User login
Anticoagulation Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving treatment options for preventing stroke, acute coronary events, deep vein thrombosis, and pulmonary embolism in at-risk patients. The Anticoagulation Hub is powered by Frontline Medical Communications.
VIDEO: A fib screening finds 5% of elderly undiagnosed
BARCELONA – Roughly 5% of people aged 75 years or older have undiagnosed atrial fibrillation, based on a population-based screening study in Sweden that has assessed nearly 7,000 people, Dr. Mårten Rosenqvist said during an interview at the annual congress of the European Society of Cardiology.
Once diagnosed with atrial fibrillation, all these people immediately qualified for anticoagulant treatment because of their age-related stroke risk. The StrokeStop study will follow all the screened people for 5 years, as well as a concurrently assembled cohort of unscreened controls, to determine the benefit from screening for preventing strokes. “If we can reduce the rate of stroke, it would be a reason to implement a national atrial fibrillation screening program” for all people aged 75 years and older, said Dr. Rosenqvist, professor of cardiology at the Karolinska Institute in Stockholm.
Dr. Rosenqvist said that he is a consultant to Zenicor, a company that markets an ECG-based device for diagnosing atrial fibrillation being used in the StrokeStop study. He also is a consultant to several drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
BARCELONA – Roughly 5% of people aged 75 years or older have undiagnosed atrial fibrillation, based on a population-based screening study in Sweden that has assessed nearly 7,000 people, Dr. Mårten Rosenqvist said during an interview at the annual congress of the European Society of Cardiology.
Once diagnosed with atrial fibrillation, all these people immediately qualified for anticoagulant treatment because of their age-related stroke risk. The StrokeStop study will follow all the screened people for 5 years, as well as a concurrently assembled cohort of unscreened controls, to determine the benefit from screening for preventing strokes. “If we can reduce the rate of stroke, it would be a reason to implement a national atrial fibrillation screening program” for all people aged 75 years and older, said Dr. Rosenqvist, professor of cardiology at the Karolinska Institute in Stockholm.
Dr. Rosenqvist said that he is a consultant to Zenicor, a company that markets an ECG-based device for diagnosing atrial fibrillation being used in the StrokeStop study. He also is a consultant to several drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
BARCELONA – Roughly 5% of people aged 75 years or older have undiagnosed atrial fibrillation, based on a population-based screening study in Sweden that has assessed nearly 7,000 people, Dr. Mårten Rosenqvist said during an interview at the annual congress of the European Society of Cardiology.
Once diagnosed with atrial fibrillation, all these people immediately qualified for anticoagulant treatment because of their age-related stroke risk. The StrokeStop study will follow all the screened people for 5 years, as well as a concurrently assembled cohort of unscreened controls, to determine the benefit from screening for preventing strokes. “If we can reduce the rate of stroke, it would be a reason to implement a national atrial fibrillation screening program” for all people aged 75 years and older, said Dr. Rosenqvist, professor of cardiology at the Karolinska Institute in Stockholm.
Dr. Rosenqvist said that he is a consultant to Zenicor, a company that markets an ECG-based device for diagnosing atrial fibrillation being used in the StrokeStop study. He also is a consultant to several drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter@mitchelzoler
AT THE ESC CONGRESS 2014
Pulmonary vein isolation alone may be best ablative procedure for persistent atrial fibrillation
BARCELONA – More extensive catheter ablation procedures offered no benefit over pulmonary vein isolation alone for persistent atrial fibrillation in the largest-ever randomized trial examining outcomes of the three most popular ablation strategies.
"This study, the STAR AF 2 trial, will force a change in thinking both in the guidelines as well as in clinical practice," Dr. Atul Verma predicted, in presenting the study findings at the annual congress of the European Society of Cardiology.
Because of a widespread belief that catheter ablation success rates are probably lower in persistent AF than in paroxysmal AF, guidelines suggest "operators should consider more ablation based on linear lesions or complex fractionated electrograms," in addition to pulmonary vein isolation, in treating patients with persistent AF (Heart Rhythm 2012;9:632-96). The guidelines noted, however, that there is little evidence to support this recommendation.
The STAR AF 2 trial was conducted to learn if more complex ablation procedures really do provide greater efficacy than pulmonary vein isolation (PVI) alone. The study included 589 patients at 48 centers in 12 countries. All patients had persistent AF refractory to at least one antiarrhythmic drug and were about to undergo their first-ever catheter ablation.
Participants were randomized 1:4:4 to PVI alone with the procedural endpoint of entrance and exit block by circular mapping catheter, or PVI plus mapping and ablation of complex fractionated electrograms during AF identified using a validated 3-D mapping system, or PVI plus a left atrial roof line and another line along the mitral valve isthmus with the endpoint of bidirectional block confirmed by prespecified pacing maneuvers.
Patients remained blinded as to which of the three treatments they received. They were prospectively followed with 24-hour Holter monitoring at 3, 6, 9, 12, and 18 months along with weekly transtelephonic monitoring transmissions or at any time they felt symptoms.
Successful PVI was achieved in 97% of patients, complex fractionated electrograms were eliminated in 80% of patients assigned to that strategy, and both target lines were blocked in 74% of patients who underwent linear ablation.
The primary outcome was freedom from a documented episode of AF lasting more than 30 seconds after one procedure with or without antiarrhythmic medication through 18 months. The rates were 59% with PVI only, 48% with PVI plus complex fractionated electrograms, and 44% with PVI and linear ablation. These rates weren’t significantly different.
There were downsides to the two more elaborate ablation strategies. Procedural times were roughly 1 hour longer. Moreover, mean fluoroscopy time was 29 minutes in the PVI-only group, compared with 41 and 42 minutes with the more complex procedures. That translates to 44% more radiation exposure for both operators and patients, with absolutely no resultant added benefit over PVI alone, noted Dr. Verma, an electrophysiologist at Southlake Regional Health Center in Newmarket, Ont.
Complication rates across the board in STAR AF 2 were among the lowest ever reported in a multicenter clinical trial of catheter ablation. Of note, however, the sole fatal complication was the result of an atrial esophageal fistula in a patient assigned to PVI plus electrogram ablation.
Discussant Dr. Jagmeet P. Singh, director of the cardiac resynchronization therapy program at Massachusetts General Hospital, Boston, called STAR AF 2 "a fantastic trial."
"This study surely advocates that less ablation is more – and less works quite well," he said, noting that the roughly 50% success rate at 18 months with PVI alone is comparable to prior published success rates in paroxysmal AF.
Discussant Dr. Paulus Kirchhof said his own recent informal survey of high-volume catheter ablation centers in the United States and Europe indicated roughly one-third do PVI alone for patients with persistent AF, one-third do PVI plus ablation of complex fractionated electrograms, and one-third do PVI plus linear ablation.
"So I would say this was a question at equipoise," added Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham (England).
Zeroing in on the added fluoroscopy time associated with the more complex ablation procedures, he noted that observational data suggest lengthier fluoroscopy may be associated with silent, subclinical brain lesions. Based upon the STAR 2 AF results, therefore, a reasonable strategy now for persistent AF is to do PVI alone, then wait and see what happens before considering additional ablation procedures later, he said.
"More importantly, I think this study shows we have to go back to the drawing board. The time pattern of AF – its duration, whether it’s paroxysmal or persistent, the left atrial size – all these things we believe identify patients who need more therapy, they may not actually help us. We just have to accept that not all patients with AF are the same, and that the pattern of AF does not discriminate so well. I think what we can really learn from this trial moving forward is that we need a clinical classification of AF patients. We have to define the patient who would benefit before we continue to develop ever-more intensive interventional strategies," he commented.
Factors worthy of further study as potential tools for separating AF patients into subgroups for treatment purposes include markers of atrial fibrosis, whether by imaging, blood, or ECG patterns; markers of parasympathetic/sympathetic imbalance; clinical markers of abnormal calcium metabolism; or blood markers, Dr. Kirchhof added.
The STAR AF 2 trial was funded by St. Jude Medical. Dr. Verma, Dr. Singh, and Dr. Kirchhof reported receiving grant support from St. Jude Medical as well as other pharmaceutical and medical device companies. In addition, Dr. Verma and Dr. Singh have served on advisory boards for St. Jude.
BARCELONA – More extensive catheter ablation procedures offered no benefit over pulmonary vein isolation alone for persistent atrial fibrillation in the largest-ever randomized trial examining outcomes of the three most popular ablation strategies.
"This study, the STAR AF 2 trial, will force a change in thinking both in the guidelines as well as in clinical practice," Dr. Atul Verma predicted, in presenting the study findings at the annual congress of the European Society of Cardiology.
Because of a widespread belief that catheter ablation success rates are probably lower in persistent AF than in paroxysmal AF, guidelines suggest "operators should consider more ablation based on linear lesions or complex fractionated electrograms," in addition to pulmonary vein isolation, in treating patients with persistent AF (Heart Rhythm 2012;9:632-96). The guidelines noted, however, that there is little evidence to support this recommendation.
The STAR AF 2 trial was conducted to learn if more complex ablation procedures really do provide greater efficacy than pulmonary vein isolation (PVI) alone. The study included 589 patients at 48 centers in 12 countries. All patients had persistent AF refractory to at least one antiarrhythmic drug and were about to undergo their first-ever catheter ablation.
Participants were randomized 1:4:4 to PVI alone with the procedural endpoint of entrance and exit block by circular mapping catheter, or PVI plus mapping and ablation of complex fractionated electrograms during AF identified using a validated 3-D mapping system, or PVI plus a left atrial roof line and another line along the mitral valve isthmus with the endpoint of bidirectional block confirmed by prespecified pacing maneuvers.
Patients remained blinded as to which of the three treatments they received. They were prospectively followed with 24-hour Holter monitoring at 3, 6, 9, 12, and 18 months along with weekly transtelephonic monitoring transmissions or at any time they felt symptoms.
Successful PVI was achieved in 97% of patients, complex fractionated electrograms were eliminated in 80% of patients assigned to that strategy, and both target lines were blocked in 74% of patients who underwent linear ablation.
The primary outcome was freedom from a documented episode of AF lasting more than 30 seconds after one procedure with or without antiarrhythmic medication through 18 months. The rates were 59% with PVI only, 48% with PVI plus complex fractionated electrograms, and 44% with PVI and linear ablation. These rates weren’t significantly different.
There were downsides to the two more elaborate ablation strategies. Procedural times were roughly 1 hour longer. Moreover, mean fluoroscopy time was 29 minutes in the PVI-only group, compared with 41 and 42 minutes with the more complex procedures. That translates to 44% more radiation exposure for both operators and patients, with absolutely no resultant added benefit over PVI alone, noted Dr. Verma, an electrophysiologist at Southlake Regional Health Center in Newmarket, Ont.
Complication rates across the board in STAR AF 2 were among the lowest ever reported in a multicenter clinical trial of catheter ablation. Of note, however, the sole fatal complication was the result of an atrial esophageal fistula in a patient assigned to PVI plus electrogram ablation.
Discussant Dr. Jagmeet P. Singh, director of the cardiac resynchronization therapy program at Massachusetts General Hospital, Boston, called STAR AF 2 "a fantastic trial."
"This study surely advocates that less ablation is more – and less works quite well," he said, noting that the roughly 50% success rate at 18 months with PVI alone is comparable to prior published success rates in paroxysmal AF.
Discussant Dr. Paulus Kirchhof said his own recent informal survey of high-volume catheter ablation centers in the United States and Europe indicated roughly one-third do PVI alone for patients with persistent AF, one-third do PVI plus ablation of complex fractionated electrograms, and one-third do PVI plus linear ablation.
"So I would say this was a question at equipoise," added Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham (England).
Zeroing in on the added fluoroscopy time associated with the more complex ablation procedures, he noted that observational data suggest lengthier fluoroscopy may be associated with silent, subclinical brain lesions. Based upon the STAR 2 AF results, therefore, a reasonable strategy now for persistent AF is to do PVI alone, then wait and see what happens before considering additional ablation procedures later, he said.
"More importantly, I think this study shows we have to go back to the drawing board. The time pattern of AF – its duration, whether it’s paroxysmal or persistent, the left atrial size – all these things we believe identify patients who need more therapy, they may not actually help us. We just have to accept that not all patients with AF are the same, and that the pattern of AF does not discriminate so well. I think what we can really learn from this trial moving forward is that we need a clinical classification of AF patients. We have to define the patient who would benefit before we continue to develop ever-more intensive interventional strategies," he commented.
Factors worthy of further study as potential tools for separating AF patients into subgroups for treatment purposes include markers of atrial fibrosis, whether by imaging, blood, or ECG patterns; markers of parasympathetic/sympathetic imbalance; clinical markers of abnormal calcium metabolism; or blood markers, Dr. Kirchhof added.
The STAR AF 2 trial was funded by St. Jude Medical. Dr. Verma, Dr. Singh, and Dr. Kirchhof reported receiving grant support from St. Jude Medical as well as other pharmaceutical and medical device companies. In addition, Dr. Verma and Dr. Singh have served on advisory boards for St. Jude.
BARCELONA – More extensive catheter ablation procedures offered no benefit over pulmonary vein isolation alone for persistent atrial fibrillation in the largest-ever randomized trial examining outcomes of the three most popular ablation strategies.
"This study, the STAR AF 2 trial, will force a change in thinking both in the guidelines as well as in clinical practice," Dr. Atul Verma predicted, in presenting the study findings at the annual congress of the European Society of Cardiology.
Because of a widespread belief that catheter ablation success rates are probably lower in persistent AF than in paroxysmal AF, guidelines suggest "operators should consider more ablation based on linear lesions or complex fractionated electrograms," in addition to pulmonary vein isolation, in treating patients with persistent AF (Heart Rhythm 2012;9:632-96). The guidelines noted, however, that there is little evidence to support this recommendation.
The STAR AF 2 trial was conducted to learn if more complex ablation procedures really do provide greater efficacy than pulmonary vein isolation (PVI) alone. The study included 589 patients at 48 centers in 12 countries. All patients had persistent AF refractory to at least one antiarrhythmic drug and were about to undergo their first-ever catheter ablation.
Participants were randomized 1:4:4 to PVI alone with the procedural endpoint of entrance and exit block by circular mapping catheter, or PVI plus mapping and ablation of complex fractionated electrograms during AF identified using a validated 3-D mapping system, or PVI plus a left atrial roof line and another line along the mitral valve isthmus with the endpoint of bidirectional block confirmed by prespecified pacing maneuvers.
Patients remained blinded as to which of the three treatments they received. They were prospectively followed with 24-hour Holter monitoring at 3, 6, 9, 12, and 18 months along with weekly transtelephonic monitoring transmissions or at any time they felt symptoms.
Successful PVI was achieved in 97% of patients, complex fractionated electrograms were eliminated in 80% of patients assigned to that strategy, and both target lines were blocked in 74% of patients who underwent linear ablation.
The primary outcome was freedom from a documented episode of AF lasting more than 30 seconds after one procedure with or without antiarrhythmic medication through 18 months. The rates were 59% with PVI only, 48% with PVI plus complex fractionated electrograms, and 44% with PVI and linear ablation. These rates weren’t significantly different.
There were downsides to the two more elaborate ablation strategies. Procedural times were roughly 1 hour longer. Moreover, mean fluoroscopy time was 29 minutes in the PVI-only group, compared with 41 and 42 minutes with the more complex procedures. That translates to 44% more radiation exposure for both operators and patients, with absolutely no resultant added benefit over PVI alone, noted Dr. Verma, an electrophysiologist at Southlake Regional Health Center in Newmarket, Ont.
Complication rates across the board in STAR AF 2 were among the lowest ever reported in a multicenter clinical trial of catheter ablation. Of note, however, the sole fatal complication was the result of an atrial esophageal fistula in a patient assigned to PVI plus electrogram ablation.
Discussant Dr. Jagmeet P. Singh, director of the cardiac resynchronization therapy program at Massachusetts General Hospital, Boston, called STAR AF 2 "a fantastic trial."
"This study surely advocates that less ablation is more – and less works quite well," he said, noting that the roughly 50% success rate at 18 months with PVI alone is comparable to prior published success rates in paroxysmal AF.
Discussant Dr. Paulus Kirchhof said his own recent informal survey of high-volume catheter ablation centers in the United States and Europe indicated roughly one-third do PVI alone for patients with persistent AF, one-third do PVI plus ablation of complex fractionated electrograms, and one-third do PVI plus linear ablation.
"So I would say this was a question at equipoise," added Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham (England).
Zeroing in on the added fluoroscopy time associated with the more complex ablation procedures, he noted that observational data suggest lengthier fluoroscopy may be associated with silent, subclinical brain lesions. Based upon the STAR 2 AF results, therefore, a reasonable strategy now for persistent AF is to do PVI alone, then wait and see what happens before considering additional ablation procedures later, he said.
"More importantly, I think this study shows we have to go back to the drawing board. The time pattern of AF – its duration, whether it’s paroxysmal or persistent, the left atrial size – all these things we believe identify patients who need more therapy, they may not actually help us. We just have to accept that not all patients with AF are the same, and that the pattern of AF does not discriminate so well. I think what we can really learn from this trial moving forward is that we need a clinical classification of AF patients. We have to define the patient who would benefit before we continue to develop ever-more intensive interventional strategies," he commented.
Factors worthy of further study as potential tools for separating AF patients into subgroups for treatment purposes include markers of atrial fibrosis, whether by imaging, blood, or ECG patterns; markers of parasympathetic/sympathetic imbalance; clinical markers of abnormal calcium metabolism; or blood markers, Dr. Kirchhof added.
The STAR AF 2 trial was funded by St. Jude Medical. Dr. Verma, Dr. Singh, and Dr. Kirchhof reported receiving grant support from St. Jude Medical as well as other pharmaceutical and medical device companies. In addition, Dr. Verma and Dr. Singh have served on advisory boards for St. Jude.
AT THE ESC CONGRESS 2014
Key clinical point: Pulmonary vein isolation alone may offer advantages over more elaborate procedures for persistent atrial fibrillation.
Major finding: The rates of freedom from a documented episode of AF lasting more than 30 seconds were 59% with pulmonary vein isolation only, 48% with PVI plus complex fractionated electrograms, and 44% with PVI and linear ablation.
Data source: The STAR AF 2 trial was a randomized, multicenter prospective study in which 589 patients with persistent AF were randomized to one of three popular catheter ablation strategies.
Disclosures: The study was funded by St. Jude Medical. The presenter has received research grants from and served on advisory boards for St. Jude and other medical device and pharmaceutical companies.
High-dose statins don’t prevent postop AF
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.
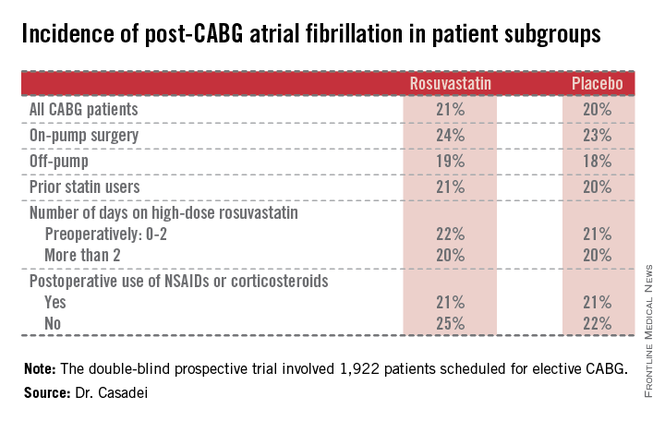
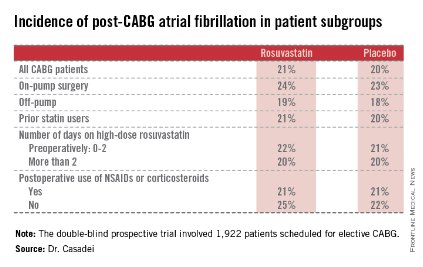
The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.

|
| Dr. Hiren Shah |
There are two key lessons from the results of the STICS trial. First, extrapolation of results from biochemical pathways and measured cellular markers does not always translate into meaningful clinical outcomes. Thus, it has long been known from several large trials that statin therapy effectively and rapidly lowers CRP levels both in hyper- and normocholesterolemic patients and that statins are effective in decreasing systemic inflammation. It has also been known that inflammation contributes to the development and maintenance of AF, so it was postulated that by improving endothelial nitric oxide availability, reducing inflammation, and decreasing oxidative stress, and through neurohormonal activation, statins would reduce the incidence of post-op AF. This link was so strong that clinical guidelines adopted limited data from small trials to make treatment recommendations.
This leads us to consider the second key lesson from this study. Trials with small sample size, even when combined across many other trials (1,300 patients were involved across 14 trials in this case), do not always yield reliable results, especially when they have significant limitations, notably not always being blind and having been performed in statin-naive patients only. The large, randomized, and well-designed STICS trial puts to rest an important issue, given the high prevalence of AF after cardiac surgery, which is associated with a longer length of stay, an increased risk of stroke, higher mortality, and greater costs, and should prompt us to consider further evaluation of different strategies to reduce this significant complication.
Dr. Hiren Shah is medical director of the medicine and cardiac telemetry hospitalist unit at Northwestern Memorial Hospital in Chicago and an adviser to Hospitalist News. He is the national chair of the Clinician Committee for ACP’s Initiative on Stroke Prevention and Atrial Fibrillation and is the lead physician for the Society of Hospital Medicine’s National Atrial Fibrillation Initiative.
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.

The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.
BARCELONA – Intensive perioperative statin therapy in patients undergoing CABG surgery doesn’t protect against postop atrial fibrillation or myocardial injury, according to a large randomized clinical trial hailed as the "definitive" study addressing this issue.
"There are many reasons why these patients should be put on statin treatment, but the prevention of postop complications is not one of them," Dr. Barbara Casadei said in presenting the findings of the Statin Therapy in Cardiac Surgery (STICS) trial at the annual congress of the European Society of Cardiology.

The STICS results are at odds with conventional wisdom. ESC guidelines give a favorable class IIa, level of evidence B recommendation that "statins should be considered for prevention of new-onset atrial fibrillation after coronary artery bypass grafting, either isolated or in combination with valvular interventions."
"STICS was a very carefully conducted, large scale, robust study that I think has definitely closed the door on this issue," commented Dr. Keith A.A. Fox, professor of cardiology at the University of Edinburgh and chair of the scientific and clinical program committee at ESC Congress 2014.
STICS was a double-blind prospective trial in which 1,922 patients scheduled for elective CABG were randomized to 20 mg per day of rosuvastatin (Crestor) or placebo starting up to 8 days prior to surgery and continued for 5 days postop. All participants were in sinus rhythm preoperatively, with no history of AF, said Dr. Casadei, professor of cardiovascular medicine at the University of Oxford, England.
The two coprimary endpoints in STICS were the incidence of new-onset AF during 5 days of postop Holter monitoring, and evidence of postop myocardial injury as demonstrated in serial troponin I assays.
Postop AF occurred in 21% of those given high-intensity therapy with rosuvastatin and 20% of placebo-treated controls. There was no subgroup where rosuvastatin was protective (see graphic).
Troponin I measurements obtained 6, 24, 48, and 120 hours postop showed areas under the curve that were superimposable in the two study groups, meaning perioperative high-dose statin therapy provided absolutely no protection against postop cardiac muscle injury.
Mean hospital length of stay and ICU time didn’t differ between the two groups, either.
The impetus for conducting STICS was recognition that the guidelines’ endorsement of perioperative high-dose statin therapy in conjunction with cardiac surgery was based upon a series of small randomized trials with serious limitations. Although the results of a meta-analysis of the 14 prior trials looked impressive at first glance – a 17% incidence of postop AF in statin-treated patients, compared with 30% in controls, for a near-halving of the risk of this important complication – these 14 studies totaled 1,300 patients, and there were many methodologic shortcomings.
The STICS researchers hypothesized that a large, well-designed trial – bigger than all previous studies combined – would shore up the previously shaky supporting evidence and perhaps provide grounds for statins to win a new indication from regulatory agencies. Post-CABG AF is associated with a doubled risk of stroke and mortality, and excess hospital costs of $8,000-$18,000 dollars per patient.
Discussant Dr. Paulus Kirchhof, a member of the task force that developed the current ESC guidelines (Europace 2010;12:1360-420), said those guidelines now clearly need to be revisited. Beyond that, he added, STICS provides important new contributions in understanding the pathophysiology of AF.
"We know that AF is caused by several vicious circles, and we believe that inflammation could influence those and cause AF. And we also thought that postop AF was the condition where inflammation plays the biggest role. Based upon the negative results with this anti-inflammatory intervention, I think we have to question this concept a bit," said Dr. Kirchhof, professor of cardiovascular sciences at the University of Birmingham, England.
Dr. Casadei countered that she’s not ready to write off postop inflammation entirely as a major trigger of new-onset AF following CABG.
"The inflammation is there. We know from experimental work in animals that there is a strong association between inflammation and postop atrial fibrillation, but whether the association is causal, I think, is still debated. However, it may be that the anti-inflammatory effect of statins is not sufficiently strong to actually prevent this complication," she said.
Discussant Dr. Steven Nissen praised STICS as "an outstanding trial."
"I also think there’s a terribly important lesson here, which is the power of self-delusion in medicine. When we base our guidelines on small, poorly controlled trials, we are often making mistakes. This is one of countless examples where when someone finally does a careful, thoughtful trial, we find out that something that people believe just isn’t true. We can’t cut corners with evidence. We need good randomized trials," declared Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
The STICS trial was funded primarily by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. In addition, Dr. Casadei reported receiving an unrestricted grant from AstraZeneca in conjunction with the trial.
AT THE ESC CONGRESS 2014
Key clinical point: Perioperative statin therapy in patients undergoing CABG failed to protect against new-onset postop atrial fibrillation.
Major finding: The incidence of postop atrial fibrillation within 5 days post-CABG was 21% in patients randomized to 20 mg/day of rosuvastatin and 20% in placebo-treated controls.
Data source: The multicenter STICS trial included 1,922 randomized patients scheduled for elective CABG.
Disclosures: STICS was funded by the British Heart Foundation, the Oxford Biomedical Research Center, and the UK Medical Research Council. The presenter reported having received a research grant from AstraZeneca.
VIDEO: Rivaroxaban provides advantages for cardioversion in AF
BARCELONA – The first-ever prospective, randomized trial of a novel oral anticoagulant in patients with atrial fibrillation undergoing elective cardioversion showed oral rivaroxaban at 20 mg once daily to be an effective and safe alternative to standard-of-care warfarin. But the study, known as X-VeRT, also showed rivaroxaban offers something in addition: more expeditious cardioversion amenable to reliable scheduling.
Dr. Riccardo Cappato, who presented the X-VeRT results at the annual congress of the European Society of Cardiology, explains in this video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – The first-ever prospective, randomized trial of a novel oral anticoagulant in patients with atrial fibrillation undergoing elective cardioversion showed oral rivaroxaban at 20 mg once daily to be an effective and safe alternative to standard-of-care warfarin. But the study, known as X-VeRT, also showed rivaroxaban offers something in addition: more expeditious cardioversion amenable to reliable scheduling.
Dr. Riccardo Cappato, who presented the X-VeRT results at the annual congress of the European Society of Cardiology, explains in this video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
BARCELONA – The first-ever prospective, randomized trial of a novel oral anticoagulant in patients with atrial fibrillation undergoing elective cardioversion showed oral rivaroxaban at 20 mg once daily to be an effective and safe alternative to standard-of-care warfarin. But the study, known as X-VeRT, also showed rivaroxaban offers something in addition: more expeditious cardioversion amenable to reliable scheduling.
Dr. Riccardo Cappato, who presented the X-VeRT results at the annual congress of the European Society of Cardiology, explains in this video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ESC CONGRESS 2014
Rivaroxaban shows advantages in AF cardioversion
BARCELONA – Rivaroxaban appears to be a safe and effective alternative to warfarin in patients with atrial fibrillation undergoing elective cardioversion, according to the findings of the first-ever prospective, randomized trial of a novel oral anticoagulant for this application.
Additionally, the X-VeRT trial showed that rivaroxaban (Xarelto) may offer an attractive, highly practical advantage over warfarin, the current guideline-recommended standard of care for cardioversion: Namely, rivaroxaban enabled patients to undergo the procedure more expeditiously, Dr. Riccardo Cappato noted in presenting the X-VeRT findings at the annual congress of the European Society of Cardiology.
Indeed, patients in the early-cardioversion arm of the trial safely underwent the procedure as early as 4 hours after taking their first 20-mg dose of rivaroxaban.
Moreover, patients in the delayed-conversion study arm, which required at least 3 consecutive weeks of effective oral anticoagulation preprocedurally, underwent cardioversion an average of 8 days earlier if randomized to rivaroxaban rather than to warfarin or another vitamin K antagonist. That’s because many warfarin-treated patients couldn’t maintain their international normalized ratio (INR) within the target range of 2.0-3.0 for 3 straight weeks, explained Dr. Cappato, professor of electrophysiology and chief of the arrhythmia and electrophysiology center at the University of Milan’s San Donato Polyclinic Hospital.
X-VeRT included 1,504 patients with nonvalvular atrial fibrillation (AF) at 141 centers in 16 countries. All were scheduled for elective cardioversion. They were randomized 2:1 to rivaroxaban 20 mg once daily or to warfarin at a dose adjusted to maintain an INR of 2.0-3.0.
Of those patients, 872 were assigned to an early-cardioversion strategy. Their cardioversion took place after 1-5 days on study medication, provided transesophageal echocardiography had ruled out a left atrial thrombus or they were already on chronic warfarin with their last three INRs in the target range. The other 632 patients were cardioverted using a delayed strategy in which they had to be on study medication for 3-8 weeks before the procedure.
The primary efficacy outcome in X-VeRT was the composite rate of stroke, TIA, peripheral embolism, MI, and cardiovascular death. The incidence was 0.51% in the rivaroxaban group and not statistically different at 1.02% in the warfarin group. The primary safety outcome – major bleeding – occurred in 0.6% of rivaroxaban-treated patients and 0.8% on warfarin.
The median time to cardioversion in the early-cardioversion strategy arm was similar regardless of which drug was used. Of note, however, in the delayed-strategy group, the median time to cardioversion was 22 days in patients on rivaroxaban, compared with 30 days with warfarin.
Of patients in the delayed-strategy group, 77% of those on rivaroxaban were cardioverted as scheduled, compared with 36% on warfarin. Only one patient in the rivaroxaban group was unable to undergo cardioversion before the 8-week cutoff because of inadequate anticoagulation as defined by less than 80% compliance in pill taking; in contrast, 95 warfarin-treated patients in the delayed-strategy group weren’t cardioverted because they missed the 8-week cutoff because of problematic INRs.
In an interview, ESC spokesman Dr. Jurrien Ten Berg predicted X-VeRT will be practice changing. He believes that on the basis of these study results, many physicians will view elective cardioversion as an excellent time to switch patients from warfarin, with all its inherent problems, to rivaroxaban, especially if they qualify for early cardioversion.
"I think this will absolutely change our policy. Here we’re talking about a once-daily pill that makes it possible to do a cardioversion early, and I think that’s a major advantage. If you delay the cardioversion, anything can happen. We know the vitamin K antagonists are unreliable, especially in the first weeks, when even if the INR is fine you don’t really know if the patient is well anticoagulated. For me, the totally of evidence, including the retrospective analyses of the large atrial fibrillation stroke prevention trials, is enough now to use a NOAC for several days and then do an early cardioversion," said Dr. Ten Berg, a cardiologist at St. Antonius Hospital, Nieuwegein, the Netherlands.
Discussant Dr. Christoph Bode called X-VeRT "a landmark trial" and "brilliant work."
"This should be included in the next update of the guidelines," said Dr. Bode, professor and chair of internal medicine and cardiology at the University of Freiburg, Germany.
However, Dr. Steven Nissen took a more skeptical, albeit clearly a minority, view.
"I think this study shows neither safety nor efficacy for this regimen. There were just a handful of events in both groups, too few to draw any conclusions. I don’t think the guidelines should be changed. I think the proper interpretation of the study is that it’s inconclusive," said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
Dr. Cappato noted in response that he’d stated at the outset that X-VeRT, even at more than 1,500 patients, was underpowered statistically. Event rates in well-anticoagulated patients undergoing cardioversion are so low that a study to establish noninferiority for rivaroxaban would require 25,000-30,000 participants, which is not going to happen.
"Many physicians are already switching to NOACs [novel oral anticoagulants] for cardioversion despite the absence of good evidence. We thought bringing forward this solid, methodologically sound information from X-VeRT would provide more consistent support for those who are doing this or considering it," the cardiologist said.
Simultaneous with his presentation in Barcelona, the X-VeRT (Explore the Efficacy and Safety of Once-Daily Oral Rivaroxaban for the Prevention of Cardiovascular Events in Patients With Nonvalvular Atrial Fibrillation Scheduled for Cardioversion) study was published online (Eur. Heart J. 2014 [doi:10.1093/eurheart/ehu367]).
Dr. Cappato reported receiving investigator fees from and serving as a consultant to and on speakers bureaus for numerous pharmaceutical companies, including Bayer HealthCare, which sponsored X-VeRT. Dr. Bode has received honoraria from Bayer HealthCare. Dr. Ten Berg and Dr. Nissen reported having no financial conflicts.
BARCELONA – Rivaroxaban appears to be a safe and effective alternative to warfarin in patients with atrial fibrillation undergoing elective cardioversion, according to the findings of the first-ever prospective, randomized trial of a novel oral anticoagulant for this application.
Additionally, the X-VeRT trial showed that rivaroxaban (Xarelto) may offer an attractive, highly practical advantage over warfarin, the current guideline-recommended standard of care for cardioversion: Namely, rivaroxaban enabled patients to undergo the procedure more expeditiously, Dr. Riccardo Cappato noted in presenting the X-VeRT findings at the annual congress of the European Society of Cardiology.
Indeed, patients in the early-cardioversion arm of the trial safely underwent the procedure as early as 4 hours after taking their first 20-mg dose of rivaroxaban.
Moreover, patients in the delayed-conversion study arm, which required at least 3 consecutive weeks of effective oral anticoagulation preprocedurally, underwent cardioversion an average of 8 days earlier if randomized to rivaroxaban rather than to warfarin or another vitamin K antagonist. That’s because many warfarin-treated patients couldn’t maintain their international normalized ratio (INR) within the target range of 2.0-3.0 for 3 straight weeks, explained Dr. Cappato, professor of electrophysiology and chief of the arrhythmia and electrophysiology center at the University of Milan’s San Donato Polyclinic Hospital.
X-VeRT included 1,504 patients with nonvalvular atrial fibrillation (AF) at 141 centers in 16 countries. All were scheduled for elective cardioversion. They were randomized 2:1 to rivaroxaban 20 mg once daily or to warfarin at a dose adjusted to maintain an INR of 2.0-3.0.
Of those patients, 872 were assigned to an early-cardioversion strategy. Their cardioversion took place after 1-5 days on study medication, provided transesophageal echocardiography had ruled out a left atrial thrombus or they were already on chronic warfarin with their last three INRs in the target range. The other 632 patients were cardioverted using a delayed strategy in which they had to be on study medication for 3-8 weeks before the procedure.
The primary efficacy outcome in X-VeRT was the composite rate of stroke, TIA, peripheral embolism, MI, and cardiovascular death. The incidence was 0.51% in the rivaroxaban group and not statistically different at 1.02% in the warfarin group. The primary safety outcome – major bleeding – occurred in 0.6% of rivaroxaban-treated patients and 0.8% on warfarin.
The median time to cardioversion in the early-cardioversion strategy arm was similar regardless of which drug was used. Of note, however, in the delayed-strategy group, the median time to cardioversion was 22 days in patients on rivaroxaban, compared with 30 days with warfarin.
Of patients in the delayed-strategy group, 77% of those on rivaroxaban were cardioverted as scheduled, compared with 36% on warfarin. Only one patient in the rivaroxaban group was unable to undergo cardioversion before the 8-week cutoff because of inadequate anticoagulation as defined by less than 80% compliance in pill taking; in contrast, 95 warfarin-treated patients in the delayed-strategy group weren’t cardioverted because they missed the 8-week cutoff because of problematic INRs.
In an interview, ESC spokesman Dr. Jurrien Ten Berg predicted X-VeRT will be practice changing. He believes that on the basis of these study results, many physicians will view elective cardioversion as an excellent time to switch patients from warfarin, with all its inherent problems, to rivaroxaban, especially if they qualify for early cardioversion.
"I think this will absolutely change our policy. Here we’re talking about a once-daily pill that makes it possible to do a cardioversion early, and I think that’s a major advantage. If you delay the cardioversion, anything can happen. We know the vitamin K antagonists are unreliable, especially in the first weeks, when even if the INR is fine you don’t really know if the patient is well anticoagulated. For me, the totally of evidence, including the retrospective analyses of the large atrial fibrillation stroke prevention trials, is enough now to use a NOAC for several days and then do an early cardioversion," said Dr. Ten Berg, a cardiologist at St. Antonius Hospital, Nieuwegein, the Netherlands.
Discussant Dr. Christoph Bode called X-VeRT "a landmark trial" and "brilliant work."
"This should be included in the next update of the guidelines," said Dr. Bode, professor and chair of internal medicine and cardiology at the University of Freiburg, Germany.
However, Dr. Steven Nissen took a more skeptical, albeit clearly a minority, view.
"I think this study shows neither safety nor efficacy for this regimen. There were just a handful of events in both groups, too few to draw any conclusions. I don’t think the guidelines should be changed. I think the proper interpretation of the study is that it’s inconclusive," said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
Dr. Cappato noted in response that he’d stated at the outset that X-VeRT, even at more than 1,500 patients, was underpowered statistically. Event rates in well-anticoagulated patients undergoing cardioversion are so low that a study to establish noninferiority for rivaroxaban would require 25,000-30,000 participants, which is not going to happen.
"Many physicians are already switching to NOACs [novel oral anticoagulants] for cardioversion despite the absence of good evidence. We thought bringing forward this solid, methodologically sound information from X-VeRT would provide more consistent support for those who are doing this or considering it," the cardiologist said.
Simultaneous with his presentation in Barcelona, the X-VeRT (Explore the Efficacy and Safety of Once-Daily Oral Rivaroxaban for the Prevention of Cardiovascular Events in Patients With Nonvalvular Atrial Fibrillation Scheduled for Cardioversion) study was published online (Eur. Heart J. 2014 [doi:10.1093/eurheart/ehu367]).
Dr. Cappato reported receiving investigator fees from and serving as a consultant to and on speakers bureaus for numerous pharmaceutical companies, including Bayer HealthCare, which sponsored X-VeRT. Dr. Bode has received honoraria from Bayer HealthCare. Dr. Ten Berg and Dr. Nissen reported having no financial conflicts.
BARCELONA – Rivaroxaban appears to be a safe and effective alternative to warfarin in patients with atrial fibrillation undergoing elective cardioversion, according to the findings of the first-ever prospective, randomized trial of a novel oral anticoagulant for this application.
Additionally, the X-VeRT trial showed that rivaroxaban (Xarelto) may offer an attractive, highly practical advantage over warfarin, the current guideline-recommended standard of care for cardioversion: Namely, rivaroxaban enabled patients to undergo the procedure more expeditiously, Dr. Riccardo Cappato noted in presenting the X-VeRT findings at the annual congress of the European Society of Cardiology.
Indeed, patients in the early-cardioversion arm of the trial safely underwent the procedure as early as 4 hours after taking their first 20-mg dose of rivaroxaban.
Moreover, patients in the delayed-conversion study arm, which required at least 3 consecutive weeks of effective oral anticoagulation preprocedurally, underwent cardioversion an average of 8 days earlier if randomized to rivaroxaban rather than to warfarin or another vitamin K antagonist. That’s because many warfarin-treated patients couldn’t maintain their international normalized ratio (INR) within the target range of 2.0-3.0 for 3 straight weeks, explained Dr. Cappato, professor of electrophysiology and chief of the arrhythmia and electrophysiology center at the University of Milan’s San Donato Polyclinic Hospital.
X-VeRT included 1,504 patients with nonvalvular atrial fibrillation (AF) at 141 centers in 16 countries. All were scheduled for elective cardioversion. They were randomized 2:1 to rivaroxaban 20 mg once daily or to warfarin at a dose adjusted to maintain an INR of 2.0-3.0.
Of those patients, 872 were assigned to an early-cardioversion strategy. Their cardioversion took place after 1-5 days on study medication, provided transesophageal echocardiography had ruled out a left atrial thrombus or they were already on chronic warfarin with their last three INRs in the target range. The other 632 patients were cardioverted using a delayed strategy in which they had to be on study medication for 3-8 weeks before the procedure.
The primary efficacy outcome in X-VeRT was the composite rate of stroke, TIA, peripheral embolism, MI, and cardiovascular death. The incidence was 0.51% in the rivaroxaban group and not statistically different at 1.02% in the warfarin group. The primary safety outcome – major bleeding – occurred in 0.6% of rivaroxaban-treated patients and 0.8% on warfarin.
The median time to cardioversion in the early-cardioversion strategy arm was similar regardless of which drug was used. Of note, however, in the delayed-strategy group, the median time to cardioversion was 22 days in patients on rivaroxaban, compared with 30 days with warfarin.
Of patients in the delayed-strategy group, 77% of those on rivaroxaban were cardioverted as scheduled, compared with 36% on warfarin. Only one patient in the rivaroxaban group was unable to undergo cardioversion before the 8-week cutoff because of inadequate anticoagulation as defined by less than 80% compliance in pill taking; in contrast, 95 warfarin-treated patients in the delayed-strategy group weren’t cardioverted because they missed the 8-week cutoff because of problematic INRs.
In an interview, ESC spokesman Dr. Jurrien Ten Berg predicted X-VeRT will be practice changing. He believes that on the basis of these study results, many physicians will view elective cardioversion as an excellent time to switch patients from warfarin, with all its inherent problems, to rivaroxaban, especially if they qualify for early cardioversion.
"I think this will absolutely change our policy. Here we’re talking about a once-daily pill that makes it possible to do a cardioversion early, and I think that’s a major advantage. If you delay the cardioversion, anything can happen. We know the vitamin K antagonists are unreliable, especially in the first weeks, when even if the INR is fine you don’t really know if the patient is well anticoagulated. For me, the totally of evidence, including the retrospective analyses of the large atrial fibrillation stroke prevention trials, is enough now to use a NOAC for several days and then do an early cardioversion," said Dr. Ten Berg, a cardiologist at St. Antonius Hospital, Nieuwegein, the Netherlands.
Discussant Dr. Christoph Bode called X-VeRT "a landmark trial" and "brilliant work."
"This should be included in the next update of the guidelines," said Dr. Bode, professor and chair of internal medicine and cardiology at the University of Freiburg, Germany.
However, Dr. Steven Nissen took a more skeptical, albeit clearly a minority, view.
"I think this study shows neither safety nor efficacy for this regimen. There were just a handful of events in both groups, too few to draw any conclusions. I don’t think the guidelines should be changed. I think the proper interpretation of the study is that it’s inconclusive," said Dr. Nissen, chair of the department of cardiovascular medicine at the Cleveland Clinic.
Dr. Cappato noted in response that he’d stated at the outset that X-VeRT, even at more than 1,500 patients, was underpowered statistically. Event rates in well-anticoagulated patients undergoing cardioversion are so low that a study to establish noninferiority for rivaroxaban would require 25,000-30,000 participants, which is not going to happen.
"Many physicians are already switching to NOACs [novel oral anticoagulants] for cardioversion despite the absence of good evidence. We thought bringing forward this solid, methodologically sound information from X-VeRT would provide more consistent support for those who are doing this or considering it," the cardiologist said.
Simultaneous with his presentation in Barcelona, the X-VeRT (Explore the Efficacy and Safety of Once-Daily Oral Rivaroxaban for the Prevention of Cardiovascular Events in Patients With Nonvalvular Atrial Fibrillation Scheduled for Cardioversion) study was published online (Eur. Heart J. 2014 [doi:10.1093/eurheart/ehu367]).
Dr. Cappato reported receiving investigator fees from and serving as a consultant to and on speakers bureaus for numerous pharmaceutical companies, including Bayer HealthCare, which sponsored X-VeRT. Dr. Bode has received honoraria from Bayer HealthCare. Dr. Ten Berg and Dr. Nissen reported having no financial conflicts.
AT THE ESC CONGRESS 2014
Key clinical point: Patients in atrial fibrillation were able to safely undergo cardioversion as early as 4 hours after taking their first 20-mg dose of oral rivaroxaban.
Major finding: Patients with AF assigned to rivaroxaban in conjunction with cardioversion had a 0.51% rate of major cardiovascular events, similar to the 1.02% rate in patients on warfarin or other vitamin K antagonists. These rates weren’t statistically different; nor were the major bleeding rates in the two groups.
Data source: X-VeRT was a randomized, prospective, open-label, phase IIIb clinical trial involving 1,504 patients at 141 centers in 16 countries.
Disclosures: The X-VeRT trial was sponsored by Bayer HealthCare. The presenter serves as a consultant to and on speakers bureaus for Bayer and other pharmaceutical companies.
Diabetes increases risk of atrial fibrillation
BARCELONA – Adults with diabetes mellitus are at increased risk of subsequent new-onset atrial fibrillation – and the younger the age at diabetes onset, the greater the likelihood of developing the arrhythmia.
That’s the key finding from a Danish national registry study in which all 5,168,416 Danish adults without atrial fibrillation in 1996 were followed through 2012 for development of atrial fibrillation (AF). The study population included 75,197 Danes with diabetes at baseline and another 235,327 who developed the disease during follow-up, Dr. Jannik L. Pallisgaard explained at the annual congress of the European Society of Cardiology.
During follow-up, 5.6% of those with diabetes and 3.3% of those without diabetes developed AF. The mean time from diabetes onset to AF onset was 5 years, reported Dr. Pallisgaard of the University of Copenhagen.
"What was particularly interesting, I think, is that we found the youngest patients were the group at highest risk" of developing AF, he said. "We suggest that starting at the onset of diabetes, routine pulse palpation, ECGs, and focused patient interviews asking about any signs of atrial fibrillation could prove beneficial in detecting the arrhythmia."
The incidence rate ratio for developing AF per 1,000 person-years of follow-up was roughly 2.5-fold greater in 18- to 39-year-olds with diabetes than in their nondiabetic peers. From this peak rate in young adults, the magnitude of relative risk dropped in stepwise fashion with age: The variability in risk was lower in 40- to 60-year-old diabetics than in the 18- to 39-year olds and lower still in 65- to 74-year olds. Variability in the incidence rate ratio finally bottomed out at a still statistically significant 1.3-fold increased risk of developing AF in diabetic individuals ages 75 and older compared to their nondiabetic peers.
Dr. Pallisgaard noted that while the relative risk of developing AF was greatest in the 18- to 39-year-olds, the absolute number of new cases of AF was far greater in older patients because there were so many more of them with diabetes. He cautioned that as the obesity epidemic leads to more and more patients developing type 2 diabetes at younger ages, more cases of AF can be expected in young adults.
Dr. Pallisgaard cited two likely mechanisms underlying the observed increased risk of AF in diabetic patients: left ventricular hypertrophy and vascular inflammation, which are both often present in the diabetic population.
He reported having no financial conflicts regarding this study, conducted with Danish institutional research funds.
BARCELONA – Adults with diabetes mellitus are at increased risk of subsequent new-onset atrial fibrillation – and the younger the age at diabetes onset, the greater the likelihood of developing the arrhythmia.
That’s the key finding from a Danish national registry study in which all 5,168,416 Danish adults without atrial fibrillation in 1996 were followed through 2012 for development of atrial fibrillation (AF). The study population included 75,197 Danes with diabetes at baseline and another 235,327 who developed the disease during follow-up, Dr. Jannik L. Pallisgaard explained at the annual congress of the European Society of Cardiology.
During follow-up, 5.6% of those with diabetes and 3.3% of those without diabetes developed AF. The mean time from diabetes onset to AF onset was 5 years, reported Dr. Pallisgaard of the University of Copenhagen.
"What was particularly interesting, I think, is that we found the youngest patients were the group at highest risk" of developing AF, he said. "We suggest that starting at the onset of diabetes, routine pulse palpation, ECGs, and focused patient interviews asking about any signs of atrial fibrillation could prove beneficial in detecting the arrhythmia."
The incidence rate ratio for developing AF per 1,000 person-years of follow-up was roughly 2.5-fold greater in 18- to 39-year-olds with diabetes than in their nondiabetic peers. From this peak rate in young adults, the magnitude of relative risk dropped in stepwise fashion with age: The variability in risk was lower in 40- to 60-year-old diabetics than in the 18- to 39-year olds and lower still in 65- to 74-year olds. Variability in the incidence rate ratio finally bottomed out at a still statistically significant 1.3-fold increased risk of developing AF in diabetic individuals ages 75 and older compared to their nondiabetic peers.
Dr. Pallisgaard noted that while the relative risk of developing AF was greatest in the 18- to 39-year-olds, the absolute number of new cases of AF was far greater in older patients because there were so many more of them with diabetes. He cautioned that as the obesity epidemic leads to more and more patients developing type 2 diabetes at younger ages, more cases of AF can be expected in young adults.
Dr. Pallisgaard cited two likely mechanisms underlying the observed increased risk of AF in diabetic patients: left ventricular hypertrophy and vascular inflammation, which are both often present in the diabetic population.
He reported having no financial conflicts regarding this study, conducted with Danish institutional research funds.
BARCELONA – Adults with diabetes mellitus are at increased risk of subsequent new-onset atrial fibrillation – and the younger the age at diabetes onset, the greater the likelihood of developing the arrhythmia.
That’s the key finding from a Danish national registry study in which all 5,168,416 Danish adults without atrial fibrillation in 1996 were followed through 2012 for development of atrial fibrillation (AF). The study population included 75,197 Danes with diabetes at baseline and another 235,327 who developed the disease during follow-up, Dr. Jannik L. Pallisgaard explained at the annual congress of the European Society of Cardiology.
During follow-up, 5.6% of those with diabetes and 3.3% of those without diabetes developed AF. The mean time from diabetes onset to AF onset was 5 years, reported Dr. Pallisgaard of the University of Copenhagen.
"What was particularly interesting, I think, is that we found the youngest patients were the group at highest risk" of developing AF, he said. "We suggest that starting at the onset of diabetes, routine pulse palpation, ECGs, and focused patient interviews asking about any signs of atrial fibrillation could prove beneficial in detecting the arrhythmia."
The incidence rate ratio for developing AF per 1,000 person-years of follow-up was roughly 2.5-fold greater in 18- to 39-year-olds with diabetes than in their nondiabetic peers. From this peak rate in young adults, the magnitude of relative risk dropped in stepwise fashion with age: The variability in risk was lower in 40- to 60-year-old diabetics than in the 18- to 39-year olds and lower still in 65- to 74-year olds. Variability in the incidence rate ratio finally bottomed out at a still statistically significant 1.3-fold increased risk of developing AF in diabetic individuals ages 75 and older compared to their nondiabetic peers.
Dr. Pallisgaard noted that while the relative risk of developing AF was greatest in the 18- to 39-year-olds, the absolute number of new cases of AF was far greater in older patients because there were so many more of them with diabetes. He cautioned that as the obesity epidemic leads to more and more patients developing type 2 diabetes at younger ages, more cases of AF can be expected in young adults.
Dr. Pallisgaard cited two likely mechanisms underlying the observed increased risk of AF in diabetic patients: left ventricular hypertrophy and vascular inflammation, which are both often present in the diabetic population.
He reported having no financial conflicts regarding this study, conducted with Danish institutional research funds.
AT THE ESC CONGRESS 2014
Key clinical point: Starting at the onset of diabetes, routine pulse palpation, ECGs, and patient interviews focused on signs of atrial fibrillation might improve detection of the arrhythmia.
Major finding: During follow-up, 5.6% of those with diabetes and 3.3% of those without diabetes developed AF.
Data source: This was a national registry study including all of the nearly 5.2 million Danish adults without atrial fibrillation in 1996. Follow-up ran through 2012.
Disclosures: The presenter reported having no financial conflicts regarding this study, funded by Danish institutional research grants.
Novel anticoagulants given to 60% of newly diagnosed AF patients
Novel oral anticoagulants introduced since October 2010 have been adopted into clinical practice rapidly, and within 2.5 years were prescribed for more than 60% of patients with newly diagnosed atrial fibrillation, according to a report published online in the American Journal of Medicine.
Further, the new drugs are being prescribed for a different patient population from that indicated by the clinical trials on which Food and Drug Administration (FDA) approval was based. Specifically, dabigatran, rivaroxaban, and apixaban are selectively prescribed for younger, healthier men who have high incomes and reside in wealthier communities, reported Dr. Nihar R. Desai of the division of pharmacoepidemiology and pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
In what they described as the first study to evaluate real-world use of all novel anticoagulants, researchers found that the rapid uptake of the drugs as first-line therapy for atrial fibrillation (AF) was accompanied by a marked decline in the use of warfarin. The difference in total costs between the generic warfarin and the proprietary dabigatran, rivaroxaban, or apixaban totaled $900 per patient during the first 6 months alone, which "translates into billions of dollars at the national level."
This has important economic implications for patients, payers, and the health care system. The impending FDA approval of new factor Xa inhibitors such as edoxaban and betrixaban will likely further complicate the picture, the researchers said.
The researchers analyzed nationwide medical and prescription claims data for 6,893 adults covered by Aetna who had newly diagnosed nonvalvular AF and were prescribed an oral anticoagulant between October 2010 and June 2013. The direct thrombin inhibitor dabigatran was approved in October 2010, and the factor Xa inhibitors rivaroxaban and apixaban were approved in November 2011 and December 2012.
During the study period, these patients filled 45,472 prescriptions for oral anticoagulants: 57.7% for warfarin, 32.8% for dabigatran, 9.3% for rivaroxaban, and 0.1% for apixaban. However, these figures don’t reflect the trend over time in which prescriptions for the newer agents rapidly displaced those for warfarin. Within 1 year of appearing on the market, dabigatran was equally likely to be prescribed as warfarin was for new AF patients. Its use as a first-line therapy dropped considerably a year later, after reports of excess rates of myocardial infarction and serious and fatal bleeding events in patients taking dabigatran. But at that point rivaroxaban had been introduced, and it soon overtook both dabigatran and warfarin as first-line therapy for AF. (Apixaban accounted for 2% of new anticoagulant prescriptions as of 6 months after it was approved, which is the most recent date for which such statistics were available.)
Simultaneously, the costs of oral anticoagulants rose dramatically, with the new agents accounting for 98% of that escalation. It is estimated that insurers spend $5.82 million every month for all agents combined, and that warfarin accounts for only $0.43 million of that. Similarly, patient out-of-pocket spending for all oral anticoagulants combined was estimated to be $1.3 million per month, with warfarin accounting for $3,844 of that total.
Viewed from another perspective, the average combined patient and insurer spending for anticoagulants during the first 6 months of therapy for warfarin was $122, dabigatran $1,053, and rivaroxaban $1,084. "This represents a difference of more than $900 per patient," said Dr. Desai, who is also at the Center for Outcomes Research and Evaluation, Yale-New Haven Health Services, and his associates.
The greatest benefit from novel anticoagulants is among patients at the highest risk for stroke or systemic embolization, as measured by higher scores on CHADS (Congestive Heart Failure, Hypertension, Age of 75 years or more, Diabetes Mellitus, and Stroke) and HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol) assessments. This is also the patient population targeted in the clinical trials that formed the basis for FDA approval.
But in this study, 46% of patients with low CHADS and HAS-BLED scores were initially prescribed the novel anticoagulants, compared with 26% of those with high scores. "For every 1-point increase in CHADS, patients were 20% less likely to receive a novel anticoagulant. Similarly, for every 1-point increase in HAS-BLED, patients were 18% less likely to receive a novel anticoagulant.
"In addition, women were 24% less likely to be initiated on a novel oral anticoagulant as compared with men. [And] there was a significant, stepwise increase in the likelihood of receiving a novel agent with progressively increasing neighborhood household income, compared with a median household income of $50,000 or less," the investigators said (Amer. J. Med. 2014 May 20 [doi: 10.1016/j.amjmed.2014.05.013]).
"These findings point to the need to conduct ongoing surveillance of the adoption of new agents into clinical practice, as well as the need for robust, real-world comparative-effectiveness analyses of these medications, to enable patients and providers to make informed decisions about their relative benefit, safety, and cost-effectiveness," Dr. Desai and his associates said.
This study was funded by an unrestricted research grant from CVS Caremark. Dr. Desai’s associates reported ties to CVS Caremark and Aetna.
It is not surprising that the novel oral anticoagulants appear to be supplanting warfarin as first-line therapy for nonvalvular AF. The newer drugs are much easier to use because they don’t require frequent monitoring of clotting parameters, require no dietary restrictions, and have simple and straightforward dosing.
I expect the use of these novel anticoagulants – and others soon to be approved – to increase over time, particularly once they become generic and cost gradually becomes less of an issue.
Dr. Joseph S. Alpert is professor of medicine at the University of Arizona, Tucson, and the editor-in-chief of the American Journal of Medicine. Dr. Alpert made these remarks in an editorial (Amer. J. Med. 2014 Aug. 8 [doi: 10.1016/amjmed.2014.07.028]) accompanying Dr. Desai’s report. He reported cochairing the data monitoring committees for two of the large clinical trials that led to FDA approval of rivaroxaban.
It is not surprising that the novel oral anticoagulants appear to be supplanting warfarin as first-line therapy for nonvalvular AF. The newer drugs are much easier to use because they don’t require frequent monitoring of clotting parameters, require no dietary restrictions, and have simple and straightforward dosing.
I expect the use of these novel anticoagulants – and others soon to be approved – to increase over time, particularly once they become generic and cost gradually becomes less of an issue.
Dr. Joseph S. Alpert is professor of medicine at the University of Arizona, Tucson, and the editor-in-chief of the American Journal of Medicine. Dr. Alpert made these remarks in an editorial (Amer. J. Med. 2014 Aug. 8 [doi: 10.1016/amjmed.2014.07.028]) accompanying Dr. Desai’s report. He reported cochairing the data monitoring committees for two of the large clinical trials that led to FDA approval of rivaroxaban.
It is not surprising that the novel oral anticoagulants appear to be supplanting warfarin as first-line therapy for nonvalvular AF. The newer drugs are much easier to use because they don’t require frequent monitoring of clotting parameters, require no dietary restrictions, and have simple and straightforward dosing.
I expect the use of these novel anticoagulants – and others soon to be approved – to increase over time, particularly once they become generic and cost gradually becomes less of an issue.
Dr. Joseph S. Alpert is professor of medicine at the University of Arizona, Tucson, and the editor-in-chief of the American Journal of Medicine. Dr. Alpert made these remarks in an editorial (Amer. J. Med. 2014 Aug. 8 [doi: 10.1016/amjmed.2014.07.028]) accompanying Dr. Desai’s report. He reported cochairing the data monitoring committees for two of the large clinical trials that led to FDA approval of rivaroxaban.
Novel oral anticoagulants introduced since October 2010 have been adopted into clinical practice rapidly, and within 2.5 years were prescribed for more than 60% of patients with newly diagnosed atrial fibrillation, according to a report published online in the American Journal of Medicine.
Further, the new drugs are being prescribed for a different patient population from that indicated by the clinical trials on which Food and Drug Administration (FDA) approval was based. Specifically, dabigatran, rivaroxaban, and apixaban are selectively prescribed for younger, healthier men who have high incomes and reside in wealthier communities, reported Dr. Nihar R. Desai of the division of pharmacoepidemiology and pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
In what they described as the first study to evaluate real-world use of all novel anticoagulants, researchers found that the rapid uptake of the drugs as first-line therapy for atrial fibrillation (AF) was accompanied by a marked decline in the use of warfarin. The difference in total costs between the generic warfarin and the proprietary dabigatran, rivaroxaban, or apixaban totaled $900 per patient during the first 6 months alone, which "translates into billions of dollars at the national level."
This has important economic implications for patients, payers, and the health care system. The impending FDA approval of new factor Xa inhibitors such as edoxaban and betrixaban will likely further complicate the picture, the researchers said.
The researchers analyzed nationwide medical and prescription claims data for 6,893 adults covered by Aetna who had newly diagnosed nonvalvular AF and were prescribed an oral anticoagulant between October 2010 and June 2013. The direct thrombin inhibitor dabigatran was approved in October 2010, and the factor Xa inhibitors rivaroxaban and apixaban were approved in November 2011 and December 2012.
During the study period, these patients filled 45,472 prescriptions for oral anticoagulants: 57.7% for warfarin, 32.8% for dabigatran, 9.3% for rivaroxaban, and 0.1% for apixaban. However, these figures don’t reflect the trend over time in which prescriptions for the newer agents rapidly displaced those for warfarin. Within 1 year of appearing on the market, dabigatran was equally likely to be prescribed as warfarin was for new AF patients. Its use as a first-line therapy dropped considerably a year later, after reports of excess rates of myocardial infarction and serious and fatal bleeding events in patients taking dabigatran. But at that point rivaroxaban had been introduced, and it soon overtook both dabigatran and warfarin as first-line therapy for AF. (Apixaban accounted for 2% of new anticoagulant prescriptions as of 6 months after it was approved, which is the most recent date for which such statistics were available.)
Simultaneously, the costs of oral anticoagulants rose dramatically, with the new agents accounting for 98% of that escalation. It is estimated that insurers spend $5.82 million every month for all agents combined, and that warfarin accounts for only $0.43 million of that. Similarly, patient out-of-pocket spending for all oral anticoagulants combined was estimated to be $1.3 million per month, with warfarin accounting for $3,844 of that total.
Viewed from another perspective, the average combined patient and insurer spending for anticoagulants during the first 6 months of therapy for warfarin was $122, dabigatran $1,053, and rivaroxaban $1,084. "This represents a difference of more than $900 per patient," said Dr. Desai, who is also at the Center for Outcomes Research and Evaluation, Yale-New Haven Health Services, and his associates.
The greatest benefit from novel anticoagulants is among patients at the highest risk for stroke or systemic embolization, as measured by higher scores on CHADS (Congestive Heart Failure, Hypertension, Age of 75 years or more, Diabetes Mellitus, and Stroke) and HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol) assessments. This is also the patient population targeted in the clinical trials that formed the basis for FDA approval.
But in this study, 46% of patients with low CHADS and HAS-BLED scores were initially prescribed the novel anticoagulants, compared with 26% of those with high scores. "For every 1-point increase in CHADS, patients were 20% less likely to receive a novel anticoagulant. Similarly, for every 1-point increase in HAS-BLED, patients were 18% less likely to receive a novel anticoagulant.
"In addition, women were 24% less likely to be initiated on a novel oral anticoagulant as compared with men. [And] there was a significant, stepwise increase in the likelihood of receiving a novel agent with progressively increasing neighborhood household income, compared with a median household income of $50,000 or less," the investigators said (Amer. J. Med. 2014 May 20 [doi: 10.1016/j.amjmed.2014.05.013]).
"These findings point to the need to conduct ongoing surveillance of the adoption of new agents into clinical practice, as well as the need for robust, real-world comparative-effectiveness analyses of these medications, to enable patients and providers to make informed decisions about their relative benefit, safety, and cost-effectiveness," Dr. Desai and his associates said.
This study was funded by an unrestricted research grant from CVS Caremark. Dr. Desai’s associates reported ties to CVS Caremark and Aetna.
Novel oral anticoagulants introduced since October 2010 have been adopted into clinical practice rapidly, and within 2.5 years were prescribed for more than 60% of patients with newly diagnosed atrial fibrillation, according to a report published online in the American Journal of Medicine.
Further, the new drugs are being prescribed for a different patient population from that indicated by the clinical trials on which Food and Drug Administration (FDA) approval was based. Specifically, dabigatran, rivaroxaban, and apixaban are selectively prescribed for younger, healthier men who have high incomes and reside in wealthier communities, reported Dr. Nihar R. Desai of the division of pharmacoepidemiology and pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
In what they described as the first study to evaluate real-world use of all novel anticoagulants, researchers found that the rapid uptake of the drugs as first-line therapy for atrial fibrillation (AF) was accompanied by a marked decline in the use of warfarin. The difference in total costs between the generic warfarin and the proprietary dabigatran, rivaroxaban, or apixaban totaled $900 per patient during the first 6 months alone, which "translates into billions of dollars at the national level."
This has important economic implications for patients, payers, and the health care system. The impending FDA approval of new factor Xa inhibitors such as edoxaban and betrixaban will likely further complicate the picture, the researchers said.
The researchers analyzed nationwide medical and prescription claims data for 6,893 adults covered by Aetna who had newly diagnosed nonvalvular AF and were prescribed an oral anticoagulant between October 2010 and June 2013. The direct thrombin inhibitor dabigatran was approved in October 2010, and the factor Xa inhibitors rivaroxaban and apixaban were approved in November 2011 and December 2012.
During the study period, these patients filled 45,472 prescriptions for oral anticoagulants: 57.7% for warfarin, 32.8% for dabigatran, 9.3% for rivaroxaban, and 0.1% for apixaban. However, these figures don’t reflect the trend over time in which prescriptions for the newer agents rapidly displaced those for warfarin. Within 1 year of appearing on the market, dabigatran was equally likely to be prescribed as warfarin was for new AF patients. Its use as a first-line therapy dropped considerably a year later, after reports of excess rates of myocardial infarction and serious and fatal bleeding events in patients taking dabigatran. But at that point rivaroxaban had been introduced, and it soon overtook both dabigatran and warfarin as first-line therapy for AF. (Apixaban accounted for 2% of new anticoagulant prescriptions as of 6 months after it was approved, which is the most recent date for which such statistics were available.)
Simultaneously, the costs of oral anticoagulants rose dramatically, with the new agents accounting for 98% of that escalation. It is estimated that insurers spend $5.82 million every month for all agents combined, and that warfarin accounts for only $0.43 million of that. Similarly, patient out-of-pocket spending for all oral anticoagulants combined was estimated to be $1.3 million per month, with warfarin accounting for $3,844 of that total.
Viewed from another perspective, the average combined patient and insurer spending for anticoagulants during the first 6 months of therapy for warfarin was $122, dabigatran $1,053, and rivaroxaban $1,084. "This represents a difference of more than $900 per patient," said Dr. Desai, who is also at the Center for Outcomes Research and Evaluation, Yale-New Haven Health Services, and his associates.
The greatest benefit from novel anticoagulants is among patients at the highest risk for stroke or systemic embolization, as measured by higher scores on CHADS (Congestive Heart Failure, Hypertension, Age of 75 years or more, Diabetes Mellitus, and Stroke) and HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol) assessments. This is also the patient population targeted in the clinical trials that formed the basis for FDA approval.
But in this study, 46% of patients with low CHADS and HAS-BLED scores were initially prescribed the novel anticoagulants, compared with 26% of those with high scores. "For every 1-point increase in CHADS, patients were 20% less likely to receive a novel anticoagulant. Similarly, for every 1-point increase in HAS-BLED, patients were 18% less likely to receive a novel anticoagulant.
"In addition, women were 24% less likely to be initiated on a novel oral anticoagulant as compared with men. [And] there was a significant, stepwise increase in the likelihood of receiving a novel agent with progressively increasing neighborhood household income, compared with a median household income of $50,000 or less," the investigators said (Amer. J. Med. 2014 May 20 [doi: 10.1016/j.amjmed.2014.05.013]).
"These findings point to the need to conduct ongoing surveillance of the adoption of new agents into clinical practice, as well as the need for robust, real-world comparative-effectiveness analyses of these medications, to enable patients and providers to make informed decisions about their relative benefit, safety, and cost-effectiveness," Dr. Desai and his associates said.
This study was funded by an unrestricted research grant from CVS Caremark. Dr. Desai’s associates reported ties to CVS Caremark and Aetna.
FROM THE AMERICAN JOURNAL OF MEDICINE
Key clinical point: Novel anticoagulants are being prescribed for younger men, a different patient population from that indicated by the clinical trials on which FDA approval was based.
Major finding: The average combined patient and insurer spending for oral anticoagulants during the first 6 months of therapy was $122 for warfarin, $1,053 for dabigatran, and $1,084 for rivaroxaban.
Data source: A retrospective, longitudinal analysis of nationwide Aetna prescription claims data for 6,893 adults with nonvalvular AF who initiated oral anticoagulants in 2010-2013.
Disclosures: This study was funded by an unrestricted research grant from CVS Caremark. Dr. Desai’s associates reported ties to CVS Caremark and Aetna.
Evidence questioned in debate over monitoring dabigatran levels to avert bleeds
The manufacturer of the oral anticoagulant dabigatran "withheld analyses that calculate how many major bleeds dose adjustment could prevent," the BMJ charges in an article based on internal documents released during litigation in the United States and freedom of information act requests obtained by the journal.
Boehringer Ingelheim "found that if the plasma levels of the drug were measured and the dose was adjusted accordingly major bleeds could be reduced by 30%-40%, compared with well-controlled warfarin," according to the article (BMJ 2014;349:g4670), which was published along with an editorial and an analysis. The article further stated that the manufacturer "has failed to share with regulators information about the potential benefits of monitoring anticoagulant activity and adjusting the dose to make sure the drug is working as safely and effectively as possible."
In the analysis, Thomas Moore, senior scientist at the Institute for Safe Medication Practices, Horsham, Pa., and his coauthors said that "the bleeding risk of dabigatran can be reduced and efficacy improved by individualizing the dose in patients based on plasma level, age, and kidney function" (BMJ 2014;349:g4517[doi:10.1136/bmj.g4517]).
Dr. Rita Redberg and Dr. Blake Charlton of the University of California, San Francisco, wrote in an accompanying editorial that the analysis "illuminates a lack of transparency about the safety of unmonitored dabigatran, compounded by the drug’s fickle pharmacokinetics, which can cause a fivefold variation of plasma concentration." (BMJ 2014;349:g4681 [doi:10.1136/bmj.g4681]).
The BMJ article, written by Deborah Cohen, includes responses from the manufacturer of dabigatran (Pradaxa).
Boehringer Ingelheim officials have asserted that no data were withheld. Additionally, the company has released a statement calling the BMJ article "biased" and "misleading."
Dabigatran is a direct thrombin inhibitor that was approved in the United States in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation at a fixed dose of 150 mg or 75 mg twice a day, with the lower dose recommended for those with renal impairment. Since its initial approval, dabigatran has gained indications for treating and reducing the risk of recurrence of deep venous thrombosis and pulmonary embolism.
The drug’s approval was based on the results of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial, which compared the drug to warfarin in 18,113 patients with nonvalvular atrial fibrillation and at least one other risk factor for stroke. The annual rate of stroke and systemic embolism in RE-LY was 1.5% in the patients on the 110-mg twice daily dose, 1.1% in those on the 150-mg twice daily dose, and 1.7% among those on warfarin. The risk reductions with dabigatran were 10% and 35% for the 110-mg and 150-mg doses, respectively, compared with warfarin.
"Our company has provided regulators with the complete data set and analyses of clinical evidence demonstrating Pradaxa’s benefits and safety," Boehringer Ingelheim said in its statement. "Contrary to the BMJ’s accusation that BI withheld analyses, here are the facts: In 2012, our scientists performed preliminary, exploratory simulations with mathematical models to understand whether dose adjustments based on plasma concentrations might further improve Pradaxa’s benefits and safety. Because the simulations did not offer reliable predictions of actual patient outcomes, they were not provided to regulators. However, all of the data that was used for the simulations had already been provided."
Dr. Sanjay Kaul, who was on the Food and Drug Administration’s advisory panel that recommended approval of dabigatran, said in an interview that the FDA reviewers raised questions at that meeting about the utility of using plasma concentrations to monitor individual subjects and adjust dose based on dabigatran concentrations. "But they also acknowledged an exposure-response relationship existed that demonstrated that going from the 10th to 90th percentile of dabigatran concentration (23 to 283 ng/mL) reduced the probability of having a stroke by 50% (from 1% to 0.5%) while increasing sixfold (from 0.3% to 1.8%) the probability of having major bleeding within 1 year."
It is well known that plasma concentration is one of the factors – among others, such as age, renal function, or history of stroke – that affect the benefit-risk balance, said Dr. Kaul, a cardiologist at Cedars-Sinai Medical Center, Los Angeles. Even in patients with moderate to severe renal dysfunction (up to creatinine clearance of 15-30 mL/min) and elevated plasma concentrations, benefit still exceeds risk.
"To what degree monitoring drug levels could potentially optimize benefit-risk balance remains an open question. For example, benefit-risk balance for dabigatran 150 mg vs 110 mg was not predicted by pharmacokinetic/pharmacodynamic modeling. Based on the PK/PD modeling, one stroke would be prevented at the cost of three extra major bleeds using 150 mg , compared with 110 mg. The RE-LY data indicate four strokes prevented and three major bleeding events incurred with the 150-mg dose as compared with the 110-mg dose," he said. "There is quite a discordance in predicted vs. observed benefit-risk balance."
Therefore, "while monitoring drug levels to optimize benefit-risk balance has intuitive appeal, given the complex exposure-response relationship and confounding effects of demographic variables, this should remain an area of active investigation. It is not ready for prime time to inform or guide clinical practice," Dr. Kaul said.
Dr. Kaul disclosed that he is a consultant for Boehringer Ingelheim and has equity interest in Johnson and Johnson.
At press time, the FDA had not responded to a request for a comment on the BMJ investigation.
The manufacturer of the oral anticoagulant dabigatran "withheld analyses that calculate how many major bleeds dose adjustment could prevent," the BMJ charges in an article based on internal documents released during litigation in the United States and freedom of information act requests obtained by the journal.
Boehringer Ingelheim "found that if the plasma levels of the drug were measured and the dose was adjusted accordingly major bleeds could be reduced by 30%-40%, compared with well-controlled warfarin," according to the article (BMJ 2014;349:g4670), which was published along with an editorial and an analysis. The article further stated that the manufacturer "has failed to share with regulators information about the potential benefits of monitoring anticoagulant activity and adjusting the dose to make sure the drug is working as safely and effectively as possible."
In the analysis, Thomas Moore, senior scientist at the Institute for Safe Medication Practices, Horsham, Pa., and his coauthors said that "the bleeding risk of dabigatran can be reduced and efficacy improved by individualizing the dose in patients based on plasma level, age, and kidney function" (BMJ 2014;349:g4517[doi:10.1136/bmj.g4517]).
Dr. Rita Redberg and Dr. Blake Charlton of the University of California, San Francisco, wrote in an accompanying editorial that the analysis "illuminates a lack of transparency about the safety of unmonitored dabigatran, compounded by the drug’s fickle pharmacokinetics, which can cause a fivefold variation of plasma concentration." (BMJ 2014;349:g4681 [doi:10.1136/bmj.g4681]).
The BMJ article, written by Deborah Cohen, includes responses from the manufacturer of dabigatran (Pradaxa).
Boehringer Ingelheim officials have asserted that no data were withheld. Additionally, the company has released a statement calling the BMJ article "biased" and "misleading."
Dabigatran is a direct thrombin inhibitor that was approved in the United States in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation at a fixed dose of 150 mg or 75 mg twice a day, with the lower dose recommended for those with renal impairment. Since its initial approval, dabigatran has gained indications for treating and reducing the risk of recurrence of deep venous thrombosis and pulmonary embolism.
The drug’s approval was based on the results of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial, which compared the drug to warfarin in 18,113 patients with nonvalvular atrial fibrillation and at least one other risk factor for stroke. The annual rate of stroke and systemic embolism in RE-LY was 1.5% in the patients on the 110-mg twice daily dose, 1.1% in those on the 150-mg twice daily dose, and 1.7% among those on warfarin. The risk reductions with dabigatran were 10% and 35% for the 110-mg and 150-mg doses, respectively, compared with warfarin.
"Our company has provided regulators with the complete data set and analyses of clinical evidence demonstrating Pradaxa’s benefits and safety," Boehringer Ingelheim said in its statement. "Contrary to the BMJ’s accusation that BI withheld analyses, here are the facts: In 2012, our scientists performed preliminary, exploratory simulations with mathematical models to understand whether dose adjustments based on plasma concentrations might further improve Pradaxa’s benefits and safety. Because the simulations did not offer reliable predictions of actual patient outcomes, they were not provided to regulators. However, all of the data that was used for the simulations had already been provided."
Dr. Sanjay Kaul, who was on the Food and Drug Administration’s advisory panel that recommended approval of dabigatran, said in an interview that the FDA reviewers raised questions at that meeting about the utility of using plasma concentrations to monitor individual subjects and adjust dose based on dabigatran concentrations. "But they also acknowledged an exposure-response relationship existed that demonstrated that going from the 10th to 90th percentile of dabigatran concentration (23 to 283 ng/mL) reduced the probability of having a stroke by 50% (from 1% to 0.5%) while increasing sixfold (from 0.3% to 1.8%) the probability of having major bleeding within 1 year."
It is well known that plasma concentration is one of the factors – among others, such as age, renal function, or history of stroke – that affect the benefit-risk balance, said Dr. Kaul, a cardiologist at Cedars-Sinai Medical Center, Los Angeles. Even in patients with moderate to severe renal dysfunction (up to creatinine clearance of 15-30 mL/min) and elevated plasma concentrations, benefit still exceeds risk.
"To what degree monitoring drug levels could potentially optimize benefit-risk balance remains an open question. For example, benefit-risk balance for dabigatran 150 mg vs 110 mg was not predicted by pharmacokinetic/pharmacodynamic modeling. Based on the PK/PD modeling, one stroke would be prevented at the cost of three extra major bleeds using 150 mg , compared with 110 mg. The RE-LY data indicate four strokes prevented and three major bleeding events incurred with the 150-mg dose as compared with the 110-mg dose," he said. "There is quite a discordance in predicted vs. observed benefit-risk balance."
Therefore, "while monitoring drug levels to optimize benefit-risk balance has intuitive appeal, given the complex exposure-response relationship and confounding effects of demographic variables, this should remain an area of active investigation. It is not ready for prime time to inform or guide clinical practice," Dr. Kaul said.
Dr. Kaul disclosed that he is a consultant for Boehringer Ingelheim and has equity interest in Johnson and Johnson.
At press time, the FDA had not responded to a request for a comment on the BMJ investigation.
The manufacturer of the oral anticoagulant dabigatran "withheld analyses that calculate how many major bleeds dose adjustment could prevent," the BMJ charges in an article based on internal documents released during litigation in the United States and freedom of information act requests obtained by the journal.
Boehringer Ingelheim "found that if the plasma levels of the drug were measured and the dose was adjusted accordingly major bleeds could be reduced by 30%-40%, compared with well-controlled warfarin," according to the article (BMJ 2014;349:g4670), which was published along with an editorial and an analysis. The article further stated that the manufacturer "has failed to share with regulators information about the potential benefits of monitoring anticoagulant activity and adjusting the dose to make sure the drug is working as safely and effectively as possible."
In the analysis, Thomas Moore, senior scientist at the Institute for Safe Medication Practices, Horsham, Pa., and his coauthors said that "the bleeding risk of dabigatran can be reduced and efficacy improved by individualizing the dose in patients based on plasma level, age, and kidney function" (BMJ 2014;349:g4517[doi:10.1136/bmj.g4517]).
Dr. Rita Redberg and Dr. Blake Charlton of the University of California, San Francisco, wrote in an accompanying editorial that the analysis "illuminates a lack of transparency about the safety of unmonitored dabigatran, compounded by the drug’s fickle pharmacokinetics, which can cause a fivefold variation of plasma concentration." (BMJ 2014;349:g4681 [doi:10.1136/bmj.g4681]).
The BMJ article, written by Deborah Cohen, includes responses from the manufacturer of dabigatran (Pradaxa).
Boehringer Ingelheim officials have asserted that no data were withheld. Additionally, the company has released a statement calling the BMJ article "biased" and "misleading."
Dabigatran is a direct thrombin inhibitor that was approved in the United States in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation at a fixed dose of 150 mg or 75 mg twice a day, with the lower dose recommended for those with renal impairment. Since its initial approval, dabigatran has gained indications for treating and reducing the risk of recurrence of deep venous thrombosis and pulmonary embolism.
The drug’s approval was based on the results of the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) trial, which compared the drug to warfarin in 18,113 patients with nonvalvular atrial fibrillation and at least one other risk factor for stroke. The annual rate of stroke and systemic embolism in RE-LY was 1.5% in the patients on the 110-mg twice daily dose, 1.1% in those on the 150-mg twice daily dose, and 1.7% among those on warfarin. The risk reductions with dabigatran were 10% and 35% for the 110-mg and 150-mg doses, respectively, compared with warfarin.
"Our company has provided regulators with the complete data set and analyses of clinical evidence demonstrating Pradaxa’s benefits and safety," Boehringer Ingelheim said in its statement. "Contrary to the BMJ’s accusation that BI withheld analyses, here are the facts: In 2012, our scientists performed preliminary, exploratory simulations with mathematical models to understand whether dose adjustments based on plasma concentrations might further improve Pradaxa’s benefits and safety. Because the simulations did not offer reliable predictions of actual patient outcomes, they were not provided to regulators. However, all of the data that was used for the simulations had already been provided."
Dr. Sanjay Kaul, who was on the Food and Drug Administration’s advisory panel that recommended approval of dabigatran, said in an interview that the FDA reviewers raised questions at that meeting about the utility of using plasma concentrations to monitor individual subjects and adjust dose based on dabigatran concentrations. "But they also acknowledged an exposure-response relationship existed that demonstrated that going from the 10th to 90th percentile of dabigatran concentration (23 to 283 ng/mL) reduced the probability of having a stroke by 50% (from 1% to 0.5%) while increasing sixfold (from 0.3% to 1.8%) the probability of having major bleeding within 1 year."
It is well known that plasma concentration is one of the factors – among others, such as age, renal function, or history of stroke – that affect the benefit-risk balance, said Dr. Kaul, a cardiologist at Cedars-Sinai Medical Center, Los Angeles. Even in patients with moderate to severe renal dysfunction (up to creatinine clearance of 15-30 mL/min) and elevated plasma concentrations, benefit still exceeds risk.
"To what degree monitoring drug levels could potentially optimize benefit-risk balance remains an open question. For example, benefit-risk balance for dabigatran 150 mg vs 110 mg was not predicted by pharmacokinetic/pharmacodynamic modeling. Based on the PK/PD modeling, one stroke would be prevented at the cost of three extra major bleeds using 150 mg , compared with 110 mg. The RE-LY data indicate four strokes prevented and three major bleeding events incurred with the 150-mg dose as compared with the 110-mg dose," he said. "There is quite a discordance in predicted vs. observed benefit-risk balance."
Therefore, "while monitoring drug levels to optimize benefit-risk balance has intuitive appeal, given the complex exposure-response relationship and confounding effects of demographic variables, this should remain an area of active investigation. It is not ready for prime time to inform or guide clinical practice," Dr. Kaul said.
Dr. Kaul disclosed that he is a consultant for Boehringer Ingelheim and has equity interest in Johnson and Johnson.
At press time, the FDA had not responded to a request for a comment on the BMJ investigation.
Apixaban approved for treating DVT, pulmonary embolism and reducing risk of recurrence
The oral factor Xa inhibitor apixaban is now approved for the treatment of deep vein thrombosis and pulmonary embolism, and for reducing the risk of recurrent DVT and PE following initial treatment, the manufacturers have announced.
This approval is based on the results of the AMPLIFY and AMPLIFY-EXT studies, according to the statement issued by Bristol-Myers Squibb and Pfizer Aug. 21.
Apixaban, initially approved in 2012 and marketed as Eliquis, is already approved for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation and for the prophylaxis of DVT, "which may lead to PE," after hip or knee surgery. The recommended dose for the treatment of DVT and PE is 10 mg twice a day for 7 days, followed by 5 mg twice a day. The recommended dose for reducing the risk for recurrent DVT and PE, after initial therapy, is 2.5 mg twice a day.
AMPLIFY was a noninferiority study of about 5,200 patients with symptomatic DVT or PE. It compared apixaban (10 mg twice a day for 1 week, followed by 5 mg twice a day for 6 months) with standard care using enoxaparin (1 mg/kg administered subcutaneously twice a day for at least 5 days [until the international normalized ratio was at least 2], followed by warfarin for at least 5 days [target INR range of 2.0-3.0] for 6 months). The primary efficacy endpoint, a composite of recurrent symptomatic VTE or VTE-related death over 6 months, was comparable in the two groups: 2.3% among those on apixaban and 2.7% among those on enoxaparin/warfarin, according to the prescribing information.
The primary safety endpoint, major bleeding, was 0.6% among those on apixaban vs. 1.8% among those on enoxaparin/warfarin, a statistically significant difference. Rates of clinically relevant nonmajor bleeding, a secondary safety endpoint, was 3.9% among those on apixaban vs. 8% among those on enoxaparin/warfarin.
In Amplify-EXT, almost 2,500 patients who had received anticoagulant therapy for DVT and/or PE for 6-12 months and had not had a recurrent event were randomized to treatment with apixaban 2.5 or 5 mg twice a day, or placebo, followed for a mean of almost 1 year. The rate of recurrent VTE or all-cause death was 3.8% among those on 2.5 mg twice daily and 4.2% among those on 5 mg twice daily, vs. 11.6% among those on placebo; the effects of both doses were significantly more effective in reducing risk than was placebo.
In this study, the rate of bleeding-related adverse reactions was 13.3% among those on apixaban vs. 8.7% among those on placebo. The rate of major bleeding was 0.2% among those on the 2.5 mg twice-daily dose and 0.1% among those on the 5 mg twice-daily dose, vs. 0.5% among those on placebo.
The apixaban label includes a boxed warning about an increased risk of spinal/epidural hematoma in patients undergoing neuraxial anesthesia or spinal puncture while on the drug.
Serious adverse events associated with apixaban should be reported to the FDA’s MedWatch program.
The oral factor Xa inhibitor apixaban is now approved for the treatment of deep vein thrombosis and pulmonary embolism, and for reducing the risk of recurrent DVT and PE following initial treatment, the manufacturers have announced.
This approval is based on the results of the AMPLIFY and AMPLIFY-EXT studies, according to the statement issued by Bristol-Myers Squibb and Pfizer Aug. 21.
Apixaban, initially approved in 2012 and marketed as Eliquis, is already approved for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation and for the prophylaxis of DVT, "which may lead to PE," after hip or knee surgery. The recommended dose for the treatment of DVT and PE is 10 mg twice a day for 7 days, followed by 5 mg twice a day. The recommended dose for reducing the risk for recurrent DVT and PE, after initial therapy, is 2.5 mg twice a day.
AMPLIFY was a noninferiority study of about 5,200 patients with symptomatic DVT or PE. It compared apixaban (10 mg twice a day for 1 week, followed by 5 mg twice a day for 6 months) with standard care using enoxaparin (1 mg/kg administered subcutaneously twice a day for at least 5 days [until the international normalized ratio was at least 2], followed by warfarin for at least 5 days [target INR range of 2.0-3.0] for 6 months). The primary efficacy endpoint, a composite of recurrent symptomatic VTE or VTE-related death over 6 months, was comparable in the two groups: 2.3% among those on apixaban and 2.7% among those on enoxaparin/warfarin, according to the prescribing information.
The primary safety endpoint, major bleeding, was 0.6% among those on apixaban vs. 1.8% among those on enoxaparin/warfarin, a statistically significant difference. Rates of clinically relevant nonmajor bleeding, a secondary safety endpoint, was 3.9% among those on apixaban vs. 8% among those on enoxaparin/warfarin.
In Amplify-EXT, almost 2,500 patients who had received anticoagulant therapy for DVT and/or PE for 6-12 months and had not had a recurrent event were randomized to treatment with apixaban 2.5 or 5 mg twice a day, or placebo, followed for a mean of almost 1 year. The rate of recurrent VTE or all-cause death was 3.8% among those on 2.5 mg twice daily and 4.2% among those on 5 mg twice daily, vs. 11.6% among those on placebo; the effects of both doses were significantly more effective in reducing risk than was placebo.
In this study, the rate of bleeding-related adverse reactions was 13.3% among those on apixaban vs. 8.7% among those on placebo. The rate of major bleeding was 0.2% among those on the 2.5 mg twice-daily dose and 0.1% among those on the 5 mg twice-daily dose, vs. 0.5% among those on placebo.
The apixaban label includes a boxed warning about an increased risk of spinal/epidural hematoma in patients undergoing neuraxial anesthesia or spinal puncture while on the drug.
Serious adverse events associated with apixaban should be reported to the FDA’s MedWatch program.
The oral factor Xa inhibitor apixaban is now approved for the treatment of deep vein thrombosis and pulmonary embolism, and for reducing the risk of recurrent DVT and PE following initial treatment, the manufacturers have announced.
This approval is based on the results of the AMPLIFY and AMPLIFY-EXT studies, according to the statement issued by Bristol-Myers Squibb and Pfizer Aug. 21.
Apixaban, initially approved in 2012 and marketed as Eliquis, is already approved for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation and for the prophylaxis of DVT, "which may lead to PE," after hip or knee surgery. The recommended dose for the treatment of DVT and PE is 10 mg twice a day for 7 days, followed by 5 mg twice a day. The recommended dose for reducing the risk for recurrent DVT and PE, after initial therapy, is 2.5 mg twice a day.
AMPLIFY was a noninferiority study of about 5,200 patients with symptomatic DVT or PE. It compared apixaban (10 mg twice a day for 1 week, followed by 5 mg twice a day for 6 months) with standard care using enoxaparin (1 mg/kg administered subcutaneously twice a day for at least 5 days [until the international normalized ratio was at least 2], followed by warfarin for at least 5 days [target INR range of 2.0-3.0] for 6 months). The primary efficacy endpoint, a composite of recurrent symptomatic VTE or VTE-related death over 6 months, was comparable in the two groups: 2.3% among those on apixaban and 2.7% among those on enoxaparin/warfarin, according to the prescribing information.
The primary safety endpoint, major bleeding, was 0.6% among those on apixaban vs. 1.8% among those on enoxaparin/warfarin, a statistically significant difference. Rates of clinically relevant nonmajor bleeding, a secondary safety endpoint, was 3.9% among those on apixaban vs. 8% among those on enoxaparin/warfarin.
In Amplify-EXT, almost 2,500 patients who had received anticoagulant therapy for DVT and/or PE for 6-12 months and had not had a recurrent event were randomized to treatment with apixaban 2.5 or 5 mg twice a day, or placebo, followed for a mean of almost 1 year. The rate of recurrent VTE or all-cause death was 3.8% among those on 2.5 mg twice daily and 4.2% among those on 5 mg twice daily, vs. 11.6% among those on placebo; the effects of both doses were significantly more effective in reducing risk than was placebo.
In this study, the rate of bleeding-related adverse reactions was 13.3% among those on apixaban vs. 8.7% among those on placebo. The rate of major bleeding was 0.2% among those on the 2.5 mg twice-daily dose and 0.1% among those on the 5 mg twice-daily dose, vs. 0.5% among those on placebo.
The apixaban label includes a boxed warning about an increased risk of spinal/epidural hematoma in patients undergoing neuraxial anesthesia or spinal puncture while on the drug.
Serious adverse events associated with apixaban should be reported to the FDA’s MedWatch program.
Catheter-directed thrombolysis for leg DVTs held risky
Catheter-directed thrombolysis plus anticoagulation is no more effective than anticoagulation alone in preventing in-hospital death among adults who have lower-extremity proximal deep vein thrombosis, according to a nationwide observational study reported online July 21 in JAMA Internal Medicine.
However, catheter-directed thrombolysis carries higher risks, particularly serious bleeding risks such as intracranial hemorrhage, than does anticoagulation alone, and it costs nearly three times as much money. These findings highlight the need for randomized trials "to evaluate the magnitude of the effect of catheter-directed thrombolysis on ... mortality, postthrombotic syndrome, and recurrence of DVT [deep vein thrombosis]. In the absence of such data, it may be reasonable to restrict this form of therapy to those patients who have a low bleeding risk and a high risk for postthrombotic syndrome, such as patients with iliofemoral DVT," said Dr. Riyaz Bashir of the division of cardiovascular diseases, Temple University, Philadelphia, and his associates.
Conflicting data from several small studies as to the safety and effectiveness of catheter-directed thrombolysis have led professional societies to devise conflicting recommendations for its use: CHEST (the American College of Chest Physicians) advises against using the procedure, while the American Heart Association recommends it as a first-line therapy for certain patients. "We sought to assess real-world comparative-safety outcomes in patients with proximal and caval DVT who underwent catheter-directed thrombolysis plus anticoagulation with a group treated with anticoagulation alone using risk-adjusted propensity-score matching," the investigators said.
They analyzed data from an Agency for Healthcare Research and Quality administrative database of patient discharges from approximately 1,000 nonfederal acute-care hospitals per year for a 6-year period. They identified 90,618 patients with a discharge diagnosis of proximal DVT; propensity-score matching yielded 3,594 well-matched patients in each study group. In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), Dr. Bashir and his associates said (JAMA Intern. Med. 2014 July 21 [doi:10.1001/jamainternmed.2014.3415]).
However, rates of blood transfusion (11.1% vs. 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention. And patients in the catheter-directed thrombolysis group required significantly longer hospitalizations (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs. $28,164). "It is imperative that the magnitude of benefit from catheter-directed therapy be substantial to justify the increased initial resource utilization and bleeding risks of this therapy," the investigators noted.
Dr. Steven Q. Simpson, FCCP, comments: This observational, real-world study provides more practical information, I believe, than we would obtain with a controlled trial for two drugs/techniques that are already FDA–approved for this purpose. We are able to infer how the drug/technique affects short-term mortality outcomes and financial costs beyond the strict selection criteria and adherence to a tight protocol that a trial requires. However, the study leaves us with the question of how catheter-directed thrombolysis compares with anticoagulation in the more immediately life-threatening setting of massive pulmonary embolism. Additionally, proponents of catheter-directed thrombolysis suggest that it reduces the pain and suffering of postphlebitic syndrome, an outcome not addressed by this study.
Dr. Steven Q. Simpson, FCCP, comments: This observational, real-world study provides more practical information, I believe, than we would obtain with a controlled trial for two drugs/techniques that are already FDA–approved for this purpose. We are able to infer how the drug/technique affects short-term mortality outcomes and financial costs beyond the strict selection criteria and adherence to a tight protocol that a trial requires. However, the study leaves us with the question of how catheter-directed thrombolysis compares with anticoagulation in the more immediately life-threatening setting of massive pulmonary embolism. Additionally, proponents of catheter-directed thrombolysis suggest that it reduces the pain and suffering of postphlebitic syndrome, an outcome not addressed by this study.
Dr. Steven Q. Simpson, FCCP, comments: This observational, real-world study provides more practical information, I believe, than we would obtain with a controlled trial for two drugs/techniques that are already FDA–approved for this purpose. We are able to infer how the drug/technique affects short-term mortality outcomes and financial costs beyond the strict selection criteria and adherence to a tight protocol that a trial requires. However, the study leaves us with the question of how catheter-directed thrombolysis compares with anticoagulation in the more immediately life-threatening setting of massive pulmonary embolism. Additionally, proponents of catheter-directed thrombolysis suggest that it reduces the pain and suffering of postphlebitic syndrome, an outcome not addressed by this study.
Catheter-directed thrombolysis plus anticoagulation is no more effective than anticoagulation alone in preventing in-hospital death among adults who have lower-extremity proximal deep vein thrombosis, according to a nationwide observational study reported online July 21 in JAMA Internal Medicine.
However, catheter-directed thrombolysis carries higher risks, particularly serious bleeding risks such as intracranial hemorrhage, than does anticoagulation alone, and it costs nearly three times as much money. These findings highlight the need for randomized trials "to evaluate the magnitude of the effect of catheter-directed thrombolysis on ... mortality, postthrombotic syndrome, and recurrence of DVT [deep vein thrombosis]. In the absence of such data, it may be reasonable to restrict this form of therapy to those patients who have a low bleeding risk and a high risk for postthrombotic syndrome, such as patients with iliofemoral DVT," said Dr. Riyaz Bashir of the division of cardiovascular diseases, Temple University, Philadelphia, and his associates.
Conflicting data from several small studies as to the safety and effectiveness of catheter-directed thrombolysis have led professional societies to devise conflicting recommendations for its use: CHEST (the American College of Chest Physicians) advises against using the procedure, while the American Heart Association recommends it as a first-line therapy for certain patients. "We sought to assess real-world comparative-safety outcomes in patients with proximal and caval DVT who underwent catheter-directed thrombolysis plus anticoagulation with a group treated with anticoagulation alone using risk-adjusted propensity-score matching," the investigators said.
They analyzed data from an Agency for Healthcare Research and Quality administrative database of patient discharges from approximately 1,000 nonfederal acute-care hospitals per year for a 6-year period. They identified 90,618 patients with a discharge diagnosis of proximal DVT; propensity-score matching yielded 3,594 well-matched patients in each study group. In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), Dr. Bashir and his associates said (JAMA Intern. Med. 2014 July 21 [doi:10.1001/jamainternmed.2014.3415]).
However, rates of blood transfusion (11.1% vs. 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention. And patients in the catheter-directed thrombolysis group required significantly longer hospitalizations (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs. $28,164). "It is imperative that the magnitude of benefit from catheter-directed therapy be substantial to justify the increased initial resource utilization and bleeding risks of this therapy," the investigators noted.
Catheter-directed thrombolysis plus anticoagulation is no more effective than anticoagulation alone in preventing in-hospital death among adults who have lower-extremity proximal deep vein thrombosis, according to a nationwide observational study reported online July 21 in JAMA Internal Medicine.
However, catheter-directed thrombolysis carries higher risks, particularly serious bleeding risks such as intracranial hemorrhage, than does anticoagulation alone, and it costs nearly three times as much money. These findings highlight the need for randomized trials "to evaluate the magnitude of the effect of catheter-directed thrombolysis on ... mortality, postthrombotic syndrome, and recurrence of DVT [deep vein thrombosis]. In the absence of such data, it may be reasonable to restrict this form of therapy to those patients who have a low bleeding risk and a high risk for postthrombotic syndrome, such as patients with iliofemoral DVT," said Dr. Riyaz Bashir of the division of cardiovascular diseases, Temple University, Philadelphia, and his associates.
Conflicting data from several small studies as to the safety and effectiveness of catheter-directed thrombolysis have led professional societies to devise conflicting recommendations for its use: CHEST (the American College of Chest Physicians) advises against using the procedure, while the American Heart Association recommends it as a first-line therapy for certain patients. "We sought to assess real-world comparative-safety outcomes in patients with proximal and caval DVT who underwent catheter-directed thrombolysis plus anticoagulation with a group treated with anticoagulation alone using risk-adjusted propensity-score matching," the investigators said.
They analyzed data from an Agency for Healthcare Research and Quality administrative database of patient discharges from approximately 1,000 nonfederal acute-care hospitals per year for a 6-year period. They identified 90,618 patients with a discharge diagnosis of proximal DVT; propensity-score matching yielded 3,594 well-matched patients in each study group. In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), Dr. Bashir and his associates said (JAMA Intern. Med. 2014 July 21 [doi:10.1001/jamainternmed.2014.3415]).
However, rates of blood transfusion (11.1% vs. 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention. And patients in the catheter-directed thrombolysis group required significantly longer hospitalizations (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs. $28,164). "It is imperative that the magnitude of benefit from catheter-directed therapy be substantial to justify the increased initial resource utilization and bleeding risks of this therapy," the investigators noted.
Key clinical point: Catheter-directed thrombolysis carries higher risks and may not improve outcomes for proximal DVT patients.
Major finding: In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), but rates of blood transfusion (11.1% vs 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention.
Data source: A propensity-matched analysis comparing the effectiveness and safety profiles between catheter-directed thrombolysis plus anticoagulation and anticoagulation alone in 3,594 adults across the country hospitalized with lower-extremity proximal DVT during a 6-year period.
Disclosures: This study was supported by Temple University Hospital, Philadelphia. Dr. Bashir reported no financial conflicts of interest; his associates reported ties to Covidien, Health Systems Networks, and Insight Telehealth.