User login
Endometriosis: Clinical Diagnosis and Empiric Treatment
Dr. Taylor: Endometriosis is a very common disease. Unfortunately, it's still widely under-recognized. It's estimated that perhaps up to 10% of reproductive age women have endometriosis, yet many are never diagnosed or diagnosed late. The average time that it takes for someone to be diagnosed is about 10 years; that is from the time they have classic symptoms of endometriosis until the time they get a definitive diagnosis and treatment.
I think it's very important that we recognize the clinical features of endometriosis. It's absolutely crucial if we want to shorten the time tom diagnosis. Often patients see multiple physicians before they get an accurate diagnosis. They're often dismissed by their early caregivers who are not very familiar with the disease.
For me, the most important feature is the pelvic pain. To identify endometriosis, I look specifically at the cyclic nature of the pelvic pain, and the progressive nature of the pelvic pain. Endometriosis is by far the most common reason that women have pelvic pain and initially it tends to be cyclic. It often starts as dysmenorrhea (painful periods), however it can progress to the point where pain occurs at times outside of menses. In fact, it can progress to the point where it's painful all the time. However, it almost always starts as dysmennorhea, and progresses.
Pelvic pain that someone had from their first menses on, i.e., from menarche, is less likely to be endometriosis than the pain that progresses and becomes worse or spreads to other times of the menstrual cycle. Endometriosis is a progressive disease, and that's one of the key distinctions in making me think somebody has endometriosis.
Most women with cyclic pelvic pain that gets worse over time, cyclic progressive pelvic pain, will have endometriosis. Those are the key features I look for.
It is also very important to recognize that endometriosis can have effects outside of the reproductive tract, can cause systemic inflammation, and impact other organ systems. Bowel and bladder dysfunction are very common. If that is cyclic and coincides with the pelvic pain, it’s very likely to be secondary to endometriosis. It's important not to get distracted or mislead into making other diagnoses.
What are some of the common symptoms and how does that impact your diagnosis evaluation?
Dr. Taylor: The most common symptom we see is that cyclic pelvic pain I just discussed. Pain, often starting as dysmenorrhea, can go on to include pain outside of the time of menses and become more than just dysmenorrhea- pain in other areas. The other common symptom is infertility. Many women with endometriosis do not have severe pain or may not have pain at all, but first present when they have trouble conceiving.
Endometriosis may be the cause of infertility. Sometimes we recognize that it's endometriosis when we do a physical exam or an ultrasound and find an endometrioma. Cyclic pelvic pain is the classic symptom we look for, and infertility is another key symptom that we shouldn't forget.
Can you talk a little bit about empiric treatment, primarily what that means and how prevalent it is in your current practice?
Dr. Taylor: I think one of the problems we have today with the treatment of endometriosis is that it doesn't get recognized and doesn't get treated right away. When we talk about emperic treatment, it's usually ruling out other potential etiologies of pain. We must ensure no one has have an infection, a tumor or other etiologies that are the cause of the pain, and not simply presume that any cyclic progressive pelvic pain is endometriosis – however most of the time it will be due to endometriosis.
Usually with a good history, physical exam, and sometimes with addition of a transvaginal ultrasound, you can rule out other etiologies of pain, and have a very good idea that this pain is related to endometriosis. Based on clinical presentation, and without needing surgery, we can make a clinical diagnosis of endometriosis.
Clinical diagnosis allows patients to have this disease recognized earlier. It allows them to get into treatment sooner and reverses this trend that we've seen of delayed recognition. This delay is especially difficult in younger women in formative years in their life, when they're in school, when they're in an early stage of their career. If they're held back because of this debilitating pain, these are critical times and opportunities they really can't completely make up.
It's very important that we recognize endometriosis early. If we require a laparoscopy to make the diagnosis, the threshold becomes very high and we don't make the diagnosis. We miss a lot of women with endometriosis and don't treat them early.
There was time when our medical options were limited, and we wanted to be sure of our diagnosis. But these days, I think we can make a clinical diagnosis knowing that we have several treatment options that are relatively easy and safe for patients. Our first line therapy-- the first thing I use when I make the clinical diagnosis of endometriosis-- the first emperic treatment I would use is an oral contraceptive.
I prefer giving the oral contraceptive in a continuous fashion, rather than in a cyclic fashion. If someone has dysmenorrhea, why have menses at all? Retrograde menstruation is the etiology of most endometriosis. If we can eliminate menstruation, potentially it may be reducing endometriosis in the long run.
A lot of women with endometriosis will not respond to progestins—a phenomenon called progestin resistance—therefore, not everyone who has endometriosis will respond to an oral contraceptive. Probably about a third of patients either will not initially respond or will develop a resistance to a progestin and fail to respond in the long-term. Still others have side effects due to progestin therapy-- breast tenderness, mood changes, or a feeling of bloating are very common progestin related side effects.
We now have agents like oral GnRH antagonists that we can use as a second line treatment for those that either don't respond to progestins or those who have side effects from a progestin, including a progestin based oral contraceptive. Additionally, in those with severe pain you may want to use something a little more aggressive.
GnRH antagonists are easy to use oral medications, with an immediate onset of action and are easily reversed. We have come a long way from when we had to use an injectable GnRH agonist as the second line therapy. We have much simpler, easy, second line medications that we can turn to that makes empiric treatment a lot easier.
In the past, when we made a surgical diagnosis it was often because we were afraid of committing them to a long course of Depo GnRH agonist treatment; we didn't use a lot of add back therapy and they had tremendous side effects and the risk of bone loss. Patients and their physicians were reluctant to use GnRH agonists.
Things have gotten a lot easier with oral contraceptives, that can be followed by GnRH antagonists, which are easy to use, simple medications that are very patient-friendly. I think we can make that clinical diagnosis. We can move to an empiric treatment, either first or second line with an oral contraceptive or a GnRH antagonist and easily treat these women without significant side effects. It is important that we advocate for women with cyclic pain, and recognize it more readily, clinically diagnosed it, and begin that empiric treatment. That paradigm really has a huge impact on women's lives.
What recent advancements have been made in diagnosing and treating endometriosis?
Dr. Taylor: I think one of the biggest things that I see improving is recognition. Hopefully, we're narrowing that long delay by closing that time gap from initial symptoms to recognition. As we see public awareness grow, patients are recognizing and looking for answers, and thinking to themselves that they may have endometriosis.
Years ago, people were embarrassed to talk about menstruation, painful menstruation, pain with intercourse, pain with bowel movements. Thankfully, we're a more open society now and we can talk about these things. Women are starting to realize that they may have a problem where it was just dismissed before or perhaps, they were embarrassed to talk about it. I think we have seen a huge advancement. Physicians, as well, are recognizing endometriosis even more than before.
I think we're also much more accepting of this clinical diagnosis paradigm and empiric treatment. A lot of that, as we just said, comes from having better, easier to use drugs available that are much more patient-friendly. The GnRH antagonist elagolix is currently available for treatment of endometriosis in the United States. There are two more GnRH antagonists in the pipeline-- relugolix, which we expect to be approved shortly, and linzagolix which is undergoing phase three clinical trials now. Hopefully, we will have several of these second line drugs, drugs which we can even use first line for severe endometriosis. Their availability is another huge advance.
I also think it is essential, that we don't put someone through surgery to recognize endometriosis. We must be better at taking a good history, doing an exam, and ultrasound when needed. You don't need a surgery to diagnose endometriosis.
However, we do still sometimes need surgery to treat endometriosis. Often, endometriosis will cause adhesions or scarring. These long-term sequelae of endometriosis can still require surgery. The medications we have available today are very good at stopping active disease. But the damage done from long-term endometriosis if we don’t treat may still require surgery. I'm hoping that the earlier we start treating people, the less damage will be done, and the less therapeutic surgeries needed. I think these changes are coming and are all very promising.
It would also be great if we had a non-invasive definitive diagnostic test. There are several of those under development, but nothing available yet. I suspect that we'll see those become available very shortly.
The other thing that we still need in the field is a treatment we can use for those women trying to get pregnant. We use in vitro fertilization, which works very well in the endometriosis population. But a medical therapy that can suppress endometriosis and allow people to try to conceive without needing IVF is something I hope for in the future. A specific endometriosis therapy that is not hormonal, that targets the specific pathophysiology of endometriosis, is something that I'd like to see developed and many of us are currently working on.
I think there is a lot coming, but we've already moved the needle a long way. The GnRH antagonists have given us much more confidence in moving forward with clinical diagnosis and empiric treatment of this disease. It's a huge boon for women's health, allowing early recognition and preventing long-term complications of endometriosis.
Dr. Taylor: Endometriosis is a very common disease. Unfortunately, it's still widely under-recognized. It's estimated that perhaps up to 10% of reproductive age women have endometriosis, yet many are never diagnosed or diagnosed late. The average time that it takes for someone to be diagnosed is about 10 years; that is from the time they have classic symptoms of endometriosis until the time they get a definitive diagnosis and treatment.
I think it's very important that we recognize the clinical features of endometriosis. It's absolutely crucial if we want to shorten the time tom diagnosis. Often patients see multiple physicians before they get an accurate diagnosis. They're often dismissed by their early caregivers who are not very familiar with the disease.
For me, the most important feature is the pelvic pain. To identify endometriosis, I look specifically at the cyclic nature of the pelvic pain, and the progressive nature of the pelvic pain. Endometriosis is by far the most common reason that women have pelvic pain and initially it tends to be cyclic. It often starts as dysmenorrhea (painful periods), however it can progress to the point where pain occurs at times outside of menses. In fact, it can progress to the point where it's painful all the time. However, it almost always starts as dysmennorhea, and progresses.
Pelvic pain that someone had from their first menses on, i.e., from menarche, is less likely to be endometriosis than the pain that progresses and becomes worse or spreads to other times of the menstrual cycle. Endometriosis is a progressive disease, and that's one of the key distinctions in making me think somebody has endometriosis.
Most women with cyclic pelvic pain that gets worse over time, cyclic progressive pelvic pain, will have endometriosis. Those are the key features I look for.
It is also very important to recognize that endometriosis can have effects outside of the reproductive tract, can cause systemic inflammation, and impact other organ systems. Bowel and bladder dysfunction are very common. If that is cyclic and coincides with the pelvic pain, it’s very likely to be secondary to endometriosis. It's important not to get distracted or mislead into making other diagnoses.
What are some of the common symptoms and how does that impact your diagnosis evaluation?
Dr. Taylor: The most common symptom we see is that cyclic pelvic pain I just discussed. Pain, often starting as dysmenorrhea, can go on to include pain outside of the time of menses and become more than just dysmenorrhea- pain in other areas. The other common symptom is infertility. Many women with endometriosis do not have severe pain or may not have pain at all, but first present when they have trouble conceiving.
Endometriosis may be the cause of infertility. Sometimes we recognize that it's endometriosis when we do a physical exam or an ultrasound and find an endometrioma. Cyclic pelvic pain is the classic symptom we look for, and infertility is another key symptom that we shouldn't forget.
Can you talk a little bit about empiric treatment, primarily what that means and how prevalent it is in your current practice?
Dr. Taylor: I think one of the problems we have today with the treatment of endometriosis is that it doesn't get recognized and doesn't get treated right away. When we talk about emperic treatment, it's usually ruling out other potential etiologies of pain. We must ensure no one has have an infection, a tumor or other etiologies that are the cause of the pain, and not simply presume that any cyclic progressive pelvic pain is endometriosis – however most of the time it will be due to endometriosis.
Usually with a good history, physical exam, and sometimes with addition of a transvaginal ultrasound, you can rule out other etiologies of pain, and have a very good idea that this pain is related to endometriosis. Based on clinical presentation, and without needing surgery, we can make a clinical diagnosis of endometriosis.
Clinical diagnosis allows patients to have this disease recognized earlier. It allows them to get into treatment sooner and reverses this trend that we've seen of delayed recognition. This delay is especially difficult in younger women in formative years in their life, when they're in school, when they're in an early stage of their career. If they're held back because of this debilitating pain, these are critical times and opportunities they really can't completely make up.
It's very important that we recognize endometriosis early. If we require a laparoscopy to make the diagnosis, the threshold becomes very high and we don't make the diagnosis. We miss a lot of women with endometriosis and don't treat them early.
There was time when our medical options were limited, and we wanted to be sure of our diagnosis. But these days, I think we can make a clinical diagnosis knowing that we have several treatment options that are relatively easy and safe for patients. Our first line therapy-- the first thing I use when I make the clinical diagnosis of endometriosis-- the first emperic treatment I would use is an oral contraceptive.
I prefer giving the oral contraceptive in a continuous fashion, rather than in a cyclic fashion. If someone has dysmenorrhea, why have menses at all? Retrograde menstruation is the etiology of most endometriosis. If we can eliminate menstruation, potentially it may be reducing endometriosis in the long run.
A lot of women with endometriosis will not respond to progestins—a phenomenon called progestin resistance—therefore, not everyone who has endometriosis will respond to an oral contraceptive. Probably about a third of patients either will not initially respond or will develop a resistance to a progestin and fail to respond in the long-term. Still others have side effects due to progestin therapy-- breast tenderness, mood changes, or a feeling of bloating are very common progestin related side effects.
We now have agents like oral GnRH antagonists that we can use as a second line treatment for those that either don't respond to progestins or those who have side effects from a progestin, including a progestin based oral contraceptive. Additionally, in those with severe pain you may want to use something a little more aggressive.
GnRH antagonists are easy to use oral medications, with an immediate onset of action and are easily reversed. We have come a long way from when we had to use an injectable GnRH agonist as the second line therapy. We have much simpler, easy, second line medications that we can turn to that makes empiric treatment a lot easier.
In the past, when we made a surgical diagnosis it was often because we were afraid of committing them to a long course of Depo GnRH agonist treatment; we didn't use a lot of add back therapy and they had tremendous side effects and the risk of bone loss. Patients and their physicians were reluctant to use GnRH agonists.
Things have gotten a lot easier with oral contraceptives, that can be followed by GnRH antagonists, which are easy to use, simple medications that are very patient-friendly. I think we can make that clinical diagnosis. We can move to an empiric treatment, either first or second line with an oral contraceptive or a GnRH antagonist and easily treat these women without significant side effects. It is important that we advocate for women with cyclic pain, and recognize it more readily, clinically diagnosed it, and begin that empiric treatment. That paradigm really has a huge impact on women's lives.
What recent advancements have been made in diagnosing and treating endometriosis?
Dr. Taylor: I think one of the biggest things that I see improving is recognition. Hopefully, we're narrowing that long delay by closing that time gap from initial symptoms to recognition. As we see public awareness grow, patients are recognizing and looking for answers, and thinking to themselves that they may have endometriosis.
Years ago, people were embarrassed to talk about menstruation, painful menstruation, pain with intercourse, pain with bowel movements. Thankfully, we're a more open society now and we can talk about these things. Women are starting to realize that they may have a problem where it was just dismissed before or perhaps, they were embarrassed to talk about it. I think we have seen a huge advancement. Physicians, as well, are recognizing endometriosis even more than before.
I think we're also much more accepting of this clinical diagnosis paradigm and empiric treatment. A lot of that, as we just said, comes from having better, easier to use drugs available that are much more patient-friendly. The GnRH antagonist elagolix is currently available for treatment of endometriosis in the United States. There are two more GnRH antagonists in the pipeline-- relugolix, which we expect to be approved shortly, and linzagolix which is undergoing phase three clinical trials now. Hopefully, we will have several of these second line drugs, drugs which we can even use first line for severe endometriosis. Their availability is another huge advance.
I also think it is essential, that we don't put someone through surgery to recognize endometriosis. We must be better at taking a good history, doing an exam, and ultrasound when needed. You don't need a surgery to diagnose endometriosis.
However, we do still sometimes need surgery to treat endometriosis. Often, endometriosis will cause adhesions or scarring. These long-term sequelae of endometriosis can still require surgery. The medications we have available today are very good at stopping active disease. But the damage done from long-term endometriosis if we don’t treat may still require surgery. I'm hoping that the earlier we start treating people, the less damage will be done, and the less therapeutic surgeries needed. I think these changes are coming and are all very promising.
It would also be great if we had a non-invasive definitive diagnostic test. There are several of those under development, but nothing available yet. I suspect that we'll see those become available very shortly.
The other thing that we still need in the field is a treatment we can use for those women trying to get pregnant. We use in vitro fertilization, which works very well in the endometriosis population. But a medical therapy that can suppress endometriosis and allow people to try to conceive without needing IVF is something I hope for in the future. A specific endometriosis therapy that is not hormonal, that targets the specific pathophysiology of endometriosis, is something that I'd like to see developed and many of us are currently working on.
I think there is a lot coming, but we've already moved the needle a long way. The GnRH antagonists have given us much more confidence in moving forward with clinical diagnosis and empiric treatment of this disease. It's a huge boon for women's health, allowing early recognition and preventing long-term complications of endometriosis.
Dr. Taylor: Endometriosis is a very common disease. Unfortunately, it's still widely under-recognized. It's estimated that perhaps up to 10% of reproductive age women have endometriosis, yet many are never diagnosed or diagnosed late. The average time that it takes for someone to be diagnosed is about 10 years; that is from the time they have classic symptoms of endometriosis until the time they get a definitive diagnosis and treatment.
I think it's very important that we recognize the clinical features of endometriosis. It's absolutely crucial if we want to shorten the time tom diagnosis. Often patients see multiple physicians before they get an accurate diagnosis. They're often dismissed by their early caregivers who are not very familiar with the disease.
For me, the most important feature is the pelvic pain. To identify endometriosis, I look specifically at the cyclic nature of the pelvic pain, and the progressive nature of the pelvic pain. Endometriosis is by far the most common reason that women have pelvic pain and initially it tends to be cyclic. It often starts as dysmenorrhea (painful periods), however it can progress to the point where pain occurs at times outside of menses. In fact, it can progress to the point where it's painful all the time. However, it almost always starts as dysmennorhea, and progresses.
Pelvic pain that someone had from their first menses on, i.e., from menarche, is less likely to be endometriosis than the pain that progresses and becomes worse or spreads to other times of the menstrual cycle. Endometriosis is a progressive disease, and that's one of the key distinctions in making me think somebody has endometriosis.
Most women with cyclic pelvic pain that gets worse over time, cyclic progressive pelvic pain, will have endometriosis. Those are the key features I look for.
It is also very important to recognize that endometriosis can have effects outside of the reproductive tract, can cause systemic inflammation, and impact other organ systems. Bowel and bladder dysfunction are very common. If that is cyclic and coincides with the pelvic pain, it’s very likely to be secondary to endometriosis. It's important not to get distracted or mislead into making other diagnoses.
What are some of the common symptoms and how does that impact your diagnosis evaluation?
Dr. Taylor: The most common symptom we see is that cyclic pelvic pain I just discussed. Pain, often starting as dysmenorrhea, can go on to include pain outside of the time of menses and become more than just dysmenorrhea- pain in other areas. The other common symptom is infertility. Many women with endometriosis do not have severe pain or may not have pain at all, but first present when they have trouble conceiving.
Endometriosis may be the cause of infertility. Sometimes we recognize that it's endometriosis when we do a physical exam or an ultrasound and find an endometrioma. Cyclic pelvic pain is the classic symptom we look for, and infertility is another key symptom that we shouldn't forget.
Can you talk a little bit about empiric treatment, primarily what that means and how prevalent it is in your current practice?
Dr. Taylor: I think one of the problems we have today with the treatment of endometriosis is that it doesn't get recognized and doesn't get treated right away. When we talk about emperic treatment, it's usually ruling out other potential etiologies of pain. We must ensure no one has have an infection, a tumor or other etiologies that are the cause of the pain, and not simply presume that any cyclic progressive pelvic pain is endometriosis – however most of the time it will be due to endometriosis.
Usually with a good history, physical exam, and sometimes with addition of a transvaginal ultrasound, you can rule out other etiologies of pain, and have a very good idea that this pain is related to endometriosis. Based on clinical presentation, and without needing surgery, we can make a clinical diagnosis of endometriosis.
Clinical diagnosis allows patients to have this disease recognized earlier. It allows them to get into treatment sooner and reverses this trend that we've seen of delayed recognition. This delay is especially difficult in younger women in formative years in their life, when they're in school, when they're in an early stage of their career. If they're held back because of this debilitating pain, these are critical times and opportunities they really can't completely make up.
It's very important that we recognize endometriosis early. If we require a laparoscopy to make the diagnosis, the threshold becomes very high and we don't make the diagnosis. We miss a lot of women with endometriosis and don't treat them early.
There was time when our medical options were limited, and we wanted to be sure of our diagnosis. But these days, I think we can make a clinical diagnosis knowing that we have several treatment options that are relatively easy and safe for patients. Our first line therapy-- the first thing I use when I make the clinical diagnosis of endometriosis-- the first emperic treatment I would use is an oral contraceptive.
I prefer giving the oral contraceptive in a continuous fashion, rather than in a cyclic fashion. If someone has dysmenorrhea, why have menses at all? Retrograde menstruation is the etiology of most endometriosis. If we can eliminate menstruation, potentially it may be reducing endometriosis in the long run.
A lot of women with endometriosis will not respond to progestins—a phenomenon called progestin resistance—therefore, not everyone who has endometriosis will respond to an oral contraceptive. Probably about a third of patients either will not initially respond or will develop a resistance to a progestin and fail to respond in the long-term. Still others have side effects due to progestin therapy-- breast tenderness, mood changes, or a feeling of bloating are very common progestin related side effects.
We now have agents like oral GnRH antagonists that we can use as a second line treatment for those that either don't respond to progestins or those who have side effects from a progestin, including a progestin based oral contraceptive. Additionally, in those with severe pain you may want to use something a little more aggressive.
GnRH antagonists are easy to use oral medications, with an immediate onset of action and are easily reversed. We have come a long way from when we had to use an injectable GnRH agonist as the second line therapy. We have much simpler, easy, second line medications that we can turn to that makes empiric treatment a lot easier.
In the past, when we made a surgical diagnosis it was often because we were afraid of committing them to a long course of Depo GnRH agonist treatment; we didn't use a lot of add back therapy and they had tremendous side effects and the risk of bone loss. Patients and their physicians were reluctant to use GnRH agonists.
Things have gotten a lot easier with oral contraceptives, that can be followed by GnRH antagonists, which are easy to use, simple medications that are very patient-friendly. I think we can make that clinical diagnosis. We can move to an empiric treatment, either first or second line with an oral contraceptive or a GnRH antagonist and easily treat these women without significant side effects. It is important that we advocate for women with cyclic pain, and recognize it more readily, clinically diagnosed it, and begin that empiric treatment. That paradigm really has a huge impact on women's lives.
What recent advancements have been made in diagnosing and treating endometriosis?
Dr. Taylor: I think one of the biggest things that I see improving is recognition. Hopefully, we're narrowing that long delay by closing that time gap from initial symptoms to recognition. As we see public awareness grow, patients are recognizing and looking for answers, and thinking to themselves that they may have endometriosis.
Years ago, people were embarrassed to talk about menstruation, painful menstruation, pain with intercourse, pain with bowel movements. Thankfully, we're a more open society now and we can talk about these things. Women are starting to realize that they may have a problem where it was just dismissed before or perhaps, they were embarrassed to talk about it. I think we have seen a huge advancement. Physicians, as well, are recognizing endometriosis even more than before.
I think we're also much more accepting of this clinical diagnosis paradigm and empiric treatment. A lot of that, as we just said, comes from having better, easier to use drugs available that are much more patient-friendly. The GnRH antagonist elagolix is currently available for treatment of endometriosis in the United States. There are two more GnRH antagonists in the pipeline-- relugolix, which we expect to be approved shortly, and linzagolix which is undergoing phase three clinical trials now. Hopefully, we will have several of these second line drugs, drugs which we can even use first line for severe endometriosis. Their availability is another huge advance.
I also think it is essential, that we don't put someone through surgery to recognize endometriosis. We must be better at taking a good history, doing an exam, and ultrasound when needed. You don't need a surgery to diagnose endometriosis.
However, we do still sometimes need surgery to treat endometriosis. Often, endometriosis will cause adhesions or scarring. These long-term sequelae of endometriosis can still require surgery. The medications we have available today are very good at stopping active disease. But the damage done from long-term endometriosis if we don’t treat may still require surgery. I'm hoping that the earlier we start treating people, the less damage will be done, and the less therapeutic surgeries needed. I think these changes are coming and are all very promising.
It would also be great if we had a non-invasive definitive diagnostic test. There are several of those under development, but nothing available yet. I suspect that we'll see those become available very shortly.
The other thing that we still need in the field is a treatment we can use for those women trying to get pregnant. We use in vitro fertilization, which works very well in the endometriosis population. But a medical therapy that can suppress endometriosis and allow people to try to conceive without needing IVF is something I hope for in the future. A specific endometriosis therapy that is not hormonal, that targets the specific pathophysiology of endometriosis, is something that I'd like to see developed and many of us are currently working on.
I think there is a lot coming, but we've already moved the needle a long way. The GnRH antagonists have given us much more confidence in moving forward with clinical diagnosis and empiric treatment of this disease. It's a huge boon for women's health, allowing early recognition and preventing long-term complications of endometriosis.
52-year-old man • hematemesis • history of cirrhosis • persistent fevers • Dx?
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
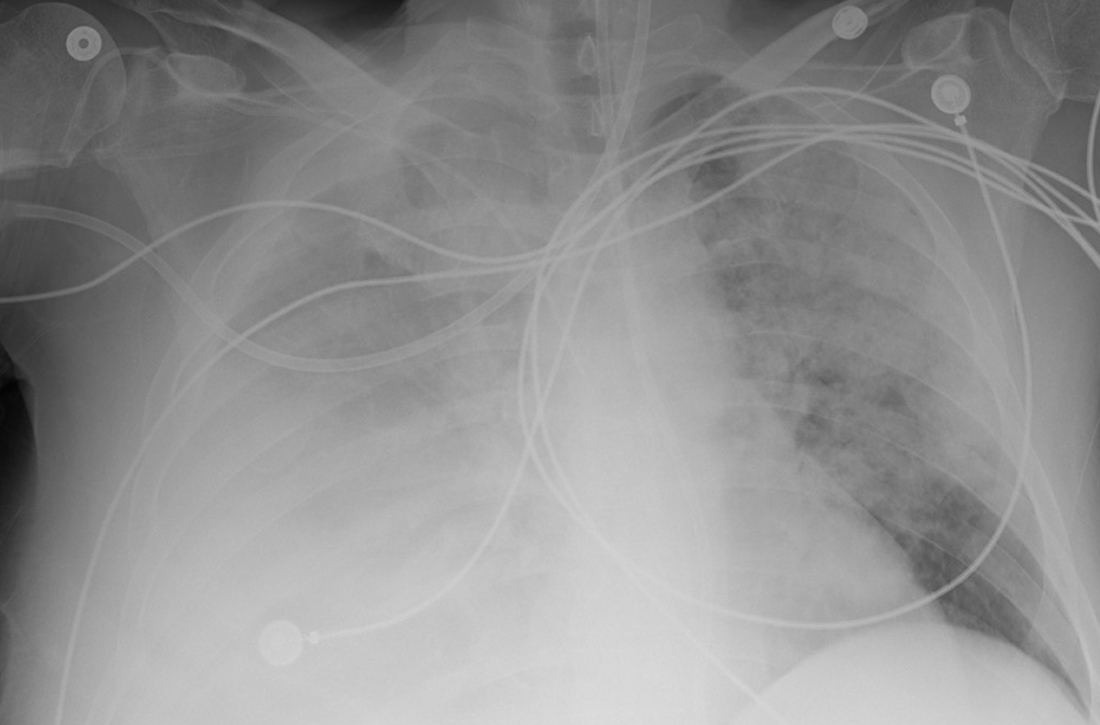
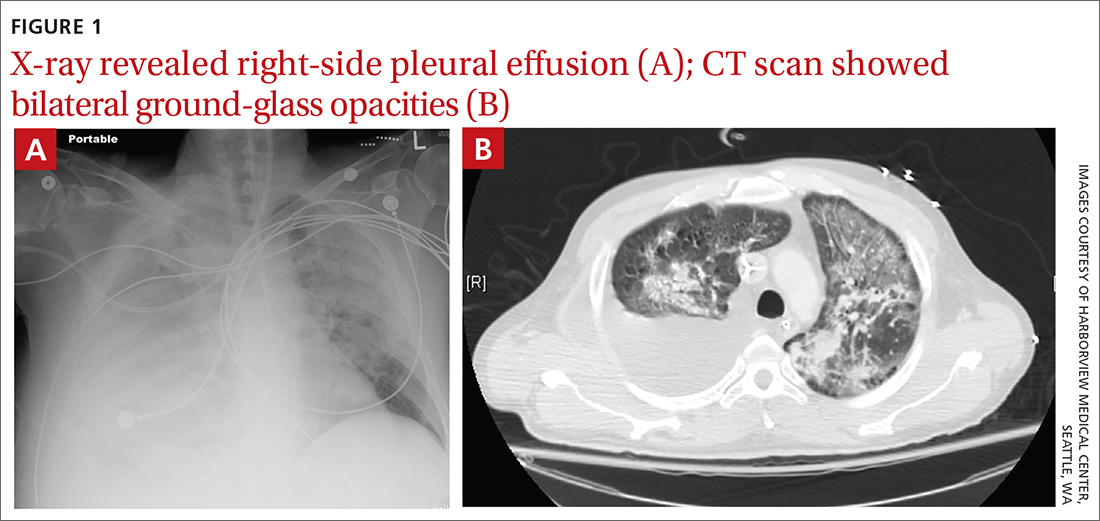
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).
Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
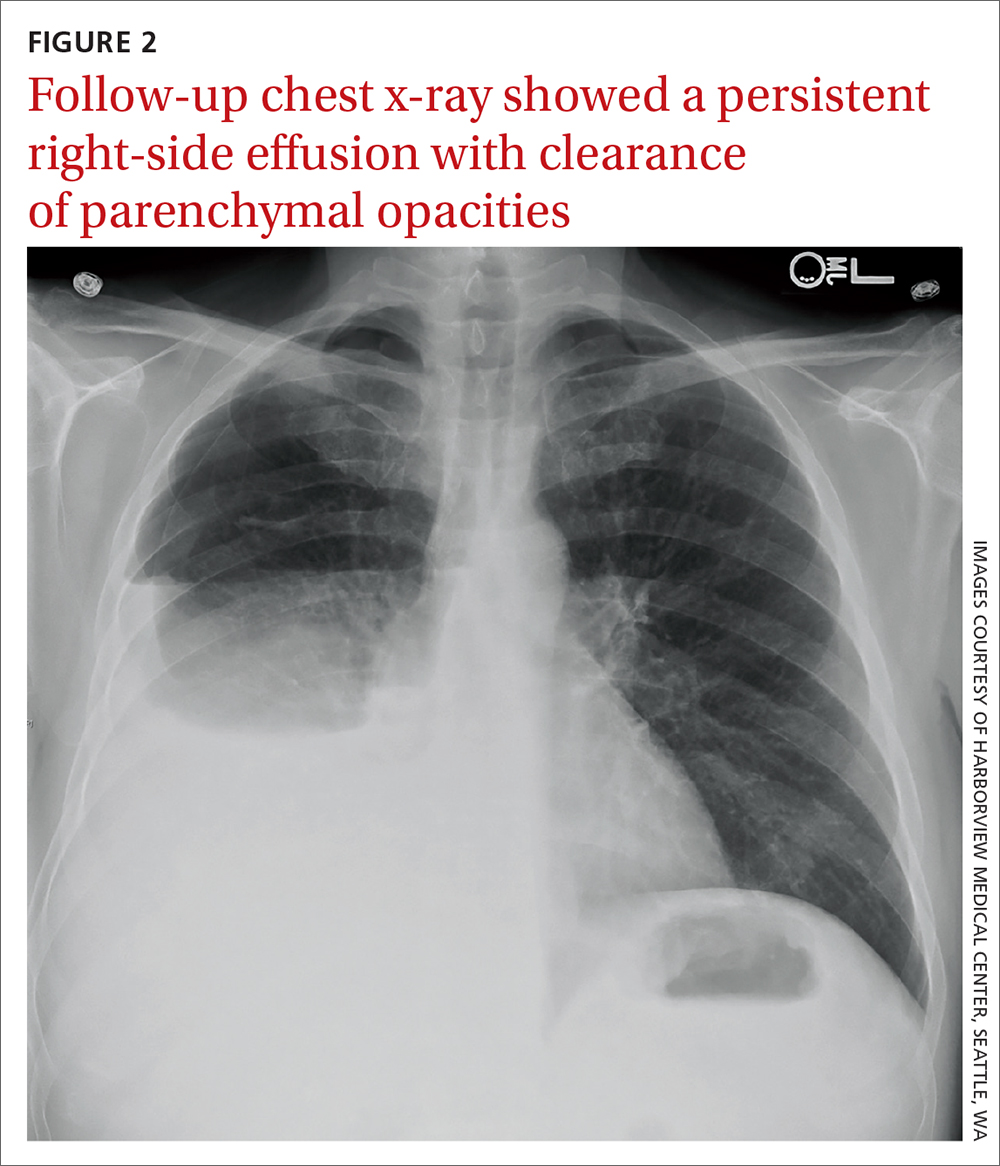
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.
THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; [email protected]
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).

Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.

THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; [email protected]
THE CASE
A 52-year-old man presented to the emergency department after vomiting a large volume of blood and was admitted to the intensive care unit. His past medical history was remarkable for untreated chronic hepatitis C resulting from injection drug use and cirrhosis without prior history of esophageal varices.
Due to ongoing hematemesis, he was intubated for airway protection and underwent esophagogastroduodenoscopy with banding of large esophageal varices on hospital day (HD) 1. He was extubated on HD 2 after clinical stability was achieved; however, he became encephalopathic over the subsequent days despite treatment with lactulose. On HD 4, the patient required re-intubation for progressive respiratory failure. Chest imaging revealed a large, simple-appearing right pleural effusion and extensive bilateral patchy ground-glass opacities (FIGURE 1).

Thoracentesis was ordered and revealed transudative pleural fluid; this finding, along with negative infectious studies, was consistent with hepatic hydrothorax. In the setting of initial decompensation, empiric treatment with vancomycin and meropenem was started for suspected hospital-acquired pneumonia.
The patient had persistent fevers that had developed during his hospital stay and pulmonary opacities, despite 72 hours of treatment with broad-spectrum antibiotics. Thus, a diagnostic bronchoscopy with bronchoalveolar lavage (BAL) was performed. BAL cell count and differential revealed 363 nucleated cells/µL, with profound eosinophilia (42% eosinophils, 44% macrophages, 14% neutrophils).
Bacterial and fungal cultures and a viral polymerase chain reaction panel were negative. HIV antibody-antigen and RNA testing were also negative. The patient had no evidence or history of underlying malignancy, autoimmune disease, or recent immunosuppressive therapy, including corticosteroids. Due to consistent imaging findings and lack of improvement with appropriate treatment for bacterial pneumonia, further work-up was pursued.
THE DIAGNOSIS
Given the consistent radiographic pattern, the differential diagnosis for this patient included pneumocystis pneumonia (PCP), a potentially life-threatening opportunistic infection. Work-up therefore included direct fluorescent antibody testing, which was positive for Pneumocystis jirovecii, a fungus that can cause PCP.
Of note, the patient’s white blood cell count was elevated on admission (11.44 × 103/µL) but low for much of his hospital stay (nadir = 1.97 × 103/µL), with associated lymphopenia (nadir = 0.22 × 103/µl). No peripheral eosinophilia was noted.
Continue to: DISCUSSION
DISCUSSION
PCP typically occurs in immunocompromised individuals and may be related to HIV infection, malignancy, or exposure to immunosuppressive therapies.1,2 While rare cases of PCP have been described in adults without predisposing factors, many of these cases occurred at the beginning of the AIDS epidemic, prior to reliable HIV testing.3-5
Uncharted territory. We were confident in our diagnosis because immunofluorescence testing has very few false-positives and a high specificity.6-8 But there were informational gaps. The eosinophilia recorded on BAL is poorly described in HIV-negative patients with PCP but well-described in HIV-positive patients, with the level of eosinophilia associated with disease severity.9,10 Eosinophils are thought to contribute to pulmonary inflammation, which may explain the severity of our patient’s course.10
A first of its kind case?
To our knowledge, this is the first report of PCP in a patient with cirrhosis from chronic hepatitis C virus infection and no other predisposing conditions or preceding immunosuppressive therapy. We suspect that his lymphopenia, which was noted during his critical illness, predisposed him to PCP.
Lymphocytes (in particular CD4+ T cells) have been shown to play an important role, along with alveolar macrophages and neutrophils, in directing the host defense against
Typical risk factors for lymphopenia had not been observed in this patient. However, cirrhosis has been associated with low CD4+ T-cell counts and disruption of cell-mediated immunity, even in HIV-seronegative patients.14,15 There are several postulated mechanisms for low CD4+ T-cell counts in cirrhosis, including splenic sequestration, impaired T-cell production (due to impaired thymopoiesis), increased T-cell consumption, and apoptosis (due to persistent immune system activation from bacterial translocation and an overall pro-inflammatory state).16,17
Continue to: Predisposing factors guide treatment
Predisposing factors guide treatment
Routine treatment for PCP in patients without HIV is a 21-day course of trimethoprim/sulfamethoxazole (Bactrim). Dosing for patients with normal renal function is 15 to 20 mg/kg orally or intravenously per day. Patients with allergy to trimethoprim/sulfamethoxazole should ideally undergo desensitization, given its effectiveness against PCP.
Due to a sulfonamide allergy, our patient was started on primaquine 30 mg/d, clindamycin 600 mg tid, and prednisone 40 mg bid. (The corticosteroid was added because of the severity of the disease.) Three days after starting treatment—and 10 days into his hospital stay—the patient had significant improvement in his respiratory status and was successfully extubated. He underwent trimethoprim/sulfamethoxazole desensitization and completed a 21-day course of treatment for PCP with complete resolution of respiratory symptoms. Follow-up chest radiograph 2 months later (FIGURE 2) confirmed clearance of opacities.

THE TAKEAWAY
PCP remains a rare disease in patients without the typical immunosuppressive risk factors. However, it should be considered in patients with cirrhosis who develop respiratory failure, especially those with compatible radiographic findings and negative microbiologic evaluation for other, more typical, organisms.
CORRESPONDENCE
Tyler Albert, MD, VA Puget Sound Healthcare System, 1660 South Columbian Way, S-111-Pulm, Seattle, WA 98108; [email protected]
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
1. Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487-2498. doi: 10.1056/NEJMra032588
2. Walzer PD, Perl DP, Krogstad DJ, et al. Pneumocystis carinii pneumonia in the United States. Epidemiologic, diagnostic, and clinical features. Ann Intern Med. 1974;80:83-93. doi: 10.7326/0003-4819-80-1-83
3. Sepkowitz KA. Pneumocystis carinii pneumonia in patients without AIDS. Clin Infect Dis. 1993;17 suppl 2:S416-422. doi: 10.1093/clinids/17.supplement_2.s416
4. Al Soub H, Taha RY, El Deeb Y, et al. Pneumocystis carinii pneumonia in a patient without a predisposing illness: case report and review. Scand J Infect Dis. 2004;36:618-621. doi: 10.1080/00365540410017608
5. Jacobs JL, Libby DM, Winters RA, et al. A cluster of Pneumocystis carinii pneumonia in adults without predisposing illnesses. N Engl J Med. 1991;324:246-250. doi: 10.1056/NEJM199101243240407
6. Ng VL, Yajko DM, McPhaul LW, et al. Evaluation of an indirect fluorescent-antibody stain for detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:975-979. doi: 10.1128/jcm.28.5.975-979.1990
7. Cregan P, Yamamoto A, Lum A, et al. Comparison of four methods for rapid detection of Pneumocystis carinii in respiratory specimens. J Clin Microbiol. 1990;28:2432-2436. doi: 10.1128/jcm.28.11.2432-2436.1990
8. Turner D, Schwarz Y, Yust I. Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J. 2003;21:204-208. doi: 10.1183/09031936.03.00035303
9. Smith RL, el-Sadr WM, Lewis ML. Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest. 1988;93:60-64. doi: 10.1378/chest.93.1.60
10. Fleury-Feith J, Van Nhieu JT, Picard C, et al. Bronchoalveolar lavage eosinophilia associated with Pneumocystis carinii pneumonitis in AIDS patients. Comparative study with non-AIDS patients. Chest. 1989;95:1198-1201. doi: 10.1378/chest.95.6.1198
11. Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol. 2007;5:298-308. doi: 10.1038/nrmicro1621
12. Toh BH, Roberts-Thomson IC, Mathews JD, et al. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973;14:193-202.
13. Mansharamani NG, Balachandran D, Vernovsky I, et al. Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest. 2000;118:712-720. doi: 10.1378/chest.118.3.712
14. McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. doi: 10.1086/509580
15. Bienvenu AL, Traore K, Plekhanova I, et al. Pneumocystis pneumonia suspected cases in 604 non-HIV and HIV patients. Int J Infect Dis. 2016;46:11-17. doi: 10.1016/j.ijid.2016.03.018
16. Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. doi: 10.1016/j.jhep.2014.08.010
17. Lario M, Muñoz L, Ubeda M, et al. Defective thymopoiesis and poor peripheral homeostatic replenishment of T-helper cells cause T-cell lymphopenia in cirrhosis. J Hepatol. 2013;59:723-730. doi: 10.1016/j.jhep.2013.05.042
Somatic symptom disorder in primary care: A collaborative approach
THE CASE
James R* is a 30-year-old man who presented for a primary care walk-in visit due to dizziness, 2 days after he visited an emergency department (ED) for the same concern. He reported episodic symptoms lasting seconds to minutes, specifically when lying down. He said he had not fallen or experienced other physical trauma, did not have blurred vision or hearing loss, and was taking no medications. He also reported panic attacks, during which he experienced palpitations, trembling, paresthesia, and fear of dying. He stated that dizziness did not occur exclusively during panic episodes. His medical history was significant for hypertension; however, he reported significant anxiety related to medical visits. All home blood pressure readings he reported were within normal limits.
Upon examination, the patient had a blood pressure reading of 142/90 mm Hg and no evidence of nystagmus at rest. A neurologic exam was normal and a Dix-Hallpike maneuver reproduced subjective vertigo without nystagmus. Laboratory findings from the patient’s ED visit were negative for troponin and drug use, and blood oxygenation levels were within normal limits. At the time of this current visit, an electrocardiogram was unremarkable, with the exception of some tachycardia.
The presumptive diagnosis was benign paroxysmal positional vertigo (BPPV). An Epley maneuver was performed in the clinic and resulted in minimal symptom improvement. The physician taught Mr. R how to perform the Epley maneuver himself, prescribed a short course of meclizine, and referred him to the integrated mental health care service to address his panic attacks and anxiety.
Over the next few months, Mr. R continued to report significant distress about the dizzy spells, which persisted even after performing the Epley maneuver, and he reported that the meclizine was causing worsening vertigo. He received an ear-nose-and-throat consultation and cognitive behavioral therapy (CBT)–based consultation/interventions. He also reported avoiding multiple activities due to concerns about his dizziness.
●
*The patient’s name and other personally identifying information have been changed to protect his identity.
Somatic symptom disorder (SSD) is characterized by one or more physical symptoms associated with “excessive thoughts, feelings, or behaviors that result in distress and/or functional impairment.”1 Individuals with SSD are preoccupied with symptom-related severity, experience high symptom-related anxiety, or devote significant time and energy to the symptoms or heath concerns. With a diagnosis of SSD, physical symptoms experienced by the patient may or may not be medically explained. The same symptom need not be continuously present as long as the overall symptomatic presentation lasts 6 months or longer.
The specifier “with predominant pain” is used when pain dominates the presentation.1 Estimated prevalence of SSD in primary care ranges from 5% to 35%.2 The true scope of SSD is difficult to assess accurately since research tends to focus on medically unexplained symptoms, rather than excessive symptom-related concerns. Furthermore, terms such as “medically unexplained symptoms” and “functional syndromes” (including fibromyalgia and irritable bowel syndrome) are frequently used when describing SSD.3
One or more factors may contribute to unexplained symptoms: limitations of medical procedures and techniques, partial clinical information, patients’ inability to follow management recommendations, challenges in differential diagnostics, and access-to-care limitations preventing regular care and appropriate diagnostic work up.
What’s important to remember is that it’s the patient’s reaction to physical symptoms, rather than the presence of symptoms per se, that defines SSD.
Considerations in the differential diagnosis
When making a diagnosis of SSD, symptoms cannot:4
- be feigned or deliberately produced as in malingering or factitious disorder.
- result from physiologic effects of a substance (eg, intoxication, withdrawal, or adverse medication effects).
- constitute somatic delusions, as occur in psychotic disorders.
- constitute symptoms or deficits affecting voluntary motor or sensory function that are better explained by neurologic, medical, or psychiatric conditions (consider conversion disorder).
- be preoccupations with physical appearance flaws, as in body dysmorphic disorder.
- be accounted for by an anxiety disorder (eg, palpitations associated with panic attacks).
Continue to: Illness anxiety disorder...
Illness anxiety disorder is also characterized by significant health-related concerns; however, physical symptoms are either mild or absent.
Possible causes of SSD are varied and complex, including genetic and biological factors, family dynamics, behavioral modeling/learning, personality traits, difficulties with emotional regulation, and awareness.5 Patients may present with ongoing requests for symptom explanations, feelings of helplessness, fear of having concerns dismissed, and low motivation for change.3
Aids in supporting a diagnosis of SSD
It’s not appropriate to rely solely on questionnaires to make the diagnosis of SSD. However, brief screening tools are a time-efficient way to capture patients’ experiences and perceptions.6 Along with other components of clinical evaluation, brief symptom screens can both support the diagnosis and help in longitudinal symptom assessment.
Patient Health Questionnaire-15 (PHQ-15), developed for self-report screening in primary care, has desirable psychometric properties including appropriate internal reliability; convergent validity with measures of functional status, disability days, and symptom-related burden; and discriminant validity from measures of depressive symptoms.7 The PHQ-15 is an open access tool that is available in several languages. The respondent is asked to rate the extent of being bothered by a range of medical symptoms in the proceeding 4 weeks. Total scores range from 0 to 30, with higher scores indicating greater symptom aggravation. Cutoffs of 5, 10, and 15 correspond to mild, moderate, and severe symptom levels.8
Somatic Symptom Disorder - B Criteria Scale (SSD-12) aims to capture SSD symptoms in line with Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria. It assesses cognitive, affective, and behavioral aspects of SSD.9 The SSD-12 is copyrighted and its use requires registration and purchase. Cutoffs by age and gender are available. SSD-12 has demonstrated appropriate reliability and validity.9
Continue to: Structured Clinical Interview for DSM Disorders
Structured Clinical Interview for DSM Disorders (SCID)10,11 is perhaps the most rigorous differential diagnostic tool. However, SCID administration requires training and skill; time for administration and cost of the materials may be prohibitive in primary care.
Finally, SSD symptoms are highly associated with depression and anxiety. Ongoing elevated screening scores for anxiety and depression refractory to interventions may indicate the possibility of overlooked SSD. Furthermore, use of SSD screening tools with anxiety and depression screening tools can provide a more comprehensive picture of impairment, as well as symptom progress.
Treatment: Avoid a split approach
Diagnosing and treating SSD can be challenging for physicians who focus on biomedically based approaches in patient care. Additional tests, studies, and prescriptions are likely to fuel (rather than pacify) patients’ concerns, as such steps divert attention from the underlying psychological needs and mechanisms which maintain SSD. Avoid using a split biopsychosocial approach—ie, beginning the inquiry and treatment planning from a biomedical perspective, and then falling back on psychosocial formulation when treatment efforts have been ineffective. Such an approach leads to understandable patient dissatisfaction and can be interpreted by them as the caregiver suggesting that physical symptoms are “all in [their] head.”12
These 4 tips can help
1. Use a biopsychosocial formulation when initiating treatment. Be familiar with biopsychosocial factors in SSD and develop a narrative for discussing this formulation with patients. For example: “Mr. R, we are going to use the following [medical tests/studies/medications] to understand the cause of your symptoms and better manage them. We also need to think about the role of stress and distress in your symptoms because these can also be at play with dizziness.” This may be particularly beneficial for a functional disorder, such as chronic pain. Incorporating patient education resources is an important step toward shared understanding (see Hunter Integrated Pain Service for chronic pain educational videos; www.tga.gov.au/chronic-pain-management-video-resource-brainman13).
2. Combine education about pathophysiology with patient-centered interviewing. Significant SSD symptom improvements were noted following a single 30-minute educational session, while motivational interviewing techniques were used to probe patients’ concerns.2
Continue to: Maintain professionalism and good clinical practice
3. Maintain professionalism and good clinical practice. Consider SSD a medical matter and address it accordingly: explore concerns fully, provide evidence-based responses, communicate empathy, and employ objective management strategies.14
4. Do not overlook the value of the relationship. A recent systematic review concluded that the relationship between the patient and care provider was central to the success of the interventions for symptom reduction.15
A controversial approach. Pharmacotherapy for SSD is controversial. While several trials of antidepressants and St. John’s wort have been positive and some authors have stated that all classes of antidepressants are effective for SSD, others maintain that questions regarding dosing, treatment duration, and sustainability of improvement have not been sufficiently addressed in research.16,17
Coordination of care issues
Primary care continues to be the de facto mental health system, and specialty services may be unavailable or declined by patients.18 CBT delivered in person or online is empirically supported as a treatment approach to medically unexplained symptoms and SSD.17,19-22
A recent meta-analysis of randomized controlled trials published by Jing and colleagues23 reported that CBT was effective for SSD symptom reduction, and that treatment gains were maintained 3 to 12 months post treatment. However, concerns about the practical implementation of CBT in primary care were raised because CBT was not shown to be effective in improving social functioning or reducing the number of medical visits. Symptom improvement was maximized with longer durations of treatment (> 10 sessions) and greater session lengths (> 50 minutes). Additionally, Abbass and colleagues24 brought up several methodologic (sampling and analysis) concerns related to Jing et al’s work.
Continue to: Overally, CBT's effect sizes...
Overall, CBT’s effect sizes are small, and patients who are open to biopsychosocial explanations for their symptoms and to receiving psychological services may differ from most patients seen in primary care practices.21 Furthermore, mental health providers may hesitate to diagnose SSD because they are concerned about missing a somatic illness.3 Therefore, when coordinating care with mental health providers, it may be beneficial to discuss the treatment approach, assess familiarity with the SSD diagnosis, and closely coordinate and collaborate on the treatment plan.
While physicians cannot be expected to function as psychotherapists, an understanding of CBT and techniques for SSD treatment can be beneficial. Integrated mental health services may hold promise in addressing SSD in primary care. Onsite availability of a behavioral health provider competent in providing evidence-based care can target SSD symptoms and support both patients and physicians.
THE CASE
Mr. R’s treatment course included multiple primary care appointments (scheduled and walk in), ED visits, and specialist visits (ENT/vestibular rehabilitation). He sought care as symptoms intensified, lasted longer, or occurred in new circumstances. He reported persistent fear of the symptoms and anxiety that serious medical causes had been overlooked. He also described distress associated with vertigo and his anxiety sensitivity (anxiety about being anxious).
The behavioral health consultant (BHC; psychologist) and physician talked to the patient about the biopsychosocial antecedents of his condition and the factors that perpetuate the anxiety and stress response. The BHC described the fight/flight/freeze response to the patient and explained its role in the physiologic stress response associated with somatic symptoms and panic. Educational materials (videos and handouts) were also provided to the patient to further illustrate these concepts. The BHC also discussed the role of interoceptive and situational avoidance and active coping (eg, engaging in safe activities); taught the patient relaxation and grounding techniques; and used cognitive disputation aimed at challenging catastrophic symptom interpretations.
The BHC and the patient’s physician established joint treatment goals that included improving functioning, promoting active coping, and decreasing distress associated with symptoms. After the initial medical and BHC visits, both vertigo and anxiety symptoms appeared to abate somewhat, but symptoms have been ongoing and distress and impairment have been variable. The patient’s family physician and BHC continue to work with him to optimize the care plan and treatment goals.
CORRESPONDENCE
Nataliya Pilipenko, PhD, ABPP, Center for Family and Community Medicine, Columbia University Vagelos College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032; [email protected]
ACKNOWLEDGEMENT
The author thanks Dr. Molly Warren for her collaboration and guidance.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
2. Johnson KK, Bennett C, Rochani H. Significant improvement of somatic symptom disorder with brief psychoeducational intervention by PMHNP in primary care. J Am Psychiatr Nurses Assoc. 2020;28:171-180. doi: 10.1177/1078390320960524
3. Weigel A, Maehder K, Witt M, et al. Psychotherapists’ perspective on the treatment of patients with somatic symptom disorders. J Psychosom Res. 2020;138:110228. doi: 10.1016/j.jpsychores.2020.110228
4. American Psychiatric Association. Handbook of Differential Diagnosis. American Psychiatric Publishing; 2014;234-235.
5. Mayo Clinic. Somatic symptom disorder. Accessed February 21, 2022. www.mayoclinic.org/diseases-conditions/somatic-symptom-disorder/symptoms-causes/syc-20377776?p=1
6. Toussaint A, Riedl B, Kehrer S, et al. Validity of the Somatic Symptom Disorder-B Criteria Scale (SSD-12) in primary care. Fam Pract. 2018;35:342-347. doi: 10.1093/fampra/cmx116
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-66. doi: 10.1097/00006842-200203000-00008
8. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. doi: 10.1016/j.genhosppsych.2010.03.006
9. Toussaint A, Löwe B, Brähler E, et al. The Somatic Symptom Disorder - B Criteria Scale (SSD-12): factorial structure, validity and population-based norms. J Psychosom Res. 2017;97:9-17. doi: 10.1016/j.jpsychores.2017.03.017
10. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Research Version. American Psychiatric Association, 2015.
11. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Publishing; 2016.
12. McDaniel SH, Hepworth J, Campbell TL, et al, eds. Family Oriented Primary Care. Springer Publishing Co; 2005:1-15.
13. Hunter Integrated Pain Service (2016). Brainman videos. Hunter New England Local Health District. New South Wales, Australia. Accessed February 21, 2022. www.tga.gov.au/chronic-pain-management-video-resource-brainman
14. Murray AM, Toussaint A, Althaus A, et al. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1-10. doi: 10.1016/j.jpsychores.2015.11.002
15. Leaviss J, Davis S, Ren S, et al. Behavioral modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-490. doi: 10.3310/hta24460
16. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888. doi: 10.1097/PSY.0b013e31815b00c4
17. Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263-271. doi: 10.1016/j.psym.2011.01.007
18. Kessler R, Stafford D. Primary care is the de facto mental health system. In Kessler R, Stafford D, eds. Collaborative Medicine Case Studies: Evidence in Practice. Springer Publishing Co, 2008; 9-21.
19. Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. doi: 10.1007/s11606-013-2392-6
20. Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86:89-98. doi: 10.1037/ccp0000248
21. van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2
22. Verdurmen MJ, Videler AC, Kamperman AM, et al. Cognitive behavioral therapy for somatic symptom disorders in later life: a prospective comparative explorative pilot study in two clinical populations. Neuropsychiatr Dis Treat. 2017;13:2331-2339. doi: 10.2147/NDT.S141208
23. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
24. Abbass A, Leichsenring F, Steinert C. Re: Jing et al., the efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;255:S0165-0327(18)33197-5. doi: 10.1016/j.jad.2019.02.055
THE CASE
James R* is a 30-year-old man who presented for a primary care walk-in visit due to dizziness, 2 days after he visited an emergency department (ED) for the same concern. He reported episodic symptoms lasting seconds to minutes, specifically when lying down. He said he had not fallen or experienced other physical trauma, did not have blurred vision or hearing loss, and was taking no medications. He also reported panic attacks, during which he experienced palpitations, trembling, paresthesia, and fear of dying. He stated that dizziness did not occur exclusively during panic episodes. His medical history was significant for hypertension; however, he reported significant anxiety related to medical visits. All home blood pressure readings he reported were within normal limits.
Upon examination, the patient had a blood pressure reading of 142/90 mm Hg and no evidence of nystagmus at rest. A neurologic exam was normal and a Dix-Hallpike maneuver reproduced subjective vertigo without nystagmus. Laboratory findings from the patient’s ED visit were negative for troponin and drug use, and blood oxygenation levels were within normal limits. At the time of this current visit, an electrocardiogram was unremarkable, with the exception of some tachycardia.
The presumptive diagnosis was benign paroxysmal positional vertigo (BPPV). An Epley maneuver was performed in the clinic and resulted in minimal symptom improvement. The physician taught Mr. R how to perform the Epley maneuver himself, prescribed a short course of meclizine, and referred him to the integrated mental health care service to address his panic attacks and anxiety.
Over the next few months, Mr. R continued to report significant distress about the dizzy spells, which persisted even after performing the Epley maneuver, and he reported that the meclizine was causing worsening vertigo. He received an ear-nose-and-throat consultation and cognitive behavioral therapy (CBT)–based consultation/interventions. He also reported avoiding multiple activities due to concerns about his dizziness.
●
*The patient’s name and other personally identifying information have been changed to protect his identity.
Somatic symptom disorder (SSD) is characterized by one or more physical symptoms associated with “excessive thoughts, feelings, or behaviors that result in distress and/or functional impairment.”1 Individuals with SSD are preoccupied with symptom-related severity, experience high symptom-related anxiety, or devote significant time and energy to the symptoms or heath concerns. With a diagnosis of SSD, physical symptoms experienced by the patient may or may not be medically explained. The same symptom need not be continuously present as long as the overall symptomatic presentation lasts 6 months or longer.
The specifier “with predominant pain” is used when pain dominates the presentation.1 Estimated prevalence of SSD in primary care ranges from 5% to 35%.2 The true scope of SSD is difficult to assess accurately since research tends to focus on medically unexplained symptoms, rather than excessive symptom-related concerns. Furthermore, terms such as “medically unexplained symptoms” and “functional syndromes” (including fibromyalgia and irritable bowel syndrome) are frequently used when describing SSD.3
One or more factors may contribute to unexplained symptoms: limitations of medical procedures and techniques, partial clinical information, patients’ inability to follow management recommendations, challenges in differential diagnostics, and access-to-care limitations preventing regular care and appropriate diagnostic work up.
What’s important to remember is that it’s the patient’s reaction to physical symptoms, rather than the presence of symptoms per se, that defines SSD.
Considerations in the differential diagnosis
When making a diagnosis of SSD, symptoms cannot:4
- be feigned or deliberately produced as in malingering or factitious disorder.
- result from physiologic effects of a substance (eg, intoxication, withdrawal, or adverse medication effects).
- constitute somatic delusions, as occur in psychotic disorders.
- constitute symptoms or deficits affecting voluntary motor or sensory function that are better explained by neurologic, medical, or psychiatric conditions (consider conversion disorder).
- be preoccupations with physical appearance flaws, as in body dysmorphic disorder.
- be accounted for by an anxiety disorder (eg, palpitations associated with panic attacks).
Continue to: Illness anxiety disorder...
Illness anxiety disorder is also characterized by significant health-related concerns; however, physical symptoms are either mild or absent.
Possible causes of SSD are varied and complex, including genetic and biological factors, family dynamics, behavioral modeling/learning, personality traits, difficulties with emotional regulation, and awareness.5 Patients may present with ongoing requests for symptom explanations, feelings of helplessness, fear of having concerns dismissed, and low motivation for change.3
Aids in supporting a diagnosis of SSD
It’s not appropriate to rely solely on questionnaires to make the diagnosis of SSD. However, brief screening tools are a time-efficient way to capture patients’ experiences and perceptions.6 Along with other components of clinical evaluation, brief symptom screens can both support the diagnosis and help in longitudinal symptom assessment.
Patient Health Questionnaire-15 (PHQ-15), developed for self-report screening in primary care, has desirable psychometric properties including appropriate internal reliability; convergent validity with measures of functional status, disability days, and symptom-related burden; and discriminant validity from measures of depressive symptoms.7 The PHQ-15 is an open access tool that is available in several languages. The respondent is asked to rate the extent of being bothered by a range of medical symptoms in the proceeding 4 weeks. Total scores range from 0 to 30, with higher scores indicating greater symptom aggravation. Cutoffs of 5, 10, and 15 correspond to mild, moderate, and severe symptom levels.8
Somatic Symptom Disorder - B Criteria Scale (SSD-12) aims to capture SSD symptoms in line with Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria. It assesses cognitive, affective, and behavioral aspects of SSD.9 The SSD-12 is copyrighted and its use requires registration and purchase. Cutoffs by age and gender are available. SSD-12 has demonstrated appropriate reliability and validity.9
Continue to: Structured Clinical Interview for DSM Disorders
Structured Clinical Interview for DSM Disorders (SCID)10,11 is perhaps the most rigorous differential diagnostic tool. However, SCID administration requires training and skill; time for administration and cost of the materials may be prohibitive in primary care.
Finally, SSD symptoms are highly associated with depression and anxiety. Ongoing elevated screening scores for anxiety and depression refractory to interventions may indicate the possibility of overlooked SSD. Furthermore, use of SSD screening tools with anxiety and depression screening tools can provide a more comprehensive picture of impairment, as well as symptom progress.
Treatment: Avoid a split approach
Diagnosing and treating SSD can be challenging for physicians who focus on biomedically based approaches in patient care. Additional tests, studies, and prescriptions are likely to fuel (rather than pacify) patients’ concerns, as such steps divert attention from the underlying psychological needs and mechanisms which maintain SSD. Avoid using a split biopsychosocial approach—ie, beginning the inquiry and treatment planning from a biomedical perspective, and then falling back on psychosocial formulation when treatment efforts have been ineffective. Such an approach leads to understandable patient dissatisfaction and can be interpreted by them as the caregiver suggesting that physical symptoms are “all in [their] head.”12
These 4 tips can help
1. Use a biopsychosocial formulation when initiating treatment. Be familiar with biopsychosocial factors in SSD and develop a narrative for discussing this formulation with patients. For example: “Mr. R, we are going to use the following [medical tests/studies/medications] to understand the cause of your symptoms and better manage them. We also need to think about the role of stress and distress in your symptoms because these can also be at play with dizziness.” This may be particularly beneficial for a functional disorder, such as chronic pain. Incorporating patient education resources is an important step toward shared understanding (see Hunter Integrated Pain Service for chronic pain educational videos; www.tga.gov.au/chronic-pain-management-video-resource-brainman13).
2. Combine education about pathophysiology with patient-centered interviewing. Significant SSD symptom improvements were noted following a single 30-minute educational session, while motivational interviewing techniques were used to probe patients’ concerns.2
Continue to: Maintain professionalism and good clinical practice
3. Maintain professionalism and good clinical practice. Consider SSD a medical matter and address it accordingly: explore concerns fully, provide evidence-based responses, communicate empathy, and employ objective management strategies.14
4. Do not overlook the value of the relationship. A recent systematic review concluded that the relationship between the patient and care provider was central to the success of the interventions for symptom reduction.15
A controversial approach. Pharmacotherapy for SSD is controversial. While several trials of antidepressants and St. John’s wort have been positive and some authors have stated that all classes of antidepressants are effective for SSD, others maintain that questions regarding dosing, treatment duration, and sustainability of improvement have not been sufficiently addressed in research.16,17
Coordination of care issues
Primary care continues to be the de facto mental health system, and specialty services may be unavailable or declined by patients.18 CBT delivered in person or online is empirically supported as a treatment approach to medically unexplained symptoms and SSD.17,19-22
A recent meta-analysis of randomized controlled trials published by Jing and colleagues23 reported that CBT was effective for SSD symptom reduction, and that treatment gains were maintained 3 to 12 months post treatment. However, concerns about the practical implementation of CBT in primary care were raised because CBT was not shown to be effective in improving social functioning or reducing the number of medical visits. Symptom improvement was maximized with longer durations of treatment (> 10 sessions) and greater session lengths (> 50 minutes). Additionally, Abbass and colleagues24 brought up several methodologic (sampling and analysis) concerns related to Jing et al’s work.
Continue to: Overally, CBT's effect sizes...
Overall, CBT’s effect sizes are small, and patients who are open to biopsychosocial explanations for their symptoms and to receiving psychological services may differ from most patients seen in primary care practices.21 Furthermore, mental health providers may hesitate to diagnose SSD because they are concerned about missing a somatic illness.3 Therefore, when coordinating care with mental health providers, it may be beneficial to discuss the treatment approach, assess familiarity with the SSD diagnosis, and closely coordinate and collaborate on the treatment plan.
While physicians cannot be expected to function as psychotherapists, an understanding of CBT and techniques for SSD treatment can be beneficial. Integrated mental health services may hold promise in addressing SSD in primary care. Onsite availability of a behavioral health provider competent in providing evidence-based care can target SSD symptoms and support both patients and physicians.
THE CASE
Mr. R’s treatment course included multiple primary care appointments (scheduled and walk in), ED visits, and specialist visits (ENT/vestibular rehabilitation). He sought care as symptoms intensified, lasted longer, or occurred in new circumstances. He reported persistent fear of the symptoms and anxiety that serious medical causes had been overlooked. He also described distress associated with vertigo and his anxiety sensitivity (anxiety about being anxious).
The behavioral health consultant (BHC; psychologist) and physician talked to the patient about the biopsychosocial antecedents of his condition and the factors that perpetuate the anxiety and stress response. The BHC described the fight/flight/freeze response to the patient and explained its role in the physiologic stress response associated with somatic symptoms and panic. Educational materials (videos and handouts) were also provided to the patient to further illustrate these concepts. The BHC also discussed the role of interoceptive and situational avoidance and active coping (eg, engaging in safe activities); taught the patient relaxation and grounding techniques; and used cognitive disputation aimed at challenging catastrophic symptom interpretations.
The BHC and the patient’s physician established joint treatment goals that included improving functioning, promoting active coping, and decreasing distress associated with symptoms. After the initial medical and BHC visits, both vertigo and anxiety symptoms appeared to abate somewhat, but symptoms have been ongoing and distress and impairment have been variable. The patient’s family physician and BHC continue to work with him to optimize the care plan and treatment goals.
CORRESPONDENCE
Nataliya Pilipenko, PhD, ABPP, Center for Family and Community Medicine, Columbia University Vagelos College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032; [email protected]
ACKNOWLEDGEMENT
The author thanks Dr. Molly Warren for her collaboration and guidance.
THE CASE
James R* is a 30-year-old man who presented for a primary care walk-in visit due to dizziness, 2 days after he visited an emergency department (ED) for the same concern. He reported episodic symptoms lasting seconds to minutes, specifically when lying down. He said he had not fallen or experienced other physical trauma, did not have blurred vision or hearing loss, and was taking no medications. He also reported panic attacks, during which he experienced palpitations, trembling, paresthesia, and fear of dying. He stated that dizziness did not occur exclusively during panic episodes. His medical history was significant for hypertension; however, he reported significant anxiety related to medical visits. All home blood pressure readings he reported were within normal limits.
Upon examination, the patient had a blood pressure reading of 142/90 mm Hg and no evidence of nystagmus at rest. A neurologic exam was normal and a Dix-Hallpike maneuver reproduced subjective vertigo without nystagmus. Laboratory findings from the patient’s ED visit were negative for troponin and drug use, and blood oxygenation levels were within normal limits. At the time of this current visit, an electrocardiogram was unremarkable, with the exception of some tachycardia.
The presumptive diagnosis was benign paroxysmal positional vertigo (BPPV). An Epley maneuver was performed in the clinic and resulted in minimal symptom improvement. The physician taught Mr. R how to perform the Epley maneuver himself, prescribed a short course of meclizine, and referred him to the integrated mental health care service to address his panic attacks and anxiety.
Over the next few months, Mr. R continued to report significant distress about the dizzy spells, which persisted even after performing the Epley maneuver, and he reported that the meclizine was causing worsening vertigo. He received an ear-nose-and-throat consultation and cognitive behavioral therapy (CBT)–based consultation/interventions. He also reported avoiding multiple activities due to concerns about his dizziness.
●
*The patient’s name and other personally identifying information have been changed to protect his identity.
Somatic symptom disorder (SSD) is characterized by one or more physical symptoms associated with “excessive thoughts, feelings, or behaviors that result in distress and/or functional impairment.”1 Individuals with SSD are preoccupied with symptom-related severity, experience high symptom-related anxiety, or devote significant time and energy to the symptoms or heath concerns. With a diagnosis of SSD, physical symptoms experienced by the patient may or may not be medically explained. The same symptom need not be continuously present as long as the overall symptomatic presentation lasts 6 months or longer.
The specifier “with predominant pain” is used when pain dominates the presentation.1 Estimated prevalence of SSD in primary care ranges from 5% to 35%.2 The true scope of SSD is difficult to assess accurately since research tends to focus on medically unexplained symptoms, rather than excessive symptom-related concerns. Furthermore, terms such as “medically unexplained symptoms” and “functional syndromes” (including fibromyalgia and irritable bowel syndrome) are frequently used when describing SSD.3
One or more factors may contribute to unexplained symptoms: limitations of medical procedures and techniques, partial clinical information, patients’ inability to follow management recommendations, challenges in differential diagnostics, and access-to-care limitations preventing regular care and appropriate diagnostic work up.
What’s important to remember is that it’s the patient’s reaction to physical symptoms, rather than the presence of symptoms per se, that defines SSD.
Considerations in the differential diagnosis
When making a diagnosis of SSD, symptoms cannot:4
- be feigned or deliberately produced as in malingering or factitious disorder.
- result from physiologic effects of a substance (eg, intoxication, withdrawal, or adverse medication effects).
- constitute somatic delusions, as occur in psychotic disorders.
- constitute symptoms or deficits affecting voluntary motor or sensory function that are better explained by neurologic, medical, or psychiatric conditions (consider conversion disorder).
- be preoccupations with physical appearance flaws, as in body dysmorphic disorder.
- be accounted for by an anxiety disorder (eg, palpitations associated with panic attacks).
Continue to: Illness anxiety disorder...
Illness anxiety disorder is also characterized by significant health-related concerns; however, physical symptoms are either mild or absent.
Possible causes of SSD are varied and complex, including genetic and biological factors, family dynamics, behavioral modeling/learning, personality traits, difficulties with emotional regulation, and awareness.5 Patients may present with ongoing requests for symptom explanations, feelings of helplessness, fear of having concerns dismissed, and low motivation for change.3
Aids in supporting a diagnosis of SSD
It’s not appropriate to rely solely on questionnaires to make the diagnosis of SSD. However, brief screening tools are a time-efficient way to capture patients’ experiences and perceptions.6 Along with other components of clinical evaluation, brief symptom screens can both support the diagnosis and help in longitudinal symptom assessment.
Patient Health Questionnaire-15 (PHQ-15), developed for self-report screening in primary care, has desirable psychometric properties including appropriate internal reliability; convergent validity with measures of functional status, disability days, and symptom-related burden; and discriminant validity from measures of depressive symptoms.7 The PHQ-15 is an open access tool that is available in several languages. The respondent is asked to rate the extent of being bothered by a range of medical symptoms in the proceeding 4 weeks. Total scores range from 0 to 30, with higher scores indicating greater symptom aggravation. Cutoffs of 5, 10, and 15 correspond to mild, moderate, and severe symptom levels.8
Somatic Symptom Disorder - B Criteria Scale (SSD-12) aims to capture SSD symptoms in line with Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria. It assesses cognitive, affective, and behavioral aspects of SSD.9 The SSD-12 is copyrighted and its use requires registration and purchase. Cutoffs by age and gender are available. SSD-12 has demonstrated appropriate reliability and validity.9
Continue to: Structured Clinical Interview for DSM Disorders
Structured Clinical Interview for DSM Disorders (SCID)10,11 is perhaps the most rigorous differential diagnostic tool. However, SCID administration requires training and skill; time for administration and cost of the materials may be prohibitive in primary care.
Finally, SSD symptoms are highly associated with depression and anxiety. Ongoing elevated screening scores for anxiety and depression refractory to interventions may indicate the possibility of overlooked SSD. Furthermore, use of SSD screening tools with anxiety and depression screening tools can provide a more comprehensive picture of impairment, as well as symptom progress.
Treatment: Avoid a split approach
Diagnosing and treating SSD can be challenging for physicians who focus on biomedically based approaches in patient care. Additional tests, studies, and prescriptions are likely to fuel (rather than pacify) patients’ concerns, as such steps divert attention from the underlying psychological needs and mechanisms which maintain SSD. Avoid using a split biopsychosocial approach—ie, beginning the inquiry and treatment planning from a biomedical perspective, and then falling back on psychosocial formulation when treatment efforts have been ineffective. Such an approach leads to understandable patient dissatisfaction and can be interpreted by them as the caregiver suggesting that physical symptoms are “all in [their] head.”12
These 4 tips can help
1. Use a biopsychosocial formulation when initiating treatment. Be familiar with biopsychosocial factors in SSD and develop a narrative for discussing this formulation with patients. For example: “Mr. R, we are going to use the following [medical tests/studies/medications] to understand the cause of your symptoms and better manage them. We also need to think about the role of stress and distress in your symptoms because these can also be at play with dizziness.” This may be particularly beneficial for a functional disorder, such as chronic pain. Incorporating patient education resources is an important step toward shared understanding (see Hunter Integrated Pain Service for chronic pain educational videos; www.tga.gov.au/chronic-pain-management-video-resource-brainman13).
2. Combine education about pathophysiology with patient-centered interviewing. Significant SSD symptom improvements were noted following a single 30-minute educational session, while motivational interviewing techniques were used to probe patients’ concerns.2
Continue to: Maintain professionalism and good clinical practice
3. Maintain professionalism and good clinical practice. Consider SSD a medical matter and address it accordingly: explore concerns fully, provide evidence-based responses, communicate empathy, and employ objective management strategies.14
4. Do not overlook the value of the relationship. A recent systematic review concluded that the relationship between the patient and care provider was central to the success of the interventions for symptom reduction.15
A controversial approach. Pharmacotherapy for SSD is controversial. While several trials of antidepressants and St. John’s wort have been positive and some authors have stated that all classes of antidepressants are effective for SSD, others maintain that questions regarding dosing, treatment duration, and sustainability of improvement have not been sufficiently addressed in research.16,17
Coordination of care issues
Primary care continues to be the de facto mental health system, and specialty services may be unavailable or declined by patients.18 CBT delivered in person or online is empirically supported as a treatment approach to medically unexplained symptoms and SSD.17,19-22
A recent meta-analysis of randomized controlled trials published by Jing and colleagues23 reported that CBT was effective for SSD symptom reduction, and that treatment gains were maintained 3 to 12 months post treatment. However, concerns about the practical implementation of CBT in primary care were raised because CBT was not shown to be effective in improving social functioning or reducing the number of medical visits. Symptom improvement was maximized with longer durations of treatment (> 10 sessions) and greater session lengths (> 50 minutes). Additionally, Abbass and colleagues24 brought up several methodologic (sampling and analysis) concerns related to Jing et al’s work.
Continue to: Overally, CBT's effect sizes...
Overall, CBT’s effect sizes are small, and patients who are open to biopsychosocial explanations for their symptoms and to receiving psychological services may differ from most patients seen in primary care practices.21 Furthermore, mental health providers may hesitate to diagnose SSD because they are concerned about missing a somatic illness.3 Therefore, when coordinating care with mental health providers, it may be beneficial to discuss the treatment approach, assess familiarity with the SSD diagnosis, and closely coordinate and collaborate on the treatment plan.
While physicians cannot be expected to function as psychotherapists, an understanding of CBT and techniques for SSD treatment can be beneficial. Integrated mental health services may hold promise in addressing SSD in primary care. Onsite availability of a behavioral health provider competent in providing evidence-based care can target SSD symptoms and support both patients and physicians.
THE CASE
Mr. R’s treatment course included multiple primary care appointments (scheduled and walk in), ED visits, and specialist visits (ENT/vestibular rehabilitation). He sought care as symptoms intensified, lasted longer, or occurred in new circumstances. He reported persistent fear of the symptoms and anxiety that serious medical causes had been overlooked. He also described distress associated with vertigo and his anxiety sensitivity (anxiety about being anxious).
The behavioral health consultant (BHC; psychologist) and physician talked to the patient about the biopsychosocial antecedents of his condition and the factors that perpetuate the anxiety and stress response. The BHC described the fight/flight/freeze response to the patient and explained its role in the physiologic stress response associated with somatic symptoms and panic. Educational materials (videos and handouts) were also provided to the patient to further illustrate these concepts. The BHC also discussed the role of interoceptive and situational avoidance and active coping (eg, engaging in safe activities); taught the patient relaxation and grounding techniques; and used cognitive disputation aimed at challenging catastrophic symptom interpretations.
The BHC and the patient’s physician established joint treatment goals that included improving functioning, promoting active coping, and decreasing distress associated with symptoms. After the initial medical and BHC visits, both vertigo and anxiety symptoms appeared to abate somewhat, but symptoms have been ongoing and distress and impairment have been variable. The patient’s family physician and BHC continue to work with him to optimize the care plan and treatment goals.
CORRESPONDENCE
Nataliya Pilipenko, PhD, ABPP, Center for Family and Community Medicine, Columbia University Vagelos College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032; [email protected]
ACKNOWLEDGEMENT
The author thanks Dr. Molly Warren for her collaboration and guidance.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
2. Johnson KK, Bennett C, Rochani H. Significant improvement of somatic symptom disorder with brief psychoeducational intervention by PMHNP in primary care. J Am Psychiatr Nurses Assoc. 2020;28:171-180. doi: 10.1177/1078390320960524
3. Weigel A, Maehder K, Witt M, et al. Psychotherapists’ perspective on the treatment of patients with somatic symptom disorders. J Psychosom Res. 2020;138:110228. doi: 10.1016/j.jpsychores.2020.110228
4. American Psychiatric Association. Handbook of Differential Diagnosis. American Psychiatric Publishing; 2014;234-235.
5. Mayo Clinic. Somatic symptom disorder. Accessed February 21, 2022. www.mayoclinic.org/diseases-conditions/somatic-symptom-disorder/symptoms-causes/syc-20377776?p=1
6. Toussaint A, Riedl B, Kehrer S, et al. Validity of the Somatic Symptom Disorder-B Criteria Scale (SSD-12) in primary care. Fam Pract. 2018;35:342-347. doi: 10.1093/fampra/cmx116
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-66. doi: 10.1097/00006842-200203000-00008
8. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. doi: 10.1016/j.genhosppsych.2010.03.006
9. Toussaint A, Löwe B, Brähler E, et al. The Somatic Symptom Disorder - B Criteria Scale (SSD-12): factorial structure, validity and population-based norms. J Psychosom Res. 2017;97:9-17. doi: 10.1016/j.jpsychores.2017.03.017
10. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Research Version. American Psychiatric Association, 2015.
11. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Publishing; 2016.
12. McDaniel SH, Hepworth J, Campbell TL, et al, eds. Family Oriented Primary Care. Springer Publishing Co; 2005:1-15.
13. Hunter Integrated Pain Service (2016). Brainman videos. Hunter New England Local Health District. New South Wales, Australia. Accessed February 21, 2022. www.tga.gov.au/chronic-pain-management-video-resource-brainman
14. Murray AM, Toussaint A, Althaus A, et al. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1-10. doi: 10.1016/j.jpsychores.2015.11.002
15. Leaviss J, Davis S, Ren S, et al. Behavioral modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-490. doi: 10.3310/hta24460
16. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888. doi: 10.1097/PSY.0b013e31815b00c4
17. Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263-271. doi: 10.1016/j.psym.2011.01.007
18. Kessler R, Stafford D. Primary care is the de facto mental health system. In Kessler R, Stafford D, eds. Collaborative Medicine Case Studies: Evidence in Practice. Springer Publishing Co, 2008; 9-21.
19. Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. doi: 10.1007/s11606-013-2392-6
20. Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86:89-98. doi: 10.1037/ccp0000248
21. van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2
22. Verdurmen MJ, Videler AC, Kamperman AM, et al. Cognitive behavioral therapy for somatic symptom disorders in later life: a prospective comparative explorative pilot study in two clinical populations. Neuropsychiatr Dis Treat. 2017;13:2331-2339. doi: 10.2147/NDT.S141208
23. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
24. Abbass A, Leichsenring F, Steinert C. Re: Jing et al., the efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;255:S0165-0327(18)33197-5. doi: 10.1016/j.jad.2019.02.055
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
2. Johnson KK, Bennett C, Rochani H. Significant improvement of somatic symptom disorder with brief psychoeducational intervention by PMHNP in primary care. J Am Psychiatr Nurses Assoc. 2020;28:171-180. doi: 10.1177/1078390320960524
3. Weigel A, Maehder K, Witt M, et al. Psychotherapists’ perspective on the treatment of patients with somatic symptom disorders. J Psychosom Res. 2020;138:110228. doi: 10.1016/j.jpsychores.2020.110228
4. American Psychiatric Association. Handbook of Differential Diagnosis. American Psychiatric Publishing; 2014;234-235.
5. Mayo Clinic. Somatic symptom disorder. Accessed February 21, 2022. www.mayoclinic.org/diseases-conditions/somatic-symptom-disorder/symptoms-causes/syc-20377776?p=1
6. Toussaint A, Riedl B, Kehrer S, et al. Validity of the Somatic Symptom Disorder-B Criteria Scale (SSD-12) in primary care. Fam Pract. 2018;35:342-347. doi: 10.1093/fampra/cmx116
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-66. doi: 10.1097/00006842-200203000-00008
8. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. doi: 10.1016/j.genhosppsych.2010.03.006
9. Toussaint A, Löwe B, Brähler E, et al. The Somatic Symptom Disorder - B Criteria Scale (SSD-12): factorial structure, validity and population-based norms. J Psychosom Res. 2017;97:9-17. doi: 10.1016/j.jpsychores.2017.03.017
10. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Research Version. American Psychiatric Association, 2015.
11. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Publishing; 2016.
12. McDaniel SH, Hepworth J, Campbell TL, et al, eds. Family Oriented Primary Care. Springer Publishing Co; 2005:1-15.
13. Hunter Integrated Pain Service (2016). Brainman videos. Hunter New England Local Health District. New South Wales, Australia. Accessed February 21, 2022. www.tga.gov.au/chronic-pain-management-video-resource-brainman
14. Murray AM, Toussaint A, Althaus A, et al. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1-10. doi: 10.1016/j.jpsychores.2015.11.002
15. Leaviss J, Davis S, Ren S, et al. Behavioral modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-490. doi: 10.3310/hta24460
16. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888. doi: 10.1097/PSY.0b013e31815b00c4
17. Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263-271. doi: 10.1016/j.psym.2011.01.007
18. Kessler R, Stafford D. Primary care is the de facto mental health system. In Kessler R, Stafford D, eds. Collaborative Medicine Case Studies: Evidence in Practice. Springer Publishing Co, 2008; 9-21.
19. Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. doi: 10.1007/s11606-013-2392-6
20. Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86:89-98. doi: 10.1037/ccp0000248
21. van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2
22. Verdurmen MJ, Videler AC, Kamperman AM, et al. Cognitive behavioral therapy for somatic symptom disorders in later life: a prospective comparative explorative pilot study in two clinical populations. Neuropsychiatr Dis Treat. 2017;13:2331-2339. doi: 10.2147/NDT.S141208
23. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
24. Abbass A, Leichsenring F, Steinert C. Re: Jing et al., the efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;255:S0165-0327(18)33197-5. doi: 10.1016/j.jad.2019.02.055
Getting a jump on recovery from sports-related concussion
ILLUSTRATIVE CASE
A 16-year-old girl with no chronic medical illness presents to your office with her parents after sustaining a head injury at a soccer game over the weekend. She collided with another player while attempting to head the ball. Immediately afterward, she was taken off the field and assessed. She was confused but had a normal level of consciousness and denied vision changes, weakness or tingling in her arms or legs, severe headache, or neck pain. Further testing revealed dizziness and abnormal balance. Her confusion and abnormal balance resolved after 1 day. She has had a mild headache and light sensitivity since the event. She otherwise feels well at rest in the office. She wants to recover quickly but safely and has heard conflicting statements about whether she should completely rest or start back to light activity now.
Sports-related concussions (SRCs) are highly prevalent in the United States, with as many as 3.8 million cases annually. Of those, 1.1 to 1.9 million cases are in children 18 years old or younger.2,3 SRCs are defined by the Concussion in Sport Group (CISG) 2017 consensus statement as involving the following criteria: (1) direct or indirect trauma anywhere on the body with force transmitted to the head; (2) rapid or delayed symptom presentation, typically with spontaneous resolution; (3) functional rather than structural injury; and (4) occurrence with or without loss of consciousness with stepwise symptom resolution.4
SRCs do not have a proven, effective treatment and can have short- or long-term consequences. Initial treatment includes removing athletes from play immediately after an event. The American Academy of Neurology recommends that athletes not return to play until the concussion is resolved, as judged by a health care provider, and the athlete is asymptomatic when off medication.2
The CISG recommends a 6-step approach, with each step taking at least 24 hours.4 The final step is a return to normal activity.4 This working group recommended extensive study of rehabilitation programs involving subsymptom threshold exercise (ie, exercise performed at a level that does not exacerbate symptoms) before implementation as routine practice. Evidence from a 2015 study suggests that following strict rest for 5 days until complete symptom resolution may prolong recovery compared with rest for only 1 to 2 days.5 Additionally, strict rest did not show a difference in neurocognitive or balance outcomes in that study, and the authors noted it may also negatively impact academic, sports, and social function in adolescents.5 This study looked at the potential benefit of subsymptom threshold exercise during recovery from SRC.1
STUDY SUMMARY
Light aerobic exercise may help speed recovery
This multicenter, prospective, parallel, randomized clinical trial compared subsymptom threshold aerobic exercise to placebo-like stretching. Patients were included if they were ages 13 to 18 years and presented within 10 days of an SRC, as diagnosed using the CISG criteria. Exclusion criteria included focal neurologic deficits; history of moderate or severe traumatic brain injury; inability to exercise due to orthopedic injury, cervical spine injury, diabetes, or heart disease; increased cardiac risk; or low postconcussion symptom severity. Patients with a diagnosis of and treatment with medication for attention-deficit/hyperactivity disorder (ADHD), depression, anxiety, or learning disorder were excluded, as were patients with a history of more than 3 previous concussions.
Patients in the aerobic exercise group were instructed to use a stationary bike or treadmill (or equivalent walking or jogging if they did not have access to this equipment) at a prescribed heart rate. The target heart rate was 80% of the heart rate achieved during initial assessment with the Buffalo Concussion Treadmill Test (BCTT).6 Patients in this group were instructed to exercise for 20 minutes or to the point at which their symptoms increased by 2 points (on a 10-point scale) from pre-exercise levels, whichever came first, with rest prescribed at all other times.
For the placebo-like group, a stretching instruction booklet was provided, with the goal of achieving a heart rate that was not significantly elevated. Participants in this group were told to perform the stretches for 20 minutes daily. Of note, researchers ensured the level of physician and research staff attention was similar for each patient, regardless of treatment group, to prevent intervention bias. Additionally, interventions were not initiated prior to 48 hours from the time of injury.
Continue to: The primary outcome...
The primary outcome was number of days to recovery since the date of injury. This was defined as symptom resolution to normal (as evaluated by a physician blinded to the study group) and by the patient’s ability to exercise to exhaustion without symptom exacerbation on the BCTT. Secondary outcomes measured the proportion of patients with delayed recovery (defined as recovery requiring > 30 days) and daily symptom scores.
Of 165 patients meeting the inclusion criteria, 52 patients were excluded prior to randomization (12 patients chose not to participate, 39 were excluded for lack of symptoms, and 1 withdrew due to severe symptoms on the BCTT). A total of 113 were randomized to either group, and 103 patients completed the study (10 patients did not complete the study or had another illness during the intervention). The study analysis included 52 patients in the aerobic exercise group and 51 in the placebo-like stretching group. The study was powered to detect a significant difference in recovery time.
Patients were about equally divided by sex, with a mean age of 15 years. Patients who had no previous concussion made up 50% of the aerobic group and 57% of the stretching group. The average time since injury was similar in the aerobic and stretching groups (4.9 days and 4.8 days, respectively). The aerobic exercise group recovered in a median of 13 days (interquartile range [IQR] = 10-18.5 days) compared with a median of 17 days (IQR = 13-23 days) for the stretching group (P = .009). The incidence of delayed recovery (> 30 days) was higher in the stretching group (n = 7) compared with the aerobic exercise group (n = 2) but was not statistically significant. Daily symptom reporting occurred at a high rate in both groups, with patients stating that they performed their prescribed exercise 89% of the time. No adverse events were reported.
WHAT’S NEW
First high-quality study to support evidence for early light activity
This is the first high-quality study of subsymptom threshold exercise for SRC. Its findings add to the growing body of evidence that early engagement in light aerobic activity that does not provoke symptoms (but not fully returning to sports activity) can aid in recovery from an SRC.
CAVEATS
Narrow study population limits application of findings
It is unclear if subsymptom threshold exercise is safe and effective in adolescents with a history of multiple concussions, as those with more than 3 concussions were excluded from this study. Additionally, patients with comorbidities such as ADHD, depression, anxiety, or learning disorders were not included in this study, which limits the application of these findings. The generalizability of this study is limited in younger children, adults, those with increased cardiovascular risk, and in patients with concussions that are not sports related.
CHALLENGES TO IMPLEMENTATION
More real-world studies needed to confirm benefit
The majority of adolescent athletes in this study completed the subsymptom threshold exercise in a monitored environment with trainers, heart rate monitors, and access to equipment, limiting the study’s generalizability. Additionally, physicians need to be familiar with the BCTT to assign heart rate goals and assess improvement. The study environment may be feasible for some but not others. Studies evaluating real-world settings with athletes self-monitoring for symptom threshold with stepwise evaluations are needed and may be more broadly applicable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Leddy JJ, Haider MN, Ellis MJ, et al. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr. 2019;173:319-325. doi: 10.1001/jamapediatrics.2018.4397
2. Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257. doi: 10.1212/WNL.0b013e31828d57dd
3. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al; Seattle Sports Concussion Research Collaborative. Sports- and recreation-related concussions in US youth. Pediatrics. 2016;138:e20154635. doi: 10.1542/peds.2015-4635
4. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi: 10.1136/bjsports-2017-097699
5. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:213-223.
6. Leddy JJ, Haider MN, Willer BS. Buffalo Concussion Treadmill Test (BCTT) – Instruction Manual. Accessed March 16, 2022. https://cdn-links.lww.com/permalink/jsm/a/jsm_2020_01_28_haider_19-313_sdc1.pdf
ILLUSTRATIVE CASE
A 16-year-old girl with no chronic medical illness presents to your office with her parents after sustaining a head injury at a soccer game over the weekend. She collided with another player while attempting to head the ball. Immediately afterward, she was taken off the field and assessed. She was confused but had a normal level of consciousness and denied vision changes, weakness or tingling in her arms or legs, severe headache, or neck pain. Further testing revealed dizziness and abnormal balance. Her confusion and abnormal balance resolved after 1 day. She has had a mild headache and light sensitivity since the event. She otherwise feels well at rest in the office. She wants to recover quickly but safely and has heard conflicting statements about whether she should completely rest or start back to light activity now.
Sports-related concussions (SRCs) are highly prevalent in the United States, with as many as 3.8 million cases annually. Of those, 1.1 to 1.9 million cases are in children 18 years old or younger.2,3 SRCs are defined by the Concussion in Sport Group (CISG) 2017 consensus statement as involving the following criteria: (1) direct or indirect trauma anywhere on the body with force transmitted to the head; (2) rapid or delayed symptom presentation, typically with spontaneous resolution; (3) functional rather than structural injury; and (4) occurrence with or without loss of consciousness with stepwise symptom resolution.4
SRCs do not have a proven, effective treatment and can have short- or long-term consequences. Initial treatment includes removing athletes from play immediately after an event. The American Academy of Neurology recommends that athletes not return to play until the concussion is resolved, as judged by a health care provider, and the athlete is asymptomatic when off medication.2
The CISG recommends a 6-step approach, with each step taking at least 24 hours.4 The final step is a return to normal activity.4 This working group recommended extensive study of rehabilitation programs involving subsymptom threshold exercise (ie, exercise performed at a level that does not exacerbate symptoms) before implementation as routine practice. Evidence from a 2015 study suggests that following strict rest for 5 days until complete symptom resolution may prolong recovery compared with rest for only 1 to 2 days.5 Additionally, strict rest did not show a difference in neurocognitive or balance outcomes in that study, and the authors noted it may also negatively impact academic, sports, and social function in adolescents.5 This study looked at the potential benefit of subsymptom threshold exercise during recovery from SRC.1
STUDY SUMMARY
Light aerobic exercise may help speed recovery
This multicenter, prospective, parallel, randomized clinical trial compared subsymptom threshold aerobic exercise to placebo-like stretching. Patients were included if they were ages 13 to 18 years and presented within 10 days of an SRC, as diagnosed using the CISG criteria. Exclusion criteria included focal neurologic deficits; history of moderate or severe traumatic brain injury; inability to exercise due to orthopedic injury, cervical spine injury, diabetes, or heart disease; increased cardiac risk; or low postconcussion symptom severity. Patients with a diagnosis of and treatment with medication for attention-deficit/hyperactivity disorder (ADHD), depression, anxiety, or learning disorder were excluded, as were patients with a history of more than 3 previous concussions.
Patients in the aerobic exercise group were instructed to use a stationary bike or treadmill (or equivalent walking or jogging if they did not have access to this equipment) at a prescribed heart rate. The target heart rate was 80% of the heart rate achieved during initial assessment with the Buffalo Concussion Treadmill Test (BCTT).6 Patients in this group were instructed to exercise for 20 minutes or to the point at which their symptoms increased by 2 points (on a 10-point scale) from pre-exercise levels, whichever came first, with rest prescribed at all other times.
For the placebo-like group, a stretching instruction booklet was provided, with the goal of achieving a heart rate that was not significantly elevated. Participants in this group were told to perform the stretches for 20 minutes daily. Of note, researchers ensured the level of physician and research staff attention was similar for each patient, regardless of treatment group, to prevent intervention bias. Additionally, interventions were not initiated prior to 48 hours from the time of injury.
Continue to: The primary outcome...
The primary outcome was number of days to recovery since the date of injury. This was defined as symptom resolution to normal (as evaluated by a physician blinded to the study group) and by the patient’s ability to exercise to exhaustion without symptom exacerbation on the BCTT. Secondary outcomes measured the proportion of patients with delayed recovery (defined as recovery requiring > 30 days) and daily symptom scores.
Of 165 patients meeting the inclusion criteria, 52 patients were excluded prior to randomization (12 patients chose not to participate, 39 were excluded for lack of symptoms, and 1 withdrew due to severe symptoms on the BCTT). A total of 113 were randomized to either group, and 103 patients completed the study (10 patients did not complete the study or had another illness during the intervention). The study analysis included 52 patients in the aerobic exercise group and 51 in the placebo-like stretching group. The study was powered to detect a significant difference in recovery time.
Patients were about equally divided by sex, with a mean age of 15 years. Patients who had no previous concussion made up 50% of the aerobic group and 57% of the stretching group. The average time since injury was similar in the aerobic and stretching groups (4.9 days and 4.8 days, respectively). The aerobic exercise group recovered in a median of 13 days (interquartile range [IQR] = 10-18.5 days) compared with a median of 17 days (IQR = 13-23 days) for the stretching group (P = .009). The incidence of delayed recovery (> 30 days) was higher in the stretching group (n = 7) compared with the aerobic exercise group (n = 2) but was not statistically significant. Daily symptom reporting occurred at a high rate in both groups, with patients stating that they performed their prescribed exercise 89% of the time. No adverse events were reported.
WHAT’S NEW
First high-quality study to support evidence for early light activity
This is the first high-quality study of subsymptom threshold exercise for SRC. Its findings add to the growing body of evidence that early engagement in light aerobic activity that does not provoke symptoms (but not fully returning to sports activity) can aid in recovery from an SRC.
CAVEATS
Narrow study population limits application of findings
It is unclear if subsymptom threshold exercise is safe and effective in adolescents with a history of multiple concussions, as those with more than 3 concussions were excluded from this study. Additionally, patients with comorbidities such as ADHD, depression, anxiety, or learning disorders were not included in this study, which limits the application of these findings. The generalizability of this study is limited in younger children, adults, those with increased cardiovascular risk, and in patients with concussions that are not sports related.
CHALLENGES TO IMPLEMENTATION
More real-world studies needed to confirm benefit
The majority of adolescent athletes in this study completed the subsymptom threshold exercise in a monitored environment with trainers, heart rate monitors, and access to equipment, limiting the study’s generalizability. Additionally, physicians need to be familiar with the BCTT to assign heart rate goals and assess improvement. The study environment may be feasible for some but not others. Studies evaluating real-world settings with athletes self-monitoring for symptom threshold with stepwise evaluations are needed and may be more broadly applicable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 16-year-old girl with no chronic medical illness presents to your office with her parents after sustaining a head injury at a soccer game over the weekend. She collided with another player while attempting to head the ball. Immediately afterward, she was taken off the field and assessed. She was confused but had a normal level of consciousness and denied vision changes, weakness or tingling in her arms or legs, severe headache, or neck pain. Further testing revealed dizziness and abnormal balance. Her confusion and abnormal balance resolved after 1 day. She has had a mild headache and light sensitivity since the event. She otherwise feels well at rest in the office. She wants to recover quickly but safely and has heard conflicting statements about whether she should completely rest or start back to light activity now.
Sports-related concussions (SRCs) are highly prevalent in the United States, with as many as 3.8 million cases annually. Of those, 1.1 to 1.9 million cases are in children 18 years old or younger.2,3 SRCs are defined by the Concussion in Sport Group (CISG) 2017 consensus statement as involving the following criteria: (1) direct or indirect trauma anywhere on the body with force transmitted to the head; (2) rapid or delayed symptom presentation, typically with spontaneous resolution; (3) functional rather than structural injury; and (4) occurrence with or without loss of consciousness with stepwise symptom resolution.4
SRCs do not have a proven, effective treatment and can have short- or long-term consequences. Initial treatment includes removing athletes from play immediately after an event. The American Academy of Neurology recommends that athletes not return to play until the concussion is resolved, as judged by a health care provider, and the athlete is asymptomatic when off medication.2
The CISG recommends a 6-step approach, with each step taking at least 24 hours.4 The final step is a return to normal activity.4 This working group recommended extensive study of rehabilitation programs involving subsymptom threshold exercise (ie, exercise performed at a level that does not exacerbate symptoms) before implementation as routine practice. Evidence from a 2015 study suggests that following strict rest for 5 days until complete symptom resolution may prolong recovery compared with rest for only 1 to 2 days.5 Additionally, strict rest did not show a difference in neurocognitive or balance outcomes in that study, and the authors noted it may also negatively impact academic, sports, and social function in adolescents.5 This study looked at the potential benefit of subsymptom threshold exercise during recovery from SRC.1
STUDY SUMMARY
Light aerobic exercise may help speed recovery
This multicenter, prospective, parallel, randomized clinical trial compared subsymptom threshold aerobic exercise to placebo-like stretching. Patients were included if they were ages 13 to 18 years and presented within 10 days of an SRC, as diagnosed using the CISG criteria. Exclusion criteria included focal neurologic deficits; history of moderate or severe traumatic brain injury; inability to exercise due to orthopedic injury, cervical spine injury, diabetes, or heart disease; increased cardiac risk; or low postconcussion symptom severity. Patients with a diagnosis of and treatment with medication for attention-deficit/hyperactivity disorder (ADHD), depression, anxiety, or learning disorder were excluded, as were patients with a history of more than 3 previous concussions.
Patients in the aerobic exercise group were instructed to use a stationary bike or treadmill (or equivalent walking or jogging if they did not have access to this equipment) at a prescribed heart rate. The target heart rate was 80% of the heart rate achieved during initial assessment with the Buffalo Concussion Treadmill Test (BCTT).6 Patients in this group were instructed to exercise for 20 minutes or to the point at which their symptoms increased by 2 points (on a 10-point scale) from pre-exercise levels, whichever came first, with rest prescribed at all other times.
For the placebo-like group, a stretching instruction booklet was provided, with the goal of achieving a heart rate that was not significantly elevated. Participants in this group were told to perform the stretches for 20 minutes daily. Of note, researchers ensured the level of physician and research staff attention was similar for each patient, regardless of treatment group, to prevent intervention bias. Additionally, interventions were not initiated prior to 48 hours from the time of injury.
Continue to: The primary outcome...
The primary outcome was number of days to recovery since the date of injury. This was defined as symptom resolution to normal (as evaluated by a physician blinded to the study group) and by the patient’s ability to exercise to exhaustion without symptom exacerbation on the BCTT. Secondary outcomes measured the proportion of patients with delayed recovery (defined as recovery requiring > 30 days) and daily symptom scores.
Of 165 patients meeting the inclusion criteria, 52 patients were excluded prior to randomization (12 patients chose not to participate, 39 were excluded for lack of symptoms, and 1 withdrew due to severe symptoms on the BCTT). A total of 113 were randomized to either group, and 103 patients completed the study (10 patients did not complete the study or had another illness during the intervention). The study analysis included 52 patients in the aerobic exercise group and 51 in the placebo-like stretching group. The study was powered to detect a significant difference in recovery time.
Patients were about equally divided by sex, with a mean age of 15 years. Patients who had no previous concussion made up 50% of the aerobic group and 57% of the stretching group. The average time since injury was similar in the aerobic and stretching groups (4.9 days and 4.8 days, respectively). The aerobic exercise group recovered in a median of 13 days (interquartile range [IQR] = 10-18.5 days) compared with a median of 17 days (IQR = 13-23 days) for the stretching group (P = .009). The incidence of delayed recovery (> 30 days) was higher in the stretching group (n = 7) compared with the aerobic exercise group (n = 2) but was not statistically significant. Daily symptom reporting occurred at a high rate in both groups, with patients stating that they performed their prescribed exercise 89% of the time. No adverse events were reported.
WHAT’S NEW
First high-quality study to support evidence for early light activity
This is the first high-quality study of subsymptom threshold exercise for SRC. Its findings add to the growing body of evidence that early engagement in light aerobic activity that does not provoke symptoms (but not fully returning to sports activity) can aid in recovery from an SRC.
CAVEATS
Narrow study population limits application of findings
It is unclear if subsymptom threshold exercise is safe and effective in adolescents with a history of multiple concussions, as those with more than 3 concussions were excluded from this study. Additionally, patients with comorbidities such as ADHD, depression, anxiety, or learning disorders were not included in this study, which limits the application of these findings. The generalizability of this study is limited in younger children, adults, those with increased cardiovascular risk, and in patients with concussions that are not sports related.
CHALLENGES TO IMPLEMENTATION
More real-world studies needed to confirm benefit
The majority of adolescent athletes in this study completed the subsymptom threshold exercise in a monitored environment with trainers, heart rate monitors, and access to equipment, limiting the study’s generalizability. Additionally, physicians need to be familiar with the BCTT to assign heart rate goals and assess improvement. The study environment may be feasible for some but not others. Studies evaluating real-world settings with athletes self-monitoring for symptom threshold with stepwise evaluations are needed and may be more broadly applicable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Leddy JJ, Haider MN, Ellis MJ, et al. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr. 2019;173:319-325. doi: 10.1001/jamapediatrics.2018.4397
2. Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257. doi: 10.1212/WNL.0b013e31828d57dd
3. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al; Seattle Sports Concussion Research Collaborative. Sports- and recreation-related concussions in US youth. Pediatrics. 2016;138:e20154635. doi: 10.1542/peds.2015-4635
4. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi: 10.1136/bjsports-2017-097699
5. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:213-223.
6. Leddy JJ, Haider MN, Willer BS. Buffalo Concussion Treadmill Test (BCTT) – Instruction Manual. Accessed March 16, 2022. https://cdn-links.lww.com/permalink/jsm/a/jsm_2020_01_28_haider_19-313_sdc1.pdf
1. Leddy JJ, Haider MN, Ellis MJ, et al. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr. 2019;173:319-325. doi: 10.1001/jamapediatrics.2018.4397
2. Giza CC, Kutcher JS, Ashwal S, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250-2257. doi: 10.1212/WNL.0b013e31828d57dd
3. Bryan MA, Rowhani-Rahbar A, Comstock RD, et al; Seattle Sports Concussion Research Collaborative. Sports- and recreation-related concussions in US youth. Pediatrics. 2016;138:e20154635. doi: 10.1542/peds.2015-4635
4. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. doi: 10.1136/bjsports-2017-097699
5. Thomas DG, Apps JN, Hoffmann RG, et al. Benefits of strict rest after acute concussion: a randomized controlled trial. Pediatrics. 2015;135:213-223.
6. Leddy JJ, Haider MN, Willer BS. Buffalo Concussion Treadmill Test (BCTT) – Instruction Manual. Accessed March 16, 2022. https://cdn-links.lww.com/permalink/jsm/a/jsm_2020_01_28_haider_19-313_sdc1.pdf
PRACTICE CHANGER
Recommend subsymptom threshold exercise in adolescents with a sports-related concussion. Early return to light aerobic activity not only seems safe but may help speed recovery compared with stretching alone in this patient population.
STRENGTH OF RECOMMENDATION
B: Based on a single multicenter, prospective, randomized clinical trial1
Leddy JJ, Haider MN, Ellis MJ, et al. Early subthreshold aerobic exercise for sport-related concussion: a randomized clinical trial. JAMA Pediatr. 2019;173:319-325. doi: 10.1001/jamapediatrics.2018.4397
Benzodiazepine and Z-hypnotic stewardship
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1
With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.

BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; [email protected]
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1

With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.

BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; [email protected]
Benzodiazepines (BZDs) and Z-hypnotics have been available for decades, yet uncertainties about their use remain. They are prescribed and overprescribed most often for anxiety and insomnia, for which they have value but also the potential for significant adverse consequences, notably physiologic dependence. Use of these agents should be limited, and planned deprescribing is a fundamental aspect of prescribing.
A brief history. BZDs are a subset of benzodiazepine receptor agonists (BZRAs), which enhance the inhibitory effect of centrally acting γ-amino butyric acid (GABA) at the GABAA receptor through allosteric modulation. In 1960, the first BZD, chlordiazepoxide, was marketed for clinical use, and as other agents in the class became available, BZDs supplanted the more toxic barbiturates, another BZRA subset (TABLE 1). By the late 1970s, BZDs had risen to the top of most prescribed medications, with one agent in particular—diazepam (Valium)—earning a reputation as “mother’s little helper,” a phrase derived from a Rolling Stones' song with that title produced in 1966.1

With recognition of the problems associated with BZDs, their popularity diminished somewhat but remained high. BZDs were listed under Schedule IV by the Drug Enforcement Administration in 1975 due to the risk for addiction, and on the American Geriatrics Society Beers Criteria list in 1991 because of significant adverse consequences in the elderly. Researchers began to question their use as early as the 1970s, and the landmark Ashton Manual, guidance for patients and clinicians alike, was published in 2002.2
Currently, there are 14 BZDs approved by the Food and Drug Administration (FDA) as well as 3 Z-hypnotics, termed such as they include the letter “z” in their generic names (TABLE 1). In recent years, BZD prescribing has risen; a 2019 study found that 1 of 8 American adults reported using a BZD in the previous year.3
Limited benefits of benzodiazepine receptor agonists
BZRAs can be of benefit in a limited range of medical conditions, including some for which they are first-line considerations. (See TABLE 2 for a list of indications for BZDs.) They are most often prescribed for anxiety and insomnia, although they are not first-line treatments for these conditions and should be prescribed only when symptoms limit a patient’s daily functioning.

BZRAs are not intended for long-term use. In recent decades, the percentage of patients prescribed BZRAs has doubled, and more than 80% of these patients indicate usage for more than 6 months.4 Evidence, however, does not support long-term daily use.
Observation periods in most studies are far shorter than the number of years over which BZDs are actually prescribed, and flawed research methodology has introduced the risk of bias. Specifically, the generalizability of reported outcomes must be qualified, since efficacy trials performed under ideal study conditions (eg, exclusion criteria to minimize confounders) differ from circumstances seen in clinical practice. Conclusions are also limited by the inherent bias of pharmaceutical industry sponsorship and unavailability of unpublished trials that may have demonstrated unfavorable results.
Continue to: Insomnia
Insomnia, a current (past 30 days) complaint in more than 40% of US adults, is associated with a variety of symptoms.5 About 20% of adults have an insomnia disorder, defined as a predominant problem for at least 1 month involving sleep initiation, maintenance, or nonrestorative sleep along with daytime function-limiting fatigue.5 Meta-analyses indicate BZRAs can reduce sleep latency (BZDs, by 4 minutes; Z-hypnotics, 22 minutes) and may increase sleep duration (BZDs, 62 minutes per limited data; Z-hypnotics, data insufficient).6,7 Definitive evidence for long-term (> 2-4 weeks) BZD benefit is lacking, and cognitive behavioral therapy for insomnia (CBT-I) is well established as first-line treatment yielding improvements that may last at least 18 months after completion of therapy. 8,9
Although CBT-I is generally provided by behavioral health specialists, elements of CBT-I and sleep hygiene measures can be effectively used by primary care clinicians.10 Data indicate other nonpharmacologic interventions are also effective,11 including acceptance and commitment therapy,12 meditation,13 and acupuncture.14
Episodic fear and anxiety are universal and essential for survival. Fear is an alarm warning of an immediate hazard. Anxiety (the emotion) paired with worry (the thought) relate to a perceived future threat. Transient (state) anxiety should not be suppressed altogether if self-management can curb its intensity and thereby allow effective problem engagement. However, when individuals are incapacitated by crisis anxiety or sporadic specific phobias such as flight anxiety, episodic BZDs do have a role.
Ongoing anxiety is a more complex treatment situation. Obsessive-compulsive disorder and posttraumatic stress disorder are no longer categorized as anxiety disorders, but they often involve anxiety. Here, BZDs have no indication aside from exceptional acute crisis presentations. Anxiety disorders are defined by a core persistent (trait) anxiety disproportionate to the actual threat, limited daily functioning, and more than 6 months’ duration. One of 3 Americans older than 13 years meet the criteria for anxiety in their lifetime; 1 of 5 meet the criteria in any single year.15
BZDs are effective in treating anxiety disorders in the short term (2-4 weeks)2,16,17; however, benefit may fade over time.18-21 For some individuals, data suggest BZDs themselves might actually generate anxiety, as evidenced by reduced symptom intensity following discontinuation.22,23 Recommended first-line medications for anxiety disorders include certain antidepressants and pregabalin, which exhibit efficacy similar to that of BZDs.24 Mindfulness and various psychotherapies have value, as well.16 Among the latter, CBT is considered first line with benefit comparable to BZDs in the short term; yet unlike BZDs, CBT gains can last 12 months or longer after the conclusion of therapy. 25,26 Because there may be a delay between the start of CBT and the onset of benefit, BZDs, which work quickly, may be used to bridge functionally impaired patients in the short term.
Continue to: Risks with benzodiazepine receptor agonists
Risks with benzodiazepine receptor agonists
Harms from BZRA use are common, tempering their utility. Sedation, dyscognition, and psychomotor impairments are often seen upon initiation of BZRA use. These adverse effects can—although not always—improve with continuous BZRA exposure, an effect known as tolerance, which is due to neuropharmacologic adaptation.
Cognitive issues include problems with memory, judgment, and decision making. These may be unrecognized or, if noted, attributed to other issues such as aging, and may become clear only when BZRAs are discontinued. Anterograde amnesia and parasomnias occur less often.
Psychomotor impairment can result in falls, fractures, and other injuries, especially in the elderly. Decrements in mood, including emergent depression and paradoxical anxiety, can occur. Some individuals experience disinhibition that is expressed through irritability, agitation, aggression, and violence.
Misuse of BZRAs is not unusual and can be related to dosing errors or attempts to ease intrusive symptoms. Nonmedical use almost always occurs in the context of an underlying use disorder, whereby BZRAs serve to amplify euphoria or ameliorate withdrawal from opioids or alcohol. Addiction per se, which entails BZRA craving and compulsive use leading to adverse consequences, is unusual.
BZRAs are associated with increased mortality, including all-cause mortality and suicide. They are respiratory depressants, although when taken alone in excess rarely result in death. They are, however, strongly implicated in opioid-related overdose fatalities, as their presence has been identified in 1 of 3 such decedents.27
Continue to: Physiologic dependence with BZRAs
Physiologic dependence with BZRAs
Among the more important adverse outcomes with ongoing BZRA exposure is physiologic dependence. This occurs primarily because of neuroadaptation of GABAA and glutaminergic receptors, but dependence probably also involves changes in the adenosine A2A, serotonergic, and peripheral benzodiazepine receptors, the latter being present on mitochondrial membranes. The hypothalamic-pituitary-adrenal axis also appears to be involved.
Physiologic dependence is expressed through BZRA tolerance and characteristic physical and psychological symptoms upon withdrawal. Tolerance refers to a reduced effect with continued substance exposure or the need for an increased dose to get the same effect. Drug withdrawal can result in manifestations distinctive to addiction-prone substances, as well as to some medications without addiction liability, such as corticosteroids and antidepressants. Tolerance and withdrawal are not applicable criteria in the diagnosis of sedative-hypnotic use disorder when BZRAs are prescribed.28
Withdrawal. Reported prevalence of BZRA physiologic dependence differs according to populations studied, criteria used, and the deprescribing process employed. Some researchers have found rates of one-third and others exceeding one-half among individuals using BZRAs for longer than a month.23,29
Deprescribing BZRAs
Because benefits are limited and adverse outcomes including physiologic dependence are common, it is recommended that clinicians urge deprescribing of BZRAs for any patient taking them consistently for more than 1 month. Published deprescribing investigations and guidance are insufficient, heterogenous, and confusing. Still, some approaches can work well, and success rates as high as 80% have been achieved among the elderly, for example.35 Brief interventions such as providing individualized advice, support, and management are effective.36,37 Abrupt discontinuation is inappropriate and can be life threatening.38 Forced cessation is also inappropriate unless significant respiratory depression is identified.
The Ashton Manual is a useful guide, readable by patients. Proceed with tapering slowly at a rate led by the patient’s response.2,39 Avoid discrediting patients’ reports of unusual withdrawal symptoms, as this can lead to misdiagnosis (eg, somatic symptom disorder) or ineffective treatment (eg, addiction recovery approaches). Adding CBT to tapering improves outcomes, and adjunctive medications may be helpful, although not without their own problems.29 Consistent support of patients by others involved in treatment (prescriber, pharmacist, behavioral health specialists, peer coach, significant others) is essential. Complex challenges generally resolve through authentic listening and response but may require referral to others with necessary skills and experience. Complete cessation may take 12 to 18 months (or longer). Even if complete cessation is not possible, the least dose necessary can be achieved.
CORRESPONDENCE
Steven Wright, MD, 1975 Ashland Mine Road, Ashland, OR 97520; [email protected]
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
- Chandler A, Whittaker A, Williams N, et al. Mother’s little helper? Contrasting accounts of benzodiazepine and methadone use among drug-dependent parents in the UK. Drugs (Abingdon Engl). 2014;21:470-475. doi: 10.3109/09687637.2014.930814Ash
- ton CH. Benzodiazepines: How They Work & How to Withdraw (the Ashton Manual). 2002. Accessed March 17, 2022. www.benzoinfo.com/ashtonmanual/
- Maust DT, Lin LA, Blow FC. Benzodiazepine use and misuse among adults in the United States. Psychiatr Serv. 2019;70:97-106. doi: 10.1176/appi.ps.201800321
- Kaufmann CN, Spira AP, Depp CA, et al. Long-term use of benzodiazepines and non-benzodiazepine hypnotics from 1999 to 2014: results from the National Health and Nutrition Examination Survey. Psychiatr Serv. 2018;69:235-238. doi: 10.1176/appi.ps.201700095
- Walsh JK, Coulouvrat C, Hajak G, et al. Nighttime insomnia symptoms and perceived health in the America Insomnia Survey (AIS). Sleep. 2011;34:997-1011. doi: 10.5665/SLEEP.1150
- Holbrook AM, Crowther R, Lotter A, et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225-233.
- Huedo-Medina TB, Kirsch I, Middlemass J, et al. Effectiveness of non-benzodiazepine hypnotics in treatment of adult insomnia: meta-analysis of data submitted to the Food and Drug Administration. BMJ. 2012;345:e8343. doi: 10.1136/bmj.e8343
- Curran HV, Collins R, Fletcher S, et al. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med. 2003;33:1223-1237. doi: 10.1017/s0033291703008213
- Geiger-Brown JM, Rogers VE, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67. doi: 10.1016/j.smrv.2014.11.007
- Sorscher AJ. Insomnia: getting to the cause, facilitating relief. J Fam Pract. 2017;66:216-225
- Laura Hrehová L, Mezian K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. 2021;75:e14084. doi: 10.1111/ijcp.14084.
- Daly-Eichenhardt A, Scott W, Howard-Jones M, et al. Changes in sleep problems and psychological flexibility following interdisciplinary acceptance and commitment therapy for chronic pain: an observational cohort study. Front Psychol. 2016;7:1326. doi: 10.3389/fpsyg.2016.01326
- Rusch HL, Rosario M, Levison LM, et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445:5-16. doi: 10.1111/nyas.13996
- Cao H, Pan X, Li H, et al. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. 2009;15:1171-1186. doi: 10.1089/acm.2009.0041
- Kessler RC, Petukhova M, Sampson NA, et al. Twelve‐month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184. doi: 10.1002/mpr.1359
- Bandelow B, Reitt M, Röver C, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183-192. doi: 10.1097/YIC.0000000000000078
- Lader M. Benzodiazepines revisited—will we ever learn? Addiction. 2011;106:2086-2109. doi: 10.1111/j.1360-0443.2011.03563.x
- Fava GA. Fading of therapeutic effects of alprazolam in agoraphobia. Case reports. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:109-112. doi: 10.1016/0278-5846(88)90066-8
- Fava GA, Grandi S, Belluardo P, et al. Benzodiazepines and anxiety sensitivity in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1163-1168. doi: 10.1016/0278-5846(94)90118-x
- Pélissolo A, Maniere F, Boutges B, et al. Anxiety and depressive disorders in 4,425 long term benzodiazepine users in general practice. Encephale. 2007;33:32-38. doi: 10.1016/s0013-7006(07)91556-0
- Gale C, Glue P, Guaiana G, et al. Influence of covariates on heterogeneity in Hamilton Anxiety Scale ratings in placebo-controlled trials of benzodiazepines in generalized anxiety disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:543-547. doi: 10.1177/0269881118822146
- Ashton CH. Benzodiazepine withdrawal: outcome in 50 patients. Br J Addict. 1987;82:655-671. Accessed February 22, 2022. www.benzo.org.uk/ashbzoc.htm
- Rickels K, Schweizer E, Case WG, et al. Long-term therapeutic use of benzodiazepines. I. Effects of abrupt discontinuation. Arch Gen Psychiatry. 1990;47:899-907. doi: 10.1001/archpsyc.1990.01810220015002
- Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84. Accessed March 17, 2022. www.wfsbp.org/fileadmin/user_upload/Treatment_Guidelines/Bandelow_et_al_01.pdf
- Imai H, Tajika A, Chen P, et al. Psychological therapies versus pharmacological interventions for panic disorder with or without agoraphobia in adults. Cochrane Database Syst Rev. 2016;10:CD011170. doi: 10.1002/14651858.CD011170.pub2
- van Dis EAM, van Veen SC, Hagenaars MA, et al. Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders. A systematic review and meta-analysis. JAMA Psychiatry. 2020;77:265-273. doi:10.1001/jamapsychiatry.2019.3986
- Chen LH, Hedegaard H, Warner M. Drug-poisoning deaths Involving opioid analgesics: United States, 1999-2011. NCHS Data Brief. 2014;(166):1-8. Accessed March 17, 2022. www.cdc.gov/nchs/data/databriefs/db166.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013:550-555.
- Marriott S, Tyrer P. Benzodiazepine dependence: avoidance and withdrawal. Drug Safety. 1993;9:93-103. doi: 10.2165/00002018-199309020-00003
- Griffiths RR, Evans SM, Guarino JJ, et al. Intravenous flumazenil following acute and repeated exposure to lorazepam in healthy volunteers: antagonism and precipitated withdrawal. J Pharmacol Exp Ther. 1993;265:1163-1174.
- Ashton H. Benzodiazepine withdrawal: an unfinished story. Br Med J. 1984;288:1135-1140. Accessed March 17, 2022. www.ncbi.nlm.nih.gov/pmc/articles/PMC1441411/pdf/bmjcred00496-0031.pdf
- Lugoboni F, Quaglio G. Exploring the dark side of the moon: the treatment of benzodiazepine tolerance. Br J Clin Pharmacol. 2014;77:239-41. doi: 10.1111/bcp.12148
- Ashton CH. Protracted withdrawal from benzodiazepines: the post-withdrawal syndrome. Psychiatr Ann. 1995;25:174-179. Accessed March 17, 2022. https://benzo.org.uk/pha-1.htm
- Fixsen AM, Ridge D. Stories of hell and healing: internet users’ construction of benzodiazepine distress and withdrawal. Qual Health Res. 2017;27:2030-2041. doi: 10.1177/1049732317728053
- Ng BJ, Le Couteur DG, Hilmer SN. Deprescribing benzodiazepines in older patients: impact of interventions targeting physicians, pharmacists, and patients. Drugs Aging. 2018;35:493-521. doi: 10.1007/s40266-018-0544-4
- Lynch T, Ryan C, Hughes CM, et al. Brief interventions targeting long-term benzodiazepine and Z-drug use in primary care: a systematic review and meta-analysis. Addiction. 2020;115:1618-1639. doi: 10.1111/add.14981
- Darker CD, Sweeney BP, Barry JM, et al. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. Cochrane Database Syst Rev. 2015;(5):CD009652. doi: 10.1002/14651858.CD009652.pub2
- Hu X. Benzodiazepine withdrawal seizures and management. J Okla State Med Assoc. 2011;104:62-65.
- Wright SL. Benzodiazepine withdrawal: clinical aspects. In Peppin J, Raffa R, Pergolizzi J, Wright SL, eds. The Benzodiazepines Crisis: The Ramifications of an Overused Drug Class. Oxford University Press. 2020:117-148.
PRACTICE RECOMMENDATIONS
› Recommend cognitive behavioral therapy as first-line treatment for anxiety and insomnia. A
› Limit benzodiazepine prescribing to ≤ 2 to 4 weeks for anxiety and insomnia. B
› Taper benzodiazepines slowly and flexibly. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Is bicarbonate therapy effective in preventing CKD progression?
Evidence summary
Bicarbonate therapy demonstrates benefit in 2 meta-analyses
Two recent meta-analyses evaluated studies of bicarbonate therapy in patients with CKD, and both found benefit.1,2
A 2020 meta-analysis included 15 RCTs (N = 2445) of adults (mean age, 61 years; range, 40.5-73.9 years) with CKD.1 Most trials enrolled patients with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2; however, 1 study (N = 80) enrolled patients who had an eGFR of 60 to 90 mL/min/1.73 m2 and albuminuria, and another (N = 74) enrolled patients with an eGFR of 15 to 89 mL/min/1.73 m2. Four studies included patients with normal baseline bicarbonate levels, while the rest enrolled patients with metabolic acidosis. The primary outcome was CKD progression at study conclusion, which ranged from 3 to 60 months (median, 12 months).
Compared to placebo or no therapy, sodium bicarbonate (variously dosed) resulted in a small reduction in the rate of loss of kidney function (defined by eGFR or creatinine clearance) from baseline to trial completion (14 trials, N = 2073; standardized mean difference [SMD] = 0.26; 95% CI, 0.13-0.40; P = .018; I2 = 50%).1
Subgroup analysis by follow-up time found a significant preservation of eGFR only in studies with follow-up > 12 months (4 trials, N = 392; weighted mean difference = 3.71 mL/min/1.73 m2; 95% CI, 0.18-7.24; P = .042; I2 = 63%).1 Duration of therapy did not affect initiation of dialysis. Another subgroup analysis found that low- and moderate-quality studies were more likely than high-quality studies to find a change in the primary outcome. Overall, there was significant heterogeneity among the trials (control intervention, follow-up duration, methods of assessment of kidney function, dosage of sodium bicarbonate), as well as underrepresentation of female, pediatric, and elderly patients.
Another meta-analysis, published in 2019 by a different research group, analyzed 7 RCTs (N = 815) that comprised a subset of those in the newer analysis.2 The 2019 analysis similarly found that, compared to placebo or usual care, oral bicarbonate therapy resulted in statistically significantly higher eGFRs at 3 to 60 months’ follow-up (mean difference = 3.1 mL/min/1.73 m²; 95% CI, 1.3-4.9).2 The authors noted that the protective effect on eGFR was not seen in studies reporting outcomes at 1 year. Progression to end-stage renal disease or initiation of dialysis were not used as outcomes.
Significant outcomes seen in 1 large study
The largest study (N = 740) included in the 2020 meta-analysis (and discussed separately due to its size and duration) was a multicenter, unblinded, pragmatic trial investigating bicarbonate therapy in CKD.3 Patients were adults (mean age, 67.8 years) with CKD stages 3 to 5 and metabolic acidosis (serum bicarbonate level of 18-24 mmol/L); mean serum creatinine was 2.3 mg/dL, and mean serum bicarbonate was 21.5 mmol/L. Patients with severe heart failure or uncontrolled hypertension were excluded.
Researchers randomized patients to oral sodium bicarbonate (titrated to a target serum concentration of 24-28 mmol/L) or standard care for a median duration of 30 months. The primary endpoint was time to doubling of serum creatinine, and secondary endpoints included all-cause mortality, time to initiation of dialysis, hospitalization rate, and hospital length of stay.
Continue to: Patients treated with...
Patients treated with bicarbonate therapy had a 64% lower risk of doubling their serum creatinine compared to those treated with standard care (hazard ratio [HR] = 0.36; 95% CI, 0.22-0.58; P < .001; NNT = 9.6).3 Bicarbonate therapy also significantly reduced the risk of dialysis (HR = 0.5; 95% CI, 0.31-0.81; P = .005; NNT = 19); all-cause mortality (HR = 0.43; 95% CI, 0.22-0.87; P = .01; NNT = 27); hospitalization rates (34.6% vs 14.2% by end of study in standard care and bicarbonate groups, respectively; P < .001); and hospital length of stay (1160 total d/y vs 400 total d/y; P < .0001).3 Inspection of Kaplan Meier curves shows outcomes beginning to diverge after 1 to 2 years of treatment. This trial was limited by the lack of blinding, placebo control, and standardization of care protocols.
Recommendations from others
The National Kidney Foundation’s 2012 Kidney Disease Outcomes Quality Initiative guidelines for the management of CKD recommend oral bicarbonate therapy for patients with CKD and serum bicarbonate concentrations < 22 mmol/L.4 The guidelines state that serum bicarbonate levels < 22 mmol/L correlate with an increased risk of CKD progression and death, whereas high bicarbonate levels (> 32 mmol/L) correlate with increased risk of death independent of level of kidney function. These guidelines cite small studies of alkali therapy slowing progression of CKD, although it was noted that the evidence base was not strong.
Editor’s takeaway
The evidence shows a small but consistent effect of bicarbonate therapy on CKD progression. For patients with CKD stages 3 to 5 and metabolic acidosis (defined by serum bicarbonate levels < 22 mmol/L), the use of supplemental oral sodium bicarbonate, which is inexpensive and safe, can delay or prevent progression of serious disease.
1. Hultin S, Hood C, Campbell KL, et al. A systematic review and meta-analysis on effects of bicarbonate therapy on kidney outcomes. Kidney Int Rep. 2020;6:695-705. doi: 10.1016/j.ekir.2020.12.019
2. Hu MK, Witham MD, Soiza RL. Oral bicarbonate therapy in non-haemodialysis dependent chronic kidney disease patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Med. 2019;8:208. doi: 10.3390/jcm8020208
3. Di Iorio BR, Bellasi A, Raphael KL, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J of Neph. 2019; 32:989-1001. doi: 10.1007/s40620-019-00656-5
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150.
Evidence summary
Bicarbonate therapy demonstrates benefit in 2 meta-analyses
Two recent meta-analyses evaluated studies of bicarbonate therapy in patients with CKD, and both found benefit.1,2
A 2020 meta-analysis included 15 RCTs (N = 2445) of adults (mean age, 61 years; range, 40.5-73.9 years) with CKD.1 Most trials enrolled patients with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2; however, 1 study (N = 80) enrolled patients who had an eGFR of 60 to 90 mL/min/1.73 m2 and albuminuria, and another (N = 74) enrolled patients with an eGFR of 15 to 89 mL/min/1.73 m2. Four studies included patients with normal baseline bicarbonate levels, while the rest enrolled patients with metabolic acidosis. The primary outcome was CKD progression at study conclusion, which ranged from 3 to 60 months (median, 12 months).
Compared to placebo or no therapy, sodium bicarbonate (variously dosed) resulted in a small reduction in the rate of loss of kidney function (defined by eGFR or creatinine clearance) from baseline to trial completion (14 trials, N = 2073; standardized mean difference [SMD] = 0.26; 95% CI, 0.13-0.40; P = .018; I2 = 50%).1
Subgroup analysis by follow-up time found a significant preservation of eGFR only in studies with follow-up > 12 months (4 trials, N = 392; weighted mean difference = 3.71 mL/min/1.73 m2; 95% CI, 0.18-7.24; P = .042; I2 = 63%).1 Duration of therapy did not affect initiation of dialysis. Another subgroup analysis found that low- and moderate-quality studies were more likely than high-quality studies to find a change in the primary outcome. Overall, there was significant heterogeneity among the trials (control intervention, follow-up duration, methods of assessment of kidney function, dosage of sodium bicarbonate), as well as underrepresentation of female, pediatric, and elderly patients.
Another meta-analysis, published in 2019 by a different research group, analyzed 7 RCTs (N = 815) that comprised a subset of those in the newer analysis.2 The 2019 analysis similarly found that, compared to placebo or usual care, oral bicarbonate therapy resulted in statistically significantly higher eGFRs at 3 to 60 months’ follow-up (mean difference = 3.1 mL/min/1.73 m²; 95% CI, 1.3-4.9).2 The authors noted that the protective effect on eGFR was not seen in studies reporting outcomes at 1 year. Progression to end-stage renal disease or initiation of dialysis were not used as outcomes.
Significant outcomes seen in 1 large study
The largest study (N = 740) included in the 2020 meta-analysis (and discussed separately due to its size and duration) was a multicenter, unblinded, pragmatic trial investigating bicarbonate therapy in CKD.3 Patients were adults (mean age, 67.8 years) with CKD stages 3 to 5 and metabolic acidosis (serum bicarbonate level of 18-24 mmol/L); mean serum creatinine was 2.3 mg/dL, and mean serum bicarbonate was 21.5 mmol/L. Patients with severe heart failure or uncontrolled hypertension were excluded.
Researchers randomized patients to oral sodium bicarbonate (titrated to a target serum concentration of 24-28 mmol/L) or standard care for a median duration of 30 months. The primary endpoint was time to doubling of serum creatinine, and secondary endpoints included all-cause mortality, time to initiation of dialysis, hospitalization rate, and hospital length of stay.
Continue to: Patients treated with...
Patients treated with bicarbonate therapy had a 64% lower risk of doubling their serum creatinine compared to those treated with standard care (hazard ratio [HR] = 0.36; 95% CI, 0.22-0.58; P < .001; NNT = 9.6).3 Bicarbonate therapy also significantly reduced the risk of dialysis (HR = 0.5; 95% CI, 0.31-0.81; P = .005; NNT = 19); all-cause mortality (HR = 0.43; 95% CI, 0.22-0.87; P = .01; NNT = 27); hospitalization rates (34.6% vs 14.2% by end of study in standard care and bicarbonate groups, respectively; P < .001); and hospital length of stay (1160 total d/y vs 400 total d/y; P < .0001).3 Inspection of Kaplan Meier curves shows outcomes beginning to diverge after 1 to 2 years of treatment. This trial was limited by the lack of blinding, placebo control, and standardization of care protocols.
Recommendations from others
The National Kidney Foundation’s 2012 Kidney Disease Outcomes Quality Initiative guidelines for the management of CKD recommend oral bicarbonate therapy for patients with CKD and serum bicarbonate concentrations < 22 mmol/L.4 The guidelines state that serum bicarbonate levels < 22 mmol/L correlate with an increased risk of CKD progression and death, whereas high bicarbonate levels (> 32 mmol/L) correlate with increased risk of death independent of level of kidney function. These guidelines cite small studies of alkali therapy slowing progression of CKD, although it was noted that the evidence base was not strong.
Editor’s takeaway
The evidence shows a small but consistent effect of bicarbonate therapy on CKD progression. For patients with CKD stages 3 to 5 and metabolic acidosis (defined by serum bicarbonate levels < 22 mmol/L), the use of supplemental oral sodium bicarbonate, which is inexpensive and safe, can delay or prevent progression of serious disease.
Evidence summary
Bicarbonate therapy demonstrates benefit in 2 meta-analyses
Two recent meta-analyses evaluated studies of bicarbonate therapy in patients with CKD, and both found benefit.1,2
A 2020 meta-analysis included 15 RCTs (N = 2445) of adults (mean age, 61 years; range, 40.5-73.9 years) with CKD.1 Most trials enrolled patients with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2; however, 1 study (N = 80) enrolled patients who had an eGFR of 60 to 90 mL/min/1.73 m2 and albuminuria, and another (N = 74) enrolled patients with an eGFR of 15 to 89 mL/min/1.73 m2. Four studies included patients with normal baseline bicarbonate levels, while the rest enrolled patients with metabolic acidosis. The primary outcome was CKD progression at study conclusion, which ranged from 3 to 60 months (median, 12 months).
Compared to placebo or no therapy, sodium bicarbonate (variously dosed) resulted in a small reduction in the rate of loss of kidney function (defined by eGFR or creatinine clearance) from baseline to trial completion (14 trials, N = 2073; standardized mean difference [SMD] = 0.26; 95% CI, 0.13-0.40; P = .018; I2 = 50%).1
Subgroup analysis by follow-up time found a significant preservation of eGFR only in studies with follow-up > 12 months (4 trials, N = 392; weighted mean difference = 3.71 mL/min/1.73 m2; 95% CI, 0.18-7.24; P = .042; I2 = 63%).1 Duration of therapy did not affect initiation of dialysis. Another subgroup analysis found that low- and moderate-quality studies were more likely than high-quality studies to find a change in the primary outcome. Overall, there was significant heterogeneity among the trials (control intervention, follow-up duration, methods of assessment of kidney function, dosage of sodium bicarbonate), as well as underrepresentation of female, pediatric, and elderly patients.
Another meta-analysis, published in 2019 by a different research group, analyzed 7 RCTs (N = 815) that comprised a subset of those in the newer analysis.2 The 2019 analysis similarly found that, compared to placebo or usual care, oral bicarbonate therapy resulted in statistically significantly higher eGFRs at 3 to 60 months’ follow-up (mean difference = 3.1 mL/min/1.73 m²; 95% CI, 1.3-4.9).2 The authors noted that the protective effect on eGFR was not seen in studies reporting outcomes at 1 year. Progression to end-stage renal disease or initiation of dialysis were not used as outcomes.
Significant outcomes seen in 1 large study
The largest study (N = 740) included in the 2020 meta-analysis (and discussed separately due to its size and duration) was a multicenter, unblinded, pragmatic trial investigating bicarbonate therapy in CKD.3 Patients were adults (mean age, 67.8 years) with CKD stages 3 to 5 and metabolic acidosis (serum bicarbonate level of 18-24 mmol/L); mean serum creatinine was 2.3 mg/dL, and mean serum bicarbonate was 21.5 mmol/L. Patients with severe heart failure or uncontrolled hypertension were excluded.
Researchers randomized patients to oral sodium bicarbonate (titrated to a target serum concentration of 24-28 mmol/L) or standard care for a median duration of 30 months. The primary endpoint was time to doubling of serum creatinine, and secondary endpoints included all-cause mortality, time to initiation of dialysis, hospitalization rate, and hospital length of stay.
Continue to: Patients treated with...
Patients treated with bicarbonate therapy had a 64% lower risk of doubling their serum creatinine compared to those treated with standard care (hazard ratio [HR] = 0.36; 95% CI, 0.22-0.58; P < .001; NNT = 9.6).3 Bicarbonate therapy also significantly reduced the risk of dialysis (HR = 0.5; 95% CI, 0.31-0.81; P = .005; NNT = 19); all-cause mortality (HR = 0.43; 95% CI, 0.22-0.87; P = .01; NNT = 27); hospitalization rates (34.6% vs 14.2% by end of study in standard care and bicarbonate groups, respectively; P < .001); and hospital length of stay (1160 total d/y vs 400 total d/y; P < .0001).3 Inspection of Kaplan Meier curves shows outcomes beginning to diverge after 1 to 2 years of treatment. This trial was limited by the lack of blinding, placebo control, and standardization of care protocols.
Recommendations from others
The National Kidney Foundation’s 2012 Kidney Disease Outcomes Quality Initiative guidelines for the management of CKD recommend oral bicarbonate therapy for patients with CKD and serum bicarbonate concentrations < 22 mmol/L.4 The guidelines state that serum bicarbonate levels < 22 mmol/L correlate with an increased risk of CKD progression and death, whereas high bicarbonate levels (> 32 mmol/L) correlate with increased risk of death independent of level of kidney function. These guidelines cite small studies of alkali therapy slowing progression of CKD, although it was noted that the evidence base was not strong.
Editor’s takeaway
The evidence shows a small but consistent effect of bicarbonate therapy on CKD progression. For patients with CKD stages 3 to 5 and metabolic acidosis (defined by serum bicarbonate levels < 22 mmol/L), the use of supplemental oral sodium bicarbonate, which is inexpensive and safe, can delay or prevent progression of serious disease.
1. Hultin S, Hood C, Campbell KL, et al. A systematic review and meta-analysis on effects of bicarbonate therapy on kidney outcomes. Kidney Int Rep. 2020;6:695-705. doi: 10.1016/j.ekir.2020.12.019
2. Hu MK, Witham MD, Soiza RL. Oral bicarbonate therapy in non-haemodialysis dependent chronic kidney disease patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Med. 2019;8:208. doi: 10.3390/jcm8020208
3. Di Iorio BR, Bellasi A, Raphael KL, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J of Neph. 2019; 32:989-1001. doi: 10.1007/s40620-019-00656-5
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150.
1. Hultin S, Hood C, Campbell KL, et al. A systematic review and meta-analysis on effects of bicarbonate therapy on kidney outcomes. Kidney Int Rep. 2020;6:695-705. doi: 10.1016/j.ekir.2020.12.019
2. Hu MK, Witham MD, Soiza RL. Oral bicarbonate therapy in non-haemodialysis dependent chronic kidney disease patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Med. 2019;8:208. doi: 10.3390/jcm8020208
3. Di Iorio BR, Bellasi A, Raphael KL, et al. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J of Neph. 2019; 32:989-1001. doi: 10.1007/s40620-019-00656-5
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1-150.
EVIDENCE-BASED ANSWER:
YES. Long-term sodium bicarbonate therapy slightly slows the loss of renal function in patients with chronic kidney disease (CKD) and may moderately reduce progression to end-stage renal disease (strength of recommendation [SOR]: B, meta-analyses of lower-quality randomized controlled trails [RCTs]). Therapy duration of 1 year or less may not be beneficial (SOR: C, secondary analyses in meta-analyses).
Colchicine use lowers T2D risk in patients with gout
Key clinical point: Colchicine use in patients with gout is associated with a lower risk for type 2 diabetes mellitus (T2D).
Major finding: Colchicine users vs. nonusers had a lower cumulative incidence of T2D (18.8% vs. 25.0%) and a significantly lower risk for T2D (adjusted hazard ratio 0.74; 95% CI 0.36-0.87), with the inverse association remaining significant across both sexes and different age groups.
Study details: The data come from a retrospective cohort study including patients newly diagnosed with gout who used (n = 3841) and did not use (n = 7682) colchicine.
Disclosures: The study received no external funding. The authors declared no conflict of interests.
Source: Chu C-C et al. Association between clinical use of colchicine and risk of type 2 diabetes mellitus among gouty patients: A nationwide cohort study. Int J Environ Res Public Health. 2022;19(6):3395 (Mar 14). Doi: 10.3390/ijerph19063395
Key clinical point: Colchicine use in patients with gout is associated with a lower risk for type 2 diabetes mellitus (T2D).
Major finding: Colchicine users vs. nonusers had a lower cumulative incidence of T2D (18.8% vs. 25.0%) and a significantly lower risk for T2D (adjusted hazard ratio 0.74; 95% CI 0.36-0.87), with the inverse association remaining significant across both sexes and different age groups.
Study details: The data come from a retrospective cohort study including patients newly diagnosed with gout who used (n = 3841) and did not use (n = 7682) colchicine.
Disclosures: The study received no external funding. The authors declared no conflict of interests.
Source: Chu C-C et al. Association between clinical use of colchicine and risk of type 2 diabetes mellitus among gouty patients: A nationwide cohort study. Int J Environ Res Public Health. 2022;19(6):3395 (Mar 14). Doi: 10.3390/ijerph19063395
Key clinical point: Colchicine use in patients with gout is associated with a lower risk for type 2 diabetes mellitus (T2D).
Major finding: Colchicine users vs. nonusers had a lower cumulative incidence of T2D (18.8% vs. 25.0%) and a significantly lower risk for T2D (adjusted hazard ratio 0.74; 95% CI 0.36-0.87), with the inverse association remaining significant across both sexes and different age groups.
Study details: The data come from a retrospective cohort study including patients newly diagnosed with gout who used (n = 3841) and did not use (n = 7682) colchicine.
Disclosures: The study received no external funding. The authors declared no conflict of interests.
Source: Chu C-C et al. Association between clinical use of colchicine and risk of type 2 diabetes mellitus among gouty patients: A nationwide cohort study. Int J Environ Res Public Health. 2022;19(6):3395 (Mar 14). Doi: 10.3390/ijerph19063395
Preventing CKD may help mitigate risk for stroke in adults with T2D
Key clinical point: In adults with type 2 diabetes (T2D), higher albuminuria and worsening chronic kidney disease (CKD) were independently associated with a higher risk for incident stroke.
Major finding: Compared with a urine albumin-to-creatinine ratio of <30 mg/g and no CKD, moderate (adjusted hazard ratio [aHR] 1.61; P = .010) and severe (aHR 2.29; P = .001) albuminuria and worsening CKD stages (stage 1: aHR 1.76; P = .020; stage 2: aHR 1.77; P = .012; and stage 3: aHR 2.03; P = .003) were significantly associated with a higher risk for stroke, respectively.
Study details: This study included 9170 adults with T2D from the ACCORD study, of which 156 experienced stroke events over a median follow-up of 4.9 years.
Disclosures: The study did not declare any source of funding. Dr. G Fonarow reported consulting for various sources.
Source: Kaze AD et al. Diabetic kidney disease and risk of incident stroke among adults with type 2 diabetes. BMC Med. 2022;20:127 (Mar 29). Doi: 10.1186/s12916-022-02317-0
Key clinical point: In adults with type 2 diabetes (T2D), higher albuminuria and worsening chronic kidney disease (CKD) were independently associated with a higher risk for incident stroke.
Major finding: Compared with a urine albumin-to-creatinine ratio of <30 mg/g and no CKD, moderate (adjusted hazard ratio [aHR] 1.61; P = .010) and severe (aHR 2.29; P = .001) albuminuria and worsening CKD stages (stage 1: aHR 1.76; P = .020; stage 2: aHR 1.77; P = .012; and stage 3: aHR 2.03; P = .003) were significantly associated with a higher risk for stroke, respectively.
Study details: This study included 9170 adults with T2D from the ACCORD study, of which 156 experienced stroke events over a median follow-up of 4.9 years.
Disclosures: The study did not declare any source of funding. Dr. G Fonarow reported consulting for various sources.
Source: Kaze AD et al. Diabetic kidney disease and risk of incident stroke among adults with type 2 diabetes. BMC Med. 2022;20:127 (Mar 29). Doi: 10.1186/s12916-022-02317-0
Key clinical point: In adults with type 2 diabetes (T2D), higher albuminuria and worsening chronic kidney disease (CKD) were independently associated with a higher risk for incident stroke.
Major finding: Compared with a urine albumin-to-creatinine ratio of <30 mg/g and no CKD, moderate (adjusted hazard ratio [aHR] 1.61; P = .010) and severe (aHR 2.29; P = .001) albuminuria and worsening CKD stages (stage 1: aHR 1.76; P = .020; stage 2: aHR 1.77; P = .012; and stage 3: aHR 2.03; P = .003) were significantly associated with a higher risk for stroke, respectively.
Study details: This study included 9170 adults with T2D from the ACCORD study, of which 156 experienced stroke events over a median follow-up of 4.9 years.
Disclosures: The study did not declare any source of funding. Dr. G Fonarow reported consulting for various sources.
Source: Kaze AD et al. Diabetic kidney disease and risk of incident stroke among adults with type 2 diabetes. BMC Med. 2022;20:127 (Mar 29). Doi: 10.1186/s12916-022-02317-0
Sustained glycemic control in T2D decreases the likelihood for long-term complications
Key clinical point: Patients with type 2 diabetes (T2D) who maintain glycosylated hemoglobin (HbA1c) levels of <7% vs. ≥7% during a 5-year post-period have a lower risk for diabetes-related complications.
Major finding: Maintaining an HbA1c level of <7% vs. ≥7% during the 5-year post-period was associated with a lower risk of developing cardiovascular disease (odds ratio [OR] 0.76; 95% CI 0.61-0.94), metabolic disease (OR 0.37; 95% CI 0.22-0.60), neuropathy (OR 0.62; 95% CI 0.45-0.84), nephropathy (OR 0.81; 95% CI 0.69-0.94), and peripheral vascular disease (OR 0.52; 95% CI 0.33-0.83).
Study details: Findings are from a retrospective study including 3067 adult patients with T2D and sustained glycemic control (HbA1c <7%; n = 2,119) or sustained suboptimal glycemic control (HbA1c ≥7%; n = 1,488).
Disclosures: This study was funded by Eli Lilly and Company. KS Boye, R Paczkowski, and VT Thieu reported being employees and shareholders of Eli Lilly and MJ Lage received personal compensation from Eli Lilly.
Source: Boye KS et al. The association between sustained HbA1c control and long-term complications among individuals with type 2 diabetes: A retrospective study. Adv Ther. 2022 (Mar 22). Doi: 10.1007/s12325-022-02106-4
Key clinical point: Patients with type 2 diabetes (T2D) who maintain glycosylated hemoglobin (HbA1c) levels of <7% vs. ≥7% during a 5-year post-period have a lower risk for diabetes-related complications.
Major finding: Maintaining an HbA1c level of <7% vs. ≥7% during the 5-year post-period was associated with a lower risk of developing cardiovascular disease (odds ratio [OR] 0.76; 95% CI 0.61-0.94), metabolic disease (OR 0.37; 95% CI 0.22-0.60), neuropathy (OR 0.62; 95% CI 0.45-0.84), nephropathy (OR 0.81; 95% CI 0.69-0.94), and peripheral vascular disease (OR 0.52; 95% CI 0.33-0.83).
Study details: Findings are from a retrospective study including 3067 adult patients with T2D and sustained glycemic control (HbA1c <7%; n = 2,119) or sustained suboptimal glycemic control (HbA1c ≥7%; n = 1,488).
Disclosures: This study was funded by Eli Lilly and Company. KS Boye, R Paczkowski, and VT Thieu reported being employees and shareholders of Eli Lilly and MJ Lage received personal compensation from Eli Lilly.
Source: Boye KS et al. The association between sustained HbA1c control and long-term complications among individuals with type 2 diabetes: A retrospective study. Adv Ther. 2022 (Mar 22). Doi: 10.1007/s12325-022-02106-4
Key clinical point: Patients with type 2 diabetes (T2D) who maintain glycosylated hemoglobin (HbA1c) levels of <7% vs. ≥7% during a 5-year post-period have a lower risk for diabetes-related complications.
Major finding: Maintaining an HbA1c level of <7% vs. ≥7% during the 5-year post-period was associated with a lower risk of developing cardiovascular disease (odds ratio [OR] 0.76; 95% CI 0.61-0.94), metabolic disease (OR 0.37; 95% CI 0.22-0.60), neuropathy (OR 0.62; 95% CI 0.45-0.84), nephropathy (OR 0.81; 95% CI 0.69-0.94), and peripheral vascular disease (OR 0.52; 95% CI 0.33-0.83).
Study details: Findings are from a retrospective study including 3067 adult patients with T2D and sustained glycemic control (HbA1c <7%; n = 2,119) or sustained suboptimal glycemic control (HbA1c ≥7%; n = 1,488).
Disclosures: This study was funded by Eli Lilly and Company. KS Boye, R Paczkowski, and VT Thieu reported being employees and shareholders of Eli Lilly and MJ Lage received personal compensation from Eli Lilly.
Source: Boye KS et al. The association between sustained HbA1c control and long-term complications among individuals with type 2 diabetes: A retrospective study. Adv Ther. 2022 (Mar 22). Doi: 10.1007/s12325-022-02106-4
Severe mental illness raises risk for serious long-term diabetic complications in T2D
Key clinical point: Patients with type 2 diabetes (T2D) and severe mental illness (SMI) are at a higher risk of developing nephropathy, lower limp amputations, and cardiovascular diseases (CVD), but not retinopathy, compared with those with T2D and without SMI.
Major finding: Compared with patients with T2D and without SMI, those with T2D and SMI had a higher incidence rate (IR) of nephropathy (IR ratio [IRR] 1.15; 95% CI 1.12-1.18), amputations (IRR 1.15; 95% CI 1.04-1.28), and CVD (men IRR 1.10; 95% CI 1.06-1.15, and women IRR 1.18; 95% CI 1.13-1.22), but a lower IR of retinopathy (IRR 0.75; 95% CI 0.70-0.81).
Study details: Findings are from a population-based dynamic cohort study including 371,625 patients with T2D, of which 30,102 had a coexisting SMI.
Disclosures: This study did not receive any funding. Some authors declared owning shares or receiving research grants from various sources.
Source: Scheuer SH et al. Severe mental illness and the risk of diabetes complications. A nationwide register-based cohort study. J Clin Endocrinol Metab. 2022 (Mar 31). Doi: 10.1210/clinem/dgac204
Key clinical point: Patients with type 2 diabetes (T2D) and severe mental illness (SMI) are at a higher risk of developing nephropathy, lower limp amputations, and cardiovascular diseases (CVD), but not retinopathy, compared with those with T2D and without SMI.
Major finding: Compared with patients with T2D and without SMI, those with T2D and SMI had a higher incidence rate (IR) of nephropathy (IR ratio [IRR] 1.15; 95% CI 1.12-1.18), amputations (IRR 1.15; 95% CI 1.04-1.28), and CVD (men IRR 1.10; 95% CI 1.06-1.15, and women IRR 1.18; 95% CI 1.13-1.22), but a lower IR of retinopathy (IRR 0.75; 95% CI 0.70-0.81).
Study details: Findings are from a population-based dynamic cohort study including 371,625 patients with T2D, of which 30,102 had a coexisting SMI.
Disclosures: This study did not receive any funding. Some authors declared owning shares or receiving research grants from various sources.
Source: Scheuer SH et al. Severe mental illness and the risk of diabetes complications. A nationwide register-based cohort study. J Clin Endocrinol Metab. 2022 (Mar 31). Doi: 10.1210/clinem/dgac204
Key clinical point: Patients with type 2 diabetes (T2D) and severe mental illness (SMI) are at a higher risk of developing nephropathy, lower limp amputations, and cardiovascular diseases (CVD), but not retinopathy, compared with those with T2D and without SMI.
Major finding: Compared with patients with T2D and without SMI, those with T2D and SMI had a higher incidence rate (IR) of nephropathy (IR ratio [IRR] 1.15; 95% CI 1.12-1.18), amputations (IRR 1.15; 95% CI 1.04-1.28), and CVD (men IRR 1.10; 95% CI 1.06-1.15, and women IRR 1.18; 95% CI 1.13-1.22), but a lower IR of retinopathy (IRR 0.75; 95% CI 0.70-0.81).
Study details: Findings are from a population-based dynamic cohort study including 371,625 patients with T2D, of which 30,102 had a coexisting SMI.
Disclosures: This study did not receive any funding. Some authors declared owning shares or receiving research grants from various sources.
Source: Scheuer SH et al. Severe mental illness and the risk of diabetes complications. A nationwide register-based cohort study. J Clin Endocrinol Metab. 2022 (Mar 31). Doi: 10.1210/clinem/dgac204