User login
Extensive Multidrug-Resistant Dermatophytosis From Trichophyton indotineae
To the Editor:
Historically, commonly available antifungal medications have been effective for treating dermatophytosis (tinea). However, recent tinea outbreaks caused by Trichophyton indotineae—a dermatophyte often resistant to terbinafine and sometimes to other antifungals—have been reported in South Asia, Europe, the Middle East, Southeast Asia, and Australia.1-5
Three confirmed cases of T indotineae dermatophytosis in the United States were reported in 2023 in New York3,6; a fourth confirmed case was reported in 2024 in Pennsylvania.7 Post hoc laboratory testing of fungal isolates in New York in 2022 and 2023 identified an additional 11 cases.8 We present a case of extensive multidrug-resistant tinea caused by T indotineae in a man in California.
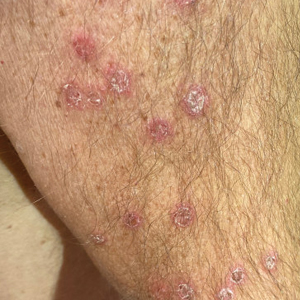
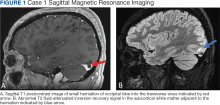
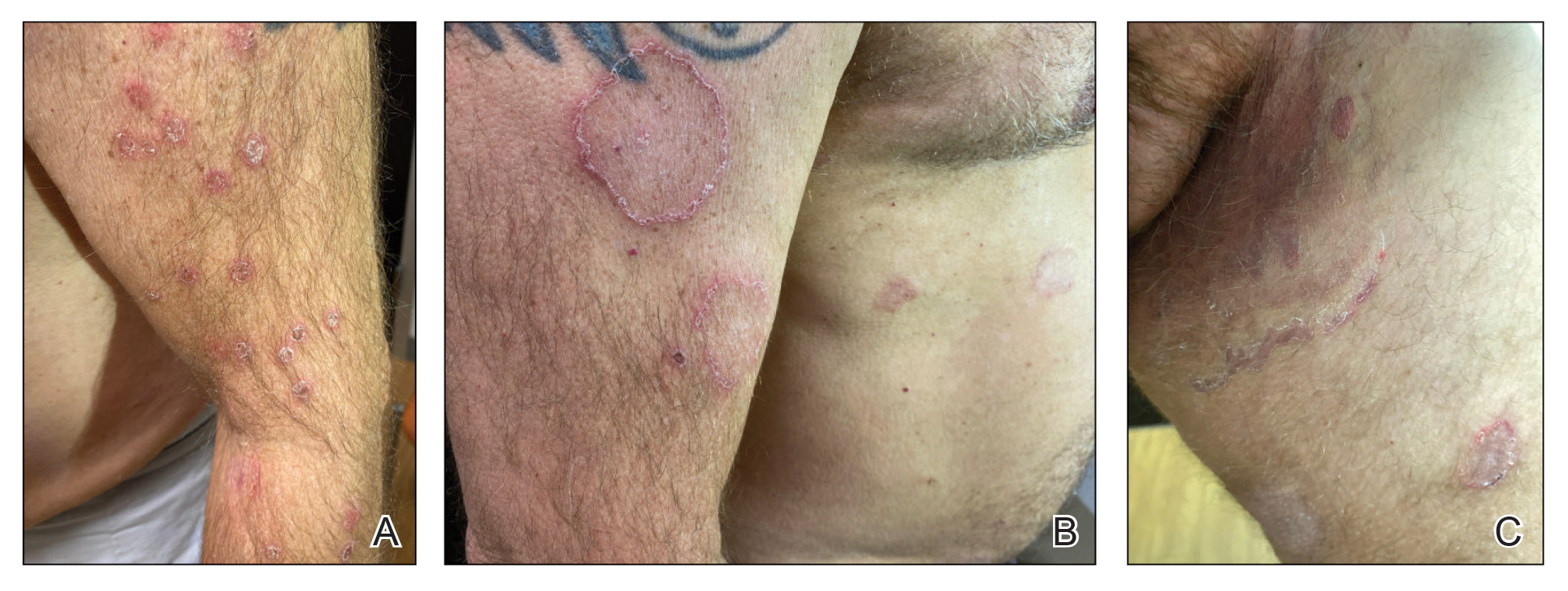
An otherwise healthy 65-year-old man who had traveled to Europe in the past 3 months presented to his primary care physician with a widespread pruritic rash (Figure 1). He was treated with 2 weeks of oral terbinafine 250 mg/d and topical medicines, including clotrimazole cream 1%, fluocinonide ointment 0.05%, and clobetasol ointment 0.05% without improvement. Subsequently, 2 weeks of oral griseofulvin microsize 500 mg/d also proved ineffective. An antibody test was negative for HIV. His hemoglobin A1c was 6.2% (reference range, ≤5.6%). The patient was referred to dermatology.
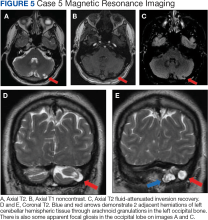
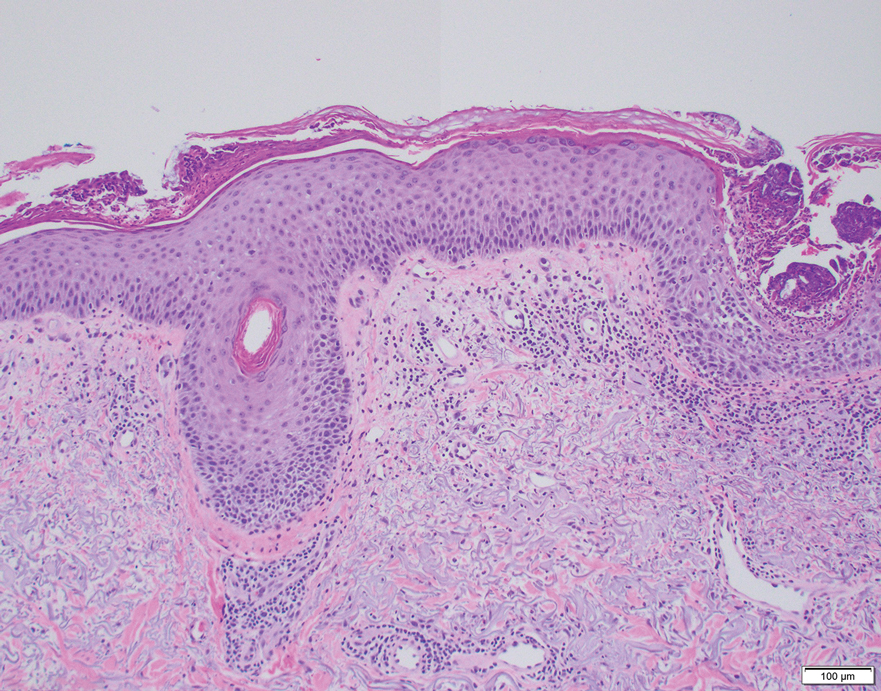
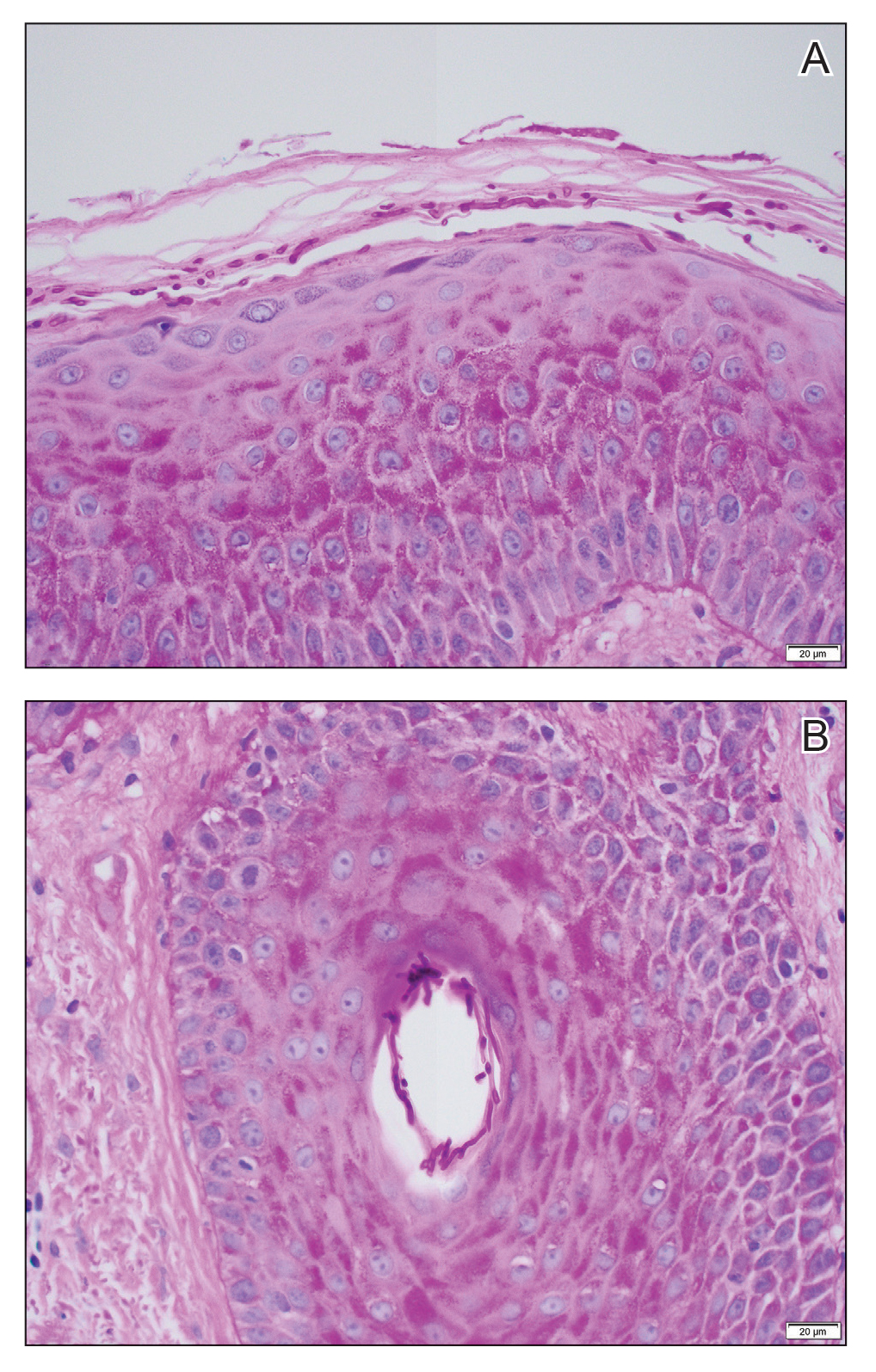
Erythematous plaques—many scaly throughout and some annular with central clearing—were present on the arms, legs, and torso as well as in the groin. Honey crust was present on some plaques on the leg. A potassium hydroxide preparation showed abundant fungal hyphae. Material for fungal and bacterial cultures was collected. The patient was treated again with oral terbinafine 250 mg/d, an oral prednisone taper starting at 60 mg/d for a presumed id reaction, and various oral antihistamines for pruritus; all were ineffective. A bacterial culture showed only mixed skin flora. Oral fluconazole 200 mg/d was prescribed. A skin biopsy specimen showed compact orthokeratosis and parakeratosis of the stratum corneum with few neutrophils and focal pustule formation (Figure 2). Superficial perivascular inflammation, including lymphocytes, histiocytes, and few neutrophils, was present. A periodic acid–Schiff stain showed fungal hyphae in the stratum corneum and a hair follicle (Figure 3). After approximately 2 weeks, mold was identified in the fungal culture. Approximately 2 weeks thereafter, the organism was reported as Trichophyton species.
The rash did not improve; resistance to terbinafine, griseofulvin, and fluconazole was suspected clinically. The fungal isolate was sent to a reference laboratory (University of Texas Health Science Center, San Antonio). Meanwhile, oral itraconazole 200 mg twice daily and ketoconazole cream 2% were prescribed; the rash began to improve. A serum itraconazole trough level obtained 4 days after treatment initiation was 0.5 μg/mL (reference range, ≥0.6 μg/mL). The evening itraconazole dose was increased to 300 mg; a subsequent trough level was 0.8 μg/mL.
Approximately 1 month after the fungal isolate was sent to the reference laboratory, T indotineae was confirmed based on polymerase chain reaction (PCR) testing of internal transcribed spacer region sequences. Minimum inhibitory concentrations (MICs) obtained through antifungal susceptibility testing (AFST) were reported for fluconazole (8 μg/mL), griseofulvin (2 μg/mL), itraconazole (≤0.03 μg/mL), posaconazole (≤0.03 μg/mL), terbinafine (≥2 μg/mL), and voriconazole (0.125 μg/mL).
Approximately 7 weeks after itraconazole and ketoconazole were started, the rash had completely resolved. Nearly 8 months later (at the time this article was written), the rash had not recurred.
We report a unique case of T indotineae in a patient residing in California. Post hoc laboratory testing of dermatophyte isolates sent to the University of Texas reference laboratory identified terbinafine-resistant T indotineae specimens from the United States and Canada dating to 2017; clinical characteristics of patients from whom those isolates were obtained were unavailable.9
Trichophyton indotineae dermatophytosis typically is more extensive, inflamed, and pruritic, as well as likely more contagious, than tinea caused by other dermatophytes.5 Previously called Trichophyton mentagrophytes genotype VIII when first isolated in 2017, the pathogen was renamed T indotineae in 2020 after important genetic differences were discovered between it and other T mentagrophytes species.5 The emergence of T indotineae has been attributed to concomitant use of topical steroids and antifungals,5,10 inappropriate prescribing of antifungals,5 and nonadherence to antifungal treatment.5
Likely risk factors for T indotineae infection include suboptimal hygiene, overcrowded conditions, hot and humid environments, and tight-fitting synthetic clothing.4 Transmission from family members appears common,5 especially when fomites are shared.4 A case reported in Pennsylvania likely was acquired through sexual contact.7 Travel to South Asia has been associated with acquisition of T indotineae infection,3,5-7 though our patient and some others had not traveled there.3,8 It is not clear whether immunosuppression and diabetes mellitus are associated with T indotineae infection.4,5,8 Trichophyton indotineae also can affect animals,11 though zoonotic transmission has not been reported.4
Not all T indotineae isolates are resistant to one or more antifungals; furthermore, antifungal resistance in other dermatophyte species has been reported.5 Terbinafine resistance in T indotineae is conferred by mutations in the gene encoding squalene epoxidase, which helps synthesize ergosterol—a component of the cell membrane in fungi.2,4,5,12 Although clinical cut-points for MIC obtained by AFST are not well established, T indotineae MICs for terbinafine of 0.5 μg/mL or more correlate with resistance.9 Resistance to azoles has been linked to overexpression of transporter genes, which increase azole efflux from cells, as well as to mutations in the gene encoding lanosterol 14α demethylase.4,12,13
Potassium hydroxide preparations and fungal cultures cannot differentiate T indotineae from other dermatophytes that typically cause tinea.5,14 Histopathologic findings in our case were no different than those of non–T indotineae dermatophytes. Only molecular testing using PCR assays to sequence internal transcribed spacer genes can confirm T indotineae infection. However, PCR assays and AFST are not available in many US laboratories.5 Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has shown promise in distinguishing T indotineae from other dermatophytes, though its clinical use is limited and it cannot assess terbinafine sensitivity.15,16 Clinicians in the United States who want to test specimens from cases suspicious for T indotineae infection should contact their local or state health department or the Centers for Disease Control and Prevention for assistance.3,5
Systemic treatment typically is necessary for T indotineae infection.5 Combinations of oral and topical azoles have been used, as well as topical ciclopirox, amorolfine (not available in the United States), and luliconazole.1,5,17-21
Itraconazole has emerged as the treatment of choice for T indotineae tinea, typically at 200 mg/d and often for courses of more than 3 months.5 Testing for serum itraconazole trough levels, as done for our patient, typically is not recommended. Clinicians should counsel patients to take itraconazole with high-fat foods and an acidic beverage to increase bioavailability.5 Potential adverse effects of itraconazole include heart failure and numerous drug-drug interactions.5,22 Patients with T indotineae dermatophytosis should avoid sharing personal belongings and having skin-to-skin contact of affected areas with others.4
Dermatologists who suspect T indotineae infection should work with public health agencies that can assist with testing and undertake infection surveillance, prevention, and control.5,23 Challenges to diagnosing and managing T indotineae infection include lack of awareness among dermatology providers, the need for specialized laboratory testing to confirm infection, lack of established clinical cut-points for MICs from AFST, the need for longer duration of treatment vs what is needed for typical tinea, and potential challenges with insurance coverage for testing and treatment. Empiric treatment with itraconazole should be considered when terbinafine-resistant dermatophytosis is suspected or when terbinafine-resistant T indotineae infection is confirmed.
Acknowledgments—Jeremy Gold, MD; Dallas J. Smith, PharmD; and Shawn Lockhart, PhD, all of the Centers for Disease Control and Prevention, Mycotic Diseases Branch (Atlanta, Georgia), provided helpful comments to the authors in preparing the manuscript of this article.
- Uhrlaß S, Verma SB, Gräser Y, al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Jabet A, Brun S, Normand A-C, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233. doi:10.3201/eid2801.210883
- Caplan AS, Chaturvedi S, Zhu Y, et al. Notes from the field. First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb Mortal Wkly Rep. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Jabet A, Normand A-C, Brun S, et al. Trichophyton indotineae, from epidemiology to therapeutic. J Mycol Med. 2023;33:101383. doi:10.1016/j.mycmed.2023.101383
- Hill RC, Caplan AS, Elewski B, et al. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am J Clin Dermatol. 2024;25:359-389. doi:10.1007/s40257-024-00848-1
- Caplan AS, Zakhem GA, Pomeranz MK. Trichophyton mentagrophytes internal transcribed spacer genotype VIII. JAMA Dermatol. 2023;159:1130. doi:10.1001/jamadermatol.2023.2645
- Spivack S, Gold JAW, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807-809. doi:10.3201/eid3004.240115
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1126
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:e0056223. doi:10.1128/jcm.00562-23
- Gupta AK, Venkataraman M, Hall DC, et al. The emergence of Trichophyton indotineae: implications for clinical practice. Int J Dermatol. 2023;62:857-861.
- Oladzad V, Nasrollahi Omran A, Haghani I, et al. Multi-drug resistance Trichophyton indotineae in a stray dog. Res Vet Sci. 2024;166:105105. doi:10.1016/j.rvsc.2023.105105
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol. 2018;9:1108. doi:10.3389/fmicb.2018.01108
- Sacheli R, Hayette MP. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel). 2021;711:983. doi:10.3390/jof7110983
- Gupta AK, Cooper EA. Dermatophytosis (tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. Humana Press; 2008:355-381.
- Normand A-C, Moreno-Sabater A, Jabet A, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. doi:10.3390/jof8101103
- De Paepe R, Normand A-C, Uhrlaß S, et al. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. doi:10.1007/s11046-024-00835-4
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468-482. doi:10.25259/IJDVL_303_20
- Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes–Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64:E01964-19. doi:10.1128/AAC.01964-19
- Burmester A, Hipler U-C, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180. doi:10.1111/myc.13150
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278. doi:10.1001/jamadermatol.2022.3745
- Itraconazole capsule. DailyMed [Internet]. Updated June 3, 2024. Accessed June 19, 2024. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2ab38a8a-3708-4b97-9f7f-8e554a15348d
- Bui TS, Katz KA. Resistant Trichophyton indotineae dermatophytosis—an emerging pandemic, now in the US. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1125
To the Editor:
Historically, commonly available antifungal medications have been effective for treating dermatophytosis (tinea). However, recent tinea outbreaks caused by Trichophyton indotineae—a dermatophyte often resistant to terbinafine and sometimes to other antifungals—have been reported in South Asia, Europe, the Middle East, Southeast Asia, and Australia.1-5
Three confirmed cases of T indotineae dermatophytosis in the United States were reported in 2023 in New York3,6; a fourth confirmed case was reported in 2024 in Pennsylvania.7 Post hoc laboratory testing of fungal isolates in New York in 2022 and 2023 identified an additional 11 cases.8 We present a case of extensive multidrug-resistant tinea caused by T indotineae in a man in California.
An otherwise healthy 65-year-old man who had traveled to Europe in the past 3 months presented to his primary care physician with a widespread pruritic rash (Figure 1). He was treated with 2 weeks of oral terbinafine 250 mg/d and topical medicines, including clotrimazole cream 1%, fluocinonide ointment 0.05%, and clobetasol ointment 0.05% without improvement. Subsequently, 2 weeks of oral griseofulvin microsize 500 mg/d also proved ineffective. An antibody test was negative for HIV. His hemoglobin A1c was 6.2% (reference range, ≤5.6%). The patient was referred to dermatology.
Erythematous plaques—many scaly throughout and some annular with central clearing—were present on the arms, legs, and torso as well as in the groin. Honey crust was present on some plaques on the leg. A potassium hydroxide preparation showed abundant fungal hyphae. Material for fungal and bacterial cultures was collected. The patient was treated again with oral terbinafine 250 mg/d, an oral prednisone taper starting at 60 mg/d for a presumed id reaction, and various oral antihistamines for pruritus; all were ineffective. A bacterial culture showed only mixed skin flora. Oral fluconazole 200 mg/d was prescribed. A skin biopsy specimen showed compact orthokeratosis and parakeratosis of the stratum corneum with few neutrophils and focal pustule formation (Figure 2). Superficial perivascular inflammation, including lymphocytes, histiocytes, and few neutrophils, was present. A periodic acid–Schiff stain showed fungal hyphae in the stratum corneum and a hair follicle (Figure 3). After approximately 2 weeks, mold was identified in the fungal culture. Approximately 2 weeks thereafter, the organism was reported as Trichophyton species.

The rash did not improve; resistance to terbinafine, griseofulvin, and fluconazole was suspected clinically. The fungal isolate was sent to a reference laboratory (University of Texas Health Science Center, San Antonio). Meanwhile, oral itraconazole 200 mg twice daily and ketoconazole cream 2% were prescribed; the rash began to improve. A serum itraconazole trough level obtained 4 days after treatment initiation was 0.5 μg/mL (reference range, ≥0.6 μg/mL). The evening itraconazole dose was increased to 300 mg; a subsequent trough level was 0.8 μg/mL.


Approximately 1 month after the fungal isolate was sent to the reference laboratory, T indotineae was confirmed based on polymerase chain reaction (PCR) testing of internal transcribed spacer region sequences. Minimum inhibitory concentrations (MICs) obtained through antifungal susceptibility testing (AFST) were reported for fluconazole (8 μg/mL), griseofulvin (2 μg/mL), itraconazole (≤0.03 μg/mL), posaconazole (≤0.03 μg/mL), terbinafine (≥2 μg/mL), and voriconazole (0.125 μg/mL).
Approximately 7 weeks after itraconazole and ketoconazole were started, the rash had completely resolved. Nearly 8 months later (at the time this article was written), the rash had not recurred.
We report a unique case of T indotineae in a patient residing in California. Post hoc laboratory testing of dermatophyte isolates sent to the University of Texas reference laboratory identified terbinafine-resistant T indotineae specimens from the United States and Canada dating to 2017; clinical characteristics of patients from whom those isolates were obtained were unavailable.9
Trichophyton indotineae dermatophytosis typically is more extensive, inflamed, and pruritic, as well as likely more contagious, than tinea caused by other dermatophytes.5 Previously called Trichophyton mentagrophytes genotype VIII when first isolated in 2017, the pathogen was renamed T indotineae in 2020 after important genetic differences were discovered between it and other T mentagrophytes species.5 The emergence of T indotineae has been attributed to concomitant use of topical steroids and antifungals,5,10 inappropriate prescribing of antifungals,5 and nonadherence to antifungal treatment.5
Likely risk factors for T indotineae infection include suboptimal hygiene, overcrowded conditions, hot and humid environments, and tight-fitting synthetic clothing.4 Transmission from family members appears common,5 especially when fomites are shared.4 A case reported in Pennsylvania likely was acquired through sexual contact.7 Travel to South Asia has been associated with acquisition of T indotineae infection,3,5-7 though our patient and some others had not traveled there.3,8 It is not clear whether immunosuppression and diabetes mellitus are associated with T indotineae infection.4,5,8 Trichophyton indotineae also can affect animals,11 though zoonotic transmission has not been reported.4
Not all T indotineae isolates are resistant to one or more antifungals; furthermore, antifungal resistance in other dermatophyte species has been reported.5 Terbinafine resistance in T indotineae is conferred by mutations in the gene encoding squalene epoxidase, which helps synthesize ergosterol—a component of the cell membrane in fungi.2,4,5,12 Although clinical cut-points for MIC obtained by AFST are not well established, T indotineae MICs for terbinafine of 0.5 μg/mL or more correlate with resistance.9 Resistance to azoles has been linked to overexpression of transporter genes, which increase azole efflux from cells, as well as to mutations in the gene encoding lanosterol 14α demethylase.4,12,13
Potassium hydroxide preparations and fungal cultures cannot differentiate T indotineae from other dermatophytes that typically cause tinea.5,14 Histopathologic findings in our case were no different than those of non–T indotineae dermatophytes. Only molecular testing using PCR assays to sequence internal transcribed spacer genes can confirm T indotineae infection. However, PCR assays and AFST are not available in many US laboratories.5 Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has shown promise in distinguishing T indotineae from other dermatophytes, though its clinical use is limited and it cannot assess terbinafine sensitivity.15,16 Clinicians in the United States who want to test specimens from cases suspicious for T indotineae infection should contact their local or state health department or the Centers for Disease Control and Prevention for assistance.3,5
Systemic treatment typically is necessary for T indotineae infection.5 Combinations of oral and topical azoles have been used, as well as topical ciclopirox, amorolfine (not available in the United States), and luliconazole.1,5,17-21
Itraconazole has emerged as the treatment of choice for T indotineae tinea, typically at 200 mg/d and often for courses of more than 3 months.5 Testing for serum itraconazole trough levels, as done for our patient, typically is not recommended. Clinicians should counsel patients to take itraconazole with high-fat foods and an acidic beverage to increase bioavailability.5 Potential adverse effects of itraconazole include heart failure and numerous drug-drug interactions.5,22 Patients with T indotineae dermatophytosis should avoid sharing personal belongings and having skin-to-skin contact of affected areas with others.4
Dermatologists who suspect T indotineae infection should work with public health agencies that can assist with testing and undertake infection surveillance, prevention, and control.5,23 Challenges to diagnosing and managing T indotineae infection include lack of awareness among dermatology providers, the need for specialized laboratory testing to confirm infection, lack of established clinical cut-points for MICs from AFST, the need for longer duration of treatment vs what is needed for typical tinea, and potential challenges with insurance coverage for testing and treatment. Empiric treatment with itraconazole should be considered when terbinafine-resistant dermatophytosis is suspected or when terbinafine-resistant T indotineae infection is confirmed.
Acknowledgments—Jeremy Gold, MD; Dallas J. Smith, PharmD; and Shawn Lockhart, PhD, all of the Centers for Disease Control and Prevention, Mycotic Diseases Branch (Atlanta, Georgia), provided helpful comments to the authors in preparing the manuscript of this article.
To the Editor:
Historically, commonly available antifungal medications have been effective for treating dermatophytosis (tinea). However, recent tinea outbreaks caused by Trichophyton indotineae—a dermatophyte often resistant to terbinafine and sometimes to other antifungals—have been reported in South Asia, Europe, the Middle East, Southeast Asia, and Australia.1-5
Three confirmed cases of T indotineae dermatophytosis in the United States were reported in 2023 in New York3,6; a fourth confirmed case was reported in 2024 in Pennsylvania.7 Post hoc laboratory testing of fungal isolates in New York in 2022 and 2023 identified an additional 11 cases.8 We present a case of extensive multidrug-resistant tinea caused by T indotineae in a man in California.
An otherwise healthy 65-year-old man who had traveled to Europe in the past 3 months presented to his primary care physician with a widespread pruritic rash (Figure 1). He was treated with 2 weeks of oral terbinafine 250 mg/d and topical medicines, including clotrimazole cream 1%, fluocinonide ointment 0.05%, and clobetasol ointment 0.05% without improvement. Subsequently, 2 weeks of oral griseofulvin microsize 500 mg/d also proved ineffective. An antibody test was negative for HIV. His hemoglobin A1c was 6.2% (reference range, ≤5.6%). The patient was referred to dermatology.
Erythematous plaques—many scaly throughout and some annular with central clearing—were present on the arms, legs, and torso as well as in the groin. Honey crust was present on some plaques on the leg. A potassium hydroxide preparation showed abundant fungal hyphae. Material for fungal and bacterial cultures was collected. The patient was treated again with oral terbinafine 250 mg/d, an oral prednisone taper starting at 60 mg/d for a presumed id reaction, and various oral antihistamines for pruritus; all were ineffective. A bacterial culture showed only mixed skin flora. Oral fluconazole 200 mg/d was prescribed. A skin biopsy specimen showed compact orthokeratosis and parakeratosis of the stratum corneum with few neutrophils and focal pustule formation (Figure 2). Superficial perivascular inflammation, including lymphocytes, histiocytes, and few neutrophils, was present. A periodic acid–Schiff stain showed fungal hyphae in the stratum corneum and a hair follicle (Figure 3). After approximately 2 weeks, mold was identified in the fungal culture. Approximately 2 weeks thereafter, the organism was reported as Trichophyton species.

The rash did not improve; resistance to terbinafine, griseofulvin, and fluconazole was suspected clinically. The fungal isolate was sent to a reference laboratory (University of Texas Health Science Center, San Antonio). Meanwhile, oral itraconazole 200 mg twice daily and ketoconazole cream 2% were prescribed; the rash began to improve. A serum itraconazole trough level obtained 4 days after treatment initiation was 0.5 μg/mL (reference range, ≥0.6 μg/mL). The evening itraconazole dose was increased to 300 mg; a subsequent trough level was 0.8 μg/mL.


Approximately 1 month after the fungal isolate was sent to the reference laboratory, T indotineae was confirmed based on polymerase chain reaction (PCR) testing of internal transcribed spacer region sequences. Minimum inhibitory concentrations (MICs) obtained through antifungal susceptibility testing (AFST) were reported for fluconazole (8 μg/mL), griseofulvin (2 μg/mL), itraconazole (≤0.03 μg/mL), posaconazole (≤0.03 μg/mL), terbinafine (≥2 μg/mL), and voriconazole (0.125 μg/mL).
Approximately 7 weeks after itraconazole and ketoconazole were started, the rash had completely resolved. Nearly 8 months later (at the time this article was written), the rash had not recurred.
We report a unique case of T indotineae in a patient residing in California. Post hoc laboratory testing of dermatophyte isolates sent to the University of Texas reference laboratory identified terbinafine-resistant T indotineae specimens from the United States and Canada dating to 2017; clinical characteristics of patients from whom those isolates were obtained were unavailable.9
Trichophyton indotineae dermatophytosis typically is more extensive, inflamed, and pruritic, as well as likely more contagious, than tinea caused by other dermatophytes.5 Previously called Trichophyton mentagrophytes genotype VIII when first isolated in 2017, the pathogen was renamed T indotineae in 2020 after important genetic differences were discovered between it and other T mentagrophytes species.5 The emergence of T indotineae has been attributed to concomitant use of topical steroids and antifungals,5,10 inappropriate prescribing of antifungals,5 and nonadherence to antifungal treatment.5
Likely risk factors for T indotineae infection include suboptimal hygiene, overcrowded conditions, hot and humid environments, and tight-fitting synthetic clothing.4 Transmission from family members appears common,5 especially when fomites are shared.4 A case reported in Pennsylvania likely was acquired through sexual contact.7 Travel to South Asia has been associated with acquisition of T indotineae infection,3,5-7 though our patient and some others had not traveled there.3,8 It is not clear whether immunosuppression and diabetes mellitus are associated with T indotineae infection.4,5,8 Trichophyton indotineae also can affect animals,11 though zoonotic transmission has not been reported.4
Not all T indotineae isolates are resistant to one or more antifungals; furthermore, antifungal resistance in other dermatophyte species has been reported.5 Terbinafine resistance in T indotineae is conferred by mutations in the gene encoding squalene epoxidase, which helps synthesize ergosterol—a component of the cell membrane in fungi.2,4,5,12 Although clinical cut-points for MIC obtained by AFST are not well established, T indotineae MICs for terbinafine of 0.5 μg/mL or more correlate with resistance.9 Resistance to azoles has been linked to overexpression of transporter genes, which increase azole efflux from cells, as well as to mutations in the gene encoding lanosterol 14α demethylase.4,12,13
Potassium hydroxide preparations and fungal cultures cannot differentiate T indotineae from other dermatophytes that typically cause tinea.5,14 Histopathologic findings in our case were no different than those of non–T indotineae dermatophytes. Only molecular testing using PCR assays to sequence internal transcribed spacer genes can confirm T indotineae infection. However, PCR assays and AFST are not available in many US laboratories.5 Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry has shown promise in distinguishing T indotineae from other dermatophytes, though its clinical use is limited and it cannot assess terbinafine sensitivity.15,16 Clinicians in the United States who want to test specimens from cases suspicious for T indotineae infection should contact their local or state health department or the Centers for Disease Control and Prevention for assistance.3,5
Systemic treatment typically is necessary for T indotineae infection.5 Combinations of oral and topical azoles have been used, as well as topical ciclopirox, amorolfine (not available in the United States), and luliconazole.1,5,17-21
Itraconazole has emerged as the treatment of choice for T indotineae tinea, typically at 200 mg/d and often for courses of more than 3 months.5 Testing for serum itraconazole trough levels, as done for our patient, typically is not recommended. Clinicians should counsel patients to take itraconazole with high-fat foods and an acidic beverage to increase bioavailability.5 Potential adverse effects of itraconazole include heart failure and numerous drug-drug interactions.5,22 Patients with T indotineae dermatophytosis should avoid sharing personal belongings and having skin-to-skin contact of affected areas with others.4
Dermatologists who suspect T indotineae infection should work with public health agencies that can assist with testing and undertake infection surveillance, prevention, and control.5,23 Challenges to diagnosing and managing T indotineae infection include lack of awareness among dermatology providers, the need for specialized laboratory testing to confirm infection, lack of established clinical cut-points for MICs from AFST, the need for longer duration of treatment vs what is needed for typical tinea, and potential challenges with insurance coverage for testing and treatment. Empiric treatment with itraconazole should be considered when terbinafine-resistant dermatophytosis is suspected or when terbinafine-resistant T indotineae infection is confirmed.
Acknowledgments—Jeremy Gold, MD; Dallas J. Smith, PharmD; and Shawn Lockhart, PhD, all of the Centers for Disease Control and Prevention, Mycotic Diseases Branch (Atlanta, Georgia), provided helpful comments to the authors in preparing the manuscript of this article.
- Uhrlaß S, Verma SB, Gräser Y, al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Jabet A, Brun S, Normand A-C, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233. doi:10.3201/eid2801.210883
- Caplan AS, Chaturvedi S, Zhu Y, et al. Notes from the field. First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb Mortal Wkly Rep. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Jabet A, Normand A-C, Brun S, et al. Trichophyton indotineae, from epidemiology to therapeutic. J Mycol Med. 2023;33:101383. doi:10.1016/j.mycmed.2023.101383
- Hill RC, Caplan AS, Elewski B, et al. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am J Clin Dermatol. 2024;25:359-389. doi:10.1007/s40257-024-00848-1
- Caplan AS, Zakhem GA, Pomeranz MK. Trichophyton mentagrophytes internal transcribed spacer genotype VIII. JAMA Dermatol. 2023;159:1130. doi:10.1001/jamadermatol.2023.2645
- Spivack S, Gold JAW, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807-809. doi:10.3201/eid3004.240115
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1126
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:e0056223. doi:10.1128/jcm.00562-23
- Gupta AK, Venkataraman M, Hall DC, et al. The emergence of Trichophyton indotineae: implications for clinical practice. Int J Dermatol. 2023;62:857-861.
- Oladzad V, Nasrollahi Omran A, Haghani I, et al. Multi-drug resistance Trichophyton indotineae in a stray dog. Res Vet Sci. 2024;166:105105. doi:10.1016/j.rvsc.2023.105105
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol. 2018;9:1108. doi:10.3389/fmicb.2018.01108
- Sacheli R, Hayette MP. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel). 2021;711:983. doi:10.3390/jof7110983
- Gupta AK, Cooper EA. Dermatophytosis (tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. Humana Press; 2008:355-381.
- Normand A-C, Moreno-Sabater A, Jabet A, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. doi:10.3390/jof8101103
- De Paepe R, Normand A-C, Uhrlaß S, et al. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. doi:10.1007/s11046-024-00835-4
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468-482. doi:10.25259/IJDVL_303_20
- Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes–Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64:E01964-19. doi:10.1128/AAC.01964-19
- Burmester A, Hipler U-C, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180. doi:10.1111/myc.13150
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278. doi:10.1001/jamadermatol.2022.3745
- Itraconazole capsule. DailyMed [Internet]. Updated June 3, 2024. Accessed June 19, 2024. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2ab38a8a-3708-4b97-9f7f-8e554a15348d
- Bui TS, Katz KA. Resistant Trichophyton indotineae dermatophytosis—an emerging pandemic, now in the US. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1125
- Uhrlaß S, Verma SB, Gräser Y, al. Trichophyton indotineae—an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—a multidimensional perspective. J Fungi (Basel). 2022;8:757. doi:10.3390/jof8070757
- Jabet A, Brun S, Normand A-C, et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022;28:229-233. doi:10.3201/eid2801.210883
- Caplan AS, Chaturvedi S, Zhu Y, et al. Notes from the field. First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City, December 2021-March 2023. MMWR Morb Mortal Wkly Rep. 2023;72:536-537. doi:10.15585/mmwr.mm7219a4
- Jabet A, Normand A-C, Brun S, et al. Trichophyton indotineae, from epidemiology to therapeutic. J Mycol Med. 2023;33:101383. doi:10.1016/j.mycmed.2023.101383
- Hill RC, Caplan AS, Elewski B, et al. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am J Clin Dermatol. 2024;25:359-389. doi:10.1007/s40257-024-00848-1
- Caplan AS, Zakhem GA, Pomeranz MK. Trichophyton mentagrophytes internal transcribed spacer genotype VIII. JAMA Dermatol. 2023;159:1130. doi:10.1001/jamadermatol.2023.2645
- Spivack S, Gold JAW, Lockhart SR, et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg Infect Dis. 2024;30:807-809. doi:10.3201/eid3004.240115
- Caplan AS, Todd GC, Zhu Y, et al. Clinical course, antifungal susceptibility, and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1126
- Cañete-Gibas CF, Mele J, Patterson HP, et al. Terbinafine-resistant dermatophytes and the presence of Trichophyton indotineae in North America. J Clin Microbiol. 2023;61:e0056223. doi:10.1128/jcm.00562-23
- Gupta AK, Venkataraman M, Hall DC, et al. The emergence of Trichophyton indotineae: implications for clinical practice. Int J Dermatol. 2023;62:857-861.
- Oladzad V, Nasrollahi Omran A, Haghani I, et al. Multi-drug resistance Trichophyton indotineae in a stray dog. Res Vet Sci. 2024;166:105105. doi:10.1016/j.rvsc.2023.105105
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, et al. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front Microbiol. 2018;9:1108. doi:10.3389/fmicb.2018.01108
- Sacheli R, Hayette MP. Antifungal resistance in dermatophytes: genetic considerations, clinical presentations and alternative therapies. J Fungi (Basel). 2021;711:983. doi:10.3390/jof7110983
- Gupta AK, Cooper EA. Dermatophytosis (tinea) and other superficial fungal infections. In: Hospenthal DR, Rinaldi MG, eds. Diagnosis and Treatment of Human Mycoses. Humana Press; 2008:355-381.
- Normand A-C, Moreno-Sabater A, Jabet A, et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J Fungi (Basel). 2022;8:1103. doi:10.3390/jof8101103
- De Paepe R, Normand A-C, Uhrlaß S, et al. Resistance profile, terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia. 2024;189:29. doi:10.1007/s11046-024-00835-4
- Rajagopalan M, Inamadar A, Mittal A, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India). BMC Dermatol. 2018;18:6. doi:10.1186/s12895-018-0073-1
- Verma SB, Panda S, Nenoff P, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468-482. doi:10.25259/IJDVL_303_20
- Shaw D, Singh S, Dogra S, et al. MIC and upper limit of wild-type distribution for 13 antifungal agents against a Trichophyton mentagrophytes–Trichophyton interdigitale complex of Indian origin. Antimicrob Agents Chemother. 2020;64:E01964-19. doi:10.1128/AAC.01964-19
- Burmester A, Hipler U-C, Uhrlaß S, et al. Indian Trichophyton mentagrophytes squalene epoxidase erg1 double mutants show high proportion of combined fluconazole and terbinafine resistance. Mycoses. 2020;63:1175-1180. doi:10.1111/myc.13150
- Khurana A, Agarwal A, Agrawal D, et al. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: a randomized clinical trial. JAMA Dermatol. 2022;158:1269-1278. doi:10.1001/jamadermatol.2022.3745
- Itraconazole capsule. DailyMed [Internet]. Updated June 3, 2024. Accessed June 19, 2024. https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2ab38a8a-3708-4b97-9f7f-8e554a15348d
- Bui TS, Katz KA. Resistant Trichophyton indotineae dermatophytosis—an emerging pandemic, now in the US. JAMA Dermatol. Published online May 15, 2024. doi:10.1001/jamadermatol.2024.1125
Practice Points
- Trichophyton indotineae can cause extensive dermatophytosis that often is resistant to terbinafine and in some cases to other antifungals.
- Only molecular testing, which is not widely available, can distinguish T indotineae from other dermatophytes.
- Suspected or confirmed cases of T indotineae dermatophytosis should be reported to public health agencies to provide assistance with testing, as well as surveillance, prevention, and control of infection.
Teaching Tips for Dermatology Residents
Dermatology residents interact with trainees of various levels throughout the workday—from undergraduate or even high school students to postgraduate fellows. Depending on the institution’s training program, residents may have responsibilities to teach through lecture series such as Grand Rounds and didactics. Therefore, it is an integral part of resident training to become educators in addition to being learners; however, formal pedagogy education is rare in dermatology programs. 1,2 Herein, I discuss several techniques that residents can apply to their practice to cultivate ideal learning environments and outcomes for trainees.
Creating Effective Teaching and Learning Experiences
Planning to teach can be as important as teaching itself. Developing learning objectives can help to create effective teaching and learning experiences. Learning objectives should be specific, time bound, attainable, and learner centered (Table 1). It is recommended that residents aim for no more than 4 objectives per hour of learning.3 By creating clear learning objectives, residents can make connections between the content and any assessments. Bloom’s taxonomy of cognitive learning objectives gives guidance on action verbs to use in writing learning objectives depending on the cognitive process being tested (Table 2).4
Creating a Safe Educational Environment
Psychological safety is the belief that a learning environment is a safe place in which to take risks.5 A clinical learning environment that is psychologically safe can support trainee well-being and learning. Cultivating a safe educational environment may include addressing microaggressions and bias in the clinical workplace. Table 3 provides examples of statements using the 6 Ds, which can be used to mitigate these issues.6 The first 4—direct, distract, delegate, and defer—represent ways to respond to racism, microaggressions, and bias, and the last 2—display discomfort and debrief—are responses that may be utilized in any problematic incident. Residents can play an important supportive role in scenarios where learners are faced with an incident that may not be regarded as psychologically safe. This is especially true if the learner is at a lower training level than the dermatology resident. We all play a role in creating a safe workplace for our teams.

Teaching in the Clinic and Hospital
There are multiple challenges to teaching in both inpatient and outpatient environments, including limited space and time; thus, more informal teaching methods are common. For example, in an outpatient dermatology clinic, the patient schedule can become a “table of contents” of potential teaching and learning opportunities. This technique is called the focused half day.3,7 By reviewing the clinic schedule, students can focus on a specific area of interest or theme throughout the course of the day.3
Priming and framing are other focused techniques that work well in both outpatient and inpatient settings.3,8,9 Priming means alerting the trainee to upcoming learning objective(s) and focusing their attention on what to observe or do during a shared visit with a patient. Framing—instructing learners to collect information that is relevant to the diagnosis and treatment—allows trainees to help move patient care forward while the resident attends to other patients.3
Modeling involves describing a thought process out loud for a learner3,10; for example, prior to starting a patient encounter, a dermatology resident may clearly state the goal of a patient conversation to the learner, describe their thought process about the topic, summarize the important points, and ask the learner if they have any questions about what was just said. Using this technique, learners may have a better understanding of why and how to go about conducting a patient encounter after the resident models one for them.
Effectively Integrating Visual Media and Presentations
Research supported by the cognitive load theory and cognitive theory of multimedia learning has led to the assertion-evidence approach for creating presentation slides that are built around messages, not topics, and messages are supported with visuals, not bullets.3,11,12 For example, slides should be constructed with 1- to 2-line assertion statements as titles and relevant illustrations or figures as supporting evidence to enhance visual memory.3
Written text on presentation slides often is redundant with spoken narration and also decreases learning because of cognitive load. Busy background colors and/or designs consume working memory and also can be detrimental to learning. Limiting these common distractors in a presentation makes for more effective delivery and retention of knowledge.3
Final Thoughts
There are multiple avenues for teaching as a resident and not all techniques may be applicable depending on the clinical or academic scenario. This column provides a starting point for residents to augment their pedagogical skills, particularly because formal teaching on pedagogy is lacking in medical education.
- Burgin S, Zhong CS, Rana J. A resident-as-teacher program increases dermatology residents’ knowledge and confidence in teaching techniques: a pilot study. J Am Acad Dermatol. 2020;83:651-653. doi:10.1016/j.jaad.2019.12.008
- Burgin S, Homayounfar G, Newman LR, et al. Instruction in teaching and teaching opportunities for residents in US dermatology programs: results of a national survey. J Am Acad Dermatol. 2017;76:703-706. doi:10.1016/j.jaad.2016.08.043
- UNM School of Medicine Continuous Professional Learning. Residents as Educators. UNM School of Medicine; 2023.
- Bloom BS. Taxonomy of Educational Objectives. Book 1, Cognitive Domain. Longman; 1979.
- McClintock AH, Fainstad T, Blau K, et al. Psychological safety in medical education: a scoping review and synthesis of the literature. Med Teach. 2023;45:1290-1299. doi:10.1080/0142159X.2023.2216863
- Ackerman-Barger K, Jacobs NN, Orozco R, et al. Addressing microaggressions in academic health: a workshop for inclusiveexcellence. MedEdPORTAL. 2021;17:11103. doi:10.15766/mep_2374-8265.11103
- Taylor C, Lipsky MS, Bauer L. Focused teaching: facilitating early clinical experience in an office setting. Fam Med. 1998;30:547-548.
- Pan Z, Kosicki G. Framing analysis: an approach to news discourse. Polit Commun. 1993;10:55-75. doi:10.1080/10584609.1993.9962963
- Price V, Tewksbury D, Powers E. Switching trains of thought: the impact of news frames on readers’ cognitive responses. Commun Res. 1997;24:481-506. doi:10.1177/009365097024005002
- Haston W. Teacher modeling as an effective teaching strategy. Music Educators J. 2007;93:26. doi:10.2307/4127130
- Alley M. Build your scientific talk on messages, not topics. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385725653
- Alley M. Support your presentation messages with visual evidence, not bullet lists. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385729603
Dermatology residents interact with trainees of various levels throughout the workday—from undergraduate or even high school students to postgraduate fellows. Depending on the institution’s training program, residents may have responsibilities to teach through lecture series such as Grand Rounds and didactics. Therefore, it is an integral part of resident training to become educators in addition to being learners; however, formal pedagogy education is rare in dermatology programs. 1,2 Herein, I discuss several techniques that residents can apply to their practice to cultivate ideal learning environments and outcomes for trainees.
Creating Effective Teaching and Learning Experiences
Planning to teach can be as important as teaching itself. Developing learning objectives can help to create effective teaching and learning experiences. Learning objectives should be specific, time bound, attainable, and learner centered (Table 1). It is recommended that residents aim for no more than 4 objectives per hour of learning.3 By creating clear learning objectives, residents can make connections between the content and any assessments. Bloom’s taxonomy of cognitive learning objectives gives guidance on action verbs to use in writing learning objectives depending on the cognitive process being tested (Table 2).4
Creating a Safe Educational Environment
Psychological safety is the belief that a learning environment is a safe place in which to take risks.5 A clinical learning environment that is psychologically safe can support trainee well-being and learning. Cultivating a safe educational environment may include addressing microaggressions and bias in the clinical workplace. Table 3 provides examples of statements using the 6 Ds, which can be used to mitigate these issues.6 The first 4—direct, distract, delegate, and defer—represent ways to respond to racism, microaggressions, and bias, and the last 2—display discomfort and debrief—are responses that may be utilized in any problematic incident. Residents can play an important supportive role in scenarios where learners are faced with an incident that may not be regarded as psychologically safe. This is especially true if the learner is at a lower training level than the dermatology resident. We all play a role in creating a safe workplace for our teams.



Teaching in the Clinic and Hospital
There are multiple challenges to teaching in both inpatient and outpatient environments, including limited space and time; thus, more informal teaching methods are common. For example, in an outpatient dermatology clinic, the patient schedule can become a “table of contents” of potential teaching and learning opportunities. This technique is called the focused half day.3,7 By reviewing the clinic schedule, students can focus on a specific area of interest or theme throughout the course of the day.3
Priming and framing are other focused techniques that work well in both outpatient and inpatient settings.3,8,9 Priming means alerting the trainee to upcoming learning objective(s) and focusing their attention on what to observe or do during a shared visit with a patient. Framing—instructing learners to collect information that is relevant to the diagnosis and treatment—allows trainees to help move patient care forward while the resident attends to other patients.3
Modeling involves describing a thought process out loud for a learner3,10; for example, prior to starting a patient encounter, a dermatology resident may clearly state the goal of a patient conversation to the learner, describe their thought process about the topic, summarize the important points, and ask the learner if they have any questions about what was just said. Using this technique, learners may have a better understanding of why and how to go about conducting a patient encounter after the resident models one for them.
Effectively Integrating Visual Media and Presentations
Research supported by the cognitive load theory and cognitive theory of multimedia learning has led to the assertion-evidence approach for creating presentation slides that are built around messages, not topics, and messages are supported with visuals, not bullets.3,11,12 For example, slides should be constructed with 1- to 2-line assertion statements as titles and relevant illustrations or figures as supporting evidence to enhance visual memory.3
Written text on presentation slides often is redundant with spoken narration and also decreases learning because of cognitive load. Busy background colors and/or designs consume working memory and also can be detrimental to learning. Limiting these common distractors in a presentation makes for more effective delivery and retention of knowledge.3
Final Thoughts
There are multiple avenues for teaching as a resident and not all techniques may be applicable depending on the clinical or academic scenario. This column provides a starting point for residents to augment their pedagogical skills, particularly because formal teaching on pedagogy is lacking in medical education.
Dermatology residents interact with trainees of various levels throughout the workday—from undergraduate or even high school students to postgraduate fellows. Depending on the institution’s training program, residents may have responsibilities to teach through lecture series such as Grand Rounds and didactics. Therefore, it is an integral part of resident training to become educators in addition to being learners; however, formal pedagogy education is rare in dermatology programs. 1,2 Herein, I discuss several techniques that residents can apply to their practice to cultivate ideal learning environments and outcomes for trainees.
Creating Effective Teaching and Learning Experiences
Planning to teach can be as important as teaching itself. Developing learning objectives can help to create effective teaching and learning experiences. Learning objectives should be specific, time bound, attainable, and learner centered (Table 1). It is recommended that residents aim for no more than 4 objectives per hour of learning.3 By creating clear learning objectives, residents can make connections between the content and any assessments. Bloom’s taxonomy of cognitive learning objectives gives guidance on action verbs to use in writing learning objectives depending on the cognitive process being tested (Table 2).4
Creating a Safe Educational Environment
Psychological safety is the belief that a learning environment is a safe place in which to take risks.5 A clinical learning environment that is psychologically safe can support trainee well-being and learning. Cultivating a safe educational environment may include addressing microaggressions and bias in the clinical workplace. Table 3 provides examples of statements using the 6 Ds, which can be used to mitigate these issues.6 The first 4—direct, distract, delegate, and defer—represent ways to respond to racism, microaggressions, and bias, and the last 2—display discomfort and debrief—are responses that may be utilized in any problematic incident. Residents can play an important supportive role in scenarios where learners are faced with an incident that may not be regarded as psychologically safe. This is especially true if the learner is at a lower training level than the dermatology resident. We all play a role in creating a safe workplace for our teams.



Teaching in the Clinic and Hospital
There are multiple challenges to teaching in both inpatient and outpatient environments, including limited space and time; thus, more informal teaching methods are common. For example, in an outpatient dermatology clinic, the patient schedule can become a “table of contents” of potential teaching and learning opportunities. This technique is called the focused half day.3,7 By reviewing the clinic schedule, students can focus on a specific area of interest or theme throughout the course of the day.3
Priming and framing are other focused techniques that work well in both outpatient and inpatient settings.3,8,9 Priming means alerting the trainee to upcoming learning objective(s) and focusing their attention on what to observe or do during a shared visit with a patient. Framing—instructing learners to collect information that is relevant to the diagnosis and treatment—allows trainees to help move patient care forward while the resident attends to other patients.3
Modeling involves describing a thought process out loud for a learner3,10; for example, prior to starting a patient encounter, a dermatology resident may clearly state the goal of a patient conversation to the learner, describe their thought process about the topic, summarize the important points, and ask the learner if they have any questions about what was just said. Using this technique, learners may have a better understanding of why and how to go about conducting a patient encounter after the resident models one for them.
Effectively Integrating Visual Media and Presentations
Research supported by the cognitive load theory and cognitive theory of multimedia learning has led to the assertion-evidence approach for creating presentation slides that are built around messages, not topics, and messages are supported with visuals, not bullets.3,11,12 For example, slides should be constructed with 1- to 2-line assertion statements as titles and relevant illustrations or figures as supporting evidence to enhance visual memory.3
Written text on presentation slides often is redundant with spoken narration and also decreases learning because of cognitive load. Busy background colors and/or designs consume working memory and also can be detrimental to learning. Limiting these common distractors in a presentation makes for more effective delivery and retention of knowledge.3
Final Thoughts
There are multiple avenues for teaching as a resident and not all techniques may be applicable depending on the clinical or academic scenario. This column provides a starting point for residents to augment their pedagogical skills, particularly because formal teaching on pedagogy is lacking in medical education.
- Burgin S, Zhong CS, Rana J. A resident-as-teacher program increases dermatology residents’ knowledge and confidence in teaching techniques: a pilot study. J Am Acad Dermatol. 2020;83:651-653. doi:10.1016/j.jaad.2019.12.008
- Burgin S, Homayounfar G, Newman LR, et al. Instruction in teaching and teaching opportunities for residents in US dermatology programs: results of a national survey. J Am Acad Dermatol. 2017;76:703-706. doi:10.1016/j.jaad.2016.08.043
- UNM School of Medicine Continuous Professional Learning. Residents as Educators. UNM School of Medicine; 2023.
- Bloom BS. Taxonomy of Educational Objectives. Book 1, Cognitive Domain. Longman; 1979.
- McClintock AH, Fainstad T, Blau K, et al. Psychological safety in medical education: a scoping review and synthesis of the literature. Med Teach. 2023;45:1290-1299. doi:10.1080/0142159X.2023.2216863
- Ackerman-Barger K, Jacobs NN, Orozco R, et al. Addressing microaggressions in academic health: a workshop for inclusiveexcellence. MedEdPORTAL. 2021;17:11103. doi:10.15766/mep_2374-8265.11103
- Taylor C, Lipsky MS, Bauer L. Focused teaching: facilitating early clinical experience in an office setting. Fam Med. 1998;30:547-548.
- Pan Z, Kosicki G. Framing analysis: an approach to news discourse. Polit Commun. 1993;10:55-75. doi:10.1080/10584609.1993.9962963
- Price V, Tewksbury D, Powers E. Switching trains of thought: the impact of news frames on readers’ cognitive responses. Commun Res. 1997;24:481-506. doi:10.1177/009365097024005002
- Haston W. Teacher modeling as an effective teaching strategy. Music Educators J. 2007;93:26. doi:10.2307/4127130
- Alley M. Build your scientific talk on messages, not topics. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385725653
- Alley M. Support your presentation messages with visual evidence, not bullet lists. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385729603
- Burgin S, Zhong CS, Rana J. A resident-as-teacher program increases dermatology residents’ knowledge and confidence in teaching techniques: a pilot study. J Am Acad Dermatol. 2020;83:651-653. doi:10.1016/j.jaad.2019.12.008
- Burgin S, Homayounfar G, Newman LR, et al. Instruction in teaching and teaching opportunities for residents in US dermatology programs: results of a national survey. J Am Acad Dermatol. 2017;76:703-706. doi:10.1016/j.jaad.2016.08.043
- UNM School of Medicine Continuous Professional Learning. Residents as Educators. UNM School of Medicine; 2023.
- Bloom BS. Taxonomy of Educational Objectives. Book 1, Cognitive Domain. Longman; 1979.
- McClintock AH, Fainstad T, Blau K, et al. Psychological safety in medical education: a scoping review and synthesis of the literature. Med Teach. 2023;45:1290-1299. doi:10.1080/0142159X.2023.2216863
- Ackerman-Barger K, Jacobs NN, Orozco R, et al. Addressing microaggressions in academic health: a workshop for inclusiveexcellence. MedEdPORTAL. 2021;17:11103. doi:10.15766/mep_2374-8265.11103
- Taylor C, Lipsky MS, Bauer L. Focused teaching: facilitating early clinical experience in an office setting. Fam Med. 1998;30:547-548.
- Pan Z, Kosicki G. Framing analysis: an approach to news discourse. Polit Commun. 1993;10:55-75. doi:10.1080/10584609.1993.9962963
- Price V, Tewksbury D, Powers E. Switching trains of thought: the impact of news frames on readers’ cognitive responses. Commun Res. 1997;24:481-506. doi:10.1177/009365097024005002
- Haston W. Teacher modeling as an effective teaching strategy. Music Educators J. 2007;93:26. doi:10.2307/4127130
- Alley M. Build your scientific talk on messages, not topics. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385725653
- Alley M. Support your presentation messages with visual evidence, not bullet lists. Vimeo website. January 18, 2020. Accessed June 14, 2024. https://vimeo.com/385729603
Resident Pearls
- Emphasizing specific learning objectives, prioritizing safety in the learning environment, utilizing clinical teaching techniques, and using multimedia to present messages all contribute to effective dermatology teaching by residents.
Anti-CD20 Therapy for Relapsing Multiple Sclerosis
Data have shown that CD20-expressing B cells are crucial to the pathogenesis of multiple sclerosis (MS). First approved by the US Food and Drug Administration for MS in 2017, anti-CD20 monoclonal antibody therapies including ocrelizumab, ofatumumab, and ublituximab have proven effective at controlling the symptoms of relapsing-remitting MS (RRMS).
In this ReCAP, Dr Fred D. Lublin, of the Mount Sinai School of Medicine, discusses recent data on anti-CD20 agents for RRMS, including results presented at the 2024 meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
He discusses a protocol examining the effect on RRMS of extending dosage intervals or stopping anti-CD20 therapy after 1 or 2 years of treatment based on results suggesting that the B cells that return post depletion are predominantly regulatory rather than pathogenic.
Next, Dr Lublin discusses a paper presented at CMSC on risks for serious infections in individuals taking ocrelizumab or ofatumumab. Major predictors were found to be progressive disease, prior use of a disease-modifying therapy, and longer duration of therapy.
Finally, he considers recent studies comparing rituximab, an anti-CD20 therapy not approved for MS in the United States but commonly used off-label internationally, with more recent therapies such as ocrelizumab. Data currently indicate that an increased risk for infections are associated with rituximab vs ocrelizumab, but further research is under way.
--
Fred D. Lublin, MD, Director, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY
Fred D. Lublin, MD, has disclosed the following relevant financial relationships:
Sources of Funding for Research: Novartis; Biogen; Sanofi; NMSS; NIH; Brainstorm Cell Therapeutics
Consulting Agreements/Advisory Boards/DSMB: Biogen; EMD Serono; Novartis; Actelion/Janssen; Sanofi/Genzyme; Roche/Genentech; Horizon Therapeutics/Amgen; Bristol Myers Squibb; Mapi Pharma; Brainstorm Cell Therapeutics; Mylan/Viatris; Immunic; Avotres; Neurogene; LabCorp; Entelexo Biotherapeutics; Neuralight; SetPoint Medical; Hexal/Sandoz; Baim Institute; Sudo Biosciences; Lapix Therapeutics; Biohaven Pharmaceuticals; Abata Therapeutics; Cognito Therapeutics; ImmPACT Bio
Speaker: Sanofi
Stock Options: Avotres; Neuralight; Lapix Therapeutics; Entelexo
I may discuss unapproved agents that are in the MS developmental pipeline without any recommendation on their use.
Data have shown that CD20-expressing B cells are crucial to the pathogenesis of multiple sclerosis (MS). First approved by the US Food and Drug Administration for MS in 2017, anti-CD20 monoclonal antibody therapies including ocrelizumab, ofatumumab, and ublituximab have proven effective at controlling the symptoms of relapsing-remitting MS (RRMS).
In this ReCAP, Dr Fred D. Lublin, of the Mount Sinai School of Medicine, discusses recent data on anti-CD20 agents for RRMS, including results presented at the 2024 meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
He discusses a protocol examining the effect on RRMS of extending dosage intervals or stopping anti-CD20 therapy after 1 or 2 years of treatment based on results suggesting that the B cells that return post depletion are predominantly regulatory rather than pathogenic.
Next, Dr Lublin discusses a paper presented at CMSC on risks for serious infections in individuals taking ocrelizumab or ofatumumab. Major predictors were found to be progressive disease, prior use of a disease-modifying therapy, and longer duration of therapy.
Finally, he considers recent studies comparing rituximab, an anti-CD20 therapy not approved for MS in the United States but commonly used off-label internationally, with more recent therapies such as ocrelizumab. Data currently indicate that an increased risk for infections are associated with rituximab vs ocrelizumab, but further research is under way.
--
Fred D. Lublin, MD, Director, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY
Fred D. Lublin, MD, has disclosed the following relevant financial relationships:
Sources of Funding for Research: Novartis; Biogen; Sanofi; NMSS; NIH; Brainstorm Cell Therapeutics
Consulting Agreements/Advisory Boards/DSMB: Biogen; EMD Serono; Novartis; Actelion/Janssen; Sanofi/Genzyme; Roche/Genentech; Horizon Therapeutics/Amgen; Bristol Myers Squibb; Mapi Pharma; Brainstorm Cell Therapeutics; Mylan/Viatris; Immunic; Avotres; Neurogene; LabCorp; Entelexo Biotherapeutics; Neuralight; SetPoint Medical; Hexal/Sandoz; Baim Institute; Sudo Biosciences; Lapix Therapeutics; Biohaven Pharmaceuticals; Abata Therapeutics; Cognito Therapeutics; ImmPACT Bio
Speaker: Sanofi
Stock Options: Avotres; Neuralight; Lapix Therapeutics; Entelexo
I may discuss unapproved agents that are in the MS developmental pipeline without any recommendation on their use.
Data have shown that CD20-expressing B cells are crucial to the pathogenesis of multiple sclerosis (MS). First approved by the US Food and Drug Administration for MS in 2017, anti-CD20 monoclonal antibody therapies including ocrelizumab, ofatumumab, and ublituximab have proven effective at controlling the symptoms of relapsing-remitting MS (RRMS).
In this ReCAP, Dr Fred D. Lublin, of the Mount Sinai School of Medicine, discusses recent data on anti-CD20 agents for RRMS, including results presented at the 2024 meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
He discusses a protocol examining the effect on RRMS of extending dosage intervals or stopping anti-CD20 therapy after 1 or 2 years of treatment based on results suggesting that the B cells that return post depletion are predominantly regulatory rather than pathogenic.
Next, Dr Lublin discusses a paper presented at CMSC on risks for serious infections in individuals taking ocrelizumab or ofatumumab. Major predictors were found to be progressive disease, prior use of a disease-modifying therapy, and longer duration of therapy.
Finally, he considers recent studies comparing rituximab, an anti-CD20 therapy not approved for MS in the United States but commonly used off-label internationally, with more recent therapies such as ocrelizumab. Data currently indicate that an increased risk for infections are associated with rituximab vs ocrelizumab, but further research is under way.
--
Fred D. Lublin, MD, Director, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY
Fred D. Lublin, MD, has disclosed the following relevant financial relationships:
Sources of Funding for Research: Novartis; Biogen; Sanofi; NMSS; NIH; Brainstorm Cell Therapeutics
Consulting Agreements/Advisory Boards/DSMB: Biogen; EMD Serono; Novartis; Actelion/Janssen; Sanofi/Genzyme; Roche/Genentech; Horizon Therapeutics/Amgen; Bristol Myers Squibb; Mapi Pharma; Brainstorm Cell Therapeutics; Mylan/Viatris; Immunic; Avotres; Neurogene; LabCorp; Entelexo Biotherapeutics; Neuralight; SetPoint Medical; Hexal/Sandoz; Baim Institute; Sudo Biosciences; Lapix Therapeutics; Biohaven Pharmaceuticals; Abata Therapeutics; Cognito Therapeutics; ImmPACT Bio
Speaker: Sanofi
Stock Options: Avotres; Neuralight; Lapix Therapeutics; Entelexo
I may discuss unapproved agents that are in the MS developmental pipeline without any recommendation on their use.

Treatment of Infantile Hemangiomas in Concomitant Tuberous Sclerosis Complex Should Prompt Evaluation for Cardiac Rhabdomyomas Prior to Initiation of Propranolol
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
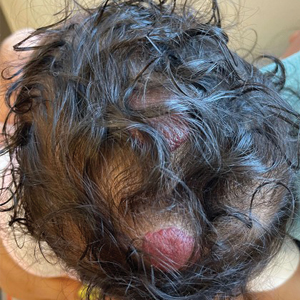
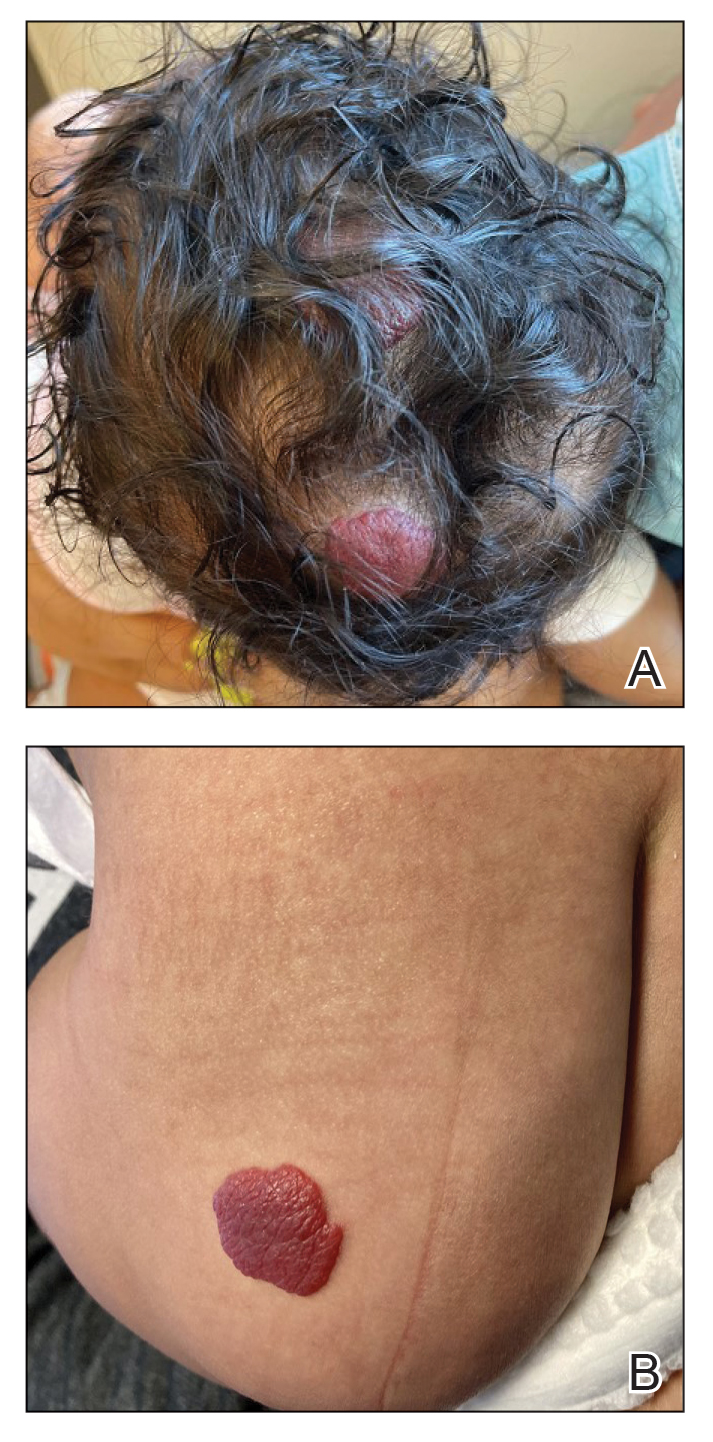
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
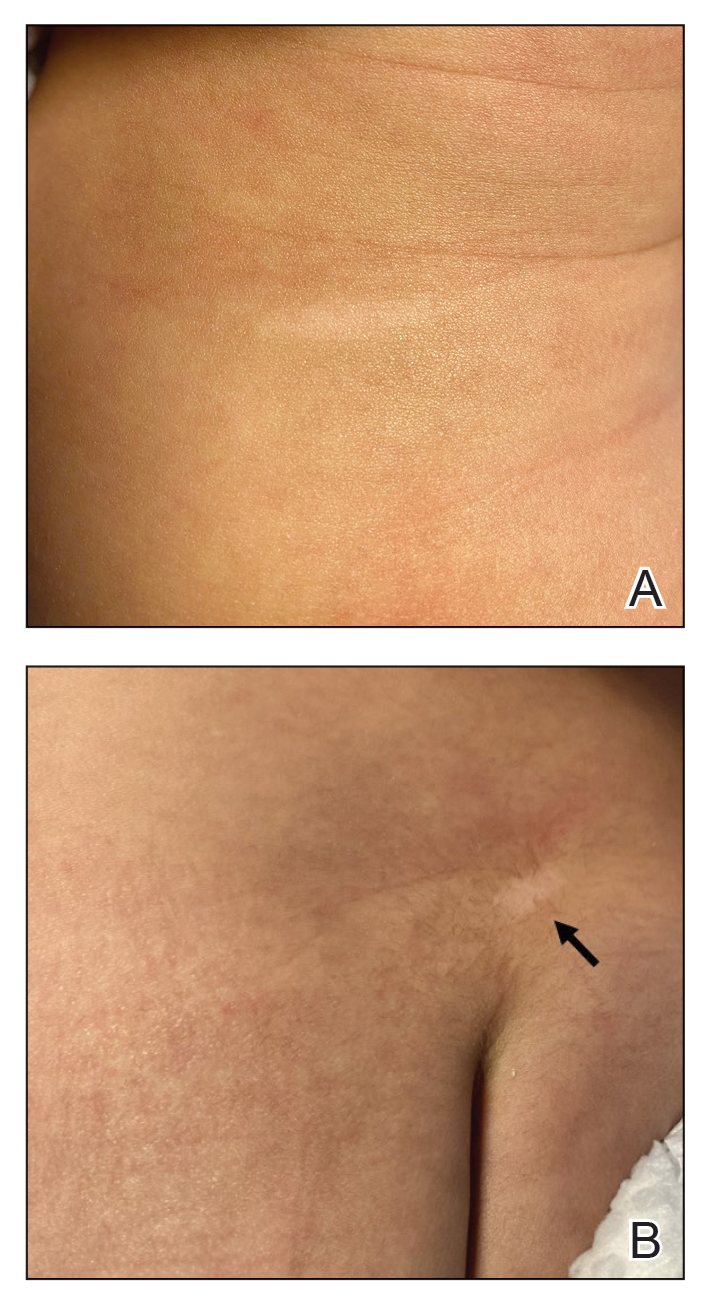
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
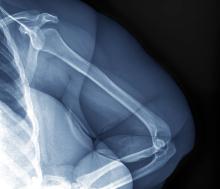
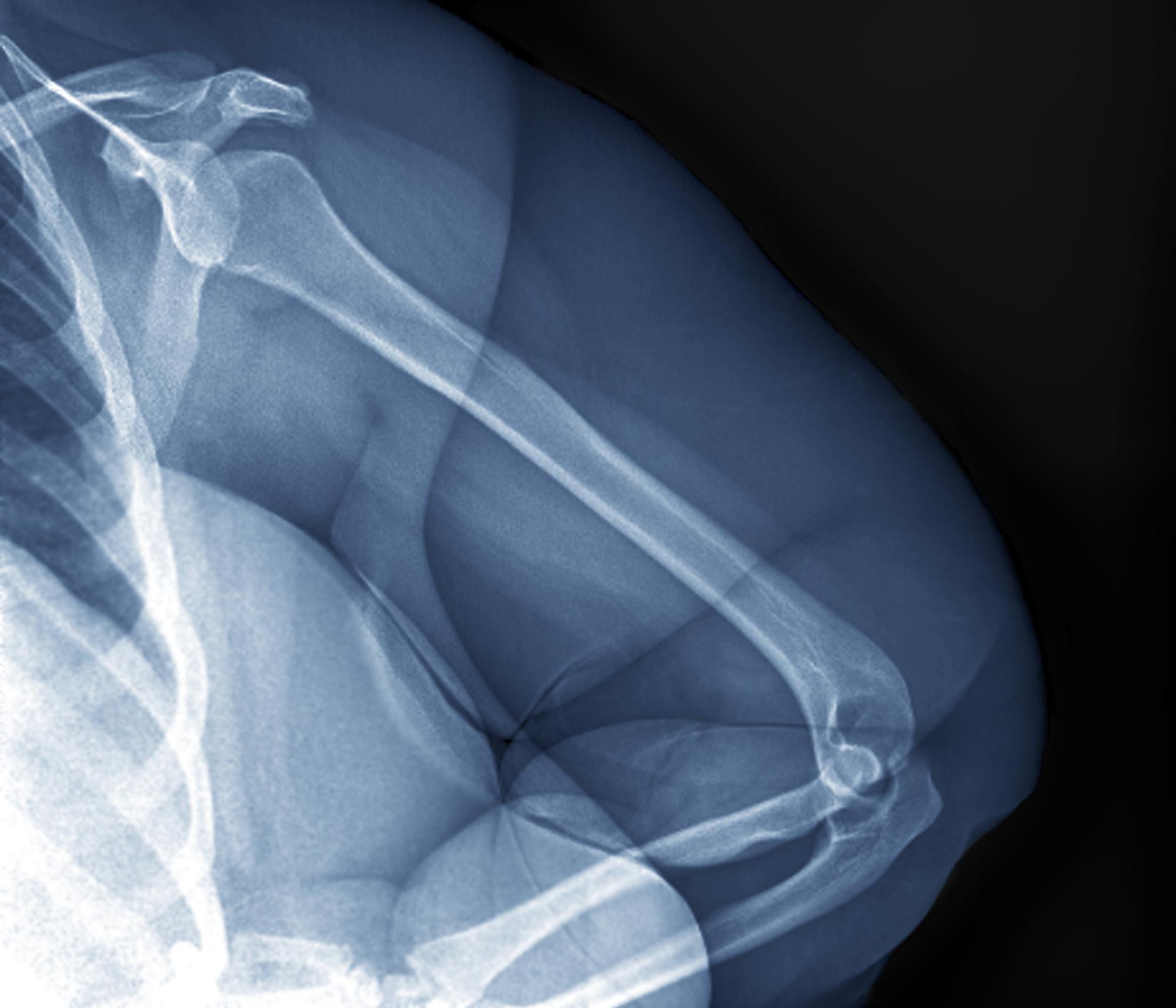
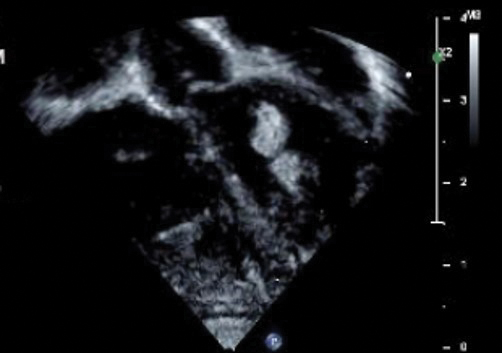
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.
Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.
Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.

Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.


Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.

Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.


Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
Practice Points
- Dermatologists may see patients with infantile hemangiomas (IHs) and tuberous sclerosis complex (TSC); therefore, they should be familiar with TSC diagnostic criteria to reach a prompt diagnosis and make appropriate referrals.
- Cardiologic evaluation is not routinely required prior to systemic treatment of IH, but knowledge of cardiac findings in TSC should prompt cardiologic clearance prior to β-blocker initiation.
Evolving Treatment of Nonradiographic Axial Spondyloarthritis
Nonradiographic axial spondyloarthritis (nr-axSpA) shares many characteristics with radiographic disease and responds to the same treatments, yet it has fewer FDA-approved options.
As Dr Marina Magrey, from Case Western Reserve University School of Medicine, in Cleveland, Ohio, explains, the TNF inhibitor certolizumab has been approved on the basis of results from the C-axSpAnd study.
Similarly, the IL-17 inhibitors secukinumab and ixekizumab are also options, and the results of the COAST-X and PREVENT studies show them to be safe and efficacious.
In closing, Dr Magrey outlines the SELECT-AXIS 2 study showing benefit from the JAK inhibitor upadacitinib for nr-axSpA, with no additional safety signals.
--
Professor of Rheumatology, Case Western Reserve University School of Medicine; Chief, Division of Rheumatology, University Hospitals, Cleveland, Ohio
Marina N. Magrey, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Novartis; AbbVie; UCB Pharma; Pfizer; Eli Lilly; Janssen; Bristol Myers Squibb
Received research grant from: AbbVie; Bristol Myers Squibb; Amgen
Nonradiographic axial spondyloarthritis (nr-axSpA) shares many characteristics with radiographic disease and responds to the same treatments, yet it has fewer FDA-approved options.
As Dr Marina Magrey, from Case Western Reserve University School of Medicine, in Cleveland, Ohio, explains, the TNF inhibitor certolizumab has been approved on the basis of results from the C-axSpAnd study.
Similarly, the IL-17 inhibitors secukinumab and ixekizumab are also options, and the results of the COAST-X and PREVENT studies show them to be safe and efficacious.
In closing, Dr Magrey outlines the SELECT-AXIS 2 study showing benefit from the JAK inhibitor upadacitinib for nr-axSpA, with no additional safety signals.
--
Professor of Rheumatology, Case Western Reserve University School of Medicine; Chief, Division of Rheumatology, University Hospitals, Cleveland, Ohio
Marina N. Magrey, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Novartis; AbbVie; UCB Pharma; Pfizer; Eli Lilly; Janssen; Bristol Myers Squibb
Received research grant from: AbbVie; Bristol Myers Squibb; Amgen
Nonradiographic axial spondyloarthritis (nr-axSpA) shares many characteristics with radiographic disease and responds to the same treatments, yet it has fewer FDA-approved options.
As Dr Marina Magrey, from Case Western Reserve University School of Medicine, in Cleveland, Ohio, explains, the TNF inhibitor certolizumab has been approved on the basis of results from the C-axSpAnd study.
Similarly, the IL-17 inhibitors secukinumab and ixekizumab are also options, and the results of the COAST-X and PREVENT studies show them to be safe and efficacious.
In closing, Dr Magrey outlines the SELECT-AXIS 2 study showing benefit from the JAK inhibitor upadacitinib for nr-axSpA, with no additional safety signals.
--
Professor of Rheumatology, Case Western Reserve University School of Medicine; Chief, Division of Rheumatology, University Hospitals, Cleveland, Ohio
Marina N. Magrey, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Novartis; AbbVie; UCB Pharma; Pfizer; Eli Lilly; Janssen; Bristol Myers Squibb
Received research grant from: AbbVie; Bristol Myers Squibb; Amgen

Knee pain on walking
Overall, persons with schizophrenia are more likely than the general population to be overweight and have cardiovascular risk factors before starting treatment with antipsychotics, and such treatment generally worsens these measures. Weight gain and associated morbidity and mortality are common side effects of antipsychotic medications. Olanzapine is associated with significant weight gain of 7% or more, higher than other second-generation antipsychotics. Olanzapine treatment is the major contributor to this patient's additional weight gain over the past 2 years. This added weight has translated to excess wear and tear on her joints, leading to evidence of osteoarthritis. Treatment with olanzapine is also independently associated with detrimental changes in cardiometabolic parameters.
Interventions to prevent or mitigate weight gain with antipsychotics are limited. In general, the American Psychiatric Association does not recommend switching antipsychotics for patients whose schizophrenia is well managed. However, there is increasing evidence that metformin may have a role in mitigating weight gain as well as beneficially modifying cardiometabolic factors in patients with schizophrenia being treated with olanzapine. A systematic review of emerging evidence with metformin in patients with schizophrenia suggests that metformin may also improve some cognitive symptoms of the illness, although further research is needed. The randomized, double-blind MELIA trial of metformin plus lifestyle intervention in antipsychotic-induced weight gain is ongoing. Starting metformin as a preventive measure at the same time as antipsychotic therapy may help to limit excess weight gain.
Research continues on the potential benefit of adding weight loss medications, including glucagon-like peptide-1 (GLP-1) receptor agonists, to antipsychotics. Daily liraglutide is most widely studied, but a published case series with weekly semaglutide also demonstrated weight loss in this setting. Liraglutide also has shown beneficial cardiometabolic effects in patients using antipsychotic medications. More studies of these drugs and of GLP-1/glucose-dependent insulinotropic polypeptide agonists are needed to elucidate the optimal use of these therapies for patients with schizophrenia.
There are few other effective ways to mitigate weight gain with olanzapine. Patients should be counseled on nutrition and lifestyle modifications. Evidence supports improvement with structured lifestyle modifications across a range of patients with less severe mental health issues, and structured programs combined with motivational interviewing were associated with reductions in antipsychotic-induced weight gain in patients with severe mental illness. As with any patient with obesity, however, the success of lifestyle modifications is heavily dependent on the individual's ability and motivation to comply with recommended interventions.
Nonpharmacologic interventions to address joint pain include heat or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints. If these measures are ineffective, nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical NSAIDs also may be useful. For more intractable joint pain, options include injecting a corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Overall, persons with schizophrenia are more likely than the general population to be overweight and have cardiovascular risk factors before starting treatment with antipsychotics, and such treatment generally worsens these measures. Weight gain and associated morbidity and mortality are common side effects of antipsychotic medications. Olanzapine is associated with significant weight gain of 7% or more, higher than other second-generation antipsychotics. Olanzapine treatment is the major contributor to this patient's additional weight gain over the past 2 years. This added weight has translated to excess wear and tear on her joints, leading to evidence of osteoarthritis. Treatment with olanzapine is also independently associated with detrimental changes in cardiometabolic parameters.
Interventions to prevent or mitigate weight gain with antipsychotics are limited. In general, the American Psychiatric Association does not recommend switching antipsychotics for patients whose schizophrenia is well managed. However, there is increasing evidence that metformin may have a role in mitigating weight gain as well as beneficially modifying cardiometabolic factors in patients with schizophrenia being treated with olanzapine. A systematic review of emerging evidence with metformin in patients with schizophrenia suggests that metformin may also improve some cognitive symptoms of the illness, although further research is needed. The randomized, double-blind MELIA trial of metformin plus lifestyle intervention in antipsychotic-induced weight gain is ongoing. Starting metformin as a preventive measure at the same time as antipsychotic therapy may help to limit excess weight gain.
Research continues on the potential benefit of adding weight loss medications, including glucagon-like peptide-1 (GLP-1) receptor agonists, to antipsychotics. Daily liraglutide is most widely studied, but a published case series with weekly semaglutide also demonstrated weight loss in this setting. Liraglutide also has shown beneficial cardiometabolic effects in patients using antipsychotic medications. More studies of these drugs and of GLP-1/glucose-dependent insulinotropic polypeptide agonists are needed to elucidate the optimal use of these therapies for patients with schizophrenia.
There are few other effective ways to mitigate weight gain with olanzapine. Patients should be counseled on nutrition and lifestyle modifications. Evidence supports improvement with structured lifestyle modifications across a range of patients with less severe mental health issues, and structured programs combined with motivational interviewing were associated with reductions in antipsychotic-induced weight gain in patients with severe mental illness. As with any patient with obesity, however, the success of lifestyle modifications is heavily dependent on the individual's ability and motivation to comply with recommended interventions.
Nonpharmacologic interventions to address joint pain include heat or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints. If these measures are ineffective, nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical NSAIDs also may be useful. For more intractable joint pain, options include injecting a corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Overall, persons with schizophrenia are more likely than the general population to be overweight and have cardiovascular risk factors before starting treatment with antipsychotics, and such treatment generally worsens these measures. Weight gain and associated morbidity and mortality are common side effects of antipsychotic medications. Olanzapine is associated with significant weight gain of 7% or more, higher than other second-generation antipsychotics. Olanzapine treatment is the major contributor to this patient's additional weight gain over the past 2 years. This added weight has translated to excess wear and tear on her joints, leading to evidence of osteoarthritis. Treatment with olanzapine is also independently associated with detrimental changes in cardiometabolic parameters.
Interventions to prevent or mitigate weight gain with antipsychotics are limited. In general, the American Psychiatric Association does not recommend switching antipsychotics for patients whose schizophrenia is well managed. However, there is increasing evidence that metformin may have a role in mitigating weight gain as well as beneficially modifying cardiometabolic factors in patients with schizophrenia being treated with olanzapine. A systematic review of emerging evidence with metformin in patients with schizophrenia suggests that metformin may also improve some cognitive symptoms of the illness, although further research is needed. The randomized, double-blind MELIA trial of metformin plus lifestyle intervention in antipsychotic-induced weight gain is ongoing. Starting metformin as a preventive measure at the same time as antipsychotic therapy may help to limit excess weight gain.
Research continues on the potential benefit of adding weight loss medications, including glucagon-like peptide-1 (GLP-1) receptor agonists, to antipsychotics. Daily liraglutide is most widely studied, but a published case series with weekly semaglutide also demonstrated weight loss in this setting. Liraglutide also has shown beneficial cardiometabolic effects in patients using antipsychotic medications. More studies of these drugs and of GLP-1/glucose-dependent insulinotropic polypeptide agonists are needed to elucidate the optimal use of these therapies for patients with schizophrenia.
There are few other effective ways to mitigate weight gain with olanzapine. Patients should be counseled on nutrition and lifestyle modifications. Evidence supports improvement with structured lifestyle modifications across a range of patients with less severe mental health issues, and structured programs combined with motivational interviewing were associated with reductions in antipsychotic-induced weight gain in patients with severe mental illness. As with any patient with obesity, however, the success of lifestyle modifications is heavily dependent on the individual's ability and motivation to comply with recommended interventions.
Nonpharmacologic interventions to address joint pain include heat or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints. If these measures are ineffective, nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical NSAIDs also may be useful. For more intractable joint pain, options include injecting a corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 32-year-old woman presents with knee pain on walking and elbow pain. She is 5 ft 6 in tall and weighs 187 lb (BMI 30.2). She was diagnosed with schizophrenia 2 years ago and began treatment with olanzapine at diagnosis; her symptoms currently are controlled, and she has tolerated the medication well.
The patient says that she has been overweight since her teenage years and weighed 170 lb (BMI ~27) at age 30. However, she remained physically active until development of painful joints over the past 18 months. She works remotely full time and lives alone. She describes her long-standing diet as heavy on meat protein and light on vegetables and snacks and says it hasn't changed; she denies binge eating or other disordered eating.
Physical exam reveals tender joints at knees and elbows and central obesity (waist circumference, 42 in). Blood pressure is 135/90 mm Hg. Lab results indicate a fasting glucose level of 115 mg/dL and a triglyceride level of 170 mg/dL. She is negative for rheumatoid factor. Radiography shows premature joint erosion at the knees and elbows.
Obesity and Pregnancy
Migraine Differential Diagnosis
Uncommon Locations for Brain Herniations Into Arachnoid Granulations: 5 Cases and Literature Review
The circulation of cerebrospinal fluid (CSF) is crucial for maintaining homeostasis for the optimal functioning of the multiple complex activities of the brain and spinal cord, including the disposal of metabolic waste products of brain and spinal cord activity into the cerebral venous drainage. Throughout the brain, the arachnoid mater forms small outpouchings or diverticula that penetrate the dura mater and communicate with the dural venous sinuses. These outpuchings are called arachnoid granulations or arachnoid villi, and most are found within the dural sinuses, primarily in the transverse sinuses and superior sagittal sinus, but can occasionally be seen extending into the inner table of the calvarium.1,2
The amount of arachnoid granulations seen in bone, particularly around the superior sagittal sinus, may increase with age.2 Arachnoid granulations are generally small but the largest ones can be seen on gross examination during intracranial procedures or autopsy.3 Magnetic resonance imaging (MRI) can detect arachnoid granulations, which are characterized as T1 hypointense and T2 hyperintense (CSF isointense), well-circumscribed, small, nonenhancing masses within the dural sinuses or in the diploic space (Figure 1). Even small arachnoid granulations < 1 mm in length can be detected.2
Smaller arachnoid granulations have been described histologically as entirely covered by a dural membrane, thus creating a subdural space that separates the body of the arachnoid granulation from the lumen of the accompanying venous sinus.4 However, larger arachnoid granulations may not be completely covered by a dural membrane, thus creating a point of contact between the arachnoid granulation and the venous sinus.4 Larger arachnoid granulations are normally filled with CSF, and their signal characteristics are similar to CSF on imaging.5,6 Arachnoid granulations also often contain vessels draining into the adjacent venous sinus.5,6
When larger arachnoid granulations are present, they may permit the protrusion of herniated brain tissue. There has been an increasing number of reports of these brain herniations into arachnoid granulations (BHAGs) in the literature.7-10 While these herniations have been associated with nonspecific neurologic symptoms like tinnitus and idiopathic intracranial hypertension, their true clinical significance remains undetermined.10,11 This article presents 5 cases of BHAG, discusses their clinical presentations and image findings, and reviews the current literature.
Case 1
A 30-year-old male with a history of multiple traumatic brain injuries presented for evaluation of seizures. The patient described the semiology of the seizures as a bright, colorful light in his right visual field, followed by loss of vision, then loss of awareness and full body convulsion. The semiology of this patient’s seizures was consistent with left temporo-occipital lobe seizure. The only abnormality seen in the brain MRI was the herniation of brain parenchyma originating from the occipital lobe into the transverse sinus, presumably through an arachnoid granulation (Figure 1). An electroencephalogram (EEG) was unremarkable, though the semiology of the seizure historically described by the patient was localized to the area of BHAG. The patient is currently taking antiseizure medications and has experienced no additional seizures.
Case 2
A male aged 53 years with a history of peripheral artery disease presented with a 6-month history of headaches and dizziness. The patient reported the onset of visual aura to his right visual field, starting as a fingernail-sized scintillating kaleidoscope light that would gradually increase in size to a round shape with fading kaleidoscope colors. This episode would last for a few minutes and was immediately followed by a headache. There was no alteration of consciousness during visual aura, although sometimes the patient would have right-sided scalp tingling. These episodes were often unprovoked, but occasionally triggered by bright lights. A single routine EEG was unremarkable. The patient reported headaches without aura, but not aura without headaches, which made occipital lobe seizure less likely. MRI demonstrated a small herniation of brain parenchyma into the inner table of the left occipital bone (Figure 2). The patient was diagnosed with migraine with aura, and the semiology of the visual aura corresponded to the location of the herniation in the left occipital region.
Case 3
A 77-year-old male with a history of left ear diving injury presented with left-sided asymmetric hearing loss and word recognition difficulty for several years. MRI obtained as part of his work-up to evaluate for possible schwannoma of the eighth left cranial nerve instead demonstrated an incidental right cerebellar herniation within an arachnoid granulation into the diploic space of the occipital bone (Figure 3). The BHAG for this patient appeared to be an incidental finding unrelated to his asymmetric hearing loss.
Case 4
A male aged 62 years with a history of metastatic esophageal cancer, substance abuse, and a prior presumed alcohol withdrawal seizure underwent an MRI for evaluation of brain metastasis after presenting to the hospital with confusion 1 day after starting chemotherapy (Figure 4). Nine years prior, the patient had an isolated generalized tonic-clonic seizure approximately 72 hours following a period of alcohol cessation. The MRI demonstrated an incidental left parasagittal herniation of left parietal lobe tissue through an arachnoid granulation into the superior sagittal sinus, in addition to metastatic brain lesions. An EEG showed mild encephalopathy without evidence of seizures. It was determined that the patient's confusion was most likely due to toxic-metabolic encephalopathy from chemotherapy.
Case 5
A 51-year-old male presented with worsening headache severity and frequency. He had a history of chronic headaches for about 20 years that occurred annually, but were now occurring twice weekly. The headaches often started with a left eye visual aura followed by pressure in the left eye, left frontal region, and left ear, with at times a cervicogenic component. No cervical spine imaging was available. An MRI revealed 2 small adjacent areas of cerebellar herniation into arachnoid granulations in the left occipital bone (Figure 5).
Discussion
Arachnoid granulations appear very early in life, although they are uncommon before age 2 years.2 Classically, they have been understood to act as 1-way valves permitting the outflow of CSF from the subarachnoid space to the dural venous sinuses. However, increasing evidence shows they may only play a minor role in that process.12 The structure of arachnoid granulations is being reexamined. A recent microscopy study demonstrated structural heterogeneity with a fine, porous lining that permits flow.13 Additionally, associated immune components in the microenvironment suggests that arachnoid granulations may function similarly to lymph nodes as part of a central nervous system lymphatic network.13 Evidence is lacking for arachnoid granulations being the primary route of CSF outflow, and newer models include CSF exit pathways along the cranial nerves and drainage through lymphatics within the dura mater.12
New MRI systems have demonstrated that the prevalence of arachnoid granulations increases with age. One study found that all subjects in the aged 40 years cohort had detectable arachnoid granulations on images obtained with a 3T MRI system, with the main site being the superior sagittal sinus.2 The prevalence increased until age 40 years and then noticeably decreased. Not only did the prevalence increase in this pattern, but the total number of detectable arachnoid granulations followed a similar pattern.2 In addition, the detectable arachnoid granulations tend to be larger in older patients. Arachnoid granulations are very common in adults, but little is known about when and why brain tissue herniates through these structures.
This case series illustrates how a small amount of adult cerebral or cerebellar matter in large arachnoid granulations can herniate into the dural sinuses and diploic space. Although arachnoid granulations extending into the dural sinuses and diploic space are a relatively common finding on MRI, BHAGs are rare in these locations.1,2,8 Improved spatial resolution afforded by newer high-field scanners with thinner sections, such as very thin (1 mm) T1- and heavily T2-weighted 3 dimensional sequences may lead to increased detection of BHAG. Some of these herniations are small and may be easily missed or confused for normal arachnoid granulations on 3 to 5 mm thickness MRIs.
Despite increased recognition, it is still uncertain to what degree these herniations contribute to the clinical presentations. Associated neurologic symptoms may include seizures, headaches, tinnitus, syncope, and increased intracranial pressure.7-10
Three cases presented in this article demonstrated abnormal signals adjacent to the herniated brain, presumably due to dysplasia of gliotic tissue. In 1 study, parenchymal signal and structural changes occurred in about one-half of the reported BHAG, all of which were cerebellar herniations.7 In Case 1, the herniation and adjacent abnormal MRI signal corresponded to localization of the seizure semiology as obtained from patient history, strongly suggesting the BHAG played a role in the presentation. Signal abnormality accompanying an adjacent BHAG may suggest a higher likelihood that the BHAG has clinical relevance. However, the patient in Case 2 had a visual aura that corresponded to the BHAG location, so a signal abnormality may not be necessary for a patient to develop symptoms. Case 1 also included a history of documented traumatic brain injuries, suggesting that perhaps head trauma may facilitate BHAG development. Regardless, there is likely also a congenital component to their formation, as BHAG has been observed in the pediatric population.14
The patient's asymmetric left-sided hearing loss in Case 3 appeared unrelated to the BHAG as its location was in the contralateral cerebellar region and did not correspond to the patient’s clinical findings. The patient in Case 4 had a limited history regarding localization details of their prior presumed alcohol withdrawal seizure, such as head movements, eye deviation, or lateralized onset of convulsions. Given this limited data, it is unclear whether their prior seizure could have been related to BHAG or not. The patient in case 5 reported worsening headaches on the left side of his head, which corresponded to BHAG occurring on the left side. However, given that the increased T2 signal occurred in the left cerebellar hemisphere with BHAG in the left occipital bone, the occipital cortex was not involved. In this case, the BHAG would not explain the patient’s visual aura as such a lesion would have been expected in the right occipital cortex rather than its actual location in this patient’s left cerebellar hemisphere.
CONCLUSIONS
Understanding the clinical impact of brain herniations is important because they are probably more common than previously thought. Improved MRI capabilities suggest that more BHAG will be detected moving forward as radiologists interpret images with higher resolution and thinner slices. Until its significance is fully understood, BHAG will continue to complicate the diagnosis of patients with neurologic complaints whose brain MRIs and EEGs are otherwise unremarkable.
There have been no cases of surgical BHAG intervention and pathology analysis that would help determine their clinical significance. A related entity, temporal lobe encephalocele, has been linked to focal temporal lobe epilepsy, which has demonstrated significant symptom improvement following surgical correction.15 However, encephaloceles have been distinguished from BHAG in part because they do not necessarily herniate through an arachnoid granulation.8 BHAG has only begun to be characterized in detail over the last decade, so more research is needed to understand how it develops and what clinical significance it truly holds.
1. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol. 1999;72(863):1046-1051. doi:10.1259/bjr.72.863.10700819
2. Rados M, Zivko M, Perisa A, Oreskovic D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi:10.3389/fnagi.2021.698865
3. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology. 1974;113(1):95-100. doi:10.1148/113.1.95
4. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724-727. doi:10.3171/jns.1972.37.6.0724
5. Leach JL, Jones BV, Tomsick TA, Stewart CA, Balko MG. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol. 1996;17(8):1523-1532.
6. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol. 1996;17(4):677-683.
7. Malekzadehlashkariani S, Wanke I, Rufenacht DA, San Millan D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58(5):443-457. doi:10.1007/s00234-016-1662-5
8. Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J. 2014;27(1):55-62. doi:10.15274/NRJ-2014-10006
9. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: clinical and neuroimaging features. J Neuroimaging. 2016;26(6):592-598. doi:10.1111/jon.12366
10. Smith ER, Caton MT, Villanueva-Meyer JE, et al. Brain herniation (encephalocele) into arachnoid granulations: Prevalence and association with pulsatile tinnitus and idiopathic intracranial hypertension. Neuroradiology. 2022;64(9):1747-1754.
11. Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26(6):1723-1731.
12. Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78(6):2429-2457.
13. Shah T, Leurgans SE, Mehta RI, et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J Exp Med. 2023;220(2).
14. Sade R, Ogul H, Polat G, Pirimoglu B, Kantarci M. Brain herniation into the transverse sinuses’ arachnoid granulations in the pediatric population investigated with 3 T MRI. Acta Neurol Belg. 2019;119(2):225-231.
15. Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology. 2015;85(17):1467-1474.
The circulation of cerebrospinal fluid (CSF) is crucial for maintaining homeostasis for the optimal functioning of the multiple complex activities of the brain and spinal cord, including the disposal of metabolic waste products of brain and spinal cord activity into the cerebral venous drainage. Throughout the brain, the arachnoid mater forms small outpouchings or diverticula that penetrate the dura mater and communicate with the dural venous sinuses. These outpuchings are called arachnoid granulations or arachnoid villi, and most are found within the dural sinuses, primarily in the transverse sinuses and superior sagittal sinus, but can occasionally be seen extending into the inner table of the calvarium.1,2
The amount of arachnoid granulations seen in bone, particularly around the superior sagittal sinus, may increase with age.2 Arachnoid granulations are generally small but the largest ones can be seen on gross examination during intracranial procedures or autopsy.3 Magnetic resonance imaging (MRI) can detect arachnoid granulations, which are characterized as T1 hypointense and T2 hyperintense (CSF isointense), well-circumscribed, small, nonenhancing masses within the dural sinuses or in the diploic space (Figure 1). Even small arachnoid granulations < 1 mm in length can be detected.2
Smaller arachnoid granulations have been described histologically as entirely covered by a dural membrane, thus creating a subdural space that separates the body of the arachnoid granulation from the lumen of the accompanying venous sinus.4 However, larger arachnoid granulations may not be completely covered by a dural membrane, thus creating a point of contact between the arachnoid granulation and the venous sinus.4 Larger arachnoid granulations are normally filled with CSF, and their signal characteristics are similar to CSF on imaging.5,6 Arachnoid granulations also often contain vessels draining into the adjacent venous sinus.5,6
When larger arachnoid granulations are present, they may permit the protrusion of herniated brain tissue. There has been an increasing number of reports of these brain herniations into arachnoid granulations (BHAGs) in the literature.7-10 While these herniations have been associated with nonspecific neurologic symptoms like tinnitus and idiopathic intracranial hypertension, their true clinical significance remains undetermined.10,11 This article presents 5 cases of BHAG, discusses their clinical presentations and image findings, and reviews the current literature.
Case 1
A 30-year-old male with a history of multiple traumatic brain injuries presented for evaluation of seizures. The patient described the semiology of the seizures as a bright, colorful light in his right visual field, followed by loss of vision, then loss of awareness and full body convulsion. The semiology of this patient’s seizures was consistent with left temporo-occipital lobe seizure. The only abnormality seen in the brain MRI was the herniation of brain parenchyma originating from the occipital lobe into the transverse sinus, presumably through an arachnoid granulation (Figure 1). An electroencephalogram (EEG) was unremarkable, though the semiology of the seizure historically described by the patient was localized to the area of BHAG. The patient is currently taking antiseizure medications and has experienced no additional seizures.
Case 2
A male aged 53 years with a history of peripheral artery disease presented with a 6-month history of headaches and dizziness. The patient reported the onset of visual aura to his right visual field, starting as a fingernail-sized scintillating kaleidoscope light that would gradually increase in size to a round shape with fading kaleidoscope colors. This episode would last for a few minutes and was immediately followed by a headache. There was no alteration of consciousness during visual aura, although sometimes the patient would have right-sided scalp tingling. These episodes were often unprovoked, but occasionally triggered by bright lights. A single routine EEG was unremarkable. The patient reported headaches without aura, but not aura without headaches, which made occipital lobe seizure less likely. MRI demonstrated a small herniation of brain parenchyma into the inner table of the left occipital bone (Figure 2). The patient was diagnosed with migraine with aura, and the semiology of the visual aura corresponded to the location of the herniation in the left occipital region.
Case 3
A 77-year-old male with a history of left ear diving injury presented with left-sided asymmetric hearing loss and word recognition difficulty for several years. MRI obtained as part of his work-up to evaluate for possible schwannoma of the eighth left cranial nerve instead demonstrated an incidental right cerebellar herniation within an arachnoid granulation into the diploic space of the occipital bone (Figure 3). The BHAG for this patient appeared to be an incidental finding unrelated to his asymmetric hearing loss.
Case 4
A male aged 62 years with a history of metastatic esophageal cancer, substance abuse, and a prior presumed alcohol withdrawal seizure underwent an MRI for evaluation of brain metastasis after presenting to the hospital with confusion 1 day after starting chemotherapy (Figure 4). Nine years prior, the patient had an isolated generalized tonic-clonic seizure approximately 72 hours following a period of alcohol cessation. The MRI demonstrated an incidental left parasagittal herniation of left parietal lobe tissue through an arachnoid granulation into the superior sagittal sinus, in addition to metastatic brain lesions. An EEG showed mild encephalopathy without evidence of seizures. It was determined that the patient's confusion was most likely due to toxic-metabolic encephalopathy from chemotherapy.
Case 5
A 51-year-old male presented with worsening headache severity and frequency. He had a history of chronic headaches for about 20 years that occurred annually, but were now occurring twice weekly. The headaches often started with a left eye visual aura followed by pressure in the left eye, left frontal region, and left ear, with at times a cervicogenic component. No cervical spine imaging was available. An MRI revealed 2 small adjacent areas of cerebellar herniation into arachnoid granulations in the left occipital bone (Figure 5).
Discussion
Arachnoid granulations appear very early in life, although they are uncommon before age 2 years.2 Classically, they have been understood to act as 1-way valves permitting the outflow of CSF from the subarachnoid space to the dural venous sinuses. However, increasing evidence shows they may only play a minor role in that process.12 The structure of arachnoid granulations is being reexamined. A recent microscopy study demonstrated structural heterogeneity with a fine, porous lining that permits flow.13 Additionally, associated immune components in the microenvironment suggests that arachnoid granulations may function similarly to lymph nodes as part of a central nervous system lymphatic network.13 Evidence is lacking for arachnoid granulations being the primary route of CSF outflow, and newer models include CSF exit pathways along the cranial nerves and drainage through lymphatics within the dura mater.12
New MRI systems have demonstrated that the prevalence of arachnoid granulations increases with age. One study found that all subjects in the aged 40 years cohort had detectable arachnoid granulations on images obtained with a 3T MRI system, with the main site being the superior sagittal sinus.2 The prevalence increased until age 40 years and then noticeably decreased. Not only did the prevalence increase in this pattern, but the total number of detectable arachnoid granulations followed a similar pattern.2 In addition, the detectable arachnoid granulations tend to be larger in older patients. Arachnoid granulations are very common in adults, but little is known about when and why brain tissue herniates through these structures.
This case series illustrates how a small amount of adult cerebral or cerebellar matter in large arachnoid granulations can herniate into the dural sinuses and diploic space. Although arachnoid granulations extending into the dural sinuses and diploic space are a relatively common finding on MRI, BHAGs are rare in these locations.1,2,8 Improved spatial resolution afforded by newer high-field scanners with thinner sections, such as very thin (1 mm) T1- and heavily T2-weighted 3 dimensional sequences may lead to increased detection of BHAG. Some of these herniations are small and may be easily missed or confused for normal arachnoid granulations on 3 to 5 mm thickness MRIs.
Despite increased recognition, it is still uncertain to what degree these herniations contribute to the clinical presentations. Associated neurologic symptoms may include seizures, headaches, tinnitus, syncope, and increased intracranial pressure.7-10
Three cases presented in this article demonstrated abnormal signals adjacent to the herniated brain, presumably due to dysplasia of gliotic tissue. In 1 study, parenchymal signal and structural changes occurred in about one-half of the reported BHAG, all of which were cerebellar herniations.7 In Case 1, the herniation and adjacent abnormal MRI signal corresponded to localization of the seizure semiology as obtained from patient history, strongly suggesting the BHAG played a role in the presentation. Signal abnormality accompanying an adjacent BHAG may suggest a higher likelihood that the BHAG has clinical relevance. However, the patient in Case 2 had a visual aura that corresponded to the BHAG location, so a signal abnormality may not be necessary for a patient to develop symptoms. Case 1 also included a history of documented traumatic brain injuries, suggesting that perhaps head trauma may facilitate BHAG development. Regardless, there is likely also a congenital component to their formation, as BHAG has been observed in the pediatric population.14
The patient's asymmetric left-sided hearing loss in Case 3 appeared unrelated to the BHAG as its location was in the contralateral cerebellar region and did not correspond to the patient’s clinical findings. The patient in Case 4 had a limited history regarding localization details of their prior presumed alcohol withdrawal seizure, such as head movements, eye deviation, or lateralized onset of convulsions. Given this limited data, it is unclear whether their prior seizure could have been related to BHAG or not. The patient in case 5 reported worsening headaches on the left side of his head, which corresponded to BHAG occurring on the left side. However, given that the increased T2 signal occurred in the left cerebellar hemisphere with BHAG in the left occipital bone, the occipital cortex was not involved. In this case, the BHAG would not explain the patient’s visual aura as such a lesion would have been expected in the right occipital cortex rather than its actual location in this patient’s left cerebellar hemisphere.
CONCLUSIONS
Understanding the clinical impact of brain herniations is important because they are probably more common than previously thought. Improved MRI capabilities suggest that more BHAG will be detected moving forward as radiologists interpret images with higher resolution and thinner slices. Until its significance is fully understood, BHAG will continue to complicate the diagnosis of patients with neurologic complaints whose brain MRIs and EEGs are otherwise unremarkable.
There have been no cases of surgical BHAG intervention and pathology analysis that would help determine their clinical significance. A related entity, temporal lobe encephalocele, has been linked to focal temporal lobe epilepsy, which has demonstrated significant symptom improvement following surgical correction.15 However, encephaloceles have been distinguished from BHAG in part because they do not necessarily herniate through an arachnoid granulation.8 BHAG has only begun to be characterized in detail over the last decade, so more research is needed to understand how it develops and what clinical significance it truly holds.
The circulation of cerebrospinal fluid (CSF) is crucial for maintaining homeostasis for the optimal functioning of the multiple complex activities of the brain and spinal cord, including the disposal of metabolic waste products of brain and spinal cord activity into the cerebral venous drainage. Throughout the brain, the arachnoid mater forms small outpouchings or diverticula that penetrate the dura mater and communicate with the dural venous sinuses. These outpuchings are called arachnoid granulations or arachnoid villi, and most are found within the dural sinuses, primarily in the transverse sinuses and superior sagittal sinus, but can occasionally be seen extending into the inner table of the calvarium.1,2
The amount of arachnoid granulations seen in bone, particularly around the superior sagittal sinus, may increase with age.2 Arachnoid granulations are generally small but the largest ones can be seen on gross examination during intracranial procedures or autopsy.3 Magnetic resonance imaging (MRI) can detect arachnoid granulations, which are characterized as T1 hypointense and T2 hyperintense (CSF isointense), well-circumscribed, small, nonenhancing masses within the dural sinuses or in the diploic space (Figure 1). Even small arachnoid granulations < 1 mm in length can be detected.2
Smaller arachnoid granulations have been described histologically as entirely covered by a dural membrane, thus creating a subdural space that separates the body of the arachnoid granulation from the lumen of the accompanying venous sinus.4 However, larger arachnoid granulations may not be completely covered by a dural membrane, thus creating a point of contact between the arachnoid granulation and the venous sinus.4 Larger arachnoid granulations are normally filled with CSF, and their signal characteristics are similar to CSF on imaging.5,6 Arachnoid granulations also often contain vessels draining into the adjacent venous sinus.5,6
When larger arachnoid granulations are present, they may permit the protrusion of herniated brain tissue. There has been an increasing number of reports of these brain herniations into arachnoid granulations (BHAGs) in the literature.7-10 While these herniations have been associated with nonspecific neurologic symptoms like tinnitus and idiopathic intracranial hypertension, their true clinical significance remains undetermined.10,11 This article presents 5 cases of BHAG, discusses their clinical presentations and image findings, and reviews the current literature.
Case 1
A 30-year-old male with a history of multiple traumatic brain injuries presented for evaluation of seizures. The patient described the semiology of the seizures as a bright, colorful light in his right visual field, followed by loss of vision, then loss of awareness and full body convulsion. The semiology of this patient’s seizures was consistent with left temporo-occipital lobe seizure. The only abnormality seen in the brain MRI was the herniation of brain parenchyma originating from the occipital lobe into the transverse sinus, presumably through an arachnoid granulation (Figure 1). An electroencephalogram (EEG) was unremarkable, though the semiology of the seizure historically described by the patient was localized to the area of BHAG. The patient is currently taking antiseizure medications and has experienced no additional seizures.
Case 2
A male aged 53 years with a history of peripheral artery disease presented with a 6-month history of headaches and dizziness. The patient reported the onset of visual aura to his right visual field, starting as a fingernail-sized scintillating kaleidoscope light that would gradually increase in size to a round shape with fading kaleidoscope colors. This episode would last for a few minutes and was immediately followed by a headache. There was no alteration of consciousness during visual aura, although sometimes the patient would have right-sided scalp tingling. These episodes were often unprovoked, but occasionally triggered by bright lights. A single routine EEG was unremarkable. The patient reported headaches without aura, but not aura without headaches, which made occipital lobe seizure less likely. MRI demonstrated a small herniation of brain parenchyma into the inner table of the left occipital bone (Figure 2). The patient was diagnosed with migraine with aura, and the semiology of the visual aura corresponded to the location of the herniation in the left occipital region.
Case 3
A 77-year-old male with a history of left ear diving injury presented with left-sided asymmetric hearing loss and word recognition difficulty for several years. MRI obtained as part of his work-up to evaluate for possible schwannoma of the eighth left cranial nerve instead demonstrated an incidental right cerebellar herniation within an arachnoid granulation into the diploic space of the occipital bone (Figure 3). The BHAG for this patient appeared to be an incidental finding unrelated to his asymmetric hearing loss.
Case 4
A male aged 62 years with a history of metastatic esophageal cancer, substance abuse, and a prior presumed alcohol withdrawal seizure underwent an MRI for evaluation of brain metastasis after presenting to the hospital with confusion 1 day after starting chemotherapy (Figure 4). Nine years prior, the patient had an isolated generalized tonic-clonic seizure approximately 72 hours following a period of alcohol cessation. The MRI demonstrated an incidental left parasagittal herniation of left parietal lobe tissue through an arachnoid granulation into the superior sagittal sinus, in addition to metastatic brain lesions. An EEG showed mild encephalopathy without evidence of seizures. It was determined that the patient's confusion was most likely due to toxic-metabolic encephalopathy from chemotherapy.
Case 5
A 51-year-old male presented with worsening headache severity and frequency. He had a history of chronic headaches for about 20 years that occurred annually, but were now occurring twice weekly. The headaches often started with a left eye visual aura followed by pressure in the left eye, left frontal region, and left ear, with at times a cervicogenic component. No cervical spine imaging was available. An MRI revealed 2 small adjacent areas of cerebellar herniation into arachnoid granulations in the left occipital bone (Figure 5).
Discussion
Arachnoid granulations appear very early in life, although they are uncommon before age 2 years.2 Classically, they have been understood to act as 1-way valves permitting the outflow of CSF from the subarachnoid space to the dural venous sinuses. However, increasing evidence shows they may only play a minor role in that process.12 The structure of arachnoid granulations is being reexamined. A recent microscopy study demonstrated structural heterogeneity with a fine, porous lining that permits flow.13 Additionally, associated immune components in the microenvironment suggests that arachnoid granulations may function similarly to lymph nodes as part of a central nervous system lymphatic network.13 Evidence is lacking for arachnoid granulations being the primary route of CSF outflow, and newer models include CSF exit pathways along the cranial nerves and drainage through lymphatics within the dura mater.12
New MRI systems have demonstrated that the prevalence of arachnoid granulations increases with age. One study found that all subjects in the aged 40 years cohort had detectable arachnoid granulations on images obtained with a 3T MRI system, with the main site being the superior sagittal sinus.2 The prevalence increased until age 40 years and then noticeably decreased. Not only did the prevalence increase in this pattern, but the total number of detectable arachnoid granulations followed a similar pattern.2 In addition, the detectable arachnoid granulations tend to be larger in older patients. Arachnoid granulations are very common in adults, but little is known about when and why brain tissue herniates through these structures.
This case series illustrates how a small amount of adult cerebral or cerebellar matter in large arachnoid granulations can herniate into the dural sinuses and diploic space. Although arachnoid granulations extending into the dural sinuses and diploic space are a relatively common finding on MRI, BHAGs are rare in these locations.1,2,8 Improved spatial resolution afforded by newer high-field scanners with thinner sections, such as very thin (1 mm) T1- and heavily T2-weighted 3 dimensional sequences may lead to increased detection of BHAG. Some of these herniations are small and may be easily missed or confused for normal arachnoid granulations on 3 to 5 mm thickness MRIs.
Despite increased recognition, it is still uncertain to what degree these herniations contribute to the clinical presentations. Associated neurologic symptoms may include seizures, headaches, tinnitus, syncope, and increased intracranial pressure.7-10
Three cases presented in this article demonstrated abnormal signals adjacent to the herniated brain, presumably due to dysplasia of gliotic tissue. In 1 study, parenchymal signal and structural changes occurred in about one-half of the reported BHAG, all of which were cerebellar herniations.7 In Case 1, the herniation and adjacent abnormal MRI signal corresponded to localization of the seizure semiology as obtained from patient history, strongly suggesting the BHAG played a role in the presentation. Signal abnormality accompanying an adjacent BHAG may suggest a higher likelihood that the BHAG has clinical relevance. However, the patient in Case 2 had a visual aura that corresponded to the BHAG location, so a signal abnormality may not be necessary for a patient to develop symptoms. Case 1 also included a history of documented traumatic brain injuries, suggesting that perhaps head trauma may facilitate BHAG development. Regardless, there is likely also a congenital component to their formation, as BHAG has been observed in the pediatric population.14
The patient's asymmetric left-sided hearing loss in Case 3 appeared unrelated to the BHAG as its location was in the contralateral cerebellar region and did not correspond to the patient’s clinical findings. The patient in Case 4 had a limited history regarding localization details of their prior presumed alcohol withdrawal seizure, such as head movements, eye deviation, or lateralized onset of convulsions. Given this limited data, it is unclear whether their prior seizure could have been related to BHAG or not. The patient in case 5 reported worsening headaches on the left side of his head, which corresponded to BHAG occurring on the left side. However, given that the increased T2 signal occurred in the left cerebellar hemisphere with BHAG in the left occipital bone, the occipital cortex was not involved. In this case, the BHAG would not explain the patient’s visual aura as such a lesion would have been expected in the right occipital cortex rather than its actual location in this patient’s left cerebellar hemisphere.
CONCLUSIONS
Understanding the clinical impact of brain herniations is important because they are probably more common than previously thought. Improved MRI capabilities suggest that more BHAG will be detected moving forward as radiologists interpret images with higher resolution and thinner slices. Until its significance is fully understood, BHAG will continue to complicate the diagnosis of patients with neurologic complaints whose brain MRIs and EEGs are otherwise unremarkable.
There have been no cases of surgical BHAG intervention and pathology analysis that would help determine their clinical significance. A related entity, temporal lobe encephalocele, has been linked to focal temporal lobe epilepsy, which has demonstrated significant symptom improvement following surgical correction.15 However, encephaloceles have been distinguished from BHAG in part because they do not necessarily herniate through an arachnoid granulation.8 BHAG has only begun to be characterized in detail over the last decade, so more research is needed to understand how it develops and what clinical significance it truly holds.
1. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol. 1999;72(863):1046-1051. doi:10.1259/bjr.72.863.10700819
2. Rados M, Zivko M, Perisa A, Oreskovic D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi:10.3389/fnagi.2021.698865
3. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology. 1974;113(1):95-100. doi:10.1148/113.1.95
4. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724-727. doi:10.3171/jns.1972.37.6.0724
5. Leach JL, Jones BV, Tomsick TA, Stewart CA, Balko MG. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol. 1996;17(8):1523-1532.
6. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol. 1996;17(4):677-683.
7. Malekzadehlashkariani S, Wanke I, Rufenacht DA, San Millan D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58(5):443-457. doi:10.1007/s00234-016-1662-5
8. Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J. 2014;27(1):55-62. doi:10.15274/NRJ-2014-10006
9. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: clinical and neuroimaging features. J Neuroimaging. 2016;26(6):592-598. doi:10.1111/jon.12366
10. Smith ER, Caton MT, Villanueva-Meyer JE, et al. Brain herniation (encephalocele) into arachnoid granulations: Prevalence and association with pulsatile tinnitus and idiopathic intracranial hypertension. Neuroradiology. 2022;64(9):1747-1754.
11. Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26(6):1723-1731.
12. Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78(6):2429-2457.
13. Shah T, Leurgans SE, Mehta RI, et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J Exp Med. 2023;220(2).
14. Sade R, Ogul H, Polat G, Pirimoglu B, Kantarci M. Brain herniation into the transverse sinuses’ arachnoid granulations in the pediatric population investigated with 3 T MRI. Acta Neurol Belg. 2019;119(2):225-231.
15. Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology. 2015;85(17):1467-1474.
1. Ikushima I, Korogi Y, Makita O, et al. MRI of arachnoid granulations within the dural sinuses using a FLAIR pulse sequence. Br J Radiol. 1999;72(863):1046-1051. doi:10.1259/bjr.72.863.10700819
2. Rados M, Zivko M, Perisa A, Oreskovic D, Klarica M. No arachnoid granulations-no problems: number, size, and distribution of arachnoid granulations from birth to 80 years of age. Front Aging Neurosci. 2021;13:698865. doi:10.3389/fnagi.2021.698865
3. Grossman CB, Potts DG. Arachnoid granulations: radiology and anatomy. Radiology. 1974;113(1):95-100. doi:10.1148/113.1.95
4. Wolpow ER, Schaumburg HH. Structure of the human arachnoid granulation. J Neurosurg. 1972;37(6):724-727. doi:10.3171/jns.1972.37.6.0724
5. Leach JL, Jones BV, Tomsick TA, Stewart CA, Balko MG. Normal appearance of arachnoid granulations on contrast-enhanced CT and MR of the brain: differentiation from dural sinus disease. AJNR Am J Neuroradiol. 1996;17(8):1523-1532.
6. Roche J, Warner D. Arachnoid granulations in the transverse and sigmoid sinuses: CT, MR, and MR angiographic appearance of a normal anatomic variation. AJNR Am J Neuroradiol. 1996;17(4):677-683.
7. Malekzadehlashkariani S, Wanke I, Rufenacht DA, San Millan D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58(5):443-457. doi:10.1007/s00234-016-1662-5
8. Battal B, Castillo M. Brain herniations into the dural venous sinuses or calvarium: MRI of a recently recognized entity. Neuroradiol J. 2014;27(1):55-62. doi:10.15274/NRJ-2014-10006
9. Liebo GB, Lane JJ, Van Gompel JJ, Eckel LJ, Schwartz KM, Lehman VT. Brain herniation into arachnoid granulations: clinical and neuroimaging features. J Neuroimaging. 2016;26(6):592-598. doi:10.1111/jon.12366
10. Smith ER, Caton MT, Villanueva-Meyer JE, et al. Brain herniation (encephalocele) into arachnoid granulations: Prevalence and association with pulsatile tinnitus and idiopathic intracranial hypertension. Neuroradiology. 2022;64(9):1747-1754.
11. Battal B, Hamcan S, Akgun V, et al. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26(6):1723-1731.
12. Proulx ST. Cerebrospinal fluid outflow: A review of the historical and contemporary evidence for arachnoid villi, perineural routes, and dural lymphatics. Cell Mol Life Sci. 2021;78(6):2429-2457.
13. Shah T, Leurgans SE, Mehta RI, et al. Arachnoid granulations are lymphatic conduits that communicate with bone marrow and dura-arachnoid stroma. J Exp Med. 2023;220(2).
14. Sade R, Ogul H, Polat G, Pirimoglu B, Kantarci M. Brain herniation into the transverse sinuses’ arachnoid granulations in the pediatric population investigated with 3 T MRI. Acta Neurol Belg. 2019;119(2):225-231.
15. Saavalainen T, Jutila L, Mervaala E, Kalviainen R, Vanninen R, Immonen A. Temporal anteroinferior encephalocele: An underrecognized etiology of temporal lobe epilepsy? Neurology. 2015;85(17):1467-1474.
Commentary: Predicting Migraine Treatment Outcomes, July 2024
Medications classified as anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb), a relatively new category of migraine therapy, have shown strong evidence of efficacy for migraine treatment and prevention. However, as these medications — which include Aimovig (erenumab), Emgality (galcanezumab), Ajovy (fremanezumab), and Vyepti (eptinezumab) — are new, their long-term outcomes are not known; in addition, they are expensive and they do not work for everyone. Patients who are doing relatively well on other medications might ask about switching to one of the anti-CGRP mAb so that they can experience the better outcomes and low side-effect profile that they've been hearing about. New research is showing some prognostic indicators that can help identify which patients might experience a better response to anti-CGRP mAb.
A prospective real-world study published in the May 2024 issue of Journal of Neurology, Neurosurgery, and Psychiatry included 5818 patients who had been treated with an anti-CGRP mAb for high-frequency episodic or chronic migraine. The researchers assessed responses after 6 months of use, defining a good response as ≥50% reduction in monthly headache days and excellent response as ≥75% reduction in monthly headache days. They found that several pretreatment baseline factors were predictors of a good or excellent 6-month response: older age, the presence of unilateral pain, the absence of depression, fewer monthly migraine days, and lower Migraine Disability Assessment (MIDAS) score. Notably, men and women experienced comparable outcomes. While it's not completely clear why these factors were associated with better responses to anti-CGRP mAb, the results could help in selecting patients who might or might not benefit from this new medication class.
Results of a prospective study published in the May 2024 in The Journal of Headache and Pain demonstrated that patients treated with eptinezumab for 3 months experienced a reduction of monthly headaches, migraines, and the use of acute medication. The patients who had previously had an inadequate response to or were unable to tolerate other anti-CGRP mAb (erenumab, galcanezumab, fremanezumab) were less likely to experience improvement with eptinezumab than patients who had not had previous unsuccessful attempts with anti-CGRP mAb. This suggests that it might not be beneficial for patents to try multiple medications in this category if they have had an inadequate response or intolerability to others in the same drug class.
Lifestyle factors can play a role in migraine outcomes and may reduce the need for medication. A study published in The Journal of Headache and Pain in May 2024 examined the relationship between migraine and the American Heart Association (AHA) Guidelines for Cardiovascular Health recommended lifestyle factors. The study included 332,895 participants, with a median follow-up of 13.58 years. Researchers found that maintaining targeted or recommended body mass index (BMI), physical activity, sleep duration, sleep pattern, and sedentary time were associated with substantial reductions in migraine risk.
Diet, another lifestyle factor, can also have an effect on migraine. Avoiding dietary triggers is a well-known adjustment that many patients are advised to make. Overall diet quality can play a role in migraine outcomes as well. According to a study published in the May 2024 issue of Nutritional Neuroscience, participants who followed a diet that qualified as having a high Carbohydrate Quality Index (CQI) had lower migraine severity and duration than participants whose diets did not qualify as high CQI. The study included 266 women (age 18-45 years), using a 147-item food frequency questionnaire to assess CQI. The CQI, a relatively new index for measuring carbohydrate quality, includes four components: glycemic index, dietary fiber intake, ratio of whole grain to total grain, ratio of solid carbohydrates to total (solid + liquid) carbohydrates.1 A low glycemic index and higher scores for the other three factors translates to a high CQI.
While the results of the AHA/migraine study and the CQI/migraine study are interesting, the physiologic reasons for the outcomes and validation of the results need further investigation. It's not clear whether the decrease in migraines that's associated with optimal carbohydrate intake is associated with outcomes such as low BMI or better sleep, or whether carbohydrate metabolism could be an independent factor.
Predictive factors can be beneficial in making migraine treatment decisions. While trial and error will always remain part of optimal migraine therapy, customizing treatment on the basis of an individual patient's characteristics can help in reaching an effective treatment and better quality of life sooner.
Additional References
1. Sawicki CM, Lichtenstein AH, Rogers GT, et al. Comparison of indices of carbohydrate quality and food sources of dietary fiber on longitudinal changes in waist circumference in the Framingham Offspring Cohort. Nutrients. 2021;13:997. Source
Medications classified as anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb), a relatively new category of migraine therapy, have shown strong evidence of efficacy for migraine treatment and prevention. However, as these medications — which include Aimovig (erenumab), Emgality (galcanezumab), Ajovy (fremanezumab), and Vyepti (eptinezumab) — are new, their long-term outcomes are not known; in addition, they are expensive and they do not work for everyone. Patients who are doing relatively well on other medications might ask about switching to one of the anti-CGRP mAb so that they can experience the better outcomes and low side-effect profile that they've been hearing about. New research is showing some prognostic indicators that can help identify which patients might experience a better response to anti-CGRP mAb.
A prospective real-world study published in the May 2024 issue of Journal of Neurology, Neurosurgery, and Psychiatry included 5818 patients who had been treated with an anti-CGRP mAb for high-frequency episodic or chronic migraine. The researchers assessed responses after 6 months of use, defining a good response as ≥50% reduction in monthly headache days and excellent response as ≥75% reduction in monthly headache days. They found that several pretreatment baseline factors were predictors of a good or excellent 6-month response: older age, the presence of unilateral pain, the absence of depression, fewer monthly migraine days, and lower Migraine Disability Assessment (MIDAS) score. Notably, men and women experienced comparable outcomes. While it's not completely clear why these factors were associated with better responses to anti-CGRP mAb, the results could help in selecting patients who might or might not benefit from this new medication class.
Results of a prospective study published in the May 2024 in The Journal of Headache and Pain demonstrated that patients treated with eptinezumab for 3 months experienced a reduction of monthly headaches, migraines, and the use of acute medication. The patients who had previously had an inadequate response to or were unable to tolerate other anti-CGRP mAb (erenumab, galcanezumab, fremanezumab) were less likely to experience improvement with eptinezumab than patients who had not had previous unsuccessful attempts with anti-CGRP mAb. This suggests that it might not be beneficial for patents to try multiple medications in this category if they have had an inadequate response or intolerability to others in the same drug class.
Lifestyle factors can play a role in migraine outcomes and may reduce the need for medication. A study published in The Journal of Headache and Pain in May 2024 examined the relationship between migraine and the American Heart Association (AHA) Guidelines for Cardiovascular Health recommended lifestyle factors. The study included 332,895 participants, with a median follow-up of 13.58 years. Researchers found that maintaining targeted or recommended body mass index (BMI), physical activity, sleep duration, sleep pattern, and sedentary time were associated with substantial reductions in migraine risk.
Diet, another lifestyle factor, can also have an effect on migraine. Avoiding dietary triggers is a well-known adjustment that many patients are advised to make. Overall diet quality can play a role in migraine outcomes as well. According to a study published in the May 2024 issue of Nutritional Neuroscience, participants who followed a diet that qualified as having a high Carbohydrate Quality Index (CQI) had lower migraine severity and duration than participants whose diets did not qualify as high CQI. The study included 266 women (age 18-45 years), using a 147-item food frequency questionnaire to assess CQI. The CQI, a relatively new index for measuring carbohydrate quality, includes four components: glycemic index, dietary fiber intake, ratio of whole grain to total grain, ratio of solid carbohydrates to total (solid + liquid) carbohydrates.1 A low glycemic index and higher scores for the other three factors translates to a high CQI.
While the results of the AHA/migraine study and the CQI/migraine study are interesting, the physiologic reasons for the outcomes and validation of the results need further investigation. It's not clear whether the decrease in migraines that's associated with optimal carbohydrate intake is associated with outcomes such as low BMI or better sleep, or whether carbohydrate metabolism could be an independent factor.
Predictive factors can be beneficial in making migraine treatment decisions. While trial and error will always remain part of optimal migraine therapy, customizing treatment on the basis of an individual patient's characteristics can help in reaching an effective treatment and better quality of life sooner.
Additional References
1. Sawicki CM, Lichtenstein AH, Rogers GT, et al. Comparison of indices of carbohydrate quality and food sources of dietary fiber on longitudinal changes in waist circumference in the Framingham Offspring Cohort. Nutrients. 2021;13:997. Source
Medications classified as anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb), a relatively new category of migraine therapy, have shown strong evidence of efficacy for migraine treatment and prevention. However, as these medications — which include Aimovig (erenumab), Emgality (galcanezumab), Ajovy (fremanezumab), and Vyepti (eptinezumab) — are new, their long-term outcomes are not known; in addition, they are expensive and they do not work for everyone. Patients who are doing relatively well on other medications might ask about switching to one of the anti-CGRP mAb so that they can experience the better outcomes and low side-effect profile that they've been hearing about. New research is showing some prognostic indicators that can help identify which patients might experience a better response to anti-CGRP mAb.
A prospective real-world study published in the May 2024 issue of Journal of Neurology, Neurosurgery, and Psychiatry included 5818 patients who had been treated with an anti-CGRP mAb for high-frequency episodic or chronic migraine. The researchers assessed responses after 6 months of use, defining a good response as ≥50% reduction in monthly headache days and excellent response as ≥75% reduction in monthly headache days. They found that several pretreatment baseline factors were predictors of a good or excellent 6-month response: older age, the presence of unilateral pain, the absence of depression, fewer monthly migraine days, and lower Migraine Disability Assessment (MIDAS) score. Notably, men and women experienced comparable outcomes. While it's not completely clear why these factors were associated with better responses to anti-CGRP mAb, the results could help in selecting patients who might or might not benefit from this new medication class.
Results of a prospective study published in the May 2024 in The Journal of Headache and Pain demonstrated that patients treated with eptinezumab for 3 months experienced a reduction of monthly headaches, migraines, and the use of acute medication. The patients who had previously had an inadequate response to or were unable to tolerate other anti-CGRP mAb (erenumab, galcanezumab, fremanezumab) were less likely to experience improvement with eptinezumab than patients who had not had previous unsuccessful attempts with anti-CGRP mAb. This suggests that it might not be beneficial for patents to try multiple medications in this category if they have had an inadequate response or intolerability to others in the same drug class.
Lifestyle factors can play a role in migraine outcomes and may reduce the need for medication. A study published in The Journal of Headache and Pain in May 2024 examined the relationship between migraine and the American Heart Association (AHA) Guidelines for Cardiovascular Health recommended lifestyle factors. The study included 332,895 participants, with a median follow-up of 13.58 years. Researchers found that maintaining targeted or recommended body mass index (BMI), physical activity, sleep duration, sleep pattern, and sedentary time were associated with substantial reductions in migraine risk.
Diet, another lifestyle factor, can also have an effect on migraine. Avoiding dietary triggers is a well-known adjustment that many patients are advised to make. Overall diet quality can play a role in migraine outcomes as well. According to a study published in the May 2024 issue of Nutritional Neuroscience, participants who followed a diet that qualified as having a high Carbohydrate Quality Index (CQI) had lower migraine severity and duration than participants whose diets did not qualify as high CQI. The study included 266 women (age 18-45 years), using a 147-item food frequency questionnaire to assess CQI. The CQI, a relatively new index for measuring carbohydrate quality, includes four components: glycemic index, dietary fiber intake, ratio of whole grain to total grain, ratio of solid carbohydrates to total (solid + liquid) carbohydrates.1 A low glycemic index and higher scores for the other three factors translates to a high CQI.
While the results of the AHA/migraine study and the CQI/migraine study are interesting, the physiologic reasons for the outcomes and validation of the results need further investigation. It's not clear whether the decrease in migraines that's associated with optimal carbohydrate intake is associated with outcomes such as low BMI or better sleep, or whether carbohydrate metabolism could be an independent factor.
Predictive factors can be beneficial in making migraine treatment decisions. While trial and error will always remain part of optimal migraine therapy, customizing treatment on the basis of an individual patient's characteristics can help in reaching an effective treatment and better quality of life sooner.
Additional References
1. Sawicki CM, Lichtenstein AH, Rogers GT, et al. Comparison of indices of carbohydrate quality and food sources of dietary fiber on longitudinal changes in waist circumference in the Framingham Offspring Cohort. Nutrients. 2021;13:997. Source