User login
Addressing supply-demand mismatch in GI
Impacts of this supply-demand mismatch are felt daily in our GI practices as we strive to expand access in our clinics and endoscopy suites, particularly in rural and urban underserved communities. In gastroenterology, increased demand for care has been driven by a perfect storm of population growth, increased patient awareness of GI health, and rising incidence of digestive diseases.
Between 2019 and 2034, the U.S. population is expected to grow by 10.6%, while the population aged 65 and older expands by over 42%. Recent increases in the CRC screening–eligible population also have contributed to unprecedented demand for GI care. Furthermore, care delivery has become more complex and time-consuming with the evolution of personalized medicine and high prevalence of comorbid conditions. At the same time, we are faced with a dwindling supply of gastroenterology providers. In 2021, there were 15,678 practicing gastroenterologists in the U.S., over half of whom were 55 years or older. This translates to 1 gastroenterologist per 20,830 people captured in the U.S. Census.
Addressing this striking supply-demand mismatch in GI requires a multi-pronged approach that addresses its complex drivers. First and foremost, we must expand the number of GI fellowship training slots to boost our pipeline. There are approximately 1,840 GI fellows currently in training, a third of whom enter the workforce each year. While the number of GI fellowship slots in the GI fellowship match has slowly increased over time (from 525 available slots across 199 programs in 2019 to 657 slots across 230 programs in 2023), this incremental growth is dwarfed by overall need. Continued advocacy for increased funding to support expansion of training slots is necessary to further move the needle – such lobbying recently led to the addition of 1,000 new Medicare-supported graduate medical education positions across specialties over a 5-year period starting in 2020, illustrating that change is possible. At the same time, we must address the factors that are causing gastroenterologists to leave the workforce prematurely through early retirement or part-time work by investing in innovative solutions to address burnout, reduce administrative burdens, enhance the efficiency of care delivery, and maintain financial viability. By investing in our physician workforce and its sustainability, we can ensure that our profession is better prepared to meet the needs of our growing and increasingly complex patient population now and in the future.
We hope you enjoy the November issue of GI & Hepatology News and have a wonderful Thanksgiving.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Impacts of this supply-demand mismatch are felt daily in our GI practices as we strive to expand access in our clinics and endoscopy suites, particularly in rural and urban underserved communities. In gastroenterology, increased demand for care has been driven by a perfect storm of population growth, increased patient awareness of GI health, and rising incidence of digestive diseases.
Between 2019 and 2034, the U.S. population is expected to grow by 10.6%, while the population aged 65 and older expands by over 42%. Recent increases in the CRC screening–eligible population also have contributed to unprecedented demand for GI care. Furthermore, care delivery has become more complex and time-consuming with the evolution of personalized medicine and high prevalence of comorbid conditions. At the same time, we are faced with a dwindling supply of gastroenterology providers. In 2021, there were 15,678 practicing gastroenterologists in the U.S., over half of whom were 55 years or older. This translates to 1 gastroenterologist per 20,830 people captured in the U.S. Census.
Addressing this striking supply-demand mismatch in GI requires a multi-pronged approach that addresses its complex drivers. First and foremost, we must expand the number of GI fellowship training slots to boost our pipeline. There are approximately 1,840 GI fellows currently in training, a third of whom enter the workforce each year. While the number of GI fellowship slots in the GI fellowship match has slowly increased over time (from 525 available slots across 199 programs in 2019 to 657 slots across 230 programs in 2023), this incremental growth is dwarfed by overall need. Continued advocacy for increased funding to support expansion of training slots is necessary to further move the needle – such lobbying recently led to the addition of 1,000 new Medicare-supported graduate medical education positions across specialties over a 5-year period starting in 2020, illustrating that change is possible. At the same time, we must address the factors that are causing gastroenterologists to leave the workforce prematurely through early retirement or part-time work by investing in innovative solutions to address burnout, reduce administrative burdens, enhance the efficiency of care delivery, and maintain financial viability. By investing in our physician workforce and its sustainability, we can ensure that our profession is better prepared to meet the needs of our growing and increasingly complex patient population now and in the future.
We hope you enjoy the November issue of GI & Hepatology News and have a wonderful Thanksgiving.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Impacts of this supply-demand mismatch are felt daily in our GI practices as we strive to expand access in our clinics and endoscopy suites, particularly in rural and urban underserved communities. In gastroenterology, increased demand for care has been driven by a perfect storm of population growth, increased patient awareness of GI health, and rising incidence of digestive diseases.
Between 2019 and 2034, the U.S. population is expected to grow by 10.6%, while the population aged 65 and older expands by over 42%. Recent increases in the CRC screening–eligible population also have contributed to unprecedented demand for GI care. Furthermore, care delivery has become more complex and time-consuming with the evolution of personalized medicine and high prevalence of comorbid conditions. At the same time, we are faced with a dwindling supply of gastroenterology providers. In 2021, there were 15,678 practicing gastroenterologists in the U.S., over half of whom were 55 years or older. This translates to 1 gastroenterologist per 20,830 people captured in the U.S. Census.
Addressing this striking supply-demand mismatch in GI requires a multi-pronged approach that addresses its complex drivers. First and foremost, we must expand the number of GI fellowship training slots to boost our pipeline. There are approximately 1,840 GI fellows currently in training, a third of whom enter the workforce each year. While the number of GI fellowship slots in the GI fellowship match has slowly increased over time (from 525 available slots across 199 programs in 2019 to 657 slots across 230 programs in 2023), this incremental growth is dwarfed by overall need. Continued advocacy for increased funding to support expansion of training slots is necessary to further move the needle – such lobbying recently led to the addition of 1,000 new Medicare-supported graduate medical education positions across specialties over a 5-year period starting in 2020, illustrating that change is possible. At the same time, we must address the factors that are causing gastroenterologists to leave the workforce prematurely through early retirement or part-time work by investing in innovative solutions to address burnout, reduce administrative burdens, enhance the efficiency of care delivery, and maintain financial viability. By investing in our physician workforce and its sustainability, we can ensure that our profession is better prepared to meet the needs of our growing and increasingly complex patient population now and in the future.
We hope you enjoy the November issue of GI & Hepatology News and have a wonderful Thanksgiving.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Selecting therapies in moderate to severe inflammatory bowel disease: Key factors in decision making
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).
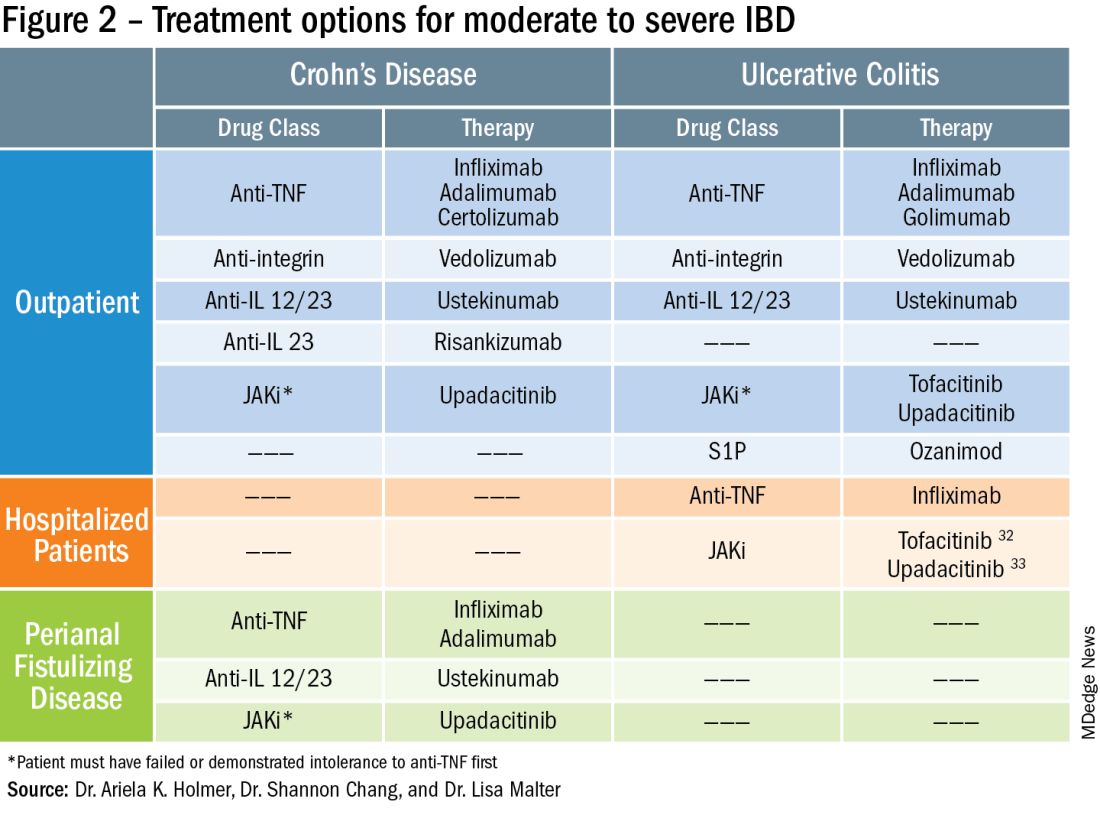
Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Despite new advances in treatment, head to head clinical trials, which are considered the gold standard when comparing therapies, remain limited. Other comparative effectiveness studies and network meta-analyses are the currently available substitutes to guide decision making.1
While efficacy is often considered first when choosing a drug, other critical factors play a role in tailoring a treatment plan. This article focuses on key considerations to help guide clinical decision making when treating patients with moderate to severe IBD (Figure 1).

Disease activity versus severity
Both disease activity and disease severity should be considered when evaluating a patient for treatment. Disease activity is a cross-sectional view of one’s signs and symptoms which can vary visit to visit. Standardized indices measure disease activity in both Crohn’s disease (CD) and ulcerative colitis (UC).2,3 Disease severity encompasses the overall prognosis of disease over time and includes factors such as the presence or absence of high risk features, prior medication exposure, history of surgery, hospitalizations and the impact on quality of life.4
To prevent disease complications, the goals of treatment should be aimed at both reducing active symptoms (disease activity) but also healing mucosal inflammation, preventing disease progression (disease severity) and downstream sequelae including cancer, hospitalization or surgery.5 Determining the best treatment option takes disease activity and severity into account, in addition to the other key factors listed below (Figure 2).

Extraintestinal manifestations
Inflammation of organs outside of the gastrointestinal tract is common and can occur in up to 50% of patients with IBD.6 The most prevalent extraintestinal manifestations (EIMs) involve the skin and joints, which will be the primary focus in this article. We will also focus on treatment options with the most evidence supporting their use. Peripheral arthritis is often associated with intestinal inflammation, and treatment of underlying IBD can simultaneously improve joint symptoms. Conversely, axial spondyloarthritis does not commonly parallel intestinal inflammation. Anti–tumor necrosis factor (TNF) agents including infliximab and adalimumab are effective for the treatment of both peripheral and axial disease.6
Ustekinumab, an interleukin (IL)-12/23 inhibitor, may be effective for peripheral arthritis, however is ineffective for the treatment of axial spondyloarthritis.6 Janus kinase (JAK) inhibitors which include tofacitinib and upadacitinib are oral small molecules used to treat peripheral and axial spondyloarthritis and have more recently been approved for moderate to severe IBD.6,7
Erythema nodosum (EN) and pyoderma gangrenosum (PG) are skin manifestations seen in patients with IBD. EN appears as subcutaneous nodules and parallels intestinal inflammation, while PG consists of violaceous, ulcerated plaques, and presents with more significant pain. Anti-TNFs are effective for both EN and PG, with infliximab being the only biologic studied in a randomized control trial of patients with PG.8 In addition, small case reports have described some benefit from ustekinumab and upadacitinib in the treatment of PG.9,10
Safety
The safety of IBD therapies is a key consideration and often the most important factor to patients when choosing a treatment option. It is important to note that untreated disease is associated with significant morbidity, and should be weighed when discussing risks of medications with patients. In general, anti-TNFs and JAK inhibitors may be associated with an increased risk of infection and malignancy, while ustekinumab, vedolizumab, risankizumab and ozanimod offer a more favorable safety profile.11 In large registries and observational studies, infliximab was associated with up to a two times greater risk of serious infection as compared to nonbiologic medications, with the most common infections being pneumonia, sepsis and herpes zoster.12 JAK inhibitors are associated with an increased risk of herpes zoster infection, with a dose dependent effect seen in the maintenance clinical trials with tofacitinib.7
Ozanimod may be associated with atrioventricular conduction delays and bradycardia, however long-term safety data has reported a low incidence of serious cardiac related adverse events.13 Overall, though risks of infection may vary with different therapies, other consistent risk factors associated with greater rates of serious infection include prolonged corticosteroid use, combination therapy with thiopurines, and disease severity. Anti-TNFs have also been associated with a somewhat increased risk of lymphoma, increased when used in combination with thiopurines. Reassuringly, however, in patients with a prior history of cancer, anti-TNFs and non-TNF biologics have not been found to increase the risk of new or recurrent cancer.14
Ultimately, in patients with a prior history of cancer, the choice of biologic or small molecule should be made in collaboration with a patient’s oncologist.
Anti-TNF exposure
Anti-TNFs were the first available biologics for the treatment of IBD. After the approval of vedolizumab in 2014, the first non-TNF biologic, many patients enrolled in clinical trials thereafter had already tried and failed anti-TNFs. In general, exposure to anti-TNFs may reduce the efficacy of a future biologic. In patients treated with vedolizumab, endoscopic and clinical outcomes were negatively impacted by prior anti-TNF exposure.15 However, in VARSITY, a head-to-head clinical trial where 20% of patients with UC were previously exposed to anti-TNFs other than adalimumab, vedolizumab had significantly higher rates of clinical remission and endoscopic improvement compared to adalimumab.16 Clinical remission rates with tofacitinib were not impacted by exposure to anti-TNF treatment, and similar findings were observed with ustekinumab.7,17 Risankizumab, a newly approved selective anti-IL23, also does not appear to be impacted by prior anti-TNF exposure by demonstrating similar rates of clinical remission regardless of biologic exposure status.18 Therefore, in patients with prior history of anti-TNF use, consideration of ustekinumab, risankizumab or JAK inhibitors as second line agents may be more favorable as compared to vedolizumab.
Perianal fistulizing disease
Perianal fistulizing disease can affect up to one-third of patients with CD and significantly impact a patient’s quality of life.19 The most robust data for the treatment of perianal fistulizing disease includes the use of infliximab with up to one-third of patients on maintenance therapy achieving complete resolution of fistula drainage. While no head-to-head trials compare combination therapy with infliximab plus immunomodulators versus infliximab alone for this indication specifically, one observational study demonstrated higher rates of fistula closure with combination therapy as compared to infliximab mono-therapy.19 In a post hoc analysis, higher infliximab concentrations at week 14 were associated with greater fistula response and remission rates.20 In patients with perianal disease, ustekinumab and vedolizumab may also be an effective treatment option by promoting resolution of fistula drainage.21
More recently, emerging data demonstrate that upadacitinib may be an excellent option as a second-line treatment for perianal disease in patients who have failed anti-TNF therapy. Use of upadacitinib was associated with greater rates of complete resolution of fistula drainage and higher rates of external fistula closure (Figure 2).22 Lastly, as an alternative to medical therapy, mesenchymal stem cell therapy has also shown to improve fistula drainage and improve external fistula openings in patients with CD.23 Stem cell therapy is only available through clinical trials at this time.
Patient preferences
Overall, data are lacking for evaluating patient preferences in treatment options for IBD especially with the recent increase in therapeutic options. One survey demonstrated that patient preferences were most impacted by the possibility of improving abdominal pain, with patients accepting additional risk of treatment side effects in order to reduce their abdominal pain.24 An oral route of administration and improving fatigue and bowel urgency were similarly important to patients. Patient preferences can also be highly variable with some valuing avoidance of corticosteroid use while others valuing avoidance of symptoms or risks of medication side effects and surgery. It is important to tailor the discussion on treatment strategies to each individual patient and inquire about the patient’s lifestyle, medical history, and value system, which may impact their treatment preferences utilizing shared decision making.
Access to treatment including the role of social determinants of health
The expanded therapeutic armamentarium has the potential to help patients achieve the current goals of care in IBD. However, these medications are not available to all patients due to numerous barriers including step therapy payer policies, prohibitive costs, insurance prior authorizations, and the role of social determinants of health and proximity to IBD expertise.25 While clinicians work with patients to determine the best treatment option, more often than not, the decision lies with the insurance payer. Step therapy is the protocol used by insurance companies that requires patients to try a lower-cost medication and fail to respond before they approve the originally requested treatment. This can lead to treatment delays, progression of disease, and disease complications. The option to incorporate the use of biosimilars, currently available for anti-TNFs, and other biologics in the near future, will reduce cost and potentially increase access.26 Additionally, working with a clinical pharmacist to navigate access and utilize patient assistance programs may help overcome cost related barriers to treatment and prevent delays in care.
Socioeconomic status has been shown to impact IBD disease outcomes, and compliance rates in treatment vary depending on race and ethnicity.27 Certain racial and ethnic groups remain vulnerable and may require additional support to achieve treatment goals. For example, disparities in health literacy in patients with IBD have been demonstrated with older black men at risk.28 Additionally, the patient’s proximity to their health care facility may impact treatment options. Most IBD centers are located in metropolitan areas and numerous “IBD deserts” exist, potentially limiting therapies for patients from more remote/rural settings.29 Access to treatment and the interplay of social determinants of health can have a large role in therapy selection.
Special considerations: Pregnancy and older adults
Certain patient populations warrant special consideration when approaching treatment strategies. Pregnancy in IBD will not be addressed in full depth in this article, however a key takeaway is that planning is critical and providers should emphasize the importance of steroid-free clinical remission for at least 3 months before conception.30 Additionally, biologic use during pregnancy has not been shown to increase adverse fetal outcomes, thus should be continued to minimize disease flare. Newer novel small molecules are generally avoided during pregnancy due to limited available safety data.
Older adults are the largest growing patient population with IBD. Frailty, or a state of decreased reserve, is more commonly observed in older patients and has been shown to increase adverse events including hospitalization and mortaility.31 Ultimately reducing polypharmacy, ensuring adequate nutrition, minimizing corticosteroid exposure and avoiding undertreatment of active IBD are all key in optimizing outcomes in an older patient with IBD.
Conclusion
When discussing treatment options with patients with IBD, it is important to individualize care and share the decision-making process with patients. Goals include improving symptoms and quality of life while working to achieve the goal of healing intestinal inflammation. In summary, this article can serve as a guide to clinicians for key factors in decision making when selecting therapies in moderate to severe IBD.
Dr. Holmer is a gastroenterologist with NYU Langone Health specializing in inflammatory bowel disease. Dr. Chang is director of clinical operations for the NYU Langone Health Inflammatory Bowel Disease Center. Dr. Malter is director of education for the Inflammatory Bowel Disease Center at NYU Langone Health and director of the inflammatory bowel disease program at Bellevue Hospital Center. Follow Dr. Holmer on X (formerly Twitter) at @HolmerMd and Dr. Chang @shannonchangmd. Dr. Holmer disclosed affiliations with Pfizer, Bristol Myers Squibb, and AvevoRx. Dr. Chang disclosed affiliations with Pfizer and Bristol Myers Squibb. Dr. Malter disclosed receiving educational grants form Abbvie, Janssen, Pfizer and Takeda, and serving on the advisory boards of AbbVie, Bristol Myers Squibb, Celltrion, Janssen, Merck, and Takeda.
References
1. Chang S et al. Am J Gastroenterol. 2023 Aug 24. doi: 10.14309/ajg.0000000000002485.
2. Harvey RF et al. The Lancet. 1980;1:514.
3. Lewis JD et al. Inflammatory Bowel Diseases. 2008;14:1660-1666.
4. Siegel CA et al. Gut. 2018;67(2):244-54.
5. Peyrin-Biroulet L et al. Am J Gastroenterol. 2015;110:1324-38
6. Rogler G et al. Gastroenterology. 2021;161:1118-32.
7. Sandborn WJ et al. N Engl J Med. 2017;376:1723-36.
8. Brooklyn TN et al. Gut. 2006;55:505-9.
9. Fahmy M et al. Am J Gastroenterol. 2012;107:794-5.
10. Van Eycken L et al. JAAD Case Rep. 2023;37:89-91.
11. Lasa JS et al. Lancet Gastroenterol Hepatol. 2022;7:161-70.
12. Lichtenstein GR et al. Inflamm Bowel Dis. 2018;24:490-501.
13. Long MD et al. Gastroenterology. 2022;162:S-5-S-6.
14. Holmer AK et al. Clin Gastroenterol Hepatol.2023;21:1598-1606.e5.
15. Sands BE et al. Gastroenterology. 2014;147:618-27.e3.
16. Sands BE et al. N Engl J Med. 2019;381:1215-26.
17. Sands BE et al. N Engl J Med. 2019;381:1201-14.
18. D’Haens G et al. Lancet. 2022;399:2015-30.
19. Bouguen G et al. Clin Gastroenterol Hepatol. 2013;11:975-81.e1-4.
20. Papamichael K et al. Am J Gastroenterol. 2021;116:1007-14.
21. Shehab M et al. Inflamm Bowel Dis. 2023;29:367-75.
22. Colombel JF et al. J Crohns Colitis. 2023;17:i620-i623.
23. Garcia-Olmo D et al. Dis Colon Rectum. 2022;65:713-20.
24. Louis E et al. J Crohns Colitis. 2023;17:231-9.
25. Rubin DT et al. Inflamm Bowel Dis. 2017;23:224-32.
26. Gulacsi L et al. Curr Med Chem. 2019;26:259-69.
27. Cai Q et al. BMC Gastroenterol. 2022;22:545.
28. Dos Santos Marques IC et al. Crohns Colitis 360. 2020 Oct;2(4):otaa076.
29. Deepak P et al. Gastroenterology. 2023;165:11-15.
30. Mahadevan U et al. Gastroenterology. 2019;156:1508-24.
31. Faye AS et al. Inflamm Bowel Dis. 2022;28:126-32.
32. Berinstein JA et al. Clin Gastroenterol Hepatol. 2021;19:2112-20.e1.
33. Levine J et al. Gastroenterology. 2023;164:S103-S104.
Cysteamine and melasma
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4 .
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4 .
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4 .
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Breastfeeding and colorectal cancer
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I, like every pediatrician I know, believe that breast milk is the best nutrition for human newborns. Its balance of nutritive elements and its role in preventing of a wide range of illnesses are so great that we are still learning the extent of their magnitude. It just makes sense that a mother’s milk is most well suited for her baby.
I am a bit less unambiguous about breastfeeding. By that I mean the process of providing breast milk to an infant directly from its mother’s breast. Before you yank my AAP membership card, let me make it clear that I think every woman should consider breastfeeding her infant. But we must accept that in a few situations, even with help from caring and enlightened health care providers and family members, breastfeeding doesn’t work as well as we would have hoped. Fortunately, there are alternatives.
My reservations about the process are few, and until recently I have had an unwaveringly positive attitude toward the safety of breast milk. The cause of my little bit of uncertainty arrived in a recent study by two researchers at the Dana Farber Institute in Boston, in which the A younger cohort within that larger group had a dramatic 40% increased risk of developing high-risk cancer before reaching age 55.
The population the investigators studied came from the large Nurses’ Health Study II, a well-known repository of longitudinal health data. The researchers reported that they included biometric data and a large collection of lifestyle factors including smoking, alcohol intake, and diet in their calculations. However, breastfeeding continued to register the highest association. Interestingly, the investigators found that women who were breastfed for 9 months or longer had twice the risk of colorectal cancer as those who breastfed for from 4 to 8 months.
The study population was all women and predominantly white. However, in the general population it is the non-Hispanic white population that is experiencing the greatest increase in incidence. Of course, the study could not answer whether this association with breastfeeding also existed in minority populations.
The researchers suspect that what they are seeing is a reflection of the Westernization of the American lifestyle. One of the researchers is interested in the gut biome of infants and plans to further the investigation in that direction. Could some substance from the environment be concentrating in breast milk? Or is something missing in breast milk? She points out that, while formulas are generally fortified with vitamin D, breast milk is not.
As concerning as the results of this study may sound, the authors are very careful to urge mothers to continue to breastfeed and choose it as their first choice for feeding their babies. I have been pleasantly surprised that this study has not gotten widespread media attention because bad news travels fast. I have chosen to share it with you because at some point you may begin getting questions from concerned parents.
While apparently well done, this study is just the beginning. Like any good research, it poses more questions than it answers. For us as pediatricians it means we should continue to recommend breast milk as the first food. But, we must stay alert as further research looks deeper into this association.
We should also take advantage of our special access to young parents, a demographic that less frequently sees a physician for preventive care. For whatever reason colorectal cancer is occurring at younger ages. When we have the opportunity we should be reminding 40-year-olds not to wait until age 50 to screen for colorectal cancer, particularly if they have a family history of the disease.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
GI symptoms during menopause deserve attention
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome back to another GI Common Concerns.
Today, I want to highlight some information about menopause.
Approximately 1.5 million women in the United States per year enter into menopause. Hysterectomy is also one of the most common surgeries for women worldwide, with an estimated 20%-40% undergoing this procedure by the age of 60.
Therefore, whether it’s because of biologic onset with age or surgical induction, menopause is a very common condition, and it’s important that we understand its symptoms and the latest information around it.
Impact on GI motility
One of the clearest functional symptoms to be aware of with menopause relates to alterations in hormonal balance. This has an impact on gastrointestinal (GI) motility by increasing abdominal muscle stimulation related to different patterns of secretion and can result in a number of symptomatic changes.
One such change that can occur is food intolerance. It is believed that menopause-associated food intolerance has multiple possible causes and may be related more to alterations to the microbiome, which can be contributed to by diet, activity, sleep cycle, and other factors.
When food intolerances are triggered in the perimenopausal or menopausal patient, it may lead you to recommend the well-established FODMAP diet, which is known to reduce symptoms. But the answer for every patient is not simply placing them on a FODMAP diet and telling them they have irritable bowel syndrome.
Other approaches can be considered for addressing food intolerance in these patients. The data are quite strong that adjunctive use of a dietitian is tremendously helpful in this particular population.
When it comes to menopausal patients, however, we need to consider other changes in their activity or adverse contributors to their mental health, such as stress or anxiety. These all contribute to more of a multifactorial composite in this population, for which irritable bowel syndrome serves as a similar example.
This means that we may need to expand our horizons rather than to focus on solely on antispasmodic or diet-related interventions.
Instead, we can start to consider more of a multidimensional treatment approach consisting of education, relaxation, cognitive-behavioral therapy, and physical activity. Certainly, there are now behavioral interventions using Internet-based digital formats to increase the acceptability and sustainability among patients.
Choosing such a multidisciplinary approach can be quite helpful.
The metabolic consequences of altering hormonal balance
Recent data from a rat model study investigated the metabolic impact of changing hormonal balance.
Investigators looked at ovariectomized rats and found that there was a biologic change in the diversity of the general GI biome. There were also noteworthy associations with weight fluctuations and dramatic changes in the spatial memory and cognitive performance characteristics of these rats, which was subsequently improved by supplemental estrogen.
This indicates that we may be able to remediate these effects with the similar use of supplemental hormone replacement treatments.
Another recent study looked at nonalcoholic fatty liver disease, which is very common in the general population and has a > 20% worldwide prevalence in postmenopausal women. Albeit small in numbers, this was a very interesting study.
Investigators looked at the delivery method for menopausal hormone therapy, which was transdermal for 75 patients and oral for 293 patients. Then, they looked at ultrasound definition of nonalcoholic fatty liver disease after 1 year as the endpoint. They found an approximate 7% reduction in the patients who received the transdermal administration compared with a 4% increase in the patients who received it orally.
Again, we have to remember this is a relatively small study, but the results indicate that the route of estrogen administration may be an important consideration in nonalcoholic fatty liver disease.
Sleep disturbances: fragmentation, duration, and quality
Sleep is something that’s near and dear to my heart and is the focus of a lot of our research.
Sleep disturbances are really part and parcel of menopause and are observed with hormonal imbalances and temperature intolerances. Disturbances such as sleep fragmentation, shorter sleep duration, and poorer sleep quality have a dramatic effect not only on the biome but also on sensory thresholds.
Therefore, as we start to look at mitigating strategies here, we need to focus on sleep and ask the right questions.
In my own practice, I try not to just ask, “How did you sleep last night?” That’s because sleep can be somewhat amnestic. You may have a cognitive awakening or a noncognitive awakening but still have experienced fragmentation.
As a result, my focus is on next-day function. I ask my patients, “When you get up in the morning, are you refreshed? Do you have the ability to perform daytime activities? Do you experience early fatigue or cognitive changes that occur?”
These questions can provide good insights into the sleep efficiency of the previous night.
The effect of the microbiome on osteoporosis
One final topic I found very interesting pertains to the effects of menopause on osteoporosis.
We certainly know that postmenopausal women have a very high prevalence of osteopenia, and that osteoporosis is a progression of that, as well as that increased bone-related disease affects fractures and related morbidity and mortality.
However, there’s accumulating evidence on the osteoporotic effects of biomarker changes in menopause, which shows that the biome regulates the pathophysiologic process of at least a large degree of osteoporosis.
This starts to make sense when you look at the pro-inflammatory factors that increase with changes in biome diversity, in particular tumor necrosis factor alpha (which is something we also see in inflammatory bowel disease), interleukin-1, and increased activated osteoclasts.
Therefore, when it comes to decreasing bone loss among patients who are perimenopausal or postmenopausal, we don’t yet have a clear answer. Hormone therapy, diet, activity, vitamin D supplementation, and other things may positively change the biome. They are worthy topics for patients to bring up with their ob.gyns. or primary care doctors.
Although it may be a little bit outside the scope of gastroenterology, in my opinion there are a number of new findings relating to menopause that we as a field need to be more proactive in addressing.
Ask the right questions when these people come in to you, irrespective of why they’re there. Start to ask about the quality of their sleep. What are their other functional symptoms? What are their other potential osteoporosis-related risks?
We must do a better job about individualizing care. Rather than treating patients as disease states, we must start to do specific patient-focused care.
I hope this gives you some provocative thoughts when you have your next session with a patient in the perimenopausal or menopausal state. There are lots of things that we continue to learn.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Va., and a past president of the American College of Gastroenterology. He serves as an adviser to ISOThrive and Johnson & Johnson.
A version of this article first appeared on Medscape.com.
Thinking about masks
I have a cold.
This is nothing new. Like most of us, I’ve probably gotten two or three a year for most of my life. I load up on Tylenol, Sudafed, cough syrup, and ginger ale (I’m not a chicken soup person), and I power through.
I may be sick, but there are patients to see. For better or worse, the idea of calling in sick never seems to apply to the health care profession. So I put on a mask to protect my patients and go ahead with my day.
But, as I blow my nose and accept my fate for the next week, I realize that I haven’t been sick with anything since 2019. Really.
I have no idea how many times in the last week I’ve told someone “I’d forgotten how much I hated being sick.” Certainly there are far worse things to have (colds are high on the “annoying” but low on the “serious” scales), but it’s odd to find myself back in the familiar pattern of coughing, sneezing, and low-grade fever that used to be a semi-annual occurrence.
So I look at myself in the mirror and wonder if the masks were that bad an idea? Certainly I have my share of patients, usually with immune diseases, who still wear them, and I see people at the store doing the same. There are countries where it was common to have them on even before the pandemic, though that was more for pollution.
I’m still pretty careful about hand washing, but that’s the nature of my job, anyway.
I keep coming back to the mask, though. Obviously, nothing is 100% successful, but certainly it puts a respiratory filter of sorts between us and the world (and vice versa). We use them in surgery and isolation rooms. It’s probably not the only reason I went 4 years without a cold, but it likely helped.
On the other hand, it has its drawbacks. A lot of my patients have hearing issues, and the mask doesn’t improve that. It also limits communication by facial expression, which is always important. It fogs up my classes (during the pandemic it became quite clear that any mask that claimed to be fog-free was lying).
I’m not saying everyone should wear them. This is up to me, that’s up to them.
But, for myself, it’s something to think about.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I have a cold.
This is nothing new. Like most of us, I’ve probably gotten two or three a year for most of my life. I load up on Tylenol, Sudafed, cough syrup, and ginger ale (I’m not a chicken soup person), and I power through.
I may be sick, but there are patients to see. For better or worse, the idea of calling in sick never seems to apply to the health care profession. So I put on a mask to protect my patients and go ahead with my day.
But, as I blow my nose and accept my fate for the next week, I realize that I haven’t been sick with anything since 2019. Really.
I have no idea how many times in the last week I’ve told someone “I’d forgotten how much I hated being sick.” Certainly there are far worse things to have (colds are high on the “annoying” but low on the “serious” scales), but it’s odd to find myself back in the familiar pattern of coughing, sneezing, and low-grade fever that used to be a semi-annual occurrence.
So I look at myself in the mirror and wonder if the masks were that bad an idea? Certainly I have my share of patients, usually with immune diseases, who still wear them, and I see people at the store doing the same. There are countries where it was common to have them on even before the pandemic, though that was more for pollution.
I’m still pretty careful about hand washing, but that’s the nature of my job, anyway.
I keep coming back to the mask, though. Obviously, nothing is 100% successful, but certainly it puts a respiratory filter of sorts between us and the world (and vice versa). We use them in surgery and isolation rooms. It’s probably not the only reason I went 4 years without a cold, but it likely helped.
On the other hand, it has its drawbacks. A lot of my patients have hearing issues, and the mask doesn’t improve that. It also limits communication by facial expression, which is always important. It fogs up my classes (during the pandemic it became quite clear that any mask that claimed to be fog-free was lying).
I’m not saying everyone should wear them. This is up to me, that’s up to them.
But, for myself, it’s something to think about.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I have a cold.
This is nothing new. Like most of us, I’ve probably gotten two or three a year for most of my life. I load up on Tylenol, Sudafed, cough syrup, and ginger ale (I’m not a chicken soup person), and I power through.
I may be sick, but there are patients to see. For better or worse, the idea of calling in sick never seems to apply to the health care profession. So I put on a mask to protect my patients and go ahead with my day.
But, as I blow my nose and accept my fate for the next week, I realize that I haven’t been sick with anything since 2019. Really.
I have no idea how many times in the last week I’ve told someone “I’d forgotten how much I hated being sick.” Certainly there are far worse things to have (colds are high on the “annoying” but low on the “serious” scales), but it’s odd to find myself back in the familiar pattern of coughing, sneezing, and low-grade fever that used to be a semi-annual occurrence.
So I look at myself in the mirror and wonder if the masks were that bad an idea? Certainly I have my share of patients, usually with immune diseases, who still wear them, and I see people at the store doing the same. There are countries where it was common to have them on even before the pandemic, though that was more for pollution.
I’m still pretty careful about hand washing, but that’s the nature of my job, anyway.
I keep coming back to the mask, though. Obviously, nothing is 100% successful, but certainly it puts a respiratory filter of sorts between us and the world (and vice versa). We use them in surgery and isolation rooms. It’s probably not the only reason I went 4 years without a cold, but it likely helped.
On the other hand, it has its drawbacks. A lot of my patients have hearing issues, and the mask doesn’t improve that. It also limits communication by facial expression, which is always important. It fogs up my classes (during the pandemic it became quite clear that any mask that claimed to be fog-free was lying).
I’m not saying everyone should wear them. This is up to me, that’s up to them.
But, for myself, it’s something to think about.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Heart rate variability: Are we ignoring a harbinger of health?
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
A very long time ago, when I ran clinical labs, one of the most ordered tests was the “sed rate” (aka ESR, the erythrocyte sedimentation rate). Easy, quick, and low cost, with high sensitivity but very low specificity. If the sed rate was normal, the patient probably did not have an infectious or inflammatory disease. If it was elevated, they probably did, but no telling what. Later, the C-reactive protein (CRP) test came into common use. Same general inferences: If the CRP was low, the patient was unlikely to have an inflammatory process; if high, they were sick, but we didn’t know what with.
Could the heart rate variability (HRV) score come to be thought of similarly? Much as the sed rate and CRP are sensitivity indicators of infectious or inflammatory diseases, might the HRV score be a sensitivity indicator for nervous system (central and autonomic) and cardiovascular (especially heart rhythm) malfunctions?
A substantial and relatively old body of heart rhythm literature ties HRV alterations to posttraumatic stress disorder, physician occupational stress, sleep disorders, depression, autonomic nervous system derangements, various cardiac arrhythmias, fatigue, overexertion, medications, and age itself.
More than 100 million Americans are now believed to use smartwatches or personal fitness monitors. Some 30%-40% of these devices measure HRV. So what? Credible research about this huge mass of accumulating data from “wearables” is lacking.
What is HRV?
HRV is the variation in time between each heartbeat, in milliseconds. HRV is influenced by the autonomic nervous system, perhaps reflecting sympathetic-parasympathetic balance. Some devices measure HRV 24/7. My Fitbit Inspire 2 reports only nighttime measures during 3 hours of sustained sleep. Most trackers report averages; some calculate the root mean squares; others calculate standard deviations. All fitness trackers warn not to use the data for medical purposes.
Normal values (reference ranges) for HRV begin at an average of 100 msec in the first decade of life and decline by approximately 10 msec per decade lived. At age 30-40, the average is 70 msec; age 60-70, it’s 40 msec; and at age 90-100, it’s 10 msec.
As a long-time lab guy, I used to teach proper use of lab tests. Fitness trackers are “lab tests” of a sort. We taught never to do a lab test unless you know what you are going to do with the result, no matter what it is. We also taught “never do anything just because you can.” Curiosity, we know, is a frequent driver of lab test ordering.
That underlying philosophy gives me a hard time when it comes to wearables. I have been enamored of watching my step count, active zone minutes, resting heart rate, active heart rate, various sleep scores, and breathing rate (and, of course, a manually entered early morning daily body weight) for several years. I even check my “readiness score” (a calculation using resting heart rate, recent sleep, recent active zone minutes, and perhaps HRV) each morning and adjust my behaviors accordingly.
Why monitor HRV?
But what should we do with HRV scores? Ignore them? Try to understand them, perhaps as a screening tool? Or monitor HRV for consistency or change? “Monitoring” is a proper and common use of lab tests.
Some say we should improve the HRV score by managing stress, getting regular exercise, eating a healthy diet, getting enough sleep, and not smoking or consuming excess alcohol. Duh! I do all of that anyway.
The claims that HRV is a “simple but powerful tool that can be used to track overall health and well-being” might turn out to be true. Proper study and sharing of data will enable that determination.
To advance understanding, I offer an n-of-1, a real-world personal anecdote about HRV.
I did not request the HRV function on my Fitbit Inspire 2. It simply appeared, and I ignored it for some time.
A year or two ago, I started noticing my HRV score every morning. Initially, I did not like to see my “low” score, until I learned that the reference range was dramatically affected by age and I was in my late 80s at the time. The vast majority of my HRV readings were in the range of 17 msec to 27 msec.
Last week, I was administered the new Moderna COVID-19 Spikevax vaccine and the old folks’ influenza vaccine simultaneously. In my case, side effects from each vaccine have been modest in the past, but I never previously had both administered at the same time. My immune response was, shall we say, robust. Chills, muscle aches, headache, fatigue, deltoid swelling, fitful sleep, and increased resting heart rate.
My nightly average HRV had been running between 17 msec and 35 msec for many months. WHOA! After the shots, my overnight HRV score plummeted from 24 msec to 10 msec, my lowest ever. Instant worry. The next day, it rebounded to 28 msec, and it has been in the high teens or low 20s since then.
Off to PubMed. A recent study of HRV on the second and 10th days after administering the Pfizer mRNA vaccine to 75 healthy volunteers found that the HRV on day 2 was dramatically lower than prevaccination levels and by day 10, it had returned to prevaccination levels. Some comfort there.
Another review article has reported a rapid fall and rapid rebound of HRV after COVID-19 vaccination. A 2010 report demonstrated a significant but not dramatic short-term lowering of HRV after influenza A vaccination and correlated it with CRP changes.
Some believe that the decline in HRV after vaccination reflects an increased immune response and sympathetic nervous activity.
I don’t plan to receive my flu and COVID vaccines on the same day again.
So, I went back to review what happened to my HRV when I had COVID in 2023. My HRV was 14 msec and 12 msec on the first 2 days of symptoms, and then returned to the 20 msec range.
I received the RSV vaccine this year without adverse effects, and my HRV scores were 29 msec, 33 msec, and 32 msec on the first 3 days after vaccination. Finally, after receiving a pneumococcal vaccine in 2023, I had no adverse effects, and my HRV scores on the 5 days after vaccination were indeterminate: 19 msec, 14 msec, 18 msec, 13 msec, and 17 msec.
Of course, correlation is not causation. Cause and effect remain undetermined. But I find these observations interesting for a potentially useful screening test.
George D. Lundberg, MD, is the Editor in Chief of Cancer Commons.
A version of this article first appeared on Medscape.com.
Q&A: Cancer screening in older patients – who to screen and when to stop
More than 1 in 10 Americans over age 60 years will be diagnosed with cancer, according to the National Cancer Institute, making screening for the disease in older patients imperative. Much of the burden of cancer screening falls on primary care physicians. This news organization spoke recently with William L. Dahut, MD, chief scientific officer of the American Cancer Society, about the particular challenges of screening in older patients.
Question: How much does cancer screening change with age? What are the considerations for clinicians – what risks and comorbidities are important to consider in older populations?
Answer: We at the American Cancer Society are giving a lot of thought to how to help primary care practices keep up with screening, particularly with respect to guidelines, but also best practices where judgment is required, such as cancer screening in their older patients.
We’ve had a lot of conversations recently about cancer risk in the young, largely because data show rates are going up for colorectal and breast cancer in this population. But it’s not one size fits all. Screening for young women who have a BRCA gene, if they have dense breasts, or if they have a strong family history of breast cancer should be different from those who are at average risk of the disease.
But statistically, there are about 15 per 100,000 breast cancer diagnoses in women under the age of 40 while over the age of 65 it’s 443 per 100,000. So, the risk significantly increases with age but we should not have an arbitrary cut-off. The life expectancy of a woman at age 75 is about 13.5 years. If you’re over the age of 70 or 75, then it’s going to be comorbidities that you look at, as well as individual patient decisions. Patients may say, “I don’t want to ever go through a mammogram again, because I don’t want to have a biopsy again, and I’m not going to get treated.” Or they may say, “My mom died of metastatic breast cancer when she was 82 and I want to know.”
Q: How should primary care physicians interpret conflicting guidance from the major medical groups? For example, the American College of Gastroenterology and your own organization recommend colorectal cancer screening start at age 45 now. But the American College of Physicians recently came out and said 50. What is a well-meaning primary care physician supposed to do?
A: We make more of guideline differences than we should. Sometimes guideline differences aren’t a reflection of different judgments, but rather what data were available when the most recent update took place. For colorectal cancer screening, the ACS dropped the age to begin screening to 45 in 2018 based on a very careful consideration of disease burden data and within several years most other guideline developers reached the same conclusion.
However, I think it’s good for family practice and internal medicine doctors to know that significant GI symptoms in a young patient could be colorectal cancer. It’s not as if nobody sees a 34-year-old or 27-year-old with colorectal cancer. They should be aware that if something goes away in a day or two, that’s fine, but persistent GI symptoms need a cancer workup – colonoscopy or referral to a gastroenterologist. So that’s why I think age 45 is the time when folks should begin screening.
Q: What are the medical-legal issues for a physician who is trying to follow guideline-based care when there are different guidelines?
A: Any physician can say, “We follow the guidelines of this particular organization.” I don’t think anyone can say that an organization’s guidelines are malpractice. For individual physicians, following a set of office-based guidelines will hopefully keep them out of legal difficulty.
Q: What are the risks of overscreening, especially in breast cancer where false positives may result in invasive testing?
A: What people think of as overscreening takes a number of different forms. What one guideline would imply is overscreening is recommended screening by another guideline. I think we would all agree that in an average-risk population, beginning screening before it is recommended would be overscreening, and continuing screening when a patient has life-limiting comorbidities would constitute overscreening. Screening too frequently can constitute overscreening.
For example, many women report that their doctors still are advising a baseline mammogram at age 35. Most guideline-developing organizations would regard this as overscreening in an average-risk population.
I think we are also getting better, certainly in prostate cancer, about knowing who needs to be treated and not treated. There are a lot of cancers that would have been treated 20-30 years ago but now are being safely followed with PSA and MRI. We may be able to get to that point with breast cancer over time, too.
Q: Are you saying that there may be breast cancers for which active surveillance is appropriate? Is that already the case?
A: We’re not there yet. I think some of the DCIS breast cancers are part of the discussion on whether hormonal treatment or surgeries are done. I think people do have those discussions in the context of morbidity and life expectancy. Over time, we’re likely to have more cancers for which we won’t need surgical treatments.
Q: Why did the American Cancer Society change the upper limit for lung cancer screening from 75 to 80 years of age?
A: For an individual older than 65, screening will now continue until the patient is 80, assuming the patient is in good health. According to the previous guideline, if a patient was 65 and more than 15 years beyond smoking cessation, then screening would end. This is exactly the time when we see lung cancers increase in the population and so a curable lung cancer would not previously have been detected by a screening CT scan. *
Q: What role do the multicancer blood and DNA tests play in screening now?
A: As you know, the Exact Sciences Cologuard test is already included in major guidelines for colorectal cancer screening and covered by insurance. Our philosophy on multicancer early detection tests is that we’re supportive of Medicare reimbursement when two things occur: 1. When we know there’s clinical benefit, and 2. When the test has been approved by the FDA.
The multicancer early detection tests in development and undergoing prospective research would not now replace screening for the cancers with established screening programs, but if they are shown to have clinical utility for the cancers in their panel, we would be able to reduce deaths from cancers that mostly are diagnosed at late stages and have poor prognoses.
There’s going to be a need for expertise in primary care practices to help interpret the tests. These are new questions, which are well beyond what even the typical oncologist is trained in, much less primary care physicians. We and other organizations are working on providing those answers.
Q: While we’re on the subject of the future, how do you envision AI helping or hindering cancer screening specifically in primary care?
A: I think AI is going to help things for a couple of reasons. The ability of AI is to get through data quickly and get you information that’s personalized and useful. If AI tools could let a patient know their individual risk of a cancer in the near and long term, that would help the primary care doctor screen in an individualized way. I think AI is going to be able to improve both diagnostic radiology and pathology, and could make a very big difference in settings outside of large cancer centers that operate at high volume every day. The data look very promising for AI to contribute to risk estimation by operating like a second reader in imaging and pathology.
Q: Anything else you’d like to say on this subject that clinicians should know?
A: The questions about whether or not patients should be screened is being pushed on family practice doctors and internists and these questions require a relationship with the patient. A hard stopping point at age 70 when lots of people will live 20 years or more doesn’t make sense.
There’s very little data from randomized clinical trials of screening people over the age of 70. We know that cancer risk does obviously increase with age, particularly prostate and breast cancer. And these are the cancers that are going to be the most common in your practices. If someone has a known mutation, I think you’re going to look differently at screening them. And first-degree family members, particularly for the more aggressive cancers, should be considered for screening.
My philosophy on cancer screening in the elderly is that I think the guidelines are guidelines. If patients have very limited life expectancy, then they shouldn’t be screened. There are calculators that estimate life expectancy in the context of current age and current health status, and these can be useful for decision making and counseling. Patients never think their life expectancy is shorter than 10 years. If their life expectancy is longer than 10 years, then I think, all things being equal, they should continue screening, but the question of ongoing screening needs to be periodically revisited.
*This story was updated on Nov. 1, 2023.
More than 1 in 10 Americans over age 60 years will be diagnosed with cancer, according to the National Cancer Institute, making screening for the disease in older patients imperative. Much of the burden of cancer screening falls on primary care physicians. This news organization spoke recently with William L. Dahut, MD, chief scientific officer of the American Cancer Society, about the particular challenges of screening in older patients.
Question: How much does cancer screening change with age? What are the considerations for clinicians – what risks and comorbidities are important to consider in older populations?
Answer: We at the American Cancer Society are giving a lot of thought to how to help primary care practices keep up with screening, particularly with respect to guidelines, but also best practices where judgment is required, such as cancer screening in their older patients.
We’ve had a lot of conversations recently about cancer risk in the young, largely because data show rates are going up for colorectal and breast cancer in this population. But it’s not one size fits all. Screening for young women who have a BRCA gene, if they have dense breasts, or if they have a strong family history of breast cancer should be different from those who are at average risk of the disease.
But statistically, there are about 15 per 100,000 breast cancer diagnoses in women under the age of 40 while over the age of 65 it’s 443 per 100,000. So, the risk significantly increases with age but we should not have an arbitrary cut-off. The life expectancy of a woman at age 75 is about 13.5 years. If you’re over the age of 70 or 75, then it’s going to be comorbidities that you look at, as well as individual patient decisions. Patients may say, “I don’t want to ever go through a mammogram again, because I don’t want to have a biopsy again, and I’m not going to get treated.” Or they may say, “My mom died of metastatic breast cancer when she was 82 and I want to know.”
Q: How should primary care physicians interpret conflicting guidance from the major medical groups? For example, the American College of Gastroenterology and your own organization recommend colorectal cancer screening start at age 45 now. But the American College of Physicians recently came out and said 50. What is a well-meaning primary care physician supposed to do?
A: We make more of guideline differences than we should. Sometimes guideline differences aren’t a reflection of different judgments, but rather what data were available when the most recent update took place. For colorectal cancer screening, the ACS dropped the age to begin screening to 45 in 2018 based on a very careful consideration of disease burden data and within several years most other guideline developers reached the same conclusion.
However, I think it’s good for family practice and internal medicine doctors to know that significant GI symptoms in a young patient could be colorectal cancer. It’s not as if nobody sees a 34-year-old or 27-year-old with colorectal cancer. They should be aware that if something goes away in a day or two, that’s fine, but persistent GI symptoms need a cancer workup – colonoscopy or referral to a gastroenterologist. So that’s why I think age 45 is the time when folks should begin screening.
Q: What are the medical-legal issues for a physician who is trying to follow guideline-based care when there are different guidelines?
A: Any physician can say, “We follow the guidelines of this particular organization.” I don’t think anyone can say that an organization’s guidelines are malpractice. For individual physicians, following a set of office-based guidelines will hopefully keep them out of legal difficulty.
Q: What are the risks of overscreening, especially in breast cancer where false positives may result in invasive testing?
A: What people think of as overscreening takes a number of different forms. What one guideline would imply is overscreening is recommended screening by another guideline. I think we would all agree that in an average-risk population, beginning screening before it is recommended would be overscreening, and continuing screening when a patient has life-limiting comorbidities would constitute overscreening. Screening too frequently can constitute overscreening.
For example, many women report that their doctors still are advising a baseline mammogram at age 35. Most guideline-developing organizations would regard this as overscreening in an average-risk population.
I think we are also getting better, certainly in prostate cancer, about knowing who needs to be treated and not treated. There are a lot of cancers that would have been treated 20-30 years ago but now are being safely followed with PSA and MRI. We may be able to get to that point with breast cancer over time, too.
Q: Are you saying that there may be breast cancers for which active surveillance is appropriate? Is that already the case?
A: We’re not there yet. I think some of the DCIS breast cancers are part of the discussion on whether hormonal treatment or surgeries are done. I think people do have those discussions in the context of morbidity and life expectancy. Over time, we’re likely to have more cancers for which we won’t need surgical treatments.
Q: Why did the American Cancer Society change the upper limit for lung cancer screening from 75 to 80 years of age?
A: For an individual older than 65, screening will now continue until the patient is 80, assuming the patient is in good health. According to the previous guideline, if a patient was 65 and more than 15 years beyond smoking cessation, then screening would end. This is exactly the time when we see lung cancers increase in the population and so a curable lung cancer would not previously have been detected by a screening CT scan. *
Q: What role do the multicancer blood and DNA tests play in screening now?
A: As you know, the Exact Sciences Cologuard test is already included in major guidelines for colorectal cancer screening and covered by insurance. Our philosophy on multicancer early detection tests is that we’re supportive of Medicare reimbursement when two things occur: 1. When we know there’s clinical benefit, and 2. When the test has been approved by the FDA.
The multicancer early detection tests in development and undergoing prospective research would not now replace screening for the cancers with established screening programs, but if they are shown to have clinical utility for the cancers in their panel, we would be able to reduce deaths from cancers that mostly are diagnosed at late stages and have poor prognoses.
There’s going to be a need for expertise in primary care practices to help interpret the tests. These are new questions, which are well beyond what even the typical oncologist is trained in, much less primary care physicians. We and other organizations are working on providing those answers.
Q: While we’re on the subject of the future, how do you envision AI helping or hindering cancer screening specifically in primary care?
A: I think AI is going to help things for a couple of reasons. The ability of AI is to get through data quickly and get you information that’s personalized and useful. If AI tools could let a patient know their individual risk of a cancer in the near and long term, that would help the primary care doctor screen in an individualized way. I think AI is going to be able to improve both diagnostic radiology and pathology, and could make a very big difference in settings outside of large cancer centers that operate at high volume every day. The data look very promising for AI to contribute to risk estimation by operating like a second reader in imaging and pathology.
Q: Anything else you’d like to say on this subject that clinicians should know?
A: The questions about whether or not patients should be screened is being pushed on family practice doctors and internists and these questions require a relationship with the patient. A hard stopping point at age 70 when lots of people will live 20 years or more doesn’t make sense.
There’s very little data from randomized clinical trials of screening people over the age of 70. We know that cancer risk does obviously increase with age, particularly prostate and breast cancer. And these are the cancers that are going to be the most common in your practices. If someone has a known mutation, I think you’re going to look differently at screening them. And first-degree family members, particularly for the more aggressive cancers, should be considered for screening.
My philosophy on cancer screening in the elderly is that I think the guidelines are guidelines. If patients have very limited life expectancy, then they shouldn’t be screened. There are calculators that estimate life expectancy in the context of current age and current health status, and these can be useful for decision making and counseling. Patients never think their life expectancy is shorter than 10 years. If their life expectancy is longer than 10 years, then I think, all things being equal, they should continue screening, but the question of ongoing screening needs to be periodically revisited.
*This story was updated on Nov. 1, 2023.
More than 1 in 10 Americans over age 60 years will be diagnosed with cancer, according to the National Cancer Institute, making screening for the disease in older patients imperative. Much of the burden of cancer screening falls on primary care physicians. This news organization spoke recently with William L. Dahut, MD, chief scientific officer of the American Cancer Society, about the particular challenges of screening in older patients.
Question: How much does cancer screening change with age? What are the considerations for clinicians – what risks and comorbidities are important to consider in older populations?
Answer: We at the American Cancer Society are giving a lot of thought to how to help primary care practices keep up with screening, particularly with respect to guidelines, but also best practices where judgment is required, such as cancer screening in their older patients.
We’ve had a lot of conversations recently about cancer risk in the young, largely because data show rates are going up for colorectal and breast cancer in this population. But it’s not one size fits all. Screening for young women who have a BRCA gene, if they have dense breasts, or if they have a strong family history of breast cancer should be different from those who are at average risk of the disease.
But statistically, there are about 15 per 100,000 breast cancer diagnoses in women under the age of 40 while over the age of 65 it’s 443 per 100,000. So, the risk significantly increases with age but we should not have an arbitrary cut-off. The life expectancy of a woman at age 75 is about 13.5 years. If you’re over the age of 70 or 75, then it’s going to be comorbidities that you look at, as well as individual patient decisions. Patients may say, “I don’t want to ever go through a mammogram again, because I don’t want to have a biopsy again, and I’m not going to get treated.” Or they may say, “My mom died of metastatic breast cancer when she was 82 and I want to know.”
Q: How should primary care physicians interpret conflicting guidance from the major medical groups? For example, the American College of Gastroenterology and your own organization recommend colorectal cancer screening start at age 45 now. But the American College of Physicians recently came out and said 50. What is a well-meaning primary care physician supposed to do?
A: We make more of guideline differences than we should. Sometimes guideline differences aren’t a reflection of different judgments, but rather what data were available when the most recent update took place. For colorectal cancer screening, the ACS dropped the age to begin screening to 45 in 2018 based on a very careful consideration of disease burden data and within several years most other guideline developers reached the same conclusion.
However, I think it’s good for family practice and internal medicine doctors to know that significant GI symptoms in a young patient could be colorectal cancer. It’s not as if nobody sees a 34-year-old or 27-year-old with colorectal cancer. They should be aware that if something goes away in a day or two, that’s fine, but persistent GI symptoms need a cancer workup – colonoscopy or referral to a gastroenterologist. So that’s why I think age 45 is the time when folks should begin screening.
Q: What are the medical-legal issues for a physician who is trying to follow guideline-based care when there are different guidelines?
A: Any physician can say, “We follow the guidelines of this particular organization.” I don’t think anyone can say that an organization’s guidelines are malpractice. For individual physicians, following a set of office-based guidelines will hopefully keep them out of legal difficulty.
Q: What are the risks of overscreening, especially in breast cancer where false positives may result in invasive testing?
A: What people think of as overscreening takes a number of different forms. What one guideline would imply is overscreening is recommended screening by another guideline. I think we would all agree that in an average-risk population, beginning screening before it is recommended would be overscreening, and continuing screening when a patient has life-limiting comorbidities would constitute overscreening. Screening too frequently can constitute overscreening.
For example, many women report that their doctors still are advising a baseline mammogram at age 35. Most guideline-developing organizations would regard this as overscreening in an average-risk population.
I think we are also getting better, certainly in prostate cancer, about knowing who needs to be treated and not treated. There are a lot of cancers that would have been treated 20-30 years ago but now are being safely followed with PSA and MRI. We may be able to get to that point with breast cancer over time, too.
Q: Are you saying that there may be breast cancers for which active surveillance is appropriate? Is that already the case?
A: We’re not there yet. I think some of the DCIS breast cancers are part of the discussion on whether hormonal treatment or surgeries are done. I think people do have those discussions in the context of morbidity and life expectancy. Over time, we’re likely to have more cancers for which we won’t need surgical treatments.
Q: Why did the American Cancer Society change the upper limit for lung cancer screening from 75 to 80 years of age?
A: For an individual older than 65, screening will now continue until the patient is 80, assuming the patient is in good health. According to the previous guideline, if a patient was 65 and more than 15 years beyond smoking cessation, then screening would end. This is exactly the time when we see lung cancers increase in the population and so a curable lung cancer would not previously have been detected by a screening CT scan. *
Q: What role do the multicancer blood and DNA tests play in screening now?
A: As you know, the Exact Sciences Cologuard test is already included in major guidelines for colorectal cancer screening and covered by insurance. Our philosophy on multicancer early detection tests is that we’re supportive of Medicare reimbursement when two things occur: 1. When we know there’s clinical benefit, and 2. When the test has been approved by the FDA.
The multicancer early detection tests in development and undergoing prospective research would not now replace screening for the cancers with established screening programs, but if they are shown to have clinical utility for the cancers in their panel, we would be able to reduce deaths from cancers that mostly are diagnosed at late stages and have poor prognoses.
There’s going to be a need for expertise in primary care practices to help interpret the tests. These are new questions, which are well beyond what even the typical oncologist is trained in, much less primary care physicians. We and other organizations are working on providing those answers.
Q: While we’re on the subject of the future, how do you envision AI helping or hindering cancer screening specifically in primary care?
A: I think AI is going to help things for a couple of reasons. The ability of AI is to get through data quickly and get you information that’s personalized and useful. If AI tools could let a patient know their individual risk of a cancer in the near and long term, that would help the primary care doctor screen in an individualized way. I think AI is going to be able to improve both diagnostic radiology and pathology, and could make a very big difference in settings outside of large cancer centers that operate at high volume every day. The data look very promising for AI to contribute to risk estimation by operating like a second reader in imaging and pathology.
Q: Anything else you’d like to say on this subject that clinicians should know?
A: The questions about whether or not patients should be screened is being pushed on family practice doctors and internists and these questions require a relationship with the patient. A hard stopping point at age 70 when lots of people will live 20 years or more doesn’t make sense.
There’s very little data from randomized clinical trials of screening people over the age of 70. We know that cancer risk does obviously increase with age, particularly prostate and breast cancer. And these are the cancers that are going to be the most common in your practices. If someone has a known mutation, I think you’re going to look differently at screening them. And first-degree family members, particularly for the more aggressive cancers, should be considered for screening.
My philosophy on cancer screening in the elderly is that I think the guidelines are guidelines. If patients have very limited life expectancy, then they shouldn’t be screened. There are calculators that estimate life expectancy in the context of current age and current health status, and these can be useful for decision making and counseling. Patients never think their life expectancy is shorter than 10 years. If their life expectancy is longer than 10 years, then I think, all things being equal, they should continue screening, but the question of ongoing screening needs to be periodically revisited.
*This story was updated on Nov. 1, 2023.
This drug works, but wait till you hear what’s in it
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.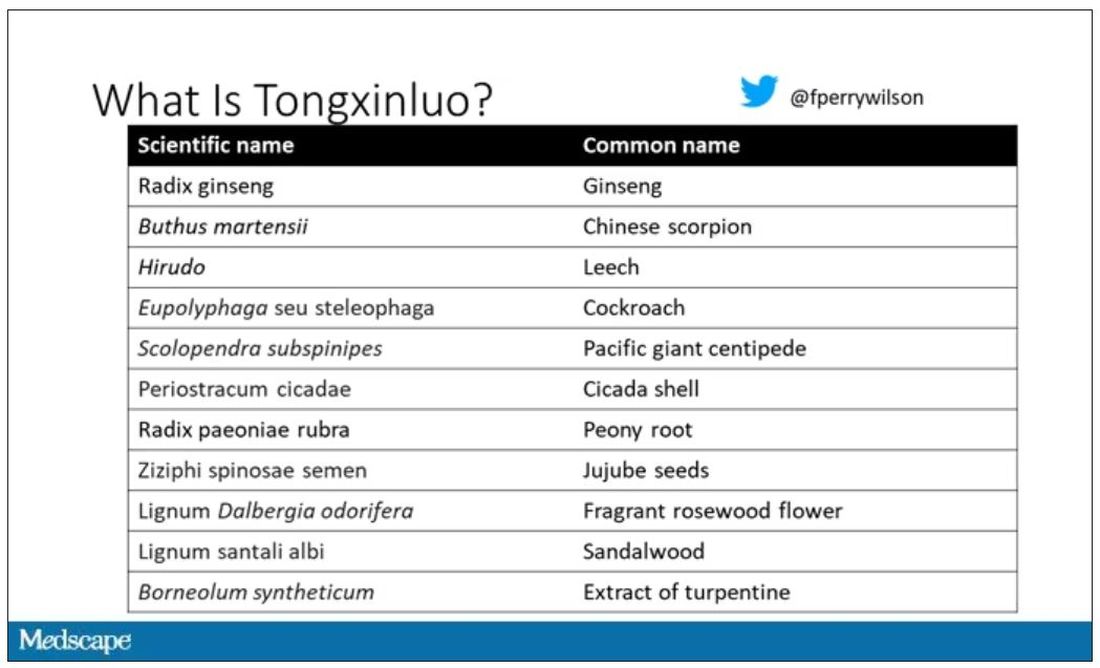
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.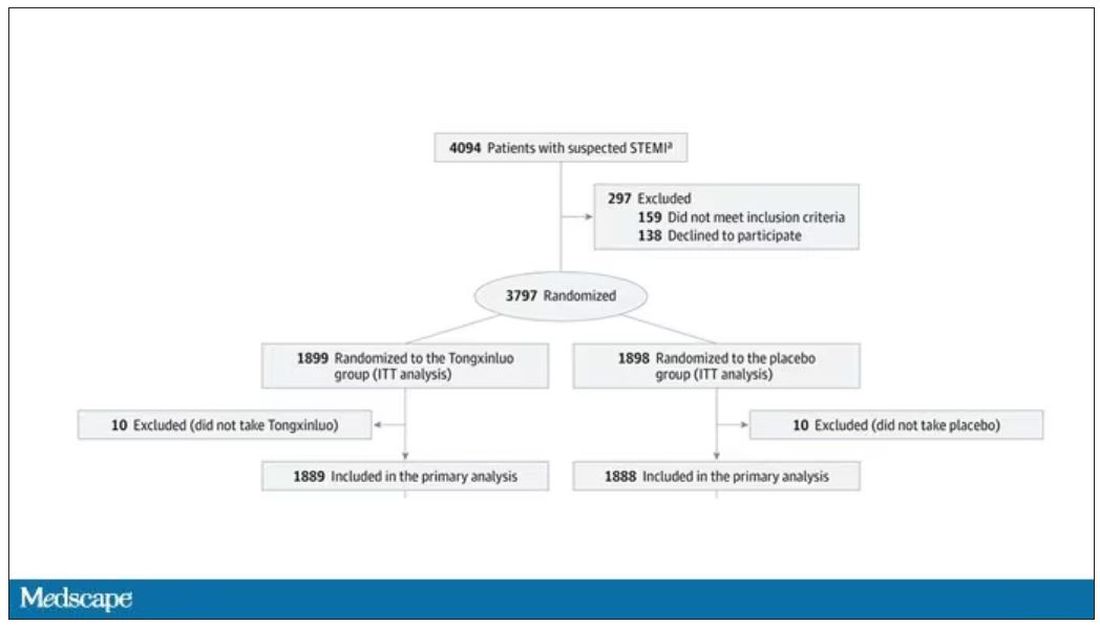
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.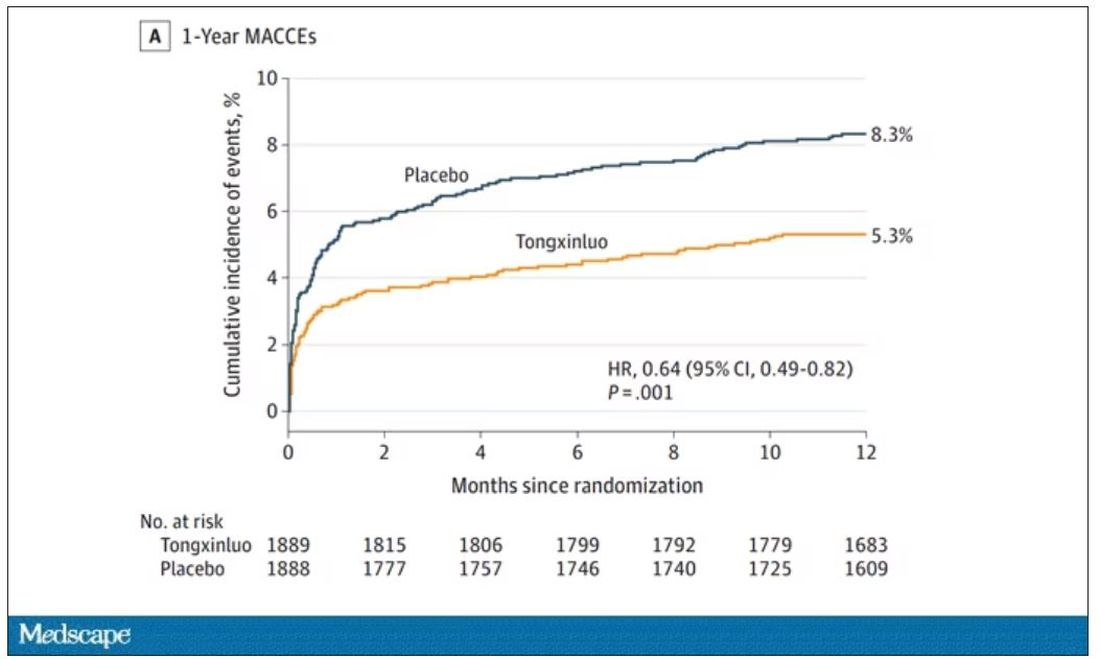
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.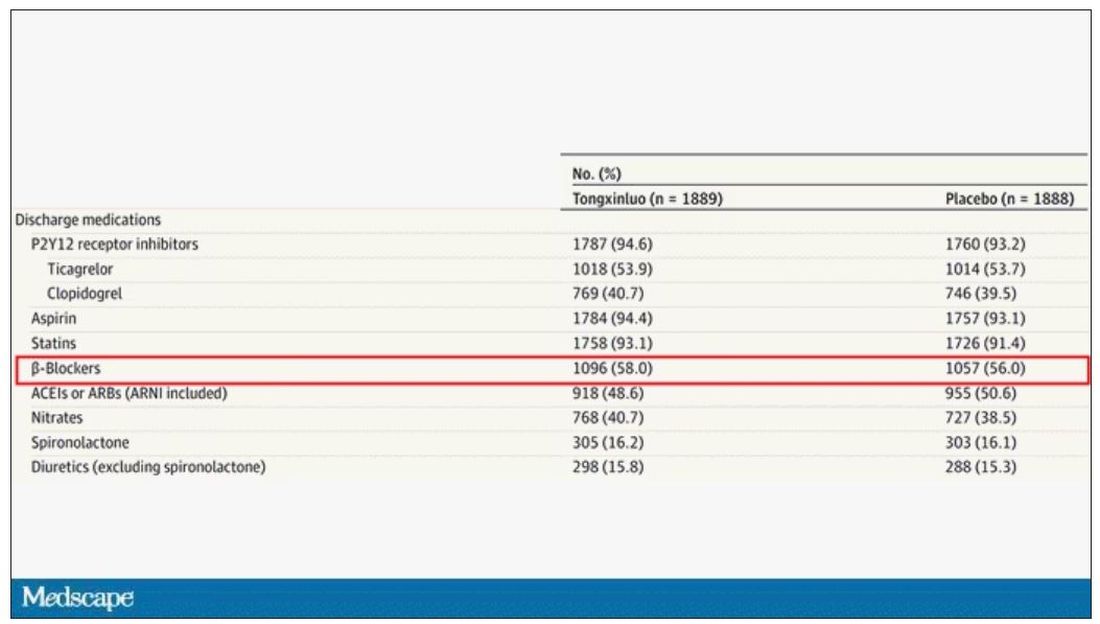
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
This transcript has been edited for clarity.
As some of you may know, I do a fair amount of clinical research developing and evaluating artificial intelligence (AI) models, particularly machine learning algorithms that predict certain outcomes.
A thorny issue that comes up as algorithms have gotten more complicated is “explainability.” The problem is that AI can be a black box. Even if you have a model that is very accurate at predicting death, clinicians don’t trust it unless you can explain how it makes its predictions – how it works. “It just works” is not good enough to build trust.
It’s easier to build trust when you’re talking about a medication rather than a computer program. When a new blood pressure drug comes out that lowers blood pressure, importantly, we know why it lowers blood pressure. Every drug has a mechanism of action and, for most of the drugs in our arsenal, we know what that mechanism is.
But what if there were a drug – or better yet, a treatment – that worked? And I can honestly say we have no idea how it works. That’s what came across my desk today in what I believe is the largest, most rigorous trial of a traditional Chinese medication in history.
“Traditional Chinese medicine” is an omnibus term that refers to a class of therapies and health practices that are fundamentally different from how we practice medicine in the West.
It’s a highly personalized practice, with practitioners using often esoteric means to choose what substance to give what patient. That personalization makes traditional Chinese medicine nearly impossible to study in the typical randomized trial framework because treatments are not chosen solely on the basis of disease states.
The lack of scientific rigor in traditional Chinese medicine means that it is rife with practices and beliefs that can legitimately be called pseudoscience. As a nephrologist who has treated someone for “Chinese herb nephropathy,” I can tell you that some of the practices may be actively harmful.
But that doesn’t mean there is nothing there. I do not subscribe to the “argument from antiquity” – the idea that because something has been done for a long time it must be correct. But at the same time, traditional and non–science-based medicine practices could still identify therapies that work.
And with that, let me introduce you to Tongxinluo. Tongxinluo literally means “to open the network of the heart,” and it is a substance that has been used for centuries by traditional Chinese medicine practitioners to treat angina but was approved by the Chinese state medicine agency for use in 1996.
Like many traditional Chinese medicine preparations, Tongxinluo is not a single chemical – far from it. It is a powder made from a variety of plant and insect parts, as you can see here.
I can’t imagine running a trial of this concoction in the United States; I just don’t see an institutional review board signing off, given the ingredient list.
But let’s set that aside and talk about the study itself.
While I don’t have access to any primary data, the write-up of the study suggests that it was highly rigorous. Chinese researchers randomized 3,797 patients with ST-elevation MI to take Tongxinluo – four capsules, three times a day for 12 months – or matching placebo. The placebo was designed to look just like the Tongxinluo capsules and, if the capsules were opened, to smell like them as well.
Researchers and participants were blinded, and the statistical analysis was done both by the primary team and an independent research agency, also in China.
And the results were pretty good. The primary outcome, 30-day major cardiovascular and cerebral events, were significantly lower in the intervention group than in the placebo group.
One-year outcomes were similarly good; 8.3% of the placebo group suffered a major cardiovascular or cerebral event in that time frame, compared with 5.3% of the Tongxinluo group. In short, if this were a pure chemical compound from a major pharmaceutical company, well, you might be seeing a new treatment for heart attack – and a boost in stock price.
But there are some issues here, generalizability being a big one. This study was done entirely in China, so its applicability to a more diverse population is unclear. Moreover, the quality of post-MI care in this study is quite a bit worse than what we’d see here in the United States, with just over 50% of patients being discharged on a beta-blocker, for example.
But issues of generalizability and potentially substandard supplementary treatments are the usual reasons we worry about new medication trials. And those concerns seem to pale before the big one I have here which is, you know – we don’t know why this works.
Is it the extract of leech in the preparation perhaps thinning the blood a bit? Or is it the antioxidants in the ginseng, or something from the Pacific centipede or the sandalwood?
This trial doesn’t read to me as a vindication of traditional Chinese medicine but rather as an example of missed opportunity. More rigorous scientific study over the centuries that Tongxinluo has been used could have identified one, or perhaps more, compounds with strong therapeutic potential.
Purity of medical substances is incredibly important. Pure substances have predictable effects and side effects. Pure substances interact with other treatments we give patients in predictable ways. Pure substances can be quantified for purity by third parties, they can be manufactured according to accepted standards, and they can be assessed for adulteration. In short, pure substances pose less risk.
Now, I know that may come off as particularly sterile. Some people will feel that a “natural” substance has some inherent benefit over pure compounds. And, of course, there is something soothing about imagining a traditional preparation handed down over centuries, being prepared with care by a single practitioner, in contrast to the sterile industrial processes of a for-profit pharmaceutical company. I get it. But natural is not the same as safe. I am glad I have access to purified aspirin and don’t have to chew willow bark. I like my pure penicillin and am glad I don’t have to make a mold slurry to treat a bacterial infection.
I applaud the researchers for subjecting Tongxinluo to the rigor of a well-designed trial. They have generated data that are incredibly exciting, but not because we have a new treatment for ST-elevation MI on our hands; it’s because we have a map to a new treatment. The next big thing in heart attack care is not the mixture that is Tongxinluo, but it might be in the mixture.
A version of this article first appeared on Medscape.com.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, “How Medicine Works and When It Doesn’t,” is available now.
Not another emergency
This country faces a broad and frightening rogues’ gallery of challenges to its health. From the recent revelation that gunshots are the leading cause of death in children to the opioid epidemic to the overworked and discouraged health care providers, the crises are so numerous it is hard to choose where we should be investing what little political will we can muster. And, where do these disasters fit against a landscape raked by natural and climate change–triggered catastrophes? How do we even begin to triage our vocabulary as we are trying to label them?
The lead article in October’s journal Pediatrics makes a heroic effort to place pediatric obesity into this pantheon of health disasters. The authors of this Pediatrics Perspective ask a simple question: Should the United States declare pediatric obesity a public health emergency? They have wisely chosen to narrow the question to the pediatric population as being a more realistic target and one that is more likely to pay bigger dividends over time.
While acknowledging that obesity prevention strategies have been largely ineffective to this point, the authors are also concerned that despite the promising development of treatment strategies, the rollout of these therapies is likely to be uneven because of funding and disparities in health care delivery.
After reviewing pros and cons for an emergency declaration, they came to the conclusion that despite the scope of the problem and the fact that health emergencies have been declared for conditions effecting fewer individuals, now is not the time. The authors observed that a declaration may serve only to hype “the problem without offering tangible solutions.” Even when as yet to be discovered effective therapies become available, the time lag before measurable improvement is likely to be so delayed that “catastrophizing” pediatric obesity may be just another exercise in wolf-crying.
A closer look
While I applaud the authors for their courage in addressing this question and their decision to discourage an emergency declaration, a few of their observations deserve a closer look. First, they are legitimately concerned that any health policy must be careful not to further perpetuate the stigmatization of children with obesity. However, they feel the recognition by all stakeholders “that obesity is a genetically and biologically driven disease are essential.” While I have supported the disease designation as a pragmatic strategy to move things forward, I would prefer their statement to read “obesity can be ... “ I don’t think we have mined the data deep enough to determine how many out of a cohort of a million obese children from across a wide span of socioeconomic strata have become obese primarily as a result of decisions made by school departments, parents, and governmental entities – all of which had the resources to make healthier decisions but failed to do so.
While a majority of the population may believe that obesity is a “condition of choice,” I think they would be more likely to support the political will for action if they saw data that acknowledges that yes, obesity can be a condition of choice, but here are the circumstances in which choice can and can’t make a difference. Language must always be chosen carefully to minimize stigmatization. However, remember we are not pointing fingers at victims; we are instead looking for teaching moments in which adults can learn to make better choices for the children under their care who are too young to make their own.
Finally, as the authors of this Pediatric Perspectives considered cons of a declaration of health care emergency, they raised the peculiarly American concern of personal autonomy. As they pointed out, there are unfortunate examples in this country in which efforts to limit personal choice have backfired and well-meaning and potentially effective methods for limiting unhealthy behaviors have been eliminated in the name of personal freedom. I’m not sure how we manage this except to wait and be judicious as we move forward addressing pediatric obesity on a national scale. I urge you to take a few minutes to read this perspective. It is a topic worth considering.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
This country faces a broad and frightening rogues’ gallery of challenges to its health. From the recent revelation that gunshots are the leading cause of death in children to the opioid epidemic to the overworked and discouraged health care providers, the crises are so numerous it is hard to choose where we should be investing what little political will we can muster. And, where do these disasters fit against a landscape raked by natural and climate change–triggered catastrophes? How do we even begin to triage our vocabulary as we are trying to label them?
The lead article in October’s journal Pediatrics makes a heroic effort to place pediatric obesity into this pantheon of health disasters. The authors of this Pediatrics Perspective ask a simple question: Should the United States declare pediatric obesity a public health emergency? They have wisely chosen to narrow the question to the pediatric population as being a more realistic target and one that is more likely to pay bigger dividends over time.
While acknowledging that obesity prevention strategies have been largely ineffective to this point, the authors are also concerned that despite the promising development of treatment strategies, the rollout of these therapies is likely to be uneven because of funding and disparities in health care delivery.
After reviewing pros and cons for an emergency declaration, they came to the conclusion that despite the scope of the problem and the fact that health emergencies have been declared for conditions effecting fewer individuals, now is not the time. The authors observed that a declaration may serve only to hype “the problem without offering tangible solutions.” Even when as yet to be discovered effective therapies become available, the time lag before measurable improvement is likely to be so delayed that “catastrophizing” pediatric obesity may be just another exercise in wolf-crying.
A closer look
While I applaud the authors for their courage in addressing this question and their decision to discourage an emergency declaration, a few of their observations deserve a closer look. First, they are legitimately concerned that any health policy must be careful not to further perpetuate the stigmatization of children with obesity. However, they feel the recognition by all stakeholders “that obesity is a genetically and biologically driven disease are essential.” While I have supported the disease designation as a pragmatic strategy to move things forward, I would prefer their statement to read “obesity can be ... “ I don’t think we have mined the data deep enough to determine how many out of a cohort of a million obese children from across a wide span of socioeconomic strata have become obese primarily as a result of decisions made by school departments, parents, and governmental entities – all of which had the resources to make healthier decisions but failed to do so.
While a majority of the population may believe that obesity is a “condition of choice,” I think they would be more likely to support the political will for action if they saw data that acknowledges that yes, obesity can be a condition of choice, but here are the circumstances in which choice can and can’t make a difference. Language must always be chosen carefully to minimize stigmatization. However, remember we are not pointing fingers at victims; we are instead looking for teaching moments in which adults can learn to make better choices for the children under their care who are too young to make their own.
Finally, as the authors of this Pediatric Perspectives considered cons of a declaration of health care emergency, they raised the peculiarly American concern of personal autonomy. As they pointed out, there are unfortunate examples in this country in which efforts to limit personal choice have backfired and well-meaning and potentially effective methods for limiting unhealthy behaviors have been eliminated in the name of personal freedom. I’m not sure how we manage this except to wait and be judicious as we move forward addressing pediatric obesity on a national scale. I urge you to take a few minutes to read this perspective. It is a topic worth considering.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
This country faces a broad and frightening rogues’ gallery of challenges to its health. From the recent revelation that gunshots are the leading cause of death in children to the opioid epidemic to the overworked and discouraged health care providers, the crises are so numerous it is hard to choose where we should be investing what little political will we can muster. And, where do these disasters fit against a landscape raked by natural and climate change–triggered catastrophes? How do we even begin to triage our vocabulary as we are trying to label them?
The lead article in October’s journal Pediatrics makes a heroic effort to place pediatric obesity into this pantheon of health disasters. The authors of this Pediatrics Perspective ask a simple question: Should the United States declare pediatric obesity a public health emergency? They have wisely chosen to narrow the question to the pediatric population as being a more realistic target and one that is more likely to pay bigger dividends over time.
While acknowledging that obesity prevention strategies have been largely ineffective to this point, the authors are also concerned that despite the promising development of treatment strategies, the rollout of these therapies is likely to be uneven because of funding and disparities in health care delivery.
After reviewing pros and cons for an emergency declaration, they came to the conclusion that despite the scope of the problem and the fact that health emergencies have been declared for conditions effecting fewer individuals, now is not the time. The authors observed that a declaration may serve only to hype “the problem without offering tangible solutions.” Even when as yet to be discovered effective therapies become available, the time lag before measurable improvement is likely to be so delayed that “catastrophizing” pediatric obesity may be just another exercise in wolf-crying.
A closer look
While I applaud the authors for their courage in addressing this question and their decision to discourage an emergency declaration, a few of their observations deserve a closer look. First, they are legitimately concerned that any health policy must be careful not to further perpetuate the stigmatization of children with obesity. However, they feel the recognition by all stakeholders “that obesity is a genetically and biologically driven disease are essential.” While I have supported the disease designation as a pragmatic strategy to move things forward, I would prefer their statement to read “obesity can be ... “ I don’t think we have mined the data deep enough to determine how many out of a cohort of a million obese children from across a wide span of socioeconomic strata have become obese primarily as a result of decisions made by school departments, parents, and governmental entities – all of which had the resources to make healthier decisions but failed to do so.
While a majority of the population may believe that obesity is a “condition of choice,” I think they would be more likely to support the political will for action if they saw data that acknowledges that yes, obesity can be a condition of choice, but here are the circumstances in which choice can and can’t make a difference. Language must always be chosen carefully to minimize stigmatization. However, remember we are not pointing fingers at victims; we are instead looking for teaching moments in which adults can learn to make better choices for the children under their care who are too young to make their own.
Finally, as the authors of this Pediatric Perspectives considered cons of a declaration of health care emergency, they raised the peculiarly American concern of personal autonomy. As they pointed out, there are unfortunate examples in this country in which efforts to limit personal choice have backfired and well-meaning and potentially effective methods for limiting unhealthy behaviors have been eliminated in the name of personal freedom. I’m not sure how we manage this except to wait and be judicious as we move forward addressing pediatric obesity on a national scale. I urge you to take a few minutes to read this perspective. It is a topic worth considering.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].












