User login
Official Newspaper of the American College of Surgeons
A third of follicular thyroid lesions of undetermined significance were malignant
CHICAGO – At least in some institutions, about a third of follicular thyroid lesions of undetermined significance are malignant, reported researchers from the University of Wisconsin, Madison.
That rate is a higher proportion than the 5%-15% rate estimated by the Bethesda System, a national standard for reporting thyroid cytopathology, said Dr. Juan Carlos Jaume at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The message here is to be aware of your local institutional rates for cancer in FLUS, because following [the Bethesda System] may mislead you and your patient" when deciding on a course of action, be it surgery, repeat biopsy, or observation, he said. "Other institutions [should be encouraged to] do similar analyses to generate more accurate local guidelines for management of FLUS," noted Dr. Jaume, senior author of the study.
Of 1,420 nodules assessed over 2 years at the thyroid clinic at the University of Wisconsin, Madison, 134 (9.4%) were reported as follicular lesions of undetermined significance (FLUS) on fine-needle aspiration. Eighty patients opted for surgery; pathology revealed that 27 (34%) actually had differentiated thyroid cancer. Cancer also was found in four more patients, but at sites different from the original fine-needle aspiration. Most of the cancers (27) were papillary, 3 were follicular, and 1 was a Hurthle cell tumor, the investigators reported.
It’s unlikely the findings were due to selection bias, with patients who were more likely to have cancer opting for surgery. More than half of the patients chose surgery after discussing risks and benefits with their providers, not because of tumor progression. Almost all the others opted for surgery because of compression symptoms or because they had a nodule larger than 4 cm.
Even if there was a bias, "the highest expectation [with Bethesda] is 15%; our rate was 34%," a large difference, Dr. Jaume said. "As soon as we had the rate available, we conveyed the information" to providers so they could more accurately counsel patients. "I think eventually we will see an increase in the number of patients deciding on surgery."
The team performed the study because providers at the university had been relying on the Bethesda estimate to guide patients, but had a hunch that their local FLUS cancer rates were higher.
The majority of the 134 FLUS patients who opted against surgery chose ultrasound monitoring. Among the 22 who chose repeat fine-needle aspiration, half were rediagnosed with benign cytology, 5 were again diagnosed with FLUS, and most of the rest were lost to follow-up.
Among the 80 surgical patients, pathology was benign in 47 and parathyroid tissue was present in 1 biopsy. Records were unavailable for the final patient.
Some cytopathologists tend to call thyroid lesions FLUS more frequently than others; possibly, that predilection has something to do with the discordance in reported cancer rates, Dr. Jaume said.
Ultimately, the solution will be genetic analysis of fine-needle aspiration samples. There is a commercial product on the market, but "we are not using [it] in our institution because the negative predictive value is high, but the positive predictive value is low," he said.
The investigators had no relevant disclosures and had no outside funding for their work.
*Correction, 8/25/2014: An earlier version of this article misspelled Dr. Jaume's name.
CHICAGO – At least in some institutions, about a third of follicular thyroid lesions of undetermined significance are malignant, reported researchers from the University of Wisconsin, Madison.
That rate is a higher proportion than the 5%-15% rate estimated by the Bethesda System, a national standard for reporting thyroid cytopathology, said Dr. Juan Carlos Jaume at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The message here is to be aware of your local institutional rates for cancer in FLUS, because following [the Bethesda System] may mislead you and your patient" when deciding on a course of action, be it surgery, repeat biopsy, or observation, he said. "Other institutions [should be encouraged to] do similar analyses to generate more accurate local guidelines for management of FLUS," noted Dr. Jaume, senior author of the study.
Of 1,420 nodules assessed over 2 years at the thyroid clinic at the University of Wisconsin, Madison, 134 (9.4%) were reported as follicular lesions of undetermined significance (FLUS) on fine-needle aspiration. Eighty patients opted for surgery; pathology revealed that 27 (34%) actually had differentiated thyroid cancer. Cancer also was found in four more patients, but at sites different from the original fine-needle aspiration. Most of the cancers (27) were papillary, 3 were follicular, and 1 was a Hurthle cell tumor, the investigators reported.
It’s unlikely the findings were due to selection bias, with patients who were more likely to have cancer opting for surgery. More than half of the patients chose surgery after discussing risks and benefits with their providers, not because of tumor progression. Almost all the others opted for surgery because of compression symptoms or because they had a nodule larger than 4 cm.
Even if there was a bias, "the highest expectation [with Bethesda] is 15%; our rate was 34%," a large difference, Dr. Jaume said. "As soon as we had the rate available, we conveyed the information" to providers so they could more accurately counsel patients. "I think eventually we will see an increase in the number of patients deciding on surgery."
The team performed the study because providers at the university had been relying on the Bethesda estimate to guide patients, but had a hunch that their local FLUS cancer rates were higher.
The majority of the 134 FLUS patients who opted against surgery chose ultrasound monitoring. Among the 22 who chose repeat fine-needle aspiration, half were rediagnosed with benign cytology, 5 were again diagnosed with FLUS, and most of the rest were lost to follow-up.
Among the 80 surgical patients, pathology was benign in 47 and parathyroid tissue was present in 1 biopsy. Records were unavailable for the final patient.
Some cytopathologists tend to call thyroid lesions FLUS more frequently than others; possibly, that predilection has something to do with the discordance in reported cancer rates, Dr. Jaume said.
Ultimately, the solution will be genetic analysis of fine-needle aspiration samples. There is a commercial product on the market, but "we are not using [it] in our institution because the negative predictive value is high, but the positive predictive value is low," he said.
The investigators had no relevant disclosures and had no outside funding for their work.
*Correction, 8/25/2014: An earlier version of this article misspelled Dr. Jaume's name.
CHICAGO – At least in some institutions, about a third of follicular thyroid lesions of undetermined significance are malignant, reported researchers from the University of Wisconsin, Madison.
That rate is a higher proportion than the 5%-15% rate estimated by the Bethesda System, a national standard for reporting thyroid cytopathology, said Dr. Juan Carlos Jaume at the joint meeting of the International Congress of Endocrinology and the Endocrine Society.
"The message here is to be aware of your local institutional rates for cancer in FLUS, because following [the Bethesda System] may mislead you and your patient" when deciding on a course of action, be it surgery, repeat biopsy, or observation, he said. "Other institutions [should be encouraged to] do similar analyses to generate more accurate local guidelines for management of FLUS," noted Dr. Jaume, senior author of the study.
Of 1,420 nodules assessed over 2 years at the thyroid clinic at the University of Wisconsin, Madison, 134 (9.4%) were reported as follicular lesions of undetermined significance (FLUS) on fine-needle aspiration. Eighty patients opted for surgery; pathology revealed that 27 (34%) actually had differentiated thyroid cancer. Cancer also was found in four more patients, but at sites different from the original fine-needle aspiration. Most of the cancers (27) were papillary, 3 were follicular, and 1 was a Hurthle cell tumor, the investigators reported.
It’s unlikely the findings were due to selection bias, with patients who were more likely to have cancer opting for surgery. More than half of the patients chose surgery after discussing risks and benefits with their providers, not because of tumor progression. Almost all the others opted for surgery because of compression symptoms or because they had a nodule larger than 4 cm.
Even if there was a bias, "the highest expectation [with Bethesda] is 15%; our rate was 34%," a large difference, Dr. Jaume said. "As soon as we had the rate available, we conveyed the information" to providers so they could more accurately counsel patients. "I think eventually we will see an increase in the number of patients deciding on surgery."
The team performed the study because providers at the university had been relying on the Bethesda estimate to guide patients, but had a hunch that their local FLUS cancer rates were higher.
The majority of the 134 FLUS patients who opted against surgery chose ultrasound monitoring. Among the 22 who chose repeat fine-needle aspiration, half were rediagnosed with benign cytology, 5 were again diagnosed with FLUS, and most of the rest were lost to follow-up.
Among the 80 surgical patients, pathology was benign in 47 and parathyroid tissue was present in 1 biopsy. Records were unavailable for the final patient.
Some cytopathologists tend to call thyroid lesions FLUS more frequently than others; possibly, that predilection has something to do with the discordance in reported cancer rates, Dr. Jaume said.
Ultimately, the solution will be genetic analysis of fine-needle aspiration samples. There is a commercial product on the market, but "we are not using [it] in our institution because the negative predictive value is high, but the positive predictive value is low," he said.
The investigators had no relevant disclosures and had no outside funding for their work.
*Correction, 8/25/2014: An earlier version of this article misspelled Dr. Jaume's name.
AT ICE/ENDO 2014
Key clinical point: National estimates of FLUS malignancy may not apply to your institution.
Major finding: Among 80 patients diagnosed with FLUS who opted for surgery, the lesion turned out to be differentiated thyroid cancer in 27 (34%).
Data source: Retrospective study of outcomes for 134 FLUS nodules.
Disclosures: The investigators had no disclosures and had no outside funding for their study.
Huge chunk of data excluded from Open Payments website
When the government publishes information on the financial relationships between physicians, teaching hospitals, and the pharmaceutical and device industries on Sept. 30 as part of the Open Payments Program, about one-third of the available payment data will be missing.
Officials at the Centers for Medicare & Medicaid Services recently confirmed that they are returning about one-third of the payment records already submitted by manufacturers and group purchasing organizations (GPOs) because of problems of "intermingled data." Those records will be included in the next public data release, scheduled for June 2015. The CMS did not provide information on the exact number of records that were being sent back for corrections.
Delaying the release of these records will allow manufacturers and GPOs to make corrections and give physicians and teaching hospitals time to review and dispute the data, according to the CMS.
But the American Medical Association said that doubts about the accuracy of one-third of the data being reported to the Open Payments system is a clear sign that more time is needed to revamp the program.
"The publication of inaccurate data can potentially harm the physician-patient relationship, which is why the AMA maintains its call for a 6-month delay of the data release," Dr. Robert M. Wah, AMA president, said in a statement.
The AMA, along with about 100 other state medical and specialty societies, has been on CMS officials to delay the start of the program, not only because of data accuracy issues, but because the registration and review processes have been confusing and time consuming.
The Open Payments program, which was mandated under the Affordable Care Act, has had a rocky rollout so far. The CMS was forced to delay the period for teaching hospitals and physicians to review and dispute payments after receiving reports that payment data were being attributed to the wrong physicians. The agency took the system offline for almost 2 weeks in early August to investigate the problems and implement fixes.
The CMS extended the deadline for physician and teaching hospitals to review and dispute data from Aug. 27 to Sept. 8, but is sticking to its plan to publish the first round of payment data on Sept. 30.
On Twitter @maryellenny
When the government publishes information on the financial relationships between physicians, teaching hospitals, and the pharmaceutical and device industries on Sept. 30 as part of the Open Payments Program, about one-third of the available payment data will be missing.
Officials at the Centers for Medicare & Medicaid Services recently confirmed that they are returning about one-third of the payment records already submitted by manufacturers and group purchasing organizations (GPOs) because of problems of "intermingled data." Those records will be included in the next public data release, scheduled for June 2015. The CMS did not provide information on the exact number of records that were being sent back for corrections.
Delaying the release of these records will allow manufacturers and GPOs to make corrections and give physicians and teaching hospitals time to review and dispute the data, according to the CMS.
But the American Medical Association said that doubts about the accuracy of one-third of the data being reported to the Open Payments system is a clear sign that more time is needed to revamp the program.
"The publication of inaccurate data can potentially harm the physician-patient relationship, which is why the AMA maintains its call for a 6-month delay of the data release," Dr. Robert M. Wah, AMA president, said in a statement.
The AMA, along with about 100 other state medical and specialty societies, has been on CMS officials to delay the start of the program, not only because of data accuracy issues, but because the registration and review processes have been confusing and time consuming.
The Open Payments program, which was mandated under the Affordable Care Act, has had a rocky rollout so far. The CMS was forced to delay the period for teaching hospitals and physicians to review and dispute payments after receiving reports that payment data were being attributed to the wrong physicians. The agency took the system offline for almost 2 weeks in early August to investigate the problems and implement fixes.
The CMS extended the deadline for physician and teaching hospitals to review and dispute data from Aug. 27 to Sept. 8, but is sticking to its plan to publish the first round of payment data on Sept. 30.
On Twitter @maryellenny
When the government publishes information on the financial relationships between physicians, teaching hospitals, and the pharmaceutical and device industries on Sept. 30 as part of the Open Payments Program, about one-third of the available payment data will be missing.
Officials at the Centers for Medicare & Medicaid Services recently confirmed that they are returning about one-third of the payment records already submitted by manufacturers and group purchasing organizations (GPOs) because of problems of "intermingled data." Those records will be included in the next public data release, scheduled for June 2015. The CMS did not provide information on the exact number of records that were being sent back for corrections.
Delaying the release of these records will allow manufacturers and GPOs to make corrections and give physicians and teaching hospitals time to review and dispute the data, according to the CMS.
But the American Medical Association said that doubts about the accuracy of one-third of the data being reported to the Open Payments system is a clear sign that more time is needed to revamp the program.
"The publication of inaccurate data can potentially harm the physician-patient relationship, which is why the AMA maintains its call for a 6-month delay of the data release," Dr. Robert M. Wah, AMA president, said in a statement.
The AMA, along with about 100 other state medical and specialty societies, has been on CMS officials to delay the start of the program, not only because of data accuracy issues, but because the registration and review processes have been confusing and time consuming.
The Open Payments program, which was mandated under the Affordable Care Act, has had a rocky rollout so far. The CMS was forced to delay the period for teaching hospitals and physicians to review and dispute payments after receiving reports that payment data were being attributed to the wrong physicians. The agency took the system offline for almost 2 weeks in early August to investigate the problems and implement fixes.
The CMS extended the deadline for physician and teaching hospitals to review and dispute data from Aug. 27 to Sept. 8, but is sticking to its plan to publish the first round of payment data on Sept. 30.
On Twitter @maryellenny
Firm policies help address staff who behave badly
CHICAGO – Strong policies and open communication within a physician practice are key to resolving office conflicts and curtailing bad behavior by staff.
"Clear communication is essential," said Dr. Joseph S. Eastern, who practices dermatology and dermatologic surgery in Belleville, N.J. "Destructive political situations are often rooted in communication failure. Policies are also essential. Predictable conflicts can be prevented if policies have been agreed upon in advance; crises often result when there is no policy in place to address the issue in question."
Dr. Eastern and other presenters discussed common challenges that arise in staff environments during the recent American Academy of Dermatology summer meeting. Frequent challenges include employees who chronically leave early, staff members who abuse sick policies, and inappropriate interoffice relationships. Texting and Internet overuse are also growing burdens facing medical practices.
Firm policies that outline acceptable behavior by staff, and potential discipline for policy violations help tackle such difficult situations before they grow out of hand, presenters said.
"Every office should have a formal policy [that limits personal cell phone and Internet use during office hours]," Dr. Eastern said in an interview. "Mine is fairly straightforward: Using office time for personal texting and Web surfing is theft – pure and simple – theft of my time. I make this crystal clear. It is never permissible to steal any office property, least of all our most marketable commodity – office time."
In some circumstances, new rules may need to be drafted or old policies revised. For instance, in the case of employees who start dating. Smaller offices may not have specific policy language that addresses such relationships, said Dr. Seemal R. Desai of the University of Texas Southwestern Medical Center in Dallas and a dermatologist in private practice in Plano. Another challenge that may not be automatically outlined is staff members who constantly seek curbside consults with physicians to ask for personal medical advice. Both issues may lend themselves to consideration and institution of new standards.
Discuss problems or complaints early with employees in a nonconfrontational way, added Dr. Desai. This could mean a one-on-one chat with a staff member or a conversation with two employees who are in conflict.
"Approach the conversation with a really open attitude to try to hear the concerns," Dr. Desai said in an interview. "Not every situation that at first seems like a negative one, really ends up being that way."
Make sure staff are aware of policies and stick to them, Dr. Eastern notes. The worst way physicians can react to an issue is to ignore it. Ongoing office politics not only can cause tension among employees but also can reduce productivity and affect patient care.
"Sentiments and feelings and themes in an office-based setting can really translate a lot into how you practice," Dr. Desai said "and how successful you are on a daily basis."
On Twitter @legal_med
CHICAGO – Strong policies and open communication within a physician practice are key to resolving office conflicts and curtailing bad behavior by staff.
"Clear communication is essential," said Dr. Joseph S. Eastern, who practices dermatology and dermatologic surgery in Belleville, N.J. "Destructive political situations are often rooted in communication failure. Policies are also essential. Predictable conflicts can be prevented if policies have been agreed upon in advance; crises often result when there is no policy in place to address the issue in question."
Dr. Eastern and other presenters discussed common challenges that arise in staff environments during the recent American Academy of Dermatology summer meeting. Frequent challenges include employees who chronically leave early, staff members who abuse sick policies, and inappropriate interoffice relationships. Texting and Internet overuse are also growing burdens facing medical practices.
Firm policies that outline acceptable behavior by staff, and potential discipline for policy violations help tackle such difficult situations before they grow out of hand, presenters said.
"Every office should have a formal policy [that limits personal cell phone and Internet use during office hours]," Dr. Eastern said in an interview. "Mine is fairly straightforward: Using office time for personal texting and Web surfing is theft – pure and simple – theft of my time. I make this crystal clear. It is never permissible to steal any office property, least of all our most marketable commodity – office time."
In some circumstances, new rules may need to be drafted or old policies revised. For instance, in the case of employees who start dating. Smaller offices may not have specific policy language that addresses such relationships, said Dr. Seemal R. Desai of the University of Texas Southwestern Medical Center in Dallas and a dermatologist in private practice in Plano. Another challenge that may not be automatically outlined is staff members who constantly seek curbside consults with physicians to ask for personal medical advice. Both issues may lend themselves to consideration and institution of new standards.
Discuss problems or complaints early with employees in a nonconfrontational way, added Dr. Desai. This could mean a one-on-one chat with a staff member or a conversation with two employees who are in conflict.
"Approach the conversation with a really open attitude to try to hear the concerns," Dr. Desai said in an interview. "Not every situation that at first seems like a negative one, really ends up being that way."
Make sure staff are aware of policies and stick to them, Dr. Eastern notes. The worst way physicians can react to an issue is to ignore it. Ongoing office politics not only can cause tension among employees but also can reduce productivity and affect patient care.
"Sentiments and feelings and themes in an office-based setting can really translate a lot into how you practice," Dr. Desai said "and how successful you are on a daily basis."
On Twitter @legal_med
CHICAGO – Strong policies and open communication within a physician practice are key to resolving office conflicts and curtailing bad behavior by staff.
"Clear communication is essential," said Dr. Joseph S. Eastern, who practices dermatology and dermatologic surgery in Belleville, N.J. "Destructive political situations are often rooted in communication failure. Policies are also essential. Predictable conflicts can be prevented if policies have been agreed upon in advance; crises often result when there is no policy in place to address the issue in question."
Dr. Eastern and other presenters discussed common challenges that arise in staff environments during the recent American Academy of Dermatology summer meeting. Frequent challenges include employees who chronically leave early, staff members who abuse sick policies, and inappropriate interoffice relationships. Texting and Internet overuse are also growing burdens facing medical practices.
Firm policies that outline acceptable behavior by staff, and potential discipline for policy violations help tackle such difficult situations before they grow out of hand, presenters said.
"Every office should have a formal policy [that limits personal cell phone and Internet use during office hours]," Dr. Eastern said in an interview. "Mine is fairly straightforward: Using office time for personal texting and Web surfing is theft – pure and simple – theft of my time. I make this crystal clear. It is never permissible to steal any office property, least of all our most marketable commodity – office time."
In some circumstances, new rules may need to be drafted or old policies revised. For instance, in the case of employees who start dating. Smaller offices may not have specific policy language that addresses such relationships, said Dr. Seemal R. Desai of the University of Texas Southwestern Medical Center in Dallas and a dermatologist in private practice in Plano. Another challenge that may not be automatically outlined is staff members who constantly seek curbside consults with physicians to ask for personal medical advice. Both issues may lend themselves to consideration and institution of new standards.
Discuss problems or complaints early with employees in a nonconfrontational way, added Dr. Desai. This could mean a one-on-one chat with a staff member or a conversation with two employees who are in conflict.
"Approach the conversation with a really open attitude to try to hear the concerns," Dr. Desai said in an interview. "Not every situation that at first seems like a negative one, really ends up being that way."
Make sure staff are aware of policies and stick to them, Dr. Eastern notes. The worst way physicians can react to an issue is to ignore it. Ongoing office politics not only can cause tension among employees but also can reduce productivity and affect patient care.
"Sentiments and feelings and themes in an office-based setting can really translate a lot into how you practice," Dr. Desai said "and how successful you are on a daily basis."
On Twitter @legal_med
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2014
Open Payments system back online; physician deadline extended
The Open Payments system is up and running again, and federal officials said the technical glitches that caused data to be linked to the wrong physicians have been fixed.
Physicians and teaching hospitals now have until Sept. 8 to review the payment information submitted to the government by drug, device, and biological manufacturers. Despite the delay, the information is still on track to be publicly released on Sept. 30, according to the Centers for Medicare & Medicaid Services.
The Open Payments Program, created by the Affordable Care Act, aims to add transparency to the financial relationships between the health care industry and physicians and teaching hospitals. But the effort to publish data on industry payments was delayed when CMS learned that some information was being incorrectly matched to physicians. The agency took the site offline on Aug. 3 to investigate.
"CMS takes data integrity very seriously and took swift action after a physician reported a problem," Dr. Shantanu Agrawal, deputy administrator and director of the Center for Program Integrity at CMS, said in a statement. "We have identified the root cause of the problem and have instituted a system fix to prevent similar errors."
Dr. Agrawal urged physicians to review their records before the Sept. 8 deadline to identify any potential errors.
The CMS investigation found that manufacturers and group purchasing organizations had submitted intermingled data, adding the wrong state license number or national provider identifier for physicians with the same first and last names. The agency implemented "system fixes," removed the inaccurate data, and revalidated the information.
Since the website used for physicians and teaching hospital to review their data was offline for several days, CMS has extended the deadline for reviewing and disputing data from Aug. 27 to Sept. 8.
On Twitter @maryellenny
The Open Payments system is up and running again, and federal officials said the technical glitches that caused data to be linked to the wrong physicians have been fixed.
Physicians and teaching hospitals now have until Sept. 8 to review the payment information submitted to the government by drug, device, and biological manufacturers. Despite the delay, the information is still on track to be publicly released on Sept. 30, according to the Centers for Medicare & Medicaid Services.
The Open Payments Program, created by the Affordable Care Act, aims to add transparency to the financial relationships between the health care industry and physicians and teaching hospitals. But the effort to publish data on industry payments was delayed when CMS learned that some information was being incorrectly matched to physicians. The agency took the site offline on Aug. 3 to investigate.
"CMS takes data integrity very seriously and took swift action after a physician reported a problem," Dr. Shantanu Agrawal, deputy administrator and director of the Center for Program Integrity at CMS, said in a statement. "We have identified the root cause of the problem and have instituted a system fix to prevent similar errors."
Dr. Agrawal urged physicians to review their records before the Sept. 8 deadline to identify any potential errors.
The CMS investigation found that manufacturers and group purchasing organizations had submitted intermingled data, adding the wrong state license number or national provider identifier for physicians with the same first and last names. The agency implemented "system fixes," removed the inaccurate data, and revalidated the information.
Since the website used for physicians and teaching hospital to review their data was offline for several days, CMS has extended the deadline for reviewing and disputing data from Aug. 27 to Sept. 8.
On Twitter @maryellenny
The Open Payments system is up and running again, and federal officials said the technical glitches that caused data to be linked to the wrong physicians have been fixed.
Physicians and teaching hospitals now have until Sept. 8 to review the payment information submitted to the government by drug, device, and biological manufacturers. Despite the delay, the information is still on track to be publicly released on Sept. 30, according to the Centers for Medicare & Medicaid Services.
The Open Payments Program, created by the Affordable Care Act, aims to add transparency to the financial relationships between the health care industry and physicians and teaching hospitals. But the effort to publish data on industry payments was delayed when CMS learned that some information was being incorrectly matched to physicians. The agency took the site offline on Aug. 3 to investigate.
"CMS takes data integrity very seriously and took swift action after a physician reported a problem," Dr. Shantanu Agrawal, deputy administrator and director of the Center for Program Integrity at CMS, said in a statement. "We have identified the root cause of the problem and have instituted a system fix to prevent similar errors."
Dr. Agrawal urged physicians to review their records before the Sept. 8 deadline to identify any potential errors.
The CMS investigation found that manufacturers and group purchasing organizations had submitted intermingled data, adding the wrong state license number or national provider identifier for physicians with the same first and last names. The agency implemented "system fixes," removed the inaccurate data, and revalidated the information.
Since the website used for physicians and teaching hospital to review their data was offline for several days, CMS has extended the deadline for reviewing and disputing data from Aug. 27 to Sept. 8.
On Twitter @maryellenny
Most Americans satisfied with cost of brand-name drugs
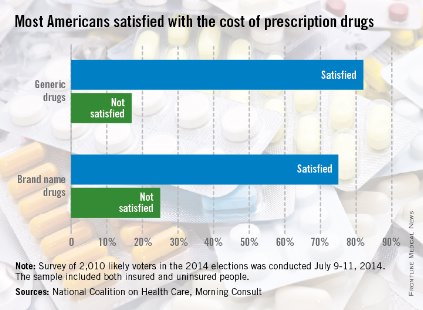
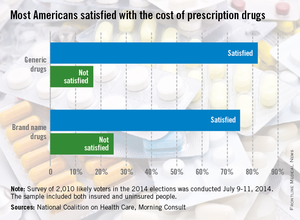
Three-quarters of American adults are satisfied with what they pay for brand-name prescription drugs, according to a survey from the National Coalition on Health Care and digital media company Morning Consult.
Generic drugs are even more popular, getting a satisfaction rate of 82% in the survey, which was conducted July 9-11, among a national sample of 2,010 people likely to vote in the 2014 elections.
When respondents were asked about the transparency of prescription drug pricing, 64% said that there was not enough transparency, 12% said there was enough, and 25% were not sure, the Morning Consult reported.
Three-quarters of American adults are satisfied with what they pay for brand-name prescription drugs, according to a survey from the National Coalition on Health Care and digital media company Morning Consult.
Generic drugs are even more popular, getting a satisfaction rate of 82% in the survey, which was conducted July 9-11, among a national sample of 2,010 people likely to vote in the 2014 elections.
When respondents were asked about the transparency of prescription drug pricing, 64% said that there was not enough transparency, 12% said there was enough, and 25% were not sure, the Morning Consult reported.

Three-quarters of American adults are satisfied with what they pay for brand-name prescription drugs, according to a survey from the National Coalition on Health Care and digital media company Morning Consult.
Generic drugs are even more popular, getting a satisfaction rate of 82% in the survey, which was conducted July 9-11, among a national sample of 2,010 people likely to vote in the 2014 elections.
When respondents were asked about the transparency of prescription drug pricing, 64% said that there was not enough transparency, 12% said there was enough, and 25% were not sure, the Morning Consult reported.

Many surgical residents consider quitting during training
A majority of general surgery residents seriously consider dropping out of their training, with female residents more likely to consider quitting, a new study in JAMA Surgery reveals.
According to a survey, 58.0% of the 288 respondents "seriously considered leaving training." The most frequent reasons cited for wanting to quit training were sleep deprivation on a specific rotation (50%), an undesirable future lifestyle (47%), and excessive work hours on a specific rotation (41.4%). Survey results were published online July 30 in JAMA Surgery (2014 [doi:10.1001/jamasurg.2014.935]).
Factors cited that ultimately keep general surgery residents from ending training are support from family or significant other (65%), support from other residents (63.5%), and perception of being better rested (58.9%).
"We believe that our survey findings highlight the fact that a desire to leave training may not be affected by job rigor alone but rather [by] program-specific or rotation-specific factors or dissatisfaction with a future career in general surgery," the report states. Dr. Edward Gifford of the department of surgery, University of California, Los Angeles, Medical Center, is the report’s lead author.
In addressing the factors that led to consideration for leaving training, the authors noted that "a potential remedy may be to identify those high work-hour rotations and modify them accordingly," though lifestyle concerns may be harder to address as practicing surgeons "continue to experience high levels of work-home conflicts and burnout."
For women specifically, another issue is "the paucity of female mentors in academic surgery," the report states. "Striving to increase the number of female faculty members within training programs and refining the mentor-mentee relationship with incoming residents may improve the outlook and productivity of future female surgeons."
Overall, while men’s thoughts of quitting decreased as their residency progressed, women’s considerations remained persistent. The report cites previous studies that reported that men and women view general surgery careers differently, including that it was not a welcoming career because of lifestyle challenges, particularly if the woman had children, limited flexible training, and lack of role models.
"These findings may explain why women in our survey continued to consider leaving residency throughout the duration of training and underscores the importance of supporting female residents through the difficult balance between motherhood and professional life," the report states.
The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
Program directors at residency programs "must take a purposeful, proactive approach from the beginning of surgery residency that shows residents how they can achieve a healthy balance of work and life, create practices over which they have control, and live happy, productive lives," Dr. Karen Deveney writes in a commentary published online July 30 in JAMA Surgery 2014 [doi:10.1001/jamasurg.2014964]).
Dr. Deveney also cautioned about current surgeons being openly critical of their chosen profession. "We have failed our younger generation if we whine and complain about our wretched lives rather than taking steps that are available to use to be proactive, take control of our own fates, and realize what a privileged position we are in as surgeons. Women residents are particularly vulnerable to worries that they may not be able to juggle competing demands of their families and their careers and need to be matched with female surgeons in practice who have managed successfully to find that balance."
Dr. Deveney works in the department of surgery at the Oregon Health and Science University in Portland.
Program directors at residency programs "must take a purposeful, proactive approach from the beginning of surgery residency that shows residents how they can achieve a healthy balance of work and life, create practices over which they have control, and live happy, productive lives," Dr. Karen Deveney writes in a commentary published online July 30 in JAMA Surgery 2014 [doi:10.1001/jamasurg.2014964]).
Dr. Deveney also cautioned about current surgeons being openly critical of their chosen profession. "We have failed our younger generation if we whine and complain about our wretched lives rather than taking steps that are available to use to be proactive, take control of our own fates, and realize what a privileged position we are in as surgeons. Women residents are particularly vulnerable to worries that they may not be able to juggle competing demands of their families and their careers and need to be matched with female surgeons in practice who have managed successfully to find that balance."
Dr. Deveney works in the department of surgery at the Oregon Health and Science University in Portland.
Program directors at residency programs "must take a purposeful, proactive approach from the beginning of surgery residency that shows residents how they can achieve a healthy balance of work and life, create practices over which they have control, and live happy, productive lives," Dr. Karen Deveney writes in a commentary published online July 30 in JAMA Surgery 2014 [doi:10.1001/jamasurg.2014964]).
Dr. Deveney also cautioned about current surgeons being openly critical of their chosen profession. "We have failed our younger generation if we whine and complain about our wretched lives rather than taking steps that are available to use to be proactive, take control of our own fates, and realize what a privileged position we are in as surgeons. Women residents are particularly vulnerable to worries that they may not be able to juggle competing demands of their families and their careers and need to be matched with female surgeons in practice who have managed successfully to find that balance."
Dr. Deveney works in the department of surgery at the Oregon Health and Science University in Portland.
A majority of general surgery residents seriously consider dropping out of their training, with female residents more likely to consider quitting, a new study in JAMA Surgery reveals.
According to a survey, 58.0% of the 288 respondents "seriously considered leaving training." The most frequent reasons cited for wanting to quit training were sleep deprivation on a specific rotation (50%), an undesirable future lifestyle (47%), and excessive work hours on a specific rotation (41.4%). Survey results were published online July 30 in JAMA Surgery (2014 [doi:10.1001/jamasurg.2014.935]).
Factors cited that ultimately keep general surgery residents from ending training are support from family or significant other (65%), support from other residents (63.5%), and perception of being better rested (58.9%).
"We believe that our survey findings highlight the fact that a desire to leave training may not be affected by job rigor alone but rather [by] program-specific or rotation-specific factors or dissatisfaction with a future career in general surgery," the report states. Dr. Edward Gifford of the department of surgery, University of California, Los Angeles, Medical Center, is the report’s lead author.
In addressing the factors that led to consideration for leaving training, the authors noted that "a potential remedy may be to identify those high work-hour rotations and modify them accordingly," though lifestyle concerns may be harder to address as practicing surgeons "continue to experience high levels of work-home conflicts and burnout."
For women specifically, another issue is "the paucity of female mentors in academic surgery," the report states. "Striving to increase the number of female faculty members within training programs and refining the mentor-mentee relationship with incoming residents may improve the outlook and productivity of future female surgeons."
Overall, while men’s thoughts of quitting decreased as their residency progressed, women’s considerations remained persistent. The report cites previous studies that reported that men and women view general surgery careers differently, including that it was not a welcoming career because of lifestyle challenges, particularly if the woman had children, limited flexible training, and lack of role models.
"These findings may explain why women in our survey continued to consider leaving residency throughout the duration of training and underscores the importance of supporting female residents through the difficult balance between motherhood and professional life," the report states.
The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
A majority of general surgery residents seriously consider dropping out of their training, with female residents more likely to consider quitting, a new study in JAMA Surgery reveals.
According to a survey, 58.0% of the 288 respondents "seriously considered leaving training." The most frequent reasons cited for wanting to quit training were sleep deprivation on a specific rotation (50%), an undesirable future lifestyle (47%), and excessive work hours on a specific rotation (41.4%). Survey results were published online July 30 in JAMA Surgery (2014 [doi:10.1001/jamasurg.2014.935]).
Factors cited that ultimately keep general surgery residents from ending training are support from family or significant other (65%), support from other residents (63.5%), and perception of being better rested (58.9%).
"We believe that our survey findings highlight the fact that a desire to leave training may not be affected by job rigor alone but rather [by] program-specific or rotation-specific factors or dissatisfaction with a future career in general surgery," the report states. Dr. Edward Gifford of the department of surgery, University of California, Los Angeles, Medical Center, is the report’s lead author.
In addressing the factors that led to consideration for leaving training, the authors noted that "a potential remedy may be to identify those high work-hour rotations and modify them accordingly," though lifestyle concerns may be harder to address as practicing surgeons "continue to experience high levels of work-home conflicts and burnout."
For women specifically, another issue is "the paucity of female mentors in academic surgery," the report states. "Striving to increase the number of female faculty members within training programs and refining the mentor-mentee relationship with incoming residents may improve the outlook and productivity of future female surgeons."
Overall, while men’s thoughts of quitting decreased as their residency progressed, women’s considerations remained persistent. The report cites previous studies that reported that men and women view general surgery careers differently, including that it was not a welcoming career because of lifestyle challenges, particularly if the woman had children, limited flexible training, and lack of role models.
"These findings may explain why women in our survey continued to consider leaving residency throughout the duration of training and underscores the importance of supporting female residents through the difficult balance between motherhood and professional life," the report states.
The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
FROM JAMA Surgery
Major finding: More than half of survey respondents (58%) considered quitting their general surgery residency, an issue more persistent with female respondents.
Data source: Analysis of 288 responses to a survey of general surgery residents in 13 residency programs across different regions (West, Southwest, Midwest, and Northeast) and training centers (university programs, independent programs, or hybrid university-affiliated programs without an onsite university or medical school).
Disclosures: The study was approved by the human subjects committee of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Los Angeles. The authors reported no conflicts of interest.
Frozen or powdered? Anticoagulation options in trauma are expanding
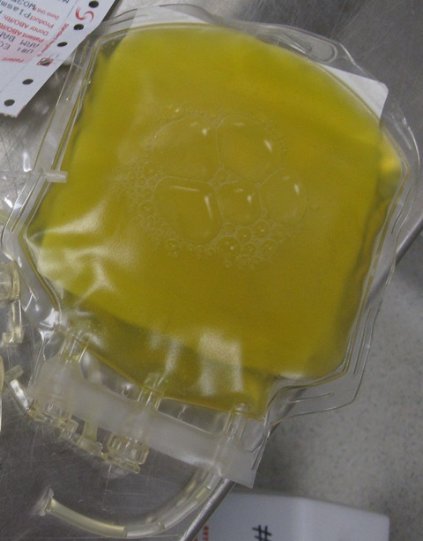
SAN DIEGO – In the next 5-10 years, reaching for a powdered form of plasma may become the normative first-line treatment for trauma patients who present with severe bleeding. That’s because the current standard of administering fresh frozen plasma is riddled with problems, Dr. Martin Schreiber said at the University of California, San Diego, Critical Care Summer Session.
For one thing, frozen plasma takes 35 minutes to thaw. "That’s a problem, and the clotting factor function of plasma deteriorates as you freeze it and thaw it," said Dr. Schreiber, professor of surgery at Oregon Health & Science University, Portland. "Also, you need it in large volumes and that’s not good for patients with congestive heart failure on Coumadin, and the availability is limited, especially in rural settings."
Enter lyophilized plasma (LP), a process developed by HemCon Medical Technologies in which whole blood is sterilely removed, and the plasma component is separated and turned into a powder. The powdered plasma is returned and reconstituted prior to transfusion. "You can put this stuff as a powder on a shelf in nearly any environment," Dr. Schreiber said. "It’s good for at least 3 years, it survives a broad range of temperatures, and you can restore it to plasma within a couple of minutes."
An initial study of LP showed encouraging results with the use of a freeze-dried form of plasma for resuscitation (J. Trauma 2008;65[5]:975-85). A later study by researchers including Dr. Schreiber evaluated the effects of the lyophilization process on plasma clotting factor levels in swine, by adding the antioxidant ascorbic acid (vitamin C) to the reconstitution solution, and by comparing the efficacy of LP with that of fresh frozen plasma and that of plasma and packed red blood cells in a 1:1 ratio (Arch. Surg. 2009;144[9]:829-34). "What we found was that if we gave LP with packed red blood cells in a 1:1 ratio we had 14% less blood loss than if it’s given as FFP with packed cells, which was significant," Dr. Schreiber said.
"The LP was better in terms of stopping hemorrhage. We also noticed that with the vitamin C, we suppressed inflammation and got reduced IL-6 [interleukin 6] expression. Now, the Germans, the Dutch, and the French are using LP in their military settings. Our special forces people are also using it. It’s under current development for common use in your hospital in 5-10 years. I think this stuff is good anywhere. With LP we can always maintain a 1:1 ratio and we don’t have to worry about the thawing process."
Tranexamic acid, a synthetic derivative of lysine, is another anticoagulant therapy that is likely to be used with increasing frequency, he predicted. This agent "binds plasminogen so plasminogen can’t break down fibrin so you can’t get fibrinolysis," said Dr. Schreiber, who has been deployed three times as a combat surgeon.
"This drug has been around forever and is extremely inexpensive. It’s approved by the FDA for use in tooth extraction and the oral form is approved for menorrhagia." Tranexamic acid has also been studied in 53 prospective, randomized studies involving some 3,800 subjects, mostly cardiac patients. "They show that if you use tranexamic acid, you use less blood. It reduces the amount of blood necessary for transfusing people in high-bleeding settings, but no difference in mortality, thrombotic events, myocardial infarction, or stroke. It does not seem to produce a hypercoagulable state."
One study of tranexamic acid use by British surgeons during Afghanistan combat operations found that soldiers who received tranexamic acid were seven times more likely to live, compared with those who did not receive the agent (Arch. Surg. 2012;147:113-19). "There was a survival of 85% in tranexamic acid group, compared with about 70% in those who did not receive it," said Dr. Schreiber. "This has resulted in a change in practice in civilian trauma centers where it is being used widely."
Another anticoagulant being used is the prothrombin complex concentrate known as Kcentra, which contains all four vitamin K–dependent coagulation factors. Distributed by CSL Behring, Kcentra is approved for warfarin reversal in adult patients with acute major bleeding and for those who require emergency surgery. The max dose is 50 units/kg. "That’s about $4,445 for a 70-kg person," Dr. Schreiber said. "Why is it so good? It’s rapidly available, you don’t have to give a lot of fluid, there’s no infectious risk, and you can very rapidly increase coagulation factor function. This is where we’re headed in the future for trauma patients."
Dr. Schreiber said that he had no relevant financial conflicts to disclose.
On Twitter @dougbrunk
SAN DIEGO – In the next 5-10 years, reaching for a powdered form of plasma may become the normative first-line treatment for trauma patients who present with severe bleeding. That’s because the current standard of administering fresh frozen plasma is riddled with problems, Dr. Martin Schreiber said at the University of California, San Diego, Critical Care Summer Session.
For one thing, frozen plasma takes 35 minutes to thaw. "That’s a problem, and the clotting factor function of plasma deteriorates as you freeze it and thaw it," said Dr. Schreiber, professor of surgery at Oregon Health & Science University, Portland. "Also, you need it in large volumes and that’s not good for patients with congestive heart failure on Coumadin, and the availability is limited, especially in rural settings."
Enter lyophilized plasma (LP), a process developed by HemCon Medical Technologies in which whole blood is sterilely removed, and the plasma component is separated and turned into a powder. The powdered plasma is returned and reconstituted prior to transfusion. "You can put this stuff as a powder on a shelf in nearly any environment," Dr. Schreiber said. "It’s good for at least 3 years, it survives a broad range of temperatures, and you can restore it to plasma within a couple of minutes."
An initial study of LP showed encouraging results with the use of a freeze-dried form of plasma for resuscitation (J. Trauma 2008;65[5]:975-85). A later study by researchers including Dr. Schreiber evaluated the effects of the lyophilization process on plasma clotting factor levels in swine, by adding the antioxidant ascorbic acid (vitamin C) to the reconstitution solution, and by comparing the efficacy of LP with that of fresh frozen plasma and that of plasma and packed red blood cells in a 1:1 ratio (Arch. Surg. 2009;144[9]:829-34). "What we found was that if we gave LP with packed red blood cells in a 1:1 ratio we had 14% less blood loss than if it’s given as FFP with packed cells, which was significant," Dr. Schreiber said.
"The LP was better in terms of stopping hemorrhage. We also noticed that with the vitamin C, we suppressed inflammation and got reduced IL-6 [interleukin 6] expression. Now, the Germans, the Dutch, and the French are using LP in their military settings. Our special forces people are also using it. It’s under current development for common use in your hospital in 5-10 years. I think this stuff is good anywhere. With LP we can always maintain a 1:1 ratio and we don’t have to worry about the thawing process."
Tranexamic acid, a synthetic derivative of lysine, is another anticoagulant therapy that is likely to be used with increasing frequency, he predicted. This agent "binds plasminogen so plasminogen can’t break down fibrin so you can’t get fibrinolysis," said Dr. Schreiber, who has been deployed three times as a combat surgeon.
"This drug has been around forever and is extremely inexpensive. It’s approved by the FDA for use in tooth extraction and the oral form is approved for menorrhagia." Tranexamic acid has also been studied in 53 prospective, randomized studies involving some 3,800 subjects, mostly cardiac patients. "They show that if you use tranexamic acid, you use less blood. It reduces the amount of blood necessary for transfusing people in high-bleeding settings, but no difference in mortality, thrombotic events, myocardial infarction, or stroke. It does not seem to produce a hypercoagulable state."
One study of tranexamic acid use by British surgeons during Afghanistan combat operations found that soldiers who received tranexamic acid were seven times more likely to live, compared with those who did not receive the agent (Arch. Surg. 2012;147:113-19). "There was a survival of 85% in tranexamic acid group, compared with about 70% in those who did not receive it," said Dr. Schreiber. "This has resulted in a change in practice in civilian trauma centers where it is being used widely."
Another anticoagulant being used is the prothrombin complex concentrate known as Kcentra, which contains all four vitamin K–dependent coagulation factors. Distributed by CSL Behring, Kcentra is approved for warfarin reversal in adult patients with acute major bleeding and for those who require emergency surgery. The max dose is 50 units/kg. "That’s about $4,445 for a 70-kg person," Dr. Schreiber said. "Why is it so good? It’s rapidly available, you don’t have to give a lot of fluid, there’s no infectious risk, and you can very rapidly increase coagulation factor function. This is where we’re headed in the future for trauma patients."
Dr. Schreiber said that he had no relevant financial conflicts to disclose.
On Twitter @dougbrunk
SAN DIEGO – In the next 5-10 years, reaching for a powdered form of plasma may become the normative first-line treatment for trauma patients who present with severe bleeding. That’s because the current standard of administering fresh frozen plasma is riddled with problems, Dr. Martin Schreiber said at the University of California, San Diego, Critical Care Summer Session.
For one thing, frozen plasma takes 35 minutes to thaw. "That’s a problem, and the clotting factor function of plasma deteriorates as you freeze it and thaw it," said Dr. Schreiber, professor of surgery at Oregon Health & Science University, Portland. "Also, you need it in large volumes and that’s not good for patients with congestive heart failure on Coumadin, and the availability is limited, especially in rural settings."
Enter lyophilized plasma (LP), a process developed by HemCon Medical Technologies in which whole blood is sterilely removed, and the plasma component is separated and turned into a powder. The powdered plasma is returned and reconstituted prior to transfusion. "You can put this stuff as a powder on a shelf in nearly any environment," Dr. Schreiber said. "It’s good for at least 3 years, it survives a broad range of temperatures, and you can restore it to plasma within a couple of minutes."
An initial study of LP showed encouraging results with the use of a freeze-dried form of plasma for resuscitation (J. Trauma 2008;65[5]:975-85). A later study by researchers including Dr. Schreiber evaluated the effects of the lyophilization process on plasma clotting factor levels in swine, by adding the antioxidant ascorbic acid (vitamin C) to the reconstitution solution, and by comparing the efficacy of LP with that of fresh frozen plasma and that of plasma and packed red blood cells in a 1:1 ratio (Arch. Surg. 2009;144[9]:829-34). "What we found was that if we gave LP with packed red blood cells in a 1:1 ratio we had 14% less blood loss than if it’s given as FFP with packed cells, which was significant," Dr. Schreiber said.
"The LP was better in terms of stopping hemorrhage. We also noticed that with the vitamin C, we suppressed inflammation and got reduced IL-6 [interleukin 6] expression. Now, the Germans, the Dutch, and the French are using LP in their military settings. Our special forces people are also using it. It’s under current development for common use in your hospital in 5-10 years. I think this stuff is good anywhere. With LP we can always maintain a 1:1 ratio and we don’t have to worry about the thawing process."
Tranexamic acid, a synthetic derivative of lysine, is another anticoagulant therapy that is likely to be used with increasing frequency, he predicted. This agent "binds plasminogen so plasminogen can’t break down fibrin so you can’t get fibrinolysis," said Dr. Schreiber, who has been deployed three times as a combat surgeon.
"This drug has been around forever and is extremely inexpensive. It’s approved by the FDA for use in tooth extraction and the oral form is approved for menorrhagia." Tranexamic acid has also been studied in 53 prospective, randomized studies involving some 3,800 subjects, mostly cardiac patients. "They show that if you use tranexamic acid, you use less blood. It reduces the amount of blood necessary for transfusing people in high-bleeding settings, but no difference in mortality, thrombotic events, myocardial infarction, or stroke. It does not seem to produce a hypercoagulable state."
One study of tranexamic acid use by British surgeons during Afghanistan combat operations found that soldiers who received tranexamic acid were seven times more likely to live, compared with those who did not receive the agent (Arch. Surg. 2012;147:113-19). "There was a survival of 85% in tranexamic acid group, compared with about 70% in those who did not receive it," said Dr. Schreiber. "This has resulted in a change in practice in civilian trauma centers where it is being used widely."
Another anticoagulant being used is the prothrombin complex concentrate known as Kcentra, which contains all four vitamin K–dependent coagulation factors. Distributed by CSL Behring, Kcentra is approved for warfarin reversal in adult patients with acute major bleeding and for those who require emergency surgery. The max dose is 50 units/kg. "That’s about $4,445 for a 70-kg person," Dr. Schreiber said. "Why is it so good? It’s rapidly available, you don’t have to give a lot of fluid, there’s no infectious risk, and you can very rapidly increase coagulation factor function. This is where we’re headed in the future for trauma patients."
Dr. Schreiber said that he had no relevant financial conflicts to disclose.
On Twitter @dougbrunk
EXPERT ANALYSIS AT THE UCSD CRITICAL CARE SUMMER SESSION
Femoral nerve blocks delay recovery after ACL reconstruction
SEATTLE – Postoperative femoral nerve blocks prolong quadricep and hamstring weakness after anterior cruciate ligament reconstruction in young athletes, and delay recovery, according to investigators from the Mayo Clinic in Rochester, Minn.
Because of that, "I’ve stopped using them," said investigator Dr. Amy McIntosh, a pediatric orthopedic surgeon in Rochester.
In a retrospective study of patients no older than 18 years, her team found that 68% (42 of 62 patients) who got the block – weight-based bupivacaine HCl in all cases – were cleared for sports at 6 months, meaning that their operated knee was at least 85% as strong as their uninjured knee, and at least 90% as functional. Among children who didn’t get the blocks after anterior cruciate ligament reconstruction (ACL), 90% (56 of 62) were ready to return to sports, a significant difference. Overall, unblocked kids were 4.4 times more likely to be cleared at 6 months.
"Kids who didn’t clear at 6 months usually took another 3-4 months. At a year, everybody looked about the same," Dr. McIntosh said at the American Orthopaedic Society for Sports Medicine annual meeting.
Also at 6 months, kids who got the nerve block had significantly greater mean deficits in fast isokinetic knee extensions, a measure of quadricep strength (17.6% in the operated knee vs. 11.2% in the uninjured knee), and fast (9.9% vs. 5.7%) and slow (13.0% vs. 8.5%) isokinetic flexion, a measure of hamstring strength. It didn’t seem to matter if they got a one-shot femoral nerve block or a continuous pump infusion.
Dr. McIntosh initially lobbied Mayo anesthesiologists to use femoral blocks in kids, opting first for the pump. "Then I saw that those kids had a lot of quad atrophy and were taking a longer time to get off their crutches, so I started going to the one-shot block, but they still had quad atrophy, and took a little longer to get off their crutches and get their normal gait back. Now, after seeing this data, I’m done with it," she said.
She’s not alone. Long considered a benign and effective method for short-term pain control, surgeons have been reconsidering the blocks because of similar findings in adults.
"I tell patients [and parents] that they have to decide if they want great pain control up front, or a little more pain in the first few days after surgery," but a quicker return to sports. When offered the choice, young athletes opt against the block because it will likely mean missing an entire season. "That’s what matters to these kids," Dr. McIntosh said.
The nerve block group and control groups were evenly matched; children in both were about 16 years old, on average, with a mean body mass index of about 24 kg/m2. There were slightly more girls in the study than boys.
Most of the kids in both groups had patellar tendon autografts, and the rest had hamstring autografts. Those who got nerve blocks had shorter tourniquet (82 vs. 93 minutes), operative (134 vs. 155 minutes.), and anesthesia times (177 vs. 200 minutes).
Dr. McIntosh said he had no relevant financial disclosures. The project was funded internally.
SEATTLE – Postoperative femoral nerve blocks prolong quadricep and hamstring weakness after anterior cruciate ligament reconstruction in young athletes, and delay recovery, according to investigators from the Mayo Clinic in Rochester, Minn.
Because of that, "I’ve stopped using them," said investigator Dr. Amy McIntosh, a pediatric orthopedic surgeon in Rochester.
In a retrospective study of patients no older than 18 years, her team found that 68% (42 of 62 patients) who got the block – weight-based bupivacaine HCl in all cases – were cleared for sports at 6 months, meaning that their operated knee was at least 85% as strong as their uninjured knee, and at least 90% as functional. Among children who didn’t get the blocks after anterior cruciate ligament reconstruction (ACL), 90% (56 of 62) were ready to return to sports, a significant difference. Overall, unblocked kids were 4.4 times more likely to be cleared at 6 months.
"Kids who didn’t clear at 6 months usually took another 3-4 months. At a year, everybody looked about the same," Dr. McIntosh said at the American Orthopaedic Society for Sports Medicine annual meeting.
Also at 6 months, kids who got the nerve block had significantly greater mean deficits in fast isokinetic knee extensions, a measure of quadricep strength (17.6% in the operated knee vs. 11.2% in the uninjured knee), and fast (9.9% vs. 5.7%) and slow (13.0% vs. 8.5%) isokinetic flexion, a measure of hamstring strength. It didn’t seem to matter if they got a one-shot femoral nerve block or a continuous pump infusion.
Dr. McIntosh initially lobbied Mayo anesthesiologists to use femoral blocks in kids, opting first for the pump. "Then I saw that those kids had a lot of quad atrophy and were taking a longer time to get off their crutches, so I started going to the one-shot block, but they still had quad atrophy, and took a little longer to get off their crutches and get their normal gait back. Now, after seeing this data, I’m done with it," she said.
She’s not alone. Long considered a benign and effective method for short-term pain control, surgeons have been reconsidering the blocks because of similar findings in adults.
"I tell patients [and parents] that they have to decide if they want great pain control up front, or a little more pain in the first few days after surgery," but a quicker return to sports. When offered the choice, young athletes opt against the block because it will likely mean missing an entire season. "That’s what matters to these kids," Dr. McIntosh said.
The nerve block group and control groups were evenly matched; children in both were about 16 years old, on average, with a mean body mass index of about 24 kg/m2. There were slightly more girls in the study than boys.
Most of the kids in both groups had patellar tendon autografts, and the rest had hamstring autografts. Those who got nerve blocks had shorter tourniquet (82 vs. 93 minutes), operative (134 vs. 155 minutes.), and anesthesia times (177 vs. 200 minutes).
Dr. McIntosh said he had no relevant financial disclosures. The project was funded internally.
SEATTLE – Postoperative femoral nerve blocks prolong quadricep and hamstring weakness after anterior cruciate ligament reconstruction in young athletes, and delay recovery, according to investigators from the Mayo Clinic in Rochester, Minn.
Because of that, "I’ve stopped using them," said investigator Dr. Amy McIntosh, a pediatric orthopedic surgeon in Rochester.
In a retrospective study of patients no older than 18 years, her team found that 68% (42 of 62 patients) who got the block – weight-based bupivacaine HCl in all cases – were cleared for sports at 6 months, meaning that their operated knee was at least 85% as strong as their uninjured knee, and at least 90% as functional. Among children who didn’t get the blocks after anterior cruciate ligament reconstruction (ACL), 90% (56 of 62) were ready to return to sports, a significant difference. Overall, unblocked kids were 4.4 times more likely to be cleared at 6 months.
"Kids who didn’t clear at 6 months usually took another 3-4 months. At a year, everybody looked about the same," Dr. McIntosh said at the American Orthopaedic Society for Sports Medicine annual meeting.
Also at 6 months, kids who got the nerve block had significantly greater mean deficits in fast isokinetic knee extensions, a measure of quadricep strength (17.6% in the operated knee vs. 11.2% in the uninjured knee), and fast (9.9% vs. 5.7%) and slow (13.0% vs. 8.5%) isokinetic flexion, a measure of hamstring strength. It didn’t seem to matter if they got a one-shot femoral nerve block or a continuous pump infusion.
Dr. McIntosh initially lobbied Mayo anesthesiologists to use femoral blocks in kids, opting first for the pump. "Then I saw that those kids had a lot of quad atrophy and were taking a longer time to get off their crutches, so I started going to the one-shot block, but they still had quad atrophy, and took a little longer to get off their crutches and get their normal gait back. Now, after seeing this data, I’m done with it," she said.
She’s not alone. Long considered a benign and effective method for short-term pain control, surgeons have been reconsidering the blocks because of similar findings in adults.
"I tell patients [and parents] that they have to decide if they want great pain control up front, or a little more pain in the first few days after surgery," but a quicker return to sports. When offered the choice, young athletes opt against the block because it will likely mean missing an entire season. "That’s what matters to these kids," Dr. McIntosh said.
The nerve block group and control groups were evenly matched; children in both were about 16 years old, on average, with a mean body mass index of about 24 kg/m2. There were slightly more girls in the study than boys.
Most of the kids in both groups had patellar tendon autografts, and the rest had hamstring autografts. Those who got nerve blocks had shorter tourniquet (82 vs. 93 minutes), operative (134 vs. 155 minutes.), and anesthesia times (177 vs. 200 minutes).
Dr. McIntosh said he had no relevant financial disclosures. The project was funded internally.
AT THE AOSSM 2014 ANNUAL MEETING
Key clinical point: When young athletes want to be back in the game at 6 months, skip femoral nerve blocks after ACL surgery.
Major finding: Ninety percent of young athletes are ready to return to sports at 6 months if they don’t get a femoral nerve block following anterior cruciate ligament reconstruction; among those who get the block, 68% are ready to get back into the game.
Data Source: A retrospective matched cohort study.
Disclosures: The work was funded internally, and the presenter said he had no disclosures.
FDA approves ex vivo lung perfusion device that preserves donor organs
A device that preserves less-than-ideal donor lungs until they are cleared for transplantation has been approved, the Food and Drug Administration announced on Aug. 12.
The ex vivo perfusion device preserves donated lungs that initially do not meet all the criteria for a transplantable lung. The device does this by warming the donor lung to "near normal body temperature," continuously flushing the lung with a sterile solution, and ventilating the lungs, "which oxygenates the cells and makes it possible for the transplant team to examine the lungs’ airways with a bronchoscope," according to the FDA statement.
The lungs can remain in the machine for up to 4 hours, providing time for the transplant team to evaluate the lungs to determine if they meet the criteria; donor lungs that meet the criteria are then transplanted into a patient.
The device, the XVIVO Perfusion System (XPS) with STEEN Solution, is manufactured by XVIVO Perfusion.
"With this approval, there may be more lungs available for transplant, which could allow more people with end stage lung disease who have exhausted all other treatment options to be able to receive a lung transplant," Christy Foreman, director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said in the statement.
About one in five donor lungs meet the standard transplantation criteria. In the United States, 1,754 lung transplants were performed in 2012 and 1,616 potential recipients were on the lung transplant waiting list at the end of 2012, according to the FDA.
In two studies, outcomes for lung-transplant recipients were similar among those who received a donor lung preserved with the device and those who received donor lungs that were considered ideal and were preserved in cold storage. "Both trials showed that recipients of the ideal and non-ideal lungs had similar survival rates up to 12 months after transplant and similar rates of organ rejection," the FDA statement said.
The manufacturer is required to conduct a long-term study of the effects of the device as a condition of approval.
This is exciting news given the shortage of available lungs which meet the current transplant criteria. Early studies showing similar 12-month survival rates and rates of organ rejection are encouraging. I would like to know if there were similar hospital lengths of stay and if there was a difference in postoperative complications. Also, how significant will the financial impact be using the device? I look forward to the results of long-term studies and hopefully this will be a viable option for our patients.
Dr. Jennifer Cox is assistant professor of pulmonary and critical care medicine critical care selective, University of South Florida, Tampa.
This is exciting news given the shortage of available lungs which meet the current transplant criteria. Early studies showing similar 12-month survival rates and rates of organ rejection are encouraging. I would like to know if there were similar hospital lengths of stay and if there was a difference in postoperative complications. Also, how significant will the financial impact be using the device? I look forward to the results of long-term studies and hopefully this will be a viable option for our patients.
Dr. Jennifer Cox is assistant professor of pulmonary and critical care medicine critical care selective, University of South Florida, Tampa.
This is exciting news given the shortage of available lungs which meet the current transplant criteria. Early studies showing similar 12-month survival rates and rates of organ rejection are encouraging. I would like to know if there were similar hospital lengths of stay and if there was a difference in postoperative complications. Also, how significant will the financial impact be using the device? I look forward to the results of long-term studies and hopefully this will be a viable option for our patients.
Dr. Jennifer Cox is assistant professor of pulmonary and critical care medicine critical care selective, University of South Florida, Tampa.
A device that preserves less-than-ideal donor lungs until they are cleared for transplantation has been approved, the Food and Drug Administration announced on Aug. 12.
The ex vivo perfusion device preserves donated lungs that initially do not meet all the criteria for a transplantable lung. The device does this by warming the donor lung to "near normal body temperature," continuously flushing the lung with a sterile solution, and ventilating the lungs, "which oxygenates the cells and makes it possible for the transplant team to examine the lungs’ airways with a bronchoscope," according to the FDA statement.
The lungs can remain in the machine for up to 4 hours, providing time for the transplant team to evaluate the lungs to determine if they meet the criteria; donor lungs that meet the criteria are then transplanted into a patient.
The device, the XVIVO Perfusion System (XPS) with STEEN Solution, is manufactured by XVIVO Perfusion.
"With this approval, there may be more lungs available for transplant, which could allow more people with end stage lung disease who have exhausted all other treatment options to be able to receive a lung transplant," Christy Foreman, director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said in the statement.
About one in five donor lungs meet the standard transplantation criteria. In the United States, 1,754 lung transplants were performed in 2012 and 1,616 potential recipients were on the lung transplant waiting list at the end of 2012, according to the FDA.
In two studies, outcomes for lung-transplant recipients were similar among those who received a donor lung preserved with the device and those who received donor lungs that were considered ideal and were preserved in cold storage. "Both trials showed that recipients of the ideal and non-ideal lungs had similar survival rates up to 12 months after transplant and similar rates of organ rejection," the FDA statement said.
The manufacturer is required to conduct a long-term study of the effects of the device as a condition of approval.
A device that preserves less-than-ideal donor lungs until they are cleared for transplantation has been approved, the Food and Drug Administration announced on Aug. 12.
The ex vivo perfusion device preserves donated lungs that initially do not meet all the criteria for a transplantable lung. The device does this by warming the donor lung to "near normal body temperature," continuously flushing the lung with a sterile solution, and ventilating the lungs, "which oxygenates the cells and makes it possible for the transplant team to examine the lungs’ airways with a bronchoscope," according to the FDA statement.
The lungs can remain in the machine for up to 4 hours, providing time for the transplant team to evaluate the lungs to determine if they meet the criteria; donor lungs that meet the criteria are then transplanted into a patient.
The device, the XVIVO Perfusion System (XPS) with STEEN Solution, is manufactured by XVIVO Perfusion.
"With this approval, there may be more lungs available for transplant, which could allow more people with end stage lung disease who have exhausted all other treatment options to be able to receive a lung transplant," Christy Foreman, director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health, Silver Spring, Md., said in the statement.
About one in five donor lungs meet the standard transplantation criteria. In the United States, 1,754 lung transplants were performed in 2012 and 1,616 potential recipients were on the lung transplant waiting list at the end of 2012, according to the FDA.
In two studies, outcomes for lung-transplant recipients were similar among those who received a donor lung preserved with the device and those who received donor lungs that were considered ideal and were preserved in cold storage. "Both trials showed that recipients of the ideal and non-ideal lungs had similar survival rates up to 12 months after transplant and similar rates of organ rejection," the FDA statement said.
The manufacturer is required to conduct a long-term study of the effects of the device as a condition of approval.
Settling a malpractice suit is seldom a straightforward decision
Sometimes it makes sense to settle a malpractice lawsuit, but making that decision is seldom straightforward, according to legal experts.
Top reasons to settle a case include indefensible actions, inadequate malpractice insurance, and an expert review that concludes a failure to meet the standard of care, Joshua R. Cohen, a medical liability defense attorney practicing in New York, said in an interview.
A low insurance limit could lead to personal assets being tapped if a high trial verdict exceeds the physician’s insurance threshold. By settling early, doctors and their attorneys generally can negotiate a discounted payment, provide for quicker compensation to an injured patient and reduce ongoing defense costs, he said.
Settling also alleviates the time and burden of participating in a malpractice trial, said George F. Indest III, president and managing partner in a law firm specializing in health law in Orlando.
Settlement brings a resolution, "including relief from the worry and stress that such litigation carries with it, the imposition on the defendant’s time that depositions, hearings, and the trial might require, and the mental anxiety which the uncertainty causes," Mr. Indest said in an interview.
One distinct disadvantage of such financial agreements is that they must be reported to the National Practitioner Data Bank (NPDB), said Michael J. Sacopulos, founder and president of a consulting and liability risk management firm in Terre Haute, Ind. The reporting requirement means settlement details can arise during new employment, professional reviews or application for privileges.
"It is in many physicians’ minds, a stain that goes with them for the rest of their career," Mr. Sacopulos said in an interview.
Settlements also are reported to state licensure boards and state departments of insurance, which may lead to separate investigations against physicians based on the facts of the settlement, Mr. Indest said. Additionally, most medical staff bylaws require that physicians report settlements to the hospital and medical staff, which may result in peer-review actions.
For physicians who believe their case is strong and want to preserve their reputations, taking a case to trial may be the best option.
"For the most part, most malpractice cases fall into a gray area," Mr. Cohen said. "There could be some issues with the charting, but the medicine itself is defensible. The question is do you want to roll the dice as to what the jury is going to decide?"
To allow for that decision, ensure that contracts with insurance companies include a "consent to settle" clause. The clauses make certain that lawsuits are not settled without a doctor’s permission. Communication with attorneys and insurers throughout the lawsuit process is essential, Mr. Sacopulos said. Attorneys and insurers should make clear relevant case updates, potential success rates and possible loss values.
In a contraction accident case, a large corporation is affected, but with a doctor, it’s personal, Mr. Cohen said. Many equate malpractice with a violation of the Hippocratic Oath, "To do no harm." Doctors want their reputations defended when they believe they didn’t do anything wrong or if there was a complication that was not a result of malpractice. They want to go to trial.
Despite the strong emotions that come with a lawsuit, the decision about whether to settle or take a suit to trial should not be rushed, Mr. Sacopulos advised. "As the case goes on, more facts come out," he said. "The decision you make today as to what’s best for you may not be the best decision down the road. You need to continue to revisit (the situation) as the facts come out."
On Twitter @legal_med
Sometimes it makes sense to settle a malpractice lawsuit, but making that decision is seldom straightforward, according to legal experts.
Top reasons to settle a case include indefensible actions, inadequate malpractice insurance, and an expert review that concludes a failure to meet the standard of care, Joshua R. Cohen, a medical liability defense attorney practicing in New York, said in an interview.
A low insurance limit could lead to personal assets being tapped if a high trial verdict exceeds the physician’s insurance threshold. By settling early, doctors and their attorneys generally can negotiate a discounted payment, provide for quicker compensation to an injured patient and reduce ongoing defense costs, he said.
Settling also alleviates the time and burden of participating in a malpractice trial, said George F. Indest III, president and managing partner in a law firm specializing in health law in Orlando.
Settlement brings a resolution, "including relief from the worry and stress that such litigation carries with it, the imposition on the defendant’s time that depositions, hearings, and the trial might require, and the mental anxiety which the uncertainty causes," Mr. Indest said in an interview.
One distinct disadvantage of such financial agreements is that they must be reported to the National Practitioner Data Bank (NPDB), said Michael J. Sacopulos, founder and president of a consulting and liability risk management firm in Terre Haute, Ind. The reporting requirement means settlement details can arise during new employment, professional reviews or application for privileges.
"It is in many physicians’ minds, a stain that goes with them for the rest of their career," Mr. Sacopulos said in an interview.
Settlements also are reported to state licensure boards and state departments of insurance, which may lead to separate investigations against physicians based on the facts of the settlement, Mr. Indest said. Additionally, most medical staff bylaws require that physicians report settlements to the hospital and medical staff, which may result in peer-review actions.
For physicians who believe their case is strong and want to preserve their reputations, taking a case to trial may be the best option.
"For the most part, most malpractice cases fall into a gray area," Mr. Cohen said. "There could be some issues with the charting, but the medicine itself is defensible. The question is do you want to roll the dice as to what the jury is going to decide?"
To allow for that decision, ensure that contracts with insurance companies include a "consent to settle" clause. The clauses make certain that lawsuits are not settled without a doctor’s permission. Communication with attorneys and insurers throughout the lawsuit process is essential, Mr. Sacopulos said. Attorneys and insurers should make clear relevant case updates, potential success rates and possible loss values.
In a contraction accident case, a large corporation is affected, but with a doctor, it’s personal, Mr. Cohen said. Many equate malpractice with a violation of the Hippocratic Oath, "To do no harm." Doctors want their reputations defended when they believe they didn’t do anything wrong or if there was a complication that was not a result of malpractice. They want to go to trial.
Despite the strong emotions that come with a lawsuit, the decision about whether to settle or take a suit to trial should not be rushed, Mr. Sacopulos advised. "As the case goes on, more facts come out," he said. "The decision you make today as to what’s best for you may not be the best decision down the road. You need to continue to revisit (the situation) as the facts come out."
On Twitter @legal_med
Sometimes it makes sense to settle a malpractice lawsuit, but making that decision is seldom straightforward, according to legal experts.
Top reasons to settle a case include indefensible actions, inadequate malpractice insurance, and an expert review that concludes a failure to meet the standard of care, Joshua R. Cohen, a medical liability defense attorney practicing in New York, said in an interview.
A low insurance limit could lead to personal assets being tapped if a high trial verdict exceeds the physician’s insurance threshold. By settling early, doctors and their attorneys generally can negotiate a discounted payment, provide for quicker compensation to an injured patient and reduce ongoing defense costs, he said.
Settling also alleviates the time and burden of participating in a malpractice trial, said George F. Indest III, president and managing partner in a law firm specializing in health law in Orlando.
Settlement brings a resolution, "including relief from the worry and stress that such litigation carries with it, the imposition on the defendant’s time that depositions, hearings, and the trial might require, and the mental anxiety which the uncertainty causes," Mr. Indest said in an interview.
One distinct disadvantage of such financial agreements is that they must be reported to the National Practitioner Data Bank (NPDB), said Michael J. Sacopulos, founder and president of a consulting and liability risk management firm in Terre Haute, Ind. The reporting requirement means settlement details can arise during new employment, professional reviews or application for privileges.
"It is in many physicians’ minds, a stain that goes with them for the rest of their career," Mr. Sacopulos said in an interview.
Settlements also are reported to state licensure boards and state departments of insurance, which may lead to separate investigations against physicians based on the facts of the settlement, Mr. Indest said. Additionally, most medical staff bylaws require that physicians report settlements to the hospital and medical staff, which may result in peer-review actions.
For physicians who believe their case is strong and want to preserve their reputations, taking a case to trial may be the best option.
"For the most part, most malpractice cases fall into a gray area," Mr. Cohen said. "There could be some issues with the charting, but the medicine itself is defensible. The question is do you want to roll the dice as to what the jury is going to decide?"
To allow for that decision, ensure that contracts with insurance companies include a "consent to settle" clause. The clauses make certain that lawsuits are not settled without a doctor’s permission. Communication with attorneys and insurers throughout the lawsuit process is essential, Mr. Sacopulos said. Attorneys and insurers should make clear relevant case updates, potential success rates and possible loss values.
In a contraction accident case, a large corporation is affected, but with a doctor, it’s personal, Mr. Cohen said. Many equate malpractice with a violation of the Hippocratic Oath, "To do no harm." Doctors want their reputations defended when they believe they didn’t do anything wrong or if there was a complication that was not a result of malpractice. They want to go to trial.
Despite the strong emotions that come with a lawsuit, the decision about whether to settle or take a suit to trial should not be rushed, Mr. Sacopulos advised. "As the case goes on, more facts come out," he said. "The decision you make today as to what’s best for you may not be the best decision down the road. You need to continue to revisit (the situation) as the facts come out."
On Twitter @legal_med