User login
Infectious disease pop quiz: Clinical challenge #12 for the ObGyn
What are the best office-based tests for the diagnosis of bacterial vaginosis?
Continue to the answer...
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their outer margin.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the best office-based tests for the diagnosis of bacterial vaginosis?
Continue to the answer...
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their outer margin.
What are the best office-based tests for the diagnosis of bacterial vaginosis?
Continue to the answer...
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their outer margin.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
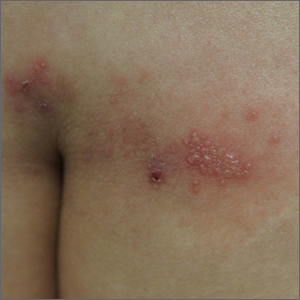
Sacral blisters
Grouped vesicles on an erythematous base should prompt concern for herpes viruses including varicella zoster (VZV) and herpes simplex (HSV). Polymerase chain reaction (PCR) testing for both VZV and HSV revealed this to be sacral HSV.
VZV classically presents in a dermatomal distribution, whereas HSV more commonly manifests along a single peripheral sensory nerve. Zosteriform presentations of HSV, however, have been reported.
Nongenital and nonoral HSV aren’t uncommon and can be associated with genital herpes, whether from self-inoculation or viremia.1 These outbreaks usually occur in the distribution of the pudendal nerve, which arises from the S2-S4 spinal nerves. There is an association of genital viral shedding even in the absence of lesions when sacral flaring manifests, and patients should be cautioned about sexual transmission or vertically transmitted perinatal infection in pregnant patients near term.
Treatment for an initial episode of genital infection with HSV is valacyclovir 1 g bid for 10 days. The regimen is ideally started within 48 to 72 hours of symptom onset.
This patient was empirically started on VZV dosing, then switched to HSV dosing when the PCR testing confirmed HSV. Knowledge of the exact pathogen is helpful in counseling the patient about the potential for spread and the risk of recurrence. With HSV, the patient may be prescribed a suppressive dose of valacyclovir 500 mg bid for 3 days, started at the onset of symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex virus infection. Proc (Bayl Univ Med Cent). 2016;29:48-49. doi:10.1080/08998280.2016.11929356

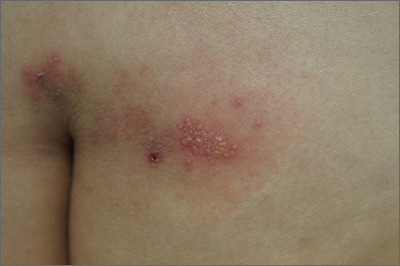
Grouped vesicles on an erythematous base should prompt concern for herpes viruses including varicella zoster (VZV) and herpes simplex (HSV). Polymerase chain reaction (PCR) testing for both VZV and HSV revealed this to be sacral HSV.
VZV classically presents in a dermatomal distribution, whereas HSV more commonly manifests along a single peripheral sensory nerve. Zosteriform presentations of HSV, however, have been reported.
Nongenital and nonoral HSV aren’t uncommon and can be associated with genital herpes, whether from self-inoculation or viremia.1 These outbreaks usually occur in the distribution of the pudendal nerve, which arises from the S2-S4 spinal nerves. There is an association of genital viral shedding even in the absence of lesions when sacral flaring manifests, and patients should be cautioned about sexual transmission or vertically transmitted perinatal infection in pregnant patients near term.
Treatment for an initial episode of genital infection with HSV is valacyclovir 1 g bid for 10 days. The regimen is ideally started within 48 to 72 hours of symptom onset.
This patient was empirically started on VZV dosing, then switched to HSV dosing when the PCR testing confirmed HSV. Knowledge of the exact pathogen is helpful in counseling the patient about the potential for spread and the risk of recurrence. With HSV, the patient may be prescribed a suppressive dose of valacyclovir 500 mg bid for 3 days, started at the onset of symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Grouped vesicles on an erythematous base should prompt concern for herpes viruses including varicella zoster (VZV) and herpes simplex (HSV). Polymerase chain reaction (PCR) testing for both VZV and HSV revealed this to be sacral HSV.
VZV classically presents in a dermatomal distribution, whereas HSV more commonly manifests along a single peripheral sensory nerve. Zosteriform presentations of HSV, however, have been reported.
Nongenital and nonoral HSV aren’t uncommon and can be associated with genital herpes, whether from self-inoculation or viremia.1 These outbreaks usually occur in the distribution of the pudendal nerve, which arises from the S2-S4 spinal nerves. There is an association of genital viral shedding even in the absence of lesions when sacral flaring manifests, and patients should be cautioned about sexual transmission or vertically transmitted perinatal infection in pregnant patients near term.
Treatment for an initial episode of genital infection with HSV is valacyclovir 1 g bid for 10 days. The regimen is ideally started within 48 to 72 hours of symptom onset.
This patient was empirically started on VZV dosing, then switched to HSV dosing when the PCR testing confirmed HSV. Knowledge of the exact pathogen is helpful in counseling the patient about the potential for spread and the risk of recurrence. With HSV, the patient may be prescribed a suppressive dose of valacyclovir 500 mg bid for 3 days, started at the onset of symptoms.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex virus infection. Proc (Bayl Univ Med Cent). 2016;29:48-49. doi:10.1080/08998280.2016.11929356
1. Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex virus infection. Proc (Bayl Univ Med Cent). 2016;29:48-49. doi:10.1080/08998280.2016.11929356
Clinical Edge Journal Scan Commentary: RA February 2022
Several recent RA studies have addressed aspects of systemic illness other than joint pain and inflammation, including sleep, fatigue, psychosocial burden, and well-being. A cohort study by Lyne et al1 evaluated sleep duration and quality in 3,265 patients in the Swedish EIRA registry from 1-12 years after RA diagnosis. About 40% had problems in at least one sleep domain and the frequency of sleep problems increased somewhat with disease duration, but the strongest correlations with poor sleep were pain and functional impairment, suggesting that the overall activity of the RA was most important. Further research on improving sleep quality with improved control of disease activity would be helpful in supporting this hypothesis.
A systematic review by Shamail et al2 examined mental health outcomes in patients with RA taking Janus kinase (JAK) inhibitors, limiting the review to studies reporting SF-36 mental health outcomes. The resulting 19 studies encompassed over 14,000 patients and did demonstrate clinically meaningful changes in SF-36 scores compared to baseline in patients treated with JAK inhibitors. When compared to changes with placebo or disease-modifying antirheumatic drug (DMARD) treatment, JAK inhibitors appeared to have a benefit, though few studies showed a clinically meaningful difference. Given that other studies have shown improvement in mental health outcomes with other classes of RA treatments, it is not clear that this is an effect of the JAK inhibitor class rather than related to overall improvement in quality of life.
Fatigue is a prevalent concern among patients with RA and may significantly impact quality of life; its origins in RA are not well-understood but thought to be related to inflammation. A UK study of an inception cohort by Ifeseman et al3 examines fatigue in early RA; about 75% of participants reported a decreased vitality score compared to the mean in the UK general population. Of the approximately 729 study participants in the longitudinal analysis, trajectory modeling was used to identify two groups of people: one with an “average” vitality score and another with a score that was significantly reduced compared to average. This group had worse disease activity scores, Health Assessment Questionnaire (HAQ) scores, and pain, though as with the other studies mentioned above, it is not clear if fatigue is a feature of worse control of RA or related to ongoing central sensitization or “non-inflammatory” mechanisms.
Doumen et al4 analyzed interaction between psychosocial variables and disease activity in an early RA cohort and found that better baseline short form-36 (SF-36) scores as well as other measures of psychosocial burden and coping were associated with sustained Disease Activity Score 28 for Rheumatoid Arthritis with C-Reactive Protein (DAS-28-CRP) remission, while negative illness perception was associated with lower probability of sustained remission. Of the 287 patients who achieved DAS-28-CRP remission at week 16, the 231 patients who had a low psychosocial burden were more likely to remain in remission. Causality and direction are not established in this small study, so while evaluating psychosocial needs is relevant, as with the other studies mentioned above, caution must be used in attributing lack of improvement in disease activity to psychosocial burden or mood disorders.
References
- Lyne L et al. Sleep problems in rheumatoid arthritis over 12 years from diagnosis: results from the Swedish EIRA study. RMD Open. 2022;8:e001800 (Jan 5).
- Shamail GMH et al. Association between janus kinase inhibitors therapy and mental health outcome in rheumatoid arthritis: A systematic review and meta-analysis. Rheumatol Ther. 2021 (Dec 13).
- Ifesemen OS et al. Fatigue in early rheumatoid arthritis: data from the Early Rheumatoid Arthritis Network. Rheumatology (Oxford). 2021;keab861 (Dec 27).
- Doumen M et al. Psychosocial burden predicts sustained remission in early rheumatoid arthritis: unraveling the complex interplay of wellbeing and disease activity. Arthritis Care Res (Hoboken). 2021 (Dec 20).
Several recent RA studies have addressed aspects of systemic illness other than joint pain and inflammation, including sleep, fatigue, psychosocial burden, and well-being. A cohort study by Lyne et al1 evaluated sleep duration and quality in 3,265 patients in the Swedish EIRA registry from 1-12 years after RA diagnosis. About 40% had problems in at least one sleep domain and the frequency of sleep problems increased somewhat with disease duration, but the strongest correlations with poor sleep were pain and functional impairment, suggesting that the overall activity of the RA was most important. Further research on improving sleep quality with improved control of disease activity would be helpful in supporting this hypothesis.
A systematic review by Shamail et al2 examined mental health outcomes in patients with RA taking Janus kinase (JAK) inhibitors, limiting the review to studies reporting SF-36 mental health outcomes. The resulting 19 studies encompassed over 14,000 patients and did demonstrate clinically meaningful changes in SF-36 scores compared to baseline in patients treated with JAK inhibitors. When compared to changes with placebo or disease-modifying antirheumatic drug (DMARD) treatment, JAK inhibitors appeared to have a benefit, though few studies showed a clinically meaningful difference. Given that other studies have shown improvement in mental health outcomes with other classes of RA treatments, it is not clear that this is an effect of the JAK inhibitor class rather than related to overall improvement in quality of life.
Fatigue is a prevalent concern among patients with RA and may significantly impact quality of life; its origins in RA are not well-understood but thought to be related to inflammation. A UK study of an inception cohort by Ifeseman et al3 examines fatigue in early RA; about 75% of participants reported a decreased vitality score compared to the mean in the UK general population. Of the approximately 729 study participants in the longitudinal analysis, trajectory modeling was used to identify two groups of people: one with an “average” vitality score and another with a score that was significantly reduced compared to average. This group had worse disease activity scores, Health Assessment Questionnaire (HAQ) scores, and pain, though as with the other studies mentioned above, it is not clear if fatigue is a feature of worse control of RA or related to ongoing central sensitization or “non-inflammatory” mechanisms.
Doumen et al4 analyzed interaction between psychosocial variables and disease activity in an early RA cohort and found that better baseline short form-36 (SF-36) scores as well as other measures of psychosocial burden and coping were associated with sustained Disease Activity Score 28 for Rheumatoid Arthritis with C-Reactive Protein (DAS-28-CRP) remission, while negative illness perception was associated with lower probability of sustained remission. Of the 287 patients who achieved DAS-28-CRP remission at week 16, the 231 patients who had a low psychosocial burden were more likely to remain in remission. Causality and direction are not established in this small study, so while evaluating psychosocial needs is relevant, as with the other studies mentioned above, caution must be used in attributing lack of improvement in disease activity to psychosocial burden or mood disorders.
References
- Lyne L et al. Sleep problems in rheumatoid arthritis over 12 years from diagnosis: results from the Swedish EIRA study. RMD Open. 2022;8:e001800 (Jan 5).
- Shamail GMH et al. Association between janus kinase inhibitors therapy and mental health outcome in rheumatoid arthritis: A systematic review and meta-analysis. Rheumatol Ther. 2021 (Dec 13).
- Ifesemen OS et al. Fatigue in early rheumatoid arthritis: data from the Early Rheumatoid Arthritis Network. Rheumatology (Oxford). 2021;keab861 (Dec 27).
- Doumen M et al. Psychosocial burden predicts sustained remission in early rheumatoid arthritis: unraveling the complex interplay of wellbeing and disease activity. Arthritis Care Res (Hoboken). 2021 (Dec 20).
Several recent RA studies have addressed aspects of systemic illness other than joint pain and inflammation, including sleep, fatigue, psychosocial burden, and well-being. A cohort study by Lyne et al1 evaluated sleep duration and quality in 3,265 patients in the Swedish EIRA registry from 1-12 years after RA diagnosis. About 40% had problems in at least one sleep domain and the frequency of sleep problems increased somewhat with disease duration, but the strongest correlations with poor sleep were pain and functional impairment, suggesting that the overall activity of the RA was most important. Further research on improving sleep quality with improved control of disease activity would be helpful in supporting this hypothesis.
A systematic review by Shamail et al2 examined mental health outcomes in patients with RA taking Janus kinase (JAK) inhibitors, limiting the review to studies reporting SF-36 mental health outcomes. The resulting 19 studies encompassed over 14,000 patients and did demonstrate clinically meaningful changes in SF-36 scores compared to baseline in patients treated with JAK inhibitors. When compared to changes with placebo or disease-modifying antirheumatic drug (DMARD) treatment, JAK inhibitors appeared to have a benefit, though few studies showed a clinically meaningful difference. Given that other studies have shown improvement in mental health outcomes with other classes of RA treatments, it is not clear that this is an effect of the JAK inhibitor class rather than related to overall improvement in quality of life.
Fatigue is a prevalent concern among patients with RA and may significantly impact quality of life; its origins in RA are not well-understood but thought to be related to inflammation. A UK study of an inception cohort by Ifeseman et al3 examines fatigue in early RA; about 75% of participants reported a decreased vitality score compared to the mean in the UK general population. Of the approximately 729 study participants in the longitudinal analysis, trajectory modeling was used to identify two groups of people: one with an “average” vitality score and another with a score that was significantly reduced compared to average. This group had worse disease activity scores, Health Assessment Questionnaire (HAQ) scores, and pain, though as with the other studies mentioned above, it is not clear if fatigue is a feature of worse control of RA or related to ongoing central sensitization or “non-inflammatory” mechanisms.
Doumen et al4 analyzed interaction between psychosocial variables and disease activity in an early RA cohort and found that better baseline short form-36 (SF-36) scores as well as other measures of psychosocial burden and coping were associated with sustained Disease Activity Score 28 for Rheumatoid Arthritis with C-Reactive Protein (DAS-28-CRP) remission, while negative illness perception was associated with lower probability of sustained remission. Of the 287 patients who achieved DAS-28-CRP remission at week 16, the 231 patients who had a low psychosocial burden were more likely to remain in remission. Causality and direction are not established in this small study, so while evaluating psychosocial needs is relevant, as with the other studies mentioned above, caution must be used in attributing lack of improvement in disease activity to psychosocial burden or mood disorders.
References
- Lyne L et al. Sleep problems in rheumatoid arthritis over 12 years from diagnosis: results from the Swedish EIRA study. RMD Open. 2022;8:e001800 (Jan 5).
- Shamail GMH et al. Association between janus kinase inhibitors therapy and mental health outcome in rheumatoid arthritis: A systematic review and meta-analysis. Rheumatol Ther. 2021 (Dec 13).
- Ifesemen OS et al. Fatigue in early rheumatoid arthritis: data from the Early Rheumatoid Arthritis Network. Rheumatology (Oxford). 2021;keab861 (Dec 27).
- Doumen M et al. Psychosocial burden predicts sustained remission in early rheumatoid arthritis: unraveling the complex interplay of wellbeing and disease activity. Arthritis Care Res (Hoboken). 2021 (Dec 20).
Clinical Edge Journal Scan Commentary: PsA February 2022
Identifying risk factors associated with transition from cutaneous psoriasis to arthritic psoriasis remains a hot area of research. In a retrospective nested case-control study using the resources of the Rochester Epidemiology Project, Karmacharya et al1 identified 164 patients with incident PsA between 2000 and 2017. Among the 158 total patients satisfying study criteria, 64 (41%) had concurrent psoriasis and PsA and 94 (59%) had onset of psoriasis before PsA. The median time from psoriasis diagnosis to the incidence of PsA was 35.5 months with age at psoriasis onset (odds ratio [OR] per 10-year decrease 1.63; 95% CI 1.26-2.11) and its severity (OR for severe vs. mild 3.65; 95% CI 1.18-11.32) being associated with having a psoriasis diagnosis >1 year prior to incident PsA. Early onset as well as severe psoriasis is associated with the HLA- C*06 allele as is longer psoriasis-PsA latency. Although not evaluated in this study, this genetic factor, or other factors such as detection bias, may underly these observations.
Once diagnosed, stratification of PsA severity is important for planning treatment. Towards this goal, Dubash et al2 demonstrated that the presence of dactylitis indicates a more severe PsA phenotype. In a study of 177 disease-modifying antirheumatic drug (DMARD)-naive patients with early PsA, they found that those with dactylitis (46%) had significantly higher tender and swollen joint counts and C-reactive protein than those with non-dactylitic PsA. Ultrasound synovitis and erosions were also significantly more prevalent in dactylitic PsA. Thus, the presence of dactylitis indicates a more severe phenotype, and patients with dactylitis should be treated aggressively to improve long-term outcomes.
Novel therapies are being frequently evaluated in PsA and a recent target is interleukin (IL)-23, a key cytokine in the T-helper 17 (Th17) pathway and in the pathogenesis of psoriatic disease. Risankizumab is a novel monoclonal antibody targeting IL-23. In the double-blind phase 3 KEEPsAKE 1 study including 964 patients with active PsA and inadequate response to one or more conventional synthetic (cs) DMARDs. They were randomly assigned to receive 150 mg risankizumab or placebo, Kristensen et al3 demonstrated that, at week 24, at least a 20% improvement in the American College of Rheumatology score (ACR20) was achieved by a significantly higher proportion of patients receiving risankizumab vs. placebo (57.3% vs. 33.5%; P < .001). Treatment-emergent adverse events were mild-to-moderate and reported at similar frequencies in the risankizumab (40.4%) and placebo (38.7%) groups. Thus, risankizumab was efficacious in reducing clinical manifestations of PsA in patients with inadequate response to csDMARDs with no new adverse events. An important question when treating patients with PsA with targeted therapies is the need for concomitant therapy with csDMARDs. In a pooled analysis of 2 phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, 1,916 patients with active PsA with an inadequate response to ≥1 non-biologic (nb) DMARDs or biologic DMARDs were randomly assigned to placebo, 15 mg upadacitinib, or 30 mg upadacitinib as monotherapy or in combination with ≤2 nbDMARDs for 24 weeks, Nash et al4 demonstrated that at week 12, ACR20 response was achieved by a similar proportion of patients receiving 15 mg upadacitinib or 30 mg upadacitinib as monotherapy (15 mg: 33.7%; 95% CI 24.4%-43.1%; 30 mg: 45.7%; 95% CI 36.9%-54.5%) or combination therapy (15 mg: 34.0%; 95% CI 27.9%-40.1%; 30 mg: 39.6%; 95% CI 33.7%-45.5%). Adverse events were generally similar between monotherapy and combination therapy. Although, we don’t have information regarding the sustainability of the response, these data indicate that upadacitinib may be used without concomitant csDMARDs in PsA.
References
- Karmacharya P et al. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin Arthritis Rheum. 2021(Dec 31):S0049-0172(21)00230-4.
- Dubash S et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021(Dec 10):annrheumdis-2021-220964.
- Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022(Feb);81(2):225-231.
- Nash P et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2021(Dec 3):keab905.
Identifying risk factors associated with transition from cutaneous psoriasis to arthritic psoriasis remains a hot area of research. In a retrospective nested case-control study using the resources of the Rochester Epidemiology Project, Karmacharya et al1 identified 164 patients with incident PsA between 2000 and 2017. Among the 158 total patients satisfying study criteria, 64 (41%) had concurrent psoriasis and PsA and 94 (59%) had onset of psoriasis before PsA. The median time from psoriasis diagnosis to the incidence of PsA was 35.5 months with age at psoriasis onset (odds ratio [OR] per 10-year decrease 1.63; 95% CI 1.26-2.11) and its severity (OR for severe vs. mild 3.65; 95% CI 1.18-11.32) being associated with having a psoriasis diagnosis >1 year prior to incident PsA. Early onset as well as severe psoriasis is associated with the HLA- C*06 allele as is longer psoriasis-PsA latency. Although not evaluated in this study, this genetic factor, or other factors such as detection bias, may underly these observations.
Once diagnosed, stratification of PsA severity is important for planning treatment. Towards this goal, Dubash et al2 demonstrated that the presence of dactylitis indicates a more severe PsA phenotype. In a study of 177 disease-modifying antirheumatic drug (DMARD)-naive patients with early PsA, they found that those with dactylitis (46%) had significantly higher tender and swollen joint counts and C-reactive protein than those with non-dactylitic PsA. Ultrasound synovitis and erosions were also significantly more prevalent in dactylitic PsA. Thus, the presence of dactylitis indicates a more severe phenotype, and patients with dactylitis should be treated aggressively to improve long-term outcomes.
Novel therapies are being frequently evaluated in PsA and a recent target is interleukin (IL)-23, a key cytokine in the T-helper 17 (Th17) pathway and in the pathogenesis of psoriatic disease. Risankizumab is a novel monoclonal antibody targeting IL-23. In the double-blind phase 3 KEEPsAKE 1 study including 964 patients with active PsA and inadequate response to one or more conventional synthetic (cs) DMARDs. They were randomly assigned to receive 150 mg risankizumab or placebo, Kristensen et al3 demonstrated that, at week 24, at least a 20% improvement in the American College of Rheumatology score (ACR20) was achieved by a significantly higher proportion of patients receiving risankizumab vs. placebo (57.3% vs. 33.5%; P < .001). Treatment-emergent adverse events were mild-to-moderate and reported at similar frequencies in the risankizumab (40.4%) and placebo (38.7%) groups. Thus, risankizumab was efficacious in reducing clinical manifestations of PsA in patients with inadequate response to csDMARDs with no new adverse events. An important question when treating patients with PsA with targeted therapies is the need for concomitant therapy with csDMARDs. In a pooled analysis of 2 phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, 1,916 patients with active PsA with an inadequate response to ≥1 non-biologic (nb) DMARDs or biologic DMARDs were randomly assigned to placebo, 15 mg upadacitinib, or 30 mg upadacitinib as monotherapy or in combination with ≤2 nbDMARDs for 24 weeks, Nash et al4 demonstrated that at week 12, ACR20 response was achieved by a similar proportion of patients receiving 15 mg upadacitinib or 30 mg upadacitinib as monotherapy (15 mg: 33.7%; 95% CI 24.4%-43.1%; 30 mg: 45.7%; 95% CI 36.9%-54.5%) or combination therapy (15 mg: 34.0%; 95% CI 27.9%-40.1%; 30 mg: 39.6%; 95% CI 33.7%-45.5%). Adverse events were generally similar between monotherapy and combination therapy. Although, we don’t have information regarding the sustainability of the response, these data indicate that upadacitinib may be used without concomitant csDMARDs in PsA.
References
- Karmacharya P et al. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin Arthritis Rheum. 2021(Dec 31):S0049-0172(21)00230-4.
- Dubash S et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021(Dec 10):annrheumdis-2021-220964.
- Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022(Feb);81(2):225-231.
- Nash P et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2021(Dec 3):keab905.
Identifying risk factors associated with transition from cutaneous psoriasis to arthritic psoriasis remains a hot area of research. In a retrospective nested case-control study using the resources of the Rochester Epidemiology Project, Karmacharya et al1 identified 164 patients with incident PsA between 2000 and 2017. Among the 158 total patients satisfying study criteria, 64 (41%) had concurrent psoriasis and PsA and 94 (59%) had onset of psoriasis before PsA. The median time from psoriasis diagnosis to the incidence of PsA was 35.5 months with age at psoriasis onset (odds ratio [OR] per 10-year decrease 1.63; 95% CI 1.26-2.11) and its severity (OR for severe vs. mild 3.65; 95% CI 1.18-11.32) being associated with having a psoriasis diagnosis >1 year prior to incident PsA. Early onset as well as severe psoriasis is associated with the HLA- C*06 allele as is longer psoriasis-PsA latency. Although not evaluated in this study, this genetic factor, or other factors such as detection bias, may underly these observations.
Once diagnosed, stratification of PsA severity is important for planning treatment. Towards this goal, Dubash et al2 demonstrated that the presence of dactylitis indicates a more severe PsA phenotype. In a study of 177 disease-modifying antirheumatic drug (DMARD)-naive patients with early PsA, they found that those with dactylitis (46%) had significantly higher tender and swollen joint counts and C-reactive protein than those with non-dactylitic PsA. Ultrasound synovitis and erosions were also significantly more prevalent in dactylitic PsA. Thus, the presence of dactylitis indicates a more severe phenotype, and patients with dactylitis should be treated aggressively to improve long-term outcomes.
Novel therapies are being frequently evaluated in PsA and a recent target is interleukin (IL)-23, a key cytokine in the T-helper 17 (Th17) pathway and in the pathogenesis of psoriatic disease. Risankizumab is a novel monoclonal antibody targeting IL-23. In the double-blind phase 3 KEEPsAKE 1 study including 964 patients with active PsA and inadequate response to one or more conventional synthetic (cs) DMARDs. They were randomly assigned to receive 150 mg risankizumab or placebo, Kristensen et al3 demonstrated that, at week 24, at least a 20% improvement in the American College of Rheumatology score (ACR20) was achieved by a significantly higher proportion of patients receiving risankizumab vs. placebo (57.3% vs. 33.5%; P < .001). Treatment-emergent adverse events were mild-to-moderate and reported at similar frequencies in the risankizumab (40.4%) and placebo (38.7%) groups. Thus, risankizumab was efficacious in reducing clinical manifestations of PsA in patients with inadequate response to csDMARDs with no new adverse events. An important question when treating patients with PsA with targeted therapies is the need for concomitant therapy with csDMARDs. In a pooled analysis of 2 phase 3 trials, SELECT-PsA 1 and SELECT-PsA 2, 1,916 patients with active PsA with an inadequate response to ≥1 non-biologic (nb) DMARDs or biologic DMARDs were randomly assigned to placebo, 15 mg upadacitinib, or 30 mg upadacitinib as monotherapy or in combination with ≤2 nbDMARDs for 24 weeks, Nash et al4 demonstrated that at week 12, ACR20 response was achieved by a similar proportion of patients receiving 15 mg upadacitinib or 30 mg upadacitinib as monotherapy (15 mg: 33.7%; 95% CI 24.4%-43.1%; 30 mg: 45.7%; 95% CI 36.9%-54.5%) or combination therapy (15 mg: 34.0%; 95% CI 27.9%-40.1%; 30 mg: 39.6%; 95% CI 33.7%-45.5%). Adverse events were generally similar between monotherapy and combination therapy. Although, we don’t have information regarding the sustainability of the response, these data indicate that upadacitinib may be used without concomitant csDMARDs in PsA.
References
- Karmacharya P et al. Time to transition from psoriasis to psoriatic arthritis: A population-based study. Semin Arthritis Rheum. 2021(Dec 31):S0049-0172(21)00230-4.
- Dubash S et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann Rheum Dis. 2021(Dec 10):annrheumdis-2021-220964.
- Kristensen LE et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 KEEPsAKE 1 trial. Ann Rheum Dis. 2022(Feb);81(2):225-231.
- Nash P et al. Upadacitinib as monotherapy and in combination with non-biologic disease-modifying antirheumatic drugs for psoriatic arthritis. Rheumatology (Oxford). 2021(Dec 3):keab905.
Management of Opioid Use Disorder in Primary Care Settings With a Focus on Long-Acting Medication Formulations
After participating in the activity, PCPs should be able to:
- Assess a patient with possible signs and symptoms of opioid use disorder
- Identify criteria necessary to make a diagnosis of opioid use disorder
- Recognize factors that should be considered to tailor treatments for patients with opioid use disorder
- Select the best treatment option for patients with opioid use disorder
Click here to access this content now
After participating in the activity, PCPs should be able to:
- Assess a patient with possible signs and symptoms of opioid use disorder
- Identify criteria necessary to make a diagnosis of opioid use disorder
- Recognize factors that should be considered to tailor treatments for patients with opioid use disorder
- Select the best treatment option for patients with opioid use disorder
Click here to access this content now
After participating in the activity, PCPs should be able to:
- Assess a patient with possible signs and symptoms of opioid use disorder
- Identify criteria necessary to make a diagnosis of opioid use disorder
- Recognize factors that should be considered to tailor treatments for patients with opioid use disorder
- Select the best treatment option for patients with opioid use disorder
Click here to access this content now
Clinical Edge Journal Scan Commentary: Breast Cancer February 2022
Breast cancer diagnosis and treatment in young women can present unique challenges based on their life stage, including potential impact on fertility and future pregnancy. The role of GnRH analogues for ovarian protection during chemotherapy has been shown in both the POEMS-SWOG S0230 and PROMISE-GIM6 studies. Zong and colleagues conducted a phase 3 trial in China among premenopausal women with stage I-III breast cancer receiving cyclophosphamide-containing chemotherapy, with randomization to GnRHa + chemotherapy vs chemotherapy alone. Among 301 patients eligible for primary endpoint analysis, the premature ovarian insufficiency rate at 12 months was 10.3% for the GnRHa group vs 44.5% for the control group (odds ratio 0.23; P < 0.001). The rate of ovarian function recovery was also 46.4% higher in the GnRHa group. Furthermore, although survival outcomes were similar between groups, in patients <35 years of age, the tumor-free survival was higher in the GnRHa group vs control (93% vs 62%, P = 0.004) (Zong et al). These data reinforce the role of GnRHa as a means to reduce POI risk and support ovarian function recovery in young women undergoing chemotherapy for breast cancer. Measures of fertility and timing of pregnancy after breast cancer diagnosis continue to be areas of active research.
The treatment landscape for early-stage HER2-positive breast cancer continues to rapidly evolve with efforts to enhance efficacy and minimize toxicity for patients. The phase 3 KAITLIN study included 1846 patients with early-stage HER2-positive breast cancer (node-positive or node-negative, hormone receptor-negative and ≥T2 primary tumor) with randomization after surgery to adjuvant AC followed by taxane + trastuzumab + pertuzumab (AC-THP) or AC followed by T-DM1 + pertuzumab (AC-KP). In both the overall and node-positive populations, there was no significant difference in IDFS between the arms (stratified HR 0.98 and 0.97, respectively). In the overall population, the 3-year IDFS was 93.1% for AC-KP and 94.2% for AC-THP. Treatment completion rates were lower for AC-KP vs AC-THP (65.0% vs 88.4%), with T-DM1 discontinuation driven mostly by lab abnormalities (elevated liver function tests and thrombocytopenia) (Krop et al). Many patients diagnosed with early HER2-positive breast cancer (specifically those with tumors >2cm or node-positive) are treated with neoadjuvant chemotherapy + HER2-targeted therapy with subsequent tailoring of adjuvant treatment pending response, including use of T-DM1 if residual disease present. Future escalation and de-escalation strategies are being explored to further optimize outcomes and decrease side effects.
The addition of CDK 4/6 inhibitors to endocrine therapy has led to improved survival outcomes for patients diagnosed with advanced HR-positive-HER2-negative breast cancer. Lu and colleagues presented exploratory updated OS results among 672 patients with extended follow-up (median 53.5 months) from MONALEESA-7, which was a phase 3 randomized trial of ribociclib + endocrine therapy vs endocrine therapy alone among peri/pre-menopausal patients with HR-positive/HER2-negative advanced breast cancer. Median OS was 58.7 months vs 48.0 months for the ribociclib and placebo arms, respectively (HR 0.76), and a more pronounced benefit was seen in patients <40 years of age (median OS 51.3 months vs 40.5 months for ribociclib vs placebo arm; HR 0.65) (Lu et al). Furthermore, there was a significant delay in time to chemotherapy with ribociclib vs placebo (50.9 months vs 36.8 months; HR 0.69) which can certainly impact quality of life. A prior pooled analysis of the various MONALEESA trials demonstrated consistent PFS benefit with ribociclib across all intrinsic breast cancer subtypes, with the exception of basal-like and a more pronounced favorable impact in HER2-enriched. Future research to elucidate differences among CDK 4/6 inhibitors, influence of breast cancer subtype on their effect and how this can be translated to routine clinical practice are warranted.
Breast cancer diagnosis and treatment in young women can present unique challenges based on their life stage, including potential impact on fertility and future pregnancy. The role of GnRH analogues for ovarian protection during chemotherapy has been shown in both the POEMS-SWOG S0230 and PROMISE-GIM6 studies. Zong and colleagues conducted a phase 3 trial in China among premenopausal women with stage I-III breast cancer receiving cyclophosphamide-containing chemotherapy, with randomization to GnRHa + chemotherapy vs chemotherapy alone. Among 301 patients eligible for primary endpoint analysis, the premature ovarian insufficiency rate at 12 months was 10.3% for the GnRHa group vs 44.5% for the control group (odds ratio 0.23; P < 0.001). The rate of ovarian function recovery was also 46.4% higher in the GnRHa group. Furthermore, although survival outcomes were similar between groups, in patients <35 years of age, the tumor-free survival was higher in the GnRHa group vs control (93% vs 62%, P = 0.004) (Zong et al). These data reinforce the role of GnRHa as a means to reduce POI risk and support ovarian function recovery in young women undergoing chemotherapy for breast cancer. Measures of fertility and timing of pregnancy after breast cancer diagnosis continue to be areas of active research.
The treatment landscape for early-stage HER2-positive breast cancer continues to rapidly evolve with efforts to enhance efficacy and minimize toxicity for patients. The phase 3 KAITLIN study included 1846 patients with early-stage HER2-positive breast cancer (node-positive or node-negative, hormone receptor-negative and ≥T2 primary tumor) with randomization after surgery to adjuvant AC followed by taxane + trastuzumab + pertuzumab (AC-THP) or AC followed by T-DM1 + pertuzumab (AC-KP). In both the overall and node-positive populations, there was no significant difference in IDFS between the arms (stratified HR 0.98 and 0.97, respectively). In the overall population, the 3-year IDFS was 93.1% for AC-KP and 94.2% for AC-THP. Treatment completion rates were lower for AC-KP vs AC-THP (65.0% vs 88.4%), with T-DM1 discontinuation driven mostly by lab abnormalities (elevated liver function tests and thrombocytopenia) (Krop et al). Many patients diagnosed with early HER2-positive breast cancer (specifically those with tumors >2cm or node-positive) are treated with neoadjuvant chemotherapy + HER2-targeted therapy with subsequent tailoring of adjuvant treatment pending response, including use of T-DM1 if residual disease present. Future escalation and de-escalation strategies are being explored to further optimize outcomes and decrease side effects.
The addition of CDK 4/6 inhibitors to endocrine therapy has led to improved survival outcomes for patients diagnosed with advanced HR-positive-HER2-negative breast cancer. Lu and colleagues presented exploratory updated OS results among 672 patients with extended follow-up (median 53.5 months) from MONALEESA-7, which was a phase 3 randomized trial of ribociclib + endocrine therapy vs endocrine therapy alone among peri/pre-menopausal patients with HR-positive/HER2-negative advanced breast cancer. Median OS was 58.7 months vs 48.0 months for the ribociclib and placebo arms, respectively (HR 0.76), and a more pronounced benefit was seen in patients <40 years of age (median OS 51.3 months vs 40.5 months for ribociclib vs placebo arm; HR 0.65) (Lu et al). Furthermore, there was a significant delay in time to chemotherapy with ribociclib vs placebo (50.9 months vs 36.8 months; HR 0.69) which can certainly impact quality of life. A prior pooled analysis of the various MONALEESA trials demonstrated consistent PFS benefit with ribociclib across all intrinsic breast cancer subtypes, with the exception of basal-like and a more pronounced favorable impact in HER2-enriched. Future research to elucidate differences among CDK 4/6 inhibitors, influence of breast cancer subtype on their effect and how this can be translated to routine clinical practice are warranted.
Breast cancer diagnosis and treatment in young women can present unique challenges based on their life stage, including potential impact on fertility and future pregnancy. The role of GnRH analogues for ovarian protection during chemotherapy has been shown in both the POEMS-SWOG S0230 and PROMISE-GIM6 studies. Zong and colleagues conducted a phase 3 trial in China among premenopausal women with stage I-III breast cancer receiving cyclophosphamide-containing chemotherapy, with randomization to GnRHa + chemotherapy vs chemotherapy alone. Among 301 patients eligible for primary endpoint analysis, the premature ovarian insufficiency rate at 12 months was 10.3% for the GnRHa group vs 44.5% for the control group (odds ratio 0.23; P < 0.001). The rate of ovarian function recovery was also 46.4% higher in the GnRHa group. Furthermore, although survival outcomes were similar between groups, in patients <35 years of age, the tumor-free survival was higher in the GnRHa group vs control (93% vs 62%, P = 0.004) (Zong et al). These data reinforce the role of GnRHa as a means to reduce POI risk and support ovarian function recovery in young women undergoing chemotherapy for breast cancer. Measures of fertility and timing of pregnancy after breast cancer diagnosis continue to be areas of active research.
The treatment landscape for early-stage HER2-positive breast cancer continues to rapidly evolve with efforts to enhance efficacy and minimize toxicity for patients. The phase 3 KAITLIN study included 1846 patients with early-stage HER2-positive breast cancer (node-positive or node-negative, hormone receptor-negative and ≥T2 primary tumor) with randomization after surgery to adjuvant AC followed by taxane + trastuzumab + pertuzumab (AC-THP) or AC followed by T-DM1 + pertuzumab (AC-KP). In both the overall and node-positive populations, there was no significant difference in IDFS between the arms (stratified HR 0.98 and 0.97, respectively). In the overall population, the 3-year IDFS was 93.1% for AC-KP and 94.2% for AC-THP. Treatment completion rates were lower for AC-KP vs AC-THP (65.0% vs 88.4%), with T-DM1 discontinuation driven mostly by lab abnormalities (elevated liver function tests and thrombocytopenia) (Krop et al). Many patients diagnosed with early HER2-positive breast cancer (specifically those with tumors >2cm or node-positive) are treated with neoadjuvant chemotherapy + HER2-targeted therapy with subsequent tailoring of adjuvant treatment pending response, including use of T-DM1 if residual disease present. Future escalation and de-escalation strategies are being explored to further optimize outcomes and decrease side effects.
The addition of CDK 4/6 inhibitors to endocrine therapy has led to improved survival outcomes for patients diagnosed with advanced HR-positive-HER2-negative breast cancer. Lu and colleagues presented exploratory updated OS results among 672 patients with extended follow-up (median 53.5 months) from MONALEESA-7, which was a phase 3 randomized trial of ribociclib + endocrine therapy vs endocrine therapy alone among peri/pre-menopausal patients with HR-positive/HER2-negative advanced breast cancer. Median OS was 58.7 months vs 48.0 months for the ribociclib and placebo arms, respectively (HR 0.76), and a more pronounced benefit was seen in patients <40 years of age (median OS 51.3 months vs 40.5 months for ribociclib vs placebo arm; HR 0.65) (Lu et al). Furthermore, there was a significant delay in time to chemotherapy with ribociclib vs placebo (50.9 months vs 36.8 months; HR 0.69) which can certainly impact quality of life. A prior pooled analysis of the various MONALEESA trials demonstrated consistent PFS benefit with ribociclib across all intrinsic breast cancer subtypes, with the exception of basal-like and a more pronounced favorable impact in HER2-enriched. Future research to elucidate differences among CDK 4/6 inhibitors, influence of breast cancer subtype on their effect and how this can be translated to routine clinical practice are warranted.
Upadacitinib inhibits structural joint damage progression in RA
Key clinical point: Upadacitinib alone or in combination with methotrexate inhibits structural joint damage progression over a year in patients with rheumatoid arthritis (RA).
Major finding: The mean change in modified total Sharp score (mTSS) at 1 year for 15 mg upadacitinib and 30 mg upadacitinib vs. methotrexate was 0.03 and 0.14 vs. 1.00 (all P < .001). In methotrexate-inadequate responders (IR), mean change in mTSS with 15 mg upadacitinib vs. placebo (both with background methotrexate) was 0.28 vs. 1.73 (P < .001).
Study details: This was an analysis of 2 phase 3 trials involving patients with active RA who were either methotrexate-naive (SELECT-EARLY) and were randomly assigned to methotrexate monotherapy, 15 mg upadacitinib, or 30 mg upadacitinib or were methotrexate-IR (SELECT-COMPARE) and were randomly assigned to either 15 mg upadacitinib, 40 mg adalimumab, or placebo, all with background methotrexate.
Disclosures: This study was funded by AbbVie. IH Song, A Friedman, T Shaw, Y Li, S Chen, and JV Enejosa reported being full-time employees or owning stocks/stock options of AbbVie, and others reported receiving research grants and consultancy or speakers fees from various sources including AbbVie.
Source: Peterfy CG et al. Rheumatology (Oxford). 2021;keab861 (Dec 13). Doi: 10.1093/rheumatology/keab861.
Key clinical point: Upadacitinib alone or in combination with methotrexate inhibits structural joint damage progression over a year in patients with rheumatoid arthritis (RA).
Major finding: The mean change in modified total Sharp score (mTSS) at 1 year for 15 mg upadacitinib and 30 mg upadacitinib vs. methotrexate was 0.03 and 0.14 vs. 1.00 (all P < .001). In methotrexate-inadequate responders (IR), mean change in mTSS with 15 mg upadacitinib vs. placebo (both with background methotrexate) was 0.28 vs. 1.73 (P < .001).
Study details: This was an analysis of 2 phase 3 trials involving patients with active RA who were either methotrexate-naive (SELECT-EARLY) and were randomly assigned to methotrexate monotherapy, 15 mg upadacitinib, or 30 mg upadacitinib or were methotrexate-IR (SELECT-COMPARE) and were randomly assigned to either 15 mg upadacitinib, 40 mg adalimumab, or placebo, all with background methotrexate.
Disclosures: This study was funded by AbbVie. IH Song, A Friedman, T Shaw, Y Li, S Chen, and JV Enejosa reported being full-time employees or owning stocks/stock options of AbbVie, and others reported receiving research grants and consultancy or speakers fees from various sources including AbbVie.
Source: Peterfy CG et al. Rheumatology (Oxford). 2021;keab861 (Dec 13). Doi: 10.1093/rheumatology/keab861.
Key clinical point: Upadacitinib alone or in combination with methotrexate inhibits structural joint damage progression over a year in patients with rheumatoid arthritis (RA).
Major finding: The mean change in modified total Sharp score (mTSS) at 1 year for 15 mg upadacitinib and 30 mg upadacitinib vs. methotrexate was 0.03 and 0.14 vs. 1.00 (all P < .001). In methotrexate-inadequate responders (IR), mean change in mTSS with 15 mg upadacitinib vs. placebo (both with background methotrexate) was 0.28 vs. 1.73 (P < .001).
Study details: This was an analysis of 2 phase 3 trials involving patients with active RA who were either methotrexate-naive (SELECT-EARLY) and were randomly assigned to methotrexate monotherapy, 15 mg upadacitinib, or 30 mg upadacitinib or were methotrexate-IR (SELECT-COMPARE) and were randomly assigned to either 15 mg upadacitinib, 40 mg adalimumab, or placebo, all with background methotrexate.
Disclosures: This study was funded by AbbVie. IH Song, A Friedman, T Shaw, Y Li, S Chen, and JV Enejosa reported being full-time employees or owning stocks/stock options of AbbVie, and others reported receiving research grants and consultancy or speakers fees from various sources including AbbVie.
Source: Peterfy CG et al. Rheumatology (Oxford). 2021;keab861 (Dec 13). Doi: 10.1093/rheumatology/keab861.
Upadacitinib inhibits structural joint damage progression in RA
Key clinical point: Upadacitinib alone or in combination with methotrexate inhibits structural joint damage progression over a year in patients with rheumatoid arthritis (RA).
Major finding: The mean change in modified total Sharp score (mTSS) at 1 year for 15 mg upadacitinib and 30 mg upadacitinib vs. methotrexate was 0.03 and 0.14 vs. 1.00 (all P < .001). In methotrexate-inadequate responders (IR), mean change in mTSS with 15 mg upadacitinib vs. placebo (both with background methotrexate) was 0.28 vs. 1.73 (P < .001).
Study details: This was an analysis of 2 phase 3 trials involving patients with active RA who were either methotrexate-naive (SELECT-EARLY) and were randomly assigned to methotrexate monotherapy, 15 mg upadacitinib, or 30 mg upadacitinib or were methotrexate-IR (SELECT-COMPARE) and were randomly assigned to either 15 mg upadacitinib, 40 mg adalimumab, or placebo, all with background methotrexate.
Disclosures: This study was funded by AbbVie. IH Song, A Friedman, T Shaw, Y Li, S Chen, and JV Enejosa reported being full-time employees or owning stocks/stock options of AbbVie, and others reported receiving research grants and consultancy or speakers fees from various sources including AbbVie.
Source: Peterfy CG et al. Rheumatology (Oxford). 2021;keab861 (Dec 13). Doi: 10.1093/rheumatology/keab861.
Key clinical point: Upadacitinib alone or in combination with methotrexate inhibits structural joint damage progression over a year in patients with rheumatoid arthritis (RA).
Major finding: The mean change in modified total Sharp score (mTSS) at 1 year for 15 mg upadacitinib and 30 mg upadacitinib vs. methotrexate was 0.03 and 0.14 vs. 1.00 (all P < .001). In methotrexate-inadequate responders (IR), mean change in mTSS with 15 mg upadacitinib vs. placebo (both with background methotrexate) was 0.28 vs. 1.73 (P < .001).
Study details: This was an analysis of 2 phase 3 trials involving patients with active RA who were either methotrexate-naive (SELECT-EARLY) and were randomly assigned to methotrexate monotherapy, 15 mg upadacitinib, or 30 mg upadacitinib or were methotrexate-IR (SELECT-COMPARE) and were randomly assigned to either 15 mg upadacitinib, 40 mg adalimumab, or placebo, all with background methotrexate.
Disclosures: This study was funded by AbbVie. IH Song, A Friedman, T Shaw, Y Li, S Chen, and JV Enejosa reported being full-time employees or owning stocks/stock options of AbbVie, and others reported receiving research grants and consultancy or speakers fees from various sources including AbbVie.
Source: Peterfy CG et al. Rheumatology (Oxford). 2021;keab861 (Dec 13). Doi: 10.1093/rheumatology/keab861.
Key clinical point: Upadacitinib alone or in combination with methotrexate inhibits structural joint damage progression over a year in patients with rheumatoid arthritis (RA).
Major finding: The mean change in modified total Sharp score (mTSS) at 1 year for 15 mg upadacitinib and 30 mg upadacitinib vs. methotrexate was 0.03 and 0.14 vs. 1.00 (all P < .001). In methotrexate-inadequate responders (IR), mean change in mTSS with 15 mg upadacitinib vs. placebo (both with background methotrexate) was 0.28 vs. 1.73 (P < .001).
Study details: This was an analysis of 2 phase 3 trials involving patients with active RA who were either methotrexate-naive (SELECT-EARLY) and were randomly assigned to methotrexate monotherapy, 15 mg upadacitinib, or 30 mg upadacitinib or were methotrexate-IR (SELECT-COMPARE) and were randomly assigned to either 15 mg upadacitinib, 40 mg adalimumab, or placebo, all with background methotrexate.
Disclosures: This study was funded by AbbVie. IH Song, A Friedman, T Shaw, Y Li, S Chen, and JV Enejosa reported being full-time employees or owning stocks/stock options of AbbVie, and others reported receiving research grants and consultancy or speakers fees from various sources including AbbVie.
Source: Peterfy CG et al. Rheumatology (Oxford). 2021;keab861 (Dec 13). Doi: 10.1093/rheumatology/keab861.
JAK inhibitors result in clinically relevant improvement in mental health in RA
Key clinical point: A clinically noteworthy improvement in mental health was observed in patients with rheumatoid arthritis (RA) who were treated with Janus kinase (JAK) inhibitors.
Major finding: The pooled incremental mean reduction in short form-36 mental component score with JAK monotherapy was 4.95 (95% CI 4.41-5.48), which was greater than the minimum clinically important difference value of 2.5, indicating significant improvement in mental health following JAK monotherapy.
Study details: This was a meta-analysis of 19 studies involving 14,323 patients with RA.
Disclosures: This study received no specific funding. The authors did not have any potential conflict of interests.
Source: Shamail GMH et al. Rheumatol Ther. 2021 (Dec 13). Doi: 10.1007/s40744-021-00409-6.
Key clinical point: A clinically noteworthy improvement in mental health was observed in patients with rheumatoid arthritis (RA) who were treated with Janus kinase (JAK) inhibitors.
Major finding: The pooled incremental mean reduction in short form-36 mental component score with JAK monotherapy was 4.95 (95% CI 4.41-5.48), which was greater than the minimum clinically important difference value of 2.5, indicating significant improvement in mental health following JAK monotherapy.
Study details: This was a meta-analysis of 19 studies involving 14,323 patients with RA.
Disclosures: This study received no specific funding. The authors did not have any potential conflict of interests.
Source: Shamail GMH et al. Rheumatol Ther. 2021 (Dec 13). Doi: 10.1007/s40744-021-00409-6.
Key clinical point: A clinically noteworthy improvement in mental health was observed in patients with rheumatoid arthritis (RA) who were treated with Janus kinase (JAK) inhibitors.
Major finding: The pooled incremental mean reduction in short form-36 mental component score with JAK monotherapy was 4.95 (95% CI 4.41-5.48), which was greater than the minimum clinically important difference value of 2.5, indicating significant improvement in mental health following JAK monotherapy.
Study details: This was a meta-analysis of 19 studies involving 14,323 patients with RA.
Disclosures: This study received no specific funding. The authors did not have any potential conflict of interests.
Source: Shamail GMH et al. Rheumatol Ther. 2021 (Dec 13). Doi: 10.1007/s40744-021-00409-6.
High psychosocial burden tied to early loss of remission in RA
Key clinical point: In a cohort of patients with early rheumatoid arthritis (RA), illness perceptions and suboptimal psychosocial well-being were associated with a lower likelihood of sustained remission.
Major finding: Among patients who were had a disease activity score in 28 joints-C-reactive protein remission at week 16, those with a low vs. high psychosocial burden profile showed a significantly longer time to first loss-of-remission (hazard ratio 0.51; P < .001).
Study details: This was a post hoc analysis of the CareRA trial involving 379 patients with early RA who received methotrexate ± additional conventional synthetic disease-modifying antirheumatic drugs or glucocorticoids.
Disclosures: This study was supported in part by a Strategic Basic Research Fellowship grant from Fonds Wetenschappelijk Onderzoek. All the authors declared no conflicts of interest.
Source: Doumen M et al. Arthritis Care Res (Hoboken). 2021 (Dec 20). Doi: 10.1002/acr.24847.
Key clinical point: In a cohort of patients with early rheumatoid arthritis (RA), illness perceptions and suboptimal psychosocial well-being were associated with a lower likelihood of sustained remission.
Major finding: Among patients who were had a disease activity score in 28 joints-C-reactive protein remission at week 16, those with a low vs. high psychosocial burden profile showed a significantly longer time to first loss-of-remission (hazard ratio 0.51; P < .001).
Study details: This was a post hoc analysis of the CareRA trial involving 379 patients with early RA who received methotrexate ± additional conventional synthetic disease-modifying antirheumatic drugs or glucocorticoids.
Disclosures: This study was supported in part by a Strategic Basic Research Fellowship grant from Fonds Wetenschappelijk Onderzoek. All the authors declared no conflicts of interest.
Source: Doumen M et al. Arthritis Care Res (Hoboken). 2021 (Dec 20). Doi: 10.1002/acr.24847.
Key clinical point: In a cohort of patients with early rheumatoid arthritis (RA), illness perceptions and suboptimal psychosocial well-being were associated with a lower likelihood of sustained remission.
Major finding: Among patients who were had a disease activity score in 28 joints-C-reactive protein remission at week 16, those with a low vs. high psychosocial burden profile showed a significantly longer time to first loss-of-remission (hazard ratio 0.51; P < .001).
Study details: This was a post hoc analysis of the CareRA trial involving 379 patients with early RA who received methotrexate ± additional conventional synthetic disease-modifying antirheumatic drugs or glucocorticoids.
Disclosures: This study was supported in part by a Strategic Basic Research Fellowship grant from Fonds Wetenschappelijk Onderzoek. All the authors declared no conflicts of interest.
Source: Doumen M et al. Arthritis Care Res (Hoboken). 2021 (Dec 20). Doi: 10.1002/acr.24847.