User login
Dr. Douglas Paauw gives updates on antihypertensives, statins, SGLT2 inhibitors
PHILADELPHIA – in a video interview at the annual meeting of the American College of Physicians.
Dr. Paauw, professor of medicine at the University of Washington, Seattle, began by discussing some of the issues that occurred with antihypertensive drugs in the past year. These included the link between hydrochlorothiazide use and the increased risk of nonmelanoma skin cancers, the recalls of many drug lots of angiotensin II receptor blockers, and a study that found an increased risk of lung cancer in people who were taking ACE inhibitors.
He then described the findings of studies that examined the links between statins and muscle pain and other new research on these drugs.
He also warned physicians to be particularity cautious about prescribing sodium-glucose cotransporter 2 inhibitors to certain kinds of patients.
Dr. Paauw concluded by explaining why clarithromycin is his most hated drug.
Dr. Paauw is also the Rathmann Family Foundation Endowed Chair for Patient-Centered Clinical Education and the medicine clerkship director at the University of Washington.
PHILADELPHIA – in a video interview at the annual meeting of the American College of Physicians.
Dr. Paauw, professor of medicine at the University of Washington, Seattle, began by discussing some of the issues that occurred with antihypertensive drugs in the past year. These included the link between hydrochlorothiazide use and the increased risk of nonmelanoma skin cancers, the recalls of many drug lots of angiotensin II receptor blockers, and a study that found an increased risk of lung cancer in people who were taking ACE inhibitors.
He then described the findings of studies that examined the links between statins and muscle pain and other new research on these drugs.
He also warned physicians to be particularity cautious about prescribing sodium-glucose cotransporter 2 inhibitors to certain kinds of patients.
Dr. Paauw concluded by explaining why clarithromycin is his most hated drug.
Dr. Paauw is also the Rathmann Family Foundation Endowed Chair for Patient-Centered Clinical Education and the medicine clerkship director at the University of Washington.
PHILADELPHIA – in a video interview at the annual meeting of the American College of Physicians.
Dr. Paauw, professor of medicine at the University of Washington, Seattle, began by discussing some of the issues that occurred with antihypertensive drugs in the past year. These included the link between hydrochlorothiazide use and the increased risk of nonmelanoma skin cancers, the recalls of many drug lots of angiotensin II receptor blockers, and a study that found an increased risk of lung cancer in people who were taking ACE inhibitors.
He then described the findings of studies that examined the links between statins and muscle pain and other new research on these drugs.
He also warned physicians to be particularity cautious about prescribing sodium-glucose cotransporter 2 inhibitors to certain kinds of patients.
Dr. Paauw concluded by explaining why clarithromycin is his most hated drug.
Dr. Paauw is also the Rathmann Family Foundation Endowed Chair for Patient-Centered Clinical Education and the medicine clerkship director at the University of Washington.
REPORTING FROM INTERNAL MEDICINE 2019
Gout Drug May Help in Metabolic Syndrome
Colchicine inhibits the formation of the Nod-like Receptor Family Pyrin Domain Containing 3 (NLRP3) inflammasome, a key component in the obesity-associated inflammatory cascade. In a retrospective study, long-term colchicine treatment had glycemic benefit in patients with gout. Other research has suggested that suppressing NLRP3 could improve peripheral insulin resistance as well as β-cell insulin production. However, no randomized controlled trial had yet investigated colchicine’s long-term effects on glucose metabolism in adults with obesity and metabolic syndrome (MetS).
The NIH researchers enrolled 40 adults to receive either colchicine or placebo; 37 completed the 3-month study. Adherence was high in both groups.
Colchicine significantly reduced multiple markers of obesity-associated inflammation, including high sensitivity C-reactive protein and erythrocyte sedimentation rate. The colchicine group also had moderate but statistically significant reductions in white blood cell count, monocytes, neutrophils, and platelets, without significant effects on lymphocyte count.
Although colchicine’s effects on the primary outcome of insulin sensitivity were not significant, some of the secondary outcomes related to glucose homeostasis—eg, insulin resistance and fasting insulin—suggest colchicine treatment may improve hepatic insulin sensitivity. Moreover, the researchers say, a trend toward improvement in disposition index suggests that the drug might potentially delay the onset of diabetes in people at risk.
While some small, short-term studies had suggested that colchicine might worsen metabolic variables by inhibiting insulin secretion, other recent retrospective studies found long-term colchicine use did not negatively affect insulin secretion or glycemic control. In this study, similarly, the researchers say, chronic colchicine use did not impair first-phase insulin response or insulin sensitivity, and other markers of metabolic health, such as hemoglobin A1c and cholesterol, were not significantly changed. However, the researchers acknowledge that their study may have been too small to confirm those differences, and say larger studies are warranted.
Colchicine inhibits the formation of the Nod-like Receptor Family Pyrin Domain Containing 3 (NLRP3) inflammasome, a key component in the obesity-associated inflammatory cascade. In a retrospective study, long-term colchicine treatment had glycemic benefit in patients with gout. Other research has suggested that suppressing NLRP3 could improve peripheral insulin resistance as well as β-cell insulin production. However, no randomized controlled trial had yet investigated colchicine’s long-term effects on glucose metabolism in adults with obesity and metabolic syndrome (MetS).
The NIH researchers enrolled 40 adults to receive either colchicine or placebo; 37 completed the 3-month study. Adherence was high in both groups.
Colchicine significantly reduced multiple markers of obesity-associated inflammation, including high sensitivity C-reactive protein and erythrocyte sedimentation rate. The colchicine group also had moderate but statistically significant reductions in white blood cell count, monocytes, neutrophils, and platelets, without significant effects on lymphocyte count.
Although colchicine’s effects on the primary outcome of insulin sensitivity were not significant, some of the secondary outcomes related to glucose homeostasis—eg, insulin resistance and fasting insulin—suggest colchicine treatment may improve hepatic insulin sensitivity. Moreover, the researchers say, a trend toward improvement in disposition index suggests that the drug might potentially delay the onset of diabetes in people at risk.
While some small, short-term studies had suggested that colchicine might worsen metabolic variables by inhibiting insulin secretion, other recent retrospective studies found long-term colchicine use did not negatively affect insulin secretion or glycemic control. In this study, similarly, the researchers say, chronic colchicine use did not impair first-phase insulin response or insulin sensitivity, and other markers of metabolic health, such as hemoglobin A1c and cholesterol, were not significantly changed. However, the researchers acknowledge that their study may have been too small to confirm those differences, and say larger studies are warranted.
Colchicine inhibits the formation of the Nod-like Receptor Family Pyrin Domain Containing 3 (NLRP3) inflammasome, a key component in the obesity-associated inflammatory cascade. In a retrospective study, long-term colchicine treatment had glycemic benefit in patients with gout. Other research has suggested that suppressing NLRP3 could improve peripheral insulin resistance as well as β-cell insulin production. However, no randomized controlled trial had yet investigated colchicine’s long-term effects on glucose metabolism in adults with obesity and metabolic syndrome (MetS).
The NIH researchers enrolled 40 adults to receive either colchicine or placebo; 37 completed the 3-month study. Adherence was high in both groups.
Colchicine significantly reduced multiple markers of obesity-associated inflammation, including high sensitivity C-reactive protein and erythrocyte sedimentation rate. The colchicine group also had moderate but statistically significant reductions in white blood cell count, monocytes, neutrophils, and platelets, without significant effects on lymphocyte count.
Although colchicine’s effects on the primary outcome of insulin sensitivity were not significant, some of the secondary outcomes related to glucose homeostasis—eg, insulin resistance and fasting insulin—suggest colchicine treatment may improve hepatic insulin sensitivity. Moreover, the researchers say, a trend toward improvement in disposition index suggests that the drug might potentially delay the onset of diabetes in people at risk.
While some small, short-term studies had suggested that colchicine might worsen metabolic variables by inhibiting insulin secretion, other recent retrospective studies found long-term colchicine use did not negatively affect insulin secretion or glycemic control. In this study, similarly, the researchers say, chronic colchicine use did not impair first-phase insulin response or insulin sensitivity, and other markers of metabolic health, such as hemoglobin A1c and cholesterol, were not significantly changed. However, the researchers acknowledge that their study may have been too small to confirm those differences, and say larger studies are warranted.
How Do These 3 Diabetes Agents Compare in Reducing Mortality?
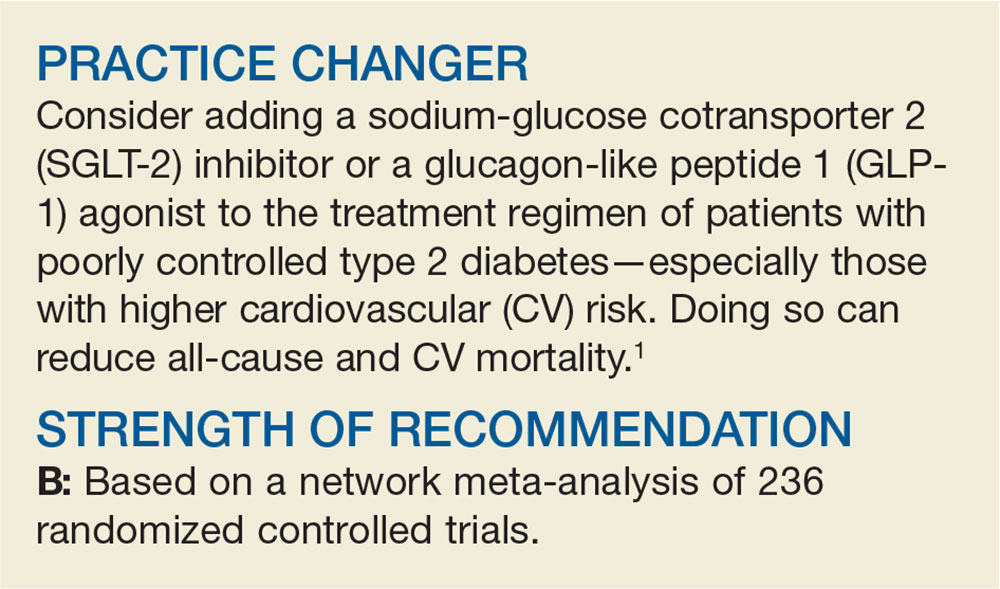
A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.

A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).

A 64-year-old man with type 2 diabetes mellitus (T2DM) presents for a follow-up visit. His point-of-care A1C is 9.5%, and he is currently taking only metformin (1000 mg bid). You are considering the addition of an SGLT-2 inhibitor, a GLP-1 agonist, or a dipeptidyl peptidase 4 (DPP-4) inhibitor to his treatment regimen. Which do you choose to better control his diabetes and reduce his all-cause and CV mortality risk?
Over the past several years, the number of patients with T2DM has continued to climb. In the United States, approximately 30 million people (1 of every 11) now struggle to reduce their blood sugar.2 As prevalence of the disease has increased, so has the number of available medications that aim to lower blood glucose and improve diabetes control.2 In particular, the introduction of SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors over the past several years has produced an area of some clinical ambiguity, due to the lack of randomized controlled trials (RCTs) comparing their efficacy.
The American Diabetes Association’s Standards of Medical Care in Diabetes points specifically to the potential roles of the SGLT-2 inhibitors empagliflozin and canagliflozin and the GLP-1 agonist liraglutide as agents that should be added to metformin and lifestyle modification for patients with established atherosclerotic CV disease. They cite data indicating that these drugs reduce major adverse CV events and CV mortality in this population.3 Deciding among these 3 medications, however, is left to providers and patients. For dual therapy in patients with T2DM without CV disease who remain hyperglycemic despite metformin and lifestyle modifications, SGLT-2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors are recommended equally, with the choice among them to be determined by “consideration of drug-specific effects and patient factors.”3
The National Institute for Health and Care Excellence (NICE) guidelines on T2DM management list both SGLT-2 inhibitors and DPP-4 inhibitors among the potential options for intensifying therapy after metformin.4 The American Association of Clinical Endocrinologists/American College of Endocrinology guidelines include a hierarchical recommendation to try a GLP-1 agonist first, followed by an SGLT-2 inhibitor, followed by a DPP-4 inhibitor, after metformin and lifestyle modifications—although the difference in the strength of recommendation for each class is noted to be small.5
STUDY SUMMARY
SGLT-2s, GLP-1s equal better mortality outcomes
Zheng and colleagues performed a network meta-analysis of 236 RCTs involving 176310 patients to compare the clinical efficacy of SGLT-2 inhibitors, GLP
A majority of the patients in both the intervention and control groups were taking additional diabetes medications (eg, metformin) prior to enrollment and during the trials. About half the patients analyzed were enrolled in trials that specifically evaluated those at elevated CV risk—notable because patients with higher CV risk ultimately derived the most benefit from the treatments studied.
The primary outcome was all-cause mortality. Secondary outcomes were CV mortality, heart failure (HF) events, myocardial infarction (MI), unstable angina, and stroke, as well as the safety outcomes of hypoglycemia and adverse events (any events, serious events, and those leading to study withdrawal).
Continue to: Results
Results. Compared with the patients in the control groups (placebo or no treatment), patients in both the SGLT-2 inhibitor and GLP-1 agonist groups had decreased all-cause mortality (SGLT-2 inhibitor group: hazard ratio [HR], 0.80; absolute risk difference [RD], –1%; number needed to treat [NNT], 100; GLP-1 agonist group: HR, 0.88; absolute RD, –0.6%; NNT, 167). Patients in the DPP-4 inhibitor group did not have a difference in mortality compared with the control groups (HR, 1.02; absolute RD, 0.1%). Both the SGLT-2 inhibitor (HR, 0.78; absolute RD, –0.9%; NNT, 111) and GLP-1 agonist (HR, 0.86; absolute RD, –0.5%; NNT, 200) groups had reduced all-cause mortality when compared with the DPP-4 inhibitor group.
CV endpoints. Similarly, the SGLT-2 inhibitor (HR, 0.79; absolute RD, –0.8%; NNT, 125) and GLP-1 agonist (HR, 0.85; absolute RD, –0.5%; NNT, 200) groups had a reduction in CV mortality compared with the control groups, while those in the DPP-4 inhibitor group experienced no effect. Additionally, those taking SGLT-2 inhibitors had lower rates of HF events (HR, 0.62; absolute RD, –1.1%; NNT, 91) and MI (HR, 0.86; absolute RD, –0.6%; NNT, 167) than those in the control groups. They also had lower rates of HF than those taking GLP-1 agonists (HR, 0.67; absolute RD, –0.9; NNT, 111) or DPP-4 inhibitors (HR, 0.55; absolute RD, –1.1%; NNT, 91). Neither the GLP-1 agonist groups nor the DPP-4 inhibitor groups had lower rates of HF or MI than the control groups.
Adverse effects. DPP-4 inhibitors, GLP-1 agonists, and SGLT-2 inhibitors were all associated with a small increased risk for hypoglycemia compared with the control groups, but there were no significant differences between drug classes. All agents resulted in an increased risk for adverse events leading to trial withdrawal compared with the control groups (GPL-1 agonists: HR, 2; absolute RD, 4.7%; number needed to harm [NNH], 21; SGLT-2 inhibitors: HR, 1.8; absolute RD, 5.8%; NNH, 17; and DPP-4 inhibitors: HR, 1.93; absolute RD, 3.1%; NNH, 32).
When compared with the control groups, the SGLT-2 inhibitor group was associated with an increased risk for genital infection (relative risk [RR], 4.19; absolute RD, 6%; NNH, 16), but not of urinary tract infection or lower limb amputation—although the authors noted high heterogeneity among studies with regard to the limb amputation outcome. DPP-4 inhibitors were associated with an increased risk for acute pancreatitis (RR, 1.58; absolute RD, 0.1%; NNH, 1000) compared with control groups.
WHAT’S NEW
SGLT-2s: Lower mortality, fewer heart failure events
This meta-analysis concludes that when compared with placebo or no treatment, the use of SGLT-2 inhibitors or GLP-1 agonists is associated with lower all-cause mortality and lower CV mortality than the use of DPP-4 inhibitors. Additionally, SGLT-2 inhibitors are associated with lower rates of HF events than GLP-1 agonists or DPP-4 inhibitors.
Continue to: CAVEATS
CAVEATS
A lack of head-to-head RCTs
This study was a network meta-analysis that included many trials, the majority of which compared SGLT-1 inhibitors, GLP-1 agonists, and DPP-4 inhibitors with controls rather than to one another. Thus, the findings are not derived from a robust base of head-to-head RCTs involving the 3 medication classes.
However, there was relatively low heterogeneity among the studies included, which lends strength to the meta-analysis.6 Patients with the highest baseline CV risk likely gleaned the greatest benefits from these treatments and may have driven much of the observed mortality reduction. This may limit the generalizability of the results to people with low CV risk. The comparative effectiveness and risk for adverse effects among individual medications within each class is unknown, because the analysis was completed by drug class in order to adequately power the study to detect treatment effects.
CHALLENGES TO IMPLEMENTATION
Cost, adverse effects, and formulation
The cost of SGLT-2 inhibitors and GLP-1 agonists may present challenges to patients wishing to use these options. Additionally, the increased risk for genital infections with SGLT-2 inhibitors and of overall adverse effects (many of which were gastrointestinal) with GLP-1 agonists must be considered. Lastly, the injectable formulation of GLP-1 agonists may present a barrier to patients’ ability and willingness to effectively administer these agents.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[2]:99-101).
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
1. Zheng S, Roddick A, Aghar-Jaffar R, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580-1591.
2. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
3. American Diabetes Association. Standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S1-S193.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. www.nice.org.uk/guidance/ng28. Accessed March 1, 2019.
5. Garber A, Abrahamson M, Barzilay J, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 executive summary. Endocr Pract. 2018;24:91-120.
6. Salanti G, Del Giovane C, Chaimani A, et al. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9:1-14.
Canagliflozin lowers kidney failure risk in T2D: CREDENCE
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Sodium-glucose cotransporter 2 inhibitors are the most promising of a number of diabetes medications that have shown potential in renoprotection through a mechanism other than glucose homeostasis.
The study suggests canagliflozin’s effects are felt both in the renal system and systemically. The initial decrease in glomerular filtration rate in the first few weeks of treatment could be the result of decreases in glomerular perfusion and intraglomerular pressure, but this effect does stabilize. Levels of angiotensin II and atrial natriuretic peptide decrease, and there is also a decrease in inflammation and an increase in intrarenal oxygenation.
These findings are good news for patients with diabetes and chronic kidney disease, and their importance cannot be overstated.
Julie R. Ingelfinger, MD, is from the Tufts University in Boston, and Clifford J. Rosen, MD, is from the Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough. These comments are adapted from an accompanying editorial (N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMe1904740).
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
Patients with type 2 diabetes and chronic kidney disease (CKD) show significantly lower incidence of kidney failure and cardiovascular events after treatment with the sodium-glucose cotransporter 2 inhibitor canagliflozin, in the CREDENCE trial.
CREDENCE (Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants With Diabetic Nephropathy) is a double-blind, placebo-controlled trial involving 4,401 patients with type 2 diabetes and albuminuric CKD, who were randomized to either 100 mg of canagliflozin daily or placebo.
After a median follow-up of 2.62 years, there was a significant 30% lower risk of the primary outcome, which was a composite of end-stage kidney disease, a doubling of serum creatinine, or death from renal or cardiovascular causes, a highly significant difference at P = .00001.
Separately, there was a 32% lower risk of end-stage kidney disease, a 20% lower risk of cardiovascular death, MI, or stroke, and a 39% lower risk of hospitalization for heart failure, both significant differences. Patients treated with canagliflozin also had a 40% lower risk of a doubling of serum creatinine, and a 28% lower risk of dialysis, kidney transplantation, or renal death.
“These findings were observed despite very modest between-group differences in blood glucose level, weight, and blood pressure, and in contrast to previous concern about the initial acute reduction in the estimated GFR [glomerular filtration rate] observed with SGLT2 inhibitors,” wrote Vlado Perkovic, MD, from the George Institute for Global Health, University of New South Wales Sydney, and his coauthors. “This suggests that the mechanism of benefit is likely to be independent of glucose levels and may possibly stem from a reduction in intraglomerular pressure, with other possible mechanisms presently being studied.”
The trial was stopped early after reaching the prespecified efficacy criteria for early cessation. The authors estimated that 21.2 patients would need to be treated with canagliflozin to prevent one primary outcome.
There were no significant differences between the two groups in the rate of adverse and serious adverse events, including the risk of lower limb amputation and fracture.
The study was supported by Janssen Research and Development. Eighteen authors declared steering committee, support and consultancies with Janssen, and thirteen also declared personal fees from other pharmaceutical and private industry. Five authors were employees of Janssen.
SOURCE: Perkovic V et al. N Engl J Med. 2019 Apr 14. doi: 10.1056/NEJMoa1811744.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
No clear winner for treating neuropathic pain
PHILADELPHIA – Nearly 7%-10% of the general population experiences neuropathic pain, but studies on treatments have not found a clear winner for reducing this “burning or electriclike pain,” explained Raymond Price, MD, during a presentation.
“It isn’t that exciting,” said Dr. Price, associate professor of neurology at the University of Pennsylvania, Philadelphia, in reference to his review of level 1-2 evidence for treatment of neuropathic pain that was presented in a study published in JAMA (2015 Nov 24;314[20]:2172-81). a few years ago. “On a scale of 1 to 10, you can reduce their pain scale by 1-2 points more than placebo,” he told his audience at the annual meeting of the American College of Physicians.
There are very limited head-to-head data as to which one is actually better,” he explained.
Given the absence of robust head-to-head trial data, Dr. Price tends to start a lot of patients on old, cheap medications like nortriptyline.
While there aren’t many head-to-head trials to guide treatment choice, the results of one prospective, randomized, open-label study of 333 patients with cryptogenic sensory polyneuropathy was presented by Barohn and colleagues at the 2018 annual meeting of the American Academy of Neurology, he said. In that study, somewhat higher efficacy rates were seen with duloxetine, a serotonin-noradrenaline reuptake inhibitor, and nortriptyline, a tricyclic antidepressant, compared with pregabalin, Dr. Price noted. Duloxetine and nortriptyline also had slightly better tolerability, as evidenced by a lower quit rate, compared with pregabalin, he added.
There was also a systematic review and meta-analysis (Lancet Neurol. 2015 Feb; 14[2]:162-73) conducted that determined the number needed to treat for neuropathic pain treatments, Dr. Price noted. In that paper, tricyclic antidepressants had a number needed to treat of 3.6, comparing favorably to 7.7 for pregabalin, 7.2 for gabapentin, and 6.4 for serotonin-noradrenaline reuptake inhibitors, mainly including duloxetine, said Dr. Price.
Regardless of the cause of neuropathic pain, the same general approach to treatment is taken, though most of the evidence comes from studies of patients with painful diabetic peripheral neuropathy or postherpetic neuralgia, he added.
For these patients, an adequate trial of a neuropathic pain treatment should be 6-12 weeks, reflecting the length of the intervention needed to demonstrate the efficacy of these agents, he said.
If that first drug doesn’t work, another can be tried, or multiple drugs can be tried together to see if the patient’s condition improves, he said.
Dr. Price reported no conflicts of interest.
SOURCE: Price R Internal Medicine 2019, Presentation MSFM 002.
PHILADELPHIA – Nearly 7%-10% of the general population experiences neuropathic pain, but studies on treatments have not found a clear winner for reducing this “burning or electriclike pain,” explained Raymond Price, MD, during a presentation.
“It isn’t that exciting,” said Dr. Price, associate professor of neurology at the University of Pennsylvania, Philadelphia, in reference to his review of level 1-2 evidence for treatment of neuropathic pain that was presented in a study published in JAMA (2015 Nov 24;314[20]:2172-81). a few years ago. “On a scale of 1 to 10, you can reduce their pain scale by 1-2 points more than placebo,” he told his audience at the annual meeting of the American College of Physicians.
There are very limited head-to-head data as to which one is actually better,” he explained.
Given the absence of robust head-to-head trial data, Dr. Price tends to start a lot of patients on old, cheap medications like nortriptyline.
While there aren’t many head-to-head trials to guide treatment choice, the results of one prospective, randomized, open-label study of 333 patients with cryptogenic sensory polyneuropathy was presented by Barohn and colleagues at the 2018 annual meeting of the American Academy of Neurology, he said. In that study, somewhat higher efficacy rates were seen with duloxetine, a serotonin-noradrenaline reuptake inhibitor, and nortriptyline, a tricyclic antidepressant, compared with pregabalin, Dr. Price noted. Duloxetine and nortriptyline also had slightly better tolerability, as evidenced by a lower quit rate, compared with pregabalin, he added.
There was also a systematic review and meta-analysis (Lancet Neurol. 2015 Feb; 14[2]:162-73) conducted that determined the number needed to treat for neuropathic pain treatments, Dr. Price noted. In that paper, tricyclic antidepressants had a number needed to treat of 3.6, comparing favorably to 7.7 for pregabalin, 7.2 for gabapentin, and 6.4 for serotonin-noradrenaline reuptake inhibitors, mainly including duloxetine, said Dr. Price.
Regardless of the cause of neuropathic pain, the same general approach to treatment is taken, though most of the evidence comes from studies of patients with painful diabetic peripheral neuropathy or postherpetic neuralgia, he added.
For these patients, an adequate trial of a neuropathic pain treatment should be 6-12 weeks, reflecting the length of the intervention needed to demonstrate the efficacy of these agents, he said.
If that first drug doesn’t work, another can be tried, or multiple drugs can be tried together to see if the patient’s condition improves, he said.
Dr. Price reported no conflicts of interest.
SOURCE: Price R Internal Medicine 2019, Presentation MSFM 002.
PHILADELPHIA – Nearly 7%-10% of the general population experiences neuropathic pain, but studies on treatments have not found a clear winner for reducing this “burning or electriclike pain,” explained Raymond Price, MD, during a presentation.
“It isn’t that exciting,” said Dr. Price, associate professor of neurology at the University of Pennsylvania, Philadelphia, in reference to his review of level 1-2 evidence for treatment of neuropathic pain that was presented in a study published in JAMA (2015 Nov 24;314[20]:2172-81). a few years ago. “On a scale of 1 to 10, you can reduce their pain scale by 1-2 points more than placebo,” he told his audience at the annual meeting of the American College of Physicians.
There are very limited head-to-head data as to which one is actually better,” he explained.
Given the absence of robust head-to-head trial data, Dr. Price tends to start a lot of patients on old, cheap medications like nortriptyline.
While there aren’t many head-to-head trials to guide treatment choice, the results of one prospective, randomized, open-label study of 333 patients with cryptogenic sensory polyneuropathy was presented by Barohn and colleagues at the 2018 annual meeting of the American Academy of Neurology, he said. In that study, somewhat higher efficacy rates were seen with duloxetine, a serotonin-noradrenaline reuptake inhibitor, and nortriptyline, a tricyclic antidepressant, compared with pregabalin, Dr. Price noted. Duloxetine and nortriptyline also had slightly better tolerability, as evidenced by a lower quit rate, compared with pregabalin, he added.
There was also a systematic review and meta-analysis (Lancet Neurol. 2015 Feb; 14[2]:162-73) conducted that determined the number needed to treat for neuropathic pain treatments, Dr. Price noted. In that paper, tricyclic antidepressants had a number needed to treat of 3.6, comparing favorably to 7.7 for pregabalin, 7.2 for gabapentin, and 6.4 for serotonin-noradrenaline reuptake inhibitors, mainly including duloxetine, said Dr. Price.
Regardless of the cause of neuropathic pain, the same general approach to treatment is taken, though most of the evidence comes from studies of patients with painful diabetic peripheral neuropathy or postherpetic neuralgia, he added.
For these patients, an adequate trial of a neuropathic pain treatment should be 6-12 weeks, reflecting the length of the intervention needed to demonstrate the efficacy of these agents, he said.
If that first drug doesn’t work, another can be tried, or multiple drugs can be tried together to see if the patient’s condition improves, he said.
Dr. Price reported no conflicts of interest.
SOURCE: Price R Internal Medicine 2019, Presentation MSFM 002.
AT INTERNAL MEDICINE 2019
Obeticholic acid reversed NASH liver fibrosis in phase 3 trial
VIENNA – making obeticholic acid the first agent proven to improve the course of this disease.
“There is no doubt that with these data we have changed the treatment” of nonalcoholic steatohepatitis (NASH), Zobair M. Younossi, MD, of Inova Fairfax Medical Campus in Falls Church, Va., said at the meeting sponsored by the European Association for the Study of the Liver. “We are at a watershed moment” in NASH treatment, Dr. Younossi added in a video interview.
Until now “we have had no effective treatments for NASH. This is the first success in a phase 3 trial; obeticholic acid looks very promising,” commented Philip N. Newsome, PhD, professor of experimental hepatology at the University of Birmingham (England).
Obeticholic acid (OCA), an agonist of the farnesoid X receptor, already has Food and Drug Administration marketing approval for the indication of primary biliary cholangitis, a much rarer disease than NASH.
The REGENERATE (Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment) trial has so far enrolled 931 patients at about 350 sites in 20 countries, including the United States, and followed them during 18 months of treatment, the prespecified time for an interim analysis. The study enrolled adults with biopsy-proven NASH and generally focused on patients with either stage 2 or 3 liver fibrosis and a nonalcoholic fatty liver disease activity score of at least 4. Enrolled patients averaged about 55 years old, slightly more than half the enrolled patients had type 2 diabetes, and more than half had stage 3 fibrosis.
The study design included two coprimary endpoints, and specified that a statistically significant finding for either outcome meant a positive trial result, but the design also prespecified that the benefit would need to meet a stringent definition of statistical significance, compared with placebo patients, with a P value of no more than .01. REGENERATE tested two different OCA dosages, 10 mg or 25 mg, once daily. The results showed a trend for benefit from the smaller dosage, but these effects did not achieve statistical significance.
For the primary endpoint of regression of liver fibrosis by at least one stage with no worsening of NASH the intention-to-treat analysis showed after 18 months a 13% rate with placebo, a 21% rate with the 10-mg dosage, and a 23% rate with the 25-mg dosage, a statistically significant improvement over placebo for the higher dosage.
The second primary endpoint was resolution of NASH without worsening liver fibrosis, which occurred in 8% of placebo patients, 11% of patients on 10 mg OCA/day and 12% of those on 25 mg/day. The differences between each of the active groups and the controls were not statistically significant for this endpoint.
Among the 931 enrolled patients 668 (72%) actually received treatment fully consistent with the study protocol, and among these per-protocol patients the benefit from 25 mg/day OCA was even more striking: a 28% rate of fibrosis regression, compared with 13% in the control patients. Regression by at least two fibrotic stages occurred in 5% of placebo patients and 13% of those on 25 mg/day OCA. Many treated patients also showed normalizations of liver enzyme levels.
Adverse events on OCA were mostly mild or moderate, with similar rates of serious adverse events in the OCA groups and in control patients. The most common adverse effect on OCA treatment was pruritus, a previously described effect, reported by 51% of patients on the 25 mg/day dosage and by 19% of control patients.
REGENERATE will continue until a goal level of endpoint events occur, and may eventually enroll as many as 2,400 patients and extend for a few more years. By then, Dr. Younossi said, he hopes that an analysis will be possible of “harder” endpoints than fibrosis, such as development of cirrhosis. He noted, however, that the FDA has designated fibrosis regression as a valid surrogate endpoint for assessing treatment efficacy for NASH.
Already on the U.S. market, a single 10-mg OCA pill currently retails for almost $230; a 25-mg formulation is not currently marketed. Dr. Younossi said that subsequent studies will assess the cost-effectiveness of OCA treatment for NASH. He also hopes that further study of patient characteristics will identify which NASH patients are most likely to respond to OCA. Eventually, OCA may be part of a multidrug strategy for treating this disease, Dr. Younossi said.
REGENERATE was sponsored by Intercept, the company that markets obeticholic acid (Ocaliva). Dr. Younossi is a consultant to and has received research funding from Intercept. He has also been a consultant to Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Novartis, Novo Nordisk, Quest, Siemens, Terns Pharmaceutical, and Viking Therapeutics. Dr. Newsome has been a consultant or speaker for Intercept as well as Boehringer Ingelheim, Dignity Sciences, Johnson & Johnson, Novo Nordisk, and Shire, and he has received research funding from Pharmaxis and Boehringer Ingelheim.
VIENNA – making obeticholic acid the first agent proven to improve the course of this disease.
“There is no doubt that with these data we have changed the treatment” of nonalcoholic steatohepatitis (NASH), Zobair M. Younossi, MD, of Inova Fairfax Medical Campus in Falls Church, Va., said at the meeting sponsored by the European Association for the Study of the Liver. “We are at a watershed moment” in NASH treatment, Dr. Younossi added in a video interview.
Until now “we have had no effective treatments for NASH. This is the first success in a phase 3 trial; obeticholic acid looks very promising,” commented Philip N. Newsome, PhD, professor of experimental hepatology at the University of Birmingham (England).
Obeticholic acid (OCA), an agonist of the farnesoid X receptor, already has Food and Drug Administration marketing approval for the indication of primary biliary cholangitis, a much rarer disease than NASH.
The REGENERATE (Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment) trial has so far enrolled 931 patients at about 350 sites in 20 countries, including the United States, and followed them during 18 months of treatment, the prespecified time for an interim analysis. The study enrolled adults with biopsy-proven NASH and generally focused on patients with either stage 2 or 3 liver fibrosis and a nonalcoholic fatty liver disease activity score of at least 4. Enrolled patients averaged about 55 years old, slightly more than half the enrolled patients had type 2 diabetes, and more than half had stage 3 fibrosis.
The study design included two coprimary endpoints, and specified that a statistically significant finding for either outcome meant a positive trial result, but the design also prespecified that the benefit would need to meet a stringent definition of statistical significance, compared with placebo patients, with a P value of no more than .01. REGENERATE tested two different OCA dosages, 10 mg or 25 mg, once daily. The results showed a trend for benefit from the smaller dosage, but these effects did not achieve statistical significance.
For the primary endpoint of regression of liver fibrosis by at least one stage with no worsening of NASH the intention-to-treat analysis showed after 18 months a 13% rate with placebo, a 21% rate with the 10-mg dosage, and a 23% rate with the 25-mg dosage, a statistically significant improvement over placebo for the higher dosage.
The second primary endpoint was resolution of NASH without worsening liver fibrosis, which occurred in 8% of placebo patients, 11% of patients on 10 mg OCA/day and 12% of those on 25 mg/day. The differences between each of the active groups and the controls were not statistically significant for this endpoint.
Among the 931 enrolled patients 668 (72%) actually received treatment fully consistent with the study protocol, and among these per-protocol patients the benefit from 25 mg/day OCA was even more striking: a 28% rate of fibrosis regression, compared with 13% in the control patients. Regression by at least two fibrotic stages occurred in 5% of placebo patients and 13% of those on 25 mg/day OCA. Many treated patients also showed normalizations of liver enzyme levels.
Adverse events on OCA were mostly mild or moderate, with similar rates of serious adverse events in the OCA groups and in control patients. The most common adverse effect on OCA treatment was pruritus, a previously described effect, reported by 51% of patients on the 25 mg/day dosage and by 19% of control patients.
REGENERATE will continue until a goal level of endpoint events occur, and may eventually enroll as many as 2,400 patients and extend for a few more years. By then, Dr. Younossi said, he hopes that an analysis will be possible of “harder” endpoints than fibrosis, such as development of cirrhosis. He noted, however, that the FDA has designated fibrosis regression as a valid surrogate endpoint for assessing treatment efficacy for NASH.
Already on the U.S. market, a single 10-mg OCA pill currently retails for almost $230; a 25-mg formulation is not currently marketed. Dr. Younossi said that subsequent studies will assess the cost-effectiveness of OCA treatment for NASH. He also hopes that further study of patient characteristics will identify which NASH patients are most likely to respond to OCA. Eventually, OCA may be part of a multidrug strategy for treating this disease, Dr. Younossi said.
REGENERATE was sponsored by Intercept, the company that markets obeticholic acid (Ocaliva). Dr. Younossi is a consultant to and has received research funding from Intercept. He has also been a consultant to Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Novartis, Novo Nordisk, Quest, Siemens, Terns Pharmaceutical, and Viking Therapeutics. Dr. Newsome has been a consultant or speaker for Intercept as well as Boehringer Ingelheim, Dignity Sciences, Johnson & Johnson, Novo Nordisk, and Shire, and he has received research funding from Pharmaxis and Boehringer Ingelheim.
VIENNA – making obeticholic acid the first agent proven to improve the course of this disease.
“There is no doubt that with these data we have changed the treatment” of nonalcoholic steatohepatitis (NASH), Zobair M. Younossi, MD, of Inova Fairfax Medical Campus in Falls Church, Va., said at the meeting sponsored by the European Association for the Study of the Liver. “We are at a watershed moment” in NASH treatment, Dr. Younossi added in a video interview.
Until now “we have had no effective treatments for NASH. This is the first success in a phase 3 trial; obeticholic acid looks very promising,” commented Philip N. Newsome, PhD, professor of experimental hepatology at the University of Birmingham (England).
Obeticholic acid (OCA), an agonist of the farnesoid X receptor, already has Food and Drug Administration marketing approval for the indication of primary biliary cholangitis, a much rarer disease than NASH.
The REGENERATE (Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid Treatment) trial has so far enrolled 931 patients at about 350 sites in 20 countries, including the United States, and followed them during 18 months of treatment, the prespecified time for an interim analysis. The study enrolled adults with biopsy-proven NASH and generally focused on patients with either stage 2 or 3 liver fibrosis and a nonalcoholic fatty liver disease activity score of at least 4. Enrolled patients averaged about 55 years old, slightly more than half the enrolled patients had type 2 diabetes, and more than half had stage 3 fibrosis.
The study design included two coprimary endpoints, and specified that a statistically significant finding for either outcome meant a positive trial result, but the design also prespecified that the benefit would need to meet a stringent definition of statistical significance, compared with placebo patients, with a P value of no more than .01. REGENERATE tested two different OCA dosages, 10 mg or 25 mg, once daily. The results showed a trend for benefit from the smaller dosage, but these effects did not achieve statistical significance.
For the primary endpoint of regression of liver fibrosis by at least one stage with no worsening of NASH the intention-to-treat analysis showed after 18 months a 13% rate with placebo, a 21% rate with the 10-mg dosage, and a 23% rate with the 25-mg dosage, a statistically significant improvement over placebo for the higher dosage.
The second primary endpoint was resolution of NASH without worsening liver fibrosis, which occurred in 8% of placebo patients, 11% of patients on 10 mg OCA/day and 12% of those on 25 mg/day. The differences between each of the active groups and the controls were not statistically significant for this endpoint.
Among the 931 enrolled patients 668 (72%) actually received treatment fully consistent with the study protocol, and among these per-protocol patients the benefit from 25 mg/day OCA was even more striking: a 28% rate of fibrosis regression, compared with 13% in the control patients. Regression by at least two fibrotic stages occurred in 5% of placebo patients and 13% of those on 25 mg/day OCA. Many treated patients also showed normalizations of liver enzyme levels.
Adverse events on OCA were mostly mild or moderate, with similar rates of serious adverse events in the OCA groups and in control patients. The most common adverse effect on OCA treatment was pruritus, a previously described effect, reported by 51% of patients on the 25 mg/day dosage and by 19% of control patients.
REGENERATE will continue until a goal level of endpoint events occur, and may eventually enroll as many as 2,400 patients and extend for a few more years. By then, Dr. Younossi said, he hopes that an analysis will be possible of “harder” endpoints than fibrosis, such as development of cirrhosis. He noted, however, that the FDA has designated fibrosis regression as a valid surrogate endpoint for assessing treatment efficacy for NASH.
Already on the U.S. market, a single 10-mg OCA pill currently retails for almost $230; a 25-mg formulation is not currently marketed. Dr. Younossi said that subsequent studies will assess the cost-effectiveness of OCA treatment for NASH. He also hopes that further study of patient characteristics will identify which NASH patients are most likely to respond to OCA. Eventually, OCA may be part of a multidrug strategy for treating this disease, Dr. Younossi said.
REGENERATE was sponsored by Intercept, the company that markets obeticholic acid (Ocaliva). Dr. Younossi is a consultant to and has received research funding from Intercept. He has also been a consultant to Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Novartis, Novo Nordisk, Quest, Siemens, Terns Pharmaceutical, and Viking Therapeutics. Dr. Newsome has been a consultant or speaker for Intercept as well as Boehringer Ingelheim, Dignity Sciences, Johnson & Johnson, Novo Nordisk, and Shire, and he has received research funding from Pharmaxis and Boehringer Ingelheim.
REPORTING FROM ILC 2019
Bipartisanship breaks out at House hearing on insulin prices
Who’s responsible for the rising cost of insulin? Manufacturers and pharmacy benefit managers pointed their fingers at each other on April 10 at a congressional hearing.
In response, Democrats and Republicans on the House Energy & Commerce Subcommittee on Oversight and Investigations said they might just take matters into their own hands.
Rebates and discounts are the drivers, according to leaders from three insulin manufacturers.
Mike Mason, a senior vice president of Eli Lilly & Co., was put on the defensive immediately by Subcommittee Chairman Diana DeGette (D-Colo.), who asked him to justify the increases in list prices during the last 10 years.
“Seventy-five percent of our list price is paid for rebates and discounts to secure access so people have affordable access,” Mr. Mason said.
He was cut off in his response as Rep. DeGette pressed further: “So that’s what’s making the price go up and up.”
To which Mr. Mason responded, “$210 of a vial of Humalog is paid for discounts and rebates.” It was noted during the hearing that a vial has a list price of $275.
Doug Langa, an executive vice president at Novo Nordisk, agreed. “There is significant demand for rebates.”
Kathleen Tregoning, an executive vice president at Sanofi, added that, as part of setting the list price, “we have to look at the dynamics of the supply chain, including the rebates.”
Leaders of several pharmacy benefit managers disagreed.
Rep. DeGette asked Thomas M. Moriarty, an executive vice president and general counsel at CVS Health whether he thought rebates were forcing manufacturers to raise list prices. His response? “I do not, no.”
Amy Bricker, a senior vice president at Express Scripts concurred. “I have no idea why list prices are high, and it’s not a result of rebates.”
Sumit Dutta, MD, a senior vice president and chief medical officer at OptumRx, added that there have been list prices rising double digits in nonrebated drugs, in generics where a manufacturer buys out the market to create a monopoly.
“We can’t see a correlation just when rebates raise list prices,” Dr. Dutta said.
While the PBMs denied the rebate system played any role in the setting of list prices, they were firm in maintaining secrecy in rebating process.
When asked by Rep. John Sarbanes (D-Md.) whether the public should be able to track the list price and see the rebates, the net prices, and the savings that are passed along to the consumer, Ms. Bricker said “we don’t believe so.”
She continued: “The reason I’m able to get the discounts that I can from the manufacturer is because it is confidential.”
And while Ms. Tregoning offered support for full transparency in every facet of the supply chain, Ms. Bricker did not. “It will hurt the consumer, Congressman, because prices will be held high.”
“I’m not buying it,” Rep. Sarbanes replied. “I think a system has been built that allows for gaming to go on and you’ve all got your talking points.”
Rep. Buddy Carter (R-Ga.), Congress’ only pharmacist, does not on the committee but was allowed to participate in the hearing. He shared with his colleagues stories of customers leaving prescriptions behind because of cost.
He asked Mr. Langa whether he thought PBM consolidation played a role in driving up rebates and list prices, to which Mr. Langa said, “I think it was a factor.”
Rep. Carter offering a sarcastic congratulations to the panel.
“You’ve done something here today that we’ve been trying to do in Congress for the 4 years and 3 months I’ve been here, and that is to create bipartisanship.”
He then cautioned PBM leaders on the panel that the status quo “is going to end. ... I have seen what you have done with the PBMs” and all the various fees that have been created over time and said that Congress will make sure rebate reform will happen, specifically for Medicare and Medicaid. But he added, “we are not going to stop there.”
During the hearing, the manufacturers, while being not completely committal, suggested that list prices could in fact come down if rebates and discounts were done away with, while the PBMs would not commit to flat administrative fees as opposed to current fees that are based on list prices.
Rep. DeGette said that work will continue until all parties come up with a viable solution. Action “is not optional and it is going to happen.”
Who’s responsible for the rising cost of insulin? Manufacturers and pharmacy benefit managers pointed their fingers at each other on April 10 at a congressional hearing.
In response, Democrats and Republicans on the House Energy & Commerce Subcommittee on Oversight and Investigations said they might just take matters into their own hands.
Rebates and discounts are the drivers, according to leaders from three insulin manufacturers.
Mike Mason, a senior vice president of Eli Lilly & Co., was put on the defensive immediately by Subcommittee Chairman Diana DeGette (D-Colo.), who asked him to justify the increases in list prices during the last 10 years.
“Seventy-five percent of our list price is paid for rebates and discounts to secure access so people have affordable access,” Mr. Mason said.
He was cut off in his response as Rep. DeGette pressed further: “So that’s what’s making the price go up and up.”
To which Mr. Mason responded, “$210 of a vial of Humalog is paid for discounts and rebates.” It was noted during the hearing that a vial has a list price of $275.
Doug Langa, an executive vice president at Novo Nordisk, agreed. “There is significant demand for rebates.”
Kathleen Tregoning, an executive vice president at Sanofi, added that, as part of setting the list price, “we have to look at the dynamics of the supply chain, including the rebates.”
Leaders of several pharmacy benefit managers disagreed.
Rep. DeGette asked Thomas M. Moriarty, an executive vice president and general counsel at CVS Health whether he thought rebates were forcing manufacturers to raise list prices. His response? “I do not, no.”
Amy Bricker, a senior vice president at Express Scripts concurred. “I have no idea why list prices are high, and it’s not a result of rebates.”
Sumit Dutta, MD, a senior vice president and chief medical officer at OptumRx, added that there have been list prices rising double digits in nonrebated drugs, in generics where a manufacturer buys out the market to create a monopoly.
“We can’t see a correlation just when rebates raise list prices,” Dr. Dutta said.
While the PBMs denied the rebate system played any role in the setting of list prices, they were firm in maintaining secrecy in rebating process.
When asked by Rep. John Sarbanes (D-Md.) whether the public should be able to track the list price and see the rebates, the net prices, and the savings that are passed along to the consumer, Ms. Bricker said “we don’t believe so.”
She continued: “The reason I’m able to get the discounts that I can from the manufacturer is because it is confidential.”
And while Ms. Tregoning offered support for full transparency in every facet of the supply chain, Ms. Bricker did not. “It will hurt the consumer, Congressman, because prices will be held high.”
“I’m not buying it,” Rep. Sarbanes replied. “I think a system has been built that allows for gaming to go on and you’ve all got your talking points.”
Rep. Buddy Carter (R-Ga.), Congress’ only pharmacist, does not on the committee but was allowed to participate in the hearing. He shared with his colleagues stories of customers leaving prescriptions behind because of cost.
He asked Mr. Langa whether he thought PBM consolidation played a role in driving up rebates and list prices, to which Mr. Langa said, “I think it was a factor.”
Rep. Carter offering a sarcastic congratulations to the panel.
“You’ve done something here today that we’ve been trying to do in Congress for the 4 years and 3 months I’ve been here, and that is to create bipartisanship.”
He then cautioned PBM leaders on the panel that the status quo “is going to end. ... I have seen what you have done with the PBMs” and all the various fees that have been created over time and said that Congress will make sure rebate reform will happen, specifically for Medicare and Medicaid. But he added, “we are not going to stop there.”
During the hearing, the manufacturers, while being not completely committal, suggested that list prices could in fact come down if rebates and discounts were done away with, while the PBMs would not commit to flat administrative fees as opposed to current fees that are based on list prices.
Rep. DeGette said that work will continue until all parties come up with a viable solution. Action “is not optional and it is going to happen.”
Who’s responsible for the rising cost of insulin? Manufacturers and pharmacy benefit managers pointed their fingers at each other on April 10 at a congressional hearing.
In response, Democrats and Republicans on the House Energy & Commerce Subcommittee on Oversight and Investigations said they might just take matters into their own hands.
Rebates and discounts are the drivers, according to leaders from three insulin manufacturers.
Mike Mason, a senior vice president of Eli Lilly & Co., was put on the defensive immediately by Subcommittee Chairman Diana DeGette (D-Colo.), who asked him to justify the increases in list prices during the last 10 years.
“Seventy-five percent of our list price is paid for rebates and discounts to secure access so people have affordable access,” Mr. Mason said.
He was cut off in his response as Rep. DeGette pressed further: “So that’s what’s making the price go up and up.”
To which Mr. Mason responded, “$210 of a vial of Humalog is paid for discounts and rebates.” It was noted during the hearing that a vial has a list price of $275.
Doug Langa, an executive vice president at Novo Nordisk, agreed. “There is significant demand for rebates.”
Kathleen Tregoning, an executive vice president at Sanofi, added that, as part of setting the list price, “we have to look at the dynamics of the supply chain, including the rebates.”
Leaders of several pharmacy benefit managers disagreed.
Rep. DeGette asked Thomas M. Moriarty, an executive vice president and general counsel at CVS Health whether he thought rebates were forcing manufacturers to raise list prices. His response? “I do not, no.”
Amy Bricker, a senior vice president at Express Scripts concurred. “I have no idea why list prices are high, and it’s not a result of rebates.”
Sumit Dutta, MD, a senior vice president and chief medical officer at OptumRx, added that there have been list prices rising double digits in nonrebated drugs, in generics where a manufacturer buys out the market to create a monopoly.
“We can’t see a correlation just when rebates raise list prices,” Dr. Dutta said.
While the PBMs denied the rebate system played any role in the setting of list prices, they were firm in maintaining secrecy in rebating process.
When asked by Rep. John Sarbanes (D-Md.) whether the public should be able to track the list price and see the rebates, the net prices, and the savings that are passed along to the consumer, Ms. Bricker said “we don’t believe so.”
She continued: “The reason I’m able to get the discounts that I can from the manufacturer is because it is confidential.”
And while Ms. Tregoning offered support for full transparency in every facet of the supply chain, Ms. Bricker did not. “It will hurt the consumer, Congressman, because prices will be held high.”
“I’m not buying it,” Rep. Sarbanes replied. “I think a system has been built that allows for gaming to go on and you’ve all got your talking points.”
Rep. Buddy Carter (R-Ga.), Congress’ only pharmacist, does not on the committee but was allowed to participate in the hearing. He shared with his colleagues stories of customers leaving prescriptions behind because of cost.
He asked Mr. Langa whether he thought PBM consolidation played a role in driving up rebates and list prices, to which Mr. Langa said, “I think it was a factor.”
Rep. Carter offering a sarcastic congratulations to the panel.
“You’ve done something here today that we’ve been trying to do in Congress for the 4 years and 3 months I’ve been here, and that is to create bipartisanship.”
He then cautioned PBM leaders on the panel that the status quo “is going to end. ... I have seen what you have done with the PBMs” and all the various fees that have been created over time and said that Congress will make sure rebate reform will happen, specifically for Medicare and Medicaid. But he added, “we are not going to stop there.”
During the hearing, the manufacturers, while being not completely committal, suggested that list prices could in fact come down if rebates and discounts were done away with, while the PBMs would not commit to flat administrative fees as opposed to current fees that are based on list prices.
Rep. DeGette said that work will continue until all parties come up with a viable solution. Action “is not optional and it is going to happen.”
REPORTING FROM A HOUSE ENERGY AND COMMERCE SUBCOMMITTEE HEARING
Dapagliflozin’s cardiovascular benefits bloom in T2D with prior MI
NEW ORLEANS – Dapagliflozin markedly reduces the risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes (T2D) and prior MI, according to a new subanalysis of the landmark DECLARE-TIMI 58 trial.
The effectiveness of dapagliflozin (Farxiga), an oral sodium glucose transporter-2 inhibitor (SGLT-2i), was particularly striking with regard to prevention of recurrent MI, Remo H.M. Furtado, MD, reported at the annual meeting of the American College of Cardiology.
“The 22% relative risk reduction in recurrent MI with dapagliflozin is comparable to other established therapies used in secondary prevention after MI, like DAPT [dual-antiplatelet therapy] and intensive lipid lowering,” observed Dr. Furtado of Brigham and Women’s Hospital, Boston.
Not bad for a drug developed as a glucose-lowering agent.
The new DECLARE-TIMI 58 subanalysis provides information that’s relevant to ACC guidelines issued in late 2018. The guidelines, in the form of an “expert consensus decision pathway,” emphatically recommend that all patients with T2D and known atherosclerotic cardiovascular disease (ASCVD) should have metformin as their first-line glucose-lowering agent, while at the same time giving serious consideration to the addition of either an oral SGLT-2i or a subcutaneously injected glucagonlike peptide–1 receptor agonist (GLP-1RA) with demonstrated cardiovascular benefit as a second glucose-lowering agent (J Am Coll Cardiol. 2018 Dec 18;72[24]:3200-23). The DECLARE-TIMI 58 subanalysis bolsters that guidance and shows, more specifically, that the cardioprotective benefits of dapagliflozin are significantly greater in T2D with prior MI than in those with known ASCVD but no history of MI, the cardiologist explained.
The main results of DECLARE-TIMI 58 have been published. The trial included 17,160 patients with T2D, 6,974 of whom had established ASCVD, while the remainder had multiple ASCVD risk factors. Participants were randomized to oral dapagliflozin at 10 mg/day or placebo on top of background guideline-directed medical therapy and followed for a median of 4.2 years. The dapagliflozin group had a 27% reduction in heart failure hospitalizations, compared with controls, but there were no significant between-group differences in the composite MACE (major adverse cardiovascular events) endpoint of cardiovascular death, MI, or ischemic stroke (N Engl J Med. 2019 Jan 24;380[4]:347-57).
Dr. Furtado presented a prespecified subgroup analysis focused on the 3,584 study participants with prior MI. Their rate of the composite endpoint of cardiovascular death, MI, or ischemic stroke was 15.2%, compared with 17.8% in controls, for a statistically significant and clinically meaningful 16% relative risk reduction and an absolute 2.6% risk reduction. Of note, the risk of recurrent MI was reduced by 22%. In contrast, there was no difference in MACE risk between the dapagliflozin and placebo groups in patients with no prior MI, even if they had established ASCVD.
A noteworthy finding was that the benefit in MACE reduction in patients with prior MI was greater in those who were closer in time to their most recent MI at enrollment in the study. Those who started on dapagliflozin within 12 months of their last MI had a 34% relative risk reduction in MACE, compared with placebo, while those who enrolled 12-24 months after their last MI enjoyed an even more robust 58% relative risk reduction on dapagliflozin. In contrast, patients who enrolled 24-36 months post MI had only a 17% relative risk reduction, and the 2,400 patients who enrolled more than 36 months after their last MI had a subsequent MACE rate no different from controls.
Session cochair Nadia R. Sutton, MD, a cardiologist at the University of Michigan, Ann Arbor, commented that she found this time-dependent benefit fascinating.
“Do you think this has anything to do with the escalation of other therapies, such as Plavix [clopidogrel]?” she asked.
Dr. Furtado replied, “This is a finding that we should interpret with a little bit of caution.” For one thing, patients in the acute phase of an MI were excluded from participation in the trial, so nothing is known about how they would fare on dapagliflozin. For another, only 844 of the 3,584 patients with T2D and prior MI had their most recent MI within 24 months of enrollment, so even though the differences were statistically significant, the confidence intervals are fairly wide.
That being said, the finding does underscore a truism about cardiovascular prevention: The higher the risk, the greater the benefit of effective therapy – and, of course, the initial months following an MI are a particularly high-risk period.
“Also, this finding is a caution to the clinicians to avoid clinical inertia in prescribing an SGLT-2i, because maybe you can get an early benefit if you prescribe the drug closer to the acute phase and not wait until some months after the patient has tried diet and exercise and so on,” he added.
With regard to the second coprimary endpoint comprising cardiovascular death or heart failure hospitalization in T2D patients with prior MI, the rate in the dapagliflozin group was 8.6%, a 19% relative risk reduction and absolute risk reduction of 1.9%, compared with the 10.5% rate with placebo.
Dr. Furtado noted that the main results of DECLARE TIMI-58 are consistent with a recent systematic review and meta-analysis of three randomized cardiovascular outcome trials of SGLT-2is for primary and secondary prevention of cardiovascular and renal outcomes in T2D. The meta-analysis included more than 34,000 patients, roughly half drawn from DECLARE-TIMI 58. SGLT-2i therapy reduced MACE by 14% in patients with established ASCVD but not significantly in those without. And the agents reduced the risk of cardiovascular death/heart failure hospitalization by 23%, regardless of whether or not patients had known ASCVD or a history of heart failure (Lancet. 2019 Jan 5;393[10166]:31-9).
Dr. Furtado reported serving as a consultant to AstraZeneca, which funded DECLARE-TIMI 58, as well as receiving direct institutional research grants from half a dozen other pharmaceutical companies.
Simultaneous with his presentation at ACC 2019 in New Orleans, the subanalysis results were published online (Circulation. 2019 Mar 18. doi: 10.1161/CIRCULATIONAHA.119.039996. [Epub ahead of print]).
NEW ORLEANS – Dapagliflozin markedly reduces the risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes (T2D) and prior MI, according to a new subanalysis of the landmark DECLARE-TIMI 58 trial.
The effectiveness of dapagliflozin (Farxiga), an oral sodium glucose transporter-2 inhibitor (SGLT-2i), was particularly striking with regard to prevention of recurrent MI, Remo H.M. Furtado, MD, reported at the annual meeting of the American College of Cardiology.
“The 22% relative risk reduction in recurrent MI with dapagliflozin is comparable to other established therapies used in secondary prevention after MI, like DAPT [dual-antiplatelet therapy] and intensive lipid lowering,” observed Dr. Furtado of Brigham and Women’s Hospital, Boston.
Not bad for a drug developed as a glucose-lowering agent.
The new DECLARE-TIMI 58 subanalysis provides information that’s relevant to ACC guidelines issued in late 2018. The guidelines, in the form of an “expert consensus decision pathway,” emphatically recommend that all patients with T2D and known atherosclerotic cardiovascular disease (ASCVD) should have metformin as their first-line glucose-lowering agent, while at the same time giving serious consideration to the addition of either an oral SGLT-2i or a subcutaneously injected glucagonlike peptide–1 receptor agonist (GLP-1RA) with demonstrated cardiovascular benefit as a second glucose-lowering agent (J Am Coll Cardiol. 2018 Dec 18;72[24]:3200-23). The DECLARE-TIMI 58 subanalysis bolsters that guidance and shows, more specifically, that the cardioprotective benefits of dapagliflozin are significantly greater in T2D with prior MI than in those with known ASCVD but no history of MI, the cardiologist explained.
The main results of DECLARE-TIMI 58 have been published. The trial included 17,160 patients with T2D, 6,974 of whom had established ASCVD, while the remainder had multiple ASCVD risk factors. Participants were randomized to oral dapagliflozin at 10 mg/day or placebo on top of background guideline-directed medical therapy and followed for a median of 4.2 years. The dapagliflozin group had a 27% reduction in heart failure hospitalizations, compared with controls, but there were no significant between-group differences in the composite MACE (major adverse cardiovascular events) endpoint of cardiovascular death, MI, or ischemic stroke (N Engl J Med. 2019 Jan 24;380[4]:347-57).
Dr. Furtado presented a prespecified subgroup analysis focused on the 3,584 study participants with prior MI. Their rate of the composite endpoint of cardiovascular death, MI, or ischemic stroke was 15.2%, compared with 17.8% in controls, for a statistically significant and clinically meaningful 16% relative risk reduction and an absolute 2.6% risk reduction. Of note, the risk of recurrent MI was reduced by 22%. In contrast, there was no difference in MACE risk between the dapagliflozin and placebo groups in patients with no prior MI, even if they had established ASCVD.
A noteworthy finding was that the benefit in MACE reduction in patients with prior MI was greater in those who were closer in time to their most recent MI at enrollment in the study. Those who started on dapagliflozin within 12 months of their last MI had a 34% relative risk reduction in MACE, compared with placebo, while those who enrolled 12-24 months after their last MI enjoyed an even more robust 58% relative risk reduction on dapagliflozin. In contrast, patients who enrolled 24-36 months post MI had only a 17% relative risk reduction, and the 2,400 patients who enrolled more than 36 months after their last MI had a subsequent MACE rate no different from controls.
Session cochair Nadia R. Sutton, MD, a cardiologist at the University of Michigan, Ann Arbor, commented that she found this time-dependent benefit fascinating.
“Do you think this has anything to do with the escalation of other therapies, such as Plavix [clopidogrel]?” she asked.
Dr. Furtado replied, “This is a finding that we should interpret with a little bit of caution.” For one thing, patients in the acute phase of an MI were excluded from participation in the trial, so nothing is known about how they would fare on dapagliflozin. For another, only 844 of the 3,584 patients with T2D and prior MI had their most recent MI within 24 months of enrollment, so even though the differences were statistically significant, the confidence intervals are fairly wide.
That being said, the finding does underscore a truism about cardiovascular prevention: The higher the risk, the greater the benefit of effective therapy – and, of course, the initial months following an MI are a particularly high-risk period.
“Also, this finding is a caution to the clinicians to avoid clinical inertia in prescribing an SGLT-2i, because maybe you can get an early benefit if you prescribe the drug closer to the acute phase and not wait until some months after the patient has tried diet and exercise and so on,” he added.
With regard to the second coprimary endpoint comprising cardiovascular death or heart failure hospitalization in T2D patients with prior MI, the rate in the dapagliflozin group was 8.6%, a 19% relative risk reduction and absolute risk reduction of 1.9%, compared with the 10.5% rate with placebo.
Dr. Furtado noted that the main results of DECLARE TIMI-58 are consistent with a recent systematic review and meta-analysis of three randomized cardiovascular outcome trials of SGLT-2is for primary and secondary prevention of cardiovascular and renal outcomes in T2D. The meta-analysis included more than 34,000 patients, roughly half drawn from DECLARE-TIMI 58. SGLT-2i therapy reduced MACE by 14% in patients with established ASCVD but not significantly in those without. And the agents reduced the risk of cardiovascular death/heart failure hospitalization by 23%, regardless of whether or not patients had known ASCVD or a history of heart failure (Lancet. 2019 Jan 5;393[10166]:31-9).
Dr. Furtado reported serving as a consultant to AstraZeneca, which funded DECLARE-TIMI 58, as well as receiving direct institutional research grants from half a dozen other pharmaceutical companies.
Simultaneous with his presentation at ACC 2019 in New Orleans, the subanalysis results were published online (Circulation. 2019 Mar 18. doi: 10.1161/CIRCULATIONAHA.119.039996. [Epub ahead of print]).
NEW ORLEANS – Dapagliflozin markedly reduces the risks of both major adverse cardiovascular events and heart failure hospitalization in the subset of patients with type 2 diabetes (T2D) and prior MI, according to a new subanalysis of the landmark DECLARE-TIMI 58 trial.
The effectiveness of dapagliflozin (Farxiga), an oral sodium glucose transporter-2 inhibitor (SGLT-2i), was particularly striking with regard to prevention of recurrent MI, Remo H.M. Furtado, MD, reported at the annual meeting of the American College of Cardiology.
“The 22% relative risk reduction in recurrent MI with dapagliflozin is comparable to other established therapies used in secondary prevention after MI, like DAPT [dual-antiplatelet therapy] and intensive lipid lowering,” observed Dr. Furtado of Brigham and Women’s Hospital, Boston.
Not bad for a drug developed as a glucose-lowering agent.
The new DECLARE-TIMI 58 subanalysis provides information that’s relevant to ACC guidelines issued in late 2018. The guidelines, in the form of an “expert consensus decision pathway,” emphatically recommend that all patients with T2D and known atherosclerotic cardiovascular disease (ASCVD) should have metformin as their first-line glucose-lowering agent, while at the same time giving serious consideration to the addition of either an oral SGLT-2i or a subcutaneously injected glucagonlike peptide–1 receptor agonist (GLP-1RA) with demonstrated cardiovascular benefit as a second glucose-lowering agent (J Am Coll Cardiol. 2018 Dec 18;72[24]:3200-23). The DECLARE-TIMI 58 subanalysis bolsters that guidance and shows, more specifically, that the cardioprotective benefits of dapagliflozin are significantly greater in T2D with prior MI than in those with known ASCVD but no history of MI, the cardiologist explained.
The main results of DECLARE-TIMI 58 have been published. The trial included 17,160 patients with T2D, 6,974 of whom had established ASCVD, while the remainder had multiple ASCVD risk factors. Participants were randomized to oral dapagliflozin at 10 mg/day or placebo on top of background guideline-directed medical therapy and followed for a median of 4.2 years. The dapagliflozin group had a 27% reduction in heart failure hospitalizations, compared with controls, but there were no significant between-group differences in the composite MACE (major adverse cardiovascular events) endpoint of cardiovascular death, MI, or ischemic stroke (N Engl J Med. 2019 Jan 24;380[4]:347-57).
Dr. Furtado presented a prespecified subgroup analysis focused on the 3,584 study participants with prior MI. Their rate of the composite endpoint of cardiovascular death, MI, or ischemic stroke was 15.2%, compared with 17.8% in controls, for a statistically significant and clinically meaningful 16% relative risk reduction and an absolute 2.6% risk reduction. Of note, the risk of recurrent MI was reduced by 22%. In contrast, there was no difference in MACE risk between the dapagliflozin and placebo groups in patients with no prior MI, even if they had established ASCVD.
A noteworthy finding was that the benefit in MACE reduction in patients with prior MI was greater in those who were closer in time to their most recent MI at enrollment in the study. Those who started on dapagliflozin within 12 months of their last MI had a 34% relative risk reduction in MACE, compared with placebo, while those who enrolled 12-24 months after their last MI enjoyed an even more robust 58% relative risk reduction on dapagliflozin. In contrast, patients who enrolled 24-36 months post MI had only a 17% relative risk reduction, and the 2,400 patients who enrolled more than 36 months after their last MI had a subsequent MACE rate no different from controls.
Session cochair Nadia R. Sutton, MD, a cardiologist at the University of Michigan, Ann Arbor, commented that she found this time-dependent benefit fascinating.
“Do you think this has anything to do with the escalation of other therapies, such as Plavix [clopidogrel]?” she asked.
Dr. Furtado replied, “This is a finding that we should interpret with a little bit of caution.” For one thing, patients in the acute phase of an MI were excluded from participation in the trial, so nothing is known about how they would fare on dapagliflozin. For another, only 844 of the 3,584 patients with T2D and prior MI had their most recent MI within 24 months of enrollment, so even though the differences were statistically significant, the confidence intervals are fairly wide.
That being said, the finding does underscore a truism about cardiovascular prevention: The higher the risk, the greater the benefit of effective therapy – and, of course, the initial months following an MI are a particularly high-risk period.
“Also, this finding is a caution to the clinicians to avoid clinical inertia in prescribing an SGLT-2i, because maybe you can get an early benefit if you prescribe the drug closer to the acute phase and not wait until some months after the patient has tried diet and exercise and so on,” he added.
With regard to the second coprimary endpoint comprising cardiovascular death or heart failure hospitalization in T2D patients with prior MI, the rate in the dapagliflozin group was 8.6%, a 19% relative risk reduction and absolute risk reduction of 1.9%, compared with the 10.5% rate with placebo.
Dr. Furtado noted that the main results of DECLARE TIMI-58 are consistent with a recent systematic review and meta-analysis of three randomized cardiovascular outcome trials of SGLT-2is for primary and secondary prevention of cardiovascular and renal outcomes in T2D. The meta-analysis included more than 34,000 patients, roughly half drawn from DECLARE-TIMI 58. SGLT-2i therapy reduced MACE by 14% in patients with established ASCVD but not significantly in those without. And the agents reduced the risk of cardiovascular death/heart failure hospitalization by 23%, regardless of whether or not patients had known ASCVD or a history of heart failure (Lancet. 2019 Jan 5;393[10166]:31-9).
Dr. Furtado reported serving as a consultant to AstraZeneca, which funded DECLARE-TIMI 58, as well as receiving direct institutional research grants from half a dozen other pharmaceutical companies.
Simultaneous with his presentation at ACC 2019 in New Orleans, the subanalysis results were published online (Circulation. 2019 Mar 18. doi: 10.1161/CIRCULATIONAHA.119.039996. [Epub ahead of print]).
REPORTING FROM ACC 19
CV disease and mortality risk higher with younger age of type 2 diabetes diagnosis
Individuals who are younger when diagnosed with type 2 diabetes are at greater risk of cardiovascular disease and death, compared with those diagnosed at an older age, according to a retrospective study involving almost 2 million people.
People diagnosed with type 2 diabetes at age 40 or younger were at greatest risk of most outcomes, reported lead author Naveed Sattar, MD, PhD, professor of metabolic medicine, University of Glasgow, Scotland, and his colleagues. “Treatment target recommendations in regards to the risk factor control may need to be more aggressive in people developing diabetes at younger ages,” they wrote in Circulation.
In contrast, developing type 2 diabetes over the age of 80 years had little impact on risks.
“[R]eassessment of treatment goals in elderly might be useful,” the investigators wrote. “Diabetes screening needs for the elderly (above 80) should also be reevaluated.”
The study involved 318,083 patients with type 2 diabetes registered in the Swedish National Diabetes Registry between 1998 and 2012. Each patient was matched with 5 individuals from the general population based on sex, age, and country of residence, providing a control population of 1,575,108. Outcomes assessed included non-cardiovascular mortality, cardiovascular mortality, all causemortality, hospitalization for heart failure, coronary heart disease, stroke, atrial fibrillation, and acute myocardial infarction. Patients were followed for cardiovascular outcomes from 1998 to December 2013, while mortality surveillance continued through 2014.
In comparison with controls, patients 40 years or less had the highest excess risk of the most outcomes. *Excess risk of heart failure was elevated almost 5-fold (hazard ratio (HR), R 4.77), and risk of coronary heart disease wasn’t far behind (HR, 4.33). Risks of acute MI (HR, 3.41), stroke (HR, 3.58), and atrial fibrillation (HR, 1.95) were also elevated. Cardiovascular-related mortality was increased almost 3-fold (HR, 2.72), while total mortality (HR, 2.05) and non-cardiovascular mortality (HR, 1.95) were raised to a lesser degree.
“Thereafter, incremental risks generally declined with each higher decade age at diagnosis” of type 2 diabetes,” the investigators wrote.
After 80 years of age, all relative mortality risk factors dropped to less than 1, indicating lower risk than controls. Although non-fatal outcomes were still greater than 1 in this age group, these risks were “substantially attenuated compared with relative incremental risks in those diagnosed with T2DM at younger ages,” the investigators wrote.
The study was funded by the Swedish Association of Local Authorities Regions, the Swedish Heart and Lung Foundation, and the Swedish Research Council.
The investigators disclosed financial relationships with Amgen, AstraZeneca, Eli Lilly, and other pharmaceutical companies.
SOURCE: Sattar et al. Circulation. 2019 Apr 8. doi:10.1161/CIRCULATIONAHA.118.037885.
Individuals who are younger when diagnosed with type 2 diabetes are at greater risk of cardiovascular disease and death, compared with those diagnosed at an older age, according to a retrospective study involving almost 2 million people.
People diagnosed with type 2 diabetes at age 40 or younger were at greatest risk of most outcomes, reported lead author Naveed Sattar, MD, PhD, professor of metabolic medicine, University of Glasgow, Scotland, and his colleagues. “Treatment target recommendations in regards to the risk factor control may need to be more aggressive in people developing diabetes at younger ages,” they wrote in Circulation.
In contrast, developing type 2 diabetes over the age of 80 years had little impact on risks.
“[R]eassessment of treatment goals in elderly might be useful,” the investigators wrote. “Diabetes screening needs for the elderly (above 80) should also be reevaluated.”
The study involved 318,083 patients with type 2 diabetes registered in the Swedish National Diabetes Registry between 1998 and 2012. Each patient was matched with 5 individuals from the general population based on sex, age, and country of residence, providing a control population of 1,575,108. Outcomes assessed included non-cardiovascular mortality, cardiovascular mortality, all causemortality, hospitalization for heart failure, coronary heart disease, stroke, atrial fibrillation, and acute myocardial infarction. Patients were followed for cardiovascular outcomes from 1998 to December 2013, while mortality surveillance continued through 2014.
In comparison with controls, patients 40 years or less had the highest excess risk of the most outcomes. *Excess risk of heart failure was elevated almost 5-fold (hazard ratio (HR), R 4.77), and risk of coronary heart disease wasn’t far behind (HR, 4.33). Risks of acute MI (HR, 3.41), stroke (HR, 3.58), and atrial fibrillation (HR, 1.95) were also elevated. Cardiovascular-related mortality was increased almost 3-fold (HR, 2.72), while total mortality (HR, 2.05) and non-cardiovascular mortality (HR, 1.95) were raised to a lesser degree.
“Thereafter, incremental risks generally declined with each higher decade age at diagnosis” of type 2 diabetes,” the investigators wrote.
After 80 years of age, all relative mortality risk factors dropped to less than 1, indicating lower risk than controls. Although non-fatal outcomes were still greater than 1 in this age group, these risks were “substantially attenuated compared with relative incremental risks in those diagnosed with T2DM at younger ages,” the investigators wrote.
The study was funded by the Swedish Association of Local Authorities Regions, the Swedish Heart and Lung Foundation, and the Swedish Research Council.
The investigators disclosed financial relationships with Amgen, AstraZeneca, Eli Lilly, and other pharmaceutical companies.
SOURCE: Sattar et al. Circulation. 2019 Apr 8. doi:10.1161/CIRCULATIONAHA.118.037885.
Individuals who are younger when diagnosed with type 2 diabetes are at greater risk of cardiovascular disease and death, compared with those diagnosed at an older age, according to a retrospective study involving almost 2 million people.
People diagnosed with type 2 diabetes at age 40 or younger were at greatest risk of most outcomes, reported lead author Naveed Sattar, MD, PhD, professor of metabolic medicine, University of Glasgow, Scotland, and his colleagues. “Treatment target recommendations in regards to the risk factor control may need to be more aggressive in people developing diabetes at younger ages,” they wrote in Circulation.
In contrast, developing type 2 diabetes over the age of 80 years had little impact on risks.
“[R]eassessment of treatment goals in elderly might be useful,” the investigators wrote. “Diabetes screening needs for the elderly (above 80) should also be reevaluated.”
The study involved 318,083 patients with type 2 diabetes registered in the Swedish National Diabetes Registry between 1998 and 2012. Each patient was matched with 5 individuals from the general population based on sex, age, and country of residence, providing a control population of 1,575,108. Outcomes assessed included non-cardiovascular mortality, cardiovascular mortality, all causemortality, hospitalization for heart failure, coronary heart disease, stroke, atrial fibrillation, and acute myocardial infarction. Patients were followed for cardiovascular outcomes from 1998 to December 2013, while mortality surveillance continued through 2014.
In comparison with controls, patients 40 years or less had the highest excess risk of the most outcomes. *Excess risk of heart failure was elevated almost 5-fold (hazard ratio (HR), R 4.77), and risk of coronary heart disease wasn’t far behind (HR, 4.33). Risks of acute MI (HR, 3.41), stroke (HR, 3.58), and atrial fibrillation (HR, 1.95) were also elevated. Cardiovascular-related mortality was increased almost 3-fold (HR, 2.72), while total mortality (HR, 2.05) and non-cardiovascular mortality (HR, 1.95) were raised to a lesser degree.
“Thereafter, incremental risks generally declined with each higher decade age at diagnosis” of type 2 diabetes,” the investigators wrote.
After 80 years of age, all relative mortality risk factors dropped to less than 1, indicating lower risk than controls. Although non-fatal outcomes were still greater than 1 in this age group, these risks were “substantially attenuated compared with relative incremental risks in those diagnosed with T2DM at younger ages,” the investigators wrote.
The study was funded by the Swedish Association of Local Authorities Regions, the Swedish Heart and Lung Foundation, and the Swedish Research Council.
The investigators disclosed financial relationships with Amgen, AstraZeneca, Eli Lilly, and other pharmaceutical companies.
SOURCE: Sattar et al. Circulation. 2019 Apr 8. doi:10.1161/CIRCULATIONAHA.118.037885.
FROM CIRCULATION
Key clinical point: Patients who are younger when diagnosed with type 2 diabetes mellitus (T2DM) are at greater risk of cardiovascular disease and death than patients diagnosed at an older age.
Major finding: Patients diagnosed with T2DM at age 40 or younger had twice the risk of death from any cause, compared with age-matched controls (hazard ratio, 2.05).
Study details: A retrospective analysis of type 2 diabetes and associations with cardiovascular and mortality risks, using data from 318,083 patients in the Swedish National Diabetes Registry.
Disclosures: The study was funded by the Swedish Association of Local Authorities Regions, the Swedish Heart and Lung Foundation, and the Swedish Research Council. The investigators disclosed financial relationships with Amgen, Astra-Zeneca, Eli Lilly, and others.
Source: Sattar et al. Circulation. 2019 Apr 8. doi:10.1161/CIRCULATIONAHA.118.037885.
Cigna, Express Scripts to offer $25 cap on 30-day insulin supply
Cigna and Express Scripts have announced
The new program, open to Cigna members who are covered in commercial plans, would cap out-of-pocket costs for a 30-day supply of insulin at $25. For plan members, the only eligibility requirement is having an out-of-pocket cost higher than $25, according to a press release.
For a member to participate in the program, the plan administrator at the member’s place of employment has to opt in to it. There are no eligibility requirements imposed on the employer, other than a willingness to opt in.
A spokeswoman for Express Scripts said that there is no charge to sign up for the program, and most plans will not see an additional cost to get the copayment to $25 for the patient.
The announcement comes in the wake of the first of two hearings by the House Committee on Energy & Commerce aimed at understanding why insulin prices have spiked in recent years. The first hearing, held on April 2, examined the impact that the high list price of insulin is having on patients, and how out-of-pocket expenses are limiting access to this life-saving drug. The second hearing, expected to occur during the week of April 8 (the date had not been scheduled as of press time), will bring together various players in the supply chain, including the three major manufacturers of insulin.
“We are confident that our new program will remove cost as a barrier for people in participating plans who need insulin,” Steve Miller, MD, executive vice president and chief clinical officer at Cigna, said in a statement.
The Express Scripts spokeswoman noted that there were more than 700,000 people in a commercially insured plan across Cigna and Express Scripts who had a claim for insulin in 2018. The average out-of-pocket cost of a 30-day supply of insulin in 2018 across this population was $41.50.
Cigna and Express Scripts have announced
The new program, open to Cigna members who are covered in commercial plans, would cap out-of-pocket costs for a 30-day supply of insulin at $25. For plan members, the only eligibility requirement is having an out-of-pocket cost higher than $25, according to a press release.
For a member to participate in the program, the plan administrator at the member’s place of employment has to opt in to it. There are no eligibility requirements imposed on the employer, other than a willingness to opt in.
A spokeswoman for Express Scripts said that there is no charge to sign up for the program, and most plans will not see an additional cost to get the copayment to $25 for the patient.
The announcement comes in the wake of the first of two hearings by the House Committee on Energy & Commerce aimed at understanding why insulin prices have spiked in recent years. The first hearing, held on April 2, examined the impact that the high list price of insulin is having on patients, and how out-of-pocket expenses are limiting access to this life-saving drug. The second hearing, expected to occur during the week of April 8 (the date had not been scheduled as of press time), will bring together various players in the supply chain, including the three major manufacturers of insulin.
“We are confident that our new program will remove cost as a barrier for people in participating plans who need insulin,” Steve Miller, MD, executive vice president and chief clinical officer at Cigna, said in a statement.
The Express Scripts spokeswoman noted that there were more than 700,000 people in a commercially insured plan across Cigna and Express Scripts who had a claim for insulin in 2018. The average out-of-pocket cost of a 30-day supply of insulin in 2018 across this population was $41.50.
Cigna and Express Scripts have announced
The new program, open to Cigna members who are covered in commercial plans, would cap out-of-pocket costs for a 30-day supply of insulin at $25. For plan members, the only eligibility requirement is having an out-of-pocket cost higher than $25, according to a press release.
For a member to participate in the program, the plan administrator at the member’s place of employment has to opt in to it. There are no eligibility requirements imposed on the employer, other than a willingness to opt in.
A spokeswoman for Express Scripts said that there is no charge to sign up for the program, and most plans will not see an additional cost to get the copayment to $25 for the patient.
The announcement comes in the wake of the first of two hearings by the House Committee on Energy & Commerce aimed at understanding why insulin prices have spiked in recent years. The first hearing, held on April 2, examined the impact that the high list price of insulin is having on patients, and how out-of-pocket expenses are limiting access to this life-saving drug. The second hearing, expected to occur during the week of April 8 (the date had not been scheduled as of press time), will bring together various players in the supply chain, including the three major manufacturers of insulin.
“We are confident that our new program will remove cost as a barrier for people in participating plans who need insulin,” Steve Miller, MD, executive vice president and chief clinical officer at Cigna, said in a statement.
The Express Scripts spokeswoman noted that there were more than 700,000 people in a commercially insured plan across Cigna and Express Scripts who had a claim for insulin in 2018. The average out-of-pocket cost of a 30-day supply of insulin in 2018 across this population was $41.50.