User login
Official Newspaper of the American College of Surgeons
Megamerger rulings may chill future consolidations
Expect a chilling effect on future health care consolidations after two health insurance megamergers were blocked by federal courts, analysts say.
A federal judge barred a merger between Aetna and Humana on Jan. 23, followed by a Feb. 8 decision that blocked Anthem and Cigna from consolidating. Both rulings called the deals anticompetitive.
“Both of these decisions are likely to chill future mergers,” Mr. Dahlquist said in an interview. “We have the FTC [Federal Trade Commission] winning on the provider side. Now we have the DOJ [Department of Justice] winning on the insurer side. While they are different players in the marketplace, the law that is being applied is very similar, if not the same. The one-sided victories by the government are creating a situation of imbalance.”
Ruling positive and negative for providers
Anthem’s proposed $54 billion merger with Cigna would have been the largest insurer consolidation in history. The merger, along with Aetna’s $37 billion plan to purchase Humana, would have reduced the number of major U.S. health insurers from five to three, reshaping the industry. The Department of Justice and a number of states immediately opposed the mergers, arguing the consolidations would significantly reduce competition to the detriment of patients. Judges ultimately agreed.
“The [insurers] already have a huge market position so they can already hammer [providers] with low rates,” Mr. Greer said in an interview. “The reason why [the rulings are] such a big win for providers, is there won’t be this huge concentration of power. Anthem may not be able to hammer them quite as much as if they would’ve merged with Cigna.”
However, the rulings may discourage future health care consolidations between insurers and among health providers, Mr. Dahlquist noted. In recent years, the government has scored a series of legal wins that have blocked provider mergers. In 2013 for example, the Federal Trade Commission successfully stopped the acquisition of Palmyra Medical Center by Phoebe Putney Memorial Hospital (Albany, Ga.), followed by a 2014 win that barred St. Luke’s Health System’s acquisition of Saltzer Medical Group (Nampa, Idaho), an independent physician practice. In 2016, the FTC successfully blocked the merger of Penn State Hershey (Pa.) Medical Center’s merger with PinnacleHealth System (Harrisburg, Penn.).
“Every merger needs to be looked at [based on] the facts of that merger, and the facts of that case, the markets they compete in, [and] the markets they want to expand into,” Mr. Dahlquist said. “That’s why you shouldn’t look at all mergers of health care providers or health insurers as inherently bad, and I think that’s what these [decisions] are unfortunately driving toward.”
A broader takeaway is that the insurance industry needs to develop a better business model, said Barak D. Richman, PhD, an antitrust expert and law professor at Duke University in Durham, N.C. Mr. Richman consulted with several state departments of insurance as they were reviewing the Anthem-Cigna mergers.
“The general story here is that the insurance industry, over a number of years, has been consolidating and has reached a point where it now has passed a threshold where antitrust scrutiny will be very real,” Mr. Richman said in an interview. “One thing that the court cases revealed is that there is a certain prevailing business model in insurance which is: Get as big as you can, balance risk through volume, and use buying leverage to negotiate favorable prices from providers. It looks like that business model has reached its limits and really is not going to be sustainable any longer.”
The current market demands business models that center on value, not volume, Mr. Richman continued. Insurance companies will need to be more deliberate and analytical about how to provide value to patients and generate cost effective care.
“That really is the takeaway, that we need a real shift in the business of health insurance,” he said.
Anthem banking on new administration
Since the rulings, Aetna and Humana have chosen not to appeal and have mutually ended their merger agreement. Cigna is seeking to terminate its agreement with Anthem, but Anthem is fighting the dissolution. The insurance giant is looking toward the Trump administration to potentially turn the case around.
To enforce the termination, Cigna is suing Anthem in the Delaware Court of Chancery seeking a declaratory judgment that Cigna has lawfully terminated the contract, according to an announcement by Cigna. Anthem, meanwhile, is seeking a temporary restraining order to enjoin Cigna from terminating its contract.
“Anthem believes there is still sufficient time and a viable path forward potentially to complete the transaction that will save millions of Americans more than $2 billion in annual medical costs and deliver significant value to shareholders,” according to a company statement. “In addition to filing this lawsuit, Anthem is pursuing an expedited appeal of the District Court’s decision and is committed to completing this value-creating merger either through a successful appeal or through settlement with the new leadership at the Department of Justice.”
Part of that new leadership could include Makan Delrahim, a former Anthem lobbyist now serving as deputy White House counsel. He is rumored to be a top contender to lead the DOJ’s antitrust division.
Records also show that Indiana insurance regulators were supportive of the Anthem-Cigna deal while Vice President Pence was governor of Indiana and that Anthem contributed $100,000 to President Trump’s inaugural committee, according to Senate lobbying reports.
“A lot of folks are talking about the possibility that Trump will appoint people at both the FTC and DOJ antitrust devision [who] are Republicans and tend to be more business friendly, which would mean that maybe some of these mergers would be more likely to make it and less likely to be challenged,” he said.
On the other hand, a couple of leadership changes will not likely reverse the country’s historic antitrust enforcement, Mr. Dahlquist said.
“I don’t believe you’re going to see a systemic shift in the antitrust enforcement policy of this country,” he said. “Even one change at the top is not going to [create] upheaval within the ranks at the FTC and DOJ. The government is on a winning streak; I think that’s going to continue in the short term.”
“I don’t expect to see any change in the government’s approach in continuing to challenge anticompetitive health care transactions,” Ms. Feinstein said during at interview at the meeting.
[email protected]
On Twitter @legal_med
Expect a chilling effect on future health care consolidations after two health insurance megamergers were blocked by federal courts, analysts say.
A federal judge barred a merger between Aetna and Humana on Jan. 23, followed by a Feb. 8 decision that blocked Anthem and Cigna from consolidating. Both rulings called the deals anticompetitive.
“Both of these decisions are likely to chill future mergers,” Mr. Dahlquist said in an interview. “We have the FTC [Federal Trade Commission] winning on the provider side. Now we have the DOJ [Department of Justice] winning on the insurer side. While they are different players in the marketplace, the law that is being applied is very similar, if not the same. The one-sided victories by the government are creating a situation of imbalance.”
Ruling positive and negative for providers
Anthem’s proposed $54 billion merger with Cigna would have been the largest insurer consolidation in history. The merger, along with Aetna’s $37 billion plan to purchase Humana, would have reduced the number of major U.S. health insurers from five to three, reshaping the industry. The Department of Justice and a number of states immediately opposed the mergers, arguing the consolidations would significantly reduce competition to the detriment of patients. Judges ultimately agreed.
“The [insurers] already have a huge market position so they can already hammer [providers] with low rates,” Mr. Greer said in an interview. “The reason why [the rulings are] such a big win for providers, is there won’t be this huge concentration of power. Anthem may not be able to hammer them quite as much as if they would’ve merged with Cigna.”
However, the rulings may discourage future health care consolidations between insurers and among health providers, Mr. Dahlquist noted. In recent years, the government has scored a series of legal wins that have blocked provider mergers. In 2013 for example, the Federal Trade Commission successfully stopped the acquisition of Palmyra Medical Center by Phoebe Putney Memorial Hospital (Albany, Ga.), followed by a 2014 win that barred St. Luke’s Health System’s acquisition of Saltzer Medical Group (Nampa, Idaho), an independent physician practice. In 2016, the FTC successfully blocked the merger of Penn State Hershey (Pa.) Medical Center’s merger with PinnacleHealth System (Harrisburg, Penn.).
“Every merger needs to be looked at [based on] the facts of that merger, and the facts of that case, the markets they compete in, [and] the markets they want to expand into,” Mr. Dahlquist said. “That’s why you shouldn’t look at all mergers of health care providers or health insurers as inherently bad, and I think that’s what these [decisions] are unfortunately driving toward.”
A broader takeaway is that the insurance industry needs to develop a better business model, said Barak D. Richman, PhD, an antitrust expert and law professor at Duke University in Durham, N.C. Mr. Richman consulted with several state departments of insurance as they were reviewing the Anthem-Cigna mergers.
“The general story here is that the insurance industry, over a number of years, has been consolidating and has reached a point where it now has passed a threshold where antitrust scrutiny will be very real,” Mr. Richman said in an interview. “One thing that the court cases revealed is that there is a certain prevailing business model in insurance which is: Get as big as you can, balance risk through volume, and use buying leverage to negotiate favorable prices from providers. It looks like that business model has reached its limits and really is not going to be sustainable any longer.”
The current market demands business models that center on value, not volume, Mr. Richman continued. Insurance companies will need to be more deliberate and analytical about how to provide value to patients and generate cost effective care.
“That really is the takeaway, that we need a real shift in the business of health insurance,” he said.
Anthem banking on new administration
Since the rulings, Aetna and Humana have chosen not to appeal and have mutually ended their merger agreement. Cigna is seeking to terminate its agreement with Anthem, but Anthem is fighting the dissolution. The insurance giant is looking toward the Trump administration to potentially turn the case around.
To enforce the termination, Cigna is suing Anthem in the Delaware Court of Chancery seeking a declaratory judgment that Cigna has lawfully terminated the contract, according to an announcement by Cigna. Anthem, meanwhile, is seeking a temporary restraining order to enjoin Cigna from terminating its contract.
“Anthem believes there is still sufficient time and a viable path forward potentially to complete the transaction that will save millions of Americans more than $2 billion in annual medical costs and deliver significant value to shareholders,” according to a company statement. “In addition to filing this lawsuit, Anthem is pursuing an expedited appeal of the District Court’s decision and is committed to completing this value-creating merger either through a successful appeal or through settlement with the new leadership at the Department of Justice.”
Part of that new leadership could include Makan Delrahim, a former Anthem lobbyist now serving as deputy White House counsel. He is rumored to be a top contender to lead the DOJ’s antitrust division.
Records also show that Indiana insurance regulators were supportive of the Anthem-Cigna deal while Vice President Pence was governor of Indiana and that Anthem contributed $100,000 to President Trump’s inaugural committee, according to Senate lobbying reports.
“A lot of folks are talking about the possibility that Trump will appoint people at both the FTC and DOJ antitrust devision [who] are Republicans and tend to be more business friendly, which would mean that maybe some of these mergers would be more likely to make it and less likely to be challenged,” he said.
On the other hand, a couple of leadership changes will not likely reverse the country’s historic antitrust enforcement, Mr. Dahlquist said.
“I don’t believe you’re going to see a systemic shift in the antitrust enforcement policy of this country,” he said. “Even one change at the top is not going to [create] upheaval within the ranks at the FTC and DOJ. The government is on a winning streak; I think that’s going to continue in the short term.”
“I don’t expect to see any change in the government’s approach in continuing to challenge anticompetitive health care transactions,” Ms. Feinstein said during at interview at the meeting.
[email protected]
On Twitter @legal_med
Expect a chilling effect on future health care consolidations after two health insurance megamergers were blocked by federal courts, analysts say.
A federal judge barred a merger between Aetna and Humana on Jan. 23, followed by a Feb. 8 decision that blocked Anthem and Cigna from consolidating. Both rulings called the deals anticompetitive.
“Both of these decisions are likely to chill future mergers,” Mr. Dahlquist said in an interview. “We have the FTC [Federal Trade Commission] winning on the provider side. Now we have the DOJ [Department of Justice] winning on the insurer side. While they are different players in the marketplace, the law that is being applied is very similar, if not the same. The one-sided victories by the government are creating a situation of imbalance.”
Ruling positive and negative for providers
Anthem’s proposed $54 billion merger with Cigna would have been the largest insurer consolidation in history. The merger, along with Aetna’s $37 billion plan to purchase Humana, would have reduced the number of major U.S. health insurers from five to three, reshaping the industry. The Department of Justice and a number of states immediately opposed the mergers, arguing the consolidations would significantly reduce competition to the detriment of patients. Judges ultimately agreed.
“The [insurers] already have a huge market position so they can already hammer [providers] with low rates,” Mr. Greer said in an interview. “The reason why [the rulings are] such a big win for providers, is there won’t be this huge concentration of power. Anthem may not be able to hammer them quite as much as if they would’ve merged with Cigna.”
However, the rulings may discourage future health care consolidations between insurers and among health providers, Mr. Dahlquist noted. In recent years, the government has scored a series of legal wins that have blocked provider mergers. In 2013 for example, the Federal Trade Commission successfully stopped the acquisition of Palmyra Medical Center by Phoebe Putney Memorial Hospital (Albany, Ga.), followed by a 2014 win that barred St. Luke’s Health System’s acquisition of Saltzer Medical Group (Nampa, Idaho), an independent physician practice. In 2016, the FTC successfully blocked the merger of Penn State Hershey (Pa.) Medical Center’s merger with PinnacleHealth System (Harrisburg, Penn.).
“Every merger needs to be looked at [based on] the facts of that merger, and the facts of that case, the markets they compete in, [and] the markets they want to expand into,” Mr. Dahlquist said. “That’s why you shouldn’t look at all mergers of health care providers or health insurers as inherently bad, and I think that’s what these [decisions] are unfortunately driving toward.”
A broader takeaway is that the insurance industry needs to develop a better business model, said Barak D. Richman, PhD, an antitrust expert and law professor at Duke University in Durham, N.C. Mr. Richman consulted with several state departments of insurance as they were reviewing the Anthem-Cigna mergers.
“The general story here is that the insurance industry, over a number of years, has been consolidating and has reached a point where it now has passed a threshold where antitrust scrutiny will be very real,” Mr. Richman said in an interview. “One thing that the court cases revealed is that there is a certain prevailing business model in insurance which is: Get as big as you can, balance risk through volume, and use buying leverage to negotiate favorable prices from providers. It looks like that business model has reached its limits and really is not going to be sustainable any longer.”
The current market demands business models that center on value, not volume, Mr. Richman continued. Insurance companies will need to be more deliberate and analytical about how to provide value to patients and generate cost effective care.
“That really is the takeaway, that we need a real shift in the business of health insurance,” he said.
Anthem banking on new administration
Since the rulings, Aetna and Humana have chosen not to appeal and have mutually ended their merger agreement. Cigna is seeking to terminate its agreement with Anthem, but Anthem is fighting the dissolution. The insurance giant is looking toward the Trump administration to potentially turn the case around.
To enforce the termination, Cigna is suing Anthem in the Delaware Court of Chancery seeking a declaratory judgment that Cigna has lawfully terminated the contract, according to an announcement by Cigna. Anthem, meanwhile, is seeking a temporary restraining order to enjoin Cigna from terminating its contract.
“Anthem believes there is still sufficient time and a viable path forward potentially to complete the transaction that will save millions of Americans more than $2 billion in annual medical costs and deliver significant value to shareholders,” according to a company statement. “In addition to filing this lawsuit, Anthem is pursuing an expedited appeal of the District Court’s decision and is committed to completing this value-creating merger either through a successful appeal or through settlement with the new leadership at the Department of Justice.”
Part of that new leadership could include Makan Delrahim, a former Anthem lobbyist now serving as deputy White House counsel. He is rumored to be a top contender to lead the DOJ’s antitrust division.
Records also show that Indiana insurance regulators were supportive of the Anthem-Cigna deal while Vice President Pence was governor of Indiana and that Anthem contributed $100,000 to President Trump’s inaugural committee, according to Senate lobbying reports.
“A lot of folks are talking about the possibility that Trump will appoint people at both the FTC and DOJ antitrust devision [who] are Republicans and tend to be more business friendly, which would mean that maybe some of these mergers would be more likely to make it and less likely to be challenged,” he said.
On the other hand, a couple of leadership changes will not likely reverse the country’s historic antitrust enforcement, Mr. Dahlquist said.
“I don’t believe you’re going to see a systemic shift in the antitrust enforcement policy of this country,” he said. “Even one change at the top is not going to [create] upheaval within the ranks at the FTC and DOJ. The government is on a winning streak; I think that’s going to continue in the short term.”
“I don’t expect to see any change in the government’s approach in continuing to challenge anticompetitive health care transactions,” Ms. Feinstein said during at interview at the meeting.
[email protected]
On Twitter @legal_med
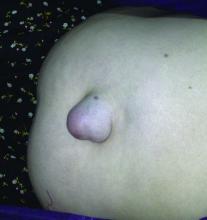
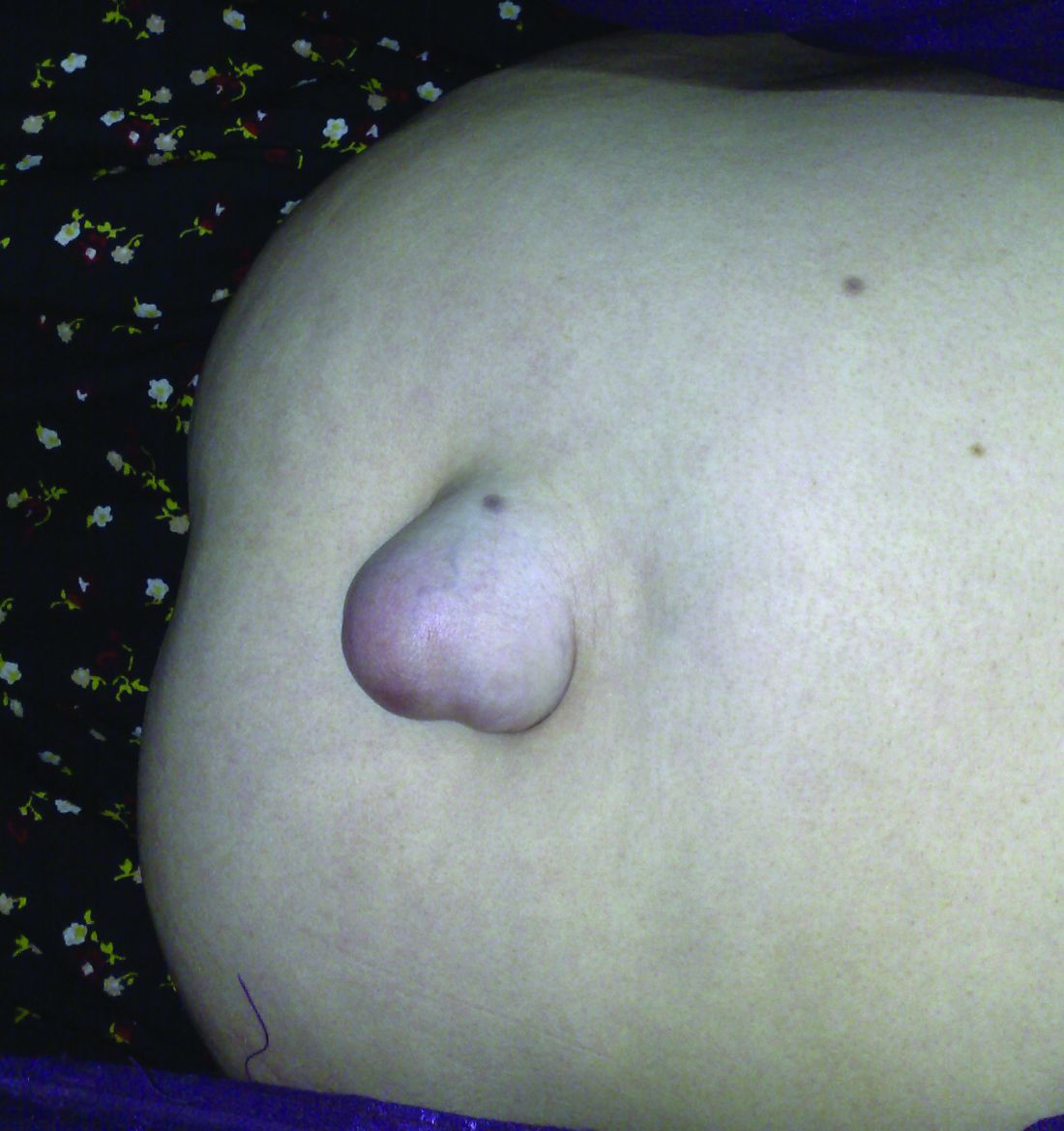
Follow-up data support using mesh for umbilical hernia repair
Patients with umbilical hernias and multiple comorbidities should be considered for mesh repair to reduce the risk of recurrence, say the authors of a study of the factors associated with umbilical hernia recurrence after repair.
The retrospective cohort study, published in JAMA Surgery, examined recurrence and mortality outcomes in 332 military veteran patients who underwent primary umbilical hernia repair between 1998 and 2008 and followed until June 2014.
The overall recurrence rate was 6% and a mean recurrence time after index repair of 3.1 years. The recurrence rate was significantly higher among patients who underwent primary suture repair, compared with those who underwent mesh repair (9.8% vs. 2.4%, P = .04). Mesh repair decreased the risk of recurrence more than threefold, compared with primary suture repair (odds ratio, 0.28; 95% confidence interval, 0.08-0.95).
Patients with ascites had a significantly greater risk of recurrence, compared with those without ascites (20% vs. 5.1%, P = .02), as did those with liver disease (35% vs. 13.8%, P = .02), and diabetes (55% vs. 32.7%).
“This information suggests that umbilical hernias should be repaired using mesh, especially if a patient has multiple comorbidities that are significantly associated with recurrence, such as obesity, diabetes, liver disease, and ascites,” wrote Divya A. Shankar, a medical student at Boston University School of Medicine, and her coauthors (JAMA Surg. 2017 Jan 25. doi: 10.1001/jamasurg.2016.5052).
Among these patients, only 1 died within 30 days, but the study captured mortality in this group over 6-16 years after their hernia surgery. Mortality rate in the group was 27% through the follow-up period. However, older individuals, smokers, patients with liver disease and ascites, and those who had to undergo emergency or semi-urgent repair or who required intraoperative bowel resection had significantly increased long-term mortality rates.
“Although there is a trend toward higher mortality rates in patients who underwent emergency repair, it is difficult to interpret whether the deaths were related to the emergency or to underlying medical conditions given that the etiology of the majority of these deaths is unknown,” the authors wrote.
Among the deaths, 43 (48%) were from unknown causes, 18 (20%) were cancer related, 12 (13%) were related to renal disease, and 3 (3.3%) were related to sepsis.
Researchers found that patients with a history of hernias were significantly less likely to have an umbilical hernia recurrence, although a greater percentage of these patients – 61% – received mesh in contrast to the 44% of patients without a history of hernia.
Defect size did not appear to affect the rate of recurrence but the authors noted that defect size was only recorded in about half of the patients in the study.
“Because there was no significant difference between these groups, we are unable to conclude whether the size of defects should play a role in a surgeon’s decision to use mesh,” they wrote.
Sixty-one patients (18%) had at least one complication within 30 days of the repair, with the most common being seroma (9.6%), surgical site infection (6.9%) and hematoma (2.4%). Two patients experienced a mesh infection and three experienced ascites leaks. The rate of complications was slightly, but not significantly, higher in the patients who received mesh repair, compared with the primary suture repair.
“A surprise finding was that patient who underwent emergent or semi-emergent repair had a 2.2 times increased odds of death. Twenty deaths occurred more than 30 days after emergent or semi-urgent repair and 12 (60%) were secondary to unknown causes and 5 (25%) were secondary to liver disease,” the investigators noted.
“Interestingly, 193 (58%) patients who underwent umbilical hernia repair had other hernias that were either repaired before the index repair or developed postoperatively. Therefore, we propose that umbilical hernias may be a type of ‘field defect’ and we support the idea of that abnormal collegan metabolism could play a role in hernia development. ...We speculate that surgeons might be more inclined to use mesh in a patients with a history of other hernias.”
Despite the potential for complications, “elective hernia repair with mesh should be considered in patients with multiple comorbidities given that the use of mesh offers protection from recurrence without major morbidity.”
The study was supported by the VA Healthcare System. No conflicts of interest were declared.
Patients with umbilical hernias and multiple comorbidities should be considered for mesh repair to reduce the risk of recurrence, say the authors of a study of the factors associated with umbilical hernia recurrence after repair.
The retrospective cohort study, published in JAMA Surgery, examined recurrence and mortality outcomes in 332 military veteran patients who underwent primary umbilical hernia repair between 1998 and 2008 and followed until June 2014.
The overall recurrence rate was 6% and a mean recurrence time after index repair of 3.1 years. The recurrence rate was significantly higher among patients who underwent primary suture repair, compared with those who underwent mesh repair (9.8% vs. 2.4%, P = .04). Mesh repair decreased the risk of recurrence more than threefold, compared with primary suture repair (odds ratio, 0.28; 95% confidence interval, 0.08-0.95).
Patients with ascites had a significantly greater risk of recurrence, compared with those without ascites (20% vs. 5.1%, P = .02), as did those with liver disease (35% vs. 13.8%, P = .02), and diabetes (55% vs. 32.7%).
“This information suggests that umbilical hernias should be repaired using mesh, especially if a patient has multiple comorbidities that are significantly associated with recurrence, such as obesity, diabetes, liver disease, and ascites,” wrote Divya A. Shankar, a medical student at Boston University School of Medicine, and her coauthors (JAMA Surg. 2017 Jan 25. doi: 10.1001/jamasurg.2016.5052).
Among these patients, only 1 died within 30 days, but the study captured mortality in this group over 6-16 years after their hernia surgery. Mortality rate in the group was 27% through the follow-up period. However, older individuals, smokers, patients with liver disease and ascites, and those who had to undergo emergency or semi-urgent repair or who required intraoperative bowel resection had significantly increased long-term mortality rates.
“Although there is a trend toward higher mortality rates in patients who underwent emergency repair, it is difficult to interpret whether the deaths were related to the emergency or to underlying medical conditions given that the etiology of the majority of these deaths is unknown,” the authors wrote.
Among the deaths, 43 (48%) were from unknown causes, 18 (20%) were cancer related, 12 (13%) were related to renal disease, and 3 (3.3%) were related to sepsis.
Researchers found that patients with a history of hernias were significantly less likely to have an umbilical hernia recurrence, although a greater percentage of these patients – 61% – received mesh in contrast to the 44% of patients without a history of hernia.
Defect size did not appear to affect the rate of recurrence but the authors noted that defect size was only recorded in about half of the patients in the study.
“Because there was no significant difference between these groups, we are unable to conclude whether the size of defects should play a role in a surgeon’s decision to use mesh,” they wrote.
Sixty-one patients (18%) had at least one complication within 30 days of the repair, with the most common being seroma (9.6%), surgical site infection (6.9%) and hematoma (2.4%). Two patients experienced a mesh infection and three experienced ascites leaks. The rate of complications was slightly, but not significantly, higher in the patients who received mesh repair, compared with the primary suture repair.
“A surprise finding was that patient who underwent emergent or semi-emergent repair had a 2.2 times increased odds of death. Twenty deaths occurred more than 30 days after emergent or semi-urgent repair and 12 (60%) were secondary to unknown causes and 5 (25%) were secondary to liver disease,” the investigators noted.
“Interestingly, 193 (58%) patients who underwent umbilical hernia repair had other hernias that were either repaired before the index repair or developed postoperatively. Therefore, we propose that umbilical hernias may be a type of ‘field defect’ and we support the idea of that abnormal collegan metabolism could play a role in hernia development. ...We speculate that surgeons might be more inclined to use mesh in a patients with a history of other hernias.”
Despite the potential for complications, “elective hernia repair with mesh should be considered in patients with multiple comorbidities given that the use of mesh offers protection from recurrence without major morbidity.”
The study was supported by the VA Healthcare System. No conflicts of interest were declared.
Patients with umbilical hernias and multiple comorbidities should be considered for mesh repair to reduce the risk of recurrence, say the authors of a study of the factors associated with umbilical hernia recurrence after repair.
The retrospective cohort study, published in JAMA Surgery, examined recurrence and mortality outcomes in 332 military veteran patients who underwent primary umbilical hernia repair between 1998 and 2008 and followed until June 2014.
The overall recurrence rate was 6% and a mean recurrence time after index repair of 3.1 years. The recurrence rate was significantly higher among patients who underwent primary suture repair, compared with those who underwent mesh repair (9.8% vs. 2.4%, P = .04). Mesh repair decreased the risk of recurrence more than threefold, compared with primary suture repair (odds ratio, 0.28; 95% confidence interval, 0.08-0.95).
Patients with ascites had a significantly greater risk of recurrence, compared with those without ascites (20% vs. 5.1%, P = .02), as did those with liver disease (35% vs. 13.8%, P = .02), and diabetes (55% vs. 32.7%).
“This information suggests that umbilical hernias should be repaired using mesh, especially if a patient has multiple comorbidities that are significantly associated with recurrence, such as obesity, diabetes, liver disease, and ascites,” wrote Divya A. Shankar, a medical student at Boston University School of Medicine, and her coauthors (JAMA Surg. 2017 Jan 25. doi: 10.1001/jamasurg.2016.5052).
Among these patients, only 1 died within 30 days, but the study captured mortality in this group over 6-16 years after their hernia surgery. Mortality rate in the group was 27% through the follow-up period. However, older individuals, smokers, patients with liver disease and ascites, and those who had to undergo emergency or semi-urgent repair or who required intraoperative bowel resection had significantly increased long-term mortality rates.
“Although there is a trend toward higher mortality rates in patients who underwent emergency repair, it is difficult to interpret whether the deaths were related to the emergency or to underlying medical conditions given that the etiology of the majority of these deaths is unknown,” the authors wrote.
Among the deaths, 43 (48%) were from unknown causes, 18 (20%) were cancer related, 12 (13%) were related to renal disease, and 3 (3.3%) were related to sepsis.
Researchers found that patients with a history of hernias were significantly less likely to have an umbilical hernia recurrence, although a greater percentage of these patients – 61% – received mesh in contrast to the 44% of patients without a history of hernia.
Defect size did not appear to affect the rate of recurrence but the authors noted that defect size was only recorded in about half of the patients in the study.
“Because there was no significant difference between these groups, we are unable to conclude whether the size of defects should play a role in a surgeon’s decision to use mesh,” they wrote.
Sixty-one patients (18%) had at least one complication within 30 days of the repair, with the most common being seroma (9.6%), surgical site infection (6.9%) and hematoma (2.4%). Two patients experienced a mesh infection and three experienced ascites leaks. The rate of complications was slightly, but not significantly, higher in the patients who received mesh repair, compared with the primary suture repair.
“A surprise finding was that patient who underwent emergent or semi-emergent repair had a 2.2 times increased odds of death. Twenty deaths occurred more than 30 days after emergent or semi-urgent repair and 12 (60%) were secondary to unknown causes and 5 (25%) were secondary to liver disease,” the investigators noted.
“Interestingly, 193 (58%) patients who underwent umbilical hernia repair had other hernias that were either repaired before the index repair or developed postoperatively. Therefore, we propose that umbilical hernias may be a type of ‘field defect’ and we support the idea of that abnormal collegan metabolism could play a role in hernia development. ...We speculate that surgeons might be more inclined to use mesh in a patients with a history of other hernias.”
Despite the potential for complications, “elective hernia repair with mesh should be considered in patients with multiple comorbidities given that the use of mesh offers protection from recurrence without major morbidity.”
The study was supported by the VA Healthcare System. No conflicts of interest were declared.
FROM JAMA SURGERY
Updated SSI prevention guidance highlights glucose control, MRSA
The guidelines for controlling surgical site infections have been updated to reflect evidence-based findings of a collaboration between surgeons and infection control experts from the American College of Surgeons, the ACS National Surgical Quality Improvement Program, and the Surgical Infection Society.
Updated strategies to reduce the risk of surgical site infections (SSIs) include perioperative glucose control in all patients and the use of oral antibiotics as an element of colon procedures, according to guidelines published in Journal of the American College of Surgeons (J Am Coll Surg. 2017;224:59-74).
Surgical site infections now account for 20% of all hospital-acquired infections, wrote lead author Kristen A. Ban, MD, a surgical resident at Loyola University Medical Center, Maywood, Ill., and her colleagues.
The most recent guidelines for preventing surgical site infections came from the Centers for Disease Control and Prevention in 1999; “the CDC has been working on an update since 2011, but this has been incredibly slow,” E. Patchen Dellinger, MD, of the University of Washington, Seattle, one of the guidelines’ authors, said in an interview. “A publication should be coming out sometime this year, but in the meantime, it was useful to have something for clinicians to refer to,” he said.
The researchers used PubMed to review specific topics in the SSI literature and address knowledge gaps.
Based on their findings, the new guidelines add recommendations to previous versions that address SSI prevention in the prehospital setting, at the hospital, and after discharge. The level of evidence to support each guideline varies; the researchers strongly recommend certain points, such as perioperative glucose control for all patients, not only those with diabetes; other recommendations such as postoperative showering 12 hours after surgery vs. delayed showering are left to the surgeon’s discretion.
“The changes/new recommendations since the 1999 guideline include the recommendation for the use of oral antibiotics with mechanical bowel prep for colon operations (in combination with intravenous prophylactic antibiotics), the control of perioperative glucose levels in ALL patients (not just diabetics), the maintenance of normothermia in the OR, the use of wound protectors for clean-contaminated cases, the use of antimicrobial sutures, and the use of increased FiO2 levels for intubated patients,” Dr. Dellinger said. These new elements also will be recommended when the updated CDC guidelines are released, and already have been recommended in recent guidelines from the World Health Organization, he added.
Guidelines for prehospital interventions include smoking cessation 4-6 weeks before surgery, preoperative bathing with chlorhexidine, glucose control for diabetes patients, MRSA screening, and bowel preparation (combining mechanical and antibiotic) for all elective colectomies.
Recommended hospital interventions include the following:
• Intraoperative normothermia.
• Use of wound protectors in open abdominal surgery.
• Use of triclosan antibiotic sutures.
• Supplemental oxygen.
• Antibiotic prophylaxis when indicated.
• Glucose control for all patients perioperatively.
• Hair removal only when necessary, avoiding a razor if possible.
• Alcohol-based skin preparation when possible.
• Surgical hand scrub.
• Facility scrub laundering and use of a skull cap if minimal hair is exposed.
• Use of double gloves and changing gloves before incision closure in colorectal cases.
• Use of new instruments for closure in colorectal cases.
• Purse string closure of stoma sites.
• Use of topical antibiotics as part of wound care.
• Using wound vacuum therapy over stapled skin.
Data on interventions after hospital discharge that may reduce SSI incidence are limited, the researchers said. No specific wound care protocols or surveillance methods have been identified. However, “promising new methods of surveillance are being explored, many of which use smartphone technology to help patients send their surgeon daily photos or updates,” they noted.
“Strategies to decrease SSI are multimodal and occur across a range of settings under the supervision of numerous providers,” the researchers wrote. “Ensuring high compliance with these risk-reduction strategies is crucial to the success of SSI reduction efforts,” they added.
However, changes to surgical practice don’t happen overnight, Dr. Dellinger said. “If all of these are actually adapted it should decrease SSI rates in all areas,” he noted. “Oral antibiotics for colorectal cases and glucose control for all patients will probably make the biggest benefit if actually adopted,” he said.
“We could use some better studies on the precise timing of parenteral prophylactic antibiotics,” said Dr. Dellinger. “One such study has been submitted from Switzerland and should be published sometime this year. Hard evidence on the best timing is missing although observational data allows some of us to come to conclusions on that,” he said. “Additional studies on perioperative oxygenation where fluid management and temperature management are better controlled would be helpful, and more and better studies are need for antimicrobial sutures,” he added.
The authors had nothing to disclose relevant to the scope of the guidelines. Outside the scope of this work, Dr. Dellinger disclosed serving on the advisory boards for 3M, Melinta, and Theravance, as well as receiving a grant from Motif for a clinical trial of iclaprim vs. vancomycin for the treatment of skin and soft tissue infections.
The guidelines for controlling surgical site infections have been updated to reflect evidence-based findings of a collaboration between surgeons and infection control experts from the American College of Surgeons, the ACS National Surgical Quality Improvement Program, and the Surgical Infection Society.
Updated strategies to reduce the risk of surgical site infections (SSIs) include perioperative glucose control in all patients and the use of oral antibiotics as an element of colon procedures, according to guidelines published in Journal of the American College of Surgeons (J Am Coll Surg. 2017;224:59-74).
Surgical site infections now account for 20% of all hospital-acquired infections, wrote lead author Kristen A. Ban, MD, a surgical resident at Loyola University Medical Center, Maywood, Ill., and her colleagues.
The most recent guidelines for preventing surgical site infections came from the Centers for Disease Control and Prevention in 1999; “the CDC has been working on an update since 2011, but this has been incredibly slow,” E. Patchen Dellinger, MD, of the University of Washington, Seattle, one of the guidelines’ authors, said in an interview. “A publication should be coming out sometime this year, but in the meantime, it was useful to have something for clinicians to refer to,” he said.
The researchers used PubMed to review specific topics in the SSI literature and address knowledge gaps.
Based on their findings, the new guidelines add recommendations to previous versions that address SSI prevention in the prehospital setting, at the hospital, and after discharge. The level of evidence to support each guideline varies; the researchers strongly recommend certain points, such as perioperative glucose control for all patients, not only those with diabetes; other recommendations such as postoperative showering 12 hours after surgery vs. delayed showering are left to the surgeon’s discretion.
“The changes/new recommendations since the 1999 guideline include the recommendation for the use of oral antibiotics with mechanical bowel prep for colon operations (in combination with intravenous prophylactic antibiotics), the control of perioperative glucose levels in ALL patients (not just diabetics), the maintenance of normothermia in the OR, the use of wound protectors for clean-contaminated cases, the use of antimicrobial sutures, and the use of increased FiO2 levels for intubated patients,” Dr. Dellinger said. These new elements also will be recommended when the updated CDC guidelines are released, and already have been recommended in recent guidelines from the World Health Organization, he added.
Guidelines for prehospital interventions include smoking cessation 4-6 weeks before surgery, preoperative bathing with chlorhexidine, glucose control for diabetes patients, MRSA screening, and bowel preparation (combining mechanical and antibiotic) for all elective colectomies.
Recommended hospital interventions include the following:
• Intraoperative normothermia.
• Use of wound protectors in open abdominal surgery.
• Use of triclosan antibiotic sutures.
• Supplemental oxygen.
• Antibiotic prophylaxis when indicated.
• Glucose control for all patients perioperatively.
• Hair removal only when necessary, avoiding a razor if possible.
• Alcohol-based skin preparation when possible.
• Surgical hand scrub.
• Facility scrub laundering and use of a skull cap if minimal hair is exposed.
• Use of double gloves and changing gloves before incision closure in colorectal cases.
• Use of new instruments for closure in colorectal cases.
• Purse string closure of stoma sites.
• Use of topical antibiotics as part of wound care.
• Using wound vacuum therapy over stapled skin.
Data on interventions after hospital discharge that may reduce SSI incidence are limited, the researchers said. No specific wound care protocols or surveillance methods have been identified. However, “promising new methods of surveillance are being explored, many of which use smartphone technology to help patients send their surgeon daily photos or updates,” they noted.
“Strategies to decrease SSI are multimodal and occur across a range of settings under the supervision of numerous providers,” the researchers wrote. “Ensuring high compliance with these risk-reduction strategies is crucial to the success of SSI reduction efforts,” they added.
However, changes to surgical practice don’t happen overnight, Dr. Dellinger said. “If all of these are actually adapted it should decrease SSI rates in all areas,” he noted. “Oral antibiotics for colorectal cases and glucose control for all patients will probably make the biggest benefit if actually adopted,” he said.
“We could use some better studies on the precise timing of parenteral prophylactic antibiotics,” said Dr. Dellinger. “One such study has been submitted from Switzerland and should be published sometime this year. Hard evidence on the best timing is missing although observational data allows some of us to come to conclusions on that,” he said. “Additional studies on perioperative oxygenation where fluid management and temperature management are better controlled would be helpful, and more and better studies are need for antimicrobial sutures,” he added.
The authors had nothing to disclose relevant to the scope of the guidelines. Outside the scope of this work, Dr. Dellinger disclosed serving on the advisory boards for 3M, Melinta, and Theravance, as well as receiving a grant from Motif for a clinical trial of iclaprim vs. vancomycin for the treatment of skin and soft tissue infections.
The guidelines for controlling surgical site infections have been updated to reflect evidence-based findings of a collaboration between surgeons and infection control experts from the American College of Surgeons, the ACS National Surgical Quality Improvement Program, and the Surgical Infection Society.
Updated strategies to reduce the risk of surgical site infections (SSIs) include perioperative glucose control in all patients and the use of oral antibiotics as an element of colon procedures, according to guidelines published in Journal of the American College of Surgeons (J Am Coll Surg. 2017;224:59-74).
Surgical site infections now account for 20% of all hospital-acquired infections, wrote lead author Kristen A. Ban, MD, a surgical resident at Loyola University Medical Center, Maywood, Ill., and her colleagues.
The most recent guidelines for preventing surgical site infections came from the Centers for Disease Control and Prevention in 1999; “the CDC has been working on an update since 2011, but this has been incredibly slow,” E. Patchen Dellinger, MD, of the University of Washington, Seattle, one of the guidelines’ authors, said in an interview. “A publication should be coming out sometime this year, but in the meantime, it was useful to have something for clinicians to refer to,” he said.
The researchers used PubMed to review specific topics in the SSI literature and address knowledge gaps.
Based on their findings, the new guidelines add recommendations to previous versions that address SSI prevention in the prehospital setting, at the hospital, and after discharge. The level of evidence to support each guideline varies; the researchers strongly recommend certain points, such as perioperative glucose control for all patients, not only those with diabetes; other recommendations such as postoperative showering 12 hours after surgery vs. delayed showering are left to the surgeon’s discretion.
“The changes/new recommendations since the 1999 guideline include the recommendation for the use of oral antibiotics with mechanical bowel prep for colon operations (in combination with intravenous prophylactic antibiotics), the control of perioperative glucose levels in ALL patients (not just diabetics), the maintenance of normothermia in the OR, the use of wound protectors for clean-contaminated cases, the use of antimicrobial sutures, and the use of increased FiO2 levels for intubated patients,” Dr. Dellinger said. These new elements also will be recommended when the updated CDC guidelines are released, and already have been recommended in recent guidelines from the World Health Organization, he added.
Guidelines for prehospital interventions include smoking cessation 4-6 weeks before surgery, preoperative bathing with chlorhexidine, glucose control for diabetes patients, MRSA screening, and bowel preparation (combining mechanical and antibiotic) for all elective colectomies.
Recommended hospital interventions include the following:
• Intraoperative normothermia.
• Use of wound protectors in open abdominal surgery.
• Use of triclosan antibiotic sutures.
• Supplemental oxygen.
• Antibiotic prophylaxis when indicated.
• Glucose control for all patients perioperatively.
• Hair removal only when necessary, avoiding a razor if possible.
• Alcohol-based skin preparation when possible.
• Surgical hand scrub.
• Facility scrub laundering and use of a skull cap if minimal hair is exposed.
• Use of double gloves and changing gloves before incision closure in colorectal cases.
• Use of new instruments for closure in colorectal cases.
• Purse string closure of stoma sites.
• Use of topical antibiotics as part of wound care.
• Using wound vacuum therapy over stapled skin.
Data on interventions after hospital discharge that may reduce SSI incidence are limited, the researchers said. No specific wound care protocols or surveillance methods have been identified. However, “promising new methods of surveillance are being explored, many of which use smartphone technology to help patients send their surgeon daily photos or updates,” they noted.
“Strategies to decrease SSI are multimodal and occur across a range of settings under the supervision of numerous providers,” the researchers wrote. “Ensuring high compliance with these risk-reduction strategies is crucial to the success of SSI reduction efforts,” they added.
However, changes to surgical practice don’t happen overnight, Dr. Dellinger said. “If all of these are actually adapted it should decrease SSI rates in all areas,” he noted. “Oral antibiotics for colorectal cases and glucose control for all patients will probably make the biggest benefit if actually adopted,” he said.
“We could use some better studies on the precise timing of parenteral prophylactic antibiotics,” said Dr. Dellinger. “One such study has been submitted from Switzerland and should be published sometime this year. Hard evidence on the best timing is missing although observational data allows some of us to come to conclusions on that,” he said. “Additional studies on perioperative oxygenation where fluid management and temperature management are better controlled would be helpful, and more and better studies are need for antimicrobial sutures,” he added.
The authors had nothing to disclose relevant to the scope of the guidelines. Outside the scope of this work, Dr. Dellinger disclosed serving on the advisory boards for 3M, Melinta, and Theravance, as well as receiving a grant from Motif for a clinical trial of iclaprim vs. vancomycin for the treatment of skin and soft tissue infections.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Prediction: LVADs will rule end-stage heart failure
SNOWMASS, COLO. – Multifaceted progress in mechanical circulatory support as long-term therapy in end-stage heart failure is happening at a brisk pace, Y. Joseph C. Woo, MD, reported at the Annual Cardiovascular Conference at Snowmass.
declared Dr. Woo, professor and chair of the department of cardiothoracic surgery at Stanford (Calif.) University.
That’s quite a prediction, especially considering the source: Stanford is where the late Dr. Norman Shumway – widely considered “the father of heart transplantation” – performed the first adult heart transplant in the United States in 1968.
Dr. Woo was coauthor of an American Heart Association policy statement on the future of cardiovascular disease in the United States, which forecast a 25% increase in heart failure between 2010 and 2030 (Circulation. 2011 Mar 1;123[8]:933-44). There is simply no way that heart transplantation can begin to meet the projected growing need for effective therapy in patients with end-stage disease.
Here’s what Dr. Woo sees as the future of MCS:
Minimally invasive implantation
At Stanford, LVAD implantations are now routinely done off-pump on a beating heart.
“We clamp only when there is a sound reason, like the presence of left ventricular thrombus, where you run the risk of embolization without the cross clamp,” the surgeon said.
Concomitant valvular surgery
At Stanford and other centers of excellence, surgeons perform additional procedures as warranted while they implant an LVAD, including atrial fibrillation ablation, revascularization of the right heart coronaries, patent foramen ovale closure, and repair of the tricuspid, pulmonic, or aortic valves.
Enhanced right ventricular management
Survival is greatly impaired if a patient with an LVAD later requires the addition of a right ventricular assist device. This realization has led to the development of multiple preoperative risk scoring systems by the Stanford group (Ann Thorac Surg. 2013 Sep;96[3]:857-63) and others, including investigators at the Deutsche Herzzentrum Berlin, the world’s busiest heart transplant center. The purpose is to identify upfront those patients who are likely to later develop right heart failure so they can receive biventricular MCS from the start.
Adjunctive biologic therapies
Intramyocardial injection of 25 million allogeneic mesenchymal precursor cells during LVAD implantation appeared to be safe and showed a promising efficacy signal in a 30-patient, multicenter, double-blind, placebo-controlled, National Institutes of Health–sponsored proof of concept study in which Dr. Woo was a coinvestigator (Circulation. 2014 Jun 3;129[22]:2287-96).
The goal of this research effort is to provide a cell therapy assist to the LVAD as a bridge to recovery of left ventricular function such that the device might eventually no longer be needed, he explained.
These cells are immune privileged. They can be transplanted into recipients without need for immunosuppressive therapy or HLA matching, basically as an off the shelf product. Rather than transforming into cardiomyocytes, it appears that the mechanism by which the donor cells enhance cardiac performance in heart failure is via secretion of a shower of growth and angiogenic factors.
Based upon the encouraging results of the initial study, a 90-patient, phase II, double-blind clinical trial is underway. In order to better evaluate efficacy, this time the patients will receive 150 million mesenchymal precursor cells rather than 25 million.
New technologies
The developmental pipeline is chock full of MCS devices. The trend is to go smaller and simpler. HeartWare is developing a miniaturized version of its approved continuous flow centrifugal force LVAD. The ReliantHeart aVAD, an intraventricular device less than 2.5 cm in diameter, is approved in Europe and under study in the U.S. The Thoratec HeartMate III is a smaller version of the HeartMate II, which is FDA-approved as destination therapy. And the Circulite Synergy micropump, designed to provide partial circulatory support to patients who don’t require a full-force LVAD, is the size of a AA battery.
Dr. Woo reported having no financial conflicts.
[email protected]
SNOWMASS, COLO. – Multifaceted progress in mechanical circulatory support as long-term therapy in end-stage heart failure is happening at a brisk pace, Y. Joseph C. Woo, MD, reported at the Annual Cardiovascular Conference at Snowmass.
declared Dr. Woo, professor and chair of the department of cardiothoracic surgery at Stanford (Calif.) University.
That’s quite a prediction, especially considering the source: Stanford is where the late Dr. Norman Shumway – widely considered “the father of heart transplantation” – performed the first adult heart transplant in the United States in 1968.
Dr. Woo was coauthor of an American Heart Association policy statement on the future of cardiovascular disease in the United States, which forecast a 25% increase in heart failure between 2010 and 2030 (Circulation. 2011 Mar 1;123[8]:933-44). There is simply no way that heart transplantation can begin to meet the projected growing need for effective therapy in patients with end-stage disease.
Here’s what Dr. Woo sees as the future of MCS:
Minimally invasive implantation
At Stanford, LVAD implantations are now routinely done off-pump on a beating heart.
“We clamp only when there is a sound reason, like the presence of left ventricular thrombus, where you run the risk of embolization without the cross clamp,” the surgeon said.
Concomitant valvular surgery
At Stanford and other centers of excellence, surgeons perform additional procedures as warranted while they implant an LVAD, including atrial fibrillation ablation, revascularization of the right heart coronaries, patent foramen ovale closure, and repair of the tricuspid, pulmonic, or aortic valves.
Enhanced right ventricular management
Survival is greatly impaired if a patient with an LVAD later requires the addition of a right ventricular assist device. This realization has led to the development of multiple preoperative risk scoring systems by the Stanford group (Ann Thorac Surg. 2013 Sep;96[3]:857-63) and others, including investigators at the Deutsche Herzzentrum Berlin, the world’s busiest heart transplant center. The purpose is to identify upfront those patients who are likely to later develop right heart failure so they can receive biventricular MCS from the start.
Adjunctive biologic therapies
Intramyocardial injection of 25 million allogeneic mesenchymal precursor cells during LVAD implantation appeared to be safe and showed a promising efficacy signal in a 30-patient, multicenter, double-blind, placebo-controlled, National Institutes of Health–sponsored proof of concept study in which Dr. Woo was a coinvestigator (Circulation. 2014 Jun 3;129[22]:2287-96).
The goal of this research effort is to provide a cell therapy assist to the LVAD as a bridge to recovery of left ventricular function such that the device might eventually no longer be needed, he explained.
These cells are immune privileged. They can be transplanted into recipients without need for immunosuppressive therapy or HLA matching, basically as an off the shelf product. Rather than transforming into cardiomyocytes, it appears that the mechanism by which the donor cells enhance cardiac performance in heart failure is via secretion of a shower of growth and angiogenic factors.
Based upon the encouraging results of the initial study, a 90-patient, phase II, double-blind clinical trial is underway. In order to better evaluate efficacy, this time the patients will receive 150 million mesenchymal precursor cells rather than 25 million.
New technologies
The developmental pipeline is chock full of MCS devices. The trend is to go smaller and simpler. HeartWare is developing a miniaturized version of its approved continuous flow centrifugal force LVAD. The ReliantHeart aVAD, an intraventricular device less than 2.5 cm in diameter, is approved in Europe and under study in the U.S. The Thoratec HeartMate III is a smaller version of the HeartMate II, which is FDA-approved as destination therapy. And the Circulite Synergy micropump, designed to provide partial circulatory support to patients who don’t require a full-force LVAD, is the size of a AA battery.
Dr. Woo reported having no financial conflicts.
[email protected]
SNOWMASS, COLO. – Multifaceted progress in mechanical circulatory support as long-term therapy in end-stage heart failure is happening at a brisk pace, Y. Joseph C. Woo, MD, reported at the Annual Cardiovascular Conference at Snowmass.
declared Dr. Woo, professor and chair of the department of cardiothoracic surgery at Stanford (Calif.) University.
That’s quite a prediction, especially considering the source: Stanford is where the late Dr. Norman Shumway – widely considered “the father of heart transplantation” – performed the first adult heart transplant in the United States in 1968.
Dr. Woo was coauthor of an American Heart Association policy statement on the future of cardiovascular disease in the United States, which forecast a 25% increase in heart failure between 2010 and 2030 (Circulation. 2011 Mar 1;123[8]:933-44). There is simply no way that heart transplantation can begin to meet the projected growing need for effective therapy in patients with end-stage disease.
Here’s what Dr. Woo sees as the future of MCS:
Minimally invasive implantation
At Stanford, LVAD implantations are now routinely done off-pump on a beating heart.
“We clamp only when there is a sound reason, like the presence of left ventricular thrombus, where you run the risk of embolization without the cross clamp,” the surgeon said.
Concomitant valvular surgery
At Stanford and other centers of excellence, surgeons perform additional procedures as warranted while they implant an LVAD, including atrial fibrillation ablation, revascularization of the right heart coronaries, patent foramen ovale closure, and repair of the tricuspid, pulmonic, or aortic valves.
Enhanced right ventricular management
Survival is greatly impaired if a patient with an LVAD later requires the addition of a right ventricular assist device. This realization has led to the development of multiple preoperative risk scoring systems by the Stanford group (Ann Thorac Surg. 2013 Sep;96[3]:857-63) and others, including investigators at the Deutsche Herzzentrum Berlin, the world’s busiest heart transplant center. The purpose is to identify upfront those patients who are likely to later develop right heart failure so they can receive biventricular MCS from the start.
Adjunctive biologic therapies
Intramyocardial injection of 25 million allogeneic mesenchymal precursor cells during LVAD implantation appeared to be safe and showed a promising efficacy signal in a 30-patient, multicenter, double-blind, placebo-controlled, National Institutes of Health–sponsored proof of concept study in which Dr. Woo was a coinvestigator (Circulation. 2014 Jun 3;129[22]:2287-96).
The goal of this research effort is to provide a cell therapy assist to the LVAD as a bridge to recovery of left ventricular function such that the device might eventually no longer be needed, he explained.
These cells are immune privileged. They can be transplanted into recipients without need for immunosuppressive therapy or HLA matching, basically as an off the shelf product. Rather than transforming into cardiomyocytes, it appears that the mechanism by which the donor cells enhance cardiac performance in heart failure is via secretion of a shower of growth and angiogenic factors.
Based upon the encouraging results of the initial study, a 90-patient, phase II, double-blind clinical trial is underway. In order to better evaluate efficacy, this time the patients will receive 150 million mesenchymal precursor cells rather than 25 million.
New technologies
The developmental pipeline is chock full of MCS devices. The trend is to go smaller and simpler. HeartWare is developing a miniaturized version of its approved continuous flow centrifugal force LVAD. The ReliantHeart aVAD, an intraventricular device less than 2.5 cm in diameter, is approved in Europe and under study in the U.S. The Thoratec HeartMate III is a smaller version of the HeartMate II, which is FDA-approved as destination therapy. And the Circulite Synergy micropump, designed to provide partial circulatory support to patients who don’t require a full-force LVAD, is the size of a AA battery.
Dr. Woo reported having no financial conflicts.
[email protected]
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Risk considerations for suicidal physicians
EXPERT ANALYSIS AT THE NPA PSYCHOPHARMACOLOGY UPDATE
LAS VEGAS – Michael F. Myers, MD, found a common thread in interviews with loved ones, friends, and colleagues of physicians who have taken their own lives: About 10%-15% of the decedents never sought help beforehand.
“They’ve gone from illness to death without any care,” Dr. Myers, a psychiatrist, said at the annual psychopharmacology update held by the Nevada Psychiatric Association.
In a new book based on the interviews, “Why Physicians Die by Suicide: Lessons Learned From Their Families and Others Who Cared” (Amazon, 2017), Dr. Myers combined stories from his own clinical practice and qualitative interviews with about 75 men and women to explore reasons why physicians might choose to take their own lives. “Our nonpsychiatric medical colleagues are really anxious to have information from us, because we’re living in an age of burnout; roughly 50% of U.S. physicians suffer from burnout,” he said. In addition to perspectives from bereaved loved ones and former medical colleagues, the book includes insights from others who are affected when doctors die by suicide: their medical training directors, employers, medical students, treating psychiatrists, and patients.
According to the American Foundation for Suicide Prevention, 300-400 physicians take their own lives every year, the equivalent of two to three medical school classes. “That’s a doctor a day we lose to suicide,” said Dr. Myers, a professor of clinical psychiatry at State University of New York, Brooklyn, who specializes in physician health. Compared with the general population, the suicide rate ratio is 2.27 among female physicians and 1.41 among male physicians (Am J Psychiatry. 2004;161[12]:2295-2302), and an estimated 85%-90% of those who carry out a suicide have a psychiatric illness such as major depressive disorder, bipolar disorder, alcohol use and substance use disorder, and borderline personality disorder. Other triggers common to physicians, Dr. Myers said, include other kinds of personality disorders, burnout, untreated anxiety disorders, substance/medication-induced depressive disorder (especially in clinicians who have been self-medicating), and posttraumatic stress disorder.
Additional risk considerations to keep in mind for physician patients include a family history of mood disorders, a sense of professional isolation, coping with lawsuits and/or medical license investigations, previous history of a depressive episode, and a previous suicide attempt. “Sometimes, it’s hard to get this information from a new physician who’s sitting opposite you,” Dr. Myers noted. “We mustn’t forget that there’s a lot of shame, embarrassment, and guilt that’s attached to previous suicide attempts.”
Suicide risk also is elevated for physicians with treatment-refractory psychiatric illness. “It troubles me when a physician is being treated by a generalist, the patient is not doing well, and he or she is not being referred for a second opinion or has never been referred to a psychopharmacologist,” he said. “It’s important that be done, because you know how difficult many of your patients can be. It’s important to have the expertise of a psychopharmacologist. I believe that physician patients will welcome that [second opinion], even if they have to travel 200 miles for it.”
The list of risk considerations for suicidal physicians also includes undiagnosed and untreated bipolar I or II disorder, rapid cycling bipolar disorder, and mixed affective states. “These are important things that can make our patients ill very quickly,” he said. Impulsivity is another consideration, as are severe sleep deprivation, circadian rhythm disturbances, and acute suicidal affective disturbance, which has emerged as a condition to consider in future revisions of DSM-5. “What you see in this condition is an individual becoming suicidal within minutes or hours,” Dr. Myers explained. “It’s usually an agitated state with insomnia and overvalued ideas or delusions that they are completely untreatable and hopeless. Physicians are no different than anyone else in that kind of state.” He emphasized that the stigma attached to mental illness in physicians is pernicious, because untreated mental illness is a key driver of suicide. After one of Dr. Myers’s patients took his own life, his son told Dr. Myers, “My father just hated being a patient. He felt so ashamed. I tried hard, too, but my support wasn’t enough.”
Inadequate treatment can occur for physician patients because of transference and countertransference dynamics “that muddle the treatment dyad,” Dr. Myers added. “We must be mindful of the many issues that are going on when we treat our own.”
In his 2005 book “Why People Die By Suicide,” psychologist Thomas Joiner, PhD, described three key reasons why people choose to take their own lives. The first is “perceived burdensomeness,” or a sense that one is a burden on others. “When I see it in my physician patients, it’s when they have a sense of being a burden on their family and feel they are no longer serving a purpose,” Dr. Myers said. The second reason is “failed belongingness,” or a sense that one does not belong to a valued social group. “This resonates with me,” Dr. Myers said. “Sometimes in therapy sessions, my physician patients will say ‘Don’t call me Dr. Smith anymore; I’m Mr. Smith.’ They’ve removed themselves from the field because they don’t feel like they belong anymore. That’s the unworthiness that physicians can feel.”
The third reason is “learned fearlessness,” or the acquired capability to enact self-injury. Dr. Myers likened it to “the kind of exposure to pain and fear that people also might learn through such experiences as mountain climbing, performing surgery, fighting in wars, or being afflicted with anorexia.”
If you suspect that a physician patient is engaged in suicidal thinking and planning, Dr. Myers advises framing your mental health assessment in the context of trust and mutual respect. “Please don’t be seduced by somebody who’s squeaky clean in the area of suicidality,” he said. “That individual may just not be sharing with you yet. You’re going to have to be firm and parental at times. There’s a lot of terror and shame that can lurk behind those symptomatic behaviors. That’s where you’ll use your expertise. I have found that there are many physicians out there who welcome you to go in that ‘dark place’ with them. For them to be able to share those scary thoughts with someone can enhance the therapeutic alliance.” The inquiry should include questions about potential means and methods, including access to firearms, stockpiled and/or self-prescribed medications, and medications ordered online or stolen/diverted from the workplace. Timely and careful documentation are essential, he said.
The plan for treating suicidal physicians should include obtaining old records and speaking with former treating professionals. “If you get pushback from the patient, say, ‘This is about me wanting to be thorough in my assessment and treatment plan with you,’ ” Dr. Myers said. “There is no substitute for a detailed mental status evaluation, collateral information, clinical intuition, experience, and consultation.” Hospitalizing the physician patient should be judicious and in consultation with others. “If you’re not sure, get a second opinion, because it can be life-saving,” he said, “but if done inappropriately, you can turn them off psychiatry for the rest of their lives. Make sure you’re not just panicking and worrying about some sort of medical-legal risk.”
In cases of split treatment, ensure regular contact with the psychotherapist and document all communication and any changes in status, medication, or psychotherapy modality change.
Dr. Myers cited many ways that psychiatrists can educate their colleagues about burnout, depression, and the risk of suicide. These include offering to give grand rounds or a lecture on the topic, serving on your local CME planning committee, joining your institute’s physician wellness team, serving on your state’s physician health program, and offering to facilitate a group for physicians after a physician colleague has died by suicide. “Postvention is prevention for the next generation,” Dr. Myers said, quoting Edwin S. Shneidman, PhD, founder of the American Association of Suicidology. “By taking care of ourselves and accepting the painful reality of physician suicide, we reach out to those left behind and make a difference. You become a change agent – someone who is part of the movement to stop doctors from killing themselves.”
Dr. Myers disclosed that he has received funding from the Medical Education Speakers Network.
EXPERT ANALYSIS AT THE NPA PSYCHOPHARMACOLOGY UPDATE
LAS VEGAS – Michael F. Myers, MD, found a common thread in interviews with loved ones, friends, and colleagues of physicians who have taken their own lives: About 10%-15% of the decedents never sought help beforehand.
“They’ve gone from illness to death without any care,” Dr. Myers, a psychiatrist, said at the annual psychopharmacology update held by the Nevada Psychiatric Association.
In a new book based on the interviews, “Why Physicians Die by Suicide: Lessons Learned From Their Families and Others Who Cared” (Amazon, 2017), Dr. Myers combined stories from his own clinical practice and qualitative interviews with about 75 men and women to explore reasons why physicians might choose to take their own lives. “Our nonpsychiatric medical colleagues are really anxious to have information from us, because we’re living in an age of burnout; roughly 50% of U.S. physicians suffer from burnout,” he said. In addition to perspectives from bereaved loved ones and former medical colleagues, the book includes insights from others who are affected when doctors die by suicide: their medical training directors, employers, medical students, treating psychiatrists, and patients.
According to the American Foundation for Suicide Prevention, 300-400 physicians take their own lives every year, the equivalent of two to three medical school classes. “That’s a doctor a day we lose to suicide,” said Dr. Myers, a professor of clinical psychiatry at State University of New York, Brooklyn, who specializes in physician health. Compared with the general population, the suicide rate ratio is 2.27 among female physicians and 1.41 among male physicians (Am J Psychiatry. 2004;161[12]:2295-2302), and an estimated 85%-90% of those who carry out a suicide have a psychiatric illness such as major depressive disorder, bipolar disorder, alcohol use and substance use disorder, and borderline personality disorder. Other triggers common to physicians, Dr. Myers said, include other kinds of personality disorders, burnout, untreated anxiety disorders, substance/medication-induced depressive disorder (especially in clinicians who have been self-medicating), and posttraumatic stress disorder.
Additional risk considerations to keep in mind for physician patients include a family history of mood disorders, a sense of professional isolation, coping with lawsuits and/or medical license investigations, previous history of a depressive episode, and a previous suicide attempt. “Sometimes, it’s hard to get this information from a new physician who’s sitting opposite you,” Dr. Myers noted. “We mustn’t forget that there’s a lot of shame, embarrassment, and guilt that’s attached to previous suicide attempts.”
Suicide risk also is elevated for physicians with treatment-refractory psychiatric illness. “It troubles me when a physician is being treated by a generalist, the patient is not doing well, and he or she is not being referred for a second opinion or has never been referred to a psychopharmacologist,” he said. “It’s important that be done, because you know how difficult many of your patients can be. It’s important to have the expertise of a psychopharmacologist. I believe that physician patients will welcome that [second opinion], even if they have to travel 200 miles for it.”
The list of risk considerations for suicidal physicians also includes undiagnosed and untreated bipolar I or II disorder, rapid cycling bipolar disorder, and mixed affective states. “These are important things that can make our patients ill very quickly,” he said. Impulsivity is another consideration, as are severe sleep deprivation, circadian rhythm disturbances, and acute suicidal affective disturbance, which has emerged as a condition to consider in future revisions of DSM-5. “What you see in this condition is an individual becoming suicidal within minutes or hours,” Dr. Myers explained. “It’s usually an agitated state with insomnia and overvalued ideas or delusions that they are completely untreatable and hopeless. Physicians are no different than anyone else in that kind of state.” He emphasized that the stigma attached to mental illness in physicians is pernicious, because untreated mental illness is a key driver of suicide. After one of Dr. Myers’s patients took his own life, his son told Dr. Myers, “My father just hated being a patient. He felt so ashamed. I tried hard, too, but my support wasn’t enough.”
Inadequate treatment can occur for physician patients because of transference and countertransference dynamics “that muddle the treatment dyad,” Dr. Myers added. “We must be mindful of the many issues that are going on when we treat our own.”
In his 2005 book “Why People Die By Suicide,” psychologist Thomas Joiner, PhD, described three key reasons why people choose to take their own lives. The first is “perceived burdensomeness,” or a sense that one is a burden on others. “When I see it in my physician patients, it’s when they have a sense of being a burden on their family and feel they are no longer serving a purpose,” Dr. Myers said. The second reason is “failed belongingness,” or a sense that one does not belong to a valued social group. “This resonates with me,” Dr. Myers said. “Sometimes in therapy sessions, my physician patients will say ‘Don’t call me Dr. Smith anymore; I’m Mr. Smith.’ They’ve removed themselves from the field because they don’t feel like they belong anymore. That’s the unworthiness that physicians can feel.”
The third reason is “learned fearlessness,” or the acquired capability to enact self-injury. Dr. Myers likened it to “the kind of exposure to pain and fear that people also might learn through such experiences as mountain climbing, performing surgery, fighting in wars, or being afflicted with anorexia.”
If you suspect that a physician patient is engaged in suicidal thinking and planning, Dr. Myers advises framing your mental health assessment in the context of trust and mutual respect. “Please don’t be seduced by somebody who’s squeaky clean in the area of suicidality,” he said. “That individual may just not be sharing with you yet. You’re going to have to be firm and parental at times. There’s a lot of terror and shame that can lurk behind those symptomatic behaviors. That’s where you’ll use your expertise. I have found that there are many physicians out there who welcome you to go in that ‘dark place’ with them. For them to be able to share those scary thoughts with someone can enhance the therapeutic alliance.” The inquiry should include questions about potential means and methods, including access to firearms, stockpiled and/or self-prescribed medications, and medications ordered online or stolen/diverted from the workplace. Timely and careful documentation are essential, he said.
The plan for treating suicidal physicians should include obtaining old records and speaking with former treating professionals. “If you get pushback from the patient, say, ‘This is about me wanting to be thorough in my assessment and treatment plan with you,’ ” Dr. Myers said. “There is no substitute for a detailed mental status evaluation, collateral information, clinical intuition, experience, and consultation.” Hospitalizing the physician patient should be judicious and in consultation with others. “If you’re not sure, get a second opinion, because it can be life-saving,” he said, “but if done inappropriately, you can turn them off psychiatry for the rest of their lives. Make sure you’re not just panicking and worrying about some sort of medical-legal risk.”
In cases of split treatment, ensure regular contact with the psychotherapist and document all communication and any changes in status, medication, or psychotherapy modality change.
Dr. Myers cited many ways that psychiatrists can educate their colleagues about burnout, depression, and the risk of suicide. These include offering to give grand rounds or a lecture on the topic, serving on your local CME planning committee, joining your institute’s physician wellness team, serving on your state’s physician health program, and offering to facilitate a group for physicians after a physician colleague has died by suicide. “Postvention is prevention for the next generation,” Dr. Myers said, quoting Edwin S. Shneidman, PhD, founder of the American Association of Suicidology. “By taking care of ourselves and accepting the painful reality of physician suicide, we reach out to those left behind and make a difference. You become a change agent – someone who is part of the movement to stop doctors from killing themselves.”
Dr. Myers disclosed that he has received funding from the Medical Education Speakers Network.
EXPERT ANALYSIS AT THE NPA PSYCHOPHARMACOLOGY UPDATE
LAS VEGAS – Michael F. Myers, MD, found a common thread in interviews with loved ones, friends, and colleagues of physicians who have taken their own lives: About 10%-15% of the decedents never sought help beforehand.
“They’ve gone from illness to death without any care,” Dr. Myers, a psychiatrist, said at the annual psychopharmacology update held by the Nevada Psychiatric Association.
In a new book based on the interviews, “Why Physicians Die by Suicide: Lessons Learned From Their Families and Others Who Cared” (Amazon, 2017), Dr. Myers combined stories from his own clinical practice and qualitative interviews with about 75 men and women to explore reasons why physicians might choose to take their own lives. “Our nonpsychiatric medical colleagues are really anxious to have information from us, because we’re living in an age of burnout; roughly 50% of U.S. physicians suffer from burnout,” he said. In addition to perspectives from bereaved loved ones and former medical colleagues, the book includes insights from others who are affected when doctors die by suicide: their medical training directors, employers, medical students, treating psychiatrists, and patients.
According to the American Foundation for Suicide Prevention, 300-400 physicians take their own lives every year, the equivalent of two to three medical school classes. “That’s a doctor a day we lose to suicide,” said Dr. Myers, a professor of clinical psychiatry at State University of New York, Brooklyn, who specializes in physician health. Compared with the general population, the suicide rate ratio is 2.27 among female physicians and 1.41 among male physicians (Am J Psychiatry. 2004;161[12]:2295-2302), and an estimated 85%-90% of those who carry out a suicide have a psychiatric illness such as major depressive disorder, bipolar disorder, alcohol use and substance use disorder, and borderline personality disorder. Other triggers common to physicians, Dr. Myers said, include other kinds of personality disorders, burnout, untreated anxiety disorders, substance/medication-induced depressive disorder (especially in clinicians who have been self-medicating), and posttraumatic stress disorder.
Additional risk considerations to keep in mind for physician patients include a family history of mood disorders, a sense of professional isolation, coping with lawsuits and/or medical license investigations, previous history of a depressive episode, and a previous suicide attempt. “Sometimes, it’s hard to get this information from a new physician who’s sitting opposite you,” Dr. Myers noted. “We mustn’t forget that there’s a lot of shame, embarrassment, and guilt that’s attached to previous suicide attempts.”
Suicide risk also is elevated for physicians with treatment-refractory psychiatric illness. “It troubles me when a physician is being treated by a generalist, the patient is not doing well, and he or she is not being referred for a second opinion or has never been referred to a psychopharmacologist,” he said. “It’s important that be done, because you know how difficult many of your patients can be. It’s important to have the expertise of a psychopharmacologist. I believe that physician patients will welcome that [second opinion], even if they have to travel 200 miles for it.”
The list of risk considerations for suicidal physicians also includes undiagnosed and untreated bipolar I or II disorder, rapid cycling bipolar disorder, and mixed affective states. “These are important things that can make our patients ill very quickly,” he said. Impulsivity is another consideration, as are severe sleep deprivation, circadian rhythm disturbances, and acute suicidal affective disturbance, which has emerged as a condition to consider in future revisions of DSM-5. “What you see in this condition is an individual becoming suicidal within minutes or hours,” Dr. Myers explained. “It’s usually an agitated state with insomnia and overvalued ideas or delusions that they are completely untreatable and hopeless. Physicians are no different than anyone else in that kind of state.” He emphasized that the stigma attached to mental illness in physicians is pernicious, because untreated mental illness is a key driver of suicide. After one of Dr. Myers’s patients took his own life, his son told Dr. Myers, “My father just hated being a patient. He felt so ashamed. I tried hard, too, but my support wasn’t enough.”
Inadequate treatment can occur for physician patients because of transference and countertransference dynamics “that muddle the treatment dyad,” Dr. Myers added. “We must be mindful of the many issues that are going on when we treat our own.”
In his 2005 book “Why People Die By Suicide,” psychologist Thomas Joiner, PhD, described three key reasons why people choose to take their own lives. The first is “perceived burdensomeness,” or a sense that one is a burden on others. “When I see it in my physician patients, it’s when they have a sense of being a burden on their family and feel they are no longer serving a purpose,” Dr. Myers said. The second reason is “failed belongingness,” or a sense that one does not belong to a valued social group. “This resonates with me,” Dr. Myers said. “Sometimes in therapy sessions, my physician patients will say ‘Don’t call me Dr. Smith anymore; I’m Mr. Smith.’ They’ve removed themselves from the field because they don’t feel like they belong anymore. That’s the unworthiness that physicians can feel.”
The third reason is “learned fearlessness,” or the acquired capability to enact self-injury. Dr. Myers likened it to “the kind of exposure to pain and fear that people also might learn through such experiences as mountain climbing, performing surgery, fighting in wars, or being afflicted with anorexia.”
If you suspect that a physician patient is engaged in suicidal thinking and planning, Dr. Myers advises framing your mental health assessment in the context of trust and mutual respect. “Please don’t be seduced by somebody who’s squeaky clean in the area of suicidality,” he said. “That individual may just not be sharing with you yet. You’re going to have to be firm and parental at times. There’s a lot of terror and shame that can lurk behind those symptomatic behaviors. That’s where you’ll use your expertise. I have found that there are many physicians out there who welcome you to go in that ‘dark place’ with them. For them to be able to share those scary thoughts with someone can enhance the therapeutic alliance.” The inquiry should include questions about potential means and methods, including access to firearms, stockpiled and/or self-prescribed medications, and medications ordered online or stolen/diverted from the workplace. Timely and careful documentation are essential, he said.
The plan for treating suicidal physicians should include obtaining old records and speaking with former treating professionals. “If you get pushback from the patient, say, ‘This is about me wanting to be thorough in my assessment and treatment plan with you,’ ” Dr. Myers said. “There is no substitute for a detailed mental status evaluation, collateral information, clinical intuition, experience, and consultation.” Hospitalizing the physician patient should be judicious and in consultation with others. “If you’re not sure, get a second opinion, because it can be life-saving,” he said, “but if done inappropriately, you can turn them off psychiatry for the rest of their lives. Make sure you’re not just panicking and worrying about some sort of medical-legal risk.”
In cases of split treatment, ensure regular contact with the psychotherapist and document all communication and any changes in status, medication, or psychotherapy modality change.
Dr. Myers cited many ways that psychiatrists can educate their colleagues about burnout, depression, and the risk of suicide. These include offering to give grand rounds or a lecture on the topic, serving on your local CME planning committee, joining your institute’s physician wellness team, serving on your state’s physician health program, and offering to facilitate a group for physicians after a physician colleague has died by suicide. “Postvention is prevention for the next generation,” Dr. Myers said, quoting Edwin S. Shneidman, PhD, founder of the American Association of Suicidology. “By taking care of ourselves and accepting the painful reality of physician suicide, we reach out to those left behind and make a difference. You become a change agent – someone who is part of the movement to stop doctors from killing themselves.”
Dr. Myers disclosed that he has received funding from the Medical Education Speakers Network.
Onlay mesh with adhesive just as safe as sublay route
While using sublay mesh continues to be standard practice when performing ventral hernia repair (VHR), a recent study shows that using onlay mesh placement with adhesive can be just as safe, at least in the short term.
“While the use of mesh during VHR is well accepted, the ideal location of mesh placement remains heavily debated,” wrote the study’s authors, adding that the “paucity of high-level data has led the choice of mesh location to reside primarily on the preference of the surgeon rather than grounded in clinical outcomes.”
The investigators identified patients in the Americas Hernia Society Quality Collaborative national registry who were undergoing open, elective VHR and had clean wounds and a wound class I designation based on Centers for Disease Control and Prevention guidelines at any point between January 2013 and January 2016. A total of 1,854 individuals were ultimately selected for inclusion in the study and were divided into two groups: one that received traditional VHR with sublay mesh and one that received onlay mesh with adhesive.
All subjects’ data were analyzed within 30 days for any adverse events related to the wounds from the surgery. These events were surgical site infections, surgical site occurrences – which could include an infection and any skin or soft tissue ischemia, necrosis, or other events – and surgical site occurrences that required procedural intervention, which were defined as any occurrences that required “opening of the wound, wound debridement, suture excision, percutaneous drainage, or partial or complete mesh removal.”
The sublay cohort numbered 1,761 (95.0%), compared with 93 (5.0%) who received the onlay technique. There was no significant difference found in the rate of 30-day adverse incidents between the two cohorts. For surgical site infections, the sublay cohort rate was 2.9%, while the onlay cohort had a 5.5% rate (P = .30). Surgical site occurrences happened in 15.2% of sublay patients versus 7.7% of those in the other group (P = .08), while surgical site occurrences that required procedural intervention were 8.2% in sublay patients but 5.5% in onlay patients (P = .42).
While both approaches fared similarly in terms of comorbidities and average Ventral Hernia Working Group grade, the investigators recommend that “the Chevrel onlay technique be used in nonobese patients without significant comorbidities, with moderate hernia defects, and whose abdominal wall vasculatures are without risk of compromise.” The data were generalizable because of the number of surgeons performing VHR and because the data sample allowed the investigators to control for the hernia width, defect size, and patient comorbidities this case, leading to this conclusion.
“Additional studies are needed to determine the long-term benefits of both approaches with respect to mesh infection rates and hernia recurrence rates, as well as the ideal mesh location for ventral hernia repairs in higher-risk patients,” the authors concluded.
No funding source was disclosed for this study. The investigators reported no relevant financial disclosures.
While using sublay mesh continues to be standard practice when performing ventral hernia repair (VHR), a recent study shows that using onlay mesh placement with adhesive can be just as safe, at least in the short term.
“While the use of mesh during VHR is well accepted, the ideal location of mesh placement remains heavily debated,” wrote the study’s authors, adding that the “paucity of high-level data has led the choice of mesh location to reside primarily on the preference of the surgeon rather than grounded in clinical outcomes.”
The investigators identified patients in the Americas Hernia Society Quality Collaborative national registry who were undergoing open, elective VHR and had clean wounds and a wound class I designation based on Centers for Disease Control and Prevention guidelines at any point between January 2013 and January 2016. A total of 1,854 individuals were ultimately selected for inclusion in the study and were divided into two groups: one that received traditional VHR with sublay mesh and one that received onlay mesh with adhesive.
All subjects’ data were analyzed within 30 days for any adverse events related to the wounds from the surgery. These events were surgical site infections, surgical site occurrences – which could include an infection and any skin or soft tissue ischemia, necrosis, or other events – and surgical site occurrences that required procedural intervention, which were defined as any occurrences that required “opening of the wound, wound debridement, suture excision, percutaneous drainage, or partial or complete mesh removal.”
The sublay cohort numbered 1,761 (95.0%), compared with 93 (5.0%) who received the onlay technique. There was no significant difference found in the rate of 30-day adverse incidents between the two cohorts. For surgical site infections, the sublay cohort rate was 2.9%, while the onlay cohort had a 5.5% rate (P = .30). Surgical site occurrences happened in 15.2% of sublay patients versus 7.7% of those in the other group (P = .08), while surgical site occurrences that required procedural intervention were 8.2% in sublay patients but 5.5% in onlay patients (P = .42).
While both approaches fared similarly in terms of comorbidities and average Ventral Hernia Working Group grade, the investigators recommend that “the Chevrel onlay technique be used in nonobese patients without significant comorbidities, with moderate hernia defects, and whose abdominal wall vasculatures are without risk of compromise.” The data were generalizable because of the number of surgeons performing VHR and because the data sample allowed the investigators to control for the hernia width, defect size, and patient comorbidities this case, leading to this conclusion.
“Additional studies are needed to determine the long-term benefits of both approaches with respect to mesh infection rates and hernia recurrence rates, as well as the ideal mesh location for ventral hernia repairs in higher-risk patients,” the authors concluded.
No funding source was disclosed for this study. The investigators reported no relevant financial disclosures.
While using sublay mesh continues to be standard practice when performing ventral hernia repair (VHR), a recent study shows that using onlay mesh placement with adhesive can be just as safe, at least in the short term.
“While the use of mesh during VHR is well accepted, the ideal location of mesh placement remains heavily debated,” wrote the study’s authors, adding that the “paucity of high-level data has led the choice of mesh location to reside primarily on the preference of the surgeon rather than grounded in clinical outcomes.”
The investigators identified patients in the Americas Hernia Society Quality Collaborative national registry who were undergoing open, elective VHR and had clean wounds and a wound class I designation based on Centers for Disease Control and Prevention guidelines at any point between January 2013 and January 2016. A total of 1,854 individuals were ultimately selected for inclusion in the study and were divided into two groups: one that received traditional VHR with sublay mesh and one that received onlay mesh with adhesive.
All subjects’ data were analyzed within 30 days for any adverse events related to the wounds from the surgery. These events were surgical site infections, surgical site occurrences – which could include an infection and any skin or soft tissue ischemia, necrosis, or other events – and surgical site occurrences that required procedural intervention, which were defined as any occurrences that required “opening of the wound, wound debridement, suture excision, percutaneous drainage, or partial or complete mesh removal.”
The sublay cohort numbered 1,761 (95.0%), compared with 93 (5.0%) who received the onlay technique. There was no significant difference found in the rate of 30-day adverse incidents between the two cohorts. For surgical site infections, the sublay cohort rate was 2.9%, while the onlay cohort had a 5.5% rate (P = .30). Surgical site occurrences happened in 15.2% of sublay patients versus 7.7% of those in the other group (P = .08), while surgical site occurrences that required procedural intervention were 8.2% in sublay patients but 5.5% in onlay patients (P = .42).
While both approaches fared similarly in terms of comorbidities and average Ventral Hernia Working Group grade, the investigators recommend that “the Chevrel onlay technique be used in nonobese patients without significant comorbidities, with moderate hernia defects, and whose abdominal wall vasculatures are without risk of compromise.” The data were generalizable because of the number of surgeons performing VHR and because the data sample allowed the investigators to control for the hernia width, defect size, and patient comorbidities this case, leading to this conclusion.
“Additional studies are needed to determine the long-term benefits of both approaches with respect to mesh infection rates and hernia recurrence rates, as well as the ideal mesh location for ventral hernia repairs in higher-risk patients,” the authors concluded.
No funding source was disclosed for this study. The investigators reported no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point:
Major finding: No significant differences were found between onlay and sublay mesh VHR patients in terms of surgical site infection (P = .30), surgical site occurrences (P = .08), and surgical site occurrences that required intervention (P = .42).
Data source: Retrospective cohort study of 1,854 VHR patients between January 2013 and January 2016.
Disclosures: No funding source disclosed. Authors reported no relevant financial disclosures.
FIRST trial: Education unaffected by flexible duty hours
Flexible duty hour policies did not affect general surgery resident performance on examinations during the prospective cluster-randomized FIRST (Flexibility in Duty Hour Requirements for Surgical Trainees) trial.
However, more years under such policies may be required to observe an effect, Eddie Blay Jr., MD, of Northwestern University, Chicago, and his colleagues reported in the Journal of the American College of Surgeons.
Further, pass rates for the QE were 90.5% and 90.4%, and for the CE were 88.6% and 86.3%, respectively, they noted.
Residents from 117 programs participated in the FIRST trial. Those in the standard policy group had daily duty hour limits as outlined by current Accreditation Council for Graduate Medical Education requirements, with a limit of 16 hours daily for interns and 28 hours for senior residents, and with at least 8 hours between daily shifts and 14 hours off after a 24-hour call. Those in the flexible policy group were exempt from these limits by a formal waiver.
The findings suggest that flexible duty hour policies do not result in significantly different educational outcomes but are limited by concerns about the generalizability of the results, and by a focus on limited aspects of surgeon training and performance, the investigators said.
Measuring actual surgeon education and training quality is challenging and might not be possible with the ABSITE, QE, and CE, they added, noting that future FIRST trial analyses will examine the impact of multiple years of flexible duty hour policies.
The FIRST trial was funded by the American Board of Surgery, the American College of Surgeons, and the ACGME. Stipends for individual authors were supported by a National Institutes of Health grant. The authors reported having no other disclosures.
Flexible duty hour policies did not affect general surgery resident performance on examinations during the prospective cluster-randomized FIRST (Flexibility in Duty Hour Requirements for Surgical Trainees) trial.
However, more years under such policies may be required to observe an effect, Eddie Blay Jr., MD, of Northwestern University, Chicago, and his colleagues reported in the Journal of the American College of Surgeons.
Further, pass rates for the QE were 90.5% and 90.4%, and for the CE were 88.6% and 86.3%, respectively, they noted.
Residents from 117 programs participated in the FIRST trial. Those in the standard policy group had daily duty hour limits as outlined by current Accreditation Council for Graduate Medical Education requirements, with a limit of 16 hours daily for interns and 28 hours for senior residents, and with at least 8 hours between daily shifts and 14 hours off after a 24-hour call. Those in the flexible policy group were exempt from these limits by a formal waiver.
The findings suggest that flexible duty hour policies do not result in significantly different educational outcomes but are limited by concerns about the generalizability of the results, and by a focus on limited aspects of surgeon training and performance, the investigators said.
Measuring actual surgeon education and training quality is challenging and might not be possible with the ABSITE, QE, and CE, they added, noting that future FIRST trial analyses will examine the impact of multiple years of flexible duty hour policies.
The FIRST trial was funded by the American Board of Surgery, the American College of Surgeons, and the ACGME. Stipends for individual authors were supported by a National Institutes of Health grant. The authors reported having no other disclosures.
Flexible duty hour policies did not affect general surgery resident performance on examinations during the prospective cluster-randomized FIRST (Flexibility in Duty Hour Requirements for Surgical Trainees) trial.
However, more years under such policies may be required to observe an effect, Eddie Blay Jr., MD, of Northwestern University, Chicago, and his colleagues reported in the Journal of the American College of Surgeons.
Further, pass rates for the QE were 90.5% and 90.4%, and for the CE were 88.6% and 86.3%, respectively, they noted.
Residents from 117 programs participated in the FIRST trial. Those in the standard policy group had daily duty hour limits as outlined by current Accreditation Council for Graduate Medical Education requirements, with a limit of 16 hours daily for interns and 28 hours for senior residents, and with at least 8 hours between daily shifts and 14 hours off after a 24-hour call. Those in the flexible policy group were exempt from these limits by a formal waiver.
The findings suggest that flexible duty hour policies do not result in significantly different educational outcomes but are limited by concerns about the generalizability of the results, and by a focus on limited aspects of surgeon training and performance, the investigators said.
Measuring actual surgeon education and training quality is challenging and might not be possible with the ABSITE, QE, and CE, they added, noting that future FIRST trial analyses will examine the impact of multiple years of flexible duty hour policies.
The FIRST trial was funded by the American Board of Surgery, the American College of Surgeons, and the ACGME. Stipends for individual authors were supported by a National Institutes of Health grant. The authors reported having no other disclosures.
FROM JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point:
Major finding: Mean overall scores on the ABSITE in 2016 were 502.7 and 502.6 in the standard policy and flexible policy groups, respectively.
Data source: The prospective cluster-randomized FIRST trial of more than 4,300 residents.
Disclosures: The FIRST trial was funded by the American Board of Surgery, the American College of Surgeons, and the ACGME. Stipends for individual authors were supported by a National Institutes of Health grant. The authors reported having no other disclosures.
Hydrogel coils improve outcomes in medium-sized intracranial aneurysms
HOUSTON – Hydrogel coils significantly outperformed bare platinum coils in treating patients with medium-sized intracranial aneurysms, reducing the incidence of adverse outcomes by about 9% in an open-label, randomized trial.
Compared against the platinum coils, the self-expandable, hydrophilic coils scored significantly better on a composite endpoint of aneurysm recurrence at 18 months, re-treatment by 18 months, serious morbidity that prevented angiographic follow-up, and death, Christian Taschner, MD, said at the International Stroke Conference sponsored by the America Heart Association.
These are the company’s second-generation products, said Dr. Taschner, a neuroradiologist at University Hospital Freiburg (Germany). The initial hydrogel-coated coils were not well received because they were too stiff, he said in an interview.
The 18-month study comprised 513 patients and was conducted in 15 centers in France and 7 in Germany. Patients with medium-sized aneurysms (4-12 mm) were randomized to coiling with either the hydrogel coils or bare platinum coils and followed for 18 months. The cohort was stratified by rupture status in the analysis.
Angiographic images of the aneurysm were obtained before treatment, immediately after treatment, at 6 months, and at 18 months. All images were reviewed by an independent laboratory that was blinded to the treatment.
In addition to the primary composite outcome, the study assessed secondary endpoints of modified Rankin Scale (mRS) score at 18 months and coil packing density.
The final analysis included 484 patients. They were a mean of 52 years old, and about 70% were women. The aneurysms were ruptured at baseline in about 43% of cases. The mean aneurysm size was 7 mm, and the mean neck size, 3.5 mm. The dome-to-neck ratio was less than 1.5 in about a third of the group. Most lesions (89%) had anterior circulation.
Most patients needed some kind of adjunctive endovascular-assisting device during the procedure. These included balloon remodeling, which was necessary in about 50% of cases to help keep the coils from extruding into the parent vessel, and stents in 22%.
Intraoperative adverse events were uncommon in both groups. They were numerically, but not significantly, less common in the hydrogel group. These included thromboembolism (8 vs. 12), intraoperative rupture (3 vs. 7), parent vessel occlusion (1 vs. 3), and vessel perforation (one in each group). There were no vessel dissections.
By 18 months, 20% of the hydrogel group and 29% of the platinum coil group had experienced the composite primary endpoint. The 9% absolute difference was statistically significant. It was largely driven by the subcomponents of major recurrence (12% vs. 18%) and re-treatment (3% vs. 6%).
An mRS of 3-5 was used as the proxy for patients whose clinical status prevented them from having angiographic follow-up. That endpoint occurred in three patients in the hydrogel group but in none of the platinum coil group. Dr. Taschner said that difference was not statistically significant.
In an analysis that stratified by baseline rupture status and by aneurysm size, the hydrogel coils were largely superior to bare platinum. However, the hydrogel coils were not significantly more effective than the platinum coils for aneurysms 10 mm or larger, Dr. Taschner noted.
There was no significant difference in the secondary endpoint of mRS at 18 months. Most patients (85% vs. 86%) did well, with an mRS of 0. Scores of 1-2 were seen in 9% of each group. An mRS of 3-5 occurred in 3% of the hydrogel group and 1% of the platinum coil group. There were 17 deaths: 7 in the hydrogel group and 10 in the platinum coil group (3% vs. 4%). This was not a significant difference.
Patients whose aneurysms were unruptured at baseline did significantly better than did those with ruptured lesions, although the mRS scores did not vary significantly between treatment groups. Among those with intact lesions, 90% of the hydrogel and 94% of the platinum coil group achieved an mRS of 0, and 2% of each group died. Among those with ruptured lesions at baseline, 78% of the hydrogel group and 75% of the platinum coil group achieved an mRS of 0. Five patients in the hydrogel group and seven in the platinum coil group died.
On the technical outcome of coil volume, hydrogel did somewhat better (0.041 cm3 vs. 0.038 cm3). The mean packing density was significantly higher (39% vs. 31%).
Dr. Taschner said the study provides good support for using the hydrogel coils in medium-sized aneurysms, and that other recent data reaffirm this.
“A recent trial in Canada and the U.S. looked at hydrogel coils in aneurysms 12 mm or larger, with broad necks, and it failed to show a benefit of the hydrogel coils.”
Additionally, a study published in January found that hydrogel coils were no better than platinum coils in 250 patients with large or recurrent aneurysms (AJNR Am J Neuroradiol. 2017 Jan 12. doi: 10.3174/ajnr.A5101).
“I myself would use them in medium-sized aneurysms with an unfavorable dome-to-neck ratio, and in those that have a broad neck. In those instances, I do think that hydrogel coils have their place. I wouldn’t use them in really large aneurysms. I don’t think they are well suited for that. In those cases, I would probably use platinum coils in combination with a flow diverter. That really provides very stable aneurysm occlusion with acceptable complication rates.”
Dr. Taschner said that he received research support from MicroVention during the study.
[email protected]
On Twitter @alz_gal
HOUSTON – Hydrogel coils significantly outperformed bare platinum coils in treating patients with medium-sized intracranial aneurysms, reducing the incidence of adverse outcomes by about 9% in an open-label, randomized trial.
Compared against the platinum coils, the self-expandable, hydrophilic coils scored significantly better on a composite endpoint of aneurysm recurrence at 18 months, re-treatment by 18 months, serious morbidity that prevented angiographic follow-up, and death, Christian Taschner, MD, said at the International Stroke Conference sponsored by the America Heart Association.
These are the company’s second-generation products, said Dr. Taschner, a neuroradiologist at University Hospital Freiburg (Germany). The initial hydrogel-coated coils were not well received because they were too stiff, he said in an interview.
The 18-month study comprised 513 patients and was conducted in 15 centers in France and 7 in Germany. Patients with medium-sized aneurysms (4-12 mm) were randomized to coiling with either the hydrogel coils or bare platinum coils and followed for 18 months. The cohort was stratified by rupture status in the analysis.
Angiographic images of the aneurysm were obtained before treatment, immediately after treatment, at 6 months, and at 18 months. All images were reviewed by an independent laboratory that was blinded to the treatment.
In addition to the primary composite outcome, the study assessed secondary endpoints of modified Rankin Scale (mRS) score at 18 months and coil packing density.
The final analysis included 484 patients. They were a mean of 52 years old, and about 70% were women. The aneurysms were ruptured at baseline in about 43% of cases. The mean aneurysm size was 7 mm, and the mean neck size, 3.5 mm. The dome-to-neck ratio was less than 1.5 in about a third of the group. Most lesions (89%) had anterior circulation.
Most patients needed some kind of adjunctive endovascular-assisting device during the procedure. These included balloon remodeling, which was necessary in about 50% of cases to help keep the coils from extruding into the parent vessel, and stents in 22%.
Intraoperative adverse events were uncommon in both groups. They were numerically, but not significantly, less common in the hydrogel group. These included thromboembolism (8 vs. 12), intraoperative rupture (3 vs. 7), parent vessel occlusion (1 vs. 3), and vessel perforation (one in each group). There were no vessel dissections.
By 18 months, 20% of the hydrogel group and 29% of the platinum coil group had experienced the composite primary endpoint. The 9% absolute difference was statistically significant. It was largely driven by the subcomponents of major recurrence (12% vs. 18%) and re-treatment (3% vs. 6%).
An mRS of 3-5 was used as the proxy for patients whose clinical status prevented them from having angiographic follow-up. That endpoint occurred in three patients in the hydrogel group but in none of the platinum coil group. Dr. Taschner said that difference was not statistically significant.
In an analysis that stratified by baseline rupture status and by aneurysm size, the hydrogel coils were largely superior to bare platinum. However, the hydrogel coils were not significantly more effective than the platinum coils for aneurysms 10 mm or larger, Dr. Taschner noted.
There was no significant difference in the secondary endpoint of mRS at 18 months. Most patients (85% vs. 86%) did well, with an mRS of 0. Scores of 1-2 were seen in 9% of each group. An mRS of 3-5 occurred in 3% of the hydrogel group and 1% of the platinum coil group. There were 17 deaths: 7 in the hydrogel group and 10 in the platinum coil group (3% vs. 4%). This was not a significant difference.
Patients whose aneurysms were unruptured at baseline did significantly better than did those with ruptured lesions, although the mRS scores did not vary significantly between treatment groups. Among those with intact lesions, 90% of the hydrogel and 94% of the platinum coil group achieved an mRS of 0, and 2% of each group died. Among those with ruptured lesions at baseline, 78% of the hydrogel group and 75% of the platinum coil group achieved an mRS of 0. Five patients in the hydrogel group and seven in the platinum coil group died.
On the technical outcome of coil volume, hydrogel did somewhat better (0.041 cm3 vs. 0.038 cm3). The mean packing density was significantly higher (39% vs. 31%).
Dr. Taschner said the study provides good support for using the hydrogel coils in medium-sized aneurysms, and that other recent data reaffirm this.
“A recent trial in Canada and the U.S. looked at hydrogel coils in aneurysms 12 mm or larger, with broad necks, and it failed to show a benefit of the hydrogel coils.”
Additionally, a study published in January found that hydrogel coils were no better than platinum coils in 250 patients with large or recurrent aneurysms (AJNR Am J Neuroradiol. 2017 Jan 12. doi: 10.3174/ajnr.A5101).
“I myself would use them in medium-sized aneurysms with an unfavorable dome-to-neck ratio, and in those that have a broad neck. In those instances, I do think that hydrogel coils have their place. I wouldn’t use them in really large aneurysms. I don’t think they are well suited for that. In those cases, I would probably use platinum coils in combination with a flow diverter. That really provides very stable aneurysm occlusion with acceptable complication rates.”
Dr. Taschner said that he received research support from MicroVention during the study.
[email protected]
On Twitter @alz_gal
HOUSTON – Hydrogel coils significantly outperformed bare platinum coils in treating patients with medium-sized intracranial aneurysms, reducing the incidence of adverse outcomes by about 9% in an open-label, randomized trial.
Compared against the platinum coils, the self-expandable, hydrophilic coils scored significantly better on a composite endpoint of aneurysm recurrence at 18 months, re-treatment by 18 months, serious morbidity that prevented angiographic follow-up, and death, Christian Taschner, MD, said at the International Stroke Conference sponsored by the America Heart Association.
These are the company’s second-generation products, said Dr. Taschner, a neuroradiologist at University Hospital Freiburg (Germany). The initial hydrogel-coated coils were not well received because they were too stiff, he said in an interview.
The 18-month study comprised 513 patients and was conducted in 15 centers in France and 7 in Germany. Patients with medium-sized aneurysms (4-12 mm) were randomized to coiling with either the hydrogel coils or bare platinum coils and followed for 18 months. The cohort was stratified by rupture status in the analysis.
Angiographic images of the aneurysm were obtained before treatment, immediately after treatment, at 6 months, and at 18 months. All images were reviewed by an independent laboratory that was blinded to the treatment.
In addition to the primary composite outcome, the study assessed secondary endpoints of modified Rankin Scale (mRS) score at 18 months and coil packing density.
The final analysis included 484 patients. They were a mean of 52 years old, and about 70% were women. The aneurysms were ruptured at baseline in about 43% of cases. The mean aneurysm size was 7 mm, and the mean neck size, 3.5 mm. The dome-to-neck ratio was less than 1.5 in about a third of the group. Most lesions (89%) had anterior circulation.
Most patients needed some kind of adjunctive endovascular-assisting device during the procedure. These included balloon remodeling, which was necessary in about 50% of cases to help keep the coils from extruding into the parent vessel, and stents in 22%.
Intraoperative adverse events were uncommon in both groups. They were numerically, but not significantly, less common in the hydrogel group. These included thromboembolism (8 vs. 12), intraoperative rupture (3 vs. 7), parent vessel occlusion (1 vs. 3), and vessel perforation (one in each group). There were no vessel dissections.
By 18 months, 20% of the hydrogel group and 29% of the platinum coil group had experienced the composite primary endpoint. The 9% absolute difference was statistically significant. It was largely driven by the subcomponents of major recurrence (12% vs. 18%) and re-treatment (3% vs. 6%).
An mRS of 3-5 was used as the proxy for patients whose clinical status prevented them from having angiographic follow-up. That endpoint occurred in three patients in the hydrogel group but in none of the platinum coil group. Dr. Taschner said that difference was not statistically significant.
In an analysis that stratified by baseline rupture status and by aneurysm size, the hydrogel coils were largely superior to bare platinum. However, the hydrogel coils were not significantly more effective than the platinum coils for aneurysms 10 mm or larger, Dr. Taschner noted.
There was no significant difference in the secondary endpoint of mRS at 18 months. Most patients (85% vs. 86%) did well, with an mRS of 0. Scores of 1-2 were seen in 9% of each group. An mRS of 3-5 occurred in 3% of the hydrogel group and 1% of the platinum coil group. There were 17 deaths: 7 in the hydrogel group and 10 in the platinum coil group (3% vs. 4%). This was not a significant difference.
Patients whose aneurysms were unruptured at baseline did significantly better than did those with ruptured lesions, although the mRS scores did not vary significantly between treatment groups. Among those with intact lesions, 90% of the hydrogel and 94% of the platinum coil group achieved an mRS of 0, and 2% of each group died. Among those with ruptured lesions at baseline, 78% of the hydrogel group and 75% of the platinum coil group achieved an mRS of 0. Five patients in the hydrogel group and seven in the platinum coil group died.
On the technical outcome of coil volume, hydrogel did somewhat better (0.041 cm3 vs. 0.038 cm3). The mean packing density was significantly higher (39% vs. 31%).
Dr. Taschner said the study provides good support for using the hydrogel coils in medium-sized aneurysms, and that other recent data reaffirm this.
“A recent trial in Canada and the U.S. looked at hydrogel coils in aneurysms 12 mm or larger, with broad necks, and it failed to show a benefit of the hydrogel coils.”
Additionally, a study published in January found that hydrogel coils were no better than platinum coils in 250 patients with large or recurrent aneurysms (AJNR Am J Neuroradiol. 2017 Jan 12. doi: 10.3174/ajnr.A5101).
“I myself would use them in medium-sized aneurysms with an unfavorable dome-to-neck ratio, and in those that have a broad neck. In those instances, I do think that hydrogel coils have their place. I wouldn’t use them in really large aneurysms. I don’t think they are well suited for that. In those cases, I would probably use platinum coils in combination with a flow diverter. That really provides very stable aneurysm occlusion with acceptable complication rates.”
Dr. Taschner said that he received research support from MicroVention during the study.
[email protected]
On Twitter @alz_gal
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: Compared with bare-metal coils, hydrogel coils reduced by 9% the incidence of a composite primary outcome of recurrence, re-treatment, morbidity, and death.
Data source: An 18-month, open-label, randomized study of 513 patients.
Disclosures: MicroVention sponsored the study. Dr. Taschner said that he received research support from the company during the study.
Antibiotic prophylaxis for artificial joints
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
A 66-year-old woman 3 years status post hip replacement is seen for dental work. The dentist contacts the clinic for an antibiotic prescription. The patient has a penicillin allergy (rash). What do you recommend?
A. Clindamycin one dose before dental work.
B. Amoxicillin one dose before dental work.
C. Amoxicillin one dose before, one dose 4 hours after dental work.
D. Clindamycin one dose before dental work, one dose 4 hours after dental work.
E. No antibiotics.
Many patients with prosthetic joints will request antibiotics to take prior to dental procedures. Sometimes this request comes from the dental office.
When I ask patients why they feel they need antibiotics, they often reply that they were told by their orthopedic surgeons or their dentist that they would need to take antibiotics before dental procedures.
In an era when Clostridium difficile infection is a common and dangerous complication in the elderly, avoidance of unnecessary antibiotics is critical. In the United States, it is estimated that there are 240,000 patients infected with C. difficile annually, with 24,000 deaths at a cost of $6 billion.1
Is there compelling evidence to justify giving antibiotic prophylaxis for dental procedures to patients with prosthetic joints?
This information has called into question the wisdom of giving antibiotic prophylaxis for dental procedures when the same patients have transient bacteremias as a regular part of day-to-day life, and mouth organisms were infrequent causes of prosthetic joint infections.
The American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) released an advisory statement 20 years ago on antibiotic prophylaxis for patients with dental replacements, which concluded: “Antibiotic prophylaxis is not indicated for dental patients with pins, plates, and screws, nor is it routinely indicated for most dental patients with total joint replacements.”4
In 2003, the AAOS and the ADA released updated guidelines that stated: “Presently, no scientific evidence supports the position that antibiotic prophylaxis to prevent hematogenous infections is required prior to dental treatment in patients with total joint prostheses. The risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotics.”5
Great confusion arose in 2009 when the AAOS published a position paper on its website that reversed this position.6 Interestingly, the statement was done by the AAOS alone, and not done in conjunction with the ADA.
In this position paper, the AAOS recommended that health care providers consider antibiotic prophylaxis prior to invasive procedures on all patients who had prosthetic joints, regardless of how long those joints have been in place. This major change in recommendations was not based on any new evidence that had been reviewed since the 2003 guidelines.
There are two studies that address outcome of patients with prosthetic joints who have and have not received prophylactic antibiotics.
Elie Berbari, MD, and colleagues reported on the results of a prospective case-control study comparing patients with prosthetic joints hospitalized with hip or knee infections with patients who had prosthetic joints hospitalized at the same time who did not have hip or knee infections.7
There was no increased risk of prosthetic hip or knee infection for patients undergoing a dental procedure who were not receiving antibiotic prophylaxis (odds ratio, 0.8; 95% confidence interval, 0.4-1.6), compared with the risk for patients not undergoing a dental procedure (OR, 0.6; 95% CI, 0.4-1.1). Antibiotic prophylaxis in patients undergoing high and low risk dental procedures did not decrease the risk of prosthetic joint infections.
In 2012, the AAOS and the ADA published updated guidelines with the following summary recommendation: “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.”8 They referenced the Berbari study as the best available evidence.
Feng-Chen Kao, MD, and colleagues published a study this year with a design very similar to the Berbari study, with similar results.9 All Taiwanese residents who had received hip or knee replacements over a 12-year period were screened. Those who had received dental procedures were matched with individuals who had not had dental procedures. The dental procedure group was subdivided into a group that received antibiotics and one that didn’t.
There was no difference in infection rates between the group that had received dental procedures and the group that did not, and no difference in infection rates between those who received prophylactic antibiotics and those who didn’t.
I think this myth can be put to rest. There is no evidence to give patients with joint prostheses prophylactic antibiotics before dental procedures.
References
1. Steckelberg J.M., Osmon D.R. Prosthetic joint infections. In: Bisno A.L., Waldvogel F.A., eds. Infections associated with indwelling medical devices. Third ed., Washington, D.C.: American Society of Microbiology Press, 2000:173-209.
2. J Dent Res. 2004 Feb;83(2):170-4.
3. J Clin Periodontol. 2006 Feb;33(6):401-7.
4. J Am Dent Assoc. 1997 Jul;128(7):1004-8.
5. J Am Dent Assoc. 2003 Jul;134(7):895-9.
6. Spec Care Dentist. 2009 Nov-Dec;29(6):229-31.
7. Clin Infect Dis. 2010 Jan 1;50(1):8-16.
8. J Dent (Shiraz). 2013 Mar;14(1):49-52.
9. Infect Control Hosp Epidemiol. 2017 Feb;38(2):154-61.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
Robotic PCI success rates higher with radial access
WASHINGTON – The clinical and technical success rates are higher among patients undergoing robotic percutaneous coronary interventions through radial than femoral access, according to registry data presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Although both the clinical and technical success rates were high with either type of access, the advantage for radial over femoral access was significant for each, reported Ali Pourdjabbar, MD, an interventional cardiologist completing his fellowship at the University of California, San Diego. However, as this was not a randomized trial, he placed emphasis on the message that robotic percutaneous coronary intervention (PCI) is safe and effective when performed through either access point.
Clinical success, defined as less than 30% residual occlusion with TIMI3 flow and no major adverse cardiovascular events, such as myocardial infarction, cardiovascular death, or revascularization, was achieved in 99.4% of the 310 patients treated through radial access and 94.7% of the 191 patients treated through femoral access (P = .002). Technical success, defined as PCI performed without any manual assistance, was achieved in 92.4% of procedures performed through radial access and 86.7% of those performed through femoral access (P = .03).
There were no significant differences in the two groups for contrast use or fluoroscopy time, but the time to completing PCI was shorter with the radial approach (57 vs. 66 minutes; P less than .04).
However, the groups did differ in baseline characteristics, according to Dr. Pourdjabbar. Patients undergoing robotic PCI through a radial approach were younger, less likely to have diabetes, and less likely to have received a prior PCI. Most importantly, they were less likely to have complex lesions. Patients treated with radial access had higher average body mass indexes.
“It is important to recognize that this was a nonrandomized, retrospective analysis,” Dr. Pourdjabbar emphasized. He noted that one reason for this analysis was to confirm that efficacy and safety was just as good with radial access, which although an approved robotic approach, was supported with fewer data at the time that the device became available.
However, it is notable that 60% of the robotic procedures were done with the radial approach, which is approximately double the proportion currently performed in the United States when done manually, according to data presented by Dr. Pourdjabbar. He noted that radial access has been more commonly used outside of the United States, but rates have also started climbing in this country, rising from less than 5% of cases in 2005 to nearly one third of cases in the most recent analysis. It is unclear why robotic procedures are performed more frequently through radial access, but Dr. Pourdjabbar speculated that centers innovating with robots might also be in the vanguard of the movement toward radial PCI.
Of reasons to consider robots, Dr. Pourdjabbar suggested that the safety advantages for the interventionalist are particularly compelling. Citing a variety of data associating cath lab radiation exposure to health risks for physicians and staff, Dr. Pourdjabbar explained that the operator performs robotic PCI from a shielded cockpit that completely eliminates exposure to radiation. A next generation robotic device, called the CorPath GRX System, is expected to further reduce opportunities for radiation exposure by allowing the operator to disengage the guide catheter in cases when this had to be done manually with the first generation CorPath 200 system.
Asked about the learning curve of PCI robotics, Dr. Pourdjabbar said that the principles appear to be grasped quickly by interventionalists, but he acknowledged that his experience as a training fellow has been limited. However, Rajesh V. Swaminathan, MD, an interventionalist affiliated with Duke University, Durham, N.C., who has experience with robotic PCI, reported that although the tactile sense of the guide wire is lost in robotic PCI, the procedure has typically proceeded more quickly in his hands once access is achieved.
“The greatest learning curve may with the staff that has to get used to not having the interventionalist at the table,” observed Dr. Swaminathan, who was a moderator of the session in which these data were presented.
WASHINGTON – The clinical and technical success rates are higher among patients undergoing robotic percutaneous coronary interventions through radial than femoral access, according to registry data presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Although both the clinical and technical success rates were high with either type of access, the advantage for radial over femoral access was significant for each, reported Ali Pourdjabbar, MD, an interventional cardiologist completing his fellowship at the University of California, San Diego. However, as this was not a randomized trial, he placed emphasis on the message that robotic percutaneous coronary intervention (PCI) is safe and effective when performed through either access point.
Clinical success, defined as less than 30% residual occlusion with TIMI3 flow and no major adverse cardiovascular events, such as myocardial infarction, cardiovascular death, or revascularization, was achieved in 99.4% of the 310 patients treated through radial access and 94.7% of the 191 patients treated through femoral access (P = .002). Technical success, defined as PCI performed without any manual assistance, was achieved in 92.4% of procedures performed through radial access and 86.7% of those performed through femoral access (P = .03).
There were no significant differences in the two groups for contrast use or fluoroscopy time, but the time to completing PCI was shorter with the radial approach (57 vs. 66 minutes; P less than .04).
However, the groups did differ in baseline characteristics, according to Dr. Pourdjabbar. Patients undergoing robotic PCI through a radial approach were younger, less likely to have diabetes, and less likely to have received a prior PCI. Most importantly, they were less likely to have complex lesions. Patients treated with radial access had higher average body mass indexes.
“It is important to recognize that this was a nonrandomized, retrospective analysis,” Dr. Pourdjabbar emphasized. He noted that one reason for this analysis was to confirm that efficacy and safety was just as good with radial access, which although an approved robotic approach, was supported with fewer data at the time that the device became available.
However, it is notable that 60% of the robotic procedures were done with the radial approach, which is approximately double the proportion currently performed in the United States when done manually, according to data presented by Dr. Pourdjabbar. He noted that radial access has been more commonly used outside of the United States, but rates have also started climbing in this country, rising from less than 5% of cases in 2005 to nearly one third of cases in the most recent analysis. It is unclear why robotic procedures are performed more frequently through radial access, but Dr. Pourdjabbar speculated that centers innovating with robots might also be in the vanguard of the movement toward radial PCI.
Of reasons to consider robots, Dr. Pourdjabbar suggested that the safety advantages for the interventionalist are particularly compelling. Citing a variety of data associating cath lab radiation exposure to health risks for physicians and staff, Dr. Pourdjabbar explained that the operator performs robotic PCI from a shielded cockpit that completely eliminates exposure to radiation. A next generation robotic device, called the CorPath GRX System, is expected to further reduce opportunities for radiation exposure by allowing the operator to disengage the guide catheter in cases when this had to be done manually with the first generation CorPath 200 system.
Asked about the learning curve of PCI robotics, Dr. Pourdjabbar said that the principles appear to be grasped quickly by interventionalists, but he acknowledged that his experience as a training fellow has been limited. However, Rajesh V. Swaminathan, MD, an interventionalist affiliated with Duke University, Durham, N.C., who has experience with robotic PCI, reported that although the tactile sense of the guide wire is lost in robotic PCI, the procedure has typically proceeded more quickly in his hands once access is achieved.
“The greatest learning curve may with the staff that has to get used to not having the interventionalist at the table,” observed Dr. Swaminathan, who was a moderator of the session in which these data were presented.
WASHINGTON – The clinical and technical success rates are higher among patients undergoing robotic percutaneous coronary interventions through radial than femoral access, according to registry data presented at CRT 2017 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Although both the clinical and technical success rates were high with either type of access, the advantage for radial over femoral access was significant for each, reported Ali Pourdjabbar, MD, an interventional cardiologist completing his fellowship at the University of California, San Diego. However, as this was not a randomized trial, he placed emphasis on the message that robotic percutaneous coronary intervention (PCI) is safe and effective when performed through either access point.
Clinical success, defined as less than 30% residual occlusion with TIMI3 flow and no major adverse cardiovascular events, such as myocardial infarction, cardiovascular death, or revascularization, was achieved in 99.4% of the 310 patients treated through radial access and 94.7% of the 191 patients treated through femoral access (P = .002). Technical success, defined as PCI performed without any manual assistance, was achieved in 92.4% of procedures performed through radial access and 86.7% of those performed through femoral access (P = .03).
There were no significant differences in the two groups for contrast use or fluoroscopy time, but the time to completing PCI was shorter with the radial approach (57 vs. 66 minutes; P less than .04).
However, the groups did differ in baseline characteristics, according to Dr. Pourdjabbar. Patients undergoing robotic PCI through a radial approach were younger, less likely to have diabetes, and less likely to have received a prior PCI. Most importantly, they were less likely to have complex lesions. Patients treated with radial access had higher average body mass indexes.
“It is important to recognize that this was a nonrandomized, retrospective analysis,” Dr. Pourdjabbar emphasized. He noted that one reason for this analysis was to confirm that efficacy and safety was just as good with radial access, which although an approved robotic approach, was supported with fewer data at the time that the device became available.
However, it is notable that 60% of the robotic procedures were done with the radial approach, which is approximately double the proportion currently performed in the United States when done manually, according to data presented by Dr. Pourdjabbar. He noted that radial access has been more commonly used outside of the United States, but rates have also started climbing in this country, rising from less than 5% of cases in 2005 to nearly one third of cases in the most recent analysis. It is unclear why robotic procedures are performed more frequently through radial access, but Dr. Pourdjabbar speculated that centers innovating with robots might also be in the vanguard of the movement toward radial PCI.
Of reasons to consider robots, Dr. Pourdjabbar suggested that the safety advantages for the interventionalist are particularly compelling. Citing a variety of data associating cath lab radiation exposure to health risks for physicians and staff, Dr. Pourdjabbar explained that the operator performs robotic PCI from a shielded cockpit that completely eliminates exposure to radiation. A next generation robotic device, called the CorPath GRX System, is expected to further reduce opportunities for radiation exposure by allowing the operator to disengage the guide catheter in cases when this had to be done manually with the first generation CorPath 200 system.
Asked about the learning curve of PCI robotics, Dr. Pourdjabbar said that the principles appear to be grasped quickly by interventionalists, but he acknowledged that his experience as a training fellow has been limited. However, Rajesh V. Swaminathan, MD, an interventionalist affiliated with Duke University, Durham, N.C., who has experience with robotic PCI, reported that although the tactile sense of the guide wire is lost in robotic PCI, the procedure has typically proceeded more quickly in his hands once access is achieved.
“The greatest learning curve may with the staff that has to get used to not having the interventionalist at the table,” observed Dr. Swaminathan, who was a moderator of the session in which these data were presented.
AT CRT 2017
Key clinical point: Registry data shows higher success rate for radial versus femoral access in robotic percutaneous coronary interventions.
Major finding: In robotic PCI, the clinical success rate was 99.4% with radial access and 94.7% (P = .002) with femoral access.
Data source: A nonrandomized, retrospective analysis.
Disclosures: Dr. Pourdjabbar reported no financial relationships to disclose.