User login
Sustained glycemic control in T2D decreases the likelihood for long-term complications
Key clinical point: Patients with type 2 diabetes (T2D) who maintain glycosylated hemoglobin (HbA1c) levels of <7% vs. ≥7% during a 5-year post-period have a lower risk for diabetes-related complications.
Major finding: Maintaining an HbA1c level of <7% vs. ≥7% during the 5-year post-period was associated with a lower risk of developing cardiovascular disease (odds ratio [OR] 0.76; 95% CI 0.61-0.94), metabolic disease (OR 0.37; 95% CI 0.22-0.60), neuropathy (OR 0.62; 95% CI 0.45-0.84), nephropathy (OR 0.81; 95% CI 0.69-0.94), and peripheral vascular disease (OR 0.52; 95% CI 0.33-0.83).
Study details: Findings are from a retrospective study including 3067 adult patients with T2D and sustained glycemic control (HbA1c <7%; n = 2,119) or sustained suboptimal glycemic control (HbA1c ≥7%; n = 1,488).
Disclosures: This study was funded by Eli Lilly and Company. KS Boye, R Paczkowski, and VT Thieu reported being employees and shareholders of Eli Lilly and MJ Lage received personal compensation from Eli Lilly.
Source: Boye KS et al. The association between sustained HbA1c control and long-term complications among individuals with type 2 diabetes: A retrospective study. Adv Ther. 2022 (Mar 22). Doi: 10.1007/s12325-022-02106-4
Key clinical point: Patients with type 2 diabetes (T2D) who maintain glycosylated hemoglobin (HbA1c) levels of <7% vs. ≥7% during a 5-year post-period have a lower risk for diabetes-related complications.
Major finding: Maintaining an HbA1c level of <7% vs. ≥7% during the 5-year post-period was associated with a lower risk of developing cardiovascular disease (odds ratio [OR] 0.76; 95% CI 0.61-0.94), metabolic disease (OR 0.37; 95% CI 0.22-0.60), neuropathy (OR 0.62; 95% CI 0.45-0.84), nephropathy (OR 0.81; 95% CI 0.69-0.94), and peripheral vascular disease (OR 0.52; 95% CI 0.33-0.83).
Study details: Findings are from a retrospective study including 3067 adult patients with T2D and sustained glycemic control (HbA1c <7%; n = 2,119) or sustained suboptimal glycemic control (HbA1c ≥7%; n = 1,488).
Disclosures: This study was funded by Eli Lilly and Company. KS Boye, R Paczkowski, and VT Thieu reported being employees and shareholders of Eli Lilly and MJ Lage received personal compensation from Eli Lilly.
Source: Boye KS et al. The association between sustained HbA1c control and long-term complications among individuals with type 2 diabetes: A retrospective study. Adv Ther. 2022 (Mar 22). Doi: 10.1007/s12325-022-02106-4
Key clinical point: Patients with type 2 diabetes (T2D) who maintain glycosylated hemoglobin (HbA1c) levels of <7% vs. ≥7% during a 5-year post-period have a lower risk for diabetes-related complications.
Major finding: Maintaining an HbA1c level of <7% vs. ≥7% during the 5-year post-period was associated with a lower risk of developing cardiovascular disease (odds ratio [OR] 0.76; 95% CI 0.61-0.94), metabolic disease (OR 0.37; 95% CI 0.22-0.60), neuropathy (OR 0.62; 95% CI 0.45-0.84), nephropathy (OR 0.81; 95% CI 0.69-0.94), and peripheral vascular disease (OR 0.52; 95% CI 0.33-0.83).
Study details: Findings are from a retrospective study including 3067 adult patients with T2D and sustained glycemic control (HbA1c <7%; n = 2,119) or sustained suboptimal glycemic control (HbA1c ≥7%; n = 1,488).
Disclosures: This study was funded by Eli Lilly and Company. KS Boye, R Paczkowski, and VT Thieu reported being employees and shareholders of Eli Lilly and MJ Lage received personal compensation from Eli Lilly.
Source: Boye KS et al. The association between sustained HbA1c control and long-term complications among individuals with type 2 diabetes: A retrospective study. Adv Ther. 2022 (Mar 22). Doi: 10.1007/s12325-022-02106-4
Severe mental illness raises risk for serious long-term diabetic complications in T2D
Key clinical point: Patients with type 2 diabetes (T2D) and severe mental illness (SMI) are at a higher risk of developing nephropathy, lower limp amputations, and cardiovascular diseases (CVD), but not retinopathy, compared with those with T2D and without SMI.
Major finding: Compared with patients with T2D and without SMI, those with T2D and SMI had a higher incidence rate (IR) of nephropathy (IR ratio [IRR] 1.15; 95% CI 1.12-1.18), amputations (IRR 1.15; 95% CI 1.04-1.28), and CVD (men IRR 1.10; 95% CI 1.06-1.15, and women IRR 1.18; 95% CI 1.13-1.22), but a lower IR of retinopathy (IRR 0.75; 95% CI 0.70-0.81).
Study details: Findings are from a population-based dynamic cohort study including 371,625 patients with T2D, of which 30,102 had a coexisting SMI.
Disclosures: This study did not receive any funding. Some authors declared owning shares or receiving research grants from various sources.
Source: Scheuer SH et al. Severe mental illness and the risk of diabetes complications. A nationwide register-based cohort study. J Clin Endocrinol Metab. 2022 (Mar 31). Doi: 10.1210/clinem/dgac204
Key clinical point: Patients with type 2 diabetes (T2D) and severe mental illness (SMI) are at a higher risk of developing nephropathy, lower limp amputations, and cardiovascular diseases (CVD), but not retinopathy, compared with those with T2D and without SMI.
Major finding: Compared with patients with T2D and without SMI, those with T2D and SMI had a higher incidence rate (IR) of nephropathy (IR ratio [IRR] 1.15; 95% CI 1.12-1.18), amputations (IRR 1.15; 95% CI 1.04-1.28), and CVD (men IRR 1.10; 95% CI 1.06-1.15, and women IRR 1.18; 95% CI 1.13-1.22), but a lower IR of retinopathy (IRR 0.75; 95% CI 0.70-0.81).
Study details: Findings are from a population-based dynamic cohort study including 371,625 patients with T2D, of which 30,102 had a coexisting SMI.
Disclosures: This study did not receive any funding. Some authors declared owning shares or receiving research grants from various sources.
Source: Scheuer SH et al. Severe mental illness and the risk of diabetes complications. A nationwide register-based cohort study. J Clin Endocrinol Metab. 2022 (Mar 31). Doi: 10.1210/clinem/dgac204
Key clinical point: Patients with type 2 diabetes (T2D) and severe mental illness (SMI) are at a higher risk of developing nephropathy, lower limp amputations, and cardiovascular diseases (CVD), but not retinopathy, compared with those with T2D and without SMI.
Major finding: Compared with patients with T2D and without SMI, those with T2D and SMI had a higher incidence rate (IR) of nephropathy (IR ratio [IRR] 1.15; 95% CI 1.12-1.18), amputations (IRR 1.15; 95% CI 1.04-1.28), and CVD (men IRR 1.10; 95% CI 1.06-1.15, and women IRR 1.18; 95% CI 1.13-1.22), but a lower IR of retinopathy (IRR 0.75; 95% CI 0.70-0.81).
Study details: Findings are from a population-based dynamic cohort study including 371,625 patients with T2D, of which 30,102 had a coexisting SMI.
Disclosures: This study did not receive any funding. Some authors declared owning shares or receiving research grants from various sources.
Source: Scheuer SH et al. Severe mental illness and the risk of diabetes complications. A nationwide register-based cohort study. J Clin Endocrinol Metab. 2022 (Mar 31). Doi: 10.1210/clinem/dgac204
Preadmission antidiabetic drug use and mortality risk in COVID-19 patients with T2D
Key clinical point: The preadmission antidiabetic medications may influence mortality outcomes in patients with COVID-19 and type 2 diabetes (T2D).
Major finding: The risk for in-hospital mortality was significantly lower among patients taking metformin (odd ratio [OR] 0.54; 95% CI 0.47-0.62), glucagon-like peptide-1 receptor agonist (OR 0.51; 95% CI 0.37-0.69), and sodium-glucose transporter-2 inhibitor (OR 0.60; 95% CI 0.40-0.88), but higher among those taking dipeptidyl peptidase-4 inhibitor (OR 1.23; 95% CI 1.07-1.42) and insulin (OR 1.70; 95% CI 1.33-2.19), compared with patients taking none of these medications. Sulfonylurea, thiazolidinedione, and alpha-glucosidase inhibitors showed neutral effects on mortality.
Study details: The data come from a meta-analysis of 61 studies including 3,061,584 patients with COVID-19 and T2D.
Disclosures: This study received no specific grant from any funding agency.
Source: Nguyen NN et al. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis. Metabolism. 2022;131:155196 (Mar 31). Doi: 10.1016/j.metabol.2022.155196
Key clinical point: The preadmission antidiabetic medications may influence mortality outcomes in patients with COVID-19 and type 2 diabetes (T2D).
Major finding: The risk for in-hospital mortality was significantly lower among patients taking metformin (odd ratio [OR] 0.54; 95% CI 0.47-0.62), glucagon-like peptide-1 receptor agonist (OR 0.51; 95% CI 0.37-0.69), and sodium-glucose transporter-2 inhibitor (OR 0.60; 95% CI 0.40-0.88), but higher among those taking dipeptidyl peptidase-4 inhibitor (OR 1.23; 95% CI 1.07-1.42) and insulin (OR 1.70; 95% CI 1.33-2.19), compared with patients taking none of these medications. Sulfonylurea, thiazolidinedione, and alpha-glucosidase inhibitors showed neutral effects on mortality.
Study details: The data come from a meta-analysis of 61 studies including 3,061,584 patients with COVID-19 and T2D.
Disclosures: This study received no specific grant from any funding agency.
Source: Nguyen NN et al. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis. Metabolism. 2022;131:155196 (Mar 31). Doi: 10.1016/j.metabol.2022.155196
Key clinical point: The preadmission antidiabetic medications may influence mortality outcomes in patients with COVID-19 and type 2 diabetes (T2D).
Major finding: The risk for in-hospital mortality was significantly lower among patients taking metformin (odd ratio [OR] 0.54; 95% CI 0.47-0.62), glucagon-like peptide-1 receptor agonist (OR 0.51; 95% CI 0.37-0.69), and sodium-glucose transporter-2 inhibitor (OR 0.60; 95% CI 0.40-0.88), but higher among those taking dipeptidyl peptidase-4 inhibitor (OR 1.23; 95% CI 1.07-1.42) and insulin (OR 1.70; 95% CI 1.33-2.19), compared with patients taking none of these medications. Sulfonylurea, thiazolidinedione, and alpha-glucosidase inhibitors showed neutral effects on mortality.
Study details: The data come from a meta-analysis of 61 studies including 3,061,584 patients with COVID-19 and T2D.
Disclosures: This study received no specific grant from any funding agency.
Source: Nguyen NN et al. Preadmission use of antidiabetic medications and mortality among patients with COVID-19 having type 2 diabetes: A meta-analysis. Metabolism. 2022;131:155196 (Mar 31). Doi: 10.1016/j.metabol.2022.155196
SGLT2is offers real-world renal protective benefits over DPP4i in T2D
Key clinical point: In patients with type 2 diabetes (T2D), the use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) vs. dipeptidyl peptidase-4 inhibitors (DPP4i) was associated with a lower risk for end-stage renal disease (ESRD) and acute renal failure (ARF) and a slower decline in the estimated glomerular filtration rate (eGFR).
Major finding: Over a median follow-up of 3.8 years, the use of SGLT2i vs. DPP4i was associated with a significantly lower risk for ESRD (hazard ratio [HR] 0.51; P < .001) and ARF (HR 0.59; P < .001) and a significantly slower decline in eGFR (−0.060 vs. −0.625 mL/min/1.73m2 per year; Pinteraction < .001).
Study details: This retrospective cohort study propensity score matched 6333 patients with T2D receiving an SGLT2i with 25,332 of those receiving a DPP4i.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Au PCM et al. Association between SGLT20iInhibitors vs DPP4 inhibitors and renal outcomes among patients with type 2 diabetes. J Clin Endocrinol Metab. 2022 (Mar 18). Doi: 10.1210/clinem/dgac164
Key clinical point: In patients with type 2 diabetes (T2D), the use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) vs. dipeptidyl peptidase-4 inhibitors (DPP4i) was associated with a lower risk for end-stage renal disease (ESRD) and acute renal failure (ARF) and a slower decline in the estimated glomerular filtration rate (eGFR).
Major finding: Over a median follow-up of 3.8 years, the use of SGLT2i vs. DPP4i was associated with a significantly lower risk for ESRD (hazard ratio [HR] 0.51; P < .001) and ARF (HR 0.59; P < .001) and a significantly slower decline in eGFR (−0.060 vs. −0.625 mL/min/1.73m2 per year; Pinteraction < .001).
Study details: This retrospective cohort study propensity score matched 6333 patients with T2D receiving an SGLT2i with 25,332 of those receiving a DPP4i.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Au PCM et al. Association between SGLT20iInhibitors vs DPP4 inhibitors and renal outcomes among patients with type 2 diabetes. J Clin Endocrinol Metab. 2022 (Mar 18). Doi: 10.1210/clinem/dgac164
Key clinical point: In patients with type 2 diabetes (T2D), the use of sodium-glucose cotransporter 2 inhibitors (SGLT2i) vs. dipeptidyl peptidase-4 inhibitors (DPP4i) was associated with a lower risk for end-stage renal disease (ESRD) and acute renal failure (ARF) and a slower decline in the estimated glomerular filtration rate (eGFR).
Major finding: Over a median follow-up of 3.8 years, the use of SGLT2i vs. DPP4i was associated with a significantly lower risk for ESRD (hazard ratio [HR] 0.51; P < .001) and ARF (HR 0.59; P < .001) and a significantly slower decline in eGFR (−0.060 vs. −0.625 mL/min/1.73m2 per year; Pinteraction < .001).
Study details: This retrospective cohort study propensity score matched 6333 patients with T2D receiving an SGLT2i with 25,332 of those receiving a DPP4i.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Au PCM et al. Association between SGLT20iInhibitors vs DPP4 inhibitors and renal outcomes among patients with type 2 diabetes. J Clin Endocrinol Metab. 2022 (Mar 18). Doi: 10.1210/clinem/dgac164
Resistance training reduces HbA1c levels in patients with T2D
Key clinical point: Resistance training (RT) effectively reduces glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2D), with RT interventions triggering a larger vs. medium or smaller improvement in muscular strength leading to a greater reduction in HbA1c.
Major finding: RT intervention vs. control treatment significantly decreased HbA1c (weighted mean difference −0.39; P < .001), with a larger vs. medium or small effect on muscular strength leading to a greater reduction in HbA1c (β −0.99; P = .0470).
Study details: Findings are from a meta-analysis of 20 trials including 1172 patients with T2DM.
Disclosures: The study received no specific funding. The authors declared no competing interests.
Source: Jansson AK et al. Effect of resistance training on HbA1c in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2022;10:e002595 (Mar 10). Doi: 10.1136/bmjdrc-2021-002595
Key clinical point: Resistance training (RT) effectively reduces glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2D), with RT interventions triggering a larger vs. medium or smaller improvement in muscular strength leading to a greater reduction in HbA1c.
Major finding: RT intervention vs. control treatment significantly decreased HbA1c (weighted mean difference −0.39; P < .001), with a larger vs. medium or small effect on muscular strength leading to a greater reduction in HbA1c (β −0.99; P = .0470).
Study details: Findings are from a meta-analysis of 20 trials including 1172 patients with T2DM.
Disclosures: The study received no specific funding. The authors declared no competing interests.
Source: Jansson AK et al. Effect of resistance training on HbA1c in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2022;10:e002595 (Mar 10). Doi: 10.1136/bmjdrc-2021-002595
Key clinical point: Resistance training (RT) effectively reduces glycosylated hemoglobin (HbA1c) in patients with type 2 diabetes mellitus (T2D), with RT interventions triggering a larger vs. medium or smaller improvement in muscular strength leading to a greater reduction in HbA1c.
Major finding: RT intervention vs. control treatment significantly decreased HbA1c (weighted mean difference −0.39; P < .001), with a larger vs. medium or small effect on muscular strength leading to a greater reduction in HbA1c (β −0.99; P = .0470).
Study details: Findings are from a meta-analysis of 20 trials including 1172 patients with T2DM.
Disclosures: The study received no specific funding. The authors declared no competing interests.
Source: Jansson AK et al. Effect of resistance training on HbA1c in adults with type 2 diabetes mellitus and the moderating effect of changes in muscular strength: a systematic review and meta-analysis. BMJ Open Diabetes Res Care. 2022;10:e002595 (Mar 10). Doi: 10.1136/bmjdrc-2021-002595
Fenofibrate improves heart failure outcomes in patients with T2D treated with simvastatin
Key clinical point: Fenofibrate reduced the composite outcome of heart failure (HF) hospitalizations or cardiovascular death in patients with type 2 diabetes (T2D) treated with simvastatin, predominantly in those who received the standard background glucose-lowering therapy.
Major finding: The composite outcome of HF hospitalization or cardiovascular death was significantly lower with fenofibrate vs. placebo (hazard ratio [HR] 0.82; P = .048), with reduction primarily observed with the standard glucose-lowering strategy (HR 0.64; 95% CI 0.48-0.85), but not with the intensive glucose-lowering strategy (HR 1.02; 95% CI 0.79-1.33; Pinteraction = .017).
Study details: Findings are from the ACCORD Lipid trial including 5518 patients with T2D who were randomly assigned to receive simvastatin plus fenofibrate (n = 2765) or simvastatin plus placebo (n = 2753).
Disclosures: The study was funded by national funds through FCT-Portuguese Foundation for
Science and Technology, under the scope of the Cardiovascular R&D Center-UnIC. Some authors declared being consultants and receiving research support or personal fees from various sources.
Source: Ferreira JP et al. Fenofibrate and heart failure outcomes in patients with type 2 diabetes: analysis from ACCORD. Diabetes Care. 2022 (Mar 23). Doi: 10.2337/dc21-1977
Key clinical point: Fenofibrate reduced the composite outcome of heart failure (HF) hospitalizations or cardiovascular death in patients with type 2 diabetes (T2D) treated with simvastatin, predominantly in those who received the standard background glucose-lowering therapy.
Major finding: The composite outcome of HF hospitalization or cardiovascular death was significantly lower with fenofibrate vs. placebo (hazard ratio [HR] 0.82; P = .048), with reduction primarily observed with the standard glucose-lowering strategy (HR 0.64; 95% CI 0.48-0.85), but not with the intensive glucose-lowering strategy (HR 1.02; 95% CI 0.79-1.33; Pinteraction = .017).
Study details: Findings are from the ACCORD Lipid trial including 5518 patients with T2D who were randomly assigned to receive simvastatin plus fenofibrate (n = 2765) or simvastatin plus placebo (n = 2753).
Disclosures: The study was funded by national funds through FCT-Portuguese Foundation for
Science and Technology, under the scope of the Cardiovascular R&D Center-UnIC. Some authors declared being consultants and receiving research support or personal fees from various sources.
Source: Ferreira JP et al. Fenofibrate and heart failure outcomes in patients with type 2 diabetes: analysis from ACCORD. Diabetes Care. 2022 (Mar 23). Doi: 10.2337/dc21-1977
Key clinical point: Fenofibrate reduced the composite outcome of heart failure (HF) hospitalizations or cardiovascular death in patients with type 2 diabetes (T2D) treated with simvastatin, predominantly in those who received the standard background glucose-lowering therapy.
Major finding: The composite outcome of HF hospitalization or cardiovascular death was significantly lower with fenofibrate vs. placebo (hazard ratio [HR] 0.82; P = .048), with reduction primarily observed with the standard glucose-lowering strategy (HR 0.64; 95% CI 0.48-0.85), but not with the intensive glucose-lowering strategy (HR 1.02; 95% CI 0.79-1.33; Pinteraction = .017).
Study details: Findings are from the ACCORD Lipid trial including 5518 patients with T2D who were randomly assigned to receive simvastatin plus fenofibrate (n = 2765) or simvastatin plus placebo (n = 2753).
Disclosures: The study was funded by national funds through FCT-Portuguese Foundation for
Science and Technology, under the scope of the Cardiovascular R&D Center-UnIC. Some authors declared being consultants and receiving research support or personal fees from various sources.
Source: Ferreira JP et al. Fenofibrate and heart failure outcomes in patients with type 2 diabetes: analysis from ACCORD. Diabetes Care. 2022 (Mar 23). Doi: 10.2337/dc21-1977
T2D: Empagliflozin improves cognitive and physical function in older adults with HFpEF
Key clinical point: Empagliflozin showed a beneficial effect on cognitive and physical impairment in frail older patients with type 2 diabetes (T2D) and heart failure with preserved ejection fraction (HFpEF).
Major finding: The mean Montreal Cognitive Assessment score significantly improved from baseline to 1 month in the empagliflozin group (19.80 vs. 22.25; P < .001) but not in the metformin (P = .26) and insulin (P = .81) groups, with empagliflozin showing a significant effect on amelioration of cognitive impairment (odds ratio 3.609; P = .03). The 5-meter gait speed improved significantly in the empagliflozin and metformin groups (both P < .05), but not in the insulin group.
Study details: This prospective observational study included 162 frail older patients aged >65 years who had T2D and HFpEF and were treated with empagliflozin (n = 52), metformin (n = 56), or insulin (n = 54).
Disclosures: The study was partly supported by the US National Institute of Diabetes and Digestive and Kidney Diseases, US National Heart, Lung, and Blood Institute, and US National Institute on Aging, among others. The authors declared no conflicts of interest.
Source: Mone P et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022 (Mar 21). Doi: 10.2337/dc21-2434
Key clinical point: Empagliflozin showed a beneficial effect on cognitive and physical impairment in frail older patients with type 2 diabetes (T2D) and heart failure with preserved ejection fraction (HFpEF).
Major finding: The mean Montreal Cognitive Assessment score significantly improved from baseline to 1 month in the empagliflozin group (19.80 vs. 22.25; P < .001) but not in the metformin (P = .26) and insulin (P = .81) groups, with empagliflozin showing a significant effect on amelioration of cognitive impairment (odds ratio 3.609; P = .03). The 5-meter gait speed improved significantly in the empagliflozin and metformin groups (both P < .05), but not in the insulin group.
Study details: This prospective observational study included 162 frail older patients aged >65 years who had T2D and HFpEF and were treated with empagliflozin (n = 52), metformin (n = 56), or insulin (n = 54).
Disclosures: The study was partly supported by the US National Institute of Diabetes and Digestive and Kidney Diseases, US National Heart, Lung, and Blood Institute, and US National Institute on Aging, among others. The authors declared no conflicts of interest.
Source: Mone P et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022 (Mar 21). Doi: 10.2337/dc21-2434
Key clinical point: Empagliflozin showed a beneficial effect on cognitive and physical impairment in frail older patients with type 2 diabetes (T2D) and heart failure with preserved ejection fraction (HFpEF).
Major finding: The mean Montreal Cognitive Assessment score significantly improved from baseline to 1 month in the empagliflozin group (19.80 vs. 22.25; P < .001) but not in the metformin (P = .26) and insulin (P = .81) groups, with empagliflozin showing a significant effect on amelioration of cognitive impairment (odds ratio 3.609; P = .03). The 5-meter gait speed improved significantly in the empagliflozin and metformin groups (both P < .05), but not in the insulin group.
Study details: This prospective observational study included 162 frail older patients aged >65 years who had T2D and HFpEF and were treated with empagliflozin (n = 52), metformin (n = 56), or insulin (n = 54).
Disclosures: The study was partly supported by the US National Institute of Diabetes and Digestive and Kidney Diseases, US National Heart, Lung, and Blood Institute, and US National Institute on Aging, among others. The authors declared no conflicts of interest.
Source: Mone P et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022 (Mar 21). Doi: 10.2337/dc21-2434
Dapagliflozin shows promise in young people with T2D in phase 3
Key clinical point: Dapagliflozin in addition to standard-of-care treatment demonstrated a clinically relevant decrease in glycated hemoglobin (HbA1c) and an acceptable safety profile in young people with type 2 diabetes (T2D).
Major finding: At 24 weeks, the adjusted mean change in HbA1c was not significantly different between the dapagliflozin and placebo groups in the intention-to-treat analysis (between-group difference [Δ] −0.75%; P = .10), but was significantly different in the sensitivity analysis in the per-protocol population (Δ −1.13%; P = .012). No new safety signals or episodes of death or diabetic ketoacidosis were recorded.
Study details: The data come from a phase 3 trial including 72 participants aged 10-24 years with T2D and HbA1c concentration of 6.5%-11% who were randomly assigned to receive oral dapagliflozin (10 mg) or placebo in addition to standard-of-care treatment for 24 weeks followed by dapagliflozin for 28 weeks
Disclosures: The study was funded by AstraZeneca. Some authors declared receiving consulting fees or research grants or serving on advisory boards for various sources, including AstraZeneca. Three authors declared being stockholders or employees of AstraZeneca.
Source: Tamborlane WV, Laffel LM et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022 (Apr 1). Doi: 10.1016/S2213-8587(22)00052-3
Key clinical point: Dapagliflozin in addition to standard-of-care treatment demonstrated a clinically relevant decrease in glycated hemoglobin (HbA1c) and an acceptable safety profile in young people with type 2 diabetes (T2D).
Major finding: At 24 weeks, the adjusted mean change in HbA1c was not significantly different between the dapagliflozin and placebo groups in the intention-to-treat analysis (between-group difference [Δ] −0.75%; P = .10), but was significantly different in the sensitivity analysis in the per-protocol population (Δ −1.13%; P = .012). No new safety signals or episodes of death or diabetic ketoacidosis were recorded.
Study details: The data come from a phase 3 trial including 72 participants aged 10-24 years with T2D and HbA1c concentration of 6.5%-11% who were randomly assigned to receive oral dapagliflozin (10 mg) or placebo in addition to standard-of-care treatment for 24 weeks followed by dapagliflozin for 28 weeks
Disclosures: The study was funded by AstraZeneca. Some authors declared receiving consulting fees or research grants or serving on advisory boards for various sources, including AstraZeneca. Three authors declared being stockholders or employees of AstraZeneca.
Source: Tamborlane WV, Laffel LM et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022 (Apr 1). Doi: 10.1016/S2213-8587(22)00052-3
Key clinical point: Dapagliflozin in addition to standard-of-care treatment demonstrated a clinically relevant decrease in glycated hemoglobin (HbA1c) and an acceptable safety profile in young people with type 2 diabetes (T2D).
Major finding: At 24 weeks, the adjusted mean change in HbA1c was not significantly different between the dapagliflozin and placebo groups in the intention-to-treat analysis (between-group difference [Δ] −0.75%; P = .10), but was significantly different in the sensitivity analysis in the per-protocol population (Δ −1.13%; P = .012). No new safety signals or episodes of death or diabetic ketoacidosis were recorded.
Study details: The data come from a phase 3 trial including 72 participants aged 10-24 years with T2D and HbA1c concentration of 6.5%-11% who were randomly assigned to receive oral dapagliflozin (10 mg) or placebo in addition to standard-of-care treatment for 24 weeks followed by dapagliflozin for 28 weeks
Disclosures: The study was funded by AstraZeneca. Some authors declared receiving consulting fees or research grants or serving on advisory boards for various sources, including AstraZeneca. Three authors declared being stockholders or employees of AstraZeneca.
Source: Tamborlane WV, Laffel LM et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022 (Apr 1). Doi: 10.1016/S2213-8587(22)00052-3
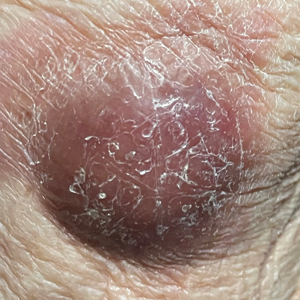
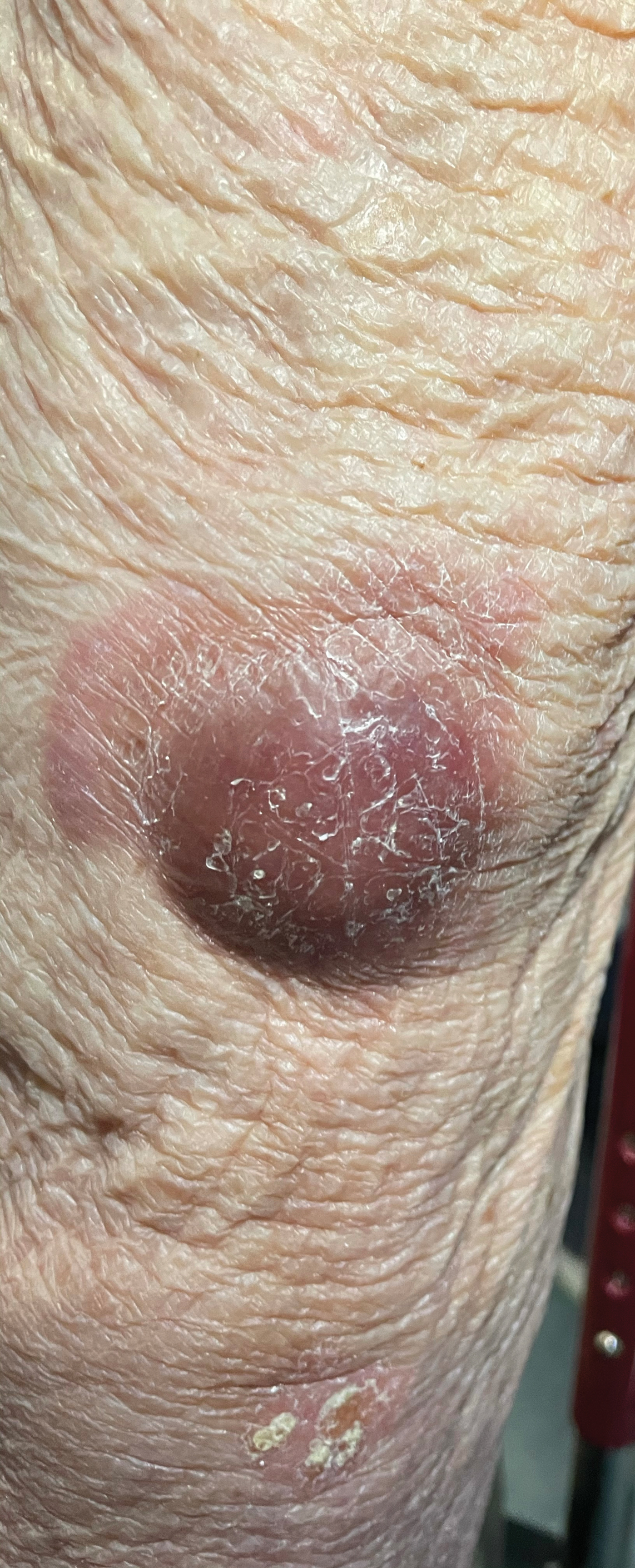
Violaceous Nodules on the Lower Leg
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.
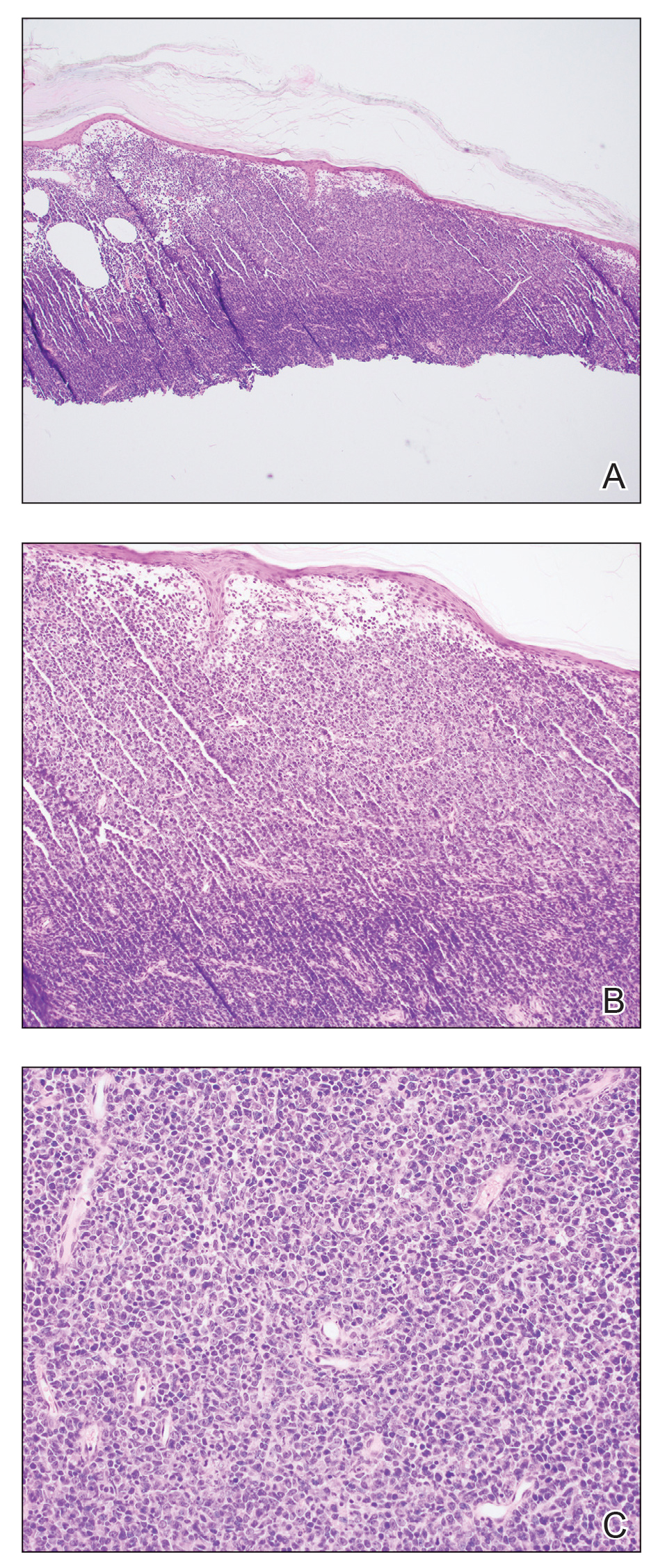
Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
A 79-year-old man presented to the dermatology clinic with 4 enlarging, asymptomatic, violaceous, desquamating nodules on the left pretibial region and calf of 3 months’ duration. He denied any constitutional symptoms such as night sweats or weight loss. His medical history included a malignant melanoma on the left ear that was excised 5 years prior. He also had a history of peripheral edema, hypertension, and rheumatoid arthritis, as well as a 50-pack-year history of smoking. Physical examination revealed 2 large nodules measuring 3.0×3.0 cm each and 2 smaller nodules measuring 1.0×1.0 cm each. There was no appreciable lymphadenopathy.
The power of the pause to prevent diagnostic error
None of us like being wrong, especially about a patient’s diagnosis. To help you avoid diagnostic errors for 4 difficult diagnoses, read and study the article in this issue of JFP by Rosen and colleagues.1 They discuss misdiagnosis of polymyalgia rheumatica, fibromyalgia, ovarian cancer, and Lewy body dementia to illustrate how we can go astray if we do not take care to pause and think through things carefully. They point out that, for quick and mostly accurate diagnoses, pattern recognition or type 1 thinking serves us well in a busy office practice. However, we must frequently pause and reflect, using type 2 thinking—especially when the puzzle pieces don’t quite fit together.
I still recall vividly a diagnostic error I made many years ago. One of my patients, whom I had diagnosed and was treating for hyperlipidemia, returned for follow-up while I was on vacation. My partner conducted the follow-up visit. To my chagrin, he noticed her puffy face and weight gain and ordered thyroid studies. Sure enough, my patient was severely hypothyroid, and her lipid levels normalized with thyroid replacement therapy.
A happier tale for me was making the correct diagnosis for a woman with chronic cough. She had been evaluated by multiple specialists during the prior year and treated with a nasal steroid for allergies, a proton pump inhibitor for reflux, and a steroid inhaler for possible asthma. None of these relieved her cough. After reviewing her medication list and noting that it included amitriptyline, which has anticholinergic adverse effects, I recommended she stop taking that medication and the cough resolved.
John Ely, MD, MPH, a family physician who has spent his academic career investigating causes of and solutions to diagnostic errors, has outlined important steps we can take. These include: (1) obtaining your own complete medical history, (2) performing a “focused and purposeful” physical exam, (3) generating initial hypotheses and differentiating them through additional history taking, exams, and diagnostic tests, (4) pausing to reflect [my emphasis], and (5) embarking on a plan (while acknowledging uncertainty) and ensuring there is a pathway for follow-up.2
To help avoid diagnostic errors, Dr. Ely developed and uses a set of checklists that cover the differential diagnosis for 72 presenting complaints/conditions, including syncope, back pain, insomnia, and headache.2 When you are faced with diagnostic uncertainty, it takes just a few minutes to run through the checklist for the patient’s presenting complaint.
1. Rosen PD, Klenzak S, Baptista S. Diagnostic challenges in primary care: identifying and avoiding cognitive bias. J Fam Pract. 2022;71:124-132.
2. Ely JW, Graber ML, Croskerry P. Checklists to reduce diagnostic errors. Acad Med. 2011;86:307-313. doi: 10.1097/ACM.0b013e31820824cd
None of us like being wrong, especially about a patient’s diagnosis. To help you avoid diagnostic errors for 4 difficult diagnoses, read and study the article in this issue of JFP by Rosen and colleagues.1 They discuss misdiagnosis of polymyalgia rheumatica, fibromyalgia, ovarian cancer, and Lewy body dementia to illustrate how we can go astray if we do not take care to pause and think through things carefully. They point out that, for quick and mostly accurate diagnoses, pattern recognition or type 1 thinking serves us well in a busy office practice. However, we must frequently pause and reflect, using type 2 thinking—especially when the puzzle pieces don’t quite fit together.
I still recall vividly a diagnostic error I made many years ago. One of my patients, whom I had diagnosed and was treating for hyperlipidemia, returned for follow-up while I was on vacation. My partner conducted the follow-up visit. To my chagrin, he noticed her puffy face and weight gain and ordered thyroid studies. Sure enough, my patient was severely hypothyroid, and her lipid levels normalized with thyroid replacement therapy.
A happier tale for me was making the correct diagnosis for a woman with chronic cough. She had been evaluated by multiple specialists during the prior year and treated with a nasal steroid for allergies, a proton pump inhibitor for reflux, and a steroid inhaler for possible asthma. None of these relieved her cough. After reviewing her medication list and noting that it included amitriptyline, which has anticholinergic adverse effects, I recommended she stop taking that medication and the cough resolved.
John Ely, MD, MPH, a family physician who has spent his academic career investigating causes of and solutions to diagnostic errors, has outlined important steps we can take. These include: (1) obtaining your own complete medical history, (2) performing a “focused and purposeful” physical exam, (3) generating initial hypotheses and differentiating them through additional history taking, exams, and diagnostic tests, (4) pausing to reflect [my emphasis], and (5) embarking on a plan (while acknowledging uncertainty) and ensuring there is a pathway for follow-up.2
To help avoid diagnostic errors, Dr. Ely developed and uses a set of checklists that cover the differential diagnosis for 72 presenting complaints/conditions, including syncope, back pain, insomnia, and headache.2 When you are faced with diagnostic uncertainty, it takes just a few minutes to run through the checklist for the patient’s presenting complaint.
None of us like being wrong, especially about a patient’s diagnosis. To help you avoid diagnostic errors for 4 difficult diagnoses, read and study the article in this issue of JFP by Rosen and colleagues.1 They discuss misdiagnosis of polymyalgia rheumatica, fibromyalgia, ovarian cancer, and Lewy body dementia to illustrate how we can go astray if we do not take care to pause and think through things carefully. They point out that, for quick and mostly accurate diagnoses, pattern recognition or type 1 thinking serves us well in a busy office practice. However, we must frequently pause and reflect, using type 2 thinking—especially when the puzzle pieces don’t quite fit together.
I still recall vividly a diagnostic error I made many years ago. One of my patients, whom I had diagnosed and was treating for hyperlipidemia, returned for follow-up while I was on vacation. My partner conducted the follow-up visit. To my chagrin, he noticed her puffy face and weight gain and ordered thyroid studies. Sure enough, my patient was severely hypothyroid, and her lipid levels normalized with thyroid replacement therapy.
A happier tale for me was making the correct diagnosis for a woman with chronic cough. She had been evaluated by multiple specialists during the prior year and treated with a nasal steroid for allergies, a proton pump inhibitor for reflux, and a steroid inhaler for possible asthma. None of these relieved her cough. After reviewing her medication list and noting that it included amitriptyline, which has anticholinergic adverse effects, I recommended she stop taking that medication and the cough resolved.
John Ely, MD, MPH, a family physician who has spent his academic career investigating causes of and solutions to diagnostic errors, has outlined important steps we can take. These include: (1) obtaining your own complete medical history, (2) performing a “focused and purposeful” physical exam, (3) generating initial hypotheses and differentiating them through additional history taking, exams, and diagnostic tests, (4) pausing to reflect [my emphasis], and (5) embarking on a plan (while acknowledging uncertainty) and ensuring there is a pathway for follow-up.2
To help avoid diagnostic errors, Dr. Ely developed and uses a set of checklists that cover the differential diagnosis for 72 presenting complaints/conditions, including syncope, back pain, insomnia, and headache.2 When you are faced with diagnostic uncertainty, it takes just a few minutes to run through the checklist for the patient’s presenting complaint.
1. Rosen PD, Klenzak S, Baptista S. Diagnostic challenges in primary care: identifying and avoiding cognitive bias. J Fam Pract. 2022;71:124-132.
2. Ely JW, Graber ML, Croskerry P. Checklists to reduce diagnostic errors. Acad Med. 2011;86:307-313. doi: 10.1097/ACM.0b013e31820824cd
1. Rosen PD, Klenzak S, Baptista S. Diagnostic challenges in primary care: identifying and avoiding cognitive bias. J Fam Pract. 2022;71:124-132.
2. Ely JW, Graber ML, Croskerry P. Checklists to reduce diagnostic errors. Acad Med. 2011;86:307-313. doi: 10.1097/ACM.0b013e31820824cd