User login
Official Newspaper of the American College of Surgeons
Obama gets do-over in ACA subsidy lawsuit
The Obama administration is getting a second chance to show that providing tax subsidies through the Affordable Care Act’s federally run insurance marketplace is legal.
On Sept. 4, the District of Columbia Circuit of the U.S. Court of Appeals granted the government’s petition for a rehearing en banc in Halbig v. Burwell. The rehearing will be heard by all 17 judges on the court and the previous ruling made by a three-judge panel of the court has been vacated.
The earlier ruling, issued on July 22, was 2-1 in favor of the plaintiffs and, had it been upheld, had the potential to strip insurance subsidies from about 5 million Americans who had purchased health plans through the federally run marketplace.
Also on July 22, the 4th Circuit of the U.S. Court of Appeals in Virginia handed down a conflicting ruling, siding unanimously with the government in a similar case, King v. Burwell. The King plaintiffs have already appealed to the Supreme Court, but the high court has not indicated whether it will consider the case.
At issue is whether the federal government has the authority to issue tax subsidies to consumers from 36 states who purchase insurance on the federal marketplace since the ACA specifies only that subsidies would be available on the state-run marketplaces. The plaintiffs have argued that the administration is overstepping its authority by also offering subsidies on the federal marketplace. But administration officials maintain that Congress intended the subsidies to be offered to all eligible consumers regardless of who operates the marketplace.
The D.C. appellate court will hear oral arguments in Halbig v. Burwell on Dec. 17.
On Twitter @maryellenny
The Obama administration is getting a second chance to show that providing tax subsidies through the Affordable Care Act’s federally run insurance marketplace is legal.
On Sept. 4, the District of Columbia Circuit of the U.S. Court of Appeals granted the government’s petition for a rehearing en banc in Halbig v. Burwell. The rehearing will be heard by all 17 judges on the court and the previous ruling made by a three-judge panel of the court has been vacated.
The earlier ruling, issued on July 22, was 2-1 in favor of the plaintiffs and, had it been upheld, had the potential to strip insurance subsidies from about 5 million Americans who had purchased health plans through the federally run marketplace.
Also on July 22, the 4th Circuit of the U.S. Court of Appeals in Virginia handed down a conflicting ruling, siding unanimously with the government in a similar case, King v. Burwell. The King plaintiffs have already appealed to the Supreme Court, but the high court has not indicated whether it will consider the case.
At issue is whether the federal government has the authority to issue tax subsidies to consumers from 36 states who purchase insurance on the federal marketplace since the ACA specifies only that subsidies would be available on the state-run marketplaces. The plaintiffs have argued that the administration is overstepping its authority by also offering subsidies on the federal marketplace. But administration officials maintain that Congress intended the subsidies to be offered to all eligible consumers regardless of who operates the marketplace.
The D.C. appellate court will hear oral arguments in Halbig v. Burwell on Dec. 17.
On Twitter @maryellenny
The Obama administration is getting a second chance to show that providing tax subsidies through the Affordable Care Act’s federally run insurance marketplace is legal.
On Sept. 4, the District of Columbia Circuit of the U.S. Court of Appeals granted the government’s petition for a rehearing en banc in Halbig v. Burwell. The rehearing will be heard by all 17 judges on the court and the previous ruling made by a three-judge panel of the court has been vacated.
The earlier ruling, issued on July 22, was 2-1 in favor of the plaintiffs and, had it been upheld, had the potential to strip insurance subsidies from about 5 million Americans who had purchased health plans through the federally run marketplace.
Also on July 22, the 4th Circuit of the U.S. Court of Appeals in Virginia handed down a conflicting ruling, siding unanimously with the government in a similar case, King v. Burwell. The King plaintiffs have already appealed to the Supreme Court, but the high court has not indicated whether it will consider the case.
At issue is whether the federal government has the authority to issue tax subsidies to consumers from 36 states who purchase insurance on the federal marketplace since the ACA specifies only that subsidies would be available on the state-run marketplaces. The plaintiffs have argued that the administration is overstepping its authority by also offering subsidies on the federal marketplace. But administration officials maintain that Congress intended the subsidies to be offered to all eligible consumers regardless of who operates the marketplace.
The D.C. appellate court will hear oral arguments in Halbig v. Burwell on Dec. 17.
On Twitter @maryellenny
Liver grafts donated after circulatory death increase early risk of diabetes
SAN FRANCISCO – The type of liver graft used in transplantation plays a large role in early development of new-onset diabetes, according to a retrospective study of 430 patients from the United Kingdom.
A team led by Dr. Hermien Hartog, an honorary clinical fellow in the Liver Unit, Queen Elizabeth Hospital, Birmingham, England, studied patients undergoing primary liver transplant between 2008 and 2012. Patients were excluded from the study if they had preexisting diabetes, had died, or had undergone retransplantation within 90 days.
The investigators assessed both the development of new-onset diabetes after transplant (NODAT), using criteria adapted from a published article (Transplantation 2013;96:58-64), and its resolution, defined as the date of cessation of antihyperglycemic therapy or the last episode of hyperglycemia.
Seventy-nine percent of the patients received grafts donated after brain death (DBD), Dr. Hartog reported at the annual meeting of the 2014 World Transplant Congress. Among the recipients of grafts donated after circulatory death (DCD), the mean warm ischemic time was 21 minutes.
With a median follow-up of 2.5 years, the cumulative 1-year incidence of NODAT was 19% in the entire cohort, with a median time to onset of 30 days. In the 44% of affected patients whose NODAT resolved, the median time to resolution was 150 days post transplantation, Dr. Hartog reported at the congress, which was sponsored by the American Society of Transplant Surgeons.
The cumulative 1-year incidence of NODAT was 23% in DCD graft recipients and 18% in DBD graft recipients, a nonsignificant difference. But when patients were stratified by graft type, "we saw an early occurrence and high peak incidence of NODAT in DCD graft recipients. Also, a larger proportion of these patients resolved their NODAT over time," she commented.
The overall temporal pattern suggested that "the effect that we see of graft type seems to be temporary and [lessens] over time when multifactorial factors come into play," according to Dr. Hartog.
In multivariate analyses, the risk of NODAT within 90 days of transplantation was higher for patients who received a DCD graft (hazard ratio, 1.8). More detailed analysis showed that the elevation of risk was greatest within the first 15 days.
"Our study confirms known associations with NODAT after liver transplantation but identifies DCD graft as a novel risk factor. This causes a temporary effect in the early post-transplant period that is independent from known risk factors," Dr. Hartog commented.
"Based on our observations, we hypothesize that hyperglycemia may be related to liver graft function through ischemia-reperfusion–induced hepatic insulin resistance," she added. "We are currently trying to confirm our data in an independent data set, which will also include postreperfusion glucose levels and correlation with the insulin receptor pathway in time-zero liver biopsies."
"The clinical relevance of our findings is as yet unknown," she acknowledged. However, they may help inform new approaches for graft optimization and selection.
Session cochair Dr. Darius Mirza, also of the University of Birmingham, asked, "Why does the pattern of recovery seem to be different in the DCDs versus the DBDs? Also, why are the cumulative incidence and the time frame so different?"
"Actually, in the literature, I have not seen any reports looking at the early post-transplant period. So most reports look at one time point, normally 1 year," Dr. Hartog replied. "What I think is that there is an early peak caused by DCD grafts that would explain why there is an early peak, but also why those patients recover later on. I think this peak is a bit obscure because there are also other factors that come into play, maybe after a while, that will obscure that first peak. If you would take those other factors out of the equation, I think you would just see a peak in the early period."
Dr. Mirza also wondered about the role of using DCD grafts that are accepted under extended criteria. "So you start off using mainly young, fit DCD livers. Now, the vast majority are extended-criteria DCD livers. Do you think that plays a role, or is it too early to say?"
"Yes, I think so," Dr. Hartog said, while adding that this phenomenon is likely not restricted to DCD grafts. "From earlier literature, there is a clear difference between a living donated graft and deceased donation. And it might also be that the extended grafts or the more steatotic grafts may exhibit this effect more than the better grafts."
Dr. Hartog disclosed no conflicts of interest relevant to the research.
SAN FRANCISCO – The type of liver graft used in transplantation plays a large role in early development of new-onset diabetes, according to a retrospective study of 430 patients from the United Kingdom.
A team led by Dr. Hermien Hartog, an honorary clinical fellow in the Liver Unit, Queen Elizabeth Hospital, Birmingham, England, studied patients undergoing primary liver transplant between 2008 and 2012. Patients were excluded from the study if they had preexisting diabetes, had died, or had undergone retransplantation within 90 days.
The investigators assessed both the development of new-onset diabetes after transplant (NODAT), using criteria adapted from a published article (Transplantation 2013;96:58-64), and its resolution, defined as the date of cessation of antihyperglycemic therapy or the last episode of hyperglycemia.
Seventy-nine percent of the patients received grafts donated after brain death (DBD), Dr. Hartog reported at the annual meeting of the 2014 World Transplant Congress. Among the recipients of grafts donated after circulatory death (DCD), the mean warm ischemic time was 21 minutes.
With a median follow-up of 2.5 years, the cumulative 1-year incidence of NODAT was 19% in the entire cohort, with a median time to onset of 30 days. In the 44% of affected patients whose NODAT resolved, the median time to resolution was 150 days post transplantation, Dr. Hartog reported at the congress, which was sponsored by the American Society of Transplant Surgeons.
The cumulative 1-year incidence of NODAT was 23% in DCD graft recipients and 18% in DBD graft recipients, a nonsignificant difference. But when patients were stratified by graft type, "we saw an early occurrence and high peak incidence of NODAT in DCD graft recipients. Also, a larger proportion of these patients resolved their NODAT over time," she commented.
The overall temporal pattern suggested that "the effect that we see of graft type seems to be temporary and [lessens] over time when multifactorial factors come into play," according to Dr. Hartog.
In multivariate analyses, the risk of NODAT within 90 days of transplantation was higher for patients who received a DCD graft (hazard ratio, 1.8). More detailed analysis showed that the elevation of risk was greatest within the first 15 days.
"Our study confirms known associations with NODAT after liver transplantation but identifies DCD graft as a novel risk factor. This causes a temporary effect in the early post-transplant period that is independent from known risk factors," Dr. Hartog commented.
"Based on our observations, we hypothesize that hyperglycemia may be related to liver graft function through ischemia-reperfusion–induced hepatic insulin resistance," she added. "We are currently trying to confirm our data in an independent data set, which will also include postreperfusion glucose levels and correlation with the insulin receptor pathway in time-zero liver biopsies."
"The clinical relevance of our findings is as yet unknown," she acknowledged. However, they may help inform new approaches for graft optimization and selection.
Session cochair Dr. Darius Mirza, also of the University of Birmingham, asked, "Why does the pattern of recovery seem to be different in the DCDs versus the DBDs? Also, why are the cumulative incidence and the time frame so different?"
"Actually, in the literature, I have not seen any reports looking at the early post-transplant period. So most reports look at one time point, normally 1 year," Dr. Hartog replied. "What I think is that there is an early peak caused by DCD grafts that would explain why there is an early peak, but also why those patients recover later on. I think this peak is a bit obscure because there are also other factors that come into play, maybe after a while, that will obscure that first peak. If you would take those other factors out of the equation, I think you would just see a peak in the early period."
Dr. Mirza also wondered about the role of using DCD grafts that are accepted under extended criteria. "So you start off using mainly young, fit DCD livers. Now, the vast majority are extended-criteria DCD livers. Do you think that plays a role, or is it too early to say?"
"Yes, I think so," Dr. Hartog said, while adding that this phenomenon is likely not restricted to DCD grafts. "From earlier literature, there is a clear difference between a living donated graft and deceased donation. And it might also be that the extended grafts or the more steatotic grafts may exhibit this effect more than the better grafts."
Dr. Hartog disclosed no conflicts of interest relevant to the research.
SAN FRANCISCO – The type of liver graft used in transplantation plays a large role in early development of new-onset diabetes, according to a retrospective study of 430 patients from the United Kingdom.
A team led by Dr. Hermien Hartog, an honorary clinical fellow in the Liver Unit, Queen Elizabeth Hospital, Birmingham, England, studied patients undergoing primary liver transplant between 2008 and 2012. Patients were excluded from the study if they had preexisting diabetes, had died, or had undergone retransplantation within 90 days.
The investigators assessed both the development of new-onset diabetes after transplant (NODAT), using criteria adapted from a published article (Transplantation 2013;96:58-64), and its resolution, defined as the date of cessation of antihyperglycemic therapy or the last episode of hyperglycemia.
Seventy-nine percent of the patients received grafts donated after brain death (DBD), Dr. Hartog reported at the annual meeting of the 2014 World Transplant Congress. Among the recipients of grafts donated after circulatory death (DCD), the mean warm ischemic time was 21 minutes.
With a median follow-up of 2.5 years, the cumulative 1-year incidence of NODAT was 19% in the entire cohort, with a median time to onset of 30 days. In the 44% of affected patients whose NODAT resolved, the median time to resolution was 150 days post transplantation, Dr. Hartog reported at the congress, which was sponsored by the American Society of Transplant Surgeons.
The cumulative 1-year incidence of NODAT was 23% in DCD graft recipients and 18% in DBD graft recipients, a nonsignificant difference. But when patients were stratified by graft type, "we saw an early occurrence and high peak incidence of NODAT in DCD graft recipients. Also, a larger proportion of these patients resolved their NODAT over time," she commented.
The overall temporal pattern suggested that "the effect that we see of graft type seems to be temporary and [lessens] over time when multifactorial factors come into play," according to Dr. Hartog.
In multivariate analyses, the risk of NODAT within 90 days of transplantation was higher for patients who received a DCD graft (hazard ratio, 1.8). More detailed analysis showed that the elevation of risk was greatest within the first 15 days.
"Our study confirms known associations with NODAT after liver transplantation but identifies DCD graft as a novel risk factor. This causes a temporary effect in the early post-transplant period that is independent from known risk factors," Dr. Hartog commented.
"Based on our observations, we hypothesize that hyperglycemia may be related to liver graft function through ischemia-reperfusion–induced hepatic insulin resistance," she added. "We are currently trying to confirm our data in an independent data set, which will also include postreperfusion glucose levels and correlation with the insulin receptor pathway in time-zero liver biopsies."
"The clinical relevance of our findings is as yet unknown," she acknowledged. However, they may help inform new approaches for graft optimization and selection.
Session cochair Dr. Darius Mirza, also of the University of Birmingham, asked, "Why does the pattern of recovery seem to be different in the DCDs versus the DBDs? Also, why are the cumulative incidence and the time frame so different?"
"Actually, in the literature, I have not seen any reports looking at the early post-transplant period. So most reports look at one time point, normally 1 year," Dr. Hartog replied. "What I think is that there is an early peak caused by DCD grafts that would explain why there is an early peak, but also why those patients recover later on. I think this peak is a bit obscure because there are also other factors that come into play, maybe after a while, that will obscure that first peak. If you would take those other factors out of the equation, I think you would just see a peak in the early period."
Dr. Mirza also wondered about the role of using DCD grafts that are accepted under extended criteria. "So you start off using mainly young, fit DCD livers. Now, the vast majority are extended-criteria DCD livers. Do you think that plays a role, or is it too early to say?"
"Yes, I think so," Dr. Hartog said, while adding that this phenomenon is likely not restricted to DCD grafts. "From earlier literature, there is a clear difference between a living donated graft and deceased donation. And it might also be that the extended grafts or the more steatotic grafts may exhibit this effect more than the better grafts."
Dr. Hartog disclosed no conflicts of interest relevant to the research.
AT THE 2014 WORLD TRANSPLANT CONGRESS
Key clinical point: Recipients of liver grafts donated after circulatory death are at a slightly higher risk for post-transplant new-onset diabetes.
Major finding: The risk of new-onset diabetes within 90 days of transplantation was 1.8-fold higher for patients who received a DCD graft than for peers who received a DBD graft.
Data source: A retrospective cohort study of 430 primary liver transplant recipients
Disclosures: Dr. Hartog disclosed no relevant conflicts of interest.
Health spending growth still low, but expected to speed up over next decade
WASHINGTON – The current trend of low growth in health care spending is expected to end in 2016 as more patients gain insurance coverage and the U.S. population continues to age, according to an analysis from the Centers for Medicare & Medicaid Services.
For the fifth year in a row, overall health care spending in 2013 grew less than 4% – significantly lower than historical growth rates, Andrea Sisko, an economist in the Office of the Actuary at the CMS, said at a press briefing. Spending growth was down, in part, because of sequestration’s 2% Medicare cut and because Medicare beneficiaries used fewer physician and hospital services. Lingering effects of the 2009 recession also meant that privately insured patients spent less on health care.
The Affordable Care Act had some impact on the growth in health spending in 2013, Ms. Sisko said. The ACA’s temporary increase in Medicaid primary care payments helped to almost double the growth in that program’s spending. Overall Medicaid spending grew almost 7% in 2013 (vs. 3% in 2012); physician payment increases by the states and enhanced benefits contributed to the overall spending growth. The analysis was published Sept. 3 in the journal Health Affairs (doi: 10.1377/hlthaff.2014.0560).
The change in Medicaid was one of few areas where the economists definitively could say that the ACA either fueled or restrained spending growth.
"We are no longer estimating or quantifying the impacts of the Affordable Care Act on national health spending," said Ms. Sisko, who noted that after 4 years, it has become difficult to tease out the law’s effects.
Overall spending on physician services grew 3.3% in 2013, but could grow by almost 6% in 2014. After a slight decline in 2015, growth is expected to be about 6% yearly from 2016 to 2023, according to the analysis.
Future growth in spending on physician services will come as more Americans gain health insurance coverage. The projected dip in 2015 is tied to the end of the temporary Medicaid pay boost, which ends in December2014. For 2016-2023, physician spending is expected to rebound as more U.S. residents become eligible for Medicare.
Spending for physician services is expected to make up about 20% of U.S. health care spending through 2023, with hospitals consuming 32% and prescription drugs 9%.
While future health care spending growth is expected to slightly outpace economic growth (6% for health care spending vs. 5% for overall spending), that growth rate is still lower than the 7% yearly increases seen in the 1990s and through the mid-2000s, Ms. Sisko said. The growth rates will be lower in part because they will be based on a historically low baseline of the last 4-5 years, Ms. Sisko added.
Drug costs, for instance, will not have the double-digit growth seen in the late 1990s, said coauthor Sean Keehan, also an economist at the CMS Office of the Actuary. Unlike in the 1990s, payers now seem to have more leverage to negotiate lower rates from physicians and hospitals, and thus keep costs and spending down.
The physician spending figures assume that Congress will override the fee cuts mandated by the Sustainable Growth Rate formula and instead keep fees flat in 2015, and then give a 0.6% increase from 2016 to 2023. They also assume that the budget sequestration will continue.
On Twitter @aliciaault
WASHINGTON – The current trend of low growth in health care spending is expected to end in 2016 as more patients gain insurance coverage and the U.S. population continues to age, according to an analysis from the Centers for Medicare & Medicaid Services.
For the fifth year in a row, overall health care spending in 2013 grew less than 4% – significantly lower than historical growth rates, Andrea Sisko, an economist in the Office of the Actuary at the CMS, said at a press briefing. Spending growth was down, in part, because of sequestration’s 2% Medicare cut and because Medicare beneficiaries used fewer physician and hospital services. Lingering effects of the 2009 recession also meant that privately insured patients spent less on health care.
The Affordable Care Act had some impact on the growth in health spending in 2013, Ms. Sisko said. The ACA’s temporary increase in Medicaid primary care payments helped to almost double the growth in that program’s spending. Overall Medicaid spending grew almost 7% in 2013 (vs. 3% in 2012); physician payment increases by the states and enhanced benefits contributed to the overall spending growth. The analysis was published Sept. 3 in the journal Health Affairs (doi: 10.1377/hlthaff.2014.0560).
The change in Medicaid was one of few areas where the economists definitively could say that the ACA either fueled or restrained spending growth.
"We are no longer estimating or quantifying the impacts of the Affordable Care Act on national health spending," said Ms. Sisko, who noted that after 4 years, it has become difficult to tease out the law’s effects.
Overall spending on physician services grew 3.3% in 2013, but could grow by almost 6% in 2014. After a slight decline in 2015, growth is expected to be about 6% yearly from 2016 to 2023, according to the analysis.
Future growth in spending on physician services will come as more Americans gain health insurance coverage. The projected dip in 2015 is tied to the end of the temporary Medicaid pay boost, which ends in December2014. For 2016-2023, physician spending is expected to rebound as more U.S. residents become eligible for Medicare.
Spending for physician services is expected to make up about 20% of U.S. health care spending through 2023, with hospitals consuming 32% and prescription drugs 9%.
While future health care spending growth is expected to slightly outpace economic growth (6% for health care spending vs. 5% for overall spending), that growth rate is still lower than the 7% yearly increases seen in the 1990s and through the mid-2000s, Ms. Sisko said. The growth rates will be lower in part because they will be based on a historically low baseline of the last 4-5 years, Ms. Sisko added.
Drug costs, for instance, will not have the double-digit growth seen in the late 1990s, said coauthor Sean Keehan, also an economist at the CMS Office of the Actuary. Unlike in the 1990s, payers now seem to have more leverage to negotiate lower rates from physicians and hospitals, and thus keep costs and spending down.
The physician spending figures assume that Congress will override the fee cuts mandated by the Sustainable Growth Rate formula and instead keep fees flat in 2015, and then give a 0.6% increase from 2016 to 2023. They also assume that the budget sequestration will continue.
On Twitter @aliciaault
WASHINGTON – The current trend of low growth in health care spending is expected to end in 2016 as more patients gain insurance coverage and the U.S. population continues to age, according to an analysis from the Centers for Medicare & Medicaid Services.
For the fifth year in a row, overall health care spending in 2013 grew less than 4% – significantly lower than historical growth rates, Andrea Sisko, an economist in the Office of the Actuary at the CMS, said at a press briefing. Spending growth was down, in part, because of sequestration’s 2% Medicare cut and because Medicare beneficiaries used fewer physician and hospital services. Lingering effects of the 2009 recession also meant that privately insured patients spent less on health care.
The Affordable Care Act had some impact on the growth in health spending in 2013, Ms. Sisko said. The ACA’s temporary increase in Medicaid primary care payments helped to almost double the growth in that program’s spending. Overall Medicaid spending grew almost 7% in 2013 (vs. 3% in 2012); physician payment increases by the states and enhanced benefits contributed to the overall spending growth. The analysis was published Sept. 3 in the journal Health Affairs (doi: 10.1377/hlthaff.2014.0560).
The change in Medicaid was one of few areas where the economists definitively could say that the ACA either fueled or restrained spending growth.
"We are no longer estimating or quantifying the impacts of the Affordable Care Act on national health spending," said Ms. Sisko, who noted that after 4 years, it has become difficult to tease out the law’s effects.
Overall spending on physician services grew 3.3% in 2013, but could grow by almost 6% in 2014. After a slight decline in 2015, growth is expected to be about 6% yearly from 2016 to 2023, according to the analysis.
Future growth in spending on physician services will come as more Americans gain health insurance coverage. The projected dip in 2015 is tied to the end of the temporary Medicaid pay boost, which ends in December2014. For 2016-2023, physician spending is expected to rebound as more U.S. residents become eligible for Medicare.
Spending for physician services is expected to make up about 20% of U.S. health care spending through 2023, with hospitals consuming 32% and prescription drugs 9%.
While future health care spending growth is expected to slightly outpace economic growth (6% for health care spending vs. 5% for overall spending), that growth rate is still lower than the 7% yearly increases seen in the 1990s and through the mid-2000s, Ms. Sisko said. The growth rates will be lower in part because they will be based on a historically low baseline of the last 4-5 years, Ms. Sisko added.
Drug costs, for instance, will not have the double-digit growth seen in the late 1990s, said coauthor Sean Keehan, also an economist at the CMS Office of the Actuary. Unlike in the 1990s, payers now seem to have more leverage to negotiate lower rates from physicians and hospitals, and thus keep costs and spending down.
The physician spending figures assume that Congress will override the fee cuts mandated by the Sustainable Growth Rate formula and instead keep fees flat in 2015, and then give a 0.6% increase from 2016 to 2023. They also assume that the budget sequestration will continue.
On Twitter @aliciaault
AT A HEALTH AFFAIRS BRIEFING
Drug black box warnings up post-Vioxx withdrawal
An examination of pharmacological and biological therapeutics approved between 1996 and 2012 revealed that the number of Food and Drug Administration–approved therapeutic agents given black box warnings skyrocketed in the wake of the 2004 withdrawal of Merck’s rofecoxib (Vioxx) from the market.
During this time, there were 522 novel therapeutics approved, including 441 pharmacological and 81 biological products, with 180 (136 pharmacological and 44 biological) receiving a black box warning. Premarket warnings were issued for 105 products, postmarket warnings were issued for 50 products, and 25 had both pre- and postmarket warnings associated with them. In total, there were 89 postmarket boxed warnings, with 11 withdrawals, most of which (81%) occurred after 2004, when the FDA launched initiatives to strengthen drug safety surveillance, Christine Cheng, Pharm.D., and her colleagues said in a report in JAMA Internal Medicine (2014 Aug. 15 [doi: 10.1001/jamainternmed.2014.4854]).
"Our study demonstrates that boxed warnings are common, affecting more than one-third of recent drug approvals. ... Our findings that half of biological products had boxed warnings is consistent with literature suggesting that biologic products pose a greater risk of serious adverse events compared to other drug types," wrote Dr. Cheng, of First Databank in San Francisco, and her colleagues.
A proposed rule issued by the FDA in June could undermine that communication of risk using the boxed warning, according to Dr. Sidney Wolfe, senior adviser at Public Citizen’s Health Research Group, Washington, D.C.
In an editorial accompanying Dr. Cheng’s report, Dr. Wolfe noted that the draft guidance would allow drug companies that "believe that the FDA-approved drug labeling information overstates the risks of their drug to tell physicians that the risks are, in fact, lower," adding that the draft guidance "may encourage companies to promote the supposed evidence of lower risk in a peer-reviewed article directly to physicians without the FDA ever having been informed, so that the agency can review the data" (2014 Aug. 15 [doi: 10.1001/jamainternmed.2014.4547]).
Dr. Wolfe called on the FDA to maintain current regulatory requirements that would have a company report evidence to the FDA first in support of risk reduction and get the label changed that way.
"Off-label risk reduction is a misguided approach," he said.
Dr. Cheng is employed by First Databank, a commercial drug knowledge vendor. No other authors of the research or the editorial reported any financial disclosures.
An examination of pharmacological and biological therapeutics approved between 1996 and 2012 revealed that the number of Food and Drug Administration–approved therapeutic agents given black box warnings skyrocketed in the wake of the 2004 withdrawal of Merck’s rofecoxib (Vioxx) from the market.
During this time, there were 522 novel therapeutics approved, including 441 pharmacological and 81 biological products, with 180 (136 pharmacological and 44 biological) receiving a black box warning. Premarket warnings were issued for 105 products, postmarket warnings were issued for 50 products, and 25 had both pre- and postmarket warnings associated with them. In total, there were 89 postmarket boxed warnings, with 11 withdrawals, most of which (81%) occurred after 2004, when the FDA launched initiatives to strengthen drug safety surveillance, Christine Cheng, Pharm.D., and her colleagues said in a report in JAMA Internal Medicine (2014 Aug. 15 [doi: 10.1001/jamainternmed.2014.4854]).
"Our study demonstrates that boxed warnings are common, affecting more than one-third of recent drug approvals. ... Our findings that half of biological products had boxed warnings is consistent with literature suggesting that biologic products pose a greater risk of serious adverse events compared to other drug types," wrote Dr. Cheng, of First Databank in San Francisco, and her colleagues.
A proposed rule issued by the FDA in June could undermine that communication of risk using the boxed warning, according to Dr. Sidney Wolfe, senior adviser at Public Citizen’s Health Research Group, Washington, D.C.
In an editorial accompanying Dr. Cheng’s report, Dr. Wolfe noted that the draft guidance would allow drug companies that "believe that the FDA-approved drug labeling information overstates the risks of their drug to tell physicians that the risks are, in fact, lower," adding that the draft guidance "may encourage companies to promote the supposed evidence of lower risk in a peer-reviewed article directly to physicians without the FDA ever having been informed, so that the agency can review the data" (2014 Aug. 15 [doi: 10.1001/jamainternmed.2014.4547]).
Dr. Wolfe called on the FDA to maintain current regulatory requirements that would have a company report evidence to the FDA first in support of risk reduction and get the label changed that way.
"Off-label risk reduction is a misguided approach," he said.
Dr. Cheng is employed by First Databank, a commercial drug knowledge vendor. No other authors of the research or the editorial reported any financial disclosures.
An examination of pharmacological and biological therapeutics approved between 1996 and 2012 revealed that the number of Food and Drug Administration–approved therapeutic agents given black box warnings skyrocketed in the wake of the 2004 withdrawal of Merck’s rofecoxib (Vioxx) from the market.
During this time, there were 522 novel therapeutics approved, including 441 pharmacological and 81 biological products, with 180 (136 pharmacological and 44 biological) receiving a black box warning. Premarket warnings were issued for 105 products, postmarket warnings were issued for 50 products, and 25 had both pre- and postmarket warnings associated with them. In total, there were 89 postmarket boxed warnings, with 11 withdrawals, most of which (81%) occurred after 2004, when the FDA launched initiatives to strengthen drug safety surveillance, Christine Cheng, Pharm.D., and her colleagues said in a report in JAMA Internal Medicine (2014 Aug. 15 [doi: 10.1001/jamainternmed.2014.4854]).
"Our study demonstrates that boxed warnings are common, affecting more than one-third of recent drug approvals. ... Our findings that half of biological products had boxed warnings is consistent with literature suggesting that biologic products pose a greater risk of serious adverse events compared to other drug types," wrote Dr. Cheng, of First Databank in San Francisco, and her colleagues.
A proposed rule issued by the FDA in June could undermine that communication of risk using the boxed warning, according to Dr. Sidney Wolfe, senior adviser at Public Citizen’s Health Research Group, Washington, D.C.
In an editorial accompanying Dr. Cheng’s report, Dr. Wolfe noted that the draft guidance would allow drug companies that "believe that the FDA-approved drug labeling information overstates the risks of their drug to tell physicians that the risks are, in fact, lower," adding that the draft guidance "may encourage companies to promote the supposed evidence of lower risk in a peer-reviewed article directly to physicians without the FDA ever having been informed, so that the agency can review the data" (2014 Aug. 15 [doi: 10.1001/jamainternmed.2014.4547]).
Dr. Wolfe called on the FDA to maintain current regulatory requirements that would have a company report evidence to the FDA first in support of risk reduction and get the label changed that way.
"Off-label risk reduction is a misguided approach," he said.
Dr. Cheng is employed by First Databank, a commercial drug knowledge vendor. No other authors of the research or the editorial reported any financial disclosures.
FROM JAMA INTERNAL MEDICINE
Major finding: Eighty-one percent of FDA boxed warnings/withdrawals of CDER-approved drugs between 1996 and 2012 came after Vioxx was withdrawn in 2004.
Data source: An analysis of therapeutics, including pharmacological and biological products, approved by the FDA Center for Drug Evaluation and Research and the associated boxed warnings issued before and after market approval to determine trends of warnings issued.
Disclosures: Dr. Cheng is employed by First Databank, a commercial drug knowledge vendor. No other authors of the research or the editorial reported any financial disclosures.
No unexpected leiomyosarcomas in chart review of TVH and LAVH cases
WASHINGTON – There were no cases of unexpected leiomyosarcomas in a retrospective chart study of about 1,600 cases of vaginal or laparoscopic-assisted vaginal hysterectomies performed over a 7-year period.
In the study, the rate of endometrial adenocarcinomas was about 1 in 200, and while the overall manual vaginal morcellation rate was 19%, no uterine malignancies were morcellated, said Dr. Pedro Maldonado of the division of female pelvic medicine and reconstructive surgery at the University of Texas Southwestern Medical Center, Dallas.
The review looked at all total vaginal hysterectomy (TVH) and laparoscopic-assisted vaginal hysterectomy (LAVH) cases performed at the three main teaching hospitals of the university from July 2006 through March 2013. Malignancies known before surgery were excluded.
There were a total of 1,608 cases: 1,091 TVH and 517 LAVH procedures. The overall morcellation rate was 19%: 32% in the TVH group and 13% in the LAVH group, Dr. Maldonado reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Among the 1,608 cases, there were no cases of leiomyosarcoma (LMS) diagnosed on pathology. There was one case of an endometrial adenosarcoma (0.06%) in a 37-year-old patient with a preoperative diagnosis of menorrhagia and fibroids, and one case of a low-grade endometrial stromal tumor (0.06%) in a 39-year-old patient also diagnosed with menorrhagia and fibroids preoperatively. Neither of these patients underwent morcellation.
Three patients (0.19%) – aged 32, 38, and 47 years – who had preoperative diagnoses of menorrhagia, fibroids, and/or anemia had a pathologic diagnosis of smooth muscle tumor of uncertain malignant potential. One of these three patients underwent morcellation.
Another 8 (0.50%) of the patients with a preoperative diagnosis of endometrial hyperplasia were diagnosed with endometrial adenocarcinoma on pathology; none of them underwent morcellation, Dr. Maldonado said.
The risk of intraperitoneal dissemination of an unexpected LMS during endoscopic power morcellation has become a major issue since the Food and Drug Administration recommended in April 2014 that the use of power morcellation during a hysterectomy or myomectomy for uterine fibroids be discouraged because of the risk of disseminating cancerous tissue and upstaging disease. The FDA estimates that about 1 in 350 women undergoing hysterectomy or myomectomy for presumed fibroids have an unsuspected uterine sarcoma and that the risk of an unsuspected LMS is about 1 in 500.
Dr. Maldonado said he had no relevant financial disclosures.
WASHINGTON – There were no cases of unexpected leiomyosarcomas in a retrospective chart study of about 1,600 cases of vaginal or laparoscopic-assisted vaginal hysterectomies performed over a 7-year period.
In the study, the rate of endometrial adenocarcinomas was about 1 in 200, and while the overall manual vaginal morcellation rate was 19%, no uterine malignancies were morcellated, said Dr. Pedro Maldonado of the division of female pelvic medicine and reconstructive surgery at the University of Texas Southwestern Medical Center, Dallas.
The review looked at all total vaginal hysterectomy (TVH) and laparoscopic-assisted vaginal hysterectomy (LAVH) cases performed at the three main teaching hospitals of the university from July 2006 through March 2013. Malignancies known before surgery were excluded.
There were a total of 1,608 cases: 1,091 TVH and 517 LAVH procedures. The overall morcellation rate was 19%: 32% in the TVH group and 13% in the LAVH group, Dr. Maldonado reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Among the 1,608 cases, there were no cases of leiomyosarcoma (LMS) diagnosed on pathology. There was one case of an endometrial adenosarcoma (0.06%) in a 37-year-old patient with a preoperative diagnosis of menorrhagia and fibroids, and one case of a low-grade endometrial stromal tumor (0.06%) in a 39-year-old patient also diagnosed with menorrhagia and fibroids preoperatively. Neither of these patients underwent morcellation.
Three patients (0.19%) – aged 32, 38, and 47 years – who had preoperative diagnoses of menorrhagia, fibroids, and/or anemia had a pathologic diagnosis of smooth muscle tumor of uncertain malignant potential. One of these three patients underwent morcellation.
Another 8 (0.50%) of the patients with a preoperative diagnosis of endometrial hyperplasia were diagnosed with endometrial adenocarcinoma on pathology; none of them underwent morcellation, Dr. Maldonado said.
The risk of intraperitoneal dissemination of an unexpected LMS during endoscopic power morcellation has become a major issue since the Food and Drug Administration recommended in April 2014 that the use of power morcellation during a hysterectomy or myomectomy for uterine fibroids be discouraged because of the risk of disseminating cancerous tissue and upstaging disease. The FDA estimates that about 1 in 350 women undergoing hysterectomy or myomectomy for presumed fibroids have an unsuspected uterine sarcoma and that the risk of an unsuspected LMS is about 1 in 500.
Dr. Maldonado said he had no relevant financial disclosures.
WASHINGTON – There were no cases of unexpected leiomyosarcomas in a retrospective chart study of about 1,600 cases of vaginal or laparoscopic-assisted vaginal hysterectomies performed over a 7-year period.
In the study, the rate of endometrial adenocarcinomas was about 1 in 200, and while the overall manual vaginal morcellation rate was 19%, no uterine malignancies were morcellated, said Dr. Pedro Maldonado of the division of female pelvic medicine and reconstructive surgery at the University of Texas Southwestern Medical Center, Dallas.
The review looked at all total vaginal hysterectomy (TVH) and laparoscopic-assisted vaginal hysterectomy (LAVH) cases performed at the three main teaching hospitals of the university from July 2006 through March 2013. Malignancies known before surgery were excluded.
There were a total of 1,608 cases: 1,091 TVH and 517 LAVH procedures. The overall morcellation rate was 19%: 32% in the TVH group and 13% in the LAVH group, Dr. Maldonado reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Among the 1,608 cases, there were no cases of leiomyosarcoma (LMS) diagnosed on pathology. There was one case of an endometrial adenosarcoma (0.06%) in a 37-year-old patient with a preoperative diagnosis of menorrhagia and fibroids, and one case of a low-grade endometrial stromal tumor (0.06%) in a 39-year-old patient also diagnosed with menorrhagia and fibroids preoperatively. Neither of these patients underwent morcellation.
Three patients (0.19%) – aged 32, 38, and 47 years – who had preoperative diagnoses of menorrhagia, fibroids, and/or anemia had a pathologic diagnosis of smooth muscle tumor of uncertain malignant potential. One of these three patients underwent morcellation.
Another 8 (0.50%) of the patients with a preoperative diagnosis of endometrial hyperplasia were diagnosed with endometrial adenocarcinoma on pathology; none of them underwent morcellation, Dr. Maldonado said.
The risk of intraperitoneal dissemination of an unexpected LMS during endoscopic power morcellation has become a major issue since the Food and Drug Administration recommended in April 2014 that the use of power morcellation during a hysterectomy or myomectomy for uterine fibroids be discouraged because of the risk of disseminating cancerous tissue and upstaging disease. The FDA estimates that about 1 in 350 women undergoing hysterectomy or myomectomy for presumed fibroids have an unsuspected uterine sarcoma and that the risk of an unsuspected LMS is about 1 in 500.
Dr. Maldonado said he had no relevant financial disclosures.
AT AUGS/IUGA 2014
Key clinical point: This study provides another estimate of the rate of unexpected leiomyosarcomas in women undergoing hysterectomy for presumably benign conditions, an issue that is currently under FDA review because of heightened concerns over the risk of spreading malignancies if morcellation also is performed during these procedures.
Major finding: There were no unexpected leiomyosarcomas, and no uterine malignancies were morcellated in a series of 1,608 cases of vaginal or laparoscopic-assisted vaginal hysterectomies performed for presumably benign conditions over a 7-year period
Data source: A retrospective study of 1,608 cases of TVH or LAVH performed over 7 years at three teaching hospitals that looked at the rate of unexpected uterine malignancies and cases of malignancies that were morcellated.
Disclosures: Dr. Maldonado said he had no disclosures.
Clinical decision support demonstration shows promise, challenges
Clinical decision support, a key component in the electronic health records meaningful use criteria, still faces some significant implementation challenges, based on the results of two demonstration projects by the Agency for Healthcare Research and Quality.
Both projects "demonstrated the ability to translate evidence-based knowledge into useful, actionable guidance for clinical care through" clinical decision support (CDS), according to a report detailing the results of the projects. Further, the projects demonstrated the value of working with professional associations and guideline developers to provide tools and guidance for improving CDS development and clinical quality reporting. They also highlighted the value of aligning clinical quality measurement with CDS implementation.
However, the projects also demonstrated that while centrally developed CDS "is feasible, customization of CDS is still required on a site-by-site basis" to account for the need to customize the CDS app to local electronic health record (EHR) systems and to follow local data-coding conventions and practices. This can be "very labor intensive," the report noted.
Other significant challenges revealed by the two projects were "major difficulties" encountered when clinical guidelines were updated. "These implementation challenges point to the need for additional work on developing standards for EHR design, terminology, and data coding," the report said.
The two projects also encountered challenges associated with local variations in clinical workflow.
"It is essential to understand early in the implementation process when in the course of clinical care the data elements needed by the CDS tool are entered into the EHR system, and when it is appropriate for the decision support to appear," the report stated. "Similar considerations will also dictate to whom the decision support should be addressed."
Legal issues related to intellectual property, liability, and other concerns "merit further discussion and policy development," the report added."
Clinical decision support, a key component in the electronic health records meaningful use criteria, still faces some significant implementation challenges, based on the results of two demonstration projects by the Agency for Healthcare Research and Quality.
Both projects "demonstrated the ability to translate evidence-based knowledge into useful, actionable guidance for clinical care through" clinical decision support (CDS), according to a report detailing the results of the projects. Further, the projects demonstrated the value of working with professional associations and guideline developers to provide tools and guidance for improving CDS development and clinical quality reporting. They also highlighted the value of aligning clinical quality measurement with CDS implementation.
However, the projects also demonstrated that while centrally developed CDS "is feasible, customization of CDS is still required on a site-by-site basis" to account for the need to customize the CDS app to local electronic health record (EHR) systems and to follow local data-coding conventions and practices. This can be "very labor intensive," the report noted.
Other significant challenges revealed by the two projects were "major difficulties" encountered when clinical guidelines were updated. "These implementation challenges point to the need for additional work on developing standards for EHR design, terminology, and data coding," the report said.
The two projects also encountered challenges associated with local variations in clinical workflow.
"It is essential to understand early in the implementation process when in the course of clinical care the data elements needed by the CDS tool are entered into the EHR system, and when it is appropriate for the decision support to appear," the report stated. "Similar considerations will also dictate to whom the decision support should be addressed."
Legal issues related to intellectual property, liability, and other concerns "merit further discussion and policy development," the report added."
Clinical decision support, a key component in the electronic health records meaningful use criteria, still faces some significant implementation challenges, based on the results of two demonstration projects by the Agency for Healthcare Research and Quality.
Both projects "demonstrated the ability to translate evidence-based knowledge into useful, actionable guidance for clinical care through" clinical decision support (CDS), according to a report detailing the results of the projects. Further, the projects demonstrated the value of working with professional associations and guideline developers to provide tools and guidance for improving CDS development and clinical quality reporting. They also highlighted the value of aligning clinical quality measurement with CDS implementation.
However, the projects also demonstrated that while centrally developed CDS "is feasible, customization of CDS is still required on a site-by-site basis" to account for the need to customize the CDS app to local electronic health record (EHR) systems and to follow local data-coding conventions and practices. This can be "very labor intensive," the report noted.
Other significant challenges revealed by the two projects were "major difficulties" encountered when clinical guidelines were updated. "These implementation challenges point to the need for additional work on developing standards for EHR design, terminology, and data coding," the report said.
The two projects also encountered challenges associated with local variations in clinical workflow.
"It is essential to understand early in the implementation process when in the course of clinical care the data elements needed by the CDS tool are entered into the EHR system, and when it is appropriate for the decision support to appear," the report stated. "Similar considerations will also dictate to whom the decision support should be addressed."
Legal issues related to intellectual property, liability, and other concerns "merit further discussion and policy development," the report added."
Meaningful use – Stage 2 (Part 1 of 2)
The words "meaningful use" have been making providers cringe for more than 2 years now. Those clinicians who worked hard to demonstrate meaningful use under the stage 1 requirements now must go on to demonstrate meaningful use under the stage 2 requirements. We recently heard one of our colleagues describe stage 2 of meaningful use as reminiscent of the 1978 movie "Jaws 2," the ads for which ran with the tagline: "Just when you thought it was safe to go back in the water..."
As you may be aware, on August 29th the Department of Health and Human Services published a final rule allowing certain eligible providers the flexibility to continue using the Stage 1 criteria for the 2014 attestation year, even if they were due to start Stage 2. This only applies to those who have been unable to obtain the 2014-certified software in time due to vendor delays. Unfortunately, this flexibility does not extend to those who can’t meet Stage 2 due to measure difficulty or procrastination in purchasing software or adopting new workflows (we recommend speaking with a meaningful use expert or consultant before attempting to take advantage of this flexibility). Regardless of stage or year, everyone is on a 90-day reporting period for 2014, but remember that 2015 will require a full year of reporting (January through December). So even if you qualify for the flexibility and opt to stick with the Stage 1 measures, you’ll need to be ready to hit the ground running with Stage 2 as soon as the ball drops on January 1st, 2015.
The government’s intent with the EHR incentive program is to ensure that practitioners use an EHR to do more than what could otherwise be done on a paper note. As we review the criteria that must be met for stage 2 of meaningful use, we will see the inclusion of menu items and quality measures that are aimed at enhancing actionable decision support to improve the quality of medical care, population management (even for patients who might not come in to the office), and physician-patient communication. By articulating these goals we can see that they are very different from what most practitioners perceive to be the main outcome of the meaningful use rules: the creation of a lot of unnecessary busywork in the office that yields very little benefit for practitioners or patients.
The EHR incentive program consists of three stages.
• Stage 1, which many practitioners have already accomplished and received incentive dollars for completing, focused on basic data capture.
• Stage 2, which focuses on more advanced processes including additional requirements for e-prescribing, incorporating lab results into the record, electronic transmission of patient summaries across systems, and increased patient engagement.
• Stage 3, which will utilize the processes put in place in the first two stages and focus on improved patient outcomes.
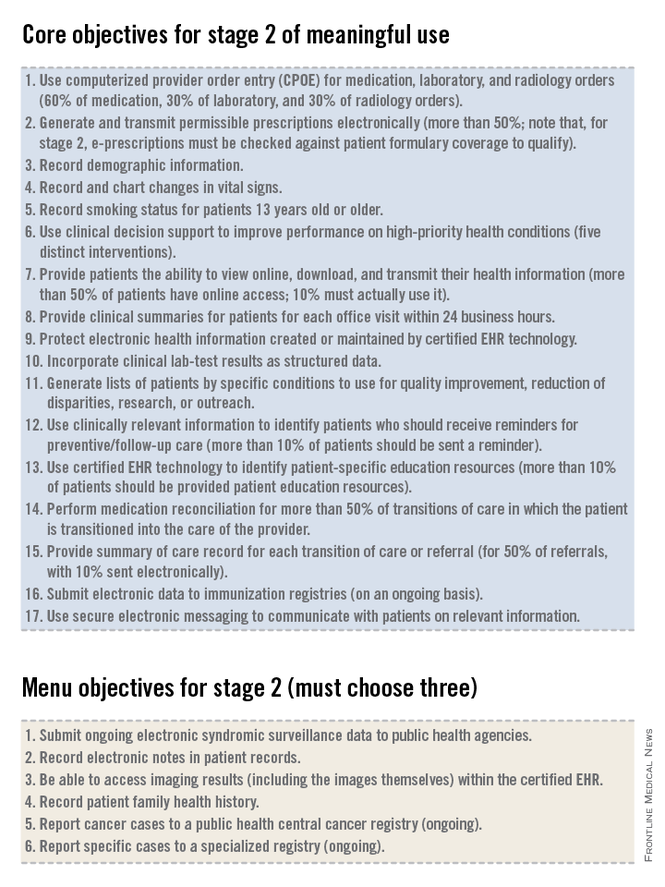
For stage 2 of meaningful use, clinicians must meet or exceed the thresholds for the 17 core objectives and 3 menu objectives, as well as report on defined Clinical Quality Measures (CQMs). Many of the objectives in stage 2 are the same as those from stage 1. Some objectives that were in the set of choices in stage 1 are now part of the mandatory core set for stage 2, required for all providers. Some objectives that were in the core set in stage 1 now have higher thresholds or percentages of patients that must meet the criteria in order to qualify for meaningful use in stage 2. The data reported to the Centers for Medicare & Medicaid Services for CQMs must originate from an EHR that has been certified for 2014 standards. This rule requires that clinicians upgrade their EHR to current technology standards, a rule that is good for EHR vendors, makes sense when we look at the system as a whole, but may be very expensive for many practitioners.
In addition to the 17 core objectives, and 3 out of 6 menu objectives, clinicians will need to report on nine CQMs. We will review the details of reporting on CQMs in next month in part 2 of our overview of Meaningful Use Stage 2.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
The words "meaningful use" have been making providers cringe for more than 2 years now. Those clinicians who worked hard to demonstrate meaningful use under the stage 1 requirements now must go on to demonstrate meaningful use under the stage 2 requirements. We recently heard one of our colleagues describe stage 2 of meaningful use as reminiscent of the 1978 movie "Jaws 2," the ads for which ran with the tagline: "Just when you thought it was safe to go back in the water..."

As you may be aware, on August 29th the Department of Health and Human Services published a final rule allowing certain eligible providers the flexibility to continue using the Stage 1 criteria for the 2014 attestation year, even if they were due to start Stage 2. This only applies to those who have been unable to obtain the 2014-certified software in time due to vendor delays. Unfortunately, this flexibility does not extend to those who can’t meet Stage 2 due to measure difficulty or procrastination in purchasing software or adopting new workflows (we recommend speaking with a meaningful use expert or consultant before attempting to take advantage of this flexibility). Regardless of stage or year, everyone is on a 90-day reporting period for 2014, but remember that 2015 will require a full year of reporting (January through December). So even if you qualify for the flexibility and opt to stick with the Stage 1 measures, you’ll need to be ready to hit the ground running with Stage 2 as soon as the ball drops on January 1st, 2015.
The government’s intent with the EHR incentive program is to ensure that practitioners use an EHR to do more than what could otherwise be done on a paper note. As we review the criteria that must be met for stage 2 of meaningful use, we will see the inclusion of menu items and quality measures that are aimed at enhancing actionable decision support to improve the quality of medical care, population management (even for patients who might not come in to the office), and physician-patient communication. By articulating these goals we can see that they are very different from what most practitioners perceive to be the main outcome of the meaningful use rules: the creation of a lot of unnecessary busywork in the office that yields very little benefit for practitioners or patients.
The EHR incentive program consists of three stages.
• Stage 1, which many practitioners have already accomplished and received incentive dollars for completing, focused on basic data capture.
• Stage 2, which focuses on more advanced processes including additional requirements for e-prescribing, incorporating lab results into the record, electronic transmission of patient summaries across systems, and increased patient engagement.
• Stage 3, which will utilize the processes put in place in the first two stages and focus on improved patient outcomes.
For stage 2 of meaningful use, clinicians must meet or exceed the thresholds for the 17 core objectives and 3 menu objectives, as well as report on defined Clinical Quality Measures (CQMs). Many of the objectives in stage 2 are the same as those from stage 1. Some objectives that were in the set of choices in stage 1 are now part of the mandatory core set for stage 2, required for all providers. Some objectives that were in the core set in stage 1 now have higher thresholds or percentages of patients that must meet the criteria in order to qualify for meaningful use in stage 2. The data reported to the Centers for Medicare & Medicaid Services for CQMs must originate from an EHR that has been certified for 2014 standards. This rule requires that clinicians upgrade their EHR to current technology standards, a rule that is good for EHR vendors, makes sense when we look at the system as a whole, but may be very expensive for many practitioners.
In addition to the 17 core objectives, and 3 out of 6 menu objectives, clinicians will need to report on nine CQMs. We will review the details of reporting on CQMs in next month in part 2 of our overview of Meaningful Use Stage 2.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
The words "meaningful use" have been making providers cringe for more than 2 years now. Those clinicians who worked hard to demonstrate meaningful use under the stage 1 requirements now must go on to demonstrate meaningful use under the stage 2 requirements. We recently heard one of our colleagues describe stage 2 of meaningful use as reminiscent of the 1978 movie "Jaws 2," the ads for which ran with the tagline: "Just when you thought it was safe to go back in the water..."

As you may be aware, on August 29th the Department of Health and Human Services published a final rule allowing certain eligible providers the flexibility to continue using the Stage 1 criteria for the 2014 attestation year, even if they were due to start Stage 2. This only applies to those who have been unable to obtain the 2014-certified software in time due to vendor delays. Unfortunately, this flexibility does not extend to those who can’t meet Stage 2 due to measure difficulty or procrastination in purchasing software or adopting new workflows (we recommend speaking with a meaningful use expert or consultant before attempting to take advantage of this flexibility). Regardless of stage or year, everyone is on a 90-day reporting period for 2014, but remember that 2015 will require a full year of reporting (January through December). So even if you qualify for the flexibility and opt to stick with the Stage 1 measures, you’ll need to be ready to hit the ground running with Stage 2 as soon as the ball drops on January 1st, 2015.
The government’s intent with the EHR incentive program is to ensure that practitioners use an EHR to do more than what could otherwise be done on a paper note. As we review the criteria that must be met for stage 2 of meaningful use, we will see the inclusion of menu items and quality measures that are aimed at enhancing actionable decision support to improve the quality of medical care, population management (even for patients who might not come in to the office), and physician-patient communication. By articulating these goals we can see that they are very different from what most practitioners perceive to be the main outcome of the meaningful use rules: the creation of a lot of unnecessary busywork in the office that yields very little benefit for practitioners or patients.
The EHR incentive program consists of three stages.
• Stage 1, which many practitioners have already accomplished and received incentive dollars for completing, focused on basic data capture.
• Stage 2, which focuses on more advanced processes including additional requirements for e-prescribing, incorporating lab results into the record, electronic transmission of patient summaries across systems, and increased patient engagement.
• Stage 3, which will utilize the processes put in place in the first two stages and focus on improved patient outcomes.
For stage 2 of meaningful use, clinicians must meet or exceed the thresholds for the 17 core objectives and 3 menu objectives, as well as report on defined Clinical Quality Measures (CQMs). Many of the objectives in stage 2 are the same as those from stage 1. Some objectives that were in the set of choices in stage 1 are now part of the mandatory core set for stage 2, required for all providers. Some objectives that were in the core set in stage 1 now have higher thresholds or percentages of patients that must meet the criteria in order to qualify for meaningful use in stage 2. The data reported to the Centers for Medicare & Medicaid Services for CQMs must originate from an EHR that has been certified for 2014 standards. This rule requires that clinicians upgrade their EHR to current technology standards, a rule that is good for EHR vendors, makes sense when we look at the system as a whole, but may be very expensive for many practitioners.
In addition to the 17 core objectives, and 3 out of 6 menu objectives, clinicians will need to report on nine CQMs. We will review the details of reporting on CQMs in next month in part 2 of our overview of Meaningful Use Stage 2.
Dr. Notte is a family physician and clinical informaticist for Abington (Pa.) Memorial Hospital. He is a partner in EHR Practice Consultants, a firm that aids physicians in adopting electronic health records. Dr. Skolnik is associate director of the family medicine residency program at Abington Memorial Hospital and professor of family and community medicine at Temple University in Philadelphia.
CMS finalizes marketplace autoenrollment rule
Patients insured through the federally operated health insurance marketplace will be automatically reenrolled in their current coverage if they fail to change their previous plan, the Centers for Medicare & Medicaid Services announced Sept. 2.
Participants in these Affordable Care Act plans will receive notices before open enrollment begins explaining how they can return to the marketplace and shop for additional assistance and new plans. Insurers will provide information regarding 2015 premiums and tax credits.
If participants do nothing, they will be autoenrolled in the same plan as their 2014 plan year with the same tax credit. Open enrollment begins Nov. 15.
According to the final rule, participants whose 2013 tax returns indicate they have high income or did not grant marketplace administrators permission to access updated tax information will be autoenrolled without financial assistance if they don’t update their plan.
"We are committed to providing a simple, familiar process for consumers to renew their coverage next year," Marilyn Tavenner, administrator of the Centers for Medicare & Medicaid Services, said in a statement. "Consumers should use this time to evaluate their health needs, browse other options, and see if they qualify for additional financial assistance. However, consumers who are happy with their plan and have no changes to their income or family situation can be auto-enrolled in their same plan next year, similar to how it is done in the employer insurance market today."
On Twitter @legal_med
Patients insured through the federally operated health insurance marketplace will be automatically reenrolled in their current coverage if they fail to change their previous plan, the Centers for Medicare & Medicaid Services announced Sept. 2.
Participants in these Affordable Care Act plans will receive notices before open enrollment begins explaining how they can return to the marketplace and shop for additional assistance and new plans. Insurers will provide information regarding 2015 premiums and tax credits.
If participants do nothing, they will be autoenrolled in the same plan as their 2014 plan year with the same tax credit. Open enrollment begins Nov. 15.
According to the final rule, participants whose 2013 tax returns indicate they have high income or did not grant marketplace administrators permission to access updated tax information will be autoenrolled without financial assistance if they don’t update their plan.
"We are committed to providing a simple, familiar process for consumers to renew their coverage next year," Marilyn Tavenner, administrator of the Centers for Medicare & Medicaid Services, said in a statement. "Consumers should use this time to evaluate their health needs, browse other options, and see if they qualify for additional financial assistance. However, consumers who are happy with their plan and have no changes to their income or family situation can be auto-enrolled in their same plan next year, similar to how it is done in the employer insurance market today."
On Twitter @legal_med
Patients insured through the federally operated health insurance marketplace will be automatically reenrolled in their current coverage if they fail to change their previous plan, the Centers for Medicare & Medicaid Services announced Sept. 2.
Participants in these Affordable Care Act plans will receive notices before open enrollment begins explaining how they can return to the marketplace and shop for additional assistance and new plans. Insurers will provide information regarding 2015 premiums and tax credits.
If participants do nothing, they will be autoenrolled in the same plan as their 2014 plan year with the same tax credit. Open enrollment begins Nov. 15.
According to the final rule, participants whose 2013 tax returns indicate they have high income or did not grant marketplace administrators permission to access updated tax information will be autoenrolled without financial assistance if they don’t update their plan.
"We are committed to providing a simple, familiar process for consumers to renew their coverage next year," Marilyn Tavenner, administrator of the Centers for Medicare & Medicaid Services, said in a statement. "Consumers should use this time to evaluate their health needs, browse other options, and see if they qualify for additional financial assistance. However, consumers who are happy with their plan and have no changes to their income or family situation can be auto-enrolled in their same plan next year, similar to how it is done in the employer insurance market today."
On Twitter @legal_med
CMS finalizes ‘flexibility’ in EHR meaningful use program
Physicians and hospitals will have more time to meet Stage 2 requirements under the federal government’s meaningful use program for the adoption of electronic health records.
Officials at the Centers for Medicare & Medicaid Services have been saying for months that they wanted to give physicians and hospitals greater flexibility in meeting the requirements of the EHR Incentive Programs, which offer bonuses for the "meaningful use" of certified EHR systems. The final rule cements a new timetable for Stage 2 of the program and allows providers to use older certified technology for longer, while vendors catch up with a backlog of demand.
Under the rule, the CMS officially extends Stage 2 of the program through 2016 for providers who were early adopters under the program, attesting to meaningful use in 2011 or 2012. Stage 3 of the meaningful use program will begin in 2017 for these providers, giving them an additional year to meet the more advanced requirements.
The rule also gives physicians more time to attest using older technology. Physicians can use the 2011 edition certified EHR technology or a combination of 2011 and 2014 edition certified product for the reporting period in 2014. All providers will be required to use 2014 edition certified products beginning in 2015.
But the American College of Physicians said the changes are "too little, too late."
Even with the increased flexibility to use older certified products, physicians will be able to attest for only Stage 1 until they have full access to 2014 edition certified technology, according to Dr. Peter Basch, chair of the ACP Medical Informatics Committee and the medical director for ambulatory EHR and health IT policy at MedStar Health in Washington. And that will impair their ability to prepare for Stage 2 attestation.
"Cut-over from their Stage 1 reporting configuration to their Stage 2 reporting configuration cannot be done overnight," Dr. Basch said. "Staffing and workflow changes take weeks, if not months, to accomplish."
And the new final rule does nothing to address other concerns raised by physicians. The American Medical Association has repeatedly called on the CMS to make more significant changes to the program, including lowering the threshold to earn incentives under the program. The AMA has urged the CMS to using a 75% pass rate as the standard for achieving meaningful use, as well as allowing physicians who meet 50% of meaningful use requirements to avoid financial penalties.
On Twitter @maryellenny
Physicians and hospitals will have more time to meet Stage 2 requirements under the federal government’s meaningful use program for the adoption of electronic health records.
Officials at the Centers for Medicare & Medicaid Services have been saying for months that they wanted to give physicians and hospitals greater flexibility in meeting the requirements of the EHR Incentive Programs, which offer bonuses for the "meaningful use" of certified EHR systems. The final rule cements a new timetable for Stage 2 of the program and allows providers to use older certified technology for longer, while vendors catch up with a backlog of demand.
Under the rule, the CMS officially extends Stage 2 of the program through 2016 for providers who were early adopters under the program, attesting to meaningful use in 2011 or 2012. Stage 3 of the meaningful use program will begin in 2017 for these providers, giving them an additional year to meet the more advanced requirements.
The rule also gives physicians more time to attest using older technology. Physicians can use the 2011 edition certified EHR technology or a combination of 2011 and 2014 edition certified product for the reporting period in 2014. All providers will be required to use 2014 edition certified products beginning in 2015.
But the American College of Physicians said the changes are "too little, too late."
Even with the increased flexibility to use older certified products, physicians will be able to attest for only Stage 1 until they have full access to 2014 edition certified technology, according to Dr. Peter Basch, chair of the ACP Medical Informatics Committee and the medical director for ambulatory EHR and health IT policy at MedStar Health in Washington. And that will impair their ability to prepare for Stage 2 attestation.
"Cut-over from their Stage 1 reporting configuration to their Stage 2 reporting configuration cannot be done overnight," Dr. Basch said. "Staffing and workflow changes take weeks, if not months, to accomplish."
And the new final rule does nothing to address other concerns raised by physicians. The American Medical Association has repeatedly called on the CMS to make more significant changes to the program, including lowering the threshold to earn incentives under the program. The AMA has urged the CMS to using a 75% pass rate as the standard for achieving meaningful use, as well as allowing physicians who meet 50% of meaningful use requirements to avoid financial penalties.
On Twitter @maryellenny
Physicians and hospitals will have more time to meet Stage 2 requirements under the federal government’s meaningful use program for the adoption of electronic health records.
Officials at the Centers for Medicare & Medicaid Services have been saying for months that they wanted to give physicians and hospitals greater flexibility in meeting the requirements of the EHR Incentive Programs, which offer bonuses for the "meaningful use" of certified EHR systems. The final rule cements a new timetable for Stage 2 of the program and allows providers to use older certified technology for longer, while vendors catch up with a backlog of demand.
Under the rule, the CMS officially extends Stage 2 of the program through 2016 for providers who were early adopters under the program, attesting to meaningful use in 2011 or 2012. Stage 3 of the meaningful use program will begin in 2017 for these providers, giving them an additional year to meet the more advanced requirements.
The rule also gives physicians more time to attest using older technology. Physicians can use the 2011 edition certified EHR technology or a combination of 2011 and 2014 edition certified product for the reporting period in 2014. All providers will be required to use 2014 edition certified products beginning in 2015.
But the American College of Physicians said the changes are "too little, too late."
Even with the increased flexibility to use older certified products, physicians will be able to attest for only Stage 1 until they have full access to 2014 edition certified technology, according to Dr. Peter Basch, chair of the ACP Medical Informatics Committee and the medical director for ambulatory EHR and health IT policy at MedStar Health in Washington. And that will impair their ability to prepare for Stage 2 attestation.
"Cut-over from their Stage 1 reporting configuration to their Stage 2 reporting configuration cannot be done overnight," Dr. Basch said. "Staffing and workflow changes take weeks, if not months, to accomplish."
And the new final rule does nothing to address other concerns raised by physicians. The American Medical Association has repeatedly called on the CMS to make more significant changes to the program, including lowering the threshold to earn incentives under the program. The AMA has urged the CMS to using a 75% pass rate as the standard for achieving meaningful use, as well as allowing physicians who meet 50% of meaningful use requirements to avoid financial penalties.
On Twitter @maryellenny
Locoregional recurrence of breast cancer less likely after neoadjuvant complete response
Patients with residual disease after neoadjuvant chemotherapy and surgery for breast cancer had a 60%-280% increased risk for locoregional recurrence, compared with patients with a pathologic complete response, an analysis of data from 12 large clinical trials found.
Investigators analyzed data on 11,955 patients with stage I-III breast cancer who underwent neoadjuvant chemotherapy in studies with long-term follow-up and information on complete pathologic response (no residual cancer in the breast and no cancer in the axillary lymph nodes after surgery). They included 5,252 patients in a multivariate analysis of predictors of locoregional recurrence a median of 5 years after treatment.
Overall, the likelihood of locoregional recurrence was low – less than 10%. Locoregional recurrence was seen in 5.5% of patients with a complete pathologic response to neoadjuvant chemotherapy and in 7.1% of patients without a complete response, a significant 60% increase in risk without a complete response, Dr. Eleftherios Mamounas reported in a press briefing held in advance of the breast cancer symposium sponsored by the American Society of Clinical Oncology.
Patients with residual cancer in the breast after surgery had a 60% higher risk for locoregional recurrence, and patients with residual cancer in the axillary lymph nodes had a 280% increased risk for locoregional recurrence, compared with patients who had a complete pathologic response, reported Dr. Mamounas, professor of surgery at the University of Central Florida, and medical director of the comprehensive breast program at the University of Florida Health Cancer Center, both in Orlando.
Breast cancer subtypes remained independent predictors of locoregional recurrence, regardless of whether patients had a pathologic complete response or not. The cancer recurred locally or regionally in 4% of patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2–) grade 1 or 2 cancer, 9% of patients with HR+/HER2– grade 3 cancer, 15% of patients with HR–/HER2+ cancer, 10% of patients with HR+/HER2+ cancer, and 12% of patients with HR–/HER2– cancer (also known as hormone receptor–negative or triple-negative breast cancer).
Those rates would be different today because of more effective treatments for HER2+ breast cancer, he noted.
"For all breast cancer subtypes except for HR+/HER2– grade 1 and 2, there was a progressive increase in the locoregional recurrence rates with decreasing rates of pathologic complete response," he said. In other words, recurrence rates went from highest to lowest in patients "having positive nodes, versus having residual disease in the breast with negative nodes, versus having complete pathologic response," he explained.
Among patients with triple-negative cancer, for example, locoregional recurrence rates went from 6.2% in those with a complete response to 11.9% in patients with residual cancer in the breast but not lymph nodes and 22.1% in those who had positive nodes after treatment.
A pathologic complete response predicted lower locoregional recurrence rates with the various cancer subtypes, regardless of whether the patient underwent lumpectomy or mastectomy, he said.
Based on these results and previously published studies, "we have a lot of evidence that pathologic complete response is predictive of outcome, both in terms of systemic recurrence and also in terms of local recurrence," Dr. Mamounas commented. A previous meta-analysis that reported conflicting results for systemic recurrence "did not quite confirm that an incremental increase in pathologic complete response will improve overall survival, but there are a lot of technical issues if you look at the different studies that were included in the meta-analysis. The bar was very high to prove that concept."
Recurrence is less likely after a pathologic complete response in patients with HER2+ breast cancer, triple-negative cancer, or highly proliferative estrogen receptor–positive breast cancer, he said. That may not be the case for patients with estrogen receptor–positive, HER2– grade 1 disease, who do very well regardless, he added.
"Our findings have clinical implications relative to further tailoring the use of adjuvant radiation therapy after neoadjuvant chemotherapy and support the conduct of ongoing clinical trials attempting to tailor locoregional therapy in this setting," Dr. Mamounas said.
Dr. Mamounas reported financial associations with Genomic Health, GE Healthcare, Celgene, Pfizer, Eisai, and Genentech/Roche. Some of his coinvestigators reported associations with multiple companies.
On Twitter @sherryboschert
This is a group of women who have higher-than-average risk, such that they are being offered up-front chemotherapy to shrink cancers even before they would go for breast surgery.
We’ve known for a long time that this clinical endpoint of complete pathologic response – that is, when you do the breast surgery, there is no evidence of residual cancer – is a powerful predictor of the cancer not recurring somewhere else in the body. These data from Dr. Mamounas show that complete pathologic response also is a predictor for not having the cancer recur within the chest wall or the breast itself. That’s very important information for the clinical team.

|
|
Dr. Mamounas’s data also point to the idea that the breast cancer subtype is very important for predicting the outcomes. In the modern era, so much of what we are thinking about in the way of managing breast cancer is driven by our understanding of these major clinical subtypes, the so-called HER2-positive breast cancers, the so-called triple-negative breast cancers (which lack estrogen receptor, progesterone receptor, and HER2), and finally the spectrum of so-called estrogen receptor–positive, HER2-negative (sometimes called luminal) cancers.
What Dr. Mamounas’s data speak to is a very complicated matrix that helps us understand the risk of local recurrence in a woman who has a greater-than-average risk of breast cancer by factoring in the type of breast cancer, the response that you see with the up-front chemotherapy, and the age of the patient. These multiplex kinds of information set the stage for a variety of trials that are looking at trying to tailor additional therapy for women who are at higher risk and, conversely, sparing women who are at lower risk the need for extra treatment – in this case, possibly the need for radiation therapy.
These are data that really resonate with radiation oncologists, surgeons, and medical oncologists, who are into the nitty-gritty of caring for women with breast cancer and need to determine who is going to need more therapy and who can be spared additional treatment.
Dr. Harold J. Burstein is associate professor of medicine at Harvard Medical School and the Dana-Farber Cancer Institute, both in Boston. He reported having no relevant financial disclosures.
This is a group of women who have higher-than-average risk, such that they are being offered up-front chemotherapy to shrink cancers even before they would go for breast surgery.
We’ve known for a long time that this clinical endpoint of complete pathologic response – that is, when you do the breast surgery, there is no evidence of residual cancer – is a powerful predictor of the cancer not recurring somewhere else in the body. These data from Dr. Mamounas show that complete pathologic response also is a predictor for not having the cancer recur within the chest wall or the breast itself. That’s very important information for the clinical team.

|
|
Dr. Mamounas’s data also point to the idea that the breast cancer subtype is very important for predicting the outcomes. In the modern era, so much of what we are thinking about in the way of managing breast cancer is driven by our understanding of these major clinical subtypes, the so-called HER2-positive breast cancers, the so-called triple-negative breast cancers (which lack estrogen receptor, progesterone receptor, and HER2), and finally the spectrum of so-called estrogen receptor–positive, HER2-negative (sometimes called luminal) cancers.
What Dr. Mamounas’s data speak to is a very complicated matrix that helps us understand the risk of local recurrence in a woman who has a greater-than-average risk of breast cancer by factoring in the type of breast cancer, the response that you see with the up-front chemotherapy, and the age of the patient. These multiplex kinds of information set the stage for a variety of trials that are looking at trying to tailor additional therapy for women who are at higher risk and, conversely, sparing women who are at lower risk the need for extra treatment – in this case, possibly the need for radiation therapy.
These are data that really resonate with radiation oncologists, surgeons, and medical oncologists, who are into the nitty-gritty of caring for women with breast cancer and need to determine who is going to need more therapy and who can be spared additional treatment.
Dr. Harold J. Burstein is associate professor of medicine at Harvard Medical School and the Dana-Farber Cancer Institute, both in Boston. He reported having no relevant financial disclosures.
This is a group of women who have higher-than-average risk, such that they are being offered up-front chemotherapy to shrink cancers even before they would go for breast surgery.
We’ve known for a long time that this clinical endpoint of complete pathologic response – that is, when you do the breast surgery, there is no evidence of residual cancer – is a powerful predictor of the cancer not recurring somewhere else in the body. These data from Dr. Mamounas show that complete pathologic response also is a predictor for not having the cancer recur within the chest wall or the breast itself. That’s very important information for the clinical team.

|
|
Dr. Mamounas’s data also point to the idea that the breast cancer subtype is very important for predicting the outcomes. In the modern era, so much of what we are thinking about in the way of managing breast cancer is driven by our understanding of these major clinical subtypes, the so-called HER2-positive breast cancers, the so-called triple-negative breast cancers (which lack estrogen receptor, progesterone receptor, and HER2), and finally the spectrum of so-called estrogen receptor–positive, HER2-negative (sometimes called luminal) cancers.
What Dr. Mamounas’s data speak to is a very complicated matrix that helps us understand the risk of local recurrence in a woman who has a greater-than-average risk of breast cancer by factoring in the type of breast cancer, the response that you see with the up-front chemotherapy, and the age of the patient. These multiplex kinds of information set the stage for a variety of trials that are looking at trying to tailor additional therapy for women who are at higher risk and, conversely, sparing women who are at lower risk the need for extra treatment – in this case, possibly the need for radiation therapy.
These are data that really resonate with radiation oncologists, surgeons, and medical oncologists, who are into the nitty-gritty of caring for women with breast cancer and need to determine who is going to need more therapy and who can be spared additional treatment.
Dr. Harold J. Burstein is associate professor of medicine at Harvard Medical School and the Dana-Farber Cancer Institute, both in Boston. He reported having no relevant financial disclosures.
Patients with residual disease after neoadjuvant chemotherapy and surgery for breast cancer had a 60%-280% increased risk for locoregional recurrence, compared with patients with a pathologic complete response, an analysis of data from 12 large clinical trials found.
Investigators analyzed data on 11,955 patients with stage I-III breast cancer who underwent neoadjuvant chemotherapy in studies with long-term follow-up and information on complete pathologic response (no residual cancer in the breast and no cancer in the axillary lymph nodes after surgery). They included 5,252 patients in a multivariate analysis of predictors of locoregional recurrence a median of 5 years after treatment.
Overall, the likelihood of locoregional recurrence was low – less than 10%. Locoregional recurrence was seen in 5.5% of patients with a complete pathologic response to neoadjuvant chemotherapy and in 7.1% of patients without a complete response, a significant 60% increase in risk without a complete response, Dr. Eleftherios Mamounas reported in a press briefing held in advance of the breast cancer symposium sponsored by the American Society of Clinical Oncology.
Patients with residual cancer in the breast after surgery had a 60% higher risk for locoregional recurrence, and patients with residual cancer in the axillary lymph nodes had a 280% increased risk for locoregional recurrence, compared with patients who had a complete pathologic response, reported Dr. Mamounas, professor of surgery at the University of Central Florida, and medical director of the comprehensive breast program at the University of Florida Health Cancer Center, both in Orlando.
Breast cancer subtypes remained independent predictors of locoregional recurrence, regardless of whether patients had a pathologic complete response or not. The cancer recurred locally or regionally in 4% of patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2–) grade 1 or 2 cancer, 9% of patients with HR+/HER2– grade 3 cancer, 15% of patients with HR–/HER2+ cancer, 10% of patients with HR+/HER2+ cancer, and 12% of patients with HR–/HER2– cancer (also known as hormone receptor–negative or triple-negative breast cancer).
Those rates would be different today because of more effective treatments for HER2+ breast cancer, he noted.
"For all breast cancer subtypes except for HR+/HER2– grade 1 and 2, there was a progressive increase in the locoregional recurrence rates with decreasing rates of pathologic complete response," he said. In other words, recurrence rates went from highest to lowest in patients "having positive nodes, versus having residual disease in the breast with negative nodes, versus having complete pathologic response," he explained.
Among patients with triple-negative cancer, for example, locoregional recurrence rates went from 6.2% in those with a complete response to 11.9% in patients with residual cancer in the breast but not lymph nodes and 22.1% in those who had positive nodes after treatment.
A pathologic complete response predicted lower locoregional recurrence rates with the various cancer subtypes, regardless of whether the patient underwent lumpectomy or mastectomy, he said.
Based on these results and previously published studies, "we have a lot of evidence that pathologic complete response is predictive of outcome, both in terms of systemic recurrence and also in terms of local recurrence," Dr. Mamounas commented. A previous meta-analysis that reported conflicting results for systemic recurrence "did not quite confirm that an incremental increase in pathologic complete response will improve overall survival, but there are a lot of technical issues if you look at the different studies that were included in the meta-analysis. The bar was very high to prove that concept."
Recurrence is less likely after a pathologic complete response in patients with HER2+ breast cancer, triple-negative cancer, or highly proliferative estrogen receptor–positive breast cancer, he said. That may not be the case for patients with estrogen receptor–positive, HER2– grade 1 disease, who do very well regardless, he added.
"Our findings have clinical implications relative to further tailoring the use of adjuvant radiation therapy after neoadjuvant chemotherapy and support the conduct of ongoing clinical trials attempting to tailor locoregional therapy in this setting," Dr. Mamounas said.
Dr. Mamounas reported financial associations with Genomic Health, GE Healthcare, Celgene, Pfizer, Eisai, and Genentech/Roche. Some of his coinvestigators reported associations with multiple companies.
On Twitter @sherryboschert
Patients with residual disease after neoadjuvant chemotherapy and surgery for breast cancer had a 60%-280% increased risk for locoregional recurrence, compared with patients with a pathologic complete response, an analysis of data from 12 large clinical trials found.
Investigators analyzed data on 11,955 patients with stage I-III breast cancer who underwent neoadjuvant chemotherapy in studies with long-term follow-up and information on complete pathologic response (no residual cancer in the breast and no cancer in the axillary lymph nodes after surgery). They included 5,252 patients in a multivariate analysis of predictors of locoregional recurrence a median of 5 years after treatment.
Overall, the likelihood of locoregional recurrence was low – less than 10%. Locoregional recurrence was seen in 5.5% of patients with a complete pathologic response to neoadjuvant chemotherapy and in 7.1% of patients without a complete response, a significant 60% increase in risk without a complete response, Dr. Eleftherios Mamounas reported in a press briefing held in advance of the breast cancer symposium sponsored by the American Society of Clinical Oncology.
Patients with residual cancer in the breast after surgery had a 60% higher risk for locoregional recurrence, and patients with residual cancer in the axillary lymph nodes had a 280% increased risk for locoregional recurrence, compared with patients who had a complete pathologic response, reported Dr. Mamounas, professor of surgery at the University of Central Florida, and medical director of the comprehensive breast program at the University of Florida Health Cancer Center, both in Orlando.
Breast cancer subtypes remained independent predictors of locoregional recurrence, regardless of whether patients had a pathologic complete response or not. The cancer recurred locally or regionally in 4% of patients with hormone receptor–positive, human epidermal growth factor receptor 2–negative (HR+/HER2–) grade 1 or 2 cancer, 9% of patients with HR+/HER2– grade 3 cancer, 15% of patients with HR–/HER2+ cancer, 10% of patients with HR+/HER2+ cancer, and 12% of patients with HR–/HER2– cancer (also known as hormone receptor–negative or triple-negative breast cancer).
Those rates would be different today because of more effective treatments for HER2+ breast cancer, he noted.
"For all breast cancer subtypes except for HR+/HER2– grade 1 and 2, there was a progressive increase in the locoregional recurrence rates with decreasing rates of pathologic complete response," he said. In other words, recurrence rates went from highest to lowest in patients "having positive nodes, versus having residual disease in the breast with negative nodes, versus having complete pathologic response," he explained.
Among patients with triple-negative cancer, for example, locoregional recurrence rates went from 6.2% in those with a complete response to 11.9% in patients with residual cancer in the breast but not lymph nodes and 22.1% in those who had positive nodes after treatment.
A pathologic complete response predicted lower locoregional recurrence rates with the various cancer subtypes, regardless of whether the patient underwent lumpectomy or mastectomy, he said.
Based on these results and previously published studies, "we have a lot of evidence that pathologic complete response is predictive of outcome, both in terms of systemic recurrence and also in terms of local recurrence," Dr. Mamounas commented. A previous meta-analysis that reported conflicting results for systemic recurrence "did not quite confirm that an incremental increase in pathologic complete response will improve overall survival, but there are a lot of technical issues if you look at the different studies that were included in the meta-analysis. The bar was very high to prove that concept."
Recurrence is less likely after a pathologic complete response in patients with HER2+ breast cancer, triple-negative cancer, or highly proliferative estrogen receptor–positive breast cancer, he said. That may not be the case for patients with estrogen receptor–positive, HER2– grade 1 disease, who do very well regardless, he added.
"Our findings have clinical implications relative to further tailoring the use of adjuvant radiation therapy after neoadjuvant chemotherapy and support the conduct of ongoing clinical trials attempting to tailor locoregional therapy in this setting," Dr. Mamounas said.
Dr. Mamounas reported financial associations with Genomic Health, GE Healthcare, Celgene, Pfizer, Eisai, and Genentech/Roche. Some of his coinvestigators reported associations with multiple companies.
On Twitter @sherryboschert
FROM THE ASCO BREAST CANCER SYMPOSIUM
Key clinical point: Response to neoadjuvant chemotherapy for breast cancer predicts the locoregional recurrence risk.
Major finding: Risk for locoregional recurrence was 60%-280% higher in patients without a pathologic complete response.
Data source: A pooled analysis of data on 11,955 patients who got neoadjuvant therapy and surgery for stage I-III breast cancer.
Disclosures: Dr. Mamounas reported financial associations with Genomic Health, GE Healthcare, Celgene, Pfizer, Eisai, and Genentech/Roche. Some of his coinvestigators reported associations with multiple companies.