. 8.
Insurers are also being asked to accept partial payment for the first month’s premium. Payment flexibility "will reduce the risk that patients will start the year without coverage," said Bob Doherty, senior vice president for governmental affairs and public policy at the American College of Physicians.
The agency is also "strongly encouraging" insurers to consider out-of-network providers as in network and to pay for prescription refills even if the drug is not covered under its plan – at least for the month of January.
The request has precedents, Michael Hash, director of the HHS office of health reform, said during the briefing. "This transition opportunity is quite common in the insurance world."
Mr. Doherty said the ACP hopes that "the insurance industry does its part to help ease the transition, especially by allowing patients who are in a course of treatment to keep seeing their doctors even when they are not in the plan’s network and to continue to get the drugs prescribed for them if not on the plan’s formulary."
Physician groups have asked that such a transition period be considered. At a White House meeting on Nov. 26, they expressed concern that patients in the midst of expensive or specialized treatment might experience a major disruption in care if forced to give up a particular physician or therapy when switching to a new plan.
Mr. Hash said insurers were open to accommodating new enrollees. He said the HHS and insurance companies are working together to "make sure that everyone seeking coverage effective Jan. 1 gets coverage."
The HHS also announced on Dec. 12 that the almost 86,000 individuals in the federal high-risk pool, known as the Pre-Existing Condition Insurance Plan (PCIP), have had their coverage extended to Jan. 31. Those policies had been set to expire Dec. 31.
The program has had its share of financial troubles, and in February, the HHS temporarily stopped enrolling new patients. House Republicans charged that the PCIP had been mismanaged and sought to give it more money. That never happened. On the call with reporters, Ms. Brooks Lasure said the program had spent only $4.74 billion of the $5 billion allotted to it under the Affordable Care Act (ACA), and thus, could afford to pay for the additional month.
The American Cancer Society Cancer Action Network (ACSCAN) applauded the PCIP extension. "Extending coverage under PCIP gives patients valuable additional time to select the marketplace plan that best meets their unique needs," ACSCAN president Chris Hansen said in a statement.
House Republicans, however, portrayed the PCIP policy change as "another delay" in ACA implementation. "The administration has known for many months that this law was not ready for prime time, and Americans who depend on high-risk pools would have been better served by the administration admitting their failures sooner and working with the Congress to protect these and other Americans being harmed by the health care law," House Energy and Commerce Committee Chairman Fred Upton (R-Mich.) said in a statement.
On Twitter @aliciaault
House budget includes SGR patch; permanent fix sails through committees
WASHINGTON – Congress has moved the ball forward on permanently replacing the Medicare Sustainable Growth Rate formula, but with time short for a fix by year’s end the House has voted to approve a temporary 3-month reprieve from the 20% cut due to take effect Jan. 1.
In a 332-94 vote, with eight abstentions, the House on Dec. 12 approved the Bipartisan Budget Act of 2013, a wide-ranging budget agreement that includes the 3-month patch. The bill also would increase physician pay by 0.5% through March.
The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. The fix would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
It also would extend the 2% sequestration cuts for Medicare providers by 2 years, from 2021 to 2023.
The Senate has yet to consider the budget package including the SGR patch; it is expected to do so before its holiday recess. President Obama has said that he supports the deal.
The agreement, brokered by House Budget Committee Chairman Paul Ryan (R-Wisc.) and Senate Budget Committee Chairman Patty Murray (D-Wash.), adds about $63 billion in discretionary federal spending over 2 years and makes targeted cuts and fee hikes to bring about overall deficit reduction of about $23 billion.
Although physician groups aren’t thrilled about the continuation of the Medicare cuts under sequestration, most favor the temporary SGR reprieve and the restoration of some funding to federal health programs.
Dr. Ardis Dee Hoven, president of the American Medical Association, said that the continuation of the sequester cuts in Medicare is "frustrating" and poses the risk of destabilizing physician practices. "The concept of the sequester is probably not the best way to rein in spending," she said.
Dr. Clifford A. Hudis, president of the American Society of Clinical Oncology, praised the agreement for providing funds that could restore cuts in medical research and cancer care at the National Institutes of Health. He expressed disappointment, however, that the bill does not reverse cuts to Medicare, including reductions in the payments for physician-administered drugs under Medicare Part B.
"Oncologists are doing everything possible to continue providing care for Medicare patients, but this reduction has forced many in private practice to send patients to hospitals for chemotherapy because they cannot afford to administer these drugs in their office," Dr. Hudis said in a statement.
Physician organizations viewed the patch as necessary while Congress continues to work on a permanent SGR fix. Both the House and Senate took steps toward that goal on Dec. 12.
The House Ways and Means Committee voted 39-0 to approve its replacement proposal, which essentially adds on to the bill approved by the House Energy and Commerce Committee in July.
"This may not be the final step, it’s a very important step forward," said Rep. Kevin Brady (R-Tex.), chairman of the Ways and Means Health Subcommittee.
The House did not address how to pay for the permanent replacement. House Ways and Means Chairman Dave Camp (R-Mich.) noted that the Congressional Budget Office has estimated that it will cost $116 billion over 10 years to repeal the SGR, which is "more than half the cost 2 years ago." Even though that is the lowest estimate ever, "I am of no illusion that finding pay-fors will be an easy task," he said.
The Senate Finance Committee also did not include a way to pay for repeal in its proposal.
The bill had widespread bipartisan support in the committee, but some Senators raised concerns about the lack of a funding mechanism. Sen. Pat Roberts (R-Kan.) said that he wouldn’t support the bill until he could see how it would be funded.
Sen. Orrin Hatch (R- Utah), the committee’s top-ranking Republican, said that the offsets would be worked out once the bill had cleared the initial policymaking phase.
"This bill will be offset, period, or it’s not going to go through both houses," he said. "This bill will be paid for."
The panel agreed to add a provision aimed at expanding access to community mental health services. The amendment, offered by Sen. Debbie Stabenow (D-Mich.) and Sen. Roy Blunt (R-Mo.), would create pilot programs in 10 states to ensure that community behavioral health clinics offer a full range of mental health services, including 24-hour crisis care, substance abuse treatment, and expanded support for families.
Physician groups praised the continued congressional action.
The AMA "strongly commends members of the House Ways and Means Committee and the Senate Finance Committee for the tremendous progress they have made toward repealing Medicare’s failed Sustainable Growth Rate (SGR) formula and creating a stronger Medicare program," Dr. Hoven said in a statement. "The AMA will continue to work collaboratively with Congress so that a bipartisan agreement can be signed into law early next year to repeal the failed SGR payment formula."
The American College of Physicians said that it, too, would work to ensure that a bill moves through Congress and gets to the White House for approval soon.
"The bills reported today ... will help ensure that Medicare patients continue to have access to their physicians," said Dr. Charles Cutler, chairman of the ACP Board of Regents. "Their efforts will work to stabilize payments, provide multiple pathways for physicians to qualify for positive updates and to participate in alternative payment models, create positive incentives for patient-centered medical homes, provide assistance to small practices, and needed funding for development of quality measures."
The American College of Cardiology said in a statement that the proposals accomplished two of its highest priorities: eliminating the SGR and including provisions that will emphasize quality of care, including "provisions that emphasize the importance of clinical data registries, quality measure development, and appropriate use criteria to promote evidence-based care."
"We caution that our final support rests upon the caveat that paying for this legislation must not cause harm to patients and the physicians who care for them," Dr. John Gordon Harold, ACC president, said in the statement.
Legislators from the Finance Committee and the Ways and Means Committee celebrated their votes in a joint statement. In the statement, Sen. Hatch also issued a word of caution.
"Now that this legislation moves out of Committee and onto the floor, we need to continue to work together to ensure that this smart policy becomes law and ensure that it doesn’t add one dime to our nation’s debt."
WASHINGTON – Congress has moved the ball forward on permanently replacing the Medicare Sustainable Growth Rate formula, but with time short for a fix by year’s end the House has voted to approve a temporary 3-month reprieve from the 20% cut due to take effect Jan. 1.
In a 332-94 vote, with eight abstentions, the House on Dec. 12 approved the Bipartisan Budget Act of 2013, a wide-ranging budget agreement that includes the 3-month patch. The bill also would increase physician pay by 0.5% through March.
The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. The fix would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
It also would extend the 2% sequestration cuts for Medicare providers by 2 years, from 2021 to 2023.
The Senate has yet to consider the budget package including the SGR patch; it is expected to do so before its holiday recess. President Obama has said that he supports the deal.
The agreement, brokered by House Budget Committee Chairman Paul Ryan (R-Wisc.) and Senate Budget Committee Chairman Patty Murray (D-Wash.), adds about $63 billion in discretionary federal spending over 2 years and makes targeted cuts and fee hikes to bring about overall deficit reduction of about $23 billion.
Although physician groups aren’t thrilled about the continuation of the Medicare cuts under sequestration, most favor the temporary SGR reprieve and the restoration of some funding to federal health programs.
Dr. Ardis Dee Hoven, president of the American Medical Association, said that the continuation of the sequester cuts in Medicare is "frustrating" and poses the risk of destabilizing physician practices. "The concept of the sequester is probably not the best way to rein in spending," she said.
Dr. Clifford A. Hudis, president of the American Society of Clinical Oncology, praised the agreement for providing funds that could restore cuts in medical research and cancer care at the National Institutes of Health. He expressed disappointment, however, that the bill does not reverse cuts to Medicare, including reductions in the payments for physician-administered drugs under Medicare Part B.
"Oncologists are doing everything possible to continue providing care for Medicare patients, but this reduction has forced many in private practice to send patients to hospitals for chemotherapy because they cannot afford to administer these drugs in their office," Dr. Hudis said in a statement.
Physician organizations viewed the patch as necessary while Congress continues to work on a permanent SGR fix. Both the House and Senate took steps toward that goal on Dec. 12.
The House Ways and Means Committee voted 39-0 to approve its replacement proposal, which essentially adds on to the bill approved by the House Energy and Commerce Committee in July.
"This may not be the final step, it’s a very important step forward," said Rep. Kevin Brady (R-Tex.), chairman of the Ways and Means Health Subcommittee.
The House did not address how to pay for the permanent replacement. House Ways and Means Chairman Dave Camp (R-Mich.) noted that the Congressional Budget Office has estimated that it will cost $116 billion over 10 years to repeal the SGR, which is "more than half the cost 2 years ago." Even though that is the lowest estimate ever, "I am of no illusion that finding pay-fors will be an easy task," he said.
The Senate Finance Committee also did not include a way to pay for repeal in its proposal.
The bill had widespread bipartisan support in the committee, but some Senators raised concerns about the lack of a funding mechanism. Sen. Pat Roberts (R-Kan.) said that he wouldn’t support the bill until he could see how it would be funded.
Sen. Orrin Hatch (R- Utah), the committee’s top-ranking Republican, said that the offsets would be worked out once the bill had cleared the initial policymaking phase.
"This bill will be offset, period, or it’s not going to go through both houses," he said. "This bill will be paid for."
The panel agreed to add a provision aimed at expanding access to community mental health services. The amendment, offered by Sen. Debbie Stabenow (D-Mich.) and Sen. Roy Blunt (R-Mo.), would create pilot programs in 10 states to ensure that community behavioral health clinics offer a full range of mental health services, including 24-hour crisis care, substance abuse treatment, and expanded support for families.
Physician groups praised the continued congressional action.
The AMA "strongly commends members of the House Ways and Means Committee and the Senate Finance Committee for the tremendous progress they have made toward repealing Medicare’s failed Sustainable Growth Rate (SGR) formula and creating a stronger Medicare program," Dr. Hoven said in a statement. "The AMA will continue to work collaboratively with Congress so that a bipartisan agreement can be signed into law early next year to repeal the failed SGR payment formula."
The American College of Physicians said that it, too, would work to ensure that a bill moves through Congress and gets to the White House for approval soon.
"The bills reported today ... will help ensure that Medicare patients continue to have access to their physicians," said Dr. Charles Cutler, chairman of the ACP Board of Regents. "Their efforts will work to stabilize payments, provide multiple pathways for physicians to qualify for positive updates and to participate in alternative payment models, create positive incentives for patient-centered medical homes, provide assistance to small practices, and needed funding for development of quality measures."
The American College of Cardiology said in a statement that the proposals accomplished two of its highest priorities: eliminating the SGR and including provisions that will emphasize quality of care, including "provisions that emphasize the importance of clinical data registries, quality measure development, and appropriate use criteria to promote evidence-based care."
"We caution that our final support rests upon the caveat that paying for this legislation must not cause harm to patients and the physicians who care for them," Dr. John Gordon Harold, ACC president, said in the statement.
Legislators from the Finance Committee and the Ways and Means Committee celebrated their votes in a joint statement. In the statement, Sen. Hatch also issued a word of caution.
"Now that this legislation moves out of Committee and onto the floor, we need to continue to work together to ensure that this smart policy becomes law and ensure that it doesn’t add one dime to our nation’s debt."
WASHINGTON – Congress has moved the ball forward on permanently replacing the Medicare Sustainable Growth Rate formula, but with time short for a fix by year’s end the House has voted to approve a temporary 3-month reprieve from the 20% cut due to take effect Jan. 1.
In a 332-94 vote, with eight abstentions, the House on Dec. 12 approved the Bipartisan Budget Act of 2013, a wide-ranging budget agreement that includes the 3-month patch. The bill also would increase physician pay by 0.5% through March.
The Congressional Budget Office estimated that the temporary fix would cost $3.3 billion in 2014 and a total of $7.3 billion through 2023. The fix would be paid for by cutting Medicaid payments for hospital-based charity care and to long-term care hospitals.
It also would extend the 2% sequestration cuts for Medicare providers by 2 years, from 2021 to 2023.
The Senate has yet to consider the budget package including the SGR patch; it is expected to do so before its holiday recess. President Obama has said that he supports the deal.
The agreement, brokered by House Budget Committee Chairman Paul Ryan (R-Wisc.) and Senate Budget Committee Chairman Patty Murray (D-Wash.), adds about $63 billion in discretionary federal spending over 2 years and makes targeted cuts and fee hikes to bring about overall deficit reduction of about $23 billion.
Although physician groups aren’t thrilled about the continuation of the Medicare cuts under sequestration, most favor the temporary SGR reprieve and the restoration of some funding to federal health programs.
Dr. Ardis Dee Hoven, president of the American Medical Association, said that the continuation of the sequester cuts in Medicare is "frustrating" and poses the risk of destabilizing physician practices. "The concept of the sequester is probably not the best way to rein in spending," she said.
Dr. Clifford A. Hudis, president of the American Society of Clinical Oncology, praised the agreement for providing funds that could restore cuts in medical research and cancer care at the National Institutes of Health. He expressed disappointment, however, that the bill does not reverse cuts to Medicare, including reductions in the payments for physician-administered drugs under Medicare Part B.
"Oncologists are doing everything possible to continue providing care for Medicare patients, but this reduction has forced many in private practice to send patients to hospitals for chemotherapy because they cannot afford to administer these drugs in their office," Dr. Hudis said in a statement.
Physician organizations viewed the patch as necessary while Congress continues to work on a permanent SGR fix. Both the House and Senate took steps toward that goal on Dec. 12.
The House Ways and Means Committee voted 39-0 to approve its replacement proposal, which essentially adds on to the bill approved by the House Energy and Commerce Committee in July.
"This may not be the final step, it’s a very important step forward," said Rep. Kevin Brady (R-Tex.), chairman of the Ways and Means Health Subcommittee.
The House did not address how to pay for the permanent replacement. House Ways and Means Chairman Dave Camp (R-Mich.) noted that the Congressional Budget Office has estimated that it will cost $116 billion over 10 years to repeal the SGR, which is "more than half the cost 2 years ago." Even though that is the lowest estimate ever, "I am of no illusion that finding pay-fors will be an easy task," he said.
The Senate Finance Committee also did not include a way to pay for repeal in its proposal.
The bill had widespread bipartisan support in the committee, but some Senators raised concerns about the lack of a funding mechanism. Sen. Pat Roberts (R-Kan.) said that he wouldn’t support the bill until he could see how it would be funded.
Sen. Orrin Hatch (R- Utah), the committee’s top-ranking Republican, said that the offsets would be worked out once the bill had cleared the initial policymaking phase.
"This bill will be offset, period, or it’s not going to go through both houses," he said. "This bill will be paid for."
The panel agreed to add a provision aimed at expanding access to community mental health services. The amendment, offered by Sen. Debbie Stabenow (D-Mich.) and Sen. Roy Blunt (R-Mo.), would create pilot programs in 10 states to ensure that community behavioral health clinics offer a full range of mental health services, including 24-hour crisis care, substance abuse treatment, and expanded support for families.
Physician groups praised the continued congressional action.
The AMA "strongly commends members of the House Ways and Means Committee and the Senate Finance Committee for the tremendous progress they have made toward repealing Medicare’s failed Sustainable Growth Rate (SGR) formula and creating a stronger Medicare program," Dr. Hoven said in a statement. "The AMA will continue to work collaboratively with Congress so that a bipartisan agreement can be signed into law early next year to repeal the failed SGR payment formula."
The American College of Physicians said that it, too, would work to ensure that a bill moves through Congress and gets to the White House for approval soon.
"The bills reported today ... will help ensure that Medicare patients continue to have access to their physicians," said Dr. Charles Cutler, chairman of the ACP Board of Regents. "Their efforts will work to stabilize payments, provide multiple pathways for physicians to qualify for positive updates and to participate in alternative payment models, create positive incentives for patient-centered medical homes, provide assistance to small practices, and needed funding for development of quality measures."
The American College of Cardiology said in a statement that the proposals accomplished two of its highest priorities: eliminating the SGR and including provisions that will emphasize quality of care, including "provisions that emphasize the importance of clinical data registries, quality measure development, and appropriate use criteria to promote evidence-based care."
"We caution that our final support rests upon the caveat that paying for this legislation must not cause harm to patients and the physicians who care for them," Dr. John Gordon Harold, ACC president, said in the statement.
Legislators from the Finance Committee and the Ways and Means Committee celebrated their votes in a joint statement. In the statement, Sen. Hatch also issued a word of caution.
"Now that this legislation moves out of Committee and onto the floor, we need to continue to work together to ensure that this smart policy becomes law and ensure that it doesn’t add one dime to our nation’s debt."
Congress poised to vote on 3-month SGR patch
WASHINGTON – Congress is preparing to vote on a proposal that would give physicians a temporary 3-month reprieve from the 20% Medicare pay cut that’s due to take effect on Jan. 1.
The proposal was quickly attached to legislation federal budget legislation that would also ameliorate some of the automatic, across-the-board spending cuts known as sequestration.
The House is scheduled to vote on the budget measure before it leaves for a month-long recess on Dec. 13. It is expected that the "doc fix" proposal could be voted on within the same time frame; however, it may not get support of the full House, even though there is bipartisan consensus to replace the Sustainable Growth Rate formula.
At a House Rules Committee hearing on Dec. 11, Democratic leaders said that they would not support the temporary SGR patch unless Republicans agreed to also vote on restoring unemployment compensation benefits for 3 months. Those benefits are due to expire at the end of December for 1.3 million Americans.
"I’m not sure that Democrats can vote for a package that adds SGR, which we support, but does not allow us to address long-term unemployment," said Rep. Nita Lowey (D-N.Y.).
The SGR amendment would increase physician fees by 0.5% for January-March 2014 and encourage Congress to keep working on a new, permanent Medicare fee system teamed with reduced administrative burdens and timely feedback on performance and to develop new payment models.
The temporary fix would be paid for by adjusting disproportionate share payments for hospitals, according to Rep. Michael Burgess (R-Tex.), an ob.gyn. who serves on the Rules Committee. The payment mechanism is noncontroversial, he said in an interview.
Physician groups have said that they would support a short-term fix, provided lawmakers continue working on a permanent repeal of the SGR.
"This is simply a pathway," Dr. Ardis Dee Hoven, president of the American Medical Association, said in an interview. "We want to keep up the momentum to get the SGR repealed. We recognize that it’s going to take a little more time."
Dr. Molly Cooke, president of the American College of Physicians, agreed. "This measure will allow Congress time to complete work early next year on comprehensive legislation to repeal the Medicare SGR formula," she said in a statement.
The Senate Finance Committee and the House Ways and Means Committee are both meeting to vote on proposals to permanently replace the SGR on Dec. 12.
On Twitter @aliciaault
WASHINGTON – Congress is preparing to vote on a proposal that would give physicians a temporary 3-month reprieve from the 20% Medicare pay cut that’s due to take effect on Jan. 1.
The proposal was quickly attached to legislation federal budget legislation that would also ameliorate some of the automatic, across-the-board spending cuts known as sequestration.
The House is scheduled to vote on the budget measure before it leaves for a month-long recess on Dec. 13. It is expected that the "doc fix" proposal could be voted on within the same time frame; however, it may not get support of the full House, even though there is bipartisan consensus to replace the Sustainable Growth Rate formula.
At a House Rules Committee hearing on Dec. 11, Democratic leaders said that they would not support the temporary SGR patch unless Republicans agreed to also vote on restoring unemployment compensation benefits for 3 months. Those benefits are due to expire at the end of December for 1.3 million Americans.
"I’m not sure that Democrats can vote for a package that adds SGR, which we support, but does not allow us to address long-term unemployment," said Rep. Nita Lowey (D-N.Y.).
The SGR amendment would increase physician fees by 0.5% for January-March 2014 and encourage Congress to keep working on a new, permanent Medicare fee system teamed with reduced administrative burdens and timely feedback on performance and to develop new payment models.
The temporary fix would be paid for by adjusting disproportionate share payments for hospitals, according to Rep. Michael Burgess (R-Tex.), an ob.gyn. who serves on the Rules Committee. The payment mechanism is noncontroversial, he said in an interview.
Physician groups have said that they would support a short-term fix, provided lawmakers continue working on a permanent repeal of the SGR.
"This is simply a pathway," Dr. Ardis Dee Hoven, president of the American Medical Association, said in an interview. "We want to keep up the momentum to get the SGR repealed. We recognize that it’s going to take a little more time."
Dr. Molly Cooke, president of the American College of Physicians, agreed. "This measure will allow Congress time to complete work early next year on comprehensive legislation to repeal the Medicare SGR formula," she said in a statement.
The Senate Finance Committee and the House Ways and Means Committee are both meeting to vote on proposals to permanently replace the SGR on Dec. 12.
On Twitter @aliciaault
WASHINGTON – Congress is preparing to vote on a proposal that would give physicians a temporary 3-month reprieve from the 20% Medicare pay cut that’s due to take effect on Jan. 1.
The proposal was quickly attached to legislation federal budget legislation that would also ameliorate some of the automatic, across-the-board spending cuts known as sequestration.
The House is scheduled to vote on the budget measure before it leaves for a month-long recess on Dec. 13. It is expected that the "doc fix" proposal could be voted on within the same time frame; however, it may not get support of the full House, even though there is bipartisan consensus to replace the Sustainable Growth Rate formula.
At a House Rules Committee hearing on Dec. 11, Democratic leaders said that they would not support the temporary SGR patch unless Republicans agreed to also vote on restoring unemployment compensation benefits for 3 months. Those benefits are due to expire at the end of December for 1.3 million Americans.
"I’m not sure that Democrats can vote for a package that adds SGR, which we support, but does not allow us to address long-term unemployment," said Rep. Nita Lowey (D-N.Y.).
The SGR amendment would increase physician fees by 0.5% for January-March 2014 and encourage Congress to keep working on a new, permanent Medicare fee system teamed with reduced administrative burdens and timely feedback on performance and to develop new payment models.
The temporary fix would be paid for by adjusting disproportionate share payments for hospitals, according to Rep. Michael Burgess (R-Tex.), an ob.gyn. who serves on the Rules Committee. The payment mechanism is noncontroversial, he said in an interview.
Physician groups have said that they would support a short-term fix, provided lawmakers continue working on a permanent repeal of the SGR.
"This is simply a pathway," Dr. Ardis Dee Hoven, president of the American Medical Association, said in an interview. "We want to keep up the momentum to get the SGR repealed. We recognize that it’s going to take a little more time."
Dr. Molly Cooke, president of the American College of Physicians, agreed. "This measure will allow Congress time to complete work early next year on comprehensive legislation to repeal the Medicare SGR formula," she said in a statement.
The Senate Finance Committee and the House Ways and Means Committee are both meeting to vote on proposals to permanently replace the SGR on Dec. 12.
On Twitter @aliciaault
AT A HOUSE RULES COMMITTEE HEARING
ASCO slams federal cuts in its annual report
Genomics and personalized medicine are transforming approaches to cancer therapy, but continuing federal budget cuts threaten to derail progress in cancer treatment, according to an annual report issued by the American Society of Clinical Oncology.
The report, "Clinical Cancer Advances 2013: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology," was published online Dec. 10 in the Journal of Clinical Oncology (doi:10.1200/JCO.2013.53.7076) and represents the 9th year in a row that ASCO has documented the successes and challenges facing oncology.
"The insights described in this report, such as how cancers hide from the immune system and why cancers may become resistant to targeted drugs, enable us to envision a future in which cancer will be even more controllable and preventable," ASCO President Clifford A. Hudis said in a message accompanying the report.
In good news, overall cancer-related death rates declined 1.5% per year from 2000 to 2009. Cancer is still the second-leading cause of death, however. In 2013, there will be 1.6 million new cancer cases in the United States, and cancer will claim 580,000 American lives, according to the report’s executive summary. The incidence of cancer is declining in men, stable in women, and on the rise in African American and Hispanic children.
The report highlighted the work of The Cancer Genome Atlas (TCGA) research network, launched in 2009 by the National Institutes of Health, and its role in charting genomic changes in more than 20 different cancer types, including reporting results of comprehensive molecular analyses of kidney and endometrial cancers as well as acute myeloid leukemia. Additionally, results from the federally funded Lung Cancer Research Consortium gave rise to genomic testing that matches lung cancer patients with the most appropriate therapies.
The approvals of nine new cancer therapies – and expanded indications for six existing therapies – by the Food and Drug Administration through Oct. 2013 reflect the agency’s efforts to bring cancer drugs to market quickly, the report said.
Yet "our position as a world leader in advancing medical knowledge and our ability to attract the most promising and talented investigators are now threatened by an acute problem: Federal funding for cancer research has steadily eroded over the past decade, and only 15% of the ever-shrinking budget is actually spent on clinical trials," Dr. Hudis said in his accompanying message. "This dismal reality threatens the pace of progress against cancer and undermines our ability to address the continuing needs of our patients."
On top of continuing cuts to the NIH budget, automatic budget cuts known as sequestration went into effect in March. Those across-the-board cuts were required by the Budget Control Act of 2011, and are due to continue through 2021, unless Congress can come up with an alternative way to reduce the deficit.
The National Cancer Institute cut its budget by 6%. According to the ASCO report, "the number of new grants supported by NIH is at its lowest level since 1998, with only one in six highly ranked grant proposals currently receiving funding." At institutions that rely on NCI funding, employees are being laid off, there are delays in translational research, and clinical trials are being postponed. Based on an August survey of ASCO members, 75% who responded said that the funding crunch is curbing their ability to do research.
ASCO is petitioning for increases in the NIH and NCI budgets for 2014, for more funding for the NCI National Clinical Trials Network, and to exempt the agencies from sequestration.
The Cancer Advances report was developed by an 18-person editorial board of prominent oncologists who reviewed peer-reviewed research published in journals or presented at major scientific meetings from Oct. 2012 to Sept. 2013. A group of expert advisers provided an additional round of review within their practice specialties. The report features 76 studies, covering prevention, screening, treatment, patient and survivor care, biomarkers, tumor biology, and cancer disparities.
On Twitter @aliciaault
Genomics and personalized medicine are transforming approaches to cancer therapy, but continuing federal budget cuts threaten to derail progress in cancer treatment, according to an annual report issued by the American Society of Clinical Oncology.
The report, "Clinical Cancer Advances 2013: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology," was published online Dec. 10 in the Journal of Clinical Oncology (doi:10.1200/JCO.2013.53.7076) and represents the 9th year in a row that ASCO has documented the successes and challenges facing oncology.
"The insights described in this report, such as how cancers hide from the immune system and why cancers may become resistant to targeted drugs, enable us to envision a future in which cancer will be even more controllable and preventable," ASCO President Clifford A. Hudis said in a message accompanying the report.
In good news, overall cancer-related death rates declined 1.5% per year from 2000 to 2009. Cancer is still the second-leading cause of death, however. In 2013, there will be 1.6 million new cancer cases in the United States, and cancer will claim 580,000 American lives, according to the report’s executive summary. The incidence of cancer is declining in men, stable in women, and on the rise in African American and Hispanic children.
The report highlighted the work of The Cancer Genome Atlas (TCGA) research network, launched in 2009 by the National Institutes of Health, and its role in charting genomic changes in more than 20 different cancer types, including reporting results of comprehensive molecular analyses of kidney and endometrial cancers as well as acute myeloid leukemia. Additionally, results from the federally funded Lung Cancer Research Consortium gave rise to genomic testing that matches lung cancer patients with the most appropriate therapies.
The approvals of nine new cancer therapies – and expanded indications for six existing therapies – by the Food and Drug Administration through Oct. 2013 reflect the agency’s efforts to bring cancer drugs to market quickly, the report said.
Yet "our position as a world leader in advancing medical knowledge and our ability to attract the most promising and talented investigators are now threatened by an acute problem: Federal funding for cancer research has steadily eroded over the past decade, and only 15% of the ever-shrinking budget is actually spent on clinical trials," Dr. Hudis said in his accompanying message. "This dismal reality threatens the pace of progress against cancer and undermines our ability to address the continuing needs of our patients."
On top of continuing cuts to the NIH budget, automatic budget cuts known as sequestration went into effect in March. Those across-the-board cuts were required by the Budget Control Act of 2011, and are due to continue through 2021, unless Congress can come up with an alternative way to reduce the deficit.
The National Cancer Institute cut its budget by 6%. According to the ASCO report, "the number of new grants supported by NIH is at its lowest level since 1998, with only one in six highly ranked grant proposals currently receiving funding." At institutions that rely on NCI funding, employees are being laid off, there are delays in translational research, and clinical trials are being postponed. Based on an August survey of ASCO members, 75% who responded said that the funding crunch is curbing their ability to do research.
ASCO is petitioning for increases in the NIH and NCI budgets for 2014, for more funding for the NCI National Clinical Trials Network, and to exempt the agencies from sequestration.
The Cancer Advances report was developed by an 18-person editorial board of prominent oncologists who reviewed peer-reviewed research published in journals or presented at major scientific meetings from Oct. 2012 to Sept. 2013. A group of expert advisers provided an additional round of review within their practice specialties. The report features 76 studies, covering prevention, screening, treatment, patient and survivor care, biomarkers, tumor biology, and cancer disparities.
On Twitter @aliciaault
Genomics and personalized medicine are transforming approaches to cancer therapy, but continuing federal budget cuts threaten to derail progress in cancer treatment, according to an annual report issued by the American Society of Clinical Oncology.
The report, "Clinical Cancer Advances 2013: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology," was published online Dec. 10 in the Journal of Clinical Oncology (doi:10.1200/JCO.2013.53.7076) and represents the 9th year in a row that ASCO has documented the successes and challenges facing oncology.
"The insights described in this report, such as how cancers hide from the immune system and why cancers may become resistant to targeted drugs, enable us to envision a future in which cancer will be even more controllable and preventable," ASCO President Clifford A. Hudis said in a message accompanying the report.
In good news, overall cancer-related death rates declined 1.5% per year from 2000 to 2009. Cancer is still the second-leading cause of death, however. In 2013, there will be 1.6 million new cancer cases in the United States, and cancer will claim 580,000 American lives, according to the report’s executive summary. The incidence of cancer is declining in men, stable in women, and on the rise in African American and Hispanic children.
The report highlighted the work of The Cancer Genome Atlas (TCGA) research network, launched in 2009 by the National Institutes of Health, and its role in charting genomic changes in more than 20 different cancer types, including reporting results of comprehensive molecular analyses of kidney and endometrial cancers as well as acute myeloid leukemia. Additionally, results from the federally funded Lung Cancer Research Consortium gave rise to genomic testing that matches lung cancer patients with the most appropriate therapies.
The approvals of nine new cancer therapies – and expanded indications for six existing therapies – by the Food and Drug Administration through Oct. 2013 reflect the agency’s efforts to bring cancer drugs to market quickly, the report said.
Yet "our position as a world leader in advancing medical knowledge and our ability to attract the most promising and talented investigators are now threatened by an acute problem: Federal funding for cancer research has steadily eroded over the past decade, and only 15% of the ever-shrinking budget is actually spent on clinical trials," Dr. Hudis said in his accompanying message. "This dismal reality threatens the pace of progress against cancer and undermines our ability to address the continuing needs of our patients."
On top of continuing cuts to the NIH budget, automatic budget cuts known as sequestration went into effect in March. Those across-the-board cuts were required by the Budget Control Act of 2011, and are due to continue through 2021, unless Congress can come up with an alternative way to reduce the deficit.
The National Cancer Institute cut its budget by 6%. According to the ASCO report, "the number of new grants supported by NIH is at its lowest level since 1998, with only one in six highly ranked grant proposals currently receiving funding." At institutions that rely on NCI funding, employees are being laid off, there are delays in translational research, and clinical trials are being postponed. Based on an August survey of ASCO members, 75% who responded said that the funding crunch is curbing their ability to do research.
ASCO is petitioning for increases in the NIH and NCI budgets for 2014, for more funding for the NCI National Clinical Trials Network, and to exempt the agencies from sequestration.
The Cancer Advances report was developed by an 18-person editorial board of prominent oncologists who reviewed peer-reviewed research published in journals or presented at major scientific meetings from Oct. 2012 to Sept. 2013. A group of expert advisers provided an additional round of review within their practice specialties. The report features 76 studies, covering prevention, screening, treatment, patient and survivor care, biomarkers, tumor biology, and cancer disparities.
On Twitter @aliciaault
FROM THE JOURNAL OF CLINICAL ONCOLOGY
ACA enrollment grows, but still less than expected
Even with a burst of activity on the state and federal health insurance exchanges during November, numbers released Dec. 11 by the Health and Human Services department indicate that the Obama administration may not be on track to meet the goal set by the Congressional Budget Office – insuring 7 million individuals by Mar. 31, 2014, the end of the current open enrollment for health insurance under the Affordable Care Act.
But Michael Hash, director of the HHS Office of Health Reform, said in a briefing with reporters, "We’re on track." Americans still have 6 months to enroll, he noted, and the expectation is that most people will sign up towards the end of that period.
According to HHS’s mid-December enrollment report, 364,682 individuals selected a health insurance plan from a state or federal exchange in October and November. The bulk of those (227,478) used a state-based exchange, while 137,204 signed up via the federal exchange, which operates in 36 states. The biggest proportion of overall sign-ups came in November, with 258,497 selecting a plan in the state or federal exchanges.
The numbers include consumers who have paid their first health insurance premium as well as those who have not.
About 2.3 million Americans have been determined to be eligible to enroll in a marketplace plan, but only 364,000 have actually selected a plan. Of the 2.3 million, 41% (944,531) are eligible for financial assistance. That number includes people who didn’t apply for financial assistance, who applied for financial assistance and were found ineligible, or those whose applications for financial assistance are pending, according to the HHS report.
Further, just over 803,000 consumers have been determined to be eligible for coverage from Medicaid or the Children’s Health Insurance Program (CHIP).
Enrollment has increased in part because of improvements to the federal exchange website, healthcare.gov. Mr. Hash said. "Healthcare.gov is night and day from where it was on October the first," he said in comments made in advance of testimony by HHS Secretary Kathleen Sebelius at a hearing called by the House Energy and Commerce Committee’s Health Subcommittee.
Overall, 83% of completed applications were done electronically, either through healthcare.gov or a state exchange website, or online when individuals applied at a community center or some other assistance location.
Mr. Hash also said that demand for exchange plans continues to grow, citing increasing visits to the federal and state websites and calls to the call centers.
Since Oct. 1, there have been 10.6 million visits to state exchanges and 1.7 million calls to state call centers. There have been 28.4 million visits to healthcare.gov and 3.4 million calls.
On Twitter @aliciaault
Even with a burst of activity on the state and federal health insurance exchanges during November, numbers released Dec. 11 by the Health and Human Services department indicate that the Obama administration may not be on track to meet the goal set by the Congressional Budget Office – insuring 7 million individuals by Mar. 31, 2014, the end of the current open enrollment for health insurance under the Affordable Care Act.
But Michael Hash, director of the HHS Office of Health Reform, said in a briefing with reporters, "We’re on track." Americans still have 6 months to enroll, he noted, and the expectation is that most people will sign up towards the end of that period.
According to HHS’s mid-December enrollment report, 364,682 individuals selected a health insurance plan from a state or federal exchange in October and November. The bulk of those (227,478) used a state-based exchange, while 137,204 signed up via the federal exchange, which operates in 36 states. The biggest proportion of overall sign-ups came in November, with 258,497 selecting a plan in the state or federal exchanges.
The numbers include consumers who have paid their first health insurance premium as well as those who have not.
About 2.3 million Americans have been determined to be eligible to enroll in a marketplace plan, but only 364,000 have actually selected a plan. Of the 2.3 million, 41% (944,531) are eligible for financial assistance. That number includes people who didn’t apply for financial assistance, who applied for financial assistance and were found ineligible, or those whose applications for financial assistance are pending, according to the HHS report.
Further, just over 803,000 consumers have been determined to be eligible for coverage from Medicaid or the Children’s Health Insurance Program (CHIP).
Enrollment has increased in part because of improvements to the federal exchange website, healthcare.gov. Mr. Hash said. "Healthcare.gov is night and day from where it was on October the first," he said in comments made in advance of testimony by HHS Secretary Kathleen Sebelius at a hearing called by the House Energy and Commerce Committee’s Health Subcommittee.
Overall, 83% of completed applications were done electronically, either through healthcare.gov or a state exchange website, or online when individuals applied at a community center or some other assistance location.
Mr. Hash also said that demand for exchange plans continues to grow, citing increasing visits to the federal and state websites and calls to the call centers.
Since Oct. 1, there have been 10.6 million visits to state exchanges and 1.7 million calls to state call centers. There have been 28.4 million visits to healthcare.gov and 3.4 million calls.
On Twitter @aliciaault
Even with a burst of activity on the state and federal health insurance exchanges during November, numbers released Dec. 11 by the Health and Human Services department indicate that the Obama administration may not be on track to meet the goal set by the Congressional Budget Office – insuring 7 million individuals by Mar. 31, 2014, the end of the current open enrollment for health insurance under the Affordable Care Act.
But Michael Hash, director of the HHS Office of Health Reform, said in a briefing with reporters, "We’re on track." Americans still have 6 months to enroll, he noted, and the expectation is that most people will sign up towards the end of that period.
According to HHS’s mid-December enrollment report, 364,682 individuals selected a health insurance plan from a state or federal exchange in October and November. The bulk of those (227,478) used a state-based exchange, while 137,204 signed up via the federal exchange, which operates in 36 states. The biggest proportion of overall sign-ups came in November, with 258,497 selecting a plan in the state or federal exchanges.
The numbers include consumers who have paid their first health insurance premium as well as those who have not.
About 2.3 million Americans have been determined to be eligible to enroll in a marketplace plan, but only 364,000 have actually selected a plan. Of the 2.3 million, 41% (944,531) are eligible for financial assistance. That number includes people who didn’t apply for financial assistance, who applied for financial assistance and were found ineligible, or those whose applications for financial assistance are pending, according to the HHS report.
Further, just over 803,000 consumers have been determined to be eligible for coverage from Medicaid or the Children’s Health Insurance Program (CHIP).
Enrollment has increased in part because of improvements to the federal exchange website, healthcare.gov. Mr. Hash said. "Healthcare.gov is night and day from where it was on October the first," he said in comments made in advance of testimony by HHS Secretary Kathleen Sebelius at a hearing called by the House Energy and Commerce Committee’s Health Subcommittee.
Overall, 83% of completed applications were done electronically, either through healthcare.gov or a state exchange website, or online when individuals applied at a community center or some other assistance location.
Mr. Hash also said that demand for exchange plans continues to grow, citing increasing visits to the federal and state websites and calls to the call centers.
Since Oct. 1, there have been 10.6 million visits to state exchanges and 1.7 million calls to state call centers. There have been 28.4 million visits to healthcare.gov and 3.4 million calls.
On Twitter @aliciaault
States take different paths in Medicaid expansion
When the Supreme Court upheld the constitutionality of the Affordable Care Act in 2012, it also ruled that states could choose whether to substantially expand their Medicaid programs. That decision has created a split across the country, with about half of the states choosing to take federal dollars to expand their programs and the others opting out.
There’s no deadline on Medicaid expansion, so those states that have opted out so far can always choose to expand at a later date.

Here’s a look at two bellwether states: California, which has accepted federal money to expand its Medi-Cal program, and Texas, which has opted out of the expansion.
California Gov. Jerry Brown (D), a strong supporter of the ACA, announced that his state would expand Medicaid to include low-income individuals up to 138% of the federal poverty level starting on Jan. 1, 2014. All of the new enrollees – likely 1 million Californians – will be added to the state’s growing Medicaid managed care program.
Conversely, Texas Gov. Rick Perry (R) deemed Medicaid expansion "a misguided, and ultimately doomed, attempt to mask the shortcomings of Obamacare."
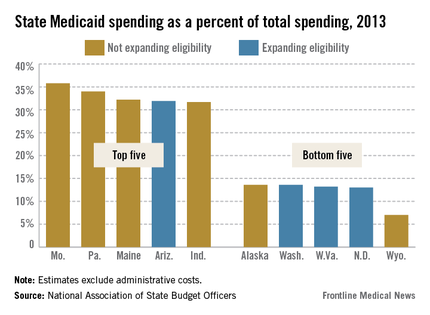
California: Pay cuts complicate expansion
Physicians in California are bracing for a significant Medicaid pay cut at the same time as about a million residents are set to join the program.
The pending cuts are just one part of a complex health care picture in California, where experts are far from certain about what the expansion of Medi-Cal will mean for physicians and patients.
"I think we’re going to have a real problem in California with all the new Medi-Cal patients," said Dr. Mark Dressner, who works at a federally qualified health center in Long Beach and is the president of the California Academy of Family Physicians. "Who is going to see them when there’s going to be so many other people in the system?"
The Kaiser Family Foundation estimates that between 990,000 and 1.4 million individuals, mostly single adults, will enter Medi-Cal by 2019 because of the ACA-permitted expansion. Currently, about 8.5 million residents are enrolled. Newly eligible individuals will be enrolled in the program’s managed care health plans.
These new patients could have a hard time finding a doctor. Medi-Cal is one of the lowest payers in the nation, paying between $18 and $24 for an office visit, according to the California Medical Association.
That’s about to be compounded by a pay cut approved by the state and the Centers for Medicare and Medicaid Services in 2011. Physicians tried unsuccessfully to fight the cut in court. Now the state is implementing it retroactively, which means that Medi-Cal payments could be cut 15%-20% over the next few years.
"It really undermines efforts to successfully implement the Affordable Care Act in California," said Lisa Folberg, vice president of medical and regulatory policy at the California Medical Association.
There are broad exceptions to the cut. It does not apply to most primary care services in either managed care plans or fee-for-service Medi-Cal, or to specialty services provided through managed care plans, Ms. Folberg said. And some managed care companies have announced that they will use their discretion in setting payment rates to shield their contracted physicians from the cuts.
"Unfortunately, that doesn’t help the cancer patient on Medi-Cal in Bakersfield who has fee-for-service Medi-Cal and can’t find an oncologist," Ms. Folberg said.
Dr. Darin Latimore, president of the California chapter of the American College of Physicians, said large health systems likely will be able to work around the cuts, using group visits and physician extenders. But small physician practices in rural areas aren’t equipped to make those changes, he said.
"They are not in a position to move work on to others in order to be more efficient and see more Medi-Cal patients," said Dr. Latimore, associate dean of medicine at the University of California Davis.
The result will be that physicians who work outside of the state’s safety net system will be less likely to participate in the Medi-Cal program or will strictly limit the number of patients they see. That will translate to longer waits to get an appointment and shorter visits for Medi-Cal patients.
Another concern: Patients entering Medi-Cal managed care plans might not have access to a broad network of physicians. While California has network adequacy laws, state oversight is inadequate, according to Ms. Folberg. For the most part, the plans are on an honor system.
There are some bright spots for the Medi-Cal expansion. The ACA includes an increase in Medicaid payments to physicians for 146 primary care services provided in 2013 and 2014. The provision temporarily raises payments up to Medicare rates, which for California physicians means a 136% hike on average, according to an analysis from the Kaiser Family Foundation.
But those payments were delayed, with the first checks going out to physicians in November 2013. That delay could cost the state in physician participation.
"I think the longer it takes to implement that, the less likely it is that it will affect any decisions about whether to participate more fully in the program than you would have otherwise," said Christopher Perrone, deputy director of the health reform and public programs initiative at the California HealthCare Foundation.
And the temporary nature of the pay increase adds to the problem, Mr. Perrone said. "I don’t see California sustaining that increase on its own and I haven’t heard anyone suggest that the federal government would sustain it after the 2 years."
Mr. Perrone said it’s more likely that physicians who are already committed to participating in Medi-Cal will use the money to invest in electronic health records and other telemedicine features, and hire medical assistants.
But with so many small and solo physicians shying away from Medi-Cal because of low payments, community clinics and health centers will have to pick up the slack.
"All these issues together really point to the importance of the community health centers and the role we are going to be playing in ensuring access," said Carmela Castellano-Garcia, president and CEO of the California Primary Care Association.
Federally qualified health centers are in a better position financially because they are paid an enhanced Medicaid rate and won’t be subject to the coming Medi-Cal cuts, Ms. Castellano-Garcia said. And the ACA has directed an influx of cash to these centers as well – more than $500 million in California alone to establish new sites, expand services, and support major capital improvement projects, according to the Health and Human Services department.
Texas: No expansion means doctors will keep feeling pressure
Texas has the highest number of uninsured residents in the United States – a quarter of its 26 million residents lacking coverage – but Gov. Rick Perry (R) refused to expand Medicaid, which could cover as many as 500,000 to 1 million Texans.
The governor’s decision will stay in place at least until 2015, when the state legislature reconvenes.
Some physicians in Texas are not upset by the decision – they consider Medicaid to be low-paying program and full of bureaucratic hassles.
Others – including many of the primary care organizations – disagree.
The Texas Medical Association, the Texas Academy of Family Physicians, and the Texas chapter of the American Congress of Obstetricians and Gynecologists all support the expansion of Medicaid allowed by the ACA.
In April, Gov. Perry reiterated his position. "Medicaid expansion is a misguided, and ultimately doomed, attempt to mask the shortcomings of Obamacare," he said in a statement. Instead of expansion, he favors flexibility for the state to manage its Medicaid program.
State Rep. John Zerwas (R-Simonton), an anesthesiologist, introduced H.B. 3791 that would give that flexibility, but it did not get consideration by the full House before the legislature adjourned in May.
The TMA supported Dr. Zerwas’ proposal, but also is in favor of expanding Medicaid, said Dr. Stephen L. Brotherton, TMA president. More people would have some type of insurance, but they might not necessarily have good access to care, he said.
That’s because Texas has a shortage of primary care physicians. The number of primary care physicians per capita is lower than the national average – at about 70/100,000 in 2011, compared with 80/100,000 nationally, according to the Texas Department of State Health Services, in the publication "Supply Trends Among Licensed Health Professions, Texas, 1980-2011. In rural areas, it’s even lower – about 50/100,000.
Then there’s the question of just how many physicians will take Medicaid. A 2012 TMA survey found that only 31% of doctors in the state were accepting new Medicaid patients.
Medicaid payment rates are so low that Dr. Brotherton, who practices in Ft. Worth, said that he treats Medicaid patients as charity care. "It’s much less expensive for me to do it for nothing as donated time," he said.
Dr. Moss Hampton, chairman of District XI of ACOG, added, "Medicaid doesn’t cover the cost of taking care of the patient."
For Medicaid expansion to eventually be successful, "there would have to be a better payment rate and less of a hassle factor," said Dr. Hampton, chairman of the obstetrics and gynecology department at Texas Tech Health Sciences Center at the Permian Basin in Odessa.
"It’s hard to get doctors to accept Medicaid because of the rates they pay," agreed Dr. Clare Hawkins, TAFP president, who added that Texas physicians also feel that it’s hard to comply with differing rules among various Medicaid managed care programs.
Even so, expansion will mean getting more patients into preventive care, and a reduction in emergency department visits and more expensive hospital care – costs that are being borne by all Texans, including physicians, said Dr. Hawkins, who is program director of the San Jacinto Methodist Hospital Family Medicine Residency Program in Baytown, Texas.
Although uninsured Texas residents are already receiving care – in emergency departments and at clinics – Medicaid expansion could bring a big uptick in office visits, especially to ob.gyn. practices, Dr. Hampton said. The need for those services is growing with a state law that went into effect on Oct. 29 that makes it prohibitive for most Planned Parenthood clinics and other community clinics that provide abortion services to stay open.
"What that’s done is cut out a very large group of providers, and now we’re trying to find providers to help take care of those folks," who normally use those clinics, she said.
In the absence of Medicaid expansion, Texas physicians are hoping to start receiving higher Medicaid payments that were due to start in Jan. 2013. An estimated 25,000 Texas doctors are eligible for Medicaid pay that will be on par with Medicare. But Texas has not begun to distribute that money and will not likely do so until March, according to the TMA.
When the Supreme Court upheld the constitutionality of the Affordable Care Act in 2012, it also ruled that states could choose whether to substantially expand their Medicaid programs. That decision has created a split across the country, with about half of the states choosing to take federal dollars to expand their programs and the others opting out.
There’s no deadline on Medicaid expansion, so those states that have opted out so far can always choose to expand at a later date.

Here’s a look at two bellwether states: California, which has accepted federal money to expand its Medi-Cal program, and Texas, which has opted out of the expansion.
California Gov. Jerry Brown (D), a strong supporter of the ACA, announced that his state would expand Medicaid to include low-income individuals up to 138% of the federal poverty level starting on Jan. 1, 2014. All of the new enrollees – likely 1 million Californians – will be added to the state’s growing Medicaid managed care program.
Conversely, Texas Gov. Rick Perry (R) deemed Medicaid expansion "a misguided, and ultimately doomed, attempt to mask the shortcomings of Obamacare."

California: Pay cuts complicate expansion
Physicians in California are bracing for a significant Medicaid pay cut at the same time as about a million residents are set to join the program.
The pending cuts are just one part of a complex health care picture in California, where experts are far from certain about what the expansion of Medi-Cal will mean for physicians and patients.
"I think we’re going to have a real problem in California with all the new Medi-Cal patients," said Dr. Mark Dressner, who works at a federally qualified health center in Long Beach and is the president of the California Academy of Family Physicians. "Who is going to see them when there’s going to be so many other people in the system?"
The Kaiser Family Foundation estimates that between 990,000 and 1.4 million individuals, mostly single adults, will enter Medi-Cal by 2019 because of the ACA-permitted expansion. Currently, about 8.5 million residents are enrolled. Newly eligible individuals will be enrolled in the program’s managed care health plans.
These new patients could have a hard time finding a doctor. Medi-Cal is one of the lowest payers in the nation, paying between $18 and $24 for an office visit, according to the California Medical Association.
That’s about to be compounded by a pay cut approved by the state and the Centers for Medicare and Medicaid Services in 2011. Physicians tried unsuccessfully to fight the cut in court. Now the state is implementing it retroactively, which means that Medi-Cal payments could be cut 15%-20% over the next few years.
"It really undermines efforts to successfully implement the Affordable Care Act in California," said Lisa Folberg, vice president of medical and regulatory policy at the California Medical Association.
There are broad exceptions to the cut. It does not apply to most primary care services in either managed care plans or fee-for-service Medi-Cal, or to specialty services provided through managed care plans, Ms. Folberg said. And some managed care companies have announced that they will use their discretion in setting payment rates to shield their contracted physicians from the cuts.
"Unfortunately, that doesn’t help the cancer patient on Medi-Cal in Bakersfield who has fee-for-service Medi-Cal and can’t find an oncologist," Ms. Folberg said.
Dr. Darin Latimore, president of the California chapter of the American College of Physicians, said large health systems likely will be able to work around the cuts, using group visits and physician extenders. But small physician practices in rural areas aren’t equipped to make those changes, he said.
"They are not in a position to move work on to others in order to be more efficient and see more Medi-Cal patients," said Dr. Latimore, associate dean of medicine at the University of California Davis.
The result will be that physicians who work outside of the state’s safety net system will be less likely to participate in the Medi-Cal program or will strictly limit the number of patients they see. That will translate to longer waits to get an appointment and shorter visits for Medi-Cal patients.
Another concern: Patients entering Medi-Cal managed care plans might not have access to a broad network of physicians. While California has network adequacy laws, state oversight is inadequate, according to Ms. Folberg. For the most part, the plans are on an honor system.
There are some bright spots for the Medi-Cal expansion. The ACA includes an increase in Medicaid payments to physicians for 146 primary care services provided in 2013 and 2014. The provision temporarily raises payments up to Medicare rates, which for California physicians means a 136% hike on average, according to an analysis from the Kaiser Family Foundation.
But those payments were delayed, with the first checks going out to physicians in November 2013. That delay could cost the state in physician participation.
"I think the longer it takes to implement that, the less likely it is that it will affect any decisions about whether to participate more fully in the program than you would have otherwise," said Christopher Perrone, deputy director of the health reform and public programs initiative at the California HealthCare Foundation.
And the temporary nature of the pay increase adds to the problem, Mr. Perrone said. "I don’t see California sustaining that increase on its own and I haven’t heard anyone suggest that the federal government would sustain it after the 2 years."
Mr. Perrone said it’s more likely that physicians who are already committed to participating in Medi-Cal will use the money to invest in electronic health records and other telemedicine features, and hire medical assistants.
But with so many small and solo physicians shying away from Medi-Cal because of low payments, community clinics and health centers will have to pick up the slack.
"All these issues together really point to the importance of the community health centers and the role we are going to be playing in ensuring access," said Carmela Castellano-Garcia, president and CEO of the California Primary Care Association.
Federally qualified health centers are in a better position financially because they are paid an enhanced Medicaid rate and won’t be subject to the coming Medi-Cal cuts, Ms. Castellano-Garcia said. And the ACA has directed an influx of cash to these centers as well – more than $500 million in California alone to establish new sites, expand services, and support major capital improvement projects, according to the Health and Human Services department.
Texas: No expansion means doctors will keep feeling pressure
Texas has the highest number of uninsured residents in the United States – a quarter of its 26 million residents lacking coverage – but Gov. Rick Perry (R) refused to expand Medicaid, which could cover as many as 500,000 to 1 million Texans.
The governor’s decision will stay in place at least until 2015, when the state legislature reconvenes.
Some physicians in Texas are not upset by the decision – they consider Medicaid to be low-paying program and full of bureaucratic hassles.
Others – including many of the primary care organizations – disagree.
The Texas Medical Association, the Texas Academy of Family Physicians, and the Texas chapter of the American Congress of Obstetricians and Gynecologists all support the expansion of Medicaid allowed by the ACA.
In April, Gov. Perry reiterated his position. "Medicaid expansion is a misguided, and ultimately doomed, attempt to mask the shortcomings of Obamacare," he said in a statement. Instead of expansion, he favors flexibility for the state to manage its Medicaid program.
State Rep. John Zerwas (R-Simonton), an anesthesiologist, introduced H.B. 3791 that would give that flexibility, but it did not get consideration by the full House before the legislature adjourned in May.
The TMA supported Dr. Zerwas’ proposal, but also is in favor of expanding Medicaid, said Dr. Stephen L. Brotherton, TMA president. More people would have some type of insurance, but they might not necessarily have good access to care, he said.
That’s because Texas has a shortage of primary care physicians. The number of primary care physicians per capita is lower than the national average – at about 70/100,000 in 2011, compared with 80/100,000 nationally, according to the Texas Department of State Health Services, in the publication "Supply Trends Among Licensed Health Professions, Texas, 1980-2011. In rural areas, it’s even lower – about 50/100,000.
Then there’s the question of just how many physicians will take Medicaid. A 2012 TMA survey found that only 31% of doctors in the state were accepting new Medicaid patients.
Medicaid payment rates are so low that Dr. Brotherton, who practices in Ft. Worth, said that he treats Medicaid patients as charity care. "It’s much less expensive for me to do it for nothing as donated time," he said.
Dr. Moss Hampton, chairman of District XI of ACOG, added, "Medicaid doesn’t cover the cost of taking care of the patient."
For Medicaid expansion to eventually be successful, "there would have to be a better payment rate and less of a hassle factor," said Dr. Hampton, chairman of the obstetrics and gynecology department at Texas Tech Health Sciences Center at the Permian Basin in Odessa.
"It’s hard to get doctors to accept Medicaid because of the rates they pay," agreed Dr. Clare Hawkins, TAFP president, who added that Texas physicians also feel that it’s hard to comply with differing rules among various Medicaid managed care programs.
Even so, expansion will mean getting more patients into preventive care, and a reduction in emergency department visits and more expensive hospital care – costs that are being borne by all Texans, including physicians, said Dr. Hawkins, who is program director of the San Jacinto Methodist Hospital Family Medicine Residency Program in Baytown, Texas.
Although uninsured Texas residents are already receiving care – in emergency departments and at clinics – Medicaid expansion could bring a big uptick in office visits, especially to ob.gyn. practices, Dr. Hampton said. The need for those services is growing with a state law that went into effect on Oct. 29 that makes it prohibitive for most Planned Parenthood clinics and other community clinics that provide abortion services to stay open.
"What that’s done is cut out a very large group of providers, and now we’re trying to find providers to help take care of those folks," who normally use those clinics, she said.
In the absence of Medicaid expansion, Texas physicians are hoping to start receiving higher Medicaid payments that were due to start in Jan. 2013. An estimated 25,000 Texas doctors are eligible for Medicaid pay that will be on par with Medicare. But Texas has not begun to distribute that money and will not likely do so until March, according to the TMA.
When the Supreme Court upheld the constitutionality of the Affordable Care Act in 2012, it also ruled that states could choose whether to substantially expand their Medicaid programs. That decision has created a split across the country, with about half of the states choosing to take federal dollars to expand their programs and the others opting out.
There’s no deadline on Medicaid expansion, so those states that have opted out so far can always choose to expand at a later date.

Here’s a look at two bellwether states: California, which has accepted federal money to expand its Medi-Cal program, and Texas, which has opted out of the expansion.
California Gov. Jerry Brown (D), a strong supporter of the ACA, announced that his state would expand Medicaid to include low-income individuals up to 138% of the federal poverty level starting on Jan. 1, 2014. All of the new enrollees – likely 1 million Californians – will be added to the state’s growing Medicaid managed care program.
Conversely, Texas Gov. Rick Perry (R) deemed Medicaid expansion "a misguided, and ultimately doomed, attempt to mask the shortcomings of Obamacare."

California: Pay cuts complicate expansion
Physicians in California are bracing for a significant Medicaid pay cut at the same time as about a million residents are set to join the program.
The pending cuts are just one part of a complex health care picture in California, where experts are far from certain about what the expansion of Medi-Cal will mean for physicians and patients.
"I think we’re going to have a real problem in California with all the new Medi-Cal patients," said Dr. Mark Dressner, who works at a federally qualified health center in Long Beach and is the president of the California Academy of Family Physicians. "Who is going to see them when there’s going to be so many other people in the system?"
The Kaiser Family Foundation estimates that between 990,000 and 1.4 million individuals, mostly single adults, will enter Medi-Cal by 2019 because of the ACA-permitted expansion. Currently, about 8.5 million residents are enrolled. Newly eligible individuals will be enrolled in the program’s managed care health plans.
These new patients could have a hard time finding a doctor. Medi-Cal is one of the lowest payers in the nation, paying between $18 and $24 for an office visit, according to the California Medical Association.
That’s about to be compounded by a pay cut approved by the state and the Centers for Medicare and Medicaid Services in 2011. Physicians tried unsuccessfully to fight the cut in court. Now the state is implementing it retroactively, which means that Medi-Cal payments could be cut 15%-20% over the next few years.
"It really undermines efforts to successfully implement the Affordable Care Act in California," said Lisa Folberg, vice president of medical and regulatory policy at the California Medical Association.
There are broad exceptions to the cut. It does not apply to most primary care services in either managed care plans or fee-for-service Medi-Cal, or to specialty services provided through managed care plans, Ms. Folberg said. And some managed care companies have announced that they will use their discretion in setting payment rates to shield their contracted physicians from the cuts.
"Unfortunately, that doesn’t help the cancer patient on Medi-Cal in Bakersfield who has fee-for-service Medi-Cal and can’t find an oncologist," Ms. Folberg said.
Dr. Darin Latimore, president of the California chapter of the American College of Physicians, said large health systems likely will be able to work around the cuts, using group visits and physician extenders. But small physician practices in rural areas aren’t equipped to make those changes, he said.
"They are not in a position to move work on to others in order to be more efficient and see more Medi-Cal patients," said Dr. Latimore, associate dean of medicine at the University of California Davis.
The result will be that physicians who work outside of the state’s safety net system will be less likely to participate in the Medi-Cal program or will strictly limit the number of patients they see. That will translate to longer waits to get an appointment and shorter visits for Medi-Cal patients.
Another concern: Patients entering Medi-Cal managed care plans might not have access to a broad network of physicians. While California has network adequacy laws, state oversight is inadequate, according to Ms. Folberg. For the most part, the plans are on an honor system.
There are some bright spots for the Medi-Cal expansion. The ACA includes an increase in Medicaid payments to physicians for 146 primary care services provided in 2013 and 2014. The provision temporarily raises payments up to Medicare rates, which for California physicians means a 136% hike on average, according to an analysis from the Kaiser Family Foundation.
But those payments were delayed, with the first checks going out to physicians in November 2013. That delay could cost the state in physician participation.
"I think the longer it takes to implement that, the less likely it is that it will affect any decisions about whether to participate more fully in the program than you would have otherwise," said Christopher Perrone, deputy director of the health reform and public programs initiative at the California HealthCare Foundation.
And the temporary nature of the pay increase adds to the problem, Mr. Perrone said. "I don’t see California sustaining that increase on its own and I haven’t heard anyone suggest that the federal government would sustain it after the 2 years."
Mr. Perrone said it’s more likely that physicians who are already committed to participating in Medi-Cal will use the money to invest in electronic health records and other telemedicine features, and hire medical assistants.
But with so many small and solo physicians shying away from Medi-Cal because of low payments, community clinics and health centers will have to pick up the slack.
"All these issues together really point to the importance of the community health centers and the role we are going to be playing in ensuring access," said Carmela Castellano-Garcia, president and CEO of the California Primary Care Association.
Federally qualified health centers are in a better position financially because they are paid an enhanced Medicaid rate and won’t be subject to the coming Medi-Cal cuts, Ms. Castellano-Garcia said. And the ACA has directed an influx of cash to these centers as well – more than $500 million in California alone to establish new sites, expand services, and support major capital improvement projects, according to the Health and Human Services department.
Texas: No expansion means doctors will keep feeling pressure
Texas has the highest number of uninsured residents in the United States – a quarter of its 26 million residents lacking coverage – but Gov. Rick Perry (R) refused to expand Medicaid, which could cover as many as 500,000 to 1 million Texans.
The governor’s decision will stay in place at least until 2015, when the state legislature reconvenes.
Some physicians in Texas are not upset by the decision – they consider Medicaid to be low-paying program and full of bureaucratic hassles.
Others – including many of the primary care organizations – disagree.
The Texas Medical Association, the Texas Academy of Family Physicians, and the Texas chapter of the American Congress of Obstetricians and Gynecologists all support the expansion of Medicaid allowed by the ACA.
In April, Gov. Perry reiterated his position. "Medicaid expansion is a misguided, and ultimately doomed, attempt to mask the shortcomings of Obamacare," he said in a statement. Instead of expansion, he favors flexibility for the state to manage its Medicaid program.
State Rep. John Zerwas (R-Simonton), an anesthesiologist, introduced H.B. 3791 that would give that flexibility, but it did not get consideration by the full House before the legislature adjourned in May.
The TMA supported Dr. Zerwas’ proposal, but also is in favor of expanding Medicaid, said Dr. Stephen L. Brotherton, TMA president. More people would have some type of insurance, but they might not necessarily have good access to care, he said.
That’s because Texas has a shortage of primary care physicians. The number of primary care physicians per capita is lower than the national average – at about 70/100,000 in 2011, compared with 80/100,000 nationally, according to the Texas Department of State Health Services, in the publication "Supply Trends Among Licensed Health Professions, Texas, 1980-2011. In rural areas, it’s even lower – about 50/100,000.
Then there’s the question of just how many physicians will take Medicaid. A 2012 TMA survey found that only 31% of doctors in the state were accepting new Medicaid patients.
Medicaid payment rates are so low that Dr. Brotherton, who practices in Ft. Worth, said that he treats Medicaid patients as charity care. "It’s much less expensive for me to do it for nothing as donated time," he said.
Dr. Moss Hampton, chairman of District XI of ACOG, added, "Medicaid doesn’t cover the cost of taking care of the patient."
For Medicaid expansion to eventually be successful, "there would have to be a better payment rate and less of a hassle factor," said Dr. Hampton, chairman of the obstetrics and gynecology department at Texas Tech Health Sciences Center at the Permian Basin in Odessa.
"It’s hard to get doctors to accept Medicaid because of the rates they pay," agreed Dr. Clare Hawkins, TAFP president, who added that Texas physicians also feel that it’s hard to comply with differing rules among various Medicaid managed care programs.
Even so, expansion will mean getting more patients into preventive care, and a reduction in emergency department visits and more expensive hospital care – costs that are being borne by all Texans, including physicians, said Dr. Hawkins, who is program director of the San Jacinto Methodist Hospital Family Medicine Residency Program in Baytown, Texas.
Although uninsured Texas residents are already receiving care – in emergency departments and at clinics – Medicaid expansion could bring a big uptick in office visits, especially to ob.gyn. practices, Dr. Hampton said. The need for those services is growing with a state law that went into effect on Oct. 29 that makes it prohibitive for most Planned Parenthood clinics and other community clinics that provide abortion services to stay open.
"What that’s done is cut out a very large group of providers, and now we’re trying to find providers to help take care of those folks," who normally use those clinics, she said.
In the absence of Medicaid expansion, Texas physicians are hoping to start receiving higher Medicaid payments that were due to start in Jan. 2013. An estimated 25,000 Texas doctors are eligible for Medicaid pay that will be on par with Medicare. But Texas has not begun to distribute that money and will not likely do so until March, according to the TMA.
FDA approves sofosbuvir for chronic hepatitis C
The Food and Drug Administration has approved sofosbuvir, a first-in-its-class antiviral, to treat chronic hepatitis C infection.
Sofosbuvir is a nucleotide analogue inhibitor of the hepatitis C virus (HCV) NS5B polymerase enzyme, which plays an important role in HCV replication. It is taken orally once a day at a 400-mg dose. It will be marketed as Sovaldi by Gilead Sciences.
The drug is approved for two chronic hepatitis C indications: In combination with pegylated interferon and ribavirin for treatment-naïve adults with genotype 1 and 4 infections, and in combination with ribavirin for adults with genotypes 2 and 3 infection.
The second indication is the first approval of an interferon-free regimen for the treatment for chronic hepatitis C.
"Today’s approval represents a significant shift in the treatment paradigm for some patients with chronic hepatitis C," Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA Center for Drug Evaluation and Research, said in a statement.
This is "truly a historic moment," Dr. Demetre Daskalakis, medical director of the HIV program at Mt. Sinai School of Medicine, New York, said at an FDA advisory committee meeting on the drug held Oct. 25. "I can’t wait to get this drug into the clinic. We are all excited," he added. The Antiviral Drugs Advisory Committee voted unanimously that day to recommend approval for sofosbuvir.
Sofosbuvir was approved based on data from six clinical trials consisting of 1,947 patients – both treatment-naïve and treatment experienced – some of whom were also HIV positive.
The most common side effects reported in clinical study participants treated with sofosbuvir and ribavirin were fatigue and headache. In participants treated with sofosbuvir, ribavirin, and peginterferon-alfa, the most common side effects reported were fatigue, headache, nausea, insomnia, and anemia, according to the FDA.
According to Gilead, sofosbuvir is also on the verge of receiving marketing approval in the European Union. Sofosbuvir is the second drug approved by the FDA in the past two weeks to treat chronic HCV infection. Simeprevir was approved Nov. 22.
Elizabeth Mechcatie contributed to this report.
The Food and Drug Administration has approved sofosbuvir, a first-in-its-class antiviral, to treat chronic hepatitis C infection.
Sofosbuvir is a nucleotide analogue inhibitor of the hepatitis C virus (HCV) NS5B polymerase enzyme, which plays an important role in HCV replication. It is taken orally once a day at a 400-mg dose. It will be marketed as Sovaldi by Gilead Sciences.
The drug is approved for two chronic hepatitis C indications: In combination with pegylated interferon and ribavirin for treatment-naïve adults with genotype 1 and 4 infections, and in combination with ribavirin for adults with genotypes 2 and 3 infection.
The second indication is the first approval of an interferon-free regimen for the treatment for chronic hepatitis C.
"Today’s approval represents a significant shift in the treatment paradigm for some patients with chronic hepatitis C," Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA Center for Drug Evaluation and Research, said in a statement.
This is "truly a historic moment," Dr. Demetre Daskalakis, medical director of the HIV program at Mt. Sinai School of Medicine, New York, said at an FDA advisory committee meeting on the drug held Oct. 25. "I can’t wait to get this drug into the clinic. We are all excited," he added. The Antiviral Drugs Advisory Committee voted unanimously that day to recommend approval for sofosbuvir.
Sofosbuvir was approved based on data from six clinical trials consisting of 1,947 patients – both treatment-naïve and treatment experienced – some of whom were also HIV positive.
The most common side effects reported in clinical study participants treated with sofosbuvir and ribavirin were fatigue and headache. In participants treated with sofosbuvir, ribavirin, and peginterferon-alfa, the most common side effects reported were fatigue, headache, nausea, insomnia, and anemia, according to the FDA.
According to Gilead, sofosbuvir is also on the verge of receiving marketing approval in the European Union. Sofosbuvir is the second drug approved by the FDA in the past two weeks to treat chronic HCV infection. Simeprevir was approved Nov. 22.
Elizabeth Mechcatie contributed to this report.
The Food and Drug Administration has approved sofosbuvir, a first-in-its-class antiviral, to treat chronic hepatitis C infection.
Sofosbuvir is a nucleotide analogue inhibitor of the hepatitis C virus (HCV) NS5B polymerase enzyme, which plays an important role in HCV replication. It is taken orally once a day at a 400-mg dose. It will be marketed as Sovaldi by Gilead Sciences.
The drug is approved for two chronic hepatitis C indications: In combination with pegylated interferon and ribavirin for treatment-naïve adults with genotype 1 and 4 infections, and in combination with ribavirin for adults with genotypes 2 and 3 infection.
The second indication is the first approval of an interferon-free regimen for the treatment for chronic hepatitis C.
"Today’s approval represents a significant shift in the treatment paradigm for some patients with chronic hepatitis C," Dr. Edward Cox, director of the Office of Antimicrobial Products in the FDA Center for Drug Evaluation and Research, said in a statement.
This is "truly a historic moment," Dr. Demetre Daskalakis, medical director of the HIV program at Mt. Sinai School of Medicine, New York, said at an FDA advisory committee meeting on the drug held Oct. 25. "I can’t wait to get this drug into the clinic. We are all excited," he added. The Antiviral Drugs Advisory Committee voted unanimously that day to recommend approval for sofosbuvir.
Sofosbuvir was approved based on data from six clinical trials consisting of 1,947 patients – both treatment-naïve and treatment experienced – some of whom were also HIV positive.
The most common side effects reported in clinical study participants treated with sofosbuvir and ribavirin were fatigue and headache. In participants treated with sofosbuvir, ribavirin, and peginterferon-alfa, the most common side effects reported were fatigue, headache, nausea, insomnia, and anemia, according to the FDA.
According to Gilead, sofosbuvir is also on the verge of receiving marketing approval in the European Union. Sofosbuvir is the second drug approved by the FDA in the past two weeks to treat chronic HCV infection. Simeprevir was approved Nov. 22.
Elizabeth Mechcatie contributed to this report.