User login
Cough and moderate hoarseness
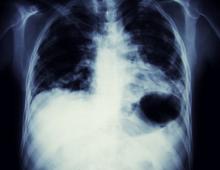
Based on the patient's presentation, history, and imaging results, the likely diagnosis is non–small cell lung cancer (NSCLC) of an adenocarcinoma subtype. NSCLC accounts for about 80% of all lung cancer cases. Adenocarcinoma, in particular, is the most common type of lung cancer in the United States, accounting for about 40% of cases. This subtype is also the most common histology among nonsmokers. Still, individuals aged 55 to 77 years with a smoking history of 30 pack-years or more are considered to be the highest-risk group for lung cancer; those who quit less than 15 years ago — like the patient in the present case — are still considered to be in this risk group. Most cases of lung cancer are diagnosed at a late stage when symptoms have already begun to manifest. However, it should be noted that women are more likely to develop adenocarcinoma, are generally younger when they present with symptoms, and are more likely to present with localized disease. It remains to be proven whether the use of HRT affects the risk for lung cancer in women. Deaths from lung cancer, and in particular NSCLC, were shown to be higher among patients undergoing HRT, though no increase in lung cancer death was reported in women receiving estrogen alone.
In addition to the imaging described in this case, workup for NSCLC should include immunohistochemical (IHC) analyses to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether anaplastic lymphoma kinase inhibitor therapy or programmed death-ligand 1 inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. Management of NSCLC is primarily informed by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes. KRAS mutations, unlike EGFR mutations, are associated with a history of smoking and are considered prognostic biomarkers. Because overlapping targetable alterations are uncommon, patients who are confirmed to be harboring KRAS mutations will likely not benefit from additional molecular testing. Presence of the KRAS mutation suggests a poor response to EGFR tyrosine kinase inhibitors, though it does not appear to impact chemotherapeutic efficacy. Although no targeted therapies are yet available for this population, immune checkpoint inhibitors appear to be beneficial. National Comprehensive Cancer Network guidelines advise that all patients with adenocarcinoma be tested for EGFR mutations and that DNA mutational analysis is the preferred method.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Based on the patient's presentation, history, and imaging results, the likely diagnosis is non–small cell lung cancer (NSCLC) of an adenocarcinoma subtype. NSCLC accounts for about 80% of all lung cancer cases. Adenocarcinoma, in particular, is the most common type of lung cancer in the United States, accounting for about 40% of cases. This subtype is also the most common histology among nonsmokers. Still, individuals aged 55 to 77 years with a smoking history of 30 pack-years or more are considered to be the highest-risk group for lung cancer; those who quit less than 15 years ago — like the patient in the present case — are still considered to be in this risk group. Most cases of lung cancer are diagnosed at a late stage when symptoms have already begun to manifest. However, it should be noted that women are more likely to develop adenocarcinoma, are generally younger when they present with symptoms, and are more likely to present with localized disease. It remains to be proven whether the use of HRT affects the risk for lung cancer in women. Deaths from lung cancer, and in particular NSCLC, were shown to be higher among patients undergoing HRT, though no increase in lung cancer death was reported in women receiving estrogen alone.
In addition to the imaging described in this case, workup for NSCLC should include immunohistochemical (IHC) analyses to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether anaplastic lymphoma kinase inhibitor therapy or programmed death-ligand 1 inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. Management of NSCLC is primarily informed by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes. KRAS mutations, unlike EGFR mutations, are associated with a history of smoking and are considered prognostic biomarkers. Because overlapping targetable alterations are uncommon, patients who are confirmed to be harboring KRAS mutations will likely not benefit from additional molecular testing. Presence of the KRAS mutation suggests a poor response to EGFR tyrosine kinase inhibitors, though it does not appear to impact chemotherapeutic efficacy. Although no targeted therapies are yet available for this population, immune checkpoint inhibitors appear to be beneficial. National Comprehensive Cancer Network guidelines advise that all patients with adenocarcinoma be tested for EGFR mutations and that DNA mutational analysis is the preferred method.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Based on the patient's presentation, history, and imaging results, the likely diagnosis is non–small cell lung cancer (NSCLC) of an adenocarcinoma subtype. NSCLC accounts for about 80% of all lung cancer cases. Adenocarcinoma, in particular, is the most common type of lung cancer in the United States, accounting for about 40% of cases. This subtype is also the most common histology among nonsmokers. Still, individuals aged 55 to 77 years with a smoking history of 30 pack-years or more are considered to be the highest-risk group for lung cancer; those who quit less than 15 years ago — like the patient in the present case — are still considered to be in this risk group. Most cases of lung cancer are diagnosed at a late stage when symptoms have already begun to manifest. However, it should be noted that women are more likely to develop adenocarcinoma, are generally younger when they present with symptoms, and are more likely to present with localized disease. It remains to be proven whether the use of HRT affects the risk for lung cancer in women. Deaths from lung cancer, and in particular NSCLC, were shown to be higher among patients undergoing HRT, though no increase in lung cancer death was reported in women receiving estrogen alone.
In addition to the imaging described in this case, workup for NSCLC should include immunohistochemical (IHC) analyses to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether anaplastic lymphoma kinase inhibitor therapy or programmed death-ligand 1 inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. Management of NSCLC is primarily informed by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes. KRAS mutations, unlike EGFR mutations, are associated with a history of smoking and are considered prognostic biomarkers. Because overlapping targetable alterations are uncommon, patients who are confirmed to be harboring KRAS mutations will likely not benefit from additional molecular testing. Presence of the KRAS mutation suggests a poor response to EGFR tyrosine kinase inhibitors, though it does not appear to impact chemotherapeutic efficacy. Although no targeted therapies are yet available for this population, immune checkpoint inhibitors appear to be beneficial. National Comprehensive Cancer Network guidelines advise that all patients with adenocarcinoma be tested for EGFR mutations and that DNA mutational analysis is the preferred method.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
A 56-year-old woman presents with dyspnea, a persistent cough, and moderate hoarseness. She has no significant medical history other than thyroiditis. Her current medications include hormone replacement therapy (HRT). Although the patient reports a 20–pack-year history of smoking tobacco, she notes that she quit 11 years ago and has not been previously screened for lung cancer. A chest radiograph is ordered, which demonstrates a mass in the upper lobe of the right lung.
Type 2 Diabetes Comorbidities
Migraine Pathophysiology
Infectious disease pop quiz: Clinical challenge #18 for the ObGyn
What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
Continue to the answer...
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
Continue to the answer...
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
Continue to the answer...
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
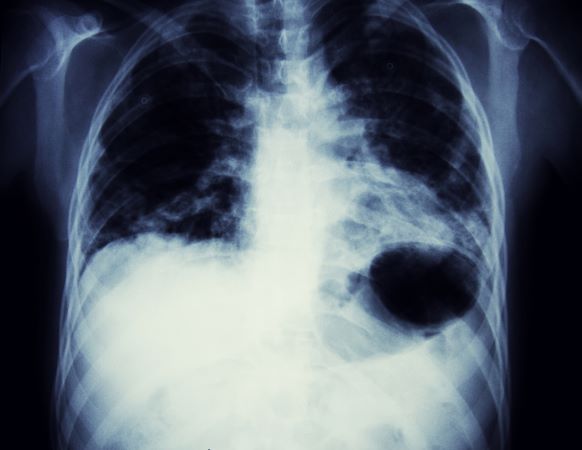
Labial growth
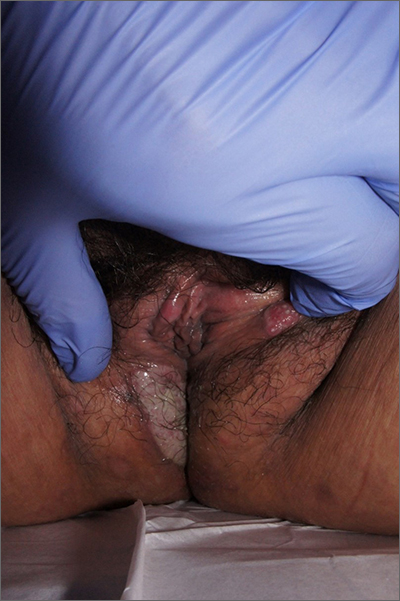
White-to-pink friable plaques occurring acutely in the vulva is concerning for one form of secondary syphilis that affects mucous membranes: condyloma lata.
Known as the great imitator for its variety of clinical presentations, syphilis is a sexually transmitted infection (STI) caused by the spirochete Treponema pallidum. Three to 10 days following contact with the spirochete, a painless ulcer or chancre forms and subsequently resolves—sometimes without notice.
Secondary syphilis develops from hematogenous spread of bacteria taking many forms—most commonly a widespread rash over the whole body of many (although sometimes faint) macules or papules up to about 1 cm in size and haphazardly spread out about every 1 cm. Palms and soles may be affected, even if faintly. Another, less common form of secondary syphilis includes the friable plaques (often in the anogenital area, as pictured) that are highly concentrated with bacteria. These occur 3 to 12 weeks after the appearance of a primary chancre and are variably symptomatic.
The differential diagnosis includes genital warts, vulvar carcinoma, and pemphigus vegetans. The relatively rapid, multifocal presentation helps to separate this disorder from vulvar carcinoma. A biopsy can distinguish the 2. However, diagnosis is better made with serology using nontreponemal tests, such as the rapid plasma reagin (RPR) test. Treponemal tests (assaying immunoglobulin [Ig]M and IgG to Treponema pallidum) are also an option and are very specific. Following this, an RPR titer can help guide treatment. Darkfield microscopy, which can reveal spirochetes directly, isn’t readily available but could be used to diagnose condyloma lata.
Patients who have been given a diagnosis of syphilis should be offered screening for other STIs, including HIV. Anyone who has had sexual contact with the patient within the previous 90 days should be notified, tested, and treated. Patients with primary or secondary syphilis should be treated with 2.4 million units of intramuscular (IM) benzathine penicillin G in a single dose—regardless of whether they test positive for HIV. To exclude tertiary syphilis, a careful neurologic exam should take place at the time of diagnosis and again 6 and 12 months after treatment (sooner if follow-up may be uncertain). Consider treatment failure if RPR titers haven’t fallen fourfold in 12 months. In 2022, the Centers for Disease Control and Prevention released a notice that COVID-19-vaccinated patients may have false-positive RPR titers performed from Bio-Rad Laboratories (BioPlex 2200 Syphilis Total & RPR kit).1
In this case, the patient tested positive for treponemal antibodies and had an RPR titer of 1:128. She was treated with IM benzathine penicillin with lasting clearance.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Centers for Disease Control and Prevention. Sexually Transmitted Infection Treatment Guidelines, 2021. Reviewed December 22, 2021. Accessed February 25, 2022. www.cdc.gov/std/treatment-guidelines/syphilis.htm

White-to-pink friable plaques occurring acutely in the vulva is concerning for one form of secondary syphilis that affects mucous membranes: condyloma lata.
Known as the great imitator for its variety of clinical presentations, syphilis is a sexually transmitted infection (STI) caused by the spirochete Treponema pallidum. Three to 10 days following contact with the spirochete, a painless ulcer or chancre forms and subsequently resolves—sometimes without notice.
Secondary syphilis develops from hematogenous spread of bacteria taking many forms—most commonly a widespread rash over the whole body of many (although sometimes faint) macules or papules up to about 1 cm in size and haphazardly spread out about every 1 cm. Palms and soles may be affected, even if faintly. Another, less common form of secondary syphilis includes the friable plaques (often in the anogenital area, as pictured) that are highly concentrated with bacteria. These occur 3 to 12 weeks after the appearance of a primary chancre and are variably symptomatic.
The differential diagnosis includes genital warts, vulvar carcinoma, and pemphigus vegetans. The relatively rapid, multifocal presentation helps to separate this disorder from vulvar carcinoma. A biopsy can distinguish the 2. However, diagnosis is better made with serology using nontreponemal tests, such as the rapid plasma reagin (RPR) test. Treponemal tests (assaying immunoglobulin [Ig]M and IgG to Treponema pallidum) are also an option and are very specific. Following this, an RPR titer can help guide treatment. Darkfield microscopy, which can reveal spirochetes directly, isn’t readily available but could be used to diagnose condyloma lata.
Patients who have been given a diagnosis of syphilis should be offered screening for other STIs, including HIV. Anyone who has had sexual contact with the patient within the previous 90 days should be notified, tested, and treated. Patients with primary or secondary syphilis should be treated with 2.4 million units of intramuscular (IM) benzathine penicillin G in a single dose—regardless of whether they test positive for HIV. To exclude tertiary syphilis, a careful neurologic exam should take place at the time of diagnosis and again 6 and 12 months after treatment (sooner if follow-up may be uncertain). Consider treatment failure if RPR titers haven’t fallen fourfold in 12 months. In 2022, the Centers for Disease Control and Prevention released a notice that COVID-19-vaccinated patients may have false-positive RPR titers performed from Bio-Rad Laboratories (BioPlex 2200 Syphilis Total & RPR kit).1
In this case, the patient tested positive for treponemal antibodies and had an RPR titer of 1:128. She was treated with IM benzathine penicillin with lasting clearance.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

White-to-pink friable plaques occurring acutely in the vulva is concerning for one form of secondary syphilis that affects mucous membranes: condyloma lata.
Known as the great imitator for its variety of clinical presentations, syphilis is a sexually transmitted infection (STI) caused by the spirochete Treponema pallidum. Three to 10 days following contact with the spirochete, a painless ulcer or chancre forms and subsequently resolves—sometimes without notice.
Secondary syphilis develops from hematogenous spread of bacteria taking many forms—most commonly a widespread rash over the whole body of many (although sometimes faint) macules or papules up to about 1 cm in size and haphazardly spread out about every 1 cm. Palms and soles may be affected, even if faintly. Another, less common form of secondary syphilis includes the friable plaques (often in the anogenital area, as pictured) that are highly concentrated with bacteria. These occur 3 to 12 weeks after the appearance of a primary chancre and are variably symptomatic.
The differential diagnosis includes genital warts, vulvar carcinoma, and pemphigus vegetans. The relatively rapid, multifocal presentation helps to separate this disorder from vulvar carcinoma. A biopsy can distinguish the 2. However, diagnosis is better made with serology using nontreponemal tests, such as the rapid plasma reagin (RPR) test. Treponemal tests (assaying immunoglobulin [Ig]M and IgG to Treponema pallidum) are also an option and are very specific. Following this, an RPR titer can help guide treatment. Darkfield microscopy, which can reveal spirochetes directly, isn’t readily available but could be used to diagnose condyloma lata.
Patients who have been given a diagnosis of syphilis should be offered screening for other STIs, including HIV. Anyone who has had sexual contact with the patient within the previous 90 days should be notified, tested, and treated. Patients with primary or secondary syphilis should be treated with 2.4 million units of intramuscular (IM) benzathine penicillin G in a single dose—regardless of whether they test positive for HIV. To exclude tertiary syphilis, a careful neurologic exam should take place at the time of diagnosis and again 6 and 12 months after treatment (sooner if follow-up may be uncertain). Consider treatment failure if RPR titers haven’t fallen fourfold in 12 months. In 2022, the Centers for Disease Control and Prevention released a notice that COVID-19-vaccinated patients may have false-positive RPR titers performed from Bio-Rad Laboratories (BioPlex 2200 Syphilis Total & RPR kit).1
In this case, the patient tested positive for treponemal antibodies and had an RPR titer of 1:128. She was treated with IM benzathine penicillin with lasting clearance.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Centers for Disease Control and Prevention. Sexually Transmitted Infection Treatment Guidelines, 2021. Reviewed December 22, 2021. Accessed February 25, 2022. www.cdc.gov/std/treatment-guidelines/syphilis.htm
1. Centers for Disease Control and Prevention. Sexually Transmitted Infection Treatment Guidelines, 2021. Reviewed December 22, 2021. Accessed February 25, 2022. www.cdc.gov/std/treatment-guidelines/syphilis.htm
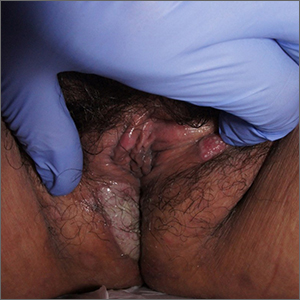
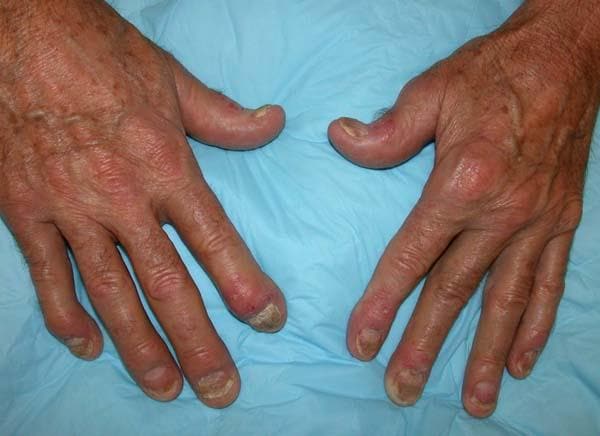
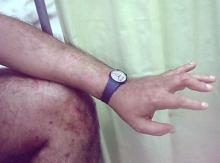
Painful swelling of fingers and toe
Although psoriatic arthritis is not the only disease associated with dactylitis — other culprits are sarcoidosis, septic arthritis, tuberculosis, and gout — dactylitis is one of the characteristic symptoms of psoriatic arthritis. Dactylitis is seen in as many as 35% of patients with psoriatic disease. Dactylitis clinically presents — as in this patient — with sausage-like swelling of the digits. It is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as one of the hallmarks of psoriatic arthritis.
Dactylitis has been thought to be a result of the concomitant swelling and inflammation of the flexor tendon sheaths of the metacarpophalangeal, metatarsophalangeal, or interphalangeal joints. Flexor tenosynovitis can be detected by examination with MRI and ultrasound. Dactylitis is associated with radiologically evident erosive damage to the joints.
Patients with psoriatic arthritis are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with psoriatic arthritis do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent, but rather indicates that different markers are needed that allow better quantification of systemic inflammation in psoriasis and psoriatic arthritis.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Although psoriatic arthritis is not the only disease associated with dactylitis — other culprits are sarcoidosis, septic arthritis, tuberculosis, and gout — dactylitis is one of the characteristic symptoms of psoriatic arthritis. Dactylitis is seen in as many as 35% of patients with psoriatic disease. Dactylitis clinically presents — as in this patient — with sausage-like swelling of the digits. It is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as one of the hallmarks of psoriatic arthritis.
Dactylitis has been thought to be a result of the concomitant swelling and inflammation of the flexor tendon sheaths of the metacarpophalangeal, metatarsophalangeal, or interphalangeal joints. Flexor tenosynovitis can be detected by examination with MRI and ultrasound. Dactylitis is associated with radiologically evident erosive damage to the joints.
Patients with psoriatic arthritis are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with psoriatic arthritis do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent, but rather indicates that different markers are needed that allow better quantification of systemic inflammation in psoriasis and psoriatic arthritis.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Although psoriatic arthritis is not the only disease associated with dactylitis — other culprits are sarcoidosis, septic arthritis, tuberculosis, and gout — dactylitis is one of the characteristic symptoms of psoriatic arthritis. Dactylitis is seen in as many as 35% of patients with psoriatic disease. Dactylitis clinically presents — as in this patient — with sausage-like swelling of the digits. It is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as one of the hallmarks of psoriatic arthritis.
Dactylitis has been thought to be a result of the concomitant swelling and inflammation of the flexor tendon sheaths of the metacarpophalangeal, metatarsophalangeal, or interphalangeal joints. Flexor tenosynovitis can be detected by examination with MRI and ultrasound. Dactylitis is associated with radiologically evident erosive damage to the joints.
Patients with psoriatic arthritis are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with psoriatic arthritis do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent, but rather indicates that different markers are needed that allow better quantification of systemic inflammation in psoriasis and psoriatic arthritis.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
A 35-year-old man presents with painful swelling of his right index and ring fingers as well as the fourth toe on his right foot, which has persisted for 5 days. He cannot perform his daily activities owing to severe pain in the affected fingers and toes. His medical history is unremarkable. His paternal uncle had psoriasis, which was successfully treated with adalimumab.
Physical assessment reveals tender, fusiform, swollen soft tissues in the affected fingertips, the fourth toe, and swollen palms. Nails are pitted. Hand radiography reveals mild edema of the soft tissue of the index and ring fingers but no significant joint abnormalities. Enthesitis is not present. Laboratory tests reveal a negative human leukocyte antigen B27 (HLA-B27) test, negative rheumatoid factor, negative antinuclear antibody, and normal C-reactive protein.
Dactylitis was diagnosed on the basis of clinical symptoms, radiographic results, and laboratory findings.
Intermittent joint aches
Fundamental changes in the initial pharmacologic approach to psoriatic arthritis were made in the 2018 American College Rheumatology/National Psoriasis (ACR/NPF) guidelines for the treatment of psoriatic arthritis. Previously, methotrexate had been widely used as the first-line agent. The 2018 guidelines recommend a tumor necrosis factor (TNF) inhibitor over methotrexate and other oral small molecules (leflunomide, cyclosporine, and apremilast).
Herein is a broad summary of the guidelines:
· Treat with TNF inhibitor over oral small molecule; may consider oral small molecule with mild psoriatic arthritis and psoriasis, patient preference, and/or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with oral small molecule over IL-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis and/or psoriatic arthritis
· Treat with oral small molecule over IL-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or psoriatic arthritis, or concomitant inflammatory bowel disease
· Treat with methotrexate over nonsteroidal anti-inflammatory drugs; may consider nonsteroidals for mild psoriatic arthritis and psoriasis
· Treat with IL-17 inhibitor over IL-12/23 inhibitor; may consider IL-12/23 inhibitor in a patient with concomitant inflammatory bowel disease
Note that these recommendations are based on conditional evidence (ie, low to very low quality). In fact, in the entire guideline document, only 6% of the recommendations are strong, whereas 96% are conditional. This emphasizes the importance of evaluating each patient individually and engaging in a discussion to choose optimal therapy.
Another set of guidelines, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), was last updated in 2015. Since then, many of the agents above have been introduced. Updated GRAPPA guidelines are expected to be released later this year.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Fundamental changes in the initial pharmacologic approach to psoriatic arthritis were made in the 2018 American College Rheumatology/National Psoriasis (ACR/NPF) guidelines for the treatment of psoriatic arthritis. Previously, methotrexate had been widely used as the first-line agent. The 2018 guidelines recommend a tumor necrosis factor (TNF) inhibitor over methotrexate and other oral small molecules (leflunomide, cyclosporine, and apremilast).
Herein is a broad summary of the guidelines:
· Treat with TNF inhibitor over oral small molecule; may consider oral small molecule with mild psoriatic arthritis and psoriasis, patient preference, and/or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with oral small molecule over IL-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis and/or psoriatic arthritis
· Treat with oral small molecule over IL-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or psoriatic arthritis, or concomitant inflammatory bowel disease
· Treat with methotrexate over nonsteroidal anti-inflammatory drugs; may consider nonsteroidals for mild psoriatic arthritis and psoriasis
· Treat with IL-17 inhibitor over IL-12/23 inhibitor; may consider IL-12/23 inhibitor in a patient with concomitant inflammatory bowel disease
Note that these recommendations are based on conditional evidence (ie, low to very low quality). In fact, in the entire guideline document, only 6% of the recommendations are strong, whereas 96% are conditional. This emphasizes the importance of evaluating each patient individually and engaging in a discussion to choose optimal therapy.
Another set of guidelines, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), was last updated in 2015. Since then, many of the agents above have been introduced. Updated GRAPPA guidelines are expected to be released later this year.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Fundamental changes in the initial pharmacologic approach to psoriatic arthritis were made in the 2018 American College Rheumatology/National Psoriasis (ACR/NPF) guidelines for the treatment of psoriatic arthritis. Previously, methotrexate had been widely used as the first-line agent. The 2018 guidelines recommend a tumor necrosis factor (TNF) inhibitor over methotrexate and other oral small molecules (leflunomide, cyclosporine, and apremilast).
Herein is a broad summary of the guidelines:
· Treat with TNF inhibitor over oral small molecule; may consider oral small molecule with mild psoriatic arthritis and psoriasis, patient preference, and/or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with TNF inhibitor over interleukin (IL)-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or contraindication to TNF inhibitor
· Treat with oral small molecule over IL-17 inhibitor; may consider IL-17 inhibitor with severe psoriasis and/or psoriatic arthritis
· Treat with oral small molecule over IL-12/23 inhibitor; may consider IL-12/23 inhibitor with severe psoriasis or psoriatic arthritis, or concomitant inflammatory bowel disease
· Treat with methotrexate over nonsteroidal anti-inflammatory drugs; may consider nonsteroidals for mild psoriatic arthritis and psoriasis
· Treat with IL-17 inhibitor over IL-12/23 inhibitor; may consider IL-12/23 inhibitor in a patient with concomitant inflammatory bowel disease
Note that these recommendations are based on conditional evidence (ie, low to very low quality). In fact, in the entire guideline document, only 6% of the recommendations are strong, whereas 96% are conditional. This emphasizes the importance of evaluating each patient individually and engaging in a discussion to choose optimal therapy.
Another set of guidelines, from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), was last updated in 2015. Since then, many of the agents above have been introduced. Updated GRAPPA guidelines are expected to be released later this year.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
A 56-year-old man presents because of intermittent joint aches and difficulty picking up his grandchild and cleaning his home. He has a 6-year history of scalp psoriasis that he has controlled with a salicylic acid shampoo. On physical examination, he has tenderness over both elbows and in his metacarpophalangeal and proximal interphalangeal (PIP) joints on both hands. Swollen joints are noted in the proximal and distal joints of the right hand. His fingernails show uniform pitting.
Neurologic examination shows no sensory deficits or hyperesthesia. Motor examination is unremarkable, and chest and abdominal findings are unremarkable. Blood pressure is 138/90 mm Hg. Radiographic imaging shows asymmetric erosive changes with very small areas of bony proliferation in the PIP joints.There is asymmetric narrowing of the joint space in the interphalangeal joints. Laboratory findings reveal an erythrocyte sedimentation rate of 35 mm/h, negative rheumatoid factor, negative antinuclear antibody, and C-reactive protein of 7 mg/dL.
These findings are consistent with a diagnosis of psoriatic arthritis. This patient has severe psoriatic arthritis based on radiographic evidence of erosive disease.
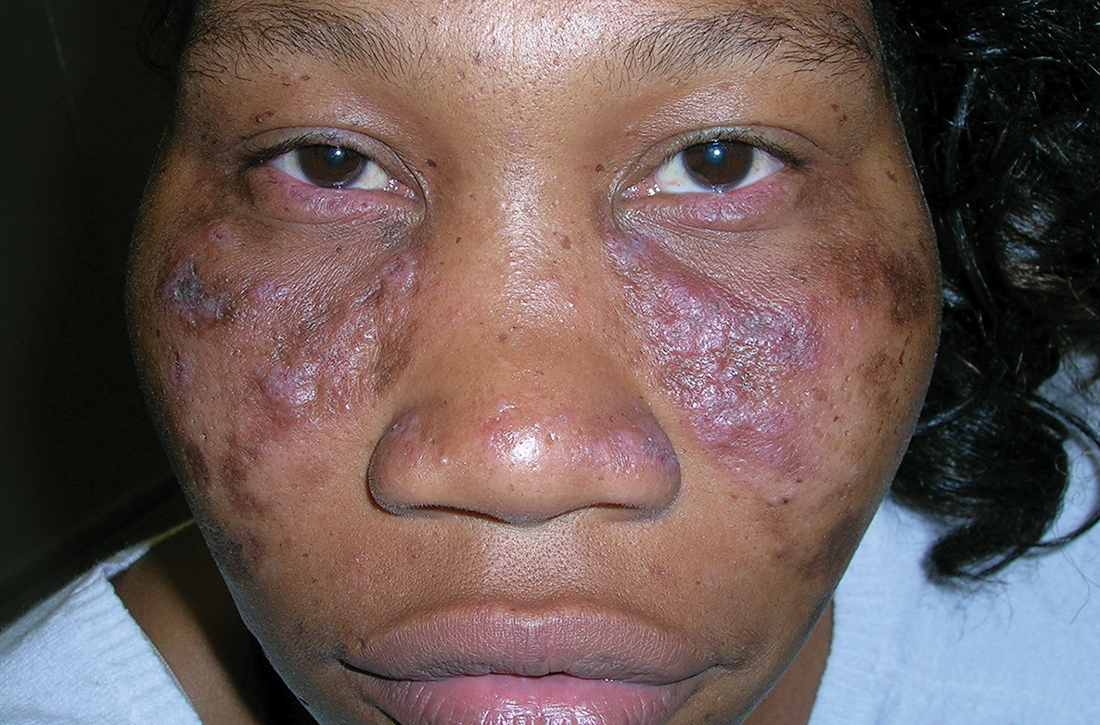
Discoid lupus
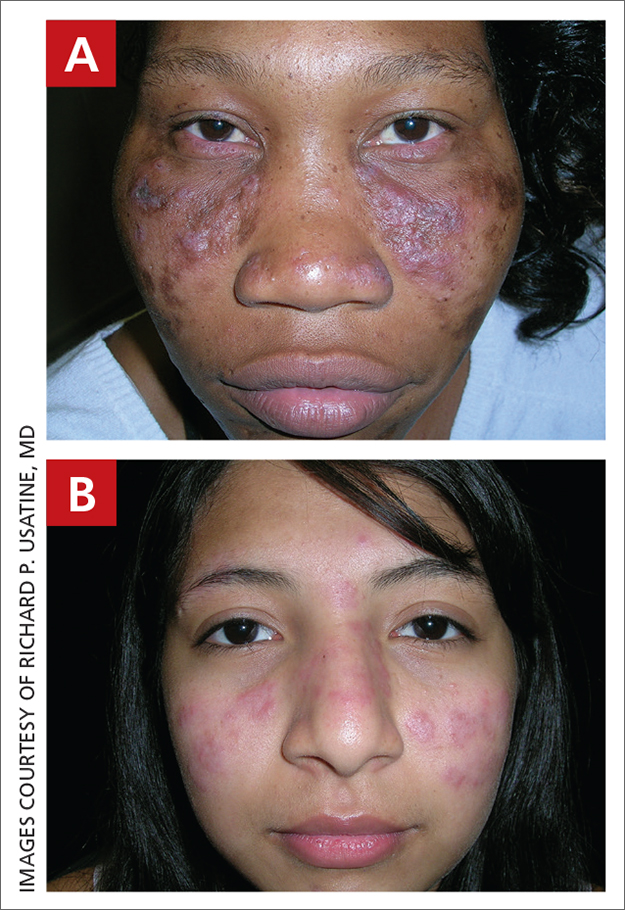
THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1
Epidemiology
DLE is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
DLE lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. DLE lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
1. Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
2. Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
3. Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
4. McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
5. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
6. Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
7. Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
8. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1

Epidemiology
DLE is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
DLE lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. DLE lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
THE COMPARISON
A Multicolored (pink, brown, and white) indurated plaques in a butterfly distribution on the face of a 30-year-old woman with a darker skin tone.
B Pink, elevated, indurated plaques with hypopigmentation in a butterfly distribution on the face of a 19-year-old woman with a lighter skin tone.
Cutaneous lupus erythematosus may occur with or without systemic lupus erythematosus. Discoid lupus erythematosus (DLE), a form of chronic cutaneous lupus, is most commonly found on the scalp, face, and ears.1

Epidemiology
DLE is most common in adult women (age range, 20–40 years).2 It occurs more frequently in women of African descent.3,4
Key clinical features in people with darker skin tones
Clinical features of DLE lesions include erythema, induration, follicular plugging, dyspigmentation, and scarring alopecia.1 In patients of African descent, lesions may be annular and hypopigmented to depigmented centrally with a border of hyperpigmentation. Active lesions may be painful and/or pruritic.2
DLE lesions occur in photodistributed areas, although not exclusively. Photoprotective clothing and sunscreen are an important part of the treatment plan.1 Although sunscreen is recommended for patients with DLE, those with darker skin tones may find some sunscreens cosmetically unappealing due to a mismatch with their normal skin color.5 Tinted sunscreens may be beneficial additions.
Worth noting
Approximately 5% to 25% of patients with cutaneous lupus go on to develop systemic lupus erythematosus.6
Health disparity highlight
Discoid lesions may cause cutaneous scars that are quite disfiguring and may negatively impact quality of life. Some patients may have a few scattered lesions, whereas others have extensive disease covering most of the scalp. DLE lesions of the scalp have classic clinical features including hair loss, erythema, hypopigmentation, and hyperpigmentation. The clinician’s comfort with performing a scalp examination with cultural humility is an important acquired skill and is especially important when the examination is performed on patients with more tightly coiled hair.7 For example, physicians may adopt the “compliment, discuss, and suggest” method when counseling patients.8
1. Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
2. Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
3. Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
4. McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
5. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
6. Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
7. Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
8. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
1. Bolognia JL, Jorizzo JJ, Schaffer JV, et al. Dermatology. 3rd ed. Elsevier; 2012.
2. Otberg N, Wu W-Y, McElwee KJ, et al. Diagnosis and management of primary cicatricial alopecia: part I. Skinmed. 2008;7:19-26. doi:10.1111/j.1540-9740.2007.07163.x
3. Callen JP. Chronic cutaneous lupus erythematosus. clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416. doi:10.1001/archderm.118.6.412
4. McCarty DJ, Manzi S, Medsger TA Jr, et al. Incidence of systemic lupus erythematosus. race and gender differences. Arthritis Rheum. 1995;38:1260-1270. doi:10.1002/art.1780380914
5. Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. In press.
6. Zhou W, Wu H, Zhao M, et al. New insights into the progression from cutaneous lupus to systemic lupus erythematosus. Expert Rev Clin Immunol. 2020;16:829-837. doi:10.1080/17446 66X.2020.1805316
7. Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
8. Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
Do Not Expect a Patient With MS to Have Just MS
By Ruth Ann Marrie, MD, PhD, FRCPC, FCAHS
Waugh Family Chair in Multiple Sclerosis, Professor of Medicine & Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba and Director, Multiple Sclerosis Clinic, Winnipeg, Manitoba, Canada.
The diseases and disorders known to coexist with multiple sclerosis (MS), overall, are not passive bystanders. While they have not been proven to cause MS – or vice versa – some of these comorbidities advance MS disease at a quicker pace; some may lead to an earlier death; and others could be, and should be, considered relevant harbingers of a diagnosis to come.
These comorbidities are not isolated to 1 organ system, but rather have been found in the endocrine, cardiovascular, respiratory, central nervous, and immune systems. The more comorbidities someone has, the higher the frequency of relapses in those with relapsing MS, the most common type of MS.1
Temporally speaking, the comorbidities can precede MS diagnosis or develop after diagnosis; they tend to increase in number with age and over time. As for their connection to MS, the very common denominator among many of these comorbidities is their inflammatory characteristic.
There are compelling reasons for specialists – endocrinologists, cardiologists, pulmonologists –and generalists, like primary care physicians, to appreciate the complexities of this disease, both in its prodromal state and beyond.
The literature shows how difficult diagnosis can be. A 2016 study of 4 MS centers found that 110 patients, 33% of the population, had been misdiagnosed for 10 years; their migraines had been misdiagnosed as MS.2 Then again, migraine and MS frequently overlap; a 2012 study reported that 43% of patients with MS also have migraine.3 Considering that females present with relapsing-remitting MS more often than males and deal more with migraines, this observation should not be a big surprise.
Patients come with histories including medical, familial, and lifestyle histories. Exploring that history informs illness; how clinicians incorporate that history is important to disease management and patient outcomes.
What follows is an overview of comorbidities and MS.
MS and the immune system
MS, for which there is no known cure, permanently disables the body and mind by progressively damaging the myelin sheath that protects axons. It is usually diagnosed in adulthood.
The words chosen to describe MS, from a scientific vantage point, include heterogeneous, complex, and multifaceted. It is likely no one who has, treats, or researches this disease would argue those points. At least 3 journal articles dating back to 2013 all described a discovery about MS as another “brick in the wall.” The latest is a Science Immunology commentary on findings that gut-barrier-protecting Th17 cells could have an evil side, expressing a ligand called dual immunoglobulin domain containing cell adhesion molecule, allowing these cells to infiltrate the blood brain barrier during neuroinflammation.4
So far, 230 loci have been implicated in modulating the risk of MS development.5 That 230 is twice the number found in rheumatoid arthritis6 and more than triple the number of genes and loci linked to psoriasis.7 The genomic map of MS, showing involvement of peripheral immune cells and microglia in susceptibility, resembles a spider web more than genetic cartography.8
One review of the literature listed more than 50 comorbid conditions found in patients with MS. While many of these conditions do not occur more often in those with MS as opposed to those without the disease, a few comorbidities certainly do.9
The comorbidities
As defined, a comorbidity is a co-existing condition not directly related to the primary, or index, disease, which in this case is MS.10 One must wonder if, as the index disease, MS defies this definition, as depression, anxiety, hypertension, hyperlipidemia, and chronic lung disease are frequently found in patients with MS: when combined, depression and anxiety are found in nearly half of patients.11,12
But MS is not dependent on aberrant genes solely for its development. The environmental and lifestyle risk factors linked to an MS diagnosis include childhood obesity, Epstein Barr virus infection (the virus that causes infectious mononucleosis), smoking, and low levels of vitamin D.13,14 A common denominator among virtually all these factors, not unlike the comorbidities themselves, is inflammation.
It is not uncommon for patients with MS to have psoriasis.7,10 Nor is it uncommon for them to have other types of autoimmune diseases, such as inflammatory bowel disease. For patients with MS, the relative risk is increased for developing some other autoimmune diseases including inflammatory bowel disease, psoriasis, and bullous pemphigoid (another skin condition).
Studies of patients with rheumatoid arthritis (RA) have shown how RA is directly or indirectly responsible for the development of other diseases, primarily due to RA’s creation of inflammatory pathways.15 In patients with RA, comorbidities tend to become fewer as the disease progresses. As already discussed, in patients with MS, comorbidities generally increase over time.15,16 As for whether a comorbidity could cause the development of MS, that question has yet to be answered.
Comorbidity specifics
There are a few comorbidities that appear in the literature more than others, with most of them falling into the vascular or the central nervous system. Diseases associated with the vascular system, including hypertension and diabetes, as they accumulate in number, will cause more physical impairment.17 A single vascular comorbidity at diagnosis was associated with a 51% increased risk of early gait disability, while 2 vascular comorbidities were associated with a 228% increased risk.18
Other comorbidities, like chronic obstructive pulmonary disease (COPD), can cause disease to progress at a quicker pace.10 COPD also can increase risk of an earlier death, as can epilepsy.10,16 People with MS, mostly women diagnosed in the prime of their lives, live 6 to 8 fewer years than those without.19
Some coexistent diseases are also linked to a longer delay to MS diagnosis and lower rate of treatment. A large study in Canada showed ischemic heart disease and anxiety were linked with a patient’s lower rate of receiving disease-modifying therapies.9
In time
While not every patient with MS has co-existing disease at the time of diagnosis, it will be highly likely that these patients will have comorbidities as the years pass. In 1 study, researchers found that the prevalence of some comorbidities, like gastrointestinal disorders, thyroid disease, and anxiety, increased as patients aged.20
When reviewing health claims data for patients with inflammatory bowel disease and RA, researchers found a similar risk of depression in both. Health claims data also show patients looking for treatment for anxiety 5 years before an MS diagnosis. Of patients who were not yet diagnosed, 19% had sought help for depression and 11% for anxiety.9
Researchers looked at 2526 patients diagnosed with MS and 9980 controls to compare the risk of developing comorbidities prior to MS diagnosis and after.16 At diagnosis, 22.7% of patients had at least one Charlson comorbidity compared with 16.8% of controls. (The Charlson comorbidity index is a weighted score comprised of several comorbidities. Scores span mild to severe, or 1 to above 5.) 21
Ten years prior to MS diagnosis, out of ~30 diseases, patients with MS were at risk to develop at least 20 of the 30, including various cancers, cardiovascular diseases, thyroid disorders, and neurologic and mental disorders. For the latter, the difference was 34.92% vs 17.87%. In the period after diagnosis, 17.23% of patients had a new comorbidity, as compared to 15.78% in the control population. The change was remarkable in the neurologic and mental disorders; prior to an MS diagnosis, there were no cases of dementia, but that changed post-diagnosis.
References
- Kowalec K, McKay KA, Patten SB, et al; CIHR Team in Epidemiology and Impact of Comorbidity on Multiple Sclerosis. Comorbidity increases the risk of relapse in multiple sclerosis: a prospective study. Neurology. 2017;89(24):2455-2461.
- Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
- Applebee A. The clinical overlap of multiple sclerosis and headache. Headache. 2012;52(Suppl.2):111-116.
- Pillai S. TH17 cells in multiple sclerosis dislodge another brick in the wall. Sci Immunol. 2022;7(68):eabo2989.
- International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365(6460):eaav7188.
- Yarwood A, Huizinga TW, Worthington J. The genetics of rheumatoid arthritis: risk and protection in different stages of the evolution of RA. Rheumatology (Oxford). 2016;55(2):199-209.
- Ran D, Cai M, Zhang X. Genetics of psoriasis: a basis for precision medicine. Precision Clinical Medicine. 2019;2(2):120-130.
- Nelson CA, Bove R, Butte AJ, Baranzini SE. Embedding electronic health records onto a knowledge network recognizes prodromal features of multiple sclerosis and predicts diagnosis. J Am Med Inform Assoc. 2022;29(3):424-434.
- Marrie RA. Comorbidity in multiple sclerosis: some answers, more questions. Int J MS Care. 2016;18(6):271-272.
- Magyari M, Sorensen PS. Comorbidity in multiple sclerosis. Front Neurol. 2020;11:851.
- Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. 2015;21(3):263-281.
- Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol. 2017;13(6):375-382.
- Fragoso YD. Modifiable environmental factors in multiple sclerosis. Arq Neuropsiquiatr. 2014;72(11):889-894.
- Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301.
- Tatangelo MR, Tomlinson G, Keystone E, Paterson JM, Bansback N, Bombardier C. Comorbidities before and after the diagnosis of rheumatoid arthritis: a matched longitudinal study. ACR Open Rheumatol. 2020;2(11):648-656.
- Chou IJ, Kuo CF, Tanasescu R, et al. Comorbidity in multiple sclerosis: its temporal relationships with disease onset and dose effect on mortality. Eur J Neurol. 2020;27(1):105-112.
- Fitzgerald KC, Damian A, Conway D, Mowry EM. Vascular comorbidity is associated with lower brain volumes and lower neuroperformance in a large multiple sclerosis cohort. Mult Scler. 2021;27(12):1914-1923.
- Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041-1047.
- Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
- Edwards NC, Munsell M, Menzin J, Phillips AL. Comorbidity in US patients with multiple sclerosis. Patient Relat Outcome Meas. 2018;9:97-102.
- Huang YQ, Gou R, Diao YS, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66.
By Ruth Ann Marrie, MD, PhD, FRCPC, FCAHS
Waugh Family Chair in Multiple Sclerosis, Professor of Medicine & Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba and Director, Multiple Sclerosis Clinic, Winnipeg, Manitoba, Canada.
The diseases and disorders known to coexist with multiple sclerosis (MS), overall, are not passive bystanders. While they have not been proven to cause MS – or vice versa – some of these comorbidities advance MS disease at a quicker pace; some may lead to an earlier death; and others could be, and should be, considered relevant harbingers of a diagnosis to come.
These comorbidities are not isolated to 1 organ system, but rather have been found in the endocrine, cardiovascular, respiratory, central nervous, and immune systems. The more comorbidities someone has, the higher the frequency of relapses in those with relapsing MS, the most common type of MS.1
Temporally speaking, the comorbidities can precede MS diagnosis or develop after diagnosis; they tend to increase in number with age and over time. As for their connection to MS, the very common denominator among many of these comorbidities is their inflammatory characteristic.
There are compelling reasons for specialists – endocrinologists, cardiologists, pulmonologists –and generalists, like primary care physicians, to appreciate the complexities of this disease, both in its prodromal state and beyond.
The literature shows how difficult diagnosis can be. A 2016 study of 4 MS centers found that 110 patients, 33% of the population, had been misdiagnosed for 10 years; their migraines had been misdiagnosed as MS.2 Then again, migraine and MS frequently overlap; a 2012 study reported that 43% of patients with MS also have migraine.3 Considering that females present with relapsing-remitting MS more often than males and deal more with migraines, this observation should not be a big surprise.
Patients come with histories including medical, familial, and lifestyle histories. Exploring that history informs illness; how clinicians incorporate that history is important to disease management and patient outcomes.
What follows is an overview of comorbidities and MS.
MS and the immune system
MS, for which there is no known cure, permanently disables the body and mind by progressively damaging the myelin sheath that protects axons. It is usually diagnosed in adulthood.
The words chosen to describe MS, from a scientific vantage point, include heterogeneous, complex, and multifaceted. It is likely no one who has, treats, or researches this disease would argue those points. At least 3 journal articles dating back to 2013 all described a discovery about MS as another “brick in the wall.” The latest is a Science Immunology commentary on findings that gut-barrier-protecting Th17 cells could have an evil side, expressing a ligand called dual immunoglobulin domain containing cell adhesion molecule, allowing these cells to infiltrate the blood brain barrier during neuroinflammation.4
So far, 230 loci have been implicated in modulating the risk of MS development.5 That 230 is twice the number found in rheumatoid arthritis6 and more than triple the number of genes and loci linked to psoriasis.7 The genomic map of MS, showing involvement of peripheral immune cells and microglia in susceptibility, resembles a spider web more than genetic cartography.8
One review of the literature listed more than 50 comorbid conditions found in patients with MS. While many of these conditions do not occur more often in those with MS as opposed to those without the disease, a few comorbidities certainly do.9
The comorbidities
As defined, a comorbidity is a co-existing condition not directly related to the primary, or index, disease, which in this case is MS.10 One must wonder if, as the index disease, MS defies this definition, as depression, anxiety, hypertension, hyperlipidemia, and chronic lung disease are frequently found in patients with MS: when combined, depression and anxiety are found in nearly half of patients.11,12
But MS is not dependent on aberrant genes solely for its development. The environmental and lifestyle risk factors linked to an MS diagnosis include childhood obesity, Epstein Barr virus infection (the virus that causes infectious mononucleosis), smoking, and low levels of vitamin D.13,14 A common denominator among virtually all these factors, not unlike the comorbidities themselves, is inflammation.
It is not uncommon for patients with MS to have psoriasis.7,10 Nor is it uncommon for them to have other types of autoimmune diseases, such as inflammatory bowel disease. For patients with MS, the relative risk is increased for developing some other autoimmune diseases including inflammatory bowel disease, psoriasis, and bullous pemphigoid (another skin condition).
Studies of patients with rheumatoid arthritis (RA) have shown how RA is directly or indirectly responsible for the development of other diseases, primarily due to RA’s creation of inflammatory pathways.15 In patients with RA, comorbidities tend to become fewer as the disease progresses. As already discussed, in patients with MS, comorbidities generally increase over time.15,16 As for whether a comorbidity could cause the development of MS, that question has yet to be answered.
Comorbidity specifics
There are a few comorbidities that appear in the literature more than others, with most of them falling into the vascular or the central nervous system. Diseases associated with the vascular system, including hypertension and diabetes, as they accumulate in number, will cause more physical impairment.17 A single vascular comorbidity at diagnosis was associated with a 51% increased risk of early gait disability, while 2 vascular comorbidities were associated with a 228% increased risk.18
Other comorbidities, like chronic obstructive pulmonary disease (COPD), can cause disease to progress at a quicker pace.10 COPD also can increase risk of an earlier death, as can epilepsy.10,16 People with MS, mostly women diagnosed in the prime of their lives, live 6 to 8 fewer years than those without.19
Some coexistent diseases are also linked to a longer delay to MS diagnosis and lower rate of treatment. A large study in Canada showed ischemic heart disease and anxiety were linked with a patient’s lower rate of receiving disease-modifying therapies.9
In time
While not every patient with MS has co-existing disease at the time of diagnosis, it will be highly likely that these patients will have comorbidities as the years pass. In 1 study, researchers found that the prevalence of some comorbidities, like gastrointestinal disorders, thyroid disease, and anxiety, increased as patients aged.20
When reviewing health claims data for patients with inflammatory bowel disease and RA, researchers found a similar risk of depression in both. Health claims data also show patients looking for treatment for anxiety 5 years before an MS diagnosis. Of patients who were not yet diagnosed, 19% had sought help for depression and 11% for anxiety.9
Researchers looked at 2526 patients diagnosed with MS and 9980 controls to compare the risk of developing comorbidities prior to MS diagnosis and after.16 At diagnosis, 22.7% of patients had at least one Charlson comorbidity compared with 16.8% of controls. (The Charlson comorbidity index is a weighted score comprised of several comorbidities. Scores span mild to severe, or 1 to above 5.) 21
Ten years prior to MS diagnosis, out of ~30 diseases, patients with MS were at risk to develop at least 20 of the 30, including various cancers, cardiovascular diseases, thyroid disorders, and neurologic and mental disorders. For the latter, the difference was 34.92% vs 17.87%. In the period after diagnosis, 17.23% of patients had a new comorbidity, as compared to 15.78% in the control population. The change was remarkable in the neurologic and mental disorders; prior to an MS diagnosis, there were no cases of dementia, but that changed post-diagnosis.
By Ruth Ann Marrie, MD, PhD, FRCPC, FCAHS
Waugh Family Chair in Multiple Sclerosis, Professor of Medicine & Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba and Director, Multiple Sclerosis Clinic, Winnipeg, Manitoba, Canada.
The diseases and disorders known to coexist with multiple sclerosis (MS), overall, are not passive bystanders. While they have not been proven to cause MS – or vice versa – some of these comorbidities advance MS disease at a quicker pace; some may lead to an earlier death; and others could be, and should be, considered relevant harbingers of a diagnosis to come.
These comorbidities are not isolated to 1 organ system, but rather have been found in the endocrine, cardiovascular, respiratory, central nervous, and immune systems. The more comorbidities someone has, the higher the frequency of relapses in those with relapsing MS, the most common type of MS.1
Temporally speaking, the comorbidities can precede MS diagnosis or develop after diagnosis; they tend to increase in number with age and over time. As for their connection to MS, the very common denominator among many of these comorbidities is their inflammatory characteristic.
There are compelling reasons for specialists – endocrinologists, cardiologists, pulmonologists –and generalists, like primary care physicians, to appreciate the complexities of this disease, both in its prodromal state and beyond.
The literature shows how difficult diagnosis can be. A 2016 study of 4 MS centers found that 110 patients, 33% of the population, had been misdiagnosed for 10 years; their migraines had been misdiagnosed as MS.2 Then again, migraine and MS frequently overlap; a 2012 study reported that 43% of patients with MS also have migraine.3 Considering that females present with relapsing-remitting MS more often than males and deal more with migraines, this observation should not be a big surprise.
Patients come with histories including medical, familial, and lifestyle histories. Exploring that history informs illness; how clinicians incorporate that history is important to disease management and patient outcomes.
What follows is an overview of comorbidities and MS.
MS and the immune system
MS, for which there is no known cure, permanently disables the body and mind by progressively damaging the myelin sheath that protects axons. It is usually diagnosed in adulthood.
The words chosen to describe MS, from a scientific vantage point, include heterogeneous, complex, and multifaceted. It is likely no one who has, treats, or researches this disease would argue those points. At least 3 journal articles dating back to 2013 all described a discovery about MS as another “brick in the wall.” The latest is a Science Immunology commentary on findings that gut-barrier-protecting Th17 cells could have an evil side, expressing a ligand called dual immunoglobulin domain containing cell adhesion molecule, allowing these cells to infiltrate the blood brain barrier during neuroinflammation.4
So far, 230 loci have been implicated in modulating the risk of MS development.5 That 230 is twice the number found in rheumatoid arthritis6 and more than triple the number of genes and loci linked to psoriasis.7 The genomic map of MS, showing involvement of peripheral immune cells and microglia in susceptibility, resembles a spider web more than genetic cartography.8
One review of the literature listed more than 50 comorbid conditions found in patients with MS. While many of these conditions do not occur more often in those with MS as opposed to those without the disease, a few comorbidities certainly do.9
The comorbidities
As defined, a comorbidity is a co-existing condition not directly related to the primary, or index, disease, which in this case is MS.10 One must wonder if, as the index disease, MS defies this definition, as depression, anxiety, hypertension, hyperlipidemia, and chronic lung disease are frequently found in patients with MS: when combined, depression and anxiety are found in nearly half of patients.11,12
But MS is not dependent on aberrant genes solely for its development. The environmental and lifestyle risk factors linked to an MS diagnosis include childhood obesity, Epstein Barr virus infection (the virus that causes infectious mononucleosis), smoking, and low levels of vitamin D.13,14 A common denominator among virtually all these factors, not unlike the comorbidities themselves, is inflammation.
It is not uncommon for patients with MS to have psoriasis.7,10 Nor is it uncommon for them to have other types of autoimmune diseases, such as inflammatory bowel disease. For patients with MS, the relative risk is increased for developing some other autoimmune diseases including inflammatory bowel disease, psoriasis, and bullous pemphigoid (another skin condition).
Studies of patients with rheumatoid arthritis (RA) have shown how RA is directly or indirectly responsible for the development of other diseases, primarily due to RA’s creation of inflammatory pathways.15 In patients with RA, comorbidities tend to become fewer as the disease progresses. As already discussed, in patients with MS, comorbidities generally increase over time.15,16 As for whether a comorbidity could cause the development of MS, that question has yet to be answered.
Comorbidity specifics
There are a few comorbidities that appear in the literature more than others, with most of them falling into the vascular or the central nervous system. Diseases associated with the vascular system, including hypertension and diabetes, as they accumulate in number, will cause more physical impairment.17 A single vascular comorbidity at diagnosis was associated with a 51% increased risk of early gait disability, while 2 vascular comorbidities were associated with a 228% increased risk.18
Other comorbidities, like chronic obstructive pulmonary disease (COPD), can cause disease to progress at a quicker pace.10 COPD also can increase risk of an earlier death, as can epilepsy.10,16 People with MS, mostly women diagnosed in the prime of their lives, live 6 to 8 fewer years than those without.19
Some coexistent diseases are also linked to a longer delay to MS diagnosis and lower rate of treatment. A large study in Canada showed ischemic heart disease and anxiety were linked with a patient’s lower rate of receiving disease-modifying therapies.9
In time
While not every patient with MS has co-existing disease at the time of diagnosis, it will be highly likely that these patients will have comorbidities as the years pass. In 1 study, researchers found that the prevalence of some comorbidities, like gastrointestinal disorders, thyroid disease, and anxiety, increased as patients aged.20
When reviewing health claims data for patients with inflammatory bowel disease and RA, researchers found a similar risk of depression in both. Health claims data also show patients looking for treatment for anxiety 5 years before an MS diagnosis. Of patients who were not yet diagnosed, 19% had sought help for depression and 11% for anxiety.9
Researchers looked at 2526 patients diagnosed with MS and 9980 controls to compare the risk of developing comorbidities prior to MS diagnosis and after.16 At diagnosis, 22.7% of patients had at least one Charlson comorbidity compared with 16.8% of controls. (The Charlson comorbidity index is a weighted score comprised of several comorbidities. Scores span mild to severe, or 1 to above 5.) 21
Ten years prior to MS diagnosis, out of ~30 diseases, patients with MS were at risk to develop at least 20 of the 30, including various cancers, cardiovascular diseases, thyroid disorders, and neurologic and mental disorders. For the latter, the difference was 34.92% vs 17.87%. In the period after diagnosis, 17.23% of patients had a new comorbidity, as compared to 15.78% in the control population. The change was remarkable in the neurologic and mental disorders; prior to an MS diagnosis, there were no cases of dementia, but that changed post-diagnosis.
References
- Kowalec K, McKay KA, Patten SB, et al; CIHR Team in Epidemiology and Impact of Comorbidity on Multiple Sclerosis. Comorbidity increases the risk of relapse in multiple sclerosis: a prospective study. Neurology. 2017;89(24):2455-2461.
- Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
- Applebee A. The clinical overlap of multiple sclerosis and headache. Headache. 2012;52(Suppl.2):111-116.
- Pillai S. TH17 cells in multiple sclerosis dislodge another brick in the wall. Sci Immunol. 2022;7(68):eabo2989.
- International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365(6460):eaav7188.
- Yarwood A, Huizinga TW, Worthington J. The genetics of rheumatoid arthritis: risk and protection in different stages of the evolution of RA. Rheumatology (Oxford). 2016;55(2):199-209.
- Ran D, Cai M, Zhang X. Genetics of psoriasis: a basis for precision medicine. Precision Clinical Medicine. 2019;2(2):120-130.
- Nelson CA, Bove R, Butte AJ, Baranzini SE. Embedding electronic health records onto a knowledge network recognizes prodromal features of multiple sclerosis and predicts diagnosis. J Am Med Inform Assoc. 2022;29(3):424-434.
- Marrie RA. Comorbidity in multiple sclerosis: some answers, more questions. Int J MS Care. 2016;18(6):271-272.
- Magyari M, Sorensen PS. Comorbidity in multiple sclerosis. Front Neurol. 2020;11:851.
- Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. 2015;21(3):263-281.
- Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol. 2017;13(6):375-382.
- Fragoso YD. Modifiable environmental factors in multiple sclerosis. Arq Neuropsiquiatr. 2014;72(11):889-894.
- Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301.
- Tatangelo MR, Tomlinson G, Keystone E, Paterson JM, Bansback N, Bombardier C. Comorbidities before and after the diagnosis of rheumatoid arthritis: a matched longitudinal study. ACR Open Rheumatol. 2020;2(11):648-656.
- Chou IJ, Kuo CF, Tanasescu R, et al. Comorbidity in multiple sclerosis: its temporal relationships with disease onset and dose effect on mortality. Eur J Neurol. 2020;27(1):105-112.
- Fitzgerald KC, Damian A, Conway D, Mowry EM. Vascular comorbidity is associated with lower brain volumes and lower neuroperformance in a large multiple sclerosis cohort. Mult Scler. 2021;27(12):1914-1923.
- Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041-1047.
- Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
- Edwards NC, Munsell M, Menzin J, Phillips AL. Comorbidity in US patients with multiple sclerosis. Patient Relat Outcome Meas. 2018;9:97-102.
- Huang YQ, Gou R, Diao YS, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66.
References
- Kowalec K, McKay KA, Patten SB, et al; CIHR Team in Epidemiology and Impact of Comorbidity on Multiple Sclerosis. Comorbidity increases the risk of relapse in multiple sclerosis: a prospective study. Neurology. 2017;89(24):2455-2461.
- Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016;87(13):1393-1399.
- Applebee A. The clinical overlap of multiple sclerosis and headache. Headache. 2012;52(Suppl.2):111-116.
- Pillai S. TH17 cells in multiple sclerosis dislodge another brick in the wall. Sci Immunol. 2022;7(68):eabo2989.
- International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365(6460):eaav7188.
- Yarwood A, Huizinga TW, Worthington J. The genetics of rheumatoid arthritis: risk and protection in different stages of the evolution of RA. Rheumatology (Oxford). 2016;55(2):199-209.
- Ran D, Cai M, Zhang X. Genetics of psoriasis: a basis for precision medicine. Precision Clinical Medicine. 2019;2(2):120-130.
- Nelson CA, Bove R, Butte AJ, Baranzini SE. Embedding electronic health records onto a knowledge network recognizes prodromal features of multiple sclerosis and predicts diagnosis. J Am Med Inform Assoc. 2022;29(3):424-434.
- Marrie RA. Comorbidity in multiple sclerosis: some answers, more questions. Int J MS Care. 2016;18(6):271-272.
- Magyari M, Sorensen PS. Comorbidity in multiple sclerosis. Front Neurol. 2020;11:851.
- Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. 2015;21(3):263-281.
- Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol. 2017;13(6):375-382.
- Fragoso YD. Modifiable environmental factors in multiple sclerosis. Arq Neuropsiquiatr. 2014;72(11):889-894.
- Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301.
- Tatangelo MR, Tomlinson G, Keystone E, Paterson JM, Bansback N, Bombardier C. Comorbidities before and after the diagnosis of rheumatoid arthritis: a matched longitudinal study. ACR Open Rheumatol. 2020;2(11):648-656.
- Chou IJ, Kuo CF, Tanasescu R, et al. Comorbidity in multiple sclerosis: its temporal relationships with disease onset and dose effect on mortality. Eur J Neurol. 2020;27(1):105-112.
- Fitzgerald KC, Damian A, Conway D, Mowry EM. Vascular comorbidity is associated with lower brain volumes and lower neuroperformance in a large multiple sclerosis cohort. Mult Scler. 2021;27(12):1914-1923.
- Marrie RA, Rudick R, Horwitz R, et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74(13):1041-1047.
- Marrie RA, Elliott L, Marriott J, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. 2015;85(3):240-247.
- Edwards NC, Munsell M, Menzin J, Phillips AL. Comorbidity in US patients with multiple sclerosis. Patient Relat Outcome Meas. 2018;9:97-102.
- Huang YQ, Gou R, Diao YS, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15(1):58-66.
Treatment Advances in Moderate to Severe Active Ulcerative Colitis
Treatment for moderate to severe active ulcerative colitis (UC) has evolved, and with more effective treatment comes higher standards for disease control.
The initial goal is clinical response, followed by clinical remission, endoscopic remission, and — the ultimate goal — histologic remission.
The majority of UC medications have been studied for clinical and endoscopic remission. Recent clinical trials, however, have evaluated the emerging targeted therapies ustekinumab and ozanimod for histologic remission and found positive results.
Dr Bincy Abraham, director of the Fondren Inflammatory Bowel Disease Program at Houston Methodist in Houston, Texas, reports on UC treatment milestones and how emerging targeted therapies can help achieve these goals.
She also discusses patient monitoring to ensure response to therapy as well as medication adjustments should response prove inadequate.
--
Professor of Clinical Medicine, Department of Internal Medicine and Gastroenterology; Director, Fondren Inflammatory Bowel Disease Program, Underwood Center for Digestive Disorders, Houston Methodist, Houston, Texas
Bincy Abraham, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; BMS; Janssen; Pfizer; Takeda; Medtronic
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; BMS; Janssen; Pfizer; Takeda
Received research grant from: Takeda; BMS; Genentech
Treatment for moderate to severe active ulcerative colitis (UC) has evolved, and with more effective treatment comes higher standards for disease control.
The initial goal is clinical response, followed by clinical remission, endoscopic remission, and — the ultimate goal — histologic remission.
The majority of UC medications have been studied for clinical and endoscopic remission. Recent clinical trials, however, have evaluated the emerging targeted therapies ustekinumab and ozanimod for histologic remission and found positive results.
Dr Bincy Abraham, director of the Fondren Inflammatory Bowel Disease Program at Houston Methodist in Houston, Texas, reports on UC treatment milestones and how emerging targeted therapies can help achieve these goals.
She also discusses patient monitoring to ensure response to therapy as well as medication adjustments should response prove inadequate.
--
Professor of Clinical Medicine, Department of Internal Medicine and Gastroenterology; Director, Fondren Inflammatory Bowel Disease Program, Underwood Center for Digestive Disorders, Houston Methodist, Houston, Texas
Bincy Abraham, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; BMS; Janssen; Pfizer; Takeda; Medtronic
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; BMS; Janssen; Pfizer; Takeda
Received research grant from: Takeda; BMS; Genentech
Treatment for moderate to severe active ulcerative colitis (UC) has evolved, and with more effective treatment comes higher standards for disease control.
The initial goal is clinical response, followed by clinical remission, endoscopic remission, and — the ultimate goal — histologic remission.
The majority of UC medications have been studied for clinical and endoscopic remission. Recent clinical trials, however, have evaluated the emerging targeted therapies ustekinumab and ozanimod for histologic remission and found positive results.
Dr Bincy Abraham, director of the Fondren Inflammatory Bowel Disease Program at Houston Methodist in Houston, Texas, reports on UC treatment milestones and how emerging targeted therapies can help achieve these goals.
She also discusses patient monitoring to ensure response to therapy as well as medication adjustments should response prove inadequate.
--
Professor of Clinical Medicine, Department of Internal Medicine and Gastroenterology; Director, Fondren Inflammatory Bowel Disease Program, Underwood Center for Digestive Disorders, Houston Methodist, Houston, Texas
Bincy Abraham, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; BMS; Janssen; Pfizer; Takeda; Medtronic
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie; BMS; Janssen; Pfizer; Takeda
Received research grant from: Takeda; BMS; Genentech