User login
Cancer Data Trends 2022: Gastrointestinal Cancers
Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol. 2021;18(6):432-443. doi: 10.1038/s41575-021-00419-3
Fitzgerald RC, di Pietro M, O'Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396(10247):333-344. doi:10.1016/S0140-6736(20)31099-0
Krishna Chandar A, Sharma A, Chak A. Novel screening alternatives for Barrett esophagus. Gastroenterol Hepatol (NY). 2020;16(5):238-245. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8132638/
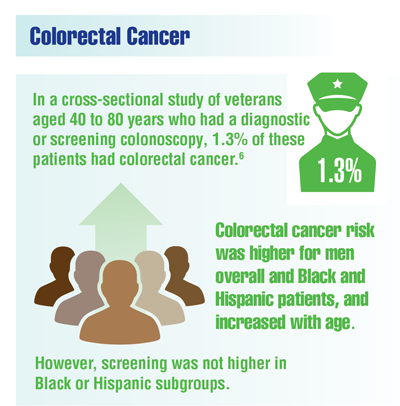
Centers for Disease Control and Prevention. Colorectal cancer awareness. Updated February 23, 2021. Accessed December 14, 2021. https://www.cdc.gov/cancer/dcpc/resources/features/colorectalawareness/index.htm
US Preventive Services Task Force. Final Recommendation Statement: Colorectal Cancer: Screening. Published May 18, 2021. Accessed January 27, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
Imperiale TF, Daggy JK, Imler TM, et al. Prevalence of advanced colorectal neoplasia in veterans: effects of age, sex, and race/ethnicity. J Clin Gastroenterol. 2021;55(10):876-883. doi:10.1097/MCG.0000000000001402
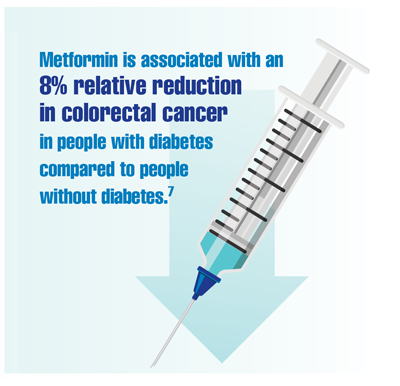
Demb J, Yaseyyedi A, Liu L, et al. Metformin is associated with reduced odds for colorectal cancer among persons with diabetes. Clin Transl Gastroenterol. 2019;10(11):e00092. doi:10.14309/ctg.0000000000000092
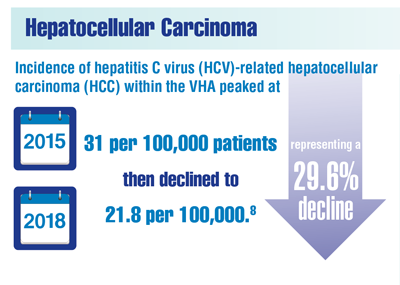
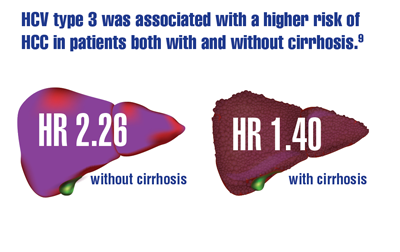
Beste LA, Green P, Berry K, et al. Hepatitis C-related hepatocellular carcinoma incidence in the Veterans Health Administration after introduction of direct-acting antivirals. JAMA. 2020;324(10):1003-1005. doi:10.1001/jama.2020.10121
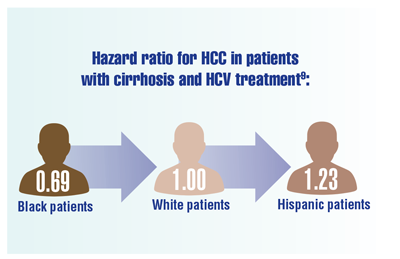
Kanwal F, Kramer JR, Asch SM, et al. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology. 2020;71(1):44-55. doi:10.1002/hep.30823
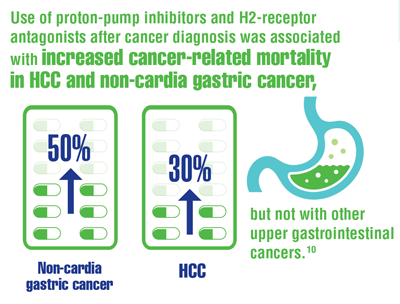
Ayyagari S, Tan MC, Liu Y, et al. Use of acid-suppressant medications after diagnosis increases mortality in a subset of gastrointestinal cancer patients. Dig Dis Sci. 2020;65(9):2691-2699. doi:10.1007/s10620-019-05984-x
Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol. 2021;18(6):432-443. doi: 10.1038/s41575-021-00419-3
Fitzgerald RC, di Pietro M, O'Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396(10247):333-344. doi:10.1016/S0140-6736(20)31099-0
Krishna Chandar A, Sharma A, Chak A. Novel screening alternatives for Barrett esophagus. Gastroenterol Hepatol (NY). 2020;16(5):238-245. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8132638/
Centers for Disease Control and Prevention. Colorectal cancer awareness. Updated February 23, 2021. Accessed December 14, 2021. https://www.cdc.gov/cancer/dcpc/resources/features/colorectalawareness/index.htm
US Preventive Services Task Force. Final Recommendation Statement: Colorectal Cancer: Screening. Published May 18, 2021. Accessed January 27, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
Imperiale TF, Daggy JK, Imler TM, et al. Prevalence of advanced colorectal neoplasia in veterans: effects of age, sex, and race/ethnicity. J Clin Gastroenterol. 2021;55(10):876-883. doi:10.1097/MCG.0000000000001402
Demb J, Yaseyyedi A, Liu L, et al. Metformin is associated with reduced odds for colorectal cancer among persons with diabetes. Clin Transl Gastroenterol. 2019;10(11):e00092. doi:10.14309/ctg.0000000000000092
Beste LA, Green P, Berry K, et al. Hepatitis C-related hepatocellular carcinoma incidence in the Veterans Health Administration after introduction of direct-acting antivirals. JAMA. 2020;324(10):1003-1005. doi:10.1001/jama.2020.10121
Kanwal F, Kramer JR, Asch SM, et al. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology. 2020;71(1):44-55. doi:10.1002/hep.30823
Ayyagari S, Tan MC, Liu Y, et al. Use of acid-suppressant medications after diagnosis increases mortality in a subset of gastrointestinal cancer patients. Dig Dis Sci. 2020;65(9):2691-2699. doi:10.1007/s10620-019-05984-x
Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat Rev Gastroenterol Hepatol. 2021;18(6):432-443. doi: 10.1038/s41575-021-00419-3
Fitzgerald RC, di Pietro M, O'Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett's oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396(10247):333-344. doi:10.1016/S0140-6736(20)31099-0
Krishna Chandar A, Sharma A, Chak A. Novel screening alternatives for Barrett esophagus. Gastroenterol Hepatol (NY). 2020;16(5):238-245. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8132638/
Centers for Disease Control and Prevention. Colorectal cancer awareness. Updated February 23, 2021. Accessed December 14, 2021. https://www.cdc.gov/cancer/dcpc/resources/features/colorectalawareness/index.htm
US Preventive Services Task Force. Final Recommendation Statement: Colorectal Cancer: Screening. Published May 18, 2021. Accessed January 27, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
Imperiale TF, Daggy JK, Imler TM, et al. Prevalence of advanced colorectal neoplasia in veterans: effects of age, sex, and race/ethnicity. J Clin Gastroenterol. 2021;55(10):876-883. doi:10.1097/MCG.0000000000001402
Demb J, Yaseyyedi A, Liu L, et al. Metformin is associated with reduced odds for colorectal cancer among persons with diabetes. Clin Transl Gastroenterol. 2019;10(11):e00092. doi:10.14309/ctg.0000000000000092
Beste LA, Green P, Berry K, et al. Hepatitis C-related hepatocellular carcinoma incidence in the Veterans Health Administration after introduction of direct-acting antivirals. JAMA. 2020;324(10):1003-1005. doi:10.1001/jama.2020.10121
Kanwal F, Kramer JR, Asch SM, et al. Long-term risk of hepatocellular carcinoma in HCV patients treated with direct acting antiviral agents. Hepatology. 2020;71(1):44-55. doi:10.1002/hep.30823
Ayyagari S, Tan MC, Liu Y, et al. Use of acid-suppressant medications after diagnosis increases mortality in a subset of gastrointestinal cancer patients. Dig Dis Sci. 2020;65(9):2691-2699. doi:10.1007/s10620-019-05984-x
Removal of Isotretinoin Gender-Based Guidelines: Inclusivity Takes Precedence
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Resident Pearls
- Major changes in the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) system recently took place, including simplifying registration categories while making the process more inclusive for patients.
- It is important to practice culturally sensitive language when discussing subjects regarding gender identification and sexual practices. Sample questions have been provided to help familiarize practitioners with optimal ways to approach these patient encounters.
- There likely will be more changes with iPLEDGE REMS in the future as the American Academy of Dermatology Association continues to work on solutions regarding decreasing monthly qualifications for patients who cannot get pregnant and possible removal of patient attestation requirements.
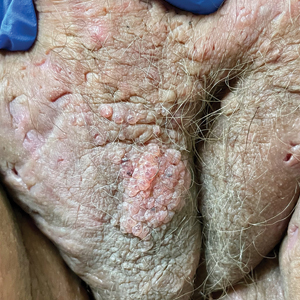
Chronic Vulvar Plaque in a Patient With Severe Hidradenitis Suppurativa
The Diagnosis: Acquired Lymphangioma Circumscriptum
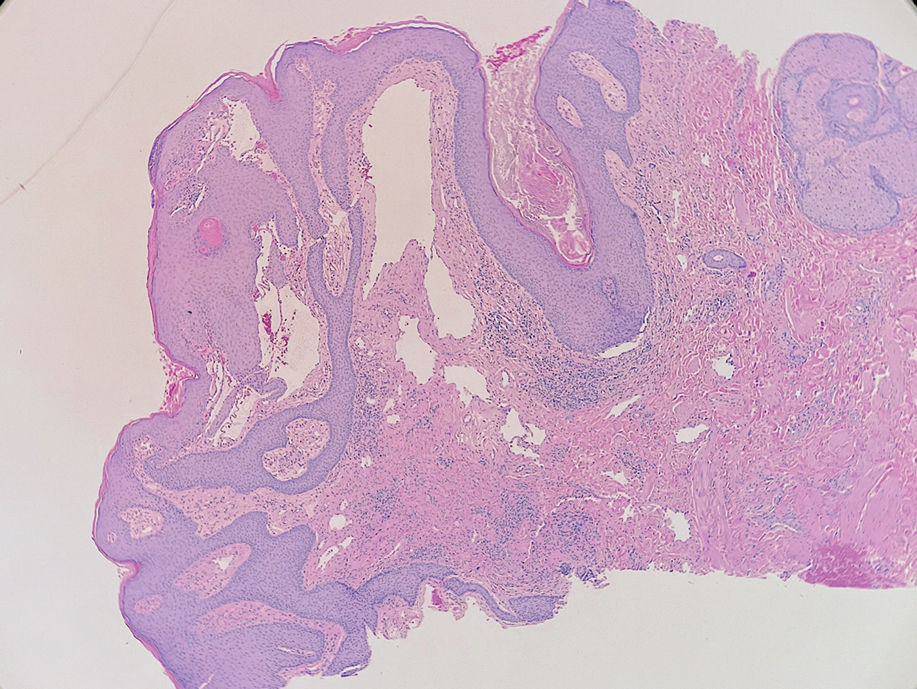
A skin biopsy of the plaque on the right labium majus showed a proliferation of well-formed, dilated lymphatic vessels lined by benign-appearing endothelial cells in the papillary dermis (Figure). These findings were consistent with a diagnosis of acquired lymphangioma circumscriptum (ALC) in the setting of severe hidradenitis suppurativa (HS).
Acquired lymphangioma circumscriptum (also known as acquired lymphangiectasia or secondary lymphangioma1) is a rare skin finding resulting from chronic lymphatic obstruction that leads to dilated lymphatic vessels within the dermis.2,3 There also is a distinct congenital form of lymphangioma circumscriptum caused by lymphatic malformations present at birth.2,4 Acquired lymphangioma circumscriptum of the vulva is a rare phenomenon.3 Identified causes include radiation or surgery for carcinoma, solid gynecologic tumors, lymphadenectomy, Crohn disease, and tuberculosis and other infections, all of which can disrupt normal lymphatics to cause ALC.2-4 Hidradenitis suppurativa is not a widely recognized cause of ALC; however, this phenomenon is reported in the literature. A long-standing history of severe HS complicated by lymphedema seems to precede the development of ALC in the reported cases, as in our patient.5-7
Acquired lymphangioma circumscriptum of the vulva can appear in women of all ages as frog spawn or cobblestone papules or vesicles, sometimes with a hyperkeratotic or verrucous appearance.2,4 Associated symptoms include serous drainage, edema, pruritus, and discomfort. The lesions may become eroded, which can predispose patients to secondary infections.1,2 Acquired lymphangioma circumscriptum of the vulva can be difficult to diagnose, as the time interval between the initial cause and the appearance of skin findings can be years, leading to the misdiagnosis of ALC as other similar-appearing genital skin conditions such as squamous cell carcinoma or condyloma.4,8 When misidentified as an infection, diagnosis can lead to substantial distress, abstinence from sexual activity, and unnecessary and painful treatments.
Skin biopsy is helpful in distinguishing ALC from other differential diagnoses such as condylomata acuminata, squamous cell carcinoma, and condyloma lata. Histopathology in ALC is notable for dilated lymphatic vessels filled with hypocellular fluid and lined with endothelial cells in the superficial dermis; the epidermis can appear hyperplastic, hyperkeratotic, or eroded.3-5,9 These lymphatic vessels stain positively for CD31 and D2-40, markers for endothelial cells and lymphatic endothelium, respectively, and negative for CD34, a marker for vascular endothelium.3,4,9 Features suggestive of condylomata acuminata such as rounded parakeratosis, hypergranulosis, and vacuolated keratinocytes9 are not present. The giant condyloma of Buschke-Löwenstein, a clinical variant of verrucous squamous cell carcinoma, also can present as a warty ulcerated papule or plaque in the genital region, but the characteristic rounded eosinophilic keratinocytes pushing down into the dermis9 are not seen in ALC. Secondary syphilis is associated with condyloma lata, which are verrucous or fleshy-appearing papules often coalescing into plaques located in the anogenital region. Pathologic features of secondary syphilis include vacuolar interface dermatitis and acanthosis with long slender rete ridges.9 Squamous cell carcinoma, which can arise from inflammation associated with long-standing HS, must be ruled out, as it is associated with a high risk of mortality in patients with HS.10
It is noteworthy to recognize the various, often confusing nomenclature used to describe cutaneous lymphatic conditions. The terms acquired lymphangioma circumscriptum, secondary lymphangioma, and lymphangiectasia are used interchangeably to describe dilated lymphatic vessels in the skin.1 The term atypical vascular lesion refers to lymphectasias of the skin of the breast due to prior radiation therapy most often used in the treatment of breast carcinoma; clinically, these present as red-brown or flesh-colored papules or telangiectatic plaques on the breast.11,12 Lymphedema also may occur alongside atypical vascular lesions, as prior radiation or surgical lymph node dissection can predispose patients to impaired lymphatic drainage.13 The lymphatic histopathologic subtype of atypical vascular lesions may appear similar to ALC; however, the vascular subtype will demonstrate collections of capillary-sized vessels and extravasated erythrocytes.11,12 Unlike ALC, the benign nature of atypical vascular lesions has been questioned, as they may be associated with a small risk for progression to angiosarcoma.11-13 It also is important to distinguish ALC from lymphangiomatosis, a generalized lymphatic anomaly that is characterized by extensive lymphatic malformations involving numerous internal organs, including the lungs and gastrointestinal tract. This condition is associated with notable morbidity and mortality.13
Although the suffix of the term lymphangioma suggests a neoplastic process, ALC is not a neoplasm and can be managed expectantly in many cases.2,3,8 However, due to cosmetic appearance, pain, discomfort, and recurrent bacterial superinfections, many patients pursue treatment. Treatment options for ALC include sclerotherapy, electrocautery, radiofrequency or carbon dioxide laser ablation, and excision, though recurrence can arise.3-5,7,8 Our patient elected to manage her asymptomatic ALC expectantly.
- Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487.
- Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
- Sims SM, McLean FW, Davis JD, et al. Vulvar lymphangioma circumscriptum: a report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010; 14:234-237.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;6:1223-1224.
- Piernick DM 2nd, Mahmood SH, Daveluy S. Acquired lymphangioma circumscriptum of the genitals in an individual with chronic hidradenitis suppurativa. JAAD Case Rep. 2018;1:64-66.
- Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;1:118-120.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2019.
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
The Diagnosis: Acquired Lymphangioma Circumscriptum
A skin biopsy of the plaque on the right labium majus showed a proliferation of well-formed, dilated lymphatic vessels lined by benign-appearing endothelial cells in the papillary dermis (Figure). These findings were consistent with a diagnosis of acquired lymphangioma circumscriptum (ALC) in the setting of severe hidradenitis suppurativa (HS).

Acquired lymphangioma circumscriptum (also known as acquired lymphangiectasia or secondary lymphangioma1) is a rare skin finding resulting from chronic lymphatic obstruction that leads to dilated lymphatic vessels within the dermis.2,3 There also is a distinct congenital form of lymphangioma circumscriptum caused by lymphatic malformations present at birth.2,4 Acquired lymphangioma circumscriptum of the vulva is a rare phenomenon.3 Identified causes include radiation or surgery for carcinoma, solid gynecologic tumors, lymphadenectomy, Crohn disease, and tuberculosis and other infections, all of which can disrupt normal lymphatics to cause ALC.2-4 Hidradenitis suppurativa is not a widely recognized cause of ALC; however, this phenomenon is reported in the literature. A long-standing history of severe HS complicated by lymphedema seems to precede the development of ALC in the reported cases, as in our patient.5-7
Acquired lymphangioma circumscriptum of the vulva can appear in women of all ages as frog spawn or cobblestone papules or vesicles, sometimes with a hyperkeratotic or verrucous appearance.2,4 Associated symptoms include serous drainage, edema, pruritus, and discomfort. The lesions may become eroded, which can predispose patients to secondary infections.1,2 Acquired lymphangioma circumscriptum of the vulva can be difficult to diagnose, as the time interval between the initial cause and the appearance of skin findings can be years, leading to the misdiagnosis of ALC as other similar-appearing genital skin conditions such as squamous cell carcinoma or condyloma.4,8 When misidentified as an infection, diagnosis can lead to substantial distress, abstinence from sexual activity, and unnecessary and painful treatments.
Skin biopsy is helpful in distinguishing ALC from other differential diagnoses such as condylomata acuminata, squamous cell carcinoma, and condyloma lata. Histopathology in ALC is notable for dilated lymphatic vessels filled with hypocellular fluid and lined with endothelial cells in the superficial dermis; the epidermis can appear hyperplastic, hyperkeratotic, or eroded.3-5,9 These lymphatic vessels stain positively for CD31 and D2-40, markers for endothelial cells and lymphatic endothelium, respectively, and negative for CD34, a marker for vascular endothelium.3,4,9 Features suggestive of condylomata acuminata such as rounded parakeratosis, hypergranulosis, and vacuolated keratinocytes9 are not present. The giant condyloma of Buschke-Löwenstein, a clinical variant of verrucous squamous cell carcinoma, also can present as a warty ulcerated papule or plaque in the genital region, but the characteristic rounded eosinophilic keratinocytes pushing down into the dermis9 are not seen in ALC. Secondary syphilis is associated with condyloma lata, which are verrucous or fleshy-appearing papules often coalescing into plaques located in the anogenital region. Pathologic features of secondary syphilis include vacuolar interface dermatitis and acanthosis with long slender rete ridges.9 Squamous cell carcinoma, which can arise from inflammation associated with long-standing HS, must be ruled out, as it is associated with a high risk of mortality in patients with HS.10
It is noteworthy to recognize the various, often confusing nomenclature used to describe cutaneous lymphatic conditions. The terms acquired lymphangioma circumscriptum, secondary lymphangioma, and lymphangiectasia are used interchangeably to describe dilated lymphatic vessels in the skin.1 The term atypical vascular lesion refers to lymphectasias of the skin of the breast due to prior radiation therapy most often used in the treatment of breast carcinoma; clinically, these present as red-brown or flesh-colored papules or telangiectatic plaques on the breast.11,12 Lymphedema also may occur alongside atypical vascular lesions, as prior radiation or surgical lymph node dissection can predispose patients to impaired lymphatic drainage.13 The lymphatic histopathologic subtype of atypical vascular lesions may appear similar to ALC; however, the vascular subtype will demonstrate collections of capillary-sized vessels and extravasated erythrocytes.11,12 Unlike ALC, the benign nature of atypical vascular lesions has been questioned, as they may be associated with a small risk for progression to angiosarcoma.11-13 It also is important to distinguish ALC from lymphangiomatosis, a generalized lymphatic anomaly that is characterized by extensive lymphatic malformations involving numerous internal organs, including the lungs and gastrointestinal tract. This condition is associated with notable morbidity and mortality.13
Although the suffix of the term lymphangioma suggests a neoplastic process, ALC is not a neoplasm and can be managed expectantly in many cases.2,3,8 However, due to cosmetic appearance, pain, discomfort, and recurrent bacterial superinfections, many patients pursue treatment. Treatment options for ALC include sclerotherapy, electrocautery, radiofrequency or carbon dioxide laser ablation, and excision, though recurrence can arise.3-5,7,8 Our patient elected to manage her asymptomatic ALC expectantly.
The Diagnosis: Acquired Lymphangioma Circumscriptum
A skin biopsy of the plaque on the right labium majus showed a proliferation of well-formed, dilated lymphatic vessels lined by benign-appearing endothelial cells in the papillary dermis (Figure). These findings were consistent with a diagnosis of acquired lymphangioma circumscriptum (ALC) in the setting of severe hidradenitis suppurativa (HS).

Acquired lymphangioma circumscriptum (also known as acquired lymphangiectasia or secondary lymphangioma1) is a rare skin finding resulting from chronic lymphatic obstruction that leads to dilated lymphatic vessels within the dermis.2,3 There also is a distinct congenital form of lymphangioma circumscriptum caused by lymphatic malformations present at birth.2,4 Acquired lymphangioma circumscriptum of the vulva is a rare phenomenon.3 Identified causes include radiation or surgery for carcinoma, solid gynecologic tumors, lymphadenectomy, Crohn disease, and tuberculosis and other infections, all of which can disrupt normal lymphatics to cause ALC.2-4 Hidradenitis suppurativa is not a widely recognized cause of ALC; however, this phenomenon is reported in the literature. A long-standing history of severe HS complicated by lymphedema seems to precede the development of ALC in the reported cases, as in our patient.5-7
Acquired lymphangioma circumscriptum of the vulva can appear in women of all ages as frog spawn or cobblestone papules or vesicles, sometimes with a hyperkeratotic or verrucous appearance.2,4 Associated symptoms include serous drainage, edema, pruritus, and discomfort. The lesions may become eroded, which can predispose patients to secondary infections.1,2 Acquired lymphangioma circumscriptum of the vulva can be difficult to diagnose, as the time interval between the initial cause and the appearance of skin findings can be years, leading to the misdiagnosis of ALC as other similar-appearing genital skin conditions such as squamous cell carcinoma or condyloma.4,8 When misidentified as an infection, diagnosis can lead to substantial distress, abstinence from sexual activity, and unnecessary and painful treatments.
Skin biopsy is helpful in distinguishing ALC from other differential diagnoses such as condylomata acuminata, squamous cell carcinoma, and condyloma lata. Histopathology in ALC is notable for dilated lymphatic vessels filled with hypocellular fluid and lined with endothelial cells in the superficial dermis; the epidermis can appear hyperplastic, hyperkeratotic, or eroded.3-5,9 These lymphatic vessels stain positively for CD31 and D2-40, markers for endothelial cells and lymphatic endothelium, respectively, and negative for CD34, a marker for vascular endothelium.3,4,9 Features suggestive of condylomata acuminata such as rounded parakeratosis, hypergranulosis, and vacuolated keratinocytes9 are not present. The giant condyloma of Buschke-Löwenstein, a clinical variant of verrucous squamous cell carcinoma, also can present as a warty ulcerated papule or plaque in the genital region, but the characteristic rounded eosinophilic keratinocytes pushing down into the dermis9 are not seen in ALC. Secondary syphilis is associated with condyloma lata, which are verrucous or fleshy-appearing papules often coalescing into plaques located in the anogenital region. Pathologic features of secondary syphilis include vacuolar interface dermatitis and acanthosis with long slender rete ridges.9 Squamous cell carcinoma, which can arise from inflammation associated with long-standing HS, must be ruled out, as it is associated with a high risk of mortality in patients with HS.10
It is noteworthy to recognize the various, often confusing nomenclature used to describe cutaneous lymphatic conditions. The terms acquired lymphangioma circumscriptum, secondary lymphangioma, and lymphangiectasia are used interchangeably to describe dilated lymphatic vessels in the skin.1 The term atypical vascular lesion refers to lymphectasias of the skin of the breast due to prior radiation therapy most often used in the treatment of breast carcinoma; clinically, these present as red-brown or flesh-colored papules or telangiectatic plaques on the breast.11,12 Lymphedema also may occur alongside atypical vascular lesions, as prior radiation or surgical lymph node dissection can predispose patients to impaired lymphatic drainage.13 The lymphatic histopathologic subtype of atypical vascular lesions may appear similar to ALC; however, the vascular subtype will demonstrate collections of capillary-sized vessels and extravasated erythrocytes.11,12 Unlike ALC, the benign nature of atypical vascular lesions has been questioned, as they may be associated with a small risk for progression to angiosarcoma.11-13 It also is important to distinguish ALC from lymphangiomatosis, a generalized lymphatic anomaly that is characterized by extensive lymphatic malformations involving numerous internal organs, including the lungs and gastrointestinal tract. This condition is associated with notable morbidity and mortality.13
Although the suffix of the term lymphangioma suggests a neoplastic process, ALC is not a neoplasm and can be managed expectantly in many cases.2,3,8 However, due to cosmetic appearance, pain, discomfort, and recurrent bacterial superinfections, many patients pursue treatment. Treatment options for ALC include sclerotherapy, electrocautery, radiofrequency or carbon dioxide laser ablation, and excision, though recurrence can arise.3-5,7,8 Our patient elected to manage her asymptomatic ALC expectantly.
- Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487.
- Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
- Sims SM, McLean FW, Davis JD, et al. Vulvar lymphangioma circumscriptum: a report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010; 14:234-237.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;6:1223-1224.
- Piernick DM 2nd, Mahmood SH, Daveluy S. Acquired lymphangioma circumscriptum of the genitals in an individual with chronic hidradenitis suppurativa. JAAD Case Rep. 2018;1:64-66.
- Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;1:118-120.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2019.
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
- Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487.
- Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
- Sims SM, McLean FW, Davis JD, et al. Vulvar lymphangioma circumscriptum: a report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010; 14:234-237.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;6:1223-1224.
- Piernick DM 2nd, Mahmood SH, Daveluy S. Acquired lymphangioma circumscriptum of the genitals in an individual with chronic hidradenitis suppurativa. JAAD Case Rep. 2018;1:64-66.
- Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;1:118-120.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2019.
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
A 38-year-old woman with long-standing severe hidradenitis suppurativa presented to our dermatology clinic with an asymptomatic, slowly enlarging growth on the right labium majus of 2 years’ duration. She also had severe persistent drainage from nodules and sinus tracts involving the abdominal pannus, inguinal folds, vulva, perineum, buttocks, and upper thighs. After treatment failure with oral antibiotics and adalimumab, her regimen included infliximab-dyyb, chronic systemic steroids, spironolactone, topical clindamycin, and benzoyl peroxide, with plans for eventual surgical intervention. Physical examination revealed the patient had numerous pink papules coalescing into a plaque on the right labium majus. She also had innumerable papulonodules, sinus tracts, and indurated scars in the inguinal folds, genitalia, and perineal region from severe hidradenitis suppurativa.
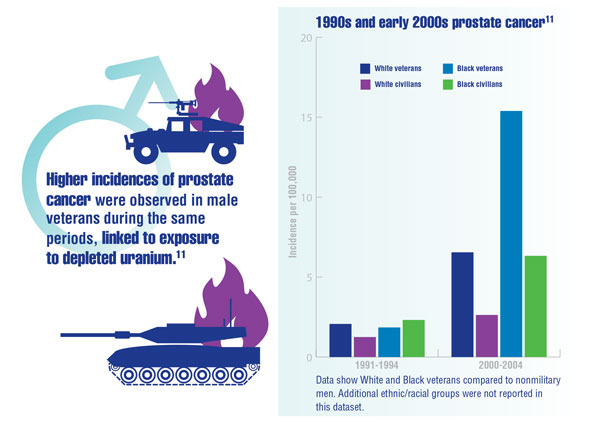
Cancer Data Trends 2022: Genitourinary Cancers
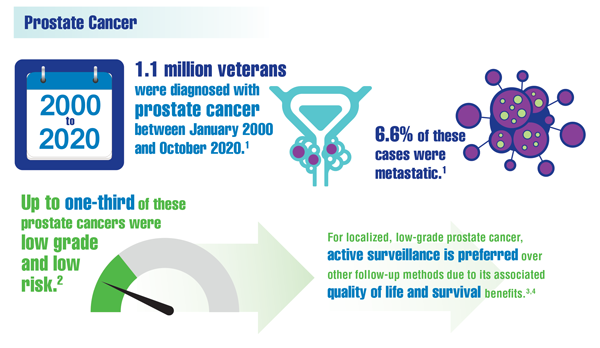
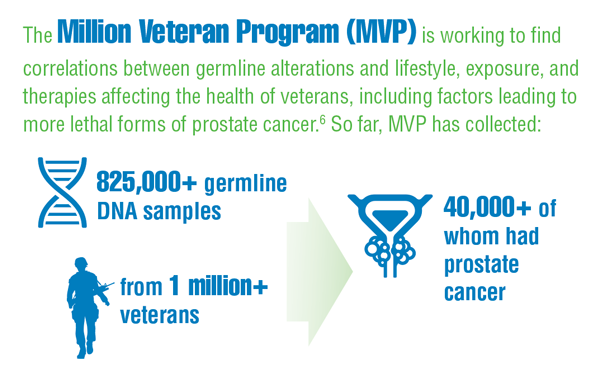
Alba PR, Gao A, Lee KM, et al. Ascertainment of veterans with metastatic prostate cancer in electronic health records: demonstrating the case for natural language processing. JCO Clin Cancer Inform. 2021;5:1005-1014. doi:10.1200/CCI.21.00030
Carter HB. Management of low (favourable)-risk prostate cancer. BJU Int. 2011;108(11):1684-1695. doi:10.1111/j.1464-410X.2010.10489.x
Hamdy FC, Donovan JL, Lane JA, et al, for the ProtecT Study Group. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. doi:10.1056/NEJMoa1606220
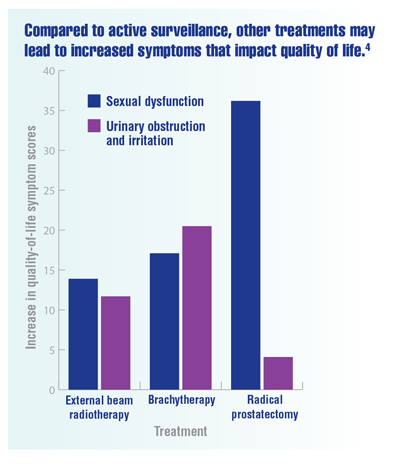
Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA. 2017;317(11):1141-1150. doi:10.1001/jama.2017.1652
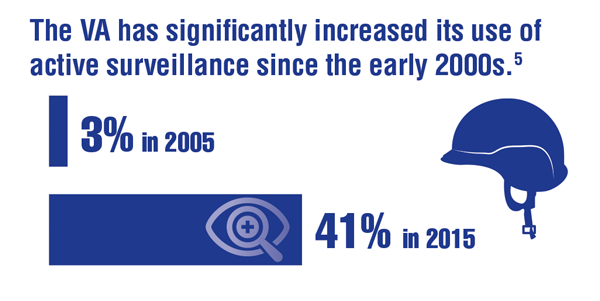
Loeb S, Byrne N, Makarov DV, et al. Use of conservative management for low-risk prostate cancer in the Veterans Affairs Integrated Health Care System from 2005-2015. JAMA. 2018;319(21):2231-2233. doi:10.1001/jama.2018.5616
Montgomery B, Rettig M, Kasten J, et al. The Precision Oncology Program for Cancer of the Prostate (POPCaP) network: a Veterans Affairs/Prostate Cancer Foundation Collaboration. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
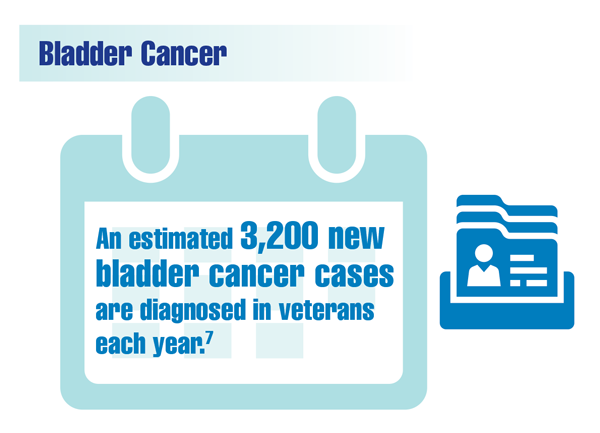
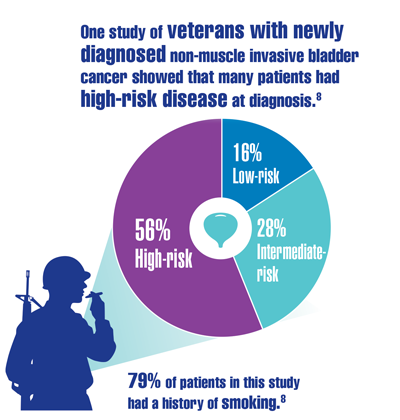
US Department of Veterans Affairs. Bladder cancer: know the signs. VAntage Point. Published June 15, 2021. Accessed December 13, 2021. https://blogs.va.gov/VAntage/90105/bladder-cancer-know-signs/
Caputo JM, Moran G, Muller B, et al. The management of newly-diagnosed non-muscle invasive bladder cancer in Veterans Integrated Services Network 02 of the Veterans Health Administration. Mil Med. 2020;185(1-2):276-281. doi:10.1093/milmed/usz166
US Department of Veterans Affairs. Agent Orange exposure and VA disability compensation. Updated June 15, 2021. Accessed December 13, 2021. https://www.va.gov/disability/eligibility/hazardous-materials-exposure/agent-orange/
Bhanegaonkar A, Pandya S, Zheng Y, et al. Real-world outcomes among US Veterans Health Administration patients newly diagnosed with metastatic renal cell carcinoma and treated with first-line monotherapy. Adv Ther. 2021;38:2644-2661. doi:10.1007/s12325-021-01657-2
Macleod LC, Tykodi SS, Holt SK, et al. Trends in metastatic kidney cancer survival from the cytokine to the targeted therapy era. Urology. 2015;86(2):262-268. doi:10.1016/j.urology.2015.05.008
Alba PR, Gao A, Lee KM, et al. Ascertainment of veterans with metastatic prostate cancer in electronic health records: demonstrating the case for natural language processing. JCO Clin Cancer Inform. 2021;5:1005-1014. doi:10.1200/CCI.21.00030
Carter HB. Management of low (favourable)-risk prostate cancer. BJU Int. 2011;108(11):1684-1695. doi:10.1111/j.1464-410X.2010.10489.x
Hamdy FC, Donovan JL, Lane JA, et al, for the ProtecT Study Group. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. doi:10.1056/NEJMoa1606220
Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA. 2017;317(11):1141-1150. doi:10.1001/jama.2017.1652
Loeb S, Byrne N, Makarov DV, et al. Use of conservative management for low-risk prostate cancer in the Veterans Affairs Integrated Health Care System from 2005-2015. JAMA. 2018;319(21):2231-2233. doi:10.1001/jama.2018.5616
Montgomery B, Rettig M, Kasten J, et al. The Precision Oncology Program for Cancer of the Prostate (POPCaP) network: a Veterans Affairs/Prostate Cancer Foundation Collaboration. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
US Department of Veterans Affairs. Bladder cancer: know the signs. VAntage Point. Published June 15, 2021. Accessed December 13, 2021. https://blogs.va.gov/VAntage/90105/bladder-cancer-know-signs/
Caputo JM, Moran G, Muller B, et al. The management of newly-diagnosed non-muscle invasive bladder cancer in Veterans Integrated Services Network 02 of the Veterans Health Administration. Mil Med. 2020;185(1-2):276-281. doi:10.1093/milmed/usz166
US Department of Veterans Affairs. Agent Orange exposure and VA disability compensation. Updated June 15, 2021. Accessed December 13, 2021. https://www.va.gov/disability/eligibility/hazardous-materials-exposure/agent-orange/
Bhanegaonkar A, Pandya S, Zheng Y, et al. Real-world outcomes among US Veterans Health Administration patients newly diagnosed with metastatic renal cell carcinoma and treated with first-line monotherapy. Adv Ther. 2021;38:2644-2661. doi:10.1007/s12325-021-01657-2
Macleod LC, Tykodi SS, Holt SK, et al. Trends in metastatic kidney cancer survival from the cytokine to the targeted therapy era. Urology. 2015;86(2):262-268. doi:10.1016/j.urology.2015.05.008
Alba PR, Gao A, Lee KM, et al. Ascertainment of veterans with metastatic prostate cancer in electronic health records: demonstrating the case for natural language processing. JCO Clin Cancer Inform. 2021;5:1005-1014. doi:10.1200/CCI.21.00030
Carter HB. Management of low (favourable)-risk prostate cancer. BJU Int. 2011;108(11):1684-1695. doi:10.1111/j.1464-410X.2010.10489.x
Hamdy FC, Donovan JL, Lane JA, et al, for the ProtecT Study Group. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. doi:10.1056/NEJMoa1606220
Chen RC, Basak R, Meyer AM, et al. Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA. 2017;317(11):1141-1150. doi:10.1001/jama.2017.1652
Loeb S, Byrne N, Makarov DV, et al. Use of conservative management for low-risk prostate cancer in the Veterans Affairs Integrated Health Care System from 2005-2015. JAMA. 2018;319(21):2231-2233. doi:10.1001/jama.2018.5616
Montgomery B, Rettig M, Kasten J, et al. The Precision Oncology Program for Cancer of the Prostate (POPCaP) network: a Veterans Affairs/Prostate Cancer Foundation Collaboration. Fed Pract. 2020;37(suppl 4):S48-S53. doi:10.12788/fp.0021
US Department of Veterans Affairs. Bladder cancer: know the signs. VAntage Point. Published June 15, 2021. Accessed December 13, 2021. https://blogs.va.gov/VAntage/90105/bladder-cancer-know-signs/
Caputo JM, Moran G, Muller B, et al. The management of newly-diagnosed non-muscle invasive bladder cancer in Veterans Integrated Services Network 02 of the Veterans Health Administration. Mil Med. 2020;185(1-2):276-281. doi:10.1093/milmed/usz166
US Department of Veterans Affairs. Agent Orange exposure and VA disability compensation. Updated June 15, 2021. Accessed December 13, 2021. https://www.va.gov/disability/eligibility/hazardous-materials-exposure/agent-orange/
Bhanegaonkar A, Pandya S, Zheng Y, et al. Real-world outcomes among US Veterans Health Administration patients newly diagnosed with metastatic renal cell carcinoma and treated with first-line monotherapy. Adv Ther. 2021;38:2644-2661. doi:10.1007/s12325-021-01657-2
Macleod LC, Tykodi SS, Holt SK, et al. Trends in metastatic kidney cancer survival from the cytokine to the targeted therapy era. Urology. 2015;86(2):262-268. doi:10.1016/j.urology.2015.05.008
Cancer Data Trends 2022: Exposure-Related Cancers
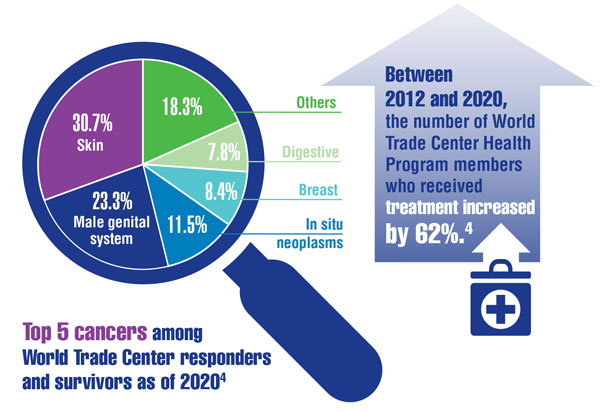
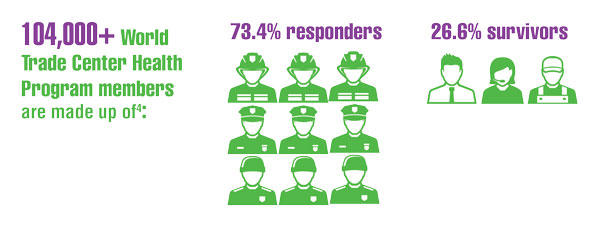
- Santiago-Colón A, Daniels R, Reissman D, et al. World Trade Center Health Program: first decade of research. Int J Environ Res Public Health. 2020;17(19):7290. doi:10.3390/ijerph17197290
- Frank Dwyer, FDNY Deputy Commissioner. Personal communication (email, December 20, 2021).
- Campbell R. New York Guard members reflect on 9/11 response. US Army News. Published September 8, 2021. Accessed December 17, 2021. https://www.army.mil/article/250057/new_york_guard_members_reflect_on_911_response
- Azofeifa A, Martin GR, Satiago-Colón A, et al. World Trade Center Health Program — United States, 2012−2020. MMWR Surveill Summ. 2021;70(4):1-21. doi:10.15585/mmwr.ss7004a1
- Lantry L, Meneses I. Expanded benefits for vets exposed to burn pits coming, but for some it's too late. ABC News. Published November 23, 2021. Accessed December 17, 2021. https://abcnews.go.com/Politics/expanded-benefits-vets-exposed-burn-pits-coming-late/story?id=81261917
- Kennedy K. “The enemy is lurking in our bodies”—Women veterans say toxic exposure caused breast cancer. The War Horse. Published October 14, 2021. Accessed December 17, 2021. https://thewarhorse.org/military-women-face-higher-breast-cancer-rates-from-exposure/
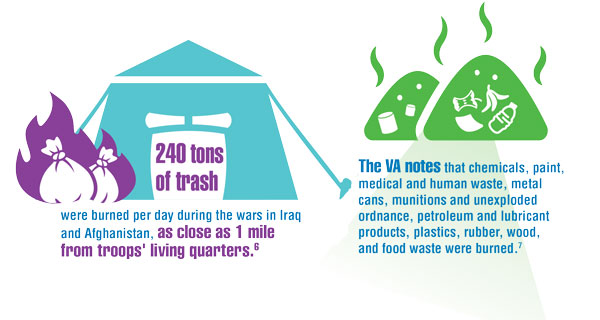
- US Department of Veteran Affairs. Airborne hazards and burn pit exposure. Updated August 5, 2021. Accessed December 20, 2021. https://www.publichealth.va.gov/exposures/burnpits/
- VA spokesperson, US Department of Veterans Affairs. Personal communication (e-mail, December 20, 2021).
- Burn Pits 360. Toxic exposure table (in reference to VA 10-03). Published 2020. Accessed December 20, 2021. https://burnpits360.org/wp-content/uploads/2021/03/Toxic-Exposure-Table-2020_V2.pdf
- Dursa EK, Cao G, Porter B, et al. The health of Gulf War and Gulf era veterans over time: US Department of Veterans Affairs’ Gulf War longitudinal study. J Occup Environ Med. 2021;63(10):889-894. doi:10.1097/JOM.0000000000002331
- Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the US military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
- Santiago-Colón A, Daniels R, Reissman D, et al. World Trade Center Health Program: first decade of research. Int J Environ Res Public Health. 2020;17(19):7290. doi:10.3390/ijerph17197290
- Frank Dwyer, FDNY Deputy Commissioner. Personal communication (email, December 20, 2021).
- Campbell R. New York Guard members reflect on 9/11 response. US Army News. Published September 8, 2021. Accessed December 17, 2021. https://www.army.mil/article/250057/new_york_guard_members_reflect_on_911_response
- Azofeifa A, Martin GR, Satiago-Colón A, et al. World Trade Center Health Program — United States, 2012−2020. MMWR Surveill Summ. 2021;70(4):1-21. doi:10.15585/mmwr.ss7004a1
- Lantry L, Meneses I. Expanded benefits for vets exposed to burn pits coming, but for some it's too late. ABC News. Published November 23, 2021. Accessed December 17, 2021. https://abcnews.go.com/Politics/expanded-benefits-vets-exposed-burn-pits-coming-late/story?id=81261917
- Kennedy K. “The enemy is lurking in our bodies”—Women veterans say toxic exposure caused breast cancer. The War Horse. Published October 14, 2021. Accessed December 17, 2021. https://thewarhorse.org/military-women-face-higher-breast-cancer-rates-from-exposure/
- US Department of Veteran Affairs. Airborne hazards and burn pit exposure. Updated August 5, 2021. Accessed December 20, 2021. https://www.publichealth.va.gov/exposures/burnpits/
- VA spokesperson, US Department of Veterans Affairs. Personal communication (e-mail, December 20, 2021).
- Burn Pits 360. Toxic exposure table (in reference to VA 10-03). Published 2020. Accessed December 20, 2021. https://burnpits360.org/wp-content/uploads/2021/03/Toxic-Exposure-Table-2020_V2.pdf
- Dursa EK, Cao G, Porter B, et al. The health of Gulf War and Gulf era veterans over time: US Department of Veterans Affairs’ Gulf War longitudinal study. J Occup Environ Med. 2021;63(10):889-894. doi:10.1097/JOM.0000000000002331
- Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the US military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
- Santiago-Colón A, Daniels R, Reissman D, et al. World Trade Center Health Program: first decade of research. Int J Environ Res Public Health. 2020;17(19):7290. doi:10.3390/ijerph17197290
- Frank Dwyer, FDNY Deputy Commissioner. Personal communication (email, December 20, 2021).
- Campbell R. New York Guard members reflect on 9/11 response. US Army News. Published September 8, 2021. Accessed December 17, 2021. https://www.army.mil/article/250057/new_york_guard_members_reflect_on_911_response
- Azofeifa A, Martin GR, Satiago-Colón A, et al. World Trade Center Health Program — United States, 2012−2020. MMWR Surveill Summ. 2021;70(4):1-21. doi:10.15585/mmwr.ss7004a1
- Lantry L, Meneses I. Expanded benefits for vets exposed to burn pits coming, but for some it's too late. ABC News. Published November 23, 2021. Accessed December 17, 2021. https://abcnews.go.com/Politics/expanded-benefits-vets-exposed-burn-pits-coming-late/story?id=81261917
- Kennedy K. “The enemy is lurking in our bodies”—Women veterans say toxic exposure caused breast cancer. The War Horse. Published October 14, 2021. Accessed December 17, 2021. https://thewarhorse.org/military-women-face-higher-breast-cancer-rates-from-exposure/
- US Department of Veteran Affairs. Airborne hazards and burn pit exposure. Updated August 5, 2021. Accessed December 20, 2021. https://www.publichealth.va.gov/exposures/burnpits/
- VA spokesperson, US Department of Veterans Affairs. Personal communication (e-mail, December 20, 2021).
- Burn Pits 360. Toxic exposure table (in reference to VA 10-03). Published 2020. Accessed December 20, 2021. https://burnpits360.org/wp-content/uploads/2021/03/Toxic-Exposure-Table-2020_V2.pdf
- Dursa EK, Cao G, Porter B, et al. The health of Gulf War and Gulf era veterans over time: US Department of Veterans Affairs’ Gulf War longitudinal study. J Occup Environ Med. 2021;63(10):889-894. doi:10.1097/JOM.0000000000002331
- Zhu K, Devesa SS, Wu H, et al. Cancer incidence in the US military population: comparison with rates from the SEER program. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1740-1745. doi:10.1158/1055-9965.EPI-09-0041
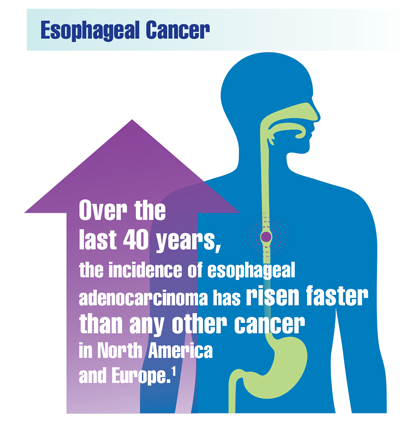
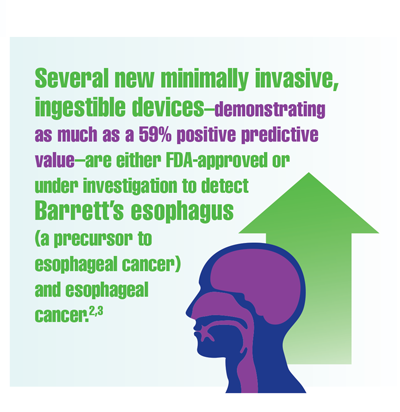
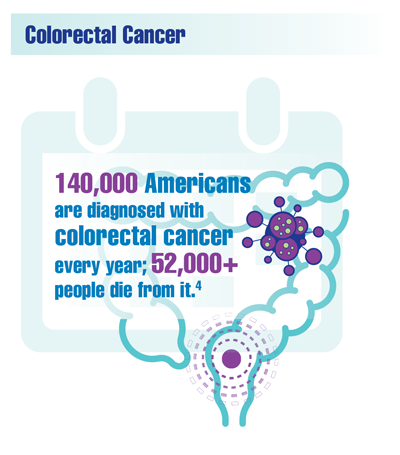
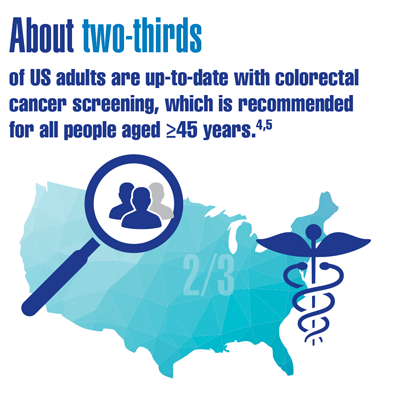
Cancer Data Trends 2022
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
Federal Practitioner, in collaboration with the Association of VA Hematology/Oncology (AVAHO), present the 2022 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- Exposure-Related Cancers
- Cancer in Women
- Genitourinary Cancers
- Gastrointestinal Cancers
- Telehealth in Oncology
- Precision Oncology
- Palliative and Hospice Care
- Alcohol and Cancer
- Lung Cancer
- Oropharyngeal Cancer
- Hematologic Cancers
Federal Practitioner and AVAHO would like to thank the following experts for their contributions to this issue:
Anita Aggarwal, DO, PhD; Sara Ahmed, PhD; Katherine Faricy-Anderson, MD; Apar Kishor Ganti, MD, MS; Solomon A Graf, MD; Kate Hendricks Thomas, PhD; Michael Kelley, MD; Mark Klein, MD, Gina McWhirter, MSN, MBA, RN; Bruce Montgomery, MD; Vida Almario Passero, MD, MBA; Thomas D Rodgers, MD; Vlad C Sandulache, MD, PhD; David H Wang, MD, PhD.
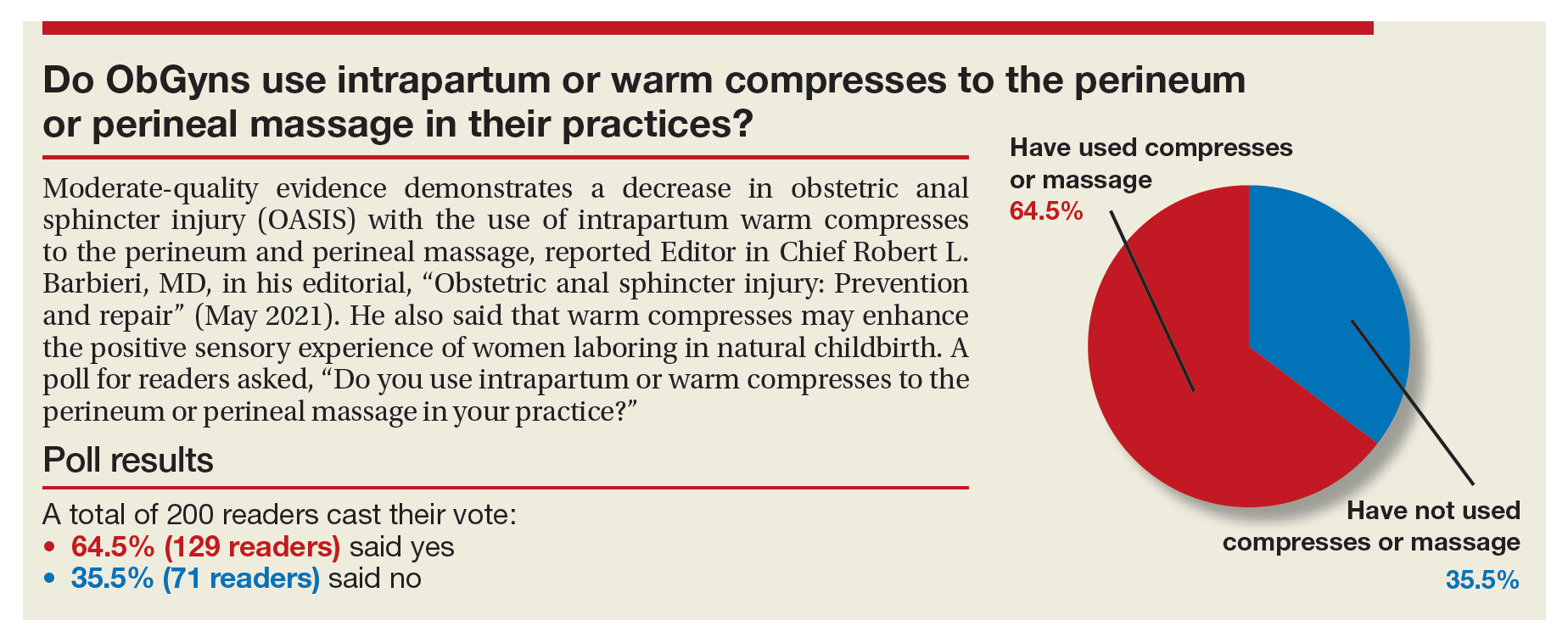
Do ObGyns use intrapartum warm compresses to the perineum or perineal massage in their practices?
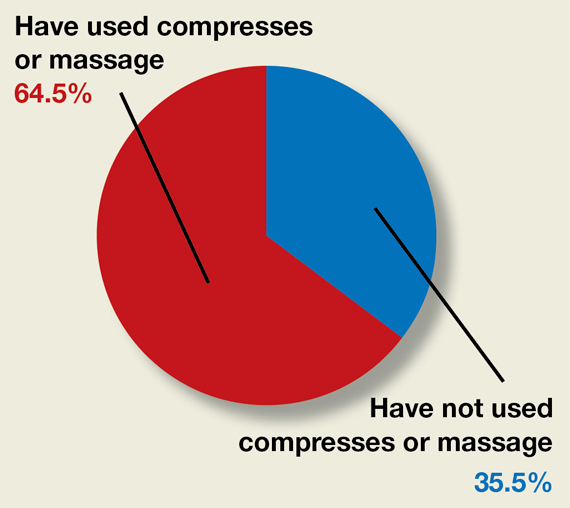
Moderate-quality evidence demonstrates a decrease in obstetric anal sphincter injury (OASIS) with the use of intrapartum warm compresses to the perineum and perineal massage, reported Editor in Chief Robert L. Barbieri, MD, in his editorial, “Obstetric anal sphincter injury: Prevention and repair” (May 2021). He also said that warm compresses may enhance the positive sensory experience of women laboring in natural childbirth. A poll for readers asked, “Do you use intrapartum or warm compresses to the perineum or perineal massage in your practice?”
A total of 200 readers cast their vote:
65.4% (129 readers)said yes
35.5% (71 readers)said no
Moderate-quality evidence demonstrates a decrease in obstetric anal sphincter injury (OASIS) with the use of intrapartum warm compresses to the perineum and perineal massage, reported Editor in Chief Robert L. Barbieri, MD, in his editorial, “Obstetric anal sphincter injury: Prevention and repair” (May 2021). He also said that warm compresses may enhance the positive sensory experience of women laboring in natural childbirth. A poll for readers asked, “Do you use intrapartum or warm compresses to the perineum or perineal massage in your practice?”
A total of 200 readers cast their vote:
65.4% (129 readers)said yes
35.5% (71 readers)said no


Moderate-quality evidence demonstrates a decrease in obstetric anal sphincter injury (OASIS) with the use of intrapartum warm compresses to the perineum and perineal massage, reported Editor in Chief Robert L. Barbieri, MD, in his editorial, “Obstetric anal sphincter injury: Prevention and repair” (May 2021). He also said that warm compresses may enhance the positive sensory experience of women laboring in natural childbirth. A poll for readers asked, “Do you use intrapartum or warm compresses to the perineum or perineal massage in your practice?”
A total of 200 readers cast their vote:
65.4% (129 readers)said yes
35.5% (71 readers)said no


Clinical Edge Journal Scan Commentary: Migraine April 2022
Neuromodulation is an up-and-coming subtype of treatments for migraine. These treatments vary significantly from transcutaneous electrical nerve stimulation (TENS)–like devices to transcranial magnetic stimulation to remote electrical stimulation of nociceptors in the arm or the vagus nerve. Some of these devices are primarily preventive in nature, whereas others are primarily for the acute treatment of migraine. Transcranial direct-current stimulation (TDCS) has recently been investigated in a number of other neurologic conditions, including multiple sclerosis and stroke, specifically for its ability to reverse manifestations of specific pathologic changes. With migraine, the question remains of whether central sensitization can similarly be reversed.
Prior studies looking at TDCS in the context of episodic migraine were mostly inconclusive. These were looking primarily at acute treatment rather than prevention. In a recent study, Hodai and colleagues took a small group of patients with treatment-refractory chronic migraine and randomly assigned them to TDCS or sham stimulation over a course of 2 months. The stimulations that the patients received were similar to protocols that have been investigated in multiple sclerosis and stroke, specifically anodal TDCS, which is thought to reverse gamma-aminobutyric acid (GABA)-ergic and glutamatergic dysregulations when the right or left cortex was stimulated.
The primary outcome of this study was decrease in baseline migraine attack frequency per month; secondary endpoints were improvement in the Headache Impact Test (HIT-6) and Migraine Disability Assessment (MIDAS) scores, the Short-Form Survey (SF-12) quality of life assessment, the Hospital Anxiety and Depression Scale (HADS) assessment, and a Clinical Global Impression (CGI) scale.
A total of 36 patients were randomly assigned to a sham or TDCS intervention. A larger reduction of migraine days per month was seen by the intervention group. The interventions were also well tolerated, and no serious adverse events were reported. None of the secondary outcomes, however, showed significance. Further analysis of responder rates showed a 50% responder rate of 36% in the intervention group vs. 14% in the sham group.
This is the first sham-controlled study investigating the use of this neuromodulation therapy for the prevention of migraine. TDCS appears to show promise even when selected for some of the most refractory situations. The question will become how this can be more practical for patient use in the future.
Prognosticating treatment effects in chronic migraine is extremely difficult to do. Most specialists have an extensive discussion with their patients that includes the likelihood of improvement in addition to the risks and benefits of the medications they are considering starting. There has been background discussion in the headache community over whether improvement with one calcitonin gene–related peptide (CGRP) antagonist medication is predictive of benefit with other medications in the class or with long-term improvement in migraine. Buse and colleagues present findings from a post hoc analysis of the PROMISE-2 study of eptinezumab for the prevention of chronic migraine.
Eptinezumab is an intravenously administered CGRP monoclonal antibody, given at either 100 mg or 300 mg every 3 months. PROMISE-2 was a randomized controlled trial that led to US Food and Drug Administration approval of eptinezumab for the prevention of chronic migraine. The authors here reviewed the data between the two intervention groups and the placebo group and then regrouped these patients according to response at month 1, defined by whether the patient was in a response group of 25%, 50%, or 75% response after 1 month of treatment. This was then compared with the patient global impact of change (PGIC) score at month 6.
This post hoc analysis did not include patients that had no response at all to either intervention or placebo at month 6. A total of 1072 patients were included in this analysis; the 100-mg, 300-mg, and placebo groups had approximately one third of patients in each.
The majority of patients in the 75% responder group continued to improve; more than half of those patients maintained the 75% response rate at month 6. More than two thirds of the 50% responders remained at a 50% response at 6 months as well. Those who responded at < 25% at month 1 were much less likely to achieve 50% response at month 6; however, the patients in the active groups were more likely to achieve a response compared with those in the placebo group.
The PGIC scores also showed significant improvement when comparing among the groups. Those who were "very much improved" at month 1 were significantly more likely to remain that way at the conclusion of the study.
Although prognosticating among different subtypes of CGRP antagonists is not yet possible, the authors here do show the ability to better inform and educate our patients when considering eptinezumab therapy for chronic migraine.
There is an age-old debate among headache specialists about overused medications: to wean or not to wean. The overuse of acute medications has long been shown to contribute to a higher frequency of migraine attacks over time, initially being called "transformed migraine" and subsequently being understood either as a subtype of chronic migraine or a separate headache disorder completely. Medication overuse headache (MOH) is something screened for by all headache providers when evaluating patients for worsening headaches. The addition of a preventive medication is the mainstay of treatment of any instance of higher frequency migraine; when MOH is a contributing factor, many practitioners will recommend complete discontinuation of the overused medications, whereas others will recommend waiting for the preventive medication to offer benefit first. As yet, there have not been any head-to-head trials investigating discontinuation vs. non-discontinuation of overused medications in this population.
Schwedt and colleagues designed a multisite trial prospectively enrolling patients with an International Classification of Headache Disorders (ICHD-3) diagnosis of both chronic migraine and MOH. Participants were told not to change their preventive medications for 4 weeks prior to enrollment. A total of 720 participants were enrolled through 14 clinics. Any patients already on preventive therapy were optimized to the best dose of that therapy or switched to other medications on the basis of the clinical investigator's judgement; all participants were randomly assigned to either discontinuation of the overused medication and given a novel acute therapy or were told to remain on their current acute therapy. No bridging therapies were recommended when switching or discontinuing acute therapies.
Of the 720 participants enrolled, 42% were already on preventive medicine. The overused medications ranged from simple analgesics for 64% of the study population to triptans, combination analgesics, and even opiates in 4% of the population. Butalbital use was included in the combination analgesic group. The primary outcome was reduction in moderate to severe migraine days, and secondary outcomes were scores for disability, depression, and quality of life (based on questionnaires).
There appeared to be no significant difference between the discontinuation and non-discontinuation groups. The authors describe this as noninferiority between the groups. To answer the age-old question of to wean or not to wean — there probably is not an answer that fits every patient. Patient adherence determines the effectiveness of anything we recommend. When evaluating patients with MOH, we have to consider whether discontinuing a medication that the patient has been depending on for months or longer will make it more or less likely for them to adhere to the other recommendations that we are making. Some patients will be very agreeable to try another acute option and stop overusing altogether. Others will be very apprehensive, and a slower, steadier approach that includes using the overused medication may be necessary. We aim always to individualize our recommendations for patients, and this should be no different.
Neuromodulation is an up-and-coming subtype of treatments for migraine. These treatments vary significantly from transcutaneous electrical nerve stimulation (TENS)–like devices to transcranial magnetic stimulation to remote electrical stimulation of nociceptors in the arm or the vagus nerve. Some of these devices are primarily preventive in nature, whereas others are primarily for the acute treatment of migraine. Transcranial direct-current stimulation (TDCS) has recently been investigated in a number of other neurologic conditions, including multiple sclerosis and stroke, specifically for its ability to reverse manifestations of specific pathologic changes. With migraine, the question remains of whether central sensitization can similarly be reversed.
Prior studies looking at TDCS in the context of episodic migraine were mostly inconclusive. These were looking primarily at acute treatment rather than prevention. In a recent study, Hodai and colleagues took a small group of patients with treatment-refractory chronic migraine and randomly assigned them to TDCS or sham stimulation over a course of 2 months. The stimulations that the patients received were similar to protocols that have been investigated in multiple sclerosis and stroke, specifically anodal TDCS, which is thought to reverse gamma-aminobutyric acid (GABA)-ergic and glutamatergic dysregulations when the right or left cortex was stimulated.
The primary outcome of this study was decrease in baseline migraine attack frequency per month; secondary endpoints were improvement in the Headache Impact Test (HIT-6) and Migraine Disability Assessment (MIDAS) scores, the Short-Form Survey (SF-12) quality of life assessment, the Hospital Anxiety and Depression Scale (HADS) assessment, and a Clinical Global Impression (CGI) scale.
A total of 36 patients were randomly assigned to a sham or TDCS intervention. A larger reduction of migraine days per month was seen by the intervention group. The interventions were also well tolerated, and no serious adverse events were reported. None of the secondary outcomes, however, showed significance. Further analysis of responder rates showed a 50% responder rate of 36% in the intervention group vs. 14% in the sham group.
This is the first sham-controlled study investigating the use of this neuromodulation therapy for the prevention of migraine. TDCS appears to show promise even when selected for some of the most refractory situations. The question will become how this can be more practical for patient use in the future.
Prognosticating treatment effects in chronic migraine is extremely difficult to do. Most specialists have an extensive discussion with their patients that includes the likelihood of improvement in addition to the risks and benefits of the medications they are considering starting. There has been background discussion in the headache community over whether improvement with one calcitonin gene–related peptide (CGRP) antagonist medication is predictive of benefit with other medications in the class or with long-term improvement in migraine. Buse and colleagues present findings from a post hoc analysis of the PROMISE-2 study of eptinezumab for the prevention of chronic migraine.
Eptinezumab is an intravenously administered CGRP monoclonal antibody, given at either 100 mg or 300 mg every 3 months. PROMISE-2 was a randomized controlled trial that led to US Food and Drug Administration approval of eptinezumab for the prevention of chronic migraine. The authors here reviewed the data between the two intervention groups and the placebo group and then regrouped these patients according to response at month 1, defined by whether the patient was in a response group of 25%, 50%, or 75% response after 1 month of treatment. This was then compared with the patient global impact of change (PGIC) score at month 6.
This post hoc analysis did not include patients that had no response at all to either intervention or placebo at month 6. A total of 1072 patients were included in this analysis; the 100-mg, 300-mg, and placebo groups had approximately one third of patients in each.
The majority of patients in the 75% responder group continued to improve; more than half of those patients maintained the 75% response rate at month 6. More than two thirds of the 50% responders remained at a 50% response at 6 months as well. Those who responded at < 25% at month 1 were much less likely to achieve 50% response at month 6; however, the patients in the active groups were more likely to achieve a response compared with those in the placebo group.
The PGIC scores also showed significant improvement when comparing among the groups. Those who were "very much improved" at month 1 were significantly more likely to remain that way at the conclusion of the study.
Although prognosticating among different subtypes of CGRP antagonists is not yet possible, the authors here do show the ability to better inform and educate our patients when considering eptinezumab therapy for chronic migraine.
There is an age-old debate among headache specialists about overused medications: to wean or not to wean. The overuse of acute medications has long been shown to contribute to a higher frequency of migraine attacks over time, initially being called "transformed migraine" and subsequently being understood either as a subtype of chronic migraine or a separate headache disorder completely. Medication overuse headache (MOH) is something screened for by all headache providers when evaluating patients for worsening headaches. The addition of a preventive medication is the mainstay of treatment of any instance of higher frequency migraine; when MOH is a contributing factor, many practitioners will recommend complete discontinuation of the overused medications, whereas others will recommend waiting for the preventive medication to offer benefit first. As yet, there have not been any head-to-head trials investigating discontinuation vs. non-discontinuation of overused medications in this population.
Schwedt and colleagues designed a multisite trial prospectively enrolling patients with an International Classification of Headache Disorders (ICHD-3) diagnosis of both chronic migraine and MOH. Participants were told not to change their preventive medications for 4 weeks prior to enrollment. A total of 720 participants were enrolled through 14 clinics. Any patients already on preventive therapy were optimized to the best dose of that therapy or switched to other medications on the basis of the clinical investigator's judgement; all participants were randomly assigned to either discontinuation of the overused medication and given a novel acute therapy or were told to remain on their current acute therapy. No bridging therapies were recommended when switching or discontinuing acute therapies.
Of the 720 participants enrolled, 42% were already on preventive medicine. The overused medications ranged from simple analgesics for 64% of the study population to triptans, combination analgesics, and even opiates in 4% of the population. Butalbital use was included in the combination analgesic group. The primary outcome was reduction in moderate to severe migraine days, and secondary outcomes were scores for disability, depression, and quality of life (based on questionnaires).
There appeared to be no significant difference between the discontinuation and non-discontinuation groups. The authors describe this as noninferiority between the groups. To answer the age-old question of to wean or not to wean — there probably is not an answer that fits every patient. Patient adherence determines the effectiveness of anything we recommend. When evaluating patients with MOH, we have to consider whether discontinuing a medication that the patient has been depending on for months or longer will make it more or less likely for them to adhere to the other recommendations that we are making. Some patients will be very agreeable to try another acute option and stop overusing altogether. Others will be very apprehensive, and a slower, steadier approach that includes using the overused medication may be necessary. We aim always to individualize our recommendations for patients, and this should be no different.
Neuromodulation is an up-and-coming subtype of treatments for migraine. These treatments vary significantly from transcutaneous electrical nerve stimulation (TENS)–like devices to transcranial magnetic stimulation to remote electrical stimulation of nociceptors in the arm or the vagus nerve. Some of these devices are primarily preventive in nature, whereas others are primarily for the acute treatment of migraine. Transcranial direct-current stimulation (TDCS) has recently been investigated in a number of other neurologic conditions, including multiple sclerosis and stroke, specifically for its ability to reverse manifestations of specific pathologic changes. With migraine, the question remains of whether central sensitization can similarly be reversed.
Prior studies looking at TDCS in the context of episodic migraine were mostly inconclusive. These were looking primarily at acute treatment rather than prevention. In a recent study, Hodai and colleagues took a small group of patients with treatment-refractory chronic migraine and randomly assigned them to TDCS or sham stimulation over a course of 2 months. The stimulations that the patients received were similar to protocols that have been investigated in multiple sclerosis and stroke, specifically anodal TDCS, which is thought to reverse gamma-aminobutyric acid (GABA)-ergic and glutamatergic dysregulations when the right or left cortex was stimulated.
The primary outcome of this study was decrease in baseline migraine attack frequency per month; secondary endpoints were improvement in the Headache Impact Test (HIT-6) and Migraine Disability Assessment (MIDAS) scores, the Short-Form Survey (SF-12) quality of life assessment, the Hospital Anxiety and Depression Scale (HADS) assessment, and a Clinical Global Impression (CGI) scale.
A total of 36 patients were randomly assigned to a sham or TDCS intervention. A larger reduction of migraine days per month was seen by the intervention group. The interventions were also well tolerated, and no serious adverse events were reported. None of the secondary outcomes, however, showed significance. Further analysis of responder rates showed a 50% responder rate of 36% in the intervention group vs. 14% in the sham group.
This is the first sham-controlled study investigating the use of this neuromodulation therapy for the prevention of migraine. TDCS appears to show promise even when selected for some of the most refractory situations. The question will become how this can be more practical for patient use in the future.
Prognosticating treatment effects in chronic migraine is extremely difficult to do. Most specialists have an extensive discussion with their patients that includes the likelihood of improvement in addition to the risks and benefits of the medications they are considering starting. There has been background discussion in the headache community over whether improvement with one calcitonin gene–related peptide (CGRP) antagonist medication is predictive of benefit with other medications in the class or with long-term improvement in migraine. Buse and colleagues present findings from a post hoc analysis of the PROMISE-2 study of eptinezumab for the prevention of chronic migraine.
Eptinezumab is an intravenously administered CGRP monoclonal antibody, given at either 100 mg or 300 mg every 3 months. PROMISE-2 was a randomized controlled trial that led to US Food and Drug Administration approval of eptinezumab for the prevention of chronic migraine. The authors here reviewed the data between the two intervention groups and the placebo group and then regrouped these patients according to response at month 1, defined by whether the patient was in a response group of 25%, 50%, or 75% response after 1 month of treatment. This was then compared with the patient global impact of change (PGIC) score at month 6.
This post hoc analysis did not include patients that had no response at all to either intervention or placebo at month 6. A total of 1072 patients were included in this analysis; the 100-mg, 300-mg, and placebo groups had approximately one third of patients in each.
The majority of patients in the 75% responder group continued to improve; more than half of those patients maintained the 75% response rate at month 6. More than two thirds of the 50% responders remained at a 50% response at 6 months as well. Those who responded at < 25% at month 1 were much less likely to achieve 50% response at month 6; however, the patients in the active groups were more likely to achieve a response compared with those in the placebo group.
The PGIC scores also showed significant improvement when comparing among the groups. Those who were "very much improved" at month 1 were significantly more likely to remain that way at the conclusion of the study.
Although prognosticating among different subtypes of CGRP antagonists is not yet possible, the authors here do show the ability to better inform and educate our patients when considering eptinezumab therapy for chronic migraine.
There is an age-old debate among headache specialists about overused medications: to wean or not to wean. The overuse of acute medications has long been shown to contribute to a higher frequency of migraine attacks over time, initially being called "transformed migraine" and subsequently being understood either as a subtype of chronic migraine or a separate headache disorder completely. Medication overuse headache (MOH) is something screened for by all headache providers when evaluating patients for worsening headaches. The addition of a preventive medication is the mainstay of treatment of any instance of higher frequency migraine; when MOH is a contributing factor, many practitioners will recommend complete discontinuation of the overused medications, whereas others will recommend waiting for the preventive medication to offer benefit first. As yet, there have not been any head-to-head trials investigating discontinuation vs. non-discontinuation of overused medications in this population.
Schwedt and colleagues designed a multisite trial prospectively enrolling patients with an International Classification of Headache Disorders (ICHD-3) diagnosis of both chronic migraine and MOH. Participants were told not to change their preventive medications for 4 weeks prior to enrollment. A total of 720 participants were enrolled through 14 clinics. Any patients already on preventive therapy were optimized to the best dose of that therapy or switched to other medications on the basis of the clinical investigator's judgement; all participants were randomly assigned to either discontinuation of the overused medication and given a novel acute therapy or were told to remain on their current acute therapy. No bridging therapies were recommended when switching or discontinuing acute therapies.
Of the 720 participants enrolled, 42% were already on preventive medicine. The overused medications ranged from simple analgesics for 64% of the study population to triptans, combination analgesics, and even opiates in 4% of the population. Butalbital use was included in the combination analgesic group. The primary outcome was reduction in moderate to severe migraine days, and secondary outcomes were scores for disability, depression, and quality of life (based on questionnaires).
There appeared to be no significant difference between the discontinuation and non-discontinuation groups. The authors describe this as noninferiority between the groups. To answer the age-old question of to wean or not to wean — there probably is not an answer that fits every patient. Patient adherence determines the effectiveness of anything we recommend. When evaluating patients with MOH, we have to consider whether discontinuing a medication that the patient has been depending on for months or longer will make it more or less likely for them to adhere to the other recommendations that we are making. Some patients will be very agreeable to try another acute option and stop overusing altogether. Others will be very apprehensive, and a slower, steadier approach that includes using the overused medication may be necessary. We aim always to individualize our recommendations for patients, and this should be no different.
Clinical Edge Journal Scan Commentary: PsA April 2022
Treatment of psoriatic arthritis (PsA) was the focus of clinical research papers published this month. Despite the advances made in treating PsA with targeted therapies, in most parts of the world, conventional disease-modifying antirheumatic drugs (DMARDs) are the first line of treatment. Methotrexate (MTX) and leflunomide (LEF) are commonly used, but there are limited data on the effectiveness of combination therapy. To address this issue, Mulder and colleagues enrolled 78 patients with active PsA who have two or more swollen joints and randomly allocated them to either 25 mg oral MTX weekly after 4 weeks of 15 mg weekly plus 20 mg LEF daily (n = 39) or MTX plus placebo (monotherapy; n = 39). At week 16, PsA disease activity score was improved significantly in the MTX + LEF vs. MTX monotherapy group (3.1 vs. 3.7; P = .025). Incidence of mild adverse events, such as nausea/vomiting (44% vs. 28%) and altered bowel habits (26% vs. 8%), was higher with MTX + LEF vs. MTX + placebo. So although less well tolerated, MTX + LEF therapy was superior to MTX monotherapy at improving disease activity in patients with PsA.
Biologics targeting tumor necrosis factor (TNF), interleukin (IL) -12/23, -23, and -17A are efficacious for the management of PsA, but questions remain about comparative effectiveness. Gossec and colleagues reported the results from their prospective observational PsABio study that evaluated real-world treatment persistence and effectiveness at 1 year after initiation of first-line to third-line IL-12/23 inhibitor ustekinumab or a TNF inhibitor (TNFi). Their study followed 893 patients. After 1 year of treatment, ustekinumab and the TNFi showed similar persistence (hazard ratio [HR] for stopping/switching treatment 0.82; 95% CI 0.60-1.13) and a similar proportion of patients achieving (on the Disease Activity Index for PsA) clinical low disease activity (odds ratio [OR] 0.80; 95% CI 0.57-1.10) and remission (OR 0.73; 95% CI 0.49-1.07), along with similar safety profiles. Thus in real-world studies, TNFi and ustekinumab seem to have similar effectiveness and safety.
Drug persistence between patients with psoriasis alone vs. those with PsA is also of interest. In a real-life study including 62 patients with psoriasis and 90 patients with PsA who initiated treatment with secukinumab and were followed up for 24 months or until discontinuation, Ortolan and colleagues demonstrated that the retention rate of secukinumab was higher in psoriasis vs. PsA at 12 (85% vs. 68%) and 24 (61% vs. 57%) months, with the risk for secukinumab discontinuation being higher among patients with PsA in the overall cohort (HR 2.43; P = .035) and in patients with obesity in the PsA cohort (P = .021). Thus, the presence of PsA and obesity lower the secukinumab retention rate.
- Despite the advent of many targeted therapies for PsA, there remain many unmet needs. Deucravacitinib is a novel oral selective inhibitor of tyrosine kinase 2 (TYK2) acting via binding to the TYK2 regulatory domain. In a phase 2 study including 203 patients with active PsA that was intolerant to at least one therapy who were randomly assigned to receive 6 mg deucravacitinib once daily, 12 mg deucravacitinib once daily, or placebo for 16 weeks, Mease and colleagues demonstrated that at week 16, American College of Rheumatology 20 (ACR20) response was significantly higher with 6 mg once-daily deucravacitinib (52.9%, adjusted OR [aOR] 2.4; P = .0134) and 12 mg (62.7%, aOR 3.6; P = .0004) vs. placebo (31.8%), with 12 mg deucravacitinib improving ACR20 response as early as at 8 weeks (P < .05). No serious adverse events were reported. Thus, TYK2 inhibition shows promise in the treatment of PsA and the results from phase 3 trials are awaited.
Treatment of psoriatic arthritis (PsA) was the focus of clinical research papers published this month. Despite the advances made in treating PsA with targeted therapies, in most parts of the world, conventional disease-modifying antirheumatic drugs (DMARDs) are the first line of treatment. Methotrexate (MTX) and leflunomide (LEF) are commonly used, but there are limited data on the effectiveness of combination therapy. To address this issue, Mulder and colleagues enrolled 78 patients with active PsA who have two or more swollen joints and randomly allocated them to either 25 mg oral MTX weekly after 4 weeks of 15 mg weekly plus 20 mg LEF daily (n = 39) or MTX plus placebo (monotherapy; n = 39). At week 16, PsA disease activity score was improved significantly in the MTX + LEF vs. MTX monotherapy group (3.1 vs. 3.7; P = .025). Incidence of mild adverse events, such as nausea/vomiting (44% vs. 28%) and altered bowel habits (26% vs. 8%), was higher with MTX + LEF vs. MTX + placebo. So although less well tolerated, MTX + LEF therapy was superior to MTX monotherapy at improving disease activity in patients with PsA.
Biologics targeting tumor necrosis factor (TNF), interleukin (IL) -12/23, -23, and -17A are efficacious for the management of PsA, but questions remain about comparative effectiveness. Gossec and colleagues reported the results from their prospective observational PsABio study that evaluated real-world treatment persistence and effectiveness at 1 year after initiation of first-line to third-line IL-12/23 inhibitor ustekinumab or a TNF inhibitor (TNFi). Their study followed 893 patients. After 1 year of treatment, ustekinumab and the TNFi showed similar persistence (hazard ratio [HR] for stopping/switching treatment 0.82; 95% CI 0.60-1.13) and a similar proportion of patients achieving (on the Disease Activity Index for PsA) clinical low disease activity (odds ratio [OR] 0.80; 95% CI 0.57-1.10) and remission (OR 0.73; 95% CI 0.49-1.07), along with similar safety profiles. Thus in real-world studies, TNFi and ustekinumab seem to have similar effectiveness and safety.
Drug persistence between patients with psoriasis alone vs. those with PsA is also of interest. In a real-life study including 62 patients with psoriasis and 90 patients with PsA who initiated treatment with secukinumab and were followed up for 24 months or until discontinuation, Ortolan and colleagues demonstrated that the retention rate of secukinumab was higher in psoriasis vs. PsA at 12 (85% vs. 68%) and 24 (61% vs. 57%) months, with the risk for secukinumab discontinuation being higher among patients with PsA in the overall cohort (HR 2.43; P = .035) and in patients with obesity in the PsA cohort (P = .021). Thus, the presence of PsA and obesity lower the secukinumab retention rate.
- Despite the advent of many targeted therapies for PsA, there remain many unmet needs. Deucravacitinib is a novel oral selective inhibitor of tyrosine kinase 2 (TYK2) acting via binding to the TYK2 regulatory domain. In a phase 2 study including 203 patients with active PsA that was intolerant to at least one therapy who were randomly assigned to receive 6 mg deucravacitinib once daily, 12 mg deucravacitinib once daily, or placebo for 16 weeks, Mease and colleagues demonstrated that at week 16, American College of Rheumatology 20 (ACR20) response was significantly higher with 6 mg once-daily deucravacitinib (52.9%, adjusted OR [aOR] 2.4; P = .0134) and 12 mg (62.7%, aOR 3.6; P = .0004) vs. placebo (31.8%), with 12 mg deucravacitinib improving ACR20 response as early as at 8 weeks (P < .05). No serious adverse events were reported. Thus, TYK2 inhibition shows promise in the treatment of PsA and the results from phase 3 trials are awaited.
Treatment of psoriatic arthritis (PsA) was the focus of clinical research papers published this month. Despite the advances made in treating PsA with targeted therapies, in most parts of the world, conventional disease-modifying antirheumatic drugs (DMARDs) are the first line of treatment. Methotrexate (MTX) and leflunomide (LEF) are commonly used, but there are limited data on the effectiveness of combination therapy. To address this issue, Mulder and colleagues enrolled 78 patients with active PsA who have two or more swollen joints and randomly allocated them to either 25 mg oral MTX weekly after 4 weeks of 15 mg weekly plus 20 mg LEF daily (n = 39) or MTX plus placebo (monotherapy; n = 39). At week 16, PsA disease activity score was improved significantly in the MTX + LEF vs. MTX monotherapy group (3.1 vs. 3.7; P = .025). Incidence of mild adverse events, such as nausea/vomiting (44% vs. 28%) and altered bowel habits (26% vs. 8%), was higher with MTX + LEF vs. MTX + placebo. So although less well tolerated, MTX + LEF therapy was superior to MTX monotherapy at improving disease activity in patients with PsA.
Biologics targeting tumor necrosis factor (TNF), interleukin (IL) -12/23, -23, and -17A are efficacious for the management of PsA, but questions remain about comparative effectiveness. Gossec and colleagues reported the results from their prospective observational PsABio study that evaluated real-world treatment persistence and effectiveness at 1 year after initiation of first-line to third-line IL-12/23 inhibitor ustekinumab or a TNF inhibitor (TNFi). Their study followed 893 patients. After 1 year of treatment, ustekinumab and the TNFi showed similar persistence (hazard ratio [HR] for stopping/switching treatment 0.82; 95% CI 0.60-1.13) and a similar proportion of patients achieving (on the Disease Activity Index for PsA) clinical low disease activity (odds ratio [OR] 0.80; 95% CI 0.57-1.10) and remission (OR 0.73; 95% CI 0.49-1.07), along with similar safety profiles. Thus in real-world studies, TNFi and ustekinumab seem to have similar effectiveness and safety.
Drug persistence between patients with psoriasis alone vs. those with PsA is also of interest. In a real-life study including 62 patients with psoriasis and 90 patients with PsA who initiated treatment with secukinumab and were followed up for 24 months or until discontinuation, Ortolan and colleagues demonstrated that the retention rate of secukinumab was higher in psoriasis vs. PsA at 12 (85% vs. 68%) and 24 (61% vs. 57%) months, with the risk for secukinumab discontinuation being higher among patients with PsA in the overall cohort (HR 2.43; P = .035) and in patients with obesity in the PsA cohort (P = .021). Thus, the presence of PsA and obesity lower the secukinumab retention rate.
- Despite the advent of many targeted therapies for PsA, there remain many unmet needs. Deucravacitinib is a novel oral selective inhibitor of tyrosine kinase 2 (TYK2) acting via binding to the TYK2 regulatory domain. In a phase 2 study including 203 patients with active PsA that was intolerant to at least one therapy who were randomly assigned to receive 6 mg deucravacitinib once daily, 12 mg deucravacitinib once daily, or placebo for 16 weeks, Mease and colleagues demonstrated that at week 16, American College of Rheumatology 20 (ACR20) response was significantly higher with 6 mg once-daily deucravacitinib (52.9%, adjusted OR [aOR] 2.4; P = .0134) and 12 mg (62.7%, aOR 3.6; P = .0004) vs. placebo (31.8%), with 12 mg deucravacitinib improving ACR20 response as early as at 8 weeks (P < .05). No serious adverse events were reported. Thus, TYK2 inhibition shows promise in the treatment of PsA and the results from phase 3 trials are awaited.