User login
A healthy lifestyle may prevent the development of RA
Key clinical point: A healthy lifestyle was associated with a reduced risk of developing rheumatoid arthritis (RA) overall, as well as for seropositive and seronegative RA.
Major finding: Each unit increase in healthy lifestyle index score was associated with a reduced risk for overall RA (hazard ratio [HR] 0.86; P < .0001), seropositive RA (HR 0.85; P < .0001), and seronegative RA (HR 0.87; P < .001).
Study details: The findings come from an analysis of 2 large prospective cohorts including 107,092 women, among which 1,219 cases of incident RA (seropositive, 776; seronegative, 443) were identified.
Disclosures: The study was supported by the National Institutes of Health and Rheumatology Research Foundation Career Development Bridge Award. The authors declared no conflicts of interest.
Source: Hahn J et al. Arthritis Care Res (Hoboken). 2022 (Jan 18). Doi: 10.1002/acr.24862
Key clinical point: A healthy lifestyle was associated with a reduced risk of developing rheumatoid arthritis (RA) overall, as well as for seropositive and seronegative RA.
Major finding: Each unit increase in healthy lifestyle index score was associated with a reduced risk for overall RA (hazard ratio [HR] 0.86; P < .0001), seropositive RA (HR 0.85; P < .0001), and seronegative RA (HR 0.87; P < .001).
Study details: The findings come from an analysis of 2 large prospective cohorts including 107,092 women, among which 1,219 cases of incident RA (seropositive, 776; seronegative, 443) were identified.
Disclosures: The study was supported by the National Institutes of Health and Rheumatology Research Foundation Career Development Bridge Award. The authors declared no conflicts of interest.
Source: Hahn J et al. Arthritis Care Res (Hoboken). 2022 (Jan 18). Doi: 10.1002/acr.24862
Key clinical point: A healthy lifestyle was associated with a reduced risk of developing rheumatoid arthritis (RA) overall, as well as for seropositive and seronegative RA.
Major finding: Each unit increase in healthy lifestyle index score was associated with a reduced risk for overall RA (hazard ratio [HR] 0.86; P < .0001), seropositive RA (HR 0.85; P < .0001), and seronegative RA (HR 0.87; P < .001).
Study details: The findings come from an analysis of 2 large prospective cohorts including 107,092 women, among which 1,219 cases of incident RA (seropositive, 776; seronegative, 443) were identified.
Disclosures: The study was supported by the National Institutes of Health and Rheumatology Research Foundation Career Development Bridge Award. The authors declared no conflicts of interest.
Source: Hahn J et al. Arthritis Care Res (Hoboken). 2022 (Jan 18). Doi: 10.1002/acr.24862
Early RA: Prevalence and factors associated with methotrexate-related adverse events
Key clinical point: Adverse events (AE), most being nonserious, are common within the first year of treatment with methotrexate in patients with early rheumatoid arthritis (RA), with gastrointestinal AEs being most common and women more likely to report AEs compared with men.
Major finding: At least 1 AE within the first year of methotrexate initiation was reported by 77.5% of patients, with gastrointestinal AEs being the most common (42%) and nausea being the most reported single event (31.2%). Women vs. men were more likely to report gastrointestinal AEs (odds ratio 2.03; 95% CI 1.49-2.75).
Study details: The findings come from a prospective cohort of 1,069 patients with RA who initiated methotrexate therapy.
Disclosures: This work was supported by the Versus Arthritis and NIHR Manchester Biomedical Research Centre. All the authors declared no conflicts of interest.
Source: Sherbini AA et al. Rheumatology (Oxford). 2022 (Jan 25). Doi: 10.1093/rheumatology/keab917
Key clinical point: Adverse events (AE), most being nonserious, are common within the first year of treatment with methotrexate in patients with early rheumatoid arthritis (RA), with gastrointestinal AEs being most common and women more likely to report AEs compared with men.
Major finding: At least 1 AE within the first year of methotrexate initiation was reported by 77.5% of patients, with gastrointestinal AEs being the most common (42%) and nausea being the most reported single event (31.2%). Women vs. men were more likely to report gastrointestinal AEs (odds ratio 2.03; 95% CI 1.49-2.75).
Study details: The findings come from a prospective cohort of 1,069 patients with RA who initiated methotrexate therapy.
Disclosures: This work was supported by the Versus Arthritis and NIHR Manchester Biomedical Research Centre. All the authors declared no conflicts of interest.
Source: Sherbini AA et al. Rheumatology (Oxford). 2022 (Jan 25). Doi: 10.1093/rheumatology/keab917
Key clinical point: Adverse events (AE), most being nonserious, are common within the first year of treatment with methotrexate in patients with early rheumatoid arthritis (RA), with gastrointestinal AEs being most common and women more likely to report AEs compared with men.
Major finding: At least 1 AE within the first year of methotrexate initiation was reported by 77.5% of patients, with gastrointestinal AEs being the most common (42%) and nausea being the most reported single event (31.2%). Women vs. men were more likely to report gastrointestinal AEs (odds ratio 2.03; 95% CI 1.49-2.75).
Study details: The findings come from a prospective cohort of 1,069 patients with RA who initiated methotrexate therapy.
Disclosures: This work was supported by the Versus Arthritis and NIHR Manchester Biomedical Research Centre. All the authors declared no conflicts of interest.
Source: Sherbini AA et al. Rheumatology (Oxford). 2022 (Jan 25). Doi: 10.1093/rheumatology/keab917
A real-world analysis finds no evidence of increased CV risk with tofacitinib vs. TNFi in RA
Key clinical point: Under real-world settings, tofacitinib was not associated with a higher risk for cardiovascular (CV) outcomes compared with tumor necrosis factor inhibitors (TNFi) in patients with rheumatoid arthritis (RA); however, the risk could not be ruled out in patients with prior CV disease.
Major finding: Tofacitinib vs. TNFi was not linked with a higher risk for composite CV outcome (pooled weighted hazard ratio [pwHR] 1.01; 95% CI 0.83-1.23); however, the pwHR for patients with and without prior CV disease was 1.27 (95% CI, 0.95-1.70) and 0.81 (95% CI 0.61-1.07), respectively.
Study details: STAR-RA is a multidatabase, population-based study including 1,02,263 patients with RA who initiated treatment with tofacitinib or TNFi.
Disclosures: This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital & Harvard Medical School, Boston, MA, USA. RJ Desai and SC Kim reported receiving research grants from various sources. All the other authors reported no conflicts of interest.
Source: Khosrow-Khavar F et al. Ann Rheum Dis. 2022 (Jan 13). Doi: 10.1136/annrheumdis-2021-221915
Key clinical point: Under real-world settings, tofacitinib was not associated with a higher risk for cardiovascular (CV) outcomes compared with tumor necrosis factor inhibitors (TNFi) in patients with rheumatoid arthritis (RA); however, the risk could not be ruled out in patients with prior CV disease.
Major finding: Tofacitinib vs. TNFi was not linked with a higher risk for composite CV outcome (pooled weighted hazard ratio [pwHR] 1.01; 95% CI 0.83-1.23); however, the pwHR for patients with and without prior CV disease was 1.27 (95% CI, 0.95-1.70) and 0.81 (95% CI 0.61-1.07), respectively.
Study details: STAR-RA is a multidatabase, population-based study including 1,02,263 patients with RA who initiated treatment with tofacitinib or TNFi.
Disclosures: This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital & Harvard Medical School, Boston, MA, USA. RJ Desai and SC Kim reported receiving research grants from various sources. All the other authors reported no conflicts of interest.
Source: Khosrow-Khavar F et al. Ann Rheum Dis. 2022 (Jan 13). Doi: 10.1136/annrheumdis-2021-221915
Key clinical point: Under real-world settings, tofacitinib was not associated with a higher risk for cardiovascular (CV) outcomes compared with tumor necrosis factor inhibitors (TNFi) in patients with rheumatoid arthritis (RA); however, the risk could not be ruled out in patients with prior CV disease.
Major finding: Tofacitinib vs. TNFi was not linked with a higher risk for composite CV outcome (pooled weighted hazard ratio [pwHR] 1.01; 95% CI 0.83-1.23); however, the pwHR for patients with and without prior CV disease was 1.27 (95% CI, 0.95-1.70) and 0.81 (95% CI 0.61-1.07), respectively.
Study details: STAR-RA is a multidatabase, population-based study including 1,02,263 patients with RA who initiated treatment with tofacitinib or TNFi.
Disclosures: This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital & Harvard Medical School, Boston, MA, USA. RJ Desai and SC Kim reported receiving research grants from various sources. All the other authors reported no conflicts of interest.
Source: Khosrow-Khavar F et al. Ann Rheum Dis. 2022 (Jan 13). Doi: 10.1136/annrheumdis-2021-221915
Acute exacerbation affects prognosis in RA-associated interstitial lung disease
Key clinical point: Almost one-third of patients with rheumatoid arthritis (RA)-associated interstitial lung disease (ILD) experience acute exacerbation (AE), which significantly affects overall survival.
Major finding: Overall, AE was experienced by 28.1% of patients with RA-ILD, with the 5-year cumulative incidence being 29.4%. The occurrence of AE was significantly associated with a higher risk for mortality (adjusted hazard ratio 2.423; P < .001).
Study details: The findings come from a retrospective analysis involving 310 patients with RA-ILD.
Disclosures: This study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science and Technology. All the authors declared no conflicts of interest.
Source: Kwon BS et al. Chest. 2022 (Jan 11). Doi: 10.1016/j.chest.2022.01.007
Key clinical point: Almost one-third of patients with rheumatoid arthritis (RA)-associated interstitial lung disease (ILD) experience acute exacerbation (AE), which significantly affects overall survival.
Major finding: Overall, AE was experienced by 28.1% of patients with RA-ILD, with the 5-year cumulative incidence being 29.4%. The occurrence of AE was significantly associated with a higher risk for mortality (adjusted hazard ratio 2.423; P < .001).
Study details: The findings come from a retrospective analysis involving 310 patients with RA-ILD.
Disclosures: This study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science and Technology. All the authors declared no conflicts of interest.
Source: Kwon BS et al. Chest. 2022 (Jan 11). Doi: 10.1016/j.chest.2022.01.007
Key clinical point: Almost one-third of patients with rheumatoid arthritis (RA)-associated interstitial lung disease (ILD) experience acute exacerbation (AE), which significantly affects overall survival.
Major finding: Overall, AE was experienced by 28.1% of patients with RA-ILD, with the 5-year cumulative incidence being 29.4%. The occurrence of AE was significantly associated with a higher risk for mortality (adjusted hazard ratio 2.423; P < .001).
Study details: The findings come from a retrospective analysis involving 310 patients with RA-ILD.
Disclosures: This study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science and Technology. All the authors declared no conflicts of interest.
Source: Kwon BS et al. Chest. 2022 (Jan 11). Doi: 10.1016/j.chest.2022.01.007
More evidence supporting ultra-low retreatment dose of rituximab in RA
Key clinical point: Retreatment with a lower rituximab dose of 200 mg or 500 mg was as effective as 1000 mg in patients with rheumatoid arthritis (RA) who responded well to standard rituximab dose.
Major finding: Treatment response was not maintained in 11%, 21%, and 13% of patients in the 1000 mg, 500 mg, and 200 mg rituximab groups, respectively. Ultra-low rituximab dosage was not associated with the presence of antidrug antibodies at 6 months, and B-cell counts were not significantly different between the dosing groups.
Study details: The data comes from a preplanned secondary analysis of the REDO trial involving 140 patients with RA who responded well to the standard rituximab dose for at least 6 months and were randomly assigned to receive 200 mg, 500 mg, or 1000 mg rituximab.
Disclosures: The REDO study was funded by health insurance companies Centraal Ziekenfonds and Menzis, and this secondary analysis did not receive any external funding. The Sint Maartenskliniek (employer of 6 authors) has a patent application filed for rituximab in the treatment of polymyalgia rheumatica.
Source: Wientjes MHM et al. Rheumatology (Oxford). 2022 (Jan 12). Doi: 10.1093/rheumatology/keac024
Key clinical point: Retreatment with a lower rituximab dose of 200 mg or 500 mg was as effective as 1000 mg in patients with rheumatoid arthritis (RA) who responded well to standard rituximab dose.
Major finding: Treatment response was not maintained in 11%, 21%, and 13% of patients in the 1000 mg, 500 mg, and 200 mg rituximab groups, respectively. Ultra-low rituximab dosage was not associated with the presence of antidrug antibodies at 6 months, and B-cell counts were not significantly different between the dosing groups.
Study details: The data comes from a preplanned secondary analysis of the REDO trial involving 140 patients with RA who responded well to the standard rituximab dose for at least 6 months and were randomly assigned to receive 200 mg, 500 mg, or 1000 mg rituximab.
Disclosures: The REDO study was funded by health insurance companies Centraal Ziekenfonds and Menzis, and this secondary analysis did not receive any external funding. The Sint Maartenskliniek (employer of 6 authors) has a patent application filed for rituximab in the treatment of polymyalgia rheumatica.
Source: Wientjes MHM et al. Rheumatology (Oxford). 2022 (Jan 12). Doi: 10.1093/rheumatology/keac024
Key clinical point: Retreatment with a lower rituximab dose of 200 mg or 500 mg was as effective as 1000 mg in patients with rheumatoid arthritis (RA) who responded well to standard rituximab dose.
Major finding: Treatment response was not maintained in 11%, 21%, and 13% of patients in the 1000 mg, 500 mg, and 200 mg rituximab groups, respectively. Ultra-low rituximab dosage was not associated with the presence of antidrug antibodies at 6 months, and B-cell counts were not significantly different between the dosing groups.
Study details: The data comes from a preplanned secondary analysis of the REDO trial involving 140 patients with RA who responded well to the standard rituximab dose for at least 6 months and were randomly assigned to receive 200 mg, 500 mg, or 1000 mg rituximab.
Disclosures: The REDO study was funded by health insurance companies Centraal Ziekenfonds and Menzis, and this secondary analysis did not receive any external funding. The Sint Maartenskliniek (employer of 6 authors) has a patent application filed for rituximab in the treatment of polymyalgia rheumatica.
Source: Wientjes MHM et al. Rheumatology (Oxford). 2022 (Jan 12). Doi: 10.1093/rheumatology/keac024
Rheumatoid arthritis: Higher risk for MACE and cancer with tofacitinib vs. TNF inhibitors
Key clinical point: Tofacitinib was associated with a higher risk for major adverse cardiovascular events (MACE) and cancer than tumor necrosis factor (TNF) inhibitors in a cardiovascular risk-enriched population of patients with active rheumatoid arthritis (RA).
Major finding: During a median follow-up of 4 years, the combined tofacitinib doses vs. TNF inhibitors were associated with a higher incidence of MACE (hazard ratio [HR] 1.33; 95% CI 0.91-1.94) and cancer (HR 1.48; 95% CI 1.04-2.09), not meeting the predefined criteria for noninferiority.
Study details: The findings come from the noninferiority, phase 3b-4, safety end-point ORAL Surveillance trial involving 4,362 patients aged 50 years or older with at least 1 additional cardiovascular risk factor who had active RA despite methotrexate treatment. The patients were randomly assigned to 5 mg or 10 mg tofacitinib twice daily or a TNF inhibitor.
Disclosures: This study was funded by Pfizer. Some of the authors declared being employees or holding stocks at Pfizer, whereas some others declared serving as a consultant or receiving grants from various sources.
Source: Ytterberg SR et al. N Engl J Med. 2022;386:316-326 (Jan 27). Doi: 10.1056/NEJMoa2109927
Key clinical point: Tofacitinib was associated with a higher risk for major adverse cardiovascular events (MACE) and cancer than tumor necrosis factor (TNF) inhibitors in a cardiovascular risk-enriched population of patients with active rheumatoid arthritis (RA).
Major finding: During a median follow-up of 4 years, the combined tofacitinib doses vs. TNF inhibitors were associated with a higher incidence of MACE (hazard ratio [HR] 1.33; 95% CI 0.91-1.94) and cancer (HR 1.48; 95% CI 1.04-2.09), not meeting the predefined criteria for noninferiority.
Study details: The findings come from the noninferiority, phase 3b-4, safety end-point ORAL Surveillance trial involving 4,362 patients aged 50 years or older with at least 1 additional cardiovascular risk factor who had active RA despite methotrexate treatment. The patients were randomly assigned to 5 mg or 10 mg tofacitinib twice daily or a TNF inhibitor.
Disclosures: This study was funded by Pfizer. Some of the authors declared being employees or holding stocks at Pfizer, whereas some others declared serving as a consultant or receiving grants from various sources.
Source: Ytterberg SR et al. N Engl J Med. 2022;386:316-326 (Jan 27). Doi: 10.1056/NEJMoa2109927
Key clinical point: Tofacitinib was associated with a higher risk for major adverse cardiovascular events (MACE) and cancer than tumor necrosis factor (TNF) inhibitors in a cardiovascular risk-enriched population of patients with active rheumatoid arthritis (RA).
Major finding: During a median follow-up of 4 years, the combined tofacitinib doses vs. TNF inhibitors were associated with a higher incidence of MACE (hazard ratio [HR] 1.33; 95% CI 0.91-1.94) and cancer (HR 1.48; 95% CI 1.04-2.09), not meeting the predefined criteria for noninferiority.
Study details: The findings come from the noninferiority, phase 3b-4, safety end-point ORAL Surveillance trial involving 4,362 patients aged 50 years or older with at least 1 additional cardiovascular risk factor who had active RA despite methotrexate treatment. The patients were randomly assigned to 5 mg or 10 mg tofacitinib twice daily or a TNF inhibitor.
Disclosures: This study was funded by Pfizer. Some of the authors declared being employees or holding stocks at Pfizer, whereas some others declared serving as a consultant or receiving grants from various sources.
Source: Ytterberg SR et al. N Engl J Med. 2022;386:316-326 (Jan 27). Doi: 10.1056/NEJMoa2109927
Clinical Edge Journal Scan Commentary: PsA March 2022
The influence of sex and gender on psoriatic arthritis (PsA) continues to be of interest. Using data from the Dutch south-west Early Psoriatic Arthritis cohort (DEPAR), Passia et al1assessed sex-related differences in demographics, disease characteristics, and evolution over 1 year in 273 men and 294 women newly diagnosed with PsA. They found that at baseline, women had a significantly longer duration of symptoms, higher tender joint count and enthesitis, higher disease activity, higher levels of pain, more severe limitations in function and worse quality of life. During the 1 year follow up, composite measures of disease activity declined in men and women, but women continued to have higher levels than men. At the end of 1 year, fewer women achieved the criteria for minimal disease activity (MDA). Thus, the disease burden of PsA was higher in women vs. men at all time points and even after 1 year of standard-of-care treatment. Sex-specific treatment strategies might help a higher proportion of women achieve MDA.
Although, enthesitis is believed to be a primary pathogenetic lesion in PsA, the relationship between active enthesitis and disease severity as measured by the presence of joint erosions is less well studied. In a cross-sectional study of 104 PsA patients, Smerilli et al2 explored the association between ultrasound (US) entheseal abnormalities and the presence of US detected bone erosions in PsA joints. At least 1 joint bone erosion was found in 45.2% of patients and was associated with power Doppler signal at enthesis (odds ratio [OR] 1.74; P < .01), entheseal bone erosions (OR 3.17; P = .01), and greyscale synovitis (OR 2.59; P = .02). Thus, Doppler signal and bone erosions at entheses indicate more severe PsA and patients with such abnormalities should therefore be treated aggressively.
Comorbidities and associated conditions were a focus of several publications last month. Venous thromboembolism (VTE) is associated with inflammatory diseases, including PsA. In a retrospective cohort study including 5,275 patients with newly diagnosed PsA, Gazitt et al3 assessed the association between PsA and VTE events using a large population-based database in Israel. During follow-up, 1.2% vs. 0.8% patients in the PsA vs. control group were diagnosed with VTE, but this association was not statistically significant after adjusting for demographic factors and comorbidities (adjusted hazard ratio [aHR] 1.27; P = .16) with only older age (aHR 1.08; P < .0001) and history of VTE (aHR 31.63; P < .0001) remaining associated with an increased risk for VTE. Thus, VTE in patients with PsA may be associated with underlying comorbidities rather than PsA per se. In another study, Harris et al4 demonstrated that PsA was associated with increased risk of endometriosis. In an analysis of 4112 patients with laparoscopically confirmed endometriosis from the Nurses’ Health Study II, they found that psoriasis with concomitant PsA was associated with increased risk for subsequent endometriosis (HR 2.01; 95% CI 1.23-3.30), which persisted even after adjusting for comorbidities. Finally, in a cross-sectional study using data from 1862 juvenile PsA (jPsA) patients (122 [6.6%] of whom developed uveitis) in the German National Pediatric Rheumatological Database, Walscheid et al5 showed that patients with jPsA were more likely to develop uveitis if they were diagnosed with PsA at a younger age or were antinuclear antibody positive, with higher disease activity being the only factor significantly associated with the presence of uveitis.
References
1. Passia E et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22 (Jan 11).
2. Smerilli G et al. Doppler signal and bone erosions at the enthesis are independently associated with ultrasound joint erosive damage in psoriatic arthritis. J Rheumatol. 2022 (Feb 1).
3. Gazitt T et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022;24(1):16 (Jan 7).
4. Harris HR et al. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 2022 (Jan 13). doi: 10.1093/aje/kwac009. Epub ahead of print. PMID: 35029650.
5. Walscheid K, Rothaus K, Niewerth M, Klotsche J, Minden K, Heiligenhaus A. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J Rheumatol. 2022 (Jan 15). doi: 10.3899/jrheum.210755. Epub ahead of print. PMID: 35034000.
The influence of sex and gender on psoriatic arthritis (PsA) continues to be of interest. Using data from the Dutch south-west Early Psoriatic Arthritis cohort (DEPAR), Passia et al1assessed sex-related differences in demographics, disease characteristics, and evolution over 1 year in 273 men and 294 women newly diagnosed with PsA. They found that at baseline, women had a significantly longer duration of symptoms, higher tender joint count and enthesitis, higher disease activity, higher levels of pain, more severe limitations in function and worse quality of life. During the 1 year follow up, composite measures of disease activity declined in men and women, but women continued to have higher levels than men. At the end of 1 year, fewer women achieved the criteria for minimal disease activity (MDA). Thus, the disease burden of PsA was higher in women vs. men at all time points and even after 1 year of standard-of-care treatment. Sex-specific treatment strategies might help a higher proportion of women achieve MDA.
Although, enthesitis is believed to be a primary pathogenetic lesion in PsA, the relationship between active enthesitis and disease severity as measured by the presence of joint erosions is less well studied. In a cross-sectional study of 104 PsA patients, Smerilli et al2 explored the association between ultrasound (US) entheseal abnormalities and the presence of US detected bone erosions in PsA joints. At least 1 joint bone erosion was found in 45.2% of patients and was associated with power Doppler signal at enthesis (odds ratio [OR] 1.74; P < .01), entheseal bone erosions (OR 3.17; P = .01), and greyscale synovitis (OR 2.59; P = .02). Thus, Doppler signal and bone erosions at entheses indicate more severe PsA and patients with such abnormalities should therefore be treated aggressively.
Comorbidities and associated conditions were a focus of several publications last month. Venous thromboembolism (VTE) is associated with inflammatory diseases, including PsA. In a retrospective cohort study including 5,275 patients with newly diagnosed PsA, Gazitt et al3 assessed the association between PsA and VTE events using a large population-based database in Israel. During follow-up, 1.2% vs. 0.8% patients in the PsA vs. control group were diagnosed with VTE, but this association was not statistically significant after adjusting for demographic factors and comorbidities (adjusted hazard ratio [aHR] 1.27; P = .16) with only older age (aHR 1.08; P < .0001) and history of VTE (aHR 31.63; P < .0001) remaining associated with an increased risk for VTE. Thus, VTE in patients with PsA may be associated with underlying comorbidities rather than PsA per se. In another study, Harris et al4 demonstrated that PsA was associated with increased risk of endometriosis. In an analysis of 4112 patients with laparoscopically confirmed endometriosis from the Nurses’ Health Study II, they found that psoriasis with concomitant PsA was associated with increased risk for subsequent endometriosis (HR 2.01; 95% CI 1.23-3.30), which persisted even after adjusting for comorbidities. Finally, in a cross-sectional study using data from 1862 juvenile PsA (jPsA) patients (122 [6.6%] of whom developed uveitis) in the German National Pediatric Rheumatological Database, Walscheid et al5 showed that patients with jPsA were more likely to develop uveitis if they were diagnosed with PsA at a younger age or were antinuclear antibody positive, with higher disease activity being the only factor significantly associated with the presence of uveitis.
References
1. Passia E et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22 (Jan 11).
2. Smerilli G et al. Doppler signal and bone erosions at the enthesis are independently associated with ultrasound joint erosive damage in psoriatic arthritis. J Rheumatol. 2022 (Feb 1).
3. Gazitt T et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022;24(1):16 (Jan 7).
4. Harris HR et al. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 2022 (Jan 13). doi: 10.1093/aje/kwac009. Epub ahead of print. PMID: 35029650.
5. Walscheid K, Rothaus K, Niewerth M, Klotsche J, Minden K, Heiligenhaus A. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J Rheumatol. 2022 (Jan 15). doi: 10.3899/jrheum.210755. Epub ahead of print. PMID: 35034000.
The influence of sex and gender on psoriatic arthritis (PsA) continues to be of interest. Using data from the Dutch south-west Early Psoriatic Arthritis cohort (DEPAR), Passia et al1assessed sex-related differences in demographics, disease characteristics, and evolution over 1 year in 273 men and 294 women newly diagnosed with PsA. They found that at baseline, women had a significantly longer duration of symptoms, higher tender joint count and enthesitis, higher disease activity, higher levels of pain, more severe limitations in function and worse quality of life. During the 1 year follow up, composite measures of disease activity declined in men and women, but women continued to have higher levels than men. At the end of 1 year, fewer women achieved the criteria for minimal disease activity (MDA). Thus, the disease burden of PsA was higher in women vs. men at all time points and even after 1 year of standard-of-care treatment. Sex-specific treatment strategies might help a higher proportion of women achieve MDA.
Although, enthesitis is believed to be a primary pathogenetic lesion in PsA, the relationship between active enthesitis and disease severity as measured by the presence of joint erosions is less well studied. In a cross-sectional study of 104 PsA patients, Smerilli et al2 explored the association between ultrasound (US) entheseal abnormalities and the presence of US detected bone erosions in PsA joints. At least 1 joint bone erosion was found in 45.2% of patients and was associated with power Doppler signal at enthesis (odds ratio [OR] 1.74; P < .01), entheseal bone erosions (OR 3.17; P = .01), and greyscale synovitis (OR 2.59; P = .02). Thus, Doppler signal and bone erosions at entheses indicate more severe PsA and patients with such abnormalities should therefore be treated aggressively.
Comorbidities and associated conditions were a focus of several publications last month. Venous thromboembolism (VTE) is associated with inflammatory diseases, including PsA. In a retrospective cohort study including 5,275 patients with newly diagnosed PsA, Gazitt et al3 assessed the association between PsA and VTE events using a large population-based database in Israel. During follow-up, 1.2% vs. 0.8% patients in the PsA vs. control group were diagnosed with VTE, but this association was not statistically significant after adjusting for demographic factors and comorbidities (adjusted hazard ratio [aHR] 1.27; P = .16) with only older age (aHR 1.08; P < .0001) and history of VTE (aHR 31.63; P < .0001) remaining associated with an increased risk for VTE. Thus, VTE in patients with PsA may be associated with underlying comorbidities rather than PsA per se. In another study, Harris et al4 demonstrated that PsA was associated with increased risk of endometriosis. In an analysis of 4112 patients with laparoscopically confirmed endometriosis from the Nurses’ Health Study II, they found that psoriasis with concomitant PsA was associated with increased risk for subsequent endometriosis (HR 2.01; 95% CI 1.23-3.30), which persisted even after adjusting for comorbidities. Finally, in a cross-sectional study using data from 1862 juvenile PsA (jPsA) patients (122 [6.6%] of whom developed uveitis) in the German National Pediatric Rheumatological Database, Walscheid et al5 showed that patients with jPsA were more likely to develop uveitis if they were diagnosed with PsA at a younger age or were antinuclear antibody positive, with higher disease activity being the only factor significantly associated with the presence of uveitis.
References
1. Passia E et al. Sex-specific differences and how to handle them in early psoriatic arthritis. Arthritis Res Ther. 2022;24(1):22 (Jan 11).
2. Smerilli G et al. Doppler signal and bone erosions at the enthesis are independently associated with ultrasound joint erosive damage in psoriatic arthritis. J Rheumatol. 2022 (Feb 1).
3. Gazitt T et al. The association between psoriatic arthritis and venous thromboembolism: a population-based cohort study. Arthritis Res Ther. 2022;24(1):16 (Jan 7).
4. Harris HR et al. Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 2022 (Jan 13). doi: 10.1093/aje/kwac009. Epub ahead of print. PMID: 35029650.
5. Walscheid K, Rothaus K, Niewerth M, Klotsche J, Minden K, Heiligenhaus A. Occurrence and risk factors of uveitis in juvenile psoriatic arthritis: Data from a population-based nationwide study in Germany. J Rheumatol. 2022 (Jan 15). doi: 10.3899/jrheum.210755. Epub ahead of print. PMID: 35034000.
Clinical Edge Journal Scan Commentary: HCC March 2022
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Clinical Edge Journal Scan Commentary: HCC March 2022
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
Kaseb et al report the results of a Phase 2 study where 27 patients with resectable HCC were randomized to receive either nivolumab alone or the combination of nivolumab and ipilimumab for 6 weeks before surgery, and then for up to 2 years after resection. Estimated median progression-free survival (PFS) was 9.4 months with nivolumab and 19.53 months with nivolumab plus ipilimumab (hazard ratio [HR] 0.99, 95% CI 0.31–2.54); median time to progression was 9.4 months in the nivolumab group and 19.53 months in the nivolumab plus ipilimumab group (HR 0.89, 95% CI 0.31–2.54). Three (23%) of 13 patients had an overall response with nivolumab monotherapy, versus none with nivolumab plus ipilimumab. Three (33%) of nine patients had a major pathological response (ie, ≥70% necrosis in the resected tumor area) with nivolumab monotherapy compared with three (27%) of 11 with nivolumab plus ipilimumab. Grade 3–4 adverse events were higher with nivolumab plus ipilimumab (six [43%] of 14 patients) than with nivolumab alone (three [23%] of 13). The authors concluded that immunotherapy is safe and feasible in patients with resectable hepatocellular carcinoma.
Marron et al. evaluated the clinical activity of cemiplimab (an anti-PD-1) in 21 patients with resectable hepatocellular carcinoma. Cemiplimab was administered twice every 3 weeks before and 8 times after surgery. Of the 20 patients with resected tumors, four (20%) had significant (>70%) tumor necrosis with 15% showing complete (100%) tumor necrosis. Three (15%) of 20 patients had a radiologic partial response, and all other patients maintained stable disease. Seven (33%) patients had grade 3 adverse events. No grade 4 or 5 events were observed. The investigators concluded that perioperative cemiplimab should be studied further in patients with resectable HCC.
Finally, Guan et al. compared outcomes of 498 patients with resected HCC who also had hepatitis B virus infection (defined as HBsAg-positivity for >90 days). Of those, 367 patients (73.69%) received at least 3 months of postoperative anti-viral treatment (AVT), while 131 (27.31%) did not (non-AVT group). Propensity score matching (PSM) analysis was performed on 206 patients. AVT was associated with better recurrence-free survival (RFS) and overall survival (OS) either before or after PSM. After PSM, the 1-, 3-, and 5-year RFS rates were 85.3%, 65.7%, and 19.1% vs. 76.7%, 46.6%, and 5.8% in the AVT and non-AVT groups, respectively (P = .001). The corresponding 1-, 3-, and 5-year OS rates were 99.0%, 89.8%, and 64.0% vs. 96.1%, 70.5%, and 43.2% in the AVT and non-AVT groups (P < .001). Risk factors that were independently associated with a poor RFS included HBV DNA positivity (P = .002), preoperative alpha fetoprotein (AFP) level of ≥20 ng/mL (P < .001), poor differentiation (P = .022), multiple tumors (P = .037), and microvascular invasion (P < .001). The conclusion was that AVT improves outcomes in patients with HBV and resectable HCC.
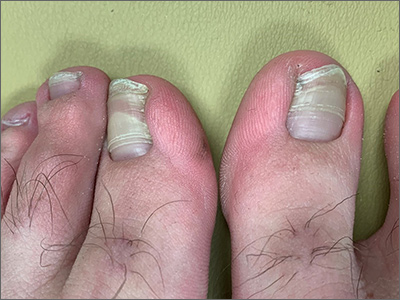
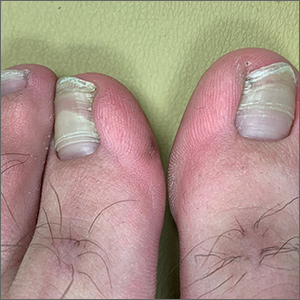
Toenail ridges
Transverse ridges that grow out with the nails are called Beau lines, also known as Beau’s ridges. This contrasts with Mees lines which are transverse white bands that grow out with the toenails, are nonpalpable, and are attributed to arsenic poisoning.
Beau lines are caused by a disruption in nail growth that can result from trauma, hypotension, or systemic or severe illness; they have also been reported in cases of COVID-19.1 Beau lines can occur on a single nail if the trauma or injury is isolated to 1 digit. If there was a systemic illness or stress, the lines can affect all 20 nails. The time of the inciting event can be approximated by how far the lines are from the cuticle. While there is some variability, it usually takes 12 to 18 months to grow an entirely new toenail. If the Beau lines have grown halfway out, then the stressor likely occurred 6 to 9 months earlier.
In this image, some asymmetry is visible between the right and left great toenails and there are some subtle distal changes, raising the possibility that there was more than 1 injury to this patient’s system (or prolonged difficulty). The patient said that to his knowledge, he had not been infected with COVID-19. However, hair and nail changes may be the only finding in some individuals who have been infected with COVID-19.1
This patient was counseled regarding the nature of this disorder and that without knowing what illness or injury caused the change, it was a benign finding. He was advised that it did not appear to be onychomycosis and did not require any medications or antifungal therapy. The patient was told to follow up if any changes developed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140. doi: 10.1016/j.jdcr.2021.05.026
Transverse ridges that grow out with the nails are called Beau lines, also known as Beau’s ridges. This contrasts with Mees lines which are transverse white bands that grow out with the toenails, are nonpalpable, and are attributed to arsenic poisoning.
Beau lines are caused by a disruption in nail growth that can result from trauma, hypotension, or systemic or severe illness; they have also been reported in cases of COVID-19.1 Beau lines can occur on a single nail if the trauma or injury is isolated to 1 digit. If there was a systemic illness or stress, the lines can affect all 20 nails. The time of the inciting event can be approximated by how far the lines are from the cuticle. While there is some variability, it usually takes 12 to 18 months to grow an entirely new toenail. If the Beau lines have grown halfway out, then the stressor likely occurred 6 to 9 months earlier.
In this image, some asymmetry is visible between the right and left great toenails and there are some subtle distal changes, raising the possibility that there was more than 1 injury to this patient’s system (or prolonged difficulty). The patient said that to his knowledge, he had not been infected with COVID-19. However, hair and nail changes may be the only finding in some individuals who have been infected with COVID-19.1
This patient was counseled regarding the nature of this disorder and that without knowing what illness or injury caused the change, it was a benign finding. He was advised that it did not appear to be onychomycosis and did not require any medications or antifungal therapy. The patient was told to follow up if any changes developed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Transverse ridges that grow out with the nails are called Beau lines, also known as Beau’s ridges. This contrasts with Mees lines which are transverse white bands that grow out with the toenails, are nonpalpable, and are attributed to arsenic poisoning.
Beau lines are caused by a disruption in nail growth that can result from trauma, hypotension, or systemic or severe illness; they have also been reported in cases of COVID-19.1 Beau lines can occur on a single nail if the trauma or injury is isolated to 1 digit. If there was a systemic illness or stress, the lines can affect all 20 nails. The time of the inciting event can be approximated by how far the lines are from the cuticle. While there is some variability, it usually takes 12 to 18 months to grow an entirely new toenail. If the Beau lines have grown halfway out, then the stressor likely occurred 6 to 9 months earlier.
In this image, some asymmetry is visible between the right and left great toenails and there are some subtle distal changes, raising the possibility that there was more than 1 injury to this patient’s system (or prolonged difficulty). The patient said that to his knowledge, he had not been infected with COVID-19. However, hair and nail changes may be the only finding in some individuals who have been infected with COVID-19.1
This patient was counseled regarding the nature of this disorder and that without knowing what illness or injury caused the change, it was a benign finding. He was advised that it did not appear to be onychomycosis and did not require any medications or antifungal therapy. The patient was told to follow up if any changes developed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140. doi: 10.1016/j.jdcr.2021.05.026
- Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140. doi: 10.1016/j.jdcr.2021.05.026