User login
Angioimmunoblastic T-cell Lymphoma Mimicking DRESS Syndrome
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
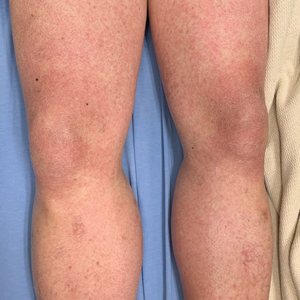
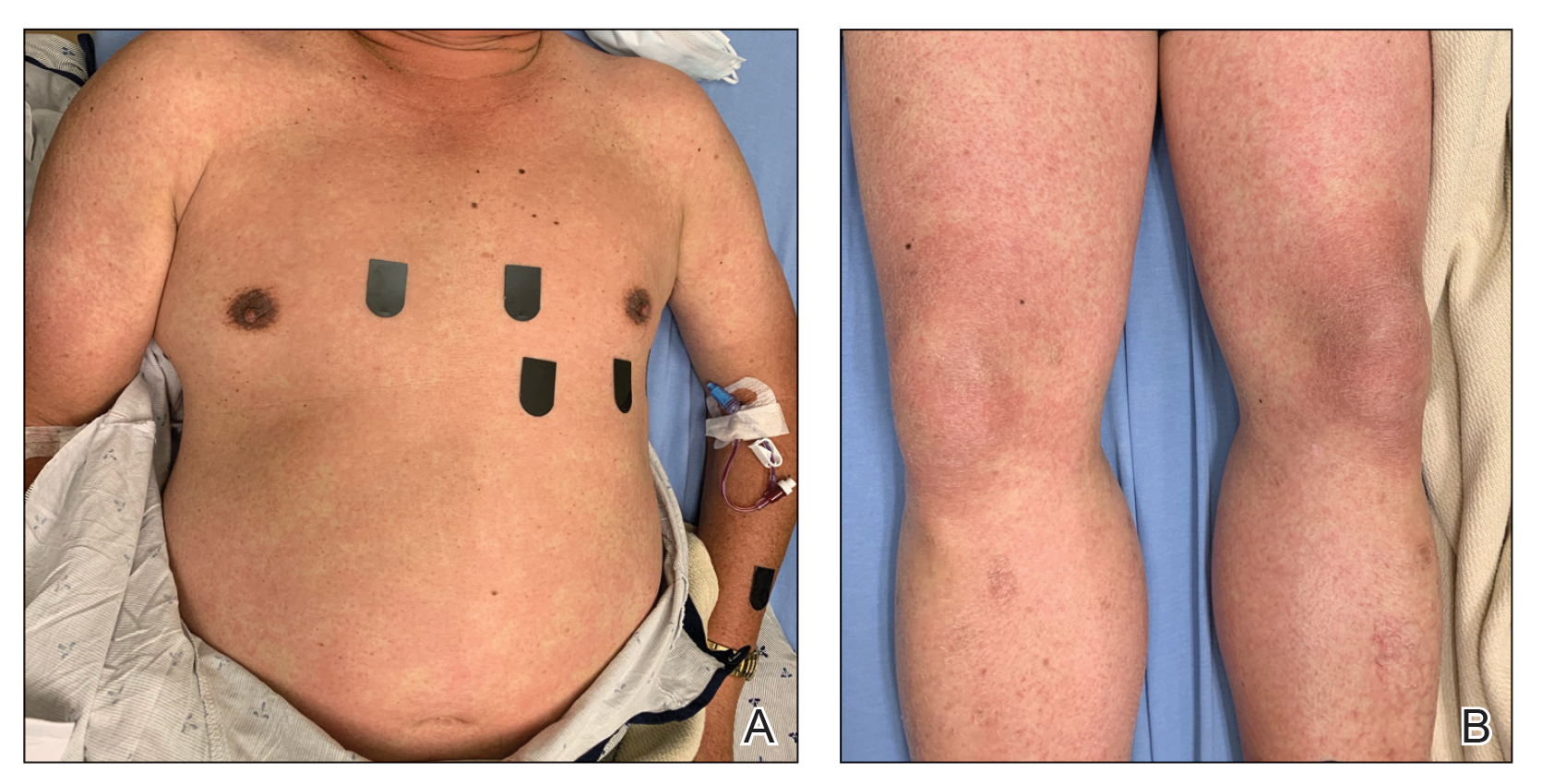
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.
Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.

Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Practice Points
- It is important to maintain a high index of suspicion for angioimmunoblastic T-cell lymphoma in older patients with a longstanding rash and no clear culprit for drug reaction with eosinophilia and systemic symptoms (DRESS syndrome).
- Consider performing a lymph node biopsy early in the course of disease in patients with presumed DRESS syndrome who do not improve with drug withdrawal and steroid therapy.
Communicating Statin Safety to Patients With Hypercholesterolemia
Dr James de Lemos, professor of internal medicine at the University of Texas Southwestern Medical Center, presents a framework to address the most common questions that patients have about the reported muscular, cognitive, and hepatotoxic side effects of statin therapy.
To start, he presents clinical data to address areas of patient concern. Next, he discusses ways to develop — from the first visit — a partnership with patients and encourage their informed decision-making by guiding them to reliable medical sources.
Finally, Dr de Lemos presents strategies that clinicians can use to improve adherence to statin therapy to reach LDL-C treatment goals
--
James de Lemos, MD, PhD
Professor, Internal Medicine, Division of Cardiology, University of Texas Southwestern Medical Center, Dallas, Texas
James de Lemos, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; Regeneron; AstraZeneca
Dr James de Lemos, professor of internal medicine at the University of Texas Southwestern Medical Center, presents a framework to address the most common questions that patients have about the reported muscular, cognitive, and hepatotoxic side effects of statin therapy.
To start, he presents clinical data to address areas of patient concern. Next, he discusses ways to develop — from the first visit — a partnership with patients and encourage their informed decision-making by guiding them to reliable medical sources.
Finally, Dr de Lemos presents strategies that clinicians can use to improve adherence to statin therapy to reach LDL-C treatment goals
--
James de Lemos, MD, PhD
Professor, Internal Medicine, Division of Cardiology, University of Texas Southwestern Medical Center, Dallas, Texas
James de Lemos, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; Regeneron; AstraZeneca
Dr James de Lemos, professor of internal medicine at the University of Texas Southwestern Medical Center, presents a framework to address the most common questions that patients have about the reported muscular, cognitive, and hepatotoxic side effects of statin therapy.
To start, he presents clinical data to address areas of patient concern. Next, he discusses ways to develop — from the first visit — a partnership with patients and encourage their informed decision-making by guiding them to reliable medical sources.
Finally, Dr de Lemos presents strategies that clinicians can use to improve adherence to statin therapy to reach LDL-C treatment goals
--
James de Lemos, MD, PhD
Professor, Internal Medicine, Division of Cardiology, University of Texas Southwestern Medical Center, Dallas, Texas
James de Lemos, MD, PhD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Amgen; Regeneron; AstraZeneca

Clinical Edge Journal Scan Commentary: RA April 2022
Vaccination strategies for people with rheumatic diseases have received significant scrutiny in regard to COVID-19 vaccines. People with rheumatoid arthritis (RA) are at higher risk for adverse outcomes related to COVID-19 but also may have reduced immunogenicity to COVID-19 vaccines; thus, temporary withdrawal of medications has been suggested. Renner Araujo and colleagues report the results of a single-center randomized study from Brazil looking at the immune response to two doses of the Sinovac-CoronaVac vaccine in 129 patients with RA. They found that those who stopped methotrexate for 2 weeks after both doses had a higher rate of seroconversion, based on immunoglobulin (Ig) G positivity (78% vs. 54%), than those who remained on methotrexate. Antibody titers were also higher in the "methotrexate-hold" group. Flare rates were higher, based on the Clinical Disease Activity Index (CDAI) — though not on the Disease Activity Scale-28 (DAS-28) — in patients who withdrew from methotrexate. This information is interesting with respect to the use of future inactivated virus vaccines, though its applicability to mRNA vaccines, other than predicting the possibility of flare, is less clear. However, the results could be useful in informed discussions and decision-making regarding withdrawing immunosuppressive drugs.
Some people with rheumatic diseases have been concerned about flares of disease activity related to COVID-19 vaccination. Tedeschi and colleagues conducted a prospective observational study of 71 patients with RA who were previously inoculated against COVID-19 with two mRNA vaccine doses or one adenovirus vector vaccine dose. Using the patient-reported Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), they measured disease activity weekly from study enrollment through 4 weeks after an additional dose. They did not find any change in mean RADAI-5 score between pre- and post additional dose, nor was disease activity different in patients who stopped disease-modifying antirheumatic drugs (DMARD) compared with those who did not. The study also examined flow cytometry of lymphocyte populations among a subset of these patients (n = 27) and found no significant differences in T peripheral helper cells, T follicular helper cells, age-associated B cells, and plasmablasts among patients before and after the additional vaccine dose. Though the flow cytometry data are difficult to generalize, given the presumed heterogeneity and small number of patients, the lack of change in RA disease activity after the additional vaccine dose is reassuring.
Achievement of remission in patients with RA is also an area of continued interest, especially because of the difficulty of reaching this target under real-world conditions. Larid and colleagues report the prognostic factors of remission and characteristics of 215 patients with RA over 7 years of follow-up at an academic hospital in France. Notably, 33% of patients were in remission at 1 year, of whom 76% remained in remission at 7 years. However, 48% of patients who were not in remission at 1 year achieved remission at 7 years; 58% of study participants were in remission in total at 7 years of follow-up. Those in remission were more frequently being prescribed both conventional synthetic DMARD (csDMARD) and biologic DMARD (bDMARD), while those not in remission at 7 years were receiving corticosteroids at higher doses. Owing to the lack of more precise treatment information, as well as the large number of patients who did not complete the 7-year follow-up visit, drawing conclusions is difficult. While we cannot say, based on these results, that use of certain medication regimens or strategies is more likely to lead to remission, the data at least lend support to the possibility of achieving remission in the long term.
Finally, Ahmad and colleagues performed a post-hoc analysis of the phase 3b AVERT trial of abatacept vs. methotrexate; 172 patients in remission were evaluated at 6 and 12 months after withdrawal of abatacept + methotrexate, abatacept monotherapy, or methotrexate monotherapy. Similar proportions of patients in all three treatment groups experienced a flare (about 58% at 6 months and 66% at 12 months). Patients with higher Health Assessment Questionnaire Disability Index (HAQ-DI) scores and evidence of erosions on MRI at withdrawal were more likely to experience a flare, consistent with prior studies. This highlights potential predictors of withdrawal from therapy. Given the lack of significant difference between the treatment groups, however, it does raise the question of why combination therapy with abatacept and methotrexate is more likely to lead to patients achieving remission in studies without as big an effect on drug-free remission after withdrawal. A more stringent definition of remission rather than DAS-28 C-reactive protein may be desirable.
Vaccination strategies for people with rheumatic diseases have received significant scrutiny in regard to COVID-19 vaccines. People with rheumatoid arthritis (RA) are at higher risk for adverse outcomes related to COVID-19 but also may have reduced immunogenicity to COVID-19 vaccines; thus, temporary withdrawal of medications has been suggested. Renner Araujo and colleagues report the results of a single-center randomized study from Brazil looking at the immune response to two doses of the Sinovac-CoronaVac vaccine in 129 patients with RA. They found that those who stopped methotrexate for 2 weeks after both doses had a higher rate of seroconversion, based on immunoglobulin (Ig) G positivity (78% vs. 54%), than those who remained on methotrexate. Antibody titers were also higher in the "methotrexate-hold" group. Flare rates were higher, based on the Clinical Disease Activity Index (CDAI) — though not on the Disease Activity Scale-28 (DAS-28) — in patients who withdrew from methotrexate. This information is interesting with respect to the use of future inactivated virus vaccines, though its applicability to mRNA vaccines, other than predicting the possibility of flare, is less clear. However, the results could be useful in informed discussions and decision-making regarding withdrawing immunosuppressive drugs.
Some people with rheumatic diseases have been concerned about flares of disease activity related to COVID-19 vaccination. Tedeschi and colleagues conducted a prospective observational study of 71 patients with RA who were previously inoculated against COVID-19 with two mRNA vaccine doses or one adenovirus vector vaccine dose. Using the patient-reported Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), they measured disease activity weekly from study enrollment through 4 weeks after an additional dose. They did not find any change in mean RADAI-5 score between pre- and post additional dose, nor was disease activity different in patients who stopped disease-modifying antirheumatic drugs (DMARD) compared with those who did not. The study also examined flow cytometry of lymphocyte populations among a subset of these patients (n = 27) and found no significant differences in T peripheral helper cells, T follicular helper cells, age-associated B cells, and plasmablasts among patients before and after the additional vaccine dose. Though the flow cytometry data are difficult to generalize, given the presumed heterogeneity and small number of patients, the lack of change in RA disease activity after the additional vaccine dose is reassuring.
Achievement of remission in patients with RA is also an area of continued interest, especially because of the difficulty of reaching this target under real-world conditions. Larid and colleagues report the prognostic factors of remission and characteristics of 215 patients with RA over 7 years of follow-up at an academic hospital in France. Notably, 33% of patients were in remission at 1 year, of whom 76% remained in remission at 7 years. However, 48% of patients who were not in remission at 1 year achieved remission at 7 years; 58% of study participants were in remission in total at 7 years of follow-up. Those in remission were more frequently being prescribed both conventional synthetic DMARD (csDMARD) and biologic DMARD (bDMARD), while those not in remission at 7 years were receiving corticosteroids at higher doses. Owing to the lack of more precise treatment information, as well as the large number of patients who did not complete the 7-year follow-up visit, drawing conclusions is difficult. While we cannot say, based on these results, that use of certain medication regimens or strategies is more likely to lead to remission, the data at least lend support to the possibility of achieving remission in the long term.
Finally, Ahmad and colleagues performed a post-hoc analysis of the phase 3b AVERT trial of abatacept vs. methotrexate; 172 patients in remission were evaluated at 6 and 12 months after withdrawal of abatacept + methotrexate, abatacept monotherapy, or methotrexate monotherapy. Similar proportions of patients in all three treatment groups experienced a flare (about 58% at 6 months and 66% at 12 months). Patients with higher Health Assessment Questionnaire Disability Index (HAQ-DI) scores and evidence of erosions on MRI at withdrawal were more likely to experience a flare, consistent with prior studies. This highlights potential predictors of withdrawal from therapy. Given the lack of significant difference between the treatment groups, however, it does raise the question of why combination therapy with abatacept and methotrexate is more likely to lead to patients achieving remission in studies without as big an effect on drug-free remission after withdrawal. A more stringent definition of remission rather than DAS-28 C-reactive protein may be desirable.
Vaccination strategies for people with rheumatic diseases have received significant scrutiny in regard to COVID-19 vaccines. People with rheumatoid arthritis (RA) are at higher risk for adverse outcomes related to COVID-19 but also may have reduced immunogenicity to COVID-19 vaccines; thus, temporary withdrawal of medications has been suggested. Renner Araujo and colleagues report the results of a single-center randomized study from Brazil looking at the immune response to two doses of the Sinovac-CoronaVac vaccine in 129 patients with RA. They found that those who stopped methotrexate for 2 weeks after both doses had a higher rate of seroconversion, based on immunoglobulin (Ig) G positivity (78% vs. 54%), than those who remained on methotrexate. Antibody titers were also higher in the "methotrexate-hold" group. Flare rates were higher, based on the Clinical Disease Activity Index (CDAI) — though not on the Disease Activity Scale-28 (DAS-28) — in patients who withdrew from methotrexate. This information is interesting with respect to the use of future inactivated virus vaccines, though its applicability to mRNA vaccines, other than predicting the possibility of flare, is less clear. However, the results could be useful in informed discussions and decision-making regarding withdrawing immunosuppressive drugs.
Some people with rheumatic diseases have been concerned about flares of disease activity related to COVID-19 vaccination. Tedeschi and colleagues conducted a prospective observational study of 71 patients with RA who were previously inoculated against COVID-19 with two mRNA vaccine doses or one adenovirus vector vaccine dose. Using the patient-reported Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), they measured disease activity weekly from study enrollment through 4 weeks after an additional dose. They did not find any change in mean RADAI-5 score between pre- and post additional dose, nor was disease activity different in patients who stopped disease-modifying antirheumatic drugs (DMARD) compared with those who did not. The study also examined flow cytometry of lymphocyte populations among a subset of these patients (n = 27) and found no significant differences in T peripheral helper cells, T follicular helper cells, age-associated B cells, and plasmablasts among patients before and after the additional vaccine dose. Though the flow cytometry data are difficult to generalize, given the presumed heterogeneity and small number of patients, the lack of change in RA disease activity after the additional vaccine dose is reassuring.
Achievement of remission in patients with RA is also an area of continued interest, especially because of the difficulty of reaching this target under real-world conditions. Larid and colleagues report the prognostic factors of remission and characteristics of 215 patients with RA over 7 years of follow-up at an academic hospital in France. Notably, 33% of patients were in remission at 1 year, of whom 76% remained in remission at 7 years. However, 48% of patients who were not in remission at 1 year achieved remission at 7 years; 58% of study participants were in remission in total at 7 years of follow-up. Those in remission were more frequently being prescribed both conventional synthetic DMARD (csDMARD) and biologic DMARD (bDMARD), while those not in remission at 7 years were receiving corticosteroids at higher doses. Owing to the lack of more precise treatment information, as well as the large number of patients who did not complete the 7-year follow-up visit, drawing conclusions is difficult. While we cannot say, based on these results, that use of certain medication regimens or strategies is more likely to lead to remission, the data at least lend support to the possibility of achieving remission in the long term.
Finally, Ahmad and colleagues performed a post-hoc analysis of the phase 3b AVERT trial of abatacept vs. methotrexate; 172 patients in remission were evaluated at 6 and 12 months after withdrawal of abatacept + methotrexate, abatacept monotherapy, or methotrexate monotherapy. Similar proportions of patients in all three treatment groups experienced a flare (about 58% at 6 months and 66% at 12 months). Patients with higher Health Assessment Questionnaire Disability Index (HAQ-DI) scores and evidence of erosions on MRI at withdrawal were more likely to experience a flare, consistent with prior studies. This highlights potential predictors of withdrawal from therapy. Given the lack of significant difference between the treatment groups, however, it does raise the question of why combination therapy with abatacept and methotrexate is more likely to lead to patients achieving remission in studies without as big an effect on drug-free remission after withdrawal. A more stringent definition of remission rather than DAS-28 C-reactive protein may be desirable.
Clinical Edge Journal Scan Commentary: HCC April 2022
Yao and colleagues confirmed that there are well-known risk factors for recurrence of HCC after surgical resection. They retrospectively analyzed 1424 patients who underwent resection with curative intent for Barcelona Clinical Liver Cancer (BCLC) stage 0/A HCC in several centers in China. Of those patients, 679 (47.7%) developed recurrence at a median follow-up of 54.8 months, including 408 (60.1%) with an early recurrence (≤ 2 years after surgery) and 271 (39.9%) with a late recurrence (> 2 years). Cirrhosis (adjusted hazard ratio [aHR] 1.49; P < .001), preoperative alpha-fetoprotein (AFP) level > 400 µg/L (aHR 1.28; P = .004), tumor size > 5 cm (aHR 1.74; P < .001), the presence of satellite nodules (aHR 1.35; P = .040), multiple tumors (aHR 1.63; P = .015), microvascular invasion (aHR 1.51; P < .001), and intraoperative blood transfusion (aHR 1.50; P = .013) were identified as independent risk factors associated with postoperative HCC recurrence. The authors concluded that those patients with risk factors for recurrence would benefit from more intensive surveillance and potentially additional liver-directed therapy with curative intent.
Not all patients with hepatitis C virus (HCV) infection and HCC are offered antiviral therapy. Takaura and colleagues confirmed that active HCV infection worsens the prognosis of patients with very early-stage HCC who undergo treatment with radiofrequency ablation (RFA). In this single-center retrospective study, 302 patients with BCLC stage 0 HCC who underwent RFA were analyzed. Of those patients, 195 had evidence of HCV, and 132 had an active infection. The authors concluded that active HCV infection was a significant risk factor for shorter overall survival (aHR 2.17; P = .003) and early recurrence of HCC (aHR 1.47; P = .022). Patients with active HCV infection had a shorter median overall survival (66 months vs 145 months) and recurrence-free survival (20 months vs 31 months) (both P < .001). Therefore, treatment of active HCV should be offered to patients even after the development of HCC.
Kuroda and colleagues retrospectively analyzed a multicenter cohort of 247 patients with unresectable HCC treated with lenvatinib between 2018 and 2020. Out of those, 63 patients who received lenvatinib and transarterial chemoembolization (TACE) sequential therapy were propensity-score matched to those receiving lenvatinib monotherapy. The overall survival and progression-free survival in the sequential group were significantly higher than those in the lenvatinib monotherapy group, 31.2 (26.4-34.3) vs 15.7 (13.1-19.4) months and 12.2 (8.5-17.3) vs 6.7 (5.3-10.2) months (P = .002 and P = .037), respectively. Multivariate analysis showed that the deep response was independently associated with the initial response to levatinib; the partial response showed an odds ratio of 13.75 (95% CI 0.41-1.32; P < .001). The authors concluded that sequential therapy might provide more clinical benefits than lenvatinib monotherapy in patients who responded to initial lenvatinib treatment, with objective response to initial lenvatinib being an independent factor predicting sequential therapy deep response.
Yao and colleagues confirmed that there are well-known risk factors for recurrence of HCC after surgical resection. They retrospectively analyzed 1424 patients who underwent resection with curative intent for Barcelona Clinical Liver Cancer (BCLC) stage 0/A HCC in several centers in China. Of those patients, 679 (47.7%) developed recurrence at a median follow-up of 54.8 months, including 408 (60.1%) with an early recurrence (≤ 2 years after surgery) and 271 (39.9%) with a late recurrence (> 2 years). Cirrhosis (adjusted hazard ratio [aHR] 1.49; P < .001), preoperative alpha-fetoprotein (AFP) level > 400 µg/L (aHR 1.28; P = .004), tumor size > 5 cm (aHR 1.74; P < .001), the presence of satellite nodules (aHR 1.35; P = .040), multiple tumors (aHR 1.63; P = .015), microvascular invasion (aHR 1.51; P < .001), and intraoperative blood transfusion (aHR 1.50; P = .013) were identified as independent risk factors associated with postoperative HCC recurrence. The authors concluded that those patients with risk factors for recurrence would benefit from more intensive surveillance and potentially additional liver-directed therapy with curative intent.
Not all patients with hepatitis C virus (HCV) infection and HCC are offered antiviral therapy. Takaura and colleagues confirmed that active HCV infection worsens the prognosis of patients with very early-stage HCC who undergo treatment with radiofrequency ablation (RFA). In this single-center retrospective study, 302 patients with BCLC stage 0 HCC who underwent RFA were analyzed. Of those patients, 195 had evidence of HCV, and 132 had an active infection. The authors concluded that active HCV infection was a significant risk factor for shorter overall survival (aHR 2.17; P = .003) and early recurrence of HCC (aHR 1.47; P = .022). Patients with active HCV infection had a shorter median overall survival (66 months vs 145 months) and recurrence-free survival (20 months vs 31 months) (both P < .001). Therefore, treatment of active HCV should be offered to patients even after the development of HCC.
Kuroda and colleagues retrospectively analyzed a multicenter cohort of 247 patients with unresectable HCC treated with lenvatinib between 2018 and 2020. Out of those, 63 patients who received lenvatinib and transarterial chemoembolization (TACE) sequential therapy were propensity-score matched to those receiving lenvatinib monotherapy. The overall survival and progression-free survival in the sequential group were significantly higher than those in the lenvatinib monotherapy group, 31.2 (26.4-34.3) vs 15.7 (13.1-19.4) months and 12.2 (8.5-17.3) vs 6.7 (5.3-10.2) months (P = .002 and P = .037), respectively. Multivariate analysis showed that the deep response was independently associated with the initial response to levatinib; the partial response showed an odds ratio of 13.75 (95% CI 0.41-1.32; P < .001). The authors concluded that sequential therapy might provide more clinical benefits than lenvatinib monotherapy in patients who responded to initial lenvatinib treatment, with objective response to initial lenvatinib being an independent factor predicting sequential therapy deep response.
Yao and colleagues confirmed that there are well-known risk factors for recurrence of HCC after surgical resection. They retrospectively analyzed 1424 patients who underwent resection with curative intent for Barcelona Clinical Liver Cancer (BCLC) stage 0/A HCC in several centers in China. Of those patients, 679 (47.7%) developed recurrence at a median follow-up of 54.8 months, including 408 (60.1%) with an early recurrence (≤ 2 years after surgery) and 271 (39.9%) with a late recurrence (> 2 years). Cirrhosis (adjusted hazard ratio [aHR] 1.49; P < .001), preoperative alpha-fetoprotein (AFP) level > 400 µg/L (aHR 1.28; P = .004), tumor size > 5 cm (aHR 1.74; P < .001), the presence of satellite nodules (aHR 1.35; P = .040), multiple tumors (aHR 1.63; P = .015), microvascular invasion (aHR 1.51; P < .001), and intraoperative blood transfusion (aHR 1.50; P = .013) were identified as independent risk factors associated with postoperative HCC recurrence. The authors concluded that those patients with risk factors for recurrence would benefit from more intensive surveillance and potentially additional liver-directed therapy with curative intent.
Not all patients with hepatitis C virus (HCV) infection and HCC are offered antiviral therapy. Takaura and colleagues confirmed that active HCV infection worsens the prognosis of patients with very early-stage HCC who undergo treatment with radiofrequency ablation (RFA). In this single-center retrospective study, 302 patients with BCLC stage 0 HCC who underwent RFA were analyzed. Of those patients, 195 had evidence of HCV, and 132 had an active infection. The authors concluded that active HCV infection was a significant risk factor for shorter overall survival (aHR 2.17; P = .003) and early recurrence of HCC (aHR 1.47; P = .022). Patients with active HCV infection had a shorter median overall survival (66 months vs 145 months) and recurrence-free survival (20 months vs 31 months) (both P < .001). Therefore, treatment of active HCV should be offered to patients even after the development of HCC.
Kuroda and colleagues retrospectively analyzed a multicenter cohort of 247 patients with unresectable HCC treated with lenvatinib between 2018 and 2020. Out of those, 63 patients who received lenvatinib and transarterial chemoembolization (TACE) sequential therapy were propensity-score matched to those receiving lenvatinib monotherapy. The overall survival and progression-free survival in the sequential group were significantly higher than those in the lenvatinib monotherapy group, 31.2 (26.4-34.3) vs 15.7 (13.1-19.4) months and 12.2 (8.5-17.3) vs 6.7 (5.3-10.2) months (P = .002 and P = .037), respectively. Multivariate analysis showed that the deep response was independently associated with the initial response to levatinib; the partial response showed an odds ratio of 13.75 (95% CI 0.41-1.32; P < .001). The authors concluded that sequential therapy might provide more clinical benefits than lenvatinib monotherapy in patients who responded to initial lenvatinib treatment, with objective response to initial lenvatinib being an independent factor predicting sequential therapy deep response.
Clinical Edge Journal Scan Commentary: HCC April 2022
Yao and colleagues confirmed that there are well-known risk factors for recurrence of HCC after surgical resection. They retrospectively analyzed 1424 patients who underwent resection with curative intent for Barcelona Clinical Liver Cancer (BCLC) stage 0/A HCC in several centers in China. Of those patients, 679 (47.7%) developed recurrence at a median follow-up of 54.8 months, including 408 (60.1%) with an early recurrence (≤ 2 years after surgery) and 271 (39.9%) with a late recurrence (> 2 years). Cirrhosis (adjusted hazard ratio [aHR] 1.49; P < .001), preoperative alpha-fetoprotein (AFP) level > 400 µg/L (aHR 1.28; P = .004), tumor size > 5 cm (aHR 1.74; P < .001), the presence of satellite nodules (aHR 1.35; P = .040), multiple tumors (aHR 1.63; P = .015), microvascular invasion (aHR 1.51; P < .001), and intraoperative blood transfusion (aHR 1.50; P = .013) were identified as independent risk factors associated with postoperative HCC recurrence. The authors concluded that those patients with risk factors for recurrence would benefit from more intensive surveillance and potentially additional liver-directed therapy with curative intent.
Not all patients with hepatitis C virus (HCV) infection and HCC are offered antiviral therapy. Takaura and colleagues confirmed that active HCV infection worsens the prognosis of patients with very early-stage HCC who undergo treatment with radiofrequency ablation (RFA). In this single-center retrospective study, 302 patients with BCLC stage 0 HCC who underwent RFA were analyzed. Of those patients, 195 had evidence of HCV, and 132 had an active infection. The authors concluded that active HCV infection was a significant risk factor for shorter overall survival (aHR 2.17; P = .003) and early recurrence of HCC (aHR 1.47; P = .022). Patients with active HCV infection had a shorter median overall survival (66 months vs 145 months) and recurrence-free survival (20 months vs 31 months) (both P < .001). Therefore, treatment of active HCV should be offered to patients even after the development of HCC.
Kuroda and colleagues retrospectively analyzed a multicenter cohort of 247 patients with unresectable HCC treated with lenvatinib between 2018 and 2020. Out of those, 63 patients who received lenvatinib and transarterial chemoembolization (TACE) sequential therapy were propensity-score matched to those receiving lenvatinib monotherapy. The overall survival and progression-free survival in the sequential group were significantly higher than those in the lenvatinib monotherapy group, 31.2 (26.4-34.3) vs 15.7 (13.1-19.4) months and 12.2 (8.5-17.3) vs 6.7 (5.3-10.2) months (P = .002 and P = .037), respectively. Multivariate analysis showed that the deep response was independently associated with the initial response to levatinib; the partial response showed an odds ratio of 13.75 (95% CI 0.41-1.32; P < .001). The authors concluded that sequential therapy might provide more clinical benefits than lenvatinib monotherapy in patients who responded to initial lenvatinib treatment, with objective response to initial lenvatinib being an independent factor predicting sequential therapy deep response.
Yao and colleagues confirmed that there are well-known risk factors for recurrence of HCC after surgical resection. They retrospectively analyzed 1424 patients who underwent resection with curative intent for Barcelona Clinical Liver Cancer (BCLC) stage 0/A HCC in several centers in China. Of those patients, 679 (47.7%) developed recurrence at a median follow-up of 54.8 months, including 408 (60.1%) with an early recurrence (≤ 2 years after surgery) and 271 (39.9%) with a late recurrence (> 2 years). Cirrhosis (adjusted hazard ratio [aHR] 1.49; P < .001), preoperative alpha-fetoprotein (AFP) level > 400 µg/L (aHR 1.28; P = .004), tumor size > 5 cm (aHR 1.74; P < .001), the presence of satellite nodules (aHR 1.35; P = .040), multiple tumors (aHR 1.63; P = .015), microvascular invasion (aHR 1.51; P < .001), and intraoperative blood transfusion (aHR 1.50; P = .013) were identified as independent risk factors associated with postoperative HCC recurrence. The authors concluded that those patients with risk factors for recurrence would benefit from more intensive surveillance and potentially additional liver-directed therapy with curative intent.
Not all patients with hepatitis C virus (HCV) infection and HCC are offered antiviral therapy. Takaura and colleagues confirmed that active HCV infection worsens the prognosis of patients with very early-stage HCC who undergo treatment with radiofrequency ablation (RFA). In this single-center retrospective study, 302 patients with BCLC stage 0 HCC who underwent RFA were analyzed. Of those patients, 195 had evidence of HCV, and 132 had an active infection. The authors concluded that active HCV infection was a significant risk factor for shorter overall survival (aHR 2.17; P = .003) and early recurrence of HCC (aHR 1.47; P = .022). Patients with active HCV infection had a shorter median overall survival (66 months vs 145 months) and recurrence-free survival (20 months vs 31 months) (both P < .001). Therefore, treatment of active HCV should be offered to patients even after the development of HCC.
Kuroda and colleagues retrospectively analyzed a multicenter cohort of 247 patients with unresectable HCC treated with lenvatinib between 2018 and 2020. Out of those, 63 patients who received lenvatinib and transarterial chemoembolization (TACE) sequential therapy were propensity-score matched to those receiving lenvatinib monotherapy. The overall survival and progression-free survival in the sequential group were significantly higher than those in the lenvatinib monotherapy group, 31.2 (26.4-34.3) vs 15.7 (13.1-19.4) months and 12.2 (8.5-17.3) vs 6.7 (5.3-10.2) months (P = .002 and P = .037), respectively. Multivariate analysis showed that the deep response was independently associated with the initial response to levatinib; the partial response showed an odds ratio of 13.75 (95% CI 0.41-1.32; P < .001). The authors concluded that sequential therapy might provide more clinical benefits than lenvatinib monotherapy in patients who responded to initial lenvatinib treatment, with objective response to initial lenvatinib being an independent factor predicting sequential therapy deep response.
Yao and colleagues confirmed that there are well-known risk factors for recurrence of HCC after surgical resection. They retrospectively analyzed 1424 patients who underwent resection with curative intent for Barcelona Clinical Liver Cancer (BCLC) stage 0/A HCC in several centers in China. Of those patients, 679 (47.7%) developed recurrence at a median follow-up of 54.8 months, including 408 (60.1%) with an early recurrence (≤ 2 years after surgery) and 271 (39.9%) with a late recurrence (> 2 years). Cirrhosis (adjusted hazard ratio [aHR] 1.49; P < .001), preoperative alpha-fetoprotein (AFP) level > 400 µg/L (aHR 1.28; P = .004), tumor size > 5 cm (aHR 1.74; P < .001), the presence of satellite nodules (aHR 1.35; P = .040), multiple tumors (aHR 1.63; P = .015), microvascular invasion (aHR 1.51; P < .001), and intraoperative blood transfusion (aHR 1.50; P = .013) were identified as independent risk factors associated with postoperative HCC recurrence. The authors concluded that those patients with risk factors for recurrence would benefit from more intensive surveillance and potentially additional liver-directed therapy with curative intent.
Not all patients with hepatitis C virus (HCV) infection and HCC are offered antiviral therapy. Takaura and colleagues confirmed that active HCV infection worsens the prognosis of patients with very early-stage HCC who undergo treatment with radiofrequency ablation (RFA). In this single-center retrospective study, 302 patients with BCLC stage 0 HCC who underwent RFA were analyzed. Of those patients, 195 had evidence of HCV, and 132 had an active infection. The authors concluded that active HCV infection was a significant risk factor for shorter overall survival (aHR 2.17; P = .003) and early recurrence of HCC (aHR 1.47; P = .022). Patients with active HCV infection had a shorter median overall survival (66 months vs 145 months) and recurrence-free survival (20 months vs 31 months) (both P < .001). Therefore, treatment of active HCV should be offered to patients even after the development of HCC.
Kuroda and colleagues retrospectively analyzed a multicenter cohort of 247 patients with unresectable HCC treated with lenvatinib between 2018 and 2020. Out of those, 63 patients who received lenvatinib and transarterial chemoembolization (TACE) sequential therapy were propensity-score matched to those receiving lenvatinib monotherapy. The overall survival and progression-free survival in the sequential group were significantly higher than those in the lenvatinib monotherapy group, 31.2 (26.4-34.3) vs 15.7 (13.1-19.4) months and 12.2 (8.5-17.3) vs 6.7 (5.3-10.2) months (P = .002 and P = .037), respectively. Multivariate analysis showed that the deep response was independently associated with the initial response to levatinib; the partial response showed an odds ratio of 13.75 (95% CI 0.41-1.32; P < .001). The authors concluded that sequential therapy might provide more clinical benefits than lenvatinib monotherapy in patients who responded to initial lenvatinib treatment, with objective response to initial lenvatinib being an independent factor predicting sequential therapy deep response.
Clinical Edge Journal Scan Commentary: Breast Cancer April 2022
Studies have demonstrated inferior survival outcomes associated with delays in time to surgery and adjuvant chemotherapy for early breast cancer. The timing of adjuvant endocrine therapy is also notable because of the favorable effect on recurrence risk and survival with these agents in early HR+ breast cancer. A cohort study from the National Cancer Database including 144,103 women demonstrated a 31% increase in the risk for death (hazard ratio [HR] 1.31; P < .001) with a time to adjuvant hormone therapy (TTH) > 150 days (6.4% of patients) compared with those with TTH ≤ 150 days (93.6% of patients). Factors associated with delay in TTH included Black race, nonprivate insurance, metropolitan residence (vs. urban or rural), community hospital setting (vs. academic), higher comorbidity index, poorly differentiated tumors, higher stage, breast conservation surgery (vs. mastectomy), and radiation therapy. This study highlights the need to avoid unnecessary delays in adjuvant hormone therapy and encourages further exploration of barriers to timely initiation of breast cancer therapies to maximize outcomes for patients.
The role of reproductive hormones in breast cancer risk and carcinogenesis has been extensively studied and hormonal therapies are an essential component of the management of HR+ breast cancer. Lan and colleagues performed a retrospective analysis including 196 premenopausal and 137 postmenopausal women treated with neoadjuvant chemotherapy for breast cancer, investigating the correlation between pretreatment levels of reproductive hormone levels with pathologic and survival outcomes. Higher likelihood of achieving pathologic complete response was seen in premenopausal women with lower vs. higher testosterone levels (odds ratio [OR] 0.996; P = .026) and in postmenopausal women with higher vs. lower follicle-stimulating hormone levels (OR 1.045; P = .005). Furthermore, lower progesterone levels in premenopausal patients was associated with inferior overall survival (OS) (3-year OS 72.9% vs. 97.4% for lowest tertile progesterone vs. higher tertiles; P = .007). These data suggest a potential role of reproductive hormones in the preoperative evaluation for breast cancer patients. Also, the complex actions of progesterone and "crosstalk" between estrogen receptors and progesterone receptors continue to be elucidated and ongoing studies are evaluating progestin combined with endocrine therapy.
The CLEOPATRA trial has established the regimen of trastuzumab plus pertuzumab plus docetaxel as first-line therapy for metastatic HER2-positive (HER2+) breast cancer with an absolute survival benefit of 16.3 months vs. trastuzumab plus docetaxel. A retrospective study conducted in Ontario, Canada, explored real-world outcomes of pertuzumab plus trastuzumab plus chemotherapy among 1158 patients and demonstrated a similar magnitude of survival improvement with the addition of pertuzumab (14.9 months) (Dai et al). The median OS was higher among patients receiving pertuzumab compared with control (40.2 vs. 25.3 months), and although the median OS was shorter in the real-world setting than in the CLEOPATRA trial, they had similar HRs for mortality reduction (0.66 for real-world and 0.69 for trial). Furthermore, there was no increase in cardiotoxicity and lower cumulative incidence of hospitalization at 1 year with pertuzumab vs. control (11.7% vs. 19.0%; P < .001). This study adds to the existing body of data supporting first-line treatment with pertuzumab plus trastuzumab plus chemotherapy for metastatic HER2+ breast cancer. The treatment landscape for these patients is certainly dynamic with the development of novel therapies and combinations in this space and resultant shifts in the current algorithm.
Recommended Additional Reading:
Hortobagyi GN et al. LBA17 Overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB). Ann Oncol. 2021;32:S1290-S1291. Doi: 10.1016/j.annonc.2021.08.2090
Chavez-MacGregor M et al. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2:322-329. Doi:10.1001/jamaoncol.2015.3856
Trabert B et al. Progesterone and breast cancer. Endocr Rev. 2020;41:320-344. Doi: 10.1210/endrev/bnz001
Swain SM et al; on behalf of the CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519-530. Doi: 10.1016/S1470-2045(19)30863-0
Studies have demonstrated inferior survival outcomes associated with delays in time to surgery and adjuvant chemotherapy for early breast cancer. The timing of adjuvant endocrine therapy is also notable because of the favorable effect on recurrence risk and survival with these agents in early HR+ breast cancer. A cohort study from the National Cancer Database including 144,103 women demonstrated a 31% increase in the risk for death (hazard ratio [HR] 1.31; P < .001) with a time to adjuvant hormone therapy (TTH) > 150 days (6.4% of patients) compared with those with TTH ≤ 150 days (93.6% of patients). Factors associated with delay in TTH included Black race, nonprivate insurance, metropolitan residence (vs. urban or rural), community hospital setting (vs. academic), higher comorbidity index, poorly differentiated tumors, higher stage, breast conservation surgery (vs. mastectomy), and radiation therapy. This study highlights the need to avoid unnecessary delays in adjuvant hormone therapy and encourages further exploration of barriers to timely initiation of breast cancer therapies to maximize outcomes for patients.
The role of reproductive hormones in breast cancer risk and carcinogenesis has been extensively studied and hormonal therapies are an essential component of the management of HR+ breast cancer. Lan and colleagues performed a retrospective analysis including 196 premenopausal and 137 postmenopausal women treated with neoadjuvant chemotherapy for breast cancer, investigating the correlation between pretreatment levels of reproductive hormone levels with pathologic and survival outcomes. Higher likelihood of achieving pathologic complete response was seen in premenopausal women with lower vs. higher testosterone levels (odds ratio [OR] 0.996; P = .026) and in postmenopausal women with higher vs. lower follicle-stimulating hormone levels (OR 1.045; P = .005). Furthermore, lower progesterone levels in premenopausal patients was associated with inferior overall survival (OS) (3-year OS 72.9% vs. 97.4% for lowest tertile progesterone vs. higher tertiles; P = .007). These data suggest a potential role of reproductive hormones in the preoperative evaluation for breast cancer patients. Also, the complex actions of progesterone and "crosstalk" between estrogen receptors and progesterone receptors continue to be elucidated and ongoing studies are evaluating progestin combined with endocrine therapy.
The CLEOPATRA trial has established the regimen of trastuzumab plus pertuzumab plus docetaxel as first-line therapy for metastatic HER2-positive (HER2+) breast cancer with an absolute survival benefit of 16.3 months vs. trastuzumab plus docetaxel. A retrospective study conducted in Ontario, Canada, explored real-world outcomes of pertuzumab plus trastuzumab plus chemotherapy among 1158 patients and demonstrated a similar magnitude of survival improvement with the addition of pertuzumab (14.9 months) (Dai et al). The median OS was higher among patients receiving pertuzumab compared with control (40.2 vs. 25.3 months), and although the median OS was shorter in the real-world setting than in the CLEOPATRA trial, they had similar HRs for mortality reduction (0.66 for real-world and 0.69 for trial). Furthermore, there was no increase in cardiotoxicity and lower cumulative incidence of hospitalization at 1 year with pertuzumab vs. control (11.7% vs. 19.0%; P < .001). This study adds to the existing body of data supporting first-line treatment with pertuzumab plus trastuzumab plus chemotherapy for metastatic HER2+ breast cancer. The treatment landscape for these patients is certainly dynamic with the development of novel therapies and combinations in this space and resultant shifts in the current algorithm.
Recommended Additional Reading:
Hortobagyi GN et al. LBA17 Overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB). Ann Oncol. 2021;32:S1290-S1291. Doi: 10.1016/j.annonc.2021.08.2090
Chavez-MacGregor M et al. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2:322-329. Doi:10.1001/jamaoncol.2015.3856
Trabert B et al. Progesterone and breast cancer. Endocr Rev. 2020;41:320-344. Doi: 10.1210/endrev/bnz001
Swain SM et al; on behalf of the CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519-530. Doi: 10.1016/S1470-2045(19)30863-0
Studies have demonstrated inferior survival outcomes associated with delays in time to surgery and adjuvant chemotherapy for early breast cancer. The timing of adjuvant endocrine therapy is also notable because of the favorable effect on recurrence risk and survival with these agents in early HR+ breast cancer. A cohort study from the National Cancer Database including 144,103 women demonstrated a 31% increase in the risk for death (hazard ratio [HR] 1.31; P < .001) with a time to adjuvant hormone therapy (TTH) > 150 days (6.4% of patients) compared with those with TTH ≤ 150 days (93.6% of patients). Factors associated with delay in TTH included Black race, nonprivate insurance, metropolitan residence (vs. urban or rural), community hospital setting (vs. academic), higher comorbidity index, poorly differentiated tumors, higher stage, breast conservation surgery (vs. mastectomy), and radiation therapy. This study highlights the need to avoid unnecessary delays in adjuvant hormone therapy and encourages further exploration of barriers to timely initiation of breast cancer therapies to maximize outcomes for patients.
The role of reproductive hormones in breast cancer risk and carcinogenesis has been extensively studied and hormonal therapies are an essential component of the management of HR+ breast cancer. Lan and colleagues performed a retrospective analysis including 196 premenopausal and 137 postmenopausal women treated with neoadjuvant chemotherapy for breast cancer, investigating the correlation between pretreatment levels of reproductive hormone levels with pathologic and survival outcomes. Higher likelihood of achieving pathologic complete response was seen in premenopausal women with lower vs. higher testosterone levels (odds ratio [OR] 0.996; P = .026) and in postmenopausal women with higher vs. lower follicle-stimulating hormone levels (OR 1.045; P = .005). Furthermore, lower progesterone levels in premenopausal patients was associated with inferior overall survival (OS) (3-year OS 72.9% vs. 97.4% for lowest tertile progesterone vs. higher tertiles; P = .007). These data suggest a potential role of reproductive hormones in the preoperative evaluation for breast cancer patients. Also, the complex actions of progesterone and "crosstalk" between estrogen receptors and progesterone receptors continue to be elucidated and ongoing studies are evaluating progestin combined with endocrine therapy.
The CLEOPATRA trial has established the regimen of trastuzumab plus pertuzumab plus docetaxel as first-line therapy for metastatic HER2-positive (HER2+) breast cancer with an absolute survival benefit of 16.3 months vs. trastuzumab plus docetaxel. A retrospective study conducted in Ontario, Canada, explored real-world outcomes of pertuzumab plus trastuzumab plus chemotherapy among 1158 patients and demonstrated a similar magnitude of survival improvement with the addition of pertuzumab (14.9 months) (Dai et al). The median OS was higher among patients receiving pertuzumab compared with control (40.2 vs. 25.3 months), and although the median OS was shorter in the real-world setting than in the CLEOPATRA trial, they had similar HRs for mortality reduction (0.66 for real-world and 0.69 for trial). Furthermore, there was no increase in cardiotoxicity and lower cumulative incidence of hospitalization at 1 year with pertuzumab vs. control (11.7% vs. 19.0%; P < .001). This study adds to the existing body of data supporting first-line treatment with pertuzumab plus trastuzumab plus chemotherapy for metastatic HER2+ breast cancer. The treatment landscape for these patients is certainly dynamic with the development of novel therapies and combinations in this space and resultant shifts in the current algorithm.
Recommended Additional Reading:
Hortobagyi GN et al. LBA17 Overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib (RIB). Ann Oncol. 2021;32:S1290-S1291. Doi: 10.1016/j.annonc.2021.08.2090
Chavez-MacGregor M et al. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. 2016;2:322-329. Doi:10.1001/jamaoncol.2015.3856
Trabert B et al. Progesterone and breast cancer. Endocr Rev. 2020;41:320-344. Doi: 10.1210/endrev/bnz001
Swain SM et al; on behalf of the CLEOPATRA study group. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519-530. Doi: 10.1016/S1470-2045(19)30863-0
Infectious disease pop quiz: Clinical challenge #20 for the ObGyn
What are the principal microorganisms that cause puerperal mastitis?
Continue to the answer...
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
What are the principal microorganisms that cause puerperal mastitis?
Continue to the answer...
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used.
What are the principal microorganisms that cause puerperal mastitis?
Continue to the answer...
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Abdominal rash
Despite his insistence that he was not scratching his abdomen, the lack of primary lesions and the appearance of horizontally oriented excoriations over the abdomen in multiple stages of healing were consistent with neurotic excoriations.
Neurotic excoriation is frequently associated with psychiatric disease, especially obsessive-compulsive disorder and depression.1 Stimulant-use, either by prescription or illicit, can lead to increased self-grooming behaviors, motor tics, and scratching. High doses of stimulants can trigger paranoia and tactile hallucinations.
In this case, the preponderance of skin lesions occurring on the left side of the patient’s abdomen fit with a right-handed individual, which the patient was. On his anterior lower legs, there were linear excoriations oriented vertically. Close observation of the patient during history taking revealed unconscious skin-picking behavior, and dead skin and debris could be noted under his fingernails. Two punch biopsies of active lesions were consistent with excoriations and excluded inflammatory causes of itching. (Careful evaluation for scabies, eczema, urticaria, and contact dermatitis was also performed.)
In this case, the patient’s psychiatrist reduced his dosage of lisdexamfetamine to a starting dose of 30 mg daily, which led to decreased skin scratching behavior. While the patient continued to have limited insight into the nature of his skin changes, progress was measured by a reduction in the number of active lesions.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi: 10.1016/j.clindermatol.2018.08.006

Despite his insistence that he was not scratching his abdomen, the lack of primary lesions and the appearance of horizontally oriented excoriations over the abdomen in multiple stages of healing were consistent with neurotic excoriations.
Neurotic excoriation is frequently associated with psychiatric disease, especially obsessive-compulsive disorder and depression.1 Stimulant-use, either by prescription or illicit, can lead to increased self-grooming behaviors, motor tics, and scratching. High doses of stimulants can trigger paranoia and tactile hallucinations.
In this case, the preponderance of skin lesions occurring on the left side of the patient’s abdomen fit with a right-handed individual, which the patient was. On his anterior lower legs, there were linear excoriations oriented vertically. Close observation of the patient during history taking revealed unconscious skin-picking behavior, and dead skin and debris could be noted under his fingernails. Two punch biopsies of active lesions were consistent with excoriations and excluded inflammatory causes of itching. (Careful evaluation for scabies, eczema, urticaria, and contact dermatitis was also performed.)
In this case, the patient’s psychiatrist reduced his dosage of lisdexamfetamine to a starting dose of 30 mg daily, which led to decreased skin scratching behavior. While the patient continued to have limited insight into the nature of his skin changes, progress was measured by a reduction in the number of active lesions.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Despite his insistence that he was not scratching his abdomen, the lack of primary lesions and the appearance of horizontally oriented excoriations over the abdomen in multiple stages of healing were consistent with neurotic excoriations.
Neurotic excoriation is frequently associated with psychiatric disease, especially obsessive-compulsive disorder and depression.1 Stimulant-use, either by prescription or illicit, can lead to increased self-grooming behaviors, motor tics, and scratching. High doses of stimulants can trigger paranoia and tactile hallucinations.
In this case, the preponderance of skin lesions occurring on the left side of the patient’s abdomen fit with a right-handed individual, which the patient was. On his anterior lower legs, there were linear excoriations oriented vertically. Close observation of the patient during history taking revealed unconscious skin-picking behavior, and dead skin and debris could be noted under his fingernails. Two punch biopsies of active lesions were consistent with excoriations and excluded inflammatory causes of itching. (Careful evaluation for scabies, eczema, urticaria, and contact dermatitis was also performed.)
In this case, the patient’s psychiatrist reduced his dosage of lisdexamfetamine to a starting dose of 30 mg daily, which led to decreased skin scratching behavior. While the patient continued to have limited insight into the nature of his skin changes, progress was measured by a reduction in the number of active lesions.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi: 10.1016/j.clindermatol.2018.08.006
1. Gupta MA, Vujcic B, Pur DR, et al. Use of antipsychotic drugs in dermatology. Clin Dermatol. 2018;36:765-773. doi: 10.1016/j.clindermatol.2018.08.006