User login
More New Therapeutics for Psoriasis
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
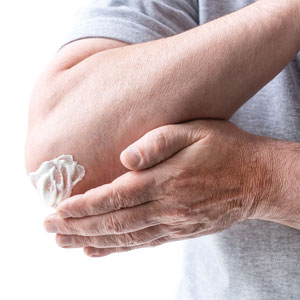
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
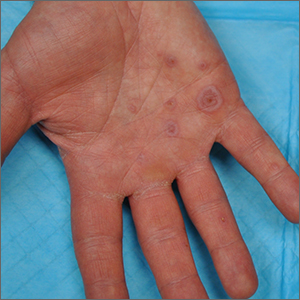
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
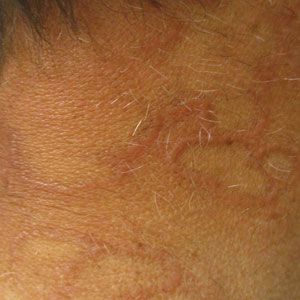
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
New treatments for psoriasis constitute an embarrassment of riches compared to any other area of dermatology. Despite the many advances over the last 25 years, additional topical and systemic treatments have recently become available. Gosh, it’s great!
In May 2022, once-daily tapinarof cream 1% was approved for the topical treatment of plaque psoriasis in adults.1 Tapinarof was identified as a metabolite made by bacteria symbiotic to a nematode, allowing the nematode to infect insects.2 Tapinarof’s anti-inflammatory effect extends to mammals. The drug works by activating the aryl hydrocarbon receptor, downregulating proinflammatory cytokines such as IL-17, and normalizing the expression of skin barrier proteins such as filaggrin.2 In two 12-week, phase 3, randomized trials with 510 and 515 patients, respectively, 35% to 40% of tapinarof-treated psoriasis patients were clear or almost clear compared with only 6% of patients in the placebo group. The drug appears safe; common adverse events (AEs) included folliculitis, nasopharyngitis, contact dermatitis, headache, upper respiratory tract infection, and pruritus.3
A second new topical treatment for plaque psoriasis was approved in July 2022—once-daily roflumilast 0.3% cream—for patients 12 years and older.4 Similar to apremilast, roflumilast is a phosphodiesterase 4 inhibitor that blocks the degradation of cAMP and reduces the downstream production of inflammatory molecules implicated in psoriasis.5 In two 8-week, phase 3 clinical trials (ClinicalTrials.gov Identifiers NCT04211363 and NCT04211389)(N=881), approximately 40% of roflumilast-treated patients were clear or almost clear vs approximately 6% in the placebo group. Topical roflumilast was well-tolerated; the most common AEs included diarrhea, headache, insomnia, nausea, application-site pain, upper respiratory tract infection, and urinary tract infection.6
We have so many patients—and many more people with psoriasis who are not yet patients—with limited psoriasis who would be amenable to topical treatment but who are not responding to current treatments. There is considerable enthusiasm for the new topicals, but it is still questionable how much they will help our patients. The main reason the current topicals fail is poor adherence to the treatment. If we give these new treatments to patients who used existing topicals and failed, thereby inadvertently selecting patients with poor adherence to topicals, it will be surprising if the new treatments live up to expectations. Perhaps tapinarof and roflumilast will revolutionize the management of localized psoriasis; perhaps their impact will be similar to topical crisaborole— exciting in trials and less practical in real life. It may be that apremilast, which is now approved for psoriasis of any severity, will make a bigger difference for patients who can access it for limited psoriasis.
Deucravacitinib is a once-daily oral selective tyrosine kinase 2 inhibitor that blocks IL-23 and type I interferon signaling. It was approved for adults with moderate to severe plaque psoriasis in September 2021.7 We know patients want oral treatment; they ask for apremilast even though injections may be much more potent. In a 16-week, phase 3 clinical trial comparing daily deucravacitinib (n=332), apremilast (n=168), and placebo (n=166), rates of clear or almost clear were approximately 55% in the deucravacitinib group, 32% in the apremilast group, and 7% with placebo. The most common AEs included nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and nausea.8 Although deucravacitinib is much more effective than apremilast, deucravacitinib will require monitoring and may have some risk for viral reactivation of herpes simplex and zoster (and hopefully not much else). Whether physicians view it as a replacement for apremilast, which requires no laboratory monitoring, remains to be seen.
Bimekizumab, a humanized monoclonal IgG1 antibody expected to receive US Food and Drug Administration approval in the coming months, inhibits both IL-17A and IL-17F and may become our most effective treatment of psoriasis. Although we are probably not hungering for a more effective psoriasis treatment (given our current embarrassment of riches), bimekizumab’s remarkably high efficacy for psoriatic arthritis may be a quantum leap forward, especially if no new safety signals are identified; bimekizumab treatment is associated with a higher risk of oral candidiasis than other currently available IL-17 antagonists.9 Biosimilars may reduce the cost of psoriasis management to the health system, but it seems unlikely that biosimilars will allow us to help patients who we cannot already help with the existing extensive psoriasis treatment armamentarium.
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
- Dermavant announces FDA approval for VTAMA® (Tapinarof) cream. International Psoriasis Council. Published May 26, 2022. Accessed January 10, 2023. https://www.psoriasiscouncil.org/treatment/dermavant-vtama/#:~:text=Dermavant%20Sciences%20announced%20that%20VTAMA,and%20Drug%20Administration%20(FDA)
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent [published online November 3, 2020]. J Am Acad Dermatol. 2021;84:1059-1067. doi:10.1016/j.jaad.2020.10.085
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- FDA approves Arcutis’ ZORYVE™ (Roflumilast) cream 0.3% for the treatment of plaque psoriasis in individuals age 12 and older. News release. Arcutis Biotherapeutics; July 29, 2022. Accessed January 10, 2023. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-cream-0-3-for-the-treatment-of-plaque-psoriasis-in-individuals-age-12-and-older/
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;17:11:21-29. doi:10.2147/PTT.S303634
- Zoryve. Package insert. Arcutis Biotherapeutics; 2022.
- Hoy SM. Deucravacitinib: first approval. Drugs. 2022;82:1671-1679. doi:10.1007/s40265-022-01796-y
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Freitas E, Blauvelt A, Torres T. Bimekizumab for the treatment of psoriasis [published online October 8, 2021]. Drugs. 2021;81:1751-1762. doi:10.1007/s40265-021-01612-z
New Treatments for Psoriasis: An Update on a Therapeutic Frontier
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
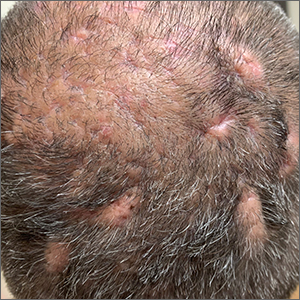
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
The landscape of psoriasis treatments has undergone rapid change within the last decade, and the dizzying speed of drug development has not slowed, with 4 notable entries into the psoriasis treatment armamentarium within the last year: tapinarof, roflumilast, deucravacitinib, and spesolimab. Several others are in late-stage development, and these therapies represent new mechanisms, pathways, and delivery systems that will meaningfully broaden the spectrum of treatment choices for our patients. However, it can be quite difficult to keep track of all of the medication options. This review aims to present the mechanisms and data on both newly available therapeutics for psoriasis and products in the pipeline that may have a major impact on our treatment paradigm for psoriasis in the near future.
Topical Treatments
Tapinarof—Tapinarof is a topical aryl hydrocarbon receptor (AhR)–modulating agent derived from a secondary metabolite produced by a bacterial symbiont of entomopathogenic nematodes.1 Tapinarof binds and activates AhR, inducing a signaling cascade that suppresses the expression of helper T cells TH17 and TH22, upregulates skin barrier protein expression, and reduces epidermal oxidative stress.2 This is a familiar mechanism, as AhR agonism is one of the pathways modulated by coal tar. Tapinarof’s overall effects on immune function, skin barrier integrity, and antioxidant activity show great promise for the treatment of plaque psoriasis.
Two phase 3 trials (N=1025) evaluated the efficacy and safety of once-daily tapinarof cream 1% for plaque psoriasis.3 A physician global assessment (PGA) score of 0/1 occurred in 35.4% to 40.2% of patients in the tapinarof group and in 6.0% of patients in the vehicle group. At week 12, 36.1% to 47.6% of patients treated with daily applications of tapinarof cream achieved a 75% reduction in their baseline psoriasis area and severity index (PASI 75) score compared with 6.9% to 10.2% in the vehicle group.3 In a long-term extension study, a substantial remittive effect of at least 4 months off tapinarof therapy was observed in patients who achieved complete clearance (PGA=0).4 Use of tapinarof cream was associated with folliculitis in up to 23.5% of patients.3,4
Roflumilast—
Topical roflumilast is a selective, highly potent PDE-4 inhibitor with greater affinity for PDE-4 compared to crisaborole and apremilast.8 Two phase 3 trials (N=881) evaluated the efficacy and safety profile of roflumilast cream for plaque psoriasis, with a particular interest in its use for intertriginous areas.9 At week 8, 37.5% to 42.4% of roflumilast-treated patients achieved investigator global assessment (IGA) success compared with 6.1% to 6.9% of vehicle-treated patients. Intertriginous IGA success was observed in 68.1% to 71.2% of patients treated with roflumilast cream compared with 13.8% to 18.5% of vehicle-treated patients. At 8-week follow-up, 39.0% to 41.6% of roflumilast-treated patients achieved PASI 75 vs 5.3% to 7.6% of patients in the vehicle group. Few stinging, burning, or application-site reactions were reported with roflumilast, along with rare instances of gastrointestinal AEs (<4%).9
Oral Therapy
Deucravacitinib—Tyrosine kinase 2 (TYK2) mediates the intracellular signaling of the TH17 and TH1 inflammatory cytokines IL-12/IL-23 and type I interferons, respectively, the former of which are critical in the development of psoriasis via the Janus kinase (JAK) signal transducer and activator of transcription pathway.10 Deucravacitinib is an oral selective TYK2 allosteric inhibitor that binds to the regulatory domain of the enzyme rather than the active catalytic domain, where other TYK2 and JAK1, JAK2, and JAK3 inhibitors bind.11 This unique inhibitory mechanism accounts for the high functional selectivity of deucravacitinib for TYK2 vs the closely related JAK1, JAK2, and JAK3 kinases, thus avoiding the pitfall of prior JAK inhibitors that were associated with major AEs, including an increased risk for serious infections, malignancies, and thrombosis.12 The selective suppression of the inflammatory TYK2 pathway has the potential to shift future therapeutic targets to a narrower range of receptors that may contribute to favorable benefit-risk profiles.
Two phase 3 trials (N=1686) compared the efficacy and safety of deucravacitinib vs placebo and apremilast in adults with moderate to severe plaque psoriasis.13,14 At week 16, 53.0% to 58.4% of deucravacitinib-treated patients achieved PASI 75 compared with 35.1% to 39.8% of apremilast-treated patients. At 16-week follow-up, static PGA response was observed in 49.5% to 53.6% of patients in the deucravacitinib group and 32.1% to 33.9% of the apremilast group. The most frequent AEs associated with deucravacitinib therapy were nasopharyngitis and upper respiratory tract infection, whereas headache, diarrhea, and nausea were more common with apremilast. Treatment with deucravacitinib caused no meaningful changes in laboratory parameters, which are known to change with JAK1, JAK2, and JAK3 inhibitors.13,14 A long-term extension study demonstrated that deucravacitinib had persistent efficacy and consistent safety for up to 2 years.15
Other TYK2 Inhibitors in the Pipeline
Novel oral allosteric TYK2 inhibitors—VTX958 and NDI-034858—and the competitive TYK2 inhibitor PF-06826647 are being developed. Theoretically, these new allosteric inhibitors possess unique structural properties to provide greater TYK2 suppression while bypassing JAK1, JAK2, and JAK3 pathways that may contribute to improved efficacy and safety profiles compared with other TYK2 inhibitors such as deucravacitinib. The results of a phase 1b trial (ClinicalTrials.gov Identifier NCT04999839) showed a dose-dependent reduction of disease severity associated with NDI-034858 treatment for patients with moderate to severe plaque psoriasis, albeit in only 26 patients. At week 4, PASI 50 was achieved in 13%, 57%, and 40% of patients in the 5-, 10-, and 30-mg groups, respectively, compared with 0% in the placebo group.16 In a phase 2 trial of 179 patients, 46.5% and 33.0% of patients treated with 400 and 200 mg of PF-06826647, respectively, achieved PASI 90 at week 16. Conversely, dose-dependent laboratory abnormalities were observed with PF-06826647, including anemia, neutropenia, and increases in creatine phosphokinase.17 At high concentrations, PF-06826647 may disrupt JAK signaling pathways involved in hematopoiesis and renal functions owing to its mode of action as a competitive inhibitor. Overall, these agents are much farther from market, and long-term studies with larger diverse patient cohorts are required to adequately assess the efficacy and safety data of these novel oral TYK2 inhibitors for patients with psoriasis.
EDP1815—EDP1815 is an oral preparation of a single strain of Prevotella histicola being developed for the treatment of inflammatory diseases, including psoriasis. EDP1815 interacts with host intestinal immune cells through the small intestinal axis (SINTAX) to suppress systemic inflammation across the TH1, TH2, and TH17 pathways. Therapy triggers broad immunomodulatory effects without causing systemic absorption, colonic colonization, or modification of the gut microbiome.18 In a phase 2 study (NCT04603027), the primary end point analysis, mean percentage change in PASI between treatment and placebo, demonstrated that at week 16, EDP1815 was superior to placebo with 80% to 90% probability across each cohort. At week 16, 25% to 32% of patients across the 3 cohorts treated with EDP1815 achieved PASI 50 compared with 12% of patients receiving placebo. Gastrointestinal AEs were comparable between treatment and placebo groups. These results suggest that SINTAX-targeted therapies may provide efficacious and safe immunomodulatory effects for patients with mild to moderate psoriasis, who often have limited treatment options. Although improvements may be mild, SINTAX-targeted therapies can be seen as a particularly attractive adjunctive treatment for patients with severe psoriasis taking other medications or as part of a treatment approach for a patient with milder psoriasis.
Biologics
Bimekizumab—Bimekizumab is a monoclonal IgG1 antibody that selectively inhibits IL-17A and IL-17F. Although IL-17A is a more potent cytokine, IL-17F may be more highly expressed in psoriatic lesional skin and independently contribute to the activation of proinflammatory signaling pathways implicated in the pathophysiology of psoriasis.19 Evidence suggests that dual inhibition of IL-17A and IL-17F may provide more complete suppression of inflammation and improved clinical responses than IL-17A inhibition alone.20
Prior bimekizumab phase 3 clinical studies have shown both rapid and durable clinical improvements in skin clearance compared with placebo.21 Three phase 3 trials—BE VIVID (N=567),22 BE SURE (N=478),23 and BE RADIANT (N=743)24—assessed the efficacy and safety of bimekizumab vs the IL-12/IL-23 inhibitor ustekinumab, the tumor necrosis factor inhibitor adalimumab, and the selective IL-17A inhibitor secukinumab, respectively. At week 4, significantly more patients treated with bimekizumab (71%–77%) achieved PASI 75 than patients treated with ustekinumab (15%; P<.0001), adalimumab (31.4%; P<.001), or secukinumab (47.3%; P<.001).22-24 After 16 weeks of treatment, PASI 90 was achieved by 85% to 86.2%, 50%, and 47.2% of patients treated with bimekizumab, ustekinumab, and adalimumab, respectively.22,23 At week 16, PASI 100 was observed in 59% to 61.7%, 21%, 23.9%, and 48.9% of patients treated with bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively. An IGA response (score of 0/1) at week 16 was achieved by 84% to 85.5%, 53%, 57.2%, and 78.6% of patients receiving bimekizumab, ustekinumab, adalimumab, and secukinumab, respectively.22-24
The most common AEs in bimekizumab-treated patients were nasopharyngitis, oral candidiasis, and upper respiratory tract infection.22-24 The dual inhibition of IL-17A and IL-17F suppresses host defenses against Candida at the oral mucosa, increasing the incidence of bimekizumab-associated oral candidiasis.25 Despite the increased risk of Candida infections, these data suggest that inhibition of both IL-17A and IL-17F with bimekizumab may provide faster and greater clinical benefit for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone and other biologic therapies, as the PASI 100 clearance rates across the multiple comparator trials and the placebo-controlled pivotal trial are consistently the highest among any biologic for the treatment of psoriasis.
Spesolimab—The IL-36 pathway and IL-36 receptor genes have been linked to the pathogenesis of generalized pustular psoriasis.26 In a phase 2 trial, 19 of 35 patients (54%) receiving an intravenous dose of spesolimab, an IL-36 receptor inhibitor, had a generalized pustular psoriasis PGA pustulation subscore of 0 (no visible pustules) at the end of week 1 vs 6% of patients in the placebo group.27 A generalized pustular psoriasis PGA total score of 0 or 1 was observed in 43% (15/35) of spesolimab-treated patients compared with 11% (2/18) of patients in the placebo group. The most common AEs in patients treated with spesolimab were minor infections.27 Two open-label phase 3 trials—NCT05200247 and NCT05239039—are underway to determine the long-term efficacy and safety of spesolimab in patients with generalized pustular psoriasis.
Conclusion
Although we have seen a renaissance in psoriasis therapies with the advent of biologics in the last 20 years, recent evidence shows that more innovation is underway. Just in the last year, 2 new mechanisms for treating psoriasis topically without steroids have come to fruition, and there have not been truly novel mechanisms for treating psoriasis topically since approvals for tazarotene and calcipotriene in the 1990s. An entirely new class—TYK2 inhibitors—was developed and landed in psoriasis first, greatly improving the efficacy measures attained with oral medications in general. Finally, an orphan diagnosis got its due with an ambitiously designed study looking at a previously unheard-of 1-week end point, but it comes for one of the few true dermatologic emergencies we encounter, generalized pustular psoriasis. We are fortunate to have so many meaningful new treatments available to us, and it is invigorating to see that even more efficacious biologics and treatments are coming, along with novel concepts such as a treatment affecting the microbiome. Now, we just need to make sure that our patients have the access they deserve to the wide array of available treatments.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
- Bissonnette R, Stein Gold L, Rubenstein DS, et al. Tapinarof in the treatment of psoriasis: a review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent. J Am Acad Dermatol. 2021;84:1059-1067.
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119.
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229.
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806.
- Card GL, England BP, Suzuki Y, et al. Structural basis for the activity of drugs that inhibit phosphodiesterases. Structure. 2004;12:2233-2247.
- Milakovic M, Gooderham MJ. Phosphodiesterase-4 inhibition in psoriasis. Psoriasis (Auckl). 2021;11:21-29.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Dong C, Virtucio C, Zemska O, et al. Treatment of skin inflammation with benzoxaborole phosphodiesterase inhibitors: selectivity, cellular activity, and effect on cytokines associated with skin inflammation and skin architecture changes. J Pharmacol Exp Ther. 2016;358:413-422.
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084.
- Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective tyk2 inhibitors. Drugs. 2020;80:341-352.
- Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973-8995.
- Chimalakonda A, Burke J, Cheng L, et al. Selectivity profile of the tyrosine kinase 2 inhibitor deucravacitinib compared with janus kinase 1/2/3 inhibitors. Dermatol Ther (Heidelb). 2021;11:1763-1776.
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program for Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51.
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39.
- Warren RB, Sofen H, Imafuku S, et al. POS1046 deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program [abstract]. Ann Rheum Dis. 2022;81(suppl 1):841.
- McElwee JJ, Garcet S, Li X, et al. Analysis of histologic, molecular and clinical improvement in moderate-to-severe psoriasis: results from a Phase 1b trial of the novel allosteric TYK2 inhibitor NDI-034858. Poster presented at: American Academy of Dermatology Annual Meeting; March 25, 2022; Boston, MA.
- Tehlirian C, Singh RSP, Pradhan V, et al. Oral tyrosine kinase 2 inhibitor PF-06826647 demonstrates efficacy and an acceptable safety profile in participants with moderate-to-severe plaque psoriasis in a phase 2b, randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2022;87:333-342.
- Hilliard-Barth K, Cormack T, Ramani K, et al. Immune mechanisms of the systemic effects of EDP1815: an orally delivered, gut-restricted microbial drug candidate for the treatment of inflammatory diseases. Poster presented at: Society for Mucosal Immunology Virtual Congress; July 20-22, 2021, Cambridge, MA.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487-498.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385:130-141.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152.
- Blauvelt A, Lebwohl MG, Bissonnette R. IL-23/IL-17A dysfunction phenotypes inform possible clinical effects from anti-IL-17A therapies. J Invest Dermatol. 2015;135:1946-1953.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440.
PRACTICE POINTS
- Roflumilast, a phosphodiesterase 4 inhibitor, and tapinarof, an aryl hydrocarbon receptor–modulating agent, are 2 novel nonsteroidal topical treatments safe for regular long-term use on all affected areas of the skin in adult patients with plaque psoriasis.
- Deucravacitinib is an oral selective tyrosine kinase 2 allosteric inhibitor that has demonstrated a favorable safety profile and greater levels of efficacy than other available oral medications for plaque psoriasis.
- The dual inhibition of IL-17A and IL-17F with bimekizumab provides faster responses and greater clinical benefits for patients with moderate to severe plaque psoriasis than inhibition of IL-17A alone, achieving higher levels of efficacy than has been reported with any other biologic therapy.
- Spesolimab, an IL-36 receptor inhibitor, is an effective, US Food and Drug Administration–approved treatment for patients with generalized pustular psoriasis.
CDC updates guidance on opioid prescribing in adults
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
The Centers for Disease Control and Prevention (CDC) recently published updated guidelines on prescribing opioids for pain that stress the need for a flexible and individual approach to pain management.1 New recommendations emphasize the use of nonopioid therapies whenever appropriate, support consideration of opioid therapy for patients with acute pain when the benefits are expected to outweigh the risks, and urge clinicians to work with patients receiving opioid therapy to determine whether it should be continued or tapered.
This revision to the agency’s 2016 guidelines is aimed at primary care clinicians who prescribe opioids to adult outpatients for treatment of pain. The recommendations are not meant for patients with sickle-cell disease or cancer-related pain, or those receiving palliative and end-of-life care.
Why an update was needed. In 2021, more than 107,000 Americans died of a drug overdose.2 Although prescription opioids caused only about 16% of these deaths, they account for a population death rate of 4:100,000—which, despite national efforts, has not changed much since 2013.3,4
Following publication of the CDC’s 2016 guidelines on prescribing opioids for chronic pain,5 there was a decline in opioid prescribing but not in related deaths. Furthermore, there appeared to have been some negative effects of reduced prescribing, including untreated and undertreated pain, and rapid tapering or sudden discontinuation of opioids in chronic users, causing withdrawal symptoms and psychological distress in these patients. To address these issues, the CDC published the new guideline in 2022.1
Categories of pain. The guideline panel classified pain into 3 categories: acute pain (duration of < 1 month), subacute pain (duration of 1-3 months), and chronic pain (duration of > 3 months).
When to prescribe opioids. The guidelines recommend a new approach to deciding whether to prescribe opioid therapy. In most cases, nonopioid options—such as nonsteroidal anti-inflammatory drugs (NSAIDs) and exercise—should be tried first, since they are as effective as opioids for many types of acute, subacute, and chronic pain. Opioids should be considered if these options fail and the potential benefits outweigh the risks. In moderate-to-severe acute pain, opioids are an option if NSAIDs are unlikely to be effective or are contraindicated.1
How to prescribe opioids. Before prescribing opioids, clinicians should discuss with the patient the known risks and benefits and offer an accompanying prescription for naloxone. Opioids should be prescribed at the lowest effective dose and for a time period limited to the expected duration of the pain. When starting opioids, immediate-release opioids should be prescribed instead of extended-release or long-acting opioids.1
Precautionary measures. Clinicians should review the patient’s history of controlled substance prescriptions via their state’s prescription drug monitoring program and consider the use of toxicology testing to determine whether the patient is receiving high-risk opioid dosages or combinations. Clinicians should be especially cautious about prescribing opioids and benzodiazepines concurrently.1
Continue or stop opioid treatment? A new recommendation advises clinicians to individually assess the benefits and risks of continuing therapy for patients who have been receiving opioids chronically. Whenever the decision is made to stop or reduce treatment, remember that opioid therapy should not be stopped abruptly or reduced quickly. The guideline panel suggests tapering by 10% per month.1
Finally, patients with opioid use disorder should be offered or referred for treatment with medications. Detoxification alone, without medication, is not recommended.1
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
1. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
2. CDC. US overdose deaths in 2021 increased half as much as in 2020—but are still up 15%. Published May 11, 2022. Accessed January 25, 2023. www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/202205.htm
3. CDC. SUDORS Dashboard: fatal overdose data. Updated December 8, 2022. Accessed January 25, 2023. www.cdc.gov/drugoverdose/fatal/dashboard/index.html
4. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths—United States, 2013–2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-207. doi: 10.15585/mmwr.mm7006a4
5. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65:1-49. doi: 10.15585/mmwr.rr6501e1:26987082
Scalp ridges

The gyrate or cerebriform pattern of inflammatory, often pus-filled, subcutaneous tracts of the scalp pointed to a diagnosis of dissecting cellulitis. This patient did not have the fluctuant tracts frequently seen in more active disease but did have the scarring and alopecia common with this disorder.
Dissecting cellulitis is similar to acne and hidradenitis suppurativa in that it starts with follicular plugging. This plugging leads to inflammation, dilation and rupture of the follicle, and purulent sinus tract formation. The sinus tracts of the scalp can be extensive. Dissecting cellulitis is most common in 18- to 40-year-olds and more common in Black individuals.1 When it occurs in conjunction with cystic acne and hidradenitis suppurativa, it is known as the follicular occlusion triad syndrome.
While oral antibiotics are an option for the treatment of dissecting cellulitis, oral isotretinoin is the first-line approach. Tumor necrosis factor alfa inhibitors have also been used with success, according to case reports.1
Given that this patient had a small area of current inflammation, he was started on oral doxycycline 100 mg twice daily for 2 months. He was scheduled for a follow-up appointment in 3 months to reassess his progress and to explore treatment with isotretinoin if the condition worsened or did not improve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Federico A, Rossi A, Caro G, et al. Are dissecting cellulitis and hidradenitis suppurativa different diseases? Clin Dermatol. 2021;39:496-499. doi: 10.1016/j.clindermatol.2021.01.002

The gyrate or cerebriform pattern of inflammatory, often pus-filled, subcutaneous tracts of the scalp pointed to a diagnosis of dissecting cellulitis. This patient did not have the fluctuant tracts frequently seen in more active disease but did have the scarring and alopecia common with this disorder.
Dissecting cellulitis is similar to acne and hidradenitis suppurativa in that it starts with follicular plugging. This plugging leads to inflammation, dilation and rupture of the follicle, and purulent sinus tract formation. The sinus tracts of the scalp can be extensive. Dissecting cellulitis is most common in 18- to 40-year-olds and more common in Black individuals.1 When it occurs in conjunction with cystic acne and hidradenitis suppurativa, it is known as the follicular occlusion triad syndrome.
While oral antibiotics are an option for the treatment of dissecting cellulitis, oral isotretinoin is the first-line approach. Tumor necrosis factor alfa inhibitors have also been used with success, according to case reports.1
Given that this patient had a small area of current inflammation, he was started on oral doxycycline 100 mg twice daily for 2 months. He was scheduled for a follow-up appointment in 3 months to reassess his progress and to explore treatment with isotretinoin if the condition worsened or did not improve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The gyrate or cerebriform pattern of inflammatory, often pus-filled, subcutaneous tracts of the scalp pointed to a diagnosis of dissecting cellulitis. This patient did not have the fluctuant tracts frequently seen in more active disease but did have the scarring and alopecia common with this disorder.
Dissecting cellulitis is similar to acne and hidradenitis suppurativa in that it starts with follicular plugging. This plugging leads to inflammation, dilation and rupture of the follicle, and purulent sinus tract formation. The sinus tracts of the scalp can be extensive. Dissecting cellulitis is most common in 18- to 40-year-olds and more common in Black individuals.1 When it occurs in conjunction with cystic acne and hidradenitis suppurativa, it is known as the follicular occlusion triad syndrome.
While oral antibiotics are an option for the treatment of dissecting cellulitis, oral isotretinoin is the first-line approach. Tumor necrosis factor alfa inhibitors have also been used with success, according to case reports.1
Given that this patient had a small area of current inflammation, he was started on oral doxycycline 100 mg twice daily for 2 months. He was scheduled for a follow-up appointment in 3 months to reassess his progress and to explore treatment with isotretinoin if the condition worsened or did not improve.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Federico A, Rossi A, Caro G, et al. Are dissecting cellulitis and hidradenitis suppurativa different diseases? Clin Dermatol. 2021;39:496-499. doi: 10.1016/j.clindermatol.2021.01.002
1. Federico A, Rossi A, Caro G, et al. Are dissecting cellulitis and hidradenitis suppurativa different diseases? Clin Dermatol. 2021;39:496-499. doi: 10.1016/j.clindermatol.2021.01.002
Palmar rash
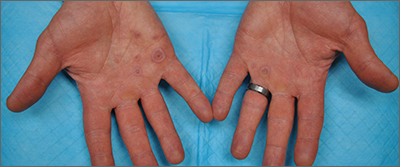
This patient’s targetoid and tingling skin lesions, in association with herpes simplex virus (HSV) infection, are a classic presentation of erythema multiforme (EM).
EM is an acute, self-limited, immune-mediated process that most commonly arises in a symmetrical pattern on acral surfaces. These lesions may be accompanied by eruptions on oral, anogenital, or ocular mucosa. EM is classified into 2 subtypes: major and minor. EM major refers to EM with significant mucosal involvement on at least 2 mucosal sites; it may also manifest with a prodrome of fevers, arthralgias, and malaise. EM minor is used to classify EM with minimal mucosal involvement.1
The term “multiforme” denotes the varied dermatologic changes, including macules, papules, and targetoid lesions with 3 identifiable zones, which are pathognomonic for EM. The classic 3 zones consist of an inner dusky, vesicular, or necrotic center; a middle elevated edematous surrounding ring; and an outer ring of macular erythema. Patients may also present with an atypical macular target lesion, characterized by fewer than 3 zones with an ill-defined border between the zones. The lesions may be asymptomatic, or patients may describe an itchy or burning sensation.
The differential diagnosis of EM includes urticaria, fixed drug eruption, subacute lupus erythematosus, Kawasaki disease, erythema annulare centrifugum, vasculitis, and Stevens-Johnson syndrome.
Infections with HSV types 1 or 2 are the leading cause of EM and are thought to involve a cell-mediated immune process directed against viral antigens in skin.2 Other infectious causes include cytomegalovirus, Epstein-Barr virus, influenza virus, and—rarely—newer strains of coronavirus.3 Pharmacologic reactions are the cause in a small percentage of patients, and may involve nonsteroidal anti-inflammatory drugs, antibiotics, sulfonamides, antiepileptics, and tumor necrosis factor-alpha inhibitors. Studies also link the development of EM to primary malignancy, autoimmune disease, and immunizations.1
The treatment of EM is dependent on the clinical course and severity of the disease. If a causative agent is identified, it should be discontinued (if a drug) or treated (if an infection). Topical antiseptic mouthwashes, antihistamines, and topical corticosteroids can be used to relieve cutaneous discomfort. Biologics and immunosuppressants can be used with patients who have severe symptoms or functional impairment. Patients who have recurrences associated with HSV should be given antiviral prophylaxis for 6 months consisting of oral acyclovir 10 mg/kg/d, valacyclovir 500 to 1000 mg/d, or famciclovir 250 mg twice daily.1
Given the recurrent nature of this patient’s disease, and its association with HSV outbreaks, he was prescribed prophylactic valacyclovir 1000 mg/d orally for 6 months to reduce HSV outbreaks and hopefully prevent future EM episodes.
Photo courtesy of Cyrelle F. Finan, MD. Text courtesy of Lynn Midani, BS, University of New Mexico School of Medicine, and Cyrelle F. Finan, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100:82-88.
2. Hafsi W, Badri T. Erythema multiforme. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated August 1, 2022. Accessed December 15, 2022. www.ncbi.nlm.nih.gov/books/NBK470259/
3. Bennardo L, Nisticò SP, Dastoli S, et al. Erythema multiforme and COVID-19: what do we know? Medicina. 2021;57:828. https://doi.org/10.3390/medicina57080828

This patient’s targetoid and tingling skin lesions, in association with herpes simplex virus (HSV) infection, are a classic presentation of erythema multiforme (EM).
EM is an acute, self-limited, immune-mediated process that most commonly arises in a symmetrical pattern on acral surfaces. These lesions may be accompanied by eruptions on oral, anogenital, or ocular mucosa. EM is classified into 2 subtypes: major and minor. EM major refers to EM with significant mucosal involvement on at least 2 mucosal sites; it may also manifest with a prodrome of fevers, arthralgias, and malaise. EM minor is used to classify EM with minimal mucosal involvement.1
The term “multiforme” denotes the varied dermatologic changes, including macules, papules, and targetoid lesions with 3 identifiable zones, which are pathognomonic for EM. The classic 3 zones consist of an inner dusky, vesicular, or necrotic center; a middle elevated edematous surrounding ring; and an outer ring of macular erythema. Patients may also present with an atypical macular target lesion, characterized by fewer than 3 zones with an ill-defined border between the zones. The lesions may be asymptomatic, or patients may describe an itchy or burning sensation.
The differential diagnosis of EM includes urticaria, fixed drug eruption, subacute lupus erythematosus, Kawasaki disease, erythema annulare centrifugum, vasculitis, and Stevens-Johnson syndrome.
Infections with HSV types 1 or 2 are the leading cause of EM and are thought to involve a cell-mediated immune process directed against viral antigens in skin.2 Other infectious causes include cytomegalovirus, Epstein-Barr virus, influenza virus, and—rarely—newer strains of coronavirus.3 Pharmacologic reactions are the cause in a small percentage of patients, and may involve nonsteroidal anti-inflammatory drugs, antibiotics, sulfonamides, antiepileptics, and tumor necrosis factor-alpha inhibitors. Studies also link the development of EM to primary malignancy, autoimmune disease, and immunizations.1
The treatment of EM is dependent on the clinical course and severity of the disease. If a causative agent is identified, it should be discontinued (if a drug) or treated (if an infection). Topical antiseptic mouthwashes, antihistamines, and topical corticosteroids can be used to relieve cutaneous discomfort. Biologics and immunosuppressants can be used with patients who have severe symptoms or functional impairment. Patients who have recurrences associated with HSV should be given antiviral prophylaxis for 6 months consisting of oral acyclovir 10 mg/kg/d, valacyclovir 500 to 1000 mg/d, or famciclovir 250 mg twice daily.1
Given the recurrent nature of this patient’s disease, and its association with HSV outbreaks, he was prescribed prophylactic valacyclovir 1000 mg/d orally for 6 months to reduce HSV outbreaks and hopefully prevent future EM episodes.
Photo courtesy of Cyrelle F. Finan, MD. Text courtesy of Lynn Midani, BS, University of New Mexico School of Medicine, and Cyrelle F. Finan, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient’s targetoid and tingling skin lesions, in association with herpes simplex virus (HSV) infection, are a classic presentation of erythema multiforme (EM).
EM is an acute, self-limited, immune-mediated process that most commonly arises in a symmetrical pattern on acral surfaces. These lesions may be accompanied by eruptions on oral, anogenital, or ocular mucosa. EM is classified into 2 subtypes: major and minor. EM major refers to EM with significant mucosal involvement on at least 2 mucosal sites; it may also manifest with a prodrome of fevers, arthralgias, and malaise. EM minor is used to classify EM with minimal mucosal involvement.1
The term “multiforme” denotes the varied dermatologic changes, including macules, papules, and targetoid lesions with 3 identifiable zones, which are pathognomonic for EM. The classic 3 zones consist of an inner dusky, vesicular, or necrotic center; a middle elevated edematous surrounding ring; and an outer ring of macular erythema. Patients may also present with an atypical macular target lesion, characterized by fewer than 3 zones with an ill-defined border between the zones. The lesions may be asymptomatic, or patients may describe an itchy or burning sensation.
The differential diagnosis of EM includes urticaria, fixed drug eruption, subacute lupus erythematosus, Kawasaki disease, erythema annulare centrifugum, vasculitis, and Stevens-Johnson syndrome.
Infections with HSV types 1 or 2 are the leading cause of EM and are thought to involve a cell-mediated immune process directed against viral antigens in skin.2 Other infectious causes include cytomegalovirus, Epstein-Barr virus, influenza virus, and—rarely—newer strains of coronavirus.3 Pharmacologic reactions are the cause in a small percentage of patients, and may involve nonsteroidal anti-inflammatory drugs, antibiotics, sulfonamides, antiepileptics, and tumor necrosis factor-alpha inhibitors. Studies also link the development of EM to primary malignancy, autoimmune disease, and immunizations.1
The treatment of EM is dependent on the clinical course and severity of the disease. If a causative agent is identified, it should be discontinued (if a drug) or treated (if an infection). Topical antiseptic mouthwashes, antihistamines, and topical corticosteroids can be used to relieve cutaneous discomfort. Biologics and immunosuppressants can be used with patients who have severe symptoms or functional impairment. Patients who have recurrences associated with HSV should be given antiviral prophylaxis for 6 months consisting of oral acyclovir 10 mg/kg/d, valacyclovir 500 to 1000 mg/d, or famciclovir 250 mg twice daily.1
Given the recurrent nature of this patient’s disease, and its association with HSV outbreaks, he was prescribed prophylactic valacyclovir 1000 mg/d orally for 6 months to reduce HSV outbreaks and hopefully prevent future EM episodes.
Photo courtesy of Cyrelle F. Finan, MD. Text courtesy of Lynn Midani, BS, University of New Mexico School of Medicine, and Cyrelle F. Finan, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100:82-88.
2. Hafsi W, Badri T. Erythema multiforme. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated August 1, 2022. Accessed December 15, 2022. www.ncbi.nlm.nih.gov/books/NBK470259/
3. Bennardo L, Nisticò SP, Dastoli S, et al. Erythema multiforme and COVID-19: what do we know? Medicina. 2021;57:828. https://doi.org/10.3390/medicina57080828
1. Trayes KP, Love G, Studdiford JS. Erythema multiforme: recognition and management. Am Fam Physician. 2019;100:82-88.
2. Hafsi W, Badri T. Erythema multiforme. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated August 1, 2022. Accessed December 15, 2022. www.ncbi.nlm.nih.gov/books/NBK470259/
3. Bennardo L, Nisticò SP, Dastoli S, et al. Erythema multiforme and COVID-19: what do we know? Medicina. 2021;57:828. https://doi.org/10.3390/medicina57080828
Annular Plaques Overlying Hyperpigmented Telangiectatic Patches on the Neck
The Diagnosis: Annular Elastolytic Giant Cell Granuloma
Histologic examination of the shave biopsies showed a granulomatous infiltrate of small lymphocytes, histiocytes, and multinucleated giant cells. The giant cells have abundant eosinophilic cytoplasm, with several also containing fragments of basophilic elastic fibers (elastophagocytosis)(Figure). Additionally, the granulomas revealed no signs of necrosis, making an infectious source unlikely, and examination under polarized light was negative for foreign material. These clinical and histologic findings were diagnostic for annular elastolytic giant cell granuloma (AEGCG).

Annular elastolytic giant cell granuloma is a rare chronic inflammatory disorder that classically presents on sun-exposed skin as annular plaques with elevated borders and atrophic centers.1-4 Histologically, AEGCG is characterized by diffuse granulomatous infiltrates composed of multinucleated giant cells, histiocytes, and lymphocytes in the dermis, along with phagocytosis of elastic fibers by multinucleated giant cells.5 The underlying etiology and pathogenesis of AEGCG remains unknown.6
Annular elastolytic giant cell granuloma commonly affects females aged 35 to 75 years; however, cases have been reported in the male and pediatric patient populations.1,2 Documented cases are known to last from 1 month to 10 years.7,8 Although the mechanisms underlying the development of AEGCG remain to be elucidated, studies have determined that the skin disorder is associated with sarcoidosis, molluscum contagiosum, amyloidosis, diabetes mellitus, and cutaneous T-cell lymphoma.9 Diabetes mellitus is the most common comorbidity associated with AEGCG, and it is theorized that diabetes contributes to the increased incidence of AEGCG in this population by inducing damage to elastic fibers in the skin.10 One study that examined 50 cases of AEGCG found that 38 patients had serum glucose levels evaluated, with 8 cases being subsequently diagnosed with diabetes mellitus and 6 cases with apparent impaired glucose tolerance, indicating that 37% of the sample population with AEGCG who were evaluated for metabolic disease were found to have definitive or latent type 2 diabetes mellitus.11 Although AEGCG is a rare disorder, a substantial number of patients diagnosed with AEGCG also have diabetes mellitus, making it important to consider screening all patients with AEGCG for diabetes given the ease and widely available resources to check glucose levels.
Actinic granuloma, granuloma annulare, atypical facial necrobiosis lipoidica, granuloma multiforme, secondary syphilis, tinea corporis, and erythema annulare centrifugum most commonly are included in the differential diagnosis with AEGCG; histopathology is the key determinant in discerning between these conditions.12 Our patient presented with typical annular plaques overlying hyperpigmented telangiectatic patches. With known type 2 diabetes mellitus and the clinical findings, granuloma annulare, erythema annulare centrifugum, and AEGCG remained high on the differential.
No standard of care exists for AEGCG due to its rare nature and tendency to spontaneously resolve. The most common first-line treatment includes topical and intralesional steroids, topical pimecrolimus, and the use of sunscreen and other sun-protective measures. UV radiation, specifically UVA, has been determined to be a causal factor for AEGCG by changing the antigenicity of elastic fibers and producing an immune response in individuals with fair skin.13 Further, resistant cases of AEGCG successfully have been treated with cyclosporine, systemic steroids, antimalarials, dapsone, and oral retinoids.14,15 Some studies reported partial regression or full resolution with topical tretinoin; adalimumab; clobetasol ointment; or a combination of corticosteroids, antihistamines, and hydroxychloroquine.2 Only 1 case series using sulfasalazine reported worsening symptoms after treatment initiation.16 Our patient deferred systemic medications and was treated with 4 weeks of topical triamcinolone followed by 4 weeks of topical tacrolimus with minimal improvement. At the time of diagnosis, our patient also was encouraged to use sun-protective measures. At 6-month follow-up, the lesions remained stable, and the decision was made to continue with photoprotection.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456. doi:10.7759/cureus.11456
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56:738-745. doi:10.1111/ijd.13502
- Raposo I, Mota F, Lobo I, et al. Annular elastolytic giant cell granuloma: a “visible” diagnosis. Dermatol Online J. 2017;23:13030/qt9rq3j927
- Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132
- Hassan R, Arunprasath P, Padmavathy L, et al. Annular elastolytic giant cell granuloma in association with Hashimoto’s thyroiditis. Indian Dermatol Online J. 2016;7:107-110. doi:10.4103/2229-5178.178087
- Kaya Erdog˘ an H, Arık D, Acer E, et al. Clinicopathological features of annular elastolytic giant cell granuloma patients. Turkish J Dermatol. 2018;12:85-89.
- Can B, Kavala M, Türkog˘ lu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxychloroquine. Int J Dermatol. 2013;52:509-511. doi:10.1111 /j.1365-4632.2011.04941.x
- Arora S, Malik A, Patil C, et al. Annular elastolytic giant cell granuloma: a report of 10 cases. Indian Dermatol Online J. 2015;6(suppl 1):S17-S20. doi:10.4103/2229-5178.171055
- Doulaveri G, Tsagroni E, Giannadaki M, et al. Annular elastolytic giant cell granuloma in a 70-year-old woman. Int J Dermatol. 2003;42:290-291. doi:10.1046/j.1365-4362.2003.01767.x
- Marmon S, O’Reilly KE, Fischer M, et al. Papular variant of annular elastolytic giant-cell granuloma. Dermatol Online J. 2012;18:23.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919. doi:10.1111 /j.1365-2230.2011.04094.x
- Liu X, Zhang W, Liu Y, et al. A case of annular elastolytic giant cell granuloma associated with syphilis. Case Rep Dermatol. 2018; 10:158-161. doi:10.1159/000489910
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33:331-341. doi:10.1016/j.det.2015.03.002
- de Oliveira FL, de Barros Silveira LK, Machado Ade M, et al. Hybrid clinical and histopathological pattern in annular lesions: an overlap between annular elastolytic giant cell granuloma and granuloma annulare? Case Rep Dermatol Med. 2012;2012:102915. doi:10.1155/2012/102915
- Wagenseller A, Larocca C, Vashi NA. Treatment of annular elastolytic giant cell granuloma with topical tretinoin. J Drugs Dermatol. 2017;16:699-700.
- Yang YW, Lehrer MD, Mangold AR, et al. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211-215. doi:10.1111/jdv.16356
The Diagnosis: Annular Elastolytic Giant Cell Granuloma
Histologic examination of the shave biopsies showed a granulomatous infiltrate of small lymphocytes, histiocytes, and multinucleated giant cells. The giant cells have abundant eosinophilic cytoplasm, with several also containing fragments of basophilic elastic fibers (elastophagocytosis)(Figure). Additionally, the granulomas revealed no signs of necrosis, making an infectious source unlikely, and examination under polarized light was negative for foreign material. These clinical and histologic findings were diagnostic for annular elastolytic giant cell granuloma (AEGCG).

Annular elastolytic giant cell granuloma is a rare chronic inflammatory disorder that classically presents on sun-exposed skin as annular plaques with elevated borders and atrophic centers.1-4 Histologically, AEGCG is characterized by diffuse granulomatous infiltrates composed of multinucleated giant cells, histiocytes, and lymphocytes in the dermis, along with phagocytosis of elastic fibers by multinucleated giant cells.5 The underlying etiology and pathogenesis of AEGCG remains unknown.6
Annular elastolytic giant cell granuloma commonly affects females aged 35 to 75 years; however, cases have been reported in the male and pediatric patient populations.1,2 Documented cases are known to last from 1 month to 10 years.7,8 Although the mechanisms underlying the development of AEGCG remain to be elucidated, studies have determined that the skin disorder is associated with sarcoidosis, molluscum contagiosum, amyloidosis, diabetes mellitus, and cutaneous T-cell lymphoma.9 Diabetes mellitus is the most common comorbidity associated with AEGCG, and it is theorized that diabetes contributes to the increased incidence of AEGCG in this population by inducing damage to elastic fibers in the skin.10 One study that examined 50 cases of AEGCG found that 38 patients had serum glucose levels evaluated, with 8 cases being subsequently diagnosed with diabetes mellitus and 6 cases with apparent impaired glucose tolerance, indicating that 37% of the sample population with AEGCG who were evaluated for metabolic disease were found to have definitive or latent type 2 diabetes mellitus.11 Although AEGCG is a rare disorder, a substantial number of patients diagnosed with AEGCG also have diabetes mellitus, making it important to consider screening all patients with AEGCG for diabetes given the ease and widely available resources to check glucose levels.
Actinic granuloma, granuloma annulare, atypical facial necrobiosis lipoidica, granuloma multiforme, secondary syphilis, tinea corporis, and erythema annulare centrifugum most commonly are included in the differential diagnosis with AEGCG; histopathology is the key determinant in discerning between these conditions.12 Our patient presented with typical annular plaques overlying hyperpigmented telangiectatic patches. With known type 2 diabetes mellitus and the clinical findings, granuloma annulare, erythema annulare centrifugum, and AEGCG remained high on the differential.
No standard of care exists for AEGCG due to its rare nature and tendency to spontaneously resolve. The most common first-line treatment includes topical and intralesional steroids, topical pimecrolimus, and the use of sunscreen and other sun-protective measures. UV radiation, specifically UVA, has been determined to be a causal factor for AEGCG by changing the antigenicity of elastic fibers and producing an immune response in individuals with fair skin.13 Further, resistant cases of AEGCG successfully have been treated with cyclosporine, systemic steroids, antimalarials, dapsone, and oral retinoids.14,15 Some studies reported partial regression or full resolution with topical tretinoin; adalimumab; clobetasol ointment; or a combination of corticosteroids, antihistamines, and hydroxychloroquine.2 Only 1 case series using sulfasalazine reported worsening symptoms after treatment initiation.16 Our patient deferred systemic medications and was treated with 4 weeks of topical triamcinolone followed by 4 weeks of topical tacrolimus with minimal improvement. At the time of diagnosis, our patient also was encouraged to use sun-protective measures. At 6-month follow-up, the lesions remained stable, and the decision was made to continue with photoprotection.
The Diagnosis: Annular Elastolytic Giant Cell Granuloma
Histologic examination of the shave biopsies showed a granulomatous infiltrate of small lymphocytes, histiocytes, and multinucleated giant cells. The giant cells have abundant eosinophilic cytoplasm, with several also containing fragments of basophilic elastic fibers (elastophagocytosis)(Figure). Additionally, the granulomas revealed no signs of necrosis, making an infectious source unlikely, and examination under polarized light was negative for foreign material. These clinical and histologic findings were diagnostic for annular elastolytic giant cell granuloma (AEGCG).

Annular elastolytic giant cell granuloma is a rare chronic inflammatory disorder that classically presents on sun-exposed skin as annular plaques with elevated borders and atrophic centers.1-4 Histologically, AEGCG is characterized by diffuse granulomatous infiltrates composed of multinucleated giant cells, histiocytes, and lymphocytes in the dermis, along with phagocytosis of elastic fibers by multinucleated giant cells.5 The underlying etiology and pathogenesis of AEGCG remains unknown.6
Annular elastolytic giant cell granuloma commonly affects females aged 35 to 75 years; however, cases have been reported in the male and pediatric patient populations.1,2 Documented cases are known to last from 1 month to 10 years.7,8 Although the mechanisms underlying the development of AEGCG remain to be elucidated, studies have determined that the skin disorder is associated with sarcoidosis, molluscum contagiosum, amyloidosis, diabetes mellitus, and cutaneous T-cell lymphoma.9 Diabetes mellitus is the most common comorbidity associated with AEGCG, and it is theorized that diabetes contributes to the increased incidence of AEGCG in this population by inducing damage to elastic fibers in the skin.10 One study that examined 50 cases of AEGCG found that 38 patients had serum glucose levels evaluated, with 8 cases being subsequently diagnosed with diabetes mellitus and 6 cases with apparent impaired glucose tolerance, indicating that 37% of the sample population with AEGCG who were evaluated for metabolic disease were found to have definitive or latent type 2 diabetes mellitus.11 Although AEGCG is a rare disorder, a substantial number of patients diagnosed with AEGCG also have diabetes mellitus, making it important to consider screening all patients with AEGCG for diabetes given the ease and widely available resources to check glucose levels.
Actinic granuloma, granuloma annulare, atypical facial necrobiosis lipoidica, granuloma multiforme, secondary syphilis, tinea corporis, and erythema annulare centrifugum most commonly are included in the differential diagnosis with AEGCG; histopathology is the key determinant in discerning between these conditions.12 Our patient presented with typical annular plaques overlying hyperpigmented telangiectatic patches. With known type 2 diabetes mellitus and the clinical findings, granuloma annulare, erythema annulare centrifugum, and AEGCG remained high on the differential.
No standard of care exists for AEGCG due to its rare nature and tendency to spontaneously resolve. The most common first-line treatment includes topical and intralesional steroids, topical pimecrolimus, and the use of sunscreen and other sun-protective measures. UV radiation, specifically UVA, has been determined to be a causal factor for AEGCG by changing the antigenicity of elastic fibers and producing an immune response in individuals with fair skin.13 Further, resistant cases of AEGCG successfully have been treated with cyclosporine, systemic steroids, antimalarials, dapsone, and oral retinoids.14,15 Some studies reported partial regression or full resolution with topical tretinoin; adalimumab; clobetasol ointment; or a combination of corticosteroids, antihistamines, and hydroxychloroquine.2 Only 1 case series using sulfasalazine reported worsening symptoms after treatment initiation.16 Our patient deferred systemic medications and was treated with 4 weeks of topical triamcinolone followed by 4 weeks of topical tacrolimus with minimal improvement. At the time of diagnosis, our patient also was encouraged to use sun-protective measures. At 6-month follow-up, the lesions remained stable, and the decision was made to continue with photoprotection.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456. doi:10.7759/cureus.11456
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56:738-745. doi:10.1111/ijd.13502
- Raposo I, Mota F, Lobo I, et al. Annular elastolytic giant cell granuloma: a “visible” diagnosis. Dermatol Online J. 2017;23:13030/qt9rq3j927
- Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132
- Hassan R, Arunprasath P, Padmavathy L, et al. Annular elastolytic giant cell granuloma in association with Hashimoto’s thyroiditis. Indian Dermatol Online J. 2016;7:107-110. doi:10.4103/2229-5178.178087
- Kaya Erdog˘ an H, Arık D, Acer E, et al. Clinicopathological features of annular elastolytic giant cell granuloma patients. Turkish J Dermatol. 2018;12:85-89.
- Can B, Kavala M, Türkog˘ lu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxychloroquine. Int J Dermatol. 2013;52:509-511. doi:10.1111 /j.1365-4632.2011.04941.x
- Arora S, Malik A, Patil C, et al. Annular elastolytic giant cell granuloma: a report of 10 cases. Indian Dermatol Online J. 2015;6(suppl 1):S17-S20. doi:10.4103/2229-5178.171055
- Doulaveri G, Tsagroni E, Giannadaki M, et al. Annular elastolytic giant cell granuloma in a 70-year-old woman. Int J Dermatol. 2003;42:290-291. doi:10.1046/j.1365-4362.2003.01767.x
- Marmon S, O’Reilly KE, Fischer M, et al. Papular variant of annular elastolytic giant-cell granuloma. Dermatol Online J. 2012;18:23.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919. doi:10.1111 /j.1365-2230.2011.04094.x
- Liu X, Zhang W, Liu Y, et al. A case of annular elastolytic giant cell granuloma associated with syphilis. Case Rep Dermatol. 2018; 10:158-161. doi:10.1159/000489910
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33:331-341. doi:10.1016/j.det.2015.03.002
- de Oliveira FL, de Barros Silveira LK, Machado Ade M, et al. Hybrid clinical and histopathological pattern in annular lesions: an overlap between annular elastolytic giant cell granuloma and granuloma annulare? Case Rep Dermatol Med. 2012;2012:102915. doi:10.1155/2012/102915
- Wagenseller A, Larocca C, Vashi NA. Treatment of annular elastolytic giant cell granuloma with topical tretinoin. J Drugs Dermatol. 2017;16:699-700.
- Yang YW, Lehrer MD, Mangold AR, et al. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211-215. doi:10.1111/jdv.16356
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456. doi:10.7759/cureus.11456
- Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56:738-745. doi:10.1111/ijd.13502
- Raposo I, Mota F, Lobo I, et al. Annular elastolytic giant cell granuloma: a “visible” diagnosis. Dermatol Online J. 2017;23:13030/qt9rq3j927
- Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132
- Hassan R, Arunprasath P, Padmavathy L, et al. Annular elastolytic giant cell granuloma in association with Hashimoto’s thyroiditis. Indian Dermatol Online J. 2016;7:107-110. doi:10.4103/2229-5178.178087
- Kaya Erdog˘ an H, Arık D, Acer E, et al. Clinicopathological features of annular elastolytic giant cell granuloma patients. Turkish J Dermatol. 2018;12:85-89.
- Can B, Kavala M, Türkog˘ lu Z, et al. Successful treatment of annular elastolytic giant cell granuloma with hydroxychloroquine. Int J Dermatol. 2013;52:509-511. doi:10.1111 /j.1365-4632.2011.04941.x
- Arora S, Malik A, Patil C, et al. Annular elastolytic giant cell granuloma: a report of 10 cases. Indian Dermatol Online J. 2015;6(suppl 1):S17-S20. doi:10.4103/2229-5178.171055
- Doulaveri G, Tsagroni E, Giannadaki M, et al. Annular elastolytic giant cell granuloma in a 70-year-old woman. Int J Dermatol. 2003;42:290-291. doi:10.1046/j.1365-4362.2003.01767.x
- Marmon S, O’Reilly KE, Fischer M, et al. Papular variant of annular elastolytic giant-cell granuloma. Dermatol Online J. 2012;18:23.
- Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36:917-919. doi:10.1111 /j.1365-2230.2011.04094.x
- Liu X, Zhang W, Liu Y, et al. A case of annular elastolytic giant cell granuloma associated with syphilis. Case Rep Dermatol. 2018; 10:158-161. doi:10.1159/000489910
- Gutiérrez-González E, Pereiro M Jr, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33:331-341. doi:10.1016/j.det.2015.03.002
- de Oliveira FL, de Barros Silveira LK, Machado Ade M, et al. Hybrid clinical and histopathological pattern in annular lesions: an overlap between annular elastolytic giant cell granuloma and granuloma annulare? Case Rep Dermatol Med. 2012;2012:102915. doi:10.1155/2012/102915
- Wagenseller A, Larocca C, Vashi NA. Treatment of annular elastolytic giant cell granuloma with topical tretinoin. J Drugs Dermatol. 2017;16:699-700.
- Yang YW, Lehrer MD, Mangold AR, et al. Treatment of granuloma annulare and related granulomatous diseases with sulphasalazine: a series of 16 cases. J Eur Acad Dermatol Venereol. 2021;35:211-215. doi:10.1111/jdv.16356
A 58-year-old man with a history of type 2 diabetes mellitus, nephrolithiasis, hypovitaminosis D, and hypercholesterolemia presented to our dermatology clinic for a follow-up total-body skin examination after a prior diagnosis of basal cell carcinoma on the vertex of the scalp. Physical examination revealed extensive photodamage and annular plaques overlying hyperpigmented telangiectatic patches on the dorsal portion of the neck. The eruption persisted for 1 year and failed to improve with clotrimazole cream. His medications included simvastatin, metformin, chlorthalidone, vitamin D, and tamsulosin. Two shave biopsies from the posterior neck were performed.

Commentary: Early Diagnosis of PsA, February 2023
Appropriate assessment of MSK symptoms and signs by dermatologists may lead to more appropriate referral to rheumatologists. MSUS is being increasingly explored for early identification of PsA. A handheld, chip-based ultrasound device (HHUD) is a novel promising instrument that can be easily implemented in clinical practice. In a prospective study including 140 patients with psoriasis who presented to dermatologists with arthralgia. Grobelski and colleagues screened for PsA using medical history, CE, and the German Psoriasis Arthritis Diagnostic PsA screening questionnaire (GEPARD) paired with MSUS examination of up to three painful joints by trained dermatologists. Nineteen patients (13.6%) were diagnosed with PsA by rheumatologists. Interestingly, in 45 of the 46 patients the preliminary diagnosis of PsA was revised to "no PsA" after MSUS. The addition of MSUS changed the sensitivity and specificity of early PsA screening strategy from 88.2% and 54.4% to 70.6% and 90.4%, respectively. The positive predictive value increased to 56.5% from 25.4% after MSUS. Thus, the use of a quick MSUS using HHUD may lead to more accurate referral to rheumatologists. The challenge is seamless integration of MSUS into busy dermatology practices.
The goal of PsA treatment is to achieve a state of remission or low disease activity. Criteria for minimal disease activity (MDA) have been established. Achieving MDA leads to better health-related quality of life (HRQOL), as well as less joint damage. In a prospective cohort study that included 240 patients with newly diagnosed disease-modifying antirheumatic drug-naive PsA, Snoeck Henkemans and colleagues demonstrate that failure to achieve MDA in the first year after the diagnosis of PsA was associated with worse HRQOL and health status, functional impairment, fatigue, pain, and higher anxiety and depression. Compared with patients who achieved sustained MDA in the first year after diagnosis, those who did not achieve MDA had higher scores for pain, fatigue, and functional ability and higher anxiety and depression during follow-up, which persisted despite treatment intensification. Thus, implementation of treat-to-target strategies with the aim of achieving sustained MDA within 1 year of diagnosis is likely to have better long-term benefits in this lifelong disease.
Another study emphasized the need for early treatment to improve long-term outcomes. In a post hoc analysis of two phase 3 trials including 1554 patients with PsA who received 300-mg or 150-mg secukinumab with or without a loading dose, Mease and colleagues showed that high baseline radiographic damage reduced the likelihood of achieving MDA.
Overall, these studies indicate that early diagnosis and treatment prior to developing joint damage with the aim to achieve sustained MDA within a year will lead to better long-term outcome for patients with PsA.
Appropriate assessment of MSK symptoms and signs by dermatologists may lead to more appropriate referral to rheumatologists. MSUS is being increasingly explored for early identification of PsA. A handheld, chip-based ultrasound device (HHUD) is a novel promising instrument that can be easily implemented in clinical practice. In a prospective study including 140 patients with psoriasis who presented to dermatologists with arthralgia. Grobelski and colleagues screened for PsA using medical history, CE, and the German Psoriasis Arthritis Diagnostic PsA screening questionnaire (GEPARD) paired with MSUS examination of up to three painful joints by trained dermatologists. Nineteen patients (13.6%) were diagnosed with PsA by rheumatologists. Interestingly, in 45 of the 46 patients the preliminary diagnosis of PsA was revised to "no PsA" after MSUS. The addition of MSUS changed the sensitivity and specificity of early PsA screening strategy from 88.2% and 54.4% to 70.6% and 90.4%, respectively. The positive predictive value increased to 56.5% from 25.4% after MSUS. Thus, the use of a quick MSUS using HHUD may lead to more accurate referral to rheumatologists. The challenge is seamless integration of MSUS into busy dermatology practices.
The goal of PsA treatment is to achieve a state of remission or low disease activity. Criteria for minimal disease activity (MDA) have been established. Achieving MDA leads to better health-related quality of life (HRQOL), as well as less joint damage. In a prospective cohort study that included 240 patients with newly diagnosed disease-modifying antirheumatic drug-naive PsA, Snoeck Henkemans and colleagues demonstrate that failure to achieve MDA in the first year after the diagnosis of PsA was associated with worse HRQOL and health status, functional impairment, fatigue, pain, and higher anxiety and depression. Compared with patients who achieved sustained MDA in the first year after diagnosis, those who did not achieve MDA had higher scores for pain, fatigue, and functional ability and higher anxiety and depression during follow-up, which persisted despite treatment intensification. Thus, implementation of treat-to-target strategies with the aim of achieving sustained MDA within 1 year of diagnosis is likely to have better long-term benefits in this lifelong disease.
Another study emphasized the need for early treatment to improve long-term outcomes. In a post hoc analysis of two phase 3 trials including 1554 patients with PsA who received 300-mg or 150-mg secukinumab with or without a loading dose, Mease and colleagues showed that high baseline radiographic damage reduced the likelihood of achieving MDA.
Overall, these studies indicate that early diagnosis and treatment prior to developing joint damage with the aim to achieve sustained MDA within a year will lead to better long-term outcome for patients with PsA.
Appropriate assessment of MSK symptoms and signs by dermatologists may lead to more appropriate referral to rheumatologists. MSUS is being increasingly explored for early identification of PsA. A handheld, chip-based ultrasound device (HHUD) is a novel promising instrument that can be easily implemented in clinical practice. In a prospective study including 140 patients with psoriasis who presented to dermatologists with arthralgia. Grobelski and colleagues screened for PsA using medical history, CE, and the German Psoriasis Arthritis Diagnostic PsA screening questionnaire (GEPARD) paired with MSUS examination of up to three painful joints by trained dermatologists. Nineteen patients (13.6%) were diagnosed with PsA by rheumatologists. Interestingly, in 45 of the 46 patients the preliminary diagnosis of PsA was revised to "no PsA" after MSUS. The addition of MSUS changed the sensitivity and specificity of early PsA screening strategy from 88.2% and 54.4% to 70.6% and 90.4%, respectively. The positive predictive value increased to 56.5% from 25.4% after MSUS. Thus, the use of a quick MSUS using HHUD may lead to more accurate referral to rheumatologists. The challenge is seamless integration of MSUS into busy dermatology practices.
The goal of PsA treatment is to achieve a state of remission or low disease activity. Criteria for minimal disease activity (MDA) have been established. Achieving MDA leads to better health-related quality of life (HRQOL), as well as less joint damage. In a prospective cohort study that included 240 patients with newly diagnosed disease-modifying antirheumatic drug-naive PsA, Snoeck Henkemans and colleagues demonstrate that failure to achieve MDA in the first year after the diagnosis of PsA was associated with worse HRQOL and health status, functional impairment, fatigue, pain, and higher anxiety and depression. Compared with patients who achieved sustained MDA in the first year after diagnosis, those who did not achieve MDA had higher scores for pain, fatigue, and functional ability and higher anxiety and depression during follow-up, which persisted despite treatment intensification. Thus, implementation of treat-to-target strategies with the aim of achieving sustained MDA within 1 year of diagnosis is likely to have better long-term benefits in this lifelong disease.
Another study emphasized the need for early treatment to improve long-term outcomes. In a post hoc analysis of two phase 3 trials including 1554 patients with PsA who received 300-mg or 150-mg secukinumab with or without a loading dose, Mease and colleagues showed that high baseline radiographic damage reduced the likelihood of achieving MDA.
Overall, these studies indicate that early diagnosis and treatment prior to developing joint damage with the aim to achieve sustained MDA within a year will lead to better long-term outcome for patients with PsA.
14-year-old boy • aching midsternal pain following a basketball injury • worsening pain with direct pressure and when the patient sneezed • Dx?
THE CASE
A 14-year-old boy sought care at our clinic for persistent chest pain after being hit in the chest with a teammate’s shoulder during a basketball game 3 weeks earlier. He had aching midsternal chest pain that worsened with direct pressure and when he sneezed, twisted, or bent forward. There was no bruising or swelling.
On examination, the patient demonstrated normal perfusion and normal work of breathing. He had focal tenderness with palpation at the manubrium with no noticeable step-off, and mild tenderness at the adjacent costochondral junctions and over his pectoral muscles. His sternal pain along the proximal sternum was reproducible with a weighted wall push-up. Although the patient maintained full range of motion in his upper extremities, he did have sternal pain with flexion, abduction, and external rotation of the bilateral upper extremities against resistance. Anteroposterior (AP) and lateral chest radiographs were unremarkable.
THE DIAGNOSIS
The unremarkable chest radiographs prompted further investigation with a diagnostic ultrasound, which revealed a small cortical defect with overlying anechoic fluid collection in the area of focal tenderness. T2-weighted magnetic resonance imaging (MRI) of the chest was performed; it revealed a transverse, nondisplaced fracture of the superior body of the sternum with surrounding bone marrow edema (FIGURE).
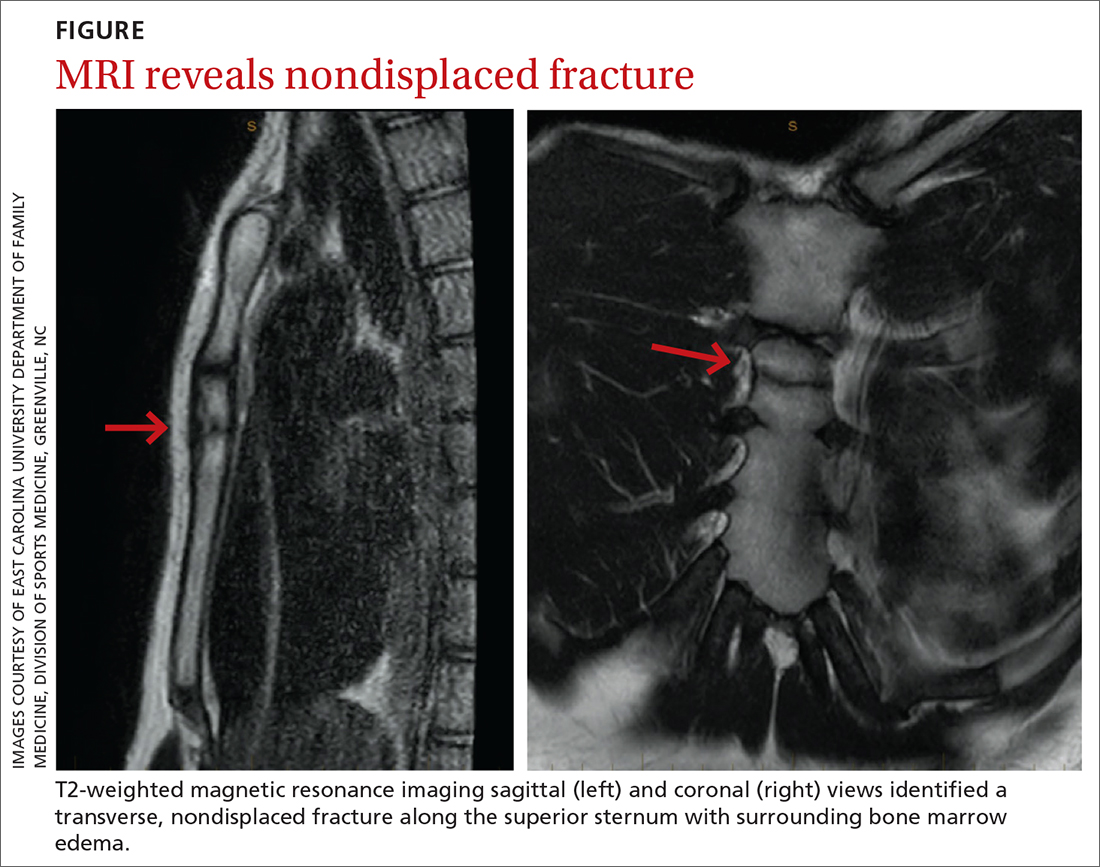
DISCUSSION
Fractures of the sternum comprise < 1% of traumatic fractures and have a low mortality rate (0.7%).1,2 The rarity of these fractures is attributed to the ribs’ elastic recoil, which protects the chest wall from anterior forces.1,3 These fractures are even more unusual in children due to the increased elasticity of their chest walls.4-6 Thus, it takes a significant amount of force for a child’s sternum to fracture.
While isolated sternum fractures can occur, two-thirds of sternum fractures are nonisolated and are associated with injuries to surrounding structures (including the heart, lungs, and vasculature) or fractures of the ribs and spine.2,3 Most often, these injuries are caused by significant blunt trauma to the anterior chest, rapid deceleration, or flexion-compression injury.2,3 They are typically transverse and localized, with 70% of fractures occurring in the mid-body and 17.6% at the manubriosternal joint.1,3,6
Athletes with a sternal fracture typically present as our patient did, with a history of blunt force trauma to the chest and with pain and tenderness over the anterior midline of the chest that increases with respiration or movement.1 A physical examination that includes chest palpation and auscultation of the heart and lungs must be performed to rule out damage to intrathoracic structures and assess the patient’s cardiac and pulmonary stability. An electrocardiogram should be performed to confirm that there are no cardiovascular complications.3,4
Initial imaging should include AP and lateral chest radiographs because any displacement will occur in the sagittal plane.1,2,4-6 If the radiograph shows no clear pathology, follow up with computed tomography, ultrasound, MRI, or technetium bone scans to gain additional information.1 Diagnosis of sternal fractures is especially difficult in children due to the presence of ossification centers for bone growth, which may be misinterpreted as a sternal fracture in the absence of a proper understanding of sternal development.5,6 On ultrasound, sternal fractures appear as a sharp step-off in the cortex, whereas in the absence of fracture, there is no cortical step-off and the cartilaginous plate between ossification centers appears in line with the cortex.7
Continue to: A self-limiting injury that requires proper pain control
A self-limiting injury that requires proper pain control
Isolated sternal fractures are typically self-limiting with a good prognosis.2 These injuries are managed supportively with rest, ice, and analgesics1; proper pain control is crucial to prevent respiratory compromise.8
Complete recovery for most patients occurs in 10 to 12 weeks.9 Recovery periods longer than 12 weeks are associated with nonisolated sternal fractures that are complicated by soft-tissue injury, injuries to the chest wall (such as sternoclavicular joint dislocation, usually from a fall on the shoulder), or fracture nonunion.1,2,5
Anterior sternoclavicular joint dislocations and stable posterior dislocations are managed with closed reduction and immobilization in a figure-of-eight brace.1 Operative management is reserved for patients with displaced fractures, sternal deformity, chest wall instability, respiratory insufficiency, uncontrolled pain, or fracture nonunion.1,3,8
A return-to-play protocol can begin once the patient is asymptomatic.1 The timeframe for a full return to play can vary from 6 weeks to 6 months, depending on the severity of the fracture.1 This process is guided by how quickly the symptoms resolve and by radiographic stability.9
Our patient was followed every 3 to 4 weeks and started physical therapy 6 weeks after his injury occurred. He was held from play for 10 weeks and gradually returned to play; he returned to full-contact activity after tolerating a practice without pain.
THE TAKEAWAY
Children typically have greater chest wall elasticity, and thus, it is unusual for them to sustain a sternal fracture. Diagnosis in children is complicated by the presence of ossification centers for bone growth on imaging. In this case, the fracture was first noticed on ultrasound and confirmed with MRI. Since these fractures can be associated with damage to surrounding structures, additional injuries should be considered when evaluating a patient with a sternum fracture.
CORRESPONDENCE
Catherine Romaine, East Carolina University, Brody School of Medicine, 600 Moye Boulevard, Greenville, NC 27834; [email protected]
1. Alent J, Narducci DM, Moran B, et al. Sternal injuries in sport: a review of the literature. Sports Med. 2018;48:2715-2724. doi: 10.1007/s40279-018-0990-5
2. Khoriati A-A, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock. 2013;6:113-116. doi: 10.4103/0974-2700.110763
3. Athanassiadi K, Gerazounis M, Moustardas M, et al. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246. doi: 10.1007/s00268-002-6511-5
4. Ferguson LP, Wilkinson AG, Beattie TF. Fracture of the sternum in children. Emerg Med J. 2003;20:518-520. doi: 10.1136/emj.20.6.518
5. Ramgopal S, Shaffiey SA, Conti KA. Pediatric sternal fractures from a Level 1 trauma center. J Pediatr Surg. 2019;54:1628-1631. doi: 10.1016/j.jpedsurg.2018.08.040
6. Sesia SB, Prüfer F, Mayr J. Sternal fracture in children: diagnosis by ultrasonography. European J Pediatr Surg Rep. 2017;5:e39-e42. doi: 10.1055/s-0037-1606197
7. Nickson C, Rippey J. Ultrasonography of sternal fractures. Australas J Ultrasound Med. 2011;14:6-11. doi: 10.1002/j.2205-0140.2011.tb00131.x
8. Bauman ZM, Yanala U, Waibel BH, et al. Sternal fixation for isolated traumatic sternal fractures improves pain and upper extremity range of motion. Eur J Trauma Emerg Surg. 2022;48:225-230. doi: 10.1007/s00068-020-01568-x
9. Culp B, Hurbanek JG, Novak J, et al. Acute traumatic sternum fracture in a female college hockey player. Orthopedics. 2010;33:683. doi: 10.3928/01477447-20100722-17
THE CASE
A 14-year-old boy sought care at our clinic for persistent chest pain after being hit in the chest with a teammate’s shoulder during a basketball game 3 weeks earlier. He had aching midsternal chest pain that worsened with direct pressure and when he sneezed, twisted, or bent forward. There was no bruising or swelling.
On examination, the patient demonstrated normal perfusion and normal work of breathing. He had focal tenderness with palpation at the manubrium with no noticeable step-off, and mild tenderness at the adjacent costochondral junctions and over his pectoral muscles. His sternal pain along the proximal sternum was reproducible with a weighted wall push-up. Although the patient maintained full range of motion in his upper extremities, he did have sternal pain with flexion, abduction, and external rotation of the bilateral upper extremities against resistance. Anteroposterior (AP) and lateral chest radiographs were unremarkable.
THE DIAGNOSIS
The unremarkable chest radiographs prompted further investigation with a diagnostic ultrasound, which revealed a small cortical defect with overlying anechoic fluid collection in the area of focal tenderness. T2-weighted magnetic resonance imaging (MRI) of the chest was performed; it revealed a transverse, nondisplaced fracture of the superior body of the sternum with surrounding bone marrow edema (FIGURE).

DISCUSSION
Fractures of the sternum comprise < 1% of traumatic fractures and have a low mortality rate (0.7%).1,2 The rarity of these fractures is attributed to the ribs’ elastic recoil, which protects the chest wall from anterior forces.1,3 These fractures are even more unusual in children due to the increased elasticity of their chest walls.4-6 Thus, it takes a significant amount of force for a child’s sternum to fracture.
While isolated sternum fractures can occur, two-thirds of sternum fractures are nonisolated and are associated with injuries to surrounding structures (including the heart, lungs, and vasculature) or fractures of the ribs and spine.2,3 Most often, these injuries are caused by significant blunt trauma to the anterior chest, rapid deceleration, or flexion-compression injury.2,3 They are typically transverse and localized, with 70% of fractures occurring in the mid-body and 17.6% at the manubriosternal joint.1,3,6
Athletes with a sternal fracture typically present as our patient did, with a history of blunt force trauma to the chest and with pain and tenderness over the anterior midline of the chest that increases with respiration or movement.1 A physical examination that includes chest palpation and auscultation of the heart and lungs must be performed to rule out damage to intrathoracic structures and assess the patient’s cardiac and pulmonary stability. An electrocardiogram should be performed to confirm that there are no cardiovascular complications.3,4
Initial imaging should include AP and lateral chest radiographs because any displacement will occur in the sagittal plane.1,2,4-6 If the radiograph shows no clear pathology, follow up with computed tomography, ultrasound, MRI, or technetium bone scans to gain additional information.1 Diagnosis of sternal fractures is especially difficult in children due to the presence of ossification centers for bone growth, which may be misinterpreted as a sternal fracture in the absence of a proper understanding of sternal development.5,6 On ultrasound, sternal fractures appear as a sharp step-off in the cortex, whereas in the absence of fracture, there is no cortical step-off and the cartilaginous plate between ossification centers appears in line with the cortex.7
Continue to: A self-limiting injury that requires proper pain control
A self-limiting injury that requires proper pain control
Isolated sternal fractures are typically self-limiting with a good prognosis.2 These injuries are managed supportively with rest, ice, and analgesics1; proper pain control is crucial to prevent respiratory compromise.8
Complete recovery for most patients occurs in 10 to 12 weeks.9 Recovery periods longer than 12 weeks are associated with nonisolated sternal fractures that are complicated by soft-tissue injury, injuries to the chest wall (such as sternoclavicular joint dislocation, usually from a fall on the shoulder), or fracture nonunion.1,2,5
Anterior sternoclavicular joint dislocations and stable posterior dislocations are managed with closed reduction and immobilization in a figure-of-eight brace.1 Operative management is reserved for patients with displaced fractures, sternal deformity, chest wall instability, respiratory insufficiency, uncontrolled pain, or fracture nonunion.1,3,8
A return-to-play protocol can begin once the patient is asymptomatic.1 The timeframe for a full return to play can vary from 6 weeks to 6 months, depending on the severity of the fracture.1 This process is guided by how quickly the symptoms resolve and by radiographic stability.9
Our patient was followed every 3 to 4 weeks and started physical therapy 6 weeks after his injury occurred. He was held from play for 10 weeks and gradually returned to play; he returned to full-contact activity after tolerating a practice without pain.
THE TAKEAWAY
Children typically have greater chest wall elasticity, and thus, it is unusual for them to sustain a sternal fracture. Diagnosis in children is complicated by the presence of ossification centers for bone growth on imaging. In this case, the fracture was first noticed on ultrasound and confirmed with MRI. Since these fractures can be associated with damage to surrounding structures, additional injuries should be considered when evaluating a patient with a sternum fracture.
CORRESPONDENCE
Catherine Romaine, East Carolina University, Brody School of Medicine, 600 Moye Boulevard, Greenville, NC 27834; [email protected]
THE CASE
A 14-year-old boy sought care at our clinic for persistent chest pain after being hit in the chest with a teammate’s shoulder during a basketball game 3 weeks earlier. He had aching midsternal chest pain that worsened with direct pressure and when he sneezed, twisted, or bent forward. There was no bruising or swelling.
On examination, the patient demonstrated normal perfusion and normal work of breathing. He had focal tenderness with palpation at the manubrium with no noticeable step-off, and mild tenderness at the adjacent costochondral junctions and over his pectoral muscles. His sternal pain along the proximal sternum was reproducible with a weighted wall push-up. Although the patient maintained full range of motion in his upper extremities, he did have sternal pain with flexion, abduction, and external rotation of the bilateral upper extremities against resistance. Anteroposterior (AP) and lateral chest radiographs were unremarkable.
THE DIAGNOSIS
The unremarkable chest radiographs prompted further investigation with a diagnostic ultrasound, which revealed a small cortical defect with overlying anechoic fluid collection in the area of focal tenderness. T2-weighted magnetic resonance imaging (MRI) of the chest was performed; it revealed a transverse, nondisplaced fracture of the superior body of the sternum with surrounding bone marrow edema (FIGURE).

DISCUSSION
Fractures of the sternum comprise < 1% of traumatic fractures and have a low mortality rate (0.7%).1,2 The rarity of these fractures is attributed to the ribs’ elastic recoil, which protects the chest wall from anterior forces.1,3 These fractures are even more unusual in children due to the increased elasticity of their chest walls.4-6 Thus, it takes a significant amount of force for a child’s sternum to fracture.
While isolated sternum fractures can occur, two-thirds of sternum fractures are nonisolated and are associated with injuries to surrounding structures (including the heart, lungs, and vasculature) or fractures of the ribs and spine.2,3 Most often, these injuries are caused by significant blunt trauma to the anterior chest, rapid deceleration, or flexion-compression injury.2,3 They are typically transverse and localized, with 70% of fractures occurring in the mid-body and 17.6% at the manubriosternal joint.1,3,6
Athletes with a sternal fracture typically present as our patient did, with a history of blunt force trauma to the chest and with pain and tenderness over the anterior midline of the chest that increases with respiration or movement.1 A physical examination that includes chest palpation and auscultation of the heart and lungs must be performed to rule out damage to intrathoracic structures and assess the patient’s cardiac and pulmonary stability. An electrocardiogram should be performed to confirm that there are no cardiovascular complications.3,4
Initial imaging should include AP and lateral chest radiographs because any displacement will occur in the sagittal plane.1,2,4-6 If the radiograph shows no clear pathology, follow up with computed tomography, ultrasound, MRI, or technetium bone scans to gain additional information.1 Diagnosis of sternal fractures is especially difficult in children due to the presence of ossification centers for bone growth, which may be misinterpreted as a sternal fracture in the absence of a proper understanding of sternal development.5,6 On ultrasound, sternal fractures appear as a sharp step-off in the cortex, whereas in the absence of fracture, there is no cortical step-off and the cartilaginous plate between ossification centers appears in line with the cortex.7
Continue to: A self-limiting injury that requires proper pain control
A self-limiting injury that requires proper pain control
Isolated sternal fractures are typically self-limiting with a good prognosis.2 These injuries are managed supportively with rest, ice, and analgesics1; proper pain control is crucial to prevent respiratory compromise.8
Complete recovery for most patients occurs in 10 to 12 weeks.9 Recovery periods longer than 12 weeks are associated with nonisolated sternal fractures that are complicated by soft-tissue injury, injuries to the chest wall (such as sternoclavicular joint dislocation, usually from a fall on the shoulder), or fracture nonunion.1,2,5
Anterior sternoclavicular joint dislocations and stable posterior dislocations are managed with closed reduction and immobilization in a figure-of-eight brace.1 Operative management is reserved for patients with displaced fractures, sternal deformity, chest wall instability, respiratory insufficiency, uncontrolled pain, or fracture nonunion.1,3,8
A return-to-play protocol can begin once the patient is asymptomatic.1 The timeframe for a full return to play can vary from 6 weeks to 6 months, depending on the severity of the fracture.1 This process is guided by how quickly the symptoms resolve and by radiographic stability.9
Our patient was followed every 3 to 4 weeks and started physical therapy 6 weeks after his injury occurred. He was held from play for 10 weeks and gradually returned to play; he returned to full-contact activity after tolerating a practice without pain.
THE TAKEAWAY
Children typically have greater chest wall elasticity, and thus, it is unusual for them to sustain a sternal fracture. Diagnosis in children is complicated by the presence of ossification centers for bone growth on imaging. In this case, the fracture was first noticed on ultrasound and confirmed with MRI. Since these fractures can be associated with damage to surrounding structures, additional injuries should be considered when evaluating a patient with a sternum fracture.
CORRESPONDENCE
Catherine Romaine, East Carolina University, Brody School of Medicine, 600 Moye Boulevard, Greenville, NC 27834; [email protected]
1. Alent J, Narducci DM, Moran B, et al. Sternal injuries in sport: a review of the literature. Sports Med. 2018;48:2715-2724. doi: 10.1007/s40279-018-0990-5
2. Khoriati A-A, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock. 2013;6:113-116. doi: 10.4103/0974-2700.110763
3. Athanassiadi K, Gerazounis M, Moustardas M, et al. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246. doi: 10.1007/s00268-002-6511-5
4. Ferguson LP, Wilkinson AG, Beattie TF. Fracture of the sternum in children. Emerg Med J. 2003;20:518-520. doi: 10.1136/emj.20.6.518
5. Ramgopal S, Shaffiey SA, Conti KA. Pediatric sternal fractures from a Level 1 trauma center. J Pediatr Surg. 2019;54:1628-1631. doi: 10.1016/j.jpedsurg.2018.08.040
6. Sesia SB, Prüfer F, Mayr J. Sternal fracture in children: diagnosis by ultrasonography. European J Pediatr Surg Rep. 2017;5:e39-e42. doi: 10.1055/s-0037-1606197
7. Nickson C, Rippey J. Ultrasonography of sternal fractures. Australas J Ultrasound Med. 2011;14:6-11. doi: 10.1002/j.2205-0140.2011.tb00131.x
8. Bauman ZM, Yanala U, Waibel BH, et al. Sternal fixation for isolated traumatic sternal fractures improves pain and upper extremity range of motion. Eur J Trauma Emerg Surg. 2022;48:225-230. doi: 10.1007/s00068-020-01568-x
9. Culp B, Hurbanek JG, Novak J, et al. Acute traumatic sternum fracture in a female college hockey player. Orthopedics. 2010;33:683. doi: 10.3928/01477447-20100722-17
1. Alent J, Narducci DM, Moran B, et al. Sternal injuries in sport: a review of the literature. Sports Med. 2018;48:2715-2724. doi: 10.1007/s40279-018-0990-5
2. Khoriati A-A, Rajakulasingam R, Shah R. Sternal fractures and their management. J Emerg Trauma Shock. 2013;6:113-116. doi: 10.4103/0974-2700.110763
3. Athanassiadi K, Gerazounis M, Moustardas M, et al. Sternal fractures: retrospective analysis of 100 cases. World J Surg. 2002;26:1243-1246. doi: 10.1007/s00268-002-6511-5
4. Ferguson LP, Wilkinson AG, Beattie TF. Fracture of the sternum in children. Emerg Med J. 2003;20:518-520. doi: 10.1136/emj.20.6.518
5. Ramgopal S, Shaffiey SA, Conti KA. Pediatric sternal fractures from a Level 1 trauma center. J Pediatr Surg. 2019;54:1628-1631. doi: 10.1016/j.jpedsurg.2018.08.040
6. Sesia SB, Prüfer F, Mayr J. Sternal fracture in children: diagnosis by ultrasonography. European J Pediatr Surg Rep. 2017;5:e39-e42. doi: 10.1055/s-0037-1606197
7. Nickson C, Rippey J. Ultrasonography of sternal fractures. Australas J Ultrasound Med. 2011;14:6-11. doi: 10.1002/j.2205-0140.2011.tb00131.x
8. Bauman ZM, Yanala U, Waibel BH, et al. Sternal fixation for isolated traumatic sternal fractures improves pain and upper extremity range of motion. Eur J Trauma Emerg Surg. 2022;48:225-230. doi: 10.1007/s00068-020-01568-x
9. Culp B, Hurbanek JG, Novak J, et al. Acute traumatic sternum fracture in a female college hockey player. Orthopedics. 2010;33:683. doi: 10.3928/01477447-20100722-17
Consider this tool to reduce antibiotic-associated adverse events in patients with sepsis
ILLUSTRATIVE CASE
A 52-year-old woman presents to the emergency department complaining of dysuria and a fever. Her work-up yields a diagnosis of sepsis secondary to pyelonephritis and bacteremia. She is admitted and started on broad-spectrum antimicrobial therapy. The patient’s symptoms improve significantly over the next 48 hours of treatment. When should antibiotic therapy be discontinued to reduce the patient’s risk for antibiotic-associated AEs and to optimize antimicrobial stewardship?
Antimicrobial resistance is a growing public health risk associated with considerable morbidity and mortality, extended hospitalization, and increased medical expenditures.2-4 Antibiotic stewardship is vital in curbing antimicrobial resistance. The predictive biomarker PCT has emerged as both a diagnostic and prognostic agent for numerous infectious diseases. It has recently received much attention as an adjunct to clinical judgment for discontinuation of antibiotic therapy in hospitalized patients with lower respiratory tract infections and/or sepsis.5-11 Indeed, use of PCT guidance in these patients has resulted in decreased AEs, as well as an enhanced survival benefit.5-15
The utility of PCT-guided early discontinuation of antibiotics had yet to be studied in an expanded population of hospitalized patients with sepsis—especially with regard to AEs associated with multidrug-resistant organisms (MDROs) and Clostridioides difficile (formerly Clostridium difficile). The Surviving Sepsis Campaign’s 2021 international guidelines support the use of PCT in conjunction with clinical evaluation for shortening the duration of antibiotic therapy (“weak recommendation, low quality of evidence”).16 They also suggest daily reassessment for de-escalation of antibiotic use (“weak recommendation, very low quality of evidence”) as a possible way to decrease MDROs and AEs but state that more and better trials are needed.15
STUDY SUMMARY
PCT-guided intervention reduced infection-associated AEs
This pragmatic, real-world, multicenter, randomized clinical trial evaluated the use of PCT-guided early discontinuation of antibiotic therapy in patients with sepsis, in hopes of decreasing infection-associated AEs related to prolonged antibiotic exposure.1 The trial took place in 7 hospitals in Athens, Greece, with 266 patients randomized to the PCT-guided intervention or the standard of care (SOC)—the 2016 international guidelines for the management of sepsis and septic shock from the Surviving Sepsis campaign.17 Study participants had sepsis, as defined by a sequential organ failure assessment (SOFA) score ≥ 2, and infections that included pneumonia, pyelonephritis, or bacteremia.16 Pregnancy, lactation, HIV infection with a low CD4 count, neutropenia, cystic fibrosis, and viral, parasitic, or tuberculosis infections were exclusion criteria. Of note, all patients were managed on general medical wards and not in intensive care units.
Serum PCT samples were collected at baseline and then at Day 5 of therapy. Discontinuation of antibiotic therapy in the PCT trial arm occurred once PCT levels were ≤ 0.5 mcg/L or were reduced by at least 80%. If PCT levels did not meet one of these criteria, the lab test would be repeated daily and antibiotic therapy would continue until the rule was met. Neither patients nor investigators were blinded to the treatment assignments, but investigators in the SOC arm were kept unaware of Day 5 PCT results. In the PCT arm, 71% of participants met Day 5 criteria for stopping antibiotics, and a retrospective analysis indicated that a near-identical 70% in the SOC arm also would have met the same criteria.
The assessment of stool colonization with either C difficile or MDROs was done by stool cultures at baseline and on Days 7, 28, and 180.
The primary outcome of infection-associated AEs, which was evaluated at 180 days, was defined as new cases of C difficile or MDRO infection, or death associated with baseline infection with either C difficile or an MDRO. Of the 133 participants allocated to each trial arm, 8 patients in the intervention group and 2 in the SOC group withdrew consent prior to treatment in the intervention group, with the remaining 125 and 131 participants, respectively, completing the interventions and not lost to follow-up.
Continue to: In an intention-to-treat analysis...
In an intention-to-treat analysis, 9 participants (7.2%; 95% CI, 3.8%-13.1%) in the PCT group compared with 20 participants (15.3%; 95% CI, 10.1%-22.4%) in the SOC group experienced the primary outcome of an antibiotic-associated AE at 180 days, resulting in a hazard ratio (HR) of 0.45 (95% CI, 0.2-0.98).
Secondary outcomes also favored the PCT arm regarding 28-day mortality (19 vs 37 patients; HR = 0.51; 95% CI, 0.29-0.89), median length of antibiotic treatment (5 days in the PCT group and 10 days in the SOC group; P < .001), and median hospitalization cost (24% greater in the SOC group; P = .05). Results for 180-day mortality were 30.4% in the PCT arm and 38.2% in the SOC arm (HR = 0.71; 95% CI, 0.42-1.19), thereby not achieving statistical significance.
WHAT'S NEW
An effective tool in reducing AEs in patients with sepsis
In this multicenter trial, PCT proved successful as a clinical decision tool for discontinuing antibiotic therapy and decreasing infection-associated AEs in patients with sepsis.
Caveats
A promising approach but its superiority is uncertain
The confidence interval for the AE hazard ratio was very wide, but significant, suggesting greater uncertainty and less precision in the chance of obtaining improved outcomes with PCT-guided intervention. However, these data also clarify that outcomes should (at least) not be worse with PCT-directed therapy.
CHALLENGES TO IMPLEMENTATION
Assay limitations and potential resistance to a new decision tool
The primary challenge to implementation is likely the availability of the PCT assay and the immediacy of turnaround time to enable physicians to make daily decisions regarding antibiotic therapy de-escalation. Additionally, as with any new knowledge, local culture and physician buy-in may limit implementation of this ever-more-valuable patient care tool.
1. Kyriazopoulou E, Liaskou-Antoniou L, Adamis G, et al. Procalcitonin to reduce long-term infection-associated adverse events in sepsis: a randomized trial. Am J Respir Crit Care Med. 2021;203:202-210. doi: 10.1164/rccm.202004-1201OC
2. European Centre for Disease Prevention and Control. US CDC report on antibiotic resistance threats in the United States, 2013. ECDC comment. September 18, 2013. Accessed December 29, 2022. www.ecdc.europa.eu/en/news-events/us-cdc-report-antibiotic-resistance-threats-united-states-2013
3. Peters L, Olson L, Khu DTK, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: a cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PloS One. 2019;14:e0215666. doi: 10.1371/journal.pone.0215666
4. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(suppl 2):S82-S89. doi: 10.1086/499406
5. Schuetz P, Beishuizen A, Broyles M, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57:1308-1318. doi: 10.1515/cclm-2018-1181
6. Schuetz P, Christ-Crain M, Thomann R, et al; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059-1066. doi: 10.1001/jama.2009.1297
7. Bouadma L, Luyt CE, Tubach F, et al; PRORATA trial group. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463-474. doi: 10.1016/S0140-6736(09)61879-1
8. Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600-607. doi: 10.1016/S0140-6736(04)15591-8
9. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84-93. doi: 10.1164/rccm.200512-1922OC
10. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16:819-827. doi: 10.1016/S1473-3099(16)00053-0
11. Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498-505. doi: 10.1164/rccm.200708-1238OC
12. Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18:95-107. doi: 10.1016/S1473-3099(17)30592-3
13. Schuetz P, Chiappa V, Briel M, et al. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171:1322-1331. doi: 10.1001/archin ternmed.2011.318
14. Wirz Y, Meier MA, Bouadma L, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. 2018;22:191. doi: 10.1186/s13054-018-2125-7
15. Elnajdy D, El-Dahiyat F. Antibiotics duration guided by biomarkers in hospitalized adult patients; a systematic review and meta-analysis. Infect Dis (Lond). 2022;54:387-402. doi: 10.1080/23744235.2022.2037701
16. Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49:e1063-e1143. doi: 10.1097/CCM.0000000000005337
17. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304-377. doi: 10.1007/s00134-017-4683-6
ILLUSTRATIVE CASE
A 52-year-old woman presents to the emergency department complaining of dysuria and a fever. Her work-up yields a diagnosis of sepsis secondary to pyelonephritis and bacteremia. She is admitted and started on broad-spectrum antimicrobial therapy. The patient’s symptoms improve significantly over the next 48 hours of treatment. When should antibiotic therapy be discontinued to reduce the patient’s risk for antibiotic-associated AEs and to optimize antimicrobial stewardship?
Antimicrobial resistance is a growing public health risk associated with considerable morbidity and mortality, extended hospitalization, and increased medical expenditures.2-4 Antibiotic stewardship is vital in curbing antimicrobial resistance. The predictive biomarker PCT has emerged as both a diagnostic and prognostic agent for numerous infectious diseases. It has recently received much attention as an adjunct to clinical judgment for discontinuation of antibiotic therapy in hospitalized patients with lower respiratory tract infections and/or sepsis.5-11 Indeed, use of PCT guidance in these patients has resulted in decreased AEs, as well as an enhanced survival benefit.5-15
The utility of PCT-guided early discontinuation of antibiotics had yet to be studied in an expanded population of hospitalized patients with sepsis—especially with regard to AEs associated with multidrug-resistant organisms (MDROs) and Clostridioides difficile (formerly Clostridium difficile). The Surviving Sepsis Campaign’s 2021 international guidelines support the use of PCT in conjunction with clinical evaluation for shortening the duration of antibiotic therapy (“weak recommendation, low quality of evidence”).16 They also suggest daily reassessment for de-escalation of antibiotic use (“weak recommendation, very low quality of evidence”) as a possible way to decrease MDROs and AEs but state that more and better trials are needed.15
STUDY SUMMARY
PCT-guided intervention reduced infection-associated AEs
This pragmatic, real-world, multicenter, randomized clinical trial evaluated the use of PCT-guided early discontinuation of antibiotic therapy in patients with sepsis, in hopes of decreasing infection-associated AEs related to prolonged antibiotic exposure.1 The trial took place in 7 hospitals in Athens, Greece, with 266 patients randomized to the PCT-guided intervention or the standard of care (SOC)—the 2016 international guidelines for the management of sepsis and septic shock from the Surviving Sepsis campaign.17 Study participants had sepsis, as defined by a sequential organ failure assessment (SOFA) score ≥ 2, and infections that included pneumonia, pyelonephritis, or bacteremia.16 Pregnancy, lactation, HIV infection with a low CD4 count, neutropenia, cystic fibrosis, and viral, parasitic, or tuberculosis infections were exclusion criteria. Of note, all patients were managed on general medical wards and not in intensive care units.
Serum PCT samples were collected at baseline and then at Day 5 of therapy. Discontinuation of antibiotic therapy in the PCT trial arm occurred once PCT levels were ≤ 0.5 mcg/L or were reduced by at least 80%. If PCT levels did not meet one of these criteria, the lab test would be repeated daily and antibiotic therapy would continue until the rule was met. Neither patients nor investigators were blinded to the treatment assignments, but investigators in the SOC arm were kept unaware of Day 5 PCT results. In the PCT arm, 71% of participants met Day 5 criteria for stopping antibiotics, and a retrospective analysis indicated that a near-identical 70% in the SOC arm also would have met the same criteria.
The assessment of stool colonization with either C difficile or MDROs was done by stool cultures at baseline and on Days 7, 28, and 180.
The primary outcome of infection-associated AEs, which was evaluated at 180 days, was defined as new cases of C difficile or MDRO infection, or death associated with baseline infection with either C difficile or an MDRO. Of the 133 participants allocated to each trial arm, 8 patients in the intervention group and 2 in the SOC group withdrew consent prior to treatment in the intervention group, with the remaining 125 and 131 participants, respectively, completing the interventions and not lost to follow-up.
Continue to: In an intention-to-treat analysis...
In an intention-to-treat analysis, 9 participants (7.2%; 95% CI, 3.8%-13.1%) in the PCT group compared with 20 participants (15.3%; 95% CI, 10.1%-22.4%) in the SOC group experienced the primary outcome of an antibiotic-associated AE at 180 days, resulting in a hazard ratio (HR) of 0.45 (95% CI, 0.2-0.98).
Secondary outcomes also favored the PCT arm regarding 28-day mortality (19 vs 37 patients; HR = 0.51; 95% CI, 0.29-0.89), median length of antibiotic treatment (5 days in the PCT group and 10 days in the SOC group; P < .001), and median hospitalization cost (24% greater in the SOC group; P = .05). Results for 180-day mortality were 30.4% in the PCT arm and 38.2% in the SOC arm (HR = 0.71; 95% CI, 0.42-1.19), thereby not achieving statistical significance.
WHAT'S NEW
An effective tool in reducing AEs in patients with sepsis
In this multicenter trial, PCT proved successful as a clinical decision tool for discontinuing antibiotic therapy and decreasing infection-associated AEs in patients with sepsis.
Caveats
A promising approach but its superiority is uncertain
The confidence interval for the AE hazard ratio was very wide, but significant, suggesting greater uncertainty and less precision in the chance of obtaining improved outcomes with PCT-guided intervention. However, these data also clarify that outcomes should (at least) not be worse with PCT-directed therapy.
CHALLENGES TO IMPLEMENTATION
Assay limitations and potential resistance to a new decision tool
The primary challenge to implementation is likely the availability of the PCT assay and the immediacy of turnaround time to enable physicians to make daily decisions regarding antibiotic therapy de-escalation. Additionally, as with any new knowledge, local culture and physician buy-in may limit implementation of this ever-more-valuable patient care tool.
ILLUSTRATIVE CASE
A 52-year-old woman presents to the emergency department complaining of dysuria and a fever. Her work-up yields a diagnosis of sepsis secondary to pyelonephritis and bacteremia. She is admitted and started on broad-spectrum antimicrobial therapy. The patient’s symptoms improve significantly over the next 48 hours of treatment. When should antibiotic therapy be discontinued to reduce the patient’s risk for antibiotic-associated AEs and to optimize antimicrobial stewardship?
Antimicrobial resistance is a growing public health risk associated with considerable morbidity and mortality, extended hospitalization, and increased medical expenditures.2-4 Antibiotic stewardship is vital in curbing antimicrobial resistance. The predictive biomarker PCT has emerged as both a diagnostic and prognostic agent for numerous infectious diseases. It has recently received much attention as an adjunct to clinical judgment for discontinuation of antibiotic therapy in hospitalized patients with lower respiratory tract infections and/or sepsis.5-11 Indeed, use of PCT guidance in these patients has resulted in decreased AEs, as well as an enhanced survival benefit.5-15
The utility of PCT-guided early discontinuation of antibiotics had yet to be studied in an expanded population of hospitalized patients with sepsis—especially with regard to AEs associated with multidrug-resistant organisms (MDROs) and Clostridioides difficile (formerly Clostridium difficile). The Surviving Sepsis Campaign’s 2021 international guidelines support the use of PCT in conjunction with clinical evaluation for shortening the duration of antibiotic therapy (“weak recommendation, low quality of evidence”).16 They also suggest daily reassessment for de-escalation of antibiotic use (“weak recommendation, very low quality of evidence”) as a possible way to decrease MDROs and AEs but state that more and better trials are needed.15
STUDY SUMMARY
PCT-guided intervention reduced infection-associated AEs
This pragmatic, real-world, multicenter, randomized clinical trial evaluated the use of PCT-guided early discontinuation of antibiotic therapy in patients with sepsis, in hopes of decreasing infection-associated AEs related to prolonged antibiotic exposure.1 The trial took place in 7 hospitals in Athens, Greece, with 266 patients randomized to the PCT-guided intervention or the standard of care (SOC)—the 2016 international guidelines for the management of sepsis and septic shock from the Surviving Sepsis campaign.17 Study participants had sepsis, as defined by a sequential organ failure assessment (SOFA) score ≥ 2, and infections that included pneumonia, pyelonephritis, or bacteremia.16 Pregnancy, lactation, HIV infection with a low CD4 count, neutropenia, cystic fibrosis, and viral, parasitic, or tuberculosis infections were exclusion criteria. Of note, all patients were managed on general medical wards and not in intensive care units.
Serum PCT samples were collected at baseline and then at Day 5 of therapy. Discontinuation of antibiotic therapy in the PCT trial arm occurred once PCT levels were ≤ 0.5 mcg/L or were reduced by at least 80%. If PCT levels did not meet one of these criteria, the lab test would be repeated daily and antibiotic therapy would continue until the rule was met. Neither patients nor investigators were blinded to the treatment assignments, but investigators in the SOC arm were kept unaware of Day 5 PCT results. In the PCT arm, 71% of participants met Day 5 criteria for stopping antibiotics, and a retrospective analysis indicated that a near-identical 70% in the SOC arm also would have met the same criteria.
The assessment of stool colonization with either C difficile or MDROs was done by stool cultures at baseline and on Days 7, 28, and 180.
The primary outcome of infection-associated AEs, which was evaluated at 180 days, was defined as new cases of C difficile or MDRO infection, or death associated with baseline infection with either C difficile or an MDRO. Of the 133 participants allocated to each trial arm, 8 patients in the intervention group and 2 in the SOC group withdrew consent prior to treatment in the intervention group, with the remaining 125 and 131 participants, respectively, completing the interventions and not lost to follow-up.
Continue to: In an intention-to-treat analysis...
In an intention-to-treat analysis, 9 participants (7.2%; 95% CI, 3.8%-13.1%) in the PCT group compared with 20 participants (15.3%; 95% CI, 10.1%-22.4%) in the SOC group experienced the primary outcome of an antibiotic-associated AE at 180 days, resulting in a hazard ratio (HR) of 0.45 (95% CI, 0.2-0.98).
Secondary outcomes also favored the PCT arm regarding 28-day mortality (19 vs 37 patients; HR = 0.51; 95% CI, 0.29-0.89), median length of antibiotic treatment (5 days in the PCT group and 10 days in the SOC group; P < .001), and median hospitalization cost (24% greater in the SOC group; P = .05). Results for 180-day mortality were 30.4% in the PCT arm and 38.2% in the SOC arm (HR = 0.71; 95% CI, 0.42-1.19), thereby not achieving statistical significance.
WHAT'S NEW
An effective tool in reducing AEs in patients with sepsis
In this multicenter trial, PCT proved successful as a clinical decision tool for discontinuing antibiotic therapy and decreasing infection-associated AEs in patients with sepsis.
Caveats
A promising approach but its superiority is uncertain
The confidence interval for the AE hazard ratio was very wide, but significant, suggesting greater uncertainty and less precision in the chance of obtaining improved outcomes with PCT-guided intervention. However, these data also clarify that outcomes should (at least) not be worse with PCT-directed therapy.
CHALLENGES TO IMPLEMENTATION
Assay limitations and potential resistance to a new decision tool
The primary challenge to implementation is likely the availability of the PCT assay and the immediacy of turnaround time to enable physicians to make daily decisions regarding antibiotic therapy de-escalation. Additionally, as with any new knowledge, local culture and physician buy-in may limit implementation of this ever-more-valuable patient care tool.
1. Kyriazopoulou E, Liaskou-Antoniou L, Adamis G, et al. Procalcitonin to reduce long-term infection-associated adverse events in sepsis: a randomized trial. Am J Respir Crit Care Med. 2021;203:202-210. doi: 10.1164/rccm.202004-1201OC
2. European Centre for Disease Prevention and Control. US CDC report on antibiotic resistance threats in the United States, 2013. ECDC comment. September 18, 2013. Accessed December 29, 2022. www.ecdc.europa.eu/en/news-events/us-cdc-report-antibiotic-resistance-threats-united-states-2013
3. Peters L, Olson L, Khu DTK, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: a cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PloS One. 2019;14:e0215666. doi: 10.1371/journal.pone.0215666
4. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(suppl 2):S82-S89. doi: 10.1086/499406
5. Schuetz P, Beishuizen A, Broyles M, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57:1308-1318. doi: 10.1515/cclm-2018-1181
6. Schuetz P, Christ-Crain M, Thomann R, et al; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059-1066. doi: 10.1001/jama.2009.1297
7. Bouadma L, Luyt CE, Tubach F, et al; PRORATA trial group. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463-474. doi: 10.1016/S0140-6736(09)61879-1
8. Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600-607. doi: 10.1016/S0140-6736(04)15591-8
9. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84-93. doi: 10.1164/rccm.200512-1922OC
10. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16:819-827. doi: 10.1016/S1473-3099(16)00053-0
11. Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498-505. doi: 10.1164/rccm.200708-1238OC
12. Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18:95-107. doi: 10.1016/S1473-3099(17)30592-3
13. Schuetz P, Chiappa V, Briel M, et al. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171:1322-1331. doi: 10.1001/archin ternmed.2011.318
14. Wirz Y, Meier MA, Bouadma L, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. 2018;22:191. doi: 10.1186/s13054-018-2125-7
15. Elnajdy D, El-Dahiyat F. Antibiotics duration guided by biomarkers in hospitalized adult patients; a systematic review and meta-analysis. Infect Dis (Lond). 2022;54:387-402. doi: 10.1080/23744235.2022.2037701
16. Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49:e1063-e1143. doi: 10.1097/CCM.0000000000005337
17. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304-377. doi: 10.1007/s00134-017-4683-6
1. Kyriazopoulou E, Liaskou-Antoniou L, Adamis G, et al. Procalcitonin to reduce long-term infection-associated adverse events in sepsis: a randomized trial. Am J Respir Crit Care Med. 2021;203:202-210. doi: 10.1164/rccm.202004-1201OC
2. European Centre for Disease Prevention and Control. US CDC report on antibiotic resistance threats in the United States, 2013. ECDC comment. September 18, 2013. Accessed December 29, 2022. www.ecdc.europa.eu/en/news-events/us-cdc-report-antibiotic-resistance-threats-united-states-2013
3. Peters L, Olson L, Khu DTK, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: a cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PloS One. 2019;14:e0215666. doi: 10.1371/journal.pone.0215666
4. Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42(suppl 2):S82-S89. doi: 10.1086/499406
5. Schuetz P, Beishuizen A, Broyles M, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57:1308-1318. doi: 10.1515/cclm-2018-1181
6. Schuetz P, Christ-Crain M, Thomann R, et al; ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059-1066. doi: 10.1001/jama.2009.1297
7. Bouadma L, Luyt CE, Tubach F, et al; PRORATA trial group. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463-474. doi: 10.1016/S0140-6736(09)61879-1
8. Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600-607. doi: 10.1016/S0140-6736(04)15591-8
9. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84-93. doi: 10.1164/rccm.200512-1922OC
10. de Jong E, van Oers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16:819-827. doi: 10.1016/S1473-3099(16)00053-0
11. Nobre V, Harbarth S, Graf JD, et al. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498-505. doi: 10.1164/rccm.200708-1238OC
12. Schuetz P, Wirz Y, Sager R, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18:95-107. doi: 10.1016/S1473-3099(17)30592-3
13. Schuetz P, Chiappa V, Briel M, et al. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171:1322-1331. doi: 10.1001/archin ternmed.2011.318
14. Wirz Y, Meier MA, Bouadma L, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. 2018;22:191. doi: 10.1186/s13054-018-2125-7
15. Elnajdy D, El-Dahiyat F. Antibiotics duration guided by biomarkers in hospitalized adult patients; a systematic review and meta-analysis. Infect Dis (Lond). 2022;54:387-402. doi: 10.1080/23744235.2022.2037701
16. Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49:e1063-e1143. doi: 10.1097/CCM.0000000000005337
17. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304-377. doi: 10.1007/s00134-017-4683-6
PRACTICE CHANGER
For patients hospitalized with sepsis, consider procalcitonin (PCT)-guided early discontinuation of antibiotic therapy for fewer infection-associated adverse events (AEs).
STRENGTH OF RECOMMENDATION
Kyriazopoulou E, Liaskou-Antoniou L, Adamis G, et al. Procalcitonin to reduce long-term infection-associated adverse events in sepsis. A randomized trial. Am J Respir Crit Care Med. 2021;203:202-210. doi: 10.1164/rccm.202004-1201OC
Does regular walking improve lipid levels in adults?
Evidence summary
Walking’s impact on cholesterol levels is modest, inconsistent
A 2022 systematic review and meta-analysis of 21 studies (n = 1129) evaluated the effects of walking on lipids and lipoproteins in women older than 18 years who were overweight or obese and were not taking any lipid-lowering medications. Median TC was 206 mg/dL and median LDL was 126 mg/dL.1
The primary outcome found that walking decreased TC and LDL levels independent of diet and weight loss. Twenty studies reported on TC and showed that walking significantly decreased TC levels compared to the control groups (raw mean difference [RMD] = 6.7 mg/dL; 95% CI, 0.4-12.9; P = .04). Fifteen studies examined LDL and showed a significant decrease in LDL levels with walking compared to control groups (RMD = 7.4 mg/dL; 95% CI, 0.3-14.5; P = .04). However, the small magnitude of the changes may have little clinical impact.1
There were no significant changes in the walking groups compared to the control groups for triglycerides (17 studies; RMD = 2.2 mg/dL; 95% CI, –8.4 to 12.8; P = .68) or high-density lipoprotein (HDL) (18 studies; RMD = 1.5 mg/dL; 95% CI, –0.4 to 3.3; P = .12). Included studies were required to be controlled but were otherwise not described. The overall risk for bias was determined to be low.1
A 2020 RCT (n = 22) assessed the effects of a walking intervention on cholesterol and cardiovascular disease (CVD) risk in individuals ages 40 to 65 years with moderate CVD risk but without diabetes or CVD.2 Moderate CVD risk was defined as a 2% to 5% 10-year risk for a CVD event using the European HeartScore, which incorporates age, sex, blood pressure, lipid levels, and smoking status3; however, study participants were not required to have hyperlipidemia. Participants were enrolled in a 12-week, nurse-led intervention of moderate-paced walking for 30 to 45 minutes 5 times weekly.
Individuals in the intervention group had significant decreases in average TC levels from baseline to follow-up (244.6 mg/dL vs 213.7 mg/dL; P = .001). As a result, participants’ average 10-year CVD risk was significantly reduced from moderate risk to low risk (2.6% vs 1.8%; P = 038) and was significantly lower in the intervention group than in the control group at follow-up (1.8% vs 3.1%; P = .019). No blinding was used, and the use of lipid-lowering medications was not reported, which could have impacted the results.2
A 2008 RCT (n = 67) examined the effect of a home-based walking program (12 weeks of brisk walking, at least 30 min/d and at least 5 d/wk, with at least 300 kcal burned per walk) vs a sedentary control group in men ages 45 to 65 years with hyperlipidemia (TC > 240 mg/dL and/or TC/HDL-C ratio ≥ 6) who were not receiving lipid-lowering medication. There were no significant changes from baseline to follow-up in the walking group compared to the control group in TC (adjusted mean difference [AMD] = –9.3 mg/dL; 95% CI, –22.8 to 4.64; P = .19), HDL-C (AMD = 2.7 mg/dL; 95% CI, –0.4 to 5.4; P = .07) or triglycerides (AMD = –26.6 mg/dL; 95% CI, –56.7 to 2.7; P = .07).4
A 2002 RCT (n = 111) of sedentary men and women (BMI, 25-35; ages, 40-65 years) with dyslipidemia (LDL of 130-190 mg/dL, or HDL < 40 mg/dL for men or < 45 mg/dL for women) examined the impact of various physical activity levels for 8 months when compared to a control group observed for 6 months. The group assigned to low-amount, moderate-intensity physical activity walked an equivalent of 12 miles per week.5
Continue to: In this group...
In this group, there was a significant decrease in average triglyceride concentrations from baseline to follow-up (mean ± standard error = 196.8 ± 30.5 mg/dL vs 145.2 ± 16.0 mg/dL; P < .001). Significance of the change compared with changes in the control group was not reported, although triglycerides in the control group increased from baseline to follow-up (132.1 ± 11.0 vs 155.8 ± 14.9 mg/dL). There were no significant changes from baseline to follow-up in TC (194 ± 4.8 vs 197.9 ± 5.4 mg/dL), LDL (122.7 ± 4.0 vs 127.8 ± 4.1 mg/dL), or HDL (42.0 ± 1.9 vs 43.1 ± 2.5 mg/dL); P values of pre-post changes and comparison to control group were not reported.5
Recommendations from others
The Physical Activity Guidelines for Americans, published by the Department of Health and Human Services and updated in 2018, cite adherence to the published guidelines as a protective factor against high LDL and total lipids in both adults and children.6 The guidelines for adults recommend 150 to 300 minutes of moderate-intensity or 75 to 150 minutes of vigorous-intensity aerobic exercise per week, as well as muscle-strengthening activities of moderate or greater intensity 2 or more days per week. Brisk walking is included as an example of a moderate-intensity activity. These same guidelines are cited and endorsed by the American College of Sports Medicine and the American Heart Association.7,8
Editor’s takeaway
The lipid reductions achieved from walking—if any—are minimal. By themselves, these small reductions will not accomplish our lipid-lowering goals. However, cholesterol goals are primarily disease oriented. This evidence does not directly inform us of important patient-oriented outcomes, such as morbidity, mortality, and vitality.
1. Ballard AM, Davis A, Wong B, et al. The effects of exclusive walking on lipids and lipoproteins in women with overweight and obesity: a systematic review and meta-analysis. Am J Health Promot. 2022;36:328-339. doi: 10.1177/08901171211048135
2. Akgöz AD, Gözüm S. Effectiveness of a nurse-led physical activity intervention to decrease cardiovascular disease risk in middle-aged adults: a pilot randomized controlled study. J Vasc Nurs. 2020;38:140-148. doi: 10.1016/j.jvn.2020.05.002
3. European Association of Preventive Cardiology. HeartScore. Accessed December 23, 2022. www.heartscore.org/en_GB
4. Coghill N, Cooper AR. The effect of a home-based walking program on risk factors for coronary heart disease in hypercholesterolaemic men: a randomized controlled trial. Prev Med. 2008; 46:545-551. doi: 10.1016/j.ypmed.2008.01.002
5. Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483-1492. doi: 10.1056/NEJMoa020194
6. US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Washington, DC: US Department of Health and Human Services; 2018. Accessed December 23, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
7. American Heart Association. Recommendations for physical activity in adults and kids. Accessed December 23, 2022. www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-adults
8. American College of Sports Medicine. Trending topic: physical activity guidelines. Accessed December 23, 2022. www.acsm.org/education-resources/trending-topics-resources/physical-activity-guidelines
Evidence summary
Walking’s impact on cholesterol levels is modest, inconsistent
A 2022 systematic review and meta-analysis of 21 studies (n = 1129) evaluated the effects of walking on lipids and lipoproteins in women older than 18 years who were overweight or obese and were not taking any lipid-lowering medications. Median TC was 206 mg/dL and median LDL was 126 mg/dL.1
The primary outcome found that walking decreased TC and LDL levels independent of diet and weight loss. Twenty studies reported on TC and showed that walking significantly decreased TC levels compared to the control groups (raw mean difference [RMD] = 6.7 mg/dL; 95% CI, 0.4-12.9; P = .04). Fifteen studies examined LDL and showed a significant decrease in LDL levels with walking compared to control groups (RMD = 7.4 mg/dL; 95% CI, 0.3-14.5; P = .04). However, the small magnitude of the changes may have little clinical impact.1
There were no significant changes in the walking groups compared to the control groups for triglycerides (17 studies; RMD = 2.2 mg/dL; 95% CI, –8.4 to 12.8; P = .68) or high-density lipoprotein (HDL) (18 studies; RMD = 1.5 mg/dL; 95% CI, –0.4 to 3.3; P = .12). Included studies were required to be controlled but were otherwise not described. The overall risk for bias was determined to be low.1
A 2020 RCT (n = 22) assessed the effects of a walking intervention on cholesterol and cardiovascular disease (CVD) risk in individuals ages 40 to 65 years with moderate CVD risk but without diabetes or CVD.2 Moderate CVD risk was defined as a 2% to 5% 10-year risk for a CVD event using the European HeartScore, which incorporates age, sex, blood pressure, lipid levels, and smoking status3; however, study participants were not required to have hyperlipidemia. Participants were enrolled in a 12-week, nurse-led intervention of moderate-paced walking for 30 to 45 minutes 5 times weekly.
Individuals in the intervention group had significant decreases in average TC levels from baseline to follow-up (244.6 mg/dL vs 213.7 mg/dL; P = .001). As a result, participants’ average 10-year CVD risk was significantly reduced from moderate risk to low risk (2.6% vs 1.8%; P = 038) and was significantly lower in the intervention group than in the control group at follow-up (1.8% vs 3.1%; P = .019). No blinding was used, and the use of lipid-lowering medications was not reported, which could have impacted the results.2
A 2008 RCT (n = 67) examined the effect of a home-based walking program (12 weeks of brisk walking, at least 30 min/d and at least 5 d/wk, with at least 300 kcal burned per walk) vs a sedentary control group in men ages 45 to 65 years with hyperlipidemia (TC > 240 mg/dL and/or TC/HDL-C ratio ≥ 6) who were not receiving lipid-lowering medication. There were no significant changes from baseline to follow-up in the walking group compared to the control group in TC (adjusted mean difference [AMD] = –9.3 mg/dL; 95% CI, –22.8 to 4.64; P = .19), HDL-C (AMD = 2.7 mg/dL; 95% CI, –0.4 to 5.4; P = .07) or triglycerides (AMD = –26.6 mg/dL; 95% CI, –56.7 to 2.7; P = .07).4
A 2002 RCT (n = 111) of sedentary men and women (BMI, 25-35; ages, 40-65 years) with dyslipidemia (LDL of 130-190 mg/dL, or HDL < 40 mg/dL for men or < 45 mg/dL for women) examined the impact of various physical activity levels for 8 months when compared to a control group observed for 6 months. The group assigned to low-amount, moderate-intensity physical activity walked an equivalent of 12 miles per week.5
Continue to: In this group...
In this group, there was a significant decrease in average triglyceride concentrations from baseline to follow-up (mean ± standard error = 196.8 ± 30.5 mg/dL vs 145.2 ± 16.0 mg/dL; P < .001). Significance of the change compared with changes in the control group was not reported, although triglycerides in the control group increased from baseline to follow-up (132.1 ± 11.0 vs 155.8 ± 14.9 mg/dL). There were no significant changes from baseline to follow-up in TC (194 ± 4.8 vs 197.9 ± 5.4 mg/dL), LDL (122.7 ± 4.0 vs 127.8 ± 4.1 mg/dL), or HDL (42.0 ± 1.9 vs 43.1 ± 2.5 mg/dL); P values of pre-post changes and comparison to control group were not reported.5
Recommendations from others
The Physical Activity Guidelines for Americans, published by the Department of Health and Human Services and updated in 2018, cite adherence to the published guidelines as a protective factor against high LDL and total lipids in both adults and children.6 The guidelines for adults recommend 150 to 300 minutes of moderate-intensity or 75 to 150 minutes of vigorous-intensity aerobic exercise per week, as well as muscle-strengthening activities of moderate or greater intensity 2 or more days per week. Brisk walking is included as an example of a moderate-intensity activity. These same guidelines are cited and endorsed by the American College of Sports Medicine and the American Heart Association.7,8
Editor’s takeaway
The lipid reductions achieved from walking—if any—are minimal. By themselves, these small reductions will not accomplish our lipid-lowering goals. However, cholesterol goals are primarily disease oriented. This evidence does not directly inform us of important patient-oriented outcomes, such as morbidity, mortality, and vitality.
Evidence summary
Walking’s impact on cholesterol levels is modest, inconsistent
A 2022 systematic review and meta-analysis of 21 studies (n = 1129) evaluated the effects of walking on lipids and lipoproteins in women older than 18 years who were overweight or obese and were not taking any lipid-lowering medications. Median TC was 206 mg/dL and median LDL was 126 mg/dL.1
The primary outcome found that walking decreased TC and LDL levels independent of diet and weight loss. Twenty studies reported on TC and showed that walking significantly decreased TC levels compared to the control groups (raw mean difference [RMD] = 6.7 mg/dL; 95% CI, 0.4-12.9; P = .04). Fifteen studies examined LDL and showed a significant decrease in LDL levels with walking compared to control groups (RMD = 7.4 mg/dL; 95% CI, 0.3-14.5; P = .04). However, the small magnitude of the changes may have little clinical impact.1
There were no significant changes in the walking groups compared to the control groups for triglycerides (17 studies; RMD = 2.2 mg/dL; 95% CI, –8.4 to 12.8; P = .68) or high-density lipoprotein (HDL) (18 studies; RMD = 1.5 mg/dL; 95% CI, –0.4 to 3.3; P = .12). Included studies were required to be controlled but were otherwise not described. The overall risk for bias was determined to be low.1
A 2020 RCT (n = 22) assessed the effects of a walking intervention on cholesterol and cardiovascular disease (CVD) risk in individuals ages 40 to 65 years with moderate CVD risk but without diabetes or CVD.2 Moderate CVD risk was defined as a 2% to 5% 10-year risk for a CVD event using the European HeartScore, which incorporates age, sex, blood pressure, lipid levels, and smoking status3; however, study participants were not required to have hyperlipidemia. Participants were enrolled in a 12-week, nurse-led intervention of moderate-paced walking for 30 to 45 minutes 5 times weekly.
Individuals in the intervention group had significant decreases in average TC levels from baseline to follow-up (244.6 mg/dL vs 213.7 mg/dL; P = .001). As a result, participants’ average 10-year CVD risk was significantly reduced from moderate risk to low risk (2.6% vs 1.8%; P = 038) and was significantly lower in the intervention group than in the control group at follow-up (1.8% vs 3.1%; P = .019). No blinding was used, and the use of lipid-lowering medications was not reported, which could have impacted the results.2
A 2008 RCT (n = 67) examined the effect of a home-based walking program (12 weeks of brisk walking, at least 30 min/d and at least 5 d/wk, with at least 300 kcal burned per walk) vs a sedentary control group in men ages 45 to 65 years with hyperlipidemia (TC > 240 mg/dL and/or TC/HDL-C ratio ≥ 6) who were not receiving lipid-lowering medication. There were no significant changes from baseline to follow-up in the walking group compared to the control group in TC (adjusted mean difference [AMD] = –9.3 mg/dL; 95% CI, –22.8 to 4.64; P = .19), HDL-C (AMD = 2.7 mg/dL; 95% CI, –0.4 to 5.4; P = .07) or triglycerides (AMD = –26.6 mg/dL; 95% CI, –56.7 to 2.7; P = .07).4
A 2002 RCT (n = 111) of sedentary men and women (BMI, 25-35; ages, 40-65 years) with dyslipidemia (LDL of 130-190 mg/dL, or HDL < 40 mg/dL for men or < 45 mg/dL for women) examined the impact of various physical activity levels for 8 months when compared to a control group observed for 6 months. The group assigned to low-amount, moderate-intensity physical activity walked an equivalent of 12 miles per week.5
Continue to: In this group...
In this group, there was a significant decrease in average triglyceride concentrations from baseline to follow-up (mean ± standard error = 196.8 ± 30.5 mg/dL vs 145.2 ± 16.0 mg/dL; P < .001). Significance of the change compared with changes in the control group was not reported, although triglycerides in the control group increased from baseline to follow-up (132.1 ± 11.0 vs 155.8 ± 14.9 mg/dL). There were no significant changes from baseline to follow-up in TC (194 ± 4.8 vs 197.9 ± 5.4 mg/dL), LDL (122.7 ± 4.0 vs 127.8 ± 4.1 mg/dL), or HDL (42.0 ± 1.9 vs 43.1 ± 2.5 mg/dL); P values of pre-post changes and comparison to control group were not reported.5
Recommendations from others
The Physical Activity Guidelines for Americans, published by the Department of Health and Human Services and updated in 2018, cite adherence to the published guidelines as a protective factor against high LDL and total lipids in both adults and children.6 The guidelines for adults recommend 150 to 300 minutes of moderate-intensity or 75 to 150 minutes of vigorous-intensity aerobic exercise per week, as well as muscle-strengthening activities of moderate or greater intensity 2 or more days per week. Brisk walking is included as an example of a moderate-intensity activity. These same guidelines are cited and endorsed by the American College of Sports Medicine and the American Heart Association.7,8
Editor’s takeaway
The lipid reductions achieved from walking—if any—are minimal. By themselves, these small reductions will not accomplish our lipid-lowering goals. However, cholesterol goals are primarily disease oriented. This evidence does not directly inform us of important patient-oriented outcomes, such as morbidity, mortality, and vitality.
1. Ballard AM, Davis A, Wong B, et al. The effects of exclusive walking on lipids and lipoproteins in women with overweight and obesity: a systematic review and meta-analysis. Am J Health Promot. 2022;36:328-339. doi: 10.1177/08901171211048135
2. Akgöz AD, Gözüm S. Effectiveness of a nurse-led physical activity intervention to decrease cardiovascular disease risk in middle-aged adults: a pilot randomized controlled study. J Vasc Nurs. 2020;38:140-148. doi: 10.1016/j.jvn.2020.05.002
3. European Association of Preventive Cardiology. HeartScore. Accessed December 23, 2022. www.heartscore.org/en_GB
4. Coghill N, Cooper AR. The effect of a home-based walking program on risk factors for coronary heart disease in hypercholesterolaemic men: a randomized controlled trial. Prev Med. 2008; 46:545-551. doi: 10.1016/j.ypmed.2008.01.002
5. Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483-1492. doi: 10.1056/NEJMoa020194
6. US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Washington, DC: US Department of Health and Human Services; 2018. Accessed December 23, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
7. American Heart Association. Recommendations for physical activity in adults and kids. Accessed December 23, 2022. www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-adults
8. American College of Sports Medicine. Trending topic: physical activity guidelines. Accessed December 23, 2022. www.acsm.org/education-resources/trending-topics-resources/physical-activity-guidelines
1. Ballard AM, Davis A, Wong B, et al. The effects of exclusive walking on lipids and lipoproteins in women with overweight and obesity: a systematic review and meta-analysis. Am J Health Promot. 2022;36:328-339. doi: 10.1177/08901171211048135
2. Akgöz AD, Gözüm S. Effectiveness of a nurse-led physical activity intervention to decrease cardiovascular disease risk in middle-aged adults: a pilot randomized controlled study. J Vasc Nurs. 2020;38:140-148. doi: 10.1016/j.jvn.2020.05.002
3. European Association of Preventive Cardiology. HeartScore. Accessed December 23, 2022. www.heartscore.org/en_GB
4. Coghill N, Cooper AR. The effect of a home-based walking program on risk factors for coronary heart disease in hypercholesterolaemic men: a randomized controlled trial. Prev Med. 2008; 46:545-551. doi: 10.1016/j.ypmed.2008.01.002
5. Kraus WE, Houmard JA, Duscha BD, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483-1492. doi: 10.1056/NEJMoa020194
6. US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Washington, DC: US Department of Health and Human Services; 2018. Accessed December 23, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
7. American Heart Association. Recommendations for physical activity in adults and kids. Accessed December 23, 2022. www.heart.org/en/healthy-living/fitness/fitness-basics/aha-recs-for-physical-activity-in-adults
8. American College of Sports Medicine. Trending topic: physical activity guidelines. Accessed December 23, 2022. www.acsm.org/education-resources/trending-topics-resources/physical-activity-guidelines
EVIDENCE-BASED ANSWER:
Minimally. Regular moderate- intensity walking for a period of 4 or more weeks minimally decreased total cholesterol (TC) and low-density lipoprotein (LDL) levels by about 7 mg/dL in women with overweight or obesity (strength of recommendation [SOR]: C, systematic review and meta-analysis on disease-oriented evidence). For adults ages 40 to 65 years, regular walking for 3 or more months inconsistently affected cholesterol and triglyceride levels (SOR: C, based on 3 randomized controlled trials [RCTs] with disease-oriented evidence).