User login
The dubious value of online reviews
I hear other doctors talk about online reviews, both good and bad.
I recently read a piece where a practice gave doctors a bonus for getting 5-star reviews, though it doesn’t say if they were penalized for getting bad reviews. I assume the latter docs got a good “talking to” by someone in administration, or marketing, or both.
I get my share of them, too, both good and bad, scattered across at least a dozen sites that profess to offer accurate ratings.
I tend to ignore all of them.
Bad ratings mean nothing. They might be reasonable. They can also be from patients whom I fired for noncompliance, or from patients I refused to give an early narcotic refill to. They can also be from people who aren’t patients, such as a neighbor angry at the way I voted at a home owners association meeting, or a person who never saw me but was upset because I don’t take their insurance, or someone at the hospital whom I had to hang up on after being put on hold for 10 minutes.
Good reviews also don’t mean much, either. They might be from patients. They could also be from well-meaning family and friends. Or the waiter I left an extra-large tip for the other night.
One of my 1-star reviews even goes on to describe me in glowing terms (the lady called my office to apologize, saying the site confused her).
There’s also a whole cottage industry around this: Like restaurants, you can pay people to give you good reviews. They’re on Craig’s list and other sites. Some are freelancers. Others are actually well-organized companies, offering to give you X number of good reviews per month for a regular fee. I see ads for the latter online, usually describing themselves as “reputation recovery services.”
There was even a recent post on Sermo about this. A doctor noted he’d gotten a string of bad reviews from nonpatients, and shortly afterward was contacted by a reputation recovery service to help. He wondered if the crappy reviews were intentionally written by that business before they called him. He also questioned if it was an unspoken blackmail tactic – pay us or we’ll write more bad reviews.
Unlike a restaurant, we can’t respond because of patient confidentiality. Unless it’s something meaninglessly generic like “thank you” or “sorry you had a bad experience.”
A friend of mine (not in medicine) said that picking your doctor from online reviews is like selecting a wine recommended by a guy who lives at the train yard.
While there are pros and cons to the whole online review thing, in medicine there are mostly cons. Many reviews are anonymous, with no way to trace them. Unless details are provided, you don’t know if the reviewer is really a patient (or even a human in this bot era). Neither does the general public, reading them and presumably making decisions about who to see.
There are minimal (if any) rules, no law enforcement, and no one knows who the good guys and bad guys really are.
And there’s nothing we can do about it, either.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I hear other doctors talk about online reviews, both good and bad.
I recently read a piece where a practice gave doctors a bonus for getting 5-star reviews, though it doesn’t say if they were penalized for getting bad reviews. I assume the latter docs got a good “talking to” by someone in administration, or marketing, or both.
I get my share of them, too, both good and bad, scattered across at least a dozen sites that profess to offer accurate ratings.
I tend to ignore all of them.
Bad ratings mean nothing. They might be reasonable. They can also be from patients whom I fired for noncompliance, or from patients I refused to give an early narcotic refill to. They can also be from people who aren’t patients, such as a neighbor angry at the way I voted at a home owners association meeting, or a person who never saw me but was upset because I don’t take their insurance, or someone at the hospital whom I had to hang up on after being put on hold for 10 minutes.
Good reviews also don’t mean much, either. They might be from patients. They could also be from well-meaning family and friends. Or the waiter I left an extra-large tip for the other night.
One of my 1-star reviews even goes on to describe me in glowing terms (the lady called my office to apologize, saying the site confused her).
There’s also a whole cottage industry around this: Like restaurants, you can pay people to give you good reviews. They’re on Craig’s list and other sites. Some are freelancers. Others are actually well-organized companies, offering to give you X number of good reviews per month for a regular fee. I see ads for the latter online, usually describing themselves as “reputation recovery services.”
There was even a recent post on Sermo about this. A doctor noted he’d gotten a string of bad reviews from nonpatients, and shortly afterward was contacted by a reputation recovery service to help. He wondered if the crappy reviews were intentionally written by that business before they called him. He also questioned if it was an unspoken blackmail tactic – pay us or we’ll write more bad reviews.
Unlike a restaurant, we can’t respond because of patient confidentiality. Unless it’s something meaninglessly generic like “thank you” or “sorry you had a bad experience.”
A friend of mine (not in medicine) said that picking your doctor from online reviews is like selecting a wine recommended by a guy who lives at the train yard.
While there are pros and cons to the whole online review thing, in medicine there are mostly cons. Many reviews are anonymous, with no way to trace them. Unless details are provided, you don’t know if the reviewer is really a patient (or even a human in this bot era). Neither does the general public, reading them and presumably making decisions about who to see.
There are minimal (if any) rules, no law enforcement, and no one knows who the good guys and bad guys really are.
And there’s nothing we can do about it, either.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I hear other doctors talk about online reviews, both good and bad.
I recently read a piece where a practice gave doctors a bonus for getting 5-star reviews, though it doesn’t say if they were penalized for getting bad reviews. I assume the latter docs got a good “talking to” by someone in administration, or marketing, or both.
I get my share of them, too, both good and bad, scattered across at least a dozen sites that profess to offer accurate ratings.
I tend to ignore all of them.
Bad ratings mean nothing. They might be reasonable. They can also be from patients whom I fired for noncompliance, or from patients I refused to give an early narcotic refill to. They can also be from people who aren’t patients, such as a neighbor angry at the way I voted at a home owners association meeting, or a person who never saw me but was upset because I don’t take their insurance, or someone at the hospital whom I had to hang up on after being put on hold for 10 minutes.
Good reviews also don’t mean much, either. They might be from patients. They could also be from well-meaning family and friends. Or the waiter I left an extra-large tip for the other night.
One of my 1-star reviews even goes on to describe me in glowing terms (the lady called my office to apologize, saying the site confused her).
There’s also a whole cottage industry around this: Like restaurants, you can pay people to give you good reviews. They’re on Craig’s list and other sites. Some are freelancers. Others are actually well-organized companies, offering to give you X number of good reviews per month for a regular fee. I see ads for the latter online, usually describing themselves as “reputation recovery services.”
There was even a recent post on Sermo about this. A doctor noted he’d gotten a string of bad reviews from nonpatients, and shortly afterward was contacted by a reputation recovery service to help. He wondered if the crappy reviews were intentionally written by that business before they called him. He also questioned if it was an unspoken blackmail tactic – pay us or we’ll write more bad reviews.
Unlike a restaurant, we can’t respond because of patient confidentiality. Unless it’s something meaninglessly generic like “thank you” or “sorry you had a bad experience.”
A friend of mine (not in medicine) said that picking your doctor from online reviews is like selecting a wine recommended by a guy who lives at the train yard.
While there are pros and cons to the whole online review thing, in medicine there are mostly cons. Many reviews are anonymous, with no way to trace them. Unless details are provided, you don’t know if the reviewer is really a patient (or even a human in this bot era). Neither does the general public, reading them and presumably making decisions about who to see.
There are minimal (if any) rules, no law enforcement, and no one knows who the good guys and bad guys really are.
And there’s nothing we can do about it, either.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Cancer as a full contact sport
John worked as a handyman and lived on a small sailboat in a marina. When he was diagnosed with metastatic kidney cancer at age 48, he quickly fell through the cracks. He failed to show to appointments and took oral anticancer treatments, but just sporadically. He had Medicaid, so insurance wasn’t the issue. It was everything else.
John was behind on his slip fees; he hadn’t been able to work for some time because of his progressive weakness and pain. He was chronically in danger of getting kicked out of his makeshift home aboard the boat. He had no reliable transportation to the clinic and so he didn’t come to appointments regularly. The specialty pharmacy refused to deliver his expensive oral chemotherapy to his address at the marina. He went days without eating full meals because he was too weak to cook for himself. Plus, he was estranged from his family who were unaware of his illness. His oncologist was overwhelmed trying to take care of him. He had a reasonable chance of achieving disease control on first-line oral therapy, but his problems seemed to hinder these chances at every turn. She was distraught – what could she do?
Enter the team approach. John’s oncologist reached out to our palliative care program for help. We recognized that this was a job too big for us alone so we connected John with the Extensivist Medicine program at UCLA Health, a high-intensity primary care program led by a physician specializing in primary care for high-risk individuals. The program provides wraparound outpatient services for chronically and seriously ill patients, like John, who are at risk for falling through the cracks. John went from receiving very little support to now having an entire team of caring professionals focused on helping him achieve his best possible outcome despite the seriousness of his disease.
He now had the support of a high-functioning team with clearly defined roles. Social work connected him with housing, food, and transportation resources. A nurse called him every day to check in and make sure he was taking medications and reminded him about his upcoming appointments. Case management helped him get needed equipment, such as grab bars and a walker. As his palliative care nurse practitioner, I counseled him on understanding his prognosis and planning ahead for medical emergencies. Our psycho-oncology clinicians helped John reconcile with his family, who were more than willing to take him in once they realized how ill he was. Once these social factors were addressed, John could more easily stay current with his oral chemotherapy, giving him the best chance possible to achieve a robust treatment response that could buy him more time.
And, John did get that time – he got 6 months of improved quality of life, during which he reconnected with his family, including his children, and rebuilt these important relationships. Eventually treatment failed him. His disease, already widely metastatic, became more active and painful. He accepted hospice care at his sister’s house and we transitioned him from our team to the hospice team. He died peacefully surrounded by family.
Interprofessional teamwork is fundamental to treat ‘total pain’
None of this would have been possible without the work of high-functioning teams. It is a commonly held belief that interprofessional teamwork is fundamental to the care of patients and families living with serious illness. But why? How did this idea come about? And what evidence is there to support teamwork?
Dame Cicely Saunders, who founded the modern hospice movement in mid-20th century England, embodied the interdisciplinary team by working first as a nurse, then a social worker, and finally as a physician. She wrote about patients’ “total pain,” the crisis of physical, spiritual, social, and emotional distress that many people have at the end of life. She understood that no single health care discipline was adequate to the task of addressing each of these domains equally well. Thus, hospice became synonymous with care provided by a quartet of specialists – physicians, nurses, social workers, and chaplains. Nowadays, there are other specialists that are added to the mix – home health aides, pharmacists, physical and occupational therapists, music and pet therapists, and so on.
But in medicine, like all areas of science, convention and tradition only go so far. What evidence is there to support the work of an interdisciplinary team in managing the distress of patients and families living with advanced illnesses? It turns out that there is good evidence to support the use of high-functioning interdisciplinary teams in the care of the seriously ill. Palliative care is associated with improved patient outcomes, including improvements in symptom control, quality of life, and end of life care, when it is delivered by an interdisciplinary team rather than by a solo practitioner.
You may think that teamwork is most useful for patients like John who have seemingly intractable social barriers. But it is also true that for even patients with many more social advantages teamwork improves quality of life. I got to see this up close recently in my own life.
Teamwork improves quality of life
My father recently passed away after a 9-month battle with advanced cancer. He had every advantage possible – financial stability, high health literacy, an incredibly devoted spouse who happens to be an RN, good insurance, and access to top-notch medical care. Yet, even he benefited from a team approach. It started small, with the oncologist and oncology NP providing excellent, patient-centered care. Then it grew to include myself as the daughter/palliative care nurse practitioner who made recommendations for treating his nausea and ensured that his advance directive was completed and uploaded to his chart. When my dad needed physical therapy, the home health agency sent a wonderful physical therapist, who brought all sorts of equipment that kept him more functional than he would have been otherwise. Other family members helped out – my sisters helped connect my dad with a priest who came to the home to provide spiritual care, which was crucial to ensuring that he was at peace. And, in his final days, my dad had the hospice team to help manage his symptoms and his family members to provide hands-on care.
The complexity of cancer care has long necessitated a team approach to planning cancer treatment – known as a tumor board – with medical oncology, radiation oncology, surgery, and pathology all weighing in. It makes sense that patients and their families would also need a team of clinicians representing different specialty areas to assist with the wide array of physical, psychosocial, practical, and spiritual concerns that arise throughout the cancer disease trajectory.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
John worked as a handyman and lived on a small sailboat in a marina. When he was diagnosed with metastatic kidney cancer at age 48, he quickly fell through the cracks. He failed to show to appointments and took oral anticancer treatments, but just sporadically. He had Medicaid, so insurance wasn’t the issue. It was everything else.
John was behind on his slip fees; he hadn’t been able to work for some time because of his progressive weakness and pain. He was chronically in danger of getting kicked out of his makeshift home aboard the boat. He had no reliable transportation to the clinic and so he didn’t come to appointments regularly. The specialty pharmacy refused to deliver his expensive oral chemotherapy to his address at the marina. He went days without eating full meals because he was too weak to cook for himself. Plus, he was estranged from his family who were unaware of his illness. His oncologist was overwhelmed trying to take care of him. He had a reasonable chance of achieving disease control on first-line oral therapy, but his problems seemed to hinder these chances at every turn. She was distraught – what could she do?
Enter the team approach. John’s oncologist reached out to our palliative care program for help. We recognized that this was a job too big for us alone so we connected John with the Extensivist Medicine program at UCLA Health, a high-intensity primary care program led by a physician specializing in primary care for high-risk individuals. The program provides wraparound outpatient services for chronically and seriously ill patients, like John, who are at risk for falling through the cracks. John went from receiving very little support to now having an entire team of caring professionals focused on helping him achieve his best possible outcome despite the seriousness of his disease.
He now had the support of a high-functioning team with clearly defined roles. Social work connected him with housing, food, and transportation resources. A nurse called him every day to check in and make sure he was taking medications and reminded him about his upcoming appointments. Case management helped him get needed equipment, such as grab bars and a walker. As his palliative care nurse practitioner, I counseled him on understanding his prognosis and planning ahead for medical emergencies. Our psycho-oncology clinicians helped John reconcile with his family, who were more than willing to take him in once they realized how ill he was. Once these social factors were addressed, John could more easily stay current with his oral chemotherapy, giving him the best chance possible to achieve a robust treatment response that could buy him more time.
And, John did get that time – he got 6 months of improved quality of life, during which he reconnected with his family, including his children, and rebuilt these important relationships. Eventually treatment failed him. His disease, already widely metastatic, became more active and painful. He accepted hospice care at his sister’s house and we transitioned him from our team to the hospice team. He died peacefully surrounded by family.
Interprofessional teamwork is fundamental to treat ‘total pain’
None of this would have been possible without the work of high-functioning teams. It is a commonly held belief that interprofessional teamwork is fundamental to the care of patients and families living with serious illness. But why? How did this idea come about? And what evidence is there to support teamwork?
Dame Cicely Saunders, who founded the modern hospice movement in mid-20th century England, embodied the interdisciplinary team by working first as a nurse, then a social worker, and finally as a physician. She wrote about patients’ “total pain,” the crisis of physical, spiritual, social, and emotional distress that many people have at the end of life. She understood that no single health care discipline was adequate to the task of addressing each of these domains equally well. Thus, hospice became synonymous with care provided by a quartet of specialists – physicians, nurses, social workers, and chaplains. Nowadays, there are other specialists that are added to the mix – home health aides, pharmacists, physical and occupational therapists, music and pet therapists, and so on.
But in medicine, like all areas of science, convention and tradition only go so far. What evidence is there to support the work of an interdisciplinary team in managing the distress of patients and families living with advanced illnesses? It turns out that there is good evidence to support the use of high-functioning interdisciplinary teams in the care of the seriously ill. Palliative care is associated with improved patient outcomes, including improvements in symptom control, quality of life, and end of life care, when it is delivered by an interdisciplinary team rather than by a solo practitioner.
You may think that teamwork is most useful for patients like John who have seemingly intractable social barriers. But it is also true that for even patients with many more social advantages teamwork improves quality of life. I got to see this up close recently in my own life.
Teamwork improves quality of life
My father recently passed away after a 9-month battle with advanced cancer. He had every advantage possible – financial stability, high health literacy, an incredibly devoted spouse who happens to be an RN, good insurance, and access to top-notch medical care. Yet, even he benefited from a team approach. It started small, with the oncologist and oncology NP providing excellent, patient-centered care. Then it grew to include myself as the daughter/palliative care nurse practitioner who made recommendations for treating his nausea and ensured that his advance directive was completed and uploaded to his chart. When my dad needed physical therapy, the home health agency sent a wonderful physical therapist, who brought all sorts of equipment that kept him more functional than he would have been otherwise. Other family members helped out – my sisters helped connect my dad with a priest who came to the home to provide spiritual care, which was crucial to ensuring that he was at peace. And, in his final days, my dad had the hospice team to help manage his symptoms and his family members to provide hands-on care.
The complexity of cancer care has long necessitated a team approach to planning cancer treatment – known as a tumor board – with medical oncology, radiation oncology, surgery, and pathology all weighing in. It makes sense that patients and their families would also need a team of clinicians representing different specialty areas to assist with the wide array of physical, psychosocial, practical, and spiritual concerns that arise throughout the cancer disease trajectory.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
John worked as a handyman and lived on a small sailboat in a marina. When he was diagnosed with metastatic kidney cancer at age 48, he quickly fell through the cracks. He failed to show to appointments and took oral anticancer treatments, but just sporadically. He had Medicaid, so insurance wasn’t the issue. It was everything else.
John was behind on his slip fees; he hadn’t been able to work for some time because of his progressive weakness and pain. He was chronically in danger of getting kicked out of his makeshift home aboard the boat. He had no reliable transportation to the clinic and so he didn’t come to appointments regularly. The specialty pharmacy refused to deliver his expensive oral chemotherapy to his address at the marina. He went days without eating full meals because he was too weak to cook for himself. Plus, he was estranged from his family who were unaware of his illness. His oncologist was overwhelmed trying to take care of him. He had a reasonable chance of achieving disease control on first-line oral therapy, but his problems seemed to hinder these chances at every turn. She was distraught – what could she do?
Enter the team approach. John’s oncologist reached out to our palliative care program for help. We recognized that this was a job too big for us alone so we connected John with the Extensivist Medicine program at UCLA Health, a high-intensity primary care program led by a physician specializing in primary care for high-risk individuals. The program provides wraparound outpatient services for chronically and seriously ill patients, like John, who are at risk for falling through the cracks. John went from receiving very little support to now having an entire team of caring professionals focused on helping him achieve his best possible outcome despite the seriousness of his disease.
He now had the support of a high-functioning team with clearly defined roles. Social work connected him with housing, food, and transportation resources. A nurse called him every day to check in and make sure he was taking medications and reminded him about his upcoming appointments. Case management helped him get needed equipment, such as grab bars and a walker. As his palliative care nurse practitioner, I counseled him on understanding his prognosis and planning ahead for medical emergencies. Our psycho-oncology clinicians helped John reconcile with his family, who were more than willing to take him in once they realized how ill he was. Once these social factors were addressed, John could more easily stay current with his oral chemotherapy, giving him the best chance possible to achieve a robust treatment response that could buy him more time.
And, John did get that time – he got 6 months of improved quality of life, during which he reconnected with his family, including his children, and rebuilt these important relationships. Eventually treatment failed him. His disease, already widely metastatic, became more active and painful. He accepted hospice care at his sister’s house and we transitioned him from our team to the hospice team. He died peacefully surrounded by family.
Interprofessional teamwork is fundamental to treat ‘total pain’
None of this would have been possible without the work of high-functioning teams. It is a commonly held belief that interprofessional teamwork is fundamental to the care of patients and families living with serious illness. But why? How did this idea come about? And what evidence is there to support teamwork?
Dame Cicely Saunders, who founded the modern hospice movement in mid-20th century England, embodied the interdisciplinary team by working first as a nurse, then a social worker, and finally as a physician. She wrote about patients’ “total pain,” the crisis of physical, spiritual, social, and emotional distress that many people have at the end of life. She understood that no single health care discipline was adequate to the task of addressing each of these domains equally well. Thus, hospice became synonymous with care provided by a quartet of specialists – physicians, nurses, social workers, and chaplains. Nowadays, there are other specialists that are added to the mix – home health aides, pharmacists, physical and occupational therapists, music and pet therapists, and so on.
But in medicine, like all areas of science, convention and tradition only go so far. What evidence is there to support the work of an interdisciplinary team in managing the distress of patients and families living with advanced illnesses? It turns out that there is good evidence to support the use of high-functioning interdisciplinary teams in the care of the seriously ill. Palliative care is associated with improved patient outcomes, including improvements in symptom control, quality of life, and end of life care, when it is delivered by an interdisciplinary team rather than by a solo practitioner.
You may think that teamwork is most useful for patients like John who have seemingly intractable social barriers. But it is also true that for even patients with many more social advantages teamwork improves quality of life. I got to see this up close recently in my own life.
Teamwork improves quality of life
My father recently passed away after a 9-month battle with advanced cancer. He had every advantage possible – financial stability, high health literacy, an incredibly devoted spouse who happens to be an RN, good insurance, and access to top-notch medical care. Yet, even he benefited from a team approach. It started small, with the oncologist and oncology NP providing excellent, patient-centered care. Then it grew to include myself as the daughter/palliative care nurse practitioner who made recommendations for treating his nausea and ensured that his advance directive was completed and uploaded to his chart. When my dad needed physical therapy, the home health agency sent a wonderful physical therapist, who brought all sorts of equipment that kept him more functional than he would have been otherwise. Other family members helped out – my sisters helped connect my dad with a priest who came to the home to provide spiritual care, which was crucial to ensuring that he was at peace. And, in his final days, my dad had the hospice team to help manage his symptoms and his family members to provide hands-on care.
The complexity of cancer care has long necessitated a team approach to planning cancer treatment – known as a tumor board – with medical oncology, radiation oncology, surgery, and pathology all weighing in. It makes sense that patients and their families would also need a team of clinicians representing different specialty areas to assist with the wide array of physical, psychosocial, practical, and spiritual concerns that arise throughout the cancer disease trajectory.
Ms. D’Ambruoso is a hospice and palliative care nurse practitioner for UCLA Health Cancer Care, Santa Monica, Calif.
Then and now: Endoscopy
In the second issue of GI & Hepatology News in February 2007, an article reviewed the disruptive forces to colonoscopy including CT colonography and the colon capsule. The article stated that “colonoscopy is still the preferred method, but the emerging technology could catch up in 3-5 years.”
While this prediction did not come to pass, the field of endoscopy has evolved in remarkable ways over the last 15 years. From the development of high-definition endoscopes to the transformation of interventional endoscopy to include “third space” procedures, previously unimaginable techniques have now become commonplace. This transformation has changed the nature and training of our field and, even more importantly, dramatically improved the care of our patients.
Just as notably, the regulatory and practice environment for endoscopy has also changed in the last 15 years, albeit at a slower pace. In January of 2007, as the first issue of GI & Hepatology News came out, Medicare announced that it would cover all screening procedures without a copay but left a loophole that charged patients if their screening colonoscopy became therapeutic. That loophole was finally fixed this year as GI & Hepatology News celebrates its 15-year anniversary.
If the past 15 years are any indication, endoscopy practice will continue to change at a humbling pace over the next 15 years. I look forward to seeing those changes unfold through the pages of GI & Hepatology News.
Dr. Gellad is associate professor of medicine and associate vice chair of ambulatory services at Duke University Medical Center, Durham, N.C. He is also a staff physician with the Durham VA Health Care system. He disclosed ties with Merck, Novo Nordisk, and Higgs Boson Health.
In the second issue of GI & Hepatology News in February 2007, an article reviewed the disruptive forces to colonoscopy including CT colonography and the colon capsule. The article stated that “colonoscopy is still the preferred method, but the emerging technology could catch up in 3-5 years.”
While this prediction did not come to pass, the field of endoscopy has evolved in remarkable ways over the last 15 years. From the development of high-definition endoscopes to the transformation of interventional endoscopy to include “third space” procedures, previously unimaginable techniques have now become commonplace. This transformation has changed the nature and training of our field and, even more importantly, dramatically improved the care of our patients.
Just as notably, the regulatory and practice environment for endoscopy has also changed in the last 15 years, albeit at a slower pace. In January of 2007, as the first issue of GI & Hepatology News came out, Medicare announced that it would cover all screening procedures without a copay but left a loophole that charged patients if their screening colonoscopy became therapeutic. That loophole was finally fixed this year as GI & Hepatology News celebrates its 15-year anniversary.
If the past 15 years are any indication, endoscopy practice will continue to change at a humbling pace over the next 15 years. I look forward to seeing those changes unfold through the pages of GI & Hepatology News.
Dr. Gellad is associate professor of medicine and associate vice chair of ambulatory services at Duke University Medical Center, Durham, N.C. He is also a staff physician with the Durham VA Health Care system. He disclosed ties with Merck, Novo Nordisk, and Higgs Boson Health.
In the second issue of GI & Hepatology News in February 2007, an article reviewed the disruptive forces to colonoscopy including CT colonography and the colon capsule. The article stated that “colonoscopy is still the preferred method, but the emerging technology could catch up in 3-5 years.”
While this prediction did not come to pass, the field of endoscopy has evolved in remarkable ways over the last 15 years. From the development of high-definition endoscopes to the transformation of interventional endoscopy to include “third space” procedures, previously unimaginable techniques have now become commonplace. This transformation has changed the nature and training of our field and, even more importantly, dramatically improved the care of our patients.
Just as notably, the regulatory and practice environment for endoscopy has also changed in the last 15 years, albeit at a slower pace. In January of 2007, as the first issue of GI & Hepatology News came out, Medicare announced that it would cover all screening procedures without a copay but left a loophole that charged patients if their screening colonoscopy became therapeutic. That loophole was finally fixed this year as GI & Hepatology News celebrates its 15-year anniversary.
If the past 15 years are any indication, endoscopy practice will continue to change at a humbling pace over the next 15 years. I look forward to seeing those changes unfold through the pages of GI & Hepatology News.
Dr. Gellad is associate professor of medicine and associate vice chair of ambulatory services at Duke University Medical Center, Durham, N.C. He is also a staff physician with the Durham VA Health Care system. He disclosed ties with Merck, Novo Nordisk, and Higgs Boson Health.
Passing the ‘baton’ with pride
I was honored to be the third Editor-in-Chief of GIHN, from 2016 through 2021. GIHN is the official newspaper of the American Gastroenterological Association and has the widest readership of any AGA publication and is one that readers told us they read cover to cover. As such, each EIC and their Board of Editors must ensure balanced content that holds the interest of a diverse readership. I was privileged to work with a talented editorial board who reviewed articles, attended leadership meetings, and offered terrific suggestions throughout our tenure. I treasured their support and friendship.
Within each of the 60 monthly issues, we sought to highlight science, practice operations, national trends, and opinions and reviews that would be most important to basic scientists, clinical researchers, and academic and community clinicians, primarily from the United States but also from a worldwide readership. I was given a 300-word section to create editorial comments on pertinent topics that were important to gastroenterologists. Having a background in both community and academic practice, I tried to bring a balanced perspective to areas that often seem worlds apart.
The period between 2016 and 2021 also was a time of political upheaval in this country – something we could not ignore. I attempted to write about current events in a balanced way that kept a focus on patients and AGA’s core constituency. Not always an easy task. Sustainability of the Affordable Care Act was very much in question because of judicial and legislative challenges; had the ACA been overturned, our practices would be very different now.
In 2016, the first private equity–backed practice platform was created in south Florida. Little did we know how much that model would change community practice. Then, on Jan. 21, 2020, the first case of COVID 19 was diagnosed in Seattle (although earlier cases likely occurred). By March, many clinics and practices were closing, and we were altering our care delivery infrastructure in ways that would forever change practice. Trying to keep current with ever-changing science and policies was a challenge.
I will always treasure my time as EIC. I was happy (and proud) to pass this baton to Megan A. Adams MD, JD, MSc, my colleague and mentee at the University of Michigan. The partnership between AGA and Frontline Medical Communications has been successful for 15 years and will continue to be so.
Dr. Allen, now retired, was professor of medicine at the University of Michigan, Ann Arbor. He is secretary/treasurer for the American Gastroenterological Association, and declares no relevant conflicts of interest.
I was honored to be the third Editor-in-Chief of GIHN, from 2016 through 2021. GIHN is the official newspaper of the American Gastroenterological Association and has the widest readership of any AGA publication and is one that readers told us they read cover to cover. As such, each EIC and their Board of Editors must ensure balanced content that holds the interest of a diverse readership. I was privileged to work with a talented editorial board who reviewed articles, attended leadership meetings, and offered terrific suggestions throughout our tenure. I treasured their support and friendship.
Within each of the 60 monthly issues, we sought to highlight science, practice operations, national trends, and opinions and reviews that would be most important to basic scientists, clinical researchers, and academic and community clinicians, primarily from the United States but also from a worldwide readership. I was given a 300-word section to create editorial comments on pertinent topics that were important to gastroenterologists. Having a background in both community and academic practice, I tried to bring a balanced perspective to areas that often seem worlds apart.
The period between 2016 and 2021 also was a time of political upheaval in this country – something we could not ignore. I attempted to write about current events in a balanced way that kept a focus on patients and AGA’s core constituency. Not always an easy task. Sustainability of the Affordable Care Act was very much in question because of judicial and legislative challenges; had the ACA been overturned, our practices would be very different now.
In 2016, the first private equity–backed practice platform was created in south Florida. Little did we know how much that model would change community practice. Then, on Jan. 21, 2020, the first case of COVID 19 was diagnosed in Seattle (although earlier cases likely occurred). By March, many clinics and practices were closing, and we were altering our care delivery infrastructure in ways that would forever change practice. Trying to keep current with ever-changing science and policies was a challenge.
I will always treasure my time as EIC. I was happy (and proud) to pass this baton to Megan A. Adams MD, JD, MSc, my colleague and mentee at the University of Michigan. The partnership between AGA and Frontline Medical Communications has been successful for 15 years and will continue to be so.
Dr. Allen, now retired, was professor of medicine at the University of Michigan, Ann Arbor. He is secretary/treasurer for the American Gastroenterological Association, and declares no relevant conflicts of interest.
I was honored to be the third Editor-in-Chief of GIHN, from 2016 through 2021. GIHN is the official newspaper of the American Gastroenterological Association and has the widest readership of any AGA publication and is one that readers told us they read cover to cover. As such, each EIC and their Board of Editors must ensure balanced content that holds the interest of a diverse readership. I was privileged to work with a talented editorial board who reviewed articles, attended leadership meetings, and offered terrific suggestions throughout our tenure. I treasured their support and friendship.
Within each of the 60 monthly issues, we sought to highlight science, practice operations, national trends, and opinions and reviews that would be most important to basic scientists, clinical researchers, and academic and community clinicians, primarily from the United States but also from a worldwide readership. I was given a 300-word section to create editorial comments on pertinent topics that were important to gastroenterologists. Having a background in both community and academic practice, I tried to bring a balanced perspective to areas that often seem worlds apart.
The period between 2016 and 2021 also was a time of political upheaval in this country – something we could not ignore. I attempted to write about current events in a balanced way that kept a focus on patients and AGA’s core constituency. Not always an easy task. Sustainability of the Affordable Care Act was very much in question because of judicial and legislative challenges; had the ACA been overturned, our practices would be very different now.
In 2016, the first private equity–backed practice platform was created in south Florida. Little did we know how much that model would change community practice. Then, on Jan. 21, 2020, the first case of COVID 19 was diagnosed in Seattle (although earlier cases likely occurred). By March, many clinics and practices were closing, and we were altering our care delivery infrastructure in ways that would forever change practice. Trying to keep current with ever-changing science and policies was a challenge.
I will always treasure my time as EIC. I was happy (and proud) to pass this baton to Megan A. Adams MD, JD, MSc, my colleague and mentee at the University of Michigan. The partnership between AGA and Frontline Medical Communications has been successful for 15 years and will continue to be so.
Dr. Allen, now retired, was professor of medicine at the University of Michigan, Ann Arbor. He is secretary/treasurer for the American Gastroenterological Association, and declares no relevant conflicts of interest.
The winding road that leads to optimal temperature management after cardiac arrest
In 2002, two landmark trials found that targeted temperature management (TTM) after out-of-hospital cardiac arrest led to improvements in neurologic outcomes. The larger of the two trials found a reduction in mortality. Such treatment benefits are hard to come by in critical care in general and in out-of-hospital cardiac arrest in particular. With the therapeutic overconfidence typical of our profession, my institution embraced TTM quickly and completely soon after these trials were published. Remember, this was “back in the day” when sepsis management included drotrecogin alfa, Cortrosyn stim tests, tight glucose control (90-120 mg/dL), and horrible over-resuscitation via the early goal-directed therapy paradigm.
If you’ve been practicing critical care medicine for more than a few years, you already know where I’m going. Most of the interventions in the preceding paragraph were adopted but discarded before 2010. Hypothermia – temperature management with a goal of 32-36° C – has been struggling to stay relevant ever since the publication of the TTM randomized controlled trial (RCT) in 2013. Then came the HYPERION trial, which brought the 32-36° C target back from the dead (pun definitely intended) in 2019. This is critical care medicine: Today’s life-saving intervention proves harmful tomorrow, but withholding it may constitute malpractice a few months down the road.
So where are we now? Good question. I’ve had seasoned neurointensivists insist that 33° C remains the standard of care and others who’ve endorsed normothermia. So much for finding an answer via my more specialized colleagues.
Let’s go to the guidelines then. Prompted largely by HYPERION, a temperature target of 32-36° C was endorsed in 2020 and 2021. Then came publication of the TTM2 trial, the largest temperature management RCT to date, which found no benefit to targeting 33° C. A network meta-analysis published in 2021 reached a similar conclusion. A recently released update by the same international guideline group now recommends targeting normothermia (< 37.7° C) and avoiding fever, and it specifically says that there is insufficient evidence to support a 32-36° C target. Okay, everyone tracking all that?
Lest I sound overly catty and nihilistic, I see all this in a positive light. Huge credit goes to the critical care medicine academic community for putting together so many RCTs. The scientific reality is that it takes “a lotta” sample size to clarify the effects of an intervention. Throw in the inevitable bevy of confounders (in- vs. out-of-hospital cardiac arrest, resuscitation time, initial rhythm, and so on), and you get a feel for the work required to understand a treatment’s true effects.
Advances in guideline science and the hard, often unpaid work of panels are also important. The guideline panel I’ve been citing came out for aggressive temperature control (32-36° C) a few months before the TTM2 RCT was published. In the past, they updated their recommendations every 5 years, but this time, they were out with a new manuscript that incorporated TTM2 in less than a year. If you’ve been involved at any level with producing guidelines, you can appreciate this achievement. Assuming that aggressive hypothermia is truly harmful, waiting 5 years to incorporate TTM2 could have led to significant morbidity.
I do take issue with you early adopters, though. Given the litany of failed therapies that have shown initial promise, and the well-documented human tendency to underestimate the impact of sample size, your rapid implementation of major interventions is puzzling. One might think you’d learned your lessons after seeing drotrecogin alfa, Cortrosyn stim tests, tight glucose control, early goal-directed therapy, and aggressive TTM come and go. Your recent enthusiasm for vitamin C after publication of a single before-after study suggests that you haven’t.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center, Bethesda, Md. He has received a research grant from Fisher-Paykel.
A version of this article first appeared on Medscape.com.
In 2002, two landmark trials found that targeted temperature management (TTM) after out-of-hospital cardiac arrest led to improvements in neurologic outcomes. The larger of the two trials found a reduction in mortality. Such treatment benefits are hard to come by in critical care in general and in out-of-hospital cardiac arrest in particular. With the therapeutic overconfidence typical of our profession, my institution embraced TTM quickly and completely soon after these trials were published. Remember, this was “back in the day” when sepsis management included drotrecogin alfa, Cortrosyn stim tests, tight glucose control (90-120 mg/dL), and horrible over-resuscitation via the early goal-directed therapy paradigm.
If you’ve been practicing critical care medicine for more than a few years, you already know where I’m going. Most of the interventions in the preceding paragraph were adopted but discarded before 2010. Hypothermia – temperature management with a goal of 32-36° C – has been struggling to stay relevant ever since the publication of the TTM randomized controlled trial (RCT) in 2013. Then came the HYPERION trial, which brought the 32-36° C target back from the dead (pun definitely intended) in 2019. This is critical care medicine: Today’s life-saving intervention proves harmful tomorrow, but withholding it may constitute malpractice a few months down the road.
So where are we now? Good question. I’ve had seasoned neurointensivists insist that 33° C remains the standard of care and others who’ve endorsed normothermia. So much for finding an answer via my more specialized colleagues.
Let’s go to the guidelines then. Prompted largely by HYPERION, a temperature target of 32-36° C was endorsed in 2020 and 2021. Then came publication of the TTM2 trial, the largest temperature management RCT to date, which found no benefit to targeting 33° C. A network meta-analysis published in 2021 reached a similar conclusion. A recently released update by the same international guideline group now recommends targeting normothermia (< 37.7° C) and avoiding fever, and it specifically says that there is insufficient evidence to support a 32-36° C target. Okay, everyone tracking all that?
Lest I sound overly catty and nihilistic, I see all this in a positive light. Huge credit goes to the critical care medicine academic community for putting together so many RCTs. The scientific reality is that it takes “a lotta” sample size to clarify the effects of an intervention. Throw in the inevitable bevy of confounders (in- vs. out-of-hospital cardiac arrest, resuscitation time, initial rhythm, and so on), and you get a feel for the work required to understand a treatment’s true effects.
Advances in guideline science and the hard, often unpaid work of panels are also important. The guideline panel I’ve been citing came out for aggressive temperature control (32-36° C) a few months before the TTM2 RCT was published. In the past, they updated their recommendations every 5 years, but this time, they were out with a new manuscript that incorporated TTM2 in less than a year. If you’ve been involved at any level with producing guidelines, you can appreciate this achievement. Assuming that aggressive hypothermia is truly harmful, waiting 5 years to incorporate TTM2 could have led to significant morbidity.
I do take issue with you early adopters, though. Given the litany of failed therapies that have shown initial promise, and the well-documented human tendency to underestimate the impact of sample size, your rapid implementation of major interventions is puzzling. One might think you’d learned your lessons after seeing drotrecogin alfa, Cortrosyn stim tests, tight glucose control, early goal-directed therapy, and aggressive TTM come and go. Your recent enthusiasm for vitamin C after publication of a single before-after study suggests that you haven’t.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center, Bethesda, Md. He has received a research grant from Fisher-Paykel.
A version of this article first appeared on Medscape.com.
In 2002, two landmark trials found that targeted temperature management (TTM) after out-of-hospital cardiac arrest led to improvements in neurologic outcomes. The larger of the two trials found a reduction in mortality. Such treatment benefits are hard to come by in critical care in general and in out-of-hospital cardiac arrest in particular. With the therapeutic overconfidence typical of our profession, my institution embraced TTM quickly and completely soon after these trials were published. Remember, this was “back in the day” when sepsis management included drotrecogin alfa, Cortrosyn stim tests, tight glucose control (90-120 mg/dL), and horrible over-resuscitation via the early goal-directed therapy paradigm.
If you’ve been practicing critical care medicine for more than a few years, you already know where I’m going. Most of the interventions in the preceding paragraph were adopted but discarded before 2010. Hypothermia – temperature management with a goal of 32-36° C – has been struggling to stay relevant ever since the publication of the TTM randomized controlled trial (RCT) in 2013. Then came the HYPERION trial, which brought the 32-36° C target back from the dead (pun definitely intended) in 2019. This is critical care medicine: Today’s life-saving intervention proves harmful tomorrow, but withholding it may constitute malpractice a few months down the road.
So where are we now? Good question. I’ve had seasoned neurointensivists insist that 33° C remains the standard of care and others who’ve endorsed normothermia. So much for finding an answer via my more specialized colleagues.
Let’s go to the guidelines then. Prompted largely by HYPERION, a temperature target of 32-36° C was endorsed in 2020 and 2021. Then came publication of the TTM2 trial, the largest temperature management RCT to date, which found no benefit to targeting 33° C. A network meta-analysis published in 2021 reached a similar conclusion. A recently released update by the same international guideline group now recommends targeting normothermia (< 37.7° C) and avoiding fever, and it specifically says that there is insufficient evidence to support a 32-36° C target. Okay, everyone tracking all that?
Lest I sound overly catty and nihilistic, I see all this in a positive light. Huge credit goes to the critical care medicine academic community for putting together so many RCTs. The scientific reality is that it takes “a lotta” sample size to clarify the effects of an intervention. Throw in the inevitable bevy of confounders (in- vs. out-of-hospital cardiac arrest, resuscitation time, initial rhythm, and so on), and you get a feel for the work required to understand a treatment’s true effects.
Advances in guideline science and the hard, often unpaid work of panels are also important. The guideline panel I’ve been citing came out for aggressive temperature control (32-36° C) a few months before the TTM2 RCT was published. In the past, they updated their recommendations every 5 years, but this time, they were out with a new manuscript that incorporated TTM2 in less than a year. If you’ve been involved at any level with producing guidelines, you can appreciate this achievement. Assuming that aggressive hypothermia is truly harmful, waiting 5 years to incorporate TTM2 could have led to significant morbidity.
I do take issue with you early adopters, though. Given the litany of failed therapies that have shown initial promise, and the well-documented human tendency to underestimate the impact of sample size, your rapid implementation of major interventions is puzzling. One might think you’d learned your lessons after seeing drotrecogin alfa, Cortrosyn stim tests, tight glucose control, early goal-directed therapy, and aggressive TTM come and go. Your recent enthusiasm for vitamin C after publication of a single before-after study suggests that you haven’t.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center, Bethesda, Md. He has received a research grant from Fisher-Paykel.
A version of this article first appeared on Medscape.com.
In progressive lung cancer second biopsies may be the norm now
Shortly after osimertinib was approved for patients with non–small cell lung cancer in 2020 by the Food and Drug Administration, a patient came to me with increasing shortness of breath. He had been on erlotinib (Tarceva) for about 2 years and had done well. Nearly all of his pulmonary lesions had resolved and he was feeling well. He enjoyed boating in the summer and visiting grandkids in California in the winter. However, on this day, it was different. He was losing weight; he was tired and didn’t feel strong enough to put his boat in the water that spring. Long story short: We ordered a CT scan and all of his lesions were progressing. Since osimertinib had just been approved, we got a second biopsy, hoping that his insurance would pay for it. It did and sure enough, a new T790M mutation was present. He was on osimertinib for another 2 years before progressing and starting chemotherapy.
Second biopsies increasingly routine
The practice of ordering a second biopsy for patients with non–small cell lung carcinoma (NSCLC) was not common practice until after 2015 when the Food and Drug Administration approved gefitinib, a tyrosine kinase inhibitor (TKI) for patients whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.
Up until then, second biopsies were not routinely done for lung cancers. But with the advent of targeted therapy and new drugs designed specifically to tackle first- and second-line treatment resistance mutations, rebiopsies have become a necessity for patients with progressive disease.
Epidermal growth factors, including HER2, ErbB2, and MET, are receptors of tyrosine kinases that control cell growth, but when in overdrive, they can lead to the development of cancers, including lung adenocarcinoma, conventional glioblastoma multiforme, glioblastoma, colon adenocarcinoma, and NSCLC.
EGFRs date back to 1962 with their discovery by Stanley Cohen. The discovery was so important that in 1986, Mr. Cohen was awarded the Nobel Prize in physiology or medicine for the discovery along with Rita Levi-Montalcini.
Now, many years later, we finally have a string of new approvals for mutations in the EGF family of receptors and several under study.
Sensitizing mutations
The more commonly used strategy for blocking EGFR signaling in lung cancer is the use of tyrosine kinase inhibitors, which compete with adenosine triphosphate (ATP) for binding to the tyrosine kinase portion of the receptor. They are located at chromosome 7p11.2. The most frequent mutations that sensitize patients to EGFR inhibitors include exon 19 deletions and L858R point mutation in exon 21, although multiple other driver mutations also exist.
The first-generation of EGFR TKIs include gefitinib and erlotinib, which bind reversibly to the EGF receptor. Second-generation inhibitors afatinib and dacomitinib bind irreversibly. Osimertinib, a third-generation EGFR TKI, which also binds irreversibly, was approved in 2020 for adjuvant therapy, and first- and second-line treatment in patients with NSCLC who have EGFR mutation–positive disease.
First-generation EGFR tyrosine kinase inhibitors
Four randomized, first-line, placebo-controlled phase 3 trials conducted with EGFR TKIs in combination with platinum-based doublet chemotherapy in an EGFR nonselected patient population failed to show a survival benefit with erlotinib or gefitinib (TRIBUTE, Tarceva Lung Cancer Investigation Trial, INTACT 1, INTACT 2).
However, a first-line study randomized patients to gefitinib or chemotherapy with carboplatin-paclitaxel, and included patients with or without an EGFR mutations. In the subgroup of patients with an EGFR mutation, progression-free survival (PFS) was significantly longer among those who received gefitinib than among those who received carboplatin–paclitaxel (hazard ratio for progression or death, 0.48), whereas in the subgroup of patients who were negative for the mutation, PFS was significantly longer among those who received chemotherapy (HR for progression or death with gefitinib, 2.85).
Numerous studies have shown that EGFR TKIs used in the first-line setting improved progression free survival, response rates, and quality of life while reducing toxicity. A recent meta-analysis of randomized clinical trials involving EGFR TKIs showed that EGFR TKI improved PFS with a HR of 0.40, compared with standard chemotherapy with fewer serious adverse events, although no benefit on overall survival was observed (HR, 0.96; 95% confidence interval, 0.83-1.10; P = .556).
T790M: The most common resistance mutation
T790M is the most common resistance mechanism to develop in patients with EGFR mutations being treated with EGFR TKIs. A randomized phase 3 trial of osimertinib vs. chemotherapy in patients with T790M-positive advanced NSCLC who had disease progression after first-line EGFR-TKI therapy, reported a median duration of progression-free survival that was significantly longer with osimertinib than with platinum therapy plus pemetrexed (10.1 months vs. 4.4 months; HR, 0.30). In addition, among 144 patients with metastases to the central nervous system, the median duration of PFS was longer among patients receiving osimertinib than among those receiving platinum therapy plus pemetrexed (8.5 months vs. 4.2 months; HR, 0.32). However, now that osimertinib has moved into the front-line setting, it has left a void for the treatment of patients with advanced disease who have failed osimertinib.
New resistance mechanisms continue to be identified
One of the most common sets of resistance mutations are insertions in exon 20 of the EGF receptor gene. These are a heterogenous group of mutations, many of which do not respond to first-, second-, or third-generation TKIs. Some, such as EGFR-A763_Y764insFQEA, may be sensitive to first- and third-generation EGFR TKIs. Other drugs targeting exon 20 insertion mutations are under development.
Newly approved by the FDA within the last year are mobocertinib and CLN-081 for adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.
Savolitinib is a receptor tyrosine kinase (MET) inhibitor currently under development for NSCLC and other cancers. Amivantamab-vmjw was approved by the FDA last year for metastatic NSCLC. It targets EGF and MET receptors in patients with EGFR exon 20 insertion mutations.
We finally have approved drugs for exon 20 insertions and c-Met amplification, even though their approvals are based on small, single arm studies with no definitive claims of improved efficacy over older therapies. as in my patient described in this article.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Shortly after osimertinib was approved for patients with non–small cell lung cancer in 2020 by the Food and Drug Administration, a patient came to me with increasing shortness of breath. He had been on erlotinib (Tarceva) for about 2 years and had done well. Nearly all of his pulmonary lesions had resolved and he was feeling well. He enjoyed boating in the summer and visiting grandkids in California in the winter. However, on this day, it was different. He was losing weight; he was tired and didn’t feel strong enough to put his boat in the water that spring. Long story short: We ordered a CT scan and all of his lesions were progressing. Since osimertinib had just been approved, we got a second biopsy, hoping that his insurance would pay for it. It did and sure enough, a new T790M mutation was present. He was on osimertinib for another 2 years before progressing and starting chemotherapy.
Second biopsies increasingly routine
The practice of ordering a second biopsy for patients with non–small cell lung carcinoma (NSCLC) was not common practice until after 2015 when the Food and Drug Administration approved gefitinib, a tyrosine kinase inhibitor (TKI) for patients whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.
Up until then, second biopsies were not routinely done for lung cancers. But with the advent of targeted therapy and new drugs designed specifically to tackle first- and second-line treatment resistance mutations, rebiopsies have become a necessity for patients with progressive disease.
Epidermal growth factors, including HER2, ErbB2, and MET, are receptors of tyrosine kinases that control cell growth, but when in overdrive, they can lead to the development of cancers, including lung adenocarcinoma, conventional glioblastoma multiforme, glioblastoma, colon adenocarcinoma, and NSCLC.
EGFRs date back to 1962 with their discovery by Stanley Cohen. The discovery was so important that in 1986, Mr. Cohen was awarded the Nobel Prize in physiology or medicine for the discovery along with Rita Levi-Montalcini.
Now, many years later, we finally have a string of new approvals for mutations in the EGF family of receptors and several under study.
Sensitizing mutations
The more commonly used strategy for blocking EGFR signaling in lung cancer is the use of tyrosine kinase inhibitors, which compete with adenosine triphosphate (ATP) for binding to the tyrosine kinase portion of the receptor. They are located at chromosome 7p11.2. The most frequent mutations that sensitize patients to EGFR inhibitors include exon 19 deletions and L858R point mutation in exon 21, although multiple other driver mutations also exist.
The first-generation of EGFR TKIs include gefitinib and erlotinib, which bind reversibly to the EGF receptor. Second-generation inhibitors afatinib and dacomitinib bind irreversibly. Osimertinib, a third-generation EGFR TKI, which also binds irreversibly, was approved in 2020 for adjuvant therapy, and first- and second-line treatment in patients with NSCLC who have EGFR mutation–positive disease.
First-generation EGFR tyrosine kinase inhibitors
Four randomized, first-line, placebo-controlled phase 3 trials conducted with EGFR TKIs in combination with platinum-based doublet chemotherapy in an EGFR nonselected patient population failed to show a survival benefit with erlotinib or gefitinib (TRIBUTE, Tarceva Lung Cancer Investigation Trial, INTACT 1, INTACT 2).
However, a first-line study randomized patients to gefitinib or chemotherapy with carboplatin-paclitaxel, and included patients with or without an EGFR mutations. In the subgroup of patients with an EGFR mutation, progression-free survival (PFS) was significantly longer among those who received gefitinib than among those who received carboplatin–paclitaxel (hazard ratio for progression or death, 0.48), whereas in the subgroup of patients who were negative for the mutation, PFS was significantly longer among those who received chemotherapy (HR for progression or death with gefitinib, 2.85).
Numerous studies have shown that EGFR TKIs used in the first-line setting improved progression free survival, response rates, and quality of life while reducing toxicity. A recent meta-analysis of randomized clinical trials involving EGFR TKIs showed that EGFR TKI improved PFS with a HR of 0.40, compared with standard chemotherapy with fewer serious adverse events, although no benefit on overall survival was observed (HR, 0.96; 95% confidence interval, 0.83-1.10; P = .556).
T790M: The most common resistance mutation
T790M is the most common resistance mechanism to develop in patients with EGFR mutations being treated with EGFR TKIs. A randomized phase 3 trial of osimertinib vs. chemotherapy in patients with T790M-positive advanced NSCLC who had disease progression after first-line EGFR-TKI therapy, reported a median duration of progression-free survival that was significantly longer with osimertinib than with platinum therapy plus pemetrexed (10.1 months vs. 4.4 months; HR, 0.30). In addition, among 144 patients with metastases to the central nervous system, the median duration of PFS was longer among patients receiving osimertinib than among those receiving platinum therapy plus pemetrexed (8.5 months vs. 4.2 months; HR, 0.32). However, now that osimertinib has moved into the front-line setting, it has left a void for the treatment of patients with advanced disease who have failed osimertinib.
New resistance mechanisms continue to be identified
One of the most common sets of resistance mutations are insertions in exon 20 of the EGF receptor gene. These are a heterogenous group of mutations, many of which do not respond to first-, second-, or third-generation TKIs. Some, such as EGFR-A763_Y764insFQEA, may be sensitive to first- and third-generation EGFR TKIs. Other drugs targeting exon 20 insertion mutations are under development.
Newly approved by the FDA within the last year are mobocertinib and CLN-081 for adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.
Savolitinib is a receptor tyrosine kinase (MET) inhibitor currently under development for NSCLC and other cancers. Amivantamab-vmjw was approved by the FDA last year for metastatic NSCLC. It targets EGF and MET receptors in patients with EGFR exon 20 insertion mutations.
We finally have approved drugs for exon 20 insertions and c-Met amplification, even though their approvals are based on small, single arm studies with no definitive claims of improved efficacy over older therapies. as in my patient described in this article.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Shortly after osimertinib was approved for patients with non–small cell lung cancer in 2020 by the Food and Drug Administration, a patient came to me with increasing shortness of breath. He had been on erlotinib (Tarceva) for about 2 years and had done well. Nearly all of his pulmonary lesions had resolved and he was feeling well. He enjoyed boating in the summer and visiting grandkids in California in the winter. However, on this day, it was different. He was losing weight; he was tired and didn’t feel strong enough to put his boat in the water that spring. Long story short: We ordered a CT scan and all of his lesions were progressing. Since osimertinib had just been approved, we got a second biopsy, hoping that his insurance would pay for it. It did and sure enough, a new T790M mutation was present. He was on osimertinib for another 2 years before progressing and starting chemotherapy.
Second biopsies increasingly routine
The practice of ordering a second biopsy for patients with non–small cell lung carcinoma (NSCLC) was not common practice until after 2015 when the Food and Drug Administration approved gefitinib, a tyrosine kinase inhibitor (TKI) for patients whose tumors have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 (L858R) substitution mutations.
Up until then, second biopsies were not routinely done for lung cancers. But with the advent of targeted therapy and new drugs designed specifically to tackle first- and second-line treatment resistance mutations, rebiopsies have become a necessity for patients with progressive disease.
Epidermal growth factors, including HER2, ErbB2, and MET, are receptors of tyrosine kinases that control cell growth, but when in overdrive, they can lead to the development of cancers, including lung adenocarcinoma, conventional glioblastoma multiforme, glioblastoma, colon adenocarcinoma, and NSCLC.
EGFRs date back to 1962 with their discovery by Stanley Cohen. The discovery was so important that in 1986, Mr. Cohen was awarded the Nobel Prize in physiology or medicine for the discovery along with Rita Levi-Montalcini.
Now, many years later, we finally have a string of new approvals for mutations in the EGF family of receptors and several under study.
Sensitizing mutations
The more commonly used strategy for blocking EGFR signaling in lung cancer is the use of tyrosine kinase inhibitors, which compete with adenosine triphosphate (ATP) for binding to the tyrosine kinase portion of the receptor. They are located at chromosome 7p11.2. The most frequent mutations that sensitize patients to EGFR inhibitors include exon 19 deletions and L858R point mutation in exon 21, although multiple other driver mutations also exist.
The first-generation of EGFR TKIs include gefitinib and erlotinib, which bind reversibly to the EGF receptor. Second-generation inhibitors afatinib and dacomitinib bind irreversibly. Osimertinib, a third-generation EGFR TKI, which also binds irreversibly, was approved in 2020 for adjuvant therapy, and first- and second-line treatment in patients with NSCLC who have EGFR mutation–positive disease.
First-generation EGFR tyrosine kinase inhibitors
Four randomized, first-line, placebo-controlled phase 3 trials conducted with EGFR TKIs in combination with platinum-based doublet chemotherapy in an EGFR nonselected patient population failed to show a survival benefit with erlotinib or gefitinib (TRIBUTE, Tarceva Lung Cancer Investigation Trial, INTACT 1, INTACT 2).
However, a first-line study randomized patients to gefitinib or chemotherapy with carboplatin-paclitaxel, and included patients with or without an EGFR mutations. In the subgroup of patients with an EGFR mutation, progression-free survival (PFS) was significantly longer among those who received gefitinib than among those who received carboplatin–paclitaxel (hazard ratio for progression or death, 0.48), whereas in the subgroup of patients who were negative for the mutation, PFS was significantly longer among those who received chemotherapy (HR for progression or death with gefitinib, 2.85).
Numerous studies have shown that EGFR TKIs used in the first-line setting improved progression free survival, response rates, and quality of life while reducing toxicity. A recent meta-analysis of randomized clinical trials involving EGFR TKIs showed that EGFR TKI improved PFS with a HR of 0.40, compared with standard chemotherapy with fewer serious adverse events, although no benefit on overall survival was observed (HR, 0.96; 95% confidence interval, 0.83-1.10; P = .556).
T790M: The most common resistance mutation
T790M is the most common resistance mechanism to develop in patients with EGFR mutations being treated with EGFR TKIs. A randomized phase 3 trial of osimertinib vs. chemotherapy in patients with T790M-positive advanced NSCLC who had disease progression after first-line EGFR-TKI therapy, reported a median duration of progression-free survival that was significantly longer with osimertinib than with platinum therapy plus pemetrexed (10.1 months vs. 4.4 months; HR, 0.30). In addition, among 144 patients with metastases to the central nervous system, the median duration of PFS was longer among patients receiving osimertinib than among those receiving platinum therapy plus pemetrexed (8.5 months vs. 4.2 months; HR, 0.32). However, now that osimertinib has moved into the front-line setting, it has left a void for the treatment of patients with advanced disease who have failed osimertinib.
New resistance mechanisms continue to be identified
One of the most common sets of resistance mutations are insertions in exon 20 of the EGF receptor gene. These are a heterogenous group of mutations, many of which do not respond to first-, second-, or third-generation TKIs. Some, such as EGFR-A763_Y764insFQEA, may be sensitive to first- and third-generation EGFR TKIs. Other drugs targeting exon 20 insertion mutations are under development.
Newly approved by the FDA within the last year are mobocertinib and CLN-081 for adult patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.
Savolitinib is a receptor tyrosine kinase (MET) inhibitor currently under development for NSCLC and other cancers. Amivantamab-vmjw was approved by the FDA last year for metastatic NSCLC. It targets EGF and MET receptors in patients with EGFR exon 20 insertion mutations.
We finally have approved drugs for exon 20 insertions and c-Met amplification, even though their approvals are based on small, single arm studies with no definitive claims of improved efficacy over older therapies. as in my patient described in this article.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Why can’t U.K. immunocompromised patients get Evusheld?
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m David Kerr, professor of cancer medicine at Oxford. As I’m gearing up to have my autumnal COVID-19 booster vaccine,
This was developed by AstraZeneca. It’s a combination of two relatively long-acting antibodies (tixagevimab and cilgavimab) that bind to the spike protein on the outside of the SARS-CoV-2 virus, the virus that causes COVID-19. The antibody binds to the spike protein and prevents it from binding to and infecting or damaging cells, so it’s what’s called preexposure prophylaxis.
Although vaccination is still the best approach to protecting against and, one would hope, conferring a degree of herd protection to our population as a whole, there are some people who cannot mount an appropriate immune response and we have to take care of these folks. Because the vaccines don’t work very well for them, the vaccine itself is not sufficient to protect them.
Evusheld, in trials that have been done hitherto, can protect people who can’t mount an immune response from being infected. Between 75% and 80% of patients treated with Evusheld didn’t get COVID-19. The duration of effect seemed to be at least for 6 months, possibly longer, so it’s a really good result. This caused our medicines regulatory authority in the United Kingdom to approve the drug in March of this year. Although the drug has been approved, it’s not yet funded and not yet available for vulnerable patients.
These are patients who, for reasons of inborn genetic diseases, cannot mount an immune response; patients who are pharmacologically immune depleted; patients who have had transplants and are on immunosuppressive drugs; and some of our cancer patients, particularly those with blood or hematologic malignancies, who can receive very heavy treatment that can pound the immune system to bits.
These are, in the population as a whole, relatively small numbers, but an important number of people who are still vulnerable to developing COVID-19 despite vaccination.
Why isn’t the drug available? We have a two-stage process in the United Kingdom. We have the scientists and regulatory authorities looking at the evidence and data and saying, “Yes, it stacks up. This drug is effective and safe to some extent.”
The second phase is a health technology assessment undertaken by NICE, our National Institute for Health and Care Excellence – something that I’ve talked about a number of times before and the sometimes seemingly arbitrary decisions that they make. NICE hasn’t evaluated the drug yet, and the British government has held out because they are arguing that we don’t have enough data.
The trials with Evusheld were done before the Omicron variant dominated, as it does now; therefore, they are looking to try to work with AstraZeneca to generate more real-world data to show that Evusheld would prevent infection from the Omicron variant of the virus. Equally as important, how long does that protection last? Is it as protective against Omicron, and what’s the duration of that protection? Those bits of work are going on now.
Some real-world data are starting to emerge, showing that Evusheld will offer some degree of protection against Omicron, but there are still question marks about duration and the proportion of the population that would benefit.
NICE aren’t due to report on this – although the drug was approved in March of this year – until next year some time. That’s what’s caused a degree of consternation in the community of patients that we serve. Some of my clinical colleagues are beating the drum, saying, “We must have this drug now.” We’re still waiting on NICE to announce.
One obvious way to go around this is the government, which has bent over backwards in the United Kingdom to do as much as it can to protect the population from COVID-19. There was fantastic vaccine rollout and an extraordinary economic package to support individuals during lockdown to maintain the workforce, to support families and people at home. They’ve done a fantastic job.
Wanting to damp down this controversy, perhaps the sensible thing would be to ask NICE to evaluate the data that they have just now, to allow AstraZeneca to present whatever real-world evidence they have, and although it may not be perfect, it may be sufficient – we don’t know – to pass the NICE health technology assessment.
Watch this space. Let’s see what happens. If I were government, that’s what I would do. I would ask NICE to bring their appraisal forward. I would ask them to work with AstraZeneca to go over every ounce and iota of data that they have to see if this drug will be sufficiently effective and sufficiently cost-effective to be used before winter comes. I think the whole world is holding its breath, expecting another COVID-19 winter surge. Now would be the time to act.
What do you think? Here we are in the United Kingdom discussing yet another quasi–”health-rationing” problem. It’s not. This is about collecting more data and being as rational as possible. Can we accelerate that process? Perhaps.
Thanks for listening. I’d be very grateful for any comments that you might choose to make.
David J. Kerr, MD, DSc, is a professor of cancer medicine at the University of Oxford (England). He disclosed financial relationships with Oxford Cancer Biomarkers, Afrox, GlaxoSmithKline, Bayer HealthCare Pharmaceuticals, Genomic Health, Merck Serono, Roche, and Celleron Therapeutics.
A version of this article first appeared on Medscape.com.
Could a vaccine (and more) fix the fentanyl crisis?
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
This discussion was recorded on Aug. 31, 2022. This transcript has been edited for clarity.
Robert Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have Dr. Paul Christo, a pain specialist in the Division of Pain Medicine at Johns Hopkins University School of Medicine in Baltimore, Maryland, and host of the national radio show Aches and Gains on SiriusXM Radio, joining us to discuss the ongoing and worsening fentanyl crisis in the U.S.
Welcome, Dr Christo.
Paul J. Christo, MD, MBA: Thanks so much for having me.
Dr. Glatter: I want to begin with a sobering statistic regarding overdoses. , based on recent data from the CDC.
Let’s start by having you explain how deadly fentanyl is in terms of its potency compared with morphine and heroin.
Dr. Christo: Fentanyl is considered a synthetic opioid. It’s not a naturally occurring opioid like morphine, for example, or codeine. We use this drug, fentanyl, often in the anesthesia well. We’ve used it for many years as an anesthetic for surgery very safely. In the chronic pain world, we’ve used it to help reduce chronic pain in the form of a patch.
What we’re seeing now, though, is something entirely different, which is the use of synthetic fentanyl as a mind- and mood-altering substance for those who don’t have pain, and essentially those who are buying this off the street. Fentanyl is about 80-100 times more potent than morphine, so you can put that in perspective in terms of its danger.
Dr. Glatter: Let me have you take us through an evolution of the opioid crisis from the 1990s, from long-acting opioid OxyContin, which was approved in 1995, to where we are now. There are different phases. If you could, educate our audience on how we got to where fentanyl is now the most common opiate involved in drug overdoses.
Dr. Christo: It really stems from the epidemic related to chronic pain. We have over 100 million people in the United States alone who suffer from chronic pain. Most chronic pain, sadly, is undertreated or untreated. In the ‘90s, in the quest to reduce chronic pain to a better extent, we saw more and more literature and studies related to the use of opioids for noncancer pain (e.g., for lower back pain).
There were many primary care doctors and pain specialists who started using opioids, probably for patients who didn’t really need it. I think it was done out of good conscience in the sense that they were trying to reduce pain. We have other methods of pain relief, but we needed more. At that time, in the ‘90s, we had a greater use of opioids to treat noncancer pain.
Then from that point, we transitioned to the use of heroin. Again, this isn’t among the chronic pain population, but it was the nonchronic pain population that starting using heroin. Today we see synthetic fentanyl.
Addressing the synthetic opioid crisis
Dr. Glatter: With fentanyl being the most common opiate we’re seeing, we’re having problems trying to save patients. We’re trying to use naloxone, but obviously in increasing amounts, and sometimes it’s not adequate and we have to intubate patients.
In terms of addressing this issue of supply, the fentanyl is coming from Mexico, China, and it’s manufactured here in the United States. How do we address this crisis? What are the steps that you would recommend we take?
Dr. Christo: I think that we need to better support law enforcement to crack down on those who are manufacturing fentanyl in the United States, and also to crack down on those who are transporting it from, say, Mexico – I think it’s primarily coming from Mexico – but from outside the United States to the United States. I feel like that’s important to do.
Two, we need to better educate those who are using these mind- and mood-altering substances. We’re seeing more and more that it’s the young-adult population, those between the ages of 13 and 25, who are starting to use these substances, and they’re very dangerous.
Dr. Glatter: Are these teens seeking out heroin and it happens to be laced with fentanyl, or are they actually seeking pure fentanyl? Are they trying to buy the colorful pills that we know about? What’s your experience in terms of the population you’re treating and what you could tell us?
Dr. Christo: I think it’s both. We’re seeing young adults who are interested in the use of fentanyl as a mind- and mood-altering substance. We’re also seeing young and older adults use other drugs, like cocaine and heroin, that are laced with fentanyl, and they don’t know it. That’s exponentially more dangerous.
Fentanyl test strips
Dr. Glatter: People are unaware that there is fentanyl in what they’re using, and it is certainly leading to overdoses and deaths. I think that parents really need to be aware of this.
Dr. Christo: Yes, for sure. I think we need better educational methods in the schools to educate that population that we’re talking about (between the ages of 13 and 25). Let them know the dangers, because I don’t think they’re aware of the danger, and how potent fentanyl is in terms of its lethality, and that you don’t need very much to take in a form of a pill or to inhale or to inject intravenously to kill yourself. That is key – education at that level – and to let those who are going to use these substances (specifically, synthetic fentanyl) know that they should consider the use of fentanyl test strips.
Fentanyl test strips would be primarily used for those who are thinking that they’re using heroin but there may be fentanyl in there, or methamphetamine and there may be fentanyl, and they don’t know. The test strip gives them that knowledge.
The other harm reduction strategies would be the use of naloxone, known as Narcan. That’s a lifesaver. You just have to spritz it into the nostril. You don’t do it yourself if you’re using the substance, but you’ve got others who can do it for you. No question, that’s a lifesaver. We need to make sure that there’s greater availability of that throughout the entire country, and we’re seeing some of that in certain states. In certain states, you don’t need a prescription to get naloxone from the pharmacy.
Dr. Glatter: I think it’s so important that it should be widely available. Certainly, the COVID-19 pandemic exacerbated the number of overdoses we saw. Are overdoses coming down or are we still at a level that’s close to 2020?
Dr. Christo: Unfortunately, we’re still seeing the same level, if not seeing it escalate. Certainly, the pandemic, because of the economic cost associated with the pandemic – loss of employment, underemployment – as well as the emotional stress of the pandemic led many people to use substances on the street in order to cope. They’re coping mechanisms, and we really haven’t seen it abate quite yet.
Dr. Glatter: Do you have a message for the lawmakers on Capitol Hill as to what we can do regarding the illegal manufacturing and distribution, how we can really crack down? Are there other approaches that we could implement that might be more tangible?
Dr. Christo: Yes. No. 1 would be to support law enforcement. No. 2 would be to create and make available more overdose prevention centers. The first was in New York City. If you look at the data on overdose prevention centers, in Canada, for example, they’ve seen a 35% reduction in overdose deaths. These are places where people who are using can go to get clean needles and clean syringes. This is where people basically oversee the use of the drug and intervene if necessary.
It seems sort of antithetical. It seems like, “Boy, why would you fund a center for people to use drugs?” The data from Canada and outside Canada are such that it can be very helpful. That would be one of my messages to lawmakers as well.
Vaccines to combat the synthetic opioid crisis
Dr. Glatter: Do you think that the legislators could approach some of these factories as a way to crack down, and have law enforcement be more aggressive? Is that another possible solution?
Dr. Christo: It is. Law enforcement needs to be supported by the government, by the Biden administration, so that we can prevent the influx of fentanyl and other drugs into the United States, and also to crack down on those in the United States who are manufacturing these drugs – synthetic fentanyl, first and foremost – because we’re seeing a lot of deaths related to synthetic fentanyl.
Also, we’re seeing — and this is pretty intriguing and interesting – the use of vaccines to help prevent overdose. The first human trial is underway right now for a vaccine against oxycodone. Not only that, but there are other vaccines that are in animal trials now against heroin, cocaine, or fentanyl. There’s hope there that we can use vaccines to also help reduce deaths related to overdose from fentanyl and other opioids.
Dr. Glatter: Do you think this would be given widely to the population or only to those at higher risk?
Dr. Christo: It would probably be targeting those who are at higher risk and have a history of drug abuse. I don’t think it would be something that would be given to the entire population, but it certainly could be effective, and we’re seeing encouraging results from the human trial right now.
Dr. Glatter: That’s very intriguing. That’s something that certainly could be quite helpful in the future.
One thing I did want to address is law enforcement and first responders who have been exposed to dust, or inhaled dust possibly, or had fentanyl on their skin. There has been lots of controversy. The recent literature has dispelled the controversy that people who had supposedly passed out and required Narcan after exposure to intact skin, or even compromised skin, had an overdose of fentanyl. Maybe you could speak to that and dispel that myth.
Dr. Christo: Yes, I’ve been asked this question a couple of times in the past. It’s not sufficient just to have contact with fentanyl on the skin to lead to an overdose. You really need to ingest it. That is, take it by mouth in the form of a pill, inhale it, or inject it intravenously. Skin contact is very unlikely going to lead to an overdose and death.
Dr. Glatter: I want to thank you for a very informative interview. Do you have one or two pearls you’d like to give our audience as a takeaway?
Dr. Christo: I would say two things. One is, don’t give up if you have chronic pain because there is hope. We have nonopioid treatments that can be effective. Two, don’t give up if you have a substance use disorder. Talk to your primary care doctor or talk to emergency room physicians if you’re in the emergency room. The Substance Abuse and Mental Health Services Administration is a good resource, too. SAMHSA has an 800 number for support and a website. Take the opportunity to use the resources that are available.
Dr. Glatter is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Hofstra University, Hempstead, N.Y. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes.
Dr. Christo is an associate professor and a pain specialist in the department of anesthesiology and critical care medicine at Johns Hopkins University, Baltimore. He also serves as director of the multidisciplinary pain fellowship program at Johns Hopkins Hospital. Christo is the author of Aches and Gains, A Comprehensive Guide to Overcoming Your Pain, and hosts an award-winning, nationally syndicated SiriusXM radio talk show on overcoming pain, called Aches and Gains.
A version of this article first appeared on Medscape.com.
Is exercise effective for constipation?
I recently presented a clinical scenario about a patient of mine named Brenda. This 35-year-old woman came to me with symptoms that had been going on for a year already. I asked for readers’ comments about my management of Brenda.
I appreciate the comments I received regarding this case. The most common suggestion was to encourage Brenda to exercise, and a systematic review of randomized clinical trials published in 2019 supports this recommendation. This review included nine studies with a total of 680 participants, and the overall effect of exercise was a twofold improvement in symptoms associated with constipation. Walking was the most common exercise intervention, and along with qigong (which combines body position, breathing, and meditation), these two modes of exercise were effective in improving constipation. However, the one study evaluating resistance training failed to demonstrate a significant effect. Importantly, the reviewers considered the collective research to be at a high risk of bias.
Exercise will probably help Brenda, although some brainstorming might be necessary to help her fit exercise into her busy schedule. Another suggestion focused on her risk for colorectal cancer, and Dr. Cooke and Dr. Boboc both astutely noted that colorectal cancer is increasingly common among adults at early middle age. This stands in contrast to a steady decline in the prevalence of colorectal cancer among U.S. adults at age 65 years or older. Whereas colorectal cancer declined by 3.3% annually among U.S. older adults from 2011 to 2016, there was a reversal of this favorable trend among individuals between 50 and 64 years of age, with rates increasing by 1% annually.
The increase in the incidence of colorectal cancer among adults 50-64 years of age has been outpaced by the increase among adults younger than 50 years, who have experienced a 2.2% increase in the incidence of colorectal cancer annually between 2012 and 2016. Previously, the increase in colorectal cancer among early middle-aged adults was driven by higher rates of rectal cancer, but more recently this trend has included higher rates of proximal and distal colon tumors. In 2020, 12% of new cases of colorectal cancer were expected to be among individuals younger than 50 years.
So how do we act on this context in the case of Brenda? Her history suggests no overt warning signs for cancer. The history did not address a family history of gastrointestinal symptoms or colorectal cancer, which is an important omission.
Although the number of cases of cancer among persons younger than 50 years may be rising, the overall prevalence of colorectal cancer among younger adults is well under 1%. At 35 years of age, it is not necessary to evaluate Brenda for colorectal cancer. However, persistent or worsening symptoms could prompt a referral for colonoscopy at a later time.
Finally, let’s address how to practically manage Brenda’s case, because many options are available. I would begin with recommendations regarding her lifestyle, including regular exercise, adequate sleep, and whatever she can achieve in the FODMAP diet. I would also recommend psyllium as a soluble fiber and expect that these changes would help her constipation. But they might be less effective for abdominal cramping, so I would also recommend peppermint oil at this time.
If Brenda commits to these recommendations, she will very likely improve. If she does not, I will be more concerned regarding anxiety and depression complicating her illness. Treating those disorders can make a big difference.
In addition, if there is an inadequate response to initial therapy, I will initiate linaclotide or lubiprostone. Plecanatide is another reasonable option. At this point, I will also consider referral to a gastroenterologist for a recalcitrant case and will certainly refer if one of these specific treatments fails in Brenda. Conditions such as pelvic floor dysfunction can mimic irritable bowel syndrome with constipation and merit consideration.
However, I really believe that Brenda will feel better. Thanks for all of the insightful and interesting comments. It is easy to see how we are all invested in improving patients’ lives.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported disclosures with McNeil Pharmaceuticals. A version of this article first appeared on Medscape.com.
I recently presented a clinical scenario about a patient of mine named Brenda. This 35-year-old woman came to me with symptoms that had been going on for a year already. I asked for readers’ comments about my management of Brenda.
I appreciate the comments I received regarding this case. The most common suggestion was to encourage Brenda to exercise, and a systematic review of randomized clinical trials published in 2019 supports this recommendation. This review included nine studies with a total of 680 participants, and the overall effect of exercise was a twofold improvement in symptoms associated with constipation. Walking was the most common exercise intervention, and along with qigong (which combines body position, breathing, and meditation), these two modes of exercise were effective in improving constipation. However, the one study evaluating resistance training failed to demonstrate a significant effect. Importantly, the reviewers considered the collective research to be at a high risk of bias.
Exercise will probably help Brenda, although some brainstorming might be necessary to help her fit exercise into her busy schedule. Another suggestion focused on her risk for colorectal cancer, and Dr. Cooke and Dr. Boboc both astutely noted that colorectal cancer is increasingly common among adults at early middle age. This stands in contrast to a steady decline in the prevalence of colorectal cancer among U.S. adults at age 65 years or older. Whereas colorectal cancer declined by 3.3% annually among U.S. older adults from 2011 to 2016, there was a reversal of this favorable trend among individuals between 50 and 64 years of age, with rates increasing by 1% annually.
The increase in the incidence of colorectal cancer among adults 50-64 years of age has been outpaced by the increase among adults younger than 50 years, who have experienced a 2.2% increase in the incidence of colorectal cancer annually between 2012 and 2016. Previously, the increase in colorectal cancer among early middle-aged adults was driven by higher rates of rectal cancer, but more recently this trend has included higher rates of proximal and distal colon tumors. In 2020, 12% of new cases of colorectal cancer were expected to be among individuals younger than 50 years.
So how do we act on this context in the case of Brenda? Her history suggests no overt warning signs for cancer. The history did not address a family history of gastrointestinal symptoms or colorectal cancer, which is an important omission.
Although the number of cases of cancer among persons younger than 50 years may be rising, the overall prevalence of colorectal cancer among younger adults is well under 1%. At 35 years of age, it is not necessary to evaluate Brenda for colorectal cancer. However, persistent or worsening symptoms could prompt a referral for colonoscopy at a later time.
Finally, let’s address how to practically manage Brenda’s case, because many options are available. I would begin with recommendations regarding her lifestyle, including regular exercise, adequate sleep, and whatever she can achieve in the FODMAP diet. I would also recommend psyllium as a soluble fiber and expect that these changes would help her constipation. But they might be less effective for abdominal cramping, so I would also recommend peppermint oil at this time.
If Brenda commits to these recommendations, she will very likely improve. If she does not, I will be more concerned regarding anxiety and depression complicating her illness. Treating those disorders can make a big difference.
In addition, if there is an inadequate response to initial therapy, I will initiate linaclotide or lubiprostone. Plecanatide is another reasonable option. At this point, I will also consider referral to a gastroenterologist for a recalcitrant case and will certainly refer if one of these specific treatments fails in Brenda. Conditions such as pelvic floor dysfunction can mimic irritable bowel syndrome with constipation and merit consideration.
However, I really believe that Brenda will feel better. Thanks for all of the insightful and interesting comments. It is easy to see how we are all invested in improving patients’ lives.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported disclosures with McNeil Pharmaceuticals. A version of this article first appeared on Medscape.com.
I recently presented a clinical scenario about a patient of mine named Brenda. This 35-year-old woman came to me with symptoms that had been going on for a year already. I asked for readers’ comments about my management of Brenda.
I appreciate the comments I received regarding this case. The most common suggestion was to encourage Brenda to exercise, and a systematic review of randomized clinical trials published in 2019 supports this recommendation. This review included nine studies with a total of 680 participants, and the overall effect of exercise was a twofold improvement in symptoms associated with constipation. Walking was the most common exercise intervention, and along with qigong (which combines body position, breathing, and meditation), these two modes of exercise were effective in improving constipation. However, the one study evaluating resistance training failed to demonstrate a significant effect. Importantly, the reviewers considered the collective research to be at a high risk of bias.
Exercise will probably help Brenda, although some brainstorming might be necessary to help her fit exercise into her busy schedule. Another suggestion focused on her risk for colorectal cancer, and Dr. Cooke and Dr. Boboc both astutely noted that colorectal cancer is increasingly common among adults at early middle age. This stands in contrast to a steady decline in the prevalence of colorectal cancer among U.S. adults at age 65 years or older. Whereas colorectal cancer declined by 3.3% annually among U.S. older adults from 2011 to 2016, there was a reversal of this favorable trend among individuals between 50 and 64 years of age, with rates increasing by 1% annually.
The increase in the incidence of colorectal cancer among adults 50-64 years of age has been outpaced by the increase among adults younger than 50 years, who have experienced a 2.2% increase in the incidence of colorectal cancer annually between 2012 and 2016. Previously, the increase in colorectal cancer among early middle-aged adults was driven by higher rates of rectal cancer, but more recently this trend has included higher rates of proximal and distal colon tumors. In 2020, 12% of new cases of colorectal cancer were expected to be among individuals younger than 50 years.
So how do we act on this context in the case of Brenda? Her history suggests no overt warning signs for cancer. The history did not address a family history of gastrointestinal symptoms or colorectal cancer, which is an important omission.
Although the number of cases of cancer among persons younger than 50 years may be rising, the overall prevalence of colorectal cancer among younger adults is well under 1%. At 35 years of age, it is not necessary to evaluate Brenda for colorectal cancer. However, persistent or worsening symptoms could prompt a referral for colonoscopy at a later time.
Finally, let’s address how to practically manage Brenda’s case, because many options are available. I would begin with recommendations regarding her lifestyle, including regular exercise, adequate sleep, and whatever she can achieve in the FODMAP diet. I would also recommend psyllium as a soluble fiber and expect that these changes would help her constipation. But they might be less effective for abdominal cramping, so I would also recommend peppermint oil at this time.
If Brenda commits to these recommendations, she will very likely improve. If she does not, I will be more concerned regarding anxiety and depression complicating her illness. Treating those disorders can make a big difference.
In addition, if there is an inadequate response to initial therapy, I will initiate linaclotide or lubiprostone. Plecanatide is another reasonable option. At this point, I will also consider referral to a gastroenterologist for a recalcitrant case and will certainly refer if one of these specific treatments fails in Brenda. Conditions such as pelvic floor dysfunction can mimic irritable bowel syndrome with constipation and merit consideration.
However, I really believe that Brenda will feel better. Thanks for all of the insightful and interesting comments. It is easy to see how we are all invested in improving patients’ lives.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported disclosures with McNeil Pharmaceuticals. A version of this article first appeared on Medscape.com.
The role of repeat uterine curettage in postmolar gestational trophoblastic neoplasia
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.
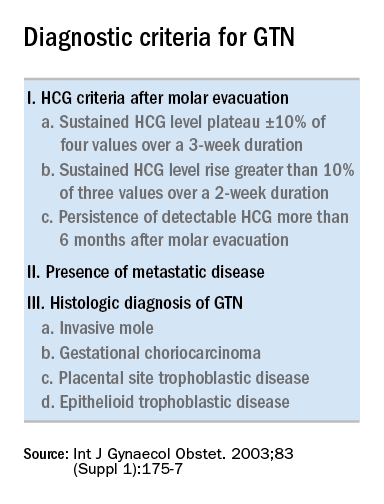
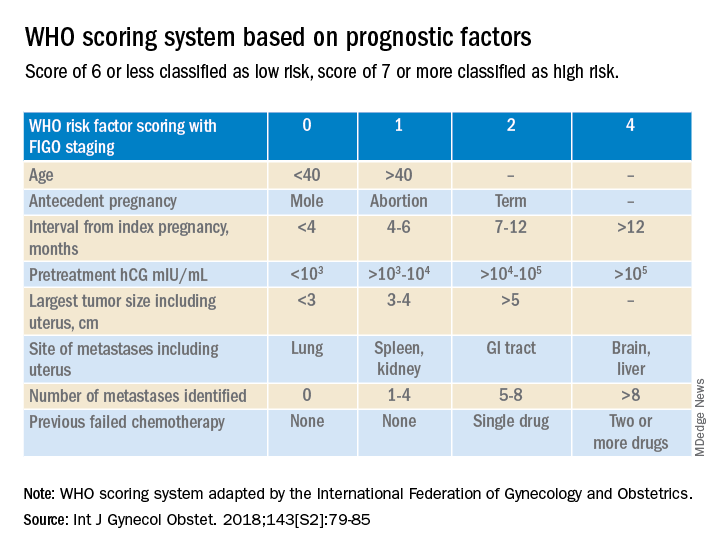
The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.


The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.
Trophoblastic tissue is responsible for formation of the placenta during pregnancy. Gestational trophoblastic disease (GTD), a group comprising benign (hydatidiform moles) and malignant tumors, occurs when gestational trophoblastic tissue behaves in an abnormal manner. Hydatidiform moles, which are thought to be caused by errors in fertilization, occur in approximately 1 in 1,200 pregnancies in the United States. Gestational trophoblastic neoplasia (GTN) refers to the subgroup of these trophoblastic or placental tumors with malignant behavior and includes postmolar GTN, invasive mole, gestational choriocarcinoma, placental-site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor. Postmolar GTN arises after evacuation of a molar pregnancy and is most frequently diagnosed by a plateau or increase in human chorionic gonadotropin (hCG).1 The risk of postmolar GTN is much higher after a complete mole (7%-30%) compared with a partial mole (2.5%-7.5%).2 Once postmolar GTN is diagnosed, a World Health Organization score is assigned to determine if patients have low- or high-risk disease.3 The primary treatment for most GTN is chemotherapy. A patient’s WHO score helps determine whether they would benefit from single-agent or multiagent chemotherapy. The standard of care for low-risk disease is single-agent chemotherapy with either methotrexate or actinomycin D.


The role of a second uterine curettage, after the diagnosis of low-risk postmolar GTN, has been controversial because of the limited data and disparate outcomes reported. In older retrospective series, a second curettage affected treatment or produced remission in only 9%-20% of patients and caused uterine perforation or major hemorrhage in 5%-8% of patients.4,5 Given relatively high rates of major complications compared with surgical cure or decreased chemotherapy cycles needed, only a limited number of patients seemed to benefit from a second procedure. On the other hand, an observational study of 544 patients who underwent second uterine evacuation after a presumed diagnosis of persistent GTD found that up to 60% of patients did not require chemotherapy afterward.6 Those with hCG levels greater than 1,500 IU/L or histologic evidence of GTD were less likely to have a surgical cure after second curettage. The indications for uterine evacuations were varied across these studies and make it nearly impossible to compare their results.
More recently, there have been two prospective trials that have tackled the question of the utility of second uterine evacuation in low-risk, nonmetastatic GTN. The Gynecologic Oncology Group performed a single-arm prospective study in the United States that enrolled patients with postmolar GTN to undergo second curettage as initial treatment of their disease.7 Of 60 eligible patients, 40% had a surgical cure (defined as normalization of hCG followed by at least 6 months of subsequent normal hCG values). Overall, 47% of patients were able to avoid chemotherapy. All surgical cures were seen in patients with WHO scores between 0 and 4. Importantly, three women were diagnosed with PSTT, which tends to be resistant to methotrexate and actinomycin D (treatment for nonmetastatic PSTT is definitive surgery with hysterectomy). The study found that hCG was a poor discriminator for achieving surgical cure. While age appeared to have an association with surgical cure (cure less likely for younger and older ages, younger than 19 and older than 40), patient numbers were too small to make a statistical conclusion. There were no uterine perforations and one patient had a grade 3 hemorrhage (requiring transfusion).
In the second prospective trial, performed in Iran, 62 patients were randomized to either second uterine evacuation or standard treatment after diagnosis of postmolar GTN.8 All patients in the surgical arm received a cervical ripening agent prior to their procedure, had their procedure under ultrasound guidance, and received misoprostol afterward to prevent uterine bleeding. Among those undergoing second uterine evacuation, 50% were cured (no need for chemotherapy). Among those needing chemotherapy after surgery, the mean number of cycles of chemotherapy needed (3.07 vs. 6.69) and the time it took to achieve negative hCG (3.23 vs. 9.19 weeks) were significantly less compared with patients who did not undergo surgery. hCG prior to second uterine evacuation could distinguish response to surgery compared with those needing chemotherapy (hCG of 1,983 IU/L or less was the level determined to best predict response). No complications related to surgery were reported.
Given prospective data available, second uterine evacuation for treatment of nonmetastatic, low-risk postmolar GTN is a reasonable treatment option and one that should be considered and discussed with patients given the potential to avoid chemotherapy or decrease the number of cycles needed. It may be prudent to limit the procedure to patients with an hCG less than 1,500-2,000 IU/L and to those between the ages of 20 and 40. While uterine hemorrhage and perforation have been reported in the literature, more recent data suggest low rates of these complications. Unfortunately, given the rarity of the disease and the historically controversial use of second curettage, little is known about the effects on future fertility that this procedure may have, including the development of uterine synechiae.
Dr. Tucker is assistant professor of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Ngan HY et al, FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2003 Oct;83 Suppl 1:175-7. Erratum in: Int J Gynaecol Obstet. 2021 Dec;155(3):563.
2. Soper JT. Obstet Gynecol. 2021 Feb.;137(2):355-70.
3. Ngan HY et al. Int J Gynecol Obstet. 2018;143:79-85.
4. Schlaerth JB et al. Am J Obstet Gynecol. 1990 Jun;162(6):1465-70.
5. van Trommel NE et al. Gynecol Oncol. 2005 Oct;99(1):6-13.
6. Pezeshki M et al. Gynecol Oncol. 2004 Dec;95(3):423-9.
7. Osborne RJ et al. Obstet Gynecol. 2016 Sep;128(3):535-42.
8. Ayatollahi H et al. Int J Womens Health. 2017 Sep 21;9:665-71.










