User login
Sunscreen Safety: 2024 Updates
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
Sunscreen is a cornerstone of skin cancer prevention. The first commercial sunscreen was developed nearly 100 years ago,1 yet questions and concerns about the safety of these essential topical photoprotective agents continue to occupy our minds. This article serves as an update on some of the big sunscreen questions, as informed by the available evidence.
Are sunscreens safe?
The story of sunscreen regulation in the United States is long and dry. The major pain point is that sunscreens are regulated by the US Food and Drug Administration (FDA) as over-the-counter drugs rather than cosmetics (as in Europe).2 Regulatory hurdles created a situation wherein no new active sunscreen ingredient has been approved by the FDA since 1999, except ecamsule for use in one product line. There is hope that changes enacted under the CARES Act will streamline and expedite the sunscreen approval process in the future.3
Amid the ongoing regulatory slog, the FDA became interested in learning more about sunscreen safety. Specifically, they sought to determine the GRASE (generally regarded as safe and effective) status of the active ingredients in sunscreens. In 2019, only the inorganic (physical/mineral) UV filters zinc oxide and titanium dioxide were considered GRASE.4 Trolamine salicylate and para-aminobenzoic acid were not GRASE, but they currently are not used in sunscreens in the United States. For all the remaining organic (chemical) filters, additional safety data were required to establish GRASE status.4 In 2024, the situation remains largely unchanged. Industry is working with the FDA on testing requirements.5
Why the focus on safety? After all, sunscreens have been used widely for decades without any major safety signals; their only well-established adverse effects are contact dermatitis and staining of clothing.6 Although preclinical studies raised concerns that chemical sunscreens could be associated with endocrine, reproductive, and neurologic toxicities, to date there are no high-quality human studies demonstrating negative effects.7,8
However, exposure patterns have evolved. Sunscreen is recommended to be applied (and reapplied) daily. Also, chemical UV filters are used in many nonsunscreen products such as cosmetics, shampoos, fragrances, and plastics. In the United States, exposure to chemical sunscreens is ubiquitous; according to data from the National Health and Nutrition Examination Survey 2003-2004, oxybenzone was detected in 97% of more than 2500 urine samples, implying systemic absorption but not harm.9
The FDA confirmed the implication of systemic absorption via 2 maximal usage trials published in 2019 and 2020.10,11 In both studies, several chemical sunscreens were applied at the recommended density of 2 mg/cm2 to 75% of the body surface area multiple times over 4 days. For all tested organic UV filters, blood levels exceeded the predetermined FDA cutoff (0.5 ng/mL), even after one application.10,11 What’s the takeaway? Simply that the FDA now requires additional safety data for chemical sunscreen filters5; the findings in no way imply any associated harm. Two potential mitigating factors are that no one applies sunscreen at 2 mg/cm2, and the FDA’s blood level cutoff was a general estimate not specific to sunscreens.4,12
Nevertheless, a good long-term safety record for sunscreens does not negate the need for enhanced safety data when there is clear evidence of systemic absorption. In the meantime, concerned patients should be counseled that the physical/mineral sunscreens containing zinc oxide and titanium dioxide are considered GRASE by the FDA; even in nanoparticle form, they generally have not been found to penetrate beneath the stratum corneum.7,13
Does sunscreen cause frontal fibrosing alopecia?
Dermatologists are confronting the conundrum of rising cases of frontal fibrosing alopecia (FFA). Several theories on the pathogenesis of this idiopathic scarring alopecia have been raised, one of which involves increased use of sunscreen. Proposed explanations for sunscreen’s role in FFA include a lichenoid reaction inducing hair follicle autoimmunity through an unclear mechanism; a T cell–mediated allergic reaction, which is unlikely according to contact dermatitis experts14; reactive oxygen species production by titanium nanoparticles, yet titanium has been detected in hair follicles of both patients with FFA and controls15; and endocrine disruption following systemic absorption, which has not been supported by any high-quality human studies.7
An association between facial sunscreen use and FFA has been reported in case-control studies16; however, they have been criticized due to methodologic issues and biases, and they provide no evidence of causality.17,18 The jury remains out on the controversial association between sunscreen and FFA, with a need for more convincing data.
Does sunscreen impact coral reef health?
Coral reefs—crucial sources of aquatic biodiversity—are under attack from several different directions including climate change and pollution. As much as 14,000 tons of sunscreen enter coral reefs each year, and chemical sunscreen filters are detectable in waterways throughout the world—even in the Arctic.19,20 Thus, sunscreen has come under scrutiny as a potential environmental threat, particularly with coral bleaching.
Bleaching is a process in which corals exposed to an environmental stressor expel their symbiotic photosynthetic algae and turn white; if conditions fail to improve, the corals are vulnerable to death. In a highly cited 2016 study, coral larvae exposed to oxybenzone in artificial laboratory conditions displayed concentration-dependent mortality and decreased chlorophyll fluorescence, which suggested bleaching.19 These findings influenced legislation in Hawaii and other localities banning sunscreens containing oxybenzone. Problematically, the study has been criticized for acutely exposing the most susceptible coral life-forms to unrealistic oxybenzone concentrations; more broadly, there is no standardized approach to coral toxicity testing.21
The bigger picture (and elephant in the room) is that the primary cause of coral bleaching is undoubtedly climate change/ocean warming.7 More recent studies suggest that oxybenzone probably adds insult to injury for corals already debilitated by ocean warming.22,23
It has been posited that a narrow focus on sunscreens detracts attention from the climate issue.24 Individuals can take a number of actions to reduce their carbon footprint in an effort to preserve our environment, specifically coral reefs.25 Concerned patients should be counseled to use sunscreens containing the physical/mineral UV filters zinc oxide and titanium dioxide, which are unlikely to contribute to coral bleaching as commercially formulated.7
Ongoing Questions
A lot of unknowns about sunscreen safety remain, and much hubbub has been made over studies that often are preliminary at best. At the time of this writing, absent a crystal ball, this author continues to wear chemical sunscreens; spends a lot more time worrying about their carbon footprint than what type of sunscreen to use at the beach; and believes the association of FFA with sunscreen is unlikely to be causal. Hopefully much-needed rigorous evidence will guide our future approach to sunscreen formulation and use.
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
- Ma Y, Yoo J. History of sunscreen: an updated view. J Cosmet Dermatol. 2021;20:1044-1049.
- Pantelic MN, Wong N, Kwa M, et al. Ultraviolet filters in the United States and European Union: a review of safety and implications for the future of US sunscreens. J Am Acad Dermatol. 2023;88:632-646.
- Mohammad TF, Lim HW. The important role of dermatologists in public education on sunscreens. JAMA Dermatol. 2021;157:509-511.
- Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regist. 2019;84:6204-6275.
- Lim HW, Mohammad TF, Wang SQ. Food and Drug Administration’s proposed sunscreen final administrative order: how does it affect sunscreens in the United States? J Am Acad Dermatol. 2022;86:E83-E84.
- Ekstein SF, Hylwa S. Sunscreens: a review of UV filters and their allergic potential. Dermatitis. 2023;34:176-190.
- Adler BL, DeLeo VA. Sunscreen safety: a review of recent studies on humans and the environment. Curr Dermatol Rep. 2020;9:1-9.
- Suh S, Pham C, Smith J, et al. The banned sunscreen ingredients and their impact on human health: a systematic review. Int J Dermatol. 2020;59:1033-1042.
- Calafat AM, Wong LY, Ye X, et al. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003-2004. Environ Health Perspect. 2008;116:893-897.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Petersen B, Wulf HC. Application of sunscreen—theory and reality. Photodermatol Photoimmunol Photomed. 2014;30:96-101.
- Mohammed YH, Holmes A, Haridass IN, et al. Support for the safe use of zinc oxide nanoparticle sunscreens: lack of skin penetration or cellular toxicity after repeated application in volunteers. J Invest Dermatol. 2019;139:308-315.
- Felmingham C, Yip L, Tam M, et al. Allergy to sunscreen and leave-on facial products is not a likely causative mechanism in frontal fibrosing alopecia: perspective from contact allergy experts. Br J Dermatol. 2020;182:481-482.
- Thompson CT, Chen ZQ, Kolivras A, et al. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181:216-217.
- Maghfour J, Ceresnie M, Olson J, et al. The association between frontal fibrosing alopecia, sunscreen, and moisturizers: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:395-396.
- Seegobin SD, Tziotzios C, Stefanato CM, et al. Frontal fibrosing alopecia:there is no statistically significant association with leave-on facial skin care products and sunscreens. Br J Dermatol. 2016;175:1407-1408.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Regarding methodologic concerns in clinical studies on frontal fibrosing alopecia. J Am Acad Dermatol. 2021;84:E207-E208.
- Downs CA, Kramarsky-Winter E, Segal R, et al. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the US Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265-288.
- National Academies of Sciences, Engineering, and Medicine. Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health. The National Academies Press; 2022.
- Mitchelmore CL, Burns EE, Conway A, et al. A critical review of organic ultraviolet filter exposure, hazard, and risk to corals. Environ Toxicol Chem. 2021;40:967-988.
- Vuckovic D, Tinoco AI, Ling L, et al. Conversion of oxybenzone sunscreen to phototoxic glucoside conjugates by sea anemones and corals. Science. 2022;376:644-648.
- Wijgerde T, van Ballegooijen M, Nijland R, et al. Adding insult to injury: effects of chronic oxybenzone exposure and elevated temperature on two reef-building corals. Sci Total Environ. 2020;733:139030.
- Sirois J. Examine all available evidence before making decisions on sunscreen ingredient bans. Sci Total Environ. 2019;674:211-212.
- United Nations. Actions for a healthy planet. Accessed April 15, 2024. https://www.un.org/en/actnow/ten-actions
Clinical Manifestation of Degos Disease: Painful Penile Ulcers
To the Editor:
A 56-year-old man was referred to our Grand Rounds by another dermatologist in our health system for evaluation of a red scaly rash on the trunk that had been present for more than a year. More recently, over the course of approximately 9 months he experienced recurrent painful penile ulcers that lasted for approximately 4 weeks and then self-resolved. He had a medical history of central retinal vein occlusion, primary hyperparathyroidism, and nonspecific colitis. A family history was notable for lung cancer in the patient’s father and myelodysplastic syndrome and breast cancer in his mother; however, there was no family history of a similar rash. A bacterial culture of the penile ulcer was negative. Testing for antibodies against HIV and herpes simplex virus (HSV) types 1 and 2 was negative. Results of a serum VDRL test were nonreactive, which ruled out syphilis. The patient was treated by the referring dermatologist with azithromycin for possible chancroid without relief.
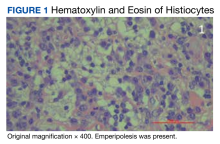
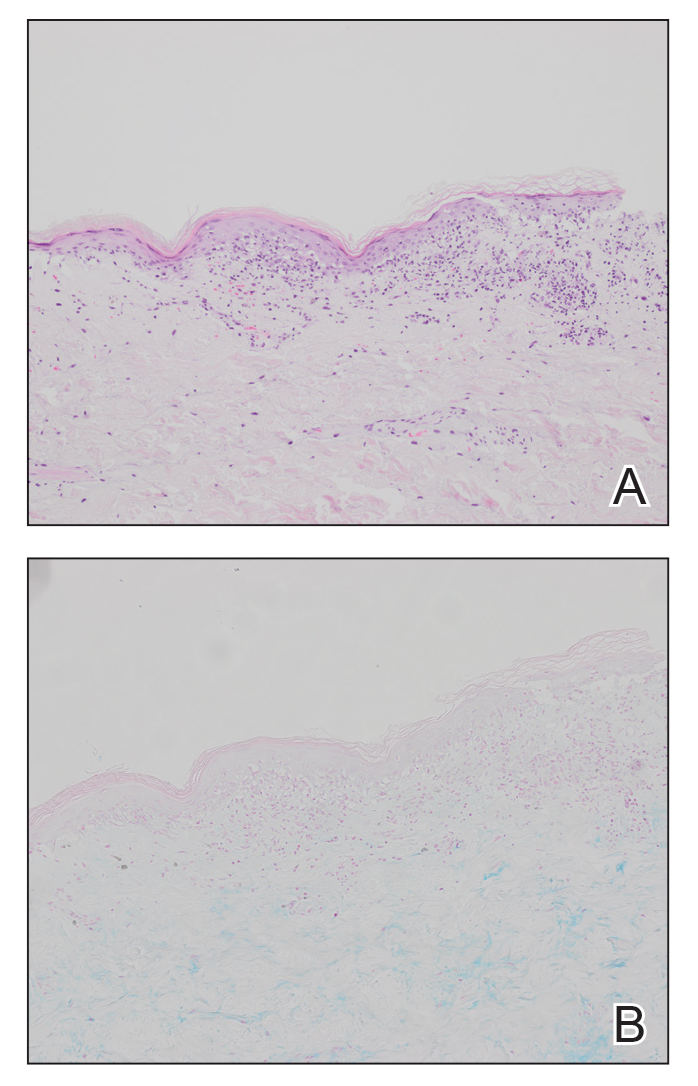
The patient was being followed by the referring dermatologist who initially was concerned for Degos disease based on clinical examination findings, prompting biopsy of a lesion on the back, which revealed vacuolar interface dermatitis, a sparse superficial perivascular lymphocytic infiltrate, and increased mucin—all highly suspicious for connective tissue disease (Figure 1). An antinuclear antibody test was positive, with a titer of 1:640. The patient was started on prednisone and referred to rheumatology; however, further evaluation by rheumatology for an autoimmune process—including anticardiolipin antibodies—was unremarkable. A few months prior to the current presentation, he also had mildly elevated liver function test results. A colonoscopy was performed, and a biopsy revealed nonspecific colitis. A biopsy of the penile ulcer also was nonspecific, showing only ulceration and acute and chronic inflammation. No epidermal interface change was seen. Results from a Grocott-Gomori methenamine-silver stain, Treponema pallidum immunostain, and HSV polymerase chain reaction were negative for fungal organisms, spirochetes, and HSV, respectively. The differential diagnosis included trauma, aphthous ulceration, and Behçet disease. Behçet disease was suspected by the referring dermatologist, and the patient was treated with colchicine, prednisone, pimecrolimus cream, and topical lidocaine; however, the lesions persisted, and he was subsequently referred to our Grand Rounds for further evaluation.
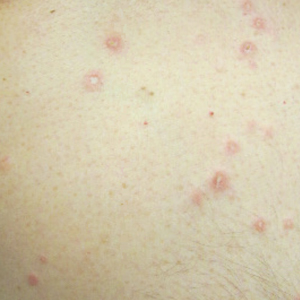
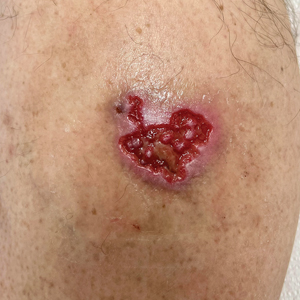
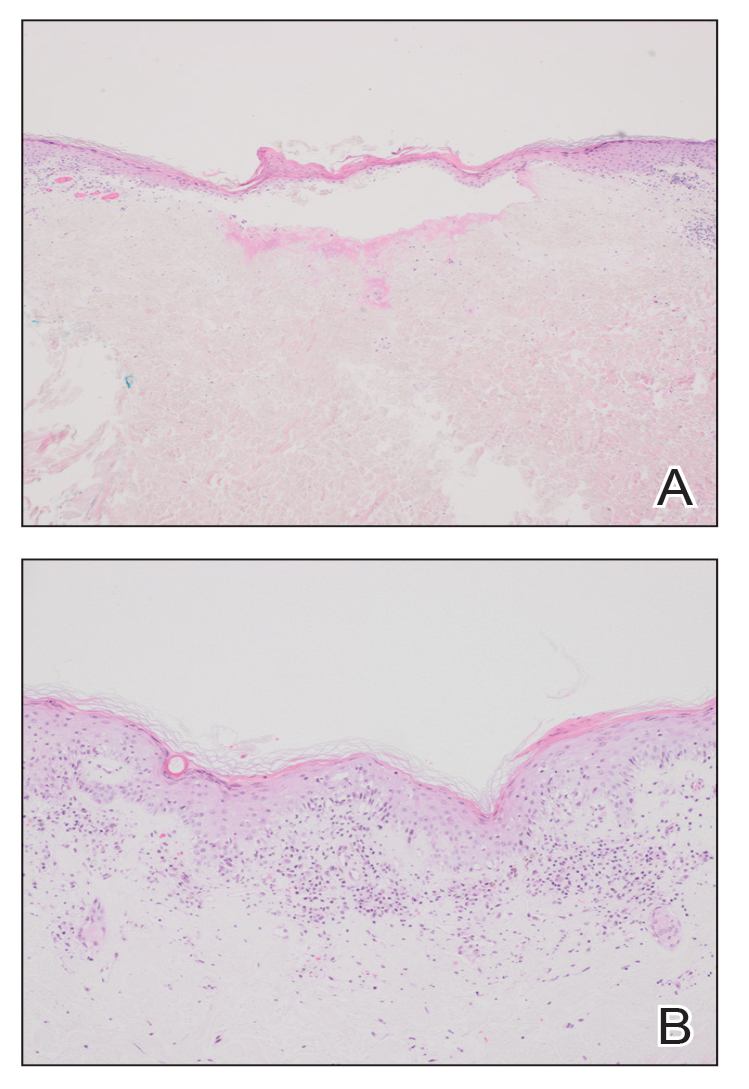
At the current presentation, physical examination revealed several small papules with white atrophic centers and erythematous rims on the trunk and extremities (Figure 2A). An ulceration was noted on the penile shaft (Figure 2B). Further evaluation for Behçet disease, including testing for pathergy and HLA-B51, was negative. Degos disease was strongly suspected clinically, and a repeat biopsy was performed of a lesion on the abdomen, which revealed central epidermal necrosis, atrophy, and parakeratosis with an underlying wedge-shaped dermal infarct surrounded by multiple small occluded dermal vessels, perivascular inflammation, and dermal edema (Figure 3). Direct immunofluorescence was performed using antibodies against IgG, IgA, IgM, fibrinogen, albumin, and C3, which was negative. These findings from direct immunofluorescence and histopathology as well as the clinical presentation were considered compatible with Degos disease. The patient was started on aspirin and pentoxifylline. Pentoxifylline 400 mg twice daily appeared to lessen some of the pain. Pain management specialists started the patient on gabapentin.

Approximately 4 months after the Grand Rounds evaluation, during which time he continued treatment with pentoxifylline, he was admitted to the hospital for intractable nausea and vomiting. His condition acutely declined due to bowel perforation, and he was started on eculizumab 1200 mg every 14 days. Because of an increased risk for meningococcal meningitis while on this medication, he also was given erythromycin 500 mg twice daily prophylactically. He was being followed by hematology for the vasculopathy, and they were planning to monitor for any disease changes with computed tomography of the chest, abdomen, and pelvis every 3 months, as well as echocardiogram every 6 months for any development of pericardial or pleural fibrosis. Approximately 1 month later, the patient was admitted to the hospital again but died after 1 week from gastrointestinal complications (approximately 22 months after the onset of the rash).
Degos disease (atrophic papulosis) is a rare small vessel vasculopathy of unknown etiology, but complement-mediated endothelial injury plays a role.1,2 It typically occurs in the fourth decade of life, with a slight female predominance.3,4 The skin lesions are characteristic and described as 5- to 10-mm papules with atrophic white centers and erythematous telangiectatic rims, most commonly on the upper body and typically sparing the head, palms, and soles.1 Penile ulceration is an uncommon cutaneous feature, with only a few cases reported in the literature.5,6 Approximately one-third of patients will have only skin lesions, but two-thirds will develop systemic involvement 1 to 2 years after onset, with the gastrointestinal tract and central nervous system most commonly involved. For those with systemic involvement, the 5-year survival rate is approximately 55%, and the most common causes of death are bowel perforation, peritonitis, and stroke.3,4 Because some patients appear to never develop systemic complications, Theodoridis et al4 proposed that the disease be classified as either malignant atrophic papulosis or benign atrophic papulosis to indicate the malignant systemic form and the benign cutaneous form, respectively.
The histopathology of Degos disease changes as the lesions evolve.7 Early lesions show a superficial and deep perivascular and periadnexal lymphocytic infiltrate, possible interface dermatitis, and dermal mucin resembling lupus. The more fully developed lesions show a greater degree of inflammation and interface change as well as lymphocytic vasculitis. This stage also may have epidermal atrophy and early papillary dermal sclerosis resembling lichen sclerosus. The late-stage lesions, clinically observed as papules with atrophic white centers and surrounding erythema, show the classic pathology of wedge-shaped dermal sclerosis and central epidermal atrophy with surrounding hyperkeratosis. Interface dermatitis and dermal mucin can be seen in all stages, though mucin is diminished in the later stage.
Effective treatment options are limited; however, antithrombotics or compounds that facilitate blood perfusion, such as aspirin or pentoxifylline, initially can be used.1 Eculizumab, a humanized monoclonal antibody that prevents the cleavage of C5, has been used for salvage therapy,8 as in our case. Treprostinil, a prostacyclin analog that causes arterial vasodilation and inhibition of platelet aggregation, has been reported to improve bowel and cutaneous lesions, functional status, and neurologic symptoms.9
Our case highlights important features of Degos disease. First, it is important for both the clinician and the pathologist to recognize that the histopathology of Degos disease changes as the lesions evolve. In our case, although the lesions were characteristic of Degos disease clinically, the initial biopsy was suspicious for connective tissue disease, which led to an autoimmune evaluation that ultimately was unremarkable. Recognizing that early lesions of Degos disease can resemble connective tissue disease histologically could have prevented this delay in diagnosis. However, Degos disease has been reported in association with autoimmune diseases.10 Second, although penile ulceration is uncommon, it can be a prominent cutaneous manifestation of the disease. Finally, eculizumab and treprostinil are therapeutic options that have shown some efficacy in improving symptoms and cutaneous lesions.8,9
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10. doi:10.1186/1750-1172-8-10
- Magro CM, Poe JC, Kim C, et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599-610. doi:10.1309/AJCP66QIMFARLZKI
- Hu P, Mao Z, Liu C, et al. Malignant atrophic papulosis with motor aphasia and intestinal perforation: a case report and review of published works. J Dermatol. 2018;45:723-726. doi:10.1111/1346-8138.14280
- Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115. doi:10.1111/bjd.12642
- Thomson KF, Highet AS. Penile ulceration in fatal malignant atrophic papulosis (Degos’ disease). Br J Dermatol. 2000;143:1320-1322. doi:10.1046/j.1365-2133.2000.03911.x
- Aydogan K, Alkan G, Karadogan Koran S, et al. Painful penile ulceration in a patient with malignant atrophic papulosis. J Eur Acad Dermatol Venereol. 2005;19:612-616. doi:10.1111/j.1468-3083.2005.01227.x
- Harvell JD, Williford PL, White WL. Benign cutaneous Degos’ disease: a case report with emphasis on histopathology as papules chronologically evolve. Am J Dermatopathol. 2001;23:116-123. doi:10.1097/00000372-200104000-00006
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277. doi:10.1016/j.jaad.2016.09.015
- Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52. doi:10.1186/1750-1172-8-52
- Burgin S, Stone JH, Shenoy-Bhangle AS, et al. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-year-old man with a rash, myalgia, and weakness. N Engl J Med. 2014;370:2327-2337. doi:10.1056/NEJMcpc1304161
To the Editor:
A 56-year-old man was referred to our Grand Rounds by another dermatologist in our health system for evaluation of a red scaly rash on the trunk that had been present for more than a year. More recently, over the course of approximately 9 months he experienced recurrent painful penile ulcers that lasted for approximately 4 weeks and then self-resolved. He had a medical history of central retinal vein occlusion, primary hyperparathyroidism, and nonspecific colitis. A family history was notable for lung cancer in the patient’s father and myelodysplastic syndrome and breast cancer in his mother; however, there was no family history of a similar rash. A bacterial culture of the penile ulcer was negative. Testing for antibodies against HIV and herpes simplex virus (HSV) types 1 and 2 was negative. Results of a serum VDRL test were nonreactive, which ruled out syphilis. The patient was treated by the referring dermatologist with azithromycin for possible chancroid without relief.
The patient was being followed by the referring dermatologist who initially was concerned for Degos disease based on clinical examination findings, prompting biopsy of a lesion on the back, which revealed vacuolar interface dermatitis, a sparse superficial perivascular lymphocytic infiltrate, and increased mucin—all highly suspicious for connective tissue disease (Figure 1). An antinuclear antibody test was positive, with a titer of 1:640. The patient was started on prednisone and referred to rheumatology; however, further evaluation by rheumatology for an autoimmune process—including anticardiolipin antibodies—was unremarkable. A few months prior to the current presentation, he also had mildly elevated liver function test results. A colonoscopy was performed, and a biopsy revealed nonspecific colitis. A biopsy of the penile ulcer also was nonspecific, showing only ulceration and acute and chronic inflammation. No epidermal interface change was seen. Results from a Grocott-Gomori methenamine-silver stain, Treponema pallidum immunostain, and HSV polymerase chain reaction were negative for fungal organisms, spirochetes, and HSV, respectively. The differential diagnosis included trauma, aphthous ulceration, and Behçet disease. Behçet disease was suspected by the referring dermatologist, and the patient was treated with colchicine, prednisone, pimecrolimus cream, and topical lidocaine; however, the lesions persisted, and he was subsequently referred to our Grand Rounds for further evaluation.

At the current presentation, physical examination revealed several small papules with white atrophic centers and erythematous rims on the trunk and extremities (Figure 2A). An ulceration was noted on the penile shaft (Figure 2B). Further evaluation for Behçet disease, including testing for pathergy and HLA-B51, was negative. Degos disease was strongly suspected clinically, and a repeat biopsy was performed of a lesion on the abdomen, which revealed central epidermal necrosis, atrophy, and parakeratosis with an underlying wedge-shaped dermal infarct surrounded by multiple small occluded dermal vessels, perivascular inflammation, and dermal edema (Figure 3). Direct immunofluorescence was performed using antibodies against IgG, IgA, IgM, fibrinogen, albumin, and C3, which was negative. These findings from direct immunofluorescence and histopathology as well as the clinical presentation were considered compatible with Degos disease. The patient was started on aspirin and pentoxifylline. Pentoxifylline 400 mg twice daily appeared to lessen some of the pain. Pain management specialists started the patient on gabapentin.

Approximately 4 months after the Grand Rounds evaluation, during which time he continued treatment with pentoxifylline, he was admitted to the hospital for intractable nausea and vomiting. His condition acutely declined due to bowel perforation, and he was started on eculizumab 1200 mg every 14 days. Because of an increased risk for meningococcal meningitis while on this medication, he also was given erythromycin 500 mg twice daily prophylactically. He was being followed by hematology for the vasculopathy, and they were planning to monitor for any disease changes with computed tomography of the chest, abdomen, and pelvis every 3 months, as well as echocardiogram every 6 months for any development of pericardial or pleural fibrosis. Approximately 1 month later, the patient was admitted to the hospital again but died after 1 week from gastrointestinal complications (approximately 22 months after the onset of the rash).

Degos disease (atrophic papulosis) is a rare small vessel vasculopathy of unknown etiology, but complement-mediated endothelial injury plays a role.1,2 It typically occurs in the fourth decade of life, with a slight female predominance.3,4 The skin lesions are characteristic and described as 5- to 10-mm papules with atrophic white centers and erythematous telangiectatic rims, most commonly on the upper body and typically sparing the head, palms, and soles.1 Penile ulceration is an uncommon cutaneous feature, with only a few cases reported in the literature.5,6 Approximately one-third of patients will have only skin lesions, but two-thirds will develop systemic involvement 1 to 2 years after onset, with the gastrointestinal tract and central nervous system most commonly involved. For those with systemic involvement, the 5-year survival rate is approximately 55%, and the most common causes of death are bowel perforation, peritonitis, and stroke.3,4 Because some patients appear to never develop systemic complications, Theodoridis et al4 proposed that the disease be classified as either malignant atrophic papulosis or benign atrophic papulosis to indicate the malignant systemic form and the benign cutaneous form, respectively.
The histopathology of Degos disease changes as the lesions evolve.7 Early lesions show a superficial and deep perivascular and periadnexal lymphocytic infiltrate, possible interface dermatitis, and dermal mucin resembling lupus. The more fully developed lesions show a greater degree of inflammation and interface change as well as lymphocytic vasculitis. This stage also may have epidermal atrophy and early papillary dermal sclerosis resembling lichen sclerosus. The late-stage lesions, clinically observed as papules with atrophic white centers and surrounding erythema, show the classic pathology of wedge-shaped dermal sclerosis and central epidermal atrophy with surrounding hyperkeratosis. Interface dermatitis and dermal mucin can be seen in all stages, though mucin is diminished in the later stage.
Effective treatment options are limited; however, antithrombotics or compounds that facilitate blood perfusion, such as aspirin or pentoxifylline, initially can be used.1 Eculizumab, a humanized monoclonal antibody that prevents the cleavage of C5, has been used for salvage therapy,8 as in our case. Treprostinil, a prostacyclin analog that causes arterial vasodilation and inhibition of platelet aggregation, has been reported to improve bowel and cutaneous lesions, functional status, and neurologic symptoms.9
Our case highlights important features of Degos disease. First, it is important for both the clinician and the pathologist to recognize that the histopathology of Degos disease changes as the lesions evolve. In our case, although the lesions were characteristic of Degos disease clinically, the initial biopsy was suspicious for connective tissue disease, which led to an autoimmune evaluation that ultimately was unremarkable. Recognizing that early lesions of Degos disease can resemble connective tissue disease histologically could have prevented this delay in diagnosis. However, Degos disease has been reported in association with autoimmune diseases.10 Second, although penile ulceration is uncommon, it can be a prominent cutaneous manifestation of the disease. Finally, eculizumab and treprostinil are therapeutic options that have shown some efficacy in improving symptoms and cutaneous lesions.8,9
To the Editor:
A 56-year-old man was referred to our Grand Rounds by another dermatologist in our health system for evaluation of a red scaly rash on the trunk that had been present for more than a year. More recently, over the course of approximately 9 months he experienced recurrent painful penile ulcers that lasted for approximately 4 weeks and then self-resolved. He had a medical history of central retinal vein occlusion, primary hyperparathyroidism, and nonspecific colitis. A family history was notable for lung cancer in the patient’s father and myelodysplastic syndrome and breast cancer in his mother; however, there was no family history of a similar rash. A bacterial culture of the penile ulcer was negative. Testing for antibodies against HIV and herpes simplex virus (HSV) types 1 and 2 was negative. Results of a serum VDRL test were nonreactive, which ruled out syphilis. The patient was treated by the referring dermatologist with azithromycin for possible chancroid without relief.
The patient was being followed by the referring dermatologist who initially was concerned for Degos disease based on clinical examination findings, prompting biopsy of a lesion on the back, which revealed vacuolar interface dermatitis, a sparse superficial perivascular lymphocytic infiltrate, and increased mucin—all highly suspicious for connective tissue disease (Figure 1). An antinuclear antibody test was positive, with a titer of 1:640. The patient was started on prednisone and referred to rheumatology; however, further evaluation by rheumatology for an autoimmune process—including anticardiolipin antibodies—was unremarkable. A few months prior to the current presentation, he also had mildly elevated liver function test results. A colonoscopy was performed, and a biopsy revealed nonspecific colitis. A biopsy of the penile ulcer also was nonspecific, showing only ulceration and acute and chronic inflammation. No epidermal interface change was seen. Results from a Grocott-Gomori methenamine-silver stain, Treponema pallidum immunostain, and HSV polymerase chain reaction were negative for fungal organisms, spirochetes, and HSV, respectively. The differential diagnosis included trauma, aphthous ulceration, and Behçet disease. Behçet disease was suspected by the referring dermatologist, and the patient was treated with colchicine, prednisone, pimecrolimus cream, and topical lidocaine; however, the lesions persisted, and he was subsequently referred to our Grand Rounds for further evaluation.

At the current presentation, physical examination revealed several small papules with white atrophic centers and erythematous rims on the trunk and extremities (Figure 2A). An ulceration was noted on the penile shaft (Figure 2B). Further evaluation for Behçet disease, including testing for pathergy and HLA-B51, was negative. Degos disease was strongly suspected clinically, and a repeat biopsy was performed of a lesion on the abdomen, which revealed central epidermal necrosis, atrophy, and parakeratosis with an underlying wedge-shaped dermal infarct surrounded by multiple small occluded dermal vessels, perivascular inflammation, and dermal edema (Figure 3). Direct immunofluorescence was performed using antibodies against IgG, IgA, IgM, fibrinogen, albumin, and C3, which was negative. These findings from direct immunofluorescence and histopathology as well as the clinical presentation were considered compatible with Degos disease. The patient was started on aspirin and pentoxifylline. Pentoxifylline 400 mg twice daily appeared to lessen some of the pain. Pain management specialists started the patient on gabapentin.

Approximately 4 months after the Grand Rounds evaluation, during which time he continued treatment with pentoxifylline, he was admitted to the hospital for intractable nausea and vomiting. His condition acutely declined due to bowel perforation, and he was started on eculizumab 1200 mg every 14 days. Because of an increased risk for meningococcal meningitis while on this medication, he also was given erythromycin 500 mg twice daily prophylactically. He was being followed by hematology for the vasculopathy, and they were planning to monitor for any disease changes with computed tomography of the chest, abdomen, and pelvis every 3 months, as well as echocardiogram every 6 months for any development of pericardial or pleural fibrosis. Approximately 1 month later, the patient was admitted to the hospital again but died after 1 week from gastrointestinal complications (approximately 22 months after the onset of the rash).

Degos disease (atrophic papulosis) is a rare small vessel vasculopathy of unknown etiology, but complement-mediated endothelial injury plays a role.1,2 It typically occurs in the fourth decade of life, with a slight female predominance.3,4 The skin lesions are characteristic and described as 5- to 10-mm papules with atrophic white centers and erythematous telangiectatic rims, most commonly on the upper body and typically sparing the head, palms, and soles.1 Penile ulceration is an uncommon cutaneous feature, with only a few cases reported in the literature.5,6 Approximately one-third of patients will have only skin lesions, but two-thirds will develop systemic involvement 1 to 2 years after onset, with the gastrointestinal tract and central nervous system most commonly involved. For those with systemic involvement, the 5-year survival rate is approximately 55%, and the most common causes of death are bowel perforation, peritonitis, and stroke.3,4 Because some patients appear to never develop systemic complications, Theodoridis et al4 proposed that the disease be classified as either malignant atrophic papulosis or benign atrophic papulosis to indicate the malignant systemic form and the benign cutaneous form, respectively.
The histopathology of Degos disease changes as the lesions evolve.7 Early lesions show a superficial and deep perivascular and periadnexal lymphocytic infiltrate, possible interface dermatitis, and dermal mucin resembling lupus. The more fully developed lesions show a greater degree of inflammation and interface change as well as lymphocytic vasculitis. This stage also may have epidermal atrophy and early papillary dermal sclerosis resembling lichen sclerosus. The late-stage lesions, clinically observed as papules with atrophic white centers and surrounding erythema, show the classic pathology of wedge-shaped dermal sclerosis and central epidermal atrophy with surrounding hyperkeratosis. Interface dermatitis and dermal mucin can be seen in all stages, though mucin is diminished in the later stage.
Effective treatment options are limited; however, antithrombotics or compounds that facilitate blood perfusion, such as aspirin or pentoxifylline, initially can be used.1 Eculizumab, a humanized monoclonal antibody that prevents the cleavage of C5, has been used for salvage therapy,8 as in our case. Treprostinil, a prostacyclin analog that causes arterial vasodilation and inhibition of platelet aggregation, has been reported to improve bowel and cutaneous lesions, functional status, and neurologic symptoms.9
Our case highlights important features of Degos disease. First, it is important for both the clinician and the pathologist to recognize that the histopathology of Degos disease changes as the lesions evolve. In our case, although the lesions were characteristic of Degos disease clinically, the initial biopsy was suspicious for connective tissue disease, which led to an autoimmune evaluation that ultimately was unremarkable. Recognizing that early lesions of Degos disease can resemble connective tissue disease histologically could have prevented this delay in diagnosis. However, Degos disease has been reported in association with autoimmune diseases.10 Second, although penile ulceration is uncommon, it can be a prominent cutaneous manifestation of the disease. Finally, eculizumab and treprostinil are therapeutic options that have shown some efficacy in improving symptoms and cutaneous lesions.8,9
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10. doi:10.1186/1750-1172-8-10
- Magro CM, Poe JC, Kim C, et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599-610. doi:10.1309/AJCP66QIMFARLZKI
- Hu P, Mao Z, Liu C, et al. Malignant atrophic papulosis with motor aphasia and intestinal perforation: a case report and review of published works. J Dermatol. 2018;45:723-726. doi:10.1111/1346-8138.14280
- Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115. doi:10.1111/bjd.12642
- Thomson KF, Highet AS. Penile ulceration in fatal malignant atrophic papulosis (Degos’ disease). Br J Dermatol. 2000;143:1320-1322. doi:10.1046/j.1365-2133.2000.03911.x
- Aydogan K, Alkan G, Karadogan Koran S, et al. Painful penile ulceration in a patient with malignant atrophic papulosis. J Eur Acad Dermatol Venereol. 2005;19:612-616. doi:10.1111/j.1468-3083.2005.01227.x
- Harvell JD, Williford PL, White WL. Benign cutaneous Degos’ disease: a case report with emphasis on histopathology as papules chronologically evolve. Am J Dermatopathol. 2001;23:116-123. doi:10.1097/00000372-200104000-00006
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277. doi:10.1016/j.jaad.2016.09.015
- Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52. doi:10.1186/1750-1172-8-52
- Burgin S, Stone JH, Shenoy-Bhangle AS, et al. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-year-old man with a rash, myalgia, and weakness. N Engl J Med. 2014;370:2327-2337. doi:10.1056/NEJMcpc1304161
- Theodoridis A, Makrantonaki E, Zouboulis CC. Malignant atrophic papulosis (Köhlmeier-Degos disease)—a review. Orphanet J Rare Dis. 2013;8:10. doi:10.1186/1750-1172-8-10
- Magro CM, Poe JC, Kim C, et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599-610. doi:10.1309/AJCP66QIMFARLZKI
- Hu P, Mao Z, Liu C, et al. Malignant atrophic papulosis with motor aphasia and intestinal perforation: a case report and review of published works. J Dermatol. 2018;45:723-726. doi:10.1111/1346-8138.14280
- Theodoridis A, Konstantinidou A, Makrantonaki E, et al. Malignant and benign forms of atrophic papulosis (Köhlmeier-Degos disease): systemic involvement determines the prognosis. Br J Dermatol. 2014;170:110-115. doi:10.1111/bjd.12642
- Thomson KF, Highet AS. Penile ulceration in fatal malignant atrophic papulosis (Degos’ disease). Br J Dermatol. 2000;143:1320-1322. doi:10.1046/j.1365-2133.2000.03911.x
- Aydogan K, Alkan G, Karadogan Koran S, et al. Painful penile ulceration in a patient with malignant atrophic papulosis. J Eur Acad Dermatol Venereol. 2005;19:612-616. doi:10.1111/j.1468-3083.2005.01227.x
- Harvell JD, Williford PL, White WL. Benign cutaneous Degos’ disease: a case report with emphasis on histopathology as papules chronologically evolve. Am J Dermatopathol. 2001;23:116-123. doi:10.1097/00000372-200104000-00006
- Oliver B, Boehm M, Rosing DR, et al. Diffuse atrophic papules and plaques, intermittent abdominal pain, paresthesias, and cardiac abnormalities in a 55-year-old woman. J Am Acad Dermatol. 2016;75:1274-1277. doi:10.1016/j.jaad.2016.09.015
- Shapiro LS, Toledo-Garcia AE, Farrell JF. Effective treatment of malignant atrophic papulosis (Köhlmeier-Degos disease) with treprostinil—early experience. Orphanet J Rare Dis. 2013;8:52. doi:10.1186/1750-1172-8-52
- Burgin S, Stone JH, Shenoy-Bhangle AS, et al. Case records of the Massachusetts General Hospital. Case 18-2014. A 32-year-old man with a rash, myalgia, and weakness. N Engl J Med. 2014;370:2327-2337. doi:10.1056/NEJMcpc1304161
PRACTICE POINTS
- Papules with atrophic white centers and erythematous telangiectatic rims are the characteristic skin lesions found in Degos disease.
- A painful penile ulceration also may occur in Degos disease, though it is uncommon.
- The histopathology of skin lesions changes as the lesions evolve. Early lesions may resemble connective tissue disease. Late lesions show the classic pathology of wedge-shaped dermal sclerosis.
Commentary: Evaluating Recent BC Treatment Trials, May 2024
The class of CDK 4/6 inhibitors represents a significant advance in the treatment of hormone receptor (HR)-positive breast cancer. All three CDK 4/6 inhibitors (palbociclib, abemaciclib, and ribociclib) are approved in combination with endocrine therapy in the metastatic setting. As drugs show promise in later-stage disease, they are then often studied in the curative space. Presently, abemaciclib is the only CDK 4/6 inhibitor that has been approved by the US Food and Drug Administration for the treatment of HR-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive, high-risk early breast cancer, based on results from the monarchE trial, which demonstrated invasive disease-free survival benefit with the addition of 2 years of abemaciclib to endocrine therapy. At 4 years, the absolute difference in invasive disease-free survival (IDFS) between the groups was 6.4% (85.8% in the abemaciclib + endocrine therapy group vs 79.4% in the endocrine therapy–alone group).[3] In contrast, the PENELOPE-B and PALLAS trials did not show benefit with the addition of palbociclib to endocrine therapy in the adjuvant setting.[4,5] The phase 3 NATALEE trial randomly assigned patients with HR-positive, HER2-negative early breast cancer to ribociclib (400 mg daily for 3 weeks followed by 1 week off for 3 years) plus a nonsteroidal aromatase inhibitor (NSAI) or an NSAI alone. At the time of prespecified interim analysis, among 5101 patients, ribociclib + NSAI led to a significant improvement in IDFS compared with endocrine therapy alone (3-year IDFS was 90.4% vs 87.1%; hazard ratio 0.75; 95% CI 0.62-0.91; P = .003). It is certainly noteworthy that the trial design, endocrine therapies, and patient populations differed between these adjuvant studies; for example, NATALEE included a lower-risk population, and all patients received an NSAI (in monarchE approximately 30% received tamoxifen). The current results of NATALEE are encouraging; an absolute benefit of 3.3% should be considered and weighed against toxicities and cost, and longer follow-up is needed to further elucidate the role of ribociclib in the adjuvant space.
The meaningful impact of achieving a pathologic complete response (pCR) has been demonstrated in various prior studies. Response to neoadjuvant chemotherapy informs prognosis and helps tailor adjuvant therapy, the latter of which is particularly relevant for the HER2-positive subtype. Strategies to identify patients who are more likely to achieve pCR and predictors of early responders may aid in improving efficacy outcomes and limiting toxicities. TRAIN-3 is a single-arm, phase 2 study that included 235 and 232 patients with stage II/III HR-/HER2+ and HR+/HER2+ breast cancer, respectively, undergoing neoadjuvant chemotherapy (weekly paclitaxel D1 and D8/carboplatin AUC 6 D1/trastuzumab D1/pertuzumab D1 every 3 weeks for up to nine cycles), and was designed to evaluate radiologic and pathologic response rates and event-free survival. Response was monitored by breast MRI every 3 cycles and lymph node biopsy. Among patients with HR-/HER2+ tumors, 84 (36%; 95% CI 30-43) achieved a radiologic complete response after one to three cycles, of whom the majority (88%; 95% CI 79-94) had pCR. Patients with HR+/HER2+ tumors did not show the same degree of benefit with an MRI-based monitoring strategy; among the 138 patients (59%; 95% CI 53-66) who had a complete radiologic response after one to nine cycles, 73 (53%; 95% CI 44-61) had pCR. Additional imaging-guided modalities being studied to tailor and optimize treatment include [18F]fluorodeoxyglucose-PET-CT and volumetric MRI, in the PHERGain and I-SPY trials, respectively.[6,7]
Additional References:
- Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918-926. doi: 10.1001/jama.2017.11470 Source
- Bartels SAL, Donker M, Poncet C, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS trial. J Clin Oncol. 2023;41:2159-2165. doi: 10.1200/JCO.22.01565 Source
- Johnston SRD, Toi M, O'Shaughnessy J, et al, on behalf of the monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77-90. doi: 10.1016/S1470-2045(22)00694-5 Source
- Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—The Penelope-B trial. J Clin Oncol. 2021;39:1518-1530. doi: 10.1200/JCO.20.03639 Source
- Gnant M, Dueck AC, Frantal S, et al, on behalf of the PALLAS groups and investigators. Adjuvant palbociclib for early breast cancer: The PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03). J Clin Oncol. 2022;40:282-293. doi: 10.1200/JCO.21.02554 Source
- Pérez-García JM, Cortés J, Ruiz-Borrego M, et al, on behalf of the PHERGain trial investigators. 3-year invasive disease-free survival with chemotherapy de-escalation using an 18F-FDG-PET-based, pathological complete response-adapted strategy in HER2-positive early breast cancer (PHERGain): A randomised, open-label, phase 2 trial. Lancet. 2024;403:1649-1659. doi: 10.1016/S0140-6736(24)00054-0 Source
- Hylton NM, Gatsonis CA, Rosen MA, et al, for the ACRIN 6657 trial team and I-SPY 1 trial investigators. Neoadjuvant chemotherapy for breast cancer: Functional tumor volume by MR imaging predicts recurrence-free survival-results from the ACRIN 6657/CALGB 150007 I-SPY 1 trial. Radiology. 2016;279:44-55. doi: 10.1148/radiol.2015150013 Source
The class of CDK 4/6 inhibitors represents a significant advance in the treatment of hormone receptor (HR)-positive breast cancer. All three CDK 4/6 inhibitors (palbociclib, abemaciclib, and ribociclib) are approved in combination with endocrine therapy in the metastatic setting. As drugs show promise in later-stage disease, they are then often studied in the curative space. Presently, abemaciclib is the only CDK 4/6 inhibitor that has been approved by the US Food and Drug Administration for the treatment of HR-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive, high-risk early breast cancer, based on results from the monarchE trial, which demonstrated invasive disease-free survival benefit with the addition of 2 years of abemaciclib to endocrine therapy. At 4 years, the absolute difference in invasive disease-free survival (IDFS) between the groups was 6.4% (85.8% in the abemaciclib + endocrine therapy group vs 79.4% in the endocrine therapy–alone group).[3] In contrast, the PENELOPE-B and PALLAS trials did not show benefit with the addition of palbociclib to endocrine therapy in the adjuvant setting.[4,5] The phase 3 NATALEE trial randomly assigned patients with HR-positive, HER2-negative early breast cancer to ribociclib (400 mg daily for 3 weeks followed by 1 week off for 3 years) plus a nonsteroidal aromatase inhibitor (NSAI) or an NSAI alone. At the time of prespecified interim analysis, among 5101 patients, ribociclib + NSAI led to a significant improvement in IDFS compared with endocrine therapy alone (3-year IDFS was 90.4% vs 87.1%; hazard ratio 0.75; 95% CI 0.62-0.91; P = .003). It is certainly noteworthy that the trial design, endocrine therapies, and patient populations differed between these adjuvant studies; for example, NATALEE included a lower-risk population, and all patients received an NSAI (in monarchE approximately 30% received tamoxifen). The current results of NATALEE are encouraging; an absolute benefit of 3.3% should be considered and weighed against toxicities and cost, and longer follow-up is needed to further elucidate the role of ribociclib in the adjuvant space.
The meaningful impact of achieving a pathologic complete response (pCR) has been demonstrated in various prior studies. Response to neoadjuvant chemotherapy informs prognosis and helps tailor adjuvant therapy, the latter of which is particularly relevant for the HER2-positive subtype. Strategies to identify patients who are more likely to achieve pCR and predictors of early responders may aid in improving efficacy outcomes and limiting toxicities. TRAIN-3 is a single-arm, phase 2 study that included 235 and 232 patients with stage II/III HR-/HER2+ and HR+/HER2+ breast cancer, respectively, undergoing neoadjuvant chemotherapy (weekly paclitaxel D1 and D8/carboplatin AUC 6 D1/trastuzumab D1/pertuzumab D1 every 3 weeks for up to nine cycles), and was designed to evaluate radiologic and pathologic response rates and event-free survival. Response was monitored by breast MRI every 3 cycles and lymph node biopsy. Among patients with HR-/HER2+ tumors, 84 (36%; 95% CI 30-43) achieved a radiologic complete response after one to three cycles, of whom the majority (88%; 95% CI 79-94) had pCR. Patients with HR+/HER2+ tumors did not show the same degree of benefit with an MRI-based monitoring strategy; among the 138 patients (59%; 95% CI 53-66) who had a complete radiologic response after one to nine cycles, 73 (53%; 95% CI 44-61) had pCR. Additional imaging-guided modalities being studied to tailor and optimize treatment include [18F]fluorodeoxyglucose-PET-CT and volumetric MRI, in the PHERGain and I-SPY trials, respectively.[6,7]
Additional References:
- Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918-926. doi: 10.1001/jama.2017.11470 Source
- Bartels SAL, Donker M, Poncet C, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS trial. J Clin Oncol. 2023;41:2159-2165. doi: 10.1200/JCO.22.01565 Source
- Johnston SRD, Toi M, O'Shaughnessy J, et al, on behalf of the monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77-90. doi: 10.1016/S1470-2045(22)00694-5 Source
- Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—The Penelope-B trial. J Clin Oncol. 2021;39:1518-1530. doi: 10.1200/JCO.20.03639 Source
- Gnant M, Dueck AC, Frantal S, et al, on behalf of the PALLAS groups and investigators. Adjuvant palbociclib for early breast cancer: The PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03). J Clin Oncol. 2022;40:282-293. doi: 10.1200/JCO.21.02554 Source
- Pérez-García JM, Cortés J, Ruiz-Borrego M, et al, on behalf of the PHERGain trial investigators. 3-year invasive disease-free survival with chemotherapy de-escalation using an 18F-FDG-PET-based, pathological complete response-adapted strategy in HER2-positive early breast cancer (PHERGain): A randomised, open-label, phase 2 trial. Lancet. 2024;403:1649-1659. doi: 10.1016/S0140-6736(24)00054-0 Source
- Hylton NM, Gatsonis CA, Rosen MA, et al, for the ACRIN 6657 trial team and I-SPY 1 trial investigators. Neoadjuvant chemotherapy for breast cancer: Functional tumor volume by MR imaging predicts recurrence-free survival-results from the ACRIN 6657/CALGB 150007 I-SPY 1 trial. Radiology. 2016;279:44-55. doi: 10.1148/radiol.2015150013 Source
The class of CDK 4/6 inhibitors represents a significant advance in the treatment of hormone receptor (HR)-positive breast cancer. All three CDK 4/6 inhibitors (palbociclib, abemaciclib, and ribociclib) are approved in combination with endocrine therapy in the metastatic setting. As drugs show promise in later-stage disease, they are then often studied in the curative space. Presently, abemaciclib is the only CDK 4/6 inhibitor that has been approved by the US Food and Drug Administration for the treatment of HR-positive, human epidermal growth factor receptor 2 (HER2)-negative, node-positive, high-risk early breast cancer, based on results from the monarchE trial, which demonstrated invasive disease-free survival benefit with the addition of 2 years of abemaciclib to endocrine therapy. At 4 years, the absolute difference in invasive disease-free survival (IDFS) between the groups was 6.4% (85.8% in the abemaciclib + endocrine therapy group vs 79.4% in the endocrine therapy–alone group).[3] In contrast, the PENELOPE-B and PALLAS trials did not show benefit with the addition of palbociclib to endocrine therapy in the adjuvant setting.[4,5] The phase 3 NATALEE trial randomly assigned patients with HR-positive, HER2-negative early breast cancer to ribociclib (400 mg daily for 3 weeks followed by 1 week off for 3 years) plus a nonsteroidal aromatase inhibitor (NSAI) or an NSAI alone. At the time of prespecified interim analysis, among 5101 patients, ribociclib + NSAI led to a significant improvement in IDFS compared with endocrine therapy alone (3-year IDFS was 90.4% vs 87.1%; hazard ratio 0.75; 95% CI 0.62-0.91; P = .003). It is certainly noteworthy that the trial design, endocrine therapies, and patient populations differed between these adjuvant studies; for example, NATALEE included a lower-risk population, and all patients received an NSAI (in monarchE approximately 30% received tamoxifen). The current results of NATALEE are encouraging; an absolute benefit of 3.3% should be considered and weighed against toxicities and cost, and longer follow-up is needed to further elucidate the role of ribociclib in the adjuvant space.
The meaningful impact of achieving a pathologic complete response (pCR) has been demonstrated in various prior studies. Response to neoadjuvant chemotherapy informs prognosis and helps tailor adjuvant therapy, the latter of which is particularly relevant for the HER2-positive subtype. Strategies to identify patients who are more likely to achieve pCR and predictors of early responders may aid in improving efficacy outcomes and limiting toxicities. TRAIN-3 is a single-arm, phase 2 study that included 235 and 232 patients with stage II/III HR-/HER2+ and HR+/HER2+ breast cancer, respectively, undergoing neoadjuvant chemotherapy (weekly paclitaxel D1 and D8/carboplatin AUC 6 D1/trastuzumab D1/pertuzumab D1 every 3 weeks for up to nine cycles), and was designed to evaluate radiologic and pathologic response rates and event-free survival. Response was monitored by breast MRI every 3 cycles and lymph node biopsy. Among patients with HR-/HER2+ tumors, 84 (36%; 95% CI 30-43) achieved a radiologic complete response after one to three cycles, of whom the majority (88%; 95% CI 79-94) had pCR. Patients with HR+/HER2+ tumors did not show the same degree of benefit with an MRI-based monitoring strategy; among the 138 patients (59%; 95% CI 53-66) who had a complete radiologic response after one to nine cycles, 73 (53%; 95% CI 44-61) had pCR. Additional imaging-guided modalities being studied to tailor and optimize treatment include [18F]fluorodeoxyglucose-PET-CT and volumetric MRI, in the PHERGain and I-SPY trials, respectively.[6,7]
Additional References:
- Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918-926. doi: 10.1001/jama.2017.11470 Source
- Bartels SAL, Donker M, Poncet C, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer: 10-year results of the randomized controlled EORTC 10981-22023 AMAROS trial. J Clin Oncol. 2023;41:2159-2165. doi: 10.1200/JCO.22.01565 Source
- Johnston SRD, Toi M, O'Shaughnessy J, et al, on behalf of the monarchE Committee Members. Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): Results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24:77-90. doi: 10.1016/S1470-2045(22)00694-5 Source
- Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—The Penelope-B trial. J Clin Oncol. 2021;39:1518-1530. doi: 10.1200/JCO.20.03639 Source
- Gnant M, Dueck AC, Frantal S, et al, on behalf of the PALLAS groups and investigators. Adjuvant palbociclib for early breast cancer: The PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03). J Clin Oncol. 2022;40:282-293. doi: 10.1200/JCO.21.02554 Source
- Pérez-García JM, Cortés J, Ruiz-Borrego M, et al, on behalf of the PHERGain trial investigators. 3-year invasive disease-free survival with chemotherapy de-escalation using an 18F-FDG-PET-based, pathological complete response-adapted strategy in HER2-positive early breast cancer (PHERGain): A randomised, open-label, phase 2 trial. Lancet. 2024;403:1649-1659. doi: 10.1016/S0140-6736(24)00054-0 Source
- Hylton NM, Gatsonis CA, Rosen MA, et al, for the ACRIN 6657 trial team and I-SPY 1 trial investigators. Neoadjuvant chemotherapy for breast cancer: Functional tumor volume by MR imaging predicts recurrence-free survival-results from the ACRIN 6657/CALGB 150007 I-SPY 1 trial. Radiology. 2016;279:44-55. doi: 10.1148/radiol.2015150013 Source
Highlights in Atherosclerosis and Lipid Management From ACC 2024
Lipid highlights from the 2024 American College of Cardiology Scientific Sessions included positive outcomes for bempedoic acid in statin-intolerant patients and a inclisiran-first strategy for patients with atherosclerosis. The presentations are reported by Dr Erin Michos, associate director of preventive cardiology, Johns Hopkins University. Dr Michos begins with an update of the CLEAR Outcomes trial, which examined bempedoic acid, a nonstatin inhibitor of cholesterol synthesis, in patients who had cardiovascular disease or were at high risk of developing it. The trial found that bempedoic acid safely reduced cardiovascular events by 13% compared with placebo — findings of particular value for patients unable or unwilling to take guideline-recommended doses of statins. Commendably, the trial enrolled 48% women and 17% Hispanic/Latinx persons. Dr Michos also reports on a trial investigating an inclisiran-first strategy vs usual care in patients with atherosclerosis. The reduction in LDL cholesterol was 60% for patients in the inclisiran upfront arm vs only 7% for patients provided with usual care. In closing, Dr Michos discusses the TACTIC trial, which was an evaluation of a technology-assisted web application used to qualify patients for nonprescription statin administration.
--
Erin D. Michos, MD, MHS, Associate Professor, Department of Medicine (Cardiology), Johns Hopkins University School of Medicine; Director of Women's Cardiovascular Health Research, Associate Director of Preventive Cardiology, Johns Hopkins Hospital, Baltimore, Maryland
Erin D. Michos, MD, MHS, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; AstraZeneca; Boehringer Ingelheim; Edwards Lifesciences; Esperion; Merck; Medtronic; New Amsterdam; Novo Nordisk; Novartis; Pfizer
Lipid highlights from the 2024 American College of Cardiology Scientific Sessions included positive outcomes for bempedoic acid in statin-intolerant patients and a inclisiran-first strategy for patients with atherosclerosis. The presentations are reported by Dr Erin Michos, associate director of preventive cardiology, Johns Hopkins University. Dr Michos begins with an update of the CLEAR Outcomes trial, which examined bempedoic acid, a nonstatin inhibitor of cholesterol synthesis, in patients who had cardiovascular disease or were at high risk of developing it. The trial found that bempedoic acid safely reduced cardiovascular events by 13% compared with placebo — findings of particular value for patients unable or unwilling to take guideline-recommended doses of statins. Commendably, the trial enrolled 48% women and 17% Hispanic/Latinx persons. Dr Michos also reports on a trial investigating an inclisiran-first strategy vs usual care in patients with atherosclerosis. The reduction in LDL cholesterol was 60% for patients in the inclisiran upfront arm vs only 7% for patients provided with usual care. In closing, Dr Michos discusses the TACTIC trial, which was an evaluation of a technology-assisted web application used to qualify patients for nonprescription statin administration.
--
Erin D. Michos, MD, MHS, Associate Professor, Department of Medicine (Cardiology), Johns Hopkins University School of Medicine; Director of Women's Cardiovascular Health Research, Associate Director of Preventive Cardiology, Johns Hopkins Hospital, Baltimore, Maryland
Erin D. Michos, MD, MHS, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; AstraZeneca; Boehringer Ingelheim; Edwards Lifesciences; Esperion; Merck; Medtronic; New Amsterdam; Novo Nordisk; Novartis; Pfizer
Lipid highlights from the 2024 American College of Cardiology Scientific Sessions included positive outcomes for bempedoic acid in statin-intolerant patients and a inclisiran-first strategy for patients with atherosclerosis. The presentations are reported by Dr Erin Michos, associate director of preventive cardiology, Johns Hopkins University. Dr Michos begins with an update of the CLEAR Outcomes trial, which examined bempedoic acid, a nonstatin inhibitor of cholesterol synthesis, in patients who had cardiovascular disease or were at high risk of developing it. The trial found that bempedoic acid safely reduced cardiovascular events by 13% compared with placebo — findings of particular value for patients unable or unwilling to take guideline-recommended doses of statins. Commendably, the trial enrolled 48% women and 17% Hispanic/Latinx persons. Dr Michos also reports on a trial investigating an inclisiran-first strategy vs usual care in patients with atherosclerosis. The reduction in LDL cholesterol was 60% for patients in the inclisiran upfront arm vs only 7% for patients provided with usual care. In closing, Dr Michos discusses the TACTIC trial, which was an evaluation of a technology-assisted web application used to qualify patients for nonprescription statin administration.
--
Erin D. Michos, MD, MHS, Associate Professor, Department of Medicine (Cardiology), Johns Hopkins University School of Medicine; Director of Women's Cardiovascular Health Research, Associate Director of Preventive Cardiology, Johns Hopkins Hospital, Baltimore, Maryland
Erin D. Michos, MD, MHS, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; AstraZeneca; Boehringer Ingelheim; Edwards Lifesciences; Esperion; Merck; Medtronic; New Amsterdam; Novo Nordisk; Novartis; Pfizer

Chronic Cribriform Ulcerated Plaque on the Left Calf
The Diagnosis: Nodular Basal Cell Carcinoma
Histopathology of the lesion showed a large basaloid lobule with focal epidermal attachment, peripheral nuclear palisading with cleft formation between the tumor and surrounding stroma, fibromyxoid stroma and mild pleomorphism, and variable mitotic activity and apoptosis (Figure). Based on the clinical presentation and histopathology, the patient was diagnosed with nodular basal cell carcinoma (BCC). He underwent a wide local excision of the affected area that was repaired with a splitthickness skin graft.
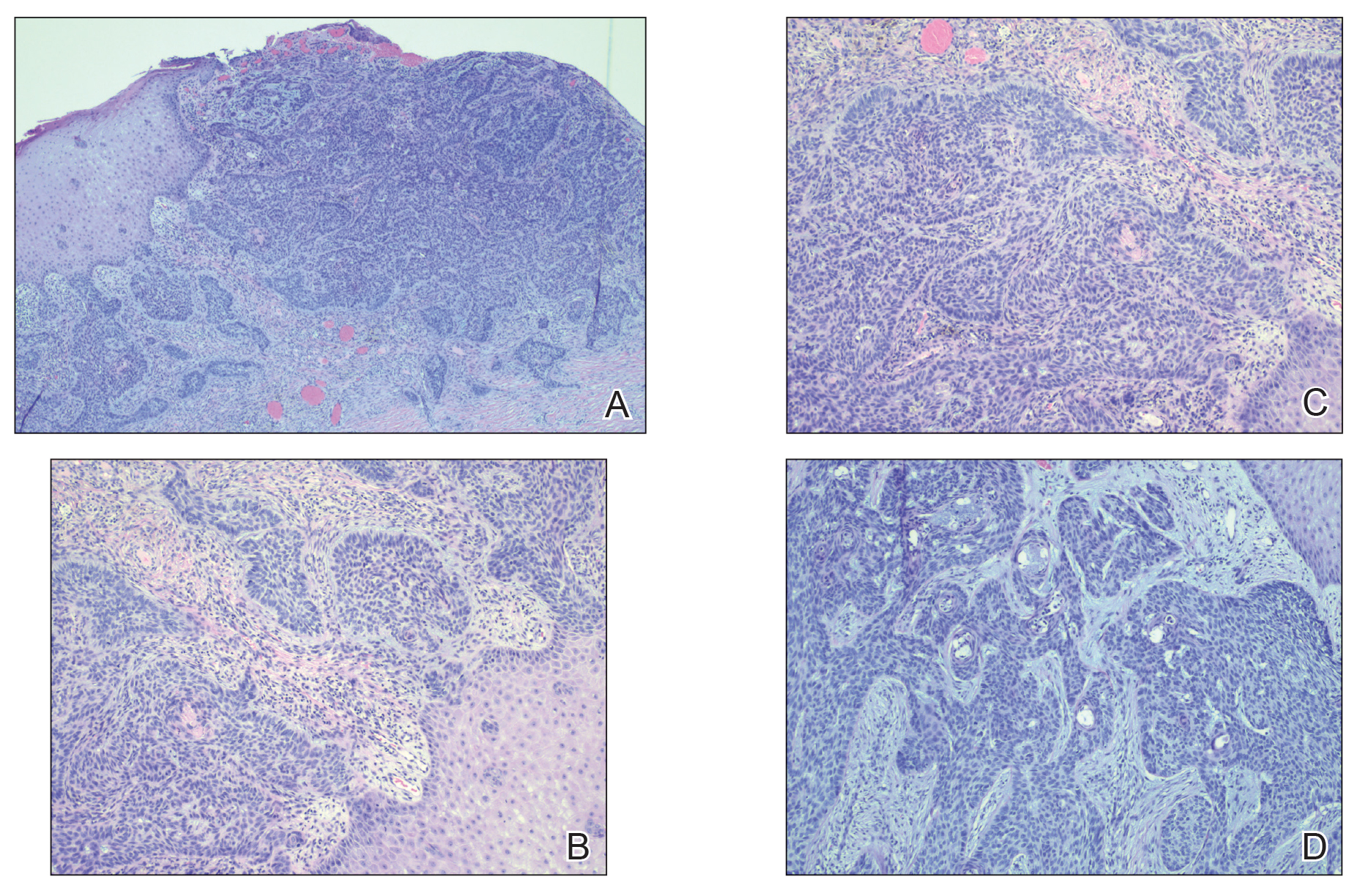
Basal cell carcinoma is the most common skin cancer worldwide and typically occurs due to years of UV radiation damage on sun-exposed skin, which accounts for a higher frequency of BCC occurring in patients residing in geographic locations with greater UV exposure (eg, higher and lower latitudes). In addition to cumulative UV dose, the duration of the exposure as well as its intensity also play a role in the development of BCC, particularly in early childhood and adolescence. Nevertheless, UV exposure is not the only risk factor, as 20% of BCCs arise in skin that is not exposed to the sun. Other risk factors include exposure to ionizing radiation and arsenic, immunosuppression, and genetic predisposition.1 Although these malignancies typically do not metastasize, growth can lead to local tissue destruction and major disfigurement if not treated in a timely fashion.2
In our patient, the differential diagnosis included pyoderma gangrenosum (PG) given the clinical appearance of the cribriform base and violaceous undermined rim of the ulcer. Pyoderma gangrenosum is a rare neutrophilic disorder that often results in ulcers that have been associated with various systemic autoimmune and inflammatory conditions, such as inflammatory bowel disease. There are 4 main subtypes of PG: the classic ulcerative type (our patient); the pustular type, which most often is seen in patients with inflammatory bowel disease; the bullous type, which can be seen in patients with an associated lymphoproliferative disorder; and the vegetative type. It frequently is thought of as both a clinical and histologic diagnosis of exclusion due to the nonspecific histopathologic features; most lesions demonstrate an infiltrate of neutrophils in the dermis. A biopsy was crucial in our patient, considering that diagnosis and treatment would have been further delayed had the patient been empirically treated with oral and topical steroids for presumed PG, which is precisely why PG is a diagnosis of exclusion. It is imperative for clinicians to rule out other pathologies, such as infection or malignancy, as demonstrated in our patient. The progressive onset and slow evolution of the lesion over years along with a lack of pain were more suggestive of BCC rather than PG. However, there is a report in the literature of PG mimicking BCC with both clinical and dermoscopic findings.3
Venous or stasis ulcers are painless, and although they rarely occur on the calf, they typically are seen lower on the leg such as on the medial ankles. Our patient endorsed occasional swelling of the affected leg and presented with edema, but overlying stasis change and other signs of venous insufficiency were absent.
Buruli ulcer is a painless chronic debilitating cutaneous disease resulting in indolent necrotizing skin as well as subcutaneous and bone lesions. It is caused by the environmental organism Mycobacterium ulcerans and typically is reported in Africa, Central/South America, the Western Pacific Region, and Australia.4 Histopathology usually demonstrates necrosis of subcutaneous tissue and dermal collagen accompanied by inflammation and acidfast bacilli highlighted by Ziehl-Neelsen stain.5 Smears of the lesions as well as culture and polymerase chain reaction for acid-fast bacilli also can be performed. Our patient reported no recent travel to any endemic areas and had no other risk factors or exposures to the pathogen responsible for this condition.
Traumatic ulcer also was included in the differential diagnosis, but the patient denied preceding trauma to the area, and the contralateral foot prosthesis did not rub on or impact the affected leg.
Basal cell carcinoma typically is treated surgically, but choice of treatment can depend on the subtype, size, tumor site, and/or patient preference.1 Other treatment modalities include electrodesiccation and curettage, cryosurgical destruction, photodynamic therapy, radiation, topical therapies, and systemic medications. Radiotherapy can be considered as a primary treatment option for BCC if surgery is contraindicated or declined by the patient, but it also is useful as an adjuvant therapy when there is perineural invasion of the tumor or positive margins. Hedgehog pathway inhibitors such as vismodegib currently are indicated for patients who are not candidates for surgery or radiation as well as for those with metastatic or locally advanced, recurrent BCC. There is no single treatment method ideal for every lesion or patient. Specific populations such as the elderly, the immunosuppressed, or those with poor baseline functional status may warrant a nonsurgical approach. The clinician must take into consideration all factors while at the same time thinking about how to best accomplish the goals of recurrencefree tumor removal, correction of any underlying functional impairment from the tumor, and maintenance of cosmesis.1
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. In: StatPearls. StatPearls; 2022.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Rosina P, Papagrigoraki A, Colato C. A case of superficial granulomatous pyoderma mimicking a basal cell carcinoma. Acta Dermatovenerol Croat. 2014;22:48-51.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256.
- Guarner J, Bartlett J, Whitney EA, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis. 2003;9:651-656.
The Diagnosis: Nodular Basal Cell Carcinoma
Histopathology of the lesion showed a large basaloid lobule with focal epidermal attachment, peripheral nuclear palisading with cleft formation between the tumor and surrounding stroma, fibromyxoid stroma and mild pleomorphism, and variable mitotic activity and apoptosis (Figure). Based on the clinical presentation and histopathology, the patient was diagnosed with nodular basal cell carcinoma (BCC). He underwent a wide local excision of the affected area that was repaired with a splitthickness skin graft.

Basal cell carcinoma is the most common skin cancer worldwide and typically occurs due to years of UV radiation damage on sun-exposed skin, which accounts for a higher frequency of BCC occurring in patients residing in geographic locations with greater UV exposure (eg, higher and lower latitudes). In addition to cumulative UV dose, the duration of the exposure as well as its intensity also play a role in the development of BCC, particularly in early childhood and adolescence. Nevertheless, UV exposure is not the only risk factor, as 20% of BCCs arise in skin that is not exposed to the sun. Other risk factors include exposure to ionizing radiation and arsenic, immunosuppression, and genetic predisposition.1 Although these malignancies typically do not metastasize, growth can lead to local tissue destruction and major disfigurement if not treated in a timely fashion.2
In our patient, the differential diagnosis included pyoderma gangrenosum (PG) given the clinical appearance of the cribriform base and violaceous undermined rim of the ulcer. Pyoderma gangrenosum is a rare neutrophilic disorder that often results in ulcers that have been associated with various systemic autoimmune and inflammatory conditions, such as inflammatory bowel disease. There are 4 main subtypes of PG: the classic ulcerative type (our patient); the pustular type, which most often is seen in patients with inflammatory bowel disease; the bullous type, which can be seen in patients with an associated lymphoproliferative disorder; and the vegetative type. It frequently is thought of as both a clinical and histologic diagnosis of exclusion due to the nonspecific histopathologic features; most lesions demonstrate an infiltrate of neutrophils in the dermis. A biopsy was crucial in our patient, considering that diagnosis and treatment would have been further delayed had the patient been empirically treated with oral and topical steroids for presumed PG, which is precisely why PG is a diagnosis of exclusion. It is imperative for clinicians to rule out other pathologies, such as infection or malignancy, as demonstrated in our patient. The progressive onset and slow evolution of the lesion over years along with a lack of pain were more suggestive of BCC rather than PG. However, there is a report in the literature of PG mimicking BCC with both clinical and dermoscopic findings.3
Venous or stasis ulcers are painless, and although they rarely occur on the calf, they typically are seen lower on the leg such as on the medial ankles. Our patient endorsed occasional swelling of the affected leg and presented with edema, but overlying stasis change and other signs of venous insufficiency were absent.
Buruli ulcer is a painless chronic debilitating cutaneous disease resulting in indolent necrotizing skin as well as subcutaneous and bone lesions. It is caused by the environmental organism Mycobacterium ulcerans and typically is reported in Africa, Central/South America, the Western Pacific Region, and Australia.4 Histopathology usually demonstrates necrosis of subcutaneous tissue and dermal collagen accompanied by inflammation and acidfast bacilli highlighted by Ziehl-Neelsen stain.5 Smears of the lesions as well as culture and polymerase chain reaction for acid-fast bacilli also can be performed. Our patient reported no recent travel to any endemic areas and had no other risk factors or exposures to the pathogen responsible for this condition.
Traumatic ulcer also was included in the differential diagnosis, but the patient denied preceding trauma to the area, and the contralateral foot prosthesis did not rub on or impact the affected leg.
Basal cell carcinoma typically is treated surgically, but choice of treatment can depend on the subtype, size, tumor site, and/or patient preference.1 Other treatment modalities include electrodesiccation and curettage, cryosurgical destruction, photodynamic therapy, radiation, topical therapies, and systemic medications. Radiotherapy can be considered as a primary treatment option for BCC if surgery is contraindicated or declined by the patient, but it also is useful as an adjuvant therapy when there is perineural invasion of the tumor or positive margins. Hedgehog pathway inhibitors such as vismodegib currently are indicated for patients who are not candidates for surgery or radiation as well as for those with metastatic or locally advanced, recurrent BCC. There is no single treatment method ideal for every lesion or patient. Specific populations such as the elderly, the immunosuppressed, or those with poor baseline functional status may warrant a nonsurgical approach. The clinician must take into consideration all factors while at the same time thinking about how to best accomplish the goals of recurrencefree tumor removal, correction of any underlying functional impairment from the tumor, and maintenance of cosmesis.1
The Diagnosis: Nodular Basal Cell Carcinoma
Histopathology of the lesion showed a large basaloid lobule with focal epidermal attachment, peripheral nuclear palisading with cleft formation between the tumor and surrounding stroma, fibromyxoid stroma and mild pleomorphism, and variable mitotic activity and apoptosis (Figure). Based on the clinical presentation and histopathology, the patient was diagnosed with nodular basal cell carcinoma (BCC). He underwent a wide local excision of the affected area that was repaired with a splitthickness skin graft.

Basal cell carcinoma is the most common skin cancer worldwide and typically occurs due to years of UV radiation damage on sun-exposed skin, which accounts for a higher frequency of BCC occurring in patients residing in geographic locations with greater UV exposure (eg, higher and lower latitudes). In addition to cumulative UV dose, the duration of the exposure as well as its intensity also play a role in the development of BCC, particularly in early childhood and adolescence. Nevertheless, UV exposure is not the only risk factor, as 20% of BCCs arise in skin that is not exposed to the sun. Other risk factors include exposure to ionizing radiation and arsenic, immunosuppression, and genetic predisposition.1 Although these malignancies typically do not metastasize, growth can lead to local tissue destruction and major disfigurement if not treated in a timely fashion.2
In our patient, the differential diagnosis included pyoderma gangrenosum (PG) given the clinical appearance of the cribriform base and violaceous undermined rim of the ulcer. Pyoderma gangrenosum is a rare neutrophilic disorder that often results in ulcers that have been associated with various systemic autoimmune and inflammatory conditions, such as inflammatory bowel disease. There are 4 main subtypes of PG: the classic ulcerative type (our patient); the pustular type, which most often is seen in patients with inflammatory bowel disease; the bullous type, which can be seen in patients with an associated lymphoproliferative disorder; and the vegetative type. It frequently is thought of as both a clinical and histologic diagnosis of exclusion due to the nonspecific histopathologic features; most lesions demonstrate an infiltrate of neutrophils in the dermis. A biopsy was crucial in our patient, considering that diagnosis and treatment would have been further delayed had the patient been empirically treated with oral and topical steroids for presumed PG, which is precisely why PG is a diagnosis of exclusion. It is imperative for clinicians to rule out other pathologies, such as infection or malignancy, as demonstrated in our patient. The progressive onset and slow evolution of the lesion over years along with a lack of pain were more suggestive of BCC rather than PG. However, there is a report in the literature of PG mimicking BCC with both clinical and dermoscopic findings.3
Venous or stasis ulcers are painless, and although they rarely occur on the calf, they typically are seen lower on the leg such as on the medial ankles. Our patient endorsed occasional swelling of the affected leg and presented with edema, but overlying stasis change and other signs of venous insufficiency were absent.
Buruli ulcer is a painless chronic debilitating cutaneous disease resulting in indolent necrotizing skin as well as subcutaneous and bone lesions. It is caused by the environmental organism Mycobacterium ulcerans and typically is reported in Africa, Central/South America, the Western Pacific Region, and Australia.4 Histopathology usually demonstrates necrosis of subcutaneous tissue and dermal collagen accompanied by inflammation and acidfast bacilli highlighted by Ziehl-Neelsen stain.5 Smears of the lesions as well as culture and polymerase chain reaction for acid-fast bacilli also can be performed. Our patient reported no recent travel to any endemic areas and had no other risk factors or exposures to the pathogen responsible for this condition.
Traumatic ulcer also was included in the differential diagnosis, but the patient denied preceding trauma to the area, and the contralateral foot prosthesis did not rub on or impact the affected leg.
Basal cell carcinoma typically is treated surgically, but choice of treatment can depend on the subtype, size, tumor site, and/or patient preference.1 Other treatment modalities include electrodesiccation and curettage, cryosurgical destruction, photodynamic therapy, radiation, topical therapies, and systemic medications. Radiotherapy can be considered as a primary treatment option for BCC if surgery is contraindicated or declined by the patient, but it also is useful as an adjuvant therapy when there is perineural invasion of the tumor or positive margins. Hedgehog pathway inhibitors such as vismodegib currently are indicated for patients who are not candidates for surgery or radiation as well as for those with metastatic or locally advanced, recurrent BCC. There is no single treatment method ideal for every lesion or patient. Specific populations such as the elderly, the immunosuppressed, or those with poor baseline functional status may warrant a nonsurgical approach. The clinician must take into consideration all factors while at the same time thinking about how to best accomplish the goals of recurrencefree tumor removal, correction of any underlying functional impairment from the tumor, and maintenance of cosmesis.1
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. In: StatPearls. StatPearls; 2022.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Rosina P, Papagrigoraki A, Colato C. A case of superficial granulomatous pyoderma mimicking a basal cell carcinoma. Acta Dermatovenerol Croat. 2014;22:48-51.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256.
- Guarner J, Bartlett J, Whitney EA, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis. 2003;9:651-656.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. In: StatPearls. StatPearls; 2022.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Rosina P, Papagrigoraki A, Colato C. A case of superficial granulomatous pyoderma mimicking a basal cell carcinoma. Acta Dermatovenerol Croat. 2014;22:48-51.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256.
- Guarner J, Bartlett J, Whitney EA, et al. Histopathologic features of Mycobacterium ulcerans infection. Emerg Infect Dis. 2003;9:651-656.
A 61-year-old man presented to the dermatology clinic for evaluation of a painless nonhealing wound on the left calf of 4 years’ duration. The patient had a history of amputation of the right foot as an infant, for which he wore an orthopedic prosthesis. He also had chronic lymphedema of the left leg, hyperlipidemia, and osteoarthritis of the right hip. There was no history of gastrointestinal tract issues. The lesion initially was small, then grew and began to ulcerate and bleed. His presentation to dermatology was delayed due to office closures during the COVID-19 pandemic. Physical examination revealed a 5-cm, erythematous, cribriform ulcer with a violaceous undermined rim. A punch biopsy was performed on the edge of the ulcer.
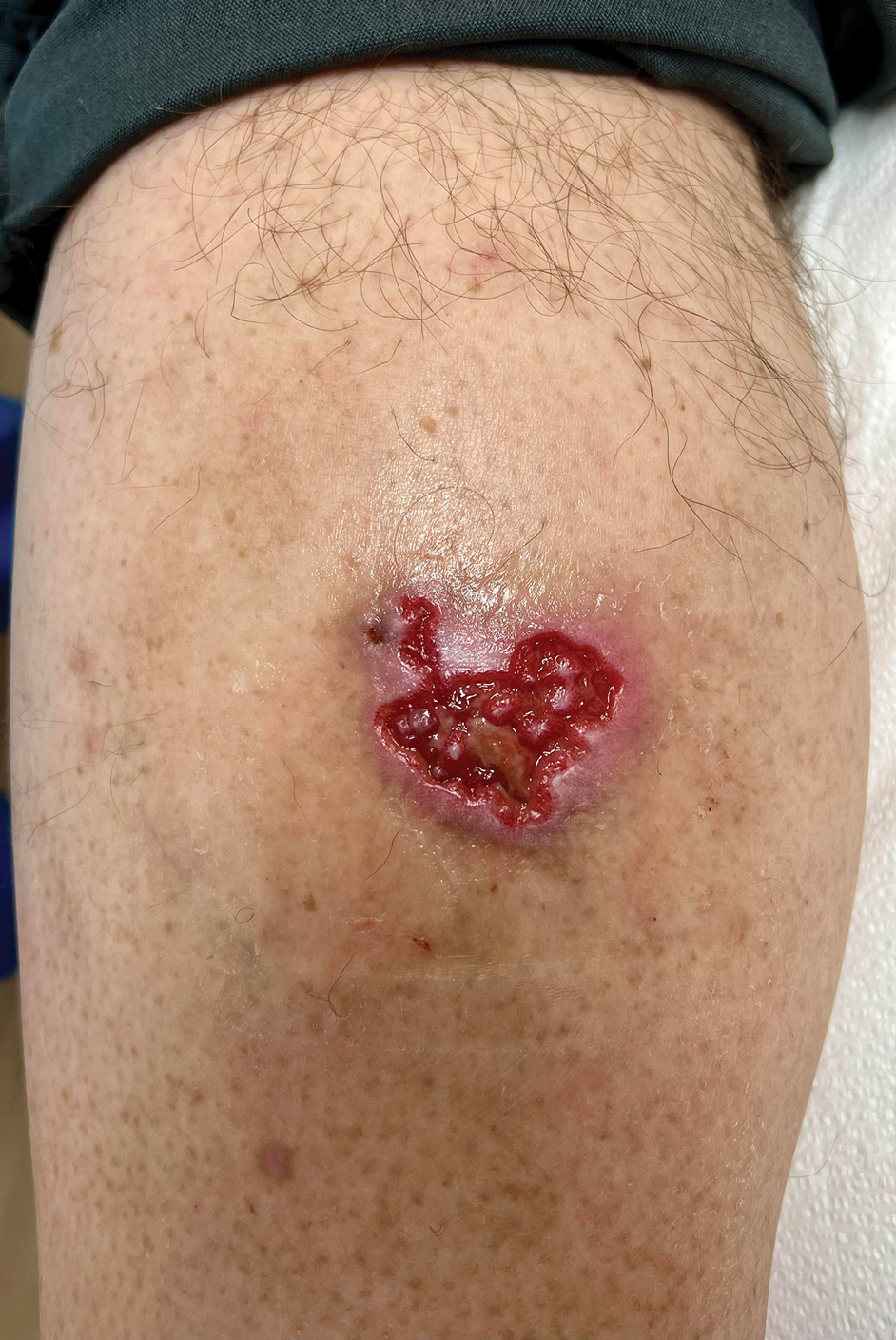
TEST TEST
test
test
test
Periorbital Changes Induced by Prostaglandin Eye Drops
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.
The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
PRACTICE POINTS
- Ask patients to provide photographs taken prior to noticed changes to assess progression if they are new to your practice.
- Take photographs of patients in good light against a solid-colored background to have a baseline. It may be helpful to update patient images annually.
- Discuss with patients the aesthetic changes that may occur with the use of prescription medications. Provide pamphlets with images to educate them on what to expect.
Recurrent Soft Tissue Rosai Dorfman Disease of Right Medial Thigh Lipoma With Lymph Node Involvement
Rosai Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis first described in 1965 by Destombes and again in 1969 by Rosai and Dorfman to depict patients who presented with massive cervical lymphadenopathy.1 The classification for histiocytosis was revised in 2016 based on new insights into the pathologic, genetic, and molecular features of RDD.2,3 Now, RDD is listed under the R group, which includes familial, sporadic, classical (nodal), extranodal RDD, and other noncutaneous, non-Langerhans cell histiocytosis.3 Cutaneous RDD is classified under the C group and typically presents as painless papules, plaques, or nodules without significant lymphadenopathy, or systemic symptoms usually seen in the presentation of RDD.4
The etiology of RDD is poorly understood, although an underlying infectious or genetic component is suspected.5 Several pathogens—including human herpesvirus 6, parvovirus B19, Epstein-Barr virus, cytomegalovirus, Brucella, and Klebsiella—have all been investigated. A link to kinase mutations has been described in nodal and extranodal RDD; however, the molecular profile of cutaneous RDD remains unknown.2 Histologic findings for RDD typically include cells that are S100 positive, CD68 positive, and CD163 positive, and CD1a and langerin (CD207) negative, thus excluding Langerhans cell histiocytosis.2 The hallmark finding of RDD is emperipolesis, which results from “histiocyte-mediated phagocytosis of intact lymphocytes and other immune cells.”6 Immunoglobulin G (Ig) G4-positive plasma cells are also common, but the significance of this finding is controversial. We present a case of a patient with recurrent RDD within a right medial thigh lipoma and include a literature review to explore the significance of histologic findings and various treatment options in the setting of emerging treatment and diagnostic criteria.
Case Presentation
A 56-year-old African American male was evaluated in the rheumatology clinic at the Central Texas Veterans Affairs Medical Center in Temple, Texas, in 2022 for a cutaneous mass of his right medial thigh. The patient previously reported the onset of a right medial thigh mass in 2005 after he had been deployed in Iraq for about 1 year. A biopsy of the mass from 2005 showed infiltration of plasma cells, lymphocytes, and histiocytes and occasional neutrophils with noted reactivity of S100 protein and CD163, but not CD1a. The patient’s original biopsy report from March 2005 was obtained secondhand from an addendum to a Dermatology Consult note. Surgical excision of the mass was not performed until 2012 and systemic therapy was not initiated.
In 2021, the mass recurred and gradually increased in size, prompting a second surgical removal. Pathology results from the 2021 mass showed a lipoma with areas of fibrosis with a mixed inflammatory cell infiltrate, including abundant lymphocytes, plasma cells, occasional hemosiderin-laden histiocytes, and clusters of enlarged histiocytes with foamy to pale eosinophilic, finely granular cytoplasm, and large, round, vesicular nuclei with prominent nucleoli. Emperipolesis was also present (Figure 1).
Special immunohistochemical staining showed most of the lymphocytes were CD20 positive B-cells with a minority of CD3 positive T-cells. Histiocytes were CD163 positive and CD68 positive with patchy reactivity for S100 protein. The plasma cells were CD138 positive. There were > 125 IgG4-positive plasma cells present in a single high-powered field and the overall IgG4:IgG plasma cell ratio was > 40%. Pertinent imaging included a whole-body positron emission tomography/computed tomography (PET/CT) hypermetabolic activity scan of a small right femoral lymph node (9 mm) and nearby medial right femoral lymph node (13 mm) (Figure 2A). A well-defined mass in the medial aspect of the right thigh (2.5 cm x 3.2 cm x 3.9 cm) and a cutaneous/subcutaneous lesion of the anterior medial aspect of the proximal right thigh superior to the mass (2.9 cm) were also evident on imaging (Figure 2B). Each area of hypermetabolic activity had decreased in size and activity when compared to a previous PET/CT obtained 1 month earlier. There was no evidence of skeletal malignancy. A physical examination did not reveal any other soft tissue masses, palpable lymphadenopathy, or areas of skin involvement. Given the patient’s reassuring imaging findings and a lack of any new physical examination findings, no systemic therapy was initiated, and following shared decision making, the patient agreed to a period of watchful waiting.
Discussion
RDD is rare with a prevalence of 1:200,000. It has been reported that multisystem involvement occurs in 19% of cases and the prognosis of RDD correlates with the number of extranodal systems involved in the disease process.7 Although sporadic RDD is usually self-limited with favorable outcomes, it is estimated that 10% of patients may die of RDD due to direct complications, infections, and amyloidosis.2,7 RDD commonly affects young male children and young adults with a mean age of 20 years and has a higher incidence among African American children.2,7,8 Although patients with RDD present bilateral, painless cervical lymphadenopathy in 90% of cases, about 43% of patients with RDD and associated adenopathy present with ≥ 1 site of extranodal involvement, and only 23% of patients with RDD present with isolated extranodal sites without adenopathy.9 As was the case with our patient, the most common extranodal sites are found in the skin and soft tissue (16%).6,9 However, histopathologic diagnosis of RDD in a lipoma is exceedingly rare. We found only 1 other case report of a patient with a history of multiple lipomas who developed a new solitary nodule that was excised and demonstrated RDD upon immunohistochemical staining.4 There has been no documented association between multiple lipomas and RDD.4
Histologically, RDD is often characterized by emperipolesis (the presence of an intact cell within the cytoplasm of anther cell) and a mixed cell infiltrate that includes S100 positive histiocytes, mononuclear cells, plasma cells, and lymphocytes.10 Despite these shared histologic features among the various phenotypes of RDD, other type-specific characteristics may also be present. When compared to nodal RDD, extranodal disease tends to demonstrate a lack of nodal architecture, more fibrosis and collagen deposition, fewer RDD cells, a lower degree of emperipolesis, and alternating pale (histiocyte rich) and dark (lymphocyte rich) regions with notable polygonal histiocytes arranged in a storiform pattern.5,10
Our patient’s histology showed an overall IgG4:IgG plasma cell ratio > 40%. RDD frequently presents with IgG4-positive plasma cells, which has confounded the diagnosis of IgG4-related diseases and hyper-IgG4 disease.11 Given this association, the Histiocyte Society revised classification recommends that all cases of RDD be evaluated for IgG4-positive cell infiltration.2,3 Further discussion on this matter was recently provided after an expert panel published a consensus statement in 2015 detailing the evaluation of IgG4. The panel advocates for stricter terminology and criteria on this issue, advises that isolated IgG4-positive plasma cells are nonspecific, and states that the diagnosis of IgG4 disease should be based on careful judgment and correlation with the clinical scenario and supportive findings.12 Therefore, while IgG4 positivity continues to be misleading in RDD cases, further evaluation for IgG4 disease is recommended.
Sporadic RDD is usually self-limited with a reported remission rate of up to 50%, according to a case series of 80 patients with RDD.13 This leads to the recommendation of a period of watchful monitoring in patients with limited disease.13 In patients with unifocal extranodal disease, surgical excision has shown positive remission results; however, local recurrence of soft tissue lesions can occur at a rate of 21.4% to 51%.14 Although initiation of systemic therapy should be considered in patients with recurrent disease, there is currently no standardized regimen or medication of choice for treatment. Treatment with steroids, including prednisone 40 to 70 mg daily or dexamethasone 8 to 20 mg daily, have been shown to be effective in reducing the nodal size and symptoms, especially in cases of nonresectable multifocal extranodal disease of the central nervous system, bone, and orbital.7,15,16 However, cases of orbital, tracheal, renal, or soft tissue RDD have reported failure in treatment with steroids.17,18
According to the consensus recommendations for the treatment of RDD released in 2018, treatment with chemotherapy has shown mixed results. Anthracycline and alkylating agents have shown minimal efficacy, but combination regimens with vinca alkaloids, methotrexate, and 6-mercaptopurine have helped patients experience remission.19,20 Due to the rarity of RDD and lack of clinical trials, the exact efficacy of these treatment regimens remains unknown and is largely limited to case reports described within the medical literature. Treatment with nucleoside analogs, such as cladribine 2.1 to 5 mg/m2 or clofarabine 25 mg/m2 per day for 5 days every 28 days for 6 months, have shown promising results and helped achieve complete remission in patients with refractory or recurrent RDD.7,21-23 Immunomodulator therapies including TNF-α inhibitor, such as thalidomide and lenalidomide, have also shown to be effective, particularly in patients with refractory disease.24,25 Low-dose thalidomide (100 mg daily) was effective for cases of refractory cutaneous RDD, though no standard dosing regimen exists. Lenalidomide has shown to be effective in patients with multiple refractory nodal or bone RDD, but is associated with more complications given that it is more myelosuppressive than thalidomide.7 Radiotherapy has also been initiated in patients with refractory soft tissue disease or persistent symptoms after resection and in patients who are not candidates for surgery or systemic therapy, though no standard doses of radiotherapy have been established.7,26,27
Conclusions
RDD is a rare histiocytic disorder that presents in a wide range of age groups, different locations in the body, and with variable disease behavior. Multidisciplinary management of the disease and research for mutations and microenvironment of RDD is needed to better understand its clinicopathological nature and improve targeted novel therapies.
Acknowledgments
The authors thank Veterans Affairs Central Texas Health Care Section Chief of Rheumatology, Swastika Jha, MD, for her guidance in this case and Bo Wang, MD, for his preparation of the pathological specimens.
1. Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348-357. Published 2020 Jan 31. doi:10.3324/haematol.2019.219626
2. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697-705. doi:10.1136/jclinpath-2020-206733
3. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi:10.1182/blood-2016-01-690636
4. Farooq U, Chacon AH, Vincek V, Elgart G. Purely cutaneous rosai-dorfman disease with immunohistochemistry. Indian J Dermatol. 2013;58(6):447-450. doi:10.4103/0019-5154.119953
5. Ma H, Zheng Y, Zhu G, Wu J, Lu C, Lai W. Rosai-dorfman disease with massive cutaneous nodule on the shoulder and back. Ann Dermatol. 2015;27(1):71-75. doi:10.5021/ad.2015.27.1.71
6. Deen IU, Chittal A, Badro N, Jones R, Haas C. Extranodal Rosai-Dorfman Disease- a Review of Diagnostic Testing and Management. J Community Hosp Intern Med Perspect. 2022;12(2):18-22. Published 2022 Apr 12. doi:10.55729/2000-9666.1032
7. Oussama A, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi: 10.1182/blood-2018-03-839753
8. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
9. Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1111-1121. doi:10.5858/2007-131-1117-MESHWM
10. Betini N, Munger AM, Rottmann D, Haims A, Costa J, Lindskog DM. Rare presentation of Rosai-Dorfman disease in soft tissue: diagnostic findings and surgical treatment. Case Rep Surg. 2022;2022:8440836. Published 2022 Mar 30. doi:10.1155/2022/8440836
11. Menon MP, Evbuomwan MO, Rosai J, Jaffe ES, Pittaluga S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: another red herring or a true association with IgG4-related disease? Histopathology. 2014;64(3):455-459. doi:10.1111/his.12274
12. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688-1699. doi:10.1002/art.39132
13. Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67-71. doi:10.1002/ajh.10008
14. Montgomery EA, Meis JM, Firzzera G. Rosai-Dorfman disease of soft tissue. Am J Surg Pathol. 1992;16(2):122-129. doi:10.1097/00000478-199202000-00004
15. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165-170. doi:10.1016/j.prp.2005.11.004
16. Shulman S, Katzenstein H, Abramowsky C, Broecker J, Wulkan M, Shehata B. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30(6):442-447. doi:10.3109/15513815.2011.61887317. Ottaviano G, Doro D, Marioni G, et al. Extranodal Rosai-Dorfman disease: involvement of eye, nose and trachea. Acta Otolaryngol. 2006;126(6):657-660. doi:10.1080/00016480500452582
18. Sakallioglu O, Gok F, Kalman S, et al. Minimal change nephropathy in a 7-year-old boy with Rosai-Dorfman disease. J Nephrol. 2006;19(2):211-214.
19. Jabali Y, Smrcka V, Pradna J. Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol. 2005;13(3):285-289. doi:10.1177/106689690501300311
20. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi:10.1182/blood-2018-03-839753
21. Konca C, Özkurt ZN, Deger M, Akı Z, Yagcı M. Extranodal multifocal Rosai-Dorfman disease: response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009;89(1):58-62. doi:10.1007/s12185-008-0192-2
22. Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52.
23. Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612-615. doi:10.1002/pbc.20668
24. Chen E, Pavlidakey P, Sami N. Rosai-Dorfman disease successfully treated with thalidomide. JAAD Case Reports. 2016;2(5):369-372. Published 2016 Sep 28. doi:10.1016/j.jdcr.2016.08.006
25. Rubinstein M, Assal A, Scherba M, et al. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am J Hematol. 2016;91(2):E1. doi:10.1002/ajh.24225
26. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165-175. doi:10.1097/MD.0000000000000030
27. Paryani NN, Daugherty LC, O’Connor MI, Jiang L. Extranodal Rosai-Dorfman disease of the bone treated with surgery and radiotherapy. Rare Tumors. 2014;6(4):5531. Published 2014 Dec 11. doi:10.4081/rt.2014.5531
Rosai Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis first described in 1965 by Destombes and again in 1969 by Rosai and Dorfman to depict patients who presented with massive cervical lymphadenopathy.1 The classification for histiocytosis was revised in 2016 based on new insights into the pathologic, genetic, and molecular features of RDD.2,3 Now, RDD is listed under the R group, which includes familial, sporadic, classical (nodal), extranodal RDD, and other noncutaneous, non-Langerhans cell histiocytosis.3 Cutaneous RDD is classified under the C group and typically presents as painless papules, plaques, or nodules without significant lymphadenopathy, or systemic symptoms usually seen in the presentation of RDD.4
The etiology of RDD is poorly understood, although an underlying infectious or genetic component is suspected.5 Several pathogens—including human herpesvirus 6, parvovirus B19, Epstein-Barr virus, cytomegalovirus, Brucella, and Klebsiella—have all been investigated. A link to kinase mutations has been described in nodal and extranodal RDD; however, the molecular profile of cutaneous RDD remains unknown.2 Histologic findings for RDD typically include cells that are S100 positive, CD68 positive, and CD163 positive, and CD1a and langerin (CD207) negative, thus excluding Langerhans cell histiocytosis.2 The hallmark finding of RDD is emperipolesis, which results from “histiocyte-mediated phagocytosis of intact lymphocytes and other immune cells.”6 Immunoglobulin G (Ig) G4-positive plasma cells are also common, but the significance of this finding is controversial. We present a case of a patient with recurrent RDD within a right medial thigh lipoma and include a literature review to explore the significance of histologic findings and various treatment options in the setting of emerging treatment and diagnostic criteria.
Case Presentation
A 56-year-old African American male was evaluated in the rheumatology clinic at the Central Texas Veterans Affairs Medical Center in Temple, Texas, in 2022 for a cutaneous mass of his right medial thigh. The patient previously reported the onset of a right medial thigh mass in 2005 after he had been deployed in Iraq for about 1 year. A biopsy of the mass from 2005 showed infiltration of plasma cells, lymphocytes, and histiocytes and occasional neutrophils with noted reactivity of S100 protein and CD163, but not CD1a. The patient’s original biopsy report from March 2005 was obtained secondhand from an addendum to a Dermatology Consult note. Surgical excision of the mass was not performed until 2012 and systemic therapy was not initiated.
In 2021, the mass recurred and gradually increased in size, prompting a second surgical removal. Pathology results from the 2021 mass showed a lipoma with areas of fibrosis with a mixed inflammatory cell infiltrate, including abundant lymphocytes, plasma cells, occasional hemosiderin-laden histiocytes, and clusters of enlarged histiocytes with foamy to pale eosinophilic, finely granular cytoplasm, and large, round, vesicular nuclei with prominent nucleoli. Emperipolesis was also present (Figure 1).
Special immunohistochemical staining showed most of the lymphocytes were CD20 positive B-cells with a minority of CD3 positive T-cells. Histiocytes were CD163 positive and CD68 positive with patchy reactivity for S100 protein. The plasma cells were CD138 positive. There were > 125 IgG4-positive plasma cells present in a single high-powered field and the overall IgG4:IgG plasma cell ratio was > 40%. Pertinent imaging included a whole-body positron emission tomography/computed tomography (PET/CT) hypermetabolic activity scan of a small right femoral lymph node (9 mm) and nearby medial right femoral lymph node (13 mm) (Figure 2A). A well-defined mass in the medial aspect of the right thigh (2.5 cm x 3.2 cm x 3.9 cm) and a cutaneous/subcutaneous lesion of the anterior medial aspect of the proximal right thigh superior to the mass (2.9 cm) were also evident on imaging (Figure 2B). Each area of hypermetabolic activity had decreased in size and activity when compared to a previous PET/CT obtained 1 month earlier. There was no evidence of skeletal malignancy. A physical examination did not reveal any other soft tissue masses, palpable lymphadenopathy, or areas of skin involvement. Given the patient’s reassuring imaging findings and a lack of any new physical examination findings, no systemic therapy was initiated, and following shared decision making, the patient agreed to a period of watchful waiting.
Discussion
RDD is rare with a prevalence of 1:200,000. It has been reported that multisystem involvement occurs in 19% of cases and the prognosis of RDD correlates with the number of extranodal systems involved in the disease process.7 Although sporadic RDD is usually self-limited with favorable outcomes, it is estimated that 10% of patients may die of RDD due to direct complications, infections, and amyloidosis.2,7 RDD commonly affects young male children and young adults with a mean age of 20 years and has a higher incidence among African American children.2,7,8 Although patients with RDD present bilateral, painless cervical lymphadenopathy in 90% of cases, about 43% of patients with RDD and associated adenopathy present with ≥ 1 site of extranodal involvement, and only 23% of patients with RDD present with isolated extranodal sites without adenopathy.9 As was the case with our patient, the most common extranodal sites are found in the skin and soft tissue (16%).6,9 However, histopathologic diagnosis of RDD in a lipoma is exceedingly rare. We found only 1 other case report of a patient with a history of multiple lipomas who developed a new solitary nodule that was excised and demonstrated RDD upon immunohistochemical staining.4 There has been no documented association between multiple lipomas and RDD.4
Histologically, RDD is often characterized by emperipolesis (the presence of an intact cell within the cytoplasm of anther cell) and a mixed cell infiltrate that includes S100 positive histiocytes, mononuclear cells, plasma cells, and lymphocytes.10 Despite these shared histologic features among the various phenotypes of RDD, other type-specific characteristics may also be present. When compared to nodal RDD, extranodal disease tends to demonstrate a lack of nodal architecture, more fibrosis and collagen deposition, fewer RDD cells, a lower degree of emperipolesis, and alternating pale (histiocyte rich) and dark (lymphocyte rich) regions with notable polygonal histiocytes arranged in a storiform pattern.5,10
Our patient’s histology showed an overall IgG4:IgG plasma cell ratio > 40%. RDD frequently presents with IgG4-positive plasma cells, which has confounded the diagnosis of IgG4-related diseases and hyper-IgG4 disease.11 Given this association, the Histiocyte Society revised classification recommends that all cases of RDD be evaluated for IgG4-positive cell infiltration.2,3 Further discussion on this matter was recently provided after an expert panel published a consensus statement in 2015 detailing the evaluation of IgG4. The panel advocates for stricter terminology and criteria on this issue, advises that isolated IgG4-positive plasma cells are nonspecific, and states that the diagnosis of IgG4 disease should be based on careful judgment and correlation with the clinical scenario and supportive findings.12 Therefore, while IgG4 positivity continues to be misleading in RDD cases, further evaluation for IgG4 disease is recommended.
Sporadic RDD is usually self-limited with a reported remission rate of up to 50%, according to a case series of 80 patients with RDD.13 This leads to the recommendation of a period of watchful monitoring in patients with limited disease.13 In patients with unifocal extranodal disease, surgical excision has shown positive remission results; however, local recurrence of soft tissue lesions can occur at a rate of 21.4% to 51%.14 Although initiation of systemic therapy should be considered in patients with recurrent disease, there is currently no standardized regimen or medication of choice for treatment. Treatment with steroids, including prednisone 40 to 70 mg daily or dexamethasone 8 to 20 mg daily, have been shown to be effective in reducing the nodal size and symptoms, especially in cases of nonresectable multifocal extranodal disease of the central nervous system, bone, and orbital.7,15,16 However, cases of orbital, tracheal, renal, or soft tissue RDD have reported failure in treatment with steroids.17,18
According to the consensus recommendations for the treatment of RDD released in 2018, treatment with chemotherapy has shown mixed results. Anthracycline and alkylating agents have shown minimal efficacy, but combination regimens with vinca alkaloids, methotrexate, and 6-mercaptopurine have helped patients experience remission.19,20 Due to the rarity of RDD and lack of clinical trials, the exact efficacy of these treatment regimens remains unknown and is largely limited to case reports described within the medical literature. Treatment with nucleoside analogs, such as cladribine 2.1 to 5 mg/m2 or clofarabine 25 mg/m2 per day for 5 days every 28 days for 6 months, have shown promising results and helped achieve complete remission in patients with refractory or recurrent RDD.7,21-23 Immunomodulator therapies including TNF-α inhibitor, such as thalidomide and lenalidomide, have also shown to be effective, particularly in patients with refractory disease.24,25 Low-dose thalidomide (100 mg daily) was effective for cases of refractory cutaneous RDD, though no standard dosing regimen exists. Lenalidomide has shown to be effective in patients with multiple refractory nodal or bone RDD, but is associated with more complications given that it is more myelosuppressive than thalidomide.7 Radiotherapy has also been initiated in patients with refractory soft tissue disease or persistent symptoms after resection and in patients who are not candidates for surgery or systemic therapy, though no standard doses of radiotherapy have been established.7,26,27
Conclusions
RDD is a rare histiocytic disorder that presents in a wide range of age groups, different locations in the body, and with variable disease behavior. Multidisciplinary management of the disease and research for mutations and microenvironment of RDD is needed to better understand its clinicopathological nature and improve targeted novel therapies.
Acknowledgments
The authors thank Veterans Affairs Central Texas Health Care Section Chief of Rheumatology, Swastika Jha, MD, for her guidance in this case and Bo Wang, MD, for his preparation of the pathological specimens.
Rosai Dorfman disease (RDD) is a rare non-Langerhans cell histiocytosis first described in 1965 by Destombes and again in 1969 by Rosai and Dorfman to depict patients who presented with massive cervical lymphadenopathy.1 The classification for histiocytosis was revised in 2016 based on new insights into the pathologic, genetic, and molecular features of RDD.2,3 Now, RDD is listed under the R group, which includes familial, sporadic, classical (nodal), extranodal RDD, and other noncutaneous, non-Langerhans cell histiocytosis.3 Cutaneous RDD is classified under the C group and typically presents as painless papules, plaques, or nodules without significant lymphadenopathy, or systemic symptoms usually seen in the presentation of RDD.4
The etiology of RDD is poorly understood, although an underlying infectious or genetic component is suspected.5 Several pathogens—including human herpesvirus 6, parvovirus B19, Epstein-Barr virus, cytomegalovirus, Brucella, and Klebsiella—have all been investigated. A link to kinase mutations has been described in nodal and extranodal RDD; however, the molecular profile of cutaneous RDD remains unknown.2 Histologic findings for RDD typically include cells that are S100 positive, CD68 positive, and CD163 positive, and CD1a and langerin (CD207) negative, thus excluding Langerhans cell histiocytosis.2 The hallmark finding of RDD is emperipolesis, which results from “histiocyte-mediated phagocytosis of intact lymphocytes and other immune cells.”6 Immunoglobulin G (Ig) G4-positive plasma cells are also common, but the significance of this finding is controversial. We present a case of a patient with recurrent RDD within a right medial thigh lipoma and include a literature review to explore the significance of histologic findings and various treatment options in the setting of emerging treatment and diagnostic criteria.
Case Presentation
A 56-year-old African American male was evaluated in the rheumatology clinic at the Central Texas Veterans Affairs Medical Center in Temple, Texas, in 2022 for a cutaneous mass of his right medial thigh. The patient previously reported the onset of a right medial thigh mass in 2005 after he had been deployed in Iraq for about 1 year. A biopsy of the mass from 2005 showed infiltration of plasma cells, lymphocytes, and histiocytes and occasional neutrophils with noted reactivity of S100 protein and CD163, but not CD1a. The patient’s original biopsy report from March 2005 was obtained secondhand from an addendum to a Dermatology Consult note. Surgical excision of the mass was not performed until 2012 and systemic therapy was not initiated.
In 2021, the mass recurred and gradually increased in size, prompting a second surgical removal. Pathology results from the 2021 mass showed a lipoma with areas of fibrosis with a mixed inflammatory cell infiltrate, including abundant lymphocytes, plasma cells, occasional hemosiderin-laden histiocytes, and clusters of enlarged histiocytes with foamy to pale eosinophilic, finely granular cytoplasm, and large, round, vesicular nuclei with prominent nucleoli. Emperipolesis was also present (Figure 1).
Special immunohistochemical staining showed most of the lymphocytes were CD20 positive B-cells with a minority of CD3 positive T-cells. Histiocytes were CD163 positive and CD68 positive with patchy reactivity for S100 protein. The plasma cells were CD138 positive. There were > 125 IgG4-positive plasma cells present in a single high-powered field and the overall IgG4:IgG plasma cell ratio was > 40%. Pertinent imaging included a whole-body positron emission tomography/computed tomography (PET/CT) hypermetabolic activity scan of a small right femoral lymph node (9 mm) and nearby medial right femoral lymph node (13 mm) (Figure 2A). A well-defined mass in the medial aspect of the right thigh (2.5 cm x 3.2 cm x 3.9 cm) and a cutaneous/subcutaneous lesion of the anterior medial aspect of the proximal right thigh superior to the mass (2.9 cm) were also evident on imaging (Figure 2B). Each area of hypermetabolic activity had decreased in size and activity when compared to a previous PET/CT obtained 1 month earlier. There was no evidence of skeletal malignancy. A physical examination did not reveal any other soft tissue masses, palpable lymphadenopathy, or areas of skin involvement. Given the patient’s reassuring imaging findings and a lack of any new physical examination findings, no systemic therapy was initiated, and following shared decision making, the patient agreed to a period of watchful waiting.
Discussion
RDD is rare with a prevalence of 1:200,000. It has been reported that multisystem involvement occurs in 19% of cases and the prognosis of RDD correlates with the number of extranodal systems involved in the disease process.7 Although sporadic RDD is usually self-limited with favorable outcomes, it is estimated that 10% of patients may die of RDD due to direct complications, infections, and amyloidosis.2,7 RDD commonly affects young male children and young adults with a mean age of 20 years and has a higher incidence among African American children.2,7,8 Although patients with RDD present bilateral, painless cervical lymphadenopathy in 90% of cases, about 43% of patients with RDD and associated adenopathy present with ≥ 1 site of extranodal involvement, and only 23% of patients with RDD present with isolated extranodal sites without adenopathy.9 As was the case with our patient, the most common extranodal sites are found in the skin and soft tissue (16%).6,9 However, histopathologic diagnosis of RDD in a lipoma is exceedingly rare. We found only 1 other case report of a patient with a history of multiple lipomas who developed a new solitary nodule that was excised and demonstrated RDD upon immunohistochemical staining.4 There has been no documented association between multiple lipomas and RDD.4
Histologically, RDD is often characterized by emperipolesis (the presence of an intact cell within the cytoplasm of anther cell) and a mixed cell infiltrate that includes S100 positive histiocytes, mononuclear cells, plasma cells, and lymphocytes.10 Despite these shared histologic features among the various phenotypes of RDD, other type-specific characteristics may also be present. When compared to nodal RDD, extranodal disease tends to demonstrate a lack of nodal architecture, more fibrosis and collagen deposition, fewer RDD cells, a lower degree of emperipolesis, and alternating pale (histiocyte rich) and dark (lymphocyte rich) regions with notable polygonal histiocytes arranged in a storiform pattern.5,10
Our patient’s histology showed an overall IgG4:IgG plasma cell ratio > 40%. RDD frequently presents with IgG4-positive plasma cells, which has confounded the diagnosis of IgG4-related diseases and hyper-IgG4 disease.11 Given this association, the Histiocyte Society revised classification recommends that all cases of RDD be evaluated for IgG4-positive cell infiltration.2,3 Further discussion on this matter was recently provided after an expert panel published a consensus statement in 2015 detailing the evaluation of IgG4. The panel advocates for stricter terminology and criteria on this issue, advises that isolated IgG4-positive plasma cells are nonspecific, and states that the diagnosis of IgG4 disease should be based on careful judgment and correlation with the clinical scenario and supportive findings.12 Therefore, while IgG4 positivity continues to be misleading in RDD cases, further evaluation for IgG4 disease is recommended.
Sporadic RDD is usually self-limited with a reported remission rate of up to 50%, according to a case series of 80 patients with RDD.13 This leads to the recommendation of a period of watchful monitoring in patients with limited disease.13 In patients with unifocal extranodal disease, surgical excision has shown positive remission results; however, local recurrence of soft tissue lesions can occur at a rate of 21.4% to 51%.14 Although initiation of systemic therapy should be considered in patients with recurrent disease, there is currently no standardized regimen or medication of choice for treatment. Treatment with steroids, including prednisone 40 to 70 mg daily or dexamethasone 8 to 20 mg daily, have been shown to be effective in reducing the nodal size and symptoms, especially in cases of nonresectable multifocal extranodal disease of the central nervous system, bone, and orbital.7,15,16 However, cases of orbital, tracheal, renal, or soft tissue RDD have reported failure in treatment with steroids.17,18
According to the consensus recommendations for the treatment of RDD released in 2018, treatment with chemotherapy has shown mixed results. Anthracycline and alkylating agents have shown minimal efficacy, but combination regimens with vinca alkaloids, methotrexate, and 6-mercaptopurine have helped patients experience remission.19,20 Due to the rarity of RDD and lack of clinical trials, the exact efficacy of these treatment regimens remains unknown and is largely limited to case reports described within the medical literature. Treatment with nucleoside analogs, such as cladribine 2.1 to 5 mg/m2 or clofarabine 25 mg/m2 per day for 5 days every 28 days for 6 months, have shown promising results and helped achieve complete remission in patients with refractory or recurrent RDD.7,21-23 Immunomodulator therapies including TNF-α inhibitor, such as thalidomide and lenalidomide, have also shown to be effective, particularly in patients with refractory disease.24,25 Low-dose thalidomide (100 mg daily) was effective for cases of refractory cutaneous RDD, though no standard dosing regimen exists. Lenalidomide has shown to be effective in patients with multiple refractory nodal or bone RDD, but is associated with more complications given that it is more myelosuppressive than thalidomide.7 Radiotherapy has also been initiated in patients with refractory soft tissue disease or persistent symptoms after resection and in patients who are not candidates for surgery or systemic therapy, though no standard doses of radiotherapy have been established.7,26,27
Conclusions
RDD is a rare histiocytic disorder that presents in a wide range of age groups, different locations in the body, and with variable disease behavior. Multidisciplinary management of the disease and research for mutations and microenvironment of RDD is needed to better understand its clinicopathological nature and improve targeted novel therapies.
Acknowledgments
The authors thank Veterans Affairs Central Texas Health Care Section Chief of Rheumatology, Swastika Jha, MD, for her guidance in this case and Bo Wang, MD, for his preparation of the pathological specimens.
1. Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348-357. Published 2020 Jan 31. doi:10.3324/haematol.2019.219626
2. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697-705. doi:10.1136/jclinpath-2020-206733
3. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi:10.1182/blood-2016-01-690636
4. Farooq U, Chacon AH, Vincek V, Elgart G. Purely cutaneous rosai-dorfman disease with immunohistochemistry. Indian J Dermatol. 2013;58(6):447-450. doi:10.4103/0019-5154.119953
5. Ma H, Zheng Y, Zhu G, Wu J, Lu C, Lai W. Rosai-dorfman disease with massive cutaneous nodule on the shoulder and back. Ann Dermatol. 2015;27(1):71-75. doi:10.5021/ad.2015.27.1.71
6. Deen IU, Chittal A, Badro N, Jones R, Haas C. Extranodal Rosai-Dorfman Disease- a Review of Diagnostic Testing and Management. J Community Hosp Intern Med Perspect. 2022;12(2):18-22. Published 2022 Apr 12. doi:10.55729/2000-9666.1032
7. Oussama A, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi: 10.1182/blood-2018-03-839753
8. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
9. Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1111-1121. doi:10.5858/2007-131-1117-MESHWM
10. Betini N, Munger AM, Rottmann D, Haims A, Costa J, Lindskog DM. Rare presentation of Rosai-Dorfman disease in soft tissue: diagnostic findings and surgical treatment. Case Rep Surg. 2022;2022:8440836. Published 2022 Mar 30. doi:10.1155/2022/8440836
11. Menon MP, Evbuomwan MO, Rosai J, Jaffe ES, Pittaluga S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: another red herring or a true association with IgG4-related disease? Histopathology. 2014;64(3):455-459. doi:10.1111/his.12274
12. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688-1699. doi:10.1002/art.39132
13. Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67-71. doi:10.1002/ajh.10008
14. Montgomery EA, Meis JM, Firzzera G. Rosai-Dorfman disease of soft tissue. Am J Surg Pathol. 1992;16(2):122-129. doi:10.1097/00000478-199202000-00004
15. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165-170. doi:10.1016/j.prp.2005.11.004
16. Shulman S, Katzenstein H, Abramowsky C, Broecker J, Wulkan M, Shehata B. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30(6):442-447. doi:10.3109/15513815.2011.61887317. Ottaviano G, Doro D, Marioni G, et al. Extranodal Rosai-Dorfman disease: involvement of eye, nose and trachea. Acta Otolaryngol. 2006;126(6):657-660. doi:10.1080/00016480500452582
18. Sakallioglu O, Gok F, Kalman S, et al. Minimal change nephropathy in a 7-year-old boy with Rosai-Dorfman disease. J Nephrol. 2006;19(2):211-214.
19. Jabali Y, Smrcka V, Pradna J. Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol. 2005;13(3):285-289. doi:10.1177/106689690501300311
20. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi:10.1182/blood-2018-03-839753
21. Konca C, Özkurt ZN, Deger M, Akı Z, Yagcı M. Extranodal multifocal Rosai-Dorfman disease: response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009;89(1):58-62. doi:10.1007/s12185-008-0192-2
22. Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52.
23. Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612-615. doi:10.1002/pbc.20668
24. Chen E, Pavlidakey P, Sami N. Rosai-Dorfman disease successfully treated with thalidomide. JAAD Case Reports. 2016;2(5):369-372. Published 2016 Sep 28. doi:10.1016/j.jdcr.2016.08.006
25. Rubinstein M, Assal A, Scherba M, et al. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am J Hematol. 2016;91(2):E1. doi:10.1002/ajh.24225
26. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165-175. doi:10.1097/MD.0000000000000030
27. Paryani NN, Daugherty LC, O’Connor MI, Jiang L. Extranodal Rosai-Dorfman disease of the bone treated with surgery and radiotherapy. Rare Tumors. 2014;6(4):5531. Published 2014 Dec 11. doi:10.4081/rt.2014.5531
1. Goyal G, Ravindran A, Young JR, et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica. 2020;105(2):348-357. Published 2020 Jan 31. doi:10.3324/haematol.2019.219626
2. Bruce-Brand C, Schneider JW, Schubert P. Rosai-Dorfman disease: an overview. J Clin Pathol. 2020;73(11):697-705. doi:10.1136/jclinpath-2020-206733
3. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-2681. doi:10.1182/blood-2016-01-690636
4. Farooq U, Chacon AH, Vincek V, Elgart G. Purely cutaneous rosai-dorfman disease with immunohistochemistry. Indian J Dermatol. 2013;58(6):447-450. doi:10.4103/0019-5154.119953
5. Ma H, Zheng Y, Zhu G, Wu J, Lu C, Lai W. Rosai-dorfman disease with massive cutaneous nodule on the shoulder and back. Ann Dermatol. 2015;27(1):71-75. doi:10.5021/ad.2015.27.1.71
6. Deen IU, Chittal A, Badro N, Jones R, Haas C. Extranodal Rosai-Dorfman Disease- a Review of Diagnostic Testing and Management. J Community Hosp Intern Med Perspect. 2022;12(2):18-22. Published 2022 Apr 12. doi:10.55729/2000-9666.1032
7. Oussama A, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi: 10.1182/blood-2018-03-839753
8. Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7(1):19-73.
9. Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131(7):1111-1121. doi:10.5858/2007-131-1117-MESHWM
10. Betini N, Munger AM, Rottmann D, Haims A, Costa J, Lindskog DM. Rare presentation of Rosai-Dorfman disease in soft tissue: diagnostic findings and surgical treatment. Case Rep Surg. 2022;2022:8440836. Published 2022 Mar 30. doi:10.1155/2022/8440836
11. Menon MP, Evbuomwan MO, Rosai J, Jaffe ES, Pittaluga S. A subset of Rosai-Dorfman disease cases show increased IgG4-positive plasma cells: another red herring or a true association with IgG4-related disease? Histopathology. 2014;64(3):455-459. doi:10.1111/his.12274
12. Khosroshahi A, Wallace ZS, Crowe JL, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67(7):1688-1699. doi:10.1002/art.39132
13. Pulsoni A, Anghel G, Falcucci P, et al. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69(1):67-71. doi:10.1002/ajh.10008
14. Montgomery EA, Meis JM, Firzzera G. Rosai-Dorfman disease of soft tissue. Am J Surg Pathol. 1992;16(2):122-129. doi:10.1097/00000478-199202000-00004
15. Z’Graggen WJ, Sturzenegger M, Mariani L, Keserue B, Kappeler A, Vajtai I. Isolated Rosai-Dorfman disease of intracranial meninges. Pathol Res Pract. 2006;202(3):165-170. doi:10.1016/j.prp.2005.11.004
16. Shulman S, Katzenstein H, Abramowsky C, Broecker J, Wulkan M, Shehata B. Unusual presentation of Rosai-Dorfman disease (RDD) in the bone in adolescents. Fetal Pediatr Pathol. 2011;30(6):442-447. doi:10.3109/15513815.2011.61887317. Ottaviano G, Doro D, Marioni G, et al. Extranodal Rosai-Dorfman disease: involvement of eye, nose and trachea. Acta Otolaryngol. 2006;126(6):657-660. doi:10.1080/00016480500452582
18. Sakallioglu O, Gok F, Kalman S, et al. Minimal change nephropathy in a 7-year-old boy with Rosai-Dorfman disease. J Nephrol. 2006;19(2):211-214.
19. Jabali Y, Smrcka V, Pradna J. Rosai-Dorfman disease: successful long-term results by combination chemotherapy with prednisone, 6-mercaptopurine, methotrexate, and vinblastine: a case report. Int J Surg Pathol. 2005;13(3):285-289. doi:10.1177/106689690501300311
20. Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877-2890. doi:10.1182/blood-2018-03-839753
21. Konca C, Özkurt ZN, Deger M, Akı Z, Yagcı M. Extranodal multifocal Rosai-Dorfman disease: response to 2-chlorodeoxyadenosine treatment. Int J Hematol. 2009;89(1):58-62. doi:10.1007/s12185-008-0192-2
22. Aouba A, Terrier B, Vasiliu V, et al. Dramatic clinical efficacy of cladribine in Rosai-Dorfman disease and evolution of the cytokine profile: towards a new therapeutic approach. Haematologica. 2006;91(12 Suppl):ECR52.
23. Tasso M, Esquembre C, Blanco E, Moscardó C, Niveiro M, Payá A. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) treated with 2-chlorodeoxyadenosine. Pediatr Blood Cancer. 2006;47(5):612-615. doi:10.1002/pbc.20668
24. Chen E, Pavlidakey P, Sami N. Rosai-Dorfman disease successfully treated with thalidomide. JAAD Case Reports. 2016;2(5):369-372. Published 2016 Sep 28. doi:10.1016/j.jdcr.2016.08.006
25. Rubinstein M, Assal A, Scherba M, et al. Lenalidomide in the treatment of Rosai Dorfman disease-a first in use report. Am J Hematol. 2016;91(2):E1. doi:10.1002/ajh.24225
26. Sandoval-Sus JD, Sandoval-Leon AC, Chapman JR, et al. Rosai-Dorfman disease of the central nervous system: report of 6 cases and review of the literature. Medicine (Baltimore). 2014;93(3):165-175. doi:10.1097/MD.0000000000000030
27. Paryani NN, Daugherty LC, O’Connor MI, Jiang L. Extranodal Rosai-Dorfman disease of the bone treated with surgery and radiotherapy. Rare Tumors. 2014;6(4):5531. Published 2014 Dec 11. doi:10.4081/rt.2014.5531
3D Printing for the Development of Palatal Defect Prosthetics
Three-dimensional (3D) printing has become a promising area of innovation in biomedical research.1,2 Previous research in orthopedic surgery has found that customized 3D printed implants, casts, orthoses, and prosthetics (eg, prosthetic hands) matched to an individual’s unique anatomy can result in more precise placement and better surgical outcomes.3-5 Customized prosthetics have also been found to lead to fewer complications.3,6
Recent advances in 3D printing technology has prompted investigation from surgeons to identify how this new tool may be incorporated into patient care.1,7 One of the most common applications of 3D printing is during preoperative planning in which surgeons gain better insight into patient-specific anatomy by using patient-specific printed models.8 Another promising application is the production of customized prosthetics suited to each patient’s unique anatomy.9 As a result, 3D printing has significantly impacted bone and cartilage restoration procedures and has the potential to completely transform the treatment of patients with debilitating musculoskeletal injuries.3,10
The potential surrounding 3D printed prosthetics has led to their adoption by several other specialties, including otolaryngology.11 The most widely used application of 3D printing among otolaryngologists is preoperative planning, and the incorporation of printed prosthetics intoreconstruction of the orbit, nasal septum, auricle, and palate has also been reported.2,12,13 Patient-specific implants might allow otolaryngologists to better rehabilitate, reconstruct, and/or regenerate craniofacial defects using more humane procedures.14
Patients with palatomaxillary cancers are treated by prosthodontists or otolaryngologists. An impression is made with a resin–which can be painful for postoperative patients–and a prosthetic is manufactured and implanted.15-17 Patients with cancer often see many specialists, though reconstructive care is a low priority. Many of these individuals also experience dynamic anatomic functional changes over time, leading to the need for multiple prothesis.
palatomaxillary prosthetics
This program aims to use patients’ previous computed tomography (CT) to tailor customized 3D printed palatomaxillary prosthetics to specifically fit their anatomy. Palatomaxillary defects are a source of profound disability for patients with head and neck cancers who are left with large anatomic defects as a direct result of treatment. Reconstruction of palatal defects poses unique challenges due to the complexity of patient anatomy.18,19
3D printed prosthetics for palatomaxillary defects have not been incorporated into patient care. We reviewed previous imaging research to determine if it could be used to assist patients who struggle with their function and appearance following treatment for head and neck cancers. The primary aim was to investigate whether 3D printing was a feasible strategy for creating patient-specific palatomaxillary prosthetics. The secondary aim is to determine whether these prosthetics should be tested in the future for use in reconstruction of maxillary defects.
Data Acquisition
This study was conducted at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) and was approved by the Stanford University Institutional Review Board (approval #28958, informed consent and patient contact excluded). A retrospective chart review was conducted on all patients with head and neck cancers who were treated at VAPAHCS from 2010 to 2022. Patients aged ≥ 18 years who had a palatomaxillary defect due to cancer treatment, had undergone a palatal resection, and who received treatment at any point from 2010 to 2022 were included in the review. CTs were not a specific inclusion criterion, though the quality of the scans was analyzed for eligible patients. Younger patients and those treated at VAPAHCS prior to 2010 were excluded.
There was no control group; all data was sourced from the US Department of Veterans Affairs (VA) imaging system database. Among the 3595 patients reviewed, 5 met inclusion criteria and the quality of their craniofacial anatomy CTs were analyzed. To maintain accurate craniofacial 3D modeling, CTs require a maximum of 1 mm slice thickness. Of the 5 patients who met the inclusion criteria, 4 were found to have variability in the quality of their CTs and severe defects not suitable for prosthetic reconstruction, which led to their exclusion from the study. One patient was investigated to demonstrate if making these prostheses was feasible. This patient was diagnosed with a malignant neoplasm of the hard palate, underwent a partial maxillectomy, and a palatal obturator was placed to cover the defect.
The primary data collected was patient identifiers as well as the gross anatomy and dimensions of the patients’ craniofacial anatomy, as seen in previous imaging research.20 Before the imaging analysis, all personal health information was removed and the dataset was deidentified to ensure patient anonymity and noninvolvement.
CT Segmentation and 3D Printing
Using CTs of the patient’s craniofacial anatomy, we developed a model of the defects. This was achieved with deidentified CTs imported into the Food and Drug Administration (FDA)-approved computerized aid design (CAD) software, Materialise Mimics. The hard palate was segmented and isolated based off the presented scan and any holes in the image were filled using the CAD software. The model was subsequently mirrored in Materialise 3-matic to replicate an original anatomical hard palate prosthesis. The final product was converted into a 3D model and imported into Formlabs preform software to generate 3D printing supports and orient it for printing. The prosthetic was printed using FDA-approved Biocompatible Denture Base Resin by a Formlabs 3B+ printer at the Palo Alto VA Simulation Center. The 3D printed prosthesis was washed using Formlabs Form Wash 80% ethyl alcohol to remove excess resin and subsequently cured to harden the malleable resin. Supports were later removed, and the prosthesis was sanded.
The primary aim of this study was to investigate whether using CTs to create patient-specific prosthetic renderings for patients with head and neck cancer could be a feasible strategy. The CTs from the patient were successfully used to generate a 3D printed prosthesis, and the prosthesis matched the original craniofacial anatomy seen in the patient's imaging (Figure). These results demonstrate that high quality CTs can be used as a template for 3D printed prostheses for mild to moderate palatomaxillary defects.
3D Printing Costs
One liter of Denture Base Resin costs $299; prostheses use about 5 mL of resin. The average annual salary of a 3D printing technician in the United States is $42,717, or $20.54 per hour.21 For an experienced 3D printing technician, the time required to segment the hard palate and prepare it for 3D printing is 1 to 2 hours. The process may exceed 2 hours if the technician is presented with a lower quality CT or if the patient has a complex craniofacial anatomy.
The average time it takes to print a palatal prosthetic is 5 hours. An additional hour is needed for postprocessing, which includes washing and sanding. Therefore, the cost of the materials and labor for an average 3D printed prosthetic is about $150. A Formlabs 3B+ printer is competitively priced around $10,000. The cost for Materialise Mimics software varies, but is estimated at $16,000 at VAPAHCS. The prices for these 2 items are not included in our price estimation but should be taken into consideration.
Prosthodontist Process and Cost
The typical process of creating a palatal prosthesis by a prosthodontist begins by examining the patient, creating a stone model, then creating a wax model. Biocompatible materials are selected and processed into a mold that is trimmed and polished to the desired shape. This is followed by another patient visit to ensure the prosthesis fits properly. Follow-up care is also necessary for maintenance and comfort.
The average cost of a palatal prosthesis varies depending on the type needed (ie, metal implant, teeth replacement), the materials used, the region in which the patient is receiving care, and the complexity of the case. For complex and customizable options like those required for patients with cancer, the prostheses typically cost several thousands of dollars. The Healthcare Common Procedure Coding System code for a palatal lift prosthesis (D5955) lists prices ranging from $4000 to $8000 per prosthetic, not including the cost of the prosthodontist visits.22,23
Discussion
This program sought to determine whether imaging studies of maxillary defects are effective templates for developing 3D printed prosthetics and whether these prosthetics should be tested for future use in reconstruction of palatomaxillary defects. Our program illustrated that CTs served as feasible templates for developing hard palate prostheses for patients with palatomaxillary defects. It is important to note the CTs used were from a newer and more modern scanner and therefore yielded detailed palatal structures with higher accuracy more suitable for 3D modeling. Lower-quality CTs from the 4 patients excluded from the program were not suitable for 3D modeling. This suggests that with high-quality imaging, 3D printed prosthesis may be a viable strategy to help patients who struggle with their function following treatment for head and neck cancers.
3D printed prosthesis may also be a more patient centered and convenient option. In the traditional prosthesis creation workflow, the patient must physically bite down onto a resin (alginate or silicone) to make an impression, a very painful postoperative process that is irritating to the raw edges of the surgical bed.15,16 Prosthodontists then create a prosthetic minus the tumor and typically secure it with clips or glue.17 Many patients also experience changes in their anatomy over time requiring them to have a new protheses created. This is particularly important in veterans with palatomaxillary defects since many VA medical centers do not have a prosthodontist on staff, making accessibility to these specialists difficult. 3D printing provides a contactless prosthetic creation process. This convenience may reduce a patient’s pain and the number of visits for which they need a specialist.
Future Directions
Additional research is needed to determine the full potential of 3D printed prosthetics. 3D printed prostheses have been effectively used for patient education in areas of presurgical planning, prosthesis creation, and trainee education.24 This research represents an early step in the development of a new technology for use in otolaryngology. Specifically, many veterans with a history of head and neck cancers have sustained changes to their craniofacial anatomy following treatment. Using imaging to create 3D printed prosthetics could be very effective for these patients. Prosthetics could improve a patient’s quality of life by restoring/approximating their anatomy after cancer treatment.
Significant time and care must be taken by cancer and reconstructive surgeons to properly fit a prosthesis. Improperly fitting prosthetics leads to mucosal ulceration that then may lead to a need for fitting a new prosthetic. The advantage of 3D printed prosthetics is that they may more precisely fit the anatomy of each patient using CT results, thus potentially reducing the time needed to fit the prosthetic as well as the risk associated with an improperly fit prosthetic. 3D printed prosthesis could be used directly in the future, however, clinical trials are needed to verify its efficacy vs prosthodontic options.
Another consideration for potential future use of 3D printed prosthetics is cost. We estimated that the cost of the materials and labor of our 3D printed prosthetic to be about $150. Pricing of current molded prosthetics varies, but is often listed at several thousand dollars. Another consideration is the durability of 3D printed prosthetics vs standard prosthetics. Since we were unable to use the prosthetic in the patient, it was difficult to determine its durability. The significant cost of the 3D printer and software necessary for 3D printed prosthetics must also be considered and may be prohibitive. While many academic hospitals are considering the purchase of 3D printers and licenses, this may be challenging for resource-constrained institutions. 3D printing may also be difficult for groups without any prior experience in the field. Outsourcing to a third party is possible, though doing so adds more cost to the project. While we recognize there is a learning curve associated with adopting any new technology, it’s equally important to note that 3D printing is being rapidly integrated and has already made significant advancements in personalized medicine.8,25,26
Limitations
This program had several limitations. First, we only obtained CTs of sufficient quality from 1 patient to generate a 3D printed prosthesis. Further research with additional patients is necessary to validate this process. Second, we were unable to trial the prosthesis in the patient because we did not have FDA approval. Additionally, it is difficult to calculate a true cost estimate for this process as materials and software costs vary dramatically across institutions as well as over time.
Conclusions
The purpose of this study was to demonstrate the possibility to develop prosthetics for the hard palate for patients suffering from palatomaxillary defects. A 3D printed prosthetic was generated that matched the patient’s craniofacial anatomy. Future research should test the feasibility of these prosthetics in patient care against a traditional prosthodontic impression. Though this is a proof-of-concept study and no prosthetics were implanted as part of this investigation, we showcase the feasibility of printing prosthetics for palatomaxillary defects. The use of 3D printed prosthetics may be a more humane process, potentially lower cost, and be more accessible to veterans.
1. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi:10.1177/0194599816678372
2. Virani FR, Chua EC, Timbang MR, Hsieh TY, Senders CW. Three-dimensional printing in cleft care: a systematic review. Cleft Palate Craniofac J. 2022;59(4):484-496. doi:10.1177/10556656211013175
3. Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9(3):260-268. doi:10.1016/j.jcot.2018.07.022
4. Vujaklija I, Farina D. 3D printed upper limb prosthetics. Expert Rev Med Devices. 2018;15(7):505-512. doi:10.1080/17434440.2018.1494568
5. Ten Kate J, Smit G, Breedveld P. 3D-printed upper limb prostheses: a review. Disabil Rehabil Assist Technol. 2017;12(3):300-314. doi:10.1080/17483107.2016.1253117
6. Thomas CN, Mavrommatis S, Schroder LK, Cole PA. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury. 2022;53(3):977-983. doi:10.1016/j.injury.2021.11.019
7. Colaco M, Igel DA, Atala A. The potential of 3D printing in urological research and patient care. Nat Rev Urol. 2018;15(4):213-221. doi:10.1038/nrurol.2018.6
8. Meyer-Szary J, Luis MS, Mikulski S, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6):3331. Published 2022 Mar 11. doi:10.3390/ijerph19063331
9. Moya D, Gobbato B, Valente S, Roca R. Use of preoperative planning and 3D printing in orthopedics and traumatology: entering a new era. Acta Ortop Mex. 2022;36(1):39-47.
10. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. Published 2021 Apr 20. doi:10.5435/JAAOSGlobal-D-20-00230
11. Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045-2052. doi:10.1002/lary.2783112. Sigron GR, Barba M, Chammartin F, Msallem B, Berg BI, Thieringer FM. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blow-out fractures) with and without the support of 3D-printed orbital anatomical models. J Clin Med. 2021;10(16):3509. Published 2021 Aug 9. doi:10.3390/jcm10163509
13. Kimura K, Davis S, Thomas E, et al. 3D Customization for microtia repair in hemifacial microsomia. Laryngoscope. 2022;132(3):545-549. doi:10.1002/lary.29823
14. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45-57. doi:10.1007/s10439-016-1668-5
15. Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo MA, et al. Evolution of oral cancer treatment in an andalusian population sample: rehabilitation with prosthetic obturation and removable partial prosthesis. J Clin Exp Dent. 2017;9(8):e1008-e1014. doi:10.4317/jced.54023
16. Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174-181. doi:10.1053/joms.2003.50044
17. Pool C, Shokri T, Vincent A, Wang W, Kadakia S, Ducic Y. Prosthetic reconstruction of the maxilla and palate. Semin Plast Surg. 2020;34(2):114-119. doi:10.1055/s-0040-1709143
18. Badhey AK, Khan MN. Palatomaxillary reconstruction: fibula or scapula. Semin Plast Surg. 2020;34(2):86-91. doi:10.1055/s-0040-1709431
19. Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the palatomaxillary structure. Semin Plast Surg. 2020;34(2):71-76. doi:10.1055/s-0040-1709430
20. Lobb DC, Cottler P, Dart D, Black JS. The use of patient-specific three-dimensional printed surgical models enhances plastic surgery resident education in craniofacial surgery. J Craniofac Surg. 2019;30(2):339-341. doi:10.1097/SCS.0000000000005322
21. 3D printing technician salary in the United States. Accessed February 27, 2024. https://www.salary.com/research/salary/posting/3d-printing-technician-salary22. US Dept of Veterans Affairs. Healthcare Common Procedure Coding System. Outpatient dental professional nationwide charges by HCPCS code. January-December 2020. Accessed February 27, 2024. https://www.va.gov/COMMUNITYCARE/docs/RO/Outpatient-DataTables/v3-27_Table-I.pdf23. Washington State Department of Labor and Industries. Professional services fee schedule HCPCS level II fees. October 1, 2020. Accessed February 27, 2024. https://lni.wa.gov/patient-care/billing-payments/marfsdocs/2020/2020FSHCPCS.pdf24. Low CM, Morris JM, Price DL, et al. Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33(6):770-781. doi:10.1177/1945892419866319
25. Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019;2019:5340616. Published 2019 Mar 21. doi:10.1155/2019/5340616
26. Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27-40. doi:10.1136/bmjstel-2017-000234
Three-dimensional (3D) printing has become a promising area of innovation in biomedical research.1,2 Previous research in orthopedic surgery has found that customized 3D printed implants, casts, orthoses, and prosthetics (eg, prosthetic hands) matched to an individual’s unique anatomy can result in more precise placement and better surgical outcomes.3-5 Customized prosthetics have also been found to lead to fewer complications.3,6
Recent advances in 3D printing technology has prompted investigation from surgeons to identify how this new tool may be incorporated into patient care.1,7 One of the most common applications of 3D printing is during preoperative planning in which surgeons gain better insight into patient-specific anatomy by using patient-specific printed models.8 Another promising application is the production of customized prosthetics suited to each patient’s unique anatomy.9 As a result, 3D printing has significantly impacted bone and cartilage restoration procedures and has the potential to completely transform the treatment of patients with debilitating musculoskeletal injuries.3,10
The potential surrounding 3D printed prosthetics has led to their adoption by several other specialties, including otolaryngology.11 The most widely used application of 3D printing among otolaryngologists is preoperative planning, and the incorporation of printed prosthetics intoreconstruction of the orbit, nasal septum, auricle, and palate has also been reported.2,12,13 Patient-specific implants might allow otolaryngologists to better rehabilitate, reconstruct, and/or regenerate craniofacial defects using more humane procedures.14
Patients with palatomaxillary cancers are treated by prosthodontists or otolaryngologists. An impression is made with a resin–which can be painful for postoperative patients–and a prosthetic is manufactured and implanted.15-17 Patients with cancer often see many specialists, though reconstructive care is a low priority. Many of these individuals also experience dynamic anatomic functional changes over time, leading to the need for multiple prothesis.
palatomaxillary prosthetics
This program aims to use patients’ previous computed tomography (CT) to tailor customized 3D printed palatomaxillary prosthetics to specifically fit their anatomy. Palatomaxillary defects are a source of profound disability for patients with head and neck cancers who are left with large anatomic defects as a direct result of treatment. Reconstruction of palatal defects poses unique challenges due to the complexity of patient anatomy.18,19
3D printed prosthetics for palatomaxillary defects have not been incorporated into patient care. We reviewed previous imaging research to determine if it could be used to assist patients who struggle with their function and appearance following treatment for head and neck cancers. The primary aim was to investigate whether 3D printing was a feasible strategy for creating patient-specific palatomaxillary prosthetics. The secondary aim is to determine whether these prosthetics should be tested in the future for use in reconstruction of maxillary defects.
Data Acquisition
This study was conducted at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) and was approved by the Stanford University Institutional Review Board (approval #28958, informed consent and patient contact excluded). A retrospective chart review was conducted on all patients with head and neck cancers who were treated at VAPAHCS from 2010 to 2022. Patients aged ≥ 18 years who had a palatomaxillary defect due to cancer treatment, had undergone a palatal resection, and who received treatment at any point from 2010 to 2022 were included in the review. CTs were not a specific inclusion criterion, though the quality of the scans was analyzed for eligible patients. Younger patients and those treated at VAPAHCS prior to 2010 were excluded.
There was no control group; all data was sourced from the US Department of Veterans Affairs (VA) imaging system database. Among the 3595 patients reviewed, 5 met inclusion criteria and the quality of their craniofacial anatomy CTs were analyzed. To maintain accurate craniofacial 3D modeling, CTs require a maximum of 1 mm slice thickness. Of the 5 patients who met the inclusion criteria, 4 were found to have variability in the quality of their CTs and severe defects not suitable for prosthetic reconstruction, which led to their exclusion from the study. One patient was investigated to demonstrate if making these prostheses was feasible. This patient was diagnosed with a malignant neoplasm of the hard palate, underwent a partial maxillectomy, and a palatal obturator was placed to cover the defect.
The primary data collected was patient identifiers as well as the gross anatomy and dimensions of the patients’ craniofacial anatomy, as seen in previous imaging research.20 Before the imaging analysis, all personal health information was removed and the dataset was deidentified to ensure patient anonymity and noninvolvement.
CT Segmentation and 3D Printing
Using CTs of the patient’s craniofacial anatomy, we developed a model of the defects. This was achieved with deidentified CTs imported into the Food and Drug Administration (FDA)-approved computerized aid design (CAD) software, Materialise Mimics. The hard palate was segmented and isolated based off the presented scan and any holes in the image were filled using the CAD software. The model was subsequently mirrored in Materialise 3-matic to replicate an original anatomical hard palate prosthesis. The final product was converted into a 3D model and imported into Formlabs preform software to generate 3D printing supports and orient it for printing. The prosthetic was printed using FDA-approved Biocompatible Denture Base Resin by a Formlabs 3B+ printer at the Palo Alto VA Simulation Center. The 3D printed prosthesis was washed using Formlabs Form Wash 80% ethyl alcohol to remove excess resin and subsequently cured to harden the malleable resin. Supports were later removed, and the prosthesis was sanded.
The primary aim of this study was to investigate whether using CTs to create patient-specific prosthetic renderings for patients with head and neck cancer could be a feasible strategy. The CTs from the patient were successfully used to generate a 3D printed prosthesis, and the prosthesis matched the original craniofacial anatomy seen in the patient's imaging (Figure). These results demonstrate that high quality CTs can be used as a template for 3D printed prostheses for mild to moderate palatomaxillary defects.
3D Printing Costs
One liter of Denture Base Resin costs $299; prostheses use about 5 mL of resin. The average annual salary of a 3D printing technician in the United States is $42,717, or $20.54 per hour.21 For an experienced 3D printing technician, the time required to segment the hard palate and prepare it for 3D printing is 1 to 2 hours. The process may exceed 2 hours if the technician is presented with a lower quality CT or if the patient has a complex craniofacial anatomy.
The average time it takes to print a palatal prosthetic is 5 hours. An additional hour is needed for postprocessing, which includes washing and sanding. Therefore, the cost of the materials and labor for an average 3D printed prosthetic is about $150. A Formlabs 3B+ printer is competitively priced around $10,000. The cost for Materialise Mimics software varies, but is estimated at $16,000 at VAPAHCS. The prices for these 2 items are not included in our price estimation but should be taken into consideration.
Prosthodontist Process and Cost
The typical process of creating a palatal prosthesis by a prosthodontist begins by examining the patient, creating a stone model, then creating a wax model. Biocompatible materials are selected and processed into a mold that is trimmed and polished to the desired shape. This is followed by another patient visit to ensure the prosthesis fits properly. Follow-up care is also necessary for maintenance and comfort.
The average cost of a palatal prosthesis varies depending on the type needed (ie, metal implant, teeth replacement), the materials used, the region in which the patient is receiving care, and the complexity of the case. For complex and customizable options like those required for patients with cancer, the prostheses typically cost several thousands of dollars. The Healthcare Common Procedure Coding System code for a palatal lift prosthesis (D5955) lists prices ranging from $4000 to $8000 per prosthetic, not including the cost of the prosthodontist visits.22,23
Discussion
This program sought to determine whether imaging studies of maxillary defects are effective templates for developing 3D printed prosthetics and whether these prosthetics should be tested for future use in reconstruction of palatomaxillary defects. Our program illustrated that CTs served as feasible templates for developing hard palate prostheses for patients with palatomaxillary defects. It is important to note the CTs used were from a newer and more modern scanner and therefore yielded detailed palatal structures with higher accuracy more suitable for 3D modeling. Lower-quality CTs from the 4 patients excluded from the program were not suitable for 3D modeling. This suggests that with high-quality imaging, 3D printed prosthesis may be a viable strategy to help patients who struggle with their function following treatment for head and neck cancers.
3D printed prosthesis may also be a more patient centered and convenient option. In the traditional prosthesis creation workflow, the patient must physically bite down onto a resin (alginate or silicone) to make an impression, a very painful postoperative process that is irritating to the raw edges of the surgical bed.15,16 Prosthodontists then create a prosthetic minus the tumor and typically secure it with clips or glue.17 Many patients also experience changes in their anatomy over time requiring them to have a new protheses created. This is particularly important in veterans with palatomaxillary defects since many VA medical centers do not have a prosthodontist on staff, making accessibility to these specialists difficult. 3D printing provides a contactless prosthetic creation process. This convenience may reduce a patient’s pain and the number of visits for which they need a specialist.
Future Directions
Additional research is needed to determine the full potential of 3D printed prosthetics. 3D printed prostheses have been effectively used for patient education in areas of presurgical planning, prosthesis creation, and trainee education.24 This research represents an early step in the development of a new technology for use in otolaryngology. Specifically, many veterans with a history of head and neck cancers have sustained changes to their craniofacial anatomy following treatment. Using imaging to create 3D printed prosthetics could be very effective for these patients. Prosthetics could improve a patient’s quality of life by restoring/approximating their anatomy after cancer treatment.
Significant time and care must be taken by cancer and reconstructive surgeons to properly fit a prosthesis. Improperly fitting prosthetics leads to mucosal ulceration that then may lead to a need for fitting a new prosthetic. The advantage of 3D printed prosthetics is that they may more precisely fit the anatomy of each patient using CT results, thus potentially reducing the time needed to fit the prosthetic as well as the risk associated with an improperly fit prosthetic. 3D printed prosthesis could be used directly in the future, however, clinical trials are needed to verify its efficacy vs prosthodontic options.
Another consideration for potential future use of 3D printed prosthetics is cost. We estimated that the cost of the materials and labor of our 3D printed prosthetic to be about $150. Pricing of current molded prosthetics varies, but is often listed at several thousand dollars. Another consideration is the durability of 3D printed prosthetics vs standard prosthetics. Since we were unable to use the prosthetic in the patient, it was difficult to determine its durability. The significant cost of the 3D printer and software necessary for 3D printed prosthetics must also be considered and may be prohibitive. While many academic hospitals are considering the purchase of 3D printers and licenses, this may be challenging for resource-constrained institutions. 3D printing may also be difficult for groups without any prior experience in the field. Outsourcing to a third party is possible, though doing so adds more cost to the project. While we recognize there is a learning curve associated with adopting any new technology, it’s equally important to note that 3D printing is being rapidly integrated and has already made significant advancements in personalized medicine.8,25,26
Limitations
This program had several limitations. First, we only obtained CTs of sufficient quality from 1 patient to generate a 3D printed prosthesis. Further research with additional patients is necessary to validate this process. Second, we were unable to trial the prosthesis in the patient because we did not have FDA approval. Additionally, it is difficult to calculate a true cost estimate for this process as materials and software costs vary dramatically across institutions as well as over time.
Conclusions
The purpose of this study was to demonstrate the possibility to develop prosthetics for the hard palate for patients suffering from palatomaxillary defects. A 3D printed prosthetic was generated that matched the patient’s craniofacial anatomy. Future research should test the feasibility of these prosthetics in patient care against a traditional prosthodontic impression. Though this is a proof-of-concept study and no prosthetics were implanted as part of this investigation, we showcase the feasibility of printing prosthetics for palatomaxillary defects. The use of 3D printed prosthetics may be a more humane process, potentially lower cost, and be more accessible to veterans.
Three-dimensional (3D) printing has become a promising area of innovation in biomedical research.1,2 Previous research in orthopedic surgery has found that customized 3D printed implants, casts, orthoses, and prosthetics (eg, prosthetic hands) matched to an individual’s unique anatomy can result in more precise placement and better surgical outcomes.3-5 Customized prosthetics have also been found to lead to fewer complications.3,6
Recent advances in 3D printing technology has prompted investigation from surgeons to identify how this new tool may be incorporated into patient care.1,7 One of the most common applications of 3D printing is during preoperative planning in which surgeons gain better insight into patient-specific anatomy by using patient-specific printed models.8 Another promising application is the production of customized prosthetics suited to each patient’s unique anatomy.9 As a result, 3D printing has significantly impacted bone and cartilage restoration procedures and has the potential to completely transform the treatment of patients with debilitating musculoskeletal injuries.3,10
The potential surrounding 3D printed prosthetics has led to their adoption by several other specialties, including otolaryngology.11 The most widely used application of 3D printing among otolaryngologists is preoperative planning, and the incorporation of printed prosthetics intoreconstruction of the orbit, nasal septum, auricle, and palate has also been reported.2,12,13 Patient-specific implants might allow otolaryngologists to better rehabilitate, reconstruct, and/or regenerate craniofacial defects using more humane procedures.14
Patients with palatomaxillary cancers are treated by prosthodontists or otolaryngologists. An impression is made with a resin–which can be painful for postoperative patients–and a prosthetic is manufactured and implanted.15-17 Patients with cancer often see many specialists, though reconstructive care is a low priority. Many of these individuals also experience dynamic anatomic functional changes over time, leading to the need for multiple prothesis.
palatomaxillary prosthetics
This program aims to use patients’ previous computed tomography (CT) to tailor customized 3D printed palatomaxillary prosthetics to specifically fit their anatomy. Palatomaxillary defects are a source of profound disability for patients with head and neck cancers who are left with large anatomic defects as a direct result of treatment. Reconstruction of palatal defects poses unique challenges due to the complexity of patient anatomy.18,19
3D printed prosthetics for palatomaxillary defects have not been incorporated into patient care. We reviewed previous imaging research to determine if it could be used to assist patients who struggle with their function and appearance following treatment for head and neck cancers. The primary aim was to investigate whether 3D printing was a feasible strategy for creating patient-specific palatomaxillary prosthetics. The secondary aim is to determine whether these prosthetics should be tested in the future for use in reconstruction of maxillary defects.
Data Acquisition
This study was conducted at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) and was approved by the Stanford University Institutional Review Board (approval #28958, informed consent and patient contact excluded). A retrospective chart review was conducted on all patients with head and neck cancers who were treated at VAPAHCS from 2010 to 2022. Patients aged ≥ 18 years who had a palatomaxillary defect due to cancer treatment, had undergone a palatal resection, and who received treatment at any point from 2010 to 2022 were included in the review. CTs were not a specific inclusion criterion, though the quality of the scans was analyzed for eligible patients. Younger patients and those treated at VAPAHCS prior to 2010 were excluded.
There was no control group; all data was sourced from the US Department of Veterans Affairs (VA) imaging system database. Among the 3595 patients reviewed, 5 met inclusion criteria and the quality of their craniofacial anatomy CTs were analyzed. To maintain accurate craniofacial 3D modeling, CTs require a maximum of 1 mm slice thickness. Of the 5 patients who met the inclusion criteria, 4 were found to have variability in the quality of their CTs and severe defects not suitable for prosthetic reconstruction, which led to their exclusion from the study. One patient was investigated to demonstrate if making these prostheses was feasible. This patient was diagnosed with a malignant neoplasm of the hard palate, underwent a partial maxillectomy, and a palatal obturator was placed to cover the defect.
The primary data collected was patient identifiers as well as the gross anatomy and dimensions of the patients’ craniofacial anatomy, as seen in previous imaging research.20 Before the imaging analysis, all personal health information was removed and the dataset was deidentified to ensure patient anonymity and noninvolvement.
CT Segmentation and 3D Printing
Using CTs of the patient’s craniofacial anatomy, we developed a model of the defects. This was achieved with deidentified CTs imported into the Food and Drug Administration (FDA)-approved computerized aid design (CAD) software, Materialise Mimics. The hard palate was segmented and isolated based off the presented scan and any holes in the image were filled using the CAD software. The model was subsequently mirrored in Materialise 3-matic to replicate an original anatomical hard palate prosthesis. The final product was converted into a 3D model and imported into Formlabs preform software to generate 3D printing supports and orient it for printing. The prosthetic was printed using FDA-approved Biocompatible Denture Base Resin by a Formlabs 3B+ printer at the Palo Alto VA Simulation Center. The 3D printed prosthesis was washed using Formlabs Form Wash 80% ethyl alcohol to remove excess resin and subsequently cured to harden the malleable resin. Supports were later removed, and the prosthesis was sanded.
The primary aim of this study was to investigate whether using CTs to create patient-specific prosthetic renderings for patients with head and neck cancer could be a feasible strategy. The CTs from the patient were successfully used to generate a 3D printed prosthesis, and the prosthesis matched the original craniofacial anatomy seen in the patient's imaging (Figure). These results demonstrate that high quality CTs can be used as a template for 3D printed prostheses for mild to moderate palatomaxillary defects.
3D Printing Costs
One liter of Denture Base Resin costs $299; prostheses use about 5 mL of resin. The average annual salary of a 3D printing technician in the United States is $42,717, or $20.54 per hour.21 For an experienced 3D printing technician, the time required to segment the hard palate and prepare it for 3D printing is 1 to 2 hours. The process may exceed 2 hours if the technician is presented with a lower quality CT or if the patient has a complex craniofacial anatomy.
The average time it takes to print a palatal prosthetic is 5 hours. An additional hour is needed for postprocessing, which includes washing and sanding. Therefore, the cost of the materials and labor for an average 3D printed prosthetic is about $150. A Formlabs 3B+ printer is competitively priced around $10,000. The cost for Materialise Mimics software varies, but is estimated at $16,000 at VAPAHCS. The prices for these 2 items are not included in our price estimation but should be taken into consideration.
Prosthodontist Process and Cost
The typical process of creating a palatal prosthesis by a prosthodontist begins by examining the patient, creating a stone model, then creating a wax model. Biocompatible materials are selected and processed into a mold that is trimmed and polished to the desired shape. This is followed by another patient visit to ensure the prosthesis fits properly. Follow-up care is also necessary for maintenance and comfort.
The average cost of a palatal prosthesis varies depending on the type needed (ie, metal implant, teeth replacement), the materials used, the region in which the patient is receiving care, and the complexity of the case. For complex and customizable options like those required for patients with cancer, the prostheses typically cost several thousands of dollars. The Healthcare Common Procedure Coding System code for a palatal lift prosthesis (D5955) lists prices ranging from $4000 to $8000 per prosthetic, not including the cost of the prosthodontist visits.22,23
Discussion
This program sought to determine whether imaging studies of maxillary defects are effective templates for developing 3D printed prosthetics and whether these prosthetics should be tested for future use in reconstruction of palatomaxillary defects. Our program illustrated that CTs served as feasible templates for developing hard palate prostheses for patients with palatomaxillary defects. It is important to note the CTs used were from a newer and more modern scanner and therefore yielded detailed palatal structures with higher accuracy more suitable for 3D modeling. Lower-quality CTs from the 4 patients excluded from the program were not suitable for 3D modeling. This suggests that with high-quality imaging, 3D printed prosthesis may be a viable strategy to help patients who struggle with their function following treatment for head and neck cancers.
3D printed prosthesis may also be a more patient centered and convenient option. In the traditional prosthesis creation workflow, the patient must physically bite down onto a resin (alginate or silicone) to make an impression, a very painful postoperative process that is irritating to the raw edges of the surgical bed.15,16 Prosthodontists then create a prosthetic minus the tumor and typically secure it with clips or glue.17 Many patients also experience changes in their anatomy over time requiring them to have a new protheses created. This is particularly important in veterans with palatomaxillary defects since many VA medical centers do not have a prosthodontist on staff, making accessibility to these specialists difficult. 3D printing provides a contactless prosthetic creation process. This convenience may reduce a patient’s pain and the number of visits for which they need a specialist.
Future Directions
Additional research is needed to determine the full potential of 3D printed prosthetics. 3D printed prostheses have been effectively used for patient education in areas of presurgical planning, prosthesis creation, and trainee education.24 This research represents an early step in the development of a new technology for use in otolaryngology. Specifically, many veterans with a history of head and neck cancers have sustained changes to their craniofacial anatomy following treatment. Using imaging to create 3D printed prosthetics could be very effective for these patients. Prosthetics could improve a patient’s quality of life by restoring/approximating their anatomy after cancer treatment.
Significant time and care must be taken by cancer and reconstructive surgeons to properly fit a prosthesis. Improperly fitting prosthetics leads to mucosal ulceration that then may lead to a need for fitting a new prosthetic. The advantage of 3D printed prosthetics is that they may more precisely fit the anatomy of each patient using CT results, thus potentially reducing the time needed to fit the prosthetic as well as the risk associated with an improperly fit prosthetic. 3D printed prosthesis could be used directly in the future, however, clinical trials are needed to verify its efficacy vs prosthodontic options.
Another consideration for potential future use of 3D printed prosthetics is cost. We estimated that the cost of the materials and labor of our 3D printed prosthetic to be about $150. Pricing of current molded prosthetics varies, but is often listed at several thousand dollars. Another consideration is the durability of 3D printed prosthetics vs standard prosthetics. Since we were unable to use the prosthetic in the patient, it was difficult to determine its durability. The significant cost of the 3D printer and software necessary for 3D printed prosthetics must also be considered and may be prohibitive. While many academic hospitals are considering the purchase of 3D printers and licenses, this may be challenging for resource-constrained institutions. 3D printing may also be difficult for groups without any prior experience in the field. Outsourcing to a third party is possible, though doing so adds more cost to the project. While we recognize there is a learning curve associated with adopting any new technology, it’s equally important to note that 3D printing is being rapidly integrated and has already made significant advancements in personalized medicine.8,25,26
Limitations
This program had several limitations. First, we only obtained CTs of sufficient quality from 1 patient to generate a 3D printed prosthesis. Further research with additional patients is necessary to validate this process. Second, we were unable to trial the prosthesis in the patient because we did not have FDA approval. Additionally, it is difficult to calculate a true cost estimate for this process as materials and software costs vary dramatically across institutions as well as over time.
Conclusions
The purpose of this study was to demonstrate the possibility to develop prosthetics for the hard palate for patients suffering from palatomaxillary defects. A 3D printed prosthetic was generated that matched the patient’s craniofacial anatomy. Future research should test the feasibility of these prosthetics in patient care against a traditional prosthodontic impression. Though this is a proof-of-concept study and no prosthetics were implanted as part of this investigation, we showcase the feasibility of printing prosthetics for palatomaxillary defects. The use of 3D printed prosthetics may be a more humane process, potentially lower cost, and be more accessible to veterans.
1. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi:10.1177/0194599816678372
2. Virani FR, Chua EC, Timbang MR, Hsieh TY, Senders CW. Three-dimensional printing in cleft care: a systematic review. Cleft Palate Craniofac J. 2022;59(4):484-496. doi:10.1177/10556656211013175
3. Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9(3):260-268. doi:10.1016/j.jcot.2018.07.022
4. Vujaklija I, Farina D. 3D printed upper limb prosthetics. Expert Rev Med Devices. 2018;15(7):505-512. doi:10.1080/17434440.2018.1494568
5. Ten Kate J, Smit G, Breedveld P. 3D-printed upper limb prostheses: a review. Disabil Rehabil Assist Technol. 2017;12(3):300-314. doi:10.1080/17483107.2016.1253117
6. Thomas CN, Mavrommatis S, Schroder LK, Cole PA. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury. 2022;53(3):977-983. doi:10.1016/j.injury.2021.11.019
7. Colaco M, Igel DA, Atala A. The potential of 3D printing in urological research and patient care. Nat Rev Urol. 2018;15(4):213-221. doi:10.1038/nrurol.2018.6
8. Meyer-Szary J, Luis MS, Mikulski S, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6):3331. Published 2022 Mar 11. doi:10.3390/ijerph19063331
9. Moya D, Gobbato B, Valente S, Roca R. Use of preoperative planning and 3D printing in orthopedics and traumatology: entering a new era. Acta Ortop Mex. 2022;36(1):39-47.
10. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. Published 2021 Apr 20. doi:10.5435/JAAOSGlobal-D-20-00230
11. Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045-2052. doi:10.1002/lary.2783112. Sigron GR, Barba M, Chammartin F, Msallem B, Berg BI, Thieringer FM. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blow-out fractures) with and without the support of 3D-printed orbital anatomical models. J Clin Med. 2021;10(16):3509. Published 2021 Aug 9. doi:10.3390/jcm10163509
13. Kimura K, Davis S, Thomas E, et al. 3D Customization for microtia repair in hemifacial microsomia. Laryngoscope. 2022;132(3):545-549. doi:10.1002/lary.29823
14. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45-57. doi:10.1007/s10439-016-1668-5
15. Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo MA, et al. Evolution of oral cancer treatment in an andalusian population sample: rehabilitation with prosthetic obturation and removable partial prosthesis. J Clin Exp Dent. 2017;9(8):e1008-e1014. doi:10.4317/jced.54023
16. Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174-181. doi:10.1053/joms.2003.50044
17. Pool C, Shokri T, Vincent A, Wang W, Kadakia S, Ducic Y. Prosthetic reconstruction of the maxilla and palate. Semin Plast Surg. 2020;34(2):114-119. doi:10.1055/s-0040-1709143
18. Badhey AK, Khan MN. Palatomaxillary reconstruction: fibula or scapula. Semin Plast Surg. 2020;34(2):86-91. doi:10.1055/s-0040-1709431
19. Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the palatomaxillary structure. Semin Plast Surg. 2020;34(2):71-76. doi:10.1055/s-0040-1709430
20. Lobb DC, Cottler P, Dart D, Black JS. The use of patient-specific three-dimensional printed surgical models enhances plastic surgery resident education in craniofacial surgery. J Craniofac Surg. 2019;30(2):339-341. doi:10.1097/SCS.0000000000005322
21. 3D printing technician salary in the United States. Accessed February 27, 2024. https://www.salary.com/research/salary/posting/3d-printing-technician-salary22. US Dept of Veterans Affairs. Healthcare Common Procedure Coding System. Outpatient dental professional nationwide charges by HCPCS code. January-December 2020. Accessed February 27, 2024. https://www.va.gov/COMMUNITYCARE/docs/RO/Outpatient-DataTables/v3-27_Table-I.pdf23. Washington State Department of Labor and Industries. Professional services fee schedule HCPCS level II fees. October 1, 2020. Accessed February 27, 2024. https://lni.wa.gov/patient-care/billing-payments/marfsdocs/2020/2020FSHCPCS.pdf24. Low CM, Morris JM, Price DL, et al. Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33(6):770-781. doi:10.1177/1945892419866319
25. Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019;2019:5340616. Published 2019 Mar 21. doi:10.1155/2019/5340616
26. Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27-40. doi:10.1136/bmjstel-2017-000234
1. Crafts TD, Ellsperman SE, Wannemuehler TJ, Bellicchi TD, Shipchandler TZ, Mantravadi AV. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol Head Neck Surg. 2017;156(6):999-1010. doi:10.1177/0194599816678372
2. Virani FR, Chua EC, Timbang MR, Hsieh TY, Senders CW. Three-dimensional printing in cleft care: a systematic review. Cleft Palate Craniofac J. 2022;59(4):484-496. doi:10.1177/10556656211013175
3. Lal H, Patralekh MK. 3D printing and its applications in orthopaedic trauma: A technological marvel. J Clin Orthop Trauma. 2018;9(3):260-268. doi:10.1016/j.jcot.2018.07.022
4. Vujaklija I, Farina D. 3D printed upper limb prosthetics. Expert Rev Med Devices. 2018;15(7):505-512. doi:10.1080/17434440.2018.1494568
5. Ten Kate J, Smit G, Breedveld P. 3D-printed upper limb prostheses: a review. Disabil Rehabil Assist Technol. 2017;12(3):300-314. doi:10.1080/17483107.2016.1253117
6. Thomas CN, Mavrommatis S, Schroder LK, Cole PA. An overview of 3D printing and the orthopaedic application of patient-specific models in malunion surgery. Injury. 2022;53(3):977-983. doi:10.1016/j.injury.2021.11.019
7. Colaco M, Igel DA, Atala A. The potential of 3D printing in urological research and patient care. Nat Rev Urol. 2018;15(4):213-221. doi:10.1038/nrurol.2018.6
8. Meyer-Szary J, Luis MS, Mikulski S, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals-cross-sectional multispecialty review. Int J Environ Res Public Health. 2022;19(6):3331. Published 2022 Mar 11. doi:10.3390/ijerph19063331
9. Moya D, Gobbato B, Valente S, Roca R. Use of preoperative planning and 3D printing in orthopedics and traumatology: entering a new era. Acta Ortop Mex. 2022;36(1):39-47.
10. Wixted CM, Peterson JR, Kadakia RJ, Adams SB. Three-dimensional printing in orthopaedic surgery: current applications and future developments. J Am Acad Orthop Surg Glob Res Rev. 2021;5(4):e20.00230-11. Published 2021 Apr 20. doi:10.5435/JAAOSGlobal-D-20-00230
11. Hong CJ, Giannopoulos AA, Hong BY, et al. Clinical applications of three-dimensional printing in otolaryngology-head and neck surgery: a systematic review. Laryngoscope. 2019;129(9):2045-2052. doi:10.1002/lary.2783112. Sigron GR, Barba M, Chammartin F, Msallem B, Berg BI, Thieringer FM. Functional and cosmetic outcome after reconstruction of isolated, unilateral orbital floor fractures (blow-out fractures) with and without the support of 3D-printed orbital anatomical models. J Clin Med. 2021;10(16):3509. Published 2021 Aug 9. doi:10.3390/jcm10163509
13. Kimura K, Davis S, Thomas E, et al. 3D Customization for microtia repair in hemifacial microsomia. Laryngoscope. 2022;132(3):545-549. doi:10.1002/lary.29823
14. Nyberg EL, Farris AL, Hung BP, et al. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann Biomed Eng. 2017;45(1):45-57. doi:10.1007/s10439-016-1668-5
15. Flores-Ruiz R, Castellanos-Cosano L, Serrera-Figallo MA, et al. Evolution of oral cancer treatment in an andalusian population sample: rehabilitation with prosthetic obturation and removable partial prosthesis. J Clin Exp Dent. 2017;9(8):e1008-e1014. doi:10.4317/jced.54023
16. Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174-181. doi:10.1053/joms.2003.50044
17. Pool C, Shokri T, Vincent A, Wang W, Kadakia S, Ducic Y. Prosthetic reconstruction of the maxilla and palate. Semin Plast Surg. 2020;34(2):114-119. doi:10.1055/s-0040-1709143
18. Badhey AK, Khan MN. Palatomaxillary reconstruction: fibula or scapula. Semin Plast Surg. 2020;34(2):86-91. doi:10.1055/s-0040-1709431
19. Jategaonkar AA, Kaul VF, Lee E, Genden EM. Surgery of the palatomaxillary structure. Semin Plast Surg. 2020;34(2):71-76. doi:10.1055/s-0040-1709430
20. Lobb DC, Cottler P, Dart D, Black JS. The use of patient-specific three-dimensional printed surgical models enhances plastic surgery resident education in craniofacial surgery. J Craniofac Surg. 2019;30(2):339-341. doi:10.1097/SCS.0000000000005322
21. 3D printing technician salary in the United States. Accessed February 27, 2024. https://www.salary.com/research/salary/posting/3d-printing-technician-salary22. US Dept of Veterans Affairs. Healthcare Common Procedure Coding System. Outpatient dental professional nationwide charges by HCPCS code. January-December 2020. Accessed February 27, 2024. https://www.va.gov/COMMUNITYCARE/docs/RO/Outpatient-DataTables/v3-27_Table-I.pdf23. Washington State Department of Labor and Industries. Professional services fee schedule HCPCS level II fees. October 1, 2020. Accessed February 27, 2024. https://lni.wa.gov/patient-care/billing-payments/marfsdocs/2020/2020FSHCPCS.pdf24. Low CM, Morris JM, Price DL, et al. Three-dimensional printing: current use in rhinology and endoscopic skull base surgery. Am J Rhinol Allergy. 2019;33(6):770-781. doi:10.1177/1945892419866319
25. Aimar A, Palermo A, Innocenti B. The role of 3D printing in medical applications: a state of the art. J Healthc Eng. 2019;2019:5340616. Published 2019 Mar 21. doi:10.1155/2019/5340616
26. Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4(1):27-40. doi:10.1136/bmjstel-2017-000234
Improving Fecal Immunochemical Test Collection for Colorectal Cancer Screening During the COVID-19 Pandemic
Colorectal cancer (CRC) is the third-most common cancer worldwide and accounts for almost 11% of all cancer diagnoses, with > 1.9 million cases reported globally.1,2 CRC is the second-most deadly cancer, responsible for about 935,000 deaths.1 Over the past several decades, a steady decline in CRC incidence and mortality has been reported in developed countries, including the US.3,4 From 2008 through 2017, an annual reduction of 3% in CRC death rates was reported in individuals aged ≥ 65 years.5 This decline can mainly be attributed to improvements made in health systems and advancements in CRC screening programs.3,5
US Preventive Services Task Force (USPSTF) recommends CRC screening in individuals aged 45 to 75 years. USPSTF recommends direct visualization tests, such as colonoscopy and flexible sigmoidoscopy for CRC screening.6 Although colonoscopy is commonly used for CRC screening, it is an invasive procedure that requires bowel preparation and sedation, and has the potential risk of colonic perforation, bleeding, and infection. Additionally, social determinants—such as health care costs, missed work, and geographic location (eg, rural communities)—may limit colonoscopy utilization.7 As a result, other cost-effective, noninvasive tests such as high-sensitivity guaiac-based fecal occult blood test (gFOBT) and fecal immunochemical test (FIT) are also used for CRC screening. These tests detect occult blood in the stool of individuals who may be at risk for CRC, helping direct them to colonoscopy if they screen positive.8
The gFOBT relies on simple oxidation and requires a stool sample to detect the presence of the heme component of blood.9 If heme is present in the stool sample, it will enable the oxidation of guaiac to form a blue-colored dye when added to hydrogen peroxide. It is important to note that the oxidation component of this test may lead to false-positive results, as it may detect dietary hemoglobin present in red meat. Medications or foods that have peroxidase properties may also result in a false-positive gFOBT result. Additionally, false-negative results may be caused by antioxidants, which may interfere with the oxidation of guaiac.
FIT uses antibodies, which bind to the intact globin component of human hemoglobin.9 The quantity of bound antibody-hemoglobin complex is detected and measured by a variety of automated quantitative techniques. This testing strategy eliminates the need for food or medication restrictions and the subjective visual assessment of change in color, as required for the gFOBT.9 A 2016 meta-analysis found that FIT performed better compared with gFOBT in terms of specificity, positivity rate, number needed to scope, and number needed to screen.8 The FIT screening method has also been found to have greater adherence rates, which is likely due to fewer stool sampling requirements and the lack of medication or dietary restrictions, compared with gFOBT.7,8
The COVID-19 pandemic had a drastic impact on CRC preventive care services. In March 2020, elective colonoscopies were temporarily ceased across the country and the US Department of Veterans Affairs (VA) deferred all elective surgeries and medical procedures, including screening and surveillance colonoscopies. In line with these recommendations, elective colonoscopies were temporarily ceased across the country.10 The National Cancer Institute’s Population-Based Research to Optimize the Screening Process consortium reported that CRC screening rates decreased by 82% across the US in 2020.11 Public health measures are likely the main reason for this decline, but other factors may include a lack of resource availability in outpatient settings and public fear of the pandemic.10
The James A. Haley Veterans Affairs Hospital (JAHVAH) in Tampa, Florida, encouraged the use of FIT in place of colonoscopies to avoid delaying preventive services. The initiative to continue CRC screening methods via FIT was scrutinized when laboratory personnel reported that in fiscal year (FY) 2020, 62% of the FIT kits that patients returned to the laboratory were missing information or had other errors (Figure 1). These improperly returned FIT kits led to delayed processing, canceled orders, increased staff workload, and more costs for FIT repetition.
Research shows many patients often fail to adhere to the instructions for proper FIT sample collection and return. Wang and colleagues reported that of 4916 FIT samples returned to the laboratory, 971 (20%) had collection errors, and 910 (94%) of those samples were missing a sample collection date.12 The sample collection date is important because hemoglobin degradation occurs over time, which may create false-negative FIT results. Although studies have found that sample return times of ≤ 10 days are not associated with a decrease in FIT positive rates, it is recommended to mail completed FITs within 24 hours of sample collection.13
Because remote screening methods like FIT were preferred during the COVID-19 pandemic, we conducted a quality improvement (QI) project to address FIT inefficiency. The aim of this initiative was to determine the root cause behind incorrectly returned FIT kits and to increase correctly collected and testable FIT kits upon initial laboratory arrival by at least 20% by the second quarter of FY 2021.
Quality Improvement Project
This QI project was conducted from July 2020 to June 2021 at the JAHVAH, which provides primary care and specialty health services to veterans in central and south Florida. The QI was designed based on the Plan-Do-Study-Act (PDSA) model of health care improvement. The QI team consisted of physicians, nurses, administrative staff, and laboratory personnel. A SIPOC (Suppliers, Input, Process, Output, Customers) map was initially designed to help clarify the different groups involved in the process of FIT kit distribution and return. This map helped the team decide who should be involved in the solution process.
The QI team performed a root cause analysis using a fishbone diagram and identified the reasons FIT kits were returned to the laboratory with errors that prevented processing. The team brainstormed potential change ideas and created an impact vs effort chart to increase the number of correctly returned and testable FIT kits upon initial arrival at the laboratory by at least 20% by the second quarter of FY 2021. We identified strengths and prioritized change ideas to improve the number of testable and correctly returned FIT kits to the hospital laboratory. These ideas included centralizing FIT kit dispersal to a new administrative group, building redundant patient reminders on kit completion and giving patients more accessible places for kit return.
Patients included in the study were adults aged 50 to 75 years seen at the JAHVAH outpatient clinic who were asked to undergo FIT CRC screening. FIT orders for other facilities were excluded. The primary endpoint of this project was to improve the number of correctly returned FITs. The number of correct and incorrect returned FITs were measured from July 2020 to June 2021. FITs returned with errors were categorized by the type of error, including: no order on file in the electronic health record (EHR), canceled test, expired test, unable to identify test, missing information, and missing collection date.
We attempted to calculate costs of FITs that were returned to the laboratory but could not be analyzed and were discarded. In FY 2020, 1568 FITs were discarded. Each FIT cost about $7.80 to process for an annualized expense of $12,230 for discarded FITs.
Root Cause Analysis
Root causes were obtained by making a fishbone diagram. From this diagram, an impact vs effort chart was created to form and prioritize ideas for our PDSA cycles. Data about correctly and incorrectly returned kits were collected monthly from laboratory personnel, then analyzed by the QI team using run charts to look for change in frequency and patterns.
To improve this process, a swim lane chart for FIT processing was assembled and later used to make a comprehensive fishbone diagram to establish the 6 main root cause errors: missing FIT EHR order, cancelled FIT EHR order, expired stool specimen, partial patient identifiers, no patient identifiers, and no stool collection date. Pareto and run charts were superimposed with the laboratory data. The most common cause of incorrectly returned FITs was no collection date.
PDSA Cycles
Beginning in January 2021, PDSA cycles from the ideas in the impact vs effort chart were used. Organization and implementation of the project occurred from July 2020 to April 2021. The team reassessed the data in April 2021 to evaluate progress after PDSA initiation. The mean rate of missing collection date dropped from 24% in FY 2020 prior to PDSA cycles to 14% in April 2021; however, the number of incorrectly returned kits was similar to the baseline level. When reviewing this discrepancy, the QI team found that although the missing collection date rate had improved, the rate of FITs with not enough information had increased from 5% in FY 2020 to 67% in April 2021 (Figure 2). After discussing with laboratory personnel, it was determined that the EHR order was missing when the process pathway changed. Our PDSA initiative changed the process pathway and different individuals were responsible for FIT dispersal. The error was quickly addressed with the help of clinical and administrative staff; a 30-day follow-up on June 21, 2021, revealed that only 9% of the patients had sent back kits with not enough information.
After troubleshooting, the team achieved a sustainable increase in the number of correctly returned FIT kits from an average of 38% before the project to 72% after 30-day follow-up.
Discussion
Proper collection and return of FIT samples are vital for process efficiency for both physicians and patients. This initiative aimed to improve the rate of correctly returned FIT kits by 20%, but its final numbers showed an improvement of 33.6%. Operational benefits from this project included early detection of CRC, improved laboratory workflow, decreased FIT kit waste, and increased patient satisfaction.
The multipronged PDSA cycle attempted to increase the rate of correctly returned FIT kits. We improved kit comprehension and laboratory accessibility, and instituted redundant return reminders for patients. We also centralized a new process pathway for FIT distribution and educated physicians and support staff. Sampling and FIT return may seem like a simple procedure, but the FIT can be cumbersome for patients and directions can be confusing. Therefore, to maximize screening participation, it is essential to minimize confusion in the collection and return of a FIT sample.14,15
This QI initiative was presented at Grand Rounds at the University of South Florida in June 2021 and has since been shared with other VA hospitals. It was also presented at the American College of Gastroenterology Conference in 2021.
Limitations
This study was a single-center QI project and focused mostly on FIT kit return rates. To fully address CRC screening, it is important to ensure that individuals with a positive screen are appropriately followed up with a colonoscopy. Although follow-up was not in the scope of this project, it is key to CRC screening in general and should be the subject of future research.
Conclusions
FIT is a useful method for CRC screening that can be particularly helpful when in-person visits are limited, as seen during the COVID-19 pandemic. This increase in demand for FITs during the pandemic revealed process deficiencies and gave JAHVAH an opportunity to improve workflow. Through the aid of a multidisciplinary team, the process to complete and return FITs improved and surpassed the goal of 20% improvement. Our goal is to continue to fine-tune the workflow and troubleshoot the system as needed.
1. Sawicki T, Ruszkowska M, Danielewicz A, Niedz′wiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025. Published 2021 Apr 22. doi:10.3390/cancers13092025
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89-103. doi:10.5114/pg.2018.81072
3. Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014;120(18):2893-2901. doi:10.1002/cncr.28794
4. Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1296-1302. doi:10.1158/1055-9965.EPI-11-0250
5. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164. doi:10.3322/caac.21601
6. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive
Colorectal cancer (CRC) is the third-most common cancer worldwide and accounts for almost 11% of all cancer diagnoses, with > 1.9 million cases reported globally.1,2 CRC is the second-most deadly cancer, responsible for about 935,000 deaths.1 Over the past several decades, a steady decline in CRC incidence and mortality has been reported in developed countries, including the US.3,4 From 2008 through 2017, an annual reduction of 3% in CRC death rates was reported in individuals aged ≥ 65 years.5 This decline can mainly be attributed to improvements made in health systems and advancements in CRC screening programs.3,5
US Preventive Services Task Force (USPSTF) recommends CRC screening in individuals aged 45 to 75 years. USPSTF recommends direct visualization tests, such as colonoscopy and flexible sigmoidoscopy for CRC screening.6 Although colonoscopy is commonly used for CRC screening, it is an invasive procedure that requires bowel preparation and sedation, and has the potential risk of colonic perforation, bleeding, and infection. Additionally, social determinants—such as health care costs, missed work, and geographic location (eg, rural communities)—may limit colonoscopy utilization.7 As a result, other cost-effective, noninvasive tests such as high-sensitivity guaiac-based fecal occult blood test (gFOBT) and fecal immunochemical test (FIT) are also used for CRC screening. These tests detect occult blood in the stool of individuals who may be at risk for CRC, helping direct them to colonoscopy if they screen positive.8
The gFOBT relies on simple oxidation and requires a stool sample to detect the presence of the heme component of blood.9 If heme is present in the stool sample, it will enable the oxidation of guaiac to form a blue-colored dye when added to hydrogen peroxide. It is important to note that the oxidation component of this test may lead to false-positive results, as it may detect dietary hemoglobin present in red meat. Medications or foods that have peroxidase properties may also result in a false-positive gFOBT result. Additionally, false-negative results may be caused by antioxidants, which may interfere with the oxidation of guaiac.
FIT uses antibodies, which bind to the intact globin component of human hemoglobin.9 The quantity of bound antibody-hemoglobin complex is detected and measured by a variety of automated quantitative techniques. This testing strategy eliminates the need for food or medication restrictions and the subjective visual assessment of change in color, as required for the gFOBT.9 A 2016 meta-analysis found that FIT performed better compared with gFOBT in terms of specificity, positivity rate, number needed to scope, and number needed to screen.8 The FIT screening method has also been found to have greater adherence rates, which is likely due to fewer stool sampling requirements and the lack of medication or dietary restrictions, compared with gFOBT.7,8
The COVID-19 pandemic had a drastic impact on CRC preventive care services. In March 2020, elective colonoscopies were temporarily ceased across the country and the US Department of Veterans Affairs (VA) deferred all elective surgeries and medical procedures, including screening and surveillance colonoscopies. In line with these recommendations, elective colonoscopies were temporarily ceased across the country.10 The National Cancer Institute’s Population-Based Research to Optimize the Screening Process consortium reported that CRC screening rates decreased by 82% across the US in 2020.11 Public health measures are likely the main reason for this decline, but other factors may include a lack of resource availability in outpatient settings and public fear of the pandemic.10
The James A. Haley Veterans Affairs Hospital (JAHVAH) in Tampa, Florida, encouraged the use of FIT in place of colonoscopies to avoid delaying preventive services. The initiative to continue CRC screening methods via FIT was scrutinized when laboratory personnel reported that in fiscal year (FY) 2020, 62% of the FIT kits that patients returned to the laboratory were missing information or had other errors (Figure 1). These improperly returned FIT kits led to delayed processing, canceled orders, increased staff workload, and more costs for FIT repetition.
Research shows many patients often fail to adhere to the instructions for proper FIT sample collection and return. Wang and colleagues reported that of 4916 FIT samples returned to the laboratory, 971 (20%) had collection errors, and 910 (94%) of those samples were missing a sample collection date.12 The sample collection date is important because hemoglobin degradation occurs over time, which may create false-negative FIT results. Although studies have found that sample return times of ≤ 10 days are not associated with a decrease in FIT positive rates, it is recommended to mail completed FITs within 24 hours of sample collection.13
Because remote screening methods like FIT were preferred during the COVID-19 pandemic, we conducted a quality improvement (QI) project to address FIT inefficiency. The aim of this initiative was to determine the root cause behind incorrectly returned FIT kits and to increase correctly collected and testable FIT kits upon initial laboratory arrival by at least 20% by the second quarter of FY 2021.
Quality Improvement Project
This QI project was conducted from July 2020 to June 2021 at the JAHVAH, which provides primary care and specialty health services to veterans in central and south Florida. The QI was designed based on the Plan-Do-Study-Act (PDSA) model of health care improvement. The QI team consisted of physicians, nurses, administrative staff, and laboratory personnel. A SIPOC (Suppliers, Input, Process, Output, Customers) map was initially designed to help clarify the different groups involved in the process of FIT kit distribution and return. This map helped the team decide who should be involved in the solution process.
The QI team performed a root cause analysis using a fishbone diagram and identified the reasons FIT kits were returned to the laboratory with errors that prevented processing. The team brainstormed potential change ideas and created an impact vs effort chart to increase the number of correctly returned and testable FIT kits upon initial arrival at the laboratory by at least 20% by the second quarter of FY 2021. We identified strengths and prioritized change ideas to improve the number of testable and correctly returned FIT kits to the hospital laboratory. These ideas included centralizing FIT kit dispersal to a new administrative group, building redundant patient reminders on kit completion and giving patients more accessible places for kit return.
Patients included in the study were adults aged 50 to 75 years seen at the JAHVAH outpatient clinic who were asked to undergo FIT CRC screening. FIT orders for other facilities were excluded. The primary endpoint of this project was to improve the number of correctly returned FITs. The number of correct and incorrect returned FITs were measured from July 2020 to June 2021. FITs returned with errors were categorized by the type of error, including: no order on file in the electronic health record (EHR), canceled test, expired test, unable to identify test, missing information, and missing collection date.
We attempted to calculate costs of FITs that were returned to the laboratory but could not be analyzed and were discarded. In FY 2020, 1568 FITs were discarded. Each FIT cost about $7.80 to process for an annualized expense of $12,230 for discarded FITs.
Root Cause Analysis
Root causes were obtained by making a fishbone diagram. From this diagram, an impact vs effort chart was created to form and prioritize ideas for our PDSA cycles. Data about correctly and incorrectly returned kits were collected monthly from laboratory personnel, then analyzed by the QI team using run charts to look for change in frequency and patterns.
To improve this process, a swim lane chart for FIT processing was assembled and later used to make a comprehensive fishbone diagram to establish the 6 main root cause errors: missing FIT EHR order, cancelled FIT EHR order, expired stool specimen, partial patient identifiers, no patient identifiers, and no stool collection date. Pareto and run charts were superimposed with the laboratory data. The most common cause of incorrectly returned FITs was no collection date.
PDSA Cycles
Beginning in January 2021, PDSA cycles from the ideas in the impact vs effort chart were used. Organization and implementation of the project occurred from July 2020 to April 2021. The team reassessed the data in April 2021 to evaluate progress after PDSA initiation. The mean rate of missing collection date dropped from 24% in FY 2020 prior to PDSA cycles to 14% in April 2021; however, the number of incorrectly returned kits was similar to the baseline level. When reviewing this discrepancy, the QI team found that although the missing collection date rate had improved, the rate of FITs with not enough information had increased from 5% in FY 2020 to 67% in April 2021 (Figure 2). After discussing with laboratory personnel, it was determined that the EHR order was missing when the process pathway changed. Our PDSA initiative changed the process pathway and different individuals were responsible for FIT dispersal. The error was quickly addressed with the help of clinical and administrative staff; a 30-day follow-up on June 21, 2021, revealed that only 9% of the patients had sent back kits with not enough information.
After troubleshooting, the team achieved a sustainable increase in the number of correctly returned FIT kits from an average of 38% before the project to 72% after 30-day follow-up.
Discussion
Proper collection and return of FIT samples are vital for process efficiency for both physicians and patients. This initiative aimed to improve the rate of correctly returned FIT kits by 20%, but its final numbers showed an improvement of 33.6%. Operational benefits from this project included early detection of CRC, improved laboratory workflow, decreased FIT kit waste, and increased patient satisfaction.
The multipronged PDSA cycle attempted to increase the rate of correctly returned FIT kits. We improved kit comprehension and laboratory accessibility, and instituted redundant return reminders for patients. We also centralized a new process pathway for FIT distribution and educated physicians and support staff. Sampling and FIT return may seem like a simple procedure, but the FIT can be cumbersome for patients and directions can be confusing. Therefore, to maximize screening participation, it is essential to minimize confusion in the collection and return of a FIT sample.14,15
This QI initiative was presented at Grand Rounds at the University of South Florida in June 2021 and has since been shared with other VA hospitals. It was also presented at the American College of Gastroenterology Conference in 2021.
Limitations
This study was a single-center QI project and focused mostly on FIT kit return rates. To fully address CRC screening, it is important to ensure that individuals with a positive screen are appropriately followed up with a colonoscopy. Although follow-up was not in the scope of this project, it is key to CRC screening in general and should be the subject of future research.
Conclusions
FIT is a useful method for CRC screening that can be particularly helpful when in-person visits are limited, as seen during the COVID-19 pandemic. This increase in demand for FITs during the pandemic revealed process deficiencies and gave JAHVAH an opportunity to improve workflow. Through the aid of a multidisciplinary team, the process to complete and return FITs improved and surpassed the goal of 20% improvement. Our goal is to continue to fine-tune the workflow and troubleshoot the system as needed.
Colorectal cancer (CRC) is the third-most common cancer worldwide and accounts for almost 11% of all cancer diagnoses, with > 1.9 million cases reported globally.1,2 CRC is the second-most deadly cancer, responsible for about 935,000 deaths.1 Over the past several decades, a steady decline in CRC incidence and mortality has been reported in developed countries, including the US.3,4 From 2008 through 2017, an annual reduction of 3% in CRC death rates was reported in individuals aged ≥ 65 years.5 This decline can mainly be attributed to improvements made in health systems and advancements in CRC screening programs.3,5
US Preventive Services Task Force (USPSTF) recommends CRC screening in individuals aged 45 to 75 years. USPSTF recommends direct visualization tests, such as colonoscopy and flexible sigmoidoscopy for CRC screening.6 Although colonoscopy is commonly used for CRC screening, it is an invasive procedure that requires bowel preparation and sedation, and has the potential risk of colonic perforation, bleeding, and infection. Additionally, social determinants—such as health care costs, missed work, and geographic location (eg, rural communities)—may limit colonoscopy utilization.7 As a result, other cost-effective, noninvasive tests such as high-sensitivity guaiac-based fecal occult blood test (gFOBT) and fecal immunochemical test (FIT) are also used for CRC screening. These tests detect occult blood in the stool of individuals who may be at risk for CRC, helping direct them to colonoscopy if they screen positive.8
The gFOBT relies on simple oxidation and requires a stool sample to detect the presence of the heme component of blood.9 If heme is present in the stool sample, it will enable the oxidation of guaiac to form a blue-colored dye when added to hydrogen peroxide. It is important to note that the oxidation component of this test may lead to false-positive results, as it may detect dietary hemoglobin present in red meat. Medications or foods that have peroxidase properties may also result in a false-positive gFOBT result. Additionally, false-negative results may be caused by antioxidants, which may interfere with the oxidation of guaiac.
FIT uses antibodies, which bind to the intact globin component of human hemoglobin.9 The quantity of bound antibody-hemoglobin complex is detected and measured by a variety of automated quantitative techniques. This testing strategy eliminates the need for food or medication restrictions and the subjective visual assessment of change in color, as required for the gFOBT.9 A 2016 meta-analysis found that FIT performed better compared with gFOBT in terms of specificity, positivity rate, number needed to scope, and number needed to screen.8 The FIT screening method has also been found to have greater adherence rates, which is likely due to fewer stool sampling requirements and the lack of medication or dietary restrictions, compared with gFOBT.7,8
The COVID-19 pandemic had a drastic impact on CRC preventive care services. In March 2020, elective colonoscopies were temporarily ceased across the country and the US Department of Veterans Affairs (VA) deferred all elective surgeries and medical procedures, including screening and surveillance colonoscopies. In line with these recommendations, elective colonoscopies were temporarily ceased across the country.10 The National Cancer Institute’s Population-Based Research to Optimize the Screening Process consortium reported that CRC screening rates decreased by 82% across the US in 2020.11 Public health measures are likely the main reason for this decline, but other factors may include a lack of resource availability in outpatient settings and public fear of the pandemic.10
The James A. Haley Veterans Affairs Hospital (JAHVAH) in Tampa, Florida, encouraged the use of FIT in place of colonoscopies to avoid delaying preventive services. The initiative to continue CRC screening methods via FIT was scrutinized when laboratory personnel reported that in fiscal year (FY) 2020, 62% of the FIT kits that patients returned to the laboratory were missing information or had other errors (Figure 1). These improperly returned FIT kits led to delayed processing, canceled orders, increased staff workload, and more costs for FIT repetition.
Research shows many patients often fail to adhere to the instructions for proper FIT sample collection and return. Wang and colleagues reported that of 4916 FIT samples returned to the laboratory, 971 (20%) had collection errors, and 910 (94%) of those samples were missing a sample collection date.12 The sample collection date is important because hemoglobin degradation occurs over time, which may create false-negative FIT results. Although studies have found that sample return times of ≤ 10 days are not associated with a decrease in FIT positive rates, it is recommended to mail completed FITs within 24 hours of sample collection.13
Because remote screening methods like FIT were preferred during the COVID-19 pandemic, we conducted a quality improvement (QI) project to address FIT inefficiency. The aim of this initiative was to determine the root cause behind incorrectly returned FIT kits and to increase correctly collected and testable FIT kits upon initial laboratory arrival by at least 20% by the second quarter of FY 2021.
Quality Improvement Project
This QI project was conducted from July 2020 to June 2021 at the JAHVAH, which provides primary care and specialty health services to veterans in central and south Florida. The QI was designed based on the Plan-Do-Study-Act (PDSA) model of health care improvement. The QI team consisted of physicians, nurses, administrative staff, and laboratory personnel. A SIPOC (Suppliers, Input, Process, Output, Customers) map was initially designed to help clarify the different groups involved in the process of FIT kit distribution and return. This map helped the team decide who should be involved in the solution process.
The QI team performed a root cause analysis using a fishbone diagram and identified the reasons FIT kits were returned to the laboratory with errors that prevented processing. The team brainstormed potential change ideas and created an impact vs effort chart to increase the number of correctly returned and testable FIT kits upon initial arrival at the laboratory by at least 20% by the second quarter of FY 2021. We identified strengths and prioritized change ideas to improve the number of testable and correctly returned FIT kits to the hospital laboratory. These ideas included centralizing FIT kit dispersal to a new administrative group, building redundant patient reminders on kit completion and giving patients more accessible places for kit return.
Patients included in the study were adults aged 50 to 75 years seen at the JAHVAH outpatient clinic who were asked to undergo FIT CRC screening. FIT orders for other facilities were excluded. The primary endpoint of this project was to improve the number of correctly returned FITs. The number of correct and incorrect returned FITs were measured from July 2020 to June 2021. FITs returned with errors were categorized by the type of error, including: no order on file in the electronic health record (EHR), canceled test, expired test, unable to identify test, missing information, and missing collection date.
We attempted to calculate costs of FITs that were returned to the laboratory but could not be analyzed and were discarded. In FY 2020, 1568 FITs were discarded. Each FIT cost about $7.80 to process for an annualized expense of $12,230 for discarded FITs.
Root Cause Analysis
Root causes were obtained by making a fishbone diagram. From this diagram, an impact vs effort chart was created to form and prioritize ideas for our PDSA cycles. Data about correctly and incorrectly returned kits were collected monthly from laboratory personnel, then analyzed by the QI team using run charts to look for change in frequency and patterns.
To improve this process, a swim lane chart for FIT processing was assembled and later used to make a comprehensive fishbone diagram to establish the 6 main root cause errors: missing FIT EHR order, cancelled FIT EHR order, expired stool specimen, partial patient identifiers, no patient identifiers, and no stool collection date. Pareto and run charts were superimposed with the laboratory data. The most common cause of incorrectly returned FITs was no collection date.
PDSA Cycles
Beginning in January 2021, PDSA cycles from the ideas in the impact vs effort chart were used. Organization and implementation of the project occurred from July 2020 to April 2021. The team reassessed the data in April 2021 to evaluate progress after PDSA initiation. The mean rate of missing collection date dropped from 24% in FY 2020 prior to PDSA cycles to 14% in April 2021; however, the number of incorrectly returned kits was similar to the baseline level. When reviewing this discrepancy, the QI team found that although the missing collection date rate had improved, the rate of FITs with not enough information had increased from 5% in FY 2020 to 67% in April 2021 (Figure 2). After discussing with laboratory personnel, it was determined that the EHR order was missing when the process pathway changed. Our PDSA initiative changed the process pathway and different individuals were responsible for FIT dispersal. The error was quickly addressed with the help of clinical and administrative staff; a 30-day follow-up on June 21, 2021, revealed that only 9% of the patients had sent back kits with not enough information.
After troubleshooting, the team achieved a sustainable increase in the number of correctly returned FIT kits from an average of 38% before the project to 72% after 30-day follow-up.
Discussion
Proper collection and return of FIT samples are vital for process efficiency for both physicians and patients. This initiative aimed to improve the rate of correctly returned FIT kits by 20%, but its final numbers showed an improvement of 33.6%. Operational benefits from this project included early detection of CRC, improved laboratory workflow, decreased FIT kit waste, and increased patient satisfaction.
The multipronged PDSA cycle attempted to increase the rate of correctly returned FIT kits. We improved kit comprehension and laboratory accessibility, and instituted redundant return reminders for patients. We also centralized a new process pathway for FIT distribution and educated physicians and support staff. Sampling and FIT return may seem like a simple procedure, but the FIT can be cumbersome for patients and directions can be confusing. Therefore, to maximize screening participation, it is essential to minimize confusion in the collection and return of a FIT sample.14,15
This QI initiative was presented at Grand Rounds at the University of South Florida in June 2021 and has since been shared with other VA hospitals. It was also presented at the American College of Gastroenterology Conference in 2021.
Limitations
This study was a single-center QI project and focused mostly on FIT kit return rates. To fully address CRC screening, it is important to ensure that individuals with a positive screen are appropriately followed up with a colonoscopy. Although follow-up was not in the scope of this project, it is key to CRC screening in general and should be the subject of future research.
Conclusions
FIT is a useful method for CRC screening that can be particularly helpful when in-person visits are limited, as seen during the COVID-19 pandemic. This increase in demand for FITs during the pandemic revealed process deficiencies and gave JAHVAH an opportunity to improve workflow. Through the aid of a multidisciplinary team, the process to complete and return FITs improved and surpassed the goal of 20% improvement. Our goal is to continue to fine-tune the workflow and troubleshoot the system as needed.
1. Sawicki T, Ruszkowska M, Danielewicz A, Niedz′wiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025. Published 2021 Apr 22. doi:10.3390/cancers13092025
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89-103. doi:10.5114/pg.2018.81072
3. Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014;120(18):2893-2901. doi:10.1002/cncr.28794
4. Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1296-1302. doi:10.1158/1055-9965.EPI-11-0250
5. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164. doi:10.3322/caac.21601
6. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive
1. Sawicki T, Ruszkowska M, Danielewicz A, Niedz′wiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel). 2021;13(9):2025. Published 2021 Apr 22. doi:10.3390/cancers13092025
2. Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14(2):89-103. doi:10.5114/pg.2018.81072
3. Yang DX, Gross CP, Soulos PR, Yu JB. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer. 2014;120(18):2893-2901. doi:10.1002/cncr.28794
4. Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1296-1302. doi:10.1158/1055-9965.EPI-11-0250
5. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164. doi:10.3322/caac.21601
6. US Preventive Services Task Force, Davidson KW, Barry MJ, et al. Screening for colorectal cancer: US Preventive