User login
Medical assistants identify strategies and barriers to clinic efficiency
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
Continue to: We developed and piloted...
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Continue to: Efficiency and competing priorities
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
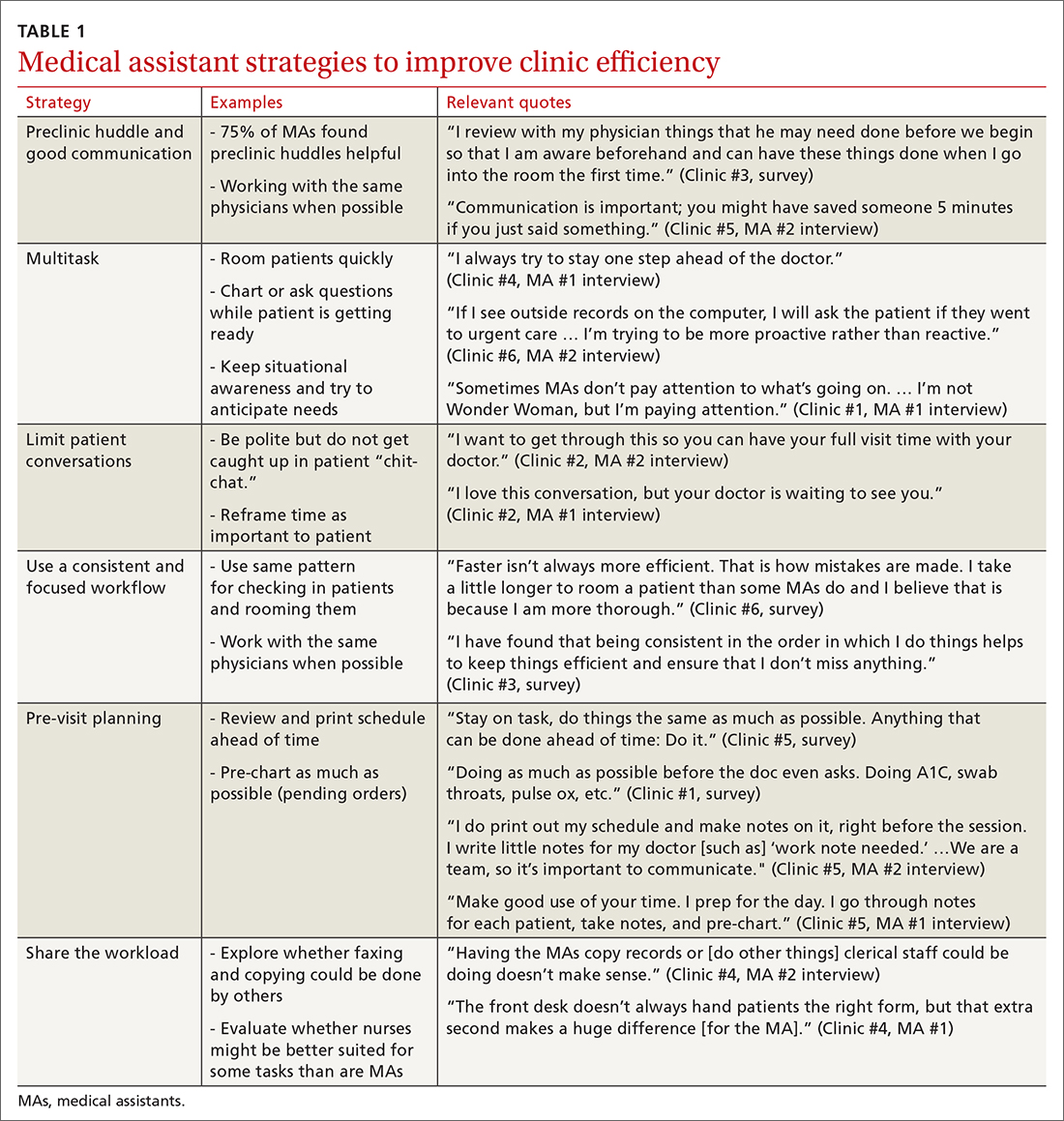
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
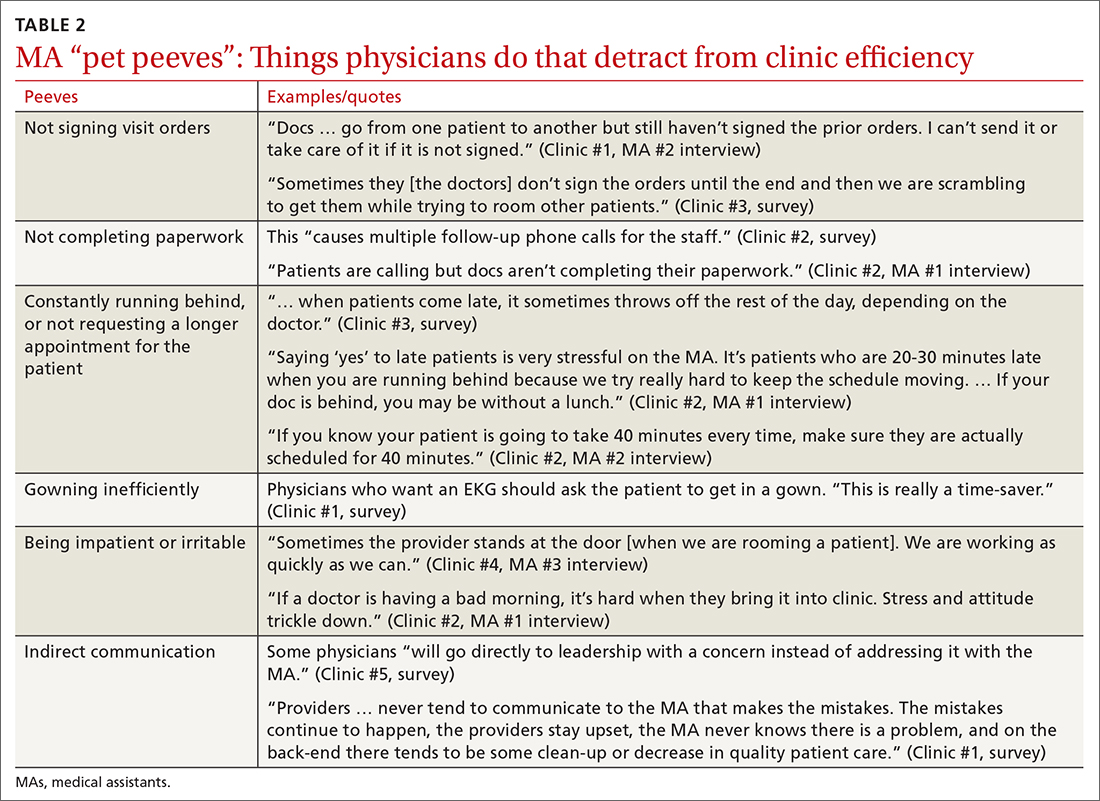
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“ Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“ [Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
Continue to: A minority of staff...
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Continue to: Almost all respondents...
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male-dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries.19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs.8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE
Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
1. Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
2. Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
3. Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/articles/medical-assisting-trends/
4. Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
5. Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medical-assisting-history/
6. Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
7. Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
8. Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
9. Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
10. STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf
11. Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
12. Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
13. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
14. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
15. Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
16. US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/oes/current/oes319092.htm
17. Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
18. Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
19. US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/healthcare/medical-assistants.htm
20. Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
21. Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
22. Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11:187-201.
23. Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
24. O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
Continue to: We developed and piloted...
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Continue to: Efficiency and competing priorities
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“ Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“ [Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
Continue to: A minority of staff...
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Continue to: Almost all respondents...
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male-dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries.19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs.8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE
Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
Continue to: We developed and piloted...
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Continue to: Efficiency and competing priorities
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.

Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)

Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“ Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“ [Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
Continue to: A minority of staff...
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Continue to: Almost all respondents...
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male-dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries.19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs.8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE
Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
1. Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
2. Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
3. Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/articles/medical-assisting-trends/
4. Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
5. Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medical-assisting-history/
6. Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
7. Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
8. Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
9. Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
10. STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf
11. Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
12. Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
13. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
14. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
15. Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
16. US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/oes/current/oes319092.htm
17. Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
18. Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
19. US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/healthcare/medical-assistants.htm
20. Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
21. Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
22. Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11:187-201.
23. Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
24. O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
1. Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
2. Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
3. Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/articles/medical-assisting-trends/
4. Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
5. Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medical-assisting-history/
6. Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
7. Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
8. Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
9. Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
10. STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf
11. Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
12. Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
13. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
14. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
15. Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
16. US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/oes/current/oes319092.htm
17. Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
18. Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
19. US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/healthcare/medical-assistants.htm
20. Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
21. Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
22. Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11:187-201.
23. Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
24. O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
Diagnostic challenges in primary care: Identifying and avoiding cognitive bias
Medical errors in all settings contributed to as many as 250,000 deaths per year in the United States between 2000 and 2008, according to a 2016 study.1 Diagnostic error, in particular, remains a leading cause of morbidity and mortality in the United States and worldwide. In 2017, 12 million patients (roughly 5% of all US adults) who sought outpatient care experienced missed, delayed, or incorrect diagnosis at least once.2
In his classic work, How Doctors Think, Jerome Groopman, MD, explored the diagnostic process with a focus on the role of cognitive bias in clinical decision-making. Groopman examined how physicians can become sidetracked in their thinking and “blinded” to potential alternative diagnoses.3 Medical error is not necessarily because of a deficiency in medical knowledge; rather, physicians become susceptible to medical error when defective and faulty reasoning distort their diagnostic ability.4
Cognitive bias in the diagnostic process has been extensively studied, and a full review is beyond the scope of this article.5 However, here we will examine pathways leading to diagnostic errors in the primary care setting, specifically the role of cognitive bias in the work-up of polymyalgia rheumatica (PMR), ovarian cancer (OC), Lewy body dementia (LBD), and fibromyalgia (FM). As these 4 disease states are seen with low-to-moderate frequency in primary care, cognitive bias can complicate accurate diagnosis. But first, a word about how to understand clinical reasoning.
There are 2 types of reasoning (and 1 is more prone to error)
Physician clinical reasoning can be divided into 2 different cognitive approaches.
Type 1 reasoning employs intuition and heuristics; this type is automatic, reflexive, and quick.5 While the use of mental shortcuts in type 1 increases the speed with which decisions are made, it also makes this form of reasoning more prone to error.
Type 2 reasoning requires conscious effort. It is goal directed and rigorous and therefore slower than type 1 reasoning. Extrapolated to the clinical context, clinicians transition from type 2 to type 1 reasoning as they gain experience and training throughout their careers and develop their own conscious and subconscious heuristics. Deviations from accurate decision-making occur in a systematic manner due to cognitive biases and result in medical error.6table 17 lists common types of cognitive bias.
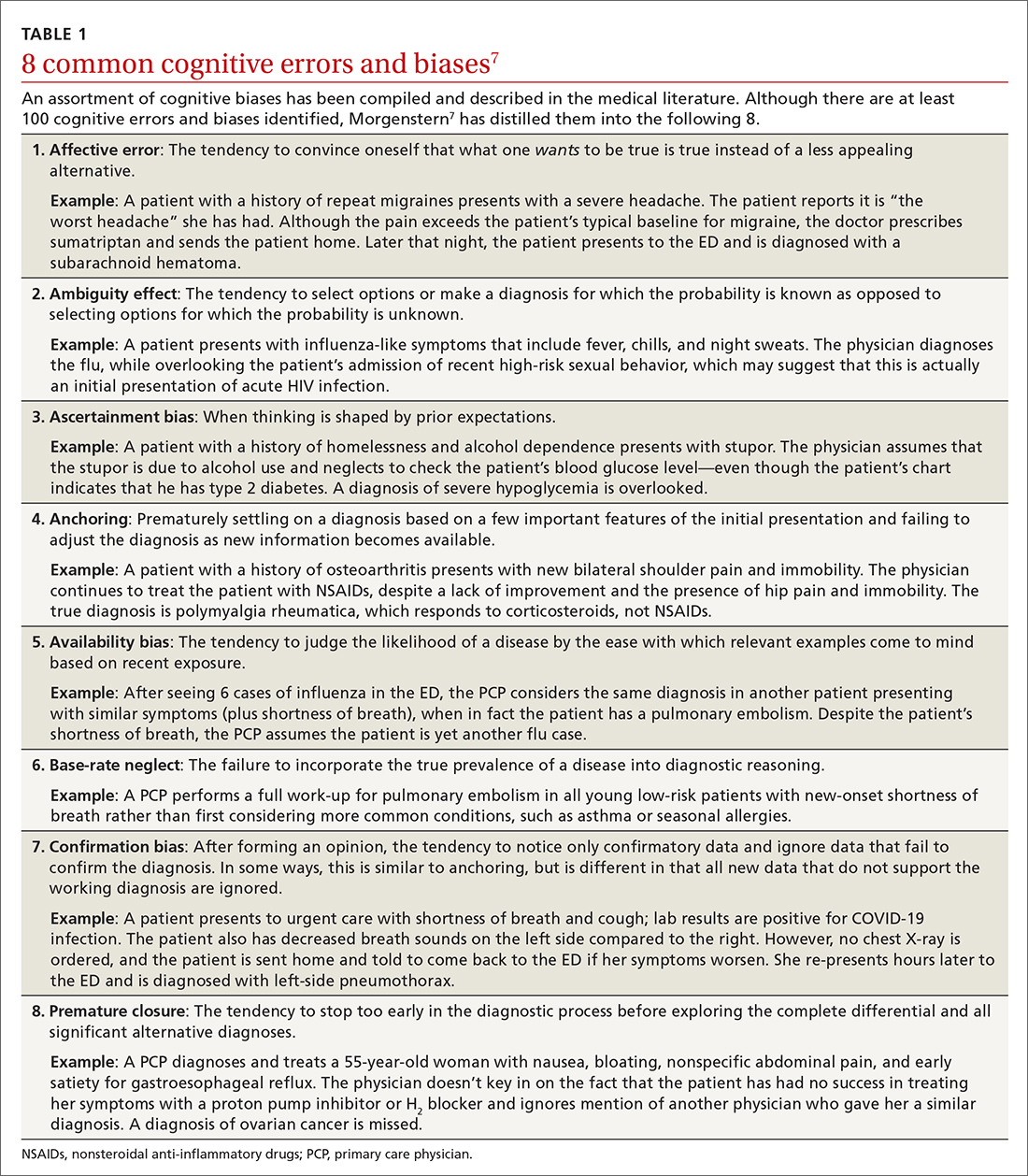
An important question to ask. Physicians tend to fall into a pattern of quick, type 1 reasoning. However, it’s important to strive to maintain a broad differential diagnosis and avoid premature closure of the diagnostic process. It’s critical that we consider alternative diagnoses (ie, consciously move from type 1 to type 2 thinking) and continue to ask ourselves, “What else?” while working through differential diagnoses. This can be a powerful debiasing technique.
Continue to: The discussion...
The discussion of the following 4 disease states demonstrates how cognitive bias can lead to diagnostic error.
The patient is barely able to ambulate and appears to be in considerable pain. She is relying heavily on her walker and is assisted by her granddaughter. The primary care physician (PCP) obtains a detailed history that includes chronic shoulder and hip pain. Given that the patient has not responded to NSAID treatment over the previous 6 months, the PCP takes a moment to reconsider the diagnosis of OA and considers other options.
In light of the high prevalence of PMR in older women, the physician pursues a more specific physical examination tailored to ferret out PMR. He had learned this diagnostic shortcut as a resident, remembered it, and adeptly applied it whenever circumstances warranted. He asks the patient to raise her arms above her head (goalpost sign). She is unable to perform this task and experiences severe bilateral shoulder pain on trial. The PCP then places the patient on the examining table and attempts to assist her in rolling toward him. The patient is also unable to perform this maneuver and experiences significant bilateral hip pain on trial.
Based primarily on the patient’s history and physical exam findings, the PCP makes a presumptive diagnosis of PMR vs OA vs combined PMR with OA, orders an erythrocyte sedimentation rate (ESR) and basic rheumatologic
PMR can be mistaken for OA
PMR is the most common inflammatory rheumatic disease in older patients.8 It is a debilitating illness with simple, effective treatment but has devastating consequences if missed or left untreated.9 PMR typically manifests in patients older than age 50, with a peak incidence at 80 years of age. It is also far more common in women.10
Approximately 80% of patients with PMR initially present to their PCP, often posing a diagnostic challenge to many clinicians.11 Due to overlap in symptoms, the condition is often misdiagnosed as OA, a more common condition seen by PCPs. Also, there are no specific diagnostic tests for PMR. An elevated ESR can help confirm the diagnosis, but one-third of patients with PMR have a normal ESR.12 Therefore, the diagnostic conundrum the physician faces is OA vs rheumatoid arthritis (RA), PMR, or another condition.
Continue to: The consequences...
The consequences of a missed and delayed PMR diagnosis range from seriously impaired quality of life to significantly increased risk of vascular events (eg, blindness, stroke) due to temporal arteritis.13 Early diagnosis is even more critical as the risk of a vascular event and death is highest during initial phases of the disease course.14
FPs often miss this Dx. A timely diagnosis relies almost exclusively on an accurate, thorough history and physical exam. However, PCPs often struggle to correctly diagnose PMR. According to a study by Bahlas and colleagues,15 the accuracy rate for correctly diagnosing PMR was 24% among a cohort of family physicians.
The differential diagnosis for PMR is broad and includes seronegative spondyloarthropathies, malignancy, Lyme disease, hypothyroidism, and both RA and OA.16
PCPs are extremely adept at correctly diagnosing RA, but not PMR. A study by Blaauw and colleagues17 comparing PCPs and rheumatologists found PCPs correctly identified 92% of RA cases but only 55% of PMR cases. When rheumatologists reviewed these same cases, they correctly identified PMR and RA almost 100% of the time.17 The difference in diagnostic accuracy between rheumatologists and PCPs suggests limited experience and gaps in fund of knowledge.
Making the diagnosis. The diagnosis of PMR is often made on empiric response to corticosteroid treatment, but doing so based solely on a patient’s response is controversial.18 There are rare instances in which patients with PMR fail to respond to treatment. On the other hand, some inflammatory conditions that mimic or share symptoms with PMR also respond to corticosteroids, potentially resulting in erroneous confirmation bias.
Some classification criteria use rapid response to low-dose prednisone/prednisolone (≤ 20 mg) to confirm the diagnosis,19 while other more recent guidelines no longer include this approach.20 If PMR continues to be suspected after a trial of steroids is unsuccessful, the PCP can try another course of higher dose steroids or consult with Rheumatology.
Continue to: A full history...
A full history and physical exam revealed a myriad of gastrointestinal (GI) complaints, such as diarrhea. But the PCP recalled a recent roundtable discussion on debiasing techniques specifically related to gynecologic disorders, including OC. Therefore, he decided to include OC in the differential diagnosis—something he would not routinely have done given the preponderance of GI symptoms. Despite the patient’s reluctance and time constraints, the PCP ordered a transvaginal ultrasound. Findings from the ultrasound study revealed stage II OC, which carries a good prognosis. The patient is currently undergoing treatment and was last reported as doing well.
Early signs of ovarian cancer can be chalked up to a “GI issue”
OC is the second most common gynecologic cancer21 and the fifth leading cause of cancer-related death22 in US women. Compared to other cancers, the prognosis for localized early-stage OC is surprisingly good, with a 5-year survival rate approaching 93%.23 However, most disease is detected in later stages, and the 5-year survival rate drops to a low of 29%.24
There remains no established screening protocol for OC. Fewer than a quarter of all cases are diagnosed in stage I, and detection of OC relies heavily on the physician’s ability to decipher vague symptomatology that overlaps with other, more common maladies. This poses an obvious diagnostic challenge and, not surprisingly, a high level of susceptibility to cognitive bias.
More than 90% of patients with OC present with some combination of the following symptoms prior to diagnosis: abdominal (77%), GI (70%), pain (58%), constitutional (50%), urinary (34%), and pelvic (26%).25 The 3 most common isolated symptoms in patients with OC are abdominal bloating, decrease in appetite, and frank abdominal pain.26 Patients with biopsy-confirmed OC experience these symptoms an average of 6 months prior to actual diagnosis.27
Knowledge gaps play a role. Studies assessing the ability of health care providers to identify presenting symptoms of OC reveal specific knowledge gaps. For instance, in a survey by Gajjar and colleagues,28 most PCPs correctly identified bloating as a key symptom of OC; however, they weren’t as good at identifying less common symptoms, such as inability to finish a meal and early satiety. Moreover, survey participants misinterpreted or missed GI symptoms as an important manifestation of early OC disease.28 These specific knowledge gaps combine with physician errors in thinking, further obscuring and extending the diagnostic process. The point prevalence for OC is relatively low, and many PCPs only encounter a few cases during their entire career.29 This low pre-test probability may also fuel the delay in diagnosis.
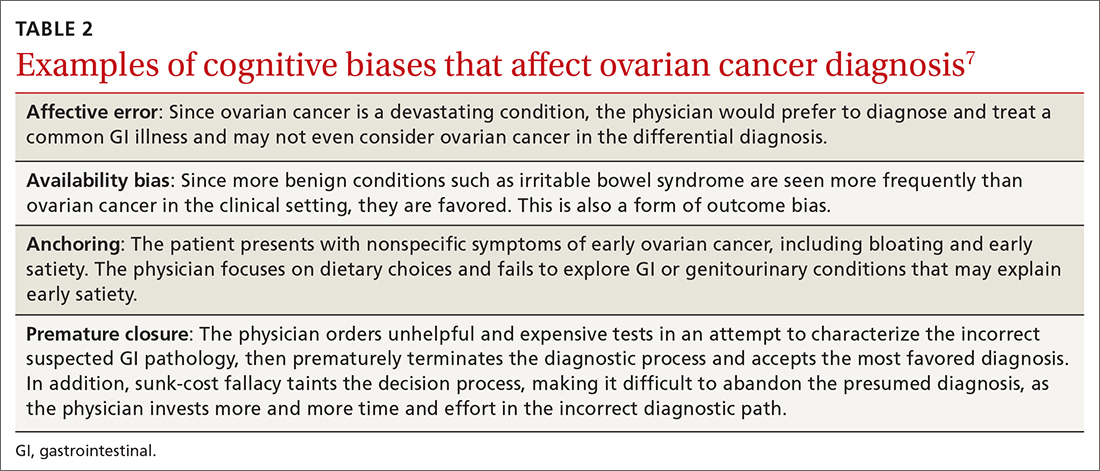
Watch for these forms of bias. Since nonspecific symptoms of early-stage OC resemble those of other more benign conditions, a form of anchoring error known as multiple alternatives bias can arise. In this scenario, clinicians investigate only 1 potential plausible diagnosis and remain focused on that single, often faulty, conclusion. This persists despite other equally plausible alternatives that arise as the investigation proceeds.28
Affective error may also play a role in missed or delayed diagnosis. For example, a physician would prefer to diagnose and treat a common GI illness than consider OC. Another distortion involves outcome bias wherein the physician gives more significance to benign conditions such as irritable bowel syndrome because they have a more favorable outcome and clear treatment path. Physicians also favor these benign conditions because they encounter them more frequently than OC in the clinic setting. (This is known as availability bias.) Outcome bias and multiple alternatives bias can result in noninvestigation of symptoms and inefficient or improper management, leading to a delay in arriving at the correct diagnosis or anchoring on a plausible but incorrect diagnosis.
Continue to: An incorrect initial diagnostic...
An incorrect initial diagnostic path often triggers a cascade of subsequent errors. The physician orders additional unhelpful and expensive tests in an effort to characterize the suspected GI pathology. This then leads the physician to prematurely terminate the work-up and accept the most favored diagnosis. Lastly, sunk-cost fallacy comes into play: The physician has “invested” time and energy investigating a particular diagnosis and rather than abandon the presumed diagnosis, continues to put more time and effort in going down an incorrect diagnostic path.
A series of failures. These biases and miscues have been observed in several studies. For example, a survey of 1725 women by Goff and colleagues30 sought to identify factors related to delayed OC diagnosis. The authors found that the following factors were significantly associated with a delayed diagnosis: omission of a pelvic exam at initial presentation, a separate exploration of a multitude of collateral symptoms, a failure to order ultrasound/computed tomography/CA-125 test, and a failure to consider age as a factor (especially if the patient was outside the norm).
Responses from the survey also revealed that physicians initially ordered work-ups related to GI etiology and only later considered a pelvic work-up. This suggests that well-known presenting signs and symptoms or a constellation of typical and atypical symptoms of OC often failed to trigger physician recognition. Understandably, patients presenting with menorrhagia or gynecologic complaints are more likely to have OC detected at an earlier stage than patients who present with GI or abdominal signs alone.31 table 27 summarizes some of the cognitive biases seen in the diagnostic path of OC.
While in the hospital, he becomes acutely upset by the hallucinations and is given haloperidol and lorazepam by house staff. In the morning, the patient exhibits severe signs of Parkinson disease that include rigidity and masked facies.
Given the patient’s poor response to haloperidol and continued confusion, the team consulted Neurology and Psychiatry. Gathering a more detailed history from the patient and family, the patient is given a diagnosis of classic LBD. The antipsychotic medications are stopped. The patient and his family receive education about LBD treatment and management, and the patient is discharged to outpatient care.
Psychiatric symptoms can be an early “misdirect” in cases of Lewy body disease
LBD, the second leading neurodegenerative dementia after Alzheimer disease (AD), affects 1.5 million Americans,32 representing about 10% of all dementia cases. LBD and AD overlap in 25% of dementia cases.33 In patients older than 85 years, the prevalence jumps to 5% of the general population and 22% of all cases of dementia.33 Despite its prevalence, a recent study showed that only 6% of PCPs correctly identified LBD as the primary diagnosis when presented with typical case examples.32
Continue to: 3 stages of presentation
3 stages of presentation. Unlike other forms of dementia, LBD typically presents first with psychiatric symptoms, then with cognitive impairment, and last with parkinsonian symptoms. Additionally, rapid eye movement sleep behavior disorder and often subtle elements of nonmemory cognitive impairment distinguish LBD from both AD and vascular dementia.32 The primary cognitive deficit in LBD is not in memory but in attention, executive functioning, and visuospatial ability.34 Only in the later stages of the disease do patients exhibit gradual and progressive memory loss.
Mistaken for many things. When evaluating patients exhibiting signs of dementia, it’s important to include LBD in the differential, with increased suspicion for patients experiencing episodes of psychosis or delirium. The uniqueness of LBD lies in its psychotic symptomatology, particularly during earlier stages of the disease. This feature helps distinguish LBD from both AD and vascular dementia. As seen in the case, LBD can also be confused with acute delirium.
Older adult patients presenting to the ED or clinic with visual hallucinations, delirium, and mental confusion may receive a false diagnosis of schizophrenia, medication- or substance-induced psychosis, Parkinson disease, or delirium of unknown etiology.35 Unfortunately, LBD is often overlooked and not considered in the differential diagnosis. Due to underrecognition, patients may receive treatment with typical antipsychotics. The addition of a neuroleptic to help control the psychotic symptoms causes patients with LBD to develop severe extrapyramidal symptoms and worsening mental status,36 leading to severe parkinsonian signs, which further muddies the diagnostic process. In addition, treatment for suspected Parkinson disease, including carbidopa-levodopa, has no benefit for patients with LBD and may increase psychotic symptoms.37
First-line treatment for LBD includes psychoeducation for the patient and family, cholinesterase inhibitors (eg, rivastigmine), and avoidance of high-potency antipsychotics, such as haloperidol. Although persistent hallucinations and psychosis remain difficult to treat in LBD, low-dose quetiapine is 1 option. Incorrectly diagnosing and prescribing treatment for another condition exacerbates symptoms in this patient population.
The patient has been experiencing chronic pain for the past few years after a motor vehicle accident. She has seen a physiatrist and another provider, both of whom found no “objective” causes of her chronic pain. They started the patient on sertraline for depression and an analgesic, both of which were ineffective.
The patient likes to exercise at a gym twice a week by doing light cardio (treadmill) exercise and light weightlifting. Lately, however, she has been unable to exercise due to the pain. At this visit, she mentions having low energy, poor sleep, frequent fatigue, and generalized soreness and pain in multiple areas of her body. The PCP recognizes the patient’s presenting symptoms as significant for FM and starts her on pregabalin and hydrotherapy, with positive results.
Continue to: Fibromyalgia skepticism may lead to a Dx of depression
Fibromyalgia skepticism may lead to a Dx of depression
FM, the second most common disorder seen in rheumatologic practice after OA, is estimated to affect approximately 1 in 20 patients (approximately 5 million Americans) in the primary care setting.38,39 The condition has a high female-to-male preponderance (3.4% vs 0.5%).40 While the primary symptom of FM is chronic pain, patients commonly present with fatigue and sleep disturbance.41 Comorbid conditions include headaches, irritable bowel syndrome, and mood disturbances (most commonly anxiety and depression).
Several studies have explored reasons for the misdiagnosis and underdiagnosis of FM. One important factor is ongoing skepticism among some physicians and the public, in general, as to whether FM is a real disease. This issue was addressed by a study by White and colleagues,42 who estimated and compared the point prevalence of FM and related disorders in Amish vs non-Amish adults. The authors hypothesized that if litigation and/or compensation availability have a major impact on FM prevalence, then there would be a near zero prevalence of FM in the Amish community. And yet, researchers found an overall age- and sex-adjusted FM prevalence of 7.3% (95% CI; 5.3%-9.7%); this was both statistically greater than zero (P < .0001) and greater than 2 control populations of non-Amish adults (both P < .05).
Many physicians consider FM fundamentally an emotional disturbance, and the high preponderance of FM in female patients may contribute to this misconception as reports of pain and emotional distress by women are often dismissed as hysteria.43 Physicians often explore the emotional aspects of FM, incorrectly diagnosing patients with depression and subsequently treating them with a psychotropic drug.39 Alternatively, they may focus on the musculoskeletal presentations of FM and prescribe analgesics or physical therapy, both of which do little to alleviate FM.
To make the correct diagnosis of FM, the American College of Rheumatology created a specific set of criteria in 1990, which was updated in 2010.44 For a diagnosis of FM, a patient must have at least a 3-month history of bilateral pain above and below the waist and along the axial skeletal spine. Although not included in the updated 2010 criteria, many clinicians continue to check for tender points, following the 1990 criteria requiring the presence of 11 of 18 points to make the diagnosis.
At least 3 cognitive biases relating to FM apply: anchoring, availability, and fundamental attribution error (see table 3).7 Anchoring occurs when the PCP settles on a psychiatric diagnosis of exaggerated pain syndrome, muscle overuse, or OA and fails to explore alternative etiology. Availability bias may obscure the true diagnosis of FM. Since PCPs see many patients with RA or OA, they may overlook or dismiss the possibility of FM. Attribution error happens when physicians dismiss the complaints of patients with FM as merely due to psychological distress, hysteria, or acting out.43
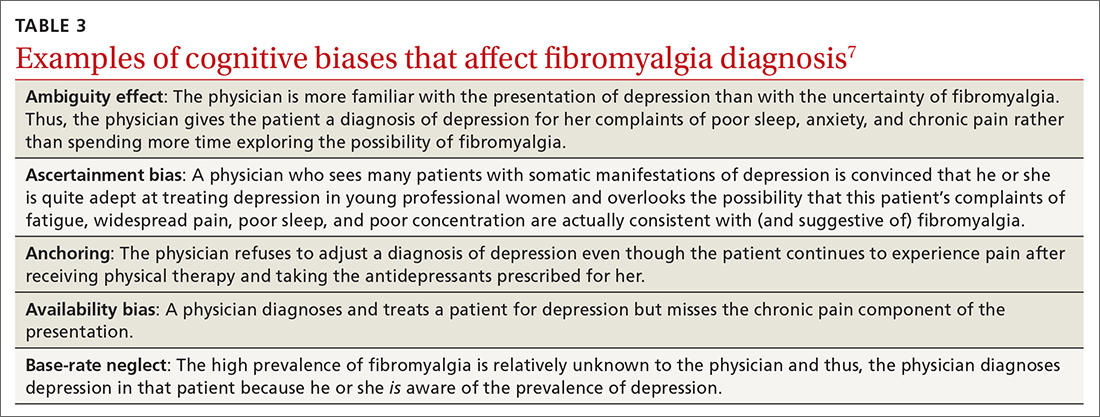
Patients with FM, who are often otherwise healthy, often present multiple times to the same PCP with a chief complaint of chronic pain. These repeat presentations can result in compassion fatigue and impact care. As Aloush and colleagues40 noted in their study, “FM patients were perceived as more difficult than RA patients, with a high level of concern and emotional response. A high proportion of physicians were reluctant to accept them because they feel emotional/psychological difficulties meeting and coping with these patients.”In response, patients with undiagnosed FM or inadequately treated FM may visit other PCPs, which may or may not result in a correct diagnosis and treatment.
We can do better
Primary care physicians face the daunting task of diagnosing and treating a wide range of common conditions while also trying to recognize less-common conditions with atypical presentations—all during a busy clinic workday. Nonetheless, we should strive to overcome internal (eg, cognitive bias and fund-of-knowledge deficits) and external (eg, time constraints, limited resources) pressures to improve diagnostic accuracy and care.
Each of the 4 disease states we’ve discussed have high rates of missed and/or delayed diagnosis. Each presents a unique set of confounders: PMR with its overlapping symptoms of many other rheumatologic diseases; OC with its often vague and misleading GI symptomatology; LBD with overlapping features of AD and Parkinson disease; and FM with skepticism. As gatekeepers to health care, it falls on PCPs to sort out these diagnostic dilemmas to avoid medical errors. Fundamental knowledge of each disease, its unique pathophysiology and symptoms, and varying presentations can be learned, internalized, and subsequently put into clinical practice to improve patient outcomes.
CORRESPONDENCE
Paul D. Rosen MD, Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Avenue, Brooklyn, New York 11201; [email protected]
1. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139
2. Van Such M, Lohr R, Beckman T, et al. Extent of diagnostic agreement among medical referrals. J Eval Clin Pract. 2017;23:870-874. doi: 10.1111/jep.12747
3. Groopman JE. How Doctors Think. Houghton Mifflin; 2007.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131. doi: 10.1126/science.185.4157.1124
5. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: Cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30. doi: 10.1097/ACM.0000000000001421
6. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. doi: 10.1097/00001888-200308000-00003
7. Morgenstern J. Cognitive errors in medicine: The common errors. First10EM blog. September 15, 2015. Updated September 22, 2019. Accessed February 8, 2022. https://first10em.com/cognitive-errors/
8. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22:40. doi: 10.1007/s11926-020-00919-2
9. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176
10. Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694-1697.
11. Barraclough K, Liddell WG, du Toit J, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract. 2008;25:328-33. doi: 10.1093/fampra/cmn044
12. Manzo C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: a case report. Diseases. 2018;6:84. doi: 10.3390/diseases6040084
13. Crowson CS, Matteson EL. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum. 2017;47:253-256. doi: 10.1016/j.semarthrit.2017.04.001
14. Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549-550. doi: 10.1136/bmj.299.6698.549
15. Bahlas S, Ramos-Remus C, Davis P. Utilisation and costs of investigations, and accuracy of diagnosis of polymyalgia rheumatica by family physicians. Clin Rheumatol. 2000;19:278-280. doi: 10.1007/s100670070045
16. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997;157:162-168.
17. Blaauw AA, Schuwirth LW, van der Vleuten CP, et al. Assessing clinical competence: recognition of case descriptions of rheumatic diseases by general practitioners. Br J Rheumatol. 1995;34:375-379. doi:10.1093/rheumatology/34.4.375
18. Mager DR. Polymylagia rheumatica: common disease, elusive diagnosis. Home Healthc Now. 2015;33:132-138. doi:10.1097/NHH.0000000000000199
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 381;63-72. doi: 10.1016/S0140-6736(12)60680-1. Published correction appears in Lancet. 20135;381:28.
20. Liew DF, Owen CE, Buchanan RR. Prescribing for polymyalgia rheumatica. Aust Prescr. 2018;41:14-19. doi: 10.18773/austprescr.2018.001
21. Ovarian cancer statistics. Centers for Disease Control and Prevention. Reviewed June 8, 2021. Accessed February 22, 2022. www.cdc.gov/cancer/ovarian/statistics/index.htm
22. Key statistics for ovarian cancer. American Cancer Society. Revised January 12, 2022. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
23. Survival rates for ovarian cancer. American Cancer Society. Revised January 25, 2021. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html
24. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9-32. doi: 10.20892/j.issn.2095-3941.2016.0084
25. Goff BA, Mandel LS, Melancon CH, et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712. doi: 10.1001/jama.291.22.2705
26. Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212-217. doi: 10.1016/s0029-7844(01)01457-0
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients: Cancer. Br J Cancer. 2005;92:1959-1970. doi: 10.1038/sj.bjc.6602587
28. Gajjar K, Ogden G, Mujahid MI, et al. Symptoms and risk factors of ovarian cancer: a survey in primary care. Obstet Gynecol. 2012:754197. doi: 10.5402/2012/754197
29. Austoker J. Diagnosis of ovarian cancer in primary care. BMJ. 2009;339:b3286. doi: 10.1136/bmj.b3286
30. Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis: results of a national ovarian cancer survey. Cancer. 2000;89:2068-2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z
31. Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727-2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8
32. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388-392. doi: 10.1016/j.parkreldis.2010.03.007
33. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6:333-341. doi: 10.31887/DCNS.2004.6.3/imckeith
34. Mrak RE, Griffin WS. Dementia with Lewy bodies: definitions, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:619-625.
35. Khotianov N, Singh R, Singh S. Lewy body dementia: case report and discussion. J Am Board Fam Pract. 2002;15:50-54.
36. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:731-742. doi: 10.1176/appi.ajp.2015.14121582
37. Velayudhan L, Ffytche D, Ballard C, et al. New therapeutic strategies of Lewy body dementias. Curr Neurol Neurosci Rep. 2017;17:68. doi: 10.1007/s11910-017-0778-2
38. Arnold LM, Clauw DJ, McCarberg BH; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457-464. doi: 10.4065/mcp.2010.0738
39. Arnold LM, Gebke KB, Choy EHS. Fibromyalgia: management strategies for primary care providers. Int J Clin Pract. 2016;70:99-112. doi: 10.1111/ijcp.12757
40. Aloush V, Niv D, Ablin JN, et al. Good pain, bad pain: illness perception and physician attitudes towards rheumatoid arthritis and fibromyalgia patients. Clin Exp Rheumatol. 2021;39(suppl 130):54-60.
41. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786-792. doi: 10.1002/acr.21896
42. White KP, Thompson J. Fibromyalgia syndrome in an Amish community: a controlled study to determine disease and symptom prevalence. J Rheumatol. 2003;30:1835-1840.
43. Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16:10.4088/PCC.14m01664. doi: 10.4088/PCC.14m01664
44. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. doi:10.1002/acr.20140
Medical errors in all settings contributed to as many as 250,000 deaths per year in the United States between 2000 and 2008, according to a 2016 study.1 Diagnostic error, in particular, remains a leading cause of morbidity and mortality in the United States and worldwide. In 2017, 12 million patients (roughly 5% of all US adults) who sought outpatient care experienced missed, delayed, or incorrect diagnosis at least once.2
In his classic work, How Doctors Think, Jerome Groopman, MD, explored the diagnostic process with a focus on the role of cognitive bias in clinical decision-making. Groopman examined how physicians can become sidetracked in their thinking and “blinded” to potential alternative diagnoses.3 Medical error is not necessarily because of a deficiency in medical knowledge; rather, physicians become susceptible to medical error when defective and faulty reasoning distort their diagnostic ability.4
Cognitive bias in the diagnostic process has been extensively studied, and a full review is beyond the scope of this article.5 However, here we will examine pathways leading to diagnostic errors in the primary care setting, specifically the role of cognitive bias in the work-up of polymyalgia rheumatica (PMR), ovarian cancer (OC), Lewy body dementia (LBD), and fibromyalgia (FM). As these 4 disease states are seen with low-to-moderate frequency in primary care, cognitive bias can complicate accurate diagnosis. But first, a word about how to understand clinical reasoning.
There are 2 types of reasoning (and 1 is more prone to error)
Physician clinical reasoning can be divided into 2 different cognitive approaches.
Type 1 reasoning employs intuition and heuristics; this type is automatic, reflexive, and quick.5 While the use of mental shortcuts in type 1 increases the speed with which decisions are made, it also makes this form of reasoning more prone to error.
Type 2 reasoning requires conscious effort. It is goal directed and rigorous and therefore slower than type 1 reasoning. Extrapolated to the clinical context, clinicians transition from type 2 to type 1 reasoning as they gain experience and training throughout their careers and develop their own conscious and subconscious heuristics. Deviations from accurate decision-making occur in a systematic manner due to cognitive biases and result in medical error.6table 17 lists common types of cognitive bias.

An important question to ask. Physicians tend to fall into a pattern of quick, type 1 reasoning. However, it’s important to strive to maintain a broad differential diagnosis and avoid premature closure of the diagnostic process. It’s critical that we consider alternative diagnoses (ie, consciously move from type 1 to type 2 thinking) and continue to ask ourselves, “What else?” while working through differential diagnoses. This can be a powerful debiasing technique.
Continue to: The discussion...
The discussion of the following 4 disease states demonstrates how cognitive bias can lead to diagnostic error.
The patient is barely able to ambulate and appears to be in considerable pain. She is relying heavily on her walker and is assisted by her granddaughter. The primary care physician (PCP) obtains a detailed history that includes chronic shoulder and hip pain. Given that the patient has not responded to NSAID treatment over the previous 6 months, the PCP takes a moment to reconsider the diagnosis of OA and considers other options.
In light of the high prevalence of PMR in older women, the physician pursues a more specific physical examination tailored to ferret out PMR. He had learned this diagnostic shortcut as a resident, remembered it, and adeptly applied it whenever circumstances warranted. He asks the patient to raise her arms above her head (goalpost sign). She is unable to perform this task and experiences severe bilateral shoulder pain on trial. The PCP then places the patient on the examining table and attempts to assist her in rolling toward him. The patient is also unable to perform this maneuver and experiences significant bilateral hip pain on trial.
Based primarily on the patient’s history and physical exam findings, the PCP makes a presumptive diagnosis of PMR vs OA vs combined PMR with OA, orders an erythrocyte sedimentation rate (ESR) and basic rheumatologic
PMR can be mistaken for OA
PMR is the most common inflammatory rheumatic disease in older patients.8 It is a debilitating illness with simple, effective treatment but has devastating consequences if missed or left untreated.9 PMR typically manifests in patients older than age 50, with a peak incidence at 80 years of age. It is also far more common in women.10
Approximately 80% of patients with PMR initially present to their PCP, often posing a diagnostic challenge to many clinicians.11 Due to overlap in symptoms, the condition is often misdiagnosed as OA, a more common condition seen by PCPs. Also, there are no specific diagnostic tests for PMR. An elevated ESR can help confirm the diagnosis, but one-third of patients with PMR have a normal ESR.12 Therefore, the diagnostic conundrum the physician faces is OA vs rheumatoid arthritis (RA), PMR, or another condition.
Continue to: The consequences...
The consequences of a missed and delayed PMR diagnosis range from seriously impaired quality of life to significantly increased risk of vascular events (eg, blindness, stroke) due to temporal arteritis.13 Early diagnosis is even more critical as the risk of a vascular event and death is highest during initial phases of the disease course.14
FPs often miss this Dx. A timely diagnosis relies almost exclusively on an accurate, thorough history and physical exam. However, PCPs often struggle to correctly diagnose PMR. According to a study by Bahlas and colleagues,15 the accuracy rate for correctly diagnosing PMR was 24% among a cohort of family physicians.
The differential diagnosis for PMR is broad and includes seronegative spondyloarthropathies, malignancy, Lyme disease, hypothyroidism, and both RA and OA.16
PCPs are extremely adept at correctly diagnosing RA, but not PMR. A study by Blaauw and colleagues17 comparing PCPs and rheumatologists found PCPs correctly identified 92% of RA cases but only 55% of PMR cases. When rheumatologists reviewed these same cases, they correctly identified PMR and RA almost 100% of the time.17 The difference in diagnostic accuracy between rheumatologists and PCPs suggests limited experience and gaps in fund of knowledge.
Making the diagnosis. The diagnosis of PMR is often made on empiric response to corticosteroid treatment, but doing so based solely on a patient’s response is controversial.18 There are rare instances in which patients with PMR fail to respond to treatment. On the other hand, some inflammatory conditions that mimic or share symptoms with PMR also respond to corticosteroids, potentially resulting in erroneous confirmation bias.
Some classification criteria use rapid response to low-dose prednisone/prednisolone (≤ 20 mg) to confirm the diagnosis,19 while other more recent guidelines no longer include this approach.20 If PMR continues to be suspected after a trial of steroids is unsuccessful, the PCP can try another course of higher dose steroids or consult with Rheumatology.
Continue to: A full history...
A full history and physical exam revealed a myriad of gastrointestinal (GI) complaints, such as diarrhea. But the PCP recalled a recent roundtable discussion on debiasing techniques specifically related to gynecologic disorders, including OC. Therefore, he decided to include OC in the differential diagnosis—something he would not routinely have done given the preponderance of GI symptoms. Despite the patient’s reluctance and time constraints, the PCP ordered a transvaginal ultrasound. Findings from the ultrasound study revealed stage II OC, which carries a good prognosis. The patient is currently undergoing treatment and was last reported as doing well.
Early signs of ovarian cancer can be chalked up to a “GI issue”
OC is the second most common gynecologic cancer21 and the fifth leading cause of cancer-related death22 in US women. Compared to other cancers, the prognosis for localized early-stage OC is surprisingly good, with a 5-year survival rate approaching 93%.23 However, most disease is detected in later stages, and the 5-year survival rate drops to a low of 29%.24
There remains no established screening protocol for OC. Fewer than a quarter of all cases are diagnosed in stage I, and detection of OC relies heavily on the physician’s ability to decipher vague symptomatology that overlaps with other, more common maladies. This poses an obvious diagnostic challenge and, not surprisingly, a high level of susceptibility to cognitive bias.
More than 90% of patients with OC present with some combination of the following symptoms prior to diagnosis: abdominal (77%), GI (70%), pain (58%), constitutional (50%), urinary (34%), and pelvic (26%).25 The 3 most common isolated symptoms in patients with OC are abdominal bloating, decrease in appetite, and frank abdominal pain.26 Patients with biopsy-confirmed OC experience these symptoms an average of 6 months prior to actual diagnosis.27
Knowledge gaps play a role. Studies assessing the ability of health care providers to identify presenting symptoms of OC reveal specific knowledge gaps. For instance, in a survey by Gajjar and colleagues,28 most PCPs correctly identified bloating as a key symptom of OC; however, they weren’t as good at identifying less common symptoms, such as inability to finish a meal and early satiety. Moreover, survey participants misinterpreted or missed GI symptoms as an important manifestation of early OC disease.28 These specific knowledge gaps combine with physician errors in thinking, further obscuring and extending the diagnostic process. The point prevalence for OC is relatively low, and many PCPs only encounter a few cases during their entire career.29 This low pre-test probability may also fuel the delay in diagnosis.
Watch for these forms of bias. Since nonspecific symptoms of early-stage OC resemble those of other more benign conditions, a form of anchoring error known as multiple alternatives bias can arise. In this scenario, clinicians investigate only 1 potential plausible diagnosis and remain focused on that single, often faulty, conclusion. This persists despite other equally plausible alternatives that arise as the investigation proceeds.28
Affective error may also play a role in missed or delayed diagnosis. For example, a physician would prefer to diagnose and treat a common GI illness than consider OC. Another distortion involves outcome bias wherein the physician gives more significance to benign conditions such as irritable bowel syndrome because they have a more favorable outcome and clear treatment path. Physicians also favor these benign conditions because they encounter them more frequently than OC in the clinic setting. (This is known as availability bias.) Outcome bias and multiple alternatives bias can result in noninvestigation of symptoms and inefficient or improper management, leading to a delay in arriving at the correct diagnosis or anchoring on a plausible but incorrect diagnosis.
Continue to: An incorrect initial diagnostic...
An incorrect initial diagnostic path often triggers a cascade of subsequent errors. The physician orders additional unhelpful and expensive tests in an effort to characterize the suspected GI pathology. This then leads the physician to prematurely terminate the work-up and accept the most favored diagnosis. Lastly, sunk-cost fallacy comes into play: The physician has “invested” time and energy investigating a particular diagnosis and rather than abandon the presumed diagnosis, continues to put more time and effort in going down an incorrect diagnostic path.
A series of failures. These biases and miscues have been observed in several studies. For example, a survey of 1725 women by Goff and colleagues30 sought to identify factors related to delayed OC diagnosis. The authors found that the following factors were significantly associated with a delayed diagnosis: omission of a pelvic exam at initial presentation, a separate exploration of a multitude of collateral symptoms, a failure to order ultrasound/computed tomography/CA-125 test, and a failure to consider age as a factor (especially if the patient was outside the norm).
Responses from the survey also revealed that physicians initially ordered work-ups related to GI etiology and only later considered a pelvic work-up. This suggests that well-known presenting signs and symptoms or a constellation of typical and atypical symptoms of OC often failed to trigger physician recognition. Understandably, patients presenting with menorrhagia or gynecologic complaints are more likely to have OC detected at an earlier stage than patients who present with GI or abdominal signs alone.31 table 27 summarizes some of the cognitive biases seen in the diagnostic path of OC.

While in the hospital, he becomes acutely upset by the hallucinations and is given haloperidol and lorazepam by house staff. In the morning, the patient exhibits severe signs of Parkinson disease that include rigidity and masked facies.
Given the patient’s poor response to haloperidol and continued confusion, the team consulted Neurology and Psychiatry. Gathering a more detailed history from the patient and family, the patient is given a diagnosis of classic LBD. The antipsychotic medications are stopped. The patient and his family receive education about LBD treatment and management, and the patient is discharged to outpatient care.
Psychiatric symptoms can be an early “misdirect” in cases of Lewy body disease
LBD, the second leading neurodegenerative dementia after Alzheimer disease (AD), affects 1.5 million Americans,32 representing about 10% of all dementia cases. LBD and AD overlap in 25% of dementia cases.33 In patients older than 85 years, the prevalence jumps to 5% of the general population and 22% of all cases of dementia.33 Despite its prevalence, a recent study showed that only 6% of PCPs correctly identified LBD as the primary diagnosis when presented with typical case examples.32
Continue to: 3 stages of presentation
3 stages of presentation. Unlike other forms of dementia, LBD typically presents first with psychiatric symptoms, then with cognitive impairment, and last with parkinsonian symptoms. Additionally, rapid eye movement sleep behavior disorder and often subtle elements of nonmemory cognitive impairment distinguish LBD from both AD and vascular dementia.32 The primary cognitive deficit in LBD is not in memory but in attention, executive functioning, and visuospatial ability.34 Only in the later stages of the disease do patients exhibit gradual and progressive memory loss.
Mistaken for many things. When evaluating patients exhibiting signs of dementia, it’s important to include LBD in the differential, with increased suspicion for patients experiencing episodes of psychosis or delirium. The uniqueness of LBD lies in its psychotic symptomatology, particularly during earlier stages of the disease. This feature helps distinguish LBD from both AD and vascular dementia. As seen in the case, LBD can also be confused with acute delirium.
Older adult patients presenting to the ED or clinic with visual hallucinations, delirium, and mental confusion may receive a false diagnosis of schizophrenia, medication- or substance-induced psychosis, Parkinson disease, or delirium of unknown etiology.35 Unfortunately, LBD is often overlooked and not considered in the differential diagnosis. Due to underrecognition, patients may receive treatment with typical antipsychotics. The addition of a neuroleptic to help control the psychotic symptoms causes patients with LBD to develop severe extrapyramidal symptoms and worsening mental status,36 leading to severe parkinsonian signs, which further muddies the diagnostic process. In addition, treatment for suspected Parkinson disease, including carbidopa-levodopa, has no benefit for patients with LBD and may increase psychotic symptoms.37
First-line treatment for LBD includes psychoeducation for the patient and family, cholinesterase inhibitors (eg, rivastigmine), and avoidance of high-potency antipsychotics, such as haloperidol. Although persistent hallucinations and psychosis remain difficult to treat in LBD, low-dose quetiapine is 1 option. Incorrectly diagnosing and prescribing treatment for another condition exacerbates symptoms in this patient population.
The patient has been experiencing chronic pain for the past few years after a motor vehicle accident. She has seen a physiatrist and another provider, both of whom found no “objective” causes of her chronic pain. They started the patient on sertraline for depression and an analgesic, both of which were ineffective.
The patient likes to exercise at a gym twice a week by doing light cardio (treadmill) exercise and light weightlifting. Lately, however, she has been unable to exercise due to the pain. At this visit, she mentions having low energy, poor sleep, frequent fatigue, and generalized soreness and pain in multiple areas of her body. The PCP recognizes the patient’s presenting symptoms as significant for FM and starts her on pregabalin and hydrotherapy, with positive results.
Continue to: Fibromyalgia skepticism may lead to a Dx of depression
Fibromyalgia skepticism may lead to a Dx of depression
FM, the second most common disorder seen in rheumatologic practice after OA, is estimated to affect approximately 1 in 20 patients (approximately 5 million Americans) in the primary care setting.38,39 The condition has a high female-to-male preponderance (3.4% vs 0.5%).40 While the primary symptom of FM is chronic pain, patients commonly present with fatigue and sleep disturbance.41 Comorbid conditions include headaches, irritable bowel syndrome, and mood disturbances (most commonly anxiety and depression).
Several studies have explored reasons for the misdiagnosis and underdiagnosis of FM. One important factor is ongoing skepticism among some physicians and the public, in general, as to whether FM is a real disease. This issue was addressed by a study by White and colleagues,42 who estimated and compared the point prevalence of FM and related disorders in Amish vs non-Amish adults. The authors hypothesized that if litigation and/or compensation availability have a major impact on FM prevalence, then there would be a near zero prevalence of FM in the Amish community. And yet, researchers found an overall age- and sex-adjusted FM prevalence of 7.3% (95% CI; 5.3%-9.7%); this was both statistically greater than zero (P < .0001) and greater than 2 control populations of non-Amish adults (both P < .05).
Many physicians consider FM fundamentally an emotional disturbance, and the high preponderance of FM in female patients may contribute to this misconception as reports of pain and emotional distress by women are often dismissed as hysteria.43 Physicians often explore the emotional aspects of FM, incorrectly diagnosing patients with depression and subsequently treating them with a psychotropic drug.39 Alternatively, they may focus on the musculoskeletal presentations of FM and prescribe analgesics or physical therapy, both of which do little to alleviate FM.
To make the correct diagnosis of FM, the American College of Rheumatology created a specific set of criteria in 1990, which was updated in 2010.44 For a diagnosis of FM, a patient must have at least a 3-month history of bilateral pain above and below the waist and along the axial skeletal spine. Although not included in the updated 2010 criteria, many clinicians continue to check for tender points, following the 1990 criteria requiring the presence of 11 of 18 points to make the diagnosis.
At least 3 cognitive biases relating to FM apply: anchoring, availability, and fundamental attribution error (see table 3).7 Anchoring occurs when the PCP settles on a psychiatric diagnosis of exaggerated pain syndrome, muscle overuse, or OA and fails to explore alternative etiology. Availability bias may obscure the true diagnosis of FM. Since PCPs see many patients with RA or OA, they may overlook or dismiss the possibility of FM. Attribution error happens when physicians dismiss the complaints of patients with FM as merely due to psychological distress, hysteria, or acting out.43

Patients with FM, who are often otherwise healthy, often present multiple times to the same PCP with a chief complaint of chronic pain. These repeat presentations can result in compassion fatigue and impact care. As Aloush and colleagues40 noted in their study, “FM patients were perceived as more difficult than RA patients, with a high level of concern and emotional response. A high proportion of physicians were reluctant to accept them because they feel emotional/psychological difficulties meeting and coping with these patients.”In response, patients with undiagnosed FM or inadequately treated FM may visit other PCPs, which may or may not result in a correct diagnosis and treatment.
We can do better
Primary care physicians face the daunting task of diagnosing and treating a wide range of common conditions while also trying to recognize less-common conditions with atypical presentations—all during a busy clinic workday. Nonetheless, we should strive to overcome internal (eg, cognitive bias and fund-of-knowledge deficits) and external (eg, time constraints, limited resources) pressures to improve diagnostic accuracy and care.
Each of the 4 disease states we’ve discussed have high rates of missed and/or delayed diagnosis. Each presents a unique set of confounders: PMR with its overlapping symptoms of many other rheumatologic diseases; OC with its often vague and misleading GI symptomatology; LBD with overlapping features of AD and Parkinson disease; and FM with skepticism. As gatekeepers to health care, it falls on PCPs to sort out these diagnostic dilemmas to avoid medical errors. Fundamental knowledge of each disease, its unique pathophysiology and symptoms, and varying presentations can be learned, internalized, and subsequently put into clinical practice to improve patient outcomes.
CORRESPONDENCE
Paul D. Rosen MD, Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Avenue, Brooklyn, New York 11201; [email protected]
Medical errors in all settings contributed to as many as 250,000 deaths per year in the United States between 2000 and 2008, according to a 2016 study.1 Diagnostic error, in particular, remains a leading cause of morbidity and mortality in the United States and worldwide. In 2017, 12 million patients (roughly 5% of all US adults) who sought outpatient care experienced missed, delayed, or incorrect diagnosis at least once.2
In his classic work, How Doctors Think, Jerome Groopman, MD, explored the diagnostic process with a focus on the role of cognitive bias in clinical decision-making. Groopman examined how physicians can become sidetracked in their thinking and “blinded” to potential alternative diagnoses.3 Medical error is not necessarily because of a deficiency in medical knowledge; rather, physicians become susceptible to medical error when defective and faulty reasoning distort their diagnostic ability.4
Cognitive bias in the diagnostic process has been extensively studied, and a full review is beyond the scope of this article.5 However, here we will examine pathways leading to diagnostic errors in the primary care setting, specifically the role of cognitive bias in the work-up of polymyalgia rheumatica (PMR), ovarian cancer (OC), Lewy body dementia (LBD), and fibromyalgia (FM). As these 4 disease states are seen with low-to-moderate frequency in primary care, cognitive bias can complicate accurate diagnosis. But first, a word about how to understand clinical reasoning.
There are 2 types of reasoning (and 1 is more prone to error)
Physician clinical reasoning can be divided into 2 different cognitive approaches.
Type 1 reasoning employs intuition and heuristics; this type is automatic, reflexive, and quick.5 While the use of mental shortcuts in type 1 increases the speed with which decisions are made, it also makes this form of reasoning more prone to error.
Type 2 reasoning requires conscious effort. It is goal directed and rigorous and therefore slower than type 1 reasoning. Extrapolated to the clinical context, clinicians transition from type 2 to type 1 reasoning as they gain experience and training throughout their careers and develop their own conscious and subconscious heuristics. Deviations from accurate decision-making occur in a systematic manner due to cognitive biases and result in medical error.6table 17 lists common types of cognitive bias.

An important question to ask. Physicians tend to fall into a pattern of quick, type 1 reasoning. However, it’s important to strive to maintain a broad differential diagnosis and avoid premature closure of the diagnostic process. It’s critical that we consider alternative diagnoses (ie, consciously move from type 1 to type 2 thinking) and continue to ask ourselves, “What else?” while working through differential diagnoses. This can be a powerful debiasing technique.
Continue to: The discussion...
The discussion of the following 4 disease states demonstrates how cognitive bias can lead to diagnostic error.
The patient is barely able to ambulate and appears to be in considerable pain. She is relying heavily on her walker and is assisted by her granddaughter. The primary care physician (PCP) obtains a detailed history that includes chronic shoulder and hip pain. Given that the patient has not responded to NSAID treatment over the previous 6 months, the PCP takes a moment to reconsider the diagnosis of OA and considers other options.
In light of the high prevalence of PMR in older women, the physician pursues a more specific physical examination tailored to ferret out PMR. He had learned this diagnostic shortcut as a resident, remembered it, and adeptly applied it whenever circumstances warranted. He asks the patient to raise her arms above her head (goalpost sign). She is unable to perform this task and experiences severe bilateral shoulder pain on trial. The PCP then places the patient on the examining table and attempts to assist her in rolling toward him. The patient is also unable to perform this maneuver and experiences significant bilateral hip pain on trial.
Based primarily on the patient’s history and physical exam findings, the PCP makes a presumptive diagnosis of PMR vs OA vs combined PMR with OA, orders an erythrocyte sedimentation rate (ESR) and basic rheumatologic
PMR can be mistaken for OA
PMR is the most common inflammatory rheumatic disease in older patients.8 It is a debilitating illness with simple, effective treatment but has devastating consequences if missed or left untreated.9 PMR typically manifests in patients older than age 50, with a peak incidence at 80 years of age. It is also far more common in women.10
Approximately 80% of patients with PMR initially present to their PCP, often posing a diagnostic challenge to many clinicians.11 Due to overlap in symptoms, the condition is often misdiagnosed as OA, a more common condition seen by PCPs. Also, there are no specific diagnostic tests for PMR. An elevated ESR can help confirm the diagnosis, but one-third of patients with PMR have a normal ESR.12 Therefore, the diagnostic conundrum the physician faces is OA vs rheumatoid arthritis (RA), PMR, or another condition.
Continue to: The consequences...
The consequences of a missed and delayed PMR diagnosis range from seriously impaired quality of life to significantly increased risk of vascular events (eg, blindness, stroke) due to temporal arteritis.13 Early diagnosis is even more critical as the risk of a vascular event and death is highest during initial phases of the disease course.14
FPs often miss this Dx. A timely diagnosis relies almost exclusively on an accurate, thorough history and physical exam. However, PCPs often struggle to correctly diagnose PMR. According to a study by Bahlas and colleagues,15 the accuracy rate for correctly diagnosing PMR was 24% among a cohort of family physicians.
The differential diagnosis for PMR is broad and includes seronegative spondyloarthropathies, malignancy, Lyme disease, hypothyroidism, and both RA and OA.16
PCPs are extremely adept at correctly diagnosing RA, but not PMR. A study by Blaauw and colleagues17 comparing PCPs and rheumatologists found PCPs correctly identified 92% of RA cases but only 55% of PMR cases. When rheumatologists reviewed these same cases, they correctly identified PMR and RA almost 100% of the time.17 The difference in diagnostic accuracy between rheumatologists and PCPs suggests limited experience and gaps in fund of knowledge.
Making the diagnosis. The diagnosis of PMR is often made on empiric response to corticosteroid treatment, but doing so based solely on a patient’s response is controversial.18 There are rare instances in which patients with PMR fail to respond to treatment. On the other hand, some inflammatory conditions that mimic or share symptoms with PMR also respond to corticosteroids, potentially resulting in erroneous confirmation bias.
Some classification criteria use rapid response to low-dose prednisone/prednisolone (≤ 20 mg) to confirm the diagnosis,19 while other more recent guidelines no longer include this approach.20 If PMR continues to be suspected after a trial of steroids is unsuccessful, the PCP can try another course of higher dose steroids or consult with Rheumatology.
Continue to: A full history...
A full history and physical exam revealed a myriad of gastrointestinal (GI) complaints, such as diarrhea. But the PCP recalled a recent roundtable discussion on debiasing techniques specifically related to gynecologic disorders, including OC. Therefore, he decided to include OC in the differential diagnosis—something he would not routinely have done given the preponderance of GI symptoms. Despite the patient’s reluctance and time constraints, the PCP ordered a transvaginal ultrasound. Findings from the ultrasound study revealed stage II OC, which carries a good prognosis. The patient is currently undergoing treatment and was last reported as doing well.
Early signs of ovarian cancer can be chalked up to a “GI issue”
OC is the second most common gynecologic cancer21 and the fifth leading cause of cancer-related death22 in US women. Compared to other cancers, the prognosis for localized early-stage OC is surprisingly good, with a 5-year survival rate approaching 93%.23 However, most disease is detected in later stages, and the 5-year survival rate drops to a low of 29%.24
There remains no established screening protocol for OC. Fewer than a quarter of all cases are diagnosed in stage I, and detection of OC relies heavily on the physician’s ability to decipher vague symptomatology that overlaps with other, more common maladies. This poses an obvious diagnostic challenge and, not surprisingly, a high level of susceptibility to cognitive bias.
More than 90% of patients with OC present with some combination of the following symptoms prior to diagnosis: abdominal (77%), GI (70%), pain (58%), constitutional (50%), urinary (34%), and pelvic (26%).25 The 3 most common isolated symptoms in patients with OC are abdominal bloating, decrease in appetite, and frank abdominal pain.26 Patients with biopsy-confirmed OC experience these symptoms an average of 6 months prior to actual diagnosis.27
Knowledge gaps play a role. Studies assessing the ability of health care providers to identify presenting symptoms of OC reveal specific knowledge gaps. For instance, in a survey by Gajjar and colleagues,28 most PCPs correctly identified bloating as a key symptom of OC; however, they weren’t as good at identifying less common symptoms, such as inability to finish a meal and early satiety. Moreover, survey participants misinterpreted or missed GI symptoms as an important manifestation of early OC disease.28 These specific knowledge gaps combine with physician errors in thinking, further obscuring and extending the diagnostic process. The point prevalence for OC is relatively low, and many PCPs only encounter a few cases during their entire career.29 This low pre-test probability may also fuel the delay in diagnosis.
Watch for these forms of bias. Since nonspecific symptoms of early-stage OC resemble those of other more benign conditions, a form of anchoring error known as multiple alternatives bias can arise. In this scenario, clinicians investigate only 1 potential plausible diagnosis and remain focused on that single, often faulty, conclusion. This persists despite other equally plausible alternatives that arise as the investigation proceeds.28
Affective error may also play a role in missed or delayed diagnosis. For example, a physician would prefer to diagnose and treat a common GI illness than consider OC. Another distortion involves outcome bias wherein the physician gives more significance to benign conditions such as irritable bowel syndrome because they have a more favorable outcome and clear treatment path. Physicians also favor these benign conditions because they encounter them more frequently than OC in the clinic setting. (This is known as availability bias.) Outcome bias and multiple alternatives bias can result in noninvestigation of symptoms and inefficient or improper management, leading to a delay in arriving at the correct diagnosis or anchoring on a plausible but incorrect diagnosis.
Continue to: An incorrect initial diagnostic...
An incorrect initial diagnostic path often triggers a cascade of subsequent errors. The physician orders additional unhelpful and expensive tests in an effort to characterize the suspected GI pathology. This then leads the physician to prematurely terminate the work-up and accept the most favored diagnosis. Lastly, sunk-cost fallacy comes into play: The physician has “invested” time and energy investigating a particular diagnosis and rather than abandon the presumed diagnosis, continues to put more time and effort in going down an incorrect diagnostic path.
A series of failures. These biases and miscues have been observed in several studies. For example, a survey of 1725 women by Goff and colleagues30 sought to identify factors related to delayed OC diagnosis. The authors found that the following factors were significantly associated with a delayed diagnosis: omission of a pelvic exam at initial presentation, a separate exploration of a multitude of collateral symptoms, a failure to order ultrasound/computed tomography/CA-125 test, and a failure to consider age as a factor (especially if the patient was outside the norm).
Responses from the survey also revealed that physicians initially ordered work-ups related to GI etiology and only later considered a pelvic work-up. This suggests that well-known presenting signs and symptoms or a constellation of typical and atypical symptoms of OC often failed to trigger physician recognition. Understandably, patients presenting with menorrhagia or gynecologic complaints are more likely to have OC detected at an earlier stage than patients who present with GI or abdominal signs alone.31 table 27 summarizes some of the cognitive biases seen in the diagnostic path of OC.

While in the hospital, he becomes acutely upset by the hallucinations and is given haloperidol and lorazepam by house staff. In the morning, the patient exhibits severe signs of Parkinson disease that include rigidity and masked facies.
Given the patient’s poor response to haloperidol and continued confusion, the team consulted Neurology and Psychiatry. Gathering a more detailed history from the patient and family, the patient is given a diagnosis of classic LBD. The antipsychotic medications are stopped. The patient and his family receive education about LBD treatment and management, and the patient is discharged to outpatient care.
Psychiatric symptoms can be an early “misdirect” in cases of Lewy body disease
LBD, the second leading neurodegenerative dementia after Alzheimer disease (AD), affects 1.5 million Americans,32 representing about 10% of all dementia cases. LBD and AD overlap in 25% of dementia cases.33 In patients older than 85 years, the prevalence jumps to 5% of the general population and 22% of all cases of dementia.33 Despite its prevalence, a recent study showed that only 6% of PCPs correctly identified LBD as the primary diagnosis when presented with typical case examples.32
Continue to: 3 stages of presentation
3 stages of presentation. Unlike other forms of dementia, LBD typically presents first with psychiatric symptoms, then with cognitive impairment, and last with parkinsonian symptoms. Additionally, rapid eye movement sleep behavior disorder and often subtle elements of nonmemory cognitive impairment distinguish LBD from both AD and vascular dementia.32 The primary cognitive deficit in LBD is not in memory but in attention, executive functioning, and visuospatial ability.34 Only in the later stages of the disease do patients exhibit gradual and progressive memory loss.
Mistaken for many things. When evaluating patients exhibiting signs of dementia, it’s important to include LBD in the differential, with increased suspicion for patients experiencing episodes of psychosis or delirium. The uniqueness of LBD lies in its psychotic symptomatology, particularly during earlier stages of the disease. This feature helps distinguish LBD from both AD and vascular dementia. As seen in the case, LBD can also be confused with acute delirium.
Older adult patients presenting to the ED or clinic with visual hallucinations, delirium, and mental confusion may receive a false diagnosis of schizophrenia, medication- or substance-induced psychosis, Parkinson disease, or delirium of unknown etiology.35 Unfortunately, LBD is often overlooked and not considered in the differential diagnosis. Due to underrecognition, patients may receive treatment with typical antipsychotics. The addition of a neuroleptic to help control the psychotic symptoms causes patients with LBD to develop severe extrapyramidal symptoms and worsening mental status,36 leading to severe parkinsonian signs, which further muddies the diagnostic process. In addition, treatment for suspected Parkinson disease, including carbidopa-levodopa, has no benefit for patients with LBD and may increase psychotic symptoms.37
First-line treatment for LBD includes psychoeducation for the patient and family, cholinesterase inhibitors (eg, rivastigmine), and avoidance of high-potency antipsychotics, such as haloperidol. Although persistent hallucinations and psychosis remain difficult to treat in LBD, low-dose quetiapine is 1 option. Incorrectly diagnosing and prescribing treatment for another condition exacerbates symptoms in this patient population.
The patient has been experiencing chronic pain for the past few years after a motor vehicle accident. She has seen a physiatrist and another provider, both of whom found no “objective” causes of her chronic pain. They started the patient on sertraline for depression and an analgesic, both of which were ineffective.
The patient likes to exercise at a gym twice a week by doing light cardio (treadmill) exercise and light weightlifting. Lately, however, she has been unable to exercise due to the pain. At this visit, she mentions having low energy, poor sleep, frequent fatigue, and generalized soreness and pain in multiple areas of her body. The PCP recognizes the patient’s presenting symptoms as significant for FM and starts her on pregabalin and hydrotherapy, with positive results.
Continue to: Fibromyalgia skepticism may lead to a Dx of depression
Fibromyalgia skepticism may lead to a Dx of depression
FM, the second most common disorder seen in rheumatologic practice after OA, is estimated to affect approximately 1 in 20 patients (approximately 5 million Americans) in the primary care setting.38,39 The condition has a high female-to-male preponderance (3.4% vs 0.5%).40 While the primary symptom of FM is chronic pain, patients commonly present with fatigue and sleep disturbance.41 Comorbid conditions include headaches, irritable bowel syndrome, and mood disturbances (most commonly anxiety and depression).
Several studies have explored reasons for the misdiagnosis and underdiagnosis of FM. One important factor is ongoing skepticism among some physicians and the public, in general, as to whether FM is a real disease. This issue was addressed by a study by White and colleagues,42 who estimated and compared the point prevalence of FM and related disorders in Amish vs non-Amish adults. The authors hypothesized that if litigation and/or compensation availability have a major impact on FM prevalence, then there would be a near zero prevalence of FM in the Amish community. And yet, researchers found an overall age- and sex-adjusted FM prevalence of 7.3% (95% CI; 5.3%-9.7%); this was both statistically greater than zero (P < .0001) and greater than 2 control populations of non-Amish adults (both P < .05).
Many physicians consider FM fundamentally an emotional disturbance, and the high preponderance of FM in female patients may contribute to this misconception as reports of pain and emotional distress by women are often dismissed as hysteria.43 Physicians often explore the emotional aspects of FM, incorrectly diagnosing patients with depression and subsequently treating them with a psychotropic drug.39 Alternatively, they may focus on the musculoskeletal presentations of FM and prescribe analgesics or physical therapy, both of which do little to alleviate FM.
To make the correct diagnosis of FM, the American College of Rheumatology created a specific set of criteria in 1990, which was updated in 2010.44 For a diagnosis of FM, a patient must have at least a 3-month history of bilateral pain above and below the waist and along the axial skeletal spine. Although not included in the updated 2010 criteria, many clinicians continue to check for tender points, following the 1990 criteria requiring the presence of 11 of 18 points to make the diagnosis.
At least 3 cognitive biases relating to FM apply: anchoring, availability, and fundamental attribution error (see table 3).7 Anchoring occurs when the PCP settles on a psychiatric diagnosis of exaggerated pain syndrome, muscle overuse, or OA and fails to explore alternative etiology. Availability bias may obscure the true diagnosis of FM. Since PCPs see many patients with RA or OA, they may overlook or dismiss the possibility of FM. Attribution error happens when physicians dismiss the complaints of patients with FM as merely due to psychological distress, hysteria, or acting out.43

Patients with FM, who are often otherwise healthy, often present multiple times to the same PCP with a chief complaint of chronic pain. These repeat presentations can result in compassion fatigue and impact care. As Aloush and colleagues40 noted in their study, “FM patients were perceived as more difficult than RA patients, with a high level of concern and emotional response. A high proportion of physicians were reluctant to accept them because they feel emotional/psychological difficulties meeting and coping with these patients.”In response, patients with undiagnosed FM or inadequately treated FM may visit other PCPs, which may or may not result in a correct diagnosis and treatment.
We can do better
Primary care physicians face the daunting task of diagnosing and treating a wide range of common conditions while also trying to recognize less-common conditions with atypical presentations—all during a busy clinic workday. Nonetheless, we should strive to overcome internal (eg, cognitive bias and fund-of-knowledge deficits) and external (eg, time constraints, limited resources) pressures to improve diagnostic accuracy and care.
Each of the 4 disease states we’ve discussed have high rates of missed and/or delayed diagnosis. Each presents a unique set of confounders: PMR with its overlapping symptoms of many other rheumatologic diseases; OC with its often vague and misleading GI symptomatology; LBD with overlapping features of AD and Parkinson disease; and FM with skepticism. As gatekeepers to health care, it falls on PCPs to sort out these diagnostic dilemmas to avoid medical errors. Fundamental knowledge of each disease, its unique pathophysiology and symptoms, and varying presentations can be learned, internalized, and subsequently put into clinical practice to improve patient outcomes.
CORRESPONDENCE
Paul D. Rosen MD, Brooklyn Hospital Center, Department of Family Medicine, 121 Dekalb Avenue, Brooklyn, New York 11201; [email protected]
1. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139
2. Van Such M, Lohr R, Beckman T, et al. Extent of diagnostic agreement among medical referrals. J Eval Clin Pract. 2017;23:870-874. doi: 10.1111/jep.12747
3. Groopman JE. How Doctors Think. Houghton Mifflin; 2007.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131. doi: 10.1126/science.185.4157.1124
5. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: Cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30. doi: 10.1097/ACM.0000000000001421
6. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. doi: 10.1097/00001888-200308000-00003
7. Morgenstern J. Cognitive errors in medicine: The common errors. First10EM blog. September 15, 2015. Updated September 22, 2019. Accessed February 8, 2022. https://first10em.com/cognitive-errors/
8. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22:40. doi: 10.1007/s11926-020-00919-2
9. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176
10. Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694-1697.
11. Barraclough K, Liddell WG, du Toit J, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract. 2008;25:328-33. doi: 10.1093/fampra/cmn044
12. Manzo C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: a case report. Diseases. 2018;6:84. doi: 10.3390/diseases6040084
13. Crowson CS, Matteson EL. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum. 2017;47:253-256. doi: 10.1016/j.semarthrit.2017.04.001
14. Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549-550. doi: 10.1136/bmj.299.6698.549
15. Bahlas S, Ramos-Remus C, Davis P. Utilisation and costs of investigations, and accuracy of diagnosis of polymyalgia rheumatica by family physicians. Clin Rheumatol. 2000;19:278-280. doi: 10.1007/s100670070045
16. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997;157:162-168.
17. Blaauw AA, Schuwirth LW, van der Vleuten CP, et al. Assessing clinical competence: recognition of case descriptions of rheumatic diseases by general practitioners. Br J Rheumatol. 1995;34:375-379. doi:10.1093/rheumatology/34.4.375
18. Mager DR. Polymylagia rheumatica: common disease, elusive diagnosis. Home Healthc Now. 2015;33:132-138. doi:10.1097/NHH.0000000000000199
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 381;63-72. doi: 10.1016/S0140-6736(12)60680-1. Published correction appears in Lancet. 20135;381:28.
20. Liew DF, Owen CE, Buchanan RR. Prescribing for polymyalgia rheumatica. Aust Prescr. 2018;41:14-19. doi: 10.18773/austprescr.2018.001
21. Ovarian cancer statistics. Centers for Disease Control and Prevention. Reviewed June 8, 2021. Accessed February 22, 2022. www.cdc.gov/cancer/ovarian/statistics/index.htm
22. Key statistics for ovarian cancer. American Cancer Society. Revised January 12, 2022. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
23. Survival rates for ovarian cancer. American Cancer Society. Revised January 25, 2021. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html
24. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9-32. doi: 10.20892/j.issn.2095-3941.2016.0084
25. Goff BA, Mandel LS, Melancon CH, et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712. doi: 10.1001/jama.291.22.2705
26. Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212-217. doi: 10.1016/s0029-7844(01)01457-0
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients: Cancer. Br J Cancer. 2005;92:1959-1970. doi: 10.1038/sj.bjc.6602587
28. Gajjar K, Ogden G, Mujahid MI, et al. Symptoms and risk factors of ovarian cancer: a survey in primary care. Obstet Gynecol. 2012:754197. doi: 10.5402/2012/754197
29. Austoker J. Diagnosis of ovarian cancer in primary care. BMJ. 2009;339:b3286. doi: 10.1136/bmj.b3286
30. Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis: results of a national ovarian cancer survey. Cancer. 2000;89:2068-2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z
31. Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727-2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8
32. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388-392. doi: 10.1016/j.parkreldis.2010.03.007
33. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6:333-341. doi: 10.31887/DCNS.2004.6.3/imckeith
34. Mrak RE, Griffin WS. Dementia with Lewy bodies: definitions, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:619-625.
35. Khotianov N, Singh R, Singh S. Lewy body dementia: case report and discussion. J Am Board Fam Pract. 2002;15:50-54.
36. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:731-742. doi: 10.1176/appi.ajp.2015.14121582
37. Velayudhan L, Ffytche D, Ballard C, et al. New therapeutic strategies of Lewy body dementias. Curr Neurol Neurosci Rep. 2017;17:68. doi: 10.1007/s11910-017-0778-2
38. Arnold LM, Clauw DJ, McCarberg BH; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457-464. doi: 10.4065/mcp.2010.0738
39. Arnold LM, Gebke KB, Choy EHS. Fibromyalgia: management strategies for primary care providers. Int J Clin Pract. 2016;70:99-112. doi: 10.1111/ijcp.12757
40. Aloush V, Niv D, Ablin JN, et al. Good pain, bad pain: illness perception and physician attitudes towards rheumatoid arthritis and fibromyalgia patients. Clin Exp Rheumatol. 2021;39(suppl 130):54-60.
41. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786-792. doi: 10.1002/acr.21896
42. White KP, Thompson J. Fibromyalgia syndrome in an Amish community: a controlled study to determine disease and symptom prevalence. J Rheumatol. 2003;30:1835-1840.
43. Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16:10.4088/PCC.14m01664. doi: 10.4088/PCC.14m01664
44. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. doi:10.1002/acr.20140
1. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139. doi: 10.1136/bmj.i2139
2. Van Such M, Lohr R, Beckman T, et al. Extent of diagnostic agreement among medical referrals. J Eval Clin Pract. 2017;23:870-874. doi: 10.1111/jep.12747
3. Groopman JE. How Doctors Think. Houghton Mifflin; 2007.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131. doi: 10.1126/science.185.4157.1124
5. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: Cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30. doi: 10.1097/ACM.0000000000001421
6. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780. doi: 10.1097/00001888-200308000-00003
7. Morgenstern J. Cognitive errors in medicine: The common errors. First10EM blog. September 15, 2015. Updated September 22, 2019. Accessed February 8, 2022. https://first10em.com/cognitive-errors/
8. Gazitt T, Zisman D, Gardner G. Polymyalgia rheumatica: a common disease in seniors. Curr Rheumatol Rep. 2020;22:40. doi: 10.1007/s11926-020-00919-2
9. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26-35. doi: 10.1002/art.23176
10. Doran MF, Crowson CS, O’Fallon WM, et al. Trends in the incidence of polymyalgia rheumatica over a 30 year period in Olmsted County, Minnesota, USA. J Rheumatol. 2002;29:1694-1697.
11. Barraclough K, Liddell WG, du Toit J, et al. Polymyalgia rheumatica in primary care: a cohort study of the diagnostic criteria and outcome. Fam Pract. 2008;25:328-33. doi: 10.1093/fampra/cmn044
12. Manzo C. Polymyalgia rheumatica (PMR) with normal values of both erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) concentration at the time of diagnosis in a centenarian man: a case report. Diseases. 2018;6:84. doi: 10.3390/diseases6040084
13. Crowson CS, Matteson EL. Contemporary prevalence estimates for giant cell arteritis and polymyalgia rheumatica, 2015. Semin Arthritis Rheum. 2017;47:253-256. doi: 10.1016/j.semarthrit.2017.04.001
14. Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549-550. doi: 10.1136/bmj.299.6698.549
15. Bahlas S, Ramos-Remus C, Davis P. Utilisation and costs of investigations, and accuracy of diagnosis of polymyalgia rheumatica by family physicians. Clin Rheumatol. 2000;19:278-280. doi: 10.1007/s100670070045
16. Brooks RC, McGee SR. Diagnostic dilemmas in polymyalgia rheumatica. Arch Intern Med. 1997;157:162-168.
17. Blaauw AA, Schuwirth LW, van der Vleuten CP, et al. Assessing clinical competence: recognition of case descriptions of rheumatic diseases by general practitioners. Br J Rheumatol. 1995;34:375-379. doi:10.1093/rheumatology/34.4.375
18. Mager DR. Polymylagia rheumatica: common disease, elusive diagnosis. Home Healthc Now. 2015;33:132-138. doi:10.1097/NHH.0000000000000199
19. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 381;63-72. doi: 10.1016/S0140-6736(12)60680-1. Published correction appears in Lancet. 20135;381:28.
20. Liew DF, Owen CE, Buchanan RR. Prescribing for polymyalgia rheumatica. Aust Prescr. 2018;41:14-19. doi: 10.18773/austprescr.2018.001
21. Ovarian cancer statistics. Centers for Disease Control and Prevention. Reviewed June 8, 2021. Accessed February 22, 2022. www.cdc.gov/cancer/ovarian/statistics/index.htm
22. Key statistics for ovarian cancer. American Cancer Society. Revised January 12, 2022. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
23. Survival rates for ovarian cancer. American Cancer Society. Revised January 25, 2021. Accessed February 22, 2022. www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html
24. Reid BM, Permuth JB, Sellers TA. Epidemiology of ovarian cancer: a review. Cancer Biol Med. 2017;14:9-32. doi: 10.20892/j.issn.2095-3941.2016.0084
25. Goff BA, Mandel LS, Melancon CH, et al. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA. 2004;291:2705-2712. doi: 10.1001/jama.291.22.2705
26. Olson SH, Mignone L, Nakraseive C, et al. Symptoms of ovarian cancer. Obstet Gynecol. 2001;98:212-217. doi: 10.1016/s0029-7844(01)01457-0
27. Allgar VL, Neal RD. Delays in the diagnosis of six cancers: analysis of data from the National Survey of NHS patients: Cancer. Br J Cancer. 2005;92:1959-1970. doi: 10.1038/sj.bjc.6602587
28. Gajjar K, Ogden G, Mujahid MI, et al. Symptoms and risk factors of ovarian cancer: a survey in primary care. Obstet Gynecol. 2012:754197. doi: 10.5402/2012/754197
29. Austoker J. Diagnosis of ovarian cancer in primary care. BMJ. 2009;339:b3286. doi: 10.1136/bmj.b3286
30. Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis: results of a national ovarian cancer survey. Cancer. 2000;89:2068-2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z
31. Smith EM, Anderson B. The effects of symptoms and delay in seeking diagnosis on stage of disease at diagnosis among women with cancers of the ovary. Cancer. 1985;56:2727-2732. doi: 10.1002/1097-0142(19851201)56:11<2727::aid-cncr2820561138>3.0.co;2-8
32. Galvin JE, Duda JE, Kaufer DI, et al. Lewy body dementia: the caregiver experience of clinical care. Parkinsonism Relat Disord. 2010;16:388-392. doi: 10.1016/j.parkreldis.2010.03.007
33. McKeith I. Dementia with Lewy bodies. Dialogues Clin Neurosci. 2004;6:333-341. doi: 10.31887/DCNS.2004.6.3/imckeith
34. Mrak RE, Griffin WS. Dementia with Lewy bodies: definitions, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatr Dis Treat. 2007;3:619-625.
35. Khotianov N, Singh R, Singh S. Lewy body dementia: case report and discussion. J Am Board Fam Pract. 2002;15:50-54.
36. Stinton C, McKeith I, Taylor JP, et al. Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry. 2015;172:731-742. doi: 10.1176/appi.ajp.2015.14121582
37. Velayudhan L, Ffytche D, Ballard C, et al. New therapeutic strategies of Lewy body dementias. Curr Neurol Neurosci Rep. 2017;17:68. doi: 10.1007/s11910-017-0778-2
38. Arnold LM, Clauw DJ, McCarberg BH; FibroCollaborative. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 2011;86:457-464. doi: 10.4065/mcp.2010.0738
39. Arnold LM, Gebke KB, Choy EHS. Fibromyalgia: management strategies for primary care providers. Int J Clin Pract. 2016;70:99-112. doi: 10.1111/ijcp.12757
40. Aloush V, Niv D, Ablin JN, et al. Good pain, bad pain: illness perception and physician attitudes towards rheumatoid arthritis and fibromyalgia patients. Clin Exp Rheumatol. 2021;39(suppl 130):54-60.
41. Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken). 2013;65:786-792. doi: 10.1002/acr.21896
42. White KP, Thompson J. Fibromyalgia syndrome in an Amish community: a controlled study to determine disease and symptom prevalence. J Rheumatol. 2003;30:1835-1840.
43. Lobo CP, Pfalzgraf AR, Giannetti V, et al. Impact of invalidation and trust in physicians on health outcomes in fibromyalgia patients. Prim Care Companion CNS Disord. 2014;16:10.4088/PCC.14m01664. doi: 10.4088/PCC.14m01664
44. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600-610. doi:10.1002/acr.20140
Strategies for improved management of hypothyroidism
The hormones thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are crucial for maintaining metabolism. A deficit of thyroid hormone production—hypothyroidism—is a common endocrine disorder seen in primary care.
Although the diagnosis and management of hypothyroidism are considered straightforward, many patients with hypothyroidism do not achieve optimal treatment goals or see an improvement in their quality of life. In this article, we address the questionable utility of screening; outline the diagnostic approach, including the central role of laboratory testing; and explain why treatment requires a precise approach to address the range of patient types.
Epidemiology and classification
Estimates are that almost 5% of Americans 12 years or older have hypothyroidism; older people and women are more likely to develop the condition. 1 In the US National Health and Nutrition Examination Survey (NHANES III) of 13,344 people without known thyroid disease or a family history, hypothyroidism was found in 4.6% (overt [clinical] in 0.3% and subclinical in 4.3%); 11% had a high serum thyroid peroxidase antibody level, which increases their risk of hypothyroidism, and is treated the same as hypothyroidism of other causes; and, overall, lower serum thyroid-stimulating hormone (TSH) levels were seen in Blacks, compared to Whites and Mexican Americans.1

Primary hypothyroidism accounts for > 95% of cases of hypothyroidism, representing a failure of the thyroid gland to produce sufficient hormone. It has been shown that, in iodine-replete countries such as the United States, the prevalence of spontaneous hypothyroidism is 1% to 2%, and it is 10 times more common in women.2,3
Central hypothyroidism is caused by insufficient stimulation of the thyroid gland by TSH, due to pituitary (secondary hypothyroidism) or hypothalamic (tertiary hypothyroidism) disease and is estimated to occur in 1 in every 20,000 to 80,000 people in the general population.4
How does hypothyroidism manifest?
Signs and symptoms. Manifestations of hypothyroidism range from life-threatening to minimal or no clinical signs and symptoms (TABLE W1). Signs and symptoms of low thyroid function vary by the degree of hypothyroidism at presentation.
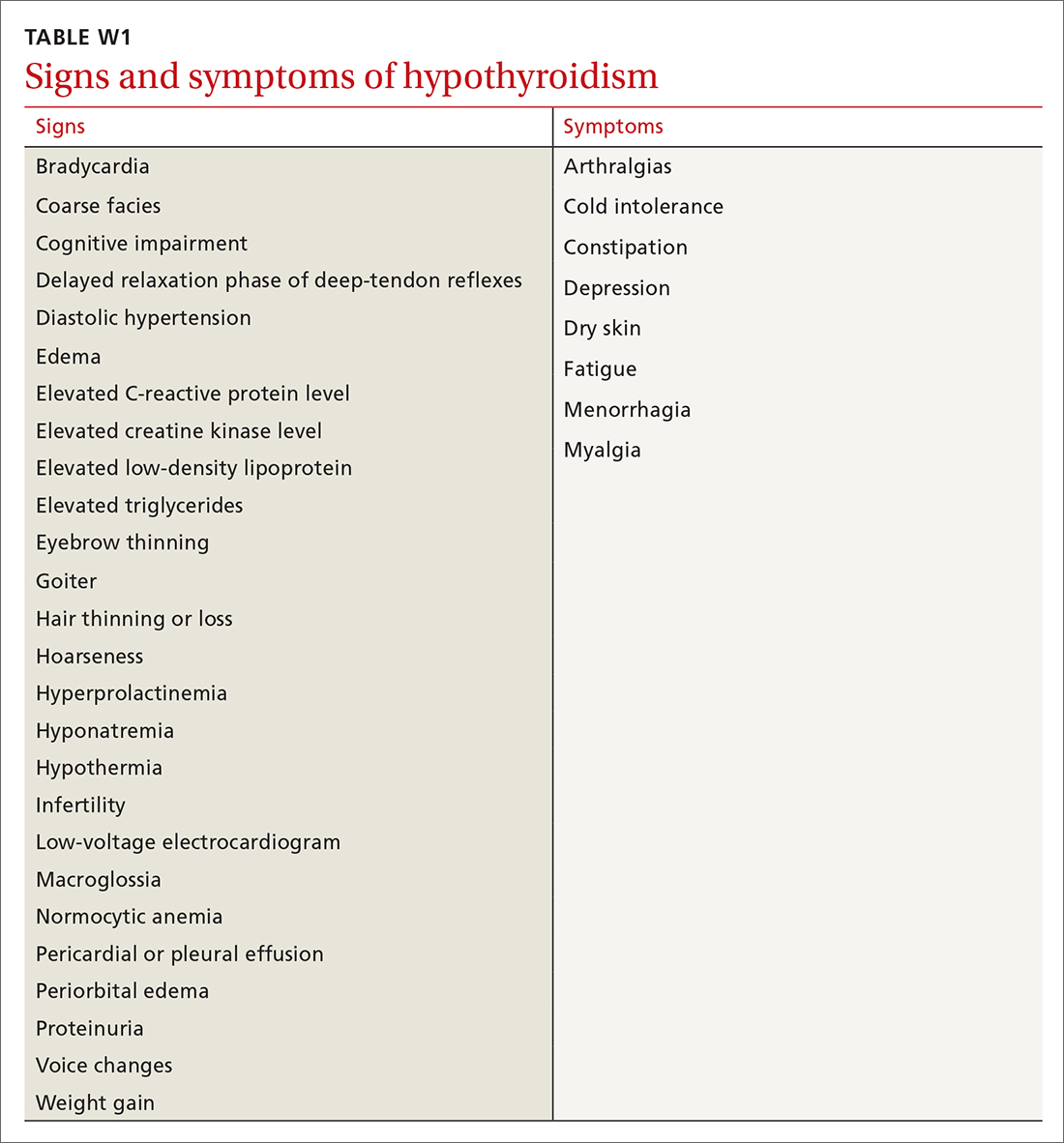
Common signs and symptoms of low thyroid function include fatigue, weight gain, dry skin, brittle hair, hair loss, morning stiffness, muscle aches, joint pain, cold intolerance, diffuse headache, constipation, difficulty concentrating, low libido, depression, and menstrual irregularities. On physical examination, a patient might present with bradycardia, hypotension, hypothermia with slow speech or movement, coarse facial appearance, goiter, diffuse hair loss, cold hands and feet, and a prolonged Achilles tendon reflex.5 Skin findings, such as keratosis pilaris, palmoplantar keratoderma (thickening of the skin), and pityriasis rubra pilar, can be associated with autoimmune hypothyroidism.6,7
Continue to: Carpal tunnel syndrome...
Carpal tunnel syndrome, plantar fasciitis, infertility or miscarriage, dyspepsia, and small intestinal bacterial overgrowth can be associated with hypothyroidism; thyroid function should therefore be assessed in patients who have any of these conditions, along with other signs and symptoms of low thyroid function.8,9 A patient with severe hypothyroidism might present with hemodynamic instability, pericardial or pleural effusion, and myxedema coma.10
Clues in the history and from the lab. A history of radiation to the head, neck, or chest area and a history or family history of autoimmune disorders are risk factors for autoimmune thyroid disease.11,12 Laboratory findings can include markers of oxidative stress, such as elevated levels of low-density lipoprotein cholesterol and serum malondialdehyde.13-15
Screening and diagnosis
Screening. The US Preventive Services Task Force has asserted that evidence is insufficient by which to evaluate the benefits and risks of routine screening for thyroid dysfunction in nonpregnant, asymptomatic adults.16 According to the American Thyroid Association and the American Association of Clinical Endocrinologists, screening should be considered in high-risk patients, including those who take medication that affects thyroid function or the results of thyroid hormone assays (TABLE W2, available at mdedge.com/familymedicine).17-20
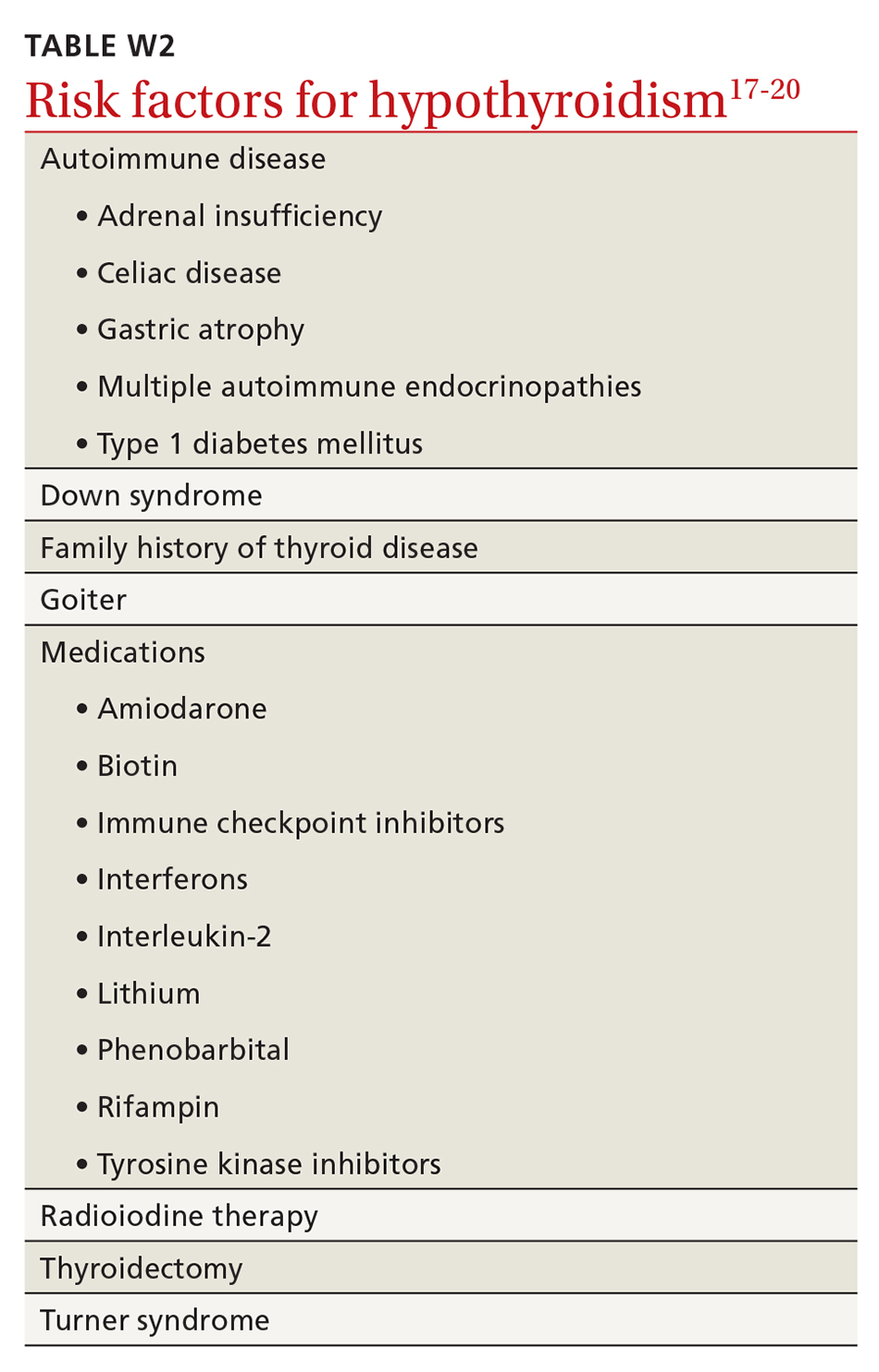
Screening inpatients is challenging and usually not recommended unless thyroid disease is strongly suspected. This is because changes in the levels of thyroid hormones, binding proteins, and the TSH concentration can occur in severe nonthyroidal illness; in addition, assay interference by antibodies and other substances can affect thyroid hormone measurement.21
Testing strategy. Generally, screening and diagnosis of hypothyroidism are based primarily on laboratory testing, because signs and symptoms are nonspecific (FIGURE 15). A serum TSH level is usually the initial test when screening for thyroid dysfunction. A normal serum TSH value ranges from 0.5-5.0 mIU/L.
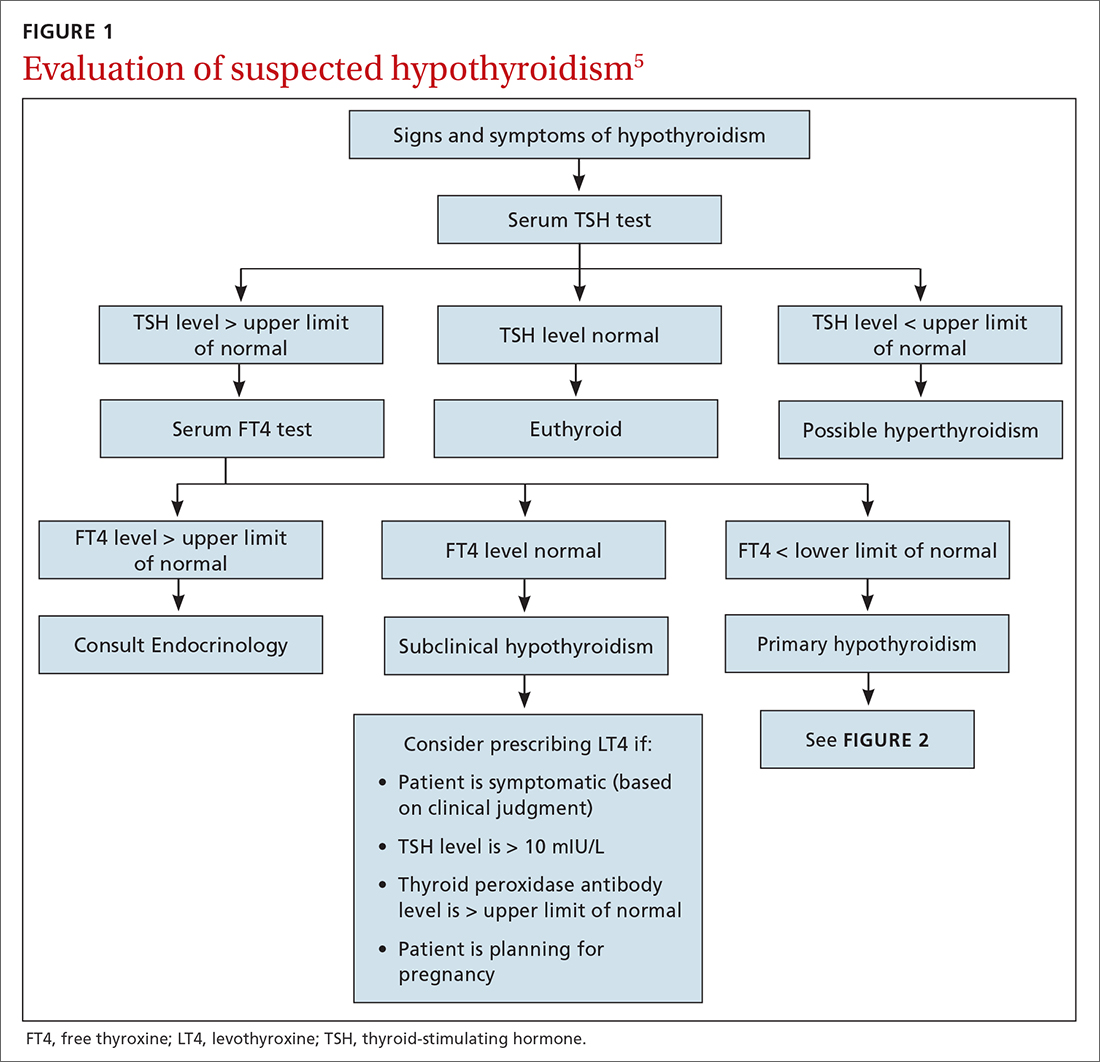
When an abnormal serum TSH level is found, further tests can be performed to investigate, including a serum free thyroxine (FT4) test. (Our preference is to order TSH and FT4 assays simultaneously to facilitate and confirm the diagnosis.) An FT4 test measures the amount of unattached, or free, thyroxine in blood by immunoassay. A normal FT4 value usually ranges from 0.7-1.9 ng/dL.
The combination of a high TSH level and a low FT4 level could be an indication of an underactive thyroid gland (ie, clinical or overt hypothyroidism). Milder, subclinical hypothyroidism is characterized by a higher-than-normal TSH level but a normal FT4 level.22 Central (secondary) hypothyroidism is characterized by a low serum FT4 level and a serum TSH level that can be below the reference range, low normal, or even slightly high.4
Continue to: These measurements...
These measurements must be interpreted within the context of the laboratory-specific normal range for each test. Third-generation serum TSH assays are more sensitive and specific than serum FT4 measurements for hypothyroidism. FT4 is usually measured by automated analogue immunoassay, which generally provides reliable results; abnormal binding proteins or other interferences occur in some patients, however, resulting in reporting of a falsely high, or falsely low, FT4 level. In such cases, FT4 by direct dialysis, or total T4, can be measured for further evaluation. In primary care, you are most likely to encounter primary hypothyroidism; secondary (central) hypothyroidism is much rarer (< 5% of cases).4
The ins and outs of treatment
For most patients, hypothyroidism is a permanent disorder requiring lifelong thyroid hormone replacement therapy—unless the disease is transient (ie, painless or subacute thyroiditis); reversible, because it is caused by medication; or responsive to medical intervention that addresses the underlying autoimmune condition.19 Goals of treatment (Figure 25,23) are to:
- normalize the TSH level to 0.5-5.0 mIU/L (the main goal), with an age-related shift toward a higher TSH goal in older patients (and an upper limit of normal of 7.5 mIU/L in patients who are ≥ 80 years of age)20
- restore the euthyroid state
- relieve symptoms
- reduce any goiter
- avoid overtreatment (iatrogenic thyrotoxicosis).
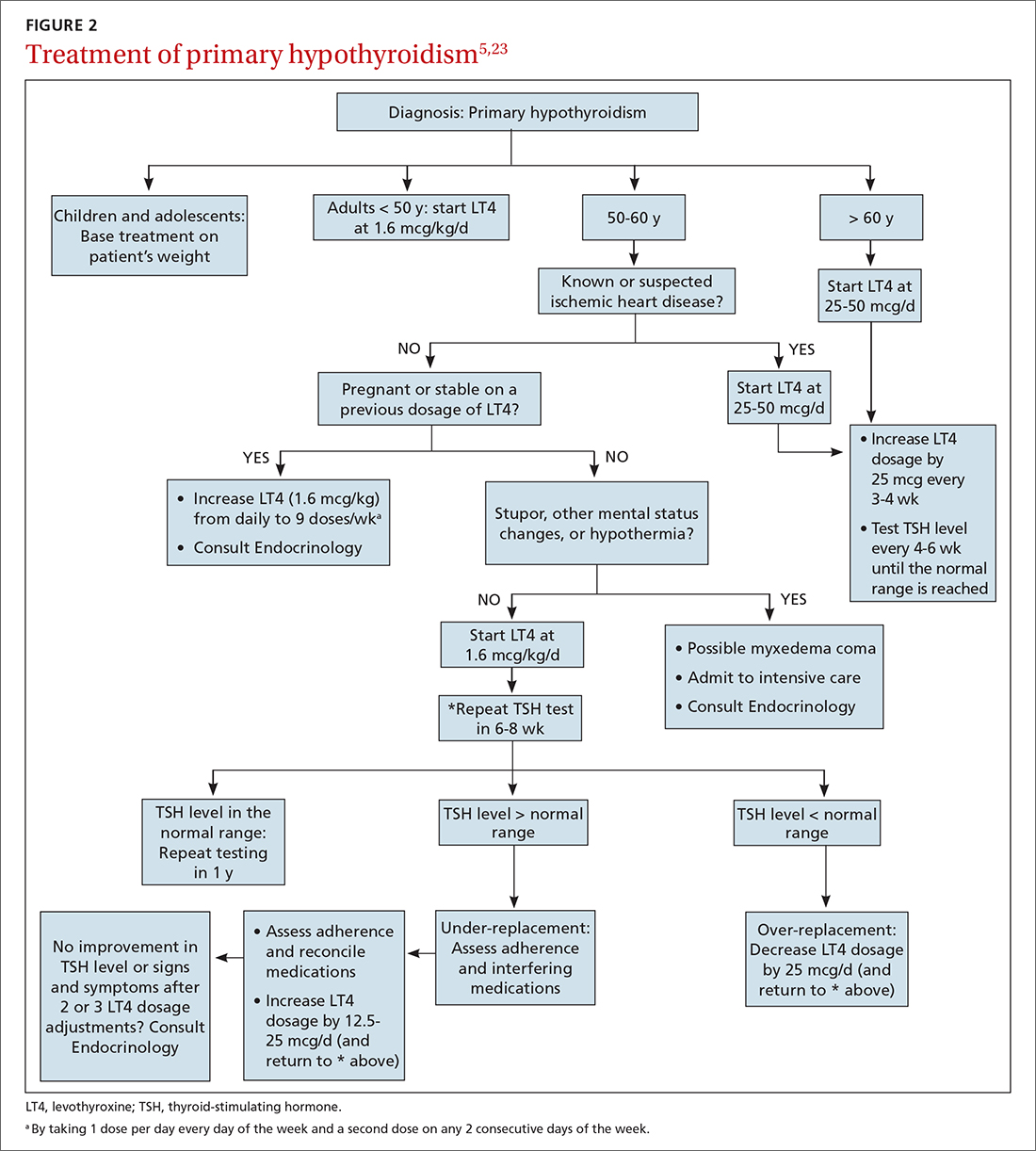
Desiccated thyroid extract (DTE), developed in the late 1880s and made from the dried thyroid gland of pigs, sheep, or cows, was the earliest treatment for hypothyroidism. The use of DTE has declined since the introduction of synthetic thyroxine (T4, or levothyroxine [here, referred to as LT4]), which is now the standard treatment.20-22 LT4 is deiodinated in peripheral tissues to form T3, the active thyroid hormone; this process accounts for 80% of total T3 production daily.24
LT4 formulations. LT4 is commercially available in tablet, soft-gel, and liquid preparations. Most patients are treated with the tablet; the soft-gel capsule or liquid is an option for patients who absorb the tablet poorly (because of atrophic gastritis, celiac disease, or gluten sensitivity or because they are post bariatric surgery). Increasing the dosage of the tablet form of LT4, with ongoing TSH monitoring, is more cost effective than moving to an alternative preparation.
If a switch of LT4 formulation is made (ie, from one manufacturer to another), test the serum TSH level to ensure that the therapeutic goal is being reached. Also, in our experience, it is best to prescribe a brand-name preparation of levothyroxine, not a generic, whenever possible, due to the variability in generic formulations and the potential presence of other (inert) ingredients.25
Dosing (TABLE 320,23). The average full replacement dosage of LT4 for a young, healthy adult is approximately 1.6 mcg/kg/d. Older patients (> 65 years) or those with coronary artery disease (CAD) should be started on a lower dosage (25-50 mcg/d) and titrated to goal accordingly.
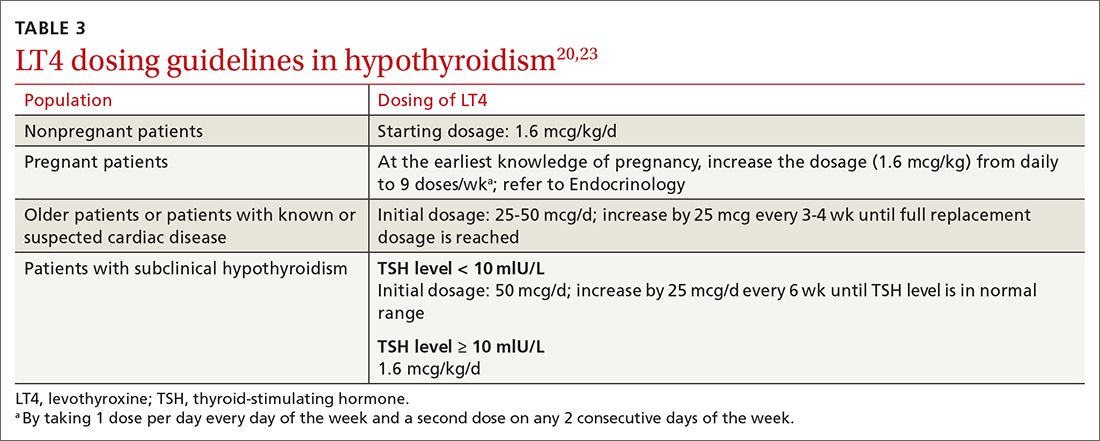
LT4 (tablets, soft-gel capsules, or liquid) should be administered on an empty stomach, with water only, 30 to 60 minutes before breakfast. Medications that interfere with LT4 absorption (eg, bile acid resins, calcium carbonate, ferrous sulfate) should be taken several hours after LT4. For patients who cannot take LT4 in the morning, taking it at bedtime (≥ 2-3 hours after the last meal) is acceptable.
Continue to: Monitoring and titrating
Monitoring and titrating. Hypothyroid symptoms usually improve after 2 or 3 weeks of LT4 treatment; in severe hypothyroidism, complete recovery might take months. Approximately 6 weeks after LT4 therapy is initiated, serum TSH should be measured. After assessing whether administration of LT4 at the starting dosage is appropriate, that dosage can be increased, or decreased, every 4 to 6 weeks until the TSH goal is reached. Once the patient is maintained at a given dosage, measure serum TSH once a year—more often if there is an abnormal result or a change in the patient’s health status.23
Adverse effects of LT4 therapy are rare, unless over-replacement occurs. Rarely, patients have an allergy to the dye or an excipient (filler) in the tablet.26-28 The white, 50-mcg tablets can be given safely to patients with dye sensitivity. For those who have an allergy to an excipient (except gelatin) or gluten intolerance, the LT4 soft-gel capsule or liquid preparation (Tirosint) can be prescribed.
Pure LT4, in a capsule made from vegetable sources, can be ordered through a compounding pharmacy for patients who are allergic to animal products.
Anemia, especially iron-deficiency anemia, can cause intolerance to LT4 therapy; in such patients, lowering the starting dosage and treating anemia are indicated.29
Persistent symptoms (despite a normal TSH level). Because many hypothyroid symptoms are nonspecific, patients might come to think that their LT4 dosage is inadequate if they feel tired or gain weight. Persistent hypothyroid symptoms despite a normal serum TSH level might be due to (1) the inability of LT4 therapy to restore tissue thyroid hormone levels to normal or (2) other variables unrelated to hypothyroidism, including disorders associated with inflammation or autoimmune disease, certain medications, diet, lifestyle, and environmental toxins.
These patients might benefit from a detailed history to identify other causes and a switch to either LT4 + liothyronine (LT3; synthetic T3) combination therapy or DTE26,30-33 (TABLE 434), although a beneficial effect of LT4 + LT3 therapy was not seen in several studies.35,36 Over-replacement with LT4 should be discouraged, due to concerns about thyrotoxicosis and its complications (eg, atrial fibrillation, accelerated bone loss).
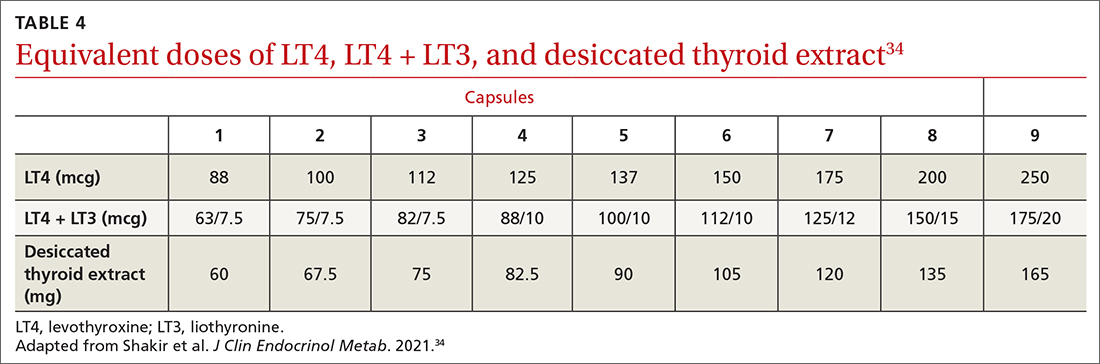
DTE and LT4 + LT3. Use of DTE has decreased since the 1970s, when LT4 became the therapy of choice. Subsequently, anecdotal evidence emerged that some patients did not feel well on LT4 and preferred to return to DTE.32,33
Continue to: Several clinical trials...
Several clinical trials addressed the question of whether residual symptoms could be resolved through LT4 + LT3 combination therapy31-39 (TABLE 434), but evidence of any consistent superiority of combination therapy was not demonstrated.35-39 In selected cases, patients might prefer the combination approach.31,33,39 The quality of life of hypothyroid patients was found to be similarly improved with LT4 or DTE, but the latter was associated with modest weight loss (approximately 4 lbs); nearly 50% of study patients preferred treatment with DTE over LT4.33 A follow-up study did not confirm weight loss with DTE, however.34
When LT4 monotherapy and LT4 + LT3 combination therapy were compared, results were mixed31-39; responsiveness to therapy containing LT3 might therefore depend on multiple variables, including genetic background, nutritional and lifestyle factors, stress, presence of comorbidities and autoimmune disorders, and other unidentified or poorly defined variables.40-48
Although combination therapy and DTE are not generally recommended over LT4 monotherapy, they might offer better options for patients who are still symptomatic when being treated with LT4 only: In a randomized, double-blind, crossover study that compared LT4 with DTE and with LT4 + LT3, one-third of the most highly symptomatic patients who had low scores on mood, cognitive, and quality-of-life assessments improved significantly after they were switched to combination therapy or DTE.34
The 2014 American Thyroid Association guidelines24 do not support routine use of LT4 + LT3 in hypothyroid patients who have residual symptoms after LT4 monotherapy; however, a therapeutic trial of LT4 + LT3, while maintaining a normal serum TSH, is reasonable in selected patients. Candidates for DTE or LT4 + LT3 might include patients who do not feel well on LT4 monotherapy, are post thyroidectomy or post radioiodine therapy, or have a low serum T3 level. DTE and combination therapy are discouraged in older patients, patients who have underlying CAD, and pregnant patients.
Special treatment circumstances
A number of patient variables have the potential to alter management strategies for hypothyroidism.18,20,23,40,49-53
Age, comorbidity. Older patients (> 65 years) and patients with cardiopulmonary disease or CAD should be treated with LT4, 25 to 50 mcg/d, initially; that dosage can be titrated upward by 12.5 to 25 mcg/d every 4 to 6 weeks until the TSH goal is reached—preferably, in the range of 4 to 8 mIU/L. An increase in the dosage of LT4 might be required in the presence of malabsorption (eg, gastrointestinal disorders, celiac disease) and in nephrotic syndrome.18,20,23
Body weight. A decrease in the dosage of LT4 might be indicated in the setting of significant weight loss (> 10% body weight).23
Continue to: Co-pharmacy
Co-pharmacy. An increase in the dosage of LT4 might be required when other drugs (eg, phenytoin, phenobarbital, rifampin, and carbamazepine) have led to an increased rate of thyroid hormone metabolism. A decrease in the dosage of LT4 might be necessary after initiation of androgen therapy.23
Pregnancy. Women with pre-existing hypothyroidism require an increase of 25% to 50% in their LT4 dosage during pregnancy to maintain a TSH level in the recommended pregnancy reference range. Thyroid function should be monitored every 4 to 6 weeks to ensure that the TSH target for each trimester is reached (first trimester, 0.1-4 mIU/L; second trimester, 0.2-4 mIU/L; third trimester, 0.3-4 mIU/L). Postpartum, LT4 can be reduced to the prepartum dosage; TSH should be checked every 4 to 6 weeks to maintain the TSH goal.23
Estrogen therapy. Hypothyroid women who are receiving estrogen therapy might require an increase in their LT4 dosage because serum thyroxine-binding globulin levels are increased by estrogens or through other mechanisms that have not been identified.23
Surgical candidacy. Observational studies show few adverse outcomes in surgical patients with mild (subclinical) hypothyroidism or moderate hypothyroidism; however, the risk of adverse surgical outcome might be increased in patients with severe hypothyroidism. For patients in whom surgery is planned and who have:
- subclinical hypothyroidism (elevated TSH and normal FT4), we recommend that surgery—urgent or elective—not be posptoned but proceed.
- moderate (overt) hypothyroidism who require urgent surgery, we recommend not postponing surgery, even though minor perioperative complications might develop. Such patients should be treated with LT4 as soon as the diagnosis for which surgery is required has been made. Alternatively, when moderate hypothyroidism is discovered in a patient who is being evaluated for elective surgery, we recommend postponing surgery until the euthyroid state is restored.
- severe hypothyroidism (myxedema coma [discussed in a bit]; severe clinical symptoms of chronic hypothyroidism, such as altered mental status, pericardial effusion, or heart failure; or a very low level of T4), surgery should be delayed until hypothyroidism has been treated. When emergency surgery is required for a severely hypothyroid patient, they should be treated with LT4 as soon as the diagnosis for which surgery is indicated has been made. When emergency surgery must be performed in a patient with myxedema coma, we recommend treatment with LT4 + LT3, rather than LT4 alone, often administered intravenously because LT4 is poorly absorbed in these patients.
Nonadherence. For patients who do not take LT4 regularly or do not respond to efforts to improve adherence, LT4 can be given weekly, instead of daily, although this interval is not ideal. Weekly dosing should not be used in older patients with CAD.23
Thyroid cancer. Patients who are post total thyroidectomy for thyroid cancer need to take LT4 to treat hypothyroidism and to prevent recurrence of thyroid cancer. The goal TSH level should be based on the cancer stage and risk of recurrence and should be monitored by an endocrinologist.
Myxedema coma. This medical emergency has high mortality. Myxedema coma occurs when severe hypothyroidism leads to any, or a combination, of the following: diminished mentation; hypothermia; bradycardia; hyponatremia; hypotension; cardiovascular, respiratory, and gastrointestinal dysfunction; and renal insufficiency. LT4, LT3, and glucocorticoids should be administered intravenously and the patient monitored closely—preferably in consultation with an endocrinologist.
Continue to: When to seek consultation
When to seek consultation
A patient with hypothyroidism should be referred to Endocrinology if they are < 18 years of age, pregnant, unresponsive to therapy, or have cardiac disease, coexisting endocrine disease, suspected myxedema coma, goiter or thyroid nodules, or a structural thyroid abnormality.
What we know about nutrition and hypothyroidism
Although it is commonly recognized that iodine is essential for production of thyroid hormone, other nutritional factors might contribute to proper production of thyroid hormones, including:
- adequate intake of iron, tyrosine, selenium, zinc, and vitamins E, B2, B3, B6, C, and D44,45
- selenium and zinc, which increase conversion of T4 to T3 and might be important in the management of hypothyroid patients40,46
- vitamin A, zinc, and regular exercise, which have been shown to improve cellular sensitivity to thyroid hormones.
Low iron stores can contribute to persistent symptoms and poor quality of life in patients with hypothyroidism, despite their being treated according to guidelines.29,47
Despite what is known about these nutritional connections, there is insufficient evidence that improving nutrition can reverse hypothyroidism.
Prevention
Prevention of hypothyroidism should take into account variables that affect or inhibit thyroid function, such as stress, infection (eg, Epstein-Barr virus), excessive fluoride intake, toxins (eg, pesticides, solvents, mercury, cadmium, and lead), autoimmune disease (eg, celiac disease), and food sensitivity.54,55 Oxidative stress can also cause thyroid impairment.40-48,54-58
Otherwise, there are, at present, no effective strategies for preventing thyroid disorders.
Subclinical hypothyroidism: Elusive management target
Subclinical hypothyroidism is defined as a normal serum FT4 level in the presence of an elevated serum TSH level. The prevalence of subclinical hypothyroidism varies from 3% to 15%, depending on the population studied; a higher incidence has been noted in women and older people.59 In the NHANES III,1 which excluded people with previously diagnosed thyroid disease, the incidence of subclinical hypothyroidism was 4.3%.
Continue to: Causes of subclinical...
Causes of subclinical hypothyroidism are the same as those of overt hypothyroidism, and include Hashimoto disease. The combination of an elevated TSH level and a normal FT4 level is associated with disorders characterized by protein-binding variations (eg, pregnancy, genetic disorders, drugs), TSH-secreting pituitary adenoma, class II and III obesity (respectively, body mass index, ≥ 35 but < 40 and ≥ 40), and assay variability.49,51
Lab diagnosis: Fraught with difficulty
The serum TSH level and either the total T4 level or the FT4 level should be measured to make a diagnosis of subclinical hypothyroidism. Most laboratories use a 1-step analogue immunoassay to determine free thyroid hormones; protein-binding variations can thus affect measurement of FT4.
Several scenarios that can result in inaccurate measurement of FT4 by radioimmunoassay include genetic disorders that affect binding proteins; pregnancy; use of certain drugs, including heparin, furosemide, antiepileptic agents, salicylate, ferrous sulfate, and cholesterol-binding resins; and some medical conditions, including cardiac surgery, critical illness, and renal failure. Variables that inhibit proper production of thyroid hormones—stress, infection, fluoride (an iodine antagonist), toxins (pesticides, mercury, cadmium, lead) and autoimmune conditions, such as celiac disease—should be considered when attempting to determine the cause of subclinical hypothyroidism.
Liquid chromatography–mass spectrometry measurement of thyroid hormones might be more accurate than immunoassay.53 Measuring serum total T4 and FT4 by dialysis, free from interfering proteins, might also be useful when measurement of FT4 by immunoassay is affected by binding-protein variations.
Features of subclinical hypothyroidism
Most patients who have subclinical hypothyroidism and a serum TSH level < 10 mIU/L are asymptomatic. Some might have nonspecific symptoms of hypothyroidism, however, such as reduced quality of life, poor cognitive function, and poor memory—symptoms that do not typically correlate with the serum TSH level.
It has been suggested that some elderly people normally have a higher level of serum TSH, and that they might have even a prolonged lifespan.51 Additionally, it has been shown that, in nonpregnant adult patients with subclinical hypothyroidism and a serum TSH level of 4.5 to 10 mIU/L, treatment with LT4 was not associated with improvement in thyroid-related symptoms or general quality of life.52
Treat, or don't treat, subclinical hypothyroidism?
It is well accepted that the goal of therapy in hypothyroid patients is to normalize the serum TSH level; however, the American Thyroid Association and the American Association of Clinical Endocrinology recommend starting LT4 in patients with a serum TSH level ≥ 10 mIU/L (TABLE 5).59-62 The principal reason for not treating subclinical hypothyroidism is the lack of benefit in reducing the risk of cardiovascular morbidity and mortality when the TSH level is between 7.5 and 10 mIU/L.62
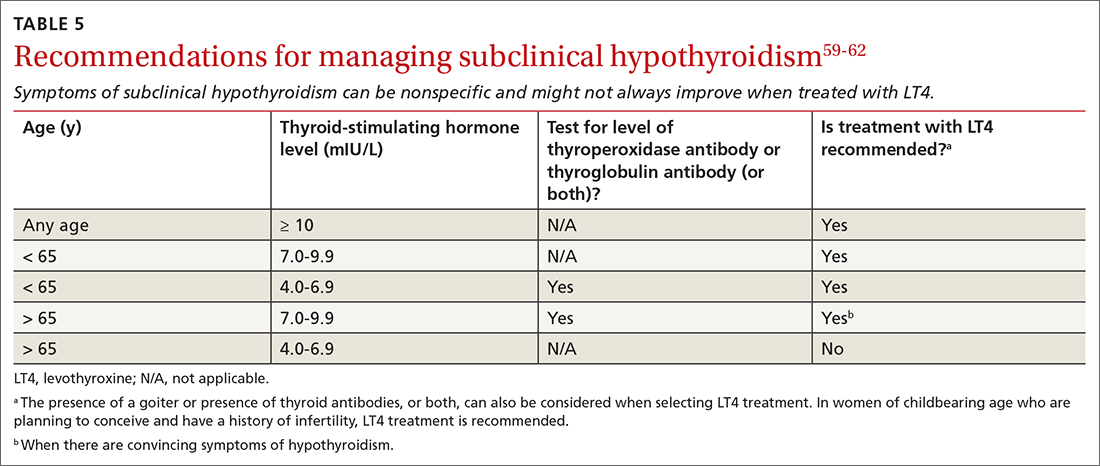
Continue to: Routine treatment
Routine treatment of patients with a serum TSH level of 4.5 to 10 mIU/L remains controversial. When TSH is 7.0 to 9.9 mIU/L, treatment is recommended for (1) patients < 65 years and (2) for older patients (> 65 years) only when there are convincing hypothyroid symptoms because of concern about unintended overtreatment.
When the TSH level is anywhere above the upper limit of normal to 6.9 mIU/L, treatment is recommended for patients < 65 years old, patients who have a high titer of thyroid peroxidase antibodies, and patients with goiter—but not for patients > 65 years (and, especially, not for octogenarians) because their upper limit of normal could be as high as 6 to 8 mIU/L, especially if they are otherwise healthy.
Treatment should be considered for women with subclinical hypothyroidism who are trying to conceive or experiencing an infertility problem.
For patients with subclinical hypothyroidism who are not being treated, monitor thyroid function every 6 to 12 months by testing TSH and FT4.
CORRESPONDENCE
Thanh D. Hoang, DO, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499. doi: 10.1210/jcem.87.2.8182
2. Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51. doi: 10.1093/bmb/ldr030
3. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
4. Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. doi: 10.1210/jc.2012-1616
5. Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am. 2012;96:203-221. doi: 10.1016/j.mcna.2012.01.005
6. Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-659. doi: 10.1067/mjd.2003.257
7. Franzotti AM, Avelar JCD, Cardoso TA, et al. Pityriasis rubra pilar and hypothyroidism. An Bras Dermatol. 2014;89:497-500. doi: 10.1590/abd1806-4841.20142994
8. Yaylali O, Kirac S, Yilmaz M, et al. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802
9. Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18:307-309.
10. Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27:117-122. doi: 10.1016/j.je.2016.04.002
11. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5. doi: 10.1016/j.radonc.2011.03.002
12. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-e9. doi: 10.1016/j.amjmed.2009.06.030
13. Cheserek MJ, Wu G-R, Ntazinda A, et al. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;34:323-331. doi: 10.2478/jomb-2014-0044
14. Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4
15. Tejovathi B, Suchitra MM, Suresh V, et al. Association of lipid oxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes. 2013;121:306-309. doi: 10.1055/s-0032-1333298
16. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
17. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562. doi: 10.1016/S0140-6736(17)30703-1
18. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801
19. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243-1251. doi: 10.1089/thy.2018.0116
20. Garber JR, Cobin RH, Gharib H, et al; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375-388. doi: 10.1016/j.ccc.2018.11.012
22. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376:2556-2565. doi: 10.1056/NEJMcp1611144
23. Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(suppl 2):59-71. doi: 10.1007/s12325-019-01079-1
24. Jonklaas J, Bianco AC, Bauer AJ, et al; . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
25. Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984;5:151-184. doi: 10.1210/edrv-5-2-151
26. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020;105:e3090-e3104. doi: 10.1210/clinem/dgaa430
27. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6:e101-e104. doi: 10.4158/ACCR-2019-0495
28. Chamorro-Pareja N, Carrillo-Martin I, Haehn DA, et al. Self-reported allergy to thyroid replacement therapy: a multicenter retrospective chart review. Endocr Pract. 2020;26:761-767. doi: 10.4158/EP-2019-0488
29. Shakir MKM, Turton D, Aprill BS, et al. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin Proc. 2000;75:189-192.
30. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31:156-182. doi: 10.1089/thy.2020.0720
31. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666-2674. doi: 10.1210/jc.2004-2111
32. Escobar-Morreale HF, Botella-Carretero JI, M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412-424. doi: 10.7326/0003-4819-142-6-200503150-00007
33. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982-1990. doi: 10.1210/jc.2012-4107
34. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106:e4400-e4413. doi: 10.1210/clinem/dgab478
35. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34:80-89. doi: 10.1080/07435800903156340
36. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543-4550. doi: 10.1210/jc.2003-030249
37. Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223-233. doi: 10.4158/EP.11.4.223
38. Sawka AM, Gerstein HC, Marriott MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551-4555. doi: 10.1210/jc.2003-030139
39. Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290:2952-2958. doi: 10.1001/jama.290.22.2952
40. Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. doi: 10.1210/jc.2010-0191
41. Winther KH, Wichman JEM, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376-385. doi: 10.1007/s12020-016-1098-z
42. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741
43. Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015,7:2485-2498. doi: 10.3390/nu7042485
44. Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet. 2016;116:302-310.e1. doi: 10.1016/j.jand.2015.09.020
45. Babiker A, Alawi A, Al Atawi M, et al. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20:13-19. doi: 10.24911/SJP.106-1587138942
46. Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769
47. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34-44. doi: 10.1017/S0029665118001192
48. Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674-678. doi: 10.4103/2230-8210.190555
49. Tseng F-Y, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730-737. doi: 10.1016/j.jacc.2012.03.047
50. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573-581. doi: 10.7326/0003-4819-145-8-200610170-00006
51. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591-2599. doi: 10.1001/jama.292.21.2591
52. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
53. Monzani F, Dardano A, Caraccio N. Does treating subclinical hypothyroidism improve markers of cardiovascular risk? Treat Endocrinol. 2006;5:65-81. doi: 10.2165/00024677-200605020-00001
54. Duntas LH. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190-191. doi: 10.1038/nrendo.2009.46
55. Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6:R52-R58. doi: 10.1530/EC-17-0021
56. Janegova A, Janega P, Rychly B, et al. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66:132-136. doi: 10.5603/EP.2015.0020
57. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761. doi: 10.1089/thy.2010.1636
58. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. doi: 10.1016/j.biocel.2006.07.001
59. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028. doi: 10.4158/EP12280.GL
60. Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. 2016;175:R255-R263. doi: 10.1530/EJE-16-0193
61. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65-68. doi: 10.1016/j.ejim.2017.11.010
62. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
The hormones thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are crucial for maintaining metabolism. A deficit of thyroid hormone production—hypothyroidism—is a common endocrine disorder seen in primary care.
Although the diagnosis and management of hypothyroidism are considered straightforward, many patients with hypothyroidism do not achieve optimal treatment goals or see an improvement in their quality of life. In this article, we address the questionable utility of screening; outline the diagnostic approach, including the central role of laboratory testing; and explain why treatment requires a precise approach to address the range of patient types.
Epidemiology and classification
Estimates are that almost 5% of Americans 12 years or older have hypothyroidism; older people and women are more likely to develop the condition. 1 In the US National Health and Nutrition Examination Survey (NHANES III) of 13,344 people without known thyroid disease or a family history, hypothyroidism was found in 4.6% (overt [clinical] in 0.3% and subclinical in 4.3%); 11% had a high serum thyroid peroxidase antibody level, which increases their risk of hypothyroidism, and is treated the same as hypothyroidism of other causes; and, overall, lower serum thyroid-stimulating hormone (TSH) levels were seen in Blacks, compared to Whites and Mexican Americans.1

Primary hypothyroidism accounts for > 95% of cases of hypothyroidism, representing a failure of the thyroid gland to produce sufficient hormone. It has been shown that, in iodine-replete countries such as the United States, the prevalence of spontaneous hypothyroidism is 1% to 2%, and it is 10 times more common in women.2,3
Central hypothyroidism is caused by insufficient stimulation of the thyroid gland by TSH, due to pituitary (secondary hypothyroidism) or hypothalamic (tertiary hypothyroidism) disease and is estimated to occur in 1 in every 20,000 to 80,000 people in the general population.4
How does hypothyroidism manifest?
Signs and symptoms. Manifestations of hypothyroidism range from life-threatening to minimal or no clinical signs and symptoms (TABLE W1). Signs and symptoms of low thyroid function vary by the degree of hypothyroidism at presentation.

Common signs and symptoms of low thyroid function include fatigue, weight gain, dry skin, brittle hair, hair loss, morning stiffness, muscle aches, joint pain, cold intolerance, diffuse headache, constipation, difficulty concentrating, low libido, depression, and menstrual irregularities. On physical examination, a patient might present with bradycardia, hypotension, hypothermia with slow speech or movement, coarse facial appearance, goiter, diffuse hair loss, cold hands and feet, and a prolonged Achilles tendon reflex.5 Skin findings, such as keratosis pilaris, palmoplantar keratoderma (thickening of the skin), and pityriasis rubra pilar, can be associated with autoimmune hypothyroidism.6,7
Continue to: Carpal tunnel syndrome...
Carpal tunnel syndrome, plantar fasciitis, infertility or miscarriage, dyspepsia, and small intestinal bacterial overgrowth can be associated with hypothyroidism; thyroid function should therefore be assessed in patients who have any of these conditions, along with other signs and symptoms of low thyroid function.8,9 A patient with severe hypothyroidism might present with hemodynamic instability, pericardial or pleural effusion, and myxedema coma.10
Clues in the history and from the lab. A history of radiation to the head, neck, or chest area and a history or family history of autoimmune disorders are risk factors for autoimmune thyroid disease.11,12 Laboratory findings can include markers of oxidative stress, such as elevated levels of low-density lipoprotein cholesterol and serum malondialdehyde.13-15
Screening and diagnosis
Screening. The US Preventive Services Task Force has asserted that evidence is insufficient by which to evaluate the benefits and risks of routine screening for thyroid dysfunction in nonpregnant, asymptomatic adults.16 According to the American Thyroid Association and the American Association of Clinical Endocrinologists, screening should be considered in high-risk patients, including those who take medication that affects thyroid function or the results of thyroid hormone assays (TABLE W2, available at mdedge.com/familymedicine).17-20

Screening inpatients is challenging and usually not recommended unless thyroid disease is strongly suspected. This is because changes in the levels of thyroid hormones, binding proteins, and the TSH concentration can occur in severe nonthyroidal illness; in addition, assay interference by antibodies and other substances can affect thyroid hormone measurement.21
Testing strategy. Generally, screening and diagnosis of hypothyroidism are based primarily on laboratory testing, because signs and symptoms are nonspecific (FIGURE 15). A serum TSH level is usually the initial test when screening for thyroid dysfunction. A normal serum TSH value ranges from 0.5-5.0 mIU/L.

When an abnormal serum TSH level is found, further tests can be performed to investigate, including a serum free thyroxine (FT4) test. (Our preference is to order TSH and FT4 assays simultaneously to facilitate and confirm the diagnosis.) An FT4 test measures the amount of unattached, or free, thyroxine in blood by immunoassay. A normal FT4 value usually ranges from 0.7-1.9 ng/dL.
The combination of a high TSH level and a low FT4 level could be an indication of an underactive thyroid gland (ie, clinical or overt hypothyroidism). Milder, subclinical hypothyroidism is characterized by a higher-than-normal TSH level but a normal FT4 level.22 Central (secondary) hypothyroidism is characterized by a low serum FT4 level and a serum TSH level that can be below the reference range, low normal, or even slightly high.4
Continue to: These measurements...
These measurements must be interpreted within the context of the laboratory-specific normal range for each test. Third-generation serum TSH assays are more sensitive and specific than serum FT4 measurements for hypothyroidism. FT4 is usually measured by automated analogue immunoassay, which generally provides reliable results; abnormal binding proteins or other interferences occur in some patients, however, resulting in reporting of a falsely high, or falsely low, FT4 level. In such cases, FT4 by direct dialysis, or total T4, can be measured for further evaluation. In primary care, you are most likely to encounter primary hypothyroidism; secondary (central) hypothyroidism is much rarer (< 5% of cases).4
The ins and outs of treatment
For most patients, hypothyroidism is a permanent disorder requiring lifelong thyroid hormone replacement therapy—unless the disease is transient (ie, painless or subacute thyroiditis); reversible, because it is caused by medication; or responsive to medical intervention that addresses the underlying autoimmune condition.19 Goals of treatment (Figure 25,23) are to:
- normalize the TSH level to 0.5-5.0 mIU/L (the main goal), with an age-related shift toward a higher TSH goal in older patients (and an upper limit of normal of 7.5 mIU/L in patients who are ≥ 80 years of age)20
- restore the euthyroid state
- relieve symptoms
- reduce any goiter
- avoid overtreatment (iatrogenic thyrotoxicosis).

Desiccated thyroid extract (DTE), developed in the late 1880s and made from the dried thyroid gland of pigs, sheep, or cows, was the earliest treatment for hypothyroidism. The use of DTE has declined since the introduction of synthetic thyroxine (T4, or levothyroxine [here, referred to as LT4]), which is now the standard treatment.20-22 LT4 is deiodinated in peripheral tissues to form T3, the active thyroid hormone; this process accounts for 80% of total T3 production daily.24
LT4 formulations. LT4 is commercially available in tablet, soft-gel, and liquid preparations. Most patients are treated with the tablet; the soft-gel capsule or liquid is an option for patients who absorb the tablet poorly (because of atrophic gastritis, celiac disease, or gluten sensitivity or because they are post bariatric surgery). Increasing the dosage of the tablet form of LT4, with ongoing TSH monitoring, is more cost effective than moving to an alternative preparation.
If a switch of LT4 formulation is made (ie, from one manufacturer to another), test the serum TSH level to ensure that the therapeutic goal is being reached. Also, in our experience, it is best to prescribe a brand-name preparation of levothyroxine, not a generic, whenever possible, due to the variability in generic formulations and the potential presence of other (inert) ingredients.25
Dosing (TABLE 320,23). The average full replacement dosage of LT4 for a young, healthy adult is approximately 1.6 mcg/kg/d. Older patients (> 65 years) or those with coronary artery disease (CAD) should be started on a lower dosage (25-50 mcg/d) and titrated to goal accordingly.

LT4 (tablets, soft-gel capsules, or liquid) should be administered on an empty stomach, with water only, 30 to 60 minutes before breakfast. Medications that interfere with LT4 absorption (eg, bile acid resins, calcium carbonate, ferrous sulfate) should be taken several hours after LT4. For patients who cannot take LT4 in the morning, taking it at bedtime (≥ 2-3 hours after the last meal) is acceptable.
Continue to: Monitoring and titrating
Monitoring and titrating. Hypothyroid symptoms usually improve after 2 or 3 weeks of LT4 treatment; in severe hypothyroidism, complete recovery might take months. Approximately 6 weeks after LT4 therapy is initiated, serum TSH should be measured. After assessing whether administration of LT4 at the starting dosage is appropriate, that dosage can be increased, or decreased, every 4 to 6 weeks until the TSH goal is reached. Once the patient is maintained at a given dosage, measure serum TSH once a year—more often if there is an abnormal result or a change in the patient’s health status.23
Adverse effects of LT4 therapy are rare, unless over-replacement occurs. Rarely, patients have an allergy to the dye or an excipient (filler) in the tablet.26-28 The white, 50-mcg tablets can be given safely to patients with dye sensitivity. For those who have an allergy to an excipient (except gelatin) or gluten intolerance, the LT4 soft-gel capsule or liquid preparation (Tirosint) can be prescribed.
Pure LT4, in a capsule made from vegetable sources, can be ordered through a compounding pharmacy for patients who are allergic to animal products.
Anemia, especially iron-deficiency anemia, can cause intolerance to LT4 therapy; in such patients, lowering the starting dosage and treating anemia are indicated.29
Persistent symptoms (despite a normal TSH level). Because many hypothyroid symptoms are nonspecific, patients might come to think that their LT4 dosage is inadequate if they feel tired or gain weight. Persistent hypothyroid symptoms despite a normal serum TSH level might be due to (1) the inability of LT4 therapy to restore tissue thyroid hormone levels to normal or (2) other variables unrelated to hypothyroidism, including disorders associated with inflammation or autoimmune disease, certain medications, diet, lifestyle, and environmental toxins.
These patients might benefit from a detailed history to identify other causes and a switch to either LT4 + liothyronine (LT3; synthetic T3) combination therapy or DTE26,30-33 (TABLE 434), although a beneficial effect of LT4 + LT3 therapy was not seen in several studies.35,36 Over-replacement with LT4 should be discouraged, due to concerns about thyrotoxicosis and its complications (eg, atrial fibrillation, accelerated bone loss).

DTE and LT4 + LT3. Use of DTE has decreased since the 1970s, when LT4 became the therapy of choice. Subsequently, anecdotal evidence emerged that some patients did not feel well on LT4 and preferred to return to DTE.32,33
Continue to: Several clinical trials...
Several clinical trials addressed the question of whether residual symptoms could be resolved through LT4 + LT3 combination therapy31-39 (TABLE 434), but evidence of any consistent superiority of combination therapy was not demonstrated.35-39 In selected cases, patients might prefer the combination approach.31,33,39 The quality of life of hypothyroid patients was found to be similarly improved with LT4 or DTE, but the latter was associated with modest weight loss (approximately 4 lbs); nearly 50% of study patients preferred treatment with DTE over LT4.33 A follow-up study did not confirm weight loss with DTE, however.34
When LT4 monotherapy and LT4 + LT3 combination therapy were compared, results were mixed31-39; responsiveness to therapy containing LT3 might therefore depend on multiple variables, including genetic background, nutritional and lifestyle factors, stress, presence of comorbidities and autoimmune disorders, and other unidentified or poorly defined variables.40-48
Although combination therapy and DTE are not generally recommended over LT4 monotherapy, they might offer better options for patients who are still symptomatic when being treated with LT4 only: In a randomized, double-blind, crossover study that compared LT4 with DTE and with LT4 + LT3, one-third of the most highly symptomatic patients who had low scores on mood, cognitive, and quality-of-life assessments improved significantly after they were switched to combination therapy or DTE.34
The 2014 American Thyroid Association guidelines24 do not support routine use of LT4 + LT3 in hypothyroid patients who have residual symptoms after LT4 monotherapy; however, a therapeutic trial of LT4 + LT3, while maintaining a normal serum TSH, is reasonable in selected patients. Candidates for DTE or LT4 + LT3 might include patients who do not feel well on LT4 monotherapy, are post thyroidectomy or post radioiodine therapy, or have a low serum T3 level. DTE and combination therapy are discouraged in older patients, patients who have underlying CAD, and pregnant patients.
Special treatment circumstances
A number of patient variables have the potential to alter management strategies for hypothyroidism.18,20,23,40,49-53
Age, comorbidity. Older patients (> 65 years) and patients with cardiopulmonary disease or CAD should be treated with LT4, 25 to 50 mcg/d, initially; that dosage can be titrated upward by 12.5 to 25 mcg/d every 4 to 6 weeks until the TSH goal is reached—preferably, in the range of 4 to 8 mIU/L. An increase in the dosage of LT4 might be required in the presence of malabsorption (eg, gastrointestinal disorders, celiac disease) and in nephrotic syndrome.18,20,23
Body weight. A decrease in the dosage of LT4 might be indicated in the setting of significant weight loss (> 10% body weight).23
Continue to: Co-pharmacy
Co-pharmacy. An increase in the dosage of LT4 might be required when other drugs (eg, phenytoin, phenobarbital, rifampin, and carbamazepine) have led to an increased rate of thyroid hormone metabolism. A decrease in the dosage of LT4 might be necessary after initiation of androgen therapy.23
Pregnancy. Women with pre-existing hypothyroidism require an increase of 25% to 50% in their LT4 dosage during pregnancy to maintain a TSH level in the recommended pregnancy reference range. Thyroid function should be monitored every 4 to 6 weeks to ensure that the TSH target for each trimester is reached (first trimester, 0.1-4 mIU/L; second trimester, 0.2-4 mIU/L; third trimester, 0.3-4 mIU/L). Postpartum, LT4 can be reduced to the prepartum dosage; TSH should be checked every 4 to 6 weeks to maintain the TSH goal.23
Estrogen therapy. Hypothyroid women who are receiving estrogen therapy might require an increase in their LT4 dosage because serum thyroxine-binding globulin levels are increased by estrogens or through other mechanisms that have not been identified.23
Surgical candidacy. Observational studies show few adverse outcomes in surgical patients with mild (subclinical) hypothyroidism or moderate hypothyroidism; however, the risk of adverse surgical outcome might be increased in patients with severe hypothyroidism. For patients in whom surgery is planned and who have:
- subclinical hypothyroidism (elevated TSH and normal FT4), we recommend that surgery—urgent or elective—not be posptoned but proceed.
- moderate (overt) hypothyroidism who require urgent surgery, we recommend not postponing surgery, even though minor perioperative complications might develop. Such patients should be treated with LT4 as soon as the diagnosis for which surgery is required has been made. Alternatively, when moderate hypothyroidism is discovered in a patient who is being evaluated for elective surgery, we recommend postponing surgery until the euthyroid state is restored.
- severe hypothyroidism (myxedema coma [discussed in a bit]; severe clinical symptoms of chronic hypothyroidism, such as altered mental status, pericardial effusion, or heart failure; or a very low level of T4), surgery should be delayed until hypothyroidism has been treated. When emergency surgery is required for a severely hypothyroid patient, they should be treated with LT4 as soon as the diagnosis for which surgery is indicated has been made. When emergency surgery must be performed in a patient with myxedema coma, we recommend treatment with LT4 + LT3, rather than LT4 alone, often administered intravenously because LT4 is poorly absorbed in these patients.
Nonadherence. For patients who do not take LT4 regularly or do not respond to efforts to improve adherence, LT4 can be given weekly, instead of daily, although this interval is not ideal. Weekly dosing should not be used in older patients with CAD.23
Thyroid cancer. Patients who are post total thyroidectomy for thyroid cancer need to take LT4 to treat hypothyroidism and to prevent recurrence of thyroid cancer. The goal TSH level should be based on the cancer stage and risk of recurrence and should be monitored by an endocrinologist.
Myxedema coma. This medical emergency has high mortality. Myxedema coma occurs when severe hypothyroidism leads to any, or a combination, of the following: diminished mentation; hypothermia; bradycardia; hyponatremia; hypotension; cardiovascular, respiratory, and gastrointestinal dysfunction; and renal insufficiency. LT4, LT3, and glucocorticoids should be administered intravenously and the patient monitored closely—preferably in consultation with an endocrinologist.
Continue to: When to seek consultation
When to seek consultation
A patient with hypothyroidism should be referred to Endocrinology if they are < 18 years of age, pregnant, unresponsive to therapy, or have cardiac disease, coexisting endocrine disease, suspected myxedema coma, goiter or thyroid nodules, or a structural thyroid abnormality.
What we know about nutrition and hypothyroidism
Although it is commonly recognized that iodine is essential for production of thyroid hormone, other nutritional factors might contribute to proper production of thyroid hormones, including:
- adequate intake of iron, tyrosine, selenium, zinc, and vitamins E, B2, B3, B6, C, and D44,45
- selenium and zinc, which increase conversion of T4 to T3 and might be important in the management of hypothyroid patients40,46
- vitamin A, zinc, and regular exercise, which have been shown to improve cellular sensitivity to thyroid hormones.
Low iron stores can contribute to persistent symptoms and poor quality of life in patients with hypothyroidism, despite their being treated according to guidelines.29,47
Despite what is known about these nutritional connections, there is insufficient evidence that improving nutrition can reverse hypothyroidism.
Prevention
Prevention of hypothyroidism should take into account variables that affect or inhibit thyroid function, such as stress, infection (eg, Epstein-Barr virus), excessive fluoride intake, toxins (eg, pesticides, solvents, mercury, cadmium, and lead), autoimmune disease (eg, celiac disease), and food sensitivity.54,55 Oxidative stress can also cause thyroid impairment.40-48,54-58
Otherwise, there are, at present, no effective strategies for preventing thyroid disorders.
Subclinical hypothyroidism: Elusive management target
Subclinical hypothyroidism is defined as a normal serum FT4 level in the presence of an elevated serum TSH level. The prevalence of subclinical hypothyroidism varies from 3% to 15%, depending on the population studied; a higher incidence has been noted in women and older people.59 In the NHANES III,1 which excluded people with previously diagnosed thyroid disease, the incidence of subclinical hypothyroidism was 4.3%.
Continue to: Causes of subclinical...
Causes of subclinical hypothyroidism are the same as those of overt hypothyroidism, and include Hashimoto disease. The combination of an elevated TSH level and a normal FT4 level is associated with disorders characterized by protein-binding variations (eg, pregnancy, genetic disorders, drugs), TSH-secreting pituitary adenoma, class II and III obesity (respectively, body mass index, ≥ 35 but < 40 and ≥ 40), and assay variability.49,51
Lab diagnosis: Fraught with difficulty
The serum TSH level and either the total T4 level or the FT4 level should be measured to make a diagnosis of subclinical hypothyroidism. Most laboratories use a 1-step analogue immunoassay to determine free thyroid hormones; protein-binding variations can thus affect measurement of FT4.
Several scenarios that can result in inaccurate measurement of FT4 by radioimmunoassay include genetic disorders that affect binding proteins; pregnancy; use of certain drugs, including heparin, furosemide, antiepileptic agents, salicylate, ferrous sulfate, and cholesterol-binding resins; and some medical conditions, including cardiac surgery, critical illness, and renal failure. Variables that inhibit proper production of thyroid hormones—stress, infection, fluoride (an iodine antagonist), toxins (pesticides, mercury, cadmium, lead) and autoimmune conditions, such as celiac disease—should be considered when attempting to determine the cause of subclinical hypothyroidism.
Liquid chromatography–mass spectrometry measurement of thyroid hormones might be more accurate than immunoassay.53 Measuring serum total T4 and FT4 by dialysis, free from interfering proteins, might also be useful when measurement of FT4 by immunoassay is affected by binding-protein variations.
Features of subclinical hypothyroidism
Most patients who have subclinical hypothyroidism and a serum TSH level < 10 mIU/L are asymptomatic. Some might have nonspecific symptoms of hypothyroidism, however, such as reduced quality of life, poor cognitive function, and poor memory—symptoms that do not typically correlate with the serum TSH level.
It has been suggested that some elderly people normally have a higher level of serum TSH, and that they might have even a prolonged lifespan.51 Additionally, it has been shown that, in nonpregnant adult patients with subclinical hypothyroidism and a serum TSH level of 4.5 to 10 mIU/L, treatment with LT4 was not associated with improvement in thyroid-related symptoms or general quality of life.52
Treat, or don't treat, subclinical hypothyroidism?
It is well accepted that the goal of therapy in hypothyroid patients is to normalize the serum TSH level; however, the American Thyroid Association and the American Association of Clinical Endocrinology recommend starting LT4 in patients with a serum TSH level ≥ 10 mIU/L (TABLE 5).59-62 The principal reason for not treating subclinical hypothyroidism is the lack of benefit in reducing the risk of cardiovascular morbidity and mortality when the TSH level is between 7.5 and 10 mIU/L.62

Continue to: Routine treatment
Routine treatment of patients with a serum TSH level of 4.5 to 10 mIU/L remains controversial. When TSH is 7.0 to 9.9 mIU/L, treatment is recommended for (1) patients < 65 years and (2) for older patients (> 65 years) only when there are convincing hypothyroid symptoms because of concern about unintended overtreatment.
When the TSH level is anywhere above the upper limit of normal to 6.9 mIU/L, treatment is recommended for patients < 65 years old, patients who have a high titer of thyroid peroxidase antibodies, and patients with goiter—but not for patients > 65 years (and, especially, not for octogenarians) because their upper limit of normal could be as high as 6 to 8 mIU/L, especially if they are otherwise healthy.
Treatment should be considered for women with subclinical hypothyroidism who are trying to conceive or experiencing an infertility problem.
For patients with subclinical hypothyroidism who are not being treated, monitor thyroid function every 6 to 12 months by testing TSH and FT4.
CORRESPONDENCE
Thanh D. Hoang, DO, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
The hormones thyroxine (T4) and triiodothyronine (T3), produced by the thyroid gland, are crucial for maintaining metabolism. A deficit of thyroid hormone production—hypothyroidism—is a common endocrine disorder seen in primary care.
Although the diagnosis and management of hypothyroidism are considered straightforward, many patients with hypothyroidism do not achieve optimal treatment goals or see an improvement in their quality of life. In this article, we address the questionable utility of screening; outline the diagnostic approach, including the central role of laboratory testing; and explain why treatment requires a precise approach to address the range of patient types.
Epidemiology and classification
Estimates are that almost 5% of Americans 12 years or older have hypothyroidism; older people and women are more likely to develop the condition. 1 In the US National Health and Nutrition Examination Survey (NHANES III) of 13,344 people without known thyroid disease or a family history, hypothyroidism was found in 4.6% (overt [clinical] in 0.3% and subclinical in 4.3%); 11% had a high serum thyroid peroxidase antibody level, which increases their risk of hypothyroidism, and is treated the same as hypothyroidism of other causes; and, overall, lower serum thyroid-stimulating hormone (TSH) levels were seen in Blacks, compared to Whites and Mexican Americans.1

Primary hypothyroidism accounts for > 95% of cases of hypothyroidism, representing a failure of the thyroid gland to produce sufficient hormone. It has been shown that, in iodine-replete countries such as the United States, the prevalence of spontaneous hypothyroidism is 1% to 2%, and it is 10 times more common in women.2,3
Central hypothyroidism is caused by insufficient stimulation of the thyroid gland by TSH, due to pituitary (secondary hypothyroidism) or hypothalamic (tertiary hypothyroidism) disease and is estimated to occur in 1 in every 20,000 to 80,000 people in the general population.4
How does hypothyroidism manifest?
Signs and symptoms. Manifestations of hypothyroidism range from life-threatening to minimal or no clinical signs and symptoms (TABLE W1). Signs and symptoms of low thyroid function vary by the degree of hypothyroidism at presentation.

Common signs and symptoms of low thyroid function include fatigue, weight gain, dry skin, brittle hair, hair loss, morning stiffness, muscle aches, joint pain, cold intolerance, diffuse headache, constipation, difficulty concentrating, low libido, depression, and menstrual irregularities. On physical examination, a patient might present with bradycardia, hypotension, hypothermia with slow speech or movement, coarse facial appearance, goiter, diffuse hair loss, cold hands and feet, and a prolonged Achilles tendon reflex.5 Skin findings, such as keratosis pilaris, palmoplantar keratoderma (thickening of the skin), and pityriasis rubra pilar, can be associated with autoimmune hypothyroidism.6,7
Continue to: Carpal tunnel syndrome...
Carpal tunnel syndrome, plantar fasciitis, infertility or miscarriage, dyspepsia, and small intestinal bacterial overgrowth can be associated with hypothyroidism; thyroid function should therefore be assessed in patients who have any of these conditions, along with other signs and symptoms of low thyroid function.8,9 A patient with severe hypothyroidism might present with hemodynamic instability, pericardial or pleural effusion, and myxedema coma.10
Clues in the history and from the lab. A history of radiation to the head, neck, or chest area and a history or family history of autoimmune disorders are risk factors for autoimmune thyroid disease.11,12 Laboratory findings can include markers of oxidative stress, such as elevated levels of low-density lipoprotein cholesterol and serum malondialdehyde.13-15
Screening and diagnosis
Screening. The US Preventive Services Task Force has asserted that evidence is insufficient by which to evaluate the benefits and risks of routine screening for thyroid dysfunction in nonpregnant, asymptomatic adults.16 According to the American Thyroid Association and the American Association of Clinical Endocrinologists, screening should be considered in high-risk patients, including those who take medication that affects thyroid function or the results of thyroid hormone assays (TABLE W2, available at mdedge.com/familymedicine).17-20

Screening inpatients is challenging and usually not recommended unless thyroid disease is strongly suspected. This is because changes in the levels of thyroid hormones, binding proteins, and the TSH concentration can occur in severe nonthyroidal illness; in addition, assay interference by antibodies and other substances can affect thyroid hormone measurement.21
Testing strategy. Generally, screening and diagnosis of hypothyroidism are based primarily on laboratory testing, because signs and symptoms are nonspecific (FIGURE 15). A serum TSH level is usually the initial test when screening for thyroid dysfunction. A normal serum TSH value ranges from 0.5-5.0 mIU/L.

When an abnormal serum TSH level is found, further tests can be performed to investigate, including a serum free thyroxine (FT4) test. (Our preference is to order TSH and FT4 assays simultaneously to facilitate and confirm the diagnosis.) An FT4 test measures the amount of unattached, or free, thyroxine in blood by immunoassay. A normal FT4 value usually ranges from 0.7-1.9 ng/dL.
The combination of a high TSH level and a low FT4 level could be an indication of an underactive thyroid gland (ie, clinical or overt hypothyroidism). Milder, subclinical hypothyroidism is characterized by a higher-than-normal TSH level but a normal FT4 level.22 Central (secondary) hypothyroidism is characterized by a low serum FT4 level and a serum TSH level that can be below the reference range, low normal, or even slightly high.4
Continue to: These measurements...
These measurements must be interpreted within the context of the laboratory-specific normal range for each test. Third-generation serum TSH assays are more sensitive and specific than serum FT4 measurements for hypothyroidism. FT4 is usually measured by automated analogue immunoassay, which generally provides reliable results; abnormal binding proteins or other interferences occur in some patients, however, resulting in reporting of a falsely high, or falsely low, FT4 level. In such cases, FT4 by direct dialysis, or total T4, can be measured for further evaluation. In primary care, you are most likely to encounter primary hypothyroidism; secondary (central) hypothyroidism is much rarer (< 5% of cases).4
The ins and outs of treatment
For most patients, hypothyroidism is a permanent disorder requiring lifelong thyroid hormone replacement therapy—unless the disease is transient (ie, painless or subacute thyroiditis); reversible, because it is caused by medication; or responsive to medical intervention that addresses the underlying autoimmune condition.19 Goals of treatment (Figure 25,23) are to:
- normalize the TSH level to 0.5-5.0 mIU/L (the main goal), with an age-related shift toward a higher TSH goal in older patients (and an upper limit of normal of 7.5 mIU/L in patients who are ≥ 80 years of age)20
- restore the euthyroid state
- relieve symptoms
- reduce any goiter
- avoid overtreatment (iatrogenic thyrotoxicosis).

Desiccated thyroid extract (DTE), developed in the late 1880s and made from the dried thyroid gland of pigs, sheep, or cows, was the earliest treatment for hypothyroidism. The use of DTE has declined since the introduction of synthetic thyroxine (T4, or levothyroxine [here, referred to as LT4]), which is now the standard treatment.20-22 LT4 is deiodinated in peripheral tissues to form T3, the active thyroid hormone; this process accounts for 80% of total T3 production daily.24
LT4 formulations. LT4 is commercially available in tablet, soft-gel, and liquid preparations. Most patients are treated with the tablet; the soft-gel capsule or liquid is an option for patients who absorb the tablet poorly (because of atrophic gastritis, celiac disease, or gluten sensitivity or because they are post bariatric surgery). Increasing the dosage of the tablet form of LT4, with ongoing TSH monitoring, is more cost effective than moving to an alternative preparation.
If a switch of LT4 formulation is made (ie, from one manufacturer to another), test the serum TSH level to ensure that the therapeutic goal is being reached. Also, in our experience, it is best to prescribe a brand-name preparation of levothyroxine, not a generic, whenever possible, due to the variability in generic formulations and the potential presence of other (inert) ingredients.25
Dosing (TABLE 320,23). The average full replacement dosage of LT4 for a young, healthy adult is approximately 1.6 mcg/kg/d. Older patients (> 65 years) or those with coronary artery disease (CAD) should be started on a lower dosage (25-50 mcg/d) and titrated to goal accordingly.

LT4 (tablets, soft-gel capsules, or liquid) should be administered on an empty stomach, with water only, 30 to 60 minutes before breakfast. Medications that interfere with LT4 absorption (eg, bile acid resins, calcium carbonate, ferrous sulfate) should be taken several hours after LT4. For patients who cannot take LT4 in the morning, taking it at bedtime (≥ 2-3 hours after the last meal) is acceptable.
Continue to: Monitoring and titrating
Monitoring and titrating. Hypothyroid symptoms usually improve after 2 or 3 weeks of LT4 treatment; in severe hypothyroidism, complete recovery might take months. Approximately 6 weeks after LT4 therapy is initiated, serum TSH should be measured. After assessing whether administration of LT4 at the starting dosage is appropriate, that dosage can be increased, or decreased, every 4 to 6 weeks until the TSH goal is reached. Once the patient is maintained at a given dosage, measure serum TSH once a year—more often if there is an abnormal result or a change in the patient’s health status.23
Adverse effects of LT4 therapy are rare, unless over-replacement occurs. Rarely, patients have an allergy to the dye or an excipient (filler) in the tablet.26-28 The white, 50-mcg tablets can be given safely to patients with dye sensitivity. For those who have an allergy to an excipient (except gelatin) or gluten intolerance, the LT4 soft-gel capsule or liquid preparation (Tirosint) can be prescribed.
Pure LT4, in a capsule made from vegetable sources, can be ordered through a compounding pharmacy for patients who are allergic to animal products.
Anemia, especially iron-deficiency anemia, can cause intolerance to LT4 therapy; in such patients, lowering the starting dosage and treating anemia are indicated.29
Persistent symptoms (despite a normal TSH level). Because many hypothyroid symptoms are nonspecific, patients might come to think that their LT4 dosage is inadequate if they feel tired or gain weight. Persistent hypothyroid symptoms despite a normal serum TSH level might be due to (1) the inability of LT4 therapy to restore tissue thyroid hormone levels to normal or (2) other variables unrelated to hypothyroidism, including disorders associated with inflammation or autoimmune disease, certain medications, diet, lifestyle, and environmental toxins.
These patients might benefit from a detailed history to identify other causes and a switch to either LT4 + liothyronine (LT3; synthetic T3) combination therapy or DTE26,30-33 (TABLE 434), although a beneficial effect of LT4 + LT3 therapy was not seen in several studies.35,36 Over-replacement with LT4 should be discouraged, due to concerns about thyrotoxicosis and its complications (eg, atrial fibrillation, accelerated bone loss).

DTE and LT4 + LT3. Use of DTE has decreased since the 1970s, when LT4 became the therapy of choice. Subsequently, anecdotal evidence emerged that some patients did not feel well on LT4 and preferred to return to DTE.32,33
Continue to: Several clinical trials...
Several clinical trials addressed the question of whether residual symptoms could be resolved through LT4 + LT3 combination therapy31-39 (TABLE 434), but evidence of any consistent superiority of combination therapy was not demonstrated.35-39 In selected cases, patients might prefer the combination approach.31,33,39 The quality of life of hypothyroid patients was found to be similarly improved with LT4 or DTE, but the latter was associated with modest weight loss (approximately 4 lbs); nearly 50% of study patients preferred treatment with DTE over LT4.33 A follow-up study did not confirm weight loss with DTE, however.34
When LT4 monotherapy and LT4 + LT3 combination therapy were compared, results were mixed31-39; responsiveness to therapy containing LT3 might therefore depend on multiple variables, including genetic background, nutritional and lifestyle factors, stress, presence of comorbidities and autoimmune disorders, and other unidentified or poorly defined variables.40-48
Although combination therapy and DTE are not generally recommended over LT4 monotherapy, they might offer better options for patients who are still symptomatic when being treated with LT4 only: In a randomized, double-blind, crossover study that compared LT4 with DTE and with LT4 + LT3, one-third of the most highly symptomatic patients who had low scores on mood, cognitive, and quality-of-life assessments improved significantly after they were switched to combination therapy or DTE.34
The 2014 American Thyroid Association guidelines24 do not support routine use of LT4 + LT3 in hypothyroid patients who have residual symptoms after LT4 monotherapy; however, a therapeutic trial of LT4 + LT3, while maintaining a normal serum TSH, is reasonable in selected patients. Candidates for DTE or LT4 + LT3 might include patients who do not feel well on LT4 monotherapy, are post thyroidectomy or post radioiodine therapy, or have a low serum T3 level. DTE and combination therapy are discouraged in older patients, patients who have underlying CAD, and pregnant patients.
Special treatment circumstances
A number of patient variables have the potential to alter management strategies for hypothyroidism.18,20,23,40,49-53
Age, comorbidity. Older patients (> 65 years) and patients with cardiopulmonary disease or CAD should be treated with LT4, 25 to 50 mcg/d, initially; that dosage can be titrated upward by 12.5 to 25 mcg/d every 4 to 6 weeks until the TSH goal is reached—preferably, in the range of 4 to 8 mIU/L. An increase in the dosage of LT4 might be required in the presence of malabsorption (eg, gastrointestinal disorders, celiac disease) and in nephrotic syndrome.18,20,23
Body weight. A decrease in the dosage of LT4 might be indicated in the setting of significant weight loss (> 10% body weight).23
Continue to: Co-pharmacy
Co-pharmacy. An increase in the dosage of LT4 might be required when other drugs (eg, phenytoin, phenobarbital, rifampin, and carbamazepine) have led to an increased rate of thyroid hormone metabolism. A decrease in the dosage of LT4 might be necessary after initiation of androgen therapy.23
Pregnancy. Women with pre-existing hypothyroidism require an increase of 25% to 50% in their LT4 dosage during pregnancy to maintain a TSH level in the recommended pregnancy reference range. Thyroid function should be monitored every 4 to 6 weeks to ensure that the TSH target for each trimester is reached (first trimester, 0.1-4 mIU/L; second trimester, 0.2-4 mIU/L; third trimester, 0.3-4 mIU/L). Postpartum, LT4 can be reduced to the prepartum dosage; TSH should be checked every 4 to 6 weeks to maintain the TSH goal.23
Estrogen therapy. Hypothyroid women who are receiving estrogen therapy might require an increase in their LT4 dosage because serum thyroxine-binding globulin levels are increased by estrogens or through other mechanisms that have not been identified.23
Surgical candidacy. Observational studies show few adverse outcomes in surgical patients with mild (subclinical) hypothyroidism or moderate hypothyroidism; however, the risk of adverse surgical outcome might be increased in patients with severe hypothyroidism. For patients in whom surgery is planned and who have:
- subclinical hypothyroidism (elevated TSH and normal FT4), we recommend that surgery—urgent or elective—not be posptoned but proceed.
- moderate (overt) hypothyroidism who require urgent surgery, we recommend not postponing surgery, even though minor perioperative complications might develop. Such patients should be treated with LT4 as soon as the diagnosis for which surgery is required has been made. Alternatively, when moderate hypothyroidism is discovered in a patient who is being evaluated for elective surgery, we recommend postponing surgery until the euthyroid state is restored.
- severe hypothyroidism (myxedema coma [discussed in a bit]; severe clinical symptoms of chronic hypothyroidism, such as altered mental status, pericardial effusion, or heart failure; or a very low level of T4), surgery should be delayed until hypothyroidism has been treated. When emergency surgery is required for a severely hypothyroid patient, they should be treated with LT4 as soon as the diagnosis for which surgery is indicated has been made. When emergency surgery must be performed in a patient with myxedema coma, we recommend treatment with LT4 + LT3, rather than LT4 alone, often administered intravenously because LT4 is poorly absorbed in these patients.
Nonadherence. For patients who do not take LT4 regularly or do not respond to efforts to improve adherence, LT4 can be given weekly, instead of daily, although this interval is not ideal. Weekly dosing should not be used in older patients with CAD.23
Thyroid cancer. Patients who are post total thyroidectomy for thyroid cancer need to take LT4 to treat hypothyroidism and to prevent recurrence of thyroid cancer. The goal TSH level should be based on the cancer stage and risk of recurrence and should be monitored by an endocrinologist.
Myxedema coma. This medical emergency has high mortality. Myxedema coma occurs when severe hypothyroidism leads to any, or a combination, of the following: diminished mentation; hypothermia; bradycardia; hyponatremia; hypotension; cardiovascular, respiratory, and gastrointestinal dysfunction; and renal insufficiency. LT4, LT3, and glucocorticoids should be administered intravenously and the patient monitored closely—preferably in consultation with an endocrinologist.
Continue to: When to seek consultation
When to seek consultation
A patient with hypothyroidism should be referred to Endocrinology if they are < 18 years of age, pregnant, unresponsive to therapy, or have cardiac disease, coexisting endocrine disease, suspected myxedema coma, goiter or thyroid nodules, or a structural thyroid abnormality.
What we know about nutrition and hypothyroidism
Although it is commonly recognized that iodine is essential for production of thyroid hormone, other nutritional factors might contribute to proper production of thyroid hormones, including:
- adequate intake of iron, tyrosine, selenium, zinc, and vitamins E, B2, B3, B6, C, and D44,45
- selenium and zinc, which increase conversion of T4 to T3 and might be important in the management of hypothyroid patients40,46
- vitamin A, zinc, and regular exercise, which have been shown to improve cellular sensitivity to thyroid hormones.
Low iron stores can contribute to persistent symptoms and poor quality of life in patients with hypothyroidism, despite their being treated according to guidelines.29,47
Despite what is known about these nutritional connections, there is insufficient evidence that improving nutrition can reverse hypothyroidism.
Prevention
Prevention of hypothyroidism should take into account variables that affect or inhibit thyroid function, such as stress, infection (eg, Epstein-Barr virus), excessive fluoride intake, toxins (eg, pesticides, solvents, mercury, cadmium, and lead), autoimmune disease (eg, celiac disease), and food sensitivity.54,55 Oxidative stress can also cause thyroid impairment.40-48,54-58
Otherwise, there are, at present, no effective strategies for preventing thyroid disorders.
Subclinical hypothyroidism: Elusive management target
Subclinical hypothyroidism is defined as a normal serum FT4 level in the presence of an elevated serum TSH level. The prevalence of subclinical hypothyroidism varies from 3% to 15%, depending on the population studied; a higher incidence has been noted in women and older people.59 In the NHANES III,1 which excluded people with previously diagnosed thyroid disease, the incidence of subclinical hypothyroidism was 4.3%.
Continue to: Causes of subclinical...
Causes of subclinical hypothyroidism are the same as those of overt hypothyroidism, and include Hashimoto disease. The combination of an elevated TSH level and a normal FT4 level is associated with disorders characterized by protein-binding variations (eg, pregnancy, genetic disorders, drugs), TSH-secreting pituitary adenoma, class II and III obesity (respectively, body mass index, ≥ 35 but < 40 and ≥ 40), and assay variability.49,51
Lab diagnosis: Fraught with difficulty
The serum TSH level and either the total T4 level or the FT4 level should be measured to make a diagnosis of subclinical hypothyroidism. Most laboratories use a 1-step analogue immunoassay to determine free thyroid hormones; protein-binding variations can thus affect measurement of FT4.
Several scenarios that can result in inaccurate measurement of FT4 by radioimmunoassay include genetic disorders that affect binding proteins; pregnancy; use of certain drugs, including heparin, furosemide, antiepileptic agents, salicylate, ferrous sulfate, and cholesterol-binding resins; and some medical conditions, including cardiac surgery, critical illness, and renal failure. Variables that inhibit proper production of thyroid hormones—stress, infection, fluoride (an iodine antagonist), toxins (pesticides, mercury, cadmium, lead) and autoimmune conditions, such as celiac disease—should be considered when attempting to determine the cause of subclinical hypothyroidism.
Liquid chromatography–mass spectrometry measurement of thyroid hormones might be more accurate than immunoassay.53 Measuring serum total T4 and FT4 by dialysis, free from interfering proteins, might also be useful when measurement of FT4 by immunoassay is affected by binding-protein variations.
Features of subclinical hypothyroidism
Most patients who have subclinical hypothyroidism and a serum TSH level < 10 mIU/L are asymptomatic. Some might have nonspecific symptoms of hypothyroidism, however, such as reduced quality of life, poor cognitive function, and poor memory—symptoms that do not typically correlate with the serum TSH level.
It has been suggested that some elderly people normally have a higher level of serum TSH, and that they might have even a prolonged lifespan.51 Additionally, it has been shown that, in nonpregnant adult patients with subclinical hypothyroidism and a serum TSH level of 4.5 to 10 mIU/L, treatment with LT4 was not associated with improvement in thyroid-related symptoms or general quality of life.52
Treat, or don't treat, subclinical hypothyroidism?
It is well accepted that the goal of therapy in hypothyroid patients is to normalize the serum TSH level; however, the American Thyroid Association and the American Association of Clinical Endocrinology recommend starting LT4 in patients with a serum TSH level ≥ 10 mIU/L (TABLE 5).59-62 The principal reason for not treating subclinical hypothyroidism is the lack of benefit in reducing the risk of cardiovascular morbidity and mortality when the TSH level is between 7.5 and 10 mIU/L.62

Continue to: Routine treatment
Routine treatment of patients with a serum TSH level of 4.5 to 10 mIU/L remains controversial. When TSH is 7.0 to 9.9 mIU/L, treatment is recommended for (1) patients < 65 years and (2) for older patients (> 65 years) only when there are convincing hypothyroid symptoms because of concern about unintended overtreatment.
When the TSH level is anywhere above the upper limit of normal to 6.9 mIU/L, treatment is recommended for patients < 65 years old, patients who have a high titer of thyroid peroxidase antibodies, and patients with goiter—but not for patients > 65 years (and, especially, not for octogenarians) because their upper limit of normal could be as high as 6 to 8 mIU/L, especially if they are otherwise healthy.
Treatment should be considered for women with subclinical hypothyroidism who are trying to conceive or experiencing an infertility problem.
For patients with subclinical hypothyroidism who are not being treated, monitor thyroid function every 6 to 12 months by testing TSH and FT4.
CORRESPONDENCE
Thanh D. Hoang, DO, Division of Endocrinology, Walter Reed National Military Medical Center, 8901 Wisconsin Avenue, Bethesda, MD 20889; [email protected]
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499. doi: 10.1210/jcem.87.2.8182
2. Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51. doi: 10.1093/bmb/ldr030
3. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
4. Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. doi: 10.1210/jc.2012-1616
5. Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am. 2012;96:203-221. doi: 10.1016/j.mcna.2012.01.005
6. Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-659. doi: 10.1067/mjd.2003.257
7. Franzotti AM, Avelar JCD, Cardoso TA, et al. Pityriasis rubra pilar and hypothyroidism. An Bras Dermatol. 2014;89:497-500. doi: 10.1590/abd1806-4841.20142994
8. Yaylali O, Kirac S, Yilmaz M, et al. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802
9. Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18:307-309.
10. Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27:117-122. doi: 10.1016/j.je.2016.04.002
11. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5. doi: 10.1016/j.radonc.2011.03.002
12. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-e9. doi: 10.1016/j.amjmed.2009.06.030
13. Cheserek MJ, Wu G-R, Ntazinda A, et al. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;34:323-331. doi: 10.2478/jomb-2014-0044
14. Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4
15. Tejovathi B, Suchitra MM, Suresh V, et al. Association of lipid oxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes. 2013;121:306-309. doi: 10.1055/s-0032-1333298
16. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
17. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562. doi: 10.1016/S0140-6736(17)30703-1
18. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801
19. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243-1251. doi: 10.1089/thy.2018.0116
20. Garber JR, Cobin RH, Gharib H, et al; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375-388. doi: 10.1016/j.ccc.2018.11.012
22. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376:2556-2565. doi: 10.1056/NEJMcp1611144
23. Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(suppl 2):59-71. doi: 10.1007/s12325-019-01079-1
24. Jonklaas J, Bianco AC, Bauer AJ, et al; . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
25. Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984;5:151-184. doi: 10.1210/edrv-5-2-151
26. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020;105:e3090-e3104. doi: 10.1210/clinem/dgaa430
27. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6:e101-e104. doi: 10.4158/ACCR-2019-0495
28. Chamorro-Pareja N, Carrillo-Martin I, Haehn DA, et al. Self-reported allergy to thyroid replacement therapy: a multicenter retrospective chart review. Endocr Pract. 2020;26:761-767. doi: 10.4158/EP-2019-0488
29. Shakir MKM, Turton D, Aprill BS, et al. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin Proc. 2000;75:189-192.
30. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31:156-182. doi: 10.1089/thy.2020.0720
31. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666-2674. doi: 10.1210/jc.2004-2111
32. Escobar-Morreale HF, Botella-Carretero JI, M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412-424. doi: 10.7326/0003-4819-142-6-200503150-00007
33. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982-1990. doi: 10.1210/jc.2012-4107
34. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106:e4400-e4413. doi: 10.1210/clinem/dgab478
35. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34:80-89. doi: 10.1080/07435800903156340
36. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543-4550. doi: 10.1210/jc.2003-030249
37. Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223-233. doi: 10.4158/EP.11.4.223
38. Sawka AM, Gerstein HC, Marriott MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551-4555. doi: 10.1210/jc.2003-030139
39. Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290:2952-2958. doi: 10.1001/jama.290.22.2952
40. Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. doi: 10.1210/jc.2010-0191
41. Winther KH, Wichman JEM, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376-385. doi: 10.1007/s12020-016-1098-z
42. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741
43. Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015,7:2485-2498. doi: 10.3390/nu7042485
44. Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet. 2016;116:302-310.e1. doi: 10.1016/j.jand.2015.09.020
45. Babiker A, Alawi A, Al Atawi M, et al. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20:13-19. doi: 10.24911/SJP.106-1587138942
46. Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769
47. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34-44. doi: 10.1017/S0029665118001192
48. Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674-678. doi: 10.4103/2230-8210.190555
49. Tseng F-Y, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730-737. doi: 10.1016/j.jacc.2012.03.047
50. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573-581. doi: 10.7326/0003-4819-145-8-200610170-00006
51. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591-2599. doi: 10.1001/jama.292.21.2591
52. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
53. Monzani F, Dardano A, Caraccio N. Does treating subclinical hypothyroidism improve markers of cardiovascular risk? Treat Endocrinol. 2006;5:65-81. doi: 10.2165/00024677-200605020-00001
54. Duntas LH. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190-191. doi: 10.1038/nrendo.2009.46
55. Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6:R52-R58. doi: 10.1530/EC-17-0021
56. Janegova A, Janega P, Rychly B, et al. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66:132-136. doi: 10.5603/EP.2015.0020
57. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761. doi: 10.1089/thy.2010.1636
58. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. doi: 10.1016/j.biocel.2006.07.001
59. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028. doi: 10.4158/EP12280.GL
60. Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. 2016;175:R255-R263. doi: 10.1530/EJE-16-0193
61. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65-68. doi: 10.1016/j.ejim.2017.11.010
62. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
1. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499. doi: 10.1210/jcem.87.2.8182
2. Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51. doi: 10.1093/bmb/ldr030
3. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
4. Persani L. Central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97:3068-3078. doi: 10.1210/jc.2012-1616
5. Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am. 2012;96:203-221. doi: 10.1016/j.mcna.2012.01.005
6. Ai J, Leonhardt JM, Heymann WR. Autoimmune thyroid diseases: etiology, pathogenesis and dermatologic manifestations. J Am Acad Dermatol. 2003;48:641-659. doi: 10.1067/mjd.2003.257
7. Franzotti AM, Avelar JCD, Cardoso TA, et al. Pityriasis rubra pilar and hypothyroidism. An Bras Dermatol. 2014;89:497-500. doi: 10.1590/abd1806-4841.20142994
8. Yaylali O, Kirac S, Yilmaz M, et al. Does hypothyroidism affect gastrointestinal motility? Gastroenterol Res Pract. 2009;2009:529802. doi: 10.1155/2009/529802
9. Patil AD. Link between hypothyroidism and small intestinal bacterial overgrowth. Indian J Endocrinol Metab. 2014;18:307-309.
10. Ono Y, Ono S, Yasunaga H, et al. Clinical characteristics and outcomes of myxedema coma: analysis of a national inpatient database in Japan. J Epidemiol. 2017;27:117-122. doi: 10.1016/j.je.2016.04.002
11. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5. doi: 10.1016/j.radonc.2011.03.002
12. Boelaert K, Newby PR, Simmonds MJ, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-e9. doi: 10.1016/j.amjmed.2009.06.030
13. Cheserek MJ, Wu G-R, Ntazinda A, et al. Association between thyroid hormones, lipids and oxidative stress markers in subclinical hypothyroidism. J Med Biochem. 2015;34:323-331. doi: 10.2478/jomb-2014-0044
14. Zha K, Zuo C, Wang A, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95. doi: 10.1186/s12944-015-0092-4
15. Tejovathi B, Suchitra MM, Suresh V, et al. Association of lipid oxidation with endothelial dysfunction in patients with overt hypothyroidism. Exp Clin Endocrinol Diabetes. 2013;121:306-309. doi: 10.1055/s-0032-1333298
16. LeFevre ML; U.S. Preventive Services Task Force. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
17. Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017;390:1550-1562. doi: 10.1016/S0140-6736(17)30703-1
18. Vaidya B, Pearce SHS. Management of hypothyroidism in adults. BMJ. 2008;337:a801. doi: 10.1136/bmj.a801
19. Iyer PC, Cabanillas ME, Waguespack SG, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid. 2018;28:1243-1251. doi: 10.1089/thy.2018.0116
20. Garber JR, Cobin RH, Gharib H, et al; American Association Of Clinical Endocrinologists And American Thyroid Association Taskforce On Hypothyroidism In Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Maiden MJ, Torpy DJ. Thyroid hormones in critical illness. Crit Care Clin. 2019;35:375-388. doi: 10.1016/j.ccc.2018.11.012
22. Peeters RP. Subclinical hypothyroidism. N Engl J Med. 2017;376:2556-2565. doi: 10.1056/NEJMcp1611144
23. Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(suppl 2):59-71. doi: 10.1007/s12325-019-01079-1
24. Jonklaas J, Bianco AC, Bauer AJ, et al; . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
25. Engler D, Burger AG. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984;5:151-184. doi: 10.1210/edrv-5-2-151
26. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: is T4 enough for everyone? J Clin Endocrinol Metab. 2020;105:e3090-e3104. doi: 10.1210/clinem/dgaa430
27. Slayden TA, Shakir MKM, Hoang TD. A bull in a pill shop: alpha-gal allergy complicating treatment options for postprocedural hypothyroidism. AACE Clin Case Rep. 2020;6:e101-e104. doi: 10.4158/ACCR-2019-0495
28. Chamorro-Pareja N, Carrillo-Martin I, Haehn DA, et al. Self-reported allergy to thyroid replacement therapy: a multicenter retrospective chart review. Endocr Pract. 2020;26:761-767. doi: 10.4158/EP-2019-0488
29. Shakir MKM, Turton D, Aprill BS, et al. Anemia: a cause of intolerance to thyroxine sodium. Mayo Clin Proc. 2000;75:189-192.
30. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31:156-182. doi: 10.1089/thy.2020.0720
31. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90:2666-2674. doi: 10.1210/jc.2004-2111
32. Escobar-Morreale HF, Botella-Carretero JI, M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142:412-424. doi: 10.7326/0003-4819-142-6-200503150-00007
33. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98:1982-1990. doi: 10.1210/jc.2012-4107
34. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021;106:e4400-e4413. doi: 10.1210/clinem/dgab478
35. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34:80-89. doi: 10.1080/07435800903156340
36. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88:4543-4550. doi: 10.1210/jc.2003-030249
37. Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11:223-233. doi: 10.4158/EP.11.4.223
38. Sawka AM, Gerstein HC, Marriott MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88:4551-4555. doi: 10.1210/jc.2003-030139
39. Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290:2952-2958. doi: 10.1001/jama.290.22.2952
40. Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab. 2010;95:5180-5188. doi: 10.1210/jc.2010-0191
41. Winther KH, Wichman JEM, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55:376-385. doi: 10.1007/s12020-016-1098-z
42. Parva NR, Tadepalli S, Singh P, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10:e2741. doi: 10.7759/cureus.2741
43. Wang J, Lv S, Chen G, et al. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015,7:2485-2498. doi: 10.3390/nu7042485
44. Wilson MM, Reedy J, Krebs-Smith SM. American diet quality: where it is, where it is heading, and what it could be. J Acad Nutr Diet. 2016;116:302-310.e1. doi: 10.1016/j.jand.2015.09.020
45. Babiker A, Alawi A, Al Atawi M, et al. The role of micronutrients in thyroid dysfunction. Sudan J Paediatr. 2020;20:13-19. doi: 10.24911/SJP.106-1587138942
46. Knezevic J, Starchl C, Tmava Berisha A, et al. Thyroid-gut-axis: How does the microbiota influence thyroid function? Nutrients. 2020;12:1769. doi: 10.3390/nu12061769
47. Rayman MP. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc Nutr Soc. 2019;78:34-44. doi: 10.1017/S0029665118001192
48. Chakrabarti SK, Ghosh S, Banerjee S, et al. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian J Endocrinol Metab. 2016;20:674-678. doi: 10.4103/2230-8210.190555
49. Tseng F-Y, Lin W-Y, Lin C-C, et al. Subclinical hypothyroidism is associated with increased risk for all-cause and cardiovascular mortality in adults. J Am Coll Cardiol. 2012;60:730-737. doi: 10.1016/j.jacc.2012.03.047
50. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573-581. doi: 10.7326/0003-4819-145-8-200610170-00006
51. Gussekloo J, van Exel E, de Craen AJM, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591-2599. doi: 10.1001/jama.292.21.2591
52. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
53. Monzani F, Dardano A, Caraccio N. Does treating subclinical hypothyroidism improve markers of cardiovascular risk? Treat Endocrinol. 2006;5:65-81. doi: 10.2165/00024677-200605020-00001
54. Duntas LH. Does celiac disease trigger autoimmune thyroiditis? Nat Rev Endocrinol. 2009;5:190-191. doi: 10.1038/nrendo.2009.46
55. Lerner A, Jeremias P, Matthias T. Gut-thyroid axis and celiac disease. Endocr Connect. 2017;6:R52-R58. doi: 10.1530/EC-17-0021
56. Janegova A, Janega P, Rychly B, et al. The role of Epstein-Barr virus infection in the development of autoimmune thyroid diseases. Endokrynol Pol. 2015;66:132-136. doi: 10.5603/EP.2015.0020
57. Brent GA. Environmental exposures and autoimmune thyroid disease. Thyroid. 2010;20:755-761. doi: 10.1089/thy.2010.1636
58. Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. doi: 10.1016/j.biocel.2006.07.001
59. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028. doi: 10.4158/EP12280.GL
60. Welsh KJ, Soldin SJ. Diagnosis of endocrine disease: How reliable are free thyroid and total T3 hormone assays? Eur J Endocrinol. 2016;175:R255-R263. doi: 10.1530/EJE-16-0193
61. Grossman A, Feldhamer I, Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur J Intern Med. 2018;50:65-68. doi: 10.1016/j.ejim.2017.11.010
62. Pearce SHS, Brabant G, Duntas LH, et al. 2013 ETA Guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
PRACTICE RECOMMENDATIONS
› Prescribe levothyroxine (LT4) to maintain thyroid-stimulating hormone (TSH) at 4 to 7 mIU/L in select patients with primary hypothyroidism for whom that range of the serum TSH level can be considered appropriate (ie, those older than 65 years and those who have underlying coronary artery disease or another debilitating chronic disorder). A
› Counsel all women of childbearing age with primary hypothyroidism that they need to have their dosage of LT4 increased as soon as pregnancy is suspected. A
› Keep in mind that treating hypothyroidism is not always necessary in older patients who have subclinical disease and a serum TSH level < 10 mIU/L. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Central Centrifugal Cicatricial Alopecia
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia (CCCA).1 Central centrifugal cicatricial alopecia (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
Central centrifugal cicatricial alopecia predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. Central centrifugal cicatricial alopecia has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7 PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
• Central centrifugal cicatricial alopecia begins in the central scalp (crown area, vertex) and spreads centrifugally.
• Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
• Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
• Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
• Central centrifugal cicatricial alopecia can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral anti-inflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/j.1600-0560.2001 .280701.x
- Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/archderm.147.12.1453
- Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111 /1346-8138.14217
- Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
- Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
- Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
- Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056 /NEJMe1900042
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j .jdcr.2019.12.008

THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia (CCCA).1 Central centrifugal cicatricial alopecia (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
Central centrifugal cicatricial alopecia predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. Central centrifugal cicatricial alopecia has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7 PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
• Central centrifugal cicatricial alopecia begins in the central scalp (crown area, vertex) and spreads centrifugally.
• Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
• Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
• Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
• Central centrifugal cicatricial alopecia can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral anti-inflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.

THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia (CCCA).1 Central centrifugal cicatricial alopecia (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
Central centrifugal cicatricial alopecia predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. Central centrifugal cicatricial alopecia has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7 PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
• Central centrifugal cicatricial alopecia begins in the central scalp (crown area, vertex) and spreads centrifugally.
• Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
• Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
• Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
• Central centrifugal cicatricial alopecia can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral anti-inflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/j.1600-0560.2001 .280701.x
- Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/archderm.147.12.1453
- Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111 /1346-8138.14217
- Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
- Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
- Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
- Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056 /NEJMe1900042
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j .jdcr.2019.12.008
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/j.1600-0560.2001 .280701.x
- Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/archderm.147.12.1453
- Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111 /1346-8138.14217
- Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
- Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
- Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
- Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056 /NEJMe1900042
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j .jdcr.2019.12.008
Ulcerating Nodule on the Foot
The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.
Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11
Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13
Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.

Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11

Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13

Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).

The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.

Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11

Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13

Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).

- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
A 59-year-old woman with a history of joint pain presented with a foot nodule that developed over the course of 2 years. Physical examination revealed a firm, mobile, mildly tender, 3-cm, deep red nodule on the dorsal aspect of the left foot (top [inset]) with an overlying central epidermal defect and thick keratinaceous debris. The remainder of the physical examination was unremarkable. Empiric treatments with oral antibiotics and intralesional corticosteroids were unsuccessful. Incisional biopsy was performed for histologic review, and tissue culture studies were negative.
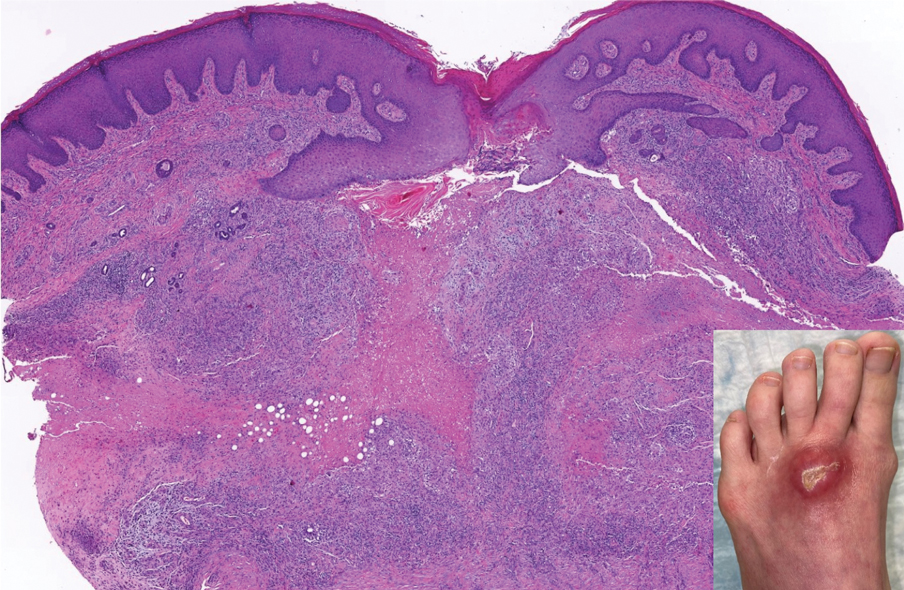
Infectious disease pop quiz: Clinical challenge #22 for the ObGyn
In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
Continue to the answer...
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
Continue to the answer...
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
Continue to the answer...
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Suing patients: Medical, ethical, and legal considerations
Although it is common to read about patients suing their hospitals, there has been increasing public and political attention given to hospitals suing their patients to collect unpaid hospital bills. KH’s story began with an emergency appendectomy. She did not have health insurance to cover the $14,000 hospital bill. The family was unable to pay the bill, and the nonprofit hospital sued them for that bill, plus some additional expenses (totaling about $17,000), plus interest was accumulating at 9% per year. The hospital won a judgment, and it garnished the husband’s pay (10% of after-taxes pay, in this case) and placed a lien on the family’s home. Years later—because of interest and additional hospital bills—the family had paid $20,000, but still owed $26,000.1
The extent of the problem
This is neither a hypothetical case nor a rare event. Studies and press reports have noted dozens of examples of hospital collection excesses. One study found that unpaid medical bill lawsuits increased by 37% in Wisconsin between 2001 and 2018, with 5% of hospitals accounting for 25% of the lawsuits.2 Another report found almost “31,000 civil cases filed by 139 hospitals in 26 New York counties from 2015 to 2019.”3 Similar to the Wisconsin report, a small number of health care providers accounted for the majority of lawsuits. In another example, one Missouri nonprofit hospital, Heartland (rebranded “Mosaic”), created its own for-profit debt collection agency (Northwest Financial Services), which filed 11,000 lawsuits from 2009 to 2013, resulting in 6,000 wage garnishments.1 The Wall Street Journal, among others, has reported for years on the difficulties created by lawsuits against patients.4 Axios and Johns Hopkins reported that “medical debt comprises 58% of all debt collections in the United States.” And although some collection actions declined early in the pandemic, it did not appear to last.5,6
Inconsistent collection policies. Collection policies vary greatly from hospital to hospital, with an increasing number of hospitals demanding up-front payments (before services). Many of these health care institutions persuade patients to put medical debt on their credit cards, sometimes as part of an up-front (before service) process.7 If using a standard credit card, this comes with a very high interest rate. There are some special health-related credit cards, such as CareCredit, that generally have better interest rates. These cards offer no-interest short-term loans, with significant interest for longer-term loans. Thus, failure to repay the full amount when due means that the “deferred interest” (about 27%) must be paid.8 Also any of the problems patients have repaying a credit card (or other loan), of course, are no longer directly related to the hospital. These “indirect collections” still burden patients with medical debt.
Where you go matters. Because there is no common collection policy or practice among hospitals, choosing the wrong hospital may result in a lawsuit. A careful study of lawsuits for medical debt or garnishments related to that debt in 2017 in Virginia showed how being treated at certain hospitals dramatically changed the odds of wage garnishment for unpaid bills.9 It revealed that 29,286 hospital lawsuits were filed to collect medical debt—9,232 of which were wage garnishments (the most aggressive form of debt collection). Five hospitals alone accounted for the majority of garnishments in the state. Notably, nonprofit hospitals accounted for 71% of the garnishment cases. On the other hand, about 50% of the hospitals in the study did not file any lawsuits to garnish wages for medical debt.9
Why is there so much hospital debt?
One would think the Affordable Care Act (ACA) and other reforms would mean fewer people do not have health insurance—and the problems experienced by the patient in the case above. Indeed, the number of insured has increased in the United States, including through the expansion of Medicaid. Nonetheless, in 2020, the Census Bureau reported that 28 million people did not have health insurance for any part of the year; that figure would be higher if those who had insurance for only part of the year were included.10
One reason for medical debt is the very high level of “under” insurance—that is, even with health insurance, copays for significant medical bills exceed what the patient can pay. Nearly half of adults (excluding the elderly) were enrolled in high-deductible health plans (in 2017).11 Among most employment-based plans, deductibles and co-pays have been going up for a decade.12 Overall, 20% of employer-provided plans had deductibles in excess of $3,000 ($5,000 for families).13 Of course, many families do not have anywhere near the resources to pay high deductibles, and that represents likely medical debt. The more modest copays of Medicare (often 20%) can be enough to push some elderly individuals beyond their capacity to pay.
“Out-of-network” care also may result in large hospital charges—and debt. Emergency care, for example, may be sought from the closest provider, even though out of network, and the insurance company may refuse to pay the charges. Another surprise form of billing is when a health care insurance company tentatively approves coverage and then after the patient receives care, determines it was unnecessary. In that case, even in-network charges may be denied, with the patient left to pay all the charges.
Continue to: How medical debt affects patients...
How medical debt affects patients
For patients, medical debt places pressure on their financial circumstances. Bankruptcy has a profound financial impact, and approximately two-thirds of bankruptcies are related to medical care costs and debt, including “indirect collection.”14 Even when the financial effect is not so devastating, it is often substantial, as the above case demonstrated. In a 2018 survey, almost 30% of those with health insurance had medical debts in some form of collection action, and 25% of those individuals said they did not know they owed the money.15 The same survey found that 20% of respondents had medical debt that adversely affected their credit scores and access to credit.15
At work, although employers are not supposed to treat employees adversely because of garnishment, some employers may not adhere to that rule. Furthermore, employees may believe or be concerned that the very existence of garnishment may penalize them at their current job or make it difficult to move to a better one.16
Lastly, patients with medical debt may be reluctant to seek needed medical care. They may be concerned about adding more medical debt or embarrassed or afraid that they would not be welcome at the hospital where they owe money.7
Public perception of hospitals
Lawsuits against patients also have a negative effect on hospitals—and it is not limited to the relatively few institutions that file many of these lawsuits each year. Press reports about lawsuits against patients garner great public interest and anger, and this tarnishes the image of heath care facilities in general because many people often do not distinguish the actions of a few institutions.
The sensitivity of health care organizations to bad publicity from debt collection practices was seen in a follow-up study of the previously discussed Virginia data. In the year following this report, there was a 59% decrease in the number of lawsuits filed, including a 66% decrease in garnishments.17 Eleven hospitals in the state that had been filing debt lawsuits stopped doing so.17
Medical debt: The obligation of nonprofit hospitals
The response seen in the Virginia follow-up study may also reflect well-founded concern from board members about political consequences and even taxation problems. The majority of hospitals, including those in these studies, are nonprofit institutions with an Internal Revenue Service (IRS) 501(c)(3) “tax-exempt” status. (Note, “nonprofit” does not mean that the organization does not make a profit, but that the profit does not accrue to individuals.) The “nonprofit” status is usually granted by states, but the federal tax-exempt status is granted by the IRS. This status exempts the institutions from paying most federal taxes, and (perhaps most importantly) qualifies donors to receive tax deductions (and similar benefits) for donations made to these hospitals. This important tax treatment is granted based on the theory that their services are so valuable to the public that advancing their work through the tax exemption ultimately benefits the public more than the tax revenue would.
In return for these benefits, the organization has obligations to work in the public interest. For years, hospitals have been criticized for not providing sufficient public benefits (compared, for example, with for-profit hospitals) to justify the tax exemption. That criticism caused the IRS to begin requiring a special Form 990, Schedule H, which is attached to the usual 501(c)(3) informational tax return, “to provide information on the activities and policies of, and community benefit provided by, its hospital facilities and other non-hospital health care facilities.”18 Part III of Schedule H asks, in part, about bad debt and collection practices.
Then the ACA Section 501(r) enhanced the obligation of nonprofit health facilities to provide charitable care in two ways. First, they must have, and make available, policies to provide free and discounted care; and second, they cannot sue for payment until they make an individualized determination as to whether the patient should have received discounted care or financial assistance.19
Thus aggressive collection practices (which should include “indirect collection”) invite special scrutiny by local officials and the IRS. In the longer-term, concern that tax-exempt hospitals are not truly operating in the public interest is undoubtedly amplified by these aggressive debt collection practices. How can a hospital claim it is truly operating in the public interest when it sues dozens of modest-income individuals each year?
Regulating medical debt and its collection
The No Surprises Act
In December 2020, Congress adopted the No Surprises Act to address some of the problems of patient debt.20 Among other things, the act protects patients “from receiving surprise medical bills when they receive most emergency services,” or when they are in an in-network hospital but receive services from out-of-network providers (such as anesthesia and radiology).21 Several states also have similar legislation, so the federal law specifically states that where state laws are more protective of patients, the state’s higher protections apply, and vice versa. The act took effect on January 1, 2022, though there is an “interim final” regulation that will be subject to change, and there is already litigation over those regulations.22 The real complexity of the rules will arise through the regulations, which are likely to change several times over the next few years. To help with this, the American Medical Association has an extensive toolkit for health care providers.23
Continue to: Additional regulations...
Additional regulations
Both the federal government and most states are likely to take additional action to reduce hospital debt lawsuits. Some proposals sound simple enough but would have significant complications. For example, governments could prohibit all lawsuits that collect hospital debt.7 Such a regulation would mean that paying hospital debts would essentially become optional. Imagine the millionaire who does not want to pay a $25,000 hospital charge; or patients with other debts who would pay those off before the hospital debt. The regulation might have income or asset limits on debt collection lawsuits and the like, but it quickly becomes complicated. Furthermore, to protect themselves, hospitals would undoubtedly become much more aggressive about requiring up-front payments—which would force the debt or prepayment onto credit cards or similar debt obligations that are not subject to the no collection lawsuit rule.
Public reporting. The follow-up study in Virginia17 suggests that requiring public reporting of the number of cases filed by or on behalf of (directly or indirectly) each hospital may help. Hospitals would, of course, have incentives to make their figures look better, perhaps by selling the debt to an agency that would be able to file suit in its name rather than the hospital’s name. These might be little more than indirect collections. For reporting purposes, any form of transferring debt might be considered filing a lawsuit. The problem, noted earlier, about requiring prepayment or credit cards would also exist.
Get the board involved. A different approach would be to ensure that a hospital’s board of trustees is involved in setting and overseeing debt collection policies. For example, the law might require boards to annually consider and adopt specific debt collection practices—including indirect collection efforts. Boards should already be doing something similar to this, but regulation might be an inexpensive way to ensure it is done—and in a manner consistent with the organization’s values. Another suggestion is to require the board to approve any legal action against specific patients.7 By making sure this is not just another item on the consent agenda, the oversight would probably reduce automatic debt collection processes.
Expand IRS reporting requirements for nonprofits. Indeed, for nonprofit hospitals with 501(c)(3) obligations, the Form 990, Schedule H already provides some information about collection actions and uncompensated care, and this is enhanced by the ACA Section 501(r). These could be expanded and perhaps include “indirect” collections. The IRS could “flag” hospitals with high total litigation and similar collection actions, and ask the hospital to provide a detailed explanation for each action and how it was consistent with the obligation to serve the public (thereby justifying the exempt taxation status, an idea proposed by the US Government Accountability Office in 2020).24
Ensure the hospital’s actions reflect their mission and values
Hospitals are created to provide medical care for people and to improve the human condition. Those who lead them should, and generally do, share that purpose. The apparent collection policies that have garnered negative public attention suggest that some of these institutions have lost focus of their ultimate mission and values. The boards and executives of these health care institutions, as well as the medical professionals and attorneys who serve them, should be continuously guided by those values.
Important decisions—including collection and prepayment processes—reflect the values of the institution. Failure to ensure these procedures are in line with the organization’s mission is an embarrassment to all health care facilities, including the majority of hospitals that do not engage in these aggressive collection practices. Not addressing these issues will likely result in political and legal action—blunt and inefficient instruments—to limit what the public sees as wrongdoing. ●
- Kiel P. From the E.R. to the courtroom: how nonprofit hospitals are seizing patients’ wages. ProPublica. December 19, 2014. Accessed March 21, 2022. https://www.propublica.org/article/how-nonprofit-hospitals-are-seizing-patients-wages
- Cooper Z, Han J, Mahoney N. Hospital lawsuits over unpaid bills increased by 37 percent in Wisconsin from 2001 to 2018. Health Affairs. 2021;40:1830-1835. Accessed March 21, 2022. https://www.healthaffairs.org/doi/full/10.1377 /hlthaff.2021.01130
- LaMantia J. New York hospitals have filed thousands of lawsuits against patients. Modern Healthcare. March 13, 2020. Accessed March 21, 2022. https://www.modernhealthcare .com/legal/new-york-hospitals-have-filed-thousands -lawsuits-against-patients
- Armour S. When patients can’t pay, many hospitals are suing. Wall Street Journal. June 25, 2019. Accessed March 21, 2022. https://www.wsj.com/articles/nonprofit-hospitals-criticized-for-debt-collection-tactics-11561467600
- McGhee M, Chase W. How America’s top hospitals hound patients with predatory billing. Axios. Accessed March 21, 2022. https://www.axios.com/hospital-billing
- Owens C. Public spotlight on hospital lawsuits may slow them down. June 14, 2021. Accessed March 22, 2022. https:// www.axios.com/hospital-lawsuits-slowing-down-media -35ce395a-9fe3-4b23-b815-d7b06cce2773.html
- Buck ID. When hospitals sue patients. Hastings L.J. 2022;73:191-232, at 209-211. Accessed March 21, 2022. https:// repository.uchastings.edu/cgi/viewcontent.cgi?article =3961&context=hastings_law_journal
- Lagasse J. Healthcare turns to zero-interest loans to give patients a better reason to pay. Healthcare Finance. May 3, 2017. Accessed March 21, 2022. https://www.healthcarefinancenews.com/news/healthcare-turns-zero-interest-loans-give-patients-better-reason-pay#:~:text=Zero%2Dinterest%20loans%20are%20finding,of%20the%20patient%2Dprovider%20relationship.
- Bruhn WE, Rutkow L, Wang P, et al. Prevalence and characteristics of Virginia hospitals suing patients and garnishing wages for unpaid medical bills. JAMA. 2019;322:691-692. doi:10.1001/jama.2019.9144
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. September 14, 2021. United States Census Bureau Current Population Reports, P60-274. US Government Publishing Office; September 2021. Accessed March 21, 2022. https://www.census.gov/content/dam /Census/library/publications/2021/demo/p60-274.pdf
- Cohen RA, Zammitti EP. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief, No. 317. August 2018. Accessed March 21, 2022. https://www.cdc.gov/nchs/data/databriefs/db317.pdf
- Kaiser Family Foundation. Employer health benefits: 2020 summary of findings. Accessed March 21, 2022. https://www.kff.org/report-section/ehbs-2020-summary-of-findings/
- Picchi A. Higher health insurance deductibles a sickening trend for Americans. CBS NEWS. June 13, 2019. Accessed March 21, 2022. https://www.cbsnews.com/news/high-health-insurance-deductibles-a-sickening-trend-thats -causing-financial-hardship/
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the Affordable Care Act. Am J Public Health. 2019;109:431-433. doi:10.2105/AJPH.2018.304901
- Rosato D. What medical debt does to your credit score. Consumer Reports. July 26, 2018. Accessed March 21, 2022. https://www.consumerreports.org/credit-scores-reports/what-medical-debt-does-to-your-credit-score/
- State laws on wage garnishments. Nolo web site. https://www.nolo.com/legal-encyclopedia/free-books/employee-rights-book/chapter2-9.html. Accessed April 1, 2022.
- Patruzo JGR, Hashim F, Dun C, et al. Trends in hospital lawsuits filed against patients for unpaid bills following published research about their activity. JAMA Network Open. 2021;4:e2121926. doi:10.1001/jamanetworkopen.2021.21926
- About Schedule H (Form 990), hospitals. IRS. Updated June 10, 2021. Accessed March 21, 2022. https://www.irs.gov/forms-pubs/about-schedule-h-form-990
- Requirements for 501(c)(3) hospitals under the Affordable Care Act – Section 501(r). Updated September 9, 2021. Accessed March 21, 2022. https://www.irs.gov/charities-non-profits/charitable-organizations/requirements-for-501c3-hospitals-under-the-affordable-care-act-section-501r
- Pub. L. No. 116-260, 134 Stat. 1182, Division BB, § 109.
- Fact sheet. No Surprises: understand your rights against surprise medical bills. Centers for Medicare and Medicaid Services. January 3, 2022. Accessed March 21, 2022. https://www.cms.gov/newsroom/fact-sheets/no-surprises-understand-your-rights-against-surprise-medical-bills
- Implementation of the No Surprises Act. Accessed March 21, 2022. https://www.ama-assn.org/delivering-care/patient-support-advocacy/implementation-no-surprises-act
- American Medical Association. Toolkit for physicians: preparing for implementation of the No Surprises Act. January 2022. Accessed March 21, 2022. https://www.ama-assn.org/system/files/ama-nsa-toolkit.pdf
- US Government Accountability Office. Tax administration: opportunities exist to improve oversight of hospitals’ taxexempt status. September 2020. Accessed March 21, 2022. https://www.gao.gov/assets/gao-20-679.pdf
Although it is common to read about patients suing their hospitals, there has been increasing public and political attention given to hospitals suing their patients to collect unpaid hospital bills. KH’s story began with an emergency appendectomy. She did not have health insurance to cover the $14,000 hospital bill. The family was unable to pay the bill, and the nonprofit hospital sued them for that bill, plus some additional expenses (totaling about $17,000), plus interest was accumulating at 9% per year. The hospital won a judgment, and it garnished the husband’s pay (10% of after-taxes pay, in this case) and placed a lien on the family’s home. Years later—because of interest and additional hospital bills—the family had paid $20,000, but still owed $26,000.1
The extent of the problem
This is neither a hypothetical case nor a rare event. Studies and press reports have noted dozens of examples of hospital collection excesses. One study found that unpaid medical bill lawsuits increased by 37% in Wisconsin between 2001 and 2018, with 5% of hospitals accounting for 25% of the lawsuits.2 Another report found almost “31,000 civil cases filed by 139 hospitals in 26 New York counties from 2015 to 2019.”3 Similar to the Wisconsin report, a small number of health care providers accounted for the majority of lawsuits. In another example, one Missouri nonprofit hospital, Heartland (rebranded “Mosaic”), created its own for-profit debt collection agency (Northwest Financial Services), which filed 11,000 lawsuits from 2009 to 2013, resulting in 6,000 wage garnishments.1 The Wall Street Journal, among others, has reported for years on the difficulties created by lawsuits against patients.4 Axios and Johns Hopkins reported that “medical debt comprises 58% of all debt collections in the United States.” And although some collection actions declined early in the pandemic, it did not appear to last.5,6
Inconsistent collection policies. Collection policies vary greatly from hospital to hospital, with an increasing number of hospitals demanding up-front payments (before services). Many of these health care institutions persuade patients to put medical debt on their credit cards, sometimes as part of an up-front (before service) process.7 If using a standard credit card, this comes with a very high interest rate. There are some special health-related credit cards, such as CareCredit, that generally have better interest rates. These cards offer no-interest short-term loans, with significant interest for longer-term loans. Thus, failure to repay the full amount when due means that the “deferred interest” (about 27%) must be paid.8 Also any of the problems patients have repaying a credit card (or other loan), of course, are no longer directly related to the hospital. These “indirect collections” still burden patients with medical debt.
Where you go matters. Because there is no common collection policy or practice among hospitals, choosing the wrong hospital may result in a lawsuit. A careful study of lawsuits for medical debt or garnishments related to that debt in 2017 in Virginia showed how being treated at certain hospitals dramatically changed the odds of wage garnishment for unpaid bills.9 It revealed that 29,286 hospital lawsuits were filed to collect medical debt—9,232 of which were wage garnishments (the most aggressive form of debt collection). Five hospitals alone accounted for the majority of garnishments in the state. Notably, nonprofit hospitals accounted for 71% of the garnishment cases. On the other hand, about 50% of the hospitals in the study did not file any lawsuits to garnish wages for medical debt.9
Why is there so much hospital debt?
One would think the Affordable Care Act (ACA) and other reforms would mean fewer people do not have health insurance—and the problems experienced by the patient in the case above. Indeed, the number of insured has increased in the United States, including through the expansion of Medicaid. Nonetheless, in 2020, the Census Bureau reported that 28 million people did not have health insurance for any part of the year; that figure would be higher if those who had insurance for only part of the year were included.10
One reason for medical debt is the very high level of “under” insurance—that is, even with health insurance, copays for significant medical bills exceed what the patient can pay. Nearly half of adults (excluding the elderly) were enrolled in high-deductible health plans (in 2017).11 Among most employment-based plans, deductibles and co-pays have been going up for a decade.12 Overall, 20% of employer-provided plans had deductibles in excess of $3,000 ($5,000 for families).13 Of course, many families do not have anywhere near the resources to pay high deductibles, and that represents likely medical debt. The more modest copays of Medicare (often 20%) can be enough to push some elderly individuals beyond their capacity to pay.
“Out-of-network” care also may result in large hospital charges—and debt. Emergency care, for example, may be sought from the closest provider, even though out of network, and the insurance company may refuse to pay the charges. Another surprise form of billing is when a health care insurance company tentatively approves coverage and then after the patient receives care, determines it was unnecessary. In that case, even in-network charges may be denied, with the patient left to pay all the charges.
Continue to: How medical debt affects patients...
How medical debt affects patients
For patients, medical debt places pressure on their financial circumstances. Bankruptcy has a profound financial impact, and approximately two-thirds of bankruptcies are related to medical care costs and debt, including “indirect collection.”14 Even when the financial effect is not so devastating, it is often substantial, as the above case demonstrated. In a 2018 survey, almost 30% of those with health insurance had medical debts in some form of collection action, and 25% of those individuals said they did not know they owed the money.15 The same survey found that 20% of respondents had medical debt that adversely affected their credit scores and access to credit.15
At work, although employers are not supposed to treat employees adversely because of garnishment, some employers may not adhere to that rule. Furthermore, employees may believe or be concerned that the very existence of garnishment may penalize them at their current job or make it difficult to move to a better one.16
Lastly, patients with medical debt may be reluctant to seek needed medical care. They may be concerned about adding more medical debt or embarrassed or afraid that they would not be welcome at the hospital where they owe money.7
Public perception of hospitals
Lawsuits against patients also have a negative effect on hospitals—and it is not limited to the relatively few institutions that file many of these lawsuits each year. Press reports about lawsuits against patients garner great public interest and anger, and this tarnishes the image of heath care facilities in general because many people often do not distinguish the actions of a few institutions.
The sensitivity of health care organizations to bad publicity from debt collection practices was seen in a follow-up study of the previously discussed Virginia data. In the year following this report, there was a 59% decrease in the number of lawsuits filed, including a 66% decrease in garnishments.17 Eleven hospitals in the state that had been filing debt lawsuits stopped doing so.17
Medical debt: The obligation of nonprofit hospitals
The response seen in the Virginia follow-up study may also reflect well-founded concern from board members about political consequences and even taxation problems. The majority of hospitals, including those in these studies, are nonprofit institutions with an Internal Revenue Service (IRS) 501(c)(3) “tax-exempt” status. (Note, “nonprofit” does not mean that the organization does not make a profit, but that the profit does not accrue to individuals.) The “nonprofit” status is usually granted by states, but the federal tax-exempt status is granted by the IRS. This status exempts the institutions from paying most federal taxes, and (perhaps most importantly) qualifies donors to receive tax deductions (and similar benefits) for donations made to these hospitals. This important tax treatment is granted based on the theory that their services are so valuable to the public that advancing their work through the tax exemption ultimately benefits the public more than the tax revenue would.
In return for these benefits, the organization has obligations to work in the public interest. For years, hospitals have been criticized for not providing sufficient public benefits (compared, for example, with for-profit hospitals) to justify the tax exemption. That criticism caused the IRS to begin requiring a special Form 990, Schedule H, which is attached to the usual 501(c)(3) informational tax return, “to provide information on the activities and policies of, and community benefit provided by, its hospital facilities and other non-hospital health care facilities.”18 Part III of Schedule H asks, in part, about bad debt and collection practices.
Then the ACA Section 501(r) enhanced the obligation of nonprofit health facilities to provide charitable care in two ways. First, they must have, and make available, policies to provide free and discounted care; and second, they cannot sue for payment until they make an individualized determination as to whether the patient should have received discounted care or financial assistance.19
Thus aggressive collection practices (which should include “indirect collection”) invite special scrutiny by local officials and the IRS. In the longer-term, concern that tax-exempt hospitals are not truly operating in the public interest is undoubtedly amplified by these aggressive debt collection practices. How can a hospital claim it is truly operating in the public interest when it sues dozens of modest-income individuals each year?
Regulating medical debt and its collection
The No Surprises Act
In December 2020, Congress adopted the No Surprises Act to address some of the problems of patient debt.20 Among other things, the act protects patients “from receiving surprise medical bills when they receive most emergency services,” or when they are in an in-network hospital but receive services from out-of-network providers (such as anesthesia and radiology).21 Several states also have similar legislation, so the federal law specifically states that where state laws are more protective of patients, the state’s higher protections apply, and vice versa. The act took effect on January 1, 2022, though there is an “interim final” regulation that will be subject to change, and there is already litigation over those regulations.22 The real complexity of the rules will arise through the regulations, which are likely to change several times over the next few years. To help with this, the American Medical Association has an extensive toolkit for health care providers.23
Continue to: Additional regulations...
Additional regulations
Both the federal government and most states are likely to take additional action to reduce hospital debt lawsuits. Some proposals sound simple enough but would have significant complications. For example, governments could prohibit all lawsuits that collect hospital debt.7 Such a regulation would mean that paying hospital debts would essentially become optional. Imagine the millionaire who does not want to pay a $25,000 hospital charge; or patients with other debts who would pay those off before the hospital debt. The regulation might have income or asset limits on debt collection lawsuits and the like, but it quickly becomes complicated. Furthermore, to protect themselves, hospitals would undoubtedly become much more aggressive about requiring up-front payments—which would force the debt or prepayment onto credit cards or similar debt obligations that are not subject to the no collection lawsuit rule.
Public reporting. The follow-up study in Virginia17 suggests that requiring public reporting of the number of cases filed by or on behalf of (directly or indirectly) each hospital may help. Hospitals would, of course, have incentives to make their figures look better, perhaps by selling the debt to an agency that would be able to file suit in its name rather than the hospital’s name. These might be little more than indirect collections. For reporting purposes, any form of transferring debt might be considered filing a lawsuit. The problem, noted earlier, about requiring prepayment or credit cards would also exist.
Get the board involved. A different approach would be to ensure that a hospital’s board of trustees is involved in setting and overseeing debt collection policies. For example, the law might require boards to annually consider and adopt specific debt collection practices—including indirect collection efforts. Boards should already be doing something similar to this, but regulation might be an inexpensive way to ensure it is done—and in a manner consistent with the organization’s values. Another suggestion is to require the board to approve any legal action against specific patients.7 By making sure this is not just another item on the consent agenda, the oversight would probably reduce automatic debt collection processes.
Expand IRS reporting requirements for nonprofits. Indeed, for nonprofit hospitals with 501(c)(3) obligations, the Form 990, Schedule H already provides some information about collection actions and uncompensated care, and this is enhanced by the ACA Section 501(r). These could be expanded and perhaps include “indirect” collections. The IRS could “flag” hospitals with high total litigation and similar collection actions, and ask the hospital to provide a detailed explanation for each action and how it was consistent with the obligation to serve the public (thereby justifying the exempt taxation status, an idea proposed by the US Government Accountability Office in 2020).24
Ensure the hospital’s actions reflect their mission and values
Hospitals are created to provide medical care for people and to improve the human condition. Those who lead them should, and generally do, share that purpose. The apparent collection policies that have garnered negative public attention suggest that some of these institutions have lost focus of their ultimate mission and values. The boards and executives of these health care institutions, as well as the medical professionals and attorneys who serve them, should be continuously guided by those values.
Important decisions—including collection and prepayment processes—reflect the values of the institution. Failure to ensure these procedures are in line with the organization’s mission is an embarrassment to all health care facilities, including the majority of hospitals that do not engage in these aggressive collection practices. Not addressing these issues will likely result in political and legal action—blunt and inefficient instruments—to limit what the public sees as wrongdoing. ●
Although it is common to read about patients suing their hospitals, there has been increasing public and political attention given to hospitals suing their patients to collect unpaid hospital bills. KH’s story began with an emergency appendectomy. She did not have health insurance to cover the $14,000 hospital bill. The family was unable to pay the bill, and the nonprofit hospital sued them for that bill, plus some additional expenses (totaling about $17,000), plus interest was accumulating at 9% per year. The hospital won a judgment, and it garnished the husband’s pay (10% of after-taxes pay, in this case) and placed a lien on the family’s home. Years later—because of interest and additional hospital bills—the family had paid $20,000, but still owed $26,000.1
The extent of the problem
This is neither a hypothetical case nor a rare event. Studies and press reports have noted dozens of examples of hospital collection excesses. One study found that unpaid medical bill lawsuits increased by 37% in Wisconsin between 2001 and 2018, with 5% of hospitals accounting for 25% of the lawsuits.2 Another report found almost “31,000 civil cases filed by 139 hospitals in 26 New York counties from 2015 to 2019.”3 Similar to the Wisconsin report, a small number of health care providers accounted for the majority of lawsuits. In another example, one Missouri nonprofit hospital, Heartland (rebranded “Mosaic”), created its own for-profit debt collection agency (Northwest Financial Services), which filed 11,000 lawsuits from 2009 to 2013, resulting in 6,000 wage garnishments.1 The Wall Street Journal, among others, has reported for years on the difficulties created by lawsuits against patients.4 Axios and Johns Hopkins reported that “medical debt comprises 58% of all debt collections in the United States.” And although some collection actions declined early in the pandemic, it did not appear to last.5,6
Inconsistent collection policies. Collection policies vary greatly from hospital to hospital, with an increasing number of hospitals demanding up-front payments (before services). Many of these health care institutions persuade patients to put medical debt on their credit cards, sometimes as part of an up-front (before service) process.7 If using a standard credit card, this comes with a very high interest rate. There are some special health-related credit cards, such as CareCredit, that generally have better interest rates. These cards offer no-interest short-term loans, with significant interest for longer-term loans. Thus, failure to repay the full amount when due means that the “deferred interest” (about 27%) must be paid.8 Also any of the problems patients have repaying a credit card (or other loan), of course, are no longer directly related to the hospital. These “indirect collections” still burden patients with medical debt.
Where you go matters. Because there is no common collection policy or practice among hospitals, choosing the wrong hospital may result in a lawsuit. A careful study of lawsuits for medical debt or garnishments related to that debt in 2017 in Virginia showed how being treated at certain hospitals dramatically changed the odds of wage garnishment for unpaid bills.9 It revealed that 29,286 hospital lawsuits were filed to collect medical debt—9,232 of which were wage garnishments (the most aggressive form of debt collection). Five hospitals alone accounted for the majority of garnishments in the state. Notably, nonprofit hospitals accounted for 71% of the garnishment cases. On the other hand, about 50% of the hospitals in the study did not file any lawsuits to garnish wages for medical debt.9
Why is there so much hospital debt?
One would think the Affordable Care Act (ACA) and other reforms would mean fewer people do not have health insurance—and the problems experienced by the patient in the case above. Indeed, the number of insured has increased in the United States, including through the expansion of Medicaid. Nonetheless, in 2020, the Census Bureau reported that 28 million people did not have health insurance for any part of the year; that figure would be higher if those who had insurance for only part of the year were included.10
One reason for medical debt is the very high level of “under” insurance—that is, even with health insurance, copays for significant medical bills exceed what the patient can pay. Nearly half of adults (excluding the elderly) were enrolled in high-deductible health plans (in 2017).11 Among most employment-based plans, deductibles and co-pays have been going up for a decade.12 Overall, 20% of employer-provided plans had deductibles in excess of $3,000 ($5,000 for families).13 Of course, many families do not have anywhere near the resources to pay high deductibles, and that represents likely medical debt. The more modest copays of Medicare (often 20%) can be enough to push some elderly individuals beyond their capacity to pay.
“Out-of-network” care also may result in large hospital charges—and debt. Emergency care, for example, may be sought from the closest provider, even though out of network, and the insurance company may refuse to pay the charges. Another surprise form of billing is when a health care insurance company tentatively approves coverage and then after the patient receives care, determines it was unnecessary. In that case, even in-network charges may be denied, with the patient left to pay all the charges.
Continue to: How medical debt affects patients...
How medical debt affects patients
For patients, medical debt places pressure on their financial circumstances. Bankruptcy has a profound financial impact, and approximately two-thirds of bankruptcies are related to medical care costs and debt, including “indirect collection.”14 Even when the financial effect is not so devastating, it is often substantial, as the above case demonstrated. In a 2018 survey, almost 30% of those with health insurance had medical debts in some form of collection action, and 25% of those individuals said they did not know they owed the money.15 The same survey found that 20% of respondents had medical debt that adversely affected their credit scores and access to credit.15
At work, although employers are not supposed to treat employees adversely because of garnishment, some employers may not adhere to that rule. Furthermore, employees may believe or be concerned that the very existence of garnishment may penalize them at their current job or make it difficult to move to a better one.16
Lastly, patients with medical debt may be reluctant to seek needed medical care. They may be concerned about adding more medical debt or embarrassed or afraid that they would not be welcome at the hospital where they owe money.7
Public perception of hospitals
Lawsuits against patients also have a negative effect on hospitals—and it is not limited to the relatively few institutions that file many of these lawsuits each year. Press reports about lawsuits against patients garner great public interest and anger, and this tarnishes the image of heath care facilities in general because many people often do not distinguish the actions of a few institutions.
The sensitivity of health care organizations to bad publicity from debt collection practices was seen in a follow-up study of the previously discussed Virginia data. In the year following this report, there was a 59% decrease in the number of lawsuits filed, including a 66% decrease in garnishments.17 Eleven hospitals in the state that had been filing debt lawsuits stopped doing so.17
Medical debt: The obligation of nonprofit hospitals
The response seen in the Virginia follow-up study may also reflect well-founded concern from board members about political consequences and even taxation problems. The majority of hospitals, including those in these studies, are nonprofit institutions with an Internal Revenue Service (IRS) 501(c)(3) “tax-exempt” status. (Note, “nonprofit” does not mean that the organization does not make a profit, but that the profit does not accrue to individuals.) The “nonprofit” status is usually granted by states, but the federal tax-exempt status is granted by the IRS. This status exempts the institutions from paying most federal taxes, and (perhaps most importantly) qualifies donors to receive tax deductions (and similar benefits) for donations made to these hospitals. This important tax treatment is granted based on the theory that their services are so valuable to the public that advancing their work through the tax exemption ultimately benefits the public more than the tax revenue would.
In return for these benefits, the organization has obligations to work in the public interest. For years, hospitals have been criticized for not providing sufficient public benefits (compared, for example, with for-profit hospitals) to justify the tax exemption. That criticism caused the IRS to begin requiring a special Form 990, Schedule H, which is attached to the usual 501(c)(3) informational tax return, “to provide information on the activities and policies of, and community benefit provided by, its hospital facilities and other non-hospital health care facilities.”18 Part III of Schedule H asks, in part, about bad debt and collection practices.
Then the ACA Section 501(r) enhanced the obligation of nonprofit health facilities to provide charitable care in two ways. First, they must have, and make available, policies to provide free and discounted care; and second, they cannot sue for payment until they make an individualized determination as to whether the patient should have received discounted care or financial assistance.19
Thus aggressive collection practices (which should include “indirect collection”) invite special scrutiny by local officials and the IRS. In the longer-term, concern that tax-exempt hospitals are not truly operating in the public interest is undoubtedly amplified by these aggressive debt collection practices. How can a hospital claim it is truly operating in the public interest when it sues dozens of modest-income individuals each year?
Regulating medical debt and its collection
The No Surprises Act
In December 2020, Congress adopted the No Surprises Act to address some of the problems of patient debt.20 Among other things, the act protects patients “from receiving surprise medical bills when they receive most emergency services,” or when they are in an in-network hospital but receive services from out-of-network providers (such as anesthesia and radiology).21 Several states also have similar legislation, so the federal law specifically states that where state laws are more protective of patients, the state’s higher protections apply, and vice versa. The act took effect on January 1, 2022, though there is an “interim final” regulation that will be subject to change, and there is already litigation over those regulations.22 The real complexity of the rules will arise through the regulations, which are likely to change several times over the next few years. To help with this, the American Medical Association has an extensive toolkit for health care providers.23
Continue to: Additional regulations...
Additional regulations
Both the federal government and most states are likely to take additional action to reduce hospital debt lawsuits. Some proposals sound simple enough but would have significant complications. For example, governments could prohibit all lawsuits that collect hospital debt.7 Such a regulation would mean that paying hospital debts would essentially become optional. Imagine the millionaire who does not want to pay a $25,000 hospital charge; or patients with other debts who would pay those off before the hospital debt. The regulation might have income or asset limits on debt collection lawsuits and the like, but it quickly becomes complicated. Furthermore, to protect themselves, hospitals would undoubtedly become much more aggressive about requiring up-front payments—which would force the debt or prepayment onto credit cards or similar debt obligations that are not subject to the no collection lawsuit rule.
Public reporting. The follow-up study in Virginia17 suggests that requiring public reporting of the number of cases filed by or on behalf of (directly or indirectly) each hospital may help. Hospitals would, of course, have incentives to make their figures look better, perhaps by selling the debt to an agency that would be able to file suit in its name rather than the hospital’s name. These might be little more than indirect collections. For reporting purposes, any form of transferring debt might be considered filing a lawsuit. The problem, noted earlier, about requiring prepayment or credit cards would also exist.
Get the board involved. A different approach would be to ensure that a hospital’s board of trustees is involved in setting and overseeing debt collection policies. For example, the law might require boards to annually consider and adopt specific debt collection practices—including indirect collection efforts. Boards should already be doing something similar to this, but regulation might be an inexpensive way to ensure it is done—and in a manner consistent with the organization’s values. Another suggestion is to require the board to approve any legal action against specific patients.7 By making sure this is not just another item on the consent agenda, the oversight would probably reduce automatic debt collection processes.
Expand IRS reporting requirements for nonprofits. Indeed, for nonprofit hospitals with 501(c)(3) obligations, the Form 990, Schedule H already provides some information about collection actions and uncompensated care, and this is enhanced by the ACA Section 501(r). These could be expanded and perhaps include “indirect” collections. The IRS could “flag” hospitals with high total litigation and similar collection actions, and ask the hospital to provide a detailed explanation for each action and how it was consistent with the obligation to serve the public (thereby justifying the exempt taxation status, an idea proposed by the US Government Accountability Office in 2020).24
Ensure the hospital’s actions reflect their mission and values
Hospitals are created to provide medical care for people and to improve the human condition. Those who lead them should, and generally do, share that purpose. The apparent collection policies that have garnered negative public attention suggest that some of these institutions have lost focus of their ultimate mission and values. The boards and executives of these health care institutions, as well as the medical professionals and attorneys who serve them, should be continuously guided by those values.
Important decisions—including collection and prepayment processes—reflect the values of the institution. Failure to ensure these procedures are in line with the organization’s mission is an embarrassment to all health care facilities, including the majority of hospitals that do not engage in these aggressive collection practices. Not addressing these issues will likely result in political and legal action—blunt and inefficient instruments—to limit what the public sees as wrongdoing. ●
- Kiel P. From the E.R. to the courtroom: how nonprofit hospitals are seizing patients’ wages. ProPublica. December 19, 2014. Accessed March 21, 2022. https://www.propublica.org/article/how-nonprofit-hospitals-are-seizing-patients-wages
- Cooper Z, Han J, Mahoney N. Hospital lawsuits over unpaid bills increased by 37 percent in Wisconsin from 2001 to 2018. Health Affairs. 2021;40:1830-1835. Accessed March 21, 2022. https://www.healthaffairs.org/doi/full/10.1377 /hlthaff.2021.01130
- LaMantia J. New York hospitals have filed thousands of lawsuits against patients. Modern Healthcare. March 13, 2020. Accessed March 21, 2022. https://www.modernhealthcare .com/legal/new-york-hospitals-have-filed-thousands -lawsuits-against-patients
- Armour S. When patients can’t pay, many hospitals are suing. Wall Street Journal. June 25, 2019. Accessed March 21, 2022. https://www.wsj.com/articles/nonprofit-hospitals-criticized-for-debt-collection-tactics-11561467600
- McGhee M, Chase W. How America’s top hospitals hound patients with predatory billing. Axios. Accessed March 21, 2022. https://www.axios.com/hospital-billing
- Owens C. Public spotlight on hospital lawsuits may slow them down. June 14, 2021. Accessed March 22, 2022. https:// www.axios.com/hospital-lawsuits-slowing-down-media -35ce395a-9fe3-4b23-b815-d7b06cce2773.html
- Buck ID. When hospitals sue patients. Hastings L.J. 2022;73:191-232, at 209-211. Accessed March 21, 2022. https:// repository.uchastings.edu/cgi/viewcontent.cgi?article =3961&context=hastings_law_journal
- Lagasse J. Healthcare turns to zero-interest loans to give patients a better reason to pay. Healthcare Finance. May 3, 2017. Accessed March 21, 2022. https://www.healthcarefinancenews.com/news/healthcare-turns-zero-interest-loans-give-patients-better-reason-pay#:~:text=Zero%2Dinterest%20loans%20are%20finding,of%20the%20patient%2Dprovider%20relationship.
- Bruhn WE, Rutkow L, Wang P, et al. Prevalence and characteristics of Virginia hospitals suing patients and garnishing wages for unpaid medical bills. JAMA. 2019;322:691-692. doi:10.1001/jama.2019.9144
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. September 14, 2021. United States Census Bureau Current Population Reports, P60-274. US Government Publishing Office; September 2021. Accessed March 21, 2022. https://www.census.gov/content/dam /Census/library/publications/2021/demo/p60-274.pdf
- Cohen RA, Zammitti EP. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief, No. 317. August 2018. Accessed March 21, 2022. https://www.cdc.gov/nchs/data/databriefs/db317.pdf
- Kaiser Family Foundation. Employer health benefits: 2020 summary of findings. Accessed March 21, 2022. https://www.kff.org/report-section/ehbs-2020-summary-of-findings/
- Picchi A. Higher health insurance deductibles a sickening trend for Americans. CBS NEWS. June 13, 2019. Accessed March 21, 2022. https://www.cbsnews.com/news/high-health-insurance-deductibles-a-sickening-trend-thats -causing-financial-hardship/
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the Affordable Care Act. Am J Public Health. 2019;109:431-433. doi:10.2105/AJPH.2018.304901
- Rosato D. What medical debt does to your credit score. Consumer Reports. July 26, 2018. Accessed March 21, 2022. https://www.consumerreports.org/credit-scores-reports/what-medical-debt-does-to-your-credit-score/
- State laws on wage garnishments. Nolo web site. https://www.nolo.com/legal-encyclopedia/free-books/employee-rights-book/chapter2-9.html. Accessed April 1, 2022.
- Patruzo JGR, Hashim F, Dun C, et al. Trends in hospital lawsuits filed against patients for unpaid bills following published research about their activity. JAMA Network Open. 2021;4:e2121926. doi:10.1001/jamanetworkopen.2021.21926
- About Schedule H (Form 990), hospitals. IRS. Updated June 10, 2021. Accessed March 21, 2022. https://www.irs.gov/forms-pubs/about-schedule-h-form-990
- Requirements for 501(c)(3) hospitals under the Affordable Care Act – Section 501(r). Updated September 9, 2021. Accessed March 21, 2022. https://www.irs.gov/charities-non-profits/charitable-organizations/requirements-for-501c3-hospitals-under-the-affordable-care-act-section-501r
- Pub. L. No. 116-260, 134 Stat. 1182, Division BB, § 109.
- Fact sheet. No Surprises: understand your rights against surprise medical bills. Centers for Medicare and Medicaid Services. January 3, 2022. Accessed March 21, 2022. https://www.cms.gov/newsroom/fact-sheets/no-surprises-understand-your-rights-against-surprise-medical-bills
- Implementation of the No Surprises Act. Accessed March 21, 2022. https://www.ama-assn.org/delivering-care/patient-support-advocacy/implementation-no-surprises-act
- American Medical Association. Toolkit for physicians: preparing for implementation of the No Surprises Act. January 2022. Accessed March 21, 2022. https://www.ama-assn.org/system/files/ama-nsa-toolkit.pdf
- US Government Accountability Office. Tax administration: opportunities exist to improve oversight of hospitals’ taxexempt status. September 2020. Accessed March 21, 2022. https://www.gao.gov/assets/gao-20-679.pdf
- Kiel P. From the E.R. to the courtroom: how nonprofit hospitals are seizing patients’ wages. ProPublica. December 19, 2014. Accessed March 21, 2022. https://www.propublica.org/article/how-nonprofit-hospitals-are-seizing-patients-wages
- Cooper Z, Han J, Mahoney N. Hospital lawsuits over unpaid bills increased by 37 percent in Wisconsin from 2001 to 2018. Health Affairs. 2021;40:1830-1835. Accessed March 21, 2022. https://www.healthaffairs.org/doi/full/10.1377 /hlthaff.2021.01130
- LaMantia J. New York hospitals have filed thousands of lawsuits against patients. Modern Healthcare. March 13, 2020. Accessed March 21, 2022. https://www.modernhealthcare .com/legal/new-york-hospitals-have-filed-thousands -lawsuits-against-patients
- Armour S. When patients can’t pay, many hospitals are suing. Wall Street Journal. June 25, 2019. Accessed March 21, 2022. https://www.wsj.com/articles/nonprofit-hospitals-criticized-for-debt-collection-tactics-11561467600
- McGhee M, Chase W. How America’s top hospitals hound patients with predatory billing. Axios. Accessed March 21, 2022. https://www.axios.com/hospital-billing
- Owens C. Public spotlight on hospital lawsuits may slow them down. June 14, 2021. Accessed March 22, 2022. https:// www.axios.com/hospital-lawsuits-slowing-down-media -35ce395a-9fe3-4b23-b815-d7b06cce2773.html
- Buck ID. When hospitals sue patients. Hastings L.J. 2022;73:191-232, at 209-211. Accessed March 21, 2022. https:// repository.uchastings.edu/cgi/viewcontent.cgi?article =3961&context=hastings_law_journal
- Lagasse J. Healthcare turns to zero-interest loans to give patients a better reason to pay. Healthcare Finance. May 3, 2017. Accessed March 21, 2022. https://www.healthcarefinancenews.com/news/healthcare-turns-zero-interest-loans-give-patients-better-reason-pay#:~:text=Zero%2Dinterest%20loans%20are%20finding,of%20the%20patient%2Dprovider%20relationship.
- Bruhn WE, Rutkow L, Wang P, et al. Prevalence and characteristics of Virginia hospitals suing patients and garnishing wages for unpaid medical bills. JAMA. 2019;322:691-692. doi:10.1001/jama.2019.9144
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020. September 14, 2021. United States Census Bureau Current Population Reports, P60-274. US Government Publishing Office; September 2021. Accessed March 21, 2022. https://www.census.gov/content/dam /Census/library/publications/2021/demo/p60-274.pdf
- Cohen RA, Zammitti EP. High-deductible health plan enrollment among adults aged 18-64 with employment-based insurance coverage. NCHS Data Brief, No. 317. August 2018. Accessed March 21, 2022. https://www.cdc.gov/nchs/data/databriefs/db317.pdf
- Kaiser Family Foundation. Employer health benefits: 2020 summary of findings. Accessed March 21, 2022. https://www.kff.org/report-section/ehbs-2020-summary-of-findings/
- Picchi A. Higher health insurance deductibles a sickening trend for Americans. CBS NEWS. June 13, 2019. Accessed March 21, 2022. https://www.cbsnews.com/news/high-health-insurance-deductibles-a-sickening-trend-thats -causing-financial-hardship/
- Himmelstein DU, Lawless RM, Thorne D, Foohey P, Woolhandler S. Medical bankruptcy: still common despite the Affordable Care Act. Am J Public Health. 2019;109:431-433. doi:10.2105/AJPH.2018.304901
- Rosato D. What medical debt does to your credit score. Consumer Reports. July 26, 2018. Accessed March 21, 2022. https://www.consumerreports.org/credit-scores-reports/what-medical-debt-does-to-your-credit-score/
- State laws on wage garnishments. Nolo web site. https://www.nolo.com/legal-encyclopedia/free-books/employee-rights-book/chapter2-9.html. Accessed April 1, 2022.
- Patruzo JGR, Hashim F, Dun C, et al. Trends in hospital lawsuits filed against patients for unpaid bills following published research about their activity. JAMA Network Open. 2021;4:e2121926. doi:10.1001/jamanetworkopen.2021.21926
- About Schedule H (Form 990), hospitals. IRS. Updated June 10, 2021. Accessed March 21, 2022. https://www.irs.gov/forms-pubs/about-schedule-h-form-990
- Requirements for 501(c)(3) hospitals under the Affordable Care Act – Section 501(r). Updated September 9, 2021. Accessed March 21, 2022. https://www.irs.gov/charities-non-profits/charitable-organizations/requirements-for-501c3-hospitals-under-the-affordable-care-act-section-501r
- Pub. L. No. 116-260, 134 Stat. 1182, Division BB, § 109.
- Fact sheet. No Surprises: understand your rights against surprise medical bills. Centers for Medicare and Medicaid Services. January 3, 2022. Accessed March 21, 2022. https://www.cms.gov/newsroom/fact-sheets/no-surprises-understand-your-rights-against-surprise-medical-bills
- Implementation of the No Surprises Act. Accessed March 21, 2022. https://www.ama-assn.org/delivering-care/patient-support-advocacy/implementation-no-surprises-act
- American Medical Association. Toolkit for physicians: preparing for implementation of the No Surprises Act. January 2022. Accessed March 21, 2022. https://www.ama-assn.org/system/files/ama-nsa-toolkit.pdf
- US Government Accountability Office. Tax administration: opportunities exist to improve oversight of hospitals’ taxexempt status. September 2020. Accessed March 21, 2022. https://www.gao.gov/assets/gao-20-679.pdf
Infectious disease pop quiz: Clinical challenges for the ObGyn
In this question-and-answer article (the third in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
21. What prophylactic antibiotic should be administered intrapartum to a pregnant woman who is colonized with group B streptococci but who has a mild allergy to penicillin?
In this situation, the drug of choice is intravenous cefazolin, 2 g initially then 1 g every 8 hours until delivery. For patients with a severe allergy to penicillin, the drugs of choice are either clindamycin, 900 mg intravenously every 8 hours (if sensitivity of the organism is confirmed), or vancomycin, 20 mg/kg intravenously every 8 hours (maximum of 2 g per single dose).
22. In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
23. What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci). ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
In this question-and-answer article (the third in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
21. What prophylactic antibiotic should be administered intrapartum to a pregnant woman who is colonized with group B streptococci but who has a mild allergy to penicillin?
In this situation, the drug of choice is intravenous cefazolin, 2 g initially then 1 g every 8 hours until delivery. For patients with a severe allergy to penicillin, the drugs of choice are either clindamycin, 900 mg intravenously every 8 hours (if sensitivity of the organism is confirmed), or vancomycin, 20 mg/kg intravenously every 8 hours (maximum of 2 g per single dose).
22. In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
23. What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci). ●
In this question-and-answer article (the third in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
21. What prophylactic antibiotic should be administered intrapartum to a pregnant woman who is colonized with group B streptococci but who has a mild allergy to penicillin?
In this situation, the drug of choice is intravenous cefazolin, 2 g initially then 1 g every 8 hours until delivery. For patients with a severe allergy to penicillin, the drugs of choice are either clindamycin, 900 mg intravenously every 8 hours (if sensitivity of the organism is confirmed), or vancomycin, 20 mg/kg intravenously every 8 hours (maximum of 2 g per single dose).
22. In a pregnant woman who has a life-threatening allergy to penicillin, what is the most appropriate treatment for syphilis?
This patient should be admitted to the hospital and rapidly desensitized to penicillin. She then can be treated with the appropriate dose of penicillin, given her stage of syphilis. Of note, in the future, the patient’s allergy to penicillin will return, despite the brief period of desensitization.
23. What are the most common organisms that cause chorioamnionitis and puerperal endometritis?
Chorioamnionitis and puerperal endometritis are polymicrobial, mixed aerobic-anaerobic infections. The dominant organisms are anaerobic gram-negative bacilli (Bacteroides and Prevotella species); anaerobic gram-positive cocci (Peptococcus species and Peptostreptococcus species); aerobic gram-negative bacilli (principally, Escherichia coli, Klebsiella pneumoniae, and Proteus species); and aerobic gram-positive cocci (enterococci, staphylococci, and group B streptococci). ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
SERMs revisited: Can they improve menopausal care?
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).
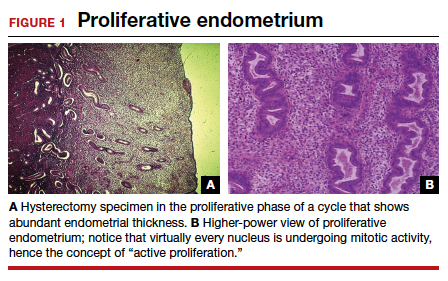
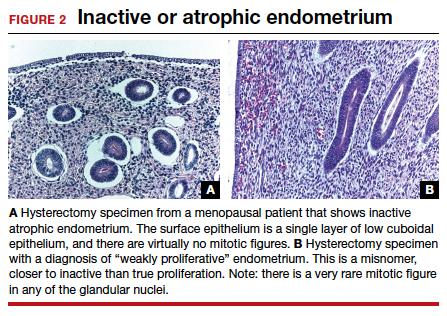
In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32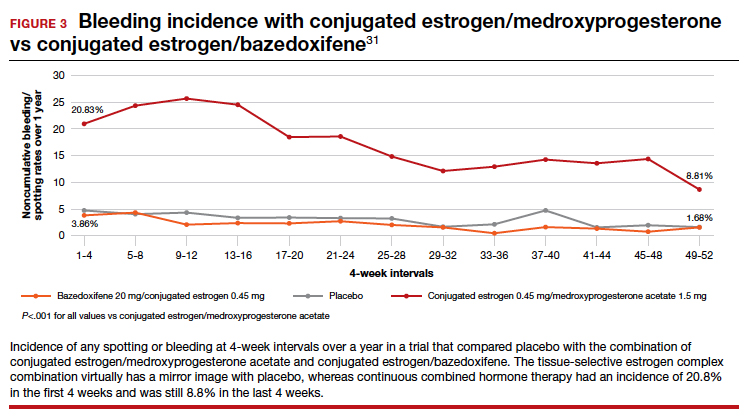
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
Selective estrogen receptor modulators (SERMs) are unique synthetic compounds that bind to the estrogen receptor and initiate either estrogenic agonistic or antagonistic activity, depending on the confirmational change they produce on binding to the receptor. Many SERMs have come to market, others have not. Unlike estrogens, which regardless of dose or route of administration all carry risks as a boxed warning on the label, referred to as class labeling,1 various SERMs exert various effects in some tissues (uterus, vagina) while they have apparent class properties in others (bone, breast).2
The first SERM, for all practical purposes, was tamoxifen (although clomiphene citrate is often considered a SERM). Tamoxifen was approved by the US Food and Drug Administration (FDA) in 1978 for the treatment of breast cancer and, subsequently, for breast cancer risk reduction. It became the most widely prescribed anticancer drug worldwide.
Subsequently, when data showed that tamoxifen could produce a small number of endometrial cancers and a larger number of endometrial polyps,3,4 there was renewed interest in raloxifene. In preclinical animal studies, raloxifene behaved differently than tamoxifen in the uterus. After clinical trials with raloxifene showed uterine safety,5 the drug was FDA approved for prevention of osteoporosis in 1997, for treatment of osteoporosis in 1999, and for breast cancer risk reduction in 2009. Most clinicians are familiar with these 2 SERMs, which have been in clinical use for more than 4 and 2 decades, respectively.
Ospemifene: A third-generation SERM and its indications
Hormone deficiency from menopause causes vulvovaginal and urogenital changes as well as a multitude of symptoms and signs, including vulvar and vaginal thinning, loss of rugal folds, diminished elasticity, increased pH, and most notably dyspareunia. The nomenclature that previously described vulvovaginal atrophy (VVA) has been expanded to include genitourinary syndrome of menopause (GSM).6 Unfortunately, many health care providers do not ask patients about GSM symptoms, and few women report their symptoms to their clinician.7 Furthermore, although low-dose local estrogens applied vaginally have been the mainstay of therapy for VVA/GSM, only 7% of symptomatic women use any pharmacologic agent,8 mainly because of fear of estrogens due to the class labeling mentioned above.
Ospemifene, a newer SERM, improved superficial cells and reduced parabasal cells as seen on a maturation index compared with placebo, according to results of multiple phase 3 clinical trials9,10; it also lowered vaginal pH and improved most bothersome symptoms (original studies were for dyspareunia). As a result, the FDA approved ospemifene for treatment of moderate to severe dyspareunia from VVA of menopause.
Subsequent studies allowed for a broadened indication to include treatment of moderate to severe dryness due to menopause.11 The ospemifene label contains a boxed warning that states, “In the endometrium, [ospemifene] has estrogen agonistic effects.”12 Although ospemifene is not an estrogen (it’s a SERM), the label goes on to state, “There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens.” This statement caused The Medical Letter to initially suggest that patients who receive ospemifene also should receive a progestational agent—a suggestion they later retracted.13,14
To understand why the ospemifene labeling might be worded in such a way, one must review the data regarding the poorly named entity “weakly proliferative endometrium.” The package labeling combines any proliferative endometrium (“weakly” plus “actively” plus “disordered”) that occurred in the clinical trial. Thus, 86.1 per 1,000 of the ospemifene-treated patients (vs 13.3 per 1,000 of those taking placebo) had any one of the proliferative types. The problem is that “actively proliferative” endometrial glands will have mitotic activity in virtually every nucleus of the gland as well as abundant glandular progression (FIGURE 1), whereas “weakly proliferative” is actually closer to inactive or atrophic endometrium with an occasional mitotic figure in only a few nuclei of each gland (FIGURE 2).


In addition, at 1 year, the incidence of active proliferation with ospemifene was 1%.15 In examining the uterine safety study for raloxifene, both doses of that agent had an active proliferation incidence of 3% at 1 year.5 Furthermore, that study had an estrogen-only arm in which, at end point, the incidence of endometrial proliferation was 39%, and hyperplasia, 23%!5 It therefore is evident that, in the endometrium, ospemifene is much more like the SERM raloxifene than it is like estrogen. The American College of Obstetricians and Gynecologists (ACOG) endorsed ospemifene (level A evidence) as a first-line therapy for dyspareunia, noting absent endometrial stimulation.16
Continue to: Ospemifene effects on breast and bone...
Ospemifene effects on breast and bone
Although ospemifene is approved for treatment of moderate to severe VVA/GSM, it has other SERM effects typical of its class. The label currently states that ospemifene “has not been adequately studied in women with breast cancer; therefore, it should not be used in women with known or suspected breast cancer.”12 We know that tamoxifen reduced breast cancer 49% in high-risk women in the Breast Cancer Prevention Trial (BCPT).17 We also know that in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene reduced breast cancer 77% in osteoporotic women,18 and in the Study of Tamoxifen and Raloxifene (STAR) trial, it performed virtually identically to tamoxifen in breast cancer prevention.19 Previous studies demonstrated that ospemifene inhibits breast cancer cell growth in in vitro cultures as well as in animal studies20 and inhibits proliferation of human breast tissue epithelial cells,21 with breast effects similar to those seen with tamoxifen and raloxifene.
Thus, although one would not choose ospemifene as a primary treatment or risk-reducing agent for a patient with breast cancer, the direction of its activity in breast tissue is indisputable and is likely the reason that in the European Union (unlike in the United States) it is approved to treat dyspareunia from VVA/GSM in women with a prior history of breast cancer.
Virtually all SERMs have estrogen agonistic activity in bone. Bone is a dynamic organ, constantly being laid down and taken away (resorption). Estrogen and SERMs are potent antiresorptives in bone metabolism. Ospemifene effectively reduced bone loss in ovariectomized rats, with activity comparable to that of estradiol and raloxifene.22 Clinical data from 3 phase 1 or 2 clinical trials found that ospemifene 60 mg/day had a positive effect on biochemical markers for bone turnover in healthy postmenopausal women, with significant improvements relative to placebo and effects comparable to those of raloxifene.23 Actual fracture or bone mineral density (BMD) data in postmenopausal women are lacking, but there is a good correlation between biochemical markers for bone turnover and the occurrence of fracture.24 Once again, women who need treatment for osteoporosis should not be treated primarily with ospemifene, but women who use ospemifene for dyspareunia can expect positive activity on bone metabolism.
Clinical application
Ospemifene is an oral SERM approved for the treatment of moderate to severe dyspareunia as well as dryness from VVA due to menopause. In addition, it appears one can safely surmise that the direction of ospemifene’s activity in bone and breast is virtually indisputable. The magnitude of that activity, however, is unstudied. Therefore, in selecting an agent to treat women with dyspareunia or vaginal dryness from VVA of menopause, determining any potential add-on benefit for that particular patient in either bone and/or breast is clinically appropriate.
The SERM bazedoxifene
A meta-analysis of 4 randomized, placebo-controlled trials showed that another SERM, bazedoxifene, can significantly decrease the incidence of vertebral fracture in postmenopausal women at follow-up of 3 and 7 years.25 That meta-analysis also confirmed the long-term favorable safety and tolerability of bazedoxifene, with no increase in adverse events, serious adverse events, myocardial infarction, stroke, venous thromboembolic events, or breast carcinoma in patients using bazedoxifene. However, bazedoxifene use did result in an increased incidence of hot flushes and leg cramps across 7 years.25 Bazedoxifene is available in a 20-mg dose for treatment of postmenopausal osteoporosis in Israel and a number of European Union countries.
Continue to: Enter the concept of tissue-selective estrogen complex (TSEC)...
Enter the concept of tissue-selective estrogen complex (TSEC)
Some postmenopausal women are extremely intolerant of any progestogen added to estrogen therapy to confer endometrial protection in those with a uterus. According to the results of a clinical trial of postmenopausal women, bazedoxifene is the only SERM shown to decrease endometrial thickness compared with placebo.26 This is the basis for thinking that perhaps a SERM like bazedoxifene, instead of a progestogen, could be used to confer endometrial protection.
A further consideration comes out of the evaluation of data derived from the 2 arms of the Women’s Health Initiative (WHI).27 In the arm that combined conjugated estrogen with medroxyprogesterone acetate through 11.3 years, there was a 25% increase in the incidence of invasive breast cancer, which was statistically significant. Contrast that with the arm in hysterectomized women who received only conjugated estrogen (often inaccurately referred to as the “estrogen only” arm of the WHI). In that study arm, the relative risk of invasive breast cancer was reduced 23%, also statistically significant. Thus, the culprit in the breast cancer incidence difference in these 2 arms appears to be the addition of the progestogen medroxyprogesterone acetate.27
Since the progestogen was used only for endometrial protection, could such endometrial protection be provided by a SERM like bazedoxifene? Preclinical trials showed that a combination of bazedoxifene and conjugated estrogen (in various estrogen doses) resulted in uterine wet weight in an ovariectomized rat model that was no different than that with placebo.28
In terms of effects on breast, preclinical models showed that conjugated estrogen use resulted in less mammary duct elongation and end bud proliferation than estradiol by itself, and that the combination of conjugated estrogen and bazedoxifene resulted in mammary duct elongation and end bud proliferation that was similar to that in the ovariectomized animals and considerably less than a combination of estradiol with bazedoxifene.29
Five phase 3 studies known as the SMART (Selective estrogens, Menopause, And Response to Therapy) trials were then conducted. Collectively, these studies examined the frequency and severity of vasomotor symptoms (VMS), BMD, bone turnover markers, lipid profiles, sleep, quality of life, breast density, and endometrial safety with conjugated estrogen/bazedoxifene treatment.30 Based on these trials with more than 7,500 women, in 2013 the FDA approved a compound of conjugated estrogen 0.45 mg and bazedoxifene 20 mg (Duavee in the United States and Duavive outside the United States).
The incidence of endometrial hyperplasia at 12 months was consistently less than 1%, which is the FDA guidance for approval of hormone therapies. The incidence of bleeding or spotting with conjugated estrogen/bazedoxifene (FIGURE 3) in each 4-week interval over 12 months mirror-imaged that of placebo and ranged from 3.9% in the first 4-week interval to 1.7% in the last 4 weeks, compared with conjugated estrogen 0.45 mg/medroxyprogesterone acetate 1.5 mg, which had a 20.8% incidence of bleeding or spotting in the first 4-week interval and was still at an 8.8% incidence in the last 4 weeks.31 This is extremely relevant in clinical practice. There was no difference from placebo in breast cancer incidence, breast pain or tenderness, abnormal mammograms, or breast density at month 12.32
In terms of frequency of VMS, there was a 74% reduction from baseline at 12 weeks compared with placebo (P<.001), as well as a 37% reduction in the VMS severity score (P<.001).32 Statistically significant improvements occurred in lumbar spine and hip BMD (P<.01) for women who were 1 to 5 years since menopause as well as for those who were more than 5 years since menopause.33
Packaging issue puts TSEC on back order
In May 2020, Pfizer voluntarily recalled its conjugated estrogen/bazedoxifene product after identifying a “flaw in the drug’s foil laminate pouch that introduced oxygen and lowered the dissolution rate of active pharmaceutical ingredient bazedoxifene acetate.”34 The manufacturer then wrote a letter to health care professionals in September 2021 stating, “Duavee continues to be out of stock due to an unexpected and complex packaging issue, resulting in manufacturing delays. This has nothing to do with the safety or quality of the product itself but could affect product stability throughout its shelf life… Given regulatory approval timelines for any new packaging, it is unlikely that Duavee will return to stock in 2022.”35
Other TSECs?
The conjugated estrogen/bazedoxifene combination is the first FDA-approved TSEC. Other attempts have been made to achieve similar results with combined raloxifene and 17β-estradiol.36 That study was meant to be a 52-week treatment trial with either raloxifene 60 mg alone or in combination with 17β-estradiol 1 mg per day to assess effects on VMS and endometrial safety. The study was stopped early because signs of endometrial stimulation were observed in the raloxifene plus estradiol group. Thus, one cannot combine any estrogen with any SERM and assume similar results.
Clinical application
The combination of conjugated estrogen/bazedoxifene is approved for treatment of VMS of menopause as well as prevention of osteoporosis. Although it is not approved for treatment of moderate to severe VVA, in younger women who initiate treatment it should prevent the development of moderate to severe symptoms of VVA.
Finally, this drug should be protective of the breast. Conjugated estrogen has clearly shown a reduction in breast cancer incidence and mortality, and bazedoxifene is a SERM. All SERMs have, as a class effect, been shown to be antiestrogens in breast tissue, and abundant preclinical data point in that direction.
This combination of conjugated estrogen/bazedoxifene, when it is once again clinically available, may well provide a new paradigm of hormone therapy that is progestogen free and has a benefit/risk ratio that tilts toward its benefits.
Potential for wider therapeutic benefits
Newer SERMs like ospemifene, approved for treatment of VVA/GSM, and bazedoxifene/conjugated estrogen combination, approved for treatment of VMS and prevention of bone loss, have other beneficial properties that can and should result in their more widespread use. ●
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
- Stuenkel CA. More evidence why the product labeling for low-dose vaginal estrogen should be changed? Menopause. 2018;25:4-6.
- Goldstein SR. Not all SERMs are created equal. Menopause. 2006;13:325-327.
- Neven P, De Muylder X, Van Belle Y, et al. Hysteroscopic follow-up during tamoxifen treatment. Eur J Obstet Gynecol Reprod Biol. 1990;35:235-238.
- Schwartz LB, Snyder J, Horan C, et al. The use of transvaginal ultrasound and saline infusion sonohysterography for the evaluation of asymptomatic postmenopausal breast cancer patients on tamoxifen. Ultrasound Obstet Gynecol. 1998;11:48-53.
- Goldstein SR, Scheele WH, Rajagopalan SK, et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet Gynecol. 2000;95:95-103.
- Portman DJ, Gass MLS. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21:1063-1068.
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health. 2013;5:437-447.
- Kingsberg SA, Krychman M, Graham S, et al. The Women’s EMPOWER Survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010;17:480-486.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013;20:623-630.
- Archer DF, Goldstein SR, Simon JA, et al. Efficacy and safety of ospemifene in postmenopausal women with moderateto-severe vaginal dryness: a phase 3, randomized, doubleblind, placebo-controlled, multicenter trial. Menopause. 2019;26:611-621.
- Osphena. Package insert. Shionogi Inc; 2018.
- Ospemifene (Osphena) for dyspareunia. Med Lett Drugs Ther. 2013;55:55-56.
- Addendum: Ospemifene (Osphena) for dyspareunia (Med Lett Drugs Ther 2013;55:55). Med Lett Drugs Ther. 2013;55:84.
- Goldstein SR, Bachmann G, Lin V, et al. Endometrial safety profile of ospemifene 60 mg when used for long-term treatment of vulvar and vaginal atrophy for up to 1 year. Abstract. Climacteric. 2011;14(suppl 1):S57.
- ACOG practice bulletin no. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371-1388.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189-2197.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
- Qu Q, Zheng H, Dahllund J, et al. Selective estrogenic effects of a novel triphenylethylene compound, FC1271a, on bone, cholesterol level, and reproductive tissues in intact and ovariectomized rats. Endocrinology. 2000;141:809-820.
- Eigeliene N, Kangas L, Hellmer C, et al. Effects of ospemifene, a novel selective estrogen-receptor modulator, on human breast tissue ex vivo. Menopause. 2016;23:719-730.
- Kangas L, Unkila M. Tissue selectivity of ospemifene: pharmacologic profile and clinical implications. Steroids. 2013;78:1273-1280.
- Constantine GD, Kagan R, Miller PD. Effects of ospemifene on bone parameters including clinical biomarkers in postmenopausal women. Menopause. 2016;23:638-644.
- Gerdhem P, Ivaska KK, Alatalo SL, et al. Biochemical markers of bone metabolism and prediction of fracture in elderly women. J Bone Miner Res. 2004;19:386-393.
- Peng L, Luo Q, Lu H. Efficacy and safety of bazedoxifene in postmenopausal women with osteoporosis: a systematic review and meta-analysis. Medicine. 2017;96(49):e8659.
- Ronkin S, Northington R, Baracat E, et al. Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol. 2005;105:1397-1404.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;13:476-486.
- Kharode Y, Bodine PV, Miller CP, et al. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology. 2008;149:6084-6091.
- Song Y, Santen RJ, Wang JP, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue-selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology. 2012;153:5706-5715.
- Umland EM, Karel L, Santoro N. Bazedoxifene and conjugated equine estrogen: a combination product for the management of vasomotor symptoms and osteoporosis prevention associated with menopause. Pharmacotherapy. 2016;36:548-561.
- Kagan R, Goldstein SR, Pickar JH, et al. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag. 2016;12:549–562.
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol. 2013;121:959-968.
- Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/ conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045-1052.
- Fierce Pharma. Pfizer continues recalls of menopause drug Duavee on faulty packaging concerns. https:// www.fiercepharma.com/manufacturing/pfizer-recallsmenopause-drug-duavive-uk-due-to-faulty-packagingworries. June 9, 2020. Accessed February 8, 2022.
- Pfizer. Letter to health care provider. Subject: Duavee (conjugated estrogens/bazedoxifene) extended drug shortage. September 10, 2021.
- Stovall DW, Utian WH, Gass MLS, et al. The effects of combined raloxifene and oral estrogen on vasomotor symptoms and endometrial safety. Menopause. 2007; 14(3 pt 1):510-517.
What placental mechanisms protect the fetus from harm in the setting of maternal COVID-19 infection?
Taglauer ES, Wachman EM, Juttukonda L, et al. Acute severe acute respiratory syndrome coronavirus 2 infection in pregnancy is associated with placental angiotensin-converting enzyme 2 shedding. Am J Pathol. 2022;192:595-603. doi.org/10.1016/j.ajpath.2021.12.011
EXPERT COMMENTARY
Although transmission of SARS-CoV-2 virus from an infected mother to her fetus is rare, placental infection with SARS-CoV-2 can occur and has been observed in association with placental damage and adverse pregnancy outcomes, including stillbirth.1 Understanding what mechanisms of defense protect the placenta and fetus from direct SARS-CoV-2 infection at the maternal-fetal interface, as well as the factors that might disturb or enhance that protection, is critical to gaining a deeper understanding of the potential impact of maternal COVID-19 on fetal well-being.
Details of the study
In a cohort of 24 pregnant individuals, Taglauer and colleagues investigated levels of placental angiotensin-converting enzyme (ACE)-2, placental ADAM17 (a disintegrin and metalloprotease domain 17) activity, and maternal serum soluble ACE2 in samples obtained at delivery from individuals with a history of second trimester COVID-19 infection, early third trimester COVID-19 infection, and no history of COVID-19 infection.
Results. Maternal COVID-19 infection in the early third trimester of pregnancy resulted in lower ACE2 protein levels in the placenta at delivery, higher ACE2 gene expression, and an increase in ADAM17 activity, compared with infection in the second trimester of pregnancy and compared with noninfected controls.
The authors postulated that increased ADAM17 activity—the enzyme responsible for ACE2 cleavage and shedding—may be responsible for lower ACE2 protein levels. Soluble ACE2 levels in maternal blood at delivery were increased in individuals with third trimester COVID-19 infection, although the source of soluble ACE2 (placental or otherwise) could not be determined with the methods employed. Levels of placental estrogen were no different between groups, which suggests that estrogen is not responsible for the observed differences.
Study strengths and limitations
ACE2 is the main receptor for the SARS-CoV-2 virus and facilitates viral entry into the cell.2 Placental villous cells that are in direct contact with maternal blood express the ACE2 protein, rendering them potentially vulnerable to SARS-CoV-2 infection.3 In this study, the authors observed lower placental ACE2 protein in term placentas from recent (early third trimester) but not remote (second trimester) maternal SARS-CoV-2 infection, arguably the result of the observed increase in ADAM17 cleavage activity. Prior studies have shown conflicting results, with equal or higher ACE2 levels noted in the setting of maternal COVID-19 infection, which may be related to differences in COVID-19 disease severity, gestational age of infection, and/or fetal sex in these cohorts.4-6
The concept that increased placental ACE2 shedding represents a protective defense mechanism that might last weeks beyond the acute infectious period is intriguing, but it requires further study. Observed differences in third but not second trimester COVID-19 infections could indicate either 1) an effect of maternal COVID-19 infection that lasts for several weeks but eventually normalizes over time, in the case of a remote infection; or 2) that second trimester maternal COVID-19 infection does not have the same pronounced effect on ACE2 levels as does third trimester infection. Observational studies of the human placenta are not able to answer this question, as directly sampling the placenta at the time of the exposure (or repeated sampling over time) in ongoing pregnancies is neither practical nor ethical. Further studies using animal or cellular models of SARS-CoV-2 infection in pregnancy may be necessary to fully understand the clinical relevance of these findings.
The study by Taglauer and colleagues provides a compelling argument for exploring how immune defenses at the maternal-fetal interface evolve over time and vary by trimester of exposure. ●
As the number of pregnancies exposed to COVID-19 continues to grow worldwide, how immune defenses at the maternal-fetal interface protect against fetal infection remains an important area of investigation.
LYDIA L. SHOOK, MD
- Pregnant people are at increased risk of more severe COVID-19 illness.
- The risk of stillbirth is 2- to 4-fold higher in women with COVID-19 infection during pregnancy.1
- COVID-19 vaccination is recommended for all people who are pregnant, lactating, or considering pregnancy.
- Pregnant and recently pregnant people up to 6 weeks postpartum should receive a third “booster” dose of a COVID-19 mRNA vaccine following completion of their initial COVID-19 vaccine or vaccine series.
- The mRNA COVID-19 vaccines are preferred over the Johnson & Johnson/Janssen COVID-19 vaccine for pregnant and lactating individuals for primary series and booster vaccination.
- Completion of a 2-dose mRNA COVID-19 vaccination series during pregnancy might help prevent COVID-19 hospitalization among infants <6 months.2
aVaccine recommendations adapted from: ACOG practice advisory: COVID-19 vaccination considerations for obstetric-gynecologic care. Last updated March 2, 2022. https://www.acog.org/clinical/ clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetricgynecologic-care. Accessed March 21, 2022.
References
1. DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morbid Mortal Wkly Rep. 2021;70:1640-1645.
2. Halasa NB, Olson SM, Staat MA, et al; Overcoming COVID-19 Investigators; Overcoming COVID-19 Network. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:264-270.
- Schwartz DA, Avvad-Portari E, Babál, et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: a study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch Pathol Lab Med. February 10, 2022. doi:10.5858/arpa.2022- 0029-SA.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181: 271-280.e8.
- Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19- positive mothers. Mod Pathol. 2020;33:2092-2103.
- Mourad M, Jacob T, Sadovsky E, et al. Placental response to maternal SARS-CoV-2 infection. Sci Rep. 2021;11:14390.
- Lu-Culligan A, Chavan AR, Vijayakumar P, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y). 2021;2:591-610.e10.
- Shook LL, Bordt EA, Meinsohn MC, et al. Placental expression of ACE2 and TMPRSS2 in maternal severe acute respiratory syndrome coronavirus 2 infection: are placental defenses mediated by fetal sex? J Infect Dis. 2021;224(suppl 6):S659.
Taglauer ES, Wachman EM, Juttukonda L, et al. Acute severe acute respiratory syndrome coronavirus 2 infection in pregnancy is associated with placental angiotensin-converting enzyme 2 shedding. Am J Pathol. 2022;192:595-603. doi.org/10.1016/j.ajpath.2021.12.011
EXPERT COMMENTARY
Although transmission of SARS-CoV-2 virus from an infected mother to her fetus is rare, placental infection with SARS-CoV-2 can occur and has been observed in association with placental damage and adverse pregnancy outcomes, including stillbirth.1 Understanding what mechanisms of defense protect the placenta and fetus from direct SARS-CoV-2 infection at the maternal-fetal interface, as well as the factors that might disturb or enhance that protection, is critical to gaining a deeper understanding of the potential impact of maternal COVID-19 on fetal well-being.
Details of the study
In a cohort of 24 pregnant individuals, Taglauer and colleagues investigated levels of placental angiotensin-converting enzyme (ACE)-2, placental ADAM17 (a disintegrin and metalloprotease domain 17) activity, and maternal serum soluble ACE2 in samples obtained at delivery from individuals with a history of second trimester COVID-19 infection, early third trimester COVID-19 infection, and no history of COVID-19 infection.
Results. Maternal COVID-19 infection in the early third trimester of pregnancy resulted in lower ACE2 protein levels in the placenta at delivery, higher ACE2 gene expression, and an increase in ADAM17 activity, compared with infection in the second trimester of pregnancy and compared with noninfected controls.
The authors postulated that increased ADAM17 activity—the enzyme responsible for ACE2 cleavage and shedding—may be responsible for lower ACE2 protein levels. Soluble ACE2 levels in maternal blood at delivery were increased in individuals with third trimester COVID-19 infection, although the source of soluble ACE2 (placental or otherwise) could not be determined with the methods employed. Levels of placental estrogen were no different between groups, which suggests that estrogen is not responsible for the observed differences.
Study strengths and limitations
ACE2 is the main receptor for the SARS-CoV-2 virus and facilitates viral entry into the cell.2 Placental villous cells that are in direct contact with maternal blood express the ACE2 protein, rendering them potentially vulnerable to SARS-CoV-2 infection.3 In this study, the authors observed lower placental ACE2 protein in term placentas from recent (early third trimester) but not remote (second trimester) maternal SARS-CoV-2 infection, arguably the result of the observed increase in ADAM17 cleavage activity. Prior studies have shown conflicting results, with equal or higher ACE2 levels noted in the setting of maternal COVID-19 infection, which may be related to differences in COVID-19 disease severity, gestational age of infection, and/or fetal sex in these cohorts.4-6
The concept that increased placental ACE2 shedding represents a protective defense mechanism that might last weeks beyond the acute infectious period is intriguing, but it requires further study. Observed differences in third but not second trimester COVID-19 infections could indicate either 1) an effect of maternal COVID-19 infection that lasts for several weeks but eventually normalizes over time, in the case of a remote infection; or 2) that second trimester maternal COVID-19 infection does not have the same pronounced effect on ACE2 levels as does third trimester infection. Observational studies of the human placenta are not able to answer this question, as directly sampling the placenta at the time of the exposure (or repeated sampling over time) in ongoing pregnancies is neither practical nor ethical. Further studies using animal or cellular models of SARS-CoV-2 infection in pregnancy may be necessary to fully understand the clinical relevance of these findings.
The study by Taglauer and colleagues provides a compelling argument for exploring how immune defenses at the maternal-fetal interface evolve over time and vary by trimester of exposure. ●
As the number of pregnancies exposed to COVID-19 continues to grow worldwide, how immune defenses at the maternal-fetal interface protect against fetal infection remains an important area of investigation.
LYDIA L. SHOOK, MD
- Pregnant people are at increased risk of more severe COVID-19 illness.
- The risk of stillbirth is 2- to 4-fold higher in women with COVID-19 infection during pregnancy.1
- COVID-19 vaccination is recommended for all people who are pregnant, lactating, or considering pregnancy.
- Pregnant and recently pregnant people up to 6 weeks postpartum should receive a third “booster” dose of a COVID-19 mRNA vaccine following completion of their initial COVID-19 vaccine or vaccine series.
- The mRNA COVID-19 vaccines are preferred over the Johnson & Johnson/Janssen COVID-19 vaccine for pregnant and lactating individuals for primary series and booster vaccination.
- Completion of a 2-dose mRNA COVID-19 vaccination series during pregnancy might help prevent COVID-19 hospitalization among infants <6 months.2
aVaccine recommendations adapted from: ACOG practice advisory: COVID-19 vaccination considerations for obstetric-gynecologic care. Last updated March 2, 2022. https://www.acog.org/clinical/ clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetricgynecologic-care. Accessed March 21, 2022.
References
1. DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morbid Mortal Wkly Rep. 2021;70:1640-1645.
2. Halasa NB, Olson SM, Staat MA, et al; Overcoming COVID-19 Investigators; Overcoming COVID-19 Network. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:264-270.
Taglauer ES, Wachman EM, Juttukonda L, et al. Acute severe acute respiratory syndrome coronavirus 2 infection in pregnancy is associated with placental angiotensin-converting enzyme 2 shedding. Am J Pathol. 2022;192:595-603. doi.org/10.1016/j.ajpath.2021.12.011
EXPERT COMMENTARY
Although transmission of SARS-CoV-2 virus from an infected mother to her fetus is rare, placental infection with SARS-CoV-2 can occur and has been observed in association with placental damage and adverse pregnancy outcomes, including stillbirth.1 Understanding what mechanisms of defense protect the placenta and fetus from direct SARS-CoV-2 infection at the maternal-fetal interface, as well as the factors that might disturb or enhance that protection, is critical to gaining a deeper understanding of the potential impact of maternal COVID-19 on fetal well-being.
Details of the study
In a cohort of 24 pregnant individuals, Taglauer and colleagues investigated levels of placental angiotensin-converting enzyme (ACE)-2, placental ADAM17 (a disintegrin and metalloprotease domain 17) activity, and maternal serum soluble ACE2 in samples obtained at delivery from individuals with a history of second trimester COVID-19 infection, early third trimester COVID-19 infection, and no history of COVID-19 infection.
Results. Maternal COVID-19 infection in the early third trimester of pregnancy resulted in lower ACE2 protein levels in the placenta at delivery, higher ACE2 gene expression, and an increase in ADAM17 activity, compared with infection in the second trimester of pregnancy and compared with noninfected controls.
The authors postulated that increased ADAM17 activity—the enzyme responsible for ACE2 cleavage and shedding—may be responsible for lower ACE2 protein levels. Soluble ACE2 levels in maternal blood at delivery were increased in individuals with third trimester COVID-19 infection, although the source of soluble ACE2 (placental or otherwise) could not be determined with the methods employed. Levels of placental estrogen were no different between groups, which suggests that estrogen is not responsible for the observed differences.
Study strengths and limitations
ACE2 is the main receptor for the SARS-CoV-2 virus and facilitates viral entry into the cell.2 Placental villous cells that are in direct contact with maternal blood express the ACE2 protein, rendering them potentially vulnerable to SARS-CoV-2 infection.3 In this study, the authors observed lower placental ACE2 protein in term placentas from recent (early third trimester) but not remote (second trimester) maternal SARS-CoV-2 infection, arguably the result of the observed increase in ADAM17 cleavage activity. Prior studies have shown conflicting results, with equal or higher ACE2 levels noted in the setting of maternal COVID-19 infection, which may be related to differences in COVID-19 disease severity, gestational age of infection, and/or fetal sex in these cohorts.4-6
The concept that increased placental ACE2 shedding represents a protective defense mechanism that might last weeks beyond the acute infectious period is intriguing, but it requires further study. Observed differences in third but not second trimester COVID-19 infections could indicate either 1) an effect of maternal COVID-19 infection that lasts for several weeks but eventually normalizes over time, in the case of a remote infection; or 2) that second trimester maternal COVID-19 infection does not have the same pronounced effect on ACE2 levels as does third trimester infection. Observational studies of the human placenta are not able to answer this question, as directly sampling the placenta at the time of the exposure (or repeated sampling over time) in ongoing pregnancies is neither practical nor ethical. Further studies using animal or cellular models of SARS-CoV-2 infection in pregnancy may be necessary to fully understand the clinical relevance of these findings.
The study by Taglauer and colleagues provides a compelling argument for exploring how immune defenses at the maternal-fetal interface evolve over time and vary by trimester of exposure. ●
As the number of pregnancies exposed to COVID-19 continues to grow worldwide, how immune defenses at the maternal-fetal interface protect against fetal infection remains an important area of investigation.
LYDIA L. SHOOK, MD
- Pregnant people are at increased risk of more severe COVID-19 illness.
- The risk of stillbirth is 2- to 4-fold higher in women with COVID-19 infection during pregnancy.1
- COVID-19 vaccination is recommended for all people who are pregnant, lactating, or considering pregnancy.
- Pregnant and recently pregnant people up to 6 weeks postpartum should receive a third “booster” dose of a COVID-19 mRNA vaccine following completion of their initial COVID-19 vaccine or vaccine series.
- The mRNA COVID-19 vaccines are preferred over the Johnson & Johnson/Janssen COVID-19 vaccine for pregnant and lactating individuals for primary series and booster vaccination.
- Completion of a 2-dose mRNA COVID-19 vaccination series during pregnancy might help prevent COVID-19 hospitalization among infants <6 months.2
aVaccine recommendations adapted from: ACOG practice advisory: COVID-19 vaccination considerations for obstetric-gynecologic care. Last updated March 2, 2022. https://www.acog.org/clinical/ clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetricgynecologic-care. Accessed March 21, 2022.
References
1. DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020–September 2021. MMWR Morbid Mortal Wkly Rep. 2021;70:1640-1645.
2. Halasa NB, Olson SM, Staat MA, et al; Overcoming COVID-19 Investigators; Overcoming COVID-19 Network. Effectiveness of maternal vaccination with mRNA COVID-19 vaccine during pregnancy against COVID-19–associated hospitalization in infants aged <6 months—17 states, July 2021–January 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:264-270.
- Schwartz DA, Avvad-Portari E, Babál, et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: a study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch Pathol Lab Med. February 10, 2022. doi:10.5858/arpa.2022- 0029-SA.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181: 271-280.e8.
- Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19- positive mothers. Mod Pathol. 2020;33:2092-2103.
- Mourad M, Jacob T, Sadovsky E, et al. Placental response to maternal SARS-CoV-2 infection. Sci Rep. 2021;11:14390.
- Lu-Culligan A, Chavan AR, Vijayakumar P, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y). 2021;2:591-610.e10.
- Shook LL, Bordt EA, Meinsohn MC, et al. Placental expression of ACE2 and TMPRSS2 in maternal severe acute respiratory syndrome coronavirus 2 infection: are placental defenses mediated by fetal sex? J Infect Dis. 2021;224(suppl 6):S659.
- Schwartz DA, Avvad-Portari E, Babál, et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: a study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch Pathol Lab Med. February 10, 2022. doi:10.5858/arpa.2022- 0029-SA.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181: 271-280.e8.
- Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19- positive mothers. Mod Pathol. 2020;33:2092-2103.
- Mourad M, Jacob T, Sadovsky E, et al. Placental response to maternal SARS-CoV-2 infection. Sci Rep. 2021;11:14390.
- Lu-Culligan A, Chavan AR, Vijayakumar P, et al. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y). 2021;2:591-610.e10.
- Shook LL, Bordt EA, Meinsohn MC, et al. Placental expression of ACE2 and TMPRSS2 in maternal severe acute respiratory syndrome coronavirus 2 infection: are placental defenses mediated by fetal sex? J Infect Dis. 2021;224(suppl 6):S659.