User login
What's your diagnosis?
The diagnosis
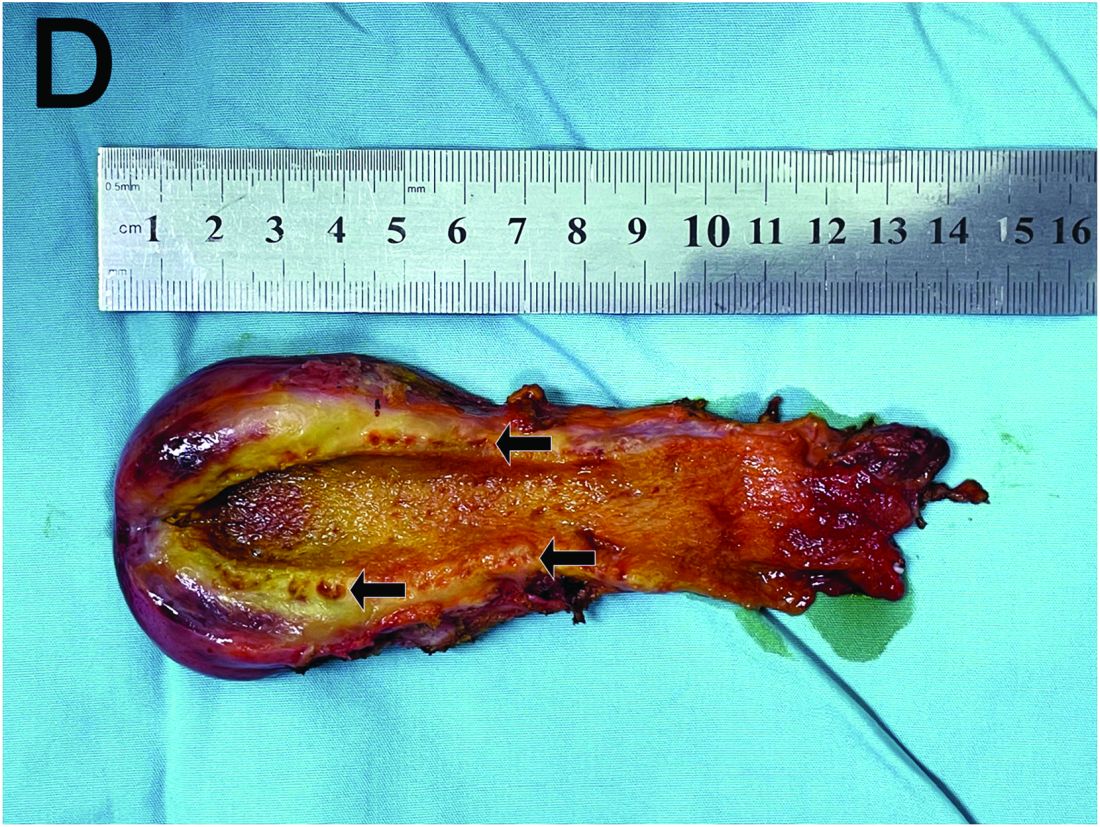
Based on the clinical and imaging findings, a diagnosis of gallbladder adenomyomatosis was made. GA is a benign and usually asymptomatic condition that occurs mainly beyond the age of 50-60 years and is very rare in childhood.1 Symptomatic gallbladder adenomyomatosis indicates cholecystectomy, considering the presence of inflammation or gallbladder stones.2 Therefore, a laparoscopic cholecystectomy was performed on our patient. Rokitansky-Aschoff sinuses were seen in the entire thickened gallbladder wall on gross pathologic examination (Figure D). Histopathologic examination confirmed the diagnosis of GA with cholecystitis. The patient was eventually diagnosed with diffuse GA. She was successfully discharged from the hospital 4 days after surgery, and 3 months of follow-up were uneventful.
References
Eroglu N et al. Diffuse adenomyomatosis of the gallbladder in a child. J Pediatr Hematol Oncol. 2016;38:e307-9.
Bonatti M. et al. Gallbladder adenomyomatosis: imaging findings, tricks and pitfalls. Insights Imaging. 2017;8:243-53.
Hammad AY et al. A literature review of radiological findings to guide the diagnosis of gallbladder adenomyomatosis. HPB (Oxford). 2016;18:129-35.
The diagnosis
Based on the clinical and imaging findings, a diagnosis of gallbladder adenomyomatosis was made. GA is a benign and usually asymptomatic condition that occurs mainly beyond the age of 50-60 years and is very rare in childhood.1 Symptomatic gallbladder adenomyomatosis indicates cholecystectomy, considering the presence of inflammation or gallbladder stones.2 Therefore, a laparoscopic cholecystectomy was performed on our patient. Rokitansky-Aschoff sinuses were seen in the entire thickened gallbladder wall on gross pathologic examination (Figure D). Histopathologic examination confirmed the diagnosis of GA with cholecystitis. The patient was eventually diagnosed with diffuse GA. She was successfully discharged from the hospital 4 days after surgery, and 3 months of follow-up were uneventful.

References
Eroglu N et al. Diffuse adenomyomatosis of the gallbladder in a child. J Pediatr Hematol Oncol. 2016;38:e307-9.
Bonatti M. et al. Gallbladder adenomyomatosis: imaging findings, tricks and pitfalls. Insights Imaging. 2017;8:243-53.
Hammad AY et al. A literature review of radiological findings to guide the diagnosis of gallbladder adenomyomatosis. HPB (Oxford). 2016;18:129-35.
The diagnosis
Based on the clinical and imaging findings, a diagnosis of gallbladder adenomyomatosis was made. GA is a benign and usually asymptomatic condition that occurs mainly beyond the age of 50-60 years and is very rare in childhood.1 Symptomatic gallbladder adenomyomatosis indicates cholecystectomy, considering the presence of inflammation or gallbladder stones.2 Therefore, a laparoscopic cholecystectomy was performed on our patient. Rokitansky-Aschoff sinuses were seen in the entire thickened gallbladder wall on gross pathologic examination (Figure D). Histopathologic examination confirmed the diagnosis of GA with cholecystitis. The patient was eventually diagnosed with diffuse GA. She was successfully discharged from the hospital 4 days after surgery, and 3 months of follow-up were uneventful.

References
Eroglu N et al. Diffuse adenomyomatosis of the gallbladder in a child. J Pediatr Hematol Oncol. 2016;38:e307-9.
Bonatti M. et al. Gallbladder adenomyomatosis: imaging findings, tricks and pitfalls. Insights Imaging. 2017;8:243-53.
Hammad AY et al. A literature review of radiological findings to guide the diagnosis of gallbladder adenomyomatosis. HPB (Oxford). 2016;18:129-35.
A 15-year-old girl presented with an 18-month history of intermittent right upper quadrant pain that appeared after meals and was relieved after rest. She denied any nausea, vomiting, chills, diarrhea, or constipation. The patient reported no trauma. At admission, physical examination showed tenderness in the right upper abdomen without rebound or guarding. Murphy's sign was also present. The laboratory tests were unremarkable.
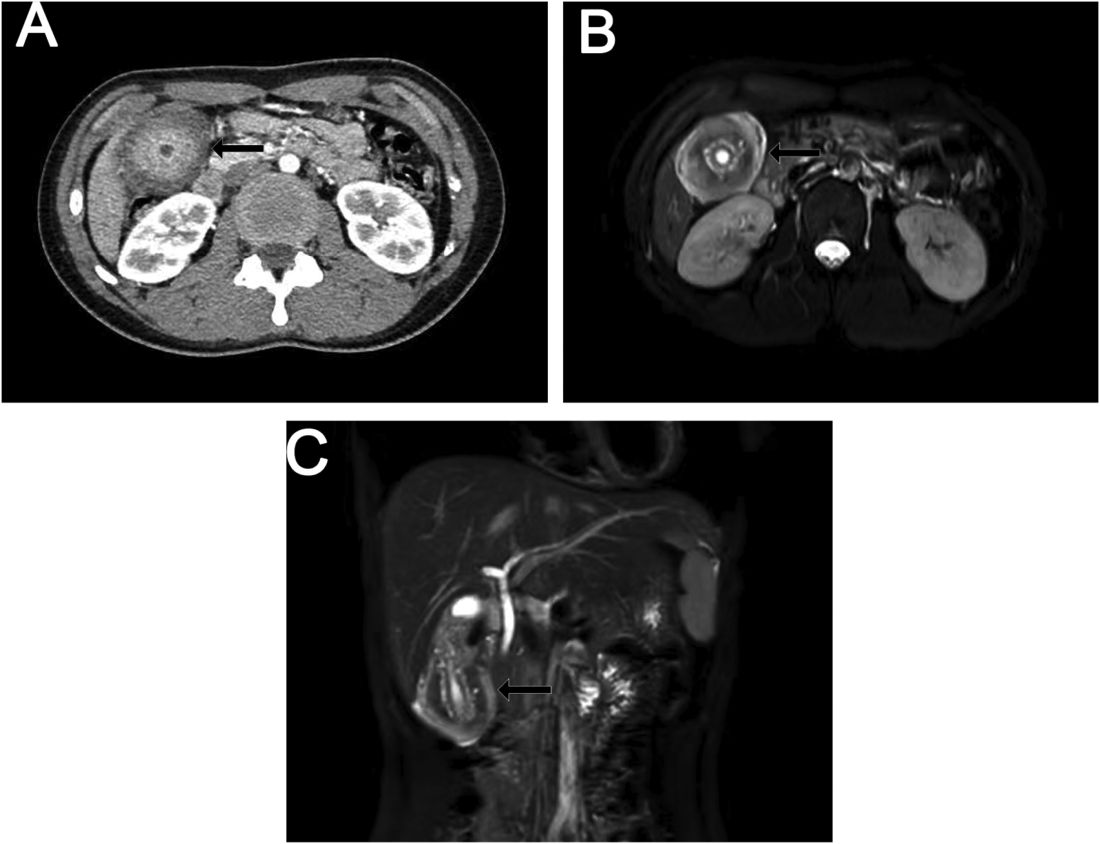
Ultrasound examination indicated gallbladder wall thickening. Furthermore, a contrast-enhanced computed tomographic scan showed marked gallbladder wall thickening with an annular unenhanced proliferative muscularis layer surrounding enhanced proliferative mucosal epithelium (Figure A), and magnetic resonance imaging showed multiple cyst-like spaces in the gallbladder wall (Figures B and C).
What is the diagnosis, and how should it be managed?
Previously published in Gastroenterology
Porocarcinoma Development in a Prior Trauma Site
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
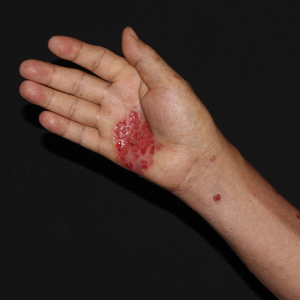
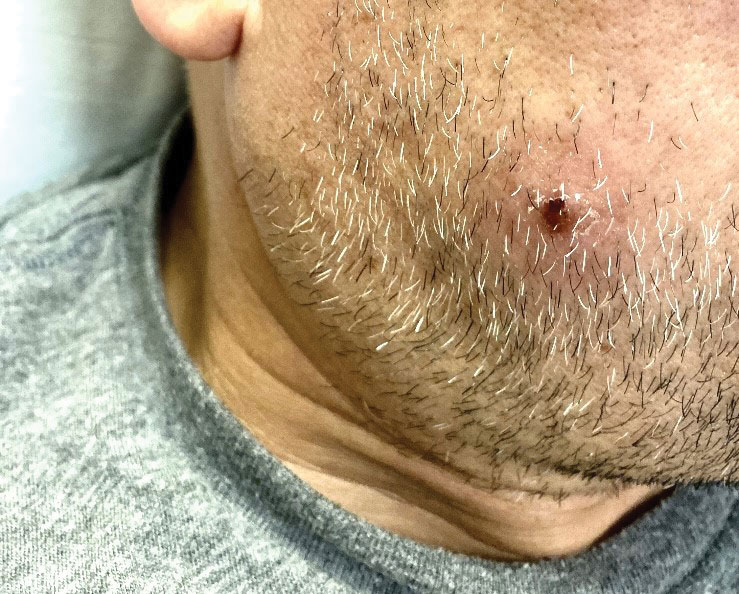
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.
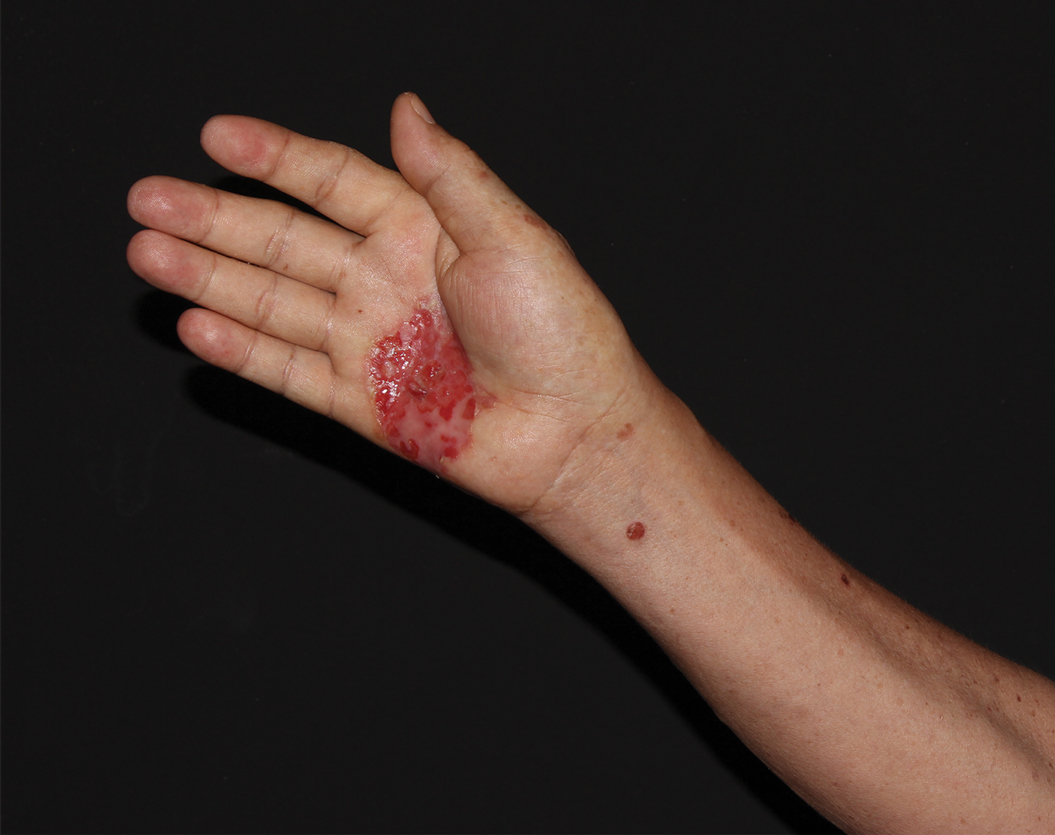
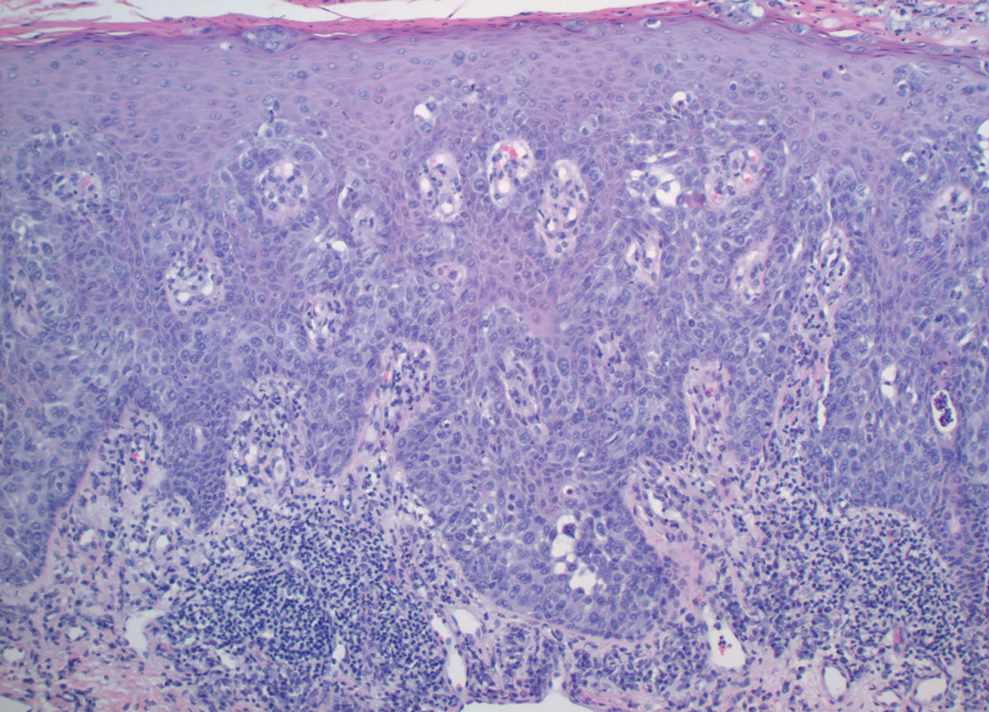
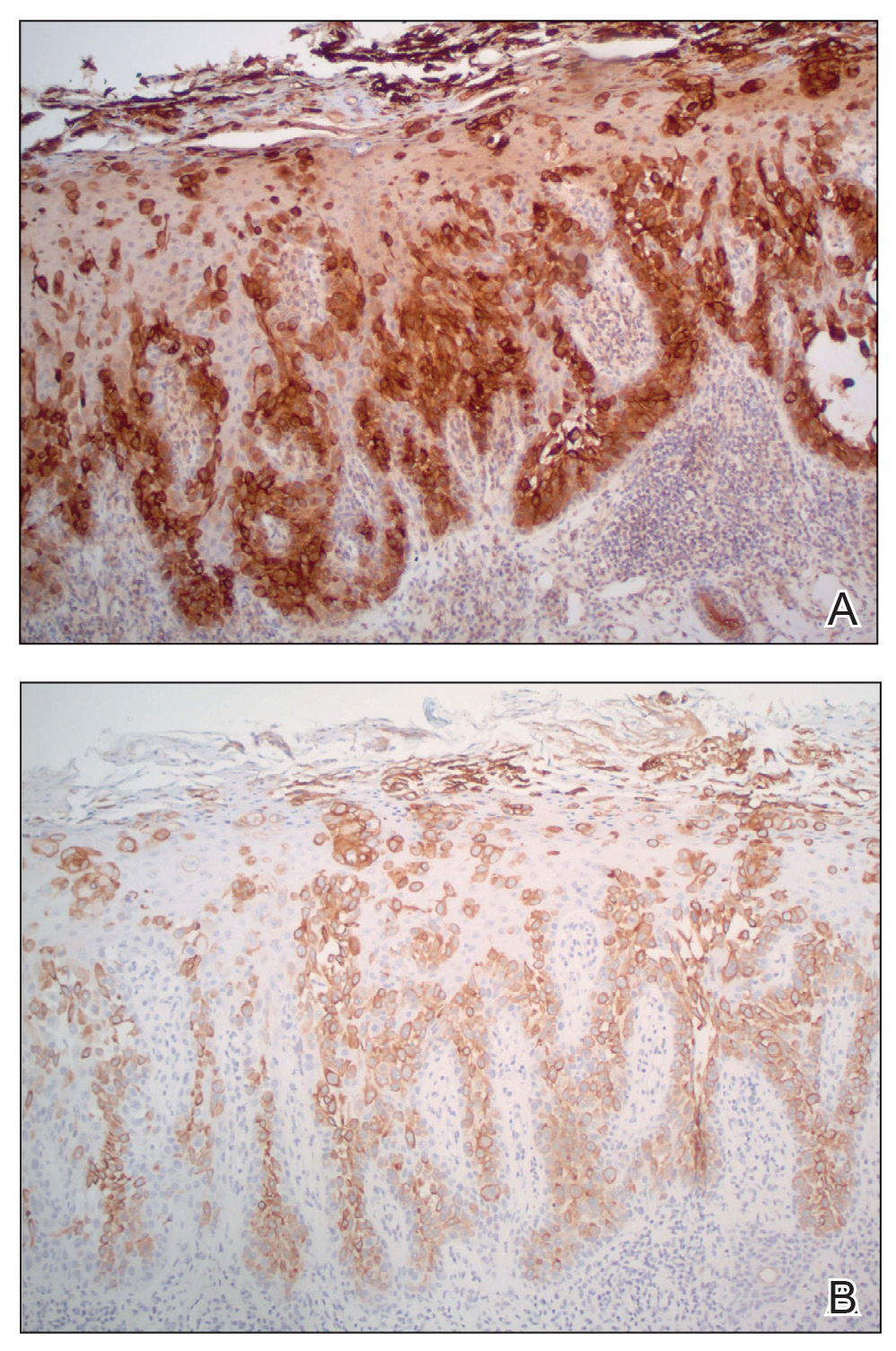
Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.
The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.
Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.

Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.

The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.

Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
To the Editor:
Porocarcinoma, or malignant poroma, is a rare adnexal malignancy of a predominantly glandular origin that comprises less than 0.01% of all cutaneous neoplasms.1,2 Although exposure to UV radiation and immunosuppression have been implicated in the malignant degeneration of benign poromas into porocarcinomas, at least half of all malignant variants will arise de novo.3,4 Patients present with an evolving nodule or plaque and often are in their seventh or eighth decade of life at the time of diagnosis.2 Localized trauma from burns or radiation exposure has been causatively linked to de novo porocarcinoma formation.2,5 These suppressive and traumatic stimuli drive increased genetic heterogeneity along with characteristic gene mutations in known tumor suppressor genes.6
A 62-year-old man presented with a nonhealing wound on the right hand of 5 years’ duration that had previously been attributed to a penetrating injury with a piece of copper from a refrigerant coolant system. The wound initially blistered and then eventually callused and developed areas of ulceration. The patient consulted multiple physicians for treatment of the intensely pruritic and ulcerated lesion. He received prescriptions for cephalexin, trimethoprim-sulfamethoxazole, doxycycline, clindamycin, and clobetasol cream, all of which offered minimal improvement. Home therapies including vitamin E and tea tree oil yielded no benefit. The lesion roughly quadrupled in size over the last 5 years.

Physical examination revealed a 7.5×4.2-cm ulcerated plaque with ragged borders and abundant central neoepithelialization on the right palmar surface (Figure 1). No gross motor or sensory defects were identified. There was no epitrochlear, axillary, cervical, or supraclavicular lymphadenopathy. A shave biopsy of the plaque’s edge was performed, which demonstrated a hyperplastic epidermis comprising atypical poroid cells with frequent mitoses, scant necrosis, and regular ductal structures confined to the epidermis (Figure 2). Immunohistochemical profiling results were positive for anticytokeratin (CAM 5.2) and Ber-EP4 (Figure 3). When evaluated in aggregate, these findings were consistent with porocarcinoma in situ.

The patient was referred to a surgical oncologist for evaluation. At that time, an exophytic mass had developed in the central lesion. Although no lymphadenopathy was identified upon examination, the patient had developed tremoring and a contracture deformity of the right hand. Extensive imaging and urgent surgical resection were recommended, but the patient did not wish to pursue these options, opting instead to continue home remedies. At a 15-month follow-up via telephone, the patient reported that the home therapy had failed and he had moved back to Vietnam. Partial limb amputation had been recommended by a local provider. Unfortunately, the patient was subsequently lost to follow-up, and his current status is unknown.

Porocarcinomas are rare tumors, comprising just 0.005% to 0.01% of all cutaneous epithelial tumors.1,2,5 They affect men and women equally, with an average age at diagnosis of 60 to 70 years.1,2 At least half of all porocarcinomas develop de novo, while 18% to 50% arise from the degeneration of an existing poroma.2,3 Exposure to UV light and immunosuppression, particularly following organ transplantation, represent 2 commonly suspected catalysts for this malignant transformation.4 De novo porocarcinomas are most causatively linked to localized trauma from burns or radiation exposure.5 Gene mutations in classic tumor suppressor genes—tumor protein p53 (TP53), phosphatase and tensin homolog (PTEN), rearranged during transfection (RET), adenomatous polyposis coli (APC)—and increased genetic heterogeneity follow these stimuli.6
The morphologic presentation of porocarcinoma is highly variable and may manifest as papules, nodules, or plaques in various states of erosion, ulceration, or excoriation. Diagnoses of basal and squamous cell carcinoma, primary adnexal tumors, seborrheic keratosis, pyogenic granuloma, and melanoma must all be considered and methodically ruled out.7 Porocarcinomas may arise nearly anywhere on the body, with a particular predilection for the lower extremities (35%), head/neck (24%), and upper extremities (14%).3,4 Primary lesions arising from the extremities, genitalia, or buttocks herald a higher risk for lymphatic invasion and distant metastasis, while head and neck tumors more commonly remain localized.8 Bleeding, ulceration, or rapid expansion of a preexisting poroma is suggestive of malignant transformation and may portend a more aggressive disease pattern.2,9
Unequivocal diagnosis relies on histological and immunohistochemical studies due to the marked clinical variance of this neoplasm.7 An irregular histologic pattern of poromatous basaloid cells with ductal differentiation and cytologic atypia commonly are seen with porocarcinomas.2,8 Nuclear pleomorphism with cellular necrosis, increased mitotic figures, and abortive ductal formation with a distinct lack of retraction around cellular aggregates often are found. Immunohistochemical staining is needed to confirm the primary tumor diagnosis. Histochemical stains commonly employed include carcinoembryonic antigen (CEA), cytokeratin AE1/AE3, epithelial membrane antigen, p53, p63, Ki67, and periodic acid-Schiff.10 The use of BerEP4 has been reported as efficacious in highlighting sweat structures, which can be particularly useful in cases when basal cell carcinoma is not in the histologic differential.11 These staining profiles afford confirmation of ductal differentiation with CEA, epithelial membrane antigen, and BerEP4, while p63 and Ki67 are used as surrogates for primary cutaneous neoplasia and cell proliferation, respectively.5,11 Porocarcinoma lesions may be most sensitive to CEA and most specific to CK19 (a component of cytokeratin AE1/AE3), though these findings have not been widely reproduced.7
The treatment and prognosis of porocarcinoma vary widely. Surgically excised lesions recur in roughly 20% of cases, though these rates likely include tumors that were incompletely resected in the primary attempt. Although wide local excision with an average 1-cm margin remains the most employed removal technique, Mohs micrographic surgery may more effectively limit recurrence and metastasis of localized disease.7,8,12 Metastatic disease foretells a mortality rate of at least 65%, which is problematic in that 10% to 20% of patients have metastatic disease at the time of diagnosis and another 20% will show metastasis following primary tumor excision.8,10 Neoplasms with high mitotic rates and depths greater than 7 mm should prompt thorough diagnostic imaging, such as positron emission tomography or magnetic resonance imaging. A sentinel lymph node biopsy should be strongly considered and discussed with the patient.10 Treatment options for nodal and distant metastases include a combination of localized surgery, lymphadenectomy, radiotherapy, and chemotherapeutic agents.2,4,5 The response to systemic treatment and radiotherapy often is quite poor, though the use of combinations of docetaxel, paclitaxel, cetuximab, and immunotherapy have been efficacious in smaller studies.8,10 The highest rates of morbidity and mortality are seen in patients with metastases on presentation or with localized tumors in the groin and buttocks.8
The diagnosis of porocarcinoma may be elusive due to its relatively rare occurrence. Therefore, it is critical to consider this neoplasm in high-risk sites in older patients who present with an evolving nodule or tumor on an extremity. Routine histology and astute histochemical profiling are necessary to exclude diseases that mimic porocarcinoma. Once diagnosis is confirmed, management with prompt excision and diagnostic imaging is recommended, including a lymph node biopsy if appropriate. Due to its high metastatic potential and associated morbidity and mortality, patients with porocarcinoma should be followed closely by a multidisciplinary care team.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
- Belin E, Ezzedine K, Stanislas S, et al. Factors in the surgical management of primary eccrine porocarcinoma: prognostic histological factors can guide the surgical procedure. Br J Dermatol. 2011;165:985-989.
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
- Spencer DM, Bigler LR, Hearne DW, et al. Pedal papule. eccrine porocarcinoma (EPC) in association with poroma. Arch Dermatol. 1995;131:211, 214.
- Salih AM, Kakamad FH, Essa RA, et al. Porocarcinoma: a systematic review of literature with a single case report. Int J Surg Case Rep. 2017;30:13-16.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Mosby Elsevier; 2018.
- Bosic M, Kirchner M, Brasanac D, et al. Targeted molecular profiling reveals genetic heterogeneity of poromas and porocarcinomas. Pathology. 2018;50:327-332.
- Mahalingam M, Richards JE, Selim MA, et al. An immunohistochemical comparison of cytokeratin 7, cytokeratin 15, cytokeratin 19, CAM 5.2, carcinoembryonic antigen, and nestin in differentiating porocarcinoma from squamous cell carcinoma. Hum Pathol. 2012;43:1265-1272.
- Nazemi A, Higgins S, Swift R, et al. Eccrine porocarcinoma: new insights and a systematic review of the literature. Dermatol Surg. 2018;44:1247-1261.
- Wen SY. Case report of eccrine porocarcinoma in situ associated with eccrine poroma on the forehead. J Dermatol. 2012;39:649-651.
- Gerber PA, Schulte KW, Ruzicka T, et al. Eccrine porocarcinoma of the head: an important differential diagnosis in the elderly patient. Dermatology. 2008;216:229-233.
- Afshar M, Deroide F, Robson A. BerEP4 is widely expressed in tumors of the sweat apparatus: a source of potential diagnostic error. J Cutan Pathol. 2013;40:259-264.
- Tolkachjov SN, Hocker TL, Camilleri MJ, et al. Treatment of porocarcinoma with Mohs micrographic surgery: the Mayo clinic experience. Dermatol Surg. 2016;42:745-750.
Practice Points
- Porocarcinoma is a rare, potentially aggressive, glandular malignancy that should be a clinical consideration in patients presenting with a cutaneous neoplasm.
- Although wide local excision historically has been the treatment of choice for porocarcinoma, Mohs micrographic surgery has demonstrated excellent cure rates.
- Patients with unresectable or metastatic porocarcinomas have a poor prognosis but may respond to combination chemotherapy regimens.
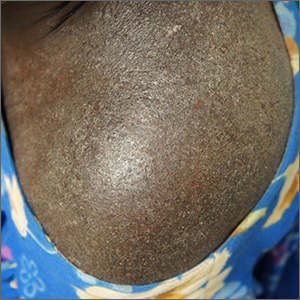
Clothing provides Dx clue

A close examination of the patient’s scalp and hair was unhelpful, but a close look at the weave and seams of her dress revealed multiple nits and lice, consistent with a diagnosis of body lice.
Head lice and body lice are 2 different ecotypes of the species Pediculus humanus and occupy different environments on the body. They differ slightly in body shape caused by variable expression of the same genes.1 Body lice primarily live and lay eggs on clothing, especially along seams and within knit weaves. They travel to the skin to feed, causing significant itching in the host from the inflammatory and allergic effects of their saliva and feces. Additionally, body lice are vectors of several serious diseases including epidemic typhus (Rickettsia prowasekii), trench fever (Bartonella quintana), and relapsing fever (Borrelia recurrentis).1
A diagnosis of body lice is a sign of severe lack of access to basic human needs—uncrowded shelter, clean clothes, and clean water for bathing. A patient who has been given this diagnosis should be offered and receive a bath or shower with generous soap and warm water. Clothes should be cleaned with hot water (up to 149 °F) or discarded. Patients also may be treated once with topical permethrin 5% cream applied from the top of the neck to the toes in the event that mites survived bathing by attaching to body hairs. Any systemic illness or fever should be evaluated for the above epidemic pathogens. Patients should also be put in touch with social services and mental health services, as appropriate.
This patient received all of the above treatments and had already accessed social services. That said, she continued to struggle with housing instability.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571. doi: 10.1016/j.pt.2012.09.003

A close examination of the patient’s scalp and hair was unhelpful, but a close look at the weave and seams of her dress revealed multiple nits and lice, consistent with a diagnosis of body lice.
Head lice and body lice are 2 different ecotypes of the species Pediculus humanus and occupy different environments on the body. They differ slightly in body shape caused by variable expression of the same genes.1 Body lice primarily live and lay eggs on clothing, especially along seams and within knit weaves. They travel to the skin to feed, causing significant itching in the host from the inflammatory and allergic effects of their saliva and feces. Additionally, body lice are vectors of several serious diseases including epidemic typhus (Rickettsia prowasekii), trench fever (Bartonella quintana), and relapsing fever (Borrelia recurrentis).1
A diagnosis of body lice is a sign of severe lack of access to basic human needs—uncrowded shelter, clean clothes, and clean water for bathing. A patient who has been given this diagnosis should be offered and receive a bath or shower with generous soap and warm water. Clothes should be cleaned with hot water (up to 149 °F) or discarded. Patients also may be treated once with topical permethrin 5% cream applied from the top of the neck to the toes in the event that mites survived bathing by attaching to body hairs. Any systemic illness or fever should be evaluated for the above epidemic pathogens. Patients should also be put in touch with social services and mental health services, as appropriate.
This patient received all of the above treatments and had already accessed social services. That said, she continued to struggle with housing instability.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A close examination of the patient’s scalp and hair was unhelpful, but a close look at the weave and seams of her dress revealed multiple nits and lice, consistent with a diagnosis of body lice.
Head lice and body lice are 2 different ecotypes of the species Pediculus humanus and occupy different environments on the body. They differ slightly in body shape caused by variable expression of the same genes.1 Body lice primarily live and lay eggs on clothing, especially along seams and within knit weaves. They travel to the skin to feed, causing significant itching in the host from the inflammatory and allergic effects of their saliva and feces. Additionally, body lice are vectors of several serious diseases including epidemic typhus (Rickettsia prowasekii), trench fever (Bartonella quintana), and relapsing fever (Borrelia recurrentis).1
A diagnosis of body lice is a sign of severe lack of access to basic human needs—uncrowded shelter, clean clothes, and clean water for bathing. A patient who has been given this diagnosis should be offered and receive a bath or shower with generous soap and warm water. Clothes should be cleaned with hot water (up to 149 °F) or discarded. Patients also may be treated once with topical permethrin 5% cream applied from the top of the neck to the toes in the event that mites survived bathing by attaching to body hairs. Any systemic illness or fever should be evaluated for the above epidemic pathogens. Patients should also be put in touch with social services and mental health services, as appropriate.
This patient received all of the above treatments and had already accessed social services. That said, she continued to struggle with housing instability.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571. doi: 10.1016/j.pt.2012.09.003
1. Veracx A, Raoult D. Biology and genetics of human head and body lice. Trends Parasitol. 2012;28:563-571. doi: 10.1016/j.pt.2012.09.003
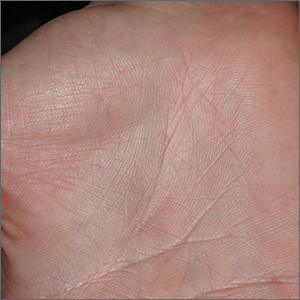
Intensely itchy normal skin
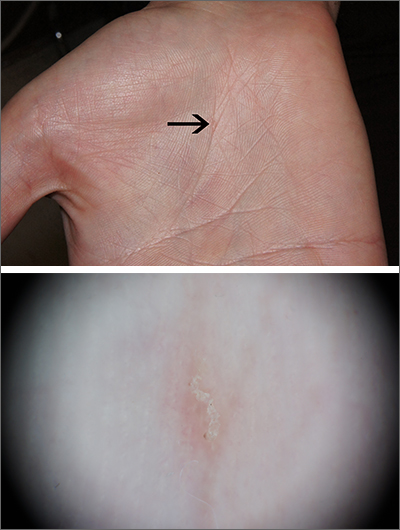
Severe itching should prompt suspicion for scabies and the hands are the highest-yield location. In this patient’s case, there weren’t findings in the web spaces and, in general, skin findings were largely absent; dermoscopy confirmed the diagnosis of scabies.
Sarcoptes scabiei, is a parasitic mite that lives and reproduces in and on human skin and is transmitted by very close contact, either skin-to-skin or by living within a household or institution with shared linens and furnishings. After infection, itching develops within days to weeks from both the physical movement and burrowing of mites within the skin and from the allergic and inflammatory response to mite bodies and their waste.1 Symptoms and infections may persist for years in the absence of treatment.
Sometimes (as in this case), burrows are few and very subtle. More often, there are widespread burrows and excoriated papules over the hands, trunk, extremities, and genitals. A burrowed mite is often adjacent to, but not directly in, an excoriation. Dermoscopy has transformed the ability to diagnose this condition quickly by enabling clinicians to visualize the triangular shape of the head and front legs of a mite (called the “delta sign”). This localization allows easy microscopic confirmation by paring the mite from the skin with a small scalpel blade. (A #11 or #15 blade works very well.)
Topical permethrin 5% cream is highly curative. The cream should be applied from the top of the neck to the tips of the patient’s toes and left on for 8 hours; the process should be repeated a week later. Very close contacts (eg, symptomatic household members or sexual partners) should be treated concurrently. A 60 g tube will treat 1 adult twice. (A 60 g tube of permethrin with a refill, therefore, will treat 2 adults twice.) Oral ivermectin 3 mg dosed at 200 mcg/kg in a single dose repeated in 1 to 2 weeks is an alternative.
Outbreaks in an institutional setting present a significant challenge and require population-based control and often the assistance of infection control specialists or local public health officials. Often this involves weekly treatment with ivermectin for all potentially affected individuals for 3 to 4 weeks and surveillance for follow-up. While there is some resistance to ivermectin, many failures relate more to reinfection from unidentified sources.
This patient received topical permethrin 5% cream dosed as noted above. Itching can be expected to persist for 3 to 4 weeks, so topical triamcinolone 0.1% cream was prescribed as needed for itching on days when permethrin wasn’t applied. At 6 weeks, this patient’s symptoms had resolved.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Richards RN. Scabies: diagnostic and therapeutic update. J Cutan Med Surg. 2021;25:95-101. doi: 10.1177/1203475420960446

Severe itching should prompt suspicion for scabies and the hands are the highest-yield location. In this patient’s case, there weren’t findings in the web spaces and, in general, skin findings were largely absent; dermoscopy confirmed the diagnosis of scabies.
Sarcoptes scabiei, is a parasitic mite that lives and reproduces in and on human skin and is transmitted by very close contact, either skin-to-skin or by living within a household or institution with shared linens and furnishings. After infection, itching develops within days to weeks from both the physical movement and burrowing of mites within the skin and from the allergic and inflammatory response to mite bodies and their waste.1 Symptoms and infections may persist for years in the absence of treatment.
Sometimes (as in this case), burrows are few and very subtle. More often, there are widespread burrows and excoriated papules over the hands, trunk, extremities, and genitals. A burrowed mite is often adjacent to, but not directly in, an excoriation. Dermoscopy has transformed the ability to diagnose this condition quickly by enabling clinicians to visualize the triangular shape of the head and front legs of a mite (called the “delta sign”). This localization allows easy microscopic confirmation by paring the mite from the skin with a small scalpel blade. (A #11 or #15 blade works very well.)
Topical permethrin 5% cream is highly curative. The cream should be applied from the top of the neck to the tips of the patient’s toes and left on for 8 hours; the process should be repeated a week later. Very close contacts (eg, symptomatic household members or sexual partners) should be treated concurrently. A 60 g tube will treat 1 adult twice. (A 60 g tube of permethrin with a refill, therefore, will treat 2 adults twice.) Oral ivermectin 3 mg dosed at 200 mcg/kg in a single dose repeated in 1 to 2 weeks is an alternative.
Outbreaks in an institutional setting present a significant challenge and require population-based control and often the assistance of infection control specialists or local public health officials. Often this involves weekly treatment with ivermectin for all potentially affected individuals for 3 to 4 weeks and surveillance for follow-up. While there is some resistance to ivermectin, many failures relate more to reinfection from unidentified sources.
This patient received topical permethrin 5% cream dosed as noted above. Itching can be expected to persist for 3 to 4 weeks, so topical triamcinolone 0.1% cream was prescribed as needed for itching on days when permethrin wasn’t applied. At 6 weeks, this patient’s symptoms had resolved.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Severe itching should prompt suspicion for scabies and the hands are the highest-yield location. In this patient’s case, there weren’t findings in the web spaces and, in general, skin findings were largely absent; dermoscopy confirmed the diagnosis of scabies.
Sarcoptes scabiei, is a parasitic mite that lives and reproduces in and on human skin and is transmitted by very close contact, either skin-to-skin or by living within a household or institution with shared linens and furnishings. After infection, itching develops within days to weeks from both the physical movement and burrowing of mites within the skin and from the allergic and inflammatory response to mite bodies and their waste.1 Symptoms and infections may persist for years in the absence of treatment.
Sometimes (as in this case), burrows are few and very subtle. More often, there are widespread burrows and excoriated papules over the hands, trunk, extremities, and genitals. A burrowed mite is often adjacent to, but not directly in, an excoriation. Dermoscopy has transformed the ability to diagnose this condition quickly by enabling clinicians to visualize the triangular shape of the head and front legs of a mite (called the “delta sign”). This localization allows easy microscopic confirmation by paring the mite from the skin with a small scalpel blade. (A #11 or #15 blade works very well.)
Topical permethrin 5% cream is highly curative. The cream should be applied from the top of the neck to the tips of the patient’s toes and left on for 8 hours; the process should be repeated a week later. Very close contacts (eg, symptomatic household members or sexual partners) should be treated concurrently. A 60 g tube will treat 1 adult twice. (A 60 g tube of permethrin with a refill, therefore, will treat 2 adults twice.) Oral ivermectin 3 mg dosed at 200 mcg/kg in a single dose repeated in 1 to 2 weeks is an alternative.
Outbreaks in an institutional setting present a significant challenge and require population-based control and often the assistance of infection control specialists or local public health officials. Often this involves weekly treatment with ivermectin for all potentially affected individuals for 3 to 4 weeks and surveillance for follow-up. While there is some resistance to ivermectin, many failures relate more to reinfection from unidentified sources.
This patient received topical permethrin 5% cream dosed as noted above. Itching can be expected to persist for 3 to 4 weeks, so topical triamcinolone 0.1% cream was prescribed as needed for itching on days when permethrin wasn’t applied. At 6 weeks, this patient’s symptoms had resolved.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Richards RN. Scabies: diagnostic and therapeutic update. J Cutan Med Surg. 2021;25:95-101. doi: 10.1177/1203475420960446
1. Richards RN. Scabies: diagnostic and therapeutic update. J Cutan Med Surg. 2021;25:95-101. doi: 10.1177/1203475420960446
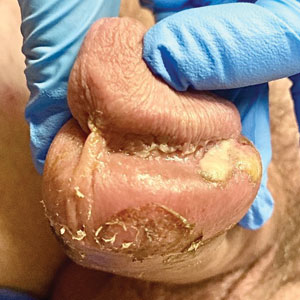
Genital Ulcerations With Swelling
The Diagnosis: Mpox (Monkeypox)
Tests for active herpes simplex virus (HHV), gonorrhea, chlamydia, HIV, and syphilis were negative. Swabs from the penile lesion demonstrated positivity for the West African clade of mpox (monkeypox) virus (MPXV) by polymerase chain reaction. The patient was treated supportively without the addition of antiviral therapy, and he experienced a complete recovery.
Mpox virus was first isolated in 1958 in a research facility and was named after the laboratory animals that were housed there. The first human documentation of the disease occurred in 1970, and it was first documented in the United States in 2003 in an infection that was traced to a shipment of small mammals from Ghana to Texas.1 The disease has always been endemic to Africa; however, the incidence has been increasing.2 A new MPXV outbreak was reported in many countries in early 2022, including the United States.1
The MPXV is a double-stranded DNA virus of the genus Orthopoxvirus, and 2 genetic clades have been identified: clade I (formerly the Central African clade) and clade II (formerly the West African clade). The virus has the capability to infect many mammals; however, its host remains unidentified.1 The exact mechanism of transmission from infected animals to humans largely is unknown; however, direct or indirect contact with infected animals likely is responsible. Human-to-human transmission can occur by many mechanisms including contact with large respiratory droplets, bodily fluids, and contaminated surfaces. The incubation period is 5 to 21 days, and the symptoms last 2 to 5 weeks.1
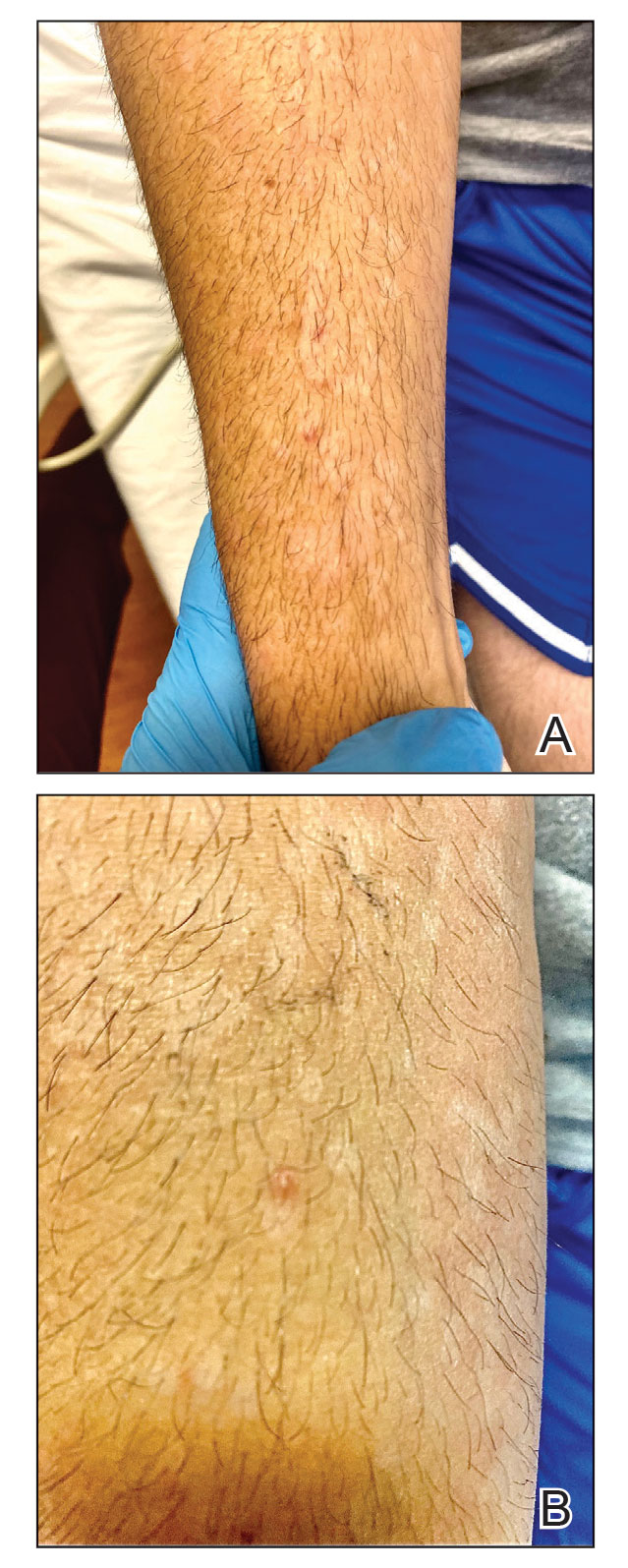
The clinical manifestations of MPXV during the most recent outbreak differ from prior outbreaks. Patients are more likely to experience minimal to no systemic symptoms, and cutaneous lesions can be few and localized to a focal area, especially on the face and in the anogenital region,3 similar to the presentation in our patient (Figure 1). Cutaneous lesions of the most recent MPXV outbreak also include painless ulcerations similar to syphilitic chancres and lesions that are in various stages of healing.3 Lesions often begin as pseudopustules, which are firm white papules with or without a necrotic center that resemble pustules; unlike true pustules, there is no identifiable purulent material within it. Bacterial superinfection of the lesions is not uncommon.4 Over time, a secondary pustular eruption resembling folliculitis also may occur,4 as noted in our patient (Figure 2).
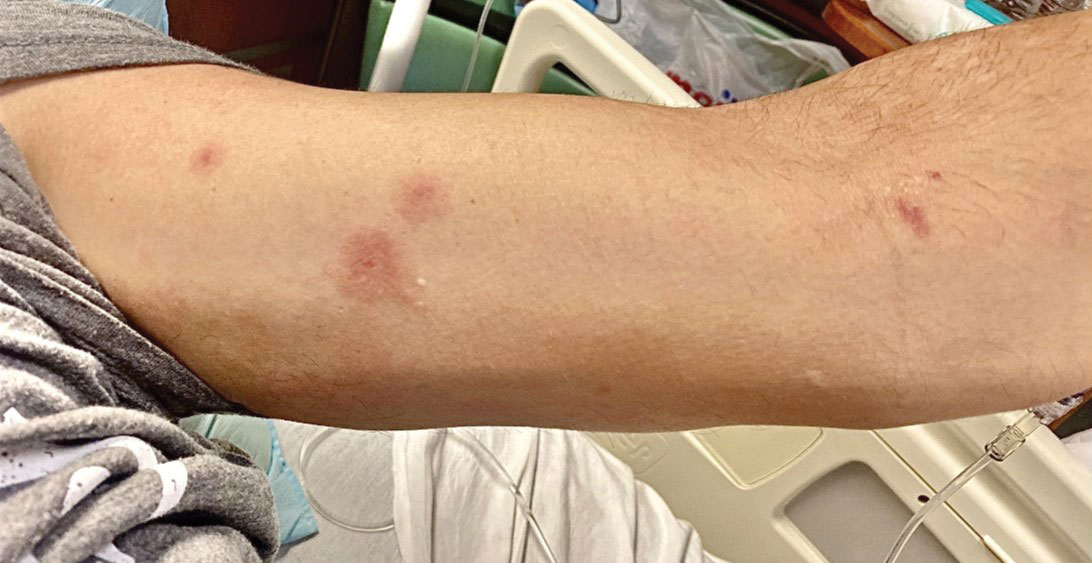
Although we did not have a biopsy to support the diagnosis of associated erythema multiforme (EM) in our patient, features supportive of this diagnosis included the classic clinical appearance of typical, well-defined, targetoid plaques with 3 distinct zones (Figure 3); the association with a known infection; the distribution on the arms with truncal sparing; and self-limited lesions. More than 90% of EM cases are associated with infection, with HHV representing the most common culprit5; therefore, the relationship with a different virus is not an unreasonable suggestion. Additionally, there have been rare reports of EM in association with MPXV.4
Histopathology of MPXV may have distinctive features. Lesions often demonstrate keratinocytic necrosis and basal layer vacuolization with an associated superficial and deep perivascular lymphohistiocytic infiltrate. When the morphology of the lesion is vesicular, histopathology reveals spongiosis and ballooning degeneration with epidermal necrosis. Viral inclusion bodies within keratinocytes may be identified.1 Death rates from MPXV has been reported from 1% to 11%, with increased mortality among high-risk populations including children and immunocompromised individuals. Treatment of the disease largely consists of supportive care and management of any associated complications including bacterial infection, pneumonia, and encephalitis.1
The differential diagnosis of MPXV includes other ulcerative lesions that can occur on the genital skin. Fixed drug eruptions often present on the penis,6 but there was no identifiable inciting drug in our patient. Herpes simplex virus infection was very high on the differential given our patient’s history of recurrent infections and association with a targetoid rash, but HHV type 1 and HHV type 2 testing of the lesion was negative. A syphilitic chancre also may present with the nontender genital ulceration7 that was seen in our patient, but serology did not support this diagnosis. Cutaneous Crohn disease also may manifest with genital ulceration even before a diagnosis of Crohn disease is made, but these lesions often present as linear knife-cut ulcerations of the anogenital region.8
Our case further supports a clinical presentation that diverges from the more traditional cases of MPXV. Additionally, associated EM may be a clue to infection, especially in cases of negative HHV and other sexually transmitted infection testing.
- Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat? a systematic review. PLoS Negl Trop Dis. 2022;16:E0010141.
- Kumar N, Acharya A, Gendelman HE, et al. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855.
- Eisenstadt R, Liszewski WJ, Nguyen CV. Recognizing minimal cutaneous involvement or systemic symptoms in monkeypox. JAMA Dermatol. 2022;158:1457-1458.
- Català A, Clavo-Escribano P, Riera-Monroig J, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases [published online August 2, 2022]. Br J Dermatol. 2022;187:765-772.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Waleryie-Allanore L, Obeid G, Revuz J. Drug reactions. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:348-375.
- Stary G, Stary A. Sexually transmitted infections. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1447-1469.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1644-1663.
The Diagnosis: Mpox (Monkeypox)
Tests for active herpes simplex virus (HHV), gonorrhea, chlamydia, HIV, and syphilis were negative. Swabs from the penile lesion demonstrated positivity for the West African clade of mpox (monkeypox) virus (MPXV) by polymerase chain reaction. The patient was treated supportively without the addition of antiviral therapy, and he experienced a complete recovery.
Mpox virus was first isolated in 1958 in a research facility and was named after the laboratory animals that were housed there. The first human documentation of the disease occurred in 1970, and it was first documented in the United States in 2003 in an infection that was traced to a shipment of small mammals from Ghana to Texas.1 The disease has always been endemic to Africa; however, the incidence has been increasing.2 A new MPXV outbreak was reported in many countries in early 2022, including the United States.1
The MPXV is a double-stranded DNA virus of the genus Orthopoxvirus, and 2 genetic clades have been identified: clade I (formerly the Central African clade) and clade II (formerly the West African clade). The virus has the capability to infect many mammals; however, its host remains unidentified.1 The exact mechanism of transmission from infected animals to humans largely is unknown; however, direct or indirect contact with infected animals likely is responsible. Human-to-human transmission can occur by many mechanisms including contact with large respiratory droplets, bodily fluids, and contaminated surfaces. The incubation period is 5 to 21 days, and the symptoms last 2 to 5 weeks.1

The clinical manifestations of MPXV during the most recent outbreak differ from prior outbreaks. Patients are more likely to experience minimal to no systemic symptoms, and cutaneous lesions can be few and localized to a focal area, especially on the face and in the anogenital region,3 similar to the presentation in our patient (Figure 1). Cutaneous lesions of the most recent MPXV outbreak also include painless ulcerations similar to syphilitic chancres and lesions that are in various stages of healing.3 Lesions often begin as pseudopustules, which are firm white papules with or without a necrotic center that resemble pustules; unlike true pustules, there is no identifiable purulent material within it. Bacterial superinfection of the lesions is not uncommon.4 Over time, a secondary pustular eruption resembling folliculitis also may occur,4 as noted in our patient (Figure 2).

Although we did not have a biopsy to support the diagnosis of associated erythema multiforme (EM) in our patient, features supportive of this diagnosis included the classic clinical appearance of typical, well-defined, targetoid plaques with 3 distinct zones (Figure 3); the association with a known infection; the distribution on the arms with truncal sparing; and self-limited lesions. More than 90% of EM cases are associated with infection, with HHV representing the most common culprit5; therefore, the relationship with a different virus is not an unreasonable suggestion. Additionally, there have been rare reports of EM in association with MPXV.4

Histopathology of MPXV may have distinctive features. Lesions often demonstrate keratinocytic necrosis and basal layer vacuolization with an associated superficial and deep perivascular lymphohistiocytic infiltrate. When the morphology of the lesion is vesicular, histopathology reveals spongiosis and ballooning degeneration with epidermal necrosis. Viral inclusion bodies within keratinocytes may be identified.1 Death rates from MPXV has been reported from 1% to 11%, with increased mortality among high-risk populations including children and immunocompromised individuals. Treatment of the disease largely consists of supportive care and management of any associated complications including bacterial infection, pneumonia, and encephalitis.1
The differential diagnosis of MPXV includes other ulcerative lesions that can occur on the genital skin. Fixed drug eruptions often present on the penis,6 but there was no identifiable inciting drug in our patient. Herpes simplex virus infection was very high on the differential given our patient’s history of recurrent infections and association with a targetoid rash, but HHV type 1 and HHV type 2 testing of the lesion was negative. A syphilitic chancre also may present with the nontender genital ulceration7 that was seen in our patient, but serology did not support this diagnosis. Cutaneous Crohn disease also may manifest with genital ulceration even before a diagnosis of Crohn disease is made, but these lesions often present as linear knife-cut ulcerations of the anogenital region.8
Our case further supports a clinical presentation that diverges from the more traditional cases of MPXV. Additionally, associated EM may be a clue to infection, especially in cases of negative HHV and other sexually transmitted infection testing.
The Diagnosis: Mpox (Monkeypox)
Tests for active herpes simplex virus (HHV), gonorrhea, chlamydia, HIV, and syphilis were negative. Swabs from the penile lesion demonstrated positivity for the West African clade of mpox (monkeypox) virus (MPXV) by polymerase chain reaction. The patient was treated supportively without the addition of antiviral therapy, and he experienced a complete recovery.
Mpox virus was first isolated in 1958 in a research facility and was named after the laboratory animals that were housed there. The first human documentation of the disease occurred in 1970, and it was first documented in the United States in 2003 in an infection that was traced to a shipment of small mammals from Ghana to Texas.1 The disease has always been endemic to Africa; however, the incidence has been increasing.2 A new MPXV outbreak was reported in many countries in early 2022, including the United States.1
The MPXV is a double-stranded DNA virus of the genus Orthopoxvirus, and 2 genetic clades have been identified: clade I (formerly the Central African clade) and clade II (formerly the West African clade). The virus has the capability to infect many mammals; however, its host remains unidentified.1 The exact mechanism of transmission from infected animals to humans largely is unknown; however, direct or indirect contact with infected animals likely is responsible. Human-to-human transmission can occur by many mechanisms including contact with large respiratory droplets, bodily fluids, and contaminated surfaces. The incubation period is 5 to 21 days, and the symptoms last 2 to 5 weeks.1

The clinical manifestations of MPXV during the most recent outbreak differ from prior outbreaks. Patients are more likely to experience minimal to no systemic symptoms, and cutaneous lesions can be few and localized to a focal area, especially on the face and in the anogenital region,3 similar to the presentation in our patient (Figure 1). Cutaneous lesions of the most recent MPXV outbreak also include painless ulcerations similar to syphilitic chancres and lesions that are in various stages of healing.3 Lesions often begin as pseudopustules, which are firm white papules with or without a necrotic center that resemble pustules; unlike true pustules, there is no identifiable purulent material within it. Bacterial superinfection of the lesions is not uncommon.4 Over time, a secondary pustular eruption resembling folliculitis also may occur,4 as noted in our patient (Figure 2).

Although we did not have a biopsy to support the diagnosis of associated erythema multiforme (EM) in our patient, features supportive of this diagnosis included the classic clinical appearance of typical, well-defined, targetoid plaques with 3 distinct zones (Figure 3); the association with a known infection; the distribution on the arms with truncal sparing; and self-limited lesions. More than 90% of EM cases are associated with infection, with HHV representing the most common culprit5; therefore, the relationship with a different virus is not an unreasonable suggestion. Additionally, there have been rare reports of EM in association with MPXV.4

Histopathology of MPXV may have distinctive features. Lesions often demonstrate keratinocytic necrosis and basal layer vacuolization with an associated superficial and deep perivascular lymphohistiocytic infiltrate. When the morphology of the lesion is vesicular, histopathology reveals spongiosis and ballooning degeneration with epidermal necrosis. Viral inclusion bodies within keratinocytes may be identified.1 Death rates from MPXV has been reported from 1% to 11%, with increased mortality among high-risk populations including children and immunocompromised individuals. Treatment of the disease largely consists of supportive care and management of any associated complications including bacterial infection, pneumonia, and encephalitis.1
The differential diagnosis of MPXV includes other ulcerative lesions that can occur on the genital skin. Fixed drug eruptions often present on the penis,6 but there was no identifiable inciting drug in our patient. Herpes simplex virus infection was very high on the differential given our patient’s history of recurrent infections and association with a targetoid rash, but HHV type 1 and HHV type 2 testing of the lesion was negative. A syphilitic chancre also may present with the nontender genital ulceration7 that was seen in our patient, but serology did not support this diagnosis. Cutaneous Crohn disease also may manifest with genital ulceration even before a diagnosis of Crohn disease is made, but these lesions often present as linear knife-cut ulcerations of the anogenital region.8
Our case further supports a clinical presentation that diverges from the more traditional cases of MPXV. Additionally, associated EM may be a clue to infection, especially in cases of negative HHV and other sexually transmitted infection testing.
- Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat? a systematic review. PLoS Negl Trop Dis. 2022;16:E0010141.
- Kumar N, Acharya A, Gendelman HE, et al. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855.
- Eisenstadt R, Liszewski WJ, Nguyen CV. Recognizing minimal cutaneous involvement or systemic symptoms in monkeypox. JAMA Dermatol. 2022;158:1457-1458.
- Català A, Clavo-Escribano P, Riera-Monroig J, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases [published online August 2, 2022]. Br J Dermatol. 2022;187:765-772.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Waleryie-Allanore L, Obeid G, Revuz J. Drug reactions. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:348-375.
- Stary G, Stary A. Sexually transmitted infections. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1447-1469.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1644-1663.
- Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—a potential threat? a systematic review. PLoS Negl Trop Dis. 2022;16:E0010141.
- Kumar N, Acharya A, Gendelman HE, et al. The 2022 outbreak and the pathobiology of the monkeypox virus. J Autoimmun. 2022;131:102855.
- Eisenstadt R, Liszewski WJ, Nguyen CV. Recognizing minimal cutaneous involvement or systemic symptoms in monkeypox. JAMA Dermatol. 2022;158:1457-1458.
- Català A, Clavo-Escribano P, Riera-Monroig J, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases [published online August 2, 2022]. Br J Dermatol. 2022;187:765-772.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Waleryie-Allanore L, Obeid G, Revuz J. Drug reactions. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:348-375.
- Stary G, Stary A. Sexually transmitted infections. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1447-1469.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. Elsevier; 2018:1644-1663.
A 50-year-old man with a history of recurrent genital herpes simplex virus infections presented to the hospital with genital lesions and swelling of 5 days’ duration. Prior to admission, the patient was treated with a course of valacyclovir by an urgent care physician without improvement. Physical examination revealed a 3-cm, nontender, shallow, ulcerative plaque with irregular borders and a purulent yellow base distributed on the distal shaft of the penis with extension into the coronal sulcus. A few other scattered erosions were noted on the distal penile shaft. He had associated diffuse nonpitting edema of the penis and scrotum as well as tender bilateral inguinal lymphadenopathy. Three days after the genital ulcerations began, the patient developed a nontender erythematous papule with a necrotic center on the right jaw followed by an eruption of erythematous papulopustules on the arms and trunk. The patient denied dysuria, purulent penile discharge, fevers, chills, headaches, myalgia, arthralgia, nausea, vomiting, or diarrhea. The patient was sexually active exclusively with females and had more than 10 partners in the prior year. Shortly after hospital admission, the patient developed red targetoid plaques on the groin, trunk, and arms. No oral mucosal lesions were identified.
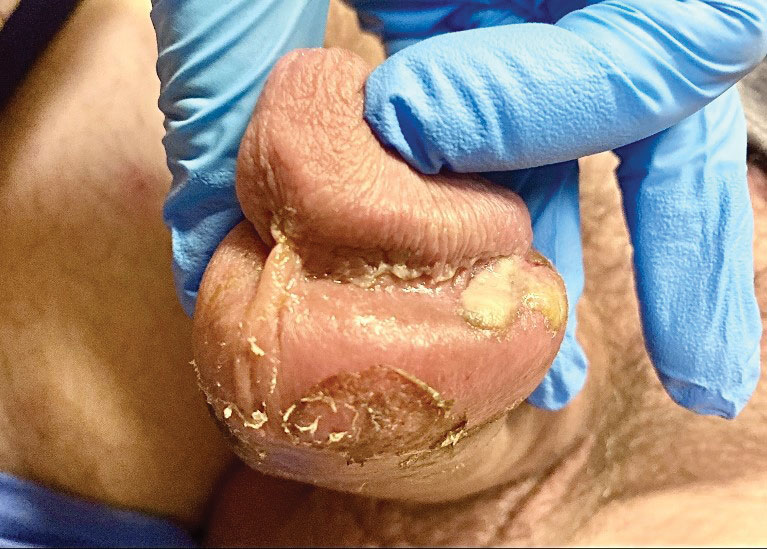
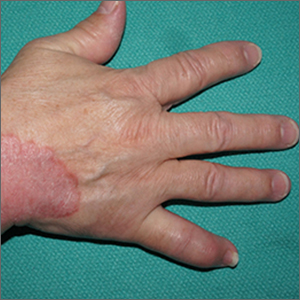
Focal plaques and finger swelling
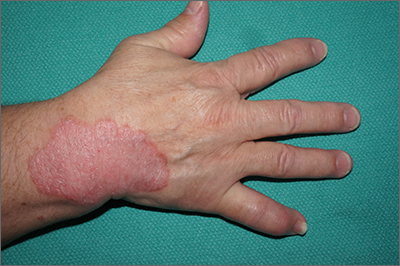
Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0

Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0
Surgeon in the C-suite

“If you don’t have a seat at the table, you are probably on the menu.” I first heard this quote in 2013, and it launched my interest in health care leadership and influenced me countless times over the last 10 years.
As Chief of Staff at Cleveland Clinic, I oversee nearly 5,000 physicians and scientists across the globe. I am involved in the physician life cycle: recruiting, hiring, privileging and credentialing, talent development, promotion, professionalism, and career transitions. I also sit at the intersection of medical care and the business of medicine. This means leading 18 clinical service lines responsible for 5.6 million visits, 161,000 surgeries, and billions of dollars in operating revenue per year. How I spend most of my time is a far cry from what I spent 11 years’ training to do—gynecologic surgery. This shift in my career was not because I changed my mind about caring for patients or that I tired of being a full-time surgeon. Nothing could be further from the truth. Women’s health remains my “why,” and my leadership journey has taught me that it is critical to have a seat at the table for the sake of ObGyns and women everywhere.
Women’s health on the menu
I will start with a concrete example of when we, as women and ObGyns, were on the menu. In late 2019, the Ohio state House of Representatives introduced a bill that subjected doctors to potential murder charges if they did not try everything to save the life of a mother and fetus, “including attempting to reimplant an ectopic pregnancy into the woman’s uterus.”1 This bill was based on 2 case reports—one from 1915 and one from 1980—which were both low quality, and the latter case was deemed to be fraudulent.2 How did this happen?
An Ohio state representative developed the bill with help from a lobbyist and without input from physicians or content experts. When asked, the representative shared that “he never researched whether re-implanting an ectopic pregnancy into a woman’s uterus was a viable medical procedure before including it in the bill.”3 He added, “I heard about it over the years. I never questioned it or gave it a lot of thought.”3
This example resonates deeply with many of us; it inspires us to speak up and act. As ObGyns, we clearly understand the consequences of legal and regulatory change in women’s health and how it directly impacts our patients and each of us as physicians. Let’s shift to something that you may feel less passion about, but I believe is equally important. This is where obstetrician-gynecologists sit in the intersection of medical care and business. This is the space where I spend most of my time, and from this vantage point, I worry about our field.
The business of medicine
Starting at the macroeconomic level, let’s think about how we as physicians are reimbursed and who makes these decisions. Looking at the national health care expenditure data, Medicare and Medicaid spending makes up nearly 40% of the total spend, and it is growing.4 Additionally, private health insurance tends to follow Centers for Medicare and Medicaid Services (CMS) decision making, further compounding its influence.4 In simple terms, CMS decides what is covered and how much we are paid. Whether you are in a solo private practice, an employer health care organization, or an academic medical center, physician reimbursement is declining.
In fact, Congress passed its year-end omnibus legislation in the final days of 2022, including a 2% Medicare physician payment cut for 2023,5 at a time when expenses to practice medicine, including nonphysician staff and supplies, are at an all-time high and we are living in a 6% inflationary state. This translates into being asked to serve more patients and cut costs. Our day-to-day feels much tighter, and this is why: Medicare physician pay increased just 11% over the past 20 years6 (2001–2021) in comparison to the cost of running a medical practice, which increased nearly 40% during that time. In other words, adjusting for inflation in practice costs, Medicare physician payment has fallen 22% over the last 20 years.7
Depending on your employment model, you may feel insulated from these changes as increases in reimbursement have occurred in other areas, such as hospitals and ambulatory surgery centers.8 In the short term, these increases help, as organizations will see additional funds. But there are 2 main issues: First, it is not nearly enough when you consider the soaring costs of running a hospital. And second, looking at our national population, we rely tremendously on self-employed doctors to serve our patients.
More than 80% of US counties lack adequate health care infrastructure.9 More than a third of the US population has less-than-adequate access to pharmacies, primary care physicians, hospitals, trauma centers, and low-cost health centers.9 To put things into perspective, more than 20% of counties in the United States are hospital deserts, where most people must drive more than 30 minutes to reach the closest hospital.9
There is good reason for this. Operating a hospital is a challenging endeavor. Even before the COVID-19 pandemic and the most recent health care financial challenges, most health care systems and large hospitals operated with very low operating margins (2%–3%). Businesses with similar margins include grocery stores and car dealerships. These low-margin businesses, including health care, rely on high volume for sustainability. High patient volumes distribute expensive hospital costs over many encounters. If physicians cannot sustain practices across the country, it is challenging to have sufficient admission and surgical volumes to justify the cost base of hospitals.
To tie this together, we have very little influence on what we are paid for our services. Reimbursement is declining, which makes it hard to have financially sustainable practices. As hospitals struggle, there is more pressure to prioritize highly profitable service lines, like orthopedics and urology, which are associated with favorable technical revenue. As hospitals are threatened, health care deserts widen, which leaves our entire health care system in jeopardy. Not surprisingly, this most likely affects those who face additional barriers to access, such as those with lower income, limited internet access, and lack of insurance. Together, these barriers further widen disparities in health care outcomes, including outcomes for women. Additionally, this death by a thousand cuts has eroded morale and increased physician burnout.
Transforming how we practice medicine is the only viable solution. I have good news: You are the leaders you have been waiting for.
Continue to: Physicians make good managers...
Physicians make good managers
To successfully transform how we practice medicine, it is critical that those leading the transformation deeply understand how medicine is practiced. The level of understanding required can be achieved only through years of medical practice, as a doctor. We understand how medical teams interact and that different sectors of our health care system are interdependent. Also, because physicians drive patient activity and ultimately reimbursement, having a seat at the table is crucial.
Some health care systems are run by businesspeople—people with finance backgrounds—and others are led by physicians. In 2017, Becker’s Hospital Review listed the chief executive officers (CEOs) of 183 nonprofit hospital and health systems.10 Of these, only 25% were led by individuals with an MD. Looking at the 115 largest hospitals in the United States, 30% are physician led.10 Considering the top 10 hospitals ranked by U.S. News & World Report for 2022, 8 of 10 have a physician at the helm.
Beyond raters and rankers, physician-led hospitals do better. Goodall compared CEOs in the top 100 best hospitals in U.S. News & World Report in 3 key medical specialties: cancer, digestive disorders, and cardiac care.11 The study explored the question: “Are hospitals’ quality ranked more highly when they are led by a medically trained doctor or non-MD professional managers?”11 Analysis revealed that hospital quality scores are about 25% higher in physician-run hospitals than in manager-run hospitals.11 Additional research shows that good management practices correlate with hospital performance, and that “the proportion of managers with a clinical degree has the largest positive effect.”12
Several theories exist as to why doctors make good managers in the health care setting.13,14 Doctors may create a more sympathetic and productive work environment for other clinicians because they are one of them. They have peer-to-peer credibility—because they have walked the walk, they have insight and perspective into how medicine is practiced.
Physicians serve as effective change agents for their organizations in several ways:
- First, physicians take a clinical approach in their leadership roles13 and focus on patient care at the center of their decisions. We see the people behind the numbers. Simply put, we humanize the operational side of health care.
- As physicians, we understand the interconnectivity in the practice of medicine. While closing certain service lines may be financially beneficial, these services are often closely linked to profitable service lines.
- Beyond physicians taking a clinical approach to leadership, we emphasize quality.13 Because we all have experienced complications and lived through bad outcomes alongside our patients, we understand deeply how important patient safety and quality is, and we are not willing to sacrifice that for financial gain. For us, this is personal. We don’t see our solution to health care challenges as an “or” situation, instead we view it as an “and” situation.
- Physician leaders often can improve medical staff engagement.13 A 2018 national survey of physicians found that those who are satisfied with their leadership are more engaged at work, have greater job satisfaction, and are less likely to experience signs of burnout.15 Physician administrators add value here.
Continue to: Surgeons as leaders...
Surgeons as leaders
What do we know about surgeons as physician leaders? Looking at the previously mentioned lists of physician leaders, surgeons are relatively absent. In the Becker’s Hospital Review study of nonprofit hospitals, only 9% of CEOs were surgeons.10 In addition, when reviewing data that associated physician leaders and hospital performance, only 3 of the CEOs were surgeons.11 Given that surgeons make up approximately 19% of US physicians, we are underrepresented.
The omission of surgeons as leaders seems inappropriate given that most hospitals are financially reliant on revenue related to surgical care and optimizing this space is an enormous opportunity. Berger and colleagues offered 3 theories as to why there are fewer surgeon leaders16:
- The relative pay of surgeons exceeds that of most other specialties, and there may be less incentive to accept the challenges presented by leadership roles. (I will add that surgeon leadership is more costly to a system.)
- The craftsmanship nature of surgery discourages the development of other career interests beginning at the trainee level.
- Surgeons have been perceived stereotypically to exhibit arrogance, a characteristic that others may not warm to.
This last observation stings. Successful leadership takes social skill and teamwork.14 Although medical care is one of the few disciplines in which lack of teamwork might cost lives, physicians are not trained to be team players. We recognize how our training has led us to be lone wolves or gunners, situations where we as individuals had to beat others to secure our spot. We have been trained in command-and-control environments, in stepping up as a leader in highly stressful situations. This part of surgical culture may handicap surgeons in their quest to be health care leaders.
Other traits, however, make us particularly great leaders in health care. Our desire to succeed, willingness to push ourselves to extremes, ability to laser focus on a task, acceptance of delayed gratification, and aptitude for making timely decisions on limited data help us succeed in leadership roles. Seven years of surgical training helped me develop the grit I use every day in the C-suite.
We need more physician and surgeon leadership to thrive in the challenging health care landscape. Berger and colleagues proposed 3 potential solutions to increase the number of surgeons in hospital leadership positions16:
Nurture future surgical leaders through exposure to management training. Given the contribution to both expense in support services and resources and revenue related to surgical care, each organization needs a content expert to guide these decisions.
Recognize the important contributions that surgeons already make regarding quality, safety, and operational efficiency. An excellent example of this is the American College of Surgeons National Surgical Quality Improvement Program. Because surgeons are content experts in this area, we are primed to lead.
Hospitals, medical schools, and academic departments of surgery should recognize administrative efforts as an important part of the overall academic mission. As the adage states, “No margin, no mission.” We need bright minds to preserve and grow our margins so that we can further invest in our missions.
This is not easy. Given the barriers, this will not happen organically. Charan and colleagues provided an outline for a leadership pathway adapted for physicians (FIGURE).17,18 It starts with the individual practitioner who is a practicing physician and spends most of their time focused on patient care. As a physician becomes more interested in leadership, they develop new skills and take on more and more responsibility. As they increase in leadership responsibility, they tend to reduce clinical time and increase time spent on strategic and business management. This framework creates a pipeline so that physicians and surgeons can be developed strategically and given increasing responsibility as they develop their capabilities and expand their skill sets.
The leadership challenge
To thrive, we must transform health care by changing how we practice medicine. As ObGyns, we are the leaders we have been waiting for. As you ponder your future, think of your current career and the opportunities you might have. Do you have a seat at the table? What table is that? How are you using your knowledge, expertise, and privilege to advance health care and medicine? I challenge you to critically evaluate this—and lead. ●
- Law T. Ohio bill suggests doctors who perform abortions could face jail, unless they perform a non-existent treatment. December 1, 2019. Time. Accessed June 12, 2023. https://time.com/5742053 /ectopic-pregnancy-ohio-abortion-bill/
- Grossman D. Ohio abortion, ectopic pregnancy bill: ‘it’s both bad medicine and bad law-making.’ May 21, 2019. Cincinnati.com–The Enquirer. Accessed June 12, 2023. https://www .cincinnati.com/story/opinion/2019/05/21/ohio-abortion-bill -john-becker-daniel-grossman-ectopic-pregnancy-false-medicine /3753610002/
- Lobbyist had hand in bill sparking ectopic pregnancy flap. December 11, 2019. Associated Press. Accessed June 12, 2023. https://apnews .com/article/03216e44405fa184ae0ab80fa85089f8
- NHE fact sheet. CMS.gov. Updated February 17, 2023. Accessed June 12, 2023. https://www.cms.gov/research-statistics-data-and -systems/statistics-trends-and-reports/nationalhealthexpenddata /nhe-fact-sheet
- Senate passes omnibus spending bill with health provisions. December 23, 2022. American Hospital Association. Accessed June 12, 2023. https://www.aha.org/special-bulletin/2022-12-20-appropriations -committees-release-omnibus-spending-bill-health-provisions
- Medicare updates compared to inflation (2001-2021). October 2021. American Medical Association. Accessed June 12, 2023. https://www .ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- Resneck Jr J. Medicare physician payment reform is long overdue. October 3, 2022. American Medical Association. Accessed June 7, 2023. https://www.ama-assn.org/about/leadership /medicare-physician-payment-reform-long-overdue
- Isenberg M. The stark reality of physician reimbursement. August 24, 2022. Zotec Partners. Accessed June 13, 2023. https://zotecpartners. com/advocacy-zpac/test-1/
- Nguyen A. Mapping healthcare deserts: 80% of the country lacks adequate access to healthcare. September 9, 2021. GoodRx Health. Accessed June 13, 2023. https://www.goodrx.com/healthcare -access/research/healthcare-deserts-80-percent-of-country-lacks -adequate-healthcare-access
- 183 nonprofit hospital and health system CEOs to know–2017. Updated June 20, 2018. Becker’s Hospital Review. Accessed June 7, 2023. https://www.beckershospitalreview.com/lists/188-nonprofit -hospital-and-health-system-ceos-to-know-2017.html
- Goodall AH. Physician-leaders and hospital performance: is there an association? Soc Sci Med. 2011;73:535-539. doi:10.1016 /j.socscimed.2011.06.025
- Bloom N, Sadun R, Van Reenen J. Does Management Matter in Healthcare? Center for Economic Performance and Harvard Business School; 2014.
- Turner J. Why healthcare C-suites should include physicians. September 3, 2019. Managed Healthcare Executive. Accessed June 13, 2023. https://www.managedhealthcareexecutive.com /view/why-healthcare-c-suites-should-include-physicians
- Stoller JK, Goodall A, Baker A. Why the best hospitals are managed by doctors. December 27, 2016. Harvard Business Review. Accessed June 13, 2023. https://hbr.org/2016/12/why-the-best-hospitals -are-managed-by-doctors
- Hayhurst C. Data confirms: leaders, physician burnout is on you. April 3, 2019. Aetnahealth. Accessed June 13, 2023. https://www .athenahealth.com/knowledge-hub/practice-management /research-confirms-leaders-burnout-you
- Berger DH, Goodall A, Tsai AY. The importance of increasing surgeon participation in hospital leadership. JAMA Surg. 2019;154:281-282. doi:10.1001/jamasurg.2018.5080
- Charan R, Drotter S, Noel J. The Leadership Pipeline: How to Build the Leadership-Powered Company. Jossey-Bass; 2001.
- Perry J, Mobley F, Brubaker M. Most doctors have little or no management training, and that’s a problem. December 15, 2017. Harvard Business Review. Accessed June 7, 2023. https://hbr.org/2017/12 /most-doctors-have-little-or-no-management-training-and-thats -a-problem

“If you don’t have a seat at the table, you are probably on the menu.” I first heard this quote in 2013, and it launched my interest in health care leadership and influenced me countless times over the last 10 years.
As Chief of Staff at Cleveland Clinic, I oversee nearly 5,000 physicians and scientists across the globe. I am involved in the physician life cycle: recruiting, hiring, privileging and credentialing, talent development, promotion, professionalism, and career transitions. I also sit at the intersection of medical care and the business of medicine. This means leading 18 clinical service lines responsible for 5.6 million visits, 161,000 surgeries, and billions of dollars in operating revenue per year. How I spend most of my time is a far cry from what I spent 11 years’ training to do—gynecologic surgery. This shift in my career was not because I changed my mind about caring for patients or that I tired of being a full-time surgeon. Nothing could be further from the truth. Women’s health remains my “why,” and my leadership journey has taught me that it is critical to have a seat at the table for the sake of ObGyns and women everywhere.
Women’s health on the menu
I will start with a concrete example of when we, as women and ObGyns, were on the menu. In late 2019, the Ohio state House of Representatives introduced a bill that subjected doctors to potential murder charges if they did not try everything to save the life of a mother and fetus, “including attempting to reimplant an ectopic pregnancy into the woman’s uterus.”1 This bill was based on 2 case reports—one from 1915 and one from 1980—which were both low quality, and the latter case was deemed to be fraudulent.2 How did this happen?
An Ohio state representative developed the bill with help from a lobbyist and without input from physicians or content experts. When asked, the representative shared that “he never researched whether re-implanting an ectopic pregnancy into a woman’s uterus was a viable medical procedure before including it in the bill.”3 He added, “I heard about it over the years. I never questioned it or gave it a lot of thought.”3
This example resonates deeply with many of us; it inspires us to speak up and act. As ObGyns, we clearly understand the consequences of legal and regulatory change in women’s health and how it directly impacts our patients and each of us as physicians. Let’s shift to something that you may feel less passion about, but I believe is equally important. This is where obstetrician-gynecologists sit in the intersection of medical care and business. This is the space where I spend most of my time, and from this vantage point, I worry about our field.
The business of medicine
Starting at the macroeconomic level, let’s think about how we as physicians are reimbursed and who makes these decisions. Looking at the national health care expenditure data, Medicare and Medicaid spending makes up nearly 40% of the total spend, and it is growing.4 Additionally, private health insurance tends to follow Centers for Medicare and Medicaid Services (CMS) decision making, further compounding its influence.4 In simple terms, CMS decides what is covered and how much we are paid. Whether you are in a solo private practice, an employer health care organization, or an academic medical center, physician reimbursement is declining.
In fact, Congress passed its year-end omnibus legislation in the final days of 2022, including a 2% Medicare physician payment cut for 2023,5 at a time when expenses to practice medicine, including nonphysician staff and supplies, are at an all-time high and we are living in a 6% inflationary state. This translates into being asked to serve more patients and cut costs. Our day-to-day feels much tighter, and this is why: Medicare physician pay increased just 11% over the past 20 years6 (2001–2021) in comparison to the cost of running a medical practice, which increased nearly 40% during that time. In other words, adjusting for inflation in practice costs, Medicare physician payment has fallen 22% over the last 20 years.7
Depending on your employment model, you may feel insulated from these changes as increases in reimbursement have occurred in other areas, such as hospitals and ambulatory surgery centers.8 In the short term, these increases help, as organizations will see additional funds. But there are 2 main issues: First, it is not nearly enough when you consider the soaring costs of running a hospital. And second, looking at our national population, we rely tremendously on self-employed doctors to serve our patients.
More than 80% of US counties lack adequate health care infrastructure.9 More than a third of the US population has less-than-adequate access to pharmacies, primary care physicians, hospitals, trauma centers, and low-cost health centers.9 To put things into perspective, more than 20% of counties in the United States are hospital deserts, where most people must drive more than 30 minutes to reach the closest hospital.9
There is good reason for this. Operating a hospital is a challenging endeavor. Even before the COVID-19 pandemic and the most recent health care financial challenges, most health care systems and large hospitals operated with very low operating margins (2%–3%). Businesses with similar margins include grocery stores and car dealerships. These low-margin businesses, including health care, rely on high volume for sustainability. High patient volumes distribute expensive hospital costs over many encounters. If physicians cannot sustain practices across the country, it is challenging to have sufficient admission and surgical volumes to justify the cost base of hospitals.
To tie this together, we have very little influence on what we are paid for our services. Reimbursement is declining, which makes it hard to have financially sustainable practices. As hospitals struggle, there is more pressure to prioritize highly profitable service lines, like orthopedics and urology, which are associated with favorable technical revenue. As hospitals are threatened, health care deserts widen, which leaves our entire health care system in jeopardy. Not surprisingly, this most likely affects those who face additional barriers to access, such as those with lower income, limited internet access, and lack of insurance. Together, these barriers further widen disparities in health care outcomes, including outcomes for women. Additionally, this death by a thousand cuts has eroded morale and increased physician burnout.
Transforming how we practice medicine is the only viable solution. I have good news: You are the leaders you have been waiting for.
Continue to: Physicians make good managers...
Physicians make good managers
To successfully transform how we practice medicine, it is critical that those leading the transformation deeply understand how medicine is practiced. The level of understanding required can be achieved only through years of medical practice, as a doctor. We understand how medical teams interact and that different sectors of our health care system are interdependent. Also, because physicians drive patient activity and ultimately reimbursement, having a seat at the table is crucial.
Some health care systems are run by businesspeople—people with finance backgrounds—and others are led by physicians. In 2017, Becker’s Hospital Review listed the chief executive officers (CEOs) of 183 nonprofit hospital and health systems.10 Of these, only 25% were led by individuals with an MD. Looking at the 115 largest hospitals in the United States, 30% are physician led.10 Considering the top 10 hospitals ranked by U.S. News & World Report for 2022, 8 of 10 have a physician at the helm.
Beyond raters and rankers, physician-led hospitals do better. Goodall compared CEOs in the top 100 best hospitals in U.S. News & World Report in 3 key medical specialties: cancer, digestive disorders, and cardiac care.11 The study explored the question: “Are hospitals’ quality ranked more highly when they are led by a medically trained doctor or non-MD professional managers?”11 Analysis revealed that hospital quality scores are about 25% higher in physician-run hospitals than in manager-run hospitals.11 Additional research shows that good management practices correlate with hospital performance, and that “the proportion of managers with a clinical degree has the largest positive effect.”12
Several theories exist as to why doctors make good managers in the health care setting.13,14 Doctors may create a more sympathetic and productive work environment for other clinicians because they are one of them. They have peer-to-peer credibility—because they have walked the walk, they have insight and perspective into how medicine is practiced.
Physicians serve as effective change agents for their organizations in several ways:
- First, physicians take a clinical approach in their leadership roles13 and focus on patient care at the center of their decisions. We see the people behind the numbers. Simply put, we humanize the operational side of health care.
- As physicians, we understand the interconnectivity in the practice of medicine. While closing certain service lines may be financially beneficial, these services are often closely linked to profitable service lines.
- Beyond physicians taking a clinical approach to leadership, we emphasize quality.13 Because we all have experienced complications and lived through bad outcomes alongside our patients, we understand deeply how important patient safety and quality is, and we are not willing to sacrifice that for financial gain. For us, this is personal. We don’t see our solution to health care challenges as an “or” situation, instead we view it as an “and” situation.
- Physician leaders often can improve medical staff engagement.13 A 2018 national survey of physicians found that those who are satisfied with their leadership are more engaged at work, have greater job satisfaction, and are less likely to experience signs of burnout.15 Physician administrators add value here.
Continue to: Surgeons as leaders...
Surgeons as leaders
What do we know about surgeons as physician leaders? Looking at the previously mentioned lists of physician leaders, surgeons are relatively absent. In the Becker’s Hospital Review study of nonprofit hospitals, only 9% of CEOs were surgeons.10 In addition, when reviewing data that associated physician leaders and hospital performance, only 3 of the CEOs were surgeons.11 Given that surgeons make up approximately 19% of US physicians, we are underrepresented.
The omission of surgeons as leaders seems inappropriate given that most hospitals are financially reliant on revenue related to surgical care and optimizing this space is an enormous opportunity. Berger and colleagues offered 3 theories as to why there are fewer surgeon leaders16:
- The relative pay of surgeons exceeds that of most other specialties, and there may be less incentive to accept the challenges presented by leadership roles. (I will add that surgeon leadership is more costly to a system.)
- The craftsmanship nature of surgery discourages the development of other career interests beginning at the trainee level.
- Surgeons have been perceived stereotypically to exhibit arrogance, a characteristic that others may not warm to.
This last observation stings. Successful leadership takes social skill and teamwork.14 Although medical care is one of the few disciplines in which lack of teamwork might cost lives, physicians are not trained to be team players. We recognize how our training has led us to be lone wolves or gunners, situations where we as individuals had to beat others to secure our spot. We have been trained in command-and-control environments, in stepping up as a leader in highly stressful situations. This part of surgical culture may handicap surgeons in their quest to be health care leaders.
Other traits, however, make us particularly great leaders in health care. Our desire to succeed, willingness to push ourselves to extremes, ability to laser focus on a task, acceptance of delayed gratification, and aptitude for making timely decisions on limited data help us succeed in leadership roles. Seven years of surgical training helped me develop the grit I use every day in the C-suite.
We need more physician and surgeon leadership to thrive in the challenging health care landscape. Berger and colleagues proposed 3 potential solutions to increase the number of surgeons in hospital leadership positions16:
Nurture future surgical leaders through exposure to management training. Given the contribution to both expense in support services and resources and revenue related to surgical care, each organization needs a content expert to guide these decisions.
Recognize the important contributions that surgeons already make regarding quality, safety, and operational efficiency. An excellent example of this is the American College of Surgeons National Surgical Quality Improvement Program. Because surgeons are content experts in this area, we are primed to lead.
Hospitals, medical schools, and academic departments of surgery should recognize administrative efforts as an important part of the overall academic mission. As the adage states, “No margin, no mission.” We need bright minds to preserve and grow our margins so that we can further invest in our missions.
This is not easy. Given the barriers, this will not happen organically. Charan and colleagues provided an outline for a leadership pathway adapted for physicians (FIGURE).17,18 It starts with the individual practitioner who is a practicing physician and spends most of their time focused on patient care. As a physician becomes more interested in leadership, they develop new skills and take on more and more responsibility. As they increase in leadership responsibility, they tend to reduce clinical time and increase time spent on strategic and business management. This framework creates a pipeline so that physicians and surgeons can be developed strategically and given increasing responsibility as they develop their capabilities and expand their skill sets.

The leadership challenge
To thrive, we must transform health care by changing how we practice medicine. As ObGyns, we are the leaders we have been waiting for. As you ponder your future, think of your current career and the opportunities you might have. Do you have a seat at the table? What table is that? How are you using your knowledge, expertise, and privilege to advance health care and medicine? I challenge you to critically evaluate this—and lead. ●

“If you don’t have a seat at the table, you are probably on the menu.” I first heard this quote in 2013, and it launched my interest in health care leadership and influenced me countless times over the last 10 years.
As Chief of Staff at Cleveland Clinic, I oversee nearly 5,000 physicians and scientists across the globe. I am involved in the physician life cycle: recruiting, hiring, privileging and credentialing, talent development, promotion, professionalism, and career transitions. I also sit at the intersection of medical care and the business of medicine. This means leading 18 clinical service lines responsible for 5.6 million visits, 161,000 surgeries, and billions of dollars in operating revenue per year. How I spend most of my time is a far cry from what I spent 11 years’ training to do—gynecologic surgery. This shift in my career was not because I changed my mind about caring for patients or that I tired of being a full-time surgeon. Nothing could be further from the truth. Women’s health remains my “why,” and my leadership journey has taught me that it is critical to have a seat at the table for the sake of ObGyns and women everywhere.
Women’s health on the menu
I will start with a concrete example of when we, as women and ObGyns, were on the menu. In late 2019, the Ohio state House of Representatives introduced a bill that subjected doctors to potential murder charges if they did not try everything to save the life of a mother and fetus, “including attempting to reimplant an ectopic pregnancy into the woman’s uterus.”1 This bill was based on 2 case reports—one from 1915 and one from 1980—which were both low quality, and the latter case was deemed to be fraudulent.2 How did this happen?
An Ohio state representative developed the bill with help from a lobbyist and without input from physicians or content experts. When asked, the representative shared that “he never researched whether re-implanting an ectopic pregnancy into a woman’s uterus was a viable medical procedure before including it in the bill.”3 He added, “I heard about it over the years. I never questioned it or gave it a lot of thought.”3
This example resonates deeply with many of us; it inspires us to speak up and act. As ObGyns, we clearly understand the consequences of legal and regulatory change in women’s health and how it directly impacts our patients and each of us as physicians. Let’s shift to something that you may feel less passion about, but I believe is equally important. This is where obstetrician-gynecologists sit in the intersection of medical care and business. This is the space where I spend most of my time, and from this vantage point, I worry about our field.
The business of medicine
Starting at the macroeconomic level, let’s think about how we as physicians are reimbursed and who makes these decisions. Looking at the national health care expenditure data, Medicare and Medicaid spending makes up nearly 40% of the total spend, and it is growing.4 Additionally, private health insurance tends to follow Centers for Medicare and Medicaid Services (CMS) decision making, further compounding its influence.4 In simple terms, CMS decides what is covered and how much we are paid. Whether you are in a solo private practice, an employer health care organization, or an academic medical center, physician reimbursement is declining.
In fact, Congress passed its year-end omnibus legislation in the final days of 2022, including a 2% Medicare physician payment cut for 2023,5 at a time when expenses to practice medicine, including nonphysician staff and supplies, are at an all-time high and we are living in a 6% inflationary state. This translates into being asked to serve more patients and cut costs. Our day-to-day feels much tighter, and this is why: Medicare physician pay increased just 11% over the past 20 years6 (2001–2021) in comparison to the cost of running a medical practice, which increased nearly 40% during that time. In other words, adjusting for inflation in practice costs, Medicare physician payment has fallen 22% over the last 20 years.7
Depending on your employment model, you may feel insulated from these changes as increases in reimbursement have occurred in other areas, such as hospitals and ambulatory surgery centers.8 In the short term, these increases help, as organizations will see additional funds. But there are 2 main issues: First, it is not nearly enough when you consider the soaring costs of running a hospital. And second, looking at our national population, we rely tremendously on self-employed doctors to serve our patients.
More than 80% of US counties lack adequate health care infrastructure.9 More than a third of the US population has less-than-adequate access to pharmacies, primary care physicians, hospitals, trauma centers, and low-cost health centers.9 To put things into perspective, more than 20% of counties in the United States are hospital deserts, where most people must drive more than 30 minutes to reach the closest hospital.9
There is good reason for this. Operating a hospital is a challenging endeavor. Even before the COVID-19 pandemic and the most recent health care financial challenges, most health care systems and large hospitals operated with very low operating margins (2%–3%). Businesses with similar margins include grocery stores and car dealerships. These low-margin businesses, including health care, rely on high volume for sustainability. High patient volumes distribute expensive hospital costs over many encounters. If physicians cannot sustain practices across the country, it is challenging to have sufficient admission and surgical volumes to justify the cost base of hospitals.
To tie this together, we have very little influence on what we are paid for our services. Reimbursement is declining, which makes it hard to have financially sustainable practices. As hospitals struggle, there is more pressure to prioritize highly profitable service lines, like orthopedics and urology, which are associated with favorable technical revenue. As hospitals are threatened, health care deserts widen, which leaves our entire health care system in jeopardy. Not surprisingly, this most likely affects those who face additional barriers to access, such as those with lower income, limited internet access, and lack of insurance. Together, these barriers further widen disparities in health care outcomes, including outcomes for women. Additionally, this death by a thousand cuts has eroded morale and increased physician burnout.
Transforming how we practice medicine is the only viable solution. I have good news: You are the leaders you have been waiting for.
Continue to: Physicians make good managers...
Physicians make good managers
To successfully transform how we practice medicine, it is critical that those leading the transformation deeply understand how medicine is practiced. The level of understanding required can be achieved only through years of medical practice, as a doctor. We understand how medical teams interact and that different sectors of our health care system are interdependent. Also, because physicians drive patient activity and ultimately reimbursement, having a seat at the table is crucial.
Some health care systems are run by businesspeople—people with finance backgrounds—and others are led by physicians. In 2017, Becker’s Hospital Review listed the chief executive officers (CEOs) of 183 nonprofit hospital and health systems.10 Of these, only 25% were led by individuals with an MD. Looking at the 115 largest hospitals in the United States, 30% are physician led.10 Considering the top 10 hospitals ranked by U.S. News & World Report for 2022, 8 of 10 have a physician at the helm.
Beyond raters and rankers, physician-led hospitals do better. Goodall compared CEOs in the top 100 best hospitals in U.S. News & World Report in 3 key medical specialties: cancer, digestive disorders, and cardiac care.11 The study explored the question: “Are hospitals’ quality ranked more highly when they are led by a medically trained doctor or non-MD professional managers?”11 Analysis revealed that hospital quality scores are about 25% higher in physician-run hospitals than in manager-run hospitals.11 Additional research shows that good management practices correlate with hospital performance, and that “the proportion of managers with a clinical degree has the largest positive effect.”12
Several theories exist as to why doctors make good managers in the health care setting.13,14 Doctors may create a more sympathetic and productive work environment for other clinicians because they are one of them. They have peer-to-peer credibility—because they have walked the walk, they have insight and perspective into how medicine is practiced.
Physicians serve as effective change agents for their organizations in several ways:
- First, physicians take a clinical approach in their leadership roles13 and focus on patient care at the center of their decisions. We see the people behind the numbers. Simply put, we humanize the operational side of health care.
- As physicians, we understand the interconnectivity in the practice of medicine. While closing certain service lines may be financially beneficial, these services are often closely linked to profitable service lines.
- Beyond physicians taking a clinical approach to leadership, we emphasize quality.13 Because we all have experienced complications and lived through bad outcomes alongside our patients, we understand deeply how important patient safety and quality is, and we are not willing to sacrifice that for financial gain. For us, this is personal. We don’t see our solution to health care challenges as an “or” situation, instead we view it as an “and” situation.
- Physician leaders often can improve medical staff engagement.13 A 2018 national survey of physicians found that those who are satisfied with their leadership are more engaged at work, have greater job satisfaction, and are less likely to experience signs of burnout.15 Physician administrators add value here.
Continue to: Surgeons as leaders...
Surgeons as leaders
What do we know about surgeons as physician leaders? Looking at the previously mentioned lists of physician leaders, surgeons are relatively absent. In the Becker’s Hospital Review study of nonprofit hospitals, only 9% of CEOs were surgeons.10 In addition, when reviewing data that associated physician leaders and hospital performance, only 3 of the CEOs were surgeons.11 Given that surgeons make up approximately 19% of US physicians, we are underrepresented.
The omission of surgeons as leaders seems inappropriate given that most hospitals are financially reliant on revenue related to surgical care and optimizing this space is an enormous opportunity. Berger and colleagues offered 3 theories as to why there are fewer surgeon leaders16:
- The relative pay of surgeons exceeds that of most other specialties, and there may be less incentive to accept the challenges presented by leadership roles. (I will add that surgeon leadership is more costly to a system.)
- The craftsmanship nature of surgery discourages the development of other career interests beginning at the trainee level.
- Surgeons have been perceived stereotypically to exhibit arrogance, a characteristic that others may not warm to.
This last observation stings. Successful leadership takes social skill and teamwork.14 Although medical care is one of the few disciplines in which lack of teamwork might cost lives, physicians are not trained to be team players. We recognize how our training has led us to be lone wolves or gunners, situations where we as individuals had to beat others to secure our spot. We have been trained in command-and-control environments, in stepping up as a leader in highly stressful situations. This part of surgical culture may handicap surgeons in their quest to be health care leaders.
Other traits, however, make us particularly great leaders in health care. Our desire to succeed, willingness to push ourselves to extremes, ability to laser focus on a task, acceptance of delayed gratification, and aptitude for making timely decisions on limited data help us succeed in leadership roles. Seven years of surgical training helped me develop the grit I use every day in the C-suite.
We need more physician and surgeon leadership to thrive in the challenging health care landscape. Berger and colleagues proposed 3 potential solutions to increase the number of surgeons in hospital leadership positions16:
Nurture future surgical leaders through exposure to management training. Given the contribution to both expense in support services and resources and revenue related to surgical care, each organization needs a content expert to guide these decisions.
Recognize the important contributions that surgeons already make regarding quality, safety, and operational efficiency. An excellent example of this is the American College of Surgeons National Surgical Quality Improvement Program. Because surgeons are content experts in this area, we are primed to lead.
Hospitals, medical schools, and academic departments of surgery should recognize administrative efforts as an important part of the overall academic mission. As the adage states, “No margin, no mission.” We need bright minds to preserve and grow our margins so that we can further invest in our missions.
This is not easy. Given the barriers, this will not happen organically. Charan and colleagues provided an outline for a leadership pathway adapted for physicians (FIGURE).17,18 It starts with the individual practitioner who is a practicing physician and spends most of their time focused on patient care. As a physician becomes more interested in leadership, they develop new skills and take on more and more responsibility. As they increase in leadership responsibility, they tend to reduce clinical time and increase time spent on strategic and business management. This framework creates a pipeline so that physicians and surgeons can be developed strategically and given increasing responsibility as they develop their capabilities and expand their skill sets.

The leadership challenge
To thrive, we must transform health care by changing how we practice medicine. As ObGyns, we are the leaders we have been waiting for. As you ponder your future, think of your current career and the opportunities you might have. Do you have a seat at the table? What table is that? How are you using your knowledge, expertise, and privilege to advance health care and medicine? I challenge you to critically evaluate this—and lead. ●
- Law T. Ohio bill suggests doctors who perform abortions could face jail, unless they perform a non-existent treatment. December 1, 2019. Time. Accessed June 12, 2023. https://time.com/5742053 /ectopic-pregnancy-ohio-abortion-bill/
- Grossman D. Ohio abortion, ectopic pregnancy bill: ‘it’s both bad medicine and bad law-making.’ May 21, 2019. Cincinnati.com–The Enquirer. Accessed June 12, 2023. https://www .cincinnati.com/story/opinion/2019/05/21/ohio-abortion-bill -john-becker-daniel-grossman-ectopic-pregnancy-false-medicine /3753610002/
- Lobbyist had hand in bill sparking ectopic pregnancy flap. December 11, 2019. Associated Press. Accessed June 12, 2023. https://apnews .com/article/03216e44405fa184ae0ab80fa85089f8
- NHE fact sheet. CMS.gov. Updated February 17, 2023. Accessed June 12, 2023. https://www.cms.gov/research-statistics-data-and -systems/statistics-trends-and-reports/nationalhealthexpenddata /nhe-fact-sheet
- Senate passes omnibus spending bill with health provisions. December 23, 2022. American Hospital Association. Accessed June 12, 2023. https://www.aha.org/special-bulletin/2022-12-20-appropriations -committees-release-omnibus-spending-bill-health-provisions
- Medicare updates compared to inflation (2001-2021). October 2021. American Medical Association. Accessed June 12, 2023. https://www .ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- Resneck Jr J. Medicare physician payment reform is long overdue. October 3, 2022. American Medical Association. Accessed June 7, 2023. https://www.ama-assn.org/about/leadership /medicare-physician-payment-reform-long-overdue
- Isenberg M. The stark reality of physician reimbursement. August 24, 2022. Zotec Partners. Accessed June 13, 2023. https://zotecpartners. com/advocacy-zpac/test-1/
- Nguyen A. Mapping healthcare deserts: 80% of the country lacks adequate access to healthcare. September 9, 2021. GoodRx Health. Accessed June 13, 2023. https://www.goodrx.com/healthcare -access/research/healthcare-deserts-80-percent-of-country-lacks -adequate-healthcare-access
- 183 nonprofit hospital and health system CEOs to know–2017. Updated June 20, 2018. Becker’s Hospital Review. Accessed June 7, 2023. https://www.beckershospitalreview.com/lists/188-nonprofit -hospital-and-health-system-ceos-to-know-2017.html
- Goodall AH. Physician-leaders and hospital performance: is there an association? Soc Sci Med. 2011;73:535-539. doi:10.1016 /j.socscimed.2011.06.025
- Bloom N, Sadun R, Van Reenen J. Does Management Matter in Healthcare? Center for Economic Performance and Harvard Business School; 2014.
- Turner J. Why healthcare C-suites should include physicians. September 3, 2019. Managed Healthcare Executive. Accessed June 13, 2023. https://www.managedhealthcareexecutive.com /view/why-healthcare-c-suites-should-include-physicians
- Stoller JK, Goodall A, Baker A. Why the best hospitals are managed by doctors. December 27, 2016. Harvard Business Review. Accessed June 13, 2023. https://hbr.org/2016/12/why-the-best-hospitals -are-managed-by-doctors
- Hayhurst C. Data confirms: leaders, physician burnout is on you. April 3, 2019. Aetnahealth. Accessed June 13, 2023. https://www .athenahealth.com/knowledge-hub/practice-management /research-confirms-leaders-burnout-you
- Berger DH, Goodall A, Tsai AY. The importance of increasing surgeon participation in hospital leadership. JAMA Surg. 2019;154:281-282. doi:10.1001/jamasurg.2018.5080
- Charan R, Drotter S, Noel J. The Leadership Pipeline: How to Build the Leadership-Powered Company. Jossey-Bass; 2001.
- Perry J, Mobley F, Brubaker M. Most doctors have little or no management training, and that’s a problem. December 15, 2017. Harvard Business Review. Accessed June 7, 2023. https://hbr.org/2017/12 /most-doctors-have-little-or-no-management-training-and-thats -a-problem
- Law T. Ohio bill suggests doctors who perform abortions could face jail, unless they perform a non-existent treatment. December 1, 2019. Time. Accessed June 12, 2023. https://time.com/5742053 /ectopic-pregnancy-ohio-abortion-bill/
- Grossman D. Ohio abortion, ectopic pregnancy bill: ‘it’s both bad medicine and bad law-making.’ May 21, 2019. Cincinnati.com–The Enquirer. Accessed June 12, 2023. https://www .cincinnati.com/story/opinion/2019/05/21/ohio-abortion-bill -john-becker-daniel-grossman-ectopic-pregnancy-false-medicine /3753610002/
- Lobbyist had hand in bill sparking ectopic pregnancy flap. December 11, 2019. Associated Press. Accessed June 12, 2023. https://apnews .com/article/03216e44405fa184ae0ab80fa85089f8
- NHE fact sheet. CMS.gov. Updated February 17, 2023. Accessed June 12, 2023. https://www.cms.gov/research-statistics-data-and -systems/statistics-trends-and-reports/nationalhealthexpenddata /nhe-fact-sheet
- Senate passes omnibus spending bill with health provisions. December 23, 2022. American Hospital Association. Accessed June 12, 2023. https://www.aha.org/special-bulletin/2022-12-20-appropriations -committees-release-omnibus-spending-bill-health-provisions
- Medicare updates compared to inflation (2001-2021). October 2021. American Medical Association. Accessed June 12, 2023. https://www .ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- Resneck Jr J. Medicare physician payment reform is long overdue. October 3, 2022. American Medical Association. Accessed June 7, 2023. https://www.ama-assn.org/about/leadership /medicare-physician-payment-reform-long-overdue
- Isenberg M. The stark reality of physician reimbursement. August 24, 2022. Zotec Partners. Accessed June 13, 2023. https://zotecpartners. com/advocacy-zpac/test-1/
- Nguyen A. Mapping healthcare deserts: 80% of the country lacks adequate access to healthcare. September 9, 2021. GoodRx Health. Accessed June 13, 2023. https://www.goodrx.com/healthcare -access/research/healthcare-deserts-80-percent-of-country-lacks -adequate-healthcare-access
- 183 nonprofit hospital and health system CEOs to know–2017. Updated June 20, 2018. Becker’s Hospital Review. Accessed June 7, 2023. https://www.beckershospitalreview.com/lists/188-nonprofit -hospital-and-health-system-ceos-to-know-2017.html
- Goodall AH. Physician-leaders and hospital performance: is there an association? Soc Sci Med. 2011;73:535-539. doi:10.1016 /j.socscimed.2011.06.025
- Bloom N, Sadun R, Van Reenen J. Does Management Matter in Healthcare? Center for Economic Performance and Harvard Business School; 2014.
- Turner J. Why healthcare C-suites should include physicians. September 3, 2019. Managed Healthcare Executive. Accessed June 13, 2023. https://www.managedhealthcareexecutive.com /view/why-healthcare-c-suites-should-include-physicians
- Stoller JK, Goodall A, Baker A. Why the best hospitals are managed by doctors. December 27, 2016. Harvard Business Review. Accessed June 13, 2023. https://hbr.org/2016/12/why-the-best-hospitals -are-managed-by-doctors
- Hayhurst C. Data confirms: leaders, physician burnout is on you. April 3, 2019. Aetnahealth. Accessed June 13, 2023. https://www .athenahealth.com/knowledge-hub/practice-management /research-confirms-leaders-burnout-you
- Berger DH, Goodall A, Tsai AY. The importance of increasing surgeon participation in hospital leadership. JAMA Surg. 2019;154:281-282. doi:10.1001/jamasurg.2018.5080
- Charan R, Drotter S, Noel J. The Leadership Pipeline: How to Build the Leadership-Powered Company. Jossey-Bass; 2001.
- Perry J, Mobley F, Brubaker M. Most doctors have little or no management training, and that’s a problem. December 15, 2017. Harvard Business Review. Accessed June 7, 2023. https://hbr.org/2017/12 /most-doctors-have-little-or-no-management-training-and-thats -a-problem
Surgical volume and outcomes for gynecologic surgery: Is more always better?
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3
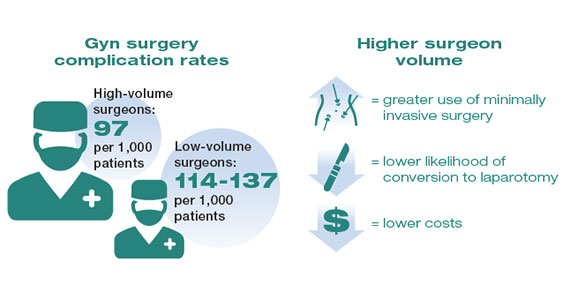
Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15
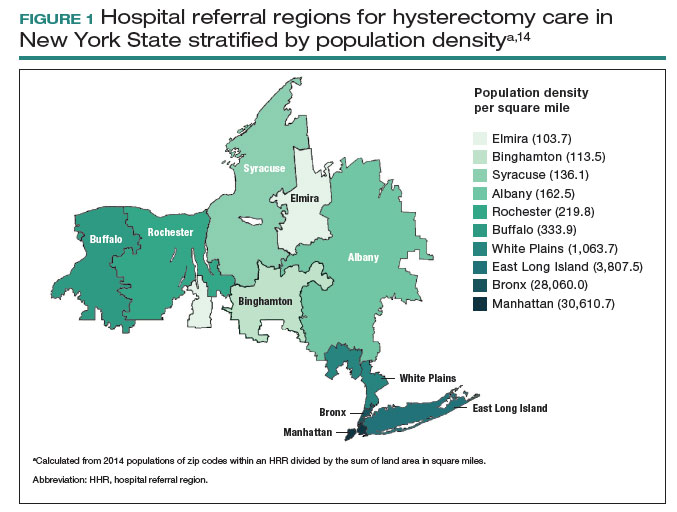
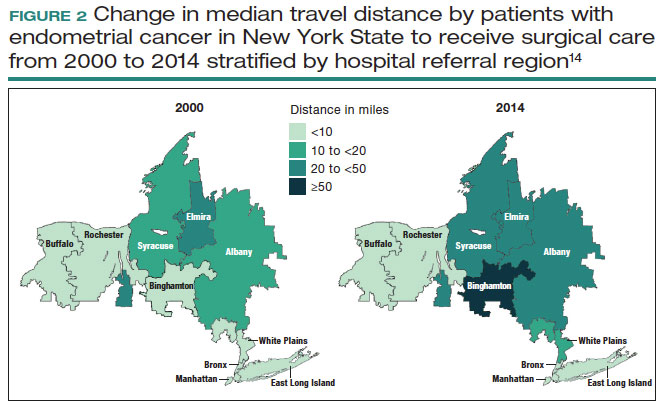
A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5
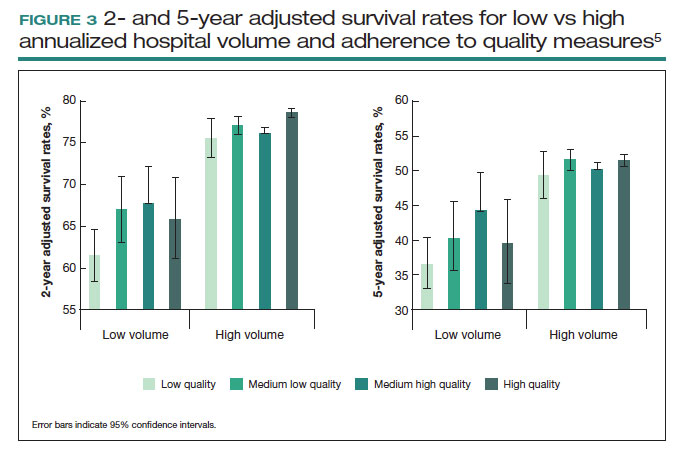
These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3

Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15


A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5

These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3

Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15


A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5

These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
Circulating Tumor DNA Testing and Liquid Biopsy: The Future for Precision Medicine and Guided Targeted Therapy for Breast Cancer?
The current standard for breast cancer screening (for non–high-risk patients) is an annual or semiannual mammogram for women aged 40 and older.1 However, mammography-based screening can give false-positive or false-negative results. This can lead to excessive use of invasive tissue biopsies and unnecessary exposure to ionizing radiation—which can also become expensive and time-consuming for patients.2
Both normal and cancerous cells shed cell-free DNA (cfDNA) into the blood circulation.3 Circulating tumor DNA (ctDNA) are fragments of DNA derived from tumor cells that circulate in the blood together with cfDNA. The ctDNA originates directly from a tumor or from circulating tumor cells (and carries information from the tumor cell genome), whereas cfDNA enters the bloodstream after apoptosis or necrosis and carries genome-wide DNA information. The amount of ctDNA in the blood has been shown to be elevated in patients with cancer.3 Different cancers release varying levels of ctDNA; the amount of ctDNA released depends on the number of tumor cells that are in senescence vs undergoing apoptosis.4
The possibility of incorporating this biomarker obtained from a “liquid biopsy” is currently being studied and will hopefully become a standard of care for breast cancer screening and monitoring. The liquid biopsy detects ctDNA that has been released into the bloodstream from tumor regions and helps identify intratumoral heterogeneity and clonal evolution.5 Additionally, sequencing tumor DNA has opened new possibilities for precision oncology.6 Detection of somatic gene mutations, amplifications, and gene fusions helps to deliver targeted therapies.6 Analysis of potential somatic mutations in ctDNA, in combination with cfDNA levels, can help capture clinically relevant information beyond single genetic alterations and tumor fraction, potentially improving the accuracy of early detection and screening for breast cancer.
Recent advances in ctDNA testing technology have made it more accurate and reliable. ctDNA testing has several benefits, including early detection of cancer (detecting ctDNA at low levels)7; monitoring of tumor dynamics, therapeutic response, and residual disease8; as well as analysis of the evolution of genetic or epigenetic alterations characterizing the tumor.9 Its noninvasiveness, rapidity, and low cost allow for longitudinal monitoring of cancer in real time, potentially capturing tumor heterogeneity.10,11
The liquid biopsy potentially can give more options for therapeutic monitoring for breast cancer and may mirror clinically relevant genetic alterations that occur in all tumor tissues. Liquid biopsy offers many advantages. It allows for the detection of minimal residual disease and micrometastatic disease that may be difficult to detect with a traditional tissue biopsy.12 Liquid biopsy detects ctDNA that has been released into the bloodstream from multiple tumor regions and allows the possibility of identifying intratumoral heterogeneity and clonal evolution.5 It can also detect small quantitative variations within the blood, enabling real-time surveillance.
The liquid biopsy can offer earlier and easier access to some tumor-based genetic information at any given timepoint and can replace a tumor tissue biopsy in some cases, helping to avoid delays and complications of a solid tumor invasive biopsy procedure. This is especially relevant in the metastatic setting, in which ctDNA might be the only available genetic material from tumors.13 Tissue biopsy can only provide a static and spatially limited view of the disease at the time of sampling; ctDNA analysis could potentially reflect the genetic alterations that occur in all metastatic breast cancer sites over time.14,15 Furthermore, machine learning of multi-gene signatures, obtained from ctDNA, can possibly identify complex biological features, including measures of tumor proliferation and estrogen receptor signaling, similar to direct tumor tissue DNA or RNA profiling.16
ctDNA testing is currently being studied to monitor patients who have been diagnosed with breast cancer. Small retrospective studies have shown that detection of ctDNA in plasma, after patients have completed therapy for early-stage breast cancer, is associated with a very high risk of relapse.17
Ongoing studies are examining the tailoring of adjuvant treatment based on ctDNA. If these trials are successful, certain aspects of adjuvant treatment could be lessened, or omitted, for patients who have undetectable ctDNA or intensified for patients who have detectable ctDNA after definitive treatments. This could personalize treatment specifically to the patient.
The detection and persistence of ctDNA in the middle of neoadjuvant systemic therapy may have the potential to negatively predict response to treatment and identify patients who will not achieve pathologic complete response. This may have the potential to aid in clinical decision-making for treatment escalation in these nonresponders.18
Despite these distinct characteristics, the low levels of ctDNA found in early-stage disease, along with the lack of ctDNA shedding from some tumors, can further complicate or impede detection of recurrence in early-stage breast cancer. Testing is further complicated by hematologic genetic alterations.5 The limitation of ctDNA approaches is that these techniques only detect known mutations in certain genes, so patients without these mutations could be overlooked, limiting the application of this technology.19
Overall, ctDNA testing represents a promising area of research for the diagnosis, treatment, and monitoring of breast cancer. While more research is needed to fully understand its potential, the advances in this technology are certainly exciting and could lead to significant improvements in patient outcomes. It is hopeful that in the near future, ctDNA testing from liquid biopsy could become a standard of care in breast cancer screening, ultimately helping clinicians to personalize treatment therapies and improve patient outcomes when treating patients with breast cancer.
1. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
2. Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20(12):E2878.
3. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-376.
4. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020;31(13):107830.
5. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172-186.
6. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895-1902.
7. Wang J, Han X, Sun Y. DNA methylation signatures in circulating cell-free DNA as biomarkers for the early detection of cancer. Sci China Life Sci. 2017;60(4):356-362.
8. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209.
9. Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586.
10. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705.
11. Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol. 2016;27(5):862-867.
12. Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with
13. Xia Y, Fan C, Hoadley KA, Parker JS, Perou CM. Genetic determinants of the molecular portraits of epithelial cancers. Nat Commun. 2019;10(1):5666.
14. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
15. Boldrin E, Nardo G, Zulato E, et al. Detection of loss of heterozygosity in cfDNA of advanced EGFR- or KRAS-mutated non-small-cell lung cancer patients. Int J Mol Sci. 2019;21(1):66.
16. Prat A, Brasó-Maristany F, Martínez-Sáez O, et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023;14(1):1157.
17. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
18. Zhou Q, Gampenrieder SP, Frantal S, et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. Clin Cancer Res. 2022;28(4):697-707.
19. Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life (Basel). 2021;11(9):890.
The current standard for breast cancer screening (for non–high-risk patients) is an annual or semiannual mammogram for women aged 40 and older.1 However, mammography-based screening can give false-positive or false-negative results. This can lead to excessive use of invasive tissue biopsies and unnecessary exposure to ionizing radiation—which can also become expensive and time-consuming for patients.2
Both normal and cancerous cells shed cell-free DNA (cfDNA) into the blood circulation.3 Circulating tumor DNA (ctDNA) are fragments of DNA derived from tumor cells that circulate in the blood together with cfDNA. The ctDNA originates directly from a tumor or from circulating tumor cells (and carries information from the tumor cell genome), whereas cfDNA enters the bloodstream after apoptosis or necrosis and carries genome-wide DNA information. The amount of ctDNA in the blood has been shown to be elevated in patients with cancer.3 Different cancers release varying levels of ctDNA; the amount of ctDNA released depends on the number of tumor cells that are in senescence vs undergoing apoptosis.4
The possibility of incorporating this biomarker obtained from a “liquid biopsy” is currently being studied and will hopefully become a standard of care for breast cancer screening and monitoring. The liquid biopsy detects ctDNA that has been released into the bloodstream from tumor regions and helps identify intratumoral heterogeneity and clonal evolution.5 Additionally, sequencing tumor DNA has opened new possibilities for precision oncology.6 Detection of somatic gene mutations, amplifications, and gene fusions helps to deliver targeted therapies.6 Analysis of potential somatic mutations in ctDNA, in combination with cfDNA levels, can help capture clinically relevant information beyond single genetic alterations and tumor fraction, potentially improving the accuracy of early detection and screening for breast cancer.
Recent advances in ctDNA testing technology have made it more accurate and reliable. ctDNA testing has several benefits, including early detection of cancer (detecting ctDNA at low levels)7; monitoring of tumor dynamics, therapeutic response, and residual disease8; as well as analysis of the evolution of genetic or epigenetic alterations characterizing the tumor.9 Its noninvasiveness, rapidity, and low cost allow for longitudinal monitoring of cancer in real time, potentially capturing tumor heterogeneity.10,11
The liquid biopsy potentially can give more options for therapeutic monitoring for breast cancer and may mirror clinically relevant genetic alterations that occur in all tumor tissues. Liquid biopsy offers many advantages. It allows for the detection of minimal residual disease and micrometastatic disease that may be difficult to detect with a traditional tissue biopsy.12 Liquid biopsy detects ctDNA that has been released into the bloodstream from multiple tumor regions and allows the possibility of identifying intratumoral heterogeneity and clonal evolution.5 It can also detect small quantitative variations within the blood, enabling real-time surveillance.
The liquid biopsy can offer earlier and easier access to some tumor-based genetic information at any given timepoint and can replace a tumor tissue biopsy in some cases, helping to avoid delays and complications of a solid tumor invasive biopsy procedure. This is especially relevant in the metastatic setting, in which ctDNA might be the only available genetic material from tumors.13 Tissue biopsy can only provide a static and spatially limited view of the disease at the time of sampling; ctDNA analysis could potentially reflect the genetic alterations that occur in all metastatic breast cancer sites over time.14,15 Furthermore, machine learning of multi-gene signatures, obtained from ctDNA, can possibly identify complex biological features, including measures of tumor proliferation and estrogen receptor signaling, similar to direct tumor tissue DNA or RNA profiling.16
ctDNA testing is currently being studied to monitor patients who have been diagnosed with breast cancer. Small retrospective studies have shown that detection of ctDNA in plasma, after patients have completed therapy for early-stage breast cancer, is associated with a very high risk of relapse.17
Ongoing studies are examining the tailoring of adjuvant treatment based on ctDNA. If these trials are successful, certain aspects of adjuvant treatment could be lessened, or omitted, for patients who have undetectable ctDNA or intensified for patients who have detectable ctDNA after definitive treatments. This could personalize treatment specifically to the patient.
The detection and persistence of ctDNA in the middle of neoadjuvant systemic therapy may have the potential to negatively predict response to treatment and identify patients who will not achieve pathologic complete response. This may have the potential to aid in clinical decision-making for treatment escalation in these nonresponders.18
Despite these distinct characteristics, the low levels of ctDNA found in early-stage disease, along with the lack of ctDNA shedding from some tumors, can further complicate or impede detection of recurrence in early-stage breast cancer. Testing is further complicated by hematologic genetic alterations.5 The limitation of ctDNA approaches is that these techniques only detect known mutations in certain genes, so patients without these mutations could be overlooked, limiting the application of this technology.19
Overall, ctDNA testing represents a promising area of research for the diagnosis, treatment, and monitoring of breast cancer. While more research is needed to fully understand its potential, the advances in this technology are certainly exciting and could lead to significant improvements in patient outcomes. It is hopeful that in the near future, ctDNA testing from liquid biopsy could become a standard of care in breast cancer screening, ultimately helping clinicians to personalize treatment therapies and improve patient outcomes when treating patients with breast cancer.
The current standard for breast cancer screening (for non–high-risk patients) is an annual or semiannual mammogram for women aged 40 and older.1 However, mammography-based screening can give false-positive or false-negative results. This can lead to excessive use of invasive tissue biopsies and unnecessary exposure to ionizing radiation—which can also become expensive and time-consuming for patients.2
Both normal and cancerous cells shed cell-free DNA (cfDNA) into the blood circulation.3 Circulating tumor DNA (ctDNA) are fragments of DNA derived from tumor cells that circulate in the blood together with cfDNA. The ctDNA originates directly from a tumor or from circulating tumor cells (and carries information from the tumor cell genome), whereas cfDNA enters the bloodstream after apoptosis or necrosis and carries genome-wide DNA information. The amount of ctDNA in the blood has been shown to be elevated in patients with cancer.3 Different cancers release varying levels of ctDNA; the amount of ctDNA released depends on the number of tumor cells that are in senescence vs undergoing apoptosis.4
The possibility of incorporating this biomarker obtained from a “liquid biopsy” is currently being studied and will hopefully become a standard of care for breast cancer screening and monitoring. The liquid biopsy detects ctDNA that has been released into the bloodstream from tumor regions and helps identify intratumoral heterogeneity and clonal evolution.5 Additionally, sequencing tumor DNA has opened new possibilities for precision oncology.6 Detection of somatic gene mutations, amplifications, and gene fusions helps to deliver targeted therapies.6 Analysis of potential somatic mutations in ctDNA, in combination with cfDNA levels, can help capture clinically relevant information beyond single genetic alterations and tumor fraction, potentially improving the accuracy of early detection and screening for breast cancer.
Recent advances in ctDNA testing technology have made it more accurate and reliable. ctDNA testing has several benefits, including early detection of cancer (detecting ctDNA at low levels)7; monitoring of tumor dynamics, therapeutic response, and residual disease8; as well as analysis of the evolution of genetic or epigenetic alterations characterizing the tumor.9 Its noninvasiveness, rapidity, and low cost allow for longitudinal monitoring of cancer in real time, potentially capturing tumor heterogeneity.10,11
The liquid biopsy potentially can give more options for therapeutic monitoring for breast cancer and may mirror clinically relevant genetic alterations that occur in all tumor tissues. Liquid biopsy offers many advantages. It allows for the detection of minimal residual disease and micrometastatic disease that may be difficult to detect with a traditional tissue biopsy.12 Liquid biopsy detects ctDNA that has been released into the bloodstream from multiple tumor regions and allows the possibility of identifying intratumoral heterogeneity and clonal evolution.5 It can also detect small quantitative variations within the blood, enabling real-time surveillance.
The liquid biopsy can offer earlier and easier access to some tumor-based genetic information at any given timepoint and can replace a tumor tissue biopsy in some cases, helping to avoid delays and complications of a solid tumor invasive biopsy procedure. This is especially relevant in the metastatic setting, in which ctDNA might be the only available genetic material from tumors.13 Tissue biopsy can only provide a static and spatially limited view of the disease at the time of sampling; ctDNA analysis could potentially reflect the genetic alterations that occur in all metastatic breast cancer sites over time.14,15 Furthermore, machine learning of multi-gene signatures, obtained from ctDNA, can possibly identify complex biological features, including measures of tumor proliferation and estrogen receptor signaling, similar to direct tumor tissue DNA or RNA profiling.16
ctDNA testing is currently being studied to monitor patients who have been diagnosed with breast cancer. Small retrospective studies have shown that detection of ctDNA in plasma, after patients have completed therapy for early-stage breast cancer, is associated with a very high risk of relapse.17
Ongoing studies are examining the tailoring of adjuvant treatment based on ctDNA. If these trials are successful, certain aspects of adjuvant treatment could be lessened, or omitted, for patients who have undetectable ctDNA or intensified for patients who have detectable ctDNA after definitive treatments. This could personalize treatment specifically to the patient.
The detection and persistence of ctDNA in the middle of neoadjuvant systemic therapy may have the potential to negatively predict response to treatment and identify patients who will not achieve pathologic complete response. This may have the potential to aid in clinical decision-making for treatment escalation in these nonresponders.18
Despite these distinct characteristics, the low levels of ctDNA found in early-stage disease, along with the lack of ctDNA shedding from some tumors, can further complicate or impede detection of recurrence in early-stage breast cancer. Testing is further complicated by hematologic genetic alterations.5 The limitation of ctDNA approaches is that these techniques only detect known mutations in certain genes, so patients without these mutations could be overlooked, limiting the application of this technology.19
Overall, ctDNA testing represents a promising area of research for the diagnosis, treatment, and monitoring of breast cancer. While more research is needed to fully understand its potential, the advances in this technology are certainly exciting and could lead to significant improvements in patient outcomes. It is hopeful that in the near future, ctDNA testing from liquid biopsy could become a standard of care in breast cancer screening, ultimately helping clinicians to personalize treatment therapies and improve patient outcomes when treating patients with breast cancer.
1. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
2. Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20(12):E2878.
3. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-376.
4. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020;31(13):107830.
5. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172-186.
6. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895-1902.
7. Wang J, Han X, Sun Y. DNA methylation signatures in circulating cell-free DNA as biomarkers for the early detection of cancer. Sci China Life Sci. 2017;60(4):356-362.
8. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209.
9. Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586.
10. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705.
11. Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol. 2016;27(5):862-867.
12. Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with
13. Xia Y, Fan C, Hoadley KA, Parker JS, Perou CM. Genetic determinants of the molecular portraits of epithelial cancers. Nat Commun. 2019;10(1):5666.
14. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
15. Boldrin E, Nardo G, Zulato E, et al. Detection of loss of heterozygosity in cfDNA of advanced EGFR- or KRAS-mutated non-small-cell lung cancer patients. Int J Mol Sci. 2019;21(1):66.
16. Prat A, Brasó-Maristany F, Martínez-Sáez O, et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023;14(1):1157.
17. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
18. Zhou Q, Gampenrieder SP, Frantal S, et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. Clin Cancer Res. 2022;28(4):697-707.
19. Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life (Basel). 2021;11(9):890.
1. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
2. Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20(12):E2878.
3. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-376.
4. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020;31(13):107830.
5. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172-186.
6. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895-1902.
7. Wang J, Han X, Sun Y. DNA methylation signatures in circulating cell-free DNA as biomarkers for the early detection of cancer. Sci China Life Sci. 2017;60(4):356-362.
8. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209.
9. Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586.
10. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705.
11. Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol. 2016;27(5):862-867.
12. Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with
13. Xia Y, Fan C, Hoadley KA, Parker JS, Perou CM. Genetic determinants of the molecular portraits of epithelial cancers. Nat Commun. 2019;10(1):5666.
14. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
15. Boldrin E, Nardo G, Zulato E, et al. Detection of loss of heterozygosity in cfDNA of advanced EGFR- or KRAS-mutated non-small-cell lung cancer patients. Int J Mol Sci. 2019;21(1):66.
16. Prat A, Brasó-Maristany F, Martínez-Sáez O, et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023;14(1):1157.
17. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
18. Zhou Q, Gampenrieder SP, Frantal S, et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. Clin Cancer Res. 2022;28(4):697-707.
19. Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life (Basel). 2021;11(9):890.
Anti-obesity medications: Breakthroughs and limitations
Obesity is a major health problem in the United States. The Centers for Disease Control and Prevention (CDC) defines the problem as weight that is higher than what is healthy for a given height, with quantitative definitions of overweight and obesity as body mass indices (BMIs) of 25 to 29.9 kg/m2 and ≥ 30 kg/m2, respectively.1 The prevalence of obesity among adults in 2017 ̶ 2018 was reported by the CDC to be 42.4%.2 Among women, the reported prevalence of obesity was lowest among Asian individuals (17.2%) and greatest among non-Hispanic Black individuals (56.9%), with White (39.8%) and Hispanic individuals (43.7%) having rates in between.2 In a meta-analysis of prospective studies that included 4 million people who were never smokers and had no chronic disease at baseline, age- and sex-adjusted mortality rates were studied over a median of 14 years of follow-up.3 Compared with those with a BMI of 20 to 25 kg/m2, people with a BMI of 30 to 34.9 kg/m2 or a BMI of 35 to 39.9 kg/m2 had increased risks of death of 46% and 94%, respectively, demonstrating that obesity increases this risk.3
The increased risk of death associated with obesity is caused by obesity-related diseases that cause early mortality, including diabetes mellitus (DM), dyslipidemia, hypertension, coronary heart disease, heart failure, atrial fibrillation, stroke, and venous thromboembolic events.4 Obesity is also associated with an increased risk of many cancers, including cancer of the endometrium, kidney, esophagus, stomach, colon, rectum, gallbladder, pancreas, liver, and breast.5 With regard to gynecologic disease, obesity is associated with an increased risk of fibroids and heavy menstrual bleeding.6 For pregnant patients, obesity is associated with increased risks of7:
- miscarriage and stillbirth
- preeclampsia and gestational hypertension
- gestational diabetes
- severe maternal morbidity
- postterm pregnancy
- venous thromboembolism
- endometritis.
For obese patients, weight loss can normalize blood pressure, reduce the risk of cardiovascular events, decrease the risk of cancer, and cure type 2 DM.8
Bariatric surgery: The gold standard treatment for reliable and sustained weight loss
All patients with obesity should be counseled to reduce caloric intake and increase physical activity. Dietary counseling provided by a nutritionist may help reinforce advice given by a provider. However, lifestyle interventions are associated with modest weight loss (<5% of bodyweight; FIGURE
In the Swedish Obese Subjects study, involving 2,010 people, following bariatric surgery the mean decrease in bodyweight was 23% at 2 years, with a slow increase in weight thereafter, resulting in a sustained mean weight loss of 18% at 10 years.8 In this study, people in the diet and exercise control group had no change in bodyweight over 10 years of follow-up.8 Not all eligible obese patients want to undergo bariatric surgery because it is an arduous sequential process involving 6 months of intensive preoperative preparation, bariatric surgery, recovery, and intensive postoperative follow-up. The perioperative mortality rate is 0.03% to 0.2%.10 Following bariatric surgery, additional operations may be necessary for more than 10% of patients.10 With recent breakthroughs in the medication management of obesity, patients who do not want bariatric surgery can achieve reliable weight loss of greater than 10% of body weight with glucagon-like peptide -1 (GLP-1) agonists.
GLP-1 agonist analogues: Practice-changing breakthrough in medication treatment
GLP-1, a 30 amino acid peptide, is produced by intestinal enteroendocrine cells and neurons in the medulla and hypothalamus.11 GLP-1 reduces hunger cravings and causes satiety, reducing daily food intake.12 GLP-1 also enhances the secretion of insulin, making GLP-1 agonists an effective treatment for type 2 DM. In humans and experimental animals, the administration of exogenous GLP-1 agonists decreases hunger cravings and causes satiety, reducing food intake, resulting in weight loss.12 The synthetic GLP-1 agonists, liraglutide (Saxenda) and semaglutide (Wegovy) are approved by the US Food and Drug Administration (FDA) as anti-obesity medications.
Native GLP-1 has a short circulating half-life of approximately 2 minutes. The synthetic GLP-1 agonist medications liraglutide and semaglutide are modified to significantly increase their half-life. Liraglutide is a modified version of GLP-1 with a palmitic acid side chain and an amino acid spacer resulting in reduced degradation and a 15-hour half-life, necessitating daily administration. Semaglutide has a steric acid diacid at Lys26, a large synthetic spacer, a modification of amino acid 8 with the addition of α-aminobutyric acid and a 165-hour half-life, permitting weekly administration.13 For weight loss, liraglutide and semaglultide are administered by subcutaneous injection. Tirzepatide (Mounjaro) is a novel GLP-1 agonist. It is also a gastric inhibitory peptide, is FDA approved to treat type 2 DM, and is awaiting FDA approval as a weight loss medication.Tirzepatide causes substantial weight loss, similar to the effect of semaglutide.14
Semaglutide and weight loss
Semaglutide is approved by the FDA for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of a weight-related comorbidity. It is also FDA approved to treat type 2 DM.
In a weight loss trial, 1,961 overweight and obese patients with a mean BMI of 38 kg/m2, were randomly assigned to semaglutide or placebo treatment for 68 weeks. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. The mean changes in body weight for the patients in the semaglutide and placebo treatment groups were -14.9% and -2.4%, respectively. The treatment difference was -12.4% (95% confidence interval [CI], -13.4% to -11.5%; P <.001). In this study, compared with placebo, semaglutide treatment resulted in a greater decrease in waist circumference, -5.3 in versus -1.6 in.15 A network meta-analysis of the efficacy of weight loss medicines indicates that semaglutide is the most effective medication currently FDA approved for weight loss, reliably producing substantial weight loss (FIGURE).9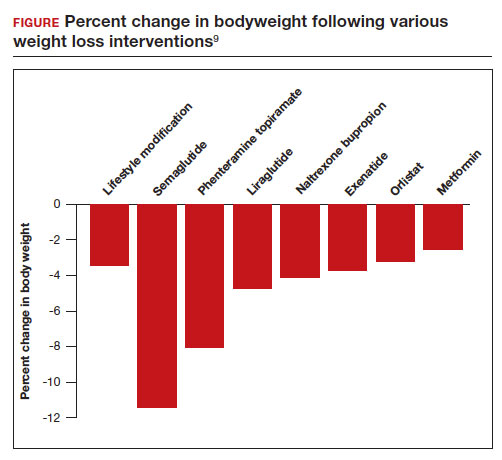
In one randomized clinical trial, investigators directly compared the efficacy of semaglutide and liraglutide in achieving weight loss. In this trial, 338 patients were assigned randomly to treatment with semaglutide 2.4 mg weekly subcutaneous injection, liraglutide 3.0 mg daily subcutaneous injection, or placebo. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity.16 After 68 weeks of treatment, the mean weight changes were -15.8%, -6.4%, and -1.9% in the semaglutide, liraglutide, and placebo groups, respectively. The difference between the semaglutide and liraglutide groups was -9.4% (95% CI, -12% to -6.8%; P <.001).16
Continue to: Semaglutide dose-escalation and contraindications...
Semaglutide dose-escalation and contraindications
For weight loss, the target dose of semaglutide is 2.4 mg once weekly subcutaneous injection achieved by sequential dose escalation. To give patients time to adjust to adverse effects caused by the medication, a standardized dose-escalation regimen is recommended. The FDA-approved escalation regimen for semaglutide treatment begins with a weekly subcutaneous dose of 0.25 mg for 4 weeks, followed by an increase in the weekly dosage every 4 weeks: 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.17 To support the dose-escalation process there are 5 unique autoinjectors that deliver the appropriate dose for the current step.
Semaglutide is contraindicated if the patient has an allergy to the medication or if there is a personal or family history of medullary thyroid cancer.17 In animal toxicology studies, semaglutide at clinically relevant dosing was associated with an increased risk of developing medullary thyroid cancer. Patients with a personal history of multiple endocrine neoplasia syndrome type 2, (medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism) should not take semaglutide. Semaglutide may cause fetal harm and the FDA recommends discontinuing semaglutide at least 2 months before pregnancy.17 According to the FDA, the safety of semaglutide during breastfeeding has not been established. In Canada, breastfeeding is a contraindication to semaglutide treatment.18
Limitations of medication treatment of obesity
There are important limitations to semaglutide treatment of obesity, including:
- weight gain after stopping treatment
- limited medical insurance supportfor an expensive medication treatment
- bothersome adverse effects.
Weight gain posttreatment. After stopping medication treatment of obesity, weight gain occurs in most patients. However, patients may remain below baseline weight for a long time after stopping medication therapy. In one trial of 803 patients, after 20 weeks of semaglutide treatment (16-week dose-escalation phase, followed by 4 weeks on a weekly dose of 2.4 mg), the participants were randomized to 48 additional weeks of semaglutide or placebo.19 All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. At the initial 20 weeks of treatment time point the mean weight change was -10.6%. Over the following 48 weeks, the patients treated with semaglutidehad an additional mean weight change of -7.9%, while the mean weight change for the placebo group was +6.9%.
Medical insurance coverage. A major barrier to semaglutide treatment of obesity is the medication’s cost. At the website GoodRx (https://www.goodrx.com/), the estimated price for a 1-month supply of semaglutide (Wegovy) is $1,350.20 By contrast, a 1-month supply of phentermine-topiramate (Qsymia) is approximately $205. Currently, many medical insurance plans do not cover the cost of semaglutide treatment for weight loss. Patent protection for liraglutide may expire in the next few years, permitting the marketing of a lower-cost generic formulation, increasing the availability of the medication. However, as noted above, compared with liraglutide, semaglutide treatment results in much greater weight loss.
The most common adverse effects associated with semaglutide treatment are nausea, vomiting, diarrhea, and constipation. In one randomized clinical trial involving 1,961 patients, the frequency of adverse effects reported by patients taking semaglutide incrementally above the frequency of the same adverse effect reported by patients on placebo was: nausea (27%), vomiting (18%), diarrhea (16%), constipation (14%), dyspepsia (7%), and abdominal pain (5%).15 In this study, treatment was discontinued due to adverse effects in 7% and 3% of the patients in the semaglutide and placebo groups, respectively. Experts believe that adverse effects can be minimized by increasing the dose slowly and decreasing the dose if adverse effects are bothersome to the patient.
Measuring the benefits of semaglutide weight loss
Overweight and obesity are prevalent problems with many adverse consequences, including an increased risk of death. In population studies, weight loss following bariatric surgery is associated with a substantial reduction in mortality, cancer, and heart disease compared with conventional therapy.21 Over the next few years, the effect of semaglutide-induced weight loss on the rate of cancer and heart disease should become clear. If semaglutide treatment of obesity is associated with a reduction in cancer and heart disease, it would be a truly breakthrough medication. ●
- Defining adult and overweight obesity. Centers for Disease Control and Prevention website. https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 19, 2023.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCH Data Brief. 2020;360. https://www.cdc.gov/nchs/data /databriefs/db360-h.pdf. Accessed June 19, 2023.
- The Global BMI Mortality Collaboration. Bodymass index and all-cause mortality: individual- participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and health life-years lost from diabetes and cardiovascular disease in the overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114-122.
- Lega IC, Lipscombe LL. Review: diabetes, obesity and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: a mendelian randomization study. PLoS Med. 19:e1003679.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and longterm adverse consequences for mother and child. BMJ. 2017;356:j1.
- Sjorstrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet. 2022;399:259-269.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879-887.
- Brierly DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems are involved in the control of eating behavior by linking food intake and satiety. Nat Metab. 2021;3:258-273.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754-762.
- Gotfredsen CF, Molck AM, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-515.
- Wilding JPH, Batterham RL, Calanna S, et al. Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1000.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327:138-150.
- Wegovy [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2021.
- Wegovy Product Monograph. Mississauga, Ontario: Novo Nordisk Canada Inc; June 30, 2022. https://pdf.hres.ca/dpd_pm/00066484.PDF
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325: 1414-1425.
- GoodRx website. https://www.goodrx.com/. Accessed June 19, 2023.
- Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and metaanalysis. PLoS Med. 2020;17:e1003206.
Obesity is a major health problem in the United States. The Centers for Disease Control and Prevention (CDC) defines the problem as weight that is higher than what is healthy for a given height, with quantitative definitions of overweight and obesity as body mass indices (BMIs) of 25 to 29.9 kg/m2 and ≥ 30 kg/m2, respectively.1 The prevalence of obesity among adults in 2017 ̶ 2018 was reported by the CDC to be 42.4%.2 Among women, the reported prevalence of obesity was lowest among Asian individuals (17.2%) and greatest among non-Hispanic Black individuals (56.9%), with White (39.8%) and Hispanic individuals (43.7%) having rates in between.2 In a meta-analysis of prospective studies that included 4 million people who were never smokers and had no chronic disease at baseline, age- and sex-adjusted mortality rates were studied over a median of 14 years of follow-up.3 Compared with those with a BMI of 20 to 25 kg/m2, people with a BMI of 30 to 34.9 kg/m2 or a BMI of 35 to 39.9 kg/m2 had increased risks of death of 46% and 94%, respectively, demonstrating that obesity increases this risk.3
The increased risk of death associated with obesity is caused by obesity-related diseases that cause early mortality, including diabetes mellitus (DM), dyslipidemia, hypertension, coronary heart disease, heart failure, atrial fibrillation, stroke, and venous thromboembolic events.4 Obesity is also associated with an increased risk of many cancers, including cancer of the endometrium, kidney, esophagus, stomach, colon, rectum, gallbladder, pancreas, liver, and breast.5 With regard to gynecologic disease, obesity is associated with an increased risk of fibroids and heavy menstrual bleeding.6 For pregnant patients, obesity is associated with increased risks of7:
- miscarriage and stillbirth
- preeclampsia and gestational hypertension
- gestational diabetes
- severe maternal morbidity
- postterm pregnancy
- venous thromboembolism
- endometritis.
For obese patients, weight loss can normalize blood pressure, reduce the risk of cardiovascular events, decrease the risk of cancer, and cure type 2 DM.8
Bariatric surgery: The gold standard treatment for reliable and sustained weight loss
All patients with obesity should be counseled to reduce caloric intake and increase physical activity. Dietary counseling provided by a nutritionist may help reinforce advice given by a provider. However, lifestyle interventions are associated with modest weight loss (<5% of bodyweight; FIGURE
In the Swedish Obese Subjects study, involving 2,010 people, following bariatric surgery the mean decrease in bodyweight was 23% at 2 years, with a slow increase in weight thereafter, resulting in a sustained mean weight loss of 18% at 10 years.8 In this study, people in the diet and exercise control group had no change in bodyweight over 10 years of follow-up.8 Not all eligible obese patients want to undergo bariatric surgery because it is an arduous sequential process involving 6 months of intensive preoperative preparation, bariatric surgery, recovery, and intensive postoperative follow-up. The perioperative mortality rate is 0.03% to 0.2%.10 Following bariatric surgery, additional operations may be necessary for more than 10% of patients.10 With recent breakthroughs in the medication management of obesity, patients who do not want bariatric surgery can achieve reliable weight loss of greater than 10% of body weight with glucagon-like peptide -1 (GLP-1) agonists.

GLP-1 agonist analogues: Practice-changing breakthrough in medication treatment
GLP-1, a 30 amino acid peptide, is produced by intestinal enteroendocrine cells and neurons in the medulla and hypothalamus.11 GLP-1 reduces hunger cravings and causes satiety, reducing daily food intake.12 GLP-1 also enhances the secretion of insulin, making GLP-1 agonists an effective treatment for type 2 DM. In humans and experimental animals, the administration of exogenous GLP-1 agonists decreases hunger cravings and causes satiety, reducing food intake, resulting in weight loss.12 The synthetic GLP-1 agonists, liraglutide (Saxenda) and semaglutide (Wegovy) are approved by the US Food and Drug Administration (FDA) as anti-obesity medications.
Native GLP-1 has a short circulating half-life of approximately 2 minutes. The synthetic GLP-1 agonist medications liraglutide and semaglutide are modified to significantly increase their half-life. Liraglutide is a modified version of GLP-1 with a palmitic acid side chain and an amino acid spacer resulting in reduced degradation and a 15-hour half-life, necessitating daily administration. Semaglutide has a steric acid diacid at Lys26, a large synthetic spacer, a modification of amino acid 8 with the addition of α-aminobutyric acid and a 165-hour half-life, permitting weekly administration.13 For weight loss, liraglutide and semaglultide are administered by subcutaneous injection. Tirzepatide (Mounjaro) is a novel GLP-1 agonist. It is also a gastric inhibitory peptide, is FDA approved to treat type 2 DM, and is awaiting FDA approval as a weight loss medication.Tirzepatide causes substantial weight loss, similar to the effect of semaglutide.14
Semaglutide and weight loss
Semaglutide is approved by the FDA for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of a weight-related comorbidity. It is also FDA approved to treat type 2 DM.
In a weight loss trial, 1,961 overweight and obese patients with a mean BMI of 38 kg/m2, were randomly assigned to semaglutide or placebo treatment for 68 weeks. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. The mean changes in body weight for the patients in the semaglutide and placebo treatment groups were -14.9% and -2.4%, respectively. The treatment difference was -12.4% (95% confidence interval [CI], -13.4% to -11.5%; P <.001). In this study, compared with placebo, semaglutide treatment resulted in a greater decrease in waist circumference, -5.3 in versus -1.6 in.15 A network meta-analysis of the efficacy of weight loss medicines indicates that semaglutide is the most effective medication currently FDA approved for weight loss, reliably producing substantial weight loss (FIGURE).9
In one randomized clinical trial, investigators directly compared the efficacy of semaglutide and liraglutide in achieving weight loss. In this trial, 338 patients were assigned randomly to treatment with semaglutide 2.4 mg weekly subcutaneous injection, liraglutide 3.0 mg daily subcutaneous injection, or placebo. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity.16 After 68 weeks of treatment, the mean weight changes were -15.8%, -6.4%, and -1.9% in the semaglutide, liraglutide, and placebo groups, respectively. The difference between the semaglutide and liraglutide groups was -9.4% (95% CI, -12% to -6.8%; P <.001).16
Continue to: Semaglutide dose-escalation and contraindications...
Semaglutide dose-escalation and contraindications
For weight loss, the target dose of semaglutide is 2.4 mg once weekly subcutaneous injection achieved by sequential dose escalation. To give patients time to adjust to adverse effects caused by the medication, a standardized dose-escalation regimen is recommended. The FDA-approved escalation regimen for semaglutide treatment begins with a weekly subcutaneous dose of 0.25 mg for 4 weeks, followed by an increase in the weekly dosage every 4 weeks: 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.17 To support the dose-escalation process there are 5 unique autoinjectors that deliver the appropriate dose for the current step.
Semaglutide is contraindicated if the patient has an allergy to the medication or if there is a personal or family history of medullary thyroid cancer.17 In animal toxicology studies, semaglutide at clinically relevant dosing was associated with an increased risk of developing medullary thyroid cancer. Patients with a personal history of multiple endocrine neoplasia syndrome type 2, (medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism) should not take semaglutide. Semaglutide may cause fetal harm and the FDA recommends discontinuing semaglutide at least 2 months before pregnancy.17 According to the FDA, the safety of semaglutide during breastfeeding has not been established. In Canada, breastfeeding is a contraindication to semaglutide treatment.18
Limitations of medication treatment of obesity
There are important limitations to semaglutide treatment of obesity, including:
- weight gain after stopping treatment
- limited medical insurance supportfor an expensive medication treatment
- bothersome adverse effects.
Weight gain posttreatment. After stopping medication treatment of obesity, weight gain occurs in most patients. However, patients may remain below baseline weight for a long time after stopping medication therapy. In one trial of 803 patients, after 20 weeks of semaglutide treatment (16-week dose-escalation phase, followed by 4 weeks on a weekly dose of 2.4 mg), the participants were randomized to 48 additional weeks of semaglutide or placebo.19 All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. At the initial 20 weeks of treatment time point the mean weight change was -10.6%. Over the following 48 weeks, the patients treated with semaglutidehad an additional mean weight change of -7.9%, while the mean weight change for the placebo group was +6.9%.
Medical insurance coverage. A major barrier to semaglutide treatment of obesity is the medication’s cost. At the website GoodRx (https://www.goodrx.com/), the estimated price for a 1-month supply of semaglutide (Wegovy) is $1,350.20 By contrast, a 1-month supply of phentermine-topiramate (Qsymia) is approximately $205. Currently, many medical insurance plans do not cover the cost of semaglutide treatment for weight loss. Patent protection for liraglutide may expire in the next few years, permitting the marketing of a lower-cost generic formulation, increasing the availability of the medication. However, as noted above, compared with liraglutide, semaglutide treatment results in much greater weight loss.
The most common adverse effects associated with semaglutide treatment are nausea, vomiting, diarrhea, and constipation. In one randomized clinical trial involving 1,961 patients, the frequency of adverse effects reported by patients taking semaglutide incrementally above the frequency of the same adverse effect reported by patients on placebo was: nausea (27%), vomiting (18%), diarrhea (16%), constipation (14%), dyspepsia (7%), and abdominal pain (5%).15 In this study, treatment was discontinued due to adverse effects in 7% and 3% of the patients in the semaglutide and placebo groups, respectively. Experts believe that adverse effects can be minimized by increasing the dose slowly and decreasing the dose if adverse effects are bothersome to the patient.
Measuring the benefits of semaglutide weight loss
Overweight and obesity are prevalent problems with many adverse consequences, including an increased risk of death. In population studies, weight loss following bariatric surgery is associated with a substantial reduction in mortality, cancer, and heart disease compared with conventional therapy.21 Over the next few years, the effect of semaglutide-induced weight loss on the rate of cancer and heart disease should become clear. If semaglutide treatment of obesity is associated with a reduction in cancer and heart disease, it would be a truly breakthrough medication. ●
Obesity is a major health problem in the United States. The Centers for Disease Control and Prevention (CDC) defines the problem as weight that is higher than what is healthy for a given height, with quantitative definitions of overweight and obesity as body mass indices (BMIs) of 25 to 29.9 kg/m2 and ≥ 30 kg/m2, respectively.1 The prevalence of obesity among adults in 2017 ̶ 2018 was reported by the CDC to be 42.4%.2 Among women, the reported prevalence of obesity was lowest among Asian individuals (17.2%) and greatest among non-Hispanic Black individuals (56.9%), with White (39.8%) and Hispanic individuals (43.7%) having rates in between.2 In a meta-analysis of prospective studies that included 4 million people who were never smokers and had no chronic disease at baseline, age- and sex-adjusted mortality rates were studied over a median of 14 years of follow-up.3 Compared with those with a BMI of 20 to 25 kg/m2, people with a BMI of 30 to 34.9 kg/m2 or a BMI of 35 to 39.9 kg/m2 had increased risks of death of 46% and 94%, respectively, demonstrating that obesity increases this risk.3
The increased risk of death associated with obesity is caused by obesity-related diseases that cause early mortality, including diabetes mellitus (DM), dyslipidemia, hypertension, coronary heart disease, heart failure, atrial fibrillation, stroke, and venous thromboembolic events.4 Obesity is also associated with an increased risk of many cancers, including cancer of the endometrium, kidney, esophagus, stomach, colon, rectum, gallbladder, pancreas, liver, and breast.5 With regard to gynecologic disease, obesity is associated with an increased risk of fibroids and heavy menstrual bleeding.6 For pregnant patients, obesity is associated with increased risks of7:
- miscarriage and stillbirth
- preeclampsia and gestational hypertension
- gestational diabetes
- severe maternal morbidity
- postterm pregnancy
- venous thromboembolism
- endometritis.
For obese patients, weight loss can normalize blood pressure, reduce the risk of cardiovascular events, decrease the risk of cancer, and cure type 2 DM.8
Bariatric surgery: The gold standard treatment for reliable and sustained weight loss
All patients with obesity should be counseled to reduce caloric intake and increase physical activity. Dietary counseling provided by a nutritionist may help reinforce advice given by a provider. However, lifestyle interventions are associated with modest weight loss (<5% of bodyweight; FIGURE
In the Swedish Obese Subjects study, involving 2,010 people, following bariatric surgery the mean decrease in bodyweight was 23% at 2 years, with a slow increase in weight thereafter, resulting in a sustained mean weight loss of 18% at 10 years.8 In this study, people in the diet and exercise control group had no change in bodyweight over 10 years of follow-up.8 Not all eligible obese patients want to undergo bariatric surgery because it is an arduous sequential process involving 6 months of intensive preoperative preparation, bariatric surgery, recovery, and intensive postoperative follow-up. The perioperative mortality rate is 0.03% to 0.2%.10 Following bariatric surgery, additional operations may be necessary for more than 10% of patients.10 With recent breakthroughs in the medication management of obesity, patients who do not want bariatric surgery can achieve reliable weight loss of greater than 10% of body weight with glucagon-like peptide -1 (GLP-1) agonists.

GLP-1 agonist analogues: Practice-changing breakthrough in medication treatment
GLP-1, a 30 amino acid peptide, is produced by intestinal enteroendocrine cells and neurons in the medulla and hypothalamus.11 GLP-1 reduces hunger cravings and causes satiety, reducing daily food intake.12 GLP-1 also enhances the secretion of insulin, making GLP-1 agonists an effective treatment for type 2 DM. In humans and experimental animals, the administration of exogenous GLP-1 agonists decreases hunger cravings and causes satiety, reducing food intake, resulting in weight loss.12 The synthetic GLP-1 agonists, liraglutide (Saxenda) and semaglutide (Wegovy) are approved by the US Food and Drug Administration (FDA) as anti-obesity medications.
Native GLP-1 has a short circulating half-life of approximately 2 minutes. The synthetic GLP-1 agonist medications liraglutide and semaglutide are modified to significantly increase their half-life. Liraglutide is a modified version of GLP-1 with a palmitic acid side chain and an amino acid spacer resulting in reduced degradation and a 15-hour half-life, necessitating daily administration. Semaglutide has a steric acid diacid at Lys26, a large synthetic spacer, a modification of amino acid 8 with the addition of α-aminobutyric acid and a 165-hour half-life, permitting weekly administration.13 For weight loss, liraglutide and semaglultide are administered by subcutaneous injection. Tirzepatide (Mounjaro) is a novel GLP-1 agonist. It is also a gastric inhibitory peptide, is FDA approved to treat type 2 DM, and is awaiting FDA approval as a weight loss medication.Tirzepatide causes substantial weight loss, similar to the effect of semaglutide.14
Semaglutide and weight loss
Semaglutide is approved by the FDA for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of a weight-related comorbidity. It is also FDA approved to treat type 2 DM.
In a weight loss trial, 1,961 overweight and obese patients with a mean BMI of 38 kg/m2, were randomly assigned to semaglutide or placebo treatment for 68 weeks. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. The mean changes in body weight for the patients in the semaglutide and placebo treatment groups were -14.9% and -2.4%, respectively. The treatment difference was -12.4% (95% confidence interval [CI], -13.4% to -11.5%; P <.001). In this study, compared with placebo, semaglutide treatment resulted in a greater decrease in waist circumference, -5.3 in versus -1.6 in.15 A network meta-analysis of the efficacy of weight loss medicines indicates that semaglutide is the most effective medication currently FDA approved for weight loss, reliably producing substantial weight loss (FIGURE).9
In one randomized clinical trial, investigators directly compared the efficacy of semaglutide and liraglutide in achieving weight loss. In this trial, 338 patients were assigned randomly to treatment with semaglutide 2.4 mg weekly subcutaneous injection, liraglutide 3.0 mg daily subcutaneous injection, or placebo. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity.16 After 68 weeks of treatment, the mean weight changes were -15.8%, -6.4%, and -1.9% in the semaglutide, liraglutide, and placebo groups, respectively. The difference between the semaglutide and liraglutide groups was -9.4% (95% CI, -12% to -6.8%; P <.001).16
Continue to: Semaglutide dose-escalation and contraindications...
Semaglutide dose-escalation and contraindications
For weight loss, the target dose of semaglutide is 2.4 mg once weekly subcutaneous injection achieved by sequential dose escalation. To give patients time to adjust to adverse effects caused by the medication, a standardized dose-escalation regimen is recommended. The FDA-approved escalation regimen for semaglutide treatment begins with a weekly subcutaneous dose of 0.25 mg for 4 weeks, followed by an increase in the weekly dosage every 4 weeks: 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.17 To support the dose-escalation process there are 5 unique autoinjectors that deliver the appropriate dose for the current step.
Semaglutide is contraindicated if the patient has an allergy to the medication or if there is a personal or family history of medullary thyroid cancer.17 In animal toxicology studies, semaglutide at clinically relevant dosing was associated with an increased risk of developing medullary thyroid cancer. Patients with a personal history of multiple endocrine neoplasia syndrome type 2, (medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism) should not take semaglutide. Semaglutide may cause fetal harm and the FDA recommends discontinuing semaglutide at least 2 months before pregnancy.17 According to the FDA, the safety of semaglutide during breastfeeding has not been established. In Canada, breastfeeding is a contraindication to semaglutide treatment.18
Limitations of medication treatment of obesity
There are important limitations to semaglutide treatment of obesity, including:
- weight gain after stopping treatment
- limited medical insurance supportfor an expensive medication treatment
- bothersome adverse effects.
Weight gain posttreatment. After stopping medication treatment of obesity, weight gain occurs in most patients. However, patients may remain below baseline weight for a long time after stopping medication therapy. In one trial of 803 patients, after 20 weeks of semaglutide treatment (16-week dose-escalation phase, followed by 4 weeks on a weekly dose of 2.4 mg), the participants were randomized to 48 additional weeks of semaglutide or placebo.19 All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. At the initial 20 weeks of treatment time point the mean weight change was -10.6%. Over the following 48 weeks, the patients treated with semaglutidehad an additional mean weight change of -7.9%, while the mean weight change for the placebo group was +6.9%.
Medical insurance coverage. A major barrier to semaglutide treatment of obesity is the medication’s cost. At the website GoodRx (https://www.goodrx.com/), the estimated price for a 1-month supply of semaglutide (Wegovy) is $1,350.20 By contrast, a 1-month supply of phentermine-topiramate (Qsymia) is approximately $205. Currently, many medical insurance plans do not cover the cost of semaglutide treatment for weight loss. Patent protection for liraglutide may expire in the next few years, permitting the marketing of a lower-cost generic formulation, increasing the availability of the medication. However, as noted above, compared with liraglutide, semaglutide treatment results in much greater weight loss.
The most common adverse effects associated with semaglutide treatment are nausea, vomiting, diarrhea, and constipation. In one randomized clinical trial involving 1,961 patients, the frequency of adverse effects reported by patients taking semaglutide incrementally above the frequency of the same adverse effect reported by patients on placebo was: nausea (27%), vomiting (18%), diarrhea (16%), constipation (14%), dyspepsia (7%), and abdominal pain (5%).15 In this study, treatment was discontinued due to adverse effects in 7% and 3% of the patients in the semaglutide and placebo groups, respectively. Experts believe that adverse effects can be minimized by increasing the dose slowly and decreasing the dose if adverse effects are bothersome to the patient.
Measuring the benefits of semaglutide weight loss
Overweight and obesity are prevalent problems with many adverse consequences, including an increased risk of death. In population studies, weight loss following bariatric surgery is associated with a substantial reduction in mortality, cancer, and heart disease compared with conventional therapy.21 Over the next few years, the effect of semaglutide-induced weight loss on the rate of cancer and heart disease should become clear. If semaglutide treatment of obesity is associated with a reduction in cancer and heart disease, it would be a truly breakthrough medication. ●
- Defining adult and overweight obesity. Centers for Disease Control and Prevention website. https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 19, 2023.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCH Data Brief. 2020;360. https://www.cdc.gov/nchs/data /databriefs/db360-h.pdf. Accessed June 19, 2023.
- The Global BMI Mortality Collaboration. Bodymass index and all-cause mortality: individual- participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and health life-years lost from diabetes and cardiovascular disease in the overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114-122.
- Lega IC, Lipscombe LL. Review: diabetes, obesity and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: a mendelian randomization study. PLoS Med. 19:e1003679.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and longterm adverse consequences for mother and child. BMJ. 2017;356:j1.
- Sjorstrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet. 2022;399:259-269.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879-887.
- Brierly DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems are involved in the control of eating behavior by linking food intake and satiety. Nat Metab. 2021;3:258-273.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754-762.
- Gotfredsen CF, Molck AM, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-515.
- Wilding JPH, Batterham RL, Calanna S, et al. Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1000.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327:138-150.
- Wegovy [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2021.
- Wegovy Product Monograph. Mississauga, Ontario: Novo Nordisk Canada Inc; June 30, 2022. https://pdf.hres.ca/dpd_pm/00066484.PDF
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325: 1414-1425.
- GoodRx website. https://www.goodrx.com/. Accessed June 19, 2023.
- Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and metaanalysis. PLoS Med. 2020;17:e1003206.
- Defining adult and overweight obesity. Centers for Disease Control and Prevention website. https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 19, 2023.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCH Data Brief. 2020;360. https://www.cdc.gov/nchs/data /databriefs/db360-h.pdf. Accessed June 19, 2023.
- The Global BMI Mortality Collaboration. Bodymass index and all-cause mortality: individual- participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and health life-years lost from diabetes and cardiovascular disease in the overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114-122.
- Lega IC, Lipscombe LL. Review: diabetes, obesity and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: a mendelian randomization study. PLoS Med. 19:e1003679.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and longterm adverse consequences for mother and child. BMJ. 2017;356:j1.
- Sjorstrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet. 2022;399:259-269.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879-887.
- Brierly DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems are involved in the control of eating behavior by linking food intake and satiety. Nat Metab. 2021;3:258-273.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754-762.
- Gotfredsen CF, Molck AM, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-515.
- Wilding JPH, Batterham RL, Calanna S, et al. Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1000.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327:138-150.
- Wegovy [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2021.
- Wegovy Product Monograph. Mississauga, Ontario: Novo Nordisk Canada Inc; June 30, 2022. https://pdf.hres.ca/dpd_pm/00066484.PDF
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325: 1414-1425.
- GoodRx website. https://www.goodrx.com/. Accessed June 19, 2023.
- Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and metaanalysis. PLoS Med. 2020;17:e1003206.