User login
New law will speed sunscreen ingredient approval
President Obama has signed into law a bill that requires speedier review of sunscreen ingredients.
The Sunscreen Innovation Act garnered support from Democrats and Republicans in the House and Senate, and from manufacturers and dermatologists as well. The House and Senate reconciled their two proposals – S. 2141 and H.R. 4250 – in mid-November, and the final bill was signed by the president on Nov. 26.
The law sets strict timetables for Food and Drug Administration action. The bill was prompted by a huge backlog of ingredients that have been awaiting review at the agency.
According to the PASS Coalition, the last new over-the-counter sunscreen ingredient was approved in the 1990s. Manufacturers have sought approval for eight new ingredients since 2002, but none has been acted on, according to PASS, an advocacy group made up of manufacturers, physicians, and organizations including the American Cancer Society Cancer Action Network, the American College of Mohs Surgery, and the Melanoma International Foundation.
The approval by Congress of the new law “signals the urgent public health need to make more effective products available to consumers, as skin cancer rates continue to rise at an alarming pace,” American Academy of Dermatology President Brett M. Coldiron said in a statement issued in mid-November. “The AADA looks forward to working closely with the FDA to implement the new law, and will be providing comments on its implementation,” said Dr. Coldiron.
Rep. Ed Whitfield (R-Ky.), a coauthor of the original House bill, said that it had been too long since the approval of a new sunscreen ingredient. “With the president’s signature, Americans will finally be able to begin purchasing products that take advantage of improved research,” said Rep. Whitfield, in a statement.
President Obama has signed into law a bill that requires speedier review of sunscreen ingredients.
The Sunscreen Innovation Act garnered support from Democrats and Republicans in the House and Senate, and from manufacturers and dermatologists as well. The House and Senate reconciled their two proposals – S. 2141 and H.R. 4250 – in mid-November, and the final bill was signed by the president on Nov. 26.
The law sets strict timetables for Food and Drug Administration action. The bill was prompted by a huge backlog of ingredients that have been awaiting review at the agency.
According to the PASS Coalition, the last new over-the-counter sunscreen ingredient was approved in the 1990s. Manufacturers have sought approval for eight new ingredients since 2002, but none has been acted on, according to PASS, an advocacy group made up of manufacturers, physicians, and organizations including the American Cancer Society Cancer Action Network, the American College of Mohs Surgery, and the Melanoma International Foundation.
The approval by Congress of the new law “signals the urgent public health need to make more effective products available to consumers, as skin cancer rates continue to rise at an alarming pace,” American Academy of Dermatology President Brett M. Coldiron said in a statement issued in mid-November. “The AADA looks forward to working closely with the FDA to implement the new law, and will be providing comments on its implementation,” said Dr. Coldiron.
Rep. Ed Whitfield (R-Ky.), a coauthor of the original House bill, said that it had been too long since the approval of a new sunscreen ingredient. “With the president’s signature, Americans will finally be able to begin purchasing products that take advantage of improved research,” said Rep. Whitfield, in a statement.
President Obama has signed into law a bill that requires speedier review of sunscreen ingredients.
The Sunscreen Innovation Act garnered support from Democrats and Republicans in the House and Senate, and from manufacturers and dermatologists as well. The House and Senate reconciled their two proposals – S. 2141 and H.R. 4250 – in mid-November, and the final bill was signed by the president on Nov. 26.
The law sets strict timetables for Food and Drug Administration action. The bill was prompted by a huge backlog of ingredients that have been awaiting review at the agency.
According to the PASS Coalition, the last new over-the-counter sunscreen ingredient was approved in the 1990s. Manufacturers have sought approval for eight new ingredients since 2002, but none has been acted on, according to PASS, an advocacy group made up of manufacturers, physicians, and organizations including the American Cancer Society Cancer Action Network, the American College of Mohs Surgery, and the Melanoma International Foundation.
The approval by Congress of the new law “signals the urgent public health need to make more effective products available to consumers, as skin cancer rates continue to rise at an alarming pace,” American Academy of Dermatology President Brett M. Coldiron said in a statement issued in mid-November. “The AADA looks forward to working closely with the FDA to implement the new law, and will be providing comments on its implementation,” said Dr. Coldiron.
Rep. Ed Whitfield (R-Ky.), a coauthor of the original House bill, said that it had been too long since the approval of a new sunscreen ingredient. “With the president’s signature, Americans will finally be able to begin purchasing products that take advantage of improved research,” said Rep. Whitfield, in a statement.
CMS proposes new network, formulary standards for 2016 ACA plans
The Obama administration is proposing to widen provider networks and increase access to prescription drugs in health plans that participate in marketplace plans in 2016.
“It is one of our many goals to strengthen the integrity of programs that fall under the Affordable Care Act to ensure the delivery of quality care with affordable options,” said Centers for Medicare & Medicaid Services Administrator Marilyn Tavenner, in a statement. “CMS is working to improve the consumer experience and promote accountability, uniformity and transparency in private health insurance.”
According to the proposal, issued on Nov. 21, the administration “continues to take great interest in ensuring strong network access.” The CMS is proposing that only hospitals and physicians that are in-network can be considered part of the network. Out-of-network providers cannot be included to demonstrate adequacy.
For now, insurers will have to follow standards issued by the agency in March, but changes could come by the time the 2016 proposal is made final.
The agency says it is waiting to see the final results of a model law on network adequacy that’s being developed by the National Association of Insurance Commissioners. A draft of that proposal was recently released and comments are due by Jan. 12.
The CMS also is proposing that new enrollees be given 30 days to transition from current physicians or hospitals to the new plan’s network.
Health plans also will be required to publish an up-to-date, accurate, and complete provider directory, including information on which providers are accepting new patients, the provider’s location, contact information, specialty, medical group, and any institutional affiliations. The guide has to be easily accessible – without having to create an account or use a policy number – to plan enrollees, prospective enrollees, states, and marketplace plans. It should be updated at least once a month, said the CMS.
The agency also is proposing changes to how marketplace plans cover prescription drugs. Currently, plans are required to cover at least one drug in each class.
But plans have had trouble meeting that requirement, and there were other problems, including that the criteria did not exactly encourage newly approved drugs to be included, according to the agency.
Instead, it is proposing that plans use pharmacy and therapeutics committees – following specific standards set out by the CMS – to review and approve drugs, and that it be an adequate amount in each class.
Drug formularies also should be easily accessible to the general public, without having to create and account or give a policy number, and it should be up to date, according to the proposal.
The proposal is open for comment until Dec. 21.
On Twitter @aliciaault
The Obama administration is proposing to widen provider networks and increase access to prescription drugs in health plans that participate in marketplace plans in 2016.
“It is one of our many goals to strengthen the integrity of programs that fall under the Affordable Care Act to ensure the delivery of quality care with affordable options,” said Centers for Medicare & Medicaid Services Administrator Marilyn Tavenner, in a statement. “CMS is working to improve the consumer experience and promote accountability, uniformity and transparency in private health insurance.”
According to the proposal, issued on Nov. 21, the administration “continues to take great interest in ensuring strong network access.” The CMS is proposing that only hospitals and physicians that are in-network can be considered part of the network. Out-of-network providers cannot be included to demonstrate adequacy.
For now, insurers will have to follow standards issued by the agency in March, but changes could come by the time the 2016 proposal is made final.
The agency says it is waiting to see the final results of a model law on network adequacy that’s being developed by the National Association of Insurance Commissioners. A draft of that proposal was recently released and comments are due by Jan. 12.
The CMS also is proposing that new enrollees be given 30 days to transition from current physicians or hospitals to the new plan’s network.
Health plans also will be required to publish an up-to-date, accurate, and complete provider directory, including information on which providers are accepting new patients, the provider’s location, contact information, specialty, medical group, and any institutional affiliations. The guide has to be easily accessible – without having to create an account or use a policy number – to plan enrollees, prospective enrollees, states, and marketplace plans. It should be updated at least once a month, said the CMS.
The agency also is proposing changes to how marketplace plans cover prescription drugs. Currently, plans are required to cover at least one drug in each class.
But plans have had trouble meeting that requirement, and there were other problems, including that the criteria did not exactly encourage newly approved drugs to be included, according to the agency.
Instead, it is proposing that plans use pharmacy and therapeutics committees – following specific standards set out by the CMS – to review and approve drugs, and that it be an adequate amount in each class.
Drug formularies also should be easily accessible to the general public, without having to create and account or give a policy number, and it should be up to date, according to the proposal.
The proposal is open for comment until Dec. 21.
On Twitter @aliciaault
The Obama administration is proposing to widen provider networks and increase access to prescription drugs in health plans that participate in marketplace plans in 2016.
“It is one of our many goals to strengthen the integrity of programs that fall under the Affordable Care Act to ensure the delivery of quality care with affordable options,” said Centers for Medicare & Medicaid Services Administrator Marilyn Tavenner, in a statement. “CMS is working to improve the consumer experience and promote accountability, uniformity and transparency in private health insurance.”
According to the proposal, issued on Nov. 21, the administration “continues to take great interest in ensuring strong network access.” The CMS is proposing that only hospitals and physicians that are in-network can be considered part of the network. Out-of-network providers cannot be included to demonstrate adequacy.
For now, insurers will have to follow standards issued by the agency in March, but changes could come by the time the 2016 proposal is made final.
The agency says it is waiting to see the final results of a model law on network adequacy that’s being developed by the National Association of Insurance Commissioners. A draft of that proposal was recently released and comments are due by Jan. 12.
The CMS also is proposing that new enrollees be given 30 days to transition from current physicians or hospitals to the new plan’s network.
Health plans also will be required to publish an up-to-date, accurate, and complete provider directory, including information on which providers are accepting new patients, the provider’s location, contact information, specialty, medical group, and any institutional affiliations. The guide has to be easily accessible – without having to create an account or use a policy number – to plan enrollees, prospective enrollees, states, and marketplace plans. It should be updated at least once a month, said the CMS.
The agency also is proposing changes to how marketplace plans cover prescription drugs. Currently, plans are required to cover at least one drug in each class.
But plans have had trouble meeting that requirement, and there were other problems, including that the criteria did not exactly encourage newly approved drugs to be included, according to the agency.
Instead, it is proposing that plans use pharmacy and therapeutics committees – following specific standards set out by the CMS – to review and approve drugs, and that it be an adequate amount in each class.
Drug formularies also should be easily accessible to the general public, without having to create and account or give a policy number, and it should be up to date, according to the proposal.
The proposal is open for comment until Dec. 21.
On Twitter @aliciaault
FDA issues final rules requiring calorie tally in restaurants
A wide range of restaurants, cafes, entertainment venues, and even vending machines would have to post calorie counts on food and beverage choices under two proposed rules issued Nov. 25 by the Food and Drug Administration.
Both the restaurant menu rule and the vending machine rule are carrying out requirements by the Affordable Care Act that certain chain restaurants and similar establishments with 20 or more locations disclose certain nutrition information for standard menu items.
“We are responding to legislation, as well as a compelling public health need,” FDA Commissioner Margaret Hamburg said during an FDA press briefing. “Obesity is a huge problem, and we need to develop better strategies to address that,” she said, adding, “a huge contributor is caloric intake.”
Americans eat and drink about one-third of their calories away from home, Dr. Hamburg added. “Making calorie information available on chain restaurant menus and vending machines is an important step for public health that will help consumers make informed choices for themselves and their families.”
The establishments covered by the restaurant rule will be required to clearly and conspicuously display calorie information for standard items on menus and menu boards, next to the name or price; such displays must be in place by Dec. 1, 2015. Menus and menu boards also must note that “2,000 calories a day is used for general nutrition advice, but calorie needs vary.”
Among those covered by the rule: sit-down and fast-food restaurants, bakeries, and coffee shops; restaurant-type foods in certain grocery and convenience stores; take-out and delivery foods such as pizza; foods that you serve yourself from a salad or hot-food bar; foods at amusement parks and movie theaters; and alcoholic drinks when they appear on a menu. Drinks ordered from a bartender, and bottles of liquor at a bar or restaurant are not covered.
In vending machines, calories would be listed on the front of the product for sale or on a sign or sticker near the food or selection button. The rules apply only to operators who control more than 20 vending machines and operators have until Dec. 1, 2016, to comply.
Several physicians praised the intent, but doubted the efficacy of the proposed rules.
“Having the information available doesn’t guarantee attention is paid to it but for those who would otherwise seek the information, the rules make it easier to find, ” said Dr. Joseph Domachowske, professor of pediatrics and professor of microbiology and immunology at the State University of New York (SUNY) Upstate Medical University, Syracuse. But, he added, “I am skeptical that the posted information will have much influence of the dietary decisions of those confronted with it.”
Dr. Lillian Beard applauded the FDA rules, but said, “I do not think it will make a meaningful difference for the population who could most benefit.” Calorie counters will take to the information readily, but “for those who do not want to be bothered, it will become more stuff for their eyes to encounter as they order what they want anyway,” said Dr. Beard, a pediatrician at George Washington University, Washington, D.C.
The American Cancer Society Cancer Action Network said it supports the rules.
“Between one-fourth and one-third of all cancers are caused by poor nutrition, physical inactivity, and excess weight,” Chris Hansen, president of ASC CAN, said in a statement.
He said the group was especially pleased that the rules covered a wide variety of establishments that serve food, and that they applied to certain alcoholic beverages, “considering that alcohol is the fifth leading source of calories in American adult diets.”
The FDA has estimated that the potential benefits of the two rules will far outweigh the costs. The agency first proposed the calorie listing requirements more than 3 years ago, in April 2011. It took that long to analyze and consider the more than 1,100 comments the FDA received, Dr. Hamburg said.
She said the FDA has no plans to use any information generated from the rules to gauge their impact on eating habits. But she predicted that the rules would create “a great deal of interest” at public health agencies, and from consumer groups and the academic community.
The two proposed rules are open for comment for 30 days after their publication in the Federal Register.
On Twitter @aliciaault
A wide range of restaurants, cafes, entertainment venues, and even vending machines would have to post calorie counts on food and beverage choices under two proposed rules issued Nov. 25 by the Food and Drug Administration.
Both the restaurant menu rule and the vending machine rule are carrying out requirements by the Affordable Care Act that certain chain restaurants and similar establishments with 20 or more locations disclose certain nutrition information for standard menu items.
“We are responding to legislation, as well as a compelling public health need,” FDA Commissioner Margaret Hamburg said during an FDA press briefing. “Obesity is a huge problem, and we need to develop better strategies to address that,” she said, adding, “a huge contributor is caloric intake.”
Americans eat and drink about one-third of their calories away from home, Dr. Hamburg added. “Making calorie information available on chain restaurant menus and vending machines is an important step for public health that will help consumers make informed choices for themselves and their families.”
The establishments covered by the restaurant rule will be required to clearly and conspicuously display calorie information for standard items on menus and menu boards, next to the name or price; such displays must be in place by Dec. 1, 2015. Menus and menu boards also must note that “2,000 calories a day is used for general nutrition advice, but calorie needs vary.”
Among those covered by the rule: sit-down and fast-food restaurants, bakeries, and coffee shops; restaurant-type foods in certain grocery and convenience stores; take-out and delivery foods such as pizza; foods that you serve yourself from a salad or hot-food bar; foods at amusement parks and movie theaters; and alcoholic drinks when they appear on a menu. Drinks ordered from a bartender, and bottles of liquor at a bar or restaurant are not covered.
In vending machines, calories would be listed on the front of the product for sale or on a sign or sticker near the food or selection button. The rules apply only to operators who control more than 20 vending machines and operators have until Dec. 1, 2016, to comply.
Several physicians praised the intent, but doubted the efficacy of the proposed rules.
“Having the information available doesn’t guarantee attention is paid to it but for those who would otherwise seek the information, the rules make it easier to find, ” said Dr. Joseph Domachowske, professor of pediatrics and professor of microbiology and immunology at the State University of New York (SUNY) Upstate Medical University, Syracuse. But, he added, “I am skeptical that the posted information will have much influence of the dietary decisions of those confronted with it.”
Dr. Lillian Beard applauded the FDA rules, but said, “I do not think it will make a meaningful difference for the population who could most benefit.” Calorie counters will take to the information readily, but “for those who do not want to be bothered, it will become more stuff for their eyes to encounter as they order what they want anyway,” said Dr. Beard, a pediatrician at George Washington University, Washington, D.C.
The American Cancer Society Cancer Action Network said it supports the rules.
“Between one-fourth and one-third of all cancers are caused by poor nutrition, physical inactivity, and excess weight,” Chris Hansen, president of ASC CAN, said in a statement.
He said the group was especially pleased that the rules covered a wide variety of establishments that serve food, and that they applied to certain alcoholic beverages, “considering that alcohol is the fifth leading source of calories in American adult diets.”
The FDA has estimated that the potential benefits of the two rules will far outweigh the costs. The agency first proposed the calorie listing requirements more than 3 years ago, in April 2011. It took that long to analyze and consider the more than 1,100 comments the FDA received, Dr. Hamburg said.
She said the FDA has no plans to use any information generated from the rules to gauge their impact on eating habits. But she predicted that the rules would create “a great deal of interest” at public health agencies, and from consumer groups and the academic community.
The two proposed rules are open for comment for 30 days after their publication in the Federal Register.
On Twitter @aliciaault
A wide range of restaurants, cafes, entertainment venues, and even vending machines would have to post calorie counts on food and beverage choices under two proposed rules issued Nov. 25 by the Food and Drug Administration.
Both the restaurant menu rule and the vending machine rule are carrying out requirements by the Affordable Care Act that certain chain restaurants and similar establishments with 20 or more locations disclose certain nutrition information for standard menu items.
“We are responding to legislation, as well as a compelling public health need,” FDA Commissioner Margaret Hamburg said during an FDA press briefing. “Obesity is a huge problem, and we need to develop better strategies to address that,” she said, adding, “a huge contributor is caloric intake.”
Americans eat and drink about one-third of their calories away from home, Dr. Hamburg added. “Making calorie information available on chain restaurant menus and vending machines is an important step for public health that will help consumers make informed choices for themselves and their families.”
The establishments covered by the restaurant rule will be required to clearly and conspicuously display calorie information for standard items on menus and menu boards, next to the name or price; such displays must be in place by Dec. 1, 2015. Menus and menu boards also must note that “2,000 calories a day is used for general nutrition advice, but calorie needs vary.”
Among those covered by the rule: sit-down and fast-food restaurants, bakeries, and coffee shops; restaurant-type foods in certain grocery and convenience stores; take-out and delivery foods such as pizza; foods that you serve yourself from a salad or hot-food bar; foods at amusement parks and movie theaters; and alcoholic drinks when they appear on a menu. Drinks ordered from a bartender, and bottles of liquor at a bar or restaurant are not covered.
In vending machines, calories would be listed on the front of the product for sale or on a sign or sticker near the food or selection button. The rules apply only to operators who control more than 20 vending machines and operators have until Dec. 1, 2016, to comply.
Several physicians praised the intent, but doubted the efficacy of the proposed rules.
“Having the information available doesn’t guarantee attention is paid to it but for those who would otherwise seek the information, the rules make it easier to find, ” said Dr. Joseph Domachowske, professor of pediatrics and professor of microbiology and immunology at the State University of New York (SUNY) Upstate Medical University, Syracuse. But, he added, “I am skeptical that the posted information will have much influence of the dietary decisions of those confronted with it.”
Dr. Lillian Beard applauded the FDA rules, but said, “I do not think it will make a meaningful difference for the population who could most benefit.” Calorie counters will take to the information readily, but “for those who do not want to be bothered, it will become more stuff for their eyes to encounter as they order what they want anyway,” said Dr. Beard, a pediatrician at George Washington University, Washington, D.C.
The American Cancer Society Cancer Action Network said it supports the rules.
“Between one-fourth and one-third of all cancers are caused by poor nutrition, physical inactivity, and excess weight,” Chris Hansen, president of ASC CAN, said in a statement.
He said the group was especially pleased that the rules covered a wide variety of establishments that serve food, and that they applied to certain alcoholic beverages, “considering that alcohol is the fifth leading source of calories in American adult diets.”
The FDA has estimated that the potential benefits of the two rules will far outweigh the costs. The agency first proposed the calorie listing requirements more than 3 years ago, in April 2011. It took that long to analyze and consider the more than 1,100 comments the FDA received, Dr. Hamburg said.
She said the FDA has no plans to use any information generated from the rules to gauge their impact on eating habits. But she predicted that the rules would create “a great deal of interest” at public health agencies, and from consumer groups and the academic community.
The two proposed rules are open for comment for 30 days after their publication in the Federal Register.
On Twitter @aliciaault
VIDEO: AMA president talks meaningful use, ICD-10, and Ebola
WASHINGTON – The recent deadline extensions for meaningful use attestation are encouraging, but not enough. The process must be more user-friendly and clinically meaningful.
That’s according to Dr. Robert Wah, president of the American Medical Association. Dr. Wah discussed the AMA’s concerns about meaningful use and health information technology in a video interview – including whether there is enough of a focus on interoperability of electronic health records.
He also touched on the transition to the ICD-10 code set, again questioning whether the move is clinically useful and ultimately helps improve patient care.
Recently, Dr. Karen DeSalvo, National Coordinator for Health Information Technology, was given an additional appointment as the acting assistant secretary for health to help with the effort to combat the Ebola epidemic. That caused concern among physicians that there could be a leadership vacuum at the Office of the National Coordinator at a time when so much is happening in health IT. Although it was later clarified that she would remain in charge of the effort, there are still some worries, Dr. Wah said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
WASHINGTON – The recent deadline extensions for meaningful use attestation are encouraging, but not enough. The process must be more user-friendly and clinically meaningful.
That’s according to Dr. Robert Wah, president of the American Medical Association. Dr. Wah discussed the AMA’s concerns about meaningful use and health information technology in a video interview – including whether there is enough of a focus on interoperability of electronic health records.
He also touched on the transition to the ICD-10 code set, again questioning whether the move is clinically useful and ultimately helps improve patient care.
Recently, Dr. Karen DeSalvo, National Coordinator for Health Information Technology, was given an additional appointment as the acting assistant secretary for health to help with the effort to combat the Ebola epidemic. That caused concern among physicians that there could be a leadership vacuum at the Office of the National Coordinator at a time when so much is happening in health IT. Although it was later clarified that she would remain in charge of the effort, there are still some worries, Dr. Wah said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
WASHINGTON – The recent deadline extensions for meaningful use attestation are encouraging, but not enough. The process must be more user-friendly and clinically meaningful.
That’s according to Dr. Robert Wah, president of the American Medical Association. Dr. Wah discussed the AMA’s concerns about meaningful use and health information technology in a video interview – including whether there is enough of a focus on interoperability of electronic health records.
He also touched on the transition to the ICD-10 code set, again questioning whether the move is clinically useful and ultimately helps improve patient care.
Recently, Dr. Karen DeSalvo, National Coordinator for Health Information Technology, was given an additional appointment as the acting assistant secretary for health to help with the effort to combat the Ebola epidemic. That caused concern among physicians that there could be a leadership vacuum at the Office of the National Coordinator at a time when so much is happening in health IT. Although it was later clarified that she would remain in charge of the effort, there are still some worries, Dr. Wah said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
VIDEO: AMA President on the ACA, narrow networks, and Medicaid expansion
WASHINGTON– With enrollment now open for the second year of Affordable Care Act coverage, what kinds of concerns do physicians have?
American Medical Association President Robert M. Wah discusses in this video interview the latest round of enrollment, and potential pluses and minuses for physicians, including the 90-day grace period. That aspect of the law gives patients who enroll in health plans through ACA marketplaces up to 90 days to pay premiums. Although insurers are liable for services rendered in the first month, physicians might be left without reimbursement for care given in the ensuing 60 days.
Another looming question as enrollment gets underway: Will patients be able to determine that their physicians are participating in a given network? The AMA and others have been pressuring the federal government and insurers to do a better job of updating network information. At its interim policy-making meeting in November, the AMA adopted a new policy urging any and all changes to be made before enrollment begins. The organization’s delegates also said that state regulators should be the primary enforcers of ensuring network adequacy.
Dr. Wah also discussed the AMA’s approach to ensuring that more Americans get health coverage through Medicaid, building on another policy passed by the delegates at the interim meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
WASHINGTON– With enrollment now open for the second year of Affordable Care Act coverage, what kinds of concerns do physicians have?
American Medical Association President Robert M. Wah discusses in this video interview the latest round of enrollment, and potential pluses and minuses for physicians, including the 90-day grace period. That aspect of the law gives patients who enroll in health plans through ACA marketplaces up to 90 days to pay premiums. Although insurers are liable for services rendered in the first month, physicians might be left without reimbursement for care given in the ensuing 60 days.
Another looming question as enrollment gets underway: Will patients be able to determine that their physicians are participating in a given network? The AMA and others have been pressuring the federal government and insurers to do a better job of updating network information. At its interim policy-making meeting in November, the AMA adopted a new policy urging any and all changes to be made before enrollment begins. The organization’s delegates also said that state regulators should be the primary enforcers of ensuring network adequacy.
Dr. Wah also discussed the AMA’s approach to ensuring that more Americans get health coverage through Medicaid, building on another policy passed by the delegates at the interim meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
WASHINGTON– With enrollment now open for the second year of Affordable Care Act coverage, what kinds of concerns do physicians have?
American Medical Association President Robert M. Wah discusses in this video interview the latest round of enrollment, and potential pluses and minuses for physicians, including the 90-day grace period. That aspect of the law gives patients who enroll in health plans through ACA marketplaces up to 90 days to pay premiums. Although insurers are liable for services rendered in the first month, physicians might be left without reimbursement for care given in the ensuing 60 days.
Another looming question as enrollment gets underway: Will patients be able to determine that their physicians are participating in a given network? The AMA and others have been pressuring the federal government and insurers to do a better job of updating network information. At its interim policy-making meeting in November, the AMA adopted a new policy urging any and all changes to be made before enrollment begins. The organization’s delegates also said that state regulators should be the primary enforcers of ensuring network adequacy.
Dr. Wah also discussed the AMA’s approach to ensuring that more Americans get health coverage through Medicaid, building on another policy passed by the delegates at the interim meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @aliciaault
VIDEO: AMA President Wah talks SGR, Medicaid parity, and IPAB
WASHINGTON – The American Medical Association is hoping – along with many other medical societies and their physician members – to convince Congress that it should repeal Medicare’s Sustainable Growth Rate formula before the end of the year.
The cost to do so is a relative bargain, and physicians have grown tired of trying to plan around a process that is predictable, yet unpredictable, according to AMA President Robert M. Wah. Why not move now, he wondered, given that there was so much progress in this Congress, including a bill that was passed by the House?
In an exclusive video interview, Dr. Wah talked about the pressing need for the SGR replacement, along with what Republican majorities in both the House and Senate in the incoming Congress might mean to physicians.
Some Republicans have said that they will press for a partial or total repeal of the Affordable Care Act. Dr. Wah touched on whether the AMA supports any part of that notion, including getting rid of the Independent Payment Advisory Board, known as the IPAB.
Dr. Wah also discussed another urgent topic: What to do about the expiring element of the ACA that gave primary care physicians who treat Medicaid patients the same rate of pay as those who serve Medicare beneficiaries. The AMA recently reiterated its position that the so-called pay bump – which ends Dec. 31 – should be extended, and that it also should include obstetricians and gynecologists, who were left out of the initial policy.
On Twitter @aliciaault
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – The American Medical Association is hoping – along with many other medical societies and their physician members – to convince Congress that it should repeal Medicare’s Sustainable Growth Rate formula before the end of the year.
The cost to do so is a relative bargain, and physicians have grown tired of trying to plan around a process that is predictable, yet unpredictable, according to AMA President Robert M. Wah. Why not move now, he wondered, given that there was so much progress in this Congress, including a bill that was passed by the House?
In an exclusive video interview, Dr. Wah talked about the pressing need for the SGR replacement, along with what Republican majorities in both the House and Senate in the incoming Congress might mean to physicians.
Some Republicans have said that they will press for a partial or total repeal of the Affordable Care Act. Dr. Wah touched on whether the AMA supports any part of that notion, including getting rid of the Independent Payment Advisory Board, known as the IPAB.
Dr. Wah also discussed another urgent topic: What to do about the expiring element of the ACA that gave primary care physicians who treat Medicaid patients the same rate of pay as those who serve Medicare beneficiaries. The AMA recently reiterated its position that the so-called pay bump – which ends Dec. 31 – should be extended, and that it also should include obstetricians and gynecologists, who were left out of the initial policy.
On Twitter @aliciaault
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – The American Medical Association is hoping – along with many other medical societies and their physician members – to convince Congress that it should repeal Medicare’s Sustainable Growth Rate formula before the end of the year.
The cost to do so is a relative bargain, and physicians have grown tired of trying to plan around a process that is predictable, yet unpredictable, according to AMA President Robert M. Wah. Why not move now, he wondered, given that there was so much progress in this Congress, including a bill that was passed by the House?
In an exclusive video interview, Dr. Wah talked about the pressing need for the SGR replacement, along with what Republican majorities in both the House and Senate in the incoming Congress might mean to physicians.
Some Republicans have said that they will press for a partial or total repeal of the Affordable Care Act. Dr. Wah touched on whether the AMA supports any part of that notion, including getting rid of the Independent Payment Advisory Board, known as the IPAB.
Dr. Wah also discussed another urgent topic: What to do about the expiring element of the ACA that gave primary care physicians who treat Medicaid patients the same rate of pay as those who serve Medicare beneficiaries. The AMA recently reiterated its position that the so-called pay bump – which ends Dec. 31 – should be extended, and that it also should include obstetricians and gynecologists, who were left out of the initial policy.
On Twitter @aliciaault
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Global survey: U.S. seniors sicker, but more likely to have advance directive
Older Americans are sicker than their peers in 11 developed countries, but are most likely to have a chronic care plan and to have discussed health-promoting behaviors with their physicians, according to a new survey.
The telephone survey analyzed responses from 15,617 adults over age 65 in the United States, Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and the United Kingdom. It’s almost an apples-to-apples comparison because Medicare beneficiaries are considered fully insured, and all the other countries have some form of universal health coverage, lead author Robin Osborn said in a briefing on the study published Nov. 19 in the journal Health Affairs.
“This new survey shows that there are areas, such as managing patients who have chronic illnesses and hospital discharge planning, where the U.S. does well compared to other countries,” Ms. Osborn, vice president and director of the International Health Policy and Practice Innovations program at the Commonwealth Fund, said in a statement.
The United States also performed well when it came to patient-physician communication, transitions from the hospital to home, and end-of-life planning (Health. Aff. [doi: 10.1377/hlthaff.2014.0947]).
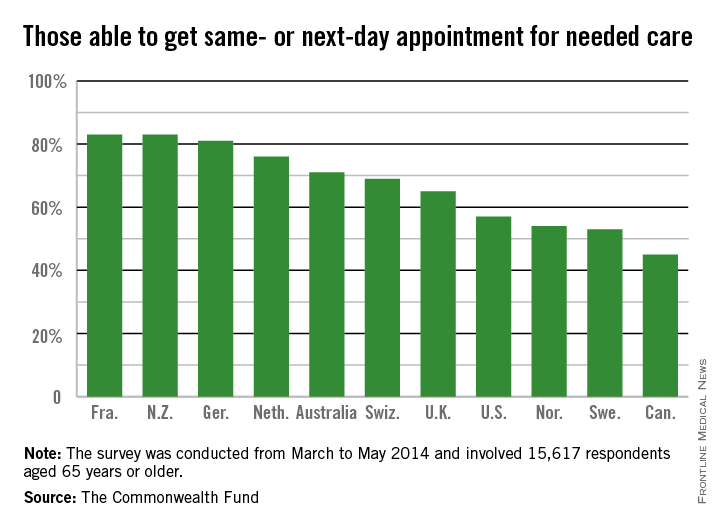
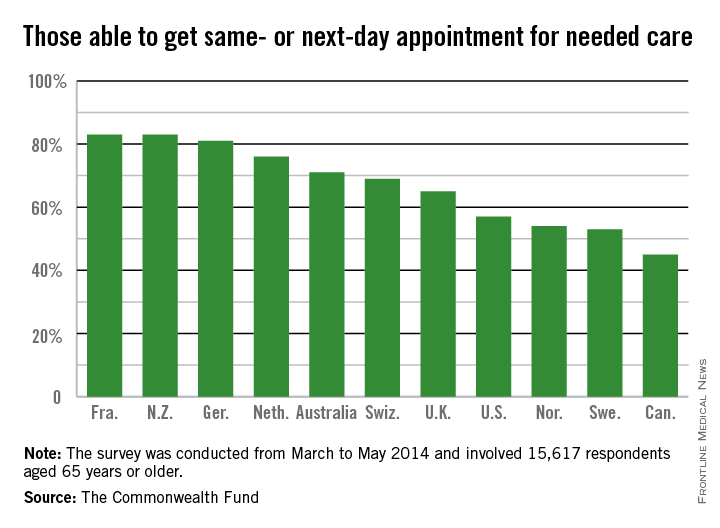
More than two-thirds (68%) of respondents in the United States had two or more chronic conditions, compared with 56% in Canada and 33% in the United Kingdom, which was at the low end of the spectrum.
The vast majority of patients in all countries reported that they thought their physician spent enough time with them, including 86% of Americans. Eighty-one percent of American patients said their physician encouraged them to ask questions – putting them on par with patients in the United Kingdom, New Zealand, Germany, and Australia – compared with a low of 40% of patients in Norway.
Hospital discharge planning got high marks in the United States, with only 28% saying that they had any gaps in care after an inpatient stay. That compares with a high of around 70% of patients reporting problems in Sweden and Norway.
End-of-life planning was another area where it appears that the United States is doing better than other nations. Seventy-eight percent of U.S. patients said they had discussed their desires with a friend, family member, or physician, while 55% said they had a written advance directive, and 67% had a health care proxy.
By comparison, only 4% of Norwegians had an advance directive, and just 5% of French patients. Only Australia, Canada, and the United Kingdom had rates near those seen in the United States.
Care coordination was a problem in all the nations surveyed. Older Americans were most likely to say that medical records or test results weren’t available at a scheduled appointment (23%) or that tests were duplicated. Patients in all nations reported receiving conflicting information from different doctors, and that primary care physicians did not speak with specialists, and vice versa.
The cost of care was a bigger issue for Americans than for patients in other countries. One in 5 (19%) of U.S. seniors had a medical problem but did not visit a doctor or skipped a test or refilling a prescription because of the cost. New Zealand was second, with 10% saying they had foregone treatment because of cost. Just 3% of French patients had cost issues.
On Twitter @aliciaault
Older Americans are sicker than their peers in 11 developed countries, but are most likely to have a chronic care plan and to have discussed health-promoting behaviors with their physicians, according to a new survey.
The telephone survey analyzed responses from 15,617 adults over age 65 in the United States, Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and the United Kingdom. It’s almost an apples-to-apples comparison because Medicare beneficiaries are considered fully insured, and all the other countries have some form of universal health coverage, lead author Robin Osborn said in a briefing on the study published Nov. 19 in the journal Health Affairs.

“This new survey shows that there are areas, such as managing patients who have chronic illnesses and hospital discharge planning, where the U.S. does well compared to other countries,” Ms. Osborn, vice president and director of the International Health Policy and Practice Innovations program at the Commonwealth Fund, said in a statement.
The United States also performed well when it came to patient-physician communication, transitions from the hospital to home, and end-of-life planning (Health. Aff. [doi: 10.1377/hlthaff.2014.0947]).
More than two-thirds (68%) of respondents in the United States had two or more chronic conditions, compared with 56% in Canada and 33% in the United Kingdom, which was at the low end of the spectrum.
The vast majority of patients in all countries reported that they thought their physician spent enough time with them, including 86% of Americans. Eighty-one percent of American patients said their physician encouraged them to ask questions – putting them on par with patients in the United Kingdom, New Zealand, Germany, and Australia – compared with a low of 40% of patients in Norway.
Hospital discharge planning got high marks in the United States, with only 28% saying that they had any gaps in care after an inpatient stay. That compares with a high of around 70% of patients reporting problems in Sweden and Norway.
End-of-life planning was another area where it appears that the United States is doing better than other nations. Seventy-eight percent of U.S. patients said they had discussed their desires with a friend, family member, or physician, while 55% said they had a written advance directive, and 67% had a health care proxy.
By comparison, only 4% of Norwegians had an advance directive, and just 5% of French patients. Only Australia, Canada, and the United Kingdom had rates near those seen in the United States.
Care coordination was a problem in all the nations surveyed. Older Americans were most likely to say that medical records or test results weren’t available at a scheduled appointment (23%) or that tests were duplicated. Patients in all nations reported receiving conflicting information from different doctors, and that primary care physicians did not speak with specialists, and vice versa.
The cost of care was a bigger issue for Americans than for patients in other countries. One in 5 (19%) of U.S. seniors had a medical problem but did not visit a doctor or skipped a test or refilling a prescription because of the cost. New Zealand was second, with 10% saying they had foregone treatment because of cost. Just 3% of French patients had cost issues.
On Twitter @aliciaault
Older Americans are sicker than their peers in 11 developed countries, but are most likely to have a chronic care plan and to have discussed health-promoting behaviors with their physicians, according to a new survey.
The telephone survey analyzed responses from 15,617 adults over age 65 in the United States, Australia, Canada, France, Germany, the Netherlands, New Zealand, Norway, Sweden, Switzerland, and the United Kingdom. It’s almost an apples-to-apples comparison because Medicare beneficiaries are considered fully insured, and all the other countries have some form of universal health coverage, lead author Robin Osborn said in a briefing on the study published Nov. 19 in the journal Health Affairs.

“This new survey shows that there are areas, such as managing patients who have chronic illnesses and hospital discharge planning, where the U.S. does well compared to other countries,” Ms. Osborn, vice president and director of the International Health Policy and Practice Innovations program at the Commonwealth Fund, said in a statement.
The United States also performed well when it came to patient-physician communication, transitions from the hospital to home, and end-of-life planning (Health. Aff. [doi: 10.1377/hlthaff.2014.0947]).
More than two-thirds (68%) of respondents in the United States had two or more chronic conditions, compared with 56% in Canada and 33% in the United Kingdom, which was at the low end of the spectrum.
The vast majority of patients in all countries reported that they thought their physician spent enough time with them, including 86% of Americans. Eighty-one percent of American patients said their physician encouraged them to ask questions – putting them on par with patients in the United Kingdom, New Zealand, Germany, and Australia – compared with a low of 40% of patients in Norway.
Hospital discharge planning got high marks in the United States, with only 28% saying that they had any gaps in care after an inpatient stay. That compares with a high of around 70% of patients reporting problems in Sweden and Norway.
End-of-life planning was another area where it appears that the United States is doing better than other nations. Seventy-eight percent of U.S. patients said they had discussed their desires with a friend, family member, or physician, while 55% said they had a written advance directive, and 67% had a health care proxy.
By comparison, only 4% of Norwegians had an advance directive, and just 5% of French patients. Only Australia, Canada, and the United Kingdom had rates near those seen in the United States.
Care coordination was a problem in all the nations surveyed. Older Americans were most likely to say that medical records or test results weren’t available at a scheduled appointment (23%) or that tests were duplicated. Patients in all nations reported receiving conflicting information from different doctors, and that primary care physicians did not speak with specialists, and vice versa.
The cost of care was a bigger issue for Americans than for patients in other countries. One in 5 (19%) of U.S. seniors had a medical problem but did not visit a doctor or skipped a test or refilling a prescription because of the cost. New Zealand was second, with 10% saying they had foregone treatment because of cost. Just 3% of French patients had cost issues.
On Twitter @aliciaault
FROM HEALTH AFFAIRS
Feds try to clarify meaningful use attestation, hardship rules
WASHINGTON – Feeling confused about how to attest to meaningful use or how to claim a hardship exemption in 2014?
You’re not alone. Federal officials say they’ve gotten lots of questions and comments in the wake of allowing flexibility on the version of certified electronic health record that can be used and on hardship exemptions for a subset of meaningful users.
Elizabeth Myers, Policy and Outreach Lead for the Centers for Medicare & Medicaid Services’ Office of E-Health Standards and Services, tried to provide some clarity to what those rules mean for practicing physicians at the annual symposium of the American Medical Informatics Association.
On the first rule on certified EHRs, “all we did is delay the expiration of 2011 edition software,” Ms. Myers said.
Physicians who have used the 2011 edition all year will be attesting to the 2013 definition of meaningful use, using those specific objectives and clinical quality measures. Those using the 2014 edition will be attesting to the 2014 definition.
The rule was “pretty vague” on what was expected for those using a combination of the 2011 and 2014 editions, Ms. Myers said. “That’s not trying to be confusing or difficult, much as it may seem that way sometimes,” she said. “It’s really on purpose, because we recognize it will be different for every provider.”
The first step for any meaningful user seeking to attest is to visit the Office of the National Coordinator’s Certified Health IT Product website. Once there, enter in all the product names used. The system will determine whether you are using the 2011 or 2014 version, or a combination.
Users of the 2011 version are taken directly to the 2013 definitions and measures, and can follow through on attestation. Those using the 2014 version are taken to the 2014 definitions and measures for attestation.
Combination users are given a unique identifier. Once that’s entered, the system asks whether the user wants to use the 2013 or 2014 measures for attestation. The meaningful user is then walked through one of those two paths, Ms. Myers said.
Hospitals have until Nov. 30 to attest. Physicians and other eligible health care providers can start attesting now. They have the option of attesting all the way through end of February, Ms. Myers said.
She also sought to clear up confusion about the hardship exemptions. For first-time participants in 2014, applying for a hardship this year was necessary to avoid a penalty in 2015.
“The reopened, extended period for hardship applications ... is for those new participants who are still struggling to get their 2014 software in place,” Ms. Myers said. “If you are unable to fully implement 2014 edition software in 2014 and you have participated in the program in the past, your application for your hardship is due in 2015 to avoid the 2016 payment adjustment.”
Those hardship applications are due April 1 for hospitals and July 1 for eligible professionals, Ms. Myers said.
In terms of what can be claimed as a hardship, the reason has to be related to the functioning of the software – not that “I didn’t feel like paying for it,” or “I was on vacation,” she said. It has to be that you were unable to implement the software to fully meet meaningful use.
Be ready to document the request, but certified letters from vendors saying it was their fault aren’t necessary. “You need the documentation that demonstrates your circumstances,” she said. That means being able to show the certified software used, your approach for meeting objectives and measures, and how the system may have failed you.
“Keep those records,” Ms. Myers said.
On Twitter @aliciaault
WASHINGTON – Feeling confused about how to attest to meaningful use or how to claim a hardship exemption in 2014?
You’re not alone. Federal officials say they’ve gotten lots of questions and comments in the wake of allowing flexibility on the version of certified electronic health record that can be used and on hardship exemptions for a subset of meaningful users.
Elizabeth Myers, Policy and Outreach Lead for the Centers for Medicare & Medicaid Services’ Office of E-Health Standards and Services, tried to provide some clarity to what those rules mean for practicing physicians at the annual symposium of the American Medical Informatics Association.
On the first rule on certified EHRs, “all we did is delay the expiration of 2011 edition software,” Ms. Myers said.
Physicians who have used the 2011 edition all year will be attesting to the 2013 definition of meaningful use, using those specific objectives and clinical quality measures. Those using the 2014 edition will be attesting to the 2014 definition.
The rule was “pretty vague” on what was expected for those using a combination of the 2011 and 2014 editions, Ms. Myers said. “That’s not trying to be confusing or difficult, much as it may seem that way sometimes,” she said. “It’s really on purpose, because we recognize it will be different for every provider.”
The first step for any meaningful user seeking to attest is to visit the Office of the National Coordinator’s Certified Health IT Product website. Once there, enter in all the product names used. The system will determine whether you are using the 2011 or 2014 version, or a combination.
Users of the 2011 version are taken directly to the 2013 definitions and measures, and can follow through on attestation. Those using the 2014 version are taken to the 2014 definitions and measures for attestation.
Combination users are given a unique identifier. Once that’s entered, the system asks whether the user wants to use the 2013 or 2014 measures for attestation. The meaningful user is then walked through one of those two paths, Ms. Myers said.
Hospitals have until Nov. 30 to attest. Physicians and other eligible health care providers can start attesting now. They have the option of attesting all the way through end of February, Ms. Myers said.
She also sought to clear up confusion about the hardship exemptions. For first-time participants in 2014, applying for a hardship this year was necessary to avoid a penalty in 2015.
“The reopened, extended period for hardship applications ... is for those new participants who are still struggling to get their 2014 software in place,” Ms. Myers said. “If you are unable to fully implement 2014 edition software in 2014 and you have participated in the program in the past, your application for your hardship is due in 2015 to avoid the 2016 payment adjustment.”
Those hardship applications are due April 1 for hospitals and July 1 for eligible professionals, Ms. Myers said.
In terms of what can be claimed as a hardship, the reason has to be related to the functioning of the software – not that “I didn’t feel like paying for it,” or “I was on vacation,” she said. It has to be that you were unable to implement the software to fully meet meaningful use.
Be ready to document the request, but certified letters from vendors saying it was their fault aren’t necessary. “You need the documentation that demonstrates your circumstances,” she said. That means being able to show the certified software used, your approach for meeting objectives and measures, and how the system may have failed you.
“Keep those records,” Ms. Myers said.
On Twitter @aliciaault
WASHINGTON – Feeling confused about how to attest to meaningful use or how to claim a hardship exemption in 2014?
You’re not alone. Federal officials say they’ve gotten lots of questions and comments in the wake of allowing flexibility on the version of certified electronic health record that can be used and on hardship exemptions for a subset of meaningful users.
Elizabeth Myers, Policy and Outreach Lead for the Centers for Medicare & Medicaid Services’ Office of E-Health Standards and Services, tried to provide some clarity to what those rules mean for practicing physicians at the annual symposium of the American Medical Informatics Association.
On the first rule on certified EHRs, “all we did is delay the expiration of 2011 edition software,” Ms. Myers said.
Physicians who have used the 2011 edition all year will be attesting to the 2013 definition of meaningful use, using those specific objectives and clinical quality measures. Those using the 2014 edition will be attesting to the 2014 definition.
The rule was “pretty vague” on what was expected for those using a combination of the 2011 and 2014 editions, Ms. Myers said. “That’s not trying to be confusing or difficult, much as it may seem that way sometimes,” she said. “It’s really on purpose, because we recognize it will be different for every provider.”
The first step for any meaningful user seeking to attest is to visit the Office of the National Coordinator’s Certified Health IT Product website. Once there, enter in all the product names used. The system will determine whether you are using the 2011 or 2014 version, or a combination.
Users of the 2011 version are taken directly to the 2013 definitions and measures, and can follow through on attestation. Those using the 2014 version are taken to the 2014 definitions and measures for attestation.
Combination users are given a unique identifier. Once that’s entered, the system asks whether the user wants to use the 2013 or 2014 measures for attestation. The meaningful user is then walked through one of those two paths, Ms. Myers said.
Hospitals have until Nov. 30 to attest. Physicians and other eligible health care providers can start attesting now. They have the option of attesting all the way through end of February, Ms. Myers said.
She also sought to clear up confusion about the hardship exemptions. For first-time participants in 2014, applying for a hardship this year was necessary to avoid a penalty in 2015.
“The reopened, extended period for hardship applications ... is for those new participants who are still struggling to get their 2014 software in place,” Ms. Myers said. “If you are unable to fully implement 2014 edition software in 2014 and you have participated in the program in the past, your application for your hardship is due in 2015 to avoid the 2016 payment adjustment.”
Those hardship applications are due April 1 for hospitals and July 1 for eligible professionals, Ms. Myers said.
In terms of what can be claimed as a hardship, the reason has to be related to the functioning of the software – not that “I didn’t feel like paying for it,” or “I was on vacation,” she said. It has to be that you were unable to implement the software to fully meet meaningful use.
Be ready to document the request, but certified letters from vendors saying it was their fault aren’t necessary. “You need the documentation that demonstrates your circumstances,” she said. That means being able to show the certified software used, your approach for meeting objectives and measures, and how the system may have failed you.
“Keep those records,” Ms. Myers said.
On Twitter @aliciaault
AT THE AMIA 2014 ANNUAL SYMPOSIUM
IOM recommends social factors to include in EHRs
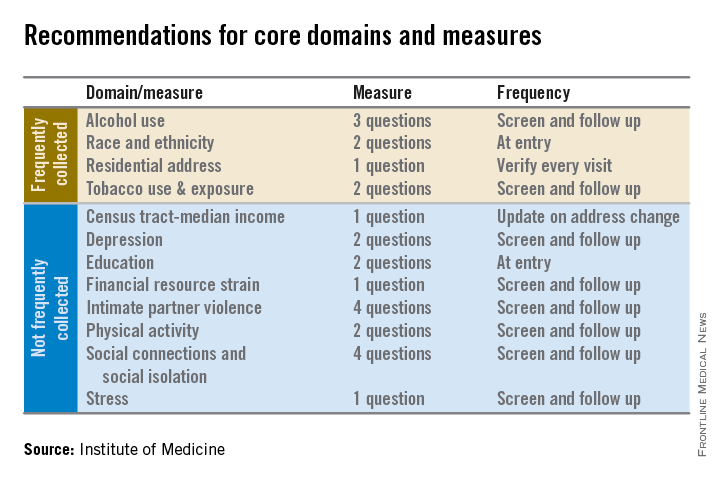
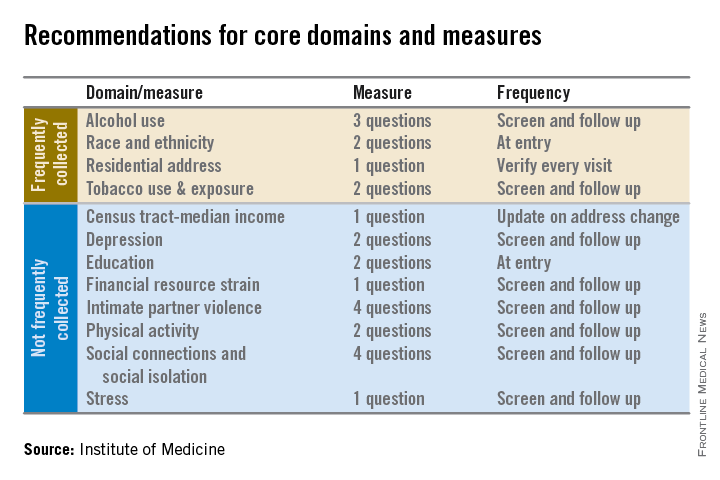
Electronic health records should be equipped to record and track 12 social and behavioral determinants of health, an Institute of Medicine committee recommended.
In addition to measures that are routinely collected now – race/ethnicity, tobacco use, alcohol use, and residential address – the committee advocated that electronic heath records (EHRs) should be able to capture:
• Educational attainment.
• Financial resource strain.
• Stress.
• Depression.
• Physical activity.
• Social isolation.
• Intimate partner violence (for women of reproductive age).
• Neighborhood median household income.
These measures can “provide crucial information about factors that influence health and the effectiveness of treatment,” collect data for researchers and policy makers, and help inform innovations that might improve health outcomes or reduce costs, according to the Nov. 13 report.
The panel aimed for what it called a “parsimonious panel of measures,” to help reduce the data collection burden for patients and health care providers, committee cochair Dr. William W. Stead, McKesson Foundation Professor of Biomedical Informatics and professor of medicine at Vanderbilt University, said during a press briefing.
The IOM report will be used by the Office of the National Coordinator for Health Information Technology (ONC) to determine what it should require from certified EHRs and from physicians who are participating in Medicare’s meaningful use incentive payment program. Physicians will be required to document social and behavioral determinants under Stage 3 of meaningful use, which begins in 2017.
Dr. Stead said that the speed of inclusion of the social and behavioral determinants in EHRs will partly be determined by whether the ONC follows the panel’s recommendations and requires them as part of meaningful use. He noted that in the past, EHR vendors and health care systems have been told by the ONC that they need to obtain certain types of information, “but then had to figure out on their own how to capture that information.
“There’s no reason why this needs to take years,” said Dr. Stead.
With the IOM recommendations, “we’re building the interoperability in from the beginning by providing a concise set of standard questions,” he said.
It will likely take less time to get the determinants into EHR packages than for health systems and physicians to figure out how to build the data collection into their workflow, Dr. Stead said.
The committee acknowledges that it will take more time during a patient encounter to collect these data. But, wrote the panel in the report, “the committee concluded that the health benefits of addressing these determinants outweigh the added burden to providers, patients, and health care systems.”
Additional recommendations in the report include:
• That the ONC’s EHR certification process be expanded to include appraisal of a vendor or product’s ability to acquire, store, transmit, and download self-reported data germane to the social and behavioral determinants of health.
• That the National Institutes of Health develops a plan for advancing research using social and behavioral determinants of health collected in electronic health records.
• That the Health & Human Services department convenes a task force within the next 3 years, and as needed thereafter, to review advances in the measurement of social and behavioral determinants and make recommendations for new standards and data elements for inclusion in electronic health records.
The committee’s work builds on draft recommendations published in April. It was sponsored by a number of federal agencies and health care foundations.
Dr. Michael E. Nelson, FCCP, comments: While physicians might agree that the recommendations of the Institute of Medicine (IOM) might enhance medical care, it is naïve to believe that this administrative burden will not make it increasingly difficult for physicians to meet Stage 3 of meaningful use should these requirements be added by the Office of the National Coordinator for Health Information Technology (ONC).

|
| Dr. Michael E. Nelson |
Assuming that EHR vendors will incorporate this information into their software, the time required to elicit and record this information is not insignificant and will put further strain on the already busy clinician.
In addition, there is a major assumption that the patient will actually provide this information willingly and that there would be implied consent to allow this information to be shared with the federal government, anonymously or otherwise.
Should the ONC adopt these recommendations from the IOM, one would hope that EHR vendors are required to add this to their software at no additional cost to physicians. Also, each question might have a button for "patient declined to answer." George Orwell might have been more prescient than credited.
Dr. Nelson is affiliated with Shawnee Mission Pulmonary Consultants in Shawnee Mission, KS.
Dr. Michael E. Nelson, FCCP, comments: While physicians might agree that the recommendations of the Institute of Medicine (IOM) might enhance medical care, it is naïve to believe that this administrative burden will not make it increasingly difficult for physicians to meet Stage 3 of meaningful use should these requirements be added by the Office of the National Coordinator for Health Information Technology (ONC).

|
| Dr. Michael E. Nelson |
Assuming that EHR vendors will incorporate this information into their software, the time required to elicit and record this information is not insignificant and will put further strain on the already busy clinician.
In addition, there is a major assumption that the patient will actually provide this information willingly and that there would be implied consent to allow this information to be shared with the federal government, anonymously or otherwise.
Should the ONC adopt these recommendations from the IOM, one would hope that EHR vendors are required to add this to their software at no additional cost to physicians. Also, each question might have a button for "patient declined to answer." George Orwell might have been more prescient than credited.
Dr. Nelson is affiliated with Shawnee Mission Pulmonary Consultants in Shawnee Mission, KS.
Dr. Michael E. Nelson, FCCP, comments: While physicians might agree that the recommendations of the Institute of Medicine (IOM) might enhance medical care, it is naïve to believe that this administrative burden will not make it increasingly difficult for physicians to meet Stage 3 of meaningful use should these requirements be added by the Office of the National Coordinator for Health Information Technology (ONC).

|
| Dr. Michael E. Nelson |
Assuming that EHR vendors will incorporate this information into their software, the time required to elicit and record this information is not insignificant and will put further strain on the already busy clinician.
In addition, there is a major assumption that the patient will actually provide this information willingly and that there would be implied consent to allow this information to be shared with the federal government, anonymously or otherwise.
Should the ONC adopt these recommendations from the IOM, one would hope that EHR vendors are required to add this to their software at no additional cost to physicians. Also, each question might have a button for "patient declined to answer." George Orwell might have been more prescient than credited.
Dr. Nelson is affiliated with Shawnee Mission Pulmonary Consultants in Shawnee Mission, KS.
Electronic health records should be equipped to record and track 12 social and behavioral determinants of health, an Institute of Medicine committee recommended.
In addition to measures that are routinely collected now – race/ethnicity, tobacco use, alcohol use, and residential address – the committee advocated that electronic heath records (EHRs) should be able to capture:
• Educational attainment.
• Financial resource strain.
• Stress.
• Depression.
• Physical activity.
• Social isolation.
• Intimate partner violence (for women of reproductive age).
• Neighborhood median household income.
These measures can “provide crucial information about factors that influence health and the effectiveness of treatment,” collect data for researchers and policy makers, and help inform innovations that might improve health outcomes or reduce costs, according to the Nov. 13 report.

The panel aimed for what it called a “parsimonious panel of measures,” to help reduce the data collection burden for patients and health care providers, committee cochair Dr. William W. Stead, McKesson Foundation Professor of Biomedical Informatics and professor of medicine at Vanderbilt University, said during a press briefing.
The IOM report will be used by the Office of the National Coordinator for Health Information Technology (ONC) to determine what it should require from certified EHRs and from physicians who are participating in Medicare’s meaningful use incentive payment program. Physicians will be required to document social and behavioral determinants under Stage 3 of meaningful use, which begins in 2017.
Dr. Stead said that the speed of inclusion of the social and behavioral determinants in EHRs will partly be determined by whether the ONC follows the panel’s recommendations and requires them as part of meaningful use. He noted that in the past, EHR vendors and health care systems have been told by the ONC that they need to obtain certain types of information, “but then had to figure out on their own how to capture that information.
“There’s no reason why this needs to take years,” said Dr. Stead.
With the IOM recommendations, “we’re building the interoperability in from the beginning by providing a concise set of standard questions,” he said.
It will likely take less time to get the determinants into EHR packages than for health systems and physicians to figure out how to build the data collection into their workflow, Dr. Stead said.
The committee acknowledges that it will take more time during a patient encounter to collect these data. But, wrote the panel in the report, “the committee concluded that the health benefits of addressing these determinants outweigh the added burden to providers, patients, and health care systems.”
Additional recommendations in the report include:
• That the ONC’s EHR certification process be expanded to include appraisal of a vendor or product’s ability to acquire, store, transmit, and download self-reported data germane to the social and behavioral determinants of health.
• That the National Institutes of Health develops a plan for advancing research using social and behavioral determinants of health collected in electronic health records.
• That the Health & Human Services department convenes a task force within the next 3 years, and as needed thereafter, to review advances in the measurement of social and behavioral determinants and make recommendations for new standards and data elements for inclusion in electronic health records.
The committee’s work builds on draft recommendations published in April. It was sponsored by a number of federal agencies and health care foundations.
Electronic health records should be equipped to record and track 12 social and behavioral determinants of health, an Institute of Medicine committee recommended.
In addition to measures that are routinely collected now – race/ethnicity, tobacco use, alcohol use, and residential address – the committee advocated that electronic heath records (EHRs) should be able to capture:
• Educational attainment.
• Financial resource strain.
• Stress.
• Depression.
• Physical activity.
• Social isolation.
• Intimate partner violence (for women of reproductive age).
• Neighborhood median household income.
These measures can “provide crucial information about factors that influence health and the effectiveness of treatment,” collect data for researchers and policy makers, and help inform innovations that might improve health outcomes or reduce costs, according to the Nov. 13 report.

The panel aimed for what it called a “parsimonious panel of measures,” to help reduce the data collection burden for patients and health care providers, committee cochair Dr. William W. Stead, McKesson Foundation Professor of Biomedical Informatics and professor of medicine at Vanderbilt University, said during a press briefing.
The IOM report will be used by the Office of the National Coordinator for Health Information Technology (ONC) to determine what it should require from certified EHRs and from physicians who are participating in Medicare’s meaningful use incentive payment program. Physicians will be required to document social and behavioral determinants under Stage 3 of meaningful use, which begins in 2017.
Dr. Stead said that the speed of inclusion of the social and behavioral determinants in EHRs will partly be determined by whether the ONC follows the panel’s recommendations and requires them as part of meaningful use. He noted that in the past, EHR vendors and health care systems have been told by the ONC that they need to obtain certain types of information, “but then had to figure out on their own how to capture that information.
“There’s no reason why this needs to take years,” said Dr. Stead.
With the IOM recommendations, “we’re building the interoperability in from the beginning by providing a concise set of standard questions,” he said.
It will likely take less time to get the determinants into EHR packages than for health systems and physicians to figure out how to build the data collection into their workflow, Dr. Stead said.
The committee acknowledges that it will take more time during a patient encounter to collect these data. But, wrote the panel in the report, “the committee concluded that the health benefits of addressing these determinants outweigh the added burden to providers, patients, and health care systems.”
Additional recommendations in the report include:
• That the ONC’s EHR certification process be expanded to include appraisal of a vendor or product’s ability to acquire, store, transmit, and download self-reported data germane to the social and behavioral determinants of health.
• That the National Institutes of Health develops a plan for advancing research using social and behavioral determinants of health collected in electronic health records.
• That the Health & Human Services department convenes a task force within the next 3 years, and as needed thereafter, to review advances in the measurement of social and behavioral determinants and make recommendations for new standards and data elements for inclusion in electronic health records.
The committee’s work builds on draft recommendations published in April. It was sponsored by a number of federal agencies and health care foundations.
Medicare expanding coverage of telehealth services
Medicare will begin paying for more care delivered by telehealth next year, but restrictions could limit the growth in these virtual or remote visits.
The program pays for telehealth services only when provided in designated Health Professional Shortage Areas; additionally, patients must receive the remote care at a hospital, doctor’s office, or another approved ambulatory care site.
Physician organizations say it’s important that Medicare continues to add services that are covered when delivered via telehealth, but that greater adoption will not happen until payment and licensure issues are addressed.
According to the 2015 Medicare physician fee schedule, doctors can be paid for providing annual wellness visits, psychoanalysis, psychotherapy, and prolonged evaluation and management via telehealth.
Currently, about 10 million patients have received some form of telehealth services, according to the American Telemedicine Association. More than half (55%) of that is the remote reading of imaging by a radiologist, 24% is remote consultations, and 15% is remote monitoring. The remainder (6%) is comprised of miscellaneous and pharmacy services.
A recent survey of hospital, group practice, and home health executives found that most (84%) said that it was important or very important to develop their telehealth capability, but that reimbursement uncertainty was holding them back. Forty-one percent said they were not being reimbursed at all for telehealth, and 21% said that the rates were lower than for in-person services.
Only 6% said their programs were “mature,” according to the survey, which was conducted by Foley & Lardner, a law firm.
The American Medical Association has made expanded coverage of telehealth a priority. “We need coverage and reimbursement of telemedicine services and fewer restrictions in Medicare,” Dr. Robert Wah, the AMA president, said at the group’s interim policy meeting. “We want patients to use it if they need it. Lift geographic restrictions. Free up its use in alternative payment models.”
Telemedicine can increase access to care, especially in rural and underserved areas, according to Dr. Reid Blackwelder, board chair of the American Academy of Family Physicians. Currently, many family physicians are delivering health care remotely – by smartphone or Skype, for instance – but they often don’t get paid, he said.
Medicare requires that physicians use a G code to bill for health care provided remotely, and that additional documentation burden may mean that physicians don’t bill for it, even if they’ve delivered the care, Dr. Blackwelder said in an interview.
The AAFP would like to see a move away from those G codes, substituting a per member per month fee instead, he said.
Although Medicare pays the same amount for telehealth services as it would for the same services delivered in a face-to-face visit, not all private payers do so, which also dampens enthusiasm for telehealth, said Dr. Blackwelder.
According to the ATA, 22 states and Washington, D.C. require telehealth coverage to be on par with in-person visits.
Licensure is also an issue. Both the AAFP and the AMA support a model license plan developed by the Federation of State Medical Boards that would allow physicians to deliver services across state lines.
The Interstate Medical Licensure Compact was issued in September. According to the FSMB, 11 state medical boards have formally endorsed it: Texas, South Dakota, Wisconsin, Washington, Maine, Nevada, Alabama, Utah, Vermont, and Wyoming.
However, for licensure requirements to change, the compact must be enacted in every state.
Physician groups also want to ensure that telehealth services are given by “providers that have ongoing and established relationship with the patient,” said Dr. Blackwelder.
Medicare will begin paying for more care delivered by telehealth next year, but restrictions could limit the growth in these virtual or remote visits.
The program pays for telehealth services only when provided in designated Health Professional Shortage Areas; additionally, patients must receive the remote care at a hospital, doctor’s office, or another approved ambulatory care site.
Physician organizations say it’s important that Medicare continues to add services that are covered when delivered via telehealth, but that greater adoption will not happen until payment and licensure issues are addressed.
According to the 2015 Medicare physician fee schedule, doctors can be paid for providing annual wellness visits, psychoanalysis, psychotherapy, and prolonged evaluation and management via telehealth.
Currently, about 10 million patients have received some form of telehealth services, according to the American Telemedicine Association. More than half (55%) of that is the remote reading of imaging by a radiologist, 24% is remote consultations, and 15% is remote monitoring. The remainder (6%) is comprised of miscellaneous and pharmacy services.
A recent survey of hospital, group practice, and home health executives found that most (84%) said that it was important or very important to develop their telehealth capability, but that reimbursement uncertainty was holding them back. Forty-one percent said they were not being reimbursed at all for telehealth, and 21% said that the rates were lower than for in-person services.
Only 6% said their programs were “mature,” according to the survey, which was conducted by Foley & Lardner, a law firm.
The American Medical Association has made expanded coverage of telehealth a priority. “We need coverage and reimbursement of telemedicine services and fewer restrictions in Medicare,” Dr. Robert Wah, the AMA president, said at the group’s interim policy meeting. “We want patients to use it if they need it. Lift geographic restrictions. Free up its use in alternative payment models.”
Telemedicine can increase access to care, especially in rural and underserved areas, according to Dr. Reid Blackwelder, board chair of the American Academy of Family Physicians. Currently, many family physicians are delivering health care remotely – by smartphone or Skype, for instance – but they often don’t get paid, he said.
Medicare requires that physicians use a G code to bill for health care provided remotely, and that additional documentation burden may mean that physicians don’t bill for it, even if they’ve delivered the care, Dr. Blackwelder said in an interview.
The AAFP would like to see a move away from those G codes, substituting a per member per month fee instead, he said.
Although Medicare pays the same amount for telehealth services as it would for the same services delivered in a face-to-face visit, not all private payers do so, which also dampens enthusiasm for telehealth, said Dr. Blackwelder.
According to the ATA, 22 states and Washington, D.C. require telehealth coverage to be on par with in-person visits.
Licensure is also an issue. Both the AAFP and the AMA support a model license plan developed by the Federation of State Medical Boards that would allow physicians to deliver services across state lines.
The Interstate Medical Licensure Compact was issued in September. According to the FSMB, 11 state medical boards have formally endorsed it: Texas, South Dakota, Wisconsin, Washington, Maine, Nevada, Alabama, Utah, Vermont, and Wyoming.
However, for licensure requirements to change, the compact must be enacted in every state.
Physician groups also want to ensure that telehealth services are given by “providers that have ongoing and established relationship with the patient,” said Dr. Blackwelder.
Medicare will begin paying for more care delivered by telehealth next year, but restrictions could limit the growth in these virtual or remote visits.
The program pays for telehealth services only when provided in designated Health Professional Shortage Areas; additionally, patients must receive the remote care at a hospital, doctor’s office, or another approved ambulatory care site.
Physician organizations say it’s important that Medicare continues to add services that are covered when delivered via telehealth, but that greater adoption will not happen until payment and licensure issues are addressed.
According to the 2015 Medicare physician fee schedule, doctors can be paid for providing annual wellness visits, psychoanalysis, psychotherapy, and prolonged evaluation and management via telehealth.
Currently, about 10 million patients have received some form of telehealth services, according to the American Telemedicine Association. More than half (55%) of that is the remote reading of imaging by a radiologist, 24% is remote consultations, and 15% is remote monitoring. The remainder (6%) is comprised of miscellaneous and pharmacy services.
A recent survey of hospital, group practice, and home health executives found that most (84%) said that it was important or very important to develop their telehealth capability, but that reimbursement uncertainty was holding them back. Forty-one percent said they were not being reimbursed at all for telehealth, and 21% said that the rates were lower than for in-person services.
Only 6% said their programs were “mature,” according to the survey, which was conducted by Foley & Lardner, a law firm.
The American Medical Association has made expanded coverage of telehealth a priority. “We need coverage and reimbursement of telemedicine services and fewer restrictions in Medicare,” Dr. Robert Wah, the AMA president, said at the group’s interim policy meeting. “We want patients to use it if they need it. Lift geographic restrictions. Free up its use in alternative payment models.”
Telemedicine can increase access to care, especially in rural and underserved areas, according to Dr. Reid Blackwelder, board chair of the American Academy of Family Physicians. Currently, many family physicians are delivering health care remotely – by smartphone or Skype, for instance – but they often don’t get paid, he said.
Medicare requires that physicians use a G code to bill for health care provided remotely, and that additional documentation burden may mean that physicians don’t bill for it, even if they’ve delivered the care, Dr. Blackwelder said in an interview.
The AAFP would like to see a move away from those G codes, substituting a per member per month fee instead, he said.
Although Medicare pays the same amount for telehealth services as it would for the same services delivered in a face-to-face visit, not all private payers do so, which also dampens enthusiasm for telehealth, said Dr. Blackwelder.
According to the ATA, 22 states and Washington, D.C. require telehealth coverage to be on par with in-person visits.
Licensure is also an issue. Both the AAFP and the AMA support a model license plan developed by the Federation of State Medical Boards that would allow physicians to deliver services across state lines.
The Interstate Medical Licensure Compact was issued in September. According to the FSMB, 11 state medical boards have formally endorsed it: Texas, South Dakota, Wisconsin, Washington, Maine, Nevada, Alabama, Utah, Vermont, and Wyoming.
However, for licensure requirements to change, the compact must be enacted in every state.
Physician groups also want to ensure that telehealth services are given by “providers that have ongoing and established relationship with the patient,” said Dr. Blackwelder.