User login
Weight-loss drug options expand, but beware cardiac risk
LOS ANGELES – Newer medications are much more powerful, but they come with cautions – insurer coverage can be a hurdle, and there are significant gaps in knowledge about their risks for patients with heart disease, Ken Fujioka, MD, told colleagues at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Fujioka, of Scripps Clinic in San Diego, shared some tips with his peers about using medications to reduce weight.
Diabetes drugs help, but may need a boost
Metformin can reduce weight by as much as 3%, Dr. Fujioka said. And there may be another benefit related to long-term weight loss maintenance, he said, citing a 15-year study of overweight or obese patients at high risk for diabetes who either received metformin, underwent an intensive lifestyle intervention, or took a placebo. Of the participants with weight loss of at least 5% after the first year, those originally assigned to receive metformin had the greatest weight loss during years 6-15. Older age, the amount of weight initially lost, and continued used of metformin were predictors of long-term weight loss maintenance, according to the researchers (Ann Intern Med. 2019 Apr 23. doi: 10.7326/M18-1605).
There are other options among diabetes drugs. Sodium-glucose cotransporter 2 (SGLT2) inhibitors – a class of drugs that includes canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have a striking effect on weight loss, Dr. Fujioka said. They can cause 300 calories to be flushed out in the urine each day. But that typically doesn’t translate into weight loss of more than 20 pounds, he said, because the body doesn’t fully adjust to fewer calories.
“The patients begin to eat more,” he said. “They have to take in more calories to make up for [the loss]. They’re not consciously trying to do this. It’s a metabolic adaptation, so 2%-3% [weight loss] is about all you’ll get. You won’t get 10% or 20%.”
To drive up weight loss, Dr. Fujioka recommended adding the glucagonlike peptide–1 [GLP1] receptor diabetes drug exenatide (Byetta; Bydureon) or the appetite suppressant phentermine (Adipex-p; Lomaira) to an SGLT2 inhibitor. Recent studies have shown that the drug combinations have a greater impact on weight loss than when taken separately (Lancet Diabetes Endocrinol. 2016 Dec;4[12]:1004-16; Diabetes Care. 2017 May;40[5]:632-9).
In regard to phentermine, which acts similarly to amphetamine, Dr. Fujioka advised colleagues to be aware that “15 mg or less is really safe, but you drive pulse and heart rate beyond that.”
Consider insurance coverage and other factors
Often, insurers will pay for GLP1-receptor and SGLT2-inhibitor medications in patients with diabetes, even if their hemoglobin A1c is in the healthy range, Dr. Fujioka said, but they’ll balk at paying for specific weight-loss medications, although that can vary by the region of the country. He added that cash discount cards are available for several weight-loss drugs.
Newer weight-loss drugs ...
Dr. Fujioka highlighted a quartet of weight-loss drugs that have been approved in recent years.
- Lorcaserin (Belviq), a selective serotonin 2C receptor agonist, has shown unique benefits in patients with diabetes. A large, multinational, randomized controlled trial found that the drug reduced the risk for incident diabetes, induced remission of hyperglycemia, and reduced the risk of microvascular complications in obese and overweight patients (Lancet. 2018 Nov 24;392[10161]:2269-79).
- Phentermine/topiramate (Qsymia), a combination of an antiseizure medication (topiramate) and an appetite suppressant (phentermine). A 2014 study found that the drug, together with lifestyle modification, effectively promoted weight loss and improved glycemic control in obese or overweight patients with type 2 diabetes (Diabetes Care. 2014 Dec;37[12]:3309-16).
- Naltrexone/bupropion (Contrave), a combination of an addiction drug (naltrexone) and an antidepressant (bupropion). Findings from a 2013 study reported that the drug “in overweight/obese patients with type 2 diabetes induced weight loss... was associated with improvements in glycemic control and select cardiovascular risk factors and was generally well tolerated with a safety profile similar to that in patients without diabetes.” (Diabetes Care. 2013 Dec;36[12]:4022-9).
- Liraglutide, an injectable GLP1 agonist that has been approved for diabetes (Victoza) and weight loss (Saxenda). Dr. Fujioka was coauthor for a study in which the findings suggested that the drug could prevent prediabetes from turning into diabetes. (Lancet. 2017 Apr 8;389[10077]:1399-409).
... but watch out for safety in patients with heart disease
Two of the newer weight-loss drugs are OK to prescribe for diabetic patients with heart disease, Dr. Fujioka said, but two are not, because no cardiac safety trials have been completed for them.
Liraglutide (at a dose of 3.0 mg) is considered safe based on previous data (Diabetes Obes Metab. 2018 Mar;20[3]:734-9), Dr. Fujioka said. Likewise, findings from a trial with lorcaserin in which 12,000 overweight or obese patients with atherosclerotic cardiovascular disease or multiple cardiovascular risk factors received either lorcaserin (10 mg twice daily) or placebo, suggested that lorcaserin helped sustain weight loss without a higher rate of major cardiovascular events compared with placebo (N Engl J Med. 2018 Sep 20;379[12]:1107-17).However, no such cardiac safety trials have been completed for naltrexone/bupropion or phentermine/topiramate, said Dr. Fujioka. As a result, he said he could not recommend either of them for patients with high-risk cardiovascular disease.
Dr. Fujioka disclosed relationships of various types with Novo Nordisk, Eisai, Gelesis, KVK Tech, Amgen, Sunovion, Boehringer Ingelheim, and Janssen Global Services.
LOS ANGELES – Newer medications are much more powerful, but they come with cautions – insurer coverage can be a hurdle, and there are significant gaps in knowledge about their risks for patients with heart disease, Ken Fujioka, MD, told colleagues at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Fujioka, of Scripps Clinic in San Diego, shared some tips with his peers about using medications to reduce weight.
Diabetes drugs help, but may need a boost
Metformin can reduce weight by as much as 3%, Dr. Fujioka said. And there may be another benefit related to long-term weight loss maintenance, he said, citing a 15-year study of overweight or obese patients at high risk for diabetes who either received metformin, underwent an intensive lifestyle intervention, or took a placebo. Of the participants with weight loss of at least 5% after the first year, those originally assigned to receive metformin had the greatest weight loss during years 6-15. Older age, the amount of weight initially lost, and continued used of metformin were predictors of long-term weight loss maintenance, according to the researchers (Ann Intern Med. 2019 Apr 23. doi: 10.7326/M18-1605).
There are other options among diabetes drugs. Sodium-glucose cotransporter 2 (SGLT2) inhibitors – a class of drugs that includes canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have a striking effect on weight loss, Dr. Fujioka said. They can cause 300 calories to be flushed out in the urine each day. But that typically doesn’t translate into weight loss of more than 20 pounds, he said, because the body doesn’t fully adjust to fewer calories.
“The patients begin to eat more,” he said. “They have to take in more calories to make up for [the loss]. They’re not consciously trying to do this. It’s a metabolic adaptation, so 2%-3% [weight loss] is about all you’ll get. You won’t get 10% or 20%.”
To drive up weight loss, Dr. Fujioka recommended adding the glucagonlike peptide–1 [GLP1] receptor diabetes drug exenatide (Byetta; Bydureon) or the appetite suppressant phentermine (Adipex-p; Lomaira) to an SGLT2 inhibitor. Recent studies have shown that the drug combinations have a greater impact on weight loss than when taken separately (Lancet Diabetes Endocrinol. 2016 Dec;4[12]:1004-16; Diabetes Care. 2017 May;40[5]:632-9).
In regard to phentermine, which acts similarly to amphetamine, Dr. Fujioka advised colleagues to be aware that “15 mg or less is really safe, but you drive pulse and heart rate beyond that.”
Consider insurance coverage and other factors
Often, insurers will pay for GLP1-receptor and SGLT2-inhibitor medications in patients with diabetes, even if their hemoglobin A1c is in the healthy range, Dr. Fujioka said, but they’ll balk at paying for specific weight-loss medications, although that can vary by the region of the country. He added that cash discount cards are available for several weight-loss drugs.
Newer weight-loss drugs ...
Dr. Fujioka highlighted a quartet of weight-loss drugs that have been approved in recent years.
- Lorcaserin (Belviq), a selective serotonin 2C receptor agonist, has shown unique benefits in patients with diabetes. A large, multinational, randomized controlled trial found that the drug reduced the risk for incident diabetes, induced remission of hyperglycemia, and reduced the risk of microvascular complications in obese and overweight patients (Lancet. 2018 Nov 24;392[10161]:2269-79).
- Phentermine/topiramate (Qsymia), a combination of an antiseizure medication (topiramate) and an appetite suppressant (phentermine). A 2014 study found that the drug, together with lifestyle modification, effectively promoted weight loss and improved glycemic control in obese or overweight patients with type 2 diabetes (Diabetes Care. 2014 Dec;37[12]:3309-16).
- Naltrexone/bupropion (Contrave), a combination of an addiction drug (naltrexone) and an antidepressant (bupropion). Findings from a 2013 study reported that the drug “in overweight/obese patients with type 2 diabetes induced weight loss... was associated with improvements in glycemic control and select cardiovascular risk factors and was generally well tolerated with a safety profile similar to that in patients without diabetes.” (Diabetes Care. 2013 Dec;36[12]:4022-9).
- Liraglutide, an injectable GLP1 agonist that has been approved for diabetes (Victoza) and weight loss (Saxenda). Dr. Fujioka was coauthor for a study in which the findings suggested that the drug could prevent prediabetes from turning into diabetes. (Lancet. 2017 Apr 8;389[10077]:1399-409).
... but watch out for safety in patients with heart disease
Two of the newer weight-loss drugs are OK to prescribe for diabetic patients with heart disease, Dr. Fujioka said, but two are not, because no cardiac safety trials have been completed for them.
Liraglutide (at a dose of 3.0 mg) is considered safe based on previous data (Diabetes Obes Metab. 2018 Mar;20[3]:734-9), Dr. Fujioka said. Likewise, findings from a trial with lorcaserin in which 12,000 overweight or obese patients with atherosclerotic cardiovascular disease or multiple cardiovascular risk factors received either lorcaserin (10 mg twice daily) or placebo, suggested that lorcaserin helped sustain weight loss without a higher rate of major cardiovascular events compared with placebo (N Engl J Med. 2018 Sep 20;379[12]:1107-17).However, no such cardiac safety trials have been completed for naltrexone/bupropion or phentermine/topiramate, said Dr. Fujioka. As a result, he said he could not recommend either of them for patients with high-risk cardiovascular disease.
Dr. Fujioka disclosed relationships of various types with Novo Nordisk, Eisai, Gelesis, KVK Tech, Amgen, Sunovion, Boehringer Ingelheim, and Janssen Global Services.
LOS ANGELES – Newer medications are much more powerful, but they come with cautions – insurer coverage can be a hurdle, and there are significant gaps in knowledge about their risks for patients with heart disease, Ken Fujioka, MD, told colleagues at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Fujioka, of Scripps Clinic in San Diego, shared some tips with his peers about using medications to reduce weight.
Diabetes drugs help, but may need a boost
Metformin can reduce weight by as much as 3%, Dr. Fujioka said. And there may be another benefit related to long-term weight loss maintenance, he said, citing a 15-year study of overweight or obese patients at high risk for diabetes who either received metformin, underwent an intensive lifestyle intervention, or took a placebo. Of the participants with weight loss of at least 5% after the first year, those originally assigned to receive metformin had the greatest weight loss during years 6-15. Older age, the amount of weight initially lost, and continued used of metformin were predictors of long-term weight loss maintenance, according to the researchers (Ann Intern Med. 2019 Apr 23. doi: 10.7326/M18-1605).
There are other options among diabetes drugs. Sodium-glucose cotransporter 2 (SGLT2) inhibitors – a class of drugs that includes canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance) – have a striking effect on weight loss, Dr. Fujioka said. They can cause 300 calories to be flushed out in the urine each day. But that typically doesn’t translate into weight loss of more than 20 pounds, he said, because the body doesn’t fully adjust to fewer calories.
“The patients begin to eat more,” he said. “They have to take in more calories to make up for [the loss]. They’re not consciously trying to do this. It’s a metabolic adaptation, so 2%-3% [weight loss] is about all you’ll get. You won’t get 10% or 20%.”
To drive up weight loss, Dr. Fujioka recommended adding the glucagonlike peptide–1 [GLP1] receptor diabetes drug exenatide (Byetta; Bydureon) or the appetite suppressant phentermine (Adipex-p; Lomaira) to an SGLT2 inhibitor. Recent studies have shown that the drug combinations have a greater impact on weight loss than when taken separately (Lancet Diabetes Endocrinol. 2016 Dec;4[12]:1004-16; Diabetes Care. 2017 May;40[5]:632-9).
In regard to phentermine, which acts similarly to amphetamine, Dr. Fujioka advised colleagues to be aware that “15 mg or less is really safe, but you drive pulse and heart rate beyond that.”
Consider insurance coverage and other factors
Often, insurers will pay for GLP1-receptor and SGLT2-inhibitor medications in patients with diabetes, even if their hemoglobin A1c is in the healthy range, Dr. Fujioka said, but they’ll balk at paying for specific weight-loss medications, although that can vary by the region of the country. He added that cash discount cards are available for several weight-loss drugs.
Newer weight-loss drugs ...
Dr. Fujioka highlighted a quartet of weight-loss drugs that have been approved in recent years.
- Lorcaserin (Belviq), a selective serotonin 2C receptor agonist, has shown unique benefits in patients with diabetes. A large, multinational, randomized controlled trial found that the drug reduced the risk for incident diabetes, induced remission of hyperglycemia, and reduced the risk of microvascular complications in obese and overweight patients (Lancet. 2018 Nov 24;392[10161]:2269-79).
- Phentermine/topiramate (Qsymia), a combination of an antiseizure medication (topiramate) and an appetite suppressant (phentermine). A 2014 study found that the drug, together with lifestyle modification, effectively promoted weight loss and improved glycemic control in obese or overweight patients with type 2 diabetes (Diabetes Care. 2014 Dec;37[12]:3309-16).
- Naltrexone/bupropion (Contrave), a combination of an addiction drug (naltrexone) and an antidepressant (bupropion). Findings from a 2013 study reported that the drug “in overweight/obese patients with type 2 diabetes induced weight loss... was associated with improvements in glycemic control and select cardiovascular risk factors and was generally well tolerated with a safety profile similar to that in patients without diabetes.” (Diabetes Care. 2013 Dec;36[12]:4022-9).
- Liraglutide, an injectable GLP1 agonist that has been approved for diabetes (Victoza) and weight loss (Saxenda). Dr. Fujioka was coauthor for a study in which the findings suggested that the drug could prevent prediabetes from turning into diabetes. (Lancet. 2017 Apr 8;389[10077]:1399-409).
... but watch out for safety in patients with heart disease
Two of the newer weight-loss drugs are OK to prescribe for diabetic patients with heart disease, Dr. Fujioka said, but two are not, because no cardiac safety trials have been completed for them.
Liraglutide (at a dose of 3.0 mg) is considered safe based on previous data (Diabetes Obes Metab. 2018 Mar;20[3]:734-9), Dr. Fujioka said. Likewise, findings from a trial with lorcaserin in which 12,000 overweight or obese patients with atherosclerotic cardiovascular disease or multiple cardiovascular risk factors received either lorcaserin (10 mg twice daily) or placebo, suggested that lorcaserin helped sustain weight loss without a higher rate of major cardiovascular events compared with placebo (N Engl J Med. 2018 Sep 20;379[12]:1107-17).However, no such cardiac safety trials have been completed for naltrexone/bupropion or phentermine/topiramate, said Dr. Fujioka. As a result, he said he could not recommend either of them for patients with high-risk cardiovascular disease.
Dr. Fujioka disclosed relationships of various types with Novo Nordisk, Eisai, Gelesis, KVK Tech, Amgen, Sunovion, Boehringer Ingelheim, and Janssen Global Services.
EXPERT ANALYSIS FROM AACE 2019
New adventures of an old device: Clinic delivers cortisol via the insulin pump
LOS ANGELES – The venerable insulin pump is being repurposed: A Detroit-area endocrinology
“We’ve seen amazing results,” said endocrinologist Opada Alzohaili, MD, MBA, of Wayne State University, Detroit, coauthor of a study released at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Alzohaili said he and his colleagues developed the approach to manage patients who are “so sick that they go to the hospital 10-12 times a year.” Oral medication just did not control their disorder.
“Most of the time, we end up way overdosing them [on oral medication] just to prevent them from going to the hospital in adrenal crisis,” he said in an interview.
Dr. Alzohaili said his clinic has tested delivering hydrocortisone via insulin pump in about 20 patients. The report presented at the conference focused on six patients who had failed oral hydrocortisone treatment for adrenal insufficiency. Testing showed that all had malabsorption of the drug.
The patients underwent training in how to use and adjust the pump, which allows dosing adjustments in increments of 1 mg. They learned how to adjust their doses based on their situation, Dr. Alzohaili said.
According to the report, the average number of adrenal crises in the patients over a 6-month period fell from a mean of 2.3 before baseline to 0.5 after treatment began. The maximum dose of hydrocortisone dose fell by 38%, while the average mean weight of patients rose from 182 pounds to 199 pounds.
In addition, the mean dose of hydrocortisone decreased with the use of the pump delivery system, from 85.8 mg with oral treatment to 32.4 mg on pump therapy, and the mean level of cortisol increased from 11.8 mcg/dL with oral treatment to 12.3 mcg/dL on pump therapy.
The researchers said that the pump provides better delivery of the medication compared with the oral route, and that the patients experienced fewer interactions with other medications.
Some patients developed skin reactions to the pump, but those adverse events were resolved by changing the pump’s location on the body and by using hypoallergenic needles, Dr. Alzohaili said.
There were fewer cases of clogging with the pumps than is normally seen when they’re used with insulin, he added.
As for expense, Dr. Alzohaili said the pumps cost thousands of dollars and supplies can cost between $100 and $150 a month. In the first couple of cases, patients paid for the treatment themselves, he said, but in later cases, insurers were willing to pay for the treatment once they learned about the results.
Other researchers have successfully used insulin pumps to deliver hydrocortisone to small numbers of patients with adrenal insufficiency, including British and U.S. teams that reported positive results in 2015 and 2018, respectively.
The next step, Dr. Alzohaili said, is to attract the interest of insulin pump manufacturers by using the treatment in more patients. “I’ve spoken to CEOs, but none of them is interested in using cortisol in their pumps,” he said. “If you don’t have the company supporting the research, it becomes difficult for it to become standard of care. So I’m trying to build awareness [of its use] and the number of patients [who use the pump].”
Dr. Alzohaili reported no financial conflicts of interest or disclosures.
LOS ANGELES – The venerable insulin pump is being repurposed: A Detroit-area endocrinology
“We’ve seen amazing results,” said endocrinologist Opada Alzohaili, MD, MBA, of Wayne State University, Detroit, coauthor of a study released at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Alzohaili said he and his colleagues developed the approach to manage patients who are “so sick that they go to the hospital 10-12 times a year.” Oral medication just did not control their disorder.
“Most of the time, we end up way overdosing them [on oral medication] just to prevent them from going to the hospital in adrenal crisis,” he said in an interview.
Dr. Alzohaili said his clinic has tested delivering hydrocortisone via insulin pump in about 20 patients. The report presented at the conference focused on six patients who had failed oral hydrocortisone treatment for adrenal insufficiency. Testing showed that all had malabsorption of the drug.
The patients underwent training in how to use and adjust the pump, which allows dosing adjustments in increments of 1 mg. They learned how to adjust their doses based on their situation, Dr. Alzohaili said.
According to the report, the average number of adrenal crises in the patients over a 6-month period fell from a mean of 2.3 before baseline to 0.5 after treatment began. The maximum dose of hydrocortisone dose fell by 38%, while the average mean weight of patients rose from 182 pounds to 199 pounds.
In addition, the mean dose of hydrocortisone decreased with the use of the pump delivery system, from 85.8 mg with oral treatment to 32.4 mg on pump therapy, and the mean level of cortisol increased from 11.8 mcg/dL with oral treatment to 12.3 mcg/dL on pump therapy.
The researchers said that the pump provides better delivery of the medication compared with the oral route, and that the patients experienced fewer interactions with other medications.
Some patients developed skin reactions to the pump, but those adverse events were resolved by changing the pump’s location on the body and by using hypoallergenic needles, Dr. Alzohaili said.
There were fewer cases of clogging with the pumps than is normally seen when they’re used with insulin, he added.
As for expense, Dr. Alzohaili said the pumps cost thousands of dollars and supplies can cost between $100 and $150 a month. In the first couple of cases, patients paid for the treatment themselves, he said, but in later cases, insurers were willing to pay for the treatment once they learned about the results.
Other researchers have successfully used insulin pumps to deliver hydrocortisone to small numbers of patients with adrenal insufficiency, including British and U.S. teams that reported positive results in 2015 and 2018, respectively.
The next step, Dr. Alzohaili said, is to attract the interest of insulin pump manufacturers by using the treatment in more patients. “I’ve spoken to CEOs, but none of them is interested in using cortisol in their pumps,” he said. “If you don’t have the company supporting the research, it becomes difficult for it to become standard of care. So I’m trying to build awareness [of its use] and the number of patients [who use the pump].”
Dr. Alzohaili reported no financial conflicts of interest or disclosures.
LOS ANGELES – The venerable insulin pump is being repurposed: A Detroit-area endocrinology
“We’ve seen amazing results,” said endocrinologist Opada Alzohaili, MD, MBA, of Wayne State University, Detroit, coauthor of a study released at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
Dr. Alzohaili said he and his colleagues developed the approach to manage patients who are “so sick that they go to the hospital 10-12 times a year.” Oral medication just did not control their disorder.
“Most of the time, we end up way overdosing them [on oral medication] just to prevent them from going to the hospital in adrenal crisis,” he said in an interview.
Dr. Alzohaili said his clinic has tested delivering hydrocortisone via insulin pump in about 20 patients. The report presented at the conference focused on six patients who had failed oral hydrocortisone treatment for adrenal insufficiency. Testing showed that all had malabsorption of the drug.
The patients underwent training in how to use and adjust the pump, which allows dosing adjustments in increments of 1 mg. They learned how to adjust their doses based on their situation, Dr. Alzohaili said.
According to the report, the average number of adrenal crises in the patients over a 6-month period fell from a mean of 2.3 before baseline to 0.5 after treatment began. The maximum dose of hydrocortisone dose fell by 38%, while the average mean weight of patients rose from 182 pounds to 199 pounds.
In addition, the mean dose of hydrocortisone decreased with the use of the pump delivery system, from 85.8 mg with oral treatment to 32.4 mg on pump therapy, and the mean level of cortisol increased from 11.8 mcg/dL with oral treatment to 12.3 mcg/dL on pump therapy.
The researchers said that the pump provides better delivery of the medication compared with the oral route, and that the patients experienced fewer interactions with other medications.
Some patients developed skin reactions to the pump, but those adverse events were resolved by changing the pump’s location on the body and by using hypoallergenic needles, Dr. Alzohaili said.
There were fewer cases of clogging with the pumps than is normally seen when they’re used with insulin, he added.
As for expense, Dr. Alzohaili said the pumps cost thousands of dollars and supplies can cost between $100 and $150 a month. In the first couple of cases, patients paid for the treatment themselves, he said, but in later cases, insurers were willing to pay for the treatment once they learned about the results.
Other researchers have successfully used insulin pumps to deliver hydrocortisone to small numbers of patients with adrenal insufficiency, including British and U.S. teams that reported positive results in 2015 and 2018, respectively.
The next step, Dr. Alzohaili said, is to attract the interest of insulin pump manufacturers by using the treatment in more patients. “I’ve spoken to CEOs, but none of them is interested in using cortisol in their pumps,” he said. “If you don’t have the company supporting the research, it becomes difficult for it to become standard of care. So I’m trying to build awareness [of its use] and the number of patients [who use the pump].”
Dr. Alzohaili reported no financial conflicts of interest or disclosures.
REPORTING FROM AACE 2019
Liraglutide seems safe, effective in children already on metformin
The addition of liraglutide to metformin shows significantly improved glycemic control in children and adolescents with type 2 diabetes, compared with metformin alone, according to data presented at the Pediatric Academic Societies annual meeting in Baltimore.
The phase 3 study, which was simultaneously published in the New England Journal of Medicine, involved 134 patients aged 10-17 years with type 2 diabetes who were managing their diabetes with diet and exercise, metformin, or insulin.
Participants were randomized either to subcutaneous liraglutide – dose-escalated up to 1.8 mg/day, depending on efficacy and side effects – or placebo for 52 weeks. The first 26 weeks were double blind and the second 26 weeks were an open-label extension period.
At 26 weeks, mean glycated hemoglobin levels in the liraglutide group had decreased by 0.64 percentage points from baseline, but in the placebo group they had increased by 0.42 percentage points, representing a treatment difference of –1.06 percentage points (P less than .001). By week 52, the treatment difference between the two groups had increased to –1.30 percentage points.
William V. Tamborlane, MD, from the department of pediatrics at Yale University, New Haven, Conn., and his coauthors wrote that metformin is the approved drug of choice for pediatric patients with type 2 diabetes, and that insulin currently is the only approved option for those who do not have an adequate response to metformin monotherapy.
“This discrepancy in available treatments for youth as compared with adults persists because of a lack of successfully completed trials needed for approval of new drugs for the treatment of type 2 diabetes in children since a trial of metformin was completed in 1999,” they wrote.
The study showed that significantly more patients in the liraglutide group (63.7%) achieved glycated hemoglobin levels below 7%, compared with 36.5% of patients in the placebo group. Fasting plasma glucose levels were decreased in the liraglutide group at both 26 and 52 weeks, but had increased in the placebo group.
Although the number of reported adverse events were similar between the two groups, there were significantly more reports of gastrointestinal adverse events – particularly nausea – in patients taking liraglutide, compared with those on placebo.
However, the study did not show a difference between liraglutide and placebo in lowering body mass index, although mean body weight decreases – which were seen in both groups – were maintained at week 52 only in the liraglutide group. The authors suggested this might be owing to the relatively small number of patients enrolled in the study and that some of the children were still growing.
Novo Nordisk, which manufactures liraglutide, supported the study. Twelve authors reported grants or support from Novo Nordisk in relation to the trial. Three authors were employees of Novo Nordisk. Eight authors reported unrelated grants and fees from Novo Nordisk and other pharmaceutical companies.
SOURCE: Tamborlane WV et al. N Engl J Med. 2019 Apr 28. doi: 10.1056/NEJMoa1903822.
The addition of liraglutide to metformin shows significantly improved glycemic control in children and adolescents with type 2 diabetes, compared with metformin alone, according to data presented at the Pediatric Academic Societies annual meeting in Baltimore.
The phase 3 study, which was simultaneously published in the New England Journal of Medicine, involved 134 patients aged 10-17 years with type 2 diabetes who were managing their diabetes with diet and exercise, metformin, or insulin.
Participants were randomized either to subcutaneous liraglutide – dose-escalated up to 1.8 mg/day, depending on efficacy and side effects – or placebo for 52 weeks. The first 26 weeks were double blind and the second 26 weeks were an open-label extension period.
At 26 weeks, mean glycated hemoglobin levels in the liraglutide group had decreased by 0.64 percentage points from baseline, but in the placebo group they had increased by 0.42 percentage points, representing a treatment difference of –1.06 percentage points (P less than .001). By week 52, the treatment difference between the two groups had increased to –1.30 percentage points.
William V. Tamborlane, MD, from the department of pediatrics at Yale University, New Haven, Conn., and his coauthors wrote that metformin is the approved drug of choice for pediatric patients with type 2 diabetes, and that insulin currently is the only approved option for those who do not have an adequate response to metformin monotherapy.
“This discrepancy in available treatments for youth as compared with adults persists because of a lack of successfully completed trials needed for approval of new drugs for the treatment of type 2 diabetes in children since a trial of metformin was completed in 1999,” they wrote.
The study showed that significantly more patients in the liraglutide group (63.7%) achieved glycated hemoglobin levels below 7%, compared with 36.5% of patients in the placebo group. Fasting plasma glucose levels were decreased in the liraglutide group at both 26 and 52 weeks, but had increased in the placebo group.
Although the number of reported adverse events were similar between the two groups, there were significantly more reports of gastrointestinal adverse events – particularly nausea – in patients taking liraglutide, compared with those on placebo.
However, the study did not show a difference between liraglutide and placebo in lowering body mass index, although mean body weight decreases – which were seen in both groups – were maintained at week 52 only in the liraglutide group. The authors suggested this might be owing to the relatively small number of patients enrolled in the study and that some of the children were still growing.
Novo Nordisk, which manufactures liraglutide, supported the study. Twelve authors reported grants or support from Novo Nordisk in relation to the trial. Three authors were employees of Novo Nordisk. Eight authors reported unrelated grants and fees from Novo Nordisk and other pharmaceutical companies.
SOURCE: Tamborlane WV et al. N Engl J Med. 2019 Apr 28. doi: 10.1056/NEJMoa1903822.
The addition of liraglutide to metformin shows significantly improved glycemic control in children and adolescents with type 2 diabetes, compared with metformin alone, according to data presented at the Pediatric Academic Societies annual meeting in Baltimore.
The phase 3 study, which was simultaneously published in the New England Journal of Medicine, involved 134 patients aged 10-17 years with type 2 diabetes who were managing their diabetes with diet and exercise, metformin, or insulin.
Participants were randomized either to subcutaneous liraglutide – dose-escalated up to 1.8 mg/day, depending on efficacy and side effects – or placebo for 52 weeks. The first 26 weeks were double blind and the second 26 weeks were an open-label extension period.
At 26 weeks, mean glycated hemoglobin levels in the liraglutide group had decreased by 0.64 percentage points from baseline, but in the placebo group they had increased by 0.42 percentage points, representing a treatment difference of –1.06 percentage points (P less than .001). By week 52, the treatment difference between the two groups had increased to –1.30 percentage points.
William V. Tamborlane, MD, from the department of pediatrics at Yale University, New Haven, Conn., and his coauthors wrote that metformin is the approved drug of choice for pediatric patients with type 2 diabetes, and that insulin currently is the only approved option for those who do not have an adequate response to metformin monotherapy.
“This discrepancy in available treatments for youth as compared with adults persists because of a lack of successfully completed trials needed for approval of new drugs for the treatment of type 2 diabetes in children since a trial of metformin was completed in 1999,” they wrote.
The study showed that significantly more patients in the liraglutide group (63.7%) achieved glycated hemoglobin levels below 7%, compared with 36.5% of patients in the placebo group. Fasting plasma glucose levels were decreased in the liraglutide group at both 26 and 52 weeks, but had increased in the placebo group.
Although the number of reported adverse events were similar between the two groups, there were significantly more reports of gastrointestinal adverse events – particularly nausea – in patients taking liraglutide, compared with those on placebo.
However, the study did not show a difference between liraglutide and placebo in lowering body mass index, although mean body weight decreases – which were seen in both groups – were maintained at week 52 only in the liraglutide group. The authors suggested this might be owing to the relatively small number of patients enrolled in the study and that some of the children were still growing.
Novo Nordisk, which manufactures liraglutide, supported the study. Twelve authors reported grants or support from Novo Nordisk in relation to the trial. Three authors were employees of Novo Nordisk. Eight authors reported unrelated grants and fees from Novo Nordisk and other pharmaceutical companies.
SOURCE: Tamborlane WV et al. N Engl J Med. 2019 Apr 28. doi: 10.1056/NEJMoa1903822.
FROM PAS 2019
Type 2 diabetes bumps up short-term risk for bone fracture
LOS ANGELES – results from a large community-based study have shown.
“Osteoporotic fractures are a significant public health burden, causing high morbidity, mortality, and associated health care costs,” Elizabeth J. Samelson, PhD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Risk of fractures are higher in patients with type 2 diabetes. Further, outcomes are worse in type 2 diabetes patients, with greater frequency of complications following a fracture.”
Given the projected increase in type 2 diabetes in the U.S. population, Dr. Samelson, an associate scientist at the Marcus Institute for Aging Research at Hebrew SeniorLife and Harvard Medical School, Boston, and colleagues set out to evaluate the short- and long-term risks of bone fractures associated with the disease. They drew from 2,105 women and 1,130 men who participated in the Framingham Original and Offspring Cohorts and whose baseline osteoporosis visit was around 1990. Type 2 diabetes was defined as having a fasting plasma glucose of greater than 125 mg/dL or being on treatment for the disease. Incident fractures excluded finger, toe, skull, face, and pathologic fractures, and the researchers used repeated measures analyses to estimate hazard ratios for the association between type 2 diabetes, type 2 diabetes medication use, and type 2 diabetes duration and incident fracture, adjusted for age, sex, height, and weight.
The mean age of the study participants was 67 years, and the mean follow-up was 9 years. The prevalence of type 2 diabetes in women and men was 7% and 13%, respectively, and 63% and 51% of those were on medication for the disease. The mean duration of diabetes was 8 years.
Dr. Samelson and colleagues found that the cumulative incidence of fracture was 37% in women with type 2 diabetes and 30% in those without the disease. Meanwhile, the cumulative incidence of fracture was 11% in men with type 2 diabetes and 16% in those without the disease. The researchers also found that type 2 diabetes was associated with 1-year fracture risk in women (hazard ratio, 2.23), but not in men.
In the entire study population, longer duration of type 2 diabetes increased the 2-year fracture risk (HR, 1.28), as did the use of any type 2 diabetes medication (HR, 1.70). The researchers observed no statistically significant differences between type 2 diabetes and long-term incidence of fracture.
“Previous studies have contributed to understanding the higher incidence of fractures and worse outcomes in type 2 diabetes, [but] the current study demonstrated that patients [with type 2 diabetes] have 50% to 100% higher short-term [1- to 2-year] risk of fracture independent of clinical risk factors, whereas long-term [10-year] risk of fracture was similar in [patients with] type 2 diabetes and those who do not have [the disease],” Dr. Samelson said. “The current study has some inherent limitations of observational studies, including a lack of definitive determination of causality and that the results are not generalizable to patients with similar demographics. The study, however, is robust in the availability of detailed clinical information, which allows for control of multiple confounding variables.”
Dr. Samelson reported having no financial disclosures. Coauthors Setareh Williams, PhD, and Rich Weiss, MD, are employees and shareholders of Radius Health Inc.
LOS ANGELES – results from a large community-based study have shown.
“Osteoporotic fractures are a significant public health burden, causing high morbidity, mortality, and associated health care costs,” Elizabeth J. Samelson, PhD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Risk of fractures are higher in patients with type 2 diabetes. Further, outcomes are worse in type 2 diabetes patients, with greater frequency of complications following a fracture.”
Given the projected increase in type 2 diabetes in the U.S. population, Dr. Samelson, an associate scientist at the Marcus Institute for Aging Research at Hebrew SeniorLife and Harvard Medical School, Boston, and colleagues set out to evaluate the short- and long-term risks of bone fractures associated with the disease. They drew from 2,105 women and 1,130 men who participated in the Framingham Original and Offspring Cohorts and whose baseline osteoporosis visit was around 1990. Type 2 diabetes was defined as having a fasting plasma glucose of greater than 125 mg/dL or being on treatment for the disease. Incident fractures excluded finger, toe, skull, face, and pathologic fractures, and the researchers used repeated measures analyses to estimate hazard ratios for the association between type 2 diabetes, type 2 diabetes medication use, and type 2 diabetes duration and incident fracture, adjusted for age, sex, height, and weight.
The mean age of the study participants was 67 years, and the mean follow-up was 9 years. The prevalence of type 2 diabetes in women and men was 7% and 13%, respectively, and 63% and 51% of those were on medication for the disease. The mean duration of diabetes was 8 years.
Dr. Samelson and colleagues found that the cumulative incidence of fracture was 37% in women with type 2 diabetes and 30% in those without the disease. Meanwhile, the cumulative incidence of fracture was 11% in men with type 2 diabetes and 16% in those without the disease. The researchers also found that type 2 diabetes was associated with 1-year fracture risk in women (hazard ratio, 2.23), but not in men.
In the entire study population, longer duration of type 2 diabetes increased the 2-year fracture risk (HR, 1.28), as did the use of any type 2 diabetes medication (HR, 1.70). The researchers observed no statistically significant differences between type 2 diabetes and long-term incidence of fracture.
“Previous studies have contributed to understanding the higher incidence of fractures and worse outcomes in type 2 diabetes, [but] the current study demonstrated that patients [with type 2 diabetes] have 50% to 100% higher short-term [1- to 2-year] risk of fracture independent of clinical risk factors, whereas long-term [10-year] risk of fracture was similar in [patients with] type 2 diabetes and those who do not have [the disease],” Dr. Samelson said. “The current study has some inherent limitations of observational studies, including a lack of definitive determination of causality and that the results are not generalizable to patients with similar demographics. The study, however, is robust in the availability of detailed clinical information, which allows for control of multiple confounding variables.”
Dr. Samelson reported having no financial disclosures. Coauthors Setareh Williams, PhD, and Rich Weiss, MD, are employees and shareholders of Radius Health Inc.
LOS ANGELES – results from a large community-based study have shown.
“Osteoporotic fractures are a significant public health burden, causing high morbidity, mortality, and associated health care costs,” Elizabeth J. Samelson, PhD, said in an interview in advance of the annual scientific and clinical congress of the American Association of Clinical Endocrinologists. “Risk of fractures are higher in patients with type 2 diabetes. Further, outcomes are worse in type 2 diabetes patients, with greater frequency of complications following a fracture.”
Given the projected increase in type 2 diabetes in the U.S. population, Dr. Samelson, an associate scientist at the Marcus Institute for Aging Research at Hebrew SeniorLife and Harvard Medical School, Boston, and colleagues set out to evaluate the short- and long-term risks of bone fractures associated with the disease. They drew from 2,105 women and 1,130 men who participated in the Framingham Original and Offspring Cohorts and whose baseline osteoporosis visit was around 1990. Type 2 diabetes was defined as having a fasting plasma glucose of greater than 125 mg/dL or being on treatment for the disease. Incident fractures excluded finger, toe, skull, face, and pathologic fractures, and the researchers used repeated measures analyses to estimate hazard ratios for the association between type 2 diabetes, type 2 diabetes medication use, and type 2 diabetes duration and incident fracture, adjusted for age, sex, height, and weight.
The mean age of the study participants was 67 years, and the mean follow-up was 9 years. The prevalence of type 2 diabetes in women and men was 7% and 13%, respectively, and 63% and 51% of those were on medication for the disease. The mean duration of diabetes was 8 years.
Dr. Samelson and colleagues found that the cumulative incidence of fracture was 37% in women with type 2 diabetes and 30% in those without the disease. Meanwhile, the cumulative incidence of fracture was 11% in men with type 2 diabetes and 16% in those without the disease. The researchers also found that type 2 diabetes was associated with 1-year fracture risk in women (hazard ratio, 2.23), but not in men.
In the entire study population, longer duration of type 2 diabetes increased the 2-year fracture risk (HR, 1.28), as did the use of any type 2 diabetes medication (HR, 1.70). The researchers observed no statistically significant differences between type 2 diabetes and long-term incidence of fracture.
“Previous studies have contributed to understanding the higher incidence of fractures and worse outcomes in type 2 diabetes, [but] the current study demonstrated that patients [with type 2 diabetes] have 50% to 100% higher short-term [1- to 2-year] risk of fracture independent of clinical risk factors, whereas long-term [10-year] risk of fracture was similar in [patients with] type 2 diabetes and those who do not have [the disease],” Dr. Samelson said. “The current study has some inherent limitations of observational studies, including a lack of definitive determination of causality and that the results are not generalizable to patients with similar demographics. The study, however, is robust in the availability of detailed clinical information, which allows for control of multiple confounding variables.”
Dr. Samelson reported having no financial disclosures. Coauthors Setareh Williams, PhD, and Rich Weiss, MD, are employees and shareholders of Radius Health Inc.
REPORTING FROM AACE 2019
Gastric outlet obstruction: A red flag, potentially manageable
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
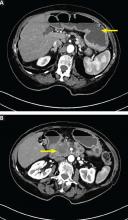
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
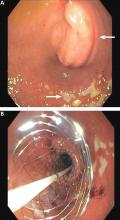
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
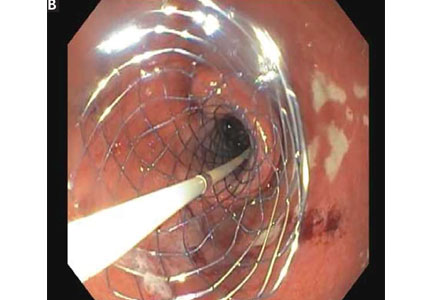
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
A 72-year-old woman presents to the emergency department with progressive nausea and vomiting. One week earlier, she developed early satiety and nausea with vomiting after eating solid food. Three days later her symptoms progressed, and she became unable to take anything by mouth. The patient also experienced a 40-lb weight loss in the previous 3 months. She denies symptoms of abdominal pain, hematemesis, or melena. Her medical history includes cholecystectomy and type 2 diabetes mellitus, diagnosed 1 year ago. She has no family history of gastrointestinal malignancy. She says she smoked 1 pack a day in her 20s. She does not consume alcohol.
On physical examination, she is normotensive with a heart rate of 105 beats per minute. The oral mucosa is dry, and the abdomen is mildly distended and tender to palpation in the epigastrium. Laboratory evaluation reveals hypokalemia and metabolic alkalosis.
Computed tomography (CT) reveals a mass 3 cm by 4 cm in the pancreatic head. The mass has invaded the medial wall of the duodenum, with obstruction of the pancreatic and common bile ducts and extension into and occlusion of the superior mesenteric vein, with soft-tissue expansion around the superior mesenteric artery. CT also reveals retained stomach contents and an air-fluid level consistent with gastric outlet obstruction.
INTRINSIC OR EXTRINSIC BLOCKAGE
Gastric outlet obstruction, also called pyloric obstruction, is caused by intrinsic or extrinsic mechanical blockage of gastric emptying, generally in the distal stomach, pyloric channel, or duodenum, with associated symptoms of nausea, vomiting, abdominal pain, and early satiety. It is encountered in both the clinic and the hospital.
Here, we review the causes, diagnosis, and management of this disorder.
BENIGN AND MALIGNANT CAUSES
In a retrospective study of 76 patients hospitalized with gastric outlet obstruction between 2006 and 2015 at our institution,2 29 cases (38%) were due to malignancy and 47 (62%) were due to benign causes. Pancreatic adenocarcinoma accounted for 13 cases (17%), while gastric adenocarcinoma accounted for 5 cases (7%); less common malignant causes were cholangiocarcinoma, cancer of the ampulla of Vater, duodenal adenocarcinoma, hepatocellular carcinoma, and metastatic disease. Of the benign causes, the most common were peptic ulcer disease (13 cases, 17%) and postoperative strictures or adhesions (11 cases, 14%).
These numbers reflect general trends around the world.
Less gastric cancer, more pancreatic cancer
The last several decades have seen a trend toward more cases due to cancer and fewer due to benign causes.3–14
In earlier studies in both developed and developing countries, gastric adenocarcinoma was the most common malignant cause of gastric outlet obstruction. Since then, it has become less common in Western countries, although it remains more common in Asia and Africa.7–14 This trend likely reflects environmental factors, including decreased prevalence of Helicobacter pylori infection, a major risk factor for gastric cancer, in Western countries.15–17
At the same time, pancreatic cancer is on the rise,16 and up to 20% of patients with pancreatic cancer develop gastric outlet obstruction.18 In a prospective observational study of 108 patients with malignant gastric outlet obstruction undergoing endoscopic stenting, pancreatic cancer was by far the most common malignancy, occurring in 54% of patients, followed by gastric cancer in 13%.19
Less peptic ulcer disease, but still common
Peptic ulcer disease used to account for up to 90% of cases of gastric outlet obstruction, and it is still the most common benign cause.
In 1990, gastric outlet obstruction was estimated to occur in 5% to 10% of all hospital admissions for ulcer-related complications, accounting for 2,000 operations annually.20,21 Gastric outlet obstruction now occurs in fewer than 5% of patients with duodenal ulcer disease and fewer than 2% of patients with gastric ulcer disease.22
Peptic ulcer disease remains an important cause of obstruction in countries with poor access to acid-suppressing drugs.23
Gastric outlet obstruction occurs in both acute and chronic peptic ulcer disease. In acute peptic ulcer disease, tissue inflammation and edema result in mechanical obstruction. Chronic peptic ulcer disease results in tissue scarring and fibrosis with strictures.20
Environmental factors, including improved diet, hygiene, physical activity, and the decreased prevalence of H pylori infection, also contribute to the decreased prevalence of peptic ulcer disease and its complications, including gastric outlet obstruction.3 The continued occurrence of peptic ulcer disease is associated with widespread use of low-dose aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs), the most common causes of peptic ulcer disease in Western countries.24,25
Other nonmalignant causes of gastric outlet obstruction are diverse and less common. They include caustic ingestion, postsurgical strictures, benign tumors of the gastrointestinal tract, Crohn disease, and pancreatic disorders including acute pancreatitis, pancreatic pseudocyst, chronic pancreatitis, and annular pancreas. Intramural duodenal hematoma may cause obstruction after blunt abdominal trauma, endoscopic biopsy, or gastrostomy tube migration, especially in the setting of a bleeding disorder or anticoagulation.26
Tuberculosis should be suspected in countries in which it is common.7 In a prospective study of 64 patients with benign gastric outlet obstruction in India,27 16 (25%) had corrosive injury, 16 (25%) had tuberculosis, and 15 (23%) had peptic ulcer disease. Compared with patients with corrosive injury and peptic ulcer disease, patients with gastroduodenal tuberculosis had the best outcomes with appropriate treatment.
Other reported causes include Bouveret syndrome (an impacted gallstone in the proximal duodenum), phytobezoar, diaphragmatic hernia, gastric volvulus, and Ladd bands (peritoneal bands associated with intestinal malrotation).7,28,29
PRESENTING SYMPTOMS
Symptoms of gastric outlet obstruction include nausea, nonbilious vomiting, epigastric pain, early satiety, abdominal distention, and weight loss.
In our patients, the most common presenting symptoms were nausea and vomiting (80%), followed by abdominal pain (72%); weight loss (15%), abdominal distention (15%), and early satiety (9%) were less common.2
Patients with gastric outlet obstruction secondary to malignancy generally present with a shorter duration of symptoms than those with peptic ulcer disease and are more likely to be older.8,13 Other conditions with an acute onset of symptoms include gastric polyp prolapse, percutaneous endoscopic gastrostomy tube migration, gastric volvulus, and gallstone impaction.
Patients with gastric outlet obstruction associated with peptic ulcer disease generally have a long-standing history of symptoms, including dyspepsia and weight loss over several years.4
SIGNS ON EXAMINATION
On examination, look for signs of chronic gastric obstruction and its consequences, such as malnutrition, cachexia, volume depletion, and dental erosions.
A succussion splash may suggest gastric outlet obstruction. This is elicited by rocking the patient back and forth by the hips or abdomen while listening over the stomach for a splash, which may be heard without a stethoscope. The test is considered positive if present 3 or more hours after drinking fluids and suggests retention of gastric materials.30,31
In thin individuals, chronic gastric outlet obstruction makes the stomach dilate and hypertrophy, which may be evident by a palpably thickened stomach with visible gastric peristalsis.4
Other notable findings on physical examination may include a palpable abdominal mass, epigastric pain, or an abnormality suggestive of metastatic gastric cancer, such as an enlarged left supraclavicular lymph node (Virchow node) or periumbilical lymph node (Sister Mary Joseph nodule). The Virchow node is at the junction of the thoracic duct and the left subclavian vein where the lymphatic circulation from the body drains into the systemic circulation, and it may be the first sign of gastric cancer.32 Sister Mary Joseph nodule (named after a surgical assistant to Dr. William James Mayo) refers to a palpable mass at the umbilicus, generally resulting from metastasis of an abdominal malignancy.33
SIGNS ON FURTHER STUDIES
Laboratory evaluation may show signs of poor oral intake and electrolyte abnormalities secondary to chronic nausea, vomiting, and dehydration, including hypochloremic metabolic alkalosis and hypokalemia.
The underlying cause of gastric outlet obstruction has major implications for treatment and prognosis and cannot be differentiated by clinical presentation alone.1,9 Diagnosis is based on clinical features and radiologic or endoscopic evaluation consistent with gastric outlet obstruction.
Plain radiography may reveal an enlarged gastric bubble, and contrast studies may be useful to determine whether the obstruction is partial or complete, depending on whether the contrast passes into the small bowel.
Upper endoscopy is often needed to establish the diagnosis and cause. Emptying the stomach with a nasogastric tube is recommended before endoscopy to minimize the risk of aspiration during the procedure, and endotracheal intubation should be considered for airway protection.34 Findings of gastric outlet obstruction on upper endoscopy include retained food and liquid. Endoscopic biopsy is important to differentiate between benign and malignant causes. For patients with malignancy, endoscopic ultrasonography is useful for diagnosis via tissue sampling with fine-needle aspiration and locoregional staging.35
A strategy. Most patients whose clinical presentation suggests gastric outlet obstruction require cross-sectional radiologic imaging, upper endoscopy, or both.36 CT is the preferred imaging study to evaluate for intestinal obstruction.36,37 Patients with suspected complete obstruction or perforation should undergo CT before upper endoscopy. Oral contrast may interfere with endoscopy and should be avoided if endoscopy is planned. Additionally, giving oral contrast may worsen patient discomfort and increase the risk of nausea, vomiting, and aspiration.36,37
Following radiographic evaluation, upper endoscopy can be performed after gastric decompression to identify the location and extent of the obstruction and to potentially provide a definitive diagnosis with biopsy.36
DIFFERENTIATE FROM GASTROPARESIS
Gastroparesis is a chronic neuromuscular disorder characterized by delayed gastric emptying without mechanical obstruction.38 The most common causes are diabetes, surgery, and idiopathy. Other causes include viral infection, connective tissue diseases, ischemia, infiltrative disorders, radiation, neurologic disorders, and paraneoplastic syndromes.39,40
Gastric outlet obstruction and gastroparesis share clinical symptoms including nausea, vomiting, abdominal pain, early satiety, and weight loss and are important to differentiate.36,38 Although abdominal pain may be present in both gastric outlet obstruction and gastroparesis, in gastroparesis it tends not to be the dominant symptom.40
Gastric scintigraphy is most commonly used to objectively quantify delayed gastric emptying.39 Upper endoscopy is imperative to exclude mechanical obstruction.39
MANAGEMENT
Initially, patients with signs and symptoms of gastric outlet obstruction should be given:
- Nothing by mouth (NPO)
- Intravenous fluids to correct volume depletion and electrolyte abnormalities
- A nasogastric tube for gastric decompression and symptom relief if symptoms persist despite being NPO
- A parenteral proton pump inhibitor, regardless of the cause of obstruction, to decrease gastric secretions41
- Medications for pain and nausea, if needed.
Definitive treatment of gastric outlet obstruction depends on the underlying cause, whether benign or malignant.
Management of benign gastric outlet obstruction
Symptoms of gastric outlet obstruction resolve spontaneously in about half of cases caused by acute peptic ulcer disease, as acute inflammation resolves.9,22
Endoscopic dilation is an important option in patients with benign gastric outlet obstruction, including peptic ulcer disease. Peptic ulcer disease-induced gastric outlet obstruction can be safely treated with endoscopic balloon dilation. This treatment almost always relieves symptoms immediately; however, the long-term response has varied from 16% to 100%, and patients may require more than 1 dilation procedure.25,42,43 The need for 2 or more dilation procedures may predict need for surgery.44 Gastric outlet obstruction after caustic ingestion or endoscopic submucosal dissection may also respond to endoscopic balloon dilation.36
Eradication of H pylori may be effective and lead to complete resolution of symptoms in patients with gastric outlet obstruction due to this infection.45–47
NSAIDs should be discontinued in patients with peptic ulcer disease and gastric outlet obstruction. These drugs damage the gastrointestinal mucosa by inhibiting cyclo-oxygenase (COX) enzymes and decreasing synthesis of prostaglandins, which are important for mucosal defense.48 Patients may be unaware of NSAIDs contained in over-the-counter medications and may have difficulty discontinuing NSAIDs taken for pain.49
These drugs are an important cause of refractory peptic ulcer disease and can be detected by platelet COX activity testing, although this test is not widely available. In a study of patients with peptic ulcer disease without definite NSAID use or H pylori infection, up to one-third had evidence of surreptitious NSAID use as detected by platelet COX activity testing.50 In another study,51 platelet COX activity testing discovered over 20% more aspirin users than clinical history alone.
Surgery for patients with benign gastric outlet obstruction is used only when medical management and endoscopic dilation fail. Ideally, surgery should relieve the obstruction and target the underlying cause, such as peptic ulcer disease. Laparoscopic surgery is generally preferred to open surgery because patients can resume oral intake sooner, have a shorter hospital stay, and have less intraoperative blood loss.52 The simplest surgical procedure to relieve obstruction is laparoscopic gastrojejunostomy.
Patients with gastric outlet obstruction and peptic ulcer disease warrant laparoscopic vagotomy and antrectomy or distal gastrectomy. This removes the obstruction and the stimulus for gastric secretion.53 An alternative is vagotomy with a drainage procedure (pyloroplasty or gastrojejunostomy), which has a similar postoperative course and reduction in gastric acid secretion compared with antrectomy or distal gastrectomy.53,54
Daily proton pump inhibitors can be used for patients with benign gastric outlet obstruction not associated with peptic ulcer disease or risk factors; for such cases, vagotomy is not required.
Management of malignant gastric outlet obstruction
Patients with malignant gastric outlet obstruction may have intractable nausea and abdominal pain secondary to retention of gastric contents. The major goal of therapy is to improve symptoms and restore tolerance of an oral diet. The short-term prognosis of malignant gastric outlet obstruction is poor, with a median survival of 3 to 4 months, as these patients often have unresectable disease.55
Surgical bypass used to be the standard of care for palliation of malignant gastric obstruction, but that was before endoscopic stenting was developed.
Endoscopic stenting allows patients to resume oral intake and get out of the hospital sooner with fewer complications than with open surgical bypass. It may be a more appropriate option for palliation of symptoms in patients with malignant obstruction who have a poor prognosis and prefer a less invasive intervention.55,56
Endoscopic duodenal stenting of malignant gastric outlet obstruction has a success rate of greater than 90%, and most patients can tolerate a mechanical soft diet afterward.34 The procedure is usually performed with a 9-cm or 12-cm self-expanding duodenal stent, 22 mm in diameter, placed over a guide wire under endoscopic and fluoroscopic guidance (Figure 2). The stent is placed by removing the outer catheter, with distal-to-proximal stent deployment.
Patients who also have biliary obstruction may require biliary stent placement, which is generally performed before duodenal stenting. For patients with an endoscopic stent who develop biliary obstruction, endoscopic retrograde cholangiopancreatography can be attempted with placement of a biliary stent; however, these patients may require biliary drain placement by percutaneous transhepatic cholangiography or by endoscopic ultrasonographically guided transduodenal or transgastric biliary drainage.
From 20% to 30% of patients require repeated endoscopic stent placement, although most patients die within several months after stenting.34 Surgical options for patients who do not respond to endoscopic stenting include open or laparoscopic gastrojejunostomy.55
Laparoscopic gastrojejunostomy may provide better long-term outcomes than duodenal stenting for patients with malignant gastric outlet obstruction and a life expectancy longer than a few months.
A 2017 retrospective study of 155 patients with gastric outlet obstruction secondary to unresectable gastric cancer suggested that those who underwent laparoscopic gastrojejunostomy had better oral intake, better tolerance of chemotherapy, and longer overall survival than those who underwent duodenal stenting. Postsurgical complications were more common in the laparoscopic gastrojejunostomy group (16%) than in the duodenal stenting group (0%).57
In most of the studies comparing endoscopic stenting with surgery, the surgery was open gastrojejunostomy; there are limited data directly comparing stenting with laparoscopic gastrojejunostomy.55 Endoscopic stenting is estimated to be significantly less costly than surgery, with a median cost of $12,000 less than gastrojejunostomy.58 As an alternative to enteral stenting and surgical gastrojejunostomy, ultrasonography-guided endoscopic gastrojejunostomy or gastroenterostomy with placement of a lumen-apposing metal stent is emerging as a third treatment option and is under active investigation.59
Patients with malignancy that is potentially curable by resection should undergo surgical evaluation before consideration of endoscopic stenting. For patients who are not candidates for surgery or endoscopic stenting, a percutaneous gastrostomy tube can be considered for gastric decompression and symptom relief.
CASE CONCLUDED
The patient underwent esophagogastroduodenoscopy with endoscopic ultrasonography for evaluation of her pancreatic mass. Before the procedure, she was intubated to minimize the risk of aspiration due to persistent nausea and retained gastric contents. A large submucosal mass was found in the duodenal bulb. Endoscopic ultrasonography showed a mass within the pancreatic head with pancreatic duct obstruction. Fine-needle aspiration biopsy was performed, and pathology study revealed pancreatic adenocarcinoma. The patient underwent stenting with a 22-mm by 12-cm WallFlex stent (Boston Scientific), which led to resolution of nausea and advancement to a mechanical soft diet on hospital discharge.
She was scheduled for follow-up in the outpatient clinic for treatment of pancreatic cancer.
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
- Johnson CD. Gastric outlet obstruction malignant until proved otherwise. Am J Gastroenterol 1995; 90(10):1740. pmid:7572886
- Koop AH, Palmer WC, Mareth K, Burton MC, Bowman A, Stancampiano F. Tu1335 - Pancreatic cancer most common cause of malignant gastric outlet obstruction at a tertiary referral center: a 10 year retrospective study [abstract]. Gastroenterology 2018; 154(6, suppl 1):S-1343.
- Hall R, Royston C, Bardhan KD. The scars of time: the disappearance of peptic ulcer-related pyloric stenosis through the 20th century. J R Coll Physicians Edinb 2014; 44(3):201–208. doi:10.4997/JRCPE.2014.303
- Kreel L, Ellis H. Pyloric stenosis in adults: a clinical and radiological study of 100 consecutive patients. Gut 1965; 6(3):253–261. pmid:18668780
- Shone DN, Nikoomanesh P, Smith-Meek MM, Bender JS. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995; 90(10):1769–1770. pmid:7572891
- Ellis H. The diagnosis of benign and malignant pyloric obstruction. Clin Oncol 1976; 2(1):11–15. pmid:1277618
- Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg 2007; 23(1):29–32.
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996; 91(8):1679. pmid:8759707
- Johnson CD, Ellis H. Gastric outlet obstruction now predicts malignancy. Br J Surg 1990; 77(9):1023–1024. pmid:2207566
- Misra SP, Dwivedi M, Misra V. Malignancy is the most common cause of gastric outlet obstruction even in a developing country. Endoscopy 1998; 30(5):484–486. doi:10.1055/s-2007-1001313
- Essoun SD, Dakubo JCB. Update of aetiological patterns of adult gastric outlet obstruction in Accra, Ghana. Int J Clin Med 2014; 5(17):1059–1064. doi:10.4236/ijcm.2014.517136
- Jaka H, Mchembe MD, Rambau PF, Chalya PL. Gastric outlet obstruction at Bugando Medical Centre in Northwestern Tanzania: a prospective review of 184 cases. BMC Surg 2013; 13:41. doi:10.1186/1471-2482-13-41
- Sukumar V, Ravindran C, Prasad RV. Demographic and etiological patterns of gastric outlet obstruction in Kerala, South India. N Am J Med Sci 2015; 7(9):403–406. doi:10.4103/1947-2714.166220
- Yoursef M, Mirza MR, Khan S. Gastric outlet obstruction. Pak J Surg 2005; 10(4):48–50.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136(5):E359–E386. doi:10.1002/ijc.29210
- Parkin DM, Stjernsward J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ 1984; 62(2):163–182. pmid:6610488
- Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014; 23(5):700–713. doi:10.1158/1055-9965.EPI-13-1057
- Jeurnink SM, Steyerberg EW, van Hooft JE, et al; Dutch SUSTENT Study Group. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT) study): a multicenter randomized trial. Gastrointest Endosc 2010; 71(3):490–499. doi:10.1016/j.gie.2009.09.042
- Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc 2014; 79(1):66–75. doi:10.1016/j.gie.2013.06.032
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009; 374(9699):1449–1461. doi:10.1016/S0140-6736(09)60938-7
- Gibson JB, Behrman SW, Fabian TC, Britt LG. Gastric outlet obstruction resulting from peptic ulcer disease requiring surgical intervention is infrequently associated with Helicobacter pylori infection. J Am Coll Surg 2000; 191(1):32–37. pmid:10898181
- Kochhar R, Kochhar S. Endoscopic balloon dilation for benign gastric outlet obstruction in adults. World J Gastrointest Endosc 2010; 2(1):29–35. doi:10.4253/wjge.v2.i1.29
- Kotisso R. Gastric outlet obstruction in Northwestern Ethiopia. East Cent Afr J Surg 2000; 5(2):25-29.
- Hamzaoui L, Bouassida M, Ben Mansour I, et al. Balloon dilatation in patients with gastric outlet obstruction related to peptic ulcer disease. Arab J Gastroenterol 2015; 16(3–4):121–124. doi:10.1016/j.ajg.2015.07.004
- Najm WI. Peptic ulcer disease. Prim Care 2011; 38(3):383–394. doi:10.1016/j.pop.2011.05.001
- Veloso N, Amaro P, Ferreira M, Romaozinho JM, Sofia C. Acute pancreatitis associated with a nontraumatic, intramural duodenal hematoma. Endoscopy 2013; 45(suppl 2):E51–E52. doi:10.1055/s-0032-1325969
- Maharshi S, Puri AS, Sachdeva S, Kumar A, Dalal A, Gupta M. Aetiological spectrum of benign gastric outlet obstruction in India: new trends. Trop Doct 2016; 46(4):186–191. doi:10.1177/0049475515626032
- Sala MA, Ligabo AN, de Arruda MC, Indiani JM, Nacif MS. Intestinal malrotation associated with duodenal obstruction secondary to Ladd’s bands. Radiol Bras 2016; 49(4):271–272. doi:10.1590/0100-3984.2015.0106
- Alibegovic E, Kurtcehajic A, Hujdurovic A, Mujagic S, Alibegovic J, Kurtcehajic D. Bouveret syndrome or gallstone ileus. Am J Med 2018; 131(4):e175. doi:10.1016/j.amjmed.2017.10.044
- Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc 1996; 43(2 Pt 1):98–101. pmid:8635729
- Ray K, Snowden C, Khatri K, McFall M. Gastric outlet obstruction from a caecal volvulus, herniated through epiploic foramen: a case report. BMJ Case Rep 2009; pii:bcr05.2009.1880. doi:10.1136/bcr.05.2009.1880
- Baumgart DC, Fischer A. Virchow’s node. Lancet 2007; 370(9598):1568. doi:10.1016/S0140-6736(07)61661-4
- Dar IH, Kamili MA, Dar SH, Kuchaai FA. Sister Mary Joseph nodule—a case report with review of literature. J Res Med Sci 2009; 14(6):385–387. pmid:21772912
- Tang SJ. Endoscopic stent placement for gastric outlet obstruction. Video Journal and Encyclopedia of GI Endoscopy 2013; 1(1):133–136.
- Valero M, Robles-Medranda C. Endoscopic ultrasound in oncology: an update of clinical applications in the gastrointestinal tract. World J Gastrointest Endosc 2017; 9(6):243–254.
- ASGE Standards of Practice Committee; Fukami N, Anderson MA, Khan K, et al. The role of endoscopy in gastroduodenal obstruction and gastroparesis. Gastrointest Endosc 2011; 74(1):13–21. doi:10.1016/j.gie.2010.12.003
- Ros PR, Huprich JE. ACR appropriateness criteria on suspected small-bowel obstruction. J Am Coll Radiol 2006; 3(11):838–841. doi:10.1016/j.jacr.2006.09.018
- Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol Clin North Am 2015; 44(1):1–7. doi:10.1016/j.gtc.2014.11.001
- Stein B, Everhart KK, Lacy BE. Gastroparesis: a review of current diagnosis and treatment options. J Clin Gastroenterol 2015; 49(7):550–558. doi:10.1097/MCG.0000000000000320
- Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L; American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol 2013; 108(1):18–37.
- Gursoy O, Memis D, Sut N. Effect of proton pump inhibitors on gastric juice volume, gastric pH and gastric intramucosal pH in critically ill patients: a randomized, double-blind, placebo-controlled study. Clin Drug Investig 2008; 28(12):777–782. doi:10.2165/0044011-200828120-00005
- Kuwada SK, Alexander GL. Long-term outcome of endoscopic dilation of nonmalignant pyloric stenosis. Gastrointest Endosc 1995; 41(1):15–17. pmid:7698619
- Kochhar R, Sethy PK, Nagi B, Wig JD. Endoscopic balloon dilatation of benign gastric outlet obstruction. J Gastroenterol Hepatol 2004; 19(4):418–422. pmid:15012779
- Perng CL, Lin HJ, Lo WC, Lai CR, Guo WS, Lee SD. Characteristics of patients with benign gastric outlet obstruction requiring surgery after endoscopic balloon dilation. Am J Gastroenterol 1996; 91(5):987–990. pmid:8633593
- Taskin V, Gurer I, Ozyilkan E, Sare M, Hilmioglu F. Effect of Helicobacter pylori eradication on peptic ulcer disease complicated with outlet obstruction. Helicobacter 2000; 5(1):38–40. pmid:10672050
- de Boer WA, Driessen WM. Resolution of gastric outlet obstruction after eradication of Helicobacter pylori. J Clin Gastroenterol 1995; 21(4):329–330. pmid:8583113
- Tursi A, Cammarota G, Papa A, Montalto M, Fedeli G, Gasbarrini G. Helicobacter pylori eradication helps resolve pyloric and duodenal stenosis. J Clin Gastroenterol 1996; 23(2):157–158. pmid:8877648
- Schmassmann A. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am J Med 1998; 104(3A):43S–51S; discussion 79S–80S. pmid:9572320
- Kim HU. Diagnostic and treatment approaches for refractory peptic ulcers. Clin Endosc 2015; 48(4):285–290. doi:10.5946/ce.2015.48.4.285
- Ong TZ, Hawkey CJ, Ho KY. Nonsteroidal anti-inflammatory drug use is a significant cause of peptic ulcer disease in a tertiary hospital in Singapore: a prospective study. J Clin Gastroenterol 2006; 40(9):795–800. doi:10.1097/01.mcg.0000225610.41105.7f
- Lanas A, Sekar MC, Hirschowitz BI. Objective evidence of aspirin use in both ulcer and nonulcer upper and lower gastrointestinal bleeding. Gastroenterology 1992; 103(3):862–869. pmid:1499936
- Zhang LP, Tabrizian P, Nguyen S, Telem D, Divino C. Laparoscopic gastrojejunostomy for the treatment of gastric outlet obstruction. JSLS 2011; 15(2):169–173. doi:10.4293/108680811X13022985132074
- Lagoo J, Pappas TN, Perez A. A relic or still relevant: the narrowing role for vagotomy in the treatment of peptic ulcer disease. Am J Surg 2014; 207(1):120–126. doi:10.1016/j.amjsurg.2013.02.012
- Csendes A, Maluenda F, Braghetto I, Schutte H, Burdiles P, Diaz JC. Prospective randomized study comparing three surgical techniques for the treatment of gastric outlet obstruction secondary to duodenal ulcer. Am J Surg 1993; 166(1):45–49. pmid:8101050
- Ly J, O’Grady G, Mittal A, Plank L, Windsor JA. A systematic review of methods to palliate malignant gastric outlet obstruction. Surg Endosc 2010; 24(2):290–297. doi:10.1007/s00464-009-0577-1
- Goldberg EM. Palliative treatment of gastric outlet obstruction in terminal patients: SEMS. Stent every malignant stricture! Gastrointest Endosc 2014; 79(1):76–78. doi:10.1016/j.gie.2013.07.056
- Min SH, Son SY, Jung DH, et al. Laparoscopic gastrojejunostomy versus duodenal stenting in unresectable gastric cancer with gastric outlet obstruction. Ann Surg Treat Res 2017; 93(3):130–136. doi:10.4174/astr.2017.93.3.130
- Roy A, Kim M, Christein J, Varadarajulu S. Stenting versus gastrojejunostomy for management of malignant gastric outlet obstruction: comparison of clinical outcomes and costs. Surg Endosc 2012; 26(11):3114–119. doi:10.1007/s00464-012-2301-9
- Amin S, Sethi A. Endoscopic ultrasound-guided gastrojejunostomy. Gastrointest Endosc Clin N Am 2017; 27(4):707–713. doi:10.1016/j.giec.2017.06.009
KEY POINTS
- Causes of gastric outlet obstruction fall into 2 categories: benign and malignant. The cause should be presumed to be malignant until proven otherwise.
- Peptic ulcer disease, a benign cause, used to account for most cases of gastric outlet obstruction. It is still common but has declined in frequency with the development of acid-suppressing drugs.
- Gastric cancer used to be the most common malignant cause but has declined in frequency in Western countries with treatment for Helicobacter pylori infection. Now, pancreatic cancer predominates.
- Endoscopic stenting is an effective, minimally invasive treatment for patients with malignant gastric outlet obstruction and poor prognosis, allowing resumption of oral intake and improving quality of life.
Pyoderma gangrenosum mistaken for diabetic ulcer
A 55-year-old man with type 2 diabetes mellitus, hypertension, anemia, and ulcerative colitis presented to the emergency department with an ulcer on his left leg (Figure 1). He said the lesion had started as a “large pimple” that ruptured one night while he was sleeping and then became drastically worse over the past week. He said the lesion was painful and was “oozing blood.”
On examination, the lesion was 7 cm by 6.5 cm, with fibrinous, necrotic tissue, purulence, and a violaceous tint at the borders. The patient’s body temperature was 100.5°F (38.1°C) and the white blood cell count was 8.1 x 109/L (reference range 4.0–11.0).
Based on the patient’s medical history, the lesion was initially diagnosed as an infected diabetic ulcer. He was admitted to the hospital and intravenous (IV) vancomycin and clindamycin were started. During this time, the lesion expanded in size, and a second lesion appeared on the right anterior thigh, in similar fashion to how the original lesion had started. The original lesion expanded to 8 cm by 8.5 cm by hospital day 2. The patient continued to have episodes of low-grade fever without leukocytosis.
Cultures of blood and tissue from the lesions were negative, ruling out bacterial infection. Magnetic resonance imaging of the left tibia was negative for osteomyelitis. Punch biopsy of the ulcer border was done on day 3 to evaluate for pyoderma gangrenosum.
On hospital day 5, the patient developed acute kidney injury, with a creatinine increase to 2.17 mg/dL over 24 hours from a baseline value of 0.82 mg/dL. The IV antibiotics were discontinued, and IV fluid hydration was started. At this time, diabetic ulcer secondary to infection and osteomyelitis were ruled out. The lesions were diagnosed as pyoderma gangrenosum.
The patient was started on prednisone 30 mg twice daily. After 2 days, the low-grade fevers resolved, both lesions began to heal, and his creatinine level returned to baseline (Figure 2). He was discharged on hospital day 10. The prednisone was tapered over 1 month, with wet-to-dry dressing changes for wound care.
After discharge, he remained adherent to his steroid regimen. At a follow-up visit to his dermatologist, the ulcers had fully closed, and the skin had begun to heal. Results of the punch biopsy study came back 2 days after the patient was discharged and further confirmed the diagnosis, with a mixed lymphocytic composition composed primarily of neutrophils.
APPROACH TO DIAGNOSIS
Pyoderma gangrenosum is rare, with an incidence of 3 to 10 cases per million people per year.1 It is a rapidly progressive ulcerative condition typically associated with inflammatory bowel disease.2 Despite its name, the condition involves neither gangrene nor infection. The ulcer typically appears on the legs and is rapidly growing, painful, and purulent, with tissue necrosis and a violaceous border.3
Pyoderma gangrenosum is often misdiagnosed as infective ulcer and inappropriately treated with antibiotics.2 It can also be mistreated with surgical debridement, which can result in severe complications such as pathergy.1
The differential diagnosis includes diabetic ulcer, peripheral vascular disease, vasculitis, bacterial infection, osteomyelitis, and malignancy. Because it presents as an open, necrotic ulcer, ruling out infection is a top priority.3 However, an initial workup to rule out infection or other conditions can delay diagnosis and treatment,1 and treatment with broad-spectrum antibiotics poses the risk of nephrotoxicity and new complications during the hospital stay.
Diagnosis requires meeting 2 major criteria—ie, presence of the characteristic ulcerous lesion, and exclusion of other causes of skin ulceration—and at least 2 minor criteria including histologic confirmation of neutrophil infiltrate at the ulcer border, the presence of a systemic disease associated with pyoderma gangrenosum, and a rapid response to steroid treatment.4,5
Our patient was at high risk for an infected diabetic ulcer. After infection was ruled out, clinical suspicion for pyoderma gangrenosum was high, given the patient’s presentation and his history of ulcerative colitis.
TREATMENT
Treatment of pyoderma gangrenosum begins with systemic corticosteroids, as was done in this patient. Additional measures depend on whether the disease is localized or extensive and can include wound care, topical treatments, immunosuppressants, and immunomodulators.1
- Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J 2012; 3(1):7–13. doi:10.4103/2229-5178.93482
- Marinopoulos S, Theofanakis C, Zacharouli T, Sotiropoulou M, Dimitrakakis C. Pyoderma gangrenosum of the breast: a case report study. Int J Surg Case Rep 2017; 31:203–205. doi:10.1016/j.ijscr.2017.01.036
- Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015; 8:285–293. doi:10.2147/CCID.S61202
- Su WP, David MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137(6):1000–1005. pmid:9470924
A 55-year-old man with type 2 diabetes mellitus, hypertension, anemia, and ulcerative colitis presented to the emergency department with an ulcer on his left leg (Figure 1). He said the lesion had started as a “large pimple” that ruptured one night while he was sleeping and then became drastically worse over the past week. He said the lesion was painful and was “oozing blood.”
On examination, the lesion was 7 cm by 6.5 cm, with fibrinous, necrotic tissue, purulence, and a violaceous tint at the borders. The patient’s body temperature was 100.5°F (38.1°C) and the white blood cell count was 8.1 x 109/L (reference range 4.0–11.0).
Based on the patient’s medical history, the lesion was initially diagnosed as an infected diabetic ulcer. He was admitted to the hospital and intravenous (IV) vancomycin and clindamycin were started. During this time, the lesion expanded in size, and a second lesion appeared on the right anterior thigh, in similar fashion to how the original lesion had started. The original lesion expanded to 8 cm by 8.5 cm by hospital day 2. The patient continued to have episodes of low-grade fever without leukocytosis.
Cultures of blood and tissue from the lesions were negative, ruling out bacterial infection. Magnetic resonance imaging of the left tibia was negative for osteomyelitis. Punch biopsy of the ulcer border was done on day 3 to evaluate for pyoderma gangrenosum.
On hospital day 5, the patient developed acute kidney injury, with a creatinine increase to 2.17 mg/dL over 24 hours from a baseline value of 0.82 mg/dL. The IV antibiotics were discontinued, and IV fluid hydration was started. At this time, diabetic ulcer secondary to infection and osteomyelitis were ruled out. The lesions were diagnosed as pyoderma gangrenosum.
The patient was started on prednisone 30 mg twice daily. After 2 days, the low-grade fevers resolved, both lesions began to heal, and his creatinine level returned to baseline (Figure 2). He was discharged on hospital day 10. The prednisone was tapered over 1 month, with wet-to-dry dressing changes for wound care.
After discharge, he remained adherent to his steroid regimen. At a follow-up visit to his dermatologist, the ulcers had fully closed, and the skin had begun to heal. Results of the punch biopsy study came back 2 days after the patient was discharged and further confirmed the diagnosis, with a mixed lymphocytic composition composed primarily of neutrophils.
APPROACH TO DIAGNOSIS
Pyoderma gangrenosum is rare, with an incidence of 3 to 10 cases per million people per year.1 It is a rapidly progressive ulcerative condition typically associated with inflammatory bowel disease.2 Despite its name, the condition involves neither gangrene nor infection. The ulcer typically appears on the legs and is rapidly growing, painful, and purulent, with tissue necrosis and a violaceous border.3
Pyoderma gangrenosum is often misdiagnosed as infective ulcer and inappropriately treated with antibiotics.2 It can also be mistreated with surgical debridement, which can result in severe complications such as pathergy.1
The differential diagnosis includes diabetic ulcer, peripheral vascular disease, vasculitis, bacterial infection, osteomyelitis, and malignancy. Because it presents as an open, necrotic ulcer, ruling out infection is a top priority.3 However, an initial workup to rule out infection or other conditions can delay diagnosis and treatment,1 and treatment with broad-spectrum antibiotics poses the risk of nephrotoxicity and new complications during the hospital stay.
Diagnosis requires meeting 2 major criteria—ie, presence of the characteristic ulcerous lesion, and exclusion of other causes of skin ulceration—and at least 2 minor criteria including histologic confirmation of neutrophil infiltrate at the ulcer border, the presence of a systemic disease associated with pyoderma gangrenosum, and a rapid response to steroid treatment.4,5
Our patient was at high risk for an infected diabetic ulcer. After infection was ruled out, clinical suspicion for pyoderma gangrenosum was high, given the patient’s presentation and his history of ulcerative colitis.
TREATMENT
Treatment of pyoderma gangrenosum begins with systemic corticosteroids, as was done in this patient. Additional measures depend on whether the disease is localized or extensive and can include wound care, topical treatments, immunosuppressants, and immunomodulators.1
A 55-year-old man with type 2 diabetes mellitus, hypertension, anemia, and ulcerative colitis presented to the emergency department with an ulcer on his left leg (Figure 1). He said the lesion had started as a “large pimple” that ruptured one night while he was sleeping and then became drastically worse over the past week. He said the lesion was painful and was “oozing blood.”
On examination, the lesion was 7 cm by 6.5 cm, with fibrinous, necrotic tissue, purulence, and a violaceous tint at the borders. The patient’s body temperature was 100.5°F (38.1°C) and the white blood cell count was 8.1 x 109/L (reference range 4.0–11.0).
Based on the patient’s medical history, the lesion was initially diagnosed as an infected diabetic ulcer. He was admitted to the hospital and intravenous (IV) vancomycin and clindamycin were started. During this time, the lesion expanded in size, and a second lesion appeared on the right anterior thigh, in similar fashion to how the original lesion had started. The original lesion expanded to 8 cm by 8.5 cm by hospital day 2. The patient continued to have episodes of low-grade fever without leukocytosis.
Cultures of blood and tissue from the lesions were negative, ruling out bacterial infection. Magnetic resonance imaging of the left tibia was negative for osteomyelitis. Punch biopsy of the ulcer border was done on day 3 to evaluate for pyoderma gangrenosum.
On hospital day 5, the patient developed acute kidney injury, with a creatinine increase to 2.17 mg/dL over 24 hours from a baseline value of 0.82 mg/dL. The IV antibiotics were discontinued, and IV fluid hydration was started. At this time, diabetic ulcer secondary to infection and osteomyelitis were ruled out. The lesions were diagnosed as pyoderma gangrenosum.
The patient was started on prednisone 30 mg twice daily. After 2 days, the low-grade fevers resolved, both lesions began to heal, and his creatinine level returned to baseline (Figure 2). He was discharged on hospital day 10. The prednisone was tapered over 1 month, with wet-to-dry dressing changes for wound care.
After discharge, he remained adherent to his steroid regimen. At a follow-up visit to his dermatologist, the ulcers had fully closed, and the skin had begun to heal. Results of the punch biopsy study came back 2 days after the patient was discharged and further confirmed the diagnosis, with a mixed lymphocytic composition composed primarily of neutrophils.
APPROACH TO DIAGNOSIS
Pyoderma gangrenosum is rare, with an incidence of 3 to 10 cases per million people per year.1 It is a rapidly progressive ulcerative condition typically associated with inflammatory bowel disease.2 Despite its name, the condition involves neither gangrene nor infection. The ulcer typically appears on the legs and is rapidly growing, painful, and purulent, with tissue necrosis and a violaceous border.3
Pyoderma gangrenosum is often misdiagnosed as infective ulcer and inappropriately treated with antibiotics.2 It can also be mistreated with surgical debridement, which can result in severe complications such as pathergy.1
The differential diagnosis includes diabetic ulcer, peripheral vascular disease, vasculitis, bacterial infection, osteomyelitis, and malignancy. Because it presents as an open, necrotic ulcer, ruling out infection is a top priority.3 However, an initial workup to rule out infection or other conditions can delay diagnosis and treatment,1 and treatment with broad-spectrum antibiotics poses the risk of nephrotoxicity and new complications during the hospital stay.
Diagnosis requires meeting 2 major criteria—ie, presence of the characteristic ulcerous lesion, and exclusion of other causes of skin ulceration—and at least 2 minor criteria including histologic confirmation of neutrophil infiltrate at the ulcer border, the presence of a systemic disease associated with pyoderma gangrenosum, and a rapid response to steroid treatment.4,5
Our patient was at high risk for an infected diabetic ulcer. After infection was ruled out, clinical suspicion for pyoderma gangrenosum was high, given the patient’s presentation and his history of ulcerative colitis.
TREATMENT
Treatment of pyoderma gangrenosum begins with systemic corticosteroids, as was done in this patient. Additional measures depend on whether the disease is localized or extensive and can include wound care, topical treatments, immunosuppressants, and immunomodulators.1
- Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J 2012; 3(1):7–13. doi:10.4103/2229-5178.93482
- Marinopoulos S, Theofanakis C, Zacharouli T, Sotiropoulou M, Dimitrakakis C. Pyoderma gangrenosum of the breast: a case report study. Int J Surg Case Rep 2017; 31:203–205. doi:10.1016/j.ijscr.2017.01.036
- Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015; 8:285–293. doi:10.2147/CCID.S61202
- Su WP, David MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137(6):1000–1005. pmid:9470924
- Bhat RM. Pyoderma gangrenosum: an update. Indian Dermatol Online J 2012; 3(1):7–13. doi:10.4103/2229-5178.93482
- Marinopoulos S, Theofanakis C, Zacharouli T, Sotiropoulou M, Dimitrakakis C. Pyoderma gangrenosum of the breast: a case report study. Int J Surg Case Rep 2017; 31:203–205. doi:10.1016/j.ijscr.2017.01.036
- Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol 2015; 8:285–293. doi:10.2147/CCID.S61202
- Su WP, David MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol 2004; 43(11):790–800. doi:10.1111/j.1365-4632.2004.02128.x
- von den Driesch P. Pyoderma gangrenosum: a report of 44 cases with follow-up. Br J Dermatol 1997; 137(6):1000–1005. pmid:9470924
‘Mammogram of the heart’: Inside coronary artery calcium scores
LOS ANGELES – according to a cardiologist who urged that endocrinologists embrace the tests when appropriate and use them to inform treatment decisions.
In the big picture, “you might want to think of this as the mammogram of the heart,” said Matthew J. Budoff, MD, professor of medicine at the University of California, Los Angeles, in a presentation at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
“If we find a lot of plaque, we act on it,” Dr. Budoff said. “If we don’t, we reassure [patients] and test them down the road.”
According to Dr. Budoff, research confirms that the tests correlate with plaque progression and atherosclerotic burden and offer important insight into treatment decisions for diabetes. “Not all people with diabetes have atherosclerosis, and not all deserve the same therapy,” he said.
In other words, not every patient with diabetes needs to be on the same regimen, such as a statin.
Dr. Budoff pointed to recent research that revealed coronary artery calcium (CAC) scores of zero Agatston units are signs of excellent cardiac health in terms of clogged arteries – regardless of whether a patient is diabetic or not.
“Even patients with a score of zero in the setting of diabetes do very well,” said Dr. Budoff, who normally wouldn’t recommend a statin for those patients even though they have diabetes. “If you see a person without coronary calcium, their cardiovascular death rate is really, really low. Maybe you don’t have to be as aggressive with atherosclerosis. You can wait 5 years after a score of zero and reassess the risk.”
And this advice holds up regardless of the gender, age, or ethnicity of a patient.
However, Dr. Budoff cautioned against waiting too long for another assessment. “I don’t think we want to wait 10 years. A lot of things change over a decade: Our blood pressure and LDL cholesterol go up, our triglycerides and [hemoglobin] A1Cs go up – our risk factors progress with age. I’d encourage you to not wait more than 5 years to retest [a patient] to see what’s going on.”
What if a CAC score is higher than zero? A score of more than 100 is a danger signal, Dr. Budoff said. “No matter how you look at the data, a patient with a high score has higher risk of cardiovascular death or dying in general.” This is especially true among women with diabetes for reasons that are not clear.
What to do if a patient’s score is over 100? “Get them on a baby aspirin and on a statin,” he said.
CAC scores lower than 100 are less worrisome in older people and more worrisome in younger people. An age-adjusted score of 5 in a 45-year-old woman, for example, is a cause for concern because any atherosclerosis is a problem at that age.
“If they have some plaque in their coronaries at age 40 or 45, it will grow over time,” he added.
Dr. Budoff offered other insights into CAC and diabetes.
First, based on CAC scores, asymptomatic, middle-aged patients with type 1 diabetes don’t seem to be at higher risk of coronary artery disease than the general population. About 70% of 1,205 patients followed for an average of 11 years had a CAC score of zero, according to findings from a study led by Dr. Budoff (JACC Cardiovasc Imaging. 2019 Mar 8. doi: 10.1016/j.jcmg.2019.01.014).
However, positive scores translate to more risk, and “the higher the score, the higher the risk,” he emphasized.
Second, CAC screening by itself can be a motivator for lifestyle changes in people with diabetes. A randomized, controlled trial reported in 2011 found that patients who were told about their scores improved on several health measures, including blood pressure, cholesterol levels, and weight (J Am Coll Cardiol. 2011 Apr 12;57[15]:1622-32).
“They were [more] willing to take their medicines. They lost weight, and they were better at diet and exercise,” Dr. Budoff said. “Showing them a calcium score and what it means was a big motivation.”
The study also found major reductions in medication and procedure cost among patients who got the CAC results. About half of them had a CAC score of zero, he said, and that means “we’re not going to run them on a treadmill or put them on a statin.”
Dr. Budoff reported receiving grant funding from GE Healthcare.
LOS ANGELES – according to a cardiologist who urged that endocrinologists embrace the tests when appropriate and use them to inform treatment decisions.
In the big picture, “you might want to think of this as the mammogram of the heart,” said Matthew J. Budoff, MD, professor of medicine at the University of California, Los Angeles, in a presentation at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
“If we find a lot of plaque, we act on it,” Dr. Budoff said. “If we don’t, we reassure [patients] and test them down the road.”
According to Dr. Budoff, research confirms that the tests correlate with plaque progression and atherosclerotic burden and offer important insight into treatment decisions for diabetes. “Not all people with diabetes have atherosclerosis, and not all deserve the same therapy,” he said.
In other words, not every patient with diabetes needs to be on the same regimen, such as a statin.
Dr. Budoff pointed to recent research that revealed coronary artery calcium (CAC) scores of zero Agatston units are signs of excellent cardiac health in terms of clogged arteries – regardless of whether a patient is diabetic or not.
“Even patients with a score of zero in the setting of diabetes do very well,” said Dr. Budoff, who normally wouldn’t recommend a statin for those patients even though they have diabetes. “If you see a person without coronary calcium, their cardiovascular death rate is really, really low. Maybe you don’t have to be as aggressive with atherosclerosis. You can wait 5 years after a score of zero and reassess the risk.”
And this advice holds up regardless of the gender, age, or ethnicity of a patient.
However, Dr. Budoff cautioned against waiting too long for another assessment. “I don’t think we want to wait 10 years. A lot of things change over a decade: Our blood pressure and LDL cholesterol go up, our triglycerides and [hemoglobin] A1Cs go up – our risk factors progress with age. I’d encourage you to not wait more than 5 years to retest [a patient] to see what’s going on.”
What if a CAC score is higher than zero? A score of more than 100 is a danger signal, Dr. Budoff said. “No matter how you look at the data, a patient with a high score has higher risk of cardiovascular death or dying in general.” This is especially true among women with diabetes for reasons that are not clear.
What to do if a patient’s score is over 100? “Get them on a baby aspirin and on a statin,” he said.
CAC scores lower than 100 are less worrisome in older people and more worrisome in younger people. An age-adjusted score of 5 in a 45-year-old woman, for example, is a cause for concern because any atherosclerosis is a problem at that age.
“If they have some plaque in their coronaries at age 40 or 45, it will grow over time,” he added.
Dr. Budoff offered other insights into CAC and diabetes.
First, based on CAC scores, asymptomatic, middle-aged patients with type 1 diabetes don’t seem to be at higher risk of coronary artery disease than the general population. About 70% of 1,205 patients followed for an average of 11 years had a CAC score of zero, according to findings from a study led by Dr. Budoff (JACC Cardiovasc Imaging. 2019 Mar 8. doi: 10.1016/j.jcmg.2019.01.014).
However, positive scores translate to more risk, and “the higher the score, the higher the risk,” he emphasized.
Second, CAC screening by itself can be a motivator for lifestyle changes in people with diabetes. A randomized, controlled trial reported in 2011 found that patients who were told about their scores improved on several health measures, including blood pressure, cholesterol levels, and weight (J Am Coll Cardiol. 2011 Apr 12;57[15]:1622-32).
“They were [more] willing to take their medicines. They lost weight, and they were better at diet and exercise,” Dr. Budoff said. “Showing them a calcium score and what it means was a big motivation.”
The study also found major reductions in medication and procedure cost among patients who got the CAC results. About half of them had a CAC score of zero, he said, and that means “we’re not going to run them on a treadmill or put them on a statin.”
Dr. Budoff reported receiving grant funding from GE Healthcare.
LOS ANGELES – according to a cardiologist who urged that endocrinologists embrace the tests when appropriate and use them to inform treatment decisions.
In the big picture, “you might want to think of this as the mammogram of the heart,” said Matthew J. Budoff, MD, professor of medicine at the University of California, Los Angeles, in a presentation at the annual scientific & clinical congress of the American Association of Clinical Endocrinologists.
“If we find a lot of plaque, we act on it,” Dr. Budoff said. “If we don’t, we reassure [patients] and test them down the road.”
According to Dr. Budoff, research confirms that the tests correlate with plaque progression and atherosclerotic burden and offer important insight into treatment decisions for diabetes. “Not all people with diabetes have atherosclerosis, and not all deserve the same therapy,” he said.
In other words, not every patient with diabetes needs to be on the same regimen, such as a statin.
Dr. Budoff pointed to recent research that revealed coronary artery calcium (CAC) scores of zero Agatston units are signs of excellent cardiac health in terms of clogged arteries – regardless of whether a patient is diabetic or not.
“Even patients with a score of zero in the setting of diabetes do very well,” said Dr. Budoff, who normally wouldn’t recommend a statin for those patients even though they have diabetes. “If you see a person without coronary calcium, their cardiovascular death rate is really, really low. Maybe you don’t have to be as aggressive with atherosclerosis. You can wait 5 years after a score of zero and reassess the risk.”
And this advice holds up regardless of the gender, age, or ethnicity of a patient.
However, Dr. Budoff cautioned against waiting too long for another assessment. “I don’t think we want to wait 10 years. A lot of things change over a decade: Our blood pressure and LDL cholesterol go up, our triglycerides and [hemoglobin] A1Cs go up – our risk factors progress with age. I’d encourage you to not wait more than 5 years to retest [a patient] to see what’s going on.”
What if a CAC score is higher than zero? A score of more than 100 is a danger signal, Dr. Budoff said. “No matter how you look at the data, a patient with a high score has higher risk of cardiovascular death or dying in general.” This is especially true among women with diabetes for reasons that are not clear.
What to do if a patient’s score is over 100? “Get them on a baby aspirin and on a statin,” he said.
CAC scores lower than 100 are less worrisome in older people and more worrisome in younger people. An age-adjusted score of 5 in a 45-year-old woman, for example, is a cause for concern because any atherosclerosis is a problem at that age.
“If they have some plaque in their coronaries at age 40 or 45, it will grow over time,” he added.
Dr. Budoff offered other insights into CAC and diabetes.
First, based on CAC scores, asymptomatic, middle-aged patients with type 1 diabetes don’t seem to be at higher risk of coronary artery disease than the general population. About 70% of 1,205 patients followed for an average of 11 years had a CAC score of zero, according to findings from a study led by Dr. Budoff (JACC Cardiovasc Imaging. 2019 Mar 8. doi: 10.1016/j.jcmg.2019.01.014).
However, positive scores translate to more risk, and “the higher the score, the higher the risk,” he emphasized.
Second, CAC screening by itself can be a motivator for lifestyle changes in people with diabetes. A randomized, controlled trial reported in 2011 found that patients who were told about their scores improved on several health measures, including blood pressure, cholesterol levels, and weight (J Am Coll Cardiol. 2011 Apr 12;57[15]:1622-32).
“They were [more] willing to take their medicines. They lost weight, and they were better at diet and exercise,” Dr. Budoff said. “Showing them a calcium score and what it means was a big motivation.”
The study also found major reductions in medication and procedure cost among patients who got the CAC results. About half of them had a CAC score of zero, he said, and that means “we’re not going to run them on a treadmill or put them on a statin.”
Dr. Budoff reported receiving grant funding from GE Healthcare.
REPORTING FROM AACE 2019
No single eating pattern stands out as best for nutritional therapy in diabetes
Although nutrition therapy is pivotal in the management of patients with diabetes or prediabetes, there’s no one correct eating pattern appropriate for all patients, according to a consensus report from an expert panel convened by the American Diabetes Association.
A one-size-fits-all eating plan would be an unrealistic expectation, given the diversity of cultural issues, personal preferences, comorbidities, and other factors that are unique to individual patients with diabetes or prediabetes, according to the report, which was published in Diabetes Care.
Instead, authors of the report outlined nine different eating patterns, along with the evidence supporting their use and their reported benefits in patients with diabetes or prediabetes.
The report reflects a commitment to developing evidence-based guidelines that are “achievable and meet people where they are” to formulate individualized nutrition plans, William T. Cefalu, MD, chief scientific, medical, and mission officer for the ADA, said in a statement.
“The importance of this consensus also lies in the fact it was authored by a group of experts who are extremely knowledgeable about numerous eating patterns, including vegan, vegetarian, and low carb,” Dr. Cefalu added.
The expert panel of 14 individuals included registered dietitians, diabetes educators, endocrinologists, a primary care physician, and a patient advocate who all answered a national call for experts, according to the ADA.
The panel reviewed more than 600 nutrition manuscripts published between 2014 and 2018 to develop the new consensus statement, which updates the ADA 2014 position statement on nutrition therapy for adults with diabetes and has been incorporated into the association’s Standards of Medical Care in Diabetes–2019 supplement as a living standards update.
All adults with type 1 or 2 diabetes should be referred to individualized, diabetes-focused medical nutrition therapy, the panel members wrote in their report.
There is no evidence suggesting an ideal percentage of calories from carbohydrate, protein, and fat in patients with prediabetes or diabetes, so macronutrient distribution should also be individualized, according to the panel.
Likewise, a variety of eating patterns are acceptable for managing diabetes, according to the report, which describes evidence for eating patterns including Mediterranean, vegetarian or vegan, low fat and very low fat, low carbohydrate and very low carbohydrate, and paleo, as well as the Dietary Approaches to Stop Hypertension diet and the Department of Agriculture Dietary Guidelines for Americans.
Not all diets have the same level of evidence, however. For prevention of prediabetes or type 2 diabetes, for example, the most robust research is available for Mediterranean-style, low-fat, and low-carbohydrate eating patterns, the panel said.
Until there’s better comparative evidence between eating patterns, health care providers should concentrate on several key factors common to a number of the eating patterns, such as limiting sugars and refined grains, emphasizing nonstarchy vegetables, and choosing whole foods over processed foods, the experts wrote.
Consensus panel participants reported disclosures with the ADA, the National Institutes of Health, the Academy of Nutrition and Dietetics, the American Medical Group Association, the University of Michigan, Novo Nordisk, Merck, Amgen, Gilead, BOYDSense, Janssen, Sanofi, Pfizer, Sunstar Foundation, New England Dairy and Dairy Farmer, the National Dairy Council, Kowa Company, and dietdoctor.com.
SOURCE: Evert AB et al. Diabetes Care. 2019 Apr 18. doi: 10.2337/dci19-0014.
Although nutrition therapy is pivotal in the management of patients with diabetes or prediabetes, there’s no one correct eating pattern appropriate for all patients, according to a consensus report from an expert panel convened by the American Diabetes Association.
A one-size-fits-all eating plan would be an unrealistic expectation, given the diversity of cultural issues, personal preferences, comorbidities, and other factors that are unique to individual patients with diabetes or prediabetes, according to the report, which was published in Diabetes Care.
Instead, authors of the report outlined nine different eating patterns, along with the evidence supporting their use and their reported benefits in patients with diabetes or prediabetes.
The report reflects a commitment to developing evidence-based guidelines that are “achievable and meet people where they are” to formulate individualized nutrition plans, William T. Cefalu, MD, chief scientific, medical, and mission officer for the ADA, said in a statement.
“The importance of this consensus also lies in the fact it was authored by a group of experts who are extremely knowledgeable about numerous eating patterns, including vegan, vegetarian, and low carb,” Dr. Cefalu added.
The expert panel of 14 individuals included registered dietitians, diabetes educators, endocrinologists, a primary care physician, and a patient advocate who all answered a national call for experts, according to the ADA.
The panel reviewed more than 600 nutrition manuscripts published between 2014 and 2018 to develop the new consensus statement, which updates the ADA 2014 position statement on nutrition therapy for adults with diabetes and has been incorporated into the association’s Standards of Medical Care in Diabetes–2019 supplement as a living standards update.
All adults with type 1 or 2 diabetes should be referred to individualized, diabetes-focused medical nutrition therapy, the panel members wrote in their report.
There is no evidence suggesting an ideal percentage of calories from carbohydrate, protein, and fat in patients with prediabetes or diabetes, so macronutrient distribution should also be individualized, according to the panel.
Likewise, a variety of eating patterns are acceptable for managing diabetes, according to the report, which describes evidence for eating patterns including Mediterranean, vegetarian or vegan, low fat and very low fat, low carbohydrate and very low carbohydrate, and paleo, as well as the Dietary Approaches to Stop Hypertension diet and the Department of Agriculture Dietary Guidelines for Americans.
Not all diets have the same level of evidence, however. For prevention of prediabetes or type 2 diabetes, for example, the most robust research is available for Mediterranean-style, low-fat, and low-carbohydrate eating patterns, the panel said.
Until there’s better comparative evidence between eating patterns, health care providers should concentrate on several key factors common to a number of the eating patterns, such as limiting sugars and refined grains, emphasizing nonstarchy vegetables, and choosing whole foods over processed foods, the experts wrote.
Consensus panel participants reported disclosures with the ADA, the National Institutes of Health, the Academy of Nutrition and Dietetics, the American Medical Group Association, the University of Michigan, Novo Nordisk, Merck, Amgen, Gilead, BOYDSense, Janssen, Sanofi, Pfizer, Sunstar Foundation, New England Dairy and Dairy Farmer, the National Dairy Council, Kowa Company, and dietdoctor.com.
SOURCE: Evert AB et al. Diabetes Care. 2019 Apr 18. doi: 10.2337/dci19-0014.
Although nutrition therapy is pivotal in the management of patients with diabetes or prediabetes, there’s no one correct eating pattern appropriate for all patients, according to a consensus report from an expert panel convened by the American Diabetes Association.
A one-size-fits-all eating plan would be an unrealistic expectation, given the diversity of cultural issues, personal preferences, comorbidities, and other factors that are unique to individual patients with diabetes or prediabetes, according to the report, which was published in Diabetes Care.
Instead, authors of the report outlined nine different eating patterns, along with the evidence supporting their use and their reported benefits in patients with diabetes or prediabetes.
The report reflects a commitment to developing evidence-based guidelines that are “achievable and meet people where they are” to formulate individualized nutrition plans, William T. Cefalu, MD, chief scientific, medical, and mission officer for the ADA, said in a statement.
“The importance of this consensus also lies in the fact it was authored by a group of experts who are extremely knowledgeable about numerous eating patterns, including vegan, vegetarian, and low carb,” Dr. Cefalu added.
The expert panel of 14 individuals included registered dietitians, diabetes educators, endocrinologists, a primary care physician, and a patient advocate who all answered a national call for experts, according to the ADA.
The panel reviewed more than 600 nutrition manuscripts published between 2014 and 2018 to develop the new consensus statement, which updates the ADA 2014 position statement on nutrition therapy for adults with diabetes and has been incorporated into the association’s Standards of Medical Care in Diabetes–2019 supplement as a living standards update.
All adults with type 1 or 2 diabetes should be referred to individualized, diabetes-focused medical nutrition therapy, the panel members wrote in their report.
There is no evidence suggesting an ideal percentage of calories from carbohydrate, protein, and fat in patients with prediabetes or diabetes, so macronutrient distribution should also be individualized, according to the panel.
Likewise, a variety of eating patterns are acceptable for managing diabetes, according to the report, which describes evidence for eating patterns including Mediterranean, vegetarian or vegan, low fat and very low fat, low carbohydrate and very low carbohydrate, and paleo, as well as the Dietary Approaches to Stop Hypertension diet and the Department of Agriculture Dietary Guidelines for Americans.
Not all diets have the same level of evidence, however. For prevention of prediabetes or type 2 diabetes, for example, the most robust research is available for Mediterranean-style, low-fat, and low-carbohydrate eating patterns, the panel said.
Until there’s better comparative evidence between eating patterns, health care providers should concentrate on several key factors common to a number of the eating patterns, such as limiting sugars and refined grains, emphasizing nonstarchy vegetables, and choosing whole foods over processed foods, the experts wrote.
Consensus panel participants reported disclosures with the ADA, the National Institutes of Health, the Academy of Nutrition and Dietetics, the American Medical Group Association, the University of Michigan, Novo Nordisk, Merck, Amgen, Gilead, BOYDSense, Janssen, Sanofi, Pfizer, Sunstar Foundation, New England Dairy and Dairy Farmer, the National Dairy Council, Kowa Company, and dietdoctor.com.
SOURCE: Evert AB et al. Diabetes Care. 2019 Apr 18. doi: 10.2337/dci19-0014.
FROM DIABETES CARE
Patients with higher HbA1c levels face greater risk for diabetic ketoacidosis
LOS ANGELES – results from a registry study have demonstrated.
The findings come from an analysis of the 2016-2017 Type 1 Diabetes Exchange Clinic Registry dataset that researchers, led by Carol Wysham, MD, presented at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
“This study is unique in that we have stratified patients based on A1c, and identified factors and patient characteristics associated with a greater risk of DKA as glycemic control diminishes,” Dr. Wysham, an endocrinologist at MultiCare Rockwood Clinic Diabetes & Endocrinology Center in Spokane, Wash., said in advance of the meeting. “Multiple interrelated factors, such as lower levels of education and household income, relationship status, access to private health insurance, being younger, and smoking, all affect a patient’s ability to properly manage insulin dosing and are associated with a higher risk of DKA. Health care providers should continue to monitor and educate all patients on the risk factors associated with developing DKA, and be vigilant in patients with an A1c of more than 9%.”
Dr. Wysham and her colleagues conducted a cross-sectional analysis of 6,242 patients in the registry. They examined associations between patient characteristics and treatment patterns with the occurrence of a DKA event in three categories of HbA1c: 7% to less than 8%, 8% to less than 9%, and 9% or greater. The researchers used chi-square or Fisher exact tests to compare DKA and non-DKA groups for categorical variables and t tests to compare continuous variables.
Of the 6,242 patients, 43% had an HbA1c from 7% to less than 8% (cohort 1), 31% had an HbA1c of 8% to less than 9% (cohort 2), and 30% had an HbA1c of 9% or greater (cohort 3). In all, 269 patients reported a DKA event. In addition, 1.7% of those in cohort 1 had a DKA episode versus 2.3% of those in cohort 2 and 9.5% of those in cohort 3.
In patients in cohort 1, the researchers observed no significant associations between individual patient demographic, socioeconomic, or treatment patterns and DKA incidence. In patients in cohort 2, race, marital status, insurance coverage, and annual household income were significantly associated with DKA incidence (P less than .01). In patients in cohort 3, DKA incidence was significantly associated with the same patterns as those in cohort 2, with the addition of age, type 1 diabetes duration, sex, education level, body mass index, and insulin delivery method (P less than .01).
On adjusted multivariate analysis, the researchers observed no significant associations between the factors studied and DKA in patients in cohort 2. In patients in cohort 3, only household income, smoking status, body mass index, and insulin delivery method (injection) were associated with DKA.
Dr. Wysham said she was surprised to learn that the patient characteristics and socioeconomic factors associated with DKA in patients with an HbA1c of more than 9% start to become less significant risk factors as patients achieved better glycemic control.
“Also, in the past, insulin pump users tended to have higher rates of DKA, compared with patients taking multiple daily insulin injections,” she said. “That phenomenon no longer seems to apply, and in this dataset, patients with an HbA1c of more than 9% and who were taking multiple daily injections had significantly higher risk of DKA than did pump users. Insulin delivery method was not a contributing factor to DKA risk at all in patients with an HbA1c of less than 9%.”
Dr. Wysham acknowledged certain limitations of the analysis, including the fact that the registry data “are collected from patients treated at diabetes centers of excellence and may not reflect the population as a whole. Data could have been collected from medical records and/or self-reported questionnaires from patients. Self-reported data are subjective and are limited to the patient’s own recollections.”
Dr. Wysham disclosed that she has received honoraria for advising, consulting, and/or speaking from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Dexcom, Novo Nordisk, and Sanofi. She also disclosed having received research funding from Mylan and Novo Nordisk that went to her institution.
LOS ANGELES – results from a registry study have demonstrated.
The findings come from an analysis of the 2016-2017 Type 1 Diabetes Exchange Clinic Registry dataset that researchers, led by Carol Wysham, MD, presented at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
“This study is unique in that we have stratified patients based on A1c, and identified factors and patient characteristics associated with a greater risk of DKA as glycemic control diminishes,” Dr. Wysham, an endocrinologist at MultiCare Rockwood Clinic Diabetes & Endocrinology Center in Spokane, Wash., said in advance of the meeting. “Multiple interrelated factors, such as lower levels of education and household income, relationship status, access to private health insurance, being younger, and smoking, all affect a patient’s ability to properly manage insulin dosing and are associated with a higher risk of DKA. Health care providers should continue to monitor and educate all patients on the risk factors associated with developing DKA, and be vigilant in patients with an A1c of more than 9%.”
Dr. Wysham and her colleagues conducted a cross-sectional analysis of 6,242 patients in the registry. They examined associations between patient characteristics and treatment patterns with the occurrence of a DKA event in three categories of HbA1c: 7% to less than 8%, 8% to less than 9%, and 9% or greater. The researchers used chi-square or Fisher exact tests to compare DKA and non-DKA groups for categorical variables and t tests to compare continuous variables.
Of the 6,242 patients, 43% had an HbA1c from 7% to less than 8% (cohort 1), 31% had an HbA1c of 8% to less than 9% (cohort 2), and 30% had an HbA1c of 9% or greater (cohort 3). In all, 269 patients reported a DKA event. In addition, 1.7% of those in cohort 1 had a DKA episode versus 2.3% of those in cohort 2 and 9.5% of those in cohort 3.
In patients in cohort 1, the researchers observed no significant associations between individual patient demographic, socioeconomic, or treatment patterns and DKA incidence. In patients in cohort 2, race, marital status, insurance coverage, and annual household income were significantly associated with DKA incidence (P less than .01). In patients in cohort 3, DKA incidence was significantly associated with the same patterns as those in cohort 2, with the addition of age, type 1 diabetes duration, sex, education level, body mass index, and insulin delivery method (P less than .01).
On adjusted multivariate analysis, the researchers observed no significant associations between the factors studied and DKA in patients in cohort 2. In patients in cohort 3, only household income, smoking status, body mass index, and insulin delivery method (injection) were associated with DKA.
Dr. Wysham said she was surprised to learn that the patient characteristics and socioeconomic factors associated with DKA in patients with an HbA1c of more than 9% start to become less significant risk factors as patients achieved better glycemic control.
“Also, in the past, insulin pump users tended to have higher rates of DKA, compared with patients taking multiple daily insulin injections,” she said. “That phenomenon no longer seems to apply, and in this dataset, patients with an HbA1c of more than 9% and who were taking multiple daily injections had significantly higher risk of DKA than did pump users. Insulin delivery method was not a contributing factor to DKA risk at all in patients with an HbA1c of less than 9%.”
Dr. Wysham acknowledged certain limitations of the analysis, including the fact that the registry data “are collected from patients treated at diabetes centers of excellence and may not reflect the population as a whole. Data could have been collected from medical records and/or self-reported questionnaires from patients. Self-reported data are subjective and are limited to the patient’s own recollections.”
Dr. Wysham disclosed that she has received honoraria for advising, consulting, and/or speaking from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Dexcom, Novo Nordisk, and Sanofi. She also disclosed having received research funding from Mylan and Novo Nordisk that went to her institution.
LOS ANGELES – results from a registry study have demonstrated.
The findings come from an analysis of the 2016-2017 Type 1 Diabetes Exchange Clinic Registry dataset that researchers, led by Carol Wysham, MD, presented at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
“This study is unique in that we have stratified patients based on A1c, and identified factors and patient characteristics associated with a greater risk of DKA as glycemic control diminishes,” Dr. Wysham, an endocrinologist at MultiCare Rockwood Clinic Diabetes & Endocrinology Center in Spokane, Wash., said in advance of the meeting. “Multiple interrelated factors, such as lower levels of education and household income, relationship status, access to private health insurance, being younger, and smoking, all affect a patient’s ability to properly manage insulin dosing and are associated with a higher risk of DKA. Health care providers should continue to monitor and educate all patients on the risk factors associated with developing DKA, and be vigilant in patients with an A1c of more than 9%.”
Dr. Wysham and her colleagues conducted a cross-sectional analysis of 6,242 patients in the registry. They examined associations between patient characteristics and treatment patterns with the occurrence of a DKA event in three categories of HbA1c: 7% to less than 8%, 8% to less than 9%, and 9% or greater. The researchers used chi-square or Fisher exact tests to compare DKA and non-DKA groups for categorical variables and t tests to compare continuous variables.
Of the 6,242 patients, 43% had an HbA1c from 7% to less than 8% (cohort 1), 31% had an HbA1c of 8% to less than 9% (cohort 2), and 30% had an HbA1c of 9% or greater (cohort 3). In all, 269 patients reported a DKA event. In addition, 1.7% of those in cohort 1 had a DKA episode versus 2.3% of those in cohort 2 and 9.5% of those in cohort 3.
In patients in cohort 1, the researchers observed no significant associations between individual patient demographic, socioeconomic, or treatment patterns and DKA incidence. In patients in cohort 2, race, marital status, insurance coverage, and annual household income were significantly associated with DKA incidence (P less than .01). In patients in cohort 3, DKA incidence was significantly associated with the same patterns as those in cohort 2, with the addition of age, type 1 diabetes duration, sex, education level, body mass index, and insulin delivery method (P less than .01).
On adjusted multivariate analysis, the researchers observed no significant associations between the factors studied and DKA in patients in cohort 2. In patients in cohort 3, only household income, smoking status, body mass index, and insulin delivery method (injection) were associated with DKA.
Dr. Wysham said she was surprised to learn that the patient characteristics and socioeconomic factors associated with DKA in patients with an HbA1c of more than 9% start to become less significant risk factors as patients achieved better glycemic control.
“Also, in the past, insulin pump users tended to have higher rates of DKA, compared with patients taking multiple daily insulin injections,” she said. “That phenomenon no longer seems to apply, and in this dataset, patients with an HbA1c of more than 9% and who were taking multiple daily injections had significantly higher risk of DKA than did pump users. Insulin delivery method was not a contributing factor to DKA risk at all in patients with an HbA1c of less than 9%.”
Dr. Wysham acknowledged certain limitations of the analysis, including the fact that the registry data “are collected from patients treated at diabetes centers of excellence and may not reflect the population as a whole. Data could have been collected from medical records and/or self-reported questionnaires from patients. Self-reported data are subjective and are limited to the patient’s own recollections.”
Dr. Wysham disclosed that she has received honoraria for advising, consulting, and/or speaking from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Dexcom, Novo Nordisk, and Sanofi. She also disclosed having received research funding from Mylan and Novo Nordisk that went to her institution.
REPORTING FROM AACE 2019
Intermittent fasting tied to positive physiological effects
PHILADELPHIA – Intermittent fasting may help improve weight status and metabolic health, but it is very challenging to adhere to and is possibly associated with certain risks, a physician with expertise in obesity and nutrition said during a presentation.
“I do not necessarily recommend intermittent fasting to my patients, but I do have a lot of patients that will come to me asking about intermittent fasting,” said Fatima Cody Stanford, MD, MPH, of Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston, at the annual meeting of the American College of Physicians.
Intermittent fasting, which can take several forms including partial-day fasting, every-other-day fasting, or fasting two days per week, has been associated with positive physiological effects in an increasing number of recent studies, Dr. Stanford said.
Those physiological effects, reported in animals or humans, have included a potentially increased lifespan, decreased mortality related to cancers or cardiovascular disease, an improved insulin sensitivity, and reduced oxidative stress and inflammation, she said.
Additionally, weight loss and improvement in other health indicators, including insulin resistance, have been demonstrated in some studies of intermittent fasting that included normal weight or overweight human subjects.
In one systematic review and meta-analysis, intermittent fasting was found to be comparable with continuous energy restriction in overweight and obese adults for short-term weight loss.
Compared with no treatment, intermittent energy restriction was associated with a 4.14-kg drop in weight (95% confidence interval, 6.30-1.99; P less than or equal to 0.001), according to that meta-analysis.
In patients with type 2 diabetes, 12 months of intermittent energy restriction resulted in glycemic control comparable with continuous energy restriction, according to results of a randomized, 137-patient, noninferiority trial.
On the flip side, intermittent fasting has been associated with possible health risks, including having “a deleterious impact on fertility” and “a negative impact on bone health,” according to Dr. Stanford.
“These are things that I bring up with my patients,” she told her audience.
Lean mass may also be in jeopardy in intermittent fasters, according to authors of one systematic review and meta-analysis of randomized controlled trials published in the International Journal of Obesity.
Those investigators found that lean mass was decreased in intermittent dieters as compared with continuous dieters in the 9 trials they included. The mean difference was –0.86 kg (95% CI, –1.62 to –0.10; P = 0.03).
Even if intermittent fasting is comparable with continuous energy restriction in weight loss, getting to that point may be more difficult because of increased hunger, at least according to researchers in one randomized 1-year trial, Dr. Sanford noted.
Subjective hunger scores were higher at 4.7 for intermittent fasters versus 3.6 for continuous restriction participants (P = 0.002), results of that trial showed.
“It’s very difficult for most of us to sustain this,” Dr. Stanford said.
Dr. Stanford reported no relevant disclosures.
PHILADELPHIA – Intermittent fasting may help improve weight status and metabolic health, but it is very challenging to adhere to and is possibly associated with certain risks, a physician with expertise in obesity and nutrition said during a presentation.
“I do not necessarily recommend intermittent fasting to my patients, but I do have a lot of patients that will come to me asking about intermittent fasting,” said Fatima Cody Stanford, MD, MPH, of Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston, at the annual meeting of the American College of Physicians.
Intermittent fasting, which can take several forms including partial-day fasting, every-other-day fasting, or fasting two days per week, has been associated with positive physiological effects in an increasing number of recent studies, Dr. Stanford said.
Those physiological effects, reported in animals or humans, have included a potentially increased lifespan, decreased mortality related to cancers or cardiovascular disease, an improved insulin sensitivity, and reduced oxidative stress and inflammation, she said.
Additionally, weight loss and improvement in other health indicators, including insulin resistance, have been demonstrated in some studies of intermittent fasting that included normal weight or overweight human subjects.
In one systematic review and meta-analysis, intermittent fasting was found to be comparable with continuous energy restriction in overweight and obese adults for short-term weight loss.
Compared with no treatment, intermittent energy restriction was associated with a 4.14-kg drop in weight (95% confidence interval, 6.30-1.99; P less than or equal to 0.001), according to that meta-analysis.
In patients with type 2 diabetes, 12 months of intermittent energy restriction resulted in glycemic control comparable with continuous energy restriction, according to results of a randomized, 137-patient, noninferiority trial.
On the flip side, intermittent fasting has been associated with possible health risks, including having “a deleterious impact on fertility” and “a negative impact on bone health,” according to Dr. Stanford.
“These are things that I bring up with my patients,” she told her audience.
Lean mass may also be in jeopardy in intermittent fasters, according to authors of one systematic review and meta-analysis of randomized controlled trials published in the International Journal of Obesity.
Those investigators found that lean mass was decreased in intermittent dieters as compared with continuous dieters in the 9 trials they included. The mean difference was –0.86 kg (95% CI, –1.62 to –0.10; P = 0.03).
Even if intermittent fasting is comparable with continuous energy restriction in weight loss, getting to that point may be more difficult because of increased hunger, at least according to researchers in one randomized 1-year trial, Dr. Sanford noted.
Subjective hunger scores were higher at 4.7 for intermittent fasters versus 3.6 for continuous restriction participants (P = 0.002), results of that trial showed.
“It’s very difficult for most of us to sustain this,” Dr. Stanford said.
Dr. Stanford reported no relevant disclosures.
PHILADELPHIA – Intermittent fasting may help improve weight status and metabolic health, but it is very challenging to adhere to and is possibly associated with certain risks, a physician with expertise in obesity and nutrition said during a presentation.
“I do not necessarily recommend intermittent fasting to my patients, but I do have a lot of patients that will come to me asking about intermittent fasting,” said Fatima Cody Stanford, MD, MPH, of Massachusetts General Hospital Weight Center and Harvard Medical School, both in Boston, at the annual meeting of the American College of Physicians.
Intermittent fasting, which can take several forms including partial-day fasting, every-other-day fasting, or fasting two days per week, has been associated with positive physiological effects in an increasing number of recent studies, Dr. Stanford said.
Those physiological effects, reported in animals or humans, have included a potentially increased lifespan, decreased mortality related to cancers or cardiovascular disease, an improved insulin sensitivity, and reduced oxidative stress and inflammation, she said.
Additionally, weight loss and improvement in other health indicators, including insulin resistance, have been demonstrated in some studies of intermittent fasting that included normal weight or overweight human subjects.
In one systematic review and meta-analysis, intermittent fasting was found to be comparable with continuous energy restriction in overweight and obese adults for short-term weight loss.
Compared with no treatment, intermittent energy restriction was associated with a 4.14-kg drop in weight (95% confidence interval, 6.30-1.99; P less than or equal to 0.001), according to that meta-analysis.
In patients with type 2 diabetes, 12 months of intermittent energy restriction resulted in glycemic control comparable with continuous energy restriction, according to results of a randomized, 137-patient, noninferiority trial.
On the flip side, intermittent fasting has been associated with possible health risks, including having “a deleterious impact on fertility” and “a negative impact on bone health,” according to Dr. Stanford.
“These are things that I bring up with my patients,” she told her audience.
Lean mass may also be in jeopardy in intermittent fasters, according to authors of one systematic review and meta-analysis of randomized controlled trials published in the International Journal of Obesity.
Those investigators found that lean mass was decreased in intermittent dieters as compared with continuous dieters in the 9 trials they included. The mean difference was –0.86 kg (95% CI, –1.62 to –0.10; P = 0.03).
Even if intermittent fasting is comparable with continuous energy restriction in weight loss, getting to that point may be more difficult because of increased hunger, at least according to researchers in one randomized 1-year trial, Dr. Sanford noted.
Subjective hunger scores were higher at 4.7 for intermittent fasters versus 3.6 for continuous restriction participants (P = 0.002), results of that trial showed.
“It’s very difficult for most of us to sustain this,” Dr. Stanford said.
Dr. Stanford reported no relevant disclosures.
EXPERT ANALYSIS FROM INTERNAL MEDICINE 2019