User login
Q&A: Clinical implications of clonal hematopoiesis
There is growing literature around clonal hematopoiesis (CH) but many questions persist about its clinical significance. On June 14, Hematology News hosted a Twitter question-and-answer session with Aaron Viny, MD, who is on the staff of the leukemia service at Memorial Sloan Kettering Cancer Center in New York and is a member of the Hematology News editorial advisory board, to answer questions about CH and interpret the latest research. The following is an edited version of the Q&A session.
Question: Can you get us started by explaining the difference between clonal hematopoiesis (CH) and clonal cytopenia of undetermined significance?
Question: So are there differences in CH depending on the gene mutated?
Dr. Viny: Hard to say. The most common mutations found in CH are DNMT3A, TET2, PPM1D, and ASXL1, as shown in the Cell Stem Cell paper by Catherine C. Coombs, MD, and Ross Levine, MD (2017 Sep 7;21[3]:374-82.e4). So in the setting of patients with acute myeloid leukemia, there are data from several groups showing that persistence of DNMT3A, TET2, and ASXL1 have less recurrence risk, compared with the field. This, of course, does not apply to CH in the absence of a hematologic disorder.
Question: Are you aware of any screening programs for clonal hematopoiesis?
Dr. Viny: Currently at Memorial Sloan Kettering, patients who undergo MSK-IMPACT testing of their solid tumor have a germ line control from blood that is also sequenced. These samples are screened for CH mutations. In fact, there are two recent papers showing one key issue with tumor sequencing and CH: A blood sample is necessary to resolve the compartment of the CH mutation (that is, not in the solid tumor). The papers are JAMA Oncol. 2018 Jun 5. doi: 10.1001/jamaoncol.2018.2297 and Clin Cancer Res. 2018 Jun 4. doi: 10.1158/1078-0432.CCR-18-1201.
Question: Will patients with CH in screened samples be notified of the results?
Dr. Viny: Yes. The patients are being referred to the Memorial Sloan Kettering clonal hematopoiesis clinic run by Dr. Levine and Kelly Bolton, MD.
Question: Once you screen and detect CH, how should these patients be followed?
Dr. Viny: First, what are the risks these patients face? Extensive work by Siddhartha Jaiswal, MD, PhD, shows that there is an increased risk of cardiovascular disease and an increased risk for leukemia. So with regards to the latter, following with serial complete blood counts seems sufficient, with a bone marrow biopsy at the detection of any cytopenias. With regard to cardiovascular risk, I consider CH akin to an unmodifiable cardiac risk factor. Patients should be counseled to exercise, and depending on any other cardiac risk factors, interventions such as blood pressure control, lipid control, and daily aspirin use should be addressed accordingly.
Question: Is this BRCA1 all over again?
Dr. Viny: Perhaps. With BRCA1, we now have a few decades of follow-up to better understand the risks and even intervene with preventive interventions. Clonal hematopoiesis needs the follow-up and research to support clinical action.
Question: Could you please refer your readers to your favorite review on clonal hematopoiesis?
Dr. Viny: An outstanding review of the mechanisms and molecular consequences of clonal hematopoiesis is in Cell Stem Cell (2018 Feb 1;22[2]:157-70).
Question: Are there any effects of previous radiation on the development of CH?
Dr. Viny: Let’s start by saying that CH in the absence of prior cancer is probably a different entity. Radiation, tobacco use, and increased age all increase the risk for detection of a CH somatic mutation.
Question: What are the clinical implications of CH?
Dr. Viny: While I think it is still too soon to say if these are the only clinical implications, both increased risk of hematologic malignancy and increased risk of cardiovascular disease are the best studied and described to date. Here’s an excellent article on the cardiovascular risk: N Engl J Med. 2017 Jul 13;377(2):111-21. Of interest, it seems that the increased risk, while being relatively low, does not plateau over time.
Question: What do you think about TET2 mutations being among the most frequent mutations in CH, but IDH being relatively rare?
Dr. Viny: Great question. Both are affecting demethylation and epigenetic instability. While I don’t think the answer is known, perhaps the IDH mutations have a more dominant effect on hematopoietic output. The majority of the CH data uses blood; maybe the marrow tells a different story.
Question: Can we cure clonal hematopoiesis with vitamin C?
Dr. Viny: You clearly know the recent work by Iannis Aifantis, PhD, showing the effects of vitamin C on TET enzyme activity, published here: Cell. 2017 Sep 7;170(6):1079-95. For CH patients with TET2 mutations, a high-dose vitamin C regimen sounds very exciting. There is also complementary work by Sean Morrison, PhD, published in Nature (2017 Sep 28;549[7673]:476-81).
Question: Is there a limit to the sequencing depth and variant allele fraction needed to identify clonal hematopoiesis? At what point is CH not CH?
Dr. Viny: So this is as much a technical question as it is a biological question. As of now we can reliably detect variant allele fraction in CH to 0.1%, at best. What is detectable and what is clinically relevant are questions that still need to be answered.
Question: There seems to be a lot of good research going on in CH. What are the big knowledge gaps that future studies should be targeting?
Dr. Viny: First, what are the functional and molecular consequences of the varying alleles in CH? Second, are there other clinical risks to CH beyond leukemia and cardiovascular disease? And third, does inflammation cause CH or does CH cause inflammation?
Dr. Viny is with the Memorial Sloan Kettering Cancer Center, New York, where he is a clinical instructor; is on the staff of the leukemia service; and is a clinical researcher in the Ross Levine Lab. Connect with him on Twitter at @TheDoctorIsVin.
There is growing literature around clonal hematopoiesis (CH) but many questions persist about its clinical significance. On June 14, Hematology News hosted a Twitter question-and-answer session with Aaron Viny, MD, who is on the staff of the leukemia service at Memorial Sloan Kettering Cancer Center in New York and is a member of the Hematology News editorial advisory board, to answer questions about CH and interpret the latest research. The following is an edited version of the Q&A session.
Question: Can you get us started by explaining the difference between clonal hematopoiesis (CH) and clonal cytopenia of undetermined significance?
Question: So are there differences in CH depending on the gene mutated?
Dr. Viny: Hard to say. The most common mutations found in CH are DNMT3A, TET2, PPM1D, and ASXL1, as shown in the Cell Stem Cell paper by Catherine C. Coombs, MD, and Ross Levine, MD (2017 Sep 7;21[3]:374-82.e4). So in the setting of patients with acute myeloid leukemia, there are data from several groups showing that persistence of DNMT3A, TET2, and ASXL1 have less recurrence risk, compared with the field. This, of course, does not apply to CH in the absence of a hematologic disorder.
Question: Are you aware of any screening programs for clonal hematopoiesis?
Dr. Viny: Currently at Memorial Sloan Kettering, patients who undergo MSK-IMPACT testing of their solid tumor have a germ line control from blood that is also sequenced. These samples are screened for CH mutations. In fact, there are two recent papers showing one key issue with tumor sequencing and CH: A blood sample is necessary to resolve the compartment of the CH mutation (that is, not in the solid tumor). The papers are JAMA Oncol. 2018 Jun 5. doi: 10.1001/jamaoncol.2018.2297 and Clin Cancer Res. 2018 Jun 4. doi: 10.1158/1078-0432.CCR-18-1201.
Question: Will patients with CH in screened samples be notified of the results?
Dr. Viny: Yes. The patients are being referred to the Memorial Sloan Kettering clonal hematopoiesis clinic run by Dr. Levine and Kelly Bolton, MD.
Question: Once you screen and detect CH, how should these patients be followed?
Dr. Viny: First, what are the risks these patients face? Extensive work by Siddhartha Jaiswal, MD, PhD, shows that there is an increased risk of cardiovascular disease and an increased risk for leukemia. So with regards to the latter, following with serial complete blood counts seems sufficient, with a bone marrow biopsy at the detection of any cytopenias. With regard to cardiovascular risk, I consider CH akin to an unmodifiable cardiac risk factor. Patients should be counseled to exercise, and depending on any other cardiac risk factors, interventions such as blood pressure control, lipid control, and daily aspirin use should be addressed accordingly.
Question: Is this BRCA1 all over again?
Dr. Viny: Perhaps. With BRCA1, we now have a few decades of follow-up to better understand the risks and even intervene with preventive interventions. Clonal hematopoiesis needs the follow-up and research to support clinical action.
Question: Could you please refer your readers to your favorite review on clonal hematopoiesis?
Dr. Viny: An outstanding review of the mechanisms and molecular consequences of clonal hematopoiesis is in Cell Stem Cell (2018 Feb 1;22[2]:157-70).
Question: Are there any effects of previous radiation on the development of CH?
Dr. Viny: Let’s start by saying that CH in the absence of prior cancer is probably a different entity. Radiation, tobacco use, and increased age all increase the risk for detection of a CH somatic mutation.
Question: What are the clinical implications of CH?
Dr. Viny: While I think it is still too soon to say if these are the only clinical implications, both increased risk of hematologic malignancy and increased risk of cardiovascular disease are the best studied and described to date. Here’s an excellent article on the cardiovascular risk: N Engl J Med. 2017 Jul 13;377(2):111-21. Of interest, it seems that the increased risk, while being relatively low, does not plateau over time.
Question: What do you think about TET2 mutations being among the most frequent mutations in CH, but IDH being relatively rare?
Dr. Viny: Great question. Both are affecting demethylation and epigenetic instability. While I don’t think the answer is known, perhaps the IDH mutations have a more dominant effect on hematopoietic output. The majority of the CH data uses blood; maybe the marrow tells a different story.
Question: Can we cure clonal hematopoiesis with vitamin C?
Dr. Viny: You clearly know the recent work by Iannis Aifantis, PhD, showing the effects of vitamin C on TET enzyme activity, published here: Cell. 2017 Sep 7;170(6):1079-95. For CH patients with TET2 mutations, a high-dose vitamin C regimen sounds very exciting. There is also complementary work by Sean Morrison, PhD, published in Nature (2017 Sep 28;549[7673]:476-81).
Question: Is there a limit to the sequencing depth and variant allele fraction needed to identify clonal hematopoiesis? At what point is CH not CH?
Dr. Viny: So this is as much a technical question as it is a biological question. As of now we can reliably detect variant allele fraction in CH to 0.1%, at best. What is detectable and what is clinically relevant are questions that still need to be answered.
Question: There seems to be a lot of good research going on in CH. What are the big knowledge gaps that future studies should be targeting?
Dr. Viny: First, what are the functional and molecular consequences of the varying alleles in CH? Second, are there other clinical risks to CH beyond leukemia and cardiovascular disease? And third, does inflammation cause CH or does CH cause inflammation?
Dr. Viny is with the Memorial Sloan Kettering Cancer Center, New York, where he is a clinical instructor; is on the staff of the leukemia service; and is a clinical researcher in the Ross Levine Lab. Connect with him on Twitter at @TheDoctorIsVin.
There is growing literature around clonal hematopoiesis (CH) but many questions persist about its clinical significance. On June 14, Hematology News hosted a Twitter question-and-answer session with Aaron Viny, MD, who is on the staff of the leukemia service at Memorial Sloan Kettering Cancer Center in New York and is a member of the Hematology News editorial advisory board, to answer questions about CH and interpret the latest research. The following is an edited version of the Q&A session.
Question: Can you get us started by explaining the difference between clonal hematopoiesis (CH) and clonal cytopenia of undetermined significance?
Question: So are there differences in CH depending on the gene mutated?
Dr. Viny: Hard to say. The most common mutations found in CH are DNMT3A, TET2, PPM1D, and ASXL1, as shown in the Cell Stem Cell paper by Catherine C. Coombs, MD, and Ross Levine, MD (2017 Sep 7;21[3]:374-82.e4). So in the setting of patients with acute myeloid leukemia, there are data from several groups showing that persistence of DNMT3A, TET2, and ASXL1 have less recurrence risk, compared with the field. This, of course, does not apply to CH in the absence of a hematologic disorder.
Question: Are you aware of any screening programs for clonal hematopoiesis?
Dr. Viny: Currently at Memorial Sloan Kettering, patients who undergo MSK-IMPACT testing of their solid tumor have a germ line control from blood that is also sequenced. These samples are screened for CH mutations. In fact, there are two recent papers showing one key issue with tumor sequencing and CH: A blood sample is necessary to resolve the compartment of the CH mutation (that is, not in the solid tumor). The papers are JAMA Oncol. 2018 Jun 5. doi: 10.1001/jamaoncol.2018.2297 and Clin Cancer Res. 2018 Jun 4. doi: 10.1158/1078-0432.CCR-18-1201.
Question: Will patients with CH in screened samples be notified of the results?
Dr. Viny: Yes. The patients are being referred to the Memorial Sloan Kettering clonal hematopoiesis clinic run by Dr. Levine and Kelly Bolton, MD.
Question: Once you screen and detect CH, how should these patients be followed?
Dr. Viny: First, what are the risks these patients face? Extensive work by Siddhartha Jaiswal, MD, PhD, shows that there is an increased risk of cardiovascular disease and an increased risk for leukemia. So with regards to the latter, following with serial complete blood counts seems sufficient, with a bone marrow biopsy at the detection of any cytopenias. With regard to cardiovascular risk, I consider CH akin to an unmodifiable cardiac risk factor. Patients should be counseled to exercise, and depending on any other cardiac risk factors, interventions such as blood pressure control, lipid control, and daily aspirin use should be addressed accordingly.
Question: Is this BRCA1 all over again?
Dr. Viny: Perhaps. With BRCA1, we now have a few decades of follow-up to better understand the risks and even intervene with preventive interventions. Clonal hematopoiesis needs the follow-up and research to support clinical action.
Question: Could you please refer your readers to your favorite review on clonal hematopoiesis?
Dr. Viny: An outstanding review of the mechanisms and molecular consequences of clonal hematopoiesis is in Cell Stem Cell (2018 Feb 1;22[2]:157-70).
Question: Are there any effects of previous radiation on the development of CH?
Dr. Viny: Let’s start by saying that CH in the absence of prior cancer is probably a different entity. Radiation, tobacco use, and increased age all increase the risk for detection of a CH somatic mutation.
Question: What are the clinical implications of CH?
Dr. Viny: While I think it is still too soon to say if these are the only clinical implications, both increased risk of hematologic malignancy and increased risk of cardiovascular disease are the best studied and described to date. Here’s an excellent article on the cardiovascular risk: N Engl J Med. 2017 Jul 13;377(2):111-21. Of interest, it seems that the increased risk, while being relatively low, does not plateau over time.
Question: What do you think about TET2 mutations being among the most frequent mutations in CH, but IDH being relatively rare?
Dr. Viny: Great question. Both are affecting demethylation and epigenetic instability. While I don’t think the answer is known, perhaps the IDH mutations have a more dominant effect on hematopoietic output. The majority of the CH data uses blood; maybe the marrow tells a different story.
Question: Can we cure clonal hematopoiesis with vitamin C?
Dr. Viny: You clearly know the recent work by Iannis Aifantis, PhD, showing the effects of vitamin C on TET enzyme activity, published here: Cell. 2017 Sep 7;170(6):1079-95. For CH patients with TET2 mutations, a high-dose vitamin C regimen sounds very exciting. There is also complementary work by Sean Morrison, PhD, published in Nature (2017 Sep 28;549[7673]:476-81).
Question: Is there a limit to the sequencing depth and variant allele fraction needed to identify clonal hematopoiesis? At what point is CH not CH?
Dr. Viny: So this is as much a technical question as it is a biological question. As of now we can reliably detect variant allele fraction in CH to 0.1%, at best. What is detectable and what is clinically relevant are questions that still need to be answered.
Question: There seems to be a lot of good research going on in CH. What are the big knowledge gaps that future studies should be targeting?
Dr. Viny: First, what are the functional and molecular consequences of the varying alleles in CH? Second, are there other clinical risks to CH beyond leukemia and cardiovascular disease? And third, does inflammation cause CH or does CH cause inflammation?
Dr. Viny is with the Memorial Sloan Kettering Cancer Center, New York, where he is a clinical instructor; is on the staff of the leukemia service; and is a clinical researcher in the Ross Levine Lab. Connect with him on Twitter at @TheDoctorIsVin.
Why contribute to your political action committee?
I got a phone call recently from a friend north of me. “Gosh, did you hear about the State of Ohio Board of Pharmacy ransacking a dermatologist’s office?” he asked. Yes, I had heard about it, and explained that the office compounding rule in Ohio, the reason behind this surprise search and practice, was the subject of an active struggle going on at the state and federal level (see “Beware the state pharmacy board,” Dermatology News, June 3, 2016). I also told him that the pharmacy board swooped into that location because the office had registered for a license to mix drugs (defined by the board as altering a prescription drug by mixing, diluting, or combining), and agreed to unannounced inspections – and that while the dermatologist was not fined, there was a list of compliance issues to be met, including recording the lot number of all samples, keeping separate paper records of each time he mixed medications, and the promise of a return visit soon.
I went on to explain that representatives – past presidents and board members – of the Ohio Dermatological Association (accompanied by Lisa Albany, director of state policy at the American Academy of Dermatology’s Washington office) had met with the state pharmacy board and explained how ridiculous these regulations were. It was a frustrating meeting.
This was obviously unacceptable, and we went on to meet with state legislators, then federal legislators, and even the Food and Drug Administration. The Ohio Dermatological Association, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the AAD all went to Washington, DC, and to the FDA last fall. The Ohio State Medical Association and the American Medical Association lined up in opposition to the rules. The state pharmacy board withdrew its rules and reopened the comment period. We are still waiting to hear back and have encouraged the pharmacy board to wait for FDA and USP (United States Pharmacopeia) guidance.
So, what has this got to do with SkinPAC, our dermatology political action committee?
When our groups went to Washington to talk to our representatives and senators, we had access to all the movers and shakers who could act on this issue because of the AAD Association’s contacts though SkinPAC.
The point I want to make is that . There are many issues directly affecting dermatology, not only compounding, but loss of global periods (see “Time for dermatologists in nine states to start submitting CPT Code 99024,” Dermatology News, July 18, 2017), MACRA reform, MACRA relief, and legislative relief for medication pricing.
So, I told my northern friend who called to attend the AAD’s legislative conference in Washington (July 15-17), regularly contribute to SkinPAC, and get five of his colleagues to sign up too. This is a solid investment of your time and money. Not participating will make it more likely that you will soon need a pharmacy license (in addition to your medical license), may have to start charging patients to remove their sutures, be forced into larger groups to demonstrate quality, and continue to have to explain why a once-cheap generic drug now costs thousands of dollars. Seems like a good investment to me.
Dr. Coldiron is vice chair of the dermatology political action committee (SkinPAC). He is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
I got a phone call recently from a friend north of me. “Gosh, did you hear about the State of Ohio Board of Pharmacy ransacking a dermatologist’s office?” he asked. Yes, I had heard about it, and explained that the office compounding rule in Ohio, the reason behind this surprise search and practice, was the subject of an active struggle going on at the state and federal level (see “Beware the state pharmacy board,” Dermatology News, June 3, 2016). I also told him that the pharmacy board swooped into that location because the office had registered for a license to mix drugs (defined by the board as altering a prescription drug by mixing, diluting, or combining), and agreed to unannounced inspections – and that while the dermatologist was not fined, there was a list of compliance issues to be met, including recording the lot number of all samples, keeping separate paper records of each time he mixed medications, and the promise of a return visit soon.
I went on to explain that representatives – past presidents and board members – of the Ohio Dermatological Association (accompanied by Lisa Albany, director of state policy at the American Academy of Dermatology’s Washington office) had met with the state pharmacy board and explained how ridiculous these regulations were. It was a frustrating meeting.
This was obviously unacceptable, and we went on to meet with state legislators, then federal legislators, and even the Food and Drug Administration. The Ohio Dermatological Association, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the AAD all went to Washington, DC, and to the FDA last fall. The Ohio State Medical Association and the American Medical Association lined up in opposition to the rules. The state pharmacy board withdrew its rules and reopened the comment period. We are still waiting to hear back and have encouraged the pharmacy board to wait for FDA and USP (United States Pharmacopeia) guidance.
So, what has this got to do with SkinPAC, our dermatology political action committee?
When our groups went to Washington to talk to our representatives and senators, we had access to all the movers and shakers who could act on this issue because of the AAD Association’s contacts though SkinPAC.
The point I want to make is that . There are many issues directly affecting dermatology, not only compounding, but loss of global periods (see “Time for dermatologists in nine states to start submitting CPT Code 99024,” Dermatology News, July 18, 2017), MACRA reform, MACRA relief, and legislative relief for medication pricing.
So, I told my northern friend who called to attend the AAD’s legislative conference in Washington (July 15-17), regularly contribute to SkinPAC, and get five of his colleagues to sign up too. This is a solid investment of your time and money. Not participating will make it more likely that you will soon need a pharmacy license (in addition to your medical license), may have to start charging patients to remove their sutures, be forced into larger groups to demonstrate quality, and continue to have to explain why a once-cheap generic drug now costs thousands of dollars. Seems like a good investment to me.
Dr. Coldiron is vice chair of the dermatology political action committee (SkinPAC). He is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
I got a phone call recently from a friend north of me. “Gosh, did you hear about the State of Ohio Board of Pharmacy ransacking a dermatologist’s office?” he asked. Yes, I had heard about it, and explained that the office compounding rule in Ohio, the reason behind this surprise search and practice, was the subject of an active struggle going on at the state and federal level (see “Beware the state pharmacy board,” Dermatology News, June 3, 2016). I also told him that the pharmacy board swooped into that location because the office had registered for a license to mix drugs (defined by the board as altering a prescription drug by mixing, diluting, or combining), and agreed to unannounced inspections – and that while the dermatologist was not fined, there was a list of compliance issues to be met, including recording the lot number of all samples, keeping separate paper records of each time he mixed medications, and the promise of a return visit soon.
I went on to explain that representatives – past presidents and board members – of the Ohio Dermatological Association (accompanied by Lisa Albany, director of state policy at the American Academy of Dermatology’s Washington office) had met with the state pharmacy board and explained how ridiculous these regulations were. It was a frustrating meeting.
This was obviously unacceptable, and we went on to meet with state legislators, then federal legislators, and even the Food and Drug Administration. The Ohio Dermatological Association, the American Society for Dermatologic Surgery, the American College of Mohs Surgery, and the AAD all went to Washington, DC, and to the FDA last fall. The Ohio State Medical Association and the American Medical Association lined up in opposition to the rules. The state pharmacy board withdrew its rules and reopened the comment period. We are still waiting to hear back and have encouraged the pharmacy board to wait for FDA and USP (United States Pharmacopeia) guidance.
So, what has this got to do with SkinPAC, our dermatology political action committee?
When our groups went to Washington to talk to our representatives and senators, we had access to all the movers and shakers who could act on this issue because of the AAD Association’s contacts though SkinPAC.
The point I want to make is that . There are many issues directly affecting dermatology, not only compounding, but loss of global periods (see “Time for dermatologists in nine states to start submitting CPT Code 99024,” Dermatology News, July 18, 2017), MACRA reform, MACRA relief, and legislative relief for medication pricing.
So, I told my northern friend who called to attend the AAD’s legislative conference in Washington (July 15-17), regularly contribute to SkinPAC, and get five of his colleagues to sign up too. This is a solid investment of your time and money. Not participating will make it more likely that you will soon need a pharmacy license (in addition to your medical license), may have to start charging patients to remove their sutures, be forced into larger groups to demonstrate quality, and continue to have to explain why a once-cheap generic drug now costs thousands of dollars. Seems like a good investment to me.
Dr. Coldiron is vice chair of the dermatology political action committee (SkinPAC). He is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
U.S. immigration policy: What harms will persist?
The Trump policy of separating children and teenagers from their parents after crossing the U.S. border has been called un-American, immoral, cruel, and inhumane. The policy thankfully has been reversed or at least subject to delay. However, as I write today, 2,300 children and their parents are separated, not in contact, lost to each other, and with no clear plan on reunification. The ultimate outcome of immigration legislation and policy is unknown and mired in partisan politics. The policy hopefully has changed permanently, but what are the harms that will persist?
1. Many if not all of the 2,300 children taken from their parents to institutional settings will have suffered acute anxiety and despair. Following data gathered by René Spitz and John Bowlby 80 years ago, children forced to separate from their parents for long hospitalizations with limited visitation went through phases of protest, despair, and if repeated or lengthy separations, “detachment” that impaired their ability to form relationships.1
2. Many of these children have suffered traumas in their country of origin and through the journey to the U.S. border. Some of this traumatic experience was mitigated by being in the presence of their parent(s). Very likely some children have psychiatric and physical disorders that will add to the level of risk. The current trauma, forcible separation by armed guards into restrictive facilities, will compound or intensify the previous traumas without the benefit of parental support.
3. Will the harms persist? Likely this level of trauma has such a strong neurologic and psychological impact that many of the children will suffer from nightmares, depression, and persistent anxiety about trusting the safety of their setting. 2
4. The parents who are jailed, have had their children removed, and do not know where they are and aren’t able to talk to them have suffered a massive trauma. We all have lost sight of a child for a minute or two in a store or on the beach. Our anxiety is immediate, and if the separation is longer, we may remember those frightening minutes for the rest of our lives. How many immigrant parents will develop depression and posttraumatic stress disorder?
5. Guards were ordered to be the front-line implementers of the policy and must have been torn between their sworn duty and their inner knowledge that what they are doing is wrong. Hearing the children crying and calling for their parents must have elicited painful feelings of what it would have been like to have their own children taken away with no way to reach them or knowing where they were taken. Implementing this policy dehumanized them, and I believe made them feel guilty or unworthy.
6. Millions of immigrants – whether lawful, dreamers, or undocumented – must have felt fearful, powerless, and angry about this policy. Millions of their children must have been worried and lost a little bit of faith in their parents and in the United States.
7. Did U.S. citizens, many from immigrant roots, wonder if this could happen to them? How many children felt a little less secure? Was the anxiety higher for descendants of the U.S. citizens remembering the trauma of the World War II Japanese internment camps? Other descendants (like me) will remember quite vividly their mother’s story of being on the St. Louis steam ship and being turned away from the United States to face a high likelihood of death in Nazi Germany. A bit of fear will replace trust in and loyalty to the United States.
Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email him at [email protected].
References:
1. Dev Psychol. 1992;28:759-75.
2. www.cdc.gov/violenceprevention/acestudy/index.html
The Trump policy of separating children and teenagers from their parents after crossing the U.S. border has been called un-American, immoral, cruel, and inhumane. The policy thankfully has been reversed or at least subject to delay. However, as I write today, 2,300 children and their parents are separated, not in contact, lost to each other, and with no clear plan on reunification. The ultimate outcome of immigration legislation and policy is unknown and mired in partisan politics. The policy hopefully has changed permanently, but what are the harms that will persist?
1. Many if not all of the 2,300 children taken from their parents to institutional settings will have suffered acute anxiety and despair. Following data gathered by René Spitz and John Bowlby 80 years ago, children forced to separate from their parents for long hospitalizations with limited visitation went through phases of protest, despair, and if repeated or lengthy separations, “detachment” that impaired their ability to form relationships.1
2. Many of these children have suffered traumas in their country of origin and through the journey to the U.S. border. Some of this traumatic experience was mitigated by being in the presence of their parent(s). Very likely some children have psychiatric and physical disorders that will add to the level of risk. The current trauma, forcible separation by armed guards into restrictive facilities, will compound or intensify the previous traumas without the benefit of parental support.
3. Will the harms persist? Likely this level of trauma has such a strong neurologic and psychological impact that many of the children will suffer from nightmares, depression, and persistent anxiety about trusting the safety of their setting. 2
4. The parents who are jailed, have had their children removed, and do not know where they are and aren’t able to talk to them have suffered a massive trauma. We all have lost sight of a child for a minute or two in a store or on the beach. Our anxiety is immediate, and if the separation is longer, we may remember those frightening minutes for the rest of our lives. How many immigrant parents will develop depression and posttraumatic stress disorder?
5. Guards were ordered to be the front-line implementers of the policy and must have been torn between their sworn duty and their inner knowledge that what they are doing is wrong. Hearing the children crying and calling for their parents must have elicited painful feelings of what it would have been like to have their own children taken away with no way to reach them or knowing where they were taken. Implementing this policy dehumanized them, and I believe made them feel guilty or unworthy.
6. Millions of immigrants – whether lawful, dreamers, or undocumented – must have felt fearful, powerless, and angry about this policy. Millions of their children must have been worried and lost a little bit of faith in their parents and in the United States.
7. Did U.S. citizens, many from immigrant roots, wonder if this could happen to them? How many children felt a little less secure? Was the anxiety higher for descendants of the U.S. citizens remembering the trauma of the World War II Japanese internment camps? Other descendants (like me) will remember quite vividly their mother’s story of being on the St. Louis steam ship and being turned away from the United States to face a high likelihood of death in Nazi Germany. A bit of fear will replace trust in and loyalty to the United States.
Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email him at [email protected].
References:
1. Dev Psychol. 1992;28:759-75.
2. www.cdc.gov/violenceprevention/acestudy/index.html
The Trump policy of separating children and teenagers from their parents after crossing the U.S. border has been called un-American, immoral, cruel, and inhumane. The policy thankfully has been reversed or at least subject to delay. However, as I write today, 2,300 children and their parents are separated, not in contact, lost to each other, and with no clear plan on reunification. The ultimate outcome of immigration legislation and policy is unknown and mired in partisan politics. The policy hopefully has changed permanently, but what are the harms that will persist?
1. Many if not all of the 2,300 children taken from their parents to institutional settings will have suffered acute anxiety and despair. Following data gathered by René Spitz and John Bowlby 80 years ago, children forced to separate from their parents for long hospitalizations with limited visitation went through phases of protest, despair, and if repeated or lengthy separations, “detachment” that impaired their ability to form relationships.1
2. Many of these children have suffered traumas in their country of origin and through the journey to the U.S. border. Some of this traumatic experience was mitigated by being in the presence of their parent(s). Very likely some children have psychiatric and physical disorders that will add to the level of risk. The current trauma, forcible separation by armed guards into restrictive facilities, will compound or intensify the previous traumas without the benefit of parental support.
3. Will the harms persist? Likely this level of trauma has such a strong neurologic and psychological impact that many of the children will suffer from nightmares, depression, and persistent anxiety about trusting the safety of their setting. 2
4. The parents who are jailed, have had their children removed, and do not know where they are and aren’t able to talk to them have suffered a massive trauma. We all have lost sight of a child for a minute or two in a store or on the beach. Our anxiety is immediate, and if the separation is longer, we may remember those frightening minutes for the rest of our lives. How many immigrant parents will develop depression and posttraumatic stress disorder?
5. Guards were ordered to be the front-line implementers of the policy and must have been torn between their sworn duty and their inner knowledge that what they are doing is wrong. Hearing the children crying and calling for their parents must have elicited painful feelings of what it would have been like to have their own children taken away with no way to reach them or knowing where they were taken. Implementing this policy dehumanized them, and I believe made them feel guilty or unworthy.
6. Millions of immigrants – whether lawful, dreamers, or undocumented – must have felt fearful, powerless, and angry about this policy. Millions of their children must have been worried and lost a little bit of faith in their parents and in the United States.
7. Did U.S. citizens, many from immigrant roots, wonder if this could happen to them? How many children felt a little less secure? Was the anxiety higher for descendants of the U.S. citizens remembering the trauma of the World War II Japanese internment camps? Other descendants (like me) will remember quite vividly their mother’s story of being on the St. Louis steam ship and being turned away from the United States to face a high likelihood of death in Nazi Germany. A bit of fear will replace trust in and loyalty to the United States.
Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email him at [email protected].
References:
1. Dev Psychol. 1992;28:759-75.
2. www.cdc.gov/violenceprevention/acestudy/index.html
Mindfulness skill can help in parenting
Behavioral parent management training (PMT), which teaches parents concrete skills to increase their attention to positive behavior and to plan for their response to undesired behavior, has abundant evidence for success for many challenging child behaviors. But sometimes parents have a hard time managing their own emotional responses in the often highly triggering situation of family conflict. Mindfulness has the potential to provide a complement to the PMT skills. Studies are beginning to explore these possibilities.
Case summary
Zoe is a bright 5-year-old who has been “strong willed” and shown intense emotional responses since early in life. The usual 2-year-old temper tantrums increased over time. She has outbursts of yelling, kicking, and hitting, especially with transitions. Her parents tried behavioral parent training, but found it frustrating. If Zoe has been yelling and hitting earlier in the day, her mother feels hurt and angry and can’t bring herself to pay warm attention when Zoe is doing better. When Zoe refuses to pick up her room, her father is flooded with thoughts about his own father hitting him for the slightest disrespect. He thinks that he is a bad, weak father, and sometimes “sees red” and ends up yelling at Zoe instead of putting into place a calm consequence.
Discussion
Mindfulness is defined by Jon Kabat-Zinn as “paying attention in a particular way – on purpose, in the present moment, and nonjudgmentally.” A central feature of mindfulness is strengthening the ability to focus our attention. We learn to pay attention to aspects of the present moment, be that breathing, the sensations in our body, or the experiences of our senses. Often the first skill in behavioral training methods is getting parents to pay attention to their children by participating in child-led play or spending attentive time with older children. This means attending to what the child is doing or talking about rather than jumping in and taking over with suggestions, instructions, or judgments. This meshes very well with this central aspect of mindfulness.
As we practice paying attention, we observe that the mind naturally jumps around from what we mean to be attending to, to a host of distractions, worries, plans, memories, thoughts, and emotions. Mindfulness encourages practitioners to notice these thoughts, to avoid criticizing or judging oneself for becoming involved with these, but instead gently lead the mind back to what you had intended to focus on. This observation of the mind’s activity gives the mindfulness practitioner a bit of space from the thought or emotion itself. We are encouraged to name the thought or emotional processes we notice: “I am worrying, I am planning, I am remembering.”
In the heat of a difficult moment with the child, parents often are flooded with intense emotions (such as anger, fear, anxiety, panic, despair) and thoughts (such as “If my child keeps acting this way he is going to go to jail when he grows up,” “I am a terrible parent,” “Why is my child doing this to me?” or “He is just like his father”). These emotions and thoughts can drive intense, impulsive responses from the parents. As they practice mindfulness, they can gain the ability to observe themselves having these thoughts; observe harsh judgments of themselves or their children or their partners; have some space from them; and realize they may change in a few minutes or realize they may be painful but don’t necessarily have to spur impulsive action. In that moment, parents can give themselves time and space to think through possible actions, and then choose one.
From a behavioral parenting standpoint, we know that parents and humans often react intensely to negative behaviors and inadvertently make them worse with intense emotional reactivity. We want parents to have a plan about how they will respond, to remain calm in the moment, and then put the plan in place. Mindfulness may enhance parents’ ability to notice their own responses and have the space to remember what the plan was and then put it into place. It also can give them space to consider what the child might be experiencing and respond in light of this awareness. This ability does require a significant amount of mindfulness practice.
The combination of mindfulness and parenting is just beginning to be studied in research trials using a range of study designs. Some of these programs have looked at the effect of mindfulness courses, especially mindfulness-based stress reduction without any specific parenting content or indices of parent stress and child behavior. Others have looked at programs which add mindfulness to standard behavioral parenting programs, and still others are specific mindfulness/parenting programs. So far, many of these studies are quasi-experimental in nature. A recent systematic review by Townshend et al. found seven randomized controlled trials of low to moderate quality with some suggestion of ability to decrease parental stress and ADHD symptoms (JBI Database System Rev Implement Rep. 2016 Mar;14[3]:139-80). There is a clear need for randomized controlled trials with larger sample sizes.
While we may not have specific, highly evidence-based mindful parenting programs available, individuals with experience in yoga, meditation, mindfulness, dialectical behavioral therapy, and acceptance and commitment therapy can be encouraged to bring these skills to bear as parents.
Zoe’s parents had pursued outside mindfulness programs. Mindfulness concepts were brought into a standard parenting program. Her parents were encouraged to engage in child-led play with Zoe in a mindful way, fully attending to her actions and experience. Zoe’s parents also were encouraged to observe their own emotional reactions and thoughts in stressful moments and to take a breathing space before taking action.
Dr. Hall is assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
Resources
“Mindful Parenting” (New York: Norton & Co., 2015).
“Integrating mindfulness with parent training: Effects of the mindfulness-enhanced strengthening families program” (Dev Psychol. 2015;51[1]:26-35).
“Everyday blessings: The inner work of mindful parenting,” (New York: Hyperion, 1997).
Behavioral parent management training (PMT), which teaches parents concrete skills to increase their attention to positive behavior and to plan for their response to undesired behavior, has abundant evidence for success for many challenging child behaviors. But sometimes parents have a hard time managing their own emotional responses in the often highly triggering situation of family conflict. Mindfulness has the potential to provide a complement to the PMT skills. Studies are beginning to explore these possibilities.
Case summary
Zoe is a bright 5-year-old who has been “strong willed” and shown intense emotional responses since early in life. The usual 2-year-old temper tantrums increased over time. She has outbursts of yelling, kicking, and hitting, especially with transitions. Her parents tried behavioral parent training, but found it frustrating. If Zoe has been yelling and hitting earlier in the day, her mother feels hurt and angry and can’t bring herself to pay warm attention when Zoe is doing better. When Zoe refuses to pick up her room, her father is flooded with thoughts about his own father hitting him for the slightest disrespect. He thinks that he is a bad, weak father, and sometimes “sees red” and ends up yelling at Zoe instead of putting into place a calm consequence.
Discussion
Mindfulness is defined by Jon Kabat-Zinn as “paying attention in a particular way – on purpose, in the present moment, and nonjudgmentally.” A central feature of mindfulness is strengthening the ability to focus our attention. We learn to pay attention to aspects of the present moment, be that breathing, the sensations in our body, or the experiences of our senses. Often the first skill in behavioral training methods is getting parents to pay attention to their children by participating in child-led play or spending attentive time with older children. This means attending to what the child is doing or talking about rather than jumping in and taking over with suggestions, instructions, or judgments. This meshes very well with this central aspect of mindfulness.
As we practice paying attention, we observe that the mind naturally jumps around from what we mean to be attending to, to a host of distractions, worries, plans, memories, thoughts, and emotions. Mindfulness encourages practitioners to notice these thoughts, to avoid criticizing or judging oneself for becoming involved with these, but instead gently lead the mind back to what you had intended to focus on. This observation of the mind’s activity gives the mindfulness practitioner a bit of space from the thought or emotion itself. We are encouraged to name the thought or emotional processes we notice: “I am worrying, I am planning, I am remembering.”
In the heat of a difficult moment with the child, parents often are flooded with intense emotions (such as anger, fear, anxiety, panic, despair) and thoughts (such as “If my child keeps acting this way he is going to go to jail when he grows up,” “I am a terrible parent,” “Why is my child doing this to me?” or “He is just like his father”). These emotions and thoughts can drive intense, impulsive responses from the parents. As they practice mindfulness, they can gain the ability to observe themselves having these thoughts; observe harsh judgments of themselves or their children or their partners; have some space from them; and realize they may change in a few minutes or realize they may be painful but don’t necessarily have to spur impulsive action. In that moment, parents can give themselves time and space to think through possible actions, and then choose one.
From a behavioral parenting standpoint, we know that parents and humans often react intensely to negative behaviors and inadvertently make them worse with intense emotional reactivity. We want parents to have a plan about how they will respond, to remain calm in the moment, and then put the plan in place. Mindfulness may enhance parents’ ability to notice their own responses and have the space to remember what the plan was and then put it into place. It also can give them space to consider what the child might be experiencing and respond in light of this awareness. This ability does require a significant amount of mindfulness practice.
The combination of mindfulness and parenting is just beginning to be studied in research trials using a range of study designs. Some of these programs have looked at the effect of mindfulness courses, especially mindfulness-based stress reduction without any specific parenting content or indices of parent stress and child behavior. Others have looked at programs which add mindfulness to standard behavioral parenting programs, and still others are specific mindfulness/parenting programs. So far, many of these studies are quasi-experimental in nature. A recent systematic review by Townshend et al. found seven randomized controlled trials of low to moderate quality with some suggestion of ability to decrease parental stress and ADHD symptoms (JBI Database System Rev Implement Rep. 2016 Mar;14[3]:139-80). There is a clear need for randomized controlled trials with larger sample sizes.
While we may not have specific, highly evidence-based mindful parenting programs available, individuals with experience in yoga, meditation, mindfulness, dialectical behavioral therapy, and acceptance and commitment therapy can be encouraged to bring these skills to bear as parents.
Zoe’s parents had pursued outside mindfulness programs. Mindfulness concepts were brought into a standard parenting program. Her parents were encouraged to engage in child-led play with Zoe in a mindful way, fully attending to her actions and experience. Zoe’s parents also were encouraged to observe their own emotional reactions and thoughts in stressful moments and to take a breathing space before taking action.
Dr. Hall is assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
Resources
“Mindful Parenting” (New York: Norton & Co., 2015).
“Integrating mindfulness with parent training: Effects of the mindfulness-enhanced strengthening families program” (Dev Psychol. 2015;51[1]:26-35).
“Everyday blessings: The inner work of mindful parenting,” (New York: Hyperion, 1997).
Behavioral parent management training (PMT), which teaches parents concrete skills to increase their attention to positive behavior and to plan for their response to undesired behavior, has abundant evidence for success for many challenging child behaviors. But sometimes parents have a hard time managing their own emotional responses in the often highly triggering situation of family conflict. Mindfulness has the potential to provide a complement to the PMT skills. Studies are beginning to explore these possibilities.
Case summary
Zoe is a bright 5-year-old who has been “strong willed” and shown intense emotional responses since early in life. The usual 2-year-old temper tantrums increased over time. She has outbursts of yelling, kicking, and hitting, especially with transitions. Her parents tried behavioral parent training, but found it frustrating. If Zoe has been yelling and hitting earlier in the day, her mother feels hurt and angry and can’t bring herself to pay warm attention when Zoe is doing better. When Zoe refuses to pick up her room, her father is flooded with thoughts about his own father hitting him for the slightest disrespect. He thinks that he is a bad, weak father, and sometimes “sees red” and ends up yelling at Zoe instead of putting into place a calm consequence.
Discussion
Mindfulness is defined by Jon Kabat-Zinn as “paying attention in a particular way – on purpose, in the present moment, and nonjudgmentally.” A central feature of mindfulness is strengthening the ability to focus our attention. We learn to pay attention to aspects of the present moment, be that breathing, the sensations in our body, or the experiences of our senses. Often the first skill in behavioral training methods is getting parents to pay attention to their children by participating in child-led play or spending attentive time with older children. This means attending to what the child is doing or talking about rather than jumping in and taking over with suggestions, instructions, or judgments. This meshes very well with this central aspect of mindfulness.
As we practice paying attention, we observe that the mind naturally jumps around from what we mean to be attending to, to a host of distractions, worries, plans, memories, thoughts, and emotions. Mindfulness encourages practitioners to notice these thoughts, to avoid criticizing or judging oneself for becoming involved with these, but instead gently lead the mind back to what you had intended to focus on. This observation of the mind’s activity gives the mindfulness practitioner a bit of space from the thought or emotion itself. We are encouraged to name the thought or emotional processes we notice: “I am worrying, I am planning, I am remembering.”
In the heat of a difficult moment with the child, parents often are flooded with intense emotions (such as anger, fear, anxiety, panic, despair) and thoughts (such as “If my child keeps acting this way he is going to go to jail when he grows up,” “I am a terrible parent,” “Why is my child doing this to me?” or “He is just like his father”). These emotions and thoughts can drive intense, impulsive responses from the parents. As they practice mindfulness, they can gain the ability to observe themselves having these thoughts; observe harsh judgments of themselves or their children or their partners; have some space from them; and realize they may change in a few minutes or realize they may be painful but don’t necessarily have to spur impulsive action. In that moment, parents can give themselves time and space to think through possible actions, and then choose one.
From a behavioral parenting standpoint, we know that parents and humans often react intensely to negative behaviors and inadvertently make them worse with intense emotional reactivity. We want parents to have a plan about how they will respond, to remain calm in the moment, and then put the plan in place. Mindfulness may enhance parents’ ability to notice their own responses and have the space to remember what the plan was and then put it into place. It also can give them space to consider what the child might be experiencing and respond in light of this awareness. This ability does require a significant amount of mindfulness practice.
The combination of mindfulness and parenting is just beginning to be studied in research trials using a range of study designs. Some of these programs have looked at the effect of mindfulness courses, especially mindfulness-based stress reduction without any specific parenting content or indices of parent stress and child behavior. Others have looked at programs which add mindfulness to standard behavioral parenting programs, and still others are specific mindfulness/parenting programs. So far, many of these studies are quasi-experimental in nature. A recent systematic review by Townshend et al. found seven randomized controlled trials of low to moderate quality with some suggestion of ability to decrease parental stress and ADHD symptoms (JBI Database System Rev Implement Rep. 2016 Mar;14[3]:139-80). There is a clear need for randomized controlled trials with larger sample sizes.
While we may not have specific, highly evidence-based mindful parenting programs available, individuals with experience in yoga, meditation, mindfulness, dialectical behavioral therapy, and acceptance and commitment therapy can be encouraged to bring these skills to bear as parents.
Zoe’s parents had pursued outside mindfulness programs. Mindfulness concepts were brought into a standard parenting program. Her parents were encouraged to engage in child-led play with Zoe in a mindful way, fully attending to her actions and experience. Zoe’s parents also were encouraged to observe their own emotional reactions and thoughts in stressful moments and to take a breathing space before taking action.
Dr. Hall is assistant professor of psychiatry and pediatrics at the University of Vermont, Burlington. She said she had no relevant financial disclosures. Email her at [email protected].
Resources
“Mindful Parenting” (New York: Norton & Co., 2015).
“Integrating mindfulness with parent training: Effects of the mindfulness-enhanced strengthening families program” (Dev Psychol. 2015;51[1]:26-35).
“Everyday blessings: The inner work of mindful parenting,” (New York: Hyperion, 1997).
Pediatric Dermatology Consult - July 2018
Frequently misdiagnosed, streptococcal intertrigo more commonly affects infants and toddlers but is rarely reported, especially compared with other Streptococcus pyogenes infections, including impetigo, erysipelas, and cellulitis.1
Intertrigo, meaning “between” (inter) and “to rub” (terere) in Latin, describes any skin disorder involving two opposing skin surfaces that touch or rub to cause friction.2 The continuous chaffing, coupled with moisture trapped within the skin folds, leads to irritation and maceration, which provides an ideal environment for pathogens to thrive. Thus, frictional dermatitides that arise may become secondarily infected with one or more microorganisms, such as Candida albicans, Staphylococcus aureus, Streptococcus pyogenes, and even organisms less commonly associated with cutaneous infection, such as Proteus mirabilis.3
Streptococcal intertrigo may affect any intertriginous area, but most commonly it affects the folds of the neck; this is likely because of the combination of the deep folds that develop in shorter, infantile necks and the moisture from drool and saliva that pools in the area.5,6 In addition to these cervical folds, other intertriginous areas commonly are affected, including the inguinal, axillary, popliteal, posterior auricular, perianal, and genital folds.
Perianal streptococcal disease may present in a similar manner as streptococcal intertrigo, manifesting as well-demarcated, beefy red plaques in the skin folds around the anus and, in females, frequently perivaginally.7 Unlike streptococcal intertrigo, perianal streptococcal disease is often characterized by pain, pruritus, and fissuring of the involved area.8 It is associated with pharyngeal colonization of group A beta-hemolytic streptococci.7
Diagnosis is straight forward and may be confirmed by a positive streptococcal rapid antigen test of swab specimens of one or more surfaces of affected skin or by culture from a skin swab yielding growth of the organism.1,5 Skin biopsy is not necessary. If the index of suspicion for candida is high, a potassium hydroxide preparation and culture may be performed. Checking serum anti-DNase B antibodies, antistreptolysin O, and pharyngeal cultures is often unrevealing.9 A urinalysis may be performed to assess for poststreptococcal glomerulonephritis if the patient later develops facial or orbital edema, hypertension, hematuria, or lethargy.9
Treatment consists of systemic antistreptococcal therapy; oral amoxicillin and penicillin frequently have been used.9 Moisture in the area should be reduced with application of absorptive powders and physical barriers, such as zinc oxide, after gentle cleansing of the area.5
Other diagnoses to consider when evaluating dermatitides affecting skin folds include: other infectious causes, which may be ruled out by fungal or bacterial culture; inverse psoriasis, which will frequently demonstrate scale; atopic dermatitis, which will be pruritic with history of atopy; irritant or contact dermatitis, which will often have correlating clinical history; seborrheic dermatitis, which will often involve greasiness and scale; and less commonly, acrodermatitis enteropathica, which will be accompanied by diarrhea and hair loss.2,9 Scabies also may be on the differential if the patient endorses severe pruritus with close contacts with similar symptoms.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university. They had no conflicts of interest or disclosures to report.
References
1. Pediatr Dermatol. 2014 Mar-Apr;31(2):e71-2.
2. Clin Dermatol. 2011 Mar-Apr;29(2):173-9.
3. Pediatrics. 2003 Dec;112(6 pt 1):1427-9.
4. BMJ Case Rep. 2018 Mar 20. doi: 10.1136/bcr-2018-224179.
5. Pediatr Infect Dis J. 2012 Aug;31(8):872-3.
6. J Pediatr. 2015 May;166(5):1318.
7. J Pediatr. 2015 Sep;167(3):687-93.e1-2.
8. Pediatrics in Review. 1991;12(8):248-55.
9. J Pediatr. 2017 May;184:230-1.e1.
Frequently misdiagnosed, streptococcal intertrigo more commonly affects infants and toddlers but is rarely reported, especially compared with other Streptococcus pyogenes infections, including impetigo, erysipelas, and cellulitis.1
Intertrigo, meaning “between” (inter) and “to rub” (terere) in Latin, describes any skin disorder involving two opposing skin surfaces that touch or rub to cause friction.2 The continuous chaffing, coupled with moisture trapped within the skin folds, leads to irritation and maceration, which provides an ideal environment for pathogens to thrive. Thus, frictional dermatitides that arise may become secondarily infected with one or more microorganisms, such as Candida albicans, Staphylococcus aureus, Streptococcus pyogenes, and even organisms less commonly associated with cutaneous infection, such as Proteus mirabilis.3
Streptococcal intertrigo may affect any intertriginous area, but most commonly it affects the folds of the neck; this is likely because of the combination of the deep folds that develop in shorter, infantile necks and the moisture from drool and saliva that pools in the area.5,6 In addition to these cervical folds, other intertriginous areas commonly are affected, including the inguinal, axillary, popliteal, posterior auricular, perianal, and genital folds.
Perianal streptococcal disease may present in a similar manner as streptococcal intertrigo, manifesting as well-demarcated, beefy red plaques in the skin folds around the anus and, in females, frequently perivaginally.7 Unlike streptococcal intertrigo, perianal streptococcal disease is often characterized by pain, pruritus, and fissuring of the involved area.8 It is associated with pharyngeal colonization of group A beta-hemolytic streptococci.7
Diagnosis is straight forward and may be confirmed by a positive streptococcal rapid antigen test of swab specimens of one or more surfaces of affected skin or by culture from a skin swab yielding growth of the organism.1,5 Skin biopsy is not necessary. If the index of suspicion for candida is high, a potassium hydroxide preparation and culture may be performed. Checking serum anti-DNase B antibodies, antistreptolysin O, and pharyngeal cultures is often unrevealing.9 A urinalysis may be performed to assess for poststreptococcal glomerulonephritis if the patient later develops facial or orbital edema, hypertension, hematuria, or lethargy.9
Treatment consists of systemic antistreptococcal therapy; oral amoxicillin and penicillin frequently have been used.9 Moisture in the area should be reduced with application of absorptive powders and physical barriers, such as zinc oxide, after gentle cleansing of the area.5
Other diagnoses to consider when evaluating dermatitides affecting skin folds include: other infectious causes, which may be ruled out by fungal or bacterial culture; inverse psoriasis, which will frequently demonstrate scale; atopic dermatitis, which will be pruritic with history of atopy; irritant or contact dermatitis, which will often have correlating clinical history; seborrheic dermatitis, which will often involve greasiness and scale; and less commonly, acrodermatitis enteropathica, which will be accompanied by diarrhea and hair loss.2,9 Scabies also may be on the differential if the patient endorses severe pruritus with close contacts with similar symptoms.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university. They had no conflicts of interest or disclosures to report.
References
1. Pediatr Dermatol. 2014 Mar-Apr;31(2):e71-2.
2. Clin Dermatol. 2011 Mar-Apr;29(2):173-9.
3. Pediatrics. 2003 Dec;112(6 pt 1):1427-9.
4. BMJ Case Rep. 2018 Mar 20. doi: 10.1136/bcr-2018-224179.
5. Pediatr Infect Dis J. 2012 Aug;31(8):872-3.
6. J Pediatr. 2015 May;166(5):1318.
7. J Pediatr. 2015 Sep;167(3):687-93.e1-2.
8. Pediatrics in Review. 1991;12(8):248-55.
9. J Pediatr. 2017 May;184:230-1.e1.
Frequently misdiagnosed, streptococcal intertrigo more commonly affects infants and toddlers but is rarely reported, especially compared with other Streptococcus pyogenes infections, including impetigo, erysipelas, and cellulitis.1
Intertrigo, meaning “between” (inter) and “to rub” (terere) in Latin, describes any skin disorder involving two opposing skin surfaces that touch or rub to cause friction.2 The continuous chaffing, coupled with moisture trapped within the skin folds, leads to irritation and maceration, which provides an ideal environment for pathogens to thrive. Thus, frictional dermatitides that arise may become secondarily infected with one or more microorganisms, such as Candida albicans, Staphylococcus aureus, Streptococcus pyogenes, and even organisms less commonly associated with cutaneous infection, such as Proteus mirabilis.3
Streptococcal intertrigo may affect any intertriginous area, but most commonly it affects the folds of the neck; this is likely because of the combination of the deep folds that develop in shorter, infantile necks and the moisture from drool and saliva that pools in the area.5,6 In addition to these cervical folds, other intertriginous areas commonly are affected, including the inguinal, axillary, popliteal, posterior auricular, perianal, and genital folds.
Perianal streptococcal disease may present in a similar manner as streptococcal intertrigo, manifesting as well-demarcated, beefy red plaques in the skin folds around the anus and, in females, frequently perivaginally.7 Unlike streptococcal intertrigo, perianal streptococcal disease is often characterized by pain, pruritus, and fissuring of the involved area.8 It is associated with pharyngeal colonization of group A beta-hemolytic streptococci.7
Diagnosis is straight forward and may be confirmed by a positive streptococcal rapid antigen test of swab specimens of one or more surfaces of affected skin or by culture from a skin swab yielding growth of the organism.1,5 Skin biopsy is not necessary. If the index of suspicion for candida is high, a potassium hydroxide preparation and culture may be performed. Checking serum anti-DNase B antibodies, antistreptolysin O, and pharyngeal cultures is often unrevealing.9 A urinalysis may be performed to assess for poststreptococcal glomerulonephritis if the patient later develops facial or orbital edema, hypertension, hematuria, or lethargy.9
Treatment consists of systemic antistreptococcal therapy; oral amoxicillin and penicillin frequently have been used.9 Moisture in the area should be reduced with application of absorptive powders and physical barriers, such as zinc oxide, after gentle cleansing of the area.5
Other diagnoses to consider when evaluating dermatitides affecting skin folds include: other infectious causes, which may be ruled out by fungal or bacterial culture; inverse psoriasis, which will frequently demonstrate scale; atopic dermatitis, which will be pruritic with history of atopy; irritant or contact dermatitis, which will often have correlating clinical history; seborrheic dermatitis, which will often involve greasiness and scale; and less commonly, acrodermatitis enteropathica, which will be accompanied by diarrhea and hair loss.2,9 Scabies also may be on the differential if the patient endorses severe pruritus with close contacts with similar symptoms.
Ms. Han is a medical student at the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university. They had no conflicts of interest or disclosures to report.
References
1. Pediatr Dermatol. 2014 Mar-Apr;31(2):e71-2.
2. Clin Dermatol. 2011 Mar-Apr;29(2):173-9.
3. Pediatrics. 2003 Dec;112(6 pt 1):1427-9.
4. BMJ Case Rep. 2018 Mar 20. doi: 10.1136/bcr-2018-224179.
5. Pediatr Infect Dis J. 2012 Aug;31(8):872-3.
6. J Pediatr. 2015 May;166(5):1318.
7. J Pediatr. 2015 Sep;167(3):687-93.e1-2.
8. Pediatrics in Review. 1991;12(8):248-55.
9. J Pediatr. 2017 May;184:230-1.e1.
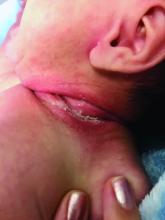
An 8-week-old male with a history of cradle cap presented for a second evaluation of an erythematous rash on the neck that started 1.5 weeks before, and it had since worsened. The parents note that their infant has been more irritable, but they otherwise deny any fever, diarrhea, constipation, or decrease in oral intake.
The patient’s first evaluation had been 3 days prior; nystatin cream was prescribed, and the parents applied it twice a day but without improvement to the rash. The patient also had a rash behind the ears bilaterally, which was treated with hydrocortisone 2.5% ointment with some improvement
On physical exam, the central neck is covered by a bright, beefy red, erythematous plaque with distinct borders and strong odor. There is faint scale and superficial desquamation between the skin folds. There are no surrounding papules or pustules. The patient’s chin is moist with drool. In the postauricular skin folds bilaterally, there are fainter but still erythematous plaques with mild scale.
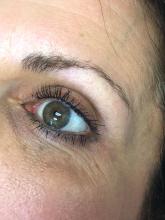
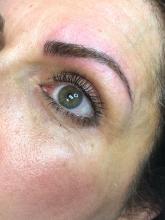
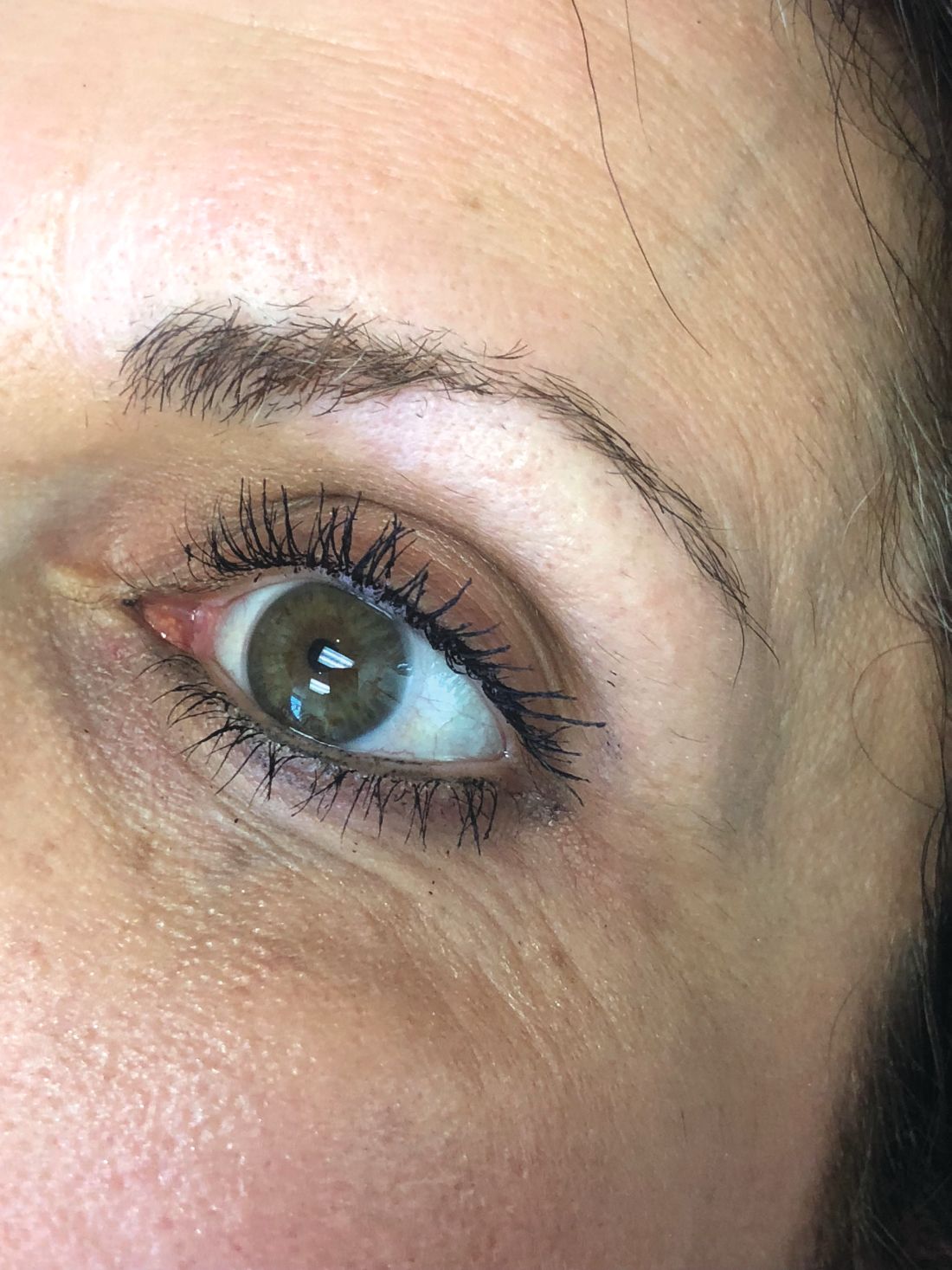
The magic of microblading
The use of permanent cosmetics dates back thousands of years in history. and has rapidly become one of the most popular cosmetic procedures in the United States. However, it has not completely replaced traditional eyebrow micropigmentation techniques: Many people may not be candidates for microblading because of how the pigment is manually deposited in the skin through tiny “tears” in the skin with this procedure.
The use of microblading has increased exponentially since 2015, as reflected by the millions of searches on popular social media sites. With the increase in the popularity and volume of tattoo artists performing these procedures, there has also been an increase in side effects and complications from microblading provided by poorly trained and unlicensed “artists,” a problem facilitated by the absence of adequate training requirements and/or regulatory oversight in many states.
Microblading is a revolutionary technique that can transform the lives of patients with hypotrichosis of the eyebrows, trichotillomania, eyebrow loss due to internal disease (such as thyroid disease), chemotherapy-induced eyebrow loss, or alopecia – or simply those seeking it for cosmetic improvement. The art of shaping the eyebrow depends on the natural growth of the brow (if any), facial symmetry, and meticulous measurement and mapping of the brow position based on facial landmarks and bone structure. The color of pigment selection is based on Fitzpatrick skin type and skin color undertones.
While dermatologists usually do not perform microblading, we may see patients with these complications. Practitioners treating patients who have had eyebrow microblading should also be aware of how to prevent premature fading of the eyebrow tattoo pigment. Tattooed eyebrows should be covered with petroleum jelly prior to the use of alpha hydroxy acids, vitamin C, chemical peels, hydroquinone, or retinols because these preparations can fade the pigment rapidly even if applied far from the microblading site. Any UV exposure, heat (such as steam from a facial), LED light exposure, or radio frequency can fade the pigment and exacerbate postinflammatory hyperpigmentation. Patients who have a history of hypertrophic scarring or keloids or are using isotretinoin concurrently should avoid microblading entirely. Resurfacing lasers and intense pulsed-light lasers should be used with caution as these aesthetic procedures will cause fading of the eyebrow pigment even if applied at a considerable distance from the eyebrow. Microbladed eyebrows should be covered with 20% zinc oxide paste prior to the use of any intense pulsed-light or resurfacing lasers.
The pigment used in eyebrow colors also may be composed of a mixture of iron oxide pigments, which should not be removed with traditional Q-switched lasers, with which not only is there potential for the pigment to darken but also postinflammatory hyper- or hypopigmentation to occur as well. Hairs can be singed, and the light absorbed by the pigment chromophore in the hair follicle can permanently damage the follicle, leading to hair loss in the area.
Despite the absolute precision and aggressive safety precautions needed for microblading, there are wide state-to-state variations in training and regulatory oversight. Infectious diseases, poor treatment outcomes, and unsterile conditions are just a few of the horrific consequences of unlicensed and untrained tattoo artists. Regulations should be imposed in every state to protect consumers and prevent serious medical complications related to microblading.
Like other cosmetic treatments, cheaper is never better.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected]. This column was written with the help and professional expertise of Emily Joy, a cosmetic tattoo artist and the founder of Dollistic in McLean.
The use of permanent cosmetics dates back thousands of years in history. and has rapidly become one of the most popular cosmetic procedures in the United States. However, it has not completely replaced traditional eyebrow micropigmentation techniques: Many people may not be candidates for microblading because of how the pigment is manually deposited in the skin through tiny “tears” in the skin with this procedure.
The use of microblading has increased exponentially since 2015, as reflected by the millions of searches on popular social media sites. With the increase in the popularity and volume of tattoo artists performing these procedures, there has also been an increase in side effects and complications from microblading provided by poorly trained and unlicensed “artists,” a problem facilitated by the absence of adequate training requirements and/or regulatory oversight in many states.
Microblading is a revolutionary technique that can transform the lives of patients with hypotrichosis of the eyebrows, trichotillomania, eyebrow loss due to internal disease (such as thyroid disease), chemotherapy-induced eyebrow loss, or alopecia – or simply those seeking it for cosmetic improvement. The art of shaping the eyebrow depends on the natural growth of the brow (if any), facial symmetry, and meticulous measurement and mapping of the brow position based on facial landmarks and bone structure. The color of pigment selection is based on Fitzpatrick skin type and skin color undertones.
While dermatologists usually do not perform microblading, we may see patients with these complications. Practitioners treating patients who have had eyebrow microblading should also be aware of how to prevent premature fading of the eyebrow tattoo pigment. Tattooed eyebrows should be covered with petroleum jelly prior to the use of alpha hydroxy acids, vitamin C, chemical peels, hydroquinone, or retinols because these preparations can fade the pigment rapidly even if applied far from the microblading site. Any UV exposure, heat (such as steam from a facial), LED light exposure, or radio frequency can fade the pigment and exacerbate postinflammatory hyperpigmentation. Patients who have a history of hypertrophic scarring or keloids or are using isotretinoin concurrently should avoid microblading entirely. Resurfacing lasers and intense pulsed-light lasers should be used with caution as these aesthetic procedures will cause fading of the eyebrow pigment even if applied at a considerable distance from the eyebrow. Microbladed eyebrows should be covered with 20% zinc oxide paste prior to the use of any intense pulsed-light or resurfacing lasers.
The pigment used in eyebrow colors also may be composed of a mixture of iron oxide pigments, which should not be removed with traditional Q-switched lasers, with which not only is there potential for the pigment to darken but also postinflammatory hyper- or hypopigmentation to occur as well. Hairs can be singed, and the light absorbed by the pigment chromophore in the hair follicle can permanently damage the follicle, leading to hair loss in the area.
Despite the absolute precision and aggressive safety precautions needed for microblading, there are wide state-to-state variations in training and regulatory oversight. Infectious diseases, poor treatment outcomes, and unsterile conditions are just a few of the horrific consequences of unlicensed and untrained tattoo artists. Regulations should be imposed in every state to protect consumers and prevent serious medical complications related to microblading.
Like other cosmetic treatments, cheaper is never better.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected]. This column was written with the help and professional expertise of Emily Joy, a cosmetic tattoo artist and the founder of Dollistic in McLean.
The use of permanent cosmetics dates back thousands of years in history. and has rapidly become one of the most popular cosmetic procedures in the United States. However, it has not completely replaced traditional eyebrow micropigmentation techniques: Many people may not be candidates for microblading because of how the pigment is manually deposited in the skin through tiny “tears” in the skin with this procedure.
The use of microblading has increased exponentially since 2015, as reflected by the millions of searches on popular social media sites. With the increase in the popularity and volume of tattoo artists performing these procedures, there has also been an increase in side effects and complications from microblading provided by poorly trained and unlicensed “artists,” a problem facilitated by the absence of adequate training requirements and/or regulatory oversight in many states.
Microblading is a revolutionary technique that can transform the lives of patients with hypotrichosis of the eyebrows, trichotillomania, eyebrow loss due to internal disease (such as thyroid disease), chemotherapy-induced eyebrow loss, or alopecia – or simply those seeking it for cosmetic improvement. The art of shaping the eyebrow depends on the natural growth of the brow (if any), facial symmetry, and meticulous measurement and mapping of the brow position based on facial landmarks and bone structure. The color of pigment selection is based on Fitzpatrick skin type and skin color undertones.
While dermatologists usually do not perform microblading, we may see patients with these complications. Practitioners treating patients who have had eyebrow microblading should also be aware of how to prevent premature fading of the eyebrow tattoo pigment. Tattooed eyebrows should be covered with petroleum jelly prior to the use of alpha hydroxy acids, vitamin C, chemical peels, hydroquinone, or retinols because these preparations can fade the pigment rapidly even if applied far from the microblading site. Any UV exposure, heat (such as steam from a facial), LED light exposure, or radio frequency can fade the pigment and exacerbate postinflammatory hyperpigmentation. Patients who have a history of hypertrophic scarring or keloids or are using isotretinoin concurrently should avoid microblading entirely. Resurfacing lasers and intense pulsed-light lasers should be used with caution as these aesthetic procedures will cause fading of the eyebrow pigment even if applied at a considerable distance from the eyebrow. Microbladed eyebrows should be covered with 20% zinc oxide paste prior to the use of any intense pulsed-light or resurfacing lasers.
The pigment used in eyebrow colors also may be composed of a mixture of iron oxide pigments, which should not be removed with traditional Q-switched lasers, with which not only is there potential for the pigment to darken but also postinflammatory hyper- or hypopigmentation to occur as well. Hairs can be singed, and the light absorbed by the pigment chromophore in the hair follicle can permanently damage the follicle, leading to hair loss in the area.
Despite the absolute precision and aggressive safety precautions needed for microblading, there are wide state-to-state variations in training and regulatory oversight. Infectious diseases, poor treatment outcomes, and unsterile conditions are just a few of the horrific consequences of unlicensed and untrained tattoo artists. Regulations should be imposed in every state to protect consumers and prevent serious medical complications related to microblading.
Like other cosmetic treatments, cheaper is never better.
Dr. Talakoub and Dr. Wesley and are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. They had no relevant disclosures. Write to them at [email protected]. This column was written with the help and professional expertise of Emily Joy, a cosmetic tattoo artist and the founder of Dollistic in McLean.
Will a cocaine epidemic follow the opioid crisis?
Ongoing efforts to understand the impact of cocaine on the brain and on behavior have gained considerable momentum over the past few decades. It was only 30 years ago when cocaine was widely considered both safe and nonaddicting – “the champagne” of drugs.1 Progress has been steady since the cocaine-dopamine depletion theory was proposed, and ultimately supported by functional and PET imaging.2,3
Additional discoveries promise further insights into the neuroscience of addiction, pleasure, and mood. While cocaine use, abuse, and dependence might seem relatively quiescent, compared with the scourge of opiate-related deaths and addiction, it remains a public health concern – and now is the second-leading cause of drug deaths. Cocaine cultivation, smuggling, use, and the number of first-time users are all escalating.4
These developments suggest that cocaine problems might get much worse and beg an important question: Will new research give us insight into better solutions?
What the neuroscience shows
As discussed, we already know quite a bit about the neuroscience behind cocaine addiction. The positron emission tomography studies conducted by Nora D. Volkow, MD, and her associates have shown long-lasting changes in abstinent cocaine addicts. Specifically, their findings clearly demonstrated that cocaine changes the brain and depletes dopamine-rich areas. Furthermore, dopamine recovery is negligible after months of abstinence.5
However, large gaps in our understanding remain. The realm of epigenetic study and protein expression behind abuse will be key in bridging our understanding of phenotype to genotype. A recent article by Eric J. Nestler, MD, PhD, and his research team, published in the Journal of Biological Psychiatry and titled “Cocaine self-administration alters transcriptome-wide responses in the brain’s reward circuitry,” offers exciting new insights (Biol Psychiatry. 2018 Apr. doi: 10.1016/jbiopsych.2018.04.009).
The study, led by Deena M. Walker, PhD, offers perhaps the most complete illumination to date of the genetic and epigenetic changes seen in the brain after cocaine self-administration and application.
Dr. Walker and her associates used a mouse model and sorted them into one of several groups. One group examined self-administered acute cocaine exposure only, with the mice immediately harvested thereafter. Two longer-term groups included one that had cocaine exposure with prolonged (30-day) withdrawal followed by context re-exposure (context re-exposure defined as being placed back in the special chamber and lighting they first received cocaine in) and another that had a cocaine exposure with prolonged withdrawal followed by both context and cocaine re-exposure.
The researchers also ran a parallel set of control groups substituting saline for cocaine with otherwise identical durations of observation and context re-exposure. Reward-related brain regions were harvested from each subject and examined with RNA-sequencing analysis to investigate the full genomic/transcriptomic profile of each. Pairwise comparison of the various experimental groups against the control groups (for example, the theoretical baseline of genetic expression in a non–cocaine-exposed brain) uncovered telling patterns, which the investigators aptly described as a comprehensive picture of transcriptome-wide change cocaine causes within the reward circuit.
The novel and creative approach used by Dr. Walker and her associates allowed them to uncover a wealth of clinically significant findings. Much could be said about their spotlighting of specific gene/protein targets for potential future pharmacological therapies toward cocaine treatment – an area that is indeed in sore need of invention. While we have highly efficacious medications for overdose and chronic treatment of opiate abuse, the landscape of treatment options for cocaine is far bleaker and shrouded in theory. With that in mind, perhaps the most salient take-home point is the evidence that cocaine, even after one exposure/withdrawal event, causes a dramatic rewiring in the very way genes are expressed across the reward circuit. The researchers found large shifts in the patterns of genetic transcription, unique and specific to discrete regions of the examined brain tissue, such as the ventral tegmental area, ventral hippocampus, and basolateral amygdala.
More interestingly, similar patterns of these genetic alternations were observed based on the exact history of the cocaine exposure. Dr. Walker and her associates concluded that the withdrawal phase and context re-exposure appear to be crucial components in the re-sculpting of the transcriptomic profile of the reward circuity.
The brain is unprepared by evolution for the reinforcing and reorganizing effects of cocaine. Clinicians, too, have learned that cocaine is addicting and can quickly replace drives such as food, water, sex, and survival. These new data from Dr. Nestler’s team reinforce the importance of prevention. In addition, they are reminders to physicians that cocaine causes changes in brain and behavior that are persistent and not necessarily reversible. Patterns of transcriptomic change are alarming enough and have only recent begun to be fleshed out, but patterns of global substance use trends suggest that we need to begin cocaine prevention activities.
Is another cocaine epidemic inevitable?
The late David F. Musto, MD, who was revered as both expert medical historian and physician at Yale University, New Haven, Conn., offered perhaps the most poignant observation in this regard: He argued that almost every opiate epidemic seems to transition into a psychostimulant epidemic.6 Experts have been looking at cocaine and methamphetamine as a way to try to understand the current opioid epidemic. Indeed, the Centers for Disease Control and Prevention’s most recent report on emerging trends in cocaine use shows numerous, concerning upticks in several realms germane to a possible emerging epidemic. One of the more upstream concerns is a gigantic spike in the shear production of coca leaves and cocaine thought to be occurring in Colombia (the principal source of cocaine in the United States). Current U.S. government estimates based on seizure rates from 2016 indicate that Colombia is producing about 910 metric tons of export quality cocaine. That represents a large increase from the 670-ton estimate the year before and the 325-ton estimate the year before that.
Similarly, a sharp rise in cocaine-related deaths, an approximate 52% increase, has been charted from 2015 to 2016. This finding is likely related to the growing presence of adulterants, such as fentanyl and carfentanil, found in seized cocaine samples. However, a rise in first-time cocaine users in the past year, which, according to the National Survey on Drug Use and Health, is up by about 12% (1.1 million people) in the 2015-2016 period, shows that the danger of cocaine-related deaths might not lie solely in adulteration but also increases in use. These signals might herald a grim return of cocaine to the center stage of public health, a development that would be an encore of the crack cocaine epidemic experienced throughout the 1980s and early 1990s.
All the above findings support cocaine as an agent of swift and massive change to our reward systems that might be poised to again surge across the United States at epidemic levels. Given this insight into just how extensively it rewires brains and the unfortunate truth that direct pharmacotherapy treatments remain mostly theoretical, it is evident that the best course of action is simply to keep cocaine from ever reaching the brain in the first place. Prevention does work, and these findings underline the importance of that message. Direct psychoeducation, awareness programs, and deterrence are the best defense we can offer to our patients at this time. In addition to these tried and true techniques, fascinating new models of prevention for cocaine abuse also are in development: vaccines. Synthesized by binding cocaine to inert proteins, these vaccines are designed to prevent addiction by training the immune system to bind cocaine and thus prevent it from crossing the blood brain barrier.7 Currently approved for clinical study in humans, these might offer a game-changing new method in the prevention of substance abuse.
In summary, continued research has enriched us with a deeper appreciation of just how profoundly cocaine, even after a single exposure, rewires the brain. Some people might have a cavalier attitude about drugs and even use terms such as experimentation to describe teen use, but cocaine is not cannabis. Not only initial cocaine self-administration, but also withdrawal and context of use (a bathroom, a bar table, a countertop) all serve to debase the natural transcriptome balance of the brain’s reward system. Our knowledge of what exactly contributes to the path of the cocaine addiction has grown, but options for how to treat cocaine overdose and addiction remain slim. This is particularly concerning, as history and data indicate a likelihood that a cocaine epidemic might come on the heels of the opiate epidemic. Now more than ever we need to emphasize the importance of preventing cocaine use – and continue to develop new interventions.
Dr. Wenzinger is a clinical fellow, PGY-4, in the department of child and adolescent psychiatry at St. Louis Children’s Hospital. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis. He also serves as chairman of the scientific advisory boards for RiverMend Health.
References
1. Yale J Biol Med. 1988 Mar-Apr;61(2):149-55.
2. Neurosci Biobehav Rev. 1985 Fall;9(3):469-77.
3. Am J Psychiatry. 1990;147(6):719-24.
4. DEA Museum. “A New Look at Old and Not So Old Drugs: A 2018 Update on Cocaine.” Drug Enforcement Administration. Retrieved from https://deamuseum.org/lecture-series/new-look-old-not-old-drugs-2018-update-cocaine.
5. J Addict Dis. 1996;15(4):55-71.
6. The American Disease: Origins of Narcotic Control (New York: Oxford University Press, 1999).
7. Br J Clin Pharmacol. 2014 Feb;77(2):368-74.
Ongoing efforts to understand the impact of cocaine on the brain and on behavior have gained considerable momentum over the past few decades. It was only 30 years ago when cocaine was widely considered both safe and nonaddicting – “the champagne” of drugs.1 Progress has been steady since the cocaine-dopamine depletion theory was proposed, and ultimately supported by functional and PET imaging.2,3
Additional discoveries promise further insights into the neuroscience of addiction, pleasure, and mood. While cocaine use, abuse, and dependence might seem relatively quiescent, compared with the scourge of opiate-related deaths and addiction, it remains a public health concern – and now is the second-leading cause of drug deaths. Cocaine cultivation, smuggling, use, and the number of first-time users are all escalating.4
These developments suggest that cocaine problems might get much worse and beg an important question: Will new research give us insight into better solutions?
What the neuroscience shows
As discussed, we already know quite a bit about the neuroscience behind cocaine addiction. The positron emission tomography studies conducted by Nora D. Volkow, MD, and her associates have shown long-lasting changes in abstinent cocaine addicts. Specifically, their findings clearly demonstrated that cocaine changes the brain and depletes dopamine-rich areas. Furthermore, dopamine recovery is negligible after months of abstinence.5
However, large gaps in our understanding remain. The realm of epigenetic study and protein expression behind abuse will be key in bridging our understanding of phenotype to genotype. A recent article by Eric J. Nestler, MD, PhD, and his research team, published in the Journal of Biological Psychiatry and titled “Cocaine self-administration alters transcriptome-wide responses in the brain’s reward circuitry,” offers exciting new insights (Biol Psychiatry. 2018 Apr. doi: 10.1016/jbiopsych.2018.04.009).
The study, led by Deena M. Walker, PhD, offers perhaps the most complete illumination to date of the genetic and epigenetic changes seen in the brain after cocaine self-administration and application.
Dr. Walker and her associates used a mouse model and sorted them into one of several groups. One group examined self-administered acute cocaine exposure only, with the mice immediately harvested thereafter. Two longer-term groups included one that had cocaine exposure with prolonged (30-day) withdrawal followed by context re-exposure (context re-exposure defined as being placed back in the special chamber and lighting they first received cocaine in) and another that had a cocaine exposure with prolonged withdrawal followed by both context and cocaine re-exposure.
The researchers also ran a parallel set of control groups substituting saline for cocaine with otherwise identical durations of observation and context re-exposure. Reward-related brain regions were harvested from each subject and examined with RNA-sequencing analysis to investigate the full genomic/transcriptomic profile of each. Pairwise comparison of the various experimental groups against the control groups (for example, the theoretical baseline of genetic expression in a non–cocaine-exposed brain) uncovered telling patterns, which the investigators aptly described as a comprehensive picture of transcriptome-wide change cocaine causes within the reward circuit.
The novel and creative approach used by Dr. Walker and her associates allowed them to uncover a wealth of clinically significant findings. Much could be said about their spotlighting of specific gene/protein targets for potential future pharmacological therapies toward cocaine treatment – an area that is indeed in sore need of invention. While we have highly efficacious medications for overdose and chronic treatment of opiate abuse, the landscape of treatment options for cocaine is far bleaker and shrouded in theory. With that in mind, perhaps the most salient take-home point is the evidence that cocaine, even after one exposure/withdrawal event, causes a dramatic rewiring in the very way genes are expressed across the reward circuit. The researchers found large shifts in the patterns of genetic transcription, unique and specific to discrete regions of the examined brain tissue, such as the ventral tegmental area, ventral hippocampus, and basolateral amygdala.
More interestingly, similar patterns of these genetic alternations were observed based on the exact history of the cocaine exposure. Dr. Walker and her associates concluded that the withdrawal phase and context re-exposure appear to be crucial components in the re-sculpting of the transcriptomic profile of the reward circuity.
The brain is unprepared by evolution for the reinforcing and reorganizing effects of cocaine. Clinicians, too, have learned that cocaine is addicting and can quickly replace drives such as food, water, sex, and survival. These new data from Dr. Nestler’s team reinforce the importance of prevention. In addition, they are reminders to physicians that cocaine causes changes in brain and behavior that are persistent and not necessarily reversible. Patterns of transcriptomic change are alarming enough and have only recent begun to be fleshed out, but patterns of global substance use trends suggest that we need to begin cocaine prevention activities.
Is another cocaine epidemic inevitable?
The late David F. Musto, MD, who was revered as both expert medical historian and physician at Yale University, New Haven, Conn., offered perhaps the most poignant observation in this regard: He argued that almost every opiate epidemic seems to transition into a psychostimulant epidemic.6 Experts have been looking at cocaine and methamphetamine as a way to try to understand the current opioid epidemic. Indeed, the Centers for Disease Control and Prevention’s most recent report on emerging trends in cocaine use shows numerous, concerning upticks in several realms germane to a possible emerging epidemic. One of the more upstream concerns is a gigantic spike in the shear production of coca leaves and cocaine thought to be occurring in Colombia (the principal source of cocaine in the United States). Current U.S. government estimates based on seizure rates from 2016 indicate that Colombia is producing about 910 metric tons of export quality cocaine. That represents a large increase from the 670-ton estimate the year before and the 325-ton estimate the year before that.
Similarly, a sharp rise in cocaine-related deaths, an approximate 52% increase, has been charted from 2015 to 2016. This finding is likely related to the growing presence of adulterants, such as fentanyl and carfentanil, found in seized cocaine samples. However, a rise in first-time cocaine users in the past year, which, according to the National Survey on Drug Use and Health, is up by about 12% (1.1 million people) in the 2015-2016 period, shows that the danger of cocaine-related deaths might not lie solely in adulteration but also increases in use. These signals might herald a grim return of cocaine to the center stage of public health, a development that would be an encore of the crack cocaine epidemic experienced throughout the 1980s and early 1990s.
All the above findings support cocaine as an agent of swift and massive change to our reward systems that might be poised to again surge across the United States at epidemic levels. Given this insight into just how extensively it rewires brains and the unfortunate truth that direct pharmacotherapy treatments remain mostly theoretical, it is evident that the best course of action is simply to keep cocaine from ever reaching the brain in the first place. Prevention does work, and these findings underline the importance of that message. Direct psychoeducation, awareness programs, and deterrence are the best defense we can offer to our patients at this time. In addition to these tried and true techniques, fascinating new models of prevention for cocaine abuse also are in development: vaccines. Synthesized by binding cocaine to inert proteins, these vaccines are designed to prevent addiction by training the immune system to bind cocaine and thus prevent it from crossing the blood brain barrier.7 Currently approved for clinical study in humans, these might offer a game-changing new method in the prevention of substance abuse.
In summary, continued research has enriched us with a deeper appreciation of just how profoundly cocaine, even after a single exposure, rewires the brain. Some people might have a cavalier attitude about drugs and even use terms such as experimentation to describe teen use, but cocaine is not cannabis. Not only initial cocaine self-administration, but also withdrawal and context of use (a bathroom, a bar table, a countertop) all serve to debase the natural transcriptome balance of the brain’s reward system. Our knowledge of what exactly contributes to the path of the cocaine addiction has grown, but options for how to treat cocaine overdose and addiction remain slim. This is particularly concerning, as history and data indicate a likelihood that a cocaine epidemic might come on the heels of the opiate epidemic. Now more than ever we need to emphasize the importance of preventing cocaine use – and continue to develop new interventions.
Dr. Wenzinger is a clinical fellow, PGY-4, in the department of child and adolescent psychiatry at St. Louis Children’s Hospital. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis. He also serves as chairman of the scientific advisory boards for RiverMend Health.
References
1. Yale J Biol Med. 1988 Mar-Apr;61(2):149-55.
2. Neurosci Biobehav Rev. 1985 Fall;9(3):469-77.
3. Am J Psychiatry. 1990;147(6):719-24.
4. DEA Museum. “A New Look at Old and Not So Old Drugs: A 2018 Update on Cocaine.” Drug Enforcement Administration. Retrieved from https://deamuseum.org/lecture-series/new-look-old-not-old-drugs-2018-update-cocaine.
5. J Addict Dis. 1996;15(4):55-71.
6. The American Disease: Origins of Narcotic Control (New York: Oxford University Press, 1999).
7. Br J Clin Pharmacol. 2014 Feb;77(2):368-74.
Ongoing efforts to understand the impact of cocaine on the brain and on behavior have gained considerable momentum over the past few decades. It was only 30 years ago when cocaine was widely considered both safe and nonaddicting – “the champagne” of drugs.1 Progress has been steady since the cocaine-dopamine depletion theory was proposed, and ultimately supported by functional and PET imaging.2,3
Additional discoveries promise further insights into the neuroscience of addiction, pleasure, and mood. While cocaine use, abuse, and dependence might seem relatively quiescent, compared with the scourge of opiate-related deaths and addiction, it remains a public health concern – and now is the second-leading cause of drug deaths. Cocaine cultivation, smuggling, use, and the number of first-time users are all escalating.4
These developments suggest that cocaine problems might get much worse and beg an important question: Will new research give us insight into better solutions?
What the neuroscience shows
As discussed, we already know quite a bit about the neuroscience behind cocaine addiction. The positron emission tomography studies conducted by Nora D. Volkow, MD, and her associates have shown long-lasting changes in abstinent cocaine addicts. Specifically, their findings clearly demonstrated that cocaine changes the brain and depletes dopamine-rich areas. Furthermore, dopamine recovery is negligible after months of abstinence.5
However, large gaps in our understanding remain. The realm of epigenetic study and protein expression behind abuse will be key in bridging our understanding of phenotype to genotype. A recent article by Eric J. Nestler, MD, PhD, and his research team, published in the Journal of Biological Psychiatry and titled “Cocaine self-administration alters transcriptome-wide responses in the brain’s reward circuitry,” offers exciting new insights (Biol Psychiatry. 2018 Apr. doi: 10.1016/jbiopsych.2018.04.009).
The study, led by Deena M. Walker, PhD, offers perhaps the most complete illumination to date of the genetic and epigenetic changes seen in the brain after cocaine self-administration and application.
Dr. Walker and her associates used a mouse model and sorted them into one of several groups. One group examined self-administered acute cocaine exposure only, with the mice immediately harvested thereafter. Two longer-term groups included one that had cocaine exposure with prolonged (30-day) withdrawal followed by context re-exposure (context re-exposure defined as being placed back in the special chamber and lighting they first received cocaine in) and another that had a cocaine exposure with prolonged withdrawal followed by both context and cocaine re-exposure.
The researchers also ran a parallel set of control groups substituting saline for cocaine with otherwise identical durations of observation and context re-exposure. Reward-related brain regions were harvested from each subject and examined with RNA-sequencing analysis to investigate the full genomic/transcriptomic profile of each. Pairwise comparison of the various experimental groups against the control groups (for example, the theoretical baseline of genetic expression in a non–cocaine-exposed brain) uncovered telling patterns, which the investigators aptly described as a comprehensive picture of transcriptome-wide change cocaine causes within the reward circuit.
The novel and creative approach used by Dr. Walker and her associates allowed them to uncover a wealth of clinically significant findings. Much could be said about their spotlighting of specific gene/protein targets for potential future pharmacological therapies toward cocaine treatment – an area that is indeed in sore need of invention. While we have highly efficacious medications for overdose and chronic treatment of opiate abuse, the landscape of treatment options for cocaine is far bleaker and shrouded in theory. With that in mind, perhaps the most salient take-home point is the evidence that cocaine, even after one exposure/withdrawal event, causes a dramatic rewiring in the very way genes are expressed across the reward circuit. The researchers found large shifts in the patterns of genetic transcription, unique and specific to discrete regions of the examined brain tissue, such as the ventral tegmental area, ventral hippocampus, and basolateral amygdala.
More interestingly, similar patterns of these genetic alternations were observed based on the exact history of the cocaine exposure. Dr. Walker and her associates concluded that the withdrawal phase and context re-exposure appear to be crucial components in the re-sculpting of the transcriptomic profile of the reward circuity.
The brain is unprepared by evolution for the reinforcing and reorganizing effects of cocaine. Clinicians, too, have learned that cocaine is addicting and can quickly replace drives such as food, water, sex, and survival. These new data from Dr. Nestler’s team reinforce the importance of prevention. In addition, they are reminders to physicians that cocaine causes changes in brain and behavior that are persistent and not necessarily reversible. Patterns of transcriptomic change are alarming enough and have only recent begun to be fleshed out, but patterns of global substance use trends suggest that we need to begin cocaine prevention activities.
Is another cocaine epidemic inevitable?
The late David F. Musto, MD, who was revered as both expert medical historian and physician at Yale University, New Haven, Conn., offered perhaps the most poignant observation in this regard: He argued that almost every opiate epidemic seems to transition into a psychostimulant epidemic.6 Experts have been looking at cocaine and methamphetamine as a way to try to understand the current opioid epidemic. Indeed, the Centers for Disease Control and Prevention’s most recent report on emerging trends in cocaine use shows numerous, concerning upticks in several realms germane to a possible emerging epidemic. One of the more upstream concerns is a gigantic spike in the shear production of coca leaves and cocaine thought to be occurring in Colombia (the principal source of cocaine in the United States). Current U.S. government estimates based on seizure rates from 2016 indicate that Colombia is producing about 910 metric tons of export quality cocaine. That represents a large increase from the 670-ton estimate the year before and the 325-ton estimate the year before that.
Similarly, a sharp rise in cocaine-related deaths, an approximate 52% increase, has been charted from 2015 to 2016. This finding is likely related to the growing presence of adulterants, such as fentanyl and carfentanil, found in seized cocaine samples. However, a rise in first-time cocaine users in the past year, which, according to the National Survey on Drug Use and Health, is up by about 12% (1.1 million people) in the 2015-2016 period, shows that the danger of cocaine-related deaths might not lie solely in adulteration but also increases in use. These signals might herald a grim return of cocaine to the center stage of public health, a development that would be an encore of the crack cocaine epidemic experienced throughout the 1980s and early 1990s.
All the above findings support cocaine as an agent of swift and massive change to our reward systems that might be poised to again surge across the United States at epidemic levels. Given this insight into just how extensively it rewires brains and the unfortunate truth that direct pharmacotherapy treatments remain mostly theoretical, it is evident that the best course of action is simply to keep cocaine from ever reaching the brain in the first place. Prevention does work, and these findings underline the importance of that message. Direct psychoeducation, awareness programs, and deterrence are the best defense we can offer to our patients at this time. In addition to these tried and true techniques, fascinating new models of prevention for cocaine abuse also are in development: vaccines. Synthesized by binding cocaine to inert proteins, these vaccines are designed to prevent addiction by training the immune system to bind cocaine and thus prevent it from crossing the blood brain barrier.7 Currently approved for clinical study in humans, these might offer a game-changing new method in the prevention of substance abuse.
In summary, continued research has enriched us with a deeper appreciation of just how profoundly cocaine, even after a single exposure, rewires the brain. Some people might have a cavalier attitude about drugs and even use terms such as experimentation to describe teen use, but cocaine is not cannabis. Not only initial cocaine self-administration, but also withdrawal and context of use (a bathroom, a bar table, a countertop) all serve to debase the natural transcriptome balance of the brain’s reward system. Our knowledge of what exactly contributes to the path of the cocaine addiction has grown, but options for how to treat cocaine overdose and addiction remain slim. This is particularly concerning, as history and data indicate a likelihood that a cocaine epidemic might come on the heels of the opiate epidemic. Now more than ever we need to emphasize the importance of preventing cocaine use – and continue to develop new interventions.
Dr. Wenzinger is a clinical fellow, PGY-4, in the department of child and adolescent psychiatry at St. Louis Children’s Hospital. Dr. Gold is the 17th Distinguished Alumni Professor at the University of Florida, Gainesville, and professor of psychiatry (adjunct) at Washington University in St. Louis. He also serves as chairman of the scientific advisory boards for RiverMend Health.
References
1. Yale J Biol Med. 1988 Mar-Apr;61(2):149-55.
2. Neurosci Biobehav Rev. 1985 Fall;9(3):469-77.
3. Am J Psychiatry. 1990;147(6):719-24.
4. DEA Museum. “A New Look at Old and Not So Old Drugs: A 2018 Update on Cocaine.” Drug Enforcement Administration. Retrieved from https://deamuseum.org/lecture-series/new-look-old-not-old-drugs-2018-update-cocaine.
5. J Addict Dis. 1996;15(4):55-71.
6. The American Disease: Origins of Narcotic Control (New York: Oxford University Press, 1999).
7. Br J Clin Pharmacol. 2014 Feb;77(2):368-74.
Is the rise of suicide a medical or societal issue?
It’s hard to ignore the recent report from the Centers for Disease Control and Prevention revealing suicide rates went up by more than 30% in half of states since 1999 (“Suicide rates rising across the U.S.,” CDC Newsroom, June 7, 2018). My professional experience with suicide is limited to one former patient who died several years after aging out of my practice. His death was not a total surprise. The cornerstones of the situation he felt he couldn’t escape were well in place when he was a freshman in high school. I’m sure there was more I could have tried to do at that early stage. Although he was anxious and mildly depressed he never admitted to being suicidal.
Just as in Ms. Roberts’ family, the CDC report has elicited a fresh round of finger pointing and introspection in our country at a time when it is already struggling to find a sense of its own identity. Is it too many guns? Or too few mental health professionals? Or a broken health delivery system?
Dr. Richard A. Friedman, a psychiatrist at Weill Cornell Medical College, writes in the New York Times that “suicide is a medical problem” and that we should declare war on it “as we’ve done with other public health threats like HIV and heart disease” (“Suicide Rates Are Rising. What Should We Do About It?” June 11, 2018). Although it may be contagious with outbreaks and clusters, particularly in the wake of celebrity suicides (“The Science Behind Suicide Contagion,” by Margot Sanger-Katz, The New York Times, Aug 13, 2014), I’m not so sure that suicide is a medical problem. Certainly, it can be spread by a vector, in this case the ubiquitous news media. With more exposure, suicide has become if not the norm, at least in certain subgroups a socially acceptable management option for an unhappy life. But while there are other features of suicide that tempt us to retreat into our comfort zone of the medical model, we need to face the more realistic and unsettling explanation that the increase in the suicide rate is the symptom of a sick society.
We already are making a mistake by interpreting the apparent rise in distractible behavior as a disease that requires medication. Let’s spread a broader net as we search for answers to the alarming suicide death statistics.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
It’s hard to ignore the recent report from the Centers for Disease Control and Prevention revealing suicide rates went up by more than 30% in half of states since 1999 (“Suicide rates rising across the U.S.,” CDC Newsroom, June 7, 2018). My professional experience with suicide is limited to one former patient who died several years after aging out of my practice. His death was not a total surprise. The cornerstones of the situation he felt he couldn’t escape were well in place when he was a freshman in high school. I’m sure there was more I could have tried to do at that early stage. Although he was anxious and mildly depressed he never admitted to being suicidal.
Just as in Ms. Roberts’ family, the CDC report has elicited a fresh round of finger pointing and introspection in our country at a time when it is already struggling to find a sense of its own identity. Is it too many guns? Or too few mental health professionals? Or a broken health delivery system?
Dr. Richard A. Friedman, a psychiatrist at Weill Cornell Medical College, writes in the New York Times that “suicide is a medical problem” and that we should declare war on it “as we’ve done with other public health threats like HIV and heart disease” (“Suicide Rates Are Rising. What Should We Do About It?” June 11, 2018). Although it may be contagious with outbreaks and clusters, particularly in the wake of celebrity suicides (“The Science Behind Suicide Contagion,” by Margot Sanger-Katz, The New York Times, Aug 13, 2014), I’m not so sure that suicide is a medical problem. Certainly, it can be spread by a vector, in this case the ubiquitous news media. With more exposure, suicide has become if not the norm, at least in certain subgroups a socially acceptable management option for an unhappy life. But while there are other features of suicide that tempt us to retreat into our comfort zone of the medical model, we need to face the more realistic and unsettling explanation that the increase in the suicide rate is the symptom of a sick society.
We already are making a mistake by interpreting the apparent rise in distractible behavior as a disease that requires medication. Let’s spread a broader net as we search for answers to the alarming suicide death statistics.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
It’s hard to ignore the recent report from the Centers for Disease Control and Prevention revealing suicide rates went up by more than 30% in half of states since 1999 (“Suicide rates rising across the U.S.,” CDC Newsroom, June 7, 2018). My professional experience with suicide is limited to one former patient who died several years after aging out of my practice. His death was not a total surprise. The cornerstones of the situation he felt he couldn’t escape were well in place when he was a freshman in high school. I’m sure there was more I could have tried to do at that early stage. Although he was anxious and mildly depressed he never admitted to being suicidal.
Just as in Ms. Roberts’ family, the CDC report has elicited a fresh round of finger pointing and introspection in our country at a time when it is already struggling to find a sense of its own identity. Is it too many guns? Or too few mental health professionals? Or a broken health delivery system?
Dr. Richard A. Friedman, a psychiatrist at Weill Cornell Medical College, writes in the New York Times that “suicide is a medical problem” and that we should declare war on it “as we’ve done with other public health threats like HIV and heart disease” (“Suicide Rates Are Rising. What Should We Do About It?” June 11, 2018). Although it may be contagious with outbreaks and clusters, particularly in the wake of celebrity suicides (“The Science Behind Suicide Contagion,” by Margot Sanger-Katz, The New York Times, Aug 13, 2014), I’m not so sure that suicide is a medical problem. Certainly, it can be spread by a vector, in this case the ubiquitous news media. With more exposure, suicide has become if not the norm, at least in certain subgroups a socially acceptable management option for an unhappy life. But while there are other features of suicide that tempt us to retreat into our comfort zone of the medical model, we need to face the more realistic and unsettling explanation that the increase in the suicide rate is the symptom of a sick society.
We already are making a mistake by interpreting the apparent rise in distractible behavior as a disease that requires medication. Let’s spread a broader net as we search for answers to the alarming suicide death statistics.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Sharpen your ax
Recently, I had a trauma call at my scenic little hospital in Maine. “Bleeding leg wound, Dr, Crosslin. We’ve got pressure on it. Come soon.” During my jog across the parking lot to the ER, I drifted into my residency mantra and started reciting the ABCs of trauma care:
Airway, Breathing, CT scan.
Airway, Breathing, C-spine collar.
Airway, Breathing, Consult with ortho.
Okay, so it’s been a while. Four years doesn’t seem like a long time, but that little span serves up a lot of change. You settle into a routine in your isolated, bucolic New England coastal town, where most trauma is related to hauling up lobster crates and having Massachusetts drivers scare the moxie out of locals in the crosswalks, and you forget about the hundreds of Level 1 traumas you managed over 5 years in Boston. The drilled-down, rapid sequence of the primary and secondary surveys gets lost, if just for a moment. Your confident swagger is replaced with a measured, humble shuffle into Trauma Bay 1. Do I scan the leg now? Did I feel for pulses in the foot? Wait, where do the major vessels branch again?
After addressing the issue at hand (or in this case, at foot), I kept thinking about the woodsman’s statement. I reflected on how I felt when I entered the trauma bay. Had I been doing enough to keep my own mental tools sharp? Well, actually, no. When did things slip just enough to allow hesitation and a bit of doubt to creep in? Probably sooner than I would care to admit. I certainly don’t think it took all of these 4 years for it to happen.
There has been some discussion of late surrounding the changes to maintenance of certification requirements from the American Board of Surgery. As with anything in surgery, we all need a chance to grumble about how things were better in the good old days. But then we grudgingly have to acknowledge that maybe – just maybe – the new approach makes some sense.
Did anyone really enjoy reporting on a 3-year cycle and taking a high-stakes, nausea-inducing exam every 10 years? I certainly wasn’t looking forward to reporting in this year about my “progress,” especially given how dull I seem to have become in so many subcategories just 4 years after graduation. But reporting every 5 years? That appeals to my inner slacker. Having a more-frequent-but-way-less-stressful examination that can be tailored to my practice? Yes, I’ll give that a shot.
It’s no secret we all are driven to care more about the things we enjoy doing, and educational science has established, quite firmly, the increased likelihood of concrete learning in higher numbers of loosely related fields when the primary subject is of particular interest to the learner. Elementary school teachers implemented that particular tidbit a long time ago. For me, the drive to excel leads me to the oncology, endocrine, and complex hernia reconstruction arenas. I do not pretend to be the world’s authority on trauma surgery, or anorectal surgery, or vascular surgery. I leave that expertise to others I secretly have judged to be far more pathological than myself. But I would be willing to glean more from reviewing those particular subjects if the overall focus is geared toward improving my knowledge and skill in cancer surgery.
In this ultramodern era, when the compendium of medical and surgical knowledge infinitely outpaces our ability to provide “one-stop shopping” services, perhaps it is time we accept the limitations of our interests and our abilities as part of the natural, beneficial evolution of good medical practice. The College’s willingness to work with the ABS to address the hot-button issue of continuing education in an interactive, relevant, timely manner should be a major point of pride. Rather than clinging to the dull ways of the past, I think we all are going to benefit from carrying a collectively sharper ax.
Dr. Crosslin is a general surgeon practicing in Rockport, Maine.
Recently, I had a trauma call at my scenic little hospital in Maine. “Bleeding leg wound, Dr, Crosslin. We’ve got pressure on it. Come soon.” During my jog across the parking lot to the ER, I drifted into my residency mantra and started reciting the ABCs of trauma care:
Airway, Breathing, CT scan.
Airway, Breathing, C-spine collar.
Airway, Breathing, Consult with ortho.
Okay, so it’s been a while. Four years doesn’t seem like a long time, but that little span serves up a lot of change. You settle into a routine in your isolated, bucolic New England coastal town, where most trauma is related to hauling up lobster crates and having Massachusetts drivers scare the moxie out of locals in the crosswalks, and you forget about the hundreds of Level 1 traumas you managed over 5 years in Boston. The drilled-down, rapid sequence of the primary and secondary surveys gets lost, if just for a moment. Your confident swagger is replaced with a measured, humble shuffle into Trauma Bay 1. Do I scan the leg now? Did I feel for pulses in the foot? Wait, where do the major vessels branch again?
After addressing the issue at hand (or in this case, at foot), I kept thinking about the woodsman’s statement. I reflected on how I felt when I entered the trauma bay. Had I been doing enough to keep my own mental tools sharp? Well, actually, no. When did things slip just enough to allow hesitation and a bit of doubt to creep in? Probably sooner than I would care to admit. I certainly don’t think it took all of these 4 years for it to happen.
There has been some discussion of late surrounding the changes to maintenance of certification requirements from the American Board of Surgery. As with anything in surgery, we all need a chance to grumble about how things were better in the good old days. But then we grudgingly have to acknowledge that maybe – just maybe – the new approach makes some sense.
Did anyone really enjoy reporting on a 3-year cycle and taking a high-stakes, nausea-inducing exam every 10 years? I certainly wasn’t looking forward to reporting in this year about my “progress,” especially given how dull I seem to have become in so many subcategories just 4 years after graduation. But reporting every 5 years? That appeals to my inner slacker. Having a more-frequent-but-way-less-stressful examination that can be tailored to my practice? Yes, I’ll give that a shot.
It’s no secret we all are driven to care more about the things we enjoy doing, and educational science has established, quite firmly, the increased likelihood of concrete learning in higher numbers of loosely related fields when the primary subject is of particular interest to the learner. Elementary school teachers implemented that particular tidbit a long time ago. For me, the drive to excel leads me to the oncology, endocrine, and complex hernia reconstruction arenas. I do not pretend to be the world’s authority on trauma surgery, or anorectal surgery, or vascular surgery. I leave that expertise to others I secretly have judged to be far more pathological than myself. But I would be willing to glean more from reviewing those particular subjects if the overall focus is geared toward improving my knowledge and skill in cancer surgery.
In this ultramodern era, when the compendium of medical and surgical knowledge infinitely outpaces our ability to provide “one-stop shopping” services, perhaps it is time we accept the limitations of our interests and our abilities as part of the natural, beneficial evolution of good medical practice. The College’s willingness to work with the ABS to address the hot-button issue of continuing education in an interactive, relevant, timely manner should be a major point of pride. Rather than clinging to the dull ways of the past, I think we all are going to benefit from carrying a collectively sharper ax.
Dr. Crosslin is a general surgeon practicing in Rockport, Maine.
Recently, I had a trauma call at my scenic little hospital in Maine. “Bleeding leg wound, Dr, Crosslin. We’ve got pressure on it. Come soon.” During my jog across the parking lot to the ER, I drifted into my residency mantra and started reciting the ABCs of trauma care:
Airway, Breathing, CT scan.
Airway, Breathing, C-spine collar.
Airway, Breathing, Consult with ortho.
Okay, so it’s been a while. Four years doesn’t seem like a long time, but that little span serves up a lot of change. You settle into a routine in your isolated, bucolic New England coastal town, where most trauma is related to hauling up lobster crates and having Massachusetts drivers scare the moxie out of locals in the crosswalks, and you forget about the hundreds of Level 1 traumas you managed over 5 years in Boston. The drilled-down, rapid sequence of the primary and secondary surveys gets lost, if just for a moment. Your confident swagger is replaced with a measured, humble shuffle into Trauma Bay 1. Do I scan the leg now? Did I feel for pulses in the foot? Wait, where do the major vessels branch again?
After addressing the issue at hand (or in this case, at foot), I kept thinking about the woodsman’s statement. I reflected on how I felt when I entered the trauma bay. Had I been doing enough to keep my own mental tools sharp? Well, actually, no. When did things slip just enough to allow hesitation and a bit of doubt to creep in? Probably sooner than I would care to admit. I certainly don’t think it took all of these 4 years for it to happen.
There has been some discussion of late surrounding the changes to maintenance of certification requirements from the American Board of Surgery. As with anything in surgery, we all need a chance to grumble about how things were better in the good old days. But then we grudgingly have to acknowledge that maybe – just maybe – the new approach makes some sense.
Did anyone really enjoy reporting on a 3-year cycle and taking a high-stakes, nausea-inducing exam every 10 years? I certainly wasn’t looking forward to reporting in this year about my “progress,” especially given how dull I seem to have become in so many subcategories just 4 years after graduation. But reporting every 5 years? That appeals to my inner slacker. Having a more-frequent-but-way-less-stressful examination that can be tailored to my practice? Yes, I’ll give that a shot.
It’s no secret we all are driven to care more about the things we enjoy doing, and educational science has established, quite firmly, the increased likelihood of concrete learning in higher numbers of loosely related fields when the primary subject is of particular interest to the learner. Elementary school teachers implemented that particular tidbit a long time ago. For me, the drive to excel leads me to the oncology, endocrine, and complex hernia reconstruction arenas. I do not pretend to be the world’s authority on trauma surgery, or anorectal surgery, or vascular surgery. I leave that expertise to others I secretly have judged to be far more pathological than myself. But I would be willing to glean more from reviewing those particular subjects if the overall focus is geared toward improving my knowledge and skill in cancer surgery.
In this ultramodern era, when the compendium of medical and surgical knowledge infinitely outpaces our ability to provide “one-stop shopping” services, perhaps it is time we accept the limitations of our interests and our abilities as part of the natural, beneficial evolution of good medical practice. The College’s willingness to work with the ABS to address the hot-button issue of continuing education in an interactive, relevant, timely manner should be a major point of pride. Rather than clinging to the dull ways of the past, I think we all are going to benefit from carrying a collectively sharper ax.
Dr. Crosslin is a general surgeon practicing in Rockport, Maine.
Breastfeeding with the FDA’s novel drugs approved in 2017, and others
The use of only one 2017 novel drug (Benznidazole) during breastfeeding has been reported. No reports describing the use of the other drugs while breastfeeding have been located. Nevertheless, exposure of a nursing infant should be considered if the mother is taking any of these drugs.
During the first 2 days after birth, nearly all drugs will be excreted into milk, but the amounts are very small and will probably have no effect on the nursing infant. After the second day, drugs with molecular weights of less than 1,000 g/mol will be excreted into milk. Some drugs with high molecular weights may also be excreted, but they may be digested in the infant’s gut. If a mother is receiving one of the drugs below and is breastfeeding, her infant should be monitored for the most common adverse effects, shown below, that were observed in nonpregnant adults.
Anti-infectives
Benznidazole (MW 260 g/mol). Abdominal pain, rash, decreased weight, headache, nausea, vomiting, neutropenia, urticaria, pruritus, eosinophilia, decreased appetite.
Delafloxacin (Baxdela) (MW 441 g/mol). Nausea, diarrhea, headache, transaminase elevations, vomiting.
Glecaprevir / Pibrentasvir (Mavyret) (MWs 839, 1,113 g/mol). Headache, fatigue.
Letermovir (Prevymis) (MW 573 g/mol). Nausea, vomiting, diarrhea, peripheral edema, cough, headache, fatigue, abdominal pain.
Meropenem / vaborbactam (Vabomere) (MWs 438, 297 g/mol). Headache, diarrhea.
Ozenoxacin cream (Xepi) (MW 363 g/mol). No relevant adverse reactions.
Sofosbuvir / Velpatasvir / Voxilaprevir (Vosevi) (MWs 529, 883, 869 g/mol). Headache, fatigue, diarrhea, nausea.
Secnidazole (Solosec) (MW 185 g/mol). Headache, nausea, dysgeusia, vomiting, diarrhea, abdominal pain. Manufacturer recommends discontinuing breastfeeding for 96 hours after administration of the drug.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, when breastfeeding.]
Abemaciclib (Verzenio) (MW 507 g/mol). Diarrhea, neutropenia, nausea, vomiting, abdominal pain, infections, fatigue, anemia, leukopenia, decreased appetite, headache, alopecia, thrombocytopenia.
Acalabrutinib (Calquence) (MW 466 g/mol). Anemia, thrombocytopenia, headache, neutropenia, diarrhea, myalgia, bruising.
Avelumab (Bavencio) (MW 147 kg/mol). Fatigue, musculoskeletal pain, diarrhea, nausea, rash, decreased appetite, peripheral edema, urinary tract infection.
Brigatinib (Alunbrig) (MW 584 g/mol). Nausea, fatigue, cough, headache.
Copanlisib (Aliqopa) (MW 480 g/mol). Hyperglycemia, diarrhea, decreased strength and energy, hypertension, leukopenia, neutropenia, nausea, lower respiratory infections, thrombocytopenia.
Durvalumab (Imfinzi) (MW 146 kg/mol). Fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, urinary tract infections, cough, upper respiratory tract infections, dyspnea, rash.
Enasidenib mesylate (Idhifa) (MW 569 g/mol). Nausea, vomiting, diarrhea, elevated bilirubin, decreased appetite.
Inotuzumab ozogamicin (Besponsa) (MW 160 kg/mol). Thrombocytopenia, neutropenia, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, increased gamma-glutamyltransferase, and hyperbilirubinemia.
Midostaurin (Rydapt) (MW 571 g/mol). Febrile neutropenia, nausea, mucositis, vomiting, headache, petechiae, musculoskeletal pain, epistaxis, hyperglycemia, vomiting, diarrhea, edema, pyrexia, dyspnea.
Neratinib (Nerlynx) (MW 557 g/mol). Diarrhea, nausea, vomiting, abdominal pain, fatigue, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, nail disorder, dry skin, abdominal distention, decreased weight, urinary tract infection.
Niraparib (Zejula) (MW 511 g/mol). Thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, vomiting, constipation, abdominal pain/distention, mucositis/stomatitis, diarrhea, dry mouth, fatigue/asthenia, decreased appetite, urinary tract infection, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, hypertension.
Ribociclib (Kisqali) (MW 553 g/mol). Neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, back pain.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046 g/mol). Thromboembolic events.
Central nervous system
Deutetrabenazine (Austedo) (MW 324 g/mol). Somnolence, diarrhea, dry mouth, fatigue, nasopharyngitis.
Edaravone (Radicava) (MW 174 g/mol). Confusion, gait disturbance, headache.
Naldemedine (Symproic) (MW 743 g/mol). Abdominal pain, diarrhea, nausea, gastroenteritis.
Ocrelizumab (Ocrevus) (MW 145 kg/mol). Upper and lower respiratory tract infections.
Safinamide (Xadago) (MW 399 g/mol). Dyskinesia, fall, nausea, insomnia.
Valbenazine (Ingrezza) (MW 419 g/mol). Somnolence.
Dermatologic
Brodalumab (Siliq) (MW 144 kg/mol). Arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, influenza, neutropenia, tinea infections.
Dupilumab (Dupixent) (MW 146.9 kg/mol). Conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye.
Guselkumab (Tremfya) (MW 143.6 kg/mol). Upper respiratory infections, headache, arthralgia, diarrhea, gastroenteritis, tinea infections, herpes simplex infections.
Endocrine / metabolic
Deflazacort (Emflaza) (MW 442 g/mol). Cushingoid appearance, weight increased, increased appetite, upper respiratory tract infection, cough, pollakiuria, hirsutism, central obesity, nasopharyngitis.
Ertugliflozin (Steglatro) (MW 566 g/mol). Female genital mycotic infections.
Etelcalcetide (Parsabiv) (MW 1,048 g/mol). Blood calcium decreased, muscle spasms, diarrhea, nausea, vomiting, headache, hypocalcemia, paresthesia.
Macimorelin (Macrilen) (MW 535 g/mol). Dysgeusia, dizziness, headache, fatigue, nausea, hunger, diarrhea, upper respiratory tract infection, feeling hot, hyperhidrosis, nasopharyngitis, sinus bradycardia.
Semaglutide (Ozempic) (MW 4,114 g/mol). Nausea, vomiting, diarrhea, abdominal pain, constipation.
Vestronidase alfa (Mepsevii) (MW 72.5 kg/mol). Diarrhea, rash, anaphylaxis, pruritus.
Gastrointestinal
Plecanatide (Trulance) (MW 1.7 kg/mol). Diarrhea.
Telotristat (Xermelo) (MW 574 g/mol). Nausea, headache, increased gamma-glutamyltransferase, depression, flatulence, decreased appetite, peripheral edema, pyrexia.
Hematologic
Betrixaban (Bevyxxa) (MW 568 g/mol). Bleeding.
Emicizumab (Hemlibra) (MW 145.6 kg/mol). Headache, arthralgia.
Immunologic
Sarilumab (Kevzara) (MW 150 kg/mol). Neutropenia, increased ALT, upper respiratory infections, urinary tract infections.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508 g/mol). All related to the eye.
Netarsudil (Rhopressa) (MW 454 g/mol). All related to the eye.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3.9 kg/mol). Hypercalciuria, dizziness, nausea, headache, palpitations, fatigue, upper abdominal pain, vertigo.
Respiratory
Benralizumab (Fasenra) (MW 150 kg/mol). Headache, pharyngitis.
The use of only one 2017 novel drug (Benznidazole) during breastfeeding has been reported. No reports describing the use of the other drugs while breastfeeding have been located. Nevertheless, exposure of a nursing infant should be considered if the mother is taking any of these drugs.
During the first 2 days after birth, nearly all drugs will be excreted into milk, but the amounts are very small and will probably have no effect on the nursing infant. After the second day, drugs with molecular weights of less than 1,000 g/mol will be excreted into milk. Some drugs with high molecular weights may also be excreted, but they may be digested in the infant’s gut. If a mother is receiving one of the drugs below and is breastfeeding, her infant should be monitored for the most common adverse effects, shown below, that were observed in nonpregnant adults.
Anti-infectives
Benznidazole (MW 260 g/mol). Abdominal pain, rash, decreased weight, headache, nausea, vomiting, neutropenia, urticaria, pruritus, eosinophilia, decreased appetite.
Delafloxacin (Baxdela) (MW 441 g/mol). Nausea, diarrhea, headache, transaminase elevations, vomiting.
Glecaprevir / Pibrentasvir (Mavyret) (MWs 839, 1,113 g/mol). Headache, fatigue.
Letermovir (Prevymis) (MW 573 g/mol). Nausea, vomiting, diarrhea, peripheral edema, cough, headache, fatigue, abdominal pain.
Meropenem / vaborbactam (Vabomere) (MWs 438, 297 g/mol). Headache, diarrhea.
Ozenoxacin cream (Xepi) (MW 363 g/mol). No relevant adverse reactions.
Sofosbuvir / Velpatasvir / Voxilaprevir (Vosevi) (MWs 529, 883, 869 g/mol). Headache, fatigue, diarrhea, nausea.
Secnidazole (Solosec) (MW 185 g/mol). Headache, nausea, dysgeusia, vomiting, diarrhea, abdominal pain. Manufacturer recommends discontinuing breastfeeding for 96 hours after administration of the drug.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, when breastfeeding.]
Abemaciclib (Verzenio) (MW 507 g/mol). Diarrhea, neutropenia, nausea, vomiting, abdominal pain, infections, fatigue, anemia, leukopenia, decreased appetite, headache, alopecia, thrombocytopenia.
Acalabrutinib (Calquence) (MW 466 g/mol). Anemia, thrombocytopenia, headache, neutropenia, diarrhea, myalgia, bruising.
Avelumab (Bavencio) (MW 147 kg/mol). Fatigue, musculoskeletal pain, diarrhea, nausea, rash, decreased appetite, peripheral edema, urinary tract infection.
Brigatinib (Alunbrig) (MW 584 g/mol). Nausea, fatigue, cough, headache.
Copanlisib (Aliqopa) (MW 480 g/mol). Hyperglycemia, diarrhea, decreased strength and energy, hypertension, leukopenia, neutropenia, nausea, lower respiratory infections, thrombocytopenia.
Durvalumab (Imfinzi) (MW 146 kg/mol). Fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, urinary tract infections, cough, upper respiratory tract infections, dyspnea, rash.
Enasidenib mesylate (Idhifa) (MW 569 g/mol). Nausea, vomiting, diarrhea, elevated bilirubin, decreased appetite.
Inotuzumab ozogamicin (Besponsa) (MW 160 kg/mol). Thrombocytopenia, neutropenia, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, increased gamma-glutamyltransferase, and hyperbilirubinemia.
Midostaurin (Rydapt) (MW 571 g/mol). Febrile neutropenia, nausea, mucositis, vomiting, headache, petechiae, musculoskeletal pain, epistaxis, hyperglycemia, vomiting, diarrhea, edema, pyrexia, dyspnea.
Neratinib (Nerlynx) (MW 557 g/mol). Diarrhea, nausea, vomiting, abdominal pain, fatigue, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, nail disorder, dry skin, abdominal distention, decreased weight, urinary tract infection.
Niraparib (Zejula) (MW 511 g/mol). Thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, vomiting, constipation, abdominal pain/distention, mucositis/stomatitis, diarrhea, dry mouth, fatigue/asthenia, decreased appetite, urinary tract infection, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, hypertension.
Ribociclib (Kisqali) (MW 553 g/mol). Neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, back pain.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046 g/mol). Thromboembolic events.
Central nervous system
Deutetrabenazine (Austedo) (MW 324 g/mol). Somnolence, diarrhea, dry mouth, fatigue, nasopharyngitis.
Edaravone (Radicava) (MW 174 g/mol). Confusion, gait disturbance, headache.
Naldemedine (Symproic) (MW 743 g/mol). Abdominal pain, diarrhea, nausea, gastroenteritis.
Ocrelizumab (Ocrevus) (MW 145 kg/mol). Upper and lower respiratory tract infections.
Safinamide (Xadago) (MW 399 g/mol). Dyskinesia, fall, nausea, insomnia.
Valbenazine (Ingrezza) (MW 419 g/mol). Somnolence.
Dermatologic
Brodalumab (Siliq) (MW 144 kg/mol). Arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, influenza, neutropenia, tinea infections.
Dupilumab (Dupixent) (MW 146.9 kg/mol). Conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye.
Guselkumab (Tremfya) (MW 143.6 kg/mol). Upper respiratory infections, headache, arthralgia, diarrhea, gastroenteritis, tinea infections, herpes simplex infections.
Endocrine / metabolic
Deflazacort (Emflaza) (MW 442 g/mol). Cushingoid appearance, weight increased, increased appetite, upper respiratory tract infection, cough, pollakiuria, hirsutism, central obesity, nasopharyngitis.
Ertugliflozin (Steglatro) (MW 566 g/mol). Female genital mycotic infections.
Etelcalcetide (Parsabiv) (MW 1,048 g/mol). Blood calcium decreased, muscle spasms, diarrhea, nausea, vomiting, headache, hypocalcemia, paresthesia.
Macimorelin (Macrilen) (MW 535 g/mol). Dysgeusia, dizziness, headache, fatigue, nausea, hunger, diarrhea, upper respiratory tract infection, feeling hot, hyperhidrosis, nasopharyngitis, sinus bradycardia.
Semaglutide (Ozempic) (MW 4,114 g/mol). Nausea, vomiting, diarrhea, abdominal pain, constipation.
Vestronidase alfa (Mepsevii) (MW 72.5 kg/mol). Diarrhea, rash, anaphylaxis, pruritus.
Gastrointestinal
Plecanatide (Trulance) (MW 1.7 kg/mol). Diarrhea.
Telotristat (Xermelo) (MW 574 g/mol). Nausea, headache, increased gamma-glutamyltransferase, depression, flatulence, decreased appetite, peripheral edema, pyrexia.
Hematologic
Betrixaban (Bevyxxa) (MW 568 g/mol). Bleeding.
Emicizumab (Hemlibra) (MW 145.6 kg/mol). Headache, arthralgia.
Immunologic
Sarilumab (Kevzara) (MW 150 kg/mol). Neutropenia, increased ALT, upper respiratory infections, urinary tract infections.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508 g/mol). All related to the eye.
Netarsudil (Rhopressa) (MW 454 g/mol). All related to the eye.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3.9 kg/mol). Hypercalciuria, dizziness, nausea, headache, palpitations, fatigue, upper abdominal pain, vertigo.
Respiratory
Benralizumab (Fasenra) (MW 150 kg/mol). Headache, pharyngitis.
The use of only one 2017 novel drug (Benznidazole) during breastfeeding has been reported. No reports describing the use of the other drugs while breastfeeding have been located. Nevertheless, exposure of a nursing infant should be considered if the mother is taking any of these drugs.
During the first 2 days after birth, nearly all drugs will be excreted into milk, but the amounts are very small and will probably have no effect on the nursing infant. After the second day, drugs with molecular weights of less than 1,000 g/mol will be excreted into milk. Some drugs with high molecular weights may also be excreted, but they may be digested in the infant’s gut. If a mother is receiving one of the drugs below and is breastfeeding, her infant should be monitored for the most common adverse effects, shown below, that were observed in nonpregnant adults.
Anti-infectives
Benznidazole (MW 260 g/mol). Abdominal pain, rash, decreased weight, headache, nausea, vomiting, neutropenia, urticaria, pruritus, eosinophilia, decreased appetite.
Delafloxacin (Baxdela) (MW 441 g/mol). Nausea, diarrhea, headache, transaminase elevations, vomiting.
Glecaprevir / Pibrentasvir (Mavyret) (MWs 839, 1,113 g/mol). Headache, fatigue.
Letermovir (Prevymis) (MW 573 g/mol). Nausea, vomiting, diarrhea, peripheral edema, cough, headache, fatigue, abdominal pain.
Meropenem / vaborbactam (Vabomere) (MWs 438, 297 g/mol). Headache, diarrhea.
Ozenoxacin cream (Xepi) (MW 363 g/mol). No relevant adverse reactions.
Sofosbuvir / Velpatasvir / Voxilaprevir (Vosevi) (MWs 529, 883, 869 g/mol). Headache, fatigue, diarrhea, nausea.
Secnidazole (Solosec) (MW 185 g/mol). Headache, nausea, dysgeusia, vomiting, diarrhea, abdominal pain. Manufacturer recommends discontinuing breastfeeding for 96 hours after administration of the drug.
Antineoplastics
[Note: All of the drugs in this category are best avoided, if possible, when breastfeeding.]
Abemaciclib (Verzenio) (MW 507 g/mol). Diarrhea, neutropenia, nausea, vomiting, abdominal pain, infections, fatigue, anemia, leukopenia, decreased appetite, headache, alopecia, thrombocytopenia.
Acalabrutinib (Calquence) (MW 466 g/mol). Anemia, thrombocytopenia, headache, neutropenia, diarrhea, myalgia, bruising.
Avelumab (Bavencio) (MW 147 kg/mol). Fatigue, musculoskeletal pain, diarrhea, nausea, rash, decreased appetite, peripheral edema, urinary tract infection.
Brigatinib (Alunbrig) (MW 584 g/mol). Nausea, fatigue, cough, headache.
Copanlisib (Aliqopa) (MW 480 g/mol). Hyperglycemia, diarrhea, decreased strength and energy, hypertension, leukopenia, neutropenia, nausea, lower respiratory infections, thrombocytopenia.
Durvalumab (Imfinzi) (MW 146 kg/mol). Fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, urinary tract infections, cough, upper respiratory tract infections, dyspnea, rash.
Enasidenib mesylate (Idhifa) (MW 569 g/mol). Nausea, vomiting, diarrhea, elevated bilirubin, decreased appetite.
Inotuzumab ozogamicin (Besponsa) (MW 160 kg/mol). Thrombocytopenia, neutropenia, anemia, leukopenia, fatigue, hemorrhage, pyrexia, nausea, headache, febrile neutropenia, transaminases increased, abdominal pain, increased gamma-glutamyltransferase, and hyperbilirubinemia.
Midostaurin (Rydapt) (MW 571 g/mol). Febrile neutropenia, nausea, mucositis, vomiting, headache, petechiae, musculoskeletal pain, epistaxis, hyperglycemia, vomiting, diarrhea, edema, pyrexia, dyspnea.
Neratinib (Nerlynx) (MW 557 g/mol). Diarrhea, nausea, vomiting, abdominal pain, fatigue, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, nail disorder, dry skin, abdominal distention, decreased weight, urinary tract infection.
Niraparib (Zejula) (MW 511 g/mol). Thrombocytopenia, anemia, neutropenia, leukopenia, palpitations, nausea, vomiting, constipation, abdominal pain/distention, mucositis/stomatitis, diarrhea, dry mouth, fatigue/asthenia, decreased appetite, urinary tract infection, myalgia, back pain, arthralgia, headache, dizziness, dysgeusia, insomnia, anxiety, nasopharyngitis, dyspnea, cough, rash, hypertension.
Ribociclib (Kisqali) (MW 553 g/mol). Neutropenia, nausea, fatigue, diarrhea, leukopenia, alopecia, vomiting, constipation, headache, back pain.
Cardiovascular
Angiotensin II (Giapreza) (MW 1,046 g/mol). Thromboembolic events.
Central nervous system
Deutetrabenazine (Austedo) (MW 324 g/mol). Somnolence, diarrhea, dry mouth, fatigue, nasopharyngitis.
Edaravone (Radicava) (MW 174 g/mol). Confusion, gait disturbance, headache.
Naldemedine (Symproic) (MW 743 g/mol). Abdominal pain, diarrhea, nausea, gastroenteritis.
Ocrelizumab (Ocrevus) (MW 145 kg/mol). Upper and lower respiratory tract infections.
Safinamide (Xadago) (MW 399 g/mol). Dyskinesia, fall, nausea, insomnia.
Valbenazine (Ingrezza) (MW 419 g/mol). Somnolence.
Dermatologic
Brodalumab (Siliq) (MW 144 kg/mol). Arthralgia, headache, fatigue, diarrhea, oropharyngeal pain, nausea, myalgia, influenza, neutropenia, tinea infections.
Dupilumab (Dupixent) (MW 146.9 kg/mol). Conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye.
Guselkumab (Tremfya) (MW 143.6 kg/mol). Upper respiratory infections, headache, arthralgia, diarrhea, gastroenteritis, tinea infections, herpes simplex infections.
Endocrine / metabolic
Deflazacort (Emflaza) (MW 442 g/mol). Cushingoid appearance, weight increased, increased appetite, upper respiratory tract infection, cough, pollakiuria, hirsutism, central obesity, nasopharyngitis.
Ertugliflozin (Steglatro) (MW 566 g/mol). Female genital mycotic infections.
Etelcalcetide (Parsabiv) (MW 1,048 g/mol). Blood calcium decreased, muscle spasms, diarrhea, nausea, vomiting, headache, hypocalcemia, paresthesia.
Macimorelin (Macrilen) (MW 535 g/mol). Dysgeusia, dizziness, headache, fatigue, nausea, hunger, diarrhea, upper respiratory tract infection, feeling hot, hyperhidrosis, nasopharyngitis, sinus bradycardia.
Semaglutide (Ozempic) (MW 4,114 g/mol). Nausea, vomiting, diarrhea, abdominal pain, constipation.
Vestronidase alfa (Mepsevii) (MW 72.5 kg/mol). Diarrhea, rash, anaphylaxis, pruritus.
Gastrointestinal
Plecanatide (Trulance) (MW 1.7 kg/mol). Diarrhea.
Telotristat (Xermelo) (MW 574 g/mol). Nausea, headache, increased gamma-glutamyltransferase, depression, flatulence, decreased appetite, peripheral edema, pyrexia.
Hematologic
Betrixaban (Bevyxxa) (MW 568 g/mol). Bleeding.
Emicizumab (Hemlibra) (MW 145.6 kg/mol). Headache, arthralgia.
Immunologic
Sarilumab (Kevzara) (MW 150 kg/mol). Neutropenia, increased ALT, upper respiratory infections, urinary tract infections.
Ophthalmic
Latanoprostene bunod (Vyzulta) (MW 508 g/mol). All related to the eye.
Netarsudil (Rhopressa) (MW 454 g/mol). All related to the eye.
Parathyroid hormone
Abaloparatide (Tymlos) (MW 3.9 kg/mol). Hypercalciuria, dizziness, nausea, headache, palpitations, fatigue, upper abdominal pain, vertigo.
Respiratory
Benralizumab (Fasenra) (MW 150 kg/mol). Headache, pharyngitis.