User login
Official Newspaper of the American College of Surgeons
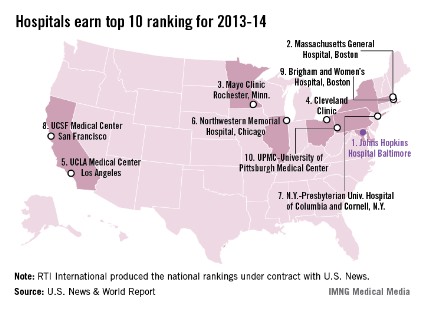
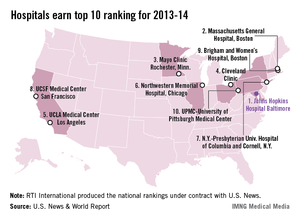
Johns Hopkins named top hospital for 2013-14
Johns Hopkins Hospital in Baltimore regained the top spot in the annual ranking of U.S. hospitals conducted by U.S. News and World Report after finishing second to Massachusetts General Hospital in Boston last year.
Massachusetts General was second this year, with the Mayo Clinic (Rochester, Minn.) ranked third, the Cleveland Clinic fourth, and the UCLA Medical Center in Los Angeles fifth, U.S. News reported.
The number six hospital, Northwestern Memorial in Chicago, made the biggest jump from last year among the top hospitals, moving up from 12th place. Also moving up six places was UCSF Medical Center in San Francisco, which went from 13th to 7th. Dropping out of the top 10 this year were Barnes-Jewish Hospital/Washington University (dropping from 6th to 15th) in St. Louis and Duke University Medical Center (from 8th to 12th) in Durham, N.C.
The ranking process initially considered 4,806 nonfederal hospitals, which were rated in 12 data-driven specialties and four reputation-only specialties. In the 12 data-driven specialties, scores were based on four elements: reputation with specialists (32.5%), survival (32.5%), patient safety (5%), and other care-related indicators (30%). The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.
Johns Hopkins Hospital in Baltimore regained the top spot in the annual ranking of U.S. hospitals conducted by U.S. News and World Report after finishing second to Massachusetts General Hospital in Boston last year.
Massachusetts General was second this year, with the Mayo Clinic (Rochester, Minn.) ranked third, the Cleveland Clinic fourth, and the UCLA Medical Center in Los Angeles fifth, U.S. News reported.
The number six hospital, Northwestern Memorial in Chicago, made the biggest jump from last year among the top hospitals, moving up from 12th place. Also moving up six places was UCSF Medical Center in San Francisco, which went from 13th to 7th. Dropping out of the top 10 this year were Barnes-Jewish Hospital/Washington University (dropping from 6th to 15th) in St. Louis and Duke University Medical Center (from 8th to 12th) in Durham, N.C.
The ranking process initially considered 4,806 nonfederal hospitals, which were rated in 12 data-driven specialties and four reputation-only specialties. In the 12 data-driven specialties, scores were based on four elements: reputation with specialists (32.5%), survival (32.5%), patient safety (5%), and other care-related indicators (30%). The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

Johns Hopkins Hospital in Baltimore regained the top spot in the annual ranking of U.S. hospitals conducted by U.S. News and World Report after finishing second to Massachusetts General Hospital in Boston last year.
Massachusetts General was second this year, with the Mayo Clinic (Rochester, Minn.) ranked third, the Cleveland Clinic fourth, and the UCLA Medical Center in Los Angeles fifth, U.S. News reported.
The number six hospital, Northwestern Memorial in Chicago, made the biggest jump from last year among the top hospitals, moving up from 12th place. Also moving up six places was UCSF Medical Center in San Francisco, which went from 13th to 7th. Dropping out of the top 10 this year were Barnes-Jewish Hospital/Washington University (dropping from 6th to 15th) in St. Louis and Duke University Medical Center (from 8th to 12th) in Durham, N.C.
The ranking process initially considered 4,806 nonfederal hospitals, which were rated in 12 data-driven specialties and four reputation-only specialties. In the 12 data-driven specialties, scores were based on four elements: reputation with specialists (32.5%), survival (32.5%), patient safety (5%), and other care-related indicators (30%). The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

Year 1: Pioneer ACOs deliver quality, some drop out
In the first year of Medicare’s most aggressive test of the accountable care organization model, less than half of participating organizations cut costs enough to share the savings with the government, though all slowed overall costs and improved quality across the-board.
Of the 32 Pioneer ACOs, 13 generated enough savings in 2012 to share in the earnings with the Medicare program, though only two generated shared losses, according to first-year results released by Medicare July 16.
Overall, the successful ACOs took home about $76 million in shared savings, while Medicare earned nearly $33 million from shared savings and about $4 million from shared losses.
The Pioneers were also successful in lowering the overall cost growth for the 669,000 Medicare beneficiaries associated with their ACOs. Costs for those beneficiaries grew by 0.3% in 2012 compared with 0.8% for similar beneficiaries during the same time period.
"If you want evidence of bending the cost curve, that’s a clear win," said Rob Lazerow, practice manager at The Advisory Board Company, a consulting firm specializing in health care.
The Pioneer ACO model, launched January 2012 under the Affordable Care Act, is a 3-year program that incentivizes health care systems to improve care and coordination to save money. Successful systems share in the savings; unsuccessful ones share in the losses.
To share in the savings, the Pioneer ACOs must meet quality standards and generate a minimum savings rate between 1% and 2.7% to account for normal variation in Medicare spending. The shared savings are determined by comparing the ACO’s performance to a benchmark, which is based on Medicare’s past spending for the group of patients associated with the ACO.
During the third year of the program, Pioneer ACOs that have achieved savings during the first 2 years can opt to move to a population-based payment model where they would receive a per-beneficiary per month payment to replace some or all of their Medicare fee-for-service payments.
In this first year, decreased hospital admissions and readmissions were partly responsible for the savings, according to officials at the Centers for Medicare and Medicaid Services. In terms of readmissions, 25 of the 32 Pioneer ACOs generated lower risk-adjusted readmission rates for their beneficiaries compared to the benchmark rate for all Medicare fee-for-service beneficiaries.
The Pioneer ACOs also outperformed other managed care populations on hypertension and cholesterol control. The median rate for hypertension control for diabetic beneficiaries was 68% at Pioneer ACOs, compared to 55% in adult diabetic patients in 10 managed care plans across seven states from 2000 to 2001. Similarly, the median rate for low density lipoprotein (LDL) control among diabetic beneficiaries was 57% in the Pioneer ACOs, compared to 48% among adult diabetic patients among the managed care plans from 2000 to 2001.
Overall, the Pioneer ACOs exceeded the published performance of fee-for-service Medicare for 15 clinical quality measures in which there were data to make comparisons. Another seven measures didn’t have comparable data in the published literature, according to the CMS.
The results are encouraging, especially for a new program, said Blair Childs, senior vice president of public affairs for the Premier health care alliance.
"The fact that these improvements were achieved, so quickly, is a real testament to the participating health systems, all of whom stepped up to assume significant risk and make major investments in ACO infrastructure, health information technology, governance, and care delivery models," Mr. Childs said. "In many cases, participating Pioneers had to take a ‘leap of faith,’ and the fact that so many goals were reached is helpful in terms of building the evidence base to support this new model of care as an effective way to improve quality and reduce costs."
The CMS also announced that nine of the ACOs will be leaving the Pioneer program. Of those, two are leaving the program completely while seven have notified the CMS that they intend to apply to the Medicare Shared Savings Program, a different ACO model with less aggressive risk sharing.
The University of Michigan is one of the seven organizations that will be moving out of the Pioneer ACO model and into the Medicare Shared Savings Program. Officials at the university said they are seeking to move to a program that will have simpler administrative requirements but will allow them to continue participation in the ACO environment.
"We remain firmly committed to the concept of improving health care and containing cost growth via the population health model that drives all ACOs," Dr. David Spahlinger, executive director of the University of Michigan Medical School’s Faculty Group Practice, said in a statement.
Conversely, the Phoenix-based Banner Health Network plans to stay in the program and is already recruiting new physicians for the third year of the program, which begins in 2014.
"Through our experience, we believe the value-based Pioneer ACO model has merit, and that it has the potential to diminish the predominance of fee-for-service plans in government and private sectors," Chuck Lehn, CEO of the Banner Health Network, said in a statement. "It is the best solution at this time for creating sustainability for the Medicare program, and could be the basis for historic change in the U.S. health care industry."
The departure of nine ACOs from the Pioneer model isn’t bad news for the program, Mr. Childs said. "In our view, this is an organic shift, and one that should be expected."
Mr. Childs predicted that even those ACOs that chose to leave the program completely will continue to experiment with the model with private payers.
"Remember that providers have made tremendous investments in getting their ACOs off the ground and they will want and need to continue to leverage these investments, most likely in a setting that offers more favorable terms that are more amenable to their operations and local patient populations," he said. "In our view, the churn out of Pioneer and into other flavors of ACO payment is more about an individual organization’s appetite for risk than it is a statement about the program."
In the first year of Medicare’s most aggressive test of the accountable care organization model, less than half of participating organizations cut costs enough to share the savings with the government, though all slowed overall costs and improved quality across the-board.
Of the 32 Pioneer ACOs, 13 generated enough savings in 2012 to share in the earnings with the Medicare program, though only two generated shared losses, according to first-year results released by Medicare July 16.
Overall, the successful ACOs took home about $76 million in shared savings, while Medicare earned nearly $33 million from shared savings and about $4 million from shared losses.
The Pioneers were also successful in lowering the overall cost growth for the 669,000 Medicare beneficiaries associated with their ACOs. Costs for those beneficiaries grew by 0.3% in 2012 compared with 0.8% for similar beneficiaries during the same time period.
"If you want evidence of bending the cost curve, that’s a clear win," said Rob Lazerow, practice manager at The Advisory Board Company, a consulting firm specializing in health care.
The Pioneer ACO model, launched January 2012 under the Affordable Care Act, is a 3-year program that incentivizes health care systems to improve care and coordination to save money. Successful systems share in the savings; unsuccessful ones share in the losses.
To share in the savings, the Pioneer ACOs must meet quality standards and generate a minimum savings rate between 1% and 2.7% to account for normal variation in Medicare spending. The shared savings are determined by comparing the ACO’s performance to a benchmark, which is based on Medicare’s past spending for the group of patients associated with the ACO.
During the third year of the program, Pioneer ACOs that have achieved savings during the first 2 years can opt to move to a population-based payment model where they would receive a per-beneficiary per month payment to replace some or all of their Medicare fee-for-service payments.
In this first year, decreased hospital admissions and readmissions were partly responsible for the savings, according to officials at the Centers for Medicare and Medicaid Services. In terms of readmissions, 25 of the 32 Pioneer ACOs generated lower risk-adjusted readmission rates for their beneficiaries compared to the benchmark rate for all Medicare fee-for-service beneficiaries.
The Pioneer ACOs also outperformed other managed care populations on hypertension and cholesterol control. The median rate for hypertension control for diabetic beneficiaries was 68% at Pioneer ACOs, compared to 55% in adult diabetic patients in 10 managed care plans across seven states from 2000 to 2001. Similarly, the median rate for low density lipoprotein (LDL) control among diabetic beneficiaries was 57% in the Pioneer ACOs, compared to 48% among adult diabetic patients among the managed care plans from 2000 to 2001.
Overall, the Pioneer ACOs exceeded the published performance of fee-for-service Medicare for 15 clinical quality measures in which there were data to make comparisons. Another seven measures didn’t have comparable data in the published literature, according to the CMS.
The results are encouraging, especially for a new program, said Blair Childs, senior vice president of public affairs for the Premier health care alliance.
"The fact that these improvements were achieved, so quickly, is a real testament to the participating health systems, all of whom stepped up to assume significant risk and make major investments in ACO infrastructure, health information technology, governance, and care delivery models," Mr. Childs said. "In many cases, participating Pioneers had to take a ‘leap of faith,’ and the fact that so many goals were reached is helpful in terms of building the evidence base to support this new model of care as an effective way to improve quality and reduce costs."
The CMS also announced that nine of the ACOs will be leaving the Pioneer program. Of those, two are leaving the program completely while seven have notified the CMS that they intend to apply to the Medicare Shared Savings Program, a different ACO model with less aggressive risk sharing.
The University of Michigan is one of the seven organizations that will be moving out of the Pioneer ACO model and into the Medicare Shared Savings Program. Officials at the university said they are seeking to move to a program that will have simpler administrative requirements but will allow them to continue participation in the ACO environment.
"We remain firmly committed to the concept of improving health care and containing cost growth via the population health model that drives all ACOs," Dr. David Spahlinger, executive director of the University of Michigan Medical School’s Faculty Group Practice, said in a statement.
Conversely, the Phoenix-based Banner Health Network plans to stay in the program and is already recruiting new physicians for the third year of the program, which begins in 2014.
"Through our experience, we believe the value-based Pioneer ACO model has merit, and that it has the potential to diminish the predominance of fee-for-service plans in government and private sectors," Chuck Lehn, CEO of the Banner Health Network, said in a statement. "It is the best solution at this time for creating sustainability for the Medicare program, and could be the basis for historic change in the U.S. health care industry."
The departure of nine ACOs from the Pioneer model isn’t bad news for the program, Mr. Childs said. "In our view, this is an organic shift, and one that should be expected."
Mr. Childs predicted that even those ACOs that chose to leave the program completely will continue to experiment with the model with private payers.
"Remember that providers have made tremendous investments in getting their ACOs off the ground and they will want and need to continue to leverage these investments, most likely in a setting that offers more favorable terms that are more amenable to their operations and local patient populations," he said. "In our view, the churn out of Pioneer and into other flavors of ACO payment is more about an individual organization’s appetite for risk than it is a statement about the program."
In the first year of Medicare’s most aggressive test of the accountable care organization model, less than half of participating organizations cut costs enough to share the savings with the government, though all slowed overall costs and improved quality across the-board.
Of the 32 Pioneer ACOs, 13 generated enough savings in 2012 to share in the earnings with the Medicare program, though only two generated shared losses, according to first-year results released by Medicare July 16.
Overall, the successful ACOs took home about $76 million in shared savings, while Medicare earned nearly $33 million from shared savings and about $4 million from shared losses.
The Pioneers were also successful in lowering the overall cost growth for the 669,000 Medicare beneficiaries associated with their ACOs. Costs for those beneficiaries grew by 0.3% in 2012 compared with 0.8% for similar beneficiaries during the same time period.
"If you want evidence of bending the cost curve, that’s a clear win," said Rob Lazerow, practice manager at The Advisory Board Company, a consulting firm specializing in health care.
The Pioneer ACO model, launched January 2012 under the Affordable Care Act, is a 3-year program that incentivizes health care systems to improve care and coordination to save money. Successful systems share in the savings; unsuccessful ones share in the losses.
To share in the savings, the Pioneer ACOs must meet quality standards and generate a minimum savings rate between 1% and 2.7% to account for normal variation in Medicare spending. The shared savings are determined by comparing the ACO’s performance to a benchmark, which is based on Medicare’s past spending for the group of patients associated with the ACO.
During the third year of the program, Pioneer ACOs that have achieved savings during the first 2 years can opt to move to a population-based payment model where they would receive a per-beneficiary per month payment to replace some or all of their Medicare fee-for-service payments.
In this first year, decreased hospital admissions and readmissions were partly responsible for the savings, according to officials at the Centers for Medicare and Medicaid Services. In terms of readmissions, 25 of the 32 Pioneer ACOs generated lower risk-adjusted readmission rates for their beneficiaries compared to the benchmark rate for all Medicare fee-for-service beneficiaries.
The Pioneer ACOs also outperformed other managed care populations on hypertension and cholesterol control. The median rate for hypertension control for diabetic beneficiaries was 68% at Pioneer ACOs, compared to 55% in adult diabetic patients in 10 managed care plans across seven states from 2000 to 2001. Similarly, the median rate for low density lipoprotein (LDL) control among diabetic beneficiaries was 57% in the Pioneer ACOs, compared to 48% among adult diabetic patients among the managed care plans from 2000 to 2001.
Overall, the Pioneer ACOs exceeded the published performance of fee-for-service Medicare for 15 clinical quality measures in which there were data to make comparisons. Another seven measures didn’t have comparable data in the published literature, according to the CMS.
The results are encouraging, especially for a new program, said Blair Childs, senior vice president of public affairs for the Premier health care alliance.
"The fact that these improvements were achieved, so quickly, is a real testament to the participating health systems, all of whom stepped up to assume significant risk and make major investments in ACO infrastructure, health information technology, governance, and care delivery models," Mr. Childs said. "In many cases, participating Pioneers had to take a ‘leap of faith,’ and the fact that so many goals were reached is helpful in terms of building the evidence base to support this new model of care as an effective way to improve quality and reduce costs."
The CMS also announced that nine of the ACOs will be leaving the Pioneer program. Of those, two are leaving the program completely while seven have notified the CMS that they intend to apply to the Medicare Shared Savings Program, a different ACO model with less aggressive risk sharing.
The University of Michigan is one of the seven organizations that will be moving out of the Pioneer ACO model and into the Medicare Shared Savings Program. Officials at the university said they are seeking to move to a program that will have simpler administrative requirements but will allow them to continue participation in the ACO environment.
"We remain firmly committed to the concept of improving health care and containing cost growth via the population health model that drives all ACOs," Dr. David Spahlinger, executive director of the University of Michigan Medical School’s Faculty Group Practice, said in a statement.
Conversely, the Phoenix-based Banner Health Network plans to stay in the program and is already recruiting new physicians for the third year of the program, which begins in 2014.
"Through our experience, we believe the value-based Pioneer ACO model has merit, and that it has the potential to diminish the predominance of fee-for-service plans in government and private sectors," Chuck Lehn, CEO of the Banner Health Network, said in a statement. "It is the best solution at this time for creating sustainability for the Medicare program, and could be the basis for historic change in the U.S. health care industry."
The departure of nine ACOs from the Pioneer model isn’t bad news for the program, Mr. Childs said. "In our view, this is an organic shift, and one that should be expected."
Mr. Childs predicted that even those ACOs that chose to leave the program completely will continue to experiment with the model with private payers.
"Remember that providers have made tremendous investments in getting their ACOs off the ground and they will want and need to continue to leverage these investments, most likely in a setting that offers more favorable terms that are more amenable to their operations and local patient populations," he said. "In our view, the churn out of Pioneer and into other flavors of ACO payment is more about an individual organization’s appetite for risk than it is a statement about the program."
Endometriomas do not negatively affect ability to conceive
LONDON – Endometrial ovarian cysts do not reduce the rate of spontaneous ovulation and the potential ability of women to conceive, according to a prospective observational study.
Dr. Umberto Leone Roberti Maggiore of San Martino Hospital and the University of Genoa (Italy) reported the results of a study comparing the healthy and affected ovaries of 214 women with endometriosis. The overall rate of spontaneous ovulation was similar for affected and healthy ovaries over the course of six ovulatory cycles, at 50.3% vs. 49.7% (P = .919).
A total of 1,311 ovulatory cycles were examined during the study, with similar rates of ovulation observed regardless of the side, number, or size of endometriomas, he reported at the annual meeting of the European Society of Human Reproduction and Embryology.
"Over the last years, great attention has been given to the impact of endometriomas on ovarian physiology," Dr. Maggiore said. "Different studies have investigated whether the presence of endometriomas affect ovarian reserve and the outcome of assisted reproductive technologies."
Data from one study in particular suggested that the presence of endometriomas reduced the rate of spontaneous ovulation (Hum. Reprod. 2009;24:2183-6).
"The objective of the current study was to investigate the rate of spontaneous ovulation between the healthy and the affected ovary in women with unilateral endometriomas," Dr. Maggiore explained. Women were recruited into the study at an academic referral center between September 2009 and June 2013. For inclusion, they had to have ultrasound-confirmed endometrioma(s) of a single ovary of 20 mm or more in size, regular menstrual cycles (24-35 days), and a desire to conceive a child.
Women were excluded if they had previous adnexal surgery, had used hormonal therapies in the past 3 months, were pregnant or had breastfed their infants in the past 6 months, had a history of infertility, or had diagnoses of any of the following: hydrosalpinx, pelvic inflammatory disease, polycystic ovary syndrome, thyroid disorders, or psychiatric disturbances.
Transvaginal ultrasound was used to assess the side, number, largest diameter, and volume of the endometriomas. Ovarian reserve was assessed by measuring levels of anti-Müllerian hormone and basal follicle-stimulating hormone. The level of CA-125 was also measured.
The mean age of recruited women was 34 years; 55% of women had endometriomas of the right ovary, the majority (81.1%) had only one endometrial cyst, with 15.2% having two and 3.7% having three endometriomas. The largest diameter of the endometriomas at baseline was a mean of 4.5 cm, with 55.5% of women having a cyst equal to or greater than 4 cm and 15.1% a cyst equal to or greater than 6 cm. The total volume of endometriomas in the same ovary at baseline was a mean of 54.9 cm2.
In terms of pregnancy outcomes, 43% of women conceived during the study period. Of these 63.8% were at term, 20% of patients had an ongoing pregnancy, 3.8% had been delivered preterm, 1.9% voluntarily terminated their pregnancy at the second trimester, and 10.5% had miscarriages.
No correlation was found between levels of follicle-stimulating hormone, anti-Müllerian hormone, or CA-125 and the total endometrial volume, the largest diameter of the endometrioma, or the number of endometriomas. However, the size and volume of the endometriomas by the sixth ovarian cycle were seen to increase from baseline values by a respective 3.9% and 8.1% (both P values less than .001).
Dr. Maggiore reported that 40.2% of women had a 0.1%-5% increase in the total volume of endometriomas over the course of the study, with 29.1% experiencing a volume increase of 5.1%-10%, a further 19.7% a volume increase of 10.1%-25%, and 4.7% an increase of 25.1% or more. Only 6.3% of women experienced a decrease in total endometrioma volume.
"Normal ovulatory function and the potential decrease of ovarian reserve should be considered before suggesting the surgical treatment of endometriomas," said Dr. Maggiore. While surgical removal of these cysts might not be necessary purely to improve fertility, it is too early to say if they will change practice.
"I think [the study] shows that the mere presence of endometrioma is not sufficient to operate," Dr. Thomas D’Hooghe of the Leuven (Belgium) University fertility center said in an interview.
However, Dr. D’Hooghe, who was not involved in the study, noted that an increase in endometrioma volume within 6 months of observation was not an insignificant finding. "If you extrapolate that to say 1 year or 2 years after baseline, there may be a larger increase in volume. ... The endometrioma may rupture and cause adhesions" at that point, he said.
"The fact that endometriomas appear to be progressive might suggest that women who want to conceive should perhaps undergo cystectomy as early as possible," Dr. D’Hooghe suggested. "I would think that if the endometrioma increases in size, sooner or later it may affect fertility."
Dr. Maggiore and Dr. D’Hooghe said they had no relevant financial disclosures.
LONDON – Endometrial ovarian cysts do not reduce the rate of spontaneous ovulation and the potential ability of women to conceive, according to a prospective observational study.
Dr. Umberto Leone Roberti Maggiore of San Martino Hospital and the University of Genoa (Italy) reported the results of a study comparing the healthy and affected ovaries of 214 women with endometriosis. The overall rate of spontaneous ovulation was similar for affected and healthy ovaries over the course of six ovulatory cycles, at 50.3% vs. 49.7% (P = .919).
A total of 1,311 ovulatory cycles were examined during the study, with similar rates of ovulation observed regardless of the side, number, or size of endometriomas, he reported at the annual meeting of the European Society of Human Reproduction and Embryology.
"Over the last years, great attention has been given to the impact of endometriomas on ovarian physiology," Dr. Maggiore said. "Different studies have investigated whether the presence of endometriomas affect ovarian reserve and the outcome of assisted reproductive technologies."
Data from one study in particular suggested that the presence of endometriomas reduced the rate of spontaneous ovulation (Hum. Reprod. 2009;24:2183-6).
"The objective of the current study was to investigate the rate of spontaneous ovulation between the healthy and the affected ovary in women with unilateral endometriomas," Dr. Maggiore explained. Women were recruited into the study at an academic referral center between September 2009 and June 2013. For inclusion, they had to have ultrasound-confirmed endometrioma(s) of a single ovary of 20 mm or more in size, regular menstrual cycles (24-35 days), and a desire to conceive a child.
Women were excluded if they had previous adnexal surgery, had used hormonal therapies in the past 3 months, were pregnant or had breastfed their infants in the past 6 months, had a history of infertility, or had diagnoses of any of the following: hydrosalpinx, pelvic inflammatory disease, polycystic ovary syndrome, thyroid disorders, or psychiatric disturbances.
Transvaginal ultrasound was used to assess the side, number, largest diameter, and volume of the endometriomas. Ovarian reserve was assessed by measuring levels of anti-Müllerian hormone and basal follicle-stimulating hormone. The level of CA-125 was also measured.
The mean age of recruited women was 34 years; 55% of women had endometriomas of the right ovary, the majority (81.1%) had only one endometrial cyst, with 15.2% having two and 3.7% having three endometriomas. The largest diameter of the endometriomas at baseline was a mean of 4.5 cm, with 55.5% of women having a cyst equal to or greater than 4 cm and 15.1% a cyst equal to or greater than 6 cm. The total volume of endometriomas in the same ovary at baseline was a mean of 54.9 cm2.
In terms of pregnancy outcomes, 43% of women conceived during the study period. Of these 63.8% were at term, 20% of patients had an ongoing pregnancy, 3.8% had been delivered preterm, 1.9% voluntarily terminated their pregnancy at the second trimester, and 10.5% had miscarriages.
No correlation was found between levels of follicle-stimulating hormone, anti-Müllerian hormone, or CA-125 and the total endometrial volume, the largest diameter of the endometrioma, or the number of endometriomas. However, the size and volume of the endometriomas by the sixth ovarian cycle were seen to increase from baseline values by a respective 3.9% and 8.1% (both P values less than .001).
Dr. Maggiore reported that 40.2% of women had a 0.1%-5% increase in the total volume of endometriomas over the course of the study, with 29.1% experiencing a volume increase of 5.1%-10%, a further 19.7% a volume increase of 10.1%-25%, and 4.7% an increase of 25.1% or more. Only 6.3% of women experienced a decrease in total endometrioma volume.
"Normal ovulatory function and the potential decrease of ovarian reserve should be considered before suggesting the surgical treatment of endometriomas," said Dr. Maggiore. While surgical removal of these cysts might not be necessary purely to improve fertility, it is too early to say if they will change practice.
"I think [the study] shows that the mere presence of endometrioma is not sufficient to operate," Dr. Thomas D’Hooghe of the Leuven (Belgium) University fertility center said in an interview.
However, Dr. D’Hooghe, who was not involved in the study, noted that an increase in endometrioma volume within 6 months of observation was not an insignificant finding. "If you extrapolate that to say 1 year or 2 years after baseline, there may be a larger increase in volume. ... The endometrioma may rupture and cause adhesions" at that point, he said.
"The fact that endometriomas appear to be progressive might suggest that women who want to conceive should perhaps undergo cystectomy as early as possible," Dr. D’Hooghe suggested. "I would think that if the endometrioma increases in size, sooner or later it may affect fertility."
Dr. Maggiore and Dr. D’Hooghe said they had no relevant financial disclosures.
LONDON – Endometrial ovarian cysts do not reduce the rate of spontaneous ovulation and the potential ability of women to conceive, according to a prospective observational study.
Dr. Umberto Leone Roberti Maggiore of San Martino Hospital and the University of Genoa (Italy) reported the results of a study comparing the healthy and affected ovaries of 214 women with endometriosis. The overall rate of spontaneous ovulation was similar for affected and healthy ovaries over the course of six ovulatory cycles, at 50.3% vs. 49.7% (P = .919).
A total of 1,311 ovulatory cycles were examined during the study, with similar rates of ovulation observed regardless of the side, number, or size of endometriomas, he reported at the annual meeting of the European Society of Human Reproduction and Embryology.
"Over the last years, great attention has been given to the impact of endometriomas on ovarian physiology," Dr. Maggiore said. "Different studies have investigated whether the presence of endometriomas affect ovarian reserve and the outcome of assisted reproductive technologies."
Data from one study in particular suggested that the presence of endometriomas reduced the rate of spontaneous ovulation (Hum. Reprod. 2009;24:2183-6).
"The objective of the current study was to investigate the rate of spontaneous ovulation between the healthy and the affected ovary in women with unilateral endometriomas," Dr. Maggiore explained. Women were recruited into the study at an academic referral center between September 2009 and June 2013. For inclusion, they had to have ultrasound-confirmed endometrioma(s) of a single ovary of 20 mm or more in size, regular menstrual cycles (24-35 days), and a desire to conceive a child.
Women were excluded if they had previous adnexal surgery, had used hormonal therapies in the past 3 months, were pregnant or had breastfed their infants in the past 6 months, had a history of infertility, or had diagnoses of any of the following: hydrosalpinx, pelvic inflammatory disease, polycystic ovary syndrome, thyroid disorders, or psychiatric disturbances.
Transvaginal ultrasound was used to assess the side, number, largest diameter, and volume of the endometriomas. Ovarian reserve was assessed by measuring levels of anti-Müllerian hormone and basal follicle-stimulating hormone. The level of CA-125 was also measured.
The mean age of recruited women was 34 years; 55% of women had endometriomas of the right ovary, the majority (81.1%) had only one endometrial cyst, with 15.2% having two and 3.7% having three endometriomas. The largest diameter of the endometriomas at baseline was a mean of 4.5 cm, with 55.5% of women having a cyst equal to or greater than 4 cm and 15.1% a cyst equal to or greater than 6 cm. The total volume of endometriomas in the same ovary at baseline was a mean of 54.9 cm2.
In terms of pregnancy outcomes, 43% of women conceived during the study period. Of these 63.8% were at term, 20% of patients had an ongoing pregnancy, 3.8% had been delivered preterm, 1.9% voluntarily terminated their pregnancy at the second trimester, and 10.5% had miscarriages.
No correlation was found between levels of follicle-stimulating hormone, anti-Müllerian hormone, or CA-125 and the total endometrial volume, the largest diameter of the endometrioma, or the number of endometriomas. However, the size and volume of the endometriomas by the sixth ovarian cycle were seen to increase from baseline values by a respective 3.9% and 8.1% (both P values less than .001).
Dr. Maggiore reported that 40.2% of women had a 0.1%-5% increase in the total volume of endometriomas over the course of the study, with 29.1% experiencing a volume increase of 5.1%-10%, a further 19.7% a volume increase of 10.1%-25%, and 4.7% an increase of 25.1% or more. Only 6.3% of women experienced a decrease in total endometrioma volume.
"Normal ovulatory function and the potential decrease of ovarian reserve should be considered before suggesting the surgical treatment of endometriomas," said Dr. Maggiore. While surgical removal of these cysts might not be necessary purely to improve fertility, it is too early to say if they will change practice.
"I think [the study] shows that the mere presence of endometrioma is not sufficient to operate," Dr. Thomas D’Hooghe of the Leuven (Belgium) University fertility center said in an interview.
However, Dr. D’Hooghe, who was not involved in the study, noted that an increase in endometrioma volume within 6 months of observation was not an insignificant finding. "If you extrapolate that to say 1 year or 2 years after baseline, there may be a larger increase in volume. ... The endometrioma may rupture and cause adhesions" at that point, he said.
"The fact that endometriomas appear to be progressive might suggest that women who want to conceive should perhaps undergo cystectomy as early as possible," Dr. D’Hooghe suggested. "I would think that if the endometrioma increases in size, sooner or later it may affect fertility."
Dr. Maggiore and Dr. D’Hooghe said they had no relevant financial disclosures.
AT THE ANNUAL MEETING OF ESHRE
Major finding: Spontaneous ovulation occurred in 50.3% of affected ovaries and 49.7% of healthy ovaries over the course of six ovulatory cycles.
Data source: A prospective observational study of 214 women with endometriosis who wanted to conceive.
Disclosures: Dr. Maggiore and Dr. D’Hooghe said they had no relevant financial disclosures.
Will a novel antibody fix the anticoagulant-bleeding problem?
It seems inescapable: If patients are made less able to form blood clots, they bleed more.
Bleeding is the perennial problem for anticoagulants. Whether it’s the traditional anticoagulants (heparin, warfarin, and the low-molecular-weight heparins) or new drugs (fondaparinux, dabigatran, rivaroxaban, and apixaban), as the anticoagulant’s potency or dosage increases to stop blood clots from forming, the inevitable downside is increased bleeding.
Maybe not.
A newly developed, synthetic human IgG antibody appears, in animal and in vitro models, to allow normal clotting to occur and stop bleeding at vessel tears and cuts, while short-circuiting pathologic clotting in intravascular spaces – the sorts of clots that cause venous thromboembolisms, myocardial infarctions, and strokes.
"It seems too good to be true. It’s beyond comprehension," said Dr. Trevor Baglin, the University of Cambridge, England, hematologist who discovered the first identified, naturally occurring example of this antibody, in IgA form, in a patient he initially saw in 2008. "All we can do is go forward and see if it genuinely is as good as it seems," he said while presenting his group’s initial animal findings with the antibody at the Congress of the International Society on Thrombosis and Haemostasis in Amsterdam earlier this month.
The antibody – which has been patented, synthesized, and is in extensive preclinical testing – has been named ichorcumab. In Greek mythology, "ichor" was the blood factor in gods that made them immortal.
The secret behind ichorcumab is that it binds to and inactivates exosite 1, the part of the thrombin molecule that cleaves fibrinogen into fibrin, an effective brake on clotting. Study results suggest that whether the exosite 1 portion of thrombin is exposed or hidden at various body sites accounts for ichorcumab’s varied effects.
"Our hypothesis is that exosite 1 is protected from the antibody [when a thrombin molecule sits] on a cell or clot surface, so hemostasis is unaffected, but thrombosis occurs in the luminal space, where exosite 1 is exposed an available to the antibody," Dr. Baglin explained.
"While before we thought of just one type of clot, [the work with ichorcumab so far] suggests there is not one clotting mechanism but two," he noted, one that leads to clot formation that stops bleeding, and a second mechanism that produces clots that cause thrombosis. Ichorcumab blocks the bad clots but not the good ones, because the clots form at different locations that affect the way that exosite 1 on thrombin is exposed.
It may sound farfetched, but it’s a way for the researchers to explain the curious patient whom Dr. Baglin first met in 2008, a 53-year old woman who spontaneously makes and carries the IgA prototype of ichorcumab in her blood.
Dr. Baglin said that he consulted on her case after a preprocedural clotting screen revealed that her blood was unclottable by standard tests, yet she had no history of any bleeding disorder. In fact, her history showed that she had undergone knee surgery (when no clotting screen had been done) 5 months before Dr. Baglin first saw her without any hint of a bleeding incident. She subsequently cut the tip of a finger while slicing with a mandolin, but her bleeding stopped spontaneously.
The patient goes through life with this antibody in her blood at a level of about 3 g/L with no bleeding problems whatsoever; yet in a mouse model, a substantially lower level of the mimic antibody, ichorcumab, effectively blocked thrombosis. In the mouse model, this effective dose of ichorcumab does not cause bleeding if the mouse’s tail is cut.
Dr. Baglin and his associates started a company in Cambridge, XO1, to fund the preclinical work and eventually commercialize ichorcumab. They believe it will be another 2 years before any person receives a dose of the antibody.
–BY MITCHEL L. ZOLER
On Twitter @mitchelzoler
It seems inescapable: If patients are made less able to form blood clots, they bleed more.
Bleeding is the perennial problem for anticoagulants. Whether it’s the traditional anticoagulants (heparin, warfarin, and the low-molecular-weight heparins) or new drugs (fondaparinux, dabigatran, rivaroxaban, and apixaban), as the anticoagulant’s potency or dosage increases to stop blood clots from forming, the inevitable downside is increased bleeding.
Maybe not.
A newly developed, synthetic human IgG antibody appears, in animal and in vitro models, to allow normal clotting to occur and stop bleeding at vessel tears and cuts, while short-circuiting pathologic clotting in intravascular spaces – the sorts of clots that cause venous thromboembolisms, myocardial infarctions, and strokes.
"It seems too good to be true. It’s beyond comprehension," said Dr. Trevor Baglin, the University of Cambridge, England, hematologist who discovered the first identified, naturally occurring example of this antibody, in IgA form, in a patient he initially saw in 2008. "All we can do is go forward and see if it genuinely is as good as it seems," he said while presenting his group’s initial animal findings with the antibody at the Congress of the International Society on Thrombosis and Haemostasis in Amsterdam earlier this month.
The antibody – which has been patented, synthesized, and is in extensive preclinical testing – has been named ichorcumab. In Greek mythology, "ichor" was the blood factor in gods that made them immortal.
The secret behind ichorcumab is that it binds to and inactivates exosite 1, the part of the thrombin molecule that cleaves fibrinogen into fibrin, an effective brake on clotting. Study results suggest that whether the exosite 1 portion of thrombin is exposed or hidden at various body sites accounts for ichorcumab’s varied effects.
"Our hypothesis is that exosite 1 is protected from the antibody [when a thrombin molecule sits] on a cell or clot surface, so hemostasis is unaffected, but thrombosis occurs in the luminal space, where exosite 1 is exposed an available to the antibody," Dr. Baglin explained.
"While before we thought of just one type of clot, [the work with ichorcumab so far] suggests there is not one clotting mechanism but two," he noted, one that leads to clot formation that stops bleeding, and a second mechanism that produces clots that cause thrombosis. Ichorcumab blocks the bad clots but not the good ones, because the clots form at different locations that affect the way that exosite 1 on thrombin is exposed.
It may sound farfetched, but it’s a way for the researchers to explain the curious patient whom Dr. Baglin first met in 2008, a 53-year old woman who spontaneously makes and carries the IgA prototype of ichorcumab in her blood.
Dr. Baglin said that he consulted on her case after a preprocedural clotting screen revealed that her blood was unclottable by standard tests, yet she had no history of any bleeding disorder. In fact, her history showed that she had undergone knee surgery (when no clotting screen had been done) 5 months before Dr. Baglin first saw her without any hint of a bleeding incident. She subsequently cut the tip of a finger while slicing with a mandolin, but her bleeding stopped spontaneously.
The patient goes through life with this antibody in her blood at a level of about 3 g/L with no bleeding problems whatsoever; yet in a mouse model, a substantially lower level of the mimic antibody, ichorcumab, effectively blocked thrombosis. In the mouse model, this effective dose of ichorcumab does not cause bleeding if the mouse’s tail is cut.
Dr. Baglin and his associates started a company in Cambridge, XO1, to fund the preclinical work and eventually commercialize ichorcumab. They believe it will be another 2 years before any person receives a dose of the antibody.
–BY MITCHEL L. ZOLER
On Twitter @mitchelzoler
It seems inescapable: If patients are made less able to form blood clots, they bleed more.
Bleeding is the perennial problem for anticoagulants. Whether it’s the traditional anticoagulants (heparin, warfarin, and the low-molecular-weight heparins) or new drugs (fondaparinux, dabigatran, rivaroxaban, and apixaban), as the anticoagulant’s potency or dosage increases to stop blood clots from forming, the inevitable downside is increased bleeding.
Maybe not.
A newly developed, synthetic human IgG antibody appears, in animal and in vitro models, to allow normal clotting to occur and stop bleeding at vessel tears and cuts, while short-circuiting pathologic clotting in intravascular spaces – the sorts of clots that cause venous thromboembolisms, myocardial infarctions, and strokes.
"It seems too good to be true. It’s beyond comprehension," said Dr. Trevor Baglin, the University of Cambridge, England, hematologist who discovered the first identified, naturally occurring example of this antibody, in IgA form, in a patient he initially saw in 2008. "All we can do is go forward and see if it genuinely is as good as it seems," he said while presenting his group’s initial animal findings with the antibody at the Congress of the International Society on Thrombosis and Haemostasis in Amsterdam earlier this month.
The antibody – which has been patented, synthesized, and is in extensive preclinical testing – has been named ichorcumab. In Greek mythology, "ichor" was the blood factor in gods that made them immortal.
The secret behind ichorcumab is that it binds to and inactivates exosite 1, the part of the thrombin molecule that cleaves fibrinogen into fibrin, an effective brake on clotting. Study results suggest that whether the exosite 1 portion of thrombin is exposed or hidden at various body sites accounts for ichorcumab’s varied effects.
"Our hypothesis is that exosite 1 is protected from the antibody [when a thrombin molecule sits] on a cell or clot surface, so hemostasis is unaffected, but thrombosis occurs in the luminal space, where exosite 1 is exposed an available to the antibody," Dr. Baglin explained.
"While before we thought of just one type of clot, [the work with ichorcumab so far] suggests there is not one clotting mechanism but two," he noted, one that leads to clot formation that stops bleeding, and a second mechanism that produces clots that cause thrombosis. Ichorcumab blocks the bad clots but not the good ones, because the clots form at different locations that affect the way that exosite 1 on thrombin is exposed.
It may sound farfetched, but it’s a way for the researchers to explain the curious patient whom Dr. Baglin first met in 2008, a 53-year old woman who spontaneously makes and carries the IgA prototype of ichorcumab in her blood.
Dr. Baglin said that he consulted on her case after a preprocedural clotting screen revealed that her blood was unclottable by standard tests, yet she had no history of any bleeding disorder. In fact, her history showed that she had undergone knee surgery (when no clotting screen had been done) 5 months before Dr. Baglin first saw her without any hint of a bleeding incident. She subsequently cut the tip of a finger while slicing with a mandolin, but her bleeding stopped spontaneously.
The patient goes through life with this antibody in her blood at a level of about 3 g/L with no bleeding problems whatsoever; yet in a mouse model, a substantially lower level of the mimic antibody, ichorcumab, effectively blocked thrombosis. In the mouse model, this effective dose of ichorcumab does not cause bleeding if the mouse’s tail is cut.
Dr. Baglin and his associates started a company in Cambridge, XO1, to fund the preclinical work and eventually commercialize ichorcumab. They believe it will be another 2 years before any person receives a dose of the antibody.
–BY MITCHEL L. ZOLER
On Twitter @mitchelzoler
Prolaris test eyed as predictor of prostate cancer outcomes
CHICAGO – Prostate cancer outcomes were predicted by a test that measures the expression of cell cycle progression genes, according to results from a retrospective analysis of prostate tissue samples from five patient cohorts.
The Prolaris test gives each tissue sample a cell cycle progression (CCP) score based on measures of 31 CCP genes, normalized to 15 "housekeeper" genes. A unit change in the test is defined as a doubling in CCP genes. For each unit increase in the test’s score, there was a two- to threefold increase in the risk of disease progression, Dr. Jack M. Cuzick reported at the annual meeting of the American Society of Clinical Oncology.
The CCP signature of Myriad Genetics’ Prolaris test was a highly significant predictor of outcome, said Dr. Cuzick of the Wolfson Institute of Preventive Medicine, London. In all five studies, the hazard ratio per unit change in the CCP score was similar, ranging from 1.89 to 2.92. The findings indicate that the effect size for the CCP score is robust in multiple patient cohorts and diverse clinical settings.
The test provides information for differentiating aggressive and indolent disease beyond that available from clinicopathologic variables, he said. As the natural history of prostate cancer can be variable and difficult to predict, the Prolaris test could help to match treatment more appropriately to each individual’s risk of progression.
In the study that examined the test’s predictive value, five patient groups were evaluated. Formalin-fixed tissue samples were obtained from two English patient cohorts that were conservatively managed (n = 337 and 349), two U.S. patient cohorts that underwent radical prostatectomy (366 men treated at Scott & White Hospital, Temple, Tex.; and 413 men treated at the University of California, San Francisco), and one U.S. cohort that underwent external beam radiation therapy (141 men treated at the Durham, N.C., VA Medical Center).
The cohort of conservatively managed English patients was from the late 1990s and had more than 15 years of follow up. In the 337-patient cohort diagnosed via transurethral resection of the prostate (TURP) and conservatively managed, there were 57 deaths from prostate cancer. In the 349-patient cohort diagnosed via needle biopsy and conservatively managed, there were 90 deaths from prostate cancer.
For each unit increase in the CCP score, the hazard ratio for the cohort diagnosed via TURP was 2.9 and the hazard ratio for those diagnosed via needle biopsy was 2. The CCP score was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy). In both studies, the CCP score remained highly significant in multivariate analysis and was a stronger predictor of disease-specific mortality than other prognostic variables, he said.
In the U.S. prostatectomy cohorts, there were 132 biochemical recurrences (BCRs) in the first cohort and 83 BCRs in the second cohort. With each unit increase in the CCP score, there was a doubling of risk for recurrence. After prostatectomy, the CCP score predicted BCR in univariate analysis (Scott & White: P = 5.6 x 10–9; University of California: P = 2.23 x 10–6) and provided additional prognostic information in multivariate analysis (Scott & White: P = 3.3 x 10–6; University of California: P = 9.5 x10–5).
After radiation therapy, the CCP score predicted BCR in univariate (P = .0017) and multivariate (P = .034) analysis. In the 141-patient cohort that was diagnosed by needle biopsy and underwent external beam radiation, there were 19 prostate cancer deaths and more than a doubling of risk with each unit increase in CCP score.
CCP scores only modestly correlated with the Gleason score and prostate-specific antigen (PSA) value. The test adds value beyond those measures, Dr. Cuzick said.
CCP scores predict patient outcome in multiple clinical settings, provide independent information beyond clinicopathological variables, and help to further differentiate aggressive from indolent prostate cancer. With low-grade Gleason 6 cancers, the results can aid in telling who is at low risk and who needs aggressive therapy, he concluded.
The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
The ability to improve clinical management by finding prostate cancer patients who would benefit from more – or less – therapy is much needed. Clinicians are concerned that many prostate cancer patients are now overtreated, but they lack reliable prognostic guides.
Cell cycle progression (CCP) scores are interesting retrospectively, but how much are they able to improve on CAPRA (Cancer of the Prostate Risk Assessment) scores for predicting prognosis?
Prostate cancer is uniquely multifocal, with most men having multiple independent foci of cancer. In the example of the conservatively managed patients, if one is looking at men with indolent disease who have low-volume disease as a single core of one or two foci, is one really going to be able to predict the biologic outcome of the cancer? Those who fail after a surveillance approach often do so early and had undersampling of their disease. So it hasn’t been proven yet that this test can predict the behavior of cancer that hasn’t been sampled.
The CCP results proved to be statistically significant, but that finding does not indicate clinical utility. It’s not known whether the novel biomarkers in this test improve on existing markers. You find yourself asking what you would do differently in a patient whose risk of progression goes from 7% to 12%.
Even if a test independently predicts outcome, that doesn’t necessarily indicate it has clinical utility. The ability to improve clinical management is key to the adoption of new prognostic tests. The real question is whether CCP results improve on the existing model. Does the test improve on CAPRA for prognosis?
Dr. Scott Tomlins is with the department of urology at the University of Michigan Health System, Ann Arbor. He was the invited discussant of the paper at the meeting. Dr. Tomlins disclosed that he is a consultant to and receives honoraria from Ventana Medical Systems/Roche. He has patents via the University of Michigan on several diagnostic genetic tests.
The ability to improve clinical management by finding prostate cancer patients who would benefit from more – or less – therapy is much needed. Clinicians are concerned that many prostate cancer patients are now overtreated, but they lack reliable prognostic guides.
Cell cycle progression (CCP) scores are interesting retrospectively, but how much are they able to improve on CAPRA (Cancer of the Prostate Risk Assessment) scores for predicting prognosis?
Prostate cancer is uniquely multifocal, with most men having multiple independent foci of cancer. In the example of the conservatively managed patients, if one is looking at men with indolent disease who have low-volume disease as a single core of one or two foci, is one really going to be able to predict the biologic outcome of the cancer? Those who fail after a surveillance approach often do so early and had undersampling of their disease. So it hasn’t been proven yet that this test can predict the behavior of cancer that hasn’t been sampled.
The CCP results proved to be statistically significant, but that finding does not indicate clinical utility. It’s not known whether the novel biomarkers in this test improve on existing markers. You find yourself asking what you would do differently in a patient whose risk of progression goes from 7% to 12%.
Even if a test independently predicts outcome, that doesn’t necessarily indicate it has clinical utility. The ability to improve clinical management is key to the adoption of new prognostic tests. The real question is whether CCP results improve on the existing model. Does the test improve on CAPRA for prognosis?
Dr. Scott Tomlins is with the department of urology at the University of Michigan Health System, Ann Arbor. He was the invited discussant of the paper at the meeting. Dr. Tomlins disclosed that he is a consultant to and receives honoraria from Ventana Medical Systems/Roche. He has patents via the University of Michigan on several diagnostic genetic tests.
The ability to improve clinical management by finding prostate cancer patients who would benefit from more – or less – therapy is much needed. Clinicians are concerned that many prostate cancer patients are now overtreated, but they lack reliable prognostic guides.
Cell cycle progression (CCP) scores are interesting retrospectively, but how much are they able to improve on CAPRA (Cancer of the Prostate Risk Assessment) scores for predicting prognosis?
Prostate cancer is uniquely multifocal, with most men having multiple independent foci of cancer. In the example of the conservatively managed patients, if one is looking at men with indolent disease who have low-volume disease as a single core of one or two foci, is one really going to be able to predict the biologic outcome of the cancer? Those who fail after a surveillance approach often do so early and had undersampling of their disease. So it hasn’t been proven yet that this test can predict the behavior of cancer that hasn’t been sampled.
The CCP results proved to be statistically significant, but that finding does not indicate clinical utility. It’s not known whether the novel biomarkers in this test improve on existing markers. You find yourself asking what you would do differently in a patient whose risk of progression goes from 7% to 12%.
Even if a test independently predicts outcome, that doesn’t necessarily indicate it has clinical utility. The ability to improve clinical management is key to the adoption of new prognostic tests. The real question is whether CCP results improve on the existing model. Does the test improve on CAPRA for prognosis?
Dr. Scott Tomlins is with the department of urology at the University of Michigan Health System, Ann Arbor. He was the invited discussant of the paper at the meeting. Dr. Tomlins disclosed that he is a consultant to and receives honoraria from Ventana Medical Systems/Roche. He has patents via the University of Michigan on several diagnostic genetic tests.
CHICAGO – Prostate cancer outcomes were predicted by a test that measures the expression of cell cycle progression genes, according to results from a retrospective analysis of prostate tissue samples from five patient cohorts.
The Prolaris test gives each tissue sample a cell cycle progression (CCP) score based on measures of 31 CCP genes, normalized to 15 "housekeeper" genes. A unit change in the test is defined as a doubling in CCP genes. For each unit increase in the test’s score, there was a two- to threefold increase in the risk of disease progression, Dr. Jack M. Cuzick reported at the annual meeting of the American Society of Clinical Oncology.
The CCP signature of Myriad Genetics’ Prolaris test was a highly significant predictor of outcome, said Dr. Cuzick of the Wolfson Institute of Preventive Medicine, London. In all five studies, the hazard ratio per unit change in the CCP score was similar, ranging from 1.89 to 2.92. The findings indicate that the effect size for the CCP score is robust in multiple patient cohorts and diverse clinical settings.
The test provides information for differentiating aggressive and indolent disease beyond that available from clinicopathologic variables, he said. As the natural history of prostate cancer can be variable and difficult to predict, the Prolaris test could help to match treatment more appropriately to each individual’s risk of progression.
In the study that examined the test’s predictive value, five patient groups were evaluated. Formalin-fixed tissue samples were obtained from two English patient cohorts that were conservatively managed (n = 337 and 349), two U.S. patient cohorts that underwent radical prostatectomy (366 men treated at Scott & White Hospital, Temple, Tex.; and 413 men treated at the University of California, San Francisco), and one U.S. cohort that underwent external beam radiation therapy (141 men treated at the Durham, N.C., VA Medical Center).
The cohort of conservatively managed English patients was from the late 1990s and had more than 15 years of follow up. In the 337-patient cohort diagnosed via transurethral resection of the prostate (TURP) and conservatively managed, there were 57 deaths from prostate cancer. In the 349-patient cohort diagnosed via needle biopsy and conservatively managed, there were 90 deaths from prostate cancer.
For each unit increase in the CCP score, the hazard ratio for the cohort diagnosed via TURP was 2.9 and the hazard ratio for those diagnosed via needle biopsy was 2. The CCP score was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy). In both studies, the CCP score remained highly significant in multivariate analysis and was a stronger predictor of disease-specific mortality than other prognostic variables, he said.
In the U.S. prostatectomy cohorts, there were 132 biochemical recurrences (BCRs) in the first cohort and 83 BCRs in the second cohort. With each unit increase in the CCP score, there was a doubling of risk for recurrence. After prostatectomy, the CCP score predicted BCR in univariate analysis (Scott & White: P = 5.6 x 10–9; University of California: P = 2.23 x 10–6) and provided additional prognostic information in multivariate analysis (Scott & White: P = 3.3 x 10–6; University of California: P = 9.5 x10–5).
After radiation therapy, the CCP score predicted BCR in univariate (P = .0017) and multivariate (P = .034) analysis. In the 141-patient cohort that was diagnosed by needle biopsy and underwent external beam radiation, there were 19 prostate cancer deaths and more than a doubling of risk with each unit increase in CCP score.
CCP scores only modestly correlated with the Gleason score and prostate-specific antigen (PSA) value. The test adds value beyond those measures, Dr. Cuzick said.
CCP scores predict patient outcome in multiple clinical settings, provide independent information beyond clinicopathological variables, and help to further differentiate aggressive from indolent prostate cancer. With low-grade Gleason 6 cancers, the results can aid in telling who is at low risk and who needs aggressive therapy, he concluded.
The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
CHICAGO – Prostate cancer outcomes were predicted by a test that measures the expression of cell cycle progression genes, according to results from a retrospective analysis of prostate tissue samples from five patient cohorts.
The Prolaris test gives each tissue sample a cell cycle progression (CCP) score based on measures of 31 CCP genes, normalized to 15 "housekeeper" genes. A unit change in the test is defined as a doubling in CCP genes. For each unit increase in the test’s score, there was a two- to threefold increase in the risk of disease progression, Dr. Jack M. Cuzick reported at the annual meeting of the American Society of Clinical Oncology.
The CCP signature of Myriad Genetics’ Prolaris test was a highly significant predictor of outcome, said Dr. Cuzick of the Wolfson Institute of Preventive Medicine, London. In all five studies, the hazard ratio per unit change in the CCP score was similar, ranging from 1.89 to 2.92. The findings indicate that the effect size for the CCP score is robust in multiple patient cohorts and diverse clinical settings.
The test provides information for differentiating aggressive and indolent disease beyond that available from clinicopathologic variables, he said. As the natural history of prostate cancer can be variable and difficult to predict, the Prolaris test could help to match treatment more appropriately to each individual’s risk of progression.
In the study that examined the test’s predictive value, five patient groups were evaluated. Formalin-fixed tissue samples were obtained from two English patient cohorts that were conservatively managed (n = 337 and 349), two U.S. patient cohorts that underwent radical prostatectomy (366 men treated at Scott & White Hospital, Temple, Tex.; and 413 men treated at the University of California, San Francisco), and one U.S. cohort that underwent external beam radiation therapy (141 men treated at the Durham, N.C., VA Medical Center).
The cohort of conservatively managed English patients was from the late 1990s and had more than 15 years of follow up. In the 337-patient cohort diagnosed via transurethral resection of the prostate (TURP) and conservatively managed, there were 57 deaths from prostate cancer. In the 349-patient cohort diagnosed via needle biopsy and conservatively managed, there were 90 deaths from prostate cancer.
For each unit increase in the CCP score, the hazard ratio for the cohort diagnosed via TURP was 2.9 and the hazard ratio for those diagnosed via needle biopsy was 2. The CCP score was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy). In both studies, the CCP score remained highly significant in multivariate analysis and was a stronger predictor of disease-specific mortality than other prognostic variables, he said.
In the U.S. prostatectomy cohorts, there were 132 biochemical recurrences (BCRs) in the first cohort and 83 BCRs in the second cohort. With each unit increase in the CCP score, there was a doubling of risk for recurrence. After prostatectomy, the CCP score predicted BCR in univariate analysis (Scott & White: P = 5.6 x 10–9; University of California: P = 2.23 x 10–6) and provided additional prognostic information in multivariate analysis (Scott & White: P = 3.3 x 10–6; University of California: P = 9.5 x10–5).
After radiation therapy, the CCP score predicted BCR in univariate (P = .0017) and multivariate (P = .034) analysis. In the 141-patient cohort that was diagnosed by needle biopsy and underwent external beam radiation, there were 19 prostate cancer deaths and more than a doubling of risk with each unit increase in CCP score.
CCP scores only modestly correlated with the Gleason score and prostate-specific antigen (PSA) value. The test adds value beyond those measures, Dr. Cuzick said.
CCP scores predict patient outcome in multiple clinical settings, provide independent information beyond clinicopathological variables, and help to further differentiate aggressive from indolent prostate cancer. With low-grade Gleason 6 cancers, the results can aid in telling who is at low risk and who needs aggressive therapy, he concluded.
The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
AT THE ASCO ANNUAL MEETING 2013
Major finding: In conservatively managed prostate cancer patients, the cell cycle progression score in tissue samples was the dominant variable for predicting death from prostate cancer in univariate analysis (P = 6.1 x 10–22 after diagnosis via TURP, and P = 8.6 x 10–10 after diagnosis via needle biopsy).
Data source: A retrospective study of tissue samples from more than 1,600 patients in five patient cohorts who were either managed conservatively, underwent prostatectomy, or received external beam radiotherapy.
Disclosures: The study was funded by Myriad Genetics, the maker of the Prolaris test. Dr. Cuzick received honoraria and research support from Myriad.
ICU stay may be unnecessary after elective endovascular aneurysm treatment
SAN DIEGO – In a retrospective review of almost 700 patients who underwent elective endovascular aneurysm treatment, 4% experienced postoperative complications, mostly within the first 4 hours post procedure. Almost half of the complications were groin hematomas or retroperitoneal hematomas that extended the length of inpatient stay but required no further treatment. The results suggest that patients undergoing endovascular aneurysm treatment may be transferred to a less resource-intensive environment once they have been followed closely for 4 hours, according to Bhuvic Patel, who presented the findings at the annual meeting of the American Society of Neuroradiology.
The 687 patients had unruptured intracranial aneurysms and underwent elective endovascular treatment from March 2002 to June 2012. Most patients underwent coiling alone (329) or stent-assisted coiling (242), although other patients underwent balloon-assisted coiling, Onyx HD 500 occlusion, or treatment with a pipeline embolization device with or without coiling. Nine patients experienced a complication during the procedure and were excluded from further analysis. After the procedure, patients were monitored for at least 24 hours in a neurologic intensive care unit or postanesthesia care unit.
In total, 4% had postprocedural complications (27/678). These included three intracerebral hemorrhages, six ischemic strokes, four cardiac events, five retroperitoneal hematomas, and nine groin hematomas.
Looking at the timing of the complications, 74% were detected within the first 4 hours following the procedure. These included one hemorrhage, four ischemic strokes, all four of the cardiac events, all nine of the hematomas, and two retroperitoneal hematomas.
"As you get further away from the procedures, fewer events were detected," said Mr. Patel, who is currently a medical student at the Washington University School of Medicine, St. Louis. Four complications (14.8%) were noted between 4 and 12 hours post procedure, one (3.7%) between 12 and 24 hours, and two (7.4%) more than 24 hours post procedure.
Of the two hemorrhages that were discovered 4 hours or more post procedure, both were diagnosed by head CT after patients complained of headache and neither resulted in any permanent significant deficit. For two patients who had ischemic stroke detected by MRI after 4 hours, deficits were considered minor although one patient was discharged to a skilled nursing facility.
"The complications that were detected more than 4 hours post procedure could all have been managed in a floor setting. We think it is reasonable for patients to be monitored in a postoperative intensive care setting for the first 4 hours following routine endovascular aneurysm treatment and then be transferred to be floor bed. This can translate to a lot of cost savings," says Mr. Patel.
Dr. Patel has no relevant financial disclosures.
SAN DIEGO – In a retrospective review of almost 700 patients who underwent elective endovascular aneurysm treatment, 4% experienced postoperative complications, mostly within the first 4 hours post procedure. Almost half of the complications were groin hematomas or retroperitoneal hematomas that extended the length of inpatient stay but required no further treatment. The results suggest that patients undergoing endovascular aneurysm treatment may be transferred to a less resource-intensive environment once they have been followed closely for 4 hours, according to Bhuvic Patel, who presented the findings at the annual meeting of the American Society of Neuroradiology.
The 687 patients had unruptured intracranial aneurysms and underwent elective endovascular treatment from March 2002 to June 2012. Most patients underwent coiling alone (329) or stent-assisted coiling (242), although other patients underwent balloon-assisted coiling, Onyx HD 500 occlusion, or treatment with a pipeline embolization device with or without coiling. Nine patients experienced a complication during the procedure and were excluded from further analysis. After the procedure, patients were monitored for at least 24 hours in a neurologic intensive care unit or postanesthesia care unit.
In total, 4% had postprocedural complications (27/678). These included three intracerebral hemorrhages, six ischemic strokes, four cardiac events, five retroperitoneal hematomas, and nine groin hematomas.
Looking at the timing of the complications, 74% were detected within the first 4 hours following the procedure. These included one hemorrhage, four ischemic strokes, all four of the cardiac events, all nine of the hematomas, and two retroperitoneal hematomas.
"As you get further away from the procedures, fewer events were detected," said Mr. Patel, who is currently a medical student at the Washington University School of Medicine, St. Louis. Four complications (14.8%) were noted between 4 and 12 hours post procedure, one (3.7%) between 12 and 24 hours, and two (7.4%) more than 24 hours post procedure.
Of the two hemorrhages that were discovered 4 hours or more post procedure, both were diagnosed by head CT after patients complained of headache and neither resulted in any permanent significant deficit. For two patients who had ischemic stroke detected by MRI after 4 hours, deficits were considered minor although one patient was discharged to a skilled nursing facility.
"The complications that were detected more than 4 hours post procedure could all have been managed in a floor setting. We think it is reasonable for patients to be monitored in a postoperative intensive care setting for the first 4 hours following routine endovascular aneurysm treatment and then be transferred to be floor bed. This can translate to a lot of cost savings," says Mr. Patel.
Dr. Patel has no relevant financial disclosures.
SAN DIEGO – In a retrospective review of almost 700 patients who underwent elective endovascular aneurysm treatment, 4% experienced postoperative complications, mostly within the first 4 hours post procedure. Almost half of the complications were groin hematomas or retroperitoneal hematomas that extended the length of inpatient stay but required no further treatment. The results suggest that patients undergoing endovascular aneurysm treatment may be transferred to a less resource-intensive environment once they have been followed closely for 4 hours, according to Bhuvic Patel, who presented the findings at the annual meeting of the American Society of Neuroradiology.
The 687 patients had unruptured intracranial aneurysms and underwent elective endovascular treatment from March 2002 to June 2012. Most patients underwent coiling alone (329) or stent-assisted coiling (242), although other patients underwent balloon-assisted coiling, Onyx HD 500 occlusion, or treatment with a pipeline embolization device with or without coiling. Nine patients experienced a complication during the procedure and were excluded from further analysis. After the procedure, patients were monitored for at least 24 hours in a neurologic intensive care unit or postanesthesia care unit.
In total, 4% had postprocedural complications (27/678). These included three intracerebral hemorrhages, six ischemic strokes, four cardiac events, five retroperitoneal hematomas, and nine groin hematomas.
Looking at the timing of the complications, 74% were detected within the first 4 hours following the procedure. These included one hemorrhage, four ischemic strokes, all four of the cardiac events, all nine of the hematomas, and two retroperitoneal hematomas.
"As you get further away from the procedures, fewer events were detected," said Mr. Patel, who is currently a medical student at the Washington University School of Medicine, St. Louis. Four complications (14.8%) were noted between 4 and 12 hours post procedure, one (3.7%) between 12 and 24 hours, and two (7.4%) more than 24 hours post procedure.
Of the two hemorrhages that were discovered 4 hours or more post procedure, both were diagnosed by head CT after patients complained of headache and neither resulted in any permanent significant deficit. For two patients who had ischemic stroke detected by MRI after 4 hours, deficits were considered minor although one patient was discharged to a skilled nursing facility.
"The complications that were detected more than 4 hours post procedure could all have been managed in a floor setting. We think it is reasonable for patients to be monitored in a postoperative intensive care setting for the first 4 hours following routine endovascular aneurysm treatment and then be transferred to be floor bed. This can translate to a lot of cost savings," says Mr. Patel.
Dr. Patel has no relevant financial disclosures.
AT THE ASNR ANNUAL MEETING
Major finding: Only 4% of 687 patients undergoing routine endovascular aneurysm treatment experienced postoperative complications and almost three-quarters of these complications occurred within 4 hours of the procedure.
Data source: Retrospective review.
Disclosures: Dr. Patel has no relevant financial disclosures.
AMPLIFY: Apixaban beat warfarin on safety in acute VTE
AMSTERDAM – In patients with acute venous thromboembolism, 6 months of treatment with the oral-anticoagulant apixaban was as effective as was standard therapy with subcutaneous enoxaparin for a week followed by oral warfarin, and apixaban caused significantly fewer major bleeding complications in a randomized, multicenter trial with more than 5,000 patients.
But in addition to apixaban’s sterling individual performance in this pivotal trial, which seems to clear the way for the drug to eventually receive a labeled indication for acute venous thromboembolism, the results also appeared to further anoint the new, oral anticoagulant roster of dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis) as the thrombotic-disease troika to be reckoned with, the newcomers whose time has come.
Ever since rivaroxaban became the first of the trio to gain acute VTE labeling, last November, physicians who manage patients with acute VTE had to wrestle with the question of how to integrate this option into their practices. The new findings on apixaban suggest that physicians will soon have to think about deciding between rivaroxaban and apixaban for this indication. And since recent results from other major trials also established dabigatran as the equal of warfarin for efficacy when treating acute VTE patients and with superior safety, dabigatran’s entry into acute VTE management seems imminent (N. Engl. J. Med. 2013;368:709-18).
Propelling this new anticoagulant era are the indications of efficacy that’s equivalent with heparin, but safer, and with far easier drug delivery as the need for anticoagulation clinics and regular measurement of international normalized ratio (INR) is eliminated by all three new drugs.
"An oral regimen without laboratory monitoring will simplify therapy," Dr. Giancarlo Agnelli noted when he presented the new apixaban findings at the congress of the International Society on Thrombosis and Haemostasis. Concurrently with his report at the meeting, the results were published online (N. Engl. J. Med. 2013;doi:10.1056/nejmoa1302507).
"I think the argument is overwhelming" to use one of the new drugs instead of warfarin. "They are oral drugs where you do not need a blood draw every 2 or 3 weeks, they are a lot easier to use, and they are at least as good as warfarin and at least as safe," said Dr. Frits R. Rosendaal, professor of clinical epidemiology in hemostasis and thrombosis at Leiden (The Netherlands) University.
The Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY) trial randomized 5,400 acute VTE patients at 358 centers in 28 countries. Patients received either apixaban starting with a 10-mg b.i.d. dosage for 7 days, followed by a dosage of 5 mg b.i.d. for 6 months, or enoxaparin at a dosage of 1 mg/kg every 12 hours for a median of 7 days followed by warfarin for 6 months with a target INR of 2.0-3.0.
The study’s primary efficacy endpoint was the combined rate of recurrent, symptomatic VTE or death related to VTE. This occurred in 59 of 2,609 patients (2.3%) who received apixaban, and in 71 of 2,635 (2.7%) patients who received enoxaparin followed by warfarin. These results met the study’s prespecified criterion for apixaban’s noninferiority to standard treatment reported Dr. Agnelli, professor of medicine at the University of Perugia, Italy.
Major bleeding events occurred in 15 of 2,676 (0.6%) patients on apixaban and in 49 of 2,689 (1.8%) patients on enoxaparin and warfarin, a statistically significant difference. A composite safety outcome of major bleeds plus clinically relevant nonmajor bleeds occurred in 4.3% of the apixaban patients and in 9.7% of the patients on standard therapy, a statistically significant difference. Aside from bleeding events, the rates of all other adverse events were similar in the two treatment arms.
The trial was sponsored by Pfizer and Bristol-Myers Squibb, which market apixaban (Eliquis). Dr. Agnelli disclosed ties to Pfizer, Boehringer Ingelheim, Sanofi, Daiichi Sankyo, and Bayer. Dr. Rosendaal said that he had no disclosures.
The new anticoagulant era for patients with acute venous thromboembolism began late last year, when the Food and Drug Administration gave rivaroxaban this new labeled indication. Each physician who enters this new era, by opting to prescribe rivaroxaban instead of warfarin, needs to carefully think through which types of patients the evidence supports treating.
Clinicians who manage patients with acute venous thromboembolism (VTE) need to decide how to bring rivaroxaban into their practices, and eventually they will need to make similar decisions about dabigatran and apixaban once those drugs receive an acute VTE indication.

|
|
A few weeks after rivaroxaban’s approval for acute VTE, I got together with my colleagues at the University of Vermont in Burlington who manage these patients to draw up a framework for how we’ll decide which patients should be treated with rivaroxaban and which ones we should still treat with warfarin. I included the six main elements of that framework in an editorial I wrote about the AMPLIFY results reported by Dr. Agnelli (N. Engl. J. Med. 2013;doi:10.1056/nejme1307413).
One factor we urged clinicians to keep in mind was that the new-anticoagulant trials use selected patients. For example, the AMPLIFY trial excluded patients with provoked VTE because they are usually not treated for 6 months, the treatment duration studied in the trial. The new drugs are not suitable for every patient; patients on dialysis shouldn’t get them because these agents all involve renal clearance. Very old patients, those aged beyond 80 years, are an uncertain group because the trials have so far included few such patients, but on the other hand, eliminating the need for regular anticoagulation monitoring may make the new drugs more suitable for some elderly patients. Patients with cancer are another uncertain subgroup because of their low representation in the trials. My colleagues and I have for the time being decided not to use rivaroxaban on cancer patients with acute VTE because we judged the current evidence base too limited.
Rivaroxaban, apixaban, and dabigatran each have their own unique list of drug interactions and contraindications. None of them has as many caveats as warfarin, but it’s still essential to be familiar with each drug’s label and which clinical situations to avoid.
Another issue is how likely a patient will be to adhere to the prescribed regimen. A limitation of the new drugs is their short half-life. A patient just needs to miss a couple of doses and the anticoagulation effect starts to fade. In contrast, warfarin’s effect lingers much longer; a patient on a stable warfarin regimen can go several days without treatment before the international normalized ratio begins to fall out of the target range. Plus, patients on the new drugs provide physicians with no compliance feedback from monitoring results. That means patients should only be started on a new oral anticoagulant if they understand this limitation and the need for commitment to full compliance. There are also issues of cost and insurance coverage with the new drugs. Some patients have insurers that require preapproval for treatment of VTE with rivaroxaban, but then the payers deny the preapproval when we request it.
Warfarin has been the top anticoagulant for the past 60 years, much longer than my entire career in medicine. Physicians now face the challenge of applying the results from carefully controlled studies of the new anticoagulants – where selected cohorts of patients received their care from a small number of anticoagulation management experts – to a much broader and more diverse spectrum of patients in a variety of clinical settings. How exactly that gets done needs careful consideration.
Dr. Mary Cushman is a hematologist and professor of medicine at the University of Vermont in Burlington. She made these comments in an interview. She said that she had no disclosures.
The new anticoagulant era for patients with acute venous thromboembolism began late last year, when the Food and Drug Administration gave rivaroxaban this new labeled indication. Each physician who enters this new era, by opting to prescribe rivaroxaban instead of warfarin, needs to carefully think through which types of patients the evidence supports treating.
Clinicians who manage patients with acute venous thromboembolism (VTE) need to decide how to bring rivaroxaban into their practices, and eventually they will need to make similar decisions about dabigatran and apixaban once those drugs receive an acute VTE indication.

|
|
A few weeks after rivaroxaban’s approval for acute VTE, I got together with my colleagues at the University of Vermont in Burlington who manage these patients to draw up a framework for how we’ll decide which patients should be treated with rivaroxaban and which ones we should still treat with warfarin. I included the six main elements of that framework in an editorial I wrote about the AMPLIFY results reported by Dr. Agnelli (N. Engl. J. Med. 2013;doi:10.1056/nejme1307413).
One factor we urged clinicians to keep in mind was that the new-anticoagulant trials use selected patients. For example, the AMPLIFY trial excluded patients with provoked VTE because they are usually not treated for 6 months, the treatment duration studied in the trial. The new drugs are not suitable for every patient; patients on dialysis shouldn’t get them because these agents all involve renal clearance. Very old patients, those aged beyond 80 years, are an uncertain group because the trials have so far included few such patients, but on the other hand, eliminating the need for regular anticoagulation monitoring may make the new drugs more suitable for some elderly patients. Patients with cancer are another uncertain subgroup because of their low representation in the trials. My colleagues and I have for the time being decided not to use rivaroxaban on cancer patients with acute VTE because we judged the current evidence base too limited.
Rivaroxaban, apixaban, and dabigatran each have their own unique list of drug interactions and contraindications. None of them has as many caveats as warfarin, but it’s still essential to be familiar with each drug’s label and which clinical situations to avoid.
Another issue is how likely a patient will be to adhere to the prescribed regimen. A limitation of the new drugs is their short half-life. A patient just needs to miss a couple of doses and the anticoagulation effect starts to fade. In contrast, warfarin’s effect lingers much longer; a patient on a stable warfarin regimen can go several days without treatment before the international normalized ratio begins to fall out of the target range. Plus, patients on the new drugs provide physicians with no compliance feedback from monitoring results. That means patients should only be started on a new oral anticoagulant if they understand this limitation and the need for commitment to full compliance. There are also issues of cost and insurance coverage with the new drugs. Some patients have insurers that require preapproval for treatment of VTE with rivaroxaban, but then the payers deny the preapproval when we request it.
Warfarin has been the top anticoagulant for the past 60 years, much longer than my entire career in medicine. Physicians now face the challenge of applying the results from carefully controlled studies of the new anticoagulants – where selected cohorts of patients received their care from a small number of anticoagulation management experts – to a much broader and more diverse spectrum of patients in a variety of clinical settings. How exactly that gets done needs careful consideration.
Dr. Mary Cushman is a hematologist and professor of medicine at the University of Vermont in Burlington. She made these comments in an interview. She said that she had no disclosures.
The new anticoagulant era for patients with acute venous thromboembolism began late last year, when the Food and Drug Administration gave rivaroxaban this new labeled indication. Each physician who enters this new era, by opting to prescribe rivaroxaban instead of warfarin, needs to carefully think through which types of patients the evidence supports treating.
Clinicians who manage patients with acute venous thromboembolism (VTE) need to decide how to bring rivaroxaban into their practices, and eventually they will need to make similar decisions about dabigatran and apixaban once those drugs receive an acute VTE indication.

|
|
A few weeks after rivaroxaban’s approval for acute VTE, I got together with my colleagues at the University of Vermont in Burlington who manage these patients to draw up a framework for how we’ll decide which patients should be treated with rivaroxaban and which ones we should still treat with warfarin. I included the six main elements of that framework in an editorial I wrote about the AMPLIFY results reported by Dr. Agnelli (N. Engl. J. Med. 2013;doi:10.1056/nejme1307413).
One factor we urged clinicians to keep in mind was that the new-anticoagulant trials use selected patients. For example, the AMPLIFY trial excluded patients with provoked VTE because they are usually not treated for 6 months, the treatment duration studied in the trial. The new drugs are not suitable for every patient; patients on dialysis shouldn’t get them because these agents all involve renal clearance. Very old patients, those aged beyond 80 years, are an uncertain group because the trials have so far included few such patients, but on the other hand, eliminating the need for regular anticoagulation monitoring may make the new drugs more suitable for some elderly patients. Patients with cancer are another uncertain subgroup because of their low representation in the trials. My colleagues and I have for the time being decided not to use rivaroxaban on cancer patients with acute VTE because we judged the current evidence base too limited.
Rivaroxaban, apixaban, and dabigatran each have their own unique list of drug interactions and contraindications. None of them has as many caveats as warfarin, but it’s still essential to be familiar with each drug’s label and which clinical situations to avoid.
Another issue is how likely a patient will be to adhere to the prescribed regimen. A limitation of the new drugs is their short half-life. A patient just needs to miss a couple of doses and the anticoagulation effect starts to fade. In contrast, warfarin’s effect lingers much longer; a patient on a stable warfarin regimen can go several days without treatment before the international normalized ratio begins to fall out of the target range. Plus, patients on the new drugs provide physicians with no compliance feedback from monitoring results. That means patients should only be started on a new oral anticoagulant if they understand this limitation and the need for commitment to full compliance. There are also issues of cost and insurance coverage with the new drugs. Some patients have insurers that require preapproval for treatment of VTE with rivaroxaban, but then the payers deny the preapproval when we request it.
Warfarin has been the top anticoagulant for the past 60 years, much longer than my entire career in medicine. Physicians now face the challenge of applying the results from carefully controlled studies of the new anticoagulants – where selected cohorts of patients received their care from a small number of anticoagulation management experts – to a much broader and more diverse spectrum of patients in a variety of clinical settings. How exactly that gets done needs careful consideration.
Dr. Mary Cushman is a hematologist and professor of medicine at the University of Vermont in Burlington. She made these comments in an interview. She said that she had no disclosures.
AMSTERDAM – In patients with acute venous thromboembolism, 6 months of treatment with the oral-anticoagulant apixaban was as effective as was standard therapy with subcutaneous enoxaparin for a week followed by oral warfarin, and apixaban caused significantly fewer major bleeding complications in a randomized, multicenter trial with more than 5,000 patients.
But in addition to apixaban’s sterling individual performance in this pivotal trial, which seems to clear the way for the drug to eventually receive a labeled indication for acute venous thromboembolism, the results also appeared to further anoint the new, oral anticoagulant roster of dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis) as the thrombotic-disease troika to be reckoned with, the newcomers whose time has come.
Ever since rivaroxaban became the first of the trio to gain acute VTE labeling, last November, physicians who manage patients with acute VTE had to wrestle with the question of how to integrate this option into their practices. The new findings on apixaban suggest that physicians will soon have to think about deciding between rivaroxaban and apixaban for this indication. And since recent results from other major trials also established dabigatran as the equal of warfarin for efficacy when treating acute VTE patients and with superior safety, dabigatran’s entry into acute VTE management seems imminent (N. Engl. J. Med. 2013;368:709-18).
Propelling this new anticoagulant era are the indications of efficacy that’s equivalent with heparin, but safer, and with far easier drug delivery as the need for anticoagulation clinics and regular measurement of international normalized ratio (INR) is eliminated by all three new drugs.
"An oral regimen without laboratory monitoring will simplify therapy," Dr. Giancarlo Agnelli noted when he presented the new apixaban findings at the congress of the International Society on Thrombosis and Haemostasis. Concurrently with his report at the meeting, the results were published online (N. Engl. J. Med. 2013;doi:10.1056/nejmoa1302507).
"I think the argument is overwhelming" to use one of the new drugs instead of warfarin. "They are oral drugs where you do not need a blood draw every 2 or 3 weeks, they are a lot easier to use, and they are at least as good as warfarin and at least as safe," said Dr. Frits R. Rosendaal, professor of clinical epidemiology in hemostasis and thrombosis at Leiden (The Netherlands) University.
The Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY) trial randomized 5,400 acute VTE patients at 358 centers in 28 countries. Patients received either apixaban starting with a 10-mg b.i.d. dosage for 7 days, followed by a dosage of 5 mg b.i.d. for 6 months, or enoxaparin at a dosage of 1 mg/kg every 12 hours for a median of 7 days followed by warfarin for 6 months with a target INR of 2.0-3.0.
The study’s primary efficacy endpoint was the combined rate of recurrent, symptomatic VTE or death related to VTE. This occurred in 59 of 2,609 patients (2.3%) who received apixaban, and in 71 of 2,635 (2.7%) patients who received enoxaparin followed by warfarin. These results met the study’s prespecified criterion for apixaban’s noninferiority to standard treatment reported Dr. Agnelli, professor of medicine at the University of Perugia, Italy.
Major bleeding events occurred in 15 of 2,676 (0.6%) patients on apixaban and in 49 of 2,689 (1.8%) patients on enoxaparin and warfarin, a statistically significant difference. A composite safety outcome of major bleeds plus clinically relevant nonmajor bleeds occurred in 4.3% of the apixaban patients and in 9.7% of the patients on standard therapy, a statistically significant difference. Aside from bleeding events, the rates of all other adverse events were similar in the two treatment arms.
The trial was sponsored by Pfizer and Bristol-Myers Squibb, which market apixaban (Eliquis). Dr. Agnelli disclosed ties to Pfizer, Boehringer Ingelheim, Sanofi, Daiichi Sankyo, and Bayer. Dr. Rosendaal said that he had no disclosures.
AMSTERDAM – In patients with acute venous thromboembolism, 6 months of treatment with the oral-anticoagulant apixaban was as effective as was standard therapy with subcutaneous enoxaparin for a week followed by oral warfarin, and apixaban caused significantly fewer major bleeding complications in a randomized, multicenter trial with more than 5,000 patients.
But in addition to apixaban’s sterling individual performance in this pivotal trial, which seems to clear the way for the drug to eventually receive a labeled indication for acute venous thromboembolism, the results also appeared to further anoint the new, oral anticoagulant roster of dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis) as the thrombotic-disease troika to be reckoned with, the newcomers whose time has come.
Ever since rivaroxaban became the first of the trio to gain acute VTE labeling, last November, physicians who manage patients with acute VTE had to wrestle with the question of how to integrate this option into their practices. The new findings on apixaban suggest that physicians will soon have to think about deciding between rivaroxaban and apixaban for this indication. And since recent results from other major trials also established dabigatran as the equal of warfarin for efficacy when treating acute VTE patients and with superior safety, dabigatran’s entry into acute VTE management seems imminent (N. Engl. J. Med. 2013;368:709-18).
Propelling this new anticoagulant era are the indications of efficacy that’s equivalent with heparin, but safer, and with far easier drug delivery as the need for anticoagulation clinics and regular measurement of international normalized ratio (INR) is eliminated by all three new drugs.
"An oral regimen without laboratory monitoring will simplify therapy," Dr. Giancarlo Agnelli noted when he presented the new apixaban findings at the congress of the International Society on Thrombosis and Haemostasis. Concurrently with his report at the meeting, the results were published online (N. Engl. J. Med. 2013;doi:10.1056/nejmoa1302507).
"I think the argument is overwhelming" to use one of the new drugs instead of warfarin. "They are oral drugs where you do not need a blood draw every 2 or 3 weeks, they are a lot easier to use, and they are at least as good as warfarin and at least as safe," said Dr. Frits R. Rosendaal, professor of clinical epidemiology in hemostasis and thrombosis at Leiden (The Netherlands) University.
The Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy (AMPLIFY) trial randomized 5,400 acute VTE patients at 358 centers in 28 countries. Patients received either apixaban starting with a 10-mg b.i.d. dosage for 7 days, followed by a dosage of 5 mg b.i.d. for 6 months, or enoxaparin at a dosage of 1 mg/kg every 12 hours for a median of 7 days followed by warfarin for 6 months with a target INR of 2.0-3.0.
The study’s primary efficacy endpoint was the combined rate of recurrent, symptomatic VTE or death related to VTE. This occurred in 59 of 2,609 patients (2.3%) who received apixaban, and in 71 of 2,635 (2.7%) patients who received enoxaparin followed by warfarin. These results met the study’s prespecified criterion for apixaban’s noninferiority to standard treatment reported Dr. Agnelli, professor of medicine at the University of Perugia, Italy.
Major bleeding events occurred in 15 of 2,676 (0.6%) patients on apixaban and in 49 of 2,689 (1.8%) patients on enoxaparin and warfarin, a statistically significant difference. A composite safety outcome of major bleeds plus clinically relevant nonmajor bleeds occurred in 4.3% of the apixaban patients and in 9.7% of the patients on standard therapy, a statistically significant difference. Aside from bleeding events, the rates of all other adverse events were similar in the two treatment arms.
The trial was sponsored by Pfizer and Bristol-Myers Squibb, which market apixaban (Eliquis). Dr. Agnelli disclosed ties to Pfizer, Boehringer Ingelheim, Sanofi, Daiichi Sankyo, and Bayer. Dr. Rosendaal said that he had no disclosures.
AT THE 2013 ISTH CONGRESS
Major finding: Major bleeds occurred in 0.6% of patients on apixaban and 1.8% of patients on warfarin, with similar efficacy.
Data source: The AMPLIFY study, which randomized 5,400 patients with acute venous thromboembolism to treatment with apixaban or enoxaparin followed by warfarin for 6 months.
Disclosures: The trial was sponsored by Pfizer and Bristol-Myers Squibb, which market apixaban (Eliquis). Dr. Agnelli disclosed ties to Pfizer, Boehringer Ingelheim, Sanofi, Daiichi Sankyo, and Bayer. Dr. Rosendaal said that he had no disclosures.
PAM50 assay aids prediction of metastasis in early node-positive breast cancer
CHICAGO – The multigene PAM50 assay identifies postmenopausal women with node-positive early breast cancer who have a low risk of distant metastases and may be able to skip adjuvant chemotherapy.
The 543 women studied had one to three positive nodes and low- or intermediate-grade tumors; all received adjuvant endocrine therapy but no chemotherapy in a pair of randomized trials: the ABCSG-8 (Austrian Breast Cancer Study Group 8) trial and the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial.
Results showed that three PAM50 parameters – the risk of recurrence (ROR) score, ROR risk group, and intrinsic subtype – significantly added to clinicopathologic factors in predicting the 10-year risk of metastases.
"A significant proportion of early breast cancer patients with only one positive node has a limited long-term risk of metastases, as well as at least some patients with two or three positive nodes," first author Dr. Michael Gnant commented in presenting the findings at the annual meeting of the American Society of Clinical Oncology.
In particular, among patients with only one positive node, those falling into the ROR low-risk group (41% of the one-node subset) and those having the luminal A intrinsic subtype (71% of the one-node subset) had 10-year risks of metastases that were less than 9%.
"Based on their low risk of metastases, these patients could – and I would personally add should – be spared the side effects of adjuvant chemotherapy despite having node-positive early breast cancer," maintained Dr. Gnant, who is a professor of surgery at the Medical University of Vienna, Austria.
"In the adjuvant treatment of hormone receptor–positive breast cancer, we every day face the dilemma of undertreatment versus overtreatment. Assessing an individual patient’s risk of metastasis as accurately as possible is an important goal in early breast cancer, and it directly impacts on treatment decisions," he added. "Still, risk assessment is mainly based on clinicopathological variables, of which nodal status is considered to be most important. As a consequence, most node-positive patients with hormone receptor–positive breast cancer globally still receive adjuvant chemotherapy."
In the study, the researchers assessed the added prognostic value derived from NanoString Technologies’ PAM50, a 50-gene assay designed to identify intrinsic subtypes of breast cancer. The subtypes are combined with a proliferation score and tumor size to yield the ROR score, and scores are used to classify patients into ROR-based risk groups.
The clinical usefulness of the PAM50 assay and other similar molecular assays, such as Genomic Health’s Oncotype DX and Agendia’s MammaPrint, has not been defined in node-positive patients, he noted.
The node-positive patients studied were drawn from the ABCSG-8 and ATAC trials. The former trial assigned women either to tamoxifen for 5 years or to 2 years of tamoxifen followed by 3 years of the aromatase inhibitor anastrozole (Arimidex); the latter assigned patients to 5 years of adjuvant anastrozole alone, tamoxifen alone, or the (subsequently discontinued) combination.
For each patient, the investigators analyzed tumor tissue to determine the PAM50 ROR score, ROR risk group, and intrinsic subtype. In addition, they derived the clinical treatment score, which is an overall integrated measure of clinicopathologic factors.
The median follow-up was 11 years, according to Dr. Gnant. As the ROR score increased from 0 to 100, so did the 10-year risk of metastases.
When added to the clinical treatment score alone, the ROR score significantly improved on the prediction of metastasis (chi-square for the difference in likelihood ratios, 32.45; P less than .0001).
The ROR risk groups also stratified patients well. The risk of metastases was about 30% in the high-risk group, 14% in the intermediate-risk group, and 8% in the low-risk group.
When added to the clinical treatment score, the luminal subtype significantly improved on prediction of metastasis (chi-square for the difference in likelihood ratios, 20.48; P less than .0001).
The estimated 10-year risk of metastasis was 11% in the luminal A group and 31% in the luminal B group.
In a combined analysis, the risk of metastases was less than 10% in several subsets of patients: those with one or two positive nodes in the ROR low-risk group (6.6% and 7.2% risks, respectively) and those with one positive node having the luminal A subtype (8.4% risk).
Finally, each factor – ROR score, ROR risk group, and intrinsic subtype – significantly improved on the prediction of risk above and beyond clinicopathologic factors in patients with one positive node, two positive nodes, and two or three positive nodes individually.
One session attendee asked, "Was there any opportunity to look at the interaction [of PAM50 parameters] with therapy?"
"Given the rather limited overall difference between anastrozole and tamoxifen both in the ATAC trial and in the sequencing study, ABCSG-8 – tamoxifen versus tamoxifen followed by anastrozole – I don’t think that we can reasonably comment on the interaction between these scores and treatment," Dr. Gnant replied.
Another attendee, Dr. Carlos Arteaga of the Vanderbilt-Ingram Cancer Center in Nashville, Tenn., asked, "Until we all start doing these genomic tests, can you tell us whether the Ki-67 had equal power in your study? It’s a simple test, and we are all using 14% as a cutoff for luminal A and B [intrinsic subtypes]. How did that pan out in this cohort?
"We are trying to have central review for Ki-67 and bring it into the multivariate model. Hopefully, later this year we will be able to answer that question," Dr. Gnant replied.
Dr. Gnant disclosed ties to several companies including Sanofi-Aventis, Novartis, and Roche, as well as NanoString Technologies, the maker of the PAM50 assay.
The ATAC population was used in this study for training and validation of PAM50, which could lead to overestimation of the test’s prognostic value. That said, the data looked pretty impressive.
Is the assay ready for prime time in node-positive patients? In my mind, probably not quite yet. The present analysis involved the testing of multiple comparisons, and in the end, even with 543 specimens it was limited by a relatively small number of events.
PAM50 classification approaches are probably useful, but which approaches are best – the ROR score, the intrinsic subtype, or the ROR group – remains uncertain, and a confirmatory analysis would certainly be welcomed.
Dr. Eric P. Winer is with the Dana-Farber Cancer Institute in Boston. He was the invited discussant of the study. Dr. Winer disclosed ties to AstraZeneca, Merrimack, Pfizer, and other companies.
The ATAC population was used in this study for training and validation of PAM50, which could lead to overestimation of the test’s prognostic value. That said, the data looked pretty impressive.
Is the assay ready for prime time in node-positive patients? In my mind, probably not quite yet. The present analysis involved the testing of multiple comparisons, and in the end, even with 543 specimens it was limited by a relatively small number of events.
PAM50 classification approaches are probably useful, but which approaches are best – the ROR score, the intrinsic subtype, or the ROR group – remains uncertain, and a confirmatory analysis would certainly be welcomed.
Dr. Eric P. Winer is with the Dana-Farber Cancer Institute in Boston. He was the invited discussant of the study. Dr. Winer disclosed ties to AstraZeneca, Merrimack, Pfizer, and other companies.
The ATAC population was used in this study for training and validation of PAM50, which could lead to overestimation of the test’s prognostic value. That said, the data looked pretty impressive.
Is the assay ready for prime time in node-positive patients? In my mind, probably not quite yet. The present analysis involved the testing of multiple comparisons, and in the end, even with 543 specimens it was limited by a relatively small number of events.
PAM50 classification approaches are probably useful, but which approaches are best – the ROR score, the intrinsic subtype, or the ROR group – remains uncertain, and a confirmatory analysis would certainly be welcomed.
Dr. Eric P. Winer is with the Dana-Farber Cancer Institute in Boston. He was the invited discussant of the study. Dr. Winer disclosed ties to AstraZeneca, Merrimack, Pfizer, and other companies.
CHICAGO – The multigene PAM50 assay identifies postmenopausal women with node-positive early breast cancer who have a low risk of distant metastases and may be able to skip adjuvant chemotherapy.
The 543 women studied had one to three positive nodes and low- or intermediate-grade tumors; all received adjuvant endocrine therapy but no chemotherapy in a pair of randomized trials: the ABCSG-8 (Austrian Breast Cancer Study Group 8) trial and the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial.
Results showed that three PAM50 parameters – the risk of recurrence (ROR) score, ROR risk group, and intrinsic subtype – significantly added to clinicopathologic factors in predicting the 10-year risk of metastases.
"A significant proportion of early breast cancer patients with only one positive node has a limited long-term risk of metastases, as well as at least some patients with two or three positive nodes," first author Dr. Michael Gnant commented in presenting the findings at the annual meeting of the American Society of Clinical Oncology.
In particular, among patients with only one positive node, those falling into the ROR low-risk group (41% of the one-node subset) and those having the luminal A intrinsic subtype (71% of the one-node subset) had 10-year risks of metastases that were less than 9%.
"Based on their low risk of metastases, these patients could – and I would personally add should – be spared the side effects of adjuvant chemotherapy despite having node-positive early breast cancer," maintained Dr. Gnant, who is a professor of surgery at the Medical University of Vienna, Austria.
"In the adjuvant treatment of hormone receptor–positive breast cancer, we every day face the dilemma of undertreatment versus overtreatment. Assessing an individual patient’s risk of metastasis as accurately as possible is an important goal in early breast cancer, and it directly impacts on treatment decisions," he added. "Still, risk assessment is mainly based on clinicopathological variables, of which nodal status is considered to be most important. As a consequence, most node-positive patients with hormone receptor–positive breast cancer globally still receive adjuvant chemotherapy."
In the study, the researchers assessed the added prognostic value derived from NanoString Technologies’ PAM50, a 50-gene assay designed to identify intrinsic subtypes of breast cancer. The subtypes are combined with a proliferation score and tumor size to yield the ROR score, and scores are used to classify patients into ROR-based risk groups.
The clinical usefulness of the PAM50 assay and other similar molecular assays, such as Genomic Health’s Oncotype DX and Agendia’s MammaPrint, has not been defined in node-positive patients, he noted.
The node-positive patients studied were drawn from the ABCSG-8 and ATAC trials. The former trial assigned women either to tamoxifen for 5 years or to 2 years of tamoxifen followed by 3 years of the aromatase inhibitor anastrozole (Arimidex); the latter assigned patients to 5 years of adjuvant anastrozole alone, tamoxifen alone, or the (subsequently discontinued) combination.
For each patient, the investigators analyzed tumor tissue to determine the PAM50 ROR score, ROR risk group, and intrinsic subtype. In addition, they derived the clinical treatment score, which is an overall integrated measure of clinicopathologic factors.
The median follow-up was 11 years, according to Dr. Gnant. As the ROR score increased from 0 to 100, so did the 10-year risk of metastases.
When added to the clinical treatment score alone, the ROR score significantly improved on the prediction of metastasis (chi-square for the difference in likelihood ratios, 32.45; P less than .0001).
The ROR risk groups also stratified patients well. The risk of metastases was about 30% in the high-risk group, 14% in the intermediate-risk group, and 8% in the low-risk group.
When added to the clinical treatment score, the luminal subtype significantly improved on prediction of metastasis (chi-square for the difference in likelihood ratios, 20.48; P less than .0001).
The estimated 10-year risk of metastasis was 11% in the luminal A group and 31% in the luminal B group.
In a combined analysis, the risk of metastases was less than 10% in several subsets of patients: those with one or two positive nodes in the ROR low-risk group (6.6% and 7.2% risks, respectively) and those with one positive node having the luminal A subtype (8.4% risk).
Finally, each factor – ROR score, ROR risk group, and intrinsic subtype – significantly improved on the prediction of risk above and beyond clinicopathologic factors in patients with one positive node, two positive nodes, and two or three positive nodes individually.
One session attendee asked, "Was there any opportunity to look at the interaction [of PAM50 parameters] with therapy?"
"Given the rather limited overall difference between anastrozole and tamoxifen both in the ATAC trial and in the sequencing study, ABCSG-8 – tamoxifen versus tamoxifen followed by anastrozole – I don’t think that we can reasonably comment on the interaction between these scores and treatment," Dr. Gnant replied.
Another attendee, Dr. Carlos Arteaga of the Vanderbilt-Ingram Cancer Center in Nashville, Tenn., asked, "Until we all start doing these genomic tests, can you tell us whether the Ki-67 had equal power in your study? It’s a simple test, and we are all using 14% as a cutoff for luminal A and B [intrinsic subtypes]. How did that pan out in this cohort?
"We are trying to have central review for Ki-67 and bring it into the multivariate model. Hopefully, later this year we will be able to answer that question," Dr. Gnant replied.
Dr. Gnant disclosed ties to several companies including Sanofi-Aventis, Novartis, and Roche, as well as NanoString Technologies, the maker of the PAM50 assay.
CHICAGO – The multigene PAM50 assay identifies postmenopausal women with node-positive early breast cancer who have a low risk of distant metastases and may be able to skip adjuvant chemotherapy.
The 543 women studied had one to three positive nodes and low- or intermediate-grade tumors; all received adjuvant endocrine therapy but no chemotherapy in a pair of randomized trials: the ABCSG-8 (Austrian Breast Cancer Study Group 8) trial and the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial.
Results showed that three PAM50 parameters – the risk of recurrence (ROR) score, ROR risk group, and intrinsic subtype – significantly added to clinicopathologic factors in predicting the 10-year risk of metastases.
"A significant proportion of early breast cancer patients with only one positive node has a limited long-term risk of metastases, as well as at least some patients with two or three positive nodes," first author Dr. Michael Gnant commented in presenting the findings at the annual meeting of the American Society of Clinical Oncology.
In particular, among patients with only one positive node, those falling into the ROR low-risk group (41% of the one-node subset) and those having the luminal A intrinsic subtype (71% of the one-node subset) had 10-year risks of metastases that were less than 9%.
"Based on their low risk of metastases, these patients could – and I would personally add should – be spared the side effects of adjuvant chemotherapy despite having node-positive early breast cancer," maintained Dr. Gnant, who is a professor of surgery at the Medical University of Vienna, Austria.
"In the adjuvant treatment of hormone receptor–positive breast cancer, we every day face the dilemma of undertreatment versus overtreatment. Assessing an individual patient’s risk of metastasis as accurately as possible is an important goal in early breast cancer, and it directly impacts on treatment decisions," he added. "Still, risk assessment is mainly based on clinicopathological variables, of which nodal status is considered to be most important. As a consequence, most node-positive patients with hormone receptor–positive breast cancer globally still receive adjuvant chemotherapy."
In the study, the researchers assessed the added prognostic value derived from NanoString Technologies’ PAM50, a 50-gene assay designed to identify intrinsic subtypes of breast cancer. The subtypes are combined with a proliferation score and tumor size to yield the ROR score, and scores are used to classify patients into ROR-based risk groups.
The clinical usefulness of the PAM50 assay and other similar molecular assays, such as Genomic Health’s Oncotype DX and Agendia’s MammaPrint, has not been defined in node-positive patients, he noted.
The node-positive patients studied were drawn from the ABCSG-8 and ATAC trials. The former trial assigned women either to tamoxifen for 5 years or to 2 years of tamoxifen followed by 3 years of the aromatase inhibitor anastrozole (Arimidex); the latter assigned patients to 5 years of adjuvant anastrozole alone, tamoxifen alone, or the (subsequently discontinued) combination.
For each patient, the investigators analyzed tumor tissue to determine the PAM50 ROR score, ROR risk group, and intrinsic subtype. In addition, they derived the clinical treatment score, which is an overall integrated measure of clinicopathologic factors.
The median follow-up was 11 years, according to Dr. Gnant. As the ROR score increased from 0 to 100, so did the 10-year risk of metastases.
When added to the clinical treatment score alone, the ROR score significantly improved on the prediction of metastasis (chi-square for the difference in likelihood ratios, 32.45; P less than .0001).
The ROR risk groups also stratified patients well. The risk of metastases was about 30% in the high-risk group, 14% in the intermediate-risk group, and 8% in the low-risk group.
When added to the clinical treatment score, the luminal subtype significantly improved on prediction of metastasis (chi-square for the difference in likelihood ratios, 20.48; P less than .0001).
The estimated 10-year risk of metastasis was 11% in the luminal A group and 31% in the luminal B group.
In a combined analysis, the risk of metastases was less than 10% in several subsets of patients: those with one or two positive nodes in the ROR low-risk group (6.6% and 7.2% risks, respectively) and those with one positive node having the luminal A subtype (8.4% risk).
Finally, each factor – ROR score, ROR risk group, and intrinsic subtype – significantly improved on the prediction of risk above and beyond clinicopathologic factors in patients with one positive node, two positive nodes, and two or three positive nodes individually.
One session attendee asked, "Was there any opportunity to look at the interaction [of PAM50 parameters] with therapy?"
"Given the rather limited overall difference between anastrozole and tamoxifen both in the ATAC trial and in the sequencing study, ABCSG-8 – tamoxifen versus tamoxifen followed by anastrozole – I don’t think that we can reasonably comment on the interaction between these scores and treatment," Dr. Gnant replied.
Another attendee, Dr. Carlos Arteaga of the Vanderbilt-Ingram Cancer Center in Nashville, Tenn., asked, "Until we all start doing these genomic tests, can you tell us whether the Ki-67 had equal power in your study? It’s a simple test, and we are all using 14% as a cutoff for luminal A and B [intrinsic subtypes]. How did that pan out in this cohort?
"We are trying to have central review for Ki-67 and bring it into the multivariate model. Hopefully, later this year we will be able to answer that question," Dr. Gnant replied.
Dr. Gnant disclosed ties to several companies including Sanofi-Aventis, Novartis, and Roche, as well as NanoString Technologies, the maker of the PAM50 assay.
AT THE ASCO ANNUAL MEETING 2013
Major finding: Among patients with one positive node, those falling into the ROR low-risk group (41% of the one-node subset) and those having the luminal A intrinsic subtype (71% of the one-node subset) had 10-year risks of metastases that were less than 9%.
Data source: A combined analysis of 543 postmenopausal patients with node-positive, hormone receptor–positive, early-stage breast cancer who received adjuvant endocrine therapy in the ABCSG-8 and ATAC trials.
Disclosures: Dr. Gnant disclosed ties to several companies including Sanofi-Aventis, Novartis, and Roche, as well as NanoString Technologies, the maker of the PAM50 assay.
Enteral nutrition linked to in-hospital mortality
CHICAGO – Hyperglycemia is common in patients receiving enteral nutrition and is a significant risk factor for hospital mortality, according to a retrospective study involving 157 patients.
Nearly 60% of patients had capillary blood glucose (CBG) values that exceeded 140 mg/dL, and nearly one-third exceeded 180 mg/dL.
More important, almost 40% of patients had a mean CBG of more than 180 mg/dL for the entire duration of their tube feedings.
At the threshold glucose of 180 mg/dL or more, the odds of dying in the hospital during tube feeding are approximately three times higher than for patients who had better glycemic control, Dr. Michael Jakoby said at the annual scientific sessions of the American Diabetes Association.
"We look at this body of work as just a stepping point to the next logical extension, which is to investigate ways that we can improve glycemic control for patients receiving any artificial nutrition, whether it’s parenteral or enteral," said Dr. Jakoby, chief of endocrinology at St. John’s Hospital in Springfield, Ill.
He previously reported that using an insulin protocol that determined insulin doses based on carbohydrate delivery and CBG was superior to ad hoc insulin dosing in the management of parenteral nutrition–induced hyperglycemia (JPEN J. Parenter. Enteral. Nutr. 2012;36:183-8).
Malnutrition among nonsurgical patients is high, and more than 40% of general surgery service patients have been reported to be malnourished (Am. J. Clin. Nutr. 1997;66:1232-9). Five studies since 2005 have established a link between parenteral nutrition and increased morbidity and in-hospital mortality, but little is available on outcomes with enteral nutrition, Dr. Jakoby explained.
The current analysis involved 157 patients receiving enteral nutrition in 2011 at St. John’s, a 350-bed hospital where each year an estimated 11,000 patients receive artificial nutrition for 2 weeks.
Of those patients, 58 were diagnosed with hyperglycemia (37%), defined as a mean CBG of 180 mg/dL or more for the duration of tube feedings.
Hyperglycemic patients were significantly older than controls (75 vs. 67), twice as likely to have a preexisting diagnosis of diabetes (81% vs. 41%), had 2 fewer days of enteral nutrition (7 vs. 9), and were less likely to be in the ICU during their tube feedings (62% vs. 83%), he said.
The two groups were well matched with regard to the amount of protein, fat, and total energy they received; however, hyperglycemic patients received significantly fewer carbohydrates than did controls (2.2 g/kg per day vs. 2.7 g/kg per day).
"With the documentation available to us, it seemed that this was an adaptive response to help combat the hyperglycemia that was happening during pure enteral nutrition," Dr. Jakoby said.
Cardiac complications – a composite of myocardial infarction, arrhythmia, and cardiac arrest – were increased in hyperglycemic patients (34% vs. 22%), but this difference was not significant in univariate analysis (odds ratio, 1.84; P = .13).
The risk of hospital mortality, however, was significantly higher in hyperglycemic patients in univariate analysis (36% vs. 16%; OR, 2.94), and remained significant in multivariate analysis after adjustment for age, sex, and preexisting diagnosis of diabetes (OR, 3.28), he noted.
During a discussion of the results, Dr. Jakoby said analyses are forthcoming looking at the impact of glycemic variability during hospitalization, observing that in a prior study he conducted, glycemic variability in the noncritical care setting strongly correlated with hospital stay and a greater likelihood of going from hospital to nursing home or the morgue.
Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
CHICAGO – Hyperglycemia is common in patients receiving enteral nutrition and is a significant risk factor for hospital mortality, according to a retrospective study involving 157 patients.
Nearly 60% of patients had capillary blood glucose (CBG) values that exceeded 140 mg/dL, and nearly one-third exceeded 180 mg/dL.
More important, almost 40% of patients had a mean CBG of more than 180 mg/dL for the entire duration of their tube feedings.
At the threshold glucose of 180 mg/dL or more, the odds of dying in the hospital during tube feeding are approximately three times higher than for patients who had better glycemic control, Dr. Michael Jakoby said at the annual scientific sessions of the American Diabetes Association.
"We look at this body of work as just a stepping point to the next logical extension, which is to investigate ways that we can improve glycemic control for patients receiving any artificial nutrition, whether it’s parenteral or enteral," said Dr. Jakoby, chief of endocrinology at St. John’s Hospital in Springfield, Ill.
He previously reported that using an insulin protocol that determined insulin doses based on carbohydrate delivery and CBG was superior to ad hoc insulin dosing in the management of parenteral nutrition–induced hyperglycemia (JPEN J. Parenter. Enteral. Nutr. 2012;36:183-8).
Malnutrition among nonsurgical patients is high, and more than 40% of general surgery service patients have been reported to be malnourished (Am. J. Clin. Nutr. 1997;66:1232-9). Five studies since 2005 have established a link between parenteral nutrition and increased morbidity and in-hospital mortality, but little is available on outcomes with enteral nutrition, Dr. Jakoby explained.
The current analysis involved 157 patients receiving enteral nutrition in 2011 at St. John’s, a 350-bed hospital where each year an estimated 11,000 patients receive artificial nutrition for 2 weeks.
Of those patients, 58 were diagnosed with hyperglycemia (37%), defined as a mean CBG of 180 mg/dL or more for the duration of tube feedings.
Hyperglycemic patients were significantly older than controls (75 vs. 67), twice as likely to have a preexisting diagnosis of diabetes (81% vs. 41%), had 2 fewer days of enteral nutrition (7 vs. 9), and were less likely to be in the ICU during their tube feedings (62% vs. 83%), he said.
The two groups were well matched with regard to the amount of protein, fat, and total energy they received; however, hyperglycemic patients received significantly fewer carbohydrates than did controls (2.2 g/kg per day vs. 2.7 g/kg per day).
"With the documentation available to us, it seemed that this was an adaptive response to help combat the hyperglycemia that was happening during pure enteral nutrition," Dr. Jakoby said.
Cardiac complications – a composite of myocardial infarction, arrhythmia, and cardiac arrest – were increased in hyperglycemic patients (34% vs. 22%), but this difference was not significant in univariate analysis (odds ratio, 1.84; P = .13).
The risk of hospital mortality, however, was significantly higher in hyperglycemic patients in univariate analysis (36% vs. 16%; OR, 2.94), and remained significant in multivariate analysis after adjustment for age, sex, and preexisting diagnosis of diabetes (OR, 3.28), he noted.
During a discussion of the results, Dr. Jakoby said analyses are forthcoming looking at the impact of glycemic variability during hospitalization, observing that in a prior study he conducted, glycemic variability in the noncritical care setting strongly correlated with hospital stay and a greater likelihood of going from hospital to nursing home or the morgue.
Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
CHICAGO – Hyperglycemia is common in patients receiving enteral nutrition and is a significant risk factor for hospital mortality, according to a retrospective study involving 157 patients.
Nearly 60% of patients had capillary blood glucose (CBG) values that exceeded 140 mg/dL, and nearly one-third exceeded 180 mg/dL.
More important, almost 40% of patients had a mean CBG of more than 180 mg/dL for the entire duration of their tube feedings.
At the threshold glucose of 180 mg/dL or more, the odds of dying in the hospital during tube feeding are approximately three times higher than for patients who had better glycemic control, Dr. Michael Jakoby said at the annual scientific sessions of the American Diabetes Association.
"We look at this body of work as just a stepping point to the next logical extension, which is to investigate ways that we can improve glycemic control for patients receiving any artificial nutrition, whether it’s parenteral or enteral," said Dr. Jakoby, chief of endocrinology at St. John’s Hospital in Springfield, Ill.
He previously reported that using an insulin protocol that determined insulin doses based on carbohydrate delivery and CBG was superior to ad hoc insulin dosing in the management of parenteral nutrition–induced hyperglycemia (JPEN J. Parenter. Enteral. Nutr. 2012;36:183-8).
Malnutrition among nonsurgical patients is high, and more than 40% of general surgery service patients have been reported to be malnourished (Am. J. Clin. Nutr. 1997;66:1232-9). Five studies since 2005 have established a link between parenteral nutrition and increased morbidity and in-hospital mortality, but little is available on outcomes with enteral nutrition, Dr. Jakoby explained.
The current analysis involved 157 patients receiving enteral nutrition in 2011 at St. John’s, a 350-bed hospital where each year an estimated 11,000 patients receive artificial nutrition for 2 weeks.
Of those patients, 58 were diagnosed with hyperglycemia (37%), defined as a mean CBG of 180 mg/dL or more for the duration of tube feedings.
Hyperglycemic patients were significantly older than controls (75 vs. 67), twice as likely to have a preexisting diagnosis of diabetes (81% vs. 41%), had 2 fewer days of enteral nutrition (7 vs. 9), and were less likely to be in the ICU during their tube feedings (62% vs. 83%), he said.
The two groups were well matched with regard to the amount of protein, fat, and total energy they received; however, hyperglycemic patients received significantly fewer carbohydrates than did controls (2.2 g/kg per day vs. 2.7 g/kg per day).
"With the documentation available to us, it seemed that this was an adaptive response to help combat the hyperglycemia that was happening during pure enteral nutrition," Dr. Jakoby said.
Cardiac complications – a composite of myocardial infarction, arrhythmia, and cardiac arrest – were increased in hyperglycemic patients (34% vs. 22%), but this difference was not significant in univariate analysis (odds ratio, 1.84; P = .13).
The risk of hospital mortality, however, was significantly higher in hyperglycemic patients in univariate analysis (36% vs. 16%; OR, 2.94), and remained significant in multivariate analysis after adjustment for age, sex, and preexisting diagnosis of diabetes (OR, 3.28), he noted.
During a discussion of the results, Dr. Jakoby said analyses are forthcoming looking at the impact of glycemic variability during hospitalization, observing that in a prior study he conducted, glycemic variability in the noncritical care setting strongly correlated with hospital stay and a greater likelihood of going from hospital to nursing home or the morgue.
Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
AT THE ANNUAL ADA SCIENTIFIC SESSIONS
Major finding: The mortality rate was 36% in hyperglycemic patients vs. 16% in controls (unadjusted OR 2.94; adjusted 3.28), a significant difference.
Data source: Retrospective analysis of 157 hospitalized patients receiving enteral nutrition.
Disclosures: Dr. Jakoby reported serving as a speaker for Sanofi-Aventis, Merck, and Novo Nordisk.
CMS bundles payments to outpatient departments, adds quality measures
Medicare officials are seeking to package more hospital outpatient department services into a single payment while also beefing up quality reporting programs in hospitals and ambulatory surgical centers.
The plans are part of the 2014 proposed rule for the Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System, announced July 8 and to be published in the Federal Register on July 19.
Overall, officials at the Centers for Medicare and Medicaid Services (CMS) are proposing a 1.8% payment increase for outpatient departments in 2014. But the agency wants to change the way these hospital departments are paid by bundling more services into a single payment.
Currently, the CMS offers a single payment for a variety of services including anesthesia, surgical supplies, imaging processing services, and implantable biologicals. The proposed rule would add seven new categories of services to that package.
The categories include:
• Drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure.
• Drugs and biologicals that function as supplies or devices when used in a surgical procedure.
• Certain clinical diagnostic laboratory tests.
• Procedures described by add-on codes.
• Ancillary services such as chest x-rays that are assigned the status indicator "X."
• Diagnostic tests on the bypass list.
• Device removal procedures.
The CMS will continue to pay separately for these types of services if they are reported alone on a claim.
Medicare will also continue to pay the average sales price plus 6% for non-pass-through drugs and biologicals that are paid separately under the prospective payment system.
In a change that would have a major impact on hospital emergency departments, the CMS is also seeking to streamline payment for outpatient visit codes. In the rule, the CMS is proposing to replace the current five levels of outpatient visit codes with three Level II Healthcare Common Procedure Coding System (HCPCS) codes representing a single level of payment. A Level II HCPCS code would be available for each type of visit: clinic, type A ED, and type B ED. Rates for the new codes will be based on the total mean costs of the Level 1 through 5 visit codes from 2012 claims data, according to the proposal.
"By collapsing the current five levels of codes to one level, the CMS believes this proposal will remove incentives hospitals may have to provide medically unnecessary services and expend additional, unnecessary resources to achieve a higher level of visit payment," the agency wrote in a fact sheet on the proposal. CMS officials also predicted that the change would reduce administration burden and be able to be easily adopted by hospitals.
The proposed rule would also expand Medicare’s quality reporting programs. The agency is proposing to add five new measures to the Hospital Outpatient Quality Reporting Program.
The new measures include influenza vaccination coverage among health care workers, complications within 30 days following cataract surgery requiring additional procedures, improvement in a patient’s visual function within 90 days after cataract surgery, appropriate follow-up interval for normal colonoscopy in average-risk patients, and colonoscopy interval for patients with a history of adenomatous polyps.
Data collection on the new measures would begin in January 2014, but payments would not be affected until 2016.
Agency officials also want to remove two measures from the program because they are overly burdensome on providers. They propose eliminating the use of the measure on "transition record with specified elements received by discharged ED patients" and a cardiac rehabilitation measure on "patient referral from an outpatient setting."
Ambulatory surgery centers will also be asked to report more quality information next year. The CMS is proposing to add four new measures to the ASC Quality Reporting Program, which are similar to those being proposed for the Hospital Outpatient Quality Reporting Program.
The measures include complications within 30 days following cataract surgery requiring additional procedures, improvement in a patient’s visual function within 90 days after cataract surgery, appropriate follow-up interval for normal colonoscopy in average-risk patients, and colonoscopy interval for patients with a history of adenomatous polyps. The data will be collected in 2014 to determine payments in 2016.
The CMS will accept public comment on the rule until Sept. 6, and a final regulation is expected to be published by November.
Medicare officials are seeking to package more hospital outpatient department services into a single payment while also beefing up quality reporting programs in hospitals and ambulatory surgical centers.
The plans are part of the 2014 proposed rule for the Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System, announced July 8 and to be published in the Federal Register on July 19.
Overall, officials at the Centers for Medicare and Medicaid Services (CMS) are proposing a 1.8% payment increase for outpatient departments in 2014. But the agency wants to change the way these hospital departments are paid by bundling more services into a single payment.
Currently, the CMS offers a single payment for a variety of services including anesthesia, surgical supplies, imaging processing services, and implantable biologicals. The proposed rule would add seven new categories of services to that package.
The categories include:
• Drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure.
• Drugs and biologicals that function as supplies or devices when used in a surgical procedure.
• Certain clinical diagnostic laboratory tests.
• Procedures described by add-on codes.
• Ancillary services such as chest x-rays that are assigned the status indicator "X."
• Diagnostic tests on the bypass list.
• Device removal procedures.
The CMS will continue to pay separately for these types of services if they are reported alone on a claim.
Medicare will also continue to pay the average sales price plus 6% for non-pass-through drugs and biologicals that are paid separately under the prospective payment system.
In a change that would have a major impact on hospital emergency departments, the CMS is also seeking to streamline payment for outpatient visit codes. In the rule, the CMS is proposing to replace the current five levels of outpatient visit codes with three Level II Healthcare Common Procedure Coding System (HCPCS) codes representing a single level of payment. A Level II HCPCS code would be available for each type of visit: clinic, type A ED, and type B ED. Rates for the new codes will be based on the total mean costs of the Level 1 through 5 visit codes from 2012 claims data, according to the proposal.
"By collapsing the current five levels of codes to one level, the CMS believes this proposal will remove incentives hospitals may have to provide medically unnecessary services and expend additional, unnecessary resources to achieve a higher level of visit payment," the agency wrote in a fact sheet on the proposal. CMS officials also predicted that the change would reduce administration burden and be able to be easily adopted by hospitals.
The proposed rule would also expand Medicare’s quality reporting programs. The agency is proposing to add five new measures to the Hospital Outpatient Quality Reporting Program.
The new measures include influenza vaccination coverage among health care workers, complications within 30 days following cataract surgery requiring additional procedures, improvement in a patient’s visual function within 90 days after cataract surgery, appropriate follow-up interval for normal colonoscopy in average-risk patients, and colonoscopy interval for patients with a history of adenomatous polyps.
Data collection on the new measures would begin in January 2014, but payments would not be affected until 2016.
Agency officials also want to remove two measures from the program because they are overly burdensome on providers. They propose eliminating the use of the measure on "transition record with specified elements received by discharged ED patients" and a cardiac rehabilitation measure on "patient referral from an outpatient setting."
Ambulatory surgery centers will also be asked to report more quality information next year. The CMS is proposing to add four new measures to the ASC Quality Reporting Program, which are similar to those being proposed for the Hospital Outpatient Quality Reporting Program.
The measures include complications within 30 days following cataract surgery requiring additional procedures, improvement in a patient’s visual function within 90 days after cataract surgery, appropriate follow-up interval for normal colonoscopy in average-risk patients, and colonoscopy interval for patients with a history of adenomatous polyps. The data will be collected in 2014 to determine payments in 2016.
The CMS will accept public comment on the rule until Sept. 6, and a final regulation is expected to be published by November.
Medicare officials are seeking to package more hospital outpatient department services into a single payment while also beefing up quality reporting programs in hospitals and ambulatory surgical centers.
The plans are part of the 2014 proposed rule for the Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System, announced July 8 and to be published in the Federal Register on July 19.
Overall, officials at the Centers for Medicare and Medicaid Services (CMS) are proposing a 1.8% payment increase for outpatient departments in 2014. But the agency wants to change the way these hospital departments are paid by bundling more services into a single payment.
Currently, the CMS offers a single payment for a variety of services including anesthesia, surgical supplies, imaging processing services, and implantable biologicals. The proposed rule would add seven new categories of services to that package.
The categories include:
• Drugs, biologicals, and radiopharmaceuticals that function as supplies when used in a diagnostic test or procedure.
• Drugs and biologicals that function as supplies or devices when used in a surgical procedure.
• Certain clinical diagnostic laboratory tests.
• Procedures described by add-on codes.
• Ancillary services such as chest x-rays that are assigned the status indicator "X."
• Diagnostic tests on the bypass list.
• Device removal procedures.
The CMS will continue to pay separately for these types of services if they are reported alone on a claim.
Medicare will also continue to pay the average sales price plus 6% for non-pass-through drugs and biologicals that are paid separately under the prospective payment system.
In a change that would have a major impact on hospital emergency departments, the CMS is also seeking to streamline payment for outpatient visit codes. In the rule, the CMS is proposing to replace the current five levels of outpatient visit codes with three Level II Healthcare Common Procedure Coding System (HCPCS) codes representing a single level of payment. A Level II HCPCS code would be available for each type of visit: clinic, type A ED, and type B ED. Rates for the new codes will be based on the total mean costs of the Level 1 through 5 visit codes from 2012 claims data, according to the proposal.
"By collapsing the current five levels of codes to one level, the CMS believes this proposal will remove incentives hospitals may have to provide medically unnecessary services and expend additional, unnecessary resources to achieve a higher level of visit payment," the agency wrote in a fact sheet on the proposal. CMS officials also predicted that the change would reduce administration burden and be able to be easily adopted by hospitals.
The proposed rule would also expand Medicare’s quality reporting programs. The agency is proposing to add five new measures to the Hospital Outpatient Quality Reporting Program.
The new measures include influenza vaccination coverage among health care workers, complications within 30 days following cataract surgery requiring additional procedures, improvement in a patient’s visual function within 90 days after cataract surgery, appropriate follow-up interval for normal colonoscopy in average-risk patients, and colonoscopy interval for patients with a history of adenomatous polyps.
Data collection on the new measures would begin in January 2014, but payments would not be affected until 2016.
Agency officials also want to remove two measures from the program because they are overly burdensome on providers. They propose eliminating the use of the measure on "transition record with specified elements received by discharged ED patients" and a cardiac rehabilitation measure on "patient referral from an outpatient setting."
Ambulatory surgery centers will also be asked to report more quality information next year. The CMS is proposing to add four new measures to the ASC Quality Reporting Program, which are similar to those being proposed for the Hospital Outpatient Quality Reporting Program.
The measures include complications within 30 days following cataract surgery requiring additional procedures, improvement in a patient’s visual function within 90 days after cataract surgery, appropriate follow-up interval for normal colonoscopy in average-risk patients, and colonoscopy interval for patients with a history of adenomatous polyps. The data will be collected in 2014 to determine payments in 2016.
The CMS will accept public comment on the rule until Sept. 6, and a final regulation is expected to be published by November.