User login
Comorbidities, Racial Disparities, and Geographic Differences in Asthma
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
Rising Incidence of Bronchiectasis and Associated Burdens
- Goeminne PC, Hernandez F, Diel R, et al. The economic burden of bronchiectasis – known and unknown: a systematic review. BMC Pulm Med. 2019;19(1):54. doi:10.1186/s12890-019-0818-6
- Cohen R, Shteinberg M. Diagnosis and evaluation of bronchiectasis. Clin Chest Med. 2022;43(1):7-22. doi:10.1016/j.ccm.2021.11.001
- Emmons EE. Bronchiectasis. Medscape. Updated September 15, 2020. Accessed June 24, 2022. https://emedicine.medscape.com/article/296961-overview
- World Populating Ageing 2019: highlights (ST/ESA/SER.A/430). United Nations Department of Economic and Social Affairs, Population Division. Published 2019. Accessed July 28, 2022. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
- O’Donnell AE. Bronchiectasis update. Curr Opin Infect Dis. 2018;31(2):194-198. doi:10.1097/QCO.0000000000000445
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi:10.1183/13993003.00629-2017
- Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377-384. doi:10.1177/1479972317709649
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142(2):432-439. doi:10.1378/chest.11-2209
- Bronchiectasis statistics. British Lung Foundation. Accessed June 24, 2022. https://statistics.blf.org.uk/bronchiectasis
- Ringshausen FC, Rademacher J, Pink I, et al. Increasing bronchiectasis prevalence in Germany, 2009-2017: a population-based cohort study. Eur Respir J. 2019;54(6):1900499. doi:10.1183/13993003.00499-2019
- Aliberti S, Sotigiu G, Lapi F, Gramegna A, Cricelli C, Blasi F. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med. 2020;20(1):15. doi:10.1186/s12890-020-1050-0
- Park DI, Kang S, Choi S. Evaluating the prevalence and incidence of bronchiectasis and nontuberculous mycobacteria in South Korea using the nationwide population data. Int J Environ Res Public Health. 2021;18(17):9029. doi:10.3390/ijerph18179029
- Feng J, Sun L, Sun X, et al. Increasing prevalence and burden of bronchiectasis in urban Chinese adults, 2013-2017: a nationwide population-based cohort study. Respir Res. 2022;23:111. doi:10.1186/s12931-022-02023-8
- Hayoung Choi, H, Yang, B, N. Hyewon et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. Aug 2019, 54 (2) 1900194; doi:10.1183/13993003.00194-2019.
- Goeminne PC, Hernandez F, Diel R, et al. The economic burden of bronchiectasis – known and unknown: a systematic review. BMC Pulm Med. 2019;19(1):54. doi:10.1186/s12890-019-0818-6
- Cohen R, Shteinberg M. Diagnosis and evaluation of bronchiectasis. Clin Chest Med. 2022;43(1):7-22. doi:10.1016/j.ccm.2021.11.001
- Emmons EE. Bronchiectasis. Medscape. Updated September 15, 2020. Accessed June 24, 2022. https://emedicine.medscape.com/article/296961-overview
- World Populating Ageing 2019: highlights (ST/ESA/SER.A/430). United Nations Department of Economic and Social Affairs, Population Division. Published 2019. Accessed July 28, 2022. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
- O’Donnell AE. Bronchiectasis update. Curr Opin Infect Dis. 2018;31(2):194-198. doi:10.1097/QCO.0000000000000445
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi:10.1183/13993003.00629-2017
- Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377-384. doi:10.1177/1479972317709649
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142(2):432-439. doi:10.1378/chest.11-2209
- Bronchiectasis statistics. British Lung Foundation. Accessed June 24, 2022. https://statistics.blf.org.uk/bronchiectasis
- Ringshausen FC, Rademacher J, Pink I, et al. Increasing bronchiectasis prevalence in Germany, 2009-2017: a population-based cohort study. Eur Respir J. 2019;54(6):1900499. doi:10.1183/13993003.00499-2019
- Aliberti S, Sotigiu G, Lapi F, Gramegna A, Cricelli C, Blasi F. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med. 2020;20(1):15. doi:10.1186/s12890-020-1050-0
- Park DI, Kang S, Choi S. Evaluating the prevalence and incidence of bronchiectasis and nontuberculous mycobacteria in South Korea using the nationwide population data. Int J Environ Res Public Health. 2021;18(17):9029. doi:10.3390/ijerph18179029
- Feng J, Sun L, Sun X, et al. Increasing prevalence and burden of bronchiectasis in urban Chinese adults, 2013-2017: a nationwide population-based cohort study. Respir Res. 2022;23:111. doi:10.1186/s12931-022-02023-8
- Hayoung Choi, H, Yang, B, N. Hyewon et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. Aug 2019, 54 (2) 1900194; doi:10.1183/13993003.00194-2019.
- Goeminne PC, Hernandez F, Diel R, et al. The economic burden of bronchiectasis – known and unknown: a systematic review. BMC Pulm Med. 2019;19(1):54. doi:10.1186/s12890-019-0818-6
- Cohen R, Shteinberg M. Diagnosis and evaluation of bronchiectasis. Clin Chest Med. 2022;43(1):7-22. doi:10.1016/j.ccm.2021.11.001
- Emmons EE. Bronchiectasis. Medscape. Updated September 15, 2020. Accessed June 24, 2022. https://emedicine.medscape.com/article/296961-overview
- World Populating Ageing 2019: highlights (ST/ESA/SER.A/430). United Nations Department of Economic and Social Affairs, Population Division. Published 2019. Accessed July 28, 2022. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
- O’Donnell AE. Bronchiectasis update. Curr Opin Infect Dis. 2018;31(2):194-198. doi:10.1097/QCO.0000000000000445
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi:10.1183/13993003.00629-2017
- Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377-384. doi:10.1177/1479972317709649
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142(2):432-439. doi:10.1378/chest.11-2209
- Bronchiectasis statistics. British Lung Foundation. Accessed June 24, 2022. https://statistics.blf.org.uk/bronchiectasis
- Ringshausen FC, Rademacher J, Pink I, et al. Increasing bronchiectasis prevalence in Germany, 2009-2017: a population-based cohort study. Eur Respir J. 2019;54(6):1900499. doi:10.1183/13993003.00499-2019
- Aliberti S, Sotigiu G, Lapi F, Gramegna A, Cricelli C, Blasi F. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med. 2020;20(1):15. doi:10.1186/s12890-020-1050-0
- Park DI, Kang S, Choi S. Evaluating the prevalence and incidence of bronchiectasis and nontuberculous mycobacteria in South Korea using the nationwide population data. Int J Environ Res Public Health. 2021;18(17):9029. doi:10.3390/ijerph18179029
- Feng J, Sun L, Sun X, et al. Increasing prevalence and burden of bronchiectasis in urban Chinese adults, 2013-2017: a nationwide population-based cohort study. Respir Res. 2022;23:111. doi:10.1186/s12931-022-02023-8
- Hayoung Choi, H, Yang, B, N. Hyewon et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. Aug 2019, 54 (2) 1900194; doi:10.1183/13993003.00194-2019.
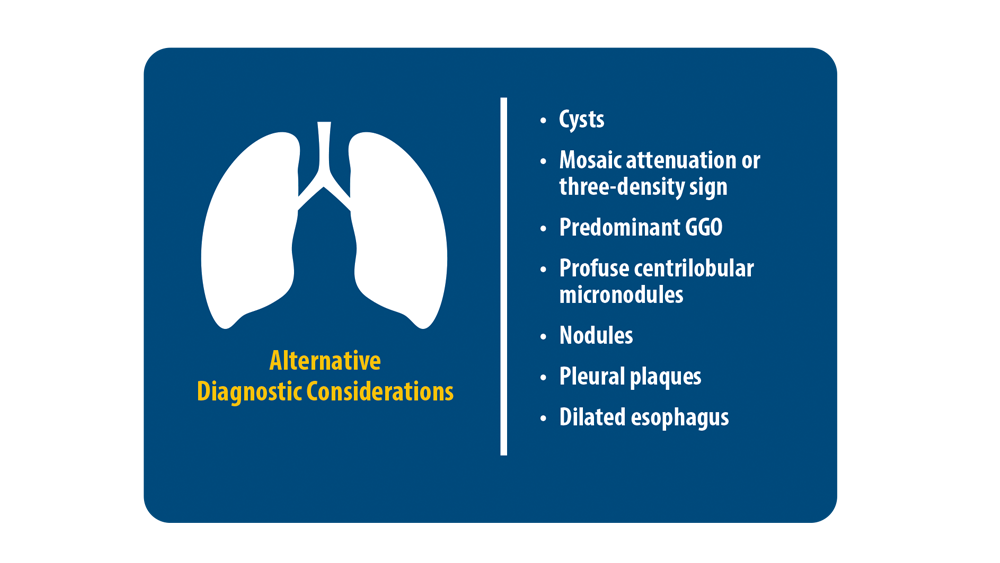
ILD: Diagnostic Considerations and Socioeconomic Barriers
1. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/ rccm.202202-0399ST
2. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report (podcast). Chest. 2021;160(2). Published August 5, 2021. Accessed July 11, 2022. https://www.podbean.com/ew/pb-jgzb7-10980b0
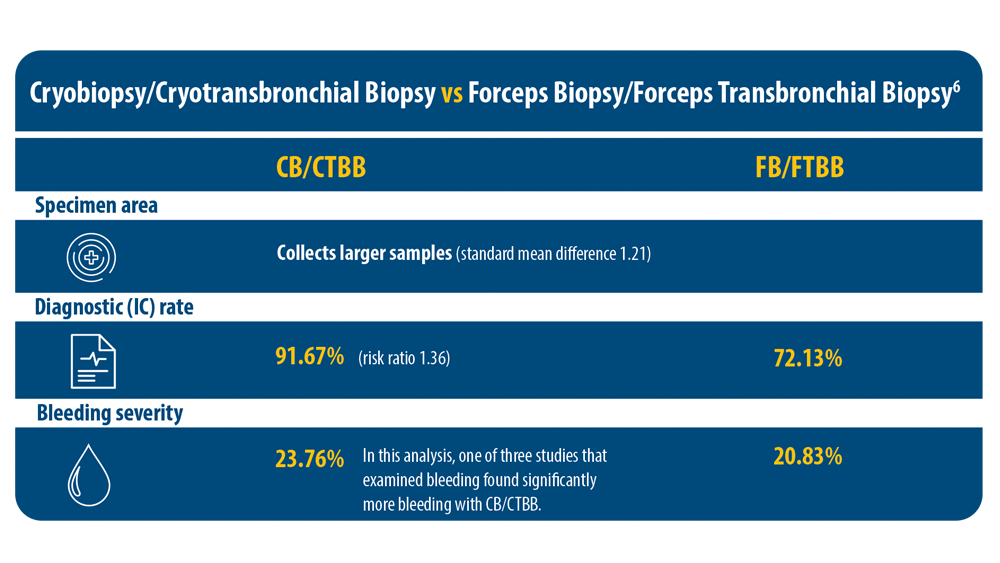
3. Kheir F, Uribe Becerra JP, Bissell B, et al. Transbronchial lung cryobiopsy in patients with interstitial lung disease: a systematic review. Ann Am Thorac Soc. 2022;19(7):1193-1202. doi:10.1513/ AnnalsATS.202102-198OC
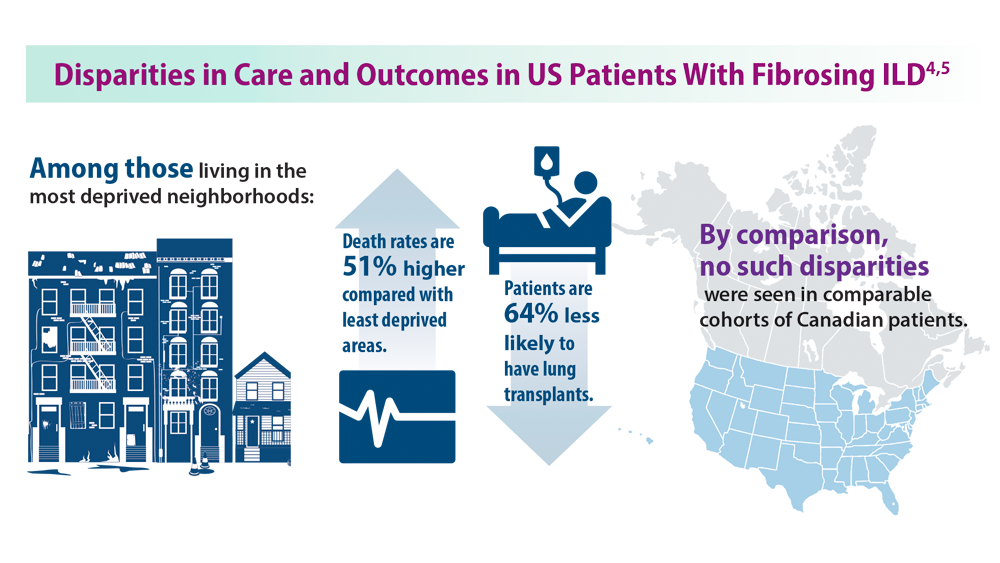
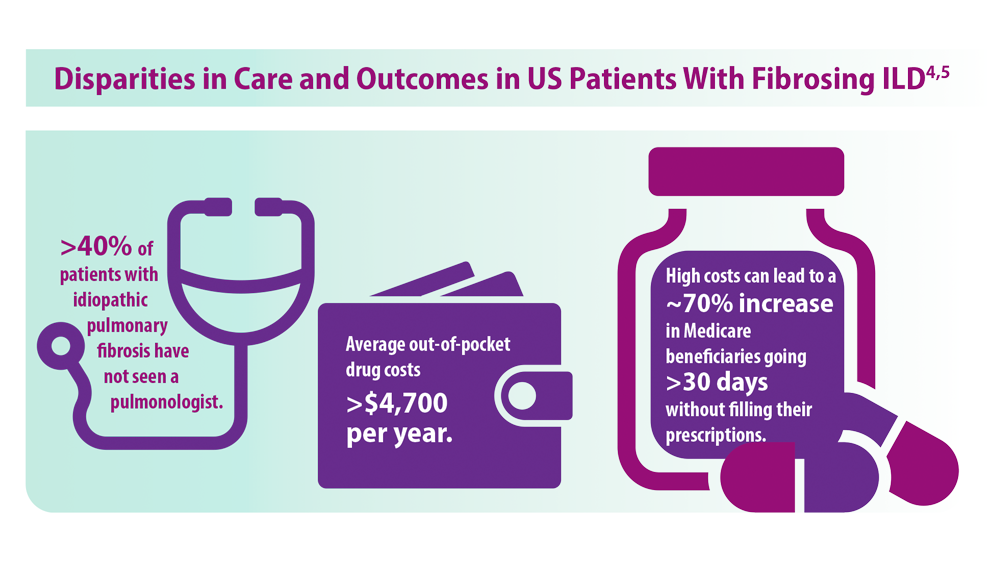
4. Goobie GC, Ryerson CJ, Johannson KA, et al. Neighborhoodlevel disadvantage impacts on patients with fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2022;205(4):459-467. doi:10.1164/rccm.202109-2065OC
5. Gaffney AW, Podolanczuk AJ. Inequity and the interstitium: pushing back on disparities in fibrosing lung disease in the United States and Canada. Am J Respir Crit Care Med. 2022;205(4):385-387. doi:10.1164/rccm.202111-2652ED
6. Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology. 2016;21(5):834-841. doi:10.1111/resp.12770
1. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/ rccm.202202-0399ST
2. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report (podcast). Chest. 2021;160(2). Published August 5, 2021. Accessed July 11, 2022. https://www.podbean.com/ew/pb-jgzb7-10980b0
3. Kheir F, Uribe Becerra JP, Bissell B, et al. Transbronchial lung cryobiopsy in patients with interstitial lung disease: a systematic review. Ann Am Thorac Soc. 2022;19(7):1193-1202. doi:10.1513/ AnnalsATS.202102-198OC
4. Goobie GC, Ryerson CJ, Johannson KA, et al. Neighborhoodlevel disadvantage impacts on patients with fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2022;205(4):459-467. doi:10.1164/rccm.202109-2065OC
5. Gaffney AW, Podolanczuk AJ. Inequity and the interstitium: pushing back on disparities in fibrosing lung disease in the United States and Canada. Am J Respir Crit Care Med. 2022;205(4):385-387. doi:10.1164/rccm.202111-2652ED
6. Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology. 2016;21(5):834-841. doi:10.1111/resp.12770
1. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/ rccm.202202-0399ST
2. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report (podcast). Chest. 2021;160(2). Published August 5, 2021. Accessed July 11, 2022. https://www.podbean.com/ew/pb-jgzb7-10980b0
3. Kheir F, Uribe Becerra JP, Bissell B, et al. Transbronchial lung cryobiopsy in patients with interstitial lung disease: a systematic review. Ann Am Thorac Soc. 2022;19(7):1193-1202. doi:10.1513/ AnnalsATS.202102-198OC
4. Goobie GC, Ryerson CJ, Johannson KA, et al. Neighborhoodlevel disadvantage impacts on patients with fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2022;205(4):459-467. doi:10.1164/rccm.202109-2065OC
5. Gaffney AW, Podolanczuk AJ. Inequity and the interstitium: pushing back on disparities in fibrosing lung disease in the United States and Canada. Am J Respir Crit Care Med. 2022;205(4):385-387. doi:10.1164/rccm.202111-2652ED
6. Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology. 2016;21(5):834-841. doi:10.1111/resp.12770
Key Presentations in Lung Cancer From CHEST 2022
The 2022 CHEST Annual Meeting had several important studies on lung cancer.
Douglas Arenberg, MD, FCCP from the University of Michigan Northville Health Center, reports on content from two papers that focus on the first million persons to have been screened for lung cancer after the initial launch of the American College of Radiology Lung Cancer Screening Registry. The research showed that the medical community is, in fact, doing well in some areas of lung cancer screening but that improvements need to be made in order to reach former tobacco users, who would greatly benefit from these screenings.
He also highlights a series of studies that were discussed regarding smoking cessation at lung evaluations known as the SCALE Collaboration. Effective smoking interventions could enhance the benefits of lung cancer screening by reducing mortality and morbidity resulting from lung cancer.
Finally, Dr Arenberg shares a series of presentations that highlight how the surgical treatment of early-stage lung cancer is creating significant changes in the standard of care.
--
Douglas Arenberg, MD, FCCP Professor of Medicine, Department of Internal Medicine, Division of Pulmonary & Critical Care, University of Michigan; Director of Bronchoscopy and Medical Director for the Lung Cancer Screening and Lung Nodule Clinics, University of Michigan, Ann Arbor, Michigan
Douglas Arenberg, MD, FCCP has disclosed no relevant financial relationships.
The 2022 CHEST Annual Meeting had several important studies on lung cancer.
Douglas Arenberg, MD, FCCP from the University of Michigan Northville Health Center, reports on content from two papers that focus on the first million persons to have been screened for lung cancer after the initial launch of the American College of Radiology Lung Cancer Screening Registry. The research showed that the medical community is, in fact, doing well in some areas of lung cancer screening but that improvements need to be made in order to reach former tobacco users, who would greatly benefit from these screenings.
He also highlights a series of studies that were discussed regarding smoking cessation at lung evaluations known as the SCALE Collaboration. Effective smoking interventions could enhance the benefits of lung cancer screening by reducing mortality and morbidity resulting from lung cancer.
Finally, Dr Arenberg shares a series of presentations that highlight how the surgical treatment of early-stage lung cancer is creating significant changes in the standard of care.
--
Douglas Arenberg, MD, FCCP Professor of Medicine, Department of Internal Medicine, Division of Pulmonary & Critical Care, University of Michigan; Director of Bronchoscopy and Medical Director for the Lung Cancer Screening and Lung Nodule Clinics, University of Michigan, Ann Arbor, Michigan
Douglas Arenberg, MD, FCCP has disclosed no relevant financial relationships.
The 2022 CHEST Annual Meeting had several important studies on lung cancer.
Douglas Arenberg, MD, FCCP from the University of Michigan Northville Health Center, reports on content from two papers that focus on the first million persons to have been screened for lung cancer after the initial launch of the American College of Radiology Lung Cancer Screening Registry. The research showed that the medical community is, in fact, doing well in some areas of lung cancer screening but that improvements need to be made in order to reach former tobacco users, who would greatly benefit from these screenings.
He also highlights a series of studies that were discussed regarding smoking cessation at lung evaluations known as the SCALE Collaboration. Effective smoking interventions could enhance the benefits of lung cancer screening by reducing mortality and morbidity resulting from lung cancer.
Finally, Dr Arenberg shares a series of presentations that highlight how the surgical treatment of early-stage lung cancer is creating significant changes in the standard of care.
--
Douglas Arenberg, MD, FCCP Professor of Medicine, Department of Internal Medicine, Division of Pulmonary & Critical Care, University of Michigan; Director of Bronchoscopy and Medical Director for the Lung Cancer Screening and Lung Nodule Clinics, University of Michigan, Ann Arbor, Michigan
Douglas Arenberg, MD, FCCP has disclosed no relevant financial relationships.

Should every scheduled cesarean birth use an Enhanced Recovery after Surgery (ERAS) pathway?

Cesarean birth is one of the most common major surgical procedures performed in developed countries1 with over 1,170,000 cesarean births in the United States in 2021.2 Many surgeons and anesthesiologists believe that Enhanced Recovery after Surgery (ERAS) pathways improve surgical outcomes.3,4 Important goals of ERAS include setting patient expectations for the surgical procedure, accelerating patient recovery to full function, and minimizing perioperative complications such as severe nausea, aspiration, surgical site infection, wound complications, and perioperative anemia. The ERAS Society in 20185-7 and the Society for Obstetric Anesthesia and Perinatology (SOAP) in 20218 proposed ERAS pathways for cesarean birth. Both societies recommended that obstetric units consider adopting an ERAS pathway compatible with local clinical resources. In addition, the American College of Obstetricians and Gynecologists (ACOG) has provided guidance for implementing ERAS pathways for gynecologic surgery.9 The consistent use of standardized protocols to improve surgical care in obstetrics should lead to a reduction in care variation and improve health equity outcomes.
The clinical interventions recommended for ERAS cesarean birth occur sequentially in the preoperative, intraoperative, and postoperative phases of care. The recommendations associated with each of these phases are reviewed below. It is important to note that each obstetric unit should use a multidisciplinary process to develop an ERAS pathway that best supports local practice given clinician preferences, patient characteristics, and resource availability.
Preoperative components of ERAS
Standardized patient education (SPE). SPE is an important component of ERAS, although evidence to support the recommendation is limited. At a minimum a written handout describing steps in the cesarean birth process, or a patient-education video should be part of patient education. The University of Michigan Medical Center has produced a 3-minute video for patients explaining ERAS cesarean birth.10 The University of Maryland Medical Center has produced a 2.5-minute video in English and Spanish, explaining ERAS cesarean birth for patients.11 Some surgeons place a telephone call to patients the evening before surgery to help orient the patient to ERAS cesarean birth.
Breastfeeding education. An important goal of obstetric care is to optimize the rate of exclusive breastfeeding at birth. Breastfeeding education, including a commitment to support the initiation of breastfeeding within 1 hour of birth, may enhance the rate of exclusive breastfeeding. There are numerous videos available for patients about breastfeeding after cesarean birth (as an example, see: https://www.youtube.com/watch?v=9iOGn85NdTg).
Limit fasting. In the past, surgical guidelines recommended fasting after midnight prior to surgery. The ERAS Society recommends that patients should be encouraged to drink clear fluids up to 2 hours before surgery and may have a light meal up to 6 hours before surgery (Part 1).
Carbohydrate loading. Surgery causes a metabolic stress that is increased by fasting. Carbohydrate loading prior to surgery reduces the magnitude of the catabolic state caused by the combination of surgery and fasting.12 SOAP and the ERAS Society recommend oral carbohydrate fluid supplementation 2 hours before surgery for nondiabetic patients. SOAP suggests 32 oz of Gatorade or 16 oz of clear apple juice as options for carbohydrate loading. For diabetic patients, the carbohydrate load can be omitted. In fasting pregnant patients at term, gastric emptying was near complete 2 hours after consumption of 400 mL of a carbohydrate drink.13 In one study, consumption of 400 mL of a carbohydrate drink 2 hours before cesarean resulted in a 7% increase in the newborn blood glucose level at 20 min after delivery.14
Minimize preoperative anemia. Approximately 50% of pregnant women are iron deficient and approximately 10% are anemic in the third trimester.15,16 Cesarean birth is associated with significant blood loss necessitating the need to optimize red blood cell mass before surgery. Measuring ferritin to identify patients with iron deficiency and aggressive iron replacement, including intravenous iron if necessary, will reduce the prevalence of anemia prior to cesarean birth.17 Another cause of anemia in pregnancy is vitamin B12 (cobalamin) deficiency. Low vitamin B12 is especially common in pregnant patients who have previously had bariatric surgery. One study reported that, of 113 pregnant patients who were, on average, 3 years from a bariatric surgery procedure, 12% had vitamin B12 circulating levels < 130 pg/mL.18 Among pregnant patients who are anemic, and do not have a hemoglobinopathy, measuring ferritin, folic acid, and vitamin B12 will help identify the cause of anemia and guide treatment.19
Optimize preoperative physical condition. Improving healthy behaviors and reducing unhealthy behaviors preoperatively may enhance patient recovery to full function. In the weeks before scheduled cesarean birth, cessation of the use of tobacco products, optimizing activity and improving diet quality, including increasing protein intake, may best prepare patients for the metabolic stress of surgery.
Continue to: Intraoperative components of ERAS...
Intraoperative components of ERAS
Reduce the risk of surgical site infection (SSI) and wound complications. Bundles that include antibiotics, chlorhexidine (or an alternative antibacterial soap) and clippers have been shown to reduce SSI.20 Routine administration of preoperative antibiotics is a consensus recommendation and there is high adherence with this recommendation in the United States. Chlorhexidine-alcohol is the preferred solution for skin preparation. Vaginal preparation with povidine-iodine or chlorhexidine may be considered.6
Surgical technique. Blunt extension of a transverse hysterotomy may reduce blood loss. Closure of the hysterotomy incision in 2 layers is recommended to reduce uterine scar dehiscence in a subsequent pregnancy. If the patient has ≥2 cm of subcutaneous tissue, this layer should be approximated with sutures. Skin closure should be with subcuticular suture.6
Optimize uterotonic administration. Routine use of uterotonics reduces the risk of blood loss, transfusion, and postoperative anemia. There is high adherence with the use of uterotonic administration after birth in the United States.6,8
Ensure normothermia. Many patients become hypothermic during a cesarean birth. Active warming of the patient with an in-line IV fluid warmer and forced air warming over the patient’s body can reduce the risk of hypothermia.8
Initiate multimodal anesthesia. Anesthesiologists often use intrathecal or epidural morphine to enhance analgesia. Ketorolac administration prior to completion of the cesarean procedure and perioperative administration of acetaminophen may reduce postoperative pain.8 The use of preoperative antiemetics will reduce intraoperative and postoperative nausea and vomiting.
Initiate VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.6
Postoperative components of ERAS
Patient education to prepare for discharge home when ready. Patient education focused on home when ready is important in preparing the patient for discharge home.7 Completion of required newborn testing, lactation education, and contraception planning plus coordination of newborn pediatric follow-up is necessary before discharge.
Support early return of bowel function. Early return of bowel function is best supported by a multimodal approach including initiation of clear fluid intake immediately following surgery, encouraging consumption of a regular diet within 27 to 4 hours8 following surgery. Gum chewing for at least 5 minutes 3 times daily accelerates return of bowel function.8 In a meta-analysis of 10 randomized studies examining the effect of gum chewing after cesarean, the investigators reported that gum chewing shortened the time to passage of flatus and defecation.21
Early ambulation.
Sequentially advanced activity, starting with sitting on the edge of the bed, sitting in a chair, and ambulation within 8 hours of surgery, is recommended to facilitate faster recovery, reduce rates of complications, and enable transition to home.8
Early removal of the urinary catheter. It is recommended that the urinary catheter be removed within 12 hours after cesarean birth.8 Early removal of the urinary catheter increases patient mobility and reduces the length of hospitalization. Early removal of the urinary catheter may be associated with postoperative urinary retention and recatheterization in a small number of patients.
Prescribe routinely scheduled acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and ketorolac. A key component of ERAS cesarean birth is the standardized administration of nonopioid pain medicines, alternating doses of acetaminophen and an NSAID. ERAS cesarean birth is likely to result in a reduction in inpatient and postdischarge opioid use.22-24
VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.8
Auditing and reporting adherence with components of ERAS
In clinical practice there may be a gap between a clinician’s subjective perception of their performance and an independent audit of their clinical performance. ERAS pathways should be implemented with a commitment to performing audits and providing quantitative feedback to clinicians. Consistent use of measurement, feedback, and coaching can improve performance and reduce variation among individual clinicians. As an example, in one study of the use of a surgical safety checklist, 99% of the surgeons reported that they routinely used a surgical safety checklist, but the audit showed that the checklist was used in only 60% of cases.25 Gaps between self-reported performance and audited performance are common in clinical practice. Audits with feedback are critical to improving adherence with the components of an ERAS pathway.
Three independent systematic reviews and meta-analyses report that ERAS pathways reduce hospital length of stay without increasing the readmission rate.26-28 One meta-analysis reported that ERAS may also reduce time to first mobilization and result in earlier removal of the urinary catheter.26 ERAS pathways also may reduce postoperative complications, lower pain scores, and decrease opioid use.27 The general consensus among quality and safety experts is that reducing variation through standardization of pathways is generally associated with improved quality and enhanced safety. ERAS pathways have been widely accepted in multiple surgical fields. ERAS pathways should become the standard for performing cesarean procedures.●
1. Molina G, Weiser RG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314:2263-2270.
2. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. Vital Statistics Release; No. 20. Hyattsville, MD: National Center for Health Statistics. May 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf.
3. Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;486-493.
4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-298.
5. Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 1). Am J Obstet Gynecol. 2018;219:523.e1-523.e15.
6. Caughey AB, Wood SL, Macones GA, et al Guidelines for intraoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 2). Am J Obstet Gynecol. 2018;219:533-544.
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 3). Am J Obstet Gynecol. 2019;221:247.e1-247.e9.
8. Bollag L, Lim G, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology: Consensus statement and recommendations for enhanced recovery after cesarean. Anesth Analg. 2021;132:1362-1377.
9. Perioperative pathways: enhanced recovery after surgery. ACOG Committee Opinion No 750. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e120-130.
10. University of Michigan. ERAS: A patient education video. https://www.youtube.com/watch?v=CoFtgdluBc0. Accessed October 24, 2022.
11. University of Maryland. ERAS. https://www.umms.org/ummc/health-services/womens-health/ostetrics-gynecology/pregnancy-childbirth/labor-delivery/enhanced-recovery-after-cesarean. Accessed October 24, 2022.
12. Bilku DK, Dennison AR, Hall TC, et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
13. Popivanov P, Irwin R, Walsh M, et al. Gastric emptying of carbohydrate drinks in term parturients before elective caesarean surgery: an observational study. Int J Obstet Anesth. 2020;41:29-34.
14. He Y, Liu C, Han Y, et al. The impact of carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes- a randomized clinical trial. BMC Pregnancy Childbirth. 2021;21:682.
15. Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
16. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
17. Nour N, Barbieri RL. Optimize detection and treatment of iron deficiency in pregnancy. OBG Manag. 2022;34:9-11.
18. Mead NC, Sakkatos P, Sakellaropoulos GC, et al. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis. 2014;10:1166-1173.
19. Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin and folate. Blood. 2017;129:940-949.
20. Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:735-746.
21. Wen Z, Shen M, Wu C, et al. Chewing gum for intestinal function recovery after caesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:105.
22. McCoy JA, Gutman S, Hamm RF, et al. The association between implementation of an enhanced recovery after cesarean pathway with standardized discharge prescriptions and opioid use and pain experience after cesarean delivery. Am J Perinatol. 2021;38:1341-1347.
23. Mullman L, Hilden P, Goral J, et al. Improved outcomes with an enhanced recovery approach to cesarean delivery. Obstet Gynecol. 2020;136:685-691.
24. Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
25. Sendlhofer G, Lumenta DB, Leitgeb K, et al. The gap between individual perception and compliance: a quantitative follow-up study of the surgical safety checklist application. PLoS One. 2016;11:e0149212.
26. Sultan P, Sharawi N, Blake L, et al. Impact of enhanced recovery after cesarean delivery on maternal outcomes: a systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2021;40:100935.
27. Meng X, Chen K, Yang C, et al. The clinical efficacy and safety of enhanced recovery after surgery for cesarean section: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Med. 2021;8:694385.
28. Corson E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols and an umbrella review of systematic reviews. BMC Pregnancy Childbirth. 2017;17:91.

Cesarean birth is one of the most common major surgical procedures performed in developed countries1 with over 1,170,000 cesarean births in the United States in 2021.2 Many surgeons and anesthesiologists believe that Enhanced Recovery after Surgery (ERAS) pathways improve surgical outcomes.3,4 Important goals of ERAS include setting patient expectations for the surgical procedure, accelerating patient recovery to full function, and minimizing perioperative complications such as severe nausea, aspiration, surgical site infection, wound complications, and perioperative anemia. The ERAS Society in 20185-7 and the Society for Obstetric Anesthesia and Perinatology (SOAP) in 20218 proposed ERAS pathways for cesarean birth. Both societies recommended that obstetric units consider adopting an ERAS pathway compatible with local clinical resources. In addition, the American College of Obstetricians and Gynecologists (ACOG) has provided guidance for implementing ERAS pathways for gynecologic surgery.9 The consistent use of standardized protocols to improve surgical care in obstetrics should lead to a reduction in care variation and improve health equity outcomes.
The clinical interventions recommended for ERAS cesarean birth occur sequentially in the preoperative, intraoperative, and postoperative phases of care. The recommendations associated with each of these phases are reviewed below. It is important to note that each obstetric unit should use a multidisciplinary process to develop an ERAS pathway that best supports local practice given clinician preferences, patient characteristics, and resource availability.
Preoperative components of ERAS
Standardized patient education (SPE). SPE is an important component of ERAS, although evidence to support the recommendation is limited. At a minimum a written handout describing steps in the cesarean birth process, or a patient-education video should be part of patient education. The University of Michigan Medical Center has produced a 3-minute video for patients explaining ERAS cesarean birth.10 The University of Maryland Medical Center has produced a 2.5-minute video in English and Spanish, explaining ERAS cesarean birth for patients.11 Some surgeons place a telephone call to patients the evening before surgery to help orient the patient to ERAS cesarean birth.
Breastfeeding education. An important goal of obstetric care is to optimize the rate of exclusive breastfeeding at birth. Breastfeeding education, including a commitment to support the initiation of breastfeeding within 1 hour of birth, may enhance the rate of exclusive breastfeeding. There are numerous videos available for patients about breastfeeding after cesarean birth (as an example, see: https://www.youtube.com/watch?v=9iOGn85NdTg).
Limit fasting. In the past, surgical guidelines recommended fasting after midnight prior to surgery. The ERAS Society recommends that patients should be encouraged to drink clear fluids up to 2 hours before surgery and may have a light meal up to 6 hours before surgery (Part 1).
Carbohydrate loading. Surgery causes a metabolic stress that is increased by fasting. Carbohydrate loading prior to surgery reduces the magnitude of the catabolic state caused by the combination of surgery and fasting.12 SOAP and the ERAS Society recommend oral carbohydrate fluid supplementation 2 hours before surgery for nondiabetic patients. SOAP suggests 32 oz of Gatorade or 16 oz of clear apple juice as options for carbohydrate loading. For diabetic patients, the carbohydrate load can be omitted. In fasting pregnant patients at term, gastric emptying was near complete 2 hours after consumption of 400 mL of a carbohydrate drink.13 In one study, consumption of 400 mL of a carbohydrate drink 2 hours before cesarean resulted in a 7% increase in the newborn blood glucose level at 20 min after delivery.14
Minimize preoperative anemia. Approximately 50% of pregnant women are iron deficient and approximately 10% are anemic in the third trimester.15,16 Cesarean birth is associated with significant blood loss necessitating the need to optimize red blood cell mass before surgery. Measuring ferritin to identify patients with iron deficiency and aggressive iron replacement, including intravenous iron if necessary, will reduce the prevalence of anemia prior to cesarean birth.17 Another cause of anemia in pregnancy is vitamin B12 (cobalamin) deficiency. Low vitamin B12 is especially common in pregnant patients who have previously had bariatric surgery. One study reported that, of 113 pregnant patients who were, on average, 3 years from a bariatric surgery procedure, 12% had vitamin B12 circulating levels < 130 pg/mL.18 Among pregnant patients who are anemic, and do not have a hemoglobinopathy, measuring ferritin, folic acid, and vitamin B12 will help identify the cause of anemia and guide treatment.19
Optimize preoperative physical condition. Improving healthy behaviors and reducing unhealthy behaviors preoperatively may enhance patient recovery to full function. In the weeks before scheduled cesarean birth, cessation of the use of tobacco products, optimizing activity and improving diet quality, including increasing protein intake, may best prepare patients for the metabolic stress of surgery.
Continue to: Intraoperative components of ERAS...
Intraoperative components of ERAS
Reduce the risk of surgical site infection (SSI) and wound complications. Bundles that include antibiotics, chlorhexidine (or an alternative antibacterial soap) and clippers have been shown to reduce SSI.20 Routine administration of preoperative antibiotics is a consensus recommendation and there is high adherence with this recommendation in the United States. Chlorhexidine-alcohol is the preferred solution for skin preparation. Vaginal preparation with povidine-iodine or chlorhexidine may be considered.6
Surgical technique. Blunt extension of a transverse hysterotomy may reduce blood loss. Closure of the hysterotomy incision in 2 layers is recommended to reduce uterine scar dehiscence in a subsequent pregnancy. If the patient has ≥2 cm of subcutaneous tissue, this layer should be approximated with sutures. Skin closure should be with subcuticular suture.6
Optimize uterotonic administration. Routine use of uterotonics reduces the risk of blood loss, transfusion, and postoperative anemia. There is high adherence with the use of uterotonic administration after birth in the United States.6,8
Ensure normothermia. Many patients become hypothermic during a cesarean birth. Active warming of the patient with an in-line IV fluid warmer and forced air warming over the patient’s body can reduce the risk of hypothermia.8
Initiate multimodal anesthesia. Anesthesiologists often use intrathecal or epidural morphine to enhance analgesia. Ketorolac administration prior to completion of the cesarean procedure and perioperative administration of acetaminophen may reduce postoperative pain.8 The use of preoperative antiemetics will reduce intraoperative and postoperative nausea and vomiting.
Initiate VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.6
Postoperative components of ERAS
Patient education to prepare for discharge home when ready. Patient education focused on home when ready is important in preparing the patient for discharge home.7 Completion of required newborn testing, lactation education, and contraception planning plus coordination of newborn pediatric follow-up is necessary before discharge.
Support early return of bowel function. Early return of bowel function is best supported by a multimodal approach including initiation of clear fluid intake immediately following surgery, encouraging consumption of a regular diet within 27 to 4 hours8 following surgery. Gum chewing for at least 5 minutes 3 times daily accelerates return of bowel function.8 In a meta-analysis of 10 randomized studies examining the effect of gum chewing after cesarean, the investigators reported that gum chewing shortened the time to passage of flatus and defecation.21
Early ambulation.
Sequentially advanced activity, starting with sitting on the edge of the bed, sitting in a chair, and ambulation within 8 hours of surgery, is recommended to facilitate faster recovery, reduce rates of complications, and enable transition to home.8
Early removal of the urinary catheter. It is recommended that the urinary catheter be removed within 12 hours after cesarean birth.8 Early removal of the urinary catheter increases patient mobility and reduces the length of hospitalization. Early removal of the urinary catheter may be associated with postoperative urinary retention and recatheterization in a small number of patients.
Prescribe routinely scheduled acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and ketorolac. A key component of ERAS cesarean birth is the standardized administration of nonopioid pain medicines, alternating doses of acetaminophen and an NSAID. ERAS cesarean birth is likely to result in a reduction in inpatient and postdischarge opioid use.22-24
VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.8
Auditing and reporting adherence with components of ERAS
In clinical practice there may be a gap between a clinician’s subjective perception of their performance and an independent audit of their clinical performance. ERAS pathways should be implemented with a commitment to performing audits and providing quantitative feedback to clinicians. Consistent use of measurement, feedback, and coaching can improve performance and reduce variation among individual clinicians. As an example, in one study of the use of a surgical safety checklist, 99% of the surgeons reported that they routinely used a surgical safety checklist, but the audit showed that the checklist was used in only 60% of cases.25 Gaps between self-reported performance and audited performance are common in clinical practice. Audits with feedback are critical to improving adherence with the components of an ERAS pathway.
Three independent systematic reviews and meta-analyses report that ERAS pathways reduce hospital length of stay without increasing the readmission rate.26-28 One meta-analysis reported that ERAS may also reduce time to first mobilization and result in earlier removal of the urinary catheter.26 ERAS pathways also may reduce postoperative complications, lower pain scores, and decrease opioid use.27 The general consensus among quality and safety experts is that reducing variation through standardization of pathways is generally associated with improved quality and enhanced safety. ERAS pathways have been widely accepted in multiple surgical fields. ERAS pathways should become the standard for performing cesarean procedures.●

Cesarean birth is one of the most common major surgical procedures performed in developed countries1 with over 1,170,000 cesarean births in the United States in 2021.2 Many surgeons and anesthesiologists believe that Enhanced Recovery after Surgery (ERAS) pathways improve surgical outcomes.3,4 Important goals of ERAS include setting patient expectations for the surgical procedure, accelerating patient recovery to full function, and minimizing perioperative complications such as severe nausea, aspiration, surgical site infection, wound complications, and perioperative anemia. The ERAS Society in 20185-7 and the Society for Obstetric Anesthesia and Perinatology (SOAP) in 20218 proposed ERAS pathways for cesarean birth. Both societies recommended that obstetric units consider adopting an ERAS pathway compatible with local clinical resources. In addition, the American College of Obstetricians and Gynecologists (ACOG) has provided guidance for implementing ERAS pathways for gynecologic surgery.9 The consistent use of standardized protocols to improve surgical care in obstetrics should lead to a reduction in care variation and improve health equity outcomes.
The clinical interventions recommended for ERAS cesarean birth occur sequentially in the preoperative, intraoperative, and postoperative phases of care. The recommendations associated with each of these phases are reviewed below. It is important to note that each obstetric unit should use a multidisciplinary process to develop an ERAS pathway that best supports local practice given clinician preferences, patient characteristics, and resource availability.
Preoperative components of ERAS
Standardized patient education (SPE). SPE is an important component of ERAS, although evidence to support the recommendation is limited. At a minimum a written handout describing steps in the cesarean birth process, or a patient-education video should be part of patient education. The University of Michigan Medical Center has produced a 3-minute video for patients explaining ERAS cesarean birth.10 The University of Maryland Medical Center has produced a 2.5-minute video in English and Spanish, explaining ERAS cesarean birth for patients.11 Some surgeons place a telephone call to patients the evening before surgery to help orient the patient to ERAS cesarean birth.
Breastfeeding education. An important goal of obstetric care is to optimize the rate of exclusive breastfeeding at birth. Breastfeeding education, including a commitment to support the initiation of breastfeeding within 1 hour of birth, may enhance the rate of exclusive breastfeeding. There are numerous videos available for patients about breastfeeding after cesarean birth (as an example, see: https://www.youtube.com/watch?v=9iOGn85NdTg).
Limit fasting. In the past, surgical guidelines recommended fasting after midnight prior to surgery. The ERAS Society recommends that patients should be encouraged to drink clear fluids up to 2 hours before surgery and may have a light meal up to 6 hours before surgery (Part 1).
Carbohydrate loading. Surgery causes a metabolic stress that is increased by fasting. Carbohydrate loading prior to surgery reduces the magnitude of the catabolic state caused by the combination of surgery and fasting.12 SOAP and the ERAS Society recommend oral carbohydrate fluid supplementation 2 hours before surgery for nondiabetic patients. SOAP suggests 32 oz of Gatorade or 16 oz of clear apple juice as options for carbohydrate loading. For diabetic patients, the carbohydrate load can be omitted. In fasting pregnant patients at term, gastric emptying was near complete 2 hours after consumption of 400 mL of a carbohydrate drink.13 In one study, consumption of 400 mL of a carbohydrate drink 2 hours before cesarean resulted in a 7% increase in the newborn blood glucose level at 20 min after delivery.14
Minimize preoperative anemia. Approximately 50% of pregnant women are iron deficient and approximately 10% are anemic in the third trimester.15,16 Cesarean birth is associated with significant blood loss necessitating the need to optimize red blood cell mass before surgery. Measuring ferritin to identify patients with iron deficiency and aggressive iron replacement, including intravenous iron if necessary, will reduce the prevalence of anemia prior to cesarean birth.17 Another cause of anemia in pregnancy is vitamin B12 (cobalamin) deficiency. Low vitamin B12 is especially common in pregnant patients who have previously had bariatric surgery. One study reported that, of 113 pregnant patients who were, on average, 3 years from a bariatric surgery procedure, 12% had vitamin B12 circulating levels < 130 pg/mL.18 Among pregnant patients who are anemic, and do not have a hemoglobinopathy, measuring ferritin, folic acid, and vitamin B12 will help identify the cause of anemia and guide treatment.19
Optimize preoperative physical condition. Improving healthy behaviors and reducing unhealthy behaviors preoperatively may enhance patient recovery to full function. In the weeks before scheduled cesarean birth, cessation of the use of tobacco products, optimizing activity and improving diet quality, including increasing protein intake, may best prepare patients for the metabolic stress of surgery.
Continue to: Intraoperative components of ERAS...
Intraoperative components of ERAS
Reduce the risk of surgical site infection (SSI) and wound complications. Bundles that include antibiotics, chlorhexidine (or an alternative antibacterial soap) and clippers have been shown to reduce SSI.20 Routine administration of preoperative antibiotics is a consensus recommendation and there is high adherence with this recommendation in the United States. Chlorhexidine-alcohol is the preferred solution for skin preparation. Vaginal preparation with povidine-iodine or chlorhexidine may be considered.6
Surgical technique. Blunt extension of a transverse hysterotomy may reduce blood loss. Closure of the hysterotomy incision in 2 layers is recommended to reduce uterine scar dehiscence in a subsequent pregnancy. If the patient has ≥2 cm of subcutaneous tissue, this layer should be approximated with sutures. Skin closure should be with subcuticular suture.6
Optimize uterotonic administration. Routine use of uterotonics reduces the risk of blood loss, transfusion, and postoperative anemia. There is high adherence with the use of uterotonic administration after birth in the United States.6,8
Ensure normothermia. Many patients become hypothermic during a cesarean birth. Active warming of the patient with an in-line IV fluid warmer and forced air warming over the patient’s body can reduce the risk of hypothermia.8
Initiate multimodal anesthesia. Anesthesiologists often use intrathecal or epidural morphine to enhance analgesia. Ketorolac administration prior to completion of the cesarean procedure and perioperative administration of acetaminophen may reduce postoperative pain.8 The use of preoperative antiemetics will reduce intraoperative and postoperative nausea and vomiting.
Initiate VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.6
Postoperative components of ERAS
Patient education to prepare for discharge home when ready. Patient education focused on home when ready is important in preparing the patient for discharge home.7 Completion of required newborn testing, lactation education, and contraception planning plus coordination of newborn pediatric follow-up is necessary before discharge.
Support early return of bowel function. Early return of bowel function is best supported by a multimodal approach including initiation of clear fluid intake immediately following surgery, encouraging consumption of a regular diet within 27 to 4 hours8 following surgery. Gum chewing for at least 5 minutes 3 times daily accelerates return of bowel function.8 In a meta-analysis of 10 randomized studies examining the effect of gum chewing after cesarean, the investigators reported that gum chewing shortened the time to passage of flatus and defecation.21
Early ambulation.
Sequentially advanced activity, starting with sitting on the edge of the bed, sitting in a chair, and ambulation within 8 hours of surgery, is recommended to facilitate faster recovery, reduce rates of complications, and enable transition to home.8
Early removal of the urinary catheter. It is recommended that the urinary catheter be removed within 12 hours after cesarean birth.8 Early removal of the urinary catheter increases patient mobility and reduces the length of hospitalization. Early removal of the urinary catheter may be associated with postoperative urinary retention and recatheterization in a small number of patients.
Prescribe routinely scheduled acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and ketorolac. A key component of ERAS cesarean birth is the standardized administration of nonopioid pain medicines, alternating doses of acetaminophen and an NSAID. ERAS cesarean birth is likely to result in a reduction in inpatient and postdischarge opioid use.22-24
VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.8
Auditing and reporting adherence with components of ERAS
In clinical practice there may be a gap between a clinician’s subjective perception of their performance and an independent audit of their clinical performance. ERAS pathways should be implemented with a commitment to performing audits and providing quantitative feedback to clinicians. Consistent use of measurement, feedback, and coaching can improve performance and reduce variation among individual clinicians. As an example, in one study of the use of a surgical safety checklist, 99% of the surgeons reported that they routinely used a surgical safety checklist, but the audit showed that the checklist was used in only 60% of cases.25 Gaps between self-reported performance and audited performance are common in clinical practice. Audits with feedback are critical to improving adherence with the components of an ERAS pathway.
Three independent systematic reviews and meta-analyses report that ERAS pathways reduce hospital length of stay without increasing the readmission rate.26-28 One meta-analysis reported that ERAS may also reduce time to first mobilization and result in earlier removal of the urinary catheter.26 ERAS pathways also may reduce postoperative complications, lower pain scores, and decrease opioid use.27 The general consensus among quality and safety experts is that reducing variation through standardization of pathways is generally associated with improved quality and enhanced safety. ERAS pathways have been widely accepted in multiple surgical fields. ERAS pathways should become the standard for performing cesarean procedures.●
1. Molina G, Weiser RG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314:2263-2270.
2. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. Vital Statistics Release; No. 20. Hyattsville, MD: National Center for Health Statistics. May 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf.
3. Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;486-493.
4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-298.
5. Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 1). Am J Obstet Gynecol. 2018;219:523.e1-523.e15.
6. Caughey AB, Wood SL, Macones GA, et al Guidelines for intraoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 2). Am J Obstet Gynecol. 2018;219:533-544.
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 3). Am J Obstet Gynecol. 2019;221:247.e1-247.e9.
8. Bollag L, Lim G, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology: Consensus statement and recommendations for enhanced recovery after cesarean. Anesth Analg. 2021;132:1362-1377.
9. Perioperative pathways: enhanced recovery after surgery. ACOG Committee Opinion No 750. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e120-130.
10. University of Michigan. ERAS: A patient education video. https://www.youtube.com/watch?v=CoFtgdluBc0. Accessed October 24, 2022.
11. University of Maryland. ERAS. https://www.umms.org/ummc/health-services/womens-health/ostetrics-gynecology/pregnancy-childbirth/labor-delivery/enhanced-recovery-after-cesarean. Accessed October 24, 2022.
12. Bilku DK, Dennison AR, Hall TC, et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
13. Popivanov P, Irwin R, Walsh M, et al. Gastric emptying of carbohydrate drinks in term parturients before elective caesarean surgery: an observational study. Int J Obstet Anesth. 2020;41:29-34.
14. He Y, Liu C, Han Y, et al. The impact of carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes- a randomized clinical trial. BMC Pregnancy Childbirth. 2021;21:682.
15. Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
16. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
17. Nour N, Barbieri RL. Optimize detection and treatment of iron deficiency in pregnancy. OBG Manag. 2022;34:9-11.
18. Mead NC, Sakkatos P, Sakellaropoulos GC, et al. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis. 2014;10:1166-1173.
19. Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin and folate. Blood. 2017;129:940-949.
20. Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:735-746.
21. Wen Z, Shen M, Wu C, et al. Chewing gum for intestinal function recovery after caesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:105.
22. McCoy JA, Gutman S, Hamm RF, et al. The association between implementation of an enhanced recovery after cesarean pathway with standardized discharge prescriptions and opioid use and pain experience after cesarean delivery. Am J Perinatol. 2021;38:1341-1347.
23. Mullman L, Hilden P, Goral J, et al. Improved outcomes with an enhanced recovery approach to cesarean delivery. Obstet Gynecol. 2020;136:685-691.
24. Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
25. Sendlhofer G, Lumenta DB, Leitgeb K, et al. The gap between individual perception and compliance: a quantitative follow-up study of the surgical safety checklist application. PLoS One. 2016;11:e0149212.
26. Sultan P, Sharawi N, Blake L, et al. Impact of enhanced recovery after cesarean delivery on maternal outcomes: a systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2021;40:100935.
27. Meng X, Chen K, Yang C, et al. The clinical efficacy and safety of enhanced recovery after surgery for cesarean section: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Med. 2021;8:694385.
28. Corson E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols and an umbrella review of systematic reviews. BMC Pregnancy Childbirth. 2017;17:91.
1. Molina G, Weiser RG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314:2263-2270.
2. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. Vital Statistics Release; No. 20. Hyattsville, MD: National Center for Health Statistics. May 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf.
3. Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;486-493.
4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-298.
5. Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 1). Am J Obstet Gynecol. 2018;219:523.e1-523.e15.
6. Caughey AB, Wood SL, Macones GA, et al Guidelines for intraoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 2). Am J Obstet Gynecol. 2018;219:533-544.
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 3). Am J Obstet Gynecol. 2019;221:247.e1-247.e9.
8. Bollag L, Lim G, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology: Consensus statement and recommendations for enhanced recovery after cesarean. Anesth Analg. 2021;132:1362-1377.
9. Perioperative pathways: enhanced recovery after surgery. ACOG Committee Opinion No 750. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e120-130.
10. University of Michigan. ERAS: A patient education video. https://www.youtube.com/watch?v=CoFtgdluBc0. Accessed October 24, 2022.
11. University of Maryland. ERAS. https://www.umms.org/ummc/health-services/womens-health/ostetrics-gynecology/pregnancy-childbirth/labor-delivery/enhanced-recovery-after-cesarean. Accessed October 24, 2022.
12. Bilku DK, Dennison AR, Hall TC, et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
13. Popivanov P, Irwin R, Walsh M, et al. Gastric emptying of carbohydrate drinks in term parturients before elective caesarean surgery: an observational study. Int J Obstet Anesth. 2020;41:29-34.
14. He Y, Liu C, Han Y, et al. The impact of carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes- a randomized clinical trial. BMC Pregnancy Childbirth. 2021;21:682.
15. Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
16. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
17. Nour N, Barbieri RL. Optimize detection and treatment of iron deficiency in pregnancy. OBG Manag. 2022;34:9-11.
18. Mead NC, Sakkatos P, Sakellaropoulos GC, et al. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis. 2014;10:1166-1173.
19. Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin and folate. Blood. 2017;129:940-949.
20. Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:735-746.
21. Wen Z, Shen M, Wu C, et al. Chewing gum for intestinal function recovery after caesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:105.
22. McCoy JA, Gutman S, Hamm RF, et al. The association between implementation of an enhanced recovery after cesarean pathway with standardized discharge prescriptions and opioid use and pain experience after cesarean delivery. Am J Perinatol. 2021;38:1341-1347.
23. Mullman L, Hilden P, Goral J, et al. Improved outcomes with an enhanced recovery approach to cesarean delivery. Obstet Gynecol. 2020;136:685-691.
24. Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
25. Sendlhofer G, Lumenta DB, Leitgeb K, et al. The gap between individual perception and compliance: a quantitative follow-up study of the surgical safety checklist application. PLoS One. 2016;11:e0149212.
26. Sultan P, Sharawi N, Blake L, et al. Impact of enhanced recovery after cesarean delivery on maternal outcomes: a systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2021;40:100935.
27. Meng X, Chen K, Yang C, et al. The clinical efficacy and safety of enhanced recovery after surgery for cesarean section: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Med. 2021;8:694385.
28. Corson E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols and an umbrella review of systematic reviews. BMC Pregnancy Childbirth. 2017;17:91.
Chagas disease: An unusual and dangerous infection for both mother and baby
CASE Pregnant woman with a suspected parasitic infection
A 20-year-old, previously healthy, primigravid woman at 24 weeks’ gestation immigrated from Bolivia to the United States 3 days ago. On the morning of her international flight, she awoke to discover a small insect bite just below her left eye. She sought medical evaluation because her eyelid is now significantly swollen, and she has a headache, anorexia, fatigue, and a fever of 38.4° C. The examining physician ordered a polymerase chain reaction (PCR) test for Trypanosoma cruzi, and the test is positive.
- How should this patient be treated during, and after, her delivery?
- Does this infection pose a risk to the newborn baby?
- What type of surveillance and treatment is indicated for the baby?
Chagas disease is common in South America, Central America, and Mexico and is well known to physicians in those countries. Clinicians who practice in the United States are much less familiar with the condition, but it is becoming increasingly common as a result of international travel within the Americas.
In this article, we review the interesting microbiology and epidemiology of Chagas disease, focus on its clinical manifestations, and discuss the most useful diagnostic tests for the illness. We conclude with a summary of preventive and treatment measures, with particular emphasis on managing the disease in pregnancy.
How Chagas disease is transmitted and who is at risk
Chagas disease was named in honor of a Brazilian physician, Carlos Chagas, who first described the condition in 1909. The disease is endemic in South America, Central America, and Mexico, and, recently, its prevalence has increased in the southern United States. Approximately 300,000 people in the United States are infected.1,2
The illness is caused by the parasite Trypanosoma cruzi, and it is also known as American trypanosomiasis. The parasite is spread primarily by the bite of triatomine insects (“kissing bugs”). Approximately 60% of these insects are infected with the parasite. The insects live and thrive in the interspaces of mud walls (adobe homes) and thatched roofs. At night, the insects leave their darkened spaces and feed on the exposed skin of sleeping persons. They are particularly likely to bite the moist skin surfaces near the eye and mouth, and, as they do, they defecate and excrete the parasite into the blood vessels beneath the skin. Within the blood, the trypomastigotes invade various host cells. Inside the host cells, the organism transforms into an amastigote, which is the replicative form of the parasite. After several rounds of replication, the amastigote transforms back into a trypomastigote, bursts from the cell, and goes on to infect other host cells.1
In addition to transmission by the insect vector, the parasite also can be transmitted by blood transfusion and organ donation. When contaminated blood is transfused, the risk of transmission is approximately 10% to 25% for each unit. Following implementation of effective screening programs by blood banks in Central America, South America, Mexico, and the United States, the risk of transmission from undetected infection is now approximately 1:200,000 per unit.
When a transplant procedure with an infected heart is performed, the risk of transmission is 75% to 100%. For liver transplants, the frequency of transmission is 0% to 29%; for kidney transplants, the risk of transmission is 0% to 19%.
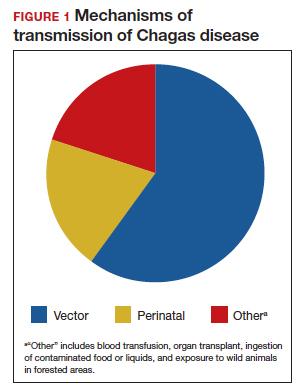
Consumption of contaminated food or drink, particularly nonpasteurized items sold by street vendors, is also an important mechanism of transmission. In addition, transmission can occur as a result of laboratory exposure and by exposure to wild animals (racoons, opossums, marmosets, bats, armadillos) in forested areas. Finally, perinatal transmission now accounts for about 22% of infections. As effective vector control programs have been introduced in endemic areas, the proportion of cases caused by the insect vector has steadily decreased1-3 (FIGURE 1).
Continue to: Clinical manifestations of Chagas disease...
Clinical manifestations of Chagas disease
Chagas disease occurs in 2 stages, acute and chronic.1,2,4 In patients who are infected via an insect vector, the acute stage typically begins 1 to 2 weeks after the insect bite. This phase of the illness usually lasts 4 to 8 weeks and almost always resolves without treatment.
Some infected patients will be completely free of symptoms. Others will have manifestations such as:
- fever
- malaise
- headache
- hepatosplenomegaly
- lymphadenopathy
- swollen nodule at the site of infection
—Romaña’s sign, when the lesion is on the eyelid
—Chagoma, when the lesion is elsewhere on the skin.
Fortunately, less than 5% of patients will have severe illness, manifested by myocarditis, pericarditis, encephalitis, or meningitis.
People infected by ingestion of the parasite in food or drink often become more severely ill within 3 weeks. Their clinical manifestations include fever, vomiting, dyspnea, cough, chest pain, abdominal pain, and myalgias. Individuals infected through organ transplant or blood transfusion present more like those infected by the insect vector, but their illness may not develop until several weeks to 5 months after exposure.
In the absence of effective treatment, approximately 40% of patients with acute infection will develop chronic infection, often several decades later. The most common, and most ominous, feature of chronic illness is cardiac disease, experienced by about 30% of patients. Cardiac disease may be manifested as a serious arrhythmia, chest pain, congestive heart failure, or thromboembolism.
The other organ system that is likely to be adversely affected in patients with chronic disease is the gastrointestinal (GI) system, and approximately 10% of chronically infected patients experience this complication. Patients may develop a dilated esophagus, which leads to odynophagia and dysphagia. Diminished motility in other areas of the GI tract also may result in chronic constipation and even bowel obstruction. Chronically infected patients who are immunosuppressed due to HIV infection may become gravely ill as a result of encephalitis and brain abscesses. Cardiac and GI dysfunction is due to the parasite’s massive destruction of nerve endings.
Continue to: Making the diagnosis...
Making the diagnosis
The diagnosis of Chagas disease begins with screening patients who have epidemiologic risk factors that place them at high risk for contracting the infection and at significantly increased risk for morbidity and mortality as a result of either the acute infection or the later chronic stage of infection. A thorough history is vital in the evaluation because the acute illness can have such vague clinical manifestations, and many patients remain asymptomatic until signs of chronic infection appear.
Risk factors that warrant screening include being born in a country endemic for Chagas disease, living in an endemic country for more than 6 months, living with someone who has a confirmed diagnosis, residing in a house made of natural materials (mud walls, thatched roof) in an endemic area, and a history of discovering the triatomine bug in the household.
Screening options include serology, microscopy, and PCR testing. Screening with a single, highly sensitive immunoglobulin G (IgG) serologic test is recommended for nonendemic clinical or community settings. In patients who were born in or who lived in an endemic area for more than 6 months, special consideration should be given to screening women of reproductive age, patients of all ages who were born to a mother with a confirmed diagnosis, individuals who were exposed to a triatomine insect, and people who are immunocompromised.5
A positive serologic test should be confirmed with a second assay based on a different antigen. Currently, 4 IgG tests have US Food and Drug Administration (FDA) approval for diagnosis. If a patient has 2 positive serologic tests, the diagnosis is confirmed, regardless of clinical presentation. Discordant results warrant a third test to differentiate between positive and negative results (FIGURE 2).5 All patients with a confirmed diagnosis should have an electrocardiogram, echocardiogram, and abdominal computed tomography (CT) scan to assess for cardiac or GI abnormalities.
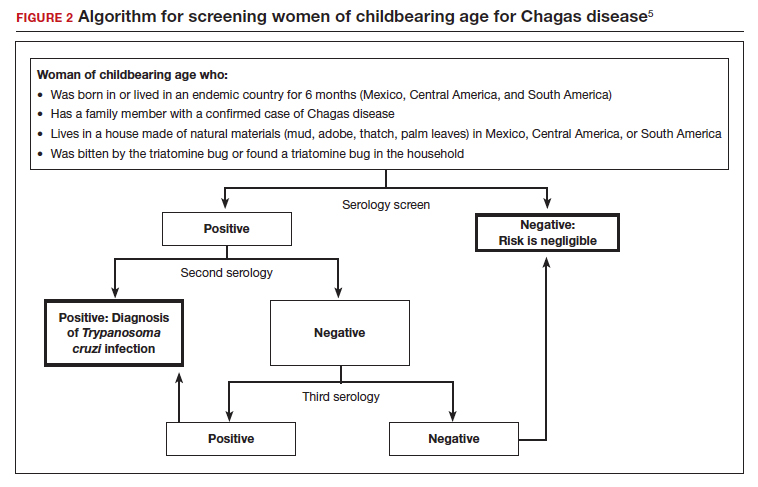
Neonates and infants of mothers with suspected or confirmed infection merit special attention. These children may demonstrate hepatomegaly, splenomegaly, anemia, thrombocytopenia, pneumonitis, heart failure, cardiac arrhythmias, or meningoencephalitis. Newborns delivered to infected mothers will invariably have positive tests for IgG antibody because of transplacental transfer of maternal antibody. Therefore, they should be evaluated by PCR or by direct microscopic examination of the blood for trypomastigotes. In neonates with a negative initial result, repeat testing should be performed by PCR at 4 to 6 weeks of age. Even if the second screening test is negative, the infant should be retested at 9 to 12 months. At this point, maternal IgG no longer should be circulating in the infant’s blood. Three negative tests should effectively rule out T cruzi infection (FIGURE 3).5-7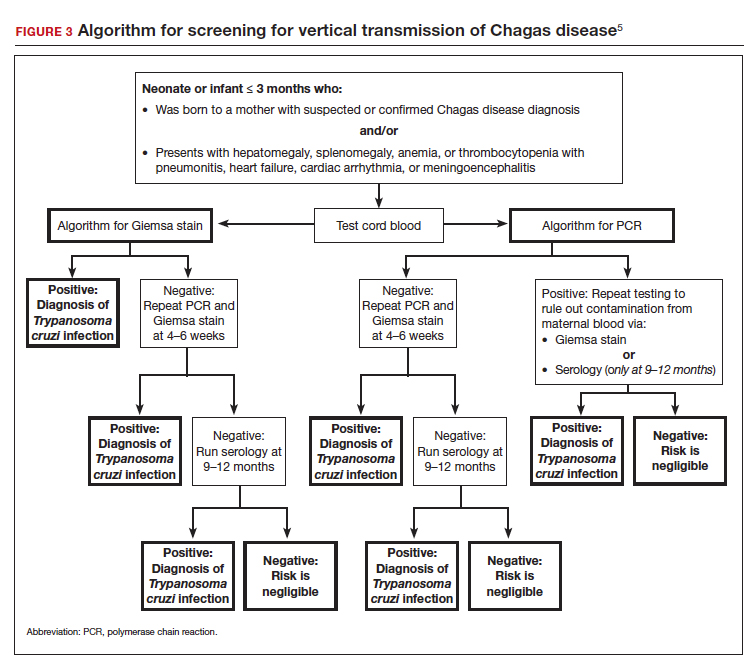
Organ recipients merit special consideration because, in these individuals, the late stages of Chagas disease may be fatal. In these patients, the preferred diagnostic test is PCR. For transplant patients, monitoring should occur every week for 2 months, bimonthly for the third month, and monthly for 6 months after transplantation. Routine monitoring is not recommended in patients with HIV infection who show no clinical signs of Chagas disease and who are not from endemic areas.
Treatment options
No vaccine or hyperimmune globulin can be used to treat Chagas disease. At this time, 2 antiparasitic drugs are available to treat the condition. One is benznidazole, which inhibits DNA, RNA, and protein synthesis within the microorganism. The medication is given in a dose of 5 to 8 mg/kg per day, divided into 2 doses, for 60 days. Benznidazole is FDA approved for the treatment of individuals older than age 2. It has been used off-label in children younger than 2 years of age. The drug is commercially available at http://www.benznidazoletablets.com.
Benznidazole causes multiple minor side effects and several very serious adverse effects. The serious adverse effects include acute generalized exanthematous pustulosis, toxic epidermal necrolysis, peripheral neuropathy, marrow suppression, and hepatotoxicity. Benznidazole has been teratogenic and carcinogenic in animal studies and should not be used in pregnancy.1,3,6
The second drug is nifurtimox. This drug is FDA approved for the treatment of Chagas disease in adults and for newborns and young children. It is commercially available for pharmacies to purchase from several drug wholesalers. Nifurtimox produces reactive oxygen species and toxic intermediates that induce DNA damage and cause cell death of the microorganism. The appropriate oral dose is 8 to 10 mg/kg per day, divided into 3 to 4 equal doses. The duration of treatment is 60 to 90 days, depending on the patient’s response. Like benznidazole, nifurtimox also is highly toxic. Severe adverse effects include a hypersensitivity reaction, anaphylaxis, angioedema, syncope, seizures, and psychosis. Nifurtimox also is teratogenic and is contraindicated in pregnancy.1,3,6
Clinicians who have questions about the use of either of these medications should contact the Centers for Disease Control and Prevention, Division of Parasitic Diseases public inquiries telephone line at (404) 718-4745.
Potential for cure. When either benznidazole or nifurtimox is administered early in the course of a patient’s acute infection, the chance for complete cure is excellent. The same is true for early treatment of the infected neonate. When treatment is delayed, or if it cannot be completed because of intolerable adverse effects, the prognosis for complete cure is diminished.
In adults who have chronic disease, antiparasitic treatment is unlikely to be effective. In such a situation, secondary treatment is directed toward correction of heart failure, control of cardiac rhythm disturbances, and control of GI motility disorders. For both cardiac and GI conditions, medication and surgery may be indicated. Antiparasitic treatment is more effective in children with chronic disease but it is still not uniformly effective.1,3,5,6
Preventing infection
Vector control is the key to preventing infection in areas where Chagas disease is endemic. One important, but often financially unaffordable, measure is construction of homes with building materials that do not support the growth of the triatomine insects that transmit the disease. A second critical preventive measure is the spraying of mud and thatched homes and surrounding areas with long-lasting insecticides. Pyrethroids are the preferred agents today. Alternative agents include fenitrothion and bendiocarb.1
Other important preventive measures include:
- screening the blood supply for T cruzi and eliminating units contaminated with the parasite
- screening for the parasite in organs targeted for transplant
- screening infected women of reproductive age in endemic areas and treating those who are positive before they become pregnant; this measure may be almost 95% effective in preventing congenital infection
- using mosquito netting when housing is insecure and air conditioning is not available
- in endemic areas, avoiding unpasteurized fruit drinks and unwashed fruits and vegetables.
Unique considerations in pregnancy
Chagas disease does not cause specific anatomic birth defects. However, infected women are more likely to experience spontaneous abortion, preterm premature rupture of membranes, preterm labor, and fetal growth restriction. Overall, the risk of perinatal transmission is approximately 5%, but it may be higher in women who have a very high parasite load. Infected neonates who remain untreated are at risk for developing the serious sequelae of chronic infection. At least half of neonates who are infected will initially be asymptomatic. Therefore, screening of at-risk neonates is essential in order to implement effective treatment.3,6
As noted earlier, the usual drugs used for treating Chagas disease should not be used in pregnancy. Nevertheless, it is still important to screen certain individuals for infection and, subsequently, target them and their neonates for treatment immediately following delivery. The following pregnant patients should be screened5,6:
- women with clinical manifestations that suggest acute or chronic infection
- women from areas of the world in which Chagas disease is endemic, namely, from the southern United States to northern Chile and Argentina. Although the disease is endemic in 21 countries, the countries with the highest prevalence are Bolivia, Argentina, and Paraguay.
- newborns delivered to mothers who have been identified as infected.
As mentioned, several tests are available for screening: PCR, antibody assays, and examination of peripheral blood smears. At least 2 test results should be positive to confirm the diagnosis of infection. Neonates should be followed for 9 to 12 months after delivery to determine if perinatal transmission has occurred. Treatment with antiparasitic drugs is indicated for all infected children.5
CASE Continue surveillance during pregnancy, treat after delivery
This patient should not be treated during pregnancy because the 2 major antiparasitic drugs are teratogenic. Antenatally, she should be followed for evidence of preterm labor and fetal growth restriction. She also should have an electrocardiogram and echocardiogram to evaluate for cardiac disease. Immediately after delivery, the patient should be treated with benznidazole for 60 days. Breastfeeding is acceptable. Her neonate should be screened for infection for up to 9 months, following the algorithm outlined earlier (FIGURE 3), and treated if the surveillance tests are positive. ●
- Chagas disease is caused by the parasite Trypanosoma cruzi, which is spread by the bite of the triatomine insect (the “kissing bug”).
- The condition is widespread among impoverished populations in South America, Central America, and Mexico, but it is rare in the United States except in individuals who immigrated here from endemic areas.
- Chagas disease evolves through 2 phases: acute and chronic. Manifestations of acute infection include fever, malaise, headache, hepatosplenomegaly, lymphadenopathy, and swelling at the site of the insect bite. The chronic phase is manifested by serious cardiac and gastrointestinal dysfunction.
- The diagnosis can be established by identifying the organism in a blood smear and by detecting antibody or antigen in the blood.
- The 2 drugs of choice for treatment of Chagas disease are benznidazole and nifurtimox. These drugs are teratogenic and are contraindicated in pregnancy.
- Women at risk for infection should be screened prior to, or during, pregnancy. Infants of infected mothers should be screened for infection for up to 9 to 12 months after delivery and treated if they test positive. Treatment of the infant is almost 100% effective in preventing chronic illness.
- Bern C. Chagas disease: epidemiology, screening, and prevention. UpToDate. Updated April 8, 2022. Accessed October 6, 2022. https://www.uptodate.com/contents /chagas-disease-epidemiology-screening-and-prevention
- Chagas disease. Cleveland Clinic. Reviewed October 8, 2021. Accessed October 6, 2022. https://my.clevelandclinic.org /health/diseases/21876-chagas-disease
- Howard EJ, Xiong X, Carlier Y, et al. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22-33.
- Chagas disease. Mayo Clinic. November 12, 2020. Accessed October 6, 2022. https://www.mayoclinic.org/diseases -conditions/chagas-disease/symptoms-causes/syc-20356212
- Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2022;225:1601-1610.
- Torrico F, Alonso-Vega C, Suarez E. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201-209.
- Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415-421.
CASE Pregnant woman with a suspected parasitic infection
A 20-year-old, previously healthy, primigravid woman at 24 weeks’ gestation immigrated from Bolivia to the United States 3 days ago. On the morning of her international flight, she awoke to discover a small insect bite just below her left eye. She sought medical evaluation because her eyelid is now significantly swollen, and she has a headache, anorexia, fatigue, and a fever of 38.4° C. The examining physician ordered a polymerase chain reaction (PCR) test for Trypanosoma cruzi, and the test is positive.
- How should this patient be treated during, and after, her delivery?
- Does this infection pose a risk to the newborn baby?
- What type of surveillance and treatment is indicated for the baby?
Chagas disease is common in South America, Central America, and Mexico and is well known to physicians in those countries. Clinicians who practice in the United States are much less familiar with the condition, but it is becoming increasingly common as a result of international travel within the Americas.
In this article, we review the interesting microbiology and epidemiology of Chagas disease, focus on its clinical manifestations, and discuss the most useful diagnostic tests for the illness. We conclude with a summary of preventive and treatment measures, with particular emphasis on managing the disease in pregnancy.
How Chagas disease is transmitted and who is at risk
Chagas disease was named in honor of a Brazilian physician, Carlos Chagas, who first described the condition in 1909. The disease is endemic in South America, Central America, and Mexico, and, recently, its prevalence has increased in the southern United States. Approximately 300,000 people in the United States are infected.1,2
The illness is caused by the parasite Trypanosoma cruzi, and it is also known as American trypanosomiasis. The parasite is spread primarily by the bite of triatomine insects (“kissing bugs”). Approximately 60% of these insects are infected with the parasite. The insects live and thrive in the interspaces of mud walls (adobe homes) and thatched roofs. At night, the insects leave their darkened spaces and feed on the exposed skin of sleeping persons. They are particularly likely to bite the moist skin surfaces near the eye and mouth, and, as they do, they defecate and excrete the parasite into the blood vessels beneath the skin. Within the blood, the trypomastigotes invade various host cells. Inside the host cells, the organism transforms into an amastigote, which is the replicative form of the parasite. After several rounds of replication, the amastigote transforms back into a trypomastigote, bursts from the cell, and goes on to infect other host cells.1
In addition to transmission by the insect vector, the parasite also can be transmitted by blood transfusion and organ donation. When contaminated blood is transfused, the risk of transmission is approximately 10% to 25% for each unit. Following implementation of effective screening programs by blood banks in Central America, South America, Mexico, and the United States, the risk of transmission from undetected infection is now approximately 1:200,000 per unit.
When a transplant procedure with an infected heart is performed, the risk of transmission is 75% to 100%. For liver transplants, the frequency of transmission is 0% to 29%; for kidney transplants, the risk of transmission is 0% to 19%.
Consumption of contaminated food or drink, particularly nonpasteurized items sold by street vendors, is also an important mechanism of transmission. In addition, transmission can occur as a result of laboratory exposure and by exposure to wild animals (racoons, opossums, marmosets, bats, armadillos) in forested areas. Finally, perinatal transmission now accounts for about 22% of infections. As effective vector control programs have been introduced in endemic areas, the proportion of cases caused by the insect vector has steadily decreased1-3 (FIGURE 1).
Continue to: Clinical manifestations of Chagas disease...
Clinical manifestations of Chagas disease
Chagas disease occurs in 2 stages, acute and chronic.1,2,4 In patients who are infected via an insect vector, the acute stage typically begins 1 to 2 weeks after the insect bite. This phase of the illness usually lasts 4 to 8 weeks and almost always resolves without treatment.
Some infected patients will be completely free of symptoms. Others will have manifestations such as:
- fever
- malaise
- headache
- hepatosplenomegaly
- lymphadenopathy
- swollen nodule at the site of infection
—Romaña’s sign, when the lesion is on the eyelid
—Chagoma, when the lesion is elsewhere on the skin.
Fortunately, less than 5% of patients will have severe illness, manifested by myocarditis, pericarditis, encephalitis, or meningitis.
People infected by ingestion of the parasite in food or drink often become more severely ill within 3 weeks. Their clinical manifestations include fever, vomiting, dyspnea, cough, chest pain, abdominal pain, and myalgias. Individuals infected through organ transplant or blood transfusion present more like those infected by the insect vector, but their illness may not develop until several weeks to 5 months after exposure.
In the absence of effective treatment, approximately 40% of patients with acute infection will develop chronic infection, often several decades later. The most common, and most ominous, feature of chronic illness is cardiac disease, experienced by about 30% of patients. Cardiac disease may be manifested as a serious arrhythmia, chest pain, congestive heart failure, or thromboembolism.
The other organ system that is likely to be adversely affected in patients with chronic disease is the gastrointestinal (GI) system, and approximately 10% of chronically infected patients experience this complication. Patients may develop a dilated esophagus, which leads to odynophagia and dysphagia. Diminished motility in other areas of the GI tract also may result in chronic constipation and even bowel obstruction. Chronically infected patients who are immunosuppressed due to HIV infection may become gravely ill as a result of encephalitis and brain abscesses. Cardiac and GI dysfunction is due to the parasite’s massive destruction of nerve endings.
Continue to: Making the diagnosis...
Making the diagnosis
The diagnosis of Chagas disease begins with screening patients who have epidemiologic risk factors that place them at high risk for contracting the infection and at significantly increased risk for morbidity and mortality as a result of either the acute infection or the later chronic stage of infection. A thorough history is vital in the evaluation because the acute illness can have such vague clinical manifestations, and many patients remain asymptomatic until signs of chronic infection appear.
Risk factors that warrant screening include being born in a country endemic for Chagas disease, living in an endemic country for more than 6 months, living with someone who has a confirmed diagnosis, residing in a house made of natural materials (mud walls, thatched roof) in an endemic area, and a history of discovering the triatomine bug in the household.
Screening options include serology, microscopy, and PCR testing. Screening with a single, highly sensitive immunoglobulin G (IgG) serologic test is recommended for nonendemic clinical or community settings. In patients who were born in or who lived in an endemic area for more than 6 months, special consideration should be given to screening women of reproductive age, patients of all ages who were born to a mother with a confirmed diagnosis, individuals who were exposed to a triatomine insect, and people who are immunocompromised.5
A positive serologic test should be confirmed with a second assay based on a different antigen. Currently, 4 IgG tests have US Food and Drug Administration (FDA) approval for diagnosis. If a patient has 2 positive serologic tests, the diagnosis is confirmed, regardless of clinical presentation. Discordant results warrant a third test to differentiate between positive and negative results (FIGURE 2).5 All patients with a confirmed diagnosis should have an electrocardiogram, echocardiogram, and abdominal computed tomography (CT) scan to assess for cardiac or GI abnormalities.

Neonates and infants of mothers with suspected or confirmed infection merit special attention. These children may demonstrate hepatomegaly, splenomegaly, anemia, thrombocytopenia, pneumonitis, heart failure, cardiac arrhythmias, or meningoencephalitis. Newborns delivered to infected mothers will invariably have positive tests for IgG antibody because of transplacental transfer of maternal antibody. Therefore, they should be evaluated by PCR or by direct microscopic examination of the blood for trypomastigotes. In neonates with a negative initial result, repeat testing should be performed by PCR at 4 to 6 weeks of age. Even if the second screening test is negative, the infant should be retested at 9 to 12 months. At this point, maternal IgG no longer should be circulating in the infant’s blood. Three negative tests should effectively rule out T cruzi infection (FIGURE 3).5-7
Organ recipients merit special consideration because, in these individuals, the late stages of Chagas disease may be fatal. In these patients, the preferred diagnostic test is PCR. For transplant patients, monitoring should occur every week for 2 months, bimonthly for the third month, and monthly for 6 months after transplantation. Routine monitoring is not recommended in patients with HIV infection who show no clinical signs of Chagas disease and who are not from endemic areas.
Treatment options
No vaccine or hyperimmune globulin can be used to treat Chagas disease. At this time, 2 antiparasitic drugs are available to treat the condition. One is benznidazole, which inhibits DNA, RNA, and protein synthesis within the microorganism. The medication is given in a dose of 5 to 8 mg/kg per day, divided into 2 doses, for 60 days. Benznidazole is FDA approved for the treatment of individuals older than age 2. It has been used off-label in children younger than 2 years of age. The drug is commercially available at http://www.benznidazoletablets.com.
Benznidazole causes multiple minor side effects and several very serious adverse effects. The serious adverse effects include acute generalized exanthematous pustulosis, toxic epidermal necrolysis, peripheral neuropathy, marrow suppression, and hepatotoxicity. Benznidazole has been teratogenic and carcinogenic in animal studies and should not be used in pregnancy.1,3,6
The second drug is nifurtimox. This drug is FDA approved for the treatment of Chagas disease in adults and for newborns and young children. It is commercially available for pharmacies to purchase from several drug wholesalers. Nifurtimox produces reactive oxygen species and toxic intermediates that induce DNA damage and cause cell death of the microorganism. The appropriate oral dose is 8 to 10 mg/kg per day, divided into 3 to 4 equal doses. The duration of treatment is 60 to 90 days, depending on the patient’s response. Like benznidazole, nifurtimox also is highly toxic. Severe adverse effects include a hypersensitivity reaction, anaphylaxis, angioedema, syncope, seizures, and psychosis. Nifurtimox also is teratogenic and is contraindicated in pregnancy.1,3,6
Clinicians who have questions about the use of either of these medications should contact the Centers for Disease Control and Prevention, Division of Parasitic Diseases public inquiries telephone line at (404) 718-4745.
Potential for cure. When either benznidazole or nifurtimox is administered early in the course of a patient’s acute infection, the chance for complete cure is excellent. The same is true for early treatment of the infected neonate. When treatment is delayed, or if it cannot be completed because of intolerable adverse effects, the prognosis for complete cure is diminished.
In adults who have chronic disease, antiparasitic treatment is unlikely to be effective. In such a situation, secondary treatment is directed toward correction of heart failure, control of cardiac rhythm disturbances, and control of GI motility disorders. For both cardiac and GI conditions, medication and surgery may be indicated. Antiparasitic treatment is more effective in children with chronic disease but it is still not uniformly effective.1,3,5,6
Preventing infection
Vector control is the key to preventing infection in areas where Chagas disease is endemic. One important, but often financially unaffordable, measure is construction of homes with building materials that do not support the growth of the triatomine insects that transmit the disease. A second critical preventive measure is the spraying of mud and thatched homes and surrounding areas with long-lasting insecticides. Pyrethroids are the preferred agents today. Alternative agents include fenitrothion and bendiocarb.1
Other important preventive measures include:
- screening the blood supply for T cruzi and eliminating units contaminated with the parasite
- screening for the parasite in organs targeted for transplant
- screening infected women of reproductive age in endemic areas and treating those who are positive before they become pregnant; this measure may be almost 95% effective in preventing congenital infection
- using mosquito netting when housing is insecure and air conditioning is not available
- in endemic areas, avoiding unpasteurized fruit drinks and unwashed fruits and vegetables.
Unique considerations in pregnancy
Chagas disease does not cause specific anatomic birth defects. However, infected women are more likely to experience spontaneous abortion, preterm premature rupture of membranes, preterm labor, and fetal growth restriction. Overall, the risk of perinatal transmission is approximately 5%, but it may be higher in women who have a very high parasite load. Infected neonates who remain untreated are at risk for developing the serious sequelae of chronic infection. At least half of neonates who are infected will initially be asymptomatic. Therefore, screening of at-risk neonates is essential in order to implement effective treatment.3,6
As noted earlier, the usual drugs used for treating Chagas disease should not be used in pregnancy. Nevertheless, it is still important to screen certain individuals for infection and, subsequently, target them and their neonates for treatment immediately following delivery. The following pregnant patients should be screened5,6:
- women with clinical manifestations that suggest acute or chronic infection
- women from areas of the world in which Chagas disease is endemic, namely, from the southern United States to northern Chile and Argentina. Although the disease is endemic in 21 countries, the countries with the highest prevalence are Bolivia, Argentina, and Paraguay.
- newborns delivered to mothers who have been identified as infected.
As mentioned, several tests are available for screening: PCR, antibody assays, and examination of peripheral blood smears. At least 2 test results should be positive to confirm the diagnosis of infection. Neonates should be followed for 9 to 12 months after delivery to determine if perinatal transmission has occurred. Treatment with antiparasitic drugs is indicated for all infected children.5
CASE Continue surveillance during pregnancy, treat after delivery
This patient should not be treated during pregnancy because the 2 major antiparasitic drugs are teratogenic. Antenatally, she should be followed for evidence of preterm labor and fetal growth restriction. She also should have an electrocardiogram and echocardiogram to evaluate for cardiac disease. Immediately after delivery, the patient should be treated with benznidazole for 60 days. Breastfeeding is acceptable. Her neonate should be screened for infection for up to 9 months, following the algorithm outlined earlier (FIGURE 3), and treated if the surveillance tests are positive. ●
- Chagas disease is caused by the parasite Trypanosoma cruzi, which is spread by the bite of the triatomine insect (the “kissing bug”).
- The condition is widespread among impoverished populations in South America, Central America, and Mexico, but it is rare in the United States except in individuals who immigrated here from endemic areas.
- Chagas disease evolves through 2 phases: acute and chronic. Manifestations of acute infection include fever, malaise, headache, hepatosplenomegaly, lymphadenopathy, and swelling at the site of the insect bite. The chronic phase is manifested by serious cardiac and gastrointestinal dysfunction.
- The diagnosis can be established by identifying the organism in a blood smear and by detecting antibody or antigen in the blood.
- The 2 drugs of choice for treatment of Chagas disease are benznidazole and nifurtimox. These drugs are teratogenic and are contraindicated in pregnancy.
- Women at risk for infection should be screened prior to, or during, pregnancy. Infants of infected mothers should be screened for infection for up to 9 to 12 months after delivery and treated if they test positive. Treatment of the infant is almost 100% effective in preventing chronic illness.
CASE Pregnant woman with a suspected parasitic infection
A 20-year-old, previously healthy, primigravid woman at 24 weeks’ gestation immigrated from Bolivia to the United States 3 days ago. On the morning of her international flight, she awoke to discover a small insect bite just below her left eye. She sought medical evaluation because her eyelid is now significantly swollen, and she has a headache, anorexia, fatigue, and a fever of 38.4° C. The examining physician ordered a polymerase chain reaction (PCR) test for Trypanosoma cruzi, and the test is positive.
- How should this patient be treated during, and after, her delivery?
- Does this infection pose a risk to the newborn baby?
- What type of surveillance and treatment is indicated for the baby?
Chagas disease is common in South America, Central America, and Mexico and is well known to physicians in those countries. Clinicians who practice in the United States are much less familiar with the condition, but it is becoming increasingly common as a result of international travel within the Americas.
In this article, we review the interesting microbiology and epidemiology of Chagas disease, focus on its clinical manifestations, and discuss the most useful diagnostic tests for the illness. We conclude with a summary of preventive and treatment measures, with particular emphasis on managing the disease in pregnancy.
How Chagas disease is transmitted and who is at risk
Chagas disease was named in honor of a Brazilian physician, Carlos Chagas, who first described the condition in 1909. The disease is endemic in South America, Central America, and Mexico, and, recently, its prevalence has increased in the southern United States. Approximately 300,000 people in the United States are infected.1,2
The illness is caused by the parasite Trypanosoma cruzi, and it is also known as American trypanosomiasis. The parasite is spread primarily by the bite of triatomine insects (“kissing bugs”). Approximately 60% of these insects are infected with the parasite. The insects live and thrive in the interspaces of mud walls (adobe homes) and thatched roofs. At night, the insects leave their darkened spaces and feed on the exposed skin of sleeping persons. They are particularly likely to bite the moist skin surfaces near the eye and mouth, and, as they do, they defecate and excrete the parasite into the blood vessels beneath the skin. Within the blood, the trypomastigotes invade various host cells. Inside the host cells, the organism transforms into an amastigote, which is the replicative form of the parasite. After several rounds of replication, the amastigote transforms back into a trypomastigote, bursts from the cell, and goes on to infect other host cells.1
In addition to transmission by the insect vector, the parasite also can be transmitted by blood transfusion and organ donation. When contaminated blood is transfused, the risk of transmission is approximately 10% to 25% for each unit. Following implementation of effective screening programs by blood banks in Central America, South America, Mexico, and the United States, the risk of transmission from undetected infection is now approximately 1:200,000 per unit.
When a transplant procedure with an infected heart is performed, the risk of transmission is 75% to 100%. For liver transplants, the frequency of transmission is 0% to 29%; for kidney transplants, the risk of transmission is 0% to 19%.
Consumption of contaminated food or drink, particularly nonpasteurized items sold by street vendors, is also an important mechanism of transmission. In addition, transmission can occur as a result of laboratory exposure and by exposure to wild animals (racoons, opossums, marmosets, bats, armadillos) in forested areas. Finally, perinatal transmission now accounts for about 22% of infections. As effective vector control programs have been introduced in endemic areas, the proportion of cases caused by the insect vector has steadily decreased1-3 (FIGURE 1).
Continue to: Clinical manifestations of Chagas disease...
Clinical manifestations of Chagas disease
Chagas disease occurs in 2 stages, acute and chronic.1,2,4 In patients who are infected via an insect vector, the acute stage typically begins 1 to 2 weeks after the insect bite. This phase of the illness usually lasts 4 to 8 weeks and almost always resolves without treatment.
Some infected patients will be completely free of symptoms. Others will have manifestations such as:
- fever
- malaise
- headache
- hepatosplenomegaly
- lymphadenopathy
- swollen nodule at the site of infection
—Romaña’s sign, when the lesion is on the eyelid
—Chagoma, when the lesion is elsewhere on the skin.
Fortunately, less than 5% of patients will have severe illness, manifested by myocarditis, pericarditis, encephalitis, or meningitis.
People infected by ingestion of the parasite in food or drink often become more severely ill within 3 weeks. Their clinical manifestations include fever, vomiting, dyspnea, cough, chest pain, abdominal pain, and myalgias. Individuals infected through organ transplant or blood transfusion present more like those infected by the insect vector, but their illness may not develop until several weeks to 5 months after exposure.
In the absence of effective treatment, approximately 40% of patients with acute infection will develop chronic infection, often several decades later. The most common, and most ominous, feature of chronic illness is cardiac disease, experienced by about 30% of patients. Cardiac disease may be manifested as a serious arrhythmia, chest pain, congestive heart failure, or thromboembolism.
The other organ system that is likely to be adversely affected in patients with chronic disease is the gastrointestinal (GI) system, and approximately 10% of chronically infected patients experience this complication. Patients may develop a dilated esophagus, which leads to odynophagia and dysphagia. Diminished motility in other areas of the GI tract also may result in chronic constipation and even bowel obstruction. Chronically infected patients who are immunosuppressed due to HIV infection may become gravely ill as a result of encephalitis and brain abscesses. Cardiac and GI dysfunction is due to the parasite’s massive destruction of nerve endings.
Continue to: Making the diagnosis...
Making the diagnosis
The diagnosis of Chagas disease begins with screening patients who have epidemiologic risk factors that place them at high risk for contracting the infection and at significantly increased risk for morbidity and mortality as a result of either the acute infection or the later chronic stage of infection. A thorough history is vital in the evaluation because the acute illness can have such vague clinical manifestations, and many patients remain asymptomatic until signs of chronic infection appear.
Risk factors that warrant screening include being born in a country endemic for Chagas disease, living in an endemic country for more than 6 months, living with someone who has a confirmed diagnosis, residing in a house made of natural materials (mud walls, thatched roof) in an endemic area, and a history of discovering the triatomine bug in the household.
Screening options include serology, microscopy, and PCR testing. Screening with a single, highly sensitive immunoglobulin G (IgG) serologic test is recommended for nonendemic clinical or community settings. In patients who were born in or who lived in an endemic area for more than 6 months, special consideration should be given to screening women of reproductive age, patients of all ages who were born to a mother with a confirmed diagnosis, individuals who were exposed to a triatomine insect, and people who are immunocompromised.5
A positive serologic test should be confirmed with a second assay based on a different antigen. Currently, 4 IgG tests have US Food and Drug Administration (FDA) approval for diagnosis. If a patient has 2 positive serologic tests, the diagnosis is confirmed, regardless of clinical presentation. Discordant results warrant a third test to differentiate between positive and negative results (FIGURE 2).5 All patients with a confirmed diagnosis should have an electrocardiogram, echocardiogram, and abdominal computed tomography (CT) scan to assess for cardiac or GI abnormalities.

Neonates and infants of mothers with suspected or confirmed infection merit special attention. These children may demonstrate hepatomegaly, splenomegaly, anemia, thrombocytopenia, pneumonitis, heart failure, cardiac arrhythmias, or meningoencephalitis. Newborns delivered to infected mothers will invariably have positive tests for IgG antibody because of transplacental transfer of maternal antibody. Therefore, they should be evaluated by PCR or by direct microscopic examination of the blood for trypomastigotes. In neonates with a negative initial result, repeat testing should be performed by PCR at 4 to 6 weeks of age. Even if the second screening test is negative, the infant should be retested at 9 to 12 months. At this point, maternal IgG no longer should be circulating in the infant’s blood. Three negative tests should effectively rule out T cruzi infection (FIGURE 3).5-7
Organ recipients merit special consideration because, in these individuals, the late stages of Chagas disease may be fatal. In these patients, the preferred diagnostic test is PCR. For transplant patients, monitoring should occur every week for 2 months, bimonthly for the third month, and monthly for 6 months after transplantation. Routine monitoring is not recommended in patients with HIV infection who show no clinical signs of Chagas disease and who are not from endemic areas.
Treatment options
No vaccine or hyperimmune globulin can be used to treat Chagas disease. At this time, 2 antiparasitic drugs are available to treat the condition. One is benznidazole, which inhibits DNA, RNA, and protein synthesis within the microorganism. The medication is given in a dose of 5 to 8 mg/kg per day, divided into 2 doses, for 60 days. Benznidazole is FDA approved for the treatment of individuals older than age 2. It has been used off-label in children younger than 2 years of age. The drug is commercially available at http://www.benznidazoletablets.com.
Benznidazole causes multiple minor side effects and several very serious adverse effects. The serious adverse effects include acute generalized exanthematous pustulosis, toxic epidermal necrolysis, peripheral neuropathy, marrow suppression, and hepatotoxicity. Benznidazole has been teratogenic and carcinogenic in animal studies and should not be used in pregnancy.1,3,6
The second drug is nifurtimox. This drug is FDA approved for the treatment of Chagas disease in adults and for newborns and young children. It is commercially available for pharmacies to purchase from several drug wholesalers. Nifurtimox produces reactive oxygen species and toxic intermediates that induce DNA damage and cause cell death of the microorganism. The appropriate oral dose is 8 to 10 mg/kg per day, divided into 3 to 4 equal doses. The duration of treatment is 60 to 90 days, depending on the patient’s response. Like benznidazole, nifurtimox also is highly toxic. Severe adverse effects include a hypersensitivity reaction, anaphylaxis, angioedema, syncope, seizures, and psychosis. Nifurtimox also is teratogenic and is contraindicated in pregnancy.1,3,6
Clinicians who have questions about the use of either of these medications should contact the Centers for Disease Control and Prevention, Division of Parasitic Diseases public inquiries telephone line at (404) 718-4745.
Potential for cure. When either benznidazole or nifurtimox is administered early in the course of a patient’s acute infection, the chance for complete cure is excellent. The same is true for early treatment of the infected neonate. When treatment is delayed, or if it cannot be completed because of intolerable adverse effects, the prognosis for complete cure is diminished.
In adults who have chronic disease, antiparasitic treatment is unlikely to be effective. In such a situation, secondary treatment is directed toward correction of heart failure, control of cardiac rhythm disturbances, and control of GI motility disorders. For both cardiac and GI conditions, medication and surgery may be indicated. Antiparasitic treatment is more effective in children with chronic disease but it is still not uniformly effective.1,3,5,6
Preventing infection
Vector control is the key to preventing infection in areas where Chagas disease is endemic. One important, but often financially unaffordable, measure is construction of homes with building materials that do not support the growth of the triatomine insects that transmit the disease. A second critical preventive measure is the spraying of mud and thatched homes and surrounding areas with long-lasting insecticides. Pyrethroids are the preferred agents today. Alternative agents include fenitrothion and bendiocarb.1
Other important preventive measures include:
- screening the blood supply for T cruzi and eliminating units contaminated with the parasite
- screening for the parasite in organs targeted for transplant
- screening infected women of reproductive age in endemic areas and treating those who are positive before they become pregnant; this measure may be almost 95% effective in preventing congenital infection
- using mosquito netting when housing is insecure and air conditioning is not available
- in endemic areas, avoiding unpasteurized fruit drinks and unwashed fruits and vegetables.
Unique considerations in pregnancy
Chagas disease does not cause specific anatomic birth defects. However, infected women are more likely to experience spontaneous abortion, preterm premature rupture of membranes, preterm labor, and fetal growth restriction. Overall, the risk of perinatal transmission is approximately 5%, but it may be higher in women who have a very high parasite load. Infected neonates who remain untreated are at risk for developing the serious sequelae of chronic infection. At least half of neonates who are infected will initially be asymptomatic. Therefore, screening of at-risk neonates is essential in order to implement effective treatment.3,6
As noted earlier, the usual drugs used for treating Chagas disease should not be used in pregnancy. Nevertheless, it is still important to screen certain individuals for infection and, subsequently, target them and their neonates for treatment immediately following delivery. The following pregnant patients should be screened5,6:
- women with clinical manifestations that suggest acute or chronic infection
- women from areas of the world in which Chagas disease is endemic, namely, from the southern United States to northern Chile and Argentina. Although the disease is endemic in 21 countries, the countries with the highest prevalence are Bolivia, Argentina, and Paraguay.
- newborns delivered to mothers who have been identified as infected.
As mentioned, several tests are available for screening: PCR, antibody assays, and examination of peripheral blood smears. At least 2 test results should be positive to confirm the diagnosis of infection. Neonates should be followed for 9 to 12 months after delivery to determine if perinatal transmission has occurred. Treatment with antiparasitic drugs is indicated for all infected children.5
CASE Continue surveillance during pregnancy, treat after delivery
This patient should not be treated during pregnancy because the 2 major antiparasitic drugs are teratogenic. Antenatally, she should be followed for evidence of preterm labor and fetal growth restriction. She also should have an electrocardiogram and echocardiogram to evaluate for cardiac disease. Immediately after delivery, the patient should be treated with benznidazole for 60 days. Breastfeeding is acceptable. Her neonate should be screened for infection for up to 9 months, following the algorithm outlined earlier (FIGURE 3), and treated if the surveillance tests are positive. ●
- Chagas disease is caused by the parasite Trypanosoma cruzi, which is spread by the bite of the triatomine insect (the “kissing bug”).
- The condition is widespread among impoverished populations in South America, Central America, and Mexico, but it is rare in the United States except in individuals who immigrated here from endemic areas.
- Chagas disease evolves through 2 phases: acute and chronic. Manifestations of acute infection include fever, malaise, headache, hepatosplenomegaly, lymphadenopathy, and swelling at the site of the insect bite. The chronic phase is manifested by serious cardiac and gastrointestinal dysfunction.
- The diagnosis can be established by identifying the organism in a blood smear and by detecting antibody or antigen in the blood.
- The 2 drugs of choice for treatment of Chagas disease are benznidazole and nifurtimox. These drugs are teratogenic and are contraindicated in pregnancy.
- Women at risk for infection should be screened prior to, or during, pregnancy. Infants of infected mothers should be screened for infection for up to 9 to 12 months after delivery and treated if they test positive. Treatment of the infant is almost 100% effective in preventing chronic illness.
- Bern C. Chagas disease: epidemiology, screening, and prevention. UpToDate. Updated April 8, 2022. Accessed October 6, 2022. https://www.uptodate.com/contents /chagas-disease-epidemiology-screening-and-prevention
- Chagas disease. Cleveland Clinic. Reviewed October 8, 2021. Accessed October 6, 2022. https://my.clevelandclinic.org /health/diseases/21876-chagas-disease
- Howard EJ, Xiong X, Carlier Y, et al. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22-33.
- Chagas disease. Mayo Clinic. November 12, 2020. Accessed October 6, 2022. https://www.mayoclinic.org/diseases -conditions/chagas-disease/symptoms-causes/syc-20356212
- Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2022;225:1601-1610.
- Torrico F, Alonso-Vega C, Suarez E. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201-209.
- Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415-421.
- Bern C. Chagas disease: epidemiology, screening, and prevention. UpToDate. Updated April 8, 2022. Accessed October 6, 2022. https://www.uptodate.com/contents /chagas-disease-epidemiology-screening-and-prevention
- Chagas disease. Cleveland Clinic. Reviewed October 8, 2021. Accessed October 6, 2022. https://my.clevelandclinic.org /health/diseases/21876-chagas-disease
- Howard EJ, Xiong X, Carlier Y, et al. Frequency of the congenital transmission of Trypanosoma cruzi: a systematic review and meta-analysis. BJOG. 2014;121:22-33.
- Chagas disease. Mayo Clinic. November 12, 2020. Accessed October 6, 2022. https://www.mayoclinic.org/diseases -conditions/chagas-disease/symptoms-causes/syc-20356212
- Forsyth CJ, Manne-Goehler J, Bern C, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2022;225:1601-1610.
- Torrico F, Alonso-Vega C, Suarez E. et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201-209.
- Messenger LA, Bern C. Congenital Chagas disease: current diagnostics, limitations and future perspectives. Curr Opin Infect Dis. 2018;31:415-421.
Treating recurrent vulvovaginal candidiasis

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.
Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.

Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●

Recurrent vulvovaginal candidiasis (RVVC) is a common cause of vaginitis and gynecologic morbidity in the United States and globally.1 RVVC is defined as at least 3 laboratory-confirmed (for example, culture, nucleic acid amplification test [NAAT]) symptomatic episodes in the previous 12 months.2 Common symptoms include vulvar pruritus, erythema, local skin and mucosal irritation, and abnormal discharge that may be thick and white or thin and watery.
The true incidence of RVVC is difficult to determine due to clinical diagnostic inaccuracy that results in over- and underdiagnosis of VVC and the general availability of over-the-counter topical antifungal medications that individuals who self-diagnose use to treat VVC.3
Causative organisms
Vulvovaginal yeast infections are caused by Candida species, a family of ubiquitous fungi that are a part of normal genitourinary and gastrointestinal flora.4 As such, these infections are commonly termed VVC. The presence of Candida species in the vagina without evidence of inflammation is not considered an infection but rather is more consistent with vaginal colonization. Inflammation in the setting of Candida species is what characterizes a true VVC infection.4
Candida albicans is responsible for the vast majority of VVC cases in the United States, with Candida glabrata accounting for most of the remaining infections.5 The majority of RVVC infections that are caused by C albicans are due to azole-sensitive strains (85%–95% of infections).2C glabrata, by contrast, is intrinsically resistant to azoles, which is thought primarily to be due to overexpression of drug efflux pumps that remove active drug from the cell.6,7
Why does VVC reoccur?
The pathogenesis of RVVC is not well understood. Predisposing factors may include frequent or recent antibiotic use, poorly controlled diabetes, immunodeficiency, and other host factors. However, many cases of RVVC are idiopathic and no predisposing or underlying conditions are identified.7
The role of genetic factors in predisposing to or triggering RVVC is unclear and is an area of ongoing investigation.2 Longitudinal DNA-typing studies suggest that recurrent disease is usually due to relapse from a persistent vaginal reservoir of organisms (that is, vaginal colonization) or endogenous reinfection with identical strains of susceptible C albicans.8,9 Symptomatic VVC likely results when the symbiotic balance between yeast and the normal vaginal microbiota is disrupted (by either Candida species overgrowth or changes in host immune factors).2 Less commonly, “recurrent” infections may in fact be due to azole-resistant Candida and non-Candida species.2
Clinical aspects and diagnosis of VVC
Signs and symptoms suggestive of VVC include vulvovaginal erythema, edema, vaginal discharge, vulvovaginal pruritus, and irritation. Given the lack of specificity of individual clinical findings in diagnosing VVC, or for distinguishing between other common causes of vaginitis (such as bacterial vaginosis and trichomoniasis), laboratory testing (that is, microscopy) should be performed in combination with a clinical exam in order to make a confident diagnosis of VVC.10 Self-diagnosis of VVC is inaccurate and is not recommended, as misdiagnosis and inappropriate treatment is cost ineffective, delays accurate diagnoses, and may contribute to growing azole resistance.
In patients with signs and symptoms of VVC, saline and potassium hydroxide microscopy should be performed.7 TABLE 1 summarizes other major diagnostic techniques for VVC.

Diagnostic considerations
Non-albicans Candida species, such as C glabrata, may be associated with minimally symptomatic or completely asymptomatic infections and may not be identified easily on wet mount as it does not form pseudohyphae or hyphae.11 Therefore, culture and susceptibility or NAAT testing is highly recommended for patients who remain symptomatic and/or have a nondiagnostic microscopy and a normal vaginal pH.7
Treatment options
Prior to May 2022, there had been no drugs approved by the US Food and Drug Administration (FDA) to treat RVVC. The mainstay of treatment is long-term maintenance therapy to achieve mycologic remission (TABLE 2).

In general, recurrent episodes of VVC should be treated with a longer duration of therapy (for example, oral fluconazole 150 mg every 72 hours for a total of 3 doses or topical azole for 7–14 days).7 If recurrent maintenance/suppressive therapy is started, the induction phase should be longer as well, at least 10 to 14 days with a topical or oral azole followed by a 6-month or longer course of weekly oral or topical azole therapy (such as 6–12 months).12,13
Patients with underlying immunodeficiency (such as poorly controlled diabetes, chronic corticosteroid treatment) may need prolonged courses of therapy. Correction of modifiable conditions and optimization of comorbidities should be prioritized—for example, optimized glucose control, weight loss, durable viral suppression, and so on. Of note, symptomatic VVC is more frequent among individuals with HIV and correlates with severity of immunodeficiency. Pharmacologic options for RVVC for individuals with HIV do not differ from standard recommendations.14
Fluconazole
Fluconazole is a safe, affordable, and convenient prescription oral medication that can be used for initial and maintenance/suppressive therapy.2 Fluconazole levels in vaginal secretions remain at therapeutic concentrations for at least 72 hours after a 150-mg dose.15 Induction therapy consists of oral fluconazole 150 mg every 72 hours for a total of 3 doses, followed by a maintenance regimen of a once-weekly dose of oral fluconazole 150 mg for a total of 6 months. Unfortunately, up to 55% of patients will experience a relapse in symptoms.12
Routine liver function test monitoring is not indicated for fluconazole maintenance therapy, but it should be performed if patients are treated with daily or long-term alternative oral azole medications, such as ketoconazole and itraconazole.
During pregnancy, only topical azole therapy is recommended for use, given the potential risk for adverse fetal outcomes, such as spontaneous abortion and congenital malformations, with fetal exposure to oral fluconazole ingested by the pregnant person.16 Fluconazole is present in breast milk, but it is safe to use during lactation when used at recommended doses.17
Continue to: Options for fluconazole-resistant C albicans infection...
Options for fluconazole-resistant C albicans infection
Patients who have RVVC with frequent and/or prolonged use of fluconazole are at risk for developing azole-resistant isolates of C albicans.12 For patients found to have azole-resistant infections, treatment options include increasing the azole dose based on isolate minimal inhibitory concentrations (MIC) to various antifungals, therapy with a non-fluconazole azole regimen, or switching to a different therapeutic drug class altogether.7
Options for non- albicans Candida species infection
Given the intrinsic resistance to azole therapy in some non-albicans Candida species (specifically C glabrata and Candida krusei), boric acid or nystatin regimens can be used. An induction course of vaginal boric acid is given as 600 mg per vagina daily for up to 14 days and is associated with a 70% rate of mycologic control.7 Boric acid is known to cause local irritation and dermatitis for both the patient and any sexual partners. If ingested orally, boric acid is associated with significant toxicity and even death.7
Vaginal nystatin also may be considered, with an induction course of 100,000 U for 14 days, with a similar regimen recommended for maintenance therapy. However, data are limited on maintenance regimens for RVVC due to non-albicans Candida species.2
Gentian violet
Gentian violet is a topical antiseptic agent that is available over the counter. Use of this agent is uncommon given the availability of highly effective azole-based therapy. Although useful due to its antipruritic properties, gentian violet can be messy to use and tends to stain clothing permanently.
Gentian violet use may be considered in cases of refractory RVVC with or without azole-resistant infections; it is applied as a 1% or 2% solution directly to affected areas for 10 to 14 days.18
Lactobacilli probiotics and dietary changes
Data that support the oral and/or vaginal use of probiotics that contain live lactobacilli are conflicting. In the absence of conclusive evidence to support probiotic use to treat and prevent RVVC, as well as variable quality of available products, use of these agents is not recommended.19
No controlled studies have evaluated the role of various diets in preventing RVVC; thus, no specific dietary changes are recommended.
Behavioral therapy
Available evidence does not support the treatment of sexual partners of patients with RVVC.7
Continue to: What’s new in treatment?...
What’s new in treatment?
Until recently, the main standard of care for RVVC has been oral fluconazole-based therapy. For patients whose symptoms do not respond to oral fluconazole therapy, oteseconazole is now available as a noninferior treatment option to fluconazole for both induction and maintenance therapy. Like other azoles, oteseconazole works by inhibiting a fungal enzyme (CYP51) that is essential in fungal cell membrane integrity and fungal growth.20 Oteseconazole is a more selective inhibitor of the fungal CYP51 enzyme and has demonstrated excellent potency against Candida species in in vitro pharmacologic studies.21
In a phase 3 study that evaluated the safety and efficacy of oteseconazole in the treatment and prevention of RVVC, oteseconazole was found to be both safe and efficacious in both the induction and maintenance phases of treatment for RVVC.20 In this trial, induction and maintenance with oteseconazole was compared with induction with fluconazole and placebo maintenance. Among the 185 participants with culture-verified RVVC, the oteseconazole regimen (n = 123) was associated with fewer recurrences of culture-verified VVC infections than was the fluconazole induction/placebo maintenance regimen (n = 62) during the 48-week maintenance phase of therapy (5% vs 42%).20
Single- and dual-drug dosing regimens of oteseconazole are recommended based on previous trial data that compared safety and efficacy of oteseconazole versus fluconazole induction therapy and oteseconazole versus placebo maintenance therapy.22 However, widespread use of oteseconazole regimens are limited due to its higher costs and limited access to the drug outside of a research setting.20
Single-drug induction therapy with oteseconazole consists of a single 600-mg oral dose on day 1 followed by a second dose of 450 mg orally on day 2. Starting on day 14, maintenance therapy starts with a single oral dose of 150 mg and is continued weekly for 11 weeks.22
Dual-drug induction therapy consists of oral fluconazole 150 mg on days 1, 4, and 7 followed by daily dosing of oral oteseconazole 150 mg on days 14 through 20. Then, starting on day 28, weekly dosing of oral oteseconazole 150 mg is continued for 11 weeks.22
Effects on pregnancy and lactation. Concerns of oteseconazole’s fetal teratogenicity are based on animal reproduction studies that reported ocular abnormalities from in utero exposure. Human data are insufficient to determine if oteseconazole is excreted in breast milk or what its effects are on milk production. Among breastfed infants whose mothers were exposed to oteseconazole during lactation, no adverse outcomes were reported, but follow up of oteseconazole-exposed infants was limited. 22 Therefore, use of oteseconazole among pregnant and/or lactating persons with RVVC is contraindicated at this time. The long-half life (approximately 138 days) of oteseconazole may preclude use among persons attempting pregnancy. 22
Other therapies. The other common classes of antifungal therapy used in the treatment of RVVC include the polyenes (for example, amphotericin B) and echinocandins (such as caspofungin) drug classes. Emerging azole-resistance among Candida species has been recognized as a significant concern from the Centers for Disease Control and Prevention. 7 Echinocandins, which are generally better tolerated and have a lower adverse side effect profile than polyenes, are a promising therapeutic class, but currently they are limited to intravenous options. SCY-078, a novel oral echinocandin in development, has shown in vitro fungicidal activity against multiple albicans and non-albicans Candida species in pharmacokinetic/pharmacodynamic studies.23
Continued development of alternative, non-azole-based therapies for Candida species is needed.●
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 1985;152(7 pt 2):924-935. doi:10.1016/S0002-9378(85)80003-x
- Sobel JD. Recurrent vulvovaginal candidiasis. Am J Obstet Gynecol. 2016;214:15-21. doi:10.1016/j.ajog.2015.06.067
- Rathod SD, Buffler PA. Highly-cited estimates of the cumulative incidence and recurrence of vulvovaginal candidiasis are inadequately documented. BMC Womens Health. 2014;14:43. doi:10.1186/1472-6874-14-43
- Eckert LO, Lentz GM. Genital tract infections: vulva, vagina, cervix, toxic shock syndrome, endometritis, and salpingitis. In: Gershenson DM, Lentz GM, Valea FA, et al, eds. Comprehensive Gynecology. 8th ed. Elsevier; 2022:515-542.
- Gonçalves B, Ferreira C, Alves CT, et al. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42:905-927. doi:10.3109/1040841X.2015.1091805
- Sobel JD, Sobel R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin Pharmacother. 2018;19:971-977. doi:10.1080/14656566.2018.1476490
- Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187. doi:10.15585/mmwr.rr7004a1
- Vazquez JA, Sobel JD, Demitriou R, et al. Karyotyping of Candida albicans isolates obtained longitudinally in women with recurrent vulvovaginal candidiasis. J Infect Dis. 1994;170:1566-1569. doi:10.1093/infdis/170.6.1566
- Lockhart SR, Reed BD, Pierson CL, et al. Most frequent scenario for recurrent Candida vaginitis is strain maintenance with “substrain shuffling”: demonstration by sequential DNA fingerprinting with probes Ca3, C1, and CARE2. J Clin Microbiol. 1996;34:767-777. doi:10.1128/jcm.34.4.767-777.1996
- Anderson MR, Klink K, Cohrssen A. Evaluation of vaginal complaints. JAMA. 2004;291:1368-1379. doi:10.1001/jama.291.11.1368
- Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961-1971. doi:10.1016/S0140-6736(07)60917-9
- Collins LM, Moore R, Sobel JD. Prognosis and long-term outcome of women with idiopathic recurrent vulvovaginal candidiasis caused by Candida albicans. J Low Genit Tract Dis. 2020;24:48-52. doi:10.1097/LGT.0000000000000496
- Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1-50. doi:10.1093/cid/civ933
- Duerr A, Heilig CM, Meikle SF, et al; HER Study Group. Incident and persistent vulvovaginal candidiasis among human immunodeficiency virus–infected women: risk factors and severity. Obstet Gynecol. 2003;101:548-556. doi:10.1016/s0029-7844(02)02729-1
- Houang ET, Chappatte O, Byrne D, et al. Fluconazole levels in plasma and vaginal secretions of patients after a 150-milligram single oral dose and rate of eradication of infection in vaginal candidiasis. Antimicrob Agents Chemother. 1990;34:909-910. doi:10.1128/AAC.34.5.909
- Bérard A, Sheehy O, Zhao JP, et al. Associations between low- and high-dose oral fluconazole and pregnancy outcomes: 3 nested case-control studies. CMAJ. 2019;191:E179-E187. doi:10.1503/cmaj.180963
- Fluconazole. In: Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2006. Revised October 31, 2018. Accessed September 23, 2022. http://www.ncbi.nlm.nih.gov/books/NBK501223/
- White DJ, Johnson EM, Warnock DW. Management of persistent vulvo vaginal candidosis due to azole-resistant Candida glabrata. Genitourin Med. 1993;69:112-114. doi:10.1136/sti.69.2.112
- Falagas ME, Betsi GI, Athanasiou S. Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother. 2006;58:266-272. doi:10.1093/jac/dkl246
- Martens MG, Maximos B, Degenhardt T, et al. Phase 3 study evaluating the safety and efficacy of oteseconazole in the treatment of recurrent vulvovaginal candidiasis and acute vulvovaginal candidiasis infections. Am J Obstet Gynecol. 2022:S0002-9378(22)005774. doi:10.1016/j.ajog.2022.07.023
- Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol. 2021;16:1453-1461. doi:10.2217/fmb-2021-0173
- Vivjoa (oteseconazole). Prescribing information. Mycovia Pharmaceuticals, Inc. April 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215888s000lbl.pdf
- Scorneaux B, Angulo D, Borroto-Esoda K, et al. SCY-078 is fungicidal against Candida species in time-kill studies. Antimicrob Agents Chemother. 2017;61:e01961-16. doi:10.1128/AAC.01961-16
- Schwebke JR, Taylor SN, Ackerman R, et al. Clinical validation of the Aptima bacterial vaginosis and Aptima Candida/Trichomonas vaginitis assays: results from a prospective multicenter clinical study. J Clin Microbiol. 2020;58:e01643-19. doi:10.1128/JCM.01643-19
- Schwebke JR, Gaydos CA, Nyirjesy P, et al. Diagnostic performance of a molecular test versus clinician assessment of vaginitis. J Clin Microbiol. 2018;56:e00252-18. doi:10.1128/JCM.00252-18
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859. doi:10.1097/AOG.0000000000004592
“Blind” endometrial sampling: A call to end the practice
Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.
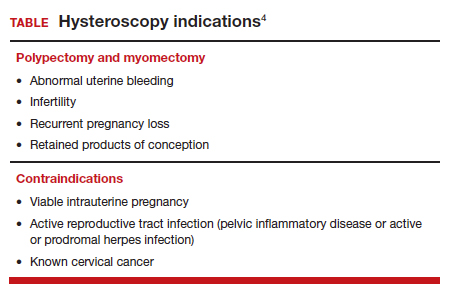
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.
Cutaneous and Subcutaneous Perineuriomas in 2 Pediatric Patients
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.
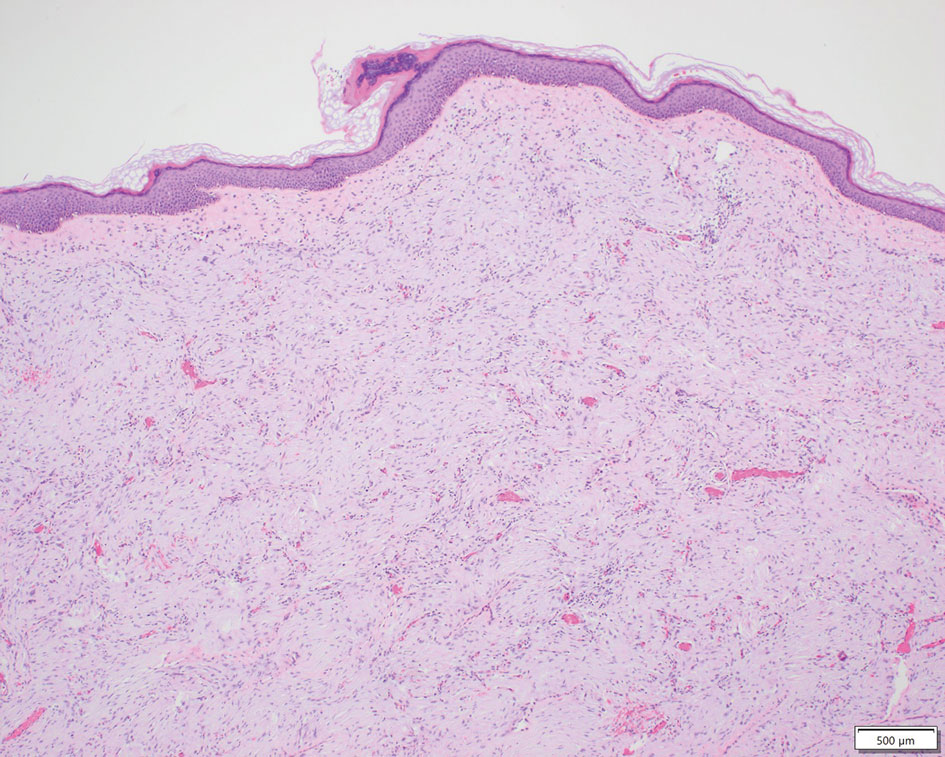
Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.
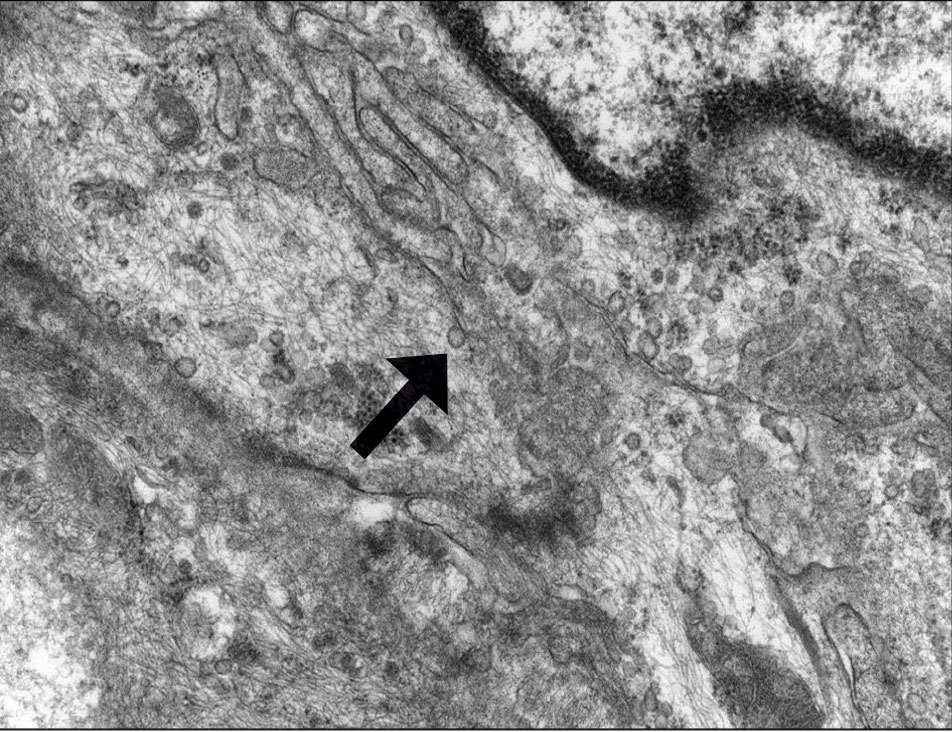
Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.
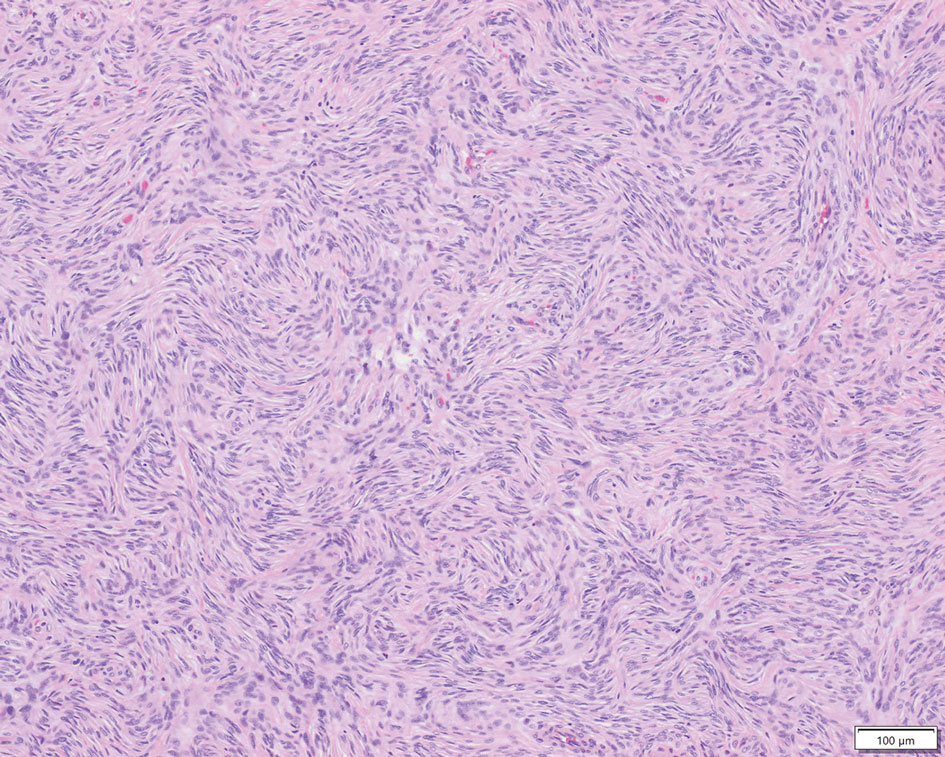
Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3
Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.

Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.

Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.

Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3

Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.

Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.

Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.

Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3

Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
Practice Points
- Perineuriomas are rare benign peripheral nerve sheath tumors that most commonly occur in young to middle-aged adults but rarely can present in children.
- Immunohistochemically, perineuriomas show positive staining with epithelial membrane antigen, GLUT1, claudin-1, and frequently with CD34; they are negative for S-100 and glial fibrillary acidic protein.
- Perineuriomas should be considered in the differential diagnosis in children who present with a well-circumscribed nodular lesion in the subcutaneous tissue.
Past, Present, and Future of Pediatric Atopic Dermatitis Management
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
PRACTICE POINTS
- Pediatric atopic dermatitis (AD) therapeutics have rapidly evolved over the last decade and dermatologists should be aware of new tools in their treatment arsenal.
- New topical nonsteroidal agents serve as useful alternatives to topical corticosteroids through mitigating adverse effects from current standard therapy and potentially simplifying topical regimens.
- Monoclonal antibodies and Janus kinase inhibitors are part of an important set of new systemic therapeutics for pediatric AD.
- Long-term data on these new therapeutics is required to better understand their impact on pediatric AD comorbidities and impact on the longitudinal disease course.