User login
Anticoagulation Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving treatment options for preventing stroke, acute coronary events, deep vein thrombosis, and pulmonary embolism in at-risk patients. The Anticoagulation Hub is powered by Frontline Medical Communications.
Plan helped atrial fibrillation patients safely switch oral anticoagulants
A detailed transition plan protected most atrial fibrillation patients from strokes and major bleeding when they switched oral anticoagulants, researchers reported in the August issue of the Journal of the American College of Cardiology.
Following such a plan could help patients safely switch anticoagulants in clinical practice, said Dr. Christian Ruff at Harvard Medical School in Boston and his associates.
They reported an analysis from the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which randomized more than 21,000 AF patients at high risk of stroke to either the investigational factor Xa inhibitor edoxaban or warfarin. Edoxaban was shown noninferior to warfarin in preventing stroke or systemic embolism (N. Engl. J. Med. 2013; 369:2093-104).
In the past, researchers from the ROCKET AF and ARISTOTLE trials reported excess strokes and bleeding events after patients were switched from blinded factor Xa inhibitors to open-label antithrombotics, the investigators said.
In response, ENGAGE AF-TIMI 48 patients and their physicians decided together whether to switch to an open-label vitamin K antagonist (VKA) or a newer oral anticoagulant at the end of the trial, the researchers said. Patients then received a transition kit with a 14-day supply of either a half-dose edoxaban for patients switching from blinded edoxaban to open warfarin, or a placebo for patients switching from blinded to open warfarin. Patients also underwent at least three international normalized ratio (INR) tests during the first 2 weeks of the transition period and were dosed based on a VKA titration algorithm designed to quickly reach therapeutic INR, they said.
Among 13,642 ENGAGE AF-TIMI patients alive at the end of the trial, 68.2% transitioned to a VKA and 31.2% to a new oral anticoagulant, said the researchers.
Thirty days later, rates of therapeutic INR, stroke, and bleeding events were similar regardless of whether patients had taken low-dose edoxaban, high-dose edoxaban, or warfarin during the trial, they reported. In all, 98.7% of patients switched from high-dose edoxaban had at least one INR of 2 or more, compared with 98.9% of patients switched from low-dose edoxaban and 99.4% of patients switched from warfarin, they said (J. Am. Coll. Cardiol. 2014;64:576-84).
Post-transition rates of strokes (1.85% to 1.90% per year) and major bleeding (2.69% to 4.76% per year) also were similar among the three trial groups, they added.
The excess ischemic events in earlier trials probably resulted from relative delays in achieving a therapeutic INR when patients were switched from a newer oral anticoagulant to an open-label VKA, the researchers noted, adding that "VKAs are effective as long as a therapeutic INR can be rapidly reached and maintained."
Daiichi Sankyo funded the ENGAGE AF-TIMI trial. Dr. Ruff reported having been a consultant and receiving honoraria from Daiichi Sankyo. Two coauthors reported financial relationships, six reported having received grant support, and two reported employment with Daiichi Sankyo.
These results appear to set a new standard for managing patients switching from a new oral anticoagulant to open-label warfarin at the end of blinded anticoagulation trials.
The data reveal ways to manage patients who need their anticoagulation interrupted. Bridging anticoagulation can prevent thromboembolic events when combined with rigorous INR monitoring and algorithm-based warfarin dosing.
Furthermore, the lack of excess bleeding with the half-dose edoxaban bridging regimen raises the possibility that lowering the dose of the anticoagulant can improve safety without compromising efficacy. In past observational studies, bridging with a fast-acting parenteral anticoagulant until INR reached 2.0 increased the risk of bleeding probably because of dose overlap between the bridging agent and warfarin. Also, annualized stroke rates during the transition phase slightly exceeded those during the rest of the trial, reminding clinicians that even when carefully managed, switching from one anticoagulant to another is not without risk.
Remaining questions include whether to apply bridging uniformly, whether a half-dose of edoxaban is sufficient, and whether results for lower edoxaban dosing can be extrapolated to other new oral anticoagulants, said the researchers. Finally, evaluation of the transition kit in the ENGAGE AF-TIMI 48 trial was not randomized, and our conclusions regarding the efficacy and safety of this approach compared with no bridging or a more limited bridging strategy are based on indirect comparisons across trials and cannot be considered definitive. In the meantime several large ongoing trials are evaluating bridging regimens for surgical patients who need their warfarin therapy interrupted.
Dr. John Eikelboom, Dr. Thomas. Vanassche, and Dr. Stuart Connolly, are cardiologists with Hamilton General Hospital and McMaster University in Ontario, Canada. Dr. Eikelboom has received financial support from companies that make and market non–vitamin K antagonist oral anticoagulants, including Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Pfizer. Dr. Connolly has been an adviser, consultant, or speaker for Bayer HealthCare Pharmaceuticals, BI, BMS, Pfizer, and Portola Pharmaceuticals. Dr. Vanassche reported no financial conflicts of interest. These remarks were excerpted from their editorial accompanying Dr. Ruff’s report (J. Am. Coll. Cardiol. 2014;64:585-7).
These results appear to set a new standard for managing patients switching from a new oral anticoagulant to open-label warfarin at the end of blinded anticoagulation trials.
The data reveal ways to manage patients who need their anticoagulation interrupted. Bridging anticoagulation can prevent thromboembolic events when combined with rigorous INR monitoring and algorithm-based warfarin dosing.
Furthermore, the lack of excess bleeding with the half-dose edoxaban bridging regimen raises the possibility that lowering the dose of the anticoagulant can improve safety without compromising efficacy. In past observational studies, bridging with a fast-acting parenteral anticoagulant until INR reached 2.0 increased the risk of bleeding probably because of dose overlap between the bridging agent and warfarin. Also, annualized stroke rates during the transition phase slightly exceeded those during the rest of the trial, reminding clinicians that even when carefully managed, switching from one anticoagulant to another is not without risk.
Remaining questions include whether to apply bridging uniformly, whether a half-dose of edoxaban is sufficient, and whether results for lower edoxaban dosing can be extrapolated to other new oral anticoagulants, said the researchers. Finally, evaluation of the transition kit in the ENGAGE AF-TIMI 48 trial was not randomized, and our conclusions regarding the efficacy and safety of this approach compared with no bridging or a more limited bridging strategy are based on indirect comparisons across trials and cannot be considered definitive. In the meantime several large ongoing trials are evaluating bridging regimens for surgical patients who need their warfarin therapy interrupted.
Dr. John Eikelboom, Dr. Thomas. Vanassche, and Dr. Stuart Connolly, are cardiologists with Hamilton General Hospital and McMaster University in Ontario, Canada. Dr. Eikelboom has received financial support from companies that make and market non–vitamin K antagonist oral anticoagulants, including Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Pfizer. Dr. Connolly has been an adviser, consultant, or speaker for Bayer HealthCare Pharmaceuticals, BI, BMS, Pfizer, and Portola Pharmaceuticals. Dr. Vanassche reported no financial conflicts of interest. These remarks were excerpted from their editorial accompanying Dr. Ruff’s report (J. Am. Coll. Cardiol. 2014;64:585-7).
These results appear to set a new standard for managing patients switching from a new oral anticoagulant to open-label warfarin at the end of blinded anticoagulation trials.
The data reveal ways to manage patients who need their anticoagulation interrupted. Bridging anticoagulation can prevent thromboembolic events when combined with rigorous INR monitoring and algorithm-based warfarin dosing.
Furthermore, the lack of excess bleeding with the half-dose edoxaban bridging regimen raises the possibility that lowering the dose of the anticoagulant can improve safety without compromising efficacy. In past observational studies, bridging with a fast-acting parenteral anticoagulant until INR reached 2.0 increased the risk of bleeding probably because of dose overlap between the bridging agent and warfarin. Also, annualized stroke rates during the transition phase slightly exceeded those during the rest of the trial, reminding clinicians that even when carefully managed, switching from one anticoagulant to another is not without risk.
Remaining questions include whether to apply bridging uniformly, whether a half-dose of edoxaban is sufficient, and whether results for lower edoxaban dosing can be extrapolated to other new oral anticoagulants, said the researchers. Finally, evaluation of the transition kit in the ENGAGE AF-TIMI 48 trial was not randomized, and our conclusions regarding the efficacy and safety of this approach compared with no bridging or a more limited bridging strategy are based on indirect comparisons across trials and cannot be considered definitive. In the meantime several large ongoing trials are evaluating bridging regimens for surgical patients who need their warfarin therapy interrupted.
Dr. John Eikelboom, Dr. Thomas. Vanassche, and Dr. Stuart Connolly, are cardiologists with Hamilton General Hospital and McMaster University in Ontario, Canada. Dr. Eikelboom has received financial support from companies that make and market non–vitamin K antagonist oral anticoagulants, including Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Pfizer. Dr. Connolly has been an adviser, consultant, or speaker for Bayer HealthCare Pharmaceuticals, BI, BMS, Pfizer, and Portola Pharmaceuticals. Dr. Vanassche reported no financial conflicts of interest. These remarks were excerpted from their editorial accompanying Dr. Ruff’s report (J. Am. Coll. Cardiol. 2014;64:585-7).
A detailed transition plan protected most atrial fibrillation patients from strokes and major bleeding when they switched oral anticoagulants, researchers reported in the August issue of the Journal of the American College of Cardiology.
Following such a plan could help patients safely switch anticoagulants in clinical practice, said Dr. Christian Ruff at Harvard Medical School in Boston and his associates.
They reported an analysis from the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which randomized more than 21,000 AF patients at high risk of stroke to either the investigational factor Xa inhibitor edoxaban or warfarin. Edoxaban was shown noninferior to warfarin in preventing stroke or systemic embolism (N. Engl. J. Med. 2013; 369:2093-104).
In the past, researchers from the ROCKET AF and ARISTOTLE trials reported excess strokes and bleeding events after patients were switched from blinded factor Xa inhibitors to open-label antithrombotics, the investigators said.
In response, ENGAGE AF-TIMI 48 patients and their physicians decided together whether to switch to an open-label vitamin K antagonist (VKA) or a newer oral anticoagulant at the end of the trial, the researchers said. Patients then received a transition kit with a 14-day supply of either a half-dose edoxaban for patients switching from blinded edoxaban to open warfarin, or a placebo for patients switching from blinded to open warfarin. Patients also underwent at least three international normalized ratio (INR) tests during the first 2 weeks of the transition period and were dosed based on a VKA titration algorithm designed to quickly reach therapeutic INR, they said.
Among 13,642 ENGAGE AF-TIMI patients alive at the end of the trial, 68.2% transitioned to a VKA and 31.2% to a new oral anticoagulant, said the researchers.
Thirty days later, rates of therapeutic INR, stroke, and bleeding events were similar regardless of whether patients had taken low-dose edoxaban, high-dose edoxaban, or warfarin during the trial, they reported. In all, 98.7% of patients switched from high-dose edoxaban had at least one INR of 2 or more, compared with 98.9% of patients switched from low-dose edoxaban and 99.4% of patients switched from warfarin, they said (J. Am. Coll. Cardiol. 2014;64:576-84).
Post-transition rates of strokes (1.85% to 1.90% per year) and major bleeding (2.69% to 4.76% per year) also were similar among the three trial groups, they added.
The excess ischemic events in earlier trials probably resulted from relative delays in achieving a therapeutic INR when patients were switched from a newer oral anticoagulant to an open-label VKA, the researchers noted, adding that "VKAs are effective as long as a therapeutic INR can be rapidly reached and maintained."
Daiichi Sankyo funded the ENGAGE AF-TIMI trial. Dr. Ruff reported having been a consultant and receiving honoraria from Daiichi Sankyo. Two coauthors reported financial relationships, six reported having received grant support, and two reported employment with Daiichi Sankyo.
A detailed transition plan protected most atrial fibrillation patients from strokes and major bleeding when they switched oral anticoagulants, researchers reported in the August issue of the Journal of the American College of Cardiology.
Following such a plan could help patients safely switch anticoagulants in clinical practice, said Dr. Christian Ruff at Harvard Medical School in Boston and his associates.
They reported an analysis from the ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48) trial, which randomized more than 21,000 AF patients at high risk of stroke to either the investigational factor Xa inhibitor edoxaban or warfarin. Edoxaban was shown noninferior to warfarin in preventing stroke or systemic embolism (N. Engl. J. Med. 2013; 369:2093-104).
In the past, researchers from the ROCKET AF and ARISTOTLE trials reported excess strokes and bleeding events after patients were switched from blinded factor Xa inhibitors to open-label antithrombotics, the investigators said.
In response, ENGAGE AF-TIMI 48 patients and their physicians decided together whether to switch to an open-label vitamin K antagonist (VKA) or a newer oral anticoagulant at the end of the trial, the researchers said. Patients then received a transition kit with a 14-day supply of either a half-dose edoxaban for patients switching from blinded edoxaban to open warfarin, or a placebo for patients switching from blinded to open warfarin. Patients also underwent at least three international normalized ratio (INR) tests during the first 2 weeks of the transition period and were dosed based on a VKA titration algorithm designed to quickly reach therapeutic INR, they said.
Among 13,642 ENGAGE AF-TIMI patients alive at the end of the trial, 68.2% transitioned to a VKA and 31.2% to a new oral anticoagulant, said the researchers.
Thirty days later, rates of therapeutic INR, stroke, and bleeding events were similar regardless of whether patients had taken low-dose edoxaban, high-dose edoxaban, or warfarin during the trial, they reported. In all, 98.7% of patients switched from high-dose edoxaban had at least one INR of 2 or more, compared with 98.9% of patients switched from low-dose edoxaban and 99.4% of patients switched from warfarin, they said (J. Am. Coll. Cardiol. 2014;64:576-84).
Post-transition rates of strokes (1.85% to 1.90% per year) and major bleeding (2.69% to 4.76% per year) also were similar among the three trial groups, they added.
The excess ischemic events in earlier trials probably resulted from relative delays in achieving a therapeutic INR when patients were switched from a newer oral anticoagulant to an open-label VKA, the researchers noted, adding that "VKAs are effective as long as a therapeutic INR can be rapidly reached and maintained."
Daiichi Sankyo funded the ENGAGE AF-TIMI trial. Dr. Ruff reported having been a consultant and receiving honoraria from Daiichi Sankyo. Two coauthors reported financial relationships, six reported having received grant support, and two reported employment with Daiichi Sankyo.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Following a prescribed transition plan when switching oral anticoagulants protected most AF patients from strokes and major bleeding.
Major finding: Thirty days after transitioning to a new oral anticoagulant, 98.7% of patients who were switched from high-dose edoxaban had at least one therapeutic INR, compared with 98.9% of patients switched from low-dose edoxaban and 99.4% of patients switched from warfarin. Post-transition rates of stroke and major bleeding also were similar among all three groups.
Data source: Randomized, open-label study of 13,642 patients with AF from the ENGAGE AF-TIMI 48 trial. At the end of the trial, 68.2% of patients transitioned to an open-label vitamin K antagonist, and 31.2% were switched to new oral anticoagulant.
Disclosures: Daiichi Sankyo funded the ENGAGE AF-TIMI trial. Dr. Ruff reported having been a consultant and receiving honoraria from Daiichi Sankyo. Two coauthors reported financial relationships, six reported having received grant support, and two reported employment with Daiichi Sankyo.
Risks scream caution for catheter-directed thrombolysis for proximal DVT
Catheter-directed thrombolysis plus anticoagulation is no more effective than anticoagulation alone in preventing in-hospital death among adults who have lower-extremity proximal deep vein thrombosis, according to a nationwide observational study reported online July 21 in JAMA Internal Medicine.
However, catheter-directed thrombolysis carries higher risks, particularly serious bleeding risks such as intracranial hemorrhage, than does anticoagulation alone, and it costs nearly three times as much money. These findings highlight the need for randomized trials "to evaluate the magnitude of the effect of catheter-directed thrombolysis on ... mortality, postthrombotic syndrome, and recurrence of DVT [deep vein thrombosis]. In the absence of such data, it may be reasonable to restrict this form of therapy to those patients who have a low bleeding risk and a high risk for postthrombotic syndrome, such as patients with iliofemoral DVT," said Dr. Riyaz Bashir of the division of cardiovascular diseases, Temple University, Philadelphia, and his associates.
Conflicting data from several small studies as to the safety and effectiveness of catheter-directed thrombolysis have led professional societies to devise conflicting recommendations for its use: the American College of Chest Physicians advises against using the procedure, while the American Heart Association recommends it as a first-line therapy for certain patients. "We sought to assess real-world comparative-safety outcomes in patients proximal and caval DVT who underwent catheter-directed thrombolysis plus anticoagulation with a group treated with anticoagulation alone using risk-adjusted propensity-score matching," the investigators said.
They analyzed data from an Agency for Healthcare Research and Quality administrative database of patient discharges from approximately 1,000 nonfederal acute-care hospitals per year for a 6-year period. They identified 90,618 patients with a discharge diagnosis of proximal DVT; propensity-score matching yielded 3,594 well-matched patients in each study group. In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), Dr. Bashir and his associates wrote (JAMA Intern. Med. 2014 July 21 [doi:10.1001/jamainternmed.2014.3415]).
However, rates of blood transfusion (11.1% vs. 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention. And patients in the catheter-directed thrombolysis group required significantly longer hospitalizations (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs. $28,164). "It is imperative that the magnitude of benefit from catheter-directed therapy be substantial to justify the increased initial resource utilization and bleeding risks of this therapy," the investigators noted.
Catheter-directed thrombolysis plus anticoagulation is no more effective than anticoagulation alone in preventing in-hospital death among adults who have lower-extremity proximal deep vein thrombosis, according to a nationwide observational study reported online July 21 in JAMA Internal Medicine.
However, catheter-directed thrombolysis carries higher risks, particularly serious bleeding risks such as intracranial hemorrhage, than does anticoagulation alone, and it costs nearly three times as much money. These findings highlight the need for randomized trials "to evaluate the magnitude of the effect of catheter-directed thrombolysis on ... mortality, postthrombotic syndrome, and recurrence of DVT [deep vein thrombosis]. In the absence of such data, it may be reasonable to restrict this form of therapy to those patients who have a low bleeding risk and a high risk for postthrombotic syndrome, such as patients with iliofemoral DVT," said Dr. Riyaz Bashir of the division of cardiovascular diseases, Temple University, Philadelphia, and his associates.
Conflicting data from several small studies as to the safety and effectiveness of catheter-directed thrombolysis have led professional societies to devise conflicting recommendations for its use: the American College of Chest Physicians advises against using the procedure, while the American Heart Association recommends it as a first-line therapy for certain patients. "We sought to assess real-world comparative-safety outcomes in patients proximal and caval DVT who underwent catheter-directed thrombolysis plus anticoagulation with a group treated with anticoagulation alone using risk-adjusted propensity-score matching," the investigators said.
They analyzed data from an Agency for Healthcare Research and Quality administrative database of patient discharges from approximately 1,000 nonfederal acute-care hospitals per year for a 6-year period. They identified 90,618 patients with a discharge diagnosis of proximal DVT; propensity-score matching yielded 3,594 well-matched patients in each study group. In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), Dr. Bashir and his associates wrote (JAMA Intern. Med. 2014 July 21 [doi:10.1001/jamainternmed.2014.3415]).
However, rates of blood transfusion (11.1% vs. 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention. And patients in the catheter-directed thrombolysis group required significantly longer hospitalizations (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs. $28,164). "It is imperative that the magnitude of benefit from catheter-directed therapy be substantial to justify the increased initial resource utilization and bleeding risks of this therapy," the investigators noted.
Catheter-directed thrombolysis plus anticoagulation is no more effective than anticoagulation alone in preventing in-hospital death among adults who have lower-extremity proximal deep vein thrombosis, according to a nationwide observational study reported online July 21 in JAMA Internal Medicine.
However, catheter-directed thrombolysis carries higher risks, particularly serious bleeding risks such as intracranial hemorrhage, than does anticoagulation alone, and it costs nearly three times as much money. These findings highlight the need for randomized trials "to evaluate the magnitude of the effect of catheter-directed thrombolysis on ... mortality, postthrombotic syndrome, and recurrence of DVT [deep vein thrombosis]. In the absence of such data, it may be reasonable to restrict this form of therapy to those patients who have a low bleeding risk and a high risk for postthrombotic syndrome, such as patients with iliofemoral DVT," said Dr. Riyaz Bashir of the division of cardiovascular diseases, Temple University, Philadelphia, and his associates.
Conflicting data from several small studies as to the safety and effectiveness of catheter-directed thrombolysis have led professional societies to devise conflicting recommendations for its use: the American College of Chest Physicians advises against using the procedure, while the American Heart Association recommends it as a first-line therapy for certain patients. "We sought to assess real-world comparative-safety outcomes in patients proximal and caval DVT who underwent catheter-directed thrombolysis plus anticoagulation with a group treated with anticoagulation alone using risk-adjusted propensity-score matching," the investigators said.
They analyzed data from an Agency for Healthcare Research and Quality administrative database of patient discharges from approximately 1,000 nonfederal acute-care hospitals per year for a 6-year period. They identified 90,618 patients with a discharge diagnosis of proximal DVT; propensity-score matching yielded 3,594 well-matched patients in each study group. In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), Dr. Bashir and his associates wrote (JAMA Intern. Med. 2014 July 21 [doi:10.1001/jamainternmed.2014.3415]).
However, rates of blood transfusion (11.1% vs. 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention. And patients in the catheter-directed thrombolysis group required significantly longer hospitalizations (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs. $28,164). "It is imperative that the magnitude of benefit from catheter-directed therapy be substantial to justify the increased initial resource utilization and bleeding risks of this therapy," the investigators noted.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Catheter-directed thrombolysis carries higher risks and may not improve outcomes for proximal DVT patients.
Major finding: In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), but rates of blood transfusion (11.1% vs 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention.
Data source: A propensity-matched analysis comparing the effectiveness and safety profiles between catheter-directed thrombolysis plus anticoagulation and anticoagulation alone in 3,594 adults across the country hospitalized with lower-extremity proximal DVT during a 6-year period.
Disclosures: This study was supported by Temple University Hospital, Philadelphia. Dr. Bashir reported no financial conflicts of interest; his associates reported ties to Covidien, Health Systems Networks, and Insight Telehealth.
Risks scream caution for catheter-directed thrombolysis for proximal DVT
Catheter-directed thrombolysis plus anticoagulation is no more effective than anticoagulation alone in preventing in-hospital death among adults who have lower-extremity proximal deep vein thrombosis, according to a nationwide observational study reported online July 21 in JAMA Internal Medicine.
However, catheter-directed thrombolysis carries higher risks, particularly serious bleeding risks such as intracranial hemorrhage, than does anticoagulation alone, and it costs nearly three times as much money. These findings highlight the need for randomized trials "to evaluate the magnitude of the effect of catheter-directed thrombolysis on ... mortality, postthrombotic syndrome, and recurrence of DVT [deep vein thrombosis]. In the absence of such data, it may be reasonable to restrict this form of therapy to those patients who have a low bleeding risk and a high risk for postthrombotic syndrome, such as patients with iliofemoral DVT," said Dr. Riyaz Bashir of the division of cardiovascular diseases, Temple University, Philadelphia, and his associates.
Conflicting data from several small studies as to the safety and effectiveness of catheter-directed thrombolysis have led professional societies to devise conflicting recommendations for its use: the American College of Chest Physicians advises against using the procedure, while the American Heart Association recommends it as a first-line therapy for certain patients. "We sought to assess real-world comparative-safety outcomes in patients proximal and caval DVT who underwent catheter-directed thrombolysis plus anticoagulation with a group treated with anticoagulation alone using risk-adjusted propensity-score matching," the investigators said.
They analyzed data from an Agency for Healthcare Research and Quality administrative database of patient discharges from approximately 1,000 nonfederal acute-care hospitals per year for a 6-year period. They identified 90,618 patients with a discharge diagnosis of proximal DVT; propensity-score matching yielded 3,594 well-matched patients in each study group. In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), Dr. Bashir and his associates wrote (JAMA Intern. Med. 2014 July 21 [doi:10.1001/jamainternmed.2014.3415]).
However, rates of blood transfusion (11.1% vs. 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention. And patients in the catheter-directed thrombolysis group required significantly longer hospitalizations (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs. $28,164). "It is imperative that the magnitude of benefit from catheter-directed therapy be substantial to justify the increased initial resource utilization and bleeding risks of this therapy," the investigators noted.
Catheter-directed thrombolysis plus anticoagulation is no more effective than anticoagulation alone in preventing in-hospital death among adults who have lower-extremity proximal deep vein thrombosis, according to a nationwide observational study reported online July 21 in JAMA Internal Medicine.
However, catheter-directed thrombolysis carries higher risks, particularly serious bleeding risks such as intracranial hemorrhage, than does anticoagulation alone, and it costs nearly three times as much money. These findings highlight the need for randomized trials "to evaluate the magnitude of the effect of catheter-directed thrombolysis on ... mortality, postthrombotic syndrome, and recurrence of DVT [deep vein thrombosis]. In the absence of such data, it may be reasonable to restrict this form of therapy to those patients who have a low bleeding risk and a high risk for postthrombotic syndrome, such as patients with iliofemoral DVT," said Dr. Riyaz Bashir of the division of cardiovascular diseases, Temple University, Philadelphia, and his associates.
Conflicting data from several small studies as to the safety and effectiveness of catheter-directed thrombolysis have led professional societies to devise conflicting recommendations for its use: the American College of Chest Physicians advises against using the procedure, while the American Heart Association recommends it as a first-line therapy for certain patients. "We sought to assess real-world comparative-safety outcomes in patients proximal and caval DVT who underwent catheter-directed thrombolysis plus anticoagulation with a group treated with anticoagulation alone using risk-adjusted propensity-score matching," the investigators said.
They analyzed data from an Agency for Healthcare Research and Quality administrative database of patient discharges from approximately 1,000 nonfederal acute-care hospitals per year for a 6-year period. They identified 90,618 patients with a discharge diagnosis of proximal DVT; propensity-score matching yielded 3,594 well-matched patients in each study group. In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), Dr. Bashir and his associates wrote (JAMA Intern. Med. 2014 July 21 [doi:10.1001/jamainternmed.2014.3415]).
However, rates of blood transfusion (11.1% vs. 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention. And patients in the catheter-directed thrombolysis group required significantly longer hospitalizations (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs. $28,164). "It is imperative that the magnitude of benefit from catheter-directed therapy be substantial to justify the increased initial resource utilization and bleeding risks of this therapy," the investigators noted.
Catheter-directed thrombolysis plus anticoagulation is no more effective than anticoagulation alone in preventing in-hospital death among adults who have lower-extremity proximal deep vein thrombosis, according to a nationwide observational study reported online July 21 in JAMA Internal Medicine.
However, catheter-directed thrombolysis carries higher risks, particularly serious bleeding risks such as intracranial hemorrhage, than does anticoagulation alone, and it costs nearly three times as much money. These findings highlight the need for randomized trials "to evaluate the magnitude of the effect of catheter-directed thrombolysis on ... mortality, postthrombotic syndrome, and recurrence of DVT [deep vein thrombosis]. In the absence of such data, it may be reasonable to restrict this form of therapy to those patients who have a low bleeding risk and a high risk for postthrombotic syndrome, such as patients with iliofemoral DVT," said Dr. Riyaz Bashir of the division of cardiovascular diseases, Temple University, Philadelphia, and his associates.
Conflicting data from several small studies as to the safety and effectiveness of catheter-directed thrombolysis have led professional societies to devise conflicting recommendations for its use: the American College of Chest Physicians advises against using the procedure, while the American Heart Association recommends it as a first-line therapy for certain patients. "We sought to assess real-world comparative-safety outcomes in patients proximal and caval DVT who underwent catheter-directed thrombolysis plus anticoagulation with a group treated with anticoagulation alone using risk-adjusted propensity-score matching," the investigators said.
They analyzed data from an Agency for Healthcare Research and Quality administrative database of patient discharges from approximately 1,000 nonfederal acute-care hospitals per year for a 6-year period. They identified 90,618 patients with a discharge diagnosis of proximal DVT; propensity-score matching yielded 3,594 well-matched patients in each study group. In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), Dr. Bashir and his associates wrote (JAMA Intern. Med. 2014 July 21 [doi:10.1001/jamainternmed.2014.3415]).
However, rates of blood transfusion (11.1% vs. 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention. And patients in the catheter-directed thrombolysis group required significantly longer hospitalizations (7.2 vs. 5.0 days) and incurred significantly higher hospital expenses ($85,094 vs. $28,164). "It is imperative that the magnitude of benefit from catheter-directed therapy be substantial to justify the increased initial resource utilization and bleeding risks of this therapy," the investigators noted.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Catheter-directed thrombolysis carries higher risks and may not improve outcomes for proximal DVT patients.
Major finding: In-hospital mortality was not significantly different between patients who had catheter-directed thrombolysis plus anticoagulation (1.2%) and those who had anticoagulation alone (0.9%), but rates of blood transfusion (11.1% vs 6.5%), pulmonary embolism (17.9% vs 11.4%), and intracranial hemorrhage (0.9% vs 0.3%) were significantly higher with the invasive intervention.
Data source: A propensity-matched analysis comparing the effectiveness and safety profiles between catheter-directed thrombolysis plus anticoagulation and anticoagulation alone in 3,594 adults across the country hospitalized with lower-extremity proximal DVT during a 6-year period.
Disclosures: This study was supported by Temple University Hospital, Philadelphia. Dr. Bashir reported no financial conflicts of interest; his associates reported ties to Covidien, Health Systems Networks, and Insight Telehealth.
Thrombolysis may offer benefit in stable pulmonary embolism
Thrombolytic therapy decreased all-cause mortality in patients with hemodynamically stable pulmonary embolism associated with right ventricular dysfunction – those at "intermediate risk," according to a meta-analysis published online June 17 in JAMA.
The investigators described their study of 16 randomized, controlled clinical trials involving 2,115 patients as "the first analysis of thrombolysis in PE that has sufficient statistical power to detect associations with a meaningful mortality reduction." If their findings are confirmed in future randomized clinical trials, "there may be a shift in the treatment of selected patients with intermediate-risk PE using thrombolytics."
However, "the optimism regarding this clinical advantage must be tempered by [our] finding of significantly increased risk of major bleeding and intracranial hemorrhage associated with thrombolytic therapy, particularly for patients older than 65 years," said Dr. Saurav Chatterjee of the division of cardiology, St. Luke’s-Roosevelt Hospital Center of the Mount Sinai Health System, New York, and his associates (JAMA 2014;311:2414-21).
The study population included 1,499 patients who had hemodynamically stable PE associated with right ventricular dysfunction, the largest subset of patients seen in clinical practice and the group for whom the risks and benefits of thrombolysis are the most unclear.
After a mean follow-up of 82 days, overall mortality was 2.17% in patients who received thrombolysis, compared with 3.89% in those who received anticoagulation. In addition, the risk of recurrent PE was significantly lower with thrombolytic therapy (1.17%) than with anticoagulation (3.04%).
However, the rate of major bleeding was 9.24% for thrombolytic therapy, compared with 3.42% for anticoagulation. And the rate of intracranial hemorrhage was 1.46% for thrombolysis, compared with 0.19% for anticoagulation, the investigators said.
The bleeding risk was especially high in patients aged 65 years and older. Attenuation of this risk in younger patients suggests that they may be considered stronger candidates for thrombolytic therapy, Dr. Chatterjee and his associates said.
Dr. Chatterjee reported no financial conflicts; his associates reported ties to AstraZeneca, Boston Scientific, Cardiostem, Cordis, EKOS Corporation, Embolitech, GenWay, Johnson & Johnson, Soteria, and Vascular Magnetics.

|
|
Dr. Chatterjee and his associates calculated the net clinical benefit of thrombolysis, and their result "suggests evidence of modest efficacy in intermediate-risk PE," said Dr. Joshua A. Beckman.
But their findings do not yet add up to a change in the standard of care. Each clinician must decide on an individualized basis which of these patients should receive thrombolytic therapy, based on clinical presentation, comorbid conditions, and both the physician’s and the patient’s tolerance of risk.
Dr. Beckman is in the cardiovascular division at Brigham and Women’s Hospital, Boston. He reported being a board member for Vascular Interventional Advances; receiving grant funding from Bristol-Myers Squibb; and consulting for AstraZeneca, Boston Scientific, Ferring, Merck, and Novartis. These remarks were taken from his editorial accompanying Dr. Chatterjee’s report (JAMA 2014;311:2385-6).

|
|
Dr. Chatterjee and his associates calculated the net clinical benefit of thrombolysis, and their result "suggests evidence of modest efficacy in intermediate-risk PE," said Dr. Joshua A. Beckman.
But their findings do not yet add up to a change in the standard of care. Each clinician must decide on an individualized basis which of these patients should receive thrombolytic therapy, based on clinical presentation, comorbid conditions, and both the physician’s and the patient’s tolerance of risk.
Dr. Beckman is in the cardiovascular division at Brigham and Women’s Hospital, Boston. He reported being a board member for Vascular Interventional Advances; receiving grant funding from Bristol-Myers Squibb; and consulting for AstraZeneca, Boston Scientific, Ferring, Merck, and Novartis. These remarks were taken from his editorial accompanying Dr. Chatterjee’s report (JAMA 2014;311:2385-6).

|
|
Dr. Chatterjee and his associates calculated the net clinical benefit of thrombolysis, and their result "suggests evidence of modest efficacy in intermediate-risk PE," said Dr. Joshua A. Beckman.
But their findings do not yet add up to a change in the standard of care. Each clinician must decide on an individualized basis which of these patients should receive thrombolytic therapy, based on clinical presentation, comorbid conditions, and both the physician’s and the patient’s tolerance of risk.
Dr. Beckman is in the cardiovascular division at Brigham and Women’s Hospital, Boston. He reported being a board member for Vascular Interventional Advances; receiving grant funding from Bristol-Myers Squibb; and consulting for AstraZeneca, Boston Scientific, Ferring, Merck, and Novartis. These remarks were taken from his editorial accompanying Dr. Chatterjee’s report (JAMA 2014;311:2385-6).
Thrombolytic therapy decreased all-cause mortality in patients with hemodynamically stable pulmonary embolism associated with right ventricular dysfunction – those at "intermediate risk," according to a meta-analysis published online June 17 in JAMA.
The investigators described their study of 16 randomized, controlled clinical trials involving 2,115 patients as "the first analysis of thrombolysis in PE that has sufficient statistical power to detect associations with a meaningful mortality reduction." If their findings are confirmed in future randomized clinical trials, "there may be a shift in the treatment of selected patients with intermediate-risk PE using thrombolytics."
However, "the optimism regarding this clinical advantage must be tempered by [our] finding of significantly increased risk of major bleeding and intracranial hemorrhage associated with thrombolytic therapy, particularly for patients older than 65 years," said Dr. Saurav Chatterjee of the division of cardiology, St. Luke’s-Roosevelt Hospital Center of the Mount Sinai Health System, New York, and his associates (JAMA 2014;311:2414-21).
The study population included 1,499 patients who had hemodynamically stable PE associated with right ventricular dysfunction, the largest subset of patients seen in clinical practice and the group for whom the risks and benefits of thrombolysis are the most unclear.
After a mean follow-up of 82 days, overall mortality was 2.17% in patients who received thrombolysis, compared with 3.89% in those who received anticoagulation. In addition, the risk of recurrent PE was significantly lower with thrombolytic therapy (1.17%) than with anticoagulation (3.04%).
However, the rate of major bleeding was 9.24% for thrombolytic therapy, compared with 3.42% for anticoagulation. And the rate of intracranial hemorrhage was 1.46% for thrombolysis, compared with 0.19% for anticoagulation, the investigators said.
The bleeding risk was especially high in patients aged 65 years and older. Attenuation of this risk in younger patients suggests that they may be considered stronger candidates for thrombolytic therapy, Dr. Chatterjee and his associates said.
Dr. Chatterjee reported no financial conflicts; his associates reported ties to AstraZeneca, Boston Scientific, Cardiostem, Cordis, EKOS Corporation, Embolitech, GenWay, Johnson & Johnson, Soteria, and Vascular Magnetics.
Thrombolytic therapy decreased all-cause mortality in patients with hemodynamically stable pulmonary embolism associated with right ventricular dysfunction – those at "intermediate risk," according to a meta-analysis published online June 17 in JAMA.
The investigators described their study of 16 randomized, controlled clinical trials involving 2,115 patients as "the first analysis of thrombolysis in PE that has sufficient statistical power to detect associations with a meaningful mortality reduction." If their findings are confirmed in future randomized clinical trials, "there may be a shift in the treatment of selected patients with intermediate-risk PE using thrombolytics."
However, "the optimism regarding this clinical advantage must be tempered by [our] finding of significantly increased risk of major bleeding and intracranial hemorrhage associated with thrombolytic therapy, particularly for patients older than 65 years," said Dr. Saurav Chatterjee of the division of cardiology, St. Luke’s-Roosevelt Hospital Center of the Mount Sinai Health System, New York, and his associates (JAMA 2014;311:2414-21).
The study population included 1,499 patients who had hemodynamically stable PE associated with right ventricular dysfunction, the largest subset of patients seen in clinical practice and the group for whom the risks and benefits of thrombolysis are the most unclear.
After a mean follow-up of 82 days, overall mortality was 2.17% in patients who received thrombolysis, compared with 3.89% in those who received anticoagulation. In addition, the risk of recurrent PE was significantly lower with thrombolytic therapy (1.17%) than with anticoagulation (3.04%).
However, the rate of major bleeding was 9.24% for thrombolytic therapy, compared with 3.42% for anticoagulation. And the rate of intracranial hemorrhage was 1.46% for thrombolysis, compared with 0.19% for anticoagulation, the investigators said.
The bleeding risk was especially high in patients aged 65 years and older. Attenuation of this risk in younger patients suggests that they may be considered stronger candidates for thrombolytic therapy, Dr. Chatterjee and his associates said.
Dr. Chatterjee reported no financial conflicts; his associates reported ties to AstraZeneca, Boston Scientific, Cardiostem, Cordis, EKOS Corporation, Embolitech, GenWay, Johnson & Johnson, Soteria, and Vascular Magnetics.
FROM JAMA
Key clinical point: Thrombolysis may be a therapeutic alternative to anticoagulation in some patients with stable, intermediate-risk pulmonary embolism.
Major finding: Mortality was 2.17% in PE patients who received thrombolysis, compared with 3.89% in those who received anticoagulation; the risk of recurrent PE also was significantly lower with thrombolytic therapy (1.17%) than with anticoagulation (3.04%).
Data source: A meta-analysis of 16 randomized, controlled trials involving 2,115 patients with PE, including 1,499 with intermediate-risk PE, who were followed for a mean of 82 days.
Disclosures: Dr. Chatterjee reported no financial conflicts; his associates reported ties to AstraZeneca, Boston Scientific, Cardiostem, Cordis, EKOS Corporation, Embolitech, GenWay, Johnson & Johnson, Soteria, and Vascular Magnetics.
FDA declines to approve IV antiplatelet drug cangrelor
The Food and Drug Administration has rejected the approval of the intravenous antiplatelet drug cangrelor, suggesting that the company provide more data, according to the drug’s manufacturer.
The Medicines Company applied for approval of cangrelor for two indications: the reduction of death, MI, stent thrombosis, and ischemic-driven revascularization in patients who have not been recently treated with a thienopyridine and who are undergoing PCI; and for patients with stents who are at an increased risk for thrombotic events, such as stent thrombosis, when oral P2Y12 therapy is interrupted for surgery.
At a meeting earlier this year, the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 7-2 against approval of the first indication, and unanimously voted against approval of the second indication, citing numerous issues with clinical trials.
The company’s April 30 statement announcing the FDA decision said that the agency had suggested that the company conduct "a series of data analyses" from the CHAMPION PHOENIX study that was the basis of the PCI indication (N. Engl. J. Med. 2013;368:1303-13).
The FDA also concluded that a prospective controlled study to evaluate the risk-benefit of cangrelor for the second bridging indication with outcomes that included bleeding was needed, the statement said. For this indication, the company had submitted only a small pharmacodynamic study, which showed that a cangrelor infusion could maintain platelet inhibition similar to that achieved with clopidogrel.
In the statement Dr. Clive A. Meanwell, the company’s chairman and chief executive officer, said that "the next steps of review will focus on additional analyses in response to the FDA."
Cangrelor is a platelet P2Y12 inhibitor administered intravenously; with a half-life of 3-6 minutes, platelet function returns to normal within 1 hour of stopping the infusion of cangrelor, according to the company. The oral P2Y12 inhibitor clopidogrel has a more delayed action, with activity that lasts for days after is stopped.
The Food and Drug Administration has rejected the approval of the intravenous antiplatelet drug cangrelor, suggesting that the company provide more data, according to the drug’s manufacturer.
The Medicines Company applied for approval of cangrelor for two indications: the reduction of death, MI, stent thrombosis, and ischemic-driven revascularization in patients who have not been recently treated with a thienopyridine and who are undergoing PCI; and for patients with stents who are at an increased risk for thrombotic events, such as stent thrombosis, when oral P2Y12 therapy is interrupted for surgery.
At a meeting earlier this year, the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 7-2 against approval of the first indication, and unanimously voted against approval of the second indication, citing numerous issues with clinical trials.
The company’s April 30 statement announcing the FDA decision said that the agency had suggested that the company conduct "a series of data analyses" from the CHAMPION PHOENIX study that was the basis of the PCI indication (N. Engl. J. Med. 2013;368:1303-13).
The FDA also concluded that a prospective controlled study to evaluate the risk-benefit of cangrelor for the second bridging indication with outcomes that included bleeding was needed, the statement said. For this indication, the company had submitted only a small pharmacodynamic study, which showed that a cangrelor infusion could maintain platelet inhibition similar to that achieved with clopidogrel.
In the statement Dr. Clive A. Meanwell, the company’s chairman and chief executive officer, said that "the next steps of review will focus on additional analyses in response to the FDA."
Cangrelor is a platelet P2Y12 inhibitor administered intravenously; with a half-life of 3-6 minutes, platelet function returns to normal within 1 hour of stopping the infusion of cangrelor, according to the company. The oral P2Y12 inhibitor clopidogrel has a more delayed action, with activity that lasts for days after is stopped.
The Food and Drug Administration has rejected the approval of the intravenous antiplatelet drug cangrelor, suggesting that the company provide more data, according to the drug’s manufacturer.
The Medicines Company applied for approval of cangrelor for two indications: the reduction of death, MI, stent thrombosis, and ischemic-driven revascularization in patients who have not been recently treated with a thienopyridine and who are undergoing PCI; and for patients with stents who are at an increased risk for thrombotic events, such as stent thrombosis, when oral P2Y12 therapy is interrupted for surgery.
At a meeting earlier this year, the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 7-2 against approval of the first indication, and unanimously voted against approval of the second indication, citing numerous issues with clinical trials.
The company’s April 30 statement announcing the FDA decision said that the agency had suggested that the company conduct "a series of data analyses" from the CHAMPION PHOENIX study that was the basis of the PCI indication (N. Engl. J. Med. 2013;368:1303-13).
The FDA also concluded that a prospective controlled study to evaluate the risk-benefit of cangrelor for the second bridging indication with outcomes that included bleeding was needed, the statement said. For this indication, the company had submitted only a small pharmacodynamic study, which showed that a cangrelor infusion could maintain platelet inhibition similar to that achieved with clopidogrel.
In the statement Dr. Clive A. Meanwell, the company’s chairman and chief executive officer, said that "the next steps of review will focus on additional analyses in response to the FDA."
Cangrelor is a platelet P2Y12 inhibitor administered intravenously; with a half-life of 3-6 minutes, platelet function returns to normal within 1 hour of stopping the infusion of cangrelor, according to the company. The oral P2Y12 inhibitor clopidogrel has a more delayed action, with activity that lasts for days after is stopped.
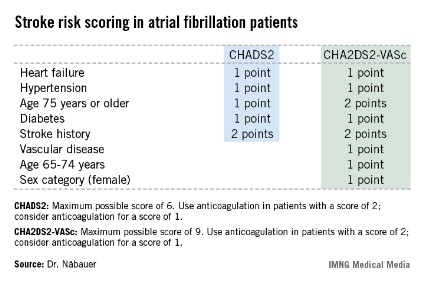
CHA2DS2-VASc score performs best in assessing atrial fibrillation stroke risk
AMSTERDAM – Use of the CHA2DS2-VASc score markedly improves classification of atrial fibrillation patients who are truly at low risk of stroke, compared with the commonly used CHADS2 score, a German national study found.
"We do not feel that a CHADS2 risk score of 0 or 1 is suitable to identify low-risk patients. The CHA2DS2-VASc score provides a more refined risk stratification in low-risk patients. In the real life, prospective German AFNET [German Competence Network on Atrial Fibrillation] registry, a CHA2DS2-VASc score of 0 identifies a subgroup of patients with very low stroke risk unlikely to benefit from oral anticoagulation therapy," Dr. Michael Näbauer said at the annual congress of the European Society of Cardiology.
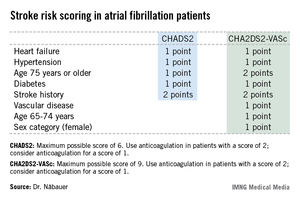
Among 795 patients in the AFNET registry who had a CHA2DS2-VASc score of 0, only 8 correctly categorized patients had a nonprocedurally related stroke, transient ischemic attack (TIA), or thromboembolism during 5 years of prospective follow-up, reported Dr. Näbauer, head of the echocardiographic unit at Ludwig Maximilians University Hospital, Munich.
A transcontinental split exists at present regarding the best clinical decision tool for assessing stroke risk in patients with atrial fibrillation (AF), and thus identifying those in whom oral anticoagulation is or is not warranted. Current American guidelines recommend using the CHADS2 score, while more recent ESC guidelines released last year advocate superseding CHADS2 with the newer CHA2DS2-VASc scoring system (Eur. Heart J. 2012;33:2719-47).
Dr. Näbauer’s report from the German AFNET registry highlighted the advantages of using CHA2DS2-VASc. Among 8,847 patients with nonvalvular AF participating in the registry run by physicians having a special interest in atrial fibrillation, 16.2% were assigned a CHADS2 score of 0 and 31.5% had a score of 1, meaning their stroke risk going forward was too low to justify the routine use of prophylactic oral anticoagulation therapy, with its attendant bleeding risk.
Here’s the deal killer for the CHADS2 scoring system, he said: Of the 403 stroke, TIA, and thromboembolic events that occurred in the nearly 9,000 AF patients during 5 years of prospective follow-up, 36% occurred in patients with a CHADS2 score of 0 or 1.
"This finding suggests that CHADS2 classes 0 and 1 contain subgroups of patients with significant stroke risk that may be identified by refined stroke risk classification," the cardiologist noted.
Application of the CHA2DS2-VASc score to the AFNET population resulted in reclassification of 126 of the 145 CHADS2 class 0 or 1 patients who had a stroke, TIA, or thromboembolism to a higher-risk CHA2DS2-VASc category where oral anticoagulation is appropriate.
Of the 45 stroke events that occurred among 1,430 patients who were CHADS2 class 0, 12 events occurred in patients who were CHA2DS2-VASc class 2 and 14 in CHA2DS2-VASc class 1 – groups in which oral anticoagulation is recommended.
Moreover, 4 of the 19 stroke events occurring in the 795 patients who were CHA2DS2-VASc class 0 happened in association with AF ablation or cardioversion procedures, when oral anticoagulation is temporarily discontinued. Another seven stroke events occurred in patients whose true CHA2DS2-VASc score had increased from 0 during follow-up, mainly because of advancing age. So ultimately only 8 of 795 patients correctly classified as CHA2DS2-VASc 0 had a stroke event unrelated to a cardiac procedure during 5 years of follow-up.
Session cochair Dr. Robert Hatala said that the AFNET experience highlights an important clinical lesson: Stroke risk in AF patients is not static. It changes over time, and periodic reassessment is essential.
"All of the risk scores are imperfect. It’s really very important to relook at your patients and not give them a fixed stamp forever. The risk scores change over time as patients get older, perhaps receive a diagnosis of hypertension, or develop congestive heart failure, maybe with preserved systolic function. So restratify," urged Dr. Hatala, head of cardiology and director of the arrhythmia and pacing center at Slovak Medical University, Bratislava, Slovakia.
Dr. Näbauer and Dr. Hatala reported having no relevant financial conflicts.
AMSTERDAM – Use of the CHA2DS2-VASc score markedly improves classification of atrial fibrillation patients who are truly at low risk of stroke, compared with the commonly used CHADS2 score, a German national study found.
"We do not feel that a CHADS2 risk score of 0 or 1 is suitable to identify low-risk patients. The CHA2DS2-VASc score provides a more refined risk stratification in low-risk patients. In the real life, prospective German AFNET [German Competence Network on Atrial Fibrillation] registry, a CHA2DS2-VASc score of 0 identifies a subgroup of patients with very low stroke risk unlikely to benefit from oral anticoagulation therapy," Dr. Michael Näbauer said at the annual congress of the European Society of Cardiology.

Among 795 patients in the AFNET registry who had a CHA2DS2-VASc score of 0, only 8 correctly categorized patients had a nonprocedurally related stroke, transient ischemic attack (TIA), or thromboembolism during 5 years of prospective follow-up, reported Dr. Näbauer, head of the echocardiographic unit at Ludwig Maximilians University Hospital, Munich.
A transcontinental split exists at present regarding the best clinical decision tool for assessing stroke risk in patients with atrial fibrillation (AF), and thus identifying those in whom oral anticoagulation is or is not warranted. Current American guidelines recommend using the CHADS2 score, while more recent ESC guidelines released last year advocate superseding CHADS2 with the newer CHA2DS2-VASc scoring system (Eur. Heart J. 2012;33:2719-47).
Dr. Näbauer’s report from the German AFNET registry highlighted the advantages of using CHA2DS2-VASc. Among 8,847 patients with nonvalvular AF participating in the registry run by physicians having a special interest in atrial fibrillation, 16.2% were assigned a CHADS2 score of 0 and 31.5% had a score of 1, meaning their stroke risk going forward was too low to justify the routine use of prophylactic oral anticoagulation therapy, with its attendant bleeding risk.
Here’s the deal killer for the CHADS2 scoring system, he said: Of the 403 stroke, TIA, and thromboembolic events that occurred in the nearly 9,000 AF patients during 5 years of prospective follow-up, 36% occurred in patients with a CHADS2 score of 0 or 1.
"This finding suggests that CHADS2 classes 0 and 1 contain subgroups of patients with significant stroke risk that may be identified by refined stroke risk classification," the cardiologist noted.
Application of the CHA2DS2-VASc score to the AFNET population resulted in reclassification of 126 of the 145 CHADS2 class 0 or 1 patients who had a stroke, TIA, or thromboembolism to a higher-risk CHA2DS2-VASc category where oral anticoagulation is appropriate.
Of the 45 stroke events that occurred among 1,430 patients who were CHADS2 class 0, 12 events occurred in patients who were CHA2DS2-VASc class 2 and 14 in CHA2DS2-VASc class 1 – groups in which oral anticoagulation is recommended.
Moreover, 4 of the 19 stroke events occurring in the 795 patients who were CHA2DS2-VASc class 0 happened in association with AF ablation or cardioversion procedures, when oral anticoagulation is temporarily discontinued. Another seven stroke events occurred in patients whose true CHA2DS2-VASc score had increased from 0 during follow-up, mainly because of advancing age. So ultimately only 8 of 795 patients correctly classified as CHA2DS2-VASc 0 had a stroke event unrelated to a cardiac procedure during 5 years of follow-up.
Session cochair Dr. Robert Hatala said that the AFNET experience highlights an important clinical lesson: Stroke risk in AF patients is not static. It changes over time, and periodic reassessment is essential.
"All of the risk scores are imperfect. It’s really very important to relook at your patients and not give them a fixed stamp forever. The risk scores change over time as patients get older, perhaps receive a diagnosis of hypertension, or develop congestive heart failure, maybe with preserved systolic function. So restratify," urged Dr. Hatala, head of cardiology and director of the arrhythmia and pacing center at Slovak Medical University, Bratislava, Slovakia.
Dr. Näbauer and Dr. Hatala reported having no relevant financial conflicts.
AMSTERDAM – Use of the CHA2DS2-VASc score markedly improves classification of atrial fibrillation patients who are truly at low risk of stroke, compared with the commonly used CHADS2 score, a German national study found.
"We do not feel that a CHADS2 risk score of 0 or 1 is suitable to identify low-risk patients. The CHA2DS2-VASc score provides a more refined risk stratification in low-risk patients. In the real life, prospective German AFNET [German Competence Network on Atrial Fibrillation] registry, a CHA2DS2-VASc score of 0 identifies a subgroup of patients with very low stroke risk unlikely to benefit from oral anticoagulation therapy," Dr. Michael Näbauer said at the annual congress of the European Society of Cardiology.

Among 795 patients in the AFNET registry who had a CHA2DS2-VASc score of 0, only 8 correctly categorized patients had a nonprocedurally related stroke, transient ischemic attack (TIA), or thromboembolism during 5 years of prospective follow-up, reported Dr. Näbauer, head of the echocardiographic unit at Ludwig Maximilians University Hospital, Munich.
A transcontinental split exists at present regarding the best clinical decision tool for assessing stroke risk in patients with atrial fibrillation (AF), and thus identifying those in whom oral anticoagulation is or is not warranted. Current American guidelines recommend using the CHADS2 score, while more recent ESC guidelines released last year advocate superseding CHADS2 with the newer CHA2DS2-VASc scoring system (Eur. Heart J. 2012;33:2719-47).
Dr. Näbauer’s report from the German AFNET registry highlighted the advantages of using CHA2DS2-VASc. Among 8,847 patients with nonvalvular AF participating in the registry run by physicians having a special interest in atrial fibrillation, 16.2% were assigned a CHADS2 score of 0 and 31.5% had a score of 1, meaning their stroke risk going forward was too low to justify the routine use of prophylactic oral anticoagulation therapy, with its attendant bleeding risk.
Here’s the deal killer for the CHADS2 scoring system, he said: Of the 403 stroke, TIA, and thromboembolic events that occurred in the nearly 9,000 AF patients during 5 years of prospective follow-up, 36% occurred in patients with a CHADS2 score of 0 or 1.
"This finding suggests that CHADS2 classes 0 and 1 contain subgroups of patients with significant stroke risk that may be identified by refined stroke risk classification," the cardiologist noted.
Application of the CHA2DS2-VASc score to the AFNET population resulted in reclassification of 126 of the 145 CHADS2 class 0 or 1 patients who had a stroke, TIA, or thromboembolism to a higher-risk CHA2DS2-VASc category where oral anticoagulation is appropriate.
Of the 45 stroke events that occurred among 1,430 patients who were CHADS2 class 0, 12 events occurred in patients who were CHA2DS2-VASc class 2 and 14 in CHA2DS2-VASc class 1 – groups in which oral anticoagulation is recommended.
Moreover, 4 of the 19 stroke events occurring in the 795 patients who were CHA2DS2-VASc class 0 happened in association with AF ablation or cardioversion procedures, when oral anticoagulation is temporarily discontinued. Another seven stroke events occurred in patients whose true CHA2DS2-VASc score had increased from 0 during follow-up, mainly because of advancing age. So ultimately only 8 of 795 patients correctly classified as CHA2DS2-VASc 0 had a stroke event unrelated to a cardiac procedure during 5 years of follow-up.
Session cochair Dr. Robert Hatala said that the AFNET experience highlights an important clinical lesson: Stroke risk in AF patients is not static. It changes over time, and periodic reassessment is essential.
"All of the risk scores are imperfect. It’s really very important to relook at your patients and not give them a fixed stamp forever. The risk scores change over time as patients get older, perhaps receive a diagnosis of hypertension, or develop congestive heart failure, maybe with preserved systolic function. So restratify," urged Dr. Hatala, head of cardiology and director of the arrhythmia and pacing center at Slovak Medical University, Bratislava, Slovakia.
Dr. Näbauer and Dr. Hatala reported having no relevant financial conflicts.
AT THE ESC CONGRESS 2013
Major finding: During 5 years of prospective follow-up of patients with nonvalvular atrial fibrillation, 145 patients who had a stroke, transient ischemic attack, or thromboembolism also had a CHADS2 score indicating a low risk for stroke, compared with only 19 who had one of those events and were classified as low risk by the CHA2DS2-VASc stroke risk scoring system.
Data source: The German AFNET registry, a real-world, prospective national registry including 8,847 patients with nonvalvular atrial fibrillation.
Disclosures: The AFNET registry is publically funded by the German Federal Ministry for Education and Research. Dr. Näbauer and Dr. Hatala reported having no relevant financial conflicts.
Statins for A-fib are ready for prime time
The risk for atrial fibrillation increases with age and the presence of structural heart disease. AF exerts an enormous financial burden on the U.S. health care system. The overall prevalence of AF is 1%, and 70% of people with AF are 65 years of age or older. Inclusive of inpatient and outpatient expenditures, costs for the first episode of atrial fibrillation are estimated to be $15,000.
Perhaps we are all too familiar with the staggering resources consumed by patients who, despite adequate rate control, remain symptomatic. In these cases, an ounce of prevention could literally have been thousands of dollars of cure.
So, can we prevent A-fib?
Statins have been proposed as a way to do this. So, what’s the most recent evidence telling us about its efficacy?
Researchers in France conducted an updated systematic review of the literature to determine the benefit of statins for the prevention of AF (Curr. Opin. Cardiol. 2013;28:7-18). Studies were selected for inclusion if they were randomized, controlled clinical trials including a direct comparison between a statin and control condition or placebo.
Thirty-two studies were included, which enrolled a total of 71,005 patients. Statin use was significantly associated with a decreased risk of AF (odds ratio, 0.69; 95% CI: 0.57-0.83). The benefit of statin therapy was significant for the prevention of postoperative AF (OR, 0.37; 95% CI: 0.28-0.51) and secondary prevention of AF (OR, 0.57; 95% CI: 0.36-0.91). No clear benefit of statins for new-onset AF was identified, and no difference was observed between intensive and standard therapy.
The mechanism of action is hypothesized to be exerted through the anti-inflammatory and antioxidant effects of statins.
Some of these patients may already be on statins. But for those who are not and could tolerate them, the use of statins decreased the odds of postoperative and secondary AF by 40%-60%. This could result in enormous potential cost savings to the U.S. health care system.
The evidence is strong, so we need to ask ourselves, why are we not doing this already?
Dr. Ebbert is professor of medicine and a primary care clinician at the Mayo Clinic in Rochester, Minn. He reported having no relevant financial conflicts. The opinions expressed are those of the author.
The risk for atrial fibrillation increases with age and the presence of structural heart disease. AF exerts an enormous financial burden on the U.S. health care system. The overall prevalence of AF is 1%, and 70% of people with AF are 65 years of age or older. Inclusive of inpatient and outpatient expenditures, costs for the first episode of atrial fibrillation are estimated to be $15,000.
Perhaps we are all too familiar with the staggering resources consumed by patients who, despite adequate rate control, remain symptomatic. In these cases, an ounce of prevention could literally have been thousands of dollars of cure.
So, can we prevent A-fib?
Statins have been proposed as a way to do this. So, what’s the most recent evidence telling us about its efficacy?
Researchers in France conducted an updated systematic review of the literature to determine the benefit of statins for the prevention of AF (Curr. Opin. Cardiol. 2013;28:7-18). Studies were selected for inclusion if they were randomized, controlled clinical trials including a direct comparison between a statin and control condition or placebo.
Thirty-two studies were included, which enrolled a total of 71,005 patients. Statin use was significantly associated with a decreased risk of AF (odds ratio, 0.69; 95% CI: 0.57-0.83). The benefit of statin therapy was significant for the prevention of postoperative AF (OR, 0.37; 95% CI: 0.28-0.51) and secondary prevention of AF (OR, 0.57; 95% CI: 0.36-0.91). No clear benefit of statins for new-onset AF was identified, and no difference was observed between intensive and standard therapy.
The mechanism of action is hypothesized to be exerted through the anti-inflammatory and antioxidant effects of statins.
Some of these patients may already be on statins. But for those who are not and could tolerate them, the use of statins decreased the odds of postoperative and secondary AF by 40%-60%. This could result in enormous potential cost savings to the U.S. health care system.
The evidence is strong, so we need to ask ourselves, why are we not doing this already?
Dr. Ebbert is professor of medicine and a primary care clinician at the Mayo Clinic in Rochester, Minn. He reported having no relevant financial conflicts. The opinions expressed are those of the author.
The risk for atrial fibrillation increases with age and the presence of structural heart disease. AF exerts an enormous financial burden on the U.S. health care system. The overall prevalence of AF is 1%, and 70% of people with AF are 65 years of age or older. Inclusive of inpatient and outpatient expenditures, costs for the first episode of atrial fibrillation are estimated to be $15,000.
Perhaps we are all too familiar with the staggering resources consumed by patients who, despite adequate rate control, remain symptomatic. In these cases, an ounce of prevention could literally have been thousands of dollars of cure.
So, can we prevent A-fib?
Statins have been proposed as a way to do this. So, what’s the most recent evidence telling us about its efficacy?
Researchers in France conducted an updated systematic review of the literature to determine the benefit of statins for the prevention of AF (Curr. Opin. Cardiol. 2013;28:7-18). Studies were selected for inclusion if they were randomized, controlled clinical trials including a direct comparison between a statin and control condition or placebo.
Thirty-two studies were included, which enrolled a total of 71,005 patients. Statin use was significantly associated with a decreased risk of AF (odds ratio, 0.69; 95% CI: 0.57-0.83). The benefit of statin therapy was significant for the prevention of postoperative AF (OR, 0.37; 95% CI: 0.28-0.51) and secondary prevention of AF (OR, 0.57; 95% CI: 0.36-0.91). No clear benefit of statins for new-onset AF was identified, and no difference was observed between intensive and standard therapy.
The mechanism of action is hypothesized to be exerted through the anti-inflammatory and antioxidant effects of statins.
Some of these patients may already be on statins. But for those who are not and could tolerate them, the use of statins decreased the odds of postoperative and secondary AF by 40%-60%. This could result in enormous potential cost savings to the U.S. health care system.
The evidence is strong, so we need to ask ourselves, why are we not doing this already?
Dr. Ebbert is professor of medicine and a primary care clinician at the Mayo Clinic in Rochester, Minn. He reported having no relevant financial conflicts. The opinions expressed are those of the author.
Good news for apixaban in recurrent VTE prevention
ATLANTA – An extra year of apixaban reduced the risk of recurrent events in patients with venous thromboembolism by 80%, while keeping major bleeding rates in line with placebo in the randomized AMPLIFY-EXT trial.
The number needed to treat with apixaban (Eliquis) to prevent one fatal or nonfatal recurrent VTE was only 14, while the number needed to treat to cause one episode of major or clinically relevant nonmajor bleeding was 200, Dr. Giancarlo Agnelli reported in a late-breaking abstract at the annual meeting of the American Society of Hematology.
"We really believe this study, for its design and results, is a remarkable achievement, and [may lead to a] change in clinical practice," he said during a press briefing at the meeting.
Apixaban was approved by the Food and Drug Administration in late December for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation, based largely on data demonstrating superiority to warfarin in patients with AF in the ARISTOTLE trial.*
In the meantime, the results of AMPLIFY-EXT (Apixaban After the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis with First-Line Therapy–Extended Treatment) provide some guidance for physicians uncertain about whether to extend or stop standard anticoagulation therapy in patients with VTE in the absence of recurrent events. Stopping warfarin therapy increases the risk of recurrent VTE by up to 10% in patients without reversible risk factors, but also requires frequent laboratory monitoring and increases the risk of bleeding.
Apixaban, an oral factor Xa inhibitor, is given in fixed doses without the need for laboratory monitoring, said Dr. Agnelli, director of the internal and cardiovascular medicine/stroke unit at the University of Perugia, Italy.
Given the efficacy demonstrated in AMPLIFY-EXT, apixaban may also be an attractive option for those VTE patients with renal impairment, because it is the least dependent on renal clearance compared with two other fixed-dose anticoagulants, rivaroxaban (Xarelto) and dabigatran (Pradaxa), said press briefing moderator Dr. Agnes Lee, medical director of the thrombosis program and associate professor of medicine at the University of British Columbia, Vancouver, and Vancouver Coastal Health.
Notably, a recent prespecified substudy of ARISTOLE demonstrated that apixaban produced 35%-52% fewer major bleeding events in patients with renal dysfunction and atrial fibrillation.
The double-blind AMPLIFY-EXT trial randomized 842 patients to apixaban 2.5 mg, 815 to apixaban 5 mg, and 829 to placebo, all twice daily for 12 months. Three-fourths had an initial diagnosis of deep vein thrombosis and one-fourth pulmonary embolism. All had received 6-12 months of anticoagulation therapy and reached clinical equipoise about the continuation or cessation of anticoagulation therapy.
VTE was associated with a transient or reversible risk factor in less than 10% of patients. Two patients from each apixaban group were excluded from the intention-to-treat efficacy analysis. Their average age was roughly 56.
The composite primary efficacy endpoint of symptomatic VTE recurrence or all-cause death occurred in 3.8% of patients on apixaban 2.5 mg and in 4.2% of patients on apixaban 5 mg, compared with 11.6% of patients given placebo, Dr. Agnelli said.
Symptomatic recurrent VTE or death from VTE occurred in 1.7% of patients in both apixaban groups vs. 8.8% of placebo-treated patients.
Major bleeding was reported in 0.2% of the 2.5-mg apixaban group, 0.1% of the 5-mg group, and 0.5% of the placebo group. Clinically relevant nonmajor bleeding rates were slightly higher at 3.0% and 4.2% in the apixaban groups vs. 2.3% in the placebo group, he said.
Further study will be needed to determine if the results can be directly applied to cancer patients who face an increased risk of VTE because of the disease, as only about 2% of the study population had active cancer, Dr. Agnelli said in an interview.
The study was simultaneously published in the New England Journal of Medicine (2012 Dec. 8 [doi: 10.1056/NEJMoa1207541]).
AMPLIFY-EXT was funded by Bristol-Myers Squibb and Pfizer. Dr. Agnelli reported commercial relationships with Bristol-Myers Squibb, Daiichi Sankyo, and other companies. His coauthors reported relationships with the study sponsors. Dr. Lee disclosed consulting for Bristol-Myers Squibb.
*This article was updated January 2, 2013.
ATLANTA – An extra year of apixaban reduced the risk of recurrent events in patients with venous thromboembolism by 80%, while keeping major bleeding rates in line with placebo in the randomized AMPLIFY-EXT trial.
The number needed to treat with apixaban (Eliquis) to prevent one fatal or nonfatal recurrent VTE was only 14, while the number needed to treat to cause one episode of major or clinically relevant nonmajor bleeding was 200, Dr. Giancarlo Agnelli reported in a late-breaking abstract at the annual meeting of the American Society of Hematology.
"We really believe this study, for its design and results, is a remarkable achievement, and [may lead to a] change in clinical practice," he said during a press briefing at the meeting.
Apixaban was approved by the Food and Drug Administration in late December for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation, based largely on data demonstrating superiority to warfarin in patients with AF in the ARISTOTLE trial.*
In the meantime, the results of AMPLIFY-EXT (Apixaban After the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis with First-Line Therapy–Extended Treatment) provide some guidance for physicians uncertain about whether to extend or stop standard anticoagulation therapy in patients with VTE in the absence of recurrent events. Stopping warfarin therapy increases the risk of recurrent VTE by up to 10% in patients without reversible risk factors, but also requires frequent laboratory monitoring and increases the risk of bleeding.
Apixaban, an oral factor Xa inhibitor, is given in fixed doses without the need for laboratory monitoring, said Dr. Agnelli, director of the internal and cardiovascular medicine/stroke unit at the University of Perugia, Italy.
Given the efficacy demonstrated in AMPLIFY-EXT, apixaban may also be an attractive option for those VTE patients with renal impairment, because it is the least dependent on renal clearance compared with two other fixed-dose anticoagulants, rivaroxaban (Xarelto) and dabigatran (Pradaxa), said press briefing moderator Dr. Agnes Lee, medical director of the thrombosis program and associate professor of medicine at the University of British Columbia, Vancouver, and Vancouver Coastal Health.
Notably, a recent prespecified substudy of ARISTOLE demonstrated that apixaban produced 35%-52% fewer major bleeding events in patients with renal dysfunction and atrial fibrillation.
The double-blind AMPLIFY-EXT trial randomized 842 patients to apixaban 2.5 mg, 815 to apixaban 5 mg, and 829 to placebo, all twice daily for 12 months. Three-fourths had an initial diagnosis of deep vein thrombosis and one-fourth pulmonary embolism. All had received 6-12 months of anticoagulation therapy and reached clinical equipoise about the continuation or cessation of anticoagulation therapy.
VTE was associated with a transient or reversible risk factor in less than 10% of patients. Two patients from each apixaban group were excluded from the intention-to-treat efficacy analysis. Their average age was roughly 56.
The composite primary efficacy endpoint of symptomatic VTE recurrence or all-cause death occurred in 3.8% of patients on apixaban 2.5 mg and in 4.2% of patients on apixaban 5 mg, compared with 11.6% of patients given placebo, Dr. Agnelli said.
Symptomatic recurrent VTE or death from VTE occurred in 1.7% of patients in both apixaban groups vs. 8.8% of placebo-treated patients.
Major bleeding was reported in 0.2% of the 2.5-mg apixaban group, 0.1% of the 5-mg group, and 0.5% of the placebo group. Clinically relevant nonmajor bleeding rates were slightly higher at 3.0% and 4.2% in the apixaban groups vs. 2.3% in the placebo group, he said.
Further study will be needed to determine if the results can be directly applied to cancer patients who face an increased risk of VTE because of the disease, as only about 2% of the study population had active cancer, Dr. Agnelli said in an interview.
The study was simultaneously published in the New England Journal of Medicine (2012 Dec. 8 [doi: 10.1056/NEJMoa1207541]).
AMPLIFY-EXT was funded by Bristol-Myers Squibb and Pfizer. Dr. Agnelli reported commercial relationships with Bristol-Myers Squibb, Daiichi Sankyo, and other companies. His coauthors reported relationships with the study sponsors. Dr. Lee disclosed consulting for Bristol-Myers Squibb.
*This article was updated January 2, 2013.
ATLANTA – An extra year of apixaban reduced the risk of recurrent events in patients with venous thromboembolism by 80%, while keeping major bleeding rates in line with placebo in the randomized AMPLIFY-EXT trial.
The number needed to treat with apixaban (Eliquis) to prevent one fatal or nonfatal recurrent VTE was only 14, while the number needed to treat to cause one episode of major or clinically relevant nonmajor bleeding was 200, Dr. Giancarlo Agnelli reported in a late-breaking abstract at the annual meeting of the American Society of Hematology.
"We really believe this study, for its design and results, is a remarkable achievement, and [may lead to a] change in clinical practice," he said during a press briefing at the meeting.
Apixaban was approved by the Food and Drug Administration in late December for the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation, based largely on data demonstrating superiority to warfarin in patients with AF in the ARISTOTLE trial.*
In the meantime, the results of AMPLIFY-EXT (Apixaban After the Initial Management of Pulmonary Embolism and Deep Vein Thrombosis with First-Line Therapy–Extended Treatment) provide some guidance for physicians uncertain about whether to extend or stop standard anticoagulation therapy in patients with VTE in the absence of recurrent events. Stopping warfarin therapy increases the risk of recurrent VTE by up to 10% in patients without reversible risk factors, but also requires frequent laboratory monitoring and increases the risk of bleeding.
Apixaban, an oral factor Xa inhibitor, is given in fixed doses without the need for laboratory monitoring, said Dr. Agnelli, director of the internal and cardiovascular medicine/stroke unit at the University of Perugia, Italy.
Given the efficacy demonstrated in AMPLIFY-EXT, apixaban may also be an attractive option for those VTE patients with renal impairment, because it is the least dependent on renal clearance compared with two other fixed-dose anticoagulants, rivaroxaban (Xarelto) and dabigatran (Pradaxa), said press briefing moderator Dr. Agnes Lee, medical director of the thrombosis program and associate professor of medicine at the University of British Columbia, Vancouver, and Vancouver Coastal Health.
Notably, a recent prespecified substudy of ARISTOLE demonstrated that apixaban produced 35%-52% fewer major bleeding events in patients with renal dysfunction and atrial fibrillation.
The double-blind AMPLIFY-EXT trial randomized 842 patients to apixaban 2.5 mg, 815 to apixaban 5 mg, and 829 to placebo, all twice daily for 12 months. Three-fourths had an initial diagnosis of deep vein thrombosis and one-fourth pulmonary embolism. All had received 6-12 months of anticoagulation therapy and reached clinical equipoise about the continuation or cessation of anticoagulation therapy.
VTE was associated with a transient or reversible risk factor in less than 10% of patients. Two patients from each apixaban group were excluded from the intention-to-treat efficacy analysis. Their average age was roughly 56.
The composite primary efficacy endpoint of symptomatic VTE recurrence or all-cause death occurred in 3.8% of patients on apixaban 2.5 mg and in 4.2% of patients on apixaban 5 mg, compared with 11.6% of patients given placebo, Dr. Agnelli said.
Symptomatic recurrent VTE or death from VTE occurred in 1.7% of patients in both apixaban groups vs. 8.8% of placebo-treated patients.
Major bleeding was reported in 0.2% of the 2.5-mg apixaban group, 0.1% of the 5-mg group, and 0.5% of the placebo group. Clinically relevant nonmajor bleeding rates were slightly higher at 3.0% and 4.2% in the apixaban groups vs. 2.3% in the placebo group, he said.
Further study will be needed to determine if the results can be directly applied to cancer patients who face an increased risk of VTE because of the disease, as only about 2% of the study population had active cancer, Dr. Agnelli said in an interview.
The study was simultaneously published in the New England Journal of Medicine (2012 Dec. 8 [doi: 10.1056/NEJMoa1207541]).
AMPLIFY-EXT was funded by Bristol-Myers Squibb and Pfizer. Dr. Agnelli reported commercial relationships with Bristol-Myers Squibb, Daiichi Sankyo, and other companies. His coauthors reported relationships with the study sponsors. Dr. Lee disclosed consulting for Bristol-Myers Squibb.
*This article was updated January 2, 2013.
AT THE ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY
Major Finding: The composite primary efficacy endpoint of symptomatic VTE recurrence or all-cause death occurred in 3.8% of patients on apixaban 2.5 mg and 4.2% of patients on apixaban 5 mg, compared with 11.6% of patients given placebo.
Data Source: Double-blind, randomized trial in 2,486 patients with VTE.
Disclosures: AMPLIFY-EXT was funded by Bristol-Myers Squibb and Pfizer. Dr. Agnelli reported commercial relationships with Bristol-Myers Squibb, Daiichi Sankyo, and other companies. His coauthors reported relationships with the study sponsors. Dr. Lee disclosed consulting for Bristol-Myers Squibb.